UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
ANNUAL REPORT
(Mark One)
þAnnual Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
For the fiscal year ended December 31, 2012
ùTransition Report under Section 13 or 15(d) of the Securities Exchange Act of 1934
For the transition period from to
SurePure, Inc.
(Exact name of registrant as specified in its charter)
Nevada (State or other jurisdiction of
Incorporation or organization) | | 3556 (Primary Standard Industrial
Classification Code Number) | | 26-3550286 (I.R.S. Employer Identification No.) |
405 Lexington Avenue, 25th Floor
New York, New York 10174
Registrant’s telephone number, including area code: (917) 368-8480
Securities registered pursuant to section 12(g) of the Act:
Title of Class: Common stock, par value $0.001 per share
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes¨ Nox
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. Yes¨ Nox
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yesx No¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data file required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes¨ Nox
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. Yes¨ Nox
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| ¨Large Accelerated Filer | ¨Accelerated Filer | ¨ Non-Accelerated Filer | x Smaller Reporting Company |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes¨ Nox
The aggregate market value of voting and non-voting common equity held by non-affiliates computed by reference to the price at which the common equity was sold, or the average bid and asked price of such common equity, as of the last business day of the registrant’s most recently completed second fiscal quarter: $9,967,400
The number of shares of the registrant’s Common Stock, par value $0.001 per share, outstanding as of March 29, 2013 was 23,890,184.
DOCUMENTS INCORPORATED BY REFERENCE
None
SUREPURE, INC.
| PART I | | |
| | | |
| Item 1. | Business | 8 |
| | | |
| Item 1A. | Risk Factors | 22 |
| | | |
| Item 1B. | Unresolved Staff Comments | 36 |
| | | |
| Item 2. | Properties | 36 |
| | | |
| Item 3. | Legal Proceedings | 36 |
| | | |
| Item 4. | Mine Safety Disclosures | 36 |
| | | |
| PART II | | |
| | | |
| Item 5. | Market for the Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities | 37 |
| | | |
| Item 6. | Selected Financial Data | 38 |
| | | |
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations | 39 |
| | | |
| Item 7A. | Quantitative and Qualitative Disclosures About Market Risk | 44 |
| | | |
| Item 8. | Financial Statements and Supplementary Data | 44 |
| | | |
| Item 9. | Changes in and Disagreements With Accountants on Accounting and Financial Disclosure | 44 |
| | | |
| Item 9A. | Controls and Procedures | 44 |
| | | |
| Item 9B. | Other Information | 45 |
| | | |
| PART III | | |
| | | |
| Item 10. | Directors and Executive Officers and Corporate Governance | 46 |
| | | |
| Item 11. | Executive Compensation | 51 |
| | | |
| Item 12. | Security Ownership Of Certain Beneficial Owners and Management and Related Stockholder Matters | 57 |
| | | |
| Item 13. | Certain Relationships and Related Transactions, and Director Independence | 58 |
| | | |
| Item 14. | Principal Accountant Fees and Services | 72 |
| | | |
| PART IV | | |
| | | |
| Item 15. | Exhibits and Financial Statement Schedules | 74 |
Cautionary Note Regarding Forward-Looking Statements
Certain statements included in this annual report on Form 10-K, other than statements of historical fact, are forward-looking statements within the meaning of the federal securities laws. Forward looking statements contained in this annual report can be identified by the use of terms and phrases such as “believe,” “plan,” “intend,” “anticipate,” “target,” “estimate,” “expect,” and the like, and/or future-tense or conditional constructions “may,” “could,” “should,” etc. In addition, statements that contemplate or make assumptions about actual or potential future sales, market size, collaborations, and trends or operating results also constitute forward-looking statements. These forward-looking statements involve risks and uncertainties and are based on the beliefs and assumptions of our management and on information available at the time these statements and disclosures were prepared. Forward-looking statements include, but are not limited to:
| · | the availability of equity or debt financing and access to capital to operate our business; and |
| · | the rate at which the Company’s proprietary technology is commercialized and generates significant revenues. |
In addition to the risks identified under the heading “Risk Factors” and above, many important factors affect the Company’s ability to achieve our plans and objectives and to successfully develop and commercialize our technology and the products that utilize it, including, among other things our ability:
| · | to obtain additional funds to operate the business and pay other expenses as well; |
| · | to enforce our patents against infringers; |
| · | to demonstrate the safety and effectiveness of our technology in the purification of liquids; |
| · | to meet applicable regulatory standards and receive required regulatory approvals; |
| · | to manufacture and distribute Turbulator systems in commercial quantities at reasonable costs; and |
| · | to compete successfully against other products and to market products in a profitable manner. |
Therefore, current and prospective security holders are cautioned that there also can be no assurance that the forward-looking statements included in this annual report will prove to be accurate. In light of the significant uncertainties inherent to the forward-looking statements included herein, the inclusion of such information should not be regarded as a representation or warranty by the Company or any other person that the objectives and plans of the Company will be achieved in any specified time frame, if at all. Except to the extent required by applicable laws or rules, the Company does not undertake any obligation to update any forward looking statements or to announce revisions to any of the forward-looking statements.
CERTAIN TERMS USED IN THIS REPORT
As used in this annual report, unless the context otherwise requires, “SurePure,” the “Company,” “we,” “us” and “our” refer to SurePure, Inc., a Nevada corporation, as well as its subsidiaries, SurePure Investment Holding AG, a Swiss corporation, SurePure Operations AG, a Swiss corporation, SurePure Participations AG, a Swiss Corporation and SurePure Latin America, a Brazilian private company, and also refer to SurePure Holdings South Africa (Pty) Ltd. and SurePure Marketing South Africa (Pty ) Ltd., which are included in the consolidated financial statements of SurePure, Inc. as variable interest entities. The “Company,” “we,” “us” and “our” refer to and also refer to the operations of SurePure Investment Holding AG, a Swiss corporation, SurePure Operations AG, a Swiss corporation, SurePure Participations, AG, a Swiss Corporation and SurePure Latin America, a Brazilian private company, and the South African variable interest entities referred to in the preceding sentence prior to the exchange of shares that occurred on December 12, 2012 and that resulted in both SurePure Investment Holding AG and SurePure Participations AG becoming wholly-owned subsidiaries of SurePure, Inc.
The following names refer to the following entities:
| “SPI” | means SurePure Investment Holding AG, a Swiss corporation that serves as the holding company and contains our international administrative and financial functions |
| “SPO” | means SurePure Operations AG, a Swiss corporation that is our principal operating business and holds all of our patents other than the patent issued in South Africa |
| “SPLAM” | means SurePure Latin America, a Brazilian private company that commercializes our technology in Latin America |
| “SPP” | means SurePure Participations AG, a Swiss corporation, a company formed by XOptics to hold certain shares of SPI |
| “SPHSA” | means SurePure Holdings South Africa (Pty) Ltd., a South African private company that serves as the holding company for SPMSA and owns the patent issued in South Africa |
| “SPMSA” | means SurePure Marketing South Africa (Pty) Ltd., a South African private company that commercializes our technology in South Africa and contains the administrative and financial functions of our business in South Africa |
SPI, SPO, SPP and SPLAM are subsidiaries of SurePure, Inc. SPHSA and SPMSA are variable interest entities and are not subsidiaries of any of SurePure, Inc., SPI, SPO or SPLAM.
We use other defined terms throughout this annual report, including the following:
| · | “Commission” means the U.S. Securities and Exchange Commission; |
| · | “Securities Act” means the U.S. Securities Act of 1933, as amended; |
| · | “Exchange Act” means the U.S. Securities Exchange Act of 1934, as amended; |
| · | “Nonvoting Convertible Preferred Stock” means our Nonvoting Convertible Preferred Stock, par value $.01 per share; |
| · | “Share Exchange” means the exchange of shares of SPI for shares of our common stock, which occurred on December 12, 2012, under the terms and conditions of the Share Exchange Agreement; |
| · | “Share Exchange Agreement” means the Amended and Restated Share Exchange Agreement that we entered into on December 12, 2012, under which we are obligated to issue shares of our common stock to the shareholders of SPI; and |
| · | “XOptics” means XOptics (PTC), Ltd., a private company formed under the laws of the British Virgin Islands. |
All references in this Annual report to “$” shall be to U.S. Dollars. All references in this annual report to “CHF” shall be to Swiss Francs. All references in this annual report to “ZAR” shall be to South African Rands.
Item 1. Description of the Business
Overview
The Companydesigns, manufactures, markets, sells or licenses and maintains its proprietary Turbulator systems forinnovative liquid photo-purification technology in the global marketplace (“SurePure Photopurification Technology” or our “Technology”). We have been engaged in these activities since 2005. Our Technology uses Ultraviolet (“UV”) light in the C band (“UVC”) to process, preserve and sustain the natural quality of food and beverage products, such as dairy products, fruit juice and concentrates, sugar syrup bases and alcoholic beverages. In addition to beverage products, our SurePure Photopurification Technology can also be used to reduce the microbial loads in turbid liquids with industrial applications, such as diesel, bio-ethanol and ship bilge, as well as pharmaceutical applications such as eye preparations and saline drips.
The use of UVC light energy with a specific wavelength to disinfect food surfaces, water and other beverages is well accepted. In 2001, the U.S. Food and Drug Administration (“FDA”) amended its regulations to permit the use of UVC light as a means of purification for water and fruit juices under certain conditions. There are multiple systems that effectively use UVC to purify or disinfect water and transparent liquids. Beverages and other liquids, such as milk and fruit juice, and water and other liquids used in industrial processes, are cloudy, opaque and contain suspended matter, and are often referred to as “turbid.” The ability to use UVC to purify turbid liquids requires technology that passes limited amounts of liquid at high flow rates very close to the UVC light source so that microbes and other pathogens in the liquid are exposed most directly and intensely to UVC waves.
It is our goal to secure approval for our Technology in 2013 and 2014 in those markets where approval is a precondition for the commercial implementation, such as with our current dairy and wine test clients, as well as to market and distribute our SurePure Photopurification Technology on a worldwide basis. After concluding research and successfully testing at various research institutions during the past several years, we are seeking approval from the FDA and EU Novel Food Clearance for use of the SurePure Photopurification Technology as an adjunct to and in certain cases as an alternative for, pasteurization in dairy applications and from theInternational Organisation of Vine and Winefor the purification of wine. We are simultaneously increasing the general awareness of our Technology through demonstrations at trade shows and exhibitions of our Technology to selected customers who are successfully running internal tests.
Based on research that we have sponsored, we believe that our SurePure Photopurification Technology improves beverage and liquid food quality and safety by safelydisinfecting these products beyond existingtechnology, such as conventional pasteurization.
Because our Technology does not require heat to be generated for purification to occur and therefore requires less energy to operate, we also believe that it enables processors of food, beverages and industrial liquids that use our Technology to reduce their operating expenditures. Our research also leads us to believe that our SurePure Photopurification Technology can be applied to virtually every consumable beverage, many industrial and processing liquids and any liquid pharmaceutical products that may suffer from microbiological contamination. Consequently, the markets that we serve can encompass all industries that manufacture or distribute consumable beverages, industries that use water or other liquids in industrial processes in the manufacturing or processing of foods and beverages and industries that produce liquids that must meet standards for high levels of purification.
Our Industry
Currently, conventional means for purifying dairy products, juices, wine, beer and other beverages are pasteurization and chemical. Pasteurization was developed in the nineteenth century and received widespread adoption in the late nineteenth and twentieth centuries. Pasteurization operates by heating milk or other liquid to a specific temperature level (generally, 73° C or 164° F, although the exact temperature will vary according to need) and holding the temperature constant for a specified period of time, during which period pathogen cells are ruptured as a result of the heat transferred by the liquid. As a result of the heating process, pasteurization transforms components of the liquid, including taste and other sensory attributes, and does not remove the ruptured microbes from the fluid. In order to create sufficient heat to raise the temperature of the liquid to meet pasteurization standards, large amounts of energy must be consumed and CO2emissions are created.
Another principal means for purifying liquids has been the addition of certain chemicals, such as sulfites and other preservatives. The addition of these elements often affects the taste and consistency of the liquid or food to be treated. Published research concludes that the additives used to achieve purification or preservation may lead to unhealthy or otherwise undesirable side effects among certain consumers.
Notwithstanding the general effectiveness of pasteurization and other means of purification, certain spore-forming bacteria, such asBacillus, remain a serious concern in the food industry, since they can survive heat, chemicals, desiccation and radiation and can cause food poisoning and spoilage. Certain species ofBacillus, such asB. sporothermodurans, are heat resistant and can survive the process of pasteurization. We believe that the food and dairy industries are seeking alternative methods to render their products microbiologically safe for consumption against the widest possible range of microorganisms.
Our Technology
UVC occupiesthe light spectrum with wavelengths of 10 nanometers to 290 nanometers. UVChas been successfully used as a process for the disinfection of air, water and surfaces. In recent years, scientists have studiedUVC irradiation technology as an alternative to heat (pasteurization) or chemically-based processes (preservatives) that currently are being used in the purification and/or sterilization or preservation of foods and liquids.Our Technology uses UVC waves to purify microbiologically sensitive turbid (cloudy or hazy fluids caused by individual particles (or suspended solids)) that are generally invisible to the naked eye byinactivating contaminating microorganisms, such as bacteria, yeasts, moulds and viruses in treated liquids. The UV wavelength most effective for killing microorganisms is between 254 and 260nanometers. Based on this, our Technology employs UVC radiation at the germicidal wavelength of 254nanometers of sufficient energy and duration to inactivate contaminating pathogens, including viruses. On the basis of research that we have sponsored, we believe that since our Technology transfers energy directly to microbial DNA, it also limits secondary effects on liquid proteins, enzymes and other components and concomitantly minimizes effects on nutritional, sensory and marketing attributes of the product being purified.
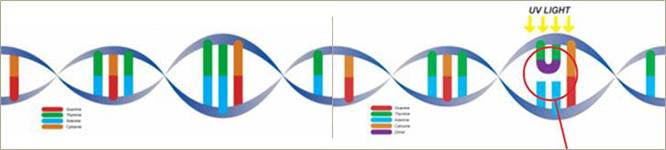
UVC light can penetrate the outer cell membranes of microorganisms, such as viruses, bacteria, mold and mildew. Exposure to UVC causes the DNA molecules of the microorganisms to become fused, or mutated. The mutations further result in the death of the organisms as their metabolic functions such as replication cannot be completed after the mutation results. In addition to being safe, our process is non-thermal, chemical-free, fast and replicable.
Our Product
We provide the core of our Technology, the Turbulator, to commercial clients for use in their plants. The Turbulator is a mechanical tube system within a double-walled stainless steel cylinder that creates turbulation in the fluid, moves the liquid to be purified in spiral flows over the source of the UVC at a specified distance from the source which maximizes the efficacy of the UVC waves, and ensures that all the liquid is exposed to the UVC source. Existing laminar flow systems, in which the liquid passes across a light source in sheets, do not allow for the sustained, controlled exposure that is necessary for effective purification, particularly for turbid liquids. The Turbulator system can be installed in parallel or series, resulting in a system that uses multiple lamps, with a modular approach enabling the treatment of a number of liquids requiring varying levels of exposure and flow rates. The proprietary design of the Turbulator is based on an internal lamp which is inserted into a quartz sleeve which in turn is placed inside a metal housing. Because of the proximity of the light source to the target liquid within the housing, there is a very high level of energy transfer to individual micro-organisms within the target liquid. The Turbulator system includes a data recording component which records quality assurance and other critical data relating to its operation.
We have designed the Turbulator system to maximize the range of liquids that our Technology can purify.The color and turbidity of the liquid being treated plays a role as UVC can only penetrate roughly 1 mm into juice and even less so into milk. The efficacy of the UVC is therefore dependent on the properties of the liquids being treated, including the color, viscosity, absorbance and presence of suspended solids. The Turbulator has been designed so that the necessary adjustments can be made to optimize its effectiveness in relation to the properties of the liquids being treated. Moreover, because of the design of the Turbulator and its components, our system can treat liquids where the use of heat as a purifying agent would be detrimental to the product. The Turbulator also allows the processor to set the precise exposure of a treated liquid to the UVC source, so that the exposure can match the exact purification requirements of the liquid.
The principal benefit of our SurePure Photopurification Technology is that it purifies liquids by inactivating pathogens without the use of heat energy. Because of the proprietary characteristics of the Turbulator system that control the flow of liquid over the UVC source, the Technology has been effective on all pathogens for which we have performed tests, including those that we believe are resistant to heat. In addition, we believe that our product has other benefits as well:
| · | Because our Turbulator systems do not rely on the generation of heat or other intense energy to perform purification, they consume substantially less power and therefore lead to less power-related expense, both in terms of power inputs and cooling needs; |
| · | Because our Turbulator systems require substantially less power than conventional means of heat-based purification, they substantially reduce the carbon footprint of the process; |
| · | Because the liquids that have been purified have not been subject to significant change in temperature, the quality of the end product remains intact; |
| · | For most liquids treated by our Technology, shelf life improves because we have inactivated the microbes and spores that result in spoilage; |
| · | Because our Turbulator systems are effective in destroying pathogens, the need to use preservatives in the liquid product that has been treated is substantially reduced; |
| · | Because our Turbulator systems effectively purify liquids used in industrial processes for washing and rinsing, among other purposes, those liquids can then be reused, which substantially reduces water consumption and the need to dispose of waste products; and |
| · | Because our Technology does not rely on heat, our Turbulator systems can possibly create new products in situations in which the application of heat has been a limiting factor in their purification. |
Approximately 400 Turbulator systems, including test systems have been installed since our inception in 2005 and are in operation in the following locations world-wide: USA, Europe, South America and South Africa. The installed systems are comprised of one to 40 separate Turbulators each. We continue to evaluate the performance of our Turbulator systems closely. As we gain customer feedback, we incorporate it into the design of new Turbulator installations.
In certain geographic markets and for applications to certain liquids, we have not obtained or are not required to obtain governmental approvals for the sale or distribution of our Turbulator products. Accordingly, we or our distributors can market our Technology at the present time. In those markets and for those applications where governmental or similar approvals are required, our timelines for market entry are estimated to be between one and three years. See “Regulation” below under this heading. We believe that we do not require further product development. Rather, our principal task is to fund research by credible and competent third parties that will generate the scientific data that underpin our requests for clearance and convincing demonstrate what we believe to be the advantages of our Technology, as well as its efficacy. We believe that the aggregate cost of these reports will be approximately $250,000.
We do not, and do not expect in the near term, to manufacture Turbulator systems through a facility of our own. Rather, we rely on third-party manufacturers to build our Turbulator components to our specifications and for our final assembly. Our current manufacturers are located in South Africa. Our products are generally delivered to our customers at their particular industrial sites where our own personnel install and test the systems, and then provide training to the customers. The materials used to manufacture and assemble Turbulator systems are commodities and, at the current time, are not in short supply. If any of our suppliers are unable to provide components, there are sufficient backup sources for components that would permit us to maintain our pricing intact.
Our Competitive Strengths
Based on its proprietary patented Turbid Fluid Photopurification Technology, SurePure is attempting to become an industry leader in the liquids purification and treatment industry. Its competitive strengths include the following:
| · | Although UV light has long been used as a surface sterilizer and has been successfully applied to clear water, we have developed the first solution that can process opaque or turbid liquids: |
| § | Our patented Turbulator design increases the liquid’s exposure to UVC, enabling greater efficacy and consistency in purification |
| § | The turbulent flow of the liquid over the UVC source for an extended period at controlled distances from the source ensures a non-fouling, self-cleaning system |
| § | The multiple-lamp system used in the Turbulator system provides greater flexibility |
| · | The Technology is an alternative to comparable heat - or chemical-based processes and has a greater microbiological efficacy than conventional, laminar-flow UV systems in turbid liquid applications. |
| · | The Turbulator uses a low maintenance, non-fouling technology that offers significant process and energy savings. |
The principal commercial benefits of our Technology and the systems that incorporate it include:
| · | an effective alternative to pasteurization; |
| · | replacement of chemical and other preservatives; |
| · | significant savings of energy and water; |
| · | extended shelf life of treated products; |
| · | preservation of the taste, smell and look of the raw materials or finished products; and |
| · | delivery of replicable, predictable germicidal efficacy. |
Notwithstanding these strengths, we nonetheless encounter and therefore must overcome serious challenges to widespread adoption and implementation of our Technology. As is the case with any business that seeks to replace an existing conventional modality, such as purification heat or preservatives, we have the burden of persuading customers and others of the superiority of the results that we produce and the value to our customers of the return on their investment in our technology. In applications, such as industrial application, where there is no regulatory involvement, we rely on various “cost/benefit” analyses to substantiate the case for acquiring and installing our system. In applications, such as dairy or foods, where there is regulatory involvement, we must demonstrate to the regulators that our Technology and its use of UVC either fits within the scope and application of existing rules and regulations, or, more often, we must persuade the regulators, by evidence they deem to be satisfactory, that they can amend or vary their regulations so that customers can employ our Technology. In many circumstances in which our technology is regulated, we prefer to present initially our Technology, as an adjunct to, and not as a replacement of, existing purification technologies. Although we believe that adjunct use will eventually lead to replacement, we do not yet have an established track record in this respect.
Sales
We have focused our sales efforts on “early adopter” global clients with multiple processing plants who have a substantial market share in their industries. Currently, we generate minimal revenues under a few existing customer agreements.Most of our sales activity takes place within our subsidiary SPO, as that company owns and administers all of our patents other than the South African patent. Most of our current sales arrangements are with SPO.
To date, we have focused on the brewing industry and its need to purify liquid inputs into its final products, such as simple sugar syrup, as well as producers of fruit juice and wine. For example, we have focused on the brewing industry in emerging economies because of the strong possibilities for our customers’ growth and growth in demand for their products in those regions. In 2009, we sold our Technology to a South African facility of a major international brewer. Depending on regulatory developments (see "Regulation" below), we will target businesses that both produce and use liquids in their production processes and require the highest levels of purification of those liquids. The customers that we serve in one particular geographic market may not be the same type of customers that we serve in another geographic market, as we expect to continue to be opportunistic with respect to sales and market penetration.
We anticipate expanding the network of distributors that we currently have established, and in certain areas we will continue to grow our own direct sales force. To date, we have selected distributors by type of liquid and by geographic region and have sought out existing distributors of engineered systems. Currently, we are represented by approximately ten distributors, one of which is based in South Africa and two distributors elsewhere in Africa. In October 2012, we entered into an agreement with a distributor for the wine industry in Spain and Italy. Putting into place a distribution network requires that we identify the best players to serve the specific industries in which our end users operate, that we provide them with that equipment and training that enables them to both demonstrate and explain the advantages of our Technology and that we design our Turbulator systems so that they are a compelling offering that permits our distributors to effectively utilize their resources in placing our products. The customer sales cycle is long for any new technology and our distributors may face resistance as a result of the historical investment that customers have made in existing facilities. Nevertheless, we believe that during 2013 we will identify those priority markets in which we must have distributors and that we will enter into appropriate contracts with distributors in those markets. See“Risk Factors —“We do not have an expansive or well developed distribution network.” ”
We also must raise sufficient working capital to support any distribution network that we establish. For example, we believe that each distributor will require at least $50,000 of equipment on hand for demonstration and similar purposes. In addition, our distribution network will require sales support. Optimally, we would provide the support of a microbiologist/biochemist and an expert in the design and construction of engineered processes to our distributors to assist them in their sales efforts. In addition, we will require working capital to pay the general and administrative costs relating to in-house staff additions such as sales trainers and regulatory experts.
During 2012, the following revenue-generating contracts were in place with customers:
| · | a contract with a producer of fruit juice in South Africa under which SPMSA maintains Turbulator systems at the producer’s facility, and SPMSA provides on-site maintenance services to the UV equipment, including, repair services and engineering improvements, the term of which contract expires on October 27, 2013; |
| · | a contract with a South African dairy under which SPMSA is paid on a usage basis for a standalone system containing UV equipment for use on dairy milk products, the term of which contract expires on October 28, 2014; and |
| · | an exclusive world-wide license with a global equipment manufacturer located in the State of Illinois, United States of America, for sterilizing milk and milk components to be used in the feeding of cow calves, which license is worldwide subject to the licensee’s meeting stated minimum sales guarantees and which becomes royalty-generating, the term of which license expires April 30, 2018 and is renewable in five one-year terms. |
During 2012, our revenue for these three contracts has been approximately $200,000 under contracts managed from Switzerland. The license referred to above provided most of our 2012 revenues.
We also have entered into a patent license agreement with a major dairy operator in the United Kingdom under which the licensee is evaluating our technology in connection with the extension of the shelf life of milk. The license was entered into in October 2010, at which time the licensee paid us a one-time fee of approximately $40,000. The territory of the license includes both the United Kingdom and the Republic of Ireland. The license permits the licensee to evaluate our Technology and to use that evaluation to pursue a Novel Food Application with the European Union Commission. The license provides that if the European Union Commission approves the Novel Foods Application, then the licensee may, at its option, extend the license so that it is able to produce liquid milk commercially for a period of 24 months after the approval, contingent on the licensee’s agreeing to purchase from us one purification system with at least 200 Turbulators. If the licensee elects to produce milk commercially using our Technology, the parties have agreed to negotiate the terms under which equipment may be purchased from us, subject to a limit on equipment prices that the parties have agreed to. If not terminated earlier by mutual agreement, the license remains in force until nine months after a final decision of the European Union Commission on the Novel Foods Application for the Technology or, if the licensee has exercised its right to option to extend the license, for 24 months after the final decision of the European Union Commission.
We are currently in discussions with a number of customers, and we expect to enter into between 6 and 10 revenue-generating contracts with customers during 2013. However, because our sales cycle is long and often requires negotiation with multiple levels and groups within a customer, we cannot be certain of the timing of these contracts and the amount of revenues to be generated either on a period-by-period basis or over the life of the contract. See “Risk Factors--“We face significant obstacles to the commercialization of SurePure Photopurification Technology and our Turbulator systems.” ”
Marketing
To maximize our marketing funds, we primarily utilize public relations, trade shows and customer trials to convert awareness into interest and purchase.
Our current working strategy is to locate markets and market participants who require effective means for purifying liquids. Since inception, we have focused on producers of milk and other dairy products, fruit juices, wine and sugar syrups in the United States, the United Kingdom, the European Union, South Africa and India. In each case, we seek to approach the strongest market participants and explain the advantages of our Technology as an adjunct to or as a replacement of existing conventional processes. To the extent that permitted uses of our Technology is subject to regulation that limits, restricts or prohibits the use of UVC as a means of purification of a specific liquid or food, we work with the customer in various tests to support an application to amend or replace the applicable regulation.
Our technology was developed in South Africa. Since our management team is familiar with the dairy, fruit juice and wine industries in South Africa, we initially focused on the South African market. During the last five years, however, we have been working with customers in the United States, the United Kingdom and the European Union, as well as Australia, New Zealand, South America and Canada. We also continue to explore emerging economies, such as India.
Regulatory Matters
The application of our SurePure Photopurification Technology to purify or disinfect milk, other dairy products, wine and juice generally requires that we take various steps to obtain regulatory clearances in each of the jurisdictions in which we plan to market our Technology. The compliance measures that we are required to take differ based on whether we are seeking to have the Technology sold as an adjunct to pasteurization or as an alternative to pasteurization, the latter having a higher standard for clearance. Other uses of our Technology—industrial uses, for example—do not require compliance with regulations governing food or dairy products. To date, we have pursued compliance efforts principally in the United States of America, the European Union and the Republic of South Africa.
United States of America
The FDA amended its regulations to permit the use of UVC to purify water and certain fruit juices in 2001, as long as certain performance characteristics are complied with. With regard to milk and other dairy products in the USA, we plan to market our Technology first as an adjunct to pasteurization. Specifically, we are applying to treat raw milk, which is milk prior to any pasteurization or any other similar process. Although we have not committed to doing so, we plan to pursue clearance from the FDA as an alternative to thermal pasteurization in the future. The timeline for this process may be as long as four to six years and will likely require that we devote additional resources to fund the necessary testing.
The FDA classifies the use of UVC to treat milk or other food as a food additive, the use of which must be expressly permitted. The current FDA regulation that permits the use of ultraviolet radiation for the processing and treatment of food does not include the use of UV light as a means of purification of dairy products, although it does permit the use of UV light in treating potable water and juice products, as well as high fat-content food. In June 2012, in compliance with the FDA’s procedures, we submitted a draft application to amend the FDA’s food radiation regulation to permit use of UV light. We filed a draft of our petition to amend the regulation to the Office of Food Additive Safety (“OFAS”) of the FDA’s Center for Food Safety and Applied Nutrition (“CFSAN”). In August 2012, CFSAN confirmed our view that an amendment of the FDA’s food radiation regulation would be required and noted certain deficiencies in our draft petition for the use of our Technology in the pasteurization of milk. When we have successfully completed tests that generate the necessary data, we intend to submit those data and studies to address directly CFSAN’s comments in an effort to establish the safety of our treatment process. Once OFAS receives our formal petition, it must act within 15 days to determine whether it is suitable for filing, in which case we are so notified and OFAS publishes a notice to that effect in the Federal Register. At that time, OFAS also determines whether there is any need for review by agencies in addition to CFSAN, and formal review by OFAS commences. If all required safety information has been included in our petition, the scientific review should be completed within approximately six months. The wording of our suggested amendment to the food radiation regulation also undergoes a review for approximately six months, at which point OFAS drafts a final rule, which we will then review. When OFAS has completed its full consideration of our petition, and if that review is positive, the FDA will publish a final rule in the Federal Register. We understand that the timeframe for publication is two to three months and that the average length of time between submission of a final petition and publication of the rule is 24 months. Accordingly, full approval is possible in late 2015 or 2016.
In addition, the U.S. Food Drug and Cosmetic Act, which prohibits the manufacture or sale of misbranded foods, provides that any food that purports to be or is represented as pasteurized must have been subjected to a safe process or treatment that meets certain standards. We must satisfy these standards to the satisfaction of the FDA if our purification process does not satisfy the applicable FDA regulations. The submissions that we have made to date to the FDA have not produced a determination that our Technology satisfies its standards. We plan to continue our dialogue with the FDA and expect to provide reports and data which will satisfy its requirements. There cannot be any assurance, however, that the reports and data that we do provide will result in a finding that is favorable to us.
We also are required to obtain FDA approval for the use of our Technology for wines or other alcoholic beverages before theAlcohol & Tobacco Tax & Trade Bureau will make any determinations with respect to the application of our Technology to purify those products.
European Union
In the European Union, before our Technology can be used commercially with respect to foods, food ingredients and beverages, it must receive clearance under Regulation (EC) No. 258/97 of the European Parliament and of the Council of 27 January 1997 Concerning Novel Foods and Novel Food Ingredients. The application pertaining to our Technology has been submitted by a member company of the EU dairy industry that is a customer of ours. The matter is being reviewed by the Novel Foods, Additives and Supplements branch of the EU’s Food Standards Agency. In the application regarding our Technology, our customer has claimed that its use significantly increases the levels of Vitamin D in milk and extends the shelf life of dairy foods. The purpose of the EU Novel Foods review is to determine whether the use of our Technology presents a danger to the consumer, misleads the consumer or results in dairy products that are nutritionally disadvantageous to the consumer. The EU process requires the party who will be distributing the product to submit a request to an individual member state of the EU (the “Member State”), which, in our case, is the United Kingdom. The Member State designates an official committee to review the application, which determines whether additional assessment is required. At the same time, the request to distribute the novel food is forwarded to the European Commission. We understand that the initial assessment of the application should be completed within three months of the initial request to the Member State, not counting any time required to complete the submission if it is deemed incomplete in some respect. If the official committee requests additional assessment or if there are objections from other Member States, the Commission then asks the European Food Safety Authority to render an opinion. When the opinion is rendered, the Commission and the Standing Committee of the Food Chain and Animal Health make their decision. We understand that the entire process is unlikely to take less than one year from submission of the request, unless the initial report of the Member State does not require additional assessment. At this time, we do not know whether additional assessment will be required. One of our customers has submitted a request to the Food Safety Authority in the United Kingdom. We anticipate that the earliest time at which we may receive the necessary approval is during 2013.
Republic of South Africa
In South Africa, we are engaged in a process to draft or amend a series of regulations of the Ministry of Health that apply to various foods and foodstuffs. In May 2012 we filed an application to amend certain regulations relating to Milk and Dairy Products GN R1555 of 21 November 1998 applicable to milk and dairy products, wine, fruit juice and other microbiologically sensitive liquids to permit our Product to be used as an alternative to pasteurization. In the case of certain regulations, draft regulations have been published for comment in the government’s official publication. In other cases, publication of the regulations may not occur until we have responded to requests for further information from the government’s Directorate: Food Control, including information that relates to the disposition of applications that we have submitted in the US and the EU. The Directorate of Food Control, Department of Health is not prepared to make an application to the Minister of Health to amend the regulation until such time that we have received either FDA or Novel Food Approval. In addition, the Directorate has advised us that the Agricultural Products Standards Act will have to be amended to conform with any amendments to South Africa’s regulations relating to milk and dairy products. Accordingly, the agency has requested that we send it peer-reviewed pasteurization reports or studies for our Technology for use an as alternative to pasteurization. Apart from the Minister being required to publish proposed new and/or amendments to existing regulations in theGovernment Gazette for public comments, allowing a three month period for this, South Africa is under obligation to notify the World Trade Organization (“WTO”) of such legislation being published. The notification will be shared with all the member countries of the WTO, who will have under the relevant provisions of the WTO an opportunity to also comment on the legislation published by South Africa. Accordingly, for this process, a six-month comment period is required.
Competition
Generally, we compete with a number of engineered services businesses who design and, in some cases, install purification systems for beverages, dairies, wineries and other manufacturers or processors of beverages. We also compete with those businesses that manufacture the systems that are use to pasteurize or otherwise purify beverages and other liquids. Both the service providers and manufacturers with which we compete generally are private companies, although a number are associated with large international conglomerates.
We believe that the principal competitive factors in our industry that create barriers to entry include, but are not limited to, reliability, effectiveness of technology to achieve requisite levels of purification, reputation, availability of resources and regulatory compliance for certain applications.
We believe that any current thermal or chemical preservative process for liquids is a direct competitor to our business. Other developing processes, such as ultrasound, high-pressure or sterile filtration, are also a source of competitive threat.
Our strategy for dealing with our competition is to gain broad commercial acceptance of our Technology by pursuing those applications, such as dairy, that have rigorous regulatory standards that must be met, and at the same time work with large international business to receive their backing for our Technology. We also focus our efforts to gain acceptance in emerging growth economies where we believe that our Technology has competitive advantages such as requiring the use of less power than other means of purification and where there are a large number of “Greenfield” installations for our Technology.
Intellectual Property
Our patented Turbid Fluid Photopurification Technology is protected by patents which have been granted in 66 countries throughout the world, including South Africa, United States, China (PRC), Japan, Australia and the countries of the European Union. A predecessor entity to SPHSA applied for our patents in October 2000. Each of the patents is based on the same international patent application and relates to the same invention. The patents will expire in the various countries on October 12, 2020. Our United States patent was granted on July 12, 2005 and will expire on October 12, 2020. Patent applications for our invention remain outstanding in Brazil and Cyprus.
The applications for these patents were examined independently by each of the patent offices where patent applications were filed, and each of the examiners in the various countries conducted an independent international novelty search for related published technology and then assessed whether or not the invention was new and inventive and thus qualified for a patent. Each examiner decided independently at the end of his examination that a patent should be granted. Accordingly, we believe that it is unlikely that a third party could successfully challenge the validity of any of our patents, and we further believe that our patents are enforceable with respect to the claims that they describe. The costs of enforcing a patent against an alleged infringer vary significantly from the relatively minor cost of sending a simple cease-and-desist letter which could result in cessation of the infringement, to the significant cost of bringing and maintaining a full patent infringement action. We believe that a third party may be infringing our Brazilian patent right and using our trade secrets in Brazil. We have engaged Brazilian counsel to advise us of our rights in this matter and whether we have an effective remedy available to us. See under the heading “Risk Factors —“We rely heavily upon our patents and other intellectual property.””
We plan to continue to develop our Technology with a view to applying for further patents.
We also own the registered trademark “SurePure” in various formats in South Africa. We have filed trademark applications for “Turbulator” in the United States, South Africa, Switzerland and the EU.
Research
We have contracted for and funded research efforts regarding the use of UVC as employed by our SurePure Photopurification Technology in the purification of liquids generally, and foods and beverages in particular, at academic institutions throughout the world. Generally, we have commissioned research in areas such a flow dynamics, biochemistry and microbiology. We, and in one or two cases, our customers, have provided funding for the studies, which generally covered both direct costs as well as overhead, and we also have provided our equipment to the institutions. In certain cases, we have provided annual grants to academic institutions to study the effects of UVC energy on foods and beverages. The academic institutions that contracted to perform studies of our Technology or that received grants from us include:
| Ø | University of Wisconsin – Madison, Wisconsin |
| Ø | Cornell University – Ithaca, New York |
| Ø | Queens College – Belfast, Northern Ireland |
| Ø | Campdens BRI – Gloucestershire, United Kingdom |
| Ø | University of Birmingham – Birmingham, United Kingdom |
| Ø | University of Stellenbosch – Stellenbosch , South Africa |
| Ø | University of the Western Cape – South Africa |
| Ø | Cape Peninsula University of Technology – Cape Town, South Africa |
By way of example, results of studies conducted at these institutions include the following:
| o | A 2011 published study that explains the effects of UVC radiation on microorganisms found in grape juice and wine products which found that our Technology “clearly indicated significant germicidal effect against the wine specific microorganisms; therefore, UVC radiation may stabilize grape juice and wine microbiologically in conjunction with reduced [sodium dioxide] levels” and that UVC radiation effectively inactivates wine-associated microorganisms such asBrettanomyces,Saccharomyces,Acetobacter, Lactrobacillus, Pediococcus andOenococcus. |
| o | A 2010 unpublished study of the use of our Technology to produce grape juice and wine that is free of microorganisms that found that using the UVC treatment on grape juice and wine samples in five different wineries could provide the wine significantly free of microorganisms (i.e., to the extent of less than 1 colony-forming unit per milliliter). |
| o | A 2012 published study that examined the use of UVC light to inactivate microorganisms that found a 100% improvement in predicted microbial kill due to the novel swirl design of our Turbulator. |
| o | A 2011 published study that investigated the efficacy of UVC radiation technology to inactivate microorganisms in milk and concluded that UVC treatments produced microbial reductions similar to traditional heat pasteurization. The study also noted that UVC has the advantage of not producing chemical residues, by-products or radiation; is a cold process requiring very low maintenance at low cost; and does not require energy to produce heat. |
| o | A 2008 published study that concluded that UVC radiation was successful in reducing bacterial counts in raw bovine milk, as well as apple juice, guava juice, pineapple juice and orange juice, while preserving the nutrients of the beverages being treated. |
| o | A 2011 unpublished study currently under peer review that investigated whether UVC treatment could reduce the number ofAlicyclobacillus acidoterrestriscells in water, fruit concentrate and grape concentrate and found that our Technology is a viable way to control contamination of juice concentrate by species of that bacterium. |
We believe that these studies were undertaken by qualified scientists who were employed by academic institutions with experience in the areas of scientific endeavor that relates to our Technology. We also believe that these scientists performed their research in accordance with the ethical and scientific guidelines of their respective universities. Prior to being published, the results were reviewed by scientific peers and presented by journals with international following. Accordingly, we believe that we and others can rely on these studies as proof of the efficacy of our Technology as presented in the studies. We have presented these studies to regulators as evidence of the efficacy of our Technology.
In addition to evaluating the results of our Technology as a means of purification, these studies also provided information with respect to certain critical process parameters, such as optimal dosage and flow rates. We plan to commission additional studies to gather additional information on these topics as well as certain toxicological and chemical safety studies. The methods to be used for our studies must comply with various regulatory standards. Any academic institutions with whom we might deal are likely to require that we fund these studies, and, accordingly, we will require additional financing so that we are able to pay all of the expenses and overhead costs that we are required to pay.
Employees
As of December 31, 2012, we employed 12 people directly, 11 of whom are based at our operational headquarters near Cape Town, South Africa. We continue to utilize the services of key academic and professional consultants in the areas of microbiology, biochemistry process engineering and manufacturing to enable us to grow the business and service our customer base on a global basis.
Item 1A. RISK FACTORS
Our operations and securities are subject to a number of risks. Below we have identified and discussed the material risks that we are likely to face. Should any of the following risks occur, they may adversely affect our business, financial condition, and/or results of operations as well as the future trading price and/or the value of our securities.
Risks Relating to our Business, Financial Condition and Corporate Structure
We have a history of significant operating losses for the past seven years and these losses may continue in the future.
We have incurred net losses of approximately $25,192,000 for the period from inception through December 31, 2012. We have been without significant revenue since inception and our current agreements provide relatively limited amounts of sales and royalty income. Our historical losses are expected to continue into the future unless we are able to generate significant amounts of revenue in our business. Our history of significant operating losses has made it difficult for us to raise additional working capital.
We require additional capital to fund our operations, and we may not be able to obtain sufficient capital and may be forced to limit the scope of our operation and cease implementation of our strategic plan.
As of the date of this annual report, we have not been able to raise significant amounts of additional capital from the institutional investors with whom we had dealt in late 2012 and early 2013. If we are unable to secure adequate additional financing on reasonable terms, or on any terms, we may not be able to pay our operating expenses and operate our business. There is no assurance that additional financing will be available to us.
In connection with our growth strategies, we may experience increased capital needs and accordingly, we may not have sufficient capital to fund our future operations without additional capital investments. Our capital needs will depend on numerous factors, including (i) our profitability; (ii) the release of competitive products by our competition; (iii) the level of our investment in research development; and (iv) our need to obtain certain regulatory approvals. We cannot assure you that we will be able to obtain capital in the future to meet our needs.
In recent years, the securities markets in the United States and elsewhere in the world have experienced a high levels of price and volume volatility, and the market price of securities of many companies have experienced wide fluctuations that have not necessarily been related to the operations, performances, underlying asset values or prospects of such companies. For these reasons, our securities can also be expected to be subject to volatility resulting from purely market forces over which we will have no control. If we need additional funding we will, most likely, seek such funding in the United States (although we may also seek funding internationally) and the market fluctuations affect on our stock price could limit our ability to obtain equity financing.
If we cannot obtain additional funding, we may be required to limit our expansion or limit our marketing efforts. Such reductions could materially adversely affect our business and our ability to compete.
Even if we do find a source of additional capital, we may not be able to negotiate terms and conditions for receiving the additional capital that are favorable to us. Any future capital investments could dilute or otherwise materially and adversely affect the holdings or rights of our existing shareholders. In addition, new equity or convertible debt securities issued by us to obtain financing could have rights, preferences and privileges senior to the units. We cannot provide any assurance that any additional financing will be available to us, or if available, will be on terms favorable to us.
We do not current have any independent directors on our Board.
As of March 27, 2013, we ceased to have any independent directors. As of the date of this annual report, we have two members of our board and they both are members of management. Until an independent director becomes a member of our board, all board decisions will be made by these two members. Because we do not have an independent director, we also do not have a functioning Audit Committee of our Board.
Our success is dependent on our ability to commercialize our SurePure Photopurification Technology and our Turbulator photopurification systems to generate sufficient revenues to sustain and expand our operations.
The execution of our business plan in the near-term is wholly dependent on our ability to commercialize our Technology through the use of our Turbulator line of photopurification systems to produce sufficient revenues to cover our operating expenses. The success of these endeavors will require that sufficient funding is available to us to finance the manufacture, marketing and sale of our current Turbulator systems and the development of new photopurification products and applications for those systems. Should we be unable to improve our financial condition through debt or equity offerings, our ability to successfully advance our business plan will be severely limited and we could be forced to discontinue or liquidate our business. Were this to occur, our shareholders would not be likely to receive any significant return, or any return at all, on their investment.
We face significant obstacles to the commercialization of SurePure Photopurification Technology and our Turbulator systems.
The industries in which we intend to commercialize our Technology are characterized by existing processes and technologies that have been used for very long periods, such as pasteurization, filtration and chemical and other preservatives. It is difficult for us as a business with limited resources to displace these existing processes and technologies and to gain acceptance even as an adjunct to the existing modalities. In the case of businesses with existing facilities, a change to our Technology may require the abandonment in whole or in part of existing facilities which have been in place for an extended period of time. In the case of businesses about to build or invest in new facilities, we face the challenge of championing a newer and often lesser-known technology.
In addition, the principal industries that we have targeted—dairy products, juice products, beer, wine and food—are regulated by government agencies or subject to standards promulgated by industry bodies. These agencies and bodies operate under and enforce regulations which often do not permit UVC purification without change in applicable legislation or regulations or both or classify UVC purification as a food additive. As a result, we are not permitted to address these industries in major markets, such as the United States of America, the European Union or South Africa, until the restrictive regulations have been changed to permit the use of our Technology or until our Technology has been approved for use in conjunction with foods and beverages. The processes that we have undertaken in our attempts to change legislation and other regulation are often prolonged and difficult. See below under “Regulatory Matters” under the caption “Business.”
To overcome these challenges, we plan to continue to create broad awareness of the advantages of our Turbulator systems among those businesses and others that have purification requirements in their processes. We also plan to continue our efforts to obtain regulatory and similar approvals where required. At the same time, we plan to keep pace with outside technological developments, to respond adequately to technological developments and to seek out additional industries whose liquid purification needs are not being met adequately or at all. As a result, we believe that we will be required to expend significant amounts to hire or engage and manage those persons with the networks, skills, experience and resources to execute these plans. We believe we must also continue to fund independent research at qualified academic and similar institutions to investigate fully the scientific basis of our Technology. The financial and other resources that we currently have available are not adequate for all of these purposes, and we will require significant additions to enable us to execute these plans. If we are not able to secure additional funding, we may not be able to address these challenges adequately or at all and therefore could be forced to discontinue our business. Were this to occur, our shareholders would not likely receive any significant return, or any return at all, on their investment.
Our SurePure Photopurification Technology may become obsolete.
The ability of UVC to destroy microbes and other pathogens has been known for several years. The principal advantage of our Turbulator systems is that they employ UVC’s purification properties in an extremely efficient and effective manner specifically as it relates to turbid liquids. There is no assurance that another business could not discover and commercialize a system that would use UVC or some other energy source in a manner that would be even more efficient or effective, or both, in which case our Technology might be rendered obsolete. Were this obsolescence to occur, we could encounter significant difficulty in selling or otherwise deploying our systems and could be forced to write off the value of our investment in our Technology. In addition, if we were not able to deploy technology or systems that met the challenge of these developments, we could be forced to discontinue our business. Were this to occur, our shareholders would not likely receive any significant return, or any return at all, on their investment.
We have not fully developed our business model for revenue generation and therefore do not have a uniform pricing or distribution strategy.
In the past, we have distributed our Technology both by selling systems and by entering into royalty agreements for processes that incorporate our Technology. Certain customers have preferred to purchase our Turbulator systems for a single, non-recurring payment. We also have been able to put in place certain patent and intellectual property license agreements that require payments over time based on the amount of liquid or other product processed. Because we choose to continue to be flexible in the marketplace, we do not yet have a single model for generating our revenue. This may place us at a competitive disadvantage with our competitors and with other technology businesses, which may have simpler or more easily understood revenue or cost structures. This lack of clarity may also make it more difficult for us to attract and engage distributors for our Turbulator systems and to explain our business to investors.
We compete with established purification processes with wide market acceptance and installed bases.
Since we acquired the SurePure Purification Technology in 2005, we have faced considerable difficulty entering the marketplace, despite what we believe to be our considerable advantages. The success of our business is likely to depend principally upon our ability to convincingly demonstrate the technical and cost advantages of our Turbulator systems and secure a reliable base of customers who are willing to install our Technology together with or in replacement of the existing purification technologies. It is possible that, despite our ability to prove our effectiveness and our cost efficiencies across a range of industries, our customers will, because of their reluctance to adopt our technology, prefer to fully amortize their investment in existing purification systems or, because of simple inertia, remain unwilling to purchase our Turbulator systems or otherwise acquire our Technology.
At the same time, many of the businesses that sell and install the existing processes have considerable resources and sophisticated marketing plans and operating distribution networks. These factors have made and may continue to make it difficult for us to compete with these businesses. In addition, many of our potential customers engage and rely on industrial engineering firms to recommend and select purification systems for their facilities. The fact that we are a relatively new business with a newly developed technology and with very limited resources may make it difficult for these engineering firms to recommend our Technology over the existing modalities.
In addition, competitors could develop technologies or systems similar to or better than our own, finish development of new technologies in advance of us, or be more successful at marketing new products, any of which factors may hurt our prospects for success.
If we are not able to deal effectively with these competitive factors either directly or by securing geographic markets or industrial markets where these competitive factors are less intense, we may not be able to generate revenues for our business. As a result, without revenues, we may be forced to discontinue or sell our business. Were this to occur, our shareholders would not likely receive any significant return, or any return at all, on their investment.
General economic conditions will affect our operations.
Changes in the general domestic and international climate may adversely affect the financial performance of the Company. Factors that may contribute to a change in the general economic climate include industrial disputes, interest rates, inflation, international currency fluctuations and political and social reform. Further, the delayed revival of the global economy is not conducive to rapid growth, particularly of technology companies with newly commercialized products.
To date, we have chosen to direct our marketing efforts to markets in emerging economies, which economies may exhibit less stability and predictability than developed economies.
Since we acquired our Technology, we have chosen to devote a considerable amount of our resources to marketing it in South Africa and other emerging economies. We believe that emerging economies tend to offer substantial opportunities in, for example, the construction of new breweries and dairies to produce beverages for their rapidly expanding consumer populations. We also believe that it may be easier for the owner or operator of a facility that is about to be built to adopt our Technology than for the owner or operator of an existing facility. Doing business in emerging economies, however, is subject to substantial risks. Among other things, economic conditions in those countries may be volatile. The role of governmental agencies and other regulators may be subject to change without advance notice or without any notice. The plans of our current and potential customers to construct or complete the planned construction of their facilities therefore may change. As a result, the investment that we have made with respect to the projects may not have the return that we anticipated or any return at all.
We do not have an expansive or well-developed distribution network.
To date, we have relied on distributors to sell our Technology on a country-by-country or region-by-region basis without putting into place a coordinated international distribution network. We believe that the establishment and maintenance of an effective worldwide distribution network will require a substantial amount of financing which has not yet been available to us. If we are not able to secure adequate equity or debt financing in the future, we may not be able to create channels of distribution for our systems, and, as a result, our revenues may not increase.
We rely on third parties to manufacture our Turbulator systems and do not have our own manufacturing facilities.
Although our own employees design, assemble and install our Turbulator systems in conjunction with our distributors and customers, we have contracted the manufacturing of the components of our Turbulator systems to third parties. As a result, we do not directly control these components and are subject to risks, including late or non-delivery, defective manufacture, breakage in transit and insufficient supply. Any of these problems, as well as other problems deriving from outsourced manufacture of components, could lead us to default on our obligations to our customers, which could have adverse financial consequences for us and as well as adverse consequences for our business reputation.
We rely heavily upon our patent and other intellectual property.
We rely on a combination of the counterparts of our issued patent, trade secrets and licenses, and, to a lesser extent, trademarks, together with non-disclosure and confidentiality agreements, to establish and protect proprietary rights to our Technology. See below under “Intellectual Property” under the heading “Business”. Our success depends, in part, on our ability to obtain additional United States and foreign patent protection for our products, their uses and our processes to preserve our trade secrets and to maintain the patents that we hold. We do not know whether any of the claims in our issued patents or pending applications will provide us with any significant protection against competitive products or otherwise be commercially valuable. Legal standards regarding the validity of patents and the proper scope of their claims are still evolving, and there is no consistent law or policy regarding the valid breadth of claims. Additionally, there may be third-party patents, patent applications and other intellectual property relevant to our products and technology which are not known to us and that block or compete with our products. Since we do business in emerging economies where legal means for effectively addressing infringement of our patent may not be available, we may not be able to prevent infringement or to obtain judicial relief when infringement occurs.
We may not be able to effectively manage our growth.
We expect considerable future growth in our business. This growth may come from the market acceptance of our Technology, increased licensing and commercialization of our Turbulator systems and expanding applications and markets for our Technology. To achieve growth in an efficient and timely manner, we will have to maintain strict controls over our internal management, technical, accounting, marketing, and research and development functions in all of our companies, both in the United States and elsewhere. We believe that we will continue to employ sufficient quality personnel to manage our anticipated future growth. Should we be unable to successfully manage our anticipated future growth by employing or otherwise engaging personnel and professionals who will maintain these standards, our costs may increase, our growth could be impaired and our ability to penetrate the marketplaces that we address may be impaired.
Failure to receive regulatory approval and other governmental action may adversely affect our business.
We have developed our SurePure Photopurification Technology such that it can be used by industries without being subject to prior regulatory approval as well as in those industries where regulatory approval is essential. Should our Technology not receive regulatory approval, the applications of our Technology will become limited to those applications where regulatory approval is not required, and our business and financial results would thereby be seriously harmed.
In those markets and industries in which regulatory approval is required, the approval process is frequently long, unpredictable and costly. See below under “Regulatory Matters” under the heading “Business”. Although we believe that we have accumulated sufficient scientific data to prove that our Technology is both safe and effective, governmental agencies frequently request additional or updated data. To satisfy these requests may require us to expend significant amounts and delay our entry into the market place, both of which adversely affect our business. The regulatory standards that we seek to satisfy are in large part subjective, and therefore we cannot be certain that our evidence will be satisfactory in any given case until the regulatory body so determines.
Although regulatory bodies in different jurisdictions may consider approval in one jurisdiction to be persuasive on certain issues of concern, in general, we must address each regulatory body separately and attempt to satisfy its own set of standards in each case. This process is extremely time-consuming and costly. Frequently delays arise because of, among other reasons, changes in the staffing of regulatory agencies or because of changes in priorities within the regulatory agencies. Although we have employees dedicated to regulatory issues, in almost all cases we must rely on outside consultants for each jurisdiction in which we are seeking regulatory approval. Although these consultants have frequent contact and wide experience with the government agency in question, these consultants are expensive and cannot assure us of a favorable outcome or that delays will be avoided.
In certain jurisdictions, regulatory approval requires that an existing law or regulation be modified. Typically, the existing laws or regulations limit the means by which foods and beverages can be purified to the existing modalities. The modification process requires publication or debate of the intended change. Publication and debate give our competition and those businesses that have vested interests in maintaining the exclusivity of the existing modalities the opportunity to oppose our efforts to modify the existing limitations. As a result, we may be forced to spend significant resources to counter these efforts, and we may not be successful in doing so. If we are not successful, then we could be prohibited from selling or distributing our Technology in that market until we later become successful in modifying existing laws and regulations. In addition, any contention will delay the time at which we can enter a market for the regulated application.
Moreover, because we are regulated in certain markets as a food additive, we are subject to ongoing scrutiny and subject to changes in regulatory actions even after approval has been obtained. Furthermore, changes in legislation and governmental policy could also negatively impact us.
Because our Technology is applied to food and beverage products to be consumed by the public, we may face liability claims.
Although we have conducted exhaustive testing programs to identify potential material defects in our Technology and have relied on published, peer-reviewed studies of UVC by independent investigators, any undetected defects could harm our business reputation, diminish our customer base, shrink revenues and expose us to product liability claims. We carry product liability insurance in amounts that we consider to be adequate for the size of our business. Nonetheless, any determination of liability that is not covered by insurance or is in excess of insurance coverage could have a material adverse effect on our business, results of operations and financial condition.
Our group of companies contains companies that are not essential to our operation and which add to our general administrative expense.
Our corporate structure includes companies which are holding companies and which we intend to eliminate and combine with our operating companies. Until we are able to do so, these companies will add to our cost structure because of applicable reporting and similar requirements which will be reflected as general and administrative expenses in our consolidated statements of operations. Until we remove the non-essential structures, these costs may place us at a competitive disadvantage to companies that are more streamlined than we are.
Consumer advocates and others may question the safety of the effects of our Technology.
We are aware that certain organizations that purport to be consumer advocates and others may question the safety of irradiation for purposes of preservation and avoiding spoilage. Often, these persons question the safety testing that has been done with respect to the consumption of foods and beverages that have been irradiated and attempt to establish a link between irradiated food and cancer, despite the FDA’s efforts to educate the public on questions relating to irradiation. These persons may attempt to use the Internet or other media to bring pressure on governmental agencies and generate publicity against the use of irradiation. As a result, and because UVC may be characterized as a form of radiation, their concerns may adversely affect the reputation of our Technology and our ability to increase our revenues. Were their concerns to become pervasive, our business could be damaged. In that case, we might have to restrict our marketing and sales efforts to markets and industries that were not affected by governmental actions or publicity.
Need for additional employees.
Our future success also depends upon our continuing ability to attract and retain highly qualified personnel. Expansion of our business and the management and operation will require additional managers and employees with industry experience, and our success will be highly dependent on our ability to attract and retain skilled management personnel and other employees. There can be no assurance that we will be able to attract or retain highly qualified personnel. Competition for skilled personnel in our industry and in the locations in which we operate is significant. This competition may make it more difficult and expensive to attract, hire and retain qualified managers and employees.
The elimination of monetary liability against our directors, officers and employees under Nevada law and the existence of indemnification rights to our directors, officers and employees may result in substantial expenditures by us and may discourage lawsuits against our directors, officers and employees.
Our certificate of incorporation contains a specific provision that eliminates the liability of directors for monetary damages that may be awarded to us and our shareholders; further, we provide indemnification to our directors and officers to the extent provided by Nevada law. We may also have contractual indemnification obligations under our employment agreements with our executive officers. The foregoing indemnification obligations could result in our incurring substantial expenditures to cover the cost of settlement or damage awards against our directors and officers, which we may be unable to recoup. These provisions and resultant costs may also discourage us from bringing lawsuits against our directors and officers for breaches of their fiduciary duties and may similarly discourage the filing of derivative litigation by our shareholders against our directors and officers, even though such actions, if successful, might otherwise benefit us and our shareholders.
We Did Not Obtain Shareholder Approval for a Transaction with a Former Shareholder.
As required by the Share Exchange Agreement, on December 12, 2012 we redeemed and then cancelled 23,180,000 outstanding shares of our Common Stock then held by Ratree Yabamrung and Kotchaporn Bousing, two of our officers, directors and shareholders prior to the Share Exchange. We redeemed Ms. Yabamrung’s shares by assigning to her all of our assets (of which there were none at that time) and by her assumption of all of our liabilities immediately prior to the closing. We did not obtain the approval of our shareholders for the transaction that we executed with Ms. Yabamrung. Although we believe that shareholder approval was not required under Nevada law for the transaction that we completed with her, it is possible that persons who were shareholders at the time of the Share Exchange may claim that their approval was required, in which case litigation might follow. Although we believe that there would be meritorious defenses to the claim and that few shareholders would have an incentive to bring such a claim, nevertheless defense of the claim may be difficult and protracted and require the expenditure of substantial funds.
Risks Relating to our Securities
Shares of our Common Stock are very thinly traded, and the price may not reflect our value and there can be no assurance that there will be an active market for shares of our Common Stock either now or in the future.
Although our Common Stock is quoted on the OTCBB, shares of our Common Stock are very thinly traded, and the price of our Common Stock, if traded, may not reflect our value. OTCBB securities are not listed and traded on the floor of an organized national or regional stock exchange. Instead, OTCBB securities transactions are conducted through a telephone and computer network connecting dealers in stocks. OTCBB stocks are traditionally smaller companies that do not meet the financial and other listing requirements of a regional or national stock exchange. As a result, there can be no assurance that there will be an active market for shares of our Common Stock either now or in the future. Market liquidity will depend on the perception of our operating business and any steps that our management might take to bring us to the awareness of investors. There can be no assurance given that there will be any awareness generated. Consequently, shareholders may not be able to liquidate their investment in us or liquidate it at a price that reflects the value of our business. As a result holders of our securities may not find purchasers for our securities should they desire to sell the securities that they hold. Consequently, only investors having no need for liquidity in their investment and who can hold our securities for an indefinite period of time should purchase our securities.
If a more active market should develop, the price of shares of our Common Stock may be highly volatile. Because there may be a low price for shares of our Common Stock, many brokerage firms may not be willing to effect transactions in our securities. Even if an investor finds a broker willing to effect a transaction in the shares of our Common Stock, the combination of brokerage commissions, transfer fees, taxes, if any, and any other selling costs may exceed the selling price.
Further, many lending institutions will not permit the use of shares of our Common Stock as collateral for any loans.
Our shares may lose their status as “DTC-eligible.”
The Depositary Trust Company (“DTC”), a financial industry service provider that immobilizes and makes "book-entry" changes to ownership of securities, advised us that shares of our Common Stock became “DTC-eligible”. This means that our shares can be electronically transferred between brokerage accounts. It is, however, possible that in the future DTC could change the status of our shares to other than “DTC-eligible” or otherwise subject them to a limited status that DTC refers to as a “chill.” Were either or both of these events to occur, the process for transferring our shares between brokerage accounts would become manual. Manual processing may take several days and is not a favored option for companies such as us that rely on broker dealers for stock transactions. Unless and until we again became DTC-eligible, our shares may not be able to trade with any volume.
We cannot assure our shareholders that an active market for shares of our Common Stock can be sustained.
We cannot assure our shareholders that an active trading market for our Common Stock can be sustained. A shareholder may find it difficult to dispose of shares or obtain accurate quotations as to the market value of his securities on the OTCBB. Securities quoted on the OTCBB may be subject to a Commission rule that imposes various practice requirements on broker-dealers who sell securities governed by the rule to persons other than established customers and accredited investors. Consequently, this rule may deter broker-dealers from recommending or selling such securities, which may further limit its liquidity. If applicable, this rule could also make it more difficult for us to raise additional capital.
The securities issued in the Share Exchange are “restricted securities” of a former “shell company” and, as such, may not be sold except in limited circumstances.
None of the shares of our Common Stock that were issued to former shareholders of SPI, the shares of our Nonvoting Convertible Preferred Stock or the shares of our Common Stock issuable upon conversion of our Nonvoting Convertible Preferred Stock (collectively, the “SurePure US Securities”) were, at the time of the Share Exchange, registered under the Securities Act, or registered or qualified under any state securities laws. The SurePure US Securities will be sold and issued pursuant to exemptions contained in and under those laws. Accordingly, the SurePure US Securities are “restricted securities” as defined in Rule 144 under the Securities Act and must, therefore, be held unless registered under applicable federal and state securities laws, or an exemption from the registration requirements of those laws is available. The certificates representing the SurePure US Securities contain legends reflecting their restricted status.
Although we will register for resale certain of the shares of our Common Stock issued in the Share Exchange, we cannot assure our shareholders that the Commission will declare the registration statement effective, thereby enabling those shares of our Common Stock to be freely tradable.
Rule 144 under the Securities Act, which permits the resale, subject to various terms and conditions, of limited amounts of restricted securities after they have been held for six months will not immediately apply to our Common Stock because SurePure US is designated as a former “shell company” under regulations of the Commission. Pursuant to Rule 144(i), securities issued by a current or former shell company that otherwise meet the holding period and other requirements of Rule 144 nevertheless cannot be sold in reliance on Rule 144 until one year after the date on which the issuer filed current “Form 10 information” (as defined in Rule 144(i)) with the Commission reflecting that it ceased being a shell company, and provided that at the time of a proposed sale pursuant to Rule 144, the issuer has satisfied certain reporting requirements under the Exchange Act. Because SurePure US will be a former shell company, the reporting requirements of Rule 144(i) will apply regardless of holding period, and the restrictive legends on certificates for the shares of our Common Stock issued in connection with the Share Exchange cannot be removed except in connection with an actual sale that is subject to an effective registration statement under, or an applicable exemption from the registration requirements of, the Securities Act.
We are incurring increased costs and demands upon management as a result of complying with the laws and regulations affecting public companies, which could harm our operating results.
As a public company, we are likely to incur significant legal, accounting and other expenses, including costs associated with public company reporting requirements. We will also incur substantial expenses in connection with a pending registration statement that has been filed with the Commission to permit the sale of our shares that have not been registered by certain or our shareholders and with our responses to the Commission’s comments in connection with its review of that registration statement. We also incur costs associated with current corporate governance requirements, including requirements under Section 404 and other provisions of the Sarbanes-Oxley Act, as well as rules implemented by the Commission or any stock exchange or quotation system on which our Common Stock may be listed in the future. The expenses incurred by public companies for reporting and corporate governance purposes have increased dramatically in recent years. We expect these rules and regulations to substantially increase our legal and financial compliance costs and to make some activities more time-consuming and costly. We are unable to currently estimate these costs with any degree of certainty. We also expect these new rules and regulations may make it difficult and expensive for us to obtain director and officer liability insurance, and, if we are able to obtain such insurance, we may be required to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage available to privately-held companies. As a result, it may be more difficult for us to attract and retain qualified individuals to serve on our board of directors or as our executive officers.
If we fail to maintain proper and effective internal controls, our ability to produce accurate and timely financial statements could be impaired, which could harm our operating results, our ability to operate our business and investors’ views of us.
Ensuring that we have adequate internal financial and accounting controls and procedures in place so that we can produce accurate financial statements on a timely basis is a costly and time-consuming effort that will need to be evaluated frequently. Our failure to maintain the effectiveness of our internal controls in accordance with the requirements of the Sarbanes-Oxley Act could have a material adverse effect on our business. We could lose investor confidence in the accuracy and completeness of our financial reports, which could have an adverse effect on the price of our Common Stock. In addition, if our efforts to comply with new or changed laws, regulations, and standards differ from the activities intended by regulatory or governing bodies due to ambiguities related to practice, regulatory authorities may initiate legal proceedings against us and our business may be harmed.
Because we became public by means of a reverse acquisition, we may not be able to attract the attention of major brokerage firms.
Additional risks may exist as a result of our having become a public reporting company through a “reverse acquisition.” Security analysts of major brokerage firms may not cover us or our stock. Because we became public through a reverse acquisition, there may be less incentive to brokerage firms to recommend the purchase of our Common Stock. No assurance can be given that brokerage firms will want to provide analyst coverage of us or our stock in the future, which may result in less liquidity and lower trading prices for our shareholders.
We are subject to Sarbanes-Oxley and Dodd Frank and the reporting requirements of federal securities laws, which can be expensive and time-consuming.
We are subject to the Sarbanes-Oxley Act of 2002 and the Dodd Frank Wall Street Reform and Consumer Protection Act (“Dodd Frank”), as well as related rules implemented by the Commission and reporting requirements of the Exchange Act and other federal securities laws. The costs of compliance with the Sarbanes-Oxley Act and of preparing and filing annual and quarterly reports, proxy statements and other information with the Commission, and furnishing audited reports to shareholders, will cause our expenses to be higher than they would be if we had remained privately held and did not consummate the Share Exchange.
None of our executive officers has any experience managing a public company subject to U.S. securities laws, which could adversely impact our ability to comply with the reporting requirements of those laws.
None of our executive officers has had any previous experience in managing a public company subject to U.S. securities laws, which could adversely impact our ability to comply with legal, regulatory, and reporting requirements of the those laws. Our management may not be able to implement programs and policies in an effective and timely manner to adequately respond to such legal, regulatory and reporting requirements, including the establishment and maintenance of internal control over financial reporting. Any such deficiencies, weaknesses or lack of compliance could have a materially adverse effect on our ability to comply with the reporting requirements of the Exchange Act, which are necessary to maintain public company status. If we were to fail to fulfill those obligations, our ability to operate as a public company would be in jeopardy, in which event the shareholders could lose their investment. Our ability to operate successfully may depend on our ability to attract and retain qualified personnel with appropriate experience in the management of a public company. Our ability to find and retain qualified personnel on our terms and budget may be very limited.
We have never paid dividends on our Common Stock, and we do not intend to do so for the foreseeable future.
We have never paid dividends on our Common Stock, and we do not anticipate that we will pay any dividends on our Common Stock for the foreseeable future. Accordingly, any return on an investment in our Common Stock will be realized, if at all, only when a shareholder sells shares of our Common Stock. In addition, our failure to pay dividends may make our stock less attractive to investors, adversely impacting trading volume and price.
Recently adopted Commission rules prohibit a reverse acquisition company from listing on a national securities exchange until the company has been in the U.S. over-the-counter market or on another regulated U.S. or foreign exchange for at least one complete fiscal year.
Recently adopted Commission rules seek to improve the reliability of the reported financial results of reverse acquisition companies by requiring a pre-listing “seasoning period” during which the post- reverse acquisition public company must produce financial and other information in connection with its required Commission filings. The company also must maintain a requisite minimum share price for at least 30 of the most recent 60 trading days prior to the date of the initial listing application and the date of listing on any national securities exchange. As a result of these rules, it is unlikely that we will be eligible to list on a national securities exchange for at least one year following the Share Exchange, and only if our stock trades above the requisite minimum price in accordance with the listing requirements of the applicable national securities exchange.
A significant portion of the total outstanding shares of our Common Stock may be sold into the public market in the near future under a pending registration statement that has been filed with the Commission, which could cause the market price to drop significantly, even if our business is doing well.
We have filed this annual report for the resale of 16,200,000 shares of our Common Stock issued in the Share Exchange, including shares issued under the Subscription Agreement and the Additional Subscription Agreement. We may seek to increase the number of shares that we are registering for resale. Once the shares that are subject to the registration statement have been registered, they can be freely sold in the public market. Sales of a substantial number of shares of our Common Stock in the public market, or the perception in the market that the holders of a large number of shares intend to sell shares, could reduce the trading price of our Common Stock or limit increases in the trading price.
We are controlled by our current officers, directors and principal shareholders.
Our directors and executive officers and their affiliates beneficially own approximately 9.76% of the outstanding shares of our Common Stock. See below under the heading “Security Ownership of Certain Beneficial Owners and Management” in this annual report. Accordingly, our directors, executive officers and principal shareholders will have substantial influence over, and may have the ability to control, the election of our board of directors and the outcome of issues submitted to a vote of our shareholders.
Because it may be considered a “penny stock,” our shareholders may have difficulty selling shares of our Common Stock.
Our Common Stock may be considered a “penny stock” if our stock price drops below $5.00 per share and we do not meet certain net asset or revenue thresholds. These thresholds include the possession of net tangible assets (i.e., total assets less intangible assets and liabilities) in excess of $2,000,000 if we have been operating for at least three years or $5,000,000 if we have been operating for fewer than three years, and the recognition of average revenues equal to at least $6,000,000 for each of the last three years. If our shares are considered to be a “penny stock,”, they will be subject to the requirements of Rule 15g-9 under the Exchange Act. Under this rule, broker-dealers who recommend penny stocks to persons other than established customers and accredited investors must satisfy special sales practice requirements. The broker-dealer must make an individualized written suitability determination for the purchaser, considering such purchaser’s financial situation, investment experience and investment objectives, with respect to penny stock transactions, and receive the purchaser’s written consent prior to the transaction.
The penny stock rules severely limit the liquidity of securities in the secondary market, and many brokers choose not to participate in penny stock transactions. As a result, there is generally less trading in penny stocks. If you become a holder of our Common Stock, you may not always be able to resell shares of our Common Stock publicly at the time and price that a shareholder believes are fair or appropriate.
Item 1B. UNRESOLVED STAFF COMMENTS
As a smaller reporting company, as defined in Rule 12b-2 of the Exchange Act, and in Item 10(f)(1) of Regulation S-K, we are electing scaled disclosure reporting obligations and therefore are not required to provide the information requested by this Item.
Item 2. PROPERTIES
SPHSA leases two premises in South Africa under separate operating leases. One is an office facility of approximately 1500 square feet in Cape Town, South Africa which houses the management of our business, and the other is a workshop of approximately 3550 square feet also located near Cape Town, South Africa. The lease for the office facility expires on February 28, 2014. The lease for the workshop facility expires September 30, 2013. We believe that there is adequate alternative space for a workshop in a location which is convenient to us if we are not able to extend the lease for the workshop facility.
Item 3. LEGAL PROCEEDINGS
We are not involved in any material legal proceedings that are pending as of the date of this report. From time to time, we may become involved in various lawsuits and legal proceedings which arise in the ordinary course of business. Currently, SPHSA is the subject of a lawsuit relating to a license of equipment in South Africa in which a settlement has been agreed but was not completed as of December 31, 2012. Litigation is subject to inherent uncertainties, and adverse results in contested matters that may arise from time to time may harm our business.
Item 4. MINE SAFETY DISCLOSURES
Not Applicable
PART II
Item 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market for Common Stock
Our common stock has been approved for quotation on The OTC Bulletin Board under the symbol “SURP.” The Company stock began trading on January 30, 2011. The table below sets forth below shows the high and low bid prices for our common stock for the six quarters ended December 31, 2012 as reported on the OTCBB website. The information reflects inter-dealer prices, without retail mark-ups, mark-downs or commissions and may not necessarily represent actual transactions. See “Risk Factors—“Shares of Our Common Stock are very thinly traded, and the price may not reflect our value and there can be no assurance that there will be an active market for shares of our Common Stock either now or in the future.”
| | | Common Stock | | | Market Price | |
| Quarter Ended | | Bid High ($) | | | Bid Low ($) | |
| December 31, 2012 | | | 1.30 | | | | 1.00 | |
| September 30, 2012 | | | 1.52 | | | | 0.84 | |
| June 30, 2012 | | | 1.35 | | | | 1.00 | |
| March 31, 2012 | | | 1.38 | | | | 1.05 | |
| December 31, 2011 | | | 1.45 | | | | 1.05 | |
| September 30, 2011 | | | 1.60 | | | | 1.45 | |
As of December 31, 2012, 23,542,184 shares of our Common Stock were issued and outstanding, an additional 22,665,447 shares of our Common Stock were issuable upon the conversion of the issued and outstanding shares of our Nonvoting Convertible Preferred Stock, and an additional 2,765,000 shares of our Common Stock were issuable under the terms and conditions of the Additional Subscription Agreement. We also have reserved 3,000,000 shares of our Common Stock for future issuance under the 2012 Stock Plan. Of the 23,542,184 shares that were issued and outstanding as of December 31, 2012, 9,272,000 shares were held by our pre-Share Exchange shareholders of SurePure US, and the remaining 14,270,184 shares were held by former shareholders of SPI, including the holders of shares purchased under the Subscription Agreement and Additional Subscription Agreement. Those shares had been exchanged into shares of our Common Stock as provided by the Share Exchange Agreement after the closing of the Share Exchange on December 12, 2012.
Record Holders
There are approximately 50 holders of record of our Common Stock, after giving effect to the anticipated exchanges of shares of SPI for shares of our Common Stock as provided in the Share Exchange Agreement.
DTC Eligibility
DTC, a financial industry service provider that immobilizes and makes "book-entry" changes to ownership of securities, has advised us that shares of our Common Stock became “DTC eligible”. This means that shares of our Common Stock can be electronically transferred between brokerage accounts. It is, however, possible that in the future DTC could change the status of our shares to other than “DTC eligible” or otherwise subject them to a limited status that DTC refers to as a “chill.” Were either or both of these events to occur, the process for transferring our shares between brokerage accounts would become manual. Manual processing may take several days and is not a favored option for companies such as us that rely on broker dealers for stock transactions. Unless and until we again became DTC eligible, our shares might not be able to trade with any volume. See “Risk Factors—“Our shares may lose their status as “DTC-eligible.” ”
Dividend Policy
We have never paid or declared any dividends on our shares of common stock. Any future decisions regarding dividends will be made by our board of directors. We currently intend to retain and use any future earnings for the development and expansion of our business and do not anticipate paying any cash dividends in the foreseeable future. Our board of directors has complete discretion on whether to pay dividends. Even if our board of directors decides to pay dividends, the form, frequency and amount will depend upon our future operations and earnings, capital requirements and surplus, general financial condition, contractual restrictions and other factors that the board of directors may deem relevant.
Performance Graph
As a smaller reporting company, as defined in Rule 12b-2 promulgated under the Exchange Act, and in Item 10(f)(1) of Regulation S-K, we are electing scaled disclosure reporting obligations and therefore are not required to provide this the information requested by this Item.
Item 6. SELECTED FINANCIAL DATA
As a smaller reporting company, as defined in Rule 12b-2 promulgated under the Exchange Act, and in Item 10(f)(1) of Regulation S-K, we are electing scaled disclosure reporting obligations and therefore are not required to provide the information requested by this Item.
Item 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATION
This “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and other parts of this current report contain forward-looking statements that involve risks and uncertainties. Forward-looking statements can also be identified by words such as “anticipates,” “expects,” “believes,” “plans,” “predicts,” and similar terms. Forward-looking statements are not guarantees of future performance and our actual results may differ significantly from the results discussed in the forward-looking statements. Factors that might cause such differences include, but are not limited to, those discussed in the item of this annual report encaptioned “Cautionary Note Regarding Forward-Looking Statements.”
The results presented in this “Management’s Discussion and Analysis of Financial Conditions and Results of Operations” are those of the SurePure, Inc., its subsidiaries and two variable interest entities (all of which are collectively referred to in this section as the “Group”) as reflected in the Group’s consolidated financial statements included in this report. The following discussion should be read in conjunction with such consolidated financial statements and the notes thereto. The results presented below pertain to the consolidated audited annual financial statements for the years ended December 31, 2012 and December 31, 2011.
Additional Funding
The Group experienced a shortfall in funding from investors during the end of 2012 and the beginning of 2013. As a result, the Group requires additional funding to maintain its operations and execute its business plan. Should the Group be unable to secure financing from outside sources, it will be unable to meet its milestones and may scale back or even discontinue its operations until such time, if ever that it is able to secure financing. Notwithstanding the Company’s efforts to secure financing, there is no assurance that financing will be available on acceptable terms or at all.
Plan of Operation
During the next twelve months, the Company will seek to expand the commercial acceptance of its SurePure Turbulator purification systems. To achieve this goal, the Company expects to execute the following principal steps:
| · | Attend and promote the Product at three or more international trade shows during late calendar 2013 for the brewing industry and for the dairy/fruit juice industry. The purpose of the Company’s exhibitions at these venues will be to reach out to industry participants; including distributors of engineered systems, to make them aware of the Product’s advantages and the expanded applications of the Product that have been developed over the past eighteen months. |
| · | Continue to build the Company’s technical sales support team by hiring qualified sales engineers and scientists. Since the Product is an engineered system, the Company believes that both its marketing and sales efforts will be enhanced by having access to qualified technical support. |
| · | Continue to pursue regulatory clearances and approvals for applications, such as dairy products, that are required for the Product to be sold in those industries. During this period, the Company expects to take the following actions with regulatory agencies: |
| a. | To request a GRAS (i.e., Generally Regarded as Safe) review of the SurePure technology by the US Food and Drug Administration (“FDA”) for a number of applications; |
| b. | To receive approval of the material that the Company and a UK customer submitted to the UK Food Standard Authority in June 2011 in pursuance of EU Novel Food approval ; |
| c. | To receive approval from the OIV (International Organization of Vine and Wine) for the use of the SurePure technology for wine industry applications ; and |
| d. | To receive approval from the Director General: Health of South Africa of the Company’s application for the amendment of South African regulations relating to milk and dairy products permitting the use of the Product to purify milk. |
| · | Seek to market and sell the Product for industrial applications, |
| · | Create one or more distribution networks for the Products by identifying distributors according to geographic market, expertise with specific liquids and existing client relationships and negotiating agreements with the identified distributors on terms acceptable to both the distributors and the Company, |
| · | Seek to expand the Company’s management team by hiring a relevant staff with industry expertise in those geographic territories in which the Company plans to sell the Product, |
| · | Obtain additional equity financing to support the Company’s growth, under an existing stock purchase agreement with an institutional investor or otherwise, plus, if appropriate, a working capital financing to provide capital to support and augment its operations. |
Growth Strategy
The Group intends to grow rapidly over the next five years through the use of working capital and equity financing. On realizing sufficient financing, and given our strategy of targeting strategic global regions with multiple potential clients, it is feasible for us to meet our expectations. However, the Group will carefully monitor the risks associated with achieving the goals in each scenario to ensure that the Group can meet client expectations while remaining financially solvent.
Results of Operations
During the year ended December 31, 2012, the Group focused on the following developments:
| · | Continuing with commercial trials at key customer sites |
| · | Working with regulatory authorities to progress the applications for use of the Group’s technology in key areas |
| · | Evaluating new product applications for the Group’s technology |
| · | Preparing documentation and finalizing processes related to the conclusion of the Share Exchange agreement and subsequent closing |
| · | Nurturing relationships with key Investors and progressing equity financing plans |
Subsequent to December 31, 2012, the Group has and continues to focus on the following developments:
| · | Continuing with commercial trial at key customer sites |
| · | Expanding the Group’s distributor network internationally |
| · | Continuing to work with regulatory authorities to progress the applications for use of the Group’s technology in key areas |
| · | Continuation with evaluation of new product applications for the Group’s technology |
| · | Finalizing documentation and processes related to the conclusion of the Share Exchange agreement and subsequent closing |
| · | Finalizing equity financing agreements |
Results of Operations
The following tabulation summarizes the company’s results of operations for the Year Ended December 31, 2012 compared with the year ended December 31, 2011.
| | | Years Ended: | | | | | | | |
| | | Dec 31, 2012 | | | Dec 31, 2011 | | | Variance | | | % | |
| | | | | | | | | | | | | |
| Revenues | | | 594,000 | | | | 344,000 | | | | 250,000 | | | | 73 | % |
| | | | | | | | | | | | | | | | | |
| General and Admin Expenses | | | 4,103,000 | | | | 3,351,000 | | | | 752,000 | | | | 22 | % |
| | | | | | | | | | | | | | | | | |
| Research and Development | | | 297,000 | | | | 319,000 | | | | (22,000 | ) | | | -7 | % |
| | | | | | | | | | | | | | | | | |
| Interest Expense | | | 409,000 | | | | 429,000 | | | | (20,000 | ) | | | -5 | % |
| | | | | | | | | | | | | | | | | |
| Net Loss | | | 4,301,000 | | | | 4,010,000 | | | | 291,000 | | | | 7 | % |
| | | | | | | | | | | | | | | | | |
| Cash | | | 142,000 | | | | 35,000 | | | | 107,000 | | | | 306 | % |
| | | | | | | | | | | | | | | | | |
| Other Current Assets | | | 149,000 | | | | 152,000 | | | | (3,000 | ) | | | -2 | % |
| | | | | | | | | | | | | | | | | |
| Current Liabilities | | | 1,039,000 | | | | 1,211,000 | | | | (172,000 | ) | | | -14 | % |
| | | | | | | | | | | | | | | | | |
| Long term Liabilities | | | 3,461,000 | | | | 5,731,000 | | | | (2,270,000 | ) | | | -40 | % |
| | | | | | | | | | | | | | | | | |
| Cash from operating activities | | | (4,182,000 | ) | | | (3,772,000 | ) | | | (410,000 | ) | | | 11 | % |
| | | | | | | | | | | | | | | | | |
| Cash from financing activities | | | 4,154,000 | | | | 2,957,000 | | | | 1,197,000 | | | | 40 | % |
Revenues
The company recorded revenue of approximately $594,000 during the year ended December 31, 2012, an increase of 73 % on the prior year. This increase was largely attributable to the sale of a number of pieces of equipment to key customers.
The Group expects that the amount of revenue realized from sales and royalty income will increase in line with its commercialization efforts, related to the introduction of its technologies to a broader range of clients and geographic areas. The business model is largely royalty related and it is expected that royalty fee income will increase in line with the increased level of commercialization.
General and Administrative Expenses
General and Administrative expenses for the year ended December 31, 2012 were approximately $4,103,000, an increase of 22% over the year ended December 31, 2011 largely as a result of the professional fees associated with the closing of the Share Exchange and the Company’s filing of reports and other documents with the Commission. General and Administrative expenses are largely attributable to employment costs, professional fees, consultants and travel expenses.
The Group expects that expenses will increase in future periods, as the increased level of commercialization of the Group’s technology will require increased levels of staffing and associated expenditures.
Research and Development Expenses
Research and Development expenses for the year ended December 31, 2012 were approximately $297,000 which was marginally lower than in the year to December 31, 2011. A large portion of these expenditures relate to work undertaken in pursuance of the FDA and EU Novel Food approval processes.
The Group expects that Research and Development expenses will increase in future periods, as the Group embarks on new applications for the technology. In addition, it is anticipated that certain additional research work may be required to support the Company’s GRAS applications to the FDA.
Net Losses
For the period from August 24, 2005 (inception) until December 31, 2013, the Group incurred net losses of approximately $25,192,000. Net losses for the year ended December 31, 2012 were approximately $4,301,000 as compared to approximately $4,010,000 for the year ended December 31, 2011, an increase of 7%. The increase in net losses over the comparative periods can be primarily attributed to increases in expenditures related to professional fees incurred related to completion of the Share Exchange, offset by the higher sales revenues.
The Group has not as yet generated sufficient revenue to fund operations and expects net losses to continue until such time as commercialization efforts related to its technologies produce an increase in revenues sufficient to meet the cost of operations. We expect to continue to operate at a loss through fiscal 2013.
Income Tax Expense (Benefit)
The Group has increased net operating losses in Switzerland, South Africa, the United States and Brazil. The Group may have a prospective income tax benefit resulting from the net operating loss carry-forward that can offset future operating profits into the extent of any unexpired net operating losses within each company.
Impact of Inflation
The Group believes that inflation has not had a material effect on operations for the period from August 24, 2005 (inception) to December 31, 2012.
Capital Expenditures
The Group has spent approximately $182,000 dollars on property and equipment for the period from August 24, 2005 (inception) to December 31, 2012.
Liquidity and Capital Resources – the Group
As noted above in this section under the heading “Additional Funding” and as also noted below under this heading, the Group’s current cash resources are extremely limited.
The Group has been in the development stage since inception. As of December 31, 2012 the Group had cash of approximately $142,000 and other current assets of $149,000 consisting accounts receivable and prepaid expenses and total assets of $424,541 consisting of current assets, patents and plant and equipment. As of December 31, 2012 the Group had current liabilities of $1,038,598, consisting of accounts payable, accounts payable to related parties and accrued liabilities. Long-term liabilities totaled approximately $3,461,000. Stockholder’s deficit in the Group was approximately $2,740,000 as of December 31, 2012.
For the period from August 24, 2005 (inception) until December 31, 2012, the Group’s cash used in operating activities was approximately $22,776,000. Cash used in operating activities for the year ended December 31, 2012, was approximately $4,182,000 compared to approximately $3,772,000 for the year ended December 31, 2011. Cash used in operating activities during the current period can be attributed to net losses from operations.
We expect to continue to incur negative cash flows in operating activities until such time as we fully commercialize the Group’s technology.
For the period from August 24, 2005 (inception) until December 31, 2012, the Group’s cash flow used in investing activities was approximately $911,000, primarily for purchase of patents and fixed assets.
These negative cash flows from operations and investing activities have largely been funded during the period since inception by the shareholders through capital injections of approximately $13,658,000 and shareholder loans of approximately $9,422,000. We expect to continue to have cash provided by financing activities as the Group completes new rounds of financing as it seeks increase the level of commercialization of the Group’s technology.
Our current assets are insufficient to meet our current obligations or to satisfy our cash needs over the next twelve months and, as such, the Group will require additional financing. We are pursuing a number of prospective sources that include shareholder loans, the sale of equity, the procurement of long term debt and working capital finance as required to allow the group to meets its commercialization targets. We face certain financial obstacles to attracting new financing due to our historical and current record of net losses and working capital deficits. Therefore, despite our efforts, we can provide no assurance that we will be able to obtain the financing required to meet our stated objectives or even to continue as a going concern.
The Group does not expect to pay cash dividends in the foreseeable future.
The Group has a defined stock option plan and contractual commitments with all of its officers and directors.
The Group has plans to increase the number of employees in order to meet the anticipated demand on wider commercialization for the Group’s technology.
Off Balance Sheet Arrangements
As of December 31, 2012, the Group had no off-balance sheet arrangements.
Item 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURE ABOUT MARKET RISK
As a smaller reporting company, as defined in Rule 12b-2 promulgated under the Exchange Act, and in Item 10(f)(1) of Regulation S-K, we are electing scaled disclosure reporting obligations and therefore are not required to provide the information requested by this Item.
Item 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The consolidated financial statements and supplementary data required by this item are included at page F-1 herein.
Item 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None
Item 9A. CONTROLS AND PROCEDURES
Disclosure Controls and Procedures
We designed our disclosure controls and procedures, as such term is defined in Rule 13a-15(e) and 15d-15(e) under the Exchange Act, to provide reasonable assurance that information required to be disclosed by us in reports we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms, and is accumulated and communicated to our management, including our principal executive officer and principal financial officer, as appropriate to allow timely decisions regarding required disclosures.
Under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, we conducted an evaluation of our disclosure controls and procedures as of the end of the period covered by this report. Based on this evaluation, our principal executive officer and our principal financial officer concluded that our disclosure controls and procedures were effective to provide such reasonable assurance.
In designing and evaluating the disclosure controls and procedures, management recognized that such controls and procedures, as any controls and procedures, can provide only reasonable assurance of achieving the desired control objectives. In addition, the design of disclosure controls and procedures must reflect the fact that there are resource constraints and that management is required to apply its judgment in evaluating the benefits of possible controls and procedures relative to their costs.
Management’s Annual Report on Internal Control Over Financial Reporting
The management of the Company is responsible for establishing and maintaining adequate internal control over financial reporting for the Company. Internal control over financial reporting is defined in Rules 13a-15(f) and 15d-15(f) promulgated under the Exchange Act.
The Company’s management assessed the effectiveness of the Company’s internal control over financial reporting as of December 31, 2012. In making this assessment, the Company’s management used the criteria set forth by the Committee of Sponsoring Organization of the Treadway Commission (“COSO”) in “Internal Control-Integrated Framework.” Based on this assessment, management concluded that as of December 31, 2012, the Company’s internal control over financial reporting is effective.
As a smaller reporting company, the Company is not required to include in this annual report a report on the effectiveness of internal control over financial reporting by the Company’s independent registered public accounting firm.
Changes in Internal Control Over Financial Reporting
During management’s assessment of the effectiveness of the Company’s internal control over financial reporting, management did not identify any change that occurred during the last quarter of 2012 that has materially affected or is reasonably likely to materially affect the Company’s internal control over financial reporting.
Item 9B. OTHER INFORMATION
None
Item 10. DIRECTORS AND EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
In connection with the Share Exchange, our board of directors was reconstituted by the resignation of Ms. Yambamrung and Mr. Bousing from their roles as former directors of SurePure US and the appointment of Nigel Brunette, Guy Kebble, and Stephen Robinson as directors (of whom only Mr. Robinson was a director of SPI immediately prior to the Share Exchange). Our executive management team was also reconstituted following the resignation of Ms. Yambamrung as the Company’s president and treasurer by the appointment of Guy Kebble as the Company’s president and chief executive officer, Stephen Robinson as the Company’s chief financial officer and chief accounting officer and Stephen Miller as the Company’s vice president-sales and marketing.
The Company
The following table sets forth the names and ages of the Company’s directors and executive officers as of December 31, 2012. Our board of directors has no nominating or compensation committee.
| Name | Age | Position with the Company | Officer/Director
Since |
| | | | |
| Nigel Brunette | 59 | Director; Chairman | January 6, 2013 |
| | | | |
| Guy Kebble (1) | 46 | Chief Executive Officer | December 12, 2012 |
| | | President | December 12, 2012 |
| | | Director | January 6, 2013 |
| | | | |
| Stephen Robinson | 49 | Chief Financial Officer; | December 12, 2012 |
| | | Chief Accounting Officer | |
| | | Treasurer | December 12, 2012 |
| | | Secretary | December 12, 2012 |
| | | Director | December 12, 2012 |
| | | | |
| Stephen Miller | 50 | Vice President-Sales and Marketing | December 12, 2012 |
Mr. Kebble became our president and chief executive officer upon the occurrence of the Share Exchange on December 12, 2012. He became a member of our board of directors on January 6, 2013.
On March 27, 2013, Mr. Brunette resigned as a director and as chairman of the board.
Term of Office
Our directors and executive officers hold office until the earlier of their death, resignation, removal or until their successors have been duly elected and qualified. Our executive officers are employed by us on full time basis and are appointed by the board of directors and serve at the discretion of the board, subject to the provisions of the employment and consulting agreements referred to below under “Employment and Consulting Agreements” under the heading “Executive Compensation”. There are no family relationships among our directors and executive officers.
Agreements and Understandings
Our executive officers have entered into employment or consulting contracts with SPO and SPMSA. The terms and conditions of those agreements are described more fully below under “below under “Employment and Consulting Agreements” under the heading “Executive Compensation.” Except as disclosed below under “Employment and Consulting Agreements” under the heading “Executive Compensation,” there are no arrangements or understandings between any of our directors and officers, and any other person, pursuant to which he serves as our officer or director.
Background and Business Experience
The business experience during the past five years of the person(s) listed above is as follows:
Stephen Robinson —Prior to the Share Exchange, Mr. Robinson served as the chief financial officer of the SurePure group of companies since January 2008. Mr. Robinson also is a member of the board of directors of each of the companies in the SurePure group. Mr. Robinson’s role incorporates the following key areas of responsibility: finance and accounting support, legal and compliance; governance and operational support.
Mr. Robinson is a Chartered Accountant and attained his Bachelor of Accounting degree from the University of the Witwatersrand in South Africa in 1989. He has been registered with the South African Institute of Chartered Accountants since 1990. Mr. Robinson has over 20 years of financial executive experience in the mining and technology sectors during which he acquired wide ranging financial and general management skills honed in international corporate and medium sized companies in the Technology, Mining, Manufacturing, Logistics and Shared Service sectors. From September 2004 through July 2007, Mr. Robinson was employed as group finance manager for the Trans Hex Group Ltd., a South African diamond mining concern.
Mr. Robinson was appointed as a member of the board of directors due to his strong experience in management, structuring and finance planning of international businesses. In addition to his extensive familiarity with SurePure group of companies, he had been employed by Samancor (then part of the BHP Billiton Group) for ten years where he became group finance manager and was involved with international joint ventures, corporate treasury and international reporting, as well as the corporate governance and risk management portfolios. For two years, he was employed by the largest mining section in the Anglo Platinum Group to develop and manage a multidisciplinary services unit, which deployed logistical, engineering, financial and human resources support to four key operating units accounting for a substantial portion of Anglo Platinum’s outputs. Mr. Robinson is a citizen of the United Kingdom and a resident of Switzerland.
Guy Kebble —Prior to the Share Exchange, Mr. Kebble served as the chief executive officer and a director of SPHSA and SPMSA since the inception of the SurePure business in 2005, both of which companies are variable interest entities of SurePure US. Mr. Kebble’s role at SurePure incorporates the following key areas of responsibility: shareholder interaction, strategic development, brand and market assessment, key stakeholder relationships and team building.
Mr. Kebble began his career in 1990 working for Natal Rugby Union as a professional rugby player. From 1996 to 2004 he managed private property portfolio managed investments. His involvement as a financier in the South African mining business led to the SurePure technology being brought forward as a project. Mr. Kebble is a citizen and resident of South Africa. His enthusiasm, passion and drive have been instrumental in bringing the technology to the current stage of technical development and market awareness. These qualifications, among others, led to the conclusion that Mr. Kebble should serve as a member of our board of directors.
Stephen Miller —Prior to the Share Exchange, Mr. Miller served as the chief marketing officer of the SurePure group of companies since October 2008. From October 2005 through September 2008, Mr. Miller was a Group Brand Executive of AVI Limited of Johannesburg, South Africa. Mr. Miller’s role at SurePure incorporates the following key areas of responsibility: brand development, market identification and development, distributor sourcing and management, customer relationship building and support, sales process and support.
Mr. Miller holds a Bachelors of Arts Honors degree from Rhodes University, Eastern Cape, South Africa. During his business career, Mr. Miller was employed for seven years by Unilever, where he became Marketing Manager for Playtex in South Africa. He also was employed for 10 years by various design houses and managing promotions agencies, including Ogilvy, where he became that company’s strategic planning director for its communications group in South Africa. Mr. Miller gained significant branding experience while serving as the global innovations director for SAB Miller plc where he set innovation strategy, improved innovation competence and created and developed global brands for its constituent companies around the world. In 2008, Mr Miller was voted one of South Africa’s top ten brand builders by The Annual, Southern Africa’s advertising and marketing “bible.” Mr. Miller is a citizen and resident of South Africa.
Nigel Brunette —Mr. Brunette has over 20 years of experience in the senior management of international businesses and holds two law degrees. Currently he is the chairman of Gold Dragon Resources Corporation of Vancouver, British Columbia, a privately-held business. From 2006 to 2010 he served as the independent chairman of Simmer & Jack Mines Limited of Johannesburg, South Africa and First Uranium Corporation of Toronto, Canada. He also served as a director of Fralex Limited and Randgold and Exploration Limited, both of which were listed on the Johannesburg Stock Exchange, as well as several private companies. Prior to 1998, Mr. Brunette was a senior banker with Rand Merchant Bank, Johannesburg, South Africa. Currently, Mr. Brunette is self-employed and operates significant farming operations in the Eastern Cape Province of South Africa. Mr. Brunette is a citizen and resident of South Africa. Prior to the Share Exchange, Mr. Brunette had no relationship with SPI or SurePure.
Family Relationships
None of our officers or directors is related to any other officer or director.
Involvement in Certain Legal Proceedings
During the past ten years no director, executive officer, promoter or control person of SurePure US, SPI or any of their subsidiaries, and no person who became a director of SurePure US in January 2013, has:
| · | been convicted in a criminal proceeding or been subject to a pending criminal proceeding (excluding traffic violations and other minor offenses); |
| · | had any bankruptcy petition filed by or against the business or property of the person, or of any partnership, corporation or business association of which he was a general partner or executive officer, either at the time of the bankruptcy filing or within two years prior to that time; |
| · | been subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction or federal or state authority, permanently or temporarily enjoining, barring, suspending or otherwise limiting, his involvement in any type of business, securities, futures, commodities, investment, banking, savings and loan, or insurance activities, or to be associated with persons engaged in any such activity; |
| · | been found by a court of competent jurisdiction in a civil action or by the Commission or the Commodity Futures Trading Commission to have violated a federal or state securities or commodities law, and the judgment has not been reversed, suspended, or vacated; |
| · | been the subject of, or a party to, any federal or state judicial or administrative order, judgment, decree, or finding, not subsequently reversed, suspended or vacated (not including any settlement of a civil proceeding among private litigants), relating to an alleged violation of any federal or state securities or commodities law or regulation, any law or regulation respecting financial institutions or insurance companies including, but not limited to, a temporary or permanent injunction, order of disgorgement or restitution, civil money penalty or temporary or permanent cease-and-desist order, or removal or prohibition order, or any law or regulation prohibiting mail or wire fraud or fraud in connection with any business entity; or |
| · | been the subject of, or a party to, any sanction or order, not subsequently reversed, suspended or vacated, of any self-regulatory organization (as defined in Section 3(a)(26) of the Exchange Act), any registered entity (as defined in Section 1(a)(29) of the Commodity Exchange Act), or any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with a member. |
Procedures for Security Holders to Nominate Candidates to the Board of Directors
During 2012, there were no changes made to the procedures by which shareholders may nominate candidates to our board of directors. The full board of directors will consider all director candidates nominated by shareholders during such times as the Company is actively considering appointment of new directors. Candidates recommended by shareholders will be evaluated based on the same criteria described above. Shareholders desiring to suggest a candidate for consideration should send a letter to the Company’s Corporate Secretary and include:
| · | a statement that the writer is a shareholder (providing evidence if the person’s shares are held in street name) and is proposing a candidate for consideration; |
| · | the name and contact information for the candidate; |
| · | a statement of the candidate’s business and educational experience; |
| · | information regarding the candidate’s qualifications to be director, including but not limited to an evaluation of the factors discussed above which the board would consider in evaluating a candidate; |
| · | information regarding any relationship or understanding between the proposing shareholder and the candidate; |
| · | information regarding potential conflicts of interest; and |
| · | a statement that the candidate is willing to be considered and willing to serve as director if nominated and elected. |
There is no assurance that all shareholder proposed candidates will be fully considered, that all candidates will be considered equally, or that the proponent of any candidate or the proposed candidate will be contacted by the Company or the board, and no undertaking to do so is implied by the willingness to consider candidates proposed by shareholders.
Our bylaws do not set forth procedures by which our shareholders may recommend nominees to our board of directors.
Board Committees
Audit Committee
The primary functions of the Audit Committee are to: (a) review the financial reports and other financial information prepared by the Company for submission to any governmental or regulatory body or the public and monitor the integrity of such financial reports; (b) review the Company’s systems of internal controls established for finance, accounting, legal compliance and ethics; (c) review the Company’s accounting and financial reporting processes and the audits of the financial statements of the Company; (d) monitor compliance with legal regulatory requirements; (e) monitor the independence and performance of the Company’s registered independent public accounting firm; and (f) provide effective communication between the board, senior and financial management and the Company’s registered independent public accounting firm.
Until his resignation on March 27, 2013, Nigel Brunette was the sole member of our Audit Committee.
Executive Compensation, and Director Nomination and Corporate Governance Function
We do not have a compensation committee, nominating or corporate governance committee. Instead, our independent director performs the functions customarily delegated to such committees. As of March 27, 2013, however, we ceased to have an independent director. In addition, we do not have a charter that relates to the functions traditionally performed by such committees. During 2013, our board of directors is expected to appoint an independent director, appoint a compensation committee and nominating and governance committee and adopt charters relative to each such committee.
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Exchange Act requires our directors and executive officers, and any persons who own more than 10% of our Common Stock Company to file with the Commission reports of beneficial ownership and changes in beneficial ownership of common stock. Officers and directors are required by the SEC regulations of the Commission to furnish us with copies of all Section 16(a) forms they file. Based solely on our review of the copies of such reports furnished to us or written representations that no other reports were required, we believe that during the fiscal year ended December 31, 2012 all filing requirements applicable to our officers, directors, greater than 10% shareholders or any other person subject to Section 16 of the Exchange Act were met on a timely basis, except that Ratree Yambamrung and Kotchaporn Bousing did not file reports with respect to the shares of our Common Stock that they assigned back to us or surrendered for cancellation as part of the Share Exchange, nor did they file reports upon their resignations from SurePure.
Code of Ethics
We have a Code of Ethics and Business Conduct (the “Code”) that applies to all directors, officers and employees, which will be posted on its website or can be obtained by writing to us at 405 Lexington Avenue, 25th/26th Floor, New York, New York 10174 c/o Corporate Secretary. All of our directors, officers and employees are expected to be familiar with the Code and to adhere to those principles and procedures set forth in the Code that apply to them. The Company will post any amendments to the Code, as well as any waivers that are required to be disclosed by the rules of the Commission, on the Company’s web site.
Item 11. EXECUTIVE COMPENSATION
In determining executive compensation, we consider the skills and added value of the individuals concerned, as well as relative cost of living and the statutory requirements in the jurisdiction in which the individual provides his services. Since our executives provide their services to various of our subsidiaries, in some cases we use multiple employment contracts. As of July 1, 2012, we introduced updated remuneration levels, resulting in a 15 – 20% reduction in executive compensation from that date. In addition, to ensure a smooth transition to full commercialization, the executives and the Company have agreed to a minimum term of employment through December 31, 2014.
Summary Compensation Table
The following table sets forth, for the periods indicated, all of the compensation awarded to, earned by or paid to (i) each individual serving as a principal executive officer of SurePure or any of the subsidiaries or related operating entities of SurePure during our last completed fiscal year; and (ii) each other individual that served as an executive officer or significant employee of SurePure or any of the subsidiaries or related operating entities of SurePuew at the conclusion of the fiscal year ended December 31, 2012 and who received in excess of $100,000 in compensation during such fiscal year (collectively, the “named executive officers”).
| Name and Principal Position | | Year | | Salary | | | Bonus | | | Total | |
| Ratree Yabamrung, | | 2012 | | | - | | | | - | | | | - | |
| Director, President and Treasurer (1) | | 2011 | | $ | 2,400 | | | | - | | | $ | 2,400 | |
| Kotchaporn Bousing, | | 2012 | | | - | | | | - | | | | - | |
| Director and Secretary (1) | | 2011 | | | - | | | | - | | | | - | |
| Guy Kebble, Director, President and Chief | | 2012 | | $ | 331,512 | | | $ | 20,000 | | | $ | 351,512 | |
| Executive Officer (2) | | 2011 | | $ | 360,000 | | | $ | 40,000 | | | $ | 400,000 | |
| Stephen Robinson, Director and Chief | | 2012 | | $ | 475,790 | | | $ | 20,000 | | | $ | 495,790 | |
| Financial Officer (3) | | 2011 | | $ | 560,817 | | | $ | 40,000 | | | $ | 600,817 | |
| Stephen Miller, Vice President-Sales and | | 2012 | | $ | 289,512 | | | $ | 20,000 | | | $ | 309,512 | |
| Marketing Manager (4) | | 2011 | | $ | 284,000 | | | $ | 40,000 | | | $ | 324,000 | |
| (1) | No longer serves as an officer of SurePure US as of the Share Exchange. |
| (2) | Has served as chief executive officer of the companies controlled by SPI and as Chairman of SPMSA since January 1, 2010. |
| (3) | Has served as chief financial officer of the companies controlled by SPI and as the sole director of SPI, SPO and SPP and as a director of SPMSA and SPHSA since January 1, 2008. |
| (4) | Has served as sales and marketing manager for SPO and SPMSA since October 1, 2008. |
In 2012, Messrs. Kebble, Miller and Robinson agreed to reduce their compensation effective July 1, 2012. As a result, their respective compensations are now paid at the annual rates of $300,000 for Mr. Kebble, $433,000 for Mr. Robinson and $264,000 for Mr. Miller.
Employment and Consulting Agreements
Guy Kebble and SPMSA entered into an updated Service Agreement dated July 1, 2012, under which SPMSA appointed Mr. Kebble as a permanent employee in the position of chief executive officer. The appointment is of indefinite duration, but may be terminated either by the employer or by Mr. Kebble on not less than three months’ prior written notice to the other and may also be summarily terminated by the employer if the executive commits a material breach of his contractual obligations under the agreement or in any circumstances justifying such termination at law. As chief executive officer, Mr. Kebble reports to the board of directors. His compensation under the agreement is ZAR 960,000 per annum (approximately $114,000) and is to be reviewed annually by the board of directors with a view to granting inflation-related or other increases. Mr. Kebble is also to be reimbursed for expenses incurred on behalf of SPMSA. The agreement provides for 20 days of vacation per year.
Mr. Kebble also has entered into an updated Consulting Agreement with SPO, dated July 1, 2012, under which he provides his services as an independent contractor to develop markets globally for those products and services marketed by SPO and to provide such other assistance to SPO as agreed with the directors of SPO from time to time. Under this agreement, Mr. Kebble has agreed to serve as the chief executive officer of SurePure US and its subsidiaries. Mr. Kebble’s remuneration from SPO is $186,000 per annum payable in equal monthly installments. Either party to the consulting agreement may terminate it for convenience on three months’ prior written notice. SPO has the right to terminate the agreement for cause with immediate notice. The agreement further provides that during the term of the agreement and for one year after the completion of his services to SPO, Mr. Kebble will not provide any services relating to the products or services of SPO to its clients. The agreement further provides that Mr. Kebble may not disclose any of SPO’s confidential information or other trade secrets and may not solicit or contact any employee, advisor or other individual working on behalf of clients in connection with SPO’s products or services for the purpose of obtaining or executing new work for the same 12-month period. The agreement is governed by Swiss law.
Stephen Robinson has entered into an updated Employment Agreement with SPO, dated July 1, 2012, under which he is employed as the chief financial officer of SPO. In performing his duties, Mr. Robinson reports to the chief executive officer and board of directors of SPO. His duties are to be determined by the board of directors and include those normally associated with the position of chief financial officer. Subject to provisions of applicable law which permit termination for cause, Mr. Robinson’s appointment may be terminated either by SPO or by Mr. Robinson on not less than three months’ prior written notice to the other. Mr. Robinson’s remuneration from SPO is CHF 396,000 (approximately $433,000) per annum payable in equal monthly installments and is to be reviewed annually by the board of directors with a view to granting inflation-related or other increases. In addition, the employer agrees to make contributions for social security and other compulsory expenses, plus the cost of certain airfare, and other local travel expenses in Switzerland. Mr. Robinson also is entitled to receive an allowance of CHF 500 (approximately $525) per month. The agreement further provides that Mr. Robinson may not disclose any of SPO’s confidential information or other trade secrets.
Stephen Miller and SPMSA entered into a Service Agreement, dated July 1, 2012, under which SPMSA appointed Mr. Miller as a full time employee in the position of sales and marketing executive. The appointment has no termination date but may be terminated either by SPMSA or by Mr. Miller on not less than three months’ prior written notice to the other and may also be summarily terminated by the employer without compensation if the executive commits a material breach of his contractual obligations under the agreement or in any circumstances justifying such termination at law. Mr. Miller’s duties are those ordinarily associated with the sales and marketing manager of a company and he reports to the chief executive officer of SPMSA. His compensation under the agreement is ZAR 960,000 per annum (approximately $114,000) and is to be reviewed annually by the board of directors with a view to granting inflation-related or other increases. Mr. Miller is also to be reimbursed for expenses incurred on behalf of SPMSA. The agreement provides for 20 days of vacation per year.
Mr. Miller also has entered into a Consulting Agreement with SPO, dated July 1, 2012, under which he provides his services as an independent contractor to develop markets globally for those products and services marketed by SPO and to provide such other assistance to SPO as agreed with the chief executive officer of the group from time to time. Mr. Miller’s remuneration from SPO is $150,000 per annum paid in equal monthly installments. Either party to the consulting agreement may terminate it for convenience on three months’ prior written notice. SPO has the right to terminate the agreement for cause without notice. Mr. Miller has agreed that during the term of the agreement and for one year after the completion of his services to SPO, he will not provide any services relating to SPO products or services to clients of SPO’s business. The agreement further provides that Mr. Miller may not disclose any of SPO’s confidential information or other trade secrets and may not solicit or contact any employee, advisor or other individual working on behalf of clients in connection with SPO’s products or services for the purpose of obtaining or executing new work for the same 12-month period. The agreement is governed by Swiss law.
Outstanding Equity Awards at Fiscal Year-End
We adopted the 2012 Nonqualified Stock Option Plan (the “2012 Stock Plan”) on December 12, 2012. There are no outstanding grants under the 2012 Stock Plan as of December 31, 2012.
2012 Stock Plan
On December 12, 2012, our directors and a majority of our shareholders, acting by written consent, approved the 2012 Stock Plan. We currently have no options to purchase shares of our Common Stock issued and outstanding under the 2012 Stock Plan, and we have reserved 3,000,000 shares of our Common Stock for future issuance under the 2012 Stock Plan.
The 2012 Stock Plan authorizes the granting of non-qualified stock options to our directors and to any independent consultants. The Plan will be administered by our board, or a committee appointed by our board (in either case, the “Committee”). The Committee may determine persons eligible for grants and the timing, type, amount, fair market value and other provisions of such grants. The Committee will have authority, subject to the express provisions of the Plan, to construe the Plan and the option agreements granted pursuant to the Plan, to prescribe, amend and rescind rules and regulations relating to the Plan, and to make all other determinations in the judgment of the Committee necessary or desirable for the administration of the Plan. The Committee may correct any defect or supply any omission or reconcile any inconsistency in the Plan or in any option agreement in the manner and to the extent it shall deem expedient to promote the best interests of the Company. Our board may suspend or terminate the Plan at any time.
The Plan provides that the determination of the option price per share for any option rests in the sole and unfettered discretion of the Committee. Options granted under the Plan must be granted within ten years from the date on which the Plan has been adopted. No options granted under the Plan may be exercisable after ten years from the date of grant. The Committee may establish installment exercise terms for an option such that the option becomes fully exercisable in a series of accumulating portions. The Committee may also accelerate the exercise of any option. Options may be exercised by delivery of a written notice of exercise and payment of the full price of the Common Stock underlying the option. The exercise price of an option may be paid in cash, or, at the discretion of the Committee, through the delivery of fully paid and nonassessable shares of Common Stock having an aggregate fair market value as of the date of exercise equal to the option price, or by a combination of both. The Committee has the right to determine acceptable methods for tendering shares of shares of Common Stock as payment upon exercise and may impose such limitations and prohibitions on the use of Common Stock to exercise an option as it deems appropriate. With the consent of the optionee, the Committee may cancel any options issued under the Plan and issue a new option to the optionee. Except by will or the laws of descent and distribution, or with the written consent of the Committee, no optionee may assign his interest in any option and no interest of any optionee in a stock option may become subject to any lien or obligation of the optionee. Options shall be exercisable during the optionee's lifetime only by the optionee or his assignees or by the duly appointed legal representative of an incompetent optionee or after the optionee's lifetime only by the duly appointed legal representative of the deceased optionee.
If the Company becomes a party to any merger, consolidation, reorganization or sale of assets, each outstanding option applies to the securities or property which a holder of the Company’s Common Stock be entitled to receive pursuant to such transaction. Whenever a change in control occurs, certain optionees are entitled to receive, in lieu of the exercise of their options, a cash payment equal to the difference between the exercise price of the option and the final offer price per share paid for common stock or such lower price as the Committee may determine to conform an option to preserve its status or the aggregate fair market value of the Common Stock underlying the stock options.
At any time and from time to time the Company’s board of directors may suspend or terminate the Plan in whole or in part or amend the Plan in such respects as the board may deem appropriate and in the best interest of the company. No amendment, suspension or termination of the Plan shall, however, without the optionee's consent, alter or impair any of the rights or obligations granted under the Plan.
Each option granted under the plan is to be embodied in a written stock option agreement and shall be signed by the optionee and an officer of the Company. Option agreements are to be subject to applicable provisions of the Plan and such other provisions as the Committee may adopt.
The Plan will terminate on December 9, 2022.
Director Compensation
We have not awarded any compensation to the members of our board of directors who took office beginning with the Share Exchange, nor have we yet determined any policy for the payment of compensation to our directors in their capacities as directors. During the remainder of 2013 and thereafter, we expect to make grants of options under the 2012 Stock Plan to all directors.
Our directors have been, and will continue to be, reimbursed for the reasonable out-of-pocket costs incurred by them in connection with travel to and from board meetings.
Item 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The following table summarizes certain information regarding the beneficial ownership (as such term is defined in Rule 13d-3 under the Exchange Act) of our outstanding Common Stock as of March 29, 2013 by (i) each person known by us to be the beneficial owner of more than 5% of our outstanding Common Stock, (ii) each of our directors, (iii) each of our named executive officers (as defined in Item 402(m) of Regulation S-K under the Securities Act), and (iv) all executive officers and directors as a group. Shares of our Common Stock subject to options, warrants, or other rights currently exercisable or exercisable within 60 days of the date of the Share Exchange are deemed to be beneficially owned and outstanding for purposes of computing the share ownership and percentage of the person holding such options, warrants or other rights, but are not deemed outstanding for computing the percentage of any other person. An asterisk (*) denotes less than 1%.
| Name of Beneficial Owner | | Number of Shares Beneficially Owned (1) | | | Percent Beneficially Owned (1) | |
| Mr. Quinton George | | | | | | | | |
| Mr. James Fitzpatrick | | | | | | | | |
| Mr. Graham Fisher | | | | | | | | |
| Trinity Asset Management(Proprietary) Ltd. (2) | | | | | | | | |
| Block F, the Terraces, 1 Silverwood Close, Steenberg Office Park, Tokai, Cape Town, South Africa | | | 3,094,737 | | | | 10.51 | % |
| RD Active Capital Ltd. (3) | | | | | | | | |
| 133 Brewhouse Lane Putney London, England SW15 2JX | | | | | | | | |
| United Kingdom | | | 3,000,000 | | | | 9.32 | % |
| Guy Kebble | | | | | | | | |
| P.O. Box 71, Milnerton, Cape Town, South Africa, 7435 | | | 1,000,000 | | | | 4.25 | % |
| Stephen Robinson | | | | | | | | |
| Dammstrasse 19, CH-6301, Zug, Switzerland | | | 813,637 | | | | 3.46 | % |
| Stephen Miller | | | | | | | | |
| P.O. Box 71, Milnerton, Cape Town, South Africa, 7435 | | | 682,670 | | | | 2.90 | % |
| All Officers and Directors as a Group | | | 2,496,307 | | | | 10.60 | % |
| (1) | Based on the sum of (a) 23,517,184 shares issued and outstanding as of the Share Exchange, and (b) as to each listed beneficial owner, any additional rights to acquire shares of our Common Stock that are exercisable within the 60 days after the date of the Share Exchange. |
| (2) | Comprised of 3,094,737 shares of our Common Stock acquired by the named beneficial owner under the Subscription Agreement. |
| (3) | Comprised of 583,000 shares of our Common Stock that have been purchased as of March 29, 2013 and 2,417,000 shares as to which RD Active Capital holds the right to purchase under the subscription agreement referred to below under “Certain Relationships and Related Transactions” under the heading “Financing Transactions with Other Related Persons--Additional Subscription Agreement for Shares." |
Outstanding Equity Awards at Fiscal Year-End
We adopted the 2012 Nonqualified Stock Option Plan (the “2012 Stock Plan”) on December 11, 2012. As of December 31, 2012, no options to purchase any shares of our Common Stock had been awarded under the 2012 Stock Plan.
Item 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
Transactions with Related Persons—The Share Exchange
On December 12, 2012, we entered into an Amended and Restated Share Exchange Agreement (the “Amended and Restated Share Exchange Agreement”) with all of the shareholders of SPI, including XOptics, that provided for the exchange of all shares of capital stock of SPI, par value CHF 0.01, for shares of our common stock, par value $.001 per share (our “Common Stock”), and for shares of our Nonvoting Convertible Preferred Stock, par value $.01 per share (the “Nonvoting Convertible Preferred Stock”), each share of which will be convertible, subject to certain terms and conditions, into one share of our Common Stock. The Amended and Restated Share Exchange Agreement amended and restated the Share Exchange Agreement (the “Original Share Exchange Agreement”), dated October 28, 2011, which we had entered into with the shareholders of SPI at that time. The Original Share Exchange Agreement was entered into to replace the Agreement and Plan of Merger that we had entered into with SPI on July 8, 2011, and which the parties thereafter terminated and replaced due to unanticipated regulatory and tax complications that made a merger transaction between them impractical. The Amended and Restated Share Exchange Agreement contained customary representations, warranties and conditions to closing. The Amended and Restated Share Exchange Agreement required that, as a condition to closing, we first have redeemed and then canceled 23,180,000 outstanding shares of our common stock held by Ratree Yabamrung and Kotchaporn Bousing, two of our officers, directors and shareholders prior to the Share Exchange. By its approval of the Amended and Restated Share Exchange Agreement, our board of directors authorized and directed us to redeem Ms. Yabamrung’s shares immediately prior to the closing in exchange for her being assigned all of our assets (of which there were none on the date of the Share Exchange) and her assumption of all our liabilities. Ms. Bousing tendered all of her shares to us for no consideration, and we then took the initial steps to cancel all of the shares owned by each Ms. Bousing and Ms. Yabamrung. The principal effect of the cancellations of these shares is that the existing shareholders of SPI acquired greater percentage ownership interests in SurePure US and may have acquired more influence or control and greater ability to delay, defer or prevent any potential changes in control. We did not obtain the approval of our shareholders for the transaction that we executed with Ms. Yabamrung. Although we believe that shareholder approval was not required under Nevada law for the transaction with her, it is possible that persons who were shareholders at the time of the Share Exchange may claim that their approval was required, in which case litigation might follow. See “Risk Factors—“We Did Not Obtain Shareholder Approval for a Transaction with a Former Shareholder,”” above in this Report.
Transactions with Related Persons—XOptics
Since 2007, XOptics has been the majority shareholder of SPI and since March 2011 had been a shareholder of SurePure, Inc. Immediately prior to the Share Exchange, XOptics owned 673,770 shares of our Common Stock, or approximately 2.89% of our issued and outstanding Common Stock immediately after the closing of the Share Exchange. As part of the Share Exchange, XOptics also acquired 22,765,447 shares of our Nonvoting Convertible Preferred Stock. Since January 1, 2009, XOptics has engaged in a series of transactions with our shareholders, with SPI and its subsidiaries, with other shareholders of SPI and its subsidiaries and with officers and directors of SPI. Those transactions are generally described below in this item of this annual report.
Acquisition of Shares of our Nonvoting Convertible Preferred Stock by XOptics
As part of the Share Exchange, XOptics also acquired 22,765,447 shares of our Nonvoting Convertible Preferred Stock, which, subject to the limitations and conditions set forth therein, are convertible into 22,765,447 shares of our Common Stock, subject to certain important limitations and restrictions. First, neither XOptics nor any other holder may convert any shares of our Nonvoting Convertible Preferred Stock if, after giving effect to the conversion, the holder and its affiliates would, in the aggregate, own beneficially or exercise control over that number of shares of our Common Stock which is 4.99% or greater of our total Common Stock on the date of conversion, immediately after giving effect to the conversion (this limitation is referred to as the "Beneficial Ownership Limitation"). Notwithstanding this restriction, however, whenever XOptics or any other holder of our Nonvoting Convertible Preferred Stock provides us with at least 61 days’ prior written notice stating that the holder elects to waive the Beneficial Ownership Limitation, the Beneficial Ownership Limitation terminates as of the end of the 61st day referenced in the notice. The Beneficial Ownership Limitation also automatically terminates three business days prior to any change of control of the Company. In this context, the term “change in control” of the Company means any acquisition by a third party (excluding any affiliate of ours) of 50% or more of our issued and outstanding voting securities by merger, consolidation or purchase of shares or of all or substantially all of our. Second, the terms of our Nonvoting Convertible Preferred Stock provide for proportionate adjustments to the conversion ratio whenever we may declare a stock dividend, effect a stock split or reverse stock split or otherwise combine shares of our Common Stock so that upon conversion XOptics or any other holder of shares of our Nonvoting Convertible Preferred Stock will receive the same relative proportion of shares of our Common Stock. We have agreed to reserve and keep available out of the authorized but unissued shares of our Common Stock that number of shares as is sufficient to effect the conversion of all unconverted shares of our Nonvoting Convertible Preferred Stock into Common Stock. If at any time the number of shares is not sufficient to effect such conversion, then we are obligated to take such corporate actions as may be necessary to increase our authorized but unissued shares to that number which will be sufficient, including obtaining shareholder approval to amend our articles of incorporation.
Shares of our Nonvoting Convertible Preferred Stock have the following rights and limitations:
| · | the holders of the shares may not vote on any matters, except to approve (i) the authorization, creation and issue of any shares of any other class or series of which would create a dividend or liquidation preference senior to the shares of our Nonvoting Convertible Preferred Stock or (ii) any action to amend or repeal any provision of our Articles of Incorporation or bylaws that adversely affects the rights or privileges of the shares of our Nonvoting Convertible Preferred Stock, and the consent or affirmative vote of the holders of at least 80% of the shares of our Nonvoting Convertible Preferred Stock is required in both cases; |
| · | the shares of our Nonvoting Convertible Preferred Stock do not have any preference or priority on the liquidation, dissolution or winding up of the Company; and |
| · | the holders of the shares of our Nonvoting Convertible Preferred Stock do not have any preference or priority as to any cash dividends or distributions, but are entitled to receive dividends and distributions pro rata with holders of our Common Stock on an “as converted” basis. |
The issued shares of our Nonvoting Convertible Preferred Stock automatically convert, at the applicable conversion ratio, into shares of our Common Stock upon the assignment, sale or other transfer of shares to any person other than an affiliate of the holder of the seller. Any assignee, purchaser or other transferee may surrender certificates representing the assigned shares to us and will receive shares of our Common Stock in return. If an assignee wishes to continue to hold the assigned shares as Nonvoting Convertible Preferred Stock, we will comply with its request in that regard.
The terms of our Nonvoting Convertible Preferred Stock also provide that no holder of shares of our Nonvoting Convertible Preferred Stock which, on a fully converted basis, when combined with shares of our Common Stock, would constitute the holder of the preferred shares as the beneficial holder of more than 4.99% of shares of our Common Stock may enter into any transaction with us or with any of our subsidiaries without the prior approval of a majority of our independent directors of SurePure US or, if we have no independent directors, the prior approval of a majority of the shares of our Common Stock not owned by the beneficial holder in question and its affiliates.
During December 2012, XOptics sold 100,000 shares of our Nonvoting Convertible Preferred Stock to another SurePure shareholder. The share automatically converted into shares of our Common Stock upon their transfer to the shareholder.
Our pending acquisition of SPHSA is described more fully below under this item of this annual report under the heading “Financing Transactions with Other Related Persons--Pending Acquisition of SPHSA.” If all conditions precedent to the pending acquisition of SPHSA under the governing agreement are satisfied or waived and the pending acquisition of SPHSA is completed, XOptics will effectively be able to exchange shares of SPHSA for 8,389,835 shares of our Nonvoting Convertible Preferred Stock, and, assuming that it neither sells nor otherwise transfers any additional shares, XOptics will then hold a total of 31,055,282 shares of our Nonvoting Convertible Preferred Stock. Also, we believe that, effective as of the Share Exchange, as a result of a change in control of XOptics, XOptics no longer is an affiliate or related party of SurePure.
Transactions between XOptics and Certain of the Shareholders of SurePure US
On or about March 3, 2011, XOptics purchased 2,906,937 shares of our Common Stock (after giving effect to the 15.2:1 stock split that we effected on June 14, 2011) from our shareholders in a resale by those shareholders under our registration statement which was initially filed with the Commission on March 23, 2009. The aggregate purchase price for the shares was $145,347, and XOptics paid the purchase price to the selling shareholders in cash from its working capital.
In the second and third quarters of 2011, XOptics sold 1,857,000 of our shares in approximately 19 transactions at prices ranging from $1.00 to $1.25 per share for aggregate proceeds of approximately $1,970,500. None of the buyers of these shares were U.S. Persons (as defined in Regulation S promulgated by the Commission) or were resident in the United States. Upon completing a sale of shares on June 15, 2011, XOptics held less than 5% of the outstanding shares of our Common Stock.
During the fourth quarter of 2011 and the first four months of 2012, XOptics sold a total of 376,167 shares of our Common Stock in approximately 11 transactions at prices ranging from $1.10 to $1.26 per share for aggregate proceeds of approximately $425,400. None of the buyers of these shares were persons who were U.S. Persons or were resident in the United States. XOptics has not sold any shares of our Common Stock since April 2012.
As a result of the foregoing sale transactions, as of December 31, 2012, XOptics beneficially owned 673,770 shares of our Common Stock.
Transactions between XOptics and SPI and its Subsidiaries
In October 2007, XOptics and its 100%-owned subsidiary SPP purchased 18,360,000 shares of the common shares of SPI for approximately $174,000, or approximately $0.01 per share, as part of the initial capitalization of SPI.
In September 2008, XOptics acquired an additional 163,347 shares from SPI at a price of $2.45 per share.
In December 2010, as part of a sale of 2,781,818 shares to its then existing shareholders at $0.01 per share in proportion to their shareholdings at that time, SPI sold 1,298,182 of its common shares to XOptics and 370,909 of its common shares to SPP.
From time to time since January 1, 2011, XOptics has advanced funds to SPI. The advances were not documented and did not state either a rate of interest or maturity date. There was, however, a written subordination agreement that provided that the advances would not be classified as current liabilities of SPI or be payable within one year if doing so would have caused SPI to be considered insolvent. The funds that XOptics advanced to SPI were principally derived from the sale of SPI shares by XOptics in a series of private transactions, all of which occurred outside of the United States. The advances made were as follows:
| Quarter and Year | | Amount of Advance | |
| 2nd Quarter 2011 | | $ | 1,686,815 | |
| 3rd Quarter 2011 | | $ | 370,000 | |
| 4th Quarter 2011 | | $ | 1,084,523 | |
| 1st Quarter 2012 | | $ | 894,500 | |
| 2nd Quarter 2012 | | $ | 1,043,500 | |
| 3rd Quarter 2012 | | $ | 0 | |
| 4th Quarter 2012 | | $ | 0 | |
| Total | | $ | 5,079,338 | |
The loan balance was reduced by a loan conversion of $1,390,130 for 1,390,130 shares of SPI in November 2011. The loan balance also included loans outstanding to SPI in the aggregate principal amount of $300,000 that XOptics purchased from three other shareholders of SPI. None of the shareholders that sold the loans to XOptics was an affiliate of XOptics. The acquired loans were included in the loan balance that was converted in July 2012 as discussed in the following paragraph.
The loan balance was paid in full by the conversion of the remaining balance of $3,689,208 into 7,378,416 common shares of SPI at the rate of $0.50 per share in July 2012. In order to satisfy a condition to the investment by the investor referred to below in this item of this annual report under the heading "Financing Transactions with Other Related Persons -- Subscription Agreement for Shares," SPI convened an annual general meeting on July 18, 2012. All of the shareholders of SPI attended by proxy and at the meeting there was unanimous approval for an increase in the authorized share capital of SPI for the purpose of, among other matters, satisfying the proposed conversion of outstanding shareholder loans into common shares of SPI. The shareholders of SPI further authorized the board of directors to determine the issue price of the new shares to be issued. Based on this authorization, the board of directors of SPI resolved to issue 7,378,416 common shares to XOptics to convert the existing shareholder loans due to XOptics in the amount of $3,689,208 at the rate of $0.50 per share. As part of the Share Exchange, XOptics exchanged the common shares of SPI issued in the conversion of the shareholder loans, together with other common shares of SPI owned by it at the time, for shares of our Nonvoting Convertible Preferred Stock at the rate of one share of our Nonvoting Convertible Preferred Stock for each common share of SPI owned by it. For additional information relating to the rights and limitations of our Nonvoting Convertible Preferred Stock, see below under the heading “Description of Securities” We believe that the sale and issue of these shares of our Nonvoting Convertible Preferred Stock to XOptics were exempt from the registration requirements of the Securities Act under Regulation S thereunder.
Contemplated Transactions between XOptics and the Majority Shareholder of SPHSA
Contingent upon and prior to the closing of the pending acquisition of SPHSA, which is expected to occur during the first half of 2013 if all governmental approvals are obtained, XOptics plans to acquire 8,389,835 shares of SPHSA from the majority shareholder of that company at the same price per share (a value of approximately ZAR 4,000, or $500, per share) that we expect to pay for the shares of SPHSA. The 8,389,835 shares include shares of SPHSA received on the conversion of loans that the majority shareholder had made to SPHSA since 2005. Under the terms of the Share Exchange Agreement, as assumed by us at the time of the Share Exchange, XOptics will then be entitled to receive 8,389,835 shares of our Nonvoting Convertible Preferred Stock, which will bring its total holdings to 31,055,282 shares of our Nonvoting Convertible Preferred Stock, based on its holdings as of March 29, 2013. For additional information relating to these transactions, see below in this item of this annual report under the heading “Financing Transactions with Other Related Persons--Pending Acquisition of SPHSA.”
Ownership of Our Shares by XOptics Immediately Post-Share Exchange
As of the Share Exchange, XOptics beneficially owned 22,765,447 common shares of SPI. Upon the closing of the Share Exchange, these shares were exchanged for 22,765,447 shares of our Nonvoting Convertible Preferred Stock.
The terms of our Nonvoting Convertible Preferred Stock provide that no holder of shares of our Nonvoting Convertible Preferred Stock which, on a fully converted basis, and when combined with shares of our Common Stock, would constitute the holder thereof the beneficial holder of more than 5.0% of shares of our Common Stock, may enter into any transaction with us or any of our subsidiaries without the prior approval of a majority of our independent directors or, if we have no independent directors, the prior approval of a majority of the shares of our Common Stock not owned by the beneficial holder in question and its affiliates.
Transactions by Officers and Directors of SPI
Prior to the Share Exchange and while SPI was a privately-owned company, XOptics made certain transfers of shares to the key executives of the business, and in one case to a family member of a key executive, for no or for nominal consideration. Stephen Robinson, a member of our board of directors and our chief financial officer, acquired 463,637 common shares of SPI from XOptics on December 20, 2010. Stephen Miller, our vice president sales and marketing, also acquired 463,637 shares of SPI from XOptics on December 20, 2010. In February 2011, Mr. Robinson acquired 210,000 common shares of SPI and Mr. Miller acquired 110,000 common shares of SPI from XOptics. As a result of these transfers, after giving effect to the Share Exchange, Mr. Robinson and Mr. Miller owned 673,637 and 573,637 shares respectively of our Common Stock in addition to the 140,000 and 109,033 shares of our Common Stock that they respectively owned prior to the Share Exchange, having acquired these shares directly from SurePure US in March 2011. On November 1, 2012 XOptics transferred 1,000,000 common shares of SPI to Guy Kebble, a member of our board of directors and our chief executive officer, and 70,000 shares of SPI to Oliver Kebble, who is the adult son of Guy Kebble.
Financing Transactions with Other Related Persons
Subscription Agreement for Shares
On July 23, 2012, SPI entered into a subscription agreement (the "Subscription Agreement") with Trinity Asset Management (PTE) Ltd., an institutional investor located in Cape Town, South Africa under which Trinity agreed, subject to the terms and conditions of the Subscription Agreement, to purchase 5,000,000 common shares of SPI from SPI and 1,000,000 common shares of SPI from XOptics in a series of six share installments, all at an issue price of $1.00 per share. The first two installments were for 500,000 shares each; the other four installments were for 1,000,000 shares each. The Subscription Agreement provided that not less than 75% of each installment shall be for the shares to be issued by SPI or by SurePure US, as SPI’s successor under the Subscription Agreement. Consequently not more than 25% of each installment could be for the purchase of shares from XOptics. Also, XOptics could not sell more than 1,000,000 of its shares to Trinity under the Subscription Agreement.
Prior to the Share Exchange, Trinity had purchased 3,094,737 of the 6,000,000 shares covered by the Subscription Agreement all at the price of $1.00 per share, as follows:
| Date of Purchase | | Total Shares Purchased | | | Shares Purchased from SPI | | | Shares Purchased from XOptics | |
| July 30, 2012 | | | 500,000 | | | | 375,000 | | | | 125,000 | |
| August 17, 2012 | | | 500,000 | | | | 375,000 | | | | 125,000 | |
| September 28, 2012 | | | 1,767,737 | | | | 1,517,737 | | | | 250,000 | |
| October 9, 2012 | | | 327,000 | | | | 232,263 | | | | 94,737 | |
| Total | | | 3,094,737 | | | | 2,500,000 | | | | 594,737 | |
As a result, 2,500,000 additional common shares of SPI were issued after June 30, 2012. In accordance with the terms and conditions of the Share Exchange Agreement, shares of SPI that are owned by Trinity, by persons on whose behalf Trinity purchased shares from SPI or XOptics or by persons to whom Trinity assigned its rights under the Subscription Agreement are to be exchanged for common shares of SurePure US on the basis of the Exchange Ratio. Trinity and SPI agreed that the installments due on August 31, 2012 and September 30, 2012 could be paid together and SPI waived any penalty resulting from the investor’s late performance.
Under the terms of the Subscription Agreement, three installments of shares remained to be purchased on each of October 31, November 30 and December 31, 2012, all at the price of $1.00 per share. Of the 2,905,263 shares remaining to be purchased by Trinity, 2,500,000 were to have been purchased from SPI and 405,263 were to have been purchased from XOptics.
Trinity failed to purchase shares as agreed on October 31, 2012. Exercising its rights under the Subscription Agreement, SPI terminated the Subscription Agreement and all rights of the investor to purchase additional shares, including both the shares referenced in the table above as well as additional shares as to which SPI had granted Trinity three purchase options, together with Trinity’s right to appoint directors. The termination of the Subscription Agreement also applied to the obligation of Trinity to purchase shares from XOptics. In November 2012, SPI entered into an additional subscription agreement with an investment manager, as described below in this item of this annual report under the heading “Financing Transactions with Other Related Persons -- Additional Subscription Agreement for Shares.”
As required by the Share Exchange Agreement, we have assumed the obligations of SPI under the Subscription Agreement, as terminated. Accordingly, we have agreed that we will register for resale to the public all of the Common Shares that Trinity and its permitted assignees have purchased by filing a registration statement under the Securities Act with the Commission within 120 days after the closing of the purchase of the shares. We also have agreed to use commercially reasonable efforts to cause the Commission to declare that registration statement effective reasonably promptly after its filing and to maintain the effectiveness of the registration statement that we have filed for a period of three years or until all of the shares so registered have been sold. Trinity and its permitted assignees have agreed to cooperate fully with us to enable us to discharge our obligations to register Common Shares, to provide all needed information and to enter into such further agreements as may be required to have the registration statement declared effective by the Commission and thereafter maintained effective.
Trinity represented to SPI that it was a registered asset manager doing business in South Africa, had no offices or presence in the United States of America and none of the accounts that it manages were beneficially owned by citizens or residents of the United States of America.
The Subscription Agreement provided that, for purposes of the Securities Act, the shares sold under the Subscription Agreement were restricted securities and may not be resold to any person who is a “U.S. Person” (as defined by the rules of the Commission) or by any means of commerce connected to the United States. Moreover, until one year and four days after the Share Exchange, Trinity has agreed not to sell or transfer the shares without our prior written consent or in a transaction which has been notified to us and does not involve any U.S. Person or any means of commerce in the United States. Similarly, any shares which are not included in the registration statement which we are obligated to file may not be sold without our prior written consent or in a transaction of which the Company has been notified and does not involve any U.S. Person or any means of commerce in the United States.
In order to comply with the regulations of the Commission that provide for exemption from the registration requirements of the Securities Act, Trinity represented to us, among other matters, that:
| · | it understood that the shares of our Common Stock that we have issued and that we will issue to it and its assignees are being issued in reliance on an exemption from registration contained in Regulation S; |
| · | no purchaser under the Subscription Agreement had engaged in any “directed selling efforts” as defined in Regulation S; |
| · | no purchaser of our shares under the Subscription Agreement is part of a plan or scheme to evade the registration requirements of the Securities Act; |
| · | no purchaser of our shares under the Subscription Agreement is a “US Person" and each purchaser will be the sole beneficial owner of the stock purchased under the Subscription Agreement; |
| · | each purchaser is outside of the United States at the time of the Share Exchange; and |
| · | the acquired shares cannot be sold unless in accordance with Regulation S, pursuant to registration under the Securities Act or pursuant to an available exemption from registration. |
Reliance on these representations may not, however, guarantee compliance with Regulation S.
In addition, Trinity agreed not to maintain any short position in our shares during the 12 months after issuance and not to engage in any hedging transactions with respect to our shares unless in compliance with law. The shares of our Common Stock issued under the Share Exchange Agreement to Trinity and its purchasers bear legends restricting their transferability until such shares have been sold as part of a registered public offering.
Pending Acquisition of SPHSA
On August 16, 2012, SPI entered into an agreement (the "Acquisition Agreement") with SPHSA providing for the acquisition of 100% of the outstanding shares of SPHSA from its shareholders. As noted below, we have assumed the executory obligations of SPI under the Acquisition Agreement, including the obligation to issue shares of our Common Stock and shares of our Nonvoting Convertible Preferred Stock upon the closing of the contemplated acquisition.
Currently, SPHSA is accounted for as a subsidiary of SPI under variable interest accounting rules. SPHSA owns the South African patent on the purification system sold by the Company, which is a counterpart of the patents owned by SPI. The shareholdings in SPHSA have remained largely unchanged since the formation of that company in 2005. Since October 2007, SPHSA has also provided various management services for SPI and SPO. In addition, our subsidiary SPO has loaned approximately $2,000,000 to SPHSA, all of which is due on demand. There have been and continue to be various intercompany transactions relating to the sale and purchase of goods and services between SPI and SPHSA. If we are able to complete the transaction, we believe that we will be able to operate SPHSA and SPMSA as wholly-owned subsidiaries and to fully consolidate the financial statements of these entities with our financial statements. In addition, upon completion the acquisition will allow us to directly control the intellectual property of these entities and allow their shareholders to have a direct equity stake in our full international business, rather than only South Africa. ccordingly, the boards of directors of both SPI and SPHSA believed it to be in the best interest of their respective companies to combine such that SPHSA would become an indirect wholly-owned subsidiary of SPI and SPO would convert its loans and advances to SPHSA into equity, thereby improving the financial condition of SPHSA.
Subject to the terms and conditions of the Acquisition Agreement, on August 21, 2012, SPI made its offer, which initially was irrevocable for a period of 20 business days after the date of the offer to purchase from each of the shareholders of SPHSA all of their shares of SPHSA at a closing to be held September 19, 2012, which was the 21st business day after the date of the offer. The shares of SPHSA subject to SPI’s offer to purchase included shares that reflected the value of shareholder loans outstanding at the time of the offer. On September 21, 2012, SPI extended the period during which its offer remained open until November 26, 2012 to permit the satisfaction of conditions to the closing of the offer. On November 30, 2012, SPI and SPHSA agreed to amend the Acquisition Agreement to provide that the offer would remain open until February 28, 2013, so as to permit the fulfillment of certain closing conditions. The offer has been extended again to remain open until June 28, 2013. The purchase price for the shares of SPHSA under the Offer was ZAR 4,000 (approximately $500.00) per share of SPHSA. The Acquisition Agreement provides that SPI will settle the purchase price it is paying for the shares of SPHSA in shares of SPI in the ratios of (i) 1,000 common shares of SPI for each share of SPHSA and (ii) 1,000 common shares of SPI for each ZAR 4,000 in principal amount of loans owed by SPHSA to its shareholders.
For purposes of valuing the shares of SPHSA, the boards of both companies considered a number of factors, including the following:
| · | the purchase price being paid by Trinity for common shares from SPI and common shares from XOptics was $1.00 per share. Trinity, however, received certain additional rights along with the shares, including the right to nominate one or two board members and the right to purchase substantial amounts of additional shares at a purchase price possibly below market. Accordingly, the board concluded that the purchase price being paid by Trinity represented a premium to the value at which SPI offered its shares to the shareholders and loanholders of SPHSA; and |
| · | those shareholders of SPHSA who were signatories to an August 2005 shareholders agreement held certain rights under that agreement. As a condition to the completion of SPI’s pending acquisition of SPHSA, those shareholders waived their rights. The acquisition of SPHSA effectively terminated these rights. Therefore, to compensate the shareholders of SPHSA for rights which they will waive as part of the transactions under the Acquisition Agreement, including certain minority protections, the directors believed that the shareholders of SPHSA were entitled to acquire shares of SPI at a favorable price, relative to the price paid by third parties. |
The average exchange rate of the ZAR to one US Dollar during the first seven months of 2012, when the transaction was negotiated, was approximately 8:1.
As of the date of this annual report, SPHSA had not yet received a final determination from the South African Reserve Bank as to whether the transaction could proceed.
As required by the Share Exchange Agreement, at the time of the Share Exchange SPI assigned its rights under the Acquisition Agreement to us, and we assumed the obligations of SPI to issue shares to the shareholders of SPHSA at such time, if any, that the pending acquisition of SPHSA was completed. As a result, we are bound by the terms and conditions of the Acquisition Agreement as the same relate to SPI.
Since we have assumed the Acquisition Agreement, at the time of the closing of the pending acquisition of their shares of SPHSA the former shareholders of SPHSA will become entitled to receive shares of our Common Stock in place of the common shares of SPI that they would have received under the Share Exchange Agreement had the acquisition closed prior to the Share Exchange. In this connection, the former SPHSA shareholders made the following representations, among others, to us with respect to the shares of our Common Stock that they have the right to acquire under the Share Exchange Agreement:
| · | they understood that the shares of our Common Stock that we have issued and that we will issue to them are being issued in reliance on an exemption from registration contained in Regulation S; |
| · | their agreement to purchase of our shares is not part of a plan or scheme to evade the registration requirements of the Securities Act; |
| · | none of the shareholders is a “US Person" and each will be the sole beneficial owner of the stock to be purchased by it; |
| · | except as disclosed to us, they are outside of the United States at the time of the Share Exchange; and |
| · | the acquired shares cannot be sold unless in accordance with Regulation S, pursuant to registration under the Securities Act or pursuant to an available exemption from registration. |
Consequently, we believe that none of the shareholders of SPHSA are residents of the United States or persons who would be considered to be “US Persons” under Regulation S promulgated by the SEC. Reliance on these representations may not, however, guarantee compliance with Regulation S. Reliance on these representations may not, however, guarantee compliance with Regulation S.
In addition, the shareholders agreed that they will not maintain any short position in our shares during the 12 months after issuance and not to engage in any hedging transactions with respect to our shares unless in compliance with law. The shares of our Common Stock that we will issue to the shareholders will bear legends restricting their transferability until they have been sold as part of a registered public offering.
Additional Subscription Agreement for Shares
On November 26, 2012, SPI entered into a Subscription Agreement (the “Additional Subscription Agreement”) with RD Active Capital Limited, a United Kingdom-based investment manager (“RD Active”), in which RD Active agreed to purchase up to 300,000 new common shares over the period ending January 31, 2013 and under which RD Active has the right to purchase up to 2,700,000 new common shares from the Company during the period ending March 29, 2013 as long as RD Active has purchased the initial 300,000 common shares which it agreed to purchase. All common shares are to be purchased and sold at an issue price of US $1.00 per share. RD Active agreed to purchase the 300,000 new common shares on an installment basis, with 100,000 shares to be purchased on or before each of November 28, 2012, December 21, 2012 and January 31, 2013. As of March 31, 2013, RD Active and its other purchasers had purchased 583,000 shares, with 2,417,000 shares remaining available for purchase under the Additional Subscription Agreement. On March 28, 2013, we and RD Active agreed to extend the termination date for the Additional Subscription Agreement to April 12, 2013.
Under the terms of the Additional Subscription Agreement, RD Active may exercise the additional purchase right on its own behalf and then resell to certain other purchasers the shares that it has purchased, or, in the alternative, RD Active may place the additional shares directly with other purchasers. If RD Active intends to exercise its additional purchase right, it must send us a written notice providing certain information with respect to the purchaser that has offered to purchase the shares, together with a joinder agreement to the Additional Subscription Agreement. We have the right to object to a purchaser on the grounds that we do not believe that the purchaser is a suitable investor or that the information provided is incomplete. The purchaser then has the right to correct the deficiencies noted and, if the deficiencies are corrected, the purchase of shares will be completed. The Additional Subscription Agreement provides that time is of the essence in connection with the performance of the purchase obligations.
At such time as RD Active and any other purchasers under the Additional Subscription Agreement have completed and paid for 3,000,000 shares under the Additional Subscription Agreement, RD Active and the other purchasers have the right to appoint an additional director to our board of directors.
As required by the Share Exchange Agreement, we assumed the obligations of SPI under the Additional Subscription Agreement. Specifically, we agreed that the following terms and conditions will govern the exchange of shares acquired by RD Active and its other purchasers: (i) we will not have any issued and outstanding securities other than our Common Stock and our Nonvoting Convertible Preferred Stock; (ii) shares of our Common Stock will continue to be quoted on the OTCBB or another comparable interdealer quotation system as long as we file all reports with the Commission that are required to be filed; and (iii) the remainder of the shares to be acquired under the Additional Subscription Agreement will be shares of our Common Stock and SPI will no longer have any obligations to issue its common shares.
As part of the Additional Subscription Agreement, we agreed that we will use our best efforts to register for resale to the public all of our Common Shares that RD Active and any other purchasers under the Additional Subscription Agreement have purchased by using our best efforts to file a registration statement under the Securities Act with the Commission prior to January 11, 2013. We filed the registration statement on January 30, 2013. We also agreed to use commercially reasonable efforts to cause the Commission to declare that registration statement effective reasonably promptly after its filing and to maintain the effectiveness of the registration statement that we have filed for a period of three years or until all of the shares so registered have been sold. We also agreed that, to the extent that RD Active and its other purchasers purchased our shares after the date on which we have filed a registration statement with the Commission, we will use our best efforts to amend the registration statement as filed, consistent with the rules and regulations of the Commission, to include the additional shares that have been purchased. RD Active and the other purchasers agreed to cooperate fully with us to enable us to discharge our obligations to register Common Shares, to provide all needed information and to enter into such further agreements as may be required to have the registration statement declared effective by the Commission and thereafter maintained effective.
Each purchase of shares under the Additional Subscription Agreement was subject to the accuracy of certain representations and warranties made by the purchasers of the shares. Among the representations and warranties made, RD Active represented that it has no offices or presence in the United States of America and none of the accounts that it manages are beneficially owned by citizens or residents of the United States of America.
The Additional Subscription Agreement provides that for purposes of the Securities Act, the shares sold under the Additional Subscription Agreement are restricted securities and may not be resold to any person who is a “U.S. Person” (as defined by the rules of the Commission) or by any means of commerce connected to the United States. Moreover, until one year and four days after the Share Exchange, the investor has agreed not to sell or transfer the shares without our prior written consent or in a transaction which has been notified to us and does not involve any U.S. Person or any means of commerce in the United States. Similarly, any shares which are not included in the registration statement which we are obligated to file may not be sold without our prior written consent or in a transaction of which the Company has been notified and does not involve any U.S. Person or any means of commerce in the United States.
To comply with the regulations of the Commission that provide for exemption from the registration requirements of the Securities Act, in the Share Exchange Agreement, RD Active and each other purchaser who becomes a party to the Additional Subscription Agreement, represented to us, among other matters, that:
| · | the purchaser understands that the shares of our Common Stock that we have issued to it and to the other purchasers under the Additional Subscription Agreement are being issued in reliance on an exemption from registration contained in Regulation S; |
| · | no purchaser has engaged in any “directed selling efforts” as defined in Regulation S; |
| · | their purchase of our shares is not part of a plan or scheme to evade the registration requirements of the Securities Act; |
| · | none of the purchasers is a “US Person" and each will be the sole beneficial owner of the stock purchased and to be purchased by them; |
| · | each purchaser is outside of the United States at the time of the Share Exchange; and |
| · | the acquired shares cannot be sold unless in accordance with Regulation S, pursuant to registration under the Securities Act or pursuant to an available exemption from registration. |
Consequently, we believe that neither RD Active nor any other purchasers under the Additional Subscription Agreement are residents of the United States or persons who would be considered to be “US Persons” under Regulation S. Reliance on these representations may not, however, guarantee compliance with Regulation S.
The shares of our Common Stock that we have issued and that we will issue to RD Active and the other purchasers under the Additional Subscription Agreement will bear legends restricting their transferability until such shares have been sold as part of a registered public offering.
Consulting and Employment Agreements
For a more complete discussion of those agreements under which our directors and officers provide their services, see above under “Employment and Consulting Agreements” under the heading “Executive Compensation.”
Director Independence
We are not currently a “listed company” under the rules of the Commission and are therefore not required to have a board comprised of a majority of independent directors or separate committees comprised of independent directors. We use the definition of “independence” under Rule 5605 of the Nasdaq Stock Market Rules, as applicable and as may be modified or supplemented from time to time and the interpretations thereunder, to determine if the members of our board are independent. In making this determination, our board considers, among other things, transactions and relationships between each director and his immediate family and the Company, including those reported above in this annual report under item captioned “Executive Compensation.” The purpose of this review is to determine whether any such relationships or transactions are material and, therefore, inconsistent with a determination that the directors are independent. On the basis of its review and its understanding of such relationships and transactions, our board has determined that of our board members only Mr. Brunette is an independent director. Until his resignation on March 27, 2013, Mr. Brunette was the sole member of our Audit Committee. At December 3, 2012, our board had no committees other than the Audit Committee.
Mr. Brunette did not enter into any transactions, relationship or arrangements during 2011 or 2012 in which we were a participant and in which any related person had or will have a direct or indirect material interest.
In accordance with our written policies and procedures our independent director is charged with monitoring and reviewing issues involving potential conflicts of interests and reviewing and approving all related party transactions. In general, for purposes of our policy, a related party transaction is a transaction, or a material amendment to any such transaction, involving a related person as defined in Rule 404 of Regulation S-K (Instructions to Item 404(a))and us involving any amount that exceeds the lesser of $120,000 or 1% of the average of our total assets at year end for the last two completed fiscal years. Because of the size and composition of our board of directors, our policy requires our independent director to review and approve related party transactions. In reviewing and approving any related party transaction or material amendment to any such transaction, our independent director must satisfy himself that he has been fully informed as to the related party’s relationship to us and interest in the transaction and as to the material facts of the transaction, and must determine that the related party transaction is fair to the Company. As of March 27, 2013, we ceased to have any independent directors.
Item 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
Rosen Seymour Shapss Martin & Company LLP (“RSSM”) served as the our independent registered public accounting firm for the fiscal year ended December 31, 2012, from and after the closing of the Share Exchange, and is expected to serve in that capacity for the 2013 fiscal year. RSSM served as the independent certified public accounting firm for SPI for 2011 and 2012. Total principal accounting fees for professional services rendered for us and for SPI by RSSM for the twelve months ended December 31, 2012 and December 31, 2011, are summarized as follows:
| | | Fiscal 2012 | | | Fiscal 2011 | |
| | | | | | | |
| Audit | | $ | 387,622 | | | $ | 172,512 | |
| Tax | | | --- | | | | --- | |
| Total | | $ | 387,622 | | | $ | 172,512 | |
Seale & Beers CPAs (“Seale”) served as the our independent registered public accounting firm for the fiscal year ended December 31, 2011 and for the fiscal year 2012 until the closing of the Share Exchange. Total principal accounting fees for professional services rendered for us by Seale during these periods are summarized as follows:
| | | Fiscal 2012 to | | | Fiscal 2011 | |
| | | December 12, 2012 | | | | |
| | | | | | | |
| Audit | | $ | 8,500 | | | $ | 10,625 | |
| Tax | | | --- | | | | --- | |
| Total | | $ | 8,500 | | | $ | 10,625 | |
Audit Fees. RSSM’s 2011 audit fees were for professional services rendered in connection with the audit and review of SPI’s audited 2010 and 2011 consolidated financial statements included in our current report on Form 8-K filed on December 13, 2012 and its review of SPI’s 2010 and 2011 quarterly financial statements. RSSM’s 2012 audit fees were for professional services rendered in connection with the audit of SurePure’s 2012 consolidated financial statements included in this annual report and RSSM’s review of SPI’s quarterly financial statements during 2012. We believe that the 2011 and 2012 audit fees of Seale & Beers were incurred for its audit of the 2010 and 2011 year-end financial statements of SurePure and for its review of the financial statements included in our quarterly reports on Form 10-Q during 2011 and 2012 and for services normally provided by our independent registered public accounting firm in connection with statutory and regulatory filings or engagements..
Tax Fees. There were no tax fees for professional services related to tax compliance, tax advice and tax planning within the United States.
Board of Directors Pre-Approval Policies and Procedures. At its regularly scheduled meetings, the board of directors, in lieu of an established audit committee, considers and pre-approves any audit and non-audit services to be performed by our independent registered public accounting firm. The board of directors has the authority to grant pre-approvals of non-audit services.
The board of directors has not, as of the time of filing this annual report on Form 10-K with the Commission, adopted policies and procedures for pre-approving audit or permissible non-audit services performed by our independent auditors. Instead, the board of directors as a whole has pre-approved all such services. In the future, our board of directors may approve the services of our independent registered public accounting firm pursuant to pre-approval policies and procedures adopted by the board of directors, provided the policies and procedures are detailed as to the particular service, the board of directors is informed of each service, and such policies and procedures do not include delegation of the board of director’s responsibilities to our management.
The board of directors has determined that the provision of services by RSSM described above is compatible with maintaining RSSM’s independence at our independent registered public accounting firm.
Part IV
Item 15. Exhibits and Financial Statement Schedules.
Financial Statement Schedules
Required information is included in the footnotes to the consolidated financial statements.
EXHIBIT INDEX
| Exhibit No. | | Description |
| 3.1 | | Articles of Incorporation of SurePure, Inc., as amended. (1) |
| | | |
| 3.2 | | Bylaws of SurePure, Inc. (2) |
| | | |
| 10.2 | | Amended and Restated Share Exchange Agreement, dated December 12, 2012, by and among SurePure, Inc. (formerly SOEFL Inc.), XOptics (PTY) Limited and the holders of all shares in SurePure Investment Holdings AG (3) |
| | | |
| 10.3 | | Acquisition Agreement, dated August 16, 2012, between SurePure Investment Holding AG and SurePure Holdings South Africa (Pty) Ltd. (3) |
| | | |
| 10.4 | | Amendment to Acquisition Agreement, dated November 26, 2012 (3) |
| | | |
| 10.5 | | 2012 Nonqualified Stock Option Plan. (3) |
| | | |
| 10.6 | | Subscription Agreement, dated July 23, 2012, between SurePure Investment Holding AG and Trinity Investment Management (Pte) Limited. (3) |
| | | |
| 10.7 | | Termination Letter, dated November 22, 2012, from SurePure Investment Holding AG to Trinity Investment Management (Pte) Limited. (3) |
| | | |
| 10.8 | | Employment Agreement dated July 1, 2012, between SurePure Marketing South Africa Ltd. and Guy Kebble. (3) |
| | | |
| 10.9 | | Consulting Agreement, dated July 1, 2012, between SurePure Operations AG and Guy Kebble. (3) |
| | | |
| 10.10 | | Employment Agreement, dated July 1, 2012, between SurePure Operations AG and Stephen Robinson. (3) |
| | | |
| 10.11 | | Employment Agreement dated July 1, 2012, between SurePure Marketing South Africa Ltd. and Stephen Miller. (3) |
| | | |
| 10.12 | | Consulting Agreement, dated July 1, 2012, between SurePure Operations AG and Stephen Miller. (3) |
| | | |
| 10.13 | | Agreement of Lease dated February 8, 2012, between Anglo African Development (Pty) Ltd. and SurePure Marketing South Africa (Pty) Ltd., effective March 1, 2012 until February 28, 2014, for the premises located at One Lagoon Beach, Unit 204, Lagoon Beach Milnerton, South Africa. (3) |
| | | |
| 10.14 | | Memorandum of Agreement of Lease, dated April 25, 2012, between HY Jack Property Holdings CC and SurePure Marketing South Africa Ltd., effective May 1, 2008, for the premises located at 4 Acteon Street, Unit 2, Paarden Eiland, South Africa. (3) |
| | | |
| 10.15 | | Agreement of Extension of Lease, dated April 19, 2012, between HY Jack Property Holdings CC and SurePure Marketing South Africa Ltd, effective January 5, 2012 until November 11, 2012, for the premises located at 4 Acteon Street, Unit 2, Paarden Eiland, South Africa. (3) |
| | | |
| 10.17 | | Form of Indemnification Agreement. (3) |
| | | |
| 10.18 | | Subscription Agreement, dated November 26, 2012, between SurePure Investment Holding AG and RD Active Capital Limited. (3) |
| | | |
| 16.2 | | Letter from Seale & Beers, CPAs, dated December 21, 2012, to the Securities and Exchange Commission. (4) |
| | | |
| 21.1 | | Subsidiaries of the Registrant. |
| | | |
| 24.1 | | Power of Attorney (included on signature page). |
| | | |
| 31.1 | | Certification of Principal Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| | | |
| 31.2 | | Certification of Principal Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| | | |
| 32.1 | | Certification of Principal Executive Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
| | | |
| 32.2 | | Certification of Principal Financial Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
| | | |
| 101 | | The following materials from SurePure, Inc. Form 10-K for the year ended December 31, 2012, formatted in Extensible Business Reporting Language (XBRL): (i) Balance Sheets at December 31, 2012 and December 31, 2011, (ii) Statements of Operations for the years ended December 31, 2012 and 2011 (iii) Statements of Changes in Stockholders’ Equity (Deficiency) for the years ended December 31, 2012 and 2011, (iv) Statements of Cash Flows for the years ended December 31, 2012 and 2011 and (v) Notes to the Financial Statements.*** |
| | | (1) Incorporated by reference from Exhibit 3 to Form S-1 filed on March 23, 2009, Exhibit 3.1 to Form 8-K filed on July 29, 2011 and Exhibit 3.3 to Form 8-K filed on December 16, 2011. |
| | | |
| | | (2) Incorporated by reference to Exhibit 3-2 to the registration statement of SOEFL Inc. on Form S-1, as filed with the Securities and Exchange Commission on March 23, 2009. |
| | | |
| | | (3) Incorporated herein by reference to the Company’s Current Report on Form 8-K filed with the Commission on December 12, 2012. |
| | | |
| | | (4) Incorporated herein by reference to the Company’s Current Report on Form 8-K/A filed with the Commission on December 21, 2012. |
SIGNATURES
Pursuant to the requirements of sections 13 or 15(d) of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of New York, State of New York, on the 1st day of April, 2013.
| | SUREPURE, INC. |
| | |
| | By: | /s/ Stephen M. Robinson |
| | | Stephen M. Robinson |
| | | Chief Financial Officer |
| �� | | (Principal Financial Officer) |
Each person whose signature appears below constitutes and appoints Stephen M. Robinson as his true and lawful attorneys-in-fact and agent, with full power of substitution and resubstitution, as their true and lawful attorneys and agents, to do any and all acts and things in their name and behalf in their capacities as directors and officers and to execute any and all instruments for them and in their names in the capacities indicated below, which said attorneys and agents, may deem necessary or advisable to enable said corporation to comply with the Securities Exchange Act of 1934, as amended, and any rules, regulations and requirements of the Securities and Exchange Commission, in connection with this Annual Report on Form 10-K, including specifically but without limitation, power and authority to sign for them or any of them in their names in the capacities indicated below, any and all amendments hereto, and they do hereby ratify and confirm all that said attorneys and agents, or either of them, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
| Signature | Title | Date |
| | | |
| /s/ Guy R. Kebble | Chief Executive Officer; Director | April 1, 2013 |
| Guy R. Kebble | (Principal Executive Officer) | |
| | | |
| /s/ Stephen M. Robinson | Chief Financial Officer | April 1, 2013 |
| Stephen M. Robinson | (Principal Financial Officer and Principal Accounting Officer) | |
EXHIBIT INDEX
The following exhibits are filed as part of, or are incorporated by reference into, this Annual Report:
| Exhibit No. | | Description |
| 3.1 | | Articles of Incorporation of SurePure, Inc., as amended. Incorporated by reference from Exhibit 3 to Form S-1 filed on March 23, 2009, Exhibit 3.1 to Form 8-K filed on July 29, 2011 and Exhibit 3.3 to Form 8-K filed on December 16, 2011. |
| | | |
| 3.2 | | Bylaws of SurePure, Inc. Incorporated by reference to Exhibit 3-2 to the registration statement of SOEFL Inc. on Form S-1, as filed with the Securities and Exchange Commission on March 23, 2009. |
| | | |
| 10.2 | | Amended and Restated Share Exchange Agreement, dated December 12, 2012, by and among SurePure, Inc. (formerly SOEFL Inc.), XOptics (PTY) Limited and the holders of all shares in SurePure Investment Holdings AG |
| | | |
| 10.3 | | Acquisition Agreement, dated August 16, 2012, between SurePure Investment Holding AG and SurePure Holdings South Africa (Pty) Ltd. |
| | | |
| 10.4 | | Amendment to Acquisition Agreement, dated November 26, 2012 |
| | | |
| 10.5 | | 2012 Nonqualified Stock Option Plan. |
| | | |
| 10.6 | | Subscription Agreement, dated July 23, 2012, between SurePure Investment Holding AG and Trinity Investment Management (Pte) Limited. |
| | | |
| 10.7 | | Termination Letter, dated November 22, 2012, from SurePure Investment Holding AG to Trinity Investment Management (Pte) Limited. |
| | | |
| 10.8 | | Employment Agreement dated July 1, 2012, between SurePure Marketing South Africa Ltd. and Guy Kebble. |
| | | |
| 10.9 | | Consulting Agreement, dated July 1, 2012, between SurePure Operations AG and Guy Kebble. |
| | | |
| 10.10 | | Employment Agreement, dated July 1, 2012, between SurePure Operations AG and Stephen Robinson. |
| | | |
| 10.11 | | Employment Agreement dated July 1, 2012, between SurePure Marketing South Africa Ltd. and Stephen Miller. |
| | | |
| 10.12 | | Consulting Agreement, dated July 1, 2012, between SurePure Operations AG and Stephen Miller. |
| | | |
| 10.13 | | Agreement of Lease dated February 8, 2012, between Anglo African Development (Pty) Ltd. and SurePure Marketing South Africa (Pty) Ltd., effective March 1, 2012 until February 28, 2014, for the premises located at One Lagoon Beach, Unit 204, Lagoon Beach Milnerton, South Africa. |
| | | |
| 10.14 | | Memorandum of Agreement of Lease, dated April 25, 2012, between HY Jack Property Holdings CC and SurePure Marketing South Africa Ltd., effective May 1, 2008, for the premises located at 4 Acteon Street, Unit 2, Paarden Eiland, South Africa. |
| | | |
| 10.15 | | Agreement of Extension of Lease, dated April 19, 2012, between HY Jack Property Holdings CC and SurePure Marketing South Africa Ltd, effective January 5, 2012 until November 11, 2012, for the premises located at 4 Acteon Street, Unit 2, Paarden Eiland, South Africa. |
| | | |
| 10.17 | | Form of Indemnification Agreement. |
| | | |
| 10.18 | | Subscription Agreement, dated November 26, 2012, between SurePure Investment Holding AG and RD Active Capital Limited. |
| | | |
| 16.2 | | Letter from Seale & Beers, CPAs, dated December 21, 2012, to the Securities and Exchange Commission. |
| | | |
| 21.1 | | Subsidiaries of the Registrant. |
| | | |
| 31.1 | | Certification of Principal Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| | | |
| 31.2 | | Certification of Principal Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| | | |
| 32.1 | | Certification of Principal Executive Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
| | | |
| 32.2 | | Certification of Principal Financial Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
| | | |
| 101 | | The following materials from SurePure, Inc. Form 10-K for the year ended December 31, 2012, formatted in Extensible Business Reporting Language (XBRL): (i) Balance Sheets at December 31, 2012 and December 31, 2011, (ii) Statements of Operations for the years ended December 31, 2012 and 2011 (iii) Statements of Changes in Stockholders’ Equity (Deficiency) for the years ended December 31, 2012 and 2011, (iv) Statements of Cash Flows for the years ended December 31, 2012 and 2011 and (v) Notes to the Financial Statements.* |
* Pursuant to Rule 406T of Regulation S-T, the Interactive Data Files on Exhibit 101 hereto are deemed not filed or part of a registration statement or prospectus for purposes of Sections 11 or 12 of the Securities Act of 1933, as amended, are deemed not filed for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, and otherwise are not subject to liability under those sections.
SurePure, Inc. and Subsidiaries (ADevelopment Stage Company) Consolidated Financial Statements Years Ended December 31, 2012 and 2011 and From August 24, 2005 (inception) to December 31, 2012 WITH REPORT OF INDEPENDENT
REGISTERED PUBLIC ACCOUNTANTS |
December 31, 2012 and 2011
| | Page |
| | |
| Report of Independent Registered Public Accounting Firm | F-1 |
| | |
| | |
| Consolidated Financial Statements | |
| | |
| Consolidated Balance Sheets | F-2 |
| | |
| Consolidated Statements of Operations | F-3 |
| | |
| Consolidated Statements of Other Comprehensive Income (Loss) | F-4 |
| | |
| Consolidated Statements of Stockholders’ Deficit | F-5 |
| | |
| Consolidated Statements of Cash Flows | F-6 |
| | |
| Notes to Consolidated Financial Statements | F-7 - F-25 |
Report of Independent Registered Public Accounting Firm
Board of Directors
SurePure, Inc. and Subsidiaries
New York, NY
We have audited the accompanying consolidated balance sheets of SurePure, Inc. and Subsidiaries (“SurePure” or the “Company”) (a development stage company) as of December 31, 2012 and 2011 and the related consolidated statements of operations, stockholders’ deficit, other comprehensive income (loss) and cash flows for each of two years then ended and for the period from August 24, 2005 (inception) to December 31, 2012. These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the consolidated financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall consolidated financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the consolidated financial position of SurePure, Inc. and Subsidiaries at December 31, 2012 and 2011, and the results of its consolidated operations and its cash flows for each of the two years then ended and for the period from August 24, 2005 (inception) to December 31, 2012 in conformity with accounting principles generally accepted in the United States of America.
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As shown in the consolidated financial statements, the Company has experienced significant losses and negative cash flows, resulting in increased accumulated deficits during its development stage. These conditions raise substantial doubt about its ability to continue as a going concern. Management’s plans regarding those matters are described in Note 15. The accompanying consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ Rosen Seymour Shapss Martin & Company LLP
CERTIFIED PUBLIC ACCOUNTANTS
New York, New York
April 1, 2013
| SUREPURE, INC. AND SUBSIDIARIES |
| (A Development Stage Group) |
Consolidated Balance Sheets
December 31, 2012 and 2011
| | | 2012 | | | 2011 | |
| Assets | | | | | | | | |
| | | | | | | | | |
| Current assets: | | | | | | | | |
| Cash | | $ | 142,373 | | | $ | 35,475 | |
| Accounts receivable, net | | | 19,948 | | | | 80,200 | |
| Prepaid expenses and other current assets | | | 128,646 | | | | 71,603 | |
| | | | | | | | | |
| Total current assets | | | 290,967 | | | | 187,278 | |
| | | | | | | | | |
| Property and equipment | | | 4,616 | | | | 12,608 | |
| | | | | | | | | |
| Other assets: | | | | | | | | |
| Intangible assets, net | | | 128,958 | | | | 145,627 | |
| | | | | | | | | |
| Total assets | | $ | 424,541 | | | $ | 345,513 | |
| | | | | | | | | |
| | | | | | | | | |
| Liabilities and Equity (Deficit) | | | | | | | | |
| | | | | | | | | |
| Current liabilities: | | | | | | | | |
| Accounts payable and other current liabilities | | $ | 790,930 | | | $ | 736,953 | |
| Due to officers/stockholders | | | 247,259 | | | | 473,129 | |
| Income taxes payable | | | 409 | | | | 717 | |
| | | | | | | | | |
| Total current liabilities | | | 1,038,598 | | | | 1,210,799 | |
| | | | | | | | | |
| Long-term liabilities: | | | | | | | | |
| Loans from stockholders | | | 3,241,768 | | | | 5,431,122 | |
| Other loans payable | | | 219,549 | | | | 300,000 | |
| | | | | | | | | |
| Total long-term liabilities | | | 3,461,317 | | | | 5,731,122 | |
| | | | | | | | | |
| Total liabilities | | | 4,499,915 | | | | 6,941,921 | |
| | | | | | | | | |
| Commitments and contingencies | | | | | | | | |
| | | | | | | | | |
| Equity (deficit): | | | | | | | | |
| Stockholder’s equity (deficit): | | | | | | | | |
| Common stock | | | 23,542 | | | | 257,431 | |
| Preferred stock | | | 226,654 | | | | - | |
| Additional paid-in capital | | | 20,920,817 | | | | 14,067,931 | |
| Equity of variable interest entities | | | 920,980 | | | | 1,004,150 | |
| Other comprehensive income | | | 360,464 | | | | 264,002 | |
| Deficit accumulated during the development stage | | | (25,192,142 | ) | | | (20,891,469 | ) |
| | | | (2,739,685 | ) | | | (5,297,955 | ) |
| Stock subscription receivable | | | - | | | | (42,141 | ) |
| | | | | | | | | |
| Total stockholders’ equity (deficit) | | | (2,739,685 | ) | | | (5,340,096 | ) |
| | | | | | | | | |
| Noncontrolling interest | | | (1,335,689 | ) | | | (1,256,312 | ) |
| | | | | | | | | |
| Total equity (deficit) | | | (4,075,374 | ) | | | (6,596,408 | ) |
| | | | | | | | | |
| Total liabilities and equity (deficit) | | $ | 424,541 | | | $ | 345,513 | |
See notes to consolidated financial statements.
| SUREPURE, INC. AND SUBSIDIARIES |
| (A Development Stage Group) |
Consolidated Statements of Operations
Years Ended December 31, 2012 and 2011 and Inception to December 31, 2012
| | | | | | | | | Cumulative from | |
| | | | | | | | | August 24, 2005 | |
| | | | | | | | | (inception) to | |
| | | 2012 | | | 2011 | | | December 31, 2012 | |
| | | | | | | | | | |
| Sales | | $ | 594,445 | | | $ | 344,416 | | | $ | 2,264,410 | |
| | | | | | | | | | | | | |
| Cost of sales | | | 92,338 | | | | 185,222 | | | | 1,537,496 | |
| | | | | | | | | | | | | |
| Gross profit | | | 502,107 | | | | 159,194 | | | | 726,914 | |
| | | | | | | | | | | | | |
| Expenses: | | | | | | | | | | | | |
| General and administrative expenses | | | 4,103,317 | | | | 3,350,695 | | | | 20,392,507 | |
| Promotion and marketing | | | 83,046 | | | | 62,491 | | | | 591,386 | |
| Research and development | | | 296,844 | | | | 319,025 | | | | 3,629,300 | |
| Depreciation and amortization | | | 24,661 | | | | 23,469 | | | | 176,099 | |
| Impairment of patent | | | - | | | | - | | | | 537,631 | |
| | | | | | | | | | | | | |
| Total expenses | | | 4,507,868 | | | | 3,755,680 | | | | 25,326,923 | |
| | | | | | | | | | | | | |
| Loss from operations | | | (4,005,761 | ) | | | (3,596,486 | ) | | | (24,600,009 | ) |
| | | | | | | | | | | | | |
| Other income (expense): | | | | | | | | | | | | |
| Interest income | | | 49 | | | | 64 | | | | 371,858 | |
| Interest expense | | | (408,863 | ) | | | (429,499 | ) | | | (2,457,226 | ) |
| Exchange rate gains and losses | | | (3,884 | ) | | | (17,636 | ) | | | (25,387 | ) |
| Loss on disposition of fixed assets | | | - | | | | (7,405 | ) | | | (64,172 | ) |
| | | | | | | | | | | | | |
| Total other (expense) income | | | (412,698 | ) | | | (454,476 | ) | | | (2,174,927 | ) |
| | | | | | | | | | | | | |
| Loss before provision for taxes | | | (4,418,459 | ) | | | (4,050,962 | ) | | | (26,774,936 | ) |
| | | | | | | | | | | | | |
| Provision for income taxes | | | - | | | | - | | | | 32,673 | |
| | | | | | | | | | | | | |
| Net loss | | | (4,418,459 | ) | | | (4,050,962 | ) | | | (26,807,609 | ) |
| | | | | | | | | | | | | |
| Net loss attributable to non-controlling interest | | | (117,786 | ) | | | (41,235 | ) | | | (1,615,467 | ) |
| | | | | | | | | | | | | |
| Net loss attributable to SurePure | | $ | (4,300,673 | ) | | $ | (4,009,727 | ) | | $ | (25,192,142 | ) |
| | | | | | | | | | | | | |
| Loss per share – basic and diluted attributable | | | | | | | | | | | | |
| to SurePure common stockholders | | $ | (0.11 | ) | | $ | (0.13 | ) | | $ | (0.94 | ) |
| | | | | | | | | | | | | |
| Weighted average shares outstanding - | | | | | | | | | | | | |
| basic and diluted | | | 38,395,001 | | | | 30,929,203 | | | | 26,668,718 | |
See notes to consolidated financial statements.
| SUREPURE, INC. AND SUBSIDIARIES |
| (A Development Stage Group) |
Consolidated Statements of Other Comprehensive Income (Loss)
Years Ended December 31, 2012 and 2011 and Inception to December 31, 2012
| | | | | | | | | Cumulative from | |
| | | | | | | | | August 24, 2005 | |
| | | | | | | | | (Inception) to | |
| | | 2012 | | | 2011 | | | December 31, 2012 | |
| | | | | | | | | | |
| Net loss | | $ | (4,418,459 | ) | | $ | (4,050,962 | ) | | $ | (26,807,609 | ) |
| | | | | | | | | | | | | |
| Other comprehensive income,net of tax | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| Unrealized gain on foreign | | | | | | | | | | | | |
| currency translation | | | 134,871 | | | | 803,314 | | | | 446,683 | |
| | | | | | | | | | | | | |
| Comprehensive loss | | | (4,283,588 | ) | | | (3,247,648 | ) | | | (26,360,926 | ) |
| | | | | | | | | | | | | |
| Less: Comprehensive (loss) income attributable | | | | | | | | | | | | |
| to noncontrolling interest | | | (79,377 | ) | | | 168,823 | | | | (1,529,248 | ) |
| | | | | | | | | | | | | |
| Comprehensive loss attributable | | | | | | | | | | | | |
| to SurePure,net of tax | | $ | (4,204,211 | ) | | $ | (3,416,471 | ) | | $ | (24,831,678 | ) |
See notes to consolidated financial statements.
| SUREPURE, INC. AND SUBSIDIARIES |
| (A Development Stage Group) |
Consolidated Statements of Other Comprehensive Income (Loss)
Years Ended December 31, 2012 and 2011 and Inception to December 31, 2012
| | | | | | | | | | | | | | | | | | | | | | | | Deficit | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | Equity in | | | Accumulated | | | | | | Accumulated | | | | | | |
| | | Common | | | Range of | | | | | | | | | Additional | | | | | | Variable | | | During The | | | | | | Other | | | Stock | | | |
| | | Shares | | | Price Per | | | Common | | | Preferred | | | Paid-in | | | Total | | | Interest | | | Development | | | Noncontrolling | | | Comprehensive | | | Subscription | | | |
| | | Issued | | | Share | | | Stock | | | Stock | | | Capital | | | Consideration | | | Entities | | | Stage | | | Interest | | | Income | | | Receivable | | | Total |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balances – January 1, 2011 | | | 26,822,215 | | | | | | | $ | 257,431 | | | $ | - | | | $ | 12,275,997 | | | | | | | $ | 1,004,150 | | | $ | (16,881,742 | ) | | $ | (1,425,135 | ) | | $ | (329,254 | ) | | $ | (42,141 | ) | $ | (5,140,694) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Conversion of stockholder’s loans to additional paid-in capital | | | | | | | | | | | | | | | | | | | 1,376,229 | | | | | | | | | | | | | | | | | | | | | | | | | | | 1,376,229 |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Imputed interest on stockholder loans | | | | | | | | | | | | | | | | | | | 415,705 | | | | | | | | | | | | | | | | | | | | | | | | | | | 415,705 |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net (loss) for the year | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | (4,009,727 | ) | | | (41,235 | ) | | | | | | | | | | (4,050,962) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Unrealized gain on foreign currency translation adjustment | | | - | | | | | | | | - | | | | - | | | | - | | | | | | | | - | | | | - | | | | 210,058 | | | | 593,256 | | | | - | | | 803,314 |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balances – December 31, 2011 | | | 26,822,215 | | | | | | | | 257,431 | | | | - | | | | 14,067,931 | | | | | | | | 1,004,150 | | | | (20,891,469 | ) | | | (1,256,312 | ) | | | 264,002 | | | | (42,141 | ) | | (6,596,408) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of common stock, pre-merger | | | 2,500,000 | | | $ | 1.00 | | | | 25,501 | | | | | | | | 2,474,499 | | | $ | 2,500,000 | | | | | | | | | | | | | | | | | | | | | | | 2,500,000 |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Conversion of stockholder loans | | | 7,378,416 | | | $ | 0.50 | | | | 75,206 | | | | | | | | 3,614,002 | | | | 3,689,208 | | | | | | | | | | | | | | | | | | | | | | | 3,689,208 |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Imputed interest on stockholder loans | | | - | | | | | | | | - | | | | | | | | 380,414 | | | | | | | | | | | | | | | | | | | | | | | | | | | 380,414 |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Adjustment for reverse merger | | | (13,393,447 | ) | | | | | | | (334,831 | ) | | | 226,654 | | | | 149,206 | | | | - | | | | (83,170 | ) | | | | | | | | | | | | | | | 42,141 | | | - |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of common stock, post-merger | | | 235,000 | | | $ | 1.00 | | | | 235 | | | | | | | | 234,765 | | | | 235,000 | | | | | | | | | | | | | | | | | | | | | | | 235,000 |
| | | | | | | | | | | | | | | | | | | | | | | $ | 6,424,208 | | | | | | | | | | | | | | | | | | | | | | | |
| Net (loss) for the year | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | (4,300,673 | ) | | | (117,786 | ) | | | | | | | | | | (4,418,459) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Unrealized gain on foreign currency translation adjustment | | | - | | | | | | | | - | | | | - | | | | - | | | | | | | | - | | | | - | | | | 38,409 | | | | 96,462 | | | | - | | | 134,871 |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balances – December 31, 2012 | | | 23,542,184 | | | | | | | $ | 23,542 | | | $ | 226,654 | | | $ | 20,920,817 | | | | | | | $ | 920,980 | | | $ | (25,192,142 | ) | | $ | (1,335,689 | ) | | $ | 360,464 | | | $ | - | | $ | (4,075,374) |
See notes to consolidated financial statements.
| SUREPURE, INC. AND SUBSIDIARIES |
| (A Development Stage Group) |
Consolidated Statements of Cash Flows
Years Ended December 31, 2012 and 2011 and Inception to December 31, 2012
| | | | | | | | | Cumulative During the | |
| | | | | | | | | Development Stage – | |
| | | | | | | | | from August 24, 2005 | |
| | | | | | | | | (inception) to | |
| | | 2012 | | | 2011 | | | December 31, 2012 | |
| Cash from operating activities: | | | | | | | | | | | | |
| Net loss | | $ | (4,418,459 | ) | | $ | (4,050,962 | ) | | $ | (26,807,609 | ) |
| Adjustments to reconcile net loss to cash used in operating activities: | | | | | | | | | | | | |
| Depreciation and amortization | | | 24,661 | | | | 23,469 | | | | 176,099 | |
| Impairment of patent | | | - | | | | - | | | | 537,631 | |
| Loss on sale of property and equipment | | | - | | | | 7,405 | | | | 64,172 | |
| Imputed interest on loans from stockholders | | | 380,414 | | | | 415,705 | | | | 2,363,937 | |
| Changes in assets and liabilities: | | | | | | | | | | | | |
| Accounts receivable | | | 60,252 | | | | (77,328 | ) | | | (19,948 | ) |
| Prepaid expenses and other current assets | | | (57,043 | ) | | | 6,250 | | | | (128,646 | ) |
| Accounts payable | | | 53,977 | | | | (370,359 | ) | | | 790,930 | |
| Due to officers | | | (225,870 | ) | | | 300,351 | | | | 247,259 | |
| Income taxes payable | | | (308 | ) | | | (26,683 | ) | | | 409 | |
| | | | | | | | | | | | | |
| Total cash used in operating activities | | | (4,182,376 | ) | | | (3,772,152 | ) | | | (22,775,766 | ) |
| | | | | | | | | | | | | |
| Cash from investing activities: | | | | | | | | | | | | |
| Purchase of property and equipment | | | - | | | | - | | | | (181,760 | ) |
| Proceeds from sales of property and equipment | | | - | | | | 1,332 | | | | 16,860 | |
| Acquisition of patents | | | - | | | | - | | | | (746,576 | ) |
| | | | | | | | | | | | | |
| Total cash provided by (used in) investing activities | | | - | | | | 1,332 | | | | (911,476 | ) |
| | | | | | | | | | | | | |
| Cash from financing activities: | | | | | | | | | | | | |
| Proceeds from sale of equity | | | 2,735,000 | | | | - | | | | 13,658,469 | |
| Proceeds from equity of variable interest entities | | | - | | | | - | | | | 83,309 | |
| Proceeds from loans from stockholders | | | 1,499,854 | | | | 2,656,518 | | | | 9,421,605 | |
| (Payments of) proceeds from other loans payable | | | (80,451 | ) | | | 300,000 | | | | 219,549 | |
| | | | | | | | | | | | | |
| Total cash provided by financing activities | | | 4,154,403 | | | | 2,956,518 | | | | 23,382,932 | |
| | | | | | | | | | | | | |
| Effect of exchange rate changes on cash and cash equivalents | | | 134,871 | | | | 803,314 | | | | 446,683 | |
| | | | | | | | | | | | | |
| Net increase (decrease) in cash | | | 106,898 | | | | (10,988 | ) | | | 142,373 | |
| | | | | | | | | | | | | |
| Cash, beginning of period | | | 35,475 | | | | 46,463 | | | | - | |
| | | | | | | | | | | | | |
| Cash, end of period | | $ | 142,373 | | | $ | 35,475 | | | $ | 142,373 | |
| | | | | | | | | | | | | |
| Supplemental disclosures: | | | | | | | | | | | | |
| Interest paid | | $ | 28,449 | | | $ | 13,794 | | | $ | 93,289 | |
| | | | | | | | | | | | | |
| Income taxes paid | | $ | - | | | $ | - | | | $ | 32,673 | |
| | | | | | | | | | | | | |
| Conversion of stockholders' loans to equity | | $ | 3,689,208 | | | $ | 1,376,229 | | | $ | 5,065,437 | |
| | | | | | | | | | | | | |
| Conversion of other loans payable to stockholders' loans | | $ | 300,000 | | | $ | - | | | $ | 300,000 | |
| | | | | | | | | | | | | |
| Conversion of stockholders' loans to equity of variable interest entities | | $ | - | | | $ | - | | | $ | 1,114,400 | |
| | | | | | | | | | | | | |
| Imputed interest on stockholders' loans reported as an | | | | | | | | | | | | |
| increase to additional paid-in capital | | $ | 380,414 | | | $ | 415,705 | | | $ | 2,363,937 | |
See notes to consolidated financial statements.
| SUREPURE, INC. AND SUBSIDIARIES |
| (A Development Stage Group) |
Notes To Consolidated financial Statements
Years Ended December 31, 2012 and 2011
| 1. | Organization and Significant Accounting Policies |
Description of Business
SurePure Investment Holding AG (“SPI”) was incorporated in Switzerland in 2007. From 2007 to December 12, 2012 SPI was the holding company of the SurePure Group (the “Group”), which included subsidiaries and other entities whose activities primarily benefit the Group. On December 12, 2012, SPI entered into an Amended and Restated Share Exchange Agreement with SurePure, Inc. (“SurePure US” or the “Company”) pursuant to which SurePure US acquired SPI in a share exchange (“Share Exchange”) and became the holding company for the Group, including SPI. Although SurePure US is the legal acquirer of SPI, SPI is treated as the acquirer for accounting and financial reporting purposes and under this method, SurePure US retains SPI’s financial reporting history.
Under the Share Exchange, each share of the capital stock of SPI was exchanged for one share of SurePure US common stock, par value $.001 per share (“Common Stock”), and, in the case of one shareholder of SPI, one share of Nonvoting Convertible Preferred Stock, par value $.01 per share (the “Nonvoting Convertible Preferred Stock”).
The Group has developed the technology for using shortwave ultraviolet light (“UV-C”) to purify turbid liquids such as wine, fruit juice and milk. Although initially designed to treat food-grade applications, it has successfully been applied to liquids such as bovine blood plasma, water, brines and sugar syrup solutions. The Group holds international patents for this technology. The Group has been engaged in raising capital, continuing research and development of its technologies and processes and developing markets for its products.
Basis of Presentation and Consolidation
These consolidated financial statements include the accounts of SurePure US and its wholly-owned subsidiaries, including SPI, and associated variable interest entities (“VIE’s”). As a development stage entity, the Company is devoting most of its efforts to establishing its business; therefore, the accompanying consolidated statements of operations, comprehensive income (loss), and cash flows present cumulative amounts since inception.
The Company’s wholly-owned subsidiaries are follows:
| · | SurePure Operations AG (“SPO”), markets the products of the Group and earns it revenue by selling equipment utilizing the Group technology globally. SPO owns a patent for its technology in a number of countries. |
| · | SurePure Latin America Maqinas de Purificasao UVC Ltda. (“SPLAM”) conducts no operations currently. |
| SUREPURE, INC. AND SUBSIDIARIES |
| (A Development Stage Group) |
Notes To Consolidated financial Statements (Continued)
Years Ended December 31, 2012 and 2011
| · | SurePure Participations AG (“SPP”) was a minority stockholder of SPI and is part of the common holding structure of the Group. SPP has no operations and all of its expenses have been and will continue to be paid by SPI. Formerly a VIE, SPP became a subsidiary with the Share Exchange. |
VIE’s are entities whose activities primarily benefit the Company and are primarily supported by the Company. The Company continues to have a variable interest in the following entities:SurePure Holdings South Africa (Pty) Ltd. (“SPHSA”) and its wholly-owned subsidiary SurePure Marketing South Africa (Pty) Ltd. (“SPMSA”), which hold the South African patent, market the products of the Group and earn revenue from selling equipment utilizing the SurePure technology.
The Group’s reporting currency is the United States Dollar (“USD”) and these consolidated financial statements are presented in USD or “$.”
Use of Estimates
The preparation of consolidated financial statements in conformity with generally accepted accounting principal (“GAAP”) requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the consolidated financial statements, and the reported amounts of revenues and expenses during the reporting period. Actual results could differ materially from those estimates.
Income Taxes
The Group accounts for income taxes using the liability method. Under this method, deferred income taxes are recognized based on the tax effects of temporary differences between the financial statement and tax bases of assets and liabilities, as measured by current enacted tax rates. Valuation allowances are recorded to reduce the deferred tax assets to an amount that will more likely than not provide a future tax benefit.
GAAP requires that, in applying the liability method, the consolidated financial statement effects of an uncertain tax position be recognized based on the outcome that is more likely than not to occur. Under this criterion, the most likely resolution of an uncertain tax position should be analyzed based on technical merits and on that will likely be sustained under examination. There are no uncertain tax positions requiring adjustment to or disclosure in these consolidated financial statements.
Accounts Receivable
The Group performs regular credit evaluations of customers to which it provides sales on credit terms, and adjusts credit limits based on the customer’s payment history and reassessments of their creditworthiness. The Group continuously monitors its collections and establishes a provision for estimated doubtful accounts, if necessary. No allowance for doubtful accounts was deemed to be necessary at December 31, 2012 and 2011.
| SUREPURE, INC. AND SUBSIDIARIES |
| (A Development Stage Group) |
Notes To Consolidated financial Statements (Continued)
Years Ended December 31, 2012 and 2011
Property, Equipment and Related Depreciation
Property and equipment are recorded at cost, less accumulated depreciation. Cost includes the price paid to acquire the asset and any expenditures that substantially increase the asset’s value or extend the useful life of an existing asset. Depreciation is computed using the straight-line method over the estimated useful lives of the property and equipment. Major repairs and betterments that significantly extend original useful lives or improve productivity are capitalized and depreciated over the period benefited. Expenditures for routine repairs and maintenance are expensed as incurred. When property and equipment are retired or otherwise disposed of, the related cost and accumulated depreciation are removed from the respective accounts and any gain or loss is recognized in operations.
Depreciation is provided over the following estimated useful lives:
| Plant machinery | | 3 to 5 years |
| Furniture and fixtures | | 3 to 5 years |
| Motor vehicles | | 5 years |
| Office and computer equipment | | 3 to 5 years |
Intangible Assets
Intangible assets consist of patents in various countries around the world for the Company’s UV-C purification technology. The patents were initially recognized at their cost and are being amortized on a straight-line basis over their remaining estimated useful lives of twelve years.
The Group evaluates the carrying value of its intangible assets for impairment at least annually or when events or changes in circumstances are identified by management that indicate that such carrying values may not be fully recoverable. The evaluation involves estimating the future undiscounted cash flows expected to be derived from the assets to assess whether or not a potential impairment exists. As a result of its evaluations, management determined that it was not necessary to recognize a loss on impairment of its intangible assets for the years ended December 31, 2012 and 2011. However, impairment losses of $537,631 were recognized prior to 2011.
Fair Value of Financial Instruments
Financial instruments include accounts receivable, accounts payable and accrued expenses. As of December 31, 2012 and 2011, the carrying values of the financial instruments approximated their fair values due to the short-term nature of these instruments.
| SUREPURE, INC. AND SUBSIDIARIES |
| (A Development Stage Group) |
Notes To Consolidated financial Statements (Continued)
Years Ended December 31, 2012 and 2011
Revenue
Revenue is earned from sales of equipment that uses the Company’s patented technology and is recognized, net of returns and discounts, when persuasive evidence of an arrangement exists, delivery has occurred, the sales price is fixed or determinable and collectability is reasonably assured. These criteria are usually met upon delivery of the product to the customer, which is also when the risk of ownership and title passes to the customer.
Research and Development
Research and development costs are charged to expense as incurred.
Foreign Currency Translations
These consolidated financial statements are presented in USD, which is the Group’s reporting currency. The consolidated financial statements of the Group members have been translated into USD in accordance with GAAP. All assets and liability accounts on the consolidated balance sheets have been translated using the exchange rate in effect at the consolidated balance sheet date. Equity accounts have been translated at their historical rates when the capital transaction occurred. Income and expenses have been translated at the average exchange rates for the periods presented. Adjustments resulting from the translation of the Group’s consolidated financial statements are included in the consolidated statement of other comprehensive income (loss). Actual transaction gains and losses are included in the consolidated statements of operations as incurred.
The functional currencies of the companies included in the Group are their respective local currencies. Accordingly, the Group is exposed to transaction gains and losses that result from changes in various foreign currency exchange rates.
Applicable functional currencies are:
| SPI, SPO, and SPP | | Swiss francs – CHF |
| SPLAM | | Brazilian Real – BRL |
| SPMSA and SPHSA | | South African Rand – ZAR |
| SUREPURE, INC. AND SUBSIDIARIES |
| (A Development Stage Group) |
Notes To Consolidated financial Statements (Continued)
Years Ended December 31, 2012 and 2011
Exchange rates used for conversion of foreign items to USD at the end of each period and the average for the period were:
| | | 2012 | | | 2011 | |
| CHF: | | | | | | | | |
| Reporting date | | | 1.0942 | | | | 1.0640 | |
| Average for period | | | 1.0667 | | | | 1.1331 | |
| | | | | | | | | |
| BRL: | | | | | | | | |
| Reporting date | | | 0.4880 | | | | 0.5357 | |
| Average for period | | | 0.5133 | | | | 0.5998 | |
| | | | | | | | | |
| ZAR: | | | | | | | | |
| Reporting date | | | 0.1178 | | | | 0.1228 | |
| Average for period | | | 0.1219 | | | | 0.1385 | |
Fair Value of Financial Instruments
GAAP has established a framework for measuring fair value that is based on a hierarchy which prioritizes the inputs to valuation techniques according to the degree of objectivity necessary. The fair value hierarchy of the inputs to valuation techniques used to measure fair value is divided into three broad levels of objectivity:
Level 1 inputs to the valuation methodology are quoted prices (unadjusted) for identical assets or liabilities in active markets.
Level 2 inputs to the valuation methodology include quoted prices for similar assets and liabilities in active markets, and inputs that are observable for the asset or liability, either directly or indirectly, for substantially the full term of the financial instrument.
Level 3 inputs to the valuation methodology are unobservable and significant to the fair value measurement. They are based on best information available in the absence of level 1 and 2 inputs.
The following methods and assumptions were used to estimate the fair value of each class of financial instruments for which it is practicable to estimate that value as required by GAAP:
Cash:The carrying amount is the fair value because it is the basic financial instrument used to express fair value.
| SUREPURE, INC. AND SUBSIDIARIES |
| (A Development Stage Group) |
Notes To Consolidated financial Statements (Continued)
Years Ended December 31, 2012 and 2011
Accounts receivable and accounts payable: The carrying amounts approximate fair value because of the short-term duration of those instruments.
Loans payable:The carrying amount approximates fair value based on current market conditions and interest rates available to the Group for similar financial instruments.
Earnings (Loss) per Share
Basic and diluted earnings (loss) per share is computed by dividing net income or loss by the weighted average number of common shares outstanding during the period. Diluted earnings (loss) per share reflects the potential dilution that could occur if securities or other contracts to issue common stock, such as options, convertible notes and convertible preferred stock, were exercised or converted into common stock or could otherwise cause the issuance of common stock that then would share in earnings (losses). Such potential additional common shares are included in the computation of diluted earnings per share. The Company has no securities or other contracts to issue common stock that could cause any dilution of earnings. In addition, when there is a loss, diluted loss per share is not computed because any potential additional common shares would reduce the reported loss per share and therefore have an anti-dilutive effect.
Property and equipment consists of the following:
| | | 2012 | | | 2011 | |
| | | | | | | | | |
| Machinery and equipment | | $ | 5,010 | | | $ | 5,010 | |
| Furniture and fixtures | | | 12,753 | | | | 12,753 | |
| Motor vehicles | | | 14,400 | | | | 14,400 | |
| Office and computer equipment | | | 12,647 | | | | 12,647 | |
| | | | 44,810 | | | | 44,810 | |
| Less: accumulated depreciation | | | 40,194 | | | | 32,202 | |
| | | | | | | | | |
| Property and equipment, net | | $ | 4,616 | | | $ | 12,608 | |
Depreciation expense was $7,992, $7,003, and $92,653 for the years ended December 31, 2012 and 2011 and for the period from inception to December 31, 2012, respectively.
| SUREPURE, INC. AND SUBSIDIARIES |
| (A Development Stage Group) |
Notes To Consolidated financial Statements (Continued)
Years Ended December 31, 2012 and 2011
Intangible assets consist of the following:
| | | 2012 | | | 2011 | |
| | | | | | | | | |
| Patents | | $ | 208,943 | | | $ | 208,943 | |
| Less: accumulated amortization | | | 79,985 | | | | 63,316 | |
| | | | | | | | | |
| Intangible assets, net | | $ | 128,958 | | | $ | 145,627 | |
Amortization expense was $16,669, $16,466, and $83,446 for the years ended December 31, 2012 and 2011 and for the period from inception to December 31, 2012, respectively.
| 4. | Due to Officers/Stockholders |
Due to officers/stockholders consists of unpaid salaries, accrued leave and advances from the three executives of the Group entities totaling $247,259 and $473,129 at December 31, 2012 and 2011, respectively.
| 5. | Stockholders and Other Loans Payable |
Stockholder and other loans payable consist of advances by individuals and companies to the Group. Certain of the lenders are either stockholders or are related to stockholders. None of these loans are supported by notes and none have a provision for interest or repayment. The Company has imputed interest on these loans. The rates of interest used to impute interest on these loans range from 4% per annum to 15% per annum during the periods in which these loans were outstanding and represent management’s best estimate of the interest rates that would be applicable to Company in a third-party marketplace. For the years ended December 31, 2012 and 2011 and for the period from inception to December 31, 2012, the imputed interest on these loans was $380,414, $415,705 and $2,363,937, respectively. These amounts are included in interest expense in the accompanying consolidated statements of operations and are reflected as an increase in additional paid-in capital in the accompanying consolidated statements of stockholders’ deficit.
The Group has obtained subordination agreements from all of the lenders with respect to these loans, the terms of which provide that the loans will not be classified as current or be payable within one year if doing so would cause a Group member to be considered insolvent in accordance with the
applicable local laws. Therefore, these loans are presented as long-term liabilities in the accompanying consolidated balance sheets.
| SUREPURE, INC. AND SUBSIDIARIES |
| (A Development Stage Group) |
Notes To Consolidated financial Statements (Continued)
Years Ended December 31, 2012 and 2011
On August 16, 2012, one of the lenders converted its entire loan balance of $3,689,208 into 7,348,416 common shares of SPI. In October, 2012, the Company and the three lenders whose balances are reflected in Other Loans Payable revised their agreements orally and these agreements were later formalized in writing effective January 2013. The Company agreed to pay interest at 5% per annum on the outstanding balances retroactive to the time that the loans were made in 2011 and repaid these lenders $105,000 in October, 2012. Interest on these loans through December 31, 2012 was $24,593. In addition, the Company agreed to make monthly payments of $10,000 to each of the three lenders commencing February, 2013. The agreements further provided that if the entire loan balance was not paid by October 31, 2013, then a 10% per annum interest rate would be applied to the entire loan balance from the inception of the loan. As of April 1, 2013, the Company has not made the monthly payments pursuant to these agreements.
Common stock through the date of the Share Exchange consisted of the common stock of SPI. The amounts presented for periods prior to the Share Exchange were denominated in CHF and have been translated from CHF to USD using the exchange rates in effect on the date of each issuance. For all of 2011, SPI had 26,822,215 common shares issued and outstanding. During 2012 prior to the Share Exchange, SPI issued 2,500,000 shares in connection with the Subscription Agreement and 7,378,416 shares in connection with the conversion of SPI stockholder loans to common shares, resulting in 36,700,631 common shares outstanding immediately prior to the Share Exchange.
On June 14, 2011, a majority of the Company’s shareholders and the directors approved a special resolution to undertake a forward split of the the Company’s common stock resulting in an increase in the number of outstanding shares on that date from 2,135,000 to 32,452,000. Of these shares, 23,180,000 shares that were held by the former directors and officers were redeemed and cancelled as a connection to the Share Exchange, leaving 9,272,000 shares of SurePure US outstanding immediately prior to the Share Exchange. No preferred shares were issued prior to the date of the Share Exchange.
In determining the annual number of outstanding common shares on a weighted-average basis, the 9,272,000 common shares held by former SurePure US stockholders are considered to be outstanding from July 25, 2011, the date that the Company and SPI entered into an Agreement and Plan of Merger.
| SUREPURE, INC. AND SUBSIDIARIES |
| (A Development Stage Group) |
Notes To Consolidated financial Statements (Continued)
Years Ended December 31, 2012 and 2011
On December 12, 2012, the Company designated 31,155,282 of the authorized shares of preferred stock as Nonvoting Convertible Preferred Stock. Under the terms of the Certificate of Designation (the “Certificate”), each share of Nonvoting Convertible Preferred Stock is convertible into one share of Common Stock, subject to certain limitations and restrictions as defined in the Certificate.
The issued shares of Nonvoting Convertible Preferred Stock automatically convert, at the applicable conversion ratio as defined in the Certificate, into shares of the Company’s Common Stock upon the assignment, sale or other transfer of shares to any person other than an affiliate of the holder of the seller. Any assignee, purchaser or other transferee may surrender certificates representing the assigned shares to us and will receive shares of our Common Stock in return.
At December 31, 2012 and 2011, 23,542,184 and 26,822,215, respectively, common shares were issued and outstanding. There were 22,665,447 issued and outstanding preferred shares at December 31, 2012 and no issued preferred shares at December 31, 2011.
All references in these consolidated financial statements to the number of common shares, price per share and weighted average number of common shares outstanding prior to the June 2011 forward split have been adjusted to reflect the stock split on a retroactive basis unless otherwise noted. As of December 31, 2012, the Company has not granted any stock options and has not recorded any stock-based compensation.
| SUREPURE, INC. AND SUBSIDIARIES |
| (A Development Stage Group) |
Notes To Consolidated financial Statements (Continued)
Years Ended December 31, 2012 and 2011
| 7. | Stock Subscription Agreement |
On July 23, 2012, the Company entered into a Subscription Agreement with Trinity Asset Management, (Pty) Limited (“Trinity”) in which Trinity agreed to purchase up to five million new common shares in a series of transactions. Under this agreement, Trinity purchased new common shares at a price of $1.00 per share and had agreed to pay the total subscription price of five million dollars on or before December 31, 2012. The Company received a total of $2,500,000 from Trinity pursuant to this agreement during the period July 23 through October 9, 2012. Thereafter, through October 31, 2012, Trinity failed to purchase shares in accordance to the terms of the Subscription Agreement. On November 22, 2012, the Company exercised its rights to terminate the Subscription Agreement.
As a part of the above Subscription Agreement, Trinity had also agreed to purchase up to one million common shares from the Company’s principal stockholder (“Investor Shares”). The termination of the Subscription Agreement also applied to the agreed-upon purchase of Investor Shares.
The following presents the information regarding the actual purchases of shares up to the point atwhich the Subscription Agreement was terminated:
| | | Total | | | Shares | | | | |
| | | Shares | | | Purchased | | | Investor | |
| Date of Purchase | | Purchased | | | from SPI | | | Shares | |
| | | | | | | | | | |
| July 30, 2012 | | | 500,000 | | | | 375,000 | | | | 125,000 | |
| August 17, 2012 | | | 500,000 | | | | 375,000 | | | | 125,000 | |
| September 28, 2012 | | | 1,767,737 | | | | 1,517,737 | | | | 250,000 | |
| October 9, 2012 | | | 327,000 | | | | 232,263 | | | | 94,737 | |
| | | | | | | | | | | | | |
| Total through December 31, 2012 | | | 3,094,737 | | | | 2,500,000 | | | | 594,737 | |
On November 26, 2012, the Company entered into a Subscription Agreement with RD Active Capital Limited, a United Kingdom-based investment manager (“RD Active”), in which RD Active agreed to purchase up to 300,000 new common shares over the period ending January 31, 2013 and acquired the right to purchase up to 2,700,000 new common shares through March 31, 2013 as long as RD Active purchased the 300,000 new common shares. The right to purchase shares was extended on March 28, 2013 to April 12, 2013. All common shares were purchased and sold, and are to be purchased and sold, at an issue price of $1.00 per share.. Under the terms of the Subscription Agreement, RD Active may exercise the additional purchase right on its own behalf and resell to other purchasers or may place the additional shares directly with other purchasers. At such time as RD Active and any other purchasers have completed and paid for 3,000,000 shares under the Subscription Agreement, RD Active and the other purchasers under the Subscription Agreement have the right to appoint an additional director to the board of directors of the Company. Pursuant to the terms and conditions of the Share Exchange, the Company has assumed the obligations of SPI under the Subscription Agreement.
| SUREPURE, INC. AND SUBSIDIARIES |
| (A Development Stage Group) |
Notes To Consolidated financial Statements (Continued)
Years Ended December 31, 2012 and 2011
As of December 31, 2012, RD Active had purchased 235,000 common shares under the Subscription Agreement. Subsequent to December 31, 2012, the Company received $348,000 pursuant to the Subscription Agreement, and the Company issued 348,000 additional common shares.
The consolidated stockholders’ equity of the Group consists of the consolidated equity of the Group attributable to the parent which includes the common stock, additional paid-in capital, the retained deficit, and the accumulated other comprehensive income plus the equity of the noncontrolling interest of the VIE’s.
The South African VIE’s (SPHSA and SPMSA) that are part of the Group are managed by the same executive management as that of SPI, and the major shareholder of the South African VIE’s and SPI are related parties. In the accompanying consolidated financial statements, the 82.64% controlling interest in the South African VIE’s is reported in the consolidated statements of stockholders’ deficit together with the entire interest of SPP (prior to the Share Exchange) as the Equity in Variable Interest Entities. The 17.36% portion of the equity of South African entities that is not owned by this controlling stockholder is reported as the Noncontrolling Interest in the VIE’s. The investment interest of SPP in SPI was eliminated in consolidation and was reported as a Stock Subscription Receivable.
| SUREPURE, INC. AND SUBSIDIARIES |
| (A Development Stage Group) |
Notes To Consolidated financial Statements (Continued)
Years Ended December 31, 2012 and 2011
The Company and group members file income tax returns in Switzerland, South Africa, the United States and Brazil. The components of income (loss) from operations before income taxes, by jurisdiction, are as follows:
| | | | | | | | | Cumulative from | |
| | | | | | | | | August 24, 2005 | |
| | | | | | | | | (Inception) to | |
| | | 2012 | | | 2011 | | | December 31, 2012 | |
| | | | | | | | | | |
| Switzerland | | $ | (3,724,792 | ) | | $ | (3,890,732 | ) | | $ | (16,515,885 | ) |
| South Africa | | | (678,233 | ) | | | (237,447 | ) | | | (9,302,119 | ) |
| United States | | | (15,434 | ) | | | | | | | (15,434 | ) |
| Brazil | | | - | | | | 77,217 | | | | (941,498 | ) |
| | | | | | | | | | | | | |
| Total | | $ | (4,418,459 | ) | | $ | (4,050,962 | ) | | $ | (26,774,936 | ) |
The provision for income taxes shown in the accompanying consolidated statements of operations consists of the following for the years ended December 31, 2012 and 2011 and for the period from inception to December 31, 2012:
| | | | | | | | | Cumulative from | |
| | | | | | | | | August 24, 2005 | |
| | | | | | | | | (Inception) to | |
| | | 2012 | | | 2011 | | | December 31, 2012 | |
| Current tax provision: | | | | | | | | | | | | |
| Switzerland | | $ | - | | | $ | - | | | $ | 32,673 | |
| South Africa | | | - | | | | - | | | | - | |
| United States | | | | | | | | | | | | |
| Brazil | | | - | | | | - | | | | - | |
| | | | | | | | | | | | | |
| Total current tax provision | | | - | | | | - | | | | 32,673 | |
| | | | | | | | | | | | | |
| Deferred tax provision: | | | | | | | | | | | | |
| Switzerland | | | (532,307 | ) | | | (635,283 | ) | | | (2,830,210 | ) |
| South Africa | | | (103,576 | ) | | | 25,283 | | | | (1,821,549 | ) |
| United States | | | (5,402 | ) | | | | | | | (5,402 | ) |
| Brazil | | | - | | | | 19,304 | | | | (235,375 | ) |
| Change in valuation allowance | | | 641,285 | | | | 590,696 | | | | 4,892,536 | |
| | | | | | | | | | | | | |
| Total deferred provision | | | - | | | | - | | | | - | |
| | | | | | | | | | | | | |
| Total | | $ | - | | | $ | - | | | $ | 32,673 | |
| SUREPURE, INC. AND SUBSIDIARIES |
| (A Development Stage Group) |
Notes To Consolidated financial Statements (Continued)
Years Ended December 31, 2012 and 2011
The Company has determined that the future tax benefits from net operating losses are not likely to be realized in future periods and a full valuation allowance has been provided for all periods.
The income tax effect of each type of temporary difference giving rise to the net deferred tax asset at December 31, 2012 and 2011 is as follows:
| | | 2012 | | | 2011 | |
| Deferred tax assets: | | | | | | | | |
| Net operating losses | | $ | 4,892,536 | | | $ | 4,251,251 | |
| Less: valuation allowance | | | (4,892,536 | ) | | | (4,251,251 | ) |
| | | | | | | | | |
| Total | | $ | - | | | $ | - | |
The following reconciles the effective income tax rates with the statutory rates for the years ended December 31, 2012 and 2011 and for the period from inception to December 31, 2012:
| | | | | | South | | | United | | | | | | | |
| | | Switzerland | | | Africa | | | States | | | Brazil | | | Total | |
| | | | | | | | | | | | | | | | | | | | | |
| Statutory rate of tax | | | 8.5%/18% | | | | 28.0 | % | | | 35.0 | % | | | 25.0 | % | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| 2012: | | | | | | | | | | | | | | | | | | | | |
| Net (loss) income from operations | | | | | | | | | | | | | | | | | | | | |
| before taxes | | $ | (3,724,792 | ) | | $ | (678,233 | ) | | $ | (15,434 | ) | | $ | - | | | $ | (4,418,459 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| As calculated at the statutory rate | | $ | (531,639 | ) | | $ | (189,905 | ) | | $ | (5,402 | ) | | $ | - | | | $ | (726,946 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Permanent differences | | | (668 | ) | | | 86,329 | | | | - | | | | - | | | | 85,661 | |
| Change in valuation reserves | | | 532,307 | | | | 103,576 | | | | 5,402 | | | | - | | | | 641,285 | |
| | | | | | | | | | | | | | | | | | | | | |
| Provision for income taxes | | $ | - | | | $ | - | | | $ | - | | | $ | - | | | $ | - | |
| | | | | | | | | | | | | | | | | | | | | |
| 2011: | | | | | | | | | | | | | | | | | | | | |
| Net (loss) from operations before taxes | | $ | (3,890,732 | ) | | $ | (237,447 | ) | | $ | - | | | $ | 77,217 | | | $ | (4,050,962 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| As calculated at the statutory rate | | $ | (648,277 | ) | | $ | (66,485 | ) | | $ | - | | | $ | 19,304 | | | $ | (695,458 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Permanent differences | | | 12,994 | | | | 91,768 | | | | - | | | | - | | | | 104,762 | |
| Change in valuation reserves | | | 635,283 | | | | (25,283 | ) | | | - | | | | (19,304 | ) | | | 590,696 | |
| | | | | | | | | | | | | | | | | | | | | |
| Provision for income taxes | | $ | - | | | $ | - | | | $ | - | | | $ | - | | | $ | - | |
| | | | | | | | | | | | | | | | | | | | | |
| From August 24, 2005 (inception) | | | | | | | | | | | | | | | | | | | | |
| to December 31, 2012: | | | | | | | | | | | | | | | | | | | | |
| Net (loss) from operations before taxes | | $ | (16,515,885 | ) | | $ | (9,302,119 | ) | | $ | (15,434 | ) | | $ | (941,498 | ) | | $ | (26,774,936 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| As calculated at the statutory rate | | $ | (2,809,862 | ) | | $ | (2,604,593 | ) | | $ | (5,402 | ) | | $ | (235,375 | ) | | $ | (5,655,232 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Permanent differences | | | 12,325 | | | | 783,044 | | | | - | | | | - | | | | 795,369 | |
| Change in valuation reserves | | | 2,830,210 | | | | 1,821,549 | | | | 5,402 | | | | 235,375 | | | | 4,892,536 | |
| | | | | | | | | | | | | | | | | | | | | |
| Provision for income taxes | | $ | 32,673 | | | $ | - | | | $ | - | | | $ | - | | | $ | 32,673 | |
| SUREPURE, INC. AND SUBSIDIARIES |
| (A Development Stage Group) |
Notes To Consolidated financial Statements (Continued)
Years Ended December 31, 2012 and 2011
Permanent differences are principally related to loss on disposal of property and equipment, interest and penalties and unallowable expenses.
The Company and group members remain subject to tax examinations for the years ended December 31, 2012 and 2011 in Switzerland and South Africa, for the three years ended December 31, 2012 and in the U.S and Brazil, for the four years ended December 31, 2012.
| 10. | Concentrations of Credit Risk |
Financial instruments that potentially subject the Company to concentrations of credit risk consist principally of cash and trade accounts receivables. Cash is maintained in financial institutions in foreign countries that do not insure the balances in the accounts. The credit risk for customer accounts is concentrated because accounts receivable consists of the balance due from one customer. However, the customer’s account typically is collected within a short period of time and, based on its assessment of current conditions, management believes there is no risk of loss. Management continuously monitors these conditions.
| 11. | Pending Acquisition of SPHSA |
On August 16, 2012 SPI entered into an agreement (the "Acquisition Agreement") with SPHSA providing for the acquisition of 100% of the outstanding shares of SPHSA from its shareholders. The Company has assumed the executory obligations of SPI under the Acquisition Agreement.
SPHSA is accounted for as a subsidiary of SPI under variable interest accounting rules. The shareholdings in SPHSA have remained largely unchanged since the formation of that company in 2005. Since October 2007, SPHSA has also provided various management services for SPI and SPO. In addition, our subsidiary SPO has loaned approximately $2,000,000 to SPHSA, all of which is due on demand. There have been and continue to be various intercompany transactions relating to the sale and purchase of goods and services between SPI and SPHSA. Accordingly, the boards of directors of both SPI and SPHSA believed it to be in the best interest of their respective companies to combine such that SPHSA would become a wholly-owned subsidiary of SPI and SPO would convert its loans and advances to SPHSA into equity, thereby improving the financial condition of SPHSA.
Subject to the terms and conditions of the Acquisition Agreement, on August 21, 2012, SPI made its offer, which initially was irrevocable for a period of 20 business days after the date of the offer to purchase from each of the shareholders of SPHSA all of their shares of SPHSA at a closing to be held September 19, 2012, which was the 21st business day after the date of the offer. The shares of SPHSA subject to SPI’s offer to purchase included shares that reflected the value of shareholder loans outstanding at the time of the offer. On September 21, 2012, SPI extended the period during which its offer remained open until November 26, 2012 to permit the satisfaction of conditions to the closing of the offer. On November 30, 2012, SPI and SPHSA agreed to amend the Acquisition Agreement to provide that the offer would remain open until February 28, 2013, so as to permit the fulfillment of certain closing conditions. On March 8, 2013, SPI extended the date that the offer would remain open to June 28, 2013. The purchase price for the shares of SPHSA under the Offer was ZAR 4,000 (approximately $500.00) per share of SPHSA. The Acquisition Agreement provides that SPI will settle the purchase price it is paying for the shares of SPHSA in shares of SPI in the ratios of (i) 1,000 common shares of SPI for each share of SPHSA and (ii) 1,000 common shares of SPI for each ZAR 4,000 in principal amount of loans owed by SPHSA to its shareholders. As of the date of the auditors’ report, SPHSA had not yet received a final determination from the South African Reserve Bank as to whether the transaction could proceed.
| SUREPURE, INC. AND SUBSIDIARIES |
| (A Development Stage Group) |
Notes To Consolidated financial Statements (Continued)
Years Ended December 31, 2012 and 2011
Acting under the terms of the Share Exchange, at the time of the Share Exchange SPI assigned its rights under the Acquisition Agreement to SurePure US, and SurePure US assumed the obligations of SPI to issue shares to the shareholders of SPHSA at such time, if any, that the pending acquisition of SPHSA is completed.
| 12. | 2012 Stock Option Plan |
On December 11, 2012, the Company adopted the 2012 Stock Plan (“the Plan”). Under the terms of the Plan, 3,000,000 shares of our Common Stock have been reserved for future issuance. The Plan authorizes the granting of non-qualified stock options to our directors and to any independent consultants. The Plan will be administered by the Company’s board, or a committee appointed by the board (the “Committee”). The Committee may determine persons eligible for grants and the timing, type, amount, fair market value and other provisions of such grants. The Committee will have authority, subject to the express provisions of the Plan, to construe the Plan and the option agreements granted pursuant to the Plan, to prescribe, amend and rescind rules and regulations relating to the Plan, and to make all other determinations in the judgment of the Committee necessary or desirable for the administration of the Plan. The Committee may correct any defect or supply any omission or reconcile any inconsistency in the Plan or in any option agreement in the manner and to the extent it shall deem expedient to promote the best interests of the Company. The Plan will terminate on December 9, 2022.
The Plan provides that the determination of the option price per share for any option rests in discretion of the Committee. Options granted under the Plan must be granted within ten years from the date on which the Plan has been adopted. No options granted under the Plan may be exercisable after ten years from the date of grant. The Company’s board of directors may suspend or terminate the Plan in whole or in part or amend the Plan in such respects as the board may deem appropriate and in the best interest of the company. No amendment, suspension or termination of the Plan shall, however, without the optionee's consent, alter or impair any of the rights or obligations granted under the Plan.
| SUREPURE, INC. AND SUBSIDIARIES |
| (A Development Stage Group) |
Notes To Consolidated financial Statements (Continued)
Years Ended December 31, 2012 and 2011
There are no outstanding grants under the Plan as of December 31, 2012.
| 13. | Commitments, Contingencies and Tax Obligation |
Lease Commitments
The Group leases two premises in South Africa under operating leases. One location is an office facility and the other is a workshop. The office facility lease was originally scheduled to expire on April 30, 2012 with a monthly rent of $4,689 but has been renegotiated to extend the lease term to February 28, 2014 at a monthly rent of $2,677 for the first year and $2,946 for the second year. The lease for the Group’s workshop facility originally expired on April 30, 2012 with a monthly rent of $2,460. This lease term was then extended to November 30, 2012 and has been extended again to September 30, 2013 with a monthly rent of $2,595 through April 30, 2013 and a monthly rent of $2,855 thereafter. In addition to the payments required under the preceding leases, payments are made for space rentals on an as-needed basis. The total rent expense for the years ended December 31, 2012 and 2011 and for the period from inception to December 31, 2012 was approximately $76,000, $89,000 and $777,000, respectively. Rent expense is included in general and administrative expenses in the accompanying consolidated statement of operations.
The future rent commitments under the above leases are as follows:
| 2013 | | $ | 62,756 | |
| 2014 | | | 5,891 | |
| | | $ | 68,647 | |
Payroll Commitments
The Group’s employees in South Africa have employment contracts that provide for one month of notice before the employee can be terminated. As of December 31, 2012, the total monthly salary commitment applicable to these employees was approximately $19,000.
| SUREPURE, INC. AND SUBSIDIARIES |
| (A Development Stage Group) |
Notes To Consolidated financial Statements (Continued)
Years Ended December 31, 2012 and 2011
Payroll Tax Obligation
During February 2012, SPM received notification from the South African Revenue Service regarding unpaid payroll taxes of approximately $185,000. SPM requested additional time to arrange a payment plan that is suitable to both parties and has commenced making monthly payments against this balance. During the Company’s review of this matter, it was discovered that an additional amount of tax was due of approximately $62,000. During the year ended December 31, 2012, the Company has accrued approximately $42,000 of interest on this liability and has made payments of approximately $102,000 towards the balance due. At December 31, 2012 and 2011, the unpaid balance of this liability was approximately $187,000 and $185,000, respectively and is included in accounts payable and other current liabilities in the accompanying consolidated balance sheets.
Employment and Consulting Agreements
The Group has entered into agreements to secure the services of three executives. These agreements provide for annual compensation and require a termination notice period by the Group of three months. The Executives are all stockholders of the Group.
The total compensation of these executives for the years ended December 31, 2012 and 2011 and for the period from inception to December 31, 2012 was approximately $1,086,000, $1,325,000 and $5,092,000, respectively. These amounts are included in general and administrative expenses.
During March 2012, the Group entered into additional agreements with unrelated third party consultants. These agreements can be terminated by either party with between two weeks and thirty days written notice or immediately if for cause. The amounts due to these consultants are approximately $32,500 per month.
The Company has evaluated its subsequent events from the balance sheet date. Other than as disclosed in Notes 7, 11 and 15, the Company has determined that there were no material subsequent events requiring adjustment to or disclosure in these consolidated financial statements.
| SUREPURE, INC. AND SUBSIDIARIES |
| (A Development Stage Group) |
Notes To Consolidated financial Statements (Continued)
Years Ended December 31, 2012 and 2011
The accompanying consolidated financial statements have been prepared on a going concern basis, which contemplates the continuation of the Group as a going concern. Due to the start-up nature of its business, the Group has generated recurring losses and expects to incur additional losses as it expands the UV-C technology and develops marketing, sales and financial plans. As reflected in the accompanying consolidated financial statements, the Group’s total liabilities exceed total assets at December 31, 2012 and 2011 by $4,075,374 and $6,596,408, respectively, and the Group has incurred cumulative operating losses since the date of inception. To date, the Group’s cash flow requirements have been met with cash investments by the stockholders, certain third parties and to a lesser extent, sales and interest income.
The Group will require additional capital to continue its development and to achieve sufficient revenues to support its operations. The Group’s future capital requirements will depend on many factors, including its ability to grow and maintain revenues and its ability to manage expenses and expected capital expenditures. The Group will require additional financing either through borrowings or the sale of additional equity to support its operations.
The Group’s access to additional financing will depend on a variety of factors many over which the Group has little or no control. These factors include market conditions, the general availability of credit, the overall availability of credit to the Group’s industry, its credit ratings and credit capacity, the actual financial and operational results of the Group as well as the lenders’ or investors’ perception of the Group’s short-term and long-term financial prospects. If future financing is not available on acceptable terms, the Group may not be able to continue as a going concern.
| SUREPURE, INC. AND SUBSIDIARIES |
| (A Development Stage Group) |
Notes To Consolidated financial Statements (Continued)
Years Ended December 31, 2012 and 2011
Management is currently pursuing various plans to meet the cash flow requirements of the Company for the year ending December 31, 2013. As disclosed in note 7 above, the Company is a party to the Subscription Agreement with RD Active. Subsequent to December 31, 2012, the company received $338,000 under this Subscription Agreement. An additional 2,417,000 shares of common stock remain available for purchase under the Subscription Agreement at $1,00 per share through the current termination date of April 12, 2013. In addition, there are ongoing discussions with current and other potential investors and the Company is also considering an additional common stock offering during 2013. In addition, the Company projects that, increase in the commercialization of the Company’s technology will provide additional cash flow for operations.
The consolidated financial statements do not include any adjustments that might be necessary should the Company be unable to continue as a going concern.
