Free signup for more
- Track your favorite companies
- Receive email alerts for new filings
- Personalized dashboard of news and more
- Access all data and search results
Filing tables
Filing exhibits
PDSB similar filings
- 2 Oct 24 88% (15/17) of patients had a complete metabolic response
- 16 Sep 24 Other Events
- 16 Sep 24 PDS Biotech Announces Updated Results from VERSATILE-002 Phase 2 Clinical Trial Presented at ESMO 2024
- 13 Aug 24 Entry into a Material Definitive Agreement
- 13 Aug 24 PDS Biotech Provides Business Update and Reports Second Quarter 2024 Financial Results
Filing view
External links
Exhibit 99.1

Transforming How the Immune System Targets and Fights Cancer to Promote Survival NASDAQ: PDSB September 2024 Precision Designed Science For Immunotherapy

Forward-Looking Statements This communication contains forward-looking statements (including within the meaning of Section 27E of the United States Securities Exchange Act of 1934, as amended, and Section 27A of the United States Securities Act of 1933, as amended) concerning PDS Biotechnology Corporation (the "Company") and other matters. These statements may discuss goals, intentions and expectations as to future plans, trends, events, results of operations or financial condition, or otherwise, based on current beliefs of the Company's management, as well as assumptions made by, and information currently available to, management. Forward-looking statements generally include statements that are predictive in nature and depend upon or refer to future events or conditions and include words such as "may," "will," "should," "would," "expect," "anticipate," "plan," "likely," "believe," "estimate," "project,“ "intend," "forecast," "guidance", "outlook“ and other similar expressions among others. Forward-looking statements are based on current beliefs and assumptions that are subject to risks and uncertainties and are not guarantees of future performance. Actual results could differ materially from those contained in any forward-looking statement as a result of various factors, including, without limitation: the Company's ability to protect its intellectual property rights; the Company's anticipated capital requirements, including the Company's anticipated cash runway and the Company's current expectations regarding its plans for future equity financings; the Company's dependence on additional financing to fund its operations and complete the development and commercialization of its product candidates, and the risks that raising such additional capital may restrict the Company's operations or require the Company to relinquish rights to the Company's technologies or product candidates; the Company's limited operating history in the Company's current line of business, which makes it difficult to evaluate the Company's prospects, the Company's business plan or the likelihood of the Company's successful implementation of such business plan; the timing for the Company or its partners to initiate the planned clinical trials for PDS01ADC, Versamune® HPV and other Versamune® and lnfectimune® based product candidates; the future success of such trials; the successful implementation of the Company's research and development programs and collaborations, including any collaboration studies concerning PDS01ADC, Versamune® HPV and other Versamune® and lnfectimune® based product candidates and the Company's interpretation of the results and findings of such programs and collaborations and whether such results are sufficient to support the future success of the Company's product candidates; the success, timing and cost of the Company's ongoing clinical trials and anticipated clinical trials for the Company's current product candidates, including statements regarding the timing of initiation, pace of enrollment and completion of the trials (including the Company's ability to fully fund its disclosed clinical trials, which assumes no material changes to the Company's currently projected expenses), futility analyses, presentations at conferences and data reported in an abstract, and receipt of interim or preliminary results (including, without limitation, any preclinical results or data), which are not necessarily indicative of the final results of the Company's ongoing clinical trials; any Company statements about its understanding of product candidates mechanisms of action and interpretation of preclinical and early clinical results from its clinical development programs and any collaboration studies; to aid in the development of the Versamune® platform; and other factors, including legislative, regulatory, political and economic developments not within the Company's control. The foregoing review of important factors that could cause actual events to differ from expectations should not be construed as exhaustive and should be read in conjunction with statements that are included herein and elsewhere, including the risk factors included in the Company's annual, quarterly and periodic reports filed with the Securities and Exchange Commission (“SEC”). The forward-looking statements are made only as of the date of this press release and, except as required by applicable law, the Company undertakes no obligation to revise or update any forward-looking statement, or to make any other forward-looking statements, whether as a result of new information, future events or otherwise. This presentation shall not constitute an offer to sell or the solicitation of an offer to buy any securities, nor shall there be any sale of any securities in any state or jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction. Versamune® and lnfectimune® are registered trademarks of PDS Biotechnology Corporation. KEYTRUDA® is a registered trademark of Merck Sharp and Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.

Late-Stage Head and Neck Cancer Program as Value Catalyst Potent Long-Lasting “Memory” T Cells High-Value Lead Program with strong KOL support Promising Phase 2 Data Phase 3 Design 30 months median Overall Survival (mOS) 77% Disease Control Rate (DCR) Well tolerated: 9% Grade 3, 1% Grade 4 AE Targeted immunotherapy in HPV16-positive Recurrent and/or Metastatic Head & Neck Squamous Cell Carcinoma (R/M HNSCC) Fast Track designation for Versamune® HPV in HNSCC Alignment with FDA on Phase 3 study design Trial initiation planned for Q4-2024 Induction of right type and quantity of potent tumor-accumulating killer T cells observed in comprehensive preclinical and human studies
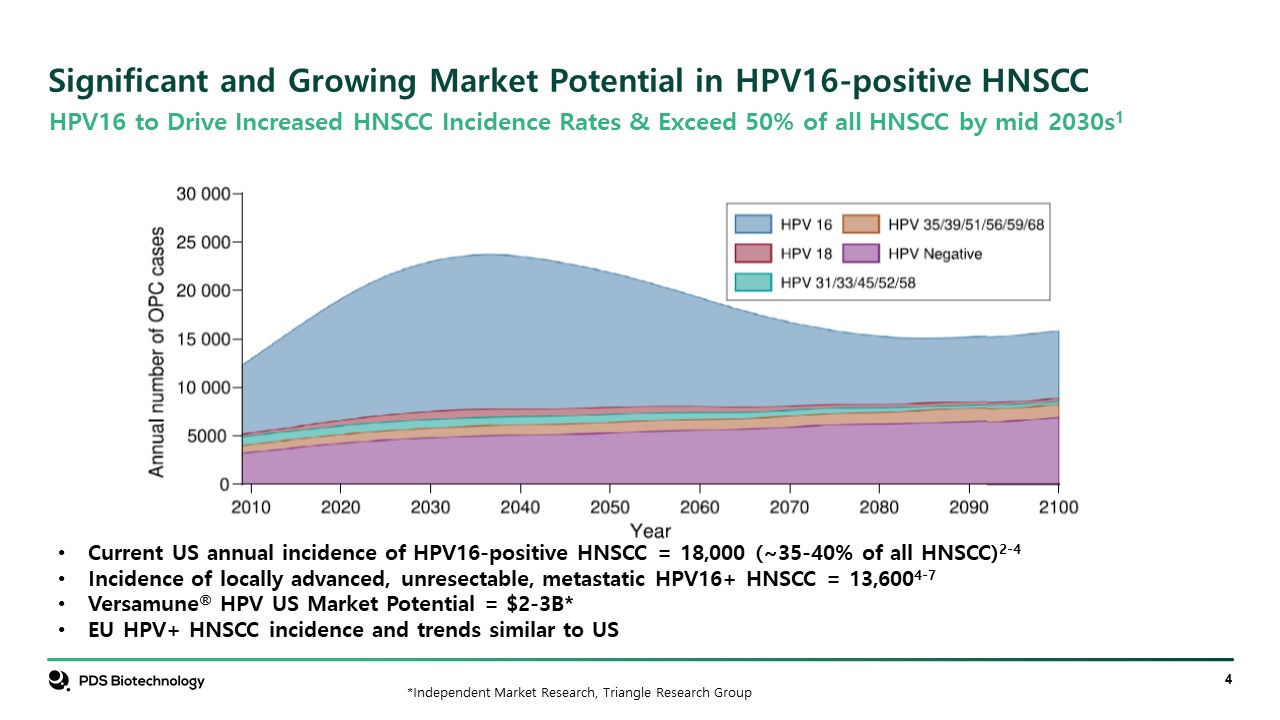
Significant and Growing Market Potential in HPV16-positive HNSCC HPV16 to Drive Increased HNSCC Incidence Rates & Exceed 50% of all HNSCC by mid 2030s1 Current US annual incidence of HPV16-positive HNSCC = 18,000 (~35-40% of all HNSCC)2-4 Incidence of locally advanced, unresectable, metastatic HPV16+ HNSCC = 13,6004-7 Versamune® HPV US Market Potential = $2-3B* EU HPV+ HNSCC incidence and trends similar to US *Independent Market Research, Triangle Research Group
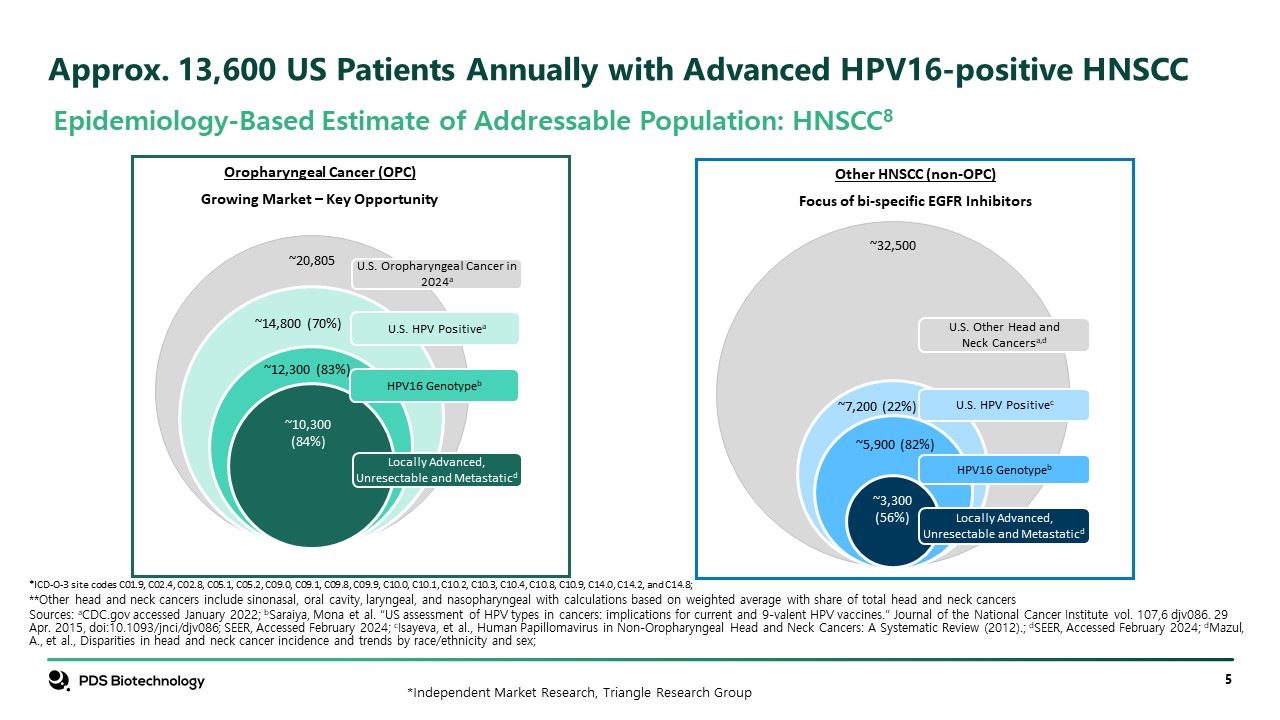
Approx. 13,600 US Patients Annually with Advanced HPV16-positive HNSCC Epidemiology-Based Estimate of Addressable Population: HNSCC8 U.S. Other Head and Neck Cancersa,d U.S. HPV Positivec HPV16 Genotypeb Locally Advanced, Unresectable and Metastaticd ~3,300 (56%) ~5,900 (82%) ~7,200 (22%) ~32,500 U.S. Oropharyngeal Cancer in 2024a U.S. HPV Positivea HPV16 Genotypeb Locally Advanced, Unresectable and Metastaticd ~10,300 (84%) ~12,300 (83%) ~14,800 (70%) ~20,805 Oropharyngeal Cancer (OPC) Growing Market – Key Opportunity Other HNSCC (non-OPC) Focus of bi-specific EGFR Inhibitors *ICD-O-3 site codes C01.9, C02.4, C02.8, C05.1, C05.2, C09.0, C09.1, C09.8, C09.9, C10.0, C10.1, C10.2, C10.3, C10.4, C10.8, C10.9, C14.0, C14.2, and C14.8; **Other head and neck cancers include sinonasal, oral cavity, laryngeal, and nasopharyngeal with calculations based on weighted average with share of total head and neck cancers Sources: aCDC.gov accessed January 2022; bSaraiya, Mona et al. “US assessment of HPV types in cancers: implications for current and 9-valent HPV vaccines.” Journal of the National Cancer Institute vol. 107,6 djv086. 29 Apr. 2015, doi:10.1093/jnci/djv086; SEER, Accessed February 2024; cIsayeva, et al., Human Papillomavirus in Non-Oropharyngeal Head and Neck Cancers: A Systematic Review (2012).; dSEER, Accessed February 2024; dMazul, A., et al., Disparities in head and neck cancer incidence and trends by race/ethnicity and sex; *Independent Market Research, Triangle Research Group

Significant Unmet Needs Remain in Recurrent/Metastatic HPV16 HNSCC KEYTRUDA® KEYTRUDA® Plus Chemo Chemotherapy + EGFR Inhibitor Objective Response Rate (ORR) 19% 36% 35% Progression Free Survival (PFS) 3.2 mos 5.0 mos 5.0 mos Median Overall Survival (OS) 12.3 mos 13.6 mos 10.3 mos Treatment Related Grade 3+ Toxicities 17% 72% 69% HPV16-Specificity: Need Targeted treatment option to address the growing population of HPV16-positive HNSCC and improve outcomes Improved Survival: Need Novel MOA that provides enhanced survival Improved Durability: Need Novel MOA that is clinically effective and provides more durable (long-term) responses. Improved Safety: Need Safe treatments that may be used with or in place of current standard of care and chemotherapy Oncologists10 – Stated Unmet Medical Needs in HPV16 HNSCC * No control or comparative studies have been conducted between immune checkpoint inhibitors, EGFR inhibitors, chemotherapy and Versamune® HPV Standard of Care for Recurrent or Metastatic HNSCC – Published Results*9
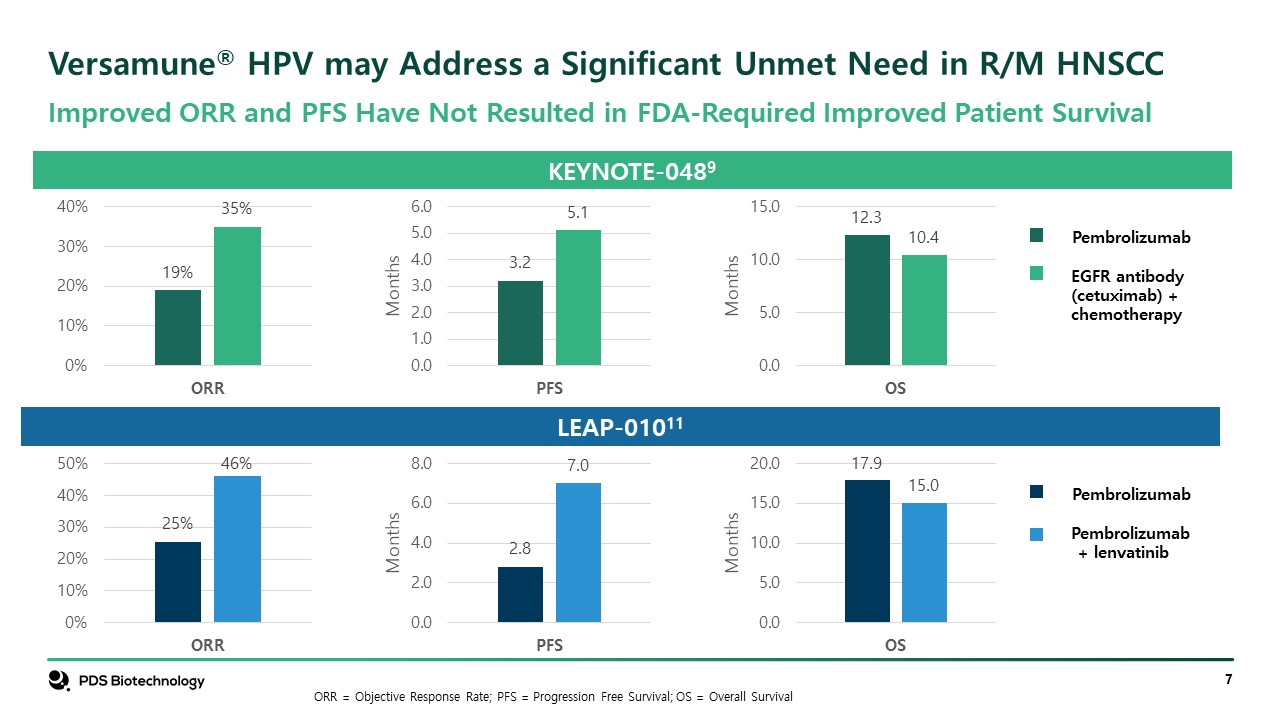
Versamune® HPV may Address a Significant Unmet Need in R/M HNSCC KEYNOTE-0489 Pembrolizumab EGFR antibody (cetuximab) + chemotherapy LEAP-01011 Pembrolizumab Pembrolizumab + lenvatinib Improved ORR and PFS Have Not Resulted in FDA-Required Improved Patient Survival ORR = Objective Response Rate; PFS = Progression Free Survival; OS = Overall Survival
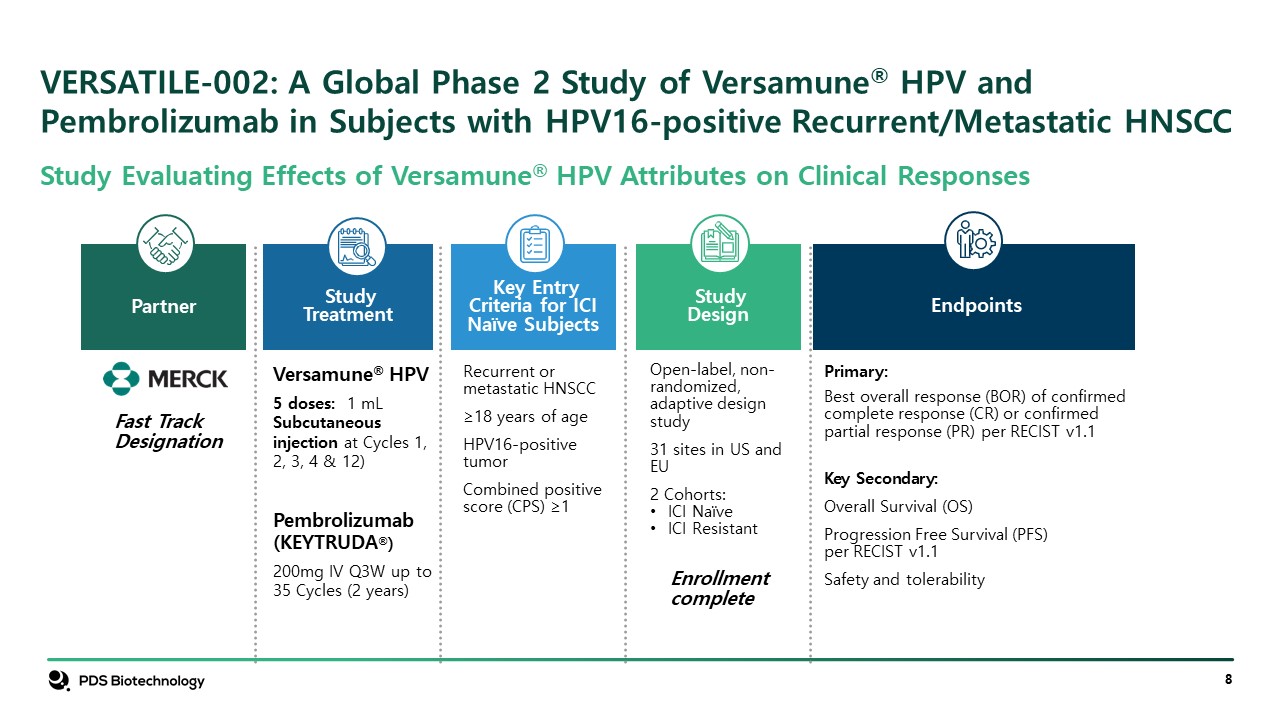
VERSATILE-002: A Global Phase 2 Study of Versamune® HPV and Pembrolizumab in Subjects with HPV16-positive Recurrent/Metastatic HNSCC Partner StudyDesign Open-label, non-randomized, adaptive design study 31 sites in US and EU 2 Cohorts: ICI Naïve ICI Resistant Enrollment complete Key Entry Criteria for ICI Naïve Subjects Recurrent or metastatic HNSCC ≥18 years of age HPV16-positive tumor Combined positive score (CPS) ≥1 Versamune® HPV 5 doses: 1 mL Subcutaneous injection at Cycles 1, 2, 3, 4 & 12) Pembrolizumab (KEYTRUDA®) 200mg IV Q3W up to 35 Cycles (2 years) Study Treatment Primary: Best overall response (BOR) of confirmed complete response (CR) or confirmed partial response (PR) per RECIST v1.1 Key Secondary: Overall Survival (OS) Progression Free Survival (PFS) per RECIST v1.1 Safety and tolerability Endpoints Fast Track Designation Study Evaluating Effects of Versamune® HPV Attributes on Clinical Responses
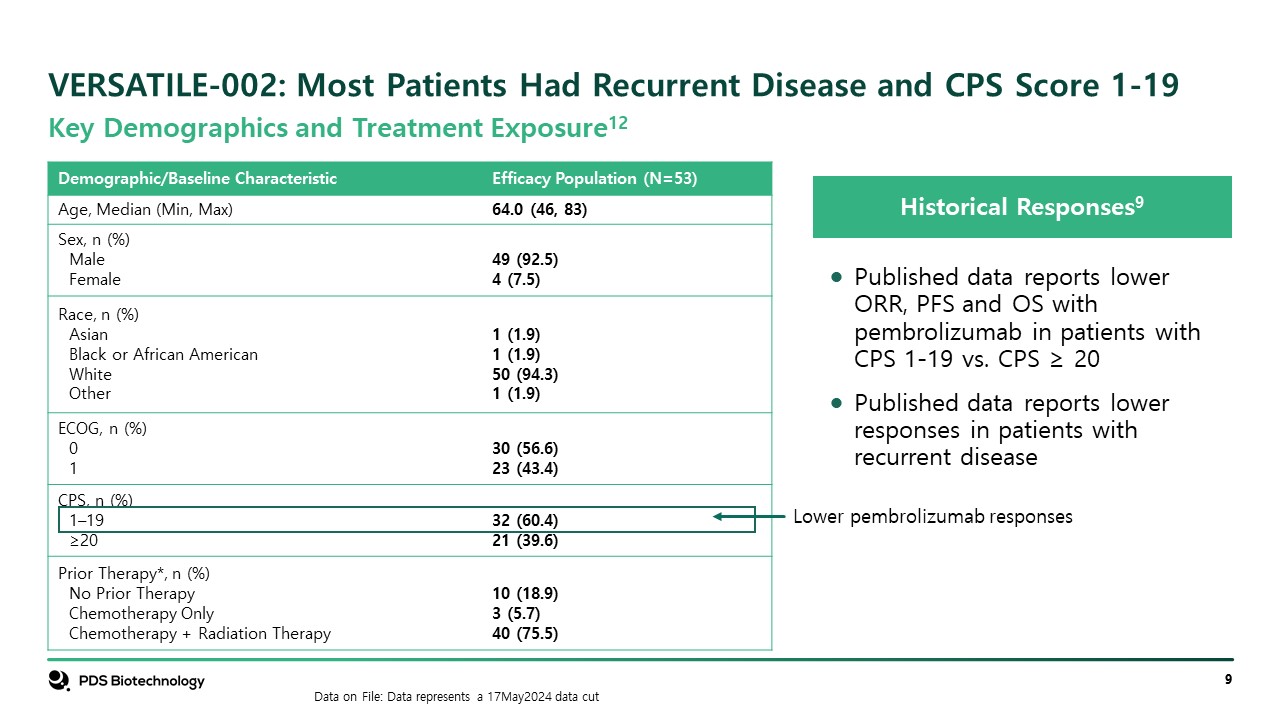
VERSATILE-002: Most Patients Had Recurrent Disease and CPS Score 1-19 Historical Responses9 Published data reports lower ORR, PFS and OS with pembrolizumab in patients with CPS 1-19 vs. CPS ≥ 20 Published data reports lower responses in patients with recurrent disease Data on File: Data represents a 17May2024 data cut Key Demographics and Treatment Exposure12 Demographic/Baseline Characteristic Efficacy Population (N=53) Age, Median (Min, Max) 64.0 (46, 83) Sex, n (%) Male Female 49 (92.5) 4 (7.5) Race, n (%) Asian Black or African American White Other 1 (1.9) 1 (1.9) 50 (94.3) 1 (1.9) ECOG, n (%) 0 1 30 (56.6) 23 (43.4) CPS, n (%) 1–19 ≥20 32 (60.4) 21 (39.6) Prior Therapy*, n (%) No Prior Therapy Chemotherapy Only Chemotherapy + Radiation Therapy 10 (18.9) 3 (5.7) 40 (75.5) Lower pembrolizumab responses
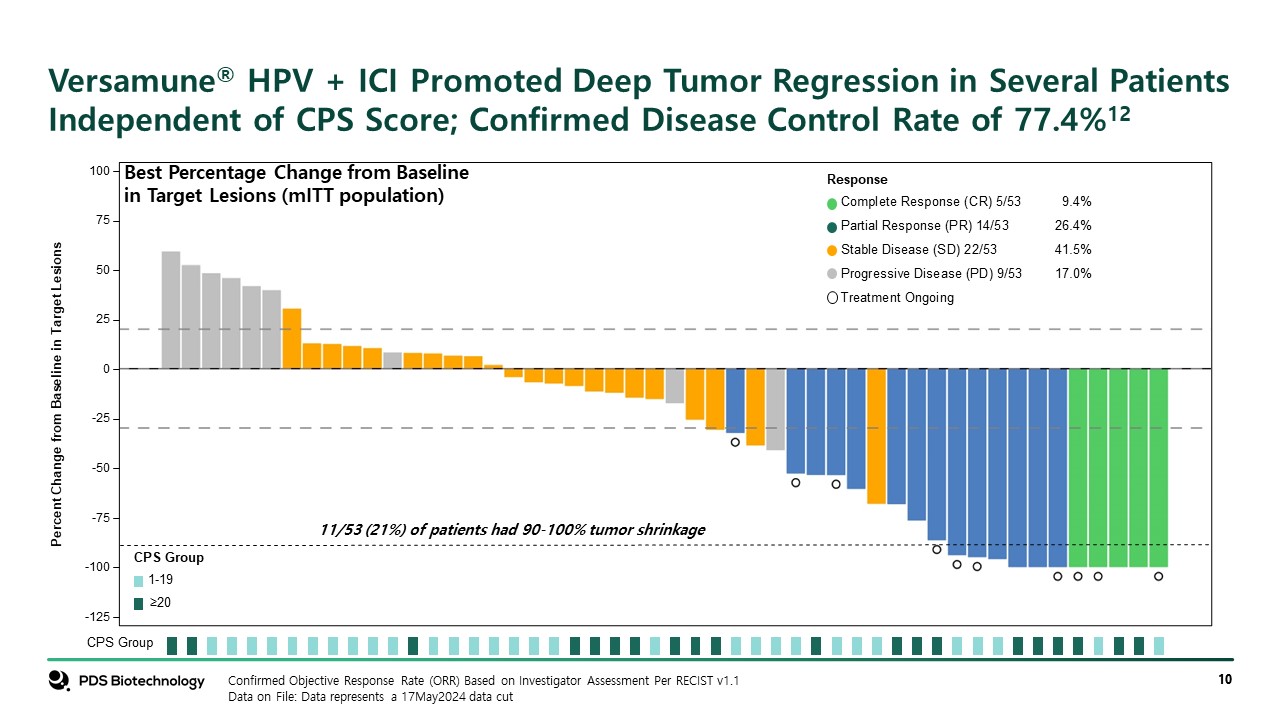
Percent Change from Baseline in Target Lesions -50 -25 25 50 75 100 0 -75 -100 -125 CPS Group 1-19 ≥20 CPS Group Response Complete Response (CR) 5/53 9.4% Partial Response (PR) 14/53 26.4% Stable Disease (SD) 22/53 41.5% Progressive Disease (PD) 9/53 17.0% Treatment Ongoing Confirmed Objective Response Rate (ORR) Based on Investigator Assessment Per RECIST v1.1 Data on File: Data represents a 17May2024 data cut Best Percentage Change from Baseline in Target Lesions (mITT population) 11/53 (21%) of patients had 90-100% tumor shrinkage Versamune® HPV + ICI Promoted Deep Tumor Regression in Several Patients Independent of CPS Score; Confirmed Disease Control Rate of 77.4%12
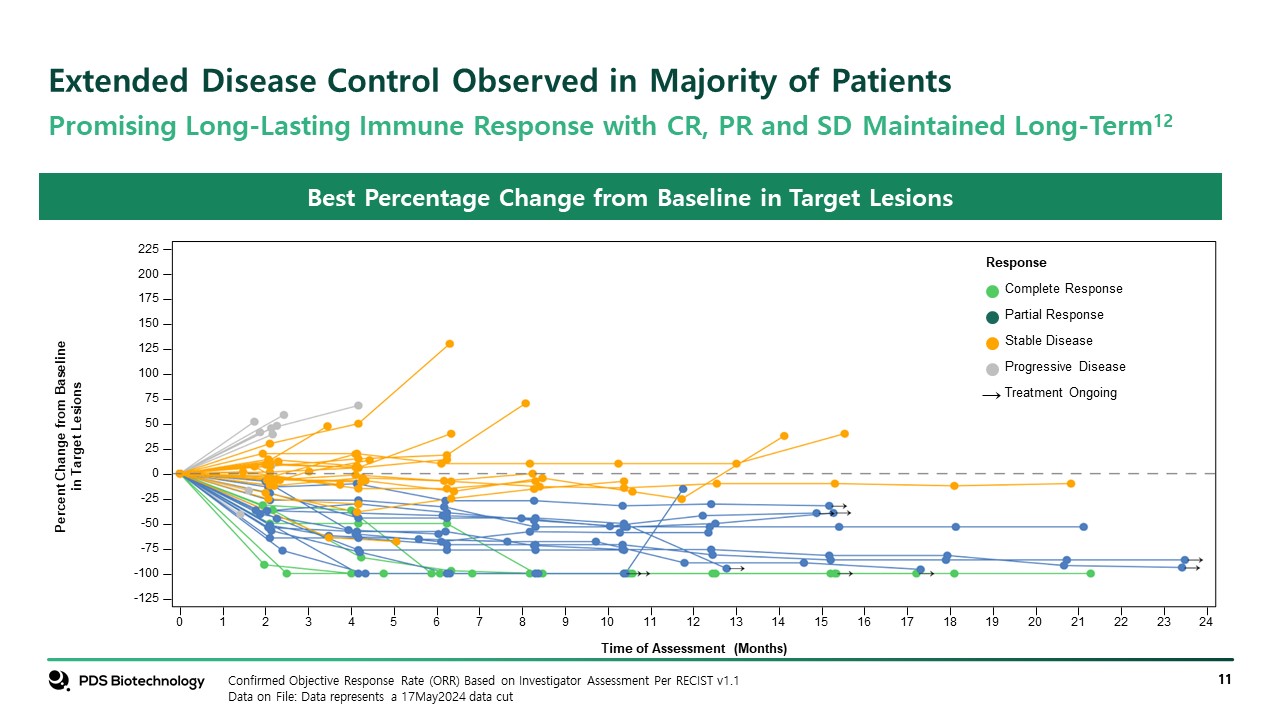
Extended Disease Control Observed in Majority of Patients Confirmed Objective Response Rate (ORR) Based on Investigator Assessment Per RECIST v1.1 Data on File: Data represents a 17May2024 data cut Best Percentage Change from Baseline in Target Lesions Promising Long-Lasting Immune Response with CR, PR and SD Maintained Long-Term12 Percent Change from Baselinein Target Lesions Time of Assessment (Months) 0 2 4 6 8 10 12 14 16 18 20 22 24 1 3 5 7 9 11 13 15 17 19 21 23 -125 -100 150 175 200 225 50 75 100 125 -50 -25 0 25 -75 Response Complete Response Partial Response Stable Disease Progressive Disease Treatment Ongoing
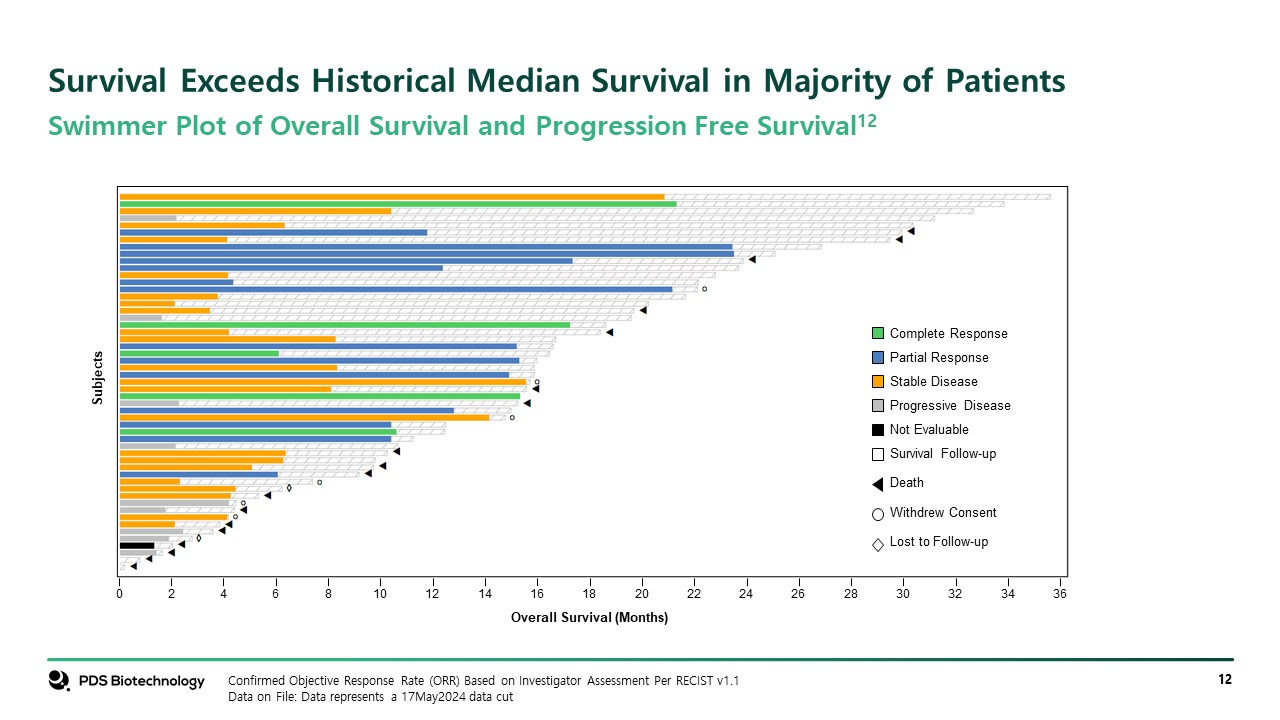
Survival Exceeds Historical Median Survival in Majority of Patients Confirmed Objective Response Rate (ORR) Based on Investigator Assessment Per RECIST v1.1 Data on File: Data represents a 17May2024 data cut Swimmer Plot of Overall Survival and Progression Free Survival12 Subjects 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 34 36 Overall Survival (Months) Complete Response Partial Response Stable Disease Progressive Disease Not Evaluable Survival Follow-up Death Withdrew Consent Lost to Follow-up
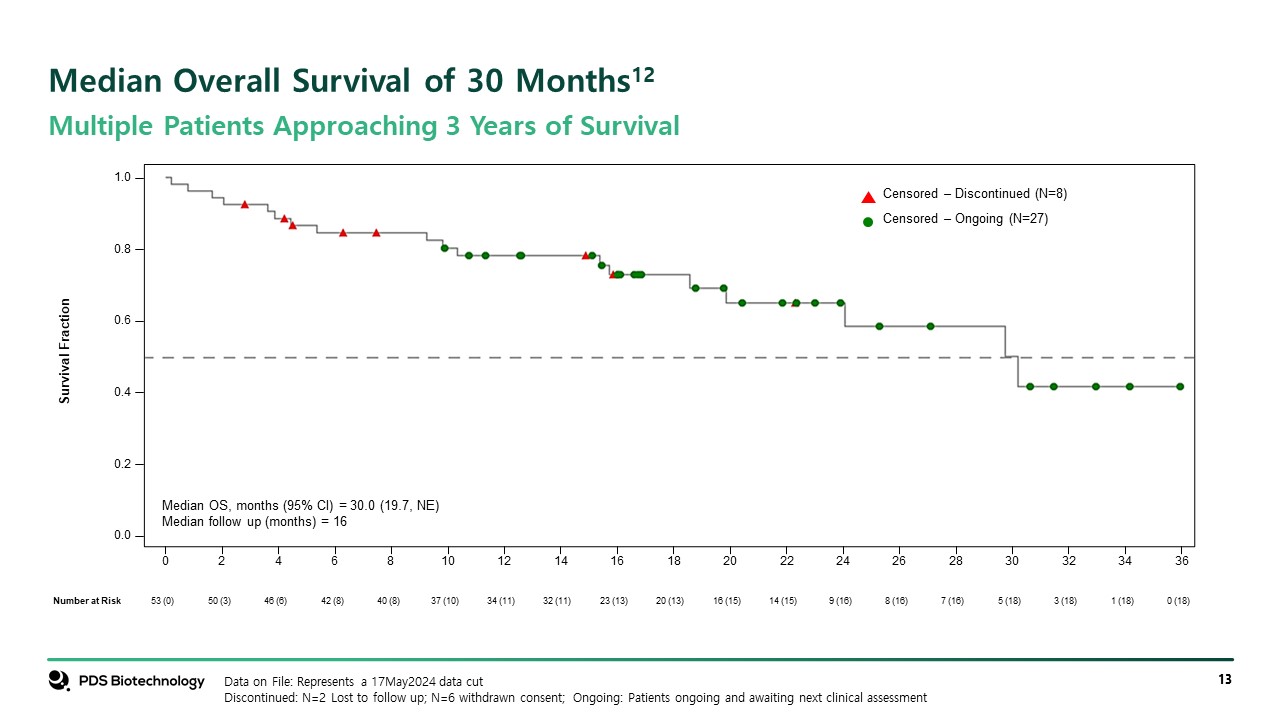
Survival Fraction 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 34 36 0.0 0.2 0.4 0.6 0.8 1.0 Censored ‒ Ongoing (N=27) Censored ‒ Discontinued (N=8) Data on File: Represents a 17May2024 data cut Discontinued: N=2 Lost to follow up; N=6 withdrawn consent; Ongoing: Patients ongoing and awaiting next clinical assessment Median Overall Survival of 30 Months12 Multiple Patients Approaching 3 Years of Survival 53 (0) 50 (3) 46 (6) 42 (8) 40 (8) 37 (10) 34 (11) 32 (11) 23 (13) 20 (13) 16 (15) 14 (15) 9 (16) 8 (16) 7 (16) 5 (18) 3 (18) 1 (18) 0 (18) Number at Risk Median OS, months (95% CI) = 30.0 (19.7, NE) Median follow up (months) = 16

Versamune® HPV Plus Pembrolizumab Appears to be Well Tolerated Protocol stipulates 5 subcutaneous injections of Versamune® HPV: 4 injections over 2 months and a final injection after an additional 6 months *Grade 3 Combination-TRAE were: Fatigue (2), Rash, Alanine aminotransferase increased, Blood alkaline phosphatase increased, Lymphocyte count decreased, Autoimmune colitis, Colitis, Headache, Acute kidney injury, Hyponatremia, Hyperglycemia, **Grade 4 Combination-TRAE: encephalitis (case recorded approx. one year after last Versamune® HPV dose) * TRAE = Treatment Related Adverse Event Data on File: Data represents a 17May2024 data cut, and includes ICI naïve and ICI resistant HPV16-positive patients 8/87 (9%) Patients had a Grade 3 TRAE*; 1/87 (1%) had a Grade 4 TRAE** TRAEs by Grade n (%) Any Combination TRAE 76 (87.4) Grade 1 40 (46.0) Grade 2 26 (29.9) Grade 3 8 (9.2) Grade 4 1 (1.1) Grade 5 0 Non-Injection Site TRAEs ≥ 5% n (%) Fatigue 30 (34.5) Headache 13 (14.9) Diarrhea 10 (11.5) Pruritis 9 (10.3) Rash 7 (8.0) Malaise 6 (6.9) Pyrexia 6 (6.9) Pain 5 (5.7) Cough 5 (5.7)
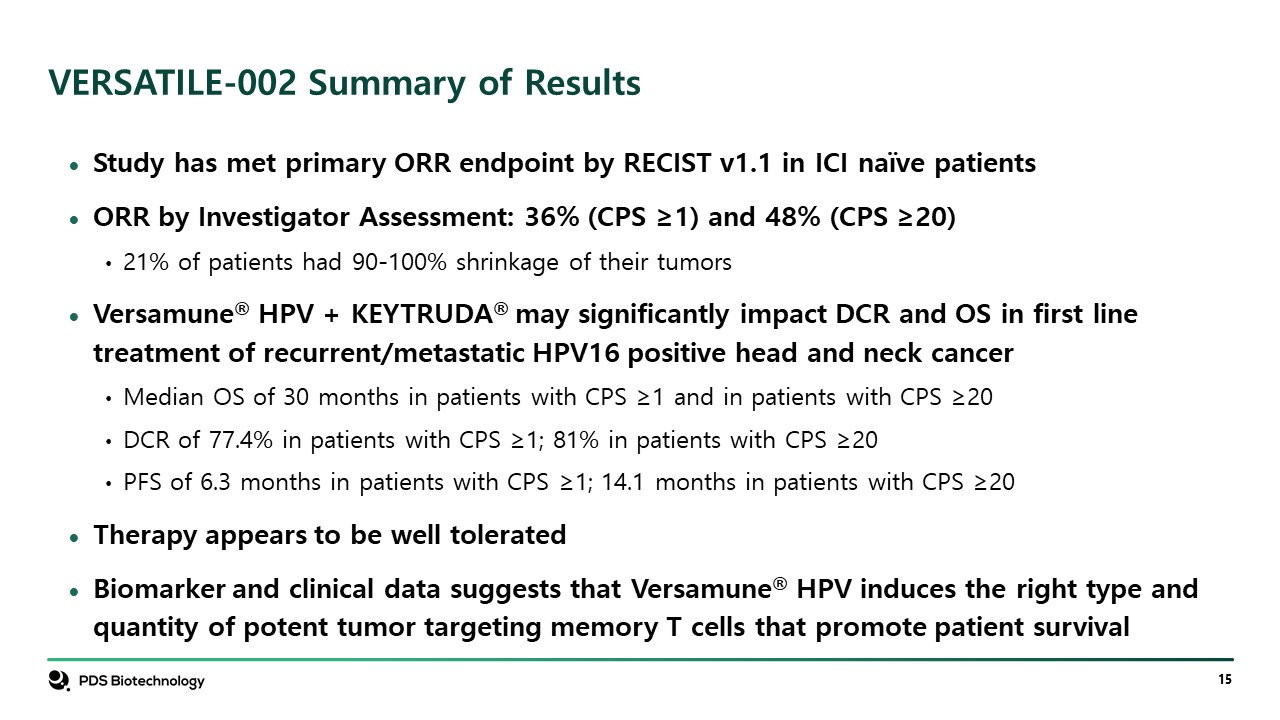
VERSATILE-002 Summary of Results Study has met primary ORR endpoint by RECIST v1.1 in ICI naïve patients ORR by Investigator Assessment: 36% (CPS ≥1) and 48% (CPS ≥20) 21% of patients had 90-100% shrinkage of their tumors Versamune® HPV + KEYTRUDA® may significantly impact DCR and OS in first line treatment of recurrent/metastatic HPV16 positive head and neck cancer Median OS of 30 months in patients with CPS ≥1 and in patients with CPS ≥20 DCR of 77.4% in patients with CPS ≥1; 81% in patients with CPS ≥20 PFS of 6.3 months in patients with CPS ≥1; 14.1 months in patients with CPS ≥20 Therapy appears to be well tolerated Biomarker and clinical data suggests that Versamune® HPV induces the right type and quantity of potent tumor targeting memory T cells that promote patient survival
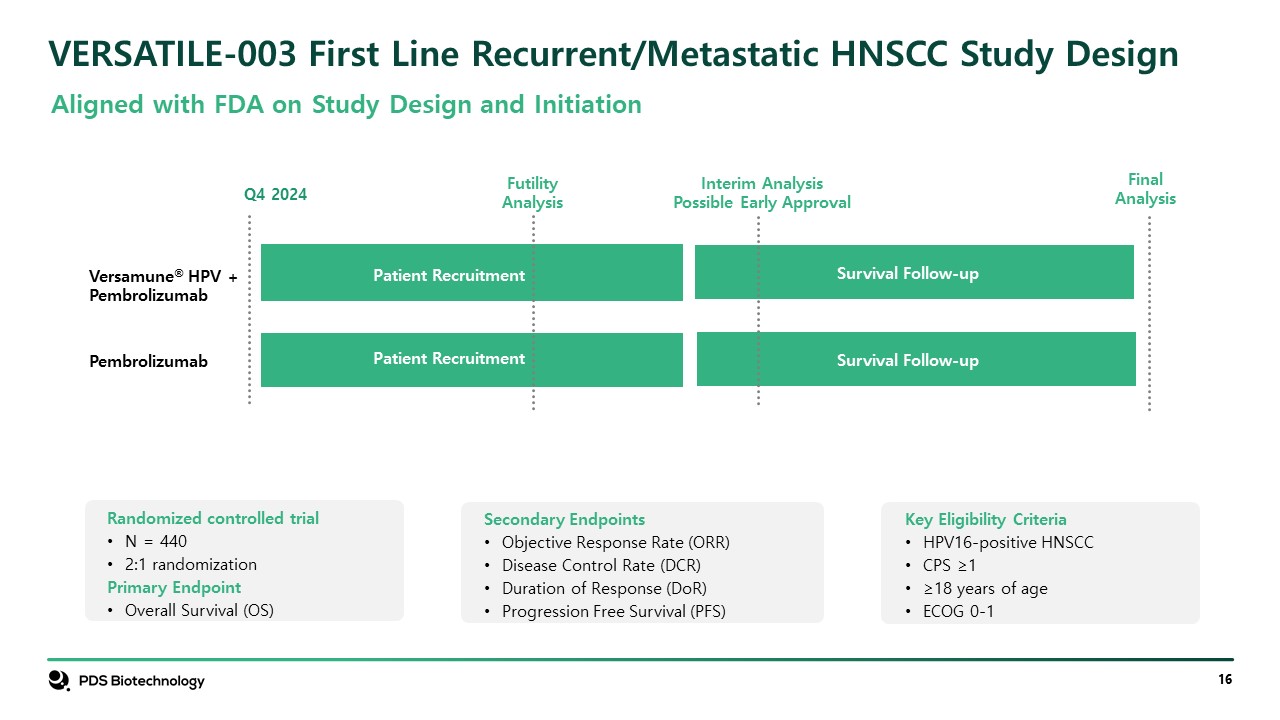
VERSATILE-003 First Line Recurrent/Metastatic HNSCC Study Design Interim Analysis Possible Early Approval Versamune® HPV + PDS01ADC + pembrolizumab Q4 2024 Versamune® HPV + Pembrolizumab Pembrolizumab Aligned with FDA on Study Design and Initiation Patient Recruitment Patient Recruitment Survival Follow-up Survival Follow-up Key Eligibility Criteria HPV16-positive HNSCC CPS ≥1 ≥18 years of age ECOG 0-1 Secondary Endpoints Objective Response Rate (ORR) Disease Control Rate (DCR) Duration of Response (DoR) Progression Free Survival (PFS) Randomized controlled trial N = 440 2:1 randomization Primary Endpoint Overall Survival (OS) FinalAnalysis FutilityAnalysis

Enabling Q4 2024 Patient Enrollment CRO engaged in site selection and preparation, investigator agreements, etc. Approx. 130 sites Site locations: US, Canada, UK, EU, Latin America 18-24 months estimated time to full enrollment 18 months estimated time to futility analysis Interim analysis for OS following event trigger VERSATILE-003 Trial Implementation
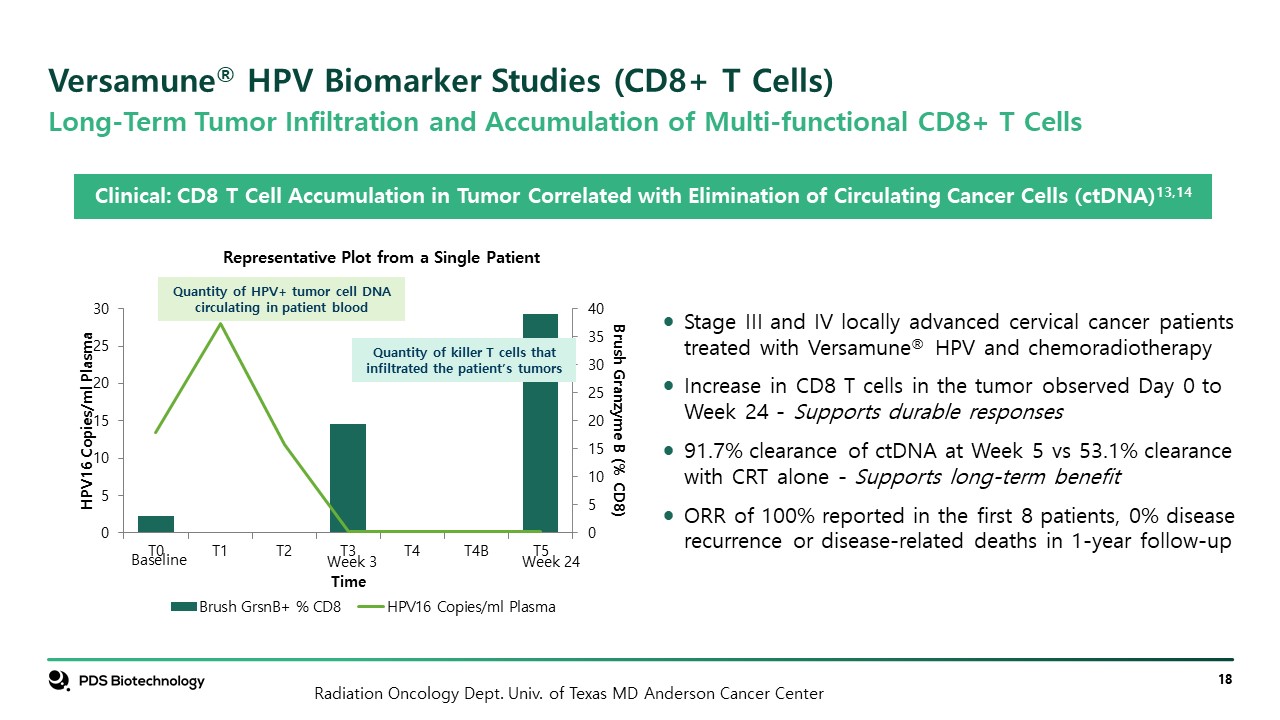
Versamune® HPV Biomarker Studies (CD8+ T Cells) Stage III and IV locally advanced cervical cancer patients treated with Versamune® HPV and chemoradiotherapy Increase in CD8 T cells in the tumor observed Day 0 to Week 24 - Supports durable responses 91.7% clearance of ctDNA at Week 5 vs 53.1% clearance with CRT alone - Supports long-term benefit ORR of 100% reported in the first 8 patients, 0% disease recurrence or disease-related deaths in 1-year follow-up Quantity of killer T cells that infiltrated the patient’s tumors Quantity of HPV+ tumor cell DNA circulating in patient blood Clinical: CD8 T Cell Accumulation in Tumor Correlated with Elimination of Circulating Cancer Cells (ctDNA)13,14 Long-Term Tumor Infiltration and Accumulation of Multi-functional CD8+ T Cells Representative Plot from a Single Patient Week 24 Week 3 Baseline Radiation Oncology Dept. Univ. of Texas MD Anderson Cancer Center
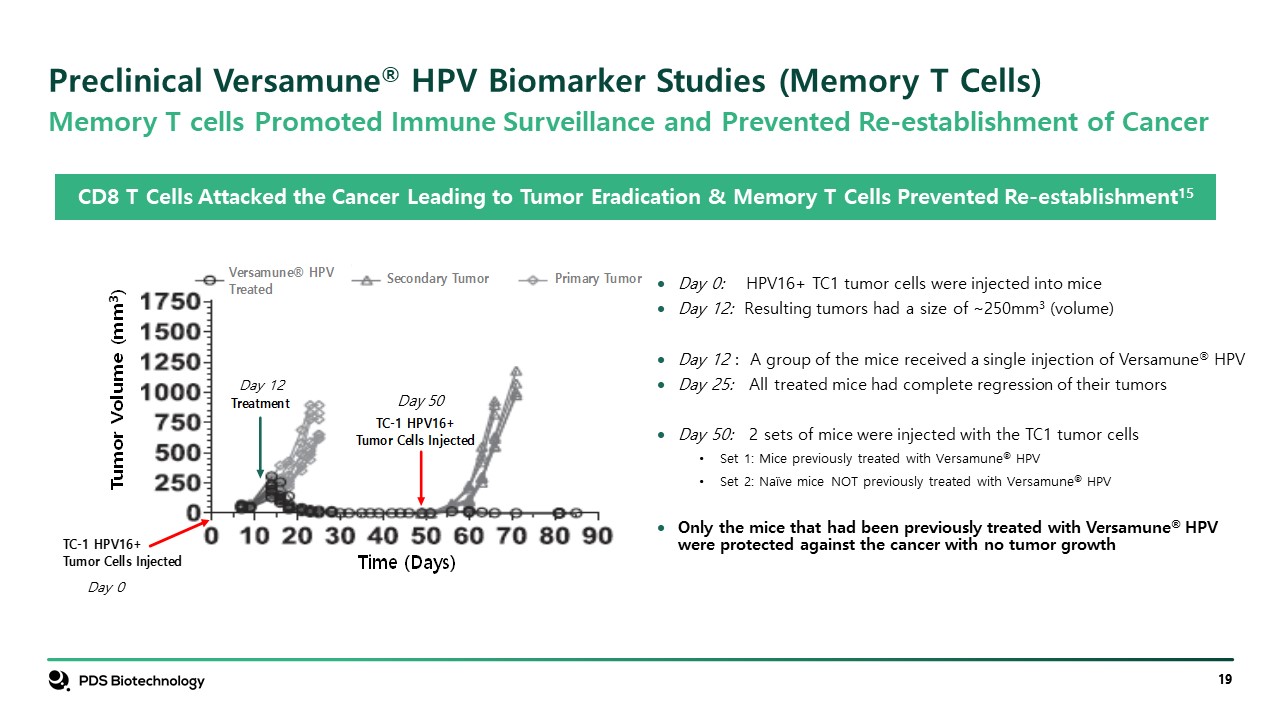
Day 0: HPV16+ TC1 tumor cells were injected into mice Day 12: Resulting tumors had a size of ~250mm3 (volume) Day 12 : A group of the mice received a single injection of Versamune® HPV Day 25: All treated mice had complete regression of their tumors Day 50: 2 sets of mice were injected with the TC1 tumor cells Set 1: Mice previously treated with Versamune® HPV Set 2: Naïve mice NOT previously treated with Versamune® HPV Only the mice that had been previously treated with Versamune® HPV were protected against the cancer with no tumor growth CD8 T Cells Attacked the Cancer Leading to Tumor Eradication & Memory T Cells Prevented Re-establishment15 Memory T cells Promoted Immune Surveillance and Prevented Re-establishment of Cancer Day 0 Day 12 Day 50 Preclinical Versamune® HPV Biomarker Studies (Memory T Cells)
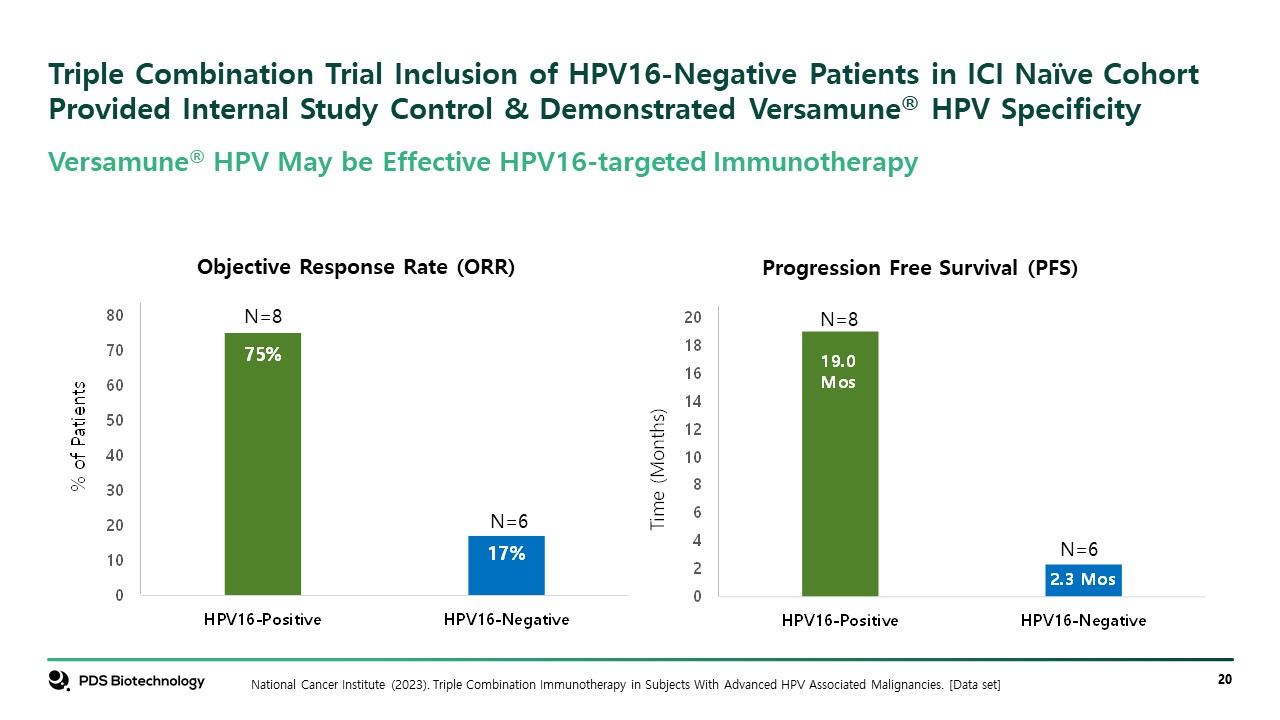
Triple Combination Trial Inclusion of HPV16-Negative Patients in ICI Naïve Cohort Provided Internal Study Control & Demonstrated Versamune® HPV Specificity National Cancer Institute (2023). Triple Combination Immunotherapy in Subjects With Advanced HPV Associated Malignancies. [Data set] Objective Response Rate (ORR) Progression Free Survival (PFS) Versamune® HPV May be Effective HPV16-targeted Immunotherapy N=8 N=8 N=6 N=6
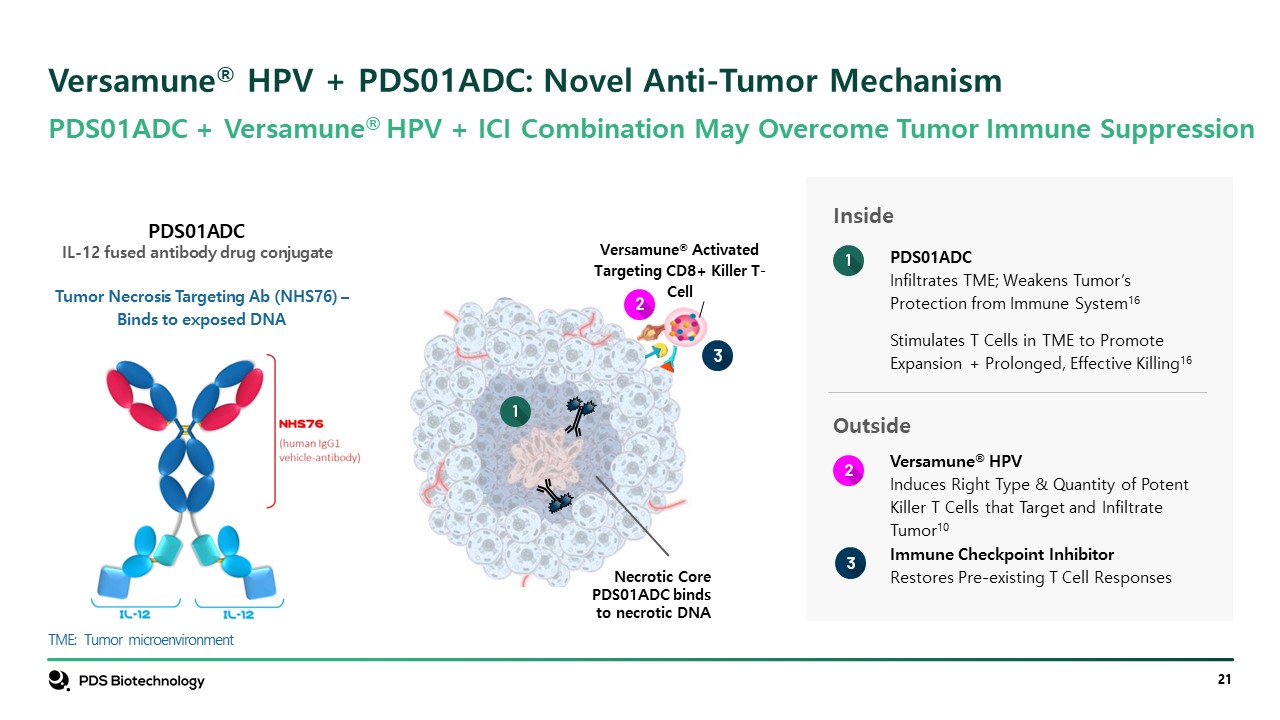
PDS01ADC + Versamune® HPV + ICI Combination May Overcome Tumor Immune Suppression TME: Tumor microenvironment Versamune® HPV + PDS01ADC: Novel Anti-Tumor Mechanism Necrotic Core PDS01ADC binds to necrotic DNA Versamune® Activated Targeting CD8+ Killer T-Cell Inside PDS01ADC Infiltrates TME; Weakens Tumor’s Protection from Immune System16 Stimulates T Cells in TME to Promote Expansion + Prolonged, Effective Killing16 Outside Versamune® HPV Induces Right Type & Quantity of Potent Killer T Cells that Target and Infiltrate Tumor10 Immune Checkpoint Inhibitor Restores Pre-existing T Cell Responses Tumor Necrosis Targeting Ab (NHS76) – Binds to exposed DNA PDS01ADC IL-12 fused antibody drug conjugate
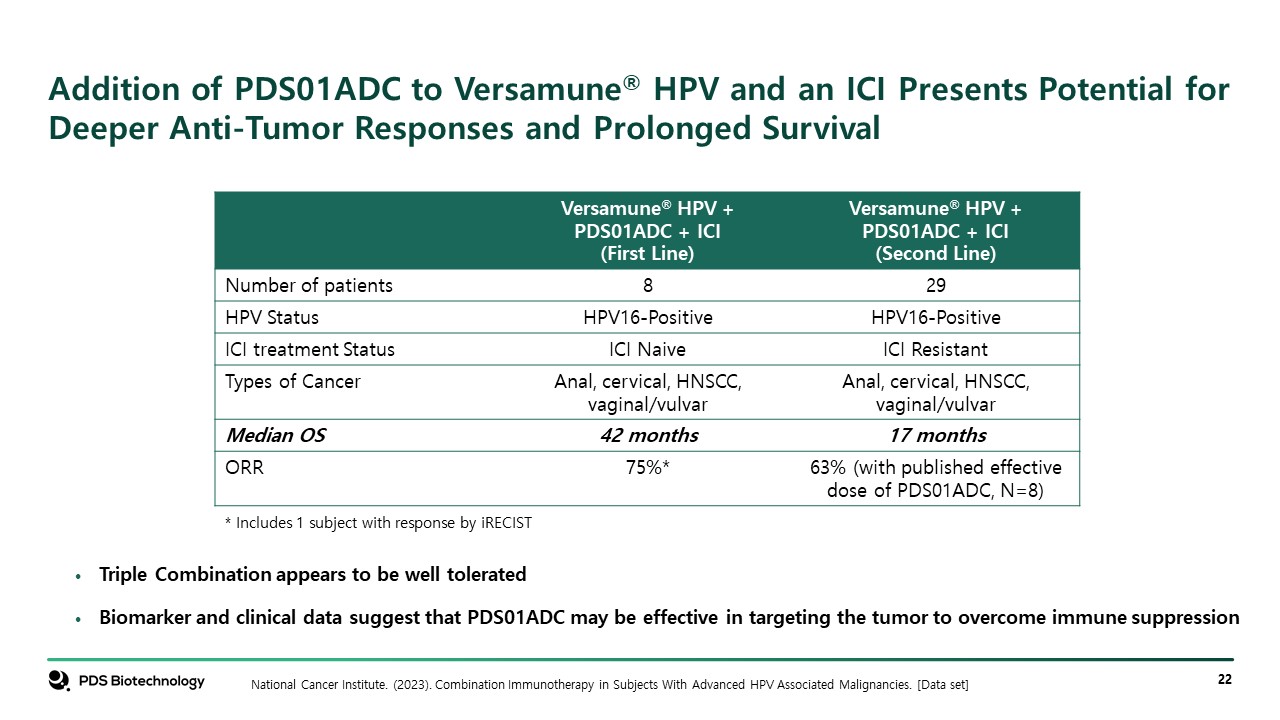
Addition of PDS01ADC to Versamune® HPV and an ICI Presents Potential for Deeper Anti-Tumor Responses and Prolonged Survival Versamune® HPV + PDS01ADC + ICI (First Line) Versamune® HPV + PDS01ADC + ICI (Second Line) Number of patients 8 29 HPV Status HPV16-Positive HPV16-Positive ICI treatment Status ICI Naive ICI Resistant Types of Cancer Anal, cervical, HNSCC, vaginal/vulvar Anal, cervical, HNSCC, vaginal/vulvar Median OS 42 months 17 months ORR 75%* 63% (with published effective dose of PDS01ADC, N=8) Triple Combination appears to be well tolerated Biomarker and clinical data suggest that PDS01ADC may be effective in targeting the tumor to overcome immune suppression National Cancer Institute. (2023). Combination Immunotherapy in Subjects With Advanced HPV Associated Malignancies. [Data set] * Includes 1 subject with response by iRECIST

Upcoming Milestones 2024-2025 Q3 2024 Q4 2024 1H 2025 2H 2025 Regulatory Confirmation of VERSATILE-003 Study Design Initiate VERSATILE-003 Pivotal Study in HNSCC IMMUNOCERV Trial Update in Cervical Cancer File IND for Versamune® MUC1 in MUC1+ Cancers Preliminary data readout: Neoadjuvant Study in Oral Cancer Initiate MUC1 Study Data readouts: Multiple NCI Phase 2 studies of PDS01ADC þ

Candidate/ Study Indication PC P1 P2 P3 Partner Versamune® Versamune® HPV + pembrolizumab Recurrent or metastatic HPV16-positive HNSCC Versamune® HPV + chemo (IMMUNOCERV)* 1st-line treatment of locally advanced(IB3-IVA) cervical cancer Versamune® HPV +/- pembrolizumab* Neo-adjuvant treatment of locally advanced HPV-positive oropharyngeal cancer (OPSCC) Versamune®+PDS01ADC Versamune® HPV + PDS01ADC + ICI* Recurrent or metastatic HPV16-positive HNSCC Versamune® MUC1 + PDS01ADC + ICI (Phase 1/2 anticipated 2024) Recurrent or metastatic MUC1+ cancer Pipeline Continues to Validate Platforms, Drive Future Opportunities Fast Track *Investigator Initiated Trials (IIT)
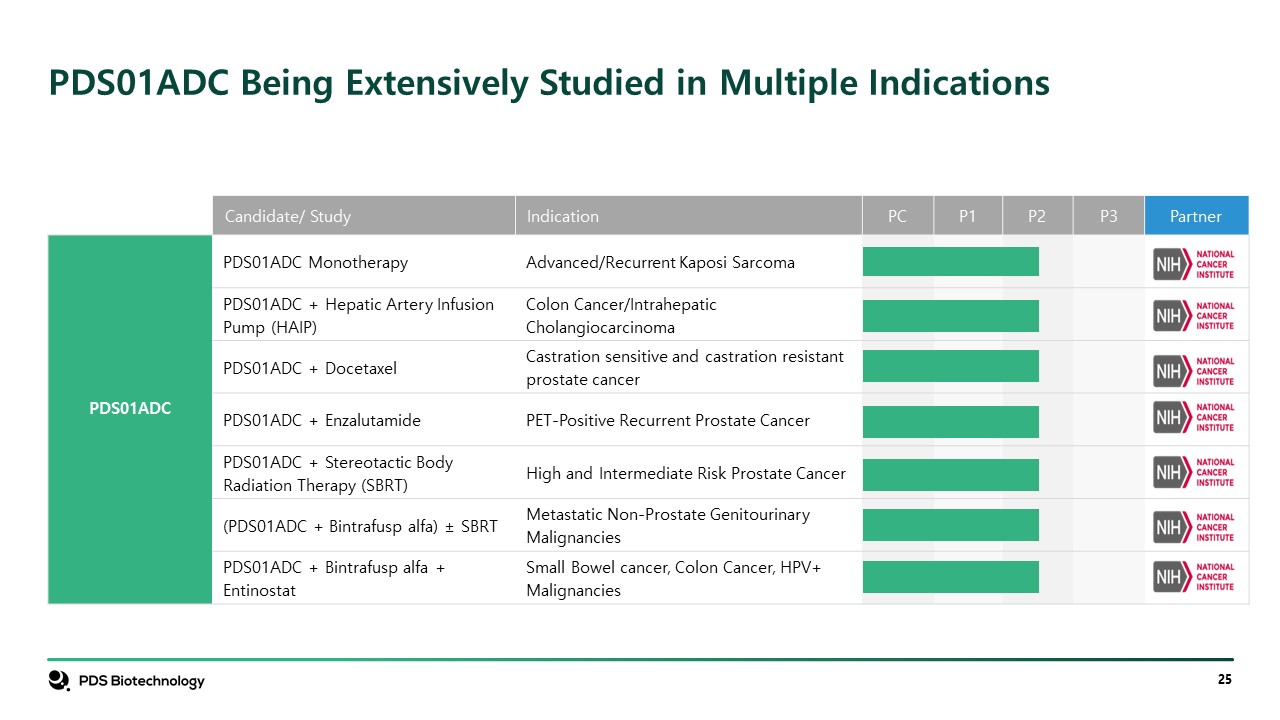
Candidate/ Study Indication PC P1 P2 P3 Partner PDS01ADC PDS01ADC Monotherapy Advanced/Recurrent Kaposi Sarcoma PDS01ADC + Hepatic Artery Infusion Pump (HAIP) Colon Cancer/Intrahepatic Cholangiocarcinoma PDS01ADC + Docetaxel Castration sensitive and castration resistant prostate cancer PDS01ADC + Enzalutamide PET-Positive Recurrent Prostate Cancer PDS01ADC + Stereotactic Body Radiation Therapy (SBRT) High and Intermediate Risk Prostate Cancer (PDS01ADC + Bintrafusp alfa) ± SBRT Metastatic Non-Prostate Genitourinary Malignancies PDS01ADC + Bintrafusp alfa + Entinostat Small Bowel cancer, Colon Cancer, HPV+ Malignancies PDS01ADC Being Extensively Studied in Multiple Indications

Thank You NASDAQ: PDSB

References Damgacioglu H., et al. The Lancet Regional Health – Americas. 2022;8:100143 https://www.cdc.gov/cancer/hpv/oropharyngeal-cancer.html. Accessed August 26, 2024 Lechner M. et al. Nature Reviews Clinical Oncl. 2022, 19, 306-327 CDC.gov accessed January 2022; Saraiya, Mona et al. “US assessment of HPV types in cancers: implications for current and 9-valent HPV vaccines.” Journal of the National Cancer Institute vol. 107,6 djv086. 29 Apr. 2015, doi:10.1093/jnci/djv086; SEER, Accessed February 2024 Isayeva, et al., Human Papillomavirus in Non-Oropharyngeal Head and Neck Cancers: A Systematic Review (2012).; Mazul, A., et al., 2023, Disparities in head and neck cancer incidence and trends by race/ethnicity and sex Market Research 2024; Triangle Research Group Harrington K. et al. J Clin Oncol. 2022 ascopubs.org/journal/jco on October 11, 2022: DOI https://doi.org/10.1200/JCO.21.02508 Market Research 2022; Triangle Research Group Licitra L. et al. 2024, International Journal of Radiation Oncology Volume 118, Issue 5e2-e3April 01 Weiss J, et al. VERSATILE-002: Survival with First-Line Treatment with PDS0101 Therapeutic Vaccine and Pembrolizumab in HPV16-positive Recurrent/Metastatic Head and Neck Squamous Cell Carcinoma (HNSCC). Poster presented at: ESMO 2024; September 13-17, 2024; Barcelona, Spain. Yoshida-Court et al: (2022) IMMUNOCERV an ongoing Phase 2 trial combining PDS0101 an HPV-specific T cell immunotherapy with chemotherapy and radiation for treatment of locally advanced cervical cancer, SITC (NCT04580771) Xiao W et al: (2023) HPV Circulating Cell-Free DNA Kinetics in Cervical Cancer Patients Undergoing Definitive Chemoradiation, ASTRO, 65th Annual Meeting Gandhapudi et al; (2019) J. Immunology, June 15, 202 (12) 3524 CM Minnar et al; (2024) Front. Oncol. 13:1321318.doi: 10.3389/fonc.2023.1321318