Exhibit 99.1

Corporate Presentation January 2021

Neos Therapeutics I January 2021 Additional Information about the Proposed Merger Transaction This presentation discusses the proposed merger transaction pursuant to the terms of the Agreement and Plan of Merger, dated as of December 10, 2020, by and among Neos Therapeutics, Inc. (“Neos”), Aytu Bioscience Inc. (“ Aytu ”), and Neutron Acquisition Sub, Inc. In connection with the proposed merger transaction, Aytu is expected to file with the United States Securities and Exchange Commission (the “SEC”) a registration statement on Form S - 4 that will include a joint proxy statement of Aytu and Neos that also constitutes a prospectus of Aytu , which joint proxy statement/prospectus will be mailed or otherwise disseminated to Aytu stockholders and Neos stockholders when it becomes available. Aytu and Neos also plan to file other relevant documents with the SEC regarding the proposed merger transaction. INVESTORS ARE URGED TO READ THE JOINT PROXY STATEMENT/PROSPECTUS AND OTHER RELEVANT DOCUMENTS FILED WITH THE SEC IF AND WHEN THE Y BECOME AVAILABLE BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION ABOUT THE PROPOSED MERGER TRANSACTION. You may obtain a free copy of the joint proxy statement/prospectus and other relevant documents (if and when they become avai lab le) filed by Aytu or Neos with the SEC at the SEC’s website at www.sec.gov. Copies of the documents filed by Aytu with the SEC will be available free of charge on Aytu’s website at www.aytubio.com or by contacting Aytu’s Investor Relations at james@haydenir.com. Copies of the documents filed by Neos with the SEC will be available free of charge on Neos’ website at www. investors.neostx.com or by contacting Neos’ Investor Relations at (972) 408 - 1300. Certain Information Regarding Participants Neos and Aytu and their respective directors, executive officers and other members of management and employees may be deemed to be particip an ts in the solicitation of proxies in respect of the proposed merger transaction. You can find information about Neos’ executive officer s a nd directors in Neos’ definitive proxy statement filed with the SEC on April 21, 2020 in connection with Neos’ 2020 annual meeting of stockholders. You can fi nd information about Aytu’s executive officers and directors in Aytu’s definitive proxy statement filed with the SEC on March 4, 2020 in connection with Aytu’s 2020 annual meeting of stockholders. Additional information regarding the interests of such potential participants will be included in the joint pro xy statement/prospectus and other relevant documents filed with the SEC if and when they become available. You may obtain free copies of these documents from N eos or Aytu using the sources indicated above. No Offer or Solicitation This presentation does not constitute an offer to sell, or the solicitation of an offer to buy, any securities, nor a solicit ati on of any vote or approval with respect to the proposed merger transaction or otherwise. No offering of securities shall be made except by means of a prospectus meet ing the requirements of Section 10 of the Securities Act of 1933, as amended (the “Securities Act”) and otherwise in accordance with applicable law. 2

Neos Therapeutics I January 2021 Forward - Looking Statements This presentation and other related materials contain forward - looking statements within the meaning of the Private Securities Li tigation Reform Act of 1995 and other federal securities laws, including, but not limited to, statements concerning: the expected timetable for completing the prop ose d merger transaction, the results, effects, benefits and synergies of the proposed merger transaction, future, opportunities for the combined company, future fi nan cial performance and condition, the executive and board structure of with Aytu ; the ability of Neos to successfully commercialize Adzenys XR - ODT®, Cotempla XR - ODT®, Adzenys ER® (the “Approved ADHD Products”) and its generic Tussionex®; its ability to successfully advance its pipeline of product candidates, including li censed product candidates; its ability to maintain and protect its intellectual property; the outcome or success of its clinical trials; the rate and degree of market acc eptance of its products; and its ability to develop sales and marketing capabilities. In some cases, you can identify forward - looking statements by terms such as “may,” ”w ill,” “should,” “expect,” “plan,” “aim,” “anticipate,” “could,” “intend,” “target,” “project,” “contemplate,” “believe,” “estimate,” “predict,” “potential” or “contin ue” or the negative of these terms or other similar expressions. The forward - looking statements of this presentation are only predictions and are subject to a number of ris ks, uncertainties and assumptions, including, without limitation, ( i ) the outcome of any legal proceedings that may be instituted against the companies related to the proposed merger transaction; (ii) unanticipated difficulties or expenditures relating to the proposed merger transaction, the response of business partners a nd competitors to the announcement of the proposed merger transaction, and/or potential difficulties in employee retention as a result of the announcement and pend enc y of the proposed merger transaction; (iii) risks associated with the companies’ ability to obtain the stockholder approvals required to consummate th e p roposed merger transaction and the timing of the closing of the proposed merger transaction, including the risks that a condition to closing would not be satisf ied within the expected timeframe or at all or that the closing of the proposed merger transaction will not occur; (iv) the impact of COVID - 19 on prescriptions for the Neos ’ products and on its business, revenues, results of operations and financial condition; (v) Neos’ commercialization strategy for the Approved ADHD Products and other products that may be approved; (vi) the timing of any such approval; (vii) Neos’ ability to market and sell the Approved ADHD Products and any oth er products that may be approved; (viii) Neos’ ability to successfully compete in the market for medications indicated for ADHD; (ix) the manufacture of the Approved ADH D Products or Neos’ other product candidates; (x) the therapeutic potential of the Approved ADHD Products or Neos’ other product candidates; (xi) our ability t o i nitiate and complete trials for NT0502; and (xii) other risks set forth under the caption “Risk Factors” in Neos’ most recent Annual Report on Form 10 - K, as updated by Neos’ most recent Quarterly Report on Form 10 - Q, and its other SEC filings. Moreover, Neos operates in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for Neos management to predict all risks, nor can Neos assess the impact of all factors on its business or the exten t t o which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward - looking statements it may make. In li ght of these risks, uncertainties and assumptions, the forward - looking events and circumstances discussed in this presentation may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward - looking statements. You should not rely upon forward - looking statements as predictio ns of future events. Although Neos believes that the expectations reflected in the forward - looking statements are reasonable, it cannot guarantee that the future r esults, levels of activity, performance or events and circumstances reflected in the forward - looking statements will be achieved or occur. Moreover, except as required by law, neither Neos nor any other person assumes responsibility for the accuracy and completeness of the forward - looking statements. Forward - looking statements in this presentation represent Neos’ views only as of the date of this presentation. Neos undertakes no obligation to update or review any forward - looking statement, whether as a result of new information, future developments or otherwise, except as required by law. This presentation may contain trade names, trademar ks or service marks of other companies. Neos does not intend the use or display of other parties’ trade names, trademarks or service marks to imply a rela tio nship with, or endorsement or sponsorship of, these other parties. Solely for convenience, the trade names, trademarks or service marks in this presentatio n a re referred to without the symbols ® and ™, but such references should not be construed as any indicator that their respective owners will not assert, to the full est extent under applicable law, their rights thereto. 3
Neos Therapeutics and AYTU Definitive Merger Agreement: Transaction Highlights & Strategic Rationale x Transaction creates highly diversified specialty pharmaceutical company with $100M of pro forma net revenue (1) ‒ Brand Marketing (10 products), Pediatric, Primary Care, and Consumer Healthcare products x Accelerates transformation to profitability with estimated annual cost synergies of approximately $15M by 2022 x Adds Aytu pediatric and primary care products to Neos established, high - performing, multi - brand ADHD portfolio, expanding footprint in pediatrics and adjacent specialty areas x Opportunity to leverage and further enhance Neos RxConnect , a best - in - class patient support program, for Aytu’s product portfolio of clinically differentiated prescription therapeutics x Broad pipeline platform anchored by lead product candidate for sialorrhea and novel microparticle technology technology delivery platform x Company well - positioned for continued growth through business development 4 (1) For the 12 - month period ended September 30, 2020. Neos Therapeutics I January 2021
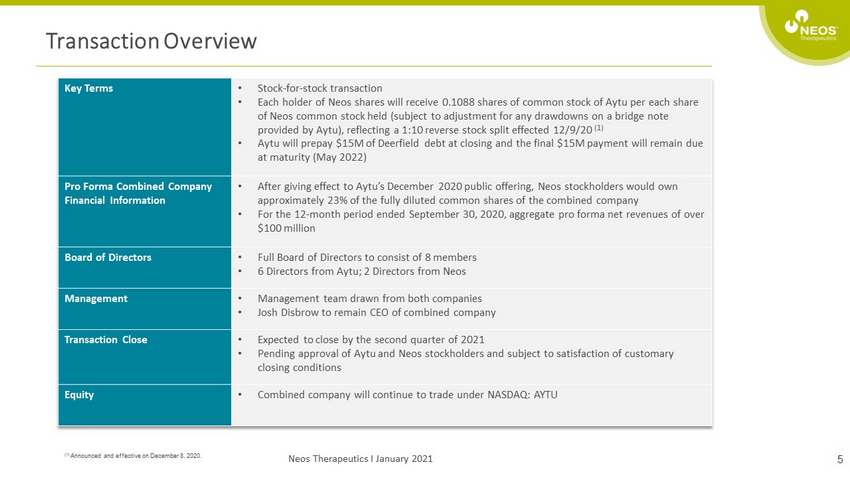
Transaction Overview 5 Key Terms • Stock - for - stock transaction • Each holder of Neos shares will receive 0.1088 shares of common stock of Aytu per each share of Neos common stock held (subject to adjustment for any drawdowns on a bridge note provided by Aytu ), reflecting a 1:10 reverse stock split effected 12/9/20 (1) • Aytu will prepay $15M of Deerfield debt at closing and the final $15M payment will remain due at maturity (May 2022) Pro Forma Combined Company Financial Information • After giving effect to Aytu’s December 2020 public offering, Neos stockholders would own approximately 23% of the fully diluted common shares of the combined company • For the 12 - month period ended September 30, 2020, aggregate pro forma net revenues of over $100 million Board of Directors • Full Board of Directors to consist of 8 members • 6 Directors from Aytu ; 2 Directors from Neos Management • Management team drawn from both companies • Josh Disbrow to remain CEO of combined company Transaction Close • Expected to close by the second quarter of 2021 • Pending approval of Aytu and Neos stockholders and subject to satisfaction of customary closing conditions Equity • Combined company will continue to trade under NASDAQ: AYTU (1) Announced and effective on December 8, 2020. Neos Therapeutics I January 2021

AYTU’s Assertive M&A Has Created Platform to Drive Growth and Support Acquisitions 6 Unique opportunity to acquire assets as a consolidator in a market ripe for integration Entered Pediatric Specialty Pharmaceutical Market Diversified into Consumer Health with Purchase of ~$30M Portfolio Expansion of Pediatric Footprint and Scale to $100M in Pro Forma Revenue Acquired Pediatric Rx Portfolio from Cerecor Closed 11/2019 Acquired Innovus Pharmaceuticals Closed 2/2020 Planned Acquisition of Neos Therapeutics (NASDAQ: NEOS) Announced 12/2020* *Announced 12/10/20; Pending S - 4 filing and stockholder votes of both Aytu BioScience and Neos and customary closing conditions Neos Therapeutics I January 2021
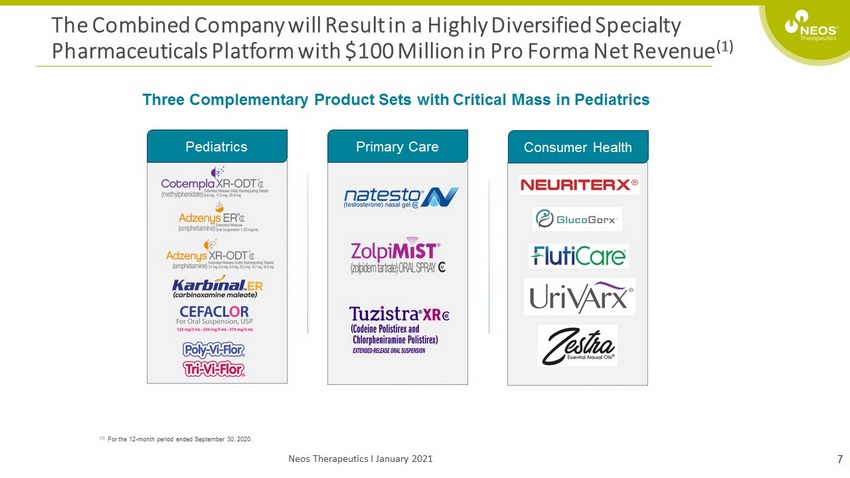
The Combined Company will Result in a Highly Diversified Specialty Pharmaceuticals Platform with $100 Million in Pro Forma Net Revenue (1) 7 (1) For the 12 - month period ended September 30, 2020. Pediatrics Primary Care Consumer Health Three Complementary Product Sets with Critical Mass in Pediatrics Neos Therapeutics I January 2021

vision We are focused on significantly improving daily living for patients by expanding and improving access to Neos medications 8
Neos Therapeutics I January 2021 Neos Today: Remain Focused on Driving Growth 9 Focused on maximizing the cash generated from the ADHD portfolio Facilitating access to Neos medicines through Neos RxConnect • Scalable beyond ADHD portfolio Leveraging Neos ’ advanced analytics platform and commercial organization Established, Multi - Brand ADHD Portfolio New Product Opportunities NT0502 in Phase 1 clinical development for treatment of chronic sialorrhea (excessive drooling) • Significant unmet need among 1.4M patients in U.S. for an effective therapy with an improved tolerability profile and dosing regimen Leverage commercial model with additional product opportunities

Neos Therapeutics I January 2021 2020 Financial Overview NET PRODUCT SALES Q3 2020 Adzenys XR - ODT $6.2M Cotempla XR - ODT $5.6M Adzenys ER * Generic Tussionex $0.7M $12.5M Fiscal Quarter Ended September 30, 2020 (in 000s) Net Product Sales $12,535 Gross Profit $7,415 Loss from Operations ($2,916) Weighted Average Shares 49,755 Cash, Cash Equivalents & ST Investments $12,744 • As of September 30, 2020, the Company had $34.4M in principal debt outstanding with Deerfield and $ 7.3 M outstanding under a senior secured revolving credit agreement . 10 * Adzenys ER revenue was negligible in the 3Q of 2020.

ADHD Product Portfolio

Large and Growing ADHD Market Drug Type Annual Prescriptions 1 (Million) Annual Gross Revenue 4 (Billion) Amphetamine 48.5 $5.6 Methylphenidate 19.6 $2.6 Non - Stimulants 6.9 $0.3 Total 75.1 $8.8 ▪ 75.1 million prescriptions written annually for ADHD medications 1 ▪ Estimated to affect 11.0% of children ages 4 - 17 2 and 4.4% of adults in the U.S. 3 ▪ ADHD prescriptions grew by 4.2% in recent 12 months 1 1. IQVIA: National Prescription Audit – trailing 12 month data as of December 2019 2. 2011 - 2012 National Survey of Children’s Health (US - DHHS) http://www.cdc.gov/nchs/slaits/nsch.htm . Accessed March 19, 2015. 3. Brus ML, et al. J. Psychiatr Pract . 2014; (6):428 - 37. 4. IQVIA; National Sales Perspective – trailing 12 month data as of December 2019 12 Neos Therapeutics I January 2021

Approved for patients 6+ years, both pediatric and adult Focused on adults – the fastest growing segment of the market Approved for patients ages 6 - 17 Compelling clinical efficacy data demonstrating symptom control at 1 hour after dosing and sustained through 12 hours Our ADHD Products – The Difference is in the Delivery 13 Neos Therapeutics I January 2021

150,000 170,000 190,000 210,000 230,000 250,000 270,000 290,000 310,000 MPH Market Comparison 2019 Vs 2020 MPH 2019 MPH 2020 ADHD TRx Market Rebounding from 1H2020 COVID - 19 Impact ▪ Extended - release ADHD TRx’s in 2020 vs. same weeks in 2019 1 ( - 2.4%): • Methylphenidate (MPH) market - 6.5% • Amphetamine (AMP) market essentially flat ▪ Pediatric segment - 10.7% vs 2019 2 1 Source: IQVIA Rapid Weekly Summary report. W/E 12/4/20 2 Source: IQVIA NPA+Patient Insights current 8 weeks ending 11/27/20 300,000 350,000 400,000 450,000 500,000 550,000 600,000 AMP Market Comparison 2019 Vs 2020 AMP 2019 AMP 2020 Neos Therapeutics I January 2021 14

19.0% 17.2% 21.7% 8.9% 8.0% 14.7% 0.0% 5.0% 10.0% 15.0% 20.0% 25.0% Neos Portfolio/ Market Adzenys XR ODT/AMP Cotempla XR ODT/MPH Neos TRx vs Branded Market 2 TRx 3Mo vs 3Mo Neos Branded Market Recent ADHD Brand TRx Performance Neos Brands Outpaced Market During 2020 Back - to - School Season ▪ Neos portfolio outperforming the market during BTS 1 • 3 - month portfolio TRx growth: o +19% (~ 2x market growth) • Both products have shown growth rates exceeding market 1 Source: IQVIA NPA ; May – July 2020 vs Aug – Oct 2020 2 Source: Branded Market include: AMP Dyanavel , Evekeo , Mydayis , Vyvanse; MPH Adhansia , Daytrana , Jornay , Quillichew , Quillivant Neos Therapeutics I January 2021 15
Neos RxConnect Addresses Key Barriers to Patient Care ▪ Offers predictability and enhances access to Neos brands for 100% of commercially insured patients, regardless of individual insurance coverage • Allows prescribers to focus on best therapeutic option, rather than on individual insurance coverage ▪ Reduces prescriber hassles that they may face when prescribing branded medications for their patients • No prior authorizations required to access Neos brands • Potentially reduces office administrative burden ▪ Affordable for all commercially insured patients • Even when enrolled in high - deductible plans 16 Neos Therapeutics I January 2021
Neos RxConnect Positioned to Support Growth of Neos ADHD Brands ▪ Neos RxConnect network growth outpaced retail growth by 17% during Q3 2020 1 • Exceeded 26k claims during Q3 2020 which was an all - time quarterly high ▪ Sound foundation and footprint • 900+ locations • 100% territory coverage ▪ Strategic growth driver for 2021 • Build greater awareness and utilization amongst key ADHD prescribers • Increase current prescriber depth of prescribing and expand prescriber breadth of utilization Unlocking future patient access growth opportunities and Neos brand adoption Source: 1 ConnectiveRx claims. Q2 2020 vs Q3 2020 Neos Therapeutics I January 2021 17

Development Pipeline
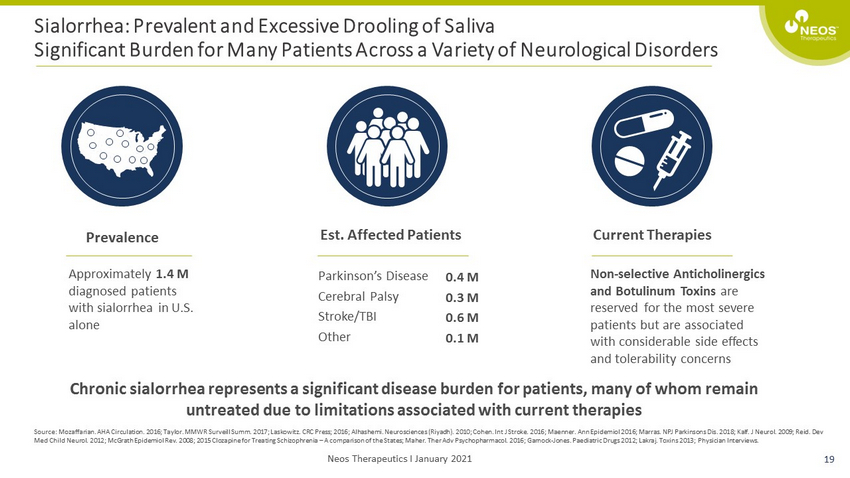
Sialorrhea: Prevalent and Excessive Drooling of Saliva Significant Burden for Many Patients Across a Variety of Neurological Disorders Source: Mozaffarian. AHA Circulation. 2016; Taylor. MMWR Surveill Summ. 2017; Laskowitz. CRC Press; 2016; Alhashemi. Neurosci enc es (Riyadh). 2010; Cohen. Int J Stroke. 2016; Maenner. Ann Epidemiol 2016; Marras. NPJ Parkinsons Dis. 2018; Kalf. J Neurol. 200 9; Reid. Dev Med Child Neurol. 2012; McGrath Epidemiol Rev. 2008; 2015 Clozapine for Treating Schizophrenia – A comparison of the States; Mah er. Ther Adv Psychopharmacol. 2016; Garnock - Jones. Paediatric Drugs 2012; Lakraj. Toxins 2013; Physician Interviews. Prevalence Est. Affected Patients Current Therapies Approximately 1.4 M diagnosed patients with sialorrhea in U.S. alone Non - selective Anticholinergics and Botulinum Toxins are reserved for the most severe patients but are associated with considerable side effects and tolerability concerns Parkinson’s Disease Cerebral Palsy Stroke/TBI Other 0.4 M 0.3 M 0.6 M 0.1 M Chronic sialorrhea represents a significant disease burden for patients, many of whom remain untreated due to limitations associated with current therapies 19 Neos Therapeutics I January 2021

NT0502: Developing a Potential New Treatment Option Aiming to Meet the Needs of Patients with Sialorrhea Selective Pharmacological Profile Based on Preclinical Data ▪ NT0502 is a new chemical entity and anticholinergic agent that is preferentially selective for blocking muscarinic receptor subtypes predominant in salivary glands Potential for Fewer Systemic Side Effects Compared to Existing Treatment Options ▪ A targeted therapy may provide improved tolerability which is important when treating complex neurologic patients Phase 1 Clinical Development Plan ▪ Top - line pharmacokinetic data in pilot pharmacokinetic study supports further clinical development ▪ Planned initiation of Phase 1 single ascending and multiple ascending dose studies (SAD/MAD) Patent Protection Through 2032 ▪ Issued patent in April 2020 directed to methods of treating sialorrhea by administering N - desethyloxybutynin 20 Neos Therapeutics I January 2021

Key Takeaways

Neos Therapeutics I January 2021 Key Takeaways Recent Highlights x Announced definitive merger agreement with Aytu in December 2020 x Neos RxConnect surpassed 900 pharmacies and accounted for 40% of Neos ADHD TRx’s in 3Q 2020 x Neos brands sequential growth in 3Q 2020 for Adzenys XR - ODT (+9.9%) and Cotempla XR - ODT (+6.5%) outperformed the overall market (+4.1%) 22 Near - term Goals ▪ Capitalize on ADHD market recovery and current Neos ADHD prescription momentum ▪ Build greater awareness and utilization of Neos RxConnect amongst key ADHD prescribers ▪ Close merger with AYTU by 2Q 2021

Corporate Presentation January 2021






















