
Novan Webcast August 2, 2017 Exhibit 99.1
Agenda Purpose of Webcast Introductions Scientific Foundation Clinical Overview Financial Update Summary Q&A 1 2 3 4 5 6 7 Page 1
Agenda Purpose of Webcast Introductions Scientific Foundation Clinical Overview Financial Update Summary Q&A 1 2 3 4 5 6 7 Page 2
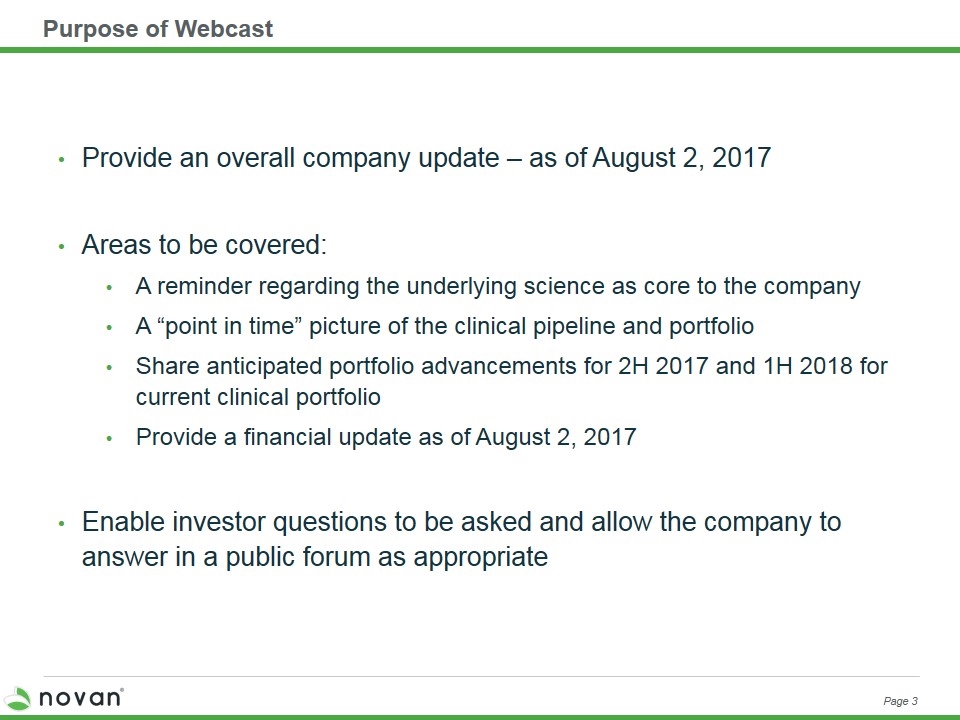
Purpose of Webcast Provide an overall company update – as of August 2, 2017 Areas to be covered: A reminder regarding the underlying science as core to the company A “point in time” picture of the clinical pipeline and portfolio Share anticipated portfolio advancements for 2H 2017 and 1H 2018 for current clinical portfolio Provide a financial update as of August 2, 2017 Enable investor questions to be asked and allow the company to answer in a public forum as appropriate Page 3
Agenda Purpose of Webcast Introductions Scientific Foundation Clinical Overview Financial Update Summary Q&A 1 2 3 4 5 6 7 Page 4

Introductions Participants on webcast Robert Ingram – Executive Chairman Nate Stasko – Co-Founder, Chief Scientific Officer and President Paula Brown Stafford – Chief Development Officer Kelly Martin – Interim Chief Executive Officer Others available to participate in the investor Q&A Stan Hollenbach – Senior VP of R&D Jeff Hunter – VP of Technical Operations Carri Geer – Director of Chemical and Analytical Development Kevin Barber – VP of Regulatory Affairs William Hodges – Interim Chief Financial Officer Andrew Novak – Director of Financial Reporting & Analysis Page 5
Agenda Purpose of Webcast Introductions Scientific Foundation Clinical Overview Financial Update Summary Q&A 1 2 3 4 5 6 7 Page 6
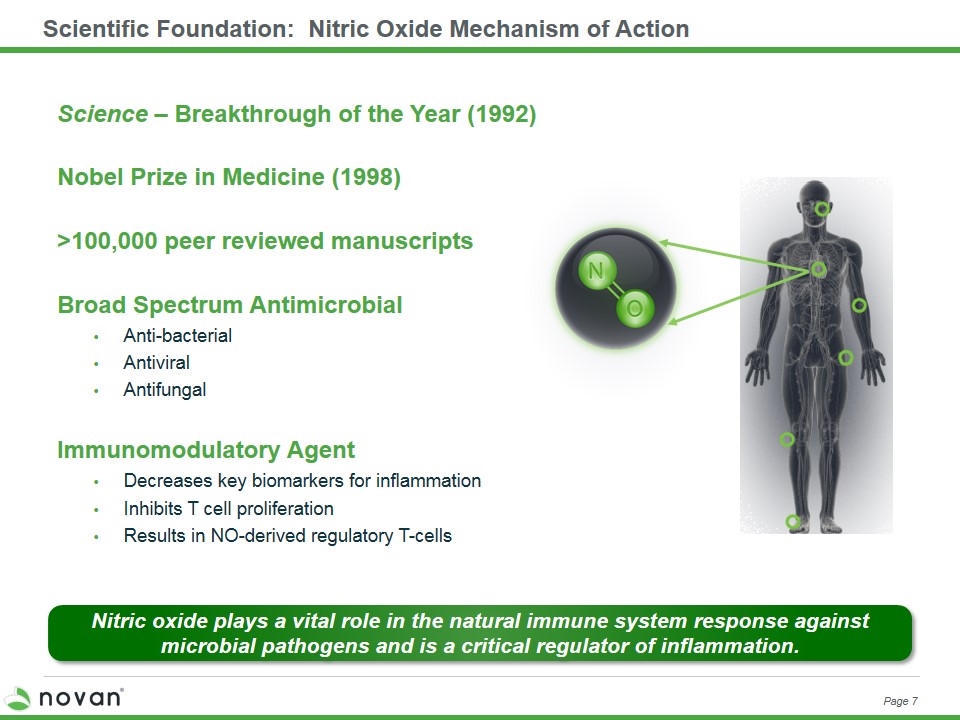
Scientific Foundation: Nitric Oxide Mechanism of Action Science – Breakthrough of the Year (1992) Nobel Prize in Medicine (1998) >100,000 peer reviewed manuscripts Broad Spectrum Antimicrobial Anti-bacterial Antiviral Antifungal Immunomodulatory Agent Decreases key biomarkers for inflammation Inhibits T cell proliferation Results in NO-derived regulatory T-cells Nitric oxide plays a vital role in the natural immune system response against microbial pathogens and is a critical regulator of inflammation. Page 7
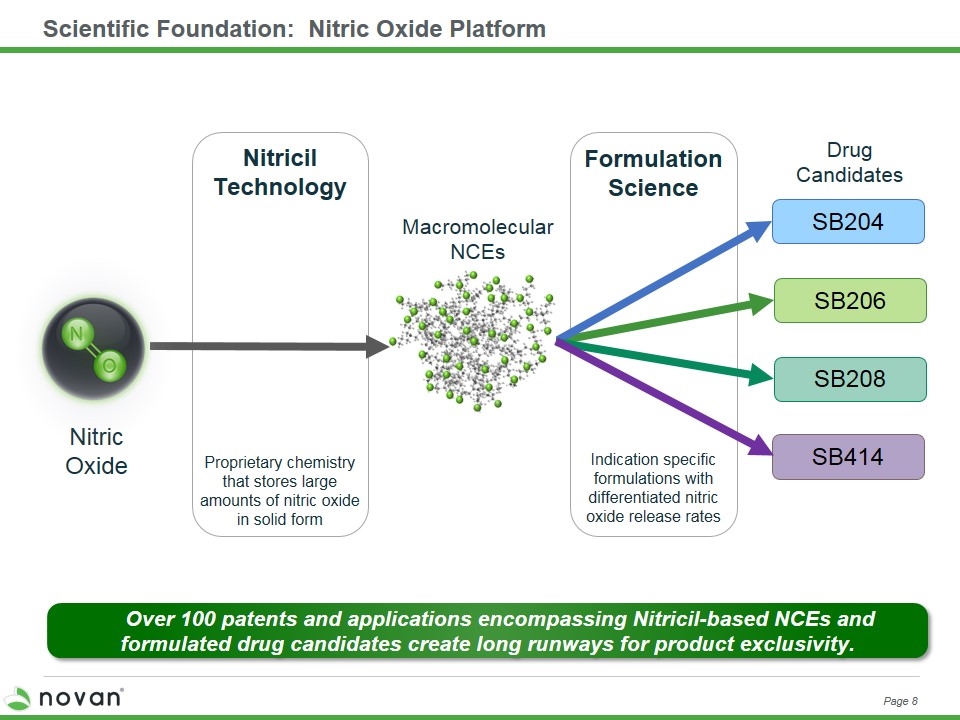
Scientific Foundation: Nitric Oxide Platform Page 8 NVN1000 Nitric Oxide Proprietary chemistry that stores large amounts of nitric oxide in solid form Macromolecular NCEs Drug Candidates Indication specific formulations with differentiated nitric oxide release rates Formulation Science Nitricil Technology SB204 SB206 Over 100 patents and applications encompassing Nitricil-based NCEs and formulated drug candidates create long runways for product exclusivity. SB208 SB414
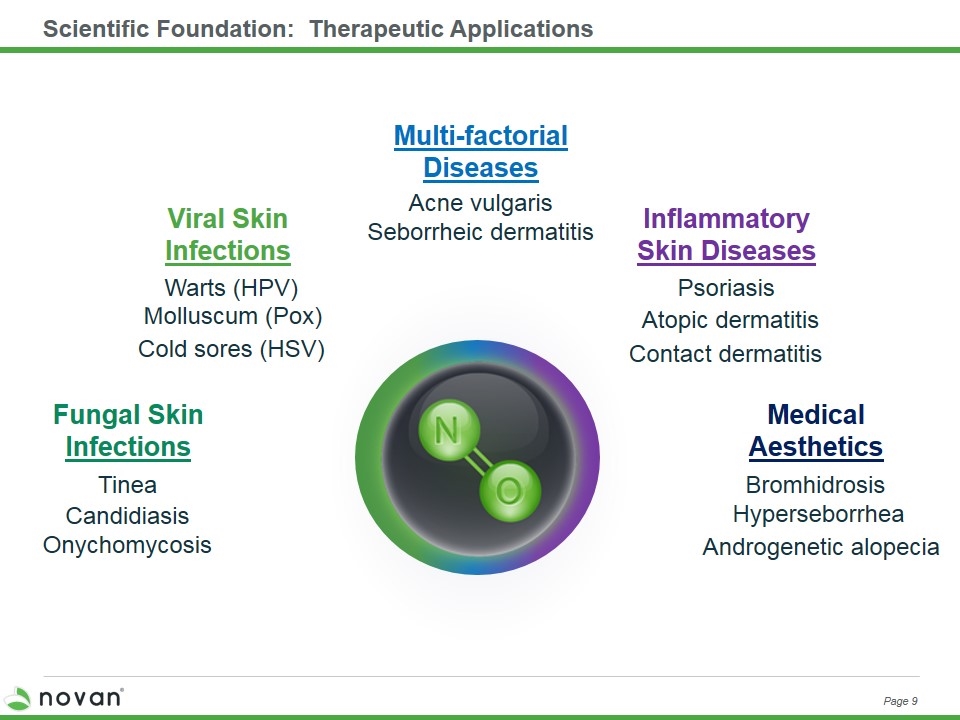
Scientific Foundation: Therapeutic Applications Androgenetic alopecia Bromhidrosis Hyperseborrhea Medical Aesthetics Contact dermatitis Psoriasis Atopic dermatitis Inflammatory Skin Diseases Warts (HPV) Cold sores (HSV) Molluscum (Pox) Viral Skin Infections Multi-factorial Diseases Acne vulgaris Seborrheic dermatitis Onychomycosis Tinea Fungal Skin Infections Candidiasis Page 9
Agenda Purpose of Webcast Introductions Scientific Foundation Clinical Overview Financial Update Summary Q&A 1 2 3 4 5 6 7 Page 10
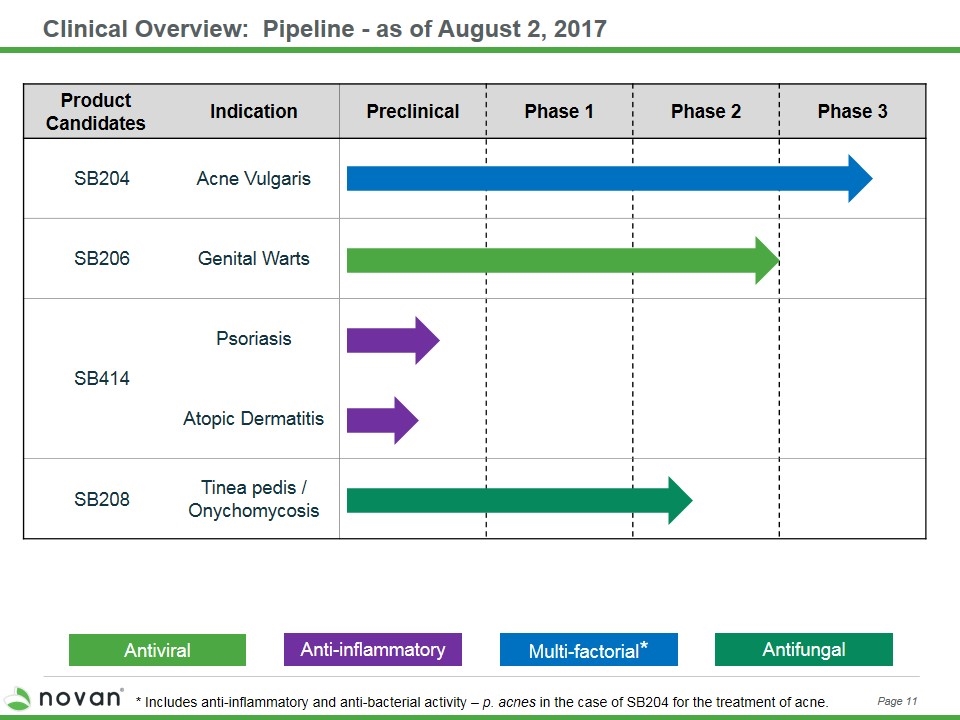
Clinical Overview: Pipeline - as of August 2, 2017 Multi-factorial* Anti-inflammatory Antiviral Product Candidates Indication Preclinical Phase 1 Phase 2 Phase 3 SB204 Acne Vulgaris SB206 Genital Warts SB414 Psoriasis Atopic Dermatitis SB208 Tinea pedis / Onychomycosis Antifungal Page 11 * Includes anti-inflammatory and anti-bacterial activity – p. acnes in the case of SB204 for the treatment of acne.
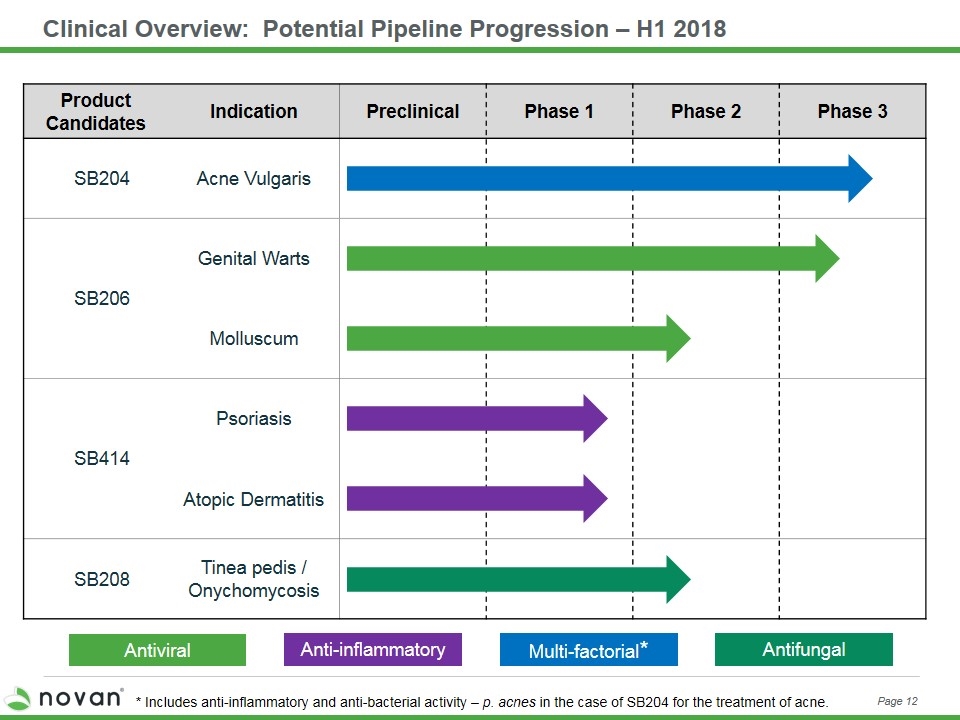
Clinical Overview: Potential Pipeline Progression – H1 2018 Multi-factorial* Anti-inflammatory Antiviral Product Candidates Indication Preclinical Phase 1 Phase 2 Phase 3 SB204 Acne Vulgaris SB206 Genital Warts Molluscum SB414 Psoriasis Atopic Dermatitis SB208 Tinea pedis / Onychomycosis Antifungal Page 12 * Includes anti-inflammatory and anti-bacterial activity – p. acnes in the case of SB204 for the treatment of acne.
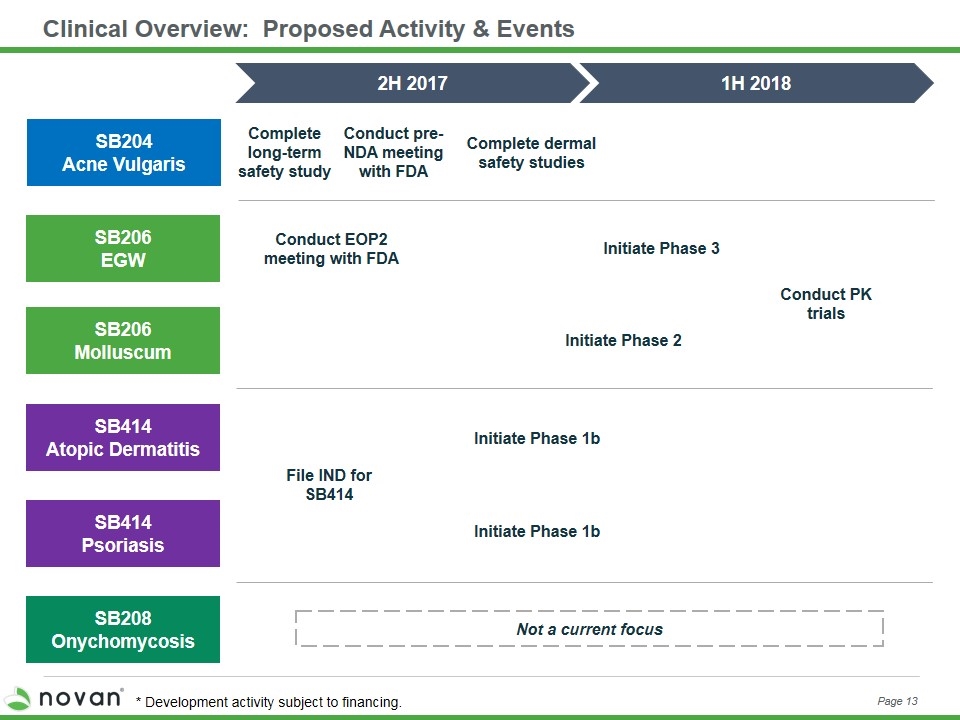
Clinical Overview: Proposed Activity & Events SB206 EGW SB206 Molluscum SB414 Atopic Dermatitis SB208 Onychomycosis 2H 2017 1H 2018 Not a current focus SB204 Acne Vulgaris SB414 Psoriasis Initiate Phase 3 Initiate Phase 2 Conduct PK trials Complete long-term safety study Complete dermal safety studies File IND for SB414 Conduct pre-NDA meeting with FDA Conduct EOP2 meeting with FDA Initiate Phase 1b Initiate Phase 1b Page 13 * Development activity subject to financing.

Clinical Overview: SB204, Acne Vulgaris Program Completed Long Term Safety Study: no significant adverse events observed Continue to do work on behalf of Sato – our Japanese partner on SB204 FDA granted meeting to be held in 3Q 2017 Goal of FDA meeting: seek programmatic clarity regarding possible clinical and regulatory path forward SB204 Acne program remains of interest from Novan perspective and from external strategic perspective Page 14
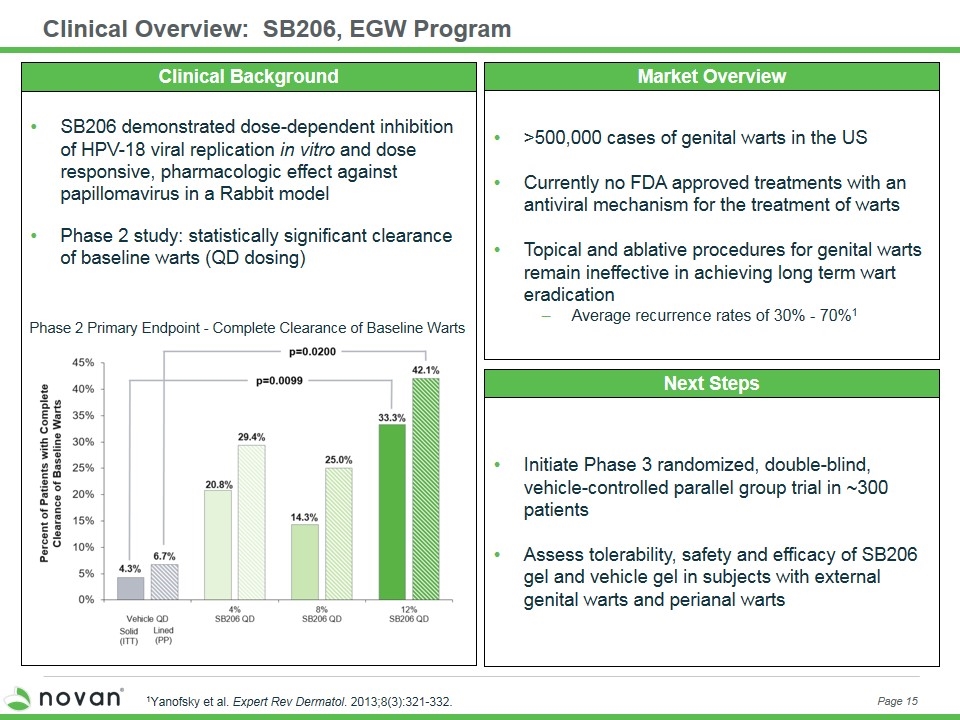
Clinical Overview: SB206, EGW Program Page 15 Market Overview Next Steps >500,000 cases of genital warts in the US Currently no FDA approved treatments with an antiviral mechanism for the treatment of warts Topical and ablative procedures for genital warts remain ineffective in achieving long term wart eradication Average recurrence rates of 30% - 70%1 1Yanofsky et al. Expert Rev Dermatol. 2013;8(3):321-332. Initiate Phase 3 randomized, double-blind, vehicle-controlled parallel group trial in ~300 patients Assess tolerability, safety and efficacy of SB206 gel and vehicle gel in subjects with external genital warts and perianal warts Phase 2 Primary Endpoint - Complete Clearance of Baseline Warts SB206 demonstrated dose-dependent inhibition of HPV-18 viral replication in vitro and dose responsive, pharmacologic effect against papillomavirus in a Rabbit model Phase 2 study: statistically significant clearance of baseline warts (QD dosing) Clinical Background
Clinical Overview: Molluscum Program Page 16 Next Steps Clinical Background Molluscum is a contagious skin infection caused by the molluscipoxvirus Affects ~6M Americans, mostly children Greatest incidence in children aged 1 to 12 years Average time to resolution is 13 months1 30% of children have lesions that may not resolve in 18 months 13% of cases unresolved after 24 months There is no FDA-approved treatment for Molluscum Recent regulatory discussion regarding medical / therapeutic need Discussions constructive Initiate Phase 2 trial utilizing SB206 for the treatment of Molluscum no later than 2Q 2018 1 Olsen JR et al. Lancet Infect Dis. 2015;15:190-5. 2 Data on file, Novan, Inc. Images courtesy of https://www.cdc.gov/poxvirus/molluscum-contagiosum/index.html Observational learnings from in-licensed sodium nitrite/citric acid topical treatment Clinically meaningful complete clearance rates from sodium nitrite/citric acid study High-dose active treatment showed 48% complete clearance of baseline lesions vs 29% in the placebo group (not stat. sig. on the primary efficacy endpoint due to only 30 subjects per arm (p=.092)2 Learnings combined with SB206 program knowledge provides logical pathway to Molluscum indication Market Overview and Opportunity
Clinical Overview: SB414, Psoriasis and Atopic Dermatitis Page 17 Market Overview Next Steps Clinical Background Nitric oxide has the potential to disrupt the propagation of IL-17 locally in the skin through its ability to disrupt the assembly and activity of the NLRP3 inflammasome¹ SB414 displayed potent anti-staphylococcal activity and dose-dependent inhibition of inflammation comparable to betamethasone in in vivo atopic dermatitis models SB414 significantly (p<0.05) reduced composite psoriasis scores and inhibited production of pro-inflammatory cytokines, including IL-17a and IL-17f in vivo Current systemic therapies are indicated only for patients with moderate-to-severe disease² ~80% of patients have mild-to-moderate disease and are treated with topical therapies (corticosteroids, retinoids and vitamin D3). All have side effects and are not well-suited for chronic use³ Phase 1b trials for Psoriasis and Atopic Dermatitis in 2H 2017 Evaluation of systemic exposure via pharmacokinetic assessment of NVN1000 Assessment of key biomarkers of inflammation Assessments of safety and cutaneous tolerability 1Speeckaert R et.al. Br J Dermatol. 2016 Nov; 175(5):892-901. 2American Academy of Dermatology . "Psoriasis." https://www.aad.org/media/stats/conditions/psoriasis ( Nov. 15, 2016 ). 3National Institutes of Health . "Questions and Answers about Psoriasis." http://www.niams.nih.gov/Health_Info/Psoriasis/ ( Nov. 15, 2016 ).
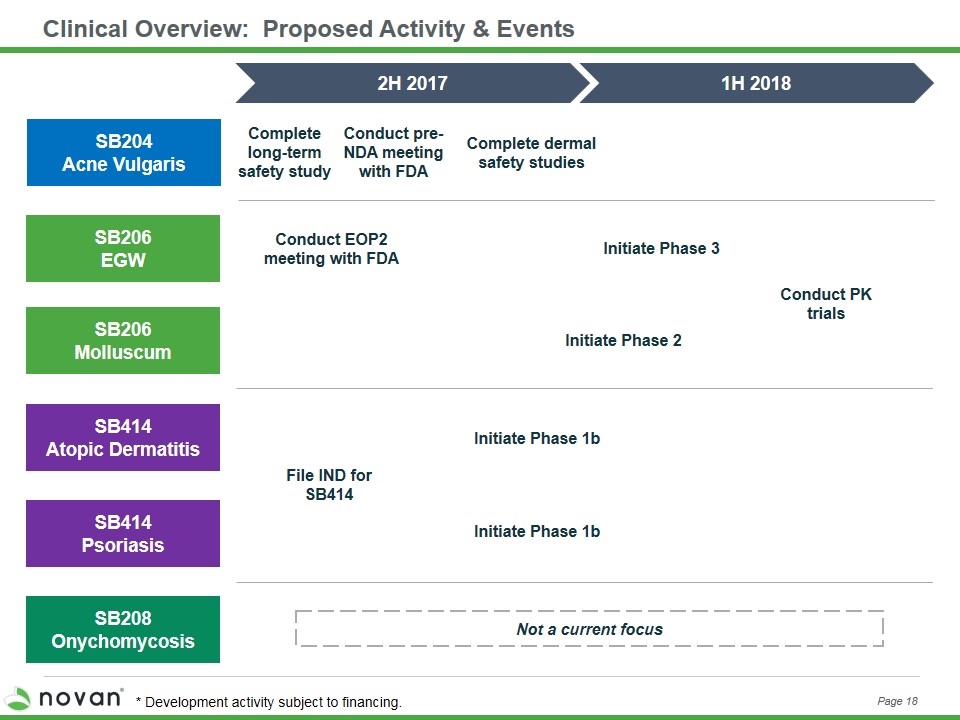
Clinical Overview: Proposed Activity & Events SB206 EGW SB206 Molluscum SB414 Atopic Dermatitis SB208 Onychomycosis 2H 2017 1H 2018 Not a current focus SB204 Acne Vulgaris SB414 Psoriasis Initiate Phase 3 Initiate Phase 2 Conduct PK trials Complete long-term safety study Complete dermal safety studies File IND for SB414 Conduct pre-NDA meeting with FDA Conduct EOP2 meeting with FDA Initiate Phase 1b Initiate Phase 1b Page 18 * Development activity subject to financing.
Agenda Purpose of Webcast Introductions Scientific Foundation Clinical Overview Financial Update Summary Q&A 1 2 3 4 5 6 7 Page 19
Financial Update Page 20 We expect to file our 10Q for the quarterly period ended June 30, 2017 on August 11, 2017 Cash at June 30, 2017 of over $19 million Steps have been taken to focus activity and resources, align talent and capability, and extend the operating and financial runway Strengthening of the balance sheet is a near-term goal to enable an increase in operating runway
Agenda Purpose of Webcast Introductions Scientific Foundation Clinical Overview Financial Update Summary Q&A 1 2 3 4 5 6 7 Page 21

Summary Focus for the company: high quality execution and moving the science/clinical assets forward Near term goal to enable increase in operating “runway” Primary clinical focus over the next 24 months: antiviral clinical work in EGW and Molluscum Secondary clinical and nitric oxide mechanistic focus is anti-inflammatory with work in Psoriasis and Atopic Dermatitis Acne indication and path forward will be largely driven by regulatory clarity Early stages of “thinking through” New Chemical Entity (NCEs) component of the company’s future Page 22

Q & A Page 23

This presentation includes forward-looking statements that reflect our current views with respect to, among other things, our operations and financial performance. These forward-looking statements are included throughout this presentation. We have used the words “anticipate,” “assume,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “future,” “will,” “seek,” “foreseeable” and similar terms and phrases to identify forward-looking statements in this presentation. The forward-looking statements contained in this presentation are based on management’s current expectations and are subject to uncertainty and changes in circumstances. We cannot assure you that future developments affecting us will be those that we have anticipated. Actual results may differ materially from these expectations due to changes in global, regional or local economic, business, competitive, market, regulatory and other factors, many of which are beyond our control. We believe that these factors include but are not limited to those described in our annual report filed with the SEC on Form 10-K for the twelve months ended Dec. 31, 2016, and in any subsequent filings with the SEC. Should one or more of these risks or uncertainties materialize, or should any of our assumptions prove incorrect, our actual results may vary in material respects from those projected in these forward-looking statements. Any forward-looking statement made by us in this presentation speaks only as of the date of this presentation. Factors or events that could cause our actual results to differ may emerge from time to time, and it is not possible for us to predict all of them. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements and you should not place undue reliance on our forward-looking statements. Our forward-looking statements do not reflect the potential impact of any future acquisitions, mergers, dispositions, joint ventures, investments or other strategic transactions we may make. We undertake no obligation to publicly update or review any forward-looking statement, whether as a result of new information, future developments or otherwise, except as may be required by any applicable securities laws. Page 24 Forward Looking Statements
























