 850f – Late Breaking Abstract Session DDW, May 6, 2014 Prabhavathi Fernandes , Taishi Hashiguchi , Masato Fujii , Hiroyuki Yoneyama Cempra Inc., NC, USA. Stelic Institute & Co. Inc., Tokyo, Japan Anti-NASH Effects of Solithromycin in NASH-HCC Mouse Model Exhibit 99.1 1 2 2 1 2 2 |
 Forward Looking Statement This presentation contains forward-looking statements regarding future events. These statements are just predictions and are subject to risks and uncertainties that could cause the actual events or results to differ materially. These risks and uncertainties include, among others: risks related to the costs, timing, regulatory review and results of our studies and clinical trials; our need to obtain additional funding and our ability to obtain future funding on acceptable terms; our anticipated capital expenditures and our estimates regarding our capital requirements; our and our strategic partners’ ability to obtain FDA and foreign regulatory approval of our product candidates; our dependence on the success of solithromycin and TAKSTA; the possible impairment of, or inability to obtain, intellectual property rights and the costs of obtaining such rights from third parties; the unpredictability of the size of the markets for, and market acceptance of, any of our products, including solithromycin and TAKSTA; our ability to produce and sell any approved products and the price we are able to realize for those products; our ability to retain and hire necessary employees and to staff our operations appropriately; our ability to compete in our industry; innovation by our competitors; and our ability to stay abreast of and comply with new or modified laws and regulations that currently apply or become applicable to our business. Please refer to the documents that we file from time to time with the Securities and Exchange Commission. 2 |
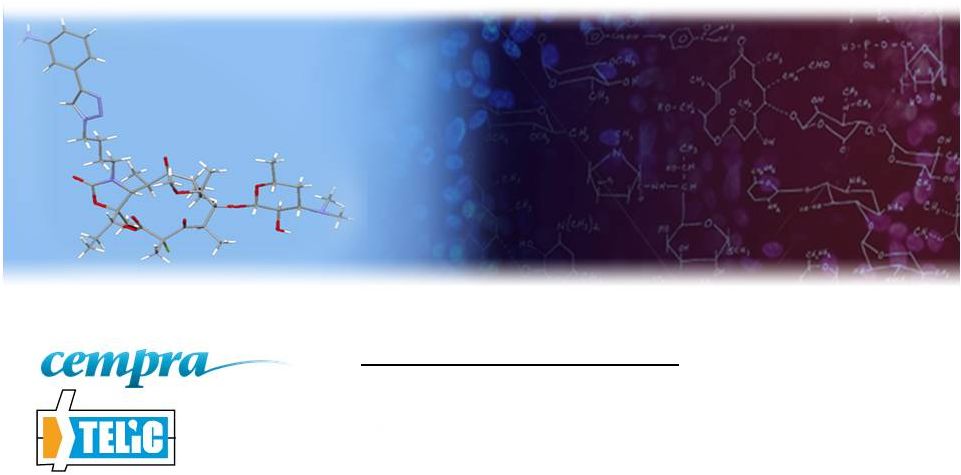
 3 Solithromycin – the first fluoroketolide Currently Approved Macrolide Antibiotics What is Solithromycin? More potent than older macrolides Activity against resistant strains Better stability, better PK Oral, intravenous and pediatric suspension Well tolerated so far, Phase 1 and Phase 2 2 Global Phase 3 trials for pneumonia (CABP) enrolling Solithromycin |
 Solithromycin’s Broad Use Potential 4 HAP, Simple RTI’s, Pharyngitis, Sinusitis, Bronchitis, Acute Exacerbation of Chronic Bronchitis (AECB) Respiratory Tract Infections (RTI) Anti-inflammatory COPD, Cystic fibrosis, Panbronchiolitis Special Populations BARDA funded Pediatrics and Pregnancy Pediatric suspension with broad potential in development Infections in Pregnancy – neonatal sepsis Infections in Utero – premature, cerebral palsy, autism Biodefense BARDA funded Multiple Unidentified Pathogens Anthrax, Tularemia Sexually Transmitted Diseases Genital Infections Major Public Health Crisis – multi drug resistance, no oral therapy. Most common reportable infectious diseases. GI & Others Ophthalmic Helicobacter Gastritis, Campylobacteria, Tick and Insect Borne Diseases, Diarrhea, and Ophthalmic Drops CABP Primary Indication Other Infections |
 Anti-inflammatory Mechanism of Solithromycin in COPD 5 Macrolides are used as anti-inflammatories in treating COPD patients Solithromycin has been shown to have stronger anti-inflammatory properties than the older macrolide antibiotics Macrolides upregulate HDAC2 promoter – which results in a decrease in cytokine production A Novel Macrolide/fluoroketolide, Solithromycin (CEM- 101), Reverses Corticosteroid Insensitivity Via Phosphoinositide 3-kinase Pathway Inhibition |
 Why Test Solithromycin in NASH? The strong anti-inflammatory effects of solithromycin will be tested in a Phase 2 study in COPD Solithromycin achieves high liver concentrations (as in the lung) Since inflammation is known to play a key role in NASH we decided to study the effect of solithromycin in NASH Large and growing body of safety data on solithromycin: – Well tolerated in approx. 1000 patients and subjects to date – oral capsules, intravenous, pediatric suspension in development – Well tolerated in hepatic insufficiency patients – mild, moderate and severe. No dose adjustments needed. – No QT effect unlike older macrolides – TQT study complete – 90 day toxicology in rat and NHP complete 6 |
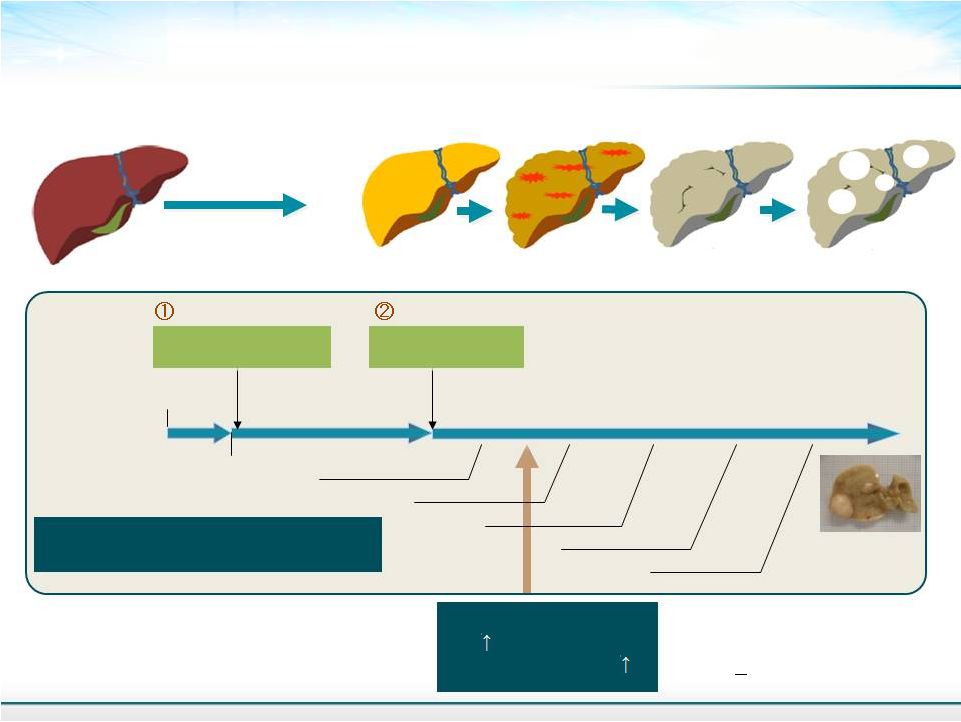 STAM : In Vivo Predictive Pharmacology Model for NASH Fujii, M. et al. Med. Mol. Morphol. 2013;46: 141-152. 7 Birth 0w Pregnant C57BL/6J mice 1st Hit - Low dose streptozotocin - 4w 5w 7w 9w Steatosis evident NASH evident Fibrosis evident 16w HCC evident 12w Nodule evident Fibrosis NASH Steatosis 100% Steatosis (+) ALT NAFLD activity score All mice at 6 weeks have a NAFLD Activity Score (NAS) >5 Similar to human NASH CHEMICAL DIET Continuous 2nd Hit - High fat diet feeding - 6w Following the initial diabetes model evolves into a model for NASH 100% 100% HCC Healthy Liver TM |
 Kleiner DE, et al. Hepatology 2005;41:1313–21 NAFLD Activity Score (NAS) 8 Histological feature Score Category definition Steatosis 0 <5% 1 5–33% 2 34–66% 3 >66% Plus Hepatocyte ballooning 0 None 1 Few 2 Many Plus Inflammation 0 None 1 1–2 foci per ×20 field 2 2–4 foci per ×20 field 3 >4 foci per ×20 field NAS total 0–8 Histological feature Score Category definition Fibrosis 0 No fibrosis 1a Zone 3 mild perisinusoidal fibrosis 1b Zone 3 moderate perisinusoidal fibrosis 1c Periportal/portal fibrosis only 2 Zone 3+periportal/portal fibrosis 3 Bridging fibrosis 4 Cirrhosis Fibrosis score 0-4 A score of 5 with steatosis and hepatocyte ballooning is generally considered diagnostic of NASH |
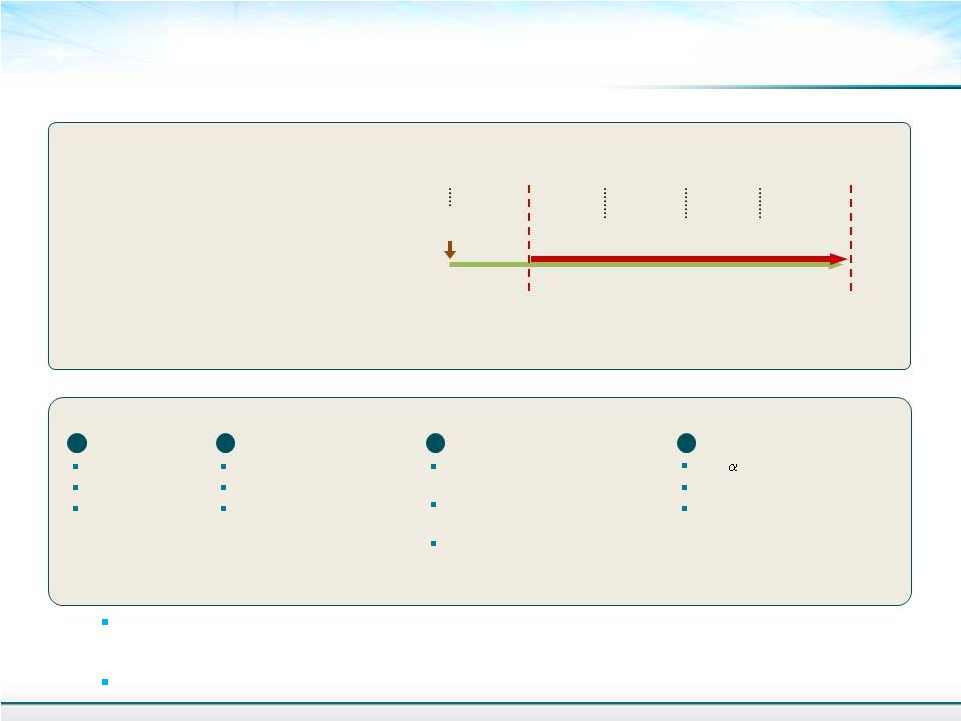 Study Plan for Assessing the Anti-NASH/fibrosis Effects of Solithromycin in STAM TM Model of NASH 9 4 Gene expression assay TNF- MCP-1 MMP-9 3 Histopathological assay HE staining (NAFLD Activity score) Sirius red staining (Fibrosis area) Immunohistochemistry for F4/80 (Inflammation area) 1 General Body weight Liver weight Liver-to-body 2 Biochemistry Whole blood glucose Plasma ALT Liver TG Analyses STAM TM + Solithromycin 50 mg/kg 4 wks 9 wks 7 wks Sacrifice total = 8 mice/group HFD Feeding 5 wks 8 wks (n=8) Oral, QD for 4 weeks 6 wks 50 mg/kg mouse dose = 240 mg adult human equivalent dose (Conversion of animal doses to HEDs based on body surface area FDA guidance 2012) 800 mg LD/400mg MD is being used in our current pneumonia clinical trials weight ratio |
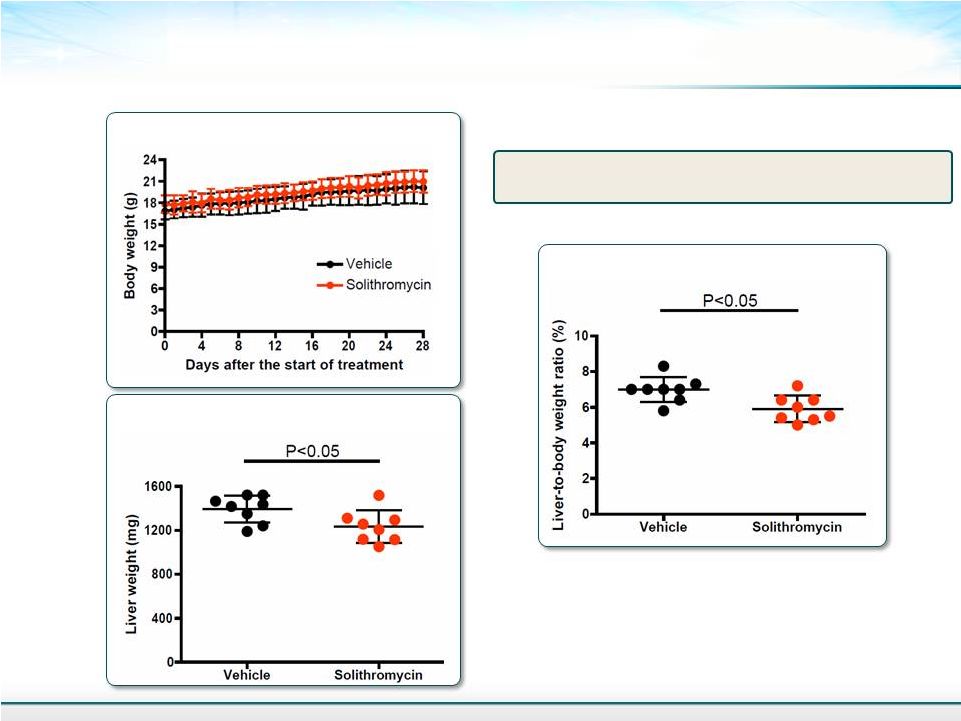 Mouse Weight and Liver Weight on Week 9 10 Steatosis seen in week 9 in the model Mean ± SD Student’s T-test Liver-to-body Weight Ratio Body Weight Liver Weight |
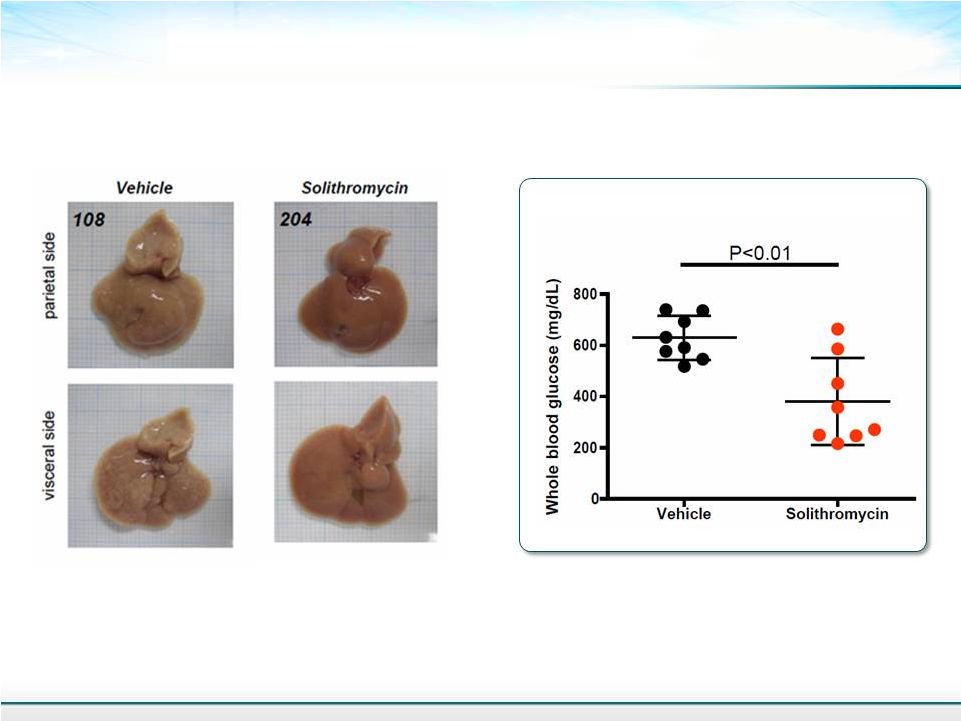 Liver Morphology and Function 11 Macroscopic Appearance Blood Glucose Mean ± SD Student’s T-test |
 Additional Chemistry 12 ALT not decreased No effect on liver triglyceride concentration Mean ± SD Student’s T-test Parameter (mean ± SD) Vehicle (n=8) Solithromycin (n=8) Plasma ALT (U/L) 48 ± 11 50 ± 9 Liver triglyceride (mg/g liver) 43.3 ± 7.0 36.8 ± 13.7 |
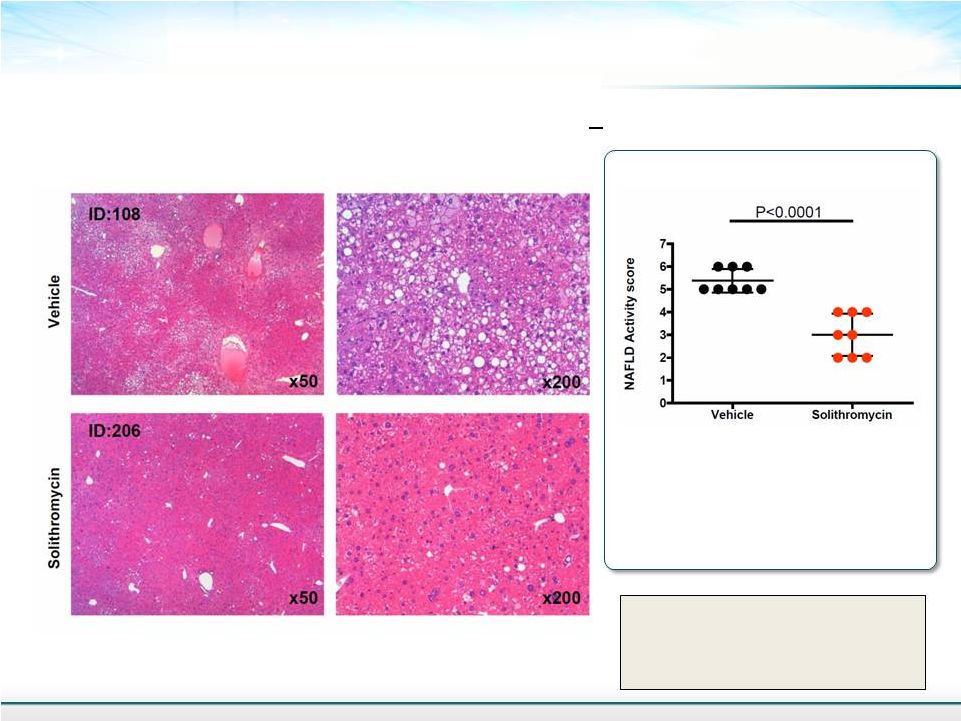 H & E Stained Liver Sections 13 NAFLD Activity score (NAS) calculated according to Kleiner DE et al. Hepatology. 2005;41:1313-1321 . All of the untreated mice had a NAFLD score of >5 and none of the soli treated mice had this score Mean ± SD Student’s T-test NAFLD Activity Score |
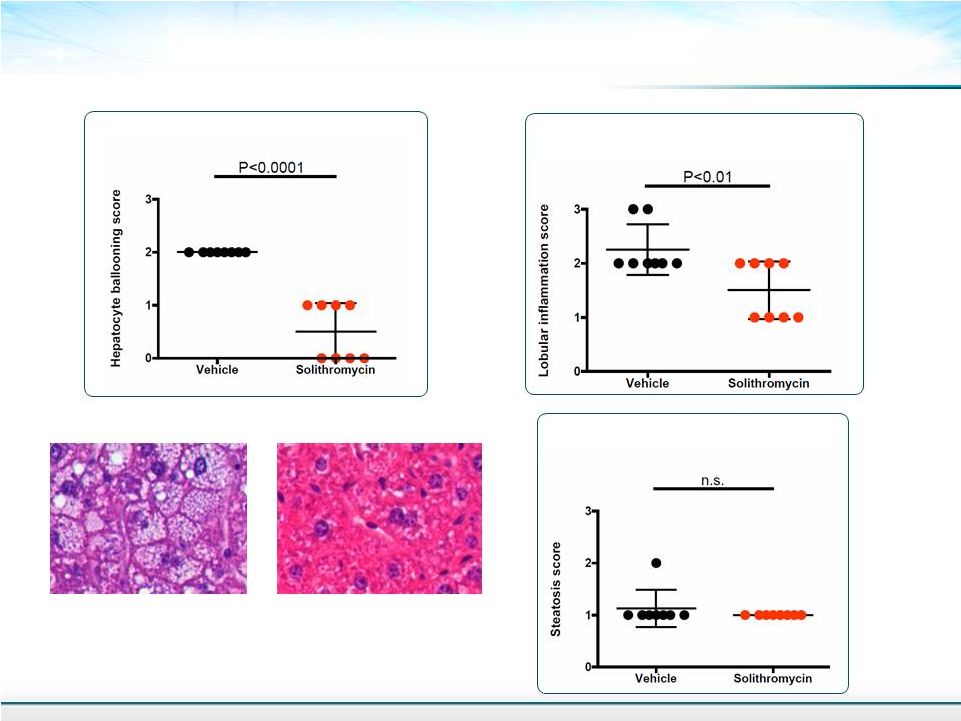 Components of NAFLD Activity Score 14 Steatosis Score Lobular Inflammation Score Hepatocyte Ballooning Score Mean ± SD Student’s T-test Treated Control untreated |
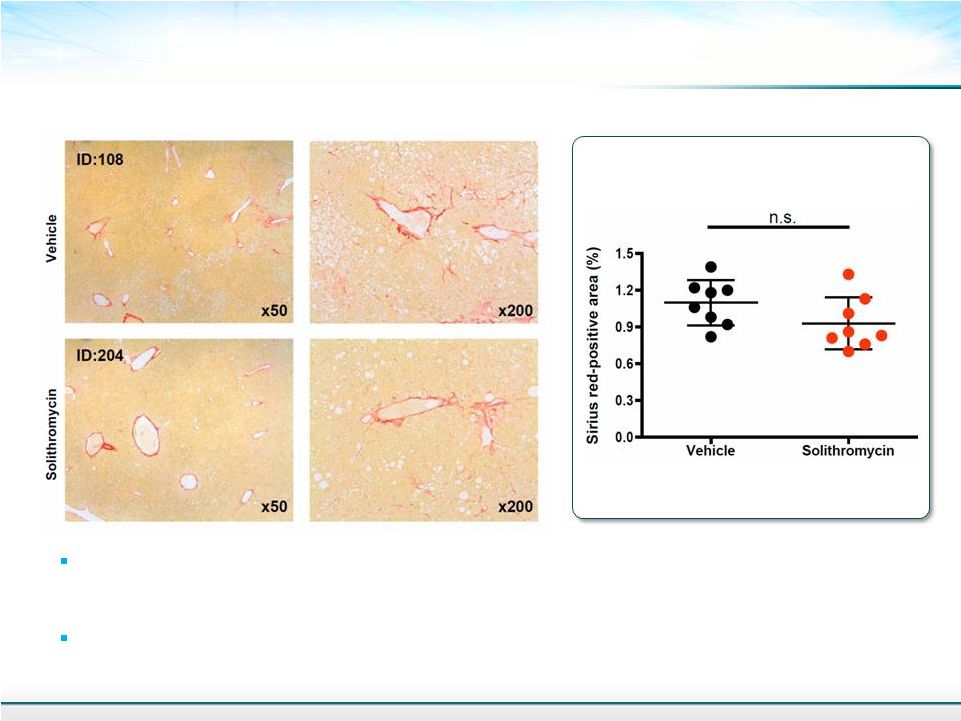 Sirius Red Stained Liver Sections 15 Percent of Fibrosis Area Short duration of treatment is not expected to improve fibrosis. Fibrosis is not massive even in vehicle treated controls in this study. Continued treatment, with continued improvement in inflammation could provide a reduction in fibrosis. Mean ± SD Student’s T-test |
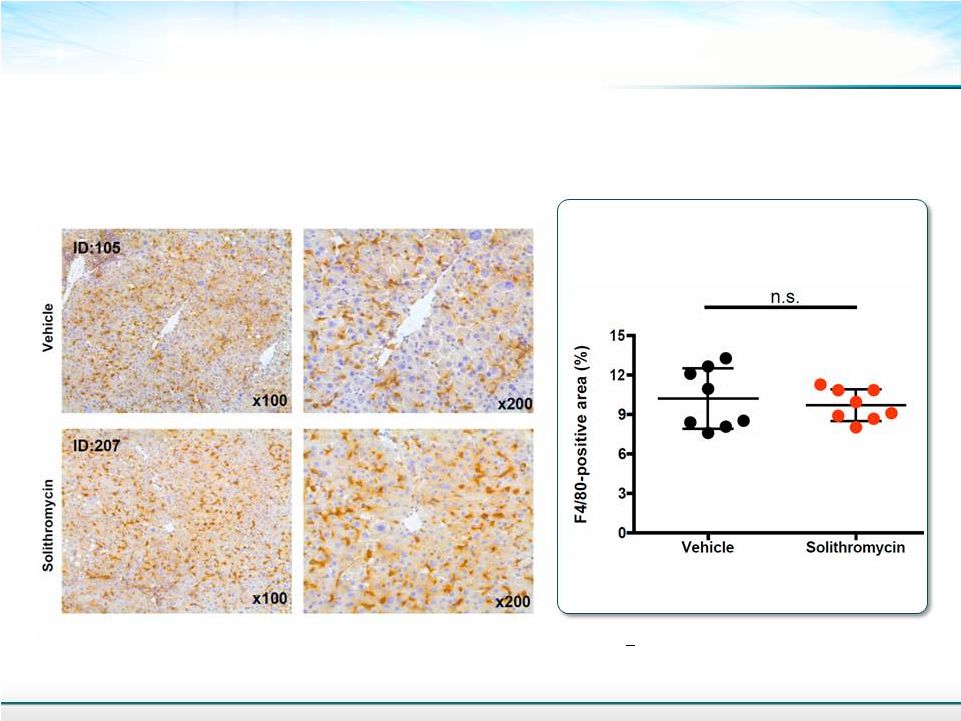 Photomicrographs of F4/80 Immunostained Liver Sections 16 F4/80 antigen is a macrophage-restricted cell surface glycoprotein and the stain is very specific for macrophages Mean + SD Student’s T-test Morphometric Analysis: Macrophage Density |
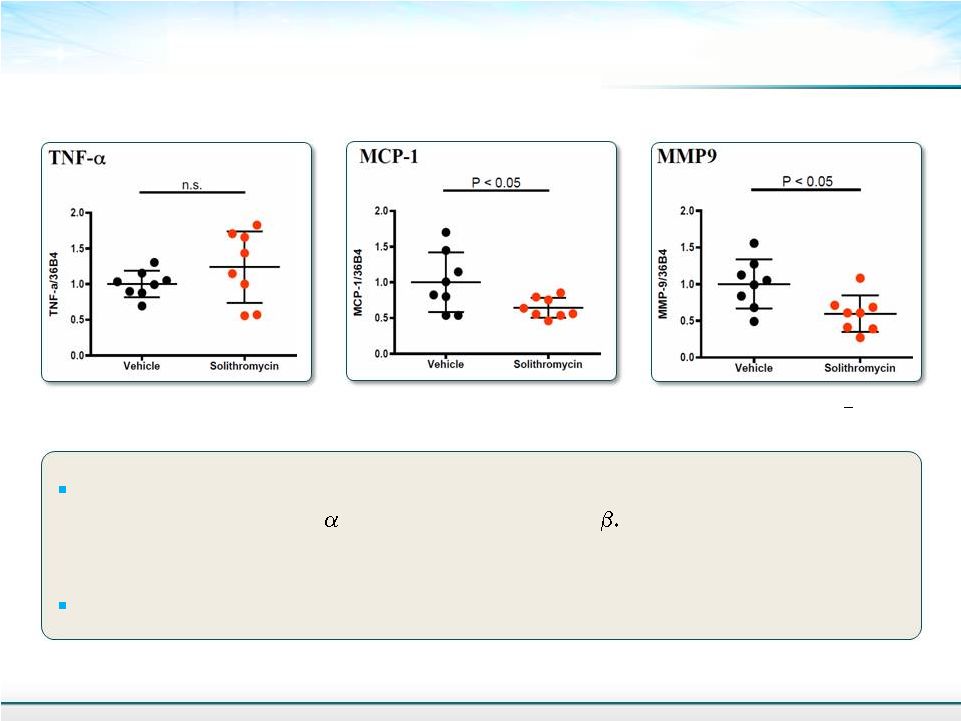 Relative Gene Expression in the Liver: Quantitative RT-PCR 17 Mean + SD Student’s T-test Future experiments will look at other inflammatory markers will be examined - Collagen Type 1, -SMA, TIMP-1 and TGF- - CK-18 biomarker Insulin resistance markers |
 18 Possible Mechanism of Action |
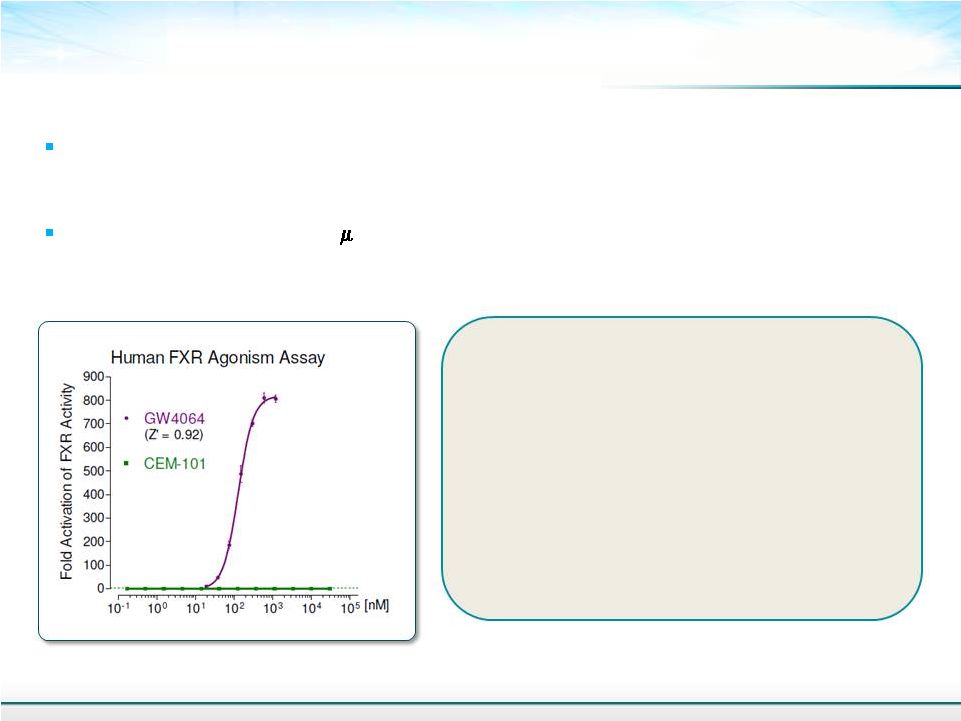 Solithromycin Has No Effect On FXR Signal Pathway 19 FXR agonist and antagonist activity measured in hybrid human FXR receptor expressing reporter cell assays Solithromycin up to 30 M Unlike Obeticholic acid from Intercept, Solithromycin (CEM-101) does not show agonistic activity nor significant antagonistic activity in the human FXR assays Solithromycin does not show evidence of cytotoxicity in the antagonist assays |
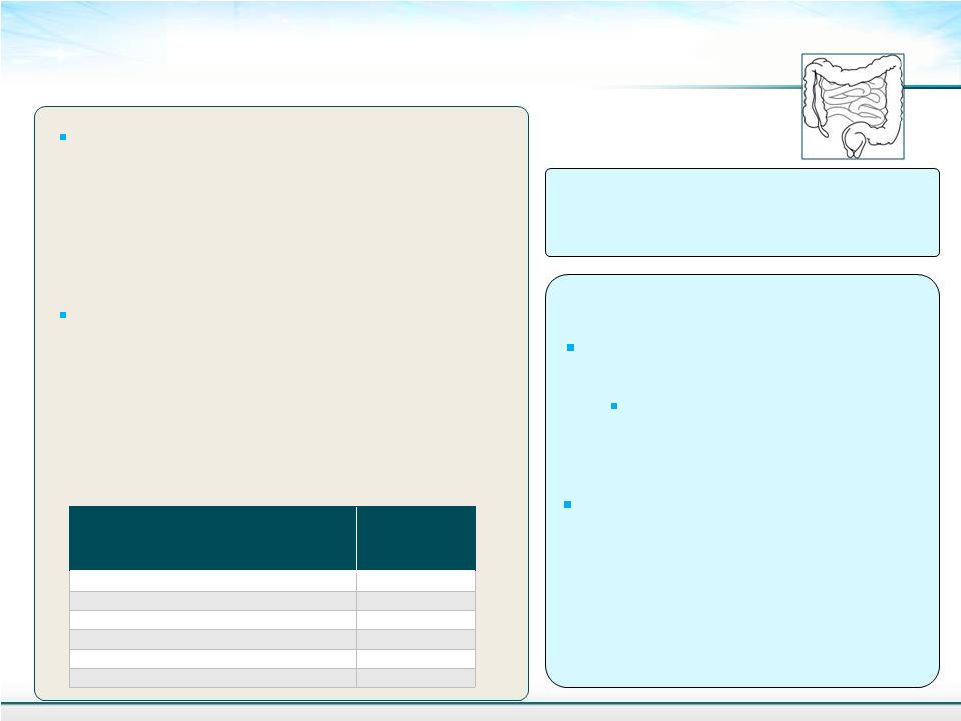 Does Solithromycin Act Through It’s Antibacterial Effects in NASH? 20 Susceptibility of Anaerobic Intestinal Bacteria MIC 90 (mcg/mL) Bacteroides spp. including B. fragilis (22) >64 Prevotella spp. (10) 4 Porphyromonas spp. (10) 0.06 Peptostreptococcus spp. (10) 0.25 Clostridium spp. (10) 0.06 C. difficile (10) >64 Endotoxin is not likely to be released by solithromycin - It does not effect aerobic or anaerobic Gram-negative intestinal bacteria - Minimal effect on bowel flora Solithromycin is well absorbed after oral administration (78%) and has very little active solithromycin is in the intestinal tract - <15% of unchanged solithromycin is Antibacterial Effect on Intestinal microflora – Unlikely Anti-inflammatory Mechanism Approximately 70% of the oral dose is metabolized and excreted by the liver Very high liver concentrations of solithromycin Organism (No.) It is possible that solithromycin is exerting its anti-inflammatory properties in the liver as it does in the lung in COPD found in the feces |
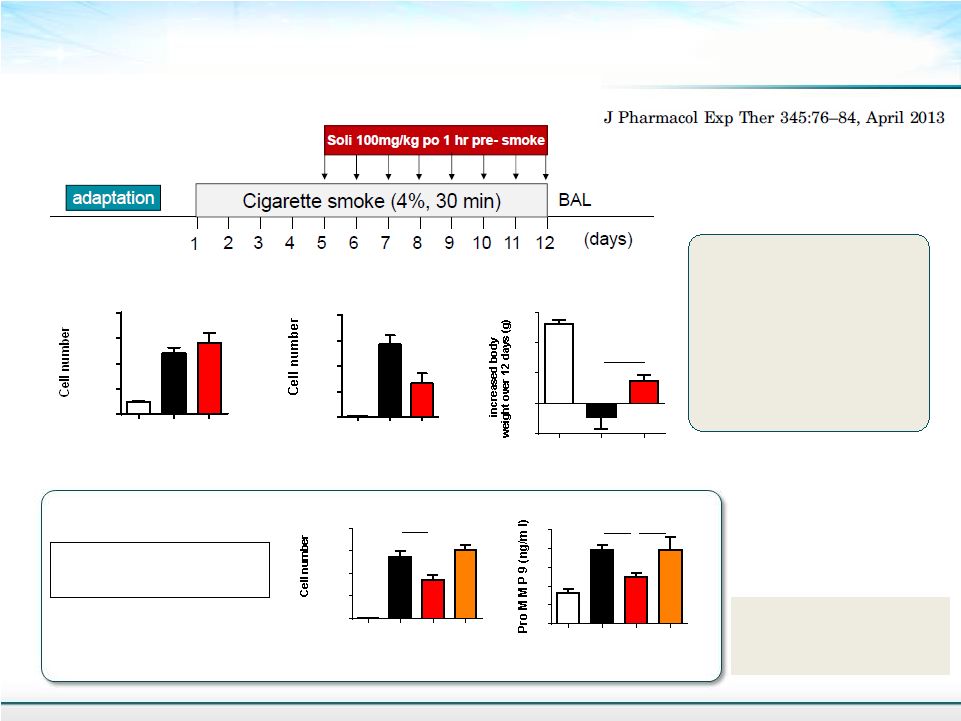 21 Anti-inflammatory Effect of Solithromycin in a mouse COPD Model Macrophage 0 100000 200000 300000 400000 Air SM + Veh SM + SOL 4 days of treatment with smoke exposure Neutrophil and pro- MMP9 production are inhibited by solithromycin in cigarette-smoke exposed murine lung Body Weight SM= Cigarette smoke EM = Erythromycin SOL = Solithromycin -0.5 0.0 0.5 1.0 1.5 p<0.05 Air SM + Veh SM + SOL MMP9 0 1 2 3 4 5 Air SM + Veh SM + SOL SM + EM NS p<0.05 Neutrophil 0 2000 4000 6000 8000 p<0.05 Air SM + Veh SM + SOL Neutrophil 0 5000 10000 15000 20000 Air SM + Veh SM + SOL SM + EM p<0.05 |
 22 Solithromycin Clinical Development Status to Date |
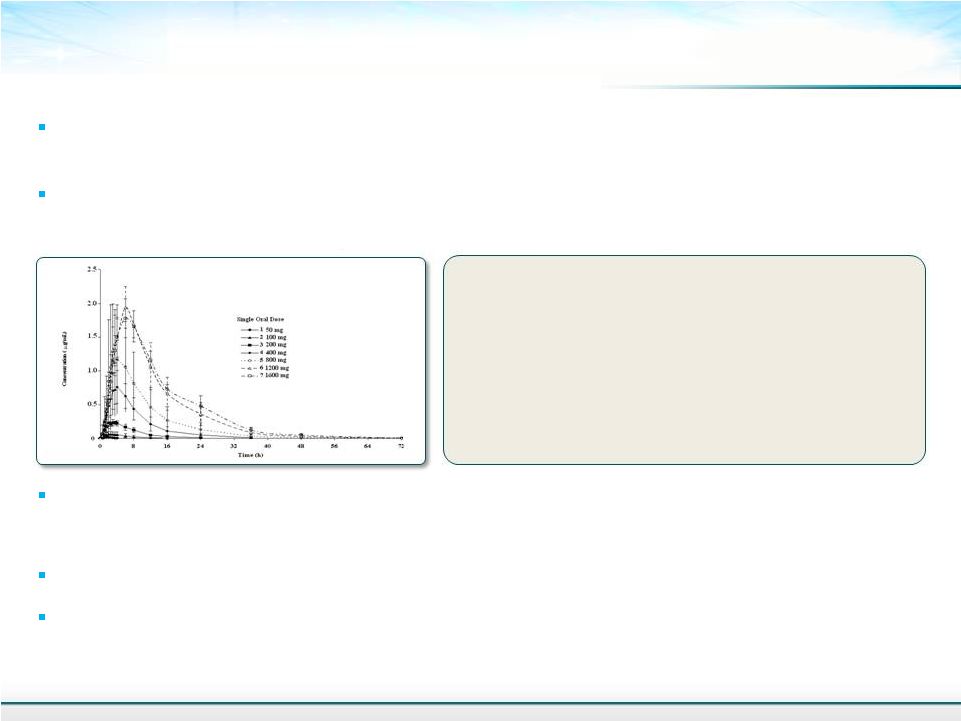 Solithromycin: Clinical Development Status 23 Two global Phase 3 studies for community acquired bacterial pneumonia (CABP) enrolling –~800 patients each Phase 1 SD, MD, Food effect, DDI, TQT, hepatic insufficiency complete – No food effect and no dose adjustment needed in hepatic insufficiency patients. No QT effect. Phase 2 CABP – Effective and well tolerated. 5 days QD 800mg LD / 400mg MD – Oldach, O., et al. Antimicrob Agents Chemother. 2013;57:2526-2534. All toxicology studies complete for NDA for CABP – 3 month toxicology in rat and NHP complete – 125 mg/kg/day Mean Cmax: 0.02 to 1.96 µg/mL Mean AUC inf : 0.06 µg•h/mL to 28.9 µg•h/mL Mean t max : 1.5 to 6.0 hours Mean T1/2: 3.2 to 7.4 hours Safe and well tolerated – Tested up to 1600 mg SD Still, J.G., et al . Antimicrob Agents Chemother. 2011;55:1997- 2003. Approx. 1000 patients or subjects have had up to 5-7 days treatment |
 Next Steps STAM TM Mouse model is being repeated with dose titration Determination of lowest optimal dose and duration of treatment Determination of duration of effect Characterization of additional inflammatory markers MOA in NASH studies We are planning a Phase 2 dose ranging study in NASH patients 3 month treatment period – possible with current toxicology data Chemical synthesis program to modify solithromycin to find a new compound without antibacterial properties Next generation product 24 |
 Key Take Away Messages Solithromycin, a novel fluoroketolide, has shown statistically significant effects in decreasing the NAFLD score, including drastically reducing ballooning degeneration and inflammation in the STAM mouse model Data indicates that the mechanism is mediated by the anti-inflammatory effects of solithromycin Solithromycin is well tolerated in clinical trials in bacterial pneumonia – approx. 1000 patients/subjects have been exposed for multiple days We expect to follow up these exciting mouse data by starting a Phase 2 study in NASH 25 TM |