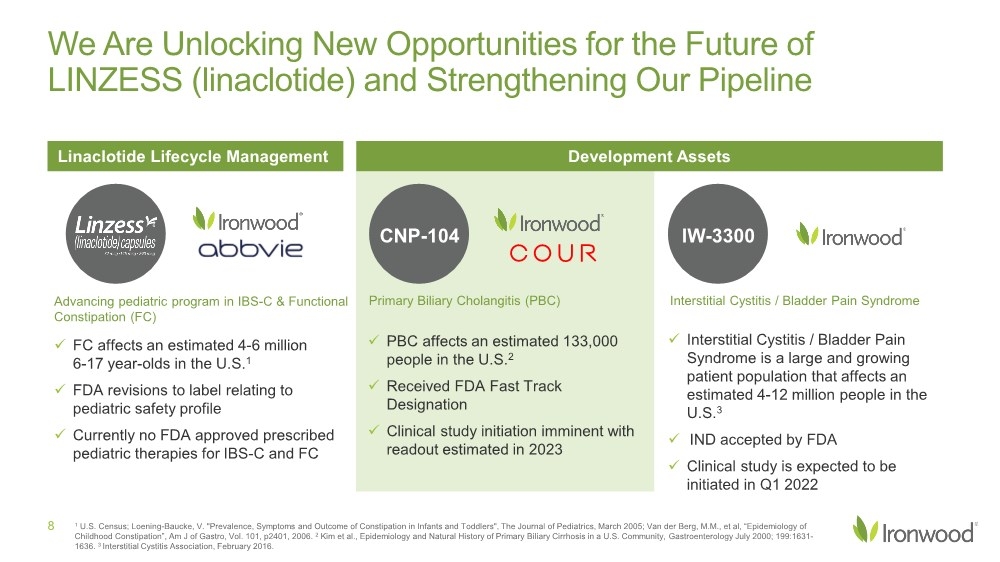
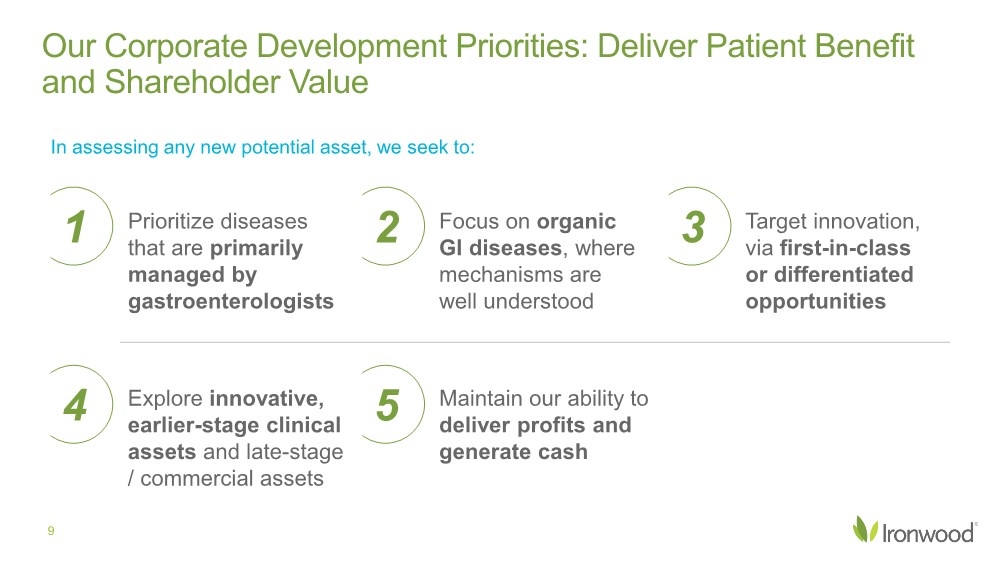
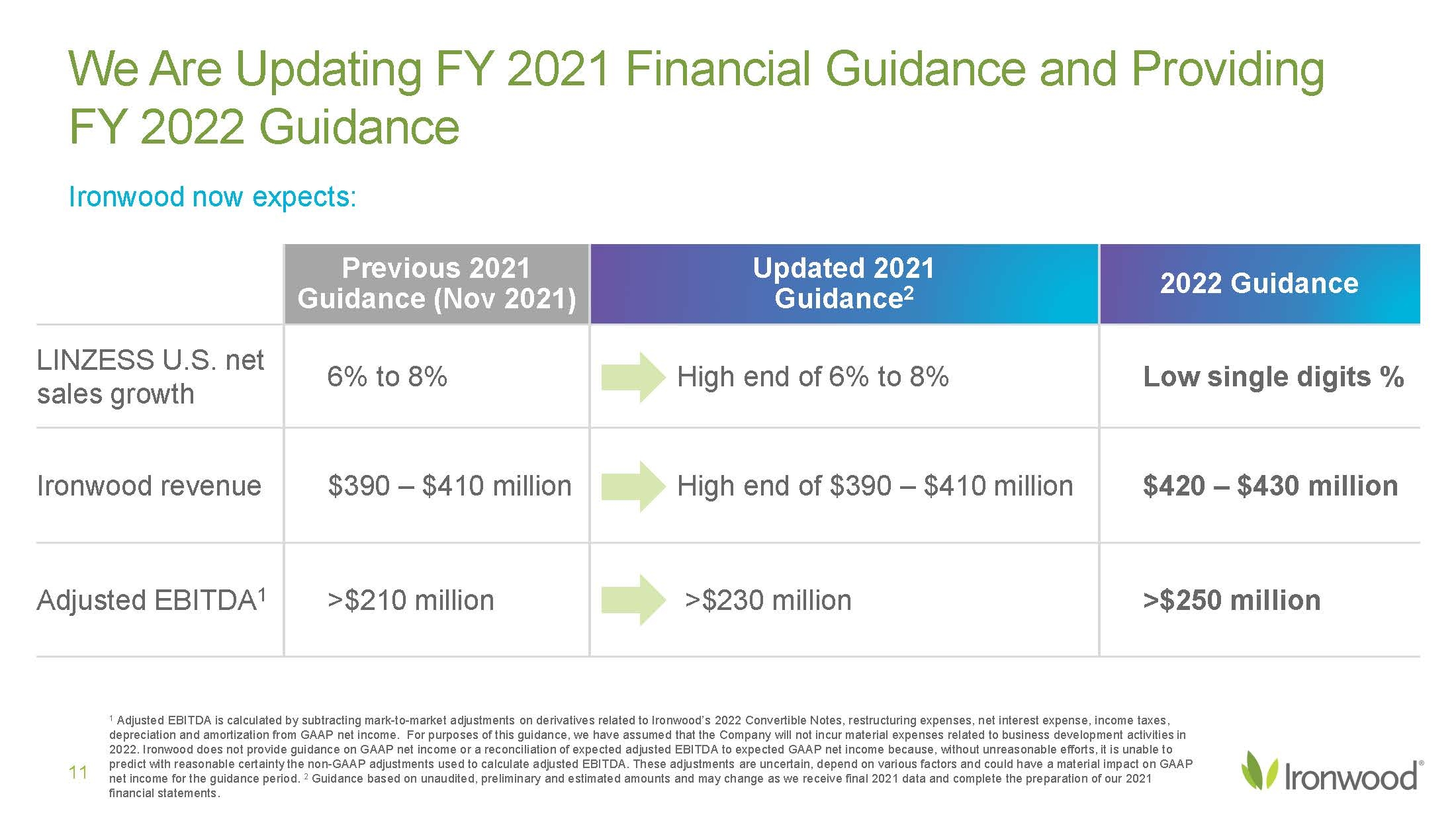
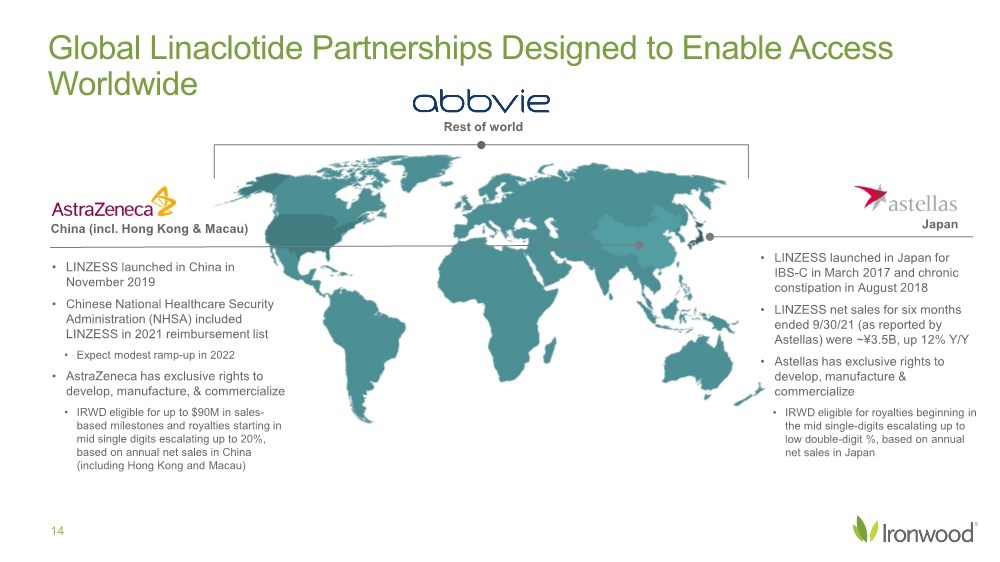
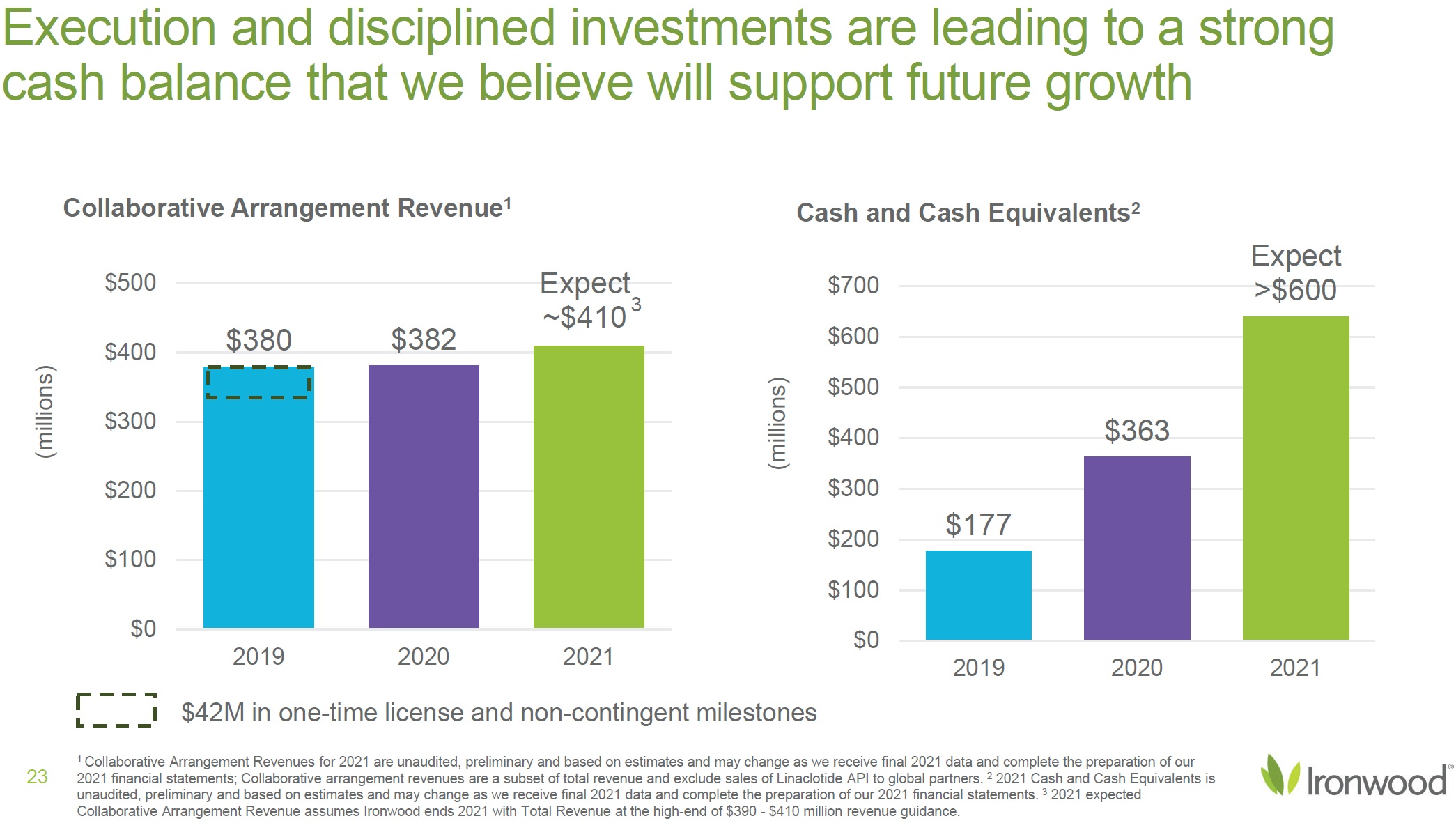
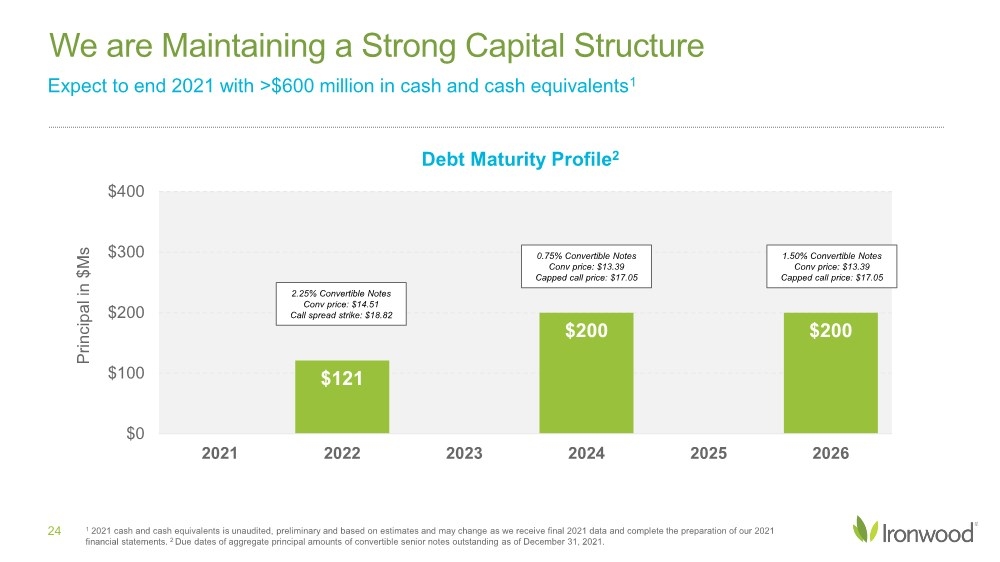
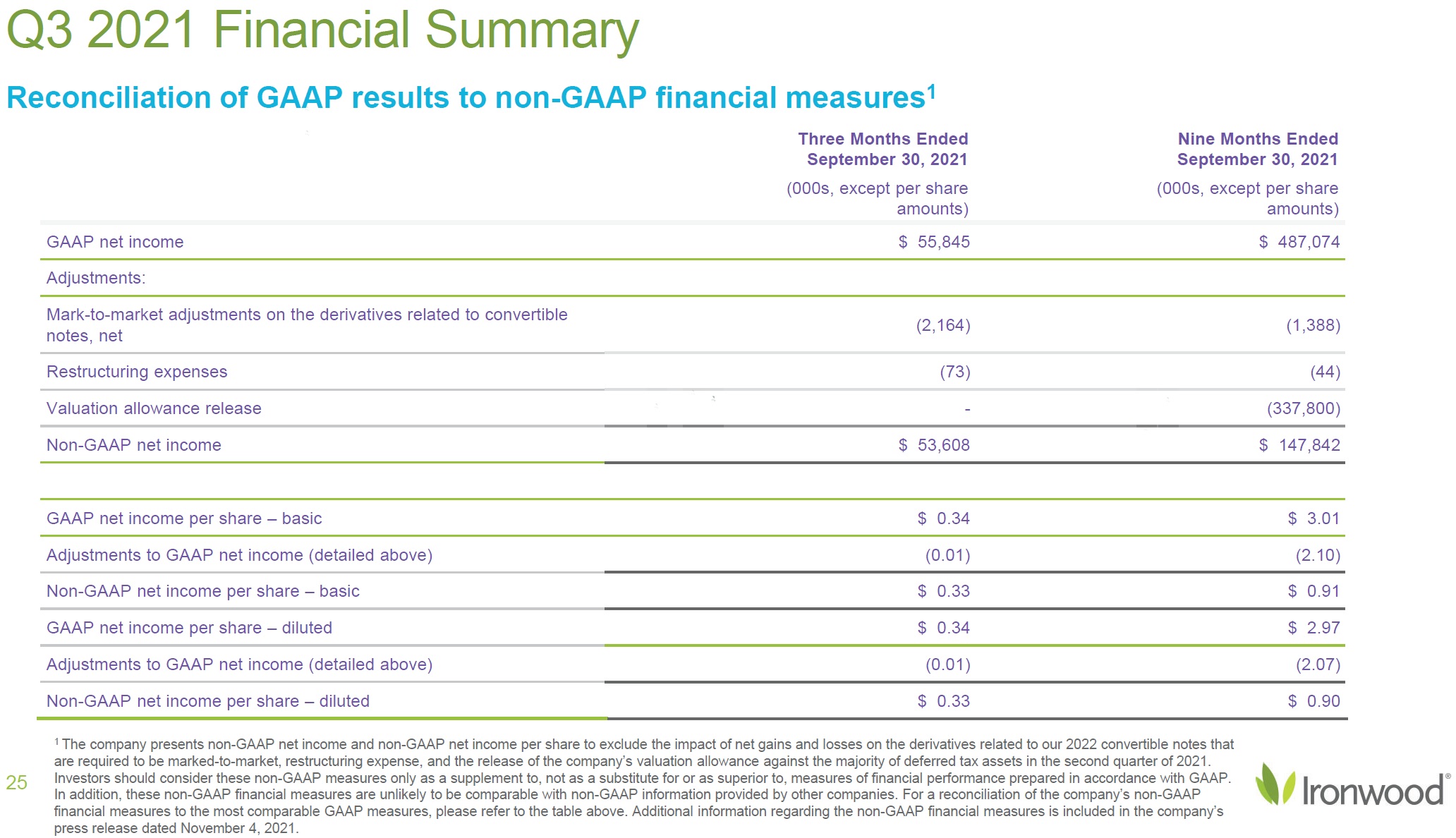
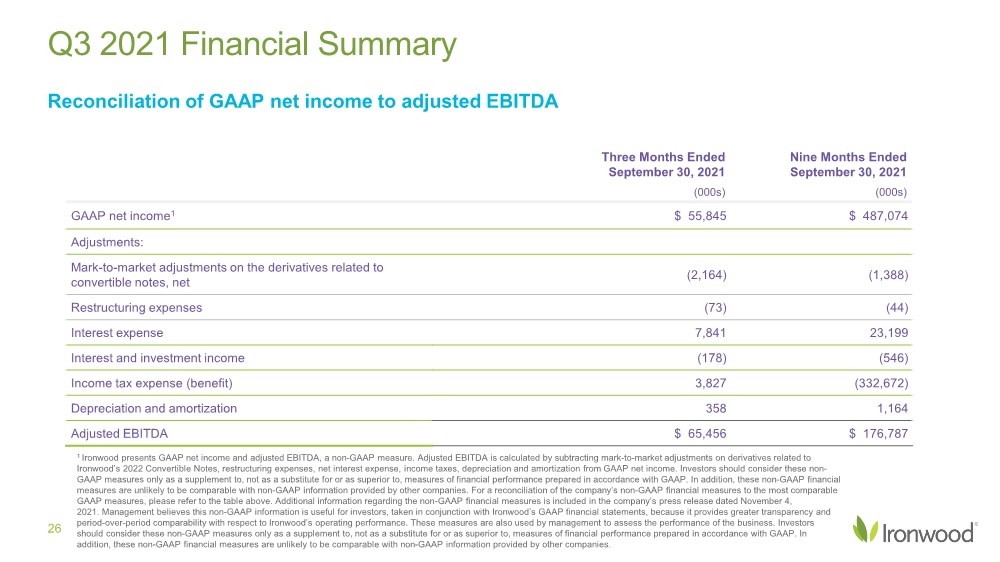
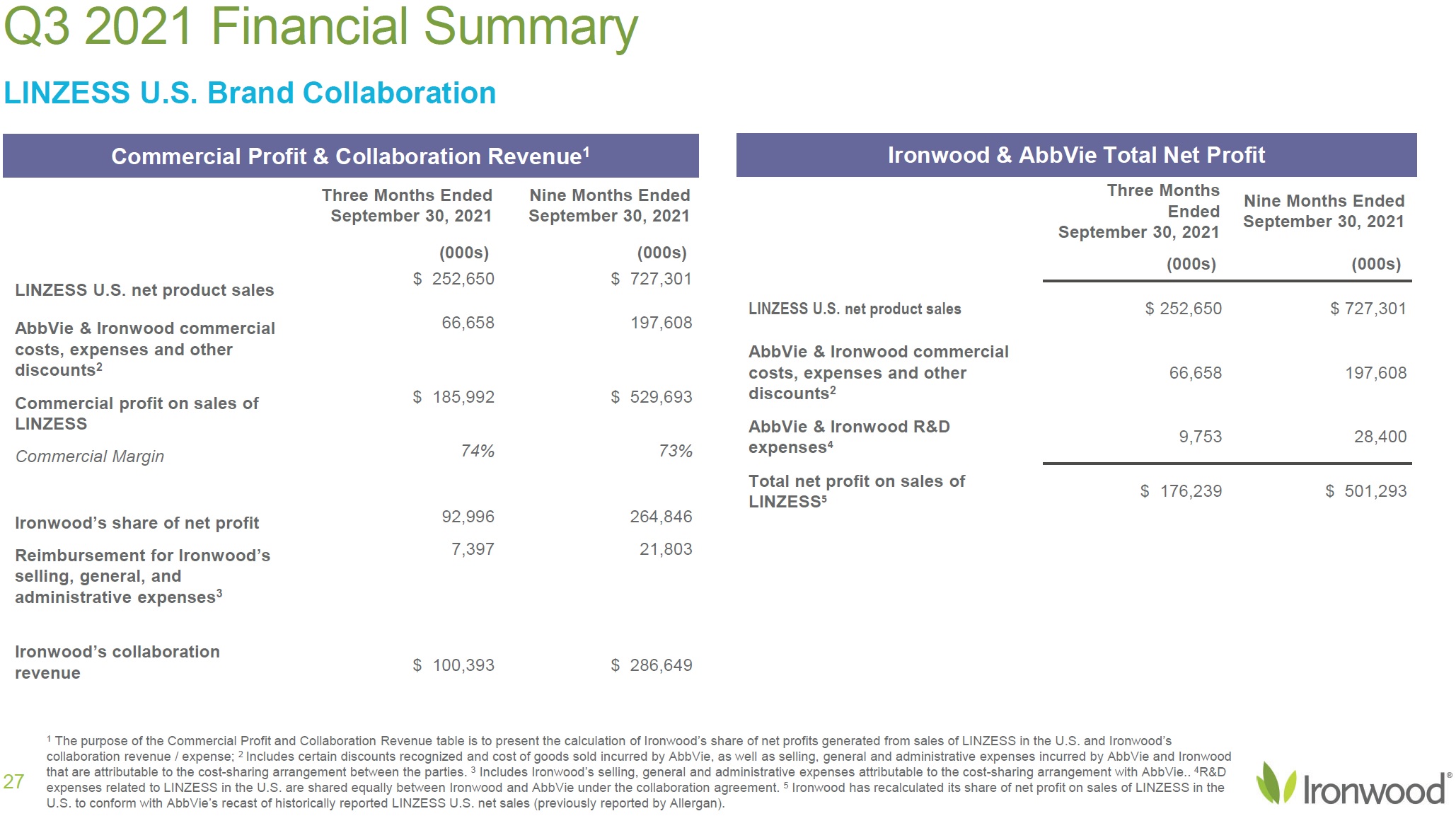
| Safe Harbor Statement 2 This presentation contains forward-looking statements. Investors are cautioned not to place undue reliance on these forward-looking statements, including statements about our ability to execute on our vision and mission; our strategy, business, financial position and operations, including with respect to maximizing LINZESS® (linaclotide), strengthening an innovative GI pipeline and delivering sustained profits and generating cash flow; the demand, development, commercial availability and commercial potential of linaclotide and the drivers, timing, impact and results thereof; the potential indications for, and benefits of, linaclotide and our ability to drive LINZESS growth; our ability to successfully execute and the value-creation potential of our strategic priorities, including our efforts to drive LINZESS growth in demand and net sales, enhance linaclotide clinical utility through robust lifecycle management opportunities, advance treatments for serious, organic GI diseases and other prioritized criteria focused on value creation, deliver sustained profits and generate cash flow, and apply thoughtful and disciplined capital allocation decisions; our option to acquire an exclusive license to develop and commercialize CNP-104 in the U.S.; the timing of initiating a PoC to evaluate the safety, tolerability, pharmacodynamic effects and efficacy of CNP-104 in PBC patients; the timing, achievement and payment of certain milestones and royalties under our agreement with COUR; the opportunity for COUR’s nanoparticle delivery platform to treat PBC and potentially eliminate the immune cell bile duct destruction present in PBC; the potential for CNP-104 to transform the treatment of PBC in the U.S.; the strength of the Company’s balance sheet and the Company’s ability to return cash to shareholders, including the potential that we return capital to shareholders via a share repurchase program; the potential of IW-3300 to be an effective treatment of visceral pain conditions and the size of the IC/BPS and endometriosis populations, as well as our plans to advance IW-3300 into clinical development (including the timing and results thereof); the status of our development program to investigate the safety and efficacy of LINZESS for children; expectations regarding our global collaborations; and our financial performance and results, and guidance and expectations related thereto, including, without limitation, expectations related to LINZESS U.S. net sales growth, Ironwood revenue and adjusted EBITDA in 2021 and 2022, as well as expected cash and cash equivalents in 2021, EUTRx and Rx demand growth and brand margins in 2022, and price erosion in 2022 and 2023. These forward-looking statements speak only as of the date of this presentation, and Ironwood undertakes no obligation to update these forward- looking statements. Each forward-looking statement is subject to risks and uncertainties that could cause actual results to differ materially from those expressed or implied in such statement. Applicable risks and uncertainties include those related to the effectiveness of development and commercialization efforts by us and our partners; preclinical and clinical development, manufacturing and formulation development of linaclotide, CNP-104 and our product candidates; the risk that clinical programs and studies may not progress or develop as anticipated, including that studies are delayed or discontinued for any reason, such as safety, tolerability, enrollment, manufacturing, economic or other reasons; the risk that findings from our completed nonclinical and clinical studies may not be replicated in later studies; the risk that we or our partners are unable to obtain, maintain or manufacture sufficient LINZESS or our product candidates, or otherwise experience difficulties with respect to supply or manufacturing; the efficacy, safety and tolerability of linaclotide and our product candidates; the risk that the therapeutic opportunities for LINZESS or our product candidates are not as we expect; decisions by regulatory and judicial authorities; the risk we may never get additional patent protection for linaclotide and other product candidates; the risk that we may never get sufficient patent protection for linaclotide and other product candidates, that patents for linaclotide or other products may not provide adequate protection from competition, or that we are not able to successfully protect such patents; the risk that we are unable to manage our expenses or cash use, or are unable to commercialize our products as expected; the impact of the COVID-19 pandemic; the risk that we may elect to not exercise our option to acquire the exclusive license for CNP-104; the risk that the development of either CNP-104 and/or IW-3300 is not successful or that any of our product candidates is not successfully commercialized; the risk that the clinical trial for CNP-104 is delayed or not initiated by COUR; outcomes in legal proceedings to protect or enforce the patents relating to our products and product candidates, including abbreviated new drug application litigation; the risk that financial and operating results may differ from our projections; developments in the intellectual property landscape; challenges from and rights of competitors or potential competitors; the risk that our planned investments do not have the anticipated effect on our company revenues; developments in accounting guidance or practice; Ironwood’s or AbbVie’s accounting practices, including reporting and settlement practices as between Ironwood and AbbVie; the risk that we are unable to manage our expenses or cash use, or are unable to commercialize our products as expected; the impact of the COVID-19 pandemic; and the risks listed under the heading "Risk Factors" and elsewhere in Ironwood's Quarterly Report on Form 10-Q for the quarter ended September 30, 2021, and in our subsequent SEC filings. Ironwood uses non-GAAP financial measures in this presentation, which should be considered only a supplement to, and not a substitute for or superior to, GAAP measures. Refer to the Reconciliation of Non-GAAP Financial Measures to GAAP Results table and to the Reconciliation of Adjusted EBITDA to GAAP net income table and related footnotes on pages 25 and 26 of this presentation. Further, Ironwood considers the net profit for the U.S. LINZESS brand collaboration with AbbVie in assessing the product’s performance and calculates it based on inputs from both Ironwood and AbbVie. This figure should not be considered a substitute for Ironwood’s GAAP financial results. An explanation of our calculation of this figure is provided in the U.S. LINZESS Brand Collaboration table and related footnotes on page 27 of this presentation. LINZESS® is a registered trademark of Ironwood Pharmaceuticals, Inc. Any other trademarks referred to in this presentation are the property of their respective owners. All rights reserved. |