
Nitric Oxide-Based Medicine N A S D A Q : N O V N | n o v a n . c o m C O R P O R AT E U P D AT E / S E P T E M B E R 9 , 2 0 2 1 Exhibit 99.2

NOVAN.COM FORWARD-LOOKING STATEMENTS This presentation contains forward-looking statements including, but not limited to, statements related to the potential therapeutic value of the Company’s NITRICIL™ platform technology, the Company’s pharmaceutical development of nitric oxide-releasing product candidates, including SB206, the Company’s strategic direction for development, the potential timing of FDA submission(s), the potential market opportunity for our product candidates, plans for launch and commercialization of SB206, if approved, the Company’s expected cash runway and the Company’s intention to partner with third parties. These forward-looking statements are included throughout this presentation, and the Company uses the words “believe,” “expect,” “target,” “anticipate,” “may,” “plan,” “potential,” “will,” and similar expressions to identify forward-looking statements in this presentation. Such statements are based on the Company’s current beliefs and expectations. Forward-looking statements are subject to a number of risks and uncertainties that could cause actual results to differ materially from the Company’s expectations, including, but not limited to, risks related to the regulatory approval process, which is lengthy, time-consuming and inherently unpredictable, including the risk that the FDA will not agree with the Company’s approach to a potential NDA submission, that the Company’s product candidates may not be approved or that additional studies may be required for approval or other delays may occur, that the Company may not have sufficient quantities of drug substance and/or drug product to support regulatory submissions and that the Company may not obtain funding sufficient to complete the regulatory or development process; the Company’s limited experience as a company in obtaining regulatory approvals and commercializing pharmaceutical products; changes in the size and nature of the market for our product candidates, including potential competition; risks and uncertainties in the Company’s ongoing or future product development activities and preclinical studies, which may not prove successful in demonstrating proof-of concept, or may show adverse toxicological findings, and even if successful may not necessarily predict that subsequent clinical trials will show the requisite safety and efficacy of the Company’s product candidates; any operational or other disruptions as a result of the COVID-19 pandemic; the Company’s ability to obtain additional funding or enter into strategic or other business relationships necessary or useful for the further development or commercialization of the Company’s product candidates; risks related to the manufacture of raw materials, including the Company’s active pharmaceutical ingredient and drug product components utilized in clinical trial materials, including supply chain disruptions or delays, failure to transfer technology and processes to third parties effectively or failure of those third parties (or the Company in connection with the upfit of the Company’s new facility) to obtain approval of and maintain compliance with the FDA or comparable regulatory authorities; the Company’s reliance on arrangements with third parties to support its operations and development efforts and the risk that such parties will not successfully carry out their contractual duties or meet expected deadlines; and other risks and uncertainties described in the Company’s annual report filed with the Securities and Exchange Commission on Form 10-K for the twelve months ended December 31, 2020, and in the Company’s subsequent filings with the Securities and Exchange Commission. Such forward-looking statements speak only as of the date of this presentation, and Novan disclaims any intent or obligation to update these forward-looking statements to reflect events or circumstances after the date of such statements, except as may be required by law. Certain information contained in this presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and the Company’s own estimates and research. While the Company believes these third-party sources to be reliable as of the date of this presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of or that any independent source has verified, any information obtained from third-party sources. 2

A world leader in nitric oxide-based science, technology, and clinical translation in support of delivering safe and efficacious therapies
NOVAN.COM AGENDA 4 A Compelling Investment Opportunity Our History of R&D and Innovation Our Near-Term Strategy Our Commercialization Plan Our Full Opportunity Why Novan – Why Now

NOVAN.COM A COMPELLING INVESTMENT OPPORTUNITY Clinically proven platform technology, NITRICIL™ Robust body of data with nearly 4,000 treated subjects supporting anti-microbial and anti-inflammatory properties Positive topline results from pivotal Phase 3 study of SB206 in patients with molluscum contagiosum Previous results from pivotal Phase 3 study of SB204 in acne vulgaris give confidence to prioritize as second lead candidate Positive preclinical data of SB019 in COVID-19 Management team with proven track record of execution Potential to address multi-billion-dollar collective U.S. market 5

NOVAN.COM THREE NDAs PLANNED OVER THREE YEARS 6 Product Candidate Indication Pre-IND Phase 1 Phase 2 Phase 3 Approval NDA Submission Targeted Novan Revenue Retention2 DERMATOLOGY SB206 Molluscum Q3 2022 ~85% N. America3 >95% ROW (ex. Japan4) SB204 Acne Vulgaris 20241 ~95% N. America >95% ROW (ex. Japan4) INFECTIOUS DISEASE SB019 SARS-CoV-2 20241 >95% Worldwide 1. Company has expected cash runway into Q4 2022. 2. Net sales as defined by our existing license and research & development arrangements. 3. Based on aggregate net sales royalty rates, excludes maximum potential milestone payments of ~$21m. 4. Based on Sato Pharmaceutical Co., Ltd. amended license agreement for SB206 and SB204 in Japan.

NOVAN.COM Potential Value Creation 7 Product Candidate SB206 SB204 SB019 Type Topical Gel Topical Gel Nasal Suspension Indication Molluscum Acne COVID-19 Potential Global Addressable Market ~12 Million1 ~733 Million2 ~134 Million3 Current Treatment Options In-office procedures; off label scripts Antibiotics; tretinoins; combination products Vaccines or antibody cocktails Novan’s Pending Solution Once per day in-home up to 12 weeks Once per day up to 12 weeks with no antibiotic resistance Prophylactic or early treatment in mild to moderate cases 1. Molluscum Contagiosum - Epidemiology Forecast – 2028. Seven Major Markets (U.S., Germany, Spain, Italy, France, the United Kingdom, and Japan from 2017 – 2028). 2. Br J Dermatol. 2015 Jul;172 Suppl 1:3-12. doi: 10.1111/bjd.13462. "A global perspective on the epidemiology of acne" J K L Tan, K Bhate (Estimate of global population affected by acne - 9.4%) 3. Syneos Health Consulting - Preliminary Estimated Target Global Patient Population 2024 (Mild/Moderate) COVID cases per year.

NOVAN.COM NITRICIL™ 8 Clinically proven proprietary nitric oxide (NO)-based technology platform Macromolecular New Chemical Entities (NCEs) Storage: ability to store therapeutic quantities of NO Tunability: pH-controlled NO release profiles Targeted: delivery of NO to site of infection or inflammation Stability: druggable form of NO with shelf-life stability Indication specific formulations with differentiated NO release rates Dual phase: coadministration of the active gel with a pH-buffered hydrogel promotes NO release Delivers therapeutic amounts of NO to the site of infection or inflammation with what we believe is an attractive safety profile Berdazimer Sodium (NVN1000) Real-Time NO Release Burst Phase Sustained PhaseRe al -T im e N O Re le as e (n m ol ) Time (Hr) New Chemical Entity (NCE)

NOVAN.COM VALUE DRIVERS BEYOND 2024 9 Product Candidate Indication Preclinical Phase 1 Phase 2 Phase 3 DERMATOLOGY SB414 Atopic Dermatitis Psoriasis SB208 Tinea Pedis MEN’S AND WOMEN’S HEALTH SB207 Genital Warts WH504 High-Risk HPV WH602 High-Risk HPV COMPANION ANIMAL NVN4100 Various Ongoing Ongoing Supported by U.S. DoD CDMRP grant Supported by NIH grant Planned next phase of development subject to obtaining additional financing or strategic partnering All activities on hold Provides Opportunity for Global Pipeline Expansion, Business Development and Collaboration

STRATEGY TOWARDS DRIVING NEAR-TERM STAKEHOLDER VALUE Leverage core synergies of science, capital, resources and patient needs to create value by bringing new nitric oxide-based medicines to market

NOVAN.COM SB206 Molluscum Contagiosum 11 Positive topline results from pivotal Phase 3 with NDA submission targeted Q3 2022

NOVAN.COM MOLLUSCUM MARKET POTENTIAL FOR SB206 Contagious skin infection caused by the molluscipoxvirus, a double-stranded DNA virus 12 High unmet medical need for topical at-home solution Prevalence rate in children 0-16 years old is 5.1% - 11.5% in the U.S.1 U.S. addressable market of ~6 million2 The most used molluscum treatments (procedures) are reimbursed on the medical benefit side We believe reimbursement for SB206 (on formulary) is expected given lack of competitors and favorable efficacy, safety profile 1. Prevalence in the US of 5.1% to 11.5% in children aged 0-16 years. (Fam Pract. 2014 Apr;31(2):130-6). 2. Molluscum Contagiosum - Epidemiology Forecast – 2028. Seven Major Markets (U.S., Germany, Spain, Italy, France, the United Kingdom, and Japan from 2017 – 2028).
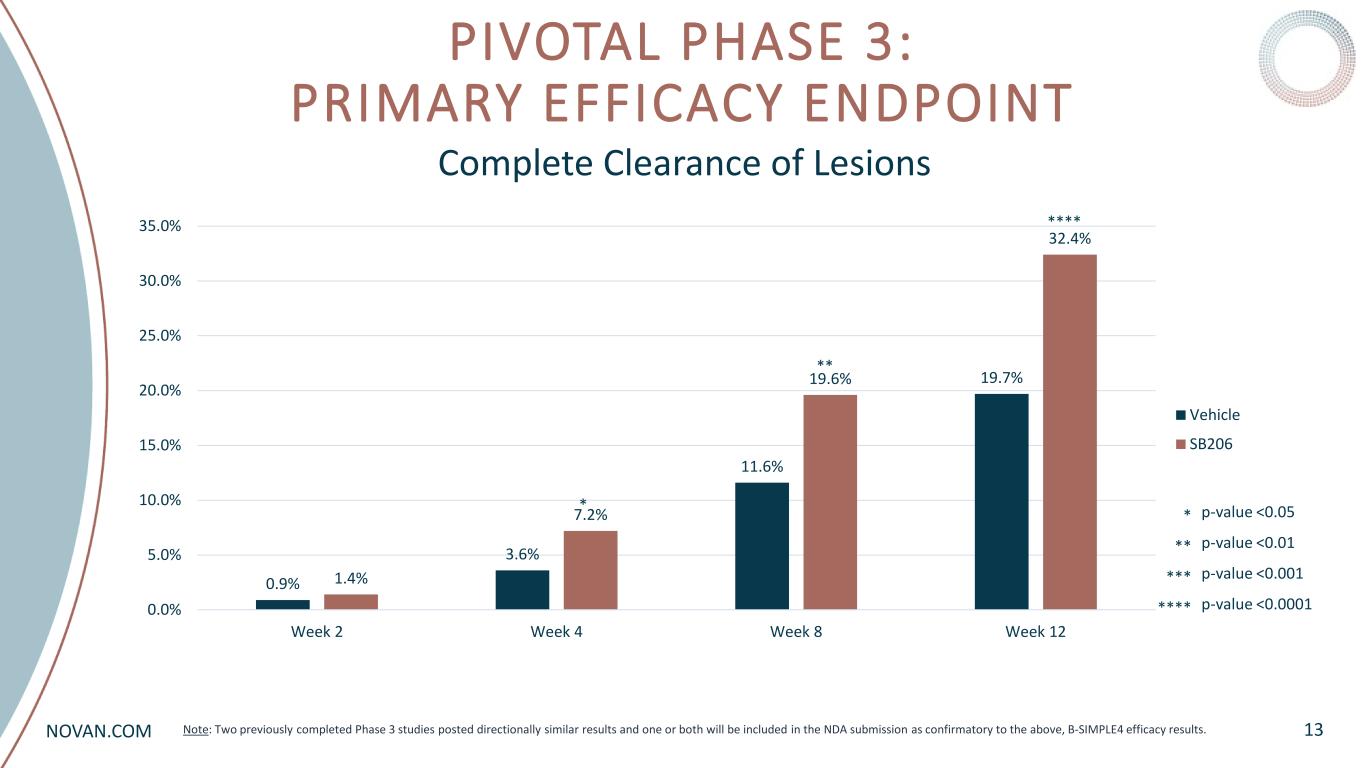
NOVAN.COM PIVOTAL PHASE 3: PRIMARY EFFICACY ENDPOINT 13 Complete Clearance of Lesions 0.9% 3.6% 11.6% 19.7% 1.4% 7.2% 19.6% 32.4% 0.0% 5.0% 10.0% 15.0% 20.0% 25.0% 30.0% 35.0% Week 2 Week 4 Week 8 Week 12 Vehicle SB206 p-value <0.05 p-value <0.01 p-value <0.001 p-value <0.0001 * ** *** **** **** ** * Note: Two previously completed Phase 3 studies posted directionally similar results and one or both will be included in the NDA submission as confirmatory to the above, B-SIMPLE4 efficacy results.

NOVAN.COM PIVOTAL PHASE 3: SECONDARY ENDPOINTS 14 90% Clearance and 0 or 1 Remaining Lesions at Week 12 0.00% 5.00% 10.00% 15.00% 20.00% 25.00% 30.00% 35.00% 40.00% 45.00% 50.00% 90% Clearance at Week 12 0 or 1 Lesions at Week 12 Vehicle SB206 ******** p-value <0.0001****

NOVAN.COM PIVOTAL PHASE 3: SECONDARY ENDPOINT - CHANGE AT WEEK 4 15 -60% -50% -40% -30% -20% -10% 0% BL Week 2 Week 4 Week 8 Week 12 Vehicle SB206 p-value <0.05 p-value <0.01 p-value <0.001 p-value <0.0001 * ** *** **** **** **** **** *** % Change From Baseline Lesion Count
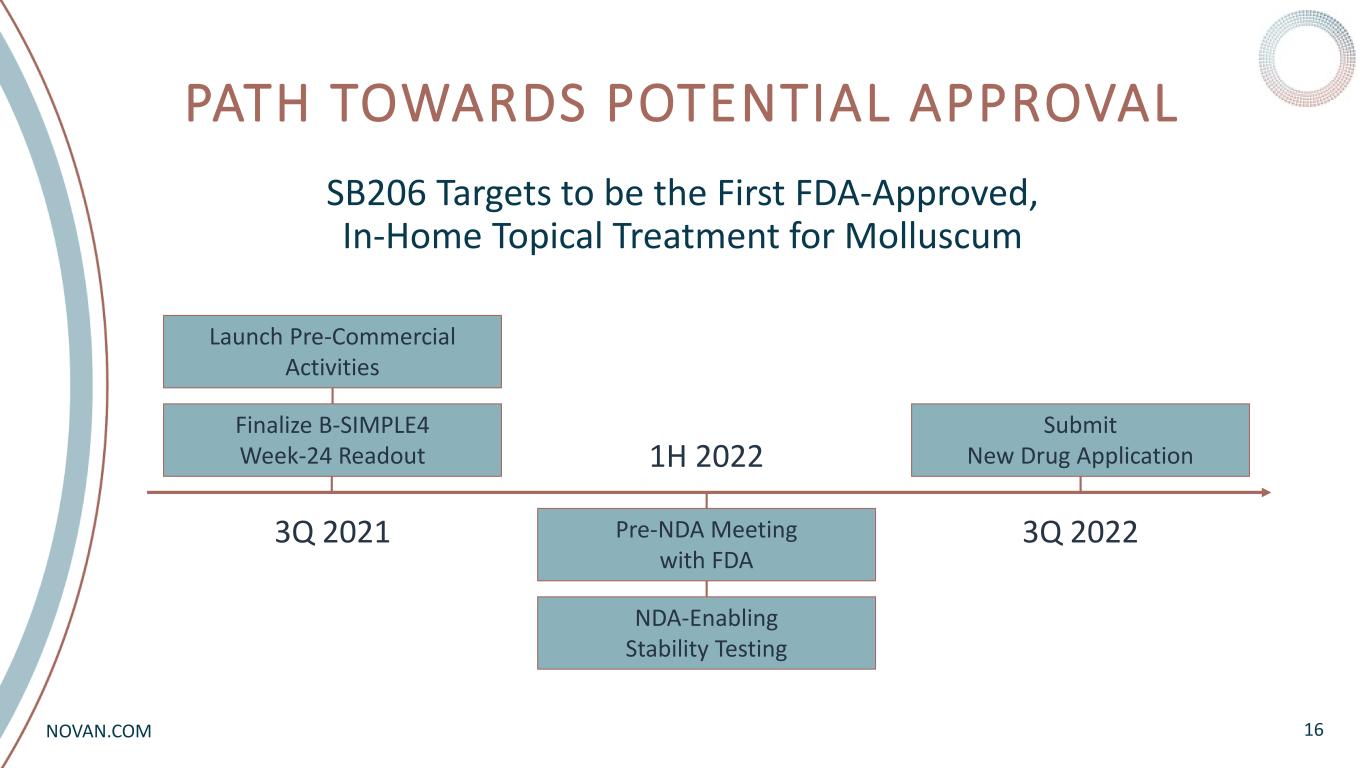
NOVAN.COM PATH TOWARDS POTENTIAL APPROVAL 16 SB206 Targets to be the First FDA-Approved, In-Home Topical Treatment for Molluscum Finalize B-SIMPLE4 Week-24 Readout 3Q 2021 1H 2022 3Q 2022 Launch Pre-Commercial Activities NDA-Enabling Stability Testing Pre-NDA Meeting with FDA Submit New Drug Application

NOVAN.COMNOVAN.COM 17 SB206 COMMERCIALIZATION PLAN Commercialization of SB206 with Syneos Health’s Integrated Support Model, if Approved End-to-end commercial solution provider with proven track record of supporting successful commercial launches Provides immediate access to robust, existing commercial infrastructure with deep dermatology expertise Analytical approach to market research, patient journey and segmentation work to strategically position SB206 Novan will utilize a small group of internal experts to provide oversight and control of commercial execution

NOVAN.COM SB206 COMMERCIAL APPROACH 18 • Communication • Education • Evidence Base • Early Engagement • Economic Burden • Commercial Formulary • Digital Health • Build the brand • Establish Differentiation • Efficiently Target Stakeholders • Amplify Reach Generate data and build the holistic evidence story Engage stakeholders to build awareness, advocacy and access Execute in the marketplace to drive adoption
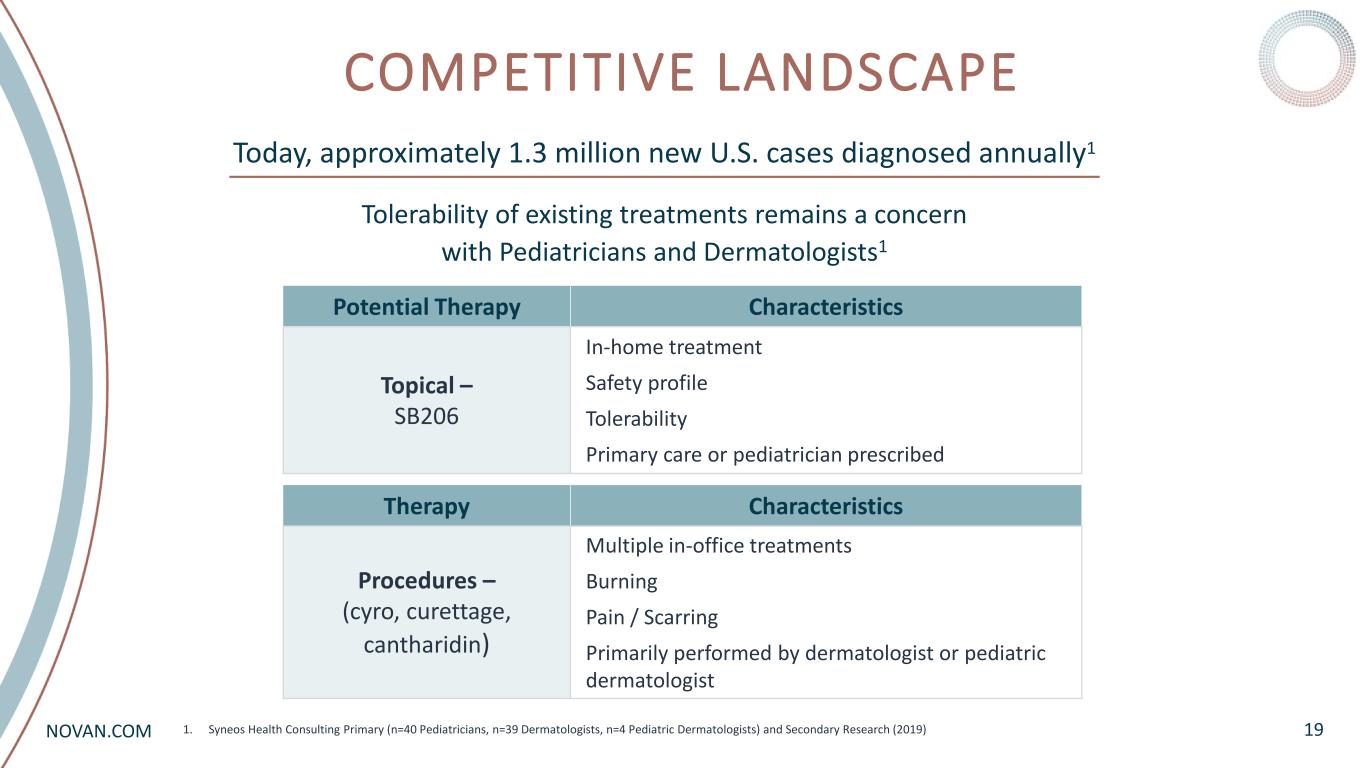
NOVAN.COM COMPETITIVE LANDSCAPE 191. Syneos Health Consulting Primary (n=40 Pediatricians, n=39 Dermatologists, n=4 Pediatric Dermatologists) and Secondary Research (2019) Today, approximately 1.3 million new U.S. cases diagnosed annually1 Potential Therapy Characteristics Topical – SB206 In-home treatment Safety profile Tolerability Primary care or pediatrician prescribed Therapy Characteristics Procedures – (cyro, curettage, cantharidin) Multiple in-office treatments Burning Pain / Scarring Primarily performed by dermatologist or pediatric dermatologist Tolerability of existing treatments remains a concern with Pediatricians and Dermatologists1

NOVAN.COM SB204 Acne Vulgaris Late-stage asset with two completed Phase 3 studies Priority Pipeline Expansion 20

NOVAN.COM SB204: ADVANCE DEVELOPMENT FOR ACNE VULGARIS Potential advantages over other topical therapies for the most common form of acne 21 Multi-factorial mechanism of action, including anti-bacterial and anti-inflammatory Convenience of once daily application Excellent local tolerability and favorable safety profile Non-bleaching, non-staining and non-irritating Elimination of patient, parental and societal concerns arising from antibiotic resistance Same active pharmaceutical ingredient as used in SB206 for molluscum yet formulated specifically for acne

NOVAN.COM DATA FROM POSITIVE PHASE 3 STUDY 22*Analysis includes all patients who were randomized. Absolute changes in lesion count reduction are shown as LSMean NI-AC302 ITT* (n = 1327) Non-Inflammatory Lesion Reduction SB204 -14.8 Vehicle -12.1 p-value p=0.001 Inflammatory Lesion Reduction SB204 -12.8 Vehicle -10.5 p-value p<0.001 Investigator Global Assessment Success SB204 18.9% Vehicle 14.3% p-value p=0.038

NOVAN.COM SB204 Acne Vulgaris: Why we are planning for success 23 FDA GUIDANCE: JUST ONE MORE PIVOTAL PHASE 3 TRIAL AC302 was a successful pivotal Phase 3 trial across all three co-primary endpoints Consistent results across AC301 and AC302 for two of three co-primary endpoints Positive B-SIMPLE4 results validated our technology and our effective study execution New study design and execution to be optimized through learnings across previous Phase 3 studies

NOVAN.COM REGULATORY PATH FOR SB204 IN ACNE 24 Targeted Timeline: Two Phase 3 studies completed in 2017 One pivotal Phase 3 study to be conducted Size of trial estimated to include >1,000 patients 3 co-primary endpoints (non-inflammatory lesions, inflammatory lesions, IGA success) Prepare for Pivotal Study Execute Pivotal Study Submit NDA 2022 2023 2024
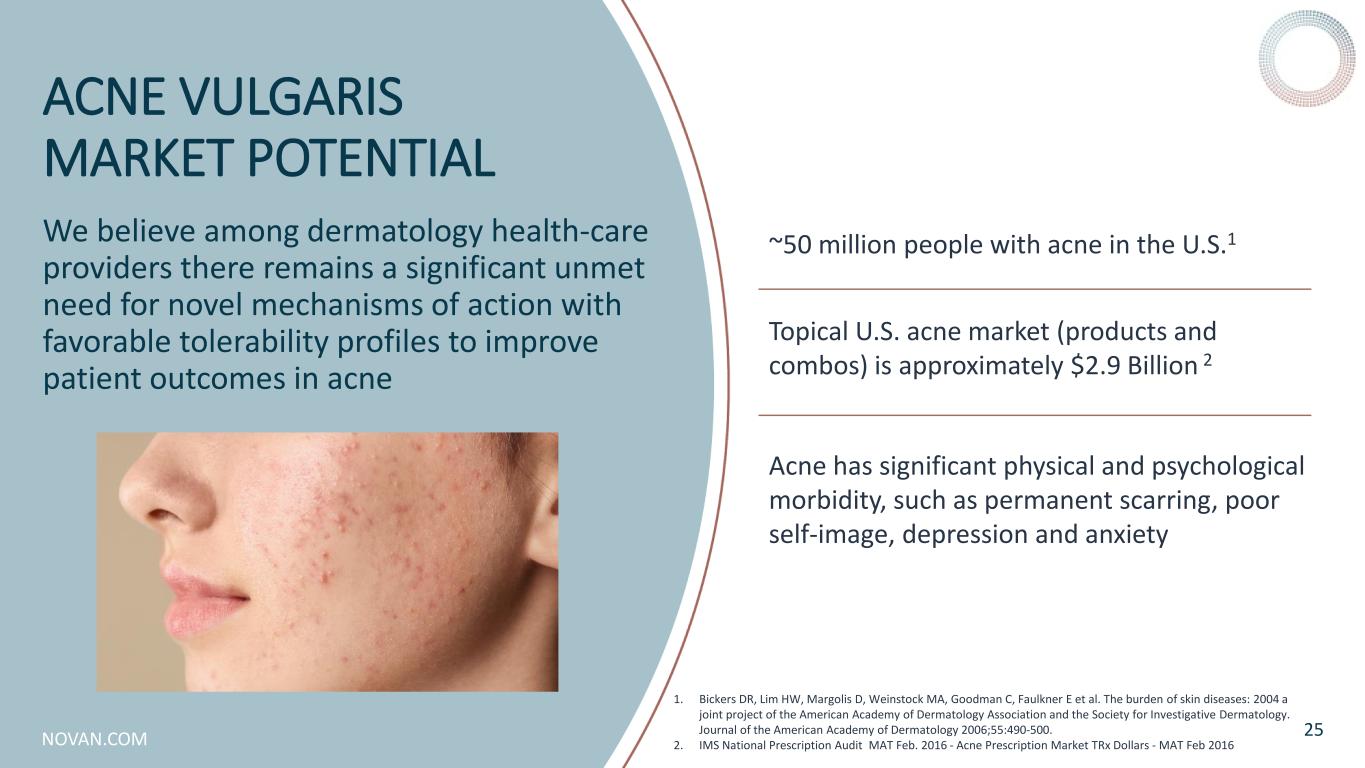
NOVAN.COM ACNE VULGARIS MARKET POTENTIAL We believe among dermatology health-care providers there remains a significant unmet need for novel mechanisms of action with favorable tolerability profiles to improve patient outcomes in acne 25 ~50 million people with acne in the U.S.1 Topical U.S. acne market (products and combos) is approximately $2.9 Billion 2 Acne has significant physical and psychological morbidity, such as permanent scarring, poor self-image, depression and anxiety 1. Bickers DR, Lim HW, Margolis D, Weinstock MA, Goodman C, Faulkner E et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. Journal of the American Academy of Dermatology 2006;55:490-500. 2. IMS National Prescription Audit MAT Feb. 2016 - Acne Prescription Market TRx Dollars - MAT Feb 2016

NOVAN.COM SB019 COVID-19 26 Phase 1 in healthy volunteers in 1H 2022

NOVAN.COM SB019: Advance Development for COVID-19 Intranasal administration may reduce viral burden in the nasal epithelium and lead to reduced transmission, disrupted disease progression, and alleviated symptoms 27 Nitric oxide known to have potent anti-viral properties Strong preclinical and clinical data demonstrate anti-viral effect of berdazimer sodium against multiple viruses In vitro and in vivo studies have demonstrated anti-viral effect of berdazimer sodium against SARS-CoV-2 Public health need to reduce breakthrough infections and transmission

NOVAN.COM REGULATORY PATH FOR SB019 IN COVID-19 28 Pre-clinical IND enabling studies completed1 Candidate formulation administered intranasally in animals Berdazimer sodium well-tolerated up to the maximum dose tested Drug-device combination in development for potential Phase 2/3 study(s) 1. Assumes pre-clinical studies completed to date are all that is required to initiate Phase 1 in human volunteers. Targeted Timeline: Execute Phase 1 Study in Healthy Volunteers 2022 2023 2024Execute Phase 2/3 Study(s) in Patients Submit New Drug Application

NOVAN.COM The Full Opportunity Building a fully integrated dermatology and anti-infectives company 29
NOVAN.COM ROBUST IP PROTECTION Over 35 issued US patents, over 125 issued non-US patents and over 85 pending applications throughout the world 30 NITRICIL™ Technology Patent Expiration1 Composition of Matter to 2026 Method of Manufacture to 2032 Formulation Science Formulation Composition and Manufacture to 2032 or 2033 Admixture Composition to 2034 Therapeutic Uses Methods of Reducing Sebum Production to 2031 Topical Antiviral Compositions and Methods of Using the Same to 2035 1. Expiry dates do not take into account any patent term extensions that may be available to Novan.
NOVAN.COM MILESTONES IN NEXT 12 MONTHS 31 2H 2021 1H 2022 Final Safety Results B-SIMPLE4 for SB206 Launch Pre-Commercial Activities for SB206 Submit SB019 Pre-IND Initiate Phase 1 with SB019 SB206 Pre-NDA Meeting with FDA Submit SB206 NDA

NOVAN.COM FULLY INTEGRATED TEAM SPECIFICALLY DESIGNED TO EXECUTE STRATEGY 32 Tomoko Maeda-Chubachi, MD, Ph.D., MBA Chief Medical Officer John M. Gay, CPA Chief Financial Officer Carri Geer, Ph.D. Chief Technology Officer Paula Brown Stafford, MPH Chairman and Chief Executive Officer Leveraging Extensive Network of Partners and Collaborators
NOVAN.COM NOVAN PRIDE UNDERPINS OUR EFFORTS 33

NOVAN.COM Why Novan, Why Now A world leader in nitric oxide-based science, technology, and clinical translation in support of delivering safe and efficacious therapies 34 NITRICIL™ technology platform allows stable, tunable, and targeted delivery of therapeutic quantities of NO Robust body of data with nearly 4,000 treated subjects supporting anti-microbial and anti-inflammatory properties Significant potential market opportunity with SB206 for the treatment of molluscum contagiosum Confidence to prioritize SB204 for the treatment of acne vulgaris as second lead candidate Near-term inflection point with SB019 pre-IND submission and Phase 1 initiation Significant upside potential with the full platform Three NDAs Planned Over Three Years

Nitric Oxide-Based Medicine N A S D A Q : N O V N | n o v a n . c o m INVESTOR RELATIONS JTC Team 833.475.8247 novn@jtcir.com Thank You!


































