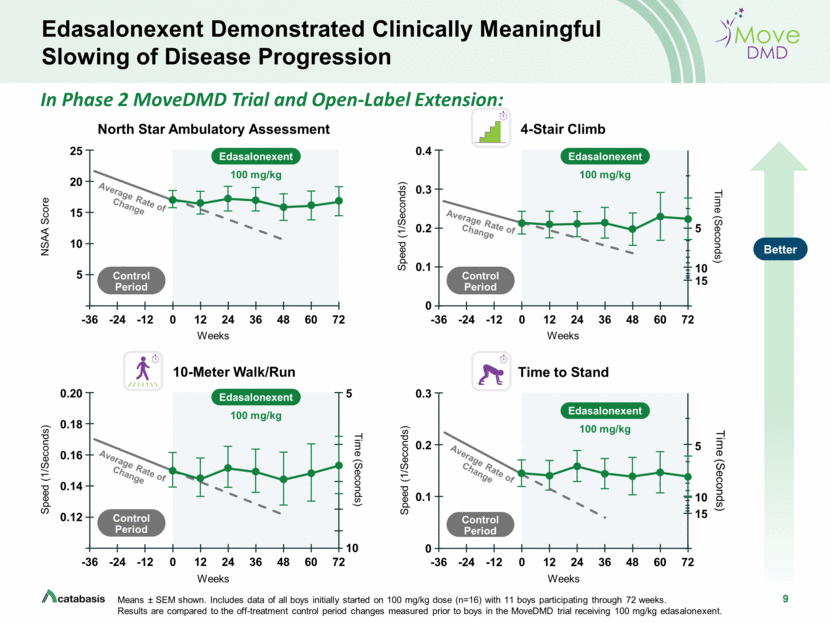
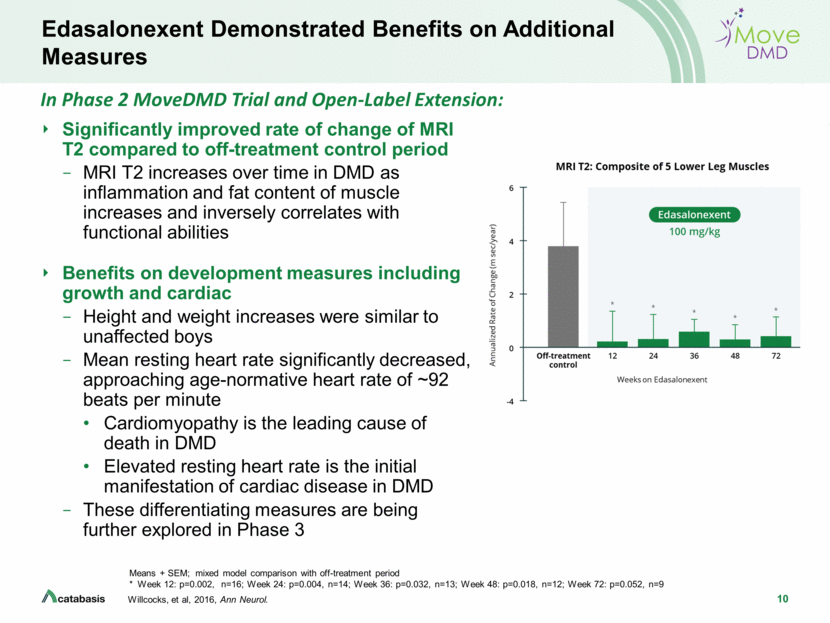
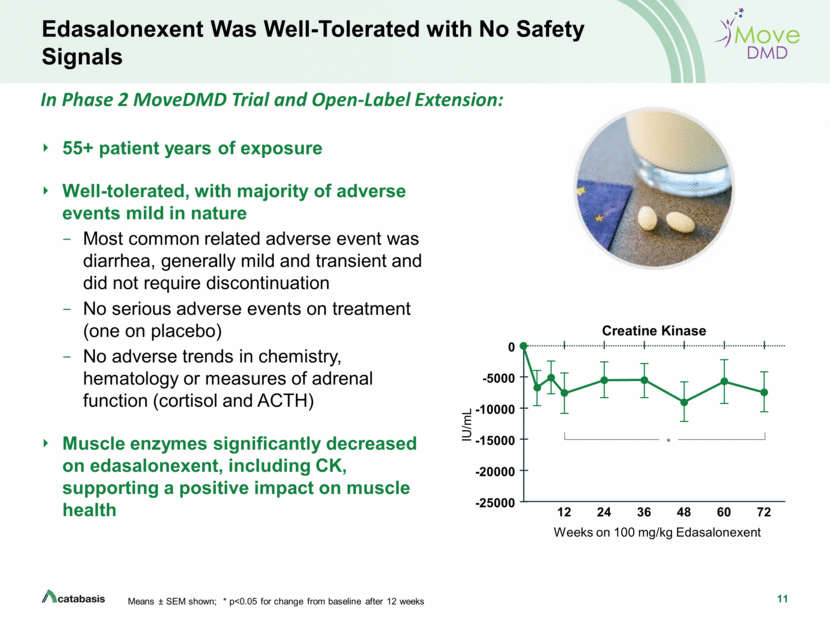
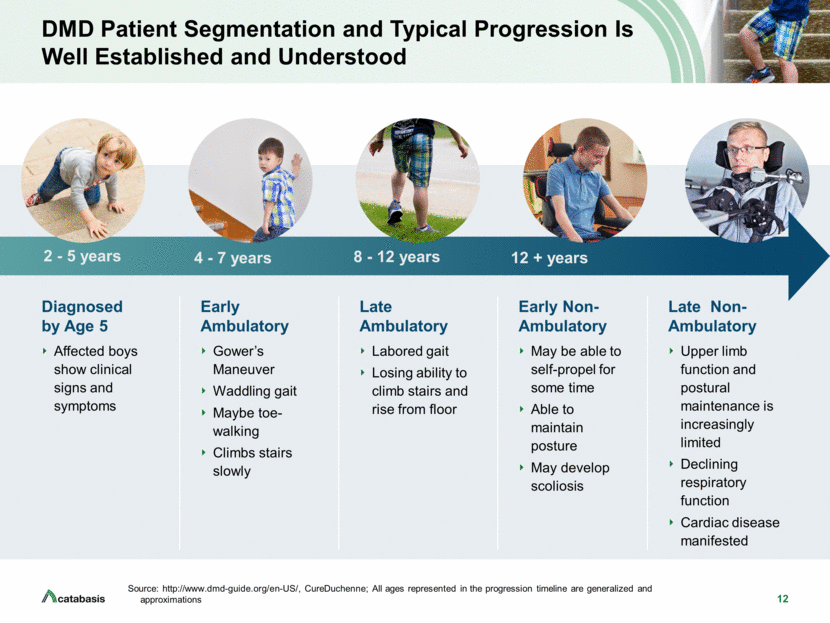
2 Forward Looking Statements This presentation contains, and any oral remarks made in connection with such presentation may contain, forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995, including statements regarding our expectations and beliefs about our business, future financial and operating performance, clinical trial plans, product development plans and prospects, including statements about future clinical trial plans including, among other things, statements about our single global Phase 3 PolarisDMD trial in Duchenne muscular dystrophy, or DMD, to evaluate the efficacy and safety of edasalonexent for registration purposes, our plans to continue to evaluate data from the open-label extension of our MoveDMD® clinical trial and from our GalaxyDMD open-label extension trial of edasalonexent for the treatment of DMD, and our plans to combine edasalonexent treatment with other DMD treatments such as gene therapy and other dystrophin-targeted approaches. The words “believe”, “anticipate”, “plans,” “expect”, “could”, “should”, “will”, “would”, “may”, “intend” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. The forward-looking statements contained in this presentation and in remarks made during this presentation and the following Q&A session are subject to important risks and uncertainties that may cause actual events or results to differ materially from our current expectations and beliefs, including: uncertainties inherent in the initiation and completion of preclinical studies and clinical trials and clinical development of our product candidates; availability and timing of results from preclinical studies and clinical trials; whether interim results from a clinical trial will be predictive of the final results of the trial or the results of future trials; expectations for regulatory approvals to conduct trials or to market products, including our expected target product profile for edasalonexent in DMD; availability of funding sufficient for our foreseeable and unforeseeable operating expenses and capital expenditure requirements; other matters that could affect the availability or commercial potential of our product candidates; and general economic and market conditions and other factors discussed in the “Risk Factors” section of our Quarterly Report on Form 10-Q for the period ended June 30, 2019, which is on file with the Securities and Exchange Commission, and in other filings that we may make with the Securities and Exchange Commission in the future. In addition, the forward-looking statements included in this presentation represent our views as of the date of this presentation. We anticipate that subsequent events and developments will cause our views to change. However, while we may elect to update these forward-looking statements at some point in the future, we specifically disclaim any obligation to do so. These forward-looking statements should not be relied upon as representing our views as of any date subsequent to the date of this presentation.