Exhibit 99.2

Teva Announces the Acquisition of Auspex
March 30, 2015

Cautionary Statement Regarding Forward-Looking 2 Statements
This presentation contains forward-looking statements, which are based on management’s current beliefs and expectations and involve a number of known and unknown risks and uncertainties that could cause our future results, performance or achievements to differ significantly from the results, performance or achievements expressed or implied by such forward-looking statements. All statements, other than statements of historical fact, are statements that could be deemed forward-looking statements, including statements about the planned acquisition of Auspex, the expected financial impact and benefits to us of such acquisition, and anticipated milestones and other expectations regarding Auspex’s product development activities and clinical trials, including the on-going clinical trials for SD-809. Important factors that could cause or contribute to such differences include risks relating to: our ability to complete the planned acquisition of Auspex, including the satisfaction of the minimum tender and other closing conditions; uncertainties as to the timing of the acquisition; the possibility that we may not realize the expected benefits of the acquisition in a timely manner or at all; our ability to successfully integrate Auspex into our business; future results of on-going or later clinical trials for Auspex’s product candidates, including SD-809; the ability to obtain regulatory approval of Auspex’s product candidates in the planned indications; the ability to commercialize Auspex’s product candidates and market acceptance of such products; our ability to achieve expected results from the research and development efforts invested in our pipeline of specialty and other products; and other factors that are discussed in our Annual Report on Form 20-F for the year ended December 31, 2014, Auspex’s Annual Report on Form 10-K for the year ended December
31, 2014, and such parties’ other filings with the United States Securities and Exchange Commission (“SEC”). Forward-looking statements speak only as of the date on which they are made and we assume no obligation to update or revise any forward-looking statement, whether as a result of new information, future events or otherwise. This presentation was provided on March 30, 2015 as part of an oral presentation and is qualified thereby.
Additional Information and Where to Find It
THE TENDER OFFER DESCRIBED IN THIS DOCUMENT HAS NOT YET COMMENCED. THIS DOCUMENT IS NEITHER AN OFFER TO PURCHASE NOR A SOLICITATION OF AN OFFER TO SELL SHARES OF AUSPEX. At the time the offer is commenced, an affiliate of Teva will file a Tender Offer Statement on Schedule TO with the SEC, and Auspex will file a Solicitation/Recommendation Statement on Schedule 14D-9 with respect to the tender offer. The Offer to Purchase, the related Letter of Transmittal and certain other offer documents, as well as the Solicitation/Recommendation
Statement, will be made available to all stockholders of Auspex at no expense to them. The Tender Offer Statement and the Solicitation/Recommendation Statement will be available at no expense on the SEC’s web site at http://www.sec.gov. Free copies of these materials and certain other offering documents will be made available by the information agent for the offer.
AUSPEX STOCKHOLDERS AND OTHER INVESTORS ARE URGED TO READ THE TENDER OFFER MATERIALS (INCLUDING THE OFFER TO PURCHASE, RELATED LETTER OF TRANSMITTAL AND CERTAIN OTHER OFFER DOCUMENTS) AND THE SOLICITATION/RECOMMENDATION STATEMENT WHEN AVAILABLE, INCLUDING ALL AMENDMENTS TO THOSE MATERIALS. SUCH DOCUMENTS WILL
CONTAIN IMPORTANT INFORMATION, WHICH SHOULD BE READ CAREFULLY BEFORE ANY DECISION IS MADE WITH RESPECT TO THE TENDER OFFER.
In addition to the Solicitation/Recommendation Statement, Auspex files annual, quarterly and current reports, proxy statements and other information with the SEC. You may read and copy any reports, statements or other information filed by Auspex at the SEC’s public reference room at 100 F Street, N.E., Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for further information on the public reference room. Auspex’s filings with the SEC are also available to the public from commercial document-retrieval services and at the SEC’s website, http://www.sec.gov.
3
Transaction Overview
Teva Announces the Acquisition of Auspex Pharmaceuticals
Agreement to acquire Auspex Pharmaceuticals for $101.00 per share in cash
Emerging leader in movement disorders that will expand and strengthen the leadership position of our core CNS franchise
Attractive close-to-market and pipeline assets in areas with substantial unmet needs
Enhances Specialty portfolio and continues Teva’s business model transformation
Demonstrates our commitment to generate growth and create value through strong focus on business development
4
Strategic Rationale
A Compelling Value-Enhancing Transaction That Fits Our Long-Term Strategy
Excellent strategic fit strengthening Teva’s leadership in central nervous system disorders
Builds on our infrastructure, capabilities and strong commercial and R&D position
Opportunity to establish leadership in movement disorders
Auspex’s therapies target attractive and underserved markets with substantial unmet needs
Numerous programs under development supporting multiple horizons for growth
SD-809 nearing registration for Huntington’s Disease and PIII read-out for tardive dyskinesia; additional movement disorder indications to follow; 60 molecules in patent portfolio
Innovative and proven deuteration technology provides platform value
Technology offers potential to create novel therapies with differentiated safety and efficacy characteristics
Enhances Teva’s long-term growth profile, profitability, and business mix
Enhances mid to long-term growth profile; accretive to non-GAAP EPS in 2017 and onwards; minimal dilution to non-GAAP EPS in H2 2015 and 2016; increases mix from Specialty
5
Alignment with Teva’s Business Development Strategy
Auspex Brings to Teva Attractive Close-to-Market and Pipeline Assets in CNS
Targeting a Unique Space In The Industry
Growth Markets
Complex/Hard to Produce Assets or Technologies
Generics
Unique Health Solutions,
Technologies, Services
Attractive Pipeline Assets/ Portfolios
In-Market or Close to Market Assets in Core TAs
Specialty

6
Company Overview
An Innovative Leader in Movement Disorders
Auspex Pharmaceuticals Background & History
Innovative biopharma company specializing in applying deuterium chemistry to known molecules to create novel therapies with improved safety and efficacy profiles Founded in 2001 Headquartered in La Jolla, California Listed on the Nasdaq (ticker: ASPX)
Key Products & Pipeline
SD-809 for Huntington’s disease
SD-809 for Tardive dyskinesia SD-809 for Tourette syndrome
SD-560 for Idiopathic pulmonary fibrosis
SD-1077 for Parkinson’s disease
Post Phase III (NDA filing by mid-2015) Phase II/III
Phase I Phase I Preclinical
7
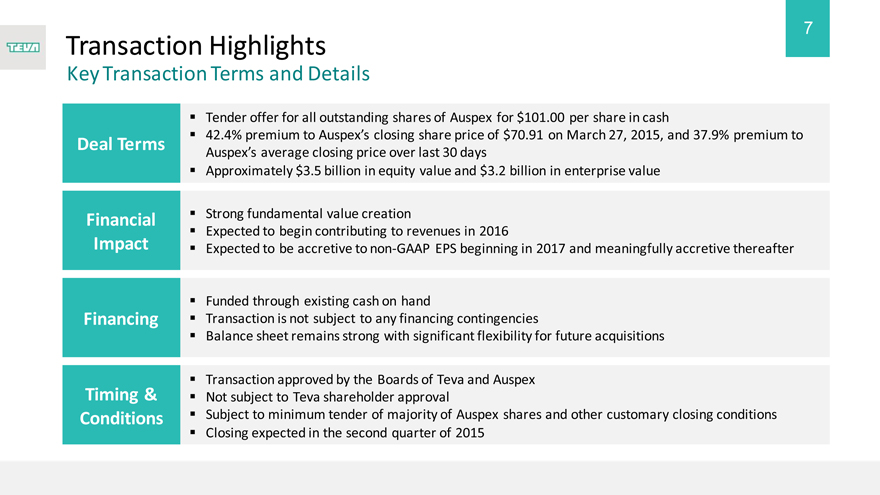
Transaction Highlights
Key Transaction Terms and Details
Deal Terms
Financial Impact
Financing
Timing & Conditions
Tender offer for all outstanding shares of Auspex for $101.00 per share in cash
42.4% premium to Auspex’s closing share price of $70.91 on March 27, 2015, and 37.9% premium to Auspex’s average closing price over last 30 days
Approximately $3.5 billion in equity value and $3.2 billion in enterprise value
Strong fundamental value creation
Expected to begin contributing to revenues in 2016
Expected to be accretive to non-GAAP EPS beginning in 2017 and meaningfully accretive thereafter
Funded through existing cash on hand
Transaction is not subject to any financing contingencies
Balance sheet remains strong with significant flexibility for future acquisitions
Transaction approved by the Boards of Teva and Auspex Not subject to Teva shareholder approval
Subject to minimum tender of majority of Auspex shares and other customary closing conditions
Closing expected in the second quarter of 2015
8
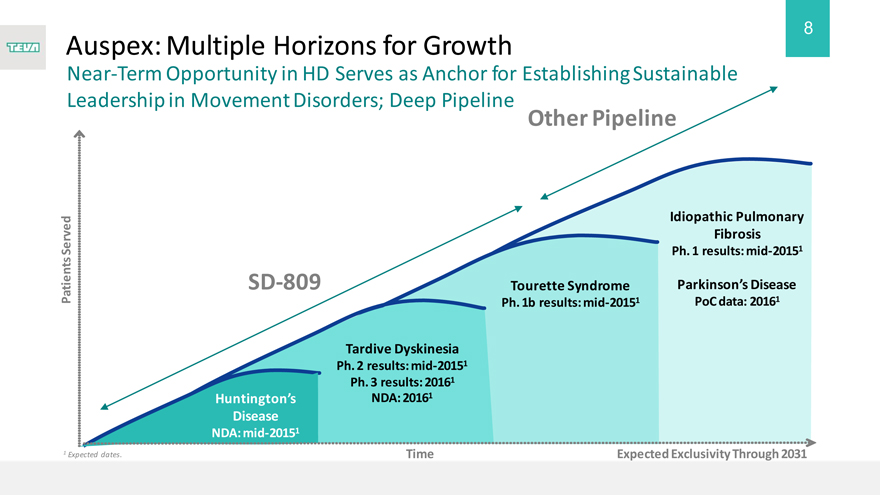
Auspex: Multiple Horizons for Growth
Near-Term Opportunity in HD Serves as Anchor for Establishing Sustainable Leadership in Movement Disorders; Deep Pipeline Pipeline
Other
Patients Served
SD-809
Huntington’s
Disease
NDA: mid-20151
1 Expected dates.
Tardive Dyskinesia
Ph. 2 results: mid-20151 Ph. 3 results: 20161 NDA: 20161
Time
Tourette Syndrome
Ph. 1b results: mid-20151
Idiopathic Pulmonary Fibrosis
Ph. 1 results: mid-20151
Parkinson’s Disease
PoC data: 20161
Expected Exclusivity Through 2031
9
The Opportunity in Movement Disorders
Serious Medical Conditions That Share Common Underlying Mechanism
Major movement disorders:
Huntington’s disease (HD)
Tardive dyskinesia (TD) Tourette syndrome (TS)
Common symptoms: Tics, chorea, dyskinesia, and dystonia
Significant unmet medical need: Social distress, disability, physical injury, loss of independence, and interference with employment and activities of daily living
Significantly underserved conditions:
HD: One FDA-approved drug (Tetrabenazine) ; significant limitations; ~ 5% of patients treated TD: No approved drug in the US
TS: One approved drug (Aripiprazole) with significant limitations
10
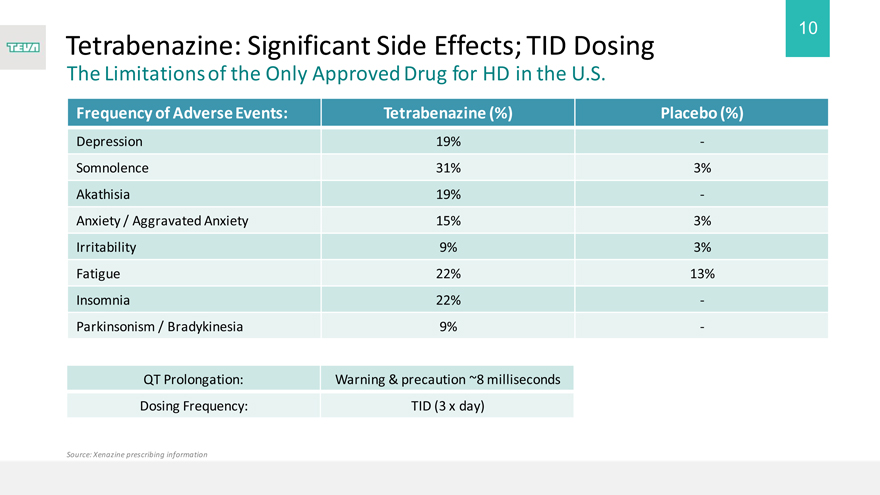
Tetrabenazine: Significant Side Effects; TID Dosing
The Limitations of the Only Approved Drug for HD in the U.S.
Frequency of Adverse Events:
Depression
Somnolence
Akathisia
Anxiety / Aggravated Anxiety Irritability Fatigue Insomnia Parkinsonism / Bradykinesia
QT Prolongation:
Dosing Frequency:
Source: Xenazine prescribing information
Tetrabenazine (%)
19%
31% 19% 15% 9% 22% 22% 9%
Warning & precaution ~8 milliseconds
TID (3 x day)
Placebo (%)
-
3%—3% 3% 13%—
-
11
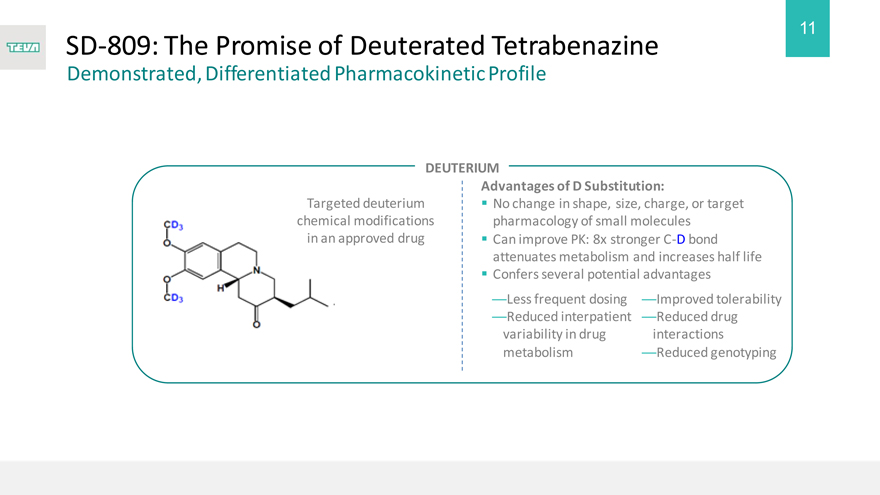
SD-809: The Promise of Deuterated Tetrabenazine
Demonstrated, Differentiated Pharmacokinetic Profile
Targeted deuterium chemical modifications in an approved drug
DEUTERIUM
Advantages of D Substitution:
No change in shape, size, charge, or target pharmacology of small molecules Can improve PK: 8x stronger C-D bond attenuates metabolism and increases half life Confers several potential advantages
—Less frequent dosing —Improved tolerability
—Reduced interpatient —Reduced drug variability in drug interactions metabolism —Reduced genotyping
12
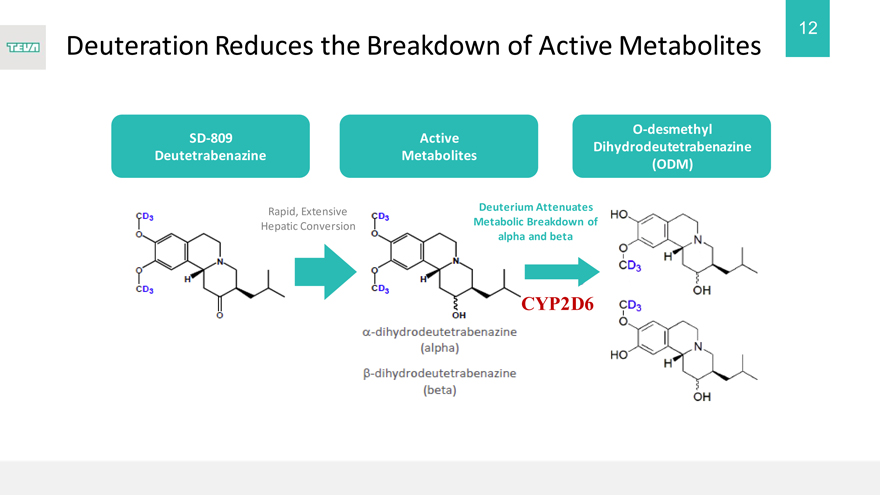
Deuteration Reduces the Breakdown of Active Metabolites
SD-809
Deutetrabenazine
Rapid, Extensive Hepatic Conversion
Active
Metabolites
Deuterium Attenuates Metabolic Breakdown of alpha and beta
CYP2D6
O-desmethyl Dihydrodeutetrabenazine (ODM)
13
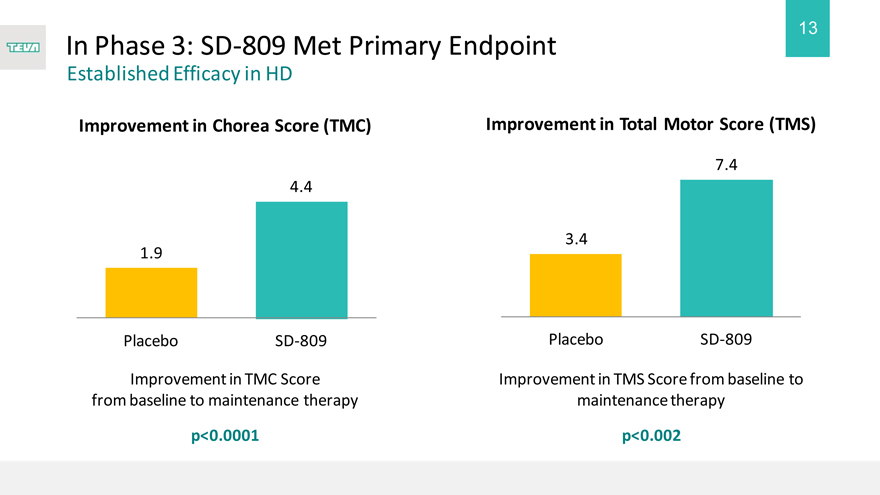
In Phase 3: SD-809 Met Primary Endpoint
Established Efficacy in HD
Improvement in Chorea Score (TMC) Improvement in Total Motor Score (TMS)
4.4
1.9
Placebo SD-809
Improvement in TMC Score from baseline to maintenance therapy
p<0.0001
7.4
3.4
Placebo SD-809
Improvement in TMS Score from baseline to maintenance therapy
p<0.002
14
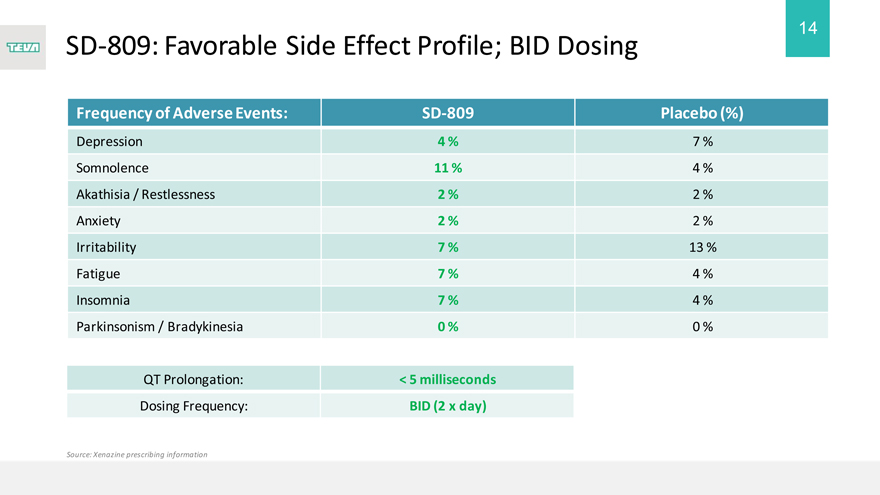
SD-809: Favorable Side Effect Profile; BID Dosing
Frequency of Adverse Events:
Depression
Somnolence
Akathisia / Restlessness Anxiety Irritability Fatigue Insomnia Parkinsonism / Bradykinesia
QT Prolongation:
Dosing Frequency:
Source: Xenazine prescribing information
SD-809
4 %
11 %
2 %
2 %
7 %
7 %
7 %
0 %
< 5 milliseconds BID (2 x day)
Placebo (%)
7 %
4 %
2 %
2 %
13 %
4 %
4 %
0 %
15
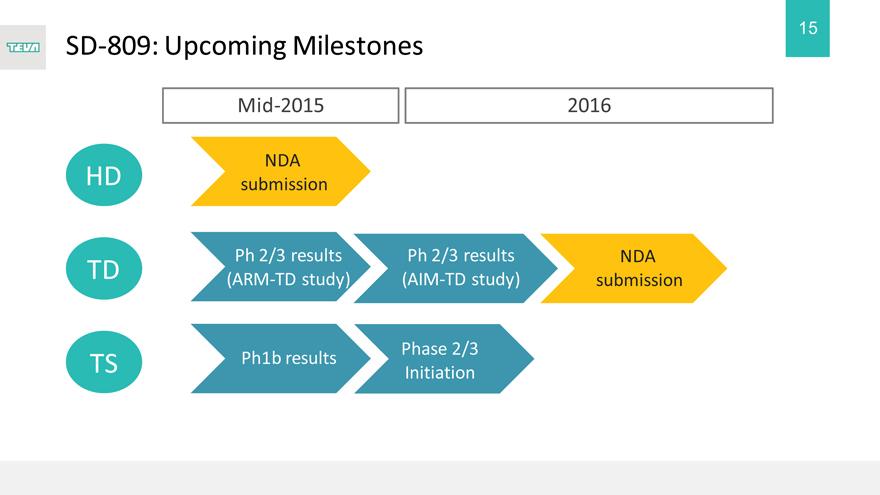
SD-809: Upcoming Milestones
Mid-2015 2016
HD TD TS
NDA submission
Ph 2/3 results (ARM-TD study)
Ph1b results
Ph 2/3 results (AIM-TD study)
Phase 2/3 Initiation
NDA submission
16
Additional Pipeline Assets
SD-560
Indication: Idiopathic Pulmonary Fibrosis In Phase 1 results in mid-2015
SD-1077
Indication: Parkinson’s Disease
Pre-clinical phase 1 POC results in 2016
~ 60 drug candidates identified based on deuteration platform

17
Highly Complementary to Teva’s Existing Specialty Pipeline
Phase 1
TV-46763 (abuse deterrent)
Pain
TV-46139 (abuse deterrent)
Pain
Fluticasone Salmeterol Spiromax EU
Asthma, COPD
Reslizumab SC
Asthma
Fluticasone Salmeterol (MDI) EU
Asthma, COPD
TEV-90110
HIV
TEV-90112
HIV
Phase 2
Laquinimod
Multiple sclerosis (progressive forms)
Laquinimod
Huntington’s disease
Pridopidine
Huntington’s disease
TV-45070 Topical
Osteoarthritis pain
TV-45070 Topical
Neuropathic pain
TEV-48125
Chronic and episodic migraine
CEP-41750 (mesenchymal precursor cell) Acute myocardial infarction
Albutropin
Growth hormone deficiency
Phase 3
Laquinimod
Multiple sclerosis (relapsing remitting)
Fluticasone Propionate RespiClick US
Asthma
Fluticasone Salmeterol RespiClick US
Asthma
QVAR® (BAI) US
Asthma
Reslizumab IV
Asthma
CEP-41750 (mesenchymal precursor cell) Chronic heart failure
Registration
CEP-33237 ER Hydrocodone (abuse det.) US—Pain Copaxone® 40mg 3w ROW
Multiple sclerosis
Copaxone® 20mg per Day Japan
Multiple sclerosis
ProAir® RespiClick US
Asthma, exercise ind. bronchospasm
Auspex Early Assets
SD-1077
Parkinson’s disease
+ multiple early CNS: depression (1), Pain (5),
Schizophrenia (1), MS (1), others Multiple early candidates in
Respiratory: IPF, PAH, others
SD-809
Tourette syndrome SD-560
Idiopathic pulmonary fibrosis
SD-809
Tardive dyskinesia
SD-809
HD (Mid-2015 NDA filing)
Note: Pipeline correct as of March 1, 2015. Phase 1 includes also projects designated for IND filing
CNS & Pain Respiratory Other
18
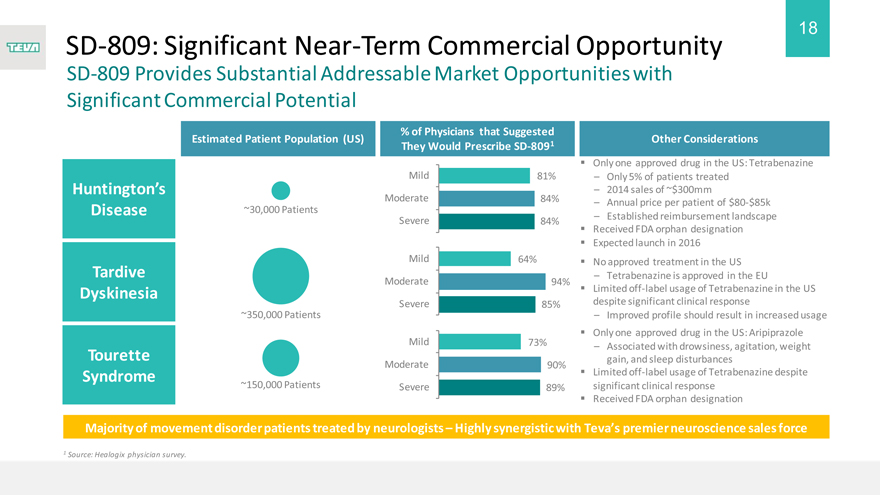
SD-809: Significant Near-Term Commercial Opportunity
SD-809 Provides Substantial Addressable Market Opportunities with Significant Commercial Potential
Huntington’s
Disease
Tardive Dyskinesia
Tourette Syndrome
Estimated Patient Population (US)
~30,000 Patients ~350,000 Patients ~150,000 Patients
% of Physicians that Suggested They Would Prescribe SD-8091
Mild 81% Moderate 84% Severe 84%
Mild 64% Moderate 94% Severe 85%
Mild 73% Moderate 90% Severe 89%
Other Considerations
Only one approved drug in the US: Tetrabenazine
– Only 5% of patients treated
– 2014 sales of ~$300mm
– Annual price per patient of $80-$85k
– Established reimbursement landscape Received FDA orphan designation Expected launch in 2016
No approved treatment in the US
– Tetrabenazine is approved in the EU
Limited off-label usage of Tetrabenazine in the US despite significant clinical response
– Improved profile should result in increased usage Only one approved drug in the US: Aripiprazole
– Associated with drowsiness, agitation, weight gain, and sleep disturbances Limited off-label usage of Tetrabenazine despite significant clinical response Received FDA orphan designation
Majority of movement disorder patients treated by neurologists – Highly synergistic with Teva’s premier neuroscience sales force
1 Source: Healogix physician survey.
19
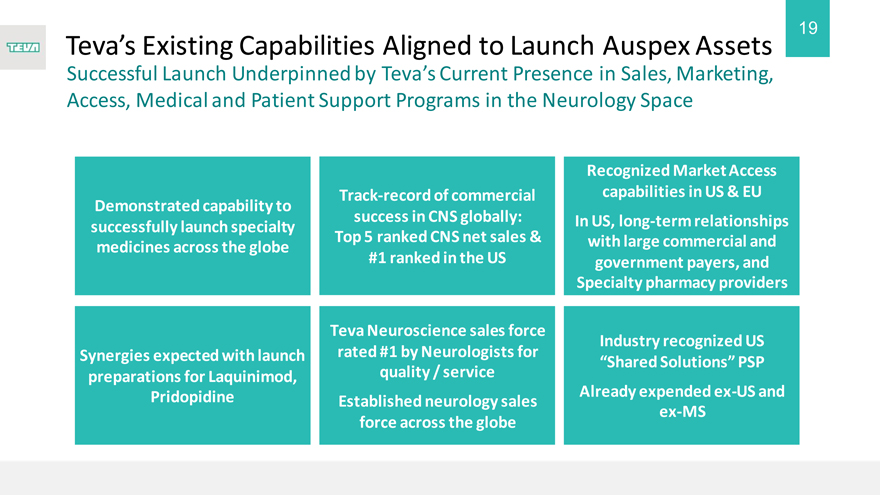
Teva’s Existing Capabilities Aligned to Launch Auspex Assets
Successful Launch Underpinned by Teva’s Current Presence in Sales, Marketing, Access, Medical and Patient Support Programs in the Neurology Space
Demonstrated capability to successfully launch specialty medicines across the globe
Synergies expected with launch preparations for Laquinimod, Pridopidine
Track-record of commercial success in CNS globally: Top 5 ranked CNS net sales & #1 ranked in the US
Teva Neuroscience sales force rated #1 by Neurologists for quality / service Established neurology sales force across the globe
Recognized Market Access capabilities in US & EU
In US, long-term relationships with large commercial and government payers, and Specialty pharmacy providers
Industry recognized US
“Shared Solutions” PSP
Already expended ex-US and ex-MS
20
Final Remarks

21
Q&A

Thank You





















