
Corporate Presentation November 2016 Exhibit 99.1

This presentation includes forward-looking statements that reflect our current views with respect to, among other things, our operations and financial performance. These forward-looking statements are included throughout this presentation. We have used the words “anticipate,” “assume,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “future,” “will,” “seek,” “foreseeable” and similar terms and phrases to identify forward-looking statements in this presentation. The forward-looking statements contained in this presentation are based on management’s current expectations and are subject to uncertainty and changes in circumstances. We cannot assure you that future developments affecting us will be those that we have anticipated. Actual results may differ materially from these expectations due to changes in global, regional or local economic, business, competitive, market, regulatory and other factors, many of which are beyond our control. We believe that these factors include but are not limited to those described in our prospectus dated September 20, 2016, filed with the Securities and Exchange Commission (the “SEC”), in our quarterly report filed with the SEC on Form 10-Q for the three months ended Sept. 30, 2016, and in any subsequent filings with the SEC. Should one or more of these risks or uncertainties materialize, or should any of our assumptions prove incorrect, our actual results may vary in material respects from those projected in these forward-looking statements. Any forward-looking statement made by us in this presentation speaks only as of the date of this presentation. Factors or events that could cause our actual results to differ may emerge from time to time, and it is not possible for us to predict all of them. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements and you should not place undue reliance on our forward-looking statements. Our forward-looking statements do not reflect the potential impact of any future acquisitions, mergers, dispositions, joint ventures, investments or other strategic transactions we may make. We undertake no obligation to publicly update or review any forward-looking statement, whether as a result of new information, future developments or otherwise, except as may be required by any applicable securities laws. Forward Looking Statements

Technology platform for transforming nitric oxide into new chemical entities and product candidates for a host of skin diseases SB204 – Phase 3 asset with top-line results expected 1Q 2017 Parallel Phase 3 pivotal trials initiated in 1Q 2016; NDA submission targeted for end of 2017 Robust clinical development program with two replicate Phase 2 studies with SB204 Aiming to be the first New Chemical Entity developed for the treatment of acne in over 20 years Pipeline of product candidates designed to leverage nitric oxide’s multiple mechanisms of action for dermatological indications SB206 – Anti-viral (Genital Warts → EOP2 Meeting with FDA planned for 1H 2017) SB208 – Anti-fungal (Onychomycosis / Tinea pedis → Phase 2 TLRs 1H 2017) SB414 – Anti-inflammatory (Psoriasis → Phase 2 program initiation 2Q 2017) Senior leadership team and experienced board of directors positioned to create a commercially successful leader in dermatology Overview

Experienced Leadership Management Team Nathan Stasko, PhD Chief Executive Officer, Director Richard Peterson Chief Financial Officer Brian Johnson, MBA Chief Commercial Officer M. Joyce Rico, MD, MBA Chief Medical Officer Stan Hollenbach, JD VP, Research and Nonclinical Development Kevin Barber, PhD, RAC VP, Regulatory Affairs Board of Directors Robert Ingram Chairman W. Kent Geer Audit Committee Chair Robert Keegan Comp Committee Chair Kelly Martin Independent Director Sean Murphy Independent Director John Palmour, PhD Independent Director
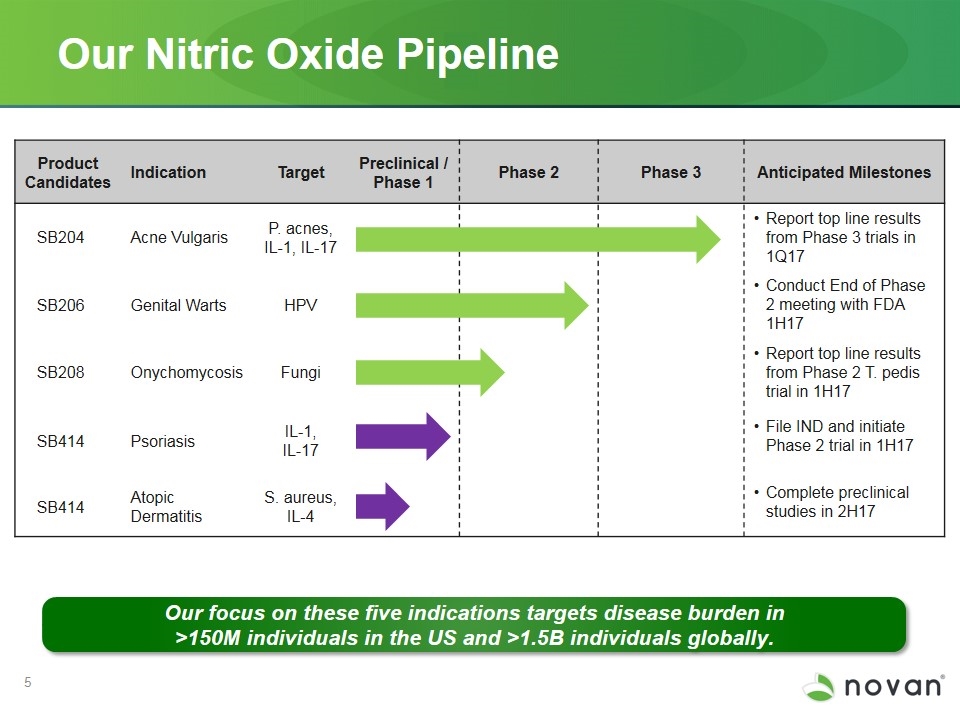
Our Nitric Oxide Pipeline Our focus on these five indications targets disease burden in >150M individuals in the US and >1.5B individuals globally. Product Candidates Indication Target Preclinical / Phase 1 Phase 2 Phase 3 Anticipated Milestones SB204 Acne Vulgaris P. acnes, IL-1, IL-17 Report top line results from Phase 3 trials in 1Q17 SB206 Genital Warts HPV Conduct End of Phase 2 meeting with FDA 1H17 SB208 Onychomycosis Fungi Report top line results from Phase 2 T. pedis trial in 1H17 SB414 Psoriasis IL-1, IL-17 File IND and initiate Phase 2 trial in 1H17 Complete preclinical studies in 2H17 SB414 Atopic Dermatitis S. aureus, IL-4

Nitric Oxide Platform Overview
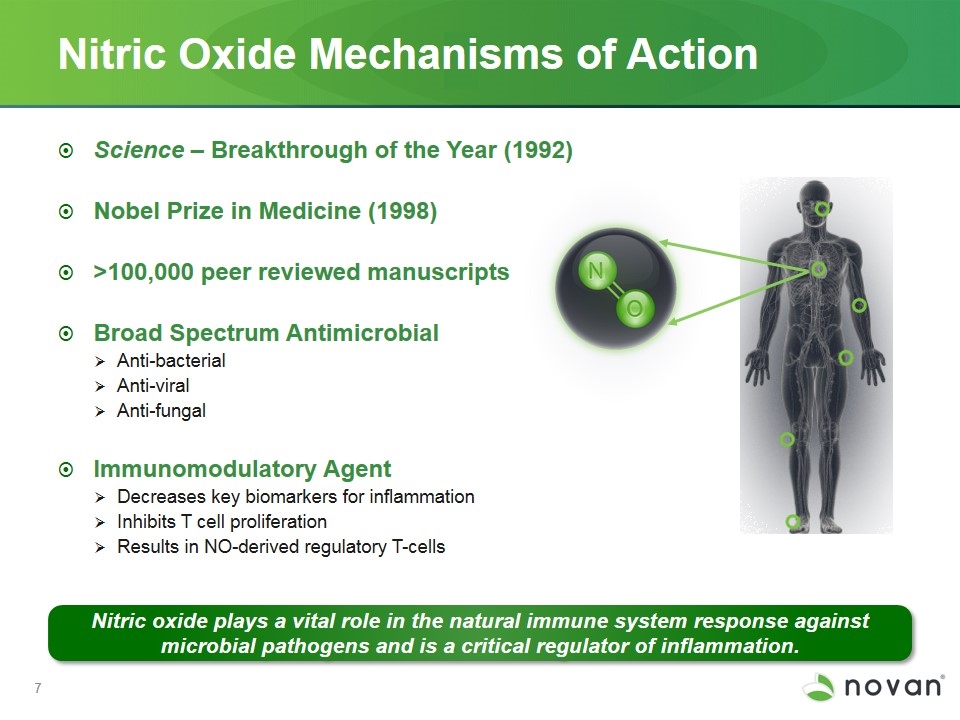
Science – Breakthrough of the Year (1992) Nobel Prize in Medicine (1998) >100,000 peer reviewed manuscripts Broad Spectrum Antimicrobial Anti-bacterial Anti-viral Anti-fungal Immunomodulatory Agent Decreases key biomarkers for inflammation Inhibits T cell proliferation Results in NO-derived regulatory T-cells Nitric Oxide Mechanisms of Action Nitric oxide plays a vital role in the natural immune system response against microbial pathogens and is a critical regulator of inflammation.
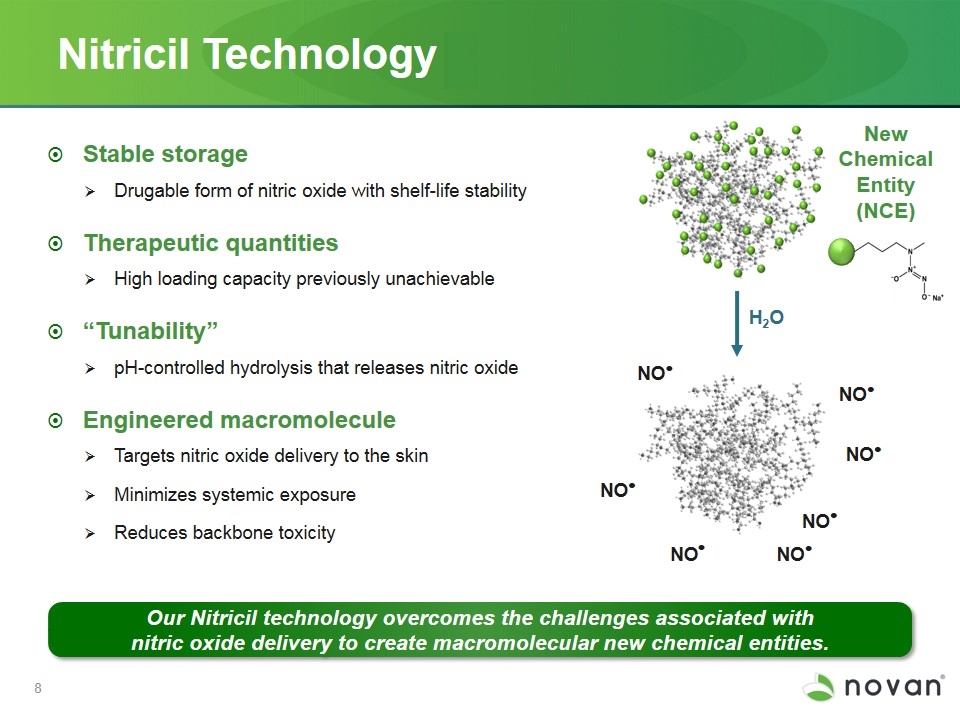
Nitricil Technology Stable storage Drugable form of nitric oxide with shelf-life stability Therapeutic quantities High loading capacity previously unachievable “Tunability” pH-controlled hydrolysis that releases nitric oxide Engineered macromolecule Targets nitric oxide delivery to the skin Minimizes systemic exposure Reduces backbone toxicity Our Nitricil technology overcomes the challenges associated with nitric oxide delivery to create macromolecular new chemical entities. H2O NO . NO . NO . NO . NO . NO . NO . New Chemical Entity (NCE)
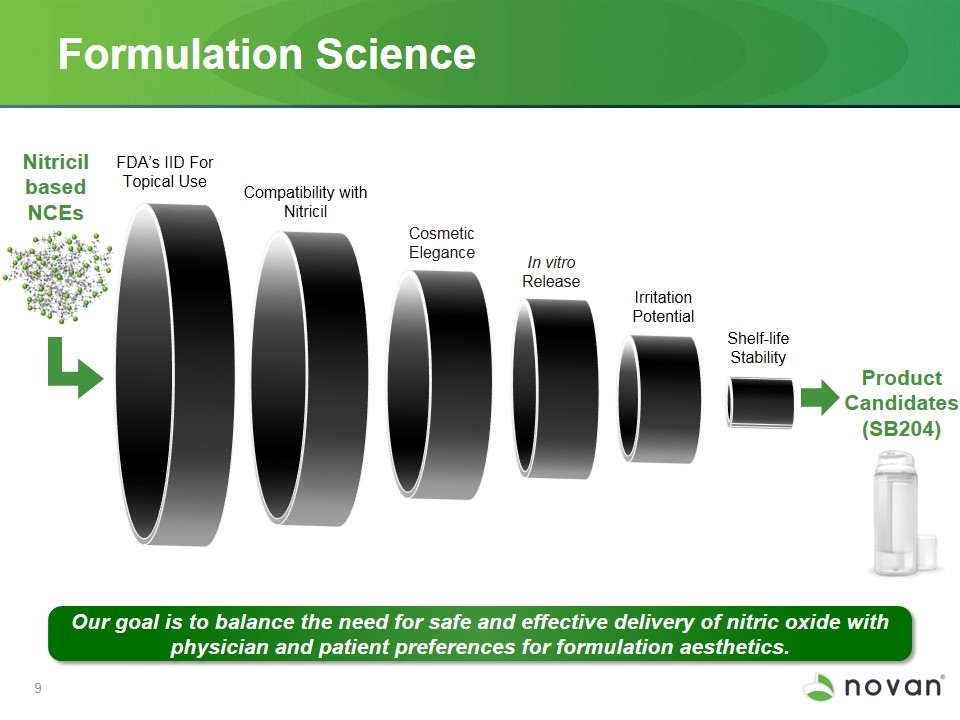
Formulation Science Cosmetic Elegance FDA’s IID For Topical Use Irritation Potential Compatibility with Nitricil In vitro Release Shelf-life Stability Product Candidates (SB204) Nitricil based NCEs Our goal is to balance the need for safe and effective delivery of nitric oxide with physician and patient preferences for formulation aesthetics.
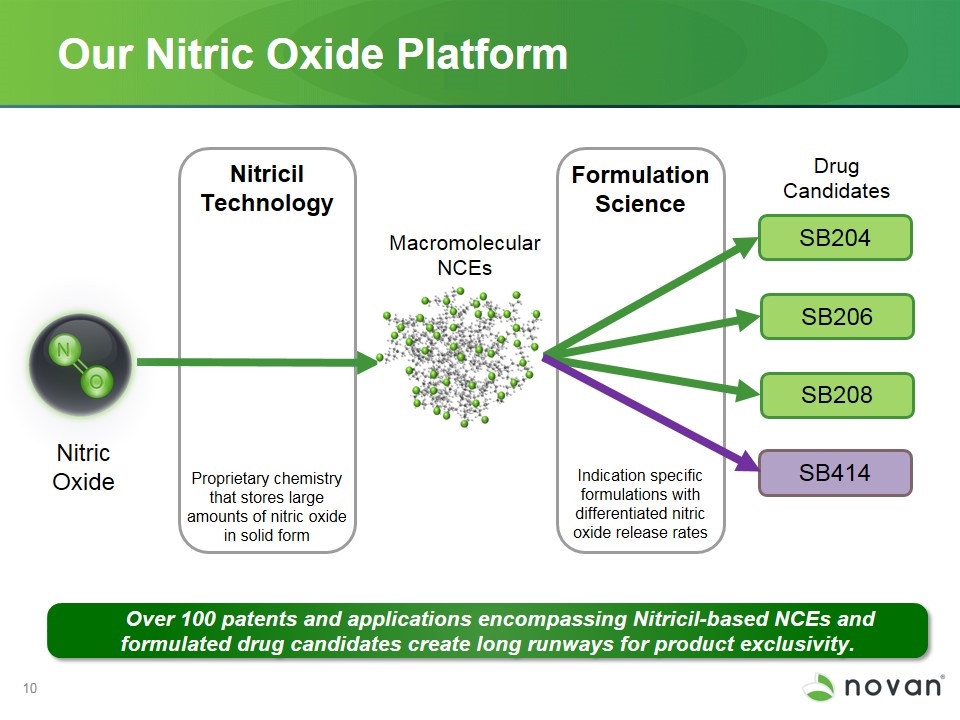
Our Nitric Oxide Platform NVN1000 Nitric Oxide Proprietary chemistry that stores large amounts of nitric oxide in solid form Macromolecular NCEs Drug Candidates Indication specific formulations with differentiated nitric oxide release rates Formulation Science Nitricil Technology SB204 SB206 Over 100 patents and applications encompassing Nitricil-based NCEs and formulated drug candidates create long runways for product exclusivity. SB208 SB414
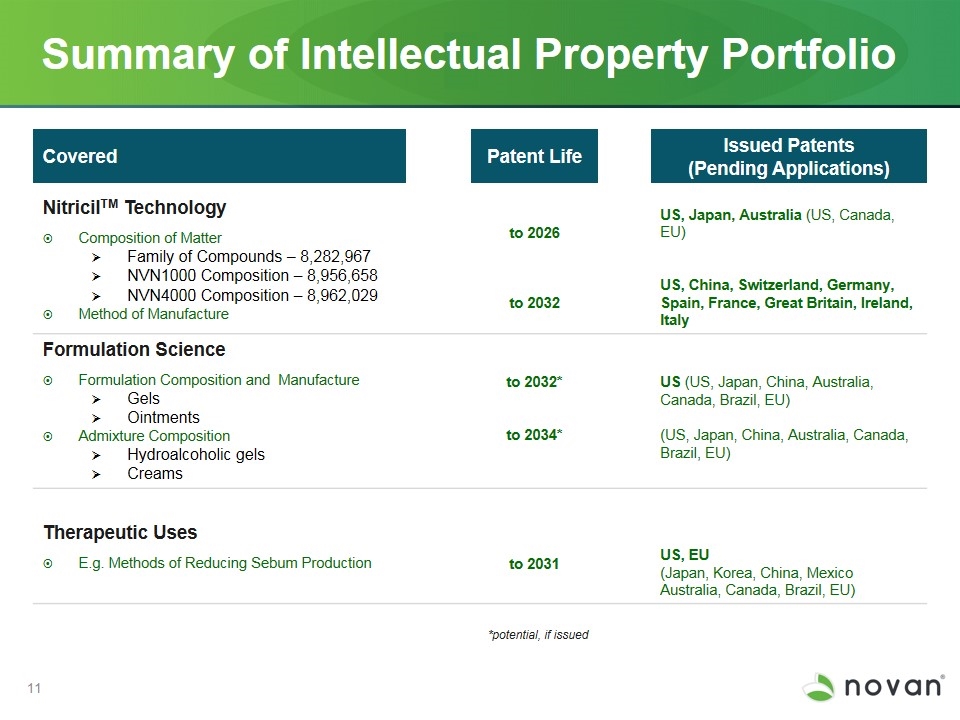
Summary of Intellectual Property Portfolio Covered Patent Life Issued Patents (Pending Applications) NitricilTM Technology Composition of Matter Family of Compounds – 8,282,967 NVN1000 Composition – 8,956,658 NVN4000 Composition – 8,962,029 Method of Manufacture to 2026 to 2032 US, Japan, Australia (US, Canada, EU) US, China, Switzerland, Germany, Spain, France, Great Britain, Ireland, Italy Formulation Science Formulation Composition and Manufacture Gels Ointments Admixture Composition Hydroalcoholic gels Creams to 2032* to 2034* US (US, Japan, China, Australia, Canada, Brazil, EU) (US, Japan, China, Australia, Canada, Brazil, EU) Therapeutic Uses E.g. Methods of Reducing Sebum Production to 2031 US, EU (Japan, Korea, China, Mexico Australia, Canada, Brazil, EU) *potential, if issued

SB204 Potential First-in-Class Topical for the Treatment of Acne
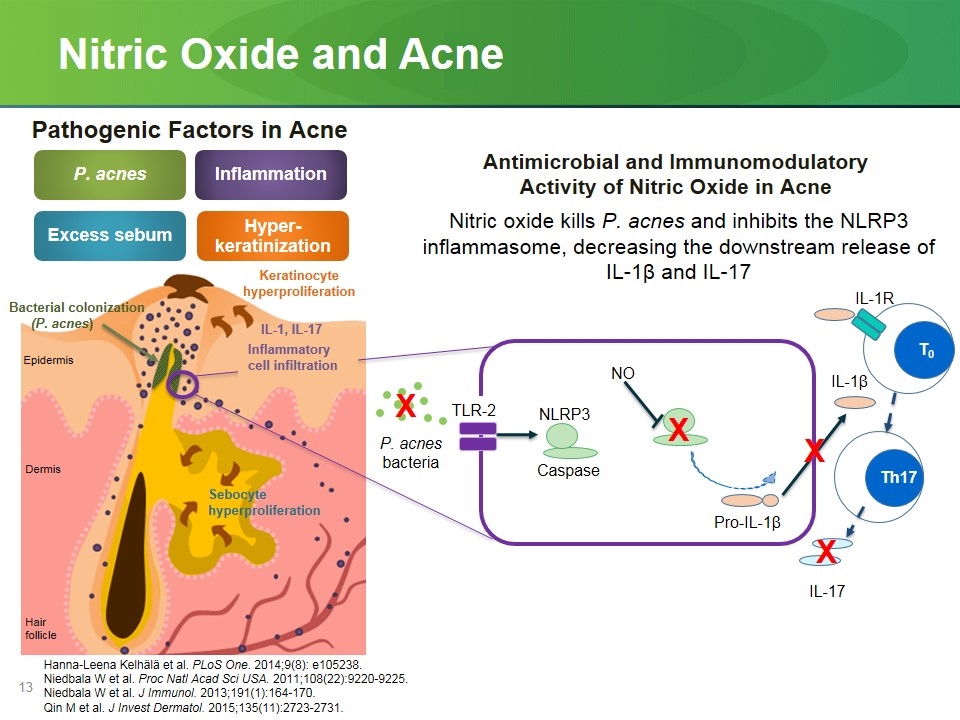
Nitric Oxide and Acne Hanna-Leena Kelhälä et al. PLoS One. 2014;9(8): e105238. Niedbala W et al. Proc Natl Acad Sci USA. 2011;108(22):9220-9225. Niedbala W et al. J Immunol. 2013;191(1):164-170. Qin M et al. J Invest Dermatol. 2015;135(11):2723-2731. P. acnes Excess sebum Inflammation Hyper-keratinization Keratinocyte hyperproliferation Bacterial colonization (P. acnes) Epidermis Dermis Hair follicle Sebocyte hyperproliferation IL-1, IL-17 Pathogenic Factors in Acne NLRP3 Th17 TLR-2 IL-1R P. acnes bacteria IL-17 IL-1β X T0 NO X Pro-IL-1β Antimicrobial and Immunomodulatory Activity of Nitric Oxide in Acne Nitric oxide kills P. acnes and inhibits the NLRP3 inflammasome, decreasing the downstream release of IL-1β and IL-17 Inflammatory cell infiltration Caspase X X

SB204 SB204 Activating Hydrogel NVN1000 Alcohol Gel SB204 is a once-daily, fast-acting, cosmetically elegant topical gel currently in Phase 3 clinical development for the treatment of acne vulgaris Potential first-in-class topical treatment that has shown evidence of efficacy against both inflammatory and non-inflammatory acne lesions in clinical studies to date Statistically significant efficacy against both inflammatory and non-inflammatory lesions in back-to-back Phase 2 clinical trials Good cutaneous tolerability and safety profile with no treatment-related SAEs in >350 patients dosed to date Kills P. acnes bacteria with no apparent risk of antibiotic resistance in preclinical studies No systemic exposure or cardiovascular safety concerns in studies to date

1,300 patients per trial x 2 pivotals = 2,600 patients total 1:1 randomization, SB204 4% Once Daily vs Vehicle Once Daily 12 weeks of treatment Inclusion Criteria Ages 9 and up Minimum 20 and no more than 40 inflammatory lesions on the face Minimum 25 and no more than 70 non-inflammatory lesions on the face IGA scores of either “moderate”, numerical score of 3, or “severe,” numerical score of 4 Co-Primary Endpoints Absolute change in inflammatory lesion counts from baseline to week 12; Absolute change in non-inflammatory lesion counts from baseline to week 12; Proportion of success according to the dichotomized IGA A patient will be considered a success if the IGA at week 12 is either “clear”, with a score of 0, or “almost clear”, with a score of 1, and is at least two grades below the baseline score Secondary Endpoints Percent change in inflammatory and non-inflammatory lesion counts from baseline at week 12 Median time to improvement ClinicalTrials.gov Identifier: NCT02672332, NCT02667444 Phase 3 Pivotal Program Trial Design

SB206 Product Candidate for the Treatment of Viral Skin Infections
Human Papilloma Virus (HPV) Associated Warts Prevalence HPV affects nearly 80 million Americans 14 million people become infected each year Over 100 subtypes, low and high risk strains Prevalence of warts in the US: Genital warts exceeds 500,000 people Common warts exceeds 3,000,000 people Infections can persist up to 2 years Currently, no FDA approved treatments with an anti-viral mechanism of action for the treatment of warts Both topical therapies and ablative procedures for genital warts remain largely ineffective in achieving long-term wart eradication Average recurrence rates range from 30% to 70%1 1Yanofsky et al. Expert Rev Dermatol. 2013;8(3):321-332.
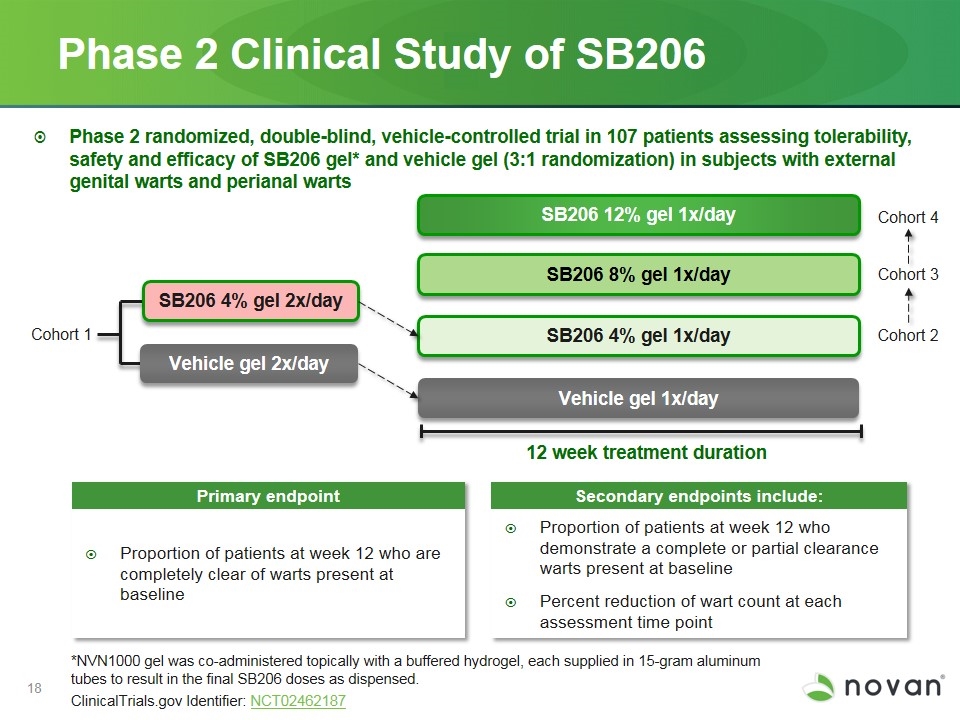
Phase 2 Clinical Study of SB206 12 week treatment duration SB206 4% gel 2x/day SB206 4% gel 1x/day Vehicle gel 1x/day SB206 8% gel 1x/day Primary endpoint Secondary endpoints include: Proportion of patients at week 12 who are completely clear of warts present at baseline Proportion of patients at week 12 who demonstrate a complete or partial clearance warts present at baseline Percent reduction of wart count at each assessment time point Phase 2 randomized, double-blind, vehicle-controlled trial in 107 patients assessing tolerability, safety and efficacy of SB206 gel* and vehicle gel (3:1 randomization) in subjects with external genital warts and perianal warts SB206 12% gel 1x/day *NVN1000 gel was co-administered topically with a buffered hydrogel, each supplied in 15-gram aluminum tubes to result in the final SB206 doses as dispensed. ClinicalTrials.gov Identifier: NCT02462187 Vehicle gel 2x/day Cohort 1 Cohort 2 Cohort 3 Cohort 4
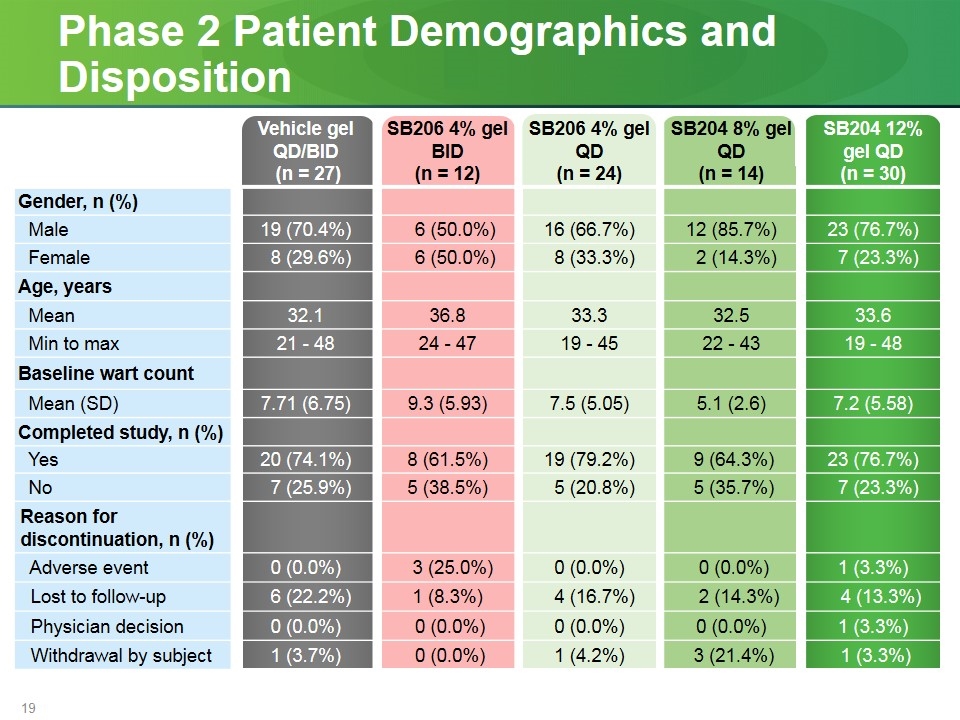
Phase 2 Patient Demographics and Disposition Vehicle gel QD/BID (n = 27) SB206 4% gel BID (n = 12) SB206 4% gel QD (n = 24) SB204 8% gel QD (n = 14) SB204 12% gel QD (n = 30) Gender, n (%) Male 19 (70.4%) 6 (50.0%) 16 (66.7%) 12 (85.7%) 23 (76.7%) Female 8 (29.6%) 6 (50.0%) 8 (33.3%) 2 (14.3%) 7 (23.3%) Age, years Mean 32.1 36.8 33.3 32.5 33.6 Min to max 21 - 48 24 - 47 19 - 45 22 - 43 19 - 48 Baseline wart count Mean (SD) 7.71 (6.75) 9.3 (5.93) 7.5 (5.05) 5.1 (2.6) 7.2 (5.58) Completed study, n (%) Yes 20 (74.1%) 8 (61.5%) 19 (79.2%) 9 (64.3%) 23 (76.7%) No 7 (25.9%) 5 (38.5%) 5 (20.8%) 5 (35.7%) 7 (23.3%) Reason for discontinuation, n (%) Adverse event 0 (0.0%) 3 (25.0%) 0 (0.0%) 0 (0.0%) 1 (3.3%) Lost to follow-up 6 (22.2%) 1 (8.3%) 4 (16.7%) 2 (14.3%) 4 (13.3%) Physician decision 0 (0.0%) 0 (0.0%) 0 (0.0%) 0 (0.0%) 1 (3.3%) Withdrawal by subject 1 (3.7%) 0 (0.0%) 1 (4.2%) 3 (21.4%) 1 (3.3%)
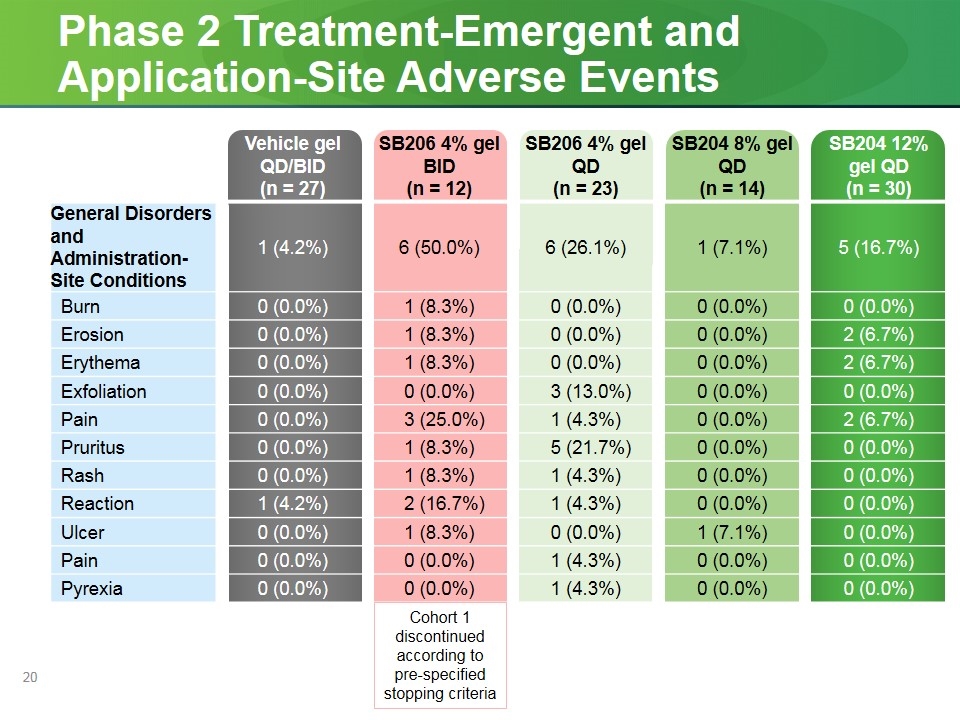
Phase 2 Treatment-Emergent and Application-Site Adverse Events Vehicle gel QD/BID (n = 27) SB206 4% gel BID (n = 12) SB206 4% gel QD (n = 23) SB204 8% gel QD (n = 14) SB204 12% gel QD (n = 30) General Disorders and Administration-Site Conditions 1 (4.2%) 6 (50.0%) 6 (26.1%) 1 (7.1%) 5 (16.7%) Burn 0 (0.0%) 1 (8.3%) 0 (0.0%) 0 (0.0%) 0 (0.0%) Erosion 0 (0.0%) 1 (8.3%) 0 (0.0%) 0 (0.0%) 2 (6.7%) Erythema 0 (0.0%) 1 (8.3%) 0 (0.0%) 0 (0.0%) 2 (6.7%) Exfoliation 0 (0.0%) 0 (0.0%) 3 (13.0%) 0 (0.0%) 0 (0.0%) Pain 0 (0.0%) 3 (25.0%) 1 (4.3%) 0 (0.0%) 2 (6.7%) Pruritus 0 (0.0%) 1 (8.3%) 5 (21.7%) 0 (0.0%) 0 (0.0%) Rash 0 (0.0%) 1 (8.3%) 1 (4.3%) 0 (0.0%) 0 (0.0%) Reaction 1 (4.2%) 2 (16.7%) 1 (4.3%) 0 (0.0%) 0 (0.0%) Ulcer 0 (0.0%) 1 (8.3%) 0 (0.0%) 1 (7.1%) 0 (0.0%) Pain 0 (0.0%) 0 (0.0%) 1 (4.3%) 0 (0.0%) 0 (0.0%) Pyrexia 0 (0.0%) 0 (0.0%) 1 (4.3%) 0 (0.0%) 0 (0.0%) Cohort 1 discontinued according to pre-specified stopping criteria
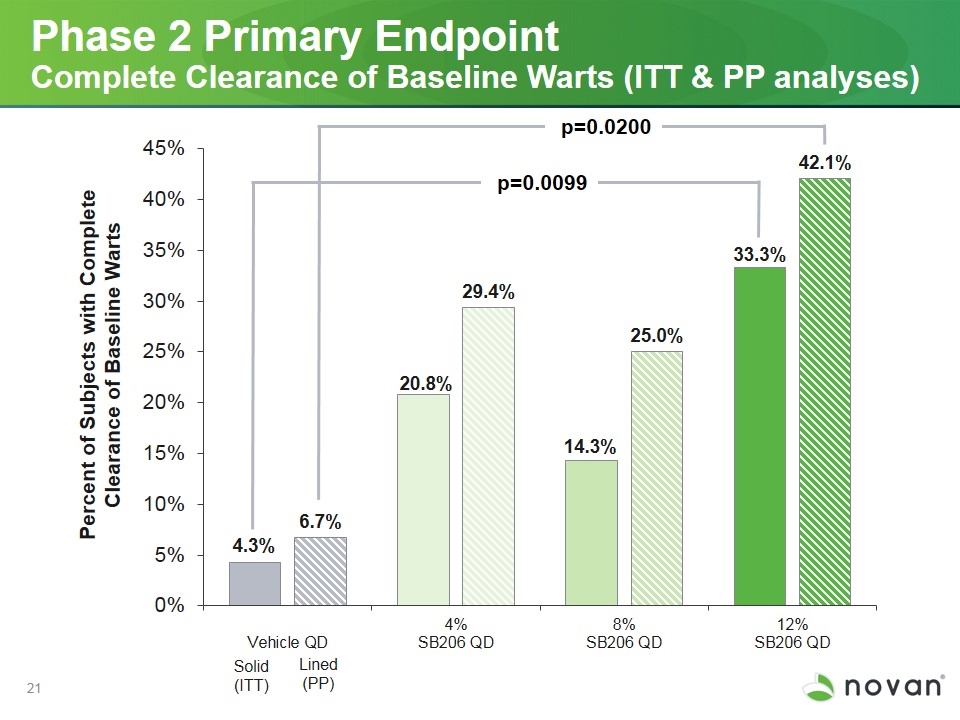
Phase 2 Primary Endpoint Complete Clearance of Baseline Warts (ITT & PP analyses) p=0.0099 p=0.0200 Solid (ITT) Lined (PP)

SB206 Research Collaborations In vitro and in vivo data generated with collaborators who are at the cutting edge of HPV disease pathology research. Dr. Neil Christensen, Professor of Pathology, and Microbiology and Immunology; Medical Director, Jake Gittlen Cancer Research Foundation Research Interest: Immunity and pathogenesis of papillomavirus infections Involved in the early testing of Gardasil prior to FDA approval Drs. Thomas Broker and Louise Chow, Department of Biochemistry & Molecular Genetics at UAB Research Interest: Human papillomavirus DNA replication and pathogenesis The Broker-Chow research program has been focused on HPV for over 30 years Developed the only in vitro model that mimics the entire HPV lifecycle in human cells
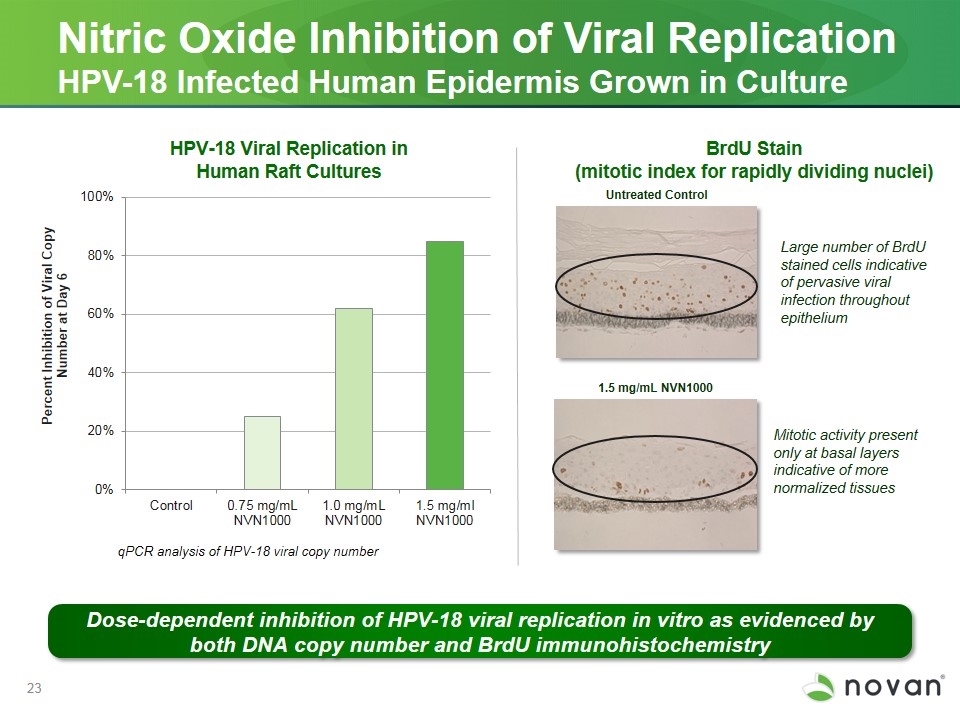
Nitric Oxide Inhibition of Viral Replication HPV-18 Infected Human Epidermis Grown in Culture BrdU Stain (mitotic index for rapidly dividing nuclei) Untreated Control 1.5 mg/mL NVN1000 qPCR analysis of HPV-18 viral copy number Large number of BrdU stained cells indicative of pervasive viral infection throughout epithelium Mitotic activity present only at basal layers indicative of more normalized tissues HPV-18 Viral Replication in Human Raft Cultures Dose-dependent inhibition of HPV-18 viral replication in vitro as evidenced by both DNA copy number and BrdU immunohistochemistry
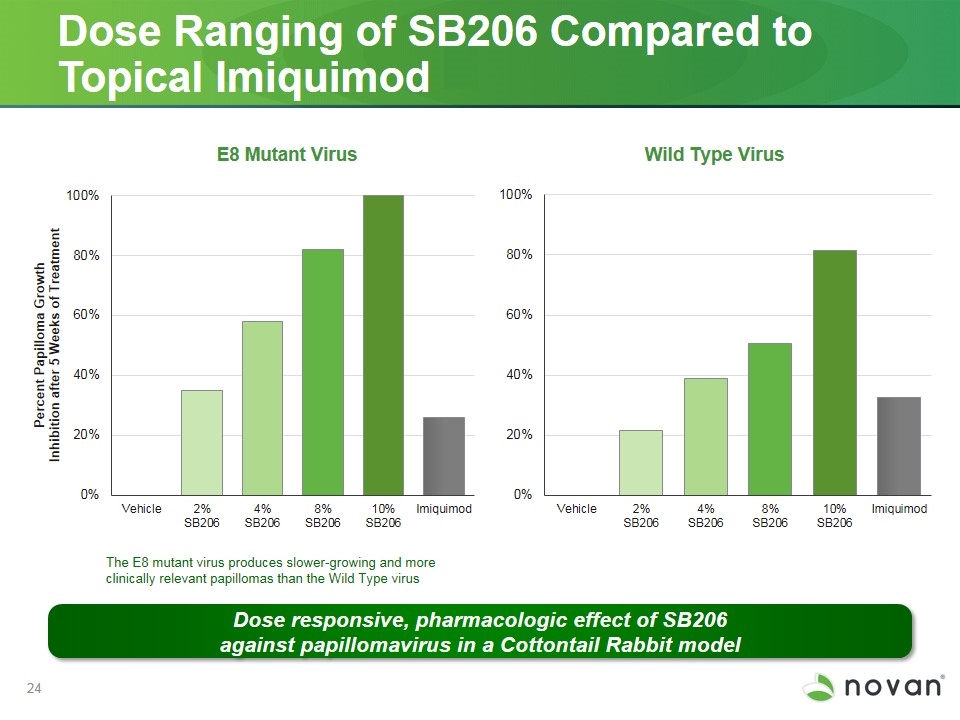
Dose Ranging of SB206 Compared to Topical Imiquimod Wild Type Virus E8 Mutant Virus The E8 mutant virus produces slower-growing and more clinically relevant papillomas than the Wild Type virus Dose responsive, pharmacologic effect of SB206 against papillomavirus in a Cottontail Rabbit model
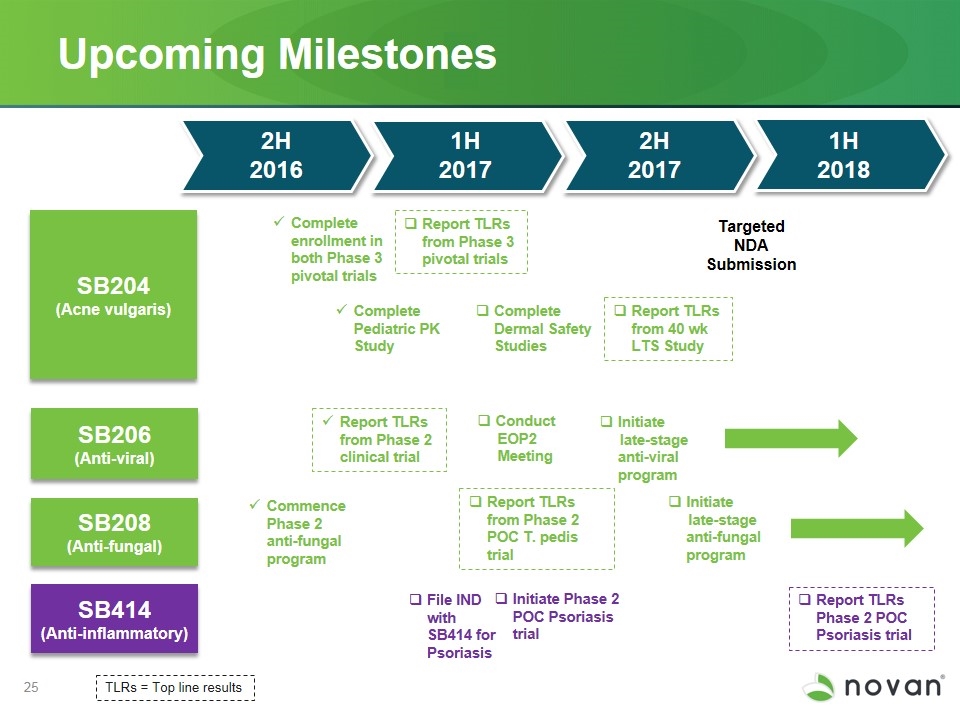
Upcoming Milestones SB204 (Acne vulgaris) SB206 (Anti-viral) SB208 (Anti-fungal) Report TLRs from Phase 3 pivotal trials Report TLRs from Phase 2 clinical trial Commence Phase 2 anti-fungal program Targeted NDA Submission Report TLRs from 40 wk LTS Study Complete enrollment in both Phase 3 pivotal trials TLRs = Top line results Report TLRs from Phase 2 POC T. pedis trial Conduct EOP2 Meeting Complete Pediatric PK Study Complete Dermal Safety Studies 2H 2016 1H 2017 2H 2017 2H 2017 1H 2017 SB414 (Anti-inflammatory) Report TLRs Phase 2 POC Psoriasis trial 1H 2018 Initiate Phase 2 POC Psoriasis trial Initiate late-stage anti-viral program File IND with SB414 for Psoriasis Initiate late-stage anti-fungal program
























