
Exhibit 99.1 April 2023 Developing next-generation immunotherapies that address cancer immune resistance KA (Nasdaq)

Disclaimers and other information Cautionary Statements Regarding Forward-Looking Statements This presentation contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. The use of words such as, but not limited to, “believe,” “expect,” “estimate,” “project,” “intend,” “future,” “potential,” “continue,” “may,” “might,” “plan,” “will,” “should,” “seek,” “anticipate,” or “could” and other similar words or expressions are intended to identify forward-looking statements. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based on Kineta’s current beliefs, expectations and assumptions regarding the future of Kineta’s business, future plans and strategies, clinical results and other future conditions. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. No representations or warranties (expressed or implied) are made about the accuracy of any such forward-looking statements. Such forward-looking statements are subject to a number of material risks and uncertainties including, but not limited to: the adequacy of Kineta’s capital to support its future operations and its ability to successfully initiate and complete clinical trials; the difficulty in predicting the time and cost of development of Kineta’s product candidates; Kineta’s plans to research, develop and commercialize its current and future product candidates, including, but not limited to, KVA12123; the timing and anticipated results of Kineta’s planned pre-clinical studies and clinical trials and the risk that the results of Kineta’s pre-clinical studies and clinical trials may not be predictive of future results in connection with future studies or clinical trials; the timing of the availability of data from Kineta’s clinical trials; the timing of any planned investigational new drug application or new drug application; the risk of cessation or delay of any ongoing or planned clinical trials of Kineta or its collaborators; the clinical utility, potential benefits and market acceptance of Kineta’s product candidates; Kineta’s commercialization, marketing and manufacturing capabilities and strategy; developments and projections relating to Kineta’s competitors and its industry; the impact of government laws and regulations; the timing and outcome of Kineta’s planned interactions with regulatory authorities; Kineta’s ability to protect its intellectual property position; Kineta’s estimates regarding future revenue, expenses, capital requirements and need for additional financing; and those risks set forth under the caption “Risk Factors” in Kineta’s most recent Annual Report on Form 10-K, as well as discussions of potential risks, uncertainties and other important factors in Kineta’s subsequent filings with the Securities and Exchange Commission. Any forward-looking statement speaks only as of the date on which it was made. Except as required by law, Kineta undertakes no obligation to publicly update or revise any forward-looking statement, whether as result of new information, future events or otherwise. 2
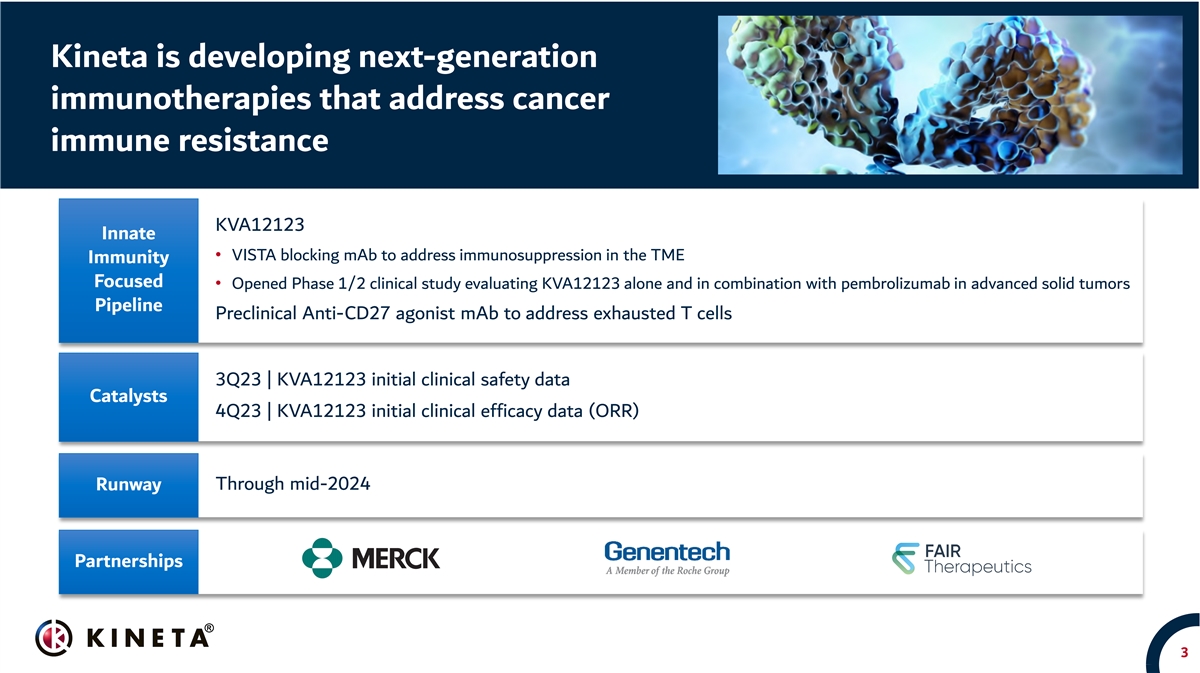
Kineta is developing next-generation immunotherapies that address cancer immune resistance KVA12123 Innate • VISTA blocking mAb to address immunosuppression in the TME Immunity Focused • Opened Phase 1/2 clinical study evaluating KVA12123 alone and in combination with pembrolizumab in advanced solid tumors Pipeline Preclinical Anti-CD27 agonist mAb to address exhausted T cells 3Q23 | KVA12123 initial clinical safety data Catalysts 4Q23 | KVA12123 initial clinical efficacy data (ORR) Through mid-2024 Runway Partnerships 3
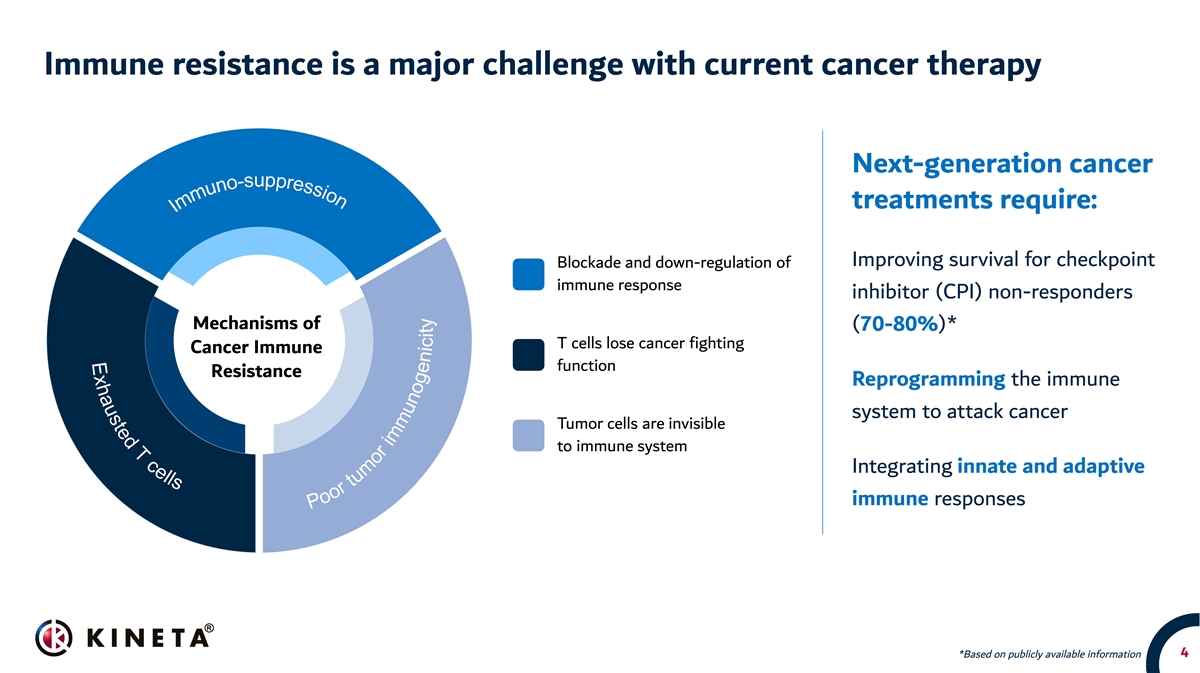
Immune resistance is a major challenge with current cancer therapy Next-generation cancer treatments require: Improving survival for checkpoint Blockade and down-regulation of immune response inhibitor (CPI) non-responders Mechanisms of (70-80%)* T cells lose cancer fighting Cancer Immune function Resistance Reprogramming the immune system to attack cancer Tumor cells are invisible to immune system Integrating innate and adaptive immune responses 4 *Based on publicly available information
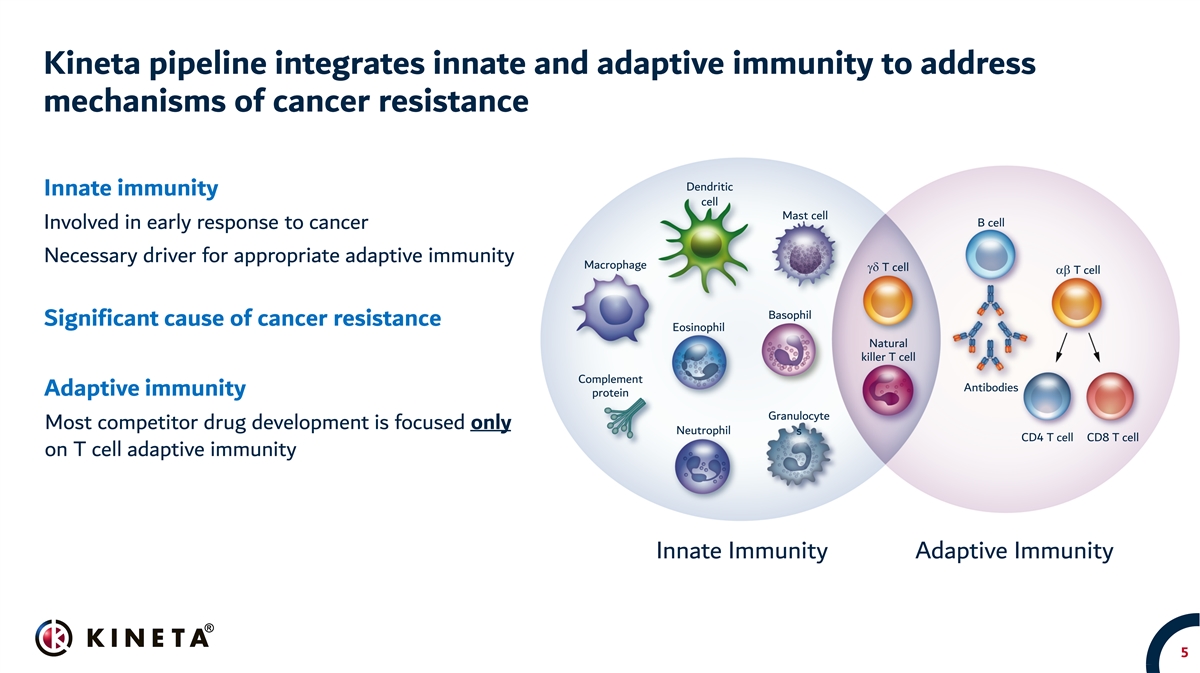
Kineta pipeline integrates innate and adaptive immunity to address mechanisms of cancer resistance Dendritic Innate immunity cell Mast cell B cell Involved in early response to cancer Necessary driver for appropriate adaptive immunity Macrophage gd T cell ab T cell Basophil Significant cause of cancer resistance Eosinophil Natural killer T cell Complement Antibodies Adaptive immunity protein Granulocyte Most competitor drug development is focused only Neutrophil s CD4 T cell CD8 T cell on T cell adaptive immunity Innate Immunity Adaptive Immunity 5
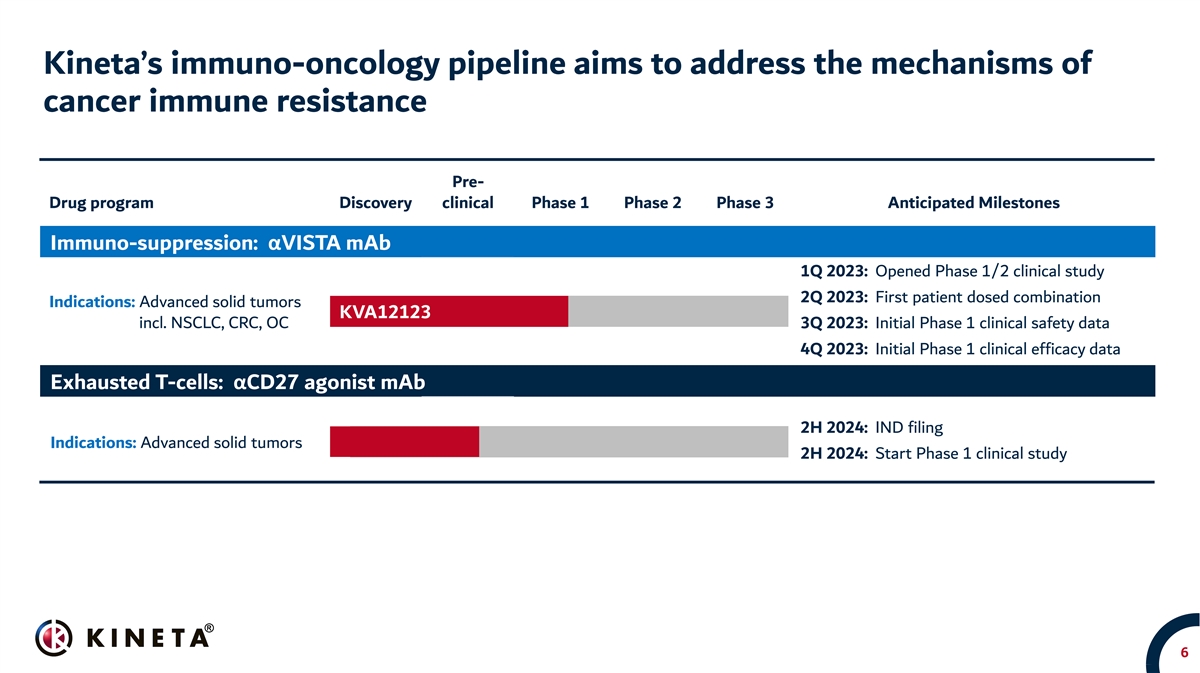
Kineta’s immuno-oncology pipeline aims to address the mechanisms of cancer immune resistance Pre- Drug program Discovery clinical Phase 1 Phase 2 Phase 3 Anticipated Milestones Immuno-suppression: αVISTA mAb 1Q 2023: Opened Phase 1/2 clinical study 2Q 2023: First patient dosed combination Indications: Advanced solid tumors KVA12123 incl. NSCLC, CRC, OC 3Q 2023: Initial Phase 1 clinical safety data 4Q 2023: Initial Phase 1 clinical efficacy data Exhausted T-cells: αCD27 agonist mAb 2H 2024: IND filing Indications: Advanced solid tumors 2H 2024: Start Phase 1 clinical study 6

KVA12123 Potentially differentiated VISTA blocking immunotherapy 7 7
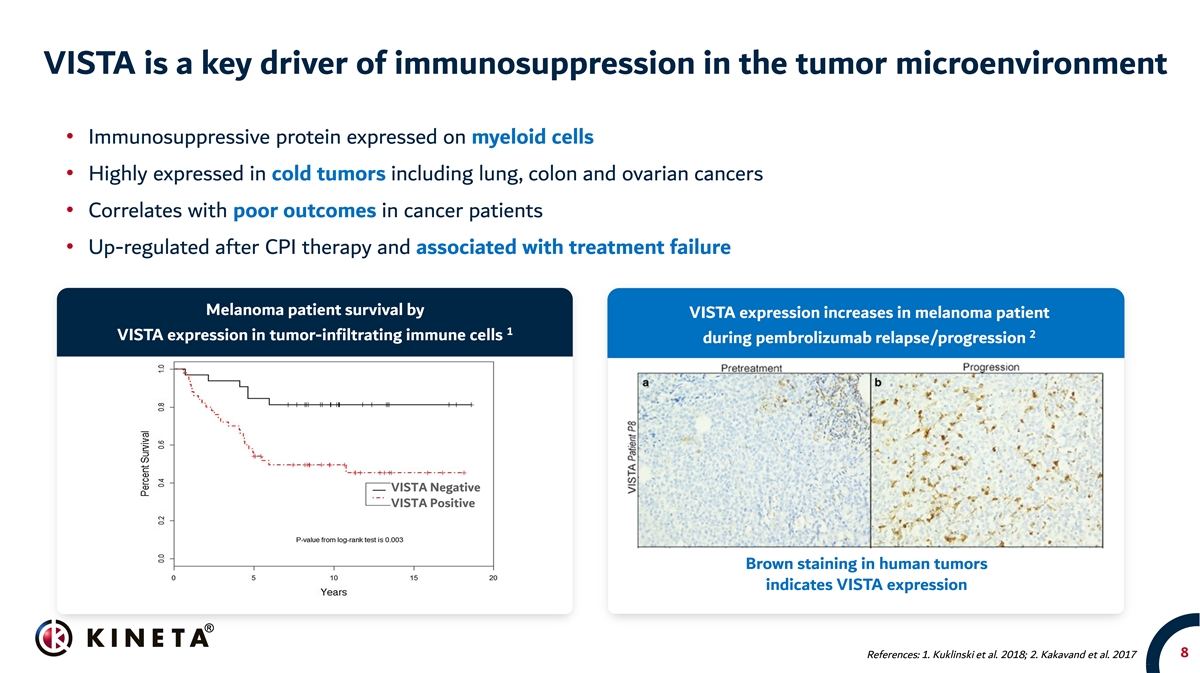
VISTA is a key driver of immunosuppression in the tumor microenvironment • Immunosuppressive protein expressed on myeloid cells • Highly expressed in cold tumors including lung, colon and ovarian cancers • Correlates with poor outcomes in cancer patients • Up-regulated after CPI therapy and associated with treatment failure Melanoma patient survival by VISTA expression increases in melanoma patient 1 2 VISTA expression in tumor-infiltrating immune cells during pembrolizumab relapse/progression VISTA Negative VISTA Positive Brown staining in human tumors indicates VISTA expression 8 References: 1. Kuklinski et al. 2018; 2. Kakavand et al. 2017
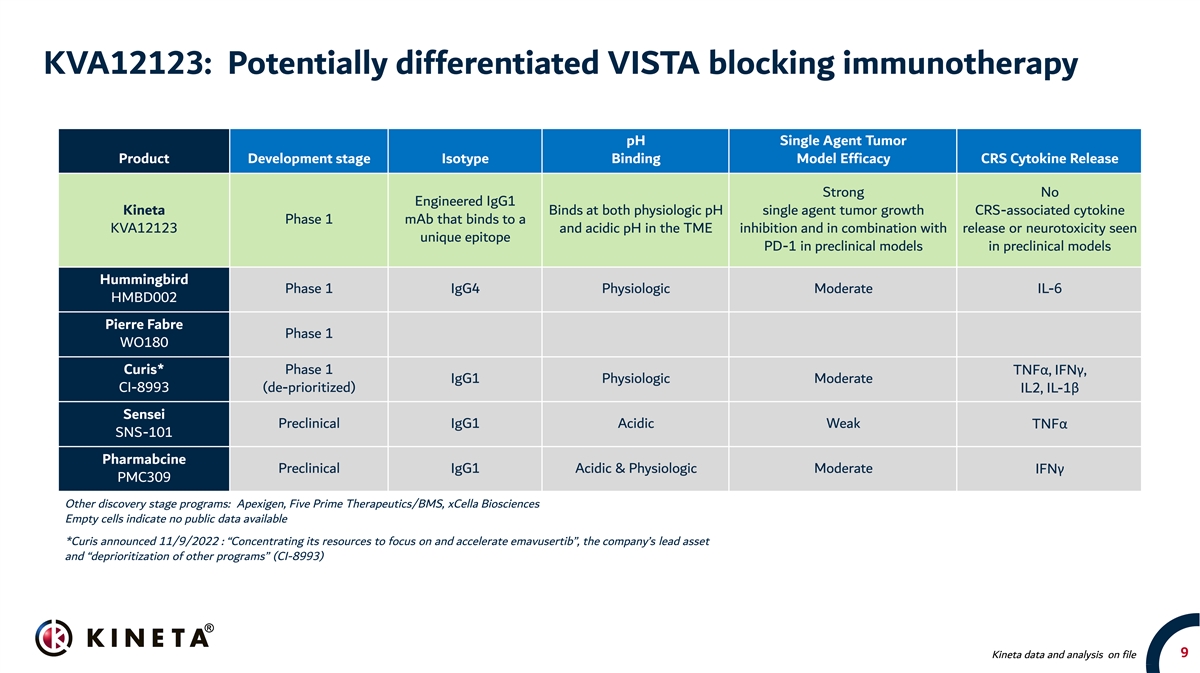
KVA12123: Potentially differentiated VISTA blocking immunotherapy pH Single Agent Tumor Product Development stage Isotype Binding Model Efficacy CRS Cytokine Release Strong No Engineered IgG1 Kineta Binds at both physiologic pH single agent tumor growth CRS-associated cytokine Phase 1 mAb that binds to a KVA12123 and acidic pH in the TME inhibition and in combination with release or neurotoxicity seen unique epitope PD-1 in preclinical models in preclinical models Hummingbird Phase 1 IgG4 Physiologic Moderate IL-6 HMBD002 Pierre Fabre Phase 1 WO180 Curis* Phase 1 TNFα, IFNγ, IgG1 Physiologic Moderate CI-8993 (de-prioritized) IL2, IL-1β Sensei Preclinical IgG1 Acidic Weak TNFα SNS-101 Pharmabcine Preclinical IgG1 Acidic & Physiologic Moderate IFNγ PMC309 Other discovery stage programs: Apexigen, Five Prime Therapeutics/BMS, xCella Biosciences Empty cells indicate no public data available *Curis announced 11/9/2022 : “Concentrating its resources to focus on and accelerate emavusertib”, the company’s lead asset and “deprioritization of other programs” (CI-8993) 9 Kineta data and analysis on file
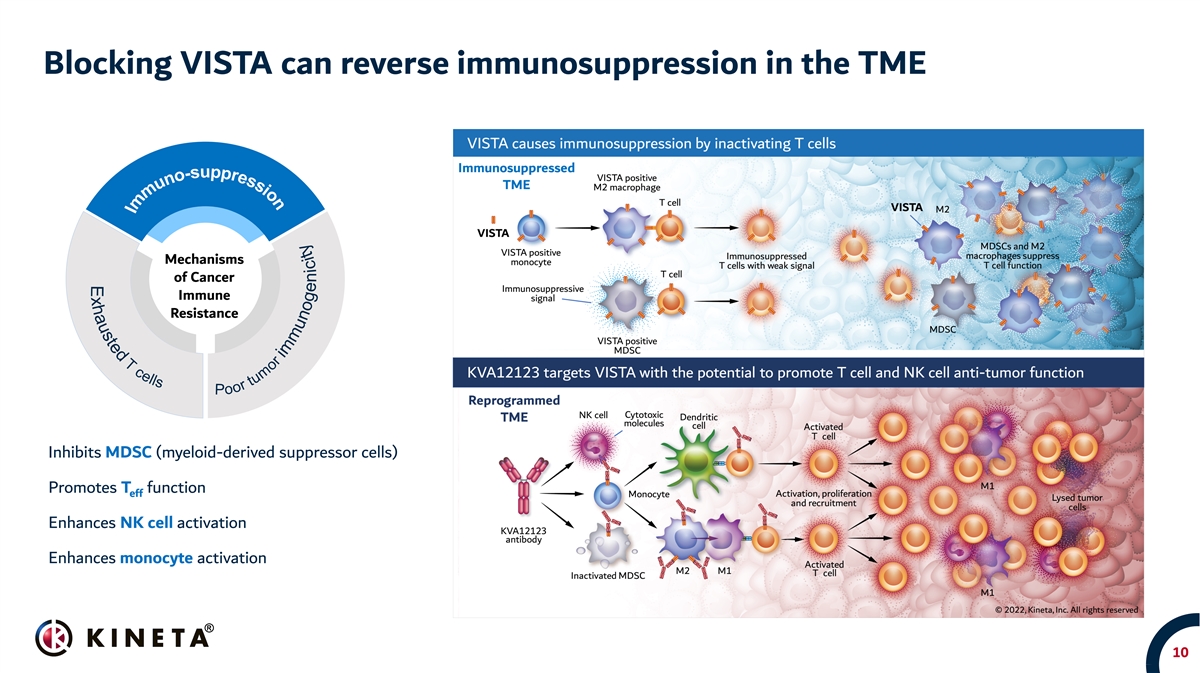
Blocking VISTA can reverse immunosuppression in the TME Mechanisms of Cancer Immune Resistance Inhibits MDSC (myeloid-derived suppressor cells) Promotes T function eff Enhances NK cell activation Enhances monocyte activation 10
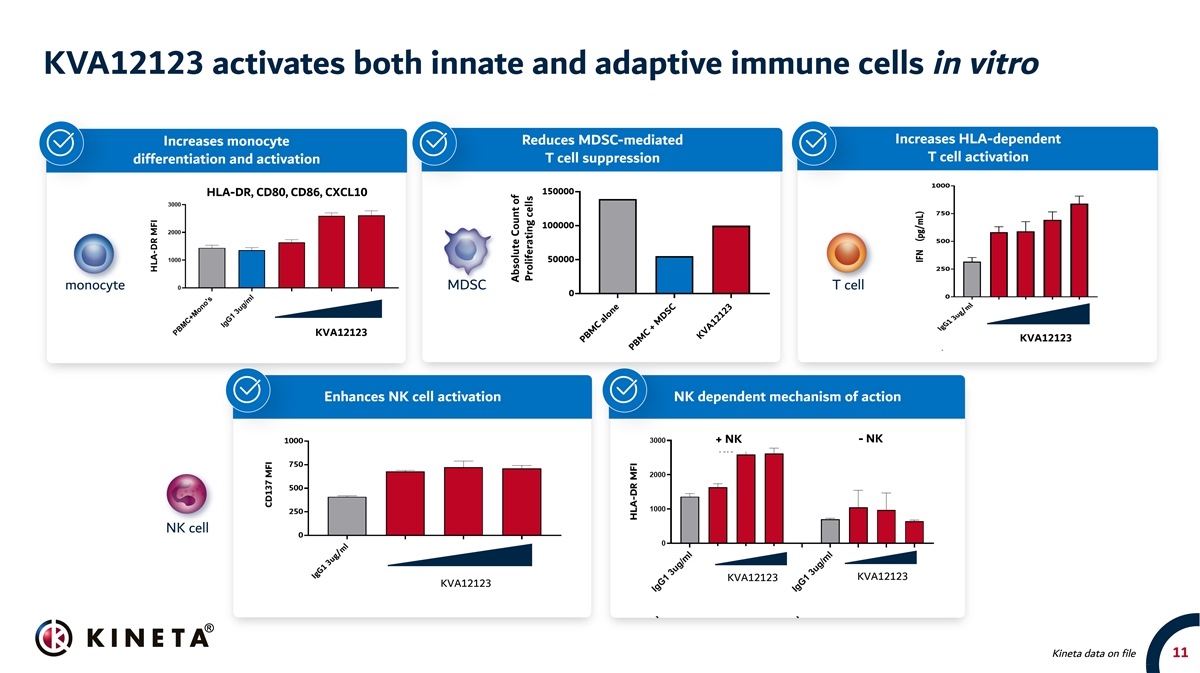
KVA12123 activates both innate and adaptive immune cells in vitro Increases HLA-dependent Reduces MDSC-mediated Increases monocyte T cell activation T cell suppression differentiation and activation HLA-DR, CD80, CD86, CXCL10 monocyte MDSC T cell KVA12123 KVA12123 Enhances NK cell activation NK dependent mechanism of action - NK + NK NK cell KVA12123 KVA12123 KVA12123 Kineta data on file 11
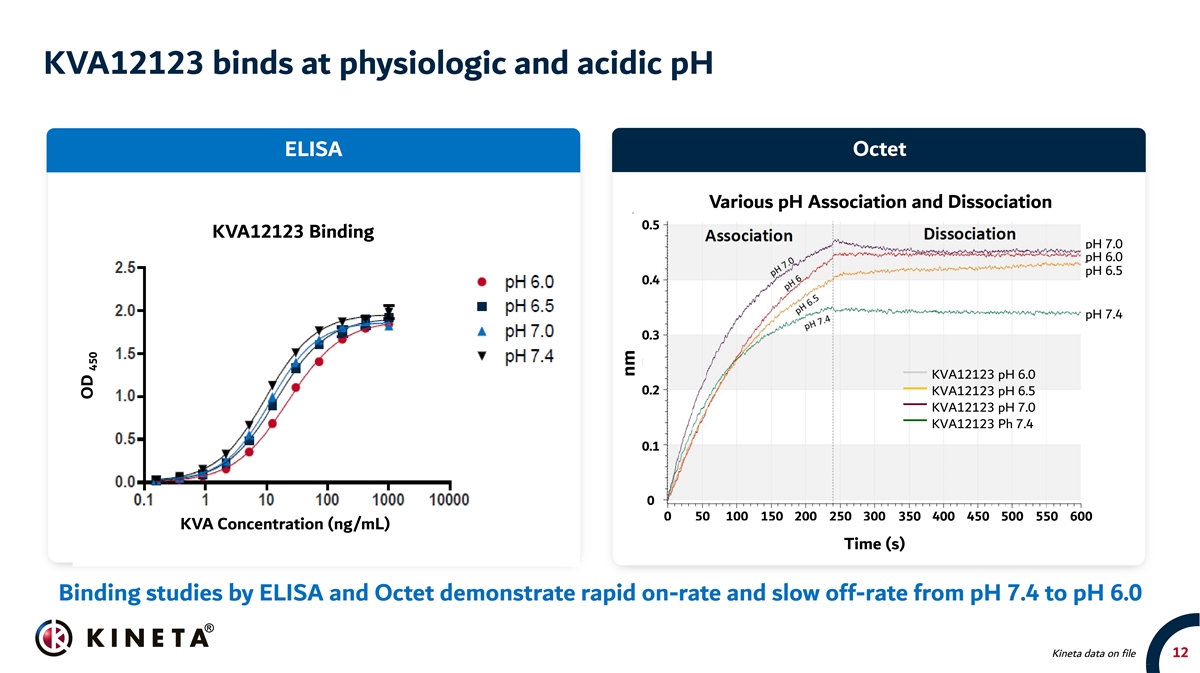
KVA12123 binds at physiologic and acidic pH ELISA Octet Various pH Association and Dissociation 0.5 KVA12123 Binding pH 7.0 pH 6.0 pH 6.5 0.4 pH 7.4 0.3 KVA12123 pH 6.0 0.2 KVA12123 pH 6.5 KVA12123 pH 7.0 KVA12123 Ph 7.4 0.1 0 0 50 100 150 200 250 300 350 400 450 500 550 600 KVA Concentration (ng/mL) Time (s) Binding studies by ELISA and Octet demonstrate rapid on-rate and slow off-rate from pH 7.4 to pH 6.0 Kineta data on file 12 OD 450 nm
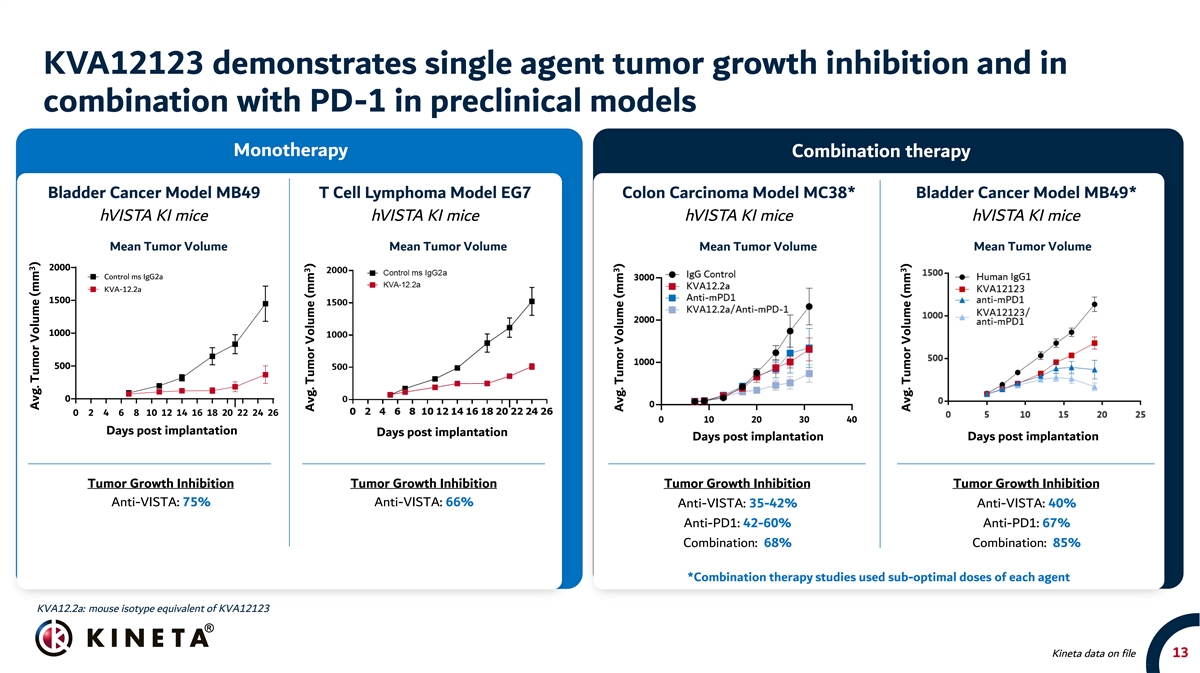
KVA12123 demonstrates single agent tumor growth inhibition and in combination with PD-1 in preclinical models Monotherapy Combination therapy Bladder Cancer Model MB49 T Cell Lymphoma Model EG7 Colon Carcinoma Model MC38* Bladder Cancer Model MB49* hVISTA KI mice hVISTA KI mice hVISTA KI mice hVISTA KI mice Mean Tumor Volume Mean Tumor Volume Mean Tumor Volume Mean Tumor Volume Mean Tumor Volume 2000 Control ms IgG2a KVA-12.2a 1500 1000 500 0 0 2 4 6 8 10 12 14 16 18 20 22 24 26 Days post implantation Days post implantation Days Post Implantation Days post implantation Days post implantation Tumor Growth Inhibition Tumor Growth Inhibition Tumor Growth Inhibition Tumor Growth Inhibition Anti-VISTA: 75% Anti-VISTA: 66% Anti-VISTA: 35-42% Anti-VISTA: 40% Anti-PD1: 42-60% Anti-PD1: 67% Combination: 68% Combination: 85% *Combination therapy studies used sub-optimal doses of each agent KVA12.2a: mouse isotype equivalent of KVA12123 Kineta data on file 13 3 Avg. Tumor Volume (mm 3 ) Avg. Tumor Volume (mm ) 3 Avg. Tumor Volume (mm ) 3 Avg. Tumor Volume (mm ) 3 Avg. Tumor Volume (mm )
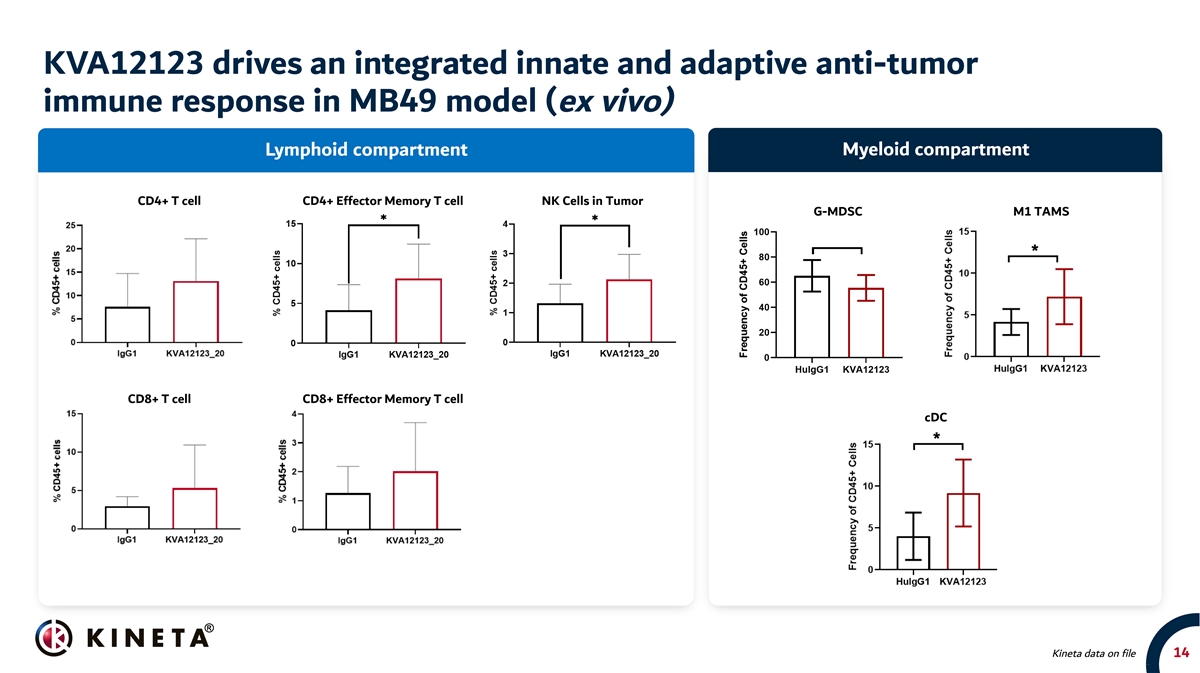
KVA12123 drives an integrated innate and adaptive anti-tumor immune response in MB49 model (ex vivo) Lymphoid compartment Myeloid compartment CD4+ T cell CD4+ Effector Memory T cell NK Cells in Tumor G-MDSC M1 TAMS CD8+ T cell CD8+ Effector Memory T cell cDC Kineta data on file 14
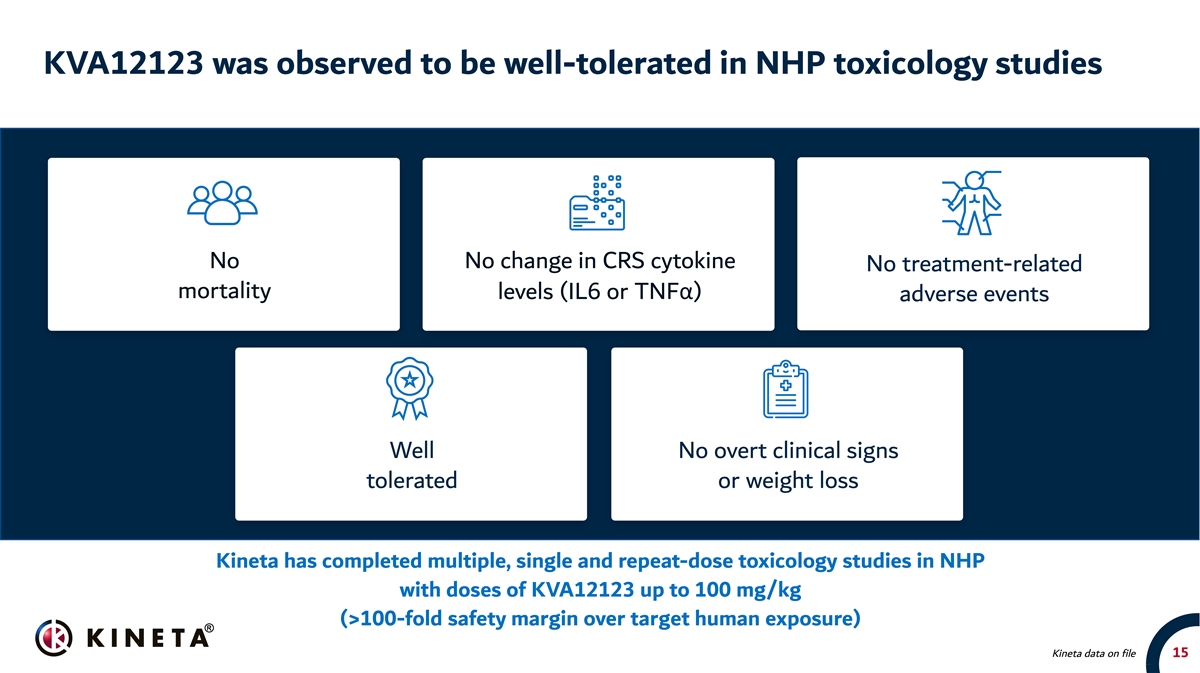
KVA12123 was observed to be well-tolerated in NHP toxicology studies No No change in CRS cytokine No treatment-related mortality levels (IL6 or TNFα) adverse events Well No overt clinical signs tolerated or weight loss Kineta has completed multiple, single and repeat-dose toxicology studies in NHP with doses of KVA12123 up to 100 mg/kg (>100-fold safety margin over target human exposure) Kineta data on file 15
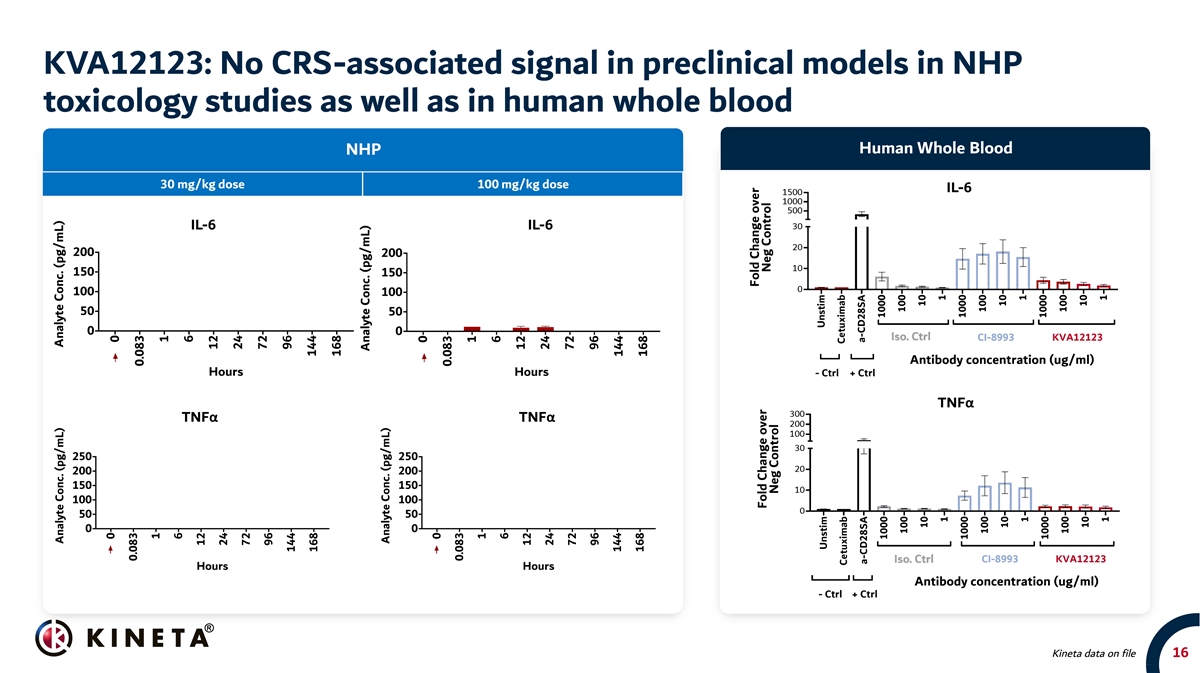
KVA12123: No CRS-associated signal in preclinical models in NHP toxicology studies as well as in human whole blood Human Whole Blood NHP 30 mg/kg dose 100 mg/kg dose IL-6 IL-6 IL-6 TNFα TNFα TNFα Kineta data on file 16
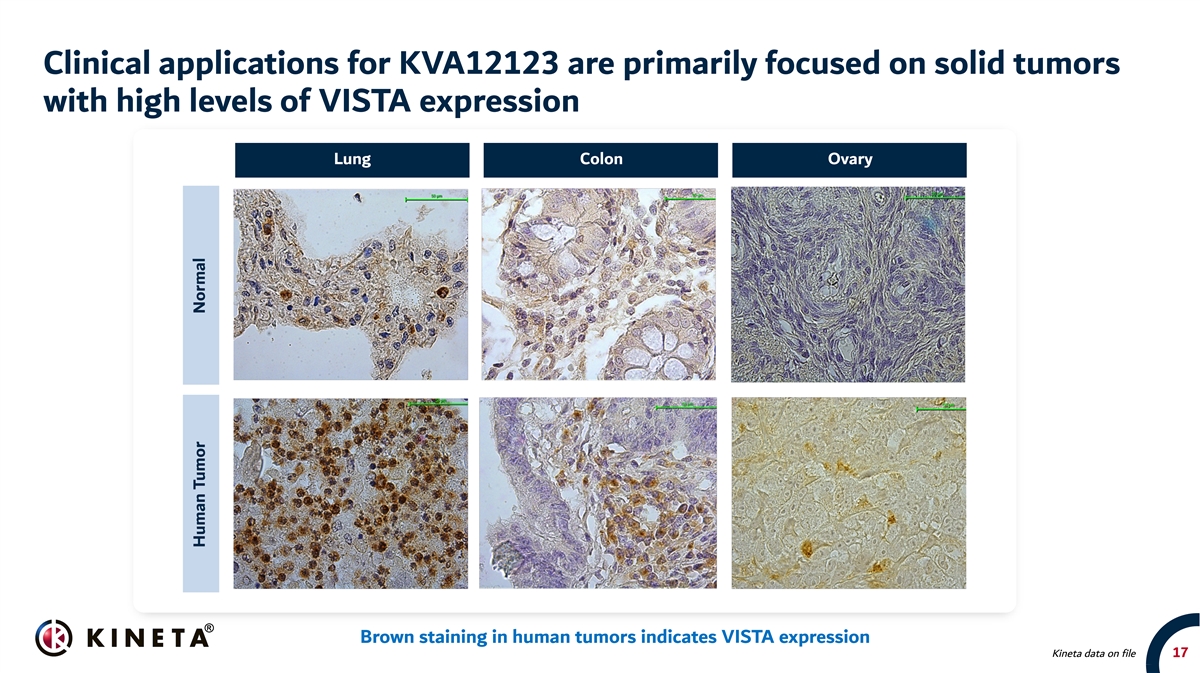
Clinical applications for KVA12123 are primarily focused on solid tumors with high levels of VISTA expression Lung Colon Ovary 20x 20x 20x Brown staining in human tumors indicates VISTA expression Kineta data on file 17 Human Tumor Normal

Phase 1 / 2 open-label clinical trial of KVA12123 alone and in combination with pembrolizumab in patients with advanced solid tumors Patient population: • Phase 1 basket trial in patients with advanced solid tumors (up to 60 patients) • Phase 2 in NSCLC, HNSCC, OC, CRC, RCC and TBD other patients Study objectives: • Primary: Safety and tolerability, recommended Phase 2 dose (RP2D) or maximum tolerated dose (MTD) of KVA12123 • Secondary: Pharmacokinetics, immunogenicity, tumor response in subjects with advanced solid tumors per iRECIST (ORR) • Exploratory: Biomarker and receptor occupancy 18
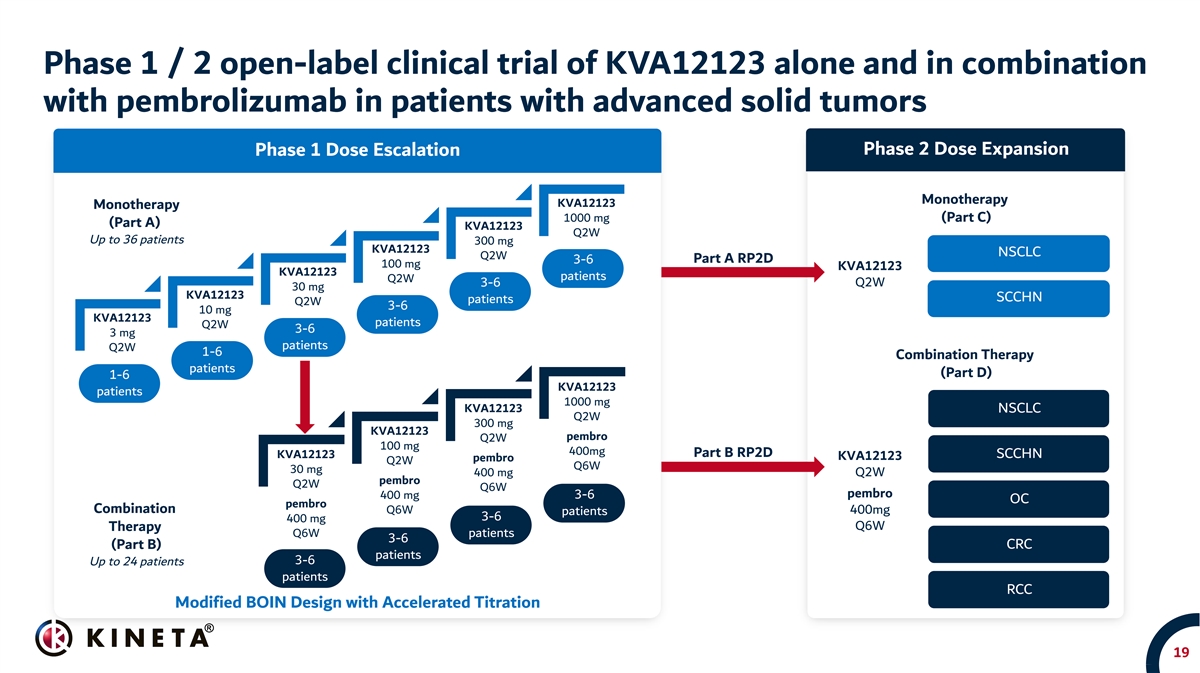
Phase 1 / 2 open-label clinical trial of KVA12123 alone and in combination with pembrolizumab in patients with advanced solid tumors Phase 2 Dose Expansion Phase 1 Dose Escalation Monotherapy KVA12123 Monotherapy 1000 mg (Part C) (Part A) KVA12123 Q2W Up to 36 patients 300 mg KVA12123 NSCLC Q2W 3-6 Part A RP2D 100 mg KVA12123 KVA12123 patients Q2W 3-6 Q2W 30 mg KVA12123 SCCHN patients Q2W 3-6 10 mg KVA12123 patients Q2W 3-6 3 mg patients Q2W 1-6 Combination Therapy patients (Part D) 1-6 KVA12123 patients 1000 mg KVA12123 NSCLC Q2W 300 mg KVA12123 pembro Q2W 100 mg 400mg Part B RP2D KVA12123 SCCHN KVA12123 pembro Q2W Q6W 30 mg 400 mg Q2W pembro Q2W Q6W 3-6 pembro 400 mg OC pembro Combination Q6W 400mg patients 3-6 400 mg Therapy Q6W Q6W patients 3-6 (Part B) CRC patients 3-6 Up to 24 patients patients RCC Modified BOIN Design with Accelerated Titration 19
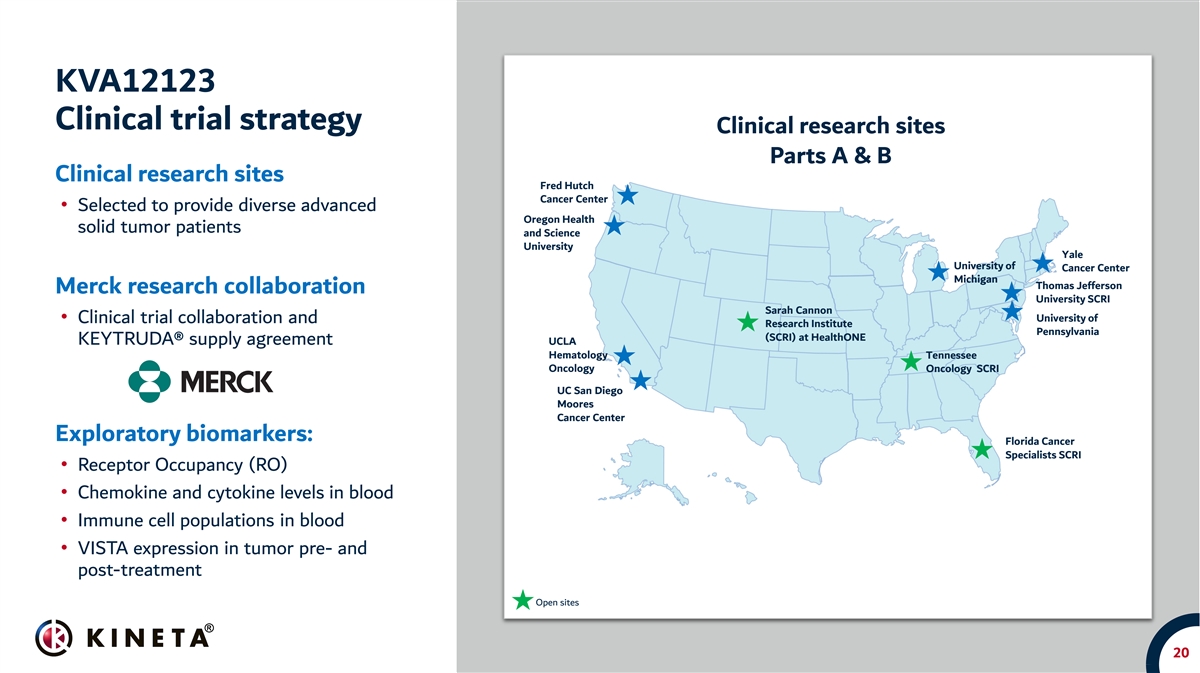
KVA12123 Clinical trial strategy Clinical research sites Parts A & B Clinical research sites Fred Hutch Cancer Center • Selected to provide diverse advanced Oregon Health solid tumor patients and Science University Yale University of Cancer Center Michigan Thomas Jefferson Merck research collaboration University SCRI Sarah Cannon University of • Clinical trial collaboration and Research Institute Pennsylvania (SCRI) at HealthONE KEYTRUDA® supply agreement UCLA Hematology Tennessee Oncology Oncology SCRI UC San Diego Moores Cancer Center Exploratory biomarkers: Florida Cancer Specialists SCRI • Receptor Occupancy (RO) • Chemokine and cytokine levels in blood • Immune cell populations in blood • VISTA expression in tumor pre- and post-treatment Open sites 20
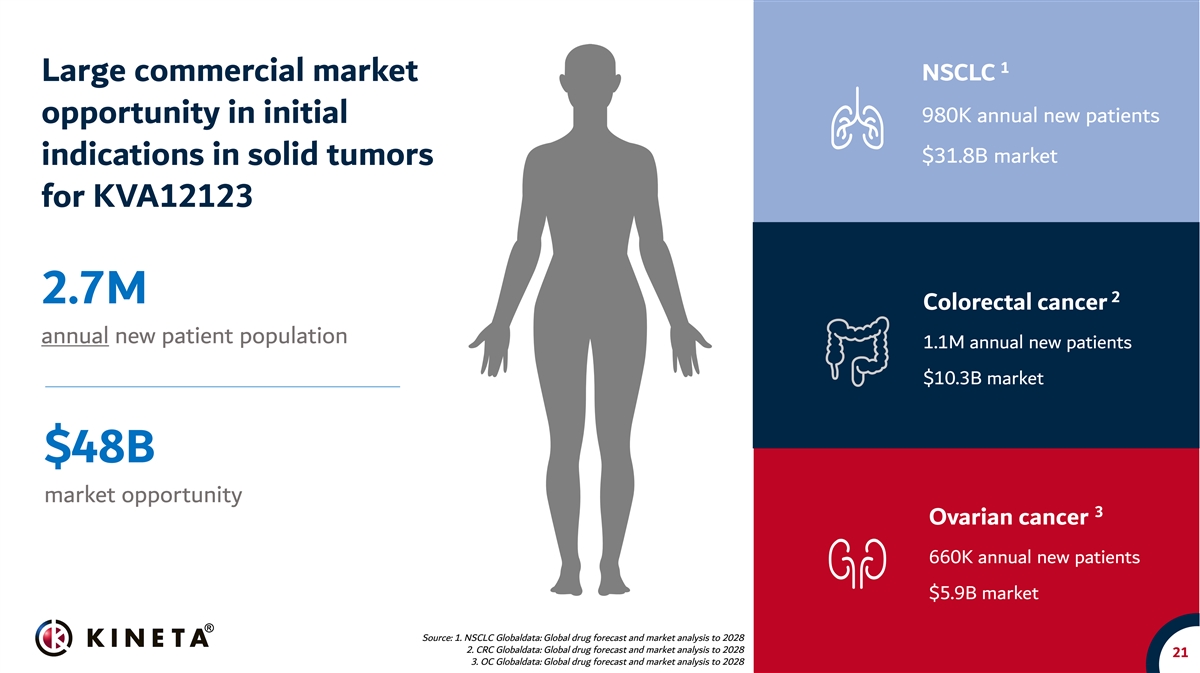
1 Large commercial market NSCLC 980K annual new patients opportunity in initial $31.8B market indications in solid tumors for KVA12123 2 2.7M Colorectal cancer annual new patient population 1.1M annual new patients $10.3B market $48B market opportunity 3 Ovarian cancer 660K annual new patients $5.9B market Source: 1. NSCLC Globaldata: Global drug forecast and market analysis to 2028 2. CRC Globaldata: Global drug forecast and market analysis to 2028 21 3. OC Globaldata: Global drug forecast and market analysis to 2028

Anti-CD27 agonist mAb immunotherapy 22
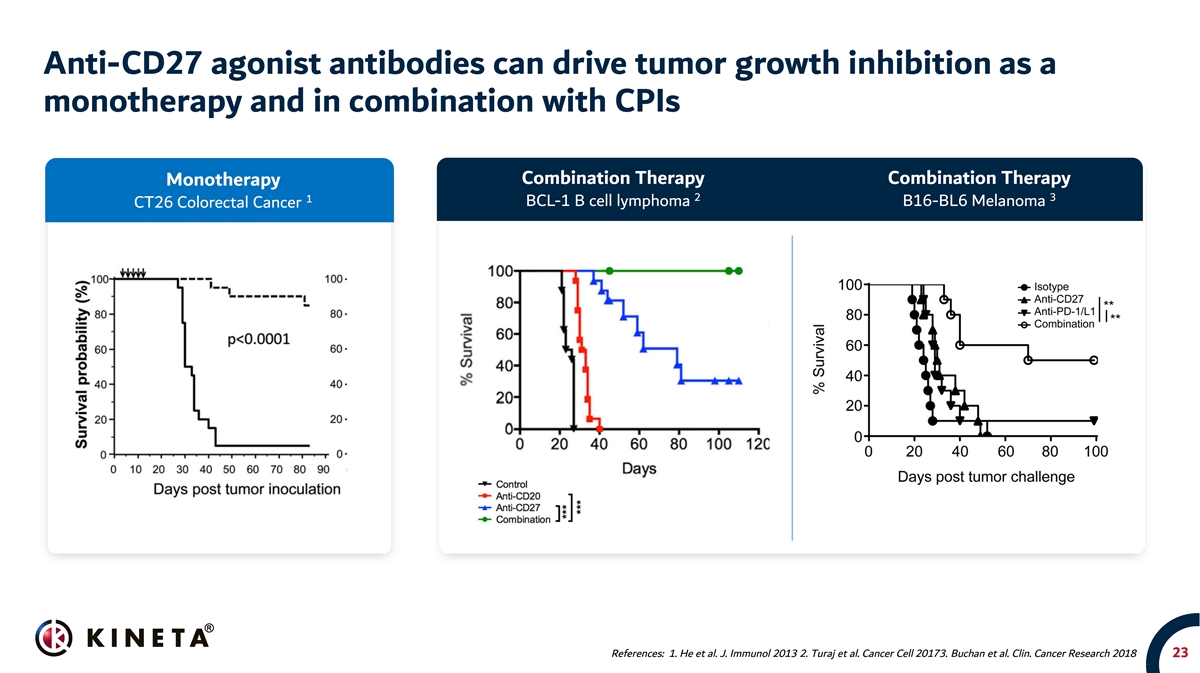
Anti-CD27 agonist antibodies can drive tumor growth inhibition as a monotherapy and in combination with CPIs Combination Therapy Combination Therapy Monotherapy 2 3 1 BCL-1 B cell lymphoma B16-BL6 Melanoma CT26 Colorectal Cancer References: 1. He et al. J. Immunol 2013 2. Turaj et al. Cancer Cell 20173. Buchan et al. Clin. Cancer Research 2018 23
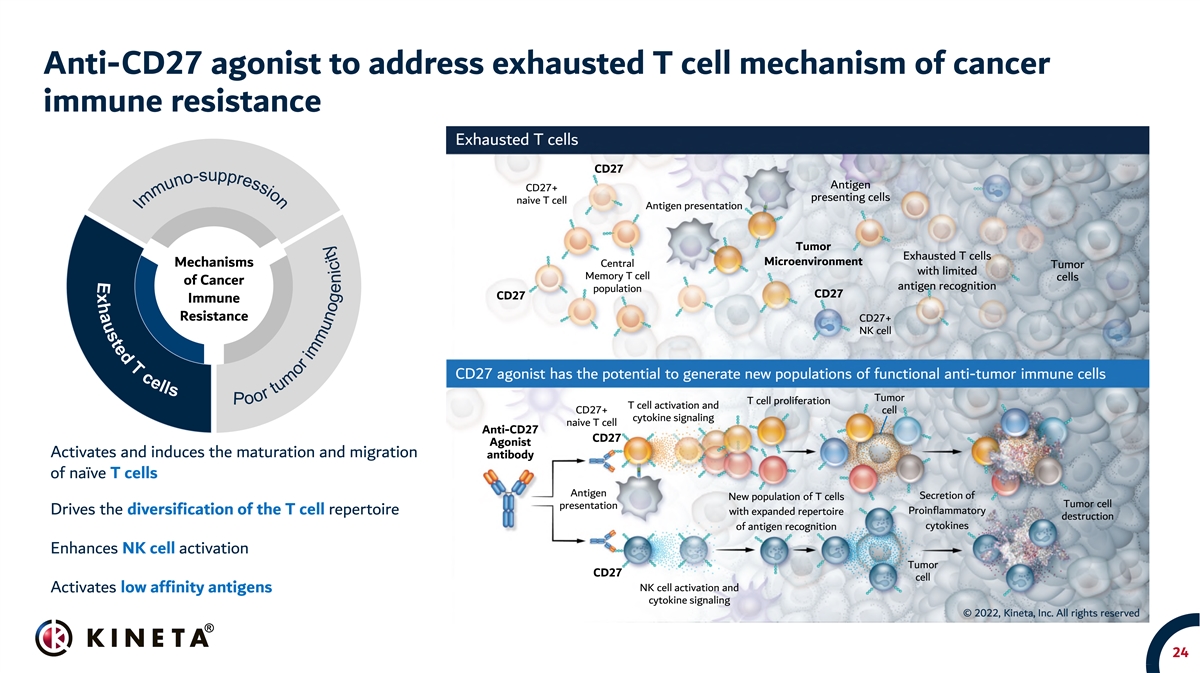
Anti-CD27 agonist to address exhausted T cell mechanism of cancer immune resistance Exhausted T cells CD27 Antigen CD27+ presenting cells naive T cell Antigen presentation Tumor Exhausted T cells Microenvironment Mechanisms Central Tumor with limited Memory T cell cells of Cancer antigen recognition population CD27 CD27 Immune Resistance CD27+ NK cell CD27 agonist has the potential to generate new populations of functional anti-tumor immune cells Tumor T cell proliferation T cell activation and CD27+ cell cytokine signaling naive T cell Anti-CD27 CD27 Agonist Activates and induces the maturation and migration antibody of naïve T cells Antigen Secretion of New population of T cells Tumor cell presentation Drives the diversification of the T cell repertoire Proinflammatory with expanded repertoire destruction cytokines of antigen recognition Enhances NK cell activation Tumor CD27 cell NK cell activation and Activates low affinity antigens cytokine signaling © 2022, Kineta, Inc. All rights reserved 24
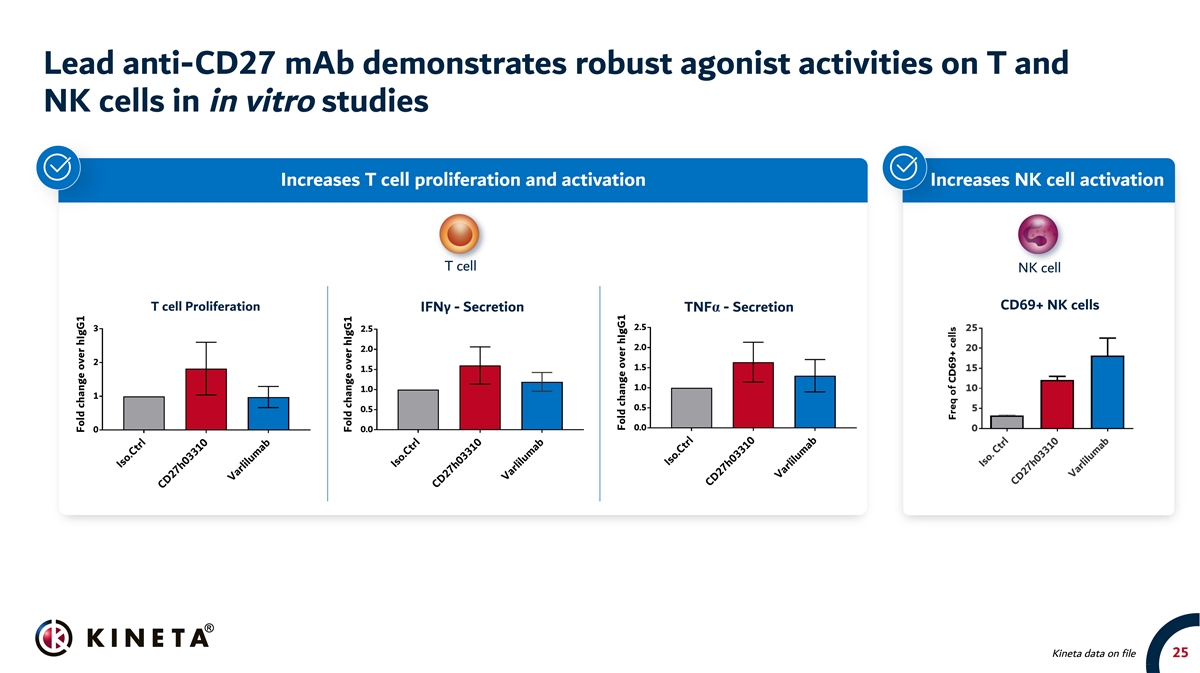
Lead anti-CD27 mAb demonstrates robust agonist activities on T and NK cells in in vitro studies Increases T cell proliferation and activation Increases NK cell activation T cell NK cell CD69+ NK cells T cell Proliferation IFNγ - Secretion TNFα - Secretion Kineta data on file 25
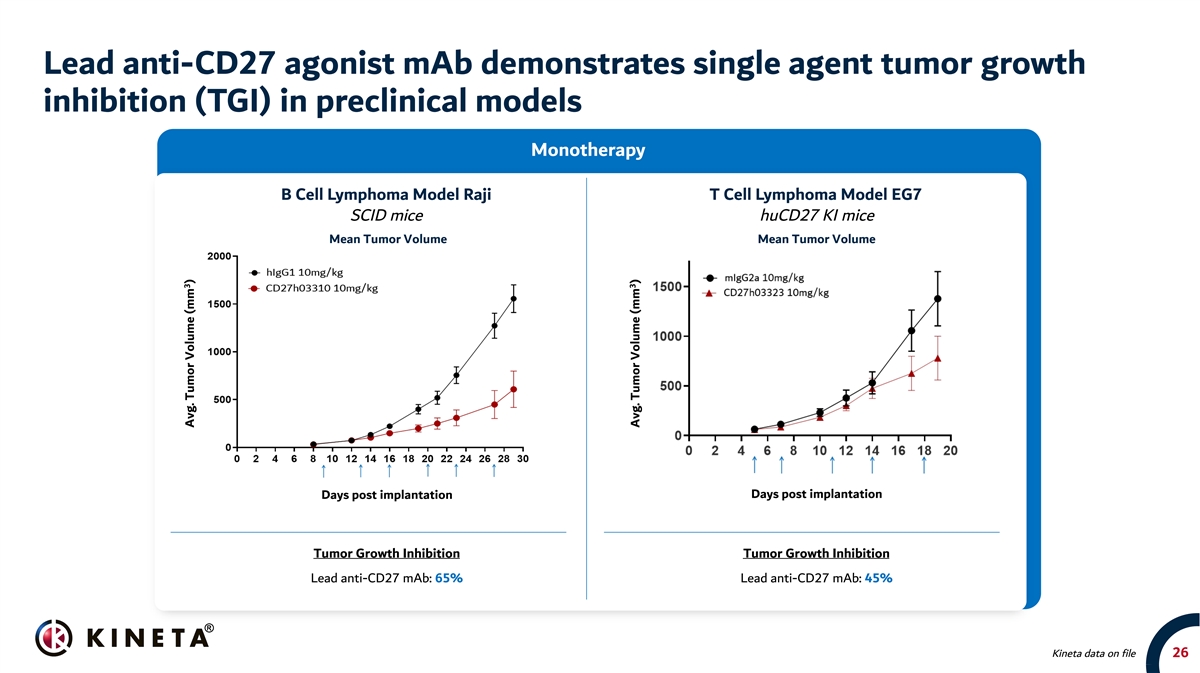
Lead anti-CD27 agonist mAb demonstrates single agent tumor growth inhibition (TGI) in preclinical models Monotherapy B Cell Lymphoma Model Raji T Cell Lymphoma Model EG7 SCID mice huCD27 KI mice Mean Tumor Volume Mean Tumor Volume Days post implantation Days post implantation Tumor Growth Inhibition Tumor Growth Inhibition Lead anti-CD27 mAb: 65% Lead anti-CD27 mAb: 45% Kineta data on file 26 3 Avg. Tumor Volume (mm ) 3 Avg. Tumor Volume (mm )
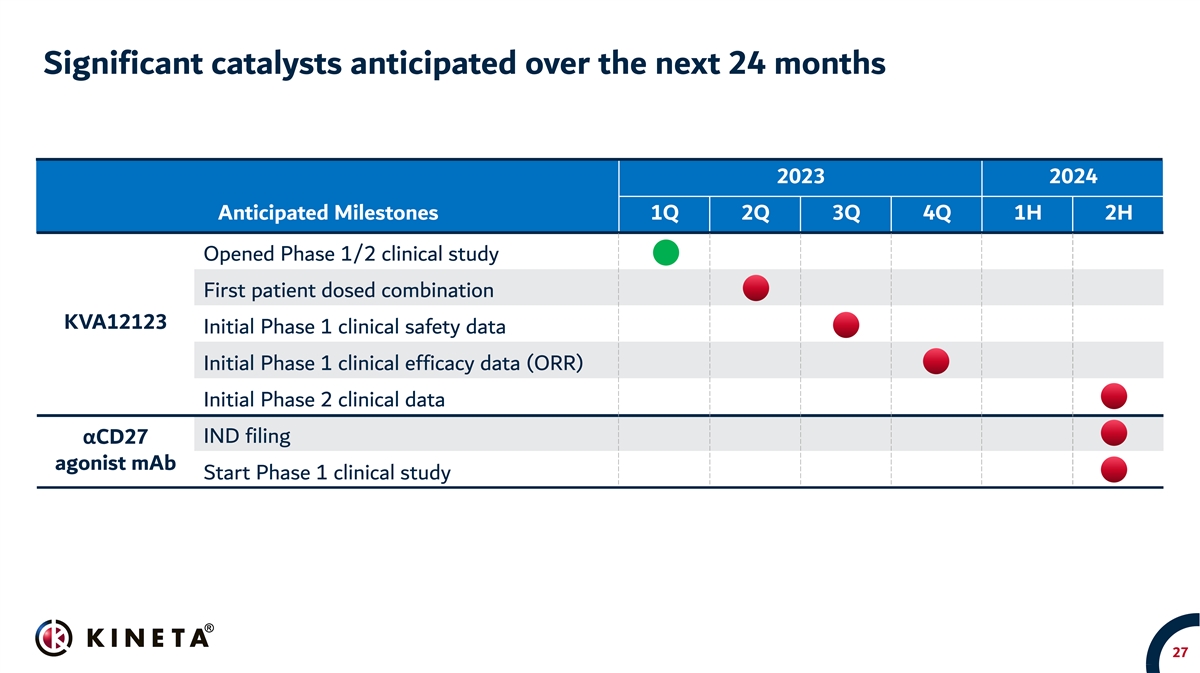
Significant catalysts anticipated over the next 24 months 2023 2024 Anticipated Milestones 1Q 2Q 3Q 4Q 1H 2H Opened Phase 1/2 clinical study First patient dosed combination KVA12123 Initial Phase 1 clinical safety data Initial Phase 1 clinical efficacy data (ORR) Initial Phase 2 clinical data IND filing αCD27 agonist mAb Start Phase 1 clinical study 27

Experienced leadership team Craig Philips Shawn Iadonato, PhD President Chief Executive Officer Keith Baker Thierry Guillaudeux, PhD Chief Financial Officer Chief Scientific Officer Jacques Bouchy Pauline Kenny EVP Investor Relations General Counsel & Business Development 28
Kineta is developing next-generation immunotherapies that address cancer immune resistance KVA12123 Innate • VISTA blocking mAb to address immunosuppression in the TME Immunity Focused • Opened Phase 1/2 clinical study evaluating KVA12123 alone and in combination with pembrolizumab in advanced solid tumors Pipeline Preclinical Anti-CD27 agonist mAb to address exhausted T cells 3Q23 | KVA12123 initial clinical safety data Catalysts 4Q23 | KVA12123 initial clinical efficacy data (ORR) Through mid-2024 Runway Partnerships 29

Developing next generation immunotherapies for cancer patients www.kinetabio.com 30

Appendix 31
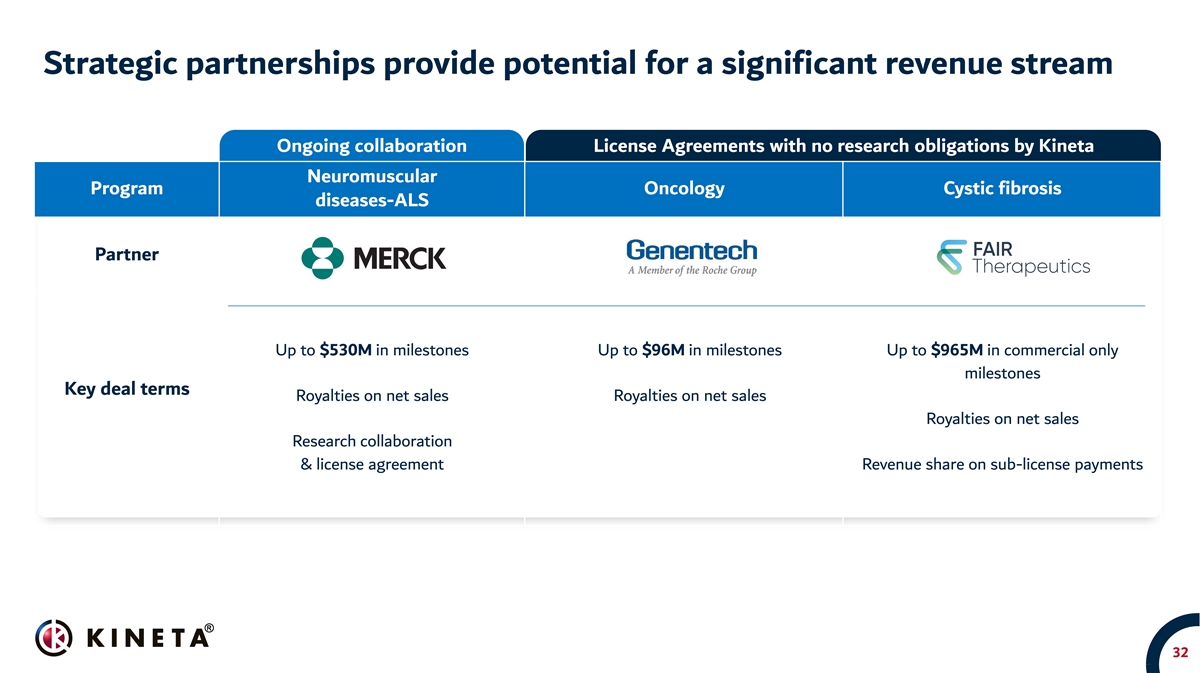
Strategic partnerships provide potential for a significant revenue stream Ongoing collaboration License Agreements with no research obligations by Kineta Neuromuscular Program Oncology Cystic fibrosis diseases-ALS Partner Up to $530M in milestones Up to $96M in milestones Up to $965M in commercial only milestones Key deal terms Royalties on net sales Royalties on net sales Royalties on net sales Research collaboration & license agreement Revenue share on sub-license payments 32































