Exhibit 99.1
|
Developing Well-Differentiated Antibiotics to Meet Medical Needs
The First Fluoroketolide, Solithromycin, In Development
To Meet the Need for a New Macrolide
July 2012
|
Forward Looking Statement
This presentation contains forward-looking statements regarding future events. These statements are just predictions and are subject to risks and uncertainties that could cause the actual events or results to differ materially. These risks and uncertainties include, among others: risks related to the costs, timing, regulatory review and results of our studies and clinical trials; our ability to obtain FDA approval of our product candidates; our dependence on the success of Solithromycin; our need to obtain additional funding and our ability to obtain future funding on acceptable terms; our anticipated capital expenditures and our estimates regarding our capital requirements; the possible impairment of, or inability to obtain, intellectual property rights and the costs of obtaining such rights from third parties; the unpredictability of the size of the markets for, and market acceptance of, any of our products, including Solithromycin; our ability to produce and sell any approved products and the price we are able realize for those products; our ability to retain and hire necessary employees and to staff our operations appropriately; our ability to compete in our industry; innovation by our competitors; and our ability to stay abreast of and comply with new or modified laws and regulations that currently apply or become applicable to our business. Please refer to the documents that we file from time to time with the Securities and Exchange Commission.
2 |
|
|
Developing Differentiated Antibiotics In a Large Market to Meet Significant Needs
Significant need for new treatment driven by:
Resistance
Adverse events/lack of tolerability Inappropriate spectrum
Lack of IV-oral
Lack of pediatric dosing formulation Acceptability for long term use
The growing need:
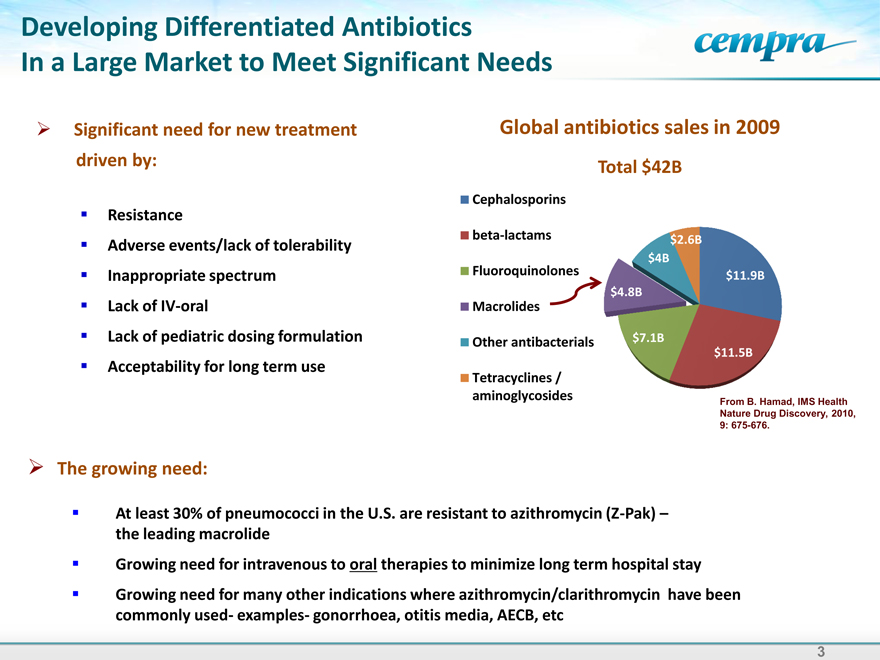
At least 30% of pneumococci in the U.S. are resistant to azithromycin (Z-Pak) – the leading macrolide
Growing need for intravenous to oral therapies to minimize long term hospital stay
Growing need for many other indications where azithromycin/clarithromycin have been commonly used- examples- gonorrhoea, otitis media, AECB, etc
Global antibiotics sales in 2009
Total $42B
Cephalosporins beta-lactams Fluoroquinolones
Macrolides Other antibacterials
Tetracyclines / aminoglycosids
$2.6B
$11.9B
$4.8B
$4B
$7.1B
$11.5B
From B. Hamad, IMS Health Nature Drug Discovery, 2010,
9: 675-676.
3 |
|
|
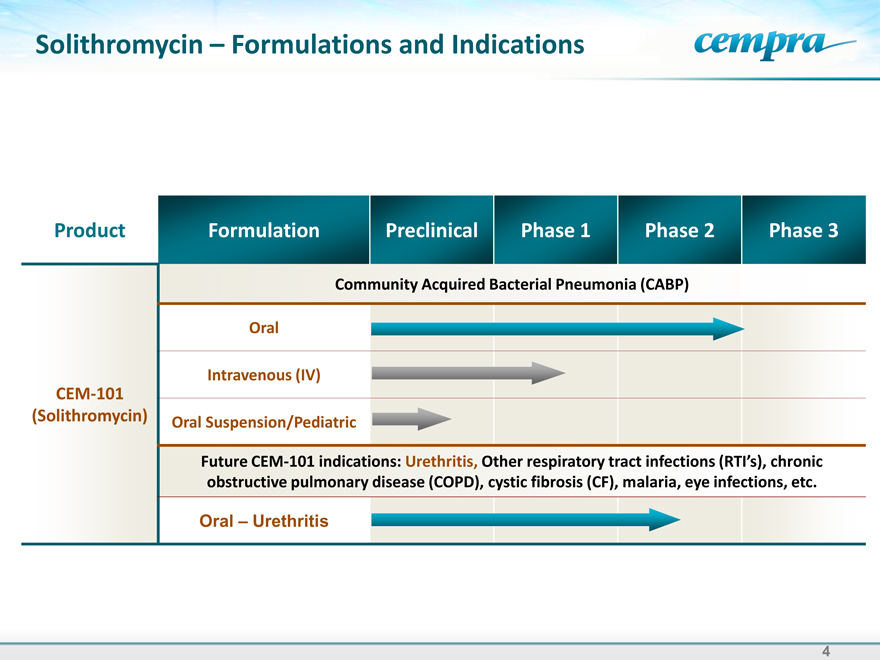
Solithromycin – Formulations and Indications
Product Formulation Preclinical Phase 1 Phase 2 Phase 3
Community Acquired Bacterial Pneumonia (CABP)
Oral
Intravenous (IV)
CEM-101
(Solithromycin) Oral Suspension/Pediatric
Future CEM-101 indications: Urethritis, Other respiratory tract
obstructive pulmonary disease (COPD), cystic fibrosis (CF), malaria, eye infections, etc.
Oral –Urethritis
4 |
|
|
Macrolide – Major Use in RTI’s
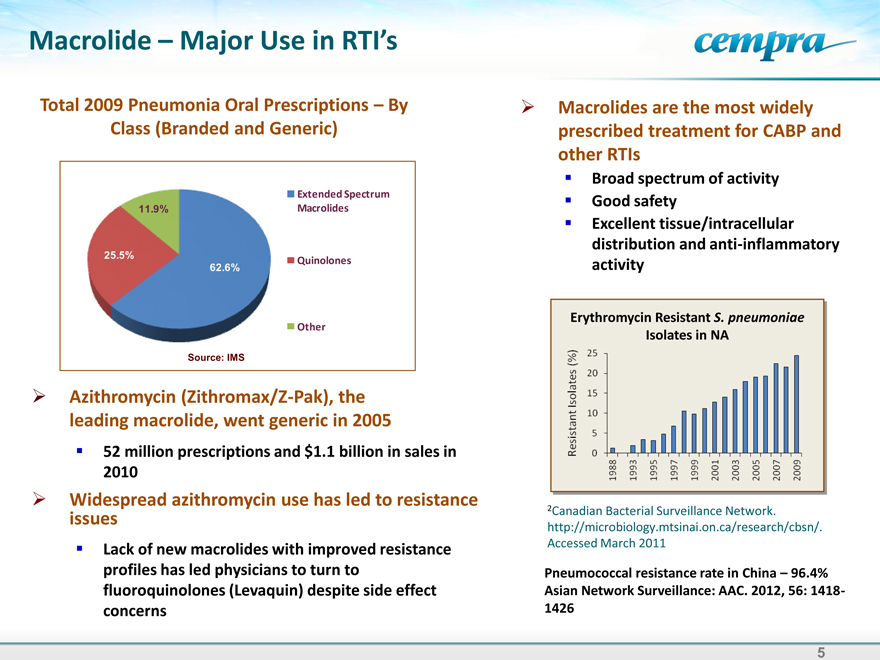
Total 2009 Pneumonia Oral Prescriptions – By Class (Branded and Generic)
Extended Spectrum
11.9% Macrolides
25.5% Quinolones
62.6%
Other
Source: IMS
Azithromycin (Zithromax/Z-Pak), the leading macrolide, went generic in 2005 52 million prescriptions and $1.1 billion in sales in 2010 Widespread azithromycin use has led to resistance issues Lack of new macrolides with improved resistance profiles has led physicians to turn to fluoroquinolones (Levaquin) despite side effect concerns
Macrolides are the most widely prescribed treatment for CABP and other RTIs
Broad spectrum of activity
Good safety
Excellent tissue/intracellular distribution and anti-inflammatory activity
Erythromycin Resistant S. pneumoniae Isolates in NA
Resistant Isolates (%0)
0
5 |
|
10
15
20
25
1988
1993
1995
1997
1999
2001
2003
2005
2007
2009
2Canadian Bacterial Surveillance Network. http://microbiology.mtsinai.on.ca/research/cbsn/. Accessed March 2011
Pneumococcal resistance rate in China – 96.4% Asian Network Surveillance: AAC. 2012, 56: 1418-1426
5 |
|
|
Community-Acquired Bacterial Pneumonia (CABP)
No. 1 cause of death due to infection
Pneumococci is the most common cause of fatal CABP
Most common cause of chronic bronchitis, sinusitis, meningitis and middle ear infection
5-6 million cases/year
~1 million hospital admissions/year
1.6 million fatal cases of pneumococcal disease worldwide annually
Pneumococcal diseases cause more deaths per year in U.S. than breast or prostate cancer
Xu. et al. Deaths: Final Data for 2007. Natl Vital Stat Rep. 2010; 58: 1-51
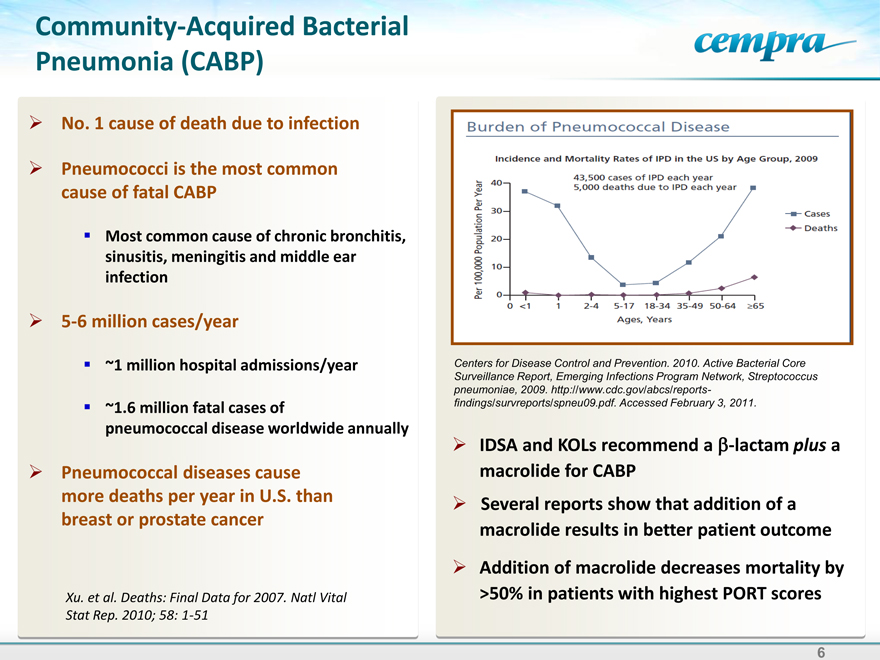
Burden of Pneumococcal disease
Incidence and Mortality Rates of IPD in the US by Age Group, 2009
43,500 cases of IPD each year
5,000 deaths due to IPD each year
Per 100,000 Population Per year
Cases
Deaths
0
10
20
30
40
0
1 |
|
1 |
|
2-4
5-17
18-34
35-49
50-64
65
Ages, Years
Centers for Disease Control and Prevention. 2010. Active Bacterial Core Surveillance Report, Emerging Infections Program Network, Streptococcus pneumoniae, 2009. http://www.cdc.gov/abcs/reports-findings/survreports/spneu09.pdf. Accessed February 3, 2011.
IDSA and KOLs recommend a plus a macrolide for CABP
Several reports show that addition of a macrolide results in better patient outcome
Addition of macrolide decreases mortality by >50% in patients with highest PORT scores
6 |
|
|
Solithromycin Opportunity – Need for a New Macrolide to Treat CABP
Impact of macrolide therapy on mortality for patients with severe pneumonia
Cumulative survival
+macrolide
-macrolide
a)
1.0
0.9
0.8
0.7
0.6
0
20
40
60
80
100
Restrepo, MI. et al. Eur Resp. J. 33: 153-159, 2009
Asthma and COPD Patient Forecasts
Patilent Population
Asthma
COPD
50 million
45 million
40 million
35 million
30 million
25 million
20 million
15 million
5 |
| million |
0
2006
2007
2008
2009
2010
2011
2012
2013
2014
2015
2016
Drug Discovery News May 2012
Report forecasts increases in respiratory disease incidence, market.
Survival of CABP patients treated in accordance with IDSA/ATS guideline using combinations with a macrolide or a quinolone
Probability of survival
1,0
,8
,6
,4
,2
0,0
0
20
40
60
Days
Macrolides
Fluoroquinolones
Martin-Loeches I., Intensive Care Med., 2010, 36: 612-620.
Azithromycin’s long, low blood/tissue levels favors selection of resistant bacteria
7 |
|
|
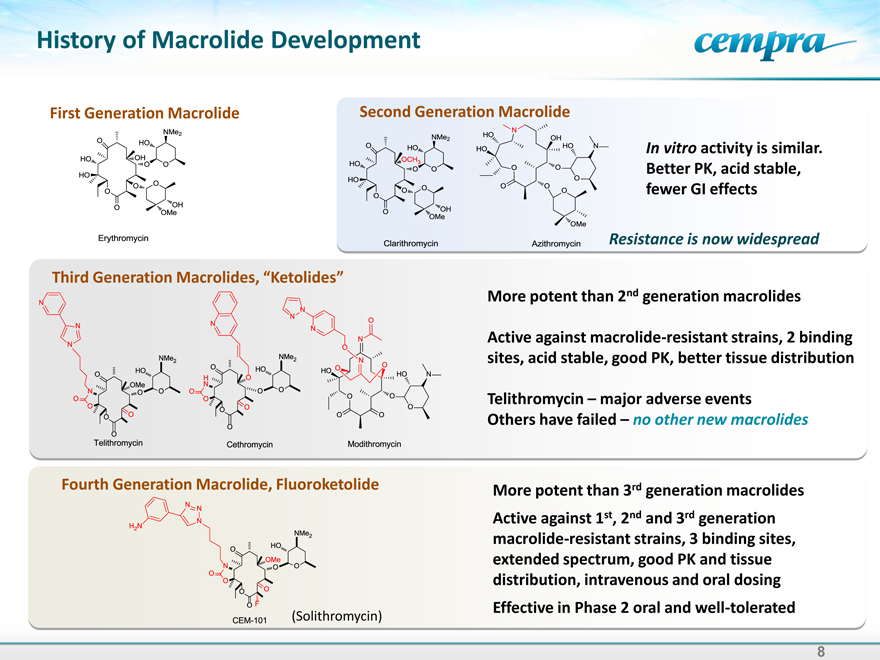
History of Macrolide Development
First Generation Macrolide
Second Generation Macrolide
In vitro activity is similar. Better PK, acid stable, fewer GI effects
Resistance is now widespread
Third Generation Macrolides, “Ketolides”
More potent than 2nd generation macrolides
Active against macrolide-resistant strains, 2 binding sites, acid stable, good PK, better tissue distribution
Telithromycin – major adverse events Others have failed – no other new macrolides
Fourth Generation Macrolide, Fluoroketolide
More potent than 3rd generation macrolides Active against 1st, 2nd and 3rd generation macrolide-resistant strains, 3 binding sites, extended spectrum, good PK and tissue distribution, intravenous and oral dosing Effective in Phase 2 oral and well-tolerated
Erythomycin
NMe2
HO
OH
OMe
HO
HO
Claithromycin
Azithromycin
Telithromycin
Cethromycin
Modithromycin
CEM-101 (Solithromycin)
8 |
|
|
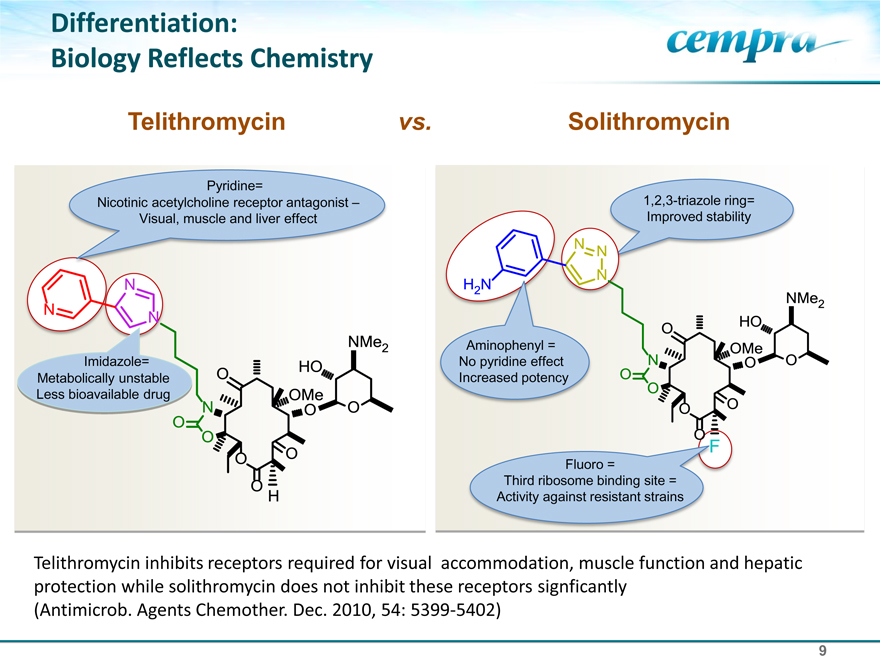
Differentiation:
Biology Reflects Chemistry
Telithromycin vs. Solithromycin
Pyridine=
Nicotinic acetylcholine receptor antagonist – Visual, muscle and liver effect
Imidazole= Metabolically unstable Less bioavailable drug
1,2,3-triazole ring= Improved stability
Aminophenyl = No pyridine effect Increased potency
Fluoro =
Third ribosome binding site = Activity against resistant strains
Telithromycin inhibits receptors required for visual accommodation, muscle function and hepatic protection while solithromycin does not inhibit these receptors signficantly (Antimicrob. Agents Chemother. Dec. 2010, 54: 5399-5402)
9
|
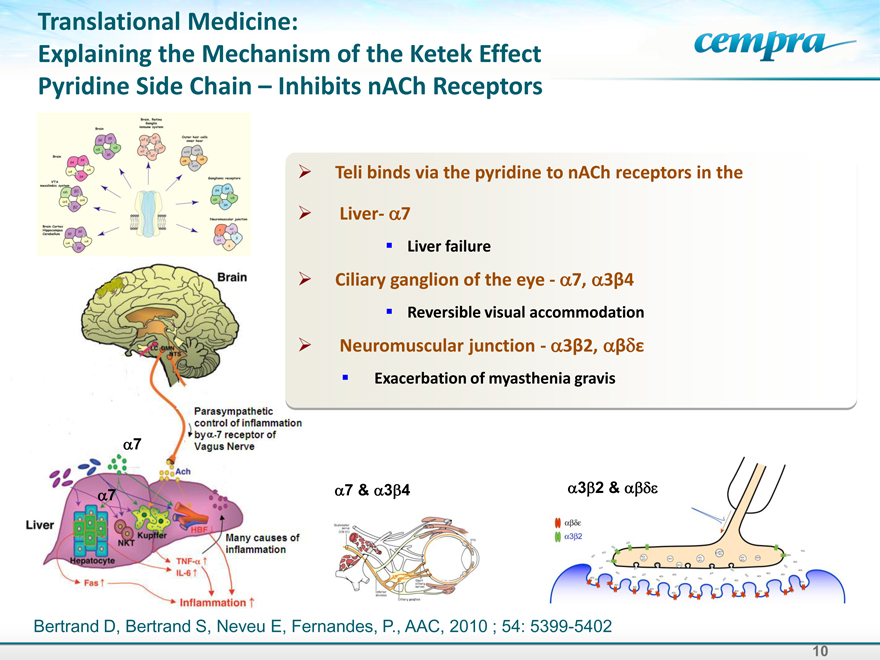
Translational Medicine:
Explaining the Mechanism of the Ketek Effect Pyridine Side Chain – Inhibits nACh Receptors
Teli binds via the pyridine to nACh receptors in the
Liver-a7
Liver failure
Ciliary ganglion of the eye—a7,a3b4
Reversible visual accommodation
Neuromuscular junction—a3b2,abde
Exacerbation of myasthenia gravis
NMe
Bertrand D, Bertrand S, Neveu E, Fernandes, P., AAC, 2010 ; 54: 5399-5402
Brain
Parasympathetic control of information bya-7 receptor of Vagus Nerve
Many casuses of inflammation
inflammation
hepatocyte
NKT
Kupfter
Ach
Liver
Fas
TNF-a
IL-6
10
|
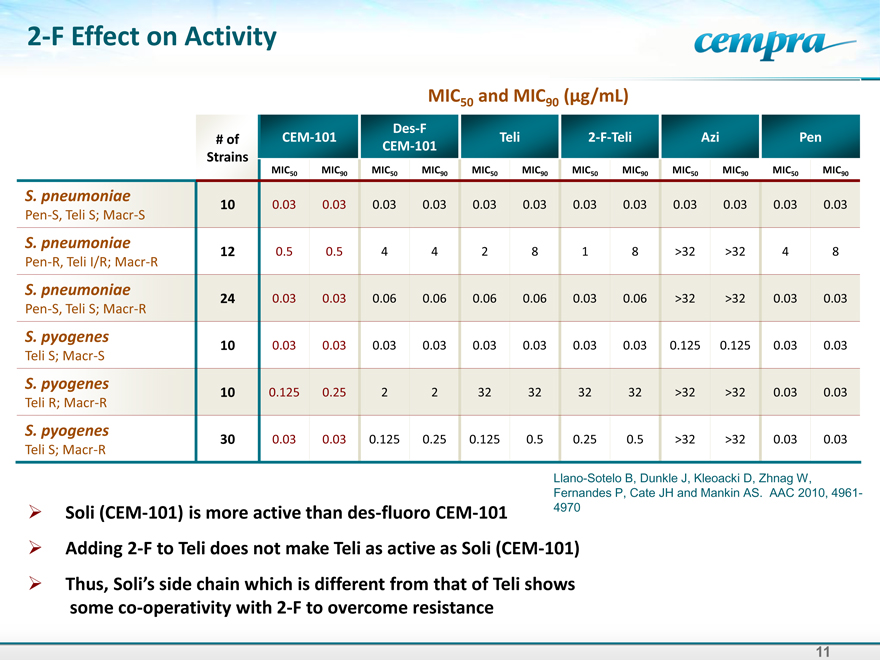
2-F Effect on Activity
MIC50 and MIC90 (g/mL)
Des-F
# of CEM-101 Teli 2-F-Teli Azi Pen
CEM-101
Strains
MIC50 MIC90 MIC50 MIC90 MIC50 MIC90 MIC50 MIC90 MIC50 MIC90 MIC50 MIC90
S. pneumoniae 10 0.03 0.03 0.03 0.03 0.03 0.03 0.03 0.03 0.03 0.03 0.03 0.03
Pen-S, Teli S; Macr-S
S. pneumoniae 12 0.5 0.5 4 4 2 8 1 8 >32 >32 4 8
Pen-R, Teli I/R; Macr-R
S. pneumoniae 24 0.03 0.03 0.06 0.06 0.06 0.06 0.03 0.06 >32 >32 0.03 0.03
Pen-S, Teli S; Macr-R
S. pyogenes 10 0.03 0.03 0.03 0.03 0.03 0.03 0.03 0.03 0.125 0.125 0.03 0.03
Teli S; Macr-S
S. pyogenes 10 0.125 0.25 2 2 32 32 32 32 >32 >32 0.03 0.03
Teli R; Macr-R
S. pyogenes 30 0.03 0.03 0.125 0.25 0.125 0.5 0.25 0.5 >32 >32 0.03 0.03
Teli S; Macr-R
Llano-Sotelo B, Dunkle J, Kleoacki D, Zhnag W,
Fernandes P, Cate JH and Mankin AS. AAC 2010, 4961-4970
Soli (CEM-101) is more active than des-fluoro CEM-101
Adding 2-F to Teli does not make Teli as active as Soli (CEM-101)
Thus, Soli’sside chain which is different from that of Teli shows
some co-operativity with 2-F to overcome resistance
11
|
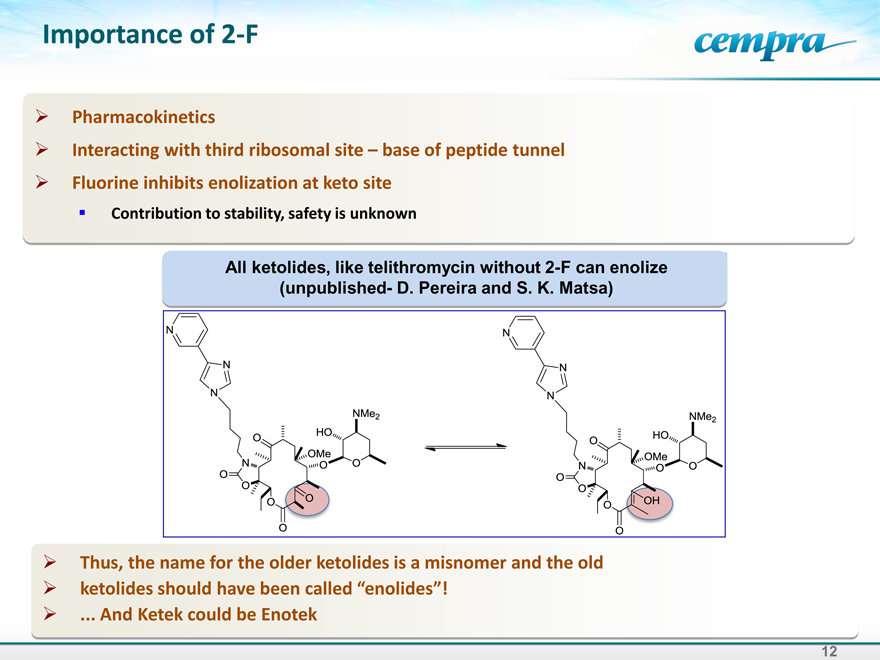
Importance of 2-F
Pharmacokinetics
Interacting with third ribosomal site – base of peptide tunnel Fluorine inhibits enolization at keto site
Contribution to stability, safety is unknown
All ketolides, like telithromycin without 2-F can enolize (unpublished- D. Pereira and S. K. Matsa)
Thus, the name for the older ketolides is a misnomer and the old ketolides should have been called “enolides”!
And Ketek could be Enotek
N N N O O O O O N O O O O HO OMe NMe2
N N N N O O O O O O OMe HO NMe2 OH
12
|
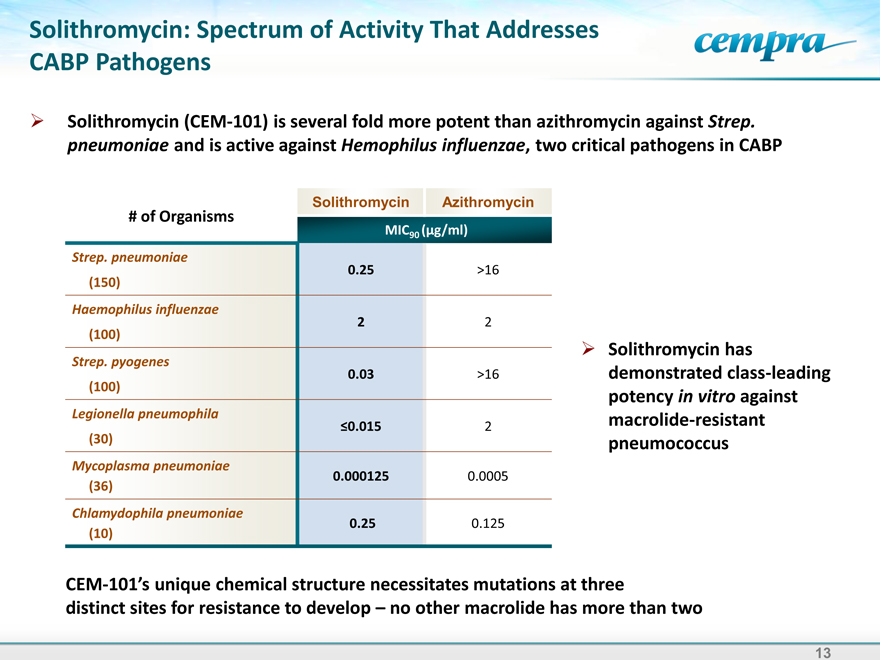
Solithromycin: Spectrum of Activity That Addresses CABP Pathogens
Solithromycin (CEM-101) is several fold more potent than azithromycin against Strep. pneumoniae and is active against Hemophilus influenzae, two critical pathogens in CABP
Solithromycin Azithromycin
# of Organisms
MIC90 (g/ml)
Strep. pneumoniae
0.25 >16
(150)
Haemophilus influenzae
2 |
| 2 |
(100)
Strep. pyogenes
0.03 >16
(100)
Legionella pneumophila
0.015 2
£(30) |
|
Mycoplasma pneumoniae
0.000125 0.0005
(36) |
|
Chlamydophila pneumoniae
0.25 0.125
(10) |
|
Solithromycin has demonstrated class-leading potency in vitro against macrolide-resistant pneumococcus
CEM-101’s unique chemical structure
distinct sites for resistance to develop –no other macrolide has more than two
13
|
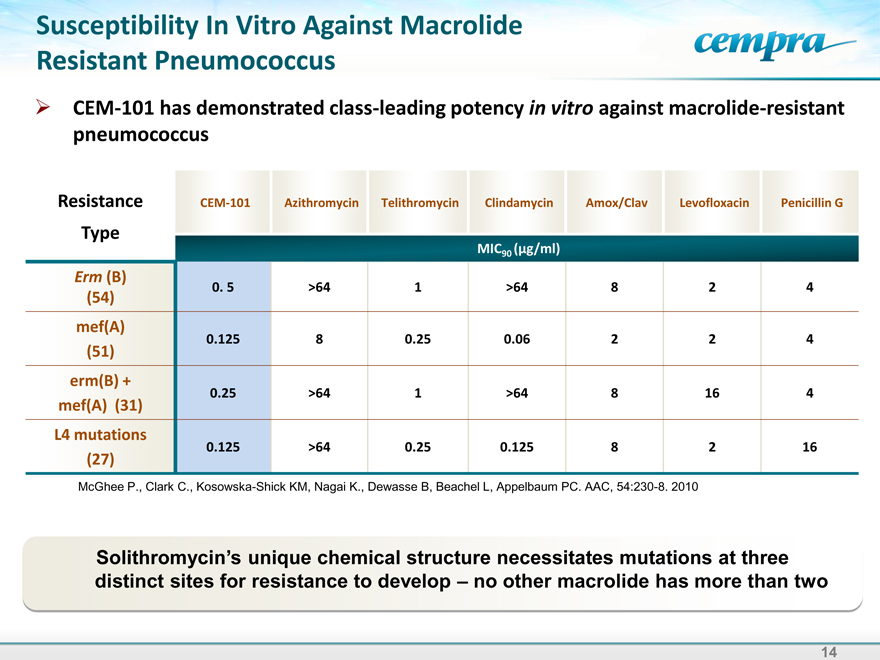
Susceptibility In Vitro Against Macrolide Resistant Pneumococcus
CEM-101 has demonstrated class-leading potency in vitro against macrolide-resistant pneumococcus
Resistance CEM-101 Azithromycin Telithromycin Clindamycin Amox/Clav Levofloxacin Penicillin G
Type
MIC90 (g/ml)
Erm (B)
0. 5 >64 1 >64 8 2 4
(54) |
|
mef(A)
0.125 8 0.25 0.06 2 2 4
(51) |
|
erm(B) +
0.25 >64 1 >64 8 16 4
mef(A) (31)
L4 mutations
0.125 >64 0.25 0.125 8 2 16
(27) |
|
McGhee P., Clark C., Kosowska-Shick KM, Nagai K., Dewasse B, Beachel L, Appelbaum PC. AAC, 54:230-8. 2010
Solithromycin’s unique chemical structure necessitates mutations at three
distinct sites for resistance to develop –no other macrolide has more than two
14
|
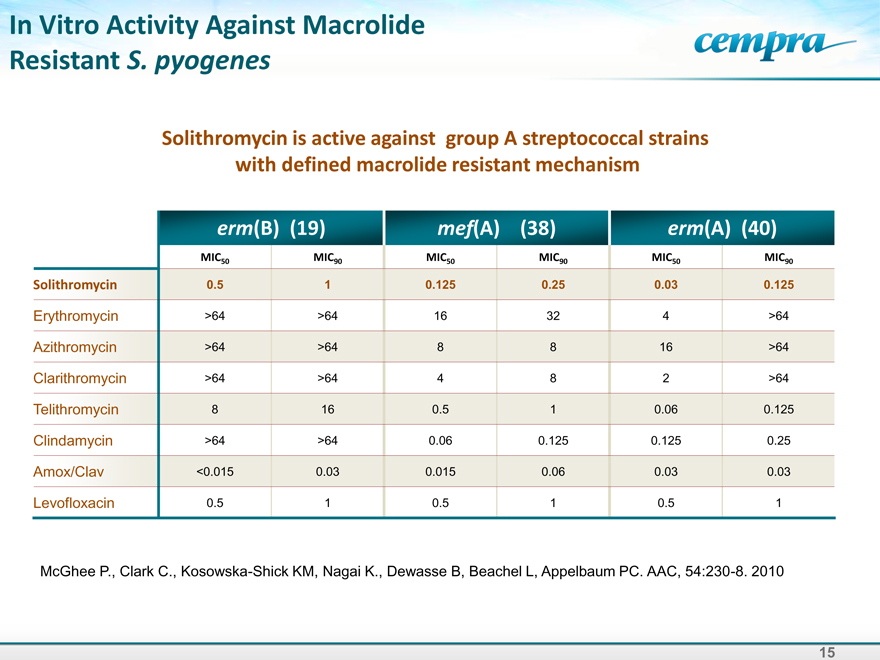
In Vitro Activity Against Macrolide Resistant S. pyogenes
Solithromycin is active against group A streptococcal strains with defined macrolide resistant mechanism
erm(B) (19) mef(A) (38) erm(A) (40)
MIC50 MIC90 MIC50 MIC90 MIC50 MIC90
Solithromycin 0.5 1 0.125 0.25 0.03 0.125
Erythromycin >64 >64 16 32 4 >64
Azithromycin >64 >64 8 8 16 >64
Clarithromycin >64 >64 4 8 2 >64
Telithromycin 8 16 0.5 1 0.06 0.125
Clindamycin >64 >64 0.06 0.125 0.125 0.25
Amox/Clav <0.015 0.03 0.015 0.06 0.03 0.03
Levofloxacin 0.5 1 0.5 1 0.5 1
McGhee P., Clark C., Kosowska-Shick KM, Nagai K., Dewasse B, Beachel L, Appelbaum PC. AAC, 54:230-8. 2010
15
|
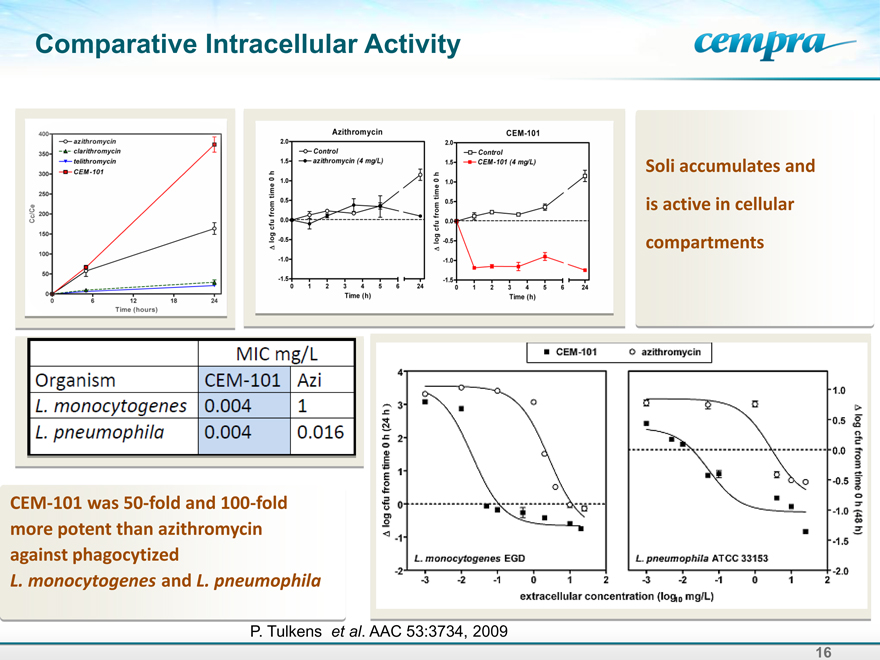
Comparative Intracellular Activity
Soli accumulates and is active in cellular compartments
MIC mg/L
Organism CEM-101 Azi
L.monocytogenes 0.004 1
L.pneumophila 0.004 0.016
400
350
300
250
200
150
100
50
0
0
6 |
|
12
18
24
Co/Ce
azithromycin
clarithromycin
telithromycin
CEM-101
Log cfu from time 0 h
2.0
1.5
1.0
0.5
0
-0.5
-1.0
-1.5
0
1 |
|
2 |
|
3 |
|
4 |
|
5 |
|
6 |
|
24
Azithoromycin
Control
Azithromycin (4 mg/L)
CEM-101
Control
CEM-101 (4 mg/L)
Log cfu from time 0 h
2.0
1.5
1.0
0.5
0
-0.5
-1.0
-1.5
0
1 |
|
2 |
|
3 |
|
4 |
|
5 |
|
6 |
|
24
CEM-101
Azithromycin
Log cfu from time 0 h (24 h)
L. monocytogenes EGD
4 |
|
3 |
|
2 |
|
1 |
|
0
-1
-2
-3
-2
-1
0
1 |
|
2 |
|
3 |
|
Log cfu from time 0 h (48 h)
L. pneumophila ATCC 33153
1.0
0.5
0
-0.5
-1.0
-1.5
-2.0
-3
-2
-1
0
1 |
|
2 |
|
Extracellular concentration (log10 mg/L)
CEM-101 was 50-fold and 100-fold more potent than azithromycin against phagocytized
L. monocytogenes and L. pneumophila
P. Tulkens et al. AAC 53:3734, 2009
16
|
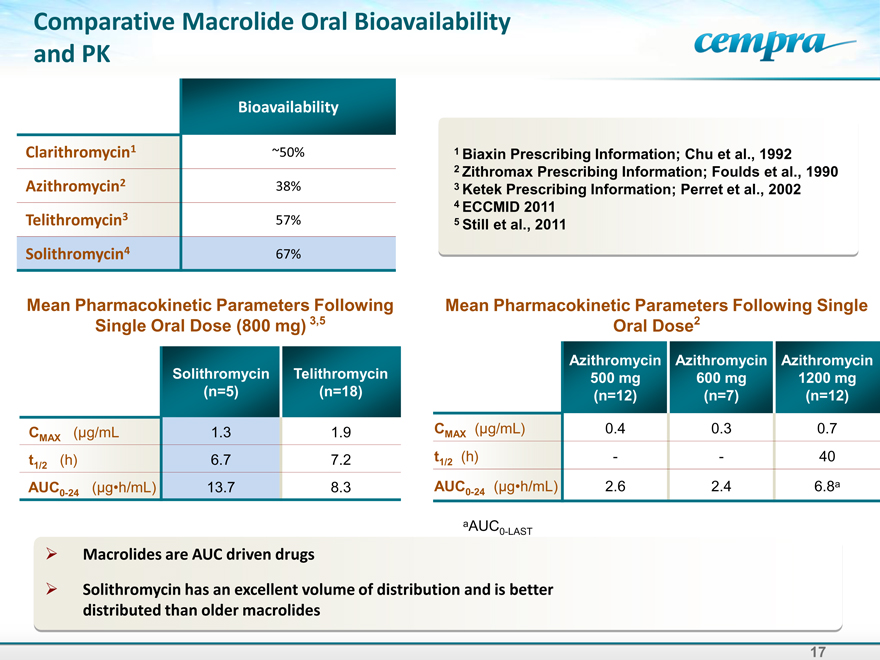
Comparative Macrolide Oral Bioavailability and PK
Bioavailability
Clarithromycin1 ~50%
Azithromycin2 38%
Telithromycin3 57%
Solithromycin4 67%
1 |
| Biaxin Prescribing Information; Chu et al., 1992 |
2 |
| Zithromax Prescribing Information; Foulds et al., 1990 |
3 |
| Ketek Prescribing Information; Perret et al., 2002 |
4 |
| ECCMID 2011 |
5 |
| Still et al., 2011 |
Mean Pharmacokinetic Parameters Following Single Oral Dose (800 mg) 3,5
Solithromycin Telithromycin
(n=5) (n=18)
CMAX (µg/mL 1.3 1.9
t1/2 (h) 6.7 7.2
AUC0-24 (g•h/mL)13.7 8.3
Mean Pharmacokinetic Parameters Following Single Oral Dose2
Azithromycin Azithromycin Azithromycin
500 mg 600 mg 1200 mg
(n=12) (n=7) (n=12)
CMAX (µg/mL) 0.4 0.3 0.7
t1/2 (h)—- 40
AUC0-24 (g•h/mL)2.6 2.4 6.8a
aAUC
0-LAST
Macrolides are AUC driven drugs
Solithromycin has an excellent volume of distribution and is better distributed than older macrolides
17
|
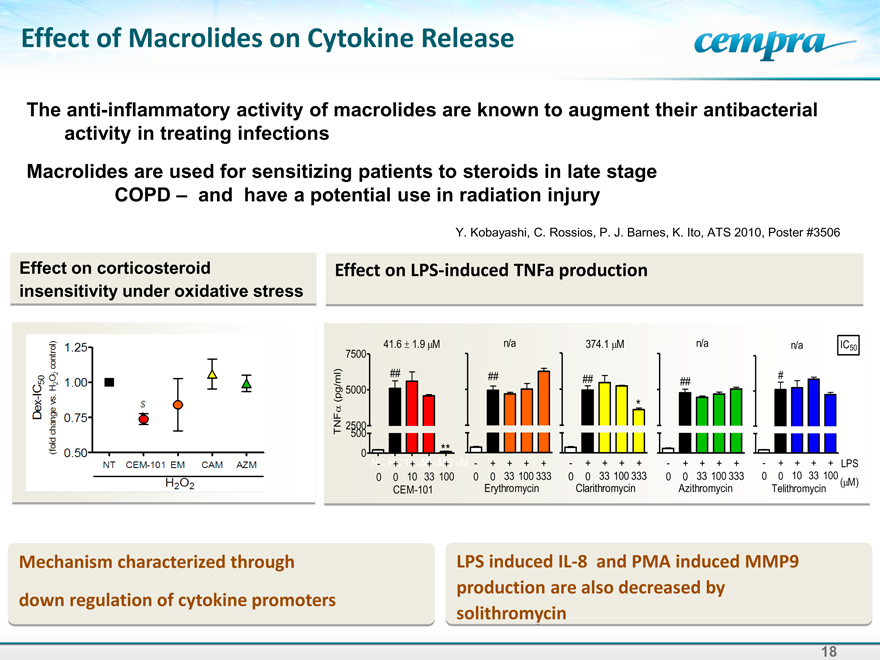
Effect of Macrolides on Cytokine Release
The anti-inflammatory activity of macrolides are known to augment their antibacterial activity in treating infections Macrolides are used for sensitizing patients to steroids in late stage COPD – and have a potential use in radiation injury
Y. Kobayashi, C. Rossios, P. J. Barnes, K. Ito, ATS 2010, Poster #3506
Effect on corticosteroid insensitivity under oxidative stress
Effect on LPS-induced TNFa production
Mechanism characterized through through down regulation of cytokine promoters
LPS induced IL-8 and PMA induced MMP9 production are also decreased by solithromycin
18
|
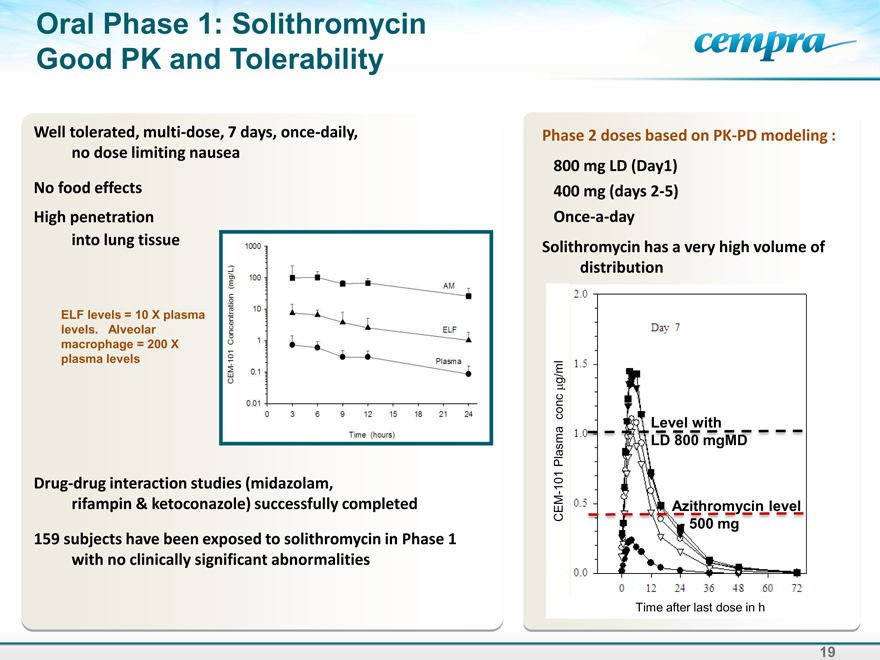
Oral Phase 1: Solithromycin Good PK and Tolerability
Well tolerated, multi-dose, 7 days, once-daily, no dose limiting nausea
No food effects
High penetration into lung tissue
ELF levels = 10 X plasma levels. Alveolar macrophage = 200 X plasma levels
Drug-drug interaction studies (midazolam, rifampin & ketoconazole) successfully completed
159 subjects have been exposed to solithromycin in Phase 1 with no clinically significant abnormalities
Phase 2 doses based on PK-PD modeling :
800 mg LD (Day1) 400 mg (days 2-5) Once-a-day
Solithromycin has a very high volume of distribution
Time after last dose in h
CEM-101 Plasma conc g/ml
Level with LD 800 mgMD
Azithromycin level —500 mg
CEM-101
Concernatraton (mg/L)
1000
100
10
1 |
|
0.1
0.01
AM
ELF
Plasma
0
3 |
|
6 |
|
9
12
15
18
21
24
19
|
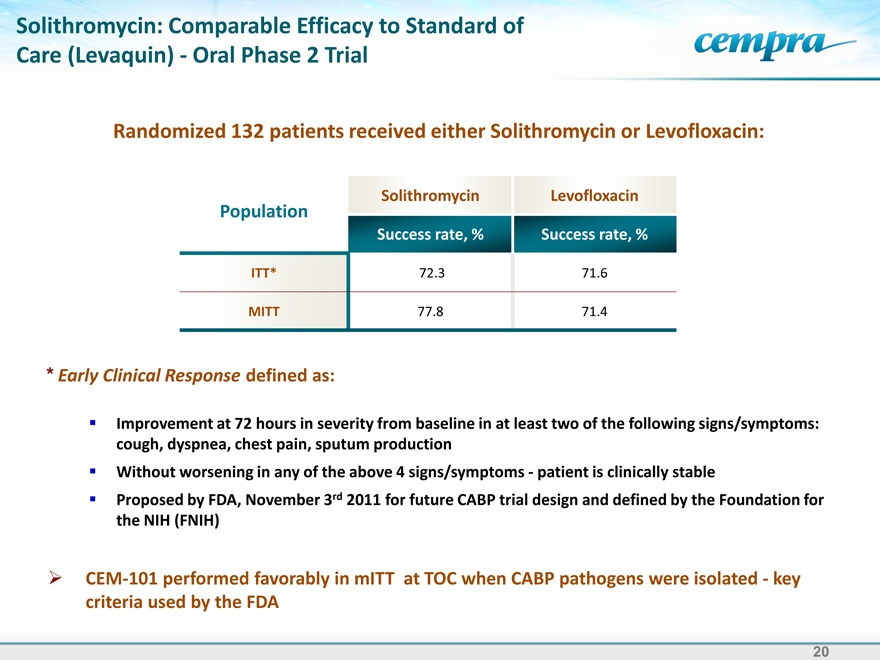
Solithromycin: Comparable Efficacy to Standard of Care (Levaquin) - Oral Phase 2 Trial
Randomized 132 patients received either Solithromycin or Levofloxacin:
Solithromycin Levofloxacin Population Success rate, % Success rate, %
ITT* 72.3 71.6
MITT 77.8 71.4
* |
| Early Clinical Response defined as: |
Improvement at 72 hours in severity from baseline in at least two of the following signs/symptoms: cough, dyspnea, chest pain, sputum production Without worsening in any of the above 4 signs/symptoms - patient is clinically stable Proposed by FDA, November 3rd 2011 for future CABP trial design and defined by the Foundation for the NIH (FNIH)
CEM-101 performed favorably in mITT at TOC when CABP pathogens were isolated - key criteria used by the FDA
20
|
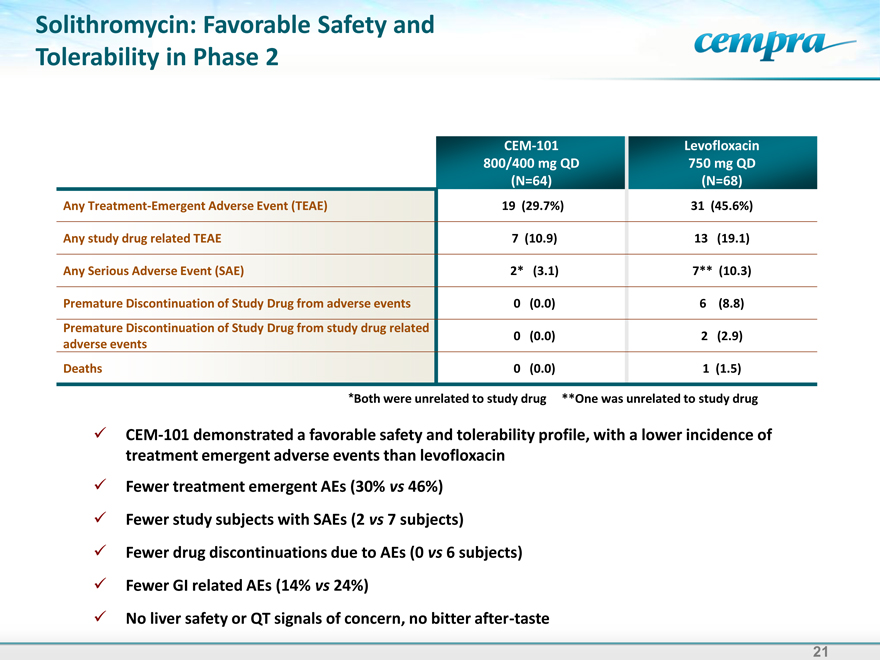
Solithromycin: Favorable Safety and Tolerability in Phase 2
CEM-101 Levofloxacin
800/400 mg QD 750 mg QD
(N=64) (N=68)
Any Treatment-Emergent Adverse Event (TEAE) 19 (29.7%) 31 (45.6%)
Any study drug related TEAE 7 (10.9) 13 (19.1)
Any Serious Adverse Event (SAE) 2* (3.1) 7** (10.3)
Premature Discontinuation of Study Drug from adverse events 0 (0.0) 6 (8.8)
Premature Discontinuation of Study Drug from study drug related
0 (0.0) 2 (2.9)
adverse events
Deaths 0 (0.0) 1 (1.5)
*Both were unrelated to study drug **One was unrelated to study drug
CEM-101 demonstrated a favorable safety and tolerability profile, with a lower incidence of treatment emergent adverse events than levofloxacin
Fewer treatment emergent AEs (30% vs 46%) Fewer study subjects with SAEs (2 vs 7 subjects) Fewer drug discontinuations due to AEs (0 vs 6 subjects) Fewer GI related AEs (14% vs 24%) No liver safety or QT signals of concern, no bitter after-taste
21
|
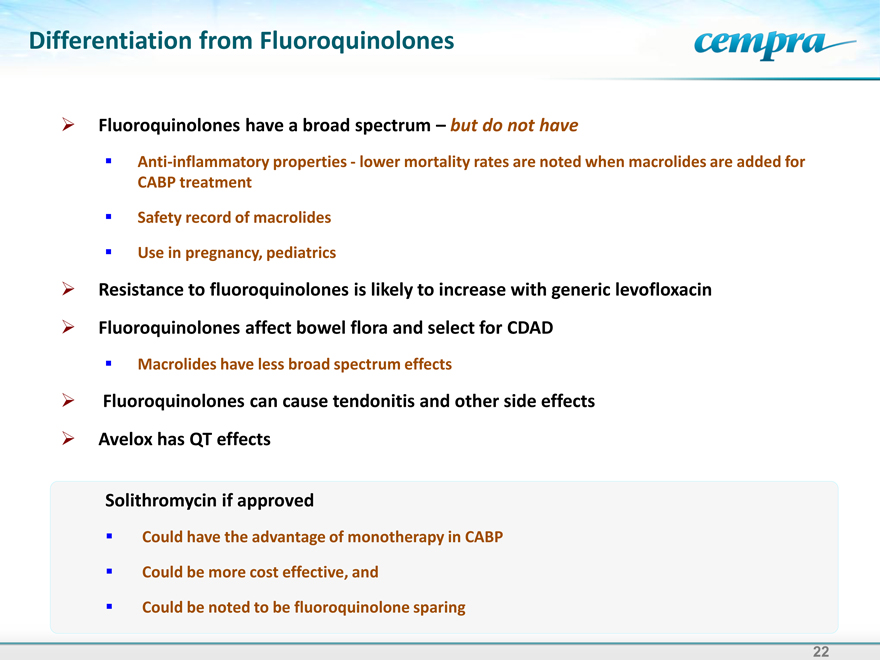
Differentiation from Fluoroquinolones
Fluoroquinolones have a broad spectrum – but do not have
Anti-inflammatory properties-lower mortality rates are noted when macrolides are added for CABP treatment
Safety record of macrolides
Use in pregnancy, pediatrics
Resistance to fluoroquinolones is likely to increase with generic levofloxacin
Fluoroquinolones affect bowel flora and select for CDAD
Macrolides have less broad spectrum effects
Fluoroquinolones can cause tendonitis and other side effects
Avelox has QT effects
Solithromycin if approved
Could have the advantage of monotherapy in CABP Could be more cost effective, and Could be noted to be fluoroquinolone sparing
22
|
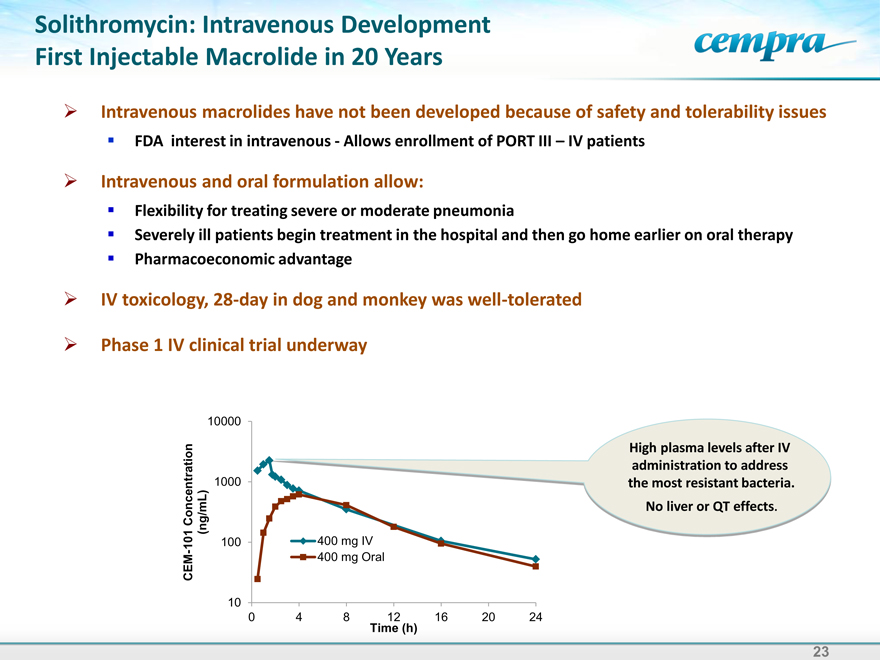
Solithromycin: Intravenous Development First Injectable Macrolide in 20 Years
Intravenous macrolides have not been developed because of safety and tolerability issues FDA interest in intravenous-Allows enrollment of PORT III – IV patients
Intravenous and oral formulation allow:
Flexibility for treating severe or moderate pneumonia
Severely ill patients begin treatment in the hospital and then go home earlier on oral therapy Pharmacoeconomic advantage
IV toxicology, 28-day in dog and monkey was well-tolerated
Phase 1 IV clinical trial underway
CEM-101 Concentration (ng/mL)
10 100 1000 10000
400 mg IV
400 mg Oral
0 4 8 Time 12 (h) 16 20 24
High plasma levels after IV administration to address the most resistant bacteria.
No liver or QT effects.
23
|
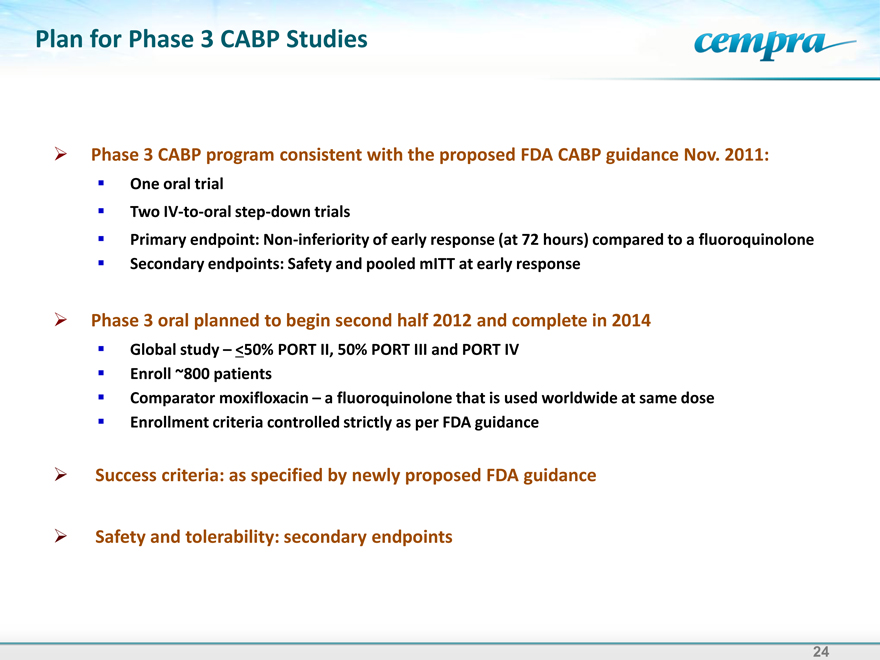
Plan for Phase 3 CABP Studies
Phase 3 CABP program consistent with the proposed FDA CABP guidance Nov. 2011: One oral trial Two IV-to-oral step-down trials Primary endpoint: Non-inferiority of early response (at 72 hours) compared to a fluoroquinolone Secondary endpoints: Safety and pooled mITT at early response
Phase 3 oral planned to begin second half 2012 and complete in 2014 Global study – <50% PORT II, 50% PORT III and PORT IV
Enroll ~800 patients
Comparator moxifloxacin – a fluoroquinolone that is used worldwide at same dose Enrollment criteria controlled strictly as per FDA guidance
Success criteria: as specified by newly proposed FDA guidance
Safety and tolerability: secondary endpoints
24
|
Community-Acquired Bacterial Pneumonia (CABP) – Standard-of-Care
Macrolides have kept a large segment of the global antibiotic market in spite of fluoroquinolones
Macrolide segment not crowded
Mostly occupied by azithromycin and clarithromycin – rising incidence of resistance
No new macrolides except solithromycin
Solithromycin is being developed for monotherapy for CABP—a cephalosporin would not be needed, eliminating side effects and costs of two drugs
Solithromycin has the spectrum of activity that provides coverage for CABP pathogens, including azithromycin-resistant bacteria
Step down IV to oral therapy could give a pharmacoeconomic advantage over current treatment options
25
|
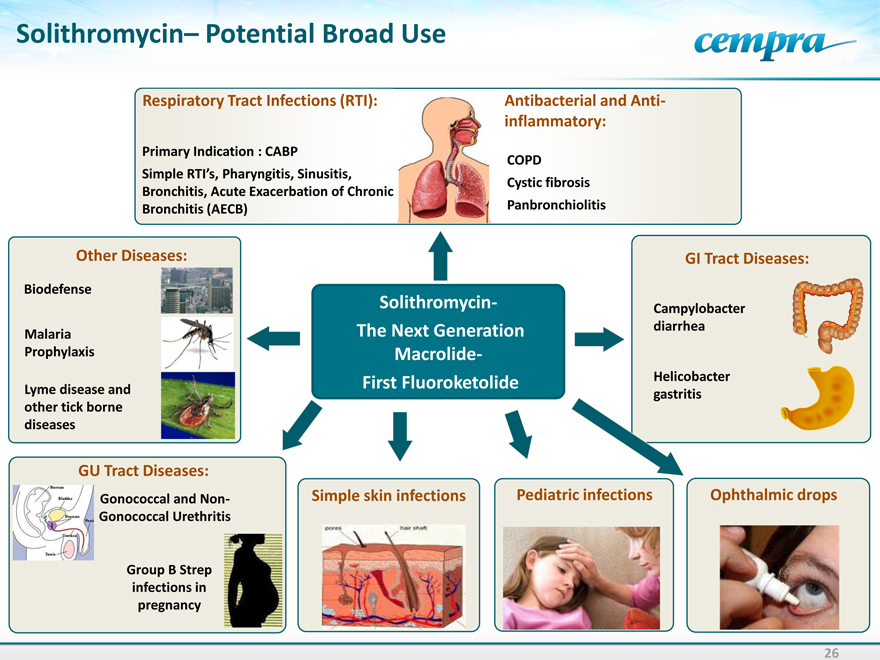
Solithromycin– Potential Broad Use
Respiratory Tract Infections (RTI):
Primary Indication : CABP
Simple RTI’s,
Bronchitis, Acute Exacerbation of Chronic Bronchitis (AECB)
Antibacterial and Anti-inflammatory:
COPD
Sinusitis,
Cystic fibrosis Panbronchiolitis
Other Diseases:
Biodefense
Malaria Prophylaxis
Lyme disease and other tick borne diseases
Solithromycin- The Next Generation Macrolide- First Fluoroketolide
GI Tract Diseases:
Campylobacter diarrhea
Helicobacter gastritis
GU Tract Diseases:
Gonococcal and Non-Gonococcal Urethritis
Group B Strep infections in pregnancy
Simple skin infections
Pediatric infections
Ophthalmic drops
26
|
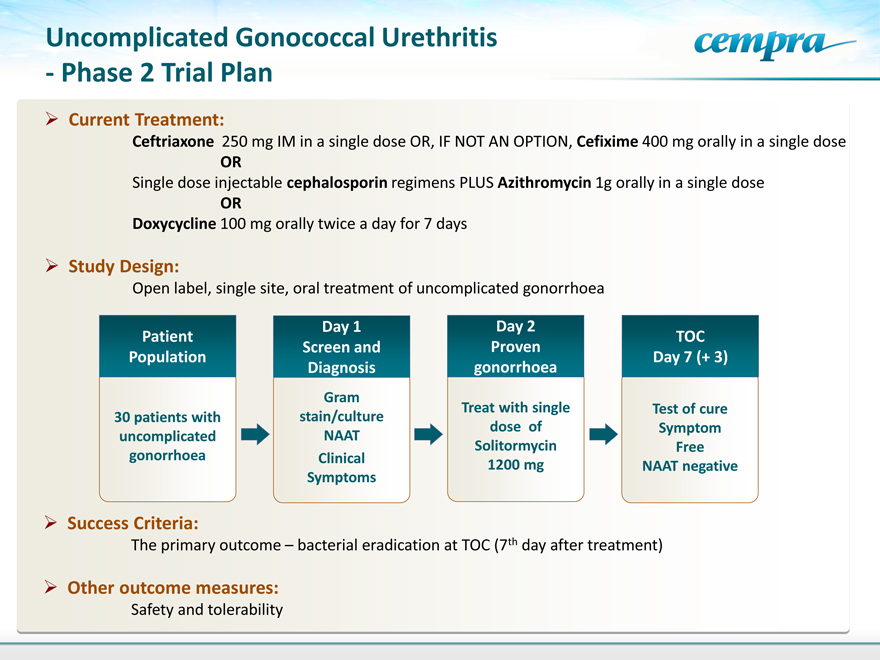
Uncomplicated Gonococcal Urethritis—Phase 2 Trial Plan
Current Treatment:
Ceftriaxone 250 mg IM in a single dose OR, IF NOT AN OPTION, Cefixime 400 mg orally in a single dose OR
Single dose injectable cephalosporin regimens PLUS Azithromycin 1g orally in a single dose OR
Doxycycline 100 mg orally twice a day for 7 days
Study Design:
Open label, single site, oral treatment of uncomplicated gonorrhoea
Patient Population
Day 1 Screen and Diagnosis
Day 2 Proven gonorrhoea
TOC Day 7 (+ 3)
30 patients with uncomplicated gonorrhoea
Gram stain/culture NAAT
Clinical Symptoms
Treat with single dose of Solitormycin 1200 mg
Test of cure Symptom Free NAAT negative
Success Criteria:
The primary outcome – bacterial eradication at TOC (7th day after treatment)
Other outcome measures:
Safety and tolerability
|
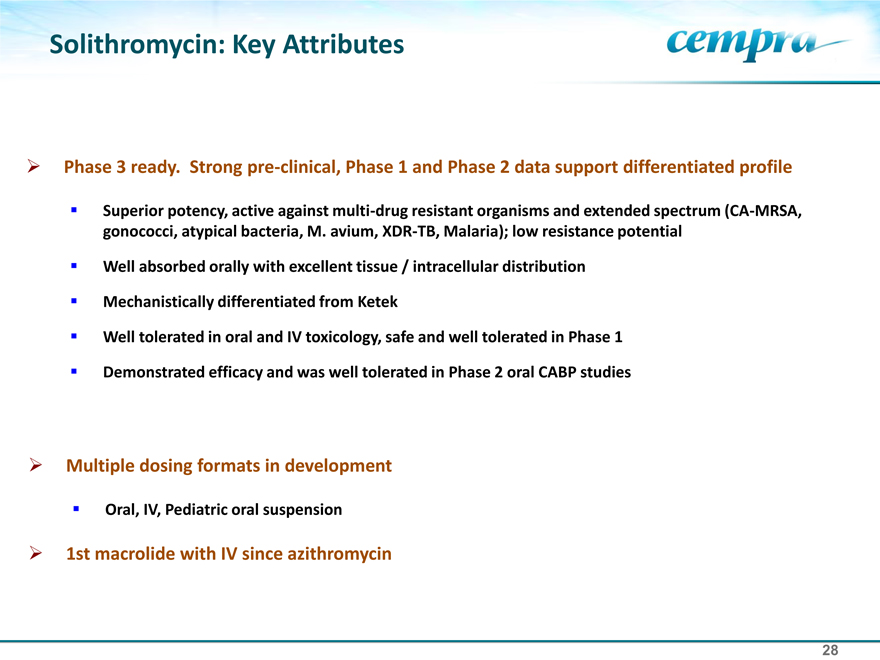
Solithromycin: Key Attributes
Phase 3 ready. Strong pre-clinical, Phase 1 and Phase 2 data support differentiated profile
Superior potency, active against multi-drug resistant organisms and extended spectrum (CA-MRSA, gonococci, atypical bacteria, M. avium, XDR-TB, Malaria); low resistance potential
Well absorbed orally with excellent tissue / intracellular distribution Mechanistically differentiated from Ketek Well tolerated in oral and IV toxicology, safe and well tolerated in Phase 1 Demonstrated efficacy and was well tolerated in Phase 2 oral CABP studies
Multiple dosing formats in development Oral, IV, Pediatric oral suspension 1st macrolide with IV since azithromycin
28