
SEL-212 Phase 2 Data Presented at ACR October 23, 2018

Safe Harbor / Disclaimer Any statements in this presentation about the future expectations, plans and prospects of Selecta Biosciences, Inc. (“the company”), including without limitation, statements regarding the progress of the Phase 2 clinical trial of SEL-212 and expectations surrounding End-of-Phase 2 meeting with the FDA, whether SEL-212 mitigates immunogenicity and enables sustained control of serum uric acid levels, low rate of gout flares and monthly dosing, the ability of SVP-Rapamycin to mitigate inflammation induced by monosodium urate crystals, the anticipated timing for advancing into Phase 3 (if at all) and expectations surrounding proposed dose regimens, whether current evaluable SEL-212 patients will be predictive of future evaluable SEL-212 patients, whether projections regarding serum uric acid control for patients who have yet to complete the 20-week study period will be consistent with actual data, whether 5-monthly combination doses of SEL-212 have the potential to extend serum uric acid control and maintain safety over the entire treatment period, whether monthly dosing of SEL-212 leads to significant reduction in uric acid deposits, the potential of SEL-212 to significantly reduce tophi/heavy urate burden and/or rapidly eliminate tissue urate burden, whether patients receiving SEL-212 will be able to complete full therapy cycles over 6 months, whether SEL-212 has the ability to reduce gout flares frequency initially and over time during SEL-212 therapy, the severity of gout flares experienced by patients receiving SEL-212, whether SEL-212 will continue to be generally well-tolerated, the design and timing of a head-to- head trial of SEL-212 and Krystexxa, the company’s commercial plans, the ability of the company’s SVP platform, including SVP-Rapamycin, to mitigate unwanted immunogenicity, unlock the full potential of biologic therapies, enable new therapies and improve the efficacy and safety of existing biologics, the potential of SEL-212 to treat severe gout patients, resolve their debilitating symptoms, and to change the chronic severe gout treatment paradigm, the company’s plan to apply its SVP platform to a range of biologics for rare and serious diseases, and other statements containing the words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “hypothesize,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “would,” and similar expressions, constitute forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors, including, but not limited to, the following: the uncertainties inherent in the initiation, completion and cost of clinical trials including their uncertain outcomes, the availability and timing of data from ongoing and future clinical trials and the results of such trials, whether preliminary results from a particular clinical trial will be predictive of the final results of that trial or whether results of early clinical trials will be indicative of the results of later clinical trials, the unproven approach of the company’s SVP technology, potential delays in enrollment of patients, undesirable side effects of the company’s product candidates, its reliance on third parties to manufacture its product candidates and to conduct its clinical trials, the company’s inability to maintain its existing or future collaborations or licenses, its inability to protect its proprietary technology and intellectual property, potential delays in regulatory approvals, the availability of funding sufficient for its foreseeable and unforeseeable operating expenses and capital expenditure requirements, substantial fluctuation in the price of its common stock, and other important factors discussed in the “Risk Factors” section of the company’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission, or SEC, on August 8, 2018, and in other filings that the company makes with the SEC. In addition, any forward-looking statements included in this presentation represent the company’s views only as of the date of its publication and should not be relied upon as representing its views as of any subsequent date. The company specifically disclaims any obligation to update any forward-looking statements included in this presentation. 2
SEL-212 Executive Summary of Available Phase 2 Data • Evaluable patients who achieved 3-month of SUA control have maintained SUA control in months 4 and 5 of combination treatment • Projection for remaining patients in 5 dose cohorts suggest approximately 66% of evaluable patients could have SUA controlled at week 20 after 5 monthly doses • Based on interim data, 5-monthly SEL-212 combination dose data has resulted in sustained SUA control & favorable tolerability over entire treatment period • SUA Control Observed • Once monthly dosing • Low flare rates • No emerging safety signals • Sustained control of SUA near 0 mg/dL may lead to reduction in uric acid deposits as measured by DECT (Dual Energy Computed Tomography) imaging • Proposed dose regimens for Phase 3 trials • 6 monthly doses of SEL-212 v. Placebo • 0.1 mg/kg of SVP-Rapamycin with 0.2 mg/kg of pegadricase* • 0.15 mg/kg of SVP-Rapamycin with 0.2 mg/kg of pegadricase 3 *Previously called pegsiticase; pegadricase is the new United States Adopted Name (USAN)
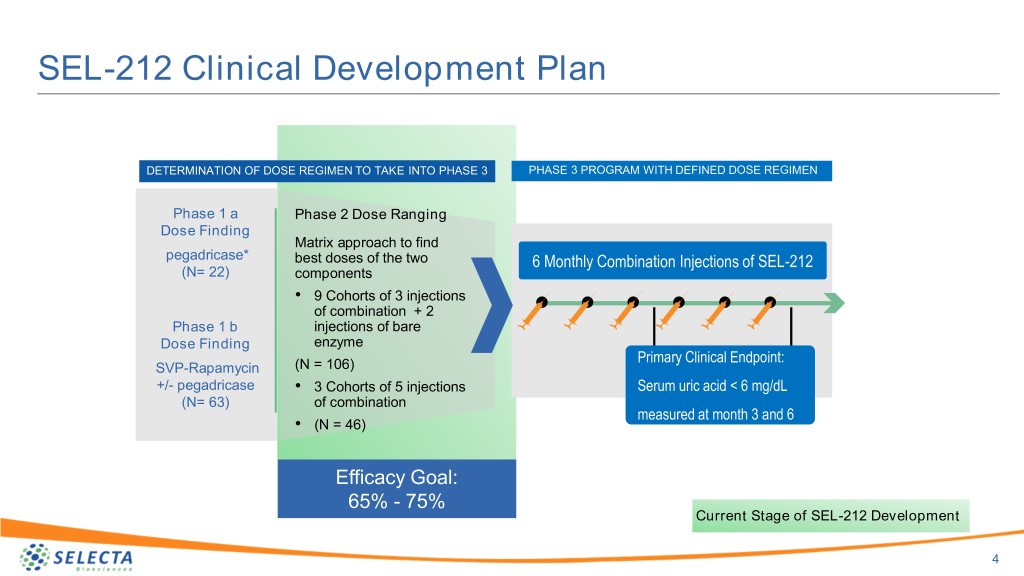
SEL-212 Clinical Development Plan DETERMINATION OF DOSE REGIMEN TO TAKE INTO PHASE 3 PHASE 3 PROGRAM WITH DEFINED DOSE REGIMEN Phase 1 a Phase 2 Dose Ranging Dose Finding Matrix approach to find pegadricase* best doses of the two 6 Monthly Combination Injections of SEL-212 (N= 22) components • 9 Cohorts of 3 injections of combination + 2 Phase 1 b injections of bare Dose Finding enzyme Primary Clinical Endpoint: SVP-Rapamycin (N = 106) +/- pegadricase • 3 Cohorts of 5 injections Serum uric acid < 6 mg/dL (N= 63) of combination measured at month 3 and 6 • (N = 46) Efficacy Goal: 65% - 75% Current Stage of SEL-212 Development 4
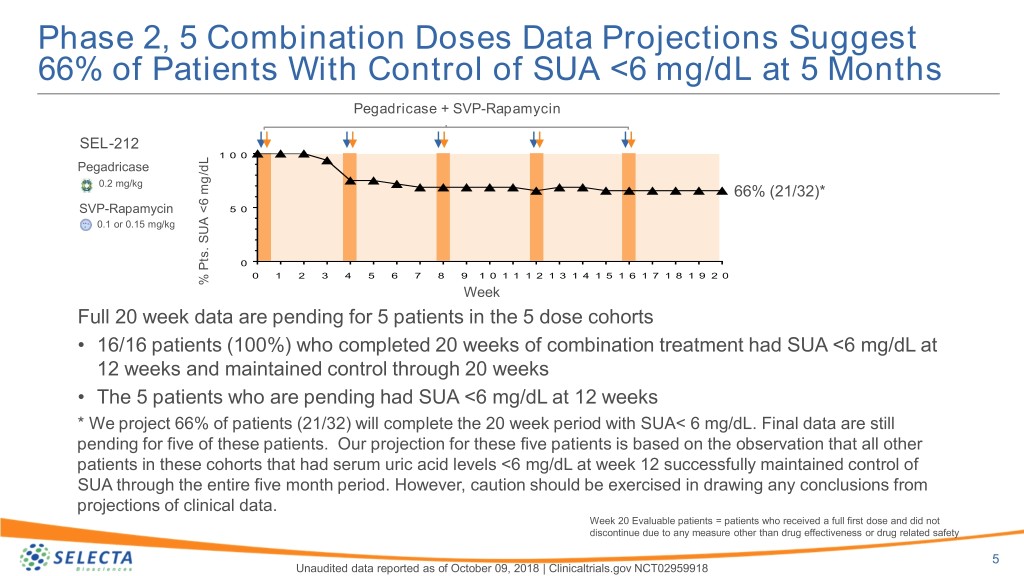
Phase 2, 5 Combination Doses Data Projections Suggest 66% of Patients With Control of SUA <6 mg/dL at 5 Months Pegadricase + SVP-Rapamycin SEL-212 1 0 0 Pegadricase 0.2 mg/kg 66% (21/32)* SVP-Rapamycin 5 0 0.1 or 0.15 mg/kg 0 0 1 2 3 4 5 6 7 8 9 1 0 1 1 1 2 1 3 1 4 1 5 1 6 1 7 1 8 1 9 2 0 % mg/dLPts.<6 SUA % Week Full 20 week data are pending for 5 patients in the 5 dose cohorts • 16/16 patients (100%) who completed 20 weeks of combination treatment had SUA <6 mg/dL at 12 weeks and maintained control through 20 weeks • The 5 patients who are pending had SUA <6 mg/dL at 12 weeks * We project 66% of patients (21/32) will complete the 20 week period with SUA< 6 mg/dL. Final data are still pending for five of these patients. Our projection for these five patients is based on the observation that all other patients in these cohorts that had serum uric acid levels <6 mg/dL at week 12 successfully maintained control of SUA through the entire five month period. However, caution should be exercised in drawing any conclusions from projections of clinical data. Week 20 Evaluable patients = patients who received a full first dose and did not discontinue due to any measure other than drug effectiveness or drug related safety 5 Unaudited data reported as of October 09, 2018 | Clinicaltrials.gov NCT02959918
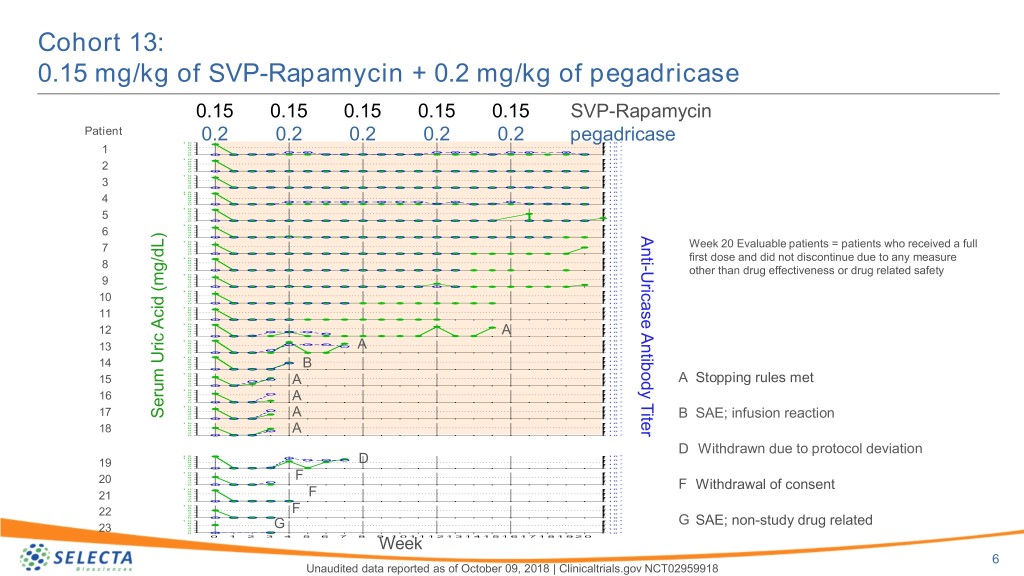
Cohort 13: 0.15 mg/kg of SVP-Rapamycin + 0.2 mg/kg of pegadricase 0.15 0.15 0.15 0.15 0.15 SVP-Rapamycin Patient 1 0 0.2 0.2 0.2 0.2 0.2 pegadricase1 0 5 8 4 6 1 0 4 1 0 3 2 1 2 0 1 0 1 0 1 0 5 8 4 6 1 0 4 1 0 3 2 2 2 0 1 0 1 0 1 0 5 8 4 6 1 0 4 1 0 3 2 3 2 0 1 0 1 2 1 0 5 1 0 8 1 0 4 6 4 1 0 3 2 4 2 0 1 0 1 0 1 0 5 8 4 6 1 0 4 1 0 3 2 5 2 0 1 0 1 0 1 0 5 8 4 6 1 0 4 1 0 3 2 Anti 6 2 0 1 0 1 0 1 0 5 8 4 6 1 0 Week 20 Evaluable patients = patients who received a full 4 1 0 3 2 7 2 0 1 0 1 0 1 0 5 8 4 first dose and did not discontinue due to any measure 6 1 0 - 4 1 0 3 8 2 2 0 1 0 Uricase Antibody Titer 1 0 1 0 5 other than drug effectiveness or drug related safety 8 4 6 1 0 4 1 0 3 9 2 0 1 0 2 1 0 1 0 5 8 4 6 1 0 4 1 0 3 10 2 0 1 0 2 1 0 1 0 5 8 4 6 1 0 4 1 0 3 11 2 0 1 0 2 1 0 1 0 5 8 4 6 1 0 4 1 0 3 12 2 A 2 0 1 0 1 0 1 0 5 8 1 0 4 6 4 1 0 3 13 2 A 0 1 0 2 1 0 1 0 5 8 1 0 4 6 4 1 0 3 14 2 0 B 1 0 2 1 0 1 0 5 8 4 6 1 0 4 1 0 3 15 2 A Stopping rules met 0 A 1 0 2 1 0 1 0 5 8 4 6 1 0 4 1 0 3 16 2 A 2 0 1 0 1 0 1 0 5 8 4 6 1 0 4 1 0 3 17 2 Serum Uric Acid (mg/dL) Acid Uric Serum A 2 0 1 0 B SAE; infusion reaction 1 0 1 0 5 8 4 6 1 0 4 1 0 3 18 2 A 2 0 1 0 D Withdrawn due to protocol deviation 1 2 1 0 5 1 0 8 1 0 4 6 4 D 1 0 3 2 19 2 0 1 0 1 0 1 0 5 8 4 6 1 0 4 F 1 0 3 2 20 2 0 1 0 1 0 1 0 5 F Withdrawal of consent 8 4 6 1 0 4 F 1 0 3 2 21 2 0 1 0 1 0 1 0 5 8 4 6 1 0 4 F 1 0 3 22 2 0 1 0 2 1 0 1 0 5 8 4 6 1 0 G SAE; non-study drug related 4 G 1 0 3 2 23 0 1 0 2 0 1 2 3 4 5 6 7 8 9Week1 0 1 1 1 2 1 3 1 4 1 5 1 6 1 7 1 8 1 9 2 0 6 Unaudited data reported as of October 09, 2018 | Clinicaltrials.gov NCT02959918
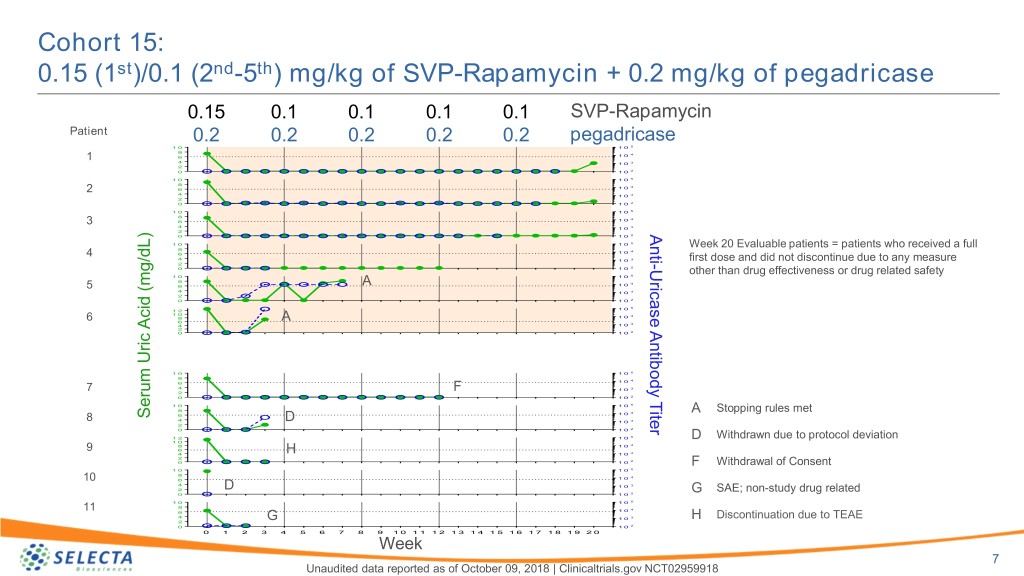
Cohort 15: 0.15 (1st)/0.1 (2nd-5th) mg/kg of SVP-Rapamycin + 0.2 mg/kg of pegadricase 0.15 0.1 0.1 0.1 0.1 SVP-Rapamycin Patient 0.2 0.2 0.2 0.2 0.2 pegadricase 1 0 1 0 5 8 4 1 6 1 0 4 1 0 3 2 0 1 0 2 1 0 1 0 5 8 4 2 6 1 0 4 1 0 3 2 0 1 0 2 1 0 1 0 5 8 4 3 6 1 0 4 1 0 3 2 Anti 0 1 0 2 1 0 1 0 5 Week 20 Evaluable patients = patients who received a full 8 4 4 6 1 0 4 1 0 3 first dose and did not discontinue due to any measure 2 - 2 0 1 0 Uricase Antibody Titer other than drug effectiveness or drug related safety 1 0 1 0 5 8 4 5 6 A 1 0 4 1 0 3 2 0 1 0 2 1 0 5 1 2 1 0 4 6 8 A 1 0 6 3 4 1 0 2 0 1 0 2 1 0 1 0 5 8 4 6 1 0 7 4 F 1 0 3 2 0 1 0 2 1 0 1 0 5 8 4 A Stopping rules met 6 1 0 Serum Uric Acid (mg/dL)Acid SerumUric 8 4 D 1 0 3 2 0 1 0 2 1 2 1 0 5 D Withdrawn due to protocol deviation 1 0 8 1 0 4 9 6 4 H 1 0 3 2 0 1 0 2 F Withdrawal of Consent 1 0 1 0 5 8 4 10 6 1 0 4 1 0 3 2 D SAE; non-study drug related 2 G 0 1 0 1 0 1 0 5 8 11 1 0 4 6 4 G 1 0 3 H Discontinuation due to TEAE 2 0 1 0 2 0 1 2 3 4 5 6 7 8 9 1 0 1 1 1 2 1 3 1 4 1 5 1 6 1 7 1 8 1 9 2 0 Week 7 Unaudited data reported as of October 09, 2018 | Clinicaltrials.gov NCT02959918
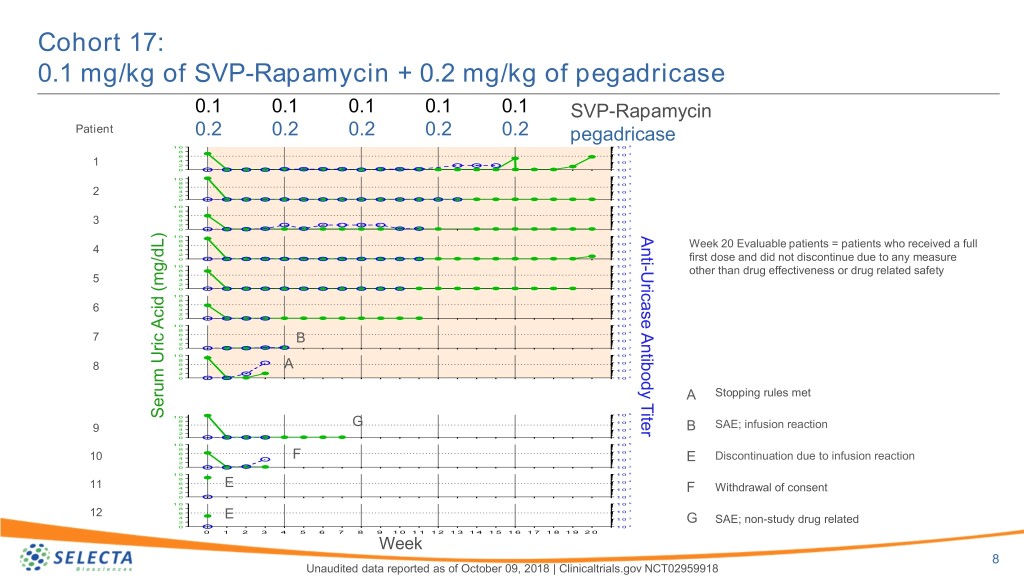
Cohort 17: 0.1 mg/kg of SVP-Rapamycin + 0.2 mg/kg of pegadricase 0.1 0.1 0.1 0.1 0.1 SVP-Rapamycin Patient 0.2 0.2 0.2 0.2 0.2 pegadricase 1 0 1 0 5 8 4 6 1 0 4 1 0 3 1 2 0 1 0 2 1 0 5 1 0 8 1 0 4 6 4 1 0 3 2 2 0 1 0 2 1 0 1 0 5 8 4 6 1 0 4 1 0 3 3 2 0 1 0 2 1 0 1 0 5 Anti 8 4 6 1 0 Week 20 Evaluable patients = patients who received a full 4 4 1 0 3 2 0 1 0 2 first dose and did not discontinue due to any measure 1 0 1 0 5 - 8 Uricase Antibody Titer 4 6 1 0 other than drug effectiveness or drug related safety 5 4 1 0 3 2 0 1 0 2 1 0 1 0 5 8 4 6 1 0 6 4 1 0 3 2 0 1 0 2 1 0 1 0 5 8 4 6 1 0 7 4 B 1 0 3 2 0 1 0 2 1 0 1 0 5 8 4 6 1 0 4 A 3 8 1 0 2 0 1 0 2 A Stopping rules met 1 0 5 Serum Uric Acid (mg/dL) Acid Uric Serum 1 0 8 1 0 4 6 G SAE; infusion reaction 9 4 1 0 3 B 2 0 1 0 2 1 0 1 0 5 8 4 6 1 0 10 4 F 1 0 3 E Discontinuation due to infusion reaction 2 0 1 0 2 1 0 1 0 5 8 4 6 1 0 E 3 11 4 1 0 2 F Withdrawal of consent 0 1 0 2 1 0 1 0 5 8 4 12 6 1 0 4 E 1 0 3 2 G SAE; non-study drug related 0 1 0 2 0 1 2 3 4 5 6 7 8 9 1 0 1 1 1 2 1 3 1 4 1 5 1 6 1 7 1 8 1 9 2 0 Week 8 Unaudited data reported as of October 09, 2018 | Clinicaltrials.gov NCT02959918
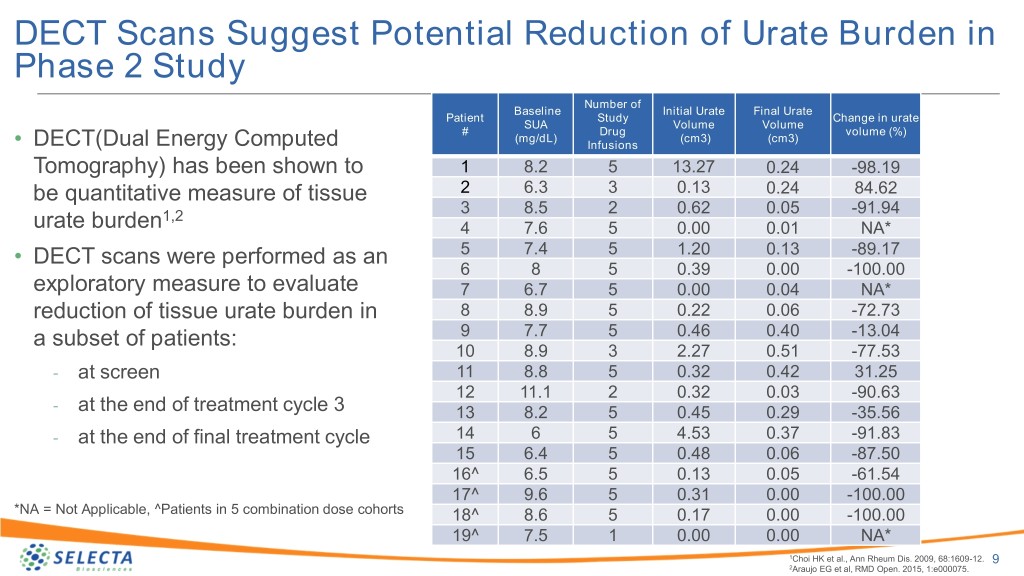
DECT Scans Suggest Potential Reduction of Urate Burden in Phase 2 Study Number of Baseline Initial Urate Final Urate Patient Study Change in urate SUA Volume Volume # Drug volume (%) (mg/dL) (cm3) (cm3) • DECT(Dual Energy Computed Infusions Tomography) has been shown to 1 8.2 5 13.27 0.24 -98.19 be quantitative measure of tissue 2 6.3 3 0.13 0.24 84.62 1,2 3 8.5 2 0.62 0.05 -91.94 urate burden 4 7.6 5 0.00 0.01 NA* • DECT scans were performed as an 5 7.4 5 1.20 0.13 -89.17 6 8 5 0.39 0.00 -100.00 exploratory measure to evaluate 7 6.7 5 0.00 0.04 NA* reduction of tissue urate burden in 8 8.9 5 0.22 0.06 -72.73 a subset of patients: 9 7.7 5 0.46 0.40 -13.04 10 8.9 3 2.27 0.51 -77.53 - at screen 11 8.8 5 0.32 0.42 31.25 12 11.1 2 0.32 0.03 -90.63 - at the end of treatment cycle 3 13 8.2 5 0.45 0.29 -35.56 - at the end of final treatment cycle 14 6 5 4.53 0.37 -91.83 15 6.4 5 0.48 0.06 -87.50 16^ 6.5 5 0.13 0.05 -61.54 17^ 9.6 5 0.31 0.00 -100.00 *NA = Not Applicable, ^Patients in 5 combination dose cohorts 18^ 8.6 5 0.17 0.00 -100.00 19^ 7.5 1 0.00 0.00 NA* 1Choi HK et al., Ann Rheum Dis. 2009, 68:1609-12. 9 2Araujo EG et al, RMD Open. 2015, 1:e000075.
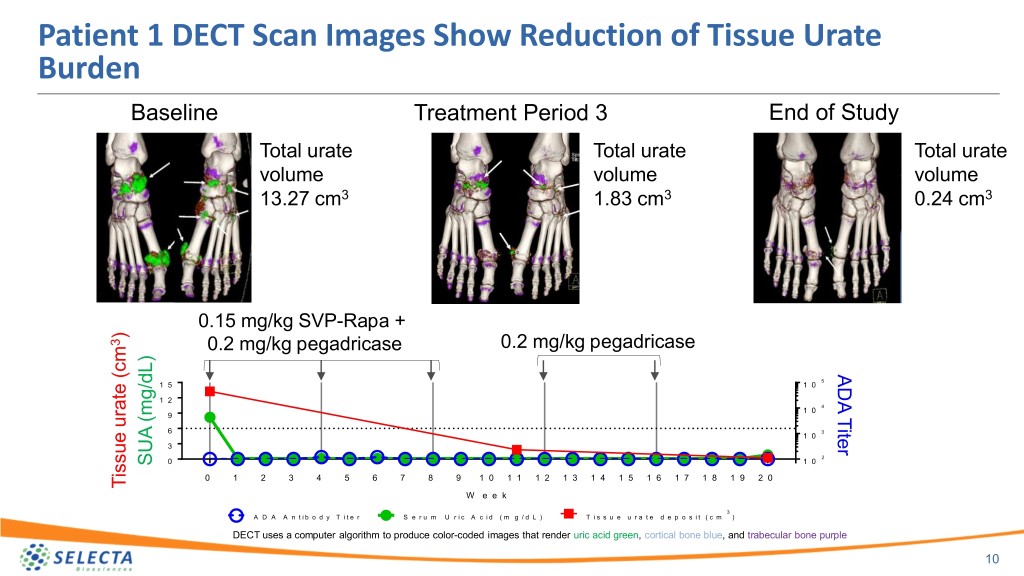
Patient 1 DECT Scan Images Show Reduction of Tissue Urate Burden Baseline Treatment Period 3 End of Study Total urate Total urate Total urate volume volume volume 13.27 cm3 1.83 cm3 0.24 cm3 0.15 mg/kg SVP-Rapa + ) 3 0.2 mg/kg pegadricase 0.2 mg/kg pegadricase ADA Titer 5 1 5 1 0 1 2 4 1 0 9 6 3 1 0 3 2 0 1 0 SUA (mg/dL) SUA 0 1 2 3 4 5 6 7 8 9 1 0 1 1 1 2 1 3 1 4 1 5 1 6 1 7 1 8 1 9 2 0 Tissue urate (cm urate Tissue W e e k 3 A D A A n t i b o d y T i t e r S e r u m U r i c A c i d ( m g / d L ) T i s s u e u r a t e d e p o s i t ( c m ) DECT uses a computer algorithm to produce color-coded images that render uric acid green, cortical bone blue, and trabecular bone purple 10
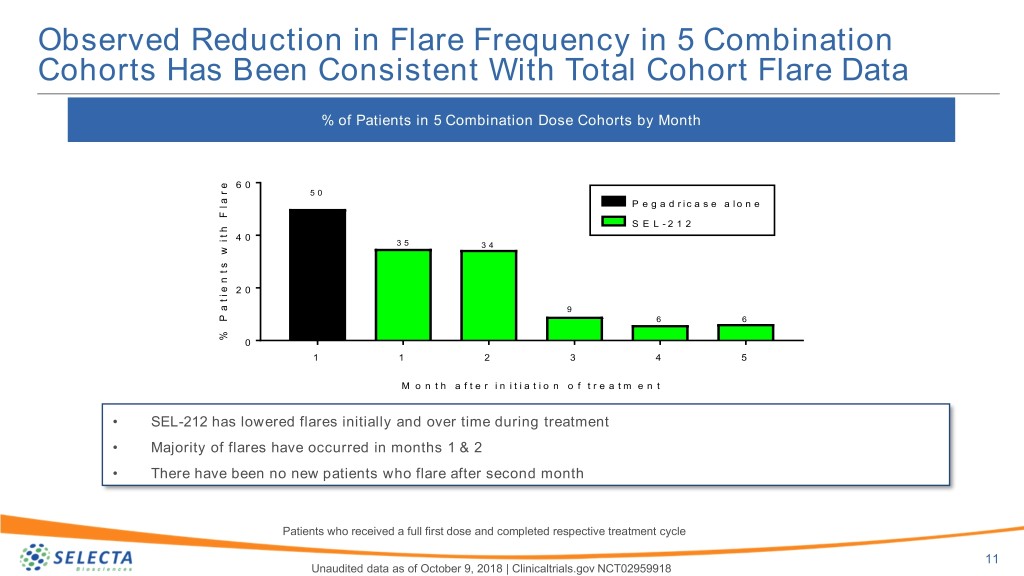
Observed Reduction in Flare Frequency in 5 Combination Cohorts Has Been Consistent With Total Cohort Flare Data % of Patients in 5 Combination Dose Cohorts by Month e r 6 0 a 5 0 l P e g a d r i c a s e a l o n e F h S E L - 2 1 2 t i 4 0 3 5 w 3 4 s t n e i 2 0 t a 9 P 6 6 % 0 1 1 2 3 4 5 M o n t h a f t e r i n i t i a t i o n o f t r e a t m e n t • SEL-212 has lowered flares initially and over time during treatment • Majority of flares have occurred in months 1 & 2 • There have been no new patients who flare after second month Patients who received a full first dose and completed respective treatment cycle 11 Unaudited data as of October 9, 2018 | Clinicaltrials.gov NCT02959918
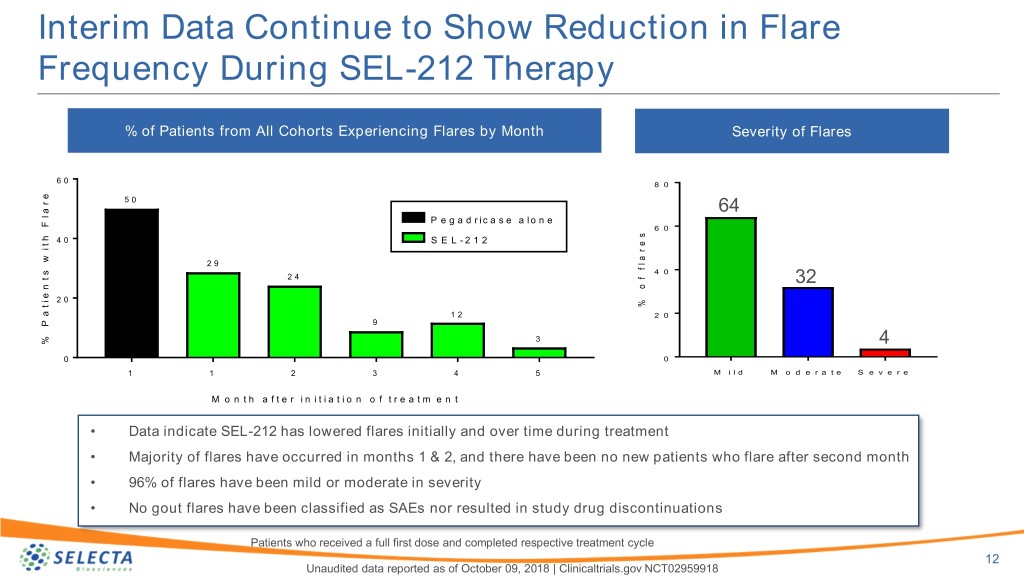
Interim Data Continue to Show Reduction in Flare Frequency During SEL-212 Therapy % of Patients from All Cohorts Experiencing Flares by Month Severity of Flares 6 0 8 0 e 5 0 r a 64 l P e g a d r i c a s e a l o n e s F e 6 0 r h 4 0 S E L - 2 1 2 t a i l f w 2 9 f s 4 0 t 2 4 o 32 n e i 2 0 % t a 1 2 2 0 P 9 3 % 4 0 0 1 1 2 3 4 5 M i l d M o d e r a t e S e v e r e M o n t h a f t e r i n i t i a t i o n o f t r e a t m e n t • Data indicate SEL-212 has lowered flares initially and over time during treatment • Majority of flares have occurred in months 1 & 2, and there have been no new patients who flare after second month • 96% of flares have been mild or moderate in severity • No gout flares have been classified as SAEs nor resulted in study drug discontinuations Patients who received a full first dose and completed respective treatment cycle 12 Unaudited data reported as of October 09, 2018 | Clinicaltrials.gov NCT02959918

5 Months Combination Treatment Has Not Shown Any Emerging Safety Signals • SEL-212 has been generally well tolerated at clinically active doses for 5- combination dose cohorts following >130 administrations • 46 patients in 5-combination dose cohorts have been dosed in the Phase 2 study as of October 9, 2018 • 5 months of combination treatment has not shown any emerging safety signals • 7 SAEs (5 individual patients) have been reported in the 5-combination dose cohorts: - 4 SAEs were reported not to be or unlikely to be related to study drug - 1 SAE was reported to be possibly related to study drug - 2 SAEs (infusion reactions) were reported as related or possibly related to study drug, both of which occurred during the infusion of SEL-212 in Treatment Period 2 - No SAEs have occurred in Treatment Periods 4 or 5 13
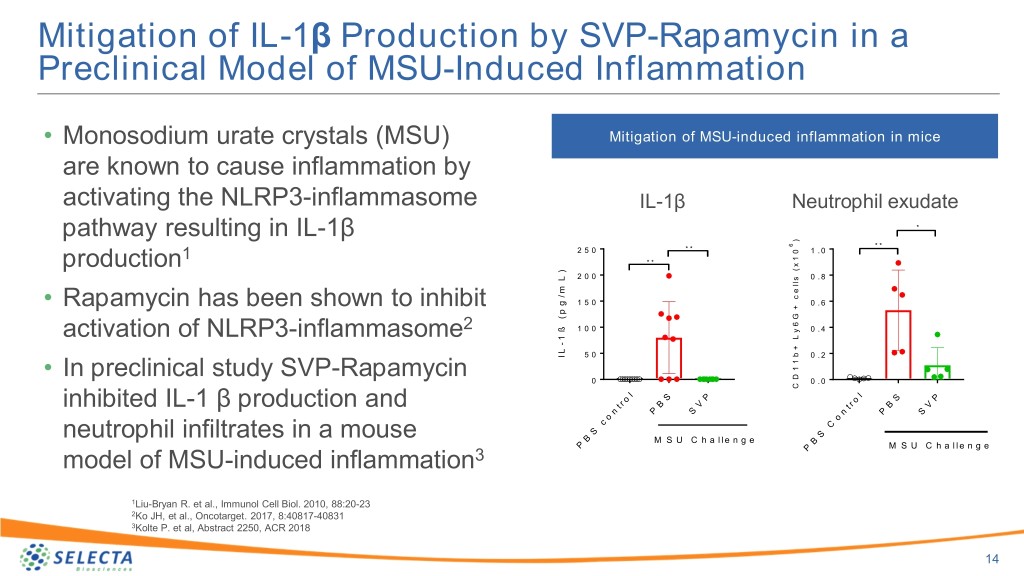
Mitigation of IL-1β Production by SVP-Rapamycin in a Preclinical Model of MSU-Induced Inflammation • Monosodium urate crystals (MSU) Mitigation of MSU-induced inflammation in mice are known to cause inflammation by activating the NLRP3-inflammasome IL-1β Neutrophil exudate pathway resulting in IL-1β * ) * * 6 * * 2 5 0 0 1 . 0 1 1 * * x production ( ) 2 0 0 s 0 . 8 L l l e m / c g 1 5 0 0 . 6 • Rapamycin has been shown to inhibit + p ( G 6 ß 2 1 0 0 y 0 . 4 L activation of NLRP3-inflammasome 1 - + L b I 5 0 0 . 2 1 1 • In preclinical study SVP-Rapamycin D 0 0 . 0 C l l o S P S P r o inhibited IL-1 β production and t B V t r B V n P S n P S o o c C neutrophil infiltrates in a mouse S S B M S U C h a l l e n g e B P M S U C h a l l e n g e model of MSU-induced inflammation3 P 1Liu-Bryan R. et al., Immunol Cell Biol. 2010, 88:20-23 2Ko JH, et al., Oncotarget. 2017, 8:40817-40831 3Kolte P. et al, Abstract 2250, ACR 2018 14

Summary and Next Steps • Projections suggest approximately 66% of patients could have SUA control during 5 months of treatment with monthly dosing of SEL-212 • Low flare rates observed to date • Potential to rapidly eliminate tissue urate burden based on DECT imaging findings • 5 month combination treatment has not shown any emerging safety signals • Proposed dose regimens for Phase 3 trials identified • End of Phase 2 meeting scheduled, and start of Phase 3 planned in 2018 • Head-to-Head trial designed and expected to be conducted in parallel with Phase 3 pivotal trials • Commercial plans accelerated 15

We thank all of the patients that have participated in our clinical program. We are very grateful to the clinical trial site investigators and their staff.

Appendix

SEL-212 Posters Presented at ACR Monday, October 22, 2018; 9:00 AM - 11:00 AM • Initial Phase 2 Clinical Data of SEL-212 in Symptomatic Gout Patients: Monthly Dosing of a Pegylated Uricase (pegadricase*) with SVP-Rapamycin Enables Sustained Reduction of Acute Gout Flares Tuesday, October 23, 2018; 9:00 AM - 11:00 AM • Mitigation of Inflammation Induced By Monosodium Urate Crystals in Mice By Treatment with SVP-Rapamycin • Update of SEL-212 Phase 2 Clinical Data in Symptomatic Gout Patients: SVP-Rapamycin Combined with pegadricase Mitigates Immunogenicity and Enables Sustained Reduction of Serum Uric Acid Levels, Low Rate of Gout Flares and Monthly Dosing • Initial Phase 2 Clinical Data of SEL-212 in Symptomatic Gout Patients: Measurement of Dissolution of Urate Deposits Associated with Monthly Dosing of a Pegylated Uricase (pegadricase) with SVP-Rapamycin By Dual Energy Computed Tomography *Previously called pegsiticase; pegadricase is the new United States Adopted Name (USAN) 18

















