As filed with the Securities and Exchange Commission on September 3, 2010
Registration No. 333-161329
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Amendment No. 11
to
Form S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
MIRION TECHNOLOGIES, INC.
(Exact Name of Registrant as Specified in Its Charter)
| | | | | |
Delaware | | 3829 | | 20-3979555 |
| (State or Other Jurisdiction of | | (Primary Standard Industrial | | (I.R.S. Employer |
Incorporation or Organization) | | Classification Code Number) | | Identification Number) |
3000 Executive Parkway, Suite 222
San Ramon, CA 94583
(925) 543-0800
(Address, Including Zip Code, and Telephone Number, Including Area Code, of Registrant’s Principal Executive Offices)
Jack A. Pacheco
Vice President and Chief Financial Officer
Mirion Technologies, Inc.
3000 Executive Parkway, Suite 222
San Ramon, CA 94583
(925) 543-0800
(Name, Address, Including Zip Code, and Telephone Number, Including Area Code, of Agent For Service)
Copies to:
| | | | | |
Alan F. Denenberg, Esq. | | Seth B. Rosen, Esq. | | Tad J. Freese, Esq. |
Davis Polk & Wardwell LLP | | General Counsel, Vice President and Secretary | | Latham & Watkins LLP |
1600 El Camino Real | | 3000 Executive Parkway, Suite 222 | | 140 Scott Drive |
Menlo Park, CA 94025 | | San Ramon, CA 94583 | | Menlo Park, CA 94025 |
(650) 752-2000 | | (925) 543-0800 | | (650) 328-4600 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after the effective date of this Registration Statement.
If any of the securities being registered on this form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. o
If this form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule12b-2 of the Exchange Act. (Check one):
| | | |
| Large accelerated filer o | Accelerated filer o | Non-accelerated filer þ | Smaller reporting company o |
(Do not check if a smaller reporting company)
CALCULATION OF REGISTRATION FEE
| | | | | | | | | | | |
| | | | Proposed Maximum
| | | | Amount of
| |
Title of Each Class
| | | Aggregate Offering
| | | | Registration
| |
| of Securities to be Registered | | | Price(1)(2) | | | | Fee(3)(4) | |
| Common stock, par value $0.001 per share | | | $ | 215,050,000 | | | | $ | 13,784 | |
| | | | | | | | | | | |
| | |
| (1) | | Estimated solely for the purpose of computing the amount of the registration fee pursuant to Rule 457(o) under the Securities Act of 1933. |
| | |
| (2) | | Includes shares of common stock which may be purchased by the underwriters to cover over-allotments, if any. |
| | |
| (3) | | The registration fee is equal to the sum of (a) the product of (i) the proposed maximum aggregate offering price of $100,000,000, as previously proposed on the initial filing of this Registration Statement on August 13, 2009 and (ii) the then-current statutory rate of $55.80 per $1,000,000 and (b) the product of (i) the marginal increase of $115,050,000 in the proposed maximum aggregate offering price hereunder and (ii) the current statutory rate of $71.30 per $1,000,000. |
| | |
| (4) | | The registration fee has been previously paid, as follows: a registration fee of $5,580 was previously paid in connection with the initial filing of this Registration Statement on August 13, 2009; an additional registration fee of $7,302 was previously paid in connection with the filing of Amendment No. 5 to this Registration Statement on March 16, 2010; and an additional registration fee of $902 was previously paid in connection with the filing of Amendment No. 6 to this Registration Statement on March 31, 2010. |
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the Registration Statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
The information in this prospectus is not complete and may be changed. We and the selling stockholders may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and neither we nor the selling stockholders are soliciting offers to buy these securities in any state where the offer or sale is not permitted.
|
SUBJECT TO COMPLETION, DATED SEPTEMBER 3, 2010
Shares
Mirion Technologies, Inc.
Common Stock
This is an initial public offering of shares of common stock of Mirion Technologies, Inc.
We are selling shares of common stock, and the selling stockholders named in this prospectus are selling additional shares of common stock. We will not receive any proceeds from the sale of shares by the selling stockholders.
Prior to this offering, there has been no public market for our common stock. We expect the initial public offering price to be between $ and $ per share. Our common stock has been approved for listing on The NASDAQ Global Market under the symbol “MION.”
The underwriters have an option to purchase a maximum of additional shares of common stock from the selling stockholders. The underwriters can exercise this option at any time within 30 days from the date of this prospectus.
Investing in our common stock involves risks. See “Risk Factors” on page 12 of this prospectus.
| | | | | | | | | |
| | | | | Underwriting
| | | | |
| | | Price to
| | Discounts and
| | Proceeds to
| | Proceeds to
|
| | | Public | | Commissions | | Us | | Selling Stockholders |
| |
| Per Share | | $ | | $ | | $ | | $ |
| Total | | $ | | $ | | $ | | $ |
Delivery of the shares of common stock in book-entry form only will be made on or about , 2010.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
| | | | | |
Credit Suisse | | BofA Merrill Lynch | | J.P. Morgan |
Baird
The date of this prospectus is , 2010
| MIRION TECHNOLOGIES Protecting people, property, and the environment A global provider of radiation detection, measurement, analysis and monitoring products and services to nuclear, defense and medical end markets. |
TABLE OF CONTENTS
You should rely only on the information contained in this prospectus and any free writing prospectus prepared by us. We, the underwriters and the selling stockholders have not authorized any other person to provide you with different information. If anyone provides you with different or inconsistent information, you should not rely on it. We, the underwriters and the selling stockholders are not making an offer of these securities in any jurisdiction where the offer is not permitted. You should not assume that the information contained in this prospectus is accurate as of any date other than the date on the front of this prospectus.
“Mirion Technologies,” “Mirion,” “GDS,” “Global Dosimetry Solutions,” “HandFoot-Fibre,” “Imaging and Sensing Technology,” “IST,” “MGP Instruments,” “MGPI,” “SPIR Ident,” “Synodys,” “TwoStep-Exit” and any corresponding logos, are our common law and registered trademarks. Solely for convenience, we refer to our trademarks in this prospectus without thetm and® symbols, but such references are not intended to indicate that we will not assert our rights to our trademarks. Other service marks, trademarks and trade names referred to in this prospectus are the property of their owners.
References to “fiscal” before any year refer to our fiscal year ending on June 30th of the year referenced.
Dealer Prospectus Delivery Obligation
Until and including , 2010 (25 days after the date of this prospectus), all dealers that effect transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers’ obligation to deliver a prospectus when acting as underwriter and with respect to unsold allotments or subscriptions.
i
PROSPECTUS SUMMARY
This summary highlights the more detailed information contained elsewhere in this prospectus. This summary does not contain all of the information that you should consider before deciding to invest in our common stock. You should read the entire prospectus, including the risk factors, the consolidated financial statements and the related notes, and the other documents to which this prospectus refers, carefully before making an investment decision. In this prospectus, “Mirion,” the “Company,” “we,” “us” or “our” refer to Mirion Technologies, Inc. and its subsidiaries, except where the context makes clear that the reference is only to Mirion Technologies, Inc. and is not inclusive of its subsidiaries.
Our Company
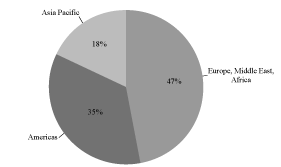
We are a global provider of radiation detection, measurement, analysis and monitoring products and services to the nuclear, defense and medical end markets. Our customers rely on our solutions to protect people, property and the environment from nuclear and radiological hazards. Our products and services include: dosimeters; contamination & clearance monitors; detection & identification instruments; radiation monitoring systems; electrical penetrations; reactor instrumentation & control equipment and systems; dosimetry services; imaging systems; and related accessories, software and services. Many of our end markets are characterized by the need to meet rigorous regulatory standards, design qualifications and operating requirements. We believe these industry dynamics create substantial barriers to entry, thereby reinforcing our market position. We have leveraged the strength of our nuclear platform to expand the commercial applications of our technologies to defense and medical end markets. The diversity of our end markets and the global nature of our customer base are illustrated in the charts below:
| | | |
Fiscal 2010 Revenue by End Markets | | Fiscal 2010 Revenue by Geography |
| | | |
 | |  |
| |
Fiscal 2010 Revenue: $228.1 Million |
For more than 50 years, we and our predecessor companies have delivered products and services that help ensure the safe and efficient operation of nuclear facilities. We believe the breadth and proven performance of our solutions support our longstanding strategic customer relationships across diverse end markets. Our products and services have been sold directly and indirectly to a variety of end-use customers including, but not limited to, all of the U.S. nuclear power producers, 400 of the global installed base of 440 active nuclear power reactors, many of the leading reactor design firms, 17 of the 28 NATO militaries, numerous international government and supranational agencies, as well as medical service providers and industrial companies worldwide.
Our broad product and services portfolio of radiation detection, measurement, analysis and monitoring solutions is supported by 168 scientists, engineers and technicians, who represented approximately 19% of our workforce as of June 30, 2010. We possess numerous product qualifications, trade secrets and patents that support our market position and our ability to deliver next generation products and services. In addition, we maintain design, manufacturing and sales capabilities across seven countries, enabling us to capitalize on growth opportunities, including the anticipated increase in demand for nuclear power and ongoing spending for defense and homeland security.
1
Our financial performance is driven by the replacement of products and the recurring provision of services into our core end markets, as well as the construction of new nuclear power plants, or NPPs, globally. Many of our products are ordered well in advance of the anticipated shipment date, providing visibility into future revenue through our backlog and deferred contract revenue, which totaled $191.7 million and $44.7 million as of June 30, 2010. We generated revenue of $228.1 million, Adjusted EBITDA of $46.3 million and net income of $6.0 million for fiscal 2010. See pages 10 and 11 of this prospectus for a definition and reconciliation of Adjusted EBITDA to cash provided by (used in) operations.
Our Market Opportunities
We sell our radiation detection, measurement, analysis and monitoring products and services into the global nuclear, defense and medical end markets. We believe that our end markets are characterized by strong fundamentals that support a robust revenue base and provide numerous growth opportunities.
Nuclear
The nuclear end market spans the entire nuclear fuel cycle, including mining, enrichment, fuel manufacturing, nuclear power generation, waste management and fuel reprocessing. Key nuclear installations include mines, fuel fabrication facilities, commercial nuclear power reactors, reprocessing facilities, research facilities, military facilities and ships, weapons facilities and waste storage facilities. We sell products and services for use in each of these types of installations, with commercial nuclear power reactors representing the majority of our sales into the nuclear end market. As of June 30, 2010, our products were installed at 91% of active nuclear power reactors globally, including all of those in the United States. We believe that the global installed base of nuclear reactors presents opportunities for replacements and upgrades of our products, as well as those of legacy suppliers, and for participation in the “decommissioning” process.
We also expect the increase in nuclear reactor construction worldwide to provide opportunities across our offerings.
Defense
Our global defense end market is driven by a combination of military, civil defense and event-driven security spending which in turn has been fueled by the unprecedented growth in global security threats.
Medical
The use of radiodiagnostic and radiotherapeutic procedures is expanding globally due to aging population demographics, technological advancements and emerging middle classes in China and India, creating a significant opportunity for us in the medical end market.
Our Competitive Strengths
Trusted radiation detection, measurement, analysis and monitoring provider. The nuclear industry is highly regulated and requires compliance with strict product specifications. Our trusted, recognized brands supported by our tradition of technical excellence, product reliability and customer service have enabled us to develop strong market share across our product and service offerings.
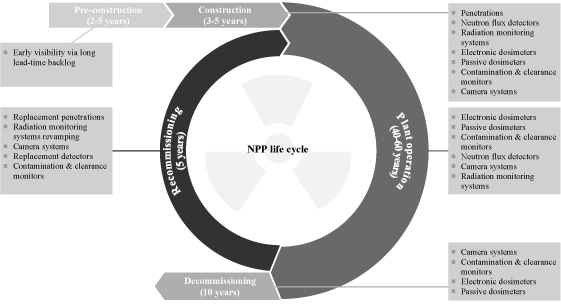
Broad and complementary product and service portfolio. We offer radiation detection, measurement, analysis and monitoring products and services to satisfy customer requirements throughout the NPP life cycle.
Large installed base drives recurring revenue. Our large installed base at active nuclear power reactors drives recurring revenue through replacement and service cycles.
Technical complexity creates high barriers to entry. We design products to meet demanding customer specifications, qualifications and regulatory requirements.
2
Global footprint designed to meet local customer needs. Our global footprint, augmented by our established network of suppliers and distributors, enables us to be responsive to our customers and provide locally customized solutions.
Seasoned management team complemented by highly skilled engineers. We are led by an experienced management team with a mix of private sector and government experience across different industries and functions.
Our Strategy
Our objective is to continue enhancing our position as a global provider of radiation detection, measurement, analysis and monitoring products and services for the nuclear, defense and medical end markets. We intend to achieve this through the following strategies:
| | |
| | • | Exploit under-penetrated market opportunities. |
| |
| | • | Expand addressable market. |
| | |
| | • | Geographic expansion. We believe we can increase our presence in the international market. For example, we intend to leverage our relationships with leading reactor design firms to capitalize on the opening of India’s nuclear end market to U.S. firms due to a recent treaty ratification. |
| |
| | • | Customer outsourcing. Some NPP operators have recently outsourced their dosimetry services in order to reduce costs. We have been able to benefit from economies of scale as well as advantages in materials procurement and processing technology to provide enhanced dosimetry services to many of these NPPs at a lower cost. |
| |
| | • | Service privatization. In some regions outside the United States, dosimetry services have historically been provided by government agencies. However, privatization of dosimetry services is accelerating in some regions, such as Europe, as providers seek to reduce costs and benefit from enhanced service offerings, providing an opportunity to leverage our expertise and North American service experience. |
| |
| | • | New applications for existing technologies. |
| | |
| | • | Develop new products and services. |
| |
| | • | Continuously improve our cost structure and productivity. |
| |
| | • | Pursue strategic acquisitions. |
Our Principal Investor
Upon completion of this offering, American Capital, Ltd. (together with American Capital Equity I, LLC and American Capital Equity II, LP, “ACAS”) will beneficially own shares of our common stock (approximately % of our outstanding common stock), which includes shares of our common stock underlying warrants, or shares of our common stock (approximately % of our common stock) if the underwriters exercise in full their over-allotment option to purchase additional shares of common stock from the selling stockholders. We are party to a number of agreements with ACAS and its affiliates. These agreements are described in the sections of this prospectus captioned, “Risk Factors—Risks Related to this Offering and Our Common Stock,” “Use of Proceeds,” “Certain Relationships and Related Party Transactions” and “Principal and Selling Stockholders.”
ACAS is a publicly traded private equity firm and global asset manager, with $15 billion in capital resources under management as of June 30, 2010.
Risk Factors
Our business is subject to many risks and uncertainties, including those highlighted in the section of this prospectus entitled “Risk Factors.” These risks could materially and adversely affect our business, financial
3
condition and results of operations, which could cause the trading price of our common stock to decline and could result in a partial or total loss of your investment. Some of these risks include:
| | |
| | • | the long and unpredictable nature of our sales cycle; |
| |
| | • | fluctuations in our financial performance; |
| |
| | • | our short operating history as a consolidated entity; |
| |
| | • | material weaknesses in our internal controls over financial reporting; |
| |
| | • | the highly competitive nature of our markets and the resources of our competitors; |
| |
| | • | the uncertain fulfillment of our backlog; |
| | |
| | • | the effect of the recent global financial crisis and worldwide economic conditions; and |
| | |
| | • | changes in our customers’ budgets for radiation detection products and services and the timing of their purchasing decisions. |
Company Information
We incorporated in Delaware in October 2005 as Global Monitoring Services, Inc., and combined our three predecessor companies under Global Monitoring Services, Inc. in December 2005. ACAS originally acquired our predecessor company Synodys SA (Synodys) in June 2004, our predecessor company Imaging and Sensing Technology Corporation (IST) in May 2004 and our predecessor company Global Dosimetry Solutions, Inc. (GDS) in September 2003. We changed our name in January 2006 to Mirion Technologies, Inc. Our principal executive offices are located at 3000 Executive Parkway, Suite 222, San Ramon, California 94583 and our telephone number is(925) 543-0800. Our website is www.mirion.com. The information that appears on our website is not part of, and is not incorporated into, this prospectus.
4
The Offering
| | |
| Common stock offered by us | | shares |
| | |
| Common stock offered by the selling stockholders | | shares |
| | |
| Common stock to be outstanding after this offering | | shares based on the number of shares of common stock outstanding as of , 2010 |
| | |
| Over-allotment option | | The underwriters have a30-day option to purchase from the selling stockholders up to an additional shares of common stock to cover over-allotments. |
| | |
| Dividend policy | | We do not anticipate paying any dividends on our common stock in the foreseeable future. See “Dividend Policy.” |
| |
| Risk factors | | Investing in our common stock involves a high degree of risk. See “Risk Factors” beginning on page 12 of this prospectus for a discussion of factors you should carefully consider before investing in our common stock. |
| | |
| Use of proceeds and benefits to be received by related persons in connection with this offering | | We estimate that the net proceeds to us from this offering, after deducting underwriting discounts and estimated offering expenses, will be approximately $ million, assuming the shares are offered at $ per share (the midpoint of the price range set forth on the cover page of this prospectus). We intend to use approximately $ million of the net proceeds from the shares that we sell in this offering to repay borrowings from ACAS and its affiliates. We intend to repay all other debt held by ACAS and its affiliates ($ million as of , 2010) with borrowings under our anticipated new bank credit facilities that we expect to enter into upon the consummation of this offering. We also intend to use net proceeds from this offering to make a one-time payment of $8.0 million to American Capital Financial Services, Inc., or ACFS, a subsidiary of ACAS, to terminate an investment banking services agreement between us and ACFS. We intend to use $0.8 million of the net proceeds from this offering to make bonus payments upon the completion of this offering to certain of our employees, including an aggregate of $0.6 million to certain of our executive officers in the respective amounts set forth on page 113 of this prospectus. ACAS is our controlling stockholder and is a selling stockholder in this offering. We will not receive any proceeds from the sale of shares of common stock held by the selling stockholders. See “Use of Proceeds” and “Principal and Selling Stockholders.” |
| | |
| | In addition, certain of our executive officers and non-ACAS director nominees will receive cash bonus payments, stock option grants and the accelerated vesting of certain stock options upon the completion of this offering. In addition, we expect certain of our equityholders that are also related persons, including ACAS, our |
5
| | |
| | principal stockholder, and Thomas D. Logan, our President, Chief Executive Officer and Chairman of the Board, to agree with us to receive cash dividends in lieu ofpaid-in-kind dividends on convertible preferred stock held by them that will accrue from and after , 2010. All warrants held by such equityholders will also become exercisable upon consummation of this offering. See “Certain Relationships and Related Party Transactions” for a discussion of the expected benefits that such related persons will receive upon the completion of this offering. |
| | |
| Proposed NASDAQ Global Market symbol | | MION |
The number of shares of common stock to be outstanding after this offering is based on 11,271,548 shares outstanding as of August 31, 2010 after giving effect to the conversion of our Series A Convertible Participating Preferred Stock pursuant to the conversion terms in our amended and restated Certificate of Incorporation and excludes:
| | |
| | • | 856,694 shares of common stock subject to outstanding options at a weighted average exercise price of $14.22 per share; |
| | |
| | • | an aggregate of 1,054,531 shares of common stock either reserved for issuance under our existing stock option plan or to be reserved for issuance under our amended and restated stock plan to become effective in connection with this offering, of which 124,448 shares are expected to be granted in the form of stock options to our employees, including options to purchase 34,008 shares to be granted to our executive officers (in the respective amounts set forth on page 113 of this prospectus), and, under the director compensation policy described on page 105 of this prospectus, to outside directors immediately following the pricing of this offering at an exercise price equal to the initial public offering price; and |
| | |
| | • | 3,420,636 shares of common stock subject to outstanding warrants at a weighted average exercise price of $0.00018 per share. These warrants only become exercisable upon a sale, liquidation or dissolution of the Company or approval by our Board of Directors. Our Board of Directors has resolved that all of these warrants will become exercisable at the option of the holder thereof upon the consummation of this offering and thereafter. |
Except as otherwise indicated, all information in this prospectus assumes that:
| | |
| | • | all of the outstanding shares of our convertible preferred stock will be converted into shares of our common stock; |
| | |
| | • | all of the outstanding shares of our Class A Voting Common Stock and Class B Non-Voting Common Stock will be converted into shares of our common stock on a one-for-one basis; and |
| | |
| | • | the underwriters will not exercise their over-allotment option. |
All share and per share information referenced throughout this prospectus have been retroactively adjusted for an 8.5-for-1 stock split of our common stock effected on March 15, 2010.
6
Summary Consolidated Financial Data
The following table summarizes the consolidated financial data for our business. You should read this summary consolidated financial data in conjunction with the sections titled “Selected Consolidated Financial Data” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and related notes, all included elsewhere in this prospectus. The summary financial data in this section is not intended to replace the consolidated financial statements and related notes included in this prospectus. The summary consolidated statements of operations data for each of the three fiscal years ending June 30, 2008, 2009 and 2010 and the consolidated balance sheet data as of June 30, 2010 are derived from our audited annual consolidated financial statements and related notes included elsewhere in this prospectus. Our historical results are not necessarily indicative of the results that should be expected in the future. The amounts below are in thousands, except percentages, share and per share data.
| | | | | | | | | | | | | |
| | | Year Ended June 30, | |
| | | 2008 | | | 2009 | | | 2010 | |
| |
Consolidated Statement of Operations Data: | | | | | | | | | | | | |
| Revenue | | $ | 191,769 | | | $ | 201,763 | | | $ | 228,124 | |
| Cost of revenue | | | 102,790 | | | | 105,954 | | | | 126,707 | |
| | | | | | | | | | | | | |
| Gross profit | | | 88,979 | | | | 95,809 | | | | 101,417 | |
| | | | | | | | | | | | | |
| % of revenue | | | 46.4 | % | | | 47.5 | % | | | 44.5 | % |
| Operating expenses | | | | | | | | | | | | |
| Selling, general and administrative expenses | | | 63,177 | | | | 65,649 | | | | 64,510 | |
| Research and development expenses | | | 14,865 | | | | 11,282 | | | | 8,729 | |
| | | | | | | | | | | | | |
| Total operating expenses | | | 78,042 | | | | 76,931 | | | | 73,239 | |
| | | | | | | | | | | | | |
| Income from operations | | | 10,937 | | | | 18,878 | | | | 28,178 | |
| Interest expense, net | | | 20,207 | | | | 17,711 | | | | 15,120 | |
| Other income, net | | | (1,759 | ) | | | (490 | ) | | | (639 | ) |
| | | | | | | | | | | | | |
| (Loss) Income before provision for income taxes | | | (7,511 | ) | | | 1,657 | | | | 13,697 | |
| Provision for income taxes | | | 5,838 | | | | 5,612 | | | | 7,732 | |
| | | | | | | | | | | | | |
| Net (loss) income | | $ | (13,349 | ) | | $ | (3,955 | ) | | $ | 5,965 | |
| Paid-in-kind preferred dividends | | | (8,993 | ) | | | (9,892 | ) | | | (10,923 | ) |
| | | | | | | | | | | | | |
| Net loss allocable to common stockholders | | $ | (22,342 | ) | | $ | (13,847 | ) | | $ | (4,958 | ) |
| | | | | | | | | | | | | |
| Pro forma net loss per common share before conversion of preferred shares — basic and diluted(1) | | | | | | | | | | $ | | |
| | | | | | | | | | | | | |
| Pro forma net income per common share — diluted(1) | | | | | | | | | | $ | | |
| | | | | | | | | | | | | |
| Pro forma net income per common share — diluted as adjusted(1) | | | | | | | | | | $ | | |
| | | | | | | | | | | | | |
| | | | | | | | | |
| | | As of June 30, 2010 |
| | | | | Pro Forma as
|
| Consolidated Balance Sheet Data: | | Actual | | Adjusted(1) |
| |
| Cash and cash equivalents(3) | | $ | 9,936 | | | $ | | |
| Total assets | | | 307,363 | | | | | |
| Notes payable to ACAS(4) | | | 183,252 | | | | | |
| New indebtedness(4)(5) | | | — | | | | | |
| Total stockholders’ equity | | | 2,190 | | | | | |
| | | | | | | | | | | | | |
| | | Year Ended June 30, | |
| | | 2008 | | | 2009 | | | 2010 | |
| |
Other Data: | | | | | | | | | | | | |
| Adjusted EBITDA(6) | | $ | 34,218 | | | $ | 40,625 | | | $ | 46,300 | |
| Amortization of intangible assets | | | 10,140 | | | | 8,144 | | | | 6,432 | |
| Capital expenditures | | | 4,953 | | | | 6,649 | | | | 10,437 | |
7
| | | | | | | | | | | | | |
| | | As of June 30, | |
| | | 2008 | | | 2009 | | | 2010 | |
| |
| Backlog(7) | | $ | 177,956 | | | $ | 183,960 | | | $ | 191.734 | |
| Deferred contract revenue | | | 53,539 | | | | 62,031 | | | | 44,730 | |
| | |
| (1) | | Pro forma net loss per common share before conversion of preferred shares — basic and diluted and pro forma net income per common share — diluted and diluted as adjusted for the year ended June 30, 2010 is calculated as follows: |
| | | | | |
| | | Year Ended
| |
| | | June 30,
| |
| | | 2010 | |
| |
Numerator: | | | | |
| Net loss allocable to common stockholders | | $ | (4,958 | ) |
| Interest expense from paydown of ACAS debt using proceeds of offering(a) | | | | |
| | | | | |
| Pro forma net loss allocable to common stockholders before conversion of preferred shares | | | | |
| Effect of preferred stock dividends(b) | | | 10,923 | |
| | | | | |
| Pro forma net income allocable to common stockholders | | | | |
| Interest expense reduction from refinancing of ACAS debt(c) | | | | |
| Compensation expense from employee stock options and call options(d) | | | | |
| | | | | |
| Pro forma net income allocable to common stockholders — as adjusted | | $ | | |
| | | | | |
| | | | | |
| | | Year Ended
| |
| | | June 30, 2010 | |
| |
Denominator: | | | | |
| Weighted average shares outstanding from conversion of Class A and B Voting Common Stock(e) | | | 405,796 | |
| Common shares issued in this offering(f) | | | | |
| | | | | |
| Shares used in computing pro forma net loss per common share before conversion of preferred shares — basic and diluted(i) | | | | |
| Weighted average shares outstanding from conversion of convertible preferred stock(g) | | | 10,233,721 | |
| | | | | |
| Shares used in computing pro forma net income per common share — basic | | | | |
| Weighted average common shares outstanding from exercise of warrants(h) | | | 3,420,636 | |
| Weighted average common share equivalents of stock option(i) | | | 73,879 | |
| | | | | |
| Shares used in computing pro forma net income per common share — diluted and diluted as adjusted | | | | |
| | | | | |
| | |
| (a) | | The pro forma reduction in interest expense assumes the repayment of $ million of ACAS debt using the net proceeds from this offering, giving effect to the elimination of the related interest expense as of the beginning of the period presented. The amount of interest expense eliminated by this adjustment is calculated from actual interest expense of $ million recorded in fiscal 2010 in connection with the specific debt arrangements that will be repaid with a portion of the net proceeds of this offering. No tax expense has been provided related to this reduction in interest expense because we are in a net operating loss position and have a full valuation allowance in the affected jurisdiction. |
| | |
| (b) | | The effect of preferred stock dividends is added back as a reduction to net loss allocable to common stockholders, assuming that all preferred stock has been converted into common shares as of the beginning of the period presented. |
| | |
| (c) | | All ACAS debt not assumed to be repaid from the net proceeds from this offering is assumed to be refinanced with a loan from a third-party bank, at interest rates averaging approximately % lower than existing average interest rates with ACAS giving effect to the elimination of the related interest expense as of the beginning of the period presented. The amount of interest expense eliminated by this adjustment is calculated by taking the difference between the actual interest expense of $ million recorded in fiscal 2010 in connection with the specific debt arrangements that will be repaid with a portion of the net proceeds of the new debt arrangements entered into concurrently with the completion of this offering and the interest expense on the new debt arrangements, calculated as the total of the new debt arrangements of $ million multiplied by the average interest rate on those arrangements of approximately % for fiscal 2010. No tax expense has been provided related to this reduction in interest expense because we are in a net operating loss position and have a full valuation allowance in the affected jurisdictions. |
| | |
| (d) | | The pro forma increase in compensation expense associated with employee stock options and call options reflects: |
| | |
| | • | A compensation charge associated with 124,448 stock options that are expected to be granted to our employees and outside directors immediately following the pricing of this offering at an exercise price equal to the initial public |
8
offering price. The value of these options was estimated to be $ million using the Black-Scholes option pricing model and key assumptions are as follows: expected option term of 7 years, risk-free interest rate will be updated at the date of grant but is currently estimated to be %, dividend yield of %, volatility will be updated at the date of grant but is currently estimated to be % and an exercise price and fair value of $ per share (the midpoint of the price range set forth on the cover page of this prospectus). Compensation expense will be recognized on these options over an expected four year vesting term, and as such, the compensation expense for fiscal 2010 was assumed to be 25% of the total value, or $ million.
| | |
| | • | A compensation charge associated with 463,794 performance-based call options with market conditions held by Thomas D. Logan, our Chief Executive Officer. These options contain vesting provisions based upon successful completion of an initial public offering or change in control and achievement by ACAS of certain internal rates of return or returns on investment. The value of these options was estimated to be $2.1 million using a Monte Carlo simulation model and key assumptions are as follows: at the January 1, 2006 modification, expected option term of 4.5 years, risk-free interest rate of 4.3%, dividend yield of 0%, volatility of 41.8%, exercise price of $10.45 per share and fair value of $2.22 per share; at the December 7, 2007 modification, expected option term of 2.6 years, risk-free interest rate of 3.1%, dividend yield of 0%, volatility of 39.8%, exercise price of $10.45 per share and fair value of $4.44 per share. The compensation expense associated with these options for fiscal 2010 was calculated to be $1.5 million based on the implied requisite service period for the market conditions (30 days for one-half of the shares, and 24 months for one-half of the shares). |
| | |
| (e) | | The weighted average common shares outstanding from the conversion of common stock assumes the conversion of all outstanding shares of Class A Voting Common Stock and Class B Non-Voting Common Stock on a one-for-one basis into 405,796 shares of common stock. |
| | |
| (f) | | Includes shares of our common stock to be sold in connection with this offering. Because distributions to ACAS, our principal stockholder, consisting of obligations under existing debt arrangements of $183.3 million as of June 30, 2010 and amounts due of $8.0 million to terminate an existing investment banking services agreement, exceed our earnings plus gross proceeds from this offering of $ million, all common shares are included in the calculation under existing rules on pro forma calculations. Following is a calculation of the deemed dividend in excess of proceeds from this offering (in thousands): |
| | | | | |
| | | For the Twelve
| |
| | | Months Ended
| |
| | | June 30, 2010 | |
| Gross proceeds from offering | | $ | | |
| Distributions to ACAS: | | | | |
| Termination of investment banking services agreement | | | | |
| Repayment of notes payable to ACAS from proceeds of offering | | | | |
| Repayment of notes payable to ACAS from new debt arrangements | | | | |
| | | | | |
| Total distribution to ACAS | | | | |
| Last twelve months earnings | | | | |
| | | | | |
| Excess of dividend to be paid over proceeds from offering | | $ | | |
| | | | | |
| | |
| (g) | | The weighted average common shares outstanding from the conversion of preferred stock assumes the conversion of all outstanding convertible preferred stock into common stock, including the conversion into common stock of all accrued and unpaidpaid-in-kind dividends on convertible preferred stock. The 10,233,721 weighted average at June 30, 2010 is comprised of 936,573A-1 preferred shares, which includes 257,769 accrued but unpaidpaid-in-kind dividends, which are convertible at a rate of 9.6288 and 137,941A-2 preferred shares, which includes 67,941 accrued but unpaidpaid-in-kind dividends, which are convertible at a rate of 8.8128. |
| | |
| (h) | | These warrants only become exercisable upon a sale, liquidation or dissolution of the Company or approval by our Board of Directors. Our Board of Directors has resolved that all of these warrants will become exercisable upon the consummation of this offering. |
| | |
| (i) | | The shares used in computing pro forma net loss per common share before conversion of preferred shares — diluted for the year ended June 30, 2010 exclude options to purchase 924,830 shares of common stock because we recorded a pro forma net loss allocable to common stockholders before conversion of preferred shares, and therefore the impact of such shares would be anti-dilutive. The shares used in computing pro forma net income per common share — diluted and diluted as adjusted for the year ended June 30, 2010 exclude options to purchase 662,353 shares of common stock because the effect would be anti-dilutive. 476,841 of these shares were excluded because the option exercise prices exceeded the average market value of our common stock during the period. 185,512 of these shares were excluded because after applying the treasury stock method of calculating earnings per share, the impact would be anti-dilutive. The shares used in computing pro forma net loss per common share before conversion of preferred shares — basic and diluted and pro forma net income per common share — diluted and diluted as adjusted for the year ended June 30, 2010 also exclude options to purchase 124,448 shares of common stock which are expected to be granted immediately following the pricing of this offering because their impact would be anti-dilutive, either because we generated a net loss or because after applying the treasury stock method of calculating earnings per share, the impact would be anti-dilutive. |
9
| | |
| (2) | | On a pro forma as adjusted basis to give effect to (i) the sale of shares of our common stock to be sold by us in this offering at an assumed initial public offering price of $ per share (the midpoint of the price range set forth on the cover page of this prospectus), (ii) the application of a portion of the net proceeds from the shares sold to repay $ million of our indebtedness to ACAS and its affiliates, to make a one-time, $8.0 million payment to ACFS and to make aggregate bonus payments of $0.8 million to certain of our employees, including an aggregate of $0.6 million to certain of our executive officers, (iii) the increase in additional paid-in capital and corresponding increase in accumulated deficit to reflect the compensation expense associated with stock options that will vest upon this offering and (iv) our intent to repay all other debt held by ACAS and its affiliates with borrowings under our anticipated new bank credit facilities that we expect to enter into upon the consummation of this offering. The compensation expense relates to unrecognized compensation expense associated with unvested employee stock options granted in August 2008, that, under the original terms of the grant, vest ratably over four years from the date of grant, with accelerated vesting of any unvested shares upon consummation of an initial public offering. We estimated the value of these options on the date of grant using the Black-Scholes model and based on the valuation assumptions detailed in Note 13 to the consolidated financial statements. |
| | |
| (3) | | As of June 30, 2010, we also had $6.6 million of restricted cash. |
| | |
| (4) | | In addition, as of June 30, 2010, we had $2.6 million of outstanding debt held by third parties not affiliated with ACAS. |
| | |
| (5) | | See “Description of Certain Indebtedness” for information about our anticipated new bank credit facilities that we expect to enter into upon the consummation of this offering. |
| | |
| (6) | | We include Adjusted EBITDA in this prospectus because (i) it is a basis upon which our management assesses our operating performance, (ii) it is a factor in the evaluation of the performance of our management in determining compensation and (iii) certain maintenance covenants under our debt agreements are tied to ratios based upon Adjusted EBITDA, as defined. Adjusted EBITDA for any period, as defined in our debt agreements, is calculated as net income (loss) for such period plus (a) without duplication and to the extent deducted in determining net income for such period, the sum of (i) interest expense for such period, (ii) income tax expense for such period, (iii) all amounts attributable to depreciation and amortization expense for such period, (iv) any extraordinary cash charges for such period in an amount not to exceed $4,000,000, (v) any extraordinary non-cash charges for such period, (vi) any other non-cash charges for such period (but excluding any non-cash charge for such period in respect of an item that was included in net income in a prior period) and (vii) any non-recurring fees, costs and expenses as reflected in our June 30, 2010 financial statements and any non-recurring fees, costs and expenses incurred in connection with this offering or in connection with the new bank credit facilities and any fees paid to ACAS and its affiliates pursuant to, or in connection with the termination of, the investment banking services agreement with ACFS after June 30, 2010 on or prior to the closing of this offeringminus(b) without duplication and to the extent included in net income, (i) any cash payments made during such period in respect of non-cash charges described in clauses (a)(vi) or (a)(vii) taken in a prior period and (ii) any extraordinary gains and any non-cash items of income for such period, all calculated on a consolidated basis in accordance with U.S. GAAPprovided that net income excludes (a) the income (or deficit) of any person (other than a subsidiary) in which we or any of our subsidiaries has an ownership interest, except to the extent that such income is actually received by us or such subsidiary in the form of dividends or similar distributions and (b) the undistributed earnings of any of our subsidiaries (other than subsidiaries party to the debt agreement) to the extent that the declaration or payment of dividends or similar distributions by such subsidiary is not at the time permitted by the terms of any contractual obligation (other than under any loan documents associated with the debt agreement) or requirement of law applicable to such subsidiary. Adjusted EBITDA is not a measure of financial performance calculated in accordance with U.S. GAAP, and should be viewed as a supplement to—not a substitute for—our results of operations presented on the basis of U.S. GAAP. Adjusted EBITDA also does not purport to represent cash flow provided by, or used in, operating activities in accordance with U.S. GAAP. Our statements of cash flows, included elsewhere in this prospectus, present our cash flow activity in accordance with U.S. GAAP. Furthermore, Adjusted EBITDA is not necessarily comparable to similarly titled measures reported by other companies. |
10
The following is a reconciliation of cash (used in) provided by operating activities to Adjusted EBITDA:
| | | | | | | | | | | | | |
| | | Year Ended June 30, | |
| | | 2008 | | | 2009 | | | 2010 | |
| |
| Cash (used in) provided by operating activities | | $ | (6,712 | ) | | $ | 10,031 | | | $ | 8,202 | |
| Interest expense, net | | | 20,207 | | | | 17,711 | | | | 15,120 | |
| Income tax expense | | | 5,838 | | | | 5,612 | | | | 7,732 | |
| Fees paid to ACFS | | | 1,625 | | | | 1,739 | | | | 1,643 | |
| Other nonrecurring charges(*) | | | 5,458 | | | | 5,867 | | | | 2,605 | |
| Actuarial (gain) loss | | | 226 | | | | (208 | ) | | | (530 | ) |
| Paid-in-kind interest expense | | | (1,904 | ) | | | (1,992 | ) | | | (2,091 | ) |
| Loss on disposal of property, plant and equipment | | | (502 | ) | | | (592 | ) | | | (859 | ) |
| Amortization of loan fees, debt discount and preferred stock discount | | | (785 | ) | | | (522 | ) | | | (458 | ) |
| Provision for doubtful accounts | | | (30 | ) | | | (140 | ) | | | (138 | ) |
| Provision for deferred income taxes | | | 1,238 | | | | 1,013 | | | | (3,062 | ) |
| Change in operating assets and liabilities | | | 9,559 | | | | 2,084 | | | | 18,136 | |
| Currency effects and other | | | — | | | | 22 | | | | — | |
| | | | | | | | | | | | | |
| Adjusted EBITDA | | $ | 34,218 | | | $ | 40,625 | | | $ | 46,300 | |
| | | | | | | | | | | | | |
| | |
| (*) | | Represents non-recurring expenses, including severance expenses and costs associated with the preparation for our initial public offering, as well as certain professional and legal expenses. |
| | |
| (7) | | Represents purchase orders or contracts received by us that have not been shipped. Amounts representing backlog are not recorded in our financial statements. |
11
RISK FACTORS
Investing in our common stock involves a high degree of risk. You should carefully consider the risks described below, together with all of the other information contained in this prospectus, before deciding whether to purchase our common stock. If any of the following risks occurs, the trading price of our common stock could decline and you could lose all or part of your investment.
Risks Related to Our Business
Our sales cycle can be long and unpredictable, and we may be unable to recognize revenue until many months or years after an order is placed. As a result, our revenue can be difficult to predict and may vary substantially from quarter to quarter, which may cause our operating results to fluctuate.
Our sales efforts for many of our products involve substantial discussion with customers regarding product customization and deployment. This process can be extremely lengthy and time consuming and typically involves a significant product evaluation process. The typical sales cycle for products whose procurement relates to the construction of new, or the refurbishment of existing, NPPs ranges from 12 to 36 months and has, in some cases, extended up to 60 months. In addition, these customers generally make a significant commitment of resources to test and evaluate our products prior to purchase. As a result, our sales process is often subject to delays associated with the lengthy approval processes that typically accompany the design, testing and adoption of new, technologically complex products. This results in our investing significant resources prior to orders being placed for our products, with no assurances that we will secure a sale.
In addition, a significant amount of time can pass before we recognize the revenue associated with an order once it has been placed. We may not recognize revenue for sales of certain of our products until the customer certifies the successful installation and operation of the product, which can be many months or, particularly with regard to our Sensing Systems and Radiation Monitoring Systems products, years following the receipt of a customer order. Additionally, under prevailing accounting guidance applicable to sales arrangements entered into prior to fiscal 2010, we may be required to delay revenue recognition on products delivered to our customers until we have completed delivery of all products associated with our arrangement with the customer. The installation of our systems are also subject to construction or scheduled outage delays unrelated to our products, which can further defer the recognition of revenue.
Our long and uncertain sales cycle and the unpredictable period of time between the placement of an order and our ability to recognize the revenue associated with the order makes revenue predictions difficult, particularly on a quarterly basis, and can cause our operating results to fluctuate significantly.
Our financial performance is unpredictable.
Our business depends on the demand for our radiation detection, measurement, analysis and monitoring products and services in the nuclear, defense and medical end markets. In the past, the demand for our products in these markets has fluctuated due to a variety of factors, many of which are beyond our control. This has caused our financial performance to fluctuate. Among the factors affecting our performance are:
| | |
| | • | general economic conditions, both domestically and internationally; |
| |
| | • | the timing, number and size of orders from, and shipments to, our customers, as well as the relative mix of those orders; |
| |
| | • | the timing of revenue recognition, which often requires customer acceptance of the delivered products; |
| |
| | • | delays, postponements or cancellations of construction or decommissioning of NPPs caused by, for example, financing difficulties or regulatory delays; |
| |
| | • | adverse economic, financialand/or political conditions in one or more of our target end markets; |
| |
| | • | variations in the volume of orders for a particular product or product line in a particular quarter; |
| |
| | • | the size and timing of new contract awards; |
| |
| | • | the timing of the release of government funds for procurement of our products; |
12
| | |
| | • | the degree to which new end markets emerge for our products; |
| |
| | • | the budget cycles of U.S. and foreign governments and commercial enterprises that affect timing of order placement for or delivery of our products; and |
| |
| | • | the tendency of commercial enterprises to fully utilize annual capital budgets prior to expiration. |
We have a short operating history as a consolidated entity and have incurred net losses since our inception.
We were formed in 2005 as a merger of several companies acquired by ACAS but operated separately. Accordingly, we rely on the employees, goodwill, brand strength, product history and qualifications of our legacy acquired companies. In addition, some of our senior executive officers have a limited history with us and no prior experience in the industries in which we compete. Furthermore, although we had $6.0 million in net income for fiscal 2010, we did not achieve positive net income in any prior period and, as of June 30, 2010, had an accumulated deficit of $96.6 million. We cannot assure you that we will achieve positive net income in any future period.
Our independent registered public accounting firm reported to us that, at each of June 30, 2008, 2009 and 2010, we had material weaknesses in our internal controls over financial reporting that, if not remediated, could result in material misstatements in our financial statements in future periods and impair our ability to comply with the accounting and reporting requirements applicable to public companies.
Our independent registered public accounting firm reported to us that at each of June 30, 2008, 2009 and 2010, we had material weaknesses in our internal controls over financial reporting that have not been remediated as of the time of this filing. Under standards established by the Public Company Accounting Oversight Board, or PCAOB, a “material weakness” is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the company’s annual or interim financial statements will not be prevented or detected on a timely basis. The material weaknesses identified were with respect to our controls in our financial accounting and reporting functions, which are necessary in order to produce U.S. generally accepted accounting principles (U.S. GAAP) compliant financial statements. These weaknesses related to a lack of sufficient accounting personnel and depth of knowledge, lack of formal policies and procedures to ensure that subsidiary financial information reflect accounting under U.S. GAAP, lack of account reconciliation procedures and untimely review and approval procedures. While we are in the process of hiring additional finance personnel, documenting and enhancing formal policies and procedures and implementing software upgrades throughout our operations, a significant number of audit adjustments continue to be necessary to appropriately reflect account balances in accordance with U.S. GAAP. Management continues to develop and implement sustainable internal controls through formalization of policies and procedures and hiring of additional qualified personnel; however, these and other remediation efforts may not enable us to remedy the material weaknesses or avoid other material weaknesses in the future. Because of these material weaknesses, there is heightened risk that a material misstatement of our annual or quarterly financial statements will not be prevented or detected. In the event that we have not adequately remedied these material weaknesses, and if we fail to maintain proper and effective internal controls in future periods, it could adversely affect our operating results, financial condition, ability to run our business effectively and our ability to timely meet our reporting requirements and could cause investors to lose confidence in our financial reporting. In addition, these and any other material weaknesses will need to be addressed as part of the evaluation of our internal controls over financial reporting pursuant to Section 404 of the Sarbanes-Oxley Act of 2002 and may impair our ability to comply with Section 404.
We operate in highly competitive markets and in some cases compete against larger companies with greater financial resources.
The market for radiation detection, measurement, analysis and monitoring products and services is fragmented, with a variety of small and large competitors, where the degree of fragmentation and the identities of our competitors vary among our target end markets. Some of our competitors have greater financial resources than do we, and they may be able to focus those resources on developing products or services that
13
are more attractive to potential customers than those that we offer, or on lobbying efforts to enhance their prospects of obtaining government contracts. Some of our competitors, for example, are substantially larger and better capitalized than we are and have the ability to combine solutions into an integrated offering at attractive prices. Our competitors may offer these solutions at prices below cost in order to improve their competitive positions. Any of these competitive factors could make it more difficult for us to attract and retain customers, cause us to lower our prices to compete, and reduce our market share and revenue, any of which could have a material adverse effect on our business, financial condition and results of operations.
Amounts included in our order backlog may not result in actual revenue or translate into profits.
Although the amount of our backlog is based on signed purchase orders or other written contractual commitments, we cannot guarantee that our order backlog will result in actual revenue in the originally anticipated period or at all. In addition, the mix of contracts included in our order backlog can greatly affect our margins in future periods, which may not be comparable to our historical product mix and operating results. Our customers may experience project delays or cancel orders due to factors beyond our control. If our order backlog fails to result in revenue in a timely manner or at all, we could experience a reduction in revenue and liquidity.
The recent global financial crisis and adverse worldwide economic conditions may have significant effects on our business, financial condition and results of operations.
The recent global financial crisis—which has included, among other things, significant reductions in available capital and liquidity, substantial reductions and fluctuations in equity and currency values, a reduction in global demand for energy and a worldwide recession, the effects of which are likely to be significant and prolonged—may have a material adverse effect on our business. We have begun to experience some weakening in demand for certain of our products and services, particularly for our high-temperature cameras used as monitoring tools in petrochemical facilities and cement kilns. Factors such as lack of business investment, government spending, the volatility and strength of the capital markets and inflation all affect the business and economic environment and, ultimately, our business, financial condition and results of operations. Continued market disruptions and broader economic downturns may affect our and our customers’ access to capital, lead to lower demand for our products and services, increase our exposure to losses from bad debts or result in our customers ceasing operations, any of which could materially adversely affect our business, financial condition and results of operations.
The credit markets have been experiencing extreme volatility and disruption for more than twelve months, and the volatility and disruption have reached unprecedented levels. In many cases, the markets have limited credit capacity for certain issuers, and lenders have requested shorter terms. The market for new debt financing is extremely limited and in some cases not available at all. In addition, the markets have increased the uncertainty that lenders will be able to comply with their previous commitments. If recent levels of market disruption and volatility continue or worsen, we may not be able to refinance our existing debt, or incur additional debt, which may require us to seek alternative funding sources to meet our liquidity needs or to fund planned expansion. Such alternative sources of funding may not be available on acceptable terms or at all.
Furthermore, the tightening of credit in financial markets may delay or prevent our customers from securing funding adequate to operate their businesses and purchase our products and services and could lead to an increase in our bad debt levels.
Unfavorable currency exchange rate fluctuations could adversely affect our profitability.
Our international sales and our operations in foreign countries expose us to risks associated with fluctuating currency values and exchange rates. Most of our sales, costs, assets and liabilities are denominated in foreign currencies. For fiscal 2010, 51.8% and 3.8% of our sales were denominated in euros and pounds sterling. Gains and losses on the conversion of accounts receivable, accounts payable and other monetary assets and liabilities to U.S. dollars may contribute to fluctuations in our results of operations. In addition, increases in the value of the U.S. dollar relative to the euro and the pound sterling could have an adverse
14
effect on our results of operations. We do not currently purchase forward contracts to hedge against the risks associated with fluctuations in exchange rates.
We may be less competitive if we fail to develop new or enhanced products and introduce them in a timely manner.
The markets in which we compete are subject to technological change, product obsolescence and evolving industry standards. Our ability to successfully compete in these markets and to continue to grow our business depends in significant part upon our ability to develop, introduce and sell new and enhanced products in a timely and cost-effective manner, and to anticipate and respond to changing customer requirements. We have experienced, and may in the future experience, delays in the development and introduction of new products. These delays could provide a competitor a first-to-market advantage and allow a competitor to achieve greater market share. Defects or errors found in our products after commencement of commercial shipment could result in delays in market acceptance of these products. Lack of market acceptance for our new products will jeopardize our ability to recoup research and development expenditures, hurt our reputation and harm our business, financial condition and results of operations. Accordingly, we can not assure you that our future product development efforts will be successful.
Our existing and future customers may reduce or halt their spending on radiation detection, measurement, analysis and monitoring products and services.
A variety of factors may cause our existing or future customers to reduce or halt their spending on radiation detection, measurement, analysis and monitoring products and services. These factors include:
| | |
| | • | disruptions in the nuclear fuel cycle, such as insufficient uranium supply or conversion; |
| |
| | • | unfavorable financial conditions and strategies of the builders, owners and operators of nuclear reactors; |
| |
| | • | civic opposition to or changes in government policies regarding nuclear operations; |
| |
| | • | a reduction in demand for nuclear generating capacity; |
| |
| | • | accidents, terrorism, natural disasters or other incidents occurring at nuclear facilities; and |
| |
| | • | the decision by one or more of our customers to acquire one of our competitors or otherwise administer the services we provide internally. |
Certain of these events could also adversely affect us to the extent that they result in the suspension or reduction of nuclear reactor construction, refurbishment or operation; the reduction of supplies of nuclear raw materials; increased regulation; increased operational costs for us or our customers; or increased liability for actual or threatened property damage or personal injury.
If we are unable to obtain adequate supplies in a timely manner, our results of operations would be adversely affected.
We are dependent upon certain sole or limited source suppliers for critical raw materials or components of some of our products. For example, we rely on the U.S. government for the enriched uranium used in certain equipment employed in our sensing systems. We also rely on limited source suppliers of certain precious metals used in some of our reactor instrumentation, scintillator materials used in our detection & identification equipment and for analog sensor tubes used in certain of our imaging products. Most of our suppliers are not required to supply us with any minimum quantities, and we cannot assure you that we will receive adequate quantities of components on a timely basis in the future. For example, a sole source supplier has recently informed us that its supplier is discontinuing the manufacture of a component that we use in certain of our dosimetry badges used by our Dosimetry Services division to provide dosimetry services to a majority of its dosimetry badge wearers. The notification prompted us to secure a final end-of-life order in an amount sufficient to meet our anticipated production requirements for the affected type of dosimetry badge at least through December 2013 and potentially through December 2015, the exact duration depending on the timing and degree to which our existing customers migrate to our other dosimetry badges that are unaffected by this discontinued component. We and our current suppliers already offer substitute dosimetry badges that
15
meet the requirements of our customers, that do not require requalification and that are available at a substantially similar cost to us. Our suppliers could have financial or other problems that could cause a disruption in the supply of components to us. In addition, were we to change suppliers of components in some of our products, we may be required to seek new qualifications for such products, which can be a time-consuming and costly process. As a result of interruption of supply, we may not be able to obtain the raw materials or components that we need to fill customer orders. The inability to fill these orders could cause delays, disruptions or reductions in product shipments, require us to negotiate alternate supply arrangements with replacement suppliers where available or require product redesigns which could, in turn, damage relationships with current or prospective customers, increase costs or prices and have a material adverse effect on our business, financial condition and results of operations.
We rely on third-party manufacturers to produce non-core components for certain of our products and services. If our manufacturers are unable to meet our demand or requirements, our business could be harmed.
We use third-party manufacturers to produce certain non-core components for some of our products. From time to time demand for our products has grown faster than the supply capabilities of these vendors. In many cases, these manufacturers have no obligation to supply products to us for any specific period, in any specific quantity or at any specific price, except as set forth in a particular purchase order. Our requirements represent a small portion of the total production capacities for many of our manufacturers, and our manufacturers may reallocate capacity to other customers, even during periods of high demand for our products or services. We have in the past experienced, and may in the future experience, quality control issues and delivery delays with our manufacturers due to factors such as high industry demand or the inability of our manufacturers to consistently meet our quality or delivery requirements. In addition, third-party manufacturers may have financial difficulties and face the risk of bankruptcy, especially in light of the current worldwide economic downturn. If one of our suppliers was to cancel or materially change a commitment with us or fail to meet the quality or delivery requirements needed to satisfy customer orders for our products, we could lose time-sensitive customer orders, be unable to develop or sell our products or services cost effectively or on a timely basis, if at all, and have significantly decreased revenue, which would harm our business, financial condition and results of operations. We may qualify additional suppliers in the future which would require time and resources. If we do not qualify additional suppliers, we may be exposed to increased risk of capacity shortages due to our dependence on our current suppliers.
Some of our suppliers and customers are also our competitors.
Some of our competitors are also our suppliers and customers. For example, Canberra, one of our chief competitors in the nuclear and defense end markets, supplies us with some of the detectors employed in the radiation monitoring systems that we supply to the nuclear end market. At the same time, Areva, the controlling shareholder of Canberra, is a customer for our radiation monitoring systems for use in its EPR reactors and in other fuel cycle industry applications. Similarly, Thermo Fisher Scientific both supplies our Dosimetry Services division with TLD crystals used in some of our thermoluminescent dosimeters that we deploy as part of providing dosimetry services to our customers, and sells products competitive with those offered by our Health Physics division. As with our other suppliers, our competitor suppliers are not required to supply us with any minimum quantities, and we cannot assure you that we will receive adequate quantities of components on a timely basis in the future. The loss of orders stemming from the actions of our supplier or customer competitors could cause delays, disruptions or reductions in product shipments or require product redesigns which could, in turn, damage relationships with current or prospective customers, increase costs or prices and have a material adverse effect on our business, financial condition and results of operations.
We could lose money if we fail to accurately estimate our costs or fail to execute within our cost estimates on fixed-price contracts.
Many of our contracts are fixed-price contracts which do not provide for price escalation in the event of unanticipated cost overruns. Under these contracts, we perform our services and provide our products at a fixed price. Fixed-price contracts carry inherent risks, including risks of losses from underestimating costs, operational difficulties and other changes that may occur over the contract period. We have in the past
16
experienced unanticipated cost overruns on some of our fixed-price contracts. If our cost estimates for a contract are inaccurate, or if we do not execute the contract within our cost estimates, we may incur losses or the contract may not be as profitable as we expected. In addition, we are sometimes required to incur costs in connection with modifications to a contract that may not be approved by the customer as to scope or price, or to incur unanticipated costs, including costs for customer-caused delays, errors in specifications or designs or contract termination, that we may not be able to recover. These, in turn, could adversely affect our business, financial condition and results of operations. The revenue, cost and gross profit realized on such contracts can vary, sometimes substantially, from the original projections due to changes in a variety of factors, such as:
| | |
| | • | failure to properly estimate, or changes in, the costs of material, components or labor; |
| |
| | • | currency exchange rate fluctuations; |
| |
| | • | unanticipated technical problems with the products or services being supplied by us, which may require that we spend our own money to remedy the problem; |
| |
| | • | our suppliers’ or subcontractors’ failure to perform; |
| |
| | • | difficulties of our customers in obtaining required governmental permits or approvals; |
| |
| | • | changes in local laws and regulations; |
| |
| | • | unanticipated delays in construction of new NPPs and decommissioning of existing NPPs; and |
| |
| | • | limited history with new products and new customers. |
Many of our large contracts have penalties for late completion.
In some cases, including many of our fixed-price contracts, we have agreed to complete a project by a scheduled date. If we fail to complete the project as scheduled, we may be held responsible for costs associated with the delay, generally in the form of liquidated damages, in some cases up to the full value of the contract. We have in the past incurred penalties associated with late completion on some of our contracts. In the event that a project is delayed, the total costs of the project could exceed our original estimates, and we could experience reduced profits or a loss for that project.
Our products and services involve the detection and monitoring of radiation, and the failure of our products or services to perform to specification could adversely affect our business, financial condition or results of operations.
Our products and services involve the detection and monitoring of radiation and are crucial components of the safety measures employed with respect to ionizing radiation. The failure of our products to perform to specification could result in personal injury or death and property damage (including environmental contamination). Legal and regulatory actions taken in response to product failure could result in significant costs to us. Additionally, the failure of our products to perform to specification could adversely affect market perception of the quality and effectiveness of our products and services, which would harm our ability to attract new customers and could cause our existing customers to cease doing business with us.
While we have attempted to secure appropriate insurance coverage at a reasonable cost, we do not insure against all risks and a claim can exceed the limits of our policies. We cannot assure you that our insurers will pay a particular claim, or that we will be able to maintain coverage at reasonable rates in the future, or at all. We may also be subject to significant deductibles.
Our contracts with customers generally seek to limit our liability in connection with product failure, but we cannot assure you that these contractual limitations on liability will be effective or sufficient in scope in all cases or that our insurance will cover the liabilities we have assumed under these contracts. The costs of defending against a claim arising out of such failure, and any damages awarded as a result of such a claim, could adversely affect our business, financial condition and results of operations.
Certain of our products and technologies for the defense end market may be eligible for designation or certification as “qualified anti-terrorism technologies” under the “SAFETY Act” provisions of The Homeland Security Act of 2002 and its implementing regulations. Under the SAFETY Act, the federal government
17
provides for certain liability limitations and a presumption that the “government contractor” defense applies if the Department of Homeland Security “designates” or “certifies” technologies or products as “qualified anti-terrorism technologies,” and if certain other conditions apply. We may seek to qualify some or all of our products and technologies under the SAFETY Act’s provisions in order to obtain such liability protections, but there is no guarantee that the Department of Homeland Security will designate or certify our products and technologies as qualified anti-terrorism technology. To date, we have not sought such designation or certification, and our products have been sold without such qualification, and we may continue to sell our products and technologies without such qualification. To the extent we do so, we will not be entitled to the benefit of the SAFETY Act’s limitations on tort liability or to any U.S. government indemnification.
We and our customers operate in a politically sensitive environment, and the public perception of nuclear power can affect our customers and us.
We and our customers operate in a politically sensitive environment. The risks associated with radioactive materials and the public perception of those risks can affect our business. Opposition by third parties can delay or prevent the construction of new NPPs and can limit the operation of nuclear reactors. Adverse public reaction to developments in the use of nuclear power, including incidents involving the discharge of radioactive materials, could directly affect our customers and indirectly affect our business. In the past, adverse public reaction, increased regulatory scrutiny and litigation have contributed to extended construction periods for new nuclear reactors, sometimes delaying construction schedules by decades or more. For example, no new reactor has been ordered in the United States since the 1970s and anti-nuclear groups in Germany successfully lobbied for the adoption of the Nuclear Exit Law in 2002, which requires the shutdown of all German NPPs by 2021. Adverse public reaction could also lead to increased regulation or limitations on the activities of our customers, more onerous operating requirements or other conditions that could have a material adverse impact on our customers and our business.
Our operations in foreign countries are subject to political, economic and other risks, which could have a material adverse effect on us.
Revenue outside of the Americas accounted for approximately 64.1%, 61.6% and 65.1% of our net sales in fiscal 2008, 2009 and 2010. We anticipate that international sales will continue to constitute a material percentage of our total net sales in future periods. As a result, our operations are subject to risks associated with global operations and sales, including:
| | |
| | • | foreign currency exchange fluctuations; |
| |
| | • | changes in regulatory requirements; |
| |
| | • | tariffs and other barriers; |
| |
| | • | timing and availability of export licenses; |
| |
| | • | difficulties in accounts receivable collections; |
| |
| | • | difficulties in protecting our intellectual property; |
| |
| | • | difficulties in staffing and managing foreign operations; |
| |
| | • | difficulties in managing sales agents, distributors and other third parties; |
| |
| | • | coordination regarding, and difficulties in obtaining, governmental approvals for products that may require certification; |
| |
| | • | rescission or termination of contracts by governmental parties without penalty and regardless of the terms of the contract; |
| |
| | • | restrictions on transfers of funds and other assets of our subsidiaries between jurisdictions; |
| |
| | • | the burden of complying with a wide variety of complex foreign laws and treaties; |
| |
| | • | potentially adverse tax consequences; and |
| |
| | • | uncertainties relative to regional political and economic circumstances. |
We are also subject to risks associated with the imposition of legislation and regulations relating to the import or export of radiation detection and monitoring technology. For example, certain export control
18
approvals of our sales, including sales to NPP operators located in Pakistan and India, were granted because of the supervision of customer sites by the International Atomic Energy Agency, or the IAEA. If the IAEA ceases to supervise such sites, our ability to sell to certain customers would be limited and our sales could be adversely affected. Furthermore, the failure to comply with export control regulations and to obtain required approvals could result in loss of the ability to export products, fines and penalties.
We cannot predict whether quotas, duties, taxes or other charges or restrictions upon the importation or exportation of our products will be implemented by the United States or other countries. Some of our customers’ purchase orders and agreements are governed by foreign laws, which often differ significantly from the laws of the United States. Therefore, we may be limited in our ability to enforce our rights under such agreements and to collect damages, if awarded. These factors may have a material adverse effect on our business, financial condition and results of operations.
Changes in our effective tax rate or adverse outcomes resulting from examination of our income tax returns could adversely affect our results.
Our effective tax rate could be adversely affected by several factors, many of which are outside of our control, including:
| | |
| | • | earnings being lower than anticipated in countries where we are taxed at lower rates as compared to the U.S. statutory tax rate; |
| |
| | • | material differences between forecasted and actual tax rates as a result of a shift in the mix of pre-tax profits and losses from one tax jurisdiction to another, our ability to use tax credits or effective tax rates by tax jurisdiction that differ from our estimates; |
| |
| | • | changing tax laws or related interpretations, accounting standards and regulations and interpretations in multiple tax jurisdictions in which we operate, as well as the requirements of certain tax rulings; |
| |
| | • | an increase in expenses not deductible for tax purposes, including certain stock-based compensation expense and impairment of goodwill; |
| |
| | • | the tax effects of purchase accounting for acquisitions and restructuring charges that may cause fluctuations between reporting periods; |
| |
| | • | changes related to our ability to ultimately realize future benefits attributed to our deferred tax assets, including those related to other-than-temporary impairments; |
| |
| | • | tax assessments resulting from income tax audits or any related tax interest or penalties that would affect our income tax expense for the period in which the settlements take place; and |
| |
| | • | a change in our decision to indefinitely reinvest foreign earnings. |
In addition, we may be subject to examination of our income tax returns by the Internal Revenue Service or other tax authorities. If tax authorities challenge the relative mix of our U.S. and international income, our future effective income tax rates could be adversely affected. While we regularly assess the likelihood of adverse outcomes from such examinations and the adequacy of our provision for income taxes, we cannot assure you that such provision is sufficient and that a determination by a tax authority will not have an adverse effect on our business, financial condition and results of operations.
Localization requirements could adversely affect our business.
Many emerging markets, including China and South Korea, impose localization requirements which favor locally based component manufacturers in the construction of NPPs and which require some degree of technology transfer to local manufacturers. Over time, such localization requirements could limit our ability to sell into such markets and could affect our ability to maintain our trade secrets. In the past, international development agencies have, as a condition of funding, imposed localization requirements that require the transfer of technology to local manufacturers, and this requirement has affected our ability to monitor and maintain control over our intellectual property. We may be subject to similar requirements as a condition of funding in the future.
19
We must comply with the U.S. Foreign Corrupt Practices Act, or FCPA. Failure to comply with the FCPA could subject us to, among other things, penalties and legal expenses that could harm our reputation and have a material adverse effect on our business, financial condition and results of operations.
We are required to comply with the United States Foreign Corrupt Practices Act, which generally prohibits U.S. companies and their intermediaries from engaging in bribery or making other prohibited payments to foreign officials for the purpose of obtaining or retaining business and/or other benefits. We operate in a number of jurisdictions that pose a high risk of potential FCPA violations, we have government customers and we utilize a number of third-party sales representatives. Although we have begun to inform our personnel and third-party sales representatives regarding the requirements of the FCPA and have developed and will continue to develop and implement systems for formalizing contracting processes, performing diligence on agents and improving our record-keeping and auditing practices, we cannot assure you that our employees, third-party sales representatives or other agents have not or will not engage in conduct for which we might be held responsible under the FCPA.
If our employees, third-party sales representatives or other agents are found to have engaged in such practices, we could suffer severe penalties, including criminal and civil penalties, disgorgement and other remedial measures (including further changes or enhancements to our procedures, policies and controls, as well as potential personnel changes and disciplinary actions), any of which could have an adverse impact on our business, financial condition, results of operations and liquidity. Any investigation of any potential violations of the FCPA or other anti-corruption laws by U.S. or foreign authorities also could have an adverse impact on our business, financial condition and results of operations.
Certain foreign companies, including some of our competitors, are not subject to prohibitions as strict as those under the FCPA or, even if subjected to strict prohibitions, such prohibitions may be laxly enforced in practice. If our competitors engage in corruption, extortion, bribery, pay-offs, theft or other fraudulent practices, they may receive preferential treatment from personnel of some companies, giving our competitors an advantage in securing business, or from government officials, who might give them priority in obtaining new licenses, which would put us at a disadvantage.
We may make acquisitions that involve numerous risks. If we are not successful in integrating the technologies, operations and personnel of acquired businesses or fail to realize the anticipated benefits of an acquisition, our operations may be adversely affected.
As part of our business and growth strategy, we may acquire or make significant investments in businesses, products or technologies that allow us to complement our existing product offerings, expand our market coverage, increase our engineering workforce or enhance our technological capabilities. Any future acquisitions or investments would expose us to many risks, including:
| | |
| | • | problems integrating the new personnel or the purchased operations, technologies or products; |
| |
| | • | difficulty securing adequate working capital; |
| |
| | • | unanticipated costs associated with the acquisition; |
| |
| | • | negative effects on our ability to generate excess free cash flow; |
| |
| | • | negative effects on profitability; |
| |
| | • | adverse effects on existing business relationships with suppliers and customers; |
| |
| | • | risks associated with entering markets in which we have no or limited prior experience; |
| |
| | • | loss of key employees of the acquired business; |
| |
| | • | litigation arising from the operations before they were acquired by us; and |
| |
| | • | difficulty completing financial statements and audits. |
Our inability to overcome problems encountered in connection with any acquisition could divert the attention of management, utilize scarce corporate resources and otherwise harm our business. In addition, we are unable to predict whether or when any prospective acquisition candidate will become available or the
20
likelihood that any acquisition will be completed. Even if we do find suitable acquisition opportunities, we may not be able to consummate the acquisitions on commercially acceptable terms or realize the anticipated benefits of any acquisitions we do undertake.
A failure to expand our manufacturing capacity and scale our capabilities to manufacture new products could constrain our ability to grow our business.
We have needed to increase our manufacturing capacity, particularly in our locations in Lamanon, France and Hamburg, Germany to support the fulfillment of certain large contracts and to put us in position to accommodate growth in our business. Accordingly, we have initiated an expansion of our manufacturing facilities in Lamanon and renovated and reorganized our facilities in Hamburg. The future growth of our business depends on our ability to successfully expand our manufacturing capacity. Expansion of our manufacturing capacity may require us to obtain regulatory approvals or additional financing. Delay in the expansion of our manufacturing capacity could constrain our ability to grow our business, which would adversely affect our business, financial condition and results of operations.
Similarly, we could have substantial difficulty in dealing with rapid growth in markets for new products that we may introduce. If demand for our new products increases rapidly, we will need to expand internal production capacity or implement additional outsourcing. Success in developing, manufacturing and supporting products manufactured in small volumes does not guarantee comparable success in operations conducted on a larger scale. Manufacturing yields and product quality may decline as production volumes increase. If we are unable to deliver products quickly and cost effectively and in the requisite volumes, our customers may decline to purchase our new products or may purchase substitute products offered by our competitors. The costs associated with implementing new manufacturing technologies, methods, and processes, including the purchase of new equipment, and any resulting delays, inefficiencies and loss of sales, could harm our results of operations.
We rely on third-party sales representatives to assist in selling our products and services, and the failure of these representatives to perform as expected could reduce our future sales.
We typically derive a portion of our revenue from sales to our customers through third-party sales representatives. We have established relationships with some of our third-party sales representatives recently, and we are unable to predict the extent to which our third-party sales representatives will be successful in marketing and selling our products and services. Moreover, many of our third-party sales representatives also market and sell competing products and services, which may affect the extent to which our third-party sales representatives promote our products and services. Even where our relationships are formalized in contracts, our third-party sales representatives often have the right to terminate their relationships with us at any time. Our future performance will also depend, in part, on our ability to attract additional third-party sales representatives who will be able to market and support our products and services effectively, especially in markets in which we have not previously sold our products and services. If we cannot retain our current third-party sales representatives or recruit additional or replacement third-party sales representatives, our business, financial condition and results of operations could be harmed.
The elimination or any modification of the Price-Anderson Act’s indemnification authority could have adverse consequences for our business.
In the United States, the Atomic Energy Act of 1954, as amended, or the AEA, comprehensively regulates the manufacture, use and storage of radioactive materials. Section 170 of the AEA, which is known as the Price-Anderson Act, supports the nuclear services industry by offering broad indemnification to commercial NPP operators and Department of Energy, or DOE, contractors for liabilities arising out of nuclear incidents at power plants licensed by the Nuclear Regulatory Commission, or NRC, and at DOE nuclear facilities. The indemnification authority of the NRC and DOE under the Price-Anderson Act was extended through 2025 by the Energy Policy Act of 2005. Some of our customers are covered by the indemnification provisions of the Price-Anderson Act. In addition, other jurisdictions have similar indemnification authority. If the indemnification authority in the United States or other countries is eliminated or adversely modified in the
21
future, our business could be adversely affected if the owners and operators of NPPs cancel or delay plans to build new plants or curtail the operations of existing plants.
Our ability to provide bid bonds, performance bonds or letters of credit is limited and could negatively affect our ability to bid on or enter into significant contracts.
We are sometimes required to provide bid or performance bonds or letters of credit to guarantee performance under contracts across our various divisions. Our ability to obtain such bonds and letters of credit depends upon several factors, including our capitalization, working capital, past performance, management expertise and reputation and external factors beyond our control, including the overall capacity of the surety market. Surety companies consider those factors in relation to the amount of our tangible net worth and other underwriting standards that may change from time to time. Events that affect surety markets generally may result in bonding becoming more difficult to obtain in the future, or being available only at a significantly higher cost. Our inability to obtain adequate bonding and, as a result, to bid for, or enter into, significant contracts, could adversely affect our future financial condition and results of operations.
As a U.S. government contractor, we are subject to a number of procurement rules and regulations.
U.S. government contractors and subcontractors must comply with specific procurement regulations and other requirements and are subject to routine audits and investigations by U.S. government agencies. If we fail to comply with these rules and regulations, we could be subject to reductions in the value of our government contracts, contract modification or termination, the assessment of penalties and fines,and/or suspension or debarment from government contracting or subcontracting for a period of time or permanently.
Furthermore, we have bid, and may in the future submit bids, for U.S. government contracts that require our employees to maintain various levels of security clearances and require us or our subsidiaries to maintain certain facility security clearances in compliance with Department of Defense and other government requirements. Obtaining and maintaining security clearances for employees involves a lengthy process, and it can be difficult to identify, recruit and retain employees who already hold security clearances. If our employees are unable to obtain or retain security clearances, or if our employees who hold security clearances stop working for us, we may face delays in fulfilling contracts, or be unable to fulfill or secure new contracts, with any customer involved in classified work. Any breach of security for which we are responsible could seriously harm our business, damage our reputation and make us ineligible to work on any classified programs.
The classified work that we currently perform at one of our facilities subjects us to the industrial security regulations of the Department of Defense and other federal agencies that are designed to safeguard against unauthorized access by foreigners and others to classified and other sensitive information. We may be subject to penalties for violations of these regulations. If we were to come under foreign ownership, control, or influence, the U.S. government could terminate our contracts with it or decide not to renew them and such a situation could also impair our ability to obtain new contracts and subcontracts. The government may also change its procurement practices or adopt new contracting rules and regulations that could be costly to satisfy or that could impair our ability to obtain new contracts.
We are subject to a variety of federal, state, local and foreign laws and regulatory regimes. Failure to comply with laws and regulations could subject us to, among other things, penalties and legal expenses which could have an adverse effect on our business.
Our business is subject to regulation by various federal, state, local and foreign governmental agencies. In the U.S., such regulation includes the radioactive material and exposure regulatory activities of the NRC, the anti-trust regulatory activities of the Federal Trade Commission and Department of Justice, the import/export regulatory activities of the Department of Commerce, the Department of State and the Department of Treasury, the regulatory activities of the Occupational Safety and Health Administration, the environmental regulatory activities of the Environmental Protection Agency, the labor regulatory activities of the Equal Employment Opportunity Commission and tax and other regulations by a variety of regulatory authorities in each of the areas in which we conduct business. We are also subject to regulation in other countries where we
22
conduct business. In certain jurisdictions, such regulatory requirements may be more stringent than in the United States. We are also subject to a variety of U.S. federal and state employment and labor laws and regulations, including the Americans with Disabilities Act, the Federal Fair Labor Standards Act, the Worker Adjustment and Restructuring Notification Act, which requires employers to give affected employees at least 60 days’ notice of a plant closing or mass layoff, and other regulations related to working conditions,wage-hour pay, overtime pay, employee benefits, anti-discrimination and termination of employment. We are also subject to the employment and labor laws and regulations of the foreign jurisdictions, including France and Germany, where the majority of our employees are located.
Noncompliance with applicable regulations or requirements could subject us to investigations, sanctions, enforcement actions, disgorgement of profits, fines, damages, civil and criminal penalties, injunctions or debarment from government contracting or subcontracting. In addition, from time to time we have received, and may in the future receive, correspondence from former employees terminated by us who threaten to bring claims against us alleging that we have violated one or more labor or employment regulations. An adverse outcome in any such litigation could require us to pay damages.
Governmental enforcement actions could harm our business, financial condition and results of operations. If any governmental sanctions are imposed, or if we do not prevail in any civil or criminal litigation, our business, financial condition and results of operations could be materially adversely affected. In addition, responding to any action could be costly and result in a significant diversion of management’s attention and resources.
Certain of our products require the use of radioactive sources or incorporate radioactive materials, which subjects us and/or our customers to regulation, related costs and delays and potential liabilities for injuries or violations of environmental, health and safety laws.
Certain products in our Health Physics, Radiation Monitoring Systems and Sensing Systems divisions require the use of radioactive sources. For certain of our products, these radioactive sources are often obtained by our customers directly from third-party providers, and for others, we directly incorporate these radioactive sources into our products. Certain of our reactor instrumentation and control equipment and systems in our Sensing Systems division incorporate radioactive materials. In all such cases, licenses for radioactive sources and materials are provided by the appropriate regulatory authority in the relevant jurisdiction and such authorities may be at the state or national level. Our failure or any customer’s failure to obtain the necessary license for radioactive sources or materials required by or incorporated into our products could result in the cancellation or delay of purchases by our customers.
While the specific process and criteria for receiving a license differ from jurisdiction to jurisdiction, it generally involves an application process in which we: identify a person or persons who have appropriate training and experience to be a health physics/radiation safety officer; specify the radioactive sources or materials sought to be licensed, their physical form (i.e., sealed or unsealed) and maximum possession limits on the amount of each type of radioactive element or compound sought under the license; specifies their intended use (e.g., calibration, testing, quality assurance, manufacturing); and, set forth written policies and procedures to ensure that we have adequate measures in place to ensure health and safety. These policies and procedures typically must be designed to ensure worker, workplace, and public safety, including emergency plans; set forth the proper handling, control and security of radioactive sources or materials on site; detail any disposal or decommissioning considerations; and adequately train personnel at the site in proper access to, and handling of, radioactive sources or materials. Our noncompliance with or failure to properly implement such policies and procedures could delay or otherwise preclude us from obtaining the necessary license for radioactive sources or materials required by or incorporated into our products, which could result in the cancellation or delay of purchases by our customers.
The particular license requirements in a given jurisdiction are normally tailored to the specific radioactive elements or compounds involved, their physical form, and possession limits. Once authorities complete their application review and any requiredfollow-up, the authority issues the site a license which imposes specific on-going compliance obligations that typically include requirements for us to pay periodic licensing fees, submit periodic written compliance reports, and agree to periodic site inspections by regulators, which may be
23
announced or unannounced. Our failure to comply with any of these on-going obligations could result in the revocation of the necessary license for radioactive sources or materials required by or incorporated into our products, which could result in the cancellation or delay of purchases by our customers.
We are subject to federal, state and local regulations governing storage, handling and disposal of these radioactive materials and waste products. Outside of the United States, we are also subject to radiation regulations that vary from country to country. The improper storage, use and disposal of such materials by usand/or our customers could result in direct or secondary liability, including penalties and fines, to us in the event of environmental contamination or physical injury. Although we believe that our safety procedures for storing, handling and disposing of these hazardous materials comply in all material respects with the standards prescribed by law and regulation, we cannot eliminate the risk of accidental contamination or injury from those hazardous materials nor can we control the practices of our customers. The sale and use of our products with radioactive sources or materials could also lead to the filing of claims if someone were to allege injury from the use of one of our products or allege that one of our products was defective. Such a claim could result in substantial damages, be costly and time-consuming to defend and adversely affect the marketability of our products and our reputation.
We and our customers operate in highly regulated industries that require us and them to obtain, and to comply with, federal, state, local and foreign government permits and approvals.
We and our customers operate in a highly regulated environment. Many of our products and services must comply with various domestic and international standards that are used by regulatory and accreditation bodies for approving such services and products. Many of our products, particularly those offered by our Radiation Monitoring Systems and Sensing Systems divisions, are subject to an array of product testing under extreme temperature, pressure, radiation and seismic conditions, known collectively as a qualification, for any given nuclear reactor design. The qualification is typically owned by the party who pays for the testing and so, in certain cases, we license such qualifications from a third party. In addition, many of our products and services, particularly those offered by our Dosimetry Services division, must be certified by the National Voluntary Laboratory Accreditation Program in the United States and by other governmental agencies in international markets. The termination of any such accreditation or our failure to obtain and maintain required qualification or accreditation for our products and services may adversely affect our revenue and results of operations.
Changes in these standards and accreditation requirements may also result in our having to incur substantial costs to adapt our products. Such adaptations may introduce quality assurance issues during transition as new features and products may not perform as expected. Additionally, changes affecting radiation protection practices, including new understandings of the hazards of radiation exposure and corresponding changes in regulations, may impact how our services are used by our customers and may, in some circumstances, cause us to alter our products and services.
In addition, our customers are required to obtain, and to comply with, federal, state, local and foreign government licenses, permits and approvals with respect to either their facilities or possession and use of radioactive sources or other radioactive materials. Any of these licenses, permits or approvals may be subject to denial, revocation or modification under various circumstances. Failure to obtain or comply with the conditions of licenses, permits or approvals may adversely affect our customers’ operations by suspending their activities or delaying or preventing the receipt of radioactive sources or other radioactive materials, and may subject them to penalties and other sanctions. Although existing licenses, permits or approvals are routinely renewed by various regulators, renewal could be denied or jeopardized by various factors, including:
| | |
| | • | failure to provide adequate financial assurance in the event of decommissioning or closure; |
| |
| | • | failure to comply with environmental and safety laws and regulations or permit conditions; |
| |
| | • | local community, political or other opposition; |
| |
| | • | executive action; and |
| |
| | • | legislative action. |
24
Furthermore, if new environmental legislation or regulations are enacted or existing laws or regulations are amended or are interpreted or enforced differently, our customers may be required to obtain additional operating licenses, permits or approvals. Regulatory issues experienced by our customers may lead to delay or cancellation of their orders for our products and services or the discontinuance of future orders. We cannot assure you that we or our customers will be able to meet all potential regulatory challenges.
We could incur substantial costs as a result of violations of or liabilities under environmental laws.
Our operations and properties are subject to a variety of federal, state, local and foreign environmental laws and regulations governing, among other things, air emissions, wastewater discharges, management and disposal of hazardous and non-hazardous materials and waste and remediation of releases of hazardous materials. Our failure to obtain any necessary permits or to comply with present and future laws, regulations or permits, or the identification of contamination at our or our predecessors’ former or current facilities or at third-party waste disposal sites, could cause us to incur substantial costs, includingclean-up costs, fines and penalties, investments to upgrade our facilities or curtailment of operations. The future identification of presently unidentified environmental conditions, more vigorous enforcement by regulatory agencies, enactment of more stringent laws, regulations or permit requirements or other unanticipated events may arise in the future and give rise to material environmental liabilities and related costs which could have a material adverse effect on our business, financial condition and results of operations.
A European Union, or EU, directive relating to the restriction of hazardous substances in electrical and electronic equipment, or RoHS directive, and an EU directive relating to waste electrical and electronic equipment, or WEEE directive, have been and are being implemented in EU member states. Among other things, the RoHS directive restricts the use of certain hazardous substances in the manufacture of electrical and electronic equipment and the WEEE directive requires producers of electrical goods to be responsible for the collection, recycling, treatment and disposal of these goods. In addition, laws similar to the RoHS and WEEE directives were passed in China in 2006 and South Korea in 2007. Governments in other countries and states, including the United States, are considering implementing similar laws or regulations.
In addition, a regulation regarding the registration, authorization and restriction of chemical substances in industrial products, or REACH, became effective in the EU in 2007. REACH and other regulations require us or our suppliers to substitute certain chemicals contained in our products with substances the EU considers less dangerous. We have assessed the impact that REACH is expected to have on us and have determined the impact at this time to be immaterial to our business and operations, but we cannot assure you that it or similar regulations will not materially affect us in the future.
The costs associated with complying with future laws and regulations could include costs associated with modifying or requalifying our products, recycling and other waste processing costs, legal and regulatory costs and insurance costs. We have recorded in the past and may be required to record in the future additional expenses for costs associated with compliance with regulations. The costs of complying with future environmental and worker health and safety laws and regulations could have a material adverse effect on our business, financial condition and results of operations.
Our future success is dependent on our ability to retain key personnel, including our executive officers, and attract qualified personnel. If we lose the services of these individuals or are unable to attract new talent, our business will be adversely affected.
Our future operating results depend in significant part upon the continued contributions of our key technical and senior management personnel, many of whom would be difficult to replace. We are particularly dependent on the continued service of Thomas D. Logan, our President, Chief Executive Officer and Chairman of the Board, W. Antony Besso, our Regional Vice President, EMEA, and President, Health Physics Division, Iain F. Wilson, our Regional Vice President, Asia, and President, Sensing Systems Division, and Jean-Louis Gouronc, our President, Radiation Monitoring Systems.
Our future operating results also depend in significant part upon our ability to attract, train and retain qualified management, manufacturing and quality assurance, engineering, marketing, sales and support personnel. In particular, engineers skilled in the analog technologies used in certain of our products are in high
25
demand and competition to attract such personnel is intense. In addition, the expected increase in construction of new NPPs may exacerbate the shortage of radiation engineers and other qualified personnel. We are continually recruiting such personnel; however, we cannot assure you that we will be successful in attracting, training or retaining such personnel now or in the future. There may be only a limited number of persons with the requisite skills to serve in these positions, and it may be increasingly difficult for us to hire such persons over time. The high demand for such personnel may increase the costs to us to recruit and retain employees.
The loss of any key employee, the failure of any key employee to perform in his or her current position, our inability to attract, train and retain skilled employees as needed or the inability of our officers and key employees to expand, train and manage our employee base could materially and adversely affect our business, financial condition and results of operations.
Our ability to compete successfully and achieve future growth will depend on our ability to protect our intellectual property and to operate without infringing the intellectual property of others.
Our business is largely dependent upon our design, engineering, manufacturing and testing know-how. We attempt to protect our intellectual property rights through trade secret laws, non-disclosure agreements, confidentiality procedures and employee disclosure and invention assignment agreements. To a lesser extent, we may also seek to protect our intellectual property through patents, trademarks and copyrights. We rely upon unpatented proprietary radiation detection expertise, continuing technological innovation and other trade secrets to develop and maintain our competitive position, some of which is licensed from third parties. Confidentiality agreements with our employees and third parties are often limited in duration and could be breached, and therefore they may not provide meaningful protection for our trade secrets or proprietary radiation detection expertise. Adequate remedies may not be available in the event of an unauthorized use or disclosure of our trade secrets. Others may obtain knowledge of our trade secrets through independent development or other access by legal means. It is possible that our efforts to protect our intellectual property rights may not:
| | |
| | • | prevent our competitors from independently developing similar products, duplicating our products or designing around the patents owned by us; |
| |
| | • | prevent third-party patents from having an adverse effect on our ability to do business; |
| |
| | • | provide adequate protection for our intellectual property rights; |
| |
| | • | prevent disputes with third parties regarding ownership of, or exclusive rights to, our intellectual property; |
| |
| | • | prevent disclosure of our trade secrets and know-how to third parties or into the public domain; |
| |
| | • | prevent the challenge, invalidation or circumvention of our existing patents; |
| |
| | • | result in patents that lead to commercially viable products or provide competitive advantages for our products; and |
| |
| | • | result in issued patents and registered trademarks from any of our pending applications. |
The laws of foreign countries also may not adequately protect our intellectual property rights. Many U.S. companies have encountered substantial infringement problems in foreign countries. Because we conduct a substantial portion of our operations and a majority of our sales have been outside of the United States, we have significant exposure to foreign intellectual property risks.
Others have in the past attempted, and may in the future attempt, to copy or otherwise obtain and use our intellectual property without our consent. Monitoring the unauthorized use of our intellectual property is difficult. There is a risk that our customers or their end users’ customers may attempt to copy or otherwise obtain and use our intellectual property without our consent. It may be necessary, from time to time, to initiate litigation against one or more third parties, including our customers or companies with whom we have manufacturing relationships, to preserve our intellectual property rights or to challenge the validity and scope of proprietary rights asserted by others, and we could face counterclaims. Legal disputes with our customers or companies with whom we have manufacturing relationships could substantially harm our relationships and
26
sales. Litigation is expensive and time consuming, and an adverse outcome could subject us to significant liability for damages or invalidate our proprietary rights.
From time to time, third parties may claim that we have infringed upon, misappropriated or misused other parties’ proprietary rights, and we may already be infringing without knowing it. Any of these events or claims could result in litigation. Such litigation, whether as plaintiff or defendant, could result in significant expense to us and divert the efforts of our technical and management personnel, whether or not such litigation is ultimately determined in our favor. In the event of an adverse result in such litigation, we could be required to pay substantial damages, cease the manufacture, use and sale of certain products, expend significant resources to develop or acquire non-infringing technology, discontinue the use of certain processes, obtain licenses to use the infringed technology, or indemnify our customers. Product development or license negotiating would likely result in significant expense to us and divert the efforts of our technical and management personnel. We cannot assure you that we would be successful in such development or acquisition or that such licenses would be available on reasonable terms, or at all.
Our obligations to indemnify our customers for the infringement by our products of the intellectual property rights of others could require us to pay substantial damages.
We currently have in effect a number of agreements in which we have agreed to defend, indemnify and hold harmless our customers and suppliers from damages and costs that may arise from the infringement by our products of third-party patents, trademarks or other proprietary rights. We may periodically have to respond to claims and initiate or participate in litigation in connection with these indemnification obligations, which may result in our paying substantial damages. Our insurance does not cover intellectual property infringement.
Some of our workforce is represented by labor unions in the United States and by works councils and trade unions in the EU, which may lead to work stoppages that could adversely affect our business.
As of June 30, 2010, approximately 33, or 12%, of our U.S. employees were unionized, and the majority of our EU employees are members of, or are represented by, works councils or trade unions. Since 1988, we have experienced only two work stoppages, at our facility in Lamanon, France. We may experience work stoppages or other labor problems in the future, which could adversely affect our business. We cannot predict how stable our relationships will be or whether we will be able to satisfy union or works council requirements without impacting our operating results and financial condition. Union and works council rules may limit our flexibility to respond to changing market conditions and the application of these rules could harm our business. The unions and works councils may also limit our flexibility in dealing with our workforce. Work stoppages and instability in our relationships could negatively impact the timely production of our products, which could strain relationships with customers and cause a loss of revenue that would adversely affect our results of operations.
Our operations could be subject to natural disasters and other business disruptions, which could materially adversely affect our business and increase our expenses.
Our operations could be subject to natural disasters and other business disruptions, which could harm our future revenue and financial condition and increase our costs and expenses. For example, our corporate headquarters in San Ramon, California and the center of operations of our Dosimetry Services division in Irvine, California are located near major earthquake fault lines. In the event of a major earthquake or other natural or manmade disaster, we could experience business interruptions, destruction of or damage to facilitiesand/or loss of life, any of which could have a material adverse effect on our business and increase our expenses.
Risks Related to this Offering and Our Common Stock
If we cannot generate sufficient operating cash flow and obtain external financing, we may be unable to make all of our planned capital expenditures and other expenses.
Our ability to fund anticipated capital expenditures and other expenses depends on generating sufficient cash flow from operations and the availability of external financing. Since our inception in 2005, ACAS and its affiliates have provided us with the capital and debt financing that we have used to fund our growth and
27
operations. Although ACAS will continue to be a significant stockholder in our company upon completion of this offering, ACAS is under no obligation to continue making capital investments in us or to provide debt financing to us and is unlikely to do so.
Our debt service obligations and our capital expenditures, together with on-going operating expenses, will be a substantial drain on our cash flow and may decrease our cash balances. The timing and amount of our capital requirements cannot be precisely determined at this moment and will depend on a number of factors, including demand for our products, product mix, changes in industry conditions and market competition. We intend to regularly assess markets for external financing opportunities, including debt and equity. Such financing may not be available when needed or, if available, may not be available on satisfactory terms, particularly in light of the limited financing available as a result of the recent global financial crisis. Any equity financing would cause further dilution to our stockholders. See “Dilution.” Our inability to obtain needed financing or to generate sufficient cash from operations may require us to abandon projects or curtail capital expenditures, and we could be materially adversely affected. If we are not able to independently generate excess free cash flow and obtain third party debt or equity financing, our ability to grow our business may be materially adversely affected.
Upon completion of this offering, ACAS will continue to have a significant influence over matters determined by our Board of Directors and will be in a position to significantly influence the outcome of all matters submitted to our stockholders for approval, which will limit your ability to influence corporate actions and may adversely affect the market price of our common stock.
Upon completion of this offering, ACAS, our principal stockholder, will beneficially own shares of our common stock (approximately % of our outstanding common stock), which includes shares of our common stock underlying warrants, or shares of our common stock (approximately % of our common stock) if the underwriters exercise in full their over-allotment option to purchase additional shares of common stock from the selling stockholders. As a result, ACAS will continue to have significant influence over the outcome of votes on all matters requiring approval by our stockholders, including the election of directors, the adoption of amendments to our amended and restated Certificate of Incorporation and Bylaws and approval of significant corporate transactions, as long as it continues to hold a significant percentage of our outstanding common stock.
On June 28, 2010, American Capital, Ltd. completed the restructuring of its unsecured revolving line of credit and its outstanding public and private notes by partially repaying and converting the line of credit into a term loan facility and partially repaying and exchanging outstanding public and private notes (the “Restructuring”). The new debt issued upon conversion of the existing line of credit and exchange of existing public and private notes is in the form of (i) secured loans made under a term loan facility for which Citicorp North America, Inc. serves as administrative agent, dated as of June 28, 2010 (the “Term Loan”) and (ii) secured notes (the “Secured Notes”) issued under an indenture with Wilmington Trust FSB, as trustee, dated as of June 28, 2010 (the “Indenture” and together with the Term Loan, the “ACAS Secured Financing”). The Term Loan and the Secured Notes are collectively secured by liens on substantially all of ACAS’s assets, including 8,419,715 shares of our outstanding common stock beneficially owned by American Capital, Ltd. prior to this offering or shares (approximately % of our outstanding shares) upon completion of this offering (or shares (approximately % of our outstanding shares) if the underwriters exercise in full their over-allotment option) (the “Pledge”). The shares of our capital stock beneficially owned by American Capital Equity I, LLC and American Capital Equity II, LP are not subject to the Pledge. If there is an event of default under the ACAS Secured Financing and either the lenders or the holders of the Secured Notes accelerate the Term Loan or the Secured Notes, as applicable, and exercise their rights under the Pledge, the purchasers of such shares, which may include the creditors secured by the Pledge, would hold a substantial percentage of our outstanding shares of common stock and would collectively become our largest stockholder. While such purchasers would not succeed to any of the rights specifically reserved to ACAS under our amended and restated Certificate of Incorporation and Bylaws, they may, under certain circumstances, succeed to the registration rights agreement into which we expect to enter upon the consummation of this offering, and they would have the ability to exert significant influence over the votes on all matters requiring approval by our stockholders by virtue of their large shareholdings.
28
Furthermore, in the event that ACAS were to sell fewer shares than contemplated hereby such that following the consummation of this offering ACAS and its affiliated funds continue to hold shares representing more than a majority of our outstanding shares, ACAS will receive the benefit of a provision of our amended and restated Bylaws that provides that at least one of the directors designated by ACAS must be part of the majority in any action taken by our Board of Directors so long as ACAS and its affiliated funds hold at least 50.1% of our outstanding common stock, other than on matters in which ACAS has a conflict of interest (as it would if it appointed a majority of our directors). Our amended and restated Bylaws also provide that ACAS will have the right to designate three of our seven directors so long as ACAS and its affiliated funds hold at least 50.1% of our outstanding common stock, two directors so long as they hold at least 25% but less than 50.1% and one director so long as they hold at least 10% but less than 25%.
ACAS will also be able to take actions that have the effect of delaying or preventing a change in control of us or discouraging others from making tender offers for our shares, which could prevent stockholders from receiving a premium for their shares. These actions may be taken even if other stockholders oppose them. In addition, this significant concentration of stock ownership may adversely affect the trading price for our common stock because investors often perceive disadvantages in owning stock in companies with controlling stockholders. This concentration of control could be disadvantageous to other stockholders with interests different from those of our principal stockholder and the trading price of our common stock could be adversely affected. See “Principal and Selling Stockholders” for a more detailed description of the ownership of our common stock.
Conflicts of interest may arise because some of our directors are principals of ACAS.
Three persons designated by ACAS will serve on our Board of Directors upon the consummation of this offering. ACAS and its affiliates may invest in entities that directly or indirectly compete with us, or companies in which they currently invest may begin competing with us. As a result of these relationships, when conflicts between the interests of ACAS and the interests of our other stockholders arise, these directors may not be disinterested.
Although our directors and officers will have a duty of loyalty to us under Delaware law and our amended and restated Certificate of Incorporation, transactions that we enter into in which a director or officer has a conflict of interest are generally permissible so long as the material facts relating to the director’s or officer’s relationship or interest as to the transaction are disclosed to our Board of Directors and a majority of our disinterested directors, or a committee consisting solely of disinterested directors, approves the transaction.
ACAS and its affiliates do not have any duty to refrain from engaging directly or indirectly in the same or similar business activities or lines of business that we do.
Under our amended and restated Certificate of Incorporation, none of ACAS or its affiliates and investment funds, or any other ACAS entity, nor any director, officer, stockholder, member, managerand/or employee of an ACAS entity, has any duty to refrain from engaging directly or indirectly in the same or similar business activities or lines of business that we do. In the event that any ACAS entity acquires knowledge of a potential transaction or matter which may be a corporate opportunity for both itself and us, the ACAS entity will not have any duty to communicate or offer the corporate opportunity to us and may pursue or acquire the corporate opportunity for itself or offer the opportunity to another person. In addition, none of ACAS’s designees on our Board of Directors will be required to offer to us any transaction opportunity of which he or she becomes aware and could take any such opportunity for him or herself or offer it to other companies (including ACAS and its other portfolio companies) in which they have an investment, unless such opportunity is expressly offered to him or her solely in his or her capacity as a director of us.
29
If ACAS were to sell fewer shares than currently contemplated, we would elect to be a “controlled company,” as defined in the NASDAQ Stock Market Rules, and, as a result, we would take advantage of exemptions from certain corporate governance requirements; in any event, we are permitted to phase-in certain corporate governance requirements over the course of one year.
If ACAS were to sell fewer shares in this offering than currently contemplated, such that following the offering ACAS owned common stock representing more than a majority of the voting power in the election of our directors, we would elect to be considered a “controlled company” within the meaning of the corporate governance standards of the NASDAQ Stock Market Rules. Under these rules, a “controlled company” may elect not to comply with certain corporate governance requirements, including the requirement that a majority of its board of directors consist of independent directors, the requirement that it have a nominating/corporate governance committee that is composed entirely of independent directors for the purposes of director nominations and the requirement that it have a compensation committee that is composed entirely of independent directors for the purposes of approving executive compensation. As a result, a majority of our directors would not be independent and our compensation and nominating and corporate governance committees would not consist entirely of independent directors. Furthermore, until one year following this offering, as permitted by applicable rules, we would not anticipate having an audit committee consisting solely of independent directors. Accordingly, you would not have the same protection afforded to stockholders of companies that are subject to all of the NASDAQ Stock Market corporate governance requirements.
Upon the consummation of this offering, ACAS, our principal stockholder, will beneficially own shares of our common stock (approximately % of our outstanding common stock), which includes shares of our common stock underlying warrants held by ACAS, or shares of our common stock (approximately % of our common stock) if the underwriters exercise in full their over-allotment option to purchase additional shares of common stock from the selling stockholders. As a result, we do not expect to be a “controlled company.” However, under the applicable rules of The Nasdaq Stock Market, we will have a one year phase-in period to comply with those corporate governance requirements of The Nasdaq Stock Market from which we would have been exempt had we continued to remain a controlled company. Accordingly, for at least one year following the consummation of this offering, you may not have the same protection afforded to stockholders of companies that are subject to all of The Nasdaq Stock Market corporate governance requirements.
The price of our common stock may be volatile and subject to wide fluctuations.
The market price of our common stock may be subject to wide fluctuations due to a number of factors. We may experience significant period-to-period fluctuations in our backlog, revenue and operating results in the future and any such variations may cause our stock price to fluctuate. It is likely that in some future period our operating results will be below the expectations of securities analysts or investors. If this occurs, our stock price could drop significantly. A number of factors, in addition to those cited in other risk factors applicable to our business, may contribute to fluctuations in our backlog, revenue and operating results, including:
| | |
| | • | the timing and volume of orders from our customers; |
| |
| | • | the rate of acceptance of our products by our customers; |
| |
| | • | the rate of adoption of our products in the end markets we target; |
| |
| | • | delays or cancellations in the construction of new NPPs by our customers; |
| |
| | • | cancellations or deferrals of customer orders in anticipation of new products or product enhancements from us or our competitors or other providers; |
| |
| | • | changes in product mix; and |
| |
| | • | the rate at which new markets emerge for products we are currently developing. |
30
Broad market and industry factors may also adversely affect the market price of our common stock, regardless of our actual operating performance. Market and industry factors that could cause fluctuations in our stock price may include, among other things:
| | |
| | • | incidents affecting the nuclear industry; |
| |
| | • | regulatory changes or legal developments affecting the nuclear industry; |
| |
| | • | changes in financial estimates by us or by any securities analysts who might cover our stock, or our failure to meet the estimates made by securities analysts; |
| |
| | • | changes in the market valuations of other companies operating in our industry; |
| |
| | • | announcements by us or our competitors of significant acquisitions, strategic partnerships or divestitures; |
| |
| | • | additions or departures of key personnel; and |
| |
| | • | a general downturn in the stock market. |
The market price of our common stock also might decline in reaction to events that affect other companies in our industry, even if these events do not directly affect us. The initial public offering price of our common stock will be determined through negotiations between the representatives of the underwriters, ACAS and us and may not be representative of the price that will prevail in the open market. You might be unable to resell your shares at or above the offering price. In the past, companies that have experienced volatility in the market price of their stock have been the subjects of securities class action litigation. If we were to become the subject of securities class action litigation, it could result in substantial costs and a diversion of management’s attention and resources.
We have and will continue to incur increased costs as a result of becoming a reporting company.
We have and will continue to face increased legal, accounting, administrative and other costs as a result of becoming a reporting company that we did not incur as a private company. The Sarbanes-Oxley Act of 2002, as well as rules subsequently implemented by the Securities and Exchange Commission, or the SEC, and the Public Company Accounting Oversight Board, have required changes in the corporate governance practices of public companies. We expect these rules and regulations to increase our legal and financial compliance costs and to make legal, accounting and administrative activities more time consuming and costly. For example, upon the consummation of this offering we will add independent directors, create additional committees of our Board of Directors and adopt policies regarding internal controls and disclosure controls and procedures. We also expect to incur substantially higher costs to obtain directors and officers’ insurance. In addition, as we gain experience with the costs associated with being a reporting company, we may identify and incur additional overhead costs.
Our internal control over financial reporting does not currently meet the standards required by Section 404 of the Sarbanes-Oxley Act, and failure to achieve and maintain effective internal control over financial reporting in accordance with Section 404 of the Sarbanes-Oxley Act could have a material adverse effect on us.
Our internal control over financial reporting does not currently meet the standards required by Section 404 of the Sarbanes-Oxley Act, standards that we will be required to meet in the course of preparing our fiscal 2012 financial statements. We currently have material weaknesses in our internal controls and do not currently have comprehensive documentation of our internal controls, nor do we document or test our compliance with these controls on a periodic basis in accordance with Section 404 of the Sarbanes-Oxley Act. Furthermore, we have not tested our internal controls in accordance with Section 404 and, due to our lack of documentation, such a test would not be possible to perform at this time.
We are in the early stages of addressing our internal control procedures to satisfy the requirements of Section 404, which requires an annual management assessment of the effectiveness of our internal control over financial reporting. If, as a public company, we are not able to implement the requirements of Section 404 in a timely manner or with adequate compliance, our independent registered public accounting firm may not be able to attest to the effectiveness of our internal control over financial reporting. If we are unable to maintain adequate internal control over financial reporting, we may be unable to report our financial information on a
31
timely basis, may suffer adverse regulatory consequences or violations of applicable stock exchange listing rules and may breach the covenants under our credit facilities. There could also be a negative reaction in the financial markets due to a loss of investor confidence in us and the reliability of our financial statements.
Future sales of shares could depress our stock price.
If our existing stockholders sell substantial amounts of our common stock in the public market following this offering, the market price of our common stock could decline. Based on 11,271,548 shares outstanding as of August 31, 2010, upon completion of this offering we will have outstanding approximately shares of common stock. Of these shares, only the shares of common stock (plus up to additional shares if the over-allotment option is exercised in full) sold in this offering will be freely tradable, without restriction, in the public market. After thelock-up agreements that ACAS has entered into, and that we expect our directors, officers and all of our other stockholders to enter into, pertaining to this offering expire, our existing stockholders will be able to sell their remaining shares in the public market, subject to legal restrictions on transfer. Upon the consummation of this offering, we will enter into a registration rights agreement that provides for registration rights with respect to the up to shares of our common stock that will be held by ACAS and its affiliates, Thomas D. Logan, W. Antony Besso and certain other stockholders following this offering. Registration of the sale of the common stock would permit their sale into the market immediately.
On June 28, 2010, American Capital, Ltd. completed the Restructuring. In connection with the Restructuring, lenders to ACAS and holders of its public and private notes received cash and new secured debt in the form of the Term Loan and Secured Notes. As of June 28, 2010, the total amount of such new secured debt was $1.31 billion. The Term Loan and the Secured Notes are collectively secured by the Pledge. The shares of our capital stock beneficially owned by American Capital Equity I, LLC and American Capital Equity II, LP are not subject to the Pledge. If there is an event of default under the ACAS Secured Financing and either the lenders or the holders of the Secured Notes accelerate the Term Loan or the Secured Notes, as applicable, and exercise their rights under the Pledge, the purchasers of such shares, which may include the creditors secured by the Pledge, would hold a substantial percentage of our outstanding shares of common stock and would collectively become our largest stockholder. While such purchasers would not succeed to any of the rights specifically reserved to ACAS under our amended and restated Certificate of Incorporation and Bylaws, they may, under certain circumstances, succeed to the registration rights agreement into which we expect to enter upon consummation of this offering, and may otherwise be free to sell their shares in the market, subject to any limitations that may be imposed by applicable securities laws. Although ACAS has entered into a lock-up agreement pertaining to this offering, we do not anticipate that the lenders under the Pledge will enter into a lock-up agreement.
If for any reason ACAS voluntarily or involuntarily sells a large number of shares, including any shares of our common stock subject to the Pledge, the market price of our common stock could decline, as these sales may, among other things, be viewed by the public as an indication of an upcoming or recently occurring shortfall in the financial performance of our company. Moreover, the perception in the public market that these investors might sell our common stock could depress the market price of the common stock. See “Shares Eligible for Future Sale” for more detailed information. Additionally, we may sell or issue additional shares of common stock in subsequent public offerings or in connection with acquisitions.
If securities or industry analysts do not publish research or reports about our business, if they adversely change their recommendations regarding our stock or if our results of operations do not meet their expectations, our stock price and trading volume could decline.
The trading market for our common stock will be influenced by the research and reports that industry or securities analysts publish about us or our business. If one or more of these analysts cease coverage of our company or fail to publish reports on us regularly, we could lose visibility in the financial markets, which in turn could cause our stock price or trading volume to decline. In addition, it is likely that in some future period our operating results will be below the expectations of securities analysts or investors. If one or more of the analysts who cover us downgrade our stock, or if our results of operations do not meet their expectations, our stock price could decline.
32
Some provisions of our amended and restated Certificate of Incorporation and Bylaws may deter third parties from acquiring us and diminish the value of our common stock.
Our amended and restated Certificate of Incorporation and Bylaws provide for, among other things:
| | |
| | • | restrictions on the ability of our stockholders to fill a vacancy on our Board of Directors; |
| |
| | • | our ability to issue preferred stock with terms that our Board of Directors may determine, without stockholder approval; |
| |
| | • | the absence of cumulative voting in the election of directors; |
| |
| | • | advance notice requirements for stockholder proposals and nominations; |
| |
| | • | our Board of Directors to be divided into three classes, with each class serving staggered terms; |
| |
| | • | the right of ACAS to designate three members of our Board of Directors so long as ACAS and its affiliated funds hold at least 50.1% of our outstanding common stock, two directors so long as they hold at least 25% but less than 50.1% of our outstanding common stock and one director so long as they hold at least 10% but less than 25% of our outstanding common stock; |
| |
| | • | the requirement that at least one of the directors designated by ACAS must be part of the majority in any action taken by our Board of Directors so long as ACAS and its affiliated funds hold at least 50.1% of our outstanding common stock, other than on matters in which ACAS has a conflict of interest (as it would if it appointed a majority of our directors); |
| |
| | • | the requirement that at least one of the directors designated by ACAS must be part of the majority in any action taken by our Board of Directors to change the number of directors of our Board of Directors so long as ACAS and its affiliated funds hold at least 10% of our outstanding common stock; and |
| |
| | • | the requirement that, so long as ACAS and its affiliated funds hold at least 10% of our outstanding common stock, ACAS must approve changes to certain provisions of the Bylaws, including, among other things, the provisions governing the size, classification and term of our Board of Directors, the right of ACAS to designate certain members of our Board of Directors and the provisions that require that at least one of the directors designated by ACAS must be part of the majority in certain actions taken by our Board of Directors, as described in the two preceding bullets above. |
These provisions may discourage, delay or prevent a transaction involving a change in control of our company that is in the best interest of our minority stockholders. Even in the absence of a takeover attempt, the existence of these provisions may adversely affect the prevailing market price of our common stock if they are viewed as discouraging future takeover attempts.
We do not anticipate paying any cash dividends for the foreseeable future.
We currently intend to retain our future earnings, if any, for the foreseeable future, to repay indebtedness and to fund the development and growth of our business. We do not intend to pay any dividends to holders of our common stock and the agreements governing our debt significantly restrict our ability to pay dividends. As a result, capital appreciation in the price of our common stock, if any, will be your only source of gain on an investment in our common stock. See “Dividend Policy.”
New investors in our common stock will experience immediate and substantial book value dilution after this offering.
The initial public offering price of our common stock will be substantially higher than the pro forma as adjusted net tangible book value per share of the outstanding common stock immediately after this offering. Based on an assumed initial public offering price of $ per share (the midpoint of the price range set forth on the cover of this prospectus) and our net tangible book value as of June 30, 2010, if you purchase our common stock in this offering, you will suffer immediate dilution in pro forma as adjusted net tangible book value per share of approximately $ . See “Dilution.”
33
FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements that are subject to risks and uncertainties. All statements other than statements of historical fact included in this prospectus are forward-looking statements. Forward-looking statements give our current expectations and projections relating to our financial condition, results of operations, plans, objectives, future performance and business. You can identify forward-looking statements by the fact that they do not relate strictly to historical or current facts. These statements may include words such as “anticipate,” “estimate,” “expect,” “project,” “plan,” “intend,” “believe,” “may,” “should,” “can have,” “likely” and other words and terms of similar meaning in connection with any discussion of the timing or nature of future operating or financial performance or other events.
The forward-looking statements contained in this prospectus are based on assumptions that we have made in light of our industry experience and on our perceptions of historical trends, current conditions, expected future developments and other factors we believe are appropriate under the circumstances. As you read and consider this prospectus, you should understand that these statements are not guarantees of performance or results. They involve assumptions as well as risks and uncertainties, some of which are beyond our control. Although we believe that these forward-looking statements are based on reasonable assumptions, you should be aware that many factors could affect our actual financial results and cause them to differ materially from those anticipated in the forward-looking statements. We believe these factors include, but are not limited to, those described under “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” Should one or more of these risks or uncertainties materialize, or should any of these assumptions prove incorrect, our actual results may vary in material respects from those projected in these forward-looking statements.
Any forward-looking statement made by us in this prospectus speaks only as of the date on which we make it. Factors or events that could cause our actual results to differ may emerge from time to time, and it is not possible for us to predict all of them. We undertake no obligation to publicly update any forward-looking statement, whether as a result of new information, future developments or otherwise, except as may be required by law.
34
USE OF PROCEEDS
We estimate that the net proceeds to us from this offering, after deducting underwriting discounts and estimated offering expenses will be approximately $ million, assuming the shares are offered at $ per share (the midpoint of the price range set forth on the cover page of this prospectus). We intend to use $ million of the net proceeds from this offering to repay certain borrowings from ACAS and its affiliates. We also intend to use net proceeds from this offering to make a one-time payment of $8.0 million to ACFS, a subsidiary of ACAS, to terminate an investment banking services agreement between us and ACFS. See “Certain Relationships and Related Party Transactions.” We also intend to use $0.8 million of the net proceeds from this offering to make bonus payments upon completion of this offering to certain of our employees, including an aggregate of $0.6 million to certain of our executive officers. See “Executive Compensation — Compensation Discussion and Analysis — Elements of Compensation.” We will not receive any proceeds from the sale of shares of common stock by selling stockholders. A $1.00 increase (decrease) in the assumed initial public offering price of $ per share would increase (decrease) the net proceeds to us from this offering by $ million, assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting underwriting discounts and commissions and estimated expenses payable by us. In addition, prior to the consummation of this offering, we expect to enter into a letter agreement with each holder of our convertible preferred stock, including ACAS and its affiliates and Thomas Logan, our President, Chief Executive Officer and Chairman of the Board, that in lieu of dividends otherwise payable in the form of additional shares of convertible preferred stock, for the period from the date of such letter agreement through the closing of this offering, we will pay cash dividends on shares of our outstanding preferred stock at a rate equal to the number of shares of common stock they would have received upon conversion of the preferred stock they would have received in dividends during that period multiplied by the initial public offering price. Assuming an initial public offering price of $ per share, the aggregate dividend payment would equal approximately $ per day from the date of such letter agreements until the closing of this offering. We will pay this amount out of our existing cash.
We intend to repay with a portion of the net proceeds from this offering the following outstanding debt of our subsidiaries that is held by our principal stockholder, ACAS, or its affiliates. For purposes of this section, EURIBOR means the Euro Interbank Offered Rate and LIBOR means the London Interbank Offered Rate.
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | Interest Rate
| | | Principal Balance at
| | | Amount to be
| |
| | | | | | Contractual
| | | at June 30,
| | | June 30,
| | | Repaid with
| |
| | | Due | | | Interest Rate | | | 2010 | | | 2010(1) | | | Net Proceeds | |
| | | | | | | | | | | | (In thousands) | | | (In thousands) | |
| Revolving Credit Facilities: | | | | | | | | | | | | | | | | | | | | |
| $20.25 million | | | July 2011 | | | | LIBOR + 4.5% | | | | 4.85% | | | $ | 20,250 | | | $ | 20,250 | |
| $14.0 million | | | July 2011 | | | | LIBOR + 5% | | | | 5.35% | | | | 13,997 | | | | 13,997 | |
| $8.2 million | | | July 2011 | | | | EURIBOR + 2% | | | | 2.43% | | | | 7,130 | | | | 7,130 | |
| Senior Term Notes: | | | | | | | | | | | | | | | | | | | | |
| $24.9 million Senior Term B | | | July 2011 | | | | EURIBOR + 3% | | | | 3.43% | | | | 24,944 | | | | 24,944 | |
| $4 million Senior Term C | | | Oct 2011 | | | | LIBOR + 9% | | | | 9.35% | | | | 4,000 | | | | 4,000 | |
| $27 million Senior Term D | | | Oct 2011 | | | | LIBOR + 6.5% | | | | 6.85% | | | | 25,785 | | | | | (2) |
| | | | | | | | | | | | | | | | | | | | | |
| Total ACAS debt to be repaid in this offering | | | | | | | | | | | | | | | | | | $ | | |
| | | | | | | | | | | | | | | | | | | | | |
| | |
| (1) | | Interest is paid by us to the lender monthly in arrears, and accordingly, the balance at each month’s end reflects only the principal balance of the related obligation. Any decrease in the principal balance between periods is due to scheduled principal repayments expected to be made by us. |
| |
| (2) | | The remaining balance outstanding on this note, consisting of both principal and paid-in-kind balances outstanding, will be repaid with borrowings under our new bank credit facilities that we expect to enter into upon the consummation of this offering. |
We intend to repay all other debt held by ACAS and its affiliates ($ million as of June 30, 2010) with borrowings under our anticipated new bank credit facilities that we expect to enter into upon the consummation of this offering.
35
DIVIDEND POLICY
We have never declared or paid any cash dividends on our common stock. We currently intend to retain our future earnings, if any, for the foreseeable future, to repay indebtedness and to fund the development and growth of our business. We do not intend to pay dividends to holders of our common stock and the agreements governing our anticipated new bank credit facilities significantly restrict our ability to pay cash dividends.
36
CAPITALIZATION
The following table sets forth our consolidated capitalization as of June 30, 2010:
| | |
| | • | on an actual basis; and |
| |
| | • | on a pro forma basis to give effect to: |
| | |
| | • | an $8.0 million increase in distribution payable to ACAS to reflect the accrual of an $8.0 million payment to ACAS to terminate an investment banking services agreement, with a corresponding increase in accumulated deficit. No tax benefit has been provided related to this expense because we are in a net operating loss position and have a full valuation allowance in the affected jurisdiction; |
| | |
| | • | a $183.3 million reduction in notes payable to ACAS to reflect the assumed distribution to ACAS in connection with this offering, with a corresponding increase in distribution payable to ACAS. Of this reduction, $ million will be paid using proceeds of the offering and $ million will be paid with proceeds from new third-party debt arrangements; |
| | |
| | • | the conversion of the outstanding shares of Series A Convertible Participating Preferred Stock into 10,697,928 shares of common stock as of June 30, 2010, based upon a conversion rate of 9.6288 forSeries A-1 preferred stock and 8.8128 forSeries A-2 preferred stock; |
| | |
| | • | the conversion of 17,773 outstanding shares of Class A Voting Common Stock and 388,023 outstanding shares of Class B Non-Voting Common Stock into 405,796 shares of common stock; and |
| | |
| | • | the adoption of our Fourth Amended and Restated Certificate of Incorporation prior to the effectiveness of this offering. |
| | |
| | • | on a pro forma as adjusted basis to give further effect to: |
| | |
| | • | the issuance and sale of shares of common stock in this offering, at an assumed initial public offering price of $ for gross proceeds of $ million, and the receipt of the net proceeds of $ million after deducting $ million of underwriting discounts and commissions and estimated offering expenses paid or payable by us. Of the $ million total offering costs paid or payable by us, $ million were previously paid by us and have been included in prepaid expenses and other current assets. The pro forma as adjusted amounts include a decrease in prepaid expenses and other current assets to reflect the reclassification of these amounts to equity at the completion of the offering and the corresponding increase in cash and cash equivalents representing the amount of the net proceeds retained by us to offset offering costs already paid. The net proceeds from this offering were used to make payments to ACAS of $8.0 million and $ million resulting in a reduction in the distribution payable to ACAS. The net proceeds from this offering were also used to make bonus payments of $0.8 million, with a corresponding increase in accumulated deficit. No tax benefit has been provided related to this expense as we are in a net operating loss position and have a full valuation allowance in the affected jurisdictions; |
| | |
| | • | a $ million increase in notes payable and a $ million increase in the current portion of notes payable, representing principal payments due within twelve months of executing the new notes, to reflect new debt arrangements entered into concurrently with the completion of this offering and the receipt of $ million of cash, net of $ million of loan origination fees incurred in connection with the new debt arrangements. These fees have been reflected as an increase in other assets; |
| | |
| | • | a $ million decrease in cash and cash equivalents and distribution payable to ACAS to reflect the distribution to ACAS made to repay debt obligations that were refinanced with a third party; |
| | |
| | • | a $ million increase in additional paid-in capital and accumulated deficit to reflect the compensation expense associated with certain employee stock options that will vest upon this offering. No tax benefit has been provided related to this expense because we are in a net operating loss position and have a full valuation allowance in the affected jurisdictions. The compensation expense relates to |
37
| | |
| | | unrecognized compensation expense associated with unvested employee stock options granted in August 2008 that, under the original terms of the grant, vest ratably over four years from the date of grant, with accelerated vesting of any unvested shares upon consummation of an initial public offering. We estimated the value of these options on the date of grant using the Black-Scholes model and based on the valuation assumptions detailed in Note 13 to the consolidated financial statements. The pro forma as adjusted balance sheet has not been adjusted to reflect the compensation expense associated with 124,448 stock options that are expected to be granted to our employees and outside directors immediately following the pricing of this offering. These options are expected to have a four year vesting term, and the compensation expense assuming the offering occurred at the end of fiscal 2010 will be recognized ratably over the following four years. Additionally, the pro forma as adjusted balance sheet has not been adjusted to reflect the compensation expense associated with performance-based call options with market conditions held by our Chief Executive Officer because the compensation expense associated with these options assuming the offering occurred at the end of fiscal 2010 will be recognized in subsequent periods based on the implied requisite service period for the market conditions (30 days subsequent to the offering for one-half of the shares, and 24 months subsequent to the offering for one-half of the shares); and |
| | |
| | • | the adoption of our Fifth Amended and Restated Certificate of Incorporation prior to the consummation of this offering solely to eliminate the designations of our Series A Convertible Participating Preferred Stock and our Class A Voting Common Stock and Class B Voting Common Stock. |
You should read this table along with “Use of Proceeds,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and the related notes appearing elsewhere in this prospectus.
| | | | | | | | | | | | | |
| | | As of June 30, 2010 | |
| | | | | | | | | Pro Forma
| |
| | | Actual | | | Pro Forma(1) | | | as Adjusted(1) | |
| | | (in thousands, except share data) | |
| |
| Total debt, including current portion and other payables to ACAS: | | | | | | | | | | | | |
| Distribution payable to ACAS | | $ | — | | | $ | | | | $ | | |
| Notes payable to ACAS | | | 183,252 | | | | | | | | | |
| | | | | | | | | | | | | |
| Total payables to ACAS | | | 183,252 | | | | | | | | | |
| Notes payable to third parties | | | 2,622 | | | | | | | | | |
| New indebtedness: | | | | | | | | | | | | |
| Short-term portion | | | — | | | | | | | | | |
| Long-term portion | | | — | | | | | | | | | |
| Stockholders’ equity: | | | | | | | | | | | | |
Series A-1 Convertible Participating Preferred Stock, $0.001 par value; 1,200,000 shares authorized, actual and pro forma; 678,804 shares issued and outstanding, actual; none issued and outstanding, pro forma; none authorized, issued and outstanding, pro forma as adjusted | | | 1 | | | | — | | | | — | |
Series A-2 Convertible Participating Preferred Stock, $0.001 par value; 300,000 shares authorized, actual and pro forma; 70,000 shares issued and outstanding, actual; none issued and outstanding, pro forma; none authorized, issued and outstanding, pro forma as adjusted | | | — | | | | — | | | | — | |
| Preferred Stock, $0.001 par value; 1,500,000 shares authorized, actual, pro forma and pro forma as adjusted; none issued and outstanding, actual, pro forma and pro forma as adjusted | | | -— | | | | — | | | | — | |
| Class A Voting Common Stock, $0.001 par value; 61,328,125 shares authorized, actual and pro forma; 17,773 shares issued and outstanding, actual; none issued and outstanding, pro forma; none authorized, issued and outstanding, pro forma as adjusted | | | — | | | | — | | | | — | |
38
| | | | | | | | | | | | | |
| | | As of June 30, 2010 | |
| | | | | | | | | Pro Forma
| |
| | | Actual | | | Pro Forma(1) | | | as Adjusted(1) | |
| | | (in thousands, except share data) | |
| |
| Class B Non-Voting Common Stock, $0.001 par value; 17,171,875 shares authorized, actual and pro forma; 388,023 shares issued and outstanding, actual; none issued and outstanding, pro forma; none authorized, issued and outstanding, pro forma as adjusted | | | — | | | | — | | | | — | |
| Common stock, $0.001 par value; 78,500,000 shares authorized, actual, pro forma and pro forma as adjusted; none issued and outstanding actual; 11,103,724 shares issued and outstanding, pro forma; issued and outstanding, pro forma as adjusted | | | — | | | | | | | | | |
| Additional paid-in capital | | | 99,585 | | | | | | | | | |
| Accumulated deficit | | | (96,596 | ) | | | | | | | | |
| Accumulated other comprehensive loss | | | (800 | ) | | | | | | | | |
| | | | | | | | | | | | | |
| Total stockholders’ equity | | | 2,190 | | | | | | | | | |
| | | | | | | | | | | | | |
| Total capitalization | | $ | 188,064 | | | $ | | | | $ | | |
| | | | | | | | | | | | | |
| | |
| (1) | | Assuming the number of shares sold by us in this offering remains the same as set forth on the cover page of this prospectus, a $1.00 increase or decrease in the assumed initial public offering price would increase or decrease, as applicable, our total cash, total stockholders’ equity and total capitalization by approximately $ million. |
The table above excludes, as of June 30, 2010:
| | |
| | • | 924,830 shares of common stock subject to outstanding options at a weighted average exercise price of $14.37 per share; |
| | |
| | • | an aggregate of 986,395 shares of common stock either reserved for issuance under our existing stock option plan or to be reserved for issuance under our amended and restated stock plan to become effective in connection with this offering, of which 124,448 shares are expected to be granted in the form of stock options to our employees, including options to purchase 34,008 shares to be granted to our executive officers (in the respective amounts set forth on page 113 of this prospectus) and, under the director compensation policy described on page 105 of this prospectus, outside directors, immediately following the pricing of this offering at an exercise price equal to the initial public offering price; and |
| | |
| | • | 3,420,636 shares of common stock subject to outstanding warrants at a weighted average exercise price of $0.00018 per share. These warrants only become exercisable upon a sale, liquidation or dissolution of the Company or approval by our Board of Directors. Our Board of Directors has resolved that all of these warrants will become exercisable at the option of the holder thereof upon the consummation of this offering and thereafter. |
39
DILUTION
If you invest in our common stock, your interest will be diluted to the extent of the difference between the public offering price per share of our common stock and the pro forma as adjusted net tangible book value per share of common stock initially upon completion of this offering. As of June 30, 2010, our net tangible book value was $( ) million, or $( ) per share of common stock. Net tangible book value per share represents the amount of our total tangible assets less total liabilities, divided by 11,103,724, the number of shares of our common stock outstanding after giving effect to the conversion of our Series A Convertible Participating Preferred Stock pursuant to the conversion terms in our amended and restated Certificate of Incorporation and the conversion of our Class A Voting Common Stock and Class B Non-Voting Common Stock on a one-for-one basis.
After giving effect to the sale of shares, assuming the shares are offered at $ per share (the midpoint of the price range set forth on the cover page of this prospectus), after deducting underwriting discounts and estimated offering expenses, the repayment of certain borrowings from ACAS and its affiliates, a one-time payment of $8.0 million to ACFS, a subsidiary of ACAS, to terminate an investment banking services agreement between us and ACFS, bonus payments upon completion of this offering to certain of our employees and the repayment of all remaining debt held by ACAS and its affiliates with borrowings under our anticipated new bank credit facilities, our pro forma as adjusted net tangible book value as of June 30, 2010 would have equaled $( ) million, or $( ) per share of common stock. This represents an immediate increase in net tangible book value of $ per share to our existing stockholders and an immediate dilution in net tangible book value of $ per share to new stockholders of common stock in this offering. If the initial public offering price is higher or lower, the dilution to new stockholders will be greater or less. The following table summarizes this per share dilution:
| | | | | | | | | |
| Assumed initial public offering price per share | | | | | | $ | | |
| Net tangible book value per share as of June 30, 2010 | | $ | ( | ) | | | | |
| Increase in pro forma tangible net book value per share as adjusted attributable to this offering from new investors | | | | | | | | |
| | | | | | | | | |
| Pro forma as adjusted net tangible book value per share after this offering | | | | | | | ( | ) |
| | | | | | | | | |
| Dilution in pro forma as adjusted net tangible book value per share to new stockholders | | | | | | $ | | |
| | | | | | | | | |
A $1.00 increase or decrease in the assumed initial public offering price of $ per share would increase or decrease, as applicable, our pro forma as adjusted net tangible book value by $ million, the pro forma as adjusted net tangible book value per share by $ per share, and the dilution in pro forma as adjusted net tangible book value per share to new stockholders by $ per share.
The following table summarizes on a pro forma basis, as of June 30, 2010, the differences between our existing stockholders and new stockholders with respect to the number of shares of common stock issued by us, the total consideration paid and the average price per share paid:
| | | | | | | | | | | | | | | | | | | | | |
| | | Shares Purchased | | Total Consideration | | Average Price
|
| | | Number | | Percent | | Amount | | Percent | | Per Share |
| | | (in thousands) | | | | (in thousands) | | | | |
| |
| Existing stockholders | | | | | | | | % | | $ | | | | | | % | | $ | | |
| New stockholders | | | | | | | | | | | | | | | | | | $ | | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | 100 | % | | $ | | | | | 100 | % | | | | |
Sales by the selling stockholders in this offering will reduce the number of shares held by existing stockholders to , or % of the total number of shares of our common stock to be outstanding after the offering, and will increase the number of shares held by new investors to , or % of the total number of shares of our common stock to be outstanding after the offering. If the underwriters exercise their over-allotment option in full, the following will occur: (1) the number of shares of common stock held by existing stockholders will represent approximately % of the total number of shares of common stock outstanding
40
after this offering; and (2) the number of shares of common stock held by new public stockholders will be increased to , or approximately % of the total number of shares of common stock outstanding after this offering.
The number of shares of common stock to be outstanding after this offering is based on 11,103,724 shares outstanding as of June 30, 2010 after giving effect to the conversion of our Series A Convertible Participating Preferred Stock pursuant to the conversion terms in our amended and restated Certificate of Incorporation and excludes:
| | |
| | • | 924,830 shares of common stock subject to outstanding options at a weighted average exercise price of $14.37 per share; |
| | |
| | • | an aggregate of 986,395 shares of common stock either reserved for issuance under our existing stock option plan or to be reserved for issuance under our amended and restated stock plan to become effective in connection with this offering, of which 124,448 shares are expected to be granted in the form of stock options to our employees, including options to purchase 34,008 shares to be granted to our executive officers (in the respective amounts set forth on page 113 of this prospectus), and, under the director compensation policy described on page 105 of this prospectus, outside directors, immediately following the pricing of this offering at an exercise price equal to the initial public offering price; and |
| | |
| | • | 3,420,636 shares of common stock subject to outstanding warrants at a weighted average exercise price of $0.00018 per share. These warrants only become exercisable upon a sale, liquidation or dissolution of the Company or approval by our Board of Directors. Our Board of Directors has resolved that all of these warrants will become exercisable at the option of the holder thereof upon the consummation of this offering and thereafter. |
Assuming that the outstanding warrants to purchase 3,420,636 shares of our common stock are exercised, the dilution to new stockholders’ net tangible book value per share would be as follows:
| | | | | | | | | |
| Assumed initial public offering price per share | | | | | | $ | | |
| Net tangible book value per share as of June 30, 2010 | | $ | ( | ) | | | | |
| Increase in pro forma tangible net book value per share as adjusted attributable to this offering from new investors | | | | | | | | |
| | | | | | | | | |
| Pro forma as adjusted net tangible book value per share after this offering | | | | | | | ( | ) |
| | | | | | | | | |
| Dilution in pro forma as adjusted net tangible book value per share to new stockholders | | | | | | $ | | |
| | | | | | | | | |
Assuming that the outstanding warrants are exercised, a $1.00 increase or decrease in the assumed initial public offering price of $ per share would increase or decrease, as applicable, our pro forma as adjusted net tangible book value by $ million, the pro forma as adjusted net tangible book value per share by $ per share, and the dilution in pro forma as adjusted net tangible book value per share to new stockholders by $ per share.
Assuming that the outstanding warrants are exercised, the following table summarizes on a pro forma basis, as of June 30, 2010, the differences between our existing stockholders and new stockholders with respect to the number of shares of common stock issued by us, the total consideration paid and the average price per share paid:
| | | | | | | | | | | | | | | | | | | | | |
| | | Shares Purchased | | | Total Consideration | | | Average Price
| |
| | | Number | | | Percent | | | Amount | | | Percent | | | Per Share | |
| | | (in thousands) | | | | | | (in thousands) | | | | | | | |
| |
| Existing stockholders | | | | | | | | % | | $ | | | | | | % | | $ | | |
| New stockholders | | | | | | | | | | | | | | | | | | $ | | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | 100 | % | | $ | | | | | 100 | % | | | | |
| | | | | | | | | | | | | | | | | | | | | |
41
Assuming that the outstanding warrants are exercised, sales by the selling stockholders in this offering will reduce the number of shares held by existing stockholders to , or %, of the total number of shares of our common stock to be outstanding after this offering, and will increase the number of shares held by new investors to , or %, of the total number of shares of our common stock to be outstanding after this offering. If the underwriters exercise their over-allotment option in full, the following will occur: (1) the number of shares of common stock held by existing stockholders will represent approximately % of the total number of shares of common stock outstanding after this offering; and (2) the number of shares of common stock held by new public stockholders will be increased to , or approximately % of the total number of shares of common stock outstanding after this offering.
To the extent that outstanding options are also exercised, there could be a further reduction in dilution to new stockholders as a result of our negative net tangible book value.
42
SELECTED CONSOLIDATED FINANCIAL DATA
You should read the following selected consolidated historical financial data below in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the consolidated financial statements, related notes and other financial information included elsewhere in this prospectus. The selected financial data in this section is not intended to replace the consolidated financial statements and related notes included elsewhere in this prospectus.
The selected consolidated statements of operations data for each of the fiscal years ending June 30, 2007, 2008, 2009 and 2010 and the consolidated balance sheet data as of June 30, 2008, 2009 and 2010 are derived from our audited annual consolidated financial statements and related notes included elsewhere in this prospectus. The consolidated statements of operations data for the fiscal year ended June 30, 2006, and the consolidated balance sheet data as of June 30, 2006 and 2007 are derived from our unaudited financial statements not included in this prospectus. Until their merger in December 2005 resulting in the formation of Mirion, we were comprised of GDS, IST and Synodys, entities which were under the common control of ACAS. Our historical results are not necessarily indicative of the results that should be expected in the future. The amounts below are in thousands, except percentages, shares and per share data.
| | | | | | | | | | | | | | | | | | | | | |
| | | Year Ended June 30, | |
| | | 2006 | | | 2007 | | | 2008 | | | 2009 | | | 2010 | |
Consolidated Statement of Operations Data: | | | | | | | | | | | | | | | | | | | | |
| Revenue | | $ | 145,770 | | | $ | 169,033 | | | $ | 191,769 | | | $ | 201,763 | | | $ | 228,124 | |
| Cost of revenue | | | 77,999 | | | | 94,321 | | | | 102,790 | | | | 105,954 | | | | 126,707 | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| Gross profit | | | 67,771 | | | | 74,712 | | | | 88,979 | | | | 95,809 | | | | 101,417 | |
| | | | | | | | | | | | | | | | | | | | | |
| % of revenue | | | 46.5 | % | | | 44.2 | % | | | 46.4 | % | | | 47.5 | % | | | 44.5 | % |
| Operating expenses | | | | | | | | | | | | | | | | | | | | |
| Selling, general and administrative expenses | | | 69,011 | | | | 59,449 | | | | 63,177 | | | | 65,649 | | | | 64,510 | |
| Research and development expenses | | | 9,726 | | | | 11,875 | | | | 14,865 | | | | 11,282 | | | | 8,729 | |
| | | | | | | | | | | | | | | | | | | | | |
| Total operating expenses | | | 78,737 | | | | 71,324 | | | | 78,042 | | | | 76,931 | | | | 73,239 | |
| | | | | | | | | | | | | | | | | | | | | |
| Income (loss) from operations | | | (10,966 | ) | | | 3,388 | | | | 10,937 | | | | 18,878 | | | | 28,178 | |
| Interest expense, net | | | 20,689 | | | | 19,153 | | | | 20,207 | | | | 17,711 | | | | 15,120 | |
| Other income (expense), net | | | (5,103 | ) | | | (1,001 | ) | | | (1,759 | ) | | | (490 | ) | | | (639 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| (Loss) Income before provision for income taxes | | | (26,552 | ) | | | (14,764 | ) | | | (7,511 | ) | | | 1,657 | | | | 13,697 | |
| Provision for income taxes | | | 1,525 | | | | 4,937 | | | | 5,838 | | | | 5,612 | | | | 7,732 | |
| | | | | | | | | | | | | | | | | | | | | |
| Net (loss) income | | $ | (28,077 | ) | | $ | (19,701 | ) | | $ | (13,349 | ) | | $ | (3,955 | ) | | $ | 5,965 | |
Paid-in-kind preferred dividends | | | (4,949 | ) | | | (8,141 | ) | | | (8,993 | ) | | | (9,892 | ) | | | (10,923 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Net loss allocable to common stockholders | | $ | (33,026 | ) | | $ | (27,842 | ) | | $ | (22,342 | ) | | $ | (13,847 | ) | | $ | (4,958 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Net loss per common share allocable to common stockholders per share — basic and diluted | | $ | (86.88 | ) | | $ | (68.79 | ) | | $ | (55.14 | ) | | $ | (34.12 | ) | | $ | (12.22 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Weighted average number of shares used in computing net loss allocable to common stockholders — basic and diluted | | | 380,120 | | | | 404,717 | | | | 405,159 | | | | 405,796 | | | | 405,796 | |
| | | | | | | | | | | | | | | | | | | | | |
| Pro forma net loss per common share before conversion of preferred shares — basic and diluted(1) | | | | | | | | | | | | | | | | | | $ | | |
| | | | | | | | | | | | | | | | | | | | | |
| Pro forma net income per common share — diluted(1) | | | | | | | | | | | | | | | | | | $ | | |
| | | | | | | | | | | | | | | | | | | | | |
| Pro forma net income per common share — diluted as adjusted(1) | | | | | | | | | | | | | | | | | | $ | | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| | | Year Ended June 30, | |
| | | 2006 | | | 2007 | | | 2008 | | | 2009 | | | 2010 | |
| |
Consolidated Balance Sheet Data: | | | | | | | | | | | | | | | | | | | | |
| Cash and cash equivalents(2) | | $ | 4,858 | | | $ | 6,561 | | | $ | 8,959 | | | $ | 5,390 | | | $ | 9,936 | |
| Total assets | | | 307,975 | | | | 310,249 | | | | 344,377 | | | | 329,754 | | | | 307,363 | |
| Notes payable to ACAS(3) | | | 148,273 | | | | 159,461 | | | | 173,186 | | | | 170,080 | | | | 183,252 | |
| Total stockholders’ equity | | | 35,419 | | | | 21,263 | | | | 19,152 | | | | 6,847 | | | | 2,190 | |
43
| | | | | | | | | | | | | |
| | | Year Ended June 30, | |
| | | 2008 | | | 2009 | | | 2010 | |
| |
Other Data: | | | | | | | | | | | | |
| Adjusted EBITDA(4) | | $ | 34,218 | | | $ | 40,625 | | | $ | 46,300 | |
| Amortization of intangible assets | | | 10,140 | | | | 8,144 | | | | 6,432 | |
| Capital expenditures | | | 4,953 | | | | 6,649 | | | | 10,437 | |
| | | | | | | | | | | | | |
| | | As of June 30, | |
| | | 2008 | | | 2009 | | | 2010 | |
| |
| Backlog(5) | | $ | 177,956 | | | $ | 183,960 | | | $ | 191,734 | |
| Deferred contract revenue | | | 53,539 | | | | 62,031 | | | | 44,730 | |
| | |
| (1) | | Pro forma net loss per common share before conversion of preferred shares — basic and diluted and pro forma net income per common share — diluted and as adjusted for the year ended June 30, 2010 is calculated as follows: |
| | | | | |
| | | Year Ended
| |
| | | June 30,
| |
| | | 2010 | |
| |
Numerator: | | | | |
| Net loss allocable to common stockholders | | $ | (4,958 | ) |
| Interest expense from paydown of ACAS debt using proceeds of offering(a) | | | | |
| | | | | |
| Pro forma net loss allocable to common stockholders before conversion of preferred shares | | | | |
| Effect of preferred stock dividends(b) | | | 10,923 | |
| | | | | |
| Pro forma net income allocable to common stockholders | | | | |
| Interest expense reduction from refinancing of ACAS debt(c) | | | | |
| Compensation expense from employee stock options and call options(d) | | | | |
| | | | | |
| Pro forma net income allocable to common stockholders — as adjusted | | $ | | |
| | | | | |
| | | | | |
| | | Year Ended
| |
| | | June 30, 2010 | |
| |
Denominator: | | | | |
| Weighted average shares outstanding from conversion of Class A and B Voting Common Stock(e) | | | 405,796 | |
| Common shares issued in this offering(f) | | | | |
| | | | | |
| Shares used in computing pro forma net loss per common share before conversion of preferred shares — basic and diluted(i) | | | | |
| Weighted average shares outstanding from conversion of convertible preferred stock(g) | | | 10,233,721 | |
| | | | | |
| Shares used in computing pro forma net income per common share — basic | | | | |
| Weighted average common shares outstanding from exercise of warrants(h) | | | 3,420,636 | |
| Weighted average common share equivalents of stock option(i) | | | 73,879 | |
| | | | | |
| Shares used in computing pro forma net income per common share — diluted and diluted as adjusted | | | | |
| | | | | |
| | |
| (a) | | The pro forma reduction in interest expense assumes the repayment of $ million of ACAS debt using the net proceeds from this offering, giving effect to the elimination of the related interest expense as of the beginning of the period presented. The amount of interest expense eliminated by this adjustment is calculated from actual interest expense of $ million and $ million recorded in fiscal 2010 in connection with the specific debt arrangements that will be repaid with a portion of the net proceeds of this offering. No tax expense has been provided related to this reduction in interest expense because we are in a net operating loss position and have a full valuation allowance in the affected jurisdiction. |
| | |
| (b) | | The effect of preferred stock dividends is added back as a reduction to net loss allocable to common stockholders, assuming that all preferred stock has been converted into common shares as of the beginning of the period presented. |
| | |
| (c) | | All ACAS debt not assumed to be repaid from the net proceeds from this offering is assumed to be refinanced with a loan from a third-party bank, at interest rates averaging approximately % lower than existing average interest rates with ACAS giving effect to the elimination of the related interest expense as of the beginning of the period presented. The amount of interest expense eliminated by this adjustment is calculated by taking the difference between the actual interest expense of $ million recorded in fiscal 2010 in connection with the specific debt arrangements that will be repaid with a portion of the net proceeds of the new debt arrangements entered into concurrently with the completion of this |
44
| | |
| | offering and the interest expense on the new debt arrangements, calculated as the total of the new debt arrangements of $ million multiplied by the average interest rate on those arrangements of approximately % for fiscal 2010. No tax expense has been provided related to this reduction in interest expense because we are in a net operating loss position and have a full valuation allowance in the affected jurisdictions. |
| | |
| (d) | | The pro forma increase in compensation expense associated with employee stock options and call options reflects: |
| | |
| • | | A compensation charge associated with 124,448 stock options that are expected to be granted to our employees and outside directors immediately following the pricing of this offering at an exercise price equal to the initial public offering price. The value of these options was estimated to be $ million using the Black-Scholes option pricing model and key assumptions are as follows: expected option term of 7 years, risk-free interest rate will be updated at the date of grant but is currently estimated to be %, dividend yield of %, volatility will be updated at the date of grant but is currently estimated to be % and an exercise price and fair value of $ per share (the midpoint of the price range set forth on the cover page of this prospectus). Compensation expense will be recognized on these options over an expected four year vesting term, and as such, the compensation expense for fiscal 2010 was assumed to be 25% of the total value, or $ million. |
| | |
| • | | A compensation charge associated with 463,794 performance-based call options with market conditions held by Thomas D. Logan, our Chief Executive Officer. These options contain vesting provisions based upon successful completion of an initial public offering or change in control and achievement by ACAS of certain internal rates of return or returns on investment. The value of these options was estimated to be $2.1 million using a Monte Carlo simulation model and key assumptions are as follows: at the January 1, 2006 modification, expected option term of 4.5 years, risk-free interest rate of 4.3%, dividend yield of 0%, volatility of 41.8%, exercise price of $10.45 per share and fair value of $2.22 per share; at the December 7, 2007 modification, expected option term of 2.6 years, risk-free interest rate of 3.1%, dividend yield of 0%, volatility of 39.8%, exercise price of $10.45 per share and fair value of $4.44 per share. The compensation expense associated with these options for fiscal 2010 was calculated to be $1.5 million, respectively, based on the implied requisite service period for the market conditions (30 days for one-half of the shares, and 24 months for one-half of the shares). |
| | |
| (e) | | The weighted average common shares outstanding from the conversion of common stock assumes the conversion of all outstanding shares of Class A Voting Common Stock and Class B Non-Voting Common Stock on a one-for-one basis into 405,796 shares of common stock. |
| | |
| (f) | | Includes shares of our common stock to be sold in connection with this offering. Because distributions to ACAS, our principal stockholder, consisting of obligations under existing debt arrangements of $183.3 million and amounts due of $8.0 million to terminate an existing investment banking services agreement, exceed our earnings plus gross proceeds from this offering of $ million, all common shares are included in the calculation under existing rules on pro forma calculations. Following is a calculation of the deemed dividend in excess of proceeds from this offering (in thousands): |
| | | | | |
| | | For the Twelve
| |
| | | Months Ended
| |
| | | June 30, 2010 | |
| Gross proceeds from offering | | | | |
| Distributions to ACAS: | | | | |
| Termination of investment banking services agreement | | | | |
| Repayment of notes payable to ACAS from proceeds of offering | | | | |
| Repayment of notes payable to ACAS from new debt arrangements | | | | |
| | | | | |
| Total distribution to ACAS | | | | |
| Last twelve months earnings | | | | |
| | | | | |
| Excess of dividend to be paid over proceeds from offering | | | | |
| | | | | |
| | |
| (g) | | The weighted average common shares outstanding from the conversion of preferred stock assumes the conversion of all outstanding convertible preferred stock into common stock, including the conversion into common stock of all accrued and unpaidpaid-in-kind dividends on convertible preferred stock. The 10,233,721 weighted average at June 30, 2010 is comprised of 936,573A-1 preferred shares, which includes 257,769 accrued but unpaidpaid-in-kind dividends, which are convertible at a rate of 9.6288 and 137,941A-2 preferred shares, which includes 67,941 accrued but unpaidpaid-in-kind dividends, which are convertible at a rate of 8.8128. |
| | |
| (h) | | These warrants only become exercisable upon a sale, liquidation or dissolution of the Company or approval by our Board of Directors. Our Board of Directors has resolved that all of these warrants will become exercisable upon the consummation of this offering. |
| | |
| (i) | | The shares used in computing pro forma net loss per common share before conversion of preferred shares — diluted for the year ended June 30, 2010 exclude options to purchase 924,830 shares of common stock because we recorded a pro forma net loss allocable to common stockholders before conversion of preferred shares, and therefore the impact of such shares would be anti-dilutive. The shares used in computing pro forma net income per common share — diluted and diluted as adjusted for the year ended June 30, 2010 exclude options to purchase 662,353 shares of common stock, because the effect would be anti-dilutive. 476,841 of these shares were excluded because the option exercise prices exceeded the average market value of our common stock during the period. 185,512 of these shares were excluded because after applying the treasury stock method of calculating earnings per share, the impact would be anti-dilutive. The |
45
| | |
| | shares used in computing pro forma net loss per common share before conversion of preferred shares — basic and diluted and pro forma net income per common share — diluted and diluted as adjusted for the year ended June 30, 2009 and fiscal 2010 also exclude options to purchase 124,448 shares of common stock which are expected to be granted immediately following the pricing of this offering because their impact would be anti-dilutive, either because we generated a net loss or because after applying the treasury stock method of calculating earnings per share, the impact would be anti-dilutive. |
| | |
| (2) | | As of June 30, 2010, we also had $6.6 million of restricted cash. |
| | |
| (3) | | In addition, as of June 30, 2010, we had $2.6 million of outstanding debt held by third parties not affiliated with ACAS. |
| | |
| (4) | | We include Adjusted EBITDA in this prospectus because (i) it is a basis upon which our management assesses our operating performance, (ii) it is a factor in the evaluation of the performance of our management in determining compensation and (iii) certain maintenance covenants under our debt agreements are tied to ratios based upon Adjusted EBITDA, as defined. Adjusted EBITDA for any period, as defined in our debt agreements, is calculated as net income (loss) for such period plus (a) without duplication and to the extent deducted in determining net income for such period, the sum of (i) interest expense for such period, (ii) income tax expense for such period, (iii) all amounts attributable to depreciation and amortization expense for such period, (iv) any extraordinary cash charges for such period in an amount not to exceed $4,000,000, (v) any extraordinary non-cash charges for such period, (vi) any other non-cash charges for such period (but excluding any non-cash charge for such period in respect of an item that was included in net income in a prior period) and (vii) any non-recurring fees, costs and expenses as reflected in our June 30, 2010 financial statements and any non-recurring fees, costs and expenses incurred in connection with this offering or in connection with the new bank credit facilities and any fees paid to ACAS and its affiliates pursuant to, or in connection with the termination of, the investment banking services agreement with ACFS after June 30, 2010 on or prior to the closing of this offeringminus (b) without duplication and to the extent included in net income, (i) any cash payments made during such period in respect of non-cash charges described in clauses (a)(vi) or (a)(vii) taken in a prior period and (ii) any extraordinary gains and any non-cash items of income for such period, all calculated on a consolidated basis in accordance with U.S. GAAPprovided that net income excludes (a) the income (or deficit) of any person (other than a subsidiary) in which we or any of our subsidiaries has an ownership interest, except to the extent that such income is actually received by us or such subsidiary in the form of dividends or similar distributions and (b) the undistributed earnings of any of our subsidiaries (other than subsidiaries party to the debt agreement) to the extent that the declaration or payment of dividends or similar distributions by such subsidiary is not at the time permitted by the terms of any contractual obligation (other than under any loan documents associated with the debt agreement) or requirement of law applicable to such subsidiary. Adjusted EBITDA is not a measure of financial performance calculated in accordance with U.S. GAAP, and should be viewed as a supplement to—not a substitute for—our results of operations presented on the basis of U.S. GAAP. Adjusted EBITDA also does not purport to represent cash flow provided by, or used in, operating activities in accordance with U.S. GAAP. Our statements of cash flows, included elsewhere in this prospectus, present our cash flow activity in accordance with U.S. GAAP. Furthermore, Adjusted EBITDA is not necessarily comparable to similarly titled measures reported by other companies. |
The following is a reconciliation of cash (used in) provided by operating activities to Adjusted EBITDA:
| | | | | | | | | | | | | |
| | | Year Ended June 30, | |
| | | 2008 | | | 2009 | | | 2010 | |
| |
| Cash (used in) provided by operating activities | | $ | (6,712 | ) | | $ | 10,031 | | | $ | 8,202 | |
| Interest expense, net | | | 20,207 | | | | 17,711 | | | | 15,120 | |
| Income tax expense | | | 5,838 | | | | 5,612 | | | | 7,732 | |
| Fees paid to ACFS | | | 1,625 | | | | 1,739 | | | | 1,643 | |
| Other nonrecurring charges(*) | | | 5,458 | | | | 5,867 | | | | 2,605 | |
| Actuarial (gain) loss | | | 226 | | | | (208 | ) | | | (530 | ) |
| Paid-in-kind interest expense | | | (1,904 | ) | | | (1,992 | ) | | | (2,091 | ) |
| Loss on disposal of property, plant and equipment | | | (502 | ) | | | (592 | ) | | | (859 | ) |
| Amortization of loan fees, debt discount and preferred stock discount | | | (785 | ) | | | (522 | ) | | | (458 | ) |
| Provision for doubtful accounts | | | (30 | ) | | | (140 | ) | | | (138 | ) |
| Provision for deferred income taxes | | | 1,238 | | | | 1,013 | | | | (3,062 | ) |
| Change in operating assets and liabilities | | | 9,559 | | | | 2,084 | | | | 18,136 | |
| Currency effects and other | | | — | | | | 22 | | | | — | |
| | | | | | | | | | | | | |
| Adjusted EBITDA | | $ | 34,218 | | | $ | 40,625 | | | $ | 46,300 | |
| | | | | | | | | | | | | |
| | |
| (*) | | Represents non-recurring expenses, including severance expenses and costs associated with the preparation for our initial public offering, as well as certain professional and legal expenses. |
| | |
| (5) | | Represents purchase orders or contracts received by us that have not been shipped. Amounts representing backlog are not recorded in our financial statements. |
46
MANAGEMENT’S DISCUSSION AND ANALYSIS
OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of the financial condition and results of our operations should be read together with “Selected Consolidated Financial Data” and the consolidated financial statements and the related notes included elsewhere in this prospectus. This discussion contains forward-looking statements, based on current expectations related to future events and our future financial performance, that involve risks and uncertainties. Our actual results may differ materially from those anticipated in these forward-looking statements as a result of many factors, including those set forth under “Risk Factors” and elsewhere in this prospectus.
Overview
We are a global provider of radiation detection, measurement, analysis and monitoring products and services to the nuclear, defense and medical end markets. Our customers rely on our solutions to protect people, property and the environment from nuclear and radiological hazards. Our products and services include: dosimeters; contamination & clearance monitors; detection & identification instruments; radiation monitoring systems; electrical penetrations; instrumentation & control equipment and systems; dosimetry services; imaging systems; and related accessories, software and services.
We provide our products and services through five segments: Health Physics, Radiation Monitoring Systems, Sensing Systems, Dosimetry Services and Imaging Systems. Our Health Physics segment derives revenue from the nuclear, defense and medical end markets. We provide our Health Physics customers, which include power and utility companies, military organizations, engineering companies as well as governmental agencies, with dosimeters, contamination & clearance monitors as well as equipment that detects and identifies radioactive isotopes. Our Radiation Monitoring Systems segment offers systems that provide process and post-event radiation monitoring to the nuclear end market. Our Radiation Monitoring Systems customers include power and utility companies, engineering companies, research laboratories, universities, as well as governmental agencies. Our Sensing Systems segment supplies electrical penetrations as well as reactor instrumentation & control equipment and systems to the builders and operators of nuclear reactors. Our Sensing Systems customers include power and utility companies, the U.S. Navy, as well as engineering companies. Our Dosimetry Services segment provides analytical services to determine occupational and environmental radiation exposure to employers of radiation workers in the nuclear and medical end markets. Our Dosimetry Services customers include power and utility companies, hospitals, governmental agencies, medical professionals, dentists and veterinarians. Our Imaging Systems segment provides specialized closed circuit camera systems used for inspection and surveillance in difficult and hazardous environments to the nuclear and other end markets. We provide these systems to power and utility companies, operators of waste management facilities, cement kilns and petrochemical facilities.
Of the $228.1 million in total revenue we generated in fiscal 2010, $73.0 million, or 32.0%, was attributable to our Health Physics segment, $60.7 million, or 26.6%, was attributable to our Radiation Monitoring Systems segment, $47.3 million, or 20.7%, was attributable to our Sensing Systems segment, $29.2 million, or 12.8%, was attributable to our Dosimetry Services segment and $17.9 million, or 7.9%, was attributable to our Imaging Systems segment. Please see Note 15 of our consolidated financial statements for additional financial information about our segments.
Despite achieving positive operating income in fiscal 2010, we have reported net losses in all other periods since our inception in 2005, due in large part to our leverage. As of June 30, 2010, we had an accumulated deficit of $96.6 million. We expect to reduce our leverage through the repayment of certain of our indebtedness with the net proceeds from this offering. See “Use of Proceeds.”
We incorporated in Delaware in October 2005 as Global Monitoring Services, Inc. Our business was formed through a series of transactions in December 2005 resulting in the combination of three companies, all owned by ACAS, our principal stockholder, and its affiliates. The three companies were GDS, a provider of dosimetry services to the nuclear and medical industries, IST, a manufacturer of electrical penetrations, reactor instrumentation & control equipment and systems and imaging systems for the nuclear, defense and other industries and Synodys, a designer and manufacturer of radiation detection, measurement, analysis and
47
monitoring equipment for the nuclear, defense and medical industries. Following these transactions, we changed our name in January 2006 to Mirion Technologies, Inc.
We are a global company with operations in Canada, China, Finland, France, Germany, the United Kingdom and the United States. Accordingly, currency exchange rates can impact our reported results of operations. Revenue outside of the Americas accounted for 64.1%, 61.6% and 65.1% of total revenue for fiscal 2008, 2009 and 2010. Please see Note 15 to our consolidated financial statements for additional financial information about geographic areas.
Our independent registered public accounting firm reported to us that at each of June 30, 2008, 2009 and 2010, we had material weaknesses in our internal controls over financial reporting which we have not yet corrected. The material weaknesses identified were with respect to our controls in our financial accounting and reporting functions, which are necessary in order to produce U.S. generally accepted accounting principles (U.S. GAAP) compliant financial statements. See “Risk Factors” for a further description of the foregoing material weaknesses.
References to “fiscal” before any year refer to our fiscal year ending on June 30th of the year referenced.
Key Indicators of Performance
In evaluating our business, our management considers Adjusted EBITDA as a key indicator of operating performance. We include Adjusted EBITDA in this prospectus because (i) it is a basis upon which our management assesses our operating performance, (ii) it is a factor in the evaluation of the performance of our management in determining compensation and (iii) certain maintenance covenants under our debt agreements are tied to ratios based upon Adjusted EBITDA, as defined. Adjusted EBITDA for any period, as defined in our debt agreements, is calculated as net income (loss) for such period plus (a) without duplication and to the extent deducted in determining net income for such period, the sum of (i) interest expense for such period, (ii) income tax expense for such period, (iii) all amounts attributable to depreciation and amortization expense for such period, (iv) any extraordinary cash charges for such period in an amount not to exceed $4,000,000, (v) any extraordinary non-cash charges for such period, (vi) any other non-cash charges for such period (but excluding any non-cash charge for such period in respect of an item that was included in net income in a prior period) and (vii) any non-recurring fees, costs and expenses as reflected in our June 30, 2010 financial statements and any non-recurring fees, costs and expenses incurred in connection with this offering or in connection with the new bank credit facilities and any fees paid to ACAS and its affiliates pursuant to, or in connection with the termination of, the investment banking services agreement with ACFS after June 30, 2010 on or prior to the closing of this offeringminus (b) without duplication and to the extent included in net income, (i) any cash payments made during such period in respect of non-cash charges described in clauses (a)(vi) or (a)(vii) taken in a prior period and (ii) any extraordinary gains and any non-cash items of income for such period, all calculated on a consolidated basis in accordance with U.S. GAAP,provided that net income excludes (a) the income (or deficit) of any person (other than a subsidiary) in which we or any of our subsidiaries has an ownership interest, except to the extent that such income is actually received by us or such subsidiary in the form of dividends or similar distributions and (b) the undistributed earnings of any of our subsidiaries (other than subsidiaries party to the debt agreement) to the extent that the declaration or payment of dividends or similar distributions by such subsidiary is not at the time permitted by the terms of any contractual obligation (other than under any loan documents associated with the debt agreement) or requirement of law applicable to such subsidiary.
We use Adjusted EBITDA as a key performance measure because we believe it facilitates operating performance comparisons from period to period by excluding potential differences caused by variations in capital structures (affecting interest expense), tax positions (such as the impact on periods or companies of changes in effective tax rates or net operating losses) and the impact of depreciation and amortization expense on definite lived intangible assets. Because Adjusted EBITDA facilitates internal comparisons of our historical operating performance on a more consistent basis, we also use Adjusted EBITDA for business planning purposes, to incentivize and compensate our management personnel, in measuring our performance relative to that of our competitors and in evaluating acquisition opportunities.
48
In addition, we believe Adjusted EBITDA and similar measures are widely used by investors, securities analysts, ratings agencies and other interested parties as a measure of financial performance and debt-service capabilities. Our use of Adjusted EBITDA has limitations as an analytical tool, and you should not consider it in isolation or as a substitute for analysis of our results as reported under U.S. GAAP. Some of these limitations are:
| | |
| | • | it does not reflect our cash expenditures for capital equipment or other contractual commitments; |
| |
| | • | although depreciation, amortization and asset impairment charges and write-offs are non-cash charges, the assets being depreciated, amortized or written off may have to be replaced in the future, and Adjusted EBITDA does not reflect cash capital expenditure requirements for such replacements; |
| |
| | • | it does not reflect changes in, or cash requirements for, our working capital needs; |
| |
| | • | it does not consider the potentially dilutive impact of issuing equity-based compensation to our management team and employees; |
| |
| | • | it does not reflect the significant interest expense or the cash requirements necessary to service interest or principal payments on our indebtedness; |
| |
| | • | it does not reflect certain tax payments that may represent a reduction in cash available to us; and |
| |
| | • | other companies, including companies in our industry, may calculate these measures differently, and as the number of differences in the way two different companies calculate these measures increases, the degree of their usefulness as a comparative measure correspondingly decreases. |
Because of these limitations, Adjusted EBITDA should not be considered as a measure of discretionary cash available to us to invest in the growth of our business. We compensate for these limitations by relying primarily on our U.S. GAAP results and using Adjusted EBITDA only supplementally. We carefully review our operating income at a segment level, which is discussed in detail in ourperiod-to-period comparison of operating results.
Components of Revenue and Expenses
Revenue and Cost of Revenue
Health Physics
We generate revenue in our Health Physics segment primarily from the sale of dosimeters, both active and passive, which measure ionizing radiation dose; contamination & clearance monitors, which detect alpha, beta, gammaand/or neutron contamination of objects of various sizes and types, from tools to trucks; and devices that detect, locate and identify radioactive isotopes. We sell our equipment either pursuant to written agreements or contracts requiring delivery of products or services over a specified time period or one-time purchase orders depending on the nature of the product and the dollar value of the sale. We typically use contracts for large installations of our equipment to power and utility companies as well as military organizations. These contracts are typically fixed price, where we bear the risk for changes in material costs as well as currency movements. The time period from receipt of a contract to the recognition of revenue generally ranges from a few months to a year. We typically use purchase orders for the sale of replacement components as well as small dollar value orders. For customer projects with customer specific acceptance criteria, we typically do not recognize revenue and the related cost of revenue until our customer has installed the equipment and certified that it is operating correctly or until we have otherwise determined that all customer-specific acceptance criteria have been met. Furthermore, customers may delay delivery or acceptance of equipment, causing postponement of revenue recognition even though we may have received payment. We record payments received from customers prior to the time we recognize revenue for associated sales as deferred contract revenue.
Revenue in our Health Physics segment has been primarily driven by product sales for new nuclear power reactor construction in Asia, replacement product sales for NPPs in the Americas and Europe, as well as replacement product sales for the defense end market.
49
Cost of revenue in our Health Physics segment primarily consists of cost of goods purchased for the manufacture of our equipment, facility costs, compensation and benefits to manufacturing employees and outsourcing costs for subcontractor services for the manufacture of various materialsub-components.
Radiation Monitoring Systems
We generate revenue in our Radiation Monitoring Systems segment from the sale of radiation monitoring systems and services to engineering firms that design and construct nuclear reactors, power and utility companies that operate NPPs and, to a lesser extent, research laboratories and universities. We generate most of the revenue in our Radiation Monitoring Systems segment from contracts with a duration greater than one year. These contracts are typically fixed price, where we bear the risk for changes in material cost as well as currency movements.
Revenue in our Radiation Monitoring Systems segment can fluctuate significantly from period to period because of customer requirements, which depend upon the operating schedules of nuclear reactors. The operating schedules of nuclear reactors are affected by, among other things, seasonality in the demand for electricity and reactor refueling and maintenance. Power and utility companies typically schedule refueling and maintenance to coincide with periods of reduced power demand, usually in the spring and fall. As a result, our revenue tends to be higher during these periods when equipment is typically installed. Revenue may also fluctuate from period to period as our equipment is installed in newly constructed nuclear reactors. Our contracts often contain multiple elements with deliveries scheduled over multiple reporting periods. Effective July 1, 2009 the Company adopted a modification to revenue recognition accounting rules that changes the requirements for establishing separate units of accounting and requires the allocation of contract revenue to each of the deliverables in the arrangement based on relative selling price. The guidance is applicable to all contracts executed or significantly modified since July 1, 2009. Under the accounting guidance applicable to contracts executed prior to July 1, 2009, revenue is allocated to each element based on relative fair value. Since the Company is often unable to establish fair value of the undelivered elements, revenue for all elements is deferred until the delivery of the final element is complete. As a result, we typically do not recognize revenue and the related cost of revenue on these contracts until our customer has installed the equipment and certified that it is operating correctly, which generally can extend to 12 months or more from shipment, or until we have otherwise determined that all customer-specific acceptance criteria have been met. Furthermore, regardless of the date of the contract, customers may delay delivery or acceptance of equipment, causing postponement of revenue recognition, even though we may have received payment. In each of the preceding circumstances, we record payments received from customers prior to the time we recognize revenue as deferred contract revenue.
The Radiation Monitoring Systems segment is a project-driven business where each project is negotiated on a stand-alone basis. Gross margins can vary significantly by project for a number of reasons, including level of competition, type of customer, and the uniqueness or complexity of the project. Accordingly, an increase or decrease in revenues from one period to another may not result in a proportional increase or decrease in gross margins or operating income due to the mix of projects completed in any given quarter or year.
Revenue in our Radiation Monitoring Systems segment has been primarily driven by new nuclear power reactor construction in Asia and Europe, as well as the retrofitting of existing reactors. Revenue in the Americas region has been primarily driven by renewed sales for the retrofitting of existing reactors.
Cost of revenue in our Radiation Monitoring Systems segment primarily consists of cost of goods purchased for the manufacture of our equipment, facility costs, compensation and benefits to employees and outsourcing costs for subcontractor services for the manufacture of various materialsub-components.
Sensing Systems
We generate revenue in our Sensing Systems segment primarily through sales of our electrical penetrations which are conduits through a nuclear reactor containment structure, as well as sales of our reactor instrumentation & control detectors, which are used in nuclear facilities to monitor radiation and temperature within a nuclear reactor core (“in-core” detectors) and in surrounding areas (“ex-core” detectors). Our Sensing Systems segment sells primarily through contracts with engineering firms that design and construct nuclear
50
reactors as well as power and utility companies that operate NPPs. These contracts are typically fixed price, where we bear the risk for changes in material costs as well as currency movements. We have had a long-term relationship with one company that provides plant design and equipment to a significant number of nuclear power plants around the world. Revenues from this customer have represented approximately 23% to 30% of the Sensing Systems segment revenue in fiscal years 2008, 2009 and 2010. If we were to lose this customer, it could have a material impact on the results of the Sensing Systems segment. We have generated the majority of the revenue in our Sensing Systems segment from contracts with a duration greater than one year.
Revenue in our Sensing Systems segment has been primarily driven by new nuclear power reactor construction in Asia and Europe for our electrical penetrations as well as by the replacement of reactor instrumentation & control equipment and systems for existing reactors in Asia, Europe and the Americas.
Cost of revenue in our Sensing Systems segment primarily consists of cost of goods purchased for the manufacture of our equipment, facility costs, compensation and benefits to employees and outsourcing costs for subcontractor services for the manufacture of various materialsub-components.
Dosimetry Services
Revenue from our Dosimetry Services segment is of a subscription nature. We provide these services to customers on anagreed-upon recurring monthly, quarterly or annual basis. Badge production, badge analysis and report preparation are all integral to the service that we provide to our customers, and therefore, we define the service period to include the provision of all of those services. We recognize revenue and related costs on a straight-line basis over the service period as the service is continuous.
Revenue in our Dosimetry Services segment has been primarily driven by the increased use of our dosimetry services in hospitals and other medical facilities resulting from increases in the incidence of radiological medical procedures, along with the increased use of our services by dental and veterinary offices in the United States.
Cost of revenue in our Dosimetry Services segment primarily consists of compensation and benefits to employees, outsourcing costs for subcontractor services and cost of goods purchased for use in our badges.
Imaging Systems
We generate revenue in our Imaging Systems segment through the sale of highly specialized closed circuit camera systems used for inspection and surveillance in difficult and hazardous environments through contracts with engineering firms that design and construct nuclear reactors, power and utility companies that operate NPPs, waste management facilities, as well as companies that operate pulp and paper recovery boilers, gas or coal-fired power boilers and cement kilns. These contracts are typically fixed price, where we bear the risk of changes in material cost and currency movements.
Revenue in our Imaging Systems segment has been primarily driven by increased demand in Asia for high radiation-tolerant cameras for use in new NPP construction, and for use in radioactive waste management and nuclear facility decommissioning projects globally.
Cost of revenue in our Imaging Systems segment primarily consists of cost of goods purchased for the manufacture of our equipment, facility costs, compensation and benefits to employees, and outsourcing costs for subcontractor services for the manufacture of various materialsub-components.
Selling, General and Administrative Expenses
Selling, general and administrative, or SG&A, expenses consist primarily of personnel costs (including salaries, performance-based bonuses, commissions and employee benefits), facilities and equipment costs, costs related to advertising and marketing and other general corporate and support costs including utilities, insurance and professional fees. SG&A expenses also include $1.6 million per year in management fees we have paid to ACFS under an investment banking services agreement. We intend to use a portion of the net proceeds from this offering to make a one-time payment of $8.0 million to ACFS upon completion of this offering to terminate the
51
agreement related to these payments. This $8.0 million payment will be included in SG&A expenses in the period paid. See “Certain Relationships and Related Party Transactions.”
Research and Development Expenses
Research and development expenses consist primarily of the costs associated with the design and testing of new products, as well as the upgrading of existing products. These costs relate primarily to compensation of personnel involved with our product development efforts, materials and outside design and testing services. Our customers sometimes compensate us separately for design and engineering work involved in developing our products for them. However, in most cases we expense product development efforts for our customers and we do not receive reimbursement.
Interest Expense, Net
Interest expense, net includes both cash and accrued interest expense and income and amortization of financing costs, as well aspaid-in-kind interest on our long-term debt.
Other Income, Net
Other income, net includes gains and losses on the sale of assets,mark-to-market gains and losses on our interest rate swap agreements and foreign exchange windows.
Paid-In-Kind Preferred Dividends
Paid-in-kind, or PIK, dividends consists of expenses attributable to dividends on our convertible preferred stock payable in additional shares of such convertible preferred stock. Our preferred stock will convert into common stock upon the completion of this offering. As a result, following the completion of this offering, we will no longer pay any additional PIK dividends.
Provision for Income Taxes
Provision for income taxes represents our estimated income tax expense for the period presented. Despite historical net losses, we have incurred income tax expense for each of the past three fiscal years, as we incur income taxes in various jurisdictions as a result of the global nature of our business and operations.
Off-Balance Sheet Arrangements
We do not have any relationships with unconsolidated entities or financial partnerships, such as entities often referred to as structured finance or special purpose entities, which would have been established for the purpose of facilitating off-balance sheet arrangements or other contractually narrow or limited purposes, nor do we have any undisclosed material transactions or commitments involving related persons or entities.
Critical Accounting Policies
This management’s discussion and analysis of financial condition and results of operations is based upon our consolidated financial statements, which have been prepared in accordance with accounting principles as set forth in the Financial Accounting Standards Board (FASB) Accounting Standards Codification (ASC), as well as the various staff accounting bulletins and other applicable guidance issued by the U.S. Securities and Exchange Commission. The preparation of these financial statements requires us to make estimates and assumptions about matters that are uncertain. These estimates and assumptions are often based on judgments that we believe to be reasonable under the circumstances, but all such estimates and assumptions are inherently uncertain and unpredictable. Actual results may differ from those estimates and assumptions, and it is possible that other professionals, applying their own judgment to the same facts and circumstances, could develop and support alternative estimates and assumptions that would result in material changes to our operating results and financial condition.
52
Critical accounting policies are those that both are important to the presentation of our financial condition and results of operations and require management’s most difficult, complex or subjective estimates and assumptions. Our critical accounting policies are discussed below.
Revenue Recognition
We recognize revenue and the related costs of revenue when there is persuasive evidence of an arrangement, product delivery has occurred or services have been provided, the sales price is fixed or determinable and collectability is reasonably assured. For sales contracts that contain customer-specific acceptance provisions, revenue and the related costs are deferred until the customer has indicated successful completion of site acceptance tests or we have otherwise determined that all customer-specific acceptance criteria have been met. In the Health Physics and Radiation Monitoring Systems segments, we perform detailed factory acceptance testing on completed products which in some instances is sufficiently extensive and reliable to demonstrate that completed products meet all of the customer-specified objective acceptance criteria set forth in its sales arrangements. In such instances, our policy is to recognize revenue based on the product’s delivery terms and prior to the receipt of notification of formal acceptance from the customer.
We enter into sales arrangements that may consist of multiple deliverables of our product and service offerings due to the requirements of our customers. For example, a customer may purchase a radiation monitoring systems solution and commissioning services. This arrangement would consist of multiple elements, with the products, as well as the commissioning services, delivered across multiple reporting periods. Another customer may purchase dosimeters, contamination and clearance monitorsand/or detection and identification devices along with commissioning services, in which all the elements are delivered within the same reporting period.
In October 2009, the FASB amended the accounting standard for multiple-deliverable revenue arrangements. The new standard changes the requirements for establishing separate units of accounting in a multiple element arrangement and requires the allocation of arrangement consideration to each deliverable to be based on the relative selling price. We adopted this standard during fiscal 2010 retroactive to the beginning of the fiscal year for new and materially modified arrangements originating on or after July 1, 2009. The adoption of the new standard was not material to consolidated revenue in fiscal 2010 primarily because our orders tend to have long lead times and most of our revenue from fiscal 2010 orders will not be recognized until fiscal 2011 or later. Beginning in fiscal 2011, we expect that the adoption of the new standard will generally result in revenue recognized in earlier reporting periods because we will be able to recognize revenue in instances where we have not established fair value for the undelivered element. We are not able to reasonably estimate the effect of adopting this standard on future financial periods, as the impact will vary based on the nature and volume of new or materially modified arrangements in any given period.
For new and materially modified arrangements originating in fiscal 2010 and future periods, when a sales arrangement contains multiple elements, we allocate revenue to each element based on a selling price hierarchy. The selling price for a deliverable is based on its vendor-specific objective evidence (“VSOE”) if available, third party evidence (“TPE”) if VSOE is not available, or estimated selling price if neither VSOE nor TPE is available. We then recognize revenue on each deliverable in accordance with our policies for product and service revenue recognition. We are not typically able to determine VSOE or TPE, and so we allocate revenue to the elements of the arrangement based on estimated selling prices. We establish our best estimate of selling price considering multiple factors including but not limited to pricing practices in different geographies and through different sales channels, costs and margin objectives, competitive pricing strategies and general market conditions.
We limit the amount of revenue recognition for delivered elements to the amount that is not contingent on the future delivery of products or services or future performance obligations or subject to customer specific cancellation rights. We evaluate each deliverable in an arrangement to determine whether they represent separate units of accounting. A deliverable constitutes a separate unit of accounting when it has stand-alone value and for an arrangement that includes a general right of return relative to the delivered products or services, delivery or performance of the undelivered product or service is considered probable and is
53
substantially controlled by us. We consider a deliverable to have standalone value if the product or service is sold separately by us or another vendor or could be resold by the customer. Further, the revenue arrangements generally do not include a general right of return relative to the delivered products. Where the aforementioned criteria for a separate unit of accounting are not met, the deliverable is combined with the undelivered element(s) and treated as a single unit of accounting for the purposes of allocation of the arrangement consideration and revenue recognition. We allocate the total arrangement consideration to each separable element of an arrangement based upon the relative selling price of each element. Allocation of the consideration is determined at arrangement inception on the basis of each unit’s relative selling price.
For transactions entered into prior to fiscal 2010 (and not otherwise materially modified in fiscal 2010 or a subsequent period), revenue for arrangements with multiple elements is recognized as each element is delivered or completed based upon its relative fair value. In instances where we have not established fair value for any undelivered element, revenue for all elements is deferred until delivery of the final element is complete and all recognition criteria are met.
Revenue from certain fixed price contracts in our Sensing Systems and Imaging Systems divisions that involve customization of equipment to customer specifications is recognized using thepercentage-of-completion method measured by thecost-to-cost method. Thecost-to-cost method is used because management considers incurred costs to be the best available measure of progress on these contracts. Contract costs include all direct materials and labor costs, as well as indirect costs related to contract performance. Changes in job performance, job conditions and estimated profitability result in revisions to costs and revenue and are recognized in the period in which the revisions are determined. Provisions for estimated losses on uncompleted contracts are made in the period in which such losses are first determined. Revenue earned in excess of billings on contracts in progress (underbillings) is classified as a current asset. Amounts billed in excess of revenue earned (advance billings) are classified as current liabilities and included in deferred contract revenue.
We derive most of our revenue in our Dosimetry Services segment from subscriptions and such revenue is continuous. We recognize revenue on a straight-line basis over a set service period that includes badge production, badge wearing, badge analysis and report preparation as the service is continuous and no other discernable pattern of recognition is evident. We provide these services to customers on anagreed-upon monthly, quarterly or annual basis that our customers choose for their wear period, payable in advance or in arrears. The amounts recorded as deferred contract revenue on our balance sheets represent customer deposits invoiced in advance for services to be rendered over the service period, net of a reserve for estimated cancellations and net of services recognized through the balance sheet date.
Recoverability of Long-Lived Assets, Including Goodwill
Goodwill represents the excess of costs over the fair value of net assets of businesses acquired. Goodwill is tested at the reporting unit level at least annually for impairment and is reviewed for impairment more frequently if events and circumstances indicate that the asset might be impaired. FASB-issued authoritative guidance on goodwill and other intangible assets requires a two-step impairment test. In the first step, we determine the fair value of the reporting unit using a discounted cash flow valuation model and compare the fair value to the reporting unit’s carrying value. If the fair value of the reporting unit exceeds its carrying value, goodwill of the reporting unit is not impaired, and no further testing is required. If the fair value does not exceed the carrying value, the second step of the goodwill impairment test is performed to measure the amount of impairment loss, if any. In the second step of the goodwill impairment test, we compare the implied fair value of the reporting unit’s goodwill to the carrying value. We determine the implied fair value of the reporting unit’s goodwill as if the reporting unit had been acquired in a business combination. If the carrying value of the reporting unit’s goodwill exceeds the implied value, we recognize an impairment loss in an amount equal to the excess.
We estimate future cash flows at the reporting unit level using a discounted cash flow methodology by assessing each major existing contract and projecting the earnings that will be recognized in future periods. We also make estimates for earnings from new contracts that we anticipate based on our evaluation of future
54
business prospects. The valuation of goodwill could be affected if actual results differ substantially from our estimates. Circumstances that could affect the valuation of goodwill include a significant change in our business climate, decisions by our customers to terminate our existing contracts and decisions by our customers to award to our competitors new contracts that we anticipated to be awarded to us.
We measure intangible assets acquired in a business combination at fair value at the date of acquisition. We assess the useful lives of other intangible assets to determine whether events or circumstances warrant a revision to the remaining period of amortization. If the estimate of an intangible asset’s remaining useful life is changed, we amortize the remaining carrying amount of the intangible asset prospectively over the revised remaining useful life. We review intangible assets for impairment whenever events or circumstances indicate that the carrying value of such assets may not be recoverable. As of June 30, 2010, we had $129.3 million of goodwill and $16.4 million of intangible assets with estimable useful lives on our consolidated balance sheets. We do not have any intangible assets with indefinite useful lives.
Intangible assets subject to amortization consist of customer relationships, backlog, qualifications, software, territorial rights, trade names, technology and non-compete agreements. We evaluate customer relationships and territorial rights, which include the fair value of acquired customer contracts, using a discounted cash flow methodology, and amortize them on an accelerated basis over a term of five to 17 years. We derive estimated future cash flows based on detailed budgets and projections prepared by management. We amortize backlog over a term of one to three years based on the estimated delivery of the backlog. We prepare the valuation of order backlog based on a discounted cash flow methodology. We evaluate qualifications using a discounted cash flow methodology and amortize them over six years. We derive estimated future cash flows based on projections prepared by management. We amortize software over a five year life and derive it by estimating the replacement cost of the software. We amortize trade names over a period of four to 13 years and derive it based on the relief from royalty method, which tries to estimate a royalty stream for the trade names derived from a benchmark for similar industrial products. We evaluate technology and non-compete agreements using a discounted cash flow methodology. We amortize intangible technology assets over a term of eight years, and non-compete agreements over a term of five years. We derive estimated future cash flows for each technology and non-compete agreement based on detailed budgets and projections prepared by management.
We review long-lived assets such as property, plant and equipment annually for impairment and whenever events or changes in circumstances indicate the carrying amount of an asset may not be recoverable. We measure recoverability of assets to be held and used by comparing the carrying amount of the asset to the estimated undiscounted future cash flows expected to be generated by the asset. If the carrying amount of an asset exceeds its estimated future cash flows, we recognize an impairment charge by the amount of excess carrying value over fair value.
Customer Concentration
No single customer represented more than 10% of consolidated revenue for fiscal 2008, 2009 and 2010.
Stock-Based Compensation Expense
Pursuant to ASC 718, Compensation — Stock Compensation, we account for equity-based compensation awards, including grants of employee stock options, based on the fair values of the equity instruments issued. We determine fair value of our equity instruments based on a valuation using an option pricing model which takes into account various assumptions that are subjective. Key assumptions used in the valuation included the expected term of the equity award taking into account both the contractual term of the award, the effects of employees’ expected exercise and post-vesting termination behavior, expected volatility, expected dividends and the risk-free interest rate for the expected term of the award. The exercise prices of our options were set at values for us consistent with the fair value of ACAS’s investment in Mirion as reported in ACAS’s publicly filed financial statements.
Effective July 1, 2005, we adopted ASC 718, Compensation — Stock Compensation, using the prospective transition method. This method requires the recognition of compensation cost for all stock-based
55
awards that are unvested as of July 1, 2005. The cost related to stock-based compensation included in the determination of consolidated net (loss) income for the years ended June 30, 2008, 2009 and 2010 includes all awards outstanding that vested during those periods. In connection with the reorganization of our three predecessor companies into Mirion in December 2005, we established the Mirion 2006 stock plan and exchanged stock options of the three predecessor companies for stock options in the newly formed company. Under this guidance, the exchange was deemed a modification, resulting in incremental compensation expense of $749,000 recorded at January 1, 2006 for those options that were vested as of January 1, 2006. For the unvested options at January 1, 2006, we recorded incremental compensation expense of $618,000 over the remaining vesting period of approximately two years.
The stock awards under the Mirion 2006 stock plan include stock awards with performance and market-based vesting (“PSA”) and time-based vesting (“TSA”). Under the terms of the PSA agreements, we grant employee stock option awards whose vesting is contingent upon meeting company-wide market goals including earnings before interest, taxes and depreciation targets or market conditions, including internal rate of return targets. The TSAs include stock options granted by us whose vesting occurs over a period of five to 60 months. The PSAs include 85,798 options for which the market-based conditions are first contingent upon a performance based goal of a qualified sale of the Company, which does not include a public offering.
In order to determine the fair value of options granted, the fair value of the underlying stock must first be determined. Following is a discussion of the methodology used in the valuation of our stock on dates when options were granted.
The valuation of our common stock was determined in accordance with the guidance set forth in the AICPA Audit and Accounting Practice Aid Series: Valuation of Privately Held Company Equity Securities Issued as Compensation. Our calculation of the fair value of our common stock was not performed contemporaneously with the grant of options because we did not have in place a process to perform such valuations at the time such options were granted. However, we considered the retrospective valuation in our calculation of the fair value of our common stock at the grant dates. The calculation reflects our best estimate of relevant variables at those dates. We considered three methods for the allocation of value among our various classes of equity: the Current Value Method, the Probability Weighted Expected Return Method, or PWERM, and the Option-Pricing Method. The Current Value Method is useful for early stage companies or when a liquidation is imminent. PWERM is useful when there are several potential future scenarios for a company to achieve a return on investment for its investors; it is future looking and incorporates future economic events and outcomes into the determination of value as of the present. The Option-Pricing Method is useful when the range of possible future outcomes is difficult to predict.
We did not use the Current Value Method in our valuation since we have not been an early stage company nor have we been near liquidation. Since our inception there have been several potential alternatives for changes in our ownership structure, including an initial public offering, a sale or merger, and retention in our current form as a private company. For most of the time since our inception, the range of possible future outcomes has been difficult to predict, and PWERM could not be used. Therefore, we used the Option-Pricing Method. However, as of the most recent valuation which was performed for June 2009, we believe that the range of possible future outcomes could be reasonably predicted, and as such used PWERM for this period.
Following is a description of the Option-Pricing Method, which we used to allocate value among the various classes of equity for the period from January 2006 through December 2008.
The first step in determining the valuation of our common stock was to determine the value of our total equity. The second step was to allocate the total equity among the different classes of stock. In determining the fair value of our total equity, we considered the three traditional approaches to valuation: the cost approach, the market approach and the income approach. The cost approach was not utilized, while the market approach and income approach were.
The market approach is based on the assumption that the value of an asset (including a company) is equal to the value of a substitute asset with the same or similar characteristics. Therefore, the value of an asset can
56
be determined by finding similar assets (or interest in similar assets) that have been sold in recent arms-length transactions.
One methodology in the market approach is the Guideline Company Method, which compares the subject company with guideline publicly-traded companies. Valuation multiples are calculated from selected guideline companies to provide an indication of how much a current investor in the marketplace would be willing to pay for a company with similar characteristics to the subject company. The Guideline Company Method is most appropriate when public companies that are reasonably similar to the subject company can be found.
Another methodology in the market approach is the Guideline Transaction Method. This method relies on data of actual transactions (such as mergers and acquisitions) that have occurred in the subject company’s industry or in related industries. As in the Guideline Company Method, valuation multiples are developed and applied to the subject company’s operating data to estimate fair value.
The income approach seeks to measure the future benefits that can be quantified in monetary terms. The income approach typically involves two general steps: the first step is to make a projection of the total cash flows expected to accrue to an investor in the asset; the second step involves discounting these cash flows to present value at a discount rate that considers the degree of risk (or uncertainty) associated with the realization of the projected monetary benefits. The discounted cash flow method is a form of the income approach often used in the valuation of entire businesses, major segments of a business or intangible property.
Once we determined our valuation using each of the various methods, we then weighted the results to arrive at a single valuation of our equity. The market approach Guideline Company Method, the market approach Guideline Transaction Method and the income approach were weighted 25%, 25% and 50%.
We then allocated this total value to the different classes of our equity using the Option-Pricing Method. As disclosed in Note 12 to our consolidated financial statements, we have issuedSeries A-1 andA-2 preferred stock, Class A and Class B common stock, stock options and warrants, over which to allocate the total value of equity. Stock options and warrants are assumed to be converted if they arein-the-money.
The Option-Pricing Method treats the preferred and common stock as call options that give their owner the right but not the obligation to buy the underlying total equity at a predetermined price. This is done by creating a series of call options with exercise prices based on the liquidation preference, participation rights and conversion behavior of the preferred stock. The value of each share of preferred and common stock can then be inferred by analyzing these options. Based on the seniority of the classes of equity in liquidation, three call options were created and valued. The first option uses an exercise price in whichSeries A-1 andA-2 preferred stock begin to receive values in liquidation. Since these are the most senior classes of equity, the exercise price is $0. The second option uses an exercise price in whichSeries A-1 andA-2 preferred stock have received their full liquidation preferences. The third option uses an exercise price in whichSeries A-1 andA-2 preferred stock will convert to common stock to share in the upside gain (the as-converted value is greater than the liquidation preference). Thus, common stock is valued as a call option with a claim on us at an exercise price equal to the remaining value beyond the preferred stock’s liquidation preference.
The following is a description of the PWERM Method, which we used to allocate value among the various classes of equity as of June 2009 and 2010.
In PWERM, our total equity valuation was developed for various potential scenarios: an initial public offering, a sale or maintaining current ownership as a private company.
In determining the fair value of our equity under each of the scenarios, the three traditional approaches to valuation were considered: the cost approach, the market approach and the income approach. The cost approach was not utilized, while the market approach and income approach were. We used the Guideline Company Method of the market approach in the valuation of our equity for the initial public offering scenario. We used the Guideline Transaction Method of the market approach to value our equity for the sale scenario. We used the income approach to value our equity in the continuing operations scenario.
After we determined the valuation of our common equity using the three methodologies above, the results were then weighted based on our estimate of expected outcomes. The initial public offering scenario, the sale scenario and the current ownership as a private company scenario were weighted 60%, 35% and 5%.
57
During fiscal 2010, we did not grant options to employees or others. During fiscal 2009, we granted options to employees to purchase a total of 390,030 shares of common stock at exercise prices ranging from $16.31 to $17.06 per share. The deemed market value of our common stock on the dates these options were granted ranged from $5.48 to $11.32 per share.
We expect to grant options to purchase 124,448 shares of our common stock immediately following the pricing of this offering to our employees and outside directors, including executive officers, at an exercise price equal to the initial public offering price. The purpose of this option grant will be to incentivize employees to work to increase stockholder value and to provide an equity interest for our outside directors. The options will be valued using the Black-Scholes option pricing model. Key assumptions used to value these options will be determined as of the grant date of the options and are expected to be as follows: expected term will be 7 years, risk-free interest rate will be updated at the date of grant but is currently estimated to be %, dividend yield will be %, volatility will be updated at the date of grant but is currently estimated to be % and the exercise price and the fair value of our common stock will be equal to the initial public offering price in this offering (assumed to be $ , the midpoint of the price range set forth on the cover page of this prospectus). Based on these assumptions, the aggregate fair value of these options to purchase 124,448 shares of our common stock is estimated to be approximately $ million, which will be recognized evenly over their four-year vesting period.
Information on employee stock options, granted since the beginning of fiscal 2009 is summarized as follows:
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | Deemed
| | | | ASC Topic 718
|
| | | Number of
| | | | Market Value
| | Intrinsic
| | Black-Scholes
|
Date of Issuance | | Options Granted | | Exercise Price | | Per Share | | Value | | Option Fair Value |
| |
| July 28, 2008(1) | | | 102,000 | | | $ | 16.31 | | | $ | 11.32 | | | $ | 0.00 | | | $ | 6.67 | |
| August 5, 2008 | | | 279,530 | | | | 17.06 | | | | 11.32 | | | | 0.00 | | | | 5.53-5.85 | |
| December 9, 2008 | | | 8,500 | | | | 16.97 | | | | 5.48 | | | | 0.00 | | | | 1.85 | |
| | |
| (1) | | The 102,000 options were a modification on July 28, 2008 of 127,500 options granted on November 5, 2007. The 102,000 options, which have time-based vesting, replaced the 127,500 options, which had performance-based vesting. |
Significant factors contributing to the changes in the deemed market value per share of our common stock at the date of each grant and through the date of this prospectus were as follows:
July 28, 2008 and August 5, 2008. We determined that the deemed market value of our common stock as of July 28, 2008 and August 5, 2008 was $11.32 per share. During this and prior periods, we used the Option-Pricing Method in order to determine the fair value of our common stock, with weighting factors and selected multiples as follows:
| | | | | | | | | | | | | |
| | | Weighting
| | | Revenue
| | | EBITDA
| |
| | | Factor | | | Multiple | | | Multiple | |
| |
| The market approach — Guideline Company Method | | | 25 | % | | | 1.7 | | | | 12.9 | |
| The market approach — Guideline Transaction Method | | | 25 | % | | | 1.9 | | | | 12.7 | |
| The income approach | | | 50 | % | | | N/A | | | | N/A | |
December 9, 2008. The deemed market value of our common stock as of December 9, 2008 decreased significantly from the prior valuation period, from $11.32 per share to $5.48 per share. Between August and December 2008, U.S. financial and stock markets suffered a severe decline in valuations. Rapidly deteriorating business conditions in the United States resulted in a freezing of capital and credit markets, which contributed to a global economic contraction. While our historical results improved over this period, our stock price
58
valuation fell due to the sharp decline in the multiples applied to our historical results. The weighting factors and selected multiples used in the December 2008 valuation were as follows:
| | | | | | | | | | | | | |
| | | Weighting
| | | Revenue
| | | EBITDA
| |
| | | Factor | | | Multiple | | | Multiple | |
| |
| The market approach — Guideline Company Method | | | 25 | % | | | 1.4 | | | | 8.5 | |
| The market approach — Guideline Transaction Method | | | 25 | % | | | 1.4 | | | | 10.0 | |
| The income approach | | | 50 | % | | | N/A | | | | N/A | |
June 30, 2009. As discussed above, in June 2009 we began using the PWERM method in order to determine the fair value of our common stock because we determined that the range of possible future outcomes for our investors could be reasonably predicted. Those possible outcomes included an initial public offering scenario, a sale of the Company scenario and a continuing operations scenario. We used the Guideline Company Method for the valuation of our common stock for the initial public offering scenario, the Guideline Transaction Method for the sale scenario and the income approach for the continuing operations scenario. There were no stock option grants or other equity-related issuances made at or near this date. The probabilities and selected multiples used in the June 2009 valuation were as follows:
| | | | | | | | | | | | | |
| | | Probability
| | | Revenue
| | | EBITDA
| |
| | | Factor | | | Multiple | | | Multiple | |
| |
| Guideline Company Method — IPO scenario high-side | | | 30 | % | | | 1.9 | | | | 10.6 | |
| Guideline Company Method — IPO scenario low-side | | | 30 | % | | | 1.2 | | | | 9.5 | |
| Guideline Transaction Method — sale scenario high-side | | | 17.5 | % | | | 1.5 | | | | 10.0 | |
| Guideline Transaction Method — sale scenario low-side | | | 17.5 | % | | | 1.3 | | | | 9.5 | |
| The income approach — continuing operations | | | 5 | % | | | N/A | | | | N/A | |
Using these assumptions, the deemed market value of our common stock as of June 30, 2009 increased significantly from the prior valuation period, from $5.48 to $12.44 per share. By June 2009, much of the turmoil in the U.S. financial and stock markets had stabilized and valuation multiples increased. Also during this period, our historical results improved as we continued to grow our business and it became increasingly probable that we would successfully complete an initial public offering.
December 31, 2009. The deemed market value of our common stock as of December 31, 2009 increased from $12.44 per share to $16.20 per share as we updated our assessment of the likelihood of a successful initial public offering given our operating performance and the state of the public equity markets. There were no stock option grants or other equity-related issuances made at or near this date. The probabilities and selected multiples used in the December 2009 valuation were as follows:
| | | | | | | | | | | | | |
| | | Probability
| | | Revenue
| | | EBITDA
| |
| | | Factor | | | Multiple | | | Multiple | |
| |
| Guideline Company Method — IPO scenario high-side | | | 40 | % | | | 2.0 | | | | 11.9 | |
| Guideline Company Method — IPO scenario low-side | | | 40 | % | | | 1.2 | | | | 9.9 | |
| Guideline Transaction Method — sale scenario high-side | | | 7.5 | % | | | 1.7 | | | | 10.0 | |
| Guideline Transaction Method — sale scenario low-side | | | 7.5 | % | | | 1.2 | | | | 9.5 | |
| The income approach — continuing operations | | | 5 | % | | | N/A | | | | N/A | |
June 30, 2010. The deemed market value of our common stock decreased slightly from $16.20 per share at December 31, 2009 to $16.16 per share at June 30, 2010. There were no stock option grants or other
59
equity-related issuances made at or near this date. The probabilities and selected multiples used in the June 30, 2010 valuation were as follows:
| | | | | | | | | | | | | |
| | | Probability
| | | Revenue
| | | EBITDA
| |
| | | Factor | | | Multiple | | | Multiple | |
| |
| Guideline Company Method — IPO scenario high-side | | | 40 | % | | | 1.7 | | | | 12.0 | |
| Guideline Company Method — IPO scenario low-side | | | 40 | % | | | 1.1 | | | | 9.9 | |
| Guideline Transaction Method — sale scenario high-side | | | 7.5 | % | | | 1.7 | | | | 10.0 | |
| Guideline Transaction Method — sale scenario low-side | | | 7.5 | % | | | 1.5 | | | | 7.8 | |
| The income approach — continuing operations | | | 5 | % | | | N/A | | | | N/A | |
We make a number of estimates and assumptions on stock-based compensation. The estimation of stock awards that will ultimately vest requires judgment, and to the extent actual results differ from our estimates, we will record such amounts as an adjustment in the period such estimates are revised. Actual results may differ substantially from these estimates. In valuing stock-based awards under this guidance, significant judgment is required in determining the expected volatility of our common stock and the expected term individuals will hold their stock-based awards prior to exercising. We base expected volatility of the stock on our peer group in the industry in which we do business, because we do not have sufficient historical volatility data for our own stock. We determine the expected term of the option based on factors including vesting period, life of option, strike price and fair market value of our common stock on the date of grant. In the future, as we gain historical data for volatility in our own stock and the actual term employees hold our options, expected volatility and expected term may change, which could substantially change the grant date fair value of future awards of stock options and ultimately the expense we record.
We recorded non-cash compensation expense of $0.2 million, $1.2 million and $1.1 million in fiscal 2008, 2009 and 2010.
In addition to the stock options we granted to our employees, our President, Chief Executive Officer and Chairman of the Board, Thomas D. Logan, entered into a Call Option Agreement on April 19, 2004 with ACAS and certain of its affiliates, in which ACAS granted time and performance-based options with market conditions to Mr. Logan to purchase shares of the common stock of two of our predecessors in connection with his services as an officer and director. The options contain vesting provisions based upon successful completion of an initial public offering or change in control, and achievement by ACAS of certain internal rates of return as discussed in detail below. Modification of these options occurred in substance on January 1, 2006, in connection with the formation of Mirion in December 2005, and was formalized in an agreement dated August 18, 2006. As a result of the modification, Mr. Logan was granted performance-based options with market conditions to purchase 463,794 shares of Mirion’s common stock held by ACAS. These options were further modified on December 7, 2007 to modify the vesting criteria of the performance based options to include, in addition to existing vesting provisions, vesting upon the achievement of certain returns on investment, as discussed in detail below. The exercise price of these options is $10.45 per share, and the total maximum value resulting from the option modification is $2.1 million. The original grant date fair value of these options was negligible. We will recognize expense on these options to the extent that we are able to either complete an effective offering in the public markets or complete a qualifying sale of the Company; and, in such instance, over the derived service period of these options, which is consistent with the periods over which the market conditions of the awards are measured, as described further below.
The performance-based options with market conditions are divided into three tranches, each of which will either vest or be cancelled in two halves upon an initial public offering (“IPO”) or change in control, depending on whether ACAS achieves certain internal rates of return or returns on investment in such an event. Upon completion of an IPO (i.e., the performance condition), vesting of these performance-based options will occur in two stages upon achievement of certain market-based conditions. The first stage occurs 30 days after the effective time of the IPO at which time 50% of the options in each tranche will vest if ACAS achieves certain minimum internal rates of return, ranging between 25–40% or certain minimum returns on investment ranging between 2.0–2.7x. If neither goal is met, the options in this tranche will be cancelled. The second stage occurs on the earlier of two years after the effective time of an IPO or upon the
60
sale by ACAS of its investments in us, at which time the remaining 50% of the options in each tranche will vest if ACAS achieves certain minimum internal rates of return ranging between 25–40% or certain minimum returns on investment of 2.0–2.7x. If neither goal is met, the options in this tranche will be cancelled. We have not recorded stock compensation expense in connection with these options because vesting is contingent upon future events.
The total compensation cost measured at the date of modification was determined to be the grant-date fair value of the original award plus the incremental cost resulting from the modification. Because these options contain market-based vesting criteria, we used a Monte Carlo simulation model, rather than a Black-Scholes model, to value such options. In the Monte Carlo simulation model, weekly stock prices were simulated until the liquidity event time using a geometric Brownian motion model. Based on the simulated stock price at the liquidity event and the vesting requirements, the number of vested shares was determined. The stock price at liquidity and the options’ exercise price were used to determine the intrinsic value per share of the options at the liquidity event. The intrinsic value was then discounted to the present at the risk free rate to determine the option value for each simulation. Fifty thousand simulations were run for each valuation date, and the sum value of those simulations was averaged to determine the value of the options. Key valuation assumptions as of the January 1, 2006 modification are an expected term of 4.5 years, volatility of 41.8%, a risk-free rate of interest of 4.3%, and a dividend yield of 0%. Key valuation assumptions as of the December 7, 2007 modification are an expected term of 2.6 years, volatility of 39.8%, a risk-free rate of interest of 3.1%, and a dividend yield of 0%.
The Call Option Agreement also provides Mr. Logan with an option to purchase 150,875 shares of our common stock held by ACAS that vest on a monthly schedule. The exercise price of these options is $10.45 per share and the total incremental value resulting from the option modification is $592,000. All such options have vested as of June 30, 2008. In connection with these options, we recorded stock compensation expense of $121,000 in fiscal 2008.
The total compensation cost measured at the date of modification was determined to be the grant-date fair value of the original award plus the incremental cost resulting from the modification. These options were valued using the Black-Scholes model. Key valuation assumptions as of the January 1, 2006 modification are an expected term of 4.5 years, volatility of 41.8%, a risk-free rate of interest of 4.3%, and a dividend yield of 0%.
All options granted by ACAS and its affiliates to Mr. Logan pursuant to the Call Option Agreement are to be reduced on an economically equivalent basis in the event we grant Mr. Logan options to purchase shares of our common stock after the date of the Call Option Agreement, provided such options are no less favorable to Mr. Logan.
In addition to the 463,794 performance-based options with market conditions granted to Mr. Logan by ACAS, there are 85,798 unvested outstanding performance-based options that we have granted to other employees. These 85,798 options are different from those granted to Mr. Logan in that they were granted by us rather than by ACAS. Furthermore, these options only vest in the event of a sale transaction, which does not include this offering. Therefore, it is not expected that we will record expense for such options.
Accounts Receivable
We evaluate the collectability of accounts receivable based on a combination of factors. In cases where we are aware of circumstances that may impair a specific customer’s ability to meet its financial obligations, we record a specific allowance against amounts due and, thereby, reduce the net recognized receivable to the amount we reasonably believe will be collected. We record increases to the allowance for bad debt as a component of general and administrative expenses.
Income Taxes
The determination of our tax provision is subject to judgments and estimates due to the complexity of the tax law that we are subject to in several tax jurisdictions. Earnings derived from our international business are
61
generally taxed at rates that are different than U.S. rates, resulting in an effective tax rate different than the U.S. statutory tax rate of 34.0%. The ability to maintain our current effective tax rate is contingent on existing tax laws in both the United States and the respective countries in which our international subsidiaries are located. In addition, a decrease in the percentage of our total earnings from international business or a change in the mix of international business among particular tax jurisdictions could alter our overall effective tax rate.
Income taxes are accounted for under the asset and liability method in accordance with ASC 740, Income Taxes. Under this method, deferred tax assets and liabilities are recognized for the estimated future tax consequences attributable to differences between the tax bases of assets and liabilities and their financial statement carrying amounts, and consideration is given to operating losses and tax credit carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on the deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. This guidance also requires that deferred tax assets be reduced by a valuation allowance if it is more likely than not that some or all of the deferred tax asset will not be realized. We have provided a valuation allowance of $31.4 million as of June 30, 2009 and $32.1 million as of June 30, 2010 on primarily our U.S. jurisdiction deferred tax assets.
We account for uncertainty in our tax positions under the revised guidance of ASC 740. The revised guidance contains a two-step approach to recognizing and measuring uncertain tax positions taken or expected to be taken in a tax return. The first step is to determine if the weight of available evidence indicates that it is more likely than not that the tax position will be sustained in an audit, including resolution of any related appeals or litigation process. The second step is to measure the tax benefit as the largest amount that is more than 50% likely to be realized upon ultimate settlement. We evaluate these uncertain tax positions on a quarterly basis. This evaluation is based on the consideration of several factors, including changes in facts or circumstances, changes in applicable tax law, settlement of issues under audit and new exposures. If we later determine that our exposure is lower or that the liability is not sufficient to cover our revised expectations, we adjust the liability and recognize the related change in our tax provision during the period in which we make such determination.
Inventory Valuation
At each balance sheet date, we evaluate our ending inventories for excess quantities and obsolescence. This evaluation includes analysis of sales levels by products. Among other factors, we consider historical demand and forecasted demand in relation to the inventory on hand, product life cycles and the number of facilities using our products when determining obsolescence and net realizable value. We adjust remaining balances to approximate the lower of our manufacturing cost or market value. We determine inventory cost on afirst-in, first-out basis and include material, labor and manufacturing overhead costs. We may be required to write-down inventory for reasons such as obsolescence, excess quantities and declines in market value below our costs.
Outlook
We expect the following factors to affect our results of operation in future periods. In addition to these factors, please refer to “Risk Factors” for additional information on what could cause our actual results to differ from our expectations.
Industry growth trends. Our performance depends on the timing and level of spending for our products by each of our customers in each of our five segments. Our success is dependent upon the continued increase in construction activity for new NPPs in Asia and Europe, as well as the operating life extension of plants in Europe, Asia and the United States. We expect defense spending to detect and prevent radiological threats to continue, as well as spending in connection with large-scale public events. The expansion of radiological medical procedures is also providing us with opportunities for continued growth. For discussion of the factors that influence spending on our products, see “Industry.”
62
Research and development expenses. We expect our research and development expenses as a percentage of revenue to decrease as we grow our business, focus our engineering activities on customer driven initiatives and benefit from the reduced engineering costs associated with optimized product lines.
SG&A expenses. We expect our SG&A expenses as a percentage of revenue to decrease as our business grows and we continue to manage expenses and as our intangible assets become fully amortized. The majority of our amortization expenses are in SG&A expenses. We have incurred expenses in the past in connection with the integration of our legacy businesses and severance costs associated with our cost reduction efforts. We do not expect these expenses to recur. We also expect to eliminate $1.6 million per year in annual management fees we have paid to ACFS pursuant to an investment banking services agreement that we intend to terminate by making a one-time payment of $8.0 million to ACFS upon completion of this offering. We expect any reduction in our SG&A expenses to be partially offset by expenses we will incur as a result of becoming a reporting company following this offering.
Interest expenses. We expect that our interest expenses will be reduced in periods following the completion of this offering reflecting the reduced level of our outstanding indebtedness.
Foreign exchange impact. We are a global company with operations in Canada, China, Finland, France, Germany, the United Kingdom and the United States. Accordingly, currency exchange rates can impact our reported results of operations.
Unamortized debt issuance costs. We expect to record a non-cash charge associated with the repayment of certain of our indebtedness with a portion of the net proceeds of this offering. This charge will consist of the write-off of unamortized debt issuance costs.
Income taxes. Despite historical net losses, we have incurred income tax expense for each of the past three fiscal years. In any given period, the jurisdictional mix of our income can vary significantly as a result of the global nature of our business and operations. The income tax rates, available deductions and credits vary significantly in the jurisdictions we do business. Accordingly, the income tax expense in any given period is a function of the effective tax rate and related income secured in a particular jurisdiction. Although we have reported historic consolidated losses, we recognized income in foreign jurisdictions and losses in the United States for the past three years. We have incurred tax expense in foreign jurisdictions due to the taxable income position. We have provided a valuation allowance on our United States based tax attributes and as a result, no tax benefit is recognized for the United States operating losses.
We have net operating loss carryforwards (“NOLs”) which we can use to reduce our United States tax expense in future periods. These NOLs are subject to elimination or reduction in the event of a change of control. We do not expect such a change of control to occur in connection with this offering. However, a future sale of our common stock by ACAS (including the exercise by ACAS’ lenders of their rights under the Pledge) could result in such a change of control.
Amortization costs related to intangible assets. Our non-cash amortization costs related to intangible assets were $10.1 million, $8.1 million and $6.4 million for fiscal 2008, 2009 and 2010. Our future amortization expense at the foreign exchange rates determined as of June 30, 2010 is as follows (in thousands):
| | | | | |
| | | Annual
| |
| | | Amortization
| |
Fiscal Year Ending June 30, | | Expense | |
| |
| 2011 | | $ | 4,446 | |
| 2012 | | | 3,465 | |
| 2013 | | | 2,588 | |
| 2014 | | | 1,584 | |
| 2015 | | | 1,348 | |
| 2016 and thereafter | | | 2,975 | |
| | | | | |
| Total | | $ | 16,406 | |
| | | | | |
63
Consolidated Results of Operations
The following table summarizes certain items of our consolidated results of operations for fiscal 2008, 2009 and 2010 (in thousands, except percentages):
| | | | | | | | | | | | | |
| | | Year Ended June 30, | |
| | | 2008 | | | 2009 | | | 2010 | |
| |
| Revenue | | $ | 191,769 | | | $ | 201,763 | | | $ | 228,124 | |
| Cost of revenue | | | 102,790 | | | | 105,954 | | | | 126,707 | |
| | | | | | | | | | | | | |
| Gross profit | | | 88,979 | | | | 95,809 | | | | 101,417 | |
| % of revenue | | | 46.4 | % | | | 47.5 | % | | | 44.5 | % |
| Operating expenses: | | | | | | | | | | | | |
| Selling, general and administrative expenses | | | 63,177 | | | | 65,649 | | | | 64,510 | |
| Research and development expenses | | | 14,865 | | | | 11,282 | | | | 8,729 | |
| | | | | | | | | | | | | |
| Total operating expenses | | | 78,042 | | | | 76,931 | | | | 73,239 | |
| | | | | | | | | | | | | |
| Income from operations | | | 10,937 | | | | 18,878 | | | | 28,178 | |
| Interest expense, net | | | 20,207 | | | | 17,711 | | | | 15,120 | |
| Other income, net | | | (1,759 | ) | | | (490 | ) | | | (639 | ) |
| | | | | | | | | | | | | |
| (Loss) Income before provision for income taxes | | | (7,511 | ) | | | 1,657 | | | | 13,697 | |
| Provision for income taxes | | | 5,838 | | | | 5,612 | | | | 7,732 | |
| | | | | | | | | | | | | |
| Net (loss) income | | $ | (13,349 | ) | | $ | (3,955 | ) | | $ | 5,965 | |
| | | | | | | | | | | | | |
Period-to-Period Analysis
As the results of operations of our business are best understood when examined on asegment-by-segment basis, we have more fully describedperiod-to-period changes in the section of this Management’s Discussion and Analysis of Financial Condition and Results of Operation entitled “Segment Results of Operations” rather than in the section immediately below.
Fiscal 2010 as Compared to Fiscal 2009
Revenue
Consolidated revenue for fiscal 2010 was $228.1 million, an increase of $26.4 million, or 13.1%, from revenue of $201.8 million for fiscal 2009. This increase was driven by revenue growth in four of our five segments:
| | |
| | • | Health Physics segment revenue increased $3.9 million, or 5.6%, to $73.0 million for the period. This increase was primarily due to an increase in sales of contamination and clearance products of $9.8 million, driven by strong demand during the first half of fiscal 2010. This increase was partially offset by a $5.9 million decrease in sales of dosimetry products, predominantly due to a reduction in dosimetry orders from military customers and U.S. nuclear power plants. |
| | |
| | • | Radiation Monitoring segment revenue increased $19.6 million, or 47.7%, to $60.7 million for the period, primarily due to the completion of several large radiation monitoring installation projects into new nuclear power plants in Asia and Europe, for which there were no comparable projects recognized in fiscal 2009. |
| | |
| | • | Sensing Systems segment revenue increased $2.3 million, or 5.1%, to $47.3 million for the period, principally due to the completion of certain long term contracts, additional spare-part orders and increased shipments of in-core and ex-core detector products. |
| | |
| | • | Imaging Systems segment revenue increased $0.8 million, or 4.9%, primarily due to an upturn in revenue from nuclear camera sales in the second half of fiscal 2010. |
| | |
| | • | The Dosimetry Service segment revenue decreased $0.3 million in fiscal 2010, primarily due to competitive pricing pressures in the medical end market. |
64
For fiscal 2010, $36.0 million, or 15.8% of revenue was attributable to sales of products into nuclear power plant facilities under construction, up 86.4% from $19.3 million for the prior year. The remaining $192.1 million of revenue for fiscal 2010 and the remaining $182.5 million of revenue for fiscal 2009 were attributable to sales other than in connection with new power plant construction, including replacement or recurring products and services, and sales of new products into existing nuclear power plants.
Gross Profit
Consolidated gross profit for fiscal 2010 was $101.4 million, an increase of $5.6 million, or 5.9%, from gross profit of $95.8 million for fiscal 2009. Gross margin decreased 3.0% to 44.5% for fiscal 2010, primarily due to lower margins in: the Health Physics segment, due to product mix; the Radiation Monitoring segment, due to geographic mix; and the Dosimetry Services segment, due to competitive pricing pressures.
Operating Expenses
Selling, general and administrative expenses for fiscal 2010 were $64.5 million, a decrease of $1.1 million, or 1.7%, compared to $65.6 million for fiscal 2009. The decrease was primarily due to an overall reduction in amortization expense as our customer relationship intangible assets become fully amortized, as well as a decrease in marketing program expenses. These decreases were partly offset by an increase in compensation expenses due to headcount increases in administrative functions of finance and information technology, professional fees associated with an expired credit agreement and activities relating to Sarbanes-Oxley preparedness work.
Research and development expenses for fiscal 2010 were $8.7 million, a decrease of $2.6 million, or 22.6%, from $11.3 million for fiscal 2009. This decrease was primarily due to the recording of $1.9 million of research and development credits (Crédit d’Impôt Recherche, or CIR) in one of our European locations, relating to fiscal 2007 and 2009. These credits were not recorded until fiscal 2010, when we received the cash, due to previous uncertainty about collectibility. We also experienced a reduction in contractor expenses and professional service fees following completion of a new product development project in the prior year.
Interest Expense, Net
Interest expense, net for fiscal 2010 was $15.1 million, a decrease of $2.6 million, or 14.6%, from $17.7 million for fiscal 2009. This decrease was primarily the result of a decline in the LIBOR and EURIBOR rates on our variable debt instruments from the previous year.
Other Income, Net
Other income, net increased from $0.5 million during fiscal 2009 to $0.6 million for fiscal 2010. This increase was primarily due to net foreign exchange gains recorded in fiscal 2010.
Income Taxes
The provision for income taxes for fiscal 2010 was $7.7 million, with an effective tax rate of 56.5%, compared with a provision for income taxes for fiscal 2009 of $5.6 million, and an effective tax rate of 338.7%. The provision for income taxes for fiscal 2009 and 2010 are comprised mainly of foreign income taxes. The increase in tax expense for fiscal 2010 was primarily attributable to higher earnings in taxable jurisdictions.
Fiscal 2009 as Compared to Fiscal 2008
Revenue
Consolidated revenue for fiscal 2009 was $201.8 million, an increase of $10.0 million, or 5.2%, from revenue of $191.8 million for fiscal 2008.
65
$19.3 million of revenue in fiscal 2009 was attributed to sales in connection with new nuclear plant construction, an increase of $2.1 million, or 12.2%, from revenue of $17.2 million in connection with new nuclear plant construction for fiscal 2008. The remaining revenue of $182.5 million for fiscal 2009 was attributed to sales other than in connection with new nuclear plant construction, including sales of replacement or recurring products and services for existing plants and customers facilities. The remaining revenue of $174.6 million for fiscal 2008 was attributed to sales other than in connection with new nuclear plant construction, which includes sales of replacement or recurring products and services.
Revenue in our Health Physics segment increased 17.8%, or $10.4 million, principally due to higher sales volumes in the following product lines:
| | |
| | • | $1.9 million increase in sales of dosimeters, principally into existing NPPs in Asia and the Americas; and |
| |
| | • | $5.4 million increase in sales of contamination & clearance monitors, principally into existing NPPs in the Americas. |
| |
| | • | $3.1 million increase in sales of detection and identification devices principally to the defense end market in Europe. |
Revenue in our Radiation Monitoring Systems segment decreased $2.1 million, or 4.8%, principally due to the negative impact of foreign currency movements of approximately $3.5 million in fiscal 2009.
Revenue in our Sensing Systems segment increased $5.1 million, or 12.8%, principally due to higher sales volumes in the following product lines:
| | |
| | • | $3.4 million increase in revenue recognized from contracts for the production of penetration products used in the construction of NPPs; and |
| |
| | • | $1.7 million increase in revenue recognized from contracts for the production of detectors used in NPPs. |
Revenue in our Dosimetry Services segment increased by $0.6 million, or 2.2%, while revenues in our Imaging Systems segment fell by $4.1 million, or 19.3%, due to weakening demand for high-temperature cameras in industrial end markets.
Revenue in fiscal 2009 was negatively impacted due to foreign currency movements by approximately $10.2 million.
Gross Profit
Consolidated gross profit for fiscal 2009 was $95.8 million, an increase of $6.8 million, or 7.7%, from gross profit of $89.0 million for fiscal 2008. Gross margin increased 1.1% to 47.5% for fiscal 2009, primarily due to lower material costs in the Sensing Systems segment. Gross profit was negatively impacted due to foreign currency movements by approximately $4.9 million.
Operating Expenses
SG&A expenses for fiscal 2009 were $65.6 million, an increase of $2.5 million, or 3.9%, from $63.2 million for fiscal 2008. This increase was primarily due to an increase in professional fees associated with preparation for our initial public offering, offset by a reduction in compensation expense, as well as an overall reduction of expense due to favorable currency exchange as our expenses in U.S. dollars were positively impacted by weaker foreign currencies, primarily the euro and the British pound.
Research and development expenses for fiscal 2009 were $11.3 million, a decrease of $3.6 million, or 24.1%, from $14.9 million for fiscal 2008. This decrease was primarily due to a decrease in compensation and subcontractor expense due to product rationalization as well as a reduction of expense due to favorable currency exchange as our expenses in U.S. dollars were positively impacted by weaker foreign currencies, principally the euro and the British pound, offset by an increase in supplies and services.
66
Interest Expense, Net
Interest expense, net for fiscal 2009 was $17.7 million, a decrease of $2.5 million, or 12.4%, from $20.2 million for fiscal 2008. This reduction was primarily the result of the decline in our interest expense on our variable rate instruments as our interest rates tied to LIBOR and EURIBOR declined from fiscal 2008.
Other Income, Net
Other income, net decreased $1.3 million, or 72.1%, to other income, net of $0.5 million for fiscal 2009, from other income, net of $1.8 million for fiscal 2008. This decrease of other income, net was primarily a result of a decrease in foreign exchange gains of $1.4 million.
Income Taxes
We recognized income tax expense of $5.6 million for fiscal 2009, a decrease of $0.2 million, or 3.9%, from fiscal 2008. The decrease was primarily due to the geographic composition of our consolidated income, with less tax expense attributable to foreign operations. In addition, the fiscal 2009 effective tax rate was impacted by losses generated in the United States where we do not record benefit for tax attributes due to the valuation allowance.
Segment Results of Operations
The following table summarizes certain items for our segments for fiscal 2008, 2009 and 2010. The amounts below are in thousands.
| | | | | | | | | | | | | |
| | | Year Ended June 30, | |
| | | 2008 | | | 2009 | | | 2010 | |
| |
| Revenue: | | | | | | | | | | | | |
| Health Physics | | $ | 58,691 | | | $ | 69,109 | | | $ | 72,992 | |
| Radiation Monitoring Systems | | | 43,201 | | | | 41,116 | | | | 60,729 | |
| Sensing Systems | | | 39,866 | | | | 44,979 | | | | 47,255 | |
| Dosimetry Services | | | 28,824 | | | | 29,457 | | | | 29,206 | |
| Imaging Systems | | | 21,187 | | | | 17,102 | | | | 17,942 | |
| | | | | | | | | | | | | |
| Total | | $ | 191,769 | | | $ | 201,763 | | | | 228,124 | |
| | | | | | | | | | | | | |
| Operating income: | | | | | | | | | | | | |
| Health Physics | | $ | (912 | ) | | $ | 6,317 | | | $ | 5,058 | |
| Radiation Monitoring Systems | | | 1,085 | | | | 4,109 | | | | 12,102 | |
| Sensing Systems | | | 10,234 | | | | 14,973 | | | | 16,587 | |
| Dosimetry Services | | | 7,746 | | | | 7,968 | | | | 7,936 | |
| Imaging Systems | | | 1,339 | | | | 1,064 | | | | 2,080 | |
| Unallocated corporate items | | | (8,555 | ) | | | (15,553 | ) | | | (15,585 | ) |
| | | | | | | | | | | | | |
| Total | | $ | 10,937 | | | $ | 18,878 | | | $ | 28,178 | |
| | | | | | | | | | | | | |
Health Physics
Fiscal 2010 as Compared to Fiscal 2009
Revenue in our Health Physics segment increased $3.9 million, or 5.6%, to $73.0 million for fiscal 2010, from $69.1 million for fiscal 2009. This increase was predominantly due to growth of $9.8 million in the contamination and clearance product line with strong growth in demand, particularly in the first half of fiscal 2010. This increase was offset by a decrease in sales of $5.8 million in the dosimetry product line due to a reduction in orders from military customers and U.S. nuclear power plants.
Operating income in our Health Physics segment decreased $1.3 million, or 19.9%, to $5.1 million for fiscal 2010 compared to $6.3 million for fiscal 2009. This decrease was primarily due to lower gross margins due to a change in product mix. The decrease in operating income from gross profit was partially offset by a
67
decrease in operating expenses for the segment by $1.2 million in fiscal 2010, due to a reduction in research and development expenses attributable to research and development credits relating to fiscal 2007 and 2009, but received and recognized in fiscal 2010, and a reduction of contractor-related expenses in favor of additional headcount at a lower overall cost.
Fiscal 2009 as Compared to Fiscal 2008
Revenue in our Health Physics segment increased $10.4 million, or 17.8%, to $69.1 million for fiscal 2009, from $58.7 million for fiscal 2008. This increase was primarily due to increased dosimeter sales of $1.9 million, principally into existing NPPs in Asia and the Americas, $5.5 million of increased sales of contamination & clearance monitors principally into existing NPPs in the Americas and $3.0 million of increased sales of detection and identification products into the defense end market, primarily in Europe. Revenue for fiscal 2009 was negatively impacted by foreign currency movements by approximately $4.0 million.
Operating income in our Health Physics segment increased $7.2 million, or 792.6%, to $6.3 million for fiscal 2009, from $(0.9) million for fiscal 2008. This increase was due to increased gross profit due to higher revenues and lower research and development expenses arising from our product rationalization efforts. During fiscal 2009, we continued to review our product portfolio and discontinued engineering work on products that were deemed to be duplicative or nearing the end of useful life. As a result we were able to eliminate a portion of our temporary and consulting engineers and correspondingly reduce engineering expense. Expenses were positively impacted by foreign currency movements, which partially offset the negative impact of currency movements on revenue.
Radiation Monitoring Systems
Fiscal 2010 as Compared to Fiscal 2009
Revenue in our Radiation Monitoring Systems segment increased $19.6 million, or 47.7%, to $60.7 million for fiscal 2010, from $41.1 million for fiscal 2009. This increase was primarily due to the completion of several large nuclear power plant construction projects in Asia and Europe where there were no comparably-sized projects completed during fiscal 2009. In addition, the segment experienced an increase in activity in the Americas.
Operating income in our Radiation Monitoring Systems segment increased $8.0 million, or 194.5%, to $12.1 million for fiscal 2010 from $4.1 million for fiscal 2009. This increase was predominantly related to higher gross profits resulting from the increase in revenue. In addition, overall operating expenses for the segment decreased by $1.1 million due to a reduction in research and development expenses attributable to research and development tax credits relating to fiscal 2007 and 2009, but received and recognized in fiscal 2010, and a decrease in contractor-related expenses in favor of additional headcount at a lower overall cost. These reductions in operating expenses were partly offset by increased selling, general and administrative expenses, which were higher in fiscal 2010 compared to fiscal 2009 due to increased headcount in the segment.
Fiscal 2009 as Compared to Fiscal 2008
Revenue in our Radiation Monitoring Systems segment decreased $2.1 million, or 4.8%, to $41.1 million for fiscal 2009, from $43.2 million for fiscal 2008. This decrease was largely due to the negative impact of foreign currency movements of approximately $3.5 million in fiscal 2009, and was partially offset by increased revenue from a higher number of customer installations in fiscal 2009.
Operating income in our Radiation Monitoring Systems segment increased $3.0 million, or 278.7%, from $1.1 million in fiscal 2008 to $4.1 million in fiscal 2009. $1.1 million of this increase was due to two projects in fiscal 2009 in Europe that had higher-than-usual gross profits due to the mix of the components sold to each customer. Both projects were for the replacement of the customers’ existing equipment. Operating income was also favorably impacted by a $0.3 million reduction in selling, general and administrative expenses due to lowered compensation-related expenses that resulted from a prior year restructuring and
68
$0.4 million reduction in research and development expenses due to the elimination of a portion of our temporary and consulting engineer positions. Operating expenses were further positively impacted by foreign currency movements by $1.1 million, which partially offset the negative impact on revenue related to currency movements.
Sensing Systems
Fiscal 2010 as Compared to Fiscal 2009
Revenue in our Sensing Systems segment increased $2.3 million, or 5.1%, to $47.3 million for fiscal 2010, from $45.0 million for fiscal 2009, due to the completion of some long term contracts and additional spare-part orders. Segment revenue also increased due to a $3.3 million, or 15.6%, increase in shipments of in-core and ex-core detector products during fiscal 2010.
Operating income in our Sensing Systems segment increased $1.6 million, or 10.8%, to $16.6 million for fiscal 2010, from $15.0 million for fiscal 2009. This increase was primarily due to higher gross profits realized from the increase in revenue in fiscal 2010, with operating expenses remaining flat from the prior year.
Fiscal 2009 as Compared to Fiscal 2008
Revenue in our Sensing Systems segment increased $5.1 million, or 12.8%, to $45.0 million for fiscal 2009, from $39.9 million for fiscal 2008. This increase was due to an increase in revenue recognized for new electrical penetrations of $3.4 million and an increase in revenue recognized for our ex-core detectors of $2.3 million, partially offset by a reduction in nuclear reactor core detector revenue of $0.6 million. Revenue in fiscal 2009 was negatively impacted due to foreign currency movements by approximately $0.8 million.
Operating income in our Sensing Systems segment increased $4.7 million, or 46.3%, to $15.0 million for fiscal 2009, from $10.2 million for fiscal 2008. This increase was primarily due to increased revenue as well as a decrease in material costs due to declining prices over fiscal 2009 for precious metals and other raw materials. Operating expenses decreased by $0.4 million, or 5.4% primarily due to lower amortization expenses.
Dosimetry Services
Fiscal 2010 as Compared to Fiscal 2009
Revenue in our Dosimetry Services segment decreased $0.3 million, or 0.9%, to $29.2 million for fiscal 2010, from $29.5 million for fiscal 2009, primarily due to decreased revenue in the Americas resulting from competitive pricing pressure in the medical end market.
Operating income in our Dosimetry Services segment decreased by 0.4%, to $7.9 million for fiscal 2010 from $8.0 million for fiscal 2009. The slight decrease in operating income was due to increases in production costs in fiscal 2010 related to our new passive dosimeter product that was introduced to the market in late fiscal 2009, offset by an overall reduction in operating expenses during fiscal 2010, primarily due to lower professional fees for research projects, and lower marketing expenses due to the timing of trade promotion activity compared to the prior fiscal year.
Fiscal 2009 as Compared to Fiscal 2008
Revenue in our Dosimetry Services segment increased $0.7 million, or 2.2%, to $29.5 million for fiscal 2009, from $28.8 million for fiscal 2008. This increase was primarily due to an increase in sales to the small medical practitioner market.
Operating income in our Dosimetry Services segment increased $0.3 million, or 2.9%, to $8.0 million for fiscal 2009, from $7.7 million for fiscal 2008. This increase was principally due to increased revenue, partially offset by increased research and development expenses related to new product development.
69
Imaging Systems
Fiscal 2010 as Compared to Fiscal 2009
Revenue in our Imaging Systems segment increased $0.8 million, or 4.9%, to $17.9 million for fiscal 2010, from $17.1 million for fiscal 2009. This increase was primarily attributable to increased sales of our nuclear cameras in the second half of fiscal 2010.
Operating income in our Imaging Systems segment increased $1.0 million, or 95.5%, to $2.1 million for fiscal 2010, from $1.1 million for fiscal 2009. This increase was the result of slightly higher gross profits driven by product mix, and lower selling, general and administrative expenses due to the declining amortization of our customer relationship intangible assets during fiscal 2010.
Fiscal 2009 as Compared to Fiscal 2008
Revenue in our Imaging Systems segment decreased $4.1 million, or 19.3%, to $17.1 million for fiscal 2009, from $21.2 million for fiscal 2008. This decrease was primarily attributable to a reduction in sales of our high temperature cameras of $3.0 million. We began to experience some weakening of demand for these products into the industrial end market, as a result of the economic climate in fiscal 2009. The products represented $7.3 million, or 42.6%, of segment sales in fiscal 2009, compared to $10.3 million, or 48.5%, of segment sales in fiscal 2008. Revenues for fiscal 2009 were negatively impacted by foreign currency movements by approximately $2.0 million, due to the weaker British pound.
Operating income in our Imaging Systems segment decreased $0.2 million, or 20.5%, to $1.1 million for fiscal 2009, from $1.3 million for fiscal 2008. This decrease was primarily attributable to the impact of a weaker British pound, with a $0.7 million positive currency impact on operating expenses partially offsetting the negative impact on revenues. Aside from the currency impact, there was a reduction of operating expenses in fiscal 2009 due to a decrease in facilities costs, primarily due to the consolidation of office facilities.
Liquidity and Capital Resources
We have financed our operations primarily through cash provided by operations and our lines of credit. As of June 30, 2010, our principal sources of liquidity consisted of $9.9 million of cash and cash equivalents and $1.1 million available under our revolving credit facilities. A substantial majority of our outstanding debt has been provided by ACAS, our principal stockholder, through senior and junior debt facilities as well as lines of credit. ACAS has also provided us with substantially all of our equity financing.
The terms of our credit agreements with ACAS require us and our subsidiaries to meet certain restrictive financial covenants and ratios computed quarterly, including a minimum fixed charge coverage ratio (adjusted EBITDA minus capital expenditures, over cash paid for interest, debt payments, tax payments and management fees) of one-to-one, maximum debt to adjusted EBITDA ratio (total debt over adjusted EBITDA) of 5.50-to-one, minimum interest coverage ratio (adjusted EBITDA over cash interest expense) of 1.50-to-one and a maximum cash-funded capital expenditure level of $7.5 million per fiscal year, with provisions for a one-year carry-forward.
Our credit agreements with ACAS are material to our consolidated business, and the financial covenants to our credit agreements are material in that any non-compliance with such covenants could result in an event of default under such credit agreements. Upon an event of default, ACAS may require us to pay penalty interest on debt under the credit agreements, accelerate any unpaid principal and interest under the credit agreements and charge a prepayment premium in the event of acceleration. In particular, in the event of an acceleration of the indebtedness held by ACAS, we may not be able to secure alternative financing on acceptable terms or at all. In such an event, we could be forced to sell assets to repay the indebtedness or take other actions that could have a material adverse effect on our business and operations. We are in compliance with all of our financial covenants as of June 30, 2010.
We previously entered into a credit agreement with JPMorgan Chase Bank, National Association, J.P. Morgan Europe Limited, J.P. Morgan Securities LLC and Fifth Third Bank in connection with our anticipated
70
new bank credit facilities, pursuant to which the lenders agreed to make loans, issue letters of credit and commit to future loans, to be effective upon the consummation of a public offering. Although the obligations and commitments of the lenders under this credit agreement terminated on June 7, 2010 in connection with the delay in our initial public offering, we expect to negotiate a new credit agreement on similar terms prior to the marketing of this offering. Professional fees of $0.6 million previously capitalized in connection with this credit agreement were recorded as SG&A expense upon termination. Our anticipated new bank credit facilities that we expect to enter into upon the consummation of this offering are expected to include financial covenants with which we will be required to comply. These expected covenants include (i) a minimum fixed charge coverage of Adjusted EBITDA minus capital expenditures, over interest expense and scheduled principal payments on indebtedness, of 1.50-to-one, (ii) a minimum net worth of 80% of our consolidated stockholders’ equity as of April 30, 2010 after giving pro forma effect to this offering and the consummation on the effective date of the transactions contemplated under the new bank credit facilities plus 50% of net income earned in each full fiscal quarter ending after March 31, 2010 and (iii) a maximum ratio of total indebtedness over Adjusted EBITDA for four consecutive fiscal quarters of 2.75-to-one, declining further to 2.25-to-one as of January 1, 2011. Given our expected substantially lower total indebtedness and reduced income expense following the offering and refinancing, we do not believe that these financial covenants will be more restrictive on our ability to operate our business and finance our operations and working capital requirements. In addition, we believe that the expected covenants in our anticipated new bank credit facilities do not pose a material risk of non-compliance to us. On a pro forma basis after giving effect to the offering and the refinancing as described in this prospectus, we would have been in compliance with all financial covenants in the new bank credit facilities as of June 30, 2010. Management has projected the likely future performance of our business for fiscal year 2011 and does not believe that there is a material risk of non-compliance with such covenants.
Our anticipated new bank credit facilities are expected to initially bear interest at rates of LIBOR or EURIBOR plus 4.50%; however, depending on our leverage ratio, the interest rate adjustment can vary from 4.00% to 5.00%. The term loans are expected to have a minimum LIBOR or EURIBOR amount of 1.50%.
During fiscal 2010, our cash and cash equivalents increased $4.5 million to $9.9 million. During this time, we had cash inflows from operating activities of $8.2 million, primarily due to revenue growth offset by offering costs that have been capitalized and included as a component of prepaids and other current assets. These costs will be reclassified to additional paid-in capital upon consummation of our initial public offering. We had cash outflows from investing activities of $11.2 million, related to purchases of property, plant and equipment and restrictions on cash, and net cash inflows from financing activities of $8.1 million. This was principally due to an increase in net borrowings under our ACAS revolving credit facilities of $12.7 million, and increases in borrowings from our third-party revolving credit facilities of $5.9 million during the period.
During fiscal 2009, our cash and cash equivalents decreased $3.6 million to $5.4 million. During this time we had cash inflows from operating activities of $10.0 million. This was offset by cash outflows from investing activities of $7.4 million, primarily for the purchase of property, plant and equipment, and financing activities of $5.8 million from payments under our revolving credit facilities.
Our anticipated refinancing will decrease our expected interest rates and provide us with substantially less total debt. We expect our liquidity to increase as our interest payments significantly decline, allowing us to repay a portion of our new debt each year. We also expect our interest expense to be significantly lower as a result of this refinancing.
Our principal need for liquidity has been, and will continue to be, for working capital, to pay down debt and for capital expenditures. We believe that our cash flow from operations, available cash and cash equivalents and available borrowings under the revolving portion of our credit facilities will be sufficient to meet our liquidity requirements for at least the next twelve months. However, our ability to make scheduled payments of principal and to pay the interest on, or to refinance, our debt and to fund planned capital expenditures will depend on our future performance. Accordingly, we may be required to raise debt or equity financing, and such financing may not be available on acceptable terms.
71
Although we currently have no specific plans to do so, if we decide to pursue one or more significant strategic acquisitions, we may incur additional debt or sell additional equity to finance the purchase of those businesses.
Historical Cash Flows
Cash Flow from Operating Activities
We used $6.7 million, and generated $10.0 million and $8.2 million, in cash flows from operating activities in fiscal 2008, 2009, and 2010, respectively.
The $1.8 million decrease in net operating cash flows for fiscal 2010 compared to fiscal 2009 was primarily due to the following: (i) decreased cash inflows from changes in cost in excess of billings on uncompleted contracts, reflecting changes in the timing of contract costs versus billings, which accounted for $6.4 million of net cash outflows for fiscal 2010 compared to net cash inflows of $2.7 million for fiscal 2009; (ii) net cash outflows for prepayments and other current assets of $12.4 million for fiscal 2010 compared to net cash outflows of $1.7 million for fiscal 2009, which reflect the increased prepayment balances related to issuance costs in connection with this offering; (iii) increased net cash outflows of $3.2 million for accrued expenses and other current liabilities; and (iv) a decrease in cash inflows from deferred cost of revenue and deferred contract revenue of $1.6 million, which was the result of timing differences on project completion activity between the two years. These decreases in operating cash flows were partially offset by: (i) an increase in cash inflows from net income, as adjusted for non cash items, of $14.2 million, due to revenue growth; and (ii) a net increase in cash inflows from changes in accounts receivable, accounts payable and other liabilities of $11.5 million.
The $16.7 million increase in cash provided from operating activities in fiscal 2009 compared to fiscal 2008 was primarily due to (i) a $9.4 million decrease in net loss for the period, due primarily to increased revenue and slightly improved gross margins in fiscal 2009; and (ii) cash inflow of $2.7 million due to a decrease in costs in excess of billings on uncompleted contracts in fiscal 2009 compared to a cash outflow of $9.5 million due to an increase in costs in excess of billings on uncompleted contracts during fiscal 2008, primarily due to timing differences on project activity between the two periods. This increase in operating cash inflows was offset by an increase in operating cash outflows of $7.8 million on accounts payable with higher purchases made towards the end of fiscal 2009 when compared to fiscal 2008.
Cash Flow from Investing Activities
We used $3.3 million, $7.4 million and $11.2 million in cash from investing activities during fiscal 2008, 2009 and 2010, respectively.
The $3.8 million increased use of cash in fiscal 2010 as compared to 2009 was primarily due to an increase of purchases of property, plant and equipment for fiscal 2010.
The $4.1 million increased use of cash in fiscal 2009 as compared to 2008 was due to higher purchases of property plant and equipment in fiscal 2009 as well as a one-time return of escrow funds that occurred in fiscal 2008.
Cash Flow from Financing Activities
We generated $13.4 million and used $5.8 million in cash from financing activities during fiscal 2008 and 2009, respectively. We generated $8.1 million in cash from financing activities during 2010.
The increase of $14.0 million in cash from financing activities from fiscal 2009 to 2010 was primarily due to an increase of $16.3 million in borrowings on our ACAS revolving credit facilities net of payments toward such facilities, from net payments of $4.0 million under these facilities during fiscal 2009 to net borrowings of $12.3 million during fiscal 2010. In fiscal 2010, $1.7 million of these borrowings were used to reduce other borrowings against third-party revolving credit facilities and other third-party notes payable
72
balances, offsetting the overall increase in cash generated from financing activities. The balance of the ACAS borrowings in fiscal 2010 was used to fund the operational and investing net cash outflows during the year.
The $19.2 million decrease in cash from financing activities in fiscal 2009 as compared to 2008 was due to a $14.2 million reduction in net borrowing on notes from ACAS as well as a $4.9 million reduction in net borrowing from third parties.
Capital Expenditures
We had capital expenditures of $5.0 million and $6.6 million in fiscal 2008 and 2009. We had capital expenditures of $10.4 million in fiscal 2010, which included mortgage-funded capital expenditures of $2.6 million for the development of new facilities in Lamanon, France. The majority of our capital expenditures has been for the replacement of existing equipment or the purchase of new equipment to support our business.
As of June 30, 2010, our contractual obligations and other commitments were as follows (in thousands):
| | | | | | | | | | | | | | | | | | | | | |
| | | Total | | | 2011 | | | 2012-2013 | | | 2014-2015 | | | Thereafter | |
| |
| Debt obligations(1) | | $ | 185,874 | | | $ | 1,068 | | | $ | 183,734 | | | $ | 504 | | | $ | 568 | |
| Operating lease obligations | | | 16,490 | | | | 3,478 | | | | 5,761 | | | | 3,719 | | | | 3,532 | |
| | | | | | | | | | | | | | | | | | | | | |
| Total | | $ | 202,364 | | | $ | 4,546 | | | $ | 189,495 | | | $ | 4,223 | | | $ | 4,100 | |
| | | | | | | | | | | | | | | | | | | | | |
| | |
| (1) | | Includes only obligations to pay principal (as described below) and does not reflect the use of net proceeds from this offering. A portion of our debt has a PIK interest feature. As a result, the principal amount of such debt increases on a periodic basis. Also does not include pensions, which are described in Note 11 of our consolidated financial statements. |
73
Credit Facilities and Long-term Debt
Our credit facilities and long-term debt with ACAS, our principal stockholder, are as follows (in thousands):
| | | | | | | | | | | | | | | |
| | | | | | | | | | | Amount
| |
| | | | | | | | Interest Rate
| | | Outstanding
| |
| | | Maturity
| | | Contractual
| | as of
| | | as of
| |
Credit Facilities and Long-term Debt | | Due | | | Interest Rate (%) | | June 30, 2010 | | | June 30, 2010 | |
| |
| Revolving credit facilities: | | | | | | | | | | | | | | |
| $20.25 million | | | July 2011 | | | LIBOR + 4.5% | | | 4.85 | % | | $ | 20,250 | |
| $14.0 million | | | July 2011 | | | LIBOR + 5% | | | 5.35 | % | | | 13,997 | |
| $8.2 million | | | July 2011 | | | EURIBOR + 2% | | | 2.43 | % | | | 7,130 | |
| Senior term notes: | | | | | | | | | | | | | | |
| $24.9 million Senior Term B | | | July 2011 | | | EURIBOR + 3% | | | 3.43 | % | | | 24,944 | |
| $7.5 million Senior Term B | | | July 2011 | | | LIBOR + 8% | | | 8.35 | % | | | 5,008 | |
| $2.0 million Senior Term B | | | July 2011 | | | LIBOR + 8% | | | 8.35 | % | | | 1,917 | |
| $4.0 million Senior Term C | | | Oct 2011 | | | LIBOR + 9% | | | 9.35 | % | | | 4,000 | |
| $4.0 million Senior Term C | | | Nov 2011 | | | LIBOR + 8.25% | | | 8.60 | % | | | 4,000 | |
| $27.0 million Senior Term D | | | Oct 2011 | | | LIBOR + 6.5% | | | 6.85 | % | | | 25,785 | |
| $15.0 million Senior Term D | | | Oct 2011 | | | LIBOR + 6.5% | | | 6.85 | % | | | 14,288 | |
| Senior subordinated notes: | | | | | | | | | | | | | | |
$7.5 millionpaid-in-kind | | | July 2011 | | | 14% | | | 14 | % | | | 8,487 | |
$8.6 millionpaid-in-kind | | | July 2011 | | | 15% | | | 15 | % | | | 9,862 | |
$12.2 millionpaid-in-kind | | | July 2011 | | | EURIBOR + 11% | | | 11.43 | % | | | 16,441 | |
| Junior subordinated notes: | | | | | | | | | | | | | | |
$4.3 millionpaid-in-kind | | | July 2011 | | | 17% | | | 17 | % | | | 5,280 | |
$4.3 millionpaid-in-kind | | | July 2011 | | | 17% | | | 17 | % | | | 5,280 | |
$1.25 millionpaid-in-kind | | | May 2012 | | | 14% | | | 14 | % | | | 1,415 | |
$4.9 millionpaid-in-kind | | | July 2011 | | | EURIBOR + 12% | | | 12.43 | % | | | 7,155 | |
| Stockholder loan: | | | | | | Three-month | | | | | | | | |
| $8.0 million | | | July 2011 | | | EURIBOR + 2% | | | 2.64 | % | | | 8,013 | |
| | | | | | | | | | | | | | | |
| Total notes payable to ACAS | | | | | | | | | | | | | 183,252 | |
| Less current portion | | | | | | | | | | | | | (420 | ) |
| | | | | | | | | | | | | | | |
| Notes payable to ACAS — long term | | | | | | | | | | | | $ | 182,832 | |
| | | | | | | | | | | | | | | |
We were in compliance with all of our financial covenants as of June 30, 2010. The credit facilities include customary events of default and affirmative, restrictive and financial covenants that, among other things, require us to maintain certain financial ratios and limit our ability to incur additional indebtedness, create liens, pay dividends, redeem capital stock or make certain other restricted payments or investments, sell assets including capital stock, engage in transactions with affiliates and effect a consolidation or merger. For more information on our financial covenants, see “—Liquidity and Capital Resources.” We are not in compliance with certain non-financial covenants that were in effect prior to the formation of Mirion. These non-financial covenants were negotiated with the predecessor companies (GDS, IST and Synodys) and were not amended at the time of the formation of Mirion. The non-financial covenants relate to matters such as changing the fiscal years or names of our subsidiaries, amending the charter documents and bylaws of our subsidiaries and the provision of audited financial statements to ACFS. We have obtained a waiver for the violations of non-financial covenants through July 1, 2011.
We have a term loan of €543,000 ($0.7 million) as of June 30, 2010, which is due November 2012 and bears interest at a rate of EURIBOR + 1%. We have entered into two additional term loans which are due March 2017 and bear interest at a rate of 4.2%. The maximum borrowing under these agreements is €3.8 million ($4.6 million). The amount due under these term loans at June 30, 2010 was €1.4 million ($1.8 million).
74
We also have various line of credit arrangements in Germany and France. The terms and use of these credit arrangements are detailed in Note 8 of our consolidated financial statements.
In connection with the consummation of this offering, we expect to enter into new bank credit facilities. See “Description of Certain Indebtedness.”
Qualitative and Quantitative Disclosures about Market Risk
Foreign Exchange Risks
We have foreign currency exposure related to our operations in France, Germany and the United Kingdom, as well as in other foreign locations. This foreign currency exposure arises primarily from the translation or re-measurement of our foreign subsidiaries’ financial statements into U.S. dollars. For example, a substantial portion of our annual revenue and operating costs are denominated in euros, and we have exposure related to revenue and operating costs increasing or decreasing based on changes in currency exchange rates. If the U.S. dollar increases in value against these foreign currencies, the value in U.S. dollars of the assets and liabilities originally recorded in these foreign currencies will decrease. Conversely, if the U.S. dollar decreases in value against these foreign currencies, the value in U.S. dollars of the assets and liabilities originally recorded in these foreign currencies will increase. Thus, increases and decreases in the value of the U.S. dollar relative to these foreign currencies have a direct impact on the value in U.S. dollars of our foreign currency denominated assets and liabilities, even if the value of these items has not changed in their original currency. At present we do not purchase forward contracts as hedging instruments, but may do so as circumstances warrant.
Interest Rate Risks
We are subject to interest rate risk in connection with our long-term debt and our revolving lines of credit. As of June 30, 2010, we had total long-term debt of $184.8 million. Our debt consists of both variable interest rate as well as fixed interest rate debt. As of June 30, 2010, we had $152.9 million of debt with variable interest rates. We swapped approximately $1.8 million of variable debt for a fixed rate of 3.865% that expires in November 2012. A 1% increase in our variable interest rates would increase our annual interest expense and decrease our cash flows and income before taxes by approximately $1.5 million per year.
Inflation
We do not believe that inflation has had a material effect on our business, financial condition or results of operations. If our costs were to become subject to significant inflationary pressures, we may not be able to fully offset such higher costs through price increases as many of our contracts are fixed-price contracts which do not provide for price escalations. Our inability or failure to do so could adversely affect our business, financial condition and results of operations.
75
INDUSTRY
We sell our radiation detection, measurement, analysis and monitoring products and services into the global nuclear, defense and medical end markets. We believe that our end markets are characterized by strong fundamentals that support an established revenue base, as well as provide numerous growth opportunities.
Nuclear
The nuclear end market spans the entire nuclear fuel cycle, including mining, enrichment, fuel manufacturing, nuclear power generation, waste management and fuel reprocessing. Key nuclear installations include mines, fuel fabrication facilities, commercial nuclear power reactors, reprocessing facilities, research facilities, military facilities and ships, weapons facilities and waste storage facilities. We sell products and services for use in each of these types of installations, with commercial nuclear power reactors representing the majority of our sales into the nuclear end market.
Increasing Global Demand For Electricity and Nuclear Power
Increasing electricity demand. The International Energy Agency, or IEA, projects a near doubling of world electricity demand from 2007 to 2030, creating the need for approximately 4,800 GWe of new generating capacity. The IEA projects this increase in electricity demand to be driven by a wide range of global trends including (i) population growth, (ii) increasing standards of living in the developing world, including in China and India and (iii) continued proliferation and commercialization of technologies dependent on the delivery of a reliable electricity supply, such as consumer electronics and information technology.
Increasing demand for nuclear power. We believe that nuclear energy is the best-positioned alternative to fossil fuels (e.g., coal, natural gas and oil) with the capability to meet electricity demand for base-load, or continuously delivered, electricity production. In addition, increased public concern regarding the effects of greenhouse gas emissions has accelerated interest in reliable, low-emissions alternatives to fossil fuels, such as nuclear power. The use of other renewable energy sources, such as wind and solar power, for base-load generation suffers from issues of intermittency while also requiring major investments to create a transmission grid capable of moving the power from the remote geographic areas where it is generated to consumers, and to adequately manage variable load-shifting requirements. We believe the existing global installed base of nuclear power reactors to be the most cost-effective and reliable source of base-load energy currently available, with relatively low marginal cost of energy production, as compared to fossil fueled generation with higher input cost volatility.
Increased public support for nuclear power also has been augmented by an increasing global desire to reduce dependency on foreign sources of fossil fuels as well as the recognition that nuclear power has maintained a very safe operating track record. Significant regulatory oversight, as well as rigorously enforced safety, quality and inspection protocols, have helped the nuclear industry achieve a favorable safety record with respect to attributable fatalities per GWe of electricity produced compared to other forms of primary energy production, including coal, natural gas and hydroelectric production. Many governments around the world are adopting policies favorable to nuclear power.
Nuclear Power Global Installed Base
According to the WNA, as of July 2010, there were 440 nuclear power reactors in operation globally. Additionally, there are 59 reactors currently under construction with an additional 149 reactors planned and 344 proposed worldwide. The average expected life cycle of an NPP (which contains one or more reactors), including planning, construction, operation and decommissioning, is between 55 and 80 years, of which the expected operating life is 40 to 60 years.
As of July 2010, nuclear power was responsible for approximately 14% of electricity generation globally and substantially more in certain nuclear-intensive countries. As shown in the table below, as of July 2010, nuclear
76
power provided 75% of the electricity output in France, over 45% in Belgium and Ukraine, over 30% in Sweden, Switzerland, South Korea and the Czech Republic and over 20% in Germany and Japan.
| | | |
| | | Percentage of National Electricity Output from
|
| Number of Operational Reactors by Country | | Nuclear Power by Country |
| |
 | |  |
Source: World Nuclear Association, as of July 2010.
Nuclear power plant re-licensing. Regulatory authorities worldwide have established timely license renewal processes and requirements to extend plant life in a manner that assures safe operation. In the United States, for instance, the Atomic Energy Act and NRC regulations limit commercial power reactor licenses to an initial term of 40 years, but also permit such licenses to be renewed for up to an additional 20 years. The NRC views the timely renewal of licenses as an important step to ensure an adequate domestic energy supply. Of the 104 nuclear power reactors in operation in the United States as of May 2010, eight are subject to license expiration within the next five years. In the United States, the NRC has approved license renewal for 59 reactors to date, with an additional 20 reactor re-licensings currently under review and 19 more reactor re-licensing applications anticipated. License renewals are generally approved for those plants where the reactor continues to operate at an efficient level and only after any necessary upgrades to the instrumentation & control equipment and systems have been made.
Nuclear power plant up-rating. NPP up-rating is a licensing, improvement and equipment modification process designed to enhance power output of existing plants by enabling reactors to operate at increased temperature and pressure levels. Utilities have used power up-rates since the 1970s as a way to generate more electricity from existing nuclear plants. In the United States alone, 129 up-rates have been approved by the NRC as of October 2009, resulting in the creation of an additional 5,726 MWe capacity within the existing nuclear footprint. Collectively, these up-rates have added generating capacity at existing plants that is equivalent to more than five new reactors, according to the NRC. In most cases, up-rating activities involve an upgrade of many critical reactor components, including instrumentation & control equipment and systems.
Nuclear power capacity factors. Increasing the capacity factor of existing plants provides a means of generating more nuclear power without building new reactors. The capacity factor of a power plant is defined as its actual power generation divided by its rated capacity. The average capacity factor for U.S. NPPs was 56% in 1980, but improved substantially to greater than 90% in 2002, according to a 2008 report of the United States Energy Information Administration. Based on this data, we estimate that the increase in capacity factor from 1990 to 2008 was equivalent to the construction of approximately 26 new reactors at 1,000 MWe capacity each. Suppliers providing reliable radiation detection, measurement, analysis and monitoring products and services have played a crucial role in the improvement of capacity factors.
New Nuclear Power Plant Construction
The re-licensing, up-rating and increased capacity factors have helped to improve the output of the existing nuclear power reactor fleet. However, in order to keep pace with increasing demand for nuclear power
77
globally, 59 new reactors are currently under construction, with an additional 149 reactors planned and 344 proposed for future development, as of July 2010.
We expect strong long-term economic growth in Asia and Eastern Europe to drive the demand for new nuclear power reactor builds, as economic growth and power usage in these regions would require additional power generation capacity. Additionally, the U.S. government has also initiated programs to provide incentives to build new reactors. The Energy Policy Act of 2005, for example, provides a tax credit of 1.8 cents perkilowatt-hour for up to 6 GWe of capacity built before 2021 and also authorizes the Department of Energy to issue loan guarantees worth approximately $18.5 billion for up to 80% of the cost of new nuclear projects.
Worldwide Nuclear Power Reactors
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Nuclear
| | | | | | | | | | | | | | | | |
| | | Electricity
| | | % of National
| | | Number of Reactors | |
| | | Generation 2009
| | | Electricity
| | | | | | Under
| | | | | | | |
Country | | (Billion KWh) | | | Output | | | Operable | | | Construction | | | Planned(1) | | | Proposed(2) | |
| |
| United States | | | 799 | | | | 20 | | | | 104 | | | | 1 | | | | 9 | | | | 22 | |
| France | | | 392 | | | | 75 | | | | 58 | | | | 1 | | | | 1 | | | | 1 | |
| Japan | | | 263 | | | | 29 | | | | 55 | | | | 2 | | | | 12 | | | | 1 | |
| Russia | | | 153 | | | | 18 | | | | 32 | | | | 10 | | | | 14 | | | | 30 | |
| South Korea | | | 141 | | | | 35 | | | | 20 | | | | 6 | | | | 6 | | | | 0 | |
| United Kingdom | | | 63 | | | | 18 | | | | 19 | | | | 0 | | | | 4 | | | | 6 | |
| Canada | | | 85 | | | | 15 | | | | 18 | | | | 2 | | | | 4 | | | | 3 | |
| India | | | 15 | | | | 2 | | | | 19 | | | | 4 | | | | 20 | | | | 40 | |
| Germany | | | 128 | | | | 26 | | | | 17 | | | | 0 | | | | 0 | | | | 0 | |
| Ukraine | | | 78 | | | | 49 | | | | 15 | | | | 0 | | | | 2 | | | | 20 | |
| China | | | 66 | | | | 2 | | | | 12 | | | | 24 | | | | 33 | | | | 120 | |
| Sweden | | | 50 | | | | 35 | | | | 10 | | | | 0 | | | | 0 | | | | 0 | |
| Spain | | | 51 | | | | 18 | | | | 8 | | | | 0 | | | | 0 | | | | 0 | |
| Belgium | | | 45 | | | | 52 | | | | 7 | | | | 0 | | | | 0 | | | | 0 | |
| Czech Republic | | | 26 | | | | 34 | | | | 6 | | | | 0 | | | | 2 | | | | 1 | |
| Rest of World | | | 205 | | | | N/A | | | | 40 | | | | 9 | | | | 42 | | | | 100 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Total | | | 2,560 | | | | 14 | | | | 440 | | | | 59 | | | | 149 | | | | 344 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Source: World Nuclear Association, as of July 2010.
| |
| (1) | Planned reactors have approvals, funding or major commitments in place, mostly expected to be in operation within eight to ten years, or with construction well advanced but suspended indefinitely. |
| |
| (2) | Proposed reactors have specific program or site proposals, with expected operation mostly within 15 years. |
Nuclear Decommissioning
Following the useful life of any nuclear reactor, it must be decommissioned and decontaminated. The decommissioning process can take ten years or more to complete, with the facility requiring ongoing radiation detection, monitoring and measurement services throughout this period. As of June 2010, 80 commercial nuclear power reactors, 45 experimental prototype reactors and over 250 research reactors had been retired from operation globally.
78
Other Nuclear Facilities
According to the WNA, there were 250 operational nuclear research reactors in 56 countries, with one more under construction, as of mid 2009. Most of these reactors reside on university campuses and are used for research and training, materials testing, medicine and industrial functions. Additionally, the WNA estimates that, as of August 2010, approximately 150 maritime vessels, primarily naval submarines, are powered by more than 220 nuclear reactors. Although not used for commercial power generation, these facilities require similar levels of radiation detection, measurement, analysis and monitoring products and services as commercial reactors.
Defense
Our global defense end market is driven by a combination of military, civil defense and event-driven security spending. The proliferation of global security threats has reached unprecedented levels, driven by an unstable geopolitical climate, the emergence and expansion of terrorist organizations and the proliferation of radiological and nuclear technologies. Taken together, these threats have the potential to cause significant human casualties and economic loss. As a result, militaries, civil defense and other security organizations have bolstered investment in the prevention and detection of radiological threats as well as in technologies capable of detecting and monitoring radiation levels in the aftermath of radiological attack.
Militaries throughout the world utilize radiation detection technologies for troop security. Spending on personnel protection and detection of radiological threats is a high priority for both NATO and non-NATO militaries and, as such, has led many countries to provide dosimeters to military personnel on a standard-issue basis. We believe that spending on these technologies will remain a high priority among armed forces globally.
Spending within the global civil defense, or homeland security, market has rapidly expanded in recent years based on increased threats presented by terrorist organizations. As a result, civil defense, first responder and other security organizations have invested in technologies and services designed both to protect civil defense personnel, civilians and domestic infrastructure from radiological threats and to detect and monitor radiation levels following a radiological incident, such as the release of a nuclear or other radiological device. In addition, homeland security organizations are increasingly focused on enhancing radiological detection capabilities at critical points of entry, such as airports, ports and borders. Within the United States, for example, the Domestic Nuclear Detection Office, or DNDO, was created within the Department of Homeland Security to implement a comprehensive inter-agency system to detect, report and respond to nuclear or radiological threats.
Additionally, large-scale public meeting events have greatly increased security measures at facilities, including rapid adoption of radiological detection technologies to address the increased threat of radiological attacks, due to their profile as high visibility targets. For example, the Olympic Games increased its security spending ten-fold from $180 million for the 2000 Sydney summer games to $1.9 billion for the 2008 Beijing summer games. We believe security spending at the Olympic Games and other public events and venues will continue to expand and increasingly incorporate radiological detection capabilities as a necessary component of crowd and facility security solutions.
Medical
Nuclear and radiological medical technologies are used for diagnostic and therapeutic procedures. These technologies provide highly accurate, cost-effective and less invasive alternatives compared to traditional techniques. Procedures where radiation exposure is most prevalent include radiodiagnostic procedures, such as x-rays and computed axial tomography (CAT) scanning, as well as radiotherapeutic procedures, such as external linear accelerator therapy, gamma knife stereotactic radiotherapy and brachytherapy. Medical imaging improves diagnosis and treatment of a variety of illnesses and conditions, including cancer, stroke, heart disease, trauma, sports injury and abdominal and neurological conditions. According to the WNA, as of May 2010, there are over 10,000 hospitals worldwide using radioisotopes in medicine, with about 90% of the
79
procedures for diagnostics. There are approximately 37 million nuclear medicine procedures performed per year globally, with the United States and Europe accounting for approximately 18 million and 10 million procedures per year, respectively.
As a result of the proliferation of radiological medical technologies, hospitals, clinics and other medical facilities rely on dosimetry systems and services to ensure the safety of both medical personnel and patients. The proliferation of nuclear and radiological medical technologies coupled with increased use of radiological medical procedures have increased the market for radiation detection and monitoring products and services. The WNA estimates that the use of radiopharmaceuticals in diagnosis continues to grow at over 10% per year.
Other
Other end markets include industrial facilities such as cement kilns, pulp and paper mills and coal/gas fired power boilers that utilize high-temperature industrial processes. These high-temperature processes are critical to plant operation and must be accurately monitored to ensure optimal operating conditions. Imaging equipment capable of withstanding the high temperatures and environmental conditions found in these facilities is employed to monitor and optimize process efficiency. Similar to the products employed in NPPs, these imaging systems require routine replacement or upgrades.
80
BUSINESS
Business Overview
We are a global provider of radiation detection, measurement, analysis and monitoring products and services to the nuclear, defense and medical end markets. Our customers rely on our solutions to protect people, property and the environment from nuclear and radiological hazards. Our products and services include: dosimeters; contamination & clearance monitors; detection & identification instruments; radiation monitoring systems; electrical penetrations; reactor instrumentation & control equipment and systems; dosimetry services; imaging systems; and related accessories, software and services. Many of our end markets are characterized by the need to meet rigorous regulatory standards, design qualifications and operating requirements. We believe these industry dynamics create substantial barriers to entry, thereby reinforcing our market position. We have successfully leveraged the strength of our nuclear platform to expand the commercial applications of our technologies to defense and other end markets. The diversity of our end markets and the global nature of our customer base are illustrated in the charts below:
| | | |
Fiscal 2010 Revenue by End Markets | | Fiscal 2010 Revenue by Geography |
| | | |
 | | 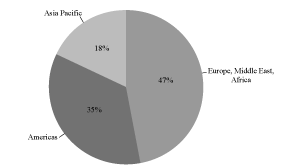 |
| |
Fiscal 2010 Revenue: $228.1 Million |
For more than 50 years, we and our predecessor companies have delivered products and services that help ensure the safe and efficient operation of nuclear facilities. We believe the breadth and proven performance of our solutions support our longstanding strategic customer relationships across diverse end markets. Our products and services have been sold directly and indirectly to a variety of end-use customers including, but not limited to, all of the U.S. nuclear power producers, 400 of the global installed base of 440 active nuclear power reactors, many of the leading reactor design firms, 17 of the 28 NATO militaries, numerous international government and supranational agencies, as well as medical service providers and industrial companies worldwide.
Our broad product and services portfolio of radiation detection, measurement, analysis and monitoring solutions is supported by our research and development organization of 168 scientists, engineers and technicians, who represented approximately 19% of our workforce as of June 30, 2010. We possess numerous product qualifications, trade secrets and patents that support our market position and our ability to deliver next generation products and services. In addition, we maintain design, manufacturing and sales capabilities across seven countries, enabling us to capitalize on growth opportunities, including the anticipated increase in demand for nuclear power and the ongoing spending for defense and homeland security.
Our financial performance is driven by the replacement of products and the recurring provision of services into our core end markets, as well as the construction of new NPPs globally. Many of our products are ordered well in advance of the anticipated shipment date, providing visibility into future revenue through our backlog and deferred contract revenue, which were $191.7 million and $44.7 million as of June 30, 2010. We generated revenue of $228.1 million, Adjusted EBITDA of $46.3 million and net income of $6.0 million for fiscal 2010. See pages 10 and 11 of this prospectus for a definition and reconciliation of Adjusted EBITDA to cash provided by (used in) operating activities.
81
Our Market Opportunity
We believe that significant opportunities for growth exist within each of our primary end markets.
Nuclear
Our legacy in the nuclear industry positions us to capitalize on the growth in demand for radiation detection, measurement, analysis and monitoring products and services in each phase of the nuclear life cycle, as outlined in the chart below.
We believe the following dynamics support the sustainability of our existing business and will drive new sources of organic growth.
Predictable upgrade, replacement and retirement cycles. Our radiation detection, measurement, analysis and monitoring products and systems have predictable life spans, typically ranging from four to 25 years. Our complex monitoring systems typically require at least one comprehensive upgrade during their useful life to optimize their functionality. In addition, many of our products require replacement parts, components and service due to normal wear during their useful lives.
Aging installed base. The existing global installed base of nuclear reactors has an average age of 26 years. This aging installed base requires frequent product replacements and upgrades over an operating life cycle that generally ranges from 40 to 60 years. Furthermore, as reactors reach the end of their useful lives, the onset of a multi-year “decommissioning” process represents a further revenue opportunity in the reactor life cycle for our products.
Large installed base of “orphaned” products and systems. Most currently operating reactors were commissioned prior to 1990. Operators of many aging NPPs often must consider new suppliers to meet their detection needs as many of the suppliers of legacy radiation detection, measurement, analysis and monitoring systems no longer service the nuclear industry.
Reduction in trade barriers. Historically closed markets, such as India, have recently opened due to enhanced globalization and free trade.
Dosimetry outsourcing. NPPs have historically managed the majority of their dosimetry service requirements internally. However, the cost benefits of outsourcing these services have become increasingly attractive to NPP operators as they focus on improving profitability and enhancing service.
82
New build opportunity. We expect the increase in the installed base of nuclear reactors worldwide to provide opportunities across our offerings. The nuclear industry is experiencing robust growth in activity related to new reactor build. As of April 2010, there were 52 reactors under construction, 143 planned and 344 proposed, according to the WNA. The first phase of this “nuclear renaissance” is occurring internationally and our global footprint positions us to capitalize on these opportunities. Since the early stages of reactor development generally represent more than 20% of our revenue opportunity over the life cycle of a reactor, we are positioned to benefit from increased global reactor construction. In addition, as new plants are added to the global nuclear fleet, we believe our recurring revenue opportunity associated with replacements, spares, software, services and system upgrades should continue to increase. Although no new commercial reactors have been ordered in the United States since the 1970s, there is some support to build new nuclear power reactors in the United States, including federal government incentives, the desire to meet long-term energy demand with reduced CO2 emissions and an increased focus on energy self-sufficiency.
Defense
Focus on military personnel. Global militaries must contend with radiological threats and the difficulties of protecting soldiers and monitoring areas of enemy engagement. The combination of our active dosimeters and telemetry technology provide a differentiated solution that addresses the radiation detection needs of modern militaries.
Increased civil defense spending on radiation detection. Civil defense and homeland security organizations are focused on preventing the illicit transportation of radiological materials across borders. The commercial application of our radiation detection expertise positions us to benefit from government spending on detection technologies.
Enhanced event specific security. The visibility of high profile events and venues has increased their value as targets of terrorist activity. In response, security spending at events, such as the Olympic Games, has increased substantially, as has the utilization of radiation detection technology, providing an expanding market opportunity for our products.
Medical
Radiological procedure growth. The use of radiodiagnostic and radiotherapeutic procedures is expanding globally due to aging population demographics, technological advancements and emerging middle classes in China and India. As the use of radiological procedures increases in the medical industry, so does our associated market opportunity.
Dosimetry outsourcing. In some regions outside the United States, dosimetry services for health care practitioners historically have been provided by government agencies. We believe that more government agencies are outsourcing dosimetry services to private providers due to favorable cost dynamics in some regions, such as Europe. This provides a market opportunity where we can leverage our technical expertise and North American service experience to expand into other regions.
Our Competitive Strengths
We believe that the following competitive strengths will enable us to maintain our position and capitalize on growth opportunities in our end markets:
Trusted radiation detection, measurement, analysis and monitoring provider. The nuclear industry is highly regulated and requires compliance with strict product specifications. Our track record in the nuclear end market enables us to gain market share across our product and service offerings. We and our predecessor companies have served the radiation detection, measurement, analysis and monitoring needs of our customers for over 50 years, having developed trusted, recognized brands supported by our tradition of technical excellence, product reliability and customer service. In addition, we have leveraged our detection expertise to commercialize applications for the defense and medical end markets. In the defense market, our products serve as critical components of personnel protection for military and civil defense applications around the
83
world while our medical products and services support important reporting and measurement requirements for medical personnel.
Broad and complementary product and service portfolio. We are one of the only companies that offers radiation detection, measurement, analysis and monitoring products and services to satisfy customer requirements throughout the NPP life cycle. Our comprehensive product line supports virtually all radiation detection and monitoring needs associated with the nuclear, defense and medical end markets. As a result, we believe that we have consistently gained market share as some of our key customers rationalize their supply chain. Furthermore, our portfolio provides us with a natural opportunity to cross-sell our products and services to our customers. For example, our relationships developed through sales of dosimeters led us to win a recently announced contract to supply radiation monitoring systems and electrical penetration adaptors to Ringhals NPP in Sweden.
Large installed base driving recurring revenue. We possess longstanding customer relationships in all of our end markets. As of June 30, 2010, our products were installed at 400 of the 440 active nuclear power reactors globally, which have an average age of 26 years. This installed base drives recurring revenue through replacement and service cycles associated with our offerings and the typical 40 to 60 year operating life cycle of an NPP. The length and quality of supplier relationships are important customer buying criteria due to high switching costs and the importance of proven product reliability. In addition, we maintain relationships with global military and government organizations that value operating longevity and technological expertise. For example, our products have been sold to 17 of the 28 NATO militaries as well as the U.S. Departments of Energy, State, Defense and Homeland Security. Our customers’ focus on personnel protection drives their recurring expenditures on service, recalibration and product upgrades in our defense end market.
Technical complexity creates high barriers to entry. Across our end markets, we design our products to meet demanding customer specifications, qualifications and regulatory requirements. In many circumstances, we design our products to be compatible with highly complex facilities and operate effectively in harsh environments. Reliability is critical for our safety-related products since a product failure may cause an unplanned nuclear power reactor shutdown resulting in costs that may exceed $1.0 million per day.
Global footprint designed to meet local customer needs. Our global footprint, augmented by our established network of suppliers and distributors, enables us to be responsive to our customers and provide locally customized solutions. We operate facilities in seven countries, accommodating the desire of certain of our customers to procure products and services from local providers. Sales outside of the United States and Canada accounted for 65.1% of total revenue for fiscal 2010. We believe that our established global infrastructure provides a scalable platform to meet the growing worldwide demand for our products and services.
Seasoned management team complemented by highly skilled engineers. We are led by an experienced management team with a mix of private sector and government experience across different industries and functions. Our five divisional presidents have an average tenure of over 20 years in the nuclear industry. Our management team has successfully integrated the legacy businesses of which we are comprised, and has positioned us as a global provider of radiation detection, measurement, analysis and monitoring. Our senior management team is complemented by a team of 168 scientists, engineers and technicians. A number of our employees are participants in international and U.S. standards setting organizations related to radiation detection in the nuclear, defense and medical end markets. Through these activities, we help define the setting of standards and preview changes that impact our products, customers and end markets.
Our Strategy
Our objective is to continue enhancing our position as a global provider of radiation detection, measurement, analysis and monitoring products and services for the global nuclear, defense and medical end markets. We intend to achieve this through the following strategies:
Exploit under-penetrated market opportunities. We believe that we can exploit historically under-penetrated segments of our end markets by leveraging our existing positions across our major product categories. For example, we have leveraged our position in active dosimetry in the North American nuclear
84
market to increase sales of our contamination & clearance monitors, as evidenced by the sale of over 100 whole body contamination monitors to Bruce Power L.P., a large Canadian nuclear power generating company.
Expand addressable market. We believe that substantial opportunities exist for us to expand our addressable market by marketing our products and services to customers in new geographic regions; providing products and services to customers moving to an outsource model; entering markets where the government is privatizing services; and introducing new applications for existing technologies.
| | |
| | • | Geographic expansion. Although we sold products and services to customers in over 90 countries between fiscal 2006 and 2010, there remain international markets where we believe we can increase our presence. One such market is India, where we intend to leverage our relationships with leading reactor design firms to capitalize on the opening of the nuclear end market to U.S. firms due to a recent treaty ratification. Other markets for expansion include the Middle East, Eastern Europe and the former Soviet Union, where we intend to increase our presence by leveraging relationships with local partners. |
| | |
| | • | Customer outsourcing. We believe we will continue to capitalize on customer outsourcing within the nuclear end market. Within the United States, several NPP operators have recently outsourced their dosimetry services in order to reduce costs. We have been able to benefit from economies of scale as well as advantages in materials procurement and processing technology to provide enhanced dosimetry services to many of these NPPs at a lower cost. |
| |
| | • | Service privatization. In regions outside the United States, dosimetry services have historically been provided by government agencies. However, privatization of dosimetry services is accelerating in some regions, such as Europe, as providers seek to reduce costs and benefit from enhanced service offerings, providing an opportunity to leverage our expertise and North American service experience. |
| |
| | • | New applications for existing technologies. A portion of our development effort is focused on adapting existing technologies to alternative applications. For example, in response to market demand, we adapted our proprietary fiber-optic detector technology used in our TwoStep-Exit whole body monitor designed for the nuclear end market to create the HandFoot-Fibre hand and foot monitor designed for both the nuclear and medical end markets. |
Develop new products and services. We believe that significant near-term opportunities exist for us to develop new products and services by capitalizing on our understanding of our customers’ needs and requirements. For example, we developed our proprietary fiber-optic technology that is used in certain of our contamination & clearance monitors through consultation with existing customers. This technology is attractive to customers because, unlike conventional contamination & clearance monitors, its detection functionality does not require a gas supply, thus reducing maintenance and total life cycle costs for end users. This technology recently helped us secure a sale for installation in two Russian utilities.
Continuously improve our cost structure and productivity. As we continue to grow our business, we have implemented a coordinated program of ongoing operating improvements, such as rationalizing costs, optimizing our product portfolio, minimizing working capital requirements, as well as reducing the use of subcontractors, that we believe will permit us to improve our operating margins. We will continue to actively pursue other continuous improvement initiatives through programs across all of our operating segments.
Pursue strategic acquisitions. We have successfully integrated acquisitions to augment our organic growth. We were formed by the merger of GDS, IST and Synodys. Since our formation, we have effectively integrated these businesses, creating a global provider of radiation detection, measurement, analysis and monitoring. We intend to further complement our organic growth with selective acquisitions that enhance our existing products and services, strengthen our position with existing customers and enable us to expand into new markets.
85
Our Segments
Our segments correspond to our five operating divisions: Health Physics, Radiation Monitoring Systems, Sensing Systems, Dosimetry Services and Imaging Systems.
Health Physics
The Health Physics division encompasses three major product lines focused on detecting radiation and protecting individuals from hazardous exposure. The dosimeters, contamination & clearance monitors, and detection & identification equipment have applications across the nuclear, defense and medical end markets. The products in our Health Physics division are summarized below:
| | | | | | | | | |
| Product Category | | End Markets | | Applications | | NPP Life Cycle Phase | | Products |
| |
| Dosimeters | | • Nuclear
• Defense
• Medical | | Pager-sized personnel monitors which monitor radiation dose rate and cumulative dose, along with readers, telemetry, software and other accessories | | • Plant operation
• Recommissioning
• Decommissioning
• Waste management | | • Active dosimeters
• Passive dosimeters
• Readers
• Calibrators
• Dosimetry software
• Telemetry systems
• Accessories
• Software
• Services |
| | | | | | | | | |
| Contamination & Clearance Monitors | | • Nuclear
• Defense
• Medical | | Stationary systems designed to detect radioactive contamination of people, waste, tools, laundry, vehicles and cargo | | • Plant operations
• Recommissioning
• Decommissioning
• Waste management | | • Body monitors
• Waste chambers
• Tool monitors
• Laundry monitors
• Vehicle monitors
• Accessories
• Software
• Services |
| | | | | | | | | |
| Detection & Identification Devices | | • Nuclear
• Defense
• Medical | | Hand-held and fixed devices used for detecting and locating ionizing radiation sources and/or spectroscopically identifying the active radioisotopes | | • Plant operations
• Recommissioning
• Waste management | | • Survey meters
• Handheld identifiers
• Spectroscopic portal monitors
• Accessories
• Software
• Services |
|
Dosimeters
Our dosimeter product line, which measures ionizing radiation dose, consists of both active and passive dosimeters. Active dosimeters detect and measure radiation levels in real time and provide warnings if the dose rate or cumulative dose exceeds specific thresholds. Passive dosimeters are worn by personnel and monitor cumulative radiation dosage.
Our active dosimeters are most often utilized in NPP and defense environments. Active dosimeters are typically pager sized, and may be worn or fix-mounted, with some models having wireless capabilities. We
86
generally sell our dosimeters as part of larger systems, which often include readers, software, telemetry and other accessories. Certain systems require commercially available, sealed radioactive sources for calibration and quality assurance purposes.
Our active dosimeters have an average lifespan of approximately seven to ten years, depending on the usage and environment. Replacement cycles can vary by country, depending on the applicable regulatory regime or customer practices. This provides recurring revenue opportunities as customers must replace and upgrade components during this timeframe. In addition, as companies upgrade their dosimeters, they often purchase upgraded readers, software, services and accessories.
We are a global provider of active dosimetry products and services to the nuclear end market. Over 68% of operating NPPs in the United States use our active dosimetry products and services. In addition, sales to the defense end market constitute a significant portion of our active dosimeter revenue. For example, 17 of the 28 NATO militaries have purchased our active dosimeters. We designed our military dosimeter to be flash-dose capable, enabling the device to effectively measure radiation dose following a nuclear event. Also, civil security forces in various countries, including first responders from France, Italy and the United States, use our active dosimeters to assess radiological risk.
We also sell passive dosimeters, which are worn by nuclear, defense and medical and industrial workers with the potential to be exposed to radiation. As with active dosimeters, we typically sell passive dosimetry equipment as a system, consisting of dosimeters, readers, accessories and software.
Contamination & Clearance Monitors
Our contamination & clearance monitors include products that detect alpha, beta, gammaand/or neutron contamination of objects of various sizes and types, from people to trucks. We have a wide range of products, ranging from small tool monitors to whole body monitors for personnel, to large portal monitors for vehicles and cargo. Our monitors utilize gas, inorganic or plastic scintillators with fiber-optic technology to detect radioactive contamination. Our patented fiber-optic technology is differentiated in the market because its detection functionality does not require a gas supply, thus reducing maintenance and total life cycle costs for end users. Certain monitors require commercially available, sealed radioactive sources for calibration and quality assurance purposes.
In the nuclear end market, our monitors are used to screen personnel, their clothing and tools, as well as vehicles entering and exiting reactor sites. In the defense end market, our products are used for homeland security applications to screen people, luggage, vehicles and cargo transiting a port or border. In the medical end market, our monitors are used to screen the hands and feet of nuclear medicine workers in hospitals and are used in the steel industry to screen scrap metal for radioactive contamination.
Detection & Identification Devices
We provide a suite of devices that detect, locate and identify radioactive isotopes. These are typically handheld or fixed devices and can also be integrated into more complex mobile systems. For example, our SPIR Ident product has been incorporated into both military vehicles and helicopters. These detection & identification devices distinguish themselves through their high level of sensitivity and their capacity to distinguish between different radioisotopes using spectroscopy identification algorithms.
For this reason, these devices are typically used in the defense end market. In homeland security and military environments, these devices are used to rapidly identify potential radiological threats originating from dangerous nuclear material, while distinguishing such threats from naturally occurring radioactive materials and medical isotopes.
87
Radiation Monitoring Systems
Our Radiation Monitoring Systems division supplies fixed and mobile systems consisting of sensors, display and processing electronics and software, which are used for barrier leak control, effluent release monitoring, radiation protection of workers, operational process monitoring and post event monitoring in nuclear installations. The products in our Radiation Monitoring Systems division are summarized below:
| | | | | | | | | |
| Product Category | | End Markets | | Applications | | NPP Life Cycle Phase | | Products |
| |
| Radiation Monitoring Systems | | • Nuclear | | Systems consisting of sensors, displays, control electronics and software which are used for barrier leak control, effluent release monitoring, radiation protection of workers, operational process monitoring and “post event” monitoring in NPPs, nuclear fuel cycle industry, reactors and military installations. | | • Construction
• Plant operation
• Recommissioning
• Decommissioning
• Waste management | | • Alpha, beta, gamma and neutron sensors
• Channels for monitoring: volume contamination (particulates, iodine, gas and liquids); dose rates (gamma and neutron); and neutron flux
• Fixed and mobile instrumentation skids
• Display and processing electronics
• Accessories
• Software
• Services |
|
We are a global provider of radiation monitoring systems, with particularly strong positions in Europe and Asia. We sell fully integrated systems that can transmit data to a central computer that tracks radiation levels continuously throughout the plant. Many of our systems incorporate or use commercially available, sealed radioactive sources for calibration and quality assurance purposes, the exact type and quantity of such sources being dependent on the requirements of the intended application. To accompany these systems, we also supply proprietary software, which allows operators to monitor trends, alarm levels, historical incident files and status reports.
Within a typical nuclear reactor, a radiation monitoring system consists of between 40 and 120 sensors and a similar number of processing and display units, all of which are generally networked to a central control system. Safety-related monitors are subject to qualifications which are time consuming and expensive to obtain. Qualification of our products often requires close cooperation by us with customers and substantial technical expertise, sometimes requiring a multi-year process and substantial expenditures of funds in advance of customer orders. Qualification is a lengthy and costly endeavor in which equipment is rigorously tested in simulated real-world environmental conditions to ensure that it meets the criteria defined in the standards applicable to the nuclear environment. Qualifications must be performed according to independent reference standards that define the methodologies, criteria and severity required. Upon achievement, qualifications are not typically subject to requalification, revocation or challenge, although a qualification may be obsoleted or required to be revised if the standards organization or regulatory changes determine that the original qualification is insufficient for its intended purpose or the standards themselves evolve, inducing changes in the methodology, criteria or severity required of the qualification. Some equipment requires lengthier qualification periods than others, but the typical period ranges from one to four years. Once a component’s qualified life has been reached, it must be replaced. The qualification process for our radiation monitoring systems typically requires one to three years.
Radiation monitoring systems are typically installed in nuclear facilities during construction, and they are replaced or upgraded upon life extensions or reactor upgrades. The expected life for a radiation monitoring system is 15 to 25 years, depending on the usage and environment, necessitating a significant upgrade of equipment at least once during a nuclear facility’s useful life. Replacement cycles can vary by country, depending on the applicable regulatory regime or customer practices. This provides recurring revenue opportunities as customers must replace and upgrade components and services during this timeframe.
88
The decommissioning of an NPP, which can take over ten years, also requires radiation monitoring systems. Typically, a larger deployment of mobile monitors is required during the decommissioning process than in normal NPP operations. The new construction, operation and decommissioning phases of the NPP life cycle each provide opportunities for sales of our radiation monitoring systems.
Radiation monitoring systems are also prevalent in the nuclear fuel cycle industry, spanning fuel fabrication, reprocessing and storage. These systems are used in many types of accelerators, including medical positron emission tomography and high-energy particle accelerators and can also be used in the operation and monitoring of nuclear military installations.
Sensing Systems
Our Sensing Systems division provides products that facilitate reactor control, safety and containment structure integrity. These products meet proprietary reactor design qualifications and are essential to the safe and efficient operation of a reactor. The products in our Sensing Systems division are summarized below:
| | | | | | | | | |
| Product Category | | End Markets | | Applications | | NPP Life Cycle Phase | | Products |
| |
| Electrical Penetrations | | • Nuclear | | Conduit systems that are used to pass electrical and fiber-optic lines through the containment structure of an NPP, without compromising the pressure or radiological integrity of the structure | | • Construction
• Recommissioning | | • Electrical penetrations containment assemblies
• Temperature sensors
• Instrumentation seals
• Thermowells
• Explosive valves |
| Reactor Instrumentation & Control Equipment and Systems | | • Nuclear
• Defense | | Sensors and electronics designed to monitor radiation and temperature within a reactor core and in surrounding areas to facilitate safe and efficient reactor operation | | • Construction
• Plant operation
• Recommissioning | | • In-core detectors
• Ex-core detectors
• Control electronics |
|
Electrical Penetrations
Electrical penetrations are conduits through a nuclear reactor containment structure. Our penetrations allow wiring for electrical and optical signals to pass safely through the containment structure wall, while maintaining the integrity of the wall and not permitting radiation or pressure to escape. Containment structures consist of concrete walls that can extend up to fourteen feet in thickness with a stainless steel liner designed to contain radioactive emissions in a confined space. The containment wall is the primary safety barrier in the reactor.
Our electrical penetrations enable the supply of power for safety systems as well as the reception of signals from neutron flux detectors, radiation monitoring detectors, cameras and other control surveillance devices. Typically, a nuclear reactor has 40 to 70 major electrical penetrations with up to 12,000 individual electrical connections, or feedthroughs.
We are a global provider of electrical penetrations. As with radiation monitoring systems, electrical penetrations must be qualified. The qualification process for our electrical penetrations typically requires three to four years, and our electrical penetrations have been qualified for installation in most major reactor designs by reactor design firms and the major utilities.
As a critical component of reactor design, electrical penetrations provide us with increased visibility into new plant builds. Our position in electrical penetrations provides us with cross-selling opportunities for other products, such as detectors, radiation monitoring systems, imaging systems and contamination & clearance monitors.
89
Reactor Instrumentation & Control Equipment and Systems
We are a global provider of reactor instrumentation & control equipment and systems. Our reactor instrumentation & control detectors are used in nuclear facilities to monitor radiation and temperature within a nuclear reactor core (“in-core” detectors) and in surrounding areas (“ex-core” detectors). Our detectors measure the distribution of neutron/gamma flux and temperature both in, and adjacent to, a reactor core and are critical components to maintaining the efficient and safe operation of a reactor. Our detectors incorporate various radioactive materials and generate a signal, giving a precise measurement of the radiation flux, which contributes to safe and efficient reactor operation.
As with radiation monitoring systems and electrical penetrations, these detectors must be qualified and such qualification is established by tests which are designed to demonstrate the intended function of a detector when subjected to conditions that simulate installed life under design service conditions. The qualification process typically requires one to two years for our detectors. Our reactor instrumentation & control detectors are qualified for all major reactor designs. Once a qualification is obtained and a contract is awarded, the supplier is well positioned for replacement revenue due to the high switching costs involved in qualifying new products and services from other suppliers.
Reactor instrumentation & control detectors are typically installed in nuclear facilities during construction and are replaced or upgraded regularly. The expected life of a detector can range from four to 25 years, depending on the type of detector and the operating environment. This provides recurring revenue opportunities as customers must replace and upgrade components during these timeframes. In addition, there are opportunities to provide more comprehensive upgrades of reactor instrumentation & control detector systems in certain existing reactors to facilitate up-rating.
Dosimetry Services
Our Dosimetry Services division provides an official “dose of record” to employers of radiation workers. The services in our Dosimetry Services division are illustrated below:
| | | | | | | | | |
| Product Category | | End Markets | | Applications | | NPP Life Cycle Phase | | Products |
| |
| Dosimetry Services | | • Nuclear
• Defense
• Medical | | An information service, which provides environmental radiation monitoring services as well as an official dose of record to employers and occupationally exposed employees | | • Plant operation
• Decommissioning
• Waste management | | • Extremity, whole body, eye, environmental and fetal monitoring reports
• Online applications for dosimetry data management
• Consulting services |
|
At the request of employers, we provide cumulative dose monitoring services to personnel at nuclear installations, research labs, government agencies, hospitals, dental offices, veterinary offices and other medical facilities where there is a potential for radiation exposure. Government regulations and industry guidelines (e.g., OSHA, NCRP, ANSI, IAEA) often require these individuals to wear dosimeters to monitor their radiation dose. We provide our customers with services such as cumulative dose reports and data management. We are a provider of dosimetry services, primarily serving the U.S. nuclear and medical end markets.
Our service uses film, thermoluminescent and track-etch dosimeters. Each of these has distinct characteristics that make them suitable for specific applications and customer types.
Dose is calculated algorithmically using filtering mechanisms to customize the dosimeter response for the type of radiation and potential exposure. Each dosimeter is identified to provide a chain of custody throughout the service cycle. We ship the dosimeters to the customer, whose personnel wear them for intervals ranging from one month to one year. As the wear period nears its end, we send the customer a new set of dosimeters, and the customer returns the original dosimeters to us for processing. After processing, we report dose information to the customer in a format that complies with relevant governing standards or regulations.
90
Reports generally take seven to ten business days to process and document each wearer’s current wear period dose, quarter-to-date dose, year-to-date dose and lifetime dose.
In fiscal 2009 90% of our dosimetry services customers, representing 65% of our dosimetry services revenue, pre-paid their annual subscriptions. In fiscal 2010, approximately 90% of our dosimetry customers, representing 66% of our dosimetry services revenue, pre-paid their annual subscriptions.
Imaging Systems
Our Imaging Systems division is a global provider of highly specialized closed circuit camera systems used for inspection and surveillance in difficult and hazardous environments. The products in our Imaging Systems division are illustrated below:
| | | | | | | | | |
| Product Category | | End Markets | | Applications | | NPP Life Cycle Phases | | Products |
| |
| Imaging Systems | | • Nuclear
• Other | | Nuclear: imaging systems for nuclear fuel handling, control, monitoring and inspection; reactor vessel maintenance; underwater surveillance; tank and vessel inspection; and cameras for remotely operated vehicles
High-temperature: kiln viewing and recovery boiler monitoring | | • Construction
• Plant operation
• Recommissioning
• Decommissioning
• Waste management | | • Radiation hardened surveillance and inspection cameras
• Video management and control systems
• Lighting systems
• Telemetry control units
• Thru-wall endoscopes
• High temperature cameras with pyrometry
• Software |
|
We have designed our imaging systems to operate in nuclear installations, with many of our cameras being radiation “hardened,” allowing them to operate in the high levels of radiation frequently found in these installations. We supply cameras for all stages of the nuclear life cycle, from construction through operation, to decommissioning and waste management. Our products are used in NPPs, nuclear reprocessing plants and waste management facilities. For example, our cameras are used during refueling shutdowns for inspecting the integrity of critical structures in nuclear reactors.
Our products are also designed for use in high temperature environments, such as pulp and paper recovery boilers, gas or coal-fired power boilers and cement kilns. In these environments, our cameras provide real time video as well as accurate temperature measurement. This enables operators to closely monitor their processes, helping to ensure plant safety and increased operational efficiency. For example, our cameras are used by two of the world’s largest cement producers to monitor flame patterns and temperature in cement kilns, helping operators maximize operational efficiency.
The expected life of our cameras typically ranges from one to five years, depending on the operating environment. This provides recurring revenue opportunities as customers must replace and upgrade components during these timeframes.
Research and Development
Our research and development efforts allow us to introduce new products to the marketplace, fulfill specific customer needs and continue to meet qualification requirements for next generation nuclear reactors and other evolving regulatory standards. Our five operating divisions are committed to both technology research and product development to fulfill their strategic objectives and are supported by our engineering and research and development organization consisting of 168 scientists, technicians and engineers, representing approximately 19% of our total workforce, as of June 30, 2010. A number of them participate in international standards setting organizations and committees. We engage in research and development activities at most of our facilities worldwide.
91
We expensed approximately $14.9 million, $11.3 million and $8.7 million on research and development for fiscal 2008, 2009 and 2010. Research and development activities range from the development of radiation tolerant electronics to the development of customized software solutions for customer-specific applications, among others. We conduct these efforts through a mix of in-house research, collaboration with academia, customers and regulatory authorities as well as selected outsourcing through external vendors. The scope and extent of the outsourced portion of research and development activities vary by division, but typically, critical hardware design, software development and project management activities are conducted in-house while specialized services such as consulting services, algorithm design, thermal analysis, complex modeling and calculations and testing services are provided by third parties.
Sales and Marketing
We sell our products and services through our direct sales organization and indirectly through our global network of independent, third-party sales representatives and distributors. Our internal sales team is organized by operating division and end market to provide a higher level of service and understanding of our customers’ unique needs. We recently instituted a key account strategy in which we have designated senior executives taking a lead role with our top customers. This enables us to systematically and actively maintain close relationships with our top customers and provide solutions that meet their specifications. We have 14 sales offices in North America, Europe and Asia, and as of June 30, 2010, our sales and marketing personnel consisted of 139 employees, which represents approximately 16% of our total workforce.
We derive a portion of our revenue from sales of our products and services through channel partners, such as independent sales representatives and distributors. In particular, our independent sales representatives are an important source of sales leads for us and augment our internal resources in remote geographies. We sell through distributors in situations in which our customers prefer to purchase from a local business entity or purchase in smaller volume.
Our marketing activities include participation in many tradeshows worldwide across our nuclear, defense and medical end markets. We advertise in technical journals, publish articles in leading industry periodicals and utilize direct mail campaigns.
We host our annual Users’ Training and Benchmarking Seminar, where customers participate in a variety of programs designed to exchange ideas and discuss occupational challenges. The event also brings together key channel partners and vendors to strengthen our sales and marketing network. Attendees gain insight into our product plans and participate in interactive sessions that give them the opportunity to better understand our current suite of products and services as well as provide feedback on our product roadmap.
Our Customers
Our principal customers include power and utility companies, reactor design firms, NPPs, government agencies, military organizations, medical service providers and industrial companies. For fiscal 2009, no single customer accounted for more than 10% of our consolidated revenue, while our top ten customers together accounted for approximately 27.3% of our consolidated sales. For fiscal 2010, no customer accounted for greater than 10% of our consolidated revenue and our top ten customers together accounted for approximately 38.4% of our consolidated sales.
We have had a long term relationship with one company that provides plant design and equipment to a significant number of nuclear power plants around the world. Revenues from this customer have represented approximately 23% to 30% of the Sensing Systems segment revenue in fiscal years 2008, 2009 and 2010. If we were to lose this customer, it could have a material impact on the results of the Sensing Systems segment. Due to the project nature of our business, there are other customers in the Sensing Systems segment, as well as in the Imaging Systems, Health Physics, and Radiation Monitoring Systems segments, that may represent approximately 3% to 8% of consolidated revenue in any one period. However, these large customer projects are continually replaced in subsequent periods with equivalent projects from different customers. We do not believe that the loss of any one or several of these major customers would have a material adverse effect on our segment results or on our consolidated financial statements.
92
Manufacturing and Supply Chain
Given the diversity of our products, we employ numerous manufacturing techniques, including high-volume process manufacturing, discrete manufacturing, cellular manufacturing and hybrid approaches. Our production personnel engage in manufacturing, procurement and logistics activities. Our production activities are located in the United States, Canada, France, Germany, Finland and the United Kingdom. As of June 30, 2010, our production personnel consisted of 423 employees, which represents approximately 48% of our total workforce.
Our manufacturing activities are focused mainly on the production of the core value-add devices and components of our products, while non-core components and sub-assemblies are generally outsourced. This strategy enables us to protect important intellectual property while minimizing the time, cost and effort to produce commoditized components. Most of the time, the design, assembly and integration of the components are performed in-house, allowing our engineers to customize the products according to customer specifications. For highly engineered nuclear products, production volumes are typically low, with a high degree of custom engineering required. For other product lines, such as passive dosimetry products, production volumes tend to be higher. We apply rigorous quality control processes and calibrate radiation detection devices internally, leading to high quality standards and customization capabilities. Most of our production sites are certified to production quality standards such as ISO 9001, 10 CFR 50 Appendix B andASME NQA-1.
The principal materials used in our manufacturing processes are commodities that are available from a variety of sources. The key metal materials used in our manufacturing processes include precious metals, tungsten, copper, aluminum, magnesium products, steel, stainless steel and various alloys, which are formed into parts such as detectors, sensors and cable assemblies. The key non-metal materials used include amorphous and crystalline scintillator materials, ceramics, epoxies, silicon and fused silica, polyethylene, polyurethane and injection molded plastic parts and components such as lenses, monitors, sensors, dosimeters, electronic boards, detectors and cables.
93
Properties
The table below lists our properties as of June 30, 2010.
| | | | | | | | | | | |
| | | | | | | | Lease
|
| | | | | Approximate
| | | | | Expiration
|
Location | | Square Feet | | | Facility Use / Description | | Date |
| |
Production facilities: | | | | | | | | |
| Canada | | Cambridge, ON | | | 31,800 | | | Sensing Systems | | July 31, 2012 |
| Finland | | Turku | | | 9,800 | | | Health Physics | | N/A(1) |
| France | | Fussy (Bourges) | | | 24,000 | | | Sensing Systems | | May 31, 2015 |
| France | | Lamanon | | | 77,700 | | | Health Physics & Radiation
Monitoring Systems | | N/A(2) |
| France | | Lamanon | | | 6,500 | | | Health Physics & Radiation
Monitoring Systems | | November 30, 2017 |
| Germany | | Hamburg | | | 33,100 | | | Health Physics | | December 31, 2016 |
| Germany | | Munich | | | 28,100 | | | Radiation Monitoring Systems | | June 30, 2015 |
| United Kingdom | | Alton | | | 27,000 | | | Imaging Systems | | December 14, 2010(3) |
| United Kingdom | | Farnborough | | | 19,976 | | | Imaging Systems | | June 24, 2014 |
| United States | | Atlanta (Smyrna), GA | | | 24,100 | | | Health Physics & Radiation
Monitoring Systems | | June 30, 2014 |
| United States | | Buffalo (Cheektowaga), NY | | | 26,200 | | | Sensing Systems | | May 31, 2013 |
| United States | | Horseheads, NY | | | 50,500 | (4) | | Sensing Systems &
Imaging Systems | | November 30, 2014 |
| United States | | Irvine, CA | | | 43,500 | | | Dosimetry Services | | August 31, 2014 |
| | | | | |
Sales / Research and Development / Administrative locations | | | | |
| China | | Beijing | | | 500 | | | Sales center | | February 28, 2011 |
| China | | Beijing | | | 2,200 | | | Sales center | | March 31, 2011 |
| Germany | | Bonn | | | 1,000 | | | Imaging Systems | | N/A(5) |
| United Kingdom | | Whitehaven, Cumbria | | | 3,000 | | | Imaging Systems | | N/A(5) |
| United States | | Pickerington, OH | | | 2,900 | | | Imaging Systems | | May 31, 2011 |
| United States | | San Ramon, CA | | | 10,300 | | | Corporate headquarters | | April 12, 2012 |
| United States | | Woodinville, WA | | | 1,000 | | | Imaging Systems | | July 31, 2012 |
| | |
| (1) | | The lease on the property we occupy in Turku, Finland is current and terminable by either party with twelve months’ notice. No notice of termination with respect to this lease has been given or received as of the date of this prospectus. |
| | |
| (2) | | We lease all listed properties except this property located in Lamanon, France, which we own. |
| | |
| (3) | | The lease on the property we occupy in Alton, United Kingdom will expire in December 2010; however, we are in the process of relocating this facility to Farnborough, UK, where we have already commenced a new lease, as listed above. |
| | |
| (4) | | Our current lease consists of a total of approximately 60,675 square feet, of which we sublet, or otherwise do not use, approximately 10,175 square feet. |
| |
| (5) | | The term of such leases are month to month. |
We believe that our properties are adequate to meet the anticipated requirements of our business for at least the next twelve months. We are currently in the process of expanding our manufacturing facilities in Lamanon, France, and we recently renovated and reorganized our facilities in Hamburg, Germany and Cambridge, Ontario, Canada. If we were to require additional space at any of our locations, we believe that it would be readily available on commercially reasonable terms.
Competition
The global markets for our products and services are competitive and continually evolving. Within each of our operating segments, we encounter a variety of competitors, ranging from small independent companies providing niche solutions to larger multi-national corporations providing a broader set of products and services to our targeted end markets. We believe that the principal bases upon which we compete in our target end markets include product
94
quality and reliability, technical capability and product qualification, strength of customer relationships, customer service and price. In particular, customers in the nuclear and defense end markets tend to emphasize product quality and reliability, technical capability and strength of supplier relationships, while customers in the medical end markets, in particular for passive dosimetry products and services, tend to make purchasing decisions on a combination of brand recognition, price, service and reliability.
We believe the primary competitors in each of our segments are as follows:
| | |
| | • | Health Physics: Thermo Fisher Scientific and Areva (Canberra). |
| |
| | • | Radiation Monitoring Systems: General Atomics (Sorrento Electronics) and Areva (Canberra). |
| |
| | • | Sensing Systems: Reuter-Stokes (General Electric), Schott and Areva. |
| |
| | • | Dosimetry Services: Landauer. |
| |
| | • | Imaging Systems: Diakont. |
Intellectual Property
We rely on a combination of intellectual property rights, including qualifications, trade secrets, patents, copyrights and trademarks, as well as contractual protections, to protect our proprietary products, methods, documentation and other technology.
As of June 30, 2010, we held 11 issued U.S. patents, 39 issued foreign counterparts of U.S. patents and three other issued foreign patents with expiration dates ranging from 2010 to 2025. In addition, we have filed one U.S. patent application, six foreign counterpart patent applications and five other foreign patent applications. We also hold exclusive and non-exclusive licenses related to patents and other intellectual property of third parties. We held 13 U.S. registered and pending trademarks, 14 international counterparts of such registered and pending trademarks and five additional international registered and pending trademarks, as of June 30, 2010.
In many instances, we rely on trade secret protection and confidentiality agreements to safeguard our interests. Due to the long useful life of certain aspects of our technology, we believe that the patent registration process, which requires public disclosure of patented claims and inventions, could harm our competitive position. We differentiate our products and technologies primarily through our proprietary know-how, technology or data that are not covered by patents or patent applications, including technical processes, equipment designs, testing and other procedures. Our employees are generally required to assign to us all of the inventions, designs and technologies they develop during the course of employment with us, either through written agreements or by operation of law, depending on the jurisdiction. Where appropriate, we require third parties with whom we deal to enter into agreements with us that address issues of confidentiality and intellectual property.
Environmental Matters
We are subject to a variety of environmental, health and safety and pollution-control laws and regulations in the jurisdictions in which we operate. We do not believe the costs of compliance with these laws and regulations will be material. We use, generate, discharge and dispose of hazardous substances, chemicals and wastes at some of our facilities in connection with our product development, testing and manufacturing activities. Any failure by us to control the use of, to remediate the presence of, or to restrict adequately the discharge of, such substances, chemicals or wastes could subject us to potentially significant liabilities,clean-up costs, monetary damages and fines or suspensions in our business operations. In addition, some of our facilities are located on properties with a history of use involving hazardous substances, chemicals and wastes and may be contaminated. Although we have not incurred, and do not currently anticipate, any material liabilities in connection with contamination, we may be required to make expenditures for environmental remediation in the future with respect to contamination at our or our predecessors’ former or current facilities or at third-party waste disposal sites. See “Risk Factors—Risks Relating to Our Business—We could incur substantial costs as a result of violations of or liabilities under environmental laws.”
95
Regulation
We are subject to a variety of laws and regulations, including but not limited to those of the United States, Canada, the EU, the EU member states and the People’s Republic of China, that impose regulatory systems that govern many aspects of our operations, including but not limited to our use, storage and disposal of radioactive materials and hazardous waste. In addition, these jurisdictions impose trade controls requirements that restrict trade to comply with applicable export controls and economic sanctions laws and requirements, and legal requirements that are intended to curtail bribery and corruption. These laws and regulations apply by virtue of the nature of our industry, end markets and products, as well as the range of potential uses of our products, the origin of the technology incorporated into our products, and the jurisdictions in which we produce and sell our products.
The multi-jurisdictional legal and regulatory environments in which we operate are subject to extensive and changing laws and regulations administered by various national, regional and local governmental agencies both within and outside the United States.
We are a federal government contractor and, as such, we are subject to Executive Order 11246 and other relevant laws and regulations. As part of our compliance obligations, we implement on an annual basis an affirmative action plan and program which, in part, include our good faith efforts to achieve in our workforce full utilization of qualified women and minorities. In addition, we have in place an affirmative action plan with respect to disabled individuals, as well as Vietnam era, disabled or other veterans.
Some of the U.S. laws affecting our operations include, but are not limited to, the AEA, the Energy Reorganization Act of 1974, or ERA, the Resource Conservation and Recovery Act of 1976 as amended by the Hazardous and Solid Waste Amendments of 1984, or RCRA, the Comprehensive Environmental Response, Compensation and Liability Act of 1980, or CERCLA, the Hazardous Materials Transportation Act, the Federal Water Pollution Control Act, or the Clean Water Act, the Toxic Substances Control Act of 1976, or TSCA, the Organized Crime Control Act of 1970, or the OCCA, and the Occupational Safety and Health Act, or OSHA, as well as the state laws governing radiation control, hazardous waste management, water quality and air quality in the states of New York, Georgia and California, each as from time to time amended. We are also subject to a variety of U.S. federal and state employment and labor laws and regulations, including the Americans with Disabilities Act, the Federal Fair Labor Standards Act, the Worker Adjustment and Restructuring Notification Act, or WARN Act, which requires employers to give affected employees at least 60 days’ notice of a plant closing or mass layoff, and other regulations related to working conditions,wage-hour pay, overtime pay, employee benefits, anti-discrimination and termination of employment. The classified work that we currently perform at one of our U.S. facilities subjects us to the industrial security regulations of the Department of Defense and other federal agencies that are designed to safeguard against unauthorized access by foreigners and others to classified and other sensitive information.
In the United States, the AEA and ERA authorize the NRC, and state authorities where applicable, to regulate the receipt, possession, use and transfer of radioactive materials. The NRC, and state authorities where applicable, sets regulatory standards for worker protection and public exposure to radioactive materials or wastes to which we are required to adhere in our operations that use radioactive materials in research and development, product manufacture, testing and calibration.
Certain products in our Health Physics, Radiation Monitoring Systems and Sensing Systems divisions require the use of radioactive sources. For certain of our products, these radioactive sources are often obtained by our customers directly from third-party providers, and for others, we directly incorporate these radioactive sources into our products. Certain of our reactor instrumentation and control equipment and systems in our Sensing Systems division incorporate radioactive materials. In all such cases, licenses for radioactive sources and materials are provided by the appropriate regulatory authority in the relevant jurisdiction and such authorities may be at the state or national level. For example, at our sites in the United States that handle radioactive sources or materials, the appropriate licenses are issued by state-level authorities which are, respectively, the New York State Department of Health, Georgia Department of Natural Resources, and California Department of Public Health; at our site in Canada, the relevant authority is the national-level Canadian Nuclear Safety Commission; at our site in Finland, the relevant authority is the national-
96
level Radiation and Nuclear Safety Authority (STUK); in Germany, the relevant authorities are the state-level Hamburg State Office for Work Safety for our Hamburg site and the Bavarian State Office for the Environment for our Munich site; and, at our site in France, the relevant authority is the national-level French Nuclear Safety Authority (ASN).
While the specific process and criteria for receiving a license differ from jurisdiction to jurisdiction, it generally involves an application process in which we: identify a person or persons who have appropriate training and experience to be a health physics/radiation safety officer; specify the radioactive sources or materials sought to be licensed, their physical form (i.e., sealed or unsealed) and maximum possession limits on the amount of each type of radioactive element or compound sought under the license; specifies their intended use (e.g., calibration, testing, quality assurance, manufacturing); and, set forth written policies and procedures to ensure that we have adequate measures in place to ensure health and safety. These policies and procedures typically must be designed to ensure worker, workplace, and public safety, including emergency plans; set forth the proper handling, control and security of radioactive sources or materials on site; detail any disposal or decommissioning considerations; and adequately train personnel at the site in proper access to, and handling of, radioactive sources or materials.
The particular license requirements in a given jurisdiction are normally tailored to the specific radioactive elements or compounds involved, their physical form, and possession limits. Once authorities complete their application review and any requiredfollow-up, the authority issues the site a license which imposes specific on-going compliance obligations that typically include requirements for us to pay periodic licensing fees, submit periodic written compliance reports, and agree to periodic site inspections by regulators, which may be announced or unannounced. Once a site has an existing license, the process for expanding or reducing the licensing scope generally is simpler than applying for a new license.
We have numerous licenses in effect at our various facilities in the United States, Canada, Finland, Germany, and France and the expiration dates of individual licenses differ by their term and effective date. Typical license terms range from two to five years, with authorities in some jurisdictions (e.g., Finland and Bavaria, Germany) issuing licenses that are perpetual subject to our on-going license compliance. While specific regulations vary by jurisdiction, generally a license may be terminated by the regulatory authority immediately upon a finding of a substantial safety violation or other material violation of licensing requirements. For more minor violations, regulatory authorities typically provide the licensee with a written statement of deficiency stating required remediation steps and a demand for proof of remediation; depending on the severity of the violation, a re-inspection of the site may be performed by the authority to ensure adequate remedial steps have been completed.
In most cases, our various sites (including our predecessors) have held, maintained and (where required) renewed their licenses for a decade or more. In all cases, the licenses we required related to radioactive sources or materials are current and in force and, to the best of our knowledge, we are not aware of any basis to expect that those of its existing licenses subject to periodic renewals will not be renewed.
RCRA provides a comprehensive framework for the regulation of hazardous and solid waste which applies to our operations. RCRA prohibits improper hazardous waste disposal and imposes criminal and civil liability for failure to comply with its requirements. TSCA provides a comprehensive framework for the management by the EPA of over 60,000 commercially produced chemical substances, some of which are used by our operations. The Clean Water Act regulates the discharge of pollutants into certain waters and may require us to apply for and obtain discharge permits, conduct sampling and monitoring and, under certain circumstances, reduce the quantity of pollutants in those discharges. The OCCA provides for the regulation of explosives, which applies in particular to our facility in Buffalo which manufactures and tests products that incorporate explosives. The OCCA establishes a framework for licensing, use, storage and sale of explosives and products containing explosives and imposes criminal and civil liability for failure to comply with its requirements. OSHA provides for the establishment of standards governing workplace safety and health requirements, including setting permissible exposure levels for hazardous chemicals. We must follow OSHA standards, including the preparation of material safety data sheets, hazardous response training and process safety management, as well as various record-keeping, disclosure and procedural requirements.
97
Our operations outside the United States are subject to similar, and sometimes more stringent, laws and regulations. For example, an EU directive relating to the restriction of hazardous substances in electrical and electronic equipment, or RoHS directive, and a directive relating to waste electrical and electronic equipment, or WEEE directive, have been and are being implemented in EU member states. Among other things, the RoHS directive restricts the use of certain hazardous substances in the manufacture of electrical and electronic equipment and the WEEE directive requires producers of electrical goods to be responsible for the collection, recycling, treatment and disposal of these goods. In addition, laws similar to the RoHS and WEEE directives were passed in China in 2006 and South Korea in 2007. Governments in other countries and states, including the United States, are considering implementing similar laws or regulations. In addition, a regulation regarding the registration, authorization and restriction of chemical substances in industrial products, or REACH, became effective in the EU in 2007. REACH and other regulations requires us or our suppliers to substitute certain chemicals contained in our products with substances the EU considers less dangerous. We have assessed the impact that REACH is expected to have on us and have determined the impact at this time to be immaterial. We have completed the chemical substitutions required by REACH and expect to complete any associated requalifications in fiscal 2011. We are also subject to the employment and labor laws and regulations of the foreign jurisdictions where the majority of our employees are located.
We deal with numerous U.S. andnon-U.S. government agencies and entities, including the U.S. military, the armed forces of many NATO countries, the U.S. Department of Defense, the U.S. Department of State, the U.S. Department of Treasury, the U.S. NRC, the U.S. Department of Homeland Security and the corresponding governmental agencies and entities in the European Union and Canada. When working with these and other government agencies and entities, we must comply with, and are affected by, laws and regulations relating to the formation, administration and performance of contracts. These laws and regulations, among other things require certification and disclosure of all cost or pricing data in connection with various contract negotiations; impose acquisition regulations that define allowable and unallowable costs and otherwise govern our right to reimbursement under various cost-based U.S. government contracts; and restrict the use and dissemination of information classified for national security purposes and the exportation of certain products and technical data.
We believe that certain of our products and technologies are eligible for designation or certification as “qualified anti-terrorism technologies” under the SAFETY Act provisions of The Homeland Security Act of 2002, and its implementing regulations. Under the SAFETY Act, the federal government provides for certain liability limitations and a presumption that the “government contractor” defense applies if the Department of Homeland Security “designates” or “certifies” technologies or products as “qualified anti-terrorism technologies,” and if certain other conditions apply. We may seek to qualify some or all of our products and technologies under the SAFETY Act’s provisions in order to obtain such liability protections, but there is no guarantee that the Department of Homeland Security will designate or certify our products and technologies as qualified anti-terrorism technology. To date, we have not sought such designation or certification as a qualified anti-terrorism technology, and our products have been sold without such qualification and we may continue to sell our products and technologies without such qualification. To the extent we do so, we will not be entitled to the benefit of the SAFETY Act’s limitations on tort liability or to any U.S. government indemnification.
Many of our products are subject to export controls of the United States, Canada and the member states of the EU, depending on a number of factors, including the nature of the product and its potential uses, the origin of the technology incorporated into the product, and the jurisdictions in which we produce and sell our products. Certain of our products are subject to U.S. export control laws and regulations, which have certain registration, licensing and recordkeeping requirements for the sale or transfer of controlled technology or information tonon-U.S. persons. These regulations include the U.S. Department of Commerce’s Export Administration Regulations, or the EAR, the U.S. Department of State’s International Traffic in Arms Regulations, or ITAR and the U.S. Nuclear Regulatory Commission regulations. Certain products that have dual-use commercial and military applications are controlled under the EAR’s Commerce Control List, and we have export compliance systems for determining the proper export licensing requirements for such products. We need to keep such export compliance systems, which include third-party service provider screening of
98
compliance lists, monitoring of Department of Commerce notifications and periodic reviews of applicable regulations, up to date and properly maintained.
U.S. laws restrict the ability of U.S. companies, U.S. citizens and U.S. permanent residents, or U.S. persons, from involvement in certain types of transactions with countries, businesses and individuals that have been targeted by U.S. economic sanctions. For example, U.S. persons are precluded from undertaking virtually any activity of any kind on the part of any U.S. person with regard to any potential or actual transactions involving Cuba, Iran and Sudan without the prior approval of the U.S. Department of Treasury’s Office of Foreign Assets Control, or OFAC. OFAC also administers U.S. sanctions against a lengthy list of entities and individuals, wherever they may be located, that the United States considers to be closely associated with these sanctioned countries or that are considered terrorists or traffickers in either narcotics or weapons of mass destruction. Furthermore, U.S. economic sanctions forbid U.S. persons from circumventing direct U.S. restrictions or from facilitating transactions bynon-U.S. persons if those activities are forbidden to U.S. persons. Penalties for violating provisions such as these can include significant civil and criminal fines, imprisonment and loss of tax credits or export privileges.
The Foreign Corrupt Practices Act of 1977, or the FCPA, as amended by the Omnibus Trade and Competitiveness Act of 1988 and the International Anti-Bribery and Fair Competition Act of 1998, makes it a criminal offense for a U.S. corporation or other U.S. domestic concern to make payments, gifts or give anything of value directly or indirectly to foreign officials for the purpose of obtaining or retaining business, or to obtain any other unfair or improper advantage. In addition, the FCPA imposes accounting standards and requirements on publicly traded U.S. corporations and their foreign affiliates, which are intended to prevent the diversion of corporate funds to the payment of bribes and other improper payments, and to prevent the establishment of “off books” slush funds from which such improper payments can be made. We are also subject to laws and regulations covering subject matter similar to that of the FCPA that have been enacted by countries outside of the United States. For example, the Convention on Combating Bribery of Foreign Public Officials in International Business Transactions was signed by the members of the Organization for Economic Cooperation and Development and certain other countries in December 1997. The Convention requires each signatory to enact legislation that prohibits local persons and firms from making payments to foreign officials for the purpose of obtaining business or securing other unfair advantages from foreign governments. Failure to comply with these laws could subject us to, among other things, penalties and legal expenses, which could harm our reputation and have a material adverse effect on our business, financial condition and results of operations.
Compliance with the myriad of export control laws of the various jurisdictions in which we do business is a challenge for any company involved in export activities within the nuclear and defense end markets. We have compliance systems in our U.S. andnon-U.S. subsidiaries to identify those products and technologies that are subject to export control regulatory restrictions and, where required, we obtain authorization from relevant regulatory authorities for sales to foreign buyers or for technology transfers to foreign consultants, companies, universities or foreign national employees. We also have a compliance system that is intended to proactively address potential compliance issues including those related to export control, trade sanctions and embargoes, as well as anti-bribery situations, and we are implementing this through such mechanisms as training, formalizing contracting processes, performing diligence on agents and continuing to improve our record-keeping and auditing practices with respect to third-party relationships and otherwise. Thus far, as part of our compliance system, for instance, we have developed a Code of Ethics and Conduct that informs all of our employees of their compliance obligations. Furthermore, we have developed an ethics and conduct training program that all of our employees are required to undertake, as well as other targeted compliance training relevant to their position, such as specific FCPA training for all of our worldwide controllers. Violations of any of the various U.S. ornon-U.S. export control laws can result in significant civil or criminal penalties, or even loss of export privileges, as mentioned above. We recognize that an effective compliance program can help protect the reputation and relationship of a regulated company with the regulatory agencies administering these laws and regulations. In the United States, each of the regulatory agencies administering these laws and regulations has a voluntary disclosure program that offers the possibility of significantly reduced penalties, if any are applicable, and we intend to use these programs as part of our overall compliance program, as necessary.
99
Backlog and Deferred Contract Revenue
Total backlog represents committed but undelivered contracts and purchase orders at period end. Backlog excludes maintenance-related activity and agreements that do not represent firm purchase orders. Customer agreements that contain cancellation for convenience terms are generally not reflected in backlog until firm purchase orders are received. Backlog is not a complete measure of our future business due to these customer agreements. Backlog can fluctuate significantly due to the timing of large project awards. In addition, annual or multi-year contracts are subject to rescheduling and cancellation by customers due to the long-term nature of the contracts.
Deferred contract revenue represents the prepayment of measuring and monitoring services. The amounts are recorded as deferred contract revenue in our balance sheets and represent customer deposits invoiced in advance for services to be rendered over the service period.
Information on backlog and deferred contract revenue follows (in thousands):
| | | | | | | | | | | | | |
| | | As of June 30, |
| | | 2008 | | 2009 | | 2010 |
| |
| Backlog | | $ | 177,956 | | | $ | 183,960 | | | $ | 191,734 | |
| Deferred contract revenue | | | 53,539 | | | | 62,031 | | | | 44,730 | |
Furthermore, we anticipate that approximately 53% of our June 30, 2010 total backlog will not be filled within the current fiscal year.
Legal Proceedings
From time to time, we are involved in various routine legal proceedings. We cannot predict the outcome of these lawsuits, legal proceedings and claims with certainty. Nevertheless, we believe that the outcome of these proceedings, even if determined adversely, would not have a material adverse effect on our business, financial condition and results of operations.
Employees
As of June 30, 2010, we had 875 employees worldwide, consisting of 139 employees in sales and marketing, 423 in production, 168 in research and development and 145 in general and administrative functions. Geographically, we had 325 employees in North America, 546 in Europe and four in Asia and other regions as of June 30, 2010. We maintain both union and non-union workforces in the United States, with unionized workforces comprising a small minority of the overall U.S. employee base. As of June 30, 2010,33 U.S.-based employees, primarily located in Horseheads and Buffalo, New York, were members of a union. Pursuant to applicable industrial relations laws, our employees located in France and Germany were represented by works councils, and our employees located in France and Finland were represented by trade unions.
100
MANAGEMENT
The following table sets forth certain information with respect to our executive officers and members of our Board of Directors:
| | | | | |
Name | | Age | | Position |
| |
| Thomas D. Logan | | 49 | | President, Chief Executive Officer and Chairman of the Board |
| Jack A. Pacheco | | 50 | | Vice President and Chief Financial Officer |
| Seth B. Rosen | | 42 | | General Counsel, Vice President, Corporate Development, and Secretary |
| W. Antony Besso | | 40 | | Regional Vice President, EMEA, and President, Health Physics Division |
| Iain F. Wilson | | 48 | | Regional Vice President, Asia, and President, Sensing Systems Division |
| Robert J. Klein(1)(2)(3)(4) | | 46 | | Director |
| Dustin G. Smith(1)(2)(4) | | 34 | | Director Nominee |
| Brian S. Graff(1) | | 45 | | Director Nominee |
| Michael T. Everett(3) | | 61 | | Director Nominee |
| Earl R. Lewis(2)(3)(4) | | 66 | | Director Nominee |
| Alfred E. Barry, Jr. | | 54 | | Director Nominee |
| | |
| (1) | | ACAS-designated representative. |
| |
| (2) | | To be a member of the Nominating and Corporate Governance Committee upon the consummation of this offering. |
| |
| (3) | | To be a member of the Audit Committee upon the consummation of this offering. |
| |
| (4) | | To be a member of the Compensation Committee upon the consummation of this offering. |
Thomas D. Loganhas been our President, Chief Executive Officer and Chairman of the Board since our formation in December 2005. From 2004 to 2007, Mr. Logan served as CEO for Global Dosimetry Solutions, one of our predecessor companies and currently a subsidiary of ours. Mr. Logan has more than 22 years of energy industry experience. In addition, he has nine years of experience within the contract manufacturing and consumer products industries. Mr. Logan holds a Bachelor of Science degree and a Master of Business Administration degree from Cornell University. Mr. Logan’s experience as our Chief Executive Officer gives him unique insight into our operations, challenges, and opportunities. His substantial executive experience, particularly leading the integration of our predecessor companies into a single global company, brings to the Board invaluable knowledge with regard to our operations, marketing, finances and business strategy.
Jack A. Pachecohas served as our Vice President and Chief Financial Officer since March 2008. From 2004 to 2008, Mr. Pacheco served as Chief Financial Officer of Smart Modular Technologies, a public company listed on the NASDAQ stock exchange. From 2001 to 2004, Mr. Pacheco served as Chief Financial Officer for Ignis Optics, Inc., an optical components startup acquired by Bookham Technology. He holds a Master of Business Administration degree from Golden Gate University and a Bachelor of Science degree in Business Administration from Washington State University.
Seth B. Rosenis our General Counsel, Vice President, Corporate Development, and Secretary, a position he has held since January 2008. In 2007, Mr. Rosen served as a business and legal consultant to a variety of existing and startup businesses. From 2006 to 2007, he was CEO of Golden Gate Energy Corporation, a solar energy startup company. From 1998 to 2006, he served as Senior Licensing Associate and then Principal Licensing Associate at the Technology Transfer Department of Lawrence Berkeley National Laboratory. From 1997 to 1998, Mr. Rosen served as Corporate Counsel at Siebel Systems, Inc. From 1994 to 1997, he was an associate at the law firm of Baker & McKenzie. Mr. Rosen received his Juris Doctor degree from Harvard Law School, his Master of Business Administration from the joint program at the Haas School of Business at
101
the University of California at Berkeley and the Graduate School of Business at Columbia University, and his Bachelor of Arts from the University of California at Berkeley.
W. Antony Bessohas been our Regional Vice President, EMEA, and President, Health Physics Division since February 2006. From 2004 to 2006, Mr. Besso acted as an advisor and interim manager for private equity firms and individual investors in a diverse range of industries. From 1996 to 2004, Mr. Besso held a series of senior management positions in the global engineering group ALSTOM SA. Mr. Besso was also a founding partner in Advention Business Partners, a leading independent consulting firm with operations in France, Germany and China. Mr. Besso holds a Bachelor of Arts degree from Queen’s University and a Master of Business Administration from Dalhousie University.
Iain F. Wilsonhas served as our Regional Vice President, Asia, and President, Sensing Systems Division since our formation in December 2005. From 2000 to 2005, Mr. Wilson was General Manager, Sensing Systems Group of IST, one of our predecessor companies. Previously, Mr. Wilson held numerous technical roles with IST, focused principally in the areas of Quality Management, Engineering and Plant Operations. He began his career as the Quality Manager for GE Reuter Stokes, Canada. Mr. Wilson holds a Bachelor of Science degree from Ryerson University, Toronto, Canada. Mr. Wilson is a member of the American Nuclear Society.
Robert J. Klein has served as a Director since our formation in December 2005. Mr. Klein has served as a Managing Director and Senior Vice President of ACAS, our principal stockholder, since 2004, where he leads the New York private equity practice. From 2002 to 2004, he served as a Principal of ACAS. Prior to joining ACAS, he was a Principal at American Securities Capital Partners. Mr. Klein received a Bachelor of Arts degree from Yale University and a Juris Doctor degree from Stanford University Law School. Mr. Klein is an ACAS-designated member of our Board of Directors. Mr. Klein’s qualifications to serve on our Board include his extensive investing and financing experience, as well as his long tenure as a Director. As Managing Director and Senior Vice President of ACAS, he was primarily responsible for the acquisition of our three predecessor companies beginning in 2004. As a Director of our company since our formation in October 2005, he has overseen the integration of our predecessor companies into a single global company and brings this experience to our Board to evaluate our operations, finances, business processes and strategies.
Dustin G. Smith has been designated to serve as a member of our Board of Directors upon the consummation of this offering. Mr. Smith has served as a Principal and Vice President in the Buyouts division of ACAS since July 2007 and serves on the Board of Directors of Halt Medical, eLynx Holdings, FreeConference.com, Inc. and DelStar Technologies. Mr. Smith joined ACAS in 2004 as an Associate and served as a Vice President from 2004 to 2007. From 2002 to 2004, Mr. Smith was with Mezzanine Management, a mezzanine and private equity fund specializing in growth financings for middle-market companies. He is a graduate of Georgetown University with both Bachelor of Science and Master of Science degrees. Mr. Smith is an ACAS-designated member of our Board of Directors. Mr. Smith’s qualifications to serve on our Board include his investing and financing experience. As Vice President of ACAS, he has been integrally involved with the acquisition of our three predecessor companies beginning in 2004 and our subsequent combined business operations, bringing to our Board valuable perspectives on evaluating our business and financing strategies.
Brian S. Graff has been designated to serve as a member of our Board of Directors upon the consummation of this offering. Mr. Graff has served as Senior Vice President of ACAS since 2004 and as a Senior Managing Director since 2008. From 2005 to 2008 he served as a Regional Managing Director of ACAS and from 2004 to 2005 he served as a Managing Director of ACAS. Mr. Graff also served as a Vice President and Principal of ACAS from 2001 to 2004. From 2000 to 2001, he was a Principal of Odyssey Investments Partners, a private equity fund. Mr. Graff is an ACAS-designated member of our Board of Directors. He holds a Bachelor of Science degree from Binghamton University, State University of New York and a Master of Business Administration degree from New York University’s Stern School of Business. Mr. Graff’s qualifications to serve on our Board include his investing and financing experience. As Senior Vice President of ACAS, he has served as a Director of our predecessor companies. This experience and his
102
accomplishments in these areas will help our Board to effectively evaluate our business and financing strategies.
Michael T. Everetthas been designated to serve as a member of our Board of Directors upon the consummation of this offering. From May 2007 until his retirement in December 2008, Mr. Everett served as a vice president of finance for Cisco Systems. From April 2003 to May 2007, Mr. Everett was Chief Financial Officer of WebEx Communications, Inc. From February 1997 to November 2000, Mr. Everett served as executive vice president and Chief Financial Officer of Netro Corporation, a wireless broadband access equipment provider. From August 1988 to August 1993, Mr. Everett served as senior vice president and Chief Financial Officer of Raychem Corporation, a telecommunications and electronics component manufacturer. Before joining Raychem Corporation, Mr. Everett served as a partner of Heller, Ehrman, White & McAuliffe LLC. Mr. Everett serves as a director and chair of the audit committee of Calix, Inc. Mr. Everett holds a Bachelor of Arts degree from Dartmouth College and a Juris Doctor degree from the University of Pennsylvania Law School. Mr. Everett qualifies as an “audit committee financial expert” under SEC guidelines. Mr. Everett has been designated to serve as a director on our Board due to his extensive background in finance and public accounting, as well as his broad managerial and legal experience. In addition, his current service on other corporate boards of directors and past service on non-profit organization boards will provide us with important perspectives on corporate governance matters.
Earl R. Lewishas been designated to serve as a member of our Board of Directors upon the consummation of this offering. Mr. Lewis has served as Chairman, President and Chief Executive Officer of FLIR Systems, Inc. since November 1, 2000. Prior to joining FLIR, Mr. Lewis served in various capacities at Thermo Instrument Systems, Inc., with his last role as President and Chief Executive Officer. Mr. Lewis is a member of the Board of Directors of Harvard BioScience, NxStage Medical, Inc. and American DG Energy, Inc. Mr. Lewis is a Trustee of Clarkson University and New Hampton School. Mr. Lewis holds a Bachelor of Science degree from Clarkson College of Technology and has attended post-graduate programs at the University of Buffalo, Northeastern University and Harvard University. Mr. Lewis has been designated to serve on our Board due to his extensive managerial and industry expertise. Mr. Lewis is a seasoned executive with extensive public and private company experience, including his tenures at FLIR Systems and Thermo Instrument Systems. His extensive experience as the chief executive of a publicly traded company and proven ability to lead complex technology companies is invaluable to our analysis and review of operations, finance and business strategies.
Alfred E. Barry, Jr. has been designated to serve as a member of our Board of Directors upon the consummation of this offering. Mr. Barry has served as Principal of Al Barry Consulting LLC since October 2009. Mr. Barry also currently serves as President of Stanlok Corporation, an industrial components manufacturer, and as a director of Wuxi REM Co., Ltd. From January 2008 to July 2009, Mr. Barry served as a director of Standard Lock Washer & Mfg. Co. Inc. From September 2001 until May 2007, Mr. Barry served as Chief Executive Officer of Central Industrial Supply Co., Inc., a mechanical hardware supplier for the computer server industry. Mr. Barry holds a Bachelor of Science degree from Worcester Polytechnic Institute, and Masters of Science degrees from Georgia Institute of Technology. Mr. Barry has been designated to serve on our Board due to his extensive operational experience in the manufacturing industry. His years of operational experience will provide valuable insight into our operations and business strategies.
Board Structure and Compensation
Our amended and restated Bylaws provide that at least one of the directors designated by ACAS must be part of the majority in any action taken by our Board of Directors so long as ACAS and its affiliated funds hold at least 50.1% of our outstanding common stock, other than on matters in which ACAS has a conflict of interest (as it would if it appointed a majority of our directors).
Our Board of Directors currently consists of two members. Upon the consummation of this offering, our Board of Directors will consist of seven members and will be divided into three classes, as follows:
| | |
| | • | Class I, which will consist of Messrs. Logan and Klein, and whose term will expire at our annual meeting of stockholders to be held in 2011; |
103
| | |
| | • | Class II, which will consist of Messrs. Smith and Everett, and whose term will expire at our annual meeting of stockholders to be held in 2012; and |
| | |
| | • | Class III, which will consist of Messrs. Graff, Lewis and Barry, and whose term will expire at our annual meeting of stockholders to be held in 2013. |
Our amended and restated Bylaws also provide that ACAS will have the exclusive right to designate up to three of our seven directors, subject to proportionate adjustment for any change to the size of our Board of Directors, as follows: one director to each of Class I, Class II and Class III so long as ACAS and its affiliated funds hold at least 50.1% of our outstanding common stock; one director to each of Class I and Class II so long as they hold at least 25% but less than 50.1% of our outstanding common stock; and one director to Class I so long as they hold at least 10% but less than 25% of our outstanding common stock. Other stockholders will not have an opportunity to vote on the election of ACAS designees.
At each annual meeting of stockholders to be held after the initial classification, the successors to directors whose terms then expire will serve until the third annual meeting following their election and until their successors are duly elected and qualified.
Our Board of Directors has determined that Messrs. Everett, Lewis and Barry are independent pursuant to the rules of The Nasdaq Stock Market andRule 10A-3 of the Exchange Act of 1934, as amended.
Our Board of Directors has the following committees:
Audit Committee
Upon the consummation of this offering, the Audit Committee shall consist of Messrs. Everett, Lewis and Klein. Our Board of Directors has determined that each member of the Audit Committee meets the requirements for financial literacy under the applicable rules of The Nasdaq Stock Market. Our Board of Directors has also determined that Mr. Everett is an “audit committee financial expert” as defined under the applicable rules and regulations of the SEC and has the accounting and related financial sophistication required by the applicable rules of The Nasdaq Stock Market. Mr. Klein is not independent under the applicable rules and regulations of The Nasdaq Stock Market or the SEC, and we intend to replace Mr. Klein on the Audit Committee with a director who is independent under the applicable rules prior to the date that is one year following the completion of this offering. Our Board of Directors has determined that Messrs. Everett and Lewis are independent. The Audit Committee reviews and, as it deems appropriate, recommends to the Board of Directors our internal accounting and financial controls and the accounting principles and auditing practices and procedures to be employed in preparation and review of our financial statements. The Audit Committee is directly responsible for the appointment, compensation, retention and oversight of the work of our independent public auditors and the scope of the audit to be undertaken by such auditors. Such auditors will report directly to the Audit Committee. Mr. Everett will serve as chairperson of the Audit Committee.
Compensation Committee
Upon the consummation of this offering, the Compensation Committee shall consist of Messrs. Lewis, Klein, and Smith. The Compensation Committee reviews and, as it deems appropriate, recommends to the Board of Directors policies, practices and procedures relating to the compensation of our officers and the establishment and administration of employee benefit plans. The Committee advises and consults with our officers as may be requested regarding managerial personnel policies. Mr. Lewis shall serve as chairperson of the Compensation Committee and our Board of Directors has determined that Mr. Lewis is independent. Our Compensation Committee will only have one independent member because neither Mr. Klein nor Mr. Smith is independent pursuant to the rules of The Nasdaq Stock Market andRule 10A-3 of the Exchange Act of 1934, as amended.
In order for our Compensation Committee to continue to make recommendations or determinations with respect to executive compensation, such committee must be composed of a majority of independent directors within ninety days from the date our common stock is listed on The Nasdaq Stock Market and entirely of independent directors within one year from the date our common stock is listed on The Nasdaq Stock Market. However, if we remain or become a “controlled company,” we will qualify for, and expect to rely on,
104
exemptions from The Nasdaq Stock Market corporate governance requirements that require such committee to be composed entirely of independent directors.
Nominating and Corporate Governance Committee
Upon the consummation of this offering, the Nominating and Corporate Governance Committee shall consist of Messrs. Klein, Smith and Lewis. The Nominating and Corporate Governance Committee reviews and, as it deems appropriate, recommends to the Board of Directors policies and procedures relating to director and board committee nominations and corporate governance policies. Mr. Klein shall serve as chairperson of the Nominating and Corporate Governance Committee. Our Board of Directors has determined that Mr. Lewis is independent. Our Nominating and Corporate Governance Committee will only have one independent member because neither Mr. Klein nor Mr. Smith is independent pursuant to the rules of The Nasdaq Stock Market andRule 10A-3 of the Exchange Act of 1934, as amended.
In order for our Nominating and Corporate Governance Committee to continue to make selections or recommendations with respect to directors, such committee must be composed of a majority of independent directors within ninety days from the date our common stock is listed on The Nasdaq Stock Market and entirely of independent directors within one year from the date our common stock is listed on The Nasdaq Stock Market. However, if we remain or become a “controlled company,” we will qualify for, and expect to rely on, exemptions from The Nasdaq Stock Market corporate governance requirements that require such committee to be composed entirely of independent directors.
Compensation Committee Interlocks and Insider Participation
None of our executive officers currently serves, or in the past year has served, as a member of the board of directors or compensation committee of any entity that has one or more executive officers serving on our Board of Directors or Compensation Committee. Additional information concerning transactions between us and entities affiliated with members of the Compensation Committee is included in this prospectus under the caption “Certain Relationships and Related Party Transactions.”
Director Compensation
During fiscal 2010, there was one non-employee director, Robert J. Klein, who is affiliated with ACAS and received no compensation for services as a member of either our Board of Directors or of the Board’s Compensation Committee. Mr. Logan’s compensation is reported below under the Summary Compensation Table, and he did not receive separate compensation for his service on our Board of Directors.
In connection with this offering, our Board of Directors will adopt a compensation policy applicable to all directors who are not employees of Mirion and who are not affiliated with ACAS. We expect that the initial compensation policy will provide that each such director will receive the following compensation:
| | |
| | • | an annual cash retainer of $50,000 for serving on the board; |
| |
| | • | an annual cash retainer of $10,000 for serving as the Chairperson of the Audit Committee and of $5,000 for serving as the Chairperson of each of the Compensation Committee and the Nominating and Corporate Governance Committee; and |
| |
| | • | upon first joining the board, an initial grant of an option to purchase 8,500 shares of our common stock, and thereafter an annual grant of an option to purchase 2,830 shares of our common stock, with each such option vesting in equal monthly installments over a four-year period following the grant and subject to accelerated vesting in the event of a change in control. |
Directors who are employees of Mirion or its subsidiaries or affiliated with ACAS will receive no compensation for services as members of either our Board of Directors or committees. Outside directors not affiliated with ACAS will each receive an initial grant of 8,500 options with an exercise price equal to the initial public offering price upon the pricing of this offering.
105
We will reimburse all directors for reasonable expenses incurred to attend meetings of our Board of Directors or committees.
Code of Ethics and Conduct
On June 12, 2008, our Board of Directors adopted a revised Code of Ethics and Conduct that establishes the standards of ethical conduct applicable to all of our directors, officers and employees. The Code of Ethics and Conduct (the “Code of Conduct”) addresses, among other things, competition and fair dealing, conflicts of interest, financial matters and external reporting, company funds and assets, confidentiality and corporate opportunity requirements and the process for reporting violations of the Code of Conduct, employee misconduct, conflicts of interest or other violations.
Our Board of Directors on March 18, 2010 adopted a revised Code of Conduct that will become effective upon the consummation of this offering that revises, as necessary, the process for reporting violations of the Code of Conduct, employee misconduct, conflicts of interest or other violations to conform with applicable legal requirements of the United States and the other jurisdictions in which Mirion operates.
Our Code of Conduct will be publicly available on our website at www.mirion.com. Any waiver of the Code of Conduct with respect to any of our executive officers or directors may only be granted by the Board of Directors and must be disclosed to our stockholders.
106
EXECUTIVE COMPENSATION
Compensation Discussion and Analysis
The following discussion specifically relates to the compensation for fiscal 2010 of our “named executive officers” set forth in the Summary Compensation Table below, as well as discussing the overall principles underlying our executive compensation policies and decisions.
Objectives of Executive Compensation Program
The objectives of our executive compensation program are to recruit and retain an executive management team with the skills necessary to achieve our business objectives and thereby create value for our stockholders. Our executive compensation program is designed to support key business goals, such as integrating acquired businesses and retaining key executives, that are particularly important to us as a company with a limited operating history.
We implement this program through a combination of fixed cash compensation, variable short-term incentive compensation (determined by our operating performance as well as achievement of individual annual performance objectives), and equity incentives designed to reward long-term performance and align interests of our executive officers with our stockholders.
As a company whose equity was not publicly traded before this offering, our compensation philosophy has focused on the achievement of performance objectives that we believe would deliver meaningful return to our investors through a public offering or a sale of our company. In connection with this offering, we have reviewed our compensation philosophy and expect to adopt a compensation philosophy and objectives that are generally more consistent with those of a public, rather than private, company.
Executive Compensation Program
Our compensation program reflects our stage of development as a company. We have a limited operating history. We were incorporated in October 2005 and consist of a series of earlier acquisitions of geographically and technologically diverse companies. We have recruited several of our executive officers from other employers, and our initial compensation for these officers generally reflects the outcome of negotiated recruitment and hiring process.
As a company with a limited operating history, retention of executive officers is a key business objective. Weathering undesirable personnel changes would be more difficult for us than for a more established company. Accordingly, our Board of Directors believes it is critical to pay sufficient base compensation and provide adequate incentives to our executive officers to ensure continuity of our management team.
The Compensation Committee of the Board of Directors was established in July 2006. During fiscal 2010, Mr. Klein was the sole member of the Compensation Committee. Our current executive compensation policies and objectives were developed and implemented by the Compensation Committee while we were a private company. The Compensation Committee has allocated compensation between long-term and short-term compensation, between cash and non-cash compensation and among different forms of non-cash compensation in a manner considered to be typical of a private equity-backed enterprise. Our compensation program has focused on offering incentives necessary to recruit and retain executives from diverse backgrounds who possess the skills necessary to achieve our business objectives. We have not adopted any formal or informal policies or guidelines for allocating compensation between long-term and short-term compensation, between cash and non-cash compensation or among different forms of non-cash compensation.
Since the inception of the Compensation Committee, it has sought to review our executive officers’ compensation packages at least annually to determine whether they provide adequate incentives to achieve our business goals. In evaluating the market, the Compensation Committee has relied generally on its experience as well as market feedback and experience derived inherently from the process of recruiting new executives to our team. Our Chief Executive Officer recommends to the Compensation Committee compensation allocations
107
for our named executive officers other than himself. The Compensation Committee sets the compensation for our Chief Executive Officer.
During fiscal 2010, the Compensation Committee of our Board of Directors did not engage an outside compensation consultant to provide recommendations with respect to compensation arrangements or to assess the level or form of executive compensation. Following this offering, we expect to retain an outside consultant to provide recommendations with respect to future compensation arrangements for both executive officers and directors.
Elements of Compensation
The following describes each element of our executive compensation program and discusses determinations regarding compensation for fiscal 2010:
Base Compensation
Our Compensation Committee sets named executive officer base salaries based on the skills, experience and scope of responsibilities of each executive. Our Compensation Committee reviews base salaries at least annually. Base salaries are adjusted from time to time to reflect each executive’s overall contribution and to conform salaries to the compensation committee’s views of market levels. Our Compensation Committee has relied primarily on its experience to negotiate base salaries. Our named executive officers’ base salaries were determined initially in the context of negotiated employment agreements. The Compensation Committee agreed to increase the base compensation of our Chief Executive Officer as of January 1, 2009 to $325,000 to be closer to what it believes to be an appropriate level for a company preparing for an initial public offering, to $400,000 on the date of the initial public offering to reflect the significant increase in responsibilities of a chief executive officer of a public company and to $450,000 on the one-year anniversary of the initial public offering. With respect to our other named executive officers, we did not make material changes to base compensation during fiscal 2010. The Compensation Committee determined these amounts primarily based on its experience with other private and public companies, as well as its experience in determining pay levels required to recruit individuals in similar roles.
Annual Incentive Bonuses
For fiscal 2010, our executive bonus program provided for executives to receive a bonus based on the following factors:
| | |
| | • | the achievement of financialand/or operational goals for the fiscal year; |
| | |
| | • | achievement of individual annual performance objectives; and |
| | |
| | • | with respect to Messrs. Logan, Besso and Wilson (our named executive officers with operational responsibilities), commitment to future growth in revenue and earnings for the subsequent fiscal year in the financial forecast approved by our Board of Directors upon recommendation of our Chief Executive Officer. |
Annual performance objectives are approved by the Compensation Committee as part of the Board of Director’s review of our prior fiscal year financial results. Our Chief Executive Officer makes recommendations to our Compensation Committee regarding individual performance objectives and awards for named executive officers other than himself. Performance objectives and awards for our Chief Executive Officer are determined solely by the Compensation Committee. The Compensation Committee retains full discretion to determine the final bonus amounts and does not rely solely on our financial results (and, as further described below, the Compensation Committee exercised this discretion with respect to Mr. Besso’s bonus). For fiscal 2010, the initial potential bonus pool for each individual was based solely on our achievement of the financial goals specified by the Compensation Committee. Then, the actual bonus pool for each individual was determined as follows, subject to increase or decrease based on our goal of decreasing our working capital requirements (as further described below):
108
For Messrs. Logan, Besso and Wilson (our named executive officers with operational responsibilities):
| | |
| | • | 50% based solely on the achievement of Adjusted EBITDA, as described below; |
| | |
| | • | 25% based on attainment of personal objectives; and |
| | |
| | • | 25% based on financial objectives specified in the following year’s plan. |
For Messrs. Pacheco and Rosen (our named executives without operational responsibilities):
| | |
| | • | 50% based solely on the achievement of Adjusted EBITDA, as described below; and |
| | |
| | • | 50% based on attainment of personal objectives. |
Fiscal 2010 financial goal. For fiscal 2010, the Compensation Committee specified that the financial goal would be achievement of Adjusted EBITDA (or, for named executive officers who are division presidents, Adjusted EBITDA for the applicable division). We calculate Adjusted EBITDA as net income (loss), less extraordinary gains and losses and interest income, plus interest expense, charges against income for taxes, depreciation expense, amortization expense, non-recurring charges, management fees paid to ACFS and all non-cash compensation expenses. For more information about the calculation of Adjusted EBITDA and a reconciliation to cash provided by (used in) operating activities, see pages 10 and 11 of this prospectus. Adjusted EBITDA results are not set forth in our audited financial statements and our calculations of these goals as a private company may differ from actual audited results. For purposes of the bonus plan, the determination of Adjusted EBITDA is made on a currency-adjusted basis, so although the calculation is derived from our audited results, the numbers will not be the same as the audited results. Although we expect the financial results to require improved performance and exceptional work each year, the goals are set to be consistent with our business plan objectives and to be achievable.
Working Capital Adjustment. For fiscal 2010, the potential bonus pool (as a percentage of base salary) set forth below was subject to increase or decrease by up to 30% depending on how the percentage change in our working capital (generally defined for this purpose as accounts receivable plus inventory, minus the sum of accounts payable and non-financial accrued liabilities) compared to our percentage change in revenue. Because the working capital modifier depends on the change in working capital from year to year compared to the change in revenue from year to year, it is a relative formula rather than working capital being set at a specific target level. That is, if there was a 20% or greater increase in working capital versus revenue, the bonus pool would be decreased by 30%; if there was between a 10% and 20% increase in working capital versus revenue, the bonus pool would be decreased by 15%; if there was less than a 10% increase in working capital versus revenue or less than a 10% decrease in working capital versus revenue, there would be no change in the bonus pool; if there was between a 10% and 20% decrease in working capital versus revenue, the bonus pool would be increased by 15%; and if there was a 20% or greater decrease in working capital versus revenue, the bonus pool would be increased by 30%.
Individual target bonus. Each named executive officer was given a target bonus (expressed as a percentage of base salary), with a minimum threshold and maximum bonus based on financial goal performance (prior to any working capital adjustment). The following table shows the target bonus pool amounts (as a percentage of base salary) for each of our named executive officers for different levels of achievement of the Adjusted EBITDA financial target (which for fiscal 2010 was $45.0 million):
| | | | | | | | | | | | | | | | | |
| | | Below
| | 90% of
| | 100% of
| | 110% of
|
| | | Threshold | | Target | | Target | | Target or Above |
| |
| Thomas D. Logan | | $ | 0 | | | | 25% salary | | | | 50% salary | | | | 100% salary | |
| Jack A. Pacheco | | $ | 0 | | | | 25% salary | | | | 50% salary | | | | 100% salary | |
| Seth B. Rosen | | $ | 0 | | | | 25% salary | | | | 40% salary | | | | 80% salary | |
109
For each division president, target levels were based on Adjusted EBITDA for the particular business unit for which that division president is responsible (approximately $12 million for the Health Physics Division for Mr. Besso and approximately $10 million for the Sensing Systems Division for Mr. Wilson):
| | | | | | | | | | | | | | | | | |
| | | Below
| | $2.0 MM
| | 100% of
| | $2.0 MM
|
| | | Threshold | | Below Target | | Target | | Above Target |
| |
| W. Antony Besso | | $ | 0 | | | | 25% salary | | | | 50% salary | | | | 80% salary | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | Below
| | $1.5 MM
| | 100% of
| | $1.5 MM
|
| | | Threshold | | Below Target | | Target | | Above Target |
| Iain F. Wilson | | $ | 0 | | | | 25% salary | | | | 40% salary | | | | 80% salary | |
Although the determination of our financial performance is an important factor in approving the actual bonus payment, our Compensation Committee believes it is preferable to retain discretion to determine awards (including above or below the amounts in the table above) based on qualitative and quantitative contributions of our named executive officers.
Individual objectives. The Compensation Committee reviewed each named executive officer’s achievement of individual annual performance objectives and addition of long-term value, particularly in carrying out our integration plan and positioning us for this offering. Many of the individual performance objectives are subjectively determined, but for any individual executive officer, each performance objective generally carries the same weight as other objectives. The individual objectives vary because they are specific for each executive officer’s particular functions. For example, for fiscal 2010:
| | |
| | • | Mr. Logan’s objectives included positioning the company to complete our initial public offering and associated credit facility, commercializing and repositioning specific business lines and products, and launching internal productivity-improvement initiatives; |
| | |
| | • | Mr. Pacheco’s objectives focused on steps to position the company to complete our initial public offering and associated credit facility, audit objectives and organizational improvement; |
| | |
| | • | Mr. Rosen’s objectives focused on positioning the company to complete our initial public offering and associated credit facility, formalizing compliance programs and supporting internal training initiatives; |
| | |
| | • | Mr. Besso’s objectives mainly related to specific products and business lines for which he is responsible and launching internal initiatives to improve productivity; and |
| | |
| | • | Mr. Wilson’s objectives included developing and implementing business unit strategies in particular locations and launching internal initiatives to improve productivity. |
Determination of fiscal 2010 bonus amounts. In August 2010, the Compensation Committee determined performance results for fiscal 2010 and awarded bonuses in the amounts set forth under “Non-Equity Incentive Plan Compensation” in the Summary Compensation Table below. In determining the amount of the bonuses for the year, the Committee first reviewed our financial results. Our Adjusted EBITDA results were above the target level at the corporate level, and so the initial eligible bonus pool for Messrs. Logan, Pacheco and Rosen was interpolated between the target and maximum levels set forth in the table above. Our Adjusted EBITDA results for our Sensing Systems division exceeded our maximum target level, so the potential bonus pool for Mr. Wilson was the maximum amount in the table above. Our Adjusted EBITDA results for our Health Physics division fell below the minimum target level, so Mr. Besso was not eligible to receive a performance bonus under this plan (although as described below, the Compensation Committee exercised its discretion to award a bonus to Mr. Besso based on qualitative performance). The Compensation Committee then reviewed the working capital achievements and determined that we achieved the second tier of working capital improvement at the corporate level (that is, there was at least a 10% decrease in working capital versus revenue), and so the potential bonus pool for each of Messrs. Logan, Pacheco and Rosen was increased by 15%. The Sensing Systems Division also achieved the second tier of working capital improvement and so Mr. Wilson’s potential bonus pool was increased by 15%. The Committee then reviewed each named executive officer’s individual performance objectives, some of which require a subjective determination. After this review, each of our chief executive officer, chief financial officer, general counsel and Sensing Systems
110
division president received a bonus greater than his target bonus, as set forth in more detail in the table below, based on the following determinations:
For Messrs. Logan and Wilson:
| | |
| | • | For the 50% of bonus based on Adjusted EBITDA, we exceeded the target levels at the corporate level and for our Sensing Systems division and so achieved 100% of this metric for Messrs. Logan and Wilson. |
| | |
| | • | For the 25% of bonus based on personal objectives, the Committee made a subjective determination based on the individual performance factors described below the table to determine the percentage achievements set forth in the table below for each individual. |
| | |
| | • | For the 25% of bonus based on whether our performance situates us for a potentially higher level of achievement for the following year, the Committee determined that Mr. Logan met 50% of this objective at the corporate level because of our commitment to increasing revenue and that Mr. Wilson met 100% of this objective for the Sensing Systems division because of our commitment to increasing revenue and continued achievement of Adjusted EBITDA levels within this division. |
For Messrs. Pacheco and Rosen:
| | |
| | • | For the 50% of bonus based on Adjusted EBITDA, we exceeded the target levels at the corporate level and so achieved 100% of this metric; and |
| | |
| | • | For the 50% of bonus based on personal objectives, the Committee made a subjective determination based on the individual performance factors described below the table to determine the percentage achievements set forth in the table below for each individual. |
Although Mr. Besso was not eligible for a bonus because his division did not meet the threshold pre-set financial goal (and so he is not included in the following table), the Compensation Committee reviewed his qualitative efforts in adding long-term value and individual performance goals for fiscal 2010 (as described below) and determined to award him a discretionary bonus of €25,000 (or approximately $34,808 based on the average exchange rate for the year).
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | Working
| | | | | | |
| | | | | | | | | Capital
| | | | | | Payout
|
| | | | | | | | | Modifier
| | Eligible
| | | | (Weighted
|
| | | | | | | | | (added to
| | Bonus Pool
| | | | Achievement
|
| | | %
| | | | Weighted
| | Baseline%
| | (as% of
| | Eligible
| | x Eligible
|
| | | Achievement | | Weight | | Achievement | | of Salary) | | Salary) | | Bonus Pool | | Bonus Pool) |
Logan | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| 2010 Financial | | | 100 | % | | | 50 | % | | | 50 | % | | | | | | | | | | | | | | | | |
| Personal Objectives | | | 100 | % | | | 25 | % | | | 25 | % | | | | | | | | | | | | | | | | |
| Future Planning | | | 50 | %(1) | | | 25 | % | | | 12.5 | % | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| TOTAL | | | | | | | | | | | 87.5 | % | | | 15 | % | | | 74.12 | %(2) | | $ | 240,884 | | | $ | 210,774 | |
Pacheco | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| 2010 Financial | | | 100 | % | | | 50 | % | | | 50 | % | | | | | | | | | | | | | | | | |
| Personal Objectives | | | 75 | % | | | 50 | % | | | 37.5 | % | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| TOTAL | | | | | | | | | | | 87.5 | % | | | 15 | % | | | 74.12 | %(2) | | $ | 206,049 | | | $ | 180,292 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Rosen | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| 2010 Financial | | | 100 | % | | | 50 | % | | | 50 | % | | | | | | | | | | | | | | | | |
| Personal Objectives | | | 100 | % | | | 50 | % | | | 50 | % | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| TOTAL | | | | | | | | | | | 100 | % | | | 15 | % | | | 62.29 | %(2) | | $ | 155,736 | | | $ | 155,736 | |
111
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | Working
| | | | | | |
| | | | | | | | | Capital
| | | | | | Payout
|
| | | | | | | | | Modifier
| | Eligible
| | | | (Weighted
|
| | | | | | | | | (added to
| | Bonus Pool
| | | | Achievement
|
| | | %
| | | | Weighted
| | Baseline%
| | (as% of
| | Eligible
| | x Eligible
|
| | | Achievement | | Weight | | Achievement | | of Salary) | | Salary) | | Bonus Pool | | Bonus Pool) |
Wilson | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| 2010 Financial | | | 100 | % | | | 50 | % | | | 50 | % | | | | | | | | | | | | | | | | |
| Personal Objectives | | | 100 | % | | | 25 | % | | | 25 | % | | | | | | | | | | | | | | | | |
| Future Planning | | | 100 | %(1) | | | 25 | % | | | 25 | % | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| TOTAL | | | | | | | | | | | 100 | % | | | 15 | % | | | 95 | % | | | Cdn 206,293 | | | | Cdn 206,293 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | $ | (195,471 | ) |
| | |
| (1) | | For Mr. Logan, the Committee determined that the future planning target for revenue was met but the Adjusted EBITDA target was not met and so should result in 50% achievement of the future planning target. For Mr. Wilson, the Committee determined that the target for both Adjusted EBITDA and revenue were met for the Sensing Systems Division and should result in achievement of 100% of the future planning target. |
| | |
| (2) | | The initial bonus pool of 59.12% of base salary for Messrs. Logan and Pacheco, and 47.29% for Mr. Rosen, was determined by interpolating between the “target” and “maximum” levels shown in the first table above based on the corporate-level achievement of 2010 Adjusted EBITDA between those levels, and the 15% working capital modifier was added to this baseline percentage. For Mr. Wilson, a 15% working capital modifier was added to his baseline percentage of the maximum target bonus percentage. |
In approving the percentage of achievement for the “personal objectives” category in the table above (and in approving Mr. Besso’s discretionary bonus), the Compensation Committee considered the following successful individual performance factors:
| | |
| | • | Mr. Logan: Good efforts to complete the initial public offering and associated credit facility by completing all regulatory and legal requirements and completing the IPO road show; commercialization of new products and repositioning of certain existing product lines; and launching internal productivity-improvement initiatives. |
| | |
| | • | Mr. Pacheco: Good efforts to complete the initial public offering and associated credit facility by completing all regulatory and legal requirements and completing the IPO road show; organizational improvements; and, the timely completion of audits. |
| | |
| | • | Mr. Rosen: Good efforts to complete the initial public offering and associated credit facility by completing all regulatory and legal requirements and completing the IPO road show; enhancement of compliance programs; and completion of internal training initiatives. |
| | |
| | • | Mr. Besso: Successful launch of specific new products and business lines; and launching internal productivity-improvement projects. |
| | |
| | • | Mr. Wilson: Development of business unit strategies and new products; organizational improvements; and launching internal productivity-improvement projects. |
Fiscal 2011 actions. Going forward, the type of financial performance target we use, and individual performance objectives, may change if our Compensation Committee determines it would be appropriate to use different types of goals as a public company. For fiscal 2011 the Compensation Committee has revised the structure of the annual incentive bonus to remove the metric tied to commitment to future growth in revenue and earnings for the subsequent fiscal year in the financial forecast and remove the working capital modifier, although bonuses will be subject to reduction unless the Company pays down its total debt (subject to equitable adjustment to neutralize the effect of fees, costs, interest changes, or debt adjustments associated with an initial public offering, debt recapitalization, acquisitions, or divestitures).
112
IPO bonuses. We will pay bonuses to some of our employees, including two of our executive officers, upon completion of this initial public offering. Mr. Logan will be eligible to receive a bonus of $125,000 and Mr. Besso will be eligible to receive a bonus of up to $507,509. The amount of Mr. Besso’s bonus was negotiated with him in conjunction with efforts to retain him by fulfilling an earlier commitment to him and as a supplement to his stock options, which vest on an initial public offering.
Equity Awards
We design our equity programs to align employees’ interests with those of our stockholders by offering employees the opportunity to acquire stock and therefore have a direct interest in helping to increase the value of our stock. The design of these equity incentives is typical of the Compensation Committee’s experience with a private equity-backed enterprise. To date, we have granted stock options to our executive officers and a limited group of other employees. Stock options permit the employee to exercise the option at a fixed price (at or above the fair market value of the stock on the grant date) in the future after the option has become vested. Grants typically are made around the time an employee is hired, and we may make additional grants following a significant change in job responsibilities or to meet other specific retention objectives. Our Compensation Committee determines the size and type of equity awards taking into account the recommendations of management and also determines the vesting schedule of the options. The terms of initial equity grants made to each named executive upon joining the company are based primarily on competitive conditions applicable to the executive officers’ specific position. We did not grant equity awards to our executive officers during fiscal 2010. We made the equity awards reflected in the compensation tables below primarily in the context of negotiated employment agreements. As a private company, the overall size of our option pool was based on a range of potential dilution levels historically used by our majority stockholder with its other private companies. The chief executive officer receives the largest share of the option pool because he has the most significant impact on value. Other executive officers’ option amounts were then determined, although individual allocations were not based on a specific formula or value. Instead, the number of options depended on a combination of share availability in the option pool, negotiations at the time of offering employment to a new officer, the Compensation Committee’s expectation for the officer’s potential impact on future value of our company and the past experience of the Compensation Committee. We have not adopted stock ownership or equity grant guidelines, but following our public offering, we may implement guidelines regarding the issuance of new equity awards in the future.
Immediately following the pricing of this offering, we expect to grant stock options covering 124,448 shares of our common stock to our employees and outside directors, including grants of 17,008 stock options to Mr. Wilson and 17,000 stock options to Mr. Rosen. The per share exercise price of these options is expected to be equal to the initial public offering price (the fair market value on the grant date), and the options will become exercisable as they vest in equal monthly installments over four years following the grant. The determination of the individual amounts for Mr. Rosen and Mr. Wilson was based on the Compensation Committee’s subjective judgment regarding increased expectations in these positions once we become a public company and in order to further align these officers’ interests with those of stockholders given that they will be executive officers of a public company. In determining to make grants to these particular individuals and not the other executive officers, the Compensation Committee reviewed the number of outstanding equity awards held by each executive officer (as set forth under “Outstanding Equity Awards” below) and determined that because these individuals currently hold fewer stock options than the other executive officers, it would be appropriate to bring the equity ownership of the executive officers into closer alignment (while not committing to provide each officer with exactly the same equity amounts). The Compensation Committee did not base its decision on a third-party study or on market competitiveness.
Severance and Change in Control Arrangements
Each of our named executive officers, with the exception of Mr. Wilson, has an employment agreement that would provide severance on specified involuntary terminations of employment. We have also agreed to accelerated vesting of Messrs. Logan’s and Pacheco’s stock options in the event of certain change in control events and of Mr. Besso in the event of this initial public offering or a change in control. The terms and
113
estimated amounts of these benefits are described below under “Employment Agreements and Potential Payments upon Termination or Change in Control.” Based on the past experience of the Compensation Committee, we believe these arrangements are competitive with arrangements offered to senior executives by companies with whom we compete for executives and are necessary to the achievement of our business objective of management retention. Given our limited operating history, our Compensation Committee believes these provisions were a key part of hiring and retaining management to ensure continuity of our management team.
Perquisites and Other Benefits
Our named executive officers are eligible to participate in our employee benefit plans provided for employees which vary by country. In the United States, these benefits include a 401(k) plan with a matching contribution, group medical and dental insurance, group life insurance and short- and long-term disability insurance. As set forth in the Summary Compensation Table below, Messrs. Besso and Wilson receive specified benefits that are typical for executives in their locations, or required by law, but these additional benefits are limited in amount and scope.
Tax and Accounting Considerations
We recognize a charge to earnings for accounting purposes for equity awards granted. As we become a public company, we expect that the Compensation Committee will consider the accounting impact of equity awards in addition to the impact to dilution and overhang when deciding on amounts and terms of equity grants. We do not require executive compensation to be tax deductible for us, but instead balance the cost and benefits of tax deductibility to comply with our executive compensation goals, including the potential future effects of Section 162(m) of the Internal Revenue Code on the compensation paid to our executive officers. Section 162(m) disallows a tax deduction for any publicly held company for individual compensation exceeding $1 million in any taxable year for our Chief Executive Officer and each of the other named executive officers (other than our Chief Financial Officer), unless the compensation is performance-based.
114
Summary Compensation Table
The following table sets forth information concerning the compensation of our Chief Executive Officer, Chief Financial Officer and three other most highly compensated executive officers for fiscal 2009 and 2010. We refer to these individuals as our “named executive officers” elsewhere in this prospectus.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | Change
| | | | |
| | | | | | | | | | | | | in Pension
| | | | |
| | | | | | | | | | | | | Value and
| | | | |
| | | | | | | | | | | | | Nonqualified
| | | | |
| | | | | | | | | | | Non-Equity
| | Deferred
| | | | |
| | | | | | | | | Option
| | Incentive Plan
| | Compensation
| | All Other
| | |
Name and
| | | | Salary
| | Bonus
| | Awards
| | Compensation
| | Earnings
| | Compensation
| | Total
|
Principal Position | | Fiscal Year | | ($) | | ($) | | ($)(1) | | ($)(2) | | ($) | | ($)(3) | | ($) |
| |
Thomas D. Logan
President, Chief
Executive Officer and Chairman | | | 2010
2009 | | | | 325,000
312,473 | | | | —
— | | | | —
— | | | | 210,774
251,094 | | | | —
— | | | | 28,053
36,884 | | | | 563,827
600,451 | |
Jack A. Pacheco
Vice President and Chief Financial Officer | | | 2010
2009 | | | | 278,000
278,000 | | | | —
— | | | | —
— | | | | 180,292
199,813 | | | | —
— | | | | 10,497
6,906 | | | | 468,789
484,719 | |
Seth B. Rosen
General Counsel,
Vice President Corporate
Development, and
Secretary | | | 2010
2009 | | | | 250,000
232,000 | | | | —
— | | | | —
99,400 | | | | 155,736
154,667 | | | | —
— | | | | 6,405
6,853 | | | | 412,141
492,920 | |
W. Antony Besso(5)
Regional Vice
President, EMEA and
President, Health Physics
Division | | | 2010
2009 | | | | 307,829
303,861 | | | | 34,808 | (2)
— | | | —
598,742 | | | | —
184,586 | | | | 5,019 | (4)
— | | | 45,283
62,806 | | | | 392,939
1,149,995 | |
Iain F. Wilson(5)
Regional Vice President, Asia and President,
Sensing Systems Division | | | 2010
2009 | | | | 205,703
185,726 | | | | —
— | | | | —
248,499 | | | | 195,471
204,298 | | | | —
— | | | | 23,412
16,749 | | | | 424,586
655,272 | |
| | |
| (1) | | The amounts in this column do not reflect dollar amounts actually received by our named executive officers. Instead, these amounts reflect the aggregate grant date fair value of each stock option granted in the fiscal year ended June 30, 2009 and 2010 computed in accordance with the provisions of FASB ASC Topic 718. Assumptions used in the calculation of these amounts are included in Note 13 to our consolidated financial statements included in this prospectus. As required by SEC rules, the amounts shown exclude the impact of estimated forfeitures related to service-based vesting conditions. Our named executive officers will only realize compensation to the extent the trading price of our common stock is greater than the exercise price of such stock options. |
| | |
| (2) | | The amounts in this column represent payments under our executive bonus program for performance during fiscal 2009 and 2010, as described above under Compensation Discussion and Analysis. |
| | |
| (3) | | The amounts in this column include our matching 401(k) contributions for the following named executive officers’ accounts in the following amounts for fiscal 2009 and 2010, respectively: Mr. Logan ($5,702 and $6,752), Mr. Pacheco ($6,906 and $10,497) and Mr. Rosen ($6,853 and $6,405). For Mr. Logan, the amount also includes car allowance ($9,217 for both fiscal 2009 and 2010) and amounts paid for accrued vacation above the maximum accrual limit ($21,965 in fiscal 2009 and $12,084 in fiscal 2010) under our annual vacation cashout policy for executive officers. For Mr. Besso, the amount consists of his car allowance ($6,259 for fiscal 2009 and $6,629 for fiscal 2010), an allowance for travel ($8,670 for fiscal 2009 and $14,515 for fiscal 2010), child school allowance ($13,743 for fiscal 2009 and $13,923 for fiscal 2010), a housing allowance ($24,050 for fiscal 2009 and $0 for fiscal 2010), which was discontinued in February 2009, and private unemployment insurance ($10,083 for fiscal 2009 and $10,216 for fiscal 2010). For Mr. Wilson, this amount consists of car allowance ($5,183 for fiscal 2009 and $5,685 for fiscal 2010), contributions to his Registered Retirement Savings Plan, a defined contribution plan in Canada |
115
| | |
| | ($7,280 for fiscal 2009 and $8,230 for fiscal 2010) and vacation pay ($4,286 for fiscal 2009 and $9,497 for fiscal 2010). |
| | |
| (4) | | This amount represents the change in value under a mandatory French pension system, as described below under Pension Benefits. |
| | |
| (5) | | Mr. Besso’s compensation, which is paid in euros, and Mr. Wilson’s compensation, which is paid in Canadian dollars, have been converted into U.S. dollars using the respective average rate of exchange for the fiscal year. |
Grants of Plan-Based Awards for Fiscal 2010
The following table sets forth information concerning grants of plan-based awards made to the executive officers named in the Summary Compensation Table during fiscal 2010.
| | | | | | | | | | | | | | | | | |
| | | | | | Estimated Future
| |
| | | | | | Payouts Under
| |
| | | | | | Non-Equity Incentive
| |
| | | | | | Plan Awards(1) | |
| | | Grant
| | | Threshold
| | | Target
| | | Maximum
| |
Name | | Date | | | ($) | | | ($) | | | ($) | |
| |
| Thomas D. Logan | | | 09/08/09 | | | | 81,250 | | | | 162,500 | | | | 325,000 | |
| Jack A. Pacheco | | | 09/08/09 | | | | 69,500 | | | | 139,000 | | | | 278,000 | |
| Seth B. Rosen | | | 09/08/09 | | | | 62,500 | | | | 100,000 | | | | 200,000 | |
| W. Antony Besso(2) | | | 09/08/09 | | | | 76,993 | | | | 153,985 | | | | 246,376 | |
| Iain F. Wilson(2) | | | 09/08/09 | | | | 51,439 | | | | 82,303 | | | | 164,607 | |
| | |
| (1) | | Threshold, Target and Maximum amounts refer to the annualized eligible bonus for each named executive officer if specified financial performance criteria were met, as more fully discussed above in the “Compensation Discussion and Analysis” and below under “Executive Bonus Program.” The actual annual performance bonus payable is subject to determination by the Compensation Committee after a review of the financial performance of Mirion or the applicable business unit, as well as each named executive officer’s achievement of their individual annual performance objectives, which may result in a higher or lower actual bonus payment. See “Compensation Discussion and Analysis—Elements of Compensation.” Actual amounts paid for fiscal 2009 and 2010 are set forth in the Summary Compensation Table above. |
| | |
| (2) | | Foreign currency amounts have been converted into U.S. dollars using the average rate of exchange for the fiscal year. |
Executive Bonus Program. As further described in the Compensation Discussion and Analysis above, the awards for fiscal 2010 were first determined based on our corporate Adjusted EBITDA performance (or business segment for each of Messrs. Besso and Wilson) and working capital, but are also subject to determination of individual performance and other factors. Each of the executive bonus awards would be 0% of base salary if the performance level was below a specified threshold of performance. Between the threshold level and target level, and between the target level (payable if 100% of plan was achieved) and the maximum level, amounts would be interpolated. The Compensation Committee retains discretion to pay amounts over the maximum level for exceptional performance. Each executive officer’s target bonus amount is set as a percentage of base salary, with target amounts for fiscal 2010 of 50% of base salary for Messrs. Logan, Pacheco and Besso and 40% of base salary for Messrs. Rosen and Wilson.
116
Outstanding Equity Awards at Fiscal Year-End June 30, 2010
The following table sets forth information concerning unexercised stock options for the executive officers named in the Summary Compensation Table as of the end of fiscal 2010. There were no unvested stock awards outstanding as of the end of the fiscal year.
| | | | | | | | | | | | | | | | | | | | | |
| | | Option Awards | |
| | | | | | | | | Number of
| | | | | | | |
| | | Numbers of
| | | Numbers of
| | | Securities
| | | | | | | |
| | | Securities
| | | Securities
| | | Underlying
| | | | | | | |
| | | Underlying
| | | Underlying
| | | Unexercised
| | | Option
| | | | |
| | | Unexercised
| | | Unexercised
| | | Unearned
| | | Exercise
| | | Option
| |
| | | Options (#)
| | | Options (#)
| | | Options
| | | Price
| | | Expiration
| |
Name | | Exercisable | | | Unexercisable | | | (#) | | | ($) | | | Date | |
| |
| Thomas D. Logan | | | 123,794 | | | | | | | | | | | $ | 10.45 | | | | 1/1/16 | |
| | | | 150,875 | (1) | | | — | | | | 463,794 | (1) | | | 10.45 | | | | 8/18/14 | |
| Jack A. Pacheco | | | 79,330 | (2) | | | 56,670 | (2) | | | | | | | 13.10 | | | | 3/31/18 | |
| Seth B. Rosen | | | 42,499 | (3) | | | 25,501 | (3) | | | | | | | 16.31 | | | | 1/7/18 | |
| | | | 8,143 | | | | 8,857 | (4) | | | | | | | 17.06 | | | | 8/5/18 | |
| W. Antony Besso | | | 75,749 | | | | 32,464 | (5) | | | | | | | 17.06 | | | | 8/5/18 | |
| Iain F. Wilson | | | 15,614 | | | | — | | | | | | | | 10.45 | | | | 1/1/16 | |
| | | | 15,614 | | | | — | | | | | | | | 10.45 | | | | 1/1/16 | |
| | | | 6,383 | (6) | | | 4,879 | (6) | | | | | | | 14.27 | | | | 9/6/17 | |
| | | | 20,366 | | | | 22,134 | (4) | | | | | | | 17.06 | | | | 8/5/18 | |
| | |
| (1) | | These options were not granted by us, and represent options to purchase shares of Mirion stock from ACAS and its affiliates. The unearned portion of these options is subject to performance and market vesting following this offering, as described further under “Certain Relationships and Related Party Transactions—Interested Transactions—Transactions with Management.” |
| |
| (2) | | Options vest in equal monthly installments over four years from March 31, 2008. |
| |
| (3) | | Options vest in equal monthly installments over four years from January 7, 2008. |
| |
| (4) | | 25% of options vest on August 5, 2009, and thereafter the remaining 75% of options vest in equal monthly installments over three years. |
| |
| (5) | | 25% of options vest on August 5, 2009, and thereafter the remaining 75% of options vest in equal quarterly installments over three years, subject to full accelerated vesting upon the consummation of this offering. |
| |
| (6) | | Options vest in equal monthly installments over five years from September 6, 2007. |
Option Exercises and Stock Vested for Fiscal 2010
No executive officers named in the Summary Compensation Table above exercised any stock options, or had any stock award become vested, during fiscal 2010.
Pension Benefits
Upon his retirement, Mr. Besso will be eligible for benefits under a mandatory French pension system, and we will pay Mr. Besso a lump-sum length of service award at his retirement based on his years of service and salary.
| | | | | | | | | | | |
| | | | | Number of
| | Present Value
| | Payments
|
| | | | | Years of
| | of Accumulated
| | During
|
Name | | Plan Name | | Credited Service | | Benefits | | Last Fiscal Year |
| |
| W. Antony Besso | | French pension system | | 4 | | $ | 10,997 | (1) | | — |
| | |
| (1) | | This amount has been converted from euros into U.S. dollars using the average rate of exchange for fiscal year 2010. For information on the valuation assumptions, see Note 11 to our consolidated financial statements. |
117
Employment Agreements and Potential Payments on Termination and Change of Control
We have entered into employment agreements with each of our named executive officers as described below. Generally, these agreements were the result of negotiations with the executive and provide that we will pay severance benefits to an executive if he is terminated without cause or resigns for good reason (which generally includes a material reduction in compensation or duties or a significant relocation), subject to the executive signing a general release of claims. In addition, our Chief Executive Officer and our Chief Financial Officer, as well as Mr. Besso, would receive accelerated vesting of some of their equity awards on change in control transactions, as further described below.
Thomas D. Logan. Under the terms of his August 2006 employment agreement, as amended through June 2010, if Mr. Logan is involuntarily terminated without cause or resigns for good reason, he will be entitled to receive the following benefits if he signs a general release:
| | |
| | • | an amount equal to his annual base salary; |
| |
| | • | a pro rata portion of his incentive bonus, if any, for the applicable period during the fiscal year in which termination occurs; and |
| |
| | • | continuation of all health benefits offered to our senior executives for one year after the date of termination. |
In addition, Mr. Logan’s Employment Agreement provides that upon a change in control, 100% of his unvested options will vest and, if applicable, he will receive reimbursement for excise taxes imposed on him as a result of Section 280G of the Internal Revenue Code, and that 50% of his unvested options will vest upon the consummation of this offering. Further, Mr. Logan’s Employment Agreement provides for a one-time bonus upon the consummation of this offering, equal to $50,000 plus an amount not to exceed $75,000, with such additional amount to be determined based upon the time taken to complete this offering.
Jack A. Pacheco. Under the terms of his March 2008 employment agreement, as amended in December 2008, if Mr. Pacheco is involuntarily terminated without cause or resigns for good reason, he will be entitled to receive the following benefits if he signs a general release:
| | |
| | • | an amount equal to his annual base salary; and |
| |
| | • | a pro rata portion of his incentive bonus, if any, for the applicable period during the fiscal year in which termination occurs. |
In addition, his employment agreement provides that 100% of his unvested options will vest in the event that either (i) ACAS or its affiliates no longer own at least 50% of the outstanding capital stock of us; provided that no such vesting shall occur as a result of the initial public offering of our capital stock or a company affiliated with us formed for the purpose of an initial public offering; or (ii) all or substantially all of our assets are sold, transferred or disposed of to a person (or group of persons acting in concert) that is not an affiliate of ACAS).
Seth B. Rosen. Under the terms of his January 2008 employment agreement, as amended in December 2008, if Mr. Rosen is involuntarily terminated without cause or resigns for good reason, he will be entitled to receive the following benefits if he signs a general release:
| | |
| | • | an amount equal to his annual base salary; |
| |
| | • | a pro rata portion of his incentive bonus, if any, for the applicable period during the fiscal year in which termination occurs; and |
| |
| | • | continued payment by us for a maximum of 12 months of his health coverage premiums under COBRA. |
Iain F. Wilson. There are no specific requirements with respect to any obligations of us in connection with a termination of Mr. Wilson’s employment under any employment agreement. Pursuant to Canadian law, executive officers may be entitled to benefits or a notice period upon termination of employment, depending on length of service and other factors.
118
W. Antony Besso. Mr. Besso’s employment agreement is governed by French law. Under the terms of his 2006 employment agreement, as amended in November 2007, if Mr. Besso is terminated, he will be entitled to receive the following benefits:
| | |
| | • | an amount equal to 12 months of remuneration, consisting of base salary, incentive bonus, and all other bonuses and benefits received by Mr. Besso during the last twelve months preceding his termination; and |
| |
| | • | any payments under the applicable collective bargaining agreement. |
Mr. Besso’s agreement provides that either party may terminate the agreement with three months notice. The agreement includes a non-competition covenant for two years following the termination of Mr. Besso’s employment if we pay to Mr. Besso an amount not to exceed6/10ths of the monthly average of specified pay and benefits from the last 12 months of his employment. In addition, Mr. Besso received stock options in August 2008 that provided for accelerated vesting upon a change in control or an initial public offering.
Potential Termination and Change in Control Benefits. The table below provides an estimate of the value of the compensation and benefits due to each of our named executive officers in the event of: (i) an involuntary termination; (ii) death or disability; or (iii) a change in control. The amounts shown assume that the specified event was effective as of June 30, 2010. The actual amounts to be paid can only be determined at the time of the termination of employment or change in control, as applicable.
| | | | | | | | | | | | | |
| | | Involuntary
| | | Disability
| | | Change in
| |
| | | Termination
| | | or Death
| | | Control
| |
Name | | ($) | | | ($) | | | ($) | |
| |
| Thomas D. Logan | | | 554,516 | (1) | | | 229,516 | (2) | | | | (3) |
| Jack A. Pacheco | | | 458,292 | (1) | | | 180,292 | (2) | | | | (3) |
| Seth B. Rosen | | | 424,478 | (1) | | | 155,736 | (2) | | | — | |
| W. Antony Besso | | | 537,698 | (4) | | | — | | | | | (3) |
| Iain F. Wilson | | | — | (5) | | | — | | | | — | |
| | |
| (1) | | Consists of payments due on a termination without cause or resignation for good reason, subject to the executive signing a release. This amount consists of (i) 100% of annual base salary, (ii) a pro rata portion of any incentive bonus (which for a termination at June 30, 2010, we have assumed to be the actual bonuses for fiscal 2010), and (iii) our payments for continued health benefits in the case of Mr. Logan and Mr. Rosen. Such amount would be payable at the same time as such payment would be made while the executive was employed with us. This amount does not include any amounts that are accrued and owing at the time of termination (such as accrued vacation and salary through the date of termination). |
| | |
| (2) | | Consists of pro rata portion of any incentive bonus (which we have assumed to be 100% of the target bonus) and, for Mr. Logan, continued health benefits for his family for 12 months. |
| | |
| (3) | | For Mr. Logan, this amount reflects (i) 100% vesting of any unvested stock options and (ii) remittance of net proceeds upon the sale by ACAS of vested and unexercised IRR Options under the Call Option Agreement. For Mr. Pacheco, this reflects 100% vesting of any unvested stock options in the event that (i) ACAS no longer owns at least 50% of our outstanding capital stock, or (ii) all or substantially all of our assets are sold, transferred or disposed of. For Mr. Besso, this reflects 100% vesting of any unvested stock options from an August 2008 grant which will vest in the event of a change in control or upon the consummation of this offering. The dollar value in each case is based on an assumed initial public offering price of $ (the midpoint of the price range set forth on the cover page of this prospectus), but otherwise assumes the transaction occurred based on unvested options at June 30, 2010. |
| | |
| (4) | | Amount includes 12 months of salary, fiscal 2009 bonus (paid in fiscal 2010) and other compensation paid for fiscal 2010. Amount does not include three months of notice and assumes we do not elect to pay for Mr. Besso’s continued non-competition agreement, as described above. |
| | |
| (5) | | Does not include amounts that may be payable as required by law. |
119
Employee Benefit Plans
Stock Plan
The following contains a summary of the material terms of our 2006 Stock Plan, which was originally approved in December 2005. On March 18, 2010, our Board of Directors and stockholders approved amendments to this plan, and we refer to the amended plan as the Stock Plan below.
Share Reserve. As of June 30, 2010, an aggregate of 1,008,865 shares of our common stock were reserved for issuance, options to purchase 924,830 shares were outstanding and 84,035 shares were available for future grant under the Stock Plan. Prior to the consummation of this offering, we expect to reserve 902,360 additional shares under the Stock Plan. In general, if options or other awards granted under the Stock Plan expire, are cancelled, forfeited or otherwise terminated before being exercised or settled, the shares subject to such options or awards will again become available for awards under the Stock Plan.
Administration of the Stock Plan. The Stock Plan will be administered by our Board of Directors or our Compensation Committee or another committee designated by our Board of Directors. The administrator has complete discretion to make all decisions relating to the interpretation and operation of the Stock Plan. The administrator will have the discretion to determine who will receive an award, the type of award, the number of shares that will be covered by the award, the vesting requirements of the award, if any, and all other features and conditions of the award. The administrator may implement rules and procedures that differ from those described below in order to adapt the Stock Plan to the requirements of countries other than the United States.
Eligibility. Any employee, consultant or non-employee director may be selected by the administrator to participate in the Stock Plan. Directors affiliated with ACAS and its affiliates are not expected to receive grants.
Type of Awards. To date, we have granted options under the Stock Plan. Following this offering, awards granted under the Stock Plan may include any of the following:
| | |
| | • | stock options to purchase shares of our common stock at a specified exercise price; |
| |
| | • | restricted stock units, representing the right to receive a specified number of shares of our common stock, the fair market value of such common stock in cash or a combination of cash and shares upon expiration of the vesting period specified for such stock units by the administrator; |
| |
| | • | restricted shares, which are shares of common stock issued to the participant subject to such forfeiture and other restrictions as the administrator, in its sole discretion, shall determine; |
| |
| | • | stock appreciation rights, which are rights to receive shares of our common stock, cash or a combination of shares and cash, the value of which is equal to the spread or excess of (i) the fair market value per share on the date of exercise over (ii) the fair market value per share on the date of grant with respect to a specified number of shares of common stock; and |
| |
| | • | other equity-based awards. |
Vesting of Awards. Equity awards vest at the time or times determined by the administrator. In most cases, our options granted to date vest over the four-year period following the date of grant, but the administrator has the discretion to determine the vesting schedule and whether the vesting will accelerate on events such as death, disability, change in control or involuntary termination of employment. In addition, the administrator may grant performance awards based on performance criteria measured over a specified period.
Other Terms of Awards. After termination of service by an employee, director or consultant, for any reason other than misconduct, he or she has a period of 30 days (or such other period as specified in an award agreement) following the date of termination during which to exercise his or her option. The administrator may, at its discretion, extend the period of time for which the option is to remain exercisable, but no option may be exercisable after the expiration of its term.
Change in Control. In the event of a merger or consolidation of us, all outstanding awards will be subject to the agreement of merger or consolidation, which may provide for the continuation or assumption of
120
outstanding awards; substitution with substantially similar awards; accelerated vesting of awards; or cancellation of awards in exchange for a cash payment equal to the fair market value of the shares over the applicable purchase price of the award.
Amendment and Termination of Plan. Our Board of Directors may amend or terminate the Stock Plan at any time. No amendment can be effective prior to its approval by our stockholders, to the extent that such approval is required by applicable legal requirements or any exchange on which our common stock is listed. The Stock Plan will continue in effect for ten years from the most recent increase in the Stock Plan’s share reserve approved by stockholders, unless our Board of Directors decides to terminate the plan earlier.
Limitation of Liability and Indemnification of Officers and Directors
Our amended and restated Certificate of Incorporation and Bylaws contain provisions that limit the personal liability of our directors for monetary damages to the fullest extent permitted by Delaware law. Consequently, our directors will not be personally liable to us or our stockholders for monetary damages or any breach of fiduciary duties as directors, except liability for:
| | |
| | • | any breach of the director’s duty of loyalty to us or our stockholders; |
| |
| | • | any act or omission not in good faith or that involves intentional misconduct or a knowing violation of law; |
| |
| | • | unlawful payments of dividends, or unlawful stock repurchases or redemptions as provided in Section 174 of the Delaware General Corporation Law; or |
| |
| | • | any transaction from which the director derived an improper personal benefit. |
Our amended and restated Certificate of Incorporation and Bylaws provide that we must indemnify our directors and officers to the fullest extent permitted by Delaware law. Our amended and restated Certificate of Incorporation and Bylaws also provide that we shall advance expenses incurred by a director or officer in advance of the final disposition of any action or proceeding, and permit us to secure insurance on behalf of any officer, director, employee or other agent for any liability arising out of his or her actions in that capacity, regardless of whether we would otherwise be permitted to indemnify him or her under the provisions of Delaware law. Upon the consummation of this offering, we expect to enter into agreements to indemnify our directors and executive officers, and other employees as determined by our Board of Directors, against expenses and liabilities to the fullest extent permitted by Delaware law. With certain exceptions, these agreements will also provide for indemnification for related expenses including, among others, attorneys’ fees, judgments, fines and settlement amounts incurred by any of these individuals in any action or proceeding. The indemnification agreements will also provide for indemnified directors and officers to select the method by which a determination of eligibility for indemnification is made. We believe that these bylaw provisions and indemnification agreements are necessary to attract and retain qualified persons as directors and officers. We also maintain directors’ and officers’ liability insurance.
The limitation of liability and indemnification provisions in our amended and restated Certificate of Incorporation and Bylaws may discourage stockholders from bringing a lawsuit against our directors for breach of their fiduciary duty of care. They may also reduce the likelihood of derivative litigation against our directors and officers, even though an action, if successful, might benefit us and other stockholders. Further, a stockholder’s investment may be adversely affected to the extent that we pay the costs of settlement and damage awards against directors and officers. At present, there is no pending litigation or proceeding involving any of our directors, officers or employees for which indemnification is sought, and we are not aware of any threatened litigation that may result in claims for indemnification.
121
PRINCIPAL AND SELLING STOCKHOLDERS
The following table sets forth certain information regarding the beneficial ownership of our common stock by (1) each person known by us to be the beneficial owner of 5% or more of the outstanding common stock, (2) each of our directors and each director nominee, (3) each of the executive officers named in the section entitled “Management” above, (4) all of our executive officers, directors and director nominees as a group and (5) all selling stockholders.
Percentage of ownership is based on 11,271,548 shares of common stock outstanding as of August 31, 2010. Beneficial ownership is calculated based on SEC requirements. These requirements also treat as outstanding all shares of common stock that a person would receive upon exercise of stock options or warrants held by that person that are immediately exercisable or exercisable within 60 days of the determination date, which in the case of the following table is August 31, 2010. Shares issuable pursuant to stock options and warrants exercisable within 60 days are deemed outstanding and held by the holder of such options or warrants for computing the percentage of the person holding such options or warrants, but are not deemed outstanding for computing the percentage of any other person. To our knowledge, except as indicated in the footnotes to this table and pursuant to applicable community property laws, the persons named in the table have sole voting and investment power with respect to all shares of common stock.
Other than as specifically noted below, the address of each of the named entities or individuals isc/o Mirion Technologies, Inc., 3000 Executive Parkway Suite 222, San Ramon, California 94583.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | Common Stock
| | | | | | | |
| | | | | | | | | | | | Beneficially Owned
| | | Common Stock
| |
| | | Common Stock
| | | Number of
| | | After the Offering
| | | Beneficially Owned
| |
| | | Beneficially Owned
| | | Shares Being Offered | | | WithoutOver-
| | | After the Offering
| |
| | | Prior to the Offering | | | Without
| | | With
| | | Allotment | | | With Over-Allotment | |
Beneficial Owner | | Number | | | Percentage | | | Overallotment | | | Overallotment | | | Number | | | Percentage | | | Number | | | Percentage | |
| |
Greater than 5% Stockholders: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| American Capital, Ltd. and affiliated entities(1) | | | 14,427,445 | | | | 99.5 | % | | | | | | | | | | | | | | | | | | | | | | | | |
2 Bethesda Metro Center
14th Floor Bethesda, MD 20814 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| American Capital Equity I, LLC(3) | | | 4,321,766 | | | | 35.3 | % | | | | | | | | | | | | | | | | | | | | | | | | |
2 Bethesda Metro Center
14th Floor Bethesda, MD 20814 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| American Capital Equity II, LP(4) | | | 1,685,964 | | | | 14.5 | % | | | | | | | | | | | | | | | | | | | | | | | | |
2 Bethesda Metro Center
14th Floor Bethesda, MD 20814 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Named Executive Officers, Directors and Director Nominees: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Thomas D. Logan(5) | | | 339,865 | | | | 2.9 | % | | | | | | | | | | | | | | | | | | | | | | | | |
| Jack A. Pacheco(6) | | | 87,830 | | | | | * | | | | | | | | | | | | | | | | | | | | | | | | |
| Seth B. Rosen(7) | | | 55,954 | | | | | * | | | | | | | | | | | | | | | | | | | | | | | | |
| W. Antony Besso(8) | | | 109,520 | | | | | * | | | | | | | | | | | | | | | | | | | | | | | | |
| Iain F. Wilson(9) | | | 61,199 | | | | | * | | | | | | | | | | | | | | | | | | | | | | | | |
| Robert J. Klein | | | 14,427,445 | | | | 99.5 | % | | | | | | | | | | | | | | | | | | | | | | | | |
| Dustin G. Smith(10) | | | 14,427,445 | | | | 99.5 | % | | | | | | | | | | | | | | | | | | | | | | | | |
| Brian S. Graff(10) | | | 14,427,445 | | | | 99.5 | % | | | | | | | | | | | | | | | | | | | | | | | | |
| Michael T. Everett | | | 0 | | | | | * | | | | | | | | | | | | | | | | | | | | | | | | |
| Earl R. Lewis | | | 0 | | | | | * | | | | | | | | | | | | | | | | | | | | | | | | |
| Alfred E. Barry, Jr. | | | 0 | | | | | * | | | | | | | | | | | | | | | | | | | | | | | | |
| All Executive Officers and Directors as a group (11 persons) | | | 15,081,813 | | | | 99.9 | % | | | | | | | | | | | | | | | | | | | | | | | | |
| | |
| (1) | | Includes 208,275 shares of Class B Non-Voting Common Stock held of record, 574,555 shares ofSeries A-1 Convertible Participating Preferred Stock and 89,737 shares ofSeries A-2 Convertible Participating Preferred |
122
| | |
| | Stock, as-converted to 6,323,114 shares of common stock. Includes a warrant to purchase 1,888,326 shares of common stock that will become exercisable upon the consummation of this offering. The members of the Board of Directors of American Capital, Ltd. are Mary Baskin, Neil Hahl, Philip Harper, John Koskinen, Stan Lundine, Kenneth Peterson, Alvin Puryear and Malon Wilkus. The Board of Directors manages these shares and exercises voting and investment power on behalf of American Capital, Ltd. As the directors of American Capital, Ltd., these individuals may be deemed to have shared voting and investment power over the shares held by American Capital, Ltd., including the power to dispose, or to direct the disposition of, such shares. Each of these individuals disclaims beneficial ownership of such shares, except to the extent of his or her pecuniary interest therein. Also includes 4,321,766 shares held directly by American Capital Equity I, LLC (“ACE I”), and 1,685,964 shares held directly by American Capital Equity II, LP (“ACE II”). See footnotes (3) and (4) below. Mr. Klein, one of our directors, is a Managing Director and Senior Vice President at American Capital, Ltd. and Messrs. Smith and Graff, two of our director nominees, are a Principal and Vice President and a Senior Vice President and Senior Managing Director at American Capital, Ltd., respectively, and each as a result may be deemed to have indirect shared voting and investment power over the shares held by American Capital, Ltd. and be deemed to be a beneficial owner for purposes of sections 13(d) and 13(g) of the Securities Exchange Act of 1934, as amended. Each of Messrs. Klein, Smith and Graff disclaim any beneficial ownership over such shares. |
| | |
| (2) | | Represents shares of our common stock to be issued upon the conversion of shares of our convertible preferred stock held by such selling stockholders. |
| | |
| (3) | | Includes 107,185 shares of Class B Non-Voting Common Stock held of record, 295,672 shares ofSeries A-1 Convertible Participating Preferred Stock and 46,179 shares ofSeries A-2 Convertible Participating Preferred Stock, as-converted to 3,253,937 shares of common stock. Includes a warrant to purchase 960,644 shares of common stock that will become exercisable upon the consummation of this offering. American Capital Equity Management, LLC (“ACEM”), a portfolio company of American Capital, Ltd., is the manager of this entity, and pursuant to an operating agreement, ACEM exercises voting power on behalf of ACE I. The members of the Board of Directors of American Capital, Ltd. are Mary Baskin, Neil Hahl, Philip Harper, John Koskinen, Stan Lundine, Kenneth Peterson, Alvin Puryear and Malon Wilkus. The Board of Directors manages these shares and exercises voting and investment power on behalf of American Capital, Ltd. As the directors of American Capital, Ltd., these individuals may be deemed to have shared voting and investment power over the shares held by American Capital, Ltd., including the power to dispose, or to direct the disposition of, such shares. Each of these individuals disclaims beneficial ownership of such shares, except to the extent of his or her pecuniary interest therein. See footnote (1). Mr. Klein, one of our directors, is a Managing Director and Senior Vice President at American Capital, Ltd. and Messrs. Smith and Graff, two of our director nominees, are a Principal and Vice President and a Senior Vice President and Senior Managing Director at American Capital, Ltd., respectively, and each as a result may be deemed to have indirect shared voting and investment power over the shares held by American Capital, Ltd. and be deemed to be a beneficial owner for purposes of sections 13(d) and 13(g) of the Securities Exchange Act of 1934, as amended. Each of Messrs. Klein, Smith and Graff disclaim any beneficial ownership over such shares. |
| | |
| (4) | | Includes 41,811 shares of Class B Non-Voting Common Stock held of record, 115,346 shares ofSeries A-1 Convertible Participating Preferred Stock and 18,014 shares ofSeries A-2 Convertible Participating Preferred Stock, as-converted to 1,269,397 shares of common stock. Includes a warrant to purchase 374,756 shares of common stock that will become exercisable upon the consummation of this offering. American Capital Equity Management II, LLC (“ACEM II”), a portfolio company of American Capital, Ltd., is the general partner of this entity, and pursuant to a management agreement, ACEM II exercises voting power on behalf of ACE II. The members of the Board of Directors of American Capital, Ltd. are Mary Baskin, Neil Hahl, Philip Harper, John Koskinen, Stan Lundine, Kenneth Peterson, Alvin Puryear and Malon Wilkus. The Board of Directors manages these shares and exercises voting and investment power on behalf of American Capital, Ltd. As the directors of American Capital, Ltd., these individuals may be deemed to have shared voting and investment power over the shares held by American Capital, Ltd., including the power to dispose, or to direct the disposition of, such shares. Each of these individuals disclaims beneficial ownership of such shares, except to the extent of his or her |
123
| | |
| | pecuniary interest therein. See footnote (1). Mr. Klein, one of our directors, is a Managing Director and Senior Vice President at American Capital, Ltd. and Messrs. Smith and Graff, two of our director nominees, are a Principal and Vice President and a Senior Vice President and Senior Managing Director at American Capital, Ltd., respectively, and each as a result may be deemed to have indirect shared voting and investment power over the shares held by American Capital, Ltd. and be deemed to be a beneficial owner for purposes of sections 13(d) and 13(g) of the Securities Exchange Act of 1934, as amended. Each of Messrs. Klein, Smith and Graff disclaim any beneficial ownership over such shares. |
| | |
| (5) | | Consists of 17,773 shares of Class A Voting Common Stock held of record, 1,338 shares ofSeries A-1 Convertible Participating Preferred Stock as-converted to 12,888 shares of common stock, options to purchase 123,794 shares of common stock that are exercisable within 60 days of August 31, 2010 and options to purchase 150,875 shares of common stock held by ACAS that are exercisable within 60 days of August 31, 2010. Includes a warrant to purchase 34,535 shares of common stock that will become exercisable upon consummation of this offering. |
| | |
| (6) | | Consists of options to purchase 87,830 shares of common stock that are exercisable within 60 days of August 31, 2010. |
| | |
| (7) | | Consists of options to purchase 55,954 shares of common stock that are exercisable within 60 days of August 31, 2010. |
| | |
| (8) | | Consists of 22,950 shares of Class B Non-Voting Common Stock held of record and options to purchase 86,570 shares of common stock that are exercisable within 60 days of August 31, 2010. |
| | |
| (9) | | Consists of options to purchase 61,199 shares of common stock that are exercisable within 60 days of August 31, 2010. |
| | |
| (10) | | Mr. Klein, one of our directors, is a Managing Director and Senior Vice President at American Capital, Ltd. and Messrs. Smith and Graff, two of our director nominees, are a Principal and Vice President and a Senior Vice President and Senior Managing Director at American Capital, Ltd., respectively, and each as a result are deemed to have indirect shared voting and investment power over the shares held by American Capital, Ltd. and may be deemed to be a beneficial owner for purposes of sections 13(d) and 13(g) of the Securities Exchange Act of 1934, as amended. Each of Messrs. Klein, Smith and Graff disclaim any beneficial ownership over such shares. |
Pledge of ACAS-owned Capital Stock
On June 28, 2010, American Capital, Ltd. completed the Restructuring. The new debt issued upon conversion of the existing line of credit and exchange of existing public and private notes is in the form of (i) secured loans made under the Term Loan and (ii) Secured Notes issued under the Indenture. The Term Loan and the Secured Notes are collectively secured by the Pledge. The shares of our capital stock beneficially owned by American Capital Equity I, LLC and American Capital Equity II, LP are not subject to the Pledge.
The acquisition by ACAS’ lenders of the shares of our capital stock subject to the Pledge would not entitle our executive officers to any potential change in control payments or other benefits or trigger the accelerated vesting of options held by Thomas D. Logan, our President, Chief Executive Officer and Chairman of the Board, to purchase shares of our common stock from ACAS pursuant to the Call Option Agreement. In addition, the pledgees would not succeed to any of the rights specifically reserved to ACAS under our amended and restated Certificate of Incorporation and Bylaws, but under certain circumstances, may succeed to the registration rights agreement which we expect to enter into upon the consummation of this offering. See “Executive Compensation — Employment Agreements and Potential Payments on Termination and Change of Control” for further information regarding the change in control benefits of our executive officers, see “Certain Relationships and Related Party Transactions — Interested Transactions — Transactions with Management” for further information regarding the Call Option Agreement and see “Description of Capital Stock” for further information regarding our amended and restated Certificate of Incorporation, Bylaws and registration rights agreement.
124
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Interest of Related Persons in the Consummation of this Offering
Executive Officer and Director Compensation
Certain of our executive officers and non-ACAS director nominees will receive certain benefits upon the completion of this offering, including cash bonus payments, stock option grants and the accelerated vesting of certain stock options, each as further described above under “Compensation Discussion and Analysis” and “Director Compensation.” The table below summarizes the expected benefits that each such executive officer and non-ACAS director nominee will receive:
| | | | | | | | | | | | | | | | | |
| | | | | | Fair
| | | Intrinsic
| | | | |
| | | | | | Value of
| | | Value of
| | | | |
| | | | | | New Option
| | | Vesting Options
| | | | |
| | | Bonus
| | | Grants (1)
| | | and Warrants
| | | Total
| |
Name and Position | | ($) | | | ($) | | | ($) | | | ($) | |
| |
| Thomas D. Logan | | | | | | | | | | | | | | | | |
| President, Chief Executive Officer and Chairman | | | 125,000 | | | | — | | | | | (2) | | | | |
| Seth B. Rosen | | | | | | | | | | | | | | | | |
| General Counsel, Vice President Corporate Development and Secretary | | | — | | | | | | | | — | | | | | |
| W. Antony Besso | | | | | | | | | | | | | | | | |
| Regional Vice President, EMEA and President, Health Physics Division | | | 507,509 | | | | — | | | | — | (3) | | | 507,509 | |
| Iain F. Wilson | | | | | | | | | | | | | | | | |
| Regional Vice President, Asia and President, Sensing Systems Division | | | — | | | | | | | | — | | | | | |
| Alfred E. Barry, Jr. | | | | | | | | | | | | | | | | |
| Director Nominee | | | — | | | | | | | | — | | | | | |
| Michael T. Everett | | | | | | | | | | | | | | | | |
| Director Nominee | | | — | | | | | | | | — | | | | | |
| Earl R. Lewis | | | | | | | | | | | | | | | | |
| Director Nominee | | | — | | | | | | | | — | | | | | |
| | |
| (1) | | Key assumptions used to value these options will be determined as of the grant date of the options and are expected to be as follows: option term of 7 years, risk-free interest rate of %, dividend yield of %, volatility of % and an exercise price and fair value of $ per share (the midpoint of the price range set forth on the cover page of this prospectus). These options vest monthly over four years. |
| | |
| (2) | | For Mr. Logan, the intrinsic value of $ is composed of the intrinsic value from call options of $ , as well as the intrinsic value from warrants of $ . The intrinsic value from call options reflects the difference between an assumed initial public offering price of $ (the midpoint of the price range set forth on the cover page of this prospectus) and the $10.45 exercise price of 231,897 of his call options that may vest 30 days after this offering, if the conditions described below under “Call Option Agreement between ACAS and Thomas D. Logan” are met. Related to any vesting that occurs for these options, we will incur an accounting charge equal to the fair value of the options, which was determined by use of a Monte Carlo model to be $ . The intrinsic value from warrants reflects the difference between an assumed initial public offering price of $ (the midpoint of the price range set forth on the cover page of this prospectus) and the $0.00118 exercise price of the 34,535 warrants. We will not incur any further accounting charge for these warrants, as the expense from these warrants was accounted for upon our formation. |
| | |
| (3) | | For Mr. Besso, the intrinsic value of $0 reflects 100% vesting of 32,470 unvested stock options from an August 2008 grant, which will vest upon the consummation of this offering. The intrinsic value is based |
125
| | |
| | upon an assumed initial public offering price of $ (the midpoint of the price range set forth on the cover page of this prospectus) but otherwise assumes the transaction occurred based upon unvested options at June 30, 2010. The exercise price of these options is $17.06, which is higher than the assumed initial public offering price, and therefore results in no intrinsic value. Related to the vesting that occurs for the previously unvested stock options, we will incur an accounting charge equal to the fair value of the previously unvested options, which was determined by use of the Black-Scholes option pricing model to be $ . Key assumptions used to value these options were as follows: expected option term of 10 years,risk-free interest rate of 4.0%, dividend yield of 0%, volatility of 45.9%, exercise price of $17.06, and fair value per share of $11.32. |
Equityholder Interests
The terms of our outstanding warrants provide that such warrants only become exercisable upon a sale, liquidation or dissolution of the Company or approval by our Board of Directors. On February 26, 2010, our Board of Directors resolved that all of these warrants will become exercisable upon the consummation of this offering and thereafter. ACAS, our principal stockholder, and its affiliates own warrants to purchase an aggregate of 3,223,726 shares of our common stock at an exercise price of $0.00012 and Thomas D. Logan, our President, Chief Executive Officer and Chairman, owns a warrant to purchase 34,535 shares of our common stock at an exercise price of $0.00118, all of which will become exercisable upon the completion of this offering and thereafter.
In addition, we entered into a letter agreement with each holder of our convertible preferred stock, including ACAS and its affiliates, ACE I and ACE II, and Mr. Logan, each dated March 18, 2010, which provided that in lieu of dividends otherwise payable in the form of additional shares of convertible preferred stock, for the period from March 1, 2010 through the closing of this offering, we would pay cash dividends on shares of our outstanding preferred stock at a rate equal to the number of shares of our common stock they would have received upon conversion of the preferred stock they would have received in dividends during that period multiplied by the initial public offering price. Pursuant to their terms, the letter agreements automatically terminated on April 30, 2010, and no cash dividends became payable. On May 5, 2010, we entered into new letter agreements with the same parties and on substantially identical terms that provided for cash dividends in lieu of stock dividends for the period from April 1, 2010 through the closing of this offering. Pursuant to their terms, the new letter agreements automatically terminated on June 1, 2010, and no cash dividends became payable. We expect to renegotiate these expired letter agreements on substantially identical terms with the same parties prior to the marketing of this offering.
Agreements with ACAS
We have entered into certain agreements with ACAS and its affiliates, which will own % of our issued and outstanding common stock after the completion of this offering, or % if the underwriters’ over-allotment option is exercised in full. One of our directors, Mr. Klein, and two of our director nominees, Messrs. Smith and Graff, are employees of ACAS. Set forth below is a brief description of the relationships and agreements between us and ACAS.
Certificate of Incorporation and Bylaws
Our amended and restated Bylaws provide that ACAS has the right to designate up to three members of our seven member Board of Directors, as set forth under “Management—Board Structure and Compensation.”
Our amended and restated Bylaws provide that at least one of the directors designated by ACAS must be part of the majority in any action taken by our Board of Directors so long as ACAS and its affiliated funds hold at least 50.1% of our outstanding common stock, other than on matters in which ACAS has a conflict of interest (as it would if it appointed a majority of our directors). Our amended and restated Bylaws also provide that ACAS will have the right to designate three of our seven directors so long as ACAS and its affiliated funds hold at least 50.1% of our outstanding common stock, two directors so long as they hold at least 25% but less than 50.1% and one director so long as they hold at least 10% but less than 25%.
126
Stockholders Agreement
In December 2005, our predecessor entered into a stockholders agreement with ACAS and certain of its affiliates, Thomas D. Logan, W. Antony Besso and certain other of our stockholders. Prior to the consummation of this offering, this agreement will terminate, and concurrently with such termination, we intend to enter into a registration rights agreement described under “— Registration Rights Agreement” and “Description of Capital Stock — Registration Rights” with such stockholders. Among other things, the stockholders agreement restricts the transfer of our securities by non-ACAS stockholders, subject to certain exceptions, and provides non-transferring stockholders with a right of first refusal (and ACAS with co-sale rights) in connection with sales of a transferring stockholder’s securities (other than transfers by ACAS). The agreement also provides ACAS with the ability to drag along the other stockholders in connection with transfers made by ACAS or changes in control relating to us that are endorsed by ACAS, and provides non-ACAS stockholders the right to tag along with certain sales or redemptions of our securities held by ACAS. Non-ACAS stockholders also have a right of first offer with respect to certain securities sold by us.
Pursuant to the stockholders agreement, if we determine to register any of our securities under the Securities Act, other than in an underwritten public offering of our common stock or certain other offerings, the holders of the registrable securities are entitled to written notice of the registration and are entitled to include all or a portion of their registrable securities in the registration, subject to certain limitations. In addition, at any time subsequent to six months following our filing of a registration statement under the Securities Act (other than on a Form S-8), these holders will have the right to require us, on no more than two occasions, to file a registration statement under the Securities Act to register all or any part of the registrable securities held by such holders, subject to certain conditions and limitations. Further, these holders may require us to register all or any portion of their registrable securities on Form S-3, when such form becomes available to us, subject to certain conditions and limitations.
The stockholders agreement also provides ACAS with the right to designate two of the seven members of our Board of Directors. In addition, the stockholders agreement gives ACAS and ACFS, under certain circumstances, the right to designate two additional members of our Board of Directors, whose seats will otherwise remain vacant.
Registration Rights Agreement
Upon the consummation of this offering, we will enter into a registration rights agreement with ACAS and certain of its affiliates, Thomas D. Logan, W. Antony Besso and certain other of our stockholders, pursuant to which such stockholders will have registration rights with respect to our common stock. Under the agreement, ACAS may from time to time require us to effect registrations of our securities held by ACAS and its affiliates, and ACAS, Thomas D. Logan, W. Antony Besso and certain other of our stockholders may join in registrations which we may effect, either for our benefit or for the benefit of other holders of our common stock. See “Description of Capital Stock—Registration Rights.”
Investment Banking Services Agreement
In December 2005, our predecessor entered into an investment banking services agreement with ACFS, a subsidiary of ACAS, pursuant to which ACFS may provide financial and advisory services to us. These services include evaluating, initiating and structuring any potential acquisitions by us, raising debt or equity financing, financial analysis and modeling, and related tasks.
The agreement also includes customary indemnification provisions in favor of ACFS, and customary limitations of each entity’s liability for services rendered under the investment banking services agreement in good faith and with reasonable care.
So long as the agreement is effective, we are required to pay to ACFS an annual management fee of $1.6 million, plus reimbursement for all reasonableout-of-pocket expenses. The management fee is payable on a quarterly basis, in advance. We incurred $1.6 million for management fees in each of fiscal 2008, 2009 and
127
2010. We and ACFS have agreed to terminate this agreement upon the consummation of this offering in return for a one-time payment by us to ACFS of $8.0 million.
Indebtedness
ACAS and its affiliates hold certain indebtedness of our subsidiaries. Such indebtedness consists of senior term notes, senior subordinated notes, junior subordinated notes, revolving notes and stockholder loans. Certain of such indebtedness is governed by Note and Equity Purchase Agreements, and is guaranteed and secured by us and our subsidiaries. See “Use of Proceeds,” “Management’s Discussion and Analysis of Financial Condition and Results of Operation—Credit Facilities And Long-Term Debt” and Note 8 of our consolidated financial statements.
The largest aggregate principal amounts outstanding under our NEPAs and stockholder loans were $173.2 million, $170.0 million and $173.1 million in fiscal 2008, 2009 and 2010. The amount outstanding (including accrued paid-in-kind interest) as of June 30, 2010 is $183.2 million. We paid $0.5 million, $0.5 million and $0.5 million in principal in fiscal 2008, 2009 and 2010, and $17.2 million, $14.8 million and $12.3 million in interest in fiscal 2008, 2009 and 2010. The interest rates under our debt are described in “Use of Proceeds.”
We expect to borrow up to $90 million and the equivalent in euros of $35 million under our anticipated new bank credit facilities, of which we expect to use approximately $ million, together with approximately $ million of the net proceeds from this offering, to repay our indebtedness to ACAS. See “Use of Proceeds.”
Dividend Rights
ACAS and its affiliates ACE I and ACE II, together hold approximately 747,426 shares of ourSeries A-1 andA-2 Convertible Participating Preferred Stock. Under the terms of our amended and restated Certificate of Incorporation, all holders of our preferred stock are entitled to receive, in preference to the holders of shares of any common stock, cumulative dividends, at a rate of 8% and 17% annually, on each quarter. Such dividends are to bepaid-in-kind, by validly issuing fully paid and non-assessable shares ofSeries A-1 andA-2 Convertible Participating Preferred Stock. ACAS and its affiliates accrued 64,050, 69,225 and 75,014 shares ofSeries A-1 preferred stockpaid-in-kind dividends in fiscal 2008, 2009 and 2010, and 16,611, 19,617 and 23,223 shares ofSeries A-2 preferred stockpaid-in-kind dividends in fiscal 2008, 2009 and 2010. The liquidation value of thepaid-in-kind dividends accrued on ourSeries A-1 preferred stock was $7.3 million, $7.8 million and $8.5 million for fiscal 2008, 2009 and 2010. The liquidation value of thepaid-in-kind dividends accrued on ourSeries A-2 preferred stock was $1.7 million, $2.0 million and $2.4 million for fiscal 2008, 2009 and 2010. See Note 12 to our consolidated financial statements.
Warrant and Preferred Stock Reissues
From October 2006 to October 2007, we cancelled and re-issued certain of our securities held by ACAS to allow ACAS to redistribute the securities between itself, ACE I and ACE II:
| | |
| | • | On October 3, 2006, we cancelled 357,271 shares of our Class B Non-Voting Common Stock held by ACAS and re-issued 250,086 shares to ACAS and 107,185 shares to ACE I. On October 3, 2007, we cancelled 250,086 shares held by ACAS and re-issued 208,275 shares to ACAS and 41,811 shares to ACE II. As a re-issuance of existing shares, we recognized no gain or loss on such transactions. |
| |
| | • | On October 3, 2006, we cancelled 677,426 shares of ourSeries A-1 Convertible Participating Preferred Stock held by ACAS and re-issued 474,198 shares to ACAS and 203,228 shares to ACE I. On October 3, 2007, we cancelled 474,198 shares held by ACAS and re-issued 394,916 shares to ACAS and 79,282 shares to ACE II. As a re-issuance of existing shares, we recognized no gain or loss on such transactions. |
| |
| | • | On October 3, 2006, we cancelled 70,000 shares of ourSeries A-2 Convertible Participating Preferred Stock held by ACAS and re-issued 49,000 shares to ACAS and 21,000 shares to ACE I. On October 3, |
128
| | |
| | | 2007, we cancelled 49,000 shares held by ACAS and re-issued 40,808 shares to ACAS and 8,192 shares to ACE II. As a re-issuance of existing shares, we recognized no gain or loss on such transactions. |
| | |
| | • | On October 3, 2006, we cancelled a warrant held by ACAS to purchase 3,223,726 shares of common stock and re-issued warrants to ACAS to purchase 2,263,082 shares and a warrant to ACE I to purchase 960,644 shares. On October 3, 2007, we cancelled a warrant held by ACAS to purchase 2,241,509 shares of common stock and reissued a warrant to ACAS to purchase 1,866,753 shares and a warrant to ACE II to purchase 374,756 shares. As a re-issuance of existing warrants, we recognized no gain or loss on such transactions. |
Interested Transactions—Transactions with Management
Call Option Agreement between ACAS and Thomas D. Logan
Our President, Chief Executive Officer and Chairman of the Board, Thomas D. Logan, entered into a Call Option Agreement with ACAS and certain of its affiliates, in which ACAS granted time and performance-based options with market conditions to Mr. Logan to purchase shares of the common stock of two of our predecessors in connection with his services as an officer and director. The options contain vesting provisions based upon successful completion of an initial public offering or change in control, and achievement by ACAS of certain internal rates of return as discussed in detail below. Modification of these options occurred in substance on January 1, 2006 in connection with the formation of Mirion in December 2005. As a result of the modification, Mr. Logan was granted performance-based options with market conditions to purchase 463,794 shares of Mirion’s Class A Common Stock held by ACAS. These options were further modified on December 7, 2007 to modify the vesting criteria of the performance based options to include, in addition to existing vesting provisions, vesting upon the achievement of certain returns on investment, as discussed in detail below. The exercise price of these options is $10.45 per share, and the total incremental value resulting from the option modification is $2.1 million and incorporates the impact of the options’ market-based conditions in the original grant date and modification date fair values. The original grant date fair value of these options was negligible. We will recognize expense on these options to the extent that we are able to either complete an effective offering in the public markets or complete a qualifying sale of the Company, and in such instance, over the derived service period of these options, which is consistent with the periods over which the market conditions are measured, as described further below.
The performance based options are divided into three tranches, each of which will either vest or become cancelled in two halves upon our IPO or change in control, depending on whether ACAS achieves certain market-based conditions, internal rates of return or returns on investment in such an event. Upon completion of this offering, vesting of the performance-based options will occur in two stages. The first stage occurs 30 days after the effective time of this offering at which time 50% of the options in each tranche will vest if ACAS achieves certain minimum internal rates of return, ranging between 25–40% or certain minimum returns on investment ranging between 2.0–2.7x. If neither goal is met, the options in this tranche will be cancelled. The second stage occurs on the earlier of two years after the effective time of this offering or upon the sale by ACAS of its investments in us, at which time the remaining 50% of the options in each tranche will vest if ACAS achieves certain minimum internal rates of return ranging between 25–40% or certain minimum returns on investment of 2.0–2.7x. If neither goal is met, the options in this tranche will be cancelled. The price of our common stock required to achieve a 25% internal rate of return is $17.58 per share, and the price required to achieve a 2.0x return on investment is $12.10 per share. The price of our common stock required to achieve a 40% internal rate of return is $31.04 per share, and the price required to achieve a 2.7x return on investment is $16.87 per share. We will record stock compensation expense in connection with these options in the event we complete an effective offering in the public markets or a qualifying sale of the Company, regardless of whether ACAS achieves the related market-based conditions.
The Call Option Agreement also provides Mr. Logan with a time-based option to purchase 150,875 shares of our common stock held by ACAS or its affiliates at an exercise price of $10.45 per share. The incremental cost resulting from the modification of the 150,875 time-based options granted to Mr. Logan is $0.6 million. All such options have vested as of June 30, 2008.
129
All options granted by ACAS and its affiliates to Mr. Logan pursuant to the Call Option Agreement are to be reduced on an economically equivalent basis in the event we grant Mr. Logan options to purchase shares of our common stock after the date of the Call Option Agreement, provided such options are no less favorable to Mr. Logan.
Indemnification and Employment Agreements
Upon the consummation of this offering, we shall enter into indemnification agreements pursuant to which we will indemnify our directors and our executive officers in certain circumstances, and hold them harmless against any expenses and liabilities incurred in the performance of their duties to us. Such indemnification includes, among other things, advancement of expenses to indemnitees, payment of fees of independent counsel and conditions of full release of liability to indemnitees in settlement. See “Executive Compensation—Limitation of Liability and Indemnification of Officers and Directors.” We have also entered into employment agreements and non-competition agreements with certain of our executive officers. See “Executive Compensation—Employment Agreements and Potential Payments on Termination and Change of Control.”
Policies and Procedures for Related Party Transactions
We do not currently have in effect a formal, written policy or procedure for the review and approval of related party transactions. However, all related party transactions are currently reviewed by our Board of Directors.
Our Board of Directors has approved a written related person transaction policy to become effective prior to the consummation of this offering setting forth the policies and procedures for the review and approval or ratification of related person transactions. This policy will cover any transaction, arrangement or relationship, or any series of similar transactions, arrangements or relationships in which we were or are to be a participant, the amount involved exceeds $120,000 and a related person had or will have a direct or indirect material interest, including, without limitation, purchases of goods or services by or from the related person or entities in which the related person has a material interest, indebtedness, guarantees of indebtedness or employment by us of a related person.
Under our amended and restated Certificate of Incorporation, none of ACAS or its affiliates and investment funds, or any other ACAS entity, nor any director, officer, stockholder, member, managerand/or employee of an ACAS entity, will have any duty to refrain from engaging directly or indirectly in the same or similar business activities or lines of business that we do, from doing business with our customers or suppliers or from employing or engaging our employees. In the event that any ACAS entity acquires knowledge of a potential transaction or matter which may be a corporate opportunity for both itself and us, the ACAS entity will not have any duty to communicate or offer the corporate opportunity to us and may pursue or acquire the corporate opportunity for itself or offer the opportunity to another person. In addition, no director or officer of ours who is also a director, officer, manager, employee or other representative of ACAS or its affiliates and investment funds will be required to offer to us any corporate opportunity belonging to us of which he or she becomes aware and could take any such opportunity for him or herself or offer it to other companies (including ACAS and its other portfolio companies) in which they have an investment. A corporate opportunity belonging to us is one that is available to (1) one of our officers, who is also a director but not an officer of ACAS or its affiliates and investment funds, unless such opportunity is expressly offered to him or her solely in his or her capacity as a director of ACAS or its affiliates and investment funds, (2) one of our outside directors, who is also a director, officer, manager, employee or other representative of ACAS or its affiliates and investment funds, if such opportunity is expressly offered to him or her solely in his or her capacity as our director or (3) an officer or director of both us and ACAS or certain affiliates and investment funds of ACAS, if such opportunity is expressly offered to him or her solely in his or her capacity as our officer or director.
130
DESCRIPTION OF CAPITAL STOCK
Following the consummation of this offering, our authorized capital stock will consist of 78,500,000 shares of common stock, par value $0.001 per share and 1,500,000 shares of preferred stock, par value $0.001 per share, undesignated as to series. As of June 30, 2010, 11,103,724 shares of common stock were issued and outstanding and held by nine stockholders of record and no shares of undesignated preferred shares were outstanding, assuming the conversion of all of our convertible preferred stock and the conversion of our Class A Voting Common Stock and Class BNon-Voting Common Stock on a one-for-one basis. In addition, as of June 30, 2010, there were 3,420,636 shares of common stock subject to outstanding warrants, which will become exercisable upon the consummation of this offering, and 924,830 shares of common stock subject to outstanding options. The following summary description relating to our capital stock does not purport to be complete and is qualified in its entirety by the amended and restated Certificate of Incorporation and Bylaws that have been filed as an exhibit to the registration statement of which this prospectus forms a part.
Common Stock
The holders of common stock are entitled to one vote per share on all matters to be voted upon by the stockholders. Subject to preferences that may be applicable to any outstanding preferred stock, the holders of common stock are entitled to receive ratably such dividends, if any, as may be declared from time to time by our Board of Directors out of funds legally available therefor. See “Dividend Policy.” In the event of our liquidation, dissolution or winding up, the holders of common stock are entitled to share ratably in all assets remaining after payment of liabilities, subject to prior distribution rights of preferred stock, if any, then outstanding. The common stock has no preemptive or conversion rights or other subscription rights. There are no redemption or sinking fund provisions applicable to the common stock. All outstanding shares of common stock are fully paid and non-assessable, and the shares of common stock to be issued upon completion of this offering will be fully paid and non-assessable.
Our amended and restated Bylaws provide that at least one of the directors designated by ACAS must be part of the majority in any action taken by our Board of Directors so long as ACAS and its affiliated funds hold at least 50.1% of our outstanding common stock, other than on matters in which ACAS has a conflict of interest (as it would if it appointed a majority of our directors). Our amended and restated Bylaws also provide that ACAS will have the right to designate three of our seven directors so long as ACAS and its affiliated funds hold at least 50.1% of our outstanding common stock, two directors so long as they hold at least 25% but less than 50.1% and one director so long as they hold at least 10% but less than 25%.
Preferred Stock
Our Board of Directors is authorized, without any action by our stockholders, to designate and issue shares of preferred stock in one or more series and to designate the powers, preferences and rights of each series, which may be greater than the rights of our common stock. It is not possible to state the actual effect of the issuance of any shares of preferred stock upon the rights of holders of our common stock until our Board of Directors determines the specific rights of the holders of such preferred stock. However, the effects might include, among other things:
| | |
| | • | impairing dividend rights of our common stock; |
| |
| | • | diluting the voting power of our common stock; |
| |
| | • | impairing the liquidation rights of our common stock; and |
| |
| | • | delaying or preventing a change of control of us without further action by our stockholders. |
Upon the consummation of this offering, no shares of our preferred stock will be outstanding.
Registration Rights
Upon the consummation of this offering and concurrently with the termination of the stockholders agreement described under “Certain Relationships and Related Party Transactions — Stockholders Agreement,” we expect to enter into a registration rights agreement with ACAS and certain of its affiliates, Thomas D. Logan,
131
W. Antony Besso and certain other of our stockholders, under which these stockholders may require us to register their shares of common stock under the securities laws for sale.
Requested Registration
The agreement shall provide that, any time following 180 days (subject to extension in the event thelock-up agreement with the underwriters is extended) after the effective date of a registration statement for this offering, ACAS and any transferee receiving at least 25% of ACAS’s registrable securities held at the time of signing the agreement, so long as the requesting party holds at least 25% of the registrable securities held by all the parties holding requested registration rights at the time of the request for registration, may require us to effect a registration under the Securities Act of 1933 of all or a portion of our securities held by them.
We may decline to honor any of these requested registrations if more than four requested registrations have already been undertaken.
Form S-3 Registration
We are also obligated in certain circumstances under the registration rights agreement to use our best efforts to effect and maintain the effectiveness of a registration onForm S-3, if ACAS, a permitted transferee of ACAS or other stockholder under the agreement holding two percent or more of our common stock requests that we make such registration and the aggregate gross proceeds of such registration are reasonably anticipated to exceed $5,000,000. Upon such a request, all other eligible holders of our securities under the registration rights agreement may also elect to register their securities under such registration statement.
We may decline to honor a requested registration 30 days prior to or 90 days immediately following the effective date of another registration statement. Furthermore, we may postpone the filing of a requested registration onForm S-3, but not more than once in any 12-month period, for a reasonable period of time if filing the registration statement would have a material adverse effect on us, including if our Board of Directors determines that a registration would materially interfere with a significant acquisition or company reorganization, require premature disclosure of material nonpublic information or render us unable to comply with requirements under the Securities Act or the Securities Exchange Act of 1934, as amended, or the Exchange Act.
Incidental Registration
In addition to our obligations with respect to requested registrations, if we propose to register any of our securities (other than a registration on FormS-8 orS-4 or successor forms to these forms and certain other limited exceptions), whether or not such registration is for our own account, ACAS, permitted transferees of ACAS and other stockholders party to the registration rights agreement will have the opportunity to participate in such registration.
If the incidental registration relates to an underwritten primary registration on our behalf and marketing factors require a limitation of the number of shares to be offered, first priority of inclusion shall be given to us and second priority will be given to ACAS and other stockholders participating in the incidental registration. If the incidental registration relates to an underwritten secondary registration on behalf of holders of our securities and marketing factors require a limitation of the number of shares to be offered, first priority of inclusion will be given to the original requesting stockholder, and second priority will be given to the remaining stockholders participating in the incidental registration.
Expenses of Registration
We will pay all expenses of registration, other than underwriting discounts and commissions, related to any requestedForm S-3 or incidental registration.
Indemnification
The agreement contains customary cross-indemnification provisions under which we are obligated to indemnify the selling stockholders in the event of material misstatements or omissions in the registration
132
statement attributable to us, and they are obligated to indemnify us for material misstatements or omissions attributable to them.
Anti-Takeover Effects of Delaware Law
Pursuant to our amended and restated Certificate of Incorporation, we have opted out of the provisions of Section 203 of the Delaware General Corporation Law regulating corporate takeovers. Section 203 prohibits a publicly held Delaware corporation from engaging, under certain circumstances, in a business combination with an interested stockholder (defined generally as a person owning 15% or more of the corporation’s voting stock) for a period of three years following the date the person became an interested stockholder unless:
| | |
| | • | prior to the date the person became an interested person, the board of directors of the corporation approved either the business combination or the transaction which resulted in the stockholder becoming an interested stockholder; |
| |
| | • | upon completion of the transaction that resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced; or |
| |
| | • | at or subsequent to the date of the transaction, the business combination is approved by the board of directors of the corporation and authorized at an annual or special meeting of stockholders, and not by written consent, by the affirmative vote of at least 662/3% of the outstanding voting stock which is not owned by the interested stockholder. |
Listing
Our common stock has been approved for listing on The NASDAQ Global Market under the symbol “MION.”
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is Computershare Trust Company, N.A.
133
DESCRIPTION OF CERTAIN INDEBTEDNESS
We previously entered into a credit agreement with JPMorgan Chase Bank, National Association, J.P. Morgan Europe Limited, J.P. Morgan Securities LLC and Fifth Third Bank in connection with our anticipated new bank credit facilities, pursuant to which the lenders agreed to make loans, issue letters of credit and commit to future loans, to be effective upon the consummation of a public offering. Although the obligations and commitments of the lenders under this credit agreement terminated on June 7, 2010 in connection with the delay in our initial public offering, we expect to negotiate a new credit agreement on similar terms prior to the marketing of this offering. The following is a summary of the anticipated terms of these new bank credit facilities. We expect the debt will have the following principal terms:
New Senior Secured Credit Facilities
In connection with the consummation of this offering, we expect to enter into new senior secured credit facilities. The new senior secured credit facilities will provide for an aggregate borrowing capacity of up to $55 million through a revolving credit facility, $35 million through a domestic term loan facility and the equivalent in euros of $35 million through a French term loan facility. The borrowers under the senior secured credit facilities will be Mirion Technologies, Inc., and our subsidiaries, Mirion Technologies (Synodys) SA and Mirion Technologies (IST France) SAS.
Revolving Credit Facility
Mirion Technologies, Inc. will be the borrower under the revolving credit facility, which will initially have an aggregate borrowing capacity of $30 million. We may elect to increase the revolving credit facility by an additional $25 million, so long as we are not in default under the terms of the credit agreement and subject to the consent of any increasing lenders. Up to $15 million of the revolving credit facility may be in the form of letters of credit, and up to $5 million of the revolving credit facility may be in the form of swingline loans. Any issuances of letters of credit and borrowing under the swingline loan will reduce availability for borrowing under the revolving credit facility. Loans under the revolving credit facility will be used to refinance our existing indebtedness to ACAS and for general corporate purposes, and will be so available for four years after entering into the new senior secured credit facilities.
Obligations of Mirion Technologies, Inc. under the revolving credit facility will be guaranteed by each of our existing and future wholly owned domestic subsidiaries, and such obligations will be secured by substantially all of the assets, including by blanket liens, of us and our domestic subsidiaries, limited, in the case of stock of our foreign subsidiaries to 65% of the capital stock of such subsidiaries.
Term Loan Facilities
Mirion Technologies, Inc. will be the domestic borrower of $35 million and Mirion Technologies (Synodys) SA and Mirion Technologies (IST France) SAS will be the French subsidiary borrowers of the equivalent in euros of $35 million under the term loan facilities of our senior secured credit facilities. The term loans will be made available in a single borrowing, and will mature four years after entering into the new senior secured credit facilities. The term loans will amortize in quarterly installments over the life of the loan, equal to 15% of the original principal balance in the first, second and third years after the closing date and 20% in the fourth year.
The term loans of Mirion Technologies, Inc. will be guaranteed by each of our existing and future wholly owned domestic subsidiaries. All obligations under term loans to Mirion Technologies, Inc. will be secured by substantially all of the assets, including by blanket liens, of us and our domestic subsidiaries, limited, in the case of stock of our foreign subsidiaries to 65% of the capital stock of such subsidiaries. The term loans of our French subsidiary borrowers will be guaranteed by us, our domestic subsidiaries and, to the extent that no material adverse tax consequences would result, our English, Canadian, French and German subsidiaries. All obligations under the term loans to our French subsidiary borrower will be secured by assets of us and several of our subsidiaries, including blanket liens on us and our domestic subsidiaries and pledges of assets or securities of our foreign subsidiaries.
134
Other Terms
Borrowings under the senior secured credit facilities will bear interest at a rate based on the prime rate, LIBOR or EURIBOR, subject to certain adjustments based on our leverage ratio, and certain fees may accrue in conjunction with the revolving credit facility, as reflected in the following table:
| | | | | | | | | | | | | |
| | | | | | | Commitment
|
Leverage Ratio: | | ABR Spread | | Eurodollar Spread | | Fee Rate |
| |
| Less than 1.25 to 1.00 | | | 3.00% | | | | 4.00% | | | | 0.35% | |
| Equal to or greater than 1.25 to 1.00 but less than 1.75 to 1.00 | | | 3.25% | | | | 4.25% | | | | 0.40% | |
| Equal to or greater than 1.75 to 1.00 but less than 2.25 to 1.00 | | | 3.50% | | | | 4.50% | | | | 0.50% | |
| Equal to or greater than 2.25 to 1.00 | | | 4.00% | | | | 5.00% | | | | 0.50% | |
However, until we deliver a compliance certificate with respect to our fiscal quarter ending September 30, 2010, the initial adjustment (i) with respect to the ABR Spread shall be 3.50%, (ii) with respect to the Eurodollar Spread shall be 4.50% and (iii) with respect to the Commitment Fee Rate shall be 0.50%.
The senior secured credit facilities will contain, among other things, covenants restricting our ability and the ability of our subsidiaries to incur indebtedness, prepay or amend other indebtedness, create liens, guarantee obligations, make certain fundamental changes including mergers or dissolutions, pay dividends and other payments in respect of capital stock, make capital expenditures, make certain investments, sell assets, change our lines of business, enter into transactions with affiliates and other corporate actions. The senior secured credit facilities will also require us to maintain a fixed charge coverage ratio (defined as the ratio of Adjusted EBITDA minus capital expenditures to interest expense and scheduled principal payments of indebtedness) of 1.50-to-one, a minimum net worth of 80% of our consolidated stockholders’ equity as of April 30, 2010 after giving pro forma effect to this offering and the consummation on the effective date of the transactions contemplated under the senior secured credit facilities plus 50% of net income earned in each full fiscal quarter ending after March 31, 2010 and a maximum leverage ratio (defined as the ratio of total indebtedness to Adjusted EBITDA for four consecutive fiscal quarters) of 2.75-to-one, stepping down to 2.25-to-one as of January 1, 2011. The senior secured credit facilities also contain mandatory prepayment provisions, which require us in certain instances to prepay obligations owing under the senior secured credit facilities in connection with asset sales, excess cash flow and subordinated debt issuances.
Borrowings under the senior secured credit facilities will also be subject to certain conditions, including the absence of any event of default and any material adverse change.
The senior secured credit facilities will also include events of default typical of these types of credit facilities and transactions, including but not limited to the nonpayment of principal, interest, fees or other amounts owing under the senior secured credit facilities (in certain cases, subject to a grace period), the violation of covenants (subject, in certain cases, to a grace period), the inaccuracy of representations and warranties, cross defaults, bankruptcy events, certain ERISA events, material judgments and a change of control. The occurrence of an event of default could result in the lenders not being required to lend any additional amounts and the acceleration of obligations under the senior secured credit facilities, causing such obligations to be due and payable immediately, which could materially and adversely affect us.
Upon the terms as presently contemplated, we anticipate that J.P. Morgan Securities LLC as lead arranger and bookrunner of our new bank credit facilities will be paid one-time fees equal to $1,025,000 and annual administrative fees equal to $25,000. It, like all other lenders under the senior credit facilities, will earn interest on its share of outstanding loans, commitment fees on its share of any unused portion of the senior credit facilities and letter of credit fees on its share of outstanding letters of credit, in each case based upon our leverage ratio in effect from time to time. In addition, each bank, including JPMorgan Chase Bank, N.A., that issues letters of credit under the senior credit facilities will earn a fronting fee on the amount of each such letter of credit at rates to be separately negotiated between us and such bank, which, in the case of JPMorgan Chase Bank, N.A., is 0.125% per annum.
135
SHARES ELIGIBLE FOR FUTURE SALE
Immediately prior to this offering, there has been no public market for our common stock. Future sales of substantial amounts of shares of our common stock in the public market could adversely affect prevailing market prices. Furthermore, since only a limited number of shares will be available for sale shortly after this offering because of contractual and legal restrictions on resale described below, sales of substantial amounts of common stock in the public market after the restrictions lapse could adversely affect the prevailing market price for our common stock, as well as our ability to raise equity capital in the future.
Based on the number of shares of common stock outstanding as of June 30, 2010, upon the completion of this offering, shares of our common stock will be outstanding, assuming no exercise of any options or warrants outstanding. All (plus up to additional shares if the over-allotment option is exercised in full) shares of common stock sold in this offering will be freely tradable unless held by one of our affiliates, as that term is defined in Rule 144 under the Securities Act.
The remaining shares of our common stock outstanding after this offering are restricted securities as such term is defined in Rule 144 under the Securities Act or are subject tolock-up agreements as described below. Following the expiration of thelock-up period, restricted securities may be sold in the public market only if registered or if they qualify for an exemption from registration under Rule 144 or 701 promulgated under the Securities Act, described in greater detail below. The shares will generally become available for sale in the public market under Rule 144 or Rule 701 upon expiration oflock-up agreements 180 days after the date of this offering, provided that shares held by affiliates will be subject to the volume limitations described below.
Rule 144
In general, under Rule 144 as currently in effect, once we have been a reporting company subject to the reporting requirements of Section 13 or Section 15(d) of the Exchange Act for 90 days, an affiliate who has beneficially owned restricted shares of our common stock for at least six months would be entitled to sell within any three-month period a number of shares that does not exceed the greater of either of the following:
| | |
| | • | 1% of the number of shares of common stock then outstanding, which will equal shares immediately after this offering; and |
| | |
| | • | the average weekly reported volume of trading of our common stock during the four calendar weeks preceding the filing of a notice on Form 144 with respect to the sale. |
However, the six month holding period increases to one year in the event we have not been a reporting company for at least 90 days. In addition, any sales by affiliates under Rule 144 are also limited by manner of sale provisions and notice requirements and the availability of current public information about us.
The volume limitation, manner of sale and notice provisions described above will not apply to sales by non-affiliates. For purposes of Rule 144, a non-affiliate is any person or entity who is not our affiliate at the time of sale and has not been our affiliate during the preceding three months. Once we have been a reporting company for 90 days, a non-affiliate who has beneficially owned restricted shares of our common stock for six months may rely on Rule 144 provided that certain public information regarding us is available. The six month holding period increases to one year in the event we have not been a reporting company for at least 90 days. However, a non-affiliate who has beneficially owned the restricted shares proposed to be sold for at least one year will not be subject to any restrictions under Rule 144 regardless of how long we have been a reporting company.
We are unable to estimate the number of shares that will be sold under Rule 144 since this will depend on the market price for our common stock, the personal circumstances of the stockholder and other factors.
Rule 701
In general, our employees, directors, officers, consultants or advisors who purchase shares from us under Rule 701 in connection with a compensatory stock or option plan or other written agreement before the
136
effective date of this offering are entitled to resell such shares 90 days after the effective date of this offering in reliance on Rule 144, without having to comply with the holding period requirements of Rule 144 described above, but subject to the lock-up agreements described below.
Registration Rights
Upon the consummation of this offering, we shall enter into a registration rights agreement with ACAS and certain of its affiliates, Thomas D. Logan, W. Antony Besso and certain other of our stockholders, under which these stockholders may require us to register their shares of common stock under the securities laws for sale. Registration of these shares under the Securities Act would result in these shares becoming freely tradable without restriction under the Securities Act immediately upon the effectiveness of the registration, except for shares purchased by affiliates.
Stock Options
As of June 30, 2010, options to purchase a total of 924,830 shares of common stock were outstanding. We expect that all of the shares subject to options will be subject tolock-up agreements. An additional 986,395 shares of common stock were either reserved for issuance under our existing stock plan or to be reserved for issuance under our amended and restated stock plan.
Upon the consummation of this offering, we intend to file a registration statement under the Securities Act covering all shares of common stock subject to outstanding options or issuable pursuant to our equity plans. Shares registered under this registration statement will be available for sale in the open market, subject to Rule 144 volume limitations applicable to affiliates, vesting restrictions with us or the contractual restrictions described below.
Lock-up Agreements
ACAS has agreed, and we expect that our officers, directors and all of our other stockholders will agree, subject to customary exceptions, not to offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, or otherwise transfer or dispose of, directly or indirectly, or enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of any shares of common stock or any securities convertible into or exercisable or exchangeable for shares of common stock for a period of 180 days after the date of this prospectus, without the prior written consent of each of Credit Suisse Securities (USA) LLC, Merrill Lynch, Pierce, Fenner & Smith Incorporated and J.P. Morgan Securities LLC. We do not anticipate that the lenders under the Pledge will enter into a lock-up agreement.
137
MATERIAL U.S. FEDERAL TAX CONSIDERATIONS FORNON-U.S. HOLDERS OF COMMON STOCK
The following is a general discussion of the material U.S. federal income and estate tax consequences of the ownership and disposition of our common stock by a beneficial owner that is a“Non-U.S. Holder,” other than aNon-U.S. Holder that owns, or has owned, actually or constructively, more than 5% of our common stock. A“Non-U.S. Holder” is a person or entity that, for U.S. federal income tax purposes, is a:
| | |
| | • | nonresident alien individual, other than a former citizen or resident of the United States subject to tax as an expatriate; |
| |
| | • | foreign corporation; or |
| |
| | • | foreign estate or trust. |
A“Non-U.S. Holder” generally does not include a nonresident alien individual who is present in the United States for 183 days or more in the taxable year of disposition of our common stock. Such an individual is urged to consult his or her own tax adviser regarding the U.S. federal income tax consequences of the sale, exchange or other disposition of our common stock.
If an entity that is classified as a partnership for U.S. federal income tax purposes holds our common stock, the U.S. federal income tax treatment of a partner will generally depend on the status of the partner and the activities of the partnership. Partnerships holding our common stock and partners in such partnerships are urged to consult their tax advisers as to the particular U.S. federal income tax consequences of holding and disposing of our common stock.
This discussion is based on the Internal Revenue Code of 1986, as amended (the “Code”), and administrative pronouncements, judicial decisions and final, temporary and proposed Treasury Regulations, changes to any of which subsequent to the date of this prospectus may affect the tax consequences described herein. This discussion does not address all aspects of U.S. federal income and estate taxation that may be relevant toNon-U.S. Holders in light of their particular circumstances and does not address any tax consequences arising under the laws of any state, local or foreign jurisdiction. Prospective holders are urged to consult their tax advisers with respect to the particular tax consequences to them of owning and disposing of our common stock, including the consequences under the laws of any state, local or foreign jurisdiction.
Dividends
As discussed under “Dividend Policy” above, we do not currently expect to pay dividends. In the event that we do pay dividends, dividends paid to aNon-U.S. Holder of our common stock generally will be subject to withholding tax at a 30% rate or a reduced rate specified by an applicable income tax treaty. In order to obtain a reduced rate of withholding, aNon-U.S. Holder will be required to provide an Internal Revenue ServiceForm W-8BEN certifying its entitlement to benefits under a treaty.
If aNon-U.S. Holder is engaged in a trade or business in the United States, and if dividends paid to theNon-U.S. Holder are effectively connected with the conduct of this trade or business (and, if required by an applicable income tax treaty, are attributable to a U.S. permanent establishment), theNon-U.S. Holder, although exempt from the withholding tax discussed in the preceding paragraph, will generally be taxed in the same manner as a U.S. person. Such aNon-U.S. Holder will be required to provide a properly executed Internal Revenue ServiceForm W-8ECI in order to claim an exemption from withholding. Anon-U.S. corporation receiving effectively connected dividends may also be subject to an additional “branch profits tax” imposed at a rate of 30% (or a lower treaty rate).
Gain on Disposition of Common Stock
Subject to the backup withholding rules discussed below, aNon-U.S. Holder generally will not be subject to U.S. federal income tax on gain realized on a sale or other disposition of our common stock unless:
| | |
| | • | the gain is effectively connected with a trade or business of thenon-U.S. holder in the United States, or |
| |
| | • | we are or have been a U.S. real property holding corporation, as defined in the Code, at any time within the five-year period preceding the disposition or theNon-U.S. Holder’s holding period, whichever period is shorter, and our common stock has ceased to be traded on an established securities market prior to the beginning of the calendar year in which the sale or disposition occurs. |
138
We believe that we are not, and do not anticipate becoming, a U.S. real property holding corporation.
If aNon-U.S. Holder is engaged in a trade or business in the United States and gain recognized by theNon-U.S. Holder on a sale or other disposition of our common stock is effectively connected with the conduct of such trade or business, theNon-U.S. Holder will generally be taxed in the same manner as a U.S. person, subject to an applicable income tax treaty providing otherwise.Non-U.S. Holders whose gain from dispositions of our common stock may be effectively connected with a conduct of a trade or business in the United States are urged to consult their own tax advisers with respect to the U.S. tax consequences of the ownership and disposition of our common stock, including the possible imposition of a branch profits tax.
Information Reporting Requirements and Backup Withholding
Information returns will be filed with the Internal Revenue Service in connection with payments of dividends on our common stock. Unless theNon-U.S. Holder complies with certification procedures to establish that it is not a U.S. person, information returns may be filed with the Internal Revenue Service in connection with the proceeds from a sale or other disposition of our common stock and theNon-U.S. Holder may be subject to backup withholding on dividend payments on our common stock or on the proceeds from a sale or other disposition of our common stock. The certification procedures required to claim a reduced rate of withholding under a treaty described above will satisfy the certification requirements necessary to avoid backup withholding as well. The amount of any backup withholding from a payment to aNon-U.S. Holder will be allowed as a credit against such holder’s U.S. federal income tax liability and may entitle such holder to a refund, provided that the required information is timely furnished to the Internal Revenue Service.
Recent Legislation
Recent legislation generally imposes a withholding tax of 30% on payments to certain foreign entities (including financial intermediaries), after December 31, 2012, of dividends on and the gross proceeds of dispositions of U.S. common stock, unless various U.S. information reporting and due diligence requirements (that are in addition to, and potentially significantly more burdensome than, the beneficial owner certification requirements described above) have been satisfied. Non-U.S. Holders should consult their tax advisers regarding the possible implications of this legislation for their investment in our common stock.
Federal Estate Tax
IndividualNon-U.S. Holders and entities the property of which is potentially includible in such an individual’s gross estate for U.S. federal estate tax purposes (for example, a trust funded by such an individual and with respect to which the individual has retained certain interests or powers), should note that, absent an applicable treaty benefit, our common stock will be treated as U.S. situs property subject to U.S. federal estate tax.
139
UNDERWRITING
Under the terms and subject to the conditions contained in an underwriting agreement dated , we and the selling stockholders have agreed to sell to the underwriters named below, for whom Credit Suisse Securities (USA) LLC, Merrill Lynch, Pierce, Fenner & Smith Incorporated and J.P. Morgan Securities LLC are acting as representatives, the following respective numbers of shares of common stock:
| | | | | |
| | | Number
| |
Underwriter | | of Shares | |
| |
| Credit Suisse Securities (USA) LLC | | | | |
Merrill Lynch, Pierce, Fenner & Smith
Incorporated | | | | |
| J.P. Morgan Securities LLC | | | | |
| Robert W. Baird & Co. Incorporated | | | | |
| | | | | |
| Total | | | | |
| | | | | |
The underwriting agreement provides that the underwriters are obligated to purchase all the shares of common stock offered by us and the selling stockholders if they purchase any shares, other than those shares covered by the over-allotment option described below. The underwriting agreement also provides that if an underwriter defaults, the purchase commitments of non-defaulting underwriters may be increased or this offering may be terminated.
The selling stockholders have granted to the underwriters a30-day option to purchase on a pro rata basis up to additional shares from the selling stockholders at the initial public offering price less the underwriting discounts and commissions. The option may be exercised only to cover any over allotments of common stock.
The underwriters propose to offer the shares of common stock initially at the public offering price on the cover page of this prospectus and to selling group members at that price less a selling concession of $ per share. The underwriters and selling group members may allow a discount of $ per share on sales to other broker/dealers. After the initial public offering the representatives may change the public offering price and concession and discount to broker/dealers.
The following table summarizes the compensation and estimated expenses we and the selling stockholders will pay:
| | | | | | | | | | | | | | | | | |
| | | Per Share | | | Total | |
| | | Without
| | | With
| | | Without
| | | With
| |
| | | Over-Allotment | | | Over-Allotment | | | Over-Allotment | | | Over-Allotment | |
| |
| Underwriting Discounts and Commissions paid by us | | $ | | | | $ | | | | $ | | | | $ | | |
| Expenses payable by us | | $ | | | | $ | | | | $ | | | | $ | | |
| Underwriting Discounts and Commissions paid by the selling stockholders | | $ | | | | $ | | | | $ | | | | $ | | |
We are paying all of the expenses of the selling stockholders in connection with this offering, other than underwriting discounts and commissions applicable to the shares sold by them.
The representatives have informed us and the selling stockholders that they do not expect sales to accounts over which the underwriters have discretionary authority to exceed 5% of the shares of common stock being offered. The underwriters will not confirm sales to any accounts over which they exercise discretionary authority without first receiving a written consent from those accounts.
We have agreed that we will not offer, sell, contract to sell, pledge or otherwise dispose of, directly or indirectly, or file with the Securities and Exchange Commission a registration statement under the Securities Act
140
relating to, any shares of our common stock or securities convertible into or exchangeable or exercisable for any shares of our common stock, or publicly disclose the intention to make any offer, sale, pledge, disposition or filing, without the prior written consent of each of Credit Suisse Securities (USA) LLC, Merrill Lynch, Pierce, Fenner & Smith Incorporated and J.P. Morgan Securities LLC for a period of 180 days after the date of this prospectus subject to customary exceptions, including a limited exception for the issuance of common stock in connection with future acquisitions or strategic investments. However, in the event that either (1) during the last 17 days of the“lock-up” period, we release earnings results or material news or a material event relating to us occurs or (2) prior to the expiration of the“lock-up” period, we announce that we will release earnings results during the16-day period beginning on the last day of the“lock-up” period, then in either case the expiration of the“lock-up” will be extended until the expiration of the18-day period beginning on the date of the release of the earnings results or the occurrence of the material news or event, as applicable, unless each of Credit Suisse Securities (USA) LLC, Merrill Lynch, Pierce, Fenner & Smith Incorporated and J.P. Morgan Securities Inc. waives, in writing, such an extension.
ACAS has agreed, and we expect that our officers, directors and all of our other stockholders will agree, that they will not offer, sell, contract to sell, pledge or otherwise dispose of, directly or indirectly, any shares of our common stock or securities convertible into or exchangeable or exercisable for any shares of our common stock, enter into a transaction that would have the same effect, or enter into any swap, hedge or other arrangement that transfers, in whole or in part, any of the economic consequences of ownership of our common stock, whether any of these transactions are to be settled by delivery of our common stock or other securities, in cash or otherwise, or publicly disclose the intention to make any offer, sale, pledge or disposition, or to enter into any transaction, swap, hedge or other arrangement, without, in each case, the prior written consent of each of Credit Suisse Securities (USA) LLC, Merrill Lynch, Pierce, Fenner & Smith Incorporated and J.P. Morgan Securities LLC for a period of 180 days after the date of this prospectus, in each case other than the shares of our common stock sold by the selling stockholders in this offering and subject to customary exceptions. We do not anticipate that the lenders under the Pledge will enter into a lock-up agreement. However, in the event that either (1) during the last 17 days of the“lock-up” period, we release earnings results or material news or a material event relating to us occurs or (2) prior to the expiration of the“lock-up” period, we announce that we will release earnings results during the16-day period beginning on the last day of the“lock-up” period, then in either case the expiration of the“lock-up” will be extended until the expiration of the18-day period beginning on the date of the release of the earnings results or the occurrence of the material news or event, as applicable, unless each of Credit Suisse Securities (USA) LLC, Merrill Lynch, Pierce, Fenner & Smith Incorporated and J.P. Morgan Securities LLC waives, in writing, such an extension.
We and the selling stockholders have agreed to indemnify the underwriters against liabilities under the Securities Act, or contribute to payments that the underwriters may be required to make in that respect.
Our common stock has been approved for listing on The NASDAQ Global Market.
In connection with this offering, the underwriters may engage in stabilizing transactions, over-allotment transactions, syndicate covering transactions and penalty bids in accordance with Regulation M under the Exchange Act.
| | |
| | • | Stabilizing transactions permit bids to purchase the underlying security so long as the stabilizing bids do not exceed a specified maximum. |
| |
| | • | Over-allotment involves sales by the underwriters of shares in excess of the number of shares the underwriters are obligated to purchase, which creates a syndicate short position. The short position may be either a covered short position or a naked short position. In a covered short position, the number of shares over-allotted by the underwriters is not greater than the number of shares that they may purchase in the over-allotment option. In a naked short position, the number of shares involved is greater than the number of shares in the over-allotment option. The underwriters may close out any covered short position by either exercising their over-allotment optionand/or purchasing shares in the open market. |
141
| | |
| | • | Syndicate covering transactions involve purchases of the common stock in the open market after the distribution has been completed in order to cover syndicate short positions. In determining the source of shares to close out the short position, the underwriters will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which they may purchase shares through the over-allotment option. If the underwriters sell more shares than could be covered by the over-allotment option, a naked short position, the position can only be closed out by buying shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that there could be downward pressure on the price of the shares in the open market after pricing that could adversely affect investors who purchase in this offering. |
| |
| | • | Penalty bids permit the representatives to reclaim a selling concession from a syndicate member when the common stock originally sold by the syndicate member is purchased in a stabilizing or syndicate covering transaction to cover syndicate short positions. |
| |
| | • | In passive market making, market makers in the common stock who are underwriters or prospective underwriters may, subject to limitations, make bids for or purchases of our common stock until the time, if any, at which a stabilizing bid is made. |
These stabilizing transactions, syndicate covering transactions and penalty bids may have the effect of raising or maintaining the market price of our common stock or preventing or retarding a decline in the market price of the common stock. As a result the price of our common stock may be higher than the price that might otherwise exist in the open market. These transactions, if commenced, may be discontinued at any time.
A prospectus in electronic format may be made available on the web sites maintained by one or more of the underwriters, or selling group members, if any, participating in this offering and one or more of the underwriters participating in this offering may distribute prospectuses electronically. The representatives may agree to allocate a number of shares to underwriters and selling group members for sale to their online brokerage account holders. Internet distributions will be allocated by the underwriters and selling group members that will make internet distributions on the same basis as other allocations.
In connection with the consummation of this offering, we expect to enter into new bank credit facilities with a group of lenders that will include some of the underwriters and affiliates of some of the underwriters, including J.P. Morgan Securities LLC. The underwriters may in the future perform investment banking, commercial banking and advising services for us and certain of our affiliates, including ACAS, from time to time for which they may in the future receive customary fees and expenses.
142
NOTICE TO CANADIAN RESIDENTS
Resale Restrictions
The distribution of the shares in Canada is being made only on a private placement basis exempt from the requirement that we prepare and file a prospectus with the securities regulatory authorities in each province where trades of the shares are made. Any resale of the shares in Canada must be made under applicable securities laws which may vary depending on the relevant jurisdiction, and which will require resales to be made under available statutory exemptions or under a discretionary exemption granted by the applicable Canadian securities regulatory authority. Purchasers are advised to seek legal advice prior to any resale of the shares.
Representations of Purchasers
By purchasing the shares in Canada and accepting delivery of a purchase confirmation a purchaser is representing to us, the selling stockholders and the dealer from whom the purchase confirmation is received that:
| | |
| | • | the purchaser is entitled under applicable provincial securities laws to purchase the Shares without the benefit of a prospectus qualified under those securities laws as it is an “accredited investor” as defined under National Instrument 45-106—Prospectus and Registration Exemptions, |
| |
| | • | the purchaser is a “permitted client” as defined in National Instrument 31-103—Registration Requirements and Exemptions, |
| |
| | • | where required by law, the purchaser is purchasing as principal and not as agent, |
| |
| | • | the purchaser has reviewed the text above under Resale Restrictions, and |
| |
| | • | the purchaser acknowledges and consents to the provision of specified information concerning the purchase of the shares to the regulatory authority that by law is entitled to collect the information, including certain personal information. For purchasers in Ontario, questions about such indirect collection of personal information should be directed to Administrative Support Clerk, Suite 1903, Box 55, 20 Queen Street West, Toronto, Ontario M5H 3S8 or to (416) 593-3684. |
Rights of Action—Ontario Purchasers Only
Under Ontario securities legislation, certain purchasers who purchase a security offered by this document during the period of distribution will have a statutory right of action for damages, or while still the owner of the shares, for rescission against us and the selling stockholders in the event that this document contains a misrepresentation without regard to whether the purchaser relied on the misrepresentation. The right of action for damages is exercisable not later than the earlier of 180 days from the date the purchaser first had knowledge of the facts giving rise to the cause of action and three years from the date on which payment is made for the shares. The right of action for rescission is exercisable not later than 180 days from the date on which payment is made for the shares. If a purchaser elects to exercise the right of action for rescission, the purchaser will have no right of action for damages against us and the selling stockholders. In no case will the amount recoverable in any action exceed the price at which the shares were offered to the purchaser and if the purchaser is shown to have purchased the securities with knowledge of the misrepresentation, we and the selling stockholders will have no liability. In the case of an action for damages, we and the selling stockholders will not be liable for all or any portion of the damages that are proven to not represent the depreciation in value of the shares as a result of the misrepresentation relied upon. These rights are in addition to, and without derogation from, any other rights or remedies available at law to an Ontario purchaser. The foregoing is a summary of the rights available to an Ontario purchaser. Ontario purchasers should refer to the complete text of the relevant statutory provisions.
143
Enforcement of Legal Rights
All of our directors and officers as well as the experts named herein and the selling stockholders may be located outside of Canada and, as a result, it may not be possible for Canadian purchasers to effect service of process within Canada upon us or those persons. All or a substantial portion of our assets and the assets of those persons may be located outside of Canada and, as a result, it may not be possible to satisfy a judgment against us or those persons in Canada or to enforce a judgment obtained in Canadian courts against us or those persons outside of Canada.
Taxation and Eligibility for Investment
Canadian purchasers of the shares should consult their own legal and tax advisors with respect to the tax consequences of an investment in the shares in their particular circumstances and about the eligibility of the investment by the purchaser under relevant Canadian legislation.
144
NOTICE TO RESIDENTS OF THE
EUROPEAN ECONOMIC AREA AND THE UNITED KINGDOM
In relation to each Member State of the European Economic Area which has implemented the Prospectus Directive (each, a “Relevant Member State”) an offer to the public of the Shares which are the subject of the offering contemplated by this prospectus may not be made in that Relevant Member State except that an offer to the public in that Relevant Member State of the Shares may be made at any time under the following exemptions under the Prospectus Directive, if they have been implemented in that Relevant Member State:
(a) to legal entities which are authorised or regulated to operate in the financial markets or, if not so authorised or regulated, whose corporate purpose is solely to invest in securities;
(b) to any legal entity which has two or more of (1) an average of at least 250 employees during the last financial year; (2) a total balance sheet of more than €43,000,000 and (3) an annual net turnover of more than €50,000,000, as shown in its last annual or consolidated accounts;
(c) by the underwriters to fewer than 100 natural or legal persons (other than “qualified investors” as defined in the Prospectus Directive) subject to obtaining the prior consent of each of Credit Suisse Securities (USA) LLC, Merrill Lynch, Pierce, Fenner & Smith Incorporated and J.P. Morgan Securities LLC for any such offer; or
(d) in any other circumstances falling within Article 3(2) of the Prospectus Directive,
provided that no such offer of the Shares shall result in a requirement for the publication by us or any underwriter of a prospectus pursuant to Article 3 of the Prospectus Directive.
Any person making or intending to make any offer within the EEA of the Shares which are the subject of the offering contemplated in this prospectus should only do so in circumstances in which no obligation arises for us or any of the underwriters to produce a prospectus for such offer. Neither we nor the underwriters have authorised, nor do they authorise, the making of any offer of the Shares through any financial intermediary, other than offers made by underwriters which constitute the final offering of Shares contemplated in this prospectus.
For the purposes of this provision, and the buyer’s representation below, the expression an “offer to the public” in relation to the Shares in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and the Shares to be offered so as to enable an investor to decide to purchase the Shares, as the same may be varied in that Relevant Member State by any measure implementing the Prospectus Directive in that Relevant Member State and the expression “Prospectus Directive” means Directive 2003/71/EC and includes any relevant implementing measure in each Relevant Member State.
Buyer’s Representation
Each person in a Relevant Member State who receives any communication in respect of, or who acquires the Shares under, the offers contemplated in this prospectus will be deemed to have represented, warranted and agreed to and with each underwriter and us that:
(a) it is a qualified investor within the meaning of the law in that Relevant Member State implementing Article 2(1)(e) of the Prospectus Directive; and
(b) in the case of the Shares acquired by it as a financial intermediary, as that term is used in Article 3(2) of the Prospectus Directive, (i) the Shares acquired by it in the offering have not been acquired on behalf of, nor have they been acquired with a view to their offer or resale to, persons in any Relevant Member State other than “qualified investors” as defined in the Prospectus Directive, or in circumstances in which the prior consent of each of Credit Suisse Securities (USA) LLC, Merrill Lynch, Pierce, Fenner & Smith Incorporated and J.P. Morgan Securities LLC has been given to the offer or resale; or (ii) where the Shares have been acquired by it on behalf of persons in any Relevant Member State other than qualified investors, the offer of the Shares to it is not treated under the Prospectus Directive as having been made to such persons.
145
NOTICE TO PROSPECTIVE INVESTORS IN SWITZERLAND
This document as well as any other material relating to the Shares which are the subject of the offering contemplated by this prospectus do not constitute an issue prospectus pursuant to Article 652a of the Swiss Code of Obligations. The Shares will not be listed on the SIX Swiss Exchange and, therefore, the documents relating to the Shares, including, but not limited to, this document, do not claim to comply with the disclosure standards of the listing rules of SIX Swiss Exchange and corresponding prospectus schemes annexed to the listing rules of the SIX Swiss Exchange.
The Shares are being offered in Switzerland by way of a private placement, i.e., to a small number of selected investors only, without any public offer and only to investors who do not purchase the Shares with the intention to distribute them to the public. The investors will be individually approached by the Issuer from time to time.
This document as well as any other material relating to the Shares is personal and confidential and do not constitute an offer to any other person. This document may only be used by those investors to whom it has been handed out in connection with the offering described herein and may neither directly nor indirectly be distributed or made available to other persons without express consent of the Issuer. It may not be used in connection with any other offer and shall in particular not be copiedand/or distributed to the public in (or from) Switzerland.
146
NOTICE TO PROSPECTIVE INVESTORS IN THE DUBAI INTERNATIONAL FINANCIAL CENTRE
This document relates to an exempt offer in accordance with the Offered Securities Rules of the Dubai Financial Services Authority. This document is intended for distribution only to persons of a type specified in those rules. It must not be delivered to, or relied on by, any other person. The Dubai Financial Services Authority has no responsibility for reviewing or verifying any documents in connection with exempt offers. The Dubai Financial Services Authority has not approved this document nor taken steps to verify the information set out in it, and has no responsibility for it. The Shares which are the subject of the offering contemplated by this prospectus may be illiquidand/or subject to restrictions on their resale. Prospective purchasers of the Shares offered should conduct their own due diligence on the Shares. If you do not understand the contents of this document you should consult an authorised financial adviser.
147
LEGAL MATTERS
The validity of the issuance of the shares of common stock offered hereby will be passed upon for us by Davis Polk & Wardwell LLP, Menlo Park, California and by Latham & Watkins LLP, Menlo Park, California for the underwriters.
EXPERTS
The consolidated financial statements of Mirion Technologies, Inc. at June 30, 2010 and 2009, and for each of the three years in the period ended June 30, 2010, appearing in this prospectus and Registration Statement have been audited by Ernst & Young LLP, independent registered public accounting firm, as set forth in their report thereon appearing elsewhere herein, and are included in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC, Washington, D.C. 20549, a Registration Statement onForm S-1 under the Securities Act with respect to the common stock offered hereby. This prospectus does not contain all of the information set forth in the registration statement and the exhibits and schedules thereto. For further information with respect to the company and its common stock, reference is made to the Registration Statement and the exhibits and any schedules filed therewith. Statements contained in this prospectus as to the contents of any contract or other document referred to are not necessarily complete and in each instance, if such contract or document is filed as an exhibit, reference is made to the copy of such contract or other document filed as an exhibit to the registration statement, each statement being qualified in all respects by such reference. A copy of the registration statement, including the exhibits and schedules thereto, may be read and copied at the SEC’s Public Reference Room at 100 F Street, N.E., Washington, D.C. 20549. Information on the operation of the Public Reference Room may be obtained by calling the SEC at1-800-SEC-0330. In addition, the SEC maintains an Internet website that contains reports, proxy statements and other information about issuers, like us, that file electronically with the SEC. The address of that site is www.sec.gov. The registration statement, including the exhibits and schedules thereto, are also available for reading and copying at the offices of NASDAQ Operations, 1735 K Street, N.W., Washington, D.C. 20006.
As a result of this offering, we will become subject to the full informational requirements of the Securities Exchange Act of 1934, as amended. We will fulfill our obligations with respect to such requirements by filing periodic reports and other information with the SEC. We intend to furnish our stockholders with annual reports containing consolidated financial statements certified by an independent public accounting firm. We also maintain an Internet site at www.mirion.com. Our website and the information contained therein or connected thereto shall not be deemed to be incorporated into this prospectus or the registration statement of which it forms a part.
148
MIRION TECHNOLOGIES, INC. AND SUBSIDIARIES
| | | | | |
| | | Page |
| |
Mirion Technologies, Inc. Consolidated Financial Statements: | | | | |
| | | F-2 | |
| | | F-3 | |
| | | F-4 | |
| | | F-5 | |
| | | F-6 | |
| | | F-7 | |
F-1
REPORT OF ERNST & YOUNG LLP,
INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Shareholders
Mirion Technologies, Inc.
We have audited the accompanying consolidated balance sheets of Mirion Technologies, Inc. and subsidiaries (the Company) as of June 30, 2010 and 2009, and the related consolidated statements of operations, stockholders’ equity, and cash flows for each of the three years in the period ended June 30, 2010. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. We were not engaged to perform an audit of the Company’s internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Mirion Technologies, Inc. and subsidiaries at June 30, 2010 and 2009, and the consolidated results of their operations and their cash flows for each of the three years in the period ended June 30, 2010, in conformity with U.S. generally accepted accounting principles.
As discussed in Note 2 to the consolidated financial statements, effective July 1, 2009, the Company changed its method of accounting for revenue recognition for arrangements with multiple elements with the adoption of Accounting Standards Update No. 2009-13, Multiple-Deliverable Revenue Arrangements, an amendment to Financial Accounting Standards Board Codification 605, Revenue Recognition.
/s/ Ernst & Young LLP
San Francisco, California
September 3, 2010
F-2
| | | | | | | | | | | | | | | | | |
| | | | | | | | | Pro Forma
| |
| | | | | | | | | Pro Forma | | | As Adjusted | |
| | | June 30, | | | June 30,
| | | June 30,
| |
| | | 2009 | | | 2010 | | | 2010 | | | 2010 | |
| | | | | | | | | (unaudited) | | | (unaudited) | |
| |
ASSETS |
| Current assets: | | | | | | | | | | | | | | | | |
| Cash and cash equivalents | | $ | 5,390 | | | $ | 9,936 | | | $ | | | | $ | | |
| Restricted cash — current | | | 1,515 | | | | 3,258 | | | | | | | | | |
| Accounts receivable — net of allowance for doubtful accounts | | | 40,165 | | | | 37,059 | | | | | | | | | |
| Costs in excess of billings on uncompleted contracts | | | 17,073 | | | | 19,474 | | | | | | | | | |
| Receivables pledged to creditors | | | 2,364 | | | | 113 | | | | | | | | | |
| Inventories — net of provision for excess and obsolete inventory | | | 33,728 | | | | 30,169 | | | | | | | | | |
| Prepaid expenses and other current assets | | | 5,253 | | | | 17,347 | | | | | | | | | |
| Deferred cost of revenue | | | 29,536 | | | | 17,072 | | | | | | | | | |
| Deferred income taxes — current | | | 5,541 | | | | 2,154 | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Total current assets | | | 140,565 | | | | 136,582 | | | | | | | | | |
| Property, plant and equipment — net | | | 18,080 | | | | 20,532 | | | | | | | | | |
| Goodwill | | | 139,021 | | | | 129,275 | | | | | | | | | |
| Intangible assets — net | | | 23,688 | | | | 16,406 | | | | | | | | | |
| Restricted cash | | | 4,532 | | | | 3,304 | | | | | | | | | |
| Deferred income taxes | | | 2,794 | | | | 65 | | | | | | | | | |
| Other assets | | | 1,074 | | | | 1,199 | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Total assets | | $ | 329,754 | | | $ | 307,363 | | | $ | | | | $ | | |
| | | | | | | | | | | | | | | | | |
| |
| LIABILITIES AND STOCKHOLDERS’ EQUITY |
| Current liabilities: | | | | | | | | | | | | | | | | |
| Accounts payable | | $ | 19,088 | | | $ | 17,183 | | | $ | | | | $ | | |
| Accrued expenses and other current liabilities | | | 35,008 | | | | 29,660 | | | | | | | | | |
| Distribution payable to ACAS | | | — | | | | — | | | | | | | | | |
| Income taxes payable | | | 1,452 | | | | 93 | | | | | | | | | |
| Deferred contract revenue | | | 62,031 | | | | 44,730 | | | | | | | | | |
| Deferred income taxes — current | | | 736 | | | | 1,714 | | | | | | | | | |
| Notes payable — current | | | 6,442 | | | | 648 | | | | | | | | | |
| Notes payable to ACAS — current | | | 520 | | | | 420 | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Total current liabilities | | | 125,277 | | | | 94,448 | | | | | | | | | |
| Notes payable to ACAS | | | 169,560 | | | | 182,832 | | | | | | | | | |
| Notes payable | | | 762 | | | | 1,974 | | | | | | | | | |
| Deferred income taxes | | | 14,339 | | | | 10,454 | | | | | | | | | |
| Other liabilities | | | 12,969 | | | | 15,465 | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Total liabilities | | | 322,907 | | | | 305,173 | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Commitments and contingencies (Note 9) | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Stockholders’ equity: | | | | | | | | | | | | | | | | |
Convertible Participating Preferred stock — $0.001 par value;Series A-1 authorized, 1,200,000 shares actual and pro forma (unaudited); issued and outstanding 678,804 shares at June 30, 2009 and 2010, liquidation preference of $110,352 at June 30, 2010; none issued and outstanding, pro forma (unaudited); none authorized, issued and outstanding, pro forma as adjusted (unaudited);Series A-2 authorized, 300,000 shares actual and pro forma (unaudited); issued and outstanding 70,000 shares at June 30, 2009 and 2010, liquidation preference of $15,506 at June 30, 2010; none issued and outstanding, pro forma (unaudited); none authorized, issued and outstanding, pro forma as adjusted (unaudited) | | | 1 | | | | 1 | | | | | | | | | |
| Preferred stock — $0.001 par value; 1,500,000 shares authorized actual, pro forma (unaudited) and pro forma as adjusted (unaudited); none issued and outstanding, actual, pro forma (unaudited) and pro forma as adjusted (unaudited) | | | — | | | | — | | | | | | | | | |
| Common stock — $0.001 par value; Class A — authorized, 61,328,125 shares actual and pro forma (unaudited); issued and outstanding, 17,773 shares at June 30, 2009 and 2010; none issued and outstanding, pro forma (unaudited); none authorized, issued and outstanding, pro forma as adjusted (unaudited); Class B — authorized, 17,171,875 shares actual and pro forma (unaudited); issued and outstanding 388,023 shares at June 30, 2009 and 2010; none issued and outstanding, pro forma (unaudited); none authorized, issued and outstanding, pro forma as adjusted (unaudited) | | | — | | | | — | | | | | | | | | |
| Common stock, $0.001 par value; 78,500,000 shares authorized, actual, pro forma (unaudited); and pro forma as adjusted (unaudited), none issued and outstanding actual; 11,103,724 shares issued and outstanding, pro forma (unaudited); shares issued and outstanding, pro forma as adjusted (unaudited) | | | — | | | | — | | | | | | | | | |
| Additional paid-in capital | | | 98,478 | | | | 99,585 | | | | | | | | | |
| Accumulated deficit | | | (102,561 | ) | | | (96,596 | ) | | | | | | | | |
| Accumulated other comprehensive income (loss) | | | 10,929 | | | | (800 | ) | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Total stockholders’ equity | | | 6,847 | | | | 2,190 | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Total liabilities and stockholders’ equity | | $ | 329,754 | | | $ | 307,363 | | | $ | | | | $ | | |
| | | | | | | | | | | | | | | | | |
See notes to consolidated financial statements.
F-3
| | | | | | | | | | | | | |
| | | Years Ended June 30, | |
| | | 2008 | | | 2009 | | | 2010 | |
| |
| Revenue | | $ | 191,769 | | | $ | 201,763 | | | $ | 228,124 | |
| Cost of revenue | | | 102,790 | | | | 105,954 | | | | 126,707 | |
| | | | | | | | | | | | | |
| Gross profit | | | 88,979 | | | | 95,809 | | | | 101,417 | |
| | | | | | | | | | | | | |
| Operating expenses: | | | | | | | | | | | | |
| Selling, general and administrative expenses | | | 63,177 | | | | 65,649 | | | | 64,510 | |
| Research and development | | | 14,865 | | | | 11,282 | | | | 8,729 | |
| | | | | | | | | | | | | |
| Total operating expenses | | | 78,042 | | | | 76,931 | | | | 73,239 | |
| | | | | | | | | | | | | |
| Income from operations | | | 10,937 | | | | 18,878 | | | | 28,178 | |
| Interest income | | | (143 | ) | | | (78 | ) | | | (24 | ) |
| Interest expense | | | 20,350 | | | | 17,789 | | | | 15,144 | |
| Other income, net | | | (1,759 | ) | | | (490 | ) | | | (639 | ) |
| | | | | | | | | | | | | |
| (Loss) income before provision for income taxes | | | (7,511 | ) | | | 1,657 | | | | 13,697 | |
| Provision for income taxes | | | 5,838 | | | | 5,612 | | | | 7,732 | |
| | | | | | | | | | | | | |
| Net (loss) income | | | (13,349 | ) | | | (3,955 | ) | | | 5,965 | |
| Paid-in-kind preferred dividends | | | (8,993 | ) | | | (9,892 | ) | | | (10,923 | ) |
| | | | | | | | | | | | | |
| Net loss allocable to common stockholders | | $ | (22,342 | ) | | $ | (13,847 | ) | | $ | (4,958 | ) |
| | | | | | | | | | | | | |
| Net loss per common share allocable to common stockholders per share — basic and diluted | | $ | (55.14 | ) | | $ | (34.12 | ) | | $ | (12.22 | ) |
| | | | | | | | | | | | | |
| Weighted average number of shares used in computing net loss allocable to common stockholders — basic and diluted | | | 405,159 | | | | 405,796 | | | | 405,796 | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| Pro forma net loss per share allocable to common stockholders before conversion of preferred shares — basic and diluted (unaudited) | | | | | | | | | | $ | | |
| | | | | | | | | | | | | |
| Pro forma net income per common share — basic (unaudited) | | | | | | | | | | $ | | |
| | | | | | | | | | | | | |
| Pro forma net income per common share — diluted (unaudited) | | | | | | | | | | $ | | |
| | | | | | | | | | | | | |
| Pro forma net income per common share — basic as adjusted (unaudited) | | | | | | | | | | $ | | |
| | | | | | | | | | | | | |
| Pro forma net income per common share — diluted as adjusted (unaudited) | | | | | | | | | | $ | | |
| | | | | | | | | | | | | |
| Shares used in computing pro forma net loss per share allocable to common stockholders before conversion of preferred stock — basic and diluted (unaudited) | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| Shares used in computing pro forma net income per share — basic and basic as adjusted (unaudited) | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| Shares used in computing pro forma net income per share — diluted and diluted as adjusted (unaudited) | | | | | | | | | | | | |
| | | | | | | | | | | | | |
See notes to consolidated financial statements.
F-4
MIRION TECHNOLOGIES, INC.
(in thousands, except share amounts)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Convertible
| | | | | | | | | | | | | | | Accumulated
| | | | |
| | | Participating
| | | | | | | | | Additional
| | | | | | Other
| | | Total
| |
| | | Preferred Stock | | | Common Stock | | | Paid-in
| | | Accumulated
| | | Comprehensive
| | | Stockholders’
| |
| | | Shares | | | Amount | | | Shares | | | Amount | | | Capital | | | Deficit | | | Income (Loss) | | | Equity | |
| BALANCE — June 30, 2007 | | | 748,804 | | | $ | 1 | | | | 404,521 | | | $ | — | | | $ | 97,056 | | | $ | (85,257 | ) | | $ | 9,463 | | | $ | 21,263 | |
| Exercise of stock options | | | — | | | | — | | | | 1,275 | | | | — | | | | 13 | | | | — | | | | — | | | | 13 | |
| Stock-based compensation | | | — | | | | — | | | | — | | | | — | | | | 248 | | | | — | | | | — | | | | 248 | |
| Preferred stock dividend | | | — | | | | — | | | | — | | | | — | | | | (8,993 | ) | | | — | | | | — | | | | (8,993 | ) |
| Dividends distributable | | | — | | | | — | | | | — | | | | — | | | | 8,993 | | | | — | | | | — | | | | 8,993 | |
| Components of comprehensive loss: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | (13,349 | ) | | | — | | | | (13,349 | ) |
| Unrecognized actuarial gains and prior service benefit, net of tax of $65 | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 161 | | | | 161 | |
| Foreign currency translation, net of tax of $0 | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 10,816 | | | | 10,816 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Total comprehensive loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (2,372 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| BALANCE — June 30, 2008 | | | 748,804 | | | | 1 | | | | 405,796 | | | | — | | | | 97,317 | | | | (98,606 | ) | | | 20,440 | | | | 19,152 | |
| Stock-based compensation | | | — | | | | — | | | | — | | | | — | | | | 1,161 | | | | — | | | | — | | | | 1,161 | |
| Preferred stock dividend | | | — | | | | — | | | | — | | | | — | | | | (9,892 | ) | | | — | | | | — | | | | (9,892 | ) |
| Dividends distributable | | | — | | | | — | | | | — | | | | — | | | | 9,892 | | | | — | | | | — | | | | 9,892 | |
| Components of comprehensive loss: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | (3,955 | ) | | | — | | | | (3,955 | ) |
| Unrecognized actuarial losses and prior service benefit, net of tax of $22 | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (192 | ) | | | (192 | ) |
| Foreign currency translation, net of tax of $0 | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (9,319 | ) | | | (9,319 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Total comprehensive loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (13,466 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| BALANCE — June 30, 2009 | | | 748,804 | | | | 1 | | | | 405,796 | | | | — | | | | 98,478 | | | | (102,561 | ) | | | 10,929 | | | | 6,847 | |
| Stock-based compensation | | | — | | | | — | | | | — | | | | — | | | | 1,107 | | | | — | | | | — | | | | 1,107 | |
| Preferred stock dividend | | | — | | | | — | | | | — | | | | — | | | | (10,923 | ) | | | — | | | | — | | | | (10,923 | ) |
| Dividends distributable | | | — | | | | — | | | | — | | | | — | | | | 10,923 | | | | — | | | | — | | | | 10,923 | |
| Components of comprehensive loss: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net income | | | — | | | | — | | | | — | | | | — | | | | — | | | | 5,965 | | | | — | | | | 5,965 | |
| Unrecognized actuarial losses and prior service benefit, net of tax of $54 | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (424 | ) | | | (424 | ) |
| Foreign currency translation, net of tax of $0 | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (11,305 | ) | | | (11,305 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Total comprehensive loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (5,764 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| BALANCE — June 30, 2010 | | | 748,804 | | | $ | 1 | | | | 405,796 | | | $ | — | | | $ | 99,585 | | | $ | (96,596 | ) | | $ | (800 | ) | | $ | 2,190 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
See notes to consolidated financial statements.
F-5
MIRION TECHNOLOGIES, INC.
(in thousands)
| | | | | | | | | | | | | |
| | | Year Ended June 30, | |
| | | 2008 | | | 2009 | | | 2010 | |
Operating activities: | | | | | | | | | | | | |
| Net (loss) income | | $ | (13,349 | ) | | $ | (3,955 | ) | | $ | 5,965 | |
| Adjustments to reconcile net (loss) income to net cash (used in) provided by operating activities: | | | | | | | | | | | | |
| Depreciation and amortization | | | 14,182 | | | | 12,479 | | | | 12,052 | |
| Actuarial (gain) loss | | | (226 | ) | | | 208 | | | | 530 | |
Paid-in-kind interest expense | | | 1,904 | | | | 1,992 | | | | 2,091 | |
| Stock-based compensation | | | 248 | | | | 1,161 | | | | 1,107 | |
| Loss on disposal of property, plant and equipment | | | 502 | | | | 592 | | | | 859 | |
| Amortization of loan fees, debt discounts and preferred stock discounts | | | 785 | | | | 522 | | | | 458 | |
| Provision for doubtful accounts | | | 30 | | | | 140 | | | | 138 | |
| Change in deferred income taxes | | | (1,238 | ) | | | (1,013 | ) | | | 3,062 | |
| Change in estimated fair value of derivative instruments | | | 9 | | | | (11 | ) | | | 76 | |
| Changes in operating assets and liabilities: | | | | | | | | | | | | |
| Accounts receivable | | | (3,712 | ) | | | (6,401 | ) | | | (911 | ) |
| Receivables pledged to creditors | | | (2,389 | ) | | | 2,273 | | | | 2,215 | |
| Prepaid expenses and other current assets | | | 1,928 | | | | (1,622 | ) | | | (12,398 | ) |
| Inventories | | | (2,236 | ) | | | (609 | ) | | | 484 | |
| Other assets | | | 350 | | | | 112 | | | | (672 | ) |
| Deferred cost of revenue | | | (6,215 | ) | | | (7,569 | ) | | | 9,859 | |
| Costs in excess of billings on uncompleted contracts | | | (9,532 | ) | | | 2,663 | | | | (6,365 | ) |
| Accounts payable | | | 4,029 | | | | (3,771 | ) | | | (90 | ) |
| Accrued expenses and other current liabilities | | | 1,809 | | | | 2,688 | | | | (3,211 | ) |
| Income taxes payable | | | (6,338 | ) | | | (1,103 | ) | | | (1,358 | ) |
| Deferred contract revenue | | | 6,671 | | | | 9,320 | | | | (9,929 | ) |
| Other liabilities | | | 6,076 | | | | 1,935 | | | | 4,240 | |
| | | | | | | | | | | | | |
| Net cash (used in) provided by operating activities | | | (6,712 | ) | | | 10,031 | | | | 8,202 | |
| | | | | | | | | | | | | |
Investing activities | | | | | | | | | | | | |
| Purchases of property, plant and equipment | | | (4,953 | ) | | | (6,649 | ) | | | (10,437 | ) |
| Return of escrow funds | | | 2,750 | | | | — | | | | — | |
| Change in restricted cash | | | (1,093 | ) | | | (779 | ) | | | (737 | ) |
| | | | | | | | | | | | | |
| Net cash used in investing activities | | | (3,296 | ) | | | (7,428 | ) | | | (11,174 | ) |
| | | | | | | | | | | | | |
Financing activities: | | | | | | | | | | | | |
| Borrowings from notes payable to ACAS | | | 13,780 | | | | 6,600 | | | | 12,717 | |
| Payments of notes payable to ACAS | | | (3,520 | ) | | | (10,576 | ) | | | (428 | ) |
| Net payments from notes payable to third parties | | | (318 | ) | | | (298 | ) | | | 1,712 | |
| Net borrowings (payments) under revolving credit facility | | | 3,396 | | | | (1,556 | ) | | | (5,861 | ) |
| Proceeds from issuance of common stock | | | 13 | | | | — | | | | — | |
| | | | | | | | | | | | | |
| Net cash provided by (used in) financing activities | | | 13,351 | | | | (5,830 | ) | | | 8,140 | |
| | | | | | | | | | | | | |
| Effect of exchange rate changes on cash and cash equivalents | | | (945 | ) | | | (342 | ) | | | (622 | ) |
| | | | | | | | | | | | | |
| Net increase (decrease) in cash and cash equivalents | | | 2,398 | | | | (3,569 | ) | | | 4,546 | |
| Cash and cash equivalents at beginning of period | | | 6,561 | | | | 8,959 | | | | 5,390 | |
| | | | | | | | | | | | | |
| Cash and cash equivalents at end of period | | $ | 8,959 | | | $ | 5,390 | | | $ | 9,936 | |
| | | | | | | | | | | | | |
Supplemental information: | | | | | | | | | | | | |
| Cash paid for interest | | $ | 16,515 | | | $ | 15,505 | | | $ | 12,514 | |
| | | | | | | | | | | | | |
| Cash paid for income taxes | | $ | 7,179 | | | $ | 6,277 | | | $ | 6,965 | |
| | | | | | | | | | | | | |
Paid-in-kind preferred dividends | | $ | 8,993 | | | $ | 9,892 | | | $ | 10,923 | |
| | | | | | | | | | | | | |
See notes to consolidated financial statements.
F-6
MIRION TECHNOLOGIES, INC.
| |
| 1. | ORGANIZATION AND OPERATIONS OF THE COMPANY |
Mirion Technologies, Inc. (“Mirion” or the “Company”) is a global provider of radiation detection, measurement, analysis and monitoring products and services to the nuclear, defense and medical end markets. Mirion was incorporated in October 2005 as Global Monitoring Systems, Inc. in the state of Delaware and subsequently changed its name to Mirion Technologies, Inc. in January 2006.
Mirion was formed through the combination of three companies: Global Dosimetry Solutions, Inc. (“GDS”), Imaging and Sensing Technology Corporation (“IST”) and Synodys SA (“Synodys”), all owned by American Capital, Ltd. and affiliates (together “ACAS”). The combination ( “Reorganization”) was effective as of December 31, 2005. Because the three companies were under the common control of ACAS, the Reorganization was accounted for by combining the assets, liabilities and accumulated deficit of the three companies at each company’s historical basis. The accompanying financial statements and related notes present the combined financial performance of the three companies.
The Company is headquartered in San Ramon, California, and has operations in the United States, Canada, the United Kingdom, France, Germany, Finland and China.
Basis of Presentation — The accompanying annual consolidated financial statements of the Company include the accounts of the Company and its wholly owned subsidiaries. All significant intercompany balances and transactions have been eliminated in consolidation.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. The Company has incurred significant losses and negative cash flows from operations since its inception. At June 30, 2010, the Company had an accumulated deficit of $96.6 million, and approximately $183.3 million of debt obligations due to American Capital Financial Services (“ACFS”), a related party. The Company has negotiated extensions on the repayment of its debt obligations with ACFS. As more fully disclosed in Note 8, debt obligations due in 2011 to ACFS total $420,000, and debt obligations due in 2012 to ACFS total approximately $182.8 million. The Company plans to continue to finance operations with a combination of operating cash flows and debt and equity issuances. There is no assurance that this additional funding will be adequate to support the Company’s long-term needs. If adequate long-term funding is not available, the Company may be required to modify its operational plan. The accompanying consolidated financial statements do not include any adjustments that might result from the outcome of these uncertainties.
Unaudited Pro Forma Information — If a public offering is consummated, all of the currently outstanding shares of Convertible Participating Preferred Stock will convert into 10,697,928 shares of common stock, and currently outstanding shares of Class A and Class B Common Stock will convert on a one-for-one basis to 405,796 shares of common stock based on the number of shares outstanding as of June 30, 2010. The accompanying consolidated balance sheets include unaudited pro forma balance sheet information to reflect the anticipated accrued distribution payable to its principal stockholder, ACAS, in addition to the conversion of the outstanding preferred and common stock. The accompanying consolidated balance sheets also include the unaudited pro forma balance sheet as adjusted to reflect the anticipated effects of the offering, the distribution payment to ACAS and new debt arrangements entered into concurrently with the offering.
F-7
MIRION TECHNOLOGIES, INC.
Notes to Consolidated Financial Statements — (Continued)
Unaudited pro forma balance sheet information has been included in the accompanying Consolidated Balance Sheets to give effect to the following adjustments:
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | Pro forma —
| |
| | | | | | | | | Pro forma | | | | | | As Adjusted | |
| | | June 30,
| | | Pro forma
| | | June 30,
| | | Additional
| | | June 30,
| |
Pro forma Balance Sheet Adjustments | | 2010 | | | Adjustments | | | 2010 | | | Adjustments | | | 2010 | |
| |
Assets: | | | | | | | | | | | | | | | | | | | | |
| Cash and cash equivalents | | | 9,936 | | | | | | | | | | | | (e | ) | | | | |
| | | | | | | | | | | | | | | | (f | ) | | | | |
| | | | | | | | | | | | | | | | (g | ) | | | | |
| Prepaid expenses and other current assets | | | 17,347 | | | | | | | | | | | | (e | ) | | | | |
| Other assets | | | 1,199 | | | | | | | | | | | | | | | | | |
| Total assets | | | 307,363 | | | | | | | | | | | | (f | ) | | | | |
Liabilities and Stockholders’ Equity: | | | | | | | | | | | | | | | | | | | | |
| Distribution payable to ACAS | | | — | | | | (a | ) | | | | | | | (e | ) | | | | |
| | | | | | | | (b | ) | | | | | | | (e | ) | | | | |
| | | | | | | | (b | ) | | | | | | | (g | ) | | | | |
| Notes payable — current | | | 648 | | | | | | | | | | | | (f | ) | | | | |
| Notes payable to ACAS — current | | | 420 | | | | (b | ) | | | | | | | | | | | | |
| Notes payable to ACAS | | | 182,832 | | | | (b | ) | | | | | | | | | | | | |
| Notes payable | | | 1,974 | | | | | | | | | | | | (f | ) | | | | |
| Total liabilities | | | 305,173 | | | | | | | | | | | | | | | | | |
| Preferred Stock | | | 1 | | | | (c | ) | | | | | | | | | | | | |
| Common stock | | | — | | | | (c | )(d) | | | | | | | | | | | | |
| Additional paid-in capital | | | 99,585 | | | | | | | | | | | | (e | ) | | | | |
| | | | | | | | | | | | | | | | (e | ) | | | | |
| | | | | | | | | | | | | | | | (h | ) | | | | |
| Accumulated deficit | | | (96,596 | ) | | | (a | ) | | | | | | | (h | ) | | | | |
| | | | | | | | | | | | | | | | (e | ) | | | | |
| | | | | | | | | | | | | | | | | | | | | |
| Accumulated other comprehensive loss | | | (800 | ) | | | | | | | | | | | | | | | | |
| Total stockholders’ equity (deficit) | | | 2,190 | | | | | | | | | | | | | | | | | |
| Total liabilities and stockholders’ equity (deficit) | | | 307,363 | | | | | | | | | | | | | | | | | |
| | |
| (a) | | An $8.0 million increase in distribution payable to ACAS to reflect the accrual of an $8.0 million payment to ACAS to terminate an investment banking services agreement, with a corresponding increase in accumulated deficit. No tax benefit has been provided related to this expense because the Company is in a net operating loss position and has a full valuation allowance in the affected jurisdiction. |
| | |
| (b) | | A $183.3 million reduction in notes payable to ACAS to reflect the assumed distribution to ACAS in connection with this offering, with a corresponding increase in distribution payable to ACAS. Of this reduction, $ million will be paid using proceeds of the offering and $ million will be paid with proceeds from new third-party debt arrangements. |
| | |
| (c) | | The conversion of the outstanding shares of Series A Convertible Participating Preferred Stock into 10,697,928 shares of common stock as of June 30, 2010, based upon a conversion rate of 9.6288 forSeries A-1 preferred stock and 8.8128 for Series A-2 preferred stock. |
| | |
| (d) | | The conversion of 17,773 outstanding shares of Class A Voting Common Stock and 388,023 outstanding shares of Class B Non-Voting Common Stock into 405,796 shares of common stock as of June 30, 2010. These shares will convert on a one-for-one basis. |
In addition, the pro forma balance sheet information reflects the effects of the adoption of our Fourth Amended and Restated Certificate of Incorporation prior to the effectiveness of this offering.
F-8
MIRION TECHNOLOGIES, INC.
Notes to Consolidated Financial Statements — (Continued)
Unaudited pro forma as adjusted balance sheet information has been included in the accompanying Consolidated Balance Sheets to give effect to the following factors in addition to those described above:
| | |
| (e) | | The issuance and sale of shares of common stock in this offering, at an assumed initial public offering price of $ for gross proceeds of $ million, and the receipt of the net proceeds of $ million after deducting $ million of underwriting discounts and commissions and estimated offering expenses paid or payable by the Company. Of the $ million total offering costs paid or payable by the Company, $ million were previously paid by the Company and have been included in prepaid expenses and other current assets. The pro forma as adjusted amounts include a decrease in prepaid expenses and other current assets to reflect the reclassification of these amounts to equity at the completion of the offering and the corresponding increase in cash and cash equivalents representing the amount of the net proceeds retained by the Company to offset offering costs already paid. The net proceeds from this offering were used to make payments to ACAS of $8.0 million and $ million resulting in a reduction in the distribution payable to ACAS. The net proceeds from this offering were also used to make bonus payments of $0.8 million, with a corresponding increase in accumulated deficit. No tax benefit has been provided related to this expense as the Company is in a net operating loss position and has a full valuation allowance in the affected jurisdictions. |
| | |
| (f) | | A $ million increase in notes payable and a $ million increase in the current portion of notes payable, representing principal payments due within twelve months of executing the new notes, to reflect new debt arrangements entered into concurrently with the completion of this offering and the receipt of $ million of cash, net of $ million of loan origination fees incurred in connection with the new debt arrangements. These fees have been reflected as an increase in other assets. |
| | |
| (g) | | A $ million decrease in cash and cash equivalents and distribution payable to ACAS to reflect the distribution to ACAS made to repay debt obligations that were refinanced with a third party. |
| | |
| (h) | | A $ million increase in additional paid-in capital and accumulated deficit to reflect the compensation expense associated with certain employee stock options that will vest upon this offering. No tax benefit has been provided related to this expense because the Company is in a net operating loss position and has a full valuation allowance in the affected jurisdictions. The compensation expense relates to unrecognized compensation expense associated with unvested employee stock options granted in August 2008 that, under the original terms of the grant, vest ratably over four years from the date of grant, with accelerated vesting of any unvested shares upon consummation of an initial public offering. We estimated the value of these options on the date of grant using the Black-Scholes model and based on the valuation assumptions detailed in Note 13 to the consolidated financial statements. The pro forma as adjusted balance sheet has not been adjusted to reflect the compensation expense associated with 124,448 stock options that are expected to be granted to our employees and outside directors immediately following the pricing of this offering. These options are expected to have a four year vesting term, and the compensation expense assuming the offering occurred at the end of fiscal 2010 will be recognized ratably over the following four years. Additionally, the pro forma as adjusted balance sheet has not been adjusted to reflect the compensation expense associated with performance-based call options with market conditions held by the Company’s Chief Executive Officer because the compensation expense associated with these options assuming the offering occurred at the end of fiscal 2010 will be recognized in subsequent periods based on the implied requisite service period for the market conditions (30 days subsequent to the offering for one-half of the shares, and 24 months subsequent to the offering for one-half of the shares). |
In addition the pro forma as adjusted balance sheet information reflects the effects of the adoption of our Fifth Amended and Restated Certificate of Incorporation prior to the consummation of this offering solely to eliminate the designations of our Series A convertible participating Preferred Stock and our Class A Voting Common Stock and Class B Voting Common Stock.
F-9
MIRION TECHNOLOGIES, INC.
Notes to Consolidated Financial Statements — (Continued)
The following table sets forth the computation of unaudited basic and diluted pro forma net loss per common share and the unaudited pro forma net income per common share-as adjusted for the periods indicated (in thousands, except share and per share amounts):
| | | | | |
| | | Year Ended
| |
| | | June 30,
| |
| | | 2010 | |
| | | (unaudited) | |
| |
Numerator: | | | | |
| Net loss allocable to common stockholders | | $ | (4,958 | ) |
| Interest expense from paydown of ACAS debt using proceeds of offering(1) | | | | |
| Pro forma net loss allocable to common stockholders before conversion of preferred shares | | | | |
| Effect of preferred stock dividends(2) | | | 10,923 | |
| Pro forma net income allocable to common stockholders | | | | |
| Interest expense reduction from refinancing of ACAS debt(3) | | | | |
| Compensation expense from employee stock options and call options(4) | | | | |
| Pro forma net income allocable to common stockholders — as adjusted | | | | |
| | | | | |
| | | Year Ended
| |
| | | June 30, 2010 | |
| | | (unaudited) | |
| |
Denominator: | | | | |
| Weighted average shares outstanding from conversion of Class A and B Voting Common Stock(5) | | | 405,796 | |
| Common shares issued in offering(6) | | | | |
| | | | | |
| Shares used in computing pro forma net loss per common share before conversion of preferred shares — basic and diluted(9) | | | | |
| Weighted average shares outstanding from conversion of convertible preferred stock(7) | | | 10,233,721 | |
| | | | | |
| Shares used in computing pro forma net income per common share — basic and basic as adjusted | | | | |
| Weighted average common shares outstanding from exercise of warrants(8) | | | 3,420,636 | |
| Weighted average common share equivalents of stock option(9) | | | 73,879 | |
| | | | | |
| Shares used in computing pro forma net income per common share — diluted and diluted as adjusted | | | | |
| | | | | |
Footnotes:
| | |
| (1) | | The pro forma reduction in interest expense assumes the repayment of $ million of ACAS debt using the net proceeds from this offering, giving effect to the elimination of the related interest expense as of the beginning of the period presented. The amount of interest expense eliminated by this adjustment is calculated from actual interest expense of $ million recorded in fiscal 2010 in connection with the specific debt arrangements that will be repaid with a portion of the net proceeds of this offering. No tax expense has been provided related to this reduction in interest expense because the Company is in a net operating loss position and has a full valuation allowance in the affected jurisdiction. |
| | |
| (2) | | The effect of preferred stock dividends is added back as a reduction to net loss allocable to common stockholders, assuming that all preferred stock has been converted into common shares as of the beginning of the period presented. |
| | |
| (3) | | All ACAS debt not assumed to be repaid from the net proceeds from this offering is assumed to be refinanced with a loan from a third-party bank, at interest rates averaging approximately % lower than existing |
F-10
MIRION TECHNOLOGIES, INC.
Notes to Consolidated Financial Statements — (Continued)
| | |
| | average interest rates with ACAS giving effect to the elimination of the related interest expense as of the beginning of the period presented. The amount of interest expense eliminated by this adjustment is calculated by taking the difference between the actual interest expense of $ million recorded in fiscal 2010 in connection with the specific debt arrangements that will be repaid with the net proceeds of the new debt arrangements entered into concurrently with the completion of this offering and the interest expense on the new debt arrangements, calculated as the total of the new debt arrangements of $ million multiplied by the average interest rate on those arrangements of approximately % for fiscal 2010. No tax expense has been provided related to this reduction in interest expense because the Company is in a net operating loss position and has a full valuation allowance in the affected jurisdictions. |
| | |
| (4) | | The pro forma increase in compensation expense associated with employee stock options and call options reflects: |
| | |
| | • | A compensation charge associated with 124,448 stock options that are expected to be granted to the Company’s employees and outside directors immediately following the pricing of this offering at an exercise price equal to the initial public offering price. The value of these options was estimated to be $ million using the Black-Scholes option pricing model and key assumptions are as follows: expected option term of years, risk-free interest rate will be updated at the date of grant but is currently estimated to be %, dividend yield of 0%, volatility will be updated at the date of grant but is currently estimated to be % and an exercise price and fair value of $ per share (the midpoint of the price range set forth on the cover page of this prospectus). Compensation expense will be recognized on these options over an expected four year vesting term, and as such, the compensation expense for fiscal 2010 was assumed to be 25% of the total value, or $ million. |
| | |
| | • | A compensation charge associated with 463,794 performance-based call options with market conditions held by Thomas D. Logan, the Company’s Chief Executive Officer. These options contain vesting provisions based upon successful completion of an initial public offering or change in control and achievement by ACAS of certain internal rates of return or returns on investment. The value of these options was estimated to be $2.1 million using a Monte Carlo simulation model and key assumptions are as follows: at the January 1, 2006 modification, expected option term of 4.5 years, risk-free interest rate of 4.3%, dividend yield of 0%, volatility of 41.8%, exercise price of $10.45 per share and fair value of $2.22 per share; at the December 7, 2007 modification, expected option term of 2.6 years, risk-free interest rate of 3.1%, dividend yield of 0%, volatility of 39.8%, exercise price of $10.45 per share and fair value of $4.44 per share. The compensation expense associated with these options for fiscal 2010 was estimated to be $1.5 million, based on the implied requisite service period for the market conditions (30 days for one-half of the shares, and 24 months for one-half of the shares). |
| | |
| (5) | | The weighted average common shares outstanding from the conversion of common stock assumes the conversion of all outstanding shares of Class A Voting Common Stock and Class B Non-Voting Common Stock on a one-for-one basis into 405,796 shares of common stock. |
| | |
| (6) | | Includes shares of the Company’s common stock to be sold in connection with this offering. Because distributions to ACAS, the Company’s principal stockholder, consisting of obligations under existing debt arrangements of $183.3 million and amounts due of $8.0 million to terminate an existing investment banking services agreement, exceed the Company’s earnings plus gross proceeds from this offering of $ million, all common shares are included in the calculation under existing rules on pro |
F-11
MIRION TECHNOLOGIES, INC.
Notes to Consolidated Financial Statements — (Continued)
| | |
| | forma calculations. Following is a calculation of the deemed dividend in excess of proceeds from this offering (in thousands): |
| | | | | |
| | | For the Twelve
| |
| | | Months Ended
| |
| | | June 30, 2010 | |
| |
| Gross proceeds from offering | | $ | | |
| Distributions to ACAS: | | | | |
| Termination of investment banking services agreement | | | | |
| Repayment of notes payable to ACAS from proceeds of offering | | | | |
| Repayment of notes payable to ACAS from new debt arrangements | | | | |
| | | | | |
| Total distribution to ACAS | | | | |
| Last twelve months earnings | | | | |
| | | | | |
| Excess of dividend to be paid over proceeds from offering | | $ | | |
| | | | | |
| | |
| (7) | | The weighted average common shares outstanding from the conversion of preferred stock assumes the conversion of all outstanding convertible preferred stock into common stock, including the conversion into common stock of all accrued and unpaidpaid-in-kind dividends on convertible preferred stock. The weighted average at June 30, 2010 is comprised of 936,573A-1 preferred shares, which includes 257,769 accrued but unpaidpaid-in-kind dividends, which are convertible at a rate of 9.6288 and 137,941A-2 preferred shares, which includes 67,941 accrued but unpaidpaid-in-kind dividends, which are convertible at a rate of 8.8128. The increase in number of preferred shares between periods is due to the monthly accrual of preferred dividends which are paid in the form of additional shares of convertible preferred stock. |
| | |
| (8) | | These warrants only become exercisable upon a sale, liquidation or dissolution of the Company or approval by the Company’s Board of Directors. Our Board of Directors has resolved that all of these warrants will become exercisable upon the consummation of this offering. |
| | |
| (9) | | The shares used in computing pro forma net loss per common share before conversion of preferred shares — diluted for the year ended June 30, 2010 exclude options to purchase 924,830 shares of common stock because the Company recorded a pro forma net loss allocable to common stockholders before conversion of preferred shares, and therefore the impact of such shares would be anti-dilutive. The shares used in computing pro forma net income per common share — diluted and diluted as adjusted for the year ended June 30, 2010 exclude options to purchase 662,353 shares of common stock because the effect would be anti-dilutive. 476,841 of these shares were excluded because the option exercise prices exceeded the average market value of our common stock during the period. 185,512 of these shares were excluded because after applying the treasury stock method of calculating earnings per share, the impact would be anti-dilutive. The shares used in computing pro forma net loss per common share before conversion of preferred shares — basic and diluted and pro forma net income per common share — diluted and diluted as adjusted for the year ended June 30, 2010 also exclude options to purchase 124,448 shares of common stock which are expected to be granted immediately following the pricing of this offering because their impact would be anti-dilutive, either because the Company generated a net loss or because after applying the treasury stock method of calculating earnings per share, the impact would be anti-dilutive. |
F-12
MIRION TECHNOLOGIES, INC.
Notes to Consolidated Financial Statements — (Continued)
| |
| 2. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES |
Use of Estimates — The Company’s consolidated financial statements are prepared in conformity with U.S. generally accepted accounting principles as set forth in the Financial Accounting Standards Board Accounting Standards Codification (ASC) and consider the various staff accounting bulletins and other applicable guidance issued by the U.S. Securities and Exchange Commission. These accounting principles require the use of estimates, judgments and assumptions that affect the reported amounts of assets and liabilities at the date of the financial statements and the reported amounts of revenue and expenses during the periods presented. Specific areas, among others, requiring the application of management’s estimates and judgment include assumptions pertaining to the valuation of fixed assets, including depreciable lives assigned, the estimated earnings on contracts in progress, accruals, stock-based compensation costs, pension and post-employment benefit costs, fair value of derivatives, future cash flows associated with impairment testing of goodwill and other long-lived assets, credit worthiness of customers, uncertain tax positions, tax valuation allowances and legal, environmental and insurance matters. Management believes that the accounting estimates employed are appropriate and the resulting balances are reasonable; however, due to the inherent uncertainties in making estimates, actual results could differ from the original estimates.
Cash and Cash Equivalents — The Company considers all cash on deposit, money market accounts and highly liquid debt instruments purchased with original maturities of three months or fewer to be cash and cash equivalents. Cash equivalents primarily consist of amounts held in interest-bearing money market accounts that are readily convertible to cash.
The Company invests its excess cash with high credit quality financial institutions. The Company reviews its investments in money market accounts or other instruments for potential impairment on a regular basis. As part of the evaluation process, the Company considers the credit ratings of these securities and its intent and ability to hold the investment for a period of time sufficient to allow for any anticipated improvement in financial condition.
Restricted Cash — The Company maintains restricted cash and cash equivalent accounts held with financial institutions to support performance bonds with irrevocable letters of credit for contractual obligations to certain customers.
Accounts Receivable and Allowance for Doubtful Accounts — Accounts receivable are recorded at the invoiced amount and do not bear interest. The allowance for doubtful accounts is based on the Company’s assessment of the collectability of customer accounts. The Company regularly reviews the allowance by considering factors such as historical experience, credit quality, the age of the accounts receivable balances and current economic conditions that may affect a customer’s ability to pay. The Company has an allowance for doubtful accounts of $135,000 and $151,000 as of June 30, 2009 and 2010.
Accounts Receivable Securitization —The Company has arrangements with several French financial institutions for borrowings which are effectively secured by the Company’s French subsidiary’s accounts receivables. Borrowings outstanding at June 30, 2009 and 2010 were $2.4 million and $0.1 million, which were equal to the accounts receivables pledged. As the financial institutions have the right to sell or repledge the collateral, the Company records these accounts receivables to “Receivables pledged to creditors” in the consolidated balance sheets.
Inventories — Inventories are stated at the lower of cost or market. Cost is computed using actual costs or standard costs that approximate actual cost, determined on afirst-in, first-out basis. The Company records inventory provisions for excess and obsolete inventories, as determined by either future demand forecasts or historical demand trends.
Deferred Cost of Revenue — Deferred cost of revenue consists of completed products shipped to customers for which the related revenue has been deferred pending completion of services or determination
F-13
MIRION TECHNOLOGIES, INC.
Notes to Consolidated Financial Statements — (Continued)
that all customer-specific acceptance criteria have been met. The deferred costs are recognized in cost of revenue in the same period that the related revenues are recognized. The Company evaluates the carrying value of deferred cost of revenue on a lower of cost or market basis, consistent with its evaluation of the carrying value of the related products.
Property, Plant and Equipment — Property, plant and equipment are stated at cost, net of accumulated depreciation and amortization. Property, plant and equipment acquired through the acquisition of a business are recorded at their estimated fair value at the date of acquisition.
Depreciation is computed when assets are placed into service using the straight-line method over the estimated useful lives of the assets. Leasehold improvements are amortized on the straight-line method over the shorter of the related lease term or estimated useful life of the improvements. Repair and maintenance costs are expensed as incurred.
Estimated useful lives of property, plant and equipment are as follows:
| | | | | |
| Buildings and leasehold improvements | | | 3–39 years | |
| Machinery and equipment | | | 5–15 years | |
| Furniture, fixtures, computer equipment and software | | | 3–10 years | |
Goodwill and Purchased Intangible Assets — Goodwill is recorded as the excess of the acquisition purchase price over the fair value of net tangible and identifiable intangible assets acquired. Goodwill is not amortized but is tested for impairment on an annual basis or more frequently if indicators of potential impairment arise. The Company has tested goodwill for impairment based on an evaluation of the Company’s five operating segments, which are also reporting units. Based on the impairment tests performed, there was no impairment of goodwill in fiscal 2008, 2009, or 2010. Purchased intangible assets other than goodwill are amortized using a straight-line or an accelerated method over their estimated useful lives, which range from three to seventeen years. Remaining useful lives of definite-lived intangible assets are evaluated on a periodic basis to determine whether events or circumstances warrant a revision to the remaining estimated amortization period.
Impairment of Long-Lived Assets — The Company reviews long-lived assets and definite-lived intangible assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Determination of recoverability of long-lived assets is based on an estimate of undiscounted future cash flows resulting from the use of the asset as compared to its carrying value. If an asset is determined to be impaired, the Company measures the amount of any impairment as the difference between the carrying amount and the fair value of the impaired asset. No impairment indicators have been identified through June 30, 2010.
Facility and Equipment Decommissioning Liabilities —The Company accounts for asset retirement obligations (primarily equipment and facility decommissioning costs) in accordance with ASC 410, Asset Retirement and Environmental Obligations. This guidance requires that the estimated fair value of liabilities for asset retirement obligations (“ARO”) be recognized in the period in which they are incurred. A corresponding increase to the carrying value of the related asset is recorded and depreciated over the useful life of the asset. The estimates are based principally on legal and regulatory requirements. The amount of the liability is subject to remeasurement at each reporting period. The Company’s estimates of its ultimate asset retirement obligation could change as a result of changes in regulations, the extent of environmental remediation required, the means of reclamation, cost estimates, or time period estimates. Changes in estimates are accounted for prospectively from the period in which the estimate is revised.
As of June 30, 2009 and 2010, the Company’s ARO liabilities totaled $422,000 and $414,000 and were included in other long-term liabilities. Accretion expense related to these liabilities was $30,000, $31,000 and $24,000, in fiscal 2008, 2009 and 2010.
F-14
MIRION TECHNOLOGIES, INC.
Notes to Consolidated Financial Statements — (Continued)
Revenue Recognition — The Company recognizes revenue from sales contracts when there is persuasive evidence of an arrangement, product delivery has occurred or services have been provided, the sales price is fixed or determinable and collectability is reasonably assured. For sales contracts that contain customer-specific acceptance provisions, revenue and the related costs are deferred until the customer has indicated successful completion of site acceptance tests or the Company has otherwise determined that all customer-specific acceptance criteria have been met. In the Health Physics and Radiation Monitoring Systems segments, the Company performs detailed factory acceptance testing on completed products which in some instances is sufficiently extensive and reliable to demonstrate that its products meet all of the customer-specified objective acceptance criteria set forth in its sales arrangements. In such instances, the Company’s policy is to recognize revenue based on the product’s delivery terms and prior to the receipt of notification of formal acceptance from the customer.
The Company enters into sales arrangements that may consist of multiple deliverables of its product and service offerings due to the requirements of its customers. For example, a customer may purchase a radiation monitoring systems solution and commissioning services. This arrangement would consist of multiple elements, with the products delivered across multiple reporting periods as well as the commissioning services. Another customer may purchase dosimeters, contamination and clearance monitorsand/or detection and identification devices along with commissioning services, in which all the elements are delivered within the same reporting period.
In October 2009, the FASB amended the accounting standard for multiple-deliverable revenue arrangements. The new standard changes the requirements for establishing separate units of accounting in a multiple element arrangement and requires the allocation of arrangement consideration to each deliverable to be based on the relative selling price. The Company adopted this standard during fiscal 2010 retroactive to the beginning of the fiscal year for new and materially modified arrangements originating on or after July 1, 2009. The adoption of the new standard was not material to consolidated revenue in fiscal 2010 primarily because the Company’s multiple element sales arrangements tend to have long lead times, and the majority of revenue from such orders will not be recognized until fiscal 2011 or later. The Company is not able to reasonably estimate the effect of adopting this standard on future financial periods as the impact will vary based on the nature and volume of new or materially modified deals in any given period.
For new and materially modified arrangements originating in fiscal 2010 and future periods, when a sales arrangement contains multiple elements, the Company allocates revenue to each element based on a selling price hierarchy. The selling price for a deliverable is based on its vendor-specific objective evidence (“VSOE”) if available, third party evidence (“TPE”) if VSOE is not available, or estimated selling price if neither VSOE nor TPE is available. The Company then recognizes revenue on each deliverable in accordance with its policies for product and service revenue recognition. The Company is not typically able to determine VSOE or TPE and therefore uses estimated selling prices to allocate revenue between the elements of the arrangement. The Company establishes its best estimate of selling price considering multiple factors including but not limited to pricing practices in different geographies and through different sales channels, costs and margin objectives, competitive pricing strategies and general market conditions.
The Company limits the amount of revenue recognition for delivered elements to the amount that is not contingent on the future delivery of products or services or future performance obligations or subject to customer specific cancellation rights. The Company evaluates each deliverable in an arrangement to determine whether they represent separate units of accounting. A deliverable constitutes a separate unit of accounting when it has stand-alone value and for an arrangement that includes a general right of return relative to the delivered products or services, delivery or performance of the undelivered product or service is considered probable and is substantially controlled by the Company. The Company considers a deliverable to have standalone value if the product or service is sold separately by the Company or another vendor or could be resold by the customer. Further, the revenue arrangements generally do not include a general right of return
F-15
MIRION TECHNOLOGIES, INC.
Notes to Consolidated Financial Statements — (Continued)
relative to the delivered products. Where the aforementioned criteria for a separate unit of accounting are not met, the deliverable is combined with the undelivered element(s) and treated as a single unit of accounting for the purposes of allocation of the arrangement consideration and revenue recognition. The Company allocates the total arrangement consideration to each separable element of an arrangement based upon the relative selling price of each element. Allocation of the consideration is determined at arrangement inception on the basis of each unit’s relative selling price.
For arrangements entered into prior to fiscal 2010, revenue for arrangements with multiple elements is recognized as each element is delivered or completed based upon its relative fair value. In instances where the Company has not established fair value for any undelivered element, revenue for all elements is deferred until delivery of the final element is complete and all recognition criteria are met.
For all arrangements, amounts billed to a customer related to shipping and handling are classified as revenue, while all costs incurred by the Company for shipping and handling are classified as cost of revenue. Provisions and allowances for discounts to customers, estimated sales returns, service cancellations and other adjustments are provided for in the same period that the related revenue is recorded.
Certain of the Company’s products are sold through distributors and third-party sales representatives under standard agreements whereby distributors purchase products from the Company and resell them to customers. These agreements give distributors the right to sell the Company’s products within certain territories and establishes minimum order requirements. These arrangements do not provide stock rotation or price protection rights. Rights of return are limited to repair or replacement of delivered products that are defective or fail to meet the Company’s published specifications. Provisions for these warranty costs are provided for in the same period that the related sale is recorded.
Revenue from certain fixed price contracts in the Sensing Systems and Imaging Systems divisions that involve customization of equipment to customer specifications is recognized in accordance with ASC 640-35-25 Accounting for Performance of Construction-Type and Certain Production-Type Contracts, using thepercentage-of-completion method measured by thecost-to-cost method. Thecost-to-cost method is used because management considers incurred costs to be the best available measure of progress on these contracts. Contract costs include all direct materials and labor costs, as well as indirect costs related to contract performance. Changes in job performance, job conditions and estimated profitability result in revisions to costs and revenue and are recognized in the period in which the revisions are determined. Provisions for estimated losses on uncompleted contracts are made in the period in which such losses are first determined. Revenue earned in excess of billings on contracts in progress (underbillings) is classified as a current asset. Amounts billed in excess of revenue earned (advance billings) are classified as a current liability and included in deferred contract revenue.
Revenue from the Company’s Dosimetry Services division is of a subscription nature, with passive dosimetry and analytical services provided to customers on anagreed-upon recurring monthly, quarterly or annual basis. Services are provided principally through film and thermo luminescent dosimeter badges that are worn by the customer’s personnel and returned to the Company for analysis. The Company believes that badge production, badge wearing, badge analysis and report preparation are all integral to the benefit that the Company provides to its customers, and therefore, the service period is defined as the period over which all of these services are provided. Revenue and the related costs are recognized on a straight-line basis over the service period as the service is continuous, and no other discernible pattern of recognition is evident. Many customers pay for these measuring and monitoring services in advance. The amounts are recorded as deferred contract revenue in the balance sheets and represent customer deposits invoiced in advance for services to be rendered over the service period, net of a reserve for estimated cancellations.
Derivative and Hedging Activities — The Company uses certain derivative financial instruments to help manage its risk or exposure to changes in interest rates in relation to variable rate debt and foreign currency exchange rate fluctuations. The Company records these derivatives at fair value in the balance sheets as either
F-16
MIRION TECHNOLOGIES, INC.
Notes to Consolidated Financial Statements — (Continued)
an asset or a liability in accordance with ASC 815, Derivatives and Hedging. The Company did not meet the hedge criteria for these existing derivatives, so any changes in fair value are recognized in earnings as incurred. As of June 30, 2009 and 2010, the Company has recorded other liabilities of $21,000 and $3,000 to reflect the fair value of the Company’s interest rate swap. The Company has recorded losses of $9,000, $83,000 and $18,000 in other income, net for fiscal 2008, 2009 and 2010. The swap had an initial notional value of $1.8 million that declines through expiration in November 2012. The notional amount was $0.7 million at June 30, 2010.
On January 1, 2009 the Company executed a series of foreign currency window contracts to mitigate currency exposure on sales contracts due to fluctuations in the euro/U.S. dollar exchange rate. Each window is an agreement to sell the U.S. dollar and purchase euros. There were a total of 17 contracts to sell $5.8 million and purchase €4.2 million from February 2, 2009 through September 18, 2009. At June 30, 2009, the Company recorded other assets of $98,000 which represented the fair value of the contracts at that date. These currency window forward contracts were not designated as hedging instruments, so the corresponding net gain of $94,000 was recognized in earnings in fiscal 2009. As of June 30, 2010, all contracts had expired and the Company recognized a net loss of $98,000 in other income, net, for fiscal 2010.
Stock-Based Payment — The Company recognizes compensation expense for the fair value of all stock-based awards granted to employees and directors in exchange for services over the requisite service period, which is typically the vesting period. The fair value of stock options with time and performance-based vesting criteria is estimated using the Black-Scholes-Merton option valuation model. The fair value of stock options with market-based vesting criteria is estimated using a Monte Carlo simulation model. This model requires the input of subjective assumptions, including estimated stock volatility, risk-free interest rate and the expected life of each award. Furthermore, this guidance requires the Company to estimate forfeitures of each award. The Company amortizes the fair value using the straight-line method over the requisite service period.
The Company adopted ASC 718, Compensation-Stock Compensation on July 1, 2005 using the modified prospective transition method. This method required the recognition of compensation cost for allstock-based payments that were unvested as of July 1, 2005. In connection with the Reorganization, on January 1, 2006 stock options of the three predecessor companies were exchanged for stock options in Mirion. The exchange was deemed a modification, resulting in incremental compensation expense of $749,000 recorded at January 1, 2006 for those options that were vested as of January 1, 2006. For the unvested options at January 1, 2006, incremental compensation expense of $618,000 was expensed over the remaining vesting period of approximately two years.
Advertising Costs — The Company expenses advertising costs in the period incurred.
Accounting for Income Taxes — The determination of the Company’s tax provision is subject to judgments and estimates due to the complexity of the tax law that the Company is subject to in several tax jurisdictions. Earnings derived from the Company’s international business are generally taxed at rates that are different than U.S. rates, resulting in an effective tax rate different than the U.S. statutory tax rate of 34%. The ability to maintain the Company’s current effective tax rate is contingent on existing tax laws in both the United States and the respective countries in which the Company’s international subsidiaries are located. In addition, a decrease in the percentage of the Company’s total earnings from international business or a change in the mix of international business among particular tax jurisdictions could alter the Company’s overall effective tax rate.
Income taxes are accounted for under the asset and liability method in accordance with ASC 740, Income Taxes. Deferred tax assets and liabilities are recognized for the estimated future tax consequences attributable to differences between the tax bases of assets and liabilities and their financial statement carrying amounts, and consideration is given to operating losses and tax credit carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on the deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. The
F-17
MIRION TECHNOLOGIES, INC.
Notes to Consolidated Financial Statements — (Continued)
Company records a valuation allowance to reduce deferred tax assets to the amount of the future tax benefit that is more likely than not to be realized.
The Company accounts for uncertainty in its tax positions using a two-step approach to recognizing and measuring uncertain tax positions taken or expected to be taken in a tax return. The first step is to determine if the weight of available evidence indicates that it is more likely than not that the tax position will be sustained in an audit, including resolution of any related appeals or litigation processes. The second step is to measure the tax benefit as the largest amount that is more than 50% likely to be realized upon ultimate settlement. The Company evaluates these uncertain tax positions on a quarterly basis. This evaluation is based on the consideration of several factors, including changes in facts or circumstances, changes in applicable tax law, settlement of issues under audit, and new exposures. If the Company later determines that its exposure is lower that the liability is not sufficient to cover its revised expectations, the Company adjusts the liability and recognizes the related change in its tax provision during the period in which it makes such determination.
Defined Benefit Pension Plans and Other Employee Benefits — The Company has defined benefit pension plans that cover the majority of its employees in France and Germany. The Company also has a postretirement plan that provides for the reimbursement of a portion of medical and life insurance premiums for certain retirees and eligible dependents. Plan liabilities are revalued annually based on assumptions relating to the long-term rate of return on plan assets, discount rates used to measure future obligations and expenses, salary-scale inflation rates, health care cost trend rates, mortality and other assumptions. The selection of assumptions is based on historical trends and known economic and market conditions at the time of valuation; however, actual results may differ substantially from the Company’s estimates. For pension plans, accumulated gains and losses and prior service costs are recognized in income when incurred.
Fair Value of Financial Instruments — The Company applies the provisions of ASC 820 Fair Value Measurements and Disclosures, to the financial instruments that are required to be carried at fair value pursuant to other accounting standards. The fair value of the Company’s cash, cash equivalents, short-term investments, accounts receivable and other current and noncurrent liabilities approximate their carrying amounts due to the relatively short maturity of these items. The fair values of the debt instruments are estimated using a discounted cash flow analysis with an interest rate similar to that of current market borrowing arrangements. The estimated fair value of the Company’s debt instruments is $162.8 million and $184.5 million as of June 30, 2009 and 2010.
Fair Value Measurement —The Company performs fair value measurements in accordance with the guidance provided by ASC 820,Fair Value Measurements and Disclosures. ASC 820 defines fair value as the price that would be received from selling an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date, with consideration given to the assumptions that market participants would use when pricing the asset or liability, such as inherent risk, transfer restrictions, and risk of nonperformance. ASC 820 establishes a fair value hierarchy that requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. An asset’s or liability’s categorization within the fair value hierarchy is based upon the lowest level of input that is significant to the fair value measurement. ASC 820 establishes three levels of inputs that may be used to measure fair value:
Level 1 —Quoted prices in active markets for identical assets or liabilities:
Level 2 —Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices in active markets for similar assets or liabilities, quoted prices for identical or similar assets or liabilities in markets that are not active, or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities; or
Level 3 :Unobservable inputs that are supported by little or no market activity and that are significant to the fair values of the assets and liabilities.
F-18
MIRION TECHNOLOGIES, INC.
Notes to Consolidated Financial Statements — (Continued)
The following table summarizes the financial assets (liabilities) of the Company that are measured at fair value on a recurring basis (in thousands):
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Fair Value Measurements at
| | Fair Value Measurements at
|
| | | June 30, 2009 Using | | June 30, 2010 Using |
| | | Level 1 | | Level 2 | | Total | | Level 1 | | Level 2 | | Total |
| |
| Assets (Long Term Liabilities): | | | | | | | | | | | | | | | | | | | | | | | | |
| FX forward rate contracts | | | — | | | $ | 94 | | | $ | 94 | | | | — | | | | — | | | | — | |
| Interest rate swaps | | | — | | | $ | (21 | ) | | $ | (21 | ) | | | — | | | $ | (3 | ) | | $ | (3 | ) |
The FX forward rate contracts are measured at fair value using information quoted by foreign exchange dealers. The interest rate swaps are measured at fair value using a pricing model, such as a discounted cash flow technique, with significant inputs derived from observable market data.
Foreign Currency Translation — Local currencies are the functional currencies for substantially all of the Company’s foreign operations. Assets and liabilities of foreign operations are translated into U.S. dollars using the exchange rates in effect at the balance sheet reporting date, while income and expenses are translated at the average monthly exchange rates during the year. The effects of the foreign currency translations are reported as a component of accumulated other comprehensive income in the accompanying consolidated balance sheets. Deferred taxes are not provided on cumulative translation adjustments where the Company expects earnings of a foreign subsidiary to be indefinitely reinvested. The income tax effect of currency translation adjustments related to foreign subsidiaries from certain subsidiaries and joint ventures that are not considered indefinitely reinvested is recorded as a component of deferred taxes with an offset to other comprehensive income.
Foreign currency transaction gains and losses, arising when the transactional currency is different from the functional currency, are included as a component of other income, net in the consolidated statements of operations.
Concentrations of Risk — Financial instruments that are potentially subject to concentration of credit risk consist primarily of cash and cash equivalents and trade receivables. The Company maintains cash in bank deposit accounts that, at times, may exceed the insured limits of the local country. The Company has not experienced any losses in such accounts and Management believes that the Company is not exposed to significant credit risk related to cash.
The Company sells its products and services mainly to large private and governmental organizations in the Americas, Europe, Middle East and Asia Pacific regions. The Company performs ongoing evaluations of its customers’ financial conditions and limits the amount of credit extended when deemed necessary. The Company generally does not require its customers to provide collateral or other security to support accounts receivable. No single customer represented more than 10% of consolidated revenue for fiscal 2008, 2009 or 2010.
Net Loss Per Common Share — Basic loss per common share is calculated by dividing net loss allocable to common stockholders by the weighted-average number of common shares outstanding for the period using the two-class method. Under the two-class method, net income is allocated between common stock and convertible preferred stock as it is deemed to be a participating security based on its participation rights. Diluted loss per common share is calculated by dividing net loss attributable to common stockholders by the weighted average number of common and potential dilutive securities outstanding during the period if the effect is dilutive. The numerator of diluted earnings per share is calculated by starting with income allocable to common stock under the two-class method and adding back income allocable to preferred stock to the extent they are dilutive. Potential common shares consist of incremental shares of common stock issuable upon the exercise of the stock options and warrants and upon conversion of preferred stock.
F-19
MIRION TECHNOLOGIES, INC.
Notes to Consolidated Financial Statements — (Continued)
The following table sets forth the computation of basic and diluted net loss per common share indicated (in thousands, except share and per share amounts):
| | | | | | | | | | | | | |
| | | Year Ended June 30, | |
| | | 2008 | | | 2009 | | | 2010 | |
| |
Numerator: | | | | | | | | | | | | |
| Net (loss) income | | $ | (13,349 | ) | | $ | (3,955 | ) | | $ | 5,965 | |
| Effect of preferred stock dividends | | | (8,993 | ) | | | (9,892 | ) | | | (10,923 | ) |
| | | | | | | | | | | | | |
| Net loss allocable to common stockholders | | $ | (22,342 | ) | | $ | (13,847 | ) | | $ | (4,958 | ) |
| | | | | | | | | | | | | |
Denominator: | | | | | | | | | | | | |
| Weighted average common shares outstanding (basic) | | | 405,159 | | | | 405,796 | | | | 405,796 | |
| Effect of dilutive securities | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | |
| Weighted average common shares outstanding (diluted) | | | 405,159 | | | | 405,796 | | | | 405,796 | |
| | | | | | | | | | | | | |
Net loss per share: | | | | | | | | | | | | |
| Basic and Diluted | | $ | (55.14 | ) | | $ | (34.12 | ) | | $ | (12.22 | ) |
| | | | | | | | | | | | | |
The computation of basic loss per share excludes the conversion of Convertible Participating Preferred Stock as the Company has net losses and therefore the effect of applying the two-class method is anti-dilutive. The computation of dilutive shares outstanding excludes the common equivalent shares related topaid-in-kind dividends, conversion of the Convertible Participating Preferred Stock, stock options and warrants as the Company had net losses and the effect would be anti-dilutive.
The following table shows the weighted-average common equivalent shares related topaid-in-kind dividends, the conversion of Convertible Participating Preferred Stock, stock options and warrants that would have been included in diluted earnings per share had the Company recorded net income as of the respective dates:
| | | | | | | | | | | | | |
| | | Year Ended June 30, | |
| | | 2008 | | | 2009 | | | 2010 | |
| |
| Weighted Average Common Share Equivalents of Potentially Dilutive Securities: | | | | | | | | | | | | |
Convertible preferred stock, includingpaid-in-kind dividends | | | 8,546,536 | | | | 9,349,119 | | | | 10,233,721 | |
| Stock options | | | 16,056 | | | | — | | | | 73,879 | |
| Warrants | | | 3,420,636 | | | | 3,420,636 | | | | 3,420,636 | |
| | | | | | | | | | | | | |
| Total | | | 11,983,228 | | | | 12,769,755 | | | | 13,728,236 | |
| | | | | | | | | | | | | |
Reclassifications — The Company has reclassified certain prior year balances within these notes to consolidated financial statements to conform to current year presentation.
F-20
MIRION TECHNOLOGIES, INC.
Notes to Consolidated Financial Statements — (Continued)
Costs and billings on uncompleted construction-type contracts consist of the following (in thousands):
| | | | | | | | | |
| | | As of June 30, | |
| | | 2009 | | | 2010 | |
| |
| Costs incurred | | $ | 34,602 | | | $ | 35,773 | |
| Estimated earnings | | | 26,329 | | | | 25,528 | |
| | | | | | | | | |
| Contracts in progress | | | 60,931 | | | | 61,301 | |
| Progress billings on contracts in progress | | | (49,132 | ) | | | (43,847 | ) |
| | | | | | | | | |
| | | $ | 11,799 | | | $ | 17,454 | |
| | | | | | | | | |
| Costs and estimated earnings in excess of billings on uncompleted contracts | | $ | 17,073 | | | $ | 19,474 | |
| Billings in excess of costs and estimated earnings on uncompleted contracts(1) | | | (5,274 | ) | | | (2,020 | ) |
| | | | | | | | | |
| | | $ | 11,799 | | | $ | 17,454 | |
| | | | | | | | | |
| | |
| (1) | | Included in deferred contract revenue within the consolidated balance sheets. |
The components of inventories consist of the following (in thousands):
| | | | | | | | | |
| | | As of June 30, | |
| | | 2009 | | | 2010 | |
| |
| Raw materials | | $ | 10,234 | | | $ | 8,237 | |
| Work in progress | | | 9,889 | | | | 10,255 | |
| Finished goods | | | 13,605 | | | | 11,677 | |
| | | | | | | | | |
| Net inventories | | $ | 33,728 | | | $ | 30,169 | |
| | | | | | | | | |
As of June 30, 2009 and 2010, inventory reserves were approximately $4.3 million and $3.4 million.
| |
| 5. | PROPERTY, PLANT AND EQUIPMENT |
Property, plant and equipment consist of the following (in thousands):
| | | | | | | | | |
| | | As of June 30, | |
| | | 2009 | | | 2010 | |
| |
| Land, buildings and leasehold improvements | | $ | 11,352 | | | $ | 10,682 | |
| Machinery and equipment | | | 24,108 | | | | 22,931 | |
| Furniture, fixtures, computer equipment and software | | | 16,951 | | | | 16,295 | |
| Construction in progress | | | 840 | | | | 4,708 | |
| | | | | | | | | |
| | | | 53,251 | | | | 54,616 | |
| Less accumulated depreciation and amortization | | | (35,171 | ) | | | (34,084 | ) |
| | | | | | | | | |
| | | $ | 18,080 | | | $ | 20,532 | |
| | | | | | | | | |
Total depreciation and amortization of property, plant and equipment was $4.1 million, $4.4 million, $5.6 million for fiscal 2008, 2009 and 2010.
F-21
MIRION TECHNOLOGIES, INC.
Notes to Consolidated Financial Statements — (Continued)
| |
| 6. | ACCRUED EXPENSES AND OTHER CURRENT LIABILITIES |
Accrued expenses and other current liabilities consist of the following (in thousands):
| | | | | | | | | |
| | | As of June 30, | |
| | | 2009 | | | 2010 | |
| |
| Compensation and related benefit costs | | $ | 14,151 | | | $ | 13,380 | |
| Customer deposits | | | 9,525 | | | | 6,136 | |
| Accrued warranty | | | 1,409 | | | | 744 | |
| Accrued legal, accounting and professional fees | | | 4,071 | | | | 4,366 | |
| Other accrued expenses | | | 5,852 | | | | 5,034 | |
| | | | | | | | | |
| | | $ | 35,008 | | | $ | 29,660 | |
| | | | | | | | | |
| |
| 7. | GOODWILL AND INTANGIBLE ASSETS |
Goodwill and intangible assets were recorded in connection with the acquisitions of GDS, IST and Synodys by ACAS as well as other subsequent acquisitions by Mirion. GDS was acquired by ACAS in October 2003 for cash of $60.8 million. IST was acquired by ACAS in May 2004 for cash of $53.5 million. Synodys was acquired by ACAS in June 2004 for cash and equity of $75.1 million. The three acquisitions by ACAS were accounted for using the purchase method of accounting which resulted in goodwill of approximately $123.9 million (including the recognition of deferred tax assets of $18.4 million associated with the acquisitions) and intangible assets of approximately $72.3 million (valued using exchange rates in effect on the date of each acquisition). The Company completed three other acquisitions during fiscal 2005 and 2006 that resulted in goodwill of approximately $8.3 million and intangible assets of approximately $12.5 million. In fiscal 2008, the Company received $2.8 million from the return of escrow funds associated with the IST acquisition. This payment was treated as a reduction in the IST purchase price, with a corresponding reduction in goodwill.
The changes in the carrying amount of goodwill allocated to the Company’s reportable segments are as follows (in thousands):
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | Radiation
| | | | | | | | | | | | | |
| | | Health
| | | Monitoring
| | | Sensing
| | | Dosimetry
| | | Imaging
| | | | |
| | | Physics | | | Systems | | | Systems | | | Services | | | Systems | | | Total | |
| |
| Balance — June 30, 2007 | | $ | 46,235 | | | $ | 19,312 | | | $ | 11,001 | | | $ | 52,413 | | | $ | 9,965 | | | $ | 138,926 | |
| Return of escrow funds | | | — | | | | — | | | | (1,367 | ) | | | — | | | | (1,383 | ) | | | (2,750 | ) |
| Translation adjustment | | | 7,972 | | | | 3,330 | | | | 200 | | | | — | | | | — | | | | 11,502 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance — June 30, 2008 | | | 54,207 | | | | 22,642 | | | | 9,834 | | | | 52,413 | | | | 8,582 | | | | 147,678 | |
| Translation adjustment | | | (6,000 | ) | | | (2,507 | ) | | | (150 | ) | | | — | | | | — | | | | (8,657 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance — June 30, 2009 | | | 48,207 | | | | 20,135 | | | | 9,684 | | | | 52,413 | | | | 8,582 | | | | 139,021 | |
| Translation adjustment | | | (6,313 | ) | | | (2,637 | ) | | | (158 | ) | | | — | | | | (638 | ) | | | (9,746 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance — June 30, 2010 | | $ | 41,894 | | | $ | 17,498 | | | $ | 9,526 | | | $ | 52,413 | | | $ | 7,944 | | | $ | 129,275 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
F-22
MIRION TECHNOLOGIES, INC.
Notes to Consolidated Financial Statements — (Continued)
Intangible assets consist of the following (in thousands):
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | As of June 30, 2009 | | | As of June 30, 2010 | |
| | | Weighted
| | Gross
| | | | | | Net
| | | Gross
| | | | | | Net
| |
| | | Average Life
| | Carrying
| | | Accumulated
| | | Book
| | | Carrying
| | | Accumulated
| | | Book
| |
| | | in Years | | Amount | | | Amortization | | | Value | | | Amount | | | Amortization | | | Value | |
| |
| Customer relationships | | | 11 | | | $ | 61,714 | | | $ | (44,069 | ) | | $ | 17,645 | | | $ | 58,346 | | | $ | (45,985 | ) | | $ | 12,361 | |
| Trade names | | | 11 | | | | 8,605 | | | | (4,153 | ) | | | 4,452 | | | | 7,895 | | | | (4,489 | ) | | | 3,406 | |
| Qualifications | | | 6 | | | | 1,600 | | | | (1,360 | ) | | | 240 | | | | 1,600 | | | | (1,600 | ) | | | — | |
| Complete technology | | | 8 | | | | 3,500 | | | | (2,394 | ) | | | 1,106 | | | | 3,378 | | | | (2,739 | ) | | | 639 | |
| Territorial rights | | | 5 | | | | 2,537 | | | | (2,292 | ) | | | 245 | | | | 2,204 | | | | (2,204 | ) | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Total | | | | | | $ | 89,204 | | | $ | (65,516 | ) | | $ | 23,688 | | | $ | 83,949 | | | $ | (67,543 | ) | | $ | 16,406 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Aggregate amortization expense for intangible assets was $10.1 million, $8.1 million and $6.4 million for fiscal 2008, 2009, and 2010. Future annual amortization expense at the current foreign exchange rate is as follows (in thousands):
| | | | | |
| | | Annual
| |
Years Ending
| | Amortization
| |
June 30, | | Expense | |
| |
| 2011 | | $ | 4,446 | |
| 2012 | | | 3,465 | |
| 2013 | | | 2,588 | |
| 2014 | | | 1,584 | |
| 2015 | | | 1,348 | |
| 2016 and thereafter | | | 2,975 | |
| | | | | |
| Total | | $ | 16,406 | |
| | | | | |
F-23
MIRION TECHNOLOGIES, INC.
Notes to Consolidated Financial Statements — (Continued)
Notes Payable to ACAS — The Company has entered into several Note and Equity Purchase Agreements (“NEPAs”) with ACFS, a subsidiary of ACAS, which contain revolving credit facilities, senior term notes payable and subordinated note agreements. The interest rates on the revolving credit facilities and notes payable are based on a fixed stated rate or a variable LIBOR or EURIBOR based rate plus additional basis points. The one-month LIBOR rates were 0.3% and 0.4%, the one-month EURIBOR rates were 0.8% and 0.4% and the three-month EURIBOR rates were 1.1% and 0.7% as of June 30, 2009 and 2010.
Notes payable to ACAS consist of the following (in thousands):
| | | | | | | | | | | | | |
| | | | | Contractual
| | As of June 30, | |
| | | Due | | Interest Rate | | 2009 | | | 2010 | |
| |
| Revolving Credit Facilities: | | | | | | | | | | | | |
| $20.25 million | | July 2011 | | LIBOR + 4.5% | | $ | 11,000 | | | $ | 20,250 | |
| $14.0 million | | July 2011 | | LIBOR + 5% | | | 13,997 | | | | 13,997 | |
| $8.2 million | | July 2011 | | EURIBOR + 2% | | | 3,631 | | | | 7,130 | |
| Senior Term Notes: | | | | | | | | | | | | |
| $24.9 million Senior Term B | | July 2011 | | EURIBOR + 3% | | | 24,944 | | | | 24,944 | |
| $7.5 million Senior Term B | | July 2011 | | LIBOR + 8% | | | 5,062 | | | | 5,008 | |
| $2.0 million Senior Term B | | July 2011 | | LIBOR + 8% | | | 1,938 | | | | 1,917 | |
| $4.0 million Senior Term C | | October 2011 | | LIBOR + 9% | | | 4,000 | | | | 4,000 | |
| $4.0 million Senior Term C | | November 2011 | | LIBOR + 8.25% | | | 4,000 | | | | 4,000 | |
| $27.0 million Senior Term D | | October 2011 | | LIBOR + 6.5% | | | 26,056 | | | | 25,785 | |
| $15.0 million Senior Term D | | October 2011 | | LIBOR + 6.5% | | | 14,437 | | | | 14,288 | |
| Senior Subordinated Notes: | | | | | | | | | | | | |
$7.5 millionpaid-in-kind | | July 2011 | | 14% | | | 8,317 | | | | 8,487 | |
$8.6 millionpaid-in-kind | | July 2011 | | 15% | | | 9,650 | | | | 9,862 | |
$12.2 millionpaid-in-kind | | July 2011 | | EURIBOR + 11% | | | 15,552 | | | | 16,441 | |
| Junior Subordinated Notes: | | | | | | | | | | | | |
$4.3 millionpaid-in-kind | | July 2011 | | 17% | | | 5,112 | | | | 5,280 | |
$4.3 millionpaid-in-kind | | July 2011 | | 17% | | | 5,112 | | | | 5,280 | |
$1.25 millionpaid-in-kind | | May 2012 | | 14% | | | 1,386 | | | | 1,415 | |
$4.9 millionpaid-in-kind | | July 2011 | | EURIBOR + 12% | | | 6,666 | | | | 7,155 | |
| Stockholder Loan: | | | | Three-month | | | | | | | | |
| $8.0 million | | July 2011 | | EURIBOR + 2% | | | 9,220 | | | | 8,013 | |
| | | | | | | | | | | | | |
| Total notes payable to ACAS | | | | | | | 170,080 | | | | 183,252 | |
| Less current portion | | | | | | | (520 | ) | | | (420 | ) |
| | | | | | | | | | | | | |
| Notes payable to ACAS — long term | | | | | | $ | 169,560 | | | $ | 182,832 | |
| | | | | | | | | | | | | |
The revolving credit facilities and notes issued under the NEPAs require the Company to maintain certain financial ratios and contain financial covenants and non-financial restrictive and affirmative covenants. As of June 30, 2009 and 2010, the Company was not in compliance with certain non-financial covenants that were in effect prior to the formation of Mirion. These non-financial covenants were negotiated with the predecessor companies (GDS, IST and Synodys) and were not amended at the time of the formation of Mirion. The non-financial covenants relate to matters such as changing the fiscal years or names of subsidiaries, amending the charter documents and by-laws of subsidiaries and the provision of audited financial statements to ACFS.The
F-24
MIRION TECHNOLOGIES, INC.
Notes to Consolidated Financial Statements — (Continued)
Company has obtained waivers from ACFS for breaches of any non-financial covenants that may occur through July 1, 2011. A description of the debt agreements payable to ACAS is as follows:
Revolving Credit Facilities — The Company has entered into three separate credit facilities with ACFS, each represented by a revolving credit note. The Revolving Credit Facilities contain various financial covenants and a first security position on all Company assets. The Company is required to pay an annual non-usage fee of 0.7% of the average annual unused balance on the Revolving Credit Facilities.
Senior Term Notes — The $24.9 million Senior Term B Note and the Senior Term C Notes provide for monthly interest only payments. The notes are secured by assets of the Company and are subordinated to the Revolving Credit Facilities and Senior Term Notes B and D. The $7.5 million and $2.0 million Senior Term B Notes provide for quarterly principal payments totaling $25,000 plus monthly payments of interest. The notes are secured by assets of the Company and are subordinated to the Revolving Credit Facilities and the Senior Term D Notes. The Senior Term D Notes provide for quarterly principal payments of $105,000 plus monthly payments of interest. The notes are secured by assets of the Company and are subordinated to the Revolving Credit Facilities.
Senior Subordinated Notes — The outstanding balances on Senior Subordinated Notes include the principal amount outstanding plus a compoundingpaid-in-kind (“PIK”) interest component calculated at rates between 2% and 5.5% per annum. The Company is required to make monthly payments of stated interest only. The notes are secured by assets of the Company and are subordinated to the Revolving Credit Facilities and Senior Term B, C and D Notes.
Junior Subordinated Notes — The outstanding balances on Junior Subordinated Notes include the principal amount outstanding plus a compounding PIK interest component calculated at rates between 2% and 7% per annum. The Company is required to make monthly payments of stated interest only. The notes are secured by assets of the Company and are subordinated to the Revolving Credit Facilities and Senior Term B, C and D Notes and Senior Subordinated Notes.
Stockholder Loan — The stockholder loan provides for quarterly interest-only payments. The loan is secured by the assets of the Company and is subordinated to all other debt.
During 2007, the NEPAs were amended to (i) increase the $20.25 million revolving credit facility from an original commitment of $5.25 million to $10.25 million, (ii) increase the $14 million revolving credit facility from a commitment of $6 million to $14 million, (iii) extend the due date on the stockholder loan from September 13, 2006 to September 23, 2008 and (iv) issue an additional $2.0 million of Senior Term B Notes on March 20, 2007 with an interest rate of LIBOR + 8% and an original due date of May 24, 2010.
During 2008, the NEPAs were further amended to (i) increase the $20.25 million revolving credit facility from a commitment amount of $10.25 million to $20.25 million and extend the due date from May 24, 2008 to October 14, 2010, (ii) extend the due date on the $8.2 million revolving credit facility from June 23, 2008 to October 14, 2010 and (iii) extend the due date on the stockholder loan from September 23, 2008 to October 14, 2010.
During 2010, the NEPAs were further amended to extend the due dates on the (i) Senior Term B Notes to July 1, 2011, (ii) Senior Subordinated Notes to July 1, 2011, (iii) Junior Subordinated Notes to July 1, 2011 except $1.25 million Junior Subordinated Notes which due date remained unchanged, (iv) Stockholder loan to June 30, 2011 and (v) Revolving Credit Facilities to July 2011. In August 2010, the stockholder loan was further amended to extend the due date to July 1, 2011.
F-25
MIRION TECHNOLOGIES, INC.
Notes to Consolidated Financial Statements — (Continued)
Third-Party Borrowings
The Company has notes payable and other borrowings to third parties as follows (in thousands):
| | | | | | | | | | | |
| | | | | As of June 30, | |
| | | Due | | 2009 | | | 2010 | |
| |
| Term loan | | November 2012 | | $ | 1,067 | | | $ | 662 | |
| Term loans | | March 2017 | | | — | | | | 1,766 | |
| Revolving line of credit | | On demand | | | 2,364 | | | | 113 | |
| Bank lines of credit | | On demand | | | 3,773 | | | | 81 | |
| | | | | | | | | | | |
| Total third-party borrowings | | | | | 7,204 | | | | 2,622 | |
| Less current portion | | | | | (6,442 | ) | | | (648 | ) |
| | | | | | | | | | | |
| Third-party borrowings-long term | | | | $ | 762 | | | $ | 1,974 | |
| | | | | | | | | | | |
The Company has a term loan with a French financial institution with an initial balance of €1.4 million as of May 2006 ($1.8 million), which is due November 2012. The note bears annual interest at the three-month EURIBOR + 1% with quarterly payments of €54,000. The amount due under this term loan at June 30, 2010 was €543,000 ($0.7 million).
The Company entered into two new term loans with two French financial institutions, as of March 2010, with a maximum borrowing under these arrangements of €3.8 million ($4.6 million). The notes bear annual interest at 4.15% and 4.17% respectively with quarterly payments of €52,000 beginning in December 2010. The amount due under these term loans at June 30, 2010 was €1.4 million ($1.8 million).
The Company has overdraft facilities of €0.9 million ($1.1 million) with certain German financial institutions. There were no amounts outstanding under these facilities at June 30, 2010.
As described in Note 2, the Company has pledged certain accounts receivable balances to several French institutions to secure a revolving line of credit. The line of credit bears interest at EURIBOR + 1%. Amounts outstanding under these arrangements are due when the related receivable is collected or upon demand.
Maturities on all notes payable as of June 30, 2010 are as follows (in thousands):
| | | | | |
Years Ending
| | | |
June 30, | | Amount | |
| |
| 2011 | | $ | 1,068 | |
| 2012 | | | 183,350 | |
| 2013 | | | 384 | |
| 2014 | | | 252 | |
| 2015 | | | 252 | |
| Thereafter | | | 568 | |
| | | | | |
| Total | | $ | 185,874 | |
| | | | | |
| |
| 9. | COMMITMENTS AND CONTINGENCIES |
Leases and Other Contractual Obligations — In the normal course of business, the Company enters into contractual arrangements with third parties for noncancelable operating lease agreements for its offices and vehicles. Under these agreements, the Company commits to provide specified payments to a lessor, based upon contractual arrangements.
F-26
MIRION TECHNOLOGIES, INC.
Notes to Consolidated Financial Statements — (Continued)
The total future minimum commitments for these and other contractual arrangements in place as of June 30, 2010, are scheduled to be paid as follows (in thousands):
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | 2011 | | | 2012 | | | 2013 | | | 2014 | | | 2015 | | | Thereafter | | | Total | |
| |
| Minimum future operating lease payments | | $ | 3,504 | | | $ | 3,135 | | | $ | 2,654 | | | $ | 2,390 | | | $ | 1,329 | | | $ | 3,532 | | | $ | 16,544 | |
| Less income from subleases | | | (26 | ) | | | (26 | ) | | | (2 | ) | | | — | | | | — | | | | — | | | | (54 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net minimum operating lease payments | | $ | 3,478 | | | $ | 3,109 | | | $ | 2,652 | | | $ | 2,390 | | | $ | 1,329 | | | $ | 3,532 | | | $ | 16,490 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total rent expense for fiscal 2008, 2009, and 2010 was $2.9 million, $3.2 million and $3.2 million.
Unconditional Purchase Obligations — The Company has entered into certain long-term unconditional purchase obligations with suppliers. These agreements are non-cancellable and specify terms including fixed or minimum quantities to be purchased, fixed or variable price provisions, and the approximate timing of payment. As of June 30, 2010, unconditional purchase obligations were $4.9 million for fiscal 2011, $1.2 million for fiscal 2012, $1.2 million for fiscal 2013, $1.2 million for fiscal 2014 and $1.1 million for fiscal 2015. Routine arrangements for other materials and goods that are entered into in the ordinary course of business are not included in these amounts.
Legal Proceedings — The Company is subject to claims and legal proceedings for matters that have arisen through the ordinary course of business. Management believes any liability that may ultimately result from the resolution of these matters will not have a material adverse effect on the Company’s consolidated financial position, operating results and cash flows.
Performance Bonds and Other Credit Facilities — The Company has entered into various line of credit arrangements with local banks in France and Germany. These arrangements provide for the issuance of documentary and standby letters of credit of up to €14.2 million ($17.3 million), subject to certain local restrictions. As of June 30, 2010, €12.3 million ($15.0 million) of the lines had been utilized to guarantee documentary and standby letters of credit, with interest rates ranging from 0.5% to 1.35%. In addition, the Company posts performance bonds with irrevocable letters of credit to support certain contractual obligations to customers for equipment delivery. These letters of credit are supported by restricted cash accounts, which totaled $6.0 million and $6.6 million as of June 30, 2009 and 2010.
F-27
MIRION TECHNOLOGIES, INC.
Notes to Consolidated Financial Statements — (Continued)
The Company’s (loss) income before income taxes and the details of the income tax provision consist of the following (in thousands):
| | | | | | | | | | | | | |
| | | Year Ended June 30, | |
| | | 2008 | | | 2009 | | | 2010 | |
| |
| (Loss) income before income taxes | | | | | | | | | | | | |
| Domestic | | $ | (14,346 | ) | | $ | (11,366 | ) | | $ | (6,240 | ) |
| Foreign | | | 6,835 | | | | 13,023 | | | | 19,937 | |
| | | | | | | | | | | | | |
| Total (loss) income before income taxes | | $ | (7,511 | ) | | $ | 1,657 | | | | 13,697 | |
| | | | | | | | | | | | | |
| Income tax provision | | | | | | | | | | | | |
| Current | | | | | | | | | | | | |
| Federal | | $ | 101 | | | $ | 24 | | | $ | 39 | |
| State | | | 17 | | | | 17 | | | | 11 | |
| Foreign | | | 5,861 | | | | 5,295 | | | | 4,620 | |
| | | | | | | | | | | | | |
| Total current provision | | | 5,979 | | | | 5,336 | | | | 4,670 | |
| Deferred | | | | | | | | | | | | |
| Federal | | | 1,205 | | | | 1,141 | | | | 1,188 | |
| State | | | (119 | ) | | | 66 | | | | 67 | |
| Foreign | | | (1,227 | ) | | | (931 | ) | | | 1,807 | |
| | | | | | | | | | | | | |
| Total deferred provision | | | (141 | ) | | | 276 | | | | 3,062 | |
| | | | | | | | | | | | | |
| Total income tax provision | | $ | 5,838 | | | $ | 5,612 | | | $ | 7,732 | |
| | | | | | | | | | | | | |
The provision for income taxes differs from the amount computed by applying the statutory federal income tax rate to (loss) income before income taxes as follows (in thousands):
| | | | | | | | | | | | | |
| | | Year Ended June 30, | |
| | | 2008 | | | 2009 | | | 2010 | |
| |
| (Loss) income before provision for income taxes | | $ | (7,511 | ) | | $ | 1,657 | | | $ | 13,697 | |
| | | | | | | | | | | | | |
| Federal income tax at statutory rate | | | (2,554 | ) | | | 563 | | | | 4,657 | |
| State income tax (net of federal benefit) | | | (517 | ) | | | (598 | ) | | | (187 | ) |
| Foreign income taxed at different rates | | | 3,625 | | | | 2,212 | | | | (126 | ) |
| Change in valuation allowance(1) | | | 5,011 | | | | 3,171 | | | | 3,546 | |
| Change in tax rates | | | 181 | | | | — | | | | — | |
| Other non-deductible expenses | | | 664 | | | | 629 | | | | 161 | |
| Benefit of tax credits | | | (939 | ) | | | (536 | ) | | | (343 | ) |
| Other | | | 367 | | | | 171 | | | | 24 | |
| | | | | | | | | | | | | |
| Total income tax provision | | $ | 5,838 | | | $ | 5,612 | | | $ | 7,732 | |
| | | | | | | | | | | | | |
| | |
| (1) | | Affecting the provision for income taxes. |
F-28
MIRION TECHNOLOGIES, INC.
Notes to Consolidated Financial Statements — (Continued)
The components of the Company’s net deferred tax assets and liabilities consisted of the following (in thousands):
| | | | | | | | | |
| | | 2009 | | | 2010 | |
| |
| Deferred tax assets: | | | | | | | | |
| Net operating losses | | $ | 16,398 | | | $ | 16,141 | |
| Tax credits | | | 7,430 | | | | 9,917 | |
| Amortization | | | 4,151 | | | | 4,237 | |
| Other reserves and accrued expenses | | | 9,027 | | | | 4,334 | |
| | | | | | | | | |
| Total deferred tax assets | | | 37,006 | | | | 34,629 | |
| Deferred tax liabilities: | | | | | | | | |
| Purchased technologies and other intangibles | | | (12,107 | ) | | | (11,770 | ) |
| Depreciation | | | (236 | ) | | | (523 | ) |
| Other liabilities | | | (10 | ) | | | (219 | ) |
| | | | | | | | | |
| Total deferred tax liabilities | | | (12,353 | ) | | | (12,512 | ) |
| Less: Valuation allowance | | | (31,393 | ) | | | (32,067 | ) |
| | | | | | | | | |
| Net deferred tax liabilities | | $ | (6,740 | ) | | $ | (9,950 | ) |
| | | | | | | | | |
Management regularly evaluates the recoverability of deferred tax assets and recognizes the tax benefit only if reassessment demonstrates that they are realizable. At such time, if it is determined that it is more likely than not that the deferred tax assets are realizable, the valuation allowance will be adjusted. As of June 30, 2009 and 2010, the Company has provided a valuation allowance for certain U.S. and foreign deferred tax assets that it believes are more likely than not unrealizable.
As of June 30, 2010, the Company had federal and state net operating loss carryforwards of approximately $57.8 million and $82.2 million. The federal net operating losses will begin to expire in 2023. The state net operating losses will begin to expire in 2013. As of June 30, 2010, the Company had tax credit carryforwards of approximately $9.8 million, available to offset foreign taxes paid for federal income tax purposes. Federal tax credit carryforwards will begin to expire in fiscal 2015.
The Company has not provided U.S. income tax on certain foreign earnings that are deemed to be indefinitely invested outside the U.S. For fiscal 2008, 2009 and 2010, the amount of accumulated unremitted earnings from the Company’s foreign subsidiaries is approximately $13.2 million, $17.3 million and $21.3 million. Determination of the amount of unrecognized deferred U.S. income tax liability is not practical due to the complexities associated with its hypothetical calculation.
The Company classifies unrecognized tax benefits as a non-current liability in the accompanying consolidated balance sheets. A reconciliation of the beginning and ending amount of unrecognized tax benefits is as follows:
| | | | | | | | | | | | | |
| | | Year Ended June 30, | |
| | | 2008 | | | 2009 | | | 2010 | |
| |
| Balance at beginning of period | | $ | 7,383 | | | $ | 11,851 | | | $ | 13,222 | |
| Additions based on tax positions taken related to prior years | | | — | | | | 451 | | | | 190 | |
| Additions based on tax positions taken related to current year | | | 3,367 | | | | 2,247 | | | | — | |
| Reductions based on settlements | | | — | | | | — | | | | — | |
| Reductions for tax positions of prior years | | | — | | | | — | | | | (197 | ) |
| Foreign currency translation adjustments | | | 1,101 | | | | (1,327 | ) | | | (679 | ) |
| | | | | | | | | | | | | |
| Balance at end of period | | $ | 11,851 | | | $ | 13,222 | | | $ | 12,536 | |
| | | | | | | | | | | | | |
F-29
MIRION TECHNOLOGIES, INC.
Notes to Consolidated Financial Statements — (Continued)
As of June 30, 2010, the Company had approximately $12.5 million of unrecognized tax benefits related to uncertain tax positions, of which $7.2 million would affect its effective tax rate if recognized. The Company does not believe the remaining amount of unrecognized tax benefits as of June 30, 2010 will materially change in the next 12 months.
The Company recognizes interest and penalties associated with uncertain tax positions in income tax expense and incurred accrued interest and penalties of $0.3 million, $0.2 million and $0.5 million for fiscal 2008, 2009 and 2010. As of June 30, 2009 and 2010, the provision for interest and penalties was $1.2 million and $1.7 million. The ultimate amount and timing of any future cash settlements cannot be predicted with reasonable certainty.
The Company conducts business globally and as a result, one or more of its subsidiaries file income tax returns in the U.S. federal jurisdiction and various state and foreign jurisdictions. In the normal course of business, the Company is subject to examination by taxing authorities throughout the world, including such major jurisdictions as the United Kingdom, France, Germany and the United States. With a few insignificant jurisdictions, the Company is no longer subject to U.S. federal, state and local, ornon-U.S. income tax examinations for years prior to fiscal 2001.
In many cases, the Company’s uncertain tax positions are related to tax years that remain subject to examination by tax authorities. The following describes open tax years by major tax jurisdictions as of June 30, 2010:
| | | | | |
| | | Years Open | |
| |
| Jurisdiction: | | | | |
| United Kingdom | | | 2008–2010 | |
| France | | | 2005–2010 | |
| Germany | | | 2005–2010 | |
| United States | | | 2005–2010 | |
| |
| 11. | EMPLOYEE BENEFIT PLANS |
Defined Benefit Pension Plans — The Company maintains contributory and noncontributory defined benefit plans for certain employees in France and Germany.
In France, the Company contributes to the national pension system, and its obligation to employees in terms of pensions is restricted to a lump-sum length of service award payable at the date that the employee reaches retirement age, such award being determined for each individual based upon years of service provided and projected final salary. The benefit obligation is unfunded. Accordingly, the fair value of plan assets is zero for the periods presented.
In Germany, plan benefits are generally based on an employee’s years of service and final salary. The benefit obligations of the German entities are unfunded. Accordingly, the fair value of plan assets is zero for the periods presented.
F-30
MIRION TECHNOLOGIES, INC.
Notes to Consolidated Financial Statements — (Continued)
The components of the pension plan benefit obligations are as follows (in thousands):
| | | | | | | | | | | | | | | | | |
| | | Year Ended June 30, | |
| | | 2009 | | | 2010 | |
| | | France | | | Germany | | | France | | | Germany | |
| |
| Change in projected benefit obligations: | | | | | | | | | | | | | | | | |
| Projected benefit obligation — at beginning of period/year | | $ | 1,375 | | | $ | 1,163 | | | $ | 1,284 | | | $ | 1,207 | |
| Foreign currency translation | | | (152 | ) | | | (128 | ) | | | (53 | ) | | | (195 | ) |
| Service cost | | | 84 | | | | 28 | | | | 99 | | | | 31 | |
| Interest cost | | | 70 | | | | 68 | | | | 79 | | | | 72 | |
| Benefit paid | | | (134 | ) | | | (21 | ) | | | (32 | ) | | | (19 | ) |
| Assumptions changes | | | (10 | ) | | | 9 | | | | 2 | | | | 13 | |
| Net actuarial loss | | | 51 | | | | 88 | | | | 267 | | | | 186 | |
| | | | | | | | | | | | | | | | | |
| Projected benefit obligation — at end of year | | $ | 1,284 | | | $ | 1,207 | | | $ | 1,646 | | | $ | 1,295 | |
| | | | | | | | | | | | | | | | | |
| Accumulated benefit obligation | | $ | 1,284 | | | $ | 1,189 | | | $ | 1,646 | | | $ | 1,295 | |
| | | | | | | | | | | | | | | | | |
Amounts recognized in the Consolidated Balance Sheets consist of (in thousands):
| | | | | | | | | | | | | | | | | |
| | | As of June 30, | |
| | | 2009 | | | 2010 | |
| | | France | | | Germany | | | France | | | Germany | |
| |
| Current liabilities | | $ | (53 | ) | | $ | (26 | ) | | $ | (64 | ) | | $ | (39 | ) |
| Other liabilities — non current | | | (1,231 | ) | | | (1,163 | ) | | | (1,582 | ) | | | (1,256 | ) |
| | | | | | | | | | | | | | | | | |
| Total | | $ | (1,284 | ) | | $ | (1,189 | ) | | $ | (1,646 | ) | | $ | (1,295 | ) |
| | | | | | | | | | | | | | | | | |
Amounts recognized in Accumulated Other Comprehensive Income consist of (in thousands):
| | | | | | | | | | | | | | | | | |
| | | Year Ended June 30, | |
| | | 2009 | | | 2010 | |
| | | France | | | Germany | | | France | | | Germany | |
| |
| Unrecognized actuarial loss (gain) | | $ | 76 | | | $ | (217 | ) | | $ | 267 | | | $ | (36 | ) |
| | | | | | | | | | | | | | | | | |
Unrecognized gains and losses are recognized over the average remaining service period of active plan participants. The estimated gains and losses that will be amortized from accumulated other comprehensive income into benefits expense over the next fiscal year is not significant.
Net periodic pension costs for the defined benefit plans consisted of the following elements (in thousands):
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Year Ended
| | | Year Ended
| | | Year Ended
| |
| | | June 30, 2008 | | | June 30, 2009 | | | June 30, 2010 | |
| | | France | | | Germany | | | France | | | Germany | | | France | | | Germany | |
| |
| Annual service cost | | $ | 91 | | | $ | 35 | | | $ | 84 | | | $ | 28 | | | $ | 99 | | | $ | 31 | |
| Interest accrued on pension obligations | | | 67 | | | | 63 | | | | 70 | | | | 68 | | | | 79 | | | | 72 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Total period pension cost | | $ | 158 | | | $ | 98 | | | $ | 154 | | | $ | 96 | | | $ | 178 | | | $ | 103 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
F-31
MIRION TECHNOLOGIES, INC.
Notes to Consolidated Financial Statements — (Continued)
The weighted-average rates used for each plan were as follows:
| | | | | | | | | | | | | | | | | |
| | | Year Ended June 30, | |
| | | 2009 | | | 2010 | |
| | | France | | | Germany | | | France | | | Germany | |
| |
| Projected benefit obligation: | | | | | | | | | | | | | | | | |
| Discount rate | | | 5.75 | % | | | 6.15 | % | | | 4.50 | % | | | 4.90 | % |
| Expected rate of return on plan assets | | | — | | | | — | | | | — | | | | — | |
| Assumed rate of compensation increase | | | 3.00 | % | | | 2.30 | % | | | 3.00 | % | | | 1.90 | % |
| Assumed rate of inflation | | | 2.00 | % | | | 2.00 | % | | | 2.00 | % | | | 2.00 | % |
| | | | | | | | | | | | | | | | | |
| Net periodic pension cost: | | | | | | | | | | | | | | | | |
| Discount rate | | | 6.00 | % | | | 6.30 | % | | | 5.75 | % | | | 6.15 | % |
| Assumed rate of compensation increase | | | 3.00 | % | | | 2.50 | % | | | 3.00 | % | | | 2.30 | % |
| Assumed rate of inflation | | | 2.00 | % | | | 2.20 | % | | | 2.00 | % | | | 2.00 | % |
Estimated future benefit payments are as follows (in thousands):
| | | | | | | | | | | | | |
| | | Amount | |
Years Ending June 30, | | France | | | Germany | | | Total | |
| |
| 2011 | | $ | 183 | | | $ | 39 | | | $ | 222 | |
| 2012 | | | 68 | | | | 57 | | | | 125 | |
| 2013 | | | 5 | | | | 60 | | | | 65 | |
| 2014 | | | 120 | | | | 62 | | | | 182 | |
| 2015 | | | 75 | | | | 65 | | | | 140 | |
| 2016-2020 | | | 930 | | | | 376 | | | | 1,306 | |
| | | | | | | | | | | | | |
| | | $ | 1,381 | | | $ | 659 | | | $ | 2,040 | |
| | | | | | | | | | | | | |
Defined Contribution Plans — The Company maintains voluntary defined contribution retirement plans for certain eligible employees. Under each plan, eligible employees may make voluntary contributions, while the Company makes contributions as defined by each plan agreement. Employee contributions in each plan are fully vested. Company contributions vest in accordance with the provision of each plan agreement. The following summarizes the features of each plan:
Retirement Plans — The Company maintains three retirement plans. Under the provisions of the plans, the Company is required to contribute a percentage of each eligible employee’s compensation. Total expense relating to these plans for fiscal 2008, 2009 and 2010 was $325,000, $288,000 and $281,000.
401(k) Savings Plan and Other Investment Savings Plans — The Company also maintains several 401(k) savings and other investment savings plans. Under these plans, both the employee and the Company make contributions to the plans and the Company contributions are based on the level of employee contributions. The amount expensed for 401(k) savings plans and other investment savings plans amounted to $439,000, $613,000 and $794,000 for fiscal 2008, 2009 and 2010.
The Company projects its contributions for all of the above employee benefit plans to be $1.4 million for the year ending June 30, 2011.
Other Postretirement Benefit Plans — The Company maintains a postretirement benefit plan for certain eligible employees. Under the provisions of the plan, certain retired employees will secure their own health insurance coverage, and the Company will reimburse the retired employee an amount specified in the plan. The premium reimbursement is only available to retirees who carried the Company’s health
F-32
MIRION TECHNOLOGIES, INC.
Notes to Consolidated Financial Statements — (Continued)
insurance at the date of retirement. This benefit obligation is unfunded and, accordingly, the fair value of plan assets is zero.
Coverage under the plan ends when the participant is eligible for Medicare benefits, which is assumed to be age 65. It is assumed that 70% of retirees eligible for coverage will select the family plan. Benefits are assumed to be available upon completion of 30 years of service and attainment of age 58 using a weighted-average discount rate of 5.0%.
The components of the Company’s postretirement benefit plan as of June 30, 2009 and 2010 are as follows (in thousands):
| | | | | | | | | |
| | | Year Ended June 30, | |
| | | 2009 | | | 2010 | |
| |
| Change in projected benefit obligations: | | | | | | | | |
| Projected benefit obligation — at beginning of year | | $ | 523 | | | $ | 626 | |
| Service cost | | | 23 | | | | 28 | |
| Interest cost | | | 39 | | | | 38 | |
| Benefit paid | | | (12 | ) | | | (9 | ) |
| Assumptions changes | | | (22 | ) | | | (9 | ) |
| Actuarial loss | | | 75 | | | | 77 | |
| | | | | | | | | |
| Projected benefit obligation — at end of year | | $ | 626 | | | $ | 751 | |
| | | | | | | | | |
| Accumulated benefit obligation | | $ | 626 | | | $ | 751 | |
| | | | | | | | | |
Amounts recognized in the Consolidated Balance Sheets as of June 30, 2009 and 2010 consist of (in thousands):
| | | | | | | | | |
| | | Year Ended June 30, | |
| | | 2009 | | | 2010 | |
| |
| Other liabilities-non current | | $ | (626 | ) | | $ | (751 | ) |
| | | | | | | | | |
Amounts recognized in Accumulated Other Comprehensive Income for fiscal 2009 and 2010 consist of (in thousands):
| | | | | | | | | |
| | | Year Ended June 30, | |
| | | 2009 | | | 2010 | |
| |
| Unrecognized actuarial gain | | $ | (240 | ) | | $ | (152 | ) |
| Unrecognized service cost | | | 87 | | | | 76 | |
| | | | | | | | | |
| | | $ | (153 | ) | | $ | (76 | ) |
| | | | | | | | | |
Unrecognized gains and losses are recognized over the average remaining service period of active plan participants. The estimated gains and losses, net that will be amortized from accumulated other comprehensive income into benefits expense over the next fiscal year is not significant.
F-33
MIRION TECHNOLOGIES, INC.
Notes to Consolidated Financial Statements — (Continued)
Estimated future benefit payments are as follows (in thousands):
| | | | | |
Years
| | | |
Ending June 30, | | Amount | |
| |
| 2011 | | $ | 58 | |
| 2012 | | | 46 | |
| 2013 | | | 66 | |
| 2014 | | | 62 | |
| 2015 | | | 46 | |
| Thereafter | | | 279 | |
| | | | | |
| Total | | $ | 557 | |
| | | | | |
Mirion was formed through the exchange of the capital stock of GDS, IST and Synodys on December 22, 2005, with the combination effective as of December 31, 2005. This transaction was considered a reorganization of entities under common control since GDS, IST and Synodys were under the common control of ACAS prior to the Reorganization. In order to effect the Reorganization, ACAS established a wholly-owned subsidiary, Global Monitoring Systems, Inc., on October 24, 2005, which was renamed Mirion Technologies, Inc. on January 4, 2006. Prior to the Reorganization, ACAS owned 90.5% of GDS, 92.5% of IST and 93.5% of Synodys. After the Reorganization, ACAS held 93.6% of Mirion. In accordance withFASB-issued authoritative guidance on exchanges of ownership interests between entities under common control andFASB-issued guidance on business combinations, the issuance of Mirion stock through the reorganization is accounted for in a manner similar to a pooling of interests which means that the historical basis of the net assets of GDS, IST and Synodys are combined for all periods prior to January 1, 2006.
Mirion Convertible Participating Preferred Stock
Mirion has 678,804 shares ofSeries A-1 Convertible Participating Preferred Stock outstanding and 70,000 shares ofSeries A-2 Convertible Participating Preferred Stock outstanding for all periods presented. The significant rights and obligations of the Mirion’s Convertible Participating Preferred stock are as follows:
Liquidation Rights — In the event of any liquidation, dissolution orwinding-up of the Company, whether voluntary or involuntary, the available funds and assets that may be legally distributed to the Company’s stockholders shall be distributed in preference to the Series A Convertible Participating Preferred Stockholders. The holders of shares of Series A Convertible Participating Preferred Stock will be entitled to receive an amount per share equal to the greater of (a) $113.28 per share in the case of theSeries A-1 Convertible Participating Preferred Stock and $103.68 per share in the case of theSeries A-2 Convertible Participating Preferred Stock as adjusted for stock splits, stock dividends or combinations plus any accrued or declared but unpaid dividends or (b) the amount the holders of Series A Convertible Participating Preferred Stock would be entitled to receive on an as-converted basis, according to the number of shares of common stock into which such shares could then be converted. If upon any liquidation, dissolution orwinding-up of the Company, the available funds and assets shall be insufficient to permit the payment to holders of the Series A Convertible Participating Preferred Stock of their full preferential amount, then the entire available funds and assets should be distributed on a pro rata basis among the holders of the outstanding Series A Convertible Participating Preferred Stock. If any assets of the Company distributed to stockholders in connection with any liquidation, dissolution orwinding-up of the Company are in a form other than cash, then the value of such assets shall be their fair market value.
F-34
MIRION TECHNOLOGIES, INC.
Notes to Consolidated Financial Statements — (Continued)
Upon completion of the distributions to the Series A Convertible Participating Preferred stockholders and the payment of all debts and liabilities of the Company, all of the remaining assets of the Company available for distribution will be distributed on a pro rata basis among the holders of common stock.
A liquidation, dissolution orwinding-up of the Company shall include the acquisition of the Company by another entity by means of a transaction or series of related transactions including without limitation any reorganization, merger or consolidation that results in the transfer of 50% or more of the outstanding voting power of the Company or the sale of all or substantially all assets of the Company.
Dividends Rights — The holders of shares ofSeries A-1 andA-2 Convertible Participating Preferred Stock, in preference to the holders of shares of any common stock, are entitled to receive cumulative dividends, at a rate of 8% and 17% per annum, payable on the first business day of each quarter commencing on January 1, 2006, by validly issuing fully paid and nonassessable shares ofSeries A-1 and A-2 Convertible Participating Preferred Stock with an aggregate liquidation preference equal to the amount of the dividends to be paid. Cumulative dividends have not been settled to date and at June 30, 2010, these cumulative dividends amounted to 295,352 shares for the holders of Series A-1 Convertible Participating Preferred Stock and 79,552 shares for the holders of Series A-2 Convertible Participating Preferred Stock. All undeclared dividends and declared but unpaid dividends including unissued additional shares ofSeries A-1 Convertible Participating Preferred Stock shall compound on a quarterly basis at theSeries A-l dividend rate, without any duplication when and if the dividends are actually paid.
No dividends should be paid on any share of common stock unless a dividend is paid with respect to all outstanding shares of Series A Convertible Participating Preferred Stock in an amount for each such share equal to or greater than the aggregate amount of such dividends for all shares of common stock into which such share of Series A Convertible Participating Preferred Stock could then be converted.
Conversion Rights — Each share of Series A Convertible Participating Preferred Stock is convertible at any time, at the option of the holder, into the number of fully paid and nonassessable shares of Class A Voting Common Stock that results from dividing the applicable original issue price per share by the split adjusted conversion price of $11.77, at a rate of 9.6288 for Series A-1 Convertible Participating Preferred Stock and a rate of 8.8128 for Series A-2 Convertible Participating Preferred Stock. The conversion price is subject to adjustments upon the occurrence of certain triggering events related to anti-dilution protection rights.
Each share of Convertible Participating Preferred Stock shall automatically convert into Class A Voting Common Stock at the conversion price at the time in effect, upon the vote or written consent of the holders of not less than a majority of the then-outstanding shares of Series A Convertible Participating Preferred Stock.
Anti-Dilution Provisions — No adjustment in the number of shares of Class A Voting Common Stock into which the shares of any Series A Convertible Participating Preferred Stock is convertible should be made, unless the effective price of additional shares of common stock is less than the conversion price in effect on the date of the issue of such additional shares of common stock.
Voting Rights — The holders of shares of Series A Convertible Participating Preferred Stock do not have voting rights of any kind. The Company cannot amend, alter or repeal any provision of the certificate of incorporation of the Company so as to adversely affect the preferences, rights, privileges or powers of the Series A Convertible Participating Preferred Stock without the consent of the holders of a majority of the outstanding shares of Series A Convertible Participating Preferred Stock. However, each holder of Series A Convertible Participating Preferred Stock would need to approve an amendment to the certificate of incorporation that reduces the dividend payable on or the liquidation preference of the Series A Convertible Participating Preferred Stock. Also, the Company cannot create, authorize or issue any securities senior to the Series A Convertible Participating Preferred Stock as to dividends and distributions upon the liquidation,winding-up and dissolution of the Company.
F-35
MIRION TECHNOLOGIES, INC.
Notes to Consolidated Financial Statements — (Continued)
Mirion Common Stock
Mirion has authorized 61,328,125 shares of Class A Voting Common Stock and 17,171,875 shares of Class B Non-Voting Common Stock. The rights and privileges of Class A and Class B common stock are the same except for voting rights. Common stock reserved for future issuance as of June 30, 2010, was as follows:
| | | | | |
| | | Number of
| |
| | | Shares | |
| |
| 2006 Stock Plan: | | | | |
| Shares authorized under the 2006 plan | | | 1,010,140 | |
| Less: Options exercised | | | 1,275 | |
| | | | | |
| Total common stock reserved for stock options | | | 1,008,865 | |
| Less: outstanding stock options | | | 924,830 | |
| | | | | |
| Reserved for future option grants | | | 84,035 | |
| | | | | |
| | | | | |
| Common stock reserved for stock options | | | 1,008,865 | |
| Warrants to purchase common stock | | | 3,420,636 | |
| Convertible preferred stock, including paid-in-kind dividends (as converted) | | | 10,697,928 | |
| | | | | |
| Total common stock reserved for future issuances | | | 15,127,429 | |
| | | | | |
Detachable Warrants
On January 1, 2006, Mirion issued warrants granting the holders the right to purchase Class A Voting Common Stock of the Company. These warrants only become exercisable upon a sale, liquidation or dissolution of the Company or approval by the Board of Directors or upon liquidation of the Company. On February 26, 2010, the Board of Directors resolved that all of these warrants will become exercisable upon the consummation of an initial public offering. The warrants expire on December 22, 2015. As of June 30, 2009 and 2010, there were 3,420,636 shares of common stock issuable upon the exercise of outstanding detachable warrants, of which 196,910 have an exercise price of $0.00118 and 3,223,726 have an exercise price of $0.00012.
| |
| 13. | STOCK-BASED COMPENSATION |
The Board of Directors approved the Mirion 2006 Stock Plan, effective as of December 22, 2005. The plan approves the granting of awards in the form of nonqualified stock options to directors, employees or consultants. The total number of shares available for distribution under the plan is 1,010,140. Under the terms of the plan, the exercise price for awards issued under the plan is determined at the sole discretion of the Board of Directors.
Following is the stock-based compensation expense (in thousands) included in the consolidated statement of operations for options granted through the Mirion 2006 Stock Plan as well as those granted by ACAS to the Company’s Chief Executive Officer and Chairman of the Board. Expense from the options granted by ACAS was $121,000 in fiscal year 2008. See Note 14.
| | | | | | | | | | | | | |
| | | Year Ended June 30, | |
| | | 2008 | | | 2009 | | | 2010 | |
| |
| Selling, general and administrative expenses | | $ | 223 | | | $ | 1,128 | | | $ | 1,075 | |
| Research and development expense | | | 25 | | | | 33 | | | | 32 | |
| | | | | | | | | | | | | |
| Total stock-based compensation expense | | $ | 248 | | | $ | 1,161 | | | $ | 1,107 | |
| | | | | | | | | | | | | |
F-36
MIRION TECHNOLOGIES, INC.
Notes to Consolidated Financial Statements — (Continued)
The stock awards under the Mirion 2006 stock plan include stock awards with performance and market-based vesting (“PSA”) and time-based vesting (“TSA”). Under the terms of the PSA agreements, Mirion grants employee stock option awards whose vesting is contingent upon meeting company-wide performance goals including earnings before interest, taxes and depreciation targets or market conditions, including internal rate of return targets. The TSAs include stock options granted by the Company whose vesting occurs over a period of five to 60 months.
The Company estimates the value of the employee stock options on the date of grant using the Black-Scholes model for TSAs and using a Monte Carlo simulation for PSAs containing market conditions. The key assumptions used in the model for valuation of options granted under the Mirion 2006 Stock Plan are provided below:
| | | | | | | |
| | | Performance-Based Vested Awards |
| | | Year Ended June 30, |
| | | 2008 | | 2009 | | 2010 |
| |
| Expected term (in years) | | 2.7–2.8 | | N/A | | N/A |
| Risk-free interest rate | | 3.7%–4.1% | | N/A | | N/A |
| Volatility | | 35% | | N/A | | N/A |
| Dividend yield | | 0% | | N/A | | N/A |
| Weighted-average fair value at grant date | | $2.53 | | N/A | | N/A |
| | | | | | | |
| | | Time-Based Vested Awards |
| | | Year Ended June 30, |
| | | 2008 | | 2009 | | 2010 |
| |
| Expected term (in years) | | 8.0–10.0 | | 10 | | N/A |
| Risk-free interest rate | | 2.9%–4.5% | | 2.7%–4.1% | | N/A |
| Volatility | | 46% | | 46%–47% | | N/A |
| Dividend yield | | 0% | | 0% | | N/A |
| Weighted-average fair value at grant date | | $5.31 | | $5.81 | | N/A |
The expected term of the option is determined based on factors including vesting period, life of option, strike price and fair market value of the Company’s stock on the date of grant. The fair market value of the Company’s stock on the date of grant was determined in a retrospective valuation. The risk-free rate of interest over the contractual life of the option is based on the U.S. Treasury yield curve in effect at the time of grant. Since the Company’s stock is not publicly traded, volatility has been determined based on the volatility of stocks of comparable companies within the Company’s industry. No dividends on the Company’s common stock have been declared in the past, and the Company does not expect to do so in the foreseeable future.
F-37
MIRION TECHNOLOGIES, INC.
Notes to Consolidated Financial Statements — (Continued)
Share option activity for the Mirion 2006 Stock Plan is as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Year Ended June 30, | |
| | | 2008 | | | 2009 | | | 2010 | |
| | | | | | Weighted-
| | | | | | Weighted-
| | | | | | Weighted-
| |
| | | | | | Average
| | | | | | Average
| | | | | | Average
| |
| | | | | | Exercise
| | | | | | Exercise
| | | | | | Exercise
| |
| | | Shares | | | Price | | | Shares | | | Price | | | Shares | | | Price | |
| |
| Outstanding — beginning of year | | | 383,677 | | | $ | 10.81 | | | | 736,989 | | | $ | 13.62 | | | | 962,944 | | | $ | 14.45 | |
| Granted | | | 504,262 | | | | 15.08 | | | | 390,030 | | | | 16.86 | | | | — | | | | — | |
| Exercised | | | (1,275 | ) | | | 10.45 | | | | — | | | | — | | | | — | | | | — | |
| Forfeited or expired | | | (149,675 | ) | | | 11.37 | | | | (164,075 | ) | | | 16.46 | | | | (38,114 | ) | | | 16.35 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Outstanding — end of year | | | 736,989 | | | $ | 13.62 | | | | 962,944 | | | $ | 14.45 | | | | 924,830 | | | $ | 14.37 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Vested or expected to vest — end of year | | | 736,989 | | | | 13.62 | | | | 962,944 | | | | 14.45 | | | | 924,830 | | | $ | 14.37 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Exercisable — end of year | | | 210,162 | | | $ | 11.18 | | | | 412,470 | | | $ | 13.51 | | | | 597,970 | | | $ | 14.22 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Options grant activity for the year ended June 30, 2009 is as follow:
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | Intrinsic
| |
| | | | | | | | | Fair Value of
| | | Value of
| |
| | | # Options
| | | Exercise Price
| | | Options on
| | | Options on
| |
Grant Date | | Granted | | | per Share | | | Grant Date | | | Grant Date | |
| |
| July 28, 2008(1) | | | 102,000 | | | $ | 16.31 | | | $ | 6.67 | | | $ | 0.00 | |
| August 5, 2008 | | | 279,530 | | | $ | 17.06 | | | $ | 5.69 | | | $ | 0.00 | |
| December 9, 2008 | | | 8,500 | | | $ | 16.97 | | | $ | 1.85 | | | $ | 0.00 | |
| | | | | | | | | | | | | | | | | |
| Total | | | 390,030 | | | | | | | | | | | $ | 0.00 | |
| | | | | | | | | | | | | | | | | |
| | |
| (1) | | The 102,000 options granted July 28, 2008 were a modification of 127,500 options granted on November 5, 2007. The 102,000 options, which have time-based vesting, replaced the 127,500 options, which had performance-based vesting. The total compensation cost measured at the date of modification was determined to be the grant-date fair value of the original award plus the incremental fair value resulting from the modification. The incremental fair value resulting from the modification was calculated using the Black-Scholes model and was determined to be $496,000. |
The fair value of Mirion’s common stock as of the grant date of all options issued has been determined in accordance with the AICPA Practice Aid “Valuation of Privately-Held Company Equity Securities Issued as Compensation.”
The following table shows the weighted-average remaining contractual term and aggregate intrinsic value for options outstanding, vested and exercisable at June 30, 2010:
| | | | | | | | | |
| | | Weighted-
| | | | |
| | | Average
| | | | |
| | | Remaining
| | | Aggregate
| |
| | | Contractual
| | | Intrinsic
| |
| | | Term | | | Value(1) | |
| | | (in years) | | | (in thousands) | |
| |
| Outstanding | | | 6.6 | | | $ | 1,915 | |
| Vested and exercisable | | | 6.2 | | | | 1,317 | |
| | |
| (1) | | Excludes options with a strike price greater than the market value of the underlying stock. |
F-38
MIRION TECHNOLOGIES, INC.
Notes to Consolidated Financial Statements — (Continued)
The aggregate intrinsic value of the share options shown in the table above was calculated using the estimated market price of common stock at June 30, 2010, which was $16.16 per share. As of June 30, 2010, there was $1.2 million of unrecognized compensation cost related to nonvested stock-based compensation arrangements granted under the Mirion 2006 Stock Plan; that cost is expected to be recognized over a weighted-average period of 1.6 years. This excludes 85,798 nonvested outstanding performance-based stock options whose fair value could be recognized upon a sale of the Company. These performance-based stock options will only vest in the event of a sale transaction, which does not include a public offering.
No tax benefit was realized for tax deductions from stock-based arrangements, including from the exercise of stock options, during fiscal 2008, 2009 and 2010. The Company did not use any cash to settle equity instruments granted under its stock-based payment arrangements for fiscal 2008, 2009 and 2010. There was no compensation cost capitalized as part of inventory or property, plant and equipment during fiscal 2008, 2009 and 2010.
| |
| 14. | RELATED-PARTY TRANSACTIONS |
The Company has NEPAs with its primary investor ACAS and its subsidiary ACFS (see Note 8). The terms of the NEPAs require the Company to pay ACFS annual management fees aggregating $1.6 million per year. In return, ACFS provides various investment banking services relating to financing arrangements, mergers and acquisitions, financial analysis and other services. The NEPAs are not cancelable by the Company as long as ACFS has an investment in the Company’s debt or equity securities. Such transactions were not consummated on terms equivalent to those that prevail in arm’s length transactions.
Amounts paid to ACFS were as follows (in thousands):
| | | | | | | | | | | | | |
| | | Year Ended June 30, | |
| | | 2008 | | | 2009 | | | 2010 | |
| |
| Expense invoices | | $ | 111 | | | $ | 114 | | | $ | 18 | |
| Loan fees | | | 201 | | | | — | | | | — | |
| Management fees | | | 1,625 | | | | 1,625 | | | | 1,625 | |
| Interest on debt | | | 17,211 | | | | 14,760 | | | | 12,356 | |
| | | | | | | | | | | | | |
| | | $ | 19,148 | | | $ | 16,499 | | | $ | 13,999 | |
| | | | | | | | | | | | | |
The Company’s President, Chief Executive Officer and Chairman of the Board, Thomas D. Logan, entered into a Call Option Agreement on April 19, 2004 with ACAS and certain of its affiliates, in which ACAS granted time and performance-based options with market conditions to Mr. Logan to purchase shares of the common stock of two of Mirion’s predecessors in connection with his services as an officer and director. The options contain vesting provisions based upon successful completion of an initial public offering or change in control, and achievement by ACAS of certain internal rates of return as described further below. Modification of these options occurred in substance on January 1, 2006, in connection with the formation of Mirion in December 2005. As a result of the modification, Mr. Logan was granted performance-based options with market conditions to purchase 463,794 shares of Mirion’s Class A Common Stock held by ACAS. These options were further modified on December 7, 2007 to modify the vesting criteria of the performance based options to include, in addition to existing vesting provisions, vesting upon the achievement of certain returns on investment, as discussed in detail below. The exercise price of these options is $10.45 per share, and the total incremental value resulting from the option modification is $2.1 million and incorporates the impact of the options’ market-based conditions in the original grant date and modification date fair values. The original grant date fair value of these options was negligible. The Company will recognize expense on these options in the period and to the extent that it satisfies the performance-based vesting conditions, which require completion of an effective offering in the public markets or a qualifying sale of the Company.
F-39
MIRION TECHNOLOGIES, INC.
Notes to Consolidated Financial Statements — (Continued)
The performance-based options are divided into three tranches, each of which will either vest or be cancelled in two halves upon an initial public offering (“IPO”) or change in control, depending on whether ACAS achieves certain market-based conditions, internal rates of return or returns on investment in such an event. Upon completion of an IPO, vesting of the performance-based options will occur in two stages. The first stage occurs 30 days after the effective time of the IPO at which time 50% of the options in each tranche will vest if ACAS achieves certain minimum internal rates of return, ranging between 25–40% or certain minimum returns on investment ranging between 2.0–2.7x. If neither goal is met, the options in this tranche will be cancelled. The second stage occurs on the earlier of two years after the effective time of an IPO or upon the sale by ACAS of its investments in the Company, at which time the remaining 50% of the options in each tranche will vest if ACAS achieves certain minimum internal rates of return ranging between 25–40% or certain minimum returns on investment of 2.0–2.7x. If neither goal is met, the options in this tranche will be cancelled. The Company will record stock compensation expense in connection with these options in the event it completes an effective offering in the public markets or a qualifying sale of the Company, regardless of whether ACAS achieves the related market-based conditions.
The total compensation cost measured at the date of modification was determined to be the grant-date fair value of the original award plus the incremental cost resulting from the modification. Because these options contain market-based vesting criteria, the Company used a Monte Carlo simulation model, as opposed to a Black-Scholes model, to value such options. In the Monte Carlo simulation model, weekly stock prices were simulated until the liquidity event time using a geometric Brownian motion model. Based on the simulated stock price at the liquidity event and the vesting requirements, the number of vested shares was determined. The stock price at liquidity and the options’ exercise price were used to determine the intrinsic value per share of the options at the liquidity event. The intrinsic value was then discounted to the present at the risk free rate to determine the option fair value for each simulation. Fifty thousand simulations were run for each valuation date, and the sum value of those simulations was averaged to determine the value of the options. Key valuation assumptions as of the January 1, 2006 modification are an expected term of 4.5 years, volatility of 41.8%, a risk-free rate of interest of 4.3%, and a dividend yield of 0%. Key valuation assumptions as of the December 7, 2007 modification are an expected term of 2.6 years, volatility of 39.8%, a risk-free rate of interest of 3.1%, and a dividend yield of 0%.
The Call Option Agreement also provides Mr. Logan with an option to purchase 150,875 shares of the Company’s common stock held by ACAS that vest on a monthly schedule. The exercise price of these options is $10.45 per share and the total incremental fair value resulting from the option modification is $592,000. All such options vested as of June 30, 2008. The total compensation cost measured at the date of modification was determined to be the grant-date fair value of the original award plus the incremental cost resulting from the modification, in which vesting of both the original and modified awards were considered to be probable. These options were valued using the Black-Scholes model. Key valuation assumptions as of the January 1, 2006 modification are an expected term of 4.5 years, volatility of 41.8%, a risk-free rate of interest of 4.3%, and a dividend yield of 0%.
All options granted by ACAS and its affiliates to Mr. Logan pursuant to the Call Option Agreement are to be reduced on an economically equivalent basis in the event the Company grants Mr. Logan options to purchase shares of Mirion common stock after the date of the Call Option Agreement, provided such options are no less favorable to Mr. Logan.
These 463,794 and 150,875 options were granted by ACAS and are supported by the Company’s stock held by ACAS. They are not part of the Mirion 2006 stock plan and are therefore not included in the “Share options activity” table presented in Note 13.
| |
| 15. | SEGMENT AND GEOGRAPHIC INFORMATION |
The Company manages its business operations through five strategic business units. Based upon the information reported to the chief operating decision maker, who is the Chief Executive Officer, the Company
F-40
MIRION TECHNOLOGIES, INC.
Notes to Consolidated Financial Statements — (Continued)
has the following reportable segments: Health Physics, Radiation Monitoring Systems, Sensing Systems, Dosimetry Services and Imaging Systems.
| | |
| | • | The Health Physics segment sells dosimeters, detection equipment and contamination & clearance monitors to power and utility companies, military organizations and governmental agencies. In the Health Physics segment, the active dosimetry product line represented 11%, 11% and 8% of consolidated revenue for fiscal 2008, 2009 and 2010. The contamination and clearance monitors product line represented 8%, 10% and 13% of consolidated revenue for fiscal 2008, 2009 and 2010. |
| | |
| | • | The Radiation Monitoring Systems segment sells radiation monitoring systems to the nuclear end market. The Radiation Monitoring Systems segment consists of a single product line and represents 23%, 20% and 27% of consolidated revenue for fiscal 2008, 2009 and 2010. |
| | |
| | • | The Sensing Systems segment supplies electrical penetration and reactor control equipment to the builders and operators of nuclear reactors. No single product line in the Sensing Systems segment represented more than 10% of consolidated revenue for fiscal 2008, 2009 and 2010. |
| | |
| | • | The Dosimetry Services segment provides dosimetry services to employers of radiation workers in the nuclear and medical end markets. The Dosimetry Services segment consists of a single product line and represents 15%, 15% and 13% of consolidated revenue for fiscal 2008, 2009 and 2010. |
| | |
| | • | The Imaging Systems segment sells specialized cameras for use in difficult and hazardous environments. No single product line in the Imaging Systems segment represented more than 10% of consolidated revenue in fiscal 2008, 2009 and 2010. |
F-41
MIRION TECHNOLOGIES, INC.
Notes to Consolidated Financial Statements — (Continued)
Summarized financial information by reporting segment was as follows (in thousands):
| | | | | | | | | | | | | |
| | | Year Ended June 30, | |
| | | 2008 | | | 2009 | | | 2010 | |
| |
| Revenue: | | | | | | | | | | | | |
| Health Physics | | $ | 58,691 | | | $ | 69,109 | | | $ | 72,992 | |
| Radiation Monitoring Systems | | | 43,201 | | | | 41,116 | | | | 60,729 | |
| Sensing Systems | | | 39,866 | | | | 44,979 | | | | 47,255 | |
| Dosimetry Services | | | 28,824 | | | | 29,457 | | | | 29,206 | |
| Imaging Systems | | | 21,187 | | | | 17,102 | | | | 17,942 | |
| | | | | | | | | | | | | |
| Total | | $ | 191,769 | | | $ | 201,763 | | | $ | 228,124 | |
| | | | | | | | | | | | | |
| (Loss) income from operations: | | | | | | | | | | | | |
| Health Physics | | $ | (912 | ) | | $ | 6,317 | | | $ | 5,058 | |
| Radiation Monitoring Systems | | | 1,085 | | | | 4,109 | | | | 12,102 | |
| Sensing Systems | | | 10,234 | | | | 14,973 | | | | 16,587 | |
| Dosimetry Services | | | 7,746 | | | | 7,968 | | | | 7,936 | |
| Imaging Systems | | | 1,339 | | | | 1,064 | | | | 2,080 | |
| Unallocated corporate items | | | (8,555 | ) | | | (15,553 | ) | | | (15,585 | ) |
| | | | | | | | | | | | | |
| Total | | $ | 10,937 | | | $ | 18,878 | | | $ | 28,178 | |
| | | | | | | | | | | | | |
| Depreciation and amortization: | | | | | | | | | | | | |
| Health Physics | | $ | 3,225 | | | $ | 2,780 | | | | 2,269 | |
| Radiation Monitoring Systems | | | 2,268 | | | | 1,927 | | | | 2,075 | |
| Sensing Systems | | | 2,172 | | | | 1,806 | | | | 1,668 | |
| Dosimetry Services | | | 5,025 | | | | 4,283 | | | | 4,130 | |
| Imaging Systems | | | 1,467 | | | | 1,410 | | | | 1,214 | |
| Unallocated corporate items | | | 25 | | | | 273 | | | | 696 | |
| | | | | | | | | | | | | |
| Total | | $ | 14,182 | | | $ | 12,479 | | | $ | 12,052 | |
| | | | | | | | | | | | | |
| Interest expense: | | | | | | | | | | | | |
| Health Physics | | $ | 3,326 | | | $ | 3,019 | | | | 2,111 | |
| Radiation Monitoring Systems | | | 3,326 | | | | 3,019 | | | | 2,137 | |
| Sensing Systems | | | 2,971 | | | | 2,408 | | | | 2,216 | |
| Dosimetry Services | | | 7,756 | | | | 6,935 | | | | 6,464 | |
| Imaging Systems | | | 2,971 | | | | 2,408 | | | | 2,216 | |
| | | | | | | | | | | | | |
| Total | | $ | 20,350 | | | $ | 17,789 | | | $ | 15,144 | |
| | | | | | | | | | | | | |
| | | | | | | | | |
| | | As of June 30, | |
| | | 2009 | | | 2010 | |
| |
| Total assets: | | | | | | | | |
| Health Physics | | $ | 94,883 | | | $ | 82,216 | |
| Radiation Monitoring Systems | | | 85,915 | | | | 67,769 | |
| Sensing Systems | | | 47,641 | | | | 50,763 | |
| Dosimetry Services | | | 71,690 | | | | 69,686 | |
| Imaging Systems | | | 24,829 | | | | 24,198 | |
| Unallocated corporate items | | | 4,796 | | | | 12,731 | |
| | | | | | | | | |
| Total | | $ | 329,754 | | | $ | 307,363 | |
| | | | | | | | | |
F-42
MIRION TECHNOLOGIES, INC.
Notes to Consolidated Financial Statements — (Continued)
The Company conducts its operations throughout the world. Revenue is attributed to each geographic location based on the locations of end customers. Assets and Long-lived assets are attributed based on where the assets are located. Revenue, assets and long-lived assets by geographic location were as follows (in thousands):
| | | | | | | | | | | | | |
| | | Year Ended June 30, | |
| | | 2008 | | | 2009 | | | 2010 | |
| |
| Revenue: | | | | | | | | | | | | |
| North American markets(1) | | $ | 68,840 | | | $ | 77,435 | | | $ | 79,593 | |
| European markets | | | 90,189 | | | | 93,977 | | | | 106,211 | |
| Asia Pacific markets | | | 32,740 | | | | 30,351 | | | | 42,320 | |
| | | | | | | | | | | | | |
| Total | | $ | 191,769 | | | $ | 201,763 | | | | 228,124 | |
| | | | | | | | | | | | | |
| | | | | | | | | |
| | | As of June 30, | |
| | | 2009 | | | 2010 | |
| |
| Assets: | | | | | | | | |
| North America | | $ | 140,927 | | | $ | 143,255 | |
| Europe | | | 188,626 | | | | 163,897 | |
| Asia Pacific | | | 201 | | | | 211 | |
| | | | | | | | | |
| Total Assets | | $ | 329,754 | | | | 307,363 | |
| | | | | | | | | |
| Long-lived assets: | | | | | | | | |
| North America | | $ | 8,593 | | | | 9,171 | |
| Europe | | | 9,486 | | | | 11,360 | |
| Asia Pacific | | | 1 | | | | 1 | |
| | | | | | | | | |
| Total | | $ | 18,080 | | | | 20,532 | |
| | | | | | | | | |
| | |
| (1) | | North American markets include all products marketed in the United States and Canada. |
F-43
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
| |
| Item 13. | Other Expenses of Issuance and Distribution. |
The following table sets forth expenses to be paid by the registrant in connection with the offering described in this Registration Statement. Each of the amounts set forth below, other than the Registration fee and the FINRA filing fee, is an estimate.
| | | | |
| | | Amount |
| |
| Registration fee | | $ | 13,784 |
| FINRA filing fee | | | 22,005 |
| NASDAQ listing fee | | | 125,000 |
| Transfer agent’s fees | | | * |
| Printing and engraving expenses | | | * |
| Legal fees and expenses | | | * |
| Accounting fees and expenses | | | * |
| Miscellaneous | | | * |
| | | | |
| Total | | $ | |
| | | | |
| | |
| * | | To be filed by amendment |
Item 4. Indemnification of Directors and Officers.
Section 145 of the Delaware General Corporation Law provides that a corporation may indemnify directors and officers as well as other employees and individuals against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by such person in connection with any threatened, pending or completed actions, suits or proceedings in which such person is made a party by reason of such person being or having been a director, officer, employee or agent to the Registrant. The Delaware General Corporation Law provides that Section 145 is not exclusive of other rights to which those seeking indemnification may be entitled under any bylaw, agreement, vote of stockholders or disinterested directors or otherwise. The amended and restated Bylaws of the Registrant provide for indemnification by the Registrant of its directors, officers and employees to the fullest extent permitted by the Delaware General Corporation Law.
Section 102(b)(7) of the Delaware General Corporation Law permits a corporation to provide in its certificate of incorporation that a director of the corporation shall not be personally liable to the corporation or its stockholders for monetary damages for breach of fiduciary duty as a director, except for liability (i) for any breach of the director’s duty of loyalty to the corporation or its stockholders, (ii) for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law, (iii) for unlawful payments of dividends or unlawful stock repurchases, redemptions or other distributions, or (iv) for any transaction from which the director derived an improper personal benefit. The Registrant’s amended and restated Certificate of Incorporation provides for such limitation of liability.
The Registrant maintains standard policies of insurance under which coverage is provided (a) to its directors and officers against loss rising from claims made by reason of breach of duty or other wrongful act, and (b) to the Registrant with respect to payments which may be made by the Registrant to such officers and directors pursuant to the above indemnification provision or otherwise as a matter of law.
The Underwriting Agreement filed as Exhibit 1.1 to this Registration Statement will provide for indemnification of the Registrant and its directors and certain officers by the underwriters of this offering. The Registration Rights Agreement filed as Exhibit 4.2 to this Registration Statement will provide for indemnification of the Registrant and its directors and certain officers against certain liabilities related to the offering of the common stock thereunder.
II-1
| |
| Item 15. | Recent Sales of Unregistered Securities. |
(a) Since September 30, 2006, the Registrant issued the following unregistered securities:
On October 3, 2006, the Registrant issued 357,271 shares of Class B Non-Voting Common Stock to ACAS and certain of its affiliates upon cancellation of a prior issuance of 357,271 shares of Class B Non-Voting Common Stock held by ACAS.
On October 3, 2006, the Registrant issued 677,426 shares ofSeries A-1 Convertible Participating Preferred Stock to ACAS and certain of its affiliates upon cancellation of a prior issuance of 677,426 shares ofSeries A-1 Convertible Participating Preferred Stock to ACAS.
On October 3, 2006, the Registrant issued 70,000 shares ofSeries A-2 Convertible Participating Preferred Stock to ACAS and certain of its affiliates upon cancellation of a prior issuance of 70,000 shares ofSeries A-2 Convertible Participating Preferred Stock to ACAS.
On October 3, 2006, the Registrant issued warrants to purchase 3,223,726 shares of common stock at an exercise price of $0.00018 per share to ACAS and certain of its affiliates upon cancellation of a prior issuance of warrants to purchase 3,223,726 shares of common stock at an exercise price of $0.00118 per share to ACAS.
On October 3, 2007, the Registrant issued 250,086 shares of Class B Non-Voting Common Stock to ACAS and certain of its affiliates upon cancellation of a prior issuance of 250,086 shares of Class B Non-Voting Common Stock to ACAS.
On October 3, 2007, the Registrant issued 474,198 shares ofSeries A-1 Convertible Participating Preferred Stock to ACAS and certain of its affiliates upon cancellation of a prior issuance of 474,198 shares ofSeries A-1 Convertible Participating Preferred Stock to ACAS.
On October 3, 2007, the Registrant issued 49,000 shares ofSeries A-2 Convertible Participating Preferred Stock to ACAS and certain of its affiliates upon cancellation of a prior issuance of 49,000 shares ofSeries A-2 Convertible Participating Preferred Stock to ACAS.
On October 3, 2007, the Registrant issued warrants to purchase 2,241,509 shares of common stock at an exercise price of $0.00012 per share to ACAS and certain of its affiliates upon cancellation of a prior issuance of warrants to purchase 2,241,509 shares of common stock at an exercise price of $0.00012 per share to ACAS.
On May 1, 2008, the Registrant issued and sold 1,275 shares of Class B Non-Voting Common Stock to a former employee upon an exercise of stock options vested under the Registrant’s 2006 Stock Plan at an exercise price of $10.45 per share, for aggregate consideration of $13,312.50.
II-2
None of the foregoing transactions involved any underwriters, underwriting discounts or commissions, or any public offering, and the registrant believes that each transaction was exempt from the registration requirements of the Securities Act pursuant to Section 4(2), with respect to items (1) through (8) above, as transactions by an issuer not involving a public offering, and Rule 701 promulgated thereunder, with respect to item (9) above, as a transaction pursuant to compensatory benefit plans and contracts relating to compensation as provided under such Rule 701. The recipients of securities in such transactions represented their intention to acquire the securities for investment only and not with a view to or for sale in connection with any distribution thereof, and appropriate legends were affixed to the share certificates and instruments issued in such transactions.
(b) Since September 30, 2006, the Registrant granted the following stock options to purchase common stock to employees, directors and consultants:
1. On October 2, 2006, the Registrant issued stock options covering an aggregate of 40,409 shares of its common stock at an exercise price of $13.84 per share and an aggregate price of $558,975 under the Registrant’s 2006 Stock Plan.
2. On September 6, 2007, the Registrant issued stock options covering an aggregate of 62,262 shares of its common stock at an exercise price of $14.27 per share and an aggregate price of $888,083 under the Registrant’s 2006 Stock Plan.
3. On November 5, 2007, the Registrant issued stock options covering an aggregate of 127,500 shares of its common stock at an exercise price of $16.31 per share and an aggregate price of $1,662,960 under the Registrant’s 2006 Stock Plan, 25,500 of which were cancelled in July 2008.
4. On January 7, 2008, the Registrant issued stock options covering an aggregate of 68,000 shares of its common stock at an exercise price of $16.31 per share and an aggregate price of $1,108,640 under the Registrant’s 2006 Stock Plan.
5. On January 8, 2008, the Registrant issued stock options covering an aggregate of 12,750 shares of its common stock at an exercise price of $16.31 per share and an aggregate price of $207,870 under the Registrant’s 2006 Stock Plan.
6. On January 22, 2008, the Registrant issued stock options covering an aggregate of 4,250 shares of its common stock at an exercise price of $16.31 per share and an aggregate price of $69,290 under the Registrant’s 2006 Stock Plan.
7. On January 28, 2008, the Registrant issued stock options covering an aggregate of 76,500 shares of its common stock at an exercise price of $16.31 per share and an aggregate price of $1,247,220 under the Registrant’s 2006 Stock Plan.
8. On March 28, 2008, the Registrant issued stock options covering an aggregate of 17,000 shares of its common stock at an exercise price of $13.10 per share and an aggregate price of $222,600 under the Registrant’s 2006 Stock Plan.
9. On March 31, 2008, the Registrant issued stock options covering an aggregate of 136,000 shares of its common stock at an exercise price of $13.10 per share and an aggregate price of $1,780,800 under the Registrant’s 2006 Stock Plan.
10. On August 5, 2008, the Registrant issued stock options covering an aggregate of 262,530 shares of its common stock at an exercise price of $17.06 per share and an aggregate price of $4,476,926 under the Registrant’s 2006 Stock Plan.
11. On August 5, 2008, the Registrant issued stock options covering an aggregate of 17,000 shares of its common stock at an exercise price of $17.06 per share and an aggregate price of $289,900 under the Registrant’s 2006 Stock Plan.
II-3
12. On December 9, 2008, the Registrant issued stock options covering an aggregate of 8,500 shares of its common stock at an exercise price of $16.97 per share and an aggregate price of $144,240 under the Registrant’s 2006 Stock Plan.
None of the foregoing transactions involved any underwriters, underwriting discounts or commissions, or any public offering, and the registrant believes each transaction was exempt from the registration requirements of the Securities Act in reliance on Rule 701 promulgated thereunder, with respect to items (1), (2), (3), (5), (7), (8), (10) and (12) above, as transactions pursuant to compensatory benefit plans and contracts relating to compensation as provided under such Rule 701, and Section 4(2) thereof and Regulation D promulgated thereunder, with respect to items (4), (9) and (11) above, as transactions by an issuer not involving a public offering. The recipients of securities in such transactions represented their intention to acquire the securities for investment only and not with a view to or for sale in connection with any distribution thereof, and appropriate legends were affixed to the share certificates and instruments issued in such transactions.
| |
| Item 16. | Exhibits and Financial Statement Schedules. |
| | | | | |
Exhibit No. | | Document |
| |
| | 1.1 | ** | | Form of Underwriting Agreement |
| | 3.1.1 | | | Form of Fourth Amended and Restated Certificate of Incorporation to be adopted prior to the effectiveness of this offering |
| | 3.1.2 | | | Form of Fifth Amended and Restated Certificate of Incorporation to be adopted prior to the consummation of this offering |
| | 3.2 | ** | | Form of Amended and Restated Bylaws |
| | 4.1 | ** | | Specimen Common Stock Certificate of the Registrant |
| | 4.2 | ** | | Form of Registration Rights Agreement |
| | 5.1 | * | | Opinion of Davis Polk & Wardwell LLP |
| | 10.1 | ** | | Shareholder Loan Agreement dated September 23, 2005 between Dosimetry Acquisitions (France) and ACAS |
| | 10.1.1 | ** | | Amendment 1 dated November 14, 2005 to Shareholder Loan Agreement |
| | 10.1.2 | ** | | Amendment No. 2 dated September 13, 2006 to Shareholder Loan Agreement |
| | 10.1.3 | ** | | Third Amendment dated May 14, 2008 to Shareholder Loan Agreement |
| | 10.1.4 | ** | | Fourth Amendment dated July 20, 2009 to Shareholder Loan Agreement |
| | 10.1.5 | | | Fifth Amendment dated July 29, 2010 to Shareholder Loan Agreement |
| | 10.2 | ** | | Note and Equity Purchase Agreement dated June 23, 2004 by and among MGP Instruments, Inc., Dosimetry Acquisitions (U.S.), Inc. and American Capital Financial Services, Inc. and various purchasers |
| | 10.2.1 | ** | | Amendment No. 1 to Note and Equity Purchase Agreement of MGP Instruments, Inc. dated October 22, 2004 |
| | 10.2.2 | ** | | Amendment No. 2 to Note and Equity Purchase Agreement of MGP Instruments, Inc. dated November 1, 2005 |
| | 10.2.3 | ** | | Amendment No. 2 and Consent to Note and Equity Purchase Agreement of MGP Instruments, Inc. dated December 22, 2005 |
| | 10.2.4 | ** | | Amendment No. 3 to Note and Equity Purchase Agreement of MGP Instruments, Inc. dated June 30, 2006 |
| | 10.2.5 | ** | | Amendment No. 4 and Waiver to Note and Equity Purchase Agreement of MGP Instruments, Inc. dated December 22, 2006 |
| | 10.2.6 | ** | | Amendment No. 4 to Note and Equity Purchase Agreement of MGP Instruments, Inc. dated May 14, 2008 |
| | 10.2.7 | ** | | Cross Guaranty of the Registrant, MGP Instruments, Inc. and Dosimetry Acquisitions (U.S.), Inc. |
| | 10.2.8 | ** | | Waiver Agreement to Note and Equity Purchase Agreement of Mirion Technologies (MGPI), Inc. (fka MGP Instruments, Inc.) dated June 15, 2009 |
II-4
| | | | | |
Exhibit No. | | Document |
| |
| | 10.2.9 | ** | | Waiver and Amendment Agreement to Note and Equity Purchase Agreement of Mirion Technologies (MGPI), Inc. dated July 31, 2009 |
| | 10.2.10 | ** | | Waiver Agreement to Note and Equity Purchase Agreement of Mirion Technologies (MGPI), Inc. dated September 17, 2009 |
| | 10.2.11 | ** | | Senior Subordinated Note dated June 23, 2004 between MGP Instruments, Inc. and American Capital Strategies, Ltd. |
| | 10.2.12 | ** | | Junior Subordinated Note dated June 23, 2004 between MGP Instruments, Inc. and American Capital Strategies, Ltd. |
| | 10.2.13 | ** | | Revolving Note dated June 23, 2004 between MGP Instruments, Inc. and American Capital Strategies, Ltd. |
| | 10.2.14 | ** | | Form of Senior Term B Note |
| | 10.2.15 | ** | | Schedule of Senior Term B Notes substantially identical in all material respects to the Form of Senior Term B Note |
| | 10.2.16 | | | Waiver Agreement to Note and Equity Purchase Agreement of Mirion Technologies (MGPI), Inc. dated March 11, 2010 |
| | 10.3 | ** | | Amended and Restated Note and Equity Purchase Agreement dated October 29, 2004 by and among IST Acquisitions, Inc., Imaging and Sensing Technology Corporation and subsidiaries and American Capital Financial Services, Inc. and various purchasers |
| | 10.3.1 | ** | | Amendment No. 1 to Amended and Restated Note and Equity Purchase Agreement of Imaging and Sensing Technology Corporation dated May 24, 2005 |
| | 10.3.2 | ** | | Amendment No. 1 to Amended and Restated Note and Equity Purchase Agreement of Imaging and Sensing Technology Corporation dated October 21, 2005 |
| | 10.3.3 | ** | | Amendment No. 2 and Consent to Amended and Restated Note and Equity Purchase Agreement of Imaging and Sensing Technology Corporation dated December 22, 2005 |
| | 10.3.4 | ** | | Amendment No. 3 to Amended and Restated Note and Equity Purchase Agreement of Imaging and Sensing Technology Corporation dated May 16, 2006 |
| | 10.3.5 | ** | | Amendment No. 3 to Amended and Restated Note and Equity Purchase Agreement of Imaging and Sensing Technology Corporation dated September 13, 2006 |
| | 10.3.6 | ** | | Amendment No. 4 and Waiver to Amended and Restated Note and Equity Purchase Agreement of Imaging and Sensing Technology Corporation dated December 22, 2006 |
| | 10.3.7 | ** | | Amendment No. 4 to Amended and Restated Note and Equity Purchase Agreement of Imaging and Sensing Technology Corporation dated July 20, 2007 |
| | 10.3.8 | ** | | Amendment No. 5 to Amended and Restated Note and Equity Purchase Agreement of Imaging and Sensing Technology Corporation dated May 14, 2008 |
| | 10.3.9 | ** | | Cross Guaranty of the Registrant and Imaging and Sensing Technology Corporation dated January 1, 2006 |
| | 10.3.10 | ** | | Waiver Agreement to Amended and Restated Note and Equity Purchase Agreement of Mirion Technologies (IST) Corporation (fka Imaging and Sensing Technology Corporation) dated June 15, 2009 |
| | 10.3.11 | ** | | Waiver and Amendment Agreement to Amended and Restated Note and Equity Purchase Agreement of Mirion Technologies (IST) Corporation dated August 4, 2009 |
| | 10.3.12 | ** | | Waiver Agreement to Note and Equity Purchase Agreement of Mirion Technologies (IST) Corporation dated September 17, 2009 |
| | 10.3.13 | ** | | Senior Term C Note dated October 29, 2004 between IST Acquisitions, Inc., Imaging and Sensing Technology Corporation, I.S. Technology de Puerto Rico, Inc., IST Instruments, Inc., Imaging and Sensing Technology International Corp., IST Conax Nuclear, Inc., Quadtek, Inc. and American Capital Strategies, Ltd. |
| | 10.3.14 | ** | | Stockholders Agreement dated May 24, 2004 between IST Acquisitions, Inc. and American Capital Strategies, Ltd. |
| | 10.3.15 | | | Waiver Agreement to Note and Equity Purchase Agreement of Mirion Technologies (IST) Corporation dated August 17, 2010 |
II-5
| | | | | |
Exhibit No. | | Document |
| |
| | 10.4 | ** | | Amended and Restated Note and Equity Purchase Agreement dated November 10, 2004 by and among Global Dosimetry Solutions, Inc. and American Capital Financial Services, Inc. and various purchasers |
| | 10.4.1 | ** | | Amendment No. 1 to Amended and Restated Note and Equity Purchase Agreement of Global Dosimetry Solutions, Inc. dated October 14, 2005 |
| | 10.4.2 | ** | | Consent to Amended and Restated Note and Equity Purchase Agreement of Global Dosimetry Solutions, Inc. dated December 22, 2005 |
| | 10.4.3 | ** | | Amendment No. 2 to Amended and Restated Note and Equity Purchase Agreement of Global Dosimetry Solutions, Inc. dated February 1, 2006 |
| | 10.4.4 | ** | | Amendment No. 3 to Amended and Restated Note and Equity Purchase Agreement of Global Dosimetry Solutions, Inc. dated March 28, 2006 |
| | 10.4.5 | ** | | Amendment No. 4 to Amended and Restated Note and Equity Purchase Agreement of Global Dosimetry Solutions, Inc. dated December 15, 2006 |
| | 10.4.6 | ** | | Amendment No. 5 and Waiver to Amended and Restated Note and Equity Purchase Agreement of Global Dosimetry Solutions, Inc. dated December 22, 2006 |
| | 10.4.7 | ** | | Cross Guaranty of the Registrant and Global Dosimetry Solutions, Inc. dated January 1, 2006 |
| | 10.4.8 | ** | | Waiver Agreement to Amended and Restated Note and Equity Purchase Agreement of Mirion Technologies (GDS), Inc. (fka Global Dosimetry Solutions, Inc.) dated June 15, 2009 |
| | 10.4.9 | ** | | Waiver and Amendment Agreement to Amended and Restated Note and Equity Purchase Agreement of Mirion Technologies (GDS), Inc. dated July 31, 2009 |
| | 10.4.10 | ** | | Waiver Agreement to Note and Equity Purchase Agreement of Mirion Technologies (GDS), Inc. dated September 17, 2009 |
| | 10.4.11 | | | Waiver Agreement to Note and Equity Purchase Agreement of Mirion Technologies (GDS), Inc. dated August 17, 2010 |
| | 10.5 | ** | | First Lien Pledge and Security Agreement made by the Registrant in favor of American Financial Services, Inc. dated January 1, 2006 |
| | 10.6.1 | ** | | Form of Indemnification Agreement for ACAS designees |
| | 10.6.2 | ** | | Form of Indemnification Agreement for other directors and officers |
| | 10.7 | ** | | Investment Banking Services Agreement dated December 25, 2005 between the Registrant and American Capital Financial Services, Inc. |
| | 10.8 | ** | | Lease Agreement dated December 28, 2005 between the Registrant and Alexander Properties Company |
| | 10.8.1 | ** | | Addendum 1 dated August 29, 2007 to Lease Agreement dated December 28, 2005 between the Registrant and Alexander Properties Company |
| | 10.8.2 | ** | | Addendum 2 dated June 10, 2008 to Lease Agreement dated December 22, 2005 between the Registrant and Alexander Properties Company |
| | 10.9 | ** | | Lease Agreement dated December 1, 1999 between the Registrant and Sonwil Development Group, L.L.C. |
| | 10.10 | ** | | Lease Agreement dated January 29, 2004 between the Registrant and The Irvine Company |
| | 10.11 | ** | | 2006 Stock Plan |
| | 10.11.1 | ** | | First Amendment to 2006 Stock Plan |
| | 10.11.2 | ** | | Amendment to 2006 Stock Plan |
| | 10.12 | ** | | Form of Stock Option Agreement under 2006 Stock Plan |
| | 10.13 | ** | | Form of Amended and Restated 2006 Stock Plan |
| | 10.14 | ** | | Form of Stock Option Agreement under Amended and Restated 2006 Stock Plan |
| | 10.15 | ** | | Second Amended and Restated Call Option Agreement among Thomas D. Logan and ACAS |
| | 10.16 | ** | | Employment Agreement dated August 15, 2006 between the Registrant and Thomas D. Logan |
| | 10.16.1 | ** | | Section 409A Amendment dated December 22, 2008 to Employment Agreement of Thomas D. Logan |
| | 10.16.2 | ** | | Amendment 2 dated January 1, 2009 to the Employment Agreement dated August 15, 2006 between the Registrant and Thomas D. Logan |
II-6
| | | | | |
Exhibit No. | | Document |
| |
| | 10.16.3 | | | Amendment 3 dated June 16, 2010 to the Employment Agreement dated August 15, 2006 between the Registrant and Thomas D. Logan |
| | 10.17 | ** | | Employment Agreement dated March 28, 2008 between the Registrant and Jack Pacheco |
| | 10.17.1 | ** | | Section 409A Amendment dated December 18, 2008 to Employment Agreement of Jack Pacheco |
| | 10.18 | ** | | Employment Agreement dated January 7, 2008 between the Registrant and Seth Rosen |
| | 10.18.1 | ** | | Section 409A Amendment dated December 18, 2008 to Employment Agreement of Seth Rosen |
| | 10.19 | ** | | Employment Agreement dated March 2, 2006 between the Registrant and W. Antony Besso |
| | 10.19.1 | ** | | Addendum dated November 26, 2007 to Employment Agreement of W. Antony Besso |
| | 10.19.2 | ** | | Bonus Agreement dated December 13, 2008 between the Registrant and W. Antony Besso |
| | 10.20 | ** | | Executive Bonus Plan |
| | 10.21 | ** | | Form of Warrant |
| | 10.22 | ** | | Schedule of warrants substantially identical in all material respects to the Form of Warrant |
| | 10.23 | ** | | Stockholders Agreement entered into as of December 22, 2005, by and among Global Monitoring Systems, Inc., American Capital Strategies, Ltd., and various stockholders |
| | 10.23.1 | ** | | First Amendment to Stockholders Agreement of Mirion Technologies, Inc. (f/k/a Global Monitoring Systems, Inc.) dated February 15, 2006 |
| | 10.23.2 | ** | | Second Amendment to Stockholders Agreement of Mirion Technologies, Inc. (f/k/a Global Monitoring Systems, Inc.) dated July 13, 2006 |
| | 10.23.3 | ** | | Third Amendment to Stockholders Agreement of Mirion Technologies, Inc. (f/k/a Global Monitoring Systems, Inc.) dated October 31, 2007 |
| | 10.23.4 | ** | | Fourth Amendment to Stockholders Agreement of Mirion Technologies, Inc. (f/k/a Global Monitoring Systems, Inc.) dated June 30, 2009 |
| | 10.24 | ** | | Form of Credit Agreement among Mirion Technologies, Inc., Mirion Technologies (Synodys) SA, Mirion Technologies (IST France) SAS, JPMorgan Chase Bank, National Association, J.P. Morgan Europe Limited, J.P. Morgan Securities LLC and Fifth Third Bank |
| | 10.25 | ** | | Form of Letter Agreement between Mirion Technologies, Inc. and each of the Preferred Stockholders |
| | 10.25.1 | ** | | Schedule of Letter Agreements substantially identical in all material respects to Form of Letter Agreement |
| | 10.26 | ** | | Form of New Letter Agreement between Mirion Technologies, Inc. and each of the Preferred Stockholders |
| | 10.26.1 | ** | | Schedule of New Letter Agreements substantially identical in all material respects to Form of New Letter Agreement |
| | 21.1 | ** | | Subsidiaries of the Registrant |
| | 23.1 | | | Consent of Ernst & Young LLP, Independent Registered Public Accounting Firm |
| | 23.2 | * | | Consent of Davis Polk & Wardwell LLP (included in Exhibit 5.1) |
| | 24.1 | ** | | Power of Attorney (included on signature page) |
| | 99.1 | ** | | Consent of Dustin G. Smith to being named as a director nominee |
| | 99.2 | ** | | Consent of Brian S. Graff to being named as a director nominee |
| | 99.3 | ** | | Consent of Michael T. Everett to being named as a director nominee |
| | 99.4 | ** | | Consent of Earl R. Lewis to being named as a director nominee |
| | 99.5 | ** | | Consent of Alfred E. Barry, Jr. to being named as a director nominee |
| | |
| * | | To be filed by amendment. |
The undersigned registrant hereby undertakes to provide to the underwriters at the closing specified in the underwriting agreement certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser.
II-7
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the provisions referenced in Item 14 of this Registration Statement, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer, or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered hereunder, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question of whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
The undersigned registrant hereby undertakes that:
(1) For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this Registration Statement in reliance upon Rule 430A and contained in a form of prospectus filed by the Registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this Registration Statement as of the time it was declared effective.
(2) For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new Registration Statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initialbona fideoffering thereof.
(3) For the purpose of determining liability under the Securities Act to any purchaser, if the registrant is subject to Rule 430C, each prospectus filed pursuant to Rule 424(b) as part of a Registration Statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the Registration Statement as of the date it is first used after effectiveness.Provided, however,that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the Registration Statement or prospectus that is part of the Registration Statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the Registration Statement or made in any such document immediately prior to such date of first use.
(4) For the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of securities, in a primary offering of securities of the registrant pursuant to this Registration Statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the registrant will be a seller to the purchaser and will be considered to offer or sell such securities to the purchaser:
(i) any preliminary prospectus or prospectus of the registrant relating to the offering required to be filed pursuant to Rule 424;
(ii) any free writing prospectus relating to the offering prepared by or on behalf of the registrant or used or referred to by the registrant;
(iii) the portion of any other free writing prospectus relating to the offering containing material information about the registrant or its securities provided by or on behalf of the registrant; and
(iv) any other communication that is an offer in the offering made by the registrant to the purchaser.
II-8
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the Registrant has duly caused this amendment number 11 to this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of San Ramon, State of California, on September 3, 2010.
MIRION TECHNOLOGIES, INC.
Name: Thomas D. Logan
| | |
| | Title: | President, Chief Executive Officer and Chairman of the Board |
Pursuant to the requirements of the Securities Act of 1933, as amended, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
| | | | | | | |
Signature | | Title | | Date |
| |
| | | | | |
/s/ Thomas D. Logan
Thomas D. Logan
(Principal Executive Officer) | | President, Chief Executive Officer
and Chairman of the Board | | September 3, 2010 |
| | | | | |
/s/ Jack A. Pacheco
Jack A. Pacheco
(Principal Financial and Accounting Officer) | | Vice President and Chief Financial Officer | | September 3, 2010 |
| | | | | |
*
Robert J. Klein | | Director | | September 3, 2010 |
| | | | | | | |
| *By: | | /s/ Thomas D. Logan
Thomas D. Logan
Attorney-in-fact | | | | |
II-9
EXHIBIT INDEX
| | | | | |
Exhibit No. | | Document |
| |
| | 1.1 | ** | | Form of Underwriting Agreement |
| | 3.1.1 | | | Form of Fourth Amended and Restated Certificate of Incorporation to be adopted prior to the effectiveness of this offering |
| | 3.1.2 | | | Form of Fifth Amended and Restated Certificate of Incorporation to be adopted prior to the consummation of this offering |
| | 3.2 | ** | | Form of Amended and Restated Bylaws |
| | 4.1 | ** | | Specimen Common Stock Certificate of the Registrant |
| | 4.2 | ** | | Form of Registration Rights Agreement |
| | 5.1 | * | | Opinion of Davis Polk & Wardwell LLP |
| | 10.1 | ** | | Shareholder Loan Agreement dated September 23, 2005 between Dosimetry Acquisitions (France) and ACAS |
| | 10.1.1 | ** | | Amendment 1 dated November 14, 2005 to Shareholder Loan Agreement |
| | 10.1.2 | ** | | Amendment No. 2 dated September 13, 2006 to Shareholder Loan Agreement |
| | 10.1.3 | ** | | Third Amendment dated May 14, 2008 to Shareholder Loan Agreement |
| | 10.1.4 | ** | | Fourth Amendment dated July 20, 2009 to Shareholder Loan Agreement |
| | 10.1.5 | | | Fifth Amendment dated July 29, 2010 to Shareholder Loan Agreement |
| | 10.2 | ** | | Note and Equity Purchase Agreement dated June 23, 2004 by and among MGP Instruments, Inc., Dosimetry Acquisitions (U.S.), Inc. and American Capital Financial Services, Inc. and various purchasers |
| | 10.2.1 | ** | | Amendment No. 1 to Note and Equity Purchase Agreement of MGP Instruments, Inc. dated October 22, 2004 |
| | 10.2.2 | ** | | Amendment No. 2 to Note and Equity Purchase Agreement of MGP Instruments, Inc. dated November 1, 2005 |
| | 10.2.3 | ** | | Amendment No. 2 and Consent to Note and Equity Purchase Agreement of MGP Instruments, Inc. dated December 22, 2005 |
| | 10.2.4 | ** | | Amendment No. 3 to Note and Equity Purchase Agreement of MGP Instruments, Inc. dated June 30, 2006 |
| | 10.2.5 | ** | | Amendment No. 4 and Waiver to Note and Equity Purchase Agreement of MGP Instruments, Inc. dated December 22, 2006 |
| | 10.2.6 | ** | | Amendment No. 4 to Note and Equity Purchase Agreement of MGP Instruments, Inc. dated May 14, 2008 |
| | 10.2.7 | ** | | Cross Guaranty of the Registrant, MGP Instruments, Inc. and Dosimetry Acquisitions (U.S.), Inc. |
| | 10.2.8 | ** | | Waiver Agreement to Note and Equity Purchase Agreement of Mirion Technologies (MGPI), Inc. (fka MGP Instruments, Inc.) dated June 15, 2009 |
| | 10.2.9 | ** | | Waiver and Amendment Agreement to Note and Equity Purchase Agreement of Mirion Technologies (MGPI), Inc. dated July 31, 2009 |
| | 10.2.10 | ** | | Waiver Agreement to Note and Equity Purchase Agreement of Mirion Technologies (MGPI), Inc. dated September 17, 2009 |
| | 10.2.11 | ** | | Senior Subordinated Note dated June 23, 2004 between MGP Instruments, Inc. and American Capital Strategies, Ltd. |
| | 10.2.12 | ** | | Junior Subordinated Note dated June 23, 2004 between MGP Instruments, Inc. and American Capital Strategies, Ltd. |
| | 10.2.13 | ** | | Revolving Note dated June 23, 2004 between MGP Instruments, Inc. and American Capital Strategies, Ltd. |
| | 10.2.14 | ** | | Form of Senior Term B Note |
| | 10.2.15 | ** | | Schedule of Senior Term B Notes substantially identical in all material respects to the Form of Senior Term B Note |
| | 10.2.16 | | | Waiver Agreement to Note and Equity Purchase Agreement of Mirion Technologies (MGPI), Inc. dated March 11, 2010 |
| | | | | |
Exhibit No. | | Document |
| |
| | 10.3 | ** | | Amended and Restated Note and Equity Purchase Agreement dated October 29, 2004 by and among IST Acquisitions, Inc., Imaging and Sensing Technology Corporation and subsidiaries and American Capital Financial Services, Inc. and various purchasers |
| | 10.3.1 | ** | | Amendment No. 1 to Amended and Restated Note and Equity Purchase Agreement of Imaging and Sensing Technology Corporation dated May 24, 2005 |
| | 10.3.2 | ** | | Amendment No. 1 to Amended and Restated Note and Equity Purchase Agreement of Imaging and Sensing Technology Corporation dated October 21, 2005 |
| | 10.3.3 | ** | | Amendment No. 2 and Consent to Amended and Restated Note and Equity Purchase Agreement of Imaging and Sensing Technology Corporation dated December 22, 2005 |
| | 10.3.4 | ** | | Amendment No. 3 to Amended and Restated Note and Equity Purchase Agreement of Imaging and Sensing Technology Corporation dated May 16, 2006 |
| | 10.3.5 | ** | | Amendment No. 3 to Amended and Restated Note and Equity Purchase Agreement of Imaging and Sensing Technology Corporation dated September 13, 2006 |
| | 10.3.6 | ** | | Amendment No. 4 and Waiver to Amended and Restated Note and Equity Purchase Agreement of Imaging and Sensing Technology Corporation dated December 22, 2006 |
| | 10.3.7 | ** | | Amendment No. 4 to Amended and Restated Note and Equity Purchase Agreement of Imaging and Sensing Technology Corporation dated July 20, 2007 |
| | 10.3.8 | ** | | Amendment No. 5 to Amended and Restated Note and Equity Purchase Agreement of Imaging and Sensing Technology Corporation dated May 14, 2008 |
| | 10.3.9 | ** | | Cross Guaranty of the Registrant and Imaging and Sensing Technology Corporation dated January 1, 2006 |
| | 10.3.10 | ** | | Waiver Agreement to Amended and Restated Note and Equity Purchase Agreement of Mirion Technologies (IST) Corporation (fka Imaging and Sensing Technology Corporation) dated June 15, 2009 |
| | 10.3.11 | ** | | Waiver and Amendment Agreement to Amended and Restated Note and Equity Purchase Agreement of Mirion Technologies (IST) Corporation dated August 4, 2009 |
| | 10.3.12 | ** | | Waiver Agreement to Note and Equity Purchase Agreement of Mirion Technologies (IST) Corporation dated September 17, 2009 |
| | 10.3.13 | ** | | Senior Term C Note dated October 29, 2004 between IST Acquisitions, Inc., Imaging and Sensing Technology Corporation, I.S. Technology de Puerto Rico, Inc., IST Instruments, Inc., Imaging and Sensing Technology International Corp., IST Conax Nuclear, Inc., Quadtek, Inc. and American Capital Strategies, Ltd. |
| | 10.3.14 | ** | | Stockholders Agreement dated May 24, 2004 between IST Acquisitions, Inc. and American Capital Strategies, Ltd. |
| | 10.3.15 | | | Waiver Agreement to Note and Equity Purchase Agreement of Mirion Technologies (IST) Corporation dated August 17, 2010 |
| | 10.4 | ** | | Amended and Restated Note and Equity Purchase Agreement dated November 10, 2004 by and among Global Dosimetry Solutions, Inc. and American Capital Financial Services, Inc. and various purchasers |
| | 10.4.1 | ** | | Amendment No. 1 to Amended and Restated Note and Equity Purchase Agreement of Global Dosimetry Solutions, Inc. dated October 14, 2005 |
| | 10.4.2 | ** | | Consent to Amended and Restated Note and Equity Purchase Agreement of Global Dosimetry Solutions, Inc. dated December 22, 2005 |
| | 10.4.3 | ** | | Amendment No. 2 to Amended and Restated Note and Equity Purchase Agreement of Global Dosimetry Solutions, Inc. dated February 1, 2006 |
| | 10.4.4 | ** | | Amendment No. 3 to Amended and Restated Note and Equity Purchase Agreement of Global Dosimetry Solutions, Inc. dated March 28, 2006 |
| | 10.4.5 | ** | | Amendment No. 4 to Amended and Restated Note and Equity Purchase Agreement of Global Dosimetry Solutions, Inc. dated December 15, 2006 |
| | 10.4.6 | ** | | Amendment No. 5 and Waiver to Amended and Restated Note and Equity Purchase Agreement of Global Dosimetry Solutions, Inc. dated December 22, 2006 |
| | 10.4.7 | ** | | Cross Guaranty of the Registrant and Global Dosimetry Solutions, Inc. dated January 1, 2006 |
| | | | | |
Exhibit No. | | Document |
| |
| | 10.4.8 | ** | | Waiver Agreement to Amended and Restated Note and Equity Purchase Agreement of Mirion Technologies (GDS), Inc. (fka Global Dosimetry Solutions, Inc.) dated June 15, 2009 |
| | 10.4.9 | ** | | Waiver and Amendment Agreement to Amended and Restated Note and Equity Purchase Agreement of Mirion Technologies (GDS), Inc. dated July 31, 2009 |
| | 10.4.10 | ** | | Waiver Agreement to Note and Equity Purchase Agreement of Mirion Technologies (GDS), Inc. dated September 17, 2009 |
| | 10.4.11 | | | Waiver Agreement to Note and Equity Purchase Agreement of Mirion Technologies (GDS), Inc. dated August 17, 2010 |
| | 10.5 | ** | | First Lien Pledge and Security Agreement made by the Registrant in favor of American Financial Services, Inc. dated January 1, 2006 |
| | 10.6.1 | ** | | Form of Indemnification Agreement for ACAS designees |
| | 10.6.2 | ** | | Form of Indemnification Agreement for other directors and officers |
| | 10.7 | ** | | Investment Banking Services Agreement dated December 25, 2005 between the Registrant and American Capital Financial Services, Inc. |
| | 10.8 | ** | | Lease Agreement dated December 28, 2005 between the Registrant and Alexander Properties Company |
| | 10.8.1 | ** | | Addendum 1 dated August 29, 2007 to Lease Agreement dated December 28, 2005 between the Registrant and Alexander Properties Company |
| | 10.8.2 | ** | | Addendum 2 dated June 10, 2008 to Lease Agreement dated December 22, 2005 between the Registrant and Alexander Properties Company |
| | 10.9 | ** | | Lease Agreement dated December 1, 1999 between the Registrant and Sonwil Development Group, L.L.C. |
| | 10.10 | ** | | Lease Agreement dated January 29, 2004 between the Registrant and The Irvine Company |
| | 10.11 | ** | | 2006 Stock Plan |
| | 10.11.1 | ** | | First Amendment to 2006 Stock Plan |
| | 10.11.2 | ** | | Amendment to 2006 Stock Plan |
| | 10.12 | ** | | Form of Stock Option Agreement under 2006 Stock Plan |
| | 10.13 | ** | | Form of Amended and Restated 2006 Stock Plan |
| | 10.14 | ** | | Form of Stock Option Agreement under Amended and Restated 2006 Stock Plan |
| | 10.15 | ** | | Second Amended and Restated Call Option Agreement among Thomas D. Logan and ACAS |
| | 10.16 | ** | | Employment Agreement dated August 15, 2006 between the Registrant and Thomas D. Logan |
| | 10.16.1 | ** | | Section 409A Amendment dated December 22, 2008 to Employment Agreement of Thomas D. Logan |
| | 10.16.2 | ** | | Amendment 2 dated January 1, 2009 to the Employment Agreement dated August 15, 2006 between the Registrant and Thomas D. Logan |
| | 10.16.3 | | | Amendment 3 dated June 16, 2010 to the Employment Agreement dated August 15, 2006 between the Registrant and Thomas D. Logan |
| | 10.17 | ** | | Employment Agreement dated March 28, 2008 between the Registrant and Jack Pacheco |
| | 10.17.1 | ** | | Section 409A Amendment dated December 18, 2008 to Employment Agreement of Jack Pacheco |
| | 10.18 | ** | | Employment Agreement dated January 7, 2008 between the Registrant and Seth Rosen |
| | 10.18.1 | ** | | Section 409A Amendment dated December 18, 2008 to Employment Agreement of Seth Rosen |
| | 10.19 | ** | | Employment Agreement dated March 2, 2006 between the Registrant and W. Antony Besso |
| | 10.19.1 | ** | | Addendum dated November 26, 2007 to Employment Agreement of W. Antony Besso |
| | 10.19.2 | ** | | Bonus Agreement dated December 13, 2008 between the Registrant and W. Antony Besso |
| | 10.20 | ** | | Executive Bonus Plan |
| | 10.21 | ** | | Form of Warrant |
| | 10.22 | ** | | Schedule of warrants substantially identical in all material respects to the Form of Warrant |
| | 10.23 | ** | | Stockholders Agreement entered into as of December 22, 2005, by and among Global Monitoring Systems, Inc., American Capital Strategies, Ltd., and various stockholders |
| | | | | |
Exhibit No. | | Document |
| |
| | 10.23.1 | ** | | First Amendment to Stockholders Agreement of Mirion Technologies, Inc. (f/k/a Global Monitoring Systems, Inc.) dated February 15, 2006 |
| | 10.23.2 | ** | | Second Amendment to Stockholders Agreement of Mirion Technologies, Inc. (f/k/a Global Monitoring Systems, Inc.) dated July 13, 2006 |
| | 10.23.3 | ** | | Third Amendment to Stockholders Agreement of Mirion Technologies, Inc. (f/k/a Global Monitoring Systems, Inc.) dated October 31, 2007 |
| | 10.23.4 | ** | | Fourth Amendment to Stockholders Agreement of Mirion Technologies, Inc. (f/k/a Global Monitoring Systems, Inc.) dated June 30, 2009 |
| | 10.24 | ** | | Form of Credit Agreement among Mirion Technologies, Inc., Mirion Technologies (Synodys) SA, Mirion Technologies (IST France) SAS, JPMorgan Chase Bank, National Association, J.P. Morgan Europe Limited, J.P. Morgan Securities LLC and Fifth Third Bank |
| | 10.25 | ** | | Form of Letter Agreement between Mirion Technologies, Inc. and each of the Preferred Stockholders |
| | 10.25.1 | ** | | Schedule of Letter Agreements substantially identical in all material respects to Form of Letter Agreement |
| | 10.26 | ** | | Form of New Letter Agreement between Mirion Technologies, Inc. and each of the Preferred Stockholders |
| | 10.26.1 | ** | | Schedule of New Letter Agreements substantially identical in all material respects to Form of New Letter Agreement |
| | 21.1 | ** | | Subsidiaries of the Registrant |
| | 23.1 | | | Consent of Ernst & Young LLP, Independent Registered Public Accounting Firm |
| | 23.2 | * | | Consent of Davis Polk & Wardwell LLP (included in Exhibit 5.1) |
| | 24.1 | ** | | Power of Attorney (included on signature page) |
| | 99.1 | ** | | Consent of Dustin G. Smith to being named as a director nominee |
| | 99.2 | ** | | Consent of Brian S. Graff to being named as a director nominee |
| | 99.3 | ** | | Consent of Michael T. Everett to being named as a director nominee |
| | 99.4 | ** | | Consent of Earl R. Lewis to being named as a director nominee |
| | 99.5 | ** | | Consent of Alfred E. Barry, Jr. to being named as a director nominee |
| | |
| * | | To be filed by amendment. |