
Arshad M. Khanani MD, MA, FASRS Director of Clinical Research at �Sierra Eye Associates Anirvan Ghosh, CEO Jamie Dananberg, CMO Lynne Sullivan, CFO UBX1325 �Phase 2 ENVISION nAMD Study�24 Week Data Exhibit 99.2

Special Note Regarding Forward-Looking Statements This presentation and the accompanying oral commentary contain forward-looking statements including statements related to Unity Biotechnology Inc.’s (“UNITY’s”) understanding of cellular senescence and the role it plays in diseases of aging, the potential for UNITY to develop therapeutics to slow, halt, or reverse diseases of aging, including for ophthalmologic and neurologic diseases, the potential for UNITY to successfully commence and complete clinical studies of UBX1325 for DME, AMD, and other ophthalmologic diseases, the expected timing of enrollment and results of the clinical trials in UBX1325, and UNITY’s expectations regarding the sufficiency of its cash runway. These statements involve substantial known and unknown risks, uncertainties, and other factors that may cause our actual results, levels of activity, performance, or achievements to be materially different from the information expressed or implied by these forward-looking statements, including the risk that the COVID-19 worldwide pandemic may continue to negatively impact the development of preclinical and clinical drug candidates, including delaying or disrupting the enrollment of patients in clinical trials, risks relating to the uncertainties inherent in the drug development process, including the risk that interim results of our clinical studies may not be indicative of future results, and risks relating to UNITY’s understanding of senescence biology. We may not actually achieve the plans, intentions, or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make. The forward-looking statements in this presentation represent our views as of the date of this release. We anticipate that subsequent events and developments will cause our views to change. However, while we may elect to update these forward-looking statements at some point in the future, we have no current intention of doing so except to the extent required by applicable law. You should, therefore, not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this release. For a further description of the risks and uncertainties that could cause actual results to differ from those expressed in these forward-looking statements, as well as risks relating to the business of UNITY in general, see UNITY’s most recent Annual Report on Form 10-K for the year ended December 31, 2022, filed with the Securities and Exchange Commission on March 15, 2023, as well as other documents that may be filed by UNITY from time to time with the Securities and Exchange Commission. This presentation concerns drug candidates that are under clinical investigation which have not yet been approved for marketing by the U.S. Food and Drug Administration. They are currently limited by Federal law to investigational use, and no representation is made as to their safety or effectiveness for the purposes for which they are being investigated. This presentation does not constitute an offer or invitation for the sale or purchase of securities and has been prepared solely for informational purposes.
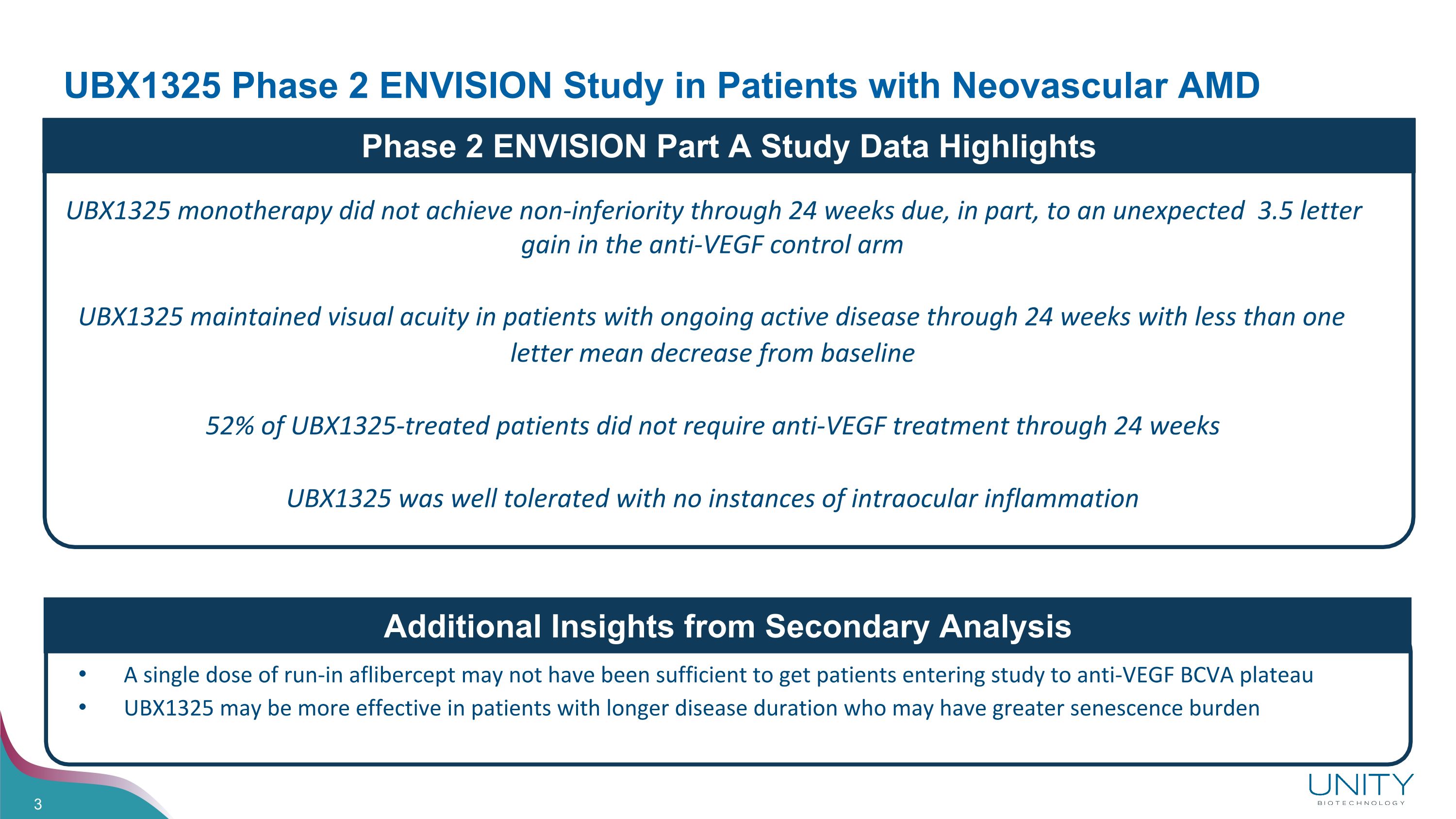
UBX1325 Phase 2 ENVISION Study in Patients with Neovascular AMD Additional Insights from Secondary Analysis UBX1325 monotherapy did not achieve non-inferiority through 24 weeks due, in part, to an unexpected 3.5 letter gain in the anti-VEGF control arm UBX1325 maintained visual acuity in patients with ongoing active disease through 24 weeks with less than one letter mean decrease from baseline 52% of UBX1325-treated patients did not require anti-VEGF treatment through 24 weeks UBX1325 was well tolerated with no instances of intraocular inflammation A single dose of run-in aflibercept may not have been sufficient to get patients entering study to anti-VEGF BCVA plateau UBX1325 may be more effective in patients with longer disease duration who may have greater senescence burden Phase 2 ENVISION Part A Study Data Highlights

Disease Pathophysiology UBX1325 Rationale and Mechanism of Action Clinical Development Plan
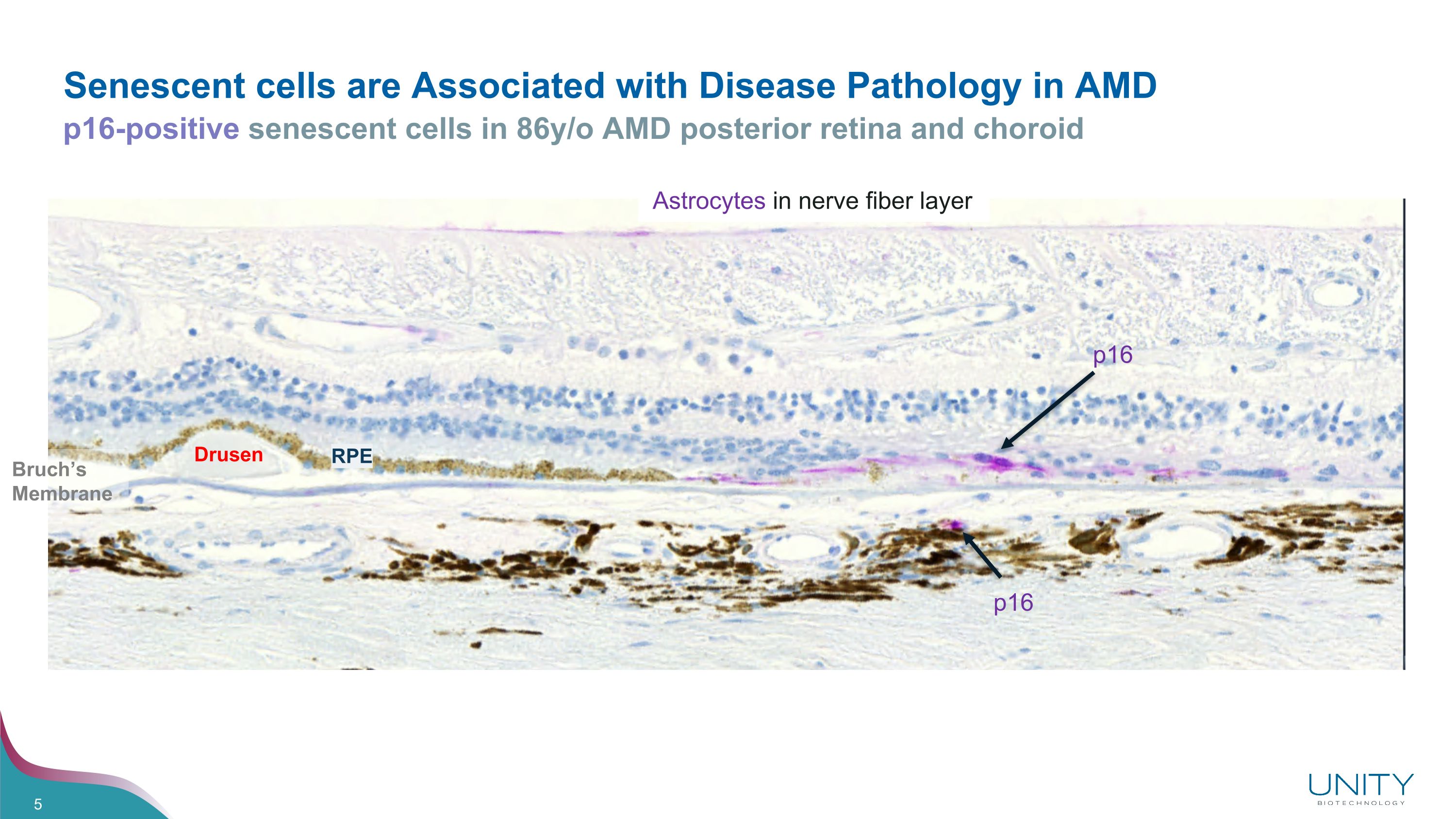
Senescent cells are Associated with Disease Pathology in AMD p16-positive senescent cells in 86y/o AMD posterior retina and choroid Drusen Astrocytes in nerve fiber layer RPE p16 p16 Bruch’s Membrane
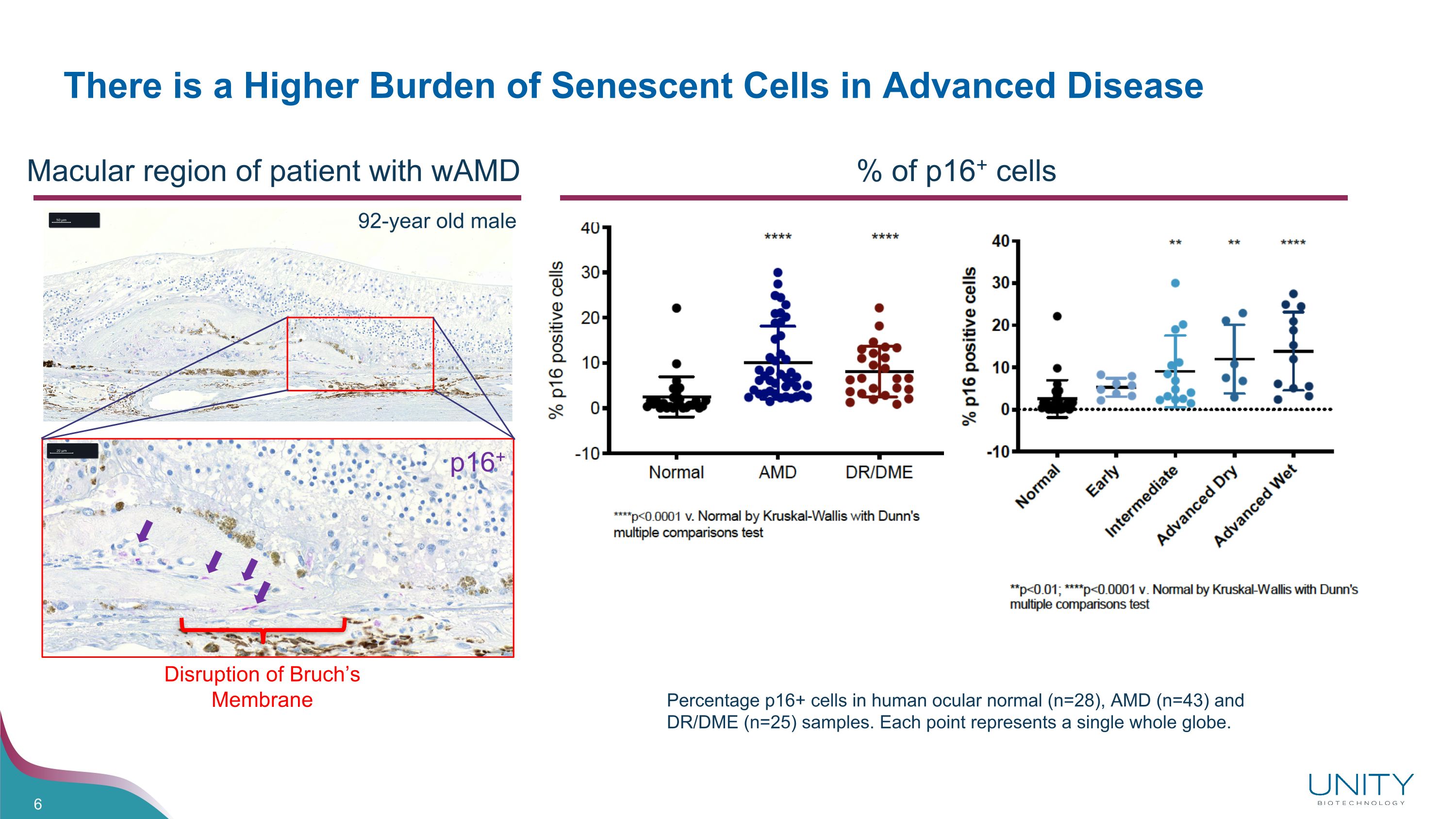
There is a Higher Burden of Senescent Cells in Advanced Disease Percentage p16+ cells in human ocular normal (n=28), AMD (n=43) and DR/DME (n=25) samples. Each point represents a single whole globe. % of p16+ cells Disruption of Bruch’s Membrane p16+ Macular region of patient with wAMD 92-year old male
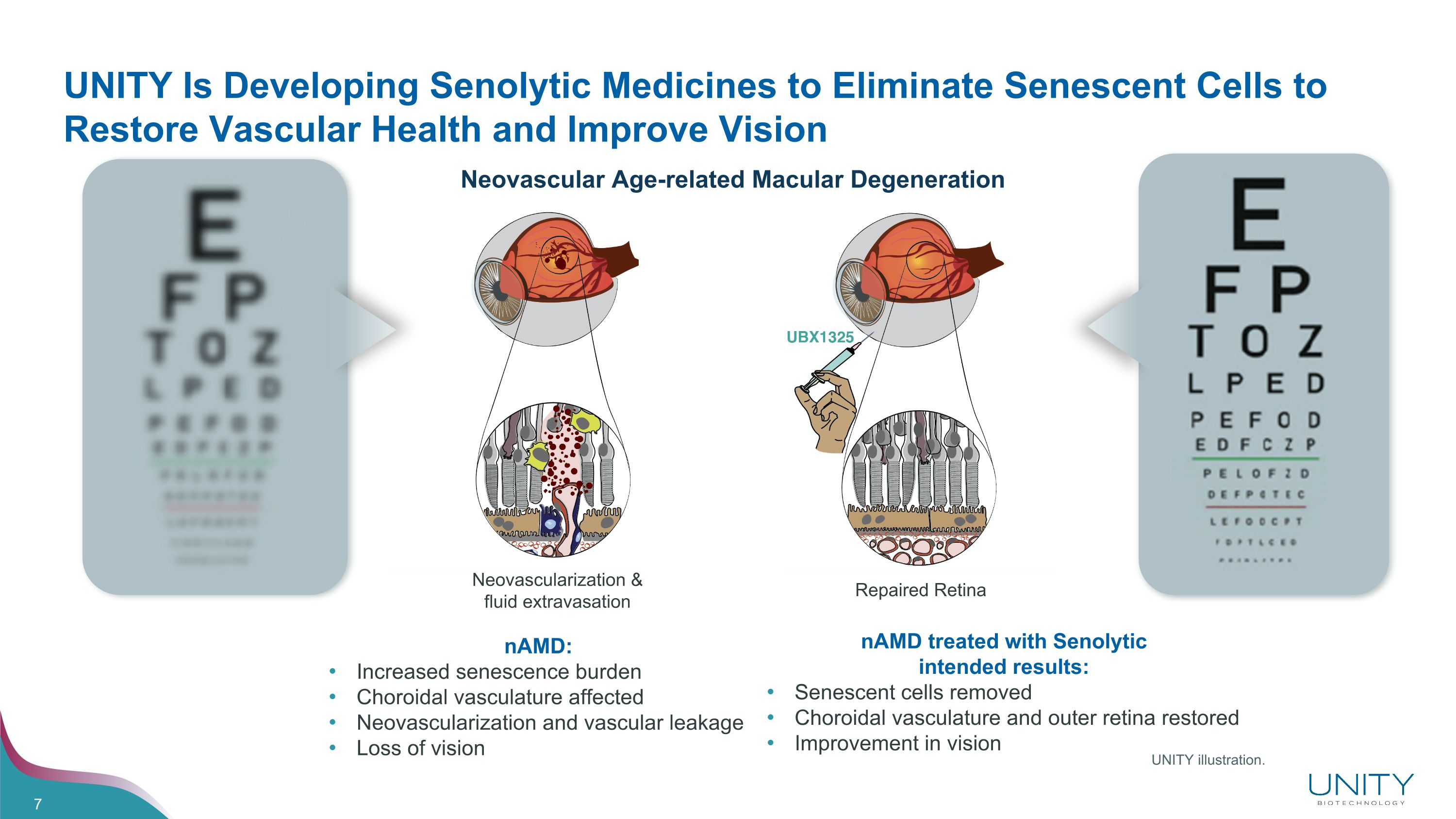
UNITY Is Developing Senolytic Medicines to Eliminate Senescent Cells to Restore Vascular Health and Improve Vision nAMD: Increased senescence burden Choroidal vasculature affected Neovascularization and vascular leakage Loss of vision nAMD treated with Senolytic intended results: Senescent cells removed Choroidal vasculature and outer retina restored Improvement in vision Neovascularization & fluid extravasation Repaired Retina UNITY illustration. Neovascular Age-related Macular Degeneration
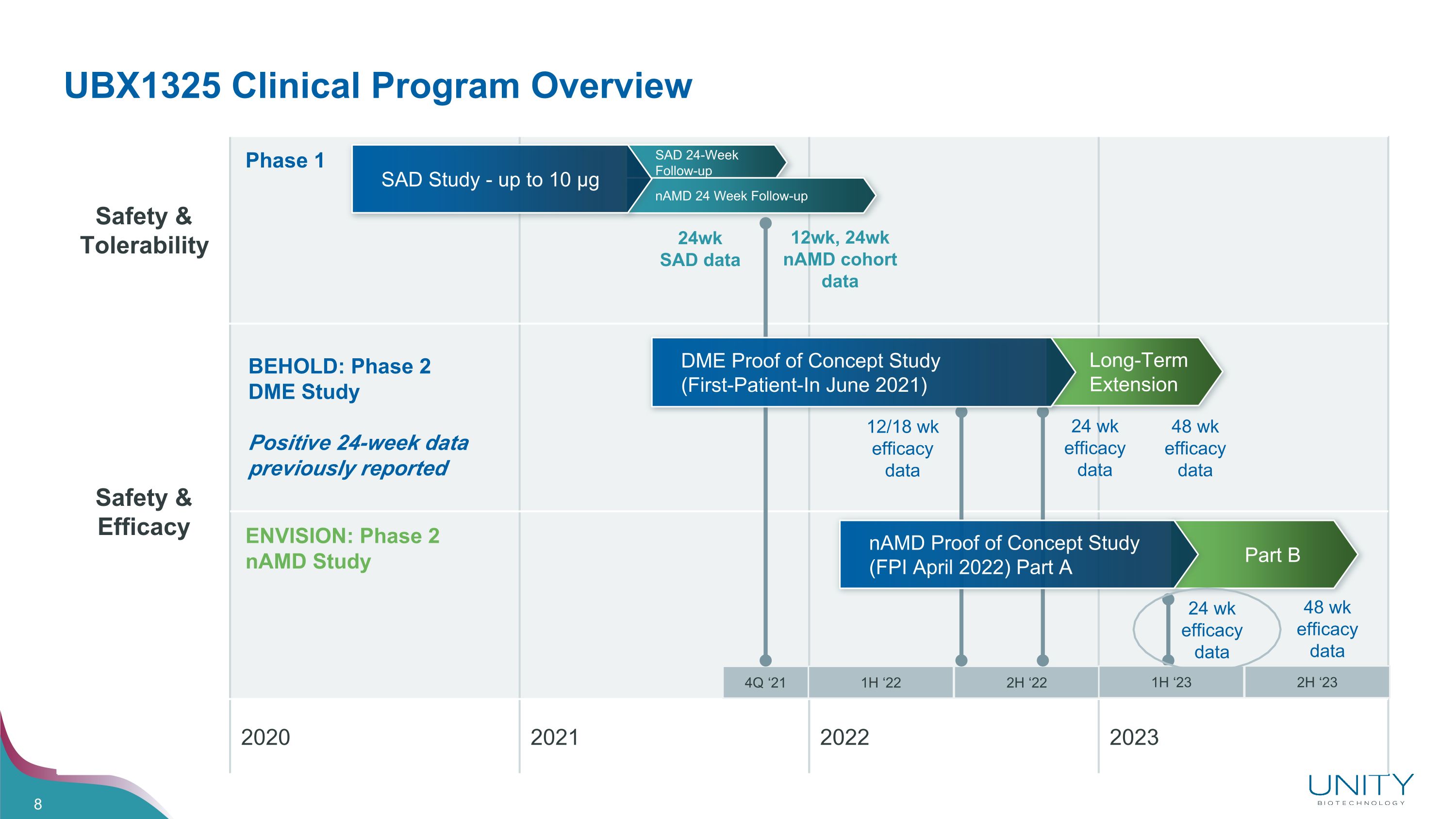
UBX1325 Clinical Program Overview Safety & Tolerability Safety & �Efficacy 2020 2021 2022 2023 Phase 1 ENVISION: Phase 2 nAMD Study BEHOLD: Phase 2 DME Study Positive 24-week data previously reported SAD 24-Week Follow-up nAMD 24 Week Follow-up SAD Study - up to 10 µg 4Q ‘21 1H ‘22 2H ‘22 Long-Term Extension DME Proof of Concept Study �(First-Patient-In June 2021) Part B nAMD Proof of Concept Study (FPI April 2022) Part A 12wk, 24wk nAMD cohort data 12/18 wk efficacy data 24 wk efficacy data 24wk SAD data 24 wk efficacy data 48 wk efficacy data 48 wk efficacy data 1H ‘23 2H ‘23

Clinical Context: Response of nAMD Patients to anti-VEGF Treatment
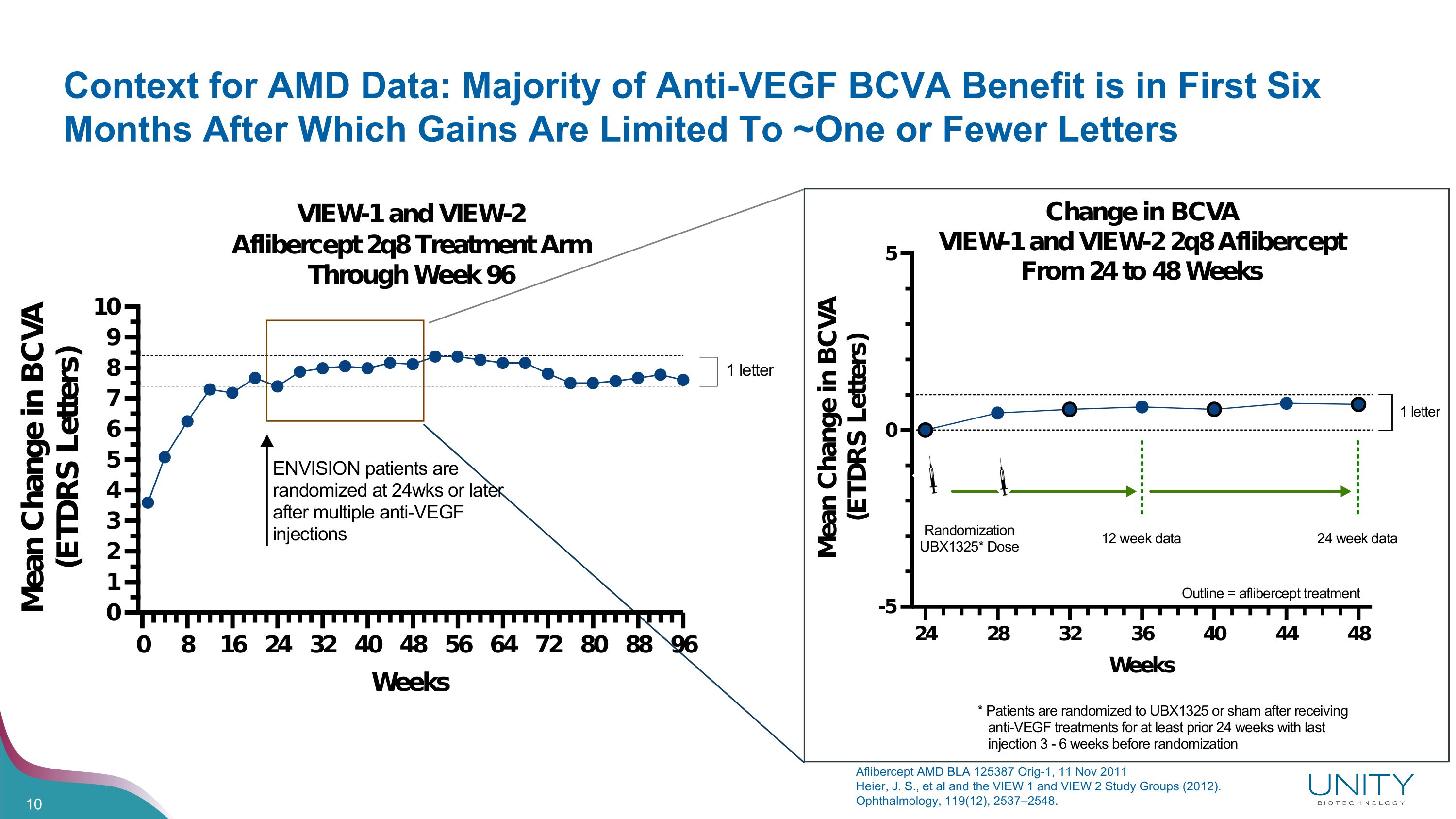
Context for AMD Data: Majority of Anti-VEGF BCVA Benefit is in First Six Months After Which Gains Are Limited To ~One or Fewer Letters Aflibercept AMD BLA 125387 Orig-1, 11 Nov 2011 Heier, J. S., et al and the VIEW 1 and VIEW 2 Study Groups (2012). Ophthalmology, 119(12), 2537–2548.

UBX1325 Phase 2 ENVISION Study 24-Week Data in Patients With nAMD 11
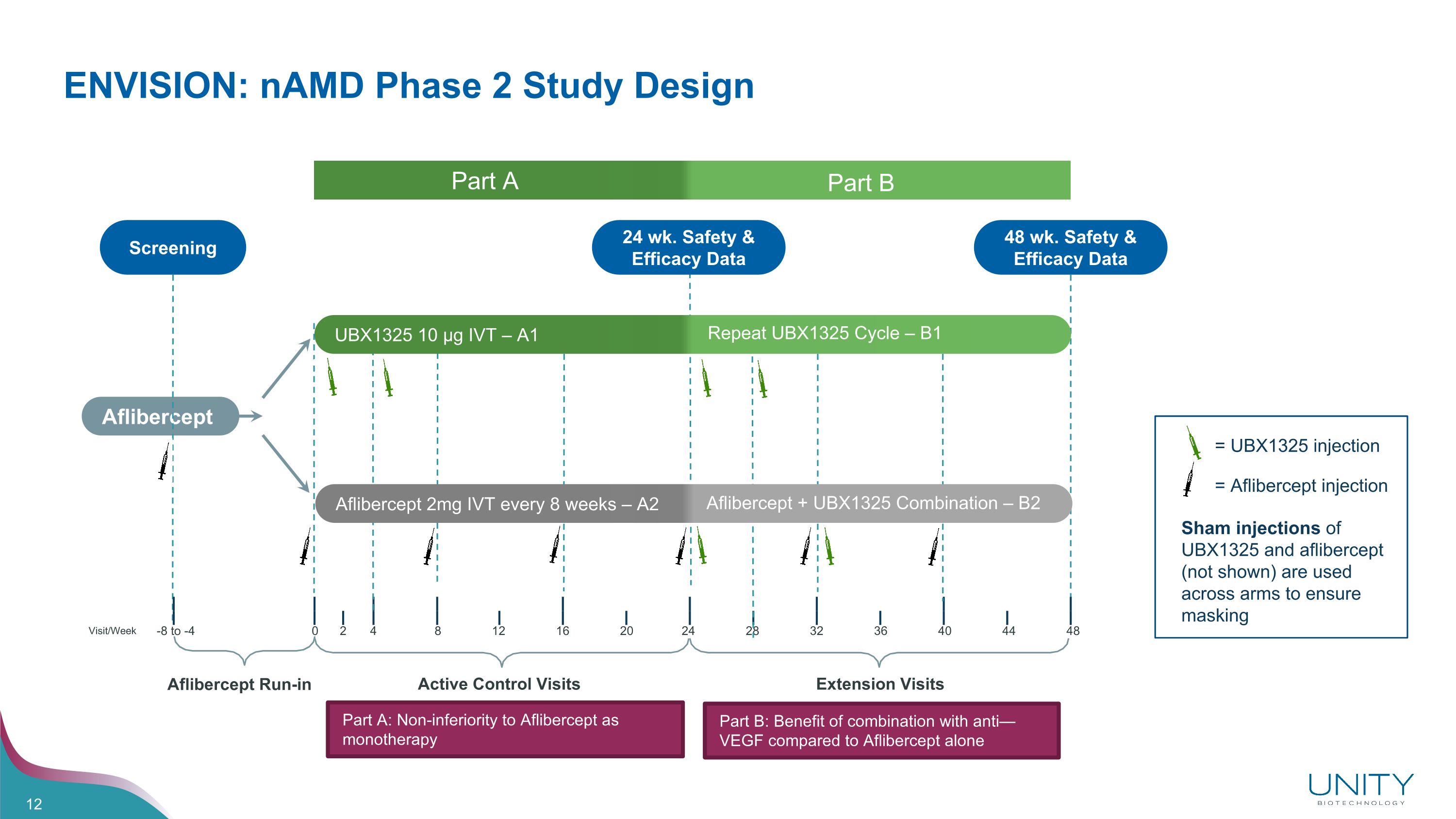
Aflibercept Run-in Active Control Visits ENVISION: nAMD Phase 2 Study Design 24 wk. Safety & Efficacy Data Aflibercept Screening = Aflibercept injection = UBX1325 injection 48 wk. Safety & Efficacy Data Extension Visits Visit/Week -8 to -4 0 2 4 8 12 16 20 24 28 32 36 40 44 48 Sham injections of UBX1325 and aflibercept (not shown) are used across arms to ensure masking Part A Part B UBX1325 10 µg IVT – A1 Repeat UBX1325 Cycle – B1 Aflibercept 2mg IVT every 8 weeks – A2 Aflibercept + UBX1325 Combination – B2 Part A: Non-inferiority to Aflibercept as monotherapy Part B: Benefit of combination with anti—VEGF compared to Aflibercept alone
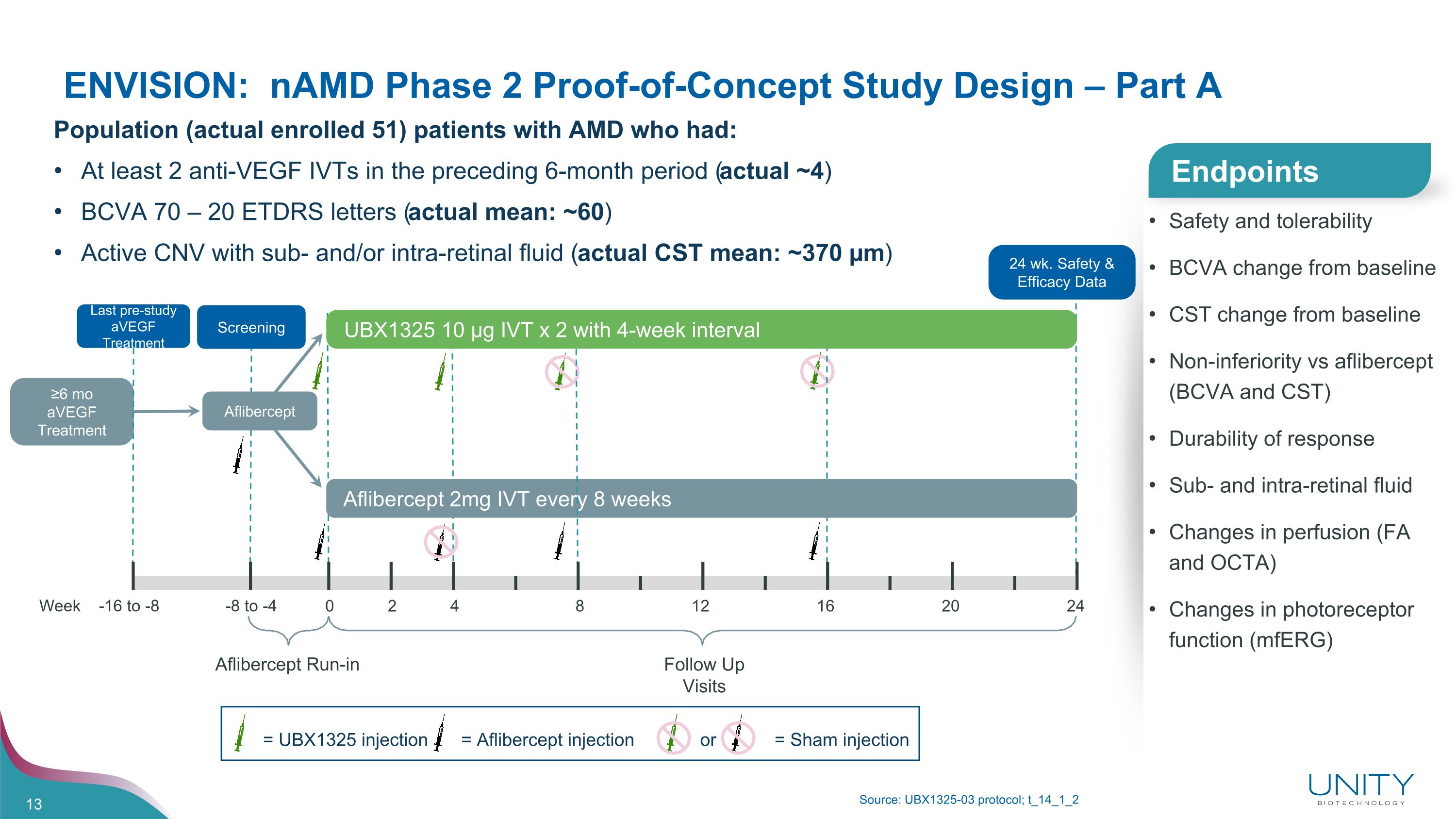
Week -16 to -8 -8 to -4 0 2 4 8 12 16 20 24 Aflibercept Run-in Follow Up Visits ENVISION: nAMD Phase 2 Proof-of-Concept Study Design – Part A Source: UBX1325-03 protocol; t_14_1_2 Safety and tolerability BCVA change from baseline CST change from baseline Non-inferiority vs aflibercept (BCVA and CST) Durability of response Sub- and intra-retinal fluid Changes in perfusion (FA and OCTA) Changes in photoreceptor function (mfERG) Endpoints Population (actual enrolled 51) patients with AMD who had: At least 2 anti-VEGF IVTs in the preceding 6-month period (actual ~4) BCVA 70 – 20 ETDRS letters (actual mean: ~60) Active CNV with sub- and/or intra-retinal fluid (actual CST mean: ~370 µm) = Aflibercept injection = Sham injection or = UBX1325 injection Screening UBX1325 10 µg IVT x 2 with 4-week interval Aflibercept 2mg IVT every 8 weeks 24 wk. Safety & Efficacy Data Aflibercept ≥6 mo aVEGF Treatment Last pre-study aVEGF Treatment
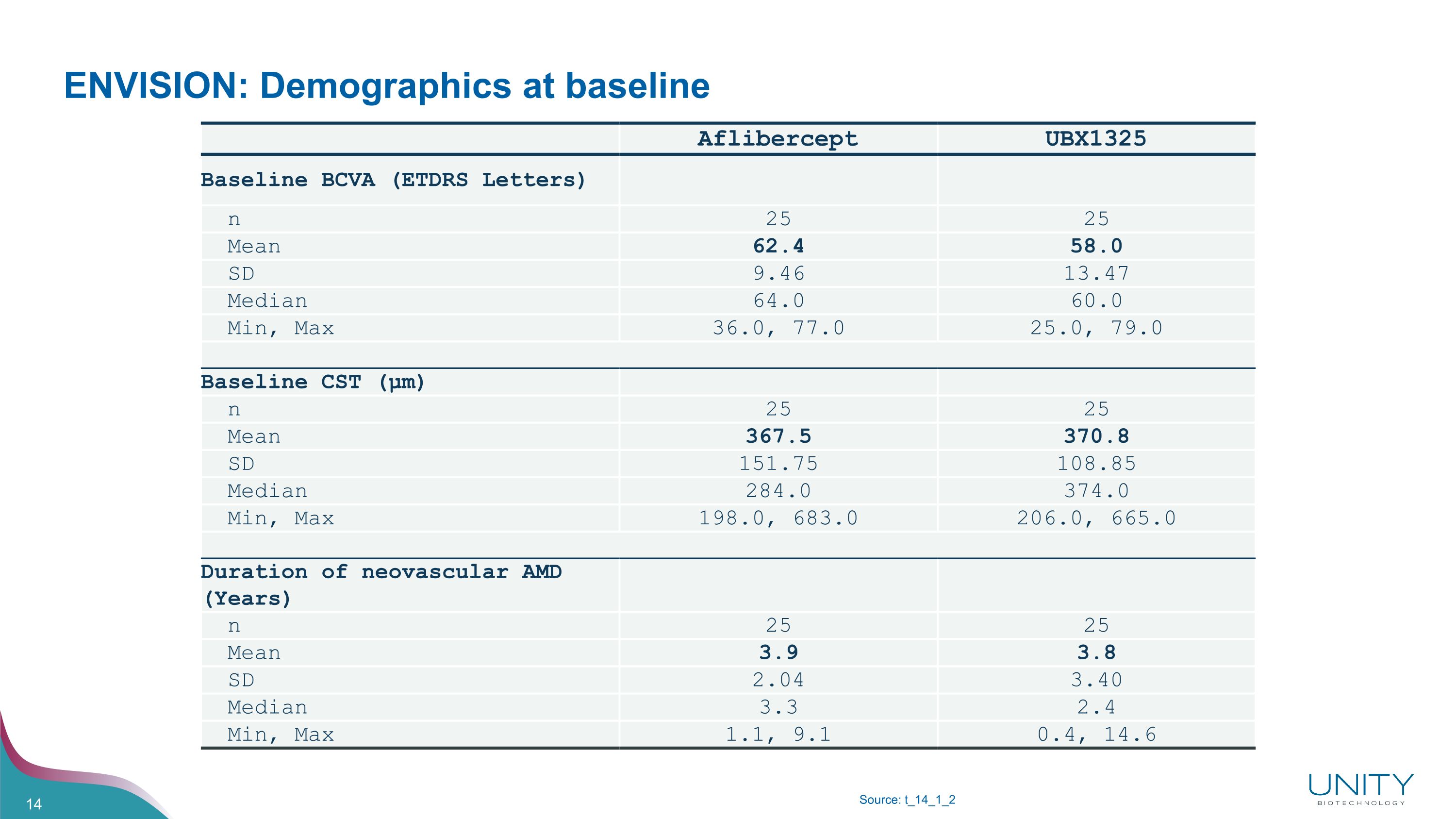
ENVISION: Demographics at baseline Source: t_14_1_2 Aflibercept UBX1325 Baseline BCVA (ETDRS Letters) n 25 25 Mean 62.4 58.0 SD 9.46 13.47 Median 64.0 60.0 Min, Max 36.0, 77.0 25.0, 79.0 Baseline CST (µm) n 25 25 Mean 367.5 370.8 SD 151.75 108.85 Median 284.0 374.0 Min, Max 198.0, 683.0 206.0, 665.0 Duration of neovascular AMD (Years) n 25 25 Mean 3.9 3.8 SD 2.04 3.40 Median 3.3 2.4 Min, Max 1.1, 9.1 0.4, 14.6
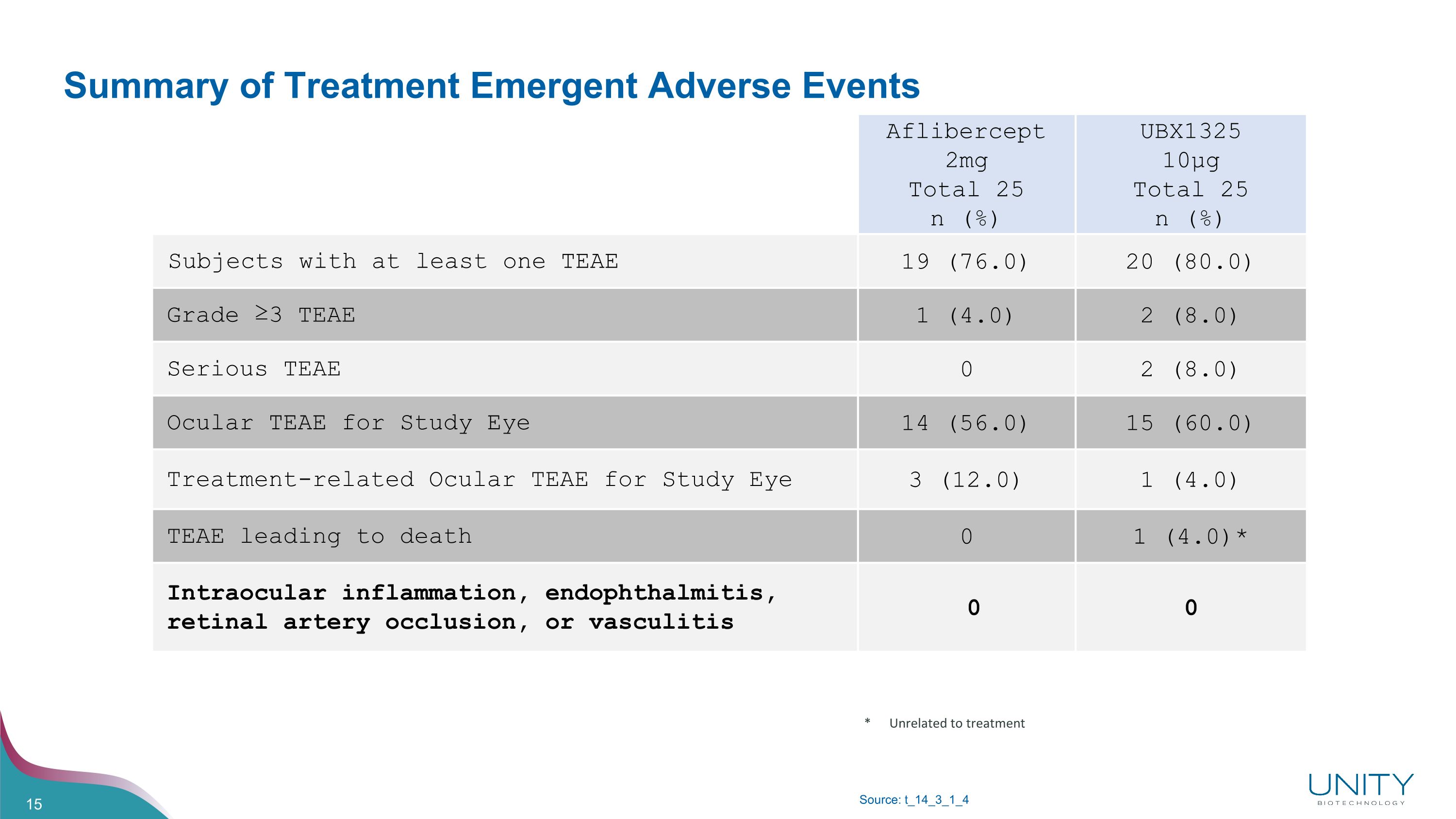
Summary of Treatment Emergent Adverse Events Source: t_14_3_1_4 * Unrelated to treatment Aflibercept 2mg�Total 25�n (%) UBX1325 10µg�Total 25�n (%) Subjects with at least one TEAE 19 (76.0) 20 (80.0) Grade ≥3 TEAE 1 (4.0) 2 (8.0) Serious TEAE 0 2 (8.0) Ocular TEAE for Study Eye 14 (56.0) 15 (60.0) Treatment-related Ocular TEAE for Study Eye 3 (12.0) 1 (4.0) TEAE leading to death 0 1 (4.0)* Intraocular inflammation, endophthalmitis, retinal artery occlusion, or vasculitis 0 0
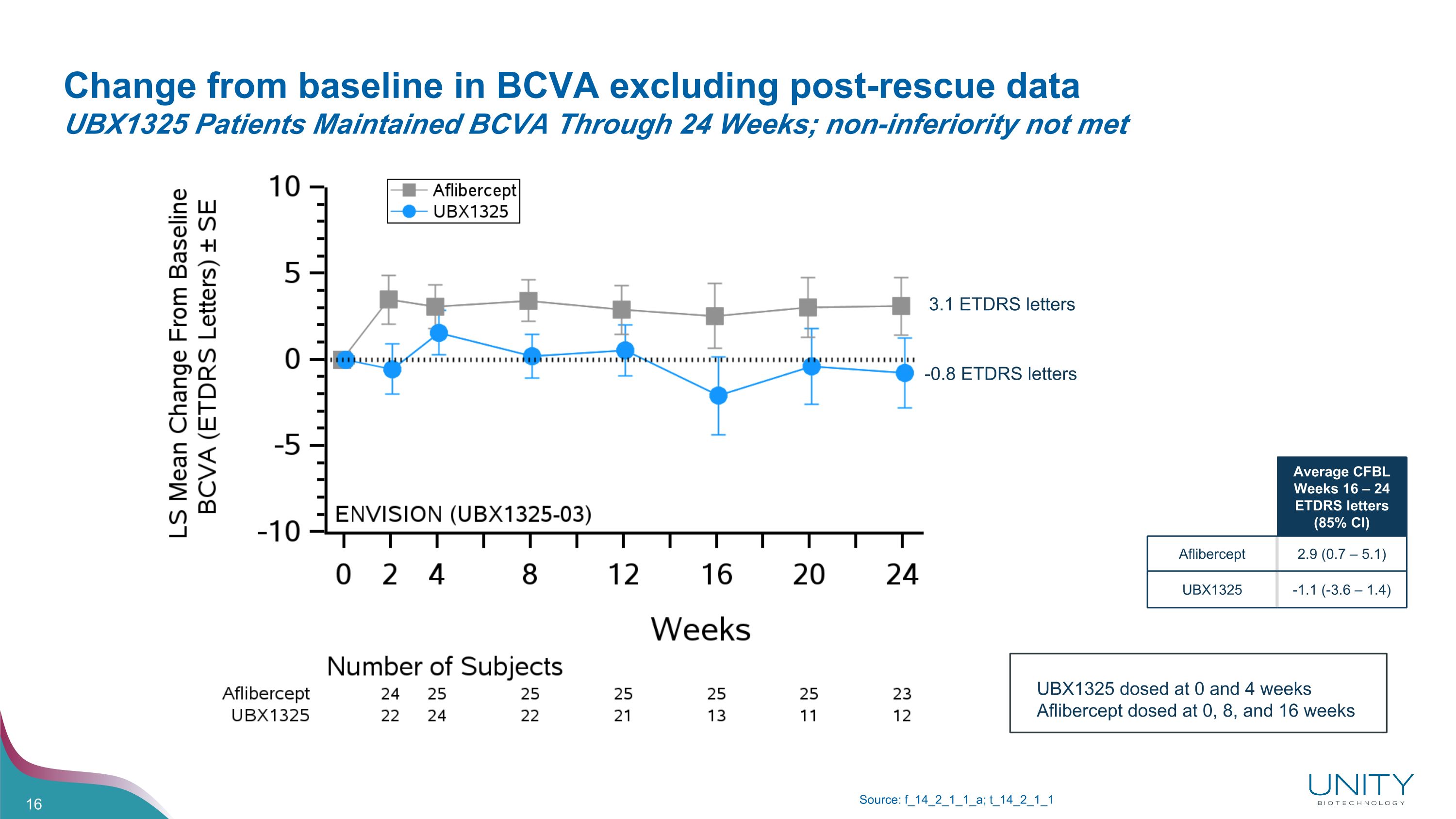
Change from baseline in BCVA excluding post-rescue data�UBX1325 Patients Maintained BCVA Through 24 Weeks; non-inferiority not met Source: f_14_2_1_1_a; t_14_2_1_1 3.1 ETDRS letters -0.8 ETDRS letters Average CFBL Weeks 16 – 24 ETDRS letters (85% CI) Aflibercept 2.9 (0.7 – 5.1) UBX1325 -1.1 (-3.6 – 1.4) UBX1325 dosed at 0 and 4 weeks Aflibercept dosed at 0, 8, and 16 weeks
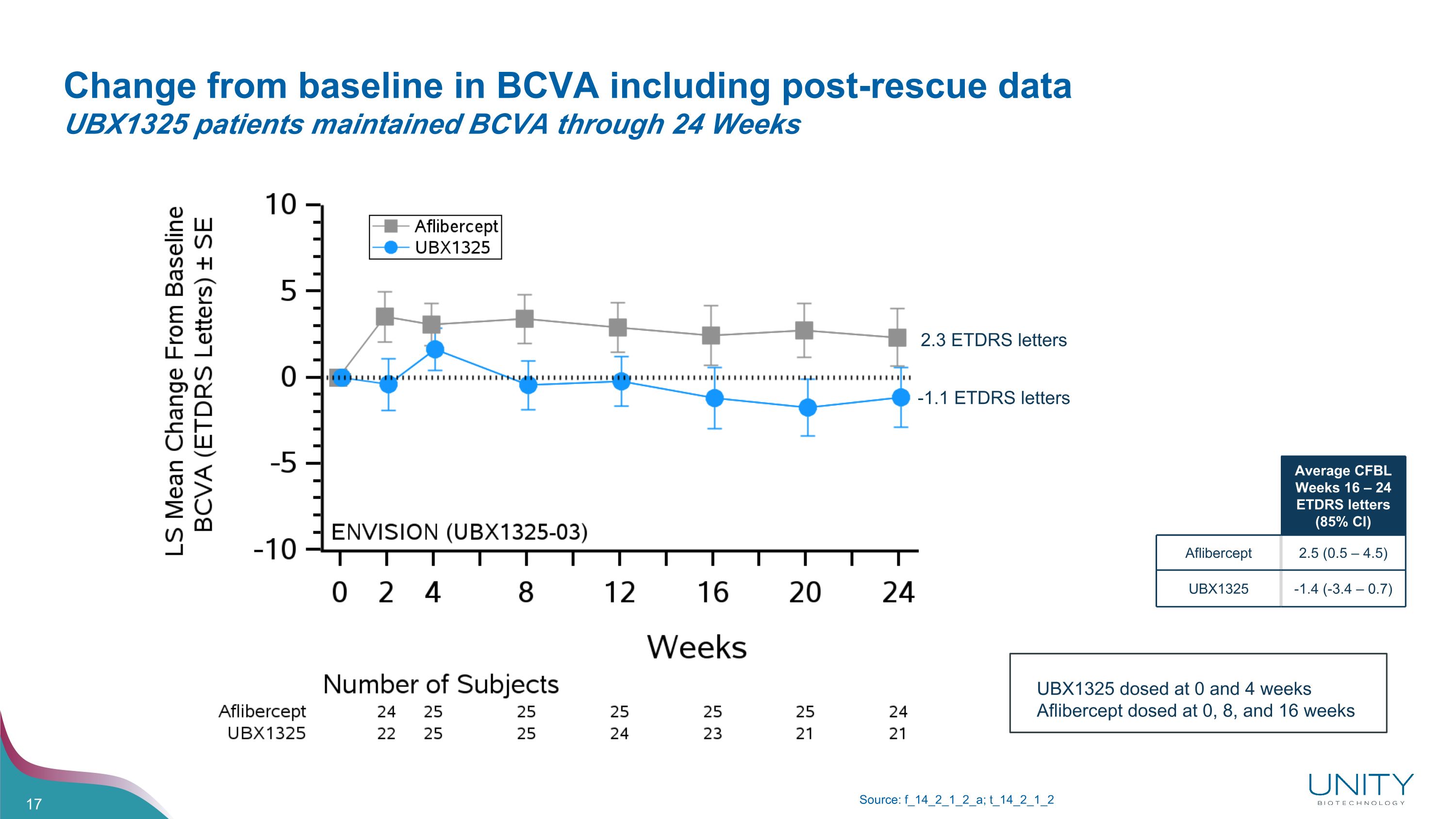
Change from baseline in BCVA including post-rescue data�UBX1325 patients maintained BCVA through 24 Weeks Source: f_14_2_1_2_a; t_14_2_1_2 Average CFBL Weeks 16 – 24 ETDRS letters (85% CI) Aflibercept 2.5 (0.5 – 4.5) UBX1325 -1.4 (-3.4 – 0.7) 2.3 ETDRS letters -1.1 ETDRS letters UBX1325 dosed at 0 and 4 weeks Aflibercept dosed at 0, 8, and 16 weeks
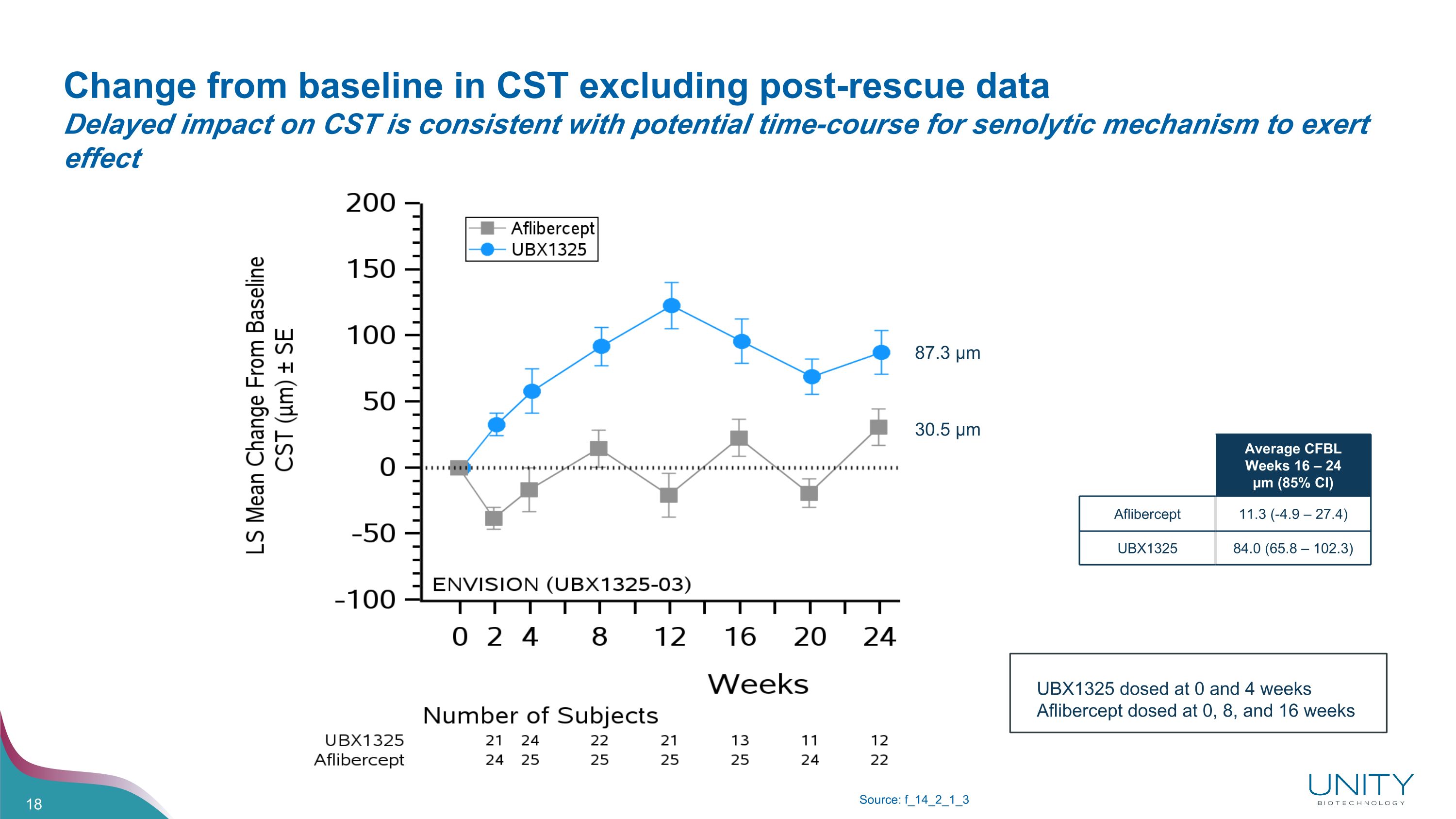
Change from baseline in CST excluding post-rescue data�Delayed impact on CST is consistent with potential time-course for senolytic mechanism to exert effect Source: f_14_2_1_3 Average CFBL Weeks 16 – 24 µm (85% CI) Aflibercept 11.3 (-4.9 – 27.4) UBX1325 84.0 (65.8 – 102.3) 87.3 µm 30.5 µm UBX1325 dosed at 0 and 4 weeks Aflibercept dosed at 0, 8, and 16 weeks
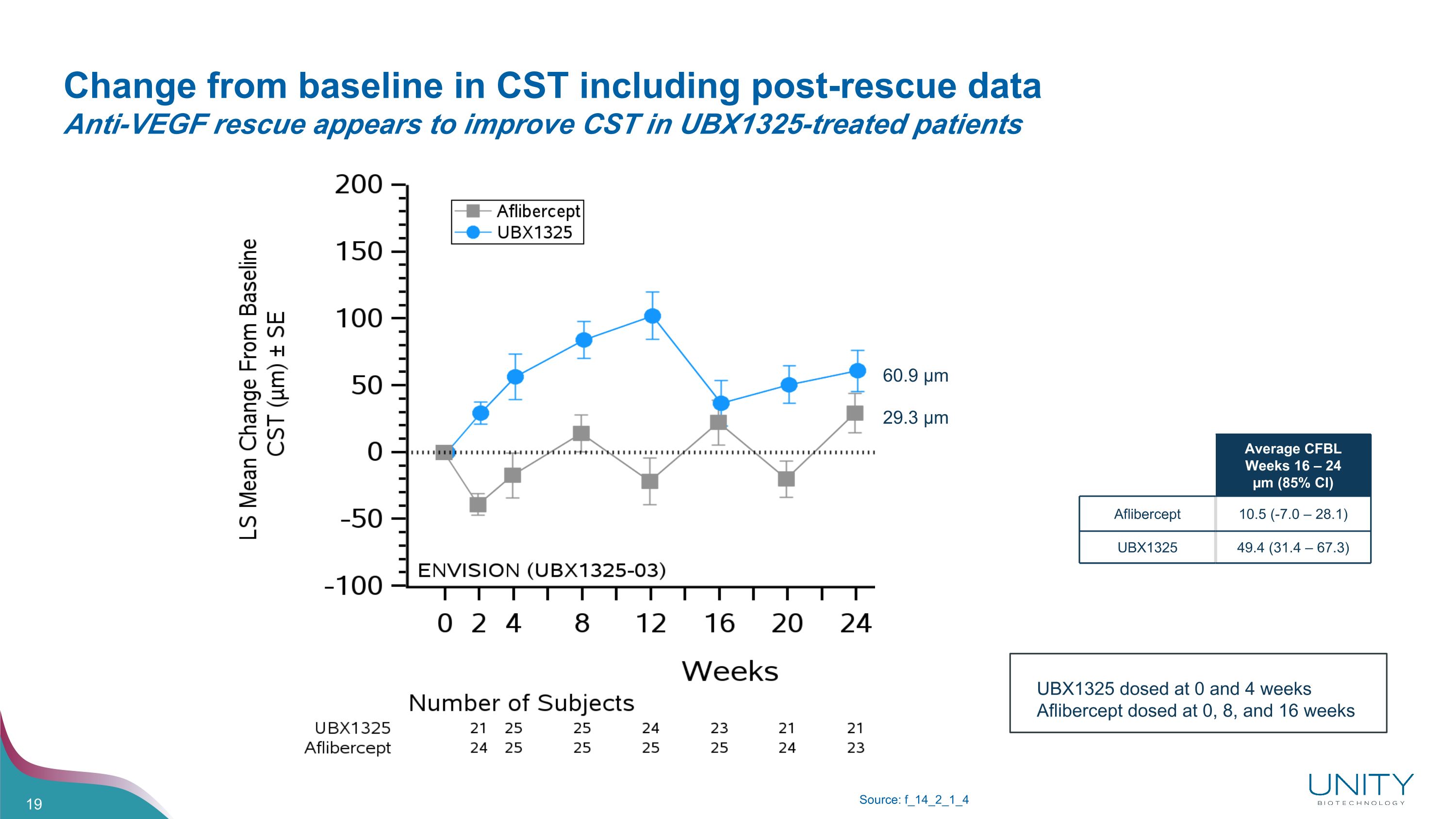
Change from baseline in CST including post-rescue data�Anti-VEGF rescue appears to improve CST in UBX1325-treated patients Source: f_14_2_1_4 Average CFBL Weeks 16 – 24 µm (85% CI) Aflibercept 10.5 (-7.0 – 28.1) UBX1325 49.4 (31.4 – 67.3) 60.9 µm 29.3 µm UBX1325 dosed at 0 and 4 weeks Aflibercept dosed at 0, 8, and 16 weeks
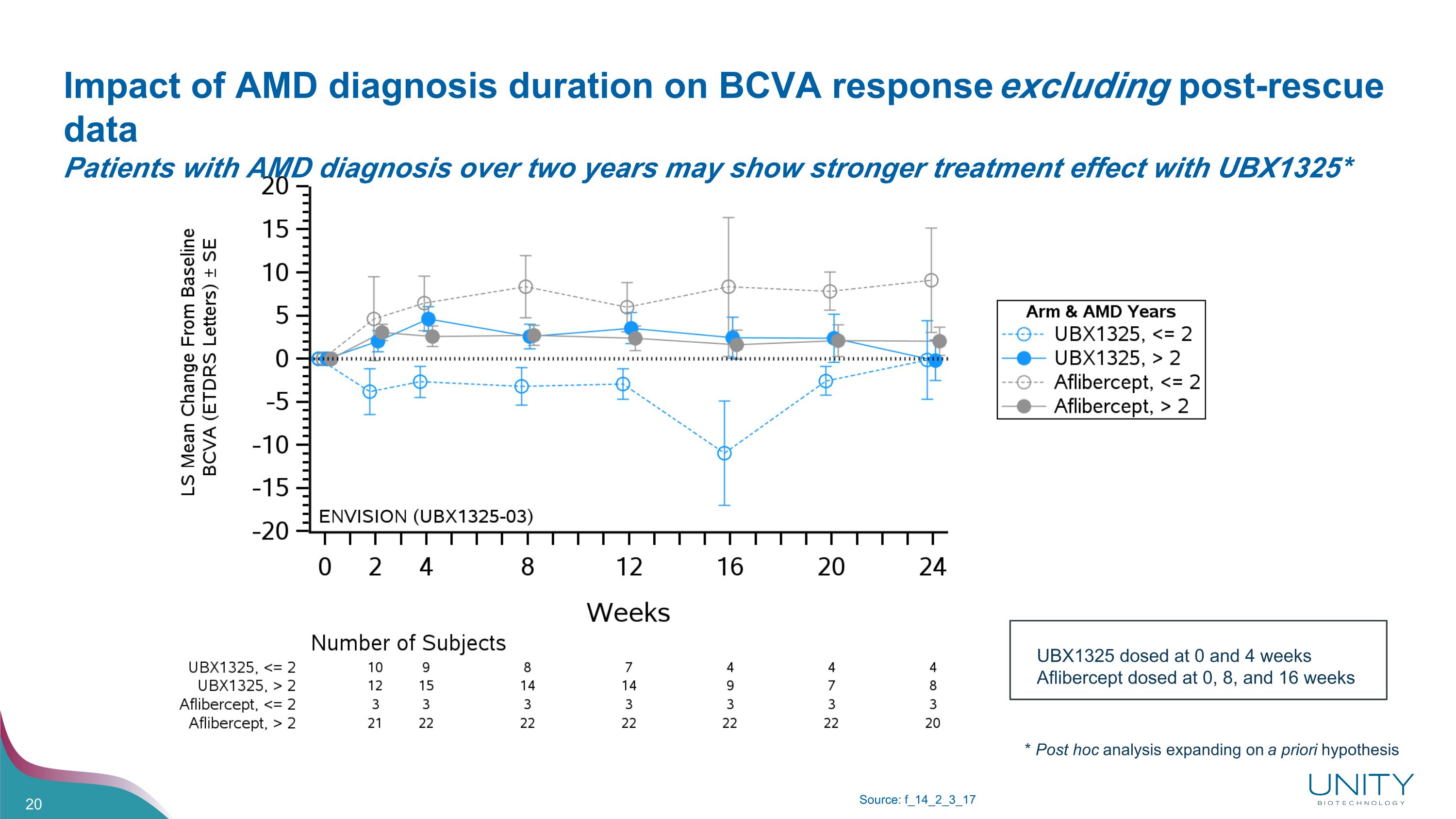
Impact of AMD diagnosis duration on BCVA response excluding post-rescue data�Patients with AMD diagnosis over two years may show stronger treatment effect with UBX1325* Source: f_14_2_3_17 UBX1325 dosed at 0 and 4 weeks Aflibercept dosed at 0, 8, and 16 weeks * Post hoc analysis expanding on a priori hypothesis
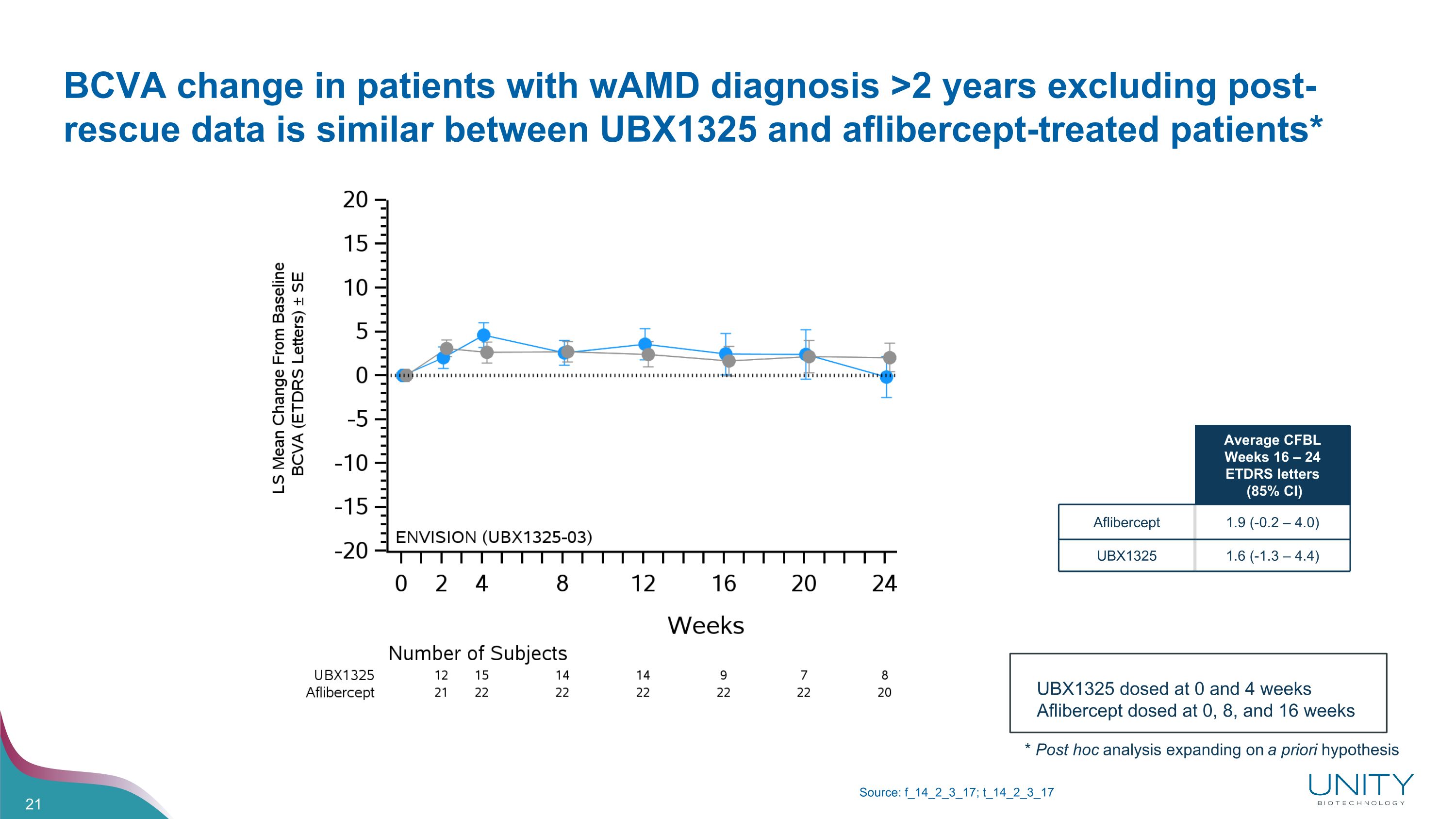
BCVA change in patients with wAMD diagnosis >2 years excluding post-rescue data is similar between UBX1325 and aflibercept-treated patients* Source: f_14_2_3_17; t_14_2_3_17 Average CFBL Weeks 16 – 24 ETDRS letters (85% CI) Aflibercept 1.9 (-0.2 – 4.0) UBX1325 1.6 (-1.3 – 4.4) UBX1325 dosed at 0 and 4 weeks Aflibercept dosed at 0, 8, and 16 weeks * Post hoc analysis expanding on a priori hypothesis
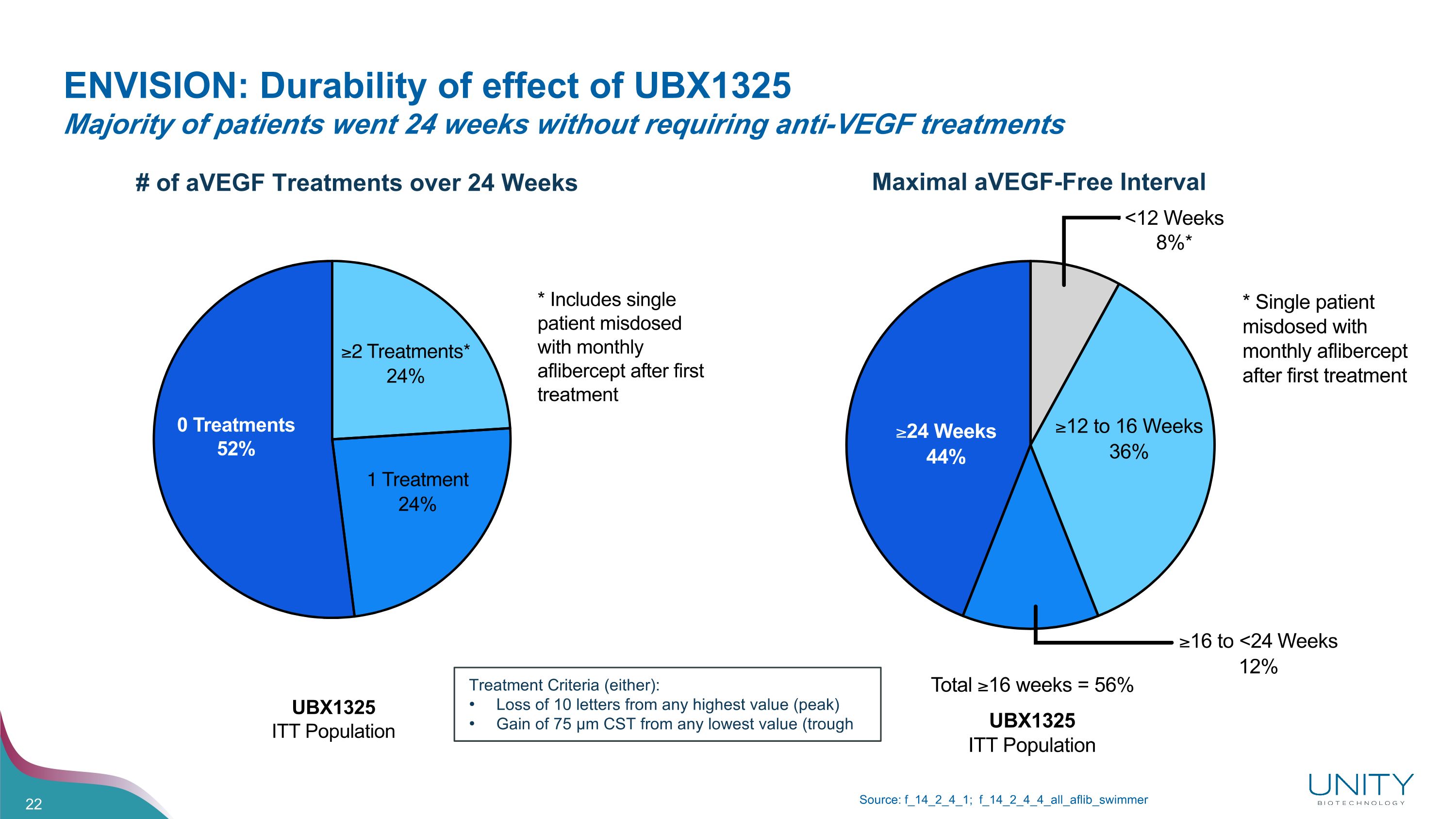
ENVISION: Durability of effect of UBX1325�Majority of patients went 24 weeks without requiring anti-VEGF treatments Source: f_14_2_4_1; f_14_2_4_4_all_aflib_swimmer Maximal aVEGF-Free Interval # of aVEGF Treatments over 24 Weeks Treatment Criteria (either): Loss of 10 letters from any highest value (peak) Gain of 75 µm CST from any lowest value (trough
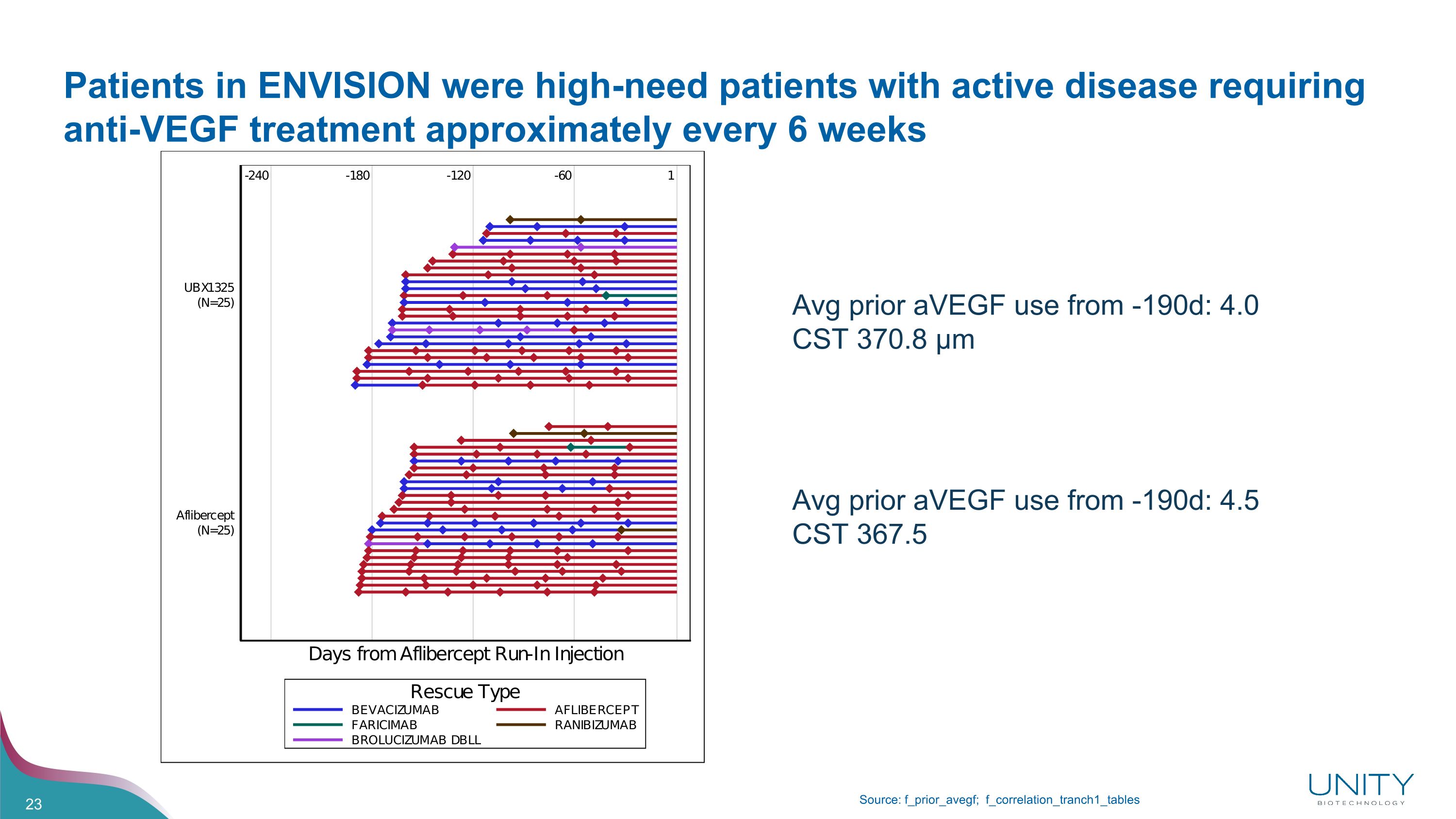
Patients in ENVISION were high-need patients with active disease requiring anti-VEGF treatment approximately every 6 weeks Source: f_prior_avegf; f_correlation_tranch1_tables Avg prior aVEGF use from -190d: 4.0 CST 370.8 µm Avg prior aVEGF use from -190d: 4.5 CST 367.5
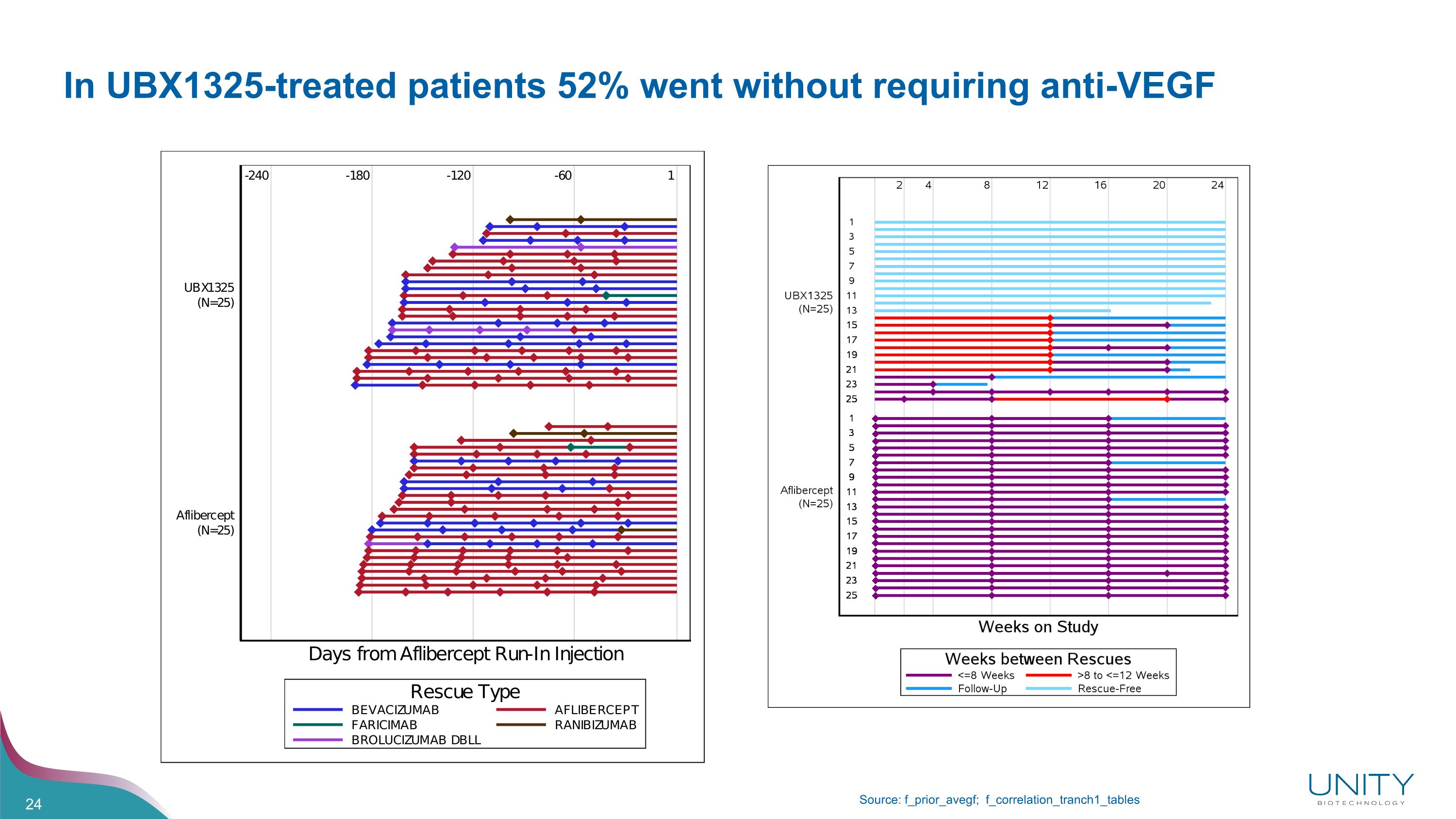
In UBX1325-treated patients 52% went without requiring anti-VEGF Source: f_prior_avegf; f_correlation_tranch1_tables
Summary of UBX1325 ENVISION Phase 2 Study in nAMD Was generally well tolerated with no intraocular inflammation Maintained visual acuity through 24 weeks in patients with active disease; there was a 3.5 letter gain in aflibercept arm at 2 weeks and non-inferiority to aflibercept was not met May have greater treatment effect in patients with AMD diagnosis greater than two years based on post-hoc assessments Allowed 52% of patients to avoid anti-VEGF treatment for at least 6 months @Vicki Can we make the this slide a little more punchy/less flat? UBX1325 In the ENVISION Study, UBX1325: Part B of ENVISION study is exploring the potential benefit of UBX1325 in combination with anti-VEGF
Summary and Development Plans for UBX1325 Additional Insights from Secondary Analysis UBX1325 monotherapy did not achieve non-inferiority through 24 weeks due, in part, to an unexpected 3.5 letter gain in the anti-VEGF control arm UBX1325 maintained visual acuity in patients with ongoing active disease through 24 weeks with less than one letter mean decrease from baseline 52% of UBX1325-treated patients did not require anti-VEGF treatment through 24 weeks UBX1325 was well tolerated with no instances of intraocular inflammation A single dose of run-in aflibercept may not have been sufficient to get patients entering study to anti-VEGF BCVA plateau UBX1325 may be more effective in patients with longer disease duration who may have greater senescence burden Phase 2 ENVISION Part A Study Data Highlights Company to share 48-week BEHOLD DME data in April and intends to initiate Phase 2b study in DME in second half of 2023

Q&A 27


























