
Corporate Overview October 23, 2017 Exhibit 99.1

This presentation includes forward-looking statements that reflect our current views with respect to, among other things, our plans to develop and commercialize our product candidates, including our interpretation of preclinical studies and the success and timing of our product development activities and clinical trials, our operations and business strategy, including our plans to enter into partnering transactions, and our financial performance and strategies and needs for additional financing. These forward-looking statements are included throughout this presentation. We have used the words “anticipate,” “assume,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “future,” “will,” “seek,” “foreseeable” and similar terms and phrases to identify forward-looking statements in this presentation. The forward-looking statements contained in this presentation are based on management’s current expectations and are subject to substantial risks, uncertainty and changes in circumstances. Actual results may differ materially from these expectations due to risks and uncertainties including, among others, those related to the success, timing and cost of ongoing or future clinical trials, the lengthy and unpredictable nature of the U.S. Food and Drug Administration’s drug approval process and our ability to enter into strategic arrangements or obtain additional capital on favorable terms to us, or at all. We believe that these risks and uncertainties include but are not limited to those described in our annual report filed with the SEC on Form 10-K for the twelve months ended Dec. 31, 2016, and in any subsequent filings with the SEC. Any forward-looking statement made by us in this presentation speaks only as of the date of this presentation. We undertake no obligation to publicly update or review any forward-looking statement, whether as a result of new information, future developments or otherwise, except as may be required by any applicable securities laws. Forward Looking Statements

Experienced Leadership Nate Stasko, PhD President and Chief Scientific Officer Paula Brown Stafford Chief Development Officer Stan Hollenbach, JD Senior VP, Research and Nonclinical Development Kevin Barber, PhD, RAC VP, Regulatory Affairs Tomoko Maeda-Chubachi. MD VP, Medical Dermatology Lawrence Eichenfield, MD Chairman Kelly Martin Chief Executive Officer William Hodges Chief Financial Officer Jeff Hunter Chief Business Officer, EVP Brian Johnson, MBA Chief Commercial Officer Carri Geer, PhD VP, Pharmaceutical Development Robert A. Ingram Executive Chairman Management Team Board of Directors Advisory Council
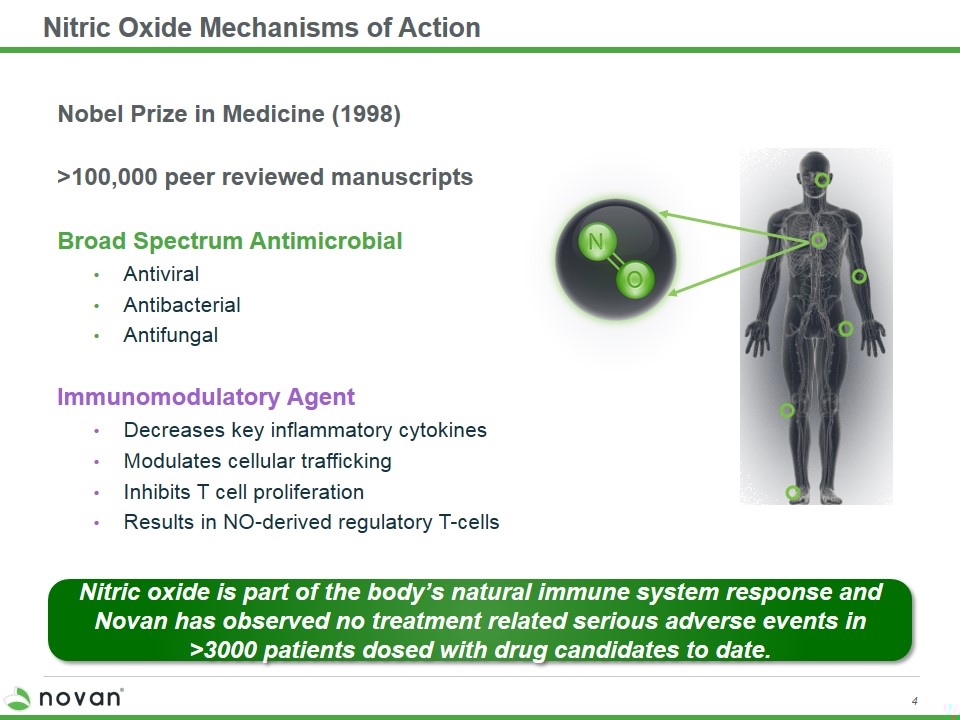
Nitric Oxide Mechanisms of Action Nobel Prize in Medicine (1998) >100,000 peer reviewed manuscripts Broad Spectrum Antimicrobial Antiviral Antibacterial Antifungal Immunomodulatory Agent Decreases key inflammatory cytokines Modulates cellular trafficking Inhibits T cell proliferation Results in NO-derived regulatory T-cells Nitric oxide is part of the body’s natural immune system response and Novan has observed no treatment related serious adverse events in >3000 patients dosed with drug candidates to date.
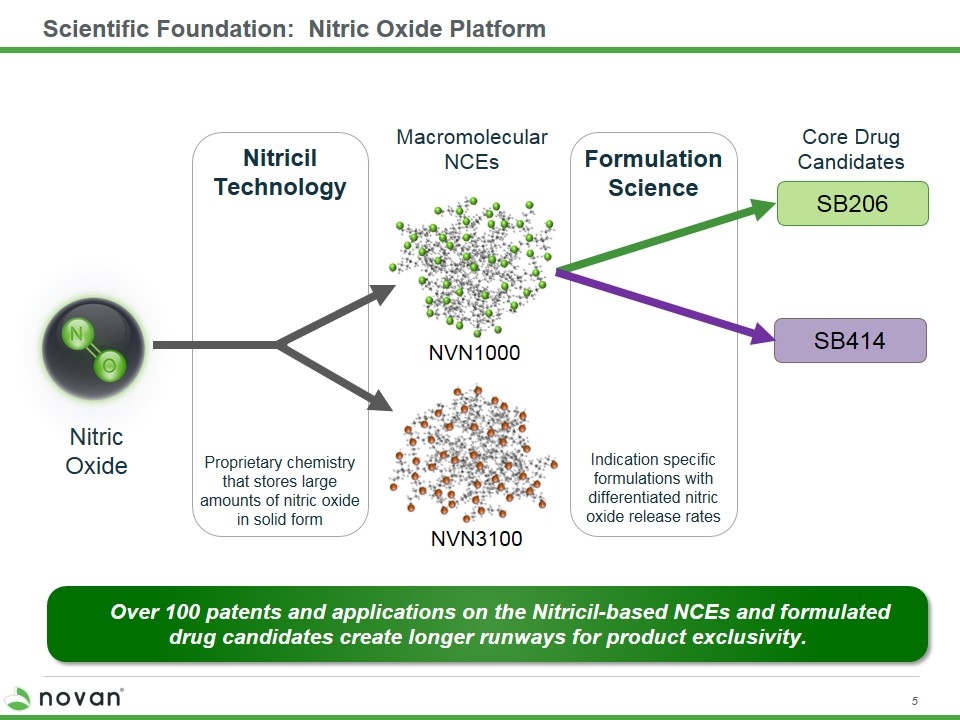
Scientific Foundation: Nitric Oxide Platform NVN1000 Nitric Oxide Proprietary chemistry that stores large amounts of nitric oxide in solid form Macromolecular NCEs Core Drug Candidates Indication specific formulations with differentiated nitric oxide release rates Formulation Science Nitricil Technology SB206 Over 100 patents and applications on the Nitricil-based NCEs and formulated drug candidates create longer runways for product exclusivity. SB414 NVN3100
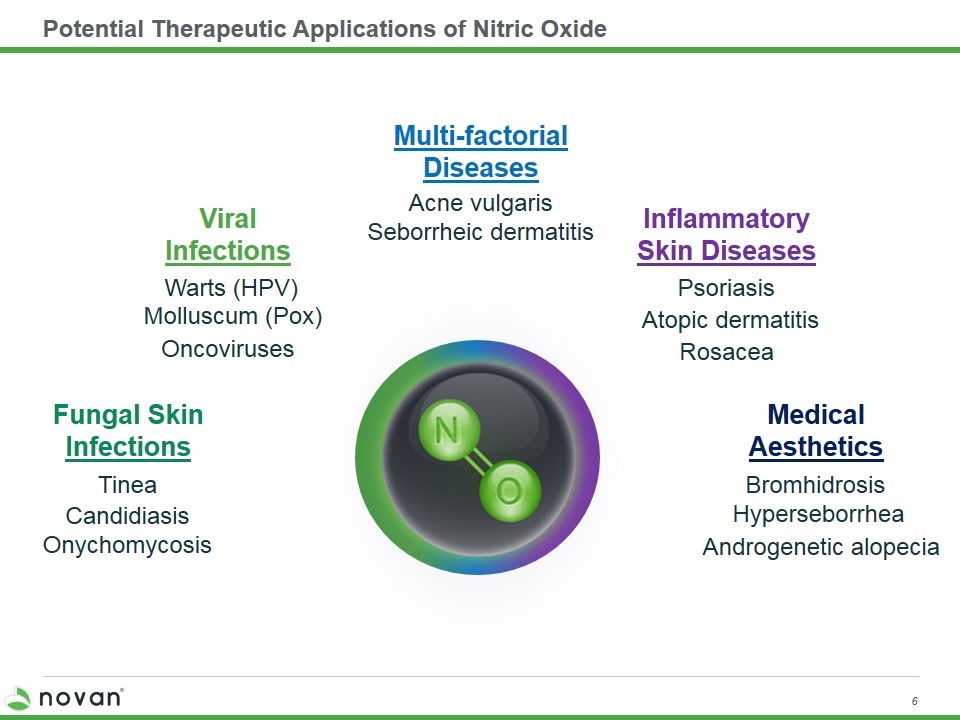
Potential Therapeutic Applications of Nitric Oxide Androgenetic alopecia Bromhidrosis Hyperseborrhea Medical Aesthetics Rosacea Psoriasis Atopic dermatitis Inflammatory Skin Diseases Warts (HPV) Oncoviruses Molluscum (Pox) Viral Infections Multi-factorial Diseases Acne vulgaris Seborrheic dermatitis Onychomycosis Tinea Fungal Skin Infections Candidiasis
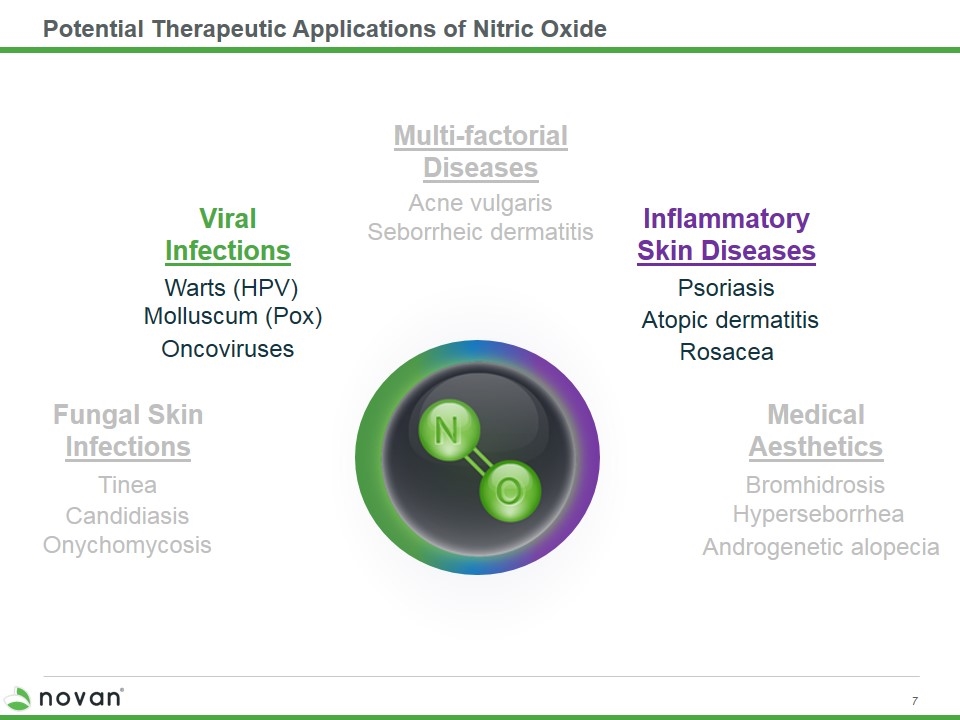
Potential Therapeutic Applications of Nitric Oxide Androgenetic alopecia Bromhidrosis Hyperseborrhea Medical Aesthetics Rosacea Psoriasis Atopic dermatitis Inflammatory Skin Diseases Warts (HPV) Oncoviruses Molluscum (Pox) Viral Infections Multi-factorial Diseases Acne vulgaris Seborrheic dermatitis Onychomycosis Tinea Fungal Skin Infections Candidiasis
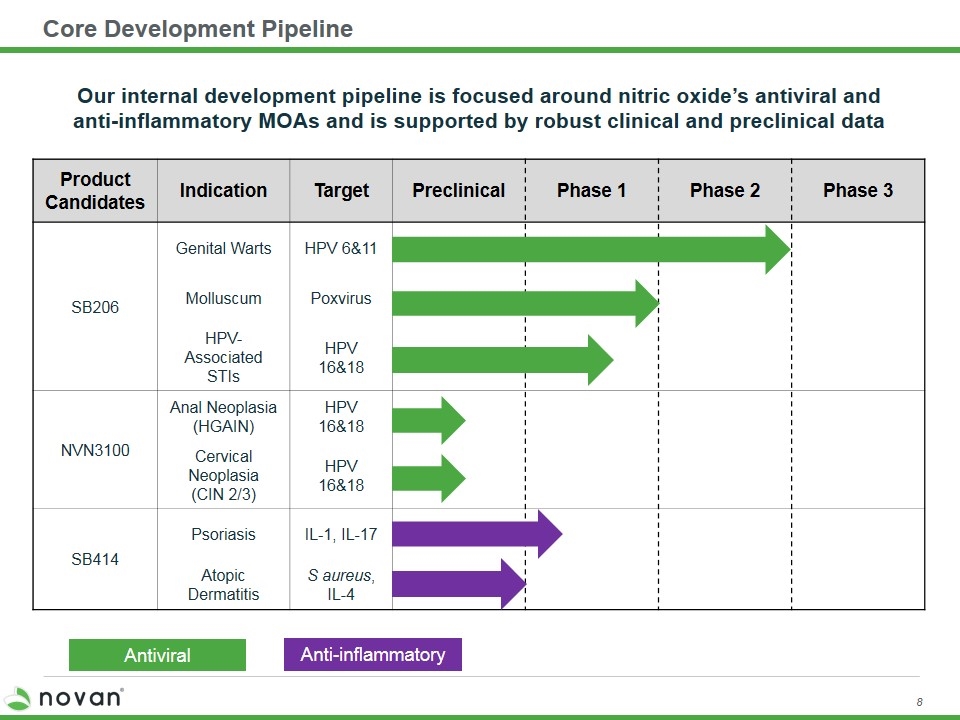
Core Development Pipeline Product Candidates Indication Target Preclinical Phase 1 Phase 2 Phase 3 SB206 Genital Warts HPV 6&11 Molluscum Poxvirus HPV-Associated STIs HPV 16&18 NVN3100 Anal Neoplasia (HGAIN) HPV 16&18 Cervical Neoplasia (CIN 2/3) HPV 16&18 SB414 Psoriasis IL-1, IL-17 Atopic Dermatitis S aureus, IL-4 Anti-inflammatory Antiviral Our internal development pipeline is focused around nitric oxide’s antiviral and anti-inflammatory MOAs and is supported by robust clinical and preclinical data

Research Collaborations Virology Immunology Thomas Broker, PhD Louise Chow, PhD Professors of Biochemistry and Molecular Genetics Richard Gallo, MD, PhD Chief of Division for Dermatology at University of California, San Diego School of Medicine Neil Christensen, PhD Professor of Pathology, and Microbiology and Immunology Associate Chief of Experimental Pathology Medical Director, Jake Gittlen Cancer Research Foundation James Krueger, MD, PhD Senior Attending Physician Director, Milstein Medical Research Program D. Martin Carter Professor in Clinical Investigation Emma Guttman, MD, PhD Associate Professor of Dermatology & Immunology Director of the Laboratory of Inflammatory Skin Diseases at the Icahn School of Medicine

SB206 – Antiviral Program Approximately 20% of human cancers worldwide are caused by oncoviruses
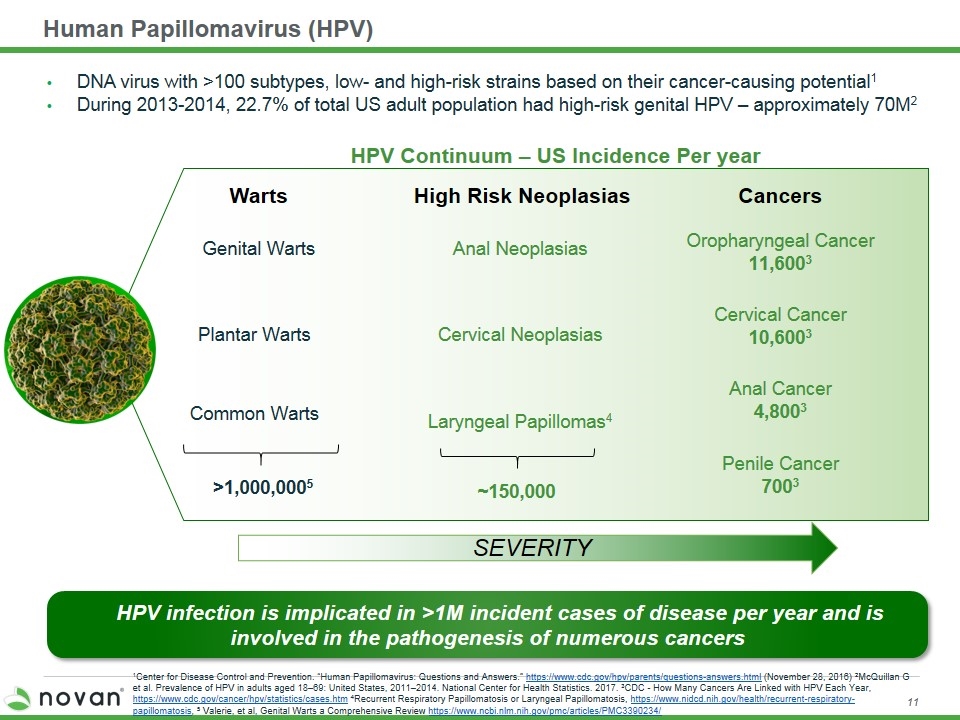
Human Papillomavirus (HPV) Cervical Neoplasias Cervical Cancer 10,6003 Anal Neoplasias Oropharyngeal Cancer 11,6003 Common Warts Genital Warts Plantar Warts DNA virus with >100 subtypes, low- and high-risk strains based on their cancer-causing potential1 During 2013-2014, 22.7% of total US adult population had high-risk genital HPV – approximately 70M2 1Center for Disease Control and Prevention. “Human Papillomavirus: Questions and Answers.” https://www.cdc.gov/hpv/parents/questions-answers.html (November 28, 2016) 2McQuillan G et al. Prevalence of HPV in adults aged 18–69: United States, 2011–2014. National Center for Health Statistics. 2017. 3CDC - How Many Cancers Are Linked with HPV Each Year, https://www.cdc.gov/cancer/hpv/statistics/cases.htm 4Recurrent Respiratory Papillomatosis or Laryngeal Papillomatosis, https://www.nidcd.nih.gov/health/recurrent-respiratory-papillomatosis, 5 Valerie, et al, Genital Warts a Comprehensive Review https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3390234/ HPV Continuum – US Incidence Per year Penile Cancer 7003 Laryngeal Papillomas4 Anal Cancer 4,8003 Warts High Risk Neoplasias Cancers SEVERITY ~150,000 >1,000,0005 HPV infection is implicated in >1M incident cases of disease per year and is involved in the pathogenesis of numerous cancers
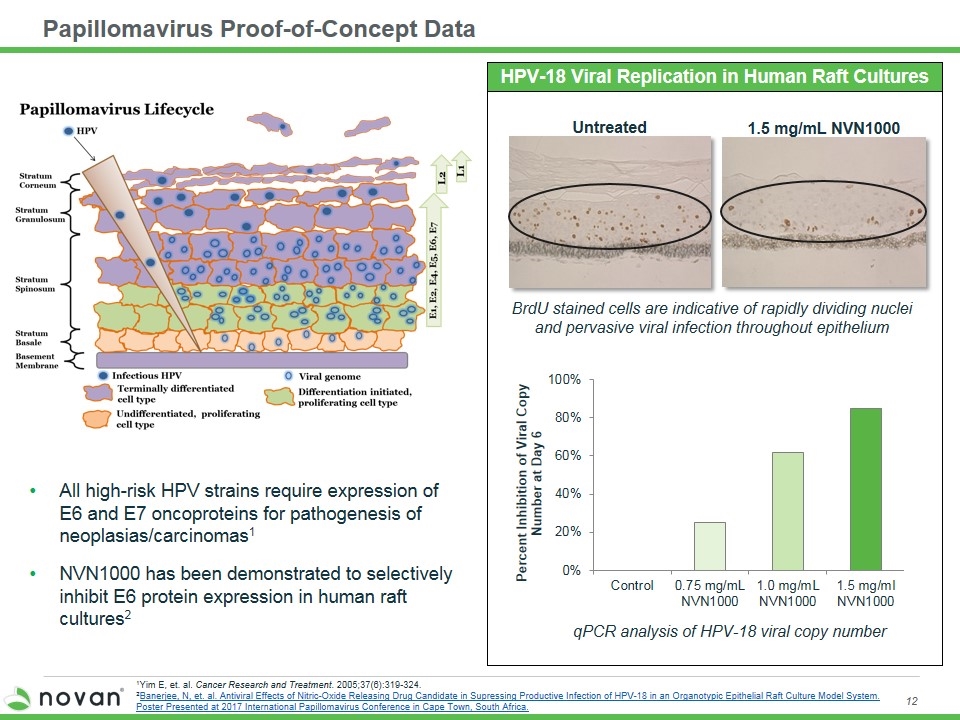
Papillomavirus Proof-of-Concept Data HPV-18 Viral Replication in Human Raft Cultures Untreated Control qPCR analysis of HPV-18 viral copy number BrdU stained cells are indicative of rapidly dividing nuclei and pervasive viral infection throughout epithelium 1.5 mg/mL NVN1000 NVN1000 All high-risk HPV strains require expression of E6 and E7 oncoproteins for pathogenesis of neoplasias/carcinomas1 NVN1000 has been demonstrated to selectively inhibit E6 protein expression in human raft cultures2 1Yim E, et. al. Cancer Research and Treatment. 2005;37(6):319-324. 2Banerjee, N, et. al. Antiviral Effects of Nitric-Oxide Releasing Drug Candidate in Supressing Productive Infection of HPV-18 in an Organotypic Epithelial Raft Culture Model System. Poster Presented at 2017 International Papillomavirus Conference in Cape Town, South Africa.
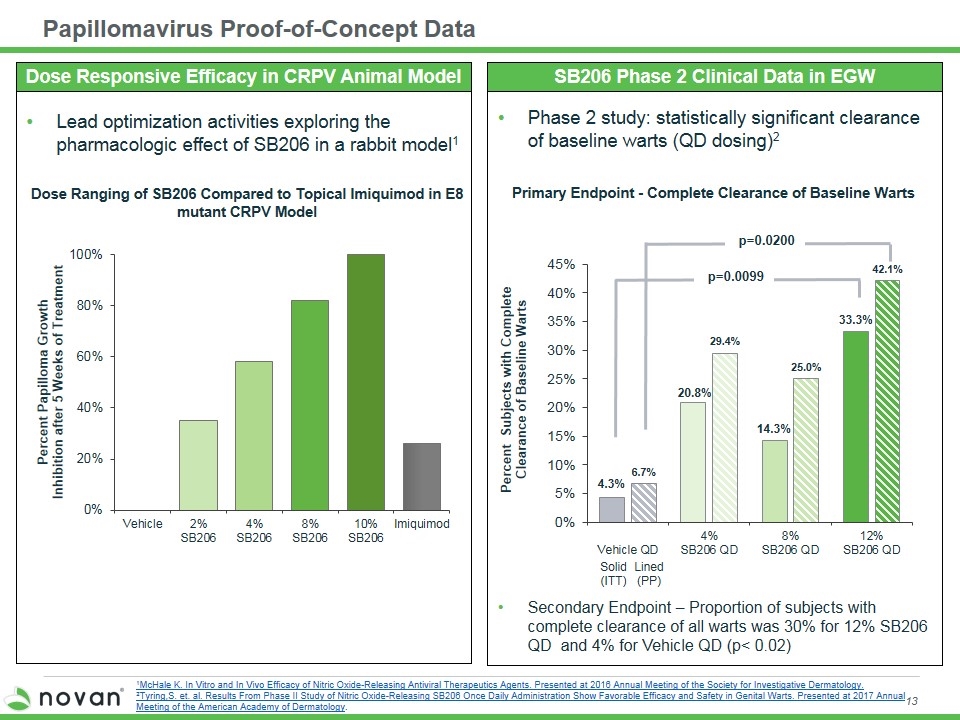
Phase 2 study: statistically significant clearance of baseline warts (QD dosing)2 Secondary Endpoint – Proportion of subjects with complete clearance of all warts was 30% for 12% SB206 QD and 4% for Vehicle QD (p< 0.02) Papillomavirus Proof-of-Concept Data Primary Endpoint - Complete Clearance of Baseline Warts SB206 Phase 2 Clinical Data in EGW Dose Ranging of SB206 Compared to Topical Imiquimod in E8 mutant CRPV Model Lead optimization activities exploring the pharmacologic effect of SB206 in a rabbit model1 Dose Responsive Efficacy in CRPV Animal Model Solid (ITT) Lined (PP) p=0.0200 p=0.0099 1McHale K. In Vitro and In Vivo Efficacy of Nitric Oxide-Releasing Antiviral Therapeutics Agents. Presented at 2016 Annual Meeting of the Society for Investigative Dermatology. 2Tyring,S. et. al. Results From Phase II Study of Nitric Oxide-Releasing SB206 Once Daily Administration Show Favorable Efficacy and Safety in Genital Warts. Presented at 2017 Annual Meeting of the American Academy of Dermatology.
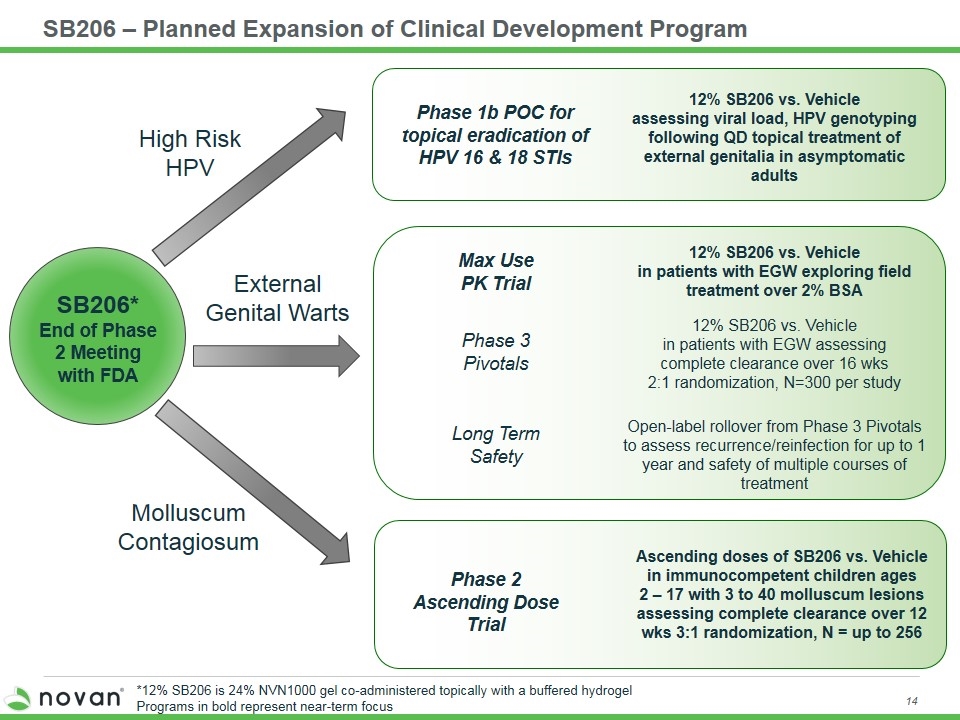
SB206 – Planned Expansion of Clinical Development Program SB206* End of Phase 2 Meeting with FDA Max Use PK Trial Phase 3 Pivotals Long Term Safety Phase 2 Ascending Dose Trial Phase 1b POC for topical eradication of HPV 16 & 18 STIs 12% SB206 vs. Vehicle assessing viral load, HPV genotyping following QD topical treatment of external genitalia in asymptomatic adults 12% SB206 vs. Vehicle in patients with EGW exploring field treatment over 2% BSA 12% SB206 vs. Vehicle in patients with EGW assessing complete clearance over 16 wks 2:1 randomization, N=300 per study Open-label rollover from Phase 3 Pivotals to assess recurrence/reinfection for up to 1 year and safety of multiple courses of treatment Molluscum Contagiosum External Genital Warts High Risk HPV Ascending doses of SB206 vs. Vehicle in immunocompetent children ages 2 – 17 with 3 to 40 molluscum lesions assessing complete clearance over 12 wks 3:1 randomization, N = up to 256 *12% SB206 is 24% NVN1000 gel co-administered topically with a buffered hydrogel Programs in bold represent near-term focus
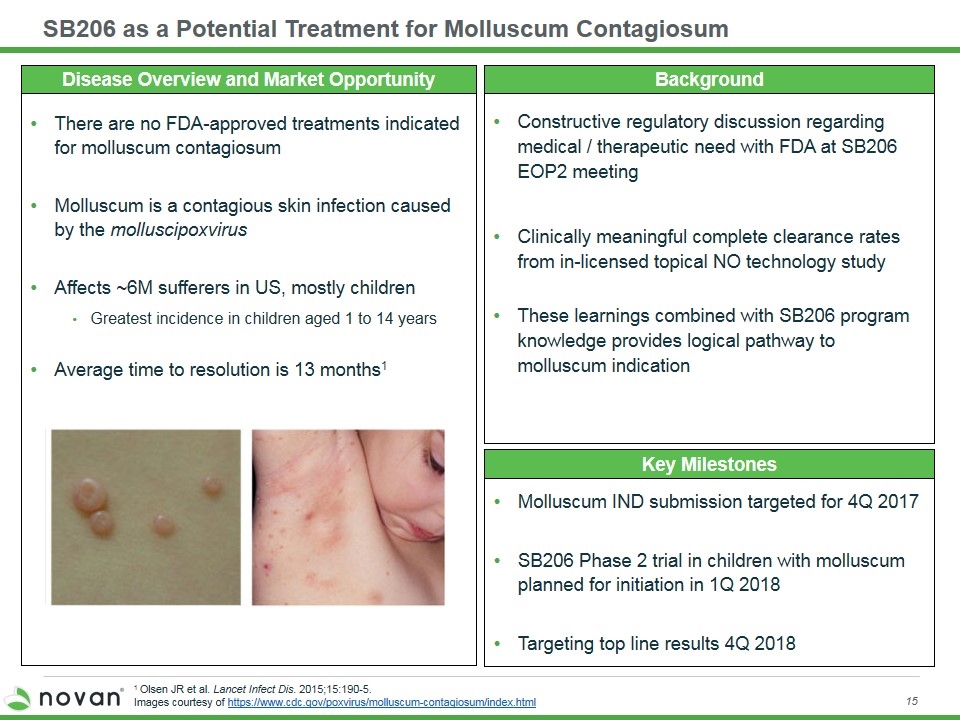
SB206 as a Potential Treatment for Molluscum Contagiosum Key Milestones Background There are no FDA-approved treatments indicated for molluscum contagiosum Molluscum is a contagious skin infection caused by the molluscipoxvirus Affects ~6M sufferers in US, mostly children Greatest incidence in children aged 1 to 14 years Average time to resolution is 13 months1 Molluscum IND submission targeted for 4Q 2017 SB206 Phase 2 trial in children with molluscum planned for initiation in 1Q 2018 Targeting top line results 4Q 2018 1 Olsen JR et al. Lancet Infect Dis. 2015;15:190-5. Images courtesy of https://www.cdc.gov/poxvirus/molluscum-contagiosum/index.html Constructive regulatory discussion regarding medical / therapeutic need with FDA at SB206 EOP2 meeting Clinically meaningful complete clearance rates from in-licensed topical NO technology study These learnings combined with SB206 program knowledge provides logical pathway to molluscum indication Disease Overview and Market Opportunity
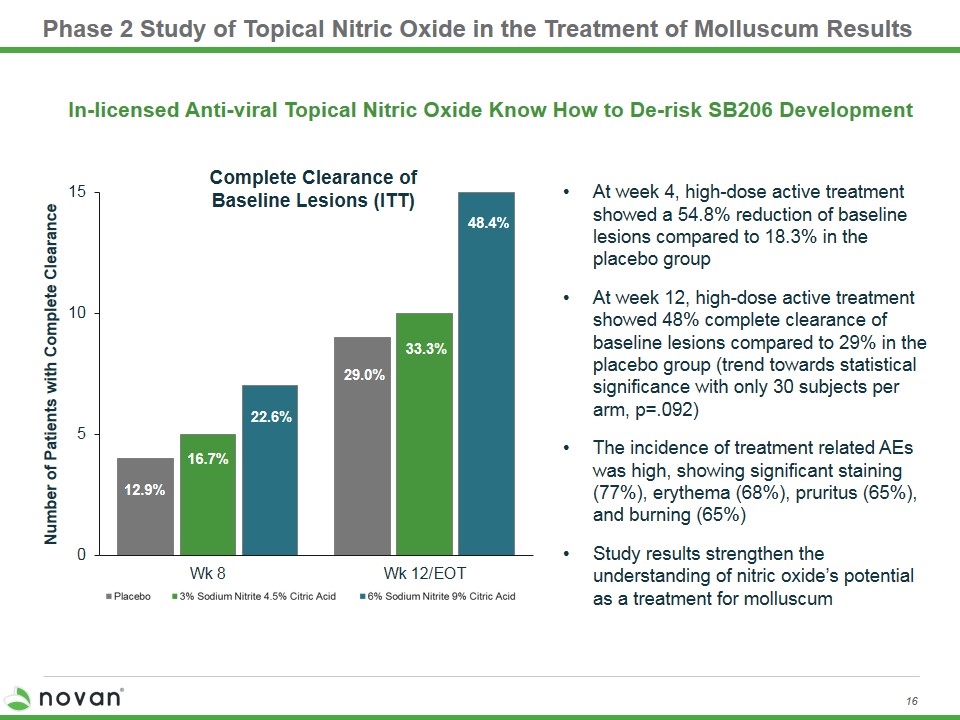
Phase 2 Study of Topical Nitric Oxide in the Treatment of Molluscum Results 12.9% 33.3% 48.4% 29.0% Number of Patients with Complete Clearance Complete Clearance of Baseline Lesions (ITT) 16.7% 22.6% In-licensed Anti-viral Topical Nitric Oxide Know How to De-risk SB206 Development At week 4, high-dose active treatment showed a 54.8% reduction of baseline lesions compared to 18.3% in the placebo group At week 12, high-dose active treatment showed 48% complete clearance of baseline lesions compared to 29% in the placebo group (trend towards statistical significance with only 30 subjects per arm, p=.092) The incidence of treatment related AEs was high, showing significant staining (77%), erythema (68%), pruritus (65%), and burning (65%) Study results strengthen the understanding of nitric oxide’s potential as a treatment for molluscum
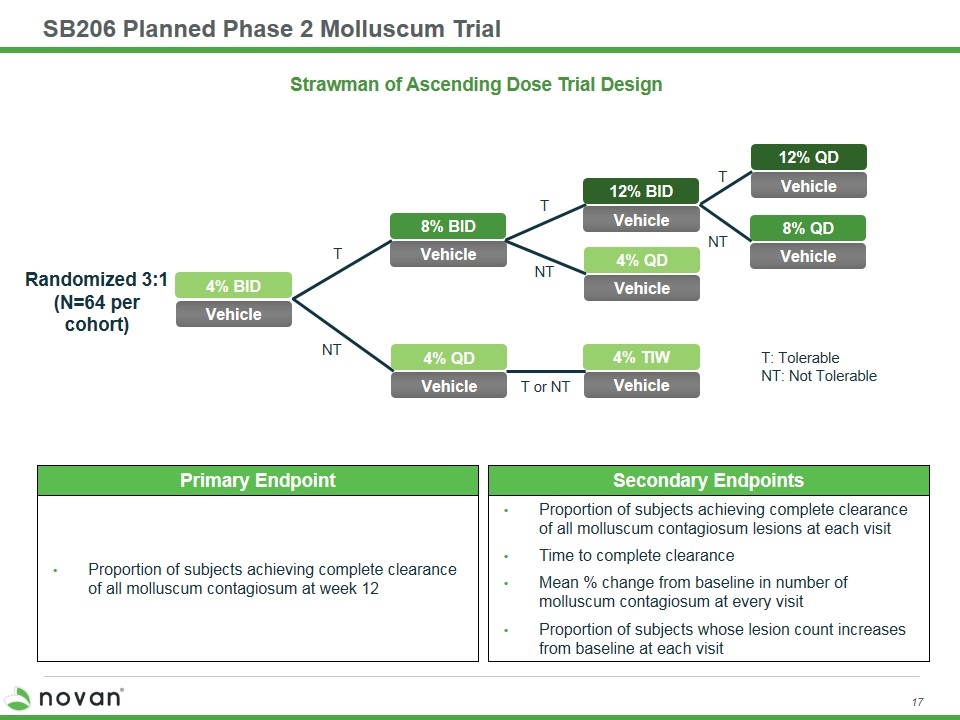
SB206 Planned Phase 2 Molluscum Trial Strawman of Ascending Dose Trial Design Randomized 3:1 (N=64 per cohort) T T T NT NT NT T or NT T: Tolerable NT: Not Tolerable 4% BID Vehicle 8% BID Vehicle 4% QD Vehicle 4% TIW Vehicle 12% BID Vehicle 4% QD Vehicle 12% QD Vehicle 8% QD Vehicle Primary Endpoint Proportion of subjects achieving complete clearance of all molluscum contagiosum at week 12 Secondary Endpoints Proportion of subjects achieving complete clearance of all molluscum contagiosum lesions at each visit Time to complete clearance Mean % change from baseline in number of molluscum contagiosum at every visit Proportion of subjects whose lesion count increases from baseline at each visit
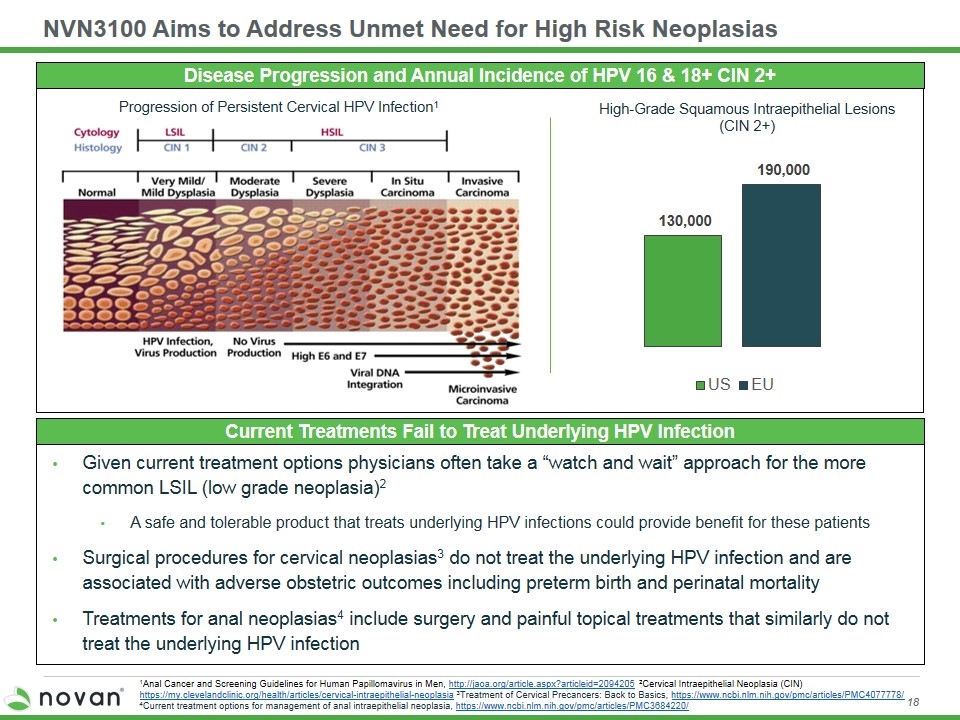
NVN3100 Aims to Address Unmet Need for High Risk Neoplasias Disease Progression and Annual Incidence of HPV 16 & 18+ CIN 2+ Current Treatments Fail to Treat Underlying HPV Infection Given current treatment options physicians often take a “watch and wait” approach for the more common LSIL (low grade neoplasia)2 A safe and tolerable product that treats underlying HPV infections could provide benefit for these patients Surgical procedures for cervical neoplasias3 do not treat the underlying HPV infection and are associated with adverse obstetric outcomes including preterm birth and perinatal mortality Treatments for anal neoplasias4 include surgery and painful topical treatments that similarly do not treat the underlying HPV infection 1Anal Cancer and Screening Guidelines for Human Papillomavirus in Men, http://jaoa.org/article.aspx?articleid=2094205 2Cervical Intraepithelial Neoplasia (CIN) https://my.clevelandclinic.org/health/articles/cervical-intraepithelial-neoplasia 3Treatment of Cervical Precancers: Back to Basics, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4077778/ 4Current treatment options for management of anal intraepithelial neoplasia, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3684220/ Progression of Persistent Cervical HPV Infection1

SB414 Inflammatory Skin Disease Program
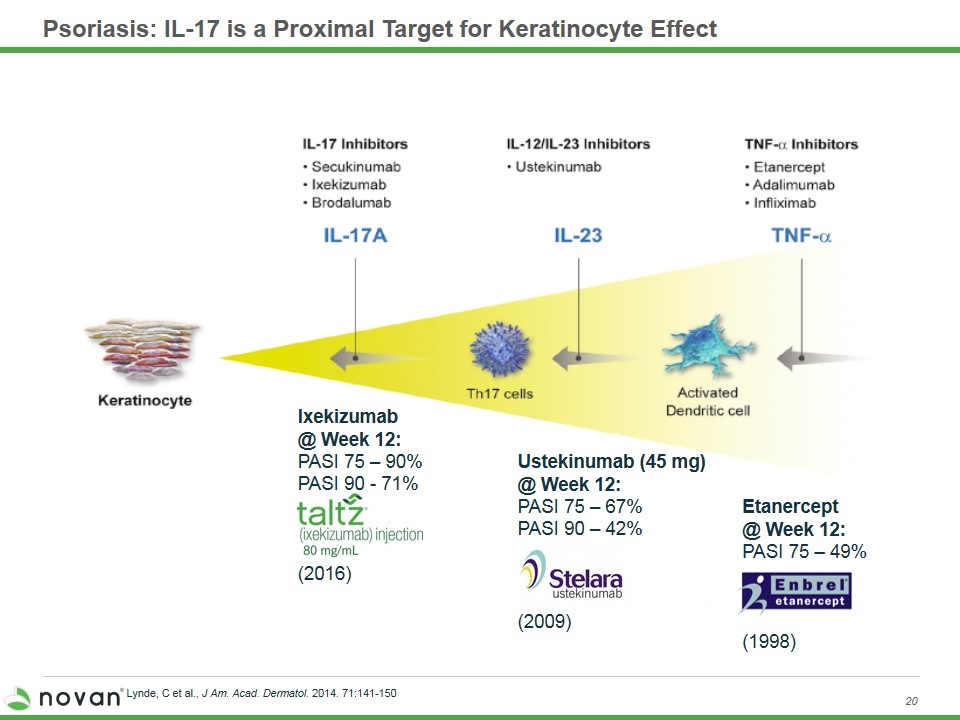
Psoriasis: IL-17 is a Proximal Target for Keratinocyte Effect Lynde, C et al., J Am. Acad. Dermatol. 2014. 71:141-150 Ixekizumab @ Week 12: PASI 75 – 90% PASI 90 - 71% (2016) Ustekinumab (45 mg) @ Week 12: PASI 75 – 67% PASI 90 – 42% (2009) Etanercept @ Week 12: PASI 75 – 49% (1998)
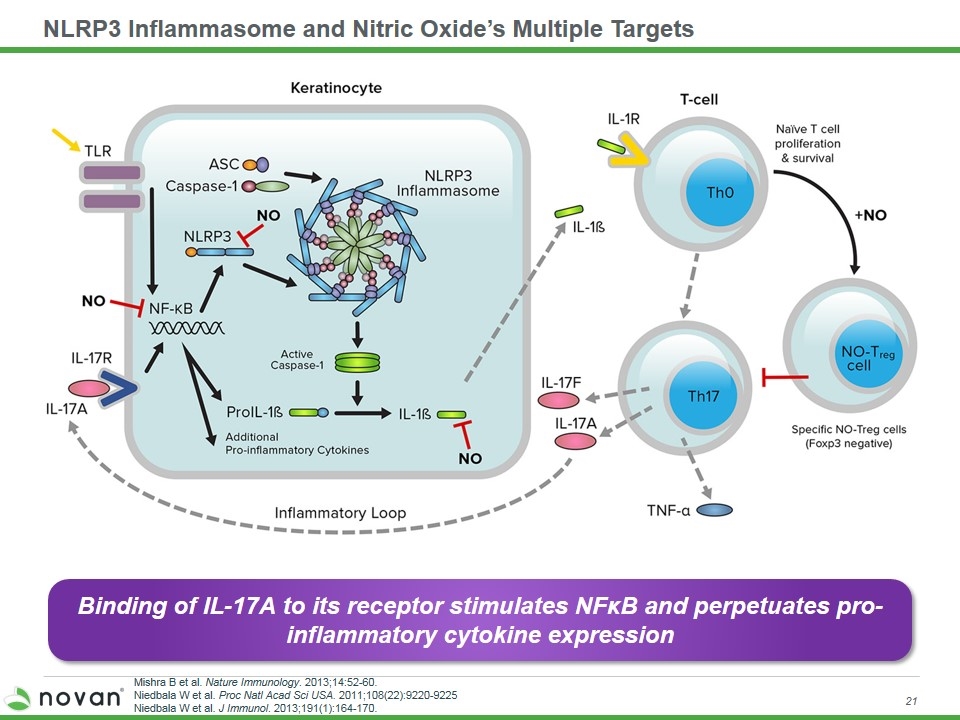
NLRP3 Inflammasome and Nitric Oxide’s Multiple Targets Mishra B et al. Nature Immunology. 2013;14:52-60. Niedbala W et al. Proc Natl Acad Sci USA. 2011;108(22):9220-9225 Niedbala W et al. J Immunol. 2013;191(1):164-170. Binding of IL-17A to its receptor stimulates NFκB and perpetuates pro-inflammatory cytokine expression
Psoriasis Market and SB414 Value Proposition Market and Disease Overview The total psoriasis market is valued at $5.4Bn with a forecasted growth to $9.1Bn in 20251 Psoriasis market is populated by a number of new entrants at the “severe” end of the disease spectrum IL-17 products are expected to account for 20% of this market and to command the top 5 brand positions by 20251 ~6M in US suffer from mild-to-moderate plaque psoriasis, and are treated first-line with topical therapies in lieu of the systemic/biologic therapies2 1Diseases Insights Psoriasis United States. IMS Health. 2016. 2Psoriasis Market Research Report, IMS Consulting Group. 2015. 3American Academy of Dermatology . "Psoriasis." https://www.aad.org/media/stats/conditions/psoriasis ( Nov. 15, 2016 ). SB414 is well positioned to leverage the investment and mechanistic understanding IL-17 antibodies seckuinumab and ixekizumab SB414 has the potential to be the first and only, topical, non-steroidal nitric oxide-based IL-17 inhibitor 2.8M topical scripts per annum3 3 drugs account for 85% of sales (Clobetasol, Taclonex, Calioptriene)2 Corticosteroid products have side effects and are not well-suited for chronic use³ Given the high price tag of biologic therapies, managed care providers have a common requirement to fail/step-through topical regimens prior to biologic therapy Non-steroidal topical therapy with an excellent safety profile has the potential to be used in conjunction with biologics Value Proposition for New Topical Mild-to-Moderate Plaque Psoriasis
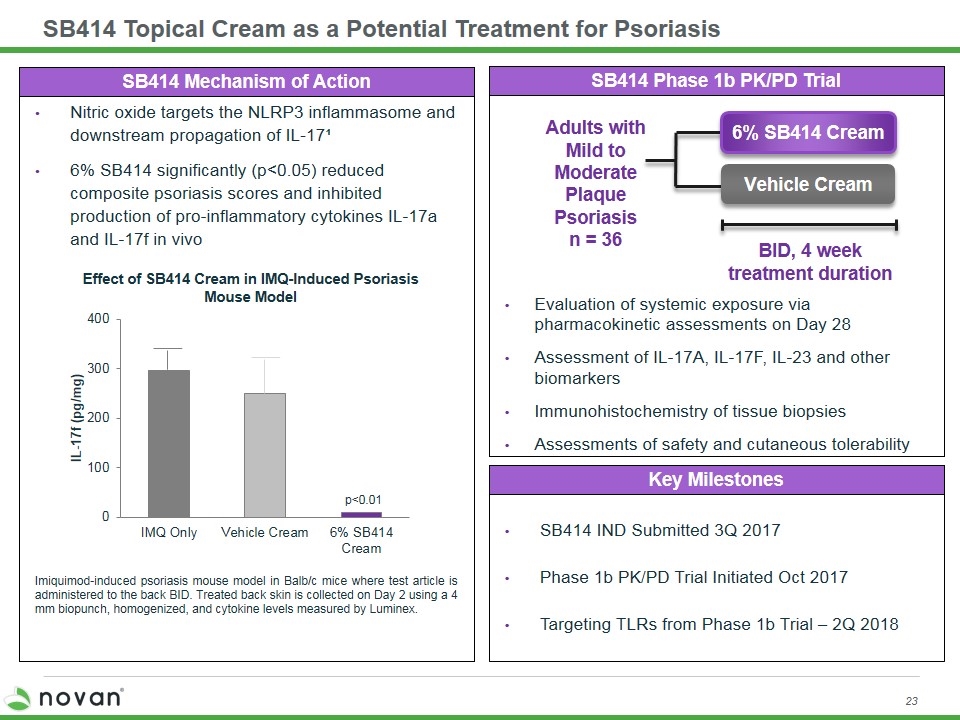
SB414 Topical Cream as a Potential Treatment for Psoriasis SB414 Phase 1b PK/PD Trial SB414 Mechanism of Action Evaluation of systemic exposure via pharmacokinetic assessments on Day 28 Assessment of IL-17A, IL-17F, IL-23 and other biomarkers Immunohistochemistry of tissue biopsies Assessments of safety and cutaneous tolerability Nitric oxide targets the NLRP3 inflammasome and downstream propagation of IL-17¹ 6% SB414 significantly (p<0.05) reduced composite psoriasis scores and inhibited production of pro-inflammatory cytokines IL-17a and IL-17f in vivo BID, 4 week treatment duration Adults with Mild to Moderate Plaque Psoriasis n = 36 6% SB414 Cream Vehicle Cream Key Milestones SB414 IND Submitted 3Q 2017 Phase 1b PK/PD Trial Initiated Oct 2017 Targeting TLRs from Phase 1b Trial – 2Q 2018 Imiquimod-induced psoriasis mouse model in Balb/c mice where test article is administered to the back BID. Treated back skin is collected on Day 2 using a 4 mm biopunch, homogenized, and cytokine levels measured by Luminex. Effect of SB414 Cream in IMQ-Induced Psoriasis Mouse Model
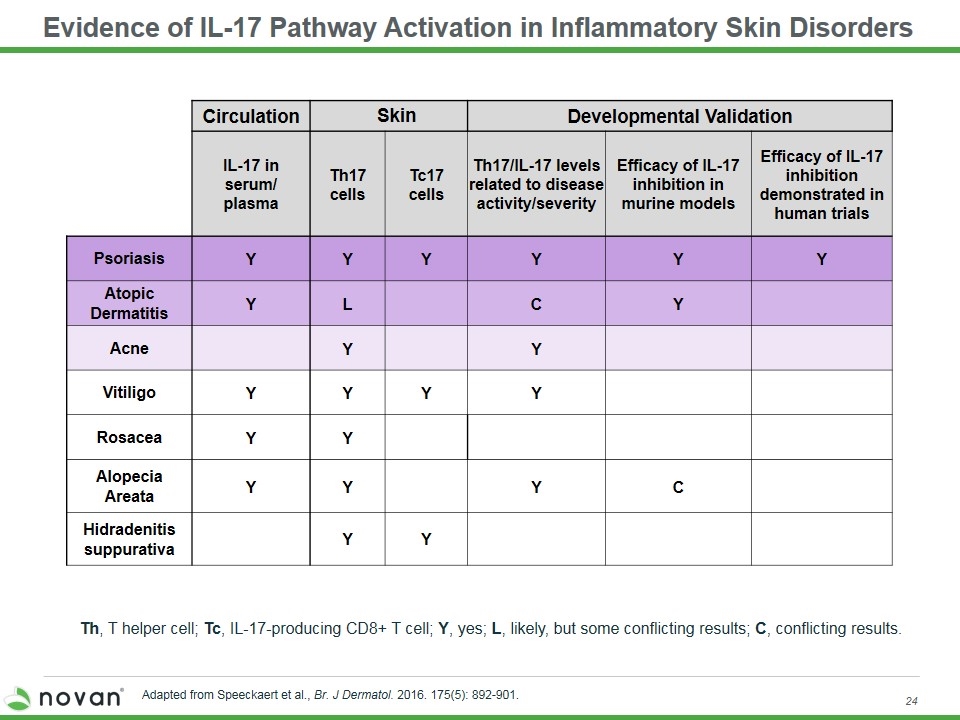
Evidence of IL-17 Pathway Activation in Inflammatory Skin Disorders Th, T helper cell; Tc, IL-17-producing CD8+ T cell; Y, yes; L, likely, but some conflicting results; C, conflicting results. Circulation Skin Developmental Validation IL-17 in serum/ plasma Th17 cells Tc17 cells Th17/IL-17 levels related to disease activity/severity Efficacy of IL-17 inhibition in murine models Efficacy of IL-17 inhibition demonstrated in human trials Psoriasis Y Y Y Y Y Y Atopic Dermatitis Y L C Y Acne Y Y Vitiligo Y Y Y Y Rosacea Y Y Alopecia Areata Y Y Y C Hidradenitis suppurativa Y Y Adapted from Speeckaert et al., Br. J Dermatol. 2016. 175(5): 892-901.
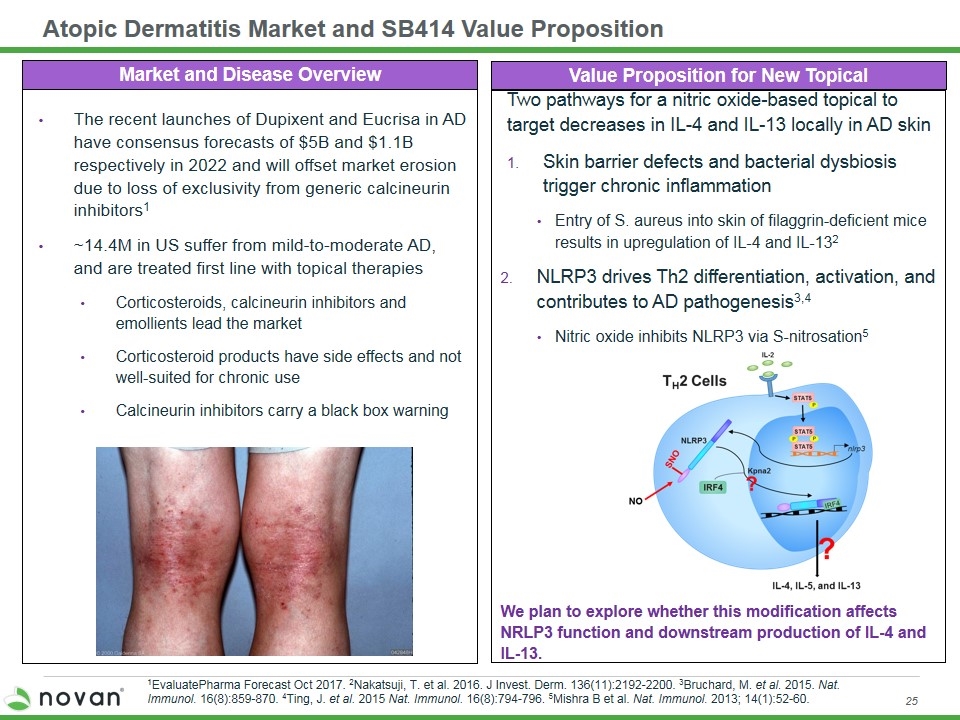
Two pathways for a nitric oxide-based topical to target decreases in IL-4 and IL-13 locally in AD skin Skin barrier defects and bacterial dysbiosis trigger chronic inflammation Entry of S. aureus into skin of filaggrin-deficient mice results in upregulation of IL-4 and IL-132 NLRP3 drives Th2 differentiation, activation, and contributes to AD pathogenesis3,4 Nitric oxide inhibits NLRP3 via S-nitrosation5 We plan to explore whether this modification affects NRLP3 function and downstream production of IL-4 and IL-13. Atopic Dermatitis Market and SB414 Value Proposition Market and Disease Overview The recent launches of Dupixent and Eucrisa in AD have consensus forecasts of $5B and $1.1B respectively in 2022 and will offset market erosion due to loss of exclusivity from generic calcineurin inhibitors1 ~14.4M in US suffer from mild-to-moderate AD, and are treated first line with topical therapies Corticosteroids, calcineurin inhibitors and emollients lead the market Corticosteroid products have side effects and not well-suited for chronic use Calcineurin inhibitors carry a black box warning Value Proposition for New Topical 1EvaluatePharma Forecast Oct 2017. 2Nakatsuji, T. et al. 2016. J Invest. Derm. 136(11):2192-2200. 3Bruchard, M. et al. 2015. Nat. Immunol. 16(8):859-870. 4Ting, J. et al. 2015 Nat. Immunol. 16(8):794-796. 5Mishra B et al. Nat. Immunol. 2013; 14(1):52-60.
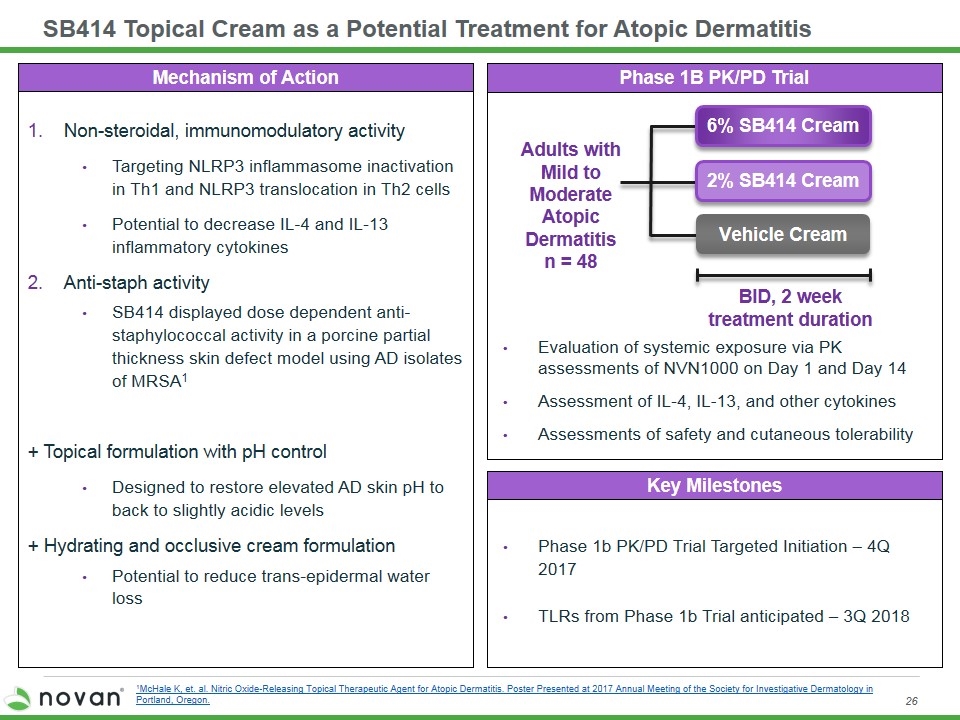
SB414 Topical Cream as a Potential Treatment for Atopic Dermatitis Mechanism of Action Key Milestones Phase 1b PK/PD Trial Targeted Initiation – 4Q 2017 TLRs from Phase 1b Trial anticipated – 3Q 2018 Evaluation of systemic exposure via PK assessments of NVN1000 on Day 1 and Day 14 Assessment of IL-4, IL-13, and other cytokines Assessments of safety and cutaneous tolerability Phase 1B PK/PD Trial BID, 2 week treatment duration Adults with Mild to Moderate Atopic Dermatitis n = 48 Vehicle Cream 2% SB414 Cream 6% SB414 Cream Non-steroidal, immunomodulatory activity Targeting NLRP3 inflammasome inactivation in Th1 and NLRP3 translocation in Th2 cells Potential to decrease IL-4 and IL-13 inflammatory cytokines Anti-staph activity SB414 displayed dose dependent anti-staphylococcal activity in a porcine partial thickness skin defect model using AD isolates of MRSA1 + Topical formulation with pH control Designed to restore elevated AD skin pH to back to slightly acidic levels + Hydrating and occlusive cream formulation Potential to reduce trans-epidermal water loss 1McHale K, et. al. Nitric Oxide-Releasing Topical Therapeutic Agent for Atopic Dermatitis. Poster Presented at 2017 Annual Meeting of the Society for Investigative Dermatology in Portland, Oregon.
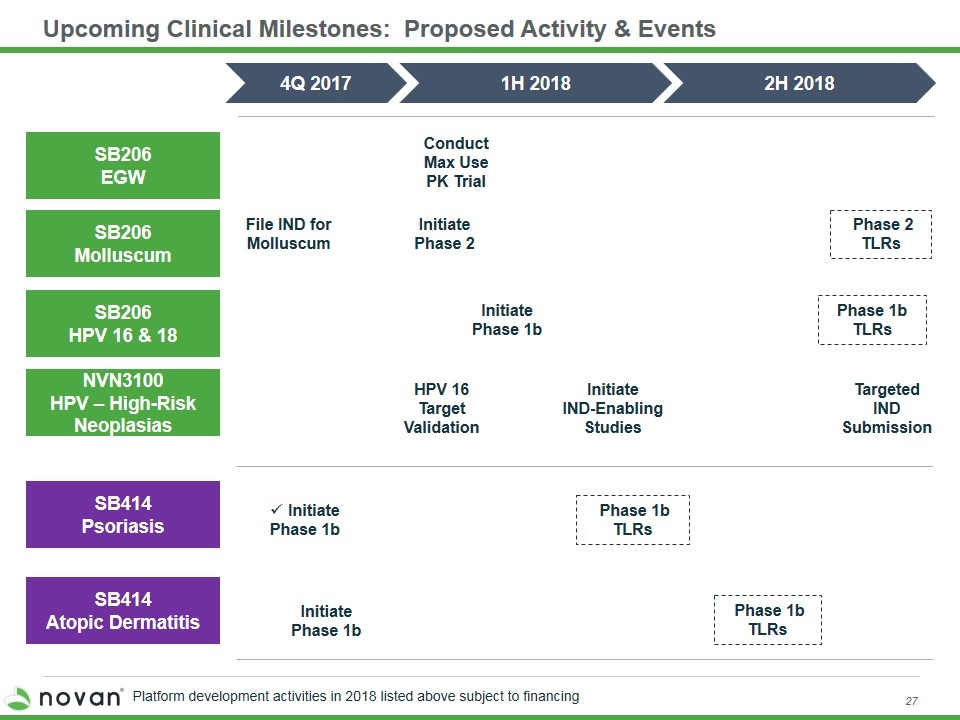
Upcoming Clinical Milestones: Proposed Activity & Events SB206 EGW SB206 Molluscum SB414 Psoriasis 4Q 2017 1H 2018 SB414 Atopic Dermatitis Initiate Phase 2 Conduct Max Use PK Trial Initiate Phase 1b Initiate Phase 1b Phase 1b TLRs SB206 HPV 16 & 18 NVN3100 HPV – High-Risk Neoplasias Initiate Phase 1b File IND for Molluscum Initiate IND-Enabling Studies HPV 16 Target Validation 2H 2018 Phase 1b TLRs Phase 2 TLRs Targeted IND Submission Phase 1b TLRs Platform development activities in 2018 listed above subject to financing
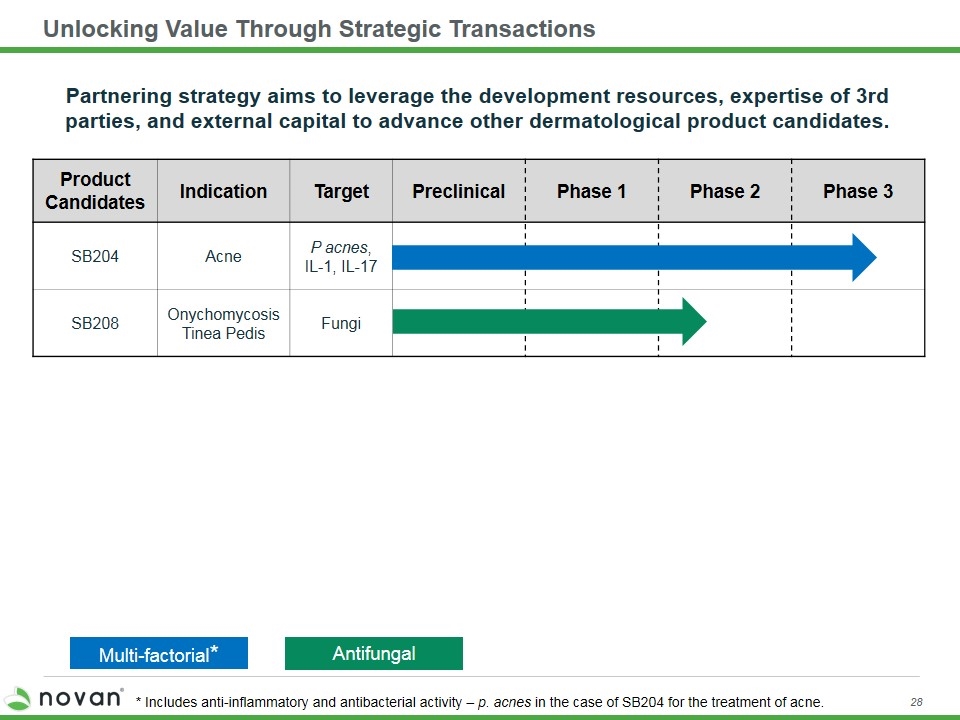
Unlocking Value Through Strategic Transactions Multi-factorial* Antifungal * Includes anti-inflammatory and antibacterial activity – p. acnes in the case of SB204 for the treatment of acne. Product Candidates Indication Target Preclinical Phase 1 Phase 2 Phase 3 SB204 Acne P acnes, IL-1, IL-17 SB208 Onychomycosis Tinea Pedis Fungi Partnering strategy aims to leverage the development resources, expertise of 3rd parties, and external capital to advance other dermatological product candidates.

SB204 Acne Program

SB204 Guidance Meeting with FDA Additional Data Shared with FDA: Long-term safety trial results (NI-AC303) showing sustained treatment benefit Analyses in relevant sub-populations (adolescents, severe subjects only) Mechanism of action data showing effect of SB204 on IL-1b in ex vivo human skin model Meeting Outcome: FDA recommended one additional pivotal study for the purposes of replication and interpretation of clinical trial findings The success criteria for IGA should be the same as the one used in the completed Phase 3 trials Based on FDA feedback, an additional Phase 3 trial should be completed prior to NDA submission No further preclinical or clinical safety studies required for NDA – safety population of >2600 patients sufficient for submission of a new molecular entity

Acne Path Forward Goal: To complete a Phase 3 pivotal trial in moderate-to-severe acne patients for the purposes of replication and interpretation of clinical trial findings Potential Partnership Models: Licensing/Collaborative Partnerships Discussions with strategic partners (corporates) ongoing Both US and ex-US market interest Third Party Financing: Capital and execution risks of Phase 3 pivotal trial borne by third party Novan retains the rights to the asset at a pre-specified multiple of capital expenditure Timeline: Internal goal to execute a term sheet with third party or strategic in 4Q 2017
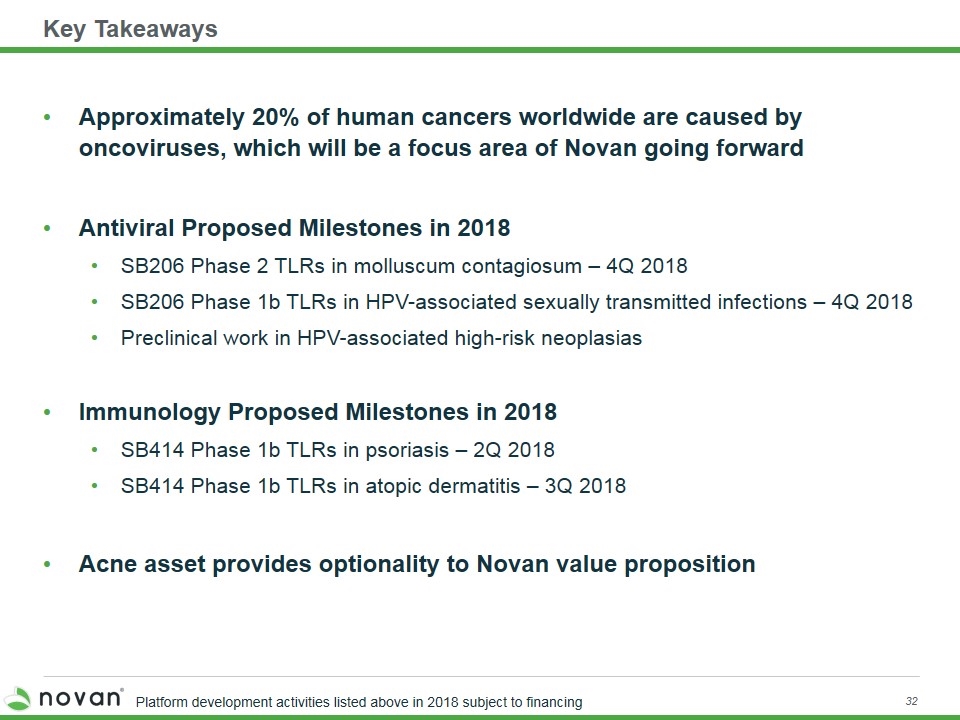
Key Takeaways Approximately 20% of human cancers worldwide are caused by oncoviruses, which will be a focus area of Novan going forward Antiviral Proposed Milestones in 2018 SB206 Phase 2 TLRs in molluscum contagiosum – 4Q 2018 SB206 Phase 1b TLRs in HPV-associated sexually transmitted infections – 4Q 2018 Preclinical work in HPV-associated high-risk neoplasias Immunology Proposed Milestones in 2018 SB414 Phase 1b TLRs in psoriasis – 2Q 2018 SB414 Phase 1b TLRs in atopic dermatitis – 3Q 2018 Acne asset provides optionality to Novan value proposition Platform development activities listed above in 2018 subject to financing
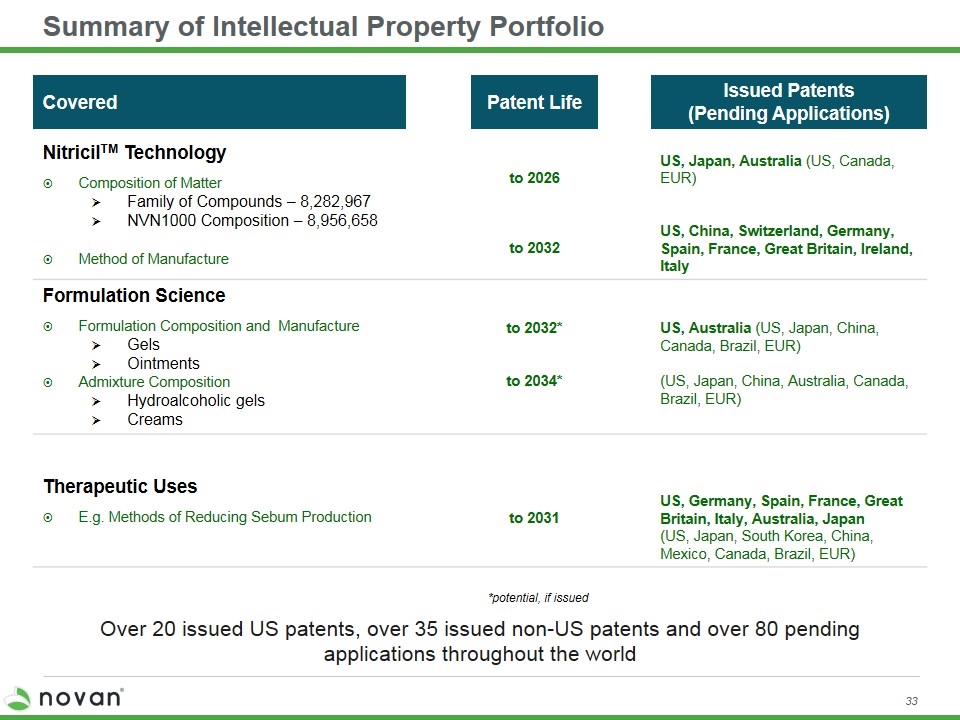
Summary of Intellectual Property Portfolio Covered Patent Life Issued Patents (Pending Applications) NitricilTM Technology Composition of Matter Family of Compounds – 8,282,967 NVN1000 Composition – 8,956,658 Method of Manufacture to 2026 to 2032 US, Japan, Australia (US, Canada, EUR) US, China, Switzerland, Germany, Spain, France, Great Britain, Ireland, Italy Formulation Science Formulation Composition and Manufacture Gels Ointments Admixture Composition Hydroalcoholic gels Creams to 2032* to 2034* US, Australia (US, Japan, China, Canada, Brazil, EUR) (US, Japan, China, Australia, Canada, Brazil, EUR) Therapeutic Uses E.g. Methods of Reducing Sebum Production to 2031 US, Germany, Spain, France, Great Britain, Italy, Australia, Japan (US, Japan, South Korea, China, Mexico, Canada, Brazil, EUR) *potential, if issued Over 20 issued US patents, over 35 issued non-US patents and over 80 pending applications throughout the world
































