UNITED STATES |
SECURITIES AND EXCHANGE COMMISSION |
Washington, D.C. 20549 |
|
SCHEDULE 14A |
|
Proxy Statement Pursuant to Section 14(a) of
the Securities Exchange Act of 1934 (Amendment No. ) |
|
Filed by the Registrant o |
|
Filed by a Party other than the Registrant x |
|
Check the appropriate box: |
o | Preliminary Proxy Statement |
o | Confidential, for Use of the Commission Only (as permitted by Rule 14a-6(e)(2)) |
o | Definitive Proxy Statement |
o | Definitive Additional Materials |
x | Soliciting Material under §240.14a-12 |
|
CORNERSTONE THERAPEUTICS INC. |
(Name of Registrant as Specified In Its Charter) |
|
CHIESI FARMACEUTICI S.P.A. CHIESI U.S. CORPORATION |
(Name of Person(s) Filing Proxy Statement, if other than the Registrant) |
|
Payment of Filing Fee (Check the appropriate box): |
x | No fee required. |
o | Fee computed on table below per Exchange Act Rules 14a-6(i)(1) and 0-11. |
| (1) | Title of each class of securities to which transaction applies: |
| | |
| (2) | Aggregate number of securities to which transaction applies: |
| | |
| (3) | Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0-11 (set forth the amount on which the filing fee is calculated and state how it was determined): |
| | |
| (4) | Proposed maximum aggregate value of transaction: |
| | |
| (5) | Total fee paid: |
| | |
o | Fee paid previously with preliminary materials. |
o | Check box if any part of the fee is offset as provided by Exchange Act Rule 0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the Form or Schedule and the date of its filing. |
| (1) | Amount Previously Paid: |
| | |
| (2) | Form, Schedule or Registration Statement No.: |
| | |
| (3) | Filing Party: |
| | |
| (4) | Date Filed: |
| | |
| | | |
This filing consists of:
1. Script for Video Presentation Prepared by Chiesi Farmaceutici S.p.A. to be Presented to Employees of Cornerstone Therapeutics Inc.
2. Powerpoint presentation delivered by Ugo Di Francesco, Chief Executive Officer of Chiesi Farmaceutici S.p.A., to Employees of Cornerstone Therapeutics Inc., regarding the Chiesi Group Profile
3. Powerpoint presentation delivered by Ugo Bettini, Head of Human Resources of Chiesi Farmaceutici S.p.A., to Employees of Cornerstone Therapeutics Inc., regarding Human Resources matters
Script for Video Presentation Prepared by Chiesi Farmaceutici S.p.A. to be Presented to Employees of Cornerstone Therapeutics Inc.
Alberto Chiesi - Chairman
Ladies and gentlemen,
I would like to express my warm ‘welcome’ to each of you and to your colleagues. This is an exciting day for all of us, and I am confident that this merger will lead us to achieve brilliant results. Today Chiesi and Cornerstone are moving forward together with a great sense of pride in what they expect to be able to achieve in the U.S. market.
Paolo Chiesi - Vice-Chairman and R&D Director
Ladies and gentleman,
I also wish to express my satisfaction for this transaction and my welcome to each of you. Together, we will advance Chiesi’s international development plans and take a significant step towards strengthening the Chiesi Group’s presence in the U.S. This merger will create a foundation for new projects in the area of respiratory, special care and orphan diseases and will provide Cornerstone access to all products that Chiesi intends to commercialize in the U.S.
Alberto Chiesi
We are confident that, with the support of the Chiesi Group and with the commitment of all of you, Cornerstone will help fulfill its mission to develop and commercialize innovative pharmaceutical solutions to improve the quality of human life. Chiesi has a strong product pipeline, including promising molecules for the treatment of respiratory, special care and orphan diseases, and will continue to develop its pipeline as it consolidates its presence in the U.S. We look forward to starting up a close co-operation program to achieve long term success.
Paolo Chiesi
We know that everyone at Cornerstone will put their skills and their enthusiasm to work and help achieve market leadership in our therapeutic areas of interest. Cornerstone’s employees are one of the primary reasons we believe this transaction is compelling. As a team, we know we have the competencies and the determination to reach this target, and our personal wish to each of you is to capitalize on this new opportunity. We look forward to officially welcoming you to the Chiesi family upon completion of the transaction. Thank you all for listening today.
Powerpoint presentation delivered by Ugo Di Francesco, Chief Executive Officer of Chiesi Farmaceutici S.p.A., to Employees of Cornerstone Therapeutics Inc., regarding the Chiesi Group Profile

| Chiesi Group Profile |

| 1 Our Mission Our aim is to be recognised as a research-focused international Group, able to develop and commercialise innovative pharmaceutical solutions to improve the quality of human life. We wish to maintain a high quality entrepreneurial team characterised by self confidence and a collaborative spirit. Our goal is to combine commitment to results with integrity, operating in a socially and environmentally responsible manner. |
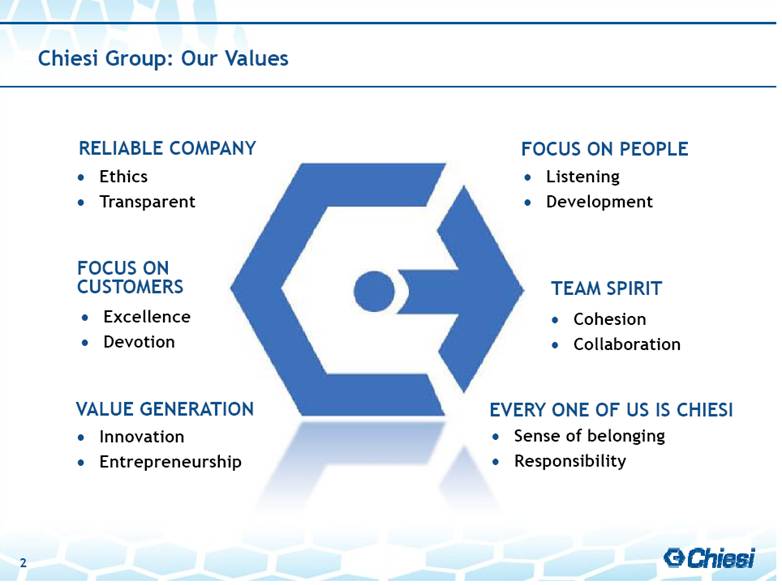
| 2 Chiesi Group: Our Values FOCUS ON PEOPLE Listening Development Excellence Devotion FOCUS ON CUSTOMERS Sense of belonging Responsibility EVERY ONE OF US IS CHIESI RELIABLE COMPANY Ethics Transparent Cohesion Collaboration TEAM SPIRIT VALUE GENERATION Innovation Entrepreneurship |
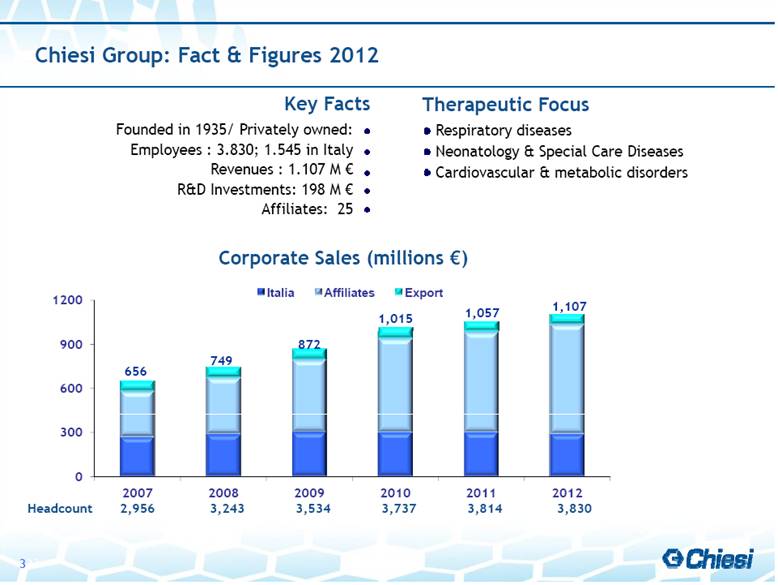
| 656 749 872 1,015 Chiesi Group: Fact & Figures 2012 Founded in 1935/ Privately owned: Employees : 3.830; 1.545 in Italy Revenues : 1.107 M € R&D Investments: 198 M € Affiliates: 25 Respiratory diseases Neonatology & Special Care Diseases Cardiovascular & metabolic disorders Key Facts Therapeutic Focus Corporate Sales (millions €) 1,057 1,107 3 Headcount 2,956 3,243 3,534 3,737 3,814 3,830 |

| 4 The Strategic View Focus Research & Development, with high level investments Development of Chiesi proprietary respiratory technologies World-wide Experience and expertise in the development of respiratory products are a core asset for the growth of the Group Respiratory The therapeutic unmet needs and the social impact of rare diseases are at the origin of the Group’s effort in this area Special Care |

| Chiesi In Europe |
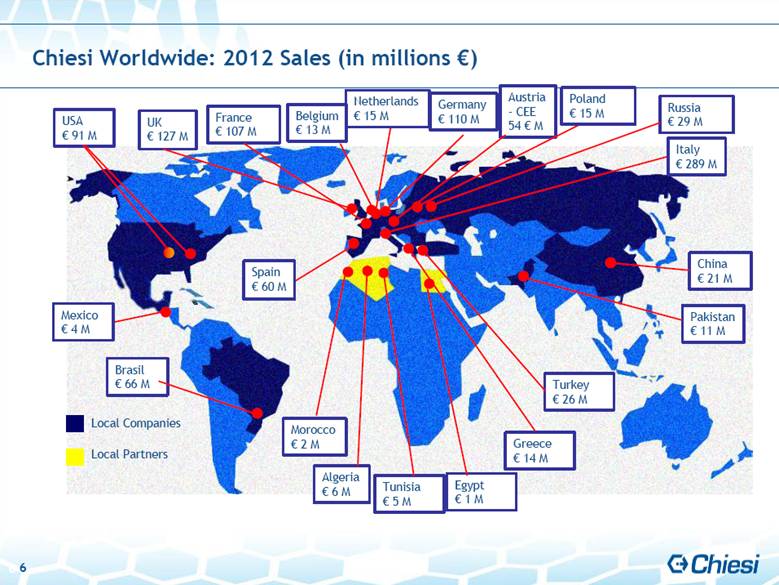
| 6 Chiesi Worldwide: 2012 Sales (in millions €) Local Companies Local Partners Russia € 29 M Germany € 110 M France € 107 M UK € 127 M Italy € 289 M Austria - CEE 54 € M Netherlands € 15 M USA € 91 M Brasil € 66 M Pakistan € 11 M China € 21 M Turkey € 26 M Greece € 14 M Egypt € 1 M Tunisia € 5 M Algeria € 6 M Morocco € 2 M Spain € 60 M Belgium € 13 M Poland € 15 M Mexico € 4 M |

| 7 Respiratory 55% Cardiovascolar 16% Musculoskeletal 5% Neonatology 13% Central Nervous System 2% Cystic Fibrosis 2% Others 7 % Chiesi Group- Sales By Therapeutic Area 2012 |

| 2004 Atimos EU 1993 Curosurf EU 1996 Iperten EU 2000 Curosurf US (partner) 2006 Foster EU 2001 Budiair EU 2007 Bramitob EU 1,107 2012 2011 Peyona International Growth & Corporate Products The geographical expansion process evolved with the development and launch of the new products 1,000 8 |

| 9 Our Main Products |
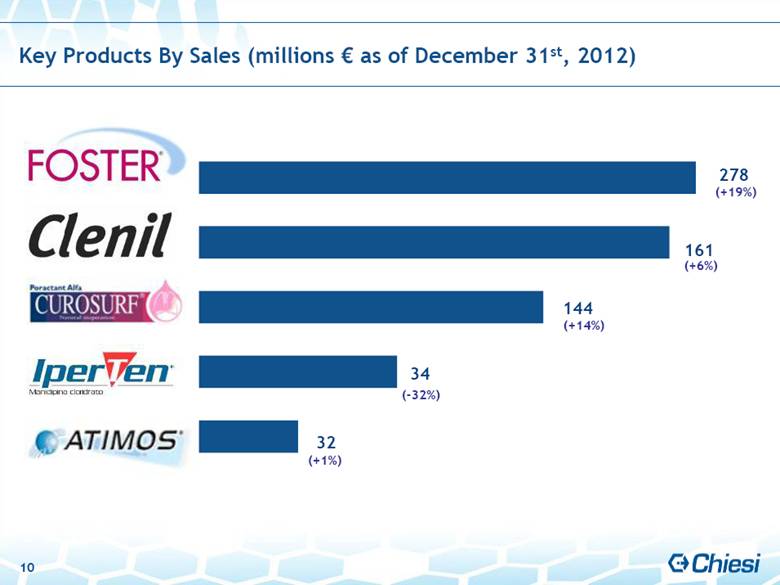
| 144 Key Products By Sales (millions € as of December 31st, 2012) (+14%) 161 34 32 278 (+6%) (+19%) (+1%) (-32%) 10 |

| 11 * BRIC = Brazil, Russia, India, China Commercial Capabilities Direct European and BRIC* presence, marketing expertise and Key Opinion Leaders networking Development Capabilities Technical, clinical, regulatory and manufacturing expertise Partnership Management Quick decision-making Chiesi Partnerships |

| Research & Development |
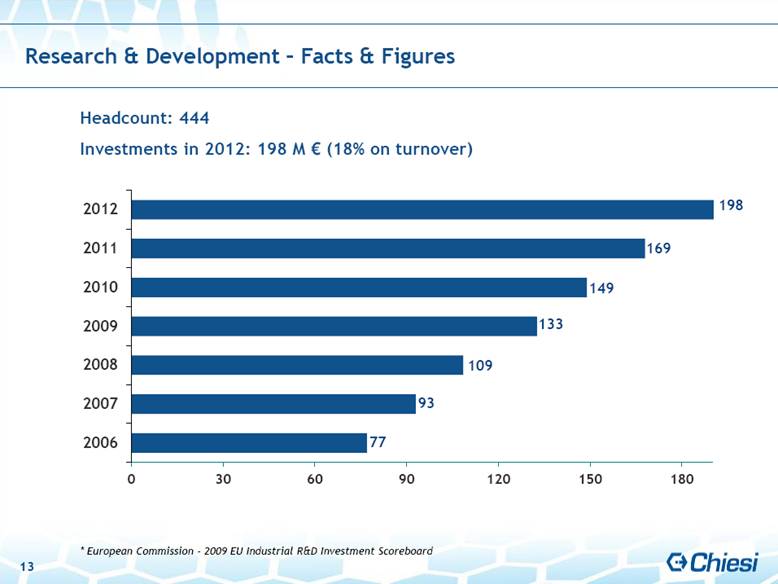
| Research & Development – Facts & Figures Headcount: 444 Investments in 2012: 198 M € (18% on turnover) * European Commission - 2009 EU Industrial R&D Investment Scoreboard 149 133 109 93 77 198 169 13 |

| 14 4 R&D sites: * European Commission - 2012 EU Industrial R&D Investment Scoreboard Investments in R&D 1st among the Italian pharmaceutical companies* 8th among the Italian companies* 12th among the European pharmaceutical companies* Parma (Italy) Paris (France) Rockville (USA) Chippenham (UK) Research & Development - International Sites |

| R&D Pipeline Now Includes Three Key Areas Respiratory Neonatology Other, Special Care R&D Pipeline |
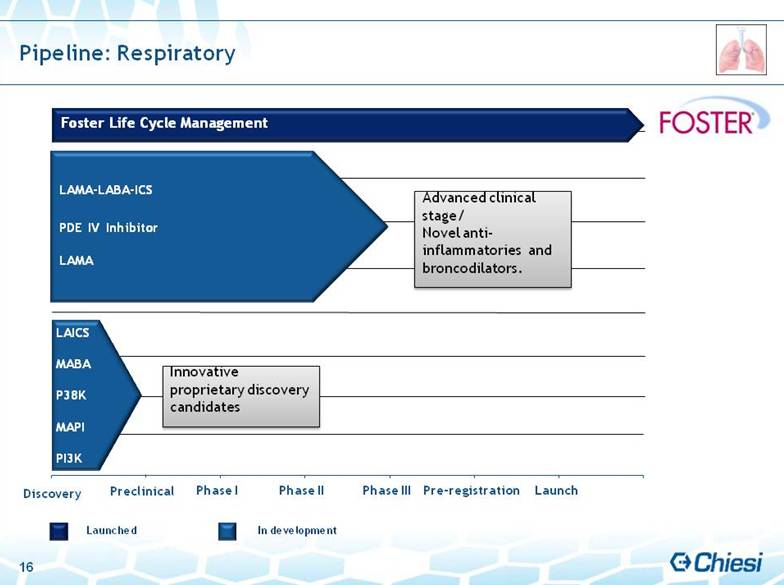
| 16 Discovery Preclinical Phase I Phase II Phase III Pre-registration Launch Pipeline: Respiratory Launched In development LAMA-LABA-ICS PDE IV Inhibitor LAMA Innovative proprietary discovery candidates Foster Life Cycle Management Advanced clinical stage/Novel anti-inflammatories and broncodilators. LAICS MABA P38K MAPI PI3K |

| 17 Synthetic Surfactant Discovery Preclinical Phase I Phase II Phase III Pre-registration Launch Caspase Inhibitor Peyona Lon Pipeline: Neonatology Launched In development Curosurf Life Cycle Management Peyona Budiair |

| 18 Discovery Preclinical Phase I Phase II Phase III Pre-registration Launch Launched In development Bramitob Life Cycle Management Holoclar LCP- Tacro Alpha1 antitrypsin Microglia Modulator In partnership with In partnership with In partnership with Pipeline: Special Care / Other |

| Research Center (Parma) Launched: October 2011 19 Research Center Awards and recognitions Facility of The Year 2012 for sustainability. Architecture Award "Rizzardi Polini” 2012. Main activities. Regulatory Clinical CMC Drug delivery technologies Preclinical Discovery Pharmacovigilance Project management & leadership Quality assurance Intellectual property & patents Portfolio management Total Capacity (people) 450 Laboratories 181 GMP Production Plants (authorized by AIFA) 2 Offices 254 Meeting rooms 32 |

| Industrial Operations |

| 21 Chiesi Group - Parma Plant Regularly and successfully inspected by the U.S. F.D.A. ISO 9001: 2008 Certified OHSAS 18001 Certified ISO 14100 Certified Total Production Area 15,650 m2 Registered into over 65 Countries Parma, Italy Production capacity 22 million packs in oral solids 13 million packs in Unit-Dose Vials (UDV) 28 million packs in Metered-Dose Inhalers (MDI) 400,000 vials in sterile suspensions 2,5 million packs in Dry Powder Inhalers (DPI)* * Started at the end of 2012 |

| 22 Chiesi Group - Other Plants Blois, France Manufacturing capacity: 13 million finished packages per year Specialised in blister packaging for capsules and tablets, and final assembly stages of the Metered-Dose Inhalers (MDIs) Ample refrigerated stores for the products Equipped to supply Group’s Affiliates and distribute directly to clients in the French and export markets. The construction of a new department to manufacture Dry Powder Inhalers (DPIs) Santana de Parnaiba, Brazil Manufacturing capacity: 14,5 million finished packages per year Solid formulations, pressurised solutions and suspensions for inhalant therapy (MDI) Santana supplies both domestic market and Group’s Affiliates and exports to licensees and distributors. |

| Projected into the future: Our Final Destination 23 A global organization able to serve the patients in multiple countries, but keeping alive the entrepreneurial spirit and the history of Chiesi Innovation Focalization Sustainability Glocal |

| Our spirit! 24 |

| Our spirit! 25 |

| Additional Information and Where to Find It Cornerstone intends to file a Proxy Statement with the Securities and Exchange Commission (the “SEC”), and Cornerstone and Chiesi intend to file a Schedule 13E-3 and other relevant materials with the SEC. A definitive proxy statement will be sent to holders of Cornerstone’s common stock seeking their approval of the proposed transaction. WE URGE INVESTORS TO READ THE PROXY STATEMENT AS WELL AS THE SCHEDULE 13E-3 AND ANY OTHER RELEVANT DOCUMENTS FILED BY CORNERSTONE OR CHIESI WHEN THEY BECOME AVAILABLE, BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION ABOUT CORNERSTONE AND THE PROPOSED MERGER. INVESTORS ARE URGED TO READ THESE DOCUMENTS CAREFULLY AND IN THEIR ENTIRETY. Investors will be able to obtain these materials (when they become available) and other documents filed with the SEC free of charge at the SEC’s website (http://www.sec.gov). In addition, these materials (when they become available) will also be available free of charge by accessing Cornerstone’s website (http://www.crtx.com). Investors may also read and copy any reports, statements and other information filed by Cornerstone with the SEC at the SEC public reference room at 100 F Street, N.E., Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 or visit the SEC’s website for further information on its public reference room. Participants in the Proxy Solicitation The directors, executive officers and other members of management and employees of Chiesi may be deemed to be participants in the solicitation of proxies from stockholders in respect of the proposed Merger. Information regarding Chiesi’s directors and executive officers is available on Chiesi’s website (http://www.chiesigroup.com). The directors, executive officers and other members of management and employees of Cornerstone may also be deemed to be participants in the solicitation of proxies from stockholders in respect of the proposed Merger. Information regarding Cornerstone’s directors and executive officers is available in its Annual Report on Form 10-K for the fiscal year ended December 31, 2012, filed with the SEC by Cornerstone on March 14, 2013, as amended by a Form 10-K/A filed with the SEC on April 11, 2013. Other information regarding the participants in the proxy solicitation and a description of their direct and indirect interests, by security holdings or otherwise, will be contained in the Proxy Statement and other relevant materials to be filed with the SEC when they become available. 26 |
Powerpoint presentation delivered by Ugo Bettini, Head of Human Resources of Chiesi Farmaceutici S.p.A., to Employees of Cornerstone Therapeutics Inc., regarding Human Resources matters

| Our Values - Ugo Bettini |

| Our Mission We want to be recognized as a research-focused international Group, developing and bringing to the market value-added innovative medical solutions. We want to build a high-quality entrepreneurial team characterized by self-confidence and a collaborative spirit. Our goal is to combine commitment to results with integrity, operating in a socially and environmentally responsible manner. We will ensure sustainable development and growth by operating according to these principles. |

| Chiesi Group: Charter of Principles FOCUS ON PEOPLE Listening Development Excellence Passion FOCUS ON CUSTOMERS Sense of belonging Responsibility EVERY ONE OF US IS CHIESI RELIABLE COMPANY Ethics Transparent Cohesion Collaboration TEAM SPIRIT VALUE GENERATION Innovation Entrepreneurship |

| [LOGO] |
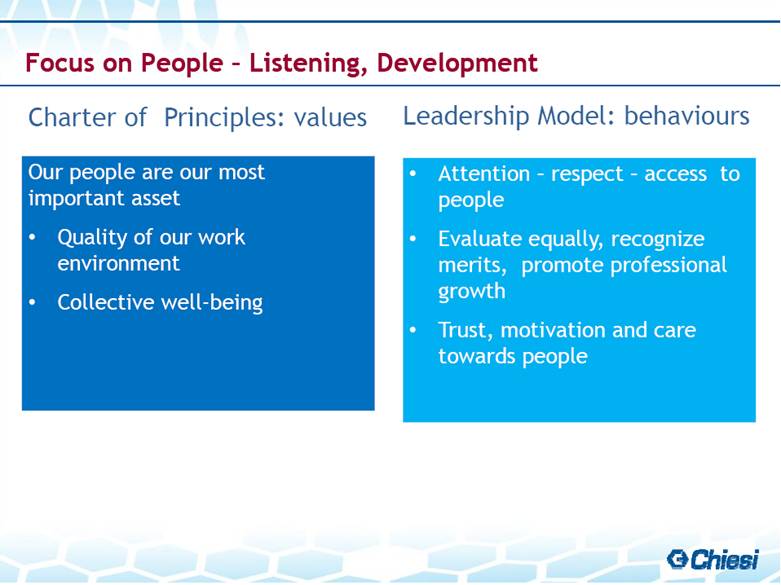
| Charter of Principles: values Leadership Model: behaviours Focus on People – Listening, Development Our people are our most important asset Quality of our work environment Collective well-being Attention – respect – access to people Evaluate equally, recognize merits, promote professional growth Trust, motivation and care towards people |

| Developing our people Chiesi Academy is the brand that identifies the Chiesi Group initiatives dedicated to people’s development. is aimed at creating the ideal context to improve our people’s managerial skills and behaviors; its projects are characterized by internationality and excellence. includes 2 programs: Corporate Chiesi Master: dedicated to High Potentials Corporate Executive Master: D.E.A.L Development for Executives And Leaders, dedicated to Key People |

| Charter of Principles: values Leadership Model: behaviours Team Spirit – Cohesion, Collaboration Encourage integration and the exchange of knowledge Recognize individual merits Demonstrate trust in Team working and celebrate successes Individual contribution to the team The success of the Team is more important than individual success |

| Charter of Principles: values Leadership Model: behaviours EVERY ONE OF US IS CHIESI - Sense of Belonging, Responsibility Act as a company’s advocate towards stakeholders. Respect and gain value from cultural diversity and from different capabilities. Promote and diffuse individual responsibility Everyone of us is part of the Company, creator of its future and advocate of the company’s reputation. |
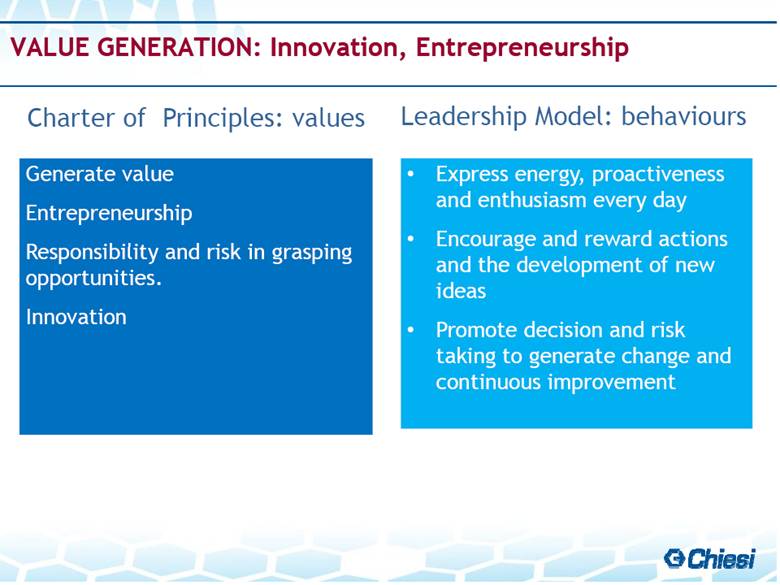
| Charter of Principles: values Leadership Model: behaviours VALUE GENERATION: Innovation, Entrepreneurship Express energy, proactiveness and enthusiasm every day Encourage and reward actions and the development of new ideas Promote decision and risk taking to generate change and continuous improvement Generate value Entrepreneurship Responsibility and risk in grasping opportunities. Innovation |

| Value generation: making our people grow in Chiesi International assignments: a process to develop international career pathways more focused on broad knowledge management than on best competence strictly needed for the position The goal is: Internationalization of competencies, values, cultures to reach more and more innovation and global knowledge, in order to grow |
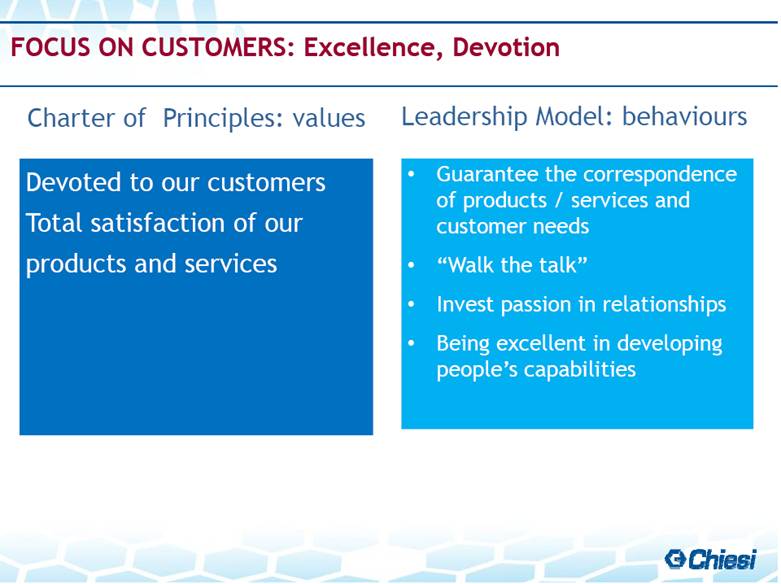
| Charter of Principles: values Leadership Model: behaviours FOCUS ON CUSTOMERS: Excellence, Devotion Guarantee the correspondence of products / services and customer needs “Walk the talk” Invest passion in relationships Being excellent in developing people’s capabilities Devoted to our customers Total satisfaction of our products and services |

| TOP EMPLOYERS: HR FOCUS ON CUSTOMERS Chiesi Farmaceutici has been certified as a Top Employer Europe 2013 in the following countries: France, Germany, Italy, Spain, United Kingdom. The Top Employers Europe certification is only awarded to organizations that meet the highest standard in HR. In the research, all critical areas of the HR management of the participating organizations were assessed. The rating showed that Chiesi Farmaceutici has outstanding employee offerings and thus qualified for the exclusive Top Employers certification. |
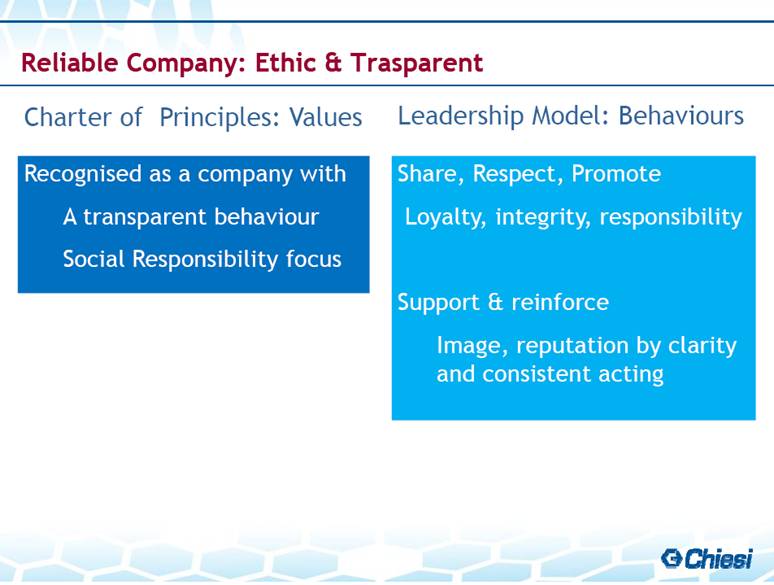
| Charter of Principles: Values Leadership Model: Behaviours Reliable Company: Ethic & Trasparent Recognised as a company with A transparent behaviour Social Responsibility focus Share, Respect, Promote Loyalty, integrity, responsibility Support & reinforce Image, reputation by clarity and consistent acting |

| Reliable Company: Ethic & Trasparent |

| A not for profit organization Focuses: respiratory, rare and neonatal diseases. The Chiesi Foundation seeks to promote health and to alleviate patients’ suffering through research, sharing of knowledge, and through medical, public and patients’ education THE PROJECTS The Chiesi Foundation funds high level research projects, in particular in chronic respiratory affections by giving specific attention to asthma and chronic obstructive pulmonary disease (COPD) and neonatal disorders. THE TOOLS Scientific research, support to young researchers and transfer of resources and expertise to improve the patients’ management by the physicians and the understanding of its needs from public and institutions. http://www.chiesifoundation.org |

| Additional Information and Where to Find It Cornerstone intends to file a Proxy Statement with the Securities and Exchange Commission (the “SEC”), and Cornerstone and Chiesi intend to file a Schedule 13E-3 and other relevant materials with the SEC. A definitive proxy statement will be sent to holders of Cornerstone’s common stock seeking their approval of the proposed transaction. WE URGE INVESTORS TO READ THE PROXY STATEMENT AS WELL AS THE SCHEDULE 13E-3 AND ANY OTHER RELEVANT DOCUMENTS FILED BY CORNERSTONE OR CHIESI WHEN THEY BECOME AVAILABLE, BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION ABOUT CORNERSTONE AND THE PROPOSED MERGER. INVESTORS ARE URGED TO READ THESE DOCUMENTS CAREFULLY AND IN THEIR ENTIRETY. Investors will be able to obtain these materials (when they become available) and other documents filed with the SEC free of charge at the SEC’s website (http://www.sec.gov). In addition, these materials (when they become available) will also be available free of charge by accessing Cornerstone’s website (http://www.crtx.com). Investors may also read and copy any reports, statements and other information filed by Cornerstone with the SEC at the SEC public reference room at 100 F Street, N.E., Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 or visit the SEC’s website for further information on its public reference room. Participants in the Proxy Solicitation The directors, executive officers and other members of management and employees of Chiesi may be deemed to be participants in the solicitation of proxies from stockholders in respect of the proposed Merger. Information regarding Chiesi’s directors and executive officers is available on Chiesi’s website (http://www.chiesigroup.com). The directors, executive officers and other members of management and employees of Cornerstone may also be deemed to be participants in the solicitation of proxies from stockholders in respect of the proposed Merger. Information regarding Cornerstone’s directors and executive officers is available in its Annual Report on Form 10-K for the fiscal year ended December 31, 2012, filed with the SEC by Cornerstone on March 14, 2013, as amended by a Form 10-K/A filed with the SEC on April 11, 2013. Other information regarding the participants in the proxy solicitation and a description of their direct and indirect interests, by security holdings or otherwise, will be contained in the Proxy Statement and other relevant materials to be filed with the SEC when they become available. |










































