AN OFFERING STATEMENT PURSUANT TO REGULATION A RELATING TO THESE SECURITIES HAS BEEN FILED WITH THE U.S. SECURITIES AND EXCHANGE COMMISSION (THE “SEC”). INFORMATION CONTAINED IN THIS PRELIMINARY OFFERING STATEMENT IS SUBJECT TO COMPLETION OR AMENDMENT. THESE SECURITIES MAY NOT BE SOLD NOR MAY OFFERS TO BUY BE ACCEPTED BEFORE THE OFFERING STATEMENT FILED WITH THE SEC IS QUALIFIED. THIS PRELIMINARY OFFERING STATEMENT SHALL NOT CONSTITUTE AN OFFER TO SELL OR THE SOLICITATION OF AN OFFER TO BUY NOR MAY THERE BE ANY SALES OF THESE SECURITIES IN ANY STATE IN WHICH SUCH OFFER, SOLICITATION OR SALE WOULD BE UNLAWFUL BEFORE REGISTRATION OR QUALIFICATION UNDER THE LAWS OF SUCH STATE. THE COMPANY MAY ELECT TO SATISFY ITS OBLIGATION TO DELIVER A FINAL OFFERING STATEMENT BY SENDING YOU A NOTICE WITHIN TWO BUSINESS DAYS AFTER THE COMPLETION OF THE COMPANY’S SALE TO YOU THAT CONTAINS THE URL WHERE THE FINAL OFFERING STATEMENT OR THE OFFERING STATEMENT IN WHICH SUCH FINAL OFFERING STATEMENT WAS FILED MAY BE OBTAINED.
PRELIMINARY OFFERING STATEMENT DATED FEBRUARY 12, 2025
HEARTSCIENCES INC.

HeartSciences Inc.
550 Reserve St, Suite 360
Southlake, Texas 76092
682-237-7781
https://heartsciences.com/
BEST EFFORTS OFFERING OF UP TO $15,000,000 OF UNITS, EACH COMPRISING 1 SHARE OF SERIES D PREFERRED STOCK AND 1 WARRANT TO PURCHASE 1 SHARE OF COMMON STOCK
UP TO 4,285,714 SHARES OF COMMON STOCK INTO WHICH THE SERIES D PREFERRED STOCK MAY CONVERT AND UP TO 4,285,714 SHARES OF COMMON STOCK, ISSUABLE UPON EXERCISE OF THE INVESTOR WARRANTS
AGENT WARRANTS FOR THE PURCHASE OF UP TO 128,571 AGENT UNITS
UP TO 128,571 SHARES OF SERIES D PREFERRED STOCK UNDERLYING AGENT UNITS AND UP TO 128,571 SHARES OF COMMON STOCK INTO WHICH SUCH SERIES D PREFERRED STOCK MAY CONVERT
UP TO 128,571 SHARES OF COMMON STOCK, ISSUABLE UPON EXERCISE OF THE AGENT UNIT WARRANTS
SEE “SECURITIES BEING OFFERED” AT PAGE 45
HeartSciences Inc, which we refer to as the “Company,” “we,” “our” and “us,” is offering up to 4,285,714 Units. Each unit (each a “Unit” and collectively the “Units”) consists of one (1) share of Series D Convertible Preferred Stock, par value $0.001 per share (the “Series D Preferred Stock”), and one (1) warrant (the “Warrants”), each to purchase one (1) share of our common stock, $0.001 par value per share (the “Common Stock”). We will not issue fractional shares. The Units will be sold at an offering price of $3.50 per Unit, for a maximum offering amount of $15,000,000 worth of Units. This offering statement also relates to the 8,571,428 shares of Common Stock issuable upon conversion of the Series D Preferred Stock and exercise of the Warrants. The Warrants are exercisable within 36 months from the date of issuance. Each Warrant will be exercisable at a price of $5.00 for one (1) share of our Common Stock, subject to customary adjustment.
In addition, this offering statement relates to (i) 128,571 agent warrants (the “Agent Warrants”) for the purchase of up to 128,571 Agent Units (the “Agent Units”), each Agent Unit consisting of one share of Series D Preferred Stock (the “Agent Shares”) and one warrant (the “Agent Unit Warrants”) to purchase one share of Common Stock (the “Agent Warrant Shares”), and (ii) up to 128,571 shares of Common Stock into which the Series D Preferred Stock underlying the Agent Units may convert (the “Agent Unit Shares”) and up to 128,571 shares of Common Stock, issuable upon exercise of the Agent Warrants (the “Agent Warrant Shares”).
There is a minimum initial investment amount per investor of $675 for the Units and any additional purchases must be made in increments of at least $675.
At any time after issuance, each share of our Series D Preferred Stock is convertible into one (1) share of our Common Stock at the option of the holder of such Series D Preferred Stock. At any time after issuance upon the occurrence of any of the following events, we shall have a right to direct the mandatory conversion of the Series D Preferred Stock: (a) a change in control of our Company, (b) if the price of the Common Stock closes at or above $5.00 per share for 10 consecutive trading days, or (c) if we consummate a firm commitment public offering of Common Stock for gross proceeds of at least $15 million at an offering price per share equal to or greater than $5.00. The shares underlying the Series D Preferred Stock will be qualified in this offering (this “Offering”). The Series D Preferred Stock and the Common Stock differ in other characteristics including voting rights. Up to 4,285,714 of Common Stock underlying the Series D Preferred Stock are being qualified in this Offering. See “Securities Being Offered” beginning on page 45 for additional details.
There is no existing public trading market for the Series D Preferred Stock. In June 2022, we completed our initial public offering (“IPO”) of units (the “IPO Units”) consisting of shares of Common Stock and warrants to purchase shares of Common Stock (the “IPO Warrants”). Our Common Stock and our IPO Warrants are listed on the Nasdaq Capital Market (“Nasdaq”) under the symbols “HSCS” and “HSCSW,” respectively. On February 11, 2025, the closing price of our Common Stock was $3.82 per share and the closing price of our IPO Warrants was $0.0405 per warrant.
The offering price of the Units is not related to, nor may it reflect the market price of the shares of our Common Stock underlying the Warrants after this Offering. The Units, Series D Preferred Stock and the Warrants are not currently listed or quoted on any exchange and we do not intend to seek a listing for them. The Units have no stand-alone rights and will not be certificated or issued as stand-alone securities. The shares of our Series D Preferred Stock and the Warrants are immediately separable and will be issued separately, but will be purchased together as a Unit in this Offering.
This Offering is being conducted on a “best efforts” basis. This Offering will terminate at the earlier of the date at which the maximum offering amount has been sold or the date at which the Offering is earlier terminated by the Company at its sole discretion, and the offering statement on Form 1-A of which this offering statement forms a part will remain qualified in accordance with Rule 251(d)(3)(i)(F) of Regulation A until the date at which all of the outstanding Warrants issued pursuant to this Offering have been exercised for shares of Common Stock, which shares of Common Stock are qualified under the Form 1-A. At least every 12 months after this Offering has been qualified by the U.S. Securities and Exchange Commission (the “SEC”), we will file a post-qualification amendment to include our then recent financial statements.
Digital Offering, LLC is a broker-dealer registered with the SEC and a member of the Financial Industry Regulatory Authority, or FINRA, and the Securities Investor Protection Corporation, or SIPC, which we refer to as the lead selling agent or managing broker-dealer, and is the lead selling agent for this Offering. The lead selling agent is selling our Units in this Offering on a best-efforts basis and is not required to sell any specific number or dollar amount of Units offered by this offering statement, but will use their best efforts to sell such Units.
We may undertake one or more closings on a rolling basis. Until we complete a closing, the proceeds for this Offering will be kept in an escrow account maintained at Enterprise Bank & Trust (the “Escrow Agent”). At each closing of this Offering, the proceeds will be distributed to us and the associated Series D Preferred Stock and Warrants will be issued to the investors participating in such closing. If there are no closings or if funds remain in the escrow account upon termination of this Offering without any corresponding closing, the funds so deposited for this Offering will be promptly returned to the applicable investors, without deduction and without interest. See “Plan of Distribution.”
| | | Price to
Public(1) | | | Selling Agent
Commissions(2) | | | Proceeds to
issuer(3) | |
| Per Unit | | $ | 3.50 | | | $ | 0.245 | | | $ | 3.255 | |
| Total Maximum of Units Offering | | $ | 15,000,000 | | | $ | 1,050,000 | | | $ | 13,950,000 | |
| Preferred Stock Contained in the Units (4,285,714 shares) | | | -- | | | | -- | | | | -- | |
| Common Stock Issuable upon Conversion of Series D Preferred Stock (4,285,714 shares) (4) | | | -- | | | | -- | | | | -- | |
| Agent Warrants (128,571 Units) (5) | | $ | 642,855 | | | | -- | | | $ | 642,855 | |
| Preferred Stock Contained in the Agent Warrants (128,571 shares) | | | -- | | | | -- | | | | -- | |
| Common Stock Issuable upon Exercise of investor Warrants (4,285,714 shares) (6) | | $ | 21,428,570 | | | | -- | | | $ | 21,428,570 | |
| Common Stock Issuable upon Exercise of Agent Unit Warrants (128,571 shares) (7) | | $ | 642,855 | | | | -- | | | $ | 642,855 | |
| Total Maximum | | $ | 37,714,280 | | | $ | 1,050,000 | | | $ | 36,664,280 | |
| 1. | Per Unit price represents the offering price for one Unit. |
| 2. | We have engaged Digital Offering, LLC (“Digital Offering”) to act as the lead selling agent to offer the Units to prospective investors in this Offering on a “best efforts” basis, which means that there is no guarantee that any minimum amount will be received by us in this Offering. In addition, the lead selling agent may engage one or more sub-agents or selected dealers to assist in its marketing efforts. Digital Offering is not purchasing the Units offered by us and is not required to sell any specific number or dollar amount of Units in this Offering before a closing occurs. We will pay a cash commission of 7.00% to Digital Offering on sales of the Units and issue warrants (the “Agent Warrants”) to Digital Offering to purchase that number of Units equal to 3% of the total number of Units sold in this Offering. The Agent Warrants and the securities comprising and underlying the Agent Warrants will not be transferable for a period of six months after the date of the final closing of the Offering (in compliance with FINRA Rule 5110(e)(1)), and the Agent Warrants will expire five years after final closing of the Offering. The price per unit for the Agent Warrants will be the amount that is 125% of the offering price of the Units, or $4.375 per Unit. The Units issuable upon exercise of the Agent Warrants will include one (1) share of Series D Preferred Stock and one (1) warrant, exercisable at $5.00 per share (the “Agent Unit Warrants”). Additionally, we paid Digital Offering an accountable due diligence fee of $25,000. Finally, we shall be responsible for Digital Offering’s reasonable legal costs up to an amount of $85,000, $25,000 of which has been paid to date. See “Plan of Distribution” on page 31 for details of compensation payable to the lead selling agent in connection with this Offering. |
| | |
| 3. | Before deducting expenses of the Offering, which are estimated to be approximately $1,220,000. See the section captioned “Plan of Distribution” on page 31 for details regarding the compensation payable in connection with this Offering. This amount represents the gross proceeds of the Offering to us, before expenses, which will be used as set out in the section captioned “Use of Proceeds to Issuer.” |
| 4. | No additional consideration or placement agent compensation will be paid in connection with the issuance of shares of Common Stock upon conversion of the Series D Preferred Stock. |
| 5. | The value of the Agent Warrants set forth in the table above is based on the number of shares of Series D Preferred Stock contained in the Units (128,571 shares) multiplied by the exercise price of $5.00 per Agent Warrant. The actual value of the Agent Warrants utilizing an options pricing model would be different from the amount indicated in the table. |
| 6. | No additional compensation will be paid in connection with the issuance of Common Stock upon exercise of the investor Warrants included in the Units offered hereby, nor will any additional commissions be paid on such shares or Warrants. No additional commissions will be paid upon exercise of any investor Warrants unless we determine to engage a placement agent for a separate warrant exercise solicitation offering. |
| 7. | Up to 128,571 of the Agent Unit Warrants will have an exercise price of $5.00 per share of Common Stock. |
Sales of these securities will commence on approximately ___________, 2025.
THE U.S. SECURITIES AND EXCHANGE COMMISSION (THE “SEC”) DOES NOT PASS UPON THE MERITS OR GIVE ITS APPROVAL OF ANY SECURITIES OFFERED OR THE TERMS OF THIS OFFERING, NOR DOES IT PASS UPON THE ACCURACY OR COMPLETENESS OF ANY OFFERING STATEMENT OR OTHER SOLICITATION MATERIALS. THESE SECURITIES ARE OFFERED PURSUANT TO AN EXEMPTION FROM REGISTRATION WITH THE SEC; HOWEVER, THE SEC HAS NOT MADE AN INDEPENDENT DETERMINATION THAT THE SECURITIES OFFERED ARE EXEMPT FROM REGISTRATION.
GENERALLY, NO SALE MAY BE MADE TO YOU IN THIS OFFERING IF THE AGGREGATE PURCHASE PRICE YOU PAY IS MORE THAN 10% OF THE GREATER OF YOUR ANNUAL INCOME OR NET WORTH. DIFFERENT RULES APPLY TO ACCREDITED INVESTORS AND NON-NATURAL PERSONS. BEFORE MAKING ANY REPRESENTATION THAT YOUR INVESTMENT DOES NOT EXCEED APPLICABLE THRESHOLDS, WE ENCOURAGE YOU TO REVIEW RULE 251(d)(2)(i)(C) OF REGULATION A. FOR GENERAL INFORMATION ON INVESTING, WE ENCOURAGE YOU TO REFER TO www.investor.gov.
This Offering is inherently risky. See “Risk Factors” on page 22.
The Company is following the format of Part I of Form S-1 pursuant to the general instructions of Part II(a)(1)(ii) of Form 1-A.
TABLE OF CONTENTS
In this offering statement, the term “HeartSciences,” the “Company,” “we,” “us” or “our” refers to HeartSciences Inc. together with its subsidiaries.
Other than in the table on the cover page, dollar amounts have been rounded to the closest whole dollar.
This offering statement contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, that are intended to be covered by the “safe harbor” created by those sections. Forward-looking statements, which are based on certain assumptions and describe our future plans, strategies and expectations, can generally be identified by the use of forward-looking terms such as “believe,” “expect,” “may,” “will,” “should,” “could,” “seek,” “intend,” “plan,” “estimate,” “anticipate”, “predicts,” “potential” or “continue” or the negative of these terms or other comparable terms.
All statements other than statements of historical facts included in this offering statement regarding our strategies, prospects, financial condition, operations, costs, plans and objectives are forward-looking statements. Examples of forward-looking statements include, among others, statements we make regarding proposed business strategy; market opportunities; regulatory approval; expectations for current and potential business relationships; expectations for revenues, cash flows and financial performance; payment of future dividends; use of our at-the-market (“ATM”) offering program; our liquidity position and capital resources; the impact of certain market risk exposures on our financial condition, results of operations or cash flows; anticipated results of research and development efforts; and completion and use of our device and all statements (other than statements of historical facts). These forward-looking statements are based on our current information and beliefs. Because forward-looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are unpredictable and many of which are outside of our control. Actual results may differ materially from what is anticipated, so you should not rely on these forward-looking statements.
Important factors that could cause actual results, developments and business decisions to differ materially from those anticipated in these forward-looking statements include, among other things:
| ● | our expectation regarding the sufficiency of our existing cash and cash equivalents to fund our current operations; and |
| | | |
| ● | our ability to receive regulatory clearance for the MyoVista wavECG (the “MyoVista”) Insights Cloud Platform and associated AI-ECG algorithms from the U.S. Food and Drug Administration (the “FDA”), state regulators, if any, or other similar foreign regulatory agencies, including approval to conduct clinical trials, the timing and scope of those trials and the prospects for regulatory approval or clearance of, or other regulatory action with respect to our current products or other future potential products; |
| | | |
| ● | our ability to further advance the development of the MyoVista wavECG, our 12-lead electrocardiograph (“ECG”) device that also incorporates an additional proprietary AI-ECG algorithm that we have been designing to detect cardiac dysfunction, as well as future potential products; |
| | | |
| ● | our ability to develop a cloud-based hardware agnostic platform and to develop and incorporate AI-ECG algorithms on that platform; |
| | | |
| ● | our ability to launch sales of the MyoVista wavECG device, MyoVista Insights Cloud Platform, and AI-ECG algorithms or any future potential products into the U.S.; |
| | | |
| ● | our assessment of the potential of the MyoVista wavECG device, MyoVista Insights Cloud Platform and AI-ECG algorithms and any future potential products; |
| | | |
| ● | our planned level of capital expenditures and liquidity; |
| | | |
| ● | our plans to continue to invest in research and development to develop technology for new products; |
| ● | our failure to maintain the continued listing requirements of Nasdaq could result in a de-listing of our shares and penny stock trading; |
| | | |
| ● | the regulatory environment and changes in the health policies and regimes in the countries in which we intend to operate, including the impact of any changes in regulation and legislation that could affect the medical device industry; |
| | | |
| ● | our ability to meet our expectations regarding the commercial supply of our current products and any future products; |
| | | |
| ● | our ability to retain key executives; |
| | | |
| ● | our ability to internally develop new inventions and intellectual property; |
| | | |
| ● | the overall global economic environment; |
| | | |
| ● | the ultimate impact of health epidemics, on our business, our clinical trials, our research programs, healthcare system or the global economy as a whole; |
| | | |
| ● | the impact of competition and new technologies; |
| | | |
| ● | general market, political and economic conditions in the countries in which we operate; |
| | | |
| ● | our ability to develop product offerings and intellectual property; |
| | | |
| ● | changes in our strategy; and |
| | | |
These statements are only current predictions and are subject to known and unknown risks, uncertainties, and other factors that may cause our or our industry’s actual results, levels of activity, performance or achievements to be materially different from those anticipated by the forward-looking statements. We discuss many of these risks in this offering statement in greater detail under the heading “Risk Factors” and elsewhere in this offering statement. You should not rely upon forward-looking statements as predictions of future events.
Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance or achievements. Except as required by law, we are under no duty to update or revise any of the forward-looking statements, whether as a result of new information, future events or otherwise, after the date of this offering statement.
We will continue to file annual, quarterly and current reports, proxy statements and other information with the SEC. Forward-looking statements speak only as of the dates specified in such filings. Except as expressly required under federal securities laws and the rules and regulations of the SEC, we do not undertake any obligation to update any forward-looking statements to reflect events or circumstances arising after any such date, whether as a result of new information or future events or otherwise. You should not place undue reliance on the forward-looking statements included in this offering statement or that may be made elsewhere from time to time by us, or on our behalf. All forward-looking statements attributable to us are expressly qualified by these cautionary statements.
Unless defined elsewhere, capitalized terms used in this offering statement are defined in the section of this offering statement titled “Glossary of Terms.”
OFFERING CIRCULAR SUMMARY
This summary highlights information contained in greater detail elsewhere in this offering circular and does not contain all of the information that you should consider in making your investment decision. Before investing in our Common Stock, you should carefully read this entire offering circular, including our financial statements and the related notes thereto included in this offering circular. You should also consider, among other things, the information set forth under the sections titled “Risk Factors” appearing elsewhere in this offering circular.
Company Overview
We are a medical technology company focused on applying innovative AI-based technology to an ECG, also known as an “EKG,” to expand and improve an ECG’s clinical usefulness. Our objective is to make an ECG a far more valuable cardiac screening tool by expanding its clinical capability to detect a broader range of cardiac indications through the development of AI-based ECG algorithms (“AI-ECG”). We are seeking to provide AI-ECG solutions in any care setting worldwide in a manner that best suits different providers, either via our cloud-based application that can receive an upload from one of the millions of ECG devices currently in clinical use or via our proprietary MyoVista wavECG device. The MyoVista wavECG, is a resting 12-lead ECG that will incorporate HeartSciences’ proprietary AI-ECG algorithm designed to provide diagnostic information related to cardiac dysfunction as well as conventional ECG information in the same test. We are also developing a cloud-based platform to host AI-ECG algorithms, both developed by us internally or by third-parties, on an ECG hardware agnostic basis (the “MyoVista Insights Cloud Platform”). In the future, we intend to deliver a range of AI-ECG algorithms, via each product. Neither the MyoVista wavECG, MyoVista Insights Cloud Platform nor any of our AI-ECG algorithms are yet cleared for marketing by the FDA.
The AI-ECG algorithms are intended to provide diagnostic information which has traditionally required cardiac imaging. We believe, the combination of a device agnostic cloud platform and MyoVista wavECG device would allow us to offer AI-ECG solutions across a wide range of healthcare settings from large heath systems to frontline or point of care environments such as primary care. The initial revenue model for the MyoVista wavECG device, which involves the use of the MyoVista hardware, associated software and consumables for each test, is expected to be “razor-razorblade” as the cable connection to the electrodes used with the MyoVista wavECG are proprietary to HeartSciences, and new electrodes are used for every test performed. As further algorithms are made commercially available via the MyoVista wavECG or the MyoVista Insights Cloud Platform, we would expect to adopt revenue models based on algorithm usage and/or recurring subscriptions. Our MyoVista Insights Cloud Platform is being designed to fit simply into existing clinical workflows and be available to host third party AI-ECG algorithms, which increases its clinical value and our speed to market as well as reducing research and development (“R&D”) costs associated with internal algorithm development.
On September 20, 2023, we entered into multiple definitive license agreements (each a “License Agreement” and collectively, the “License Agreements”) with Icahn School of Medicine at Mount Sinai (“Mount Sinai”) to commercialize a range of AI-ECG algorithms covering a range of cardiovascular conditions developed by Mount Sinai as well as a memorandum of understanding for ongoing cooperation encompassing de-identified data access, on-going research, and the evaluation of the MyoVista wavECG device.
Our future success is dependent upon receiving FDA clearances for our products and additional funding may be required as part of achieving FDA clearance and thereafter would be required to support a sales launch, provide working capital and support further R&D.
We believe that there is currently no low-cost, front-line, medical device that is effective at screening broadly for many types of heart disease. As a result, we believe that frontline physicians face a significant challenge in determining if a patient has heart disease. Although many think of the ECG as the frontline test for heart disease, in 2012, the United States Preventive Services Task Force conducted an evaluation of conventional ECG testing and stated: “There is no good evidence the test, called an ECG, helps doctors predict heart risks any better than traditional considerations such as smoking, blood pressure and cholesterol levels in people with no symptoms.”
ECG devices record the electrical signals of a patient’s heart. The ECG is a ubiquitous, relatively low-cost, simple and quick test; it is portable and can be performed in a wide range of clinical settings by a non-specialist clinician or clinical aide. There are three basic categories of heart disease: electrical (such as an arrhythmia), structural (such as valvular disease) and ischemic (such as coronary artery disease, or CAD). Conventional resting ECGs have limited sensitivity in detecting structural and ischemic disease and are typically used for diagnosing cardiac rhythm abnormalities, such as atrial fibrillation, or acute coronary syndrome, such as a myocardial infarction which is also known as a heart attack. However, traditional ECGs have a limited role in identifying cardiac dysfunction associated with structural and ischemic disease.
HeartSciences has designed or licensed algorithms to help address these limitations and extend the clinical capability of an ECG to detect cardiac dysfunction and other heart disease types.
Our first AI-ECG algorithm to be incorporated into the MyoVista wavECG device has been designed by the Company and applies AI-machine learning to the signal processed ECG signal to develop a proprietary algorithm designed to detect impaired cardiac relaxation, or cardiac dysfunction, caused by heart disease and/or age-related cardiac dysfunction. We have been adjusting the algorithm to reflect updated echo measurement thresholds in respect of ≥60 year old patients. The change was made, and agreed with the FDA, to reflect recent clinical findings which we believe will further increase the clinical value of this algorithm.
We expect the first AI-ECG algorithm to be submitted as part of the MyoVista Insights Cloud Platform will be for the detection of low ejection fraction, or systolic dysfunction, based on one of the licensed algorithms from Mount Sinai.
The editorial comment associated with the study titled “Prediction of Abnormal Myocardial Relaxation from Signal Processed Surface ECG” presented below discusses recent applications of machine learning to data derived from surface 12-lead ECGs in relation to cardiac dysfunction:
“These represent some of the most significant advances in electrocardiography since its inception, which has historically had a limited, if any, role in the evaluation of cardiac dysfunction. In the past, our cardiovascular community was resigned to the fact that surface ECGs are poor indicators for cardiac dysfunction.”
Khurram Nasir, MD, MPH, MSC, Department of Cardiology, Houston Methodist DeBakey Heart & Vascular Center, Houston, Texas, et. al., Journal of American College of Cardiology Editorial Comment Volume 76 Number 8 2020.
Almost all forms of heart disease, including CAD and structural disease, affect heart muscle, or cardiac, function prior to symptoms. Impaired cardiac function is first observed as impaired cardiac relaxation which is an early indicator of diastolic dysfunction and usually continues to increase in severity as heart disease progresses. The diastolic phase of the cardiac cycle occurs when the heart muscle relaxes (following contraction). Diastolic dysfunction may also be related to age-related cardiac dysfunction. Low ejection fraction, or systolic dysfunction, is a later stage of cardiac dysfunction and occurs when the heart pumps a reduced level of blood from the ventricles during contraction.
If we receive FDA clearances for our product candidates, our main target markets would be frontline healthcare environments in the U.S., to assist physician decision making in the cardiology referral process. Currently, cardiology referral decisions are often based on a patient’s risk factors and/or a conventional ECG test. Accordingly, many patients with heart disease are left undetected while no current treatment or intervention is required for most patients referred for cardiac imaging. We believe that adding the capability to detect a broader range of cardiac conditions to the standard 12-lead resting ECG could help improve cardiac referral pathways and be valuable for patients, physicians, health systems and third-party payors.
New Class II devices, such as our products, require FDA premarket review. The MyoVista wavECG device along with its proprietary software and hardware is classified as a Class II medical device by the FDA. Premarket review and clearance by the FDA for these devices is generally accomplished through the 510(k) premarket notification process or De Novo classification request, or petition process. We previously submitted an FDA De Novo classification request in December 2019 and, following feedback and communications with the FDA during and since that submission, we have been making modifications to our device, including our proprietary algorithm. We have finished the patient recruitment and core lab work for our FDA validation study and have been undertaking device and algorithm development testing for a revised FDA submission. We had been planning a revised submission under the De Novo pathway, however, in December 2023 the FDA confirmed that we could submit the MyoVista for clearance under the 510(k) pathway following the grant by the FDA in August 2023 of an industry-first De Novo clearance which created a new Class II product code for cardiovascular machine learning-based notification software. This was in respect of a hypertrophic cardiomyopathy algorithm and in late September 2023, the FDA cleared an algorithm for low ejection fraction (less than 40%) under the 510(k) pathway using this new product code. Accordingly, we are now preparing for a 510(k) FDA submission and are aiming for a submission in the first calendar quarter of 2025. If successful, FDA clearance would provide us the ability to market and sell the MyoVista wavECG device in the U.S. and additional funding would be required to support the sales launch of the MyoVista wavECG device in the U.S., provide working capital and support further R&D.
To date we have not yet entered into a discussion with the FDA regarding the MyoVista Insights Cloud Platform or Mount Sinai licensed AI-ECG algorithms but expect to begin that process in the current calendar year. We expect these products to fall under the 510(k) pathway and are aiming for an FDA submission of our MyoVista Insights Cloud Platform and low ejection fraction algorithm in the second half of 2025.
Heart Disease Facts and Current ECG Testing Limitations
Heart disease refers to a variety of conditions that affect the heart — including heart rhythm problems, heart valve problems, genetic defects and blood-vessel diseases such as CAD. It is often referred to as the “silent killer.” According to the American Heart Association, one in three patients are not properly diagnosed until after a heart attack occurs and 50% of men and 64% of women who died suddenly of coronary heart disease showed no previous symptoms. Statistics published by the U.S. Centers for Disease Control and Prevention (the “CDC”), show that in the United States heart disease is the leading cause of death for both men and women, across most racial and ethnic groups. According to the CDC, in the United States, one person dies from cardiovascular disease every 34 seconds. In 2021, about 20.1 million adults aged 20 and older in the United States have CAD (about 7.2%), with approximately one in five heart attacks being a silent heart attack therefore the person is not even aware of it, but the damage is done. Approximately 695,000 people in the U.S. died from heart disease in 2021, that’s one in every five deaths. The scale of the problem is similar worldwide. In 2020, the World Health Organization confirmed that heart disease has remained the leading cause of death at the global level for the last 20 years. Cardiovascular diseases are the leading cause of death globally. An estimated 17.9 million people died from cardiovascular diseases in 2019, representing 32% of all global deaths.
The 2019 National Ambulatory Medical Care Survey showed there were approximately 1 billion ambulatory care visits in the U.S. with a high incidence of patients with risk factors for heart disease (33% had hypertension, 15% had diabetes and 7% had a history of CAD, ischemic heart disease or myocardial infarction).
As heart disease progresses to more acute stages, the cost to treat patients increases significantly. Cardiovascular disease is the leading cost to the healthcare system and is estimated to be responsible for one in every six healthcare dollars spent in the United States. Heart disease cost the United States about $240 billion in each of 2018 and 2019, including the cost of health care services, medicines, and lost productivity due to death. Governments, healthcare providers and payors are motivated to shift the diagnosis and management of these conditions to earlier stages where better patient outcomes can be delivered at lower costs.
We believe that there is currently no low-cost, front-line, medical device that is effective at screening for heart disease. As a result, frontline physicians face a significant challenge in determining if a patient has heart disease. The conventional ECG is thought of by many to be the front-line tool in cardiac testing, but it has poor sensitivity in detecting CAD or structural heart disease.
Overuse of Expensive Cardiology-Based Diagnostic Testing
We believe that the absence of cost-effective front-line or primary-care-based testing has resulted in the over-use of costly cardiology-based diagnostic tests. Noninvasive cardiac tests are significant contributors to healthcare costs, accounting for greater than 40% of Medicare Part B spending on medical imaging, or over $17 billion annually according to the U.S. Centers for Medicare & Medicaid Services (“CMS”). There are a variety of effective, though expensive, diagnostic tests used for patients to detect heart disease. These diagnostic tests are typically performed in a specialist cardiology or hospital setting and may include:
| ● | Stress ECG testing, a non-invasive diagnostic test with a cost of approximately $200 with, according to the American College of Cardiology, a sensitivity of 68% in the detection of CAD; |
| ● | Echocardiogram, or echo, a non-invasive diagnostic imaging test, similar to an ultrasound, which is effective in the detection of heart disease; however, the Medicare cost of an echo in a hospital is approximately $600 and can be as much as $3,000 if performed privately; |
| ● | Cardiac imaging tests, such as nuclear stress tests and coronary computerized tomography angiograms alternatively can be conducted noninvasively, but typically cost $1,000 or more; or |
| ● | Coronary angiogram, an invasive test in which dye that is visible by X-ray is injected into the blood vessels of the heart. A coronary angiogram can cost in excess of $5,000. |
Diastolic Dysfunction, an Early Indicator of Heart Disease
The symptoms and causes of cardiac dysfunction have been researched for many years. The causes of cardiac dysfunction during the contraction (systolic) phase, also called reduced left ventricular ejection fraction, have been well understood for many years. However, according to the American Heart Association Statistics Committee report in 2013, approximately 50% of patients with heart failure (“HF”) symptoms have ejection fraction measures that are not markedly abnormal. In addition, multiple articles published by the National Institutes of Health (“NIH”), state that approximately 50% of HF cases are due to severe diastolic dysfunction, also called heart failure with preserved ejection fraction. HF with preserved ejection fraction (“HFpEF”) is a clinical syndrome in which patients have symptoms and signs of HF with normal or near-normal left ventricular ejection fraction (“LVEF”) (LVEF ≥50%). Roughly half of all patients with HF worldwide have an LVEF ≥50% and nearly half have an LVEF <50%. Thanks to the increased scientific attention about the condition and improved characterization and diagnostic tools, the incidence of HF with reduced ejection fraction (“HFrEF”) dropped while that of HFpEF has increased by 45%. As a result, understanding the causes and progression of diastolic dysfunction has become a key area of scientific and clinical interest. This research has led to the understanding that almost all patients with systolic dysfunction also have diastolic dysfunction and almost all types of heart disease including CAD, valvular disease, cardiomyopathy, hypertension, congenital heart disease, and pericardial disease induce diastolic dysfunction.
According to an article by Dr. Dalane W. Kitzman, MD and Dr. William C. Little, MD published in the February 14, 2012 issue of the Journal of the American Heart Association, diastolic performance is sensitive to nearly all of the common disease processes that affect cardiovascular function. The article indicates that left ventricular, or LV, diastolic function is impaired by all of the common disease processes that affect LV function or produce LV hypertrophy or fibrosis, including hypertension, diabetes, ischemia, myocarditis, toxins and infiltrative cardiomyopathies. LV diastolic dysfunction (“LVDD”) begins early in the heart disease process and continues to increase in severity as heart disease progresses. LVDD is now recognized as one of the earliest signs of heart disease and typical onset occurs when a patient is still asymptomatic. We believe that the early detection of diastolic dysfunction can be a clinically valuable marker for almost all forms of heart disease and age-related cardiac abnormalities that may otherwise be missed by current conventional ECG devices.
MyoVista Insights Cloud Platform and Related AI-ECG Algorithms
We are currently developing a reporting platform to host the provision of cloud-based AI-ECG algorithms. The platform is being designed to be ECG device agnostic to allow clinical institutions to access AI-ECG algorithms without the need to replace existing devices. Our objective for the MyoVista Insights Cloud Platform is to develop an AI-ECG marketplace to include integration and hosting of AI-ECG algorithms developed and FDA cleared by third parties. This would be expected to provide clinical and commercial benefit by increasing the number of available algorithms and clinical use indications, increase our speed of roll-out, and reduce the burden and cost of algorithm R&D on the Company.
We have engaged a firm with significant experience developing and delivering healthcare software platforms, including other cloud platforms. We plan to deliver the MyoVista Insights Cloud Platform on a phased basis. Phase 1 has been designed to provide an initial functional platform as a basis for FDA clearance contemporaneous with our first cloud-based AI-ECG algorithm. The Phase 1 platform is designed to receive an input ECG signal and then process and report/provide the results of an AI-ECG algorithm which in combination with the AI-ECG algorithm forms the containerized medical device for regulatory purposes. As such, FDA clearance of the Phase 1 platform would be sought contemporaneously with FDA clearance of our first cloud-based AI-ECG algorithm as the two go hand in glove for regulatory purposes. This type of algorithm cloud platform is generally well defined for regulatory purposes. We already have built a working beta version of the MyoVista Insights Cloud Platform and expect it to be complete in the first calendar quarter of 2025.
We expect our first AI-ECG cloud-based algorithm to be a low ejection fraction (LVEF ≤ 40) algorithm licensed from Mount Sinai. There is a well-defined predicate for this algorithm which provides greater clarity for the regulatory pathway which we expect to be 510(k), accordingly, we expect this process will be relatively straightforward, assuming appropriate clinical performance of the algorithm. This algorithm was developed using over 100,000 patient records and in published clinical data had strong performance comparable to the predicate. We are in the process of undertaking further work to assess and, if necessary, adjust the algorithm, and prepare for an FDA validation study and submission. We expect the validation study will be performed using retrospective data, i.e. validation using pre-existing ECG’s rather than requiring active patient recruitment, which would reduce costs and timescales compared to prospective clinical validation.
To date we have not yet entered into a discussion with the FDA regarding the MyoVista Insights Cloud Platform or Mount Sinai licensed AI-ECG algorithms but expect to begin that process in the current calendar year. We expect these products to fall under the 510(k) pathway and are aiming for an FDA submission of our MyoVista Insights Cloud Platform and low ejection fraction algorithm in the second half of 2025.
Phase 2 and beyond of the MyoVista Insights Cloud Platform would expand user functionality and connectivity and integration with electronic medical record systems, ECG management systems and direct to devices.
Following clearance of the Phase 1 platform we then expect to bring forward a pipeline of other AI-ECG algorithms. As they would be delivered on the pre-cleared MyoVista Insights Cloud Platform the regulatory focus would then be focused primarily on the clinical performance of each algorithm. We have already licensed a number of AI-ECG algorithms from Mount Sinai and have relationships with other clinical institutions which would enable us to develop further algorithms. We are also developing the MyoVista Insights Cloud Platform to be able to host third-party algorithms.
MyoVista wavECG Product and Technology
The HeartSciences developed cardiac dysfunction algorithm is being incorporated into the MyoVista wavECG device. It has been developed in response to the relatively recent understanding in cardiology that most forms of heart disease are associated with LV relaxation abnormalities and diastolic dysfunction. The MyoVista wavECG is a 12-lead resting ECG device featuring our proprietary algorithm designed to detect cardiac dysfunction in the diastolic phase, which is specifically slower than normal left ventricular relaxation rates, in accordance with recent clinical findings and the American Society of Echocardiology Guidelines.
The MyoVista wavECG also includes the capabilities of a full-featured conventional 12-lead resting ECG including analysis using the Glasgow Algorithm, also known as the Glasgow ECG Interpretation Algorithm. Developed by the University of Glasgow in the United Kingdom, the 12-lead ECG Analysis Algorithm has been relied upon for more than 35 years and is a widely used resting ECG interpretive algorithm. The Glasgow Algorithm has been improved over the years and is licensed to us pursuant to a licensing agreement with The University Court of the University of Glasgow. Under this licensing agreement, we obtained a non-exclusive, worldwide license with automatic renewal provisions and the right to license: (i) software modules for an Android-based platform for the analysis of resting 12-lead electrocardiograms and (ii) all intellectual property rights (including patents, copyright, trademarks, trade secrets and know-how) relating to the software modules to be used in the MyoVista wavECG (the “Glasgow Licensing Agreement”).
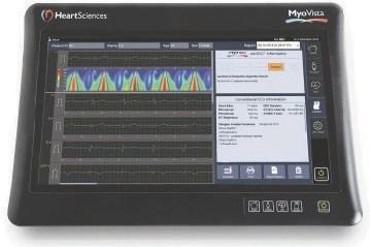
The MyoVista wavECG combines both, the conventional ECG capabilities (including the Glasgow Algorithm) and our proprietary AI-ECG algorithm, designed to detect impaired left ventricular cardiac relaxation abnormalities, as a single test with results presented separately. The MyoVista wavECG has a high-resolution touchscreen display and incorporates many intuitive features commonly associated with a tablet device. In the future we would expect to incorporate further AI-ECG algorithms in the MyoVista wavECG.
MyoVista wavECG device with 1 lead view of signal processed waveform
Market Opportunity
Diagnostic Gap
We believe that the most significant diagnostic gap in heart disease is early identification. Heart disease often remains asymptomatic for many years until it reaches an acute stage, at which point many patients have a heart attack or die without prior diagnosis of disease. For this reason, heart disease is often referred to as the “silent killer.” In 2012, the United States Preventative Services Task Force stated that there is no good evidence that an ECG helps physicians predict heart risks in people with no symptoms any better than traditional considerations such as smoking, blood pressure and cholesterol levels, acknowledging the diagnostic gap that currently exists.
According to the CDC, cardiovascular disease remains the largest cost for the U.S. healthcare system at approximately $219 billion per year. The cost of treating acute cardiac events and heart failure is especially high in comparison to preventative treatment. Governments, healthcare providers and third-party payors are focused on shifting the diagnosis and management of heart disease to earlier stages where better patient outcomes can be delivered at lower cost; however, to make substantial progress the existing diagnostic gap needs to be closed.
We believe that the scale of cardiac disease as well as changing demographics, growing ECG market, impetus to identify risks earlier through low-cost testing, along with the increasing number and type of health care settings creates a significant opportunity for a device such as the MyoVista wavECG.
Changing Demographics
Heart disease is most commonly found in individuals aged 65 and older with incidences of heart disease increasing at 65 years for men and 71.8 years for women. According to the Organization for Economic Co-operation and Development, advances in the field of medicine have led to an increase in life expectancy which, as of 2020, was estimated to average 77.3. years for a person in the U.S., up from 75.4 years in 1990. As life expectancy increases, the average age of the population is expected to increase. According to the U.S. Health and Human Services — Office of the Inspector General (the “HHS”), the population age 65 and older increased from 38.8 million in 2008 to 52.4 million in 2018 (a 35% increase) and is projected to reach 94.7 million by 2060. By 2030, more than 20 percent of U.S. residents are projected to be age 65 and over. Since heart disease is most commonly found in individuals aged 65 years and older, and that population pool is increasing, we believe there is a significant opportunity for a device such as the MyoVista wavECG as well the MyoVista Insights Cloud Platform.
Growing ECG Market
The demand for electrocardiograph devices and related supplies known as electrodes is on the rise worldwide. Despite the limitations of the conventional ECG and healthcare guidance around the world that recommends against its use for screening, in the absence of a better alternative, the ECG remains a ubiquitous and widely used test throughout healthcare including non-cardiology settings. It is estimated that 1.5 million to 3.0 million ECGs are performed worldwide every day, making it one of the most commonly used cardiovascular diagnostic tests in healthcare and a fundamental tool in clinical practice. It is estimated that more than 100 million ECGs are performed each year in the United States. The 2019 National Ambulatory Medical Care Survey indicated that office-based patient care physicians, excluding anesthesiologists, radiologists and pathologists, ordered or provided 47 million ECG tests during office visits, and the 2020 National Hospital Ambulatory Medical Care Survey showed that during ambulatory care visits to hospital emergency departments, an additional 32 million ECG tests were ordered or performed by hospital emergency departments.
With the advent of advanced technology, ECG testing market research reports demonstrate that market growth in ECG devices and use is increasing. Precedence Research, a Canada/India based market research company recently released market research on the global electrocardiograph market for 2023, the market size is expected grow significantly from $10.93 billion in 2023 to $25.56 billion by 2032.
Impetus to Identify Risks Earlier for More Effective Low-Cost Testing
A key goal of the HHS is reducing healthcare costs. This places pressure on physicians and healthcare institutions to contain healthcare costs. Additionally, one of the key objectives of HHS’s Healthy People 2030, is to increase preventive care for people of all ages. We believe that efforts towards preventive care and maintenance will lead to more testing for high-risk individuals and patients who have existing cardiac conditions. This trend, we believe, in tandem with the push to shorten hospital stays, has created an impetus to identify pre-symptomatic patients at risk more effectively at the front-line physician or clinic level and to treat recovering cardiac patients through outpatient care and rehabilitation.
It is our belief that the MyoVista wavECG device and the MyoVista Insights Cloud Platform incorporating our AI-ECG algorithms, would be well positioned to respond to the global need for more effective, low-cost ECG testing to facilitate improved referral processes for heart disease.
Changing Nature of Healthcare Providers
The delivery of healthcare in the U.S. is evolving. Alternative treatment sites, such as retail clinics, concierge medicine, urgent care clinics and ambulatory surgical centers, deliver care from qualified providers in settings outside of emergency departments, hospitals or traditional physician offices. We expect this trend to accelerate the drive to provide more effective preventative care and represents a significant opportunity for the introduction of our AI-ECG algorithms that offer an enhanced ability to screen for heart disease.
Capitation Provides an Incentive to Identify Medicare Advantage Patients
Healthcare providers are paid either through fee-for-service or capitation. Fee-for-service is a payment model where services are unbundled and paid for separately. In health care, the fee-for-service payment model incentivizes physicians to provide more treatments because payment is dependent on the quantity, rather than quality, of care. Capitation is a payment arrangement that pays a physician or group of physicians a set amount for each enrolled person assigned to them, per period of time, whether or not that person seeks care. Under capitation, the amount of remuneration is based on the average expected healthcare utilization of that patient, with greater payment for patients with a significant history of medical problems.
Approximately 48% (approximately 28 million people) of those covered by Medicare according to CMS are enrolled in a Medicare Advantage plan. With respect to these patients, CMS pays capitation to healthcare providers. CMS uses risk adjustment to adjust capitation payments to health plans, either higher or lower, to account for the differences in the health costs of individuals with ailments such as heart failure, CAD, angina and valvular heart disease. Accordingly, under CMS guidelines, risk factor adjustments per patient will provide payment that is higher for sicker patients who have conditions where diagnosis codes are documented in the medical record as a result of a face-to-face visit. Therefore, there is a financial incentive to identify those Medicare Advantage patients who are sicker, including those who have undiagnosed ailments such as heart disease. We believe that undiagnosed heart disease represents a significant problem, and we believe insurance plans that have a high number of Medicare Advantage patients could be a target market for the MyoVista cloud-based and hardware-based platforms.
Market Strategy
General
Our objective is to provide AI-ECG solutions in any care setting worldwide in a manner that best suits different providers, either via one of the millions of ECG’s currently in clinical use or via our proprietary MyoVista wavECG device in order to significantly improve front-line testing and referral processes for heart disease. Our business model is primarily focused on recurring revenues for each of the MyoVista Insights Cloud Platform and the MyoVista wavECG device.
The initial revenue model for the MyoVista wavECG device involves the capital sale of the device and recurring revenue from the sale of its proprietary supplies (electrodes) for each test. We would expect to charge for recurring software revenues from the use of AI-ECG algorithms delivered via the MyoVista Insights Cloud Platform or made available on the MyoVista wavECG device. There are estimated to be millions of ECGs in use worldwide and the MyoVista Insights Cloud Platform is intended to be device-agnostic, thereby facilitating the provision of AI-ECG algorithms to physicians from existing devices. In short, we expect to generate recurring supplies or software revenues and do not expect to rely on high initial capital or device pricing in order to encourage adoption of our AI-ECG algorithms.
Territories
Our initial sales focus will primarily be within the U.S. and European markets where we have established relationships. We intend to market our products in the U.S. using a direct sales force following FDA clearance. Outside of the U.S., for markets such as Europe and Latin America, we intend to utilize medical device distributors that have existing healthcare provider relationships and experience selling ECG devices, which will be supported by a small number of local field personnel.
Potential Markets
We believe that there is a large variety of potential markets for AI-ECG algorithms with new diagnostic capabilities that are not currently available for ECG devices. Conventional ECGs are used throughout healthcare in almost every clinical setting including clinics, doctor’s offices, urgent care centers, and hospitals. We believe that, in many of those settings, the additional information provided by the AI-ECG algorithms could be extremely valuable.
Our AI-ECG algorithms range of applications and potential uses are vast, and include providing:
| ● | Primary care — front-line cardiac testing/referral tool, heart disease screening. |
| ● | Retail Healthcare — access to ECG testing at retail sites such as CVS and Walgreens. |
| ● | Emergency Departments — enhanced ECG testing for emergency room patients. |
| ● | Cardiologists — prescreening cardiology patients. |
| ● | Hospitals — in-patient testing or testing prior to discharge, particularly cardiac wards. |
| ● | Surgery — pre-anesthesia testing, pre/post intervention. |
| ● | Life Insurance testing — ECGs when required in connection with the issuance of life insurance policies. |
| ● | Specialty Environments — screening for conditions such as, cardiomyopathy, cardiac oncology, drug trials, heart failure, and diabetes. |
| ● | Athlete testing — cardiac screening programs for athletes. |
Early Target Markets
Initially, our focus markets in the U.S. will include: cardiology; primary care providers that serve upper to middle income regions including concierge medicine providers; health systems, retail clinics; and insurers with high levels of Medicare Advantage patients. As additional algorithms obtain FDA clearance, HeartSciences will extend its sales efforts to clinics and physicians that will benefit the most from the specific AI-ECG algorithm.
Reimbursement
In addition to targeting the health care settings described above, a key element of our strategy is to ensure each algorithm qualifies for reimbursement from third-party payors such as CMS (Medicare payor). CPT codes are numbers assigned to each task or service provided by a healthcare provider including medical, surgical and diagnostic services. Insurers use the numbers to determine the procedure and the amount to pay a provider. The American Medical Association has already issued a temporary Current Procedural Terminology (CPT) Category III code for novel AI assistive algorithmic ECG risk assessment for cardiac dysfunction. These codes are designed to facilitate the use, adoption, and potential reimbursement of emerging technologies. This provides physicians and clinical institutions the ability to bill for HeartSciences algorithms that detect different types of heart dysfunction such as systolic and diastolic dysfunction. While we cannot be certain that these new codes will ultimately lead to the issuance of permanent CPT Category I codes, or that insurance coverage or payment can be obtained, if successful, this could potentially provide total reimbursement that is larger than reimbursement for conventional ECG devices, which, in turn, could provide MyoVista wavECG device and the MyoVista Insights Cloud Platform delivering AI-ECG algorithms with a competitive advantage as compared to conventional ECG testing and devices. The MyoVista wavECG device also includes conventional ECG testing capabilities and is expected to also qualify for Medicare reimbursement for existing ECG testing procedures with interpretation and report ranges from approximately $17 to $55 depending on the type of healthcare facility. These charges would go directly to the healthcare facility/physician.
Competition
The medical device industry is characterized by rapidly advancing technologies, intense competition, and a strong emphasis on proprietary products. There are many medical device companies, biotechnology companies, public and private universities and research organizations actively engaged in the research and development of products that may be similar to HeartSciences AI-ECG algorithms and MyoVista hardware. Competitors could include traditional ECG manufacturers such as GE Healthcare Technologies, Inc., (“GE Healthcare”), Koninklijke Philips N.V. (“Phillips”), Baxter International, Inc. (“Baxter”), and Nihon Kohden Corporation that may seek to innovate, and new commercial entrants to the AI ECG market, such as Anumana, Inc. or companies involved in AI healthcare, such as Tempus Labs, Inc. or VIZ.ai that also see the opportunity to bring innovation in a market that, we believe, has significant need for improved products and technology change.
Intellectual Property
Our technology is protected by a patent portfolio as well as trade secrets, which together comprise an important part of the intellectual property protection for our existing and licensed proprietary algorithms (especially when developing proprietary algorithms). We believe that the combination of patents and trade secrets creates valuable competitive barriers in favor of HeartSciences.
The USPTO has issued eight utility patents and one design patent to us, and a patent allowance for a utility patent exclusively licensed to us. The patent expiration dates range from March 2031 to August 2040. We also have fourteen international design registrations and sixteen international utility patents granted (with expiration dates ranging from September 2036 to March 2037) in jurisdictions such as China, Japan, South Korea, the United Kingdom, France, Germany, Mexico and Australia. We currently have two patent allowances in Europe, United Arab Emirates and Brazil and also have additional pending patent applications in various jurisdictions.
In addition, we have entered into two agreements that are material to our rights to the intellectual property utilized in the MyoVista wavECG:
| ● | In January 2014, we entered into an invention assignment agreement under which certain specified MyoVista wavECG technology and proprietary and intellectual property rights thereto (including patents, copyright, trademarks, trade secrets and know-how) were transferred and assigned to us by the inventor; and |
| ● | In December 2015, we entered the Glasgow Licensing Agreement with The University Court of the University of Glasgow under which we obtained a non-exclusive, worldwide license to software modules for an Android platform for analysis of resting 12-lead electrocardiograms and all intellectual property rights (including patents, copyright, trademarks, trade secrets and know-how) relating to the software modules to be used in the MyoVista wavECG. |
Research and Development
The Company’s R&D staff designs our hardware, software and internally developed AI-ECG algorithms. Hardware development assistance is provided by outside consulting firms. The Company internally develops the software for the device along with the assistance of multiple software development contractors. The data science work necessary to build the AI-ECG algorithms is performed both internally and externally using data science experts.
Incorporation of all software elements into the MyoVista wavECG hardware is performed internally. We currently employ six full-time R&D staff.
We believe, based on our research and other published research, that further algorithms could be developed for a range of additional clinical indications. To accelerate HeartSciences’ route to market with additional algorithms we entered into multiple license agreements with Mount Sinai on September 20, 2023. Please see the section, “Agreements with Mount Sinai related to Commercialization of Multiple AI-based Cardiovascular ECG Algorithms developed by Mount Sinai” for additional information regarding these license agreements. Studies involving the use of the MyoVista wavECG and proof of concept algorithms for alternative clinical indications have already been published in addition to the growing body of third-party published research in this field.
On November 29, 2022, we entered into a multi-year collaboration agreement with Rutgers, The State University of New Jersey, to research and develop additional AI-ECG algorithms.
FDA and Other Government Regulation
Our products are subject to regulation by the FDA and various other federal and state agencies, as well as by foreign governmental agencies. These agencies enforce laws and regulations that govern the development, testing, manufacturing, labeling, advertising, marketing and distribution, and market surveillance of our medical device products.
In addition to those indicated below, the only other regulations we encounter are regulations that are common to all businesses, such as employment legislation, implied warranty laws, and environmental, health and safety standards, to the extent applicable. We will also encounter in the future industry-specific government regulations that would govern our device, if and when developed for commercial use. It may become the case that other regulatory approvals will be required for the design and manufacture of our device.
FDA Requirements and Other Regulatory Approval Requirements
Our products are subject to regulation under the Federal Food, Drug, and Cosmetic Act (the “FDCA”) as implemented and enforced by the FDA. The FDA regulates the development, design, non-clinical and clinical research, manufacturing, safety, efficacy, labeling, packaging, storage, installation, servicing, recordkeeping, premarket clearance or approval, import, export, adverse event reporting, advertising, promotion, marketing and distribution of medical devices to ensure that medical devices distributed domestically are safe and effective for their intended uses and otherwise meet the requirements of the FDCA.
In addition to U.S. regulations, we are subject to a variety of regulations in the European Economic Area (the “EEA”) governing clinical trials and the commercial sales and distribution of our products. Whether or not we have or are required to obtain FDA clearance or approval for a product, we will be required to obtain authorization before commencing clinical trials and to obtain marketing authorization or approval of our products under the comparable regulatory authorities of countries outside of the United States before we can commence clinical trials or launch sales of our products in those countries. The approval process varies from country to country and the time may be longer or shorter than that required for FDA clearance or approval. Medical devices are generally subject to varying levels of regulatory control based on risk level of the device.
A clearance or authorization letter from the FDA authorizes commercial marketing of the device for one or more specific indications of use. After clearance or authorization, the Company will be required to comply with a number of post-clearance requirements, including, but not limited to, Medical Device Reporting and complaint handling, and, if applicable, reporting of corrective actions. Also, quality control and manufacturing procedures must continue to conform to the QSR. The FDA periodically inspects manufacturing facilities to assess compliance with QSRs, which impose extensive procedural, substantive, and record keeping requirements on medical device manufacturers. In addition, changes to the manufacturing process are strictly regulated, and, depending on the change, validation activities may need to be performed. Accordingly, manufacturers must continue to expend time, money and effort in the area of production and quality control to maintain compliance with the QSR and other types of regulatory controls.
The FDA and the Federal Trade Commission, or FTC, will also regulate the advertising claims of the Company’s products to ensure that the claims it makes are consistent with its regulatory clearances, that there is scientific data to substantiate the claims and that product advertising is neither false nor misleading.
FDA Clearance Process and FDA Validation Clinical Study
Unless an exemption applies, each medical device commercially distributed in the United States requires FDA clearance of a 510(k) premarket notification, granting of a de novo request, or approval of an application for premarket approval, or PMA. Under the FDCA, medical devices are classified into one of three classes — Class I, Class II or Class III — depending on the degree of risk associated with each medical device and the extent of regulatory controls needed to ensure its safety and effectiveness.
Class I includes devices with the lowest risk to the patient and are those for which safety and effectiveness can be assured by adherence to the FDA’s General Controls for medical devices, which include compliance with the applicable portions of the Quality System Regulation, or QSR, facility registration and product listing, reporting of adverse medical events, and truthful and non-misleading labeling, advertising and promotional materials.
Class II devices are subject to the FDA’s General Controls, and special controls as deemed necessary by the FDA to ensure the safety and effectiveness of the device. These special controls can include performance standards, post-market surveillance, patient registries and FDA guidance documents. While most Class I devices are exempt from the 510(k) premarket notification requirement, manufacturers of most Class II devices are required to submit to the FDA a premarket notification under Section 510(k) of the FDCA requesting permission to commercially distribute the device. The FDA’s permission to commercially distribute a device subject to class II controls is generally described as 510(k) clearance.
Devices deemed by the FDA to pose the greatest risks, such as life sustaining, life supporting or some implantable devices, or devices that have a new intended use, or use advanced technology that is not substantially equivalent to that of a legally marketed device, are placed in Class III, requiring approval of a PMA. Some pre-amendment devices are unclassified but are subject to FDA’s premarket notification and clearance process in order to be commercially distributed.
510(k) Clearance Marketing Pathway
To obtain 510(k) clearance, we must submit to the FDA a premarket notification submission demonstrating that the proposed device is “substantially equivalent” to a predicate device already on the market. A predicate device is a legally marketed device that is not subject to PMA, i.e., a device that was legally marketed prior to May 28, 1976 (pre-amendments device) and for which a PMA is not required, a device that has been reclassified from Class III to Class II or I, or a device that was found substantially equivalent through the 510(k) process. The FDA’s 510(k) clearance process usually takes from three to twelve months, but often takes longer. The FDA may require additional information, including clinical data, to make a determination regarding substantial equivalence. In addition, the FDA collects user fees for certain medical device submissions and annual fees for medical device establishments.
If the FDA agrees that the device is substantially equivalent to a predicate device currently on the market, it will grant 510(k) clearance to commercially market the device. If the FDA determines that the device is “not substantially equivalent” to a previously cleared device, the device is automatically designated as a Class III device. The device sponsor must then fulfill more rigorous PMA requirements or can request a risk-based classification determination for the device in accordance with the “De Novo” process, which is a route to market novel medical devices that are low to moderate risk and are not substantially equivalent to a predicate device.
After a device receives 510(k) clearance, any modification that could significantly affect its safety or effectiveness, or that would constitute a major change in its intended use, will require a new 510(k) or possibly a PMA. The FDA requires each manufacturer to make this determination initially, but the FDA can review any such decision and can disagree with a manufacturer’s determination. If the FDA disagrees with our determination not to seek a new 510(k) clearance, the FDA may retroactively require us to seek 510(k) clearance or possibly a PMA. The FDA could also require us to cease marketing and distribution and/or recall the modified device until 510(k) clearance or a PMA is obtained. Also, in these circumstances, we may be subject to significant regulatory fines and penalties.
PMA Approval Pathway
Class III devices require approval of a PMA before they can be marketed, although some pre-amendment Class III devices for which the FDA has not yet required a PMA are cleared through the 510(k) process. The PMA process is more demanding than the 510(k) premarket notification process. In a PMA application, the manufacturer must demonstrate that the device is safe and effective, and the PMA application must be supported by extensive data, including data from preclinical studies and human clinical trials. The PMA application must also contain a full description of the device and its components, a full description of the methods, facilities, and controls used for manufacturing, and proposed labeling. Following receipt of a PMA application, the FDA determines whether the application is sufficiently complete to permit a substantive review. If the FDA accepts the application for review, it has 180 days under the FDCA to complete its review of a PMA application, although in practice, the FDA’s review often takes significantly longer, and can take up to several years. An advisory panel of experts from outside the FDA may be convened to review and evaluate the application and provide recommendations to the FDA as to the approvability of the device. The FDA may or may not accept the panel’s recommendation. In addition, the FDA will generally conduct a pre-approval inspection of the applicant or its third-party manufacturers’ or suppliers’ manufacturing facility or facilities to ensure compliance with the QSR. PMA devices are also subject to the payment of user fees.
The FDA will approve the new device for commercial distribution if it determines that the data and information in the PMA application constitute valid scientific evidence and that there is reasonable assurance that the device is safe and effective for its intended use(s). A PMA may include post-approval conditions intended to ensure the safety and effectiveness of the device, including, among other things, restrictions on labeling, promotion, sale and distribution, and collection of long-term follow-up data from patients in the clinical study that supported the PMA or requirements to conduct additional clinical studies post-approval. The FDA may condition PMA approval on some form of post-market surveillance when deemed necessary to protect the public health or to provide additional safety and efficacy data for the device in a larger population or for a longer period of use. In such cases, the manufacturer might be required to follow certain patient groups for a number of years and to make periodic reports to the FDA on the clinical status of those patients. Failure to comply with the conditions of approval can result in material adverse enforcement action, including withdrawal of the approval.
Certain changes to an approved device, such as changes in manufacturing facilities, methods, or quality control procedures, or changes in the design performance specifications, which affect the safety or effectiveness of the device, require submission of a PMA supplement. PMA supplements often require submission of the same type of information as a PMA, except that the supplement is limited to information needed to support any changes from the device covered by the original PMA and may not require as extensive clinical data or the convening of an advisory panel. Certain other changes to an approved device require the submission of a new PMA, such as when the design change causes a different intended use, mode of operation, and technical basis of operation, or when the design change is so significant that a new generation of the device will be developed, and the data that were submitted with the original PMA are not applicable for the change in demonstrating a reasonable assurance of safety and effectiveness. None of our currently developed products require a PMA to be marketed.
De Novo Classification
Medical device types that the FDA has not previously classified as Class I, II or III are automatically classified into Class III regardless of the level of risk they pose. The Food and Drug Administration Modernization Act of 1997 established a new route to market for low to moderate risk medical devices that are automatically placed into Class III due to the absence of a predicate device, called the “Request for Evaluation of Automatic Class III Designation,” or the De Novo classification procedure.
This procedure allows a manufacturer whose novel device is automatically classified into Class III to request down-classification of its medical device into Class I or Class II on the basis that the device presents low or moderate risk, rather than requiring the submission and approval of a PMA application. Prior to the enactment of the Food and Drug Administration Safety and Innovation Act of 2012, or FDASIA, a medical device could only be eligible for De Novo classification if the manufacturer first submitted a 510(k) pre-market notification and received a determination from the FDA that the device was not substantially equivalent. FDASIA streamlined the De Novo classification pathway by permitting manufacturers to request De Novo classification directly without first submitting a 510(k) pre-market notification to the FDA and receiving a not substantially equivalent determination. Under FDASIA, the FDA is required to classify the device within 120 days following receipt of the De Novo application. If the manufacturer seeks reclassification into Class II, the manufacturer must include a draft proposal for special controls that are necessary to provide a reasonable assurance of the safety and effectiveness of the medical device. In addition, the FDA may reject the reclassification petition if it identifies a legally marketed predicate device that would be appropriate for a 510(k) or determines that the device is not low to moderate risk or that general controls would be inadequate to control the risks and special controls cannot be developed.
After initial authorization, any modification that could significantly affect its safety or effectiveness, or that would constitute a major change or modification in its intended use, will require a new 510(k) clearance or, depending on the modification, another De Novo classification request, or PMA approval. The FDA requires each manufacturer to determine whether the proposed change requires submission of a 510(k) or a PMA in the first instance, but the FDA can review any such decision and disagree with a manufacturer’s determination. If the FDA disagrees with a manufacturer’s determination, the FDA can require the manufacturer to cease marketing and/or request the recall of the modified device until 510(k) marketing clearance or PMA approval is obtained. Also, in these circumstances, the manufacturer may be subject to significant regulatory fines or penalties.
The MyoVista wavECG device along with its proprietary software and hardware is classified as a Class II medical device by the FDA. We previously submitted and intended to seek authorization to market the device through submission under the De Novo pathway, however in December 2023 the FDA confirmed that we could submit the MyoVista wavECG device for clearance under the 510(k) pathway following the grant by the FDA in August 2023 of an industry-first De Novo clearance which created a new Class II product code for cardiovascular machine learning-based notification software. Accordingly, we are now preparing for a 510(k) FDA submission and are aiming for a submission in the first calendar quarter of 2025. To date we have not yet entered into a discussion with the FDA regarding the MyoVista Insights Cloud Platform or Mount Sinai licensed AI-ECG algorithms but are aiming to start that process in the current calendar year. We expect these products to fall under the 510(k) pathway and are aiming for an FDA submission of our MyoVista Insights Cloud Platform and low ejection fraction algorithm in the second half of 2025. Delays in receipt or failure to receive the necessary clearances, or the failure to comply with existing or future regulatory requirements, could reduce our business prospects.
Clinical Trials
Clinical trials are almost always required to support a De Novo request and are sometimes required to support a 510(k) submission. All clinical investigations of investigational devices to determine safety and effectiveness must be conducted in accordance with the FDA’s investigational device exemption, or IDE, regulations which govern investigational device labeling, prohibit promotion of the investigational device, and specify an array of recordkeeping, reporting and monitoring responsibilities of study sponsors and study investigators. If the device presents a “significant risk” to human health, as defined by the FDA, the FDA requires the device sponsor to submit an IDE application to the FDA, which must become effective prior to commencing human clinical trials. A significant risk device is one that presents a potential for serious risk to the health, safety or welfare of a patient and either is implanted, used in supporting or sustaining human life, substantially important in diagnosing, curing, mitigating or treating disease or otherwise preventing impairment of human health, or otherwise presents a potential for serious risk to a subject. An IDE application must be supported by appropriate data, such as animal and laboratory test results, showing that it is safe to test the device in humans and that the testing protocol is scientifically sound. The IDE will automatically become effective 30 days after receipt by the FDA unless the FDA notifies us that the investigation may not begin. If the FDA determines that there are deficiencies or other concerns with an IDE for which it requires modification, the FDA may permit a clinical trial to proceed under a conditional approval.
In addition, the study must be approved by, and conducted under the oversight of, an Institutional Review Board, or IRB, for each clinical site. The IRB is responsible for the initial and continuing review of the IDE, and may pose additional requirements for the conduct of the study. If an IDE application is approved by the FDA and one or more IRBs, human clinical trials may begin at a specific number of investigational sites with a specific number of patients, as approved by the FDA. If the device presents a non-significant risk to the patient, a sponsor may begin the clinical trial after obtaining approval for the trial by one or more IRBs without separate approval from the FDA, but must still follow abbreviated IDE requirements, such as monitoring the investigation, ensuring that the investigators obtain informed consent, and labeling and record-keeping requirements. Acceptance of an IDE application for review does not guarantee that the FDA will allow the IDE to become effective and, if it does become effective, the FDA may or may not determine that the data derived from the trials support the safety and effectiveness of the device or warrant the continuation of clinical trials. An IDE supplement must be submitted to, and approved by, the FDA before a sponsor or investigator may make a change to the investigational plan that may affect its scientific soundness, study plan or the rights, safety or welfare of human subjects.
During a study, the sponsor is required to comply with the applicable FDA requirements, including, for example, trial monitoring, selecting clinical investigators and providing them with the investigational plan, ensuring IRB review, adverse event reporting, record keeping and prohibitions on the promotion of investigational devices or on making safety or effectiveness claims for them. The clinical investigators in the clinical study are also subject to FDA regulations and must obtain patient informed consent, rigorously follow the investigational plan and study protocol, control the disposition of the investigational device, and comply with all reporting and recordkeeping requirements. Additionally, after a trial begins, we, the FDA or the IRB could suspend or terminate a clinical trial at any time for various reasons, including a belief that the risks to study subjects outweigh the anticipated benefits.
Post-market Regulation
After a device is cleared or approved for marketing, numerous and pervasive regulatory requirements continue to apply. These include:
| ● | establishment registration and device listing with the FDA; |
| ● | QSR requirements, which require manufacturers, including third-party manufacturers, to follow stringent design, testing, control, documentation and other quality assurance procedures during all aspects of the design and manufacturing process; |
| ● | labeling and marketing regulations, which require that promotion is truthful, not misleading, fairly balanced and provide adequate directions for use and that all claims are substantiated, and also prohibit the promotion of products for unapproved or “off-label” uses and impose other restrictions on labeling; FDA guidance on off-label dissemination of information and responding to unsolicited requests for information; |
| ● | clearance or approval of product modifications to 510(k)-cleared devices, or those re-classified to 510(k) cleared devices, that could significantly affect safety or effectiveness or that would constitute a major change in intended use of one of our cleared devices, or approval of a supplement for certain modifications to PMA devices; |
| ● | medical device reporting regulations, which require that a manufacturer report to the FDA if a device it markets may have caused or contributed to a death or serious injury, or has malfunctioned and the device or a similar device that it markets would be likely to cause or contribute to a death or serious injury, if the malfunction were to recur; |
| ● | correction, removal and recall reporting regulations, which require that manufacturers report to the FDA field corrections and product recalls or removals if undertaken to reduce a risk to health posed by the device or to remedy a violation of the FDCA that may present a risk to health; |
| ● | complying with the new federal law and regulations requiring Unique Device Identifiers (UDI) on devices and also requiring the submission of certain information about each device to the FDA’s Global Unique Device Identification Database (GUDID); |
| ● | the FDA’s recall authority, whereby the agency can order device manufacturers to recall from the market a product that is in violation of governing laws and regulations; and |
| ● | post-market surveillance activities and regulations, which apply when deemed by the FDA to be necessary to protect the public health or to provide additional safety and effectiveness data for the device. |
The manufacturing processes for medical devices are required to comply with the applicable portions of the QSR, which cover the methods and the facilities and controls for the design, manufacture, testing, production, processes, controls, quality assurance, labeling, packaging, distribution, installation and servicing of finished devices intended for human use. The QSR also requires, among other things, maintenance of a device master file, device history file, and complaint files. These requirements impose certain procedural and documentation requirements upon us and our third-party manufacturers related to the methods used in and the facilities and controls used for designing, manufacturing, packaging, labeling, storing, medical devices. As a manufacturer, we will be subject to periodic scheduled or unscheduled inspections by the FDA. Following these inspections, the FDA may assert noncompliance with QSR requirements on a Form 483, which is a report of observations from an inspection, or by way of “untitled letters” or “warning letters” that could cause us or any third-party manufacturers to modify certain activities. A Form 483 notice, if issued at the conclusion of an FDA inspection, can list conditions the FDA investigators believe may have violated QSR or other FDA requirements. We cannot be certain that we or our present or any future third-party manufacturers or suppliers will be able to comply with QSR or other FDA regulatory requirements to the agency’s satisfaction. Failure to comply with these obligations may lead to possible legal or regulatory enforcement action by the FDA.
The FDA has broad regulatory compliance and enforcement powers. If the FDA determines that we failed to comply with applicable regulatory requirements, it can enforce a variety of compliance or enforcement actions, which may result in any of the following sanctions:
| ● | warning letters, untitled letters, fines, injunctions, consent decrees and civil penalties; |
| ● | recalls, withdrawals, or administrative detention or seizure of our device; |
| ● | operating restrictions or partial suspension or total shutdown of production; |
| ● | refusing or delaying requests for 510(k) marketing clearance or PMA approvals of new products or modified products; |
| ● | withdrawing 510(k) clearances or PMA approvals that have already been granted; |
| ● | refusal to grant export or import approvals for our device; or |
Advertising and Promotion
The FDA and other regulatory agencies closely regulate the post-approval marketing and promotion of medical devices, including standards and regulations for direct-to-consumer advertising, communications about unapproved uses, industry-sponsored scientific and educational activities and promotional activities involving the internet. Devices may be marketed only for the approved or cleared indications and in accordance with the provisions of the approved or cleared label.
Foreign Regulation
As we plan to market our device in the EU and other foreign markets, in addition to regulations in the United States, we will be subject to a variety of foreign regulations governing clinical trials and commercial sales and distribution of our device in foreign countries. Whether or not we obtain FDA approval for a product, we must obtain approval of a product by the comparable regulatory authorities of foreign countries before we can commence clinical trials or marketing of the product in those countries. The approval process varies from country to country, and the time may be longer or shorter than that required for FDA approval. The requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement also vary greatly from country to country and such regulatory requirements have been changing and increasing in some countries. We may be unable to maintain regulatory qualifications, clearances, approvals or CE Certificates of Conformity in these countries or to obtain clearances or approvals in other countries. We may incur significant costs in attempting to obtain, renew, or modify foreign regulatory clearances or approvals, qualifications or CE Certificates of Conformity. If we experience difficulties in receiving, maintaining, renewing or modifying necessary qualifications, clearances, approvals or CE Certificates of Conformity to market our products outside the United States, or if we fail to receive, renew, modify or maintain those qualifications, clearances, approvals or CE Certificates of Conformity, we may be unable to market our products or enhancements in certain international markets effectively, or at all.
On April 5, 2017, a regulation on medical devices was adopted to establish a modernized and more robust European Union legislative framework, with the aim of ensuring better protection of public health and patient safety: Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on medical devices, amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and repealing Council Directives 90/385/EEC and 93/42/EEC, which became applicable from May 26, 2021, (the “EU MDR”). The EU MDR repeals and replaces the EU Medical Devices Directive and unlike directives, which must be given effect through transposition into the national domestic laws of the Relevant States, regulations are directly applicable (i.e., without the need for transposition into national laws implementing them) in all Relevant States. The EU MDR is also applicable in the EEA. Regulations (as EU law instruments) must be applied in their entirety across the EU so that legal acts are automatically and uniformly applied to all EU countries as soon as they enter into force to minimize variations that may arise in transposition of EU law into national law. These modifications may have an effect on the way we design and manufacture products and conduct our business in the EU and EEA. For example, as a result of the transition towards the new regime, Notified Bodies have lengthened their review times, and product introductions or modifications could be delayed or cancelled or otherwise rejected, which could adversely affect our ability to grow our business.
The Company previously achieved a CE Mark under the EU Medical Devices Directive (the “MDD”) in February 2017. The Medical Device Directive was established on June 14, 1993 but the MDD regulatory framework, has since been replaced by EU MDR. In order to sell in member countries of the EEA, our devices must now comply with the essential requirements of the EU MDR. Our CE Mark issued under the MDD lapsed in February 2022 and we will need to establish compliance under EU MDR. An updated CE mark certificate under EU MDR, which we have not yet obtained, would entitle the Company to market the MyoVista wavECG in the European Economic Area as well as other countries for which CE Mark represents an appropriate regulatory standard.
Implications of Being an “Emerging Growth Company” and a “Smaller Reporting Company”
We qualify as an “emerging growth company” under the Jumpstart our Business Startups Act of 2012, or the JOBS Act. For so long as we remain an emerging growth company, we may take advantage of relief from certain reporting requirements and other burdens generally applicable to public companies. In particular, as an emerging growth company we:
| ● | are not required to obtain an attestation and report from our auditors on our management’s assessment of our internal control over financial reporting pursuant to the Sarbanes-Oxley Act; |
| ● | are not required to provide a detailed narrative disclosure discussing our compensation principles, objectives and elements and analyzing how those elements fit with our principles and objectives (commonly referred to as “compensation discussion and analysis”); |
| ● | are not required to obtain a non-binding advisory vote from our shareholders on executive compensation or golden parachute arrangements (commonly referred to as the “say-on-pay,” “say-on-frequency” and “say-on-golden-parachute” votes); |
| ● | are exempt from certain executive compensation disclosure provisions requiring a pay-for-performance graph and CEO pay ratio disclosure; |
| ● | may present only two years of audited financial statements and only two years of related Management’s Discussion & Analysis of Financial Condition and Results of Operations (“MD&A”); and |
| ● | are eligible to claim longer phase-in periods for the adoption of new or revised financial accounting standards under §107 of the JOBS Act. |
We intend to take advantage of these reduced reporting requirements and exemptions, including the longer phase-in periods for the adoption of new or revised financial accounting standards under §107 of the JOBS Act. Our election to use the phase-in periods may make it difficult to compare our financial statements to those of non-emerging growth companies and other emerging growth companies that have opted out of the phase-in periods under §107 of the JOBS Act. Please see “Risk Factors — We are an ‘emerging growth company,’ and any decision on our part to comply with certain reduced disclosure requirements applicable to emerging growth companies could make the Common Stock less attractive to investors.”
Under the JOBS Act, we may take advantage of the above-described reduced reporting requirements and exemptions for up to five years after our initial sale of common equity on June 17, 2022, pursuant to a registration statement declared effective under the Securities Act, or such earlier time that we no longer meet the definition of an emerging growth company. In this regard, the JOBS Act provides that we would cease to be an “emerging growth company” if we have more than $1.235 billion in annual revenue, have more than $700 million in market value of our Common Stock held by non-affiliates (and are not otherwise eligible to be a smaller reporting company), or issue more than $1 billion in principal amount of non-convertible debt over a three-year period. Further, under current SEC rules we will continue to qualify as a “smaller reporting company” for so long as we have a public float (i.e., the market value of common equity held by non-affiliates) of less than $250 million as of the last business day of our most recently completed second fiscal quarter.
Certain of the reduced reporting requirements and exemptions available to us as an “emerging growth company” are also available to us due to the fact that we also qualify as a “smaller reporting company” under the SEC rules. For instance, smaller reporting companies are not required to obtain an auditor attestation and report regarding internal control over financial reporting; are not required to provide a compensation discussion and analysis; are not required to provide a pay-for-performance graph or CEO pay ratio disclosure; and may present only two years of audited financial statements and related MD&A disclosure.
If we are a smaller reporting company at the time we cease to be an emerging growth company, we may continue to rely on exemptions from certain disclosure requirements that are available to smaller reporting companies. We will continue to be a smaller reporting company so long as (i) the market value of our stock held by non-affiliates is less than $250 million as of the last business day of our second fiscal quarter or (ii) our annual revenue was less than $100 million during our most recently completed fiscal year and the market value of our stock held by non-affiliates is less than $700 million as of the last business day of our second fiscal quarter. Specifically, as a smaller reporting company we may choose to present only the two most recent fiscal years of audited financial statements in our Annual Reports on Form 10-K and, similar to emerging growth companies, smaller reporting companies have reduced disclosure obligations regarding executive compensation.
Recent Developments
Going Concern
On July 29, 2024, our independent registered public accounting firm issued an opinion on our audited financial statements, included in our Annual Report on Form 10-K for the year ended April 30, 2024, that contained an explanatory paragraph regarding substantial doubt about our ability to continue as a going concern because we have experienced recurring losses, negative cash flows from operations, and limited capital resources. These events and conditions raise substantial doubt about our ability to continue as a going concern.
Reverse Stock Split
On May 6, 2024, we filed a Certificate of Amendment to the Amended and Restated Certificate of Formation with the Secretary of the State of Texas to effect a reverse stock split of our outstanding shares of Common Stock, effective at the market open on May 17, 2024 (the “Reverse Stock Split”). As a result of the Reverse Stock Split, equitable adjustments corresponding to the Reverse Stock Split ratio were made to our outstanding warrants and our other convertible instruments and upon the exercise or vesting of all stock options such that every 100 shares of Common Stock that may be issued upon the exercise of our warrants and stock options and conversion of our other convertible instruments held immediately prior to the Reverse Stock Split represent one share of Common Stock that may be issued upon exercise of such warrants and stock options and conversion of the other convertible instruments immediately following the Reverse Stock Split. Correspondingly, the exercise price per share of Common Stock attributable to our warrants and stock options and the conversion price of our other convertible instruments immediately prior to the Reverse Stock Split was proportionately increased by a multiple of 100 following the Reverse Stock Split.
Patents
In May 2024, we were granted a patent from the Indian Patent Office covering MyoVista wavelet technology.
In July 2024, we were granted a patent allowance from the United States Patent and Trademark Office for the detection of left ventricular and/or right ventricular dysfunction using deep learning.
FRV Note Extension
On August 19, 2024, the Company and FRV entered into Amendment No. 6 to the Loan and Security Agreement to further extend the maturity date of the FRV Note to September 30, 2025 and pay the outstanding accrued interest as follows: (i) a payment of all accrued interest outstanding within five (5) business days of execution of the agreement; (ii) a payment of accrued interest on September 30, 2024 and (iii) thereafter all accrued interest due shall be payable at maturity. In August and September 2024, the Company paid approximately $305,000 in accrued interest to FRV.
Streeterville Note Purchase Agreement and Promissory Note
On September 6, 2024, we entered into a Note Purchase Agreement (the “Note Purchase Agreement”) with Streeterville Capital, LLC (“Streeterville”), an accredited investor, pursuant to which Streeterville issued our Company an unsecured promissory note in the amount of $2,510,000 (the “Streeterville Note”), which included an original issue discount of $500,000 and reimbursement of Streeterville’s transaction expenses of $10,000. The Streeterville Note bears interest at a rate of 8.5% per annum and matures 18 months after its issuance date. From time to time, beginning six months after issuance, Streeterville may redeem a portion of the Streeterville Note, not to exceed an amount of $270,000 per month. In the event we have not reduced the outstanding balance under the Streeterville Note by at least $900,000.00 by the 12-month anniversary of following the issuance date, then the outstanding balance at such time will automatically increase by 5%. Subject to the terms and conditions set forth in the Streeterville Note, we may prepay all or any portion of the outstanding balance of the Streeterville Note at any time.
Corporate Name Change
On October 23, 2024, the Company changed its corporate name to HeartSciences Inc. pursuant to the Certificate of Amendment (the “Certificate of Amendment”) to its Amended and Restated Certificate of Formation, filed with the Secretary of State of the State of Texas on October 17, 2024 (the “Name Change”). Pursuant to the Company’s Annual Meeting, held on January 17, 2024, the Company’s shareholders approved the Certificate of Amendment to effectuate the Name Change. The Name Change does not affect the Company’s ticker symbol (HSCS) or the equity CUSIP number for the Company’s outstanding shares of common stock. Outstanding stock certificates for shares of the Company are not affected by the Name Change and continue to be valid and need not be exchanged. Other than the Name Change, there were no changes to the Company’s Amended and Restated Certificate of Formation.
Corporate Information
We are a Texas corporation based in Southlake, Texas and were incorporated in Texas in August 2007. Our principal executive offices are located at 550 Reserve Street, Suite 360, Southlake TX 76092. Our telephone number is 682-237-7781. Our website address is www.heartsciences.com. The information contained on, or that can be accessed through, our website is not part of this offering circular or the registration statement of which it forms a part. We have included our website address in this offering circular solely as an inactive textual reference.
SUMMARY
The Offering
Securities being
offered: | We are offering on a “best efforts” basis a maximum of up to 4,285,714 Units, with each Unit consisting of one (1) share of Series D Preferred Stock and one (1) Warrant, to purchase one (1) share of Common Stock at a price of $5.00 per share of our Common Stock. We will not issue fractional shares. The Units will be sold at an offering price of $3.50 per Unit, for a maximum offering amount of $15,000,000 worth of Units. This offering statement also relates to the 8,571,428 shares of Common Stock issuable upon conversion of the Series D Preferred Stock and exercise of the Warrants. The Warrants are exercisable within 36 months from the date of issuance. Four Million Two Hundred Eighty-Five Thousand Seven Hundred Fourteen (4,285,714) Warrants will each be exercisable at a price of $5.00 for one (1) share of our Common Stock, subject to customary adjustment. The 4,285,714 shares of Common Stock underlying the Series D Preferred Stock and the 4,285,714 shares of Common Stock issuable upon exercise of the Warrants are being qualified in this Offering. In addition, up to 128,571 shares of Common Stock underlying the Series D Preferred Stock and the up to 128,571 shares of Common Stock issuable upon exercise of the Agent Warrants are being qualified in this Offering. In addition, this offering statement relates to (i) 128,571 Agent Warrants for the purchase of up to 128,571 Agent Units, each Agent Unit consisting of one Agent Share and one Agent Unit Warrant to purchase one Agent Warrant Share, and (ii) up to 128,571 shares of Common Stock into which the Series D Preferred Stock underlying the Agent Unit Shares may convert and up to 128,571 shares of Common Stock, issuable upon exercise of the Agent Warrant Shares. The Units have no stand-alone rights and will not be certificated or issued as stand-alone securities. The shares of our Series D Preferred Stock, the Warrants and the Agent Warrants are immediately separable and will be issued separately, but will be purchased together as a unit in this Offering. |
| | |
| Minimum Initial Investment Amount Per Investor: | $675, or 193 Units |
Terms of Series D Preferred Stock: | | ● Ranking ⸺The Series D Preferred Stock ranks, as to dividend rights and rights upon our liquidation, dissolution, or winding up, senior to our Common Stock. The terms of the Series D Preferred Stock will not limit our ability to (i) incur indebtedness or (ii) issue additional equity securities that are equal or junior in rank to the shares of our Series D Preferred Stock as to distribution rights and rights upon our liquidation, dissolution or winding up. |
| | | |
| | | ● Liquidation Preference ⸺ The liquidation preference for each share of our Series D Preferred Stock is $3.50. Upon a liquidation, dissolution or winding up of our Company, holders of shares of our Series D Preferred Stock will be entitled to receive the liquidation preference with respect to their shares. |
| | | ● Optional Conversion – At any time each share of our Series D Preferred Stock is convertible into one (1) share of our Common Stock at the option of the holder. |
| | | |
| | | ● Mandatory Conversion -- At any time after issuance upon the occurrence of any of the following events, the Company shall have a right to direct the mandatory conversion of the Series D Preferred Stock: (a) a change in control, (b) if the price of the Common Stock closes at or above $5.00 per share for 10 consecutive trading days, or (c) if the Company consummates a firm commitment public offering of Common Stock for gross proceeds of at least $15 million at an offering price per share equal to or greater than $5.00. |
| | | |
| | | ● Further Issuances ⸺ The shares of our Series D Preferred Stock have no maturity date, and we will not be required to redeem shares of our Series D Preferred Stock at any time. Accordingly, the shares of our Series D Preferred Stock will remain outstanding indefinitely, unless the Series D Preferred Stock is converted into Common Stock. |
| | | ● Voting Rights ⸺ In the future, we may not authorize or issue any class or series of equity securities ranking senior to the Series D Preferred Stock as to dividends or distributions upon liquidation (including securities convertible into or exchangeable for any such senior equity securities) or amend our Amended and Restated Certificate of Formation, as amended (whether by merger, consolidation, or otherwise), to materially, and adversely change the terms of the Series D Preferred Stock without the affirmative vote of at least a majority of the votes entitled to be cast on such matter by holders of our outstanding shares of Series D Preferred Stock, voting together as a class. Otherwise, holders of the shares of our Series D Preferred Stock will not have any voting rights. |
| Terms of Warrants: | The Warrants will be exercisable at any time from the date of issuance through the third anniversary from the date of issuance, unless earlier redeemed. The Warrants will be exercisable to purchase one share of our Common Stock at an exercise price of $5.00 per share. |
Summary of Risk Factors
Our business is subject to numerous risks, as more fully described below in this “Risk Factors” section. The following is a summary of the most significant risks and uncertainties that we believe could adversely affect our business, financial condition, and results of operations. In addition to the following summary, you should read the other information set forth below in this “Risk Factors” section before you invest in our securities. In particular, our risks include, but are not limited to, the following:
Risks Related to Our Financial Condition and Capital Requirements:
| ● | We have a limited operating history and we have incurred significant operating losses since our inception, and anticipate that we will incur continued losses for the foreseeable future; |
| | | |
| ● | If we are unable to maintain compliance with all applicable continued listing requirements and standards of Nasdaq, our Common Stock could be delisted from Nasdaq. |
| | | |
| ● | Our future operating results are dependent on regulatory approval for the MyoVista wavECG and the MyoVista Insights Cloud Platform, which we have not received as of the date of filing of this offering statement on Form 1-A; |
| | | |
| ● | We will need to raise additional funding, which may not be available on acceptable terms, or at all. Failure to obtain funding on acceptable terms and on a timely basis may require us to curtail, delay or discontinue our development efforts and other operations; |
| | | |
| ● | All of our assets are subject to security interests; and |
| | | |
| ● | There is substantial doubt about our ability to continue as a going concern, which could prevent us from obtaining new financing either on reasonable terms or at all. |
Risks Related to Our Business and Industry:
| ● | Our future success depends on our ability to develop, receive regulatory clearance or approval for, and introduce the MyoVista wavECG device and the MyoVista Insights Cloud Platform to the market in a timely manner. If we do not obtain and maintain the regulatory registrations and clearances for our products, we will be unable to market and sell our products in the United States, Europe or other regions; |
| | | |
| ● | Our success will be dependent upon physician acceptance; |
| | | |
| ● | If third-party payors do not provide adequate coverage and reimbursement for the use of our AI-ECG algorithms, our revenue will be negatively impacted; |
| | | |
| ● | We will be dependent upon third-party manufacturers and suppliers, making us vulnerable to supply shortages and problems, increased costs and quality or compliance issues, any of which could harm our business; |
| | | |
| ● | Interruptions in computing and data management cloud systems could impair the delivery of our cardiac monitoring services; and |
| | | |
| ● | Our proprietary data analytics engine may not operate properly, which could damage our reputation, give rise to claims against us or divert application of our resources from other purposes, any of which could harm our business and operating results. |
Risks Related to Product Development and Regulatory Approval:
| ● | Our products and operations are subject to extensive government regulation and oversight both in the U.S. and abroad, and our failure to comply with applicable requirements could harm our business; |
| | | |
| ● | If and when our products are ready for sales launch into the U.S., modifications to our marketed products may require new 510(k) clearances, or may require us to cease marketing or recall the modified products until clearances or approvals are obtained; |
| | | |
| ● | Clinical studies may be necessary to support future product submissions to the FDA. The clinical trial process is lengthy and expensive with uncertain outcomes, and often requires the enrollment of large numbers of patients, and suitable patients may be difficult to identify and recruit. Delays or failures in our clinical studies will prevent us from launching sales of modified or new products into the U.S. and will adversely affect our business, operating results and prospects; |
| ● | If the third parties on which we rely to conduct our clinical studies and to assist us with pre-clinical development do not perform as required or expected, we may be delayed or unable to obtain regulatory clearance or approval for sales launch of our products in the U.S.; |
| | | |
| ● | We may encounter substantial delays in our clinical studies, or we may fail to demonstrate specificity and sensitivity to the satisfaction of applicable regulatory authorities; |
| | | |
| ● | The results of future clinical studies may not support additional or new claims for future products or may result in the discovery of adverse side effects; |
| | | |
| ● | Failure to comply with post-marketing regulatory requirements could subject us to enforcement actions, including substantial penalties, and might require us to recall or withdraw a product from the market; |
| | | |
| ● | The MyoVista wavECG device must be manufactured in accordance with federal, state and foreign regulations, and we could be forced to recall our devices or terminate production if we fail to comply with these regulations; and |
| | | |
| ● | Our products may cause or contribute to adverse medical events or be subject to failures or malfunctions that we are required to report to the FDA, and if we fail to do so, we would be subject to sanctions that could harm our reputation, business, financial condition and results of operations. |
Risks Related to Our Intellectual Property:
| ● | If we are unable to obtain and maintain effective patent rights for our products, we may not be able to compete effectively in our markets. If we are unable to protect the confidentiality of our trade secrets or know-how, such proprietary information may be used by others to compete against us; |
| | | |
| ● | Intellectual property rights of third parties could adversely affect our ability to market our products, and we might be required to litigate or obtain licenses from third parties in order to develop or market our products. Such litigation or licenses could be costly or not available on commercially reasonable terms; and |
| | | |
| ● | We may be subject to claims challenging the inventorship of our intellectual property. |
Risks Related to Our License Agreements with Mount Sinai:
| ● | We are highly dependent on the Licenses, the termination of which may prevent us from commercializing our products, and which imposes significant obligations on us; |
| | | |
| ● | Our future financial performance will depend in part on the successful integration, improvement and software updates from the Mount Sinai algorithms; and |
| | | |
| ● | Our future success depends on our ability to develop, receive regulatory clearance or approval for, and introduce the algorithms underlying the Mount Sinai Licenses to the market in a timely manner. |
Risks Related to the Ownership of our Securities:
| ● | The market price of our Common Stock may be highly volatile, and you could lose all or part of your investment; |
| | | |
| ● | Future sales of a substantial number of shares of our Common Stock by our existing shareholders could cause our stock price to decline; |
| | | |
| ● | If securities or industry analysts do not publish or cease publishing research or reports about us, our business or our market, or if they publish negative reports regarding our business or our securities, our share price and trading volume could decline; |
| | | |
| ● | We have identified weaknesses in our internal controls, and we cannot provide assurances that these weaknesses will be effectively remediated or that additional material weaknesses will not occur in the future; and |
| | | |
| ● | We are an “emerging growth company,” and any decision on our part to comply with certain reduced disclosure requirements applicable to emerging growth companies could make the Common Stock less attractive to investors. |
RISK FACTORS
Investing in our securities involves a high degree of risk. You should consider carefully the risks and uncertainties described below, together with all of the other information included in this offering statement, as well as the risk factors included in Item 1A. “Risk Factors” in our Annual Report on Form 10-K for the year ended April 30, 2024, which is incorporated by reference into this offering statement, as well as other information we include or incorporate by reference in this offering statement or include in any applicable offering statement supplement, before making a decision to invest in our securities. Our business, results of operations, financial condition and prospects could also be harmed by risks and uncertainties that are not presently known to us or that we currently believe are not material. If any of the following risks actually occur, our business, platform, reputation, brand, results of operations, financial condition and prospects could be materially and adversely affected. In such event, the market price of our securities could decline, and you could lose all or part of your investment. Additional risks and uncertainties not presently known to us or that we deem currently immaterial may also impair our business operations or adversely affect our results of operations or financial condition.
The following risks are only some of the risks that we face, and as set forth above, you should consider carefully the risks and uncertainties described below, together with all of the other risks and information described above before making a decision to invest in our securities.
Risks Related to Our Financial Condition and Capital Requirements
There is substantial doubt about our ability to continue as a going concern.
Our independent registered public accounting firm has issued an opinion on our audited financial statements incorporated by reference into this offering circular that contains an explanatory paragraph regarding substantial doubt about our ability to continue as a going concern because we have experienced recurring losses, negative cash flows from operations, and limited capital resources. These events and conditions indicate that a material uncertainty exists that may cast significant doubt on our ability to continue as a going concern. The perception that we may not be able to continue as a going concern may have a material adverse effect on our share price and our ability to raise new capital (whether it is through the issuance of equity or debt securities or otherwise), enter into critical contractual relations with third parties and otherwise execute our business objectives. Until we can generate significant recurring revenues, we expect to satisfy our future cash needs through debt or equity financing. We cannot be certain that additional funding will be available to us on acceptable terms, if at all. Our financial statements do not include any adjustments that may result from the outcome of this uncertainty.
If we are unable to continue as a going concern, we may have to liquidate our assets, and the values we receive for our assets in liquidation or dissolution could be significantly lower than the values reflected in our financial statements.
If we are unable to maintain compliance with all applicable continued listing requirements and standards of Nasdaq, our Common Stock could be delisted from Nasdaq.
Our Common Stock and IPO Warrants are currently listed on Nasdaq. In order to maintain that listing, we must satisfy minimum financial and other continued listing requirements and standards, including those regarding director independence and independent committee requirements, minimum stockholders’ equity, minimum share price, and certain corporate governance requirements.
On August 2, 2023, we received a letter from the Staff indicating that, based upon the closing bid price of the Company’s Common Stock for the last 30 consecutive business days, the Company no longer met the requirement to maintain a minimum bid price of $1 per share (the “Minimum Bid Price Requirement”). In accordance with Nasdaq listing rules, we had until January 29, 2024 to regain compliance with the Minimum Bid Price Requirement. In the event we did not regain compliance during this period, we were eligible to seek an additional 180 calendar day compliance period if we met the Nasdaq continued listing requirement for market value of publicly held shares and all other initial listing standards, with the exception of the Minimum Bid Price Requirement, and provided written notice to Nasdaq of our intent to cure the deficiency during this second compliance period.
On August 17, 2023, we attended a hearing before the Nasdaq Hearing Panel (the “Panel”), and requested the continued listing of our securities on the Nasdaq Capital Market pending our return to compliance with the Minimum Bid Price Requirement.
On January 30, 2024, we received a letter from the Panel advising that we have been granted an additional 180-day extension to July 29, 2024, to regain compliance with the Minimum Bid Price Requirement.
On May 9, 2024, the Company received a staff determination from Nasdaq to delist the Company’s securities from the Nasdaq Capital Market (the “Staff Determination”). The Staff Determination was issued because, as of May 8, 2024, the Company’s Common Stock had a closing bid price of $0.10 or less for at least ten consecutive trading days. Accordingly, the Company was subject to the provisions contemplated under Nasdaq Listing Rule 5810(c)(3)(A)(iii) (the “Low Priced Stocks Rule”). On May 17, 2024, the Company effected the Reverse Stock Split, such that as a result of the Reverse Stock Split, every 100 shares of the Company’s issued and outstanding pre-reverse split Common Stock were combined into one share of Common Stock.
On June 3, 2024, the Company received a letter from Nasdaq informing the Company that the Nasdaq Listing Qualifications staff has confirmed that the Company has regained compliance with the Minimum Bid Price Requirement, and that the Company is therefore in compliance with Nasdaq’s listing requirements. The Company’s Common Stock and public warrants continue to be listed on Nasdaq.
Nasdaq Capital Market requires us to meet certain financial, public float, bid price and liquidity standards on an ongoing basis in order to continue the listing of our Common Stock and IPO Warrants. If we fail to meet these continued listing requirements, our Common Stock or IPO Warrants may be subject to delisting. If our Common Stock or IPO Warrants are delisted and we are not able to list such Common Stock and IPO Warrants on another national securities exchange, we expect our securities would be quoted on an over-the-counter market; However, if this were to occur, our stockholders could face significant material adverse consequences, including limited availability of market quotations for our Common Stock and IPO Warrants and reduced liquidity for the trading of our securities. In addition, in the event of such delisting, we could experience a decreased ability to issue additional securities and obtain additional financing in the future. There can be no assurance that in the future we will continue to be in compliance with the Nasdaq listing rules or remain listed on Nasdaq.
Risks Related to the Ownership of our Securities
The market price of our Common Stock may be highly volatile, and you could lose all or part of your investment.
The market price of our Common Stock may be highly volatile and subject to wide fluctuations in response to a variety of factors and risks, many of which are beyond our control. This volatility could be the result of a variety of factors, which include:
| ● | whether we achieve our anticipated corporate objectives; |
| ● | actual or anticipated fluctuations in our quarterly or annual operating results; |
| ● | changes in our financial or operational estimates or projections; |
| ● | our ability to implement our operational plans; |
| ● | termination of lock-up agreements or other restrictions on the ability of our shareholders to sell shares after the IPO; |
| ● | changes in the economic performance or market valuations of companies similar to ours; |
| ● | general economic or political conditions in the U.S. or elsewhere; and |
| ● | other events or factors, including those resulting from war, incidents of terrorism or responses to these events. |
In addition, the stock market in general, and the stock of publicly-traded medical device companies in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of such companies. Broad market and industry factors may negatively affect the market price of our Common Stock and IPO Warrants, regardless of our actual operating performance, and we have little or no control over these factors.
Future sales and issuances of our Common Stock or rights to purchase our Common Stock, including pursuant to our Equity Incentive Plan and other equity securities could result in dilution of the percentage ownership of our stockholders and could cause our stock price to fall.
We expect that significant additional capital may be needed in the future to continue our planned operations, including conducting clinical trials, commercialization efforts, expanded research and development activities and costs associated with operating a public company. To raise capital, we may sell Common Stock or other equity securities in one or more transactions at prices and in a manner we determine from time to time. If we sell Common Stock or other equity securities, investors may be materially diluted by subsequent sales. Such sales may also result in material dilution to our existing stockholders, and new investors could gain rights, preferences and privileges senior to the holders of our Common Stock. As of February 5, 2025, there were 1,036,321 shares of our Common Stock outstanding. In addition, as of February 5, 2025, there were 380,440 shares of Series C Preferred Stock outstanding that, as of such date, were convertible into 84,733 shares of Common Stock and options and warrants exercisable for 236,670 shares of our Common Stock.
If securities or industry analysts do not publish or cease publishing research or reports about us, our business or our market, or if they publish negative reports regarding our business or our securities, our share price and trading volume could decline.
The trading market for the Common Stock and the IPO Warrants will be influenced by the research and reports that industry or securities analysts may publish about us, our business, our market or our competitors. We do not have any control over these analysts and we cannot provide any assurance that analysts will cover us or provide favorable coverage. If any of the analysts who may cover us adversely change their recommendation regarding the Common Stock or IPO Warrants, or provide more favorable relative recommendations about our competitors, the price of our Common Stock and IPO Warrants would likely decline. If any analyst who may cover us were to cease coverage of our Company or fail to regularly publish reports on us, we could lose visibility in the financial markets, which in turn could cause the price of our Common Stock and IPO Warrants or trading volume to decline.
We have identified weaknesses in our internal controls, and we cannot provide assurances that these weaknesses will be effectively remediated or that additional material weaknesses will not occur in the future.
Prior to the completion of the IPO, we had been a private company with limited accounting personnel to adequately execute our accounting processes and limited supervisory resources with which to address our internal control over financial reporting. While a private company, we had not designed or maintained an effective control environment as required of public companies under the rules and regulations of the SEC. Management and our independent registered public accounting firm, Haskell & White LLP, identified several material weaknesses in our internal control over financial reporting in connection with our preparation and the audits of our financial statements for our fiscal year ended April 30, 2024 (“Fiscal 2024”).
A material weakness is a deficiency, or combination of deficiencies, in internal control over financing reporting such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis. The material weaknesses we and our independent registered public accounting firms identified are listed below:
| ● | lack of proper approval processes and review of processes and documentation for such reviews; |
| ● | we did not maintain sufficient U.S. GAAP and SEC accounting resources commensurate with those required of a public company; and |
| ● | insufficient number of staff to maintain optimal segregation of duties and levels of oversight. |
These material weaknesses resulted in adjustments to our prior year financial statements primarily related to equity accounts, accruals, and inventory and could result in a misstatement of any account balances or disclosures that would result in a material misstatement to the annual or interim financial statements that would not be prevented or detected.
During Fiscal 2024, we have taken and continue to take remedial steps to improve our internal controls over financial reporting, which includes establishing a more robust process related to review of complex accounting transactions, preparation of account reconciliations, and review of journal entries. Our Chief Financial Officer frequently attends continuing education for updates on accounting policies and procedures. We cannot assure you that these measures will significantly improve or remediate the material weaknesses described above. Management is monitoring the effectiveness of these and other processes, procedures and controls and will make any further changes deemed appropriate. Management believes the foregoing actions will effectively remediate the material weaknesses, however, our material weaknesses will not be considered remediated until controls are in place for a period of time, the controls are tested, and management concludes that the controls are properly designed and operating effectively. As a result, the timing of when we will be able to fully remediate the material weaknesses is uncertain. If the steps we take do not remediate the material weaknesses in a timely manner, there could continue to be a reasonable possibility that these control deficiencies or others would result in a material misstatement of our annual or interim financial statements that would not be prevented or detected on a timely basis. This, in turn, could jeopardize our ability to comply with our reporting obligations, limit our ability to access the capital markets and adversely impact our stock price.
Our independent registered public accounting firm was not required to perform an evaluation of our internal control over financial reporting as of either April 30, 2024 or April 30, 2023 in accordance with the provisions of the Sarbanes-Oxley Act. Accordingly, we cannot assure you that we have identified all, or that we will not in the future have additional, material weaknesses. Material weaknesses may still exist when we report on the effectiveness of our internal control over financial reporting in the future as required by reporting requirements under Section 404 of the Sarbanes-Oxley Act.
If we are unable to successfully remediate the existing material weaknesses in our internal control over financial reporting, the accuracy and timing of our financial reporting, and our stock price, may be adversely affected and we may be unable to maintain compliance with the applicable stock exchange listing requirements. Implementing any appropriate changes to our internal controls may divert the attention of our officers and employees, entail substantial costs to modify our existing processes and take significant time to complete. These changes may not, however, be effective in maintaining the adequacy of our internal controls, and any failure to maintain that adequacy, or consequent inability to produce accurate financial statements on a timely basis, could increase our operating costs and harm our business. In addition, investors’ perceptions that our internal controls are adequate or that we are unable to produce accurate financial statements on a timely basis may harm our stock price and make it more difficult for us to effectively market and sell our services to new and existing customers.
Our Board of Directors is authorized to issue and designate shares of our preferred stock in additional series without shareholder approval.
Our Amended and Restated Certificate of Formation authorizes our Board of Directors, without the approval of our shareholders, to issue shares of our preferred stock, subject to limitations prescribed by applicable law, rules and regulations and the provisions of our Amended and Restated Certificate of Formation, as shares of preferred stock in series, to establish from time to time the number of shares to be included in each such series and to fix the designation, powers, preferences, privileges and rights of the shares of each such series and the qualifications, limitations or restrictions thereof. The powers, preferences and rights of these additional series of preferred stock may be senior to or on parity with our Common Stock, which may reduce its value.
As of February 5, 2025 our principal shareholders, officers and directors beneficially own approximately 7.8% of our Common Stock. They will therefore be able to exert significant influence over matters submitted to our shareholders for approval.
As of February 5, 2025, our principal shareholders, officers and directors beneficially owned approximately 7.8% of the outstanding shares of our Common Stock, including shares issuable pursuant to antidilution provisions set forth in the Series C Preferred Stock. This concentration of share ownership may adversely affect the trading price for our Common Stock because investors often perceive disadvantages in owning shares in companies with controlling shareholders. As a result, these shareholders, if they acted together, could significantly influence matters requiring approval by our shareholders, including the election of directors and the approval of mergers or other business combination transactions. The interests of these shareholders may not always coincide with our interests or the interests of other shareholders. For more information regarding the beneficial ownership of such principal shareholders, officers and directors, see “Security Ownership of Management and Certain Securityholders” and “Interest of Management and Others in Certain Transactions.”
We have incurred and will continue to incur significant costs as a result of the listing of our securities for trading on Nasdaq. As a public company in the U.S., our management is required to devote substantial time to new compliance initiatives as well as compliance with ongoing U.S. requirements.
Upon the listing of securities on Nasdaq, we became a publicly traded company in the United States and as such, we are incurring significant accounting, legal and other expenses that we did not incur before the IPO. We also are incurring costs associated with corporate governance requirements of the SEC, as well as requirements under Section 404 and other provisions of the Sarbanes-Oxley Act. We expect these rules and regulations to continue to increase our legal and financial compliance costs, introduce new costs such as investor relations, stock exchange listing fees and shareholder reporting, and to make some activities more time consuming and costly. The implementation and testing of such processes and systems may require us to hire outside consultants and incur other significant costs. Any future changes in the laws and regulations affecting public companies in the United States, including Section 404 and other provisions of the Sarbanes-Oxley Act, and the rules and regulations adopted by the SEC, for so long as they apply to us, will result in increased costs to us as we respond to such changes. These laws, rules and regulations could make it more difficult or more costly for us to obtain certain types of insurance, including director and officer liability insurance, and we may be forced to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. The impact of these requirements could also make it more difficult for us to attract and retain qualified people to serve on our Board of Directors, any committees of our Board of Directors, or as executive officers.
We may be subject to securities litigation, which is expensive and could divert management attention.
In the past, companies that have experienced volatility in the market price of their stock have been subject to securities class action litigation. We may be the target of this type of litigation in the future. Litigation of this type could result in substantial costs and diversion of management’s attention and resources, which could seriously hurt our business. Any adverse determination in litigation could also subject us to significant liabilities.
We have never paid cash dividends on our Common Stock, and we do not anticipate paying any cash dividends in the foreseeable future.
We have neither declared nor paid cash dividends, and we do not anticipate paying cash dividends in the foreseeable future. Therefore, you should not rely on an investment in Common Stock as a source for any future dividend income. Our Board of Directors has complete discretion as to when or whether to distribute dividends. Even if our Board of Directors decides to declare and pay dividends, the timing, amount and form of future dividends, if any, will depend on our future results of operations and cash flow, our capital requirements and surplus, the amount of distributions, if any, received by us from our subsidiaries, our financial condition, contractual restrictions and other factors deemed relevant by our Board of Directors.
We are an “emerging growth company” and we cannot be certain if the reduced disclosure requirements applicable to “emerging growth companies” will make our Common Stock or IPO Warrants less attractive to investors.
We are an “emerging growth company,” as defined in Section 2(a)(19) of the Securities Act, and for as long as we continue to be an emerging growth company, we may choose to take advantage of certain exemptions and relief from various reporting requirements that are applicable to other public companies that are not “emerging growth companies.” In particular, while we are an “emerging growth company,” among other exemptions, we will:
| ● | not be required to engage an independent registered public accounting firm to report on our internal controls over financial reporting pursuant to Section 404(b) of the Sarbanes-Oxley Act; |
| ● | not be required to comply with the requirement in Public Company Accounting Oversight Board Auditing Standard 3101, The Auditor’s Report on an Audit of Financial Statements When the Auditor Expresses an Unqualified Opinion, to communicate critical audit matters in the auditor’s report; |
| ● | be permitted to present only two years of audited financial statements and only two years of related “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in our periodic reports and registration statements, including in this offering statement; |
| ● | not be required to disclose certain executive compensation related items such as the correlation between executive compensation and performance and comparisons of the chief executive officer’s compensation to median employee compensation; or |
| ● | not be required to submit certain executive compensation matters to stockholder advisory votes, such as “say-on-pay,” “say-on-frequency,” and “say-on-golden parachutes.” |
In addition, the JOBS Act also permits an emerging growth company such as us to take advantage of an extended transition period to comply with new or revised accounting standards applicable to public companies, meaning that we can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have elected to use this extended transition period and, as a result, our financial statements may not be comparable with similarly situated public companies.
We will remain an “emerging growth company” until the earliest to occur of (1) our reporting of $1.235 billion or more in annual gross revenue; (2) our becoming a “large accelerated filer,” with at least $700 million of equity securities held by non-affiliates; (3) our issuance, in any three-year period, of more than $1.0 billion in non-convertible debt; and (4) the last day of our fiscal year following the fifth anniversary of the date of our first sale of common equity securities which was on June 17, 2022, pursuant to an effective registration statement.
We cannot predict if investors may find our Common Stock or IPO Warrants less attractive if we rely on the exemptions and relief granted by the JOBS Act. For example, if we do not adopt a new or revised accounting standard, our future results of operations may not be as comparable to the results of operations of certain other companies in our industry that adopted such standards. If some investors find our Common Stock or IPO Warrants less attractive as a result, there may be a less active trading market for our Common Stock or IPO Warrants and our stock price may decline and/or become more volatile.
Anti-takeover provisions could make a third party acquisition of us difficult.
Our Amended and Restated Certificate of Formation and Second Amended and Restated Bylaws eliminate the ability of shareholders to take action by less than unanimous written consent. This provision could make it more difficult for a third party to acquire us without the approval of our board. In addition, the Texas Business Organizations Code (the “TBOC”) also contains certain provisions that could make an acquisition by a third party more difficult.
Provisions of the IPO Warrants, the Remaining Bridge Warrants and our Series C Preferred Stock could discourage an acquisition of us by a third party.
In addition to the provisions of our Amended and Restated Certificate of Formation and Second Amended and Restated Bylaws, certain provisions of the IPO Warrants, the Remaining Bridge Warrants and the Series C Preferred Stock could make it more difficult or expensive for a third party to acquire us. The terms of the IPO Warrants, the Remaining Bridge Warrants and the Series C Preferred Stock prohibit us from engaging in certain transactions constituting “fundamental transactions” or a “Deemed Liquidation Event”, unless, among other things, the surviving entity assumes our obligations under the IPO Warrants, the Remaining Bridge Warrants or the Series C Preferred Stock. These and other provisions of the IPO Warrants, the Remaining Bridge Warrants or the Series C Preferred Stock could prevent or deter a third party from acquiring us even where the acquisition could be beneficial to investors.
Financial Industry Regulatory Authority, Inc. (“FINRA”) sales practice requirements may limit a stockholder’s ability to buy and sell our shares Common Stock.
FINRA has adopted rules that require that in recommending an investment to a customer, a broker-dealer must have reasonable grounds for believing that the investment is suitable for that customer. Prior to recommending speculative low-priced securities to their non-institutional customers, broker-dealers must make reasonable efforts to obtain information about the customer’s financial status, tax status, investment objectives and other information. Under interpretations of these rules, FINRA believes that there is a high probability that speculative low-priced securities will not be suitable for certain customers. FINRA requirements will likely make it more difficult for broker-dealers to recommend that their customers buy our shares of Common Stock, which may have the effect of reducing the level of trading activity in our Common Stock. As a result, fewer broker- dealers may be willing to make a market in our Common Stock, reducing a stockholder’s ability to resell shares of our Common Stock.
If our shares become subject to the penny stock rules, it would become more difficult to trade our shares.
The SEC has adopted rules that regulate broker-dealer practices in connection with transactions in penny stocks. Penny stocks are generally equity securities with a price of less than $5.00, other than securities registered on certain national securities exchanges or authorized for quotation on certain automated quotation systems, provided that current price and volume information with respect to transactions in such securities is provided by the exchange or system. If we do not retain a listing on Nasdaq and if the price of our Common Stock is less than $5.00, our Common Stock will be deemed a penny stock. The penny stock rules require a broker-dealer, before a transaction in a penny stock not otherwise exempt from those rules, to deliver a standardized risk disclosure document containing specified information. In addition, the penny stock rules require that before effecting any transaction in a penny stock not otherwise exempt from those rules, a broker-dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive (i) the purchaser’s written acknowledgment of the receipt of a risk disclosure statement; (ii) a written agreement to transactions involving penny stocks; and (iii) a signed and dated copy of a written suitability statement. These disclosure requirements may have the effect of reducing the trading activity in the secondary market for our Common Stock or IPO Warrants, and therefore shareholders may have difficulty selling their shares of Common Stock or IPO Warrants.
Risks Related to this Offering
This is a fixed price offering and the fixed offering price may not accurately represent the current value of us or our assets at any particular time. Therefore, the purchase price you pay for our Units may not be supported by the value of our assets at the time of your purchase.
This is a fixed price offering, which means that the offering price for our Units is fixed and will not vary based on the underlying value of our assets at any time. Our board of directors (our “Board of Directors”) has determined the offering price in its sole discretion. The fixed offering price for our Units has not been based on appraisals of any assets we own or may own, or of our company as a whole, nor do we intend to obtain such appraisals. Therefore, the fixed offering price established for our Units may not be supported by the current value of our company or our assets at any particular time.
If you purchase our securities being sold in this offering, you may experience substantial dilution in the net tangible book value of your shares if and when the Series D Preferred Stock underlying the Units are fully converted and if and when the Warrants underlying the Units are fully exercised. In addition, we may issue additional equity or convertible debt securities in the future, which may result in additional dilution to investors.
The price per Unit being offered in this offering may be higher than the net tangible book value per share of the outstanding shares of our Common Stock prior to this offering. For a more detailed discussion of the foregoing, see the section entitled “Dilution” below. To the extent outstanding preferred stock or convertible notes are converted or outstanding stock options or warrants (including placement agent warrants) are exercised, there will be further dilution to new investors.
We may amend our business policies without stockholder approval.
Our Board of Directors determines our growth, investment, financing, capitalization, borrowing, operations and distributions policies. Although our Board of Directors has no intention at present to change or reverse any of these policies, they may be amended or revised without notice to holders of our Series D Preferred Stock. Accordingly, holders of our Series D Preferred Stock will not have any control over changes in our policies.
Our management team will have broad discretion over the use of the net proceeds from our sale of the Units, if any, and you may not agree with how we use the proceeds and the proceeds may not be invested successfully.
Our management team will have broad discretion as to the use of the net proceeds from our sale of the Units, if any, and we could use such proceeds for purposes other than those contemplated at the time of commencement of this Offering. Accordingly, you will be relying on the judgment of our management team with regard to the use of those net proceeds, and you will not have the opportunity, as part of your investment decision, to assess whether the proceeds are being used appropriately. It is possible that, pending their use, we may invest those net proceeds in a way that does not yield a favorable, or any, return for us. The failure of our management team to use such funds effectively could have a material adverse effect on our business, financial condition, operating results and cash flows.
We cannot assure you that we will be able to redeem our Series D Preferred Stock.
Our ability to redeem our Series D Preferred Stock is dependent on our ability to operate profitably and to generate cash from our operations and the operations of our operating businesses or from raising additional capital. We cannot guarantee that we will be able to redeem our Series D Preferred Stock and may only be able to offer investors the ability to convert shares of Series D Preferred Stock into shares of our Common Stock.
We may issue additional debt and equity securities, which are senior to our Series D Preferred Stock as to distributions and in liquidation, which could materially adversely affect the value of the Series D Preferred Stock.
In the future, we may attempt to increase our capital resources by entering into additional debt or debt-like financing that is secured by all or up to all of our assets, or issuing debt or equity securities, which could include issuances of commercial paper, medium-term notes, senior notes, subordinated notes or shares. In the event of our liquidation, our lenders and holders of our debt securities would receive a distribution of our available assets before distributions to our stockholders. Any preferred securities, if issued by our company, may have a preference with respect to distributions and upon liquidation that is senior to the preference of the Series D Preferred Stock, which could further limit our ability to make distributions to our stockholders. Because our decision to incur debt and issue securities in our future offerings will depend on market conditions and other factors beyond our control, we cannot predict or estimate the amount, timing or nature of our future offerings and debt financing.
Further, market conditions could require us to accept less favorable terms for the issuance of our securities in the future. Thus, you will bear the risk of our future offerings reducing the value of your Series D Preferred Stock. In addition, we can change our leverage strategy from time to time without approval of holders of our preferred stock or Common Stock, which could materially adversely affect the value of the Series D Preferred Stock.
We are not required to raise any minimum amount in this Offering before we may utilize the funds received in this Offering. Investors should be aware that there is no assurance that any monies beside their own will be invested in this Offering.
Because there is no minimum amount of subscriptions which we must receive before accepting funds in the Offering, you will not be assured that we will have sufficient funds to execute our business plan or satisfy its working capital requirements and will bear the risk that we will be unable to secure the funds necessary to meet our current and anticipated financial obligations.
This Offering is being conducted on a “best efforts” basis without a minimum and we may not be able to execute our growth strategy if the $15,000,000 maximum is not sold.
If you invest in our Units and less than all of the offered shares of our Units are sold, the risk of losing your entire investment will be increased. We are offering our Units on a “best efforts” basis without a minimum, and we can give no assurance that all of the offered Units will be sold. If less than $15,000,000 of Units offered are sold, we may be unable to fund all the intended uses described in this offering statement from the net proceeds anticipated from this Offering without obtaining funds from alternative sources or using working capital that we generate. Alternative sources of funding may not be available to us at what we consider to be a reasonable cost, and the working capital generated by us may not be sufficient to fund any uses not financed by offering net proceeds. No assurance can be given to you that any funds will be invested in this Offering other than your own.
You will not have a vote or influence on the management of our company.
All decisions with respect to the management of the Company will be made exclusively by the officers, directors or employees of the Company. You, as an investor in our Series D Preferred Stock, have very limited voting rights and will have no ability to vote on issues of company management and will not have the right or power to take part in the management of our company and will not be represented on the Board of Directors. Accordingly, no person should purchase our Series D Preferred Stock unless he or she is willing to entrust all aspects of management to our Company. See “Securities Being Offered – Preferred Stock – Series D Preferred Stock -- Voting Rights.”
We may terminate this Offering at any time during the Offering period.
We reserve the right to terminate this Offering at any time, regardless of the number of Units sold. In the event that we terminate this Offering at any time prior to the sale of all of the Units offered hereby, whatever amount of capital that we have raised at that time will have already been utilized by our company and no funds will be returned to subscribers.
Investors in this Offering may not be entitled to a jury trial with respect to claims arising under the subscription agreement, which could result in less favorable outcomes to the plaintiff(s) in any action under the agreement.
Investors in this Offering will be bound by the subscription agreement, which includes a provision under which investors waive the right to a jury trial of any claim they may have against the company arising out of or relating to the agreement other than those arising under the federal securities laws.
If we opposed a jury trial demand based on the waiver, a court would determine whether the waiver was enforceable based on the facts and circumstances of that case in accordance with the applicable state and federal law. In determining whether to enforce a contractual pre-dispute jury trial waiver provision, courts will generally consider whether the visibility of the jury trial waiver provision within the agreement is sufficiently prominent such that a party knowingly, intelligently and voluntarily waived the right to a jury trial. We believe that this is the case with respect to the subscription agreement. You should consult legal counsel regarding the jury waiver provision before entering into the subscription agreement.
If you bring a claim not arising under the federal securities laws against the company in connection with matters arising under the subscription agreement, you may not be entitled to a jury trial with respect to those claims, which may have the effect of limiting and discouraging lawsuits against the company. If a lawsuit is brought against the company under the agreement, it may be heard only by a judge or justice of the applicable trial court, which would be conducted according to different civil procedures and may result in different outcomes than a trial by jury would have had, including results that could be less favorable to the plaintiff(s) in such an action.
Nevertheless, if the jury trial waiver provision is not permitted by applicable law, an action could proceed under the terms of the agreement with a jury trial. No condition, stipulation or provision of the subscription agreement serves as a waiver by any holder of the company’s securities or by the company of compliance with any substantive provision of the federal securities laws and the rules and regulations promulgated under those laws.
The jury trial waiver only applies to claims against our Company arising out of or related to the subscription agreement. As the provisions of the subscription agreement relate to the initial sale of the securities, subsequent transferees will not be bound by the subscription agreement and therefore to the conditions, obligations and restrictions thereunder, including the jury trial waiver.
The Units subscription agreement has a forum selection provision that requires disputes be resolved in state or federal courts in the State of Texas, regardless of convenience or cost to you, the investor.
In order to invest in this Offering, investors agree to resolve disputes arising under the subscription agreement in state or federal courts located in the State of Texas, for the purpose of any suit, action or other proceeding arising out of or based upon the agreement. This forum selection provision may limit your ability to obtain a favorable judicial forum for disputes with us. Although we believe the provision benefits us by providing increased consistency in the application of Texas law in the types of lawsuits to which it applies and in limiting our litigation costs, to the extent it is enforceable, the forum selection provision may limit investors’ ability to bring claims in judicial forums that they find favorable to such disputes, may increase investors’ costs of bringing suit and may discourage lawsuits with respect to such claims. Alternatively, if a court were to find the provision inapplicable to, or unenforceable in an action, we may incur additional costs associated with resolving such matters in other jurisdictions, which could adversely affect our business, financial condition or results of operations. You will not be deemed to have waived our compliance with the federal securities laws and the rules and regulations thereunder.
Using a credit card to purchase Units may impact the return on your investment as well as subject you to other risks inherent in this form of payment.
Investors in this Offering have the option of paying for their investment with a credit card, which is not usual in the traditional investment markets. Transaction fees charged by your credit card company (which can reach 5% of transaction value if considered a cash advance) and interest charged on unpaid card balances (which can reach almost 25% in some states) add to the effective purchase price of the shares you buy. See “Plan of Distribution.” The cost of using a credit card may also increase if you do not make the minimum monthly card payments and incur late fees. Using a credit card is a relatively new form of payment for securities and will subject you to other risks inherent in this form of payment, including that, if you fail to make credit card payments (e.g. minimum monthly payments), you risk damaging your credit score and payment by credit card may be more susceptible to abuse than other forms of payment. Moreover, where a third-party payment processor is used, as in this Offering, your recovery options in the case of disputes may be limited. The increased costs due to transaction fees and interest may reduce the return on your investment.
The SEC’s Office of Investor Education and Advocacy issued an Investor Alert dated February 14, 2018 entitled: Credit Cards and Investments – A Risky Combination, which explains these and other risks you may want to consider before using a credit card to pay for your investment.
PLAN OF DISTRIBUTION
Plan of Distribution
We are offering, on a “best efforts” basis, up to $15,000,000 worth of Units (up to 4,285,714 Units), each Unit consisting of one (1) share of Series D Preferred Stock and one (1) Warrant to purchase one (1) share of Common Stock at a price of $5.00 per share. The minimum subscription is $675, or 193 Units.
We intend to market the Units in this Offering through both online and offline means. Online marketing may take the form of contacting potential investors through electronic media and posting our offering statement on an online investment platform. This offering statement will be furnished to prospective investors via download 24 hours per day, 7 days per week on our website (https://heartsciences.com) on a landing page that relates to the Offering.
This Offering will terminate at the earlier of the date at which the maximum offering amount has been sold or the date at which the Offering is earlier terminated by us at our sole discretion, and the offering statement on Form 1-A of which this offering statement forms a part will remain qualified in accordance with Rule 251(d)(3)(i)(F) of Regulation A until the date at which all of our outstanding investor warrants issued pursuant to this Offering have been exercised for shares of Common Stock, which shares of Common Stock are qualified under the Form 1-A. At least every 12 months after this Offering has been qualified by the SEC, we will file a post-qualification amendment to include our then recent financial statements.
We intend to complete multiple closings in this Offering. After each closing, funds tendered by investors will be available to us.
Engagement Agreement with Digital Offering
We are currently party to an engagement agreement dated October 17, 2024 with Digital Offering, LLC (“Digital Offering” or the “lead selling agent”). Digital Offering has agreed to act as our lead selling agent for the Offering. Digital Offering has made no commitment to purchase all or any part of the Units being offered but has agreed to use commercially reasonable efforts to sell such Units in the Offering. As such, Digital Offering is an “underwriter” within the meaning of Section 2(a)(11) of the Securities Act. Digital Offering is under no obligation to purchase any of the Units or arrange for the sale of any specific number or dollar amount of Units. The term of the engagement agreement began on October 17, 2024 and will continue until the earliest to occur of: (a) the date that either party gives the other at least ten (10) days written notice of the termination of the engagement agreement, which termination may occur with or without cause, (b) October 31, 2025, and (c) the date that the Offering is consummated (such applicable date, the “Termination Date”). The engagement agreement provides that Digital Offering may engage other Financial Industry Regulatory Authority (“FINRA”) member broker-dealers that are registered with the SEC to participate as soliciting dealers for this Offering. We refer to these other broker-dealers as soliciting dealers or members of the selling group. Upon engagement of any such soliciting dealer, Digital Offering will be permitted to re-allow all or part of its fees and expense allowance as described below. Such soliciting dealer will also be entitled to receive the benefits of our engagement agreement with Digital Offering, including the indemnification rights arising under the engagement agreement upon their execution of a soliciting dealer agreement with Digital Offering that confirms that such soliciting dealer is so entitled. As of the date hereof, we have been advised that Digital Offering has retained DealMaker Securities LLC to participate in this Offering as soliciting dealers. We will not be responsible for paying any placement agency fees, commissions or expense reimbursements to any soliciting dealers retained by Digital Offering. None of the soliciting dealers is purchasing any of the Units in this Offering or is required to sell any specific number or dollar amount of Units, but will instead arrange for the sale of Units to investors on a “best efforts” basis, meaning that they need only use their best efforts to sell the Units. In addition to the engagement agreement, we plan to enter into a definitive selling agency agreement with Digital Offering prior to the commencement of the Offering.
Offering Expenses
We are responsible for all offering fees and expenses, including the following: (i) fees and disbursements of our legal counsel, accountants, and other professionals we engage; (ii) fees and expenses incurred in the production of offering documents, including design, printing, photograph, and written material procurement costs; (iii) all filing fees, including those charged by FINRA; and (iv) all of the legal fees related to FINRA clearance. Additionally, we have agreed to pay Digital Offering an accountable $25,000 due diligence fee considered as expenses of Digital Offering, which amount has already been advanced to Digital Offering by us. This $25,000 due diligence fee received by Digital Offering as an advance against expenses anticipated to be incurred will be reimbursed to us to the extent not actually incurred, in compliance with FINRA Rule 5110(g)(4)(a). We have also agreed to reimburse Digital Offering for its reasonable and documented legal costs up to a maximum of $85,000, $25,000 of which has been paid to date. Notwithstanding the foregoing, this advance for legal fees received by Digital Offering will be reimbursed to us to the extent not actually incurred in compliance with FINRA Rule 5110(g)(4)(a).
Reimbursable Expenses in the Event of Termination
In the event the Offering does not close or the selling agency agreement is terminated for any reason, we have agreed to reimburse Digital Offering for all actual unreimbursed, reasonable, documented, out-of-pocket fees, expenses, and disbursements, including its legal fees up to a maximum of $85,000.
Other Expenses of the Offering
The lead selling agent has engaged DealMaker Securities LLC as a soliciting dealer to assist in the placement of our Units in those states where it is registered to undertake such activities, including soliciting potential investors on a best efforts basis.
In addition, we have retained DealMaker Reach LLC (“Reach”) for marketing and advisory services. Reach, an affiliate of DealMaker Securities, LLC, will consult and advise on the design and messaging on creative assets, website design and implementation, paid media and email campaigns, advise on optimizing our campaign page to track investor progress, and advise on strategic planning, implementation, and execution of our capital raise marketing budget. We will pay Reach a monthly fee of $12,000 in cash up to a maximum of $78,000. We have also paid Reach a $30,000 launch fee. This launch fee received by Reach will be reimbursed to us to the extent not actually incurred, in compliance with FINRA Rule 5110(g)(4)(a). To the extent services under this agreement are commenced in advance of a FINRA no objection letter being received by us, such amounts shall be considered an advance against accountable expenses anticipated to be incurred, and fully refunded to extent not actually incurred, in compliance with FINRA Rule 5110(g)(4)(a). A maximum of $48,000 or three months of account management fees are payable prior to a no objection letter being received.
We have also engaged, through its agreement with Reach, Novation Solutions Inc. operating as DealMaker (“DealMaker”), an affiliate of DealMaker Securities, LLC, to create and maintain the online subscription processing platform for the Offering. After our offering statement is qualified by the SEC, the Offering will be conducted, in part, using DealMaker’s online subscription processing platform through our website, whereby investors will receive, review, execute and deliver subscription agreements electronically as well as make purchase price payments through a third-party processor by ACH debit transfer, wire transfer or credit card. Novation Solutions, Inc. has not received, is not receiving and will not receive any compensation for its services.
Selling Agents’ Commission
We have agreed that the definitive selling agency agreement will provide for us to pay a cash commission of 7% of the gross proceeds received by us in the Offering, which shall be allocated by Digital Offering to members of the selling group and soliciting dealers in its sole discretion (we sometimes refer to Digital Offering and such members and dealers collectively as the “Selling Agents”).
The following table shows the total commissions payable to Digital Offering on a per-Unit basis in connection with this Offering, assuming a fully subscribed Offering.
| | | Per Unit | |
| Public offering price | | $ | 3.50 | |
| Digital Offering commission (7.0 %)* | | $ | 0.245 | |
| Proceeds, before expenses, to us, per Unit | | $ | 3.255 | |
| * | Assuming a fully subscribed Offering, Digital Offering would receive total cash commissions of $1,050,000. |
Lead Selling Agents’ Warrants
Upon the closing of the Offering, we have agreed to issue warrants, the Agent Warrants, to the lead selling agent to purchase a number of our Units equal to 3.0% of the total number of our Units sold in the Offering (the “Agent Unit Warrants”). Each Agent Warrant will be exercisable for one Unit consisting of one share of Series D Preferred Stock and one Warrant (the “Agent Unit Warrants,”) each exercisable for the purchase of one share of Common Stock. The Agent Warrants and the Agent Unit Warrants will be exercisable from the date of issuance until the fifth anniversary of the date of commencement of sales in the Offering in accordance with FINRA Rule 5110(g)(8)(A). The price per unit for the Agents Warrants will be the amount that is 125% of the offering price, or $4.375 per Unit. The exercise price for up to 128,571 of the Agent Unit Warrants included in the Units issuable upon exercise of the Agents Warrants will be $5.00 per share. The Agents Warrants will not be redeemable. The Agent Warrants will provide for cashless exercise in the event there is not a qualified offering statement (or effective registration statement) covering the shares underlying the Selling Agent’s Warrants, the offering circular (or prospectus, as applicable) contained therein is not available for the issuance of the securities underlying the Units or we are not current in our public company reporting requirements, and immediate “piggyback” registration rights, with a duration of five years from the date of commencement of sales in the Offering (in compliance with FINRA Rule 5110(g)(8)(D)), with respect to the registration or qualification of the Agent Unit Warrants, shares of Series D Preferred Stock, shares of Common Stock into which the Series D Preferred Stock may convert and shares of Common Stock issuable upon exercise of the Agent Unit Warrants. and immediate “piggyback” registration rights, with a duration of five years from the date of commencement of sales in the Offering (in compliance with FINRA Rule 5110(g)(8)(D)), with respect to the registration or qualification of the Agent Unit Warrants, shares of Series D Preferred Stock, shares of Common Stock into which the Series D Preferred Stock may convert and shares of Common Stock issuable upon exercise of the Agent Unit Warrants. We are qualifying the Agents Warrants and the Agent Unit Warrants, shares of Series D Preferred Stock, shares of Common Stock into which the Series D Preferred Stock may convert and shares of Common Stock issuable upon exercise of the Agents Unit Warrants in the Offering.
The Agents Warrants and the Agent Unit Warrants, Series D Preferred Stock and the Common Stock underlying the Agents Unit Warrants or into which the Series D Preferred Stock may convert have been deemed compensation by FINRA and are therefore subject to a 180-day lock-up from the date of the commencement of sales in the Offering pursuant to Rule 5110(e)(1) of FINRA. The lead selling agent, or permitted assignees under such rule, may not sell, transfer, assign, pledge, or hypothecate the Agents Warrants or the Agent Unit Warrants, Series D Preferred Stock and the Common Stock underlying the Agent Unit Warrants or into which the Series D Preferred Stock may convert, nor will the lead selling agent or permitted assignees engage in any hedging, short sale, derivative, put, or call transaction that would result in the effective economic disposition of the Agent Warrants or the underlying Agent Unit Warrants, Series D Preferred Stock and the Common Stock for a period of six months after the date of the final Closing of the Offering, except that they may be transferred, in whole or in part, by operation of law or by reason of our reorganization, or to any selling agent or selected dealer participating in the Offering and their officers, partners or registered representatives if the Agent Warrants or the underlying securities so transferred remain subject to the foregoing lock-up restrictions for the remainder of the time period. The Agents Warrants will provide for adjustment in the number and price of such Agent Warrants (and the underlying Agent Unit Warrants, Series D Preferred Stock and the Common Stock) to prevent dilution in the event of a stock dividend or stock split. The Agent Warrants will also be subject to adjustment in the event of a capital reorganization or reclassification of our Common Stock.
Pricing of the Offering
Prior to the Offering, there is no public market for the Units. The offering price has been determined by negotiation between us and Digital Offering. The principal factors considered in determining the offering price include:
| ● | the information set forth in this offering statement and otherwise available to Digital Offering; |
| ● | our history and prospects and the history of and prospects for the industry in which we compete; |
| ● | our past and present financial performance; |
| ● | our prospects for future earnings and the present state of our development; |
| ● | an assessment of our management; |
| ● | the general condition of the securities markets at the time of this Offering; |
| ● | the recent market prices of, and demand for, publicly traded Common Stock of generally comparable companies; and |
| ● | other factors deemed relevant by Digital Offering and us. |
Indemnification and Control
We have agreed to indemnify the lead selling agent, its affiliates and controlling persons and members of the selling group against certain liabilities, including liabilities under the Securities Act. If we are unable to provide this indemnification, we will contribute to the payments the lead selling agent, its affiliates and controlling persons as may be required to make in respect of these liabilities.
The lead selling agent and its affiliates are engaged in various activities, which may include securities trading, commercial and investment banking, financial advisory, investment management, investment research, principal investment, hedging, financing and brokerage activities. The lead selling agent and its affiliates may in the future perform various financial advisory and investment banking services for us, for which they received or will receive customary fees and expenses.
Our Relationship with the Lead Selling Agent
In the ordinary course of their various business activities, Digital Offering and its affiliates may make or hold a broad array of investments and actively trade debt and equity securities (or related derivative securities) and financial instruments (including bank loans) for their own account and for the accounts of their customers, and such investment and securities activities may involve securities and/or instruments of the Company. Digital Offering and its affiliates may also make investment recommendations and/or publish or express independent research views in respect of such securities or instruments, or recommend to clients that they acquire, long and/or short positions in such securities and instruments.
Investment Limitations
As set forth in Title IV of the JOBS Act, there would be no limit on how many units an investor may purchase if this offering results in a listing of our Series D Preferred Stock on Nasdaq or other national securities exchange. However, our Series D Preferred Stock will not be listed on Nasdaq upon the initial qualification of this offering by the SEC and we do not intend to list our Series D Preferred Stock on Nasdaq.
For individuals who are not accredited investors, no sale may be made to you in this offering if the aggregate purchase price you pay is more than 10% of the greater of your annual income or net worth (please see under “— Procedures for Subscribing — How to Calculate Net Worth”). Different rules apply to accredited investors and non-natural persons. Before making any representation that your investment does not exceed applicable thresholds, we encourage you to review Rule 251(d)(2)(i)(C) of Regulation A. For general information on investing, we encourage you to refer to www.investor.gov.
Because this is a Tier 2, Regulation A offering, most investors in the case of trading on the over-the-counter markets must comply with the 10% limitation on investment in this offering. The only investors in this offering exempt from this limitation, if our Series D Preferred Stock is not listed on Nasdaq, are “accredited investors” as defined under Rule 501 of Regulation D under the Securities Act (each, an “Accredited Investor”). If you meet one of the following tests you should qualify as an Accredited Investor:
| (i) | You are a natural person who has had individual income in excess of $200,000 in each of the two most recent years, or joint income with your spouse in excess of $300,000 in each of these years, and have a reasonable expectation of reaching the same income level in the current year; |
| (ii) | You are a natural person and your individual net worth, or joint net worth with your spouse, exceeds $1,000,000 at the time you purchase units (please see below under “— How to Calculate Net Worth”); |
| (iii) | You are an executive officer or general partner of the issuer or a director, executive officer or general partner of the general partner of the issuer; |
| (iv) | You are a holder in good standing of the General Securities Representative license (Series 7), the Private Securities Offerings Representative license (Series 82), and the Licensed Investment Adviser Representative (Series 65), each as issued by FINRA; |
| (v) | You are a corporation, limited liability company, partnership or are an organization described in Section 501(c)(3) of the Internal Revenue Code of 1986, as amended, a corporation or similar business trust or a partnership, not formed for the specific purpose of acquiring the units, with total assets in excess of $5,000,000; |
| (vi) | You are a bank or a savings and loan association or other institution as defined in the Securities Act, a broker or dealer registered pursuant to Section 15 of the Exchange Act, an insurance company as defined by the Securities Act, an investment company registered under the Investment Company Act of 1940 (the “Investment Company Act”), or a business development company as defined in that act, any Small Business Investment Company licensed by the Small Business Investment Act of 1958 or a private business development company as defined in the Investment Advisers Act of 1940; |
| (vii) | You are an entity (including an Individual Retirement Account trust) in which each equity owner is an accredited investor; |
| (viii) | You are a trust with total assets in excess of $5,000,000, your purchase of units is directed by a person who either alone or with his purchaser representative(s) (as defined in Regulation D promulgated under the Securities Act) has such knowledge and experience in financial and business matters that he is capable of evaluating the merits and risks of the prospective investment, and you were not formed for the specific purpose of investing in the units; |
| (ix) | You are a plan established and maintained by a state, its political subdivisions, or any agency or instrumentality of a state or its political subdivisions, for the benefit of its employees, if such plan has assets in excess of $5,000,000; |
| (x) | You are a Commission or state-registered investment adviser or a federally exempt reporting adviser; |
| (xi) | You are a Rural Business Investment Company as defined in section 384A of the Consolidated Farm and Rural Development Act; |
| (xii) | You are an entity not listed above that that owns “investments,” in excess of $5 million and that was not formed for the specific purpose of investing in the securities offered; or |
| (xiii) | You are an Investor certifies that (A) it is a “family office” as defined in Rule 202(a)(11)(G)-1 under the Investment Advisers Act of 1940 (i) with at least $5 million in assets under management, (ii) not formed for the specific purpose of acquiring the securities offered and (iii) whose investment is directed by a person who has such knowledge and experience in financial and business matters that such family office is capable of evaluating the merits and risks of the prospective investment or (B) that it is a “family client” as defined in Rule 202(a)(11)(G)-1, of a family office meeting the criteria specified above. |
Procedures for Subscribing
DealMaker Securities LLC
Investors who invest through DealMaker Securities LLC may subscribe through http://invest.heartsciences.com by tendering funds by wire, credit, or debit card or ACH transfer to the escrow account to be set up at Enterprise Bank & Trust, the Escrow Agent. Tendered funds will remain in escrow until a closing has occurred. Upon each closing, funds tendered by investors will be made available to us for our use. We will not cover credit card fees on behalf of investors.
Procedures for subscribing directly through the Company’s website
The subscription procedure is summarized as follows:
| 1. | Go to the http://invest.heartsciences.com website and click on the “Invest Now” button; |
| 2. | Complete the online investment form; |
| 3. | Deliver funds directly by wire, debit card, credit card or electronic funds transfer via ACH to the specified escrow account; |
| 4. | Once funds or documentation are received an automated Anti Money Laundering (“AML”) check will be performed to verify the identity and status of the investor; |
| 5. | Once AML is verified, investor will electronically receive, review, execute and deliver to us a subscription agreement. Investors will be required to complete a subscription agreement in order to invest. The subscription agreement will include a representation by the investor to the effect that, if the investor is not an “accredited investor” as defined under securities law, the investor is investing an amount that does not exceed the greater of 10% of his or her annual income or 10% of his or her net worth (excluding the investor’s principal residence). |
Right to Reject Subscriptions
After we receive your complete, executed subscription agreement (forms of which are attached to the offering statement, of which this offering statement forms a part, as Exhibits 4.1 and 4.2 to this offering statement) and the funds required under the subscription agreement have been transferred to the HeartSciences Escrow Account or such other selected dealer designated escrow account, we have the right to review and accept or reject your subscription in whole or in part, for any reason or for no reason. If we reject your subscription, we will return all monies from your rejected subscription to you, without interest or deduction.
Acceptance of Subscriptions
Upon our acceptance of a subscription agreement, we will countersign the subscription agreement and issue the shares of subscribed for Units at closing. Once you submit the subscription agreement and it is accepted, you may not revoke or change your subscription or request your subscription funds. All accepted subscription agreements are irrevocable.
Under Rule 251 of Regulation A, unless a company’s offered securities are listed on a national securities exchange, non-accredited, non-natural person investors are subject to the investment limitation and may only invest funds which do not exceed 10% of the greater of the purchaser’s revenue or net assets (as of the purchaser’s most recent fiscal year end). As a result, non-accredited, non-natural persons may only invest funds in our Units which do not exceed 10% of the greater of the purchaser’s annual revenue or net assets (please see below on how to calculate your net worth).
How to Calculate Net Worth
For the purposes of calculating your net worth, it is defined as the difference between total assets and total liabilities. This calculation must exclude the value of your primary residence and may exclude any indebtedness secured by your primary residence (up to an amount equal to the value of your primary residence). In the case of fiduciary accounts, net worth and/or income suitability requirements may be satisfied by the beneficiary of the account or by the fiduciary, if the fiduciary directly or indirectly provides funds for the purchase of the Units.
In order to purchase the Units and prior to the acceptance of any funds from an investor, for so long as our Units are not listed on Nasdaq, an investor in our Units will be required to represent, to the Company’s satisfaction, that he or she is either an accredited investor or is in compliance with the 10% of net worth or annual income limitation on investment in this Offering.
No Minimum Offering Amount
There is no minimum offering amount in this Offering and we may close on any funds that we receive. Potential investors should be aware that there can be no assurance that any other funds will be invested in this Offering other than their own funds.
No Selling Security holders
No securities are being sold for the account of security holders; all net proceeds of this Offering will go to the Company.
Agent and Registrar
We have engaged Equiniti Trust Company, LLC, a registered transfer agent with the SEC, who will serve as transfer agent to maintain stockholder information on a book-entry basis.
Provisions of Note in our Subscription Agreement
Forum Selection Provision
The subscription agreement that investors will execute in connection with the Offering includes a forum selection provision that requires any claims against the Company based on the subscription agreement to be brought in a state or federal court of competent jurisdiction in the State of Texas, for the purpose of any suit, action or other proceeding arising out of or based upon the agreement. Although we believe the provision benefits us by providing increased consistency in the application of Texas law in the types of lawsuits to which it applies and in limiting our litigation costs, to the extent it is enforceable, the forum selection provision may limit investors’ ability to bring claims in judicial forums that they find favorable to such disputes and may discourage lawsuits with respect to such claims. The Company has adopted the provision to limit the time and expense incurred by its management to challenge any such claims. As a company with a small management team, this provision allows its officers to not lose a significant amount of time traveling to any particular forum so they may continue to focus on the operations of the Company. Section 22 of the Securities Act creates concurrent jurisdiction for federal and state courts over all suits brought to enforce any duty or liability created by the Securities Act or the rules and regulations thereunder. We believe that the exclusive forum provision applies to claims arising under the Securities Act, but there is uncertainty as to whether a court would enforce such a provision in this context. Section 27 of the Exchange Act creates exclusive federal jurisdiction over all suits brought to enforce any duty or liability created by the Exchange Act or the rules and regulations thereunder. As a result, the exclusive forum provision will not apply to suits brought to enforce any duty or liability created by the Exchange Act or any other claim for which the federal courts have exclusive jurisdiction. Investors will not be deemed to have waived the Company’s compliance with the federal securities laws and the rules and regulations thereunder.
Jury Trial Waiver
The subscription agreement that investors will execute in connection with the Offering provides that subscribers waive the right to a jury trial of any claim they may have against us arising out of or relating to the agreement, other than claims arising under federal securities laws. If we opposed a jury trial demand based on the waiver, a court would determine whether the waiver was enforceable given the facts and circumstances of that case in accordance with applicable case law. In addition, by agreeing to the provision, subscribers will not be deemed to have waived the Company’s compliance with the federal securities laws and the rules and regulations promulgated thereunder.
Offer Restrictions Outside the United States
Other than in the United States, no action has been taken by us or the lead selling agent that would permit a public offering of the securities offered by this offering statement in any jurisdiction where action for that purpose is required. The securities offered by this offering statement may not be offered or sold, directly or indirectly, nor may this offering statement or any other offering material or advertisements in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this offering statement comes are advised to inform themselves about and to observe any restrictions relating to the Offering and the distribution of this offering statement. This offering statement does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this offering statement in any jurisdiction in which such an offer or a solicitation is unlawful.
DISCLOSURE OF COMMISSION POSITION ON INDEMNIFICATION FOR SECURITIES LIABILITIES
Our Amended and Restated Certificate of Formation, as amended, and our Second Amended and Restated Bylaws, subject to the provisions of Texas law, contain provisions that allow the Company to indemnify any person against liabilities and other expenses incurred as the result of defending or administering any pending or anticipated legal issue in connection with service to us if it is determined that person acted in good faith and in a manner which he reasonably believed was in the best interest of the Company. We have also entered into indemnification agreements with each of our executive officers and directors that provide our executive officers and directors with contractual rights to indemnification, and expense advancement and reimbursement, to the fullest extent permitted under the laws of the State of Texas in effect from time to time, subject to certain exceptions contained in those agreements. Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors and officers, we have been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable.
USE OF PROCEEDS
The table below sets forth our estimated use of proceeds from the Units being offered in this offering statement, assuming we sell $15,000,000 in Units.
| | | 25% of
Maximum
Offering
Amount | | | 50% of
Maximum
Offering
Amount | | | 75% of
Maximum
Offering
Amount | | | Maximum
Offering
Amount | |
| Gross Offering Proceeds | | $ | 3,750,000 | | | $ | 7,500,000 | | | $ | 11,250,000 | | | $ | 15,000,000 | |
| Less: | | | | | | | | | | | | | | | | |
| Estimated Offering Expenses | | $ | 432,500 | | | $ | 695,000 | | | $ | 957,500 | | | $ | 1,220,000 | |
| Estimated Net Offering Proceeds | | $ | 3,317,500 | | | $ | 6,805,000 | | | $ | 10,292,500 | | | $ | 13,780,000 | |
| | | | | | | | | | | | | | | | | |
| Principal Uses of Net Proceeds | | | | | | | | | | | | | | | | |
| Sales and Marketing | | $ | 217,500 | | | $ | 605,000 | | | $ | 992,500 | | | $ | 1,380,000 | |
| Inventory & Operations | | $ | 125,000 | | | $ | 250,000 | | | $ | 375,000 | | | $ | 500,000 | |
| Administrative G&A | | $ | 1,175,000 | | | $ | 2,350,000 | | | $ | 3,525,000 | | | $ | 4,700,000 | |
| Research & Development | | $ | 1,800,000 | | | $ | 3,600,000 | | | $ | 5,400,000 | | | $ | 7,200,000 | |
| Total Use of Proceeds | | $ | 3,317,500 | | | $ | 6,805,000 | | | $ | 10,292,500 | | | $ | 13,780,000 | |
We reserve the right to change the use of proceeds at management’s discretion.
DIVIDEND POLICY
We have never declared or paid any cash dividends on our capital stock. We currently intend to retain any future earnings to fund the development and expansion of our business, and therefore we do not anticipate paying cash dividends on our Common Stock in the foreseeable future. Any future determination to pay dividends will be at the discretion of our Board of Directors and will depend on our results of operations, financial condition, capital requirements, contractual restrictions and other factors deemed relevant by our Board of Directors.
DILUTION
We do not believe there would be any material disparity between the public offering price of the Units and any effective cash cost to officers, directors, promoters and affiliated persons for shares acquired by them in a transaction during the past calendar year, or that they have a right to acquire. Accordingly, pursuant to Item 4 of Form 1-A, a comparison of the public contribution under the proposed public offering of the Units and the average effective cash contribution of such persons is not presented in this offering statement.
Security Ownership of Certain Beneficial Owners and Management
The following table sets forth information regarding beneficial ownership of our Common Stock and Series C Preferred Stock as of February 5, 2025 by:
| ● | each person, or group of affiliated persons, known to us to be the beneficial owner of more than 5% of our outstanding Common Stock or Series C Preferred Stock; |
| ● | each of our directors and executive officers; and |
| ● | all of our directors and executive officers as a group. |
Beneficial ownership is determined in accordance with the rules of the SEC and includes voting or investment power with respect to the shares of capital stock indicated. Shares of capital stock that are issuable upon (i) the conversion of Series C Preferred Stock or (ii) the exercise of options or warrants exercisable within 60 days after July 26, 2024, are deemed outstanding for the purpose of computing the percentage ownership of the person holding such Series C Preferred Stock, options or warrants, but are not deemed outstanding for the purpose of computing the percentage ownership of any other person.
We are not controlled by another corporation, by any foreign government or by any natural or legal persons except as set forth herein, and there are no arrangements known to us which would result in a change in control of our Company at a subsequent date. Except as indicated in footnotes to this table, we believe that the shareholders named in this table have sole voting and investment power with respect to all shares shown to be beneficially owned by them, based on information provided to us by such shareholders. Unless otherwise noted below, each beneficial owner’s address is c/o HeartSciences Inc., 550 Reserve Street, Suite 360, Southlake, Texas 76092.
The percentage of beneficial ownership in the table below is based on 1,036,321 shares of our Common Stock and 380,440 shares of Series C Preferred Stock outstanding as of February 5, 2025.
| | | Beneficial Ownership | |
| | | Number of Shares (1) | | | Percentages (2) | |
| Name of Beneficial Owner | | Common Stock | | | Series C Preferred Stock | | | Common Stock | | | Series C Preferred Stock | | | Combined Voting Power (3) | |
| Holders of 5% or more of each class of our securities: | | | | | | | | | | | | | | | |
| Front Range Ventures, LLC (4) | | | 34,099 | | | | 148,213 | | | | 3.2 | % | | | 39.0 | % | | | 3.0 | % |
| Matthews Holdings Southwest (5) | | | 52,419 | | | | — | | | | 5.0 | % | | | — | | | | 4.6 | % |
| Mount Sinai (6) | | | 64,798 | | | | — | | | | 6.2 | % | | | — | | | | 5.7 | % |
| | | | | | | | | | | | | | | | | | | | | |
| Directors and executive officers: | | | | | | | | | | | | | | | | | | | | |
| Andrew Simpson, President, CEO, and Chairman (7) | | | 8,518 | | | | 6,117 | | | | * | | | | 1.6 | % | | | * | |
| Mark Hilz, Coo & Secretary (8) | | | 7,694 | | | | 2,080 | | | | * | | | | * | | | | * | |
| Danielle Watson, CFO & Treasurer (9) | | | 531 | | | | — | | | | * | | | | — | | | | * | |
| Bruce Bent, Director (10) | | | 2,036 | | | | — | | | | * | | | | — | | | | * | |
| Brian Szymczak, Director (11) | | | 2,367 | | | | 400 | | | | * | | | | * | | | | * | |
| David R. Wells, Director (12) | | | 2,000 | | | | — | | | | * | | | | — | | | | * | |
| All directors and executive officers as a group (6 persons): | | | 23,146 | | | | 8,597 | | | | 2.2 | % | | | 2.3 | % | | | 2.1 | % |
| (1) | For each person named in the table, the total number of shares of capital stock beneficially owned by such person to the best knowledge of the Company, is listed opposite of such person’s name. |
| (2) | For each person named in the table, the shares of capital stock indicated opposite such person’s name represents the percentage of the total number of the shares of capital stock beneficially owned by such person as a percentage of the shares of our outstanding capital stock indicated as a class. |
| (3) | For each person named in the table, the voting percentage indicated opposite of such person’s name under the column “Combined Voting Power” represents the combined voting percentage of all shares of our Common Stock and all of our Series C Preferred Stock, on an as converted basis, owned by such person. |
| (4) | Front Range Ventures, LLC’s (“FRV”) sole member is the L. Lee Stryker Irrevocable Trust U/A/D 09/10/1974. Bohemian Asset Management, Inc. who has voting and dispositive power with respect to the shares of our Common Stock on behalf of the L. Lee Stryker Irrevocable Trust U/A/D 09/10/1974. Includes (i) 33,023 shares of our Common Stock issuable upon conversion of 148,213 shares of our Series C Preferred Stock; and (ii) 1,076 shares of our Common Stock issuable upon exercise of $1M Lender Warrants. |
| (5) | All of the shares are owned by either Matthews Holdings Southwest, Inc. or Mr. Matthews (“MSW”). Mr. Matthews, as sole controlling shareholder of Matthews Holdings Southwest, Inc., has sole voting and dispositive power over all such shares. Includes (i) 16 shares of our Common Stock issuable upon exercise of $1.5M Lender Warrants; (ii) 10,000 shares of our Common Stock issuable in aggregate upon exercise of MSW Warrants, (iii) 1,177 shares of our Common Stock issuable upon exercise of IPO Warrants and (iv) 1,500 shares of our Common Stock issuable upon the exercise of Pre-Funded Bridge Warrants. |
| (6) | All of the shares are owned by Mount Sinai and its Board of Directors has sole voting and dispositive power over all such shares. Includes (i) 9,142 shares of our Common Stock issuable upon exercise of the MTS Warrants and (ii) 7,107 shares of our Common Stock issuable upon the exercise of the MTS Pre-Funded Warrants. |
| (7) | Includes (i) 1,362 shares of our Common Stock issuable upon conversion of 6,117 shares of Series C Preferred Stock; (ii) 1 share of our Common Stock issuable upon exercise of $1.5M Lender Warrants; and (iii) options to purchase 2,773 shares of our Common Stock, which were issued as compensation for services rendered to the Company as its Chairman of the Board of Directors |
| (8) | Includes (i) 463 shares of our Common Stock issuable upon conversion of 2,080 shares of Series C Preferred Stock; (ii) 1 share of our Common Stock issuable upon exercise of $1.5M Lender Warrants; and (iii) options to purchase 2,773 shares of our Common Stock issued as compensation for services as an officer of the Company. |
| (9) | Includes options to purchase 531 shares of our Common Stock issued as compensation for services as an officer of the Company. |
| (10) | Includes (i) 17 shares of our Common Stock held by Mr. Bent’s spouse and (ii) 2,019 shares of our Common Stock issuable upon exercise of options issued as compensation for services rendered to the Company. |
| (11) | Includes (i) 89 shares of our Common Stock issuable upon conversion of 400 shares of Series C Preferred Stock held jointly with Mr. Szymczak’s spouse; (ii) 1 share of our Common Stock issuable upon exercise of $1.5M Lender Warrants; and (iii) 2,216 shares of our Common Stock issuable upon exercise of options issued as compensation for services rendered to the Company. |
| (12) | Includes 2,000 shares of our Common Stock issuable upon exercise of options issued as compensation for services rendered to the Company. |
Equity Compensation Plan Information
The following table reflects the number of shares of our Common Stock issuable upon the exercise of awards granted under our equity compensation plans approved and not approved by shareholders and the weighted average exercise price for such awards as of April 30, 2024.
| Name of Plan | | Number of shares of
Common Stock to be
issued upon exercise of
outstanding options,
warrants and rights | | | Weighted
Average Exercise Price of
Outstanding
Options ($) | | | Number of shares
remaining available for
issuance under equity
compensation plans
(excluding the shares
reflected in column (1) | |
| Equity compensation plans approved by security holders (1) | | | 18,815 | | | $ | 53.65 | | | | 74,507 | |
| Equity compensation plans not approved by security holders | | | — | | | | — | | | | — | |
| Total (1) | | | 18,815 | | | $ | 53.65 | | | | 74,507 | |
| (1) | Represents securities issued under our Equity Incentive Plan |
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
The following is a description of transactions or series of transactions since the beginning of Fiscal 2024 to which we were or will be a party, in which:
| ● | The amount involved in the transaction exceeds, or will exceed, the lesser of $120,000 or one percent of the average of our total assets for the last two completed fiscal years; and |
| ● | in which any of our executive officers, directors or holders of five percent or more of any class of our capital stock, including their immediate family members or affiliated entities, had or will have a direct or indirect material interest. |
For additional information regarding compensation arrangements for our named executive officers and directors, see “Item 11. Executive Compensation” of our Annual Report on Form 10-K, filed with the SEC on July 29, 2024 (the “2024 Annual Report”).
Related Party Transactions
$1M Loan and Security Agreement
In April 2020, we entered into a loan and security agreement (the “$1M Loan and Security Agreement”) pursuant to which a secured promissory note in the original principal amount of $500,000 was issued to each of FRV (the “FRV Note”) and John Q. Adams (the “JQA Note”), who were both shareholders of our Company at the time of issuance. John Q. Adams was also a Director of our Company at the time of entering into the $1M Loan and Security Agreement. Each party committed to lend a principal amount of $500,000, totaling $1,000,000, and the loan was drawn in three installments of $300,000 upon execution of the loan agreement, $350,000 on or about July 2, 2020 and $350,000 on or about September 4, 2020. The loan accrued interest at a rate of 12% per annum, compounded annually, payable at maturity. We also required to pay default interest at a rate of 18% per annum, compounded annually, on any unpaid amounts after the applicable due date until the loan amounts are fully re-paid. The loan is collateralized by substantially all of our assets and intellectual property, except for the secured interest on the covered technology as discussed in Note 8 to our audited financial statements included elsewhere in this offering statement.
The loan had an original maturity date of September 30, 2021, which was amended in September 2021 making the note repayable on demand. The loan was amended in November 2021, extending the maturity to September 30, 2022; further amended in May 2022 to extend the maturity to September 30, 2023; amended again in January 2023 to (i) further extend the maturity date of the FRV Note to September 30, 2024, on which date the principal amount and all accrued interest thereon would be due and payable; and (ii) amend the dates on which principal and accrued interest was due under the JQA Note, such that interest accrued since June 28, 2022 would be due and payable on September 30, 2023, and the principal amount together with all accrued interest after September 30, 2023 would be due and payable on March 31, 2024.
In connection with the amendment in May 2022, the Company agreed to pay Mr. Adams all accrued but unpaid interest on his note prior to September 30, 2022.
In October 2023, the Company issued to FRV and Mr. Adams warrants (“$1M Lender Warrants”) to purchase an aggregate of 2,000 shares of Common Stock as consideration for the extension of the interest maturity date to one lender.
On November 16, 2023, the Company entered into a note conversion letter agreement with John Q. Adams (the “Adams Note Conversion Letter Agreement”). Pursuant to the Adams Note Conversion Letter Agreement, in consideration for the conversion of the principal and interest in the amounts of $585,006 due under the JQA Note, on November 16, 2023, the Company: (1) issued 36,563 shares of Common Stock to Mr. Adams; and (2) entered into a Warrant Amendment Agreement with Mr. Adams, amending the $1M Lender Warrants owned by Mr. Adams to reduce the exercise price of an aggregate of 1,076 $1M Lender Warrants to $16.00 per share (the “Adams Warrant Amendment”).
MSW Note
On September 6, 2023, the Company entered into the MSW Note with Matthews Holdings Southwest, Inc., (the “Lender”). The MSW Note provided for an unsecured drawdown loan of up to $1.0 million, drawn in installments consisting of (i) $250,000 on or prior to September 8, 2023, (ii) $250,000 on or prior to September 20, 2023, and (iii) further drawdowns of up to $500,000 in such amounts and such times to be mutually agreed upon between the Company and Lender.
In September 2023, the Company drew $0.5 million under the MSW Note and issued warrants in lieu of a facility fee to purchase 5,000 shares of Common Stock exercisable at $100.00 per share, warrants to purchase 2,500 shares of Common Stock exercisable at $125.00 per share, and warrants to purchase 2,500 shares of Common Stock exercisable at $150.00 per share.
On November 16, 2023, the Company entered into a note conversion letter agreement with the Lender (the “MSW Note Conversion Letter Agreement”). Pursuant to the MSW Note Conversion Letter Agreement, in consideration for the conversion of the aggregate amount of $500,000 due under the MSW Note, on November 16, 2023, the Company (i) issued to the Lender 31,250 shares of Common Stock at a conversion price of $16.00 per share; and (ii) entered into a Warrant Amendment Agreement with the Lender, amending the warrants to reduce the exercise price of an aggregate of 10,000 warrants to $16.00 per share (the “MSW Warrant Amendment”). See further discussion in Note 5 to our 2024 Annual Report. In accordance with the terms, no interest was payable as the note converted prior to maturity.
Agreements with Mount Sinai
On September 20, 2023, the Company entered into multiple definitive license agreements (each a “License Agreement” and collectively, the “License Agreements”) with Mount Sinai to commercialize a range of AI cardiovascular algorithms developed by Mount Sinai as well as a memorandum of understanding for ongoing cooperation encompassing de-identified data access, on-going research, and the evaluation of the MyoVista wavECG. The License Agreements, of which there are eleven in total, cover rights to thirteen AI cardiovascular algorithms, two data science methods for use with ECG waveforms and three filed patents.
The closing of the transactions contemplated under the Securities Purchase Agreement (the “MTS Transaction”), dated as of September 20, 2023 (the “Securities Purchase Agreement”), by and between the Company and Mount Sinai, and the effectiveness of the licenses under the License Agreements, were subject to the satisfaction or waiver of certain closing conditions.
On November 16, 2023, and pursuant to the Securities Purchase agreement, the Company issued to Mount Sinai the following:
| ● | 48,549 shares of Common Stock (the “Consideration Shares”); |
| ● | pre-funded warrants to purchase up to 7,107 shares of Common Stock, with an exercise price per share of $0.001, which warrants were issued in lieu of shares of Common Stock issuable to Mount Sinai to ensure that the number of shares of Common Stock held by Mount Sinai does not exceed the Beneficial Ownership Limitation (the “MTS Pre-Funded Warrants”); and |
| ● | Common stock warrants to purchase up to 9,142 shares of Common Stock, having an exercise price per share equal to $50.60, (the “MTS Warrants” and collectively with the Consideration Shares and the MTS Pre-Funded Warrants, the “MTS Securities”). |
On December 1, 2023, the Company satisfied all material closing conditions of the Mount Sinai Securities Purchase Agreement and the MTS Warrants thereafter became fully exercisable by Mount Sinai. Registration rights related to the MTS Securities provide that on or prior to the date of one hundred and fifty days (150) days after the closing date, the Company shall prepare and file with the SEC a Registration Statement on Form S-1 (or such other form as applicable) covering the resale under the Securities Act of the MTS Securities issued to Mount Sinai, subject to any limitations imposed by the Nasdaq Rules. On March 5, 2024, the Company filed with the SEC a Registration Statement on Form S-1 registering the resale of the MTS Securities issued to Mount Sinai and the Registration Statement on Form S-1 was declared effective on March 13, 2024.
Agreements with Front Range Ventures
Pursuant to the FRV Side Letter, FRV has the right to designate a director of the Company, which has not been exercised as of the date of this Offering Statement on Form 1-A.
Policy Related to Related Party Transactions
Our Board of Directors has adopted a formal, written related party transactions policy setting forth the Company’s policies and procedures for the review, approval, or ratification of “related party transactions.” For these purposes, a “related party” is (i) any person who is or was an executive officer, director, or director nominee of the Company at any time since the beginning of the Company’s last fiscal year, (ii) a person who is or was an immediate family member of an executive officer, director, director nominee at any time since the beginning of the Company’s last fiscal year, (iii) any person who, at the time of the occurrence or existence of the transaction, is the beneficial owner of more than 5% of any class of the Company’s voting securities, (iv) any person who, at the time of the occurrence or existence of the transaction, is an immediate family member of a shareholder owning more than 5% of any class of the Company’s voting securities or (v) any entity that, at the time of the occurrence or existence of the transaction, is a an entity in which a director of the Company is a partner, shareholder or executive officer or otherwise over which such director has influence or control. This policy applies to any transaction between the Company and a related party other than the following:
| ● | Transactions available to all employees generally; and |
| ● | Transactions, which when aggregated with the amount of all similar transactions, involve less than $5,000. |
Any related party transaction subject to this policy may only be consummated or may continue only if the Audit Committee approves or ratifies such transaction in accordance with the guidelines set forth in the policy and if the transaction is on terms comparable to those that could be obtained in arm’s length dealings with an unrelated third party and the transaction is approved by the disinterested members of the Board of Directors. In addition, if the transaction involves compensation, the compensation must have been approved by the Compensation Committee.
Our Audit Committee will analyze the following factors, in addition to any other factors the members of the Audit Committee deem appropriate, in determining whether to approve a related-person transaction:
| ● | The benefits to the Company; |
| ● | The impact on a director’s independence in the event the related party is a director, an immediate family member of a director or an entity in which a director is a partner, shareholder or executive officer or otherwise over which such director has influence or control; |
| ● | The availability of other sources for comparable products or services; |
| ● | The terms of the related party transaction; and |
| ● | The terms available to unrelated third parties or to employees generally. |
Our Audit Committee shall approve only those related party transactions that are in, or are not inconsistent with, the best interests of the Company and its shareholders, as the Audit Committee determines in good faith.
Director Independence
Under Nasdaq rules, independent directors must comprise a majority of a listed company’s board of directors. In addition, Nasdaq rules require that, subject to specified exceptions, each member of a listed company’s audit, compensation and nominating and governance committees must be independent. Under Nasdaq rules, a director will only qualify as an “independent director” if, in the opinion of that Company’s Board of Directors, that person does not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. Ownership of a significant amount of our stock, by itself, does not constitute a material relationship.
Audit committee members of a listed company must also satisfy the independence criteria set forth in Rule 10A-3 under the Exchange Act. In order to be considered independent for purposes of Rule 10A-3, a member of an audit committee of a listed company may not, other than in his or her capacity as a member of the audit committee, the Board of Directors, or any other board committee: (i) accept, directly or indirectly, any consulting, advisory, or other compensatory fee from the listed company or any of its subsidiaries; or (ii) be an affiliated person of the listed company or any of its subsidiaries. We have an audit committee composed of three independent directors who each meet the Nasdaq audit committee independence standards for a listed company.
Nasdaq rules require that, subject to limited exceptions, a listed company’s compensation committee must consist of at least two members, each of whom must be independent. In affirmatively determining the independence of any director who will serve on the compensation committee, the board of directors must consider all factors specifically relevant to determining whether a director has a relationship to the listed company which is material to that director’s ability to be independent from management in connection with the duties of a compensation committee member, including, but not limited to (i) the source of compensation of such director, including any consulting, advisory or other compensatory fee paid by the listed company to such director, and (ii) whether such director is affiliated with the listed company, a subsidiary of the listed company or an affiliate of a subsidiary of the listed company. We have a compensation committee composed of three independent directors.
Nasdaq rules require that director nominees must either be selected, or recommended for the board of director’s selection, either by (i) independent directors constituting a majority of the board’s independent directors in a vote in which only independent directors participate, or (ii) a nominations committee comprised solely of independent directors. We have a nominating and governance committee composed of three independent directors that recommends to our Board of Directors nominees for election as directors.
Our Board of Directors undertook a review of its composition, the composition of its committees and the independence of each director. Based upon information requested from and provided by each director concerning his or her background, employment and affiliations, including family relationships, our Board of Directors has determined that that Bruce Bent, David R. Wells, and Brian Szymczak, representing a majority of our directors, do not have any relationships that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director and that each of these directors is “independent” as that term is defined under Nasdaq rules and Rule 10A-3 under the Exchange Act. In making these determinations, our Board of Directors considered the relationships that each non-employee director has with our Company and all other facts and circumstances our Board of Directors deemed relevant in determining their independence, including the beneficial ownership of our capital stock by each non-employee director.
SECURITIES BEING OFFERED
General
The following description summarizes the terms of our securities and certain provisions of our Amended and Restated Certificate of Formation (the “Certificate of Formation”) and our Second Amended and Restated Bylaws (the “Bylaws”). As it is only a summary, it does not contain all the information that may be important to you. For a complete description, you should refer to our Certificate of Formation and Bylaws, as in effect as of the date of filing with the SEC of this offering statement, the forms of which are included as exhibits to the registration statement of which this offering statement forms a part.
Our purpose is to engage in any lawful act or activity for which corporations may now or hereafter be organized under the TBOC. Our authorized capital stock consists of 500,000,000 shares of common stock, $0.001 par value per share (the “Common Stock”), and 20,000,000 shares of preferred stock, $0.001 par value per share (the “Preferred Stock”). As of February 5, 2025, there were 1,036,321 shares of Common Stock outstanding and held of record by 290 shareholders and 380,440 shares of Series C Preferred Stock outstanding that, as of such date, were convertible into 84,733 shares of Common Stock and held of record by 64 shareholders. Of our authorized Preferred Stock, 600,000 shares have been designated as Series C Preferred Stock, having a par value of $0.001 per share, of which 380,440 were outstanding as of February 5, 2025. Unless our Board of Directors determines otherwise, we have and will continue to issue all shares of our capital stock in uncertificated form.
Common Stock
Holders of our Common Stock are entitled to one vote for each share held of record on all matters on which shareholders are entitled to vote generally, including the election or removal of directors, subject to certain limitations. The holders of our Common Stock do not have cumulative voting rights in the election of directors. Upon our liquidation, dissolution or winding up and after payment in full of all amounts required to be paid to creditors and to the holders of preferred stock having liquidation preferences, if any, the holders of our Common Stock will be entitled to receive pro rata our remaining assets available for distribution on a pro rata basis. Holders of our Common Stock do not have preemptive, subscription, redemption or conversion rights. The Common Stock will not be subject to further calls or assessment by us. There will be no redemption or sinking fund provisions applicable to the Common Stock. All outstanding shares of our Common Stock are fully paid and non-assessable. The rights, powers, preferences and privileges of holders of our Common Stock will be subject to those of the holders of any shares of our Preferred Stock, including any Preferred Stock we may authorize and issue in the future.
As a Texas corporation, we are subject to certain restrictions on dividends under the TBOC. Generally, a Texas corporation may pay dividends to its shareholders out of its surplus (the excess of its assets over its liabilities and stated capital) unless the dividend would render the corporation insolvent.
The declaration, amount and payment of any future dividends will be at the sole discretion of our Board of Directors. Our Board of Directors may take into account general and economic conditions, our financial condition and results of operations, our available cash and current and anticipated cash needs, capital requirements, contractual, legal, tax and regulatory restrictions and implications on the payment of dividends by us to our shareholders.
We currently expect to retain all future earnings for use in the operation and expansion of our business and have no current plans to pay dividends.
Preferred Stock
Our Certificate of Formation authorizes our Board of Directors to establish one or more series of Preferred Stock (including convertible Preferred Stock). Unless required by law or by the TBOC, the authorized shares of Preferred Stock will be available for issuance without further action by our shareholders.
Our Board of Directors will be able to determine, with respect to any series of Preferred Stock, the powers including preferences and relative participations, optional or other special rights, and the qualifications, limitations or restrictions thereof, of that series, including, without limitation:
| ● | the designation of the series; |
| ● | the number of shares of the series, which our Board of Directors may, except where otherwise provided in the preferred stock designation, increase (but not above the total number of authorized shares of the class) or decrease (but not below the number of shares then outstanding); |
| ● | whether dividends, if any, will be cumulative or non-cumulative and the dividend rate of the series; |
| ● | the dates at which dividends, if any, will be payable; |
| ● | the redemption rights and price or prices, if any, for shares of the series; |
| ● | the terms and amounts of any sinking fund provided for the purchase or redemption of shares of the series; |
| ● | the amounts payable on shares of the series in the event of any voluntary or involuntary liquidation, dissolution or winding-up of the affairs of the Company; |
| ● | whether the shares of the series will be convertible into shares of any other class or series, or any other security, of the Company or any other corporation and, if so, the specification of the other class or series or other security, the conversion price or prices or rate or rates, any rate adjustments, the date or dates as of which the shares will be convertible and all other terms and conditions upon which the conversion may be made; |
| ● | restrictions on the issuance of shares of the same series or of any other class or series; and |
| ● | the voting rights, if any, of the holders of the series. |
We will be able to issue a series of Preferred Stock that could, depending on the terms of the series, impede or discourage an acquisition attempt or other transaction that some, or a majority, of the holders of our Common Stock might believe to be in their best interests or in which the holders of our Common Stock might receive a premium for their Common Stock over the market price of the Common Stock. In addition, the issuance of Preferred Stock may adversely affect the rights of holders of our Common Stock by restricting dividends on the Common Stock, diluting the voting power of the Common Stock or subordinating the liquidation rights of the Common Stock. As a result of these or other factors, the issuance of Preferred Stock may have an adverse impact on the market price of our Common Stock.
Series C Preferred Stock
As of February 5, 2025, there were 380,440 shares of Series C Preferred Stock outstanding that, as of such date, were convertible into 84,733 shares of Common Stock. As of February 5, 2025, there were no shares of Series A Preferred Stock or Series B Preferred Stock outstanding.
The Series C Preferred Stock was issued from April 2019 to October 2020 to accredited investors and has a liquidation preference to the Common Stock. As of February 5, 2025, the liquidation preference was approximately $9.5 million. Any amendment to, or waiver of rights of the Series C Preferred Stock must include the consent of FRV, so long as FRV holds at least 71,000 shares of Series C Preferred Stock. Additionally, pursuant to a letter agreement entered into by and between the Company and FRV on April 10, 2019, for so long as FRV holds at least 71,000 shares of Series C Preferred Stock, it is entitled to appoint a member of the Board of Directors as well as a board observer (the “Appointment Rights”). As of February 5, 2025, FRV has not exercised its Appointment Rights.
Voting and Dividends
The holders of the shares of the Series C Preferred Stock have voting rights equal to an equivalent number of shares of the Common Stock into which such shares of Series C Preferred Stock are convertible and vote together as one class with the Common Stock.
The holders of the Series C Preferred Stock are entitled to receive dividends at an annual rate of $1.50 per share. Such dividends shall accrue and are payable out of funds legally available, are payable only when and if declared by the Board of Directors and are noncumulative. The Company is not permitted to declare, pay or set aside any dividends on shares of any other class or series of capital stock of the Company (other than dividends on shares of the Common Stock payable in shares of Common Stock) unless the holders of the shares of the Series C Preferred Stock then outstanding first receive, or simultaneously receive, a dividend on each outstanding share of the Series C Preferred Stock in an amount at least equal to the greater of (i) the amount of the aggregate dividends then accrued on such share of the Series C Preferred Stock and not previously paid and (ii) in the case of a dividend on the Common Stock or any class or series that is convertible into Common Stock, that dividend per share of Series C Preferred Stock as would equal the product of (1) the dividend payable on each share of such class or series determined, if applicable, as if all shares of such class or series had been converted into Common Stock and (2) the number of shares of Common Stock issuable upon conversion of a share of the Series C Preferred Stock.
No dividends have been declared to date on any shares of Preferred Stock.
Liquidation
In the event of any liquidation, dissolution or winding up of the Company, either voluntarily or involuntarily, the holders of the Series C Preferred Stock are entitled to receive, prior and in preference to the holders of the Common Stock, a per share amount equal to the original issue price ($25.00 per share) plus any accrued but unpaid dividends thereon.
If upon the liquidation, dissolution or winding up of the Company, the assets of the Company that are legally available for distribution to the holders of the Series C Preferred Stock are insufficient to permit the payment to such holders of the full amounts above, then the entire assets of the Company that are legally available for distribution shall be distributed with equal priority and pro rata among the holders of the Series C Preferred Stock in proportion to what they would otherwise be entitled to receive.
After the payment of the full Series C Preferred Stock liquidation preference and unpaid accrued dividends, the holders of the Series C Preferred Stock shall participate in the distribution of the entire remaining assets of the Company legally available for distributions pro rata to holders of the Common Stock on an as converted basis. The sale of a majority of the capital stock of the Company or the sale, lease, transfer, exclusive license or other disposition, in a single transaction or series of related transactions, by the Company or any subsidiary of the Company of all or substantially all the assets of the Company and its subsidiaries taken as a whole shall be a “Deemed Liquidation Event” for the purpose of the Series C Preferred Stock.
Conversion
Each share of Series C Preferred Stock is convertible, at the option of the holder, at any time after the date of issuance of such share, into such number of fully paid and non-assessable shares of Common Stock determined by dividing the original issue price of $25.00 by the conversion price for such series in effect at the time of conversion for the Series C Preferred Stock. The conversion price for the Series C Preferred Stock is subject to adjustment in accordance with conversion provisions contained in our Certificate of Designations, Number, Voting Power, Preferences and Rights of Series C Convertible Preferred Stock dated March 12, 2019. Following this offering, the conversion price of the Series C Preferred Stock will be $29.65 per share. See “— Antidilution Provisions” below.
Each share of Series C Preferred Stock automatically converts into shares of Common Stock at the conversion price at the time in effect immediately upon the Company’s sale of its Common Stock in a public offering provided that the offering price is not less than $1,650.00 per share (as adjusted for recapitalizations, stock combinations, stock dividends, stock splits and the like) and which results in aggregate cash proceeds of not less than $20.0 million before underwriting discounts, commissions, and fees. As of the date of this offering statement, no such sale has occurred.
Series D Preferred Stock.
In connection with this Offering, our Board of Directors has designated up to 4,285,714 shares of the Series D Preferred Stock. A form of the Certificate of Designations is filed as Exhibit 2.8 to this offering statement, of which this offering statement forms a part. We will file the certificate of designations immediately prior to the initial closing in this Offering. Our Series D Preferred Stock has the following voting powers, designations, preferences and relative rights, qualifications, limitations or restrictions.
Ranking. The Series D Preferred Stock ranks, as to dividend rights and rights upon the Company’s liquidation, dissolution, or winding up, senior to all classes or series of the Company’s common stock. The terms of the Series D Preferred Stock do not limit our ability to (i) incur indebtedness or (ii) issue additional equity securities that are equal or junior in rank to the shares of our Series D Preferred Stock as to distribution rights and rights upon our liquidation, dissolution or winding up.
Stated Value. Each share of Series D Preferred Stock has an initial stated value of $3.50, which is equal to the offering price per Unit, subject to appropriate adjustment in relation to certain events, such as recapitalizations, stock dividends, stock splits, stock combinations, reclassifications or similar events affecting our Series D Preferred Stock.
Liquidation Preference. In the event of any voluntary or involuntary liquidation, dissolution or winding up of our affairs, the holders of shares of Series D Preferred Stock are entitled to be paid out of our assets legally available for distribution to our stockholders a liquidation preference of $3.50 per share, before any distribution or payment may be made to holders of shares of common stock or any other class or series of our equity stock ranking, as to liquidation rights, junior to the Series D Preferred Stock.
If, upon our voluntary or involuntary liquidation, dissolution or winding up, our available assets are insufficient to pay the full amount of the liquidating distributions on all outstanding shares of Series D Preferred Stock and the corresponding amounts payable on all shares of each other class or series of capital stock ranking, as to liquidation rights, on a parity with the Series D Preferred Stock, then the holders of the Series D Preferred Stock and each such other class or series of capital stock ranking, as to liquidation rights, on a parity with the Series D Preferred Stock will share ratably in any distribution of assets in proportion to the full liquidating distributions to which they would otherwise be respectively entitled. Holders of Series D Preferred Stock will be entitled to written notice of any liquidation no fewer than 30 days and no more than 60 days prior to the payment date. After payment of the full amount of the liquidating distributions to which they are entitled, the holders of Series D Preferred Stock will have no right or claim to any of our remaining assets.
Our consolidation, merger or conversion with or into any other entity, or the voluntary sale, lease, transfer or conveyance of all or substantially all our property and assets (which shall not, in fact, result in our voluntary or involuntary liquidation, dissolution or winding up and the distribution of our assets to stockholders), shall not be deemed to constitute a voluntary or involuntary liquidation, dissolution or winding up of the Company.
Conversion at Option of Holder. Each share of Series D Preferred Stock shall be convertible into one (1) share of Common Stock, at the option of the holder thereof, at any time following the issuance date of such share of Series D Preferred Stock and on or prior to the fifth (5th) day prior to a redemption date, if any, as may have been fixed in any redemption notice with respect to the shares of Series D Preferred Stock, at our office or any transfer agent for such stock. The conversion rate shall not be adjusted for stock splits, stock dividends, recapitalizations or similar events.
Mandatory Conversion. At any time after issuance upon the occurrence of any of the following events, the Company shall have a right to direct the mandatory conversion of the Series D Preferred Stock: (a) a change in control, (b) if the price of the Common Stock closes at or above $5.00 per share for 10 consecutive trading days, or (c) if the Company consummates a firm commitment public offering of Common Stock for gross proceeds of at least $15 million at an offering price per share equal to or greater than $5.00.
Please see the certificate of designation, which has been filed as an exhibit to the offering statement of which this offering statement forms a part, for the procedures to request a redemption.
Further Issuances. The shares of our Series D Preferred Stock have no maturity date, and we will not be required to redeem shares of our Series D Preferred Stock at any time. Accordingly, the shares of our Series D Preferred Stock will remain outstanding indefinitely, unless otherwise converted into shares of our Common Stock. The shares of Series D Preferred Stock will not be subject to any sinking fund.
Voting Rights. We may not authorize or issue any class or series of equity securities ranking senior to the Series D Preferred Stock as to dividends or distributions upon liquidation (including securities convertible into or exchangeable for any such senior equity securities) or amend our certificate of formation (whether by merger, consolidation, or otherwise) to materially and adversely change the terms of the Series D Preferred Stock without the affirmative vote of at least a majority of the votes entitled to be cast on such matter by holders of our outstanding shares of Series D Preferred Stock, voting together as a class. Otherwise, holders of the shares of our Series D Preferred Stock will not have any voting rights.
Qualification of Shares of Common Stock issuable upon conversion of the Series D Preferred Stock. In this Offering we are qualifying with the SEC up to 4,285,714 shares of Common Stock underlying the Series D Preferred Stock.
Investor Warrants to be Sold to the Public in Connection with this Offering
General. Each Unit includes one (1) investor Warrant, which is exercisable to purchase one share of Common Stock at an exercise price of $5.00 per share. The applicable exercise price will be adjusted if specific events, summarized below, occur. A holder of Warrants will not be deemed a holder of the underlying stock for any purpose until the Warrant is exercised.
Exercisability. The Warrants are exercisable at any time after their original issuance and at any time up to the date that is 36 months after their original issuance. The Warrants will be exercisable, at the option of each holder, in whole or in part by delivering to us a duly executed exercise notice and, at any time an offering statement covering the issuance of the shares of Common Stock underlying the Warrants under the Securities Act is qualified for the issuance of such shares, or an exemption from registration under the Securities Act is available for the issuance of such shares, by payment in full in immediately available funds for the number of shares of Common Stock purchased upon such exercise. No fractional shares of Common Stock will be issued in connection with the exercise of a Warrant. In lieu of fractional shares, we will pay the holder an amount in cash equal to the fractional amount multiplied by the exercise price.
Cashless Exercise. If at the time of exercise of a Warrant there is no qualified offering statement (or effective registration statement), the offering statement (or prospectus, as applicable) contained therein is not available for the issuance of the underlying Warrant shares or the Company is not current in its public company reporting obligations, the Warrant may also be exercised, in whole or in part and in the holder’s sole discretion, at such time by means of a “cashless exercise” in which case the holder would receive upon such exercise the net number of shares of Common Stock determined according to the formula set forth in the Warrant.
Exercise Price. The price per share of Common Stock purchasable upon exercise of the Warrants is $5.00 per share. The exercise price is not subject to appropriate adjustment in the event of certain stock dividends and distributions, stock splits, stock combinations, reclassifications or similar events affecting our Common Stock and also upon any distributions of assets, including cash, stock or other property to our stockholders.
Transferability. Subject to applicable laws and procedural requirements, the Warrants may be offered for sale, sold, transferred or assigned without our consent.
Adjustments in Certain Events. In the event of a capital reorganization or reclassification of our Common Stock, the Warrants will be adjusted so that thereafter each Warrant holder will be entitled to receive upon exercise the same number and kind of securities that such holder would have received if the Warrant had been exercised before the capital reorganization or reclassification of our Common Stock.
If we merge or consolidate with another corporation, or if we sell our assets as an entirety or substantially as an entirety to another corporation, we will make provisions so that Warrant holders will be entitled to receive upon exercise of a Warrant the kind and number of securities, cash or other property that would have been received as a result of the transaction by a person who was our stockholder immediately before the transaction and who owned the same number of shares of Common Stock for which the Warrant was exercisable immediately before the transaction. No adjustment to the Warrants will be made, however, if a merger or consolidation does not result in any reclassification or change in our outstanding Common Stock.
Rights as a Stockholder. Except as otherwise provided in the Warrants or by virtue of such holder’s ownership of shares of our Common Stock, the holder of a Warrant does not have the rights or privileges of a holder of our Common Stock until the holder exercises the Warrant.
Beneficial Ownership Limitation. Notwithstanding anything herein to the contrary, the Company shall not affect any exercise of any Warrant, and a holder shall not have the right exercise any investor Warrants, to the extent that, after giving effect to an attempted exercise set forth on an applicable exercise notice, such attempted exercise would result in the holder (together with such holder’s affiliates, and any other person whose beneficial ownership of Common Stock would be aggregated with the holder’s for purposes of Section 13(d) or Section 16 of the Exchange Act and the applicable regulations of the SEC), including any Attribution Parties beneficially owning a number of shares of Common Stock in excess of the Beneficial Ownership Limitation (as defined in the Warrant).
Qualification of Shares of Common Stock issuable upon exercise of the Warrants. In this Offering we are qualifying with the SEC up to 4,285,714 shares of Common Stock underlying the Warrants.
Lead Selling Agents’ Warrants
Upon the closing of this Offering, there will be up to 128,571 shares of Series D Preferred Stock issuable upon exercise of the Agent Warrants, which shares of Series D Preferred Stock may convert into up to 128,571 shares of Common Stock, and 128,571 shares of Common Stock issuable upon exercise of the Agent Unit Warrants contained within the Agent Warrants. We are qualifying with the SEC these shares in this Offering. See “Plan of Distribution — Lead Selling Agents’ Warrants” for a description of the Agent Warrants.
Other Warrants Previously Issued by Our Company
Other Investor Warrants
We issued warrants (the “Investor Warrants”) in connection with various funding transactions or as consideration, in lieu of cash, for amounts billed in respect of services rendered to us. The Investor Warrants have terms ranging from five to ten years from the date of issuance. As of February 5, 2025, there were Investor Warrants to purchase 7,794 shares of Common Stock at exercise prices ranging from $5.15 to $825.00 per share.
Warrants issued in connection with the MSW Note
In September 2023, we issued warrants in lieu of a facility fee payment in connection with entering into the MSW Note with Matthews Southwest Holdings. These warrants have a five-year term from the date of issuance. As of February 5, 2025 there were warrants to purchase 10,000 shares of our Common Stock at exercise prices ranging from $100.00 to $150.00 per share. On November 16, 2023, we entered into a Warrant Amendment Agreement with Matthews Southwest Holdings, amending the Existing MSW Warrants to reduce the exercise price of an aggregate of 10,000 Existing MSW Warrants to $16.00 per share.
Warrants issued in connection with the 2021 Bridge Financing
We issued the Bridge Warrants to originally purchase 775 shares of Common Stock in connection with the 2021 Bridge Financing. The Bridge Warrants expire five years after the date of issuance, beginning on December 22, 2026, with an initial exercise price of $908.00 per share, subject to certain adjustments. No holder of a Bridge Warrant may exercise any portion of a Bridge Warrant if after giving effect to such exercise such holder (together with its Attribution Parties) would beneficially own in excess of 4.99% of the number of shares of the Common Stock outstanding immediately after giving effect to the issuance of shares of Common Stock issuable upon exercise of such holder’s Bridge Warrant. This limitation may be waived by a holder, at its election, upon not less than 61 days’ prior notice to the Company, to change the limitation to 9.99% of the number of shares of the Common Stock outstanding immediately after giving effect to the issuance of shares of Common Stock upon exercise of such holder’s warrant. Any exercise of the Bridge Warrants resulting in a number of shares in excess of 9.99% of the number of shares of the Common Stock outstanding immediately after giving effect to the exercise shall be deemed null and void and shall be cancelled ab initio.
On September 8, 2022, we entered into an amendment to the Bridge Warrants, which we refer to as the Bridge Warrant Amendment No. 1. The Bridge Warrant Amendment No. 1 amended the Bridge Warrants by (i) increasing the number of shares of Common Stock for which the Bridge Warrants are exercisable from a total of 13,677 shares to a total of 16,849 shares, (ii) lowering the exercise price to $425.00 per share, (iii) providing that, until June 15, 2023, the exercise price will be further adjusted whenever the Company issues shares of Common Stock for consideration per share that when multiplied by 1.25 is less than the exercise price then in effect, subject to certain exceptions, (iv) confirming that, for purposes of the Bridge Warrants, the value of each share of Common Stock and each IPO Warrant was deemed to be $412.50 and $12.50, respectively, (v) providing that the number of shares of Common Stock underlying the Bridge Warrants will only be adjusted if the Company (a) pays a stock dividend on one or more classes of its then outstanding shares of Common Stock or otherwise makes a distribution on any class of capital stock that is payable in shares of Common Stock, (b) subdivides (by any stock split, stock dividend, recapitalization or otherwise) one or more classes of its then outstanding shares of Common Stock into a larger number of shares or (c) combines (by combination, reverse stock split or otherwise) one or more classes of its then outstanding shares of Common Stock into a smaller number of shares, and (vi) amending the formula for calculating Black Scholes values.
On February 3, 2023, we entered into a second amendment to the Bridge Warrants, which we refer to as the Bridge Warrant Amendment No. 2. The Bridge Warrant Amendment No. 2 amended the Bridge Warrants by (i) lowering the exercise price of $425.00 for the Limited Period, during which period the exercise price was set at $100.00, subject to adjustments set forth in the Bridge Warrant; (ii) providing that during the Limited Period, the holder was able, in its sole discretion, to elect a cashless exercise of the Bridge Warrant in whole or in part, pursuant to which the holder received a net number of shares of Common Stock equal to one-third of the total number of shares into which the Bridge Warrant could otherwise have been exercised; and (iii) removing the exercise price adjustment provisions of the Bridge Warrants with limited exceptions for transactions such as stock dividends, stock splits, stock combinations and reverse stock splits. Additionally, the Bridge Warrant Amendment No. 2 provided that in the event that the aggregate number of shares of Common Stock to be received by a holder upon an exercise of its Bridge Warrant during the Limited Period would result in such holder’s receiving shares of Common Stock in excess of its applicable Bridge Maximum Percentage, in lieu of delivery of shares of Common Stock in excess of the Bridge Maximum Percentage, the holder would receive such excess shares as pre-funded warrants substantially in the form of the Pre-Funded Bridge Warrants, with certain exercise price adjustment provisions removed. Further, the Bridge Warrant Amendment No. 2 included a waiver of Section 4(w) of the Bridge SPA, which placed certain restrictions on the Company’s ability to issue securities for a specified period of time.
During the Limited Period, Bridge Warrants were exercised for (i) a total of 11,736 shares of Common Stock at an exercise price of $100.00 per share or pursuant to cashless exercises in which the holder received a net number of shares of Common Stock equal to one-third of the total number of shares with respect to which the Bridge Warrant was exercised and (ii) the Remaining Pre-Funded Bridge Warrant to purchase 1,500 shares of Common Stock. At the end the Limited Period, Remaining Bridge Warrants to purchase a total of 2,989 shares of Common Stock remained outstanding, with the exercise price adjusted back to $425.00 per share, subject to future adjustments as set forth in the Remaining Bridge Warrants.
The exercise price of the Remaining Bridge Warrants (as amended by the Bridge Warrant Amendment No. 1 and the Bridge Warrant Amendment No. 2) is subject to adjustment for certain events such as stock dividends, splits, and reverse splits or other combinations, but not otherwise as the result of issuances of additional securities by the Company, even if such issuances are at prices below the exercise price of the Bridge Warrants. Upon an adjustment of the exercise price as a result of a stock dividend, split, reverse split, combination or similar event, the number of shares of Common Stock to be received shall be proportionately adjusted. Otherwise, there are no antidilution provisions that result in adjustments to the number of shares of Common Stock to be received upon exercise of the Bridge Warrants.
All Pre-Funded Bridge Warrants that were issued upon conversion of the Bridge Notes have been exercised in full and are no longer outstanding as of the date of this offering statement, although the Remaining Pre-Funded Bridge Warrant issued in connection with Bridge Warrant Amendment No. 2 remains outstanding. For more information regarding the Bridge Warrants, please see “ Management’s Discussion and Analysis of Financial Condition and Results of Operations — Description of Indebtedness —2021 Bridge Financing”.
$1.5M Lender Warrants
In November 2021, we issued the $1.5M Lender Warrants exercisable for 71 shares of our Common Stock to noteholders of the $1.5M Notes as consideration for the extension of the maturity of the $1.5M Notes to February 5, 2023. The $1.5M Lender Warrants expire on October 12, 2026. The exercise price of the $1.5M Lender Warrants was $289.00 per share as of February 5, 2025.
$1M Lender Warrants
In November 2021, we issued warrants to purchase 152 shares of our Common Stock, which we refer to as the $1M Lender Warrants, to the lenders of the $1M Notes as consideration for the extension of the maturity of the $1M Loan and Security Agreement to September 30, 2022. The $1M Loan and Security Agreement was further amended in May 2022 to extend the maturity date to September 30, 2023 and amended again in January 2023 to (i) further extend the maturity date of the portion of the $1M Notes issued to one lender (in the principal amount of $0.5 million) to March 31, 2024 and (ii) further extend the maturity date of the remaining portion of the $1M Notes issued to the other lender (in the principal amount of $0.5 million) to September 30, 2024. The $1M Loan and Security Agreement was further amended in September 2023 to extend the interest maturity date to one lender to December 31, 2023. In October 2023, we issued additional $1M Lender Warrants to purchase 2,000 shares of our Common Stock to lenders of the $1M Notes as consideration for the extension of the interest maturity date to one lender. On November 16, 2023, we entered into the Adams Warrant Amendment, amending the $1M Lender Warrants owned by Adams to reduce the exercise price of an aggregate of 1,076 $1M Lender Warrants to $16.00 per share. The exercise prices of the $1M Lender Warrants range from $16.00 to $289.00 per share as of February 5, 2025.
Mount Sinai Warrants
On September 20, 2023, we entered into the License Agreements with Mount Sinai to commercialize a range of AI cardiovascular algorithms developed by Mount Sinai. On November 15, 2023, we closed the transactions contemplated under the Mount Sinai Securities Purchase Agreement and the licenses under the License Agreements, which became effective on that date. As consideration, we issued pre-funded warrants to purchase up to 7,107 shares of our Common Stock with an exercise price per share of $0.001 and warrants to purchase up to 9,142 shares of our Common Stock with an exercise price per share of $50.60.
IPO Warrants
The following summary of certain terms and provisions of the IPO Warrants that were included in the IPO Units issued in the IPO, plus the additional IPO Warrants issued as a result of the exercise, in part, of the underwriter’s over-allotment option in the IPO, is not complete and is subject to, and qualified in its entirety by, the provisions of the warrant agent agreement between us and Equiniti Trust Company, LLC, as warrant agent, and the form of warrant, both of which are included as exhibits to the offering statement of which this offering statement is a part.
Exercisability. The IPO Warrants are exercisable at any time until 5:00 P.M. New York City time on June 17, 2027. The IPO Warrants are exercisable, at the option of each holder, in whole or in part by delivering to us a duly executed exercise notice and, at any time a registration statement registering the issuance of the shares of Common Stock underlying the IPO Warrants under the Securities Act, is effective and available for the issuance of such shares of Common Stock, or an exemption from registration under the Securities Act is available for the issuance of such shares of Common Stock, by payment in full in immediately available funds for the number of shares of Common Stock purchased upon such exercise. If a registration statement registering the issuance of the Common Stock underlying the IPO Warrants under the Securities Act is not effective or available and an exemption from registration under the Securities Act is not available for the issuance of such Common Stock, the holder may, in its sole discretion, elect to exercise the IPO Warrant through a cashless exercise, in which case the holder would receive upon such exercise the net number of shares of Common Stock determined according to the formula set forth in the IPO Warrant. No fractional shares of Common Stock will be issued in connection with the exercise of an IPO Warrant. In lieu of fractional shares, we will pay the holder an amount in cash equal to the fractional amount multiplied by the exercise price. We will not affect the exercise of any portion of the IPO Warrants, and the holder will not have the right to exercise any portion of the IPO Warrants, and any such exercise shall be null and void and treated as if never made, to the extent that after giving effect to such exercise, the holder together with its affiliates and certain other persons specified in the IPO Warrants collectively would own beneficially in excess of 4.99% (or, upon election by a holder prior to the issuance of any IPO Warrants, 9.99%) of the shares of Common Stock outstanding immediately after giving effect to such exercise.
Exercise Price. The exercise price per share purchasable upon exercise of the IPO Warrants is $425.00 per share. The exercise price is subject to appropriate adjustment in the event of certain stock dividends and distributions, stock splits, stock combinations, reclassifications or similar events affecting our shares of Common Stock and also upon any distributions of assets, including cash, stock or other property to our shareholders.
Transferability. Subject to applicable laws, the IPO Warrants may be offered for sale, sold, transferred or assigned without our consent.
Warrant Agent. The IPO Warrants were issued in registered form under a warrant agent agreement between Equiniti Trust Company, LLC, as warrant agent, and us. The IPO Warrants shall be represented only by one or more global warrants deposited with the warrant agent, as custodian on behalf of The Depository Trust Company (“DTC”) and registered in the name of Cede & Co., a nominee of DTC, or as otherwise directed by DTC.
Fundamental Transactions. In the event of a fundamental transaction, as described in the IPO Warrants and generally including any reorganization, recapitalization or reclassification of our ordinary shares, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger with or into another person, the acquisition of more than 50% of our outstanding shares of Common Stock, or any person or group becoming the beneficial owner of 50% of the voting power represented by our shares of Common Stock, the holders of the IPO Warrants will be entitled to receive upon exercise of the IPO Warrants the kind and amount of securities, cash or other property that the holders would have received had they exercised the IPO Warrants immediately prior to such fundamental transaction.
Rights as a Shareholder. Except as otherwise provided in the IPO Warrants or by virtue of such holder’s ownership of our shares of Common Stock, the holder of an IPO Warrant does not have the rights or privileges of a holder of our Common Stock, including any voting rights, until the holder exercises the IPO Warrant.
Governing Law. The IPO Warrants and the warrant agent agreement are governed by New York law.
IPO Underwriter Warrants
At the consummation of the IPO, we issued warrants to the underwriter, or the IPO Underwriter Warrants, to purchase 1,050 shares of Common Stock, representing 7.0% of the aggregate number of shares of Common Stock underlying the IPO Units sold in the IPO. The IPO Underwriter Warrants expire at 5:00 P.M. New York City time on June 17, 2027, have an exercise price equal to $425.00, which is equal to 100% of the public offering price per IPO Unit in the IPO, provide for a “cashless” exercise, and contain certain antidilution adjustments (but excluding any price based antidilution). The IPO Underwriter Warrants contain provisions for unlimited “piggyback” registration rights for a period of no greater than three (3) years from the date of the IPO in compliance with FINRA Rule 5110(g)(8)(D). Pursuant to FINRA Rule 5110I, the IPO Underwriter Warrants and any shares of Common Stock issued upon exercise of the IPO Underwriter Warrants may not be sold, transferred, assigned, pledged, or hypothecated, or be the subject of any hedging, short sale, derivative, put or call transaction that would result in the effective economic disposition of the securities by any person for a period of 180 days beginning on the date of commencement of sales of the IPO, except certain transfers of such securities, including: (i) by operation of law or by reason of our reorganization; (ii) to any FINRA member firm participating in the IPO and the officers or partners thereof, if all securities so transferred remain subject to lock-up restriction set forth in Section 4(a) of the IPO Underwriter Warrants for the remainder of the time period; (iii) if the aggregate amount of our securities held by the IPO underwriter or related persons do not exceed 1% of the securities offered in the IPO; (iv) that is beneficially owned on a pro-rata basis by all equity owners of an investment fund, provided that no participating member manages or otherwise directs investments by the fund and the participating members in the aggregate do not own more than 10% of the equity in the fund; or (v) the exercise or conversion of any security, if all securities remain subject to the lock-up restriction set forth in Section 4(a) of the IPO Underwriter Warrants for the remainder of the time period.
Lead Selling Agents’ Warrants
Upon the closing of this Offering, there will be up to 128,571 shares of Series D Preferred Stock issuable upon exercise of the Agent Warrants, which shares of Preferred Stock may convert into up to 128,571 shares of Common Stock, and 128,571 shares of Common Stock issuable upon exercise of the Agent Unit Warrants contained within the Agent Warrants. We are qualifying with the SEC these shares in this Offering. See “Plan of Distribution—Lead Selling Agents’ Warrants” for a description of the Agent Warrants.
Options
We previously granted certain of our employees and board members stock option awards where vesting is contingent upon a service period, as we believe that such awards better align the interests of our employees with those of our shareholders. Such stock option awards were granted with an exercise price equal to or above the market price of our Common Stock at the date of grant. Certain stock option awards provide for accelerated vesting if there is a change in control, as defined in the option agreement. Stock options may not, subject to certain limited exceptions, be exercised when an employee leaves our Company. Where option awards were granted based on service periods, they generally vest quarterly based on three years of continuous service for executive directors and employees, or 12 months continuous service for directors and have 10-year contractual terms. As of February 5, 2025, there were time-based options to purchase a total of 172,159 shares of Common Stock at an average exercise price of $17.00 per share.
We also previously granted stock option awards where vesting is contingent upon meeting various departmental and/or company-wide performance goals, including, in some instances, FDA and/or CE Mark regulatory approval and/or certain EBITDA and funding thresholds. Such performance-based stock options are expected to vest when the performance criteria and metrics have been met. These stock options have a term of ten years. As of February 5, 2025, there were performance-based options to purchase a total of 5,456 shares of Common Stock at an average exercise price of $502.00 per share.
Antidilution Provisions
As of February 5, 2025, 84,733 shares of Common Stock issuable upon conversion of the Series C Preferred Stock were subject to antidilution protection provisions. The holders of these securities may be entitled to receive additional shares of Common Stock upon conversion of the Series C Preferred Stock.
Selling Agents Lock-Up
The Agents Warrants and the Agent Unit Warrants, Series D Preferred Stock and the Common Stock underlying the Agents Unit Warrants or into which the Series D Preferred Stock may convert have been deemed compensation by FINRA and are therefore subject to a 180-day lock-up from the date of the commencement of sales in the Offering pursuant to Rule 5110(e)(1) of FINRA. The lead selling agent, or permitted assignees under such rule, may not sell, transfer, assign, pledge, or hypothecate the Agents Warrants or the Agent Unit Warrants, Series D Preferred Stock and the Common Stock underlying the Agent Unit Warrants or into which the Series D Preferred Stock may convert, nor will the lead selling agent or permitted assignees engage in any hedging, short sale, derivative, put, or call transaction that would result in the effective economic disposition of the Agent Warrants or the underlying Agent Unit Warrants, Series A Preferred Stock and the Common Stock for a period of 180 days from the date of commencement of sales in the Offering, except that they may be transferred, in whole or in part, by operation of law or by reason of our reorganization, or to any selling agent or selected dealer participating in the Offering and their officers, partners or registered representatives if the Agent Warrants or the underlying securities so transferred remain subject to the foregoing lock-up restrictions for the remainder of the time period.
Registration Rights
We previously granted certain registration rights to the holders of the Series C Preferred Stock. Under the terms of the registration rights agreement, which we refer to as the Series C Registration Rights Agreement, the holders of the Series C Preferred Stock owning not less than 30% of (i) the Common Stock issuable or issued upon conversion of the Series C Preferred Stock; and (ii) any Common Stock issued as (or issuable upon the conversion or exercise of any warrant, right, or other security that is issued as) a dividend or other distribution with respect to, or in exchange for or in replacement of, the shares referenced in clause (i) above, referred to herein as the Series C Registrable Securities, and the anticipated aggregate offering price, net of certain expenses, would exceed $10 million, may demand that we file a registration statement relating to the Series C Registrable Securities owned by the holders who have demanded such registration. In addition, if at any time when we are eligible to use a registration statement on Form S-3, we receive a request from holders of at least twenty-five percent (25%) of the Series C Registrable Securities then outstanding that we file a registration statement on Form S-3 with respect to outstanding Series C Registrable Securities of such holders having an anticipated aggregate offering price, net of certain expenses, of at least $3 million, then we will be required to file a registration statement relating to the resale of the Series C Registrable Securities owned by such holders. Finally, if we propose to register (including, for this purpose, a registration effected by us for shareholders other than the holders of the Series C Preferred Stock) any of the Common Stock under the Securities Act in connection with the public offering of such securities solely for cash, we are required to give each holder of Series C Registrable Securities notice of such registration and such holders may include their Series C Registrable Securities in such registration statement. In March 2022, we entered into written waiver agreements with the requisite holders of our Series C Preferred Stock whereby such holders agreed, on behalf of all holders of Series C Preferred Stock, to waive their right to include their Series C Registrable Securities.
Anti-takeover Effects of Certain Provisions of Our Certificate of Formation, Bylaws and Texas Law
Our Certificate of Formation and Bylaws and the TBOC contain provisions, which are summarized in the following paragraphs, which are intended to enhance the likelihood of continuity and stability in the composition of our Board of Directors. These provisions are intended to avoid costly takeover battles, reduce our vulnerability to a hostile change of control and enhance the ability of our Board of Directors to maximize shareholder value in connection with any unsolicited offer to acquire us. However, these provisions may have an anti-takeover effect and may delay, deter or prevent a merger or acquisition of the Company by means of a tender offer, a proxy contest or other takeover attempt that a shareholder might consider in its best interest, including those attempts that might result in a premium over the prevailing market price for the shares of Common Stock held by shareholders.
Authorized but unissued capital stock
Texas law does not require shareholder approval for any issuance of authorized shares. However, the listing requirements of the Nasdaq, which apply so long as our securities are listed on the Nasdaq, require shareholder approval of certain issuances equal to or exceeding 20% of the then outstanding voting power or then outstanding number of shares of Common Stock. Additional shares that may be issued in the future may be used for a variety of corporate purposes, including future public offerings, to raise additional capital or to facilitate acquisitions.
Our Board of Directors may generally issue shares of Preferred Stock on terms calculated to discourage, delay or prevent a change of control of the Company or the removal of our management. Moreover, our authorized but unissued shares of Preferred Stock are available for future issuances without shareholder approval and could be utilized for a variety of corporate purposes, including future offerings to raise additional capital, to facilitate acquisitions and employee benefit plans.
One of the effects of the existence of unissued and unreserved shares of Common Stock or Preferred Stock may be to enable our Board of Directors to issue shares to persons friendly to current management, which issuance could render more difficult or discourage an attempt to obtain control of the Company by means of a merger, tender offer, proxy contest or otherwise, and thereby protect the continuity of our management and possibly deprive our shareholders of opportunities to sell their shares of Common Stock at prices higher than prevailing market prices.
Classified Board of Directors
Our Certificate of Formation provides that our Board of Directors be divided into three classes of directors, with the classes to be as nearly equal in number as possible, and with the directors serving three-year terms. As a result, approximately one-third of our Board of Directors will be elected each year. The classification of directors will have the effect of making it more difficult for shareholders to change the composition of our Board of Directors. Our Certificate of Formation and Bylaws provide that, subject to any rights of holders of Preferred Stock to elect additional directors under specified circumstances, the number of directors will be fixed from time to time exclusively pursuant to a resolution adopted by the Board of Directors.
Removal of directors; vacancies
Under the TBOC, unless otherwise provided in our Certificate of Formation, directors serving on a classified board may be removed by the shareholders only for cause. Our Certificate of Formation provides that directors may be removed only for cause. In addition, our Certificate of Formation also provides that, subject to the rights granted to one or more series of Preferred Stock then outstanding, any vacancy occurring in our Board of Directors may be filled by election at an annual or special meeting of the shareholders called for that purpose or by the affirmative vote of a majority of the directors then in office (even if the remaining directors constitute less than a quorum of the Board of Directors), and any director so chosen shall hold office for the remainder of the term to which the director has been selected and until such director’s successor shall have been elected and qualified.
No cumulative voting
Under Texas law, the right to vote cumulatively does not exist unless the certificate of formation specifically authorizes cumulative voting. Our Certificate of Formation does not authorize cumulative voting. Therefore, shareholders holding a majority in voting power of the shares of our stock entitled to vote generally in the election of directors will be able to elect all our directors.
Special shareholder meetings
Our Certificate of Formation provides that special meetings of our shareholders may be called at any time by the Board of Directors, the chairman of the Board of Directors or the chief executive officer of the Company. Our Bylaws prohibit the conduct of any business at a special meeting other than as specified in the notice for such meeting. These provisions may have the effect of deferring, delaying or discouraging hostile takeovers, or changes in control or management of the Company.
Requirements for advance notification of director nominations and shareholder proposals
Our Bylaws establish advance notice procedures with respect to shareholder proposals and the nomination of for election as directors, other than nominations made by or at the direction of the Board of Directors or a committee of the Board of Directors. In order for any matter to be “properly brought” before a meeting, a shareholder will have to comply with advance notice requirements and provide us with certain information. Generally, to be timely, a shareholder’s notice must be received at our principal executive offices not less than 75 days nor more than 100 days prior to the first anniversary date of the immediately preceding annual meeting of shareholders. Our Bylaws also specify requirements as to the form and content of a shareholder’s notice. Our Bylaws allow the chairman of the meeting at a meeting of the shareholders to adopt rules and regulations for the conduct of meetings which may have the effect of precluding the conduct of certain business at a meeting if the rules and regulations are not followed. These provisions may also defer, delay or discourage a potential acquiror from conducting a solicitation of proxies to elect the acquiror’s own slate of directors or otherwise attempting to influence or obtain control of the Company.
Shareholder action by written consent
Our Certificate of Formation provides that any action required or permitted to be taken at an annual or special meeting of shareholders may be taken by written consent in lieu of a meeting of shareholders only with the unanimous written consent of our shareholders.
Amendment and restatement of bylaws
Our Bylaws provide that the Board of Directors is expressly authorized to make, alter, amend, change, add to, rescind or repeal, in whole or in part, our Bylaws without a shareholder vote in any matter not inconsistent with the laws of the State of Texas and our Certificate of Formation.
The combination of the classification of our Board of Directors and the lack of cumulative voting will make it more difficult for shareholders to replace our Board of Directors as well as for another party to obtain control of us by replacing our Board of Directors. Because our Board of Directors has the power to retain and discharge our officers, these provisions could also make it more difficult for existing shareholders or another party to effect a change in management.
These provisions may have the effect of deterring hostile takeovers or delaying or preventing changes in control of our management or the Company, such as a merger, reorganization or tender offer. These provisions are intended to enhance the likelihood of continued stability in the composition of our Board of Directors and its policies and to discourage certain types of transactions that may involve an actual or threatened acquisition of the Company. These provisions are designed to reduce our vulnerability to an unsolicited acquisition proposal. The provisions are also intended to discourage certain tactics that may be used in proxy fights. However, such provisions could have the effect of discouraging others from making tender offers for our shares and, as a consequence, they also may inhibit fluctuations in the market price of our shares that could result from actual or rumored takeover attempts. Such provisions may also have the effect of preventing changes in management.
Dissenters’ rights of appraisal and payment
Under the TBOC, with certain exceptions, our shareholders will have appraisal rights in connection with a merger, a sale of all or substantially all of our assets, an interest exchange or a conversion. Pursuant to the TBOC, shareholders who properly request and perfect appraisal rights in connection with such merger, sale of all or substantially all of our assets, interest exchange or conversion will have the right to receive payment of the fair value of their shares as agreed to between the shareholder and the Company or, if they are unable to reach agreement, as determined by the State District Court in Tarrant County, Texas.
Shareholders’ derivative actions
Under the TBOC, any of our shareholders may bring an action in our name to procure a judgment in our favor, also known as a derivative action, provided that the shareholder bringing the action (i) is a holder of our shares at the time of the transaction to which the action relates or such shareholder became a shareholder by operation of law from a person that was a shareholder at the time of the transaction to which the action relates and (ii) fairly and adequately represents the interests of the Company in enforcing the right of the Company.
Limitations on liability and indemnification of officers and directors
The TBOC authorizes corporations to limit or eliminate the personal liability of directors to corporations and their shareholders for monetary damages for breaches of directors’ fiduciary duties (other than breaches of the directors’ duty of loyalty to corporations or their shareholders), subject to certain exceptions. Our Certificate of Formation includes a provision that limits the personal liability of directors for monetary damages for an act or omission in the director’s capacity as a director to the fullest extent permitted by Texas law. However, exculpation will not apply to any director if the director has acted in bad faith, engaged in intentional misconduct, knowingly violated the law, authorized illegal dividends or redemptions, derived an improper benefit from his or her actions as a director or engaged in an act or omission for which the liability of the director is expressly provided by an applicable statute.
Our Certificate of Formation provides that we must indemnify our directors and officers to the fullest extent authorized by the TBOC. We also are expressly authorized to carry directors’ and officers’ liability insurance providing indemnification for our directors, officers and certain employees for some liabilities. We believe that these indemnification provisions and insurance will be useful to attract and retain qualified directors and officers.
The limitation of liability and indemnification provisions in our Certificate of Formation and Bylaws may discourage shareholders from bringing a lawsuit against directors for breach of their fiduciary duty. These provisions also may have the effect of reducing the likelihood of derivative litigation against directors and officers, even though such an action, if successful, might otherwise benefit us and our shareholders. In addition, your investment may be adversely affected to the extent we pay the costs of settlement and damage awards against directors and officers pursuant to these indemnification provisions. As of February 5, 2025, there is no pending material litigation or proceeding involving any of our directors, officers or employees for which indemnification is sought.
Business combinations
Under Title 2, Chapter 21, Subchapter M of the TBOC, we may not engage in certain “business combinations” with any “affiliated shareholder,” or any affiliate or associate of the affiliated shareholder for a three-year period following the time that the shareholder became an affiliated shareholder, unless:
| · | prior to such time, our Board of Directors approved either the business combination or the transaction which resulted in the shareholder becoming an affiliated shareholder; or |
| · | not less than six months after the affiliated shareholders’ share acquisition date, the business combination is approved by the affirmative vote at a meeting, and not by written consent, of holders of at least 2/3 of our outstanding voting shares that are not owned by the affiliated shareholder or an affiliate or associate of the affiliated shareholder. |
Generally, a “business combination” includes a merger, asset or stock sale or other similar transaction. Subject to certain exceptions, an “affiliated shareholder” is a person who beneficially owns (as determined pursuant to Title 2, Chapter 21, Subchapter M of the TBOC), or within the previous three years beneficially owned, 20% or more of our outstanding voting shares. For purposes of this section only, “voting share” has the meaning given to it in Title 2, Chapter 21, Subchapter M of the TBOC.
Under certain circumstances, this provision will make it more difficult for a person who would be an “affiliated shareholder” to effect various business combinations with the Company for a three-year period. This provision may encourage companies interested in acquiring the Company to negotiate in advance with our Board of Directors because the shareholder approval requirement would be avoided if our Board of Directors approves either the business combination or the transaction that results in such shareholder becoming an affiliated shareholder. These provisions also may have the effect of preventing changes in our Board of Directors and may make it more difficult to accomplish transactions which shareholders may otherwise deem to be in their best interests.
LEGAL MATTERS
Certain legal matters in connection with this Offering will be passed upon for us by Foley Shechter Ablovatskiy LLP.
EXPERTS
The financial statements of HeartSciences Inc. as of April 30, 2024 and 2023, and for the years then ended and related notes, are incorporated by reference into this offering statement in reliance upon the report of Haskell & White LLP, independent registered public accounting firm, and upon the authority of said firm as experts in accounting and auditing. The report on the financial statements contains an explanatory paragraph regarding the Company’s ability to continue as a going concern.
WHERE YOU CAN FIND MORE INFORMATION
For further information about us and the securities offered hereby, reference is made to the offering statement, the exhibits filed therewith and the documents incorporated by reference therein. Statements contained in this offering statement regarding the contents of any contract or any other document that is filed as an exhibit to the offering statement are not necessarily complete, and in each instance we refer you to the copy of such contract or other document filed as an exhibit to the offering statement. The SEC maintains a website that contains reports, proxy and information statements and other information regarding registrants that file electronically with the SEC. The address of the website is www.sec.gov.
We are subject to the information and reporting requirements of the Exchange Act, and, in accordance with this law, file periodic reports and other information with the SEC. These periodic reports and other information are available for inspection on the website of the SEC referred to above. We also maintain a website at www.heartsciences.com. You may access these materials free of charge as soon as reasonably practicable after they are electronically filed with, or furnished to, the SEC. Information contained on our website is not a part of this offering statement and the inclusion of our website address in this offering statement is an inactive textual reference only. You may also inspect these documents at our corporate headquarters at 550 Reserve Street, Suite 360, Southlake, Texas, during normal business hours.
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The SEC allows us to “incorporate by reference” the information we file with them. This means that we can disclose important information to you by referring you to those documents instead of having to repeat the information in this document. The information incorporated by reference is considered to be part of this offering circular, and later information that we file with the SEC will automatically update and supersede this information. We incorporate by reference the documents listed below and any future filings made with the SEC under Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act (1) after the date of the initial registration statement, as amended, and prior to effectiveness of the registration statement, and (2) after the date of this offering circular and prior to the termination of this offering. Such information will automatically update and supersede the information contained in this offering circular and the documents listed below:
| (a) | Our Annual Report on Form 10-K for the year ended April 30, 2024, filed with the SEC on July 29, 2024; |
| (b) | Our Quarterly Report on Form 10-Q for the quarter ended July 31, 2024, filed with the SEC on September 12, 2024; |
| (c) | Our Quarterly Report on Form 10-Q for the quarter ended October 31, 2024, filed with the SEC on December 16, 2024; |
| (d) | Our Current Reports on Form 8-K filed with the SEC on May 10, 2024, May 15, 2024, May 16, 2024, May 22, 2024, June 4, 2024, July 29, 2024, August 21, 2024, September 10, 2024, September 12, 2024, October 18, 2024, October 21, 2024, November 8, 2024, December 17, 2024; January 21, 2025; and |
| (e) | The description of our Common Stock and IPO Warrants, which is contained in Exhibit 4.17 to our Annual Report for the fiscal year ended April 30, 2024, filed with the SEC on July 29, 2024, and including any amendments or reports filed for the purpose of updating such description. |
Notwithstanding the foregoing, information that we elect to furnish, but not file, or have furnished, but not filed, with the SEC in accordance with SEC rules and regulations is not incorporated into this offering circular, shall not be deemed “filed” under the Securities Act, and does not constitute a part hereof.
We will provide to each person, including any beneficial owner, to whom a copy of this offering circular is delivered, a copy of any or all of the information that we have incorporated by reference into this offering circular but not delivered with this offering circular. We will provide this information upon written or oral request at no cost to the requester. You may request this information by contacting our corporate headquarters at the following address: at 550 Reserve St, Suite 360, Southlake, Texas 76092, Attn: Danielle Watson, or by calling (682) 237-7781 or at the following email address: investorrelations@heartsciences.com.
GLOSSARY OF TERMS
The following definitions shall apply to the terms used in this offering statement.
Terms Used by and for United States Federal Regulators and Regulations
“510(k)” means a premarket notification submission to the FDA for determination that a medical device is substantially equivalent to another legally U.S. marketed medical device prior to such device being marketed.
“CDC” means the U.S. Centers for Disease Control and Prevention.
“Class II” means a classification of medical devices that are subject to the FDA’s General Controls, and special controls as deemed necessary by the FDA to ensure the safety and effectiveness of the device. These special controls can include submission of a 510(k), performance standards, post-market surveillance, patient registries and FDA guidance documents.
“CMS” means U.S. Centers for Medicare & Medicaid Services.
“De Novo” means the process for obtaining authorization from the FDA of a novel medical device that is low to moderate risk for which general controls alone, or general and special controls, provide reasonable assurance of safety and effectiveness for the intended use, but for which there is no legally marketed predicate device. Devices that are classified (or re-classified) into Class II through a De Novo classification request may be marketed and used as predicates for future premarket notification 510(k) submissions, when applicable.
“FDA” means the U.S. Food and Drug Administration.
“FINRA” means the Financial Industry Regulatory Authority.
“HHS” means the U.S. Health and Human Services — Office of the Inspector General.
“JOBS Act” means the Jumpstart our Business Startups Act of 2012.
“SEC” means the U.S. Securities and Exchange Commission.
Terms Used in Jurisdictions Other Than the U.S.
“CE Mark” means Conformité Européene Mark.
Terms Used for Medical and Medical Device Related Purposes
“AI” means artificial intelligence.
“Aortic stenosis” means narrowing of the valve between the heart’s main pumping chamber and the body’s main artery.
“CAD” means coronary artery disease.
“CPT” means Current Procedural Terminology.
“Diastolic phase” means the period of the heart’s relaxation or filling phase (as opposed to the heart’s period of contraction or pumping phase called “systolic”) of a heartbeat.
“Diastolic dysfunction” means impaired left ventricular relaxation and elevated filling pressures during the diastolic phase.
“Ejection Fraction” means percentage of fluid pumped out of the ventricle during the systolic phase of the cardiac cycle.
“ECG” means electrocardiogram or electrocardiograph as appropriate, which is also known by the acronym “EKG.”
“echo” means an echocardiogram.
“Elevated ST segment” means an ECG electrocardiogram in which the portion of the ECG heartbeat record called the ST segment is elevated and may indicate a serious blockage of a coronary artery and that a section of the heart muscle is currently dying.
“Hypertrophic Cardiomyopathy” means a condition affecting the left ventricle where the walls become thick and stiff.
“LV” means left ventricular.
“LVD” means left ventricular dysfunction.
“LVDD” means left ventricular diastolic dysfunction.
“Mitral regurgitation” a disorder in which the mitral valve on the left side of the heart does not close properly and therefore leakage occurs in the direction it is designed to prevent.
“Premature Ventricular Contraction” extra heartbeats that begin in one of the heart’s two lower pumping chambers (ventricles). These extra beats disrupt the regular heart rhythm.
“Pulmonary Embolism” means sudden blockage in your pulmonary arteries, the blood vessels that send blood to your lungs.
“sensitivity” means the true positive rate or the percentage probability of a positive test result identifying patient with a condition as compared to the gold standard test which in our case is an echo.
“Systolic phase” means the heart’s period of contraction or pumping phase.
Terms Used in Connection with Our Company and Products
“$1.5M Lender Warrants” means the warrants issued to holders of the $1.5M Notes as consideration for the extension of the maturity of the $1.5M Notes.
“$1.5M Notes” means our 12% secured subordinated convertible promissory notes in the aggregate principal amount of $1.5 million issued to accredited investors between December 2020 and April 2021.
“$130K Note” means our private placement on August 12, 2019 with FRV, an accredited investor, of an unsecured drawdown convertible promissory note in the amount of $130,000.
“$1M Lender Warrants” means the warrants issued to holders of the $1M Notes as consideration for the extension of the maturity of the $1M Notes.
“$1M Loan and Security Agreement” means the Loan and Security Agreement entered into by and among the Company, FRV and John Q. Adams, Sr. in April 2020 in connection with the $1M Notes, as amended by Amendment No. 1 dated September 30, 2021, Amendment No. 2 dated November 3, 2021, Amendment No. 3 dated May 24, 2022, Amendment No. 4 dated January 24, 2023, Amendment No. 5 dated September 29, 2023 and Amendment No. 6 dated August 15, 2024.
“$1M Notes” means our 12% secured, non-convertible promissory notes payable to FRV and John Q. Adams, Sr. in the aggregate principal amount of $1 million, as amended and restated.
“Certificate of Designations” means our Certificate of Designations, Number, Voting Power, Preferences and Rights of Series C Convertible Preferred Stock of Heart Test Laboratories, Inc., as filed with the Secretary of State of the State of Texas on March 12, 2019.
“Investor Warrants” means all outstanding warrants to purchase 7,794 shares of our Common Stock issued in connection with funding or as consideration for services rendered to the Company and excludes the Bridge Warrants, Pre-Funded Bridge Warrants, $1M Lender Warrants and $1.5M Lender Warrants.
“IPO Underwriter Warrants” means the warrants to purchase an aggregate of 1,050 shares of Common Stock that were issued to the underwriter in the IPO as a portion of the underwriting compensation payable in connection with the IPO.
“IPO Warrants” means all outstanding warrants to purchase shares of our Common Stock that were issued as part of the IPO Units in the IPO plus additional warrants to purchase 2,250 shares of Common Stock that were issued in the IPO as a result of the underwriter’s exercise of its over-allotment option in part.
“IT” means our information technology. “MyoVista” means the MyoVista wavECG device.
“MyoVista Insights” or “Cloud Platform” means the platform for the delivery of AI-ECG algorithms via the cloud.
“Series A Preferred Stock” means our Series A convertible preferred stock, par value $0.001 per share, all outstanding shares of which converted to Common Stock in connection with our IPO.
“Series B Preferred Stock” means our Series B convertible preferred stock, par value $0.001 per share, all outstanding shares of which were cancelled in connection with our IPO.
“Series C Preferred Stock” means our Series C convertible preferred stock, par value $0.001 per share.
Terms Used in Connection with Our 2021 Bridge Financing
“2021 Bridge Financing” means our private placement, pursuant to a securities purchase agreement, with a lead investor and additional accredited investors of the Bridge Notes, Pre-Funded Bridge Warrants and Bridge Warrants from December 2021 through February 2022, which were issued to such lead investor and additional accredited investors in exchange for the secured subordinated convertible notes and warrants issued to them in an initial closing of a private placement in October 2021.
“2021 Bridge Securities” means, collectively, the Bridge Notes, the Pre-Funded Bridge Warrants and Bridge Warrants.
“Bridge Attribution Parties” are any Bridge Purchaser, together with its affiliates and any other person acting as a group as defined under Section 13(d) of the Exchange Act with regard to determining Bridge Maximum Percentage.
“Bridge Notes” means the 8% secured Senior Subordinated Convertible Loan Notes we sold to Bridge Purchasers pursuant to the Bridge SPA.
“Bridge Purchasers” means the accredited investors who purchased our securities pursuant to the Bridge SPA.
“Bridge SPA” means the Securities Purchase Agreement we entered into with the Bridge Purchasers in connection with the 2021 Bridge Financing.
“Bridge Warrant Amendment No. 1” means Amendment No. 1 to Bridge Warrant by and between Heart Test Laboratories, Inc. and the lead investor under the Bridge SPA, dated September 8, 2022.
“Bridge Warrant Amendment No. 2” means Amendment No. 2 to Bridge Warrant by and between Heart Test Laboratories, Inc. and the lead investor under the Bridge SPA, dated February 3, 2023.
“Bridge Warrants” means the warrants to purchase our Common Stock issued along with the Bridge Notes pursuant to the Bridge SPA. The term “Bridge Warrants” does not include the Pre-Funded Bridge Warrants.
“Bridge Maximum Percentage” means the beneficial ownership in excess of 4.99% of the number of shares of the Common Stock outstanding immediately prior to, and immediately after giving effect to, the conversion of all or any portion of the Bridge Notes as applied to Bridge Attribution Parties unless a holder has notified the Company that it has elected to increase the Bridge Maximum Percentage to 9.99%.
“Pre-Funded Bridge Warrants” means the warrants issued as a result of the number of shares of Common Stock issued to a Bridge Purchaser upon conversion of in the Bridge Notes being in excess of the Bridge Maximum Percentage.
PART III
INDEX TO EXHIBITS
The documents listed in the Exhibit Index of this offering statement are incorporated by reference or are filed with this offering statement, in each case as indicated below.
Exhibit
Number | | Description |
| 1.1* | | Selling Agency Agreement, dated as of October 17, 2024, by and between HeartSciences Inc. and Digital Offering, LLC |
| 1.2+ | | Soliciting Dealer Agreement with Digital Offering |
| 2.1 | | Amended and Restated Certificate of Formation of HeartSciences Inc. (incorporated by reference to Exhibit 3.1 to our Registration Statement on Form S-1 filed May 17, 2022) |
| 2.2 | | Certificate of Designations, Number, Voting Power, Preferences and Rights of Series C Convertible Preferred Stock of HeartSciences Inc. (incorporated by reference to Exhibit 3.2 to our Registration Statement on Form S-1 filed May 17, 2022) |
| 2.3 | | Second Amended and Restated Bylaws of HeartSciences Inc. (incorporated by reference to Exhibit 3.3 to our Registration Statement on Form S-1 filed May 17, 2022) |
| 2.4 | | Form of Certificate of Amendment to Amended and Restated Certificate of Formation of HeartSciences Inc. (incorporated by reference to Exhibit 3.4 to Amendment No. 1 to our Registration Statement on Form S-1 filed June 6, 2022) |
| 2.5 | | Certificate of Amendment to Amended and Restated Certificate of Formation of HeartSciences Inc., as amended (incorporated by reference to Exhibit 3.1 to our Current Report on Form 8-K filed June 23, 2022) |
| 2.6 | | Certificate of Amendment to the Amended and Restated Certificate of Formation, dated as of May 6, 2024 (incorporated by reference to Exhibit 3.1 to our Current Report on Form 8-K filed May 6, 2024) |
| 2.7 | | Certificate of Amendment to the Amended and Restated Certificate of Formation of the Company (Incorporated by reference to Exhibit 3.1 to the Company’s Current Report on Form 8-K, filed with the SEC on October 18, 2024). |
| 2.8+ | | Form of Certificate of Designations for Series D Convertible Preferred Stock |
| 3.1 | | Form of Registration Rights Agreement by and between HeartSciences Inc. and Buyers listed as signatories thereto, dated December 22, 2021 (incorporated by reference to Exhibit 4.2 to our Registration Statement on Form S-1 filed May 17, 2022) |
| 3.2 | | Form of Registration Rights Agreement by and among HeartSciences Inc. and the parties listed as signatories thereto related to the Series C Preferred Stock (incorporated by reference to Exhibit 4.3 to our Registration Statement on Form S-1 filed May 17, 2022) |
| 3.3 | | Form of Bridge Warrant (incorporated by reference to Exhibit 4.4 to our Registration Statement on Form S-1 filed May 17, 2022) |
| 3.4 | | Form of Pre-Funded Warrant (incorporated by reference to Exhibit 4.5 to our Registration Statement on Form S-1 filed May 17, 2022) |
| 3.5 | | Form of $1M Lender Warrant and $1.5M Lender Warrant (incorporated by reference to Exhibit 4.6 to our Registration Statement on Form S-1 filed May 17, 2022) |
| 3.6 | | Form of Investor Warrant (incorporated by reference to Exhibit 4.7 to our Registration Statement on Form S-1 filed May 17, 2022) |
| 3.7 | | Representative’s Warrant Agreement issued June 17, 2022 (incorporated by reference to Exhibit 4.1 to our Current Report on Form 8-K filed June 23, 2022) |
| 3.8 | | Warrant Agent Agreement dated June 17, 2022 between HeartSciences Inc. and American Stock Transfer & Trust Company, LLC (incorporated by reference to Exhibit 4.2 to our Current Report on Form 8-K filed June 23, 2022) |
| 3.9 | | Form of Certificated Warrant (incorporated by reference to Exhibit 4.10 to Amendment No. 2 to our Registration Statement on Form S-1 filed June 10, 2022) |
| 3.10 | | Amendment No. 1 to Bridge Warrants dated September 8, 2022 (incorporated by reference to Exhibit 4.1 to our Current Report on Form 8-K, filed with the SEC on September 9, 2022) |
| 3.11 | | Form of Amendment No. 2 to Bridge Warrants dated February 3, 2023 (incorporated by reference to Exhibit 4.1 to our Current Report on Form 8-K, filed with the SEC on February 3, 2022) |
| 3.12 | | Form of Amended and Restated Warrant to Purchase Common Stock, as amended through February 3, 2023 (incorporated by reference to Exhibit 4.1 to our Current Report on Form 8-K, filed with the SEC on February 22, 2023) |
| 3.13 | | Form of Pre-Funded Warrant, issued pursuant to Amendment No. 2 to Warrants to Purchase Common Stock (incorporated by reference to Exhibit 4.2 to our Current Report on Form 8-K/A, filed with the SEC on March 14, 2023) |
| 3.14 | | Form of Warrant to Purchase Common Stock dated September 7, 2023 (incorporated by reference to Exhibit 4.1 our Current Report on Form 8-K, filed with the SEC on September 7, 2023) |
| 3.15 | | Form of Pre-Funded Purchase Warrant dated as of September 20, 2023 (incorporated by reference to Exhibit 4.1 our Current Report on Form 8-K, filed with the SEC on September 21, 2023) |
| 3.16 | | Form of Common Stock Warrant dated September 20, 2023 (incorporated by reference to Exhibit 4.2 our Current Report on Form 8-K, filed with the SEC on September 21, 2023) |
| 3.17+ | | Form of Common Warrant |
| 3.18+ | | Form of Selling Agent’s Warrant |
| 4.1+ | | Form of Subscription Agreement |
| 4.2+ | | Form of Subscription Agreement |
| 4.3+ | | Form of Subscription Agreement |
| 6.1 | | MyoVista Technology Agreement, by and between HeartSciences Inc. and Guangren “Gary” Chen, dated December 31, 2013 (incorporated by reference to Exhibit 10.1 to our Registration Statement on Form S-1 filed May 17, 2022) |
| 6.2 | | First Amendment of MyoVista Technology Agreement by and between HeartSciences Inc. and Guangren “Gary” Chen, dated March 13, 2017 (incorporated by reference to Exhibit 10.2 to our Registration Statement on Form S-1 filed May 17, 2022) |
| 6.3 | | Master Assignment by and between HeartSciences Inc. and Guangren “Gary” Chen, dated January 1, 2014 (incorporated by reference to Exhibit 10.3 to our Registration Statement on Form S-1 filed May 17, 2022) |
| 6.4 | | Security Agreement and Pledge by and between HeartSciences Inc. and Guangren “Gary” Chen, dated March 14, 2014 (incorporated by reference to Exhibit 10.4 to our Registration Statement on Form S-1 filed May 17, 2022) |
| 6.5 | | Evaluation, Option and License Agreement by and between HeartSciences Inc. and The University Court of The University of Glasgow, dated June 2, 2015 (incorporated by reference to Exhibit 10.5 to our Registration Statement on Form S-1 filed May 17, 2022) |
| 6.6 | | Exercise of Option Agreement by and between HeartSciences Inc. and The University Court of The University of Glasgow, dated December 23, 2015 (incorporated by reference to Exhibit 10.6 to our Registration Statement on Form S-1 filed May 17, 2022) |
| 6.7 | | $130K Note by and between HeartSciences Inc. and Front Range Ventures, LLC, dated August 12, 2019 (incorporated by reference to Exhibit 10.7 to our Registration Statement on Form S-1 filed May 17, 2022) |
| 6.8 | | $1M Loan and Security Agreement by and among HeartSciences Inc., Front Range Ventures, LLC and John Q. Adams, Sr., dated April 24, 2020 (incorporated by reference to Exhibit 10.8 to our Registration Statement on Form S-1 filed May 17, 2022) |
| 6.9 | | Amendment No. 1 to the $1M Loan and Security Agreement, dated September 30, 2021 (incorporated by reference to Exhibit 10.9 to our Registration Statement on Form S-1 filed May 17, 2022) |
| 6.10 | | Amendment No. 2 to the $1M Loan and Security Agreement, dated November 3, 2021 (incorporated by reference to Exhibit 10.10 to our Registration Statement on Form S-1 filed May 17, 2022) |
| 6.11 | | Form of $1.5M Note (incorporated by reference to Exhibit 10.11 to our Registration Statement on Form S-1 filed May 17, 2022) |
| 6.12 | | Form of Amendment No. 1 to the Form of $1.5M Note by and among HeartSciences Inc. and the Requisite Noteholders, dated November 2, 2021 (incorporated by reference to Exhibit 10.12 to our Registration Statement on Form S-1 filed May 17, 2022) |
| 6.13 | | Form of Securities Purchase Agreement by and between HeartSciences Inc. and Purchasers listed as signatories thereto, dated December 22, 2021 (incorporated by reference to Exhibit 10.13 to our Registration Statement on Form S-1 filed May 17, 2022) |
| 6.14 | | Form of Bridge Note (incorporated by reference to Exhibit 10.14 to our Registration Statement on Form S-1 filed May 17, 2022) |
| 6.15 | | Consulting Agreement by and between HeartSciences Inc. and Kyngstone Limited, Inc., dated June 25, 2013 (incorporated by reference to Exhibit 10.15 to our Registration Statement on Form S-1 filed May 17, 2022) |
| 6.16 | | FRV Side Letter by and between HeartSciences Inc. and Front Range Ventures, LLC, dated April 10, 2019 (incorporated by reference to Exhibit 10.16 to Amendment No. 1 to our Registration Statement on Form S-1 filed June 6, 2022) |
| 6.17† | | Amended and Restated Employment Agreement by and between HeartSciences Inc. and Mark Hilz, dated April 5, 2022 (incorporated by reference to Exhibit 10.17 to our Registration Statement on Form S-1 filed May 17, 2022) |
| 6.18† | | Employment Agreement by and between HeartSciences Inc. and Andrew Simpson, dated April 5, 2022 (incorporated by reference to Exhibit 10.18 to our Registration Statement on Form S-1 filed May 17, 2022) |
| 6.19 | | Form of Amendment No. 3 to the $1M Loan and Security Agreement, dated May 2022 (incorporated by reference to Exhibit 10.19 to our Registration Statement on Form S-1 filed May 17, 2022) |
| 6.20 | | Form of Amendment No. 2 to the Form of $1.5M Note by and among HeartSciences Inc. and the Requisite Noteholders, dated May 2022 (incorporated by reference to Exhibit 10.20 to our Registration Statement on Form S-1 filed May 17, 2022) |
| 6.21† | | Form of Time-Based Vesting Nonstatutory Stock Option Agreement of HeartSciences Inc. (incorporated by reference to Exhibit 10.21 to our Registration Statement on Form S-1 filed May 17, 2022) |
| 6.22† | | Form of Performance-Based Vesting Nonstatutory Stock Option Agreement of HeartSciences Inc. (incorporated by reference to Exhibit 10.22 to our Registration Statement on Form S-1 filed May 17, 2022) |
| 6.23 | | Amendment No. 4 to the $1M Loan and Security Agreement, dated January 24, 2023 (incorporated by reference to Exhibit 10.1 to our Current Report on Form 8-K, filed with the SEC on January 24, 2023) |
| 6.24 | | Purchase Agreement, dated as of March 10, 2023, by and between HeartSciences Inc. and Lincoln Park (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K, filed with the SEC on March 10, 2023) |
| 6.25 | | Registration Rights Agreement, dated as of March 10, 2023, by and between HeartSciences Inc. and Lincoln Park (incorporated by reference to Exhibit 10.2 to the Current Report on Form 8-K, filed with the SEC on March 13, 2023) |
| 6.26† | | HeartSciences Inc. 2023 Equity Incentive Plan (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K, filed with the SEC on March 16, 2023) |
| 6.27† | | Form of HeartSciences Inc.’s Incentive Stock Option Agreement under HeartSciences Inc.’s 2023 Equity Incentive Plan (incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K, filed with the SEC on March 23, 2023) |
| 6.28† | | Form of HeartSciences Inc.’s Non-Qualified Stock Option Agreement under HeartSciences Inc.’s 2023 Equity Incentive Plan (incorporated by reference to Exhibit 10.2 to the Current Report on Form 8-K, filed with the SEC on March 23, 2023) |
| 6.29† | | Amendment No. 1 to the HeartSciences Inc. 2023 Equity Incentive Plan (incorporated by reference to Exhibit 10.29 to the Registration Statement on Form S-8, filed with the SEC on February 26, 2024) |
| 6.30 | | Amendment No. 2 License Agreement by and between HeartSciences Inc. and The University Court of The University of Glasgow, dated March 31, 2023 (incorporated by reference to Exhibit 10.29 to the Annual Report on Form 10-K, filed with the SEC on July 19, 2023) |
| 6.31 | | Senior Unsecured Promissory Drawdown Loan Note by and among HeartSciences Inc., and Matthews Southwest Holdings, Inc., dated September 6, 2023 and executed on September 7, 2023 (incorporated by reference to Exhibit 10.1 our Current Report on Form 8-K, filed with the SEC on September 7, 2023) |
| 6.32 | | Securities Purchase Agreement, dated as of September 20, 2023, by and between the Company and Icahn School of Medicine at Mount Sinai (incorporated by reference to Exhibit 10.1 our Current Report on Form 8-K, filed with the SEC on September 21, 2023) |
| 6.33 | | License: Pulmonary Embolism Detection From the Electrocardiogram Using Deep Learning (incorporated by reference to Exhibit 10.2 our Current Report on Form 8-K, filed with the SEC on September 21, 2023) |
| 6.34 | | License: Deep Learning Algorithm to Predict PVC-Related Cardiomyopathy (incorporated by reference to Exhibit 10.3 our Current Report on Form 8-K, filed with the SEC on September 21, 2023) |
| 6.35 | | License: Deep Learning on ECGs to Derive Left and Right Ventricular Function (incorporated by reference to Exhibit 10.4 our Current Report on Form 8-K, filed with the SEC on September 21, 2023) |
| 6.36 | | License: Prediction of right ventricular size and systolic function from the 12-lead ECG (incorporated by reference to Exhibit 10.5 our Current Report on Form 8-K, filed with the SEC on September 21, 2023) |
| 6.37 | | License: Deep learning for electrocardiograms to identify left heart valvular dysfunction – aortic stenosis (incorporated by reference to Exhibit 10.6 our Current Report on Form 8-K, filed with the SEC on September 21, 2023) |
| 6.38 | | License: Deep learning for electrocardiograms to identify left heart valvular dysfunction – mitral regurgitation (incorporated by reference to Exhibit 10.7 our Current Report on Form 8-K, filed with the SEC on September 21, 2023) |
| 6.39 | | License: HeartBEiT: Vision Transformers improve diagnostic performance for electrocardiograms (incorporated by reference to Exhibit 10.8 our Current Report on Form 8-K, filed with the SEC on September 21, 2023) |
| 6.40 | | License: Derivation of low Left Ventricular Ejection fraction based on a foundational vision transformer (HeartBEiT) (incorporated by reference to Exhibit 10.9 our Current Report on Form 8-K, filed with the SEC on September 21, 2023) |
| 6.41 | | License: Diagnosis of Hypertrophic Cardiomyopathy using a model derived from a foundational vision transformer (HeartBEiT) (incorporated by reference to Exhibit 10.10 our Current Report on Form 8-K, filed with the SEC on September 21, 2023) |
| 6.42 | | License: Diagnosis of STEMI using a model derived from a foundational vision transformer (HeartBEiT) (incorporated by reference to Exhibit 10.11 our Current Report on Form 8-K, filed with the SEC on September 21, 2023) |
| 6.43 | | License: Electrocardiogram Deep Learning Interpretability Toolbox (incorporated by reference to Exhibit 10.12 our Current Report on Form 8-K, filed with the SEC on September 21, 2023) |
| 6.44 | | Amendment No. 5 to the $1M Loan and Security Agreement, dated September 29, 2023 (incorporated by reference to Exhibit 10.45 to our Registration Statement on Form S-1 filed October 16, 2023) |
| 6.45 | | Note Conversion Letter Agreement, dated November 16, 2023, by and between HeartSciences Inc. and Matthews Southwest Holdings, Inc. (filed as Exhibit 10.1 on our Current Report on Form 8-K, filed with the SEC on November 17, 2023) |
| 6.46 | | Note Conversion Letter Agreement, dated November 16, 2023, by and between HeartSciences Inc. and John Q. Adams (filed as Exhibit 10.2 on our Current Report on Form 8-K, filed with the SEC on November 17, 2023) |
| 6.47 | | Warrant Amendment, dated November 16, 2023, by and between HeartSciences Inc. and Matthews Southwest Holdings, Inc. (filed as Exhibit 10.3 on our Current Report on Form 8-K, filed with the SEC on November 17, 2023) |
| 6.48 | | Warrant Amendment, dated November 16, 2023, by and between HeartSciences Inc. and John Q. Adams (filed as Exhibit 10.4 on our Current Report on Form 8-K, filed with the SEC on November 17, 2023) |
| 6.49 | | Amendment No. 6 to Loan and Security Agreement by and between HeartSciences Inc. and Front Range Ventures LLC dated August 19, 2024 (filed as Exhibit 10.1 on our Current Report on Form 8-K, filed with the SEC on August 19, 2024) |
| 6.50 | | No. 2 Amended and Restated Secured Promissory Note by and between HeartSciences Inc. and Front Range Ventures LLC dated August 19, 2024 (filed as Exhibit 10.1 on our Current Report on Form 8-K, filed with the SEC on August 19, 2024) |
| 6.51† | | Employment Agreement by and between HeartSciences Inc. and Danielle Watson, dated October 15, 2021 (incorporated by reference to Exhibit 10.19 to our Registration Statement on Form S-1 filed October 16, 2023) |
| 6.52 | | Note Purchase Agreement, by and between the Company and Streeterville Capital, LLC dated September 6, 2024 (filed as Exhibit 10.1 on our Current Report on Form 8-K, filed with the SEC on September 10, 2024) |
| 6.53 | | Purchase Note dated September 6, 2024 (filed as Exhibit 10.2 on our Current Report on Form 8-K, filed with the SEC on September 10, 2024) |
| 8.1+ | | Form of Escrow Agreement |
| 10.1* | | Power of Attorney (included on signature page) |
| 11.1* | | Consent of Haskell & White LLP |
| 11.2* | | Consent of Foley Shechter Ablovatskiy LLP (included in Exhibit 12.1) |
| 12.1* | | Opinion of Foley Shechter Ablovatskiy LLP |
| † | Management contract or compensatory plan or arrangement |
| + | To be filed by amendment. |
SIGNATURES
Pursuant to the requirements of Regulation A, the issuer certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form 1-A and has duly caused this offering statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Southlake, State of Texas on February 12, 2025.
| | HeartSciences Inc. |
| | | |
| | By: | /s/ Andrew Simpson |
| | | Andrew Simpson |
| | | President, Chief Executive Officer and Chairman of the Board of Directors |
POWER OF ATTORNEY
The registrant and each person whose signature appears below constitutes and appoints Andrew Simpson, Mark Hilz and Danielle Watson, and each of them singly, as his, her or its true and lawful attorneys-in-fact and agent, with full power of substitution and resubstitution, for him, her or it and in his, her or its name, place and stead, in any and all capacities, to sign and file any and all amendments (including post-effective amendments) to this offering statement on Form 1-A, with all exhibits thereto, and other documents in connection therewith, with the U.S. Securities and Exchange Commission, granting unto said attorney-in-fact and agent, and each of them, full power and authority to do and perform each and every act and thing requisite or necessary to be done in and about the premises, as fully to all intents and purposes as he, she, or it might or could do in person, hereby ratifying and confirming all that said attorney-in-fact and agent or his substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of Regulation A, this offering statement has been signed below by the following persons in the capacities and on the date indicated.
| Signature | | Title | | Date |
| | | | | |
| /s/ Andrew Simpson | | President, Chief Executive Officer and Chairman of | | February 12, 2025 |
| Andrew Simpson | | the Board of Directors (Principal Executive Officer) | | |
| | | | | |
| /s/ Danielle Watson | | Chief Financial Officer and Treasurer | | February 12, 2025 |
| Danielle Watson | | (Principal Financial and Accounting Officer) | | |
| | | | | |
| /s/ Mark Hilz | | Chief Operating Officer, Secretary and Director | | February 12, 2025 |
| Mark Hilz | | | | |
| | | | | |
| /s/ Bruce Bent | | Director | | February 12, 2025 |
| Bruce Bent | | | | |
| | | | | |
| /s/ David Wells | | Director | | February 12, 2025 |
| David R. Wells | | | | |
| | | | | |
| /s/ Brian Szymczak | | Director | | February 12, 2025 |
| Brian Szymczak | | | | |

