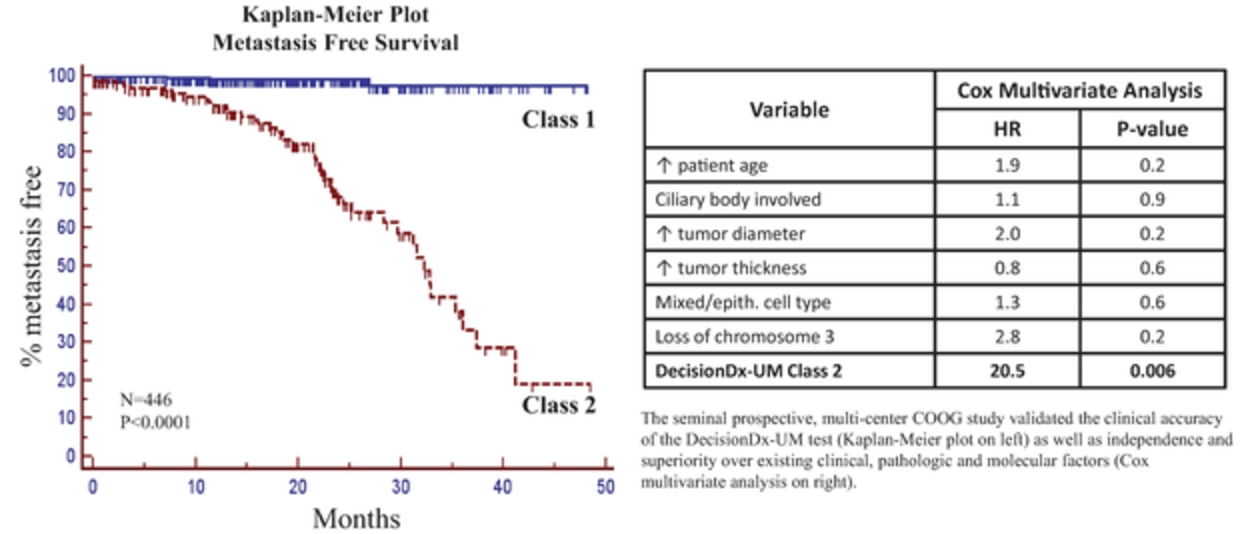
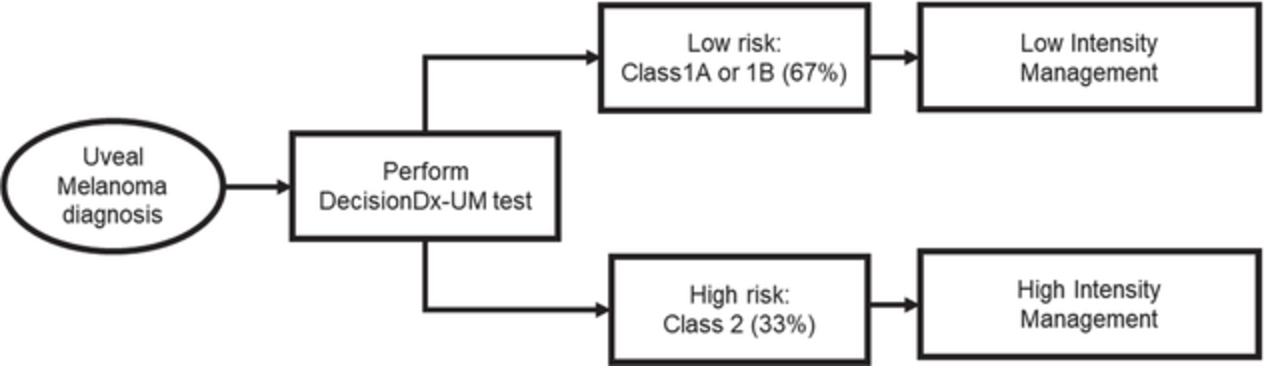
of our information technology systems. While we have confidence in these individuals, organizations and systems, agreements or security measures may be breached, and we may not have adequate remedies for any breach. In addition, our trade secrets may otherwise become known or be independently discovered by competitors. To the extent that our commercial partners, collaborators, employees and consultants use intellectual property owned by others in their work for us, disputes may arise as to the rights in related or resulting know-how and inventions.
Government Regulation and Product Approval
Regulations
Clinical Laboratory Improvement Amendments of 1988
As a clinical reference laboratory, we are required to hold certain federal, state and local licenses, certifications and permits to conduct our business. Under CLIA, we are required to hold a certificate applicable to the type of laboratory tests we perform and to comply with standards applicable to our operations, including test processes, personnel, facilities administration, equipment maintenance, recordkeeping, quality systems and proficiency testing. We must maintain CLIA compliance and certification to be eligible to bill for diagnostic services provided to Medicare beneficiaries.
To renew our CLIA certificate, we are subject to survey and inspection every two years to assess compliance with program standards. Because we are a College of American Pathologists, or CAP, accredited laboratory, CMS does not perform this survey and inspection and relies on our CAP survey and inspection. We may also be subject to additional unannounced inspections. The regulatory and compliance standards applicable to the testing we perform may change over time, and any such changes could have a material effect on our business.
Penalties for non-compliance with CLIA requirements include suspension, limitation or revocation of the laboratory’s CLIA certificate, as well as directed plan of correction, state on-site monitoring, civil money penalties, civil injunctive suit or criminal penalties.
State Laboratory Licensing
In addition to federal certification requirements of laboratories under CLIA, CLIA provides that states may adopt laboratory regulations and licensure requirements that are more stringent than those under federal law. Such laws, among other things, establish standards for the day-to-day operation of a clinical reference laboratory, including the training and skills required of personnel and quality control. We currently provide laboratory services in all 50 states and maintain out-of-state laboratory licenses in New York, California, Maryland, Pennsylvania and Rhode Island.
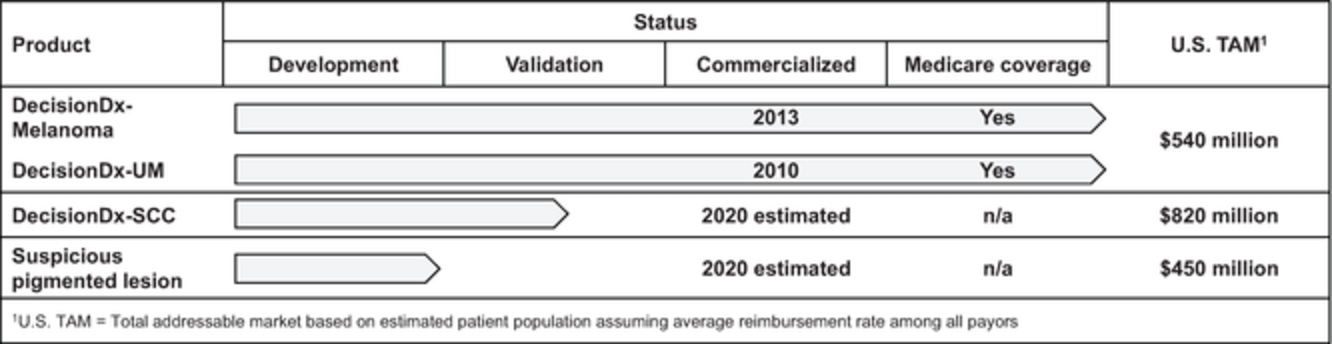
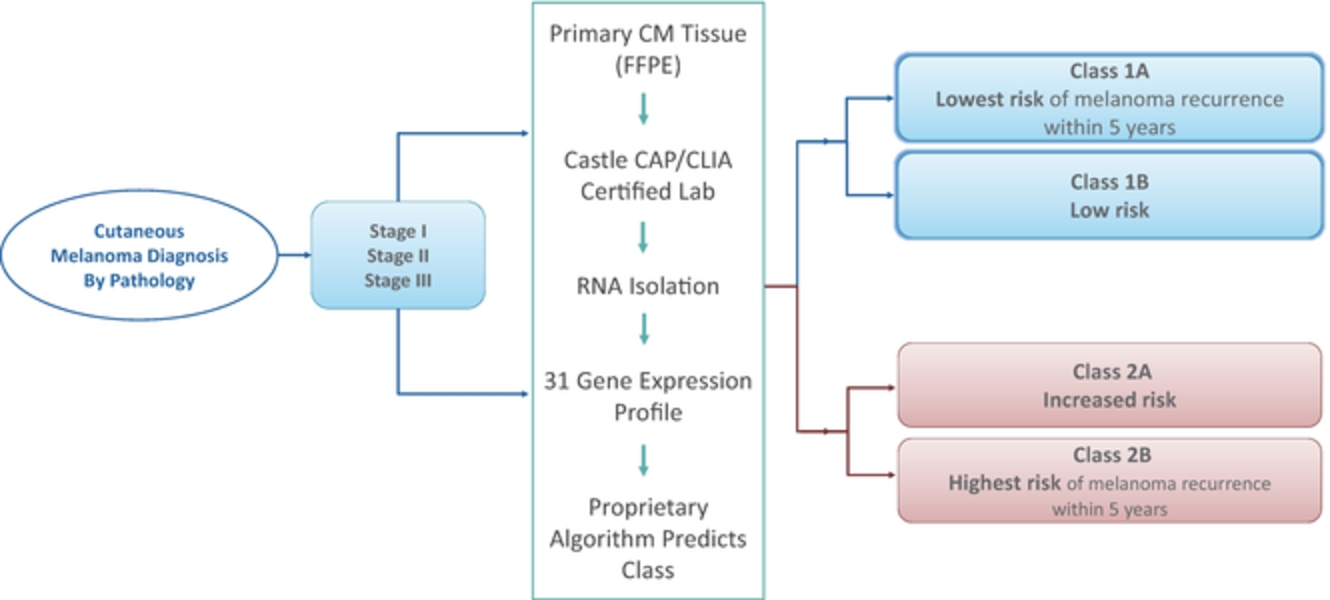
Multiple states require the licensure of out-of-state laboratories that accept specimens from those states. Because we receive specimens from New York, our clinical reference laboratory is required to be licensed by New York, under New York laws and regulations. New York law also mandates proficiency testing for laboratories licensed under New York state law, regardless of whether such laboratories are located in New York. If a laboratory is out of compliance with New York statutory or regulatory standards, the New York Department of Health, or NYDOH, may suspend, limit, revoke or annul the laboratory’s New York license, censure the holder of the license, or assess civil money penalties. We have received written approval from NYDOH to offer our proprietary DecisionDx-Melanoma DecisionDx-UM and DecisionDx-PRAME products in New York. If we were to be found out of compliance with New York laboratory requirements, we could be subject to such sanctions, which could harm our business.
Federal Oversight of Laboratory Developed Tests
The laws and regulations governing the marketing of diagnostic products are evolving, extremely complex, and in many instances, there are no significant regulatory or judicial interpretations of these laws and regulations. Clinical laboratory tests are regulated under CLIA, as administered by CMS, as well as by applicable state laws. In addition, the Federal Food, Drug and Cosmetic Act, or FDCA, defines a medical device to include any instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent, or other similar or related article, including a component part, or accessory, intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease, in man or other animals. Our in vitro testing products are considered by the FDA to be subject to regulation as medical devices. Among other things, pursuant