UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 20-F
(Mark One)
| ¨ | REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR 12(g) OF THE SECURITIES EXCHANGE ACT OF 1934 |
OR
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2009
OR
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
OR
| ¨ | SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(D) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Date of event requiring this shell company report
Commission file number: 001-34564
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
(Exact Name of Registrant as Specified in Its Charter)
N/A
(Translation of Registrant’s Name Into English)
Cayman Islands
(Jurisdiction of Incorporation or Organization)
No. 18-1 East Nanping Road
Hunnan National New & High-tech Development Zone
Shenyang, Liaoning Province
People’s Republic of China 110171
(Address of Principal Executive Offices)
Tianruo Pu, Chief Financial Officer
No. 18-1 East Nanping Road
Hunnan National New & High-tech Development Zone
Shenyang, Liaoning Province
People’s Republic of China 110171
Tel: +86-024-2469-6033
Fax: +86-024-2469-6133
E-mail: info@lnnk.net
(Name, Telephone, E-mail and/or Facsimile number and Address of Company Contact Person)
Securities registered or to be registered pursuant to Section 12(b) of the Act:
| | |
Title of Each Class | | Name of Each Exchange on Which Registered |
| American Depositary Shares, each representing eight ordinary shares, par value $0.0005 per share | | The NASDAQ Stock Market LLC (The NASDAQ Global Market) |
Securities registered or to be registered pursuant to Section 12(g) of the Act:
None
(Title of Class)
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act:
None
(Title of Class)
Indicate the number of outstanding shares of each of the issuer’s classes of capital or common stock as of the close of the period covered by the annual report.
158,420,142 ordinary shares, par value $0.0005 per share, as of December 31, 2009.
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No x
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934. Yes ¨ No x
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ¨ No ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, or a non-accelerated filer. See definition of “accelerated filer and large accelerated filer” in Rule 12b-2 of the Exchange Act. (Check one):
| | | | | | |
| Large accelerated filer | | ¨ | | Accelerated filer | | ¨ |
| | | |
| Non-accelerated filer | | x | | | | |
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing:
U.S. GAAP x International Financial Reporting Standards as issued by the International Accounting Standards Board ¨ Other ¨
* If “Other” has been checked in response to the previous question, indicate by check mark which financial statement item the registrant has elected to follow.
Item 17 ¨ Item 18 ¨
If this is an annual report, indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x
(APPLICABLE ONLY TO ISSUERS INVOLVED IN BANKRUPTCY PROCEEDINGS DURING THE PAST FIVE YEARS)
Indicate by check mark whether the registrant has filed all documents and reports required to be filed by Sections 12, 13 or 15(d) of the Securities Exchange Act of 1934 subsequent to the distribution of securities under a plan confirmed by a court. Yes ¨ No ¨
TABLE OF CONTENTS
2
INTRODUCTION
Unless the context otherwise requires, in this annual report on Form 20-F:
| | • | | “we,” “us,” “our company,” and “our” refer to China Nuokang Bio-Pharmaceutical Inc., its predecessor entities, subsidiaries and consolidated entities; |
| | • | | “China” or “PRC” refers to the People’s Republic of China, excluding Taiwan, Hong Kong and Macau; |
| | • | | “ADSs” refers to American Depositary Shares, each representing eight of our ordinary shares; |
| | • | | “Renminbi” or “RMB” refers to the legal currency of China; and |
| | • | | “$” or “U.S. dollars” refers to the legal currency of the United States. |
Names of certain companies provided in this annual report are translated or transliterated from their original Chinese legal names.
Discrepancies in any table between the amounts identified as total amounts and the sum of the amounts listed therein are due to rounding.
This annual report on Form 20-F includes our audited consolidated financial statements for the years ended December 31, 2007, 2008 and 2009.
We and certain selling shareholders of our company completed the initial public offering of 5,000,000 ADSs on December 15, 2009. On December 9, 2009, we listed our ADSs on the NASDAQ Global Market under the symbol “NKBP.”
3
FORWARD-LOOKING STATEMENTS
This annual report on Form 20-F contains forward-looking statements that involve risks and uncertainties. All statements other than statements of historical facts are forward-looking statements. These statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements to be materially different from those expressed or implied by the forward-looking statements.
The words “may,” “will,” “expect,” “anticipate,” “aim,” “estimate,” “intend,” “plan,” “believe,” “potential,” “continue,” “is/are likely to” and other similar expressions may identify forward-looking statements, but the absence of these words does not necessarily mean that a statement is not forward-looking. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our financial condition, results of operations, business strategy and financial needs. These forward-looking statements include, among other things, statements about:
| | • | | competition from other domestic and foreign pharmaceutical companies; |
| | • | | our ability to enhance existing products and develop, obtain government approvals for, and market future generations of our existing products and other new products; |
| | • | | the expected market growth for pharmaceutical products in China; |
| | • | | market acceptance of our products; |
| | • | | our expectations regarding hospital or patient demand for our products; |
| | • | | our ability to expand our production, sales and distribution network and other aspects of our operations; |
| | • | | our ability to diversify our product range; |
| | • | | our ability to effectively protect our intellectual property; |
| | • | | our ability to identify and acquire new medical technologies, pharmaceutical products and product candidates; |
| | • | | changes in the healthcare industry in China, including changes in the healthcare policies and regulations of the PRC government and changes in the healthcare insurance sector in the PRC; |
| | • | | fluctuations in general economic and business conditions in China; and |
| | • | | our ability to control Liaoning Nuokang Medicines Co., Ltd., or Nuokang Distribution, our variable interest entity, through contractual arrangements. |
You should thoroughly read this annual report and the documents that we refer to with the understanding that our actual future results may be materially different from and worse than what we expect. We qualify all of our forward-looking statements by these cautionary statements. We would like to caution you not to place undue reliance on forward-looking statements and you should read these statements in conjunction with the risk factors disclosed in “Item 3.D. Key Information — Risk Factors” of this annual report. Those risks are not exhaustive. We operate in an emerging and evolving environment. New risk factors emerge from time to time and it is impossible for our management to predict all risk factors, and we cannot assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause our actual results to materially differ from results contained in any forward-looking statement.
You should not rely upon forward-looking statements as predictions of future events. We undertake no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as required under applicable law.
4
PART I
| Item 1. | IDENTITYOF DIRECTORS, SENIOR MANAGEMENTAND ADVISERS |
Not Applicable.
| Item 2. | OFFER STATISTICSAND EXPECTED TIMETABLE |
Not Applicable.
A. Selected Financial Data
We have derived our consolidated statement of income data for the years ended December 31, 2007, 2008 and 2009 and our consolidated balance sheet data as of December 31, 2008 and 2009 from our audited consolidated financial statements included in this annual report. Our selected consolidated statement of income data for the year ended December 31, 2005 and 2006 and our consolidated balance sheet data as of December 31, 2005, 2006 and 2007 have been derived from our audited consolidated financial statements not included in this annual report. Our historical operating results presented below are not necessarily indicative of the results that may be expected for the fiscal year ending December 31, 2010 or any other future fiscal period. Our financial statements have been prepared in accordance with the accounting principles generally accepted in the United States, or U.S. GAAP. You should read the selected consolidated financial data set forth below in conjunction with “Item 5. Operating and Financial Review and Prospects – A. Operating Results” and with our consolidated financial statements and related notes included in this annual report.
| | | | | | | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2005 | | | 2006 | | | 2007 | | | 2008 | | | 2009 | |
| | | RMB | | | RMB | | | RMB | | | RMB | | | RMB | | | $ | |
| | | (in thousands, except share and per share data and percentages) | |
Consolidated Statement of Income Data: | | | | | | | | | | | | | | | | | | |
Net revenue | | 129,422 | | | 121,661 | | | 147,875 | | | 225,399 | | | 282,911 | | | 41,447 | |
Cost of revenue | | (52,826 | ) | | (44,083 | ) | | (34,745 | ) | | (30,963 | ) | | (35,625 | ) | | (5,219 | ) |
| | | | | | | | | | | | | | | | | | |
Gross profit | | 76,596 | | | 77,578 | | | 113,130 | | | 194,436 | | | 247,286 | | | 36,228 | |
Operating expenses: | | | | | | | | | | | | | | | | | | |
Research and development costs | | (6,422 | ) | | (6,054 | ) | | (7,995 | ) | | (5,585 | ) | | (8,297 | ) | | (1,216 | ) |
Sales, marketing and distribution expenses | | (22,427 | ) | | (23,173 | ) | | (52,443 | ) | | (92,404 | ) | | (110,513 | ) | | (16,190 | ) |
General and administrative expenses | | (17,275 | ) | | (14,809 | ) | | (16,796 | ) | | (31,884 | ) | | (47,582 | ) | | (6,971 | ) |
| | | | | | | | | | | | | | | | | | |
Total operating expenses | | (46,124 | ) | | (44,036 | ) | | (77,234 | ) | | (129,873 | ) | | (166,392 | ) | | (24,377 | ) |
| | | | | | | | | | | | | | | | | | |
Operating profit | | 30,472 | | | 33,542 | | | 35,896 | | | 64,563 | | | 80,894 | | | 11,851 | |
Interest income | | 44 | | | 47 | | | 196 | | | 1,259 | | | 1,372 | | | 201 | |
Interest expense | | (1,248 | ) | | (1,672 | ) | | (5,190 | ) | | (1,309 | ) | | (3,327 | ) | | (487 | ) |
Other (expenses) income, net | | (346 | ) | | (344 | ) | | 2,191 | | | 6,353 | | | 3,014 | | | 441 | |
| | | | | | | | | | | | | | | | | | |
Income before income tax expense and non-controlling interest | | 28,922 | | | 31,573 | | | 33,093 | | | 70,866 | | | 81,953 | | | 12,006 | |
5
| | | | | | | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2005 | | | 2006 | | | 2007 | | | 2008 | | | 2009 | |
| | | RMB | | | RMB | | | RMB | | | RMB | | | RMB | | | $ | |
| | | (in thousands, except share and per share data and percentages) | |
Income tax (expense) benefit | | (7,042 | ) | | (6,181 | ) | | 2,102 | | | (7,246 | ) | | (16,858 | ) | | (2,470 | ) |
Non-controlling interest | | (3,359 | ) | | 912 | | | — | | | — | | | — | | | — | |
| | | | | | | | | | | | | | | | | | |
Net income | | 18,521 | | | 26,304 | | | 35,195 | | | 63,620 | | | 65,095 | | | 9,536 | |
Accretion of Series A convertible redeemable preference shares | | — | | | — | | | (30,156 | ) | | (13,403 | ) | | (13,886 | ) | | (2,034 | ) |
Allocation to Series A convertible redeemable preference shares for participating rights to dividends | | — | | | — | | | (326 | ) | | (9,458 | ) | | — | | | — | |
| | | | | | | | | | | | | | | | | | |
Net income attributed to ordinary shares | | 18,521 | | | 26,304 | | | 4,713 | | | 40,759 | | | 51,209 | | | 7,502 | |
| | | | | | | | | | | | | | | | | | |
Net income per share | | | | | | | | | | | | | | | | | | |
Basic | | 0.19 | | | 0.26 | | | 0.05 | | | 0.43 | | | 0.52 | | | 0.08 | |
Diluted | | 0.19 | | | 0.26 | | | 0.05 | | | 0.43 | | | 0.51 | | | 0.08 | |
Shares used in net income per share computation | | | | | | | | | | | | | | | | | | |
Basic | | 100,000,000 | | | 100,000,000 | | | 99,850,334 | | | 95,447,648 | | | 98,885,045 | | | 98,885,045 | |
Diluted | | 100,000,000 | | | 100,000,000 | | | 99,850,334 | | | 95,460,841 | | | 99,725,253 | | | 99,725,253 | |
Pro forma net income per share (unaudited) | | | | | | | | | | | | | | | | | | |
Basic | | | | | | | | 0.28 | | | 0.52 | | | | | | | |
Diluted | | | | | | | | 0.28 | | | 0.52 | | | | | | | |
Shares used in pro forma net income per share computation (unaudited) | | | | | | | | | | | | | | | | | | |
Basic | | | | | | | | 125,646,996 | | | 121,244,310 | | | | | | | |
Diluted | | | | | | | | 125,646,996 | | | 121,257,503 | | | | | | | |
6
| | | | | | | | | | | | |
| | | As of December 31, |
| | | 2005 | | 2006 | | 2007 | | 2008 | | 2009 |
| | | RMB | | RMB | | RMB | | RMB | | RMB | | $ |
| | | (in thousands) |
Consolidated Balance Sheet Data: | | | | | | | | | | | | |
Cash and cash equivalents | | 18,197 | | 2,395 | | 112,589 | | 81,837 | | 351,258 | | 51,460 |
Restricted cash | | — | | 2,400 | | — | | 20,000 | | 34,200 | | 5,010 |
Accounts receivable | | 12,482 | | 14,569 | | 30,417 | | 71,312 | | 103,719 | | 15,195 |
Inventories | | 26,212 | | 21,585 | | 7,034 | | 10,610 | | 14,765 | | 2,163 |
Property, plant and equipment | | 27,294 | | 53,614 | | 119,029 | | 149,679 | | 163,915 | | 24,014 |
Total assets | | 112,783 | | 152,704 | | 320,724 | | 405,031 | | 781,454 | | 114,484 |
Short-term bank loans | | 30,580 | | 18,380 | | 50,380 | | 72,430 | | 125,618 | | 18,403 |
Total current liabilities | | 77,378 | | 78,210 | | 95,293 | | 119,760 | | 167,246 | | 24,502 |
Total shareholders’ equity | | 28,483 | | 53,872 | | 77,157 | | 127,721 | | 580,384 | | 85,027 |
Total liabilities and shareholders’ equity | | 112,783 | | 152,704 | | 320,724 | | 405,031 | | 781,454 | | 114,484 |
Exchange Rate Information
Our business is primarily conducted in China and all of our revenues are denominated in RMB. This annual report contains translations of RMB amounts into U.S. dollars at specific rates solely for the convenience of the readers. Unless otherwise noted, all translations from RMB to U.S. dollars in this annual report were made at a rate of RMB6.8259 to US$1.00, the noon buying rate in effect as of December 31, 2009 in The City of New York for cable transfers of RMB as certified for customs purposes by the Federal Reserve Bank of New York. We make no representation that any RMB or U.S. dollar amounts could have been, or could be, converted into U.S. dollars or RMB, as the case may be, at any particular rate, or at all. The PRC government imposes control over its foreign currency reserves in part through direct regulation of the conversion of RMB into foreign exchange and through restrictions on foreign trade. On May 28, 2010, the noon buying rate was RMB6.8305 to US$1.00.
The following table sets forth information concerning exchange rates between the RMB and the U.S. dollar for the periods indicated. These rates are provided solely for your convenience and are not necessarily the exchange rates that we used in this annual report or will use in the preparation of our future periodic reports or any other information to be provided to you. The source of these rates is the Federal Reserve Bank of New York.
7
| | | | | | | | |
| | | Noon Buying Rate
(RMB per US$1.00) |
Period | | Period
End | | Average(1) | | Low | | High |
2005 | | 8.0702 | | 8.1826 | | 8.2765 | | 8.0702 |
2006 | | 7.8041 | | 7.9579 | | 8.0702 | | 7.8041 |
2007 | | 7.2946 | | 7.5806 | | 7.8127 | | 7.2946 |
2008 | | 6.8225 | | 6.9193 | | 7.2946 | | 6.7800 |
2009 | | 6.8259 | | 6.8295 | | 6.8470 | | 6.8176 |
November | | 6.8265 | | 6.8271 | | 6.8300 | | 6.8255 |
December | | 6.8259 | | 6.8275 | | 6.8299 | | 6.8244 |
2010 | | | | | | | | |
January | | 6.8268 | | 6.8269 | | 6.8295 | | 6.8258 |
February | | 6.8258 | | 6.8285 | | 6.8330 | | 6.8258 |
March | | 6.8258 | | 6.8262 | | 6.8270 | | 6.8254 |
April | | 6.8247 | | 6.8256 | | 6.8275 | | 6.8229 |
May (through May 28, 2010) | | 6.8305 | | 6.8275 | | 6.8310 | | 6.8245 |
| (1) | Annual averages are calculated using the average of month-end rates of the relevant year. Monthly averages are calculated using the average of the daily rates during the relevant period. |
B. Capitalization and Indebtedness
Not Applicable.
C. Reasons for the Offer and Use of Proceeds
Not Applicable.
D. Risk Factors
Risks Related to Our Business
We are dependent and expect to continue to be dependent on the revenue contribution from our flagship product, Baquting. A reduction in Baquting sales would cause our revenues to decline and materially harm our business.
We are largely dependent on sales of our hemocoagulase product, which we market under the name of Baquting. We launched Baquting in November 2001. In 2007, 2008 and 2009, we sold 6.3 million, 9.7 million and 13.1 million units of Baquting, respectively. Revenues from Baquting sales accounted for 91.5%, 90.1% and 93.5% of our total revenues in 2007, 2008 and 2009, respectively. We plan to dedicate additional resources toward maintaining and expanding Baquting’s market share, such as for improved quality control, production technology and formulation and dosage upgrades and modifications. Sales of Baquting will continue to comprise a substantial portion of our revenues in the future.
Any reduction in revenues from Baquting will have a direct negative impact on our business, financial condition and results of operations. Baquting sales could be adversely affected by a variety of factors, including:
| | • | | new product introductions to the market; |
8
| | • | | government-imposed pricing constraints; |
| | • | | problems with raw material supplies; |
| | • | | disruptions in manufacturing or distribution; |
| | • | | newly discovered safety issues arising from misuse of or adverse reactions from our products; and |
| | • | | intellectual property issues. |
Because of our relative lack of product diversification, an investment in our company may entail more risk than investments in companies that offer a wider variety of products or services. Despite our efforts, we may be unable to develop or acquire new products that would enable us to diversify our business and reduce our dependence on Baquting.
The commercial success of our products depends upon the degree of their market acceptance by the medical community. If our products do not attain market acceptance by the medical community, our operations and profitability would be adversely affected.
The commercial success of our products depends upon the degree of market acceptance they receive from the medical community, particularly physicians and hospital administrators. Physicians may not prescribe or recommend our products to patients and hospital procurement departments may not purchase our products if physicians or hospital pharmacists do not find our products attractive. The acceptance and use of our products by the medical community depend upon a number of factors, including:
| | • | | perceptions of physicians, patients and other members of the medical community regarding the safety and effectiveness of our products; |
| | • | | the prevalence and severity of any side effects; |
| | • | | the efficacy, pharmacological benefit and potential advantages of our products relative to competing products and products under development; |
| | • | | the relative convenience and ease of administration of our products; |
| | • | | effectiveness of our and our distributors’ education, marketing and distribution efforts; |
| | • | | publicity concerning our products or competing products; and |
| | • | | the price for our products and competing products. |
In addition, the continued success of Baquting depends in part upon perceptions of blood transfusion risk and blood supply quality in China. If our products fail to gain market acceptance by the medical community, or if our currently marketed products cannot maintain market acceptance, our results of operations and profitability would be adversely affected.
9
We may not be able to obtain manufacturing or marketing approvals for our products, including Aiduo, Aiwen and Kaitong, or pass on-site inspections of our production facilities. Failure to obtain approvals for our products or pass on-site inspections of our production facilities could materially harm our operations and business prospects.
All medicines must be approved by the PRC State Food and Drug Administration, or the SFDA, before they can be manufactured, marketed or sold in the PRC. The SFDA requires a pharmaceutical manufacturer to successfully complete clinical trials of a new medicine and demonstrate its manufacturing capability before granting manufacturing approval for the new medicine. Clinical trials are expensive and their results are uncertain. In addition, the SFDA and other regulatory authorities may apply new standards for safety, manufacturing, labeling, marketing and distribution of future products. Complying with these standards may be time-consuming and expensive. Furthermore, our future products may not be efficacious or may have undesirable or unintended side effects, toxicities or other characteristics that may preclude us from obtaining approval or may prevent or limit their commercial use. As a result, we may not be able to obtain SFDA or other governmental approvals for our future products on a timely basis or at all.
In particular, Jilin Yuhua Pharmaceutical Co., Ltd., or Jilin Yuhua, our manufacturer and distributor for Kaitong, requires a permit for its production facility to produce Kaitong. In March 2010, Jilin Yuhua received a request from the SFDA for supplementary chemical manufacturing control data on Kaitong and was informed that SFDA review of Kaitong will take longer than initially anticipated due to additional review time of this supplemental data. If the production permit for Kaitong is delayed or not granted at all, Jilin Yuhua will be unable to commence its production and we will be unable to launch Kaitong in the market. In addition, we submitted our bioequivalence clinical trial data for dipyridamole aspirin sustained release capsules to the SFDA in December 2007, and we received an SFDA request in June 2009 for supplementary research data. We are in process of compiling this data and expect to receive SFDA approval for this product in 2010. However, we or Jilin Yuhua may not be able to obtain approvals for these products on a timely basis, or at all. Even if we or Jilin Yuhua do obtain these approvals, such approvals may be modified or revoked or subject to limitations on the indicated uses for which we may market the product, which may limit the size of its market. We will not be able to manufacture and market our new products or at all if we do not obtain require governmental approvals.
Furthermore, even after we obtain such approvals, we may not be able to pass the on-site inspections of our facilities required prior to the launch of our products. For example, in 2004, we entered into agreements for the acquisition of Aiduo and Aiwen from Shenyang Guangda Pharmaceutical Co., Ltd., or Shenyang Guangda, and Shenyang Wanjia Biotechnology Research Institute, or Shenyang Wanjia. We require approval from the SFDA for the transfer of the production permit for Aiduo and Aiwen from Shenyang Guangda to us and Good Manufacturing Practice, or GMP, certification for our Shenyang facility in order to complete the acquisition of Aiduo and Aiwen from Shenyang Guangda and to commence production of these products at our production facility. If we fail to pass the on-site inspection in connection with our production permit application, we will not be able to obtain the production permit and commence production. If we fail to pass the on-site inspection in connection with GMP certification, we will not be able to obtain a GMP certificate, which is required for pharmaceutical production and sale. GMP certificates are subject to review and renewal every five years. See “Item 4. Information on the Company – B. Business Overview — Certificates and Permit” for a description of our current licenses and permits. Failure to obtain or renew approvals for our existing or future products or pass on-site inspections of our facilities could materially harm our business and operations.
10
Selling prices of our products may decline over time. Our success depends on our ability to successfully develop and commercialize biopharmaceutical products. Our product development efforts may not result in commercially viable products.
As is typical in the Chinese pharmaceutical industry, the average selling prices of biopharmaceutical products may decline significantly over the life of the product. These declines principally result from increased competition and price controls. Historically, the average selling prices of our products have not declined significantly. However, there may be a material decline of selling prices in the future. We have sought to mitigate downward pricing pressure by introducing new products or enhanced versions of existing products with higher margins. We must therefore constantly identify product candidates that can be developed into cost-effective therapeutic products. We have devoted substantial resources to our research and development efforts; however, successful product development in our industry is highly uncertain, and relatively few research and development projects produce commercially viable products. If we cannot offset declines in revenues from and margins of our existing products with new products, our overall results of operations will suffer.
Our products face substantial competition. Our competitors may discover, develop, acquire or commercialize products earlier or more successfully than we do.
We operate in a highly competitive environment and our products compete with other products or treatments. Baquting competes with existing hemocoagulase products and potential new drug candidates. In China, our major competitors in the hemocoagulase market include Aohong Pharma’s Bangting, Solco Basel’s Reptilase and Lee’s Pharm’s Sulejuan, which in 2009 held approximately 28%, 23% and 12% market share, by volume, respectively. Our products may compete against products with lower prices, superior performance, greater ease of administration or other advantages compared to our products. We do not have patents of any commercial significance for many of our products and product candidates to protect these products and product candidates from direct competition. Our inability to compete effectively could reduce sales or margins, which could materially and adversely affect our results of operations.
Certain of our competitors market products or are actively engaged in research and development in areas where we have products or where we are developing product candidates or new indications, or usage purposes, for existing products. Our products compete with new drugs in development, drugs that may be approved for the same indications as our products and drugs approved for other indications that are used off-label. Additionally, an increasing number of foreign pharmaceutical companies have introduced their pharmaceutical products into the Chinese market. If less expensive alternatives to our products are dispensed or prescribed to patients, our sales may be adversely impacted.
Large Chinese state-owned and privately owned pharmaceutical companies and foreign-invested or foreign pharmaceutical companies may have greater clinical, research, regulatory, manufacturing, marketing, financial and human resources than we do. In addition, certain of our competitors may have technical or competitive advantages over us with respect to the development of technologies and processes. These resources may make it difficult for us to compete with them to successfully discover, develop and market new products and for our current products to compete with new products or indications that these competitors may bring to market. Our competitors in the pharmaceutical industry may also consolidate and acquire market share.
11
Furthermore, in order to gain market share in China, competitors may significantly increase their advertising expenditures and promotional activities or engage in irrational or predatory pricing behavior. In addition, our competitors may engage in inappropriate competitive practices or illegal acts, such as bribery. Third parties may actively engage in activities designed to undermine our brand name and product quality or to influence customer confidence in our products. Increased competition may result in price reductions, reduced margins and loss of market share, any of which could materially adversely affect our profit margins. We may not be able to compete effectively against current and future competitors, which may harm our business and operations.
Our business depends on our well-known brands such as Nuokang and Baquting, and if we are not able to maintain and enhance our brand recognition to maintain our competitive advantage, our reputation, business and operating results may be harmed.
We believe that market awareness of our Nuokang and Baquting brands has contributed significantly to our success, and that maintaining and enhancing these brands is critical to maintaining our competitive advantage. We may not be successful in promoting our brands. If we are unable to enhance our brand recognition and increase awareness of our products, or if we incur excessive expenses to maintain our brand awareness, our business and results of operations may be materially and adversely affected. Furthermore, our sales and results of operations could be adversely affected if our brands or reputation are impaired by actions taken by our distributors, competitors, third-party marketing firms or relevant regulatory authorities.
Pricing of our principal products is subject to government approval. Changes in government control on prices of our products may limit our profitability or cause us to stop manufacturing certain products.
The prices of pharmaceutical products listed in the PRC national medical insurance catalog and certain other medicines are subject to the regulation of the PRC National Development and Reform Commission, or NDRC, and relevant authorized provincial or local price control authorities, in the form of fixed prices or price ceilings. From time to time, the NDRC publishes a list of medicines subject to price controls. Our principal product, Baquting, and the product candidate under our exclusive distributorship, Kaitong, are subject to price ceilings set by the NDRC. The limitation on our ability to raise the wholesale prices of our products may prevent us from absorbing or offsetting any increase in the costs of producing our products, which would lower our margins. Additionally, we are required to file the prices of Aiduo, Aiwen and our other products with provincial price control authorities. In aggregate, seven out of 14 of our products, sales of which accounted for 97.6%, 94.2% and 96.5% of our total sales in 2007, 2008 and 2009, respectively, are subject to price controls imposed by the NDRC and provincial price control authorities. Furthermore, the prices of our products may be adjusted downward by the relevant governmental authorities in the future. In May 2007, the NDRC lowered the price ceilings of 260 medicines in China in response to a rapid increase in the medicine prices, resulting in an average reduction of 19% in retail prices of medicines affected. As a result, the maximum retail price at which hemocoagulase products such as Baquting may be sold to patients was reduced from RMB56.7 per unit to RMB51.6 per unit in May 2007. If we are required to lower wholesale prices of our principal products in the future as a result of any government-mandated reduction in the price ceilings, our future results of operation and profitability would be adversely affected. In addition, in order to access certain local or provincial-level markets, we are required to enter into competitive bidding processes with a pre-defined price range for Baquting, Aiduo, Aiwen and our other products every one or two years. The competitive bidding effectively sets price ceilings for our products, thereby limiting our profitability. We may stop manufacturing of certain products of its price range designated by the local or provincial government falls below production costs.
12
Reimbursement may not be available for our products, which could diminish our sales or affect our ability to sell our products profitably.
Market acceptance and sales of our products depend to a large extent on the reimbursement policies of the PRC government. The PRC Ministry of Labor and Social Security or provincial or local labor and social security authorities, together with other government authorities, review the inclusion or removal of drugs from the national medical insurance catalog or provincial or local medical insurance catalogs for the National Medical Insurance Program every two years, and the tier under which a drug will be classified, both of which affect the amounts reimbursable to program participants for their purchases of those medicines. These determinations are made based on a number of factors, including price and efficacy of the drug. Depending on the tier under which a drug is classified in the provincial medicine catalog, a National Medical Insurance Program participant residing in that province can be reimbursed for the full cost of Tier 1 medicine and for 80% to 90% of the cost of Tier 2 medicine. Our principal product, Baquting, and the product candidate under our exclusive distributorship, Kaitong, are currently included in the national medical insurance catalog as Tier 2 medicines. Aiduo and Aiwen are Tier 2 medicines in the Zhejiang, Hubei, Gansu, Hunan and Ningxia provincial medical insurance catalogs and the Changchun municipal medicine catalog. If our products are removed from medical insurance catalogs by relevant government authorities, sales of these products and our revenues in turn may be materially adversely affected. Furthermore, if our new products are not included in national, provincial or local medical insurance catalogs, sales of these products may be materially adversely affected.
The growth and success of our business depends on our ability to successfully market our principal products to hospitals and their selection in tender processes used by hospitals for medicine purchases.
Our future growth and success significantly depend on our ability to successfully market Baquting, Aiduo and Aiwen to hospitals as prescription medicines. In 2007, 2008 and 2009, substantially all of the end-customers of Baquting, Aiduo and Aiwen were hospitals. Hospitals may make bulk purchases of a medicine included in the national and provincial medicine catalogs only if that medicine is selected under a government-administered tender process. Every one or two years, bidding is organized on a provincial or municipal basis. A medicine manufacturer is invited to participate in the tender based on the level of interest that hospitals have in purchasing the medicine. A hospital’s interest in a medicine is evidenced by:
| | • | | the inclusion of the medicine on the hospital’s formulary, which lists medicines its physicians may prescribe; and |
| | • | | the willingness of physicians at this hospital to prescribe this medicine to their patients. |
13
We believe that effective marketing efforts are critical to maintaining hospital interest in Baquting, Aiduo and Aiwen. If our marketing efforts are ineffective, hospital administrators may not want to include Baquting, Aiduo and Aiwen in their formularies and physicians may not be interested in prescribing Baquting, Aiduo or Aiwen to their patients, which decreases our chances of being invited to and succeeding at tenders. As a result, we may find it difficult to maintain the existing level of sales of our products, and our revenues and profitability may decline.
Rapid changes in the pharmaceutical industry may render our products obsolete.
The pharmaceutical industry is characterized by rapid changes in technology, constant enhancement of industrial know-how and frequent emergence of new products. Future technological improvements and continual product developments in the pharmaceutical market may render our existing products obsolete or affect our viability and competitiveness. Therefore, our future success will largely depend on our ability to:
| | • | | improve our existing products; |
| | • | | diversify our product portfolio; and |
| | • | | develop new and competitively priced products which meet the requirements of the constantly changing market. |
If we fail to respond to this environment by improving our existing products or developing new products in a timely fashion, or if our new or improved products do not achieve adequate market acceptance, our business and profitability may be materially and adversely affected.
The recent global economic and financial market crisis has had and could continue to have a material adverse effect on our business, financial condition, results of operations and cash flow.
The recent global economic and financial market crisis has caused, among other things, a general tightening in the credit markets, lower levels of liquidity, increases in the rates of default and bankruptcy, lower consumer and business spending, and lower consumer net worth, in the United States and other parts of the world. This global economic and financial market crisis may impair our ability to raise funds through capital market transactions or enter into other financial arrangements if and when additional funds become necessary for our operations. The recent economic and financial market crisis has caused, among other things, lower customer spending across China. People may have diminished financial resources to purchase even critical medical care, which could lead to reduced demand for our products and reduced gross margins. In addition, some of our distributors and their customers, primarily hospitals and other healthcare institutions, have been adversely affected by the recent economic crisis, resulting in delays in payment to us or defaults of payments by our distributor customers. Our accounts receivable net of allowance for doubtful accounts increased from RMB71.3 million as of December 31, 2008 to RMB103.7 million ($15.2 million) as of December 31, 2009 and our receivable turnover days increased from 89 days in the year ended December 31, 2008 to 112 days in the year ended December 31, 2009. Our accounts receivable may also continue to increase in the near future. Therefore, the global economic and financial market crisis has had and could continue to have a material adverse effect on our business, financial condition, results of operations and cash flow. In addition, the timing and nature of any recovery in the economic and financial markets remains uncertain, and there can be no assurance that market conditions will improve in the near future or that our results will not continue to be materially and adversely affected.
14
Our limited operating history may not serve as an adequate basis to judge our future prospects and results of operations.
We commenced operations in 1997 and have gradually built up our research, development, manufacturing, sales and marketing capabilities. We have a limited operating history under our current business model upon which you can evaluate the viability and sustainability of our business. Accordingly, you should consider our future prospects in light of the risks and uncertainties experienced by other China-based early stage pharmaceutical companies. If we are unsuccessful in addressing any of these risks and uncertainties, our business, financial condition, results of operations and future growth would be adversely affected.
We may not achieve our projected development goals in the time frames we announce and expect.
We set goals for, and made disclosures in this annual report regarding, timing of the accomplishment of objectives material to our success, such as the commencement and completion of pre-clinical tests, clinical trials, anticipated regulatory submission and approval dates and timing of product launches. As a public U.S.-listed company, we anticipate that we will make additional announcements in our public reports and in press releases regarding these events. The actual timing of these events can vary dramatically due to factors beyond our control, such as delays or failures in our pre-clinical tests or clinical trials, the uncertainties inherent in the regulatory approval process and delays in achieving manufacturing or marketing arrangements sufficient to commercialize our products. There can be no assurance that our pre-clinical tests or clinical trials will be completed as planned or at all, that we will make regulatory submissions or receive regulatory approvals as planned or that we will be able to adhere to our current schedule for the launch of any of our products. If we fail to achieve one or more of these milestones as planned, the price of our shares could decline.
New product development in the pharmaceutical industry is time-consuming and costly and has a low rate of successful commercialization.
Our success will depend in part on our ability to enhance our existing products and to develop new products. The development process for pharmaceutical products is complex and uncertain, as well as time-consuming and costly. Relatively few research and development programs produce a commercial product. A product candidate that appears promising in the early phases of development may fail to reach the market for a number of reasons, such as:
| | • | | the failure to demonstrate safety and efficacy in preclinical and clinical trials; |
| | • | | the failure to obtain approvals for intended use from relevant regulatory bodies, such as the SFDA; |
| | • | | our inability to manufacture and commercialize sufficient quantities of the product economically; and |
15
| | • | | proprietary rights, such as patent rights, held by others to our product candidates and their refusal to sell or license such rights to us on reasonable terms, or at all. |
In addition, product development requires the accurate assessment of market trends. We cannot assure you that:
| | • | | our new product research and development efforts will be successfully and timely completed; |
| | • | | our clinical trials on humans for our product candidate will be successful; |
| | • | | SFDA or other regulatory bodies will grant necessary regulatory clearances or approvals on a timely basis, or at all; or |
| | • | | any product we develop will be commercialized or achieve market acceptance. |
Delays in any part of the development process or our inability to obtain regulatory approval of our products could adversely affect our operating results by restricting or delaying our introduction of new products. Even if we successfully commercialize new products, these products may address markets that are currently being served by our mature products and may result in a reduction in the sales volume of our mature product or vice versa. Failure to develop, obtain necessary regulatory clearances or approvals for or successfully commercialize or market potential new products or technologies could have a material adverse effect on our financial condition and results of operations.
We will not be able to commercialize our product candidates if our preclinical studies do not produce successful results or our clinical trials do not demonstrate safety and efficacy in humans.
Before obtaining regulatory approvals for the manufacturing and sale of our product candidates, we must conduct, at our own expense, extensive preclinical tests and clinical trials to demonstrate the safety and efficacy in humans of our product candidates. Preclinical and clinical testing is expensive, difficult to design and implement, can take many years to complete and is uncertain as to outcome. Success in preclinical testing and early clinical trials does not ensure that later clinical trials will be successful, and interim results of a clinical trial do not necessarily predict final results. A failure of one or more of our clinical trials can occur at any stage of testing. We may experience numerous unforeseen events during, or as a result of, preclinical testing and the clinical trial process that could delay or prevent our ability to receive regulatory approval or commercialize our product candidates, including the following:
| | • | | our preclinical tests or clinical trials may produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional preclinical testing or clinical trials, or we may abandon projects that appear promising; |
| | • | | we might have to suspend or terminate our clinical trials if the participating patients are being exposed to unacceptable health risks; |
| | • | | regulators may require that we hold, suspend or terminate clinical research for various reasons, including noncompliance with regulatory requirements or safety concerns; |
16
| | • | | the time or cost of our clinical trials may be greater than we currently anticipate; |
| | • | | any regulatory approval we ultimately obtain may be limited or subject to restrictions or post-approval commitments that render the product not commercially viable; and |
| | • | | our product candidates may produce undesirable side effects or may have other unexpected characteristics. |
If we are required to conduct additional clinical trials or other testing of our product candidates beyond those that we currently contemplate, if we are unable to successfully complete our clinical trials or other testing or if the results of these trials or tests are not positive or are only modestly positive, we may:
| | • | | be delayed in obtaining approval for our product candidates; |
| | • | | not be able to obtain approval; or |
| | • | | obtain approval for indications that are not as broad as intended. |
Our product development costs will also increase if we experience delays in testing or approvals. We do not know whether planned clinical trials will begin as planned, will need to be restructured or will be completed on schedule, if at all. Significant clinical trial delays also could allow our competitors to bring products to market before we do and impair our ability to commercialize our products or product candidates.
If we are unable to protect our products through intellectual property rights, our competitors may compete directly against us.
Our success depends, in part, on our ability to protect our products from competition by establishing, maintaining and enforcing intellectual property rights. We seek to protect the products and technology that we consider important to our business by filing PRC and international patent applications, relying on trade secrets or pharmaceutical regulatory protection or employing a combination of these methods. We have patents and patent applications relating to Baquting upgrades, adenosine for myocardial protection, Kaitong,Agkistrodon acutus hemocoagulase and lanthanum polystyrene. For more details, see “Item 4. Information on the Company – B. Business Overview —Intellectual Property.” However, the filing of a patent application does not mean that we will be issued a patent, or that any patent eventually issued will be as broad as requested in the patent application or sufficient to protect our technology. There are a number of factors that could cause our patents, if granted, to become invalid or unenforceable or that could cause our patent applications to not be granted, including known or unknown prior art, deficiencies in the patent application or the lack of originality of the technology. In addition, the PRC adopts the first-to-file system under which whoever first files a patent application will be awarded the patent. By contrast, U.S. patent law endorses the first-to-invent system under which whoever makes the first actual discovery will be awarded the patent. Under the first-to-file system, a third party may be granted a patent relating to a technology which we invented. For more details on the process for applying for and obtaining intellectual property protection in the PRC, see “Item 4. Information on the Company – B. Business Overview —Regulations—Regulation of patent and trademark protection.” Furthermore, the terms of our patents are limited. The patents we hold and patents to be issued from our currently pending patent applications have a twenty-year protection period starting from the date of application.
17
We may become involved in patent litigation against third parties to enforce our patent rights, to invalidate patents held by such third parties, or to defend against such claims. The cost to us of any patent litigation or similar proceeding could be substantial, and it may consume significant management time. We do not maintain insurance to cover intellectual property infringement.
Intellectual property rights and confidentiality protections in China may not be as effective as in the United States or other countries, because, among other reasons, of the lack of procedural rules for discovery and evidence, low damage awards and lack of judicial independence. Implementation and enforcement of PRC intellectual property laws have historically been deficient and ineffective and may be hampered by corruption and local protectionism. Policing unauthorized use of proprietary technology is difficult and expensive, and we may need to resort to litigation to enforce or defend patents issued to us or to determine the enforceability, scope and validity of our proprietary rights or those of others. The experience and capabilities of PRC courts in handling intellectual property litigation varies, and outcomes are unpredictable. Further, such litigation may require a significant expenditure of cash and may divert management’s attention, which could harm our business, financial condition and results of operations. An adverse determination in any such litigation could materially impair our intellectual property rights and may harm our business, prospects and reputation.
If our products infringe the intellectual property rights of third parties, we may incur substantial liabilities, and we may be unable to sell these products.
Our commercial success depends significantly on our ability to operate without infringing the patents and other proprietary rights of third parties. Under the PRC Patent Law, patent applications are maintained in confidence until their publication 18 months from the filing date. The publication of discoveries in the scientific or patent literature frequently occurs substantially later than the date on which the underlying discoveries were made and patent applications are filed. Even after reasonable investigation, we may not know with certainty whether any third party may have filed a patent application without our knowledge while we are still developing or producing that product. While the success of pending patent applications and applicability of any of them to our programs are uncertain, if asserted against us, we could incur substantial costs and we may have to:
| | • | | obtain licenses, which may not be available on commercially reasonable terms, if at all; |
| | • | | redesign our products or processes to avoid infringement; and |
| | • | | stop producing our products using the patents held by others, which could cause us to lose the use of one or more of our products. |
We are currently in the process of disputing an objection raised by a third party during the publication period of the registration of our “Shunxin” trademark. On May 5, 2010, we received a letter from the PRC Trademark Office indicating that there was insufficient evidence supporting Beijing Shunxin’s claims and that if Beijing Shunxun does not request a second review of its objection, we will receive registration for our “Shunxin” trademark. To date, we have not received any other claims of infringement by any third parties. If a third party claims that we infringe its proprietary rights, any of the following may occur:
| | • | | we may have to defend litigation or administrative proceedings that may be costly whether we win or lose, and which could result in a substantial diversion of management resources; |
18
| | • | | we may become liable for substantial damages for past infringement if a court decides that our technology infringes a third party’s intellectual property rights; |
| | • | | a court may prohibit us from producing and selling our product without a license from the holder of the intellectual property rights, which may not be available on commercially acceptable terms, if at all; and |
| | • | | we may have to reformulate our product so that it does not infringe the intellectual property rights of others, which may not be possible or could be very expensive and time consuming. |
Any costs incurred in connection with such events or the inability to sell our products may have a material adverse effect on our business and results of operations.
We rely on confidentiality agreements that could be breached and may be difficult to enforce, which could have a material adverse effect on our business and competitive position.
Our policy is to enter agreements relating to the non-disclosure of confidential information with third parties, including our contractors, consultants, advisors and research collaborators, as well as agreements that purport to require the disclosure and assignment to us of the rights to the ideas, developments, discoveries and inventions of our employees and consultants while we employ them. However, these agreements can be difficult and costly to enforce. Moreover, to the extent that our contractors, consultants, advisors and research collaborators apply or independently develop intellectual property in connection with any of our projects, disputes may arise as to the proprietary rights to this type of information. If a dispute arises, a court may determine that the right belongs to a third party, and enforcement of our rights can be costly and unpredictable. In addition, we rely on trade secrets and proprietary know-how that we will seek to protect in part by confidentiality agreements with our employees, contractors, consultants, advisors or others. Despite the protective measures we employ, we still face the risk that:
| | • | | These agreements may be breached; |
| | • | | These agreements may not provide adequate remedies for the applicable type of breach; or |
| | • | | our trade secrets or proprietary know-how will otherwise become known. |
Any breach of our confidentiality agreements or our failure to effectively enforce such agreements would have a material adverse effect on our business and competitive position.
19
We source our primary raw material, Bothrops atrox venom, from a single third-party supplier and any supply failure could adversely affect our ability to manufacture our products.
We source our primary raw material,Bothrops atrox venom, for the production of Baquting, from Centro De Extracão De Toxinas Animais, an unaffiliated third-party supplier in Brazil. Export ofBothrops atrox venom is subject to the laws and administrative rules and regulations related to export-control in Brazil and its import to China is also required to comply with laws, regulations and restrictions imposed by the relevant national and local authorities in China. We must receive governmental approval from PRC authorities for each shipment ofBothrops atrox venom. We may not be able to obtain such approval in time to meet our production requirements or at all. The Brazilian government may impose more stringent control over exports, and the PRC government may impose stricter restrictions on imports, which, in either case, may impair our ability to import, or prevent us from importing,Bothrops atrox venom from Brazil to China.
If there is any supply interruption for an indeterminate period of time, we may not be able to identify and obtain alternative supplies that comply with our quality standards in a timely manner. Any supply disruption could adversely affect our ability to satisfy demand for our products, and materially and adversely affect our product sales and operating results.
The manufacture of our products is an exacting and complex process, and if we or our contract manufacturers encounter problems in manufacturing our products, our business and results of operations would be materially and adversely affected.
The SFDA and foreign regulators require manufacturers to register manufacturing facilities. The SFDA and foreign regulators also inspect these facilities to confirm compliance with GMP or similar requirements that the SFDA or foreign regulators establish. We or our contract manufacturers may face manufacturing or quality control problems causing product production and shipment delays or a situation where we or contract manufacturers may not be able to maintain compliance with the SFDA’s GMP requirements, or those of foreign regulators, necessary to continue manufacturing our drug candidates. A new inspection rule for GMP certification recently became effective on January 1, 2008 that implemented more stringent GMP standards. Any failure to comply with GMP requirements or other SFDA or foreign regulatory requirements could adversely affect our ability to manufacture, market and sell our products.
We rely on a limited number of distributors for sales of our products.
We rely on a limited number of distributors for most of our net revenue. Our top five distributors in the aggregate accounted for 37.2%, 33.4% and 42.1% of our net revenue in 2007, 2008 and 2009, respectively. We expect that a relatively small number of our distributors will continue to account for a major portion of our net revenue in the near future. Our dependence on a few distributors could expose us to the risk of substantial losses if a single large distributor stops purchasing our products, purchases fewer of our products or goes out of business and we cannot find substitute distributors on equivalent terms. If any of our significant distributors reduces the quantity of the products they purchase from us or stops purchasing from us, our net revenue would be materially and adversely affected.
We do not have long-term distribution agreements with our distributors, and we compete for desired distributors with other pharmaceutical manufacturers. Consequently, maintaining relationships with existing distributors and replacing distributors may be difficult and time consuming. Any disruption of our distribution network, including failure to renew existing distribution agreements with desired distributors, could negatively affect our ability to effectively sell our products and materially and adversely affect our business, financial condition and results of operations.
20
We enter into distribution agreements with our distributors which generally provide distribution rights for our products in a designated geographic area for a period of one year. We generally have one to three distributors in each provincial-level region. If any distributor sells our products outside its designated geographic area, harmful competition among our distributors could result, which may adversely impact our product sales activities and operating results.
If we are unable to attract, train, retain and motivate our direct sales force and third-party marketing agents, sales of our products may be materially and adversely affected.
We rely on our direct sales force and third-party marketing agents, who are dispersed across China, to market our products to hospitals and other healthcare institutions. We believe that Baquting’s current leading position in the market was the result, to a significant extent, of the dedication, efforts and performance of our direct sales force and third-party marketing agents. We believe that our future success will continue to depend on these same factors. There are only limited numbers of competent and qualified marketing agents in the Chinese pharmaceutical industry. We do not provide compensation to, or contract directly with, our marketing agents. Our marketing agents are instead compensated for their marketing and promotional activities by distributors. We therefore are unable to directly incentivize the marketing agents for their abilities. Our competitors may provide commissions or other economic incentives to third-party marketing agents significantly above the market standard, which may cause such agents to cease marketing our products. If we are unable to attract, train, retain and motivate our direct sales force and marketing agents, sales of our products may be materially and adversely affected.
Anti-corruption measures taken by the government to correct corruptive practices in the pharmaceutical industry could adversely affect our sales and reputation.
The government has recently taken anti-corruption measures to correct corrupt practices. In the pharmaceutical industry, such practices include, among others, acceptance of kickbacks, bribery or other illegal gains or benefits by the hospitals and medical practitioners from pharmaceutical distributors in connection with the prescription of a certain drug. Substantially all of our sales are made to hospitals through third-party distributors. We have no control over our third-party marketing agents and distributors, who may engage in corrupt practices to promote our products. While we maintain strict anti-corruption policies applicable to our internal sales force and third-party marketing agents and distributors, these policies may not be completely effective. If our sales staff or any of our third-party marketing agents and distributors engage in such practices and the government takes enforcement action, our products may be seized and our own practices, and involvement in the distributors’ practices may be investigated. If this occurs, our sales and reputation may be materially and adversely affected.
In addition, government-sponsored anti-corruption campaigns from time to time could have an adverse effect on our efforts to reach new hospital customers. Our sales representatives primarily rely on hospital visits to better educate physicians on our products and promote our brand awareness. Recently, there have been occasions on which our sales representatives were denied access to hospitals in order to avoid the perception of corruption. If this attitude becomes widespread among our potential customers, our ability to promote our products will be adversely affected.
21
If we are unable to successfully manage our growth, there could be a material adverse impact on our business, results of operations and financial condition.
We have grown rapidly and expect to continue to grow. We expect to hire more employees, particularly in the areas of research and development, regulatory affairs and sales and marketing, and enhance our facilities and corporate infrastructure, further increasing the size of our organization and related expenses. To manage our anticipated future growth, we must continue to implement and improve our managerial, operational and financial systems, expand our facilities and continue to recruit and train additional qualified personnel. Because of our limited resources, we may not be able to effectively manage the expansion of our operations or recruit and train additional qualified personnel. The expansion of our operations may lead to significant costs and may divert our management and business development resources. Any inability on the part of our management to manage growth could delay the execution of our business plans or disrupt our operations. If we are unable to manage our growth effectively, we may be unable to use our resources in an efficient manner, which may negatively impact our business, results of operations and financial condition.
We depend upon key employees and consultants in a competitive market for skilled personnel. If we are unable to attract and retain key personnel, it could adversely affect our ability to develop and market our products.
We are highly dependent upon the principal members of our management team, especially our chairman and chief executive officer, Mr. Baizhong Xue. We have employment agreements, non-compete agreements and confidentiality agreements with these key employees. Although these agreements provide for severance payments that are contingent upon the applicable employee’s refraining from competition with us, the applicable provisions can be difficult and costly to monitor and enforce. The loss of any of these persons’ services would adversely affect our ability to develop and market our products.
We also depend in part on the continued services of our key scientific personnel and our ability to identify, hire and retain additional personnel, including marketing and sales staff. We face intense competition for qualified personnel, and the existence of non-competition agreements between prospective employees and their former employers may prevent us from hiring those individuals or subject us to suit from their former employers. While we attempt to provide competitive compensation packages to attract and retain key personnel, many of our competitors are likely to have greater resources and more experience than we have, making it difficult for us to compete successfully for key personnel.
Certain of our employees and consultants were previously employed at other biotechnology or pharmaceutical companies, including our competitors or potential competitors, or at universities or other research institutions. Although no claims against us are currently pending, we may be subject to claims that these employees or consultants have, inadvertently or otherwise, used or disclosed trade secrets or other proprietary information of their former employers. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to our management. If we fail in defending such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel.
22
Our collaborations with outside scientists and consultants may be subject to restriction and change.
We work with chemists, biologists and other scientists at academic and other institutions, and consultants who assist us in our research, development, regulatory and commercial efforts. These scientists and consultants have provided, and we expect that they will continue to provide, valuable advice on our programs. These scientists and consultants are not our employees, may have other commitments that would limit their future availability to us and typically will not enter into non-compete agreements with us. If a conflict of interest arises between their work for us and their work for another entity, we may lose their services. In addition, we will be unable to prevent them from establishing competing businesses or developing competing products. For example, if a key scientist acting as a principal investigator in any of our clinical trials identifies a potential product or compound that is more scientifically interesting to his or her professional interests, his or her availability to remain involved in such clinical trials could be restricted or eliminated.
If we acquire companies, products or technologies, we may face integration risks and costs associated with those acquisitions that could negatively impact our business, results from operations and financial condition.
If we are presented with appropriate opportunities, we may acquire or make investments in complementary companies, products or technologies. For example, we entered into a joint venture with QRxPharma Limited, an Australian company, to develop and commercialize two bleeding control products based on technologies developed by QRxPharma and the University of Queensland. We invested $5.0 million in the joint venture for it to fund the clinical trials for the product candidates, regardless of whether we are able to obtain regulatory approvals for their production or successfully commercialize them. We may not realize the anticipated benefit of any acquisition or investment. If we acquire companies or technologies, we will face risks, uncertainties and disruptions associated with the integration process, including difficulties in the integration of the operations of an acquired company, integration of acquired technology with our products, diversion of our management’s attention from other business concerns, the potential loss of key employees or customers of the acquired business, the potential involvement into any litigation related to the acquired company, and impairment charges if future acquisitions are not as successful as we originally anticipate. In addition, our operating results may suffer because of acquisition-related costs or amortization expenses or charges relating to acquired intangible assets. Any failure to successfully integrate other companies, products or technologies that we may acquire may have a material adverse effect on our business and results of operations. Furthermore, we may have to incur debt or issue equity securities to pay for any additional future acquisitions or investments, the issuance of which could be dilutive to our existing shareholders.
The pharmaceutical industry in China is highly regulated, and future government regulation may place additional burdens on our business.
The pharmaceutical industry in China is subject to extensive government regulation and supervision. The regulatory framework addresses all aspects of operating in the pharmaceutical industry, including approval, production, distribution, licensing and certification requirements and procedures, periodic renewal and reassessment processes, registration of new drugs and environmental protection. Violation of applicable laws and regulations may materially adversely affect our business. In order to manufacture pharmaceutical products in China, we are required to obtain a pharmaceutical manufacturing permit and GMP certificate for each production line from the relevant food and drug administrative authority. We are required to obtain the drug registration certificate, which includes a drug approval number, from the SFDA for each drug manufactured by us. In addition, in order to distribute any drug in China, we must obtain a pharmaceutical distribution permit and good supply practice, or GSP, certificate from the SFDA. We are required to renew the pharmaceutical manufacturing permits, the pharmaceutical distribution permits, drug registration certificates, GMP certificates and GSP certificates every five years. If we are unable to obtain or renew such permits or any other permits or licenses required for our operation, we will not be able to engage in the manufacture and distribution of our products and our business may be adversely affected.
23
The regulatory framework regarding the pharmaceutical industry in China is subject to change and amendment from time to time. Any such change or amendment may have an adverse effect on our business. Changes to the regulatory framework could materially and adversely impact our business, financial condition and results of operations. The PRC government has released a number of announcements since April 2009 that collectively outlined a comprehensive plan to reform the healthcare system in China within the next few years, with an overall objective to expand the basic medical insurance coverage and improve the quality and reliability of healthcare services. The details of the reform have yet to be announced and the specific regulatory changes under the reform still remain uncertain. The implementing measures to be issued may not be sufficiently effective to achieve the stated goals, and as a result, we may not be able to benefit from such reform to the level we expect, if at all. Moreover, the reform could give rise to regulatory developments, such as tighter control over product pricing or more burdensome administrative procedures, which may have an adverse effect on our business and prospects.
For further information regarding government regulation in China, see “Item 4. Information on the Company – B. Business Overview —Regulations.”
The pharmaceutical industry is extremely competitive and China’s entry to the WTO may intensify this competition in China.
Our business is subject to competition from other pharmaceutical manufacturers. Local and overseas pharmaceutical manufacturers engaged in the manufacture and sale of similar products to ours in China may have more capital resources, superior research and development capabilities and more experience in manufacturing and marketing their products. China joined the WTO in December 2001. Following its entry, China lowered tariffs on certain imported pharmaceutical products as part of its obligation under the WTO framework. The reduction or removal of tariffs on imported pharmaceutical products made such products more competitive with domestic pharmaceutical products. In addition, an increasing number of foreign-invested pharmaceutical manufacturers may establish operations to engage in the manufacture or distribution of pharmaceutical products in China, which would increase the number of suppliers of pharmaceutical products in the market and intensify the competition with domestic manufacturers. If the domestic pharmaceutical manufacturers are unable to distinguish their products from imported products or products produced domestically by foreign-invested pharmaceutical manufacturers, they may lose market share to imported products or products produced domestically by foreign-invested pharmaceutical manufacturers which may be of higher quality and are sold at competitive prices. Furthermore, due to the lack of capital for the research and development of new medicines, most of the domestic pharmaceuticals are imitations of foreign products. Following China’s entry to the WTO, many more companies in Europe and the U.S. have applied for patents in the PRC, thereby increasing the likelihood of litigation for Chinese domestic pharmaceutical companies whose products may be covered by PRC patents owned by foreign companies.
24
Adverse publicity associated with our company or our products or similar products manufactured by our competitors could have a material adverse effect on our results of operations.
There have been recent incidents reported in the Chinese media of a significant number of patients experiencing severe adverse health consequences following their use of biopharmaceutical products manufactured by certain biopharmaceutical companies in China. A number of patients have become ill and a number of fatalities have been reported. For example, several deaths were caused by human immunoglobulin manufactured and sold by Jiangxi Boya Biopharmaceutical Co., Ltd., a PRC biopharmaceutical product manufacturer, in May 2008. We are highly dependent upon market perceptions of the safety and quality of our products. Concerns over the safety of biopharmaceutical products manufactured in China could have an adverse effect on the sale of such products, including products manufactured by us.
We could be adversely affected if any of our products or any similar products manufactured by other companies prove to be, or are alleged to be, harmful to patients. Any negative publicity associated with severe adverse reactions or other adverse effects resulting from patients’ use or misuse of our products or any similar products manufactured by other companies could also have a material adverse impact on our results of operations. We have not, to date, experienced any significant quality control or safety problems. If in the future we become involved in incidents of the type described above, such problems could severely and adversely impact our product sales and reputation.
We are subject to environmental regulations and may be exposed to liability and potential costs for environmental compliance.
We are subject to PRC laws and regulations concerning the discharge of waste water, gaseous waste and solid waste during our manufacturing processes. We are required to establish and maintain facilities to dispose of waste and report the volume of waste to the relevant government authorities, which conduct scheduled or unscheduled inspections of our facilities and treatment of such discharge. We may not at all times comply fully with environmental regulations. Any violation of these regulations may result in substantial fines, criminal sanctions, revocations of operating permits, shutdown of our facilities and obligation to take corrective measures. Our cost of complying with current and future environmental protection laws and regulations and our liabilities which may potentially arise from the discharge of effluent water and solid waste may materially adversely affect our business, financial condition and results of operations.
The government may take steps towards the adoption of more stringent environmental regulations. Due to the possibility of unanticipated regulatory or other developments, the amount and timing of future environmental expenditures may vary substantially from those currently anticipated. If there is any unanticipated change in the environmental regulations, we may need to incur substantial capital expenditures to install, replace, upgrade or supplement our pollution control equipment or make operational changes to limit any adverse impact or potential adverse impact on the environment in order to comply with new environmental protection laws and regulations. If such costs become prohibitively expensive, we may be forced to cease certain aspects of our business operations.
25
We may be required to defend lawsuits or pay damages for product liability claims. We do not have any liability or business disruption insurance, and a claim against us, or an interruption in our business, could adversely offset our reputation and our financial results.
The development and commercialization of pharmaceutical products entails an inherent risk of harm to the patient and, therefore, product liability. Even though there are no punitive damages under the PRC law, if a product liability claim is brought against us, it may, regardless of merit or eventual outcome, result in damage to our reputation, breach of contract with our customers, decreased demand for our products, costly litigation, product recalls, loss of revenue, and the inability to commercialize some products. We currently are not aware of any existing or anticipated product liability claims with respect to our products.
Existing PRC laws and regulations do not require us to nor do we maintain liability insurance to cover product liability claims. The insurance industry in China is still at an early stage of development. Insurance companies in China offer limited business insurance products. As a result, we do not have business liability, or in particular, product liability, or disruption insurance coverage for our operations. Any business disruption, litigation or natural disaster might result in substantial costs and diversion of resources. When and if we attempt to obtain product liability insurance for clinical trials, this insurance may be prohibitively expensive, or may not fully cover our potential liabilities. Inability to obtain sufficient insurance coverage at an acceptable cost or otherwise to protect against potential product liability claims could prevent or inhibit the commercialization of products that we or our collaborators develop.
Power shortages, natural disasters, terrorist acts or other calamities could disrupt our production and have a material adverse effect on our business, financial position and results of operations.
Baquting and our other products are produced at our manufacturing facility in Penglai, China. A significant disruption at that facility, even on a short-term basis, could impair our ability to timely produce and ship products, which could have a material adverse effect on our business, financial position and results of operations.
Our manufacturing operations are vulnerable to interruption and damage from natural and other types of disasters, including earthquake, fire, floods, environmental accidents, power loss, communications failures and similar events. If any disaster were to occur, our ability to operate our business at our facilities would be seriously impaired. In addition, the nature of our production and research activities could cause significant delays in our programs and make it difficult for us to recover from a disaster. We do not maintain any insurance other than insurance for some of our properties. Accordingly, unexpected business interruptions resulting from disasters could disrupt our operations and thereby result in substantial costs and diversion of resources.
26
In addition, our production process requires a continuous supply of electricity. We have encountered power shortages historically due to restricted power supply to industrial users during summers when the usage of electricity is high and supply is limited or as a result of damage to the electricity supply network. Because the duration of those power shortages was brief, they had no material impact on our operations. Interruptions of electricity supply could result in lengthy production shutdowns, increased costs associated with restarting production and the loss of production in progress. Any major suspension or termination of electricity or other unexpected business interruptions could have a material adverse impact on our business, financial condition and results of operations.
If our lease with Shandong Penglai Pharmaceutical Plant is terminated, not renewed or suspended, or if we are required to vacate the leased premises for any reason, our operations may be materially adversely affected.
Certain production facilities of Penglai Nuokang Pharmaceutical Co., Ltd., or Penglai Nuokang, are located on land leased from a third party, Shandong Penglai Pharmaceutical Plant. The lease will expire on December 31, 2014 and upon its expiration, Penglai Nuokang and Shandong Penglai Pharmaceutical Plant may negotiate to extend the lease agreement. Shandong Penglai Pharmaceutical Plant may immediately terminate the lease if Penglai Nuokang subleases or lends the land use right or buildings to any third parties without its consent or if Penglai Nuokang violates the intended use of the land or uses the land to engage in any illegal activities. Under the lease agreement, any party that unilaterally terminates the lease without cause will pay to the other party damages equal to RMB1.0 million for each year remaining under the lease agreement. In addition, Shandong Penglai Pharmaceutical Plant has not obtained the building ownership certificates for our solid formulation plant. We cannot guarantee that it will ever be able to obtain the necessary building ownership certificates or that PRC government authorities or third parties will not challenge or invalidate its ownership. If Shandong Penglai Pharmaceutical Plant’s ownership of these buildings were challenged or invalidated, we may need to vacate our existing facilities in Penglai and move these manufacturing operations to our facilities in Shenyang or find alternative facilities. Any termination, non-renewal or suspension of the lease or our eviction from the leased premises could disrupt our operations. We might suffer losses as a result of business interruptions and our operations and financial results may be materially and adversely affected.
Our future capital needs are uncertain. As a result, we may need to raise additional funds in the future.
We may require additional cash resources in the future. Our future cash needs will depend upon:
| | • | | the extent to which our products are accepted in the market and generate cash flows; |
| | • | | the resources we devote to developing, marketing and producing our products; |
| | • | | the receipt of, and the time and expenses required to obtain and maintain, regulatory clearances and approvals; |
| | • | | our ability to identify and our desire or need to pursue acquisitions or other investments; and |
| | • | | changed business conditions or other future developments. |
27
Our revenues may not be sufficient to meet our operational needs and capital requirements, and needed financing may not be available in amounts or on terms acceptable to us, if at all. Our future capital needs and other business reasons could require us to sell additional equity or debt securities or obtain a credit facility. The sale of additional equity or equity-linked securities could result in additional dilution to our shareholders. The incurrence of indebtedness would result in increased debt service obligations and could result in operating and financing covenants that would restrict our operations or our ability to pay dividends to our shareholders. Moreover, credit arrangements in the PRC are subject to government restrictions and may not be available to us on commercially reasonable terms or at all.
If we fail to establish an effective system of internal controls, we may be unable to accurately report our financial results or prevent fraud, and investor confidence and the market price of our ADSs may be adversely impacted.
We are subject to reporting obligations under U.S. securities laws. The SEC, as required by Section 404 of the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, adopted rules requiring every public company to include a management report on the company’s internal controls over financial reporting in its annual report, which contains management’s assessment of the effectiveness of the company’s internal controls over financial reporting. In addition, an independent registered public accounting firm must attest to and report on the effectiveness of the company’s internal controls over financial reporting. These requirements will first apply to our annual report on Form 20-F for the fiscal year ending on December 31, 2010. Our reporting obligations as a public company will place a significant strain on our management, operational and financial resources and systems for the foreseeable future.
Prior to our initial public offering in December 2009, we were a private company with limited accounting personnel and other resources with which to address our internal control and procedures over financial reporting. As a result, during the preparation and external audit of our consolidated financial statements as of and for the years ended December 31, 2006, 2007, 2008 and 2009, our independent registered public accounting firm identified two material weaknesses and certain significant deficiencies in our internal controls over financial reporting, as defined in the standards established by the U.S. Public Company Accounting Oversight Board. These material weaknesses, which could result in more than a remote likelihood that a material misstatement in our annual or interim financial statements would not be prevented or detected, consisted of:
| | • | | the internal audit function had not been fully executed to assist our audit committee and management in the effective discharge of their responsibilities through independent and objective review, analysis, appraisal and reporting of recommendations on our business operations and activities; and |
| | • | | the lack of adequate personnel with sufficient understanding of U.S. GAAP and SEC reporting requirements and adequate policies and procedures to prepare financial information in accordance with U.S. GAAP and SEC regulations. |
28
Following the identification of these material weaknesses and other deficiencies, we have undertaken remedial steps to address them, including hiring additional staff, training our new and existing staff and augmenting our financial information technology systems.
We plan to continue to take additional steps to improve our internal controls to meet the deadline imposed by Section 404 of the Sarbanes-Oxley Act. We may encounter substantial difficulty attracting qualified staff with requisite experience due to the high level of competition for experienced financial professionals. If we fail to timely achieve and maintain the adequacy of our internal controls, our management may conclude that our internal control over financial reporting is not effective. Moreover, effective internal controls over financial reporting is necessary for us to produce reliable financial reports and is important to help prevent fraud. As a result, our failure to achieve and maintain effective internal controls over financial reporting could result in the loss of investor confidence in the reliability of our financial statements, which in turn could harm our business and negatively impact the market price of our ADSs.
We may be classified as a passive foreign investment company, which could result in adverse U.S. federal income tax consequences to U.S. Holders of our ADSs or ordinary shares.
Based on the market price of our ADSs, the value of our assets, and the composition of our income and assets, we do not believe that we were a “passive foreign investment company,” or PFIC, for United States federal income tax purposes for our taxable year ended December 31, 2009. A non-U.S. corporation will be a PFIC for any taxable year if either (1) at least 75% of its gross income for such year is passive income or (2) at least 50% of the value of its assets (based on an average of the quarterly values of the assets) during such year is attributable to assets that produce passive income or are held for the production of passive income. We must make a separate determination after the close of each taxable year as to whether we were a PFIC for that year. Because the value of our assets for purposes of the PFIC test will generally be determined by reference to the market price of our ADSs or ordinary shares, fluctuations in the market price of our ADSs or ordinary shares may cause us to become a PFIC. In addition, changes in the composition of our income or assets may cause us to become a PFIC. If we are a PFIC for any taxable year during which a U.S. Holder (as defined in “Item 10. Additional Information—E. Taxation—U.S. Federal Income Taxation”) holds an ADS or an ordinary share, certain adverse U.S. federal income tax consequences could apply to such U.S. Holder. For example, such U.S. Holder may incur a significantly increased U.S. federal income tax liability on the receipt of certain distributions on our ADSs or ordinary shares or on any gain recognized from a sale or other disposition of our ADSs or ordinary shares. See “Item 10. Additional Information—E. Taxation—U.S. Federal Income Taxation—Passive Foreign Investment Company.”
Risks Related to Doing Business in China
The PRC’s economic, political and social conditions, as well as governmental policies, could affect the financial markets in China, our liquidity and access to capital and our ability to operate our business.
Substantially all of our business operations are conducted in China. Accordingly, our results of operations, financial condition and prospects are subject to a significant degree to economic, political and legal developments in China. China’s economy differs from the economies of developed countries in many respects, including with respect to the amount of government involvement, level of development, growth rate, control of foreign exchange and allocation of resources. While the PRC economy has experienced significant growth in the past 30 years, growth has been uneven across different regions and among various economic sectors of China. The PRC government has implemented various measures to encourage economic development and guide the allocation of resources. Some of these measures benefit the overall PRC economy, but may have a negative effect on us. For example, our financial condition and results of operations may be adversely affected by government control over capital investments or changes in tax regulations that are applicable to us. More generally, if the business environment in China deteriorates from the perspective of domestic or international investors, our business in China may also be adversely affected.
29
Future changes in laws, regulations or enforcement policies in China could adversely affect our business.
Laws, regulations or enforcement policies in China, including those regulating healthcare and the pharmaceutical industry, are evolving and subject to frequent changes. Further, regulatory agencies in China may periodically, and sometimes abruptly, change their enforcement practices. Therefore, prior enforcement activity, or lack of enforcement activity, is not necessarily predictive of future actions. Any enforcement actions against us could have a material and adverse effect on us and the market price of our ADSs. In addition, any litigation or governmental investigation or enforcement proceedings in China may be protracted and may result in substantial cost and diversion of resources and management attention, negative publicity, damage to our reputation and decline in the price of our ADSs.
Changes in PRC government policy on foreign investment in China may adversely affect our business and results of operations.
As foreign invested enterprises, our wholly owned subsidiaries including Liaoning Nuokang Bio-pharmaceutical Co., Ltd., or Liaoning Nuokang, Shenyang Shouzheng Bio-technology Co., Ltd., or Shenyang Shouzheng, and Penglai Nuokang are subject to restrictions on foreign investment imposed by PRC laws from time to time. For instance, under the Foreign Investment Industrial Guidance Catalogue, some industries are categorized as sectors which are encouraged, restricted or prohibited for foreign investment.
According to the latest version of this Catalogue, which became effective on December 1, 2007, our business, other than the distribution of pharmaceutical products, does not belong to the prohibited or the restricted category. As this Catalogue is updated every few years, there can be no assurance that the PRC government will not change its policies in a manner that would cause part or all of our businesses to fall within the restricted or prohibited categories. If any of our businesses becomes prohibited or if we cannot obtain approval from relevant approval authorities to engage in businesses which become restricted for foreign investors, we may be forced to sell or restructure our businesses which have become restricted or prohibited for foreign investment. If we are forced to adjust our corporate structure or business line as a result of changes in government policy on foreign investment, our business, financial condition and results of operations may be materially and adversely affected.
30
Recent regulations relating to offshore investment activities by PRC residents may limit our ability to acquire PRC companies and could adversely affect our business, financial condition and results of operations.
In October 2005, State Administration of Foreign Exchange (SAFE) promulgated a regulation known as Circular No. 75 that states that if PRC residents use assets or equity interests in their PRC entities as capital contributions to establish offshore companies or inject assets or equity interests of their PRC entities into offshore companies to raise capital overseas, they must register with local SAFE branches with respect to their overseas investments in offshore companies. They must also file amendments to their registrations if their offshore companies experience material events involving capital variation, such as changes in share capital, share transfers, mergers and acquisitions, spin-off transactions, long-term equity or debt investments or guarantee. Under this regulation, failure to comply with the registration procedures set forth in such regulation may result in restrictions being imposed on the foreign exchange activities of the relevant PRC entity, including the payment of dividends and other distributions to its offshore parent, as well as restrictions on the capital inflow from the offshore entity to the PRC entity. Mr. Baizhong Xue, our chief executive officer and chairman of our board of directors, beneficially owned approximately 59.7% of our outstanding share capital as of March 31, 2010. Mr. Xue has registered with the SAFE under the relevant SAFE regulations and updated his registration upon the completion of our initial public offering in December 2009. While we believe our shareholders have complied with existing SAFE registration procedures, any future failure by any of our shareholders who is a PRC resident, or controlled by a PRC resident, to comply with relevant requirements under this regulation could subject our company to fines or sanctions imposed by the PRC government, including restrictions on our subsidiaries’ ability to pay dividends or make distributions to us and our ability to increase our investment in or to provide loans to our subsidiaries.
On December 25, 2006, the People’s Bank of China promulgated the Measures for Administration of Individual Foreign Exchange, on January 5, 2007, the SAFE promulgated Implementation Rules for those measures and on March 28, 2007, the SAFE further promulgated the Operating Procedures on Administration of Foreign Exchange regarding PRC Individuals’ Participation in Employee Share Ownership Plans and Employee Stock Option Plans of Overseas Listed Companies. According to these new foreign exchange regulations, PRC citizens who are granted shares or share options by a company listed on an overseas stock market under its employee share option or share incentive plan are required to register with the SAFE or its local counterparts by following certain procedures. We and our employees who are PRC citizens and individual beneficiary owners or have been granted restricted shares or share options may be subject to these rules. The failure of our PRC individual beneficiary owners and holders of restricted shares or share options to complete their SAFE registrations according to the requirements of local counterparts of the SAFE or the new foreign exchange rules may subject these PRC citizens to fines and legal sanctions and may also limit our ability to contribute additional capital into our PRC subsidiaries, limit our PRC subsidiaries’ ability to distribute dividends to us or otherwise materially and adversely affect our business.
Uncertainties with respect to the PRC legal system could materially and adversely affect us.
We conduct our business primarily through our subsidiaries and affiliated entities in China. PRC laws and regulations govern our operations in China. Our subsidiaries are generally subject to laws and regulations applicable to foreign investments in China and, in particular, laws applicable to wholly foreign-owned enterprises. The PRC legal system is based on written statutes. Prior court decisions may be cited for reference but have limited precedential value. Since 1979, PRC legislation and regulations have significantly enhanced the protections afforded to various forms of foreign investments in China. However, China has not developed a fully integrated legal system and recently enacted laws and regulations may not sufficiently cover all aspects of economic activities in China. In particular, because these laws and regulations are relatively new, and because of the limited volume of published decisions and their non-binding nature, the interpretation and enforcement of these laws and regulations involve uncertainties. In addition, the PRC legal system is based in part on government policies and internal rules (some of which are not published on a timely basis or at all) that may have a retroactive effect. As a result, we may not be aware of our violation of these policies and rules until some time after the violation. In addition, any litigation in China, regardless of outcome, may be protracted and result in substantial costs and diversion of resources and management attention.
31
We may not be able to pay dividends to our shareholders.
We are a holding company, and we rely on dividends paid by our wholly owned subsidiaries, Liaoning Nuokang, Shenyang Shouzheng and Penglai Nuokang, for our cash needs, including the funds necessary to pay any dividends and other cash distributions to our shareholders, service any debt we may incur and pay our operating expenses. The payment of dividends in China is subject to limitations. Regulations in the PRC currently permit payment of dividends by our PRC subsidiaries only out of accumulated profits as determined in accordance with accounting standards and regulations in China. The payment of dividends by Nuokang Distribution, our variable interest enterprise, or VIE, is determined by its sole shareholder, Mr. Baizhong Xue, our chairman and chief executive officer, subject to Penglai Nuokang’s approval. Repayment by Mr. Xue of these dividends to Penglai Nuokang under the contractual arrangements is subject to applicable PRC laws and regulations. Each of our subsidiaries is required to allocate at least 10% of its net profit after tax to its general reserve fund until the balance of such fund has reached 50% of its registered capital. These funds are not distributable in cash dividends. If our subsidiaries are unable to pay us sufficient dividends due to statutory or contractual restrictions on their ability to distribute dividends to us, we may not be able to pay dividends to our shareholders and our various other cash needs may not be met. In addition, our debt arrangements and those of our subsidiaries that we may enter into from time to time may impose operating and financial restrictions on us and our subsidiaries. These restrictions may limit our ability and the ability of our subsidiaries to pay dividends. In addition, if our subsidiaries incur debts or losses, such indebtedness or loss may impair their ability to pay dividends to us and limit our ability to pay dividends to our shareholders.
Our ability to declare dividends in relation to our shares will also depend on our future financial performance, which in turn depends on successfully implementing our strategy and on financial, competitive, regulatory, and other factors, general economic conditions, demand and prices for our services, costs of supplies and other factors specific to our industry or specific projects, many of which are beyond our control. The receipt of dividends from our subsidiaries may also be affected by the passage of new laws, adoption of new regulations or changes to, or in the interpretation or implementation of existing laws and regulations and other events outside our control. In particular, as our operating subsidiaries are located in the PRC, we may be adversely affected by implementation of capital and other exchange controls by the PRC Government. See “Item 8. Financial Information—Dividend Policy” for a discussion of our dividend policy.
32
Restrictions on currency exchange may limit our ability to receive and use our revenues effectively.
We receive all of our revenues in Renminbi, which currently is not a freely convertible currency. A portion of our revenues may be converted into other currencies to meet our foreign currency obligations, including, among others, payments of dividends declared, if any, in respect of our ordinary shares or ADSs. Under China’s existing foreign exchange regulations, our subsidiaries are able to pay dividends in foreign currencies or convert Renminbi into other currencies for use in operations without prior approval from the SAFE, by complying with certain procedural requirements. However, we cannot assure you that the PRC government will not take future measures to restrict access to foreign currencies for current account transactions.
Our PRC subsidiaries’ ability to obtain foreign exchange is subject to significant foreign exchange controls and, in the case of amounts under the capital account, requires the approval of and/or registration with PRC government authorities, including the SAFE. In particular, if we finance our PRC subsidiaries by means of foreign debt from us or other foreign lenders, the amount is not allowed to exceed the difference between the amount of total investment and the amount of the registered capital as approved by the Ministry of Commerce, or MOFCOM, and registered with the SAFE. Further, such loans must be registered with the SAFE. Currently, the amount of total investment of each of Shenyang Shouzheng and Penglai Nuokang is equal to the amount of its registered capital. If we finance Shenyang Shouzheng and Penglai Nuokang by means of foreign debt, we must obtain approvals from the local MOFCOM branches to increase the amount of their total investment first. If we fail to receive approval from the local MOFCOM branches to increase the amount of our subsidiaries’ total investment, we may not finance our PRC subsidiaries by means of foreign debts. If we finance our PRC subsidiaries by means of additional capital contributions, the amount of these capital contributions must first be approved by the relevant government approval authority. These limitations could affect the ability of our PRC subsidiaries to obtain foreign exchange through debt or equity financing.
Fluctuation in the value of the Renminbi may have a material adverse effect on your investment.
The value of the Renminbi against the U.S. dollar and other currencies may fluctuate and is affected by, among other things, changes in China’s political and economic conditions and China’s foreign exchange policies. The conversion of Renminbi into foreign currencies, including the U.S. dollar, has historically been set by the People’s Bank of China. On July 21, 2005, the PRC government changed its policy of pegging the value of the Renminbi to the U.S. dollar. Under the new policy, the Renminbi is permitted to fluctuate within a narrow and managed band against a basket of certain foreign currencies. This change in policy caused the Renminbi to appreciate approximately 21.5% against the U.S. dollar over the following three years. Since reaching a high against the U.S. dollar in July 2008, however, the Renminbi has traded within a narrow band against the U.S. dollar, remaining within 1% of its July 2008 high but never exceeding it. As a consequence, the Renminbi has fluctuated sharply since July 2008 against other freely traded currencies, in tandem with the U.S. dollar. It is difficult to predict how long the current situation may last and when and how it may change again. As we import our primary raw material from Brazil and may import certain production equipment in the future, fluctuations in the value of the Renminbi against the U.S. dollar and other currencies may affect our cost of production. In addition, as we rely entirely on dividends paid to us by our subsidiaries, any significant revaluation of the Renminbi may have a material adverse effect on our revenues and financial condition, and the value of, and any dividends payable on, our ordinary shares in foreign currency terms. For example, to the extent that we need to convert U.S. dollars into Renminbi for our operations, appreciation of the Renminbi against the U.S. dollar would reduce the Renminbi amount we receive from the conversion. Conversely, if we decide to convert our Renminbi into U.S. dollars for the purpose of making dividend payments on our ordinary shares or for other business purposes, appreciation of the U.S. dollar against the Renminbi would reduce the U.S. dollar amount available to us.
33
Our business benefits from certain government tax incentives. Expiration of, or changes to, these incentives could have a material adverse effect on our operating results by significantly increasing our tax expenses.
The PRC government has provided various tax incentives to foreign-invested companies, such as Liaoning Nuokang, Shenyang Shouzheng and Penglai Nuokang, although these incentives are subject to the new enterprise income tax law, or the new EIT law. Such tax incentives include reduced tax rates and other measures. Under the PRC tax laws effective prior to January 1, 2008, domestic companies typically were subject to an enterprise income tax rate of 33%. On March 16, 2007, the National People’s Congress of China enacted the new EIT law, and the implementation rules of such new EIT law were promulgated by the State Council of China on December 6, 2007. Under the new EIT law and its implementation rules, foreign invested enterprises, or FIEs, and domestic companies would be subject to an enterprise income tax at a uniform rate of 25%. The new EIT law and its implementation rules became effective on January 1, 2008. Under the new EIT law, enterprises that were established and already enjoyed tax holidays of two-year tax exemption and three-year tax reduction of 50% before March 16, 2007 will continue to enjoy such preferential tax treatment until the expiration of such term.
Liaoning Nuokang, Shenyang Shouzheng and Penglai Nuokang are foreign-invested companies established in 2005, 2006 and 2006, respectively, which were subject to the two-year tax exemption and three-year tax reduction of 50%. They continue to enjoy the preferential tax treatment until the expiration of such term. The tables below set forth the applicable income tax rates of our subsidiaries and Nuokang Distribution for the periods indicated.
| | | | | | | | | | | | | | | |
| Subsidiary | | 2006 | | | 2007 | | | 2008 | | | 2009 | | | 2010 | |
Liaoning Nuokang | | 0 | % | | 0 | % | | 12.5 | % | | 12.5 | % | | 12.5 | % |
Shenyang Shouzheng | | 15 | % | | 0 | % | | 0 | % | | 12.5 | % | | 12.5 | % |
Penglai Nuokang | | 0 | %(1) | | 0 | % | | 12.5 | % | | 12.5 | % | | 12.5 | % |
Nuokang Distribution | | 33 | % | | 33 | % | | 25.0 | % | | 25.0 | % | | 25.0 | % |
| (1) | Penglai Nuokang became fully exempted from enterprise income tax, or EIT, starting in September 2006. Prior to that, it was subject to EIT at a statutory rate of 33%. |
Under the new EIT law and its implementation rules, enterprises that are “high and new technology enterprises strongly supported by the State” are entitled to a reduced EIT rate of 15%. Enterprises may choose to benefit from the two-year tax exemption and three-year tax reduction of 50% tax holidays or the reduced EIT rate of 15% during the transition period before the expiration of the tax holidays. In 2008, the relevant PRC authorities recognized Liaoning Nuokang and Penglai Nuokang as “high and new technology enterprises” through 2010, after which we plan to apply for the renewal of these qualifications. We will also apply for the “high and new technology enterprise” qualification for Shenyang Shouzheng, whose existing preferential tax treatment expires in 2011. Liaoning Nuokang, Penglai Nuokang and Shenyang Shouzheng have chosen to benefit from the tax holidays during the transition period. However, after the expiration of the tax holidays, Liaoning Nuokang, Penglai Nuokang or Shenyang Shouzheng may not be qualified as “high and new technology enterprises”. Any discontinuation of preferential tax treatments or any increase of the EIT rate applicable to our subsidiaries or Nuokang Distribution could materially adversely affect our financial condition and results of operations.
34
The dividends we receive from our PRC subsidiaries and our global income may be subject to PRC tax under the new EIT law, which would have a material adverse effect on our results of operations, and dividends distributed by us to our non-PRC shareholders or any gains realized by non-PRC shareholders from transfer of our shares may be subject to PRC tax.
Under the new EIT law, dividends, interests, rents and royalties payable by a foreign-invested enterprise in the PRC to its foreign investor who is a non-resident enterprise, as well as gains on transfers of shares of a foreign-invested enterprise in the PRC by such a foreign investor, will be subject to a 10% withholding tax, unless such non-resident enterprise’s jurisdiction of incorporation has a tax treaty with the PRC that provides for a reduced rate of withholding tax. Surplus International Investments Limited, or Surplus International, the 100% shareholder of Liaoning Nuokang, Shenyang Shouzheng and Penglai Nuokang, is incorporated in Hong Kong. According to the Mainland and Hong Kong Special Administrative Region Arrangement on Avoiding Double Taxation or Evasion of Taxation on Income agreed between China and Hong Kong in August 2006, dividends paid by a foreign-invested enterprise in China to its direct holding company in Hong Kong will be subject to withholding tax at a rate of no more than 5% (if the foreign investor owns directly at least 25% of the shares of the foreign-invested enterprise). Therefore, if Surplus International is considered a non-resident enterprise for purposes of the new EIT law, this new withholding tax imposed on dividends paid to us by our PRC subsidiaries would reduce our net income in the event we decide to declare a dividend, which may have an adverse effect on our operating results.
Under the new EIT law, an enterprise established outside the PRC with its “de facto management body” within the PRC is considered a “resident enterprise” and will be subject to the enterprise income tax at the rate of 25% on its worldwide income. The “de facto management body” is defined as the organizational body that effectively exercises overall management and control over production and business operations, personnel, finance and accounting, and properties of the enterprise. It remains unclear how the PRC tax authorities will interpret such a broad definition. Substantially all of our management members are based in the PRC. If the PRC tax authorities subsequently determine that we should be classified as a resident enterprise, then our worldwide income will be subject to income tax at a uniform rate of 25%, which will decrease our earnings from operations. Notwithstanding the foregoing provision, the new EIT law also provides that, if a resident enterprise directly invests in another resident enterprise, the dividends received by the investing resident enterprise from the invested enterprise are exempted from income tax, subject to certain conditions. Therefore, if we are classified as a resident enterprise, the dividends received from our PRC subsidiaries may be exempted from income tax. However, it remains unclear how the PRC tax authorities will interpret the PRC tax resident treatment of an offshore company having ownership interest in a PRC enterprise.
In addition, because there remains uncertainty regarding the interpretation and implementation of the new EIT law and its implementation rules, if we are regarded as a PRC resident enterprise, any dividends to be distributed by us to our non-PRC shareholders or any gains realized by non-PRC shareholders or ADS holders from transfer of our shares or ADSs may be subject to PRC tax. If we are required under the new EIT law to withhold PRC income tax on the above dividends or if investors are subject to PRC income tax on gains on sale of ADSs or shares, your investment in our shares or ADSs may be materially and adversely affected.
35
If the approval of the China Securities Regulatory Commission was required in connection with our initial public offering in December 2009, we could be subject to sanctions, fines and other penalties. The regulation also establishes more complex procedures for acquisitions by foreign investors, which could make it more difficult to pursue growth through acquisitions.
On August 8, 2006, six PRC regulatory agencies, including the MOFCOM and the China Securities Regulatory Commission, or the CSRC, jointly promulgated the Regulation on Mergers and Acquisitions of Domestic Companies by Foreign Investors, which became effective on September 8, 2006. This regulation, among other things, requires offshore special purpose vehicles, formed for overseas listing purposes through acquisitions of PRC domestic companies and controlled by PRC individuals, to obtain the approval of the CSRC prior to publicly listing their securities on an overseas stock exchange. On September 21, 2006, the CSRC published a notice specifying the documents and materials that are required to be submitted for obtaining CSRC approval. Our PRC counsel, Tian Yuan Law Firm, has advised us that this regulation does not apply to us and that CSRC approval is not required because we obtained approvals from the local branches of MOFCOM for the acquisition of Liaoning Nuokang, Shenyang Shouzheng and Penglai Nuokang, our wholly owned subsidiaries in the PRC, before September 8, 2006, the effective date of this new regulation. Based on the advice we have received from our PRC counsel, we did not seek the CSRC approval in connection with our initial public offering in December 2009.
Although this regulation was adopted over three years ago, there remains uncertainty as to how this regulation will be interpreted or implemented. If the CSRC or other PRC regulatory authorities subsequently determine that the CSRC’s approval was required for our initial public offering, we may face sanctions by the CSRC or other PRC regulatory agencies. In that case, these regulatory agencies may impose fines and penalties on our operations in the PRC, limit our operating privileges in the PRC, restrict or prohibit payment or remittance of dividends by our PRC subsidiaries, or take other actions that could have a material adverse effect on our business, financial condition, results of operations, reputation and prospects, as well as the trading price of our ADSs.
The regulation also established additional procedures and requirements that could make merger and acquisition activities by foreign investors more time-consuming and complex, including requirements in some instances that the MOFCOM be notified in advance of any change-of-control transaction in which a foreign investor takes control of a PRC domestic enterprise. In the future, we may grow our business in part by acquiring complementary businesses. Complying with the requirements of this regulation to complete such transactions could be time-consuming, and any required approval processes, including obtaining approval from the MOFCOM, may delay or inhibit our ability to complete such transactions. Any delay or inability to obtain applicable approvals to complete acquisitions could affect our ability to expand our business or maintain our market share.
36
We face risks related to health epidemics and outbreaks of contagious diseases.
Our business could be adversely affected by the effects of H1N1 pandemic avian flu, SARS or other epidemics or outbreaks. China reported a number of cases of SARS in April 2004. In 2005, 2006 and 2007, there have been reports on the occurrences of avian flu in various parts of China, including a few confirmed human cases and deaths. Recently, concerns have been raised with respect to the spread of avian influenza and the H1N1 pandemic in various regions of China. Any prolonged recurrence of avian flu, SARS or other adverse public health developments in China may have a material adverse effect on our business operations. These could include temporary closure of our manufacturing facilities, as well as certain sectors of the hospital industry. Such closures or impact over business of hospitals would severely disrupt our business operations and adversely affect our results of operations. We have not adopted any written preventive measures or contingency plans to combat any future outbreak of avian flu, SARS or any other epidemic.
Risks Related to Our Corporate Structure
Our contractual arrangements with Nuokang Distribution and its shareholder may not be as effective in providing control over the entity as direct ownership.
According to the PRC Drug Administration Law and other applicable regulations, our subsidiaries in China may only sell the pharmaceutical products that we manufacture to certain distributors and we may not sell products that others manufacture. Accordingly, Mr. Baizhong Xue, our chairman and chief executive officer, established Nuokang Distribution in 1999 with four other shareholders to engage in the distribution of pharmaceutical products. Mr. Xue subsequently became the sole shareholder of Nuokang Distribution. Substantially all of our sales are conducted through Nuokang Distribution, which was the direct contracting party with most of our key distributors. We are a party to certain contractual arrangements with Nuokang Distribution which are designed to provide us with certain effective control over, and substantially all of the economic benefits of, Nuokang Distribution. If Nuokang Distribution or its sole shareholder, Mr. Baizhong Xue, refuses to make payments or otherwise refuses to perform their contractual obligations necessary for us to realize these sales contracts, we may not be able to distribute our products and our financial condition and results of operations will be materially and adversely affected.
We currently conduct our sales activities through Nuokang Distribution by means of contractual arrangements. If the PRC government determines that the contractual arrangements do not comply with applicable regulations, our business could be adversely affected.
We conduct our sales activities through contractual arrangements with Nuokang Distribution, which holds the licenses and approvals, including a pharmaceutical distribution permit that is essential for the distribution of our products. We have contractual arrangements with Nuokang Distribution and its sole shareholder, Mr. Baizhong Xue, our chairman and chief executive officer, that allow us to substantially control this entity. These contracts include business cooperation agreements, which impose certain restrictions on the conduct of Nuokang Distribution’s businesses. We cannot assure you that we will be able to enforce these contracts.
Although we believe we comply with current PRC regulations, we cannot assure you that the PRC government would agree that our arrangements with Nuokang Distribution and its sole shareholder comply with PRC licensing, registration or other regulatory requirements, with existing policies or with requirements or policies that may be adopted in the future.
37
There are substantial uncertainties regarding the interpretation and application of current and future PRC laws and regulations and the PRC government may determine that the contractual arrangements were designed to circumvent the applicable PRC laws and regulations. Accordingly, we cannot assure you that our contractual arrangements with Nuokang Distribution and its sole shareholder will be enforceable. For example, under the equity pledge agreement by and between Mr. Baizhong Xue and Penglai Nuokang, Mr. Baizhong Xue has pledged all of his equity interest in Nuokang Distribution to Penglai Nuokang to guarantee the performance of Nuokang Distribution under the relevant contractual arrangements. Under the PRC Property Law effective on October 1, 2007, an equity pledge will not become effective until it is registered with local SAIC. We are still in the process of registering the equity pledge with the SAIC Liaoning branch but are experiencing delays because the implementation rules of the PRC Property Law have only recently been issued. If the PRC government determines that we are not in compliance with applicable law, it could revoke our business and operating licenses, require us to discontinue or restrict our operations, restrict our right to collect revenues, require us to restructure our operations, impose additional conditions or requirements with which we may not be able to comply, impose restrictions on our business operations or take other regulatory or enforcement actions against us that could be harmful to our business.
The sole shareholder of Nuokang Distribution has potential conflicts of interest with us, which may adversely affect our business.
Nuokang Distribution is 100% owned by Mr. Baizhong Xue, our chairman and chief executive officer. Although Mr. Baizhong Xue owes us a duty of loyalty and care under Cayman Islands law, the potential exists for conflicts of interests between his duties to us and his ownership interests in Nuokang Distribution. In particular, Mr. Xue may be able to cause our agreements with Nuokang Distribution to be performed or amended in a manner adverse to us by, among other things, failing to remit payments to us on a timely basis or operating Nuokang Distribution so as to cause harm to our business. We can provide no assurance that if potential conflicts of interests arise, these conflicts will not result in a significant loss in corporate opportunities for us or a diversion of our resources to Nuokang Distribution, which may not be in the best interest of our company and our other shareholders.
Except for the trademarks “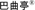 ” (Baquting) and “
” (Baquting) and “ ” (Danya) that are owned by Penglai Nuokang, all other trademarks we are currently using are owned by Nuokang Distribution. We received long term licenses from Nuokang Distribution to use these trademarks. We may need additional licenses from Nuokang Distribution in the future if we plan to use other trademarks it owns. As a result of the conflict of interest between Nuokang Distribution and us, the terms of such licenses may not be favorable to us.
” (Danya) that are owned by Penglai Nuokang, all other trademarks we are currently using are owned by Nuokang Distribution. We received long term licenses from Nuokang Distribution to use these trademarks. We may need additional licenses from Nuokang Distribution in the future if we plan to use other trademarks it owns. As a result of the conflict of interest between Nuokang Distribution and us, the terms of such licenses may not be favorable to us.
38
Our contractual arrangements with Nuokang Distribution may be subject to scrutiny by the PRC tax authorities and we could be required to pay additional taxes, which could substantially reduce our consolidated net income and the value of your investment.
Arrangements and transactions among related parties may be subject to audits or challenges by the PRC tax authorities. We could face material and adverse tax consequences if the PRC tax authorities determine that our contractual arrangements with Nuokang Distribution are not arm’s-length transactions. If this were to occur, the tax authorities could adjust Nuokang Distribution’s income in the form of a transfer pricing adjustment. A transfer pricing adjustment could, among other things, result in a reduction, for PRC tax purposes, of expense deductions recorded by Nuokang Distribution, which could in turn increase its tax liabilities. The PRC tax authorities could also impose late payment fees and other penalties on Nuokang Distribution for under-paid taxes. In addition, any challenge by the PRC tax authorities may limit the ability of Nuokang Distribution to receive any preferential tax treatments and other financial incentives. Our consolidated net income may be materially and adversely affected if Nuokang Distribution’s tax liabilities increase or if it is found to be subject to late payment fees or other penalties.
Risks Related to Our ADSs
The market price for our ADSs may be volatile.
The market price for our ADSs is likely to be volatile and subject to wide fluctuations in response to factors including the following:
| | • | | actual or anticipated fluctuations in our quarterly operating results and changes or revisions of our expected results; |
| | • | | changes in financial estimates by securities research analysts; |
| | • | | announcements of studies and reports relating to the effectiveness or safety of our products or those of our competitors; |
| | • | | negative publicity associated with pharmaceutical companies, particularly in China; |
| | • | | announcements of technological or competitive developments; |
| | • | | any litigation, governmental investigation or enforcement proceedings brought against us by authorities and industry regulators in China or elsewhere; |
| | • | | announcements regarding patent litigation or the issuance of patents to us or our competitors; |
| | • | | addition or departure of our senior management and key research and development personnel; |
| | • | | changes in the economic performance or market valuations of other pharmaceutical or health care companies; |
| | • | | economic, regulatory or political developments in China; |
| | • | | release or expiration of lock-up or other transfer restrictions on our outstanding ordinary shares or ADSs; and |
| | • | | sales of additional ordinary shares or ADSs, or the perception that such sales might occur. |
In addition, the securities market has from time to time experienced significant price and volume fluctuations that are not related to the operating performance of particular companies. These market fluctuations may also have a material adverse effect on the market price of our ADSs.
39
Substantial future sales of our ADSs in the public market, or the perception that such sales might occur, could cause the price of our ADSs to decline.
Additional sales of our ADSs in the public market, or the perception that these sales could occur, could cause the market price of our ADSs to decline. If any existing shareholder or shareholders sell a substantial amount of ordinary shares in the form of ADSs, the market price of our ADSs could decline. In addition, we may issue additional ordinary shares as considerations for future acquisitions. If we do so, your ownership interests in our company would be diluted and this in turn could have an adverse effect on the price of our ADSs.
Our principal shareholder have substantial influence over our company and their interests may not be aligned with the interests of our other shareholders.
Mr. Baizhong Xue, our chairman and chief executive officer, beneficially owned approximately 59.7% of our outstanding share capital as of March 31, 2010. Because of this high level of shareholding, Mr. Xue has substantial influence over our business, including decisions regarding mergers, consolidations and the sale of all or substantially all of our assets, election of directors and other significant corporate actions. Mr. Xue may take actions that are not in the best interest of us or our other shareholders. This concentration of ownership may discourage, delay or prevent a change in control of our company, which could deprive our shareholders of an opportunity to receive a premium for their shares as part of a sale of our company and might reduce the price of our ADSs. These actions may be taken even if they are opposed by our other shareholders.
Holders of ADSs have fewer rights than shareholders and must act through the depositary to exercise those rights.
Holders of ADSs do not have the same rights as holders of our ordinary shares and ADS holders only have such rights as are specified in the deposit agreement, which generally are more restricted than the rights of holders of ordinary shares. Under our articles of association, the minimum notice period required to convene a general meeting is at least 21 days. When a general meeting is convened, you may not receive sufficient notice of a shareholders’ meeting to permit you to withdraw your ordinary shares to allow you to cast your vote with respect to any specific matter. In addition, the depositary and its agents may not be able to send voting instructions to you or carry out your voting instructions in a timely manner. We will make all reasonable efforts to cause the depositary to extend voting rights to you in a timely manner, but we cannot assure you that you will receive the voting materials in time to ensure that you can instruct the depositary to vote your shares. Furthermore, the depositary and its agents will not be responsible for any failure to carry out any instructions to vote, for the manner in which any vote is cast or for the effect of any such vote. As a result, you may not be able to exercise your right to vote and you may lack recourse if your ordinary shares are not voted as you requested. In addition, in your capacity as an ADS holder, you will not be able to call a shareholder meeting.
40
You may be subject to limitations on transfers of your ADSs.
Your ADSs are transferable on the books of the depositary. However, the depositary may close its transfer books at any time or from time to time when it deems expedient in connection with the performance of its duties. In addition, the depositary may refuse to deliver, transfer or register transfers of ADSs generally when our books or the books of the depositary are closed, or at any time if we or the depositary deem it advisable to do so because of any requirement of law or of any government or governmental body, or under any provision of the deposit agreement, or for any other reason.
Your right to participate in any future rights offerings may be limited, which may cause dilution to your holdings.
We may from time to time distribute rights to our shareholders, including rights to acquire our securities. However, we cannot make rights available to you in the United States unless we register the rights and the securities to which the rights relate under the Securities Act or an exemption from the registration requirements is available. Also, under the deposit agreement, the depositary will not make rights available to you unless the distribution to ADS holders of both the rights and any related securities are either registered under the Securities Act, or exempted from registration under the Securities Act. We are under no obligation to file a registration statement with respect to any such rights or securities or to endeavor to cause such a registration statement to be declared effective. Moreover, we may not be able to establish an exemption from registration under the Securities Act. Accordingly, you may be unable to participate in our rights offerings and may experience dilution in your holdings.
You may not receive cash dividends if the depositary decides that it is inequitable or impractical to make them available to you.
The depositary of our ADSs has agreed to pay to you the cash dividends or other distributions it or the custodian receives on our ordinary shares or other deposited securities after deducting its fees and expenses. You will receive these distributions in proportion to the number of ordinary shares your ADSs represent. However, the depositary may, at its discretion, decide that it is inequitable or impractical to make a distribution available to any holders of ADSs. For example, the depositary may determine that it is not practicable to distribute certain property through the mail, or that the value of certain distributions may be less than the cost of mailing them. In these cases, the depositary may decide not to distribute such property, in which event you would not receive such distribution.
We are a Cayman Islands company and, because judicial precedent regarding the rights of shareholders is more limited under Cayman Islands law than under U.S. law, you may have less protection of your shareholder rights than you would under U.S. law.
Our corporate affairs are governed by our amended and restated memorandum and articles of association, the Cayman Islands Companies Law and the common law of the Cayman Islands.
The rights of shareholders to take action against the directors, the rights of minority shareholders to institute actions, and the fiduciary responsibilities of our directors to us under Cayman Islands law are to a large extent governed by the common law of the Cayman Islands. The common law of the Cayman Islands is derived in part from comparatively limited judicial precedent in the Cayman Islands as well as from English common law, the latter of which has persuasive, but not binding, authority on a court in the Cayman Islands. The rights of our shareholders and the fiduciary responsibilities of our directors under Cayman Islands law are not as clearly established as they would be under statutes or judicial precedent in the United States. In particular, because the Cayman Islands has no legislation specifically dedicated to the rights of investors in securities, and thus no statutorily defined private causes of action specific to investors in securities such as those found under the Securities Act of 1933 or the Securities Exchange Act of 1934 in the United States, it provides significantly less protection to investors. In addition, Cayman Islands companies may not have standing to initiate a shareholder derivative action before the federal courts of the United States.
41
There is uncertainty regarding whether Cayman Islands courts would:
| | • | | recognize or enforce against us or our directors or officers judgments of courts of the United States predicated upon certain civil liability provisions of U.S. securities laws; and |
| | • | | impose liability against us or our directors or officers, in original actions brought in the Cayman Islands, based on certain civil liability provisions of U.S. securities laws or laws of any state in the U.S. |
As a result of all of the above, our public shareholders may have more difficulty in protecting their interests through actions against our management, directors or major shareholders than would shareholders of a corporation incorporated in a jurisdiction in the United States.
Your ability to bring an action against us or against our directors and officers, or to enforce a judgment against us or them, will be limited because we are incorporated in the Cayman Islands, because we conduct substantially all of our operations in China and because the majority of our directors and officers reside outside of the United States.
We are incorporated in the Cayman Islands, and we conduct substantially all of our operations in China through our PRC subsidiaries. Most of our directors and officers reside, and substantially all of the assets of those persons are located, outside the United States. As a result, it may be difficult for investors to effect service of process upon these persons within the United States or to enforce against us or these persons in U.S. courts, judgments obtained in U.S. courts, including judgments based on the civil liability provisions of the federal securities laws of the United States. In addition, it may be difficult for investors to enforce, in original actions brought in courts in jurisdictions located outside the United States, liabilities based on the U.S. federal securities laws. In addition, there is uncertainty as to whether the courts of the Cayman Islands or the PRC would recognize or enforce judgments of U.S. courts against us or our officers and directors predicated upon the civil liability provisions of the securities laws of the United States or any state. Our PRC counsel has advised us that China does not have treaties with the United States or many other countries providing for the reciprocal recognition and enforcement of judgment of courts. It is also uncertain whether the Cayman Islands or PRC courts would be competent to hear original actions brought in the Cayman Islands or the PRC against us or our officers and directors predicated upon the securities laws of the United States or any state.
42
Our articles of association contains anti-takeover provisions that could have a material adverse effect on the rights of holders of our ordinary shares and ADSs.
Our amended and restated articles of association contain provisions limiting the ability of others to acquire control of our company or cause us to engage in change-of-control transactions. These provisions could have the effect of depriving our shareholders of an opportunity to sell their shares at a premium over prevailing market prices by discouraging third parties from seeking to obtain control of our company in a tender offer or similar transaction. For example, our board of directors has the authority, without further action by our shareholders, to issue preference shares in one or more series and to fix their designations, powers, preferences, privileges, and relative participating, optional or special rights and the qualifications, limitations or restrictions, including dividend rights, conversion rights, voting rights, terms of redemption and liquidation preferences, any or all of which may be greater than the rights associated with our ordinary shares, in the form of ADSs or otherwise. Preference shares could be issued quickly with terms calculated to delay or prevent a change in control of our company or make removal of management more difficult. If our board of directors decides to issue preference shares, the price of our ADSs may fall and the voting and other rights of the holders of our ordinary shares and ADSs may be materially and adversely affected.
| Item 4. | INFORMATIONONTHE COMPANY |
A. History and Development of the Company
Our legal and commercial name is China Nuokang Bio-Pharmaceutical Inc. China Nuokang Bio-Pharmaceutical Inc. was incorporated in the Cayman Islands as an exempted limited liability company in June 2006. We conduct our business operations in China through wholly owned subsidiaries and, with respect to the distribution aspects of our business, through an affiliated entity. Our business, founded in 1997, was originally conducted through Shenyang Sainuo Technology Development Co., Ltd., a PRC limited liability company, the predecessor company of Liaoning Nuokang. To conduct our research and development and manufacturing operation, two affiliated companies were formed, Shenyang Shouzheng in 2001 and Penglai Nuokang in 2002. Liaoning Nuokang, Shenyang Shouzheng and Penglai Nuokang were wholly owned by Mr. Baizhong Xue, our chairman and chief executive officer, or nominee owners on his behalf before they were acquired by Surplus International.
In order to facilitate foreign investment in our company, Surplus International was incorporated in Hong Kong in August 2005 as an offshore holding company, wholly owned by Ms. Yuhuan Zhu, the wife of Mr. Baizhong Xue, our chairman and chief executive officer. In September 2005 and July 2006, Surplus International acquired 100% of the equity interests in Liaoning Nuokang and Shenyang Shouzheng from Mr. Xue, including interests held by nominees on his behalf, for cash considerations of RMB11 million and RMB2 million, respectively. In August 2006, Surplus International acquired all of the equity interests in Penglai Nuokang from Mr. Xue for a cash consideration of RMB10 million. The Liaoning Nuokang acquisition was registered and approved by the relevant authority in December 2005. The Shenyang Shouzheng and Penglai Nuokang acquisitions were registered and approved by the relevant authorities in August 2006. This series of transactions from September 2005 to August 2006 was accounted for as a legal reorganization of entities under common control in a manner similar to a pooling of interests and the three PRC operating subsidiaries acquired by Surplus International were reorganized as wholly foreign-owned enterprises, or WFOEs. China Nuokang Bio-Pharmaceutical Inc. acquired all of the equity interests of Surplus International from Ms. Zhu in August 2006 for a cash consideration equal to its then issued capital of Surplus International of HK$10,000 ($1,464). In October 2009, we invested $5.0 million in Venomics Hong Kong Limited, a company incorporated in Hong Kong, for its 93% equity interest.
43
In December 2009, we completed our initial public offering of 5,000,000 ADSs and listed our ADSs on the NASDAQ Global Market.
Our principal executive offices are located at No. 18-1 East Nanping Road, Hunnan National New & High-tech Development Zone, Shenyang, Liaoning Province, People’s Republic of China 110171. Our telephone number at this address is +86-24-2469-6033 and our fax number is +86-24-2469-6133. Our registered office in the Cayman Islands is located at the offices of Maples Corporate Services Limited, PO Box 309, Ugland House, Grand Cayman, KY1-1104 Cayman Islands.
For information regarding our principal capital expenditures, see “–– D. Property, Plant and Equipment.”
Investor inquiries should be directed to us at the address and telephone number of our principal executive offices set forth above. Our website ishttp://www.lnnk.com. The information contained on our website does not form part of this annual report. Our agent for service of process in the United States is Law Debenture Corporate Services Inc. of 400 Madison Avenue, 4th Floor, New York, New York 10017.
B. Business Overview
Overview
We are a fully integrated, China-based biopharmaceutical company focused on the research, development, manufacture, marketing and sales of hematological and cardiovascular products. We currently sell a portfolio of fourteen products, including three principal products: (i) Baquting , our flagship bleeding control product, (ii) Aiduo
, our flagship bleeding control product, (ii) Aiduo , our cardiovascular stress imaging agent and (iii) Aiwen
, our cardiovascular stress imaging agent and (iii) Aiwen , our anti-arrhythmic agent. We have also entered into an exclusive agreement for the marketing and distribution in China of Kaitong
, our anti-arrhythmic agent. We have also entered into an exclusive agreement for the marketing and distribution in China of Kaitong , an intravenous injectable lipid emulsion of vasodilator alprostadil for the treatment of peripheral vascular diseases, cardiocerebral microcirculation disorders and post-surgery thrombosis. Our product pipeline includes four product candidates under development to address growing market and medical needs for bleeding control and hematological, cardiovascular and cerebrovascular disease diagnosis, treatment and prevention.
, an intravenous injectable lipid emulsion of vasodilator alprostadil for the treatment of peripheral vascular diseases, cardiocerebral microcirculation disorders and post-surgery thrombosis. Our product pipeline includes four product candidates under development to address growing market and medical needs for bleeding control and hematological, cardiovascular and cerebrovascular disease diagnosis, treatment and prevention.
Developed in-house and launched in 2001, Baquting is China’s leading hemocoagulase and is prescribed to hospital patients for the treatment and prevention of bleeding. A hemocoagulase is an enzyme obtained from the venom of the snake speciesBothrops atrox that can be used as a plasma clotting agent for wound and tissue repair. Baquting commanded an approximately 38% market share by volume in 2007 and maintained this market share in 2008, according to an independent study conducted by China Pharmaceutical Association, a national organization of pharmacists and pharmaceutical scientists in China. In 2007, Baquting’s market share surpassed that of Solco Basel’s Reptilase, the long-time market leader, for the first time. Reptilase, a competing Swiss brand, had an approximately 33% market share by volume in 2007. Through March 31, 2010, we had sold an aggregate of over 45 million units of Baquting to our end-customer base of over 2,400 hospitals across China. Sales of Baquting accounted for 90.1% and 93.5% of our total net revenue in 2008 and 2009, respectively. Baquting’s brand is well-recognized by the medical community in China, where traditional medicine and culture emphasize the importance of blood and limiting its loss and where there are higher clinical risks related to blood transfusions than in some Western countries. Our patent in China for Baquting with enhanced quality will expire in 2025.
44
Aiduo and Aiwen are the first adenosine products to be manufactured in China. Adenosine is a nucleoside that plays important biological roles, including dilating the coronary arteries and regulating the pacemaker activity of the heart. Aiduo is our diagnostic stress-test agent for coronary arterial diseases and Aiwen is used in the diagnosis and treatment of PSVT, an abnormal conduction of electrical signals that causes the heart to beat very rapidly. We entered into agreements to acquire Aiduo and Aiwen from two third parties, Shenyang Guangda and Shenyang Wanjia, in 2004. Aiduo and Aiwen are currently manufactured by Shenyang Guangda, and we have been the exclusive distributor and marketer of Aiduo and Aiwen in China since January 2005. In order to complete the acquisition and to commence production of Aiduo and Aiwen at our Shenyang facility, we require approval from the SFDA for the transfer of the production permit for these drugs from Shenyang Guangda to us and GMP certification for our Shenyang facility. We submitted our application for the SFDA approval in July 2008. If the SFDA approval and the GMP certification are delayed or not granted at all, we will continue to be the exclusive distributor and marketer of Aiduo and Aiwen but manufacturing will continue to be conducted by Shenyang Guangda.
In December 2008, we entered into a ten-year exclusive distribution agreement with Jilin Yuhua to market and distribute Kaitong exclusively in China with the exception of Jilin and Heilongjiang Provinces. Jilin Yuhua is in the process of obtaining a production permit for Kaitong to allow it to manufacture this product at its GMP-certified new facilities in Changchun, Jilin Province. If the production permit for Kaitong is delayed or not granted at all, Jilin Yuhua will not be able to commence its production and we will not be able to launch this product in the market.
We intend to continue to pursue product and technology in-licensing opportunities. For example, in June 2009 we entered into a joint venture with QRxPharma Limited, an Australian company, to develop and commercialize two bleeding control products based on technologies developed by QRxPharma and the University of Queensland. We invested $5.0 million in October 2009 for a 93% interest in the joint venture to fund clinical trials in China for the commercialization of Textilinin, an antifibrinolytic agent, and Haempatch, a potent pro-coagulant, under an exclusive royalty-free license.
Our product pipeline consists of four product candidates at various stages of development:
| | • | | dipyridamole aspirin sustained release capsules, which combines the anti-clotting or blood thinning properties of aspirin with the antiparticle and thrombus formation inhibition properties of dipyridamole, for the prevention of secondary strokes; |
| | • | | hemocoagulase derived from the venom of the snake species Agkistrodon acutus for bleeding control; |
| | • | | adenosine for myocardial protection; and |
| | • | | lanthanum polystyrene sulfonate for the treatment of hyperphosphatemia, or high levels of blood phosphate typically found in chronic renal failure patients undergoing dialysis. |
45
Substantially all of our sales are made to pharmaceutical distributors, which in turn sell our products primarily to hospitals and other healthcare institutions in China. Our end-customer base has expanded from approximately 100 hospitals in 2001 to over 2,400 hospitals in all 31 provinces and municipalities in China as of March 31, 2010. Our end-customer base includes approximately 80% of the Class 3A hospitals, considered the best and largest under the China Ministry of Health hospital classification system, in major Chinese cities. As of March 31, 2010, our sales force consisted of 26 direct sales offices with 300 sales personnel, complemented by 16 third-party marketing agents with over 200 sales personnel. We believe the reputation of our Nuokang brand name and our well-known branded pharmaceutical, Baquting, enable our sales and marketing team to effectively promote our principal products and help to stimulate the sales of our other pharmaceuticals. We employ a physician-targeted marketing model that is focused on promoting our products by providing physicians and hospitals with information on the benefits and differentiating clinical aspects of our products. Our sales and marketing activities receive strong academic support from a specialist network comprising approximately 100 national key opinion leaders and physicians in various clinical areas, and over 100 peer-reviewed academic articles have been published in recognized academic magazines or periodicals regarding the use of our principal products. As of March 31, 2010, our distribution network included approximately 200 distributors.
brand name and our well-known branded pharmaceutical, Baquting, enable our sales and marketing team to effectively promote our principal products and help to stimulate the sales of our other pharmaceuticals. We employ a physician-targeted marketing model that is focused on promoting our products by providing physicians and hospitals with information on the benefits and differentiating clinical aspects of our products. Our sales and marketing activities receive strong academic support from a specialist network comprising approximately 100 national key opinion leaders and physicians in various clinical areas, and over 100 peer-reviewed academic articles have been published in recognized academic magazines or periodicals regarding the use of our principal products. As of March 31, 2010, our distribution network included approximately 200 distributors.
We have built our market reputation through our strict adherence to high product safety standards and our quality control and monitoring system that spans the entire production and sales process, including inspection of raw and auxiliary materials, manufacturing, delivery of finished products, clinical testing at hospitals and ethical sales practices. Our 11,000-square-meter manufacturing facility in Penglai, China commenced operations in 2002. At this facility, we maintain five GMP-certified formulation production lines, including injectables, freeze-dried powder, aerosols, tablets and granules, and three GMP-certified active pharmaceutical ingredient production lines. In February 2008, we completed the construction of a 24,000-square-meter integrated facility in Shenyang, China. The Shenyang facility was designed to have the capacity for three additional production lines, including two formulation production lines for injectables and one active pharmaceutical ingredient production line, a new research and development center and a warehouse. Both research and development center and warehouse are operational while the GMP certification of these three production lines is currently pending.
Our net revenue was RMB147.9 million, RMB225.4 million and RMB282.9 million ($41.4 million) in 2007, 2008 and 2009, respectively. Our net income was RMB35.2 million in 2007, RMB63.6 million in 2008 and RMB65.1 million ($9.5 million) in 2009.
Our Product Portfolio
Our principal products target large markets with growing medical needs in bleeding control as well as hematological, cardiovascular and cerebrovascular disease diagnosis, treatment and prevention. We are currently focused on expanding the indication for our marketed products, developing next generation, enhanced versions of our marketed products, and bringing novel therapeutics to market.
46
The table below summarizes the development status of our principal products, product under exclusive distributorship and product candidates:
Phases I, II and III described in the table above are comparable to the similar phases of clinical trials involved in obtaining marketing approval from the U.S. Food and Drug Administration. Under the Administrative Measures on the Registration of Pharmaceutical Products promulgated by the SFDA, the three phases refer to:
“Phase I”: Preliminary evaluation of safety.
“Phase II”: Evaluation of safety, dosing and efficacy.
“Phase III”: Larger scale evaluation of safety and efficacy.
“Pre-launch”: Awaiting final SFDA approval.
Our Hematological Products and Product Candidates
Baquting
Baquting is our flagship hemocoagulase product. Baquting contains the proteolytic enzyme batroxobin, which is derived from the venom of the pit viperBothrops atrox. Batroxobin’s main function is to cleave and activate a protein called fibrinogen, and is similar in structure and function to thrombin, an endogenous human enzyme that plays a key role in the blood coagulation pathway. Baquting also contains trace amounts of a blood clotting activating factor FXA, a metalloprotease that promotes the conversion of factor X to factor Xa, which is another essential step in the coagulation pathway. We receive the venom from our South American supplier in the form of a freeze-dried powder that contains a cocktail of over 20 different active proteins and peptides, which we then purify to extract batroxobin and FXA. Batroxobin and FXA promote the blood coagulation process, and a drug containing both is more effective than either agent acting alone to reduce and stop bleeding.
Batroxobin belongs to a class of proteins known as snake venom thrombin-like enzymes and as such promotes the conversion of fibrinogen to fibrin monomer, a key step in the coagulation cascade. These effects ultimately lead to the adhesion of platelets and the formation of an interlacing fibrin monomer network. This adhesion of platelets establishes the coagulation of blood to stop bleeding. Importantly, Baquting only catalyzes the conversion of fibrinogen to fibrin in the damaged areas of blood vessels and does not promote coagulation in healthy blood vessels and therefore can be safely administered systemically.
Baquting is clinically proven to reduce and stop bleeding in various medical settings, including, but not limited to, bleeding control and bleeding disorders. It is widely used in China in the surgical setting, internal medicine, gynecology and obstetrics, ophthalmology, otorhinolaryngology and stomatology. In surgery, it is also often administered as a pre-operative prophylactic to prevent or reduce bleeding during or after an operation.
47
We developed Baquting in-house and were the first Chinese biopharmaceutical company to obtain regulatory approval for and introduce a form of injectable hemocoagulase into our domestic market. In August 2001, we received the Certificate of Chinese Chemical New Drug and the production permit for Baquting. In November 2001, we launched Baquting in the Chinese market. We originally sold Baquting in a 1 unit/vial formulation, and, in 2006, we launched two additional dosages, 0.5 unit/vial and 2 units/vial. We believe we are the first hemocoagulase manufacturer in the world to offer three distinct dosages, which provides physicians greater flexibility in prescribing the product to suit diverse patient profiles or clinical and surgical settings. We have filed a patent application in China relating to the composition of matter of Baquting. Baquting is also included as one of the hemocoagulases on the national medical insurance catalog in China, which we believe increases both its affordability for patients who have access to medical insurance and the likelihood of its prescription by physicians. In 2007, 2008 and 2009, we sold 6.3 million, 9.7 million and 13.1 million units of Baquting, respectively. According to the China Pharmaceutical Association, Baquting was the leading hemocoagulase in China with an approximately 38% market share in 2008 based on volume and 37% by volume in 2009.
Baquting can be administered systemically and locally. For systemic administration, Baquting may be injected intramuscularly or intravenously. Baquting is also approved by the SFDA to be administered locally in five ways, depending upon the indication:
| | • | | applying Baquting with absorbent gauze on wounds to stop bleeding; |
| | • | | spraying Baquting directly to the bleeding intracavitary areas with the aid of a gastroscope, bronchoscope or other similar telescopes; |
| | • | | applying Baquting locally to exposed wounds; |
| | • | | diffusing or injecting Baquting through intracavitary tubes placed in the chest, abdomen or pelvis; and |
| | • | | injecting Baquting through gastrojejunal tubes or orally taking Baquting to treat upper gastrointestinal bleeding. |
In 2000, we completed a prospective, randomized, double-blind, controlled, parallel-group, multicenter Phase III clinical trial with Baquting in 217 patients undergoing surgery. This trial compared the safety and efficacy of our product to those of Reptilase, an approved batroxobin product manufactured by Solco Basel, and Mannitol, which served as the placebo. In this trial, 97 patients received Baquting, 60 patients received Reptilase and 60 patients received Mannitol. Patients were treated with either Baquting, Reptilase or Mannitol twice, one time 16 to 18 hours before the surgery and another time thirty minutes before the surgery. The primary endpoints in the trial, including bleeding time, blood loss and blood loss per unit area, are illustrated in the following table:
| | | | | | | | | | |
| Product | | Number of
patients | | Dosage | | Bleeding time(s) | | Blood loss (g) | | Blood loss per
unit area (g/cm2) |
Baquting | | 97 | | 1 unit/vial × 2 | | 125.6±46.9 | | 9.6±2.8 | | 0.2±0.12 |
Reptilase | | 60 | | 1 unit/vial × 2 | | 118.9±40.8 | | 10.0±6.0 | | 0.2±0.15 |
Placebo | | 60 | | 30 mg/vial × 2 | | 159.2±39.2 | | 12.5±5.4 | | 0.29±0.16 |
The data demonstrated that Baquting and Reptilase had comparable bleeding time and blood loss per unit area, and Baquting outperformed the placebo in reducing bleeding time (p=0.0011) and decreasing blood loss per unit area (p=0.0016). The p value is the probability of obtaining a result at least as extreme as the one that was actually observed. A p value of less than 0.05 indicates that the two sets of data have a statistically significant difference. This trial also demonstrated that the occurrence of adverse reactions to Baquting, primarily allergic reactions, was low. The product’s positive safety profile confirms that Baquting does not promote blood coagulation in healthy blood vessels, the risk of blood clots from systemic administration of Baquting to the whole body is minimal.
48
After we launched Baquting in 2001, independent studies in various specialty fields such as oncology, gastrointestinal endoscopy, extracorporeal circulation and plastic surgery in 70 hospitals in China involved targeted clinical trials with Baquting to observe its utility, dosage, safety and efficacy in accordance with their respective clinical needs in different clinical settings. Over 60 articles have been published in connection with these clinical trials, which further validate the expanded uses and growing adoption of Baquting.
To maintain and enhance Baquting’s market leadership, we are dedicated to improving product quality and hemocoagulase production technology and to this end we are engaged in researching product modifications and enhancements. We are utilizing an FXA quantitative measurement technology that is designed to promote the bioactivity and clinical reliability of Baquting.
We believe our marketing efforts have established Baquting as a well-recognized domestic brand that has an established reputation for quality and efficacy in China. We endeavor to enhance physicians’ and hospital administrators’ knowledge of the therapeutic benefits of Baquting by using our in-house and third-party clinical study results, organizing academic seminars and conferences, and sponsoring clinical trials and testing of Baquting’s efficacy in bleeding treatment. Currently our Baquting products are primarily sold to Class 3 and Class 2 hospitals, the larger, more advanced hospitals, in big- and mid-sized cities in China.
Agkistrodon Acutus Hemocoagulase
In order to maintain our competitive advantage in the hemocoagulase market, we are developing a new type of hemocoagulase that is extracted and purified from the venom ofAgkistrodon acutus snakes, which are indigenous to China. Similar to Baquting based on theBothrops atrox venom,Agkistrodon acutushemocoagulase catalyzes fibrinogen locally in damaged blood vessels without activating the blood clotting factors in healthy blood vessels.Agkistrodon acutussnakes are prevalent in several regions in China including Guangxi, Fujian and Yunnan. We believe the domestic supplies ofAgkistrodon acutus venom would make the production costs ofAgkistrodon acutus hemocoagulase lower than those of Baquting and allow us to maintain our competitive position in the hemocoagulase market.
We co-developed theAgkistrodon acutus hemocoagulase with the Chinese Science Academy Kunming Animal Research Institute. We completed a randomized and open-label Phase I clinical trial to test the safety of this new product in 78 healthy males. Twenty-eight subjects were administered a single-dose ofAgkistrodon acutus hemocoagulase, twenty subjects were administered multiple doses and thirty subjects were involved in pharmacokinetic trials. The primary endpoints were the sustainability and safety and the rate of absorption, distribution, metabolism and excretion of this new product. These results, combined with the data from our preclinical studies, have demonstrated that this hemocoagulase was well tolerated and highlight its potential as a next generation hemocoagulase. We hold two PRC patents relating to the process technology forAgkistrodon acutus hemocoagulase that will expire in 2021. We have received SFDA approval for Phase II and Phase III clinical trials, and commenced Phase II clinical trials in January 2010. We intend to leverage our current sales and marketing network for Baquting in promoting this new product if approved.
49
Lanthanum Polystyrene Sulfonate
We are developing a lanthanum polystyrene sulfonate product candidate for the treatment of hyperphosphatemia. Hyperphosphatemia is the presence of abnormally elevated levels of phosphate in the blood. Hyperphosphatemia occurs in at least 70% of patients with renal insufficiency or acute or chronic renal failure and may lead to hyperparathyroidism, the overactivity of the parathyroid glands resulting in excess production of parathyroid hormone and abnormal metabolic activity, and a variety of other cardiovascular complications that could be life-threatening.
We believe that, when administered orally, lanthanum polystyrene sulfonate releases lanthanum ions in the gastrointestinal tract that combine with phosphate ions from food to form an insoluble, non-poisonous complex that can not be absorbed by the human body and is discharged in fecal matter. As a result, we believe this compound may have the potential to reduce the phosphate level in the blood and improve the health condition of patients with chronic renal failure and reduce the frequency of their dialysis treatments. Our lanthanum polystyrene sulfonate product candidate contains a new compound derived from resin that can be easily combined with phosphate without relying on a high pH environment and can therefore remove phosphates from various segments of gastrointestinal tracts.
We hold a patent for our lanthanum polystyrene sulfonate product candidate in China. We have completed preclinical toxicity research and pharmacodynamics studies that demonstrate the potential efficacy and safety of lanthanum polystyrene sulfonate. We are continuing to conduct long-term toxicity research and other preclinical research of our lanthanum polystyrene sulfonate product candidate.
Our Cardiovascular Products and Product Candidates
Adenosine
Aiduo and Aiwen are the first adenosine products manufactured in China. Aiduo and Aiwen consist of the same compound, but are administered at different dosages. Aiduo , our cardiovascular stress imaging agent, is administered at 30ml/90mg per dose, and Aiwen
, our cardiovascular stress imaging agent, is administered at 30ml/90mg per dose, and Aiwen , our product used in the diagnosis and treatment of PSVT, is administered at 2ml/6mg per dose. We are also developing an adenosine product for myocardial protection.
, our product used in the diagnosis and treatment of PSVT, is administered at 2ml/6mg per dose. We are also developing an adenosine product for myocardial protection.
We entered into agreements to acquire Aiduo and Aiwen from two third parties, Shenyang Guangda and Shenyang Wanjia, in 2004. Aiduo and Aiwen are currently manufactured by Shenyang Guangda, and we have been the exclusive distributor and marketer of Aiduo and Aiwen in China since 2005. In order to complete the acquisition and to commence production of Aiduo and Aiwen at our Shenyang facility, we require approval from the SFDA for the transfer of the production permit for these drugs from Shenyang Guangda to us and GMP certification for our Shenyang facility. We submitted our application for the SFDA approval in July 2008. If the SFDA approval and the GMP certification are delayed or not granted at all, we will have to continue to be the exclusive distributor and marketer of Aiduo and Aiwen that are manufactured by Shenyang Guangda.
50
Aiduo
Aiduo is an injectable coronary vasodilator that is approved for use in China as a diagnostic agent in connection with MPI stress tests. Such tests are routinely performed in a nuclear cardiology suite to diagnose coronary arterial diseases. Aiduo dilates blood vessels by activating the A2 receptor, which binds to adenosine, relaxing smooth muscles, thereby adjusting sympathetic nerve transmission and reducing blood vessel tension.
When used in MPI stress tests, adenosine significantly increases the blood flow in the healthy coronary arteries while the blood flow within the narrow segments of the coronary arteries increases insignificantly or remains the same, which changes the patterns of myocardial blood flow. A radio labeled imaging agent co-injected during cardiac imaging can therefore more effectively demonstrate the locations, scopes and levels of stenosis areas and helps the diagnosis of coronary artery disease. Adenosine is one of the only two stress agents approved by the U.S. FDA and is also recommended inGuidelines for the Clinical Use of Cardiac Radionuclide Imagingreleased by the committee comprising the American College of Cardiology, the American Heart Association and the American Society of Nuclear Cardiology (the ACC/AHA/ASNC committee) in 2003. Since 2004, the use of adenosine in MPI stress tests has gained increasing acceptance in the global medical community. TheGuidelines for Chronic Stable Angina Diagnosis and Treatmentprovided by the China Medical Association, which set forth protocol procedures to physicians, described the use of adenosine in MPI stress tests as useful and effective.
Chinese drug registration laws provided Shenyang Guangda with administrative protection for the manufacture of Aiduo as a diagnostic agent in connection with MPI stress tests in China until 2007. Administrative protection in China prevents other companies from manufacturing, importing or selling a similar drug for the same indication, unless they have received approval for the clinical trials of similar drugs prior to the commencement of the protection period. We are not aware of any other adenosine product in development in China. We believe that, given the time necessary to obtain a drug registration, Aiduo will continue to be the only adenosine product approved for use as a diagnostic agent in connection with MPI stress tests in the near term. We began sales and marketing activities for Aiduo in January 2005 and sold 13,579, 25,550 and 24,816 units in 2007, 2008 and 2009, respectively.
In 2000 and 2004, randomized, placebo-controlled, open-label multicenter clinical trials with Aiduo were conducted in 60 and 89 patients with suspected coronary arterial diseases to test the efficacy of our product in echo stress tests and MPI stress tests, respectively. In these two clinical trials, patients received Aiduo for six minutes. The endpoint was the sensitivity and specificity in the diagnosis of coronary artery disease. Our clinical trial demonstrated that our Aiduo-induced stress tests had good sensitivity and specificity.
Our marketing efforts for Aiduo products focus on its application in MPI stress tests and include promoting the drafting and application ofChinese Nuclear Cardiology Guidelines and encouraging physicians to test the performance of Aiduo based on clinical observation. To promote adenosine as the first-choice stress-test agent in China, we also provided our Aiduo products to a research project on the use of image analysis for the diagnosis and treatment of coronary artery disease as part of the state-sponsored National Eleventh Five-year Plan.
51
Aiwen
Aiwen is an intravenous anti-arrhythmic agent approved in China for the diagnosis and treatment of PSVT. Aiwen slows the conduction of electrical impulses in the heart and helps the heart to beat regularly during supraventricular tachycardia, a type of heart rhythm disorder, or a heart attack. While the Chinese market for Aiwen is still relatively underdeveloped, an example of the potential for Aiwen is that adenosine was designated as the drug of first choice to treat PSVT in theGuidelines for the Management of Patients with Supraventricular Arrhythmiasreleased by the ACC/AHA/ESC committee in 2003.
In 2000, a third party completed a randomized, controlled and multicenter clinical trial with Aiwen in 122 patients with PSVT to test the safety and efficacy of our product in treating PSVT. In this trial, 60 patients received Aiwen and 62 patients received Verapamil, a standard medicine administered by injection for treating PSVT. Patients were treated with Aiwen and Verapamil for three times with a different dosage each time within 1 or 2 minutes after each administration. The data demonstrated that patients treated with Aiwen experienced significantly shorter recovery time and had a better recovery rate compared to patients treated with Verapamil. There were 18 cases of minor adverse reactions relating to Aiwen treatment but patients recovered within one minute without discontinuing treatment.
Adenosine for Myocardial Protection
We are also developing adenosine as a myocardial protection agent in various cardiovascular-related clinical settings. We believe adenosine may be useful as a myocardial protection agent due to its ability to attenuate or prevent post-ischemic myocardial damage that occurs as a result of acute myocardial infarction, or a heart attack, and during and after heart surgery. When administered, adenosine quickly enters into the ischemic areas through the arteries, dilates the microvessel, increases blood flow in the coronary artery and protects the vascular endothelium, the layer of cells that line the interior surface of blood vessels. According to scientific literature, adenosine can effectively mitigate reperfusion injury, damage to tissue caused when blood supply returns to the area after a period of ischemia, or inadequate blood supply.
We have completed preclinical studies of adenosine for such application. We plan to apply to the SFDA for commencement of clinical trials of adenosine for myocardial protection after the transfer of the production permit for Aiduo and Aiwen from Shenyang Guangda to us is approved by the SFDA. We intend to apply for Class A new medicine status for this new indication and in April 2006 we filed a PRC patent application related to this product.
Kaitong
Kaitong is an intravenous injectable lipid emulsion of alprostadil for treatment of peripheral vascular diseases, cardiocerebral microcirculation disorders and post-surgery thrombosis. Alprostadil has been shown to dilate blood vessels, inhibit platelet aggregation, increase pulmonary blood flow, promote vasodilation and stimulate intestinal and uterine smooth muscle contraction. The total sales of alprostadil products in China was approximately RMB700 million ($102.5 million) in 2007. PICO estimates that this market may exceed RMB1.3 billion ($190.4 million) in 2011, representing a CAGR of 16.7%. Kaitong’s lipid emulsion provides it with a series of advantageous characteristics, such as specificity for the narrow segments of blood vessels, lower effective dosage and decreased side effects. It has been approved in China for (i) limb ulceration caused by chronic artery occlusion such as thromboangiitis obliterans and arteriosclerosis obliterans, pain caused by microcirculation disorders, and other cardiocerebral microcirculation disorders, (ii) use as an anti-thrombotic agent after organ transplants, and (iii) ductal-dependent congenital heart disease in the newborns, to prevent premature closure of the ductus arteriosus until surgical correction is possible.
52
In December 2008, we entered into an exclusive distribution agreement with Jilin Yuhua, amended in June and December 2009 and April 2010, under which we have the rights to exclusively market and distribute Kaitong in China with the exception of Jilin and Heilongjiang Provinces. Under this distribution agreement, Jilin Yuhua will sell Kaitong to us at a fixed wholesale price below the price at which we will sell Kaitong to our distributor customers. We do not pay Jilin Yuhua any royalties for Kaitong and instead paid an RMB16 million upfront fee to Jilin Yuhua which was fully paid by August 31, 2009. This agreement will become effective upon the earlier of December 31, 2010 and the first delivery of Kaitong by Jilin Yuhua. The term of this agreement is 10 years after it becomes effective and we have a priority right to renew this agreement upon the expiration of this agreement. Jilin Yuhua is in the process of obtaining a production permit for Kaitong to allow it to manufacture this product at its GMP-certified new facilities in Changchun, Jilin Province. In March 2010, Jilin Yuhua received a request from the SFDA for supplementary chemical manufacturing control data on Kaitong and was informed that SFDA review of Kaitong will take longer than initially anticipated. If the production permit for Kaitong is delayed or not granted at all, Jilin Yuhua will not be able to commence its production and we will not be able to launch this product in the market.
Dipyridamole Aspirin Sustained Release Capsules
We are developing dipyridamole aspirin capsules in a sustained release formulation for the prevention of strokes in patients who have previously suffered a stroke. Dipyridamole and aspirin are two different antiplatelet agents that, when used together, have been clinically demonstrated to be more effective than either agent alone in the secondary prevention of strokes.
Aspirin is an antiplatelet agent that inhibits platelet aggregation and controls active substance release in blood vessels. Dipyridamole inhibits platelet aggregation, dilates coronary vessels, significantly increases coronary blood flow and increases myocardial oxygen supply. Both drugs can inhibit platelet function and blood clot formation at different stages and can achieve better results in the secondary prevention of stroke when used together. The clinical efficacy of this product was demonstrated by the data from ESPS 2, the largest stroke prevention trial to date. This clinical study was conducted with Aggrenox, an over-the-counter dipyridamole aspirin drug that is manufactured and sold in Europe by Boehringer-Ingelheim, a German pharmaceutical company. The ESPS 2 data demonstrated that a low dose aspirin combined with an extended release dipyridamole prevented twice as many strokes as aspirin alone and also reduced the risk of stroke in patients with high blood pressure and heart disease.
In May 2007, we completed the required bioequivalence clinical trials of our dipyridamole aspirin sustained release capsules in a double-blind, double-cycle, double crossover, controlled study in 22 stroke patients. The controlled drug we used was Aggrenox which contains the same active ingredients. Our clinical trials show that both medicines are bioequivalent and there were no significant differences between the two.
53
We submitted our bioequivalence clinical trial data to the SFDA in December 2007 and received an SFDA request in June 2009 for supplementary research data. We are in the process of compiling this data. On March 2010, the SFDA advised us that it has completed all technical reviews of our dipyridamole aspirin sustained release capsules and recommended minor label modifications. We are now working with the SFDA to schedule an onsite inspection of our facilities and quality verification of this product, the final steps for it to issue a manufacturing license. We expect to receive SFDA approval for manufacturing and marketing and launch this product in the market in 2010.
Our Other Products
In addition to Baquting, Aiduo and Aiwen, we also manufacture and sell the following 11 medicines:
| | |
Medicine | | Indication |
| Salbutamol Aerosol | | Asthma and chronic obstructive pulmonary disease |
| Erythromycin Ethylsuccinate Tablets | | Infections caused by gram-positive bacteria |
| Metformin Hydrochloride Tablets | | Type 2 diabetes |
| Ribavirin Spray | | Influenza |
| “Kanglai Baby” Pediatric Paracetamol, Artificial Cow-bezoar and Chlorphenamine Maleate Granules | | Cold symptoms of children and infants, such as fever, headache and nasal congestion |
| Isoprenaline Hydrochloride Aerosol | | Asthma and complications |
| Asarone for Injection | | Asthma |
| Clemastine Fumarate Tablets | | Allergic rhinitis and urticaria |
| Gavlacon Tablets | | Pain and heartburn caused by acid reflex and chronic gastric disease |
| Lidocaine and Chlorhexine Acetate Aerosol | | Cuts, abrasions, soft tissue injuries, mosquito or insect bites, miliaria, pruritus, burns and sunburns |
| Shengxue Tablets | | Anemia |
Three of these products, salbutamol aerosol, erythromycin ethylsuccinate tablets and metformin hydrochloride tablets, have been included in the PRC National Essential Drugs List published in August 2009. Baquting and six of these products, including salbutamol aerosol, erythromycin ethylsuccinate tablets, metformin hydrochloride tablets, Ribavirin spray, isoprenaline hydrochloride aerosol and asarone for injection, are subject to price controls imposed by the NDRC and provincial price control authorities.
International Collaboration
In July 2009, we entered into a joint venture with QRxPharma Limited, a publicly listed biopharmaceutical company in Australia. QRxPharma has spun off its snake venom-related products to a new subsidiary, VPL. We have invested $500,000 for a 10% interest in VPL through our subsidiary, Surplus International. VPL in turn formed a joint venture company, VHK, in Hong Kong with our VIE, Nuokang Distribution, to develop and commercialize QRxPharma’s two bleeding control product candidates in China. Under the terms of the VHK share subscription agreement, we invested $5.0 million in October 2009 for a 93% interest in VHK. In exchange for a 7% interest, VPL granted VHK a royalty-free exclusive license to commercialize in China Textilinin, an antifibrinolytic agent, and Haempatch, a potent pro-coagulant.
54
Textilinin is a novel recombinant peptide that inhibits plasmin, a key enzyme in the fibrinolytic pathway, and has potential to reduce blood loss during major surgeries. In pre- clinical testing, Textilinin has compared favorably with other antifibrinolytic products such as Trasylol (aprotinin), a product used to reduce peri-operative blood loss in patients undergoing coronary artery bypass graft surgery.
Haempatch is a novel prothrombin activating protease, with properties similar to the FXA clotting factor. It is effective in clotting blood and stopping blood flow (haemostasis) and has compared favorably to thrombin in pre-clinical testing. Both Haempatch and the native form of Textilinin were originally isolated from the venom of the Australian Common Brown snake (Pseudonaja textilis) by researchers at the University of Queensland. QRxPharma and the University of Queensland had collaborated to develop Textilinin and Haempatch and screen Australian snake venoms for therapeutic leads.
Textilinin and Haempatch have not yet been approved for production and sale in any jurisdiction, pending the completion of clinical trials. The $5.0 million investment we made under the VHK share subscription agreement will fund the clinical trials for these product candidates in China, regardless of whether we are able to obtain regulatory approvals for their production and sale. We are not contractually committed to make any investment beyond this $5.0 million.
Sales and Marketing
We maintain our sales and marketing force in all of the 31 provinces and municipalities in China. As of March 31, 2010, our sales force consisted of 26 direct sales offices with 300 sales personnel, complemented by 16 marketing agents with over 200 sales personnel. We allocate our direct sales offices and marketing agents according to geographic areas and in certain regions such as Shanghai and Jiangsu Province with more significant market demand for our products, we maintain multiple direct sales offices. We review and improve the geographical allocation among our direct sales offices and marketing agents in response to respective market conditions and customer feedback.
Both direct sales teams and marketing agents market our principal products to physicians and hospital administrators using a physician-targeted marketing model, focusing on promoting the differentiating clinical aspects of our products. Our direct sales teams focus on raising our brand awareness and recognition by organizing academic seminars and conferences, sponsoring clinical trials and testing of our products by physicians, providing academic consulting services and developing collaborative clinical solutions. Our marketing agents focus on effective market coverage and penetration to meet rapidly growing demand for our products in their respective regions. The key management members of our current sales team have an average of over eight years of sales experience in the pharmaceutical industry. We provide in-house education and training to our sales force to improve their sales skills and efficiency and to ensure they provide our current and prospective clients with comprehensive information about our products. We employ centralized information technology to integrate market information from various sources into a unified system to increase the efficiency and effectiveness of our market data collection and analysis. Our performance-linked compensation structure and career-oriented training are key drivers that motivate our sales employees.
55
We select our marketing agents based on their reputation, market coverage, sales experience and the size of their sales force. We do not have an exclusive agency arrangement with any of our marketing agents though we believe that our marketing agents do not market competing brands. Before September 2006, we primarily relied on the joint sales and marketing efforts of our direct sales offices and marketing agents to promote our flagship product Baquting. In September 2006, we changed our sales and marketing strategy and delineated our marketing efforts into geographic regions, each covered exclusively by either our direct sales offices or third-party marketing agents.
We have established a highly integrated and well-organized sales and marketing system with five teams to manage and control our national sales and marketing activities:
| | • | | Our marketing team is responsible for developing the sales strategies and marketing plans, which are then approved by the senior management. The marketing team also provides technical support to the sales force. |
| | • | | Our sales management team collects and analyzes the latest market information in support of sales and develops strategies and marketing plans. |
| | • | | Our sales team executes the sales and marketing strategies. |
| | • | | Our agent management team focuses on the identification of new regional marketing agents and the management of existing marketing agents. |
| | • | | Our commerce team manages inventory, establishes policies relating to payment collection, receivables and other sales and distribution activities and provides pricing in regional bidding processes. |
We have established strong relationships with physicians, hospital administrators and leading experts in the hematological and cardiovascular fields. We maintain a specialist network of approximately 100 national key opinion leaders and physicians associated with national academic institutions or organizations such as the China Medical Association, China Physician Association and China Pharmaceutical Association, and provincial-level expert physicians and pharmacists in the surgery, anesthesiology, urology, neurosurgery and gastroenterology fields. Over 100 peer-reviewed academic articles have been published by recognized academic magazines or periodicals regarding the usage of our principal products. We believe our relationships with these physicians, hospital administrators and experts raise our profile, enhances awareness of our products among patients and in the medical community, provide us with valuable clinical data to improve our products and keep us abreast of industry trends and developments, all of which in turn helps us market and sell our products.
Provincial and municipal government agencies in China operate a mandatory tender process for purchases of medicines by hospitals included in provincial medicine catalogs. The tender requirement was first introduced in 2004 and has since been implemented across China. We believe effective sales and marketing efforts in promoting our products to physicians and hospitals are critical in the success of our products in the tender process. Our patents also provide our products with significant competitive advantages in the tender process in terms of pricing and success rates.
56
We maintain strict anti-corruption policies among our direct sales offices and distributors in our sales and marketing activities and we believe we will therefore be less affected by the increasingly stringent anti-corruption measures taken by the PRC government to correct corruptive practices in the pharmaceutical industry.
Distribution and Customers
Substantially all of our sales are made to pharmaceutical distributors, which in turn sell our products primarily to hospitals and other healthcare institutions in China. Currently our distribution network includes approximately 200 distributors. Our hospital coverage in China has increased from 778 hospitals in 2003 to over 2,400 hospitals as of March 31, 2010, including 80% of the Class 3A hospitals in major cities.
The tables below set forth the aggregate sales to our top five distributors, expressed in RMB and as a percentage of our total sales, for the periods indicated.
| | | | | |
| | | Year Ended
December 31, 2009 | |
| | | Sales revenue | | Sales revenue | |
| | | (RMB in
thousands) | | (% of total
sales) | |
Liaoning Pacific Medicine Co., Ltd. | | 77,687 | | 27.5 | % |
Beijing Xidan Medicine Co., Ltd. | | 17,669 | | 6.2 | % |
Chongqing Medicine Co., Ltd. | | 8,906 | | 3.1 | % |
Beijing Aixin Weiye Medicine Co., Ltd. | | 7,578 | | 2.7 | % |
Sinopharm Group Co., Ltd. | | 7,159 | | 2.5 | % |
| | | | | |
Total | | 118,999 | | 42.1 | % |
| | | | | |
Our distributors do not sell our products on an exclusive basis. We typically enter into an annual distribution agreement with each distributor which provides general terms for the distribution arrangement, such as the designated sales area, place and method for delivery, targets for annual sales volume and receivable collection. Under our standard distribution agreement, a distributor cannot sell our products outside the designated geographical area without first obtaining our written consent. The term of a distribution agreement is typically for one year, reflecting the prevailing pricing arrangement under the local competitive bidding process.
Intellectual Property
We rely primarily on a combination of patent, trademark and trade secret protections, as well as employee and third-party confidentiality agreements to protect our intellectual property. As of March 31, 2010, we owned seven invention patents and one design patent registered in the PRC, and had four pending patent applications in China and one international patent application pending. In China, patents relating to pharmaceutical inventions are effective for 20 years from the initial filing date of the patent application. Our design patent relates to the packaging box design of Baquting and will expire in July 2016. We do not own any foreign patents.
57
The following chart lists the invention patents that we own, the product or product candidate the patents relate to and their respective expiration dates:
| | | | |
Name of Patents | | Products or Product Candidates | | Expiration Dates |
Hemocoagulase ofAgkistrodon Acutus and its preparative methods | | Agkistrodon Acutushemocoagulase | | April 2021 |
A type of hemocoagulase ofAgkistrodon Acutus and its preparative methods | | Agkistrodon Acutus hemocoagulase | | April 2021 |
A type of haemostatic compound prepared from snake venom | | Baquting | | September 2025 |
A type of medication for the treatment of hyperphosphatemia and its preparative method | | Lanthanum polystyrene sulfonate | | March 2026 |
Genetic engineering batroxobin with targeted mutation and its use | | Baquting | | September 2025 |
Compound drugs for treating various disease related to the heart blood | | Aiduo | | September 2025 |
A type of bleeding control compound | | Hemocoagulase | | September 2026 |
In addition, we own 32 registered trademarks in China such as (Baquting),
(Baquting), (Aiduo) and
(Aiduo) and (Aiwen) in connection with our marketed products with expiration dates ranging from 2012 to 2019. On November 15, 2007, we received an objection raised during the publication period of our “Shunxin” trademark from Beijing Shunxin Agriculture Co., Ltd., or Beijing Shunxin, a PRC company, claiming that our “Shunxin” trademark under registration with the PRC Trademark Office imitated one of Beijing Shunxin’s trademarks used in its products. We filed our response with the PRC Trademark Office on December 13, 2007. On May 5, 2010, we received a letter from the PRC Trademark Office indicating that there was insufficient evidence supporting Beijing Shunxin’s claims and that if Beijing Shunxun does not request a second review of its objection, we will receive registration for our “Shunxin” trademark.
(Aiwen) in connection with our marketed products with expiration dates ranging from 2012 to 2019. On November 15, 2007, we received an objection raised during the publication period of our “Shunxin” trademark from Beijing Shunxin Agriculture Co., Ltd., or Beijing Shunxin, a PRC company, claiming that our “Shunxin” trademark under registration with the PRC Trademark Office imitated one of Beijing Shunxin’s trademarks used in its products. We filed our response with the PRC Trademark Office on December 13, 2007. On May 5, 2010, we received a letter from the PRC Trademark Office indicating that there was insufficient evidence supporting Beijing Shunxin’s claims and that if Beijing Shunxun does not request a second review of its objection, we will receive registration for our “Shunxin” trademark.
We also rely on trade secrets, proprietary know-how and continuing technological innovation to develop and maintain a competitive position in our product areas. We generally require our employees, consultants and advisors to enter into confidentiality agreements. These agreements provide that all confidential information developed or made known to the individual during the course of the individual’s relationship with us is to be kept confidential and not disclosed to third parties except under specific circumstances. In the case of our employees, the agreements provide that all of the technology which is conceived by the individual during the course of employment is our exclusive property. The development of our technology and many of our processes are dependent upon the knowledge, experience and skills of key scientific and technical personnel. Further, as a matter of company policy, all key scientific and technical employees have entered into agreements that generally require disclosure and assignment to us of ideas, developments, discoveries and inventions made by them.
Manufacturing
Our Penglai-based manufacturing operations consist of bulk manufacturing, and formulation, fill, and finish activities that produce products and product candidates for both clinical and commercial purposes. All of the eight production lines at our 11,000-square-meter Penglai facility are certified in accordance with current GMP, and we hold a valid GMP certificate. Three of these eight production lines are active pharmaceutical ingredient production lines while the other five are finished product production lines. We have recently expanded the production capacity of our freeze-dried powder production line at our Penglai facility by installing additional equipment and ancillary facilities. The expanded production line was GMP-certified in November 2009. As part of our overall strategy to increase our manufacturing capacity, in February 2008, we completed the construction of a 24,000-square-meter integrated facility in Shenyang, China with three additional production lines, including two formulation production lines for injectables and one active pharmaceutical ingredient production line, a new research and development center and a warehouse. We are currently awaiting GMP certification from the SFDA with regard to these three production lines. The new research and development center and warehouse are in operation and the GMP certification for these three production lines is pending.
58
We generally produce our products based on our sales plans and reasonable order forecasts. Raw materials and supplies are generally available from various suppliers in quantities adequate to meet our needs. We have not experienced any disruptions in the supply of these raw materials in the past. If any one of these supply arrangements or agreements is terminated or the ability of any one of these suppliers to perform under our agreements were to be materially adversely affected, we believe that we will be able to locate, qualify and enter into an agreement with a new supplier on a timely basis. We maintain long-term relationships with most of our suppliers and place orders from these suppliers from time to time on an as-needed basis. We expect that our existing manufacturing facilities and outside sources will allow us to meet near-term manufacturing needs for our commercial products and other products that are in clinical trials.
Quality Control and Assurance
We have our own independent quality control system and devote significant attention to quality control for the designing, manufacturing and testing of our products. We have established a strict quality control system in accordance with SFDA regulations. Our laboratories fully comply with the Chinese GMP guidelines and are staffed with highly educated and skilled technicians to ensure quality of all batches of product release. We monitor in real time our operations throughout the entire production process, from inspection of raw and auxiliary materials, manufacture, delivery of finished products, clinical testing at hospitals, to ethical sales tactics. Our quality assurance team is also responsible for ensuring that we are in compliance with all applicable regulations, standards and internal policies. Our senior management team is actively involved in setting quality policies and managing internal and external quality performance.
Raw Materials
Raw materials for the manufacturing of Baquting primarily comprise the venom ofBothrops atrox snakes. We implemented manufacturing technology upgrades for Baquting in August 2006. The technology upgrades enabled us to purify and extract batroxobin from the venom ofBothrops atrox snakes in-house and purchase the venom from the supplier in Brazil instead of purchasing the partially purified venom from third-party suppliers in China, which significantly lowered our costs of raw materials. We currently purchase all of ourBothrops atrox venom requirements from a snake farm located in Brazil through an intermediary trading company. We have entered into a supply contract with Centro De Extracão De Toxinas Animais with basic supply terms such as volumes and prices, shipment schedules, quality guarantees and defaults and claims. Unit prices are fixed, subject to annual adjustments caused by currency rate fluctuations, changes in production costs, environmental law changes and changes in government taxes and availability of the product. We face minimum annual purchase requirements over the term of the contract and are required to purchase at least $384,000, $354,000 and $236,000 worth ofBothrops atrox venom from Centro De Extracão De Toxinas Animais in 2009, 2010 and the first eight months of 2011, respectively. We purchased approximately RMB1.2 million ($179,500)worth ofBothrops atrox venom from Centro De Extracão De Toxinas Animais in 2009 due to a temporary supply disruption. Our production has not been affected as we have sufficient stock of snake venom.
59
Failure by either party to meet the minimum quantity required under the agreement would result in a penalty of 50% of the total shortfall, calculated from an average unit price based off our previous invoices. We have agreed to make full payments in advance under the contract. This supply contract runs for three years from August 2008 and is subject to automatic renewal. This fixed venom supply arrangement guarantees the consistent quality of venom we use. Our unique extraction and purification technology and large-scale production allow us to extract batroxobin of reliable quality from the venom.
Other raw materials that we use in production such as vessels, bottles, caps and other packing materials are readily available in China at reasonable costs.
Certificates and Permits
Our Penglai facility received the pharmaceutical manufacturing permit from the local SFDA in June 2002, which was renewed in January 2006 and will expire in December 2010. Our Shenyang facility received the pharmaceutical manufacturing enterprise permit from the local SFDA in May 2008, which will expire in December 2010. Nuokang Distribution’s pharmaceutical distribution permit was issued by local SFDA in 1999, renewed in December 2009 and will expire in November 2019. Nuokang Distribution’s GSP certificate was issued in September 2003, renewed in September 2008 and will expire in September 2013. The GMP certificate for our injectable production line was issued in 2005 and will expire in May 2010. The GMP certificate for our three active pharmaceutical ingredient production lines was renewed in April 2009 and will expire in April 2014. The GMP certificate for Baquting was issued by the SFDA in 2004 and expired in November 2009. The new GMP certificate for our expanded Baquting production capacity was issued in November 2009 and will expire in November 2014. The GMP certificate in relation to Aiduo and Aiwen was issued by the SFDA to a third party in 2003, renewed in 2005 and will expire in 2010. We are in the process of obtaining GMP certification for our own Aiduo and Aiwen production facility.
Competition
The market for hematological and cardiovascular products is competitive and rapidly evolving on a global basis. Increased competition has resulted in industry-wide price reductions or reduced margins. In particular, we face competition from domestic and foreign pharmaceutical companies in each of our potential product areas. Certain of these companies have substantially greater capital resources, research and development personnel, facilities and experience at conducting clinical trials and obtaining regulatory approvals, and greater experience and expertise in developing and commercializing products. In China, our flagship product Baquting competes primarily with Solco Basle’s Reptilase, Aohong Pharma’s Bangting and Lee’s Pharm’s Sulejuan. Kaitong will primarily compete with Beijing Tide Pharma’s Kaishi, Hayao Bio’s Manxintuo and Leilong Pharma’s Beichang.
60
We believe that competition and leadership in our industry are based on managerial and technological superiority, and the ability to identify and exploit commercially viable products. Other factors affecting our competitive position include time to market, patent position, product efficacy, safety, convenience, reliability, availability and pricing. We believe we are well-positioned to compete in the fast-developing Chinese biopharmaceutical market with our well recognized Nuokang and Baquting brand, diverse product portfolio, market exclusivity, proven research and development and in-licensing capabilities, established sales and marketing network, management experience and favorable cost structure.
Seasonal Variations
We experience insignificant seasonal variations in the sales of our principal products. We usually experience business declines during significant holiday seasons, such as Chinese New Year in the first quarter and National Day in the fourth quarter, both of which last over two weeks. The number of patients and surgeries tend to decrease during these holiday seasons, which in turn decreases the sales of our principal products.
Regulations
This section sets forth a summary of the most significant regulations or requirements that affect our business activities in China.
Regulation of Pharmaceutical Industry in China
Regulatory Authorities
In the PRC, the SFDA is the authority that monitors and supervises the administration of pharmaceutical products and medical appliances and equipment as well as food, health food and cosmetics. The SFDA’s predecessor, the State Drug Administration, or the SDA, was established on April 16, 1998 as an organization under the State Council to assume the responsibilities previously handled by the Ministry of Health of the PRC, or the MOH, the State Pharmaceutical Administration Bureau of the PRC and the State Administration of Traditional Chinese Medicine of the PRC. The SFDA was founded in March 2003 to replace the SDA.
The primary responsibilities of the SFDA include:
| | • | | monitoring and supervising the administration of pharmaceutical products, medical appliances and equipment as well as food, health food and cosmetics in the PRC; |
| | • | | formulating administrative rules and policies concerning the supervision and administration of food, health food, cosmetics and the pharmaceutical industry; evaluating, registering and approving of new drugs, generic drugs, imported drugs and traditional Chinese medicine, or TCM; |
| | • | | approving and issuing permits for the manufacture and export/import of pharmaceutical products and medical appliances and equipment and approving the establishment of enterprises to be engaged in the manufacture and distribution of pharmaceutical products; and |
| | • | | examining and evaluating the safety of food, health food and cosmetics and handling significant accidents involving these products. |
61
The MOH is an authority at the ministerial level under the State Council and is primarily responsible for national public health. Following the establishment of the SFDA in 2003, the MOH was put in charge of the overall administration of the national health in the PRC excluding the pharmaceutical industry. In March 2008, the State Council placed the SFDA under the management and supervision of the MOH. The MOH performs a variety of tasks in relation to the health industry such as establishing social medical institutes and producing professional codes of ethics for public medical personnel. The MOH is also responsible for overseas affairs, such as dealings with overseas companies and governments.
Healthcare System Reform
The PRC government recently promulgated several healthcare reform policies and regulations to reform the healthcare system. On March 17, 2009, the Central Committee of the PRC Communist Party and the State Council jointly issued the Guidelines on Strengthening the Reform of Healthcare System. On March 18, 2009, the State Council issued the Implementation Plan for the Recent Priorities of the Healthcare System Reform (2009-2011). On July 22, 2009, the General Office of the State Council issued the Five Main Tasks of Healthcare System Reform in 2009. On April 6, 2010, the General Office of the State Council issued the Five Main Tasks of Healthcare System Reform in 2010.
Highlights of these healthcare reform policies and regulations include the following:
| | • | | The overall objective of the reform is to establish a basic healthcare system to cover both urban and rural residents and provide the Chinese people with safe, effective, convenient and affordable healthcare services. The PRC government aims to extend basic medical insurance coverage to at least 90% of the country’s population by 2011 and increase the amount of subsidies on basic medical insurance for urban residents and rural cooperative medical insurance to RMB 120 per person per year by 2010. By 2020, a basic healthcare system covering both urban and rural residents should be established. |
| | • | | The reforms aim to promote orderly market competition and improve the efficiency and quality of the healthcare system to meet the various medical needs of the Chinese population. From 2009, basic public healthcare services such as preventive healthcare, maternal and child healthcare and health education will be provided to urban and rural residents. In the meantime, the reforms also encourage innovations by pharmaceutical companies to eliminate low-quality and duplicative products. |
| | • | | The five key tasks of the reform from 2009 to 2011 are as follows: (1) to accelerate the formation of a basic medical insurance system, (2) to establish a national essential drug system, (3) to establish a basic healthcare service system, (4) to promote equal access to basic public healthcare services, and (5) to promote the reform of public hospitals. |
62
Drug Administration Laws and Regulations
The PRC Drug Administration Law as promulgated by the Standing Committee of the National People’s Congress in 1984 and the Implementing Measures of the PRC Drug Administration Law as promulgated by the MOH in 1989 have laid down the legal framework for the establishment of pharmaceutical manufacturing enterprises, pharmaceutical trading enterprises and for the administration of pharmaceutical products including the development and manufacturing of new drugs and medicinal preparations by medical institutions. The PRC Drug Administration Law also regulates the packaging, trademarks and the advertisements of pharmaceutical products in the PRC.
Certain revisions to the PRC Drug Administration Law took effect on December 1, 2001. They were formulated to strengthen the supervision and administration of pharmaceutical products, and to ensure the quality of pharmaceutical products and the safety of pharmaceutical products for human use. The revised PRC Drug Administration Law applies to entities and individuals engaged in the development, production, trade, application, supervision and administration of pharmaceutical products. It regulates and prescribes a framework for the administration of pharmaceutical manufacturers, pharmaceutical trading companies, medicinal preparations of medical institutions and the development, research, manufacturing, distribution, packaging, pricing and advertisements of pharmaceutical products.
The PRC Drug Administration Implementation Regulations promulgated by the State Council took effect on September 15, 2002 to provide detailed implementation regulations for the revised PRC Drug Administration Law.
Examination and Approval of New Medicines
On July 10, 2007, the SFDA promulgated the Administrative Measures on the Registration of Pharmaceutical Products, or the Registration Measures, which became effective on October 1, 2007. Under the Registration Measures, new medicines generally refer to those medicines that have not yet been marketed in the PRC. In addition, certain marketed medicines may also be treated as new medicines if the type or application method of such medicines has been changed or new therapeutic functions have been added to such medicines. According to the Registration Measures, the approval of new medicines requires the following steps:
| | • | | upon completion of the pre-clinical research of the new medicine, application for registration of the new medicine will be submitted to the drug regulatory authorities at the provincial level for review in formalities. If all the formality requirements are met, the drug regulatory authorities at the provincial level will issue a notice of acceptance and conduct site inspections on the research and original data of the new medicine. The drug regulatory authorities at the provincial level will subsequently issue a preliminary opinion and notify a medical examination institute to conduct a sample examination on the new medicine (if the new medicine is a biological product); |
| | • | | the drug regulatory authorities at the provincial level will then submit their preliminary opinion, inspection report and application materials to the Drug Review Center of the SFDA and notify the applicant of the progress; |
63
| | • | | after receiving the application materials, the Drug Review Center of the SFDA will arrange for pharmaceutical, medical or other professionals to conduct a technical review on the application materials and request for supplemental materials and explanations, if necessary. After completion of the technical review, the Drug Review Center of the SFDA will issue an opinion and submit such opinion to the SFDA, along with the application materials; |
| | • | | after receiving the technical opinion from the Drug Review Center, the SFDA will assess whether or not to grant the approval for conducting the clinical research on the new medicine; |
| | • | | after obtaining the SFDA’s approval for conducting the clinical research, the applicant may proceed with the relevant clinical research (which is generally conducted in three phases for a new medicine under the Registration Measures) at institutions with appropriate qualification: |
| | • | | Phase I refers to the preliminary clinical trial for clinical pharmacology and body safety. It is conducted to observe the human body tolerance for new medicine and pharmacokinetics, so as to provide a basis for determining the prescription plan. |
| | • | | Phase II refers to the stage of preliminary evolution of clinical effectiveness. The purpose is to preliminarily evaluate the clinical effectiveness and safety of the medicine used on patients with targeted indication, as well as to provide a basis for determining the Phase III clinical trial research plan and the volume under the prescription plan. |
| | • | | Phase III is a clinical trial stage to verify the clinical effectiveness. The purpose is to test and determine the clinical effectiveness and safety of the medicine used on patients with targeted indication, to evaluate the benefits and risks thereof, and, eventually, to provide sufficient basis for review of the medicine registration application. |
| | • | | Phase IV refers the stage of surveillance and research after the new medicines is launched. The purpose is to observe the clinical effectiveness and adverse effects of the medicine over a much larger patient population and longer time period than in Phase I to III clinical trials, and evaluate the benefits and risks when it is administered to general or special patient population in larger prescription volume. |
| | • | | after completion of the relevant clinical research, the applicant shall submit its clinical research report together with the relevant supporting documents to the drug regulatory |
| | • | | authorities at the provincial level and shall provide raw materials of the standard products and research result on relevant standard products to the PRC National Institute for the Control of Pharmaceutical and Biological Products; |
| | • | | the drug regulatory authorities at the provincial level will then review the relevant documents in formalities. If all the formality requirements are met, the drug regulatory authorities at the provincial level will issue a notice of acceptance and within five days of notice and start conducting site inspections. The drug regulatory authorities at the provincial level will issue a preliminary opinion and then collect three samples of the new medicine (if the new medicine is not a biological product) and notify the relevant medicine examination institute to review the medicine standards; |
64
| | • | | the drug regulatory authorities at the provincial level will then submit their preliminary opinion, inspection report and application materials to the Drug Review Center of the SFDA and notify the applicant of the progress; |
| | • | | the medical examination institute will review the medicine standards and report its opinion to the Drug Review Center of the SFDA and send a copy of the opinion to the applicant; |
| | • | | after receiving the application materials, the Drug Review Center of the SFDA will arrange for pharmaceutical, medical or other professionals to conduct a technical review on the application materials and request for supplemental materials and explanations, if necessary. After completion of the technical review and if all the requirements are complied with, the Drug Review Center of the SFDA will report so to the Certification Center of the SFDA and notify the applicant that it may apply to the Certification Center of the SFDA for a site inspection; |
| | • | | the applicant will apply to the Certification Center of the SFDA for a site inspection within six months after receiving the notice from the Drug Review Center of the SFDA; |
| | • | | the Certification Center of the SFDA will arrange a site inspection on the process of manufacturing samples within thirty days after the application from the applicant to ensure the feasibility of the manufacturing process. The Certification Center of the SFDA will collect a sample (three samples if the new medicine is a biological product) for the medicine examination institute to examine. The Certification Center of the SFDA will prepare an inspection report within 10 days after the site inspection and submit the report to the Drug Center of the SFDA; |
| | • | | the sample(s) will be manufactured at a GMP certified workshop. The medicine examination institute will examine the sample(s) under the reviewed medicine standards and prepare a report after completion the examination and submit the report to the Drug Review Center of the SFDA. A copy of the report will be available to the applicant; |
| | • | | the Drug Review Center of the SFDA will form a comprehensive opinion based on the technical opinion previously received, the report on site inspection and the result of sample examination and submit the comprehensive opinion and the application materials to the SFDA; and |
| | • | | if all the regulatory requirements are satisfied, the SFDA will grant a new drug certificate and a pharmaceutical approval number (assuming the applicant has a valid Pharmaceutical Manufacturing Permit and the requisite production conditions for the new medicine have been met). |
65
Any applicant who is not satisfied with the SFDA’s decision to deny the application can appeal within 60 days of its receipt of the SFDA’s decision. If the applicant is dissatisfied with the result of the appeal, it may apply for an administrative review with a special committee consisting of senior officials of the SFDA or file an administrative lawsuit with a people’s court in China.
Drug Technology Transfer Regulations
On August 19, 2009, the SFDA promulgated the Administrative Regulations for Technology Transfer Registration of Drugs to standardize the registration process of drug technology transfer, which includes application for, and evaluation, examination, approval and monitoring of, drug technology transfer. Drug technology transfer refers to the transfer of drug production technology by the owner to a drug manufacturer and the application for drug registration by the transferee according to the provisions in the new regulations. Drug technology transfer includes new drug technology transfer and drug production technology transfer.
Conditions for the application for new drug technology transfer
Applications for new drug technology transfer may be submitted prior to the expiration date of the monitoring period of the new drugs with respect to:
| | • | | drugs with new drug certificates only; or |
| | • | | drugs with new drug certificates and drug approval numbers. |
For drugs with new drug certificates only and not yet in the monitoring period, or drug substances with new drug certificates, applications for new drug technology transfer should be submitted prior to the respective expiration date of the monitoring periods for each drug registration category set forth in the new regulations and after the issue date of the new drug certificates.
Conditions for the application of drug production technology transfer
Applications for drug production technology transfer may be submitted if:
| | • | | the transferor holds new drug certificates or both new drug certificates and drug approval numbers, and the monitoring period has expired or there is no monitoring period; or |
| | • | | with respect to drugs without new drug certificates, both the transferor and the transferee are legally qualified drug manufacturing enterprises, one of which holds over 50% of the equity interests in the other, or both of which are majority-owned subsidiaries of the same drug manufacturing enterprise. |
With respect to imported drugs with imported drug licenses, the original applicants for the imported drug registration may transfer these drugs to local drug manufacturing enterprises.
66
Application for, and examination and approval of, drug technology transfer
Applications for drug technology transfer should be submitted to the provincial food and drug administration. If the transferor and the transferee are located in different provinces, the provincial food and drug administration where the transferee is located should provide examination opinions. The provincial food and drug administration where the transferee is located is responsible for examining application materials for technology transfer and organizing inspections on the production facilities of the transferee. Food and drug control institutes are responsible for testing three batches of drug samples.
The Center for Drug Evaluation of the SFDA should further review the application materials, provide technical evaluation opinions and form a comprehensive evaluation opinion based on the site inspection reports and the testing results of the samples. The SFDA should determine whether to approve the application according to the comprehensive evaluation opinion of the Center for Drug Evaluation of the SFDA. An approval letter of supplementary application and a drug approval number will be issued to qualified applications. An approval letter of clinical trials will be issued when necessary. For rejected applications, a notification letter of the examination opinions will be issued with the reasons for rejection.
Permits and Licenses for Manufacturing and Registration of Drugs
Production License.To manufacture pharmaceutical products in the PRC, a pharmaceutical manufacturing enterprise must first obtain a Pharmaceutical Manufacturing Permit issued by the relevant pharmaceutical administrative authorities at the provincial level where the enterprise is located. Among other things, such a permit must set forth the permit number, the name, legal representative and registered address of the enterprise, the site and scope of production, issuing institution, date of issuance and effective period.
Each Pharmaceutical Manufacturing Permit issued to a pharmaceutical manufacturing enterprise is effective for a period of five years. Any enterprise holding a Pharmaceutical Manufacturing Permit is subject to review by the relevant regulatory authorities on an annual basis. The enterprise is required to apply for renewal of such permit within six months prior to its expiry and will be subject to reassessment by the issuing authorities in accordance with then prevailing legal and regulatory requirements for the purposes of such renewal.
Business Licenses.In addition to a Pharmaceutical Manufacturing permit, the manufacturing enterprise must also obtain a business license from the administrative bureau of industry and commerce at the local level after it has obtained the requisite Pharmaceutical Manufacturing Permit. The name, legal representative and registered address of the enterprise specified in the business license must be identical to that set forth in the Pharmaceutical Manufacturing Permit.
Registration of Pharmaceutical Products.All pharmaceutical products that are produced in the PRC must bear a registered number issued by the SFDA, with the exception of Chinese herbs and Chinese herbal medicines in soluble form. The medicine manufacturing enterprises must obtain the medicine registration number before manufacturing any medicine.
GMP Certificates.The World Health Organization encourages the adoption of good manufacturing practice, or GMP, standards in pharmaceutical production in order to minimize the risks involved in any pharmaceutical production that cannot be eliminated through testing the final products.
67
The Guidelines on Good Manufacturing Practices, as amended in 1998, or the Guidelines, took effect on August 1, 1999 and set the basic standards for the manufacture of pharmaceuticals. These Guidelines cover issues such as the production facilities, the qualification of the personnel at the management level, production plant and facilities, documentation, material packaging and labeling, inspection, production management, sales and return of products and customers’ complaints. On October 23, 2003, the SFDA issued the Notice on the Overall Implementation and Supervision of Accreditation of Good Manufacturing Practice Certificates for Pharmaceuticals, which required all pharmaceutical manufacturers to apply for the GMP certificates by June 30, 2004. Those enterprises that failed to obtain the GMP certificates by December 31, 2004 would have their Pharmaceutical Manufacturing Permit revoked by the drug administrative authorities at the provincial level. On October 24, 2007, the SFDA issued a revised Guidelines on Good Manufacturing Practices which became effective on January 1, 2008. The GMP certificate is valid for a term of five years and application for renewal must be submitted six months prior to its expiration date.
Administrative Protection and Monitoring Periods for New Drugs
According to the Registration Measures, with a view to protecting public health, the SFDA may provide for administrative monitoring periods of up to five years for new drugs approved to be manufactured, to continually monitor the safety of those new drugs.
During the monitoring period of a new drug, the SFDA will not approve any other enterprise’s application to manufacture, change the dosage of or import a similar new drug. The only exception is that the SFDA will continue to handle any application if, prior to the commencement of the monitoring period, the SFDA has already approved the applicant’s clinical trial for a similar new drug. If such application conforms to the relevant provisions, the SFDA may approve such applicant to manufacture or import the similar new drug during the remainder of the monitoring period.
Distribution of Pharmaceutical Products
According to the PRC Drug Administration Law and its implementing regulations and the Administrative Measures on Oversight of Distribution of Pharmaceutical Products, a manufacturer of pharmaceutical products in the PRC can only engage in the trading of the pharmaceutical products that the manufacturer has produced itself. In addition, such manufacturer can only sell its products to:
| | • | | wholesalers and distributors holding Pharmaceutical Distribution Permits; |
| | • | | other holders of Pharmaceutical Manufacturing Permits; or |
| | • | | medical practitioners holding Medical Practice Permits. |
A pharmaceutical manufacturer in the PRC is prohibited from selling its products to end-users, or individuals or entities other than holders of Pharmaceutical Distribution Permits, the Pharmaceutical Manufacturing Permits or the Medical Practice Permits.
68
The granting of a Pharmaceutical Distribution Permit to wholesalers shall be subject to approval of the provincial level drug regulatory authorities, while the granting of a retailer permit shall be subject to the approval of the drug regulatory authorities above the county level. Unless otherwise expressly approved, no pharmaceutical wholesaler may engage in the retail of pharmaceutical products, and neither may pharmaceutical retailers engage in wholesale.
A pharmaceutical distributor shall satisfy the following requirements:
| | • | | personnel with pharmaceutical expertise as qualified according to law; |
| | • | | business site, facilities, warehousing and sanitary environment compatible to the distributed pharmaceutical products; |
| | • | | quality management system and personnel compatible to the distributed pharmaceutical products; and |
| | • | | rules and regulations to ensure the quality of the distributed pharmaceutical products. |
Operations of pharmaceutical distributors shall be conducted in accordance with the Pharmaceutical Operation Quality Management Rules and shall be granted a GSP certificate under such rules by the SFDA.
Pharmaceutical distributors must keep true and complete records of any pharmaceutical products purchased, distributed or sold with the generic name of such products, specification, approval code, term, manufacturer, purchasing or selling party, price and date of purchase or sale. A pharmaceutical distributor must keep such record at least until one year after the expiry date of such products and in any case, such record must be kept for no less than three years. Penalties may be imposed for any violation of record-keeping.
Pharmaceutical distributors can only distribute pharmaceutical products obtained from those with a Pharmaceutical Manufacturing Permit and a Pharmaceutical Distribution Permit.
Price Control
The administration of price control of pharmaceutical products is vested in the national and provincial price administration authorities. Depending on the categories of pharmaceutical products in question, the prices of pharmaceutical products listed in the State Basic Medical Insurance Drug Catalogue, drugs with patents and other drugs whose production or trading may constitute monopolies are subject to the control of the National Development and Reform Commission of the PRC, or the NDRC, and the relevant provincial or local price administration authorities. With respect to pharmaceutical products manufactured in the PRC, the national price administration authority from time to time publishes price control lists setting out the names of pharmaceutical products and their respective price ceilings. The provincial price administration authorities also publish price control lists in respect of the pharmaceutical products which are manufactured within their respective areas. The main purpose of the price control policy is to set an upper limit to the prices of pharmaceutical products to prevent excessive increases in the prices of such products. Pursuant to the Measures for Medicine Pricing by the Government, the price ceiling is determined mainly by reference to the quality of the product, whether the products are newly developed products, and the status of implementing the GMP Guidelines by the manufacturer of the relevant product.
69
The prices of pharmaceutical products included in the price control lists are subject to adjustment upon approval by the price administration authorities from time to time. Pharmaceutical enterprises in the PRC are required to submit cost-related information such as raw material prices regularly to the relevant authorities, so that the authorities could take into account the prevailing market conditions when setting the prices. The price administration authorities may approve adjustments to the price of pharmaceutical products upon the pharmaceutical manufacturer’s request if material changes in the cost structure in producing the pharmaceutical products or significant changes in demand for these pharmaceutical products are recognized.
Tender Requirements for Hospital Purchases of Medicines
Provincial and municipal government agencies such as provincial or municipal health departments also operate a mandatory tender process for purchases by hospitals of a medicine included in provincial medicine catalogs. These government agencies organize tenders in their province or city and typically invite manufacturers of provincial catalog medicines that are on the hospitals’ formularies and are in demand by these hospitals to participate in the tender process. A government-approved committee consisting of physicians, experts and officials is delegated by these government agencies the power to review bids and select one or more medicines for the treatment of a particular medical condition. The selection is based on a number of factors, including bid price, quality and a manufacturer’s reputation and service. The bidding price of a wining medicine will become the price required for purchases of that medicine by all hospitals in that province or city. This price, however, is effective only until the next tender, where the manufacturer of the winning medicine must submit a new bid.
The tender requirement was first introduced in 2001 and has since been implemented across China. We understand that the level of present implementation of the tender requirement varies among different provinces in China. Our principal products are subject to the tender requirement.
Product Liability
In addition to the strict new drug approval process, certain PRC laws have been promulgated to protect the rights of consumers and to strengthen the control of medical products in the PRC. Under current PRC law, manufacturers and vendors of defective products in the PRC may incur liability for loss and injury caused by such products. Pursuant to the General Principles of the Civil Law of the PRC, or the PRC Civil Law, promulgated on April 12, 1986, a defective product which causes property damage or physical injury to any person may subject the manufacturer or vendor of such product to civil liability for such damage or injury.
On February 22, 1993 the Product Quality Law of the PRC, or the Product Quality Law, was promulgated to supplement the PRC Civil Law aiming to protect the legitimate rights and interests of the end-users and consumers and to strengthen the supervision and control of the quality of products. The Product Quality Law was revised by the Ninth National People’s Congress on July 8, 2000. Pursuant to the revised Product Quality Law, manufacturers who produce defective products may be subject to civil or criminal liability and have their business licenses revoked.
70
On October 31, 1993, the PRC Law on the Protection of the Rights and Interests of Consumers, or the Consumers Protection Law, was promulgated which provides further protection to the legal rights and interests of consumers in connection with the purchase or use of goods and services. At present, all business operations must observe and comply with the Consumers Protection Law when they provide their goods and/or consumer services.
Health Insurance System
According to the State Council Decision on the Establishment of the Basic Medical Insurance System of Staff in Cities and Town promulgated by the State Council in December 1998, the Ministry of Labor and Social Security assumed the responsibilities for the reform of the social medical insurance system. As part of the reform of the state basic medical insurance system for employees in the urban areas, the Ministry of Labor and Social Security, the MOH, the SDFA and various other governmental departments jointly issued the State Basic Medical Insurance Drug Catalogue with a view to enhancing the management of the use of drugs under the medical insurance system. The drugs listed in the catalogue are covered by the medical insurance system. The labor and social security authorities at the provincial level may, subject to ratification by the Ministry of Labor and Social Security, make amendments to the list of Tier 2 drugs in the catalog to a small extent to suit local needs.
Other National and Provincial Level Laws and Regulations
We are subject to changing regulations under many other laws and regulations administered by governmental authorities at the national, provincial and municipal levels, some of which are or may become applicable to our business. Our hospital customers are also subject to a wide variety of laws and regulations that could affect the nature and scope of their relationships with us.
For example, regulations control the confidentiality of patients’ medical information and the circumstances under which patient medical information may be released for inclusion in our databases, or released by us to third parties. These laws and regulations governing both the disclosure and the use of confidential patient medical information may become more restrictive in the future.
We also comply with numerous additional state and local laws relating to matters such as safe working conditions, manufacturing practices, environmental protection and fire hazard control. We believe that we are currently in compliance with these laws and regulations; however, we may be required to incur significant costs to comply with these laws and regulations in the future. Unanticipated changes in existing regulatory requirements or adoption of new requirements could therefore have a material adverse effect on our business, results of operations and financial condition.
Regulation of Patent and Trademark Protection
Patent Law
The PRC first allowed patents for the protection of proprietary rights, as set forth in the Patent Law, promulgated in 1984 and amended in 1992 and 2000. Pharmaceutical inventions were not patentable under the Patent Law until 1993. On December 27, 2008, the Standing Committee of the National People’s Congress issued the amended China Patent Law, which will become effective on October 1, 2009 (the Patent Law Amendment 2009).
71
Patents relating to pharmaceutical inventions are effective for 20 years from the initial date the patent application was filed.
Patent Prosecution
The Chinese patent prosecution system is different from the U.S. system in a number of ways. The Chinese patent system, like most countries other than the United States, adopts the principle of “first to file.” This means that, where more than one person files a patent application for the same invention, a patent will be granted to the person who first filed the application. The United States uses a principle of “first to discover” to determine the granting of patents. In addition, the PRC requires absolute novelty in order for an invention to be patentable. If a technology that is the subject of an invention patent application has been known to the public prior to the filling of the application, then such technology is not qualified to be patented as an invention. Conversely, inventors in the United States have a one-year grace period after publication of the invention in which they may file a patent. Patents issued in the PRC are not enforceable in Hong Kong, Taiwan or Macau, which each have independent patent systems. Patents are filed at the State Intellectual Property Office, or SIPO, in Beijing.
Patent Enforcement
A patent holder who believes the patent is being infringed may either file a civil legal suit or file an administrative complaint with a provincial or municipal office of SIPO. A PRC court may issue a preliminary injunction upon the patent holder’s or an interested party’s request before instituting any legal proceedings or during the proceedings. Damages for infringement are calculated as either the loss suffered by the patent holder arising from the infringement or the benefit gained by the infringer from the infringement. If it is difficult to ascertain damages in this manner, damages may be determined in the range of from one to three times of the license fee under a contractual license. If damages cannot still be determined, statutory damages from RMB10,000 to 1,000,000 may be requested. As in other jurisdictions, with one notable exception, the patent holder in the PRC has the burden of proving that the patent is being infringed. However, if the holder of a manufacturing process patent alleges infringement of such patent, the alleged infringing party has the burden of proving that there has been no infringement.
Compulsory License
Pursuant to the Patent Law Amendment 2009, SIPO may, upon the application of any entity or individual possessing the means to utilize a patented technology, grant a compulsory license to such entity or individual under the circumstances: 1) where, within three years from the date of being granted the patent right and within four years from the date of patent application, the patent holder has not utilized the patent, or has not utilized the patent adequately without any reasonable basis; or 2) where the patent holder’s act of utilizing the patent is defined as a monopoly act pursuant to the PRC laws, to eliminate or alleviate the negative impact of such act on competition. A compulsory license can also be granted where a national emergency or any extraordinary state of affairs occurs or where the public interest so requires. We do not believe a compulsory license has yet been granted by the SIPO.
72
International Patent Treaties
The PRC is also a signatory to all major intellectual property conventions, including Paris Convention for the Protection of Industrial Property, Madrid Agreement on the International Registration of Marks (Madrid Agreement) and Madrid Protocol, Patent Cooperation Treaty, Budapest Treaty on the International Recognition of the Deposit of Microorganisms for the Purposes of Patent Procedure and the Agreement on Trade-Related Aspects of Intellectual Property Rights, or TRIPs.
Although patent rights are national rights, there is also a large degree of international co-operation under the Patent Cooperation Treaty, or the PCT, to which China is a signatory. Under the PCT, applicants in one country can seek patent protection for an invention simultaneously in a number of other member countries by filing a single international patent application. The fact that a patent application is pending is no guarantee that a patent will be granted, and even if granted, the scope of a patent may not be as broad as the subject of the initial application.
Trademarks
The PRC Trademark Law was promulgated in 1982 and was amended on February 2, 1993 and October 27, 2001. The PRC Trademark Law Implementation Regulation was promulgated on August 3, 2002 and became effective on September 15, 2002. As noted above, the PRC is a signatory to the Madrid Agreement and the Madrid Protocol. These agreements provide a mechanism whereby an international registration produces the same effects as an application for registration of the mark made in each of the countries designated by the applicant.
The PRC Trademark Office is responsible for the registration and administration of trademarks throughout the country. Like patents, the PRC has adopted a “first-to-file” principle with respect to trademarks.
PRC law provides that the following acts constitute infringement of the exclusive right to use a registered trademark:
| | • | | use of a trademark that is identical with or similar to a registered trademark in respect of the same or similar commodities without the authorization of the trademark registrant; |
| | • | | sale of commodities infringing upon the exclusive right to use the trademark; |
| | • | | counterfeiting or making, without authorization, representations of a registered trademark of another person, or sale of such representations of a registered trademark as were counterfeited, or made without authorization; |
| | • | | changing a registered trademark and putting commodities on which the changed registered trademark is used into the market without the consent of the trademark registrant; and |
| | • | | otherwise infringing upon the exclusive right of another person to use a registered trademark. |
73
In the PRC, a trademark owner who believes the trademark is being infringed has three options:
| | • | | The trademark owner can provide his trademark registration certificate and other relevant evidence to the State or local Administration for Industry and Commerce, or AIC, which can, at its discretion, launch an investigation. The AIC may take such actions as: order the infringer to immediately cease the infringing behavior, seize and destroy the representations of the trademark in question and impose a fine. If the trademark owner is dissatisfied with the SAIC’s decision, he may apply to have the decision reconsidered. |
| | • | | The trademark owner may institute civil proceedings directly with the court. Civil redress for trademark infringement includes: |
| | • | | requiring the infringer to take steps to mitigate the damage (i.e., print notices in newspapers); and |
| | • | | damages (i.e. compensation for the economic loss and injury to reputation as a result of trademark infringement suffered by the trademark holder). |
| | • | | The amount of compensation is calculated according to either the gains acquired by the infringer from the infringement during the infringement, or the loss suffered by the trademark owner, including expenses incurred by the trademark holder to deter such infringement. If it is difficult to determine the gains acquired by the infringer from the infringement, or the loss suffered by the trademark owner, the court may elect to award compensation of not more than RMB500,000. |
| | • | | If the case is so serious as to constitute a crime, the trademark owner may lodge a complaint with the relevant public security organization. |
Regulation on Foreign Currency Exchange
Foreign Currency Exchange
Foreign currency exchange regulation in China is primarily governed by the following rules:
| | • | | Foreign Currency Administration Rules (1996), as amended, or the Exchange Rules; and |
| | • | | Administration Rules of the Settlement, Sale and Payment of Foreign Exchange (1996), or the Administration Rules. |
Under the Exchange Rules, the Renminbi is convertible for current account items, including the distribution of dividends, interest payments, trade and service-related foreign exchange transactions. Conversion of Renminbi for capital account items, such as direct investment, loan, security investment and repatriation of investment, however, is still subject to the approval of the PRC State Administration of Foreign Exchange, or SAFE.
74
Under the Administration Rules, foreign-invested enterprises may only buy, sell and/or remit foreign currencies at those banks authorized to conduct foreign exchange business after providing valid commercial documents and, in the case of capital account item transactions, obtaining approval from the SAFE. Capital investments by foreign-invested enterprises outside of China are also subject to limitations, which include approvals by the Ministry of Commerce, the SAFE and the NDRC.
Foreign Exchange Registration of Offshore Investment by PRC Residents
Pursuant to the SAFE’s Notice on Relevant Issues Concerning Foreign Exchange Administration for PRC Residents to Engage in Financing and Inbound Investment via Overseas Special Purpose Vehicles, generally known in China as SAFE Circular No. 75, issued on October 21, 2005 and became effective on November 1, 2005, (i) a person residing in the PRC, who is referred to as a PRC resident in SAFE Circular 75, shall register with the local branch of the SAFE before it establishes or controls an overseas special purpose vehicle, or SPV, for the purpose of overseas equity financing (including convertible debts financing); (ii) when a PRC resident contributes the assets of or its equity interests in a domestic enterprise into an SPV, or engages in overseas financing after contributing assets or equity interests into an SPV, such PRC resident shall register his or her interest in the SPV and the change thereof with the local branch of the SAFE; and (iii) when the SPV undergoes a material event outside of China, such as change in share capital or merger and acquisition, the PRC resident shall, within 30 days from the occurrence of such event, register such change with the local branch of the SAFE. PRC residents who are shareholders of SPVs established before November 1, 2005 were required to register with the local SAFE branch before March 31, 2006.
Under SAFE Circular No. 75, failure to comply with the registration procedures set forth above may result in the penalties, including imposition of restrictions on a PRC subsidiary’s foreign exchange activities and its ability to distribute dividends to the SPV. See “Item 3. Key Information – D. Risk Factors—Risks Related to Doing Business in China—Recent regulations relating to offshore investment activities by PRC residents may limit our ability to acquire PRC companies and could adversely affect our business, financial condition and results of operations.”
On December 25, 2006, the People’s Bank of China promulgated the “Measures for the Administration of Individual Foreign Exchange,” and on January 5, 2007, the SAFE further promulgated the implementation rules on those measures. Both became effective on February 1, 2007. According to the implementation rules, PRC citizens who are granted shares or share options by a company listed on an overseas stock market according to its employee share option or share incentive plan are required, through the PRC subsidiary of such overseas listed company or any other qualified PRC agent, to register with the SAFE and to complete certain other procedures related to the share option or other share incentive plan. Foreign exchange income received from the sale of shares or dividends distributed by the overseas listed company may be remitted into a foreign currency account of such PRC citizen or be exchanged into Renminbi. Our PRC citizen employees who have been granted share options are subject to the Individual Foreign Exchange Rules as a result of the listing of our ADSs on the NASDAQ Global Market.
75
Regulation on Dividend Distribution
The principal regulations governing distribution of dividends paid by wholly foreign-owned enterprises include:
| | • | | Wholly Foreign-Owned Enterprise Law (1986), as amended; and |
| | • | | Wholly Foreign-Owned Enterprise Law Implementation Rules (1990), as amended. |
Under these regulations, foreign-invested enterprises in China may pay dividends only out of their accumulated profits, if any, determined in accordance with PRC accounting standards and regulations. In addition, a wholly foreign-owned enterprise in China is required to set aside at least 10.0% of its after-tax profit based on PRC accounting standards each year to its general reserves until the accumulative amount of such reserves reach 50.0% of its registered capital. These reserves are not distributable as cash dividends. The board of directors of a foreign-invested enterprise has the discretion to allocate a portion of its after-tax profits to staff welfare and bonus funds, which may not be distributed to equity owners except in the event of liquidation.
Regulation on Overseas Listings
On August 8, 2006, six PRC regulatory agencies, including the Ministry of Commerce, or the MOFCOM, and the CSRC, jointly adopted the Regulations on Mergers and Acquisitions of Domestic Enterprises by Foreign Investors, or the M&A Rule, which became effective on September 8, 2006. The M&A Rule, among other things, purports to require an offshore special purpose vehicle, or SPV, formed for the purpose of listing the SPV’s securities on an offshore securities exchange and controlled directly or indirectly by PRC companies or individuals, to obtain the approval of the CSRC prior to such offshore listing and trading. On September 21, 2006 the CSRC published on its official website procedures specifying documents and materials required to be submitted to it by SPVs seeking CSRC approval of their overseas listings. However, substantial uncertainty remains regarding the scope and applicability of the M&A Rule to overseas listings of offshore SPVs.
Our PRC counsel, Tian Yuan Law Firm, has advised us that, based on its understanding of the current PRC laws, regulations and rules, it was not necessary for us to obtain the CSRC’s approval for our initial public offering because we obtained approvals from local branches of MOFCOM for the acquisition of Liaoning Nuokang, Shenyang Shouzheng and Penglai Nuokang, our wholly owned subsidiaries in the PRC, before September 8, 2006, the effective date of the new regulation.
See “Item 3. Key Information – D. Risk Factors—Risks Related to Doing Business in China—The approval of the China Securities Regulatory Commission may be required in connection with our initial public offering in December 2009; any failure to obtain this approval, if required, could have a material adverse effect on our business, operating results and trading price of our ADSs, and may also create uncertainties for our initial public offering. The regulation also establishes more complex procedures for acquisitions by foreign investors, which could make it more difficult to pursue growth through acquisitions.”
76
C. Organizational Structure
The following diagram illustrates our corporate structure as of the date of this annual report.
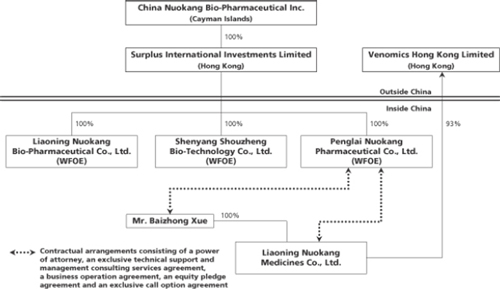
Contractual Arrangements with Nuokang Distribution and Its Sole Shareholder
PRC laws and regulations currently impose different levels of restrictions or prohibitions on investment of foreign capital in the pharmaceutical distribution industry. See “—B. Business Overview—Regulations—Distribution of pharmaceutical products.” Our WFOE subsidiaries in China may only sell the pharmaceutical products that we manufacture to certain distributors and may not sell products that others manufacture. Accordingly, we conduct our distribution activities through Nuokang Distribution, a company whose sole registered shareholder is Mr. Xue, and other third-party distributors. Through contractual arrangements with Mr. Xue and Nuokang Distribution, we bear the economic risks, and derive the economic benefits, of Nuokang Distribution. Nuokang Distribution is our VIE and we are its primary beneficiary. The financial results of Nuokang Distribution are consolidated into our financial statements despite the lack of our majority equity interest in Nuokang Distribution. We believe the consolidation is necessary to fairly present the financial position and results of operations of our company, because of the existence of a parent-subsidiary relationship by means of contractual arrangements.
Penglai Nuokang has entered into contractual arrangements with Nuokang Distribution and its shareholder, which enable us to:
| | • | | exercise effective control over Nuokang Distribution; |
| | • | | receive substantially all of the economic benefits from Nuokang Distribution as if we were its sole shareholder; and |
| | • | | have an exclusive option to purchase all of the equity interests in Nuokang Distribution. |
77
Equity Pledge Agreement
Under his equity pledge agreement with Penglai Nuokang, Mr. Xue, the sole shareholder of Nuokang Distribution, pledged all of his equity interests in Nuokang Distribution to Penglai Nuokang to secure the performance of his and Nuokang Distribution’s obligations under the business operation agreement and the exclusive technology support and management consulting service agreement described below. If Nuokang Distribution or Mr. Xue breaches any of their respective contractual obligations under these agreements, Penglai Nuokang, as pledgee, will be entitled to certain rights, including the right to sell the pledged equity interests. Mr. Xue has agreed not to transfer, sell, pledge, dispose of or otherwise create any new encumbrance on his equity interests in Nuokang Distribution without the prior written consent of Penglai Nuokang. The duration of the equity pledge agreement is the same as the term of the exclusive technology support and management consulting service agreement, which will be valid and effective as long as Nuokang Distribution is in existence.
Exclusive Call Option Agreement
Under the exclusive call option agreement among Nuokang Distribution, Mr. Xue and Penglai Nuokang, Nuokang Distribution and Mr. Xue irrevocably granted Penglai Nuokang or its designated person an exclusive option to purchase, when and to the extent permitted under PRC law, all or part of the equity interests in Nuokang Distribution. The exercise price for the option to buy all of the equity interests is the then total amount of Nuokang Distribution’s registered capital or such other minimum amount agreed to by all parties and permissible under PRC law. Such consideration received by Mr. Xue upon the exercise of the exclusive call option is required to be remitted in full to Penglai Nuokang. The exclusive call option agreement will remain valid and effective for ten years and is renewable at Penglai Nuokang’s sole discretion. This exclusive call option agreement provides that without Penglai Nuokang’s prior written consent:
| | • | | Mr. Xue may not transfer, encumber, grant security interest in, or otherwise dispose of any equity interests in Nuokang Distribution, except as required under applicable law; |
| | • | | Nuokang Distribution may not (i) transfer, grant security interest in or otherwise dispose of any assets, business, revenue or interest valued at RMB2 million or more; (ii) enter into any material contract in the amount of RMB2 million or more (except for those incurred in the ordinary course of business); or (iii) incur any liabilities (except for those incurred in the ordinary course of business); |
| | • | | Nuokang Distribution may not declare or pay any dividends and Mr. Xue must remit in full any funds received from Nuokang Distribution to Penglai Nuokang; and |
| | • | | Nuokang Distribution may not merge with any third parties, or purchase or transfer any assets or businesses from or to any third parties. |
78
Business Operation Agreement
Under the business operation agreement among Nuokang Distribution, Mr. Xue and Penglai Nuokang, Mr. Xue may not enter into any transaction that could materially affect Nuokang Distribution’s assets, liabilities, interests or operations without the prior written consent of Penglai Nuokang, and Mr. Xue must appoint Penglai Nuokang’s nominees to Nuokang Distribution’s board of directors and to act as the general manager, financial controller and other senior managers. In addition, if Nuokang Distribution requires a guarantor for its borrowings, it will first make such request to Penglai Nuokang. Despite the fact that Penglai Nuokang retains the contractual right to object to such request, it is our intention to facilitate such guarantee if and when such request is made. The term of this agreement is ten years from December 14, 2007 and is renewable at Penglai Nuokang’s sole discretion.
Power of Attorney
Mr. Xue has executed a power of attorney appointing Penglai Nuokang or Chinese natural persons designated by Penglai Nuokang to be his attorneys, and irrevocably authorizing them to vote on his behalf on all of the matters of Nuokang Distribution requiring shareholders’ approval.
Exclusive Technology Support and Management Consulting Service Agreement
Under the exclusive technology support and management consulting service agreement between Nuokang Distribution and Penglai Nuokang, Penglai Nuokang has the exclusive right to provide to Nuokang Distribution technology support and consulting services related to the business operations of Nuokang Distribution. Nuokang Distribution agrees to pay monthly service fees to Penglai Nuokang in the amount of 80% of Nuokang Distribution’s total monthly revenue after deducting sales expenses and operational costs approved by Penglai Nuokang, which is adjustable at the sole discretion of Penglai Nuokang. In addition, Penglai Nuokang agrees to provide full financial support for Nuokang Distribution’s working capital requirement for its business operations. If Nuokang Distribution is unable to repay its obligations owing to Penglai Nuokang, Penglai Nuokang agrees not to enforce these obligations. This agreement will remain valid and effective as long as Nuokang Distribution is in existence.
D. Property, Plant and Equipment
Our manufacturing facilities of 11,000 square meters are located in Penglai, Shandong Province, China. Our facilities in Penglai consist of office, research and development and manufacturing facilities. We lease the land and buildings where our Penglai manufacturing facilities are located from a third party, Shandong Penglai Pharmaceutical Plant. We intend to extend such lease when it expires in December 2014. In February 2008, we completed the construction of a 24,000 square meter integrated facility in Shenyang, China with three additional production lines, a new research and development center and a warehouse. The new research and development center and warehouse are in operation and the GMP certification from the SFDA for these three production lines is pending. We believe that our existing facilities are adequate for our current and foreseeable production requirements of our principal products.
79
| Item 4A. | UNRESOLVED STAFF COMMENTS |
None.
| Item 5. | OPERATINGAND FINANCIAL REVIEWAND PROSPECTS |
The following discussion of our financial condition and results of operations is based upon and should be read in conjunction with our consolidated financial statements and their related notes included in this annual report. This report contains forward-looking statements. See “—G. Safe Harbor.” In evaluating our business, you should carefully consider the information provided under the caption “Item 3. Key Information—D. Risk Factors” in this annual report. We caution you that our businesses and financial performance are subject to substantial risks and uncertainties.
A. Operating Results
Overview
We are a fully integrated, China-based biopharmaceutical company focused on the research, development, manufacture, marketing and sales of hematological and cardiovascular products. We currently sell a portfolio of 14 products, including three principal products Baquting , Aiduo
, Aiduo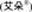 and Aiwen
and Aiwen . Our product pipeline includes four product candidates under development that seek to better address the growing market and medical needs for bleeding control and hematological, cardiovascular and cerebrovascular disease diagnosis, treatment and prevention.
. Our product pipeline includes four product candidates under development that seek to better address the growing market and medical needs for bleeding control and hematological, cardiovascular and cerebrovascular disease diagnosis, treatment and prevention.
We generate our revenues primarily from the sale of our flagship product Baquting, China’s leading hemocoagulase prescribed for treating or preventing bleeding in the hospital, Aiduo, our cardiovascular stress imaging agent and Aiwen, our anti-arrhythmic agent. In 2007, 2008 and 2009, Baquting accounted for 91.5%, 90.1% and 93.5% of our total net revenue, respectively, while Aiduo and Aiwen accounted for 2.4%, 2.8% and 2.0% of our total net revenue, respectively. Our other marketed products collectively accounted for 6.1%, 7.1% and 4.5% of our total net revenue in the same periods. We expect sales of Baquting will continue to grow but account for a decreasing percentage of our total net revenue as we generate additional revenues from new product launches. We will launch Kaitong , a lipid emulsion alprostadil product candidate for the treatment of peripheral vascular diseases, cardiocerebral microcirculation disorders and post-surgery thrombosis, subject to the ability of Jilin Yuhua, the manufacturer of Kaitong, to receive a production permit from SFDA. We also plan to launch dipyridamole aspirin sustained release capsules once we receive SFDA manufacturing and marketing approval.
, a lipid emulsion alprostadil product candidate for the treatment of peripheral vascular diseases, cardiocerebral microcirculation disorders and post-surgery thrombosis, subject to the ability of Jilin Yuhua, the manufacturer of Kaitong, to receive a production permit from SFDA. We also plan to launch dipyridamole aspirin sustained release capsules once we receive SFDA manufacturing and marketing approval.
We have grown significantly since our inception in 1997. We have established an integrated research and development, production, and sales and marketing infrastructure. Our end-customer base expanded from approximately 100 hospitals in 22 provinces in 2001 to over 2,400 hospitals in all 31 provinces in China as of March 31, 2010, including approximately 80% of the Class 3A hospitals in major Chinese cities. As of March 31, 2010, our sales force consisted of 26 direct sales offices with 300 sales personnel, complemented by 16 third-party marketing agents with over 200 sales personnel. Substantially all of our sales are made to pharmaceutical distributors, which in turn sell our products primarily to hospitals and other healthcare institutions in China. We sold 6.3 million, 9.7 million and 13.1 million units of Baquting and 16,730, 30,221 and 25,856 units of Aiduo and Aiwen in 2007, 2008 and 2009, respectively. Our pharmaceutical products are distributed through Penglai Nuokang and Nuokang Distribution, our consolidated variable interest entity.
80
Our net revenue was RMB147.9 million, RMB225.4 million and RMB282.9 million ($41.4 million) in 2007, 2008 and 2009, respectively. Our net income increased from RMB35.2 million in 2007 to RMB65.1 million ($9.5 million) in 2009.
Financial Overview
Net Revenue
We derive revenue primarily from the sale of our marketed products. Our net revenue represents our total revenue from the sales of our products, less value-added taxes, or VAT. In the PRC, VAT on the invoiced amount is collected on behalf of tax authorities in respect of the sales of goods. VAT collected from customers is set off by VAT paid for purchases, with the net amount recorded as a liability in the consolidated balance sheet until it is paid to the tax authorities.
Sales of Baquting, Aiduo and Aiwen accounted for substantially all of our net revenue in 2007, 2008 and 2009. The following table sets forth our net revenue by product, both in absolute amounts and as percentages of total net revenue, for the periods indicated.
| | | | | | | | | | | | | | |
| | | Year Ended December 31, |
| | | 2007 | | 2008 | | 2009 |
| | | RMB | | % | | RMB | | % | | RMB | | $ | | % |
| | | (in thousands, except percentages) |
Baquting | | 135,311 | | 91.5 | | 203,164 | | 90.1 | | 264,533 | | 38,754 | | 93.5 |
Aiduo/Aiwen | | 3,511 | | 2.4 | | 6,318 | | 2.8 | | 5,598 | | 820 | | 2.0 |
Others marketed products | | 9,053 | | 6.1 | | 15,917 | | 7.1 | | 12,780 | | 1,873 | | 4.5 |
| | | | | | | | | | | | | | |
Total | | 147,875 | | 100.0 | | 225,399 | | 100.0 | | 282,911 | | 41,447 | | 100.0 |
| | | | | | | | | | | | | | |
Substantially all of our sales are made to pharmaceutical distributors, which in turn sell our products primarily to hospitals and other healthcare institutions in China. We typically enter into a distribution agreement with each distributor for a one-year term that is generally renewed. As is typical in China, our distributors do not sell our products on an exclusive basis. In each of 2007, 2008 and 2009, Liaoning Pacific Medicine Co., Ltd., or Liaoning Pacific, contributed 10% or more of our total net revenue. Sales to Liaoning Pacific accounted for 27.5% of our total net revenue in the year ended December 31, 2009. Our one-year framework distribution agreement with Liaoning Pacific, similar to our agreements with other distributors, includes general terms for the distribution arrangements, such as an annual sales target, stipulated wholesale sales prices, place and method of delivery, payment terms and geographical allocation. Each purchase is governed by purchase orders that Liaoning Pacific and we enter into from time to time. Our top five distributors in the aggregate accounted for 37.2%, 33.4% and 42.1% of our net revenue in 2007, 2008 and 2009, respectively. We expect that a relatively small number of our distributors will continue to account for a major portion of our net revenue in the near future.
Our marketing efforts are delineated into geographical regions, each covered exclusively by either our direct sales offices or third-party marketing agents. We do not incur sales and marketing expenses for the regions covered by the third-party marketing agents. As we grew our business and intensified our marketing efforts, a clear delineation between internal staff and external agents allows us to focus on ensuring ethical sales strategies of our staff without the diversion of resources that would be required to monitor the day to day activities of our marketing agents. In 2007, 2008, 2009, net revenue from Baquting sales made through our direct sales offices accounted for 68.8%, 70.1% and 67.7%, respectively, of our total Baquting sales revenue, and net revenue from Baquting sales made through third-party marketing agents accounted for the balance of 31.2%, 29.9% and 32.3%, respectively.
81
Factors Impacting Our Revenues
Sales Volume
The primary factor impacting our revenues is the sales volume of our principal products, which is driven in part by market demand for our products. Market demand in turn depends on the perception and acceptance of our products by physicians and patients. We sold 6.3 million, 9.7 million and 13.1 million units of Baquting in 2007, 2008 and 2009, respectively, representing a CAGR of 44.2% from 2007 to 2009. We believe market demand for Baquting has been increasing over the years due to higher healthcare quality requirements, increasing healthcare spending of patients, increasing attention to risks associated with transfusion and blood supply shortage, and the inclusion of hemocoagulase in the national medical insurance catalog.
Our marketed products and product candidates target attractive commercial opportunities in the Chinese hospital market, focusing on the hematological market and cardiovascular markets. We believe there are growth opportunities in these markets. Factors driving the growth of our product revenue include increasing healthcare spending, including among rural households with greater healthcare awareness, an expanded health insurance coverage, government support of the pharmaceutical industry, higher incidence of lifestyle diseases, an aging population, and a higher prevalence of blood disorders among people over 35 years of age.
Product Mix
In 2007, 2008 and 2009, approximately 91.5%, 90.1% and 93.5% of the total net revenue was generated by sales of Baquting, respectively. Since we began sales and marketing activities for Aiduo and Aiwen in 2005, its sales revenue increased to account for 2.8% of our total net revenue in 2008 and 2.0% of our total net revenue in 2009. Our other marketed products accounted for approximately 6.1%, 7.1% and 4.5% of our total net revenue in 2007, 2008 and 2009, respectively. Baquting is available in three differently-priced dosages, 0.5 unit/vial, 1 unit/vial and 2 units/vial. We launched the 1 unit/vial dosage in 2001 and the 0.5 unit/vial and 2 units/vial dosages in 2006. The three distinct dosages offer physicians the flexibility in prescribing the drug to suit different patient profiles or medical situations, which we believe provides us an advantage in the tender process. On a per-unit basis, the selling price of the 0.5 unit/vial dosage tends to be slightly higher than that of the 1 unit/vial dosage, and the selling price of the 2 units/vial tends to be lower than that of the 1 unit/vial dosage. From 2007 to 2009, sales of the 0.5 unit/vial and 2 units/vial dosages increased both in absolute amounts and as percentages of total Baquting sales.
82
The following table sets forth our Baquting sales revenue by dosage, both in absolute amounts and as percentages of our total Baquting sales revenue, for the periods indicated:
| | | | | | | | | | | | | | |
| | | Year Ended December 31, |
| | | 2007 | | 2008 | | 2009 |
| | | RMB | | % | | RMB | | % | | RMB | | $ | | % |
| | | (in thousands, except percentages) |
0.5 unit/vial | | 3,669 | | 2.7 | | 8,778 | | 4.3 | | 17,184 | | 2,517 | | 6.5 |
1 unit/vial | | 128,075 | | 94.7 | | 181,106 | | 89.2 | | 219,863 | | 32,210 | | 83.1 |
2 units/vial | | 3,567 | | 2.6 | | 13,280 | | 6.5 | | 27,486 | | 4,027 | | 10.4 |
| | | | | | | | | | | | | | |
Total | | 135,311 | | 100.0 | | 203,164 | | 100.0 | | 264,533 | | 38,754 | | 100.0 |
| | | | | | | | | | | | | | |
We expect our product mix to diversify as we launch new products to market. We plan to launch Kaitong after Jilin Yuhua, the manufacturer of Kaitong, receives the production permit from SFDA. We expect to launch dipyridamole aspirin sustained release capsules once we receive the SFDA approval to manufacture and market the product. As a result, we expect future sales of Baquting to continue to grow and constitute a substantial portion of our total revenues, but its sales will account for a lower percentage of our total net revenues in the future.
Market demand for bleeding control drugs has been increasing over the years due to higher healthcare quality requirements, increasing healthcare spending by patients and increasing attention to risks associated with transfusion and blood supply shortage. Due to their proven safety and efficacy, hemocoagulase products have received increasing acceptance and recognition in the Chinese medical community, have had broader clinical applications and have increased their market shares by replacing traditional bleeding control products. We believe physicians’ and patients’ knowledge and acceptance of our principal products will be further enhanced by our established physician-targeted marketing model, focusing on detailing and promoting our products by providing physicians and hospitals with information on the benefits and differentiating clinical aspects of our products.
Product Pricing and Our Ability to Compete in the Tender Processes
Price Controls and Pricing Models. Pharmaceutical products sold in China, primarily those included in the national or provincial medical insurance catalog, are subject to price controls in the form of fixed retail prices or retail price ceilings. From time to time, the PRC government publishes and updates a list of medicines that are subject to price controls, either at the national level or the provincial or regional level. PRC government authorities impose no control over the prices at which pharmaceutical manufacturers sell their products to their distributors. Nevertheless, the prices at which pharmaceutical manufacturers, such as us, sell products to distributors are impacted by the relevant fixed retail price or retail price ceilings.
Baquting is included as one of the hemocoagulases in the national medical insurance catalog and is subject to retail price controls at the national level. Government-controlled hemocoagulase prices remained relatively stable from 2005 through 2008 in China. In 2007, the NDRC set RMB51.6 per unit as the maximum retail price at which hemocoagulase products may be sold to patients. This price ceiling continues to remain in effect. Aiduo is included in the provincial medical insurance catalog in four provinces and in the municipal medical insurance catalog in Changchun, China and is subject to price controls within the respective provinces and Changchun. We price Aiduo in these four provinces and Changchun by reference to government controlled prices. We price Aiduo in other provinces and we price other marketed products that are not subject to price controls based on the prices of competing pharmaceuticals, if any, in the market.
83
Tender Process. The end-customers of our products, hospitals and healthcare institutions, purchase Baquting and Aiduo through a government-sponsored tender process every one or two years, under which we and our competitors submit pricing and other product information to local and provincial pricing control bureaus and other related authorities. Factors considered in assessing bids include, among other things, the quality and price of the medicine and the service and reputation of the manufacturers. The collective tender process for pharmaceuticals with the same chemical composition generally will be conducted at least annually. We did not conduct sales and marketing activities for our other 11 products by ourselves and are not directly involved in the tender process for these products.
Patent and Exclusivity. Patent-protected drugs are placed in a separate category from GMP drugs and we believe, as a result, patent-protected drugs typically enjoy higher success rates in the tender process and are generally priced at a higher level. We were granted a patent in China for our Baquting with enhanced quality, which enabled us to be even more competitive in the tender processes since 2008.
Exclusivity provides us with a competitive advantage in the tender process in terms of pricing and success rates. Chinese drug registration laws provided Aiduo’s registered owner with administrative protection for the manufacture of Aiduo as a diagnostic agent in connection with MPI stress tests in China until 2007. Administrative protection in China prevents other companies from manufacturing, importing or selling a similar drug for the same indication, unless they have received approval for the clinical trials of similar drugs prior to the commencement of the protection period. We are not aware of any other adenosine product in development in China. We believe that, given the time necessary to obtain a drug registration, Aiduo will continue to be the only adenosine product approved for use as a diagnostic agent in connection with MPI stress tests in the near term.
Cost of Revenue and Operating Expenses
The following table sets forth our cost of revenue and operating expenses, both in absolute amounts and as percentages of total net revenues for the periods indicated.
| | | | | | | | | | | | | | |
| | | Year Ended December 31, |
| | | 2007 | | 2008 | | 2009 |
| | | RMB | | % | | RMB | | % | | RMB | | $ | | % |
| | | (in thousands, except percentages) |
Net revenue | | 147,875 | | 100.0 | | 225,399 | | 100.0 | | 282,911 | | 41,447 | | 100.0 |
Cost of revenue | | 34,745 | | 23.5 | | 30,963 | | 13.7 | | 35,625 | | 5,219 | | 12.6 |
Research and development costs | | 7,995 | | 5.4 | | 5,585 | | 2.5 | | 8,297 | | 1,216 | | 2.9 |
Selling, marketing and distribution expenses | | 52,443 | | 35.5 | | 92,404 | | 41.0 | | 110,513 | | 16,190 | | 39.1 |
General and administrative expenses | | 16,796 | | 11.4 | | 31,884 | | 14.1 | | 47,582 | | 6,971 | | 16.8 |
| | | | | | | | | | | | | | |
Total operating expenses | | 77,234 | | 52.2 | | 129,873 | | 57.6 | | 166,392 | | 24,377 | | 58.8 |
| | | | | | | | | | | | | | |
84
Cost of Revenue
Our cost of revenue include costs of raw materials, packaging, labor costs and manufacturing overhead. Raw material costs includes the cost of acquiringBothrops atrox venom and other raw materials. Packaging costs primarily consists of costs of packaging materials for our products. Labor costs are primarily related to salaries and benefits for our personnel involved in the production of our products. Manufacturing overhead primarily comprises allocated utilities and depreciation of our production facilities.
Our cost of revenues is affected by our ability to control raw material costs, achieve economies of scale in our operations and improve our manufacturing productivity and efficiency. Our cost of revenue has declined as a percentage of our net revenue from 2007 to 2009 primarily due to improved economies of scale and efficiencies in manufacturing processes. We expect our per unit manufacturing costs to decrease as our production yields improve. We also anticipate our depreciation charges to increase because of our new production facility in Shenyang, particularly before the full deployment of our new plant capacity.
Operating Expenses
Our operating expenses include research and development costs, selling, marketing and distribution expenses and general and administrative expenses. We expect our operating expenses to increase in line with growth in our net revenue.
Research and Development Costs.Our research and development costs primarily consist of costs associated with the research and development for upgrades and indication expansion of our principal products, costs related to acquiring or in-licensing new technologies or products, costs related to pre-clinical studies, clinical trials and regulatory filings, and costs of manufacturing technology upgrades. In addition, our research and development costs also include employee compensation, depreciation of research and development equipment and other costs that could not be allocated to any specific research project.
We expect to incur higher research and development costs as we enhance our research and development efforts and capabilities. We have also received government grants for certain of our projects, which will be recorded as reductions in our research and development costs after certain conditions or performance obligations attached to these grants have been satisfied. We received RMB0.4 million, RMB0.8 million and RMB1.9 million ($0.3 million) of government grants in 2007, 2008 and 2009, respectively.
Selling, Marketing and Distribution Expenses.Our selling, marketing and distribution expenses primarily consist of salaries and related expenses for personnel engaged in sales, marketing, distribution and customer support functions and costs associated with advertising and other marketing activities. They also include the direct costs attributable to our sales and marketing activities, such as conference and seminar hosting and attendance, travel, entertainment and advertising expenses.
We expect our selling, marketing and distribution expenses to increase in absolute amounts in the future as we continue to broaden our market reach and increase revenues and results of operations by upgrading our principal products and introducing new products and by enhancing our brand awareness and recognition. However, we plan to maintain our sales, marketing and distribution expenses in line with our growth in total revenues.
85
General and Administrative Expenses.General and administrative expenses primarily consist of the following:
| | • | | salaries, bonuses and benefits for our administrative and management personnel, including share-based compensation; |
| | • | | accounting and other professional service fees; |
| | • | | depreciations and amortizations of equipment and facilities used for administrative purposes and real estate rental expenses; and |
| | • | | taxes and fees other than VAT and income tax. |
We expect general and administrative expenses to increase as we recruit additional professionals and incur additional costs related to the growth of our business, including compliance-related costs to support our operations as a U.S. listed public company.
Non-controlling Interest
In July 2009, we entered into a cooperative arrangement with QRxPharma Limited, which has spun off its snake venom-related products to a new subsidiary, Venomics Pty Ltd., or VPL. We have invested $500,000 for a 10% interest in VPL through our subsidiary, Surplus International. VPL in turn formed a joint venture company, Venomics Hong Kong Limited, or VHK, in Hong Kong with our VIE, Nuokang Distribution, to develop and commercialize QRxPharma’s two bleeding control product candidates in China. Under the terms of the VHK share subscription agreement, we invested $5.0 million in October 2009 for acquisition of a 93% interest in VHK. VHK did not commence operations in 2009.
Taxation
The Cayman Islands and Hong Kong
We are a tax exempt company incorporated in the Cayman Islands. Surplus International, our wholly owned subsidiary incorporated in Hong Kong, is subject to Hong Kong corporate income tax of 17.5% on the estimated assessable profits arising in Hong Kong. We did not record any income tax expenses for Surplus International as it had no taxable income during the years ended December 31, 2007, 2008 and 2009. Dividend payments are not subject to withholding tax in the Cayman Islands. The new PRC enterprise income tax law effective on January 1, 2008 provides that an income tax rate of 5% will normally be applicable to dividends from domestic PRC companies to Hong Kong incorporated companies.
Enterprise Income Tax
Our three subsidiaries, Liaoning Nuokang, Shenyang Shouzheng and Penglai Nuokang, and our VIE, Nuokang Distribution, are incorporated in the PRC and therefore are subject to EIT on their taxable income as reported in their PRC statutory accounts adjusted in accordance with relevant PRC income tax laws. Prior to the effectiveness of the new EIT law on January 1, 2008, domestic companies were generally subject to the EIT at a statutory rate of 33%. The three PRC subsidiaries of our company satisfied certain condition and were granted preferential tax treatments under the tax law in effect before January 1, 2008.
86
The new EIT law imposes a uniform EIT rate of 25% on all domestic enterprises and foreign invested enterprises unless they qualify under certain exceptions.
Liaoning Nuokang and Shenyang Shouzheng were recognized by the relevant PRC authorities in December 2002 and December 2004, respectively, as “Hi-Tech Enterprises” located in the Shenyang Economic and Technology Development Zone in Liaoning Province of the PRC and were entitled to a preferential EIT rate of 15%. Furthermore, Liaoning Nuokang and Shenyang Shouzheng were also recognized as “Foreign Investment Manufacturing Enterprises” in December 2005 and October 2006 and were entitled to full exemption of EIT for the first two years and a 50% reduction in EIT for the following three years, commencing from January 2006 and January 2007, respectively.
Penglai Nuokang was recognized as a “Foreign Investment Manufacturing Enterprise” in September 2006 and was entitled to full exemption from EIT for the first two years and a 50% reduction in EIT for the following three years, commencing from September 2006.
Under the new EIT law, enterprises that were established and already enjoyed preferential tax treatments of two-year tax exemption and three-year tax reduction of 50% before March 16, 2007 will continue to enjoy them until the expiration of such term.
Under the new EIT law and the rules related to implementation of the new EIT law, enterprises that are “high and new technology enterprises” strongly supported by the state are entitled to a reduced EIT rate of 15%. Enterprises may choose to benefit from the two-year tax exemption and three-year tax reduction of 50% tax holidays or the reduced EIT rate of 15% during the transition period before the expiration of the tax holidays. In 2008, the relevant PRC authorities recognized Liaoning Nuokang and Penglai Nuokang as “high and new technology enterprises” through 2010, after which we plan to apply for the renewal of these qualifications. We will also apply for the “high and new technology enterprise” qualification for Shenyang Shouzheng, whose existing preferential tax treatment expires in 2011. Liaoning Nuokang, Penglai Nuokang and Shenyang Shouzheng have chosen to benefit from the tax holidays during the transition period. However, after the expiration of the tax holidays, Liaoning Nuokang, Penglai Nuokang and Shenyang Shouzheng may not be qualified as “high and new technology enterprises”. See “Item 3. Key Information—D. Risk Factors—Risks Related to Doing Business in China—Our business benefits from certain government incentives. Expiration of, or changes to, these incentives could have a material adverse effect on our operating results by significantly increasing our tax expenses.”
The tables below set forth the applicable income tax rates of our subsidiaries and Nuokang Distribution for the periods indicated.
| | | | | | | | | | | | | | | | | |
Subsidiary | | 2006 | | | 2007 | | | 2008 | | | 2009 | | | 2010 | |
Liaoning Nuokang | | 0 | % | | 0 | % | | 12.5 | % | | 12.5 | % | | 12.5 | % |
Shenyang Shouzheng | | 15 | % | | 0 | % | | 0 | % | | 12.5 | % | | 12.5 | % |
Penglai Nuokang | | 0 | %(1) | | 0 | % | | 12.5 | % | | 12.5 | % | | 12.5 | % |
Nuokang Distribution | | 33 | % | | 33 | % | | 25 | % | | 25 | % | | 25 | % |
| (1) | Penglai Nuokang commenced being fully exempted from EIT from September 2006. Prior to that, it was subject to EIT at a statutory rate of 33%. |
Value-added Tax
Our revenues are recorded net of VAT. In accordance with the relevant tax laws in the PRC, VAT is levied on the invoiced value of sales at a general rate of 17% and is payable by the purchaser. We are required to remit the VAT collected to the tax authority, but may deduct the VAT that we have paid on eligible purchases.
87
Critical Accounting Policies and Estimates
We prepare our financial statements in conformity with U.S. GAAP, which requires us to make judgments, estimates and assumptions that affect our reporting of, among other things, assets and liabilities, contingent assets and liabilities and revenues and expenses. We continually evaluate these estimates and assumptions based on the most recently available information, our own historical experience and various other assumptions that we believe to be reasonable under the circumstances. Since the use of estimates is an integral component of the financial reporting process, actual results could differ from those estimates. This is especially true for certain accounting policies that require higher degrees of judgment than others in their application. We consider the policies discussed below to be critical to an understanding of our audited consolidated financial statements because they involve significant reliance on our management’s judgment.
Revenue Recognition
We record revenue when the criteria of Staff Accounting Bulletin No. 104 “Revenue Recognition” (codified in FASB Accounting Standards Codification (“ASC”) Topic 605, “Revenue Recognition”) are met. We recognize revenue from the sale of pharmaceutical products when all of the following criteria are met: (1) persuasive evidence of an arrangement exists; (2) delivery has occurred; (3) the sales price is fixed or determinable; and (4) collectibility is reasonably assured. The customer takes title and assumes the risks and rewards of ownership of the products upon delivery of the products, which generally occurs at destination point. The product sales price stated in the sales contract or purchase order is final and not subject to adjustment. There are no general rights of return on delivered products and no price protection is granted to customers.
Our principal products are subject to price control by the PRC government. The maximum prices of the products are published by the PRC price administration authorities from time to time. Any changes in product pricing announced by the PRC price administration authorities affect future sales transactions and do not impact sales that have already occurred, including those for which revenues have not yet been collected.
Research and Development Costs
Research and development costs are expensed as incurred. The costs of acquired technology know-how for drugs in a development stage that are purchased from others for a particular research and development project either singly, or as part of a group of assets, or as part of a business combination, and that have no alternative future uses (in other research and development projects or otherwise), are expensed as research and development costs. We have determined that for such acquired technology know-how to have an alternative future use, it should be (a) reasonably expected that we will use the technology in an alternative manner for an economic benefit and (b) the use of the technology is not contingent on further development subsequent to acquisition (that is, it can be used in an alternative manner at the acquisition date). None of our acquired technology know-how for drugs in a development stage during the periods presented was determined to have an alternative future use at the acquisition date since technological feasibility was not established and regulatory approval from the SFDA was not obtained. Further development, including additional clinical testing, was required to obtain the necessary regulatory approval before the products could be launched into the market for sale. The determination of alternative use requires judgment about the application of developed processes and know-how as well as market acceptance of related products. Different judgments regarding alternative uses could result in a change in the timing and amount of costs capitalized as materials and equipments.
Starting 2009, FASB ASC 805, “Business Combinations”, requires the recognition of tangible and intangible assets that result from or are to be used in research and development activities and assets, irrespective of whether the acquired assets have an alternative future use. Acquired in-process R&D assets, or IPR&D assets, are required to be measured at their acquisition-date fair value. Uncertainty about the outcome of an individual project does not affect the recognition of an IPR&D asset, but is reflected in its fair value.
88
Allowance for Doubtful Accounts
Our accounts receivable balance on our balance sheet is affected by our allowance for doubtful accounts, which reflects our estimate of the expected amount of the receivables that we will not be able to collect. We maintain an allowance for doubtful accounts for estimated losses resulting from the inability of our customers to make required payments. Our allowance for doubtful accounts is based on our assessment of the collectibility of specific customer accounts, the aging of accounts receivable, our history of bad debts, and the general condition of the industry. If a major customer’s credit worthiness deteriorates, or our customers’ actual defaults exceed our historical experience, our estimates could change and impact our reported results.
Allowance for doubtful accounts amounted to RMB579,559, RMB448,103 and RMB393,860 ($57,701) as of December 31, 2007, 2008 and 2009, respectively, representing 2%, 1% and 0.3% of our accounts receivable as of those dates. This allowance reflected a reduction in accounts receivable that was charged to the bad debt provision under operating expenses.
Inventories and Allowance for Obsolescence
We state inventories at the lower of cost or market value, determined using the weighted average cost method. We provide inventory allowances when conditions indicate that the selling price is less than cost due to physical deterioration, usage, obsolescence and reductions in estimated future demand. We balance the need to maintain strategic inventory levels with the risk of obsolescence due to varying customer demand levels and changing technology, although this rarely happens. Unfavorable changes in market conditions may result in a need for additional inventory reserves that could adversely impact our gross margins. Conversely, favorable changes in demand could result in higher gross margins when we sell products.
Impairment of Long-Lived Assets
We evaluate our long-lived assets or asset group for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset or a group of long-lived assets may not be recoverable. When these events occur, we evaluate the impairment by comparing the carrying amount of the assets to future undiscounted net cash flows expected to result from the use of the assets and their eventual disposition. If the sum of the expected undiscounted cash flow is less than the carrying amount of the assets, we would recognize an impairment loss based on the excess of the carrying amount of the asset group over its fair value. We generally determine fair value by discounting the cash flows expected to be generated by the assets, when the market prices are not readily available for the long-lived assets. We did not recognize any impairment of long-lived assets for any of the periods presented.
Income Taxes
We account for income taxes using the liability method. Under this method, deferred tax assets and liabilities are determined based on the difference between the financial reporting and tax bases of assets and liabilities using enacted tax rates that will be in effect in the period in which the differences are expected to reverse. We record a valuation allowance against deferred tax assets if, based on the weight of available evidence, it is more-likely-than-not that some portion, or all, of the deferred tax assets will not be realized. The effect on deferred taxes of a change in tax rates is recognized in income, in the period that includes the enactment date.
89
Effective January 1, 2007, we adopted Financial Accounting Standards Board Interpretation No. 48, “Accounting for Uncertainty in Income Taxes an Interpretation of FASB Statement No. 109” (“FIN 48”) (codified in ASC Topic 740, “Income Taxes”). FIN 48 clarifies the accounting for uncertainty in income taxes by prescribing the recognition threshold a tax position is required to meet before being recognized in the financial statements. We record the cumulative effects of applying FIN 48, if any, as an adjustment to retained earnings as of the beginning of the period of adoption. The adoption of FIN 48 did not result in any adjustment to the opening balance of retained earnings as of January 1, 2007.
We have elected to classify interest and penalties related to an uncertain position, if and when required, as part of “interest expenses” and “other expenses”, respectively, in the consolidated statements of income. No such amounts have been incurred or accrued through December 31, 2009.
Share-based Compensation
In December 2007, we adopted a share option scheme, under which we made option grants on December 19, 2007 and October 11, 2008. As of December 31, 2009, 6,290,000 options granted to the employees (including directors) were outstanding.
In October 2008, we adopted another share option scheme. As of December 31, 2009, no options had been granted under this share option scheme.
We accounted for share options granted to employees and non-employees under SFAS No. 123(R), “Share-Based Payment” (“SFAS 123(R)”) (codified in ASC Topic 718, “Compensation—Stock Compensation”) and EITF Issue No. 96-18 “Accounting for Equity Instruments That Are Issued to Other Than Employees for Acquiring, or in Conjunction with Selling, Goods or Services” (EITF No. 96-18) (codified in ASC Topic 505-50, “Equity-Based Payments to Non-Employees”).
We determine whether a share option should be classified and accounted for as a liability award or an equity award in accordance with ASC Topic 718. All grants of share options to employees classified as equity awards are recognized in the financial statements based on their grant date fair values. All grants of share options to employees classified as liability awards are remeasured at the end of each reporting period. An adjustment for fair value is recorded to the current period expense in order to properly reflect the cumulative expense based on the current fair value of the vested options over the vesting period. We have elected to recognize compensation expense using the straight-line method for both equity and liability classified share options granted with service conditions that have a graded vesting schedule.
ASC Topic 718 requires forfeitures to be estimated at the time of grant and revised, if necessary, in the subsequent period if actual forfeitures differ from initial estimates. Forfeiture rate is estimated based on historical and future expectation of employee turnover rate and is adjusted to reflect future change in circumstances and facts, if any. We recorded share-based compensation expense net of estimated forfeitures such that expense was recorded only for those share-based awards that are expected to vest.
90
We record share-based compensation expense for awards granted to non-employees in exchange for services at fair value in accordance with the provisions of ASC Topic 505-50. For the awards granted to non-employees, we record compensation expenses equal to the fair value of the share options at the measurement date.
We engaged Jones Lang LaSalle Sallmanns, or Sallmanns, an independent appraiser, to determine the fair value of options granted to our employees and non-employees, although our management is ultimately responsible for the determination of all amounts related to share-based compensation recorded in our financial statements. Sallmanns applied the binomial option pricing model in determining the fair value of the options granted to employees and Black-Scholes pricing model in determining the fair value of the options granted to non-employees. These models require the input of highly subjective assumptions including the expected stock price volatility, the expected price multiple at which employees are likely to exercise share options. For expected volatilities, Sallmanns made reference to historical volatilities of several comparable companies. The risk-free rate for periods within the contractual life of the option was based on the yield of U.S. Treasury Bills with similar terms in effect at the time of grant.
Sallmanns used the income approach to assess our equity value at the grant date and each reporting date prior to our initial public offering in December 2009. Under this method, indications of value have been developed by discounting projected future net cash flows to their present worth at discount rates which we believe are appropriate for the risks of the business. The discount rate used is the weighted average cost of capital, or WACC, to reflect the risks of the cash flows. As we were a privately held company at the grant date and each reporting date and the discount rate used was based on WACC for publicly traded comparable companies, Sallmanns applied a further discount for lack of marketability to derive our equity value. Based on this approach, our equity value was determined to be $84.0 million as of December 31, 2007 and $100.6 million as of December 31, 2008. The discount for lack of marketability applied was 20% for the December 31, 2007 valuation date and 26% for the December 31, 2008 valuation date.
The equity value was allocated between ordinary shares and preference shares in accordance with “Valuation of Privately-Held-Company Equity Securities Issued as Compensation” developed by the American Institute of Certified Public Accountants. The fair value was allocated between the preference shares and the ordinary shares based on the probability-weighted average of expected future net cash flows or distributions to shareholders. This also took into consideration each of the possible future events and the rights and preferences of each class of shares.
An option-pricing method is used to allocate the equity value between the preference shares and ordinary shares under the liquidation and redemption scenarios. Under the option- pricing method, each class of shares is modeled as a series of call options with a distinct claim on our equity value, the value of which is estimated using the Black-Scholes model. The characteristics of each class of shares, including liquidation preference and conversion ratio of the preference shares, determine the claim of the preference shares on our equity. In applying the option-pricing method to the redemption scenario, non-participation clause beyond the redemption value of the preference shares are also included. The application of the option-pricing model also involved making estimates of the anticipated timing of a potential liquidity event such as a sale of our company or an initial public offering and estimates of the volatility of our equity securities. The anticipated timing was based on the plans of our board of directors and management. Furthermore, the volatilities applied in the allocation were based on the historical volatility of the shares of comparable companies.
91
Options Granted to Employees
We calculated the estimated fair value of the options on the grant date using the binomial option pricing model with the following assumptions:
| | | | | | | | |
| | | December 19, 2007 | | | October 11, 2008 | |
Suboptimal exercise factor | | | 1.5 | | | | 1.5 | |
Risk-free interest rates | | | 3.43% - 3.98 | % | | | 2.83% - 3.86 | % |
Expected volatility | | | 46.43% - 60.04 | % | | | 48.27% - 57.94 | % |
Expected dividend yield | | | 0 | % | | | 0 | % |
Fair value of share option | | $ | 0.343 | | | $ | 0.356 | |
Fair value per share of ordinary shares | | $ | 0.65 | | | $ | 0.78 | |
Fair value per share of preference shares | | $ | 0.85 | | | $ | 0.91 | |
We calculated the estimated fair value of the options at each reporting date before our initial public offering using the binomial option pricing model with the following assumptions:
| | | | | | | | | |
| | | December 31, 2007 | | | December 31, 2008 | | | December 9, 2009 | |
Suboptimal exercise factor | | 1.5 | | | 1.5 | | | 1.5 | |
Risk-free interest rates | | 3.43%-3.98 | % | | 1%-1.62 | % | | 1.28%-2.67 | % |
Expected volatility | | 46.34%-60.04 | % | | 57.63%-59.91 | % | | 63.10%-78.10 | % |
Expected dividend yield | | 0 | % | | 0 | % | | 0 | % |
The suboptimal exercise factor is defined as the price multiple at which our employees are likely to exercise their options. For example, an assumption of two times price multiple for senior management means senior management is assumed to be likely to exercise their options when the future stock price is double the exercise price for their options. One of the flexibilities and advantages of the binomial model is that it captures the value of early exercise via input assumption of the suboptimal exercise factor. However, different exercise factors will produce different estimate of option values, and eventually a different estimate of option expenses.
Prior to our initial public offering, we accounted for our options granted to employees as liability awards because the options are indexed to the U.S. dollar to RMB exchange rate in addition to the fair value of the underlying shares, and the exercise price is dominated in U.S. dollar while our functional currency is RMB. Upon our initial public offering, we started to account for our options granted to employees as equity awards, because our shares are publicly traded in the United States and as a result, the options are no longer considered dual-indexed. The fair value of the options granted to employees are remeasured on the date of our initial public offering, with the fair value of the options reclassified to equity and the fair value of the unvested options to be recognized over the remaining requisite service period as compensation expenses using the straight-line method.
92
As of December 31, 2009, there was RMB15.5 million ($2.3 million) of unrecognized share-based compensation cost related to share options issued to employees. Deferred costs of RMB12.5 million ($1.8 million) and RMB3.0 million ($0.4 million) are expected to be recognized over a period of 1.97 years and 2.78 years, respectively, using the straight-line method. To the extent the actual forfeiture rate is different from the original estimate, actual share-based compensation related to these awards may be different from our expectations.
Share options issued to non-employees
We record compensation expenses equal to the fair value of the share options at the measurement date. The measurement date is set upon a qualified initial public offering. Upon the completion of our initial public offering in December 2009, we incurred RMB4.3 million ($0.6 million) of share-based compensation expenses relating to the 960,000 options granted to non-employees. The compensation expense was determined using an option pricing model which takes into account a number of variables including the fair value of our ordinary shares at the measurement date.
Results of Operations
The following table sets forth a summary of our consolidated results of operations for the periods indicated. This information should be read together with our consolidated financial statements and related notes included elsewhere in this annual report. The operating results in any period are not necessarily indicative of the results that may be expected for any future period.
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2007 | | | 2008 | | | 2009 | |
| | | RMB | | | RMB | | | RMB | | | $ | |
| | | (in thousands) | |
Consolidated amount of income data | | | | | | | | | | | | |
Net revenue | | 147,875 | | | 225,399 | | | 282,911 | | | 41,447 | |
Cost of revenue | | (34,745 | ) | | (30,963 | ) | | (35,625 | ) | | (5,219 | ) |
Gross profit | | 113,130 | | | 194,436 | | | 247,286 | | | 36,228 | |
Operating expenses | | | | | | | | | | | | |
Research and development costs | | (7,995 | ) | | (5,585 | ) | | (8,297 | ) | | (1,216 | ) |
Selling, marketing and distribution expenses | | (52,443 | ) | | (92,404 | ) | | (110,513 | ) | | (16,190 | ) |
General and administrative expenses | | (16,796 | ) | | (31,884 | ) | | (47,582 | ) | | (6,971 | ) |
Total operating expenses | | (77,234 | ) | | (129,873 | ) | | (166,392 | ) | | (24,377 | ) |
Operating profit | | 35,896 | | | 64,563 | | | 80,894 | | | 11,851 | |
Interest income | | 196 | | | 1,259 | | | 1,372 | | | 201 | |
Interest expense | | (5,190 | ) | | (1,309 | ) | | (3,327 | ) | | (487 | ) |
Other income, net | | 2,191 | | | 6,353 | | | 3,014 | | | 441 | |
Income before income tax expense and non-controlling interest | | 33,093 | | | 70,866 | | | 81,953 | | | 12,006 | |
Income tax benefit (expense) | | 2,102 | | | (7,246 | ) | | (16,858 | ) | | (2,470 | ) |
Non-controlling interest | | — | | | — | | | — | | | — | |
Net income | | 35,195 | | | 63,620 | | | 65,095 | | | 9,536 | |
The following table sets forth our results of operations as a percentage of total net revenue for the periods indicated.
93
| | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2007 | | | 2008 | | | 2009 | |
Net revenue | | 100.0 | | | 100.0 | | | 100.0 | |
Cost of revenue | | (23.5 | ) | | (13.7 | ) | | (12.6 | ) |
| | | | | | | | | |
Gross profit | | 76.5 | | | 86.3 | | | 87.4 | |
Operating expenses | | | | | | | | | |
Research and development costs | | (5.4 | ) | | (2.5 | ) | | (2.9 | ) |
Selling, marketing and distribution expenses | | (35.5 | ) | | (41.0 | ) | | (39.1 | ) |
General and administrative expenses | | (11.4 | ) | | (14.1 | ) | | (16.8 | ) |
| | | | | | | | | |
Total operating expenses | | (52.2 | ) | | (57.6 | ) | | (58.8 | ) |
| | | | | | | | | |
Operating profit | | 24.3 | | | 28.6 | | | 28.6 | |
Interest income | | 0.1 | | | 0.6 | | | 0.5 | |
Interest expense | | (3.5 | ) | | (0.6 | ) | | (1.2 | ) |
Other income, net | | 1.5 | | | 2.8 | | | 1.1 | |
| | | | | | | | | |
Income before income tax expense and non-controlling interest | | 22.4 | | | 31.4 | | | 29.0 | |
Income tax benefit (expense) | | 1.4 | | | (3.2 | ) | | (6.0 | ) |
Non-controlling interest | | — | | | — | | | | |
| | | | | | | | | |
Net income | | 23.8 | | | 28.2 | | | 23.0 | |
| | | | | | | | | |
Year Ended December 31, 2009 Compared to Year Ended December 31, 2008
Net revenue. Our net revenue increased by RMB57.5 million, or 25.5%, from RMB225.4 million for the year ended December 31, 2008 to RMB282.9 million ($41.4 million) for the year ended December 31, 2009. This increase was primarily due to an increase of RMB61 million, or 30%, in sales of Baquting, partially offset by a decrease of RMB0.72 million and RMB3.3 million in sales of Aiduo and other products, respectively.
The increase in Baquting sales was primarily due to an increase in the sales volume of Baquting from 9.7 million units in the year ended December 31, 2008 to 13.1 million units in the year ended December 31, 2009, partially offset by a decrease in the average selling prices of Baquting from RMB20.99 per unit in the year ended December 31, 2008 to RMB20.24 per unit in the year ended December 31, 2009. The increase in sales volume of Baquting was attributable to the increased sales through both our direct sales offices and third-party marketing agents as a result of increased market demand. The average selling prices of Baquting decreased as a result of changes in the percentage contribution of our revenue attributable to different dosages of Baquting. Sales of 2 units/vial dosages, which tend to have a lower per-unit price compared to the other two dosages, accounted for a larger percentage of total Baquting sales in the year ended December 31, 2009 —increasing to 10.4% in the year ended December 31, 2009 from 6.5% in the year ended December 31, 2008.
The decrease in Aiduo sales was primarily due to a decrease in sales volume of Aiduo to 24,816 units in the year ended December 31, 2009 from 25,550 units in the year ended December 31, 2008, and a decrease in the average selling price of Aiduo from RMB241.46 per unit in the year ended December 31, 2008 to RMB224.23 per unit in the year ended December 31, 2009 as a result of decreased tender prices of Aiduo in the year ended December 31, 2009.
Cost of revenue.Our cost of revenue increased by RMB4.7 million, or 15.1%, from RMB31.0 million for the year ended December 31, 2008 to RMB35.6 million ($5.2 million) for the year ended December 31, 2009. However, cost of revenue as a percentage of our net revenue decreased to 12.6% in the year ended December 31, 2009 from 13.7% in the year ended December 31, 2008. The decrease in our cost of revenue as a percentage of our net revenue is primarily due to increased sales of higher margin products such as Baquting and improved economies of scale and efficiencies in manufacturing processes.
94
Operating expenses.Our operating expenses increased by RMB36.5 million, or 28.1%, from RMB129.9 million for the year ended December 31, 2008 to RMB166.4 million ($24.3 million) for the year ended December 31, 2009. Operating expenses as a percentage of our net revenue increased slightly from 57.6% to 58.8%.
| | • | | Research and Development Costs.Our research and development costs increased by 48.6% from RMB5.6 million for the year ended December 31, 2008 to RMB8.3 million ($1.2 million) for the year ended December 31, 2009. This increase was primarily attributable to an increase of RMB2.6 million in expenses associated with the development of new indications for adenosine. |
| | • | | Selling, Marketing and Distribution Expenses.Our selling, marketing and distribution expenses increased by 19.6% from RMB92.4 million for the year ended December 31, 2008 to RMB110.5 million ($16.2 million) for the year ended December 31, 2009. The increase was primarily due to an increase of RMB8.8 million in expenses for academic seminars and conferences and an increase of RMB6.1 million in salaries and other personnel-related expenses. |
| | • | | General and Administrative Expenses.Our general and administrative expenses increased by 49.2% from RMB31.9 million for the year ended December 31, 2008 to RMB47.6 million ($7.0 million) for the year ended December 31, 2009, primarily due to an increase of RMB8.1 million in share-based compensation expenses, an increase of RMB1.6 million in depreciation expenses, an increase of RMB3.3 million in salary and an increase of RMB2.7 million in other general and administrative expenses. |
Interest Income.Our interest income increased by 9.0% from RMB1.3 million for the year ended December 31, 2008 to RMB1.4 million ($0.2 million) for the year ended December 31, 2009. This increase was primarily due to an increase in the average balance of interest-bearing savings accounts.
Interest expense.Our interest expense increased by RMB2.0 million, or 154.2%, from RMB1.3 million for the year ended December 31, 2008 to RMB3.3 million ($0.5 million) for the year ended December 31, 2009. This increase was primarily due to a decrease of RMB1.6 million in interest expense reimbursements from the amortization of deferred government grants and a decrease of RMB0.4 million in capitalization of interest expense from the year ended December 31, 2008.
Other Income.Our other income decreased by RMB3.3 million from RMB6.3 million for the year ended December 31, 2008 to RMB3.0 million ($0.4 million) for the year ended December 31, 2009. Our other income decreased primarily because the foreign exchange gain from the devaluation of our U.S. dollar-denominated preference shares issued in December 2007 as a result of the depreciation of the U.S. dollar against the Renminbi in the year ended December 31, 2008 did not recur in the year ended December 31, 2009.
95
Income tax expense.Our income tax expense increased from RMB7.2 million for the year ended December 31, 2008 to RMB16.9 million ($2.5 million) for the year ended December 31, 2009. This increase was primarily due to an increase in our taxable income and an increase of non-deductible expenses, together with an increase in the tax rate of Shenyang Shouzheng in the year ended December 31, 2009.
Net Income.As a result of the foregoing, we had net income of RMB65.1 million ($9.5 million) for the year ended December 31, 2009, compared to net income of RMB63.6 million for the year ended December 31, 2008.
Year Ended December 31, 2008 Compared to Year Ended December 31, 2007
Net Revenue.Our net revenue increased by 52.4% from RMB147.9 million for the year ended December 31, 2007 to RMB225.4 million ($33.0 million) for the year ended December 31, 2008. This increase was primarily due to an increase of RMB68.1 million, or 50.4%, in net revenue from sales of Baquting, and to a lesser extent, an increase of RMB2.8 million in sales of Aiduo and an increase of RMB6.6 million in sales of 11 other products.
The increase in Baquting sales was primarily due to an increase in sales volume of Baquting from 6.3 million units in the year ended December 31, 2007 to 9.7 million units in the year ended December 31, 2008, partially offset by a decrease in average selling prices of Baquting from RMB21.3 per unit in 2007 to RMB21.0 per unit in 2008. The increase in sales volume of Baquting was attributable to the increased sales through both our direct sales offices and third-party marketing agents as a result of increased market demand. The average selling prices of Baquting decreased due to changes in the sales of different dosages of Baquting. Sales of 2 units/vial dosages, which tend to have a lower per-unit price compared to the other two dosages, accounted for a larger percentage of total Baquting sales in 2008—increasing to 6.5% in 2008 from 2.6% in 2007.
The increase in Aiduo sales was primarily due to an increase in sales volume of Aiduo to 25,550 units in 2008 from 13,579 units in 2007 from increased demand and wider acceptance by physicians of our products, partially offset by a decrease in the average selling price from RMB249.62 per unit in 2007 to RMB241.46 per unit in 2008 due to a 10.8% decrease in the bidding price of Aiduo in Guangxi Province in 2008 while the selling prices of Aiduo in other provinces remained stable.
Cost of Revenue.Our cost of revenue decreased by 10.7% from RMB34.7 million for the year ended December 31, 2007 to RMB31.0 ($4.5 million) for the year ended December 31, 2008. Cost of revenue as a percentage of our net revenue also decreased to 13.7% in 2008 from 23.5% in 2007. This decrease was due to a significant decrease of RMB7.1 million in costs of raw materials for Baquting from 2007 to 2008, primarily as a result of manufacturing technology upgrades for Baquting that enabled us to purify and extract batroxobin from snake venom in-house at lower costs compared to purchasing partially purified venom from third parties. This decrease was partially offset by an RMB0.5 million increase in other manufacturing costs related to Baquting sales, including costs of packaging materials and shipping costs as a result of the increased sales of Baquting. For other products, cost of revenue increased by RMB2.8 million due to an aggregate increase in sales volume of those products.
Operating Expenses.Our operating expenses increased by 68.4% from RMB77.2 million in the year ended December 31, 2007 to RMB130.0 million ($19.0 million) in the year ended December 31, 2008. Operating expenses as a percentage of our net revenue increased from 52.2% to 57.6%.
96
| | • | | Research and Development Costs.Our research and development costs decreased by 30.0% from RMB8.0 million in the year ended December 31, 2007 to RMB5.6 million ($0.8 million) in the year ended December 31, 2008. This decrease was primarily attributable to a decrease of RMB3.4 million in expenses associated with the research and development of hemocoagulase products from 2007 to 2008 as we completed the development of Baquting upgrades with enhanced quality at the end of 2007. The decrease was partially offset by an increase of RMB0.8 million in expenses mainly associated with the pre-clinical development of lanthanum polystyrene sulfonate. |
| | • | | Selling, Marketing and Distribution Expenses.Our selling, marketing and distribution expenses increased by 76.3% from RMB52.4 million in the year ended December 31, 2007 to RMB92.4 million ($13.5 million) in the year ended December 31, 2008. The increase was primarily due to an increase of RMB32.9 million in expenses for seminars, hosting of conferences and direct promotion of our products to targeted hospitals and physicians, an increase of RMB11.4 million in salaries and social security expenses from both increased headcount of our sales personnel and their salaries and an increase of RMB1.5 million in traveling expenses incurred by our sales personnel. |
| | • | | General and Administrative Expenses.Our general and administrative expenses increased by 89.9% from RMB16.8 million for the year ended December 31, 2007 to RMB31.9 million ($4.7 million) for the year ended December 31, 2008, primarily due to an increase of RMB3.5 million in professional service fees, an increase of RMB2.6 million in salary expenses due to both increased headcount of our administrative personnel and their salaries and bonus payments, and an increase of RMB2.3 million in stock option expenses. |
Interest Income.Our interest income increased by RMB1.1 million from RMB0.2 million for the year ended December 31, 2007 to RMB1.3 million ($0.2 million) for the year ended December 31, 2008. This increase was primarily due to an increased average balance of our cash in interest-bearing savings accounts.
Interest Expense.Our interest expense decreased by 75.0% from RMB5.2 million for the year ended December 31, 2007 to RMB1.3 million ($0.2 million) for the year ended December 31, 2008. This decrease was primarily due to government grant of interest expense reimbursement associated with our short-term bank borrowings in 2008.
Other Income.Our other income increased from RMB2.2 million for the year ended December 31, 2007 to RMB6.4 million ($0.9 million) for the year ended December 31, 2008. This increase was primarily due to the foreign exchange gains from the devaluation of our U.S. dollar–denominated preference shares issued in December 2007 as a result of the depreciation of the U.S. dollar against the Renminbi.
Income Tax (Expense)/Benefit.We recorded an income tax benefit of RMB2.1 million for the year ended December 31, 2007 and we recorded an income tax expense of RMB7.2 million ($1.1 million) for the year ended December 31, 2008. This change was primarily resulted from the expiration in December 2007 of the two-year exemption from the EIT that Liaoning Nuokang and Penglai Nuokang enjoyed. Liaoning Nuokang and Penglai Nuokang became subject to EIT at the reduced rate of 12.5% in January 2008.
97
Net Income.As a result of the foregoing, we had a net income of RMB63.6 million ($9.3 million) for the year ended December 31, 2008, compared to a net income of RMB35.2 million for the year ended December 31, 2007.
B. Liquidity and Capital Resources
We have financed our operations primarily through cash flows from operations, short-term bank borrowings, the sale of preferred shares in private placements and proceeds from our initial public offering in December 2009. We believe that our current levels of cash and cash flows from operations and bank borrowings, combined with the net proceeds from our initial public offering in December 2009, will be sufficient to meet our anticipated cash needs for at least the next 12 months. However, we may need additional cash resources in the future if we experience changed business conditions or other developments. We may also need additional cash resources in the future if we find and wish to pursue opportunities for investment, acquisition, strategic cooperation or other similar actions. If we ever determine that our cash requirements exceed our amounts of cash and cash equivalents on hand, we may seek to issue debt or equity securities or obtain a credit facility. Any issuance of equity securities could cause dilution for our shareholders. Any incurrence of indebtedness could increase our debt service obligations and cause us to be subject to restrictive operating and finance covenants. It is possible that, when we need additional cash resources, financing will only be available to us in amounts or on terms that would not be acceptable to us or financing will not be available at all.
As of December 31, 2007, 2008 and 2009, we had RMB112.6 million, RMB81.8 million and RMB351.2 million ($51.5 million), respectively, in cash and cash equivalents. Our cash and cash equivalents primarily consist of cash on hand and demand deposits with original maturities of three months or less that are placed with banks and other financial institutions.
As of December 31, 2007, 2008 and 2009, we had RMB50.4 million, RMB72.4 million and RMB125.6 million ($18.4 million) in outstanding short-term bank loans and borrowings, respectively. Our outstanding short-term bank loans and borrowings as of December 31, 2009 bore weighted-average interest rates of 5.437% per annum. These short-term bank loans and borrowings have terms ranging from three to twelve months, and expire at various times throughout the year. These facilities contain no specific renewal terms but we have historically been able to obtain extensions of certain of the facilities shortly before they mature. These loans are secured by certain of our property, plant and equipment and land use rights and guaranteed by Mr. Baizhong Xue, our chairman and chief executive officer, and other third parties whose owners are business associates of Mr. Xue.
Cash Flows and Working Capital
The following table shows our cash flow with respect to operating activities, investing activities and financial activities for the periods indicated:
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2007 | | | 2008 | | | 2009 | |
| | | RMB | | | RMB | | | RMB | | | $ | |
| | | (in thousands) | |
Net cash provided by operating activities | | 25,241 | | | 25,310 | | | 9,109 | | | 1,335 | |
Net cash used in investing activities | | (53,465 | ) | | (56,763 | ) | | (33,241 | ) | | (4,870 | ) |
Net cash provided by financing activities | | 138,418 | | | 701 | | | 293,553 | | | 43,006 | |
Net increase (decrease) in cash and cash equivalents | | 110,194 | | | (30,752 | ) | | 269,421 | | | 39,471 | |
Cash and cash equivalents at beginning of the period | | 2,395 | | | 112,589 | | | 81,837 | | | 11,989 | |
Cash and cash equivalents at end of the period | | 112,589 | | | 81,837 | | | 351,258 | | | 51,460 | |
98
Operating Activities
Net cash provided by operating activities decreased to RMB9.1 million ($1.3 million) in 2009 from RMB25.3 million in 2008. Although our growing business generated an RMB57.5 million ($8.4 million) increase in our net revenue from 2008 to 2009, we did not generate a corresponding increase in cash inflow because changes in our accounts and bills receivables increased by RMB10.1 million for 2009 compared to 2008, as our receivable turnover days increased from 89 days to 112 days as a result of our market promotion efforts and the adverse economic environment. Our cost of revenue and operating expenses, after deducting non-cash items and items that did not affect operations, increased by RMB60.2 million from 2008 to 2009. In addition, our income tax paid increased by RMB3.4 million from 2008 to 2009.
Net cash provided by operating activities increased to RMB25.3 million ($3.7 million) in 2008 from RMB25.2 million in 2007. Although our growing business generated an RMB77.5 million ($11.4 million) increase in our net revenue from 2007 to 2008, we did not generate a corresponding increase in cash inflow because changes in our accounts and bills receivables increased by RMB34.5 million for 2008 compared to 2007 as our receivable turnover days increased from 56 days to 89 days as a result of our market promotion efforts and the adverse economic environment. Our cost of revenue and operating expenses, after deducting non-cash items and items that did not affect operations, increased by RMB34.0 million from 2007 to 2008. In addition, our income tax paid increased by RMB10.2 million from 2007 to 2008.
Investing Activities
Net cash used in investing activities amounted to RMB33.2 million ($4.9 million) in the year ended December 31, 2009, mainly as a result of (1) our purchases of manufacturing and research and development equipment and construction of the new production facility in Shenyang for RMB26.8 million ($3.9 million), (2) prepayment of RMB6.0 million ($0.9 million) for exclusive distribution rights, (3) payment of RMB3.4 million ($0.5 million) for our investment in a joint venture, (4) payment of RMB2.0 million ($0.3 million) for our investment in an investment fund offset by proceeds from the disposal of plant, property and equipment of RMB1.2 million ($0.2 million) and loan repayment from a related party of RMB3.8 million ($556,703).
Net cash used in investing activities amounted to RMB56.8 million ($8.3 million) in 2008, mainly as a result of (1) our purchases of manufacturing and research and development equipment and construction of the new production facility in Shenyang for RMB51.1 million ($7.5 million), and (2) the prepayment of RMB10.0 million ($1.5 million) made for exclusive distribution rights.
99
Net cash used in investing activities amounted to RMB53.5 million in 2007, mainly as a result of our purchases of manufacturing and research and development equipment and construction of the new production facility in Shenyang for RMB59.4 million, partially offset by the loan repayment of RMB7.0 million from related parties.
Financing Activities
Net cash provided by financing activities amounted to RMB293.6 million ($43.0 million) in the year ended December 31, 2009, mainly as a result of proceeds from our initial public offering of RMB257.3 million ($37.7 million), proceeds of RMB128.4 million ($18.8 million) from renewal of short-term bank loans, primarily offset by the repayment of RMB75.2 million ($11.0 million) of short-term bank loans, the pledge of cash amounts of RMB14.2 million for guarantees related to our operations and the repayment of RMB5.8 million ($0.9 million) to a related party.
Net cash provided by financing activities amounted to RMB0.7 million ($0.1 million) in 2008, mainly as a result of repayment of RMB50.4 million ($7.4 million) of our short-term bank loans, partially offset by proceeds of RMB72.4 million ($10.6 million) from our short-term bank loans.
Net cash provided by financing activities amounted to RMB138.4 million in 2007, mainly as a result of the net proceeds of RMB124.2 million from our placement of Series A convertible redeemable preference shares in December 2007 and net proceeds of RMB32.0 million from our short-term bank loans partially offset by our repurchase of ordinary shares from a shareholder with cash consideration of RMB21.9 million.
Capital Expenditures
We had capital expenditures of RMB59.4 million, RMB51.1 million and RMB26.9 million ($3.9 million) for the years ended December 31, 2007, 2008 and 2009, respectively. Our capital expenditures were made primarily for the construction of our production facilities, obtaining land use rights and the purchases of property, plant and equipment. Our capital expenditures have been primarily funded by net cash generated from our operating activities and cash provided by financing activities. In 2010, we expect to incur capital expenditures of approximately RMB50.0 million ($7.3 million). We expect our capital expenditures in 2010 to primarily consist of the purchases of property, plant and equipment. We expect to fund these capital expenditures by cash generated from operating and financing activities as well as net proceeds from our initial public offering.
Recent Accounting Pronouncements
On April 1, 2009, the FASB approved FSP FAS 141(R)-1, “Accounting for Assets Acquired and Liabilities Assumed in a Business Combination That Arise from Contingencies”, which amends FAS 141(R), as incorporated into FASB ASC 805, and eliminates the distinction between contractual and non-contractual contingencies. Under the updated standard, an acquirer is required to recognize at fair value an asset acquired or liability assumed in a business combination that arises from a contingency if the acquisition-date fair value of that asset or liability can be determined during the measurement period. If the acquisition-date fair value cannot be determined, the acquirer applies the recognition criteria in FASB ASC 450 “Contingencies” to determine whether the contingency should be recognized as of the acquisition date or after it. This guidance is effective for assets or liabilities arising from contingencies in business combinations for which the acquisition date is on or after the beginning of the first annual reporting period beginning on or after December 15, 2008. The adoption of such standard has not had a material impact on our consolidated financial statements.
100
In April 2009, the FASB issued FSP FAS 157-4, “Determining Fair Value When the Volume and Level of Activity for the Asset or Liability Have Significantly Decreased and Identifying Transactions That Are Not Orderly” (“FSP FAS 157-4”) as incorporated into FASB ASC 820, “Fair Value Measurements and Disclosures”. The guidance relates to determining fair values when there is no active market or where the price inputs being used represent distressed sales. It reaffirms what FASB ASC 820 states is the objective of fair value measurement—to reflect how much an asset would be sold for in an orderly transaction (as opposed to a distressed or forced transaction) at the date of the financial statements under current market conditions. Specifically, it reaffirms the need to use judgment to ascertain if a formerly active market has become inactive and in determining fair values when markets have become inactive. This guidance is effective for interim and annual periods ended after June 15, 2009, but entities may early adopt this guidance for the interim and annual periods ended after March 15, 2009. The adoption of such standard has not had a material impact on our consolidated financial statements.
FSP FAS 115-2 and FAS 124-2, as incorporated into FASB ASC 320, “Investments- Debt and Equity Securities” amend the other-than-temporary impairment guidance in U.S. GAAP for debt securities to make the guidance more operational and to improve the presentation and disclosure of other-than-temporary impairments on debt and equity securities in the financial statements. It does not amend existing recognition and measurement guidance related to other-than-temporary impairments of equity securities. Disclosures for periods presented for comparative purposes at initial adoption are not required. This guidance is effective for interim and annual reporting periods ended after June 15, 2009, and shall be applied prospectively. The adoption of such standard has not had a material impact on our consolidated financial statements.
On April 9, 2009, the FASB approved FSP FAS 107-1 and APB 28-1, “Interim Disclosures about Fair Value of Financial Instruments” to require disclosures about fair value of financial instruments in interim period financial statements of publicly traded companies and in summarized financial information required by APB Opinion No. 28, “Interim Financial Reporting”. This guidance was incorporated into FASB ASC 825, “Financial Instruments” which was effective for interim and annual reporting periods ended after June 15, 2009 and was adopted starting third quarter 2009. Under the standard comparative disclosures are only required for periods ended after initial adoption.
In May 2009, the FASB issued SFAS No. 165, “Subsequent Events,” (FASB ASC 855-10) which establishes general standards of accounting for and disclosure of events that occur after the balance sheet date but before financial statements. The statement is effective for interim and annual periods ended after June 15, 2009. The standard was subsequently amended by FASB Accounting Standards Update (“FASB ASU”) 2010-09 which exempts an entity that is an SEC filer from the requirement to disclose the date through which subsequent events have been evaluated.
101
In June 2009, the FASB issued FASB ASU 2009-01, which amends FASB ASC 105, “Generally Accepted Accounting Principles” (“Statement of Financial Accounting Standards (“SFAS”) No. 168”, “The FASB Accounting Standards Codification TM and the Hierarchy of Generally Accepted Accounting Principles – a replacement of FASB Statement No. 162” ), which establishes the FASB Accounting Standards Codification TM (“Codification”) as the source of authoritative GAAP in the United States for non-governmental entities. The Codification does not change GAAP. Instead, it takes the thousands of individual pronouncements that currently comprise GAAP and reorganizes them into approximately 90 accounting Topics, and displays all Topics using a consistent structure. Contents in each Topic are further organized first by Subtopic, then Section and finally Paragraph. The Paragraph level is the only level that contains substantive content. Citing particular content in the Codification involves specifying the unique numeric path to the content through the Topic, Subtopic, Section and Paragraph structure. FASB suggests that all citations begin with “FASB ASC”. Changes to the ASC subsequent to June 30, 2009 are referred to as Accounting Standards Updates. We have implemented the Codification in the consolidated financial statements by providing references to the ASC topics.
In August 2009, the FASB issued FASB ASU 2009-5, “Measuring Liabilities at Fair Value”. FASB ASU 2009-05 amends FASB ASC 820, “Fair Value Measurements”. Specifically, FASB ASU 2009-05 provides clarification that in circumstances in which a quoted price in an active market for the identical liability is not available, a reporting entity is required to measure fair value using one or more of the following methods: 1) a valuation technique that uses a) the quoted price of the identical liability when traded as an asset or b) quoted prices for similar liabilities or similar liabilities when traded as assets and/or 2) a valuation technique that is consistent with the principles of FASB ASC 820 of the Accounting Standards Codification (e.g. an income approach or market approach). FASB ASU 2009-05 also clarifies that when estimating the fair value of a liability, a reporting entity is not required to adjust to include inputs relating to the existence of transfer restrictions on that liability. The adoption of such standard has not had a material impact on our consolidated financial statements.
In September 2009, the Emerging Issues Task Force reached final consensus on FASB ASU 2009-13, “Revenue Arrangements with Multiple Deliverables”. ASU 2009-13 addresses how to determine whether an arrangement involving multiple deliverables contains more than one unit of accounting, and how the arrangement consideration should be allocated among the separate units of accounting. This ASU will be effective prospectively for revenue arrangements entered into or materially modified in fiscal years beginning on or after June 15, 2010. Early adoption is permitted. The adoption of such standard has not had a material impact on our e consolidated financial statements.
In December 2009, the FASB issued FASB ASU 2009-17, Consolidation (“FASB ASC 810”): “Improvements to Financial Reporting by Enterprises Involved with Variable Interest Entities”. This ASU amends the FASB Accounting Standards Codification for statement 167, In June 2009, the FASB issued Statement of Financial Accounting Standards No.167, Amendments to FASB Interpretation No.46(R) (“SFAS No.167”). SFAS No.167 eliminates FASB Interpretation No.46(R)’s exceptions to consolidating qualifying special-purpose entities, contains new criteria for determining the primary beneficiary, and increases the frequency of required reassessments to determine whether a company is the primary beneficiary of a variable interest entity. SFAS No.167 is effective for fiscal years beginning after November 15, 2009, which for the Company is January 1, 2010, with earlier adoption prohibited. The Company does not expect the adoption of FSAB ASU 2009-17 to have a material effect on our consolidated financial statements.
102
In January 2010, the FASB issued ASU 2010-06, “Disclosures about Fair Value Measurements”. ASU 2010-06, which amends ASC 820, provides for disclosure of the amount of significant transfers in or out of Level 1 and Level 2, the reasons for those transfers, as well as information in the Level 3 rollforward about purchases, sales, issuances and settlements on a gross basis. ASU 2010-06 is effective for interim and annual periods beginning after December 15, 2009, except for the requirement to provide Level 3 activity on a gross basis, which is effective for all interim and annual periods beginning after December 15, 2010. The adoption of ASU 2010-06 is not expected to have a material effect on the our consolidated financial statements.
In April 2010, the FASB issued ASU 2010-13, “Effect of Denominating the Exercise Price of a Share-Based Payment Award in the Currency of the Market in Which the Underlying Equity Security Trades”, which provides amendments to ASC topic 718, Compensation-Stock Compensation, to clarify that an employee share-based payment award with an exercise price denominated in the currency of a market in which a substantial portion of the entity’s equity securities trades should not be considered to contain a condition that is not a market, performance, or service condition. Therefore, an entity would not classify such an award as a liability if it otherwise qualifies as equity. ASU 2010-13 is effective for all interim and annual periods beginning on or after December 15, 2010. The adoption of ASU 2010-13 is not expected to have a material effect on our consolidated financial statements.
C. Research and Development
As of March 31, 2010, our research and development team consisted of 45 research personnel and medical professionals. Our research and development personnel comprises medical and biopharmaceutical professionals, many of whom have experience in healthcare and biotechnological research, including experience working in research institutions and hospitals and in proceeding through the SFDA drug approval process. Our research and development management committee oversees our research and development activities and consists of senior management members, company scientists and senior scientific advisors with extensive academic and industry experiences in the healthcare and biotechnology research fields.
Currently we conduct our research and development activities in our facilities in Shenyang and Penglai, where we have the full spectrum of in-house research and development capabilities, from drug discovery to regulatory approval. Our research and development facility in Shenyang has six laboratories, including a protein extraction and analysis lab, a compound process lab, a biological engineering lab, an analysis lab, a polypeptide pharmaceutical lab and a clinical research lab. Our research and development facility in Penglai has two laboratories, including a pharmaceutical formulation lab and a pilot-scale lab. Our research and development facilities are equipped with modern branded equipment.
103
To date, our primary sources of new clinical products have been our research and development activities, including engaging in research and innovation of our key existing technologies, leveraging our key technologies in support of enhanced quality, expanded indications and clinical development of our existing products, and the acquisition and commercialization of new technologies and products from third parties through acquisitions and partnerships. Our research and development team has experience identifying, developing and commercializing product development candidates at various stages of development with commercial potential in our core therapeutic areas.
Our research and development team also maintains cooperative relationship and collaborates closely with domestic and international research institutions, laboratories and universities on new drug development. We believe by complementing our internal research and development efforts with a disciplined strategy of entering into collaborative relationships, we can further build a pipeline of diversified pharmaceuticals to drive sustainable revenue growth. We are in the process of collaborating with certain leading research laboratories in building an international, advanced research center to achieve technology breakthroughs. We are carrying out several major research projects in hematological and cardiovascular areas that were sponsored by provincial and municipal health departments and we also serve as a post-doctoral scientific research station designated by relevant local authorities in Shenyang, China. Our research and development expenses were RMB8.0 million, RMB5.6 million and RMB8.3 million ($1.2 million) in 2007, 2008 and 2009, respectively, representing 5.4%, 2.5% and 2.9% of our net revenue in those respective periods.
D. Trend Information
Other than as disclosed elsewhere in this annual report, we are not aware of any trends, uncertainties, demands, commitments or events for the period from January 1, 2009 to December 31, 2009 that are reasonably likely to have a material adverse effect on our net revenues, income, profitability, liquidity or capital resources, or that caused the disclosed financial information to be not necessarily indicative of future operating results or financial conditions.
E. Off-Balance Sheet Commitments and Arrangements
We have not entered into any financial guarantees or other commitments to guarantee the payment obligations of any third parties. We have not entered into any derivative contracts that are indexed to our shares and classified as shareholder’s equity, or that are not reflected in our consolidated financial statements. Furthermore, we do not have any retained or contingent interest in assets transferred to an unconsolidated entity that serves as credit, liquidity or market risk support to such entity. We do not have any variable interest in any unconsolidated entity that provides financing, liquidity, market risk or credit support to us or engages in leasing, hedging or research and development services with us.
F. Contractual Obligations and Commercial Commitments
The following table sets forth our contractual obligations as of December 31, 2009:
| | | | | | | | | | |
| | | Payment Due by Period |
Contractual Obligations | | Total | | Less than
1 year | | 1-3
years | | 3-5
years | | More than
5 years |
| | | (RMB in thousands) |
Use right obligations(1) | | 8,000 | | 1,600 | | 3,200 | | 3,200 | | — |
Operating lease obligations | | 906 | | 826 | | 57 | | 23 | | — |
Purchase obligations(2) | | 41,219 | | 10,856 | | 15,987 | | 14,376 | | — |
Total | | 50,125 | | 13,282 | | 19,244 | | 17,599 | | — |
| (1) | In connection with the acquisition of a non-controlling interest in Penglai Nuokang, we have a total contractual obligation of RMB8.0 million ($1.2 million) as of December 31, 2009 to Shandong Penglai Pharmaceutical Plant, of which RMB1.6 million ($0.2 million) will be paid annually until December 31, 2014. |
| (2) | Purchase obligations were incurred in connection with construction-in-progress, purchase of office equipment, purchase of snake venom and purchase of a product under our distributorship. |
104
G. Safe Harbor
This annual report on Form 20-F contains statements of a forward-looking nature. These statements are made under the “safe harbor” provisions of the U.S. Private Securities Litigation Reform Act of 1995. Forward-looking statements convey our current expectations and views of future events. The words “may,” “will,” “expect,” “anticipate,” “aim,” “estimate,” “intend,” “plan,” “believe,” “potential,” “continue,” “is/are likely to” and other similar expressions may identify forward-looking statements, but the absence of these words does not necessarily mean that a statement is not forward-looking. These forward-looking statements include, among other things, statements about:
| | • | | competition from other domestic and foreign pharmaceutical companies; |
| | • | | our ability to enhance existing products and develop, obtain government approvals for, and market future generations of our existing products and other new products; |
| | • | | the expected market growth for pharmaceutical products in China; |
| | • | | market acceptance of our products; |
| | • | | our expectations regarding hospital or patient demand for our products; |
| | • | | our ability to expand our production, sales and distribution network and other aspects of our operations; |
| | • | | our ability to diversify our product range; |
| | • | | our ability to effectively protect our intellectual property; |
| | • | | our ability to identify and acquire new medical technologies, pharmaceutical products and product candidates; |
| | • | | changes in the healthcare industry in China, including changes in the healthcare policies and regulations of the PRC government and changes in the healthcare insurance sector in the PRC; |
| | • | | fluctuations in general economic and business conditions in China; and |
| | • | | our ability to control Nuokang Distribution through contractual arrangements. |
105
Any or all of our forward-looking statements in this annual report may turn out to be inaccurate. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our financial condition, results of operations, business strategy and financial needs. They may be affected by inaccurate assumptions we might make or by known or unknown risks and uncertainties, including the risks, uncertainties and assumptions described in “Item 4. Key Information—D. Risk Factors.” In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed in this annual report may not occur as contemplated, and actual results could differ materially from those anticipated or implied by the forward-looking statements.
You should not unduly rely on these forward-looking statements, which speak only as of the date of this annual report. Unless required by law, we undertake no obligation to update or revise any forward-looking statements to reflect new information or future events or otherwise. You should, however, review the factors and risks we describe in reports we will file from time to time with the SEC after the date of this annual report. See “Item 10. Additional Information—H. Documents on Display.”
| Item 6. | DIRECTORS, SENIOR MANAGEMENTAND EMPLOYEES |
A. Directors and Senior Management
Set forth below is the name, age, position and a brief account of the business experience of each of our executive officers and directors. The business address for each of our executive officers and directors is No. 18-1 East Nanping Road, Hunnan National New & High-tech Development Zone, Shenyang, Liaoning Province, People’s Republic of China 110171.
| | | | |
Name | | Age | | Position/Title |
Baizhong Xue | | 45 | | Chairman and Chief Executive Officer |
Qiang Liu | | 44 | | Director and Vice President of Business Development |
Neil Nanpeng Shen | | 43 | | Director |
Sean Shao | | 53 | | Independent director |
William Keller | | 62 | | Independent director |
Mingde Yu | | 64 | | Independent director |
Tianruo Pu | | 42 | | Chief Financial Officer |
Hongying Wang | | 37 | | Executive Vice President |
Kevin Kaijun Li | | 40 | | Chief Scientific Officer |
Lixin Niu | | 38 | | Vice President of Finance |
Yong Xu | | 45 | | Vice President of Human Resources |
Lijun Zhang | | 42 | | Vice President of Sales & Marketing |
Yan Xue | | 33 | | Director of Research and Development |
Jiuxiang Li | | 39 | | Director of Marketing |
Shizheng Duan | | 42 | | Director of Investor Relations and Board Secretary |
Baizhong Xue is the founder of our company and has been our chairman and chief executive officer since our inception in November 1997. Mr. Xue has extensive experience in research and development, sales and marketing and corporate management. From 1994 to 1997, Mr. Xue was the chief scientist at Xinmin Biotechnology Research Institute, a company engaged in biotechnology research and development. Prior to July 1994, he conducted research related to biotechnology commercialization at Shenyang New Technology Commercialization Research Institute since 1992. Mr. Xue is currently the vice chairman of the China Biochemical Industrial Association and the executive vice chairman of Liaoning Biotechnology Association. Mr. Xue holds a bachelor’s degree in biochemistry from Jilin University, China and a master’s degree in microbiology from the Microorganism Research Institute of Shandong University, China.
106
Qiang Liu has been our vice president of business development since January 2006 and has served as our director since December 2007. Mr. Liu has over ten years of experience in sales, marketing, corporate finance and financial analysis. Since June 2006, Mr. Liu has been the founding managing director of Tangent Capital Inc., an investment firm providing equity capital and corporate finance advisory services to Chinese companies. From August 2007 to December 2008, Mr. Liu served as the president of BCTech Investments Inc., a Toronto-based environmental business service firm. Mr. Liu received his bachelor’s degree in biochemistry from Jilin University, China in 1989, a master’s degree in biotechnology from the University of New South Wales in Australia in 1996 and an MBA from the Schulich School of Business at York University, Canada in 2000.
Neil Nanpeng Shen has been our company’s director since December 2007. Mr. Shen is the founding managing partner of Sequoia Capital China. Mr. Shen co-founded Ctrip.com International Ltd., the largest travel consolidator in China, and served as its chief financial officer from 2000 to October 2005 and as its president from August 2003 to October 2005. He also co-founded Home Inns and Hotels Management, a leading economy hotel chain in China. Prior to founding Ctrip and Home Inns, Mr. Shen had worked for over eight years in the investment banking industry in New York and Hong Kong. Currently, Mr. Shen is a co-chairman of Home Inns, a director of Ctrip, a director of E-House (China) Holdings Limited, a NYSE-listed real estate service company in China, a director of China Real Estate Information Corporation, a NASDAQ-listed real estate information service company in China, a director of American Dairy Inc., a NYSE-listed infant milk company in China and a director of Peak Sport Products, a Hong Kong Stock Exchange listed sports apparel company in China. He is also an independent director of Focus Media Holding Limited, a NASDAQ-listed media advertising company based in China and a director of a number of privately owned companies based in China. Mr. Shen received a bachelor’s degree from Shanghai Jiao Tong University, China in 1988 and a master’s degree from the School of Management at Yale University in 1992.
Sean Shao has been our company’s independent director since August 2008. Mr. Shao served as the chief financial officer of Trina Solar Limited, a Chinese vertically integrated solar company, from August 2006 to June 2008. Mr. Shao was the chief financial officer of ChinaEdu Corporation, a Chinese educational service provider, from September 2005 to August 2006. Mr. Shao was the chief financial officer of Watchdata Technologies Ltd., a Chinese security software company, from August 2004 to September 2005. He was previously a senior manager at Deloitte Touche Tohmatsu Beijing from October 1998 to July 2004 and at Deloitte & Touche Toronto from December 1994 to November 1997. Mr. Shao currently serves as chairman of the audit committee of China Recycling Energy Corporation, a NASDAQ-listed energy recycling system design company in China, chairman of the audit committee of Yongye International Inc., a NASDAQ-listed Chinese agricultural company, chairman of the compensation committee of Agria Corporation, an NYSE-listed Chinese agricultural company, chairman of the audit committee of China Biologic Products, Inc., a NASDAQ-listed plasma-based biopharmaceutical company and chairman of the audit committee of Renhuang Pharmaceuticals Inc., a publicly traded China-based bio-pharmaceutical company. Mr. Shao received his master’s degree in healthcare administration from the University of California at Los Angeles in 1988 and his bachelor’s degree in art from East China Normal University in 1982. Mr. Shao holds a CPA license issued by the American Institute of Certified Public Accountants.
107
William Keller has been our company’s independent director since August 2008. Mr. Keller has over 30 years of international business and management experience in the pharmaceutical industry. Mr. Keller is the founder of Keller Pharma Consultancy, a pharmaceutical consulting firm in China. He is also a senior consultant to the Shanghai Foreign Investment Development Board and the deputy general manager of Zhangjiang Biotech & Pharmaceutical Base Development Co., Ltd. From 2007 to September 2009, Mr. Keller was the chairman of HBM Biomed China Partners, a specialized venture capital organization dedicated exclusively to life sciences in China. Before joining HBM Biomed China Partners, Mr. Keller served in various positions at Roche Group. He was the general manager of Roche China Ltd. and Shanghai Roche Pharmaceutical Ltd. from 1994 to 2002, where he formulated and developed the overall Roche strategy for China. Mr. Keller is the honorary president of the R&D-based Pharmaceutical Association, the vice chairman of the Shanghai Association of Foreign Investment Enterprises, and holds directorships in various other pharmaceutical companies, such as Cathay Industrial Biotech Ltd., Fosun Pharma Co. Ltd. and TaiGen Biopharmaceuticals Holdings Limited. Mr. Keller graduated from the School of Economics and Business Administration (Zurich) and has taken several management and leadership courses at the International Institute of Management Development in Switzerland and the London Business School.
Mingde Yu has been our company’s independent director since August 2008. Mr. Yu has extensive experience in manufacturing and distribution management in the pharmaceutical industry in China. He has held a number of senior positions both in the government and the private sectors, including as the vice-bureau chief of the Economic Operations Department of the National Development and Reform Commission from 1998 to 2003, the drug department chief of the Economic Operations Bureau of the State Economic and Trade Committee from 1997 to 1998, the bureau chief of the Liaoning Provincial Food & Drug Administration from 1991 to 1997, the bureau chief of the Fuxin City Food & Drug Administration from 1983 to 1991 and the chief technology officer and the head of manufacturing at both Liaoning Fuxin Pharmaceutical Co. and Fuxin Traditional Chinese Medicine Co. from 1978 to 1983. Mr. Yu is currently the honorary chairman and director of the Beijing Pharmaceutical Group and is the vice committee chairman for the China Pharmaceutical Enterprises Administrative Association and China Medical Entrepreneur Association. In addition, Mr. Yu holds senior consultancy roles with the China Chemical Drug Association and the National Pharmaceutical Industry & Commerce Association. Mr. Yu also serves as an independent director of 3SBio Inc., a biotechnology company in China listed on the NASDAQ Global Market, an independent director of North China Pharmaceutical Co., Ltd., a Shanghai Stock Exchange listed company, and an independent director of Shandong Wohua Pharmaceuticals Technology Corporation, a Shenzhen Stock Exchange listed company. Mr. Yu graduated from the Macromolecule Materials and Engineering Department of the Chemical Technology School at the Dalian University of Technology in China.
108
Tianruo Pu has been our chief financial officer since September 2008. Mr. Pu has over 10 years of experience in accounting, finance and management. Prior to joining our company, Mr. Pu was the chief financial officer of Global Data Solutions, a Chinese information technology services outsourcing company, which he joined in June 2006. From September 2000 to May 2006, he worked as a management consultant with Accenture, CSC Consulting Group and Mitchell Madison Consulting Group, where he provided various management consulting services. He worked as an accountant at GATX Corporation from July 1997 to May 1999, and an auditor at Bernstein Brown CPAs from June 1994 to May 1997. Mr. Pu received his MBA degree from Northwestern University Kellogg School of Management in 2000, his Master of Science degree in accounting from the University of Illinois College of Business Administration in 1995 and Bachelor of Art degree in English from China Foreign Affairs College in 1992.
Hongying Wanghas been our executive vice president since October 2007. Ms. Wang joined our company in July 1998 and has been responsible for managing our marketing department and our research and development facilities, production base and quality control system. She has also participated in the research and development of our hemocoagulase formulation project. Prior to 1998, Ms. Wang worked as a technology supervisor at Shenyang Lantian Group Xinbei Pharmaceutical Co., Ltd. Ms. Wang received a bachelor’s degree in microbiology from Liaoning University, China in 1996 and an MBA degree from Northeastern University, China in 2006.
Kevin Kaijun Li has been our chief scientific officer since August 2009. He has also been an observer on our board of directors and the chairman of our product development committee since December 2007. Dr. Li has over 10 years of professional experience in biomedical sciences, healthcare investment and life science entrepreneurship. Dr. Li was a partner of HBM BioMed China, a Shanghai-based venture capital fund focused on China-related biomedical investment opportunities from 2007 to 2009. From 2005 to 2007, he was a co-founder and vice president of Fountain Medical Development, a leading clinical contract research organization in Asia serving international clients. From 2003 to 2005, Dr. Li was an investment manager at Toucan Capital, a venture capital fund focused on biotech investments in the United States. He was a founding member and senior scientist at GenoSpectra (later acquired by Affymatrix) in California from 2001 to 2003. Dr. Li was a Damon Runyon-Walter Winchell Fellow at Stanford University from 1997 to 2000 and received his PhD in biological sciences from Indiana University in 1997.
Lixin Niu has been our vice president of finance since December 2006. Ms. Niu has more than ten years of working experience in financial management and corporate finance. From August 2002 to December 2003, Ms. Niu was the director and the chief financial officer of Beijing Dingcheng Weiye Technology Co., Ltd., an IT services company. From January 2004 to March 2005, Ms. Niu worked as the chief financial officer of Singapore City One Group (China) Telecom Limited, a telecommunications company. Prior to joining our company, from April 2005 to November 2006, Ms. Niu worked as a vice president at Liaoning Zhongping Accounting Firm. Ms. Niu received a bachelor’s degree in accounting from Shenyang Industry University, China in 1993 and is a certified public accountant in China.
Yong Xu has been our vice president of human resources since February 2006. From 2002 to 2006, Mr. Xu was the vice-general manager and manager of the retail division of Zhongguan Chuangye Technology Development Lenovo Group (China) Co., Ltd., an IT services company. Mr. Xu received a bachelor’s degree in mechanical engineering from Zhejiang University, China.
109
Lijun Zhang has been our vice president of sales and marketing since December 2006. From January 2001 to December 2006, Mr. Zhang was the general manager of Liaoning Pacific Pharmaceutical Co., Ltd., a wholesaler of chemical and pharmaceutical agents. From 1991 to 2000, Mr. Zhang was a sales manager at Shenyang Guangda Pharmaceutical Co., Ltd. Mr. Zhang received a bachelor’s degree in electronics from Liaoning University, China in 1990 and an MBA degree from Renmin University in China in 2005.
Yan Xue has been our director of research and development since October 2006. Ms. Xue joined our company in July 2002 and was the manager of research and development of Shouzheng Bio-tech from May 2003 to October 2006. Ms. Xue is our chief scientist on enzymes and is the developer of our core homeostatic technology. Ms. Xue focuses on the research of separation and extraction techniques relating to proteins and peptides. Ms. Xue is responsible for the development of homeostatic drugs, pre-clinical research and technology modifications related to our flagship product Baquting. Ms. Xue received a bachelor’s degree and a master’s degree in biochemistry from Jilin University, China in 1999 and 2002, respectively.
Jiuxiang Li joined us in January 2004 and has been our director of marketing since May 2008. Mr. Li supervises our product clinical trials, product marketing activities and key opinion leader network. Prior to joining our company, Mr. Li was a manager of Shenyang Shuangding Pharmaceutical Co., Ltd. from June 2003 to December 2003. Mr. Li received a bachelor’s degree in clinical medicine from Shenyang Medical College, China in 1995, a master’s degree in clinical medicine from Dalian Medical University, China in 2003, and a Ph.D. degree in pharmaceutical administration from Shenyang Pharmaceutical University in 2009.
Shizheng Duan has been our director of investor relations and board secretary since May 2008. Mr. Duan has held other various positions in our company, including the manager of corporate strategic planning and the manager of product strategic planning since he joined us in August 2004. From June 2001 to September 2001, Mr. Duan worked for Eli Lilly & Company (UK) as an e-business consultant to their new product launch team. From November 2002 to August 2004, Mr. Duan was the general manager of the Beijing sales and marketing center of Shenyang Guangda Pharmaceutical Co., Ltd., a Chinese biopharmaceutical company. Mr. Duan received a bachelor’s degree in technical English from Shenyang Television University in 1989 and an MBA degree from Manchester Business School, United Kingdom in 2002.
Employment Agreements
We have entered into employment agreements with our senior executive officers. Under these employment agreements, each of our executive officers is employed for a specified time period, subject to automatic extension unless either we or the executive officer gives a one-month prior notice to terminate such employment. We may terminate the employment for cause, at any time, without notice or remuneration, for certain acts of the employee, including but not limited to a conviction or plea of guilty to certain crimes, negligence or dishonesty to our detriment and failure to perform the agreed-to duties after a reasonable opportunity to cure the failure. An executive officer may terminate his employment at any time with one-month prior written notice if there is a material reduction in his authority, duties and responsibilities or if there is a material reduction in his annual salary before the next annual salary review. Furthermore, we may terminate the employment at any time without cause upon advance written notice to the executive officer. These agreements do not provide for any special termination benefits, nor do we have other arrangements with these executive officers for special termination benefits.
110
Each executive officer has agreed to hold, both during and after the employment agreement expires or is earlier terminated, in strict confidence and not to use, except as required in the performance of his duties in connection with the employment, any confidential information, trade secrets and know-how of our company or the confidential information of any third party, including our variable interest entity and our subsidiaries, received by us. In addition, each executive officer has agreed to be bound by non-competition restrictions set forth in his or her employment agreement. Specifically, each executive officer has agreed not to, for a period of two years following the termination or expiration of the employment agreement, (i) carry on or be engaged or interested, directly or indirectly, as shareholder, director, employee, partner, agent or otherwise carry on any business in direct competition with our business; (ii) solicit or entice away from us, or attempt to solicit or entice away from us, any person or entity who has been our customer, client or our representative or agent or in the habit of dealing with us within two years prior to such executive officer’s termination of employment; (iii) solicit or entice away from us, or attempt to solicit or entice away from us, any person or entity who has been our officer, manager, consultant or employee within 12 months prior to such executive officer’s termination of employment; or (iv) use a name including the word “Nuokang” or any other words used by us in our name or in the name of any of our products or services, in such a way as to be capable of or likely to be confused with our name or the name of our products or services.
B. Compensation of Directors and Executive Officers
Compensation of Directors and Executive Officers
Our directors and executive officers receive compensation in the form of annual salaries and bonuses. While we do not have a specific bonus plan setting the calculation of our annual bonuses, each director and executive officer is entitled to receive an annual discretionary bonus based upon his or her performance of such amount as shall be determined by the board of directors.
In 2009, the aggregate cash compensation we paid to our directors and executive officers named in this annual report was approximately $740,000.
Share Incentive Plans
Our board of directors and shareholders have adopted share incentive plans in December 2007 and October 2008, respectively, to attract and retain the best available personnel for positions of substantial responsibility, provide additional incentive to employees, directors and consultants and promote the success of our business. We have reserved an aggregate of 9,538,998 of our ordinary shares for issuance under our share incentive plans. Our future option grants will be made pursuant to our share incentive plans. The following paragraphs describe the principal terms of our share incentive plans.
Types of awards.The types of awards we may grant under our plans include the following:
| | • | | options to purchase our ordinary shares; and |
111
Awards may be designated in the form of ADSs instead of ordinary shares. If we designate an award in the form of ADSs, the number of shares issuable under the share incentive plans will be adjusted to reflect the ratio of ADSs to ordinary shares.
Eligibility.We may grant awards to employees, directors and consultants of our company and our subsidiaries or any entities in which we, directly or indirectly, beneficially hold a majority of the outstanding voting shares or voting power.
Plan administration.The compensation committee of our board of directors, or a committee of one or more members of the board of directors designated by the compensation committee, will administer the plan. However, awards made to our independent directors and executive officers must be approved by the board of directors. The compensation committee or the full board of directors acting by a majority of its members, as appropriate, will determine the individuals who will receive grants, the types of awards to be granted and terms and conditions of each award grant, including any vesting or forfeiture restrictions.
Award agreement.Awards granted under our plans will be evidenced by an award agreement that will set forth the terms, conditions and limitations for each award which may include the terms of the award, the provisions applicable in the event that the employee’s employment or service terminates, and our authority to unilaterally or bilaterally amend, modify, suspend, cancel or rescind the award.
Acceleration of awards upon corporate transactions.The outstanding awards will accelerate upon occurrence of a change-of-control corporate transaction where the successor entity does not assume our outstanding awards under the plans. In such event, each outstanding award will become fully vested and immediately exercisable, and the transfer restrictions on the awards will be released and any forfeiture provisions will terminate immediately before the date of the change-of-control transaction. If the successor entity assumes our outstanding awards or replaces our outstanding awards with a cash incentive program of the successor which preserves the compensation element of such award existing at the time of the corporate transaction and provides for subsequent payout in accordance with the same vesting schedule applicable to such award and later terminates the grantee’s service without cause within 12 months of the change-of-control transaction, the outstanding awards will automatically become fully vested, exercisable and payable and will be released from any restrictions on transfer, repurchase or disposal rights.
Exercise price and term of awards.In general, the compensation committee determines the exercise price of an award and sets forth the price in the award agreement. The exercise price may be fixed or variable price related to the fair market value of our ordinary shares. The compensation committee is also permitted to adjust the exercise price of the outstanding options granted under the plans with the consent of the option holders. However, incentive share options, or ISOs, may not be granted to any employee if the fair market value of the shares underlying such ISOs that are exercisable in any calendar year exceeds $100,000 or other limitations imposed by law. Also, if we grant an ISO to an employee, who, at the time of that grant, owns shares representing more than 10% of the total combined voting power of all classes of our share capital, the exercise price cannot be less than 110% of the fair market value of our ordinary shares on the date of that grant. The term of each award will be stated in the award agreement. The term of an award shall not exceed 10 years from the date of the grant, except that five years is maximum term of an ISO granted to an employee who holds more than 10% of the total combined voting power of our share capital.
112
Amendment and termination.With the approval of our board of directors, the compensation committee or the committee designated by the compensation committee may at any time amend, suspend or terminate the plans. Amendments to the plans are subject to shareholder approval to the extent required by law, or stock exchange rules or regulations. Additionally, shareholder approval will be specifically required to increase the number of shares available for issuance under the plans or to extend the term or the exercise period of an option beyond ten years from the date of grant, or if amendments result in material increases in benefits or a change in eligibility requirements. Unless terminated earlier, the plans will expire and no further awards may be granted after the tenth anniversary of the shareholders’ approval of the plans.
The following table summarizes, as of March 31, 2010, outstanding options that we granted to several of our directors and executive officers and to other individuals as a group under our share incentive plans:
| | | | | | |
| Name | | Ordinary
Shares
Underlying
Outstanding
Options | | Exercise
Price
($ per share) | | Grant Date |
Baizhong Xue | | — | | — | | — |
Neil Nanpeng Shen | | — | | — | | — |
Sean Shao | | * | | 0.7250 | | October 11, 2008 |
Mingde Yu | | * | | 0.7250 | | October 11, 2008 |
William Keller | | * | | 0.7250 | | October 11, 2008 |
Tianruo Pu | | * | | 0.7250 | | October 11, 2008 |
Hongying Wang | | * | | 0.4613 | | December 19, 2007 |
Kevin Kaijun Li | | — | | — | | — |
Qiang Liu | | * | | 0.4613 | | December 19, 2007 |
Lixin Niu | | * | | 0.4613 | | December 19, 2007 |
Yong Xu | | * | | 0.4613 | | December 19, 2007 |
Lijun Zhang | | * | | 0.4613 | | December 19, 2007 |
Yan Xue | | * | | 0.4613 | | December 19, 2007 |
Jiuxiang Li | | * | | 0.4613 | | December 19, 2007 |
Shizheng Duan | | * | | 0.4613 | | December 19, 2007 |
Directors and executive officers listed above as a group | | 2,610,000 820,000 | | 0.4613
0.7250 | | December 19, 2007 October 11, 2008 |
All other employees as a group | | 2,530,000 220,000 | | 0.4613
0.7250 | | December 19, 2007 October 11, 2008 |
| * | Upon the exercise of all share options, would beneficially own 1% or less of our ordinary shares. |
All options granted to directors and officers vest over four years in four equal installments beginning on the first anniversary of the grant date. 25% of the options granted to directors and executive officers on December 19, 2007 will expire on each of December 19, 2012, December 19, 2013, December 19, 2014 and December 19, 2015 and 25% of the options granted to directors and executive officers on October 11, 2008 will expire on each of October 11, 2013, October 11, 2014, October 11, 2015 and October 11, 2016.
113
C. Board Practices
Board of Directors
Our board of directors currently consists of six directors. A director is not required to hold any shares in our company by way of qualification. A director may vote with respect to any contract, proposed contract or arrangement in which he is materially interested as long as he or she has made a declaration of the nature of such interest. Our board of directors may exercise all the powers of our company to borrow money, to mortgage or charge its undertaking, property and uncalled capital, or any part thereof, and to issue debentures, debenture stock or other securities whether outright or as security for any debt, liability or obligation of our company or of any third party.
Committees of the Board of Directors
Our board of directors established an audit committee and a compensation committee in February 2009 and a corporate governance and nominating committee in October 2009.
Audit Committee
Our audit committee consists of Messrs. Sean Shao, Neil Nanpeng Shen and William Keller, and is chaired by Mr. Sean Shao. Messrs. Shao, Shen and Keller satisfy the independence requirements of Rule 5605 of NASDAQ Stock Market, Marketplace Rules. Messrs. Shao and Keller also satisfy the independence requirements of Rule 10A-3 under the Securities Exchange Act of 1934. Our audit committee will consist solely of independent directors that satisfy NASDAQ and SEC requirements within one year of the completion of our initial public offering in December 2009. Our board also has determined that Mr. Sean Shao qualifies as an audit committee financial expert within the meaning of the SEC rules. The audit committee oversees our accounting and financial reporting processes and the audits of the financial statements of our company. The audit committee is responsible for, among other things:
| | • | | selecting our independent auditors and pre-approving all auditing and non-auditing services permitted to be performed by our independent auditors; |
| | • | | regularly reviewing the independence of the independent auditors; |
| | • | | reviewing all related-party transactions on an ongoing basis; |
| | • | | discussing the annual audited financial statements with management and our independent auditors; |
| | • | | annually reviewing and reassessing the adequacy of our audit committee charter; |
| | • | | such other matters that are specifically delegated to our audit committee by our board of directors from time to time; |
| | • | | meeting separately and periodically with management and our internal and independent auditors; and |
| | • | | reporting regularly to the full board of directors. |
In 2009, our audit committee did not hold any meetings or pass any resolutions by unanimous written consent.
114
Compensation Committee
Our compensation committee consists of Messrs. Neil Nanpeng Shen, Sean Shao and Mingde Yu and is chaired by Mr. Neil Nanpeng Shen. Messrs. Shen, Shao and Yu satisfy the independence requirements of Rule 5605 of NASDAQ Stock Market, Marketplace Rules. Our compensation committee assists the board in reviewing and approving the compensation structure of our directors and executive officers, including all forms of compensation to be provided to our directors and executive officers. Members of the compensation committee are not prohibited from direct involvement in determining their own compensation. Our chief executive officer may not be present at any committee meeting during which his compensation is deliberated. The compensation committee is responsible for, among other things:
| | • | | reviewing the compensation plans, policies and programs adopted by the management; |
| | • | | reviewing and approving the compensation package for our executive officers; |
| | • | | reviewing and approving corporate goals and objectives relevant to the compensation of our chief executive officer, evaluating the performance of our chief executive officer in light of those goals and objectives, and setting the compensation level of our chief executive officer based on this evaluation; and |
| | • | | reviewing periodically and making recommendations to the board regarding any long-term incentive compensation or equity plans, programs or similar arrangements, annual bonuses, employee pension and welfare benefit plans. |
In 2009, our compensation committee did not hold any meetings or pass any resolutions by unanimous written consent.
Corporate Governance and Nominating Committee.
Our corporate governance and nominating committee consists of Messrs. Baizhong Xue, Sean Shao and Mingde Yu and is chaired by Mr. Baizhong Xue. Messrs. Sean Shao and Mingde Yu satisfy the independence requirements of Rule 5605 of the NASDAQ Stock Market, Marketplace Rules. The corporate governance and nominating committee assists our board of directors in identifying individuals qualified to become our directors and in determining the composition of the board and its committees. The corporate governance and nominating committee is responsible for, among other things:
| | • | | identifying and recommending to the board nominees for election or re-election to the board, or for appointment to fill any vacancy; |
| | • | | reviewing annually with the board the current composition of the board in light of the characteristics of independence, skills, experience and availability of service to us; |
| | • | | identifying and recommending to the board the names of directors to serve as members of the audit committee and the compensation committee, as well as the corporate governance and nominating committee itself; |
115
| | • | | advising the board periodically with respect to significant developments in the law and practice of corporate governance as well as our compliance with applicable laws and regulations, and making recommendations to the board on all matters of corporate governance and on any corrective action to be taken; and |
| | • | | monitoring compliance with our code of business conduct and ethics, including reviewing the adequacy and effectiveness of our procedures to ensure proper compliance. |
In 2009, our corporate governance and nominating committee did not hold any meetings or pass any resolutions by unanimous written consent.
Duties of Directors
Under Cayman Islands law, our directors have a duty of loyalty to act honestly, in good faith and with a view to our best interests. Our directors also have a duty to exercise the skill they actually possess and such care and diligence that a reasonably prudent person would exercise in comparable circumstances. In fulfilling their duty of care to us, our directors must ensure compliance with our memorandum and articles of association, as amended and re-stated from time to time. A shareholder has the right to seek damages if a duty owed by our directors is breached.
The functions and powers of our board of directors include, among others:
| | • | | convening shareholders’ annual general meetings and reporting its work to shareholders at such meetings; |
| | • | | declaring dividends and distributions; |
| | • | | appointing officers and determining the term of office of officers; |
| | • | | exercising the borrowing powers of our company and mortgaging the property of our company; and |
| | • | | approving the issuance and transfer of shares of our company, including the registering of such shares in our share register. |
Terms of Directors and Officers
All directors hold office until their successors have been duly elected and qualified. A director may only be removed by the shareholders. Officers are elected by and serve at the discretion of the board of directors.
D. Employees
We had 516, 634 and 642 employees as of December 31, 2007, 2008 and 2009, respectively. The following table sets forth the number of our employees by function as of December 31, 2009:
| | |
| | | Number of Employees |
Manufacturing and services | | 183 |
Research and development | | 45 |
General and administration | | 79 |
Sales and marketing personnel | | 295 |
Other | | 40 |
Total | | 642 |
116
We have a workers’ union that protects our employees’ rights and benefits, and assists in mediating any disputes that may arise between us and our employees. We have not had any strikes or other labor disturbances that have interfered with our operations, and we believe that we have a good working relationship with our employees.
E. Share Ownership
The following table sets forth information with respect to the beneficial ownership of our shares as of March 31, 2010 by:
| | • | | each of our directors and executive officers; and |
| | • | | each person known to us to own beneficially more than 5% of our shares. |
| | | | |
| | | Ordinary Shares
Beneficially Owned | | % |
Directors and Executive Officers: | | | | |
Baizhong Xue(1) | | 94,508,704 | | 59.65 |
Qiang Liu | | * | | * |
Neil Nanpeng Shen(2) | | 20,106,222 | | 12.69 |
Sean Shao | | * | | * |
William Keller | | * | | * |
Mingde Yu | | * | | * |
Tianruo Pu | | * | | * |
Hongying Wang | | * | | * |
Kevin Kaijun Li | | — | | — |
Lixin Niu | | * | | * |
Yong Xu | | * | | * |
Lijun Zhang | | * | | * |
Yan Xue | | * | | * |
Jiuxiang Li | | * | | * |
Shizheng Duan | | * | | * |
All directors and executive officers as a group | | 116,124,926 | | 72.60 |
| | |
Principal Shareholders: | | | | |
Anglo China Bio-technology Investment Holdings Limited(3) | | 85,447,648 | | 53.93 |
Sequoia Capital China Growth Fund I., L.P. and its affiliates(4) | | 20,106,222 | | 12.69 |
Britain Ukan Technology Investment Holdings (Group) Limited(5) | | 9,061,056 | | 5.72 |
| * | The person beneficially owns less than 1% of our outstanding ordinary shares. |
| (1) | Includes 9,061,056 shares held by Britain Ukan Technology Investment Holdings (Group) Limited, a British Virgin Islands company wholly owned by Yuhuan Zhu, Mr. Xue’s wife and a Canadian citizen, and 85,447,648 shares held by Anglo China Bio-technology Investment Holdings Limited, a British Virgin Islands company 49% owned by Mr. Xue and 51% ultimately owned by an irrevocable trust constituted under the laws of Singapore with Mrs. Yuhuan Zhu as the settler and certain family members of Mr. Xue as the beneficiaries. Mr. Xue is the sole director of Britain Ukan Technology Investment Holdings (Group) Limited and Anglo China Bio-technology Investment Holdings Limited and is deemed to be the beneficial owner, under SEC rules, of all the shares held by Britain Ukan Technology Investment Holdings (Group) Limited and Anglo China Bio-technology Investment Holdings Limited. Mr. Xue disclaims the beneficial ownership in the shares held by these companies except to the extent of his economic interest in those shares. |
117
| (2) | Includes 17,540,668 ordinary shares held by Sequoia Capital China Growth Fund I, L.P., 414,188 ordinary shares held by Sequoia Capital China Growth Partners Fund I, L.P. and 2,151,366 ordinary shares held by Sequoia Capital China GF Principals Fund I, L.P. (together with Sequoia Capital China Growth Fund I, L.P. and Sequoia Capital China GF Principals Fund I, L.P., the “Sequoia Funds”). The Sequoia Funds are managed by Sequoia Capital China Advisors Limited, a company incorporated in the Cayman Islands. The Sequoia Funds’ general partner is Sequoia Capital China Growth Fund Management I, L.P., whose general partner is SC China Holding Limited which is a company incorporated in the Cayman Islands. SC China Holding Limited is wholly owned by Max Wealth Enterprises Limited, a company wholly owned by Neil Nanpeng Shen. The registered address of Sequoia Capital China Growth Fund I., L.P. is Cricket Square, Hutchins Dr., P.O. Box 2681, Grand Cayman KY1-1111, Cayman Islands. Mr. Shen disclaims any beneficial ownership in the shares owned by the Sequoia Funds except to the extent of his pecuniary interest therein. |
| (3) | Anglo China Bio-technology Investment Holdings Limited, a British Virgin Islands company, is 49% owned by Mr. Baizhong Xue and 51% ultimately owned by an irrevocable trust constituted under the laws of Singapore with Mrs. Yuhuan Zhu as the settler and certain family members of Mr. Xue as the beneficiaries. Mr. Xue is the sole director of Anglo China Bio-technology Investment Holdings Limited and is deemed to be the beneficial owner, under SEC rules, of all the shares held by Anglo China Bio-technology Investment Holdings Limited. Mr. Xue disclaims the beneficial ownership in the shares held by this company except to the extent of his economic interest in those shares. The registered address of Anglo China Bio-technology Investment Holdings Limited is P.O. Box 957, Offshore Incorporations Center, Road Town, Tortola, British Virgin Islands. |
| (4) | Includes 17,540,668 ordinary shares held by Sequoia Capital China Growth Fund I, L.P., 414,188 ordinary shares held by Sequoia Capital China Growth Partners Fund I, L.P. and 2,151,366 ordinary shares held by Sequoia Capital China GF Principals Fund I, L.P.. The Sequoia Funds are managed by Sequoia Capital China Advisors Limited, a company incorporated in the Cayman Islands. Sequoia Capital China Growth Fund I, L.P.’s general partner is Sequoia Capital China Growth Fund Management I, L.P., whose general partner is SC China Holding Limited which is a company incorporated in the Cayman Islands. SC China Holding Limited is wholly owned by Max Wealth Enterprises Limited, a company wholly owned by Neil Nanpeng Shen. The registered address of Sequoia Capital China Growth Fund I., L.P. is Cricket Square, Hutchins Dr., P.O. Box 2681, Grand Cayman KY1-1111, Cayman Islands. |
| (5) | Britain Ukan Technology Investment Holdings (Group) Limited, a British Virgin Islands company, is wholly owned by Yuhuan Zhu, Mr. Baizhong Xue’s wife. Mr. Xue is the sole director of Britain Ukan Technology Investment Holdings (Group) Limited and is deemed to be the beneficial owner, under SEC rules, of all the shares held by Britain Ukan Technology Investment Holdings (Group) Limited. Mr. Xue disclaims the beneficial ownership in the shares held by this company except to the extent of his economic interest in those shares. The registered address of Britain Ukan Technology Investment Holdings (Group) Limited is P.O. Box 957, Offshore Incorporations Center, Road Town, Tortola, British Virgin Islands. |
As of March 31, 2009, 158,440,142 of our ordinary shares were issued and outstanding. Based on a review of the register of members maintained by our Cayman Islands registrar, we believe that, 40,960,000 ordinary shares, or approximately 25.85% of our issued and outstanding shares, were held by the record shareholders in the United States, represented by 5,120,000 ADSs held of record by JPMorgan Chase Bank, N.A., the depositary of our ADS program.
None of our shareholders has different voting rights from other shareholders as of the date of this annual report. We are currently not aware of any arrangement that may, at a subsequent date, result in a change of control of our company.
| Item 7. | MAJOR SHAREHOLDERSAND RELATED PARTY TRANSACTIONS |
A. Major Shareholders
Please refer to “Item 6. Directors, Senior Management and Employees—E. Share Ownership.”
118
B. Related Party Transactions
Transactions with Certain Directors, Shareholders and Affiliates
Transactions with Mr. Baizhong Xue
We paid consideration to Mr. Baizhong Xue, our chairman and chief executive officer, in the form of capital distribution in connection with our restructuring. See “Item 4. Information on the Company—C. Organizational Structure.” This shareholder distribution was paid by us to Mr. Xue in installments from 2005 to 2009. Mr. Xue from time to time extended loans to us and also paid business-related expenses on our behalf. We from time to time make advances in respect of business-related expenses incurred by Mr. Xue on our behalf. As of December 31, 2007, 2008 and 2009, amounts due to Mr. Xue were RMB3.3 million, RMB2.0 million and nil, respectively.
Amounts Due from Liaoning Pacific Pharmaceutical Co., Ltd.
We sold our products to Liaoning Pacific Pharmaceutical Co., Ltd., or Pacific Pharma, in which we previously owned a 35% equity interest. Pacific Pharma in turn sold our products to hospitals and other healthcare institutes in China. As of December 31, 2006, amounts due from Pacific Pharma were RMB12.6 million. The amounts due from Pacific Pharma primarily included accounts receivable and in 2006, also included an RMB10 million advance to Pacific Pharma, which had been fully repaid in 2007. In June 2007, we sold our 35% equity interest in Pacific Pharma to third parties for a cash consideration of RMB0.7 million ($0.1 million).
Loans and Guarantees
In August 2007 and 2008, Industrial Bank Co., Ltd. extended to us short-term loans of RMB25.0 million ($3.7 million) and RMB50.0 million ($7.3 million) at interest rates of 8.4240% and 8.9640%, respectively. These loans were fully repaid in August 2008 and 2009, respectively. In March 2008, Penglai Dengzhou Rural Credit Cooperatives Union extended to us a short-term loan of RMB2.0 million ($0.3 million) at the interest rate of 10.83%. This loan was fully repaid in March 2009. In March 2009, Dengzhou Credit Union extended to us a short-term loan of RMB3.0 million ($0.4 million) at the interest rate of 7.7%. In September 2009, Industrial Bank Co., Ltd. and China Merchants Bank extended to us short-term loans of RMB60.0 million ($8.8 million) and RMB15.0 million ($2.2 million), respectively, each at an interest rate of 6.372%. While these loans were secured by certain of our property, plant and equipment or land use rights, Mr. Baizhong Xue, our chairman and chief executive officer, also provided guarantees for all these loans. As of December 31, 2009, RMB78.0 million ($11.4 million) of these loans remained outstanding.
Restructuring
See “Item 4. Information on the Company—C. Organizational Structure.”
Private Placement
Share Purchase Agreement of Series A Convertible Redeemable Preference Shares
In December 2007, pursuant to a share purchase agreement, we sold an aggregate of 25,796,662 Series A convertible redeemable preference shares in a private placement at a price of $0.659 per share. The investors in our private placement were Sequoia Capital China Growth Fund I, L.P., which purchased 20,106,222 shares, and HBM BioMed China, which purchased 5,690,440 shares. The managing partner of Sequoia Capital China, Neil Nanpeng Shen, serves on our board of directors.
119
Investors’ Rights Agreement
In connection with the private placement, we and our shareholders entered into an investors’ rights agreement that imposed certain restrictions on the sale of shares by us or our ordinary shareholders, and granted preemptive rights to the preference shareholders except with respect to our initial public offering and certain other issuances. The agreement also contains certain registration rights to the preference shareholders, including demand and piggyback registration rights and Form S-3 or F-3 registration rights. Except for the registration rights, all of the other covenants under the agreement have terminated upon the completion of our initial public offering.
Right of First Refusal and Co-sale Agreement
We and our shareholders each have certain rights of first refusal and co-sale rights with respect to any proposed share transfers by any of our existing shareholders. This agreement has terminated upon the completion of our initial public offering.
Voting Agreement
In connection with the private placement, we and our shareholders entered into a voting agreement that provides that our board of directors will include one director designated by one of our shareholders, Sequoia Capital China Growth Fund I, L.P. This agreement has terminated upon the completion of our initial public offering.
Employment Agreements
See “Item 6. Directors, Senior Management and Employees—Management—Employment Agreements.”
Share Incentive Plans
See “Item 6. Directors, Senior Management and Employees—Management—Share Incentive Plans.”
C. Interests of Experts and Counsel
Not applicable.
| Item 8. | Financial Information |
A. Consolidated Statements and Other Financial Information
We have appended consolidated financial statements filed as part of this annual report.
120
Legal and Administrative Proceedings
From time to time, we may be subject to various claims and legal actions arising in the ordinary course of business. We are not aware of any proceedings being contemplated by any governmental authority.
We are currently not a party to any material legal or administrative proceedings, and we are not aware of threatened material legal or administrative proceedings against us. We may from time to time become a party to various legal or administrative proceedings arising in the ordinary course of our business.
Dividend Policy
We have never declared or paid any dividends, nor do we have any present plan to pay any cash dividends on our ordinary shares in the foreseeable future. We currently intend to retain most, if not all, of our available funds and any future earnings to operate and expand our business.
Our board of directors has complete discretion on whether to pay dividends, subject to the approval of our shareholders. Even if our board of directors decides to pay dividends, the form, frequency and amount will depend upon our future operations and earnings, capital requirements and surplus, general financial condition, contractual restrictions and other factors that the board of directors may deem relevant.
If we pay any dividends, we will pay our ADS holders to the same extent as holders of our ordinary shares, subject to the terms of the deposit agreement, including the fees and expenses payable thereunder. Cash dividends on our ordinary shares, if any, will be paid in U.S. dollars.
We are a holding company, and we rely on dividends paid by our operating subsidiaries in China for our cash needs, including the funds necessary to pay dividends and other cash distributions to our shareholders, service any debt we may incur and pay our operating expenses. The payment of dividends in China is subject to limitations. Regulations in the PRC currently permit payment of dividends by our PRC subsidiaries only out of accumulated profits as determined in accordance with accounting standards and regulations in China. Our PRC subsidiaries are required to set aside at least 10% of their after-tax profits each year to contribute to their respective reserve funds until the accumulated balance of the reserve funds reach 50% of their respective registered capital. They are also required to reserve a portion of their after-tax profits to their employee welfare and bonus fund, the amount of which is determined by their respective board of directors. These funds are not distributable in cash dividends.
B. Significant Changes
Except as disclosed elsewhere in this annual report, we have not experienced any significant changes since the date of our audited consolidated financial statements included in this annual report.
121
| Item 9. | The Offer and Listing |
A. Offering and Listing Details.
Our ADSs have been listed on the NASDAQ Global Market under the symbol “NKBP” since December 9, 2009. The following table provides the high and low trading prices for our ADSs on the NASDAQ Global Market for the indicated periods.
| | | | |
| | | Sales Price |
| | | High | | Low |
| | |
Annual High and Low | | | | |
| | |
2009 (from December 9, 2009) | | 9.30 | | 7.01 |
| | |
Quarterly High and Low | | | | |
| | |
Fourth Quarter 2009 (from December 9, 2009) | | 9.30 | | 7.01 |
First Quarter 2010 | | 9.24 | | 6.20 |
Second Quarter 2010 (through May 28, 2010) | | 6.85 | | 5.50 |
| | |
Monthly High and Low | | | | |
| | |
December 2009 | | 9.30 | | 7.01 |
January 2010 | | 9.24 | | 6.95 |
February 2010 | | 8.60 | | 7.15 |
March 2010 | | 8.69 | | 6.20 |
April 2010 | | 6.85 | | 5.54 |
May 2010 | | 6.82 | | 5.50 |
B. Plan of Distribution
Not applicable.
C. Markets
Our ADSs, each representing eight ordinary shares, have been listed on the NASDAQ Global Market since December 9, 2009 under the symbol “NKBP.”
D. Selling Shareholders
Not applicable.
E. Dilution
Not applicable.
F. Expenses of the Issue
Not applicable.
| Item 10. | Additional Information |
A. Share Capital
Not applicable.
122
B. Memorandum and Articles of Association
The following are summaries of material provisions of our amended and restated memorandum and articles of association, as well as the as provided by Section 27(2) of The Companies Law (Revised), as the same may be amended from time to time, of the Cayman Islands, which is referred to as the “Companies Law” below, insofar as they relate to the material terms of our ordinary shares. This summary is not complete, and you should read our memorandum and articles of association.
Registered Office and Objects
The Registered Office of our company is at the offices of Maples Corporate Services Limited, P.O. Box 309, Uglond House, Grand Cayman, KY1-1104, Cayman Islands or at such other place as our directors may from time to time decide. The objects for which our company is established are unrestricted and we have full power and authority to carry out any object not prohibited by the Companies Law (2009 Revision) or as the same may be revised from time to time, or any other law of the Cayman Islands.
Board of Directors
See “Item 6. Directors, Senior Management and Employees—C. Board Practices—Board of Directors.”
Ordinary Shares
All of our outstanding ordinary shares are fully paid and non-assessable. Certificates representing the ordinary shares are issued in registered form. Shareholders who are nonresidents of the Cayman Islands may freely hold and vote their shares.
Dividends
The holders of our ordinary shares are entitled to such dividends as may be declared by our board of directors subject to the Companies Law.
Voting Rights
Each ordinary share is entitled to one vote on all matters upon which the ordinary shares are entitled to vote. Voting at any shareholders’ meeting is by show of hands unless a poll is demanded. A poll may be demanded by our chairman or any shareholder holding at least 10% of the shares given a right to vote at the meeting, present in person or by proxy.
A quorum required for a meeting of shareholders consists of shareholders who hold at least one-third of the issued voting share capital at the meeting present in person or by proxy or, if a corporation or other non-natural person, by its duly authorized representative. Although not required by the Companies Law, we expect to hold annual shareholders’ meetings as required by NASDAQ and such meetings may be convened by our board of directors on its own initiative or upon a request to the directors by shareholders holding in aggregate at least one-third of our voting share capital. Advance notice of at least 21 calendar days is required for the convening of our annual general meeting and other shareholders’ meetings.
123
An ordinary resolution to be passed by the shareholders requires the affirmative vote of a simple majority of the votes attaching to the ordinary shares cast in a general meeting, while a special resolution requires the affirmative vote of no less than two-thirds of the votes cast attaching to the ordinary shares. A special resolution is required for important matters, including reducing the amount of our share capital, removing a director before the expiration of his or her term, amending the memorandum or articles of association, and changing the corporate name. Holders of the ordinary shares may effect certain changes by ordinary resolution, including increasing the amount of our authorized share capital, consolidating and dividing all or any of our share capital into shares of larger amount than our existing share capital, and canceling any shares.
Transfer of Shares
Subject to the restrictions of our memorandum and articles of association, as applicable, a shareholder may transfer all or any of his or her ordinary shares by an instrument of transfer in writing and in the usual or common form or any other form approved by our board.
Our board of directors may, in its sole discretion, decline to register any transfer of any ordinary share that is not fully paid up or on which we have a lien. Our directors may also decline to register any transfer of any ordinary share unless (a) the instrument of transfer is lodged with us, accompanied by the certificate for the ordinary shares to which it relates and such other evidence as our board of directors may reasonably require to show the right of the transferor to make the transfer; (b) the instrument of transfer is in respect of only one class of ordinary shares; (c) the instrument of transfer is properly stamped, if required; (d) in the case of a transfer to joint holders, the number of joint holders to whom the ordinary share is to be transferred does not exceed four; or (e) the shares conceded are free of any lien in favor of us.
If our directors refuse to register a transfer they must, within two months after the date on which the instrument of transfer was lodged, send to each of the transferor and the transferee notice of such refusal. The registration of transfers may, on 14 calendar days’ notice being given by advertisement in such one or more newspapers or by electronic means, be suspended and our register of members closed at such times and for such periods as our board of directors may from time to time determine. However, the registration of transfers may not be suspended, and the register may not be closed, for more than 30 calendar days in any year.
Liquidation
On a return of capital on winding up or otherwise (other than on conversion, redemption or purchase of shares), assets available for distribution among the holders of ordinary shares must be distributed among the holders of the ordinary shares on a pro rata basis. If our assets available for distribution are insufficient to repay all of the paid-up capital, the assets will be distributed so that the losses are borne by our shareholders proportionately.
Calls on Shares and Forfeiture of Shares
Our board of directors may from time to time make calls upon shareholders for any amounts unpaid on their shares in a notice served to such shareholders at least 14 days prior to the specified time and place of payment. The shares that have been called upon and remain unpaid on the specified time are subject to forfeiture.
124
Redemption of Shares
Subject to the provisions of the Companies Law, we may issue shares on terms that are subject to redemption, at our option or at the option of the holders, on such terms and in such manner as may be determined by our board, before the issue of such shares.
Variation of Rights of Shares
All or any of the special rights attached to any class of shares may, subject to the provisions of the Companies Law, be varied either with the written consent of the holders of a majority of the issued shares of that class or with the sanction of a special resolution passed at a general meeting of the holders of the shares of that class.
Inspection of Books and Records
Holders of our ordinary shares will have no general right under Cayman Islands law to inspect or obtain copies of our list of shareholders or our corporate records. However, we will provide our shareholders with annual audited financial statements. See “Item 10. Additional Information—H. Documents on Display.”
Anti-takeover Provisions in Our Memorandum and Articles of Association
Some provisions of our memorandum and articles of association may discourage, delay or prevent a change of control of our company or management that shareholders may consider favorable, including a provision that authorizes our board of directors to issue preference shares in one or more series and to designate the price, rights, preferences, privileges and restrictions of such preference shares without any further vote or action by our shareholders.
Such shares could be issued quickly with terms calculated to delay or prevent a change in control of our company or make removal of management more difficult. If our board of directors decides to issue these preference shares, the price of our ADSs may fall and the voting and other rights of the holders of our ordinary shares and ADSs may be materially and adversely affected.
However, under Cayman Islands law, our directors may only exercise the rights and powers granted to them under our memorandum and articles of association for a proper purpose and for what they believe in good faith to be in the best interests of our company.
C. Material Contracts
We have not entered into any material contracts other than in the ordinary course of business and other than those described in “Item 4. Information on the Company” or elsewhere in this annual report.
D. Exchange Controls
See “Item 4. Information on the Company—B. Business Overview—Regulation—Regulation on Foreign Currency Exchange” and “— Regulation on Dividend Distribution.”
125
E. Taxation
Cayman Islands Taxation
The Cayman Islands currently levies no taxes on individuals or corporations based upon profits, income, gains or appreciation and there is no taxation in the nature of inheritance tax or estate duty. There are no other taxes likely to be material to us levied by the Government of the Cayman Islands except for stamp duties which may be applicable on instruments executed in, or brought within the jurisdiction of the Cayman Islands. The Cayman Islands is not party to any double tax treaties. There are no exchange control regulations or currency restrictions in the Cayman Islands.
PRC Taxation
Under the former PRC Income Tax Law for Enterprises with Foreign Investment and Foreign Enterprises, any dividends payable by foreign-invested enterprises to non-PRC investors were exempt from any PRC withholding tax. In addition, any interest or dividends payable, or distributions made, by us to holders or beneficial owners of our ADSs or ordinary shares would not have been subject to any PRC tax, provided that such holders or beneficial owners, including individuals and enterprises, were not deemed to be PRC residents under the PRC tax law and had not become subject to PRC tax.
Under the new EIT law, which took effect as of January 1, 2008, enterprises established under the laws of non-PRC jurisdictions but whose “de facto management body” is located in China are considered “resident enterprises” for PRC tax purposes. Under the implementation regulations issued by the State Council relating to the new EIT law, “de facto management bodies” are defined as the bodies that have material and overall management control over the business, personnel, accounts and properties of an enterprise. Substantially all of our management are currently based in China, and may remain in China in the future. If we were treated as a “resident enterprise” for PRC tax purposes, we would be subject to PRC income tax on our worldwide income at a uniform tax rate of 25%, but dividends received by us from our PRC subsidiaries may be exempt from the income tax.
Under the new EIT law and its implementation regulations, dividends paid to a non-PRC investor are generally subject to a 10% PRC withholding tax, if such dividends are derived from sources within China and the non-PRC investor is considered to be a non-resident enterprise without any establishment or place of business within China or if the dividends paid have no connection with the non-PRC investor’s establishment or place of business within China, unless such tax is eliminated or reduced under an applicable tax treaty. Similarly, any gain realized on he transfer of ADSs or shares by such investor is also subject to a 10% PRC withholding tax if such gain is regarded as income derived from sources within China, unless such tax is eliminated or reduced under an applicable tax treaty.
If we were considered a PRC “resident enterprise,” it is possible that the dividends we pay with respect to our ADSs or ordinary shares, or the gain you may realize from the transfer of our ADSs or ordinary shares, would be treated as income derived from sources within China and be subject to the 10% PRC withholding tax.
126
U.S. Federal Income Taxation
The following discussion describes the material U.S. federal income tax consequences to U.S. Holders (as defined below) under current law of an investment in our ADSs or ordinary shares. This discussion applies only to U.S. Holders that hold the ADSs or ordinary shares as capital assets (generally, property held for investment) and that have the U.S. dollar as their functional currency. This discussion is based on the tax laws of the United States in effect as of the date of this annual report and on U.S. Treasury regulations in effect or, in some cases, proposed as of the date of this annual report, as well as judicial and administrative interpretations thereof available on or before such date. All of the foregoing authorities are subject to change, which could apply retroactively and could affect the tax consequences described below.
The following discussion does not address the tax consequences to any particular investor or to persons in special tax situations such as:
| | • | | certain financial institutions; |
| | • | | regulated investment companies; |
| | • | | real estate investment trusts; |
| | • | | traders that elect to use a mark-to-market method of accounting; |
| | • | | persons liable for alternative minimum tax; |
| | • | | persons holding ADSs or ordinary shares as part of a straddle, hedging, constructive sale, conversion or integrated transaction; |
| | • | | persons that actually or constructively own 10% or more of the voting power of our voting stock; |
| | • | | persons who acquired ADSs or ordinary shares pursuant to the exercise of any employee share option or otherwise as compensation; or |
| | • | | partnerships or other pass-through entities, or persons holding ADSs or ordinary shares through such entities. |
INVESTORS SHOULD CONSULT THEIR TAX ADVISORS REGARDING THE APPLICATION OF THE U.S. FEDERAL TAX RULES TO THEIR PARTICULAR CIRCUMSTANCES AS WELL AS THE STATE, LOCAL, NON-U.S. AND OTHER TAX CONSEQUENCES TO THEM OF THE PURCHASE, OWNERSHIP AND DISPOSITION OF OUR ADSs OR ORDINARY SHARES.
127
The discussion below of the U.S. federal income tax consequences to “U.S. Holders” will apply to you if you are a beneficial owner of our ADSs or ordinary shares and you are, for U.S. federal income tax purposes:
| | • | | an individual who is a citizen or resident of the United States; |
| | • | | a corporation (or other entity treated as a corporation for U.S. federal income tax purposes) created or organized in the United States or under the laws of the United States, any state thereof or the District of Columbia; |
| | • | | an estate, the income of which is subject to U.S. federal income taxation regardless of its source; or |
| | • | | a trust that (1) is subject to the primary supervision of a court within the United States and the control of one or more U.S. persons for all substantial decisions or (2) has a valid election in effect under applicable U.S. Treasury regulations to be treated as a U.S. person. |
The discussion below assumes that the representations contained in the deposit agreement are true and that the obligations in the deposit agreement and any related agreement have been and will be complied with in accordance with their terms. If you hold our ADSs, you should be treated as the holder of the underlying ordinary shares represented by those ADSs for U.S. federal income tax purposes.
The U.S. Treasury has expressed concerns that parties to whom ADSs are pre-released may be taking actions that are inconsistent with the claiming, by U.S. Holders of ADSs, of foreign tax credits for U.S. federal income tax purposes. Such actions would also be inconsistent with the claiming of the reduced rate of tax applicable to dividends received by certain non-corporate U.S. Holders, including individual U.S. Holders, as described below. Accordingly, the availability of foreign tax credits or the reduced tax rate for dividends received by certain non-corporate U.S. Holders, including individual U.S. Holders, could be affected by future actions that the U.S. Treasury or parties to whom ADSs are pre-released may take.
Taxation of Dividends and Other Distributions on the ADSs or Ordinary Shares
Subject to the passive foreign investment company rules discussed below, the U.S. dollar amount of the gross amount of any distribution we make to you with respect to our ADSs or ordinary shares will generally be includible in your gross income as dividend income on the date of receipt by the depositary, in the case of ADSs, or by you, in the case of ordinary shares, but only to the extent that such distribution is paid out of our current or accumulated earnings and profits (as determined under U.S. federal income tax principles). To the extent that the amount of the distribution exceeds our current and accumulated earnings and profits (as determined under U.S. federal income tax principles), such excess will be treated first as a tax-free return of your tax basis in the ADSs or ordinary shares you hold, and then, to the extent such excess exceeds your tax basis in the ADSs or ordinary shares, as capital gain. We currently do not, and we do not intend to, calculate our earnings and profits under U.S. federal income tax principles. Therefore, you should expect that any distribution we make to you will be treated as a dividend even if such distribution would otherwise be treated as a tax-free return of capital or as capital gain under the rules described above. Any dividends we pay will not be eligible for the dividends-received deduction allowed to corporations in respect of dividends received from other U.S. corporations.
128
With respect to certain non-corporate U.S. Holders, including individual U.S. Holders, for taxable years beginning before January 1, 2011, dividends will be taxed at the lower capital gains rate applicable to “qualified dividend income,” provided that (1) either (a) our ADSs or ordinary shares are readily tradable on an established securities market in the United States or (b) we are eligible for the benefits of a qualifying income tax treaty with the United States that includes an exchange of information program, (2) we are neither a passive foreign investment company nor treated as such with respect to you (as discussed below) for our taxable year in which the dividend is paid and the preceding taxable year, and (3) certain holding period requirements are met. Under U.S. Internal Revenue Service authority, common or ordinary shares, or ADSs representing such shares, are considered for the purpose of clause (1) above to be readily tradable on an established securities market in the United States if they are listed on the NASDAQ Global Market, as are our ADSs (but not our ordinary shares). If we are treated as a “resident enterprise” for PRC tax purposes under the new EIT law, we may be eligible for the benefits of the income tax treaty between the United States and the PRC. For more information regarding the new EIT law, see “Item 10. Additional Information—E. Taxation—PRC Taxation.” You should consult your tax advisors regarding the availability of the lower capital gains rate applicable to qualified dividend income for any dividends we pay with respect to the ADSs or ordinary shares.
For foreign tax credit purposes, the limitation on foreign taxes eligible for credit is calculated separately with respect to specific classes of income. For this purpose, any dividends we pay with respect to our ADSs or ordinary shares will generally constitute “passive category income” but could, in the case of certain U.S. Holders, constitute “general category income.” Any dividends we pay to you will constitute foreign source income for foreign tax credit limitation purposes. If the dividends are taxed as qualified dividend income (as discussed above), the amount of the dividend taken into account for purposes of calculating the foreign tax credit limitation will be limited to the gross amount of the dividend, multiplied by the reduced tax rate and divided by the highest tax rate normally applicable to dividends. If PRC withholding taxes apply to dividends paid to you with respect to our ADSs or ordinary shares (see “Item 10. Additional Information—E. Taxation—PRC Taxation”), subject to certain conditions and limitations, such PRC withholding taxes may be treated as foreign taxes eligible for credit against your U.S. federal income tax liability. The rules relating to the determination of the foreign tax credit are complex and you should consult your tax advisors regarding the availability of a foreign tax credit in your particular circumstances.
Taxation of Dispositions of the ADSs or Ordinary Shares
Subject to the passive foreign investment company rules discussed below, you will recognize taxable gain or loss on any sale, exchange or other taxable disposition of an ADS or ordinary share equal to the difference between the amount realized (in U.S. dollars) for the ADS or ordinary share and your tax basis (in U.S. dollars) in the ADS or ordinary share. The gain or loss will generally be capital gain or loss. If you are a non-corporate U.S. Holder, including an individual U.S. Holder, that has held the ADS or ordinary share for more than one year, you will be eligible for reduced tax rates. The deductibility of capital losses is subject to limitations. Any gain or loss that you recognize on a disposition of our ADSs or ordinary shares will generally be treated as U.S. source income or loss for foreign tax credit limitation purposes. However, if we are treated as a “resident enterprise” for PRC tax purposes, we may be eligible for the benefits of the income tax treaty between the United States and the PRC. In such event, if PRC withholding taxes were to be imposed on any gain from the disposition of the ADSs or ordinary shares (see “Item 10. Additional Information—E. Taxation—PRC Taxation”), a U.S. Holder that is eligible for the benefits of the income tax treaty between the United States and the PRC may elect to treat the gain as PRC source income. You should consult your tax advisors regarding the proper treatment of gain or loss in your particular circumstances.
129
Passive Foreign Investment Company
Based on the market price of our ADSs, the value of our assets, and the composition of our income and assets, we do not believe that we were a “passive foreign investment company,” or PFIC, for U.S. federal income tax purposes for our taxable year ended December 31, 2009. A non-U.S. corporation will be a PFIC for any taxable year if either:
| | • | | at least 75% of its gross income for such year is passive income, or |
| | • | | at least 50% of the value of its assets (based on an average of the quarterly values of the assets) during such year is attributable to assets that produce passive income or are held for the production of passive income (the “asset test”). |
For the purpose of determining whether we or any of our non-U.S. subsidiaries is a PFIC for a taxable year, each such corporation will be treated as owning its proportionate share of the assets and as earning its proportionate share of the income of any other corporation in which it owns, directly or indirectly, at least 25% (by value) of the stock. In applying this rule, however, it is not clear whether the contractual arrangements between us and our affiliated entity will be treated as ownership of stock.
We must make a separate determination after the close of each taxable year as to whether we were a PFIC for that year. Because the value of our assets for purposes of the PFIC test will generally be determined by reference to the market price of our ADSs or ordinary shares, fluctuations in the market price of our ADSs or ordinary shares may cause us to become a PFIC. In addition, changes in the composition of our income or assets may cause us to become a PFIC. If we are a PFIC for any taxable year during which you hold ADSs or ordinary shares, we will generally continue to be treated as a PFIC with respect to you for all succeeding years during which you hold the ADSs or ordinary shares, unless we cease to be a PFIC and you make a “deemed sale” election with respect to the ADSs or ordinary shares, as applicable. If such election is made, you will be deemed to have sold the ADSs or ordinary shares you hold at their fair market value and any gain from such deemed sale would be subject to the rules described in the following two paragraphs. After the deemed sale election, your ADSs or ordinary shares with respect to which such election was made will not be treated as shares in a PFIC unless we subsequently become a PFIC.
130
Any dividends we pay to you during any taxable year will not be eligible for the lower capital gains rate applicable to qualified dividend income (discussed above under “—Taxation of Dividends and Other Distributions on the ADSs or Ordinary Shares”) if we are treated as a PFIC with respect to you for either such taxable year or the previous taxable year. Additionally, for each taxable year that we are treated as a PFIC with respect to you, you will be subject to special tax rules with respect to any “excess distribution” that you receive and any gain you recognize from a sale or other disposition (including a pledge) of the ADSs or ordinary shares, unless you make a “mark-to-market” election as discussed below. Distributions you receive in a taxable year that are greater than 125% of the average annual distributions you received during the shorter of the three preceding taxable years or your holding period for the ADSs or ordinary shares will be treated as an excess distribution. Under these special tax rules:
| | • | | the excess distribution or recognized gain will be allocated ratably over your holding period for the ADSs or ordinary shares, |
| | • | | the amount allocated to the current taxable year, and any taxable years in your holding period prior to the first taxable year in which we were a PFIC, will be treated as ordinary income, and |
| | • | | the amount allocated to each other taxable year will be subject to the highest tax rate in effect for each such year, and the interest charge generally applicable to underpayments of tax will be imposed on the resulting tax attributable to each such year. |
The tax liability for amounts allocated to taxable years prior to the year of disposition or excess distribution cannot be offset by any net operating losses for such years, and gains (but not losses) from a sale or other disposition of our ADSs or ordinary shares cannot be treated as capital, even if you hold the ADSs or ordinary shares as capital assets.
If we are treated as a PFIC with respect to you for any taxable year, to the extent any of our subsidiaries are also PFICs or we make direct or indirect equity investments in other entities that are PFICs, you may be deemed to own shares in such lower-tier PFICs that are directly or indirectly owned by us in that proportion which the value of the ADSs or ordinary shares you own bears to the value of all of our ADSs and ordinary shares, and you may be subject to the rules described in the preceding two paragraphs with respect to the shares of such lower-tier PFICs that you would be deemed to own. A “mark-to-market” election (discussed below), however, cannot be made with respect to any such lower-tier PFICs. You should consult your tax advisors regarding the application of the PFIC rules to any of our subsidiaries.
A U.S. Holder of “marketable stock” (as defined below) in a PFIC may make a mark-to-market election for such stock to elect out of the PFIC rules described above regarding excess distributions and recognized gains. If you make a mark-to-market election for our ADSs or ordinary shares, you will include in income for each year that we are a PFIC an amount equal to the excess, if any, of the fair market value of the ADSs or ordinary shares you hold as of the close of your taxable year over your adjusted basis in such ADSs or ordinary shares. You will be allowed a deduction for the excess, if any, of the adjusted basis of the ADSs or ordinary shares over their fair market value as of the close of the taxable year. However, deductions will be allowable only to the extent of any net mark-to-market gains on the ADSs or ordinary shares included in your income for prior taxable years. Amounts included in your income under a mark-to-market election, as well as any gain from the actual sale or other disposition of the ADSs or ordinary shares, will be treated as ordinary income. Ordinary loss treatment will apply to the deductible portion of any mark-to-market loss on the ADSs or ordinary shares, as well as to any loss from the actual sale or other disposition of the ADSs or ordinary shares, to the extent that the amount of such loss does not exceed the net mark-to-market gains previously included for such ADSs or ordinary shares. Your basis in the ADSs or ordinary shares will be adjusted to reflect any such income or loss amounts. If you make a valid mark-to-market election, the tax rules that apply to distributions by corporations that are not PFICs would apply to any distributions we make, except that the lower capital gains rate applicable to qualified dividend income (discussed above under “—Taxation of Dividends and Other Distributions on the ADSs or Ordinary Shares”) generally would not apply.
131
The mark-to-market election is available only for “marketable stock,” which is stock that is traded in greater thande minimis quantities on at least 15 days during each calendar quarter (“regularly traded”) on a qualified exchange or other market, as defined in applicable U.S. Treasury regulations. Our ADSs are currently listed on the NASDAQ Global Market, which is a qualified exchange or other market for these purposes. Consequently, if the ADSs continue to be listed on the NASDAQ Global Market and are regularly traded, and you are a holder of the ADSs, we expect that the mark-to-market election would be available to you if we become a PFIC. Because a mark-to-market election cannot be made for equity interests in any lower-tier PFICs that we own, a U.S. Holder may continue to be subject to the PFIC rules described above regarding excess distributions and recognized gains with respect to its indirect interest in any investments held by us that are treated as equity interest in a PFIC for U.S. federal income tax purposes. You should consult your tax advisors as to the availability and desirability of a mark-to-market election, as well as the impact of such election on interests in any lower-tier PFICs.
Alternatively, a U.S. Holder of stock in a PFIC may make a “qualified electing fund” election with respect to such PFIC to elect out of the PFIC rules described above regarding excess distributions and recognized gains. A U.S. Holder that makes a valid qualified electing fund election with respect to a PFIC will generally include in income for a taxable year such holder’spro rata share of the corporation’s income for the taxable year. However, the qualified electing fund election is available only if the PFIC provides such U.S. Holder with certain tax information as required under applicable U.S. Treasury regulations. We do not intend to prepare or provide the information that would enable you to make a qualified electing fund election.
Under newly enacted legislation, unless otherwise provided by the U.S. Treasury, each U.S. shareholder of a PFIC is required to file an annual report containing such information as the U.S. Treasury may require. Prior to such legislation, a U.S. shareholder of a PFIC was required to file Internal Revenue Service Form 8621 only for each taxable year in which such shareholder received distributions from the PFIC, recognized gain on a disposition of the PFIC stock, or made a “reportable election.” If we become a PFIC, you should consult your tax advisors regarding any reporting requirements that may apply to you.
You should consult your tax advisors regarding the application of the PFIC rules to your investment in our ADSs or ordinary shares and the elections discussed above.
Information Reporting and Backup Withholding
Dividend payments with respect to ADSs or ordinary shares and proceeds from the sale, exchange or other disposition of ADSs or ordinary shares will generally be subject to information reporting to the U.S. Internal Revenue Service and possible U.S. backup withholding at a current rate of 28%. Backup withholding will not apply, however, to a U.S. Holder that furnishes a correct taxpayer identification number and makes any other required certification on U.S. Internal Revenue Service Form W-9 or that is otherwise exempt from backup withholding. U.S. Holders that are exempt from backup withholding should still complete U.S. Internal Revenue Service Form W-9 to avoid possible erroneous backup withholding. Under newly enacted legislation, for taxable years beginning after March 18, 2010, certain individuals holding the ADSs or ordinary shares other than in an account at a financial institution may be subject to additional information reporting requirements. You should consult your tax advisors regarding the application of the U.S. information reporting and backup withholding rules.
132
Backup withholding is not an additional tax. Amounts withheld as backup withholding may be credited against your U.S. federal income tax liability, and you may obtain a refund of any excess amounts withheld under the backup withholding rules by filing an appropriate claim for refund with the U.S. Internal Revenue Service and furnishing any required information in a timely manner.
F. Dividends and Paying Agents
Not applicable.
G. Statement by Experts
Not applicable.
H. Documents on Display
We have previously filed with the Commission our registration statement on Form F-1 (File Number 333-163250), as amended, and a prospectus under the Securities Act with respect to our ordinary shares represented by our ADSs. We also filed with the SEC a related registration statement on Form F-6 (File Number 333-163398) with respect to our ADSs.
We are subject to the periodic reporting and other informational requirements of the Exchange Act. Under the Exchange Act, we are required to file reports and other information with the SEC. Specifically, we are required to file annually a Form 20-F: (1) within six months after the end of each fiscal year, which is December 31, for fiscal years ending before December 15, 2011; and (2) within four months after the end of each fiscal year for fiscal years ending on or after December 15, 2011. Copies of reports and other information, when so filed, may be inspected without charge and may be obtained at prescribed rates at the public reference facilities maintained by the Securities and Exchange Commission at Judiciary Plaza, 100 F Street, N.E., Washington, D.C. 20549, and at the regional office of the Securities and Exchange Commission located at Citicorp Center, 500 West Madison Street, Suite 1400, Chicago, Illinois 60661. The public may obtain information regarding the Washington, D.C. Public Reference Room by calling the Commission at 1-800-SEC-0330. The SEC also maintains a web site athttp://www.sec.gov that contains reports, proxy and information statements, and other information regarding registrants that make electronic filings with the SEC using its EDGAR system.
As a foreign private issuer, we are exempt from the rules of the Exchange Act prescribing the furnishing and content of quarterly reports and proxy statements, and our executive officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we are not required under the Exchange Act to file periodic reports and financial statements with the SEC as frequently or as promptly as U.S. companies whose securities are registered under the Exchange Act.
133
We will furnish JPMorgan Chase Bank, N.A., the depositary of our ADSs, with all notices of shareholders’ meeting and other reports and communications that are made generally available to our shareholders. The depositary will make such notices, reports and communications available to holders of ADSs and, upon our written request, will mail to all record holders of ADSs the information contained in any notice of a shareholders’ meeting received by the depositary from us.
In accordance with NASDAQ Stock Market Rule 5250(d), we will post this annual report on Form 20-F on our website athttp://ir.nkbp.com. In addition, we will provide hard copies of our annual report free of charge to shareholders and ADS holders upon request.
I. Subsidiary Information
For a listing of our subsidiaries, see “Item 4. Information on the Company—C. Organizational Structure.”
| Item 11. | Quantitative and Qualitative Disclosures About Market Risk |
Inflation
Since our inception, inflation in China has not materially impacted our results of operations. According to the National Bureau of Statistics of China, the change of consumer price index in China was 4.8%, 5.9% and -0.7% in 2007, 2008 and 2009, respectively. Although we have not in the past been materially affected by any such inflation since our inception, we can provide no assurance that we will not be affected in the future by higher rates of inflation in China. For example, certain operating costs and expenses, such as personnel expenses, real estate leasing expenses, travel expenses and office operating expenses may increase as a result of higher inflation. Additionally, because a substantial portion of our assets consists of cash and cash equivalents and short-term investments, high inflation could significantly reduce the value and purchasing power of these assets. We are not able to hedge our exposures to higher inflation in China.
Interest Rate Risk
We are exposed to interest rate risk related to the interest income generated by excess cash invested in liquid investments with original maturities of three months or less. Such interest-earning instruments carry a degree of interest rate risk. We have not used any derivative financial instruments to manage our interest risk exposure. We have not been exposed to material risks due to changes in interest rates. However, our future interest income may be lower than expected due to changes in market interest rates. We are also subject to market risks due to fluctuations in interest rates on our debt. Increases in interest rates will increase the cost of new borrowing and our interest expense. Accordingly, fluctuations in interest can lead to significant fluctuations in the fair value of these debt instruments.
Foreign Exchange Risk
Substantially all of our revenues and most of our expenses are denominated in RMB. Our exposure to foreign exchange risk primarily relates to cash denominated in U.S. dollars as a result of our issuance of ADSs representing ordinary shares in our initial public offering. We do not believe that we currently have any significant direct foreign exchange risk and have not hedged exposures denominated in foreign currencies or any other derivative financial instruments. Although in general, our exposure to foreign exchange risks should be limited, the value of your investment in our ADSs will be affected by the foreign exchange rate between U.S. dollars and RMB because the value of our business is effectively denominated in RMB, while our ADSs are traded in U.S. dollars.
134
The value of the RMB against the U.S. dollar and other currencies may fluctuate and is affected by, among other things, changes in China’s political and economic conditions. Historically, the conversion of RMB into foreign currencies, including U.S. dollars, has been based on rates set by the People’s Bank of China. On July 21, 2005, the PRC government changed its decade-old policy of pegging the value of the RMB to the U.S. dollar. Under the new policy, the RMB is permitted to fluctuate within a narrow and managed band against a basket of certain foreign currencies. This change in policy has resulted in an approximately 21.2% appreciation of the RMB against the U.S. dollar by December 31, 2009. There remains significant international pressure on the PRC government to adopt an even more flexible currency policy, which could result in a further and more significant appreciation of the RMB against the U.S. dollar. To the extent that we need to convert U.S. dollars we received from our initial public offering into RMB for our operations, appreciation of the RMB against the U.S. dollar would have an adverse effect on the RMB amount we receive from the conversion. We have not used any forward contracts or currency borrowings to hedge our exposure to foreign currency exchange risk.
| Item 12. | Description of Securities Other than Equity Securities |
A. Debt Securities
Not applicable.
B. Warrants and Rights
Not applicable.
C. Other Securities
Not applicable.
D. American Depositary Shares
JPMorgan Chase Bank, N.A., our depositary, may charge each person to whom ADSs are issued, including, without limitation, issuances against deposits of shares, issuances in respect of share distributions, rights and other distributions, issuances pursuant to a stock dividend or stock split declared by us or issuances pursuant to a merger, exchange of securities or any other transaction or event affecting the ADSs or deposited securities, and each person surrendering ADSs for withdrawal of deposited securities in any manner permitted by the deposit agreement or whose ADRs are cancelled or reduced for any other reason, $5.00 for each 100 ADSs (or any portion thereof) issued, delivered, reduced, cancelled or surrendered, the case may be. The depositary may sell (by public or private sale) sufficient securities and property received in respect of a share distribution, rights and/or other distribution prior to such deposit to pay such charge.
135
The following additional charges shall be incurred by the ADR holders, by any party depositing or withdrawing shares or by any party surrendering ADSs or to whom ADSs are issued (including, without limitation, issuance pursuant to a stock dividend or stock split declared by us or an exchange of stock regarding the ADRs or the deposited securities or a distribution of ADSs), whichever is applicable:
| | • | | a fee of $1.50 per ADR or ADRs for transfers of certificated or direct registration ADRs; |
| | • | | a fee of up to $0.05 per ADS (or portion thereof) for any cash distribution made pursuant to the deposit agreement; |
| | • | | a fee of up to $0.05 per ADS per calendar year (or portion thereof )for services performed by the depositary in administering our ADR program (which fee may be charged on a periodic basis during each calendar year and shall be assessed against holders of ADRs as of the record date or record dates set by the depositary during each calendar year and shall be payable in the manner described in the next succeeding provision); |
| | • | | reimbursement of such fees, charges and expenses incurred by the depositary and/or any of the depositary’s agents (including, without limitation, the custodian and expenses incurred on behalf of holders in connection with compliance with foreign exchange control regulations or any law or regulation relating to foreign investment) in connection with the servicing of the shares or other deposited securities, the delivery of deposited securities or otherwise in connection with the depositary’s or its custodian’s compliance with applicable law, rules or regulations (which charge shall be assessed on a proportionate basis against holders as of the record date or dates set by the depositary and shall be payable at the sole discretion of the depositary by billing such registered holders or by deducting such charge from one or more cash dividends or other cash distributions); |
| | • | | a fee for the distribution of securities (or the sale of securities in connection with a distribution), such fee being in an amount equal to the fee for the execution and delivery of ADSs which would have been charged as a result of the deposit of such securities (treating all such securities as if they were shares) but which securities or the net cash proceeds from the sale thereof are instead distributed by the depositary to those holders entitled thereto; |
| | • | | stock transfer or other taxes and other governmental charges; |
| | • | | cable, telex and facsimile transmission and delivery charges incurred at your request; |
| | • | | transfer or registration fees for the registration of transfer of deposited securities on any applicable register in connection with the deposit or withdrawal of deposited securities; and |
| | • | | expenses of the depositary in connection with the conversion of foreign currency into U.S. dollars. |
136
We will pay all other charges and expenses of the depositary and any agent of the depositary (except the custodian) pursuant to agreements from time to time between us and the depositary. The fees described above may be amended from time to time.
Our depositary has agreed to reimburse us for certain expenses we incur that are related to establishment and maintenance of the ADR program, including investor relations expenses and exchange application and listing fees. The amount of reimbursement available to us is not based upon the amounts of fees the depositary collects from investors. The depositary collects its fees for issuance and cancellation of ADSs directly from investors depositing shares or surrendering ADSs for the purpose of withdrawal or from intermediaries acting for them. The depositary collects fees for making distributions to investors by deducting those fees from the amounts distributed or by selling a portion of distributable property to pay the fees. The depositary may collect its annual fee for depositary services by deduction from cash distributions, or by directly billin -g investors, or by charging the book-entry system accounts of participants acting for them. The depositary may generally refuse to provide services to any holder until the fees and expenses owing by such holder for those services or otherwise are paid.
PART II
| Item 13. | Defaults, Dividend Arrearages and Delinquencies |
None.
| Item 14. | Material Modifications to the Rights of Security Holders and Use of Proceeds |
A. – D. Material Modifications to the Rights of Security Holders
None.
E. Use of Proceeds
The following “Use of Proceeds” information relates to our initial public offering, at $9.00 per ADS, of 5,000,000 ADSs, each representing eight ordinary shares, of which 4,526,979 ADSs were offered by our company and 473,021 ADSs were offered by a selling shareholder. The aggregate offering price was $45,000,000, with $40,742,811 for the ADSs offered by us and $4,257,189 for the ADSs offered by the selling shareholder. The registration statement on Form F-1 (File No. 333-163250) for our initial public offering was declared effective by the SEC on December 9, 2009. On December 15, 2009, we completed our initial public offering after all of the registered securities were sold. Jefferies & Company, Inc. and Oppenheimer & Co. Inc. were the underwriters for our initial public offering.
The total expenses incurred for our company’s account in connection with our initial public offering were approximately $5.6 million, including underwriting discounts and commissions of approximately $2.9 million, SEC registration fees of $6,000, FINRA filing fees of $10,560, Nasdaq Global Market listing fees of $106,000, printing expenses of approximately $349,200, legal fees of approximately $1.3 million, audit and audit-related fees of approximately $337,900, other professional fees of approximately $357,600 and roadshow costs and expenses of approximately $198,000. None of the above expenses included direct or indirect payments to directors or officers of our company or their associates, persons owning 10% or more of our equity securities or our affiliates.
137
We received net proceeds of approximately $35.1 million from our initial public offering. From December 9, 2009, the effective date of our registration statement on Form F-1 for the offering, to December 31, 2009, we did not use any of the net proceeds from our initial public offering. We intend to use our net proceeds as follows:
| | • | | approximately $14.0 million to fund the acquisition and in-licensing of new technologies, products or companies; |
| | • | | approximately $5.0 million to fund the research and development of our product candidates and the expansion of our product pipeline, including clinical trials, of which approximately $0.7 million used to develop the manufacturing process of dipyridamole aspirin sustained release capsules, $1.2 million for Phase II and Phase III clinical trials of hemocoagulase derived from the venom of the snake species Agkistrodon acutus, $0.7 million for preclinical trials of adenosine for myocardial protection and $1.9 million for preclinical trials of lanthanum polystyrene sulfonate; |
| | • | | approximately $8.0 million to fund the improvement of our production systems, the expansion of our production facilities and the enhancement of our production lines; |
| | • | | approximately $8.0 million to fund the expansion and enhancement of our sales and marketing network; and |
| | • | | the balance for general corporate purposes. |
None of the net proceeds from our initial public offering were paid directly or indirectly to directors or officers of our company or their associates, persons owning 10% or more of our equity securities or our affiliates.
| Item 15. | Controls and Procedures |
Disclosure Controls and Procedures
Under the supervision and with the participation of our management, including our chief executive officer and our chief financial officer, we carried out an evaluation of the effectiveness of our disclosure controls and procedures, which is defined in Rules 13a-15(e) of the Exchange Act, as of the period covered by this annual report. Based on that evaluation, our chief executive officer and chief financial officer concluded that our disclosure controls and procedures were effective as of the end of the period covered by this annual report.
Internal Control over Financial Reporting
Our independent registered public accounting firm has not conducted an audit of our internal control over financial reporting. However, we and our independent registered public accounting firm, in connection with the preparation and external audit of our consolidated financial statements for 2007, 2008 and 2009, noted two material weaknesses and certain deficiencies in our internal control over financial reporting. A “material weakness” is a significant deficiency, or combination of significant deficiencies, that results in more than a remote likelihood that a material misstatement of the annual or interim financial statements will not be prevented or detected. The material weaknesses observed were:
| | • | | the internal audit function had not been fully executed in our day-to-day operations to assist our audit committee and management in the effective discharge of their responsibilities through independent and objective review, analysis, appraisal and reporting of recommendations on our business operations and activities; and |
138
| | • | | a lack of adequate personnel with sufficient understanding of U.S. GAAP and the SEC reporting requirements and adequate policies and procedures to prepare financial information in accordance with U.S. GAAP and the SEC. |
Following the identification of these material weaknesses and other deficiencies, we have taken and will continue to take remedial steps to address the above material weaknesses and other control deficiencies, including hiring additional accounting personnel and training our new and existing accounting staff. However, the process of designing and implementing an effective financial reporting system is a continuous effort that requires us to anticipate and react to changes in our business and the economic and regulatory environments and to expend significant resources to maintain a financial reporting system that is adequate to satisfy our reporting obligations. See “Item 3. Key Information—D. Risk Factors—Risks Related to Our Business—If we fail to establish an effective system of internal controls, we may be unable to accurately report our financial results or prevent fraud, and investor confidence and the market price of our ADSs may be adversely impacted.”
Management’s Report on Internal Control over Financial Reporting and Attestation Report of the Registered Public Accounting Firm
This annual report does not include a report of management’s assessment regarding internal control over financial reporting or an attestation report of our company’s registered public accounting firm due to a transition period established by rules of the SEC for newly public companies.
Changes in Internal Controls
There were no changes in our internal control over financial reporting that occurred during the year ended December 31, 2009 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
| Item 16A. | Audit Committee Financial Expert |
Mr. Sean Shao qualifies as an “audit committee financial expert” as defined in Item 16A of Form 20-F. Mr. Shao satisfies the independence requirements of Rule 5605 of NASDAQ Stock Market, Marketplace Rules and Rule 10A-3 under the Exchange Act.
139
Our board of directors adopted a code of business conduct and ethics that applies to our directors, officers and employees. We have filed our code of business conduct and ethics as an exhibit to Exhibit 99.1 of our registration statement on Form F-1 (file No. 333-163250) filed with the Securities and Exchange Commission on November 20, 2009 and posted a copy of our code of business conduct and ethics on our investor relations website athttp://ir.nkbp.com. We hereby undertake to provide to any person without charge, upon a written request, a copy of our code of business conduct and ethics.
| Item 16C. | Principal Accountant Fees and Services |
The following table sets forth the aggregate fees by categories specified below in connection with certain professional services rendered by Ernst & Young Hua Ming, our principal external auditors, for the periods indicated. We did not pay any tax related or other fees to our auditors during the periods indicated below.
| | | | | | | | |
| | | Year Ended December 31, |
| | | 2007 | | 2008 | | 2009 |
| | | RMB | | RMB | | RMB | | $ |
| | | (in thousands) |
Audit fees(1) | | 2,388 | | 5,030 | | 4,315 | | 632 |
Audit-related fees(2) | | — | | 1,194 | | 1,114 | | 163 |
Tax fees(3) | | — | | 735 | | — | | — |
| (1) | “Audit fees” means the aggregate fees billed for professional services rendered in connection with the audit or review of our financial statements. |
| (2) | “Audit-related fees” represents aggregate fees billed for professional services rendered incrementally and directly in connection with our initial public offering in 2009. |
| (3) | “Tax fees” represents the aggregated fees billed for professional services rendered in connection with tax compliance, tax advice and tax planning. |
The policy of our audit committee is to pre-approve all audit and non-audit services provided by Ernst & Young Hua Ming, including audit services, audit-related services, tax services and other services as described above, other than those forde minimus services which are approved by our audit committee prior to the completion of the audit.
| Item 16D. | Exemptions from the Listing Standards for Audit Committees |
Not applicable.
| Item 16E. | Purchases of Equity Securities by the Issuer and Affiliated Purchasers |
None.
| Item 16F. | Change in Registrant’s Certifying Accountant |
Not applicable.
| Item 16G. | Corporate Governance |
We believe that there are no significant differences between our corporate governance practices and those of U.S. domestic companies under the listing standards of the NASDAQ Global Market.
140
PART III
| Item 17. | Financial Statements |
We have elected to provide financial statements pursuant to Item 18.
| Item 18. | Financial Statements |
The consolidated financial statements of China Nuokang Bio-Pharmaceutical Inc., its subsidiaries and its variable interest entity are included at the end of this annual report.
| | |
Exhibit
Number | | Description of Document |
| |
| 1.1 | | Amended and Restated Memorandum and Articles of Association of the Registrant, as currently in effect (incorporated by reference to Exhibit 3.2 of our Registration Statement on Form F-1 (file No. 333-163250), as amended, initially filed with the Securities and Exchange Commission on November 20, 2009) |
| |
| 2.1 | | Form of Registrant’s American Depositary Receipt (included in Exhibit 2.3) |
| |
| 2.2 | | Registrant’s Specimen Certificate for Ordinary Shares (incorporated by reference to Exhibit 4.2 of our Registration Statement on Form F-1 (file No. 333-163250), as amended, initially filed with the Securities and Exchange Commission on November 20, 2009) |
| |
| 2.3 | | Form of Deposit Agreement among the Registrant, the depositary and Owners and Beneficial Owners of the American Depositary Shares issued thereunder (incorporated by reference to Exhibit 4.3 of our Registration Statement on Form F-1 (file No. 333-163250), as amended, initially filed with the Securities and Exchange Commission on November 20, 2009) |
| |
| 4.1 | | 2007 Share Incentive Plan, as amended (incorporated by reference to Exhibit 10.1 of our Registration Statement on Form F-1 (file No. 333-163250), as amended, initially filed with the Securities and Exchange Commission on November 20, 2009) |
| |
| 4.2 | | 2008 Share Incentive Plan (incorporated by reference to Exhibit 10.2 of our Registration Statement on Form F-1 (file No. 333-163250), as amended, initially filed with the Securities and Exchange Commission on November 20, 2009) |
| |
| 4.3 | | Form of Indemnification Agreement with the Registrant’s directors and officers (incorporated by reference to Exhibit 10.3 of our Registration Statement on Form F-1 (file No. 333-163250), as amended, initially filed with the Securities and Exchange Commission on November 20, 2009) |
| |
| 4.4 | | Form of Employment Agreement between the Registrant and an Executive Officer of the Registrant (incorporated by reference to Exhibit 10.4 of our Registration Statement on Form F-1 (file No. 333-163250), as amended, initially filed with the Securities and Exchange Commission on November 20, 2009) |
| |
| 4.5 | | Preference A Shares Purchase Agreement dated December 20, 2007 among the Registrant, the Management Shareholder and the Purchasers, each as defined therein (incorporated by reference to Exhibit 10.5 of our Registration Statement on Form F-1 (file No. 333-163250), as amended, initially filed with the Securities and Exchange Commission on November 20, 2009) |
| |
| 4.6 | | Investors’ Rights Agreement dated December 20, 2007 among the Registrant, Anglo China Bio-Technology Investment Holdings Limited and Britain Ukan Technology Investment Holdings (Group) Limited, the Management Shareholders and the Preference A Holders, each as defined therein (incorporated by reference to Exhibit 10.6 of our Registration Statement on Form F-1 (file No. 333-163250), as amended, initially filed with the Securities and Exchange Commission on November 20, 2009) |
141
| | |
| 4.7 | | Side Letter dated June 2, 2008 to Preference A Shares Purchase Agreement dated December 20, 2007 among the Registrant, the Management Shareholder and the Purchasers, each as defined therein (incorporated by reference to Exhibit 10.7 of our Registration Statement on Form F-1 (file No. 333-163250), as amended, initially filed with the Securities and Exchange Commission on November 20, 2009) |
| |
| 4.8 | | Voting Agreement dated December 20, 2007 among the Registrant, Anglo China Bio-Technology Investment Holdings Limited and Britain Ukan Technology Investment Holdings (Group) Limited, the Management Shareholders and the Preference A Holders, each as defined therein (incorporated by reference to Exhibit 10.8 of our Registration Statement on Form F-1 (file No. 333-163250), as amended, initially filed with the Securities and Exchange Commission on November 20, 2009) |
| |
| 4.9 | | Right of First Refusal and Co-Sale Agreement dated December 20, 2007 among the Registrant, Anglo China Bio-Technology Investment Holdings Limited and Britain Ukan Technology Investment Holdings (Group) Limited, the Management Shareholders and the Preference A Holders, each as defined therein (incorporated by reference to Exhibit 10.9 of our Registration Statement on Form F-1 (file No. 333-163250), as amended, initially filed with the Securities and Exchange Commission on November 20, 2009) |
| |
| 4.10 | | Repurchase Agreement dated December 20, 2007 between the Registrant and Anglo China Bio-Technology Investment Holdings Limited (incorporated by reference to Exhibit 10.10 of our Registration Statement on Form F-1 (file No. 333-163250), as amended, initially filed with the Securities and Exchange Commission on November 20, 2009) |
| |
| 4.11 | | English translation of Equity Pledge Agreement dated December 14, 2007 among Penglai Nuokang Pharmaceutical Co., Ltd., Liaoning Nuokang Medicines Co., Ltd. and the shareholder of Liaoning Nuokang Medicines Co., Ltd. (incorporated by reference to Exhibit 10.11 of our Registration Statement on Form F-1 (file No. 333-163250), as amended, initially filed with the Securities and Exchange Commission on November 20, 2009) |
| |
| 4.12 | | English translation of Exclusive Call Option Agreement dated December 14, 2007 among Penglai Nuokang Pharmaceutical Co., Ltd., Liaoning Nuokang Medicines Co., Ltd. and the shareholder of Liaoning Nuokang Medicines Co., Ltd. (incorporated by reference to Exhibit 10.12 of our Registration Statement on Form F-1 (file No. 333-163250), as amended, initially filed with the Securities and Exchange Commission on November 20, 2009) |
| |
| 4.13 | | English translation of Business Operation Agreement dated December 14, 2007 among Penglai Nuokang Pharmaceutical Co., Ltd., Liaoning Nuokang Medicines Co., Ltd. and the shareholder of Liaoning Nuokang Medicines Co., Ltd. (incorporated by reference to Exhibit 10.13 of our Registration Statement on Form F-1 (file No. 333-163250), as amended, initially filed with the Securities and Exchange Commission on November 20, 2009) |
| |
| 4.14 | | English translation of Power of Attorney dated December 14, 2007 from the shareholder of Liaoning Nuokang Medicines Co., Ltd. (incorporated by reference to Exhibit 10.14 of our Registration Statement on Form F-1 (file No. 333-163250), as amended, initially filed with the Securities and Exchange Commission on November 20, 2009) |
142
| | |
| 4.15 | | English translation of Exclusive Technology Support and Management Consulting Service Agreement dated December 14, 2007 among Penglai Nuokang Pharmaceutical Co., Ltd. and Liaoning Nuokang Medicines Co., Ltd. (incorporated by reference to Exhibit 10.15 of our Registration Statement on Form F-1 (file No. 333-163250), as amended, initially filed with the Securities and Exchange Commission on November 20, 2009) |
| |
| 4.16 | | English translation of Real Estate Lease Contract dated August 22, 2006 between Penglai Nuokang Pharmaceutical Co., Ltd. and Shandong Penglai Pharmaceutical Plant (incorporated by reference to Exhibit 10.16 of our Registration Statement on Form F-1 (file No. 333-163250), as amended, initially filed with the Securities and Exchange Commission on November 20, 2009) |
| |
| 4.17 | | English translation of Land Use Right Grant Contract dated September 7, 2005 between the Hunnan New District branch bureau of Shenyang City Planning and National Land Resources Bureau and Liaoning Nuokang Bio-pharmaceutical Co., Ltd. (incorporated by reference to Exhibit 10.17 of our Registration Statement on Form F-1 (file No. 333-163250), as amended, initially filed with the Securities and Exchange Commission on November 20, 2009) |
| |
| 4.18** | | Contract dated August 1, 2008 among Shenyang Nanhu Import and Export Co., Ltd., Liaoning Nuokang Bio-pharmaceutical Co., Ltd., Procamp Coml Import Export Ltda. and Ceta-Centro de Extraçåo de Toxinas Animais Ltda. (incorporated by reference to Exhibit 10.18 of our Registration Statement on Form F-1 (file No. 333-163250), as amended, initially filed with the Securities and Exchange Commission on November 20, 2009) |
| |
| 4.19** | | English translation of Exclusive Regional Distribution Agreement dated December 28, 2008 between Liaoning Nuokang Medicines Co., Ltd. and Jilin Yuhua Pharmaceutical Co., Ltd., and its Supplemental Agreement dated June 28, 2009 (incorporated by reference to Exhibit 10.19 of our Registration Statement on Form F-1 (file No. 333-163250), as amended, initially filed with the Securities and Exchange Commission on November 20, 2009) |
| |
| 4.20 | | Subscription Agreement dated July 10, 2009 between Venomics Hong Kong Limited, Liaoning Nuokang Medicines Co., Ltd., Venomics Pty Limited and QRxPharma Limited (incorporated by reference to Exhibit 10.20 of our Registration Statement on Form F-1 (file No. 333-163250), as amended, initially filed with the Securities and Exchange Commission on November 20, 2009) |
| |
| 4.21* | | English translation of Second Supplemental Agreement dated December 28, 2009 to Exclusive Regional Distribution Agreement dated December 28, 2008 between Liaoning Nuokang Medicines Co., Ltd. and Jilin Yuhua Pharmaceutical Co., Ltd. |
| |
| 4.22* | | English translation of Third Supplemental Agreement dated April 30, 2010 to Exclusive Regional Distribution Agreement dated December 28, 2008 between Liaoning Nuokang Medicines Co., Ltd. and Jilin Yuhua Pharmaceutical Co., Ltd. |
| |
| 8.1 | | Subsidiaries of the Registrant (incorporated by reference to Exhibit 21.1 of our Registration Statement on Form F-1 (file No. 333-163250), as amended, initially filed with the Securities and Exchange Commission on November 20, 2009) |
| |
| 11.1 | | Code of Business Conduct and Ethics of the Registrant (incorporated by reference to Exhibit 99.1 of our Registration Statement on Form F-1 (file No. 333-163250), as amended, initially filed with the Securities and Exchange Commission on November 20, 2009) |
| |
| 12.1* | | CEO Certification Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| |
| 12.2* | | CFO Certification Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| |
| 13.1* | | CEO Certification Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
| |
| 13.2* | | CFO Certification Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
| |
| 15.1* | | Consent of Tian Yuan Law Firm |
| |
| 15.2* | | Consent of Ernst & Young Hua Ming, Independent Registered Public Accounting Firm |
| * | Filed with this annual report on Form 20-F. |
| ** | Confidential treatment has been granted with respect to the redacted portions of this exhibit. |
143
SIGNATURES
The registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this annual report on its behalf.
| | |
| China Nuokang Bio-Pharmaceutical Inc. |
| |
| By: | | /S/ BAIZHONG XUE |
| Name: | | Baizhong Xue |
| Title: | | Chairman and Chief Executive Officer |
Date: June 2, 2010
144
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders
China Nuokang Bio-Pharmaceutical Inc.
We have audited the accompanying consolidated balance sheets of China Nuokang Bio-Pharmaceutical Inc. (the “Company”) and its subsidiaries (together, the “Group”) as of December 31, 2008 and 2009, and the related consolidated statements of income, changes in shareholders’ equity and cash flows for each of the three years in the period ended December 31, 2009. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. We were not engaged to perform an audit of the Group’s internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Group’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of China Nuokang Bio-Pharmaceutical Inc. at December 31, 2008 and 2009 and the consolidated results of its operations and its cash flows for each of the three years in the period ended December 31, 2009, in conformity with U.S. generally accepted accounting principles.
|
| /s/ Ernst & Young Hua Ming |
Ernst & Young Hua Ming Shanghai, the People’s Republic of China |
June 2, 2010
F-2
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
CONSOLIDATED BALANCE SHEETS
AS OF DECEMBER 31, 2008 and 2009
| | | | | | | | |
| | | | | December 31, |
| | | Note | | 2008 | | 2009 |
| | | | | (RMB’000) | | (RMB’000) | | (US$’000) |
| | | | |
ASSETS | | | | | | | | |
Current assets: | | | | | | | | |
Cash and cash equivalents | | | | 81,837 | | 351,258 | | 51,460 |
Restricted cash | | 5 | | 20,000 | | 34,200 | | 5,010 |
Other investment – current | | 13 | | — | | 2,000 | | 293 |
Accounts receivable (net of allowance for doubtful accounts of RMB448,103 and RMB393,860 (US$57,701) as of December 31, 2008 and 2009, respectively) | | 6 | | 71,312 | | 103,719 | | 15,195 |
Bills receivable | | 7 | | 8,134 | | 34,346 | | 5,032 |
Inventories | | 8 | | 10,610 | | 14,765 | | 2,163 |
Prepayments and other receivables | | 9 | | 22,416 | | 26,976 | | 3,952 |
Prepaid income tax | | | | 3,869 | | 6,397 | | 937 |
Deferred tax assets | | 20 | | 180 | | 1,171 | | 172 |
| | | | | | | | |
| | | | |
Total current assets | | | | 218,358 | | 574,832 | | 84,214 |
| | | | | | | | |
| | | | |
Non-current assets: | | | | | | | | |
Property, plant and equipment, net | | 10 | | 149,679 | | 163,915 | | 24,014 |
Land use rights, net | | 11 | | 26,669 | | 26,212 | | 3,840 |
Intangible assets, net | | 12 | | 6,337 | | 8,369 | | 1,226 |
Other investment – Non current | | 13 | | — | | 3,414 | | 500 |
Deferred tax assets | | 20 | | 3,988 | | 4,712 | | 690 |
| | | | | | | | |
| | | | |
Total non-current assets | | | | 186,673 | | 206,622 | | 30,270 |
| | | | | | | | |
| | | | |
TOTAL ASSETS | | | | 405,031 | | 781,454 | | 114,484 |
| | | | | | | | |
The accompanying notes are an integral part of the consolidated financial statements.
F-3
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
CONSOLIDATED BALANCE SHEETS (CONT’D)
AS OF DECEMBER 31, 2008 and 2009
| | | | | | | | | |
| | | | | | December 31, |
| | | Note | | | 2008 | | 2009 |
| | | | | | (RMB’000) | | (RMB’000) | | (US$’000) |
| | | | |
LIABILITIES, PREFERENCE SHARES AND SHAREHOLDERS’ EQUITY | | | | | | | | | |
Current liabilities: | | | | | | | | | |
Short-term bank loans | | 14 | | | 72,430 | | 125,618 | | 18,403 |
Accounts payable | | | | | 5,035 | | 1,924 | | 282 |
Bills payable | | 15 | | | 20,000 | | — | | — |
Accrued expenses and other payables | | 16 | | | 11,958 | | 29,994 | | 4,394 |
Income tax payable | | | | | 4,227 | | 8,885 | | 1,302 |
Amount due to a related party | | 23 | | | 2,026 | | — | | — |
Unrecognized tax benefits | | 20 | | | — | | 809 | | 119 |
Share-based compensation liability | | 22 | | | 4,084 | | — | | — |
Deferred tax liability | | 20 | | | — | | 16 | | 2 |
| | | | | | | | | |
| | | | |
Total current liabilities | | | | | 119,760 | | 167,246 | | 24,502 |
| | | | | | | | | |
| | | | |
Non-current liabilities: | | | | | | | | | |
Deferred tax liabilities | | 20 | | | 1,106 | | 1,858 | | 272 |
Deferred government grants | | 17 | | | 19,384 | | 19,258 | | 2,821 |
Long-term payable | | 4 | | | 9,815 | | 10,557 | | 1,547 |
| | | | | | | | | |
| | | | |
Total non-current liabilities | | | | | 30,305 | | 31,673 | | 4,640 |
| | | | | | | | | |
| | | | |
Commitments and contingencies | | 26 | | | — | | — | | — |
| | | | | | | | | |
| | | | |
Convertible redeemable preference shares | | | | | | | | | |
| | | | |
Series A preference shares (25,800,000 shares authorized and 25,796,662 shares issued and outstanding with par value of US$0.0005 as of December 31, 2008; nil authorized and outstanding as of December 31, 2009) | | 18 | | | 127,245 | | — | | — |
| | | | | | | | | |
| | | | |
Shareholders’ equity: | | | | | | | | | |
Ordinary shares (par value US$0.0005 per share, 474,200,000 shares authorized and 95,447,648 shares issued and outstanding as of December 31, 2008; 474,200,000 shares authorized and 158,420,142 share issued and outstanding as of December 31, 2009) | | | | | 382 | | 597 | | 87 |
Additional paid-in capital | | | | | 50,477 | | 451,716 | | 66,177 |
Retained earnings | | 19 | (c) | | 76,862 | | 128,071 | | 18,763 |
| | | | | | | | | |
| | | | |
Total shareholders’ equity | | | | | 127,721 | | 580,384 | | 85,027 |
| | | | | | | | | |
| | | | |
Non-controlling interest | | 25 | | | — | | 2,151 | | 315 |
| | | | | | | | | |
| | | | |
TOTAL EQUITY | | | | | 127,721 | | 582,535 | | 85,342 |
| | | | | | | | | |
| | | | |
TOTAL LIABILITIES, PREFERENCE SHARES AND SHAREHOLDERS’ EQUITY | | | | | 405,031 | | 781,454 | | 114,484 |
| | | | | | | | | |
The accompanying notes are an integral part of the consolidated financial statements.
F-4
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
CONSOLIDATED STATEMENTS OF INCOME
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
| | | | | | | | | | | | | | | |
| | | Note | | 2007 | | | 2008 | | | 2009 | |
| | | | | (RMB’000) | | | (RMB’000) | | | (RMB’000) | | | (US$’000) | |
| | | | | |
Net revenue | | | | 147,875 | | | 225,399 | | | 282,911 | | | | 41,447 | |
Cost of revenue | | | | (34,745 | ) | | (30,963 | ) | | (35,625 | ) | | | (5,219 | ) |
| | | | | | | | | | | | | | | |
| | | | | |
Gross profit | | | | 113,130 | | | 194,436 | | | 247,286 | | | | 36,228 | |
| | | | | | | | | | | | | | | |
| | | | | |
Operating expenses | | | | | | | | | | | | | | | |
Research and development costs | | | | (7,995 | ) | | (5,585 | ) | | (8,297 | ) | | | (1,216 | ) |
Selling, marketing and distribution expenses | | | | (52,443 | ) | | (92,404 | ) | | (110,513 | ) | | | (16,190 | ) |
General and administrative expenses | | | | (16,796 | ) | | (31,884 | ) | | (47,582 | ) | | | (6,971 | ) |
| | | | | | | | | | | | | | | |
| | | | | |
Total operating expenses | | | | (77,234 | ) | | (129,873 | ) | | (166,392 | ) | | | (24,377 | ) |
| | | | | | | | | | | | | | | |
| | | | | |
Operating profit | | | | 35,896 | | | 64,563 | | | 80,894 | | | | 11,851 | |
Interest income | | | | 196 | | | 1,259 | | | 1,372 | | | | 201 | |
Interest expense | | 7 | | (5,190 | ) | | (1,309 | ) | | (3,327 | ) | | | (487 | ) |
Other income, net | | | | 2,191 | | | 6,353 | | | 3,014 | | | | 441 | |
| | | | | | | | | | | | | | | |
| | | | | |
Income before income tax expense | | 20 | | 33,093 | | | 70,866 | | | 81,953 | | | | 12,006 | |
Income tax benefit (expense) | | 20 | | 2,102 | | | (7,246 | ) | | (16,858 | ) | | | (2,470 | ) |
| | | | | | | | | | | | | | | |
| | | | | |
Net Income | | | | 35,195 | | | 63,620 | | | 65,095 | | | | 9,536 | |
| | | | | | | | | | | | | | | |
| | | | | |
Net income attributable to non-controlling interest | | | | — | | | — | | | — | | | | — | |
| | | | | | | | | | | | | | | |
| | | | | |
Net income | | | | 35,195 | | | 63,620 | | | 65,095 | | | | 9,536 | |
| | | | | | | | | | | | | | | |
| | | | | |
Accretion of Series A convertible redeemable preference shares | | 21 | | (30,156 | ) | | (13,403 | ) | | (13,886 | ) | | | (2,034 | ) |
Allocation to Series A convertible redeemable preference shares for participating rights to dividends | | 21 | | (326 | ) | | (9,458 | ) | | — | | | | — | |
| | | | | | | | | | | | | | | |
| | | | | |
Net income attributed to ordinary shares | | 21 | | 4,713 | | | 40,759 | | | 51,209 | | | | 7,502 | |
| | | | | | | | | | | | | | | |
| | | | | |
Net income per share | | | | | | | | | | | | | | | |
Basic | | 21 | | RMB 0.05 | | | RMB 0.43 | | | RMB 0.52 | | | US$ | 0.08 | |
Diluted | | 21 | | RMB 0.05 | | | RMB 0.43 | | | RMB 0.51 | | | US$ | 0.08 | |
| | | | | |
Weighted average ordinary shares | | | | | | | | | | | | | | | |
Shares used in net income per share computation | | | | | | | | | | | | | | | |
Basic | | 21 | | 99,850,334 | | | 95,447,648 | | | 98,885,045 | | | | 98,885,045 | |
Diluted | | 21 | | 99,850,334 | | | 95,460,841 | | | 99,725,253 | | | | 99,725,253 | |
The accompanying notes are an integral part of the consolidated financial statements.
F-5
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
CONSOLIDATED STATEMENTS OF CHANGES IN SHAREHOLDERS’ EQUITY
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
| | | | | | | | | | | | | | | | | | | | |
| | | | | | China Nuokang Bio-Pharmaceutical Inc. | | | | | | |
| | | Note | | | Number of
ordinary
shares | | | Amount | | | Additional
paid-in
capital | | | Retained
earnings | | | Non -
controlling
Interest | | Total
shareholders’
equity | |
| | | | | | | | | (RMB’000) | | | (RMB’000) | | | (RMB’000) | | | (RMB’000) | | (RMB’000) | |
| | | | | | | |
Balance as of January 1, 2007 | | | | | 100,000,000 | | | 399 | | | 31,867 | | | 21,606 | | | — | | 53,872 | |
| | | | | | | |
Repurchase of ordinary shares | | 19 | (a) | | (4,552,352 | ) | | (17 | ) | | (21,614 | ) | | — | | | — | | (21,631 | ) |
| | | | | | | |
Shareholder’s contribution | | | | | — | | | — | | | 10,000 | | | — | | | — | | 10,000 | |
| | | | | | | |
Beneficial conversion feature on Series A convertible redeemable preference shares | | 18 | | | — | | | — | | | 29,877 | | | — | | | — | | 29,877 | |
| | | | | | | |
Accretion of Series A convertible redeemable preference shares | | 18 | | | — | | | — | | | — | | | (30,156 | ) | | — | | (30,156 | ) |
| | | | | | | |
Net income for the year | | | | | — | | | — | | | — | | | 35,195 | | | — | | 35,195 | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | |
Balance as of December 31, 2007 | | | | | 95,447,648 | | | 382 | | | 50,130 | | | 26,645 | | | — | | 77,157 | |
| | | | | | | |
Shareholder’s injection of capital | | | | | — | | | — | | | 347 | | | — | | | — | | 347 | |
| | | | | | | |
Accretion of Series A convertible redeemable preference shares | | 18 | | | — | | | — | | | — | | | (13,403 | ) | | — | | (13,403 | ) |
| | | | | | | |
Net income for the year | | | | | — | | | — | | | — | | | 63,620 | | | — | | 63,620 | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | |
Balance as of December 31, 2008 | | | | | 95,447,648 | | | 382 | | | 50,477 | | | 76,862 | | | — | | 127,721 | |
| | | | | | | |
Proceeds from issuance of common stocks upon initial public offering | | 19 | | | 36,215,832 | | | 124 | | | 239,506 | | | — | | | — | | 239,630 | |
| | | | | | | |
Conversion of preference shares | | 19 | | | 25,796,662 | | | 88 | | | 140,907 | | | — | | | — | | 140,995 | |
| | | | | | | |
Exercise of share options | | 19 | | | 960,000 | | | 3 | | | 3,020 | | | — | | | — | | 3,023 | |
| | | | | | | |
Capital contributed by non-controlling interest | | | | | — | | | — | | | — | | | — | | | 2,151 | | 2,151 | |
| | | | | | | |
Share-based compensation expense reclassification of liability awards to equity | | 22 | | | — | | | — | | | 13,096 | | | — | | | — | | 13,096 | |
| | | | | | | |
Share-based compensation expenses of equity awards | | 22 | | | — | | | — | | | 4,710 | | | — | | | — | | 4,710 | |
| | | | | | | |
Accretion of Series A convertible redeemable preference shares | | 18 | | | — | | | — | | | — | | | (13,886 | ) | | — | | (13,886 | ) |
| | | | | | | |
Net income for the year | | | | | — | | | — | | | — | | | 65,095 | | | — | | 65,095 | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | |
Balance as of December 31, 2009 | | | | | 158,420,142 | | | 597 | | | 451,716 | | | 128,071 | | | 2,151 | | 582,535 | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | |
Balance as of December 31, 2009 (US$’000) | | | | | 158,420,142 | | | 87 | | | 66,177 | | | 18,763 | | | 315 | | 85,342 | |
| | | | | | | | | | | | | | | | | | | | |
The accompanying notes are an integral part of the consolidated financial statements.
F-6
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
| | | | | | | | | | | | |
| | | For the Year Ended
December 31, | |
| | | 2007 | | | 2008 | | | 2009 | |
| | | (RMB’000) | | | (RMB’000) | | | (RMB’000) | | | (US$’000) | |
Cash flow from operating activities | | | | | | | | | | | | |
Net income | | 35,195 | | | 63,620 | | | 65,095 | | | 9,536 | |
Adjustments to reconcile net income to net cash generated from operating activities: | | | | | | | | | | | | |
Compensation expenses to shareholder | | 293 | | | — | | | — | | | — | |
Share-based compensation | | 105 | | | 3,979 | | | 13,722 | | | 2,010 | |
Reversal of allowance for doubtful accounts | | (7 | ) | | (132 | ) | | (54 | ) | | (8 | ) |
Write-down of inventories | | — | | | — | | | 51 | | | 7 | |
Depreciation of property, plant and equipment | | 2,983 | | | 6,956 | | | 10,662 | | | 1,562 | |
Loss (Gain) on disposal of property, plant and equipment | | 6 | | | (6 | ) | | (23 | ) | | (3 | ) |
Amortization of land use rights | | 419 | | | 457 | | | 457 | | | 67 | |
Amortization of intangible assets | | 540 | | | 540 | | | 540 | | | 79 | |
Investment income | | (226 | ) | | (13 | ) | | — | | | — | |
Accretion of interest for long-term payable (Note 4) | | 742 | | | 742 | | | 742 | | | 109 | |
Deferred tax benefit | | (2,643 | ) | | (479 | ) | | (1,371 | ) | | (201 | ) |
Foreign currency exchange gains | | (2,335 | ) | | (8,494 | ) | | (176 | ) | | (26 | ) |
| | | | |
Changes in operating assets and liabilities: | | | | | | | | | | | | |
Accounts and bills receivables | | (13,936 | ) | | (48,397 | ) | | (58,565 | ) | | (8,580 | ) |
Inventories | | 14,551 | | | (3,576 | ) | | (4,206 | ) | | (616 | ) |
Prepayments and other receivables | | (188 | ) | | (185 | ) | | (3,138 | ) | | (460 | ) |
Prepaid income tax | | — | | | (3,869 | ) | | (2,528 | ) | | (370 | ) |
Income tax payable | | (1,165 | ) | | (299 | ) | | 4,658 | | | 682 | |
Unrecognized tax benefits | | — | | | — | | | 809 | | | 118 | |
Accounts and bills payables | | (13,061 | ) | | 16,931 | | | (23,111 | ) | | (3,386 | ) |
Accrued expenses and other payables | | (832 | ) | | (5,329 | ) | | 5,671 | | | 833 | |
Deferred government grants | | 4,800 | | | 2,864 | | | (126 | ) | | (18 | ) |
| | | | | | | | | | | | |
| | | | |
Net cash generated from operating activities | | 25,241 | | | 25,310 | | | 9,109 | | | 1,335 | |
| | | | | | | | | | | | |
The accompanying notes are an integral part of the consolidated financial statements.
F-7
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS (CONT’D)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
| | | | | | | | | | | | |
| | | For the Year Ended
December 31, | |
| | | 2007 | | | 2008 | | | 2009 | |
| | | (RMB’000) | | | (RMB’000) | | | (RMB’000) | | | (US$’000) | |
Cash flow from investing activities | | | | | | | | | | | | |
Prepayment of investments | | (12,500 | ) | | — | | | — | | | — | |
Payment of other investments | | — | | | — | | | (5,414 | ) | | (793 | ) |
Proceeds from sales of short-term investments | | 12,579 | | | — | | | — | | | — | |
Purchase of property, plant and equipment | | (59,374 | ) | | (51,083 | ) | | (26,853 | ) | | (3,934 | ) |
Prepayment for exclusive distribution rights | | — | | | (10,000 | ) | | (6,000 | ) | | (879 | ) |
Proceeds from disposal of property, plant and equipment | | — | | | 16 | | | 1,203 | | | 176 | |
Proceeds from investments in equity securities | | 700 | | | 313 | | | — | | | — | |
Repayment from related parties | | 7,083 | | | 791 | | | 3,823 | | | 560 | |
(Loan to) repayment from third parties | | (2,133 | ) | | 3,200 | | | — | | | — | |
| | | | | | | | | | | | |
| | | | |
Net cash used in investing activities | | (53,645 | ) | | (56,763 | ) | | (33,241 | ) | | (4,870 | ) |
| | | | | | | | | | | | |
| | | | |
Cash flow from financing activities | | | | | | | | | | | | |
Release (Pledge) of restricted cash | | 2,400 | | | (20,000 | ) | | (14,200 | ) | | (2,080 | ) |
Repayment of short-term bank loans | | (18,380 | ) | | (50,380 | ) | | (75,229 | ) | | (11,021 | ) |
Proceeds from short-term bank loans | | 50,380 | | | 72,430 | | | 128,417 | | | 18,813 | |
Loan from (repayment to) a related party | | 3,708 | | | (1,696 | ) | | (5,809 | ) | | (851 | ) |
Capital contribution from a shareholder | | 10,000 | | | 347 | | | — | | | — | |
Proceeds from issuance of preference shares | | 124,234 | | | — | | | — | | | — | |
Repurchase of ordinary shares | | (21,924 | ) | | — | | | — | | | — | |
Capital distribution to shareholder | | (12,000 | ) | | — | | | — | | | — | |
Proceeds from exercise of share options | | — | | | — | | | 3,023 | | | 443 | |
Proceeds from issuance of common stocks upon initial public offering, net off initial public offering insurance costs | | — | | | — | | | 257,351 | | | 37,702 | |
| | | | | | | | | | | | |
| | | | |
Net cash generated from financing activities | | 138,418 | | | 701 | | | 293,553 | | | 43,006 | |
| | | | | | | | | | | | |
| | | | |
Net increase (decrease) in cash and cash equivalents | | 110,194 | | | (30,752 | ) | | 269,421 | | | 39,471 | |
Cash and cash equivalents at the beginning of year | | 2,395 | | | 112,589 | | | 81,837 | | | 11,989 | |
| | | | | | | | | | | | |
| | | | |
Cash and cash equivalents at the end of year | | 112,589 | | | 81,837 | | | 351,258 | | | 51,460 | |
| | | | | | | | | | | | |
| | | | |
Supplemental disclosures of cash flow information: | | | | | | | | | | | | |
Interest expense paid | | (3,360 | ) | | (4,986 | ) | | (4,563 | ) | | (668 | ) |
Income tax paid | | (1,706 | ) | | (11,893 | ) | | (15,292 | ) | | (2,240 | ) |
Supplemental disclosures of non-cash activities: | | | | | | | | | | | | |
Acquisition of property, plant and equipment included in accrued expenses and other payables | | 13,914 | | | 674 | | | 343 | | | 50 | |
Accretion of Series A convertible redeemable preference shares | | 30,156 | | | 13,403 | | | 13,886 | | | 2,034 | |
Accretion of interest for long-term payable | | 742 | | | 742 | | | 742 | | | 109 | |
Initial of public offering costs included in accrued expenses and other payables | | — | | | — | | | 8,311 | | | 1,218 | |
The accompanying notes are an integral part of the consolidated financial statements.
F-8
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
1. BASIS OF PRESENTATION
Brighter Sky Limited (“Brighter Sky”) was incorporated in the Cayman Islands on June 16, 2006 under the Cayman Islands Companies Law as an exempted company with limited liability. By a shareholder’s resolution passed on September 28, 2007 and on August 11, 2008, the name of Brighter Sky Limited was changed to China Nuokang Bio-Pharmaceutical Pty and was subsequently changed to China Nuokang Bio-Pharmaceutical Inc. (the “Company”). Upon incorporation, Brighter Sky was 90% owned by Anglo China Bio-Technology Investment Holdings Limited (“Anglo China”) and 10% owned by Britain Ukan Technology Investment Holdings (Group) Limited (“Ukan Technology”), both of which are wholly-owned by Mr. Xue Bai Zhong (“Mr. Xue”).
On December 20, 2007, the Company issued convertible redeemable preference shares to two investors (Note 18).
On December 9, 2009, the Company completed its initial public offering of 4,526,979 American Depositary Shares (“ADS”) at US$9.0 per ADS. Each ADS comprises eight ordinary shares. The net proceeds to the Company from the offering amounted to RMB239,629,419, (US$35,105,908), net of issuance costs paid and payable.
The Company, its subsidiaries and variable interest entity (“VIE”) (collectively, the “Group”) are principally engaged in research, development, manufacture and distribution of pharmaceutical products in the People’s Republic of China (the “PRC”).
As of December 31, 2008 and 2009, the consolidated financial statements of the Company include the following subsidiaries and variable interest entity:
F-9
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(CONT’D)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
1. BASIS OF PRESENTATION (CONT’D)
| | | | | | | | | | | | | | | | | | |
Name | | Date of incorporation/ establishment | | Place of incorporation/ establishment | | Percentage of
shareholding/
ownership | | | Principal activities |
| | | | | | | December 31 | | | |
| | | | | | | 2008 | | | 2009 | | | |
| | | | | | | Direct | | | Indirect | | | Direct | | | Indirect | | | |
Subsidiaries: | | | | | | | | | | | | | | | | | | |
| | | | | | | |
Surplus International Investments Limited (“Surplus International”) | | August 26, 2005 | | Hong Kong | | 100 | % | | — | | | 100 | % | | — | | | Investment holding |
| | | | | | | |
Liaoning Nuokang Bio-Pharmaceutical
Co., Ltd.
(“Liaoning Nuokang”) | | November 7, 1997 | | The PRC | | — | | | 100 | % | | — | | | 100 | % | | Sourcing and processing of raw materials for Penglai Nuokang |
| | | | | | | |
Shenyang Shouzheng Bio-Technology Co., Ltd. (“Shenyang Shouzheng”) | | October 22, 2001 | | The PRC | | — | | | 100 | % | | — | | | 100 | % | | Performing research and development activities and manufacturing of certain auxiliary raw materials for Penglai Nuokang |
| | | | | | | |
Penglai Nuokang Pharmaceutical Co., Ltd. (“Penglai Nuokang”) | | June 7, 2002 | | The PRC | | — | | | 100 | % | | — | | | 100 | % | | Manufacturing of pharmaceutical products |
| | | | | | | |
VIE: | | | | | | | | | | | | | | | | | | |
| | | | | | | |
Liaoning Nuokang Medicines Co., Ltd. (“Nuokang Distribution”) | | December 3, 1999 | | The PRC | | — | | | VIE | (f) | | — | | | VIE | (f) | | Distribution of pharmaceutical products |
| | | | | | | |
Subsidiary of VIE: | | | | | | | | | | | | | | | | | | |
| | | | | | | |
Venomics Hong Kong Limited (“VHK”) (Note 25) | | April 28, 2009 | | Hong Kong | | — | | | — | | | — | | | 93.7 | %(*) | | Investment holding |
| * | The Group owns a total of 93.7% equity interest, directly and indirectly, in VHK (93% direct ownership through VIE Nuokang Distribution, and 0.7% through a 10% equity interest in VHK’s parent Venomics Pty limited (“VPL”). |
F-10
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(CONT’D)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
1. BASIS OF PRESENTATION (CONT’D)
The above organization structure reflects the following transactions which were undertaken by the Company and Mr. Xue to restructure the Company for its initial public offering (“IPO”):
| (a) | Upon establishment, Liaoning Nuokang was controlled by Mr. Xue through his 50% direct equity interest and 20% equity interest held by a nominee owner on behalf of Mr. Xue. The remaining 30% equity interest was held by third party investors. Pursuant to a nominee ownership agreement, the nominee owner will exercise his voting rights in Liaoning Nuokang based on instructions from Mr. Xue. All dividends and distributions received by the nominee owner shall be paid to Mr. Xue. The nominee owner cannot sell or pledge his equity interest in Liaoning Nuokang without the approval of Mr. Xue. In March 2003, Mr. Xue acquired the remaining 30% equity interest in Liaoning Nuokang held by third party investors for cash consideration of RMB150,000 and accounted for the transaction using the purchase method. |
| (b) | Upon establishment, Shenyang Shouzheng was controlled by Mr. Xue through his 75% direct equity interest and 25% equity interest held by two nominee owners on behalf of Mr. Xue. Pursuant to nominee ownership agreements, the nominee owners will exercise their voting rights in Shenyang Shouzheng based on instructions from Mr. Xue. All dividends and distributions received by the nominee owners shall be paid to Mr. Xue. The nominee owners cannot sell or pledge their equity ownership in Shenyang Shouzheng without the approval of Mr. Xue. |
| (c) | Upon establishment, Penglai Nuokang was controlled by Mr. Xue through his 60% equity interest. The remaining 40% equity interest was held by a third party investor. On August 22, 2006, Mr. Xue acquired the remaining 40% equity interest in Penglai Nuokang held by the third party investor for consideration of RMB8,209,000 and accounted for the transaction using the purchase method (Note 4). |
| (d) | Surplus International was incorporated by Ms. Zhu Yu Huan, wife of Mr. Xue, on August 26, 2005. On September 20, 2005 and July 20, 2006, Surplus International acquired 100% of the equity interests in Liaoning Nuokang and Shenyang Shouzheng from Mr. Xue in exchange for cash consideration of RMB11,000,000 and RMB2,000,000, respectively. On August 17, 2006, the Company acquired 100% of the equity interest in Surplus International at purchase consideration in cash equal to the then issued capital of Surplus International of HK$10,000. |
| (e) | On August 23, 2006, Surplus International acquired all the equity interest in Penglai Nuokang from Mr. Xue in exchange for cash consideration of RMB10,000,000. |
The series of transactions noted in (d) and (e) above (together, the “Restructuring”) have been accounted for as a legal reorganization of entities under common control in a manner similar to a pooling of interest using historical cost as the entities are either controlled by Mr. Xue directly (or through respective nominee ownership agreements) or by his wife, who qualifies as an immediate family member pursuant to Emerging Issues Task Force (“EITF”) 02-5, “Definition of “Common Control” in Relation to FASB Statement No. 141”. Cash consideration for the Restructuring has been accounted for as distributions to the shareholder. Accordingly, the accompanying consolidated financial statements have been prepared as if the current corporate structure had been in existence throughout the periods presented.
F-11
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(CONT’D)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
1. BASIS OF PRESENTATION (CONT’D)
| (f) | Nuokang Distribution was established on December 3, 1999 by Mr. Xue who held 5% of the equity interest and three other third party investors who held 95% of the equity interest. On October 30, 2001, Mr. Xue, together with two nominee shareholders, acquired the 95% equity interest in Nuokang Distribution from the three third party investors in exchange for cash considerations of RMB475,000 and accounted for the transaction using the purchase method. After this acquisition, Nuokang Distribution was controlled by Mr. Xue through his 75% direct equity interest with the remaining 25% equity interest held by two nominee owners on behalf of Mr. Xue. Pursuant to nominee ownership agreements, the nominee owners will exercise their voting rights in Nuokang Distribution based on instructions from Mr. Xue. All dividends and distributions received by the nominee owners shall be paid to Mr. Xue. The nominee owners cannot sell or pledge their equity interest in Nuokang Distribution without the approval of Mr. Xue. On November 26, 2007, the nominee owners transferred their 25% equity interests in Nuokang Distribution for no consideration to Mr. Xue. |
On December 14, 2007, Nuokang Distribution, Mr. Xue and Penglai Nuokang entered into a series of contractual arrangements (the “Reorganization”), including a power of attorney agreement, an exclusive technical support and management consultancy agreement, a business operation restriction agreement, an equity pledging agreement, and an equity purchase agreement (collectively, the “VIE Agreements”), which collectively enabled Penglai Nuokang to: a) exercise effective control over Nuokang Distribution through its ability to exercise all the rights of the underlying equity interests held by Mr. Xue, including voting and transfer rights; b) provide unlimited financial support to Nuokang Distribution for its operations and agrees to forego the right to seek repayment in the event Nuokang Distribution fails to repay such funding; c) receive technical support and management consulting fees of 80% of Nuokang Distribution’s profit before tax, which is adjustable at the sole discretion of Penglai Nuokang; and d) have an exclusive option to purchase all or part of the equity interest held by Mr. Xue in Nuokang Distribution, to the extent permitted under PRC law at an amount equal to either (i) the registered capital of Nuokang Distribution at the time of purchase or (ii) the lowest permissible purchase price as set by PRC law. In addition, pursuant to these VIE agreements, Mr. Xue, as the equity interest holder of Nuokang Distribution a) cannot declare any profit distributions in any form without the prior consent of Penglai Nuokang; b) must remit in full any funds received from Nuokang Distribution to Penglai Nuokang, in the event any distributions are made by Nuokang Distribution; and c) must remit in full to Penglai Nuokang any consideration received from Penglai Nuokang upon the exercise of the equity purchase option.
The Group has adopted Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 810-10-15, “Variable Interest Entities”. FASB ASC 810-10-15 requires certain variable interest entities to be consolidated by the primary beneficiary of the entity if the equity investors in the entity do not have characteristics of a controlling financial interest or do not have sufficient equity at risk for the entity to finance its activities without additional subordinated financial support from other parties.
F-12
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(CONT’D)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
1. BASIS OF PRESENTATION (CONT’D)
The following is a summary of the various VIE agreements dated December 14, 2007:
Power of attorney agreement
Mr. Xue and Penglai Nuokang entered into the power of attorney agreement whereby Mr. Xue granted an irrevocable proxy of his rights underlying his equity interests in Nuokang Distribution to a PRC natural person designated by Penglai Nuokang, which includes, but are not limited to, all the shareholder’s rights and voting rights empowered to Mr. Xue by the company law and the Company’s Article of Association.
Exclusive technical support and management consultancy agreement
Pursuant to the exclusive technical support and management consultancy agreement between Penglai Nuokang and Nuokang Distribution, Penglai Nuokang is to a) provide exclusive technical support and management consultancy services in respect of technology support service, market development, customer maintenance and financing to Nuokang Distribution; and b) provide unlimited financial support to Nuokang Distribution for its operations and agree to forego the right to seek repayment in the event Nuokang Distribution fails to repay such funding. In return, Penglai Nuokang is to receive technical support and management consulting fees of 80% of Nuokang Distribution’s profit before tax, which is adjustable at the sole discretion of Penglai Nuokang. No consultation fees were paid for 2007 due to Nuokang Distribution’s loss before tax status, while RMB9,342,876 (US$ 1,368,739) and RMB12,860,249 (US$1,884,037) has incurred for the years ended December 31, 2008 and 2009, respectively.
Business operation restriction agreement
Penglai Nuokang, Nuokang Distribution and Mr. Xue, the sole shareholder of Nuokang Distribution, entered into a business operation restriction agreement, whereby restriction is imposed on the manner in which Nuokang Distribution is to conduct its operations such that it can fulfill its payment obligations under the exclusive technical support and management consultancy agreement. The restriction to be imposed mainly includes a) neither Nuokang Distribution nor Mr. Xue is permitted to enter into any transaction that will substantially impact the assets, obligations, rights or operations of Nuokang Distribution without the prior consent from Penglai Nuokang; b) Nuokang Distribution together with Mr. Xue agrees to accept from Penglai Nuokang any instructions relating to the hiring and terminating of personnel, daily operating and financial management processes, and any recommendations over hiring of directors, chief executive officer, chief finance officer and other senior management positions; and c) in the event that Nuokang Distribution seeks for guarantor over its borrowings, it shall make such request to Penglai Nuokang. Penglai Nuokang retains the right to objection. Despite the fact that Penglai Nuokang retains the contractual right to object to such request, it is the intention of the Company to facilitate such guarantee if and when such request is made.
Equity pledging agreement
Pursuant to the equity pledging agreement between Penglai Nuokang and Mr. Xue, Mr. Xue has pledged all his equity interests in Nuokang Distribution to guarantee the performance of Nuokang Distribution’s obligations under the exclusive technical support and management consultancy agreement and the business operation restriction agreement.
F-13
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(CONT’D)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
1. BASIS OF PRESENTATION (CONT’D)
Equity pledging agreement (Cont’d)
If Nuokang Distribution breaches its respective contractual obligations under the exclusive technology supporting and service agreement or business operating restriction agreement, Penglai Nuokang, as pledgee, will be entitled to certain rights, including the right to sell the pledged equity interests. Mr. Xue agreed not to transfer, sell, pledge, dispose of or otherwise create any new encumbrance on his equity interests in Nuokang Distribution without the prior consent of Penglai Nuokang.
Equity purchase agreement
Pursuant to the equity purchase agreement amongst Penglai Nuokang, Nuokang Distribution and Mr. Xue, Mr. Xue irrevocably granted Penglai Nuokang, or its designated person, an exclusive option to purchase all or part of the equity interest held by Mr. Xue in Nuokang Distribution, when and to the extent permitted under PRC law, at an amount equal to either a) the registered capital of Nuokang Distribution at the time of purchase or b) the lowest permissible purchase price as set by PRC law. Such consideration received by Mr. Xue upon the exercise of the equity purchase option is required to be remitted in full to Penglai Nuokang. Nuokang Distribution cannot declare any profit distributions in any form without the prior consent of Penglai Nuokang and Mr. Xue must remit in full any funds received from Nuokang Distribution to Penglai Nuokang, in the event any distributions are made by Nuokang Distribution.
Despite the lack of technical majority ownership, there exists a parent-subsidiary relationship between Penglai Nuokang and Nuokang Distribution through the irrevocable power of attorney agreement, whereby Mr. Xue effectively assigned all of his voting rights underlying his equity interest in Nuokang Distribution to Penglai Nuokang. In addition, through the other aforementioned agreements, Penglai Nuokang demonstrates its ability and intention to continue to exercise the ability to absorb substantially all of the expected losses and majority of the profits of Nuokang Distribution. Thus, Penglai Nuokang is also considered the primary beneficiary of Nuokang Distribution. As a result of the above, Nuokang Distribution is consolidated in the Company’s financial statements.
Since Nuokang Distribution and Penglai Nuokang were under common control by Mr. Xue immediately before and after the Reorganization, the transaction was accounted for as a legal reorganization of entities under common control, in a manner similar to pooling of interest using historical cost. Accordingly, the accompanying consolidated financial statements have been prepared as if the current corporate structure had been in existence throughout the periods presented.
The accompanying consolidated financial statements have been prepared in accordance with U.S. generally accepted accounting principles (“US GAAP”).
F-14
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(CONT’D)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICES
(a) Principles of Consolidation
The consolidated financial statements include the financial statements of the Company, its subsidiaries and the VIE for which a subsidiary of the Company is the primary beneficiary. All significant inter-company transactions and balances between the Company, its subsidiaries and the VIE are eliminated upon consolidation. Results of acquired subsidiaries are consolidated from the date on which control is transferred to the Group and are no longer consolidated from the date that control ceases.
(b) Use of Estimates
The preparation of financial statements in conformity with US GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the period. Significant estimates reflected in the Group’s consolidated financial statements include, but are not limited to, the recoverability of the carrying amount and estimated useful lives of long-lived assets, allowance for accounts receivable, realizable values for inventories, valuation allowance of deferred tax assets, purchase price allocation of its acquisitions and share-based compensation expenses. Changes in facts and circumstances may result in revised estimates. Actual results could differ from these estimates.
(c) Foreign Currency
The functional currency of the Group is the Renminbi (“RMB”) as determined based on the criteria of Accounting Standards Codification (“ASC”) Topic 830 “Foreign Currency”. The reporting currency of the Group is also the RMB. Transactions denominated in foreign currencies are re-measured into the functional currency at the exchange rates prevailing on the transaction dates. Foreign currency denominated financial assets and liabilities are re-measured at the balance sheet date exchange rate. Exchange gains and losses are included in foreign exchange gains and losses in the consolidated statements of income.
Amounts in United States dollars (“US$”) are presented for the convenience of the reader and are translated at the noon buying rate of US$1.00 to RMB6.8259 on December 31, 2009 in the City of New York for cable transfers of RMB as certified for customs purposes by the Federal Reserve Bank of New York. No representation is made that the RMB amounts could have been, or could be, converted into US$ at such rate.
(d) Cash and Cash Equivalents
Cash and cash equivalents consist of cash on hand and demand deposits placed with banks or other financial institutions which are unrestricted as to withdrawal and use and have original maturities less than three months. Restricted cash represents amounts held by a bank as security for the Group’s bills payable or as pledge for the Group’s short-term loan and is not available for the Group’s use.
(e) Accounts Receivable
Accounts receivable are carried at net realizable value. An allowance for doubtful accounts is recorded in the period when loss is probable based on an assessment of specific evidence indicating troubled collection, historical experience, accounts aging and other factors. An accounts receivable is written off after all collection effort has ceased.
F-15
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(CONT’D)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICES (CONT’D)
(f) Inventories
Inventories are stated at the lower of cost or market value. Cost is determined by the weighted average method. Costs of raw materials and consumables and packaging materials are based on purchase costs while costs of work-in-progress and finished goods comprise direct materials, direct labor and an allocation of manufacturing overhead costs.
(g) Financial Instrument
At times, the Company uses forward exchange contracts to manage its exposure to movements in foreign exchange rates. Pursuant to FASB ASC 815 “Derivatives and Hedging”, such forward contracts qualify as derivatives. Hedge accounting does not apply as the Company does not formally documents its hedge relationships.
Derivatives are recorded in the consolidated balance sheets at fair value in either other assets or other liabilities. Gains or losses are recorded as other income or other expense. There were no significant derivative contracts during any of the years presented.
(h) Property, Plant and Equipment
Property, plant and equipment are stated at cost and are depreciated using the straight-line method over the estimated useful lives of the assets, as follows:
| | |
Buildings | | 8 - 20 years |
Plant and machinery | | 5 - 10 years |
Motor vehicles | | 4 - 5 years |
Furniture, fixtures and office equipment | | 3 - 10 years |
Repair and maintenance costs are charged to expense when incurred, whereas the cost of renewals and betterment that extends the useful life of property, plant and equipment are capitalized as additions to the related assets. Retirement, sale and disposals of assets are recorded by removing the cost and related accumulated depreciation with any resulting gain or loss reflected in the consolidated statements of income.
Property, plant and equipment that are purchased or constructed which require a period of time before the assets are ready for their intended use are accounted for as construction-in-progress. Construction-in-progress is recorded at acquisition cost, including installation costs and interest costs. The capitalization of interest costs commences when expenditures for the asset have been made, activities that are necessary to get the asset ready for its intended use are in progress and interest cost is being incurred. The capitalization period ends when the asset is substantially complete and ready for its intended use. The Group capitalized RMB523,000, RMB480,997 and nil of interest expense for the years ended December 31, 2007, 2008 and 2009, respectively.
Certain buildings form part of the purchase consideration payable pursuant to an equity purchase agreement and are expected to be transferred to a third party eight years after the acquisition date at their then fair value. Such buildings are therefore depreciated over eight years with a residual value of RMB4,515,000 (US$661,451) which is the estimated fair value of such buildings eight years from the acquisition date (Note 4).
F-16
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(CONT’D)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICES (CONT’D)
(i) Land Use Rights and Intangible Assets
A land use right in the PRC represents an exclusive right to occupy, use, develop, lease, transfer and mortgage a piece of land during the contractual term of the land use right. Land use rights are generally paid in one lump sum at the date the right is granted and covers the entire duration period of the land use right. The lump sum advance payments are capitalized as land use right assets and then amortized on a straight-line basis over the respective terms of the rights, which range from 8 to 50 years.
One of the Group’s land use right form part of the purchase consideration payable pursuant to an equity purchase agreement and is expected to be transferred to a third party eight years after the acquisition date at its then fair value. Such land use right is therefore amortized over eight years with a residual value of RMB5,821,000 (US$852,781) which is the estimated fair value of such land use right eight years from the acquisition date (Note 4).
Intangible assets represent purchased technical know-how and are stated at the acquisition cost less accumulated amortization. Amortization expense is recognized using the straight-line method over the estimated useful life and is included as a cost of revenue. Technical know-how include (i) intangible assets acquired from the acquisition of non-controlling interest in Penglai Nuokang (Note 4) and for one of the Group’s major product, (ii) Technical know-how acquired from the acquisition of VHK (Note 25), and (iii) developed new drug technologies purchased from third parties. Developed new drug technologies are recorded as an intangible asset only if regulatory approval from the State Food and Drug Administration of China (“SFDA”) has been obtained and for which the re-registration of the developed new drug technology with the SFDA by the Group in its name is determined to be reasonably assured or perfunctory. The amortization of such intangible assets will not commence until the technology has been re-registered with the SFDA and hence ready for its intended use. Refundable deposits paid for new drug technologies for which recoverability is reasonably assured are classified as prepayments and other receivables.
The estimated useful economic lives of the identifiable long-lived assets acquired are as follows:
| | |
Land use rights | | 8 years |
Technical know-how | | 9 - 13 years |
F-17
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(CONT’D)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICES (CONT’D)
(j) Investment
The Group applies the equity method of accounting for investments in entities in which the Group has the ability to exercise significant influence but does not own a majority equity interest or otherwise controls, in accordance with Financial FASB ASC 325 “Investments-other”.
The cost method is used for investments in entities in which the Group does not have the ability to exercise significant influence, or in securities where fair value is not readily determinable.
Short-term investments include time deposits with financial institutions which have maturities of more than three months but less than one year from the date of deposit. The Group’s short-term investments are classified as held-to-maturity based on positive intent and ability to hold the investments to maturity. Income related to these investments is reported as a component of other income.
The Group monitors its investments for other-than-temporary impairment by considering factors including, but not limited to, current economic and market conditions, the operating performance of the investee companies, including current earnings trends and other company-specific information.
(k) Impairment of Long-lived Assets
The Group evaluates its long-lived assets or asset group for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset or a group of long-lived asset may not be recoverable. When these events occur, the Group evaluates the impairment by comparing the carrying amount of the assets to future undiscounted net cash flows expected to result from the use of the assets and their eventual disposition. If the sum of the expected undiscounted cash flow is less than the carrying amount of the assets, the Group would recognize an impairment loss based on the excess of the carrying amount of the asset group over its fair value. Fair value is generally determined by discounting the cash flows expected to be generated by the assets, when the market prices are not readily available for the long-lived assets. No impairment of long-lived assets was recognized for any of the periods presented.
(l) Fair Value of Financial Instruments
The carrying amounts of financial assets and liabilities, such as cash and cash equivalents, accounts receivable, bills receivable, other receivables, bank loans, accounts payable, bills payable, income tax payable, balances with related parties and other payables, approximate to their fair values because of the short maturity of these instruments. The share-based compensation liability is initially recognized at fair value on the date of grant and is subsequently remeasured at the end of each reporting period. The preference shares are initially recognized at fair value upon issuance and subsequently accreted to the redemption value using the effective interest rate method. When a beneficial conversion feature exists as of the commitment date, its intrinsic value is allocated from the carrying value of the preference shares as a contribution to additional paid-in capital. The discount resulting from the beneficial conversion feature is amortized from the date of issuance to the earliest conversion date. The preference shares are then accreted to their respective redemption values using the effective interest method. The Group utilized Jones Lang LaSalle Sallmanns Limited (“Sallmanns”), an independent third party valuation firm, to determine the fair value of the preference shares, although the Group’s management is ultimately responsible for the determination of all amounts related to share-based compensation recorded in the consolidated financial statements.
F-18
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(CONT’D)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICES (CONT’D)
(m) Revenue Recognition
The Company records revenue when the criteria of FASB ASC 605 “Revenue Recognition” are met. The Group recognizes revenue from the sale of pharmaceutical products when all of the following criteria are met: 1) persuasive evidence of an arrangement exists; 2) delivery has occurred; 3) the sales price is fixed or determinable; and 4) collectability is reasonably assured. More specifically, the Group’s sales arrangements are evidenced by individual sales agreements or purchase orders based on master sales agreements. The customer takes title and assumes the risks and rewards of ownership of the products upon delivery of the products, which generally occurs at destination point. The product sales price stated in the sales contract or purchase order is final and not subject to adjustment. The Group’s products are subject to price control by the PRC government. The maximum prices of the products are published by the PRC price administration authorities from time to time. Any changes in product pricing announced by the PRC price administration authorities affect future sales transactions and do not impact sales that have already occurred, including those for which revenues have not yet been collected. There are no general rights of return on delivered products and no price protection is granted to customers. The Group assesses a customer’s creditworthiness before accepting sales orders. Based on the above, the Group records revenue upon delivery of the product to its customers.
Revenue is recognized net of all value-added taxes imposed by governmental authorities and collected from customers concurrent with revenue-producing transactions.
The Group has not offered any significant discounts or rebates to its customers nor does it provide for refunds in its sales contracts with customers.
(n) Cost of Revenue
Cost of revenue includes direct and indirect production costs, as well as shipping and handling costs for products sold.
Upon the Reorganization, Penglai Nuokang is subject to business tax and other surcharges on the revenues earned for technical support and management consulting services provided to Nuokang Distribution, the Group’s VIE, pursuant to the VIE agreements (Note 1(f)). The current applicable rate of business tax is 5%. Such business tax and other surcharges are accrued and charged to cost of revenues as the related technical support and consulting services are rendered.
F-19
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(CONT’D)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICES (CONT’D)
(o) Research and Development Costs
Research and development costs are expensed as incurred. Prior to 2009, the costs of acquired technology know-how for drugs in a development stage as in-process research and development (“IPR&D”) that are purchased from others for a particular research and development project either singly, or as part of a group of assets, or as part of a business combination, and that have no alternative future uses (in other research and development projects or otherwise), are expensed as research and development costs. The Group has determined that for such acquired technology know-how to have an alternative future use, it should be (a) reasonably expected that the Group will use the technology in an alternative manner for an economic benefit and (b) the Group’s use of the technology is not contingent on further development subsequent to acquisition (that is, it can be used in an alternative manner at the acquisition date). None of the Group’s acquired technology know-how for drugs in a development stage during the periods presented was determined to have an alternative future use at the acquisition date since technological feasibility was not established and regulatory approval from the SFDA was not obtained. Further subsequent development, including additional clinical testing, was required to obtain the necessary regulatory approval before the products could be launched into the market for sale.
Staring 2009, FASB ASC 805 “Business combination” requires the recognition of tangible and intangible assets that result from or are to be used in research and development activities and assets, irrespective of whether the acquired assets have an alternative future use. Acquired IPR&D assets are required to be measured at their acquisition-date fair value. Uncertainly about the outcome of an individual project does not affect the recognition of an IPR&D asset, but is reflected in its fair value.
(p) Advertising Expenditures
Advertising expenditures are expensed as incurred. Advertising expenditures, included in sales, marketing and distribution expenses, amounted to RMB16,041,634, RMB8,694,673 and RMB5,913,578 (US$866,344) for the years ended December 31, 2007, 2008 and 2009 respectively.
F-20
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(CONT’D)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICES (CONT’D)
(q) Government Grants
Government grants are provided by the relevant PRC municipal government authorities to reward the Group for the business achievements, to subsidize the cost of certain research and development projects, construction of property, plant and equipment or to reimburse the interest expenses for the bank loans borrowed to finance the construction of property, plant and equipment. The amount of such government grants are determined solely at the discretion of the relevant government authorities and there is no assurance that the Group will continue to receive these government grants in the future. Government grants are initially deferred and subsequently recognized in the statement of income when the Group has complied with the conditions or performance obligations attached to the related government grants, if any, and the grants are no longer refundable. Grants that subsidize the cost of research and development expenses are recorded as a reduction of the related research and development expenses. Grants that subsidize the construction cost of property, plant and equipment are amortized over the life of the related assets, when operational, as a reduction of the related depreciation expense. To the extent the grants that reimburse the interest expenses for the bank loans borrowed to finance the construction of property, plant and equipment are capitalized, the grants are regarded as a reduction of capitalized interests, and are amortized over the life of the related assets, when operational, as a reduction of the related depreciation expense. The non-capitalized grants that reimburse the interest expenses for the bank loans borrowed are recorded as a reduction of interest expenses. Grants that are rewarded to the Group for business achievements are recorded as other income upon receipts when the Group has no requirement to comply with the conditions or performance obligations attached to the related government grants.
F-21
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(CONT’D)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
(r) Income Taxes
The Group accounts for income taxes using the liability method. Under this method, deferred tax assets and liabilities are determined based on the difference between the financial reporting and tax bases of assets and liabilities using enacted tax rates that will be in effect in the period in which the differences are expected to reverse. The Group records a valuation allowance against deferred tax assets if, based on the weight of available evidence, it is more-likely-than-not that some portion, or all, of the deferred tax assets will not be realized. The effect on deferred taxes of a change in tax rates is recognized in income in the period that includes the enactment date.
Effective January 1, 2007, the Group adopted FASB ASC 740 “Income Taxes”. FASB ASC 740 clarifies the accounting for uncertainty in income taxes by prescribing the recognition threshold a tax position is required to meet before being recognized in the financial statements. The cumulative effects of applying FASB ASC 740, if any, is recorded as an adjustment to retained earnings as of the beginning of the period of adoption. The Group’s adoption of FASB ASC 740 did not result in any adjustment to the opening balance of the Group’s retained earnings as of January 1, 2007.
The Group has elected to classify interest and penalties related to an uncertain position, if and when required, as part of “interest expenses” and “other expenses”, respectively, in the consolidated statements of income. No such amounts have been incurred or accrued through December 31, 2009 by the Group.
F-22
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(CONT’D)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICES (CONT’D)
(s) Value-added Tax (“VAT”)
In accordance with the relevant tax laws in the PRC, VAT is levied on the invoiced value of sales and is payable by the purchaser. The Group is required to remit the VAT it collects to the tax authority, but may deduct the VAT it has paid on eligible purchases. The difference between the amounts collected and paid is presented as VAT recoverable or payable balance on the balance sheet.
(t) Share-based Compensation
Share options granted to employees and non-employees are accounted for under FASB ASC 718 “Compensation-Stock Compensation” and FASB ASC 505-50 “Equity Based Payments to Non-Employees”.
In accordance with FASB ASC 718, the Company determines whether a share option should be classified and accounted for as a liability award or an equity award. All grants of share options to employees classified as equity awards are recognized in the financial statements based on their grant date fair values. All grants of share options to employees classified as liability awards are remeasured at the end of each reporting period with an adjustment for fair value recorded to the current period expense in order to properly reflect the cumulative expense based on the current fair value of the vested options over the vesting period. The Group has elected to recognize compensation expenses using the straight-line method for both equity and liability classified share options granted with service conditions that have a graded vesting schedule.
FASB ASC 718 requires forfeitures to be estimated at the time of grant and revised, if necessary, in the subsequent period if actual forfeitures differ from initial estimates. Forfeiture rate is estimated based on historical and future expectation of employee turnover rate and are adjusted to reflect future change in circumstances and facts, if any. Share-based compensation expense is recorded net of estimated forfeitures such that expense was recorded only for those share-based awards that are expected to vest.
The Group utilized Sallmanns to determine the fair values of the share options although the Group’s management is ultimately responsible for the determination of all amounts related to share based compensation recorded in the consolidated financial statements. The binomial option pricing model is applied in determining the fair value of the options granted to employees, while the Black-Scholes pricing model is utilized in determining the fair value of the options granted to non-employees.
The Group records share-based compensation expense for awards granted to non-employees in exchange for services at fair value in accordance with the provisions of FASB ASC 718. For the awards granted to non-employees, the Group has recorded compensation expenses equal to the fair value of the share options at the initial public offering date.
F-23
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(CONT’D)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICES (CONT’D)
(u) Leases
Leases are classified at the inception date as either a capital lease or an operating lease. For the lessee, a lease is a capital lease if any of the following conditions exist: a) ownership is transferred to the lessee by the end of the lease term, b) there is a bargain purchase option, c) the lease term is at least 75% of the property’s estimated remaining economic life or d) the present value of the minimum lease payments at the beginning of the lease term is 90% or more of the fair value of the leased property to the lessor at the inception date. A capital lease is accounted for as if there was an acquisition of an asset and an incurrence of an obligation at the inception of the lease. All other leases are accounted for as operating leases wherein rental payments are expensed on a straight-line basis over the periods of their respective leases. The Group has no capital lease for any of the periods stated herein in the financial statements.
(v) Net Income per Share
In accordance with FASB ASC 260 “Earnings Per Share” basic income per share is computed by dividing net income attributable to ordinary shareholders by the weighted average number of unrestricted ordinary shares outstanding during the year using the two-class method. Under the two-class method, net income is allocated between ordinary shares and other participating securities based on their participating rights. The Company’s Series A convertible redeemable preference shares (Note 18) are participating securities. Diluted income per share is calculated by dividing net income attributable to ordinary shareholders as adjusted for the effect of dilutive ordinary equivalent shares, if any, by the weighted average number of ordinary and dilutive ordinary equivalent shares outstanding during the period. Ordinary equivalent shares consist of the ordinary shares issuable upon the conversion of the Company’s convertible redeemable preference shares, using the if-converted method, and ordinary shares issuable upon the conversion of the share options (Note 22), using the treasury stock method. Ordinary share equivalents are excluded from the computation of diluted per earning share if their effects would be anti-dilutive.
F-24
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(CONT’D)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICES (CONT’D)
(w) Comprehensive Income
Comprehensive income is defined to include all changes in equity except those resulting from investments by owners and distributions to owners. The Group did not have any other component of comprehensive income except for net income for the years ended December 31, 2007, 2008 and 2009.
(x) Segment Reporting
The Group follows FASB ASC 280 “Segment Reporting” for its segment reporting.
The Group operates and manages its business as a single segment. As the Group’s long-lived assets are all located in the PRC and revenues are all derived from the PRC, no geographical segments are presented.
(y) Employee Benefit
The full-time employees of the Company’s PRC subsidiaries are entitled to staff welfare benefits including medical care, housing fund, unemployment insurance and pension benefits. These entities are required to accrue for these benefits based on certain percentages of the employees’ salaries, subject to certain ceilings, in accordance with the relevant PRC regulations, and make contributions to the state-sponsored plans out of the amounts accrued. The total amounts for such employee benefits, which were expensed as incurred, were RMB2,242,911, RMB3,832,319 and RMB4,450,133 (US$651,948) for the years ended December 31, 2007, 2008 and 2009, respectively.
(z) Recent Accounting Pronouncements
On April 1, 2009, the FASB approved FSP FAS 141(R)-1, “Accounting for Assets Acquired and Liabilities Assumed in a Business Combination That Arise from Contingencies”, which amends FAS 141(R), as incorporated into FASB ASC 805, and eliminates the distinction between contractual and non-contractual contingencies. Under the updated standard, an acquirer is required to recognize at fair value an asset acquired or liability assumed in a business combination that arises from a contingency if the acquisition-date fair value of that asset or liability can be determined during the measurement period. If the acquisition-date fair value cannot be determined, the acquirer applies the recognition criteria in FASB ASC 450 “Contingencies” to determine whether the contingency should be recognized as of the acquisition date or after it. This guidance is effective for assets or liabilities arising from contingencies in business combinations for which the acquisition date is on or after the beginning of the first annual reporting period beginning on or after December 15, 2008. The adoption of such standard has not had a material impact on the consolidated financial statements.
F-25
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(CONT’D)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICES (CONT’D)
(z) Recent Accounting Pronouncements (Cont’d)
In April 2009, the FASB issued FSP FAS 157-4, “Determining Fair Value When the Volume and Level of Activity for the Asset or Liability Have Significantly Decreased and Identifying Transactions That Are Not Orderly” (“FSP FAS 157-4”) as incorporated into FASB ASC 820, “Fair Value Measurements and Disclosures”. The guidance relates to determining fair values when there is no active market or where the price inputs being used represent distressed sales. It reaffirms what FASB ASC 820 states is the objective of fair value measurement—to reflect how much an asset would be sold for in an orderly transaction (as opposed to a distressed or forced transaction) at the date of the financial statements under current market conditions. Specifically, it reaffirms the need to use judgment to ascertain if a formerly active market has become inactive and in determining fair values when markets have become inactive. This guidance is effective for interim and annual periods ended after June 15, 2009, but entities may early adopt this guidance for the interim and annual periods ended after March 15, 2009. The adoption of such standard has not had a material impact on the consolidated financial statements.
FSP FAS 115-2 and FAS 124-2, as incorporated into FASB ASC 320, “Investments- Debt and Equity Securities” amend the other-than-temporary impairment guidance in U.S. GAAP for debt securities to make the guidance more operational and to improve the presentation and disclosure of other-than-temporary impairments on debt and equity securities in the financial statements. It does not amend existing recognition and measurement guidance related to other-than-temporary impairments of equity securities. Disclosures for periods presented for comparative purposes at initial adoption are not required. This guidance is effective for interim and annual reporting periods ended after June 15, 2009, and shall be applied prospectively. The adoption of such standard has not had a material impact on the consolidated financial statements.
On April 9, 2009, the FASB approved FSP FAS 107-1 and APB 28-1, “Interim Disclosures about Fair Value of Financial Instruments” to require disclosures about fair value of financial instruments in interim period financial statements of publicly traded companies and in summarized financial information required by APB Opinion No. 28, “Interim Financial Reporting”. This guidance was incorporated into FASB ASC 825, “Financial Instruments” which was effective for interim and annual reporting periods ended after June 15, 2009 and was adopted starting third quarter 2009. Under the standard comparative disclosures are only required for periods ended after initial adoption.
In May 2009, the FASB issued SFAS No. 165, “Subsequent Events,” (FASB ASC 855-10) which establishes general standards of accounting for and disclosure of events that occur after the balance sheet date but before financial statements. The statement is effective for interim and annual periods ended after June 15, 2009. The standard was subsequently amended by FASB Accounting Standards Update (“FASB ASU”) 2010-09 which exempts an entity that is an SEC filer from the requirement to disclose the date through which subsequent events have been evaluated.
F-26
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(CONT’D)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICES (CONT’D)
(z) Recent Accounting Pronouncements (Cont’d)
In June 2009, the FASB issued FASB ASU 2009-01, which amends FASB ASC 105, “Generally Accepted Accounting Principles” (“Statement of Financial Accounting Standards (“SFAS”) No. 168”, “The FASB Accounting Standards Codification TM and the Hierarchy of Generally Accepted Accounting Principles – a replacement of FASB Statement No. 162”), which establishes the FASB Accounting Standards Codification TM (“Codification”) as the source of authoritative GAAP in the United States for non-governmental entities. The Codification does not change GAAP. Instead, it takes the thousands of individual pronouncements that currently comprise GAAP and reorganizes them into approximately 90 accounting Topics, and displays all Topics using a consistent structure. Contents in each Topic are further organized first by Subtopic, then Section and finally Paragraph. The Paragraph level is the only level that contains substantive content. Citing particular content in the Codification involves specifying the unique numeric path to the content through the Topic, Subtopic, Section and Paragraph structure. FASB suggests that all citations begin with “FASB ASC”. Changes to the ASC subsequent to June 30, 2009 are referred to as Accounting Standards Updates. The Group has implemented the Codification in the consolidated financial statements by providing references to the ASC topics.
In August 2009, the FASB issued FASB ASU 2009-5, “Measuring Liabilities at Fair Value”. FASB ASU 2009-05 amends FASB ASC 820, “Fair Value Measurements”. Specifically, FASB ASU 2009-05 provides clarification that in circumstances in which a quoted price in an active market for the identical liability is not available, a reporting entity is required to measure fair value using one or more of the following methods: 1) a valuation technique that uses a) the quoted price of the identical liability when traded as an asset or b) quoted prices for similar liabilities or similar liabilities when traded as assets and/or 2) a valuation technique that is consistent with the principles of FASB ASC 820 of the Accounting Standards Codification (e.g. an income approach or market approach). FASB ASU 2009-05 also clarifies that when estimating the fair value of a liability, a reporting entity is not required to adjust to include inputs relating to the existence of transfer restrictions on that liability. The adoption of such standard has not had a material impact on the consolidated financial statements.
In September 2009, the Emerging Issues Task Force reached final consensus on FASB ASU 2009-13, “Revenue Arrangements with Multiple Deliverables”. ASU 2009-13 addresses how to determine whether an arrangement involving multiple deliverables contains more than one unit of accounting, and how the arrangement consideration should be allocated among the separate units of accounting. This ASU will be effective prospectively for revenue arrangements entered into or materially modified in fiscal years beginning on or after June 15, 2010. Early adoption is permitted. The adoption of such standard has not had a material impact on the consolidated financial statements.
In December 2009, the FASB issued FASB ASU 2009-17, Consolidation (“FASB ASC 810”): “Improvements to Financial Reporting by Enterprises Involved with Variable Interest Entities”. This ASU amends the FASB Accounting Standards Codification for statement 167, In June 2009, the FASB issued Statement of Financial Accounting Standards No.167, Amendments to FASB Interpretation No.46(R) (“SFAS No.167”). SFAS No.167 eliminates FASB Interpretation No.46(R)’s exceptions to consolidating qualifying special-purpose entities, contains new criteria for determining the primary beneficiary, and increases the frequency of required reassessments to determine whether a company is the primary beneficiary of a variable interest entity. SFAS No.167 is effective for fiscal years beginning after November 15, 2009, which for the Company is January 1, 2010, with earlier adoption prohibited. The Company does not expect the adoption of FSAB ASU 2009-17 to have a material effect on its consolidated financial statements.
F-27
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(CONT’D)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICES (CONT’D)
(z) Recent Accounting Pronouncements (Cont’d)
In January 2010, the FASB issued ASU 2010-06, “Disclosures about Fair Value Measurements”. ASU 2010-06, which amends ASC 820, provides for disclosure of the amount of significant transfers in or out of Level 1 and Level 2, the reasons for those transfers, as well as information in the Level 3 rollforward about purchases, sales, issuances and settlements on a gross basis. ASU 2010-06 is effective for interim and annual periods beginning after December 15, 2009, except for the requirement to provide Level 3 activity on a gross basis, which is effective for all interim and annual periods beginning after December 15, 2010. The adoption of ASU 2010-06 is not expected to have a material effect on the Company’s consolidated financial statements.
In April 2010, the FASB issued ASU 2010-13, “Effect of Denominating the Exercise Price of a Share-Based Payment Award in the Currency of the Market in Which the Underlying Equity Security Trades”, which provides amendments to ASC topic 718, Compensation-Stock Compensation, to clarify that an employee share-based payment award with an exercise price denominated in the currency of a market in which a substantial portion of the entity’s equity securities trades should not be considered to contain a condition that is not a market, performance, or service condition. Therefore, an entity would not classify such an award as a liability if it otherwise qualifies as equity. ASU 2010-13 is effective for all interim and annual periods beginning on or after December 15, 2010. The adoption of ASU 2010-13 is not expected to have a material effect on the Group’s consolidated financial statements.
F-28
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(CONT’D)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
3. CONCENTRATION OF RISK
(a) Concentration of credit risk
Assets that potentially subject the Group to significant concentration of credit risk consist primarily of accounts receivable. The Group conducts credit evaluations of its customers but does not require collateral or other forms of security from its customers. The Group makes an allowance for doubtful accounts primarily based on the age of receivables and factors surrounding the credit risk of specific customers.
(b) Concentration of customers
The Group sells its products to pharmaceutical distributors in the PRC and sales to distributors account for substantially all of the Group’s revenues. The Group does not have long-term distribution agreements and competes for desired distributors with other pharmaceutical manufacturers. Consequently, maintaining relationships with existing distributors and replacing distributors may be difficult and time-consuming. Any disruption of the Group’s distribution network, including its failure to renew existing distribution agreements with desired distributors, could negatively affect its ability to effectively sell its products and could materially and adversely affect its business, financial condition and results of operations. For the years ended December 31, 2007, 2008 and 2009, one single customer contributed 14%, 13% and 27% of the Group’s total revenues, respectively.
The Group derives substantially all of its revenue from the sales of one product, namely Hemocoagulase Atrox for Injection. Sales of the product accounted for 92%, 90% and 94% of the Group’s revenues for the years ended December 31, 2007, 2008 and 2009, respectively. As the Group expects the sales of the product to continue to comprise a substantial portion of revenues in the future, any factors adversely affecting the sales of the product will have a material adverse effect on the Group’s business, financial condition and results of operations.
(c) Concentration of suppliers
A significant portion of the Group’s raw materials are sourced from its five largest suppliers who collectively accounted for 84%, 69% and 40% of the total raw material purchases for the years ended December 31, 2007, 2008 and 2009, respectively. For the years ended December 31, 2007, 2008 and 2009, two single suppliers contributed, on an individual basis, 56% and 10%, 33% and 17%, 15% and 10%, respectively.
Failure to develop or maintain the relationships with these suppliers may cause the Group to be unable to manufacture its products. Any disruption in the supply of raw materials to the Group may adversely affect the Group’s business, financial condition and results of operations.
F-29
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(CONT’D)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
3. CONCENTRATION OF RISK (CONT’D)
(d) Price Control
Certain medical products sold in the PRC are subject to retail price controls in the form of fixed prices or price ceilings. The fixed prices or the price ceilings of such medicines are published by the national and provincial price administration authorities from time to time. Although the Group sells its products through distributors, the controls over retail prices could have a corresponding effect on the wholesale prices. The prices of medicines that are not subject to price controls are determined freely at the discretion of the respective pharmaceutical companies, subject, in certain cases, to notification to the provincial pricing authorities. Certain of the Group’s products, including its main product, Hemocoagulase Atrox for Injection, are subject to price controls and accordingly, the price of such products could not be increased at the Group’s discretion above the relevant controlled price ceiling without prior governmental approval. In addition, the price of such products may also be adjusted downward by the relevant government authorities in the future. Such price controls, especially downward price adjustment, may negatively affect the Group’s revenue and profitability.
In addition, in order to access certain local or provincial-level markets, the Group is required to enter into competitive bidding processes for certain products, including its main product, every year or every other year with a pre-defined price range. The competitive bidding in effect sets price ceilings for the Group’s products, thereby limiting its profitability.
(e) Current vulnerability due to certain other concentrations
The Group’s operations may be adversely affected by significant political, economic and social uncertainties in the PRC. Although the PRC government has been pursuing economic reform policies for more than 20 years, no assurance can be given that the PRC government will continue to pursue such policies or that such policies may not be significantly altered, especially in the event of a change in leadership, social or political disruption or unforeseen circumstances affecting the PRC’s political, economic and social conditions. There is also no guarantee that the PRC government’s pursuit of economic reforms will be consistent or effective.
The Group transacts substantially all of its business in RMB, which is not freely convertible into foreign currencies. On January 1, 1994, the PRC government abolished the dual rate system and introduced a single rate of exchange as quoted daily by the People’s Bank of China (the “PBOC”). However, the unification of the exchange rates does not imply RMB may be readily convertible into US$ or other foreign currencies. All foreign exchange transactions continue to take place either through the PBOC or other banks authorized to buy and sell foreign currencies at the exchange rates quoted by the PBOC. Approval of foreign currency payments by the PBOC or other institutions requires submitting a payment application form together with suppliers’ invoices, shipping documents and signed contracts.
Additionally, the value of RMB is subject to changes in central government policies and to international economic and political developments affecting supply and demand in the PRC foreign exchange trading system market.
F-30
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(CONT’D)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
4. ACQUISITION OF NON-CONTROLLING INTEREST
Since its establishment in 2002, Penglai Nuokang was controlled by Mr. Xue through his 60% equity interest, with the remaining 40% non-controlling interest held by a third party owner, Ronghai Group Beihai Co., Ltd. (“Ronghai Group”). In contemplation of the Restructuring (Note 1(c)), Mr. Xue sought to acquire the remaining 40% non-controlling interest from Ronghai Group, after which Mr. Xue transferred 100% of the equity interest in Penglai Nuokang to the Group.
Pursuant to an equity purchase agreement (the “Agreement”) entered into on August 22, 2006 (which was also determined to be the acquisition date), Mr. Xue acquired the remaining 40% equity interest in Penglai Nuokang from Ronghai Group in exchange for RMB4,000,000 (the “Equity Purchase Consideration”). The Equity Purchase Consideration was assumed by Penglai Nuokang on behalf of Mr. Xue and there was no intention on the part of Mr. Xue to repay this amount to Penglai Nuokang.
On August 23, 2006, Mr. Xue transferred his 100% equity interests in Penglai Nuokang to the Group for RMB10,000,000 (Note 1(e)), which was accounted for as a legal reorganization of entities under common control in a manner similar to a pooling of interest using historical cost. The carrying values of the assets and liabilities of Penglai Nuokang transferred to the Group were comprised of (i) historical carrying values of the assets and liabilities underlying the original 60% equity interest held by Mr. Xue in Penglai Nuokang, and (ii) fair values of the assets and liabilities underlying the remaining 40% equity interest acquired from Ronghai Group on August 22, 2006 using the purchase method, pursuant to ASC Subtopic 805, “Business Combinations”. The Group consolidated the financial position and results of operations of Penglai Nuokang for all the periods where common control can be established (which is since the inception of Penglai Nuokang), in accordance with ASC Subtopic 805.
The Agreement called for (a) land use right and certain property, plant and equipment (collectively the “Manufacturing Assets”) held by Penglai Nuokang to be transferred to Shandong Penglai Pharmaceutical Plant (“SDPP”), a wholly-owned subsidiary of Ronghai Group, in exchange for RMB13,900,000 (the “Manufacturing Assets Payment”); and (b) a right for Penglai Nuokang to continue to use those Manufacturing Assets for an initial period of eight years in exchange for total consideration of RMB14,100,000 (the “Manufacturing Assets Use Right Payment”), for which payments of RMB1,600,000 per annum will be made thereafter until December 31, 2014.
According to the Agreement, the Equity Purchase Consideration and the Manufacturing Assets Payment shall be settled on a net basis, with the net balance of RMB9,900,000 (“Net Manufacturing Assets Payment”) payable at anytime within ten years from the date of the Agreement at the discretion of SDPP. The Manufacturing Assets are currently pledged as security for certain bank loans of Penglai Nuokang (Note 14), and therefore, the Agreement restricts Ronghai Group from selling or otherwise disposing of the Manufacturing Assets without the consent of Penglai Nuokang within the contractual period. Penglai Nuokang also has the option to extend the period over which it can use the Manufacturing Assets for a fee to be negotiated at the time of extension. If Penglai Nuokang does not exercise the option to extend, in addition to the Manufacturing Assets, certain intellectual properties owned by Penglai Nuokang shall be transferred to SDPP for no consideration. Both Penglai Nuokang and SDPP can early terminate the right to use the Manufacturing Assets during the eight year period, though the terminating party will be obligated to pay the other party at the time of termination a penalty of RMB1,000,000 for each year of the remaining eight years. At the end of the initial eight years or, if extended, at the end of the extended period, the title and ownership of the Manufacturing Assets and certain intellectual properties will automatically be transferred to the SDPP.
F-31
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(CONT’D)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
4. ACQUISITION OF NON-CONTROLING INTEREST (CONT’D)
Since Penglai Nuokang has continuing involvement in the Manufacturing Assets, the transaction does not qualify for sales-leaseback accounting pursuant to FASB ASC 840 “Lease”.
The purchase of the 40% non-controlling interest from Ronghai Group was accounted for as a step acquisition using the purchase method for a total purchase consideration of RMB8,209,000, which equals the sum of (i) the present value of the Manufacturing Assets Use Right Payment less the Net Manufacturing Assets Payment; and (ii) the present value of the fair value at the end of eight years of the Manufacturing Assets to be given up. The Group determined the fair value of certain intellectual properties to be transferred to SDPP for no consideration should Penglai Nuokang decide not to extend the Manufacturing Assets Use Right at the end of eight years to be insignificant as no revenues are expected to be generated by the products associated with these intellectual properties in eight years.
The total purchase consideration is recorded as a long-term payable on the balance sheet with accreted interest charged to the consolidated statement of income through the dates of settlement.
Since the Manufacturing Assets do not qualify for sales-leaseback accounting, the Group continues to carry the assets on its consolidated balance sheet and depreciate and amortize such assets to the end of the eight-year contractual period. It is the intention of the Group not to extend the eight-year contractual period because (i) the Group has built new production facilities in Shenyang City and plans to centralize all of its manufacturing activities; (ii) there is no bargain renewal option as renewal terms are subject to further commercial negotiation; and (iii) the penalty that would be imposed on Penglai Nuokang should the lease not be renewed did not constitute a significant penalty that would reasonably ensure a renewal. As such the Manufacturing Assets are expected to be transferred to the SDPP at the end of the eight years, and the expected residual value of the Manufacturing Assets at the date of the acquisition is determined to be equal to their fair values at the end of the eight years.
F-32
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(CONT’D)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
5. RESTRICTED CASH
As of December 31, 2008, restricted cash of RMB20,000,000 represents amounts held by a bank as security for the Group’s bills payable (Note 15). As of December 31, 2009, restricted cash of RMB34,200,000 (US$5,010,328) represents time deposits held by a bank as security for the Group’s short-term bank loans (Note 14). Restricted cash is not available for the Group’s use.
6. ACCOUNTS RECEIVABLE, NET
| | | | | | | | | |
| | | 2008 | | | 2009 | |
| | | (RMB’000) | | | (RMB’000) | | | (US$’000) | |
| | | |
Accounts receivable | | 71,760 | | | 104,113 | | | 15,253 | |
Less: Allowance for doubtful accounts | | (448 | ) | | (394 | ) | | (58 | ) |
| | | | | | | | | |
| | | |
| | 71,312 | | | 103,719 | | | 15,195 | |
| | | | | | | | | |
As of December 31, 2008 and 2009, all accounts receivable were due from third party customers.
An analysis of the allowance for doubtful accounts is as follows:
| | | | | | | | | |
| | | 2008 | | | 2009 | |
| | | (RMB’000) | | | (RMB’000) | | | (US$’000) | |
| | | |
Balance, beginning of year | | (580 | ) | | (448 | ) | | (66 | ) |
Additions for the current year | | — | | | (135 | ) | | (20 | ) |
Deductions for the current year | | | | | | | | | |
-Reversals | | 132 | | | 189 | | | 28 | |
| | | | | | | | | |
| | | |
Balance, end of year | | (448 | ) | | (394 | ) | | (58 | ) |
| | | | | | | | | |
The additions (deductions) of allowance for doubtful accounts were charged to (reversed against) general and administration expenses for the years ended December 31, 2008 and 2009.
F-33
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(CONT’D)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
7. BILLS RECEIVABLE
To reduce the Group’s credit risk, the Group has required certain customers to pay for the purchase of the Group’s products using bills receivable. Bills receivable represent short-term notes receivable issued by financial institutions that entitle the Group to receive the full face amount from the financial institutions at maturity, which generally ranges from three to six months from the date of issuance. Historically, the Group has experienced no losses on bills receivable.
For the years ended December 31, 2007, 2008 and 2009, the Group entered into factoring arrangements with unrelated commercial banks, without recourse, on certain of its bills receivable in return for cash at a discount of their carrying value. The Group accounted for these factoring arrangements in accordance with FASB ASC Topic 860, “Transfers and Servicing”, pursuant to which transfers of bills receivable of the Group are considered sales when the Group surrenders control of the factored bills receivable. Surrender of control exists when all three criteria are met: a) there is legal isolation of the financial asset from the seller and its creditors, even in the event of bankruptcy or other receivership, b) the buyer has the unrestricted right to pledge or exchange the financial asset, and c) the seller does not retain effective control over the financial asset through agreement or otherwise enable the seller to unilaterally cause the buyer to return financial assets. These factoring transactions are recorded as a reduction to bills receivable with the difference between cash received and the carrying value of the factored bills receivable recorded as interest expense in the consolidated statement of income. For the years ended December 31, 2007, 2008 and 2009, interest expense related to factoring transactions amounted to RMB17,672, RMB220,288 and RMB633,341 (US$92,785), respectively. As of December 31, 2008 and 2009, bills receivables discounted but not yet at maturity amounted to RMB23,360,449 and RMB4,358,940 (US$638,588), respectively.
8. INVENTORIES
Inventories consist of the following:
| | | | | | |
| | | 2008 | | 2009 |
| | | (RMB’000) | | (RMB’000) | | (US$’000) |
| | | |
Raw materials | | 474 | | 911 | | 133 |
Work-in-progress | | 6,074 | | 9,426 | | 1,381 |
Finished goods | | 2,204 | | 2,456 | | 360 |
Consumables and packaging materials | | 1,858 | | 1,972 | | 289 |
| | | | | | |
| | | |
| | 10,610 | | 14,765 | | 2,163 |
| | | | | | |
The amounts of write-down of inventories recognized as an expense in cost of revenue for the years ended December 31, 2007, 2008 and 2009 were RMB1,239,097, nil, and RMB51,491 (US$7,543), respectively.
F-34
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(CONT’D)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
9. PREPAYMENTS AND OTHER RECEIVABLES
Prepayments and other receivables consist of the following:
| | | | | | |
| | | 2008 | | 2009 |
| | | (RMB’000) | | (RMB’000) | | (US$’000) |
| | | |
Prepayment for raw materials (i) | | 4,088 | | 4,300 | | 630 |
Staff advances | | 1,641 | | 3,490 | | 511 |
Prepayment for purchase of an intellectual property | | 440 | | — | | — |
Prepayment for an exclusive distribution right (ii) | | 10,000 | | 16,000 | | 2,344 |
Deferred initial public offering costs (iii) | | 5,277 | | — | | — |
Receivable for disposal of property, plant and equipment | | — | | 677 | | 99 |
Deposits | | 560 | | 560 | | 82 |
Other receivables | | 410 | | 1,949 | | 286 |
| | | | | | |
| | | |
| | 22,416 | | 26,976 | | 3,952 |
| | | | | | |
| (i) | Prepayments for raw materials represent non-interest-bearing cash deposits paid to suppliers for future purchases of raw materials. The risk of loss arising from non-performance by or bankruptcy of the suppliers is assessed prior to making the deposits and monitored for recoverability on a regular basis by the Group. A charge to cost of revenue will be recognized in the period in which the advances are determined to be unrecoverable. To date, the Group has not experienced any loss on prepayment for raw materials. |
| (ii) | The Group entered into an exclusive distribution agreement with a third party vendor to market and distribute new drug product. In accordance with the agreement, a refundable deposit of RMB10,000,000 and RMB6,000,000 was prepaid by the Group for the exclusive distribution right, representing the total contractual amount of RMB16,000,000 (US$2,344,013) in November 2008 and March 2009, respectively. This exclusive distribution right is valid for a period of 10 years commencing from the effective date being the earlier of six months from the contract signing date or the date of first product shipment received. Prior to the effective date, the contractual arrangement is cancellable at the right of the Group and the prepayment fully refundable. On June 28, 2009, a supplementary agreement was agreed between the Group and the third party vendor to extend the effective date as the earlier of December 28, 2009 or the date of first product shipment received. On December 28, 2009, an additional supplementary agreement was agreed between the Group and the third party vendor to extend the effective date as the earlier of April 30, 2010 or the date of first product shipment received. As of December 31, 2009, the contract had not yet become effective. Subsequently in March, 2010, the Group was notified by the vendor to further extend the effective date due to prolonged review and approval process by SFDA. . On April 30, 2010, a third supplementary agreement was agreed between the Group and the third party vendor to extend the effective date as the earlier of December 31, 2010 or the date of first product shipment received. |
| (iii) | The amount represents the deferred costs incurred by the Group directly attributable to the Company’s IPO, which are incremental to the Group and have been charged against the gross proceeds received from the IPO. |
F-35
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(CONT’D)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
10. PROPERTY, PLANT AND EQUIPMENT, NET
Property, plant and equipment consist of the following:
| | | | | | | | | |
| | | 2008 | | | 2009 | |
| | | (RMB’000) | | | (RMB’000) | | | (US$’000) | |
| | | |
Buildings | | 128,025 | | | 134,574 | | | 19,716 | |
Plant and machinery | | 26,027 | | | 35,552 | | | 5,208 | |
Motor vehicles | | 4,161 | | | 5,159 | | | 756 | |
Furniture, fixtures and office equipment | | 4,394 | | | 4,869 | | | 713 | |
| | | | | | | | | |
| | | |
| | 162,607 | | | 180,154 | | | 26,393 | |
Less: Accumulated depreciation | | (17,441 | ) | | (26,003 | ) | | (3,809 | ) |
| | | | | | | | | |
| | | |
| | 145,166 | | | 154,151 | | | 22,584 | |
Construction in progress | | 4,513 | | | 9,764 | | | 1,430 | |
| | | | | | | | | |
| | | |
| | 149,679 | | | 163,915 | | | 24,014 | |
| | | | | | | | | |
Depreciation expenses were RMB2,983,000, RMB6,956,192 and RMB10,661,737 (US$1,561,953) for the years ended December 31, 2007, 2008 and 2009, respectively, and were included in the following captions:
| | | | | | | | |
| | | 2007 | | 2008 | | 2009 |
| | | (RMB’000) | | (RMB’000) | | (RMB’000) | | (US$’000) |
| | | | |
Cost of revenue | | 1,851 | | 3,960 | | 5,634 | | 826 |
Research and development costs | | 255 | | 625 | | 952 | | 139 |
Selling, marketing and distribution expenses | | 202 | | 217 | | 211 | | 31 |
General and administrative expenses | | 675 | | 2,154 | | 3,865 | | 566 |
| | | | | | | | |
| | | | |
| | 2,983 | | 6,956 | | 10,662 | | 1,562 |
| | | | | | | | |
As of December 31, 2008 and 2009, certain machinery with carrying value of RMB6,647,400 and RMB7,813,035 (US$1,144,616), certain buildings with carrying value of RMB113,024,433 and RMB112,445,800 (US$16,473,403), respectively, were pledged as securities for the Group’s short-term bank loans (Note 14).
As of December 31, 2008 and 2009, certain buildings with carrying value of RMB5,859,728 and RMB5,622,462 (US$823,695), respectively, formed part of the purchase consideration payable pursuant to an equity purchase agreement and were expected to be transferred to SDPP, a wholly-owned subsidiary of the former non-controlling owner of Penglai Nuokang, in year 2014 (Note 4).
F-36
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(CONT’D)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
11. LAND USE RIGHTS, NET
| | | | | | | | | |
| | | 2008 | | | 2009 | |
| | | (RMB’000) | | | (RMB’000) | | | (US$’000) | |
| | | |
Cost | | | | | | | | | |
Beginning of year | | 28,237 | | | 28,237 | | | 4,137 | |
Additions | | — | | | — | | | — | |
| | | | | | | | | |
| | | |
End of year | | 28,237 | | | 28,237 | | | 4,137 | |
| | | | | | | | | |
| | | |
Accumulated amortization | | | | | | | | | |
Beginning of year | | (1,111 | ) | | (1,568 | ) | | (230 | ) |
Charge for the year | | (457 | ) | | (457 | ) | | (67 | ) |
| | | | | | | | | |
| | | |
End of year | | (1,568 | ) | | (2,025 | ) | | (297 | ) |
| | | | | | | | | |
| | | |
Net book value, end of year | | 26,669 | | | 26,212 | | | 3,840 | |
| | | | | | | | | |
As of December 31, 2008 and 2009, land use right with carrying value of RMB21,613,808 and RMB21,166,816 (US$3,100,956), respectively, was pledged as securities for the Group’s short-term bank loans (Note 14).
As of December 31, 2008 and 2009, certain land use right with carrying value of RMB5,892,814 and RMB5,880,189 (US$861,453), respectively, formed part of the purchase consideration payable pursuant to an equity purchase agreement and was expected to be transferred to SDPP, a wholly-owned subsidiary of the former non-controlling owner of Penglai Nuokang, in year 2014 (Note 4).
For each of the next five years starting from January 1, 2010, annual amortization expenses of the land use rights is expected to be RMB456,000 (US$66,804).
12. INTANGIBLE ASSETS, NET
Intangible assets consist of the following:
| | | | | | | | | |
| | | 2008 | | | 2009 | |
| | | (RMB’000) | | | (RMB’000) | | | (US$’000) | |
| | | |
Technical know-how | | 8,173 | | | 10,745 | | | 1,574 | |
Less: Accumulated amortization | | (1,836 | ) | | (2,376 | ) | | (348 | ) |
| | | | | | | | | |
| | | |
| | 6,337 | | | 8,369 | | | 1,226 | |
| | | | | | | | | |
For the year ended December 31, 2009, the Group acquired technical know-how from the acquisition of VHK (Note 25). Amortization expenses were recognized since date of acquisition using the straight-line method over the estimated useful life of 9 to 13 years.
As of December 31, 2008 and 2009, technical know-how with a carrying amount of RMB2,180,000 (US$319,372) is not subject to amortization as such technical know-how has not been re-registered with SFDA (Note 2(i)).
Annual amortization expenses of intangible assets are estimated to be approximately RMB789,703 (US$115,692) for the year ending December 31, 2010 and RMB1,007,703 (US$147,629) for each of the four years thereafter.
F-37
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(CONT’D)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
13. OTHER INVESTMENTS
Other investments at each year end comprised of:
| | | | | | | | | |
| | | | | | 2008 | | 2009 |
| | | | | | (RMB’000) | | (RMB’000) | | (US$’000) |
| | | | |
Investment fund, at cost | | (i | ) | | — | | 2,000 | | 293 |
| | | | |
Investment in unlisted equity, at cost | | (ii | ) | | — | | 3,414 | | 500 |
| | | | | | | | | |
| | | | |
| | | | | — | | 5,414 | | 793 |
| | | | | | | | | |
| (i) | This represents an investment with a amount of RMB2,000,000 to an investment fund which was set up on July 20, 2009. The investment is not freely tradable during the first six month commencing July 20, 2009 and its fair value is not readily determinable. The Group has redeemed the fund based on the published price with the cash consideration of RMB2,120,000 on January 25, 2010, with net gain of RMB120,000. |
| (ii) | The Group subscribed 125,000 shares of VPL, representing 10% of authorized capital of Venomics, on September 23, 2009 for cash consideration of US$500,000. The Group does not have the ability to exercise significant influence over VPL and the investment was accounted for under cost method. |
To date, the Group has not recognized any impairment losses on its investments.
14. SHORT-TERM BANK LOANS
| | | | | | |
| | | 2008 | | 2009 |
| | | (RMB’000) | | (RMB’000) | | (US$’000) |
| | | |
Bank loans | | 72,430 | | 125,618 | | 18,403 |
| | | | | | |
Bank loans were secured/guaranteed by the following:
| | | | | | |
| 2008 | | 2009 | | Secured/guaranteed by |
| (RMB’000) | | (RMB’000) | | (US$’000) | | |
| | | |
| 52,000 | | 78,000 | | 11,427 | | Secured by certain of the Group’s property, plant and equipment (Note 10), a land use right (Note 11) and guaranteed by Mr. Xue (Note 23) |
| | | |
| 12,980 | | 6,480 | | 949 | | Guaranteed by other third parties whose owners are business associates of Mr. Xue (i) |
| | | |
| 7,000 | | 7,000 | | 1,026 | | Secured by certain of the Group’s property, plant and equipment (Note 10) and a land use right (Note 11) |
| | | |
| — | | 34,138 | | 5,001 | | Secured by time deposits (Note 5) |
| | | |
| 450 | | — | | — | | Secured by bills receivable |
| | | | | | | |
| | | |
| 72,430 | | 125,618 | | 18,403 | | |
| | | | | | | |
F-38
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(CONT’D)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
14. SHORT-TERM BANK LOANS (CONT’D)
| (i) | The Group paid no service fee for the provision of guarantees provided by the third parties. |
All short-term bank loans were denominated in RMB for the year ended December 31, 2008. Short-term bank loans of RMB91,480,000 (US$13,401,896) and RMB34,137,500 (US$5,001,172) are denominated in RMB and USD, respectively, for the year ended December 31, 2009. All short-term loans were from third party commercial banks, which bore interest at fixed interest rates between 2.747% and 7.699%. The weighted average interest rates of the Group’s short-term bank loans outstanding as of December 31, 2008 and 2009 were 9.050% and 5.437% per annum, respectively. These short-term bank loans had terms of three months to one year and expired at various times throughout the year. These short-term bank loans contain no specific renewal terms and the extension is dependent on the negotiation between the Group and the banks.
The short-term bank loan agreements do not require the Group to comply with financial covenants.
15. BILLS PAYABLE
Bills payable are used for settlement of the subsidiaries’ accounts payable to suppliers. Bills payable represent short-term notes payable issued by financial institutions that entitle the supplier to receive the full face amount from the financial institutions at maturity, which generally ranges from three to six months from the date of issuance. The bills payable were secured by the Group’s restricted cash (Note 5).
16. ACCRUED EXPENSES AND OTHER PAYABLES
| | | | | | |
| | | 2008 | | 2009 |
| | | (RMB’000) | | (RMB’000) | | (US$’000) |
| | | |
Payable for purchase of property, plant and equipment | | 1,026 | | 951 | | 139 |
Accrued professional service fees | | 1,670 | | 8,311 | | 1,218 |
Value-added tax payable | | 3,092 | | 4,311 | | 632 |
Accrued advertising and consulting expenses | | 742 | | 470 | | 69 |
Accrued salaries, bonus and welfare expenses | | 2,114 | | 8,268 | | 1,211 |
Advance from customers (i) | | 746 | | 1,750 | | 256 |
Other tax payables | | 954 | | 1,641 | | 240 |
Others | | 1,614 | | 4,292 | | 629 |
| | | | | | |
| | | |
| | 11,958 | | 29,994 | | 4,394 |
| | | | | | |
| (i) | Advance from customers represents the prepayment from customers for finished goods and is non-interest-bearing. |
F-39
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(CONT’D)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
17. DEFERRED GOVERNMENT GRANTS
During the years ended December 31, 2007, 2008, and 2009, the Group received RMB5,400,000, RMB8,134,000, and RMB4,462,793 (US$653,803), respectively, in government grants from the relevant PRC government authorities.
Of the grants received in years ended December 31, 2008 and 2009, amounts of RMB6,560,000 and RMB1,000,000 (US$146,501), respectively, are required to be used towards the construction of the Group’s new manufacturing facilities located in Shenyang City of the PRC. Other than the restriction on use, these grants are not otherwise subject to adjustment or refund. The amortization of such government grants related to the Group’s new manufacturing facilities under construction has commenced as the related assets have been ready for use and commenced depreciation.
Of the grants received in years ended December 31, 2007, 2008 and 2009, amounts of RMB5,000,000, RMB800,000 and RMB1,600,000 (US$234,401), respectively, are required to be used to reimburse the interest expense incurred by Liaoning Nuokang on bank loans borrowed to finance the construction of its production facilities and one of its research and development projects. The right to recognize the grants is conditional upon the approval by the relevant government authority of the interest expenses incurred. To the extent the Group capitalizes interest expenses related to the construction of production facilities in future years, the related grants are regarded as a reduction of capitalized interests, and will be amortized to reduce depreciation expense over the estimated useful lives of the related assets. The remaining non-capitalized grants will be recognized as a reduction of interest expenses in the same period that the approval is obtained. The Group deferred all such grants during the year ended December 31, 2007 as no relevant interest expense had been incurred and approved. For the years ended December 31, 2008 and 2009, RMB4,787,417 (US$701,361) and RMB212,583 (US$31,144) of the RMB5,000,000 (US$732,504) grants received in 2007 had been approved by the relevant government authority, out of which RMB520,952 (US$76,320) and nil were related to the capitalized interests and are to be amortized to reduce the depreciation expense over the life of the related production facilities, and the non-capitalized portions of RMB4,266,465 (US$625,041) and RMB212,583 (US$31,144) were recorded as reduction of interests expenses, respectively. For the year ended December 31, 2009, amounts of RMB800,000 (US$117,201) and RMB1,600,000 (US$234,401) received in 2008 and 2009, respectively, had been approved by the relevant government authority and were recorded as reduction of interest expenses, respectively.
The remaining portion of the government grants received in the years ended December 31, 2007, 2008 and 2009 is required to be used in research and development projects as authorized by the relevant PRC government authorities. Related to this restriction on use, the Group is required to provide the results of the underlying research and development projects upon completion, regardless of the result, for formal approval by the relevant government authority. As such, these grants are recognized when the formal approvals are obtained.
F-40
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(CONT’D)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
17. DEFERRED GOVERNMENT GRANTS (CONT’D)
Movements in deferred government grants are as follows:
| | | | | | | | | |
| | | 2008 | | | 2009 | |
| | | (RMB’000) | | | (RMB’000) | | | (US$’000) | |
| | | |
Balance at beginning of year | | 16,520 | | | 19,384 | | | 2,840 | |
Additions | | 8,134 | | | 4,463 | | | 654 | |
Amortization as a reduction of research and development expense | | (774 | ) | | (1,120 | ) | | (164 | ) |
Amortization as a reduction of financial expense | | (4,266 | ) | | (2,642 | ) | | (387 | ) |
Amortization as a reduction of depreciation of manufacturing facilities | | (230 | ) | | (827 | ) | | (122 | ) |
| | | | | | | | | |
| | | |
Balance at end of year | | 19,384 | | | 19,258 | | | 2,821 | |
| | | | | | | | | |
18. SERIES A CONVERTIBLE REDEEMABLE PREFERENCE SHARES
The Company and a group of third party investors (the “Investors”) entered into a purchase agreement (the “Purchase Agreement”) on December 20, 2007 (the “Purchase Date”) whereby the Company issued in aggregate 25,796,662 Series A Redeemable Convertible Preference Shares at an issue price of US$0.6590 per share for gross proceeds of US$17,000,000 (RMB124,234,000). Significant terms of the Preference Shares are outlined below.
Voting rights
The holders of the Preference Shares shall have the same voting rights as the holders of ordinary shares. The holders of the Preference Shares and ordinary shares shall vote together as a single class on all matters. Each holder of ordinary shares shall be entitled to one vote for each ordinary share held, and each holder of Preference Shares shall be entitled to the number of votes equal to the number of ordinary shares into which such Preference Shares could be converted.
Dividends
The holders of the Preference Shares shall be entitled to receive Preference Shares dividend at a rate of 8% per annum of the original purchase price, in preference to any dividend on ordinary shares. The Preference Shares dividend is payable quarterly when, as and if declared by the board of directors of the Company. Such dividend shall not be cumulative.
F-41
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(CONT’D)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
18 SERIES A CONVERTIBLE REDEEMABLE PREFERENCE SHARES (CONT’D)
Performance linked valuation adjustment (“PLVA”)
The PLVA is a performance ratchet that adjusts the conversion ratio. Under PLVA, if the Company’s audited consolidated net income for the year ended December 31, 2008, subject to certain adjustments, was less than certain pre-determined amount, additional Preference Shares shall be issued to Preference Share holders at no cost. Such increase in shares would be proportionate with the net income shortfall, subject to a 25% cap, or 6,449,166 shares. No additional Preference Shares were issued eventually as the 2008 audited consolidated net income after taking into consideration of the agreed adjustments did not trigger PLVA.
Liquidation preferences
Upon any voluntary or involuntary liquidation, winding up or cessation of business of the Company, the Preference Shareholders shall be entitled to receive distribution of assets and funds, prior to any ordinary share holders, of an amount equal to 130% of the original purchase price plus any declared and unpaid dividends.
Conversion feature
Each Preference Share is convertible at the option of the holder, at any time after the date of issuance of such shares, into such number of ordinary shares as determined by dividing the original purchase price by the applicable conversion price.
Each Preference Share shall be automatically converted into ordinary shares at the applicable conversion price immediately upon the earlier of:
| (i) | the Company’s qualified public offering, as defined in the Purchase Agreement; or |
| (ii) | a date specified by written consent or agreement of holders of at least 75% of the then outstanding Preference Shares, voting as a separate class. |
The conversion price has been adjusted under the following two scenarios:
| (i) | if there was a share split or reverse share split, the conversion price should be adjusted proportionally; and |
| (ii) | if shares (either ordinary or preference shares) were subsequently issued at a lower price than the original conversion price (US$0.6590) of the Preference Shares, the conversion price shall be adjusted to the price of the newly issued shares. |
Upon the completion of the IPO dated on December 9, 2009, all of the Preference Share had been converted into ordinary shares with the conversion price equaled to the original purchase price of US$0.659 per share (i.e, 1:1 conversion).
F-42
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(CONT’D)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
18. SERIES A CONVERTIBLE REDEEMABLE PREFERENCE SHARES (CONT’D)
Redemption features
At any time commencing on the third anniversary of the Purchase Date, at the request of any holder of the Preference Shares then outstanding, the Company shall redeem all the Preference Shares held by such holder and such other holders who elect to participate in the redemption, at a redemption price equal to the original purchase price plus 12% per annum return (simple and not compounded) for each year the Preference Shares were outstanding.
Accounting for the Preference Shares
The Preference Shares have been classified as mezzanine equity prior to conversion because their redemption is contingent on certain events which are not within the control of the Company. The Preference Shares were not redeemable because none of the contingent redemption events occurred and over the life of the preference shares, the Company had evaluated and determined that they were not probable of occurring. The initial carrying value of the preference shares was accreted using the effective interest method to the redemption amount over the earliest redemption date.
The initial carrying value of the Preference Shares was the issuance price of the Preference Shares at the date of issuance.
The Company evaluated the Preference Shares to determine if there were any embedded derivatives requiring bifurcation and to determine if there was any beneficial conversion feature. The Company determined that the conversion option of the Preference Shares did not qualify for the scope exception of SFAS 133 paragraph 11(a) because the conversion price may be adjusted if the Company’s ordinary or preference shares are subsequently issued at a lower price than the original conversion price. However, the embedded conversion option did not qualify for derivative accounting because the Preference Shares are not readily convertible into cash as there is not a market mechanism in place for trading the underlying ordinary shares. The conversion price adjustments were no longer applicable upon the successful completion of qualified offering, at which time the Preference Shares automatically convert into ordinary shares of the Company pursuant to the Purchase Agreement. The redemption options of the Preference Shares do not qualify for derivative accounting because the options do not require or permit net settlement.
Beneficial conversion feature exists when the effective conversion price of the Preference Shares is lower than the fair value of the ordinary shares at December 20, 2007. The intrinsic value of the beneficial conversion feature is allocated from the carrying value of the Preference Shares as a contribution to additional paid-in capital. Since the conversion price of the Preference Shares is subject to adjustment arising from the PLVA, the effective conversion price used to calculate the beneficial conversion feature is determined at the commitment date as the most favorable conversion price that would be in effect at the conversion date, assuming there are no changes to the current circumstances except for the passage of time. The discount resulting from the beneficial conversion feature to the Preference Shares is then accreted to the earliest conversion date.
F-43
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(CONT’D)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
18. SERIES A CONVERTIBLE REDEEMABLE PREFERENCE SHARES (CONT’D)
The fair value of the ordinary shares as of December 20, 2007, the commitment date, was taken from the valuation report of Sallmanns. The Group’s management is ultimately responsible for the determination of the fair value of the ordinary shares. As of the commitment date, the most favorable conversion price used to calculate the amount of the beneficial conversion feature was RMB3.8371 (US$0.5617), which reflects the most dilutive adjustment resulting from the PLVA. A beneficial conversion feature of RMB29,876,209 (US$4,376,889) was recognized through a credit to additional paid-in capital because the fair value per ordinary share at the commitment date was RMB4.7641 (US$0.6975), which was more than the most favorable conversion price. The discount from recording such beneficial conversion feature was accreted from the date of issuance to the earliest conversion date which was also the issuance date, and was treated as a return to the convertible redeemable preference shareholders.
The Company incurred issuance costs of RMB2,548,000 (US$373,284) directly attributable to the Preference Shares offering. Such costs were charged against the gross proceeds of the offering. These costs were not included in the calculation of the beneficial conversion feature, except for a reimbursement of RMB798,744 (US$117,017) to the investors for their offering expenses.
Upon the completion of the IPO dated on December 9, 2009, all of the Preference Share had been converted into 25,796,662 ordinary shares. Accordingly, the carrying value of the Preference Shares of the date of conversion was reclassified to equity, which resulted in an increase of ordinary shares by RMB88,066 (US$12,902) and additional paid-in capital by RMB140,906,981 (US$2,064,299).
Net accretion charges of RMB30,156,239 (US$4,417,914), RMB13,402,897 (US$1,963,536), and RMB13,886,046 (US$2,034,317) were recorded as a reduction of net income available to ordinary shareholders for the years ended December 31, 2007, 2008 and 2009, respectively.
19. SHAREHOLDERS’ EQUITY
(a) Repurchase of Ordinary Shares
On December 20, 2007, the Company repurchased 4,552,352 ordinary shares from one of its shareholders, Anglo China, a company wholly-owned by Mr. Xue, at a purchase price of US$0.6590 per share for total consideration of RMB21,924,700 (US$3,211,987). The then fair value of these shares was US$0.6502 as determined based on an independent valuation prepared by Sallmanns. The Group’s management is ultimately responsible for the determination of the fair value of the ordinary shares. The excess amount of the total consideration over fair value of shares amounted to RMB292,627 (US$42,870) and was recorded as compensation expense in general and administration expenses for the year ended December 31, 2007. The Company cancelled these ordinary shares upon the completion of the repurchase.
F-44
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(CONT’D)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
19. SHAREHOLDERS’ EQUITY (CONT’D)
(b) Issuance of new ordinary Shares
On December 9, 2009, the Company completed its initial public offering of 36,215,832 new ordinary shares, which resulted in an increase of common stocks by RMB123,635 (US$12,902) and additional paid-in capital by RMB239,505,784 (US$35,093,006). All of the Preference Shares had been converted into ordinary shares, which resulted in an increase of common stocks by RMB88,066 (US$12,902) and additional paid in capital by RMB140,906,981 (US$2,064,299) (Note 18). All the non-employee stock options had been converted into ordinary shares, which resulted in an increase of common stocks by RMB3,277 (US$480) and additional paid-in capital by RMB3,020,356 (US$442,484) (Note 22).
(c) Statutory Reserves
PRC laws and regulations require wholly-owned foreign enterprises (“WOFE”), which includes all of the Company’s PRC subsidiaries, to provide for certain statutory reserve funds, namely, (i) general reserve fund and (ii) staff and workers’ bonus and welfare fund, which are appropriated from their annual net profit after tax (as reported in the entity’s PRC statutory accounts) but before dividend distribution. A WOFE is required to allocate at least 10% of its annual net profit after tax to the general reserve fund until the balance of such fund has reached 50% of its registered capital. Appropriation to the staff and workers’ bonus and welfare fund is at the discretion of the board of directors of the respective WOFE. The general reserve fund can only be used, upon approval by the relevant PRC authority, to offset accumulated losses or increase paid-in capital. The staff and workers’ bonus and welfare fund can only be used for special bonuses or collective welfare of employees, and any assets acquired through this fund shall not be treated as assets of the Group. The aforementioned reserves are not distributable as dividends.
Additionally, in accordance with the relevant PRC laws and regulations, the Company’s VIE (being as a domestic enterprise) is required to provide statutory general reserve at least 10% of its annual after-tax profit until such reserve has reached 50% of its respective registered capital based on the enterprise’s PRC statutory accounts. A domestic enterprise is also required to provide for discretionary surplus reserve, at the discretion of the board of directors, from the profits determined in accordance with the enterprise’s PRC statutory accounts. The aforementioned reserves can only be used for specific purposes and are not distributable as cash dividends.
As a result, for the years ended December 31, 2007, 2008 and 2009, RMB3,364,372, RMB8,508,296, and RMB10,207,946 (US$1,495,473), respectively, have been appropriated to the statutory reserves (included in the Group’s retained earnings) by the Group’s PRC subsidiaries. As of December 31, 2008 and 2009, an analysis of the Group’s retained earnings is as follows:
| | | | | | |
| | | 2008 | | 2009 |
| | | (RMB’000) | | (RMB’000) | | (US$’000) |
| | | |
Retained earnings | | | | | | |
Restricted – statutory reserves | | 20,854 | | 31,062 | | 4,551 |
Unrestricted | | 56,008 | | 97,009 | | 14,212 |
| | | | | | |
| | | |
| | 76,862 | | 128,071 | | 18,763 |
| | | | | | |
F-45
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(CONT’D)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
20. INCOME TAX EXPENSE
The Cayman Islands and Hong Kong
The Company is a tax exempt company incorporated in the Cayman Islands and conducts substantially all of its business through its subsidiaries and VIE located in the PRC.
Surplus International, the Group’s wholly owned subsidiary incorporated in Hong Kong, was subject to Hong Kong corporate income tax rate of 17.5% before 2008 and is thereafter subject to 16.5% on the estimated assessable profits arising in Hong Kong. Since Surplus International is an investment holding company, no provision for income tax has been made as it had no assessable profits during the years ended December 31, 2007, 2008 and 2009.
VHK, the subsidiary of VIE in Hong Kong, is subject to Hong Kong corporate income tax of 16.5% on the estimated assessable profits arising in Hong Kong. No provision for income tax has been made as it had no assessable profits during the year ended December 31, 2009.
PRC
The Company’s subsidiaries and VIE incorporated in the PRC are subject to PRC enterprise income tax (“EIT”) on the taxable income as reported in their PRC statutory accounts adjusted in accordance with relevant PRC income tax laws. The general applicable EIT rate is 25% from 2008 onwards while the pre-2008 general applicable EIT rate was 33%.
Liaoning Nuokang and Shenyang Shouzheng, recognized by the State Tax Bureau as “High and New Technology Enterprises” located in the Shenyang Economic and Technology Development Zone in Liaoning Province of the PRC, are entitled to a preferential EIT rate of 15%. Furthermore, being recognized as “Foreign Investment Manufacturing Enterprises” in December 2005 and October 2006, Liaoning Nuokang and Shenyang Shouzheng were entitled to full exemption of EIT for the first two years and a 50% reduction in EIT for the following three years (“Tax Holiday”), commencing from January 2006 and January 2007, respectively.
Penglai Nuokang, recognized as a “Foreign Investment Manufacturing Enterprise” in September 2006, was entitled to full exemption from EIT for the first two years and a 50% reduction in EIT for the following three years, commencing from September 2006. Despite the tax holiday started from September 2006, the calendar year 2006 was counted as the first year of such tax holiday.
During the 5th Session of the 10th National People’s Congress, which was concluded on March 16, 2007, the PRC Corporate Income Tax Law (the “New Corporate Income Tax Law”) was approved and became effective on January 1, 2008. The New Corporate Income Tax Law introduces a wide range of changes which include, but are not limited to, the unification of the income tax rates for domestic-invested and foreign-invested enterprises at 25%. Under the New Corporate Income Tax Law and the rules related to implementation of the New Corporate Income Tax Law, enterprises that are “high and new technology enterprises strongly supported by the State” are entitled to a reduced income tax rate of 15%, subject to approval by, and receipt of a qualification certificate from relevant authorities. The Group’s PRC subsidiaries were generally subject to an income tax rate of 25% in 2009, except for Liaoning Nuokang and Penglai Nuokang which have been recognized by relevant authorities as “High and New Technology Enterprises” for each of the tax years from 2008 to 2010 and were subject to a reduced income tax rate of 15%. New income tax rates have been applied in measuring deferred tax assets and liabilities resulting from temporary differences estimated to be realized after January 1, 2008.
F-46
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(CONT’D)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
| 20. | | INCOME TAX EXPENSE (CONT’D) |
Income before income taxes consists of:
| | | | | | | | | | | |
| | | 2007 | | | 2008 | | 2009 | |
| | | (RMB’000) | | | (RMB’000) | | (RMB’000) | | | (US$’000) | |
| | | | |
Cayman Islands | | (438 | ) | | 425 | | (2,850 | ) | | (417 | ) |
Hong Kong | | 30 | | | 4,593 | | (732 | ) | | (107 | ) |
The PRC | | 33,501 | | | 65,848 | | 85,535 | | | 12,530 | |
| | | | | | | | | | | |
| | | | |
| | 33,093 | | | 70,866 | | 81,953 | | | 12,006 | |
| | | | | | | | | | | |
The income tax benefit (expense) comprises the following:
| | | | | | | | | | | | |
| | | 2007 | | | 2008 | | | 2009 | |
| | | (RMB’000) | | | (RMB’000) | | | (RMB’000) | | | (US$’000) | |
| | | | |
Current | | (541 | ) | | (7,725 | ) | | (18,231 | ) | | (2,671 | ) |
Deferred | | 2,643 | | | 479 | | | 1,373 | | | 201 | |
| | | | | | | | | | | | |
| | | | |
| | 2,102 | | | (7,246 | ) | | (16,858 | ) | | (2,470 | ) |
| | | | | | | | | | | | |
The reconciliation of tax computed by applying the statutory income tax rate of 33% for the year ended December 31, 2007 and 25% for the years ended December 31, 2008 and 2009 applicable to the PRC operations to income tax (benefit) expense is as follows:
| | | | | | | | | | | | |
| | | 2007 | | | 2008 | | | 2009 | |
| | | (RMB’000) | | | (RMB’000) | | | (RMB’000) | | | (US$’000) | |
| | | | |
Income before income tax expense | | 33,093 | | | 70,866 | | | 81,953 | | | 12,006 | |
| | | | | | | | | | | | |
| | | | |
Income tax computed at PRC statutory tax rate of 33% or 25% | | 10,921 | | | 17,717 | | | 20,488 | | | 3,002 | |
Non-deductible staff costs | | 87 | | | 58 | | | 146 | | | 21 | |
Non-deductible entertainment expenses | | 1,165 | | | 680 | | | 846 | | | 124 | |
Non-deductible share based compensation expense | | — | | | — | | | 3,431 | | | 503 | |
Other non-deductible expenses | | 1,365 | | | 1,789 | | | 5,098 | | | 746 | |
Deferred tax expense | | — | | | 506 | | | — | | | — | |
Income not subject to tax | | — | | | (3,156 | ) | | (690 | ) | | (101 | ) |
Tax Holiday | | (9,080 | ) | | (9,797 | ) | | (12,169 | ) | | (1,783 | ) |
Preferential tax rates | | (3,765 | ) | | — | | | — | | | — | |
Changes in tax rates | | (2,795 | ) | | (551 | ) | | (292 | ) | | (42 | ) |
| | | | | | | | | | | | |
| | | | |
| | (2,102 | ) | | 7,246 | | | 16,858 | | | 2,470 | |
| | | | | | | | | | | | |
F-47
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(CONT’D)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
| 20. | | INCOME TAX EXPENSE (CONT’D) |
The benefit of the tax holiday per basic and diluted earnings per share is as follows:
| | | | | | | | | | | | |
| | | 2007 | | 2008 | | 2009 |
| | | | |
Basic | | RMB | 0.091 | | RMB | 0.103 | | RMB | 0.123 | | US$ | 0.018 |
| | | | | | | | | | | | |
| | | | |
Diluted | | RMB | 0.091 | | RMB | 0.103 | | RMB | 0.122 | | US$ | 0.018 |
| | | | | | | | | | | | |
The components of deferred tax assets and liabilities are as follows:
| | | | | | |
| | | 2008 | | 2009 |
| | | (RMB’000) | | (RMB’000) | | (US$’000) |
| | | |
Deferred tax assets: | | | | | | |
| | | |
Current: | | | | | | |
Bad debt provision | | 77 | | 63 | | 9 |
Write-down of inventories | | — | | 6 | | 1 |
Accruals | | 103 | | 1,102 | | 162 |
| | | | | | |
| | | |
| | 180 | | 1,171 | | 172 |
| | | | | | |
| | | |
Non-current: | | | | | | |
Property, plant and equipment | | 281 | | 542 | | 79 |
Deferred government grants | | 3,707 | | 4,170 | | 611 |
| | | | | | |
| | | |
| | 3,988 | | 4,712 | | 690 |
| | | | | | |
| | | |
Total deferred tax assets | | 4,168 | | 5,883 | | 862 |
| | | | | | |
| | |
| | | 2008 | | 2009 |
| | | (RMB’000) | | (RMB’000) | | (US$’000) |
| | | |
Deferred tax liabilities: | | | | | | |
| | | |
Current: | | | | | | |
Fair value gains on derivative instruments | | — | | 16 | | 2 |
| | | | | | |
| | | |
| | — | | 16 | | 2 |
| | | | | | |
| | | |
Non-current: | | | | | | |
Property, plant and equipment | | | | | | |
– capitalized interest | | 292 | | 278 | | 40 |
Intangible assets | | 814 | | 1,580 | | 232 |
| | | | | | |
| | | |
| | 1,106 | | 1,858 | | 272 |
| | | | | | |
| | | |
Total deferred tax liabilities | | 1,106 | | 1,874 | | 274 |
| | | | | | |
In assessing the realizability of deferred tax assets, the Group has considered whether it is more likely than not that some portion or all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. As of December 31, 2009, the Group recorded no valuation allowance as management believes is more-likely-than-not that the Group’s deferred tax assets are realizable based on the weight of all available evidence.
F-48
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(CONT’D)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
| 20. | | INCOME TAX EXPENSE (CONT’D) |
As of December 31, 2009, the Group intended to reinvest permanently the retained earnings of its PRC subsidiaries. The amount of unrecognized deferred tax liabilities for temporary differences related to investments in foreign subsidiaries is not determined because such a determination is not practicable.
As of December 31, 2009, the Group reported pre-paid income tax of RMB6,397,398 (US$937,224), related to tax due on inter-company sales between Group companies.
Effective from January 1, 2007, the Group adopted FASB ASC 740 to account for uncertainties in income taxes, which prescribes a more-likely-than-not threshold for financial statement recognition and measurement of uncertain tax positions taken in the tax return. This interpretation also provides guidance on de-recognition of income tax assets and liabilities, classification of current and deferred income tax assets and liabilities, accounting for interest and penalties associated with tax positions, accounting for income taxes in interim periods and income tax disclosures related to uncertain tax positions.
The Group’s adoption of FASB ASC 740 did not result in any adjustment to the opening balance of the Group’s retained earnings as of January 1, 2007. For the year ended December 31, 2009, the Group recorded a liability of RMB808,765 (US$118,484) for unrecognized tax benefits related to certain intercompany consulting income. It is possible that the amount of unrecognized tax benefits will change in the next 12 months. However, an estimate of the range of the possible change cannot be made at this time. The Group has elected to classify interest and penalties related to an uncertain position, if and when required, as part of interest expenses and other expenses, respectively, in the consolidated statements of income. No such amounts have been accrued through December 31, 2009 by the Group. The total unrecognized tax benefits as of December 31, 2009, if recognized, would affect the Group’s effective income tax rate. For the PRC subsidiaries, the tax years ended December 31, 2006, 2007, 2008 and 2009 remain open to examination by the tax authorities. For the HK subsidiaries, the tax years ended December 31, 2005 to 2009 remain open to examination. The following table summarizes the activity related to the Group’s unrecognized tax benefits from January 1, 2009 to December 31, 2009:
| | |
| | | RMB’000 |
| |
Balance as of January 1, 2009 | | — |
Increases related to current year tax positions | | 809 |
| | |
| |
Balance as of December 31, 2009 | | 809 |
| | |
The New Corporate Income Tax Law stipulates that a PRC resident enterprise, which includes an enterprise established outside of PRC with its effective management and control located in PRC, will be subject to PRC income tax on its worldwide income. If the PRC tax authorities subsequently determine that the Company or Surplus International which was registered outside PRC should be deemed a resident enterprise, the Company or Surplus International will be subject to PRC income tax at a rate of 25%. The Company will continue to monitor its tax status.
In addition, the New Corporate Income Tax Law provides that dividend income between qualified “resident enterprises” is exempted from the 10%, or a lower rate as provide by tax treaty or agreement if available, withholding tax on dividends paid to non-PRC enterprise shareholders. If the Company or Surplus International is considered a “non-resident enterprise,” dividends paid to Surplus International by our subsidiaries in the PRC (through our holding company structure) may be subject to the withholding tax.
F-49
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(CONT’D)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
21. NET INCOME PER SHARE
Basic and diluted net income per share for each of the period presented are calculated as follows:
| | | | | | | | | | | | | |
| | | 2007 | | | 2008 | | | 2009 | |
| | | (RMB’000) | | | (RMB’000) | | | (RMB’000) | | | (US$’000) | |
| | | | |
Numerator: | | | | | | | | | | | | | |
Net income | | 35,195 | | | 63,620 | | | 65,095 | | | | 9,535 | |
Accretion of Preference Shares | | (30,156 | ) | | (13,403 | ) | | (13,886 | ) | | | (2,034 | ) |
Allocation to Preference Shares for participating rights to dividends | | (326 | ) | | (9,458 | ) | | — | | | | — | |
| | | | | | | | | | | | | |
Income attributable to ordinary shareholders | | 4,713 | | | 40,759 | | | 51,209 | | | | 7,501 | |
| | | | | | | | | | | | | |
| | | | |
Denominator: | | | | | | | | | | | | | |
Number of shares outstanding, opening | | 100,000,000 | | | 95,447,648 | | | 95,447,648 | | | | 95,447,648 | |
Weighted average number of shares repurchased (Note 19(a)) | | (149,666 | ) | | — | | | | | | | — | |
New ordinary shares issued from IPO (36,215,832 shares issued on December 9, 2009) | | — | | | — | | | 1,984,429 | | | | 1,984,429 | |
Conversion of Preference Share (25,796,662 shares converted on December 9, 2009) | | — | | | — | | | 1,413,516 | | | | 1,413,516 | |
Effect of stock options (960,000 share options exercised on December 16, 2009) | | — | | | — | | | 39,452 | | | | 39,452 | |
| | | | | | | | | | | | | |
| | | | |
Weighted average number of shares outstanding – basic | | 99,850,334 | | | 95,447,648 | | | 98,885,045 | | | | 98,885,045 | |
| | | | |
Dilutive effect of convertible securities: | | | | | | | | | | | | | |
- Share options | | — | | | 13,193 | | | 840,208 | | | | 840,208 | |
- Preference Shares | | — | | | — | | | — | | | | — | |
| | | | | | | | | | | | | |
| | | | |
Weighted average number of shares outstanding – diluted | | 99,850,334 | | | 95,460,841 | | | 99,725,253 | | | | 99,725,253 | |
| | | | |
Basic net income per share | | RMB 0.05 | | | RMB 0.43 | | | RMB 0.52 | | | US$ | 0.08 | |
| | | | | | | | | | | | | |
| | | | |
Diluted net income per share | | RMB 0.05 | | | RMB 0.43 | | | RMB 0.51 | | | US$ | 0.08 | |
| | | | | | | | | | | | | |
F-50
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(CONT’D)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
21 NET INCOME PER SHARE (CONT’D)
The effects of share options granted on December 19, 2007 have been excluded from the computation of dilutive earnings per share for the year ended December 31, 2007, as they were anti-dilutive.
The effects of share options granted on October 11, 2008 have been excluded from the computation of dilutive earnings per share for the years ended December 31, 2008 and 2009, as they were anti-dilutive.
Preference Shares have been excluded from the computation of dilutive earnings per share for all the periods presented, as they were anti-dilutive.
22. SHARE OPTION PLAN
On December 19, 2007, the Company adopted a share option scheme (the “2007 Option Plan”) which allows the Company’s board of directors, at its discretion, to offer options to officers (including directors), employees and consultants of the Group to purchase up to 7,738,998 ordinary shares of the Company. As of December 31, 2007, 5,500,000 and 960,000 options have been granted to the employees and non-employees (consultants and former employees), respectively, at an exercise price of US$0.4613 (RMB3.1528) per share. On October 11, 2008, another 1,200,000 options have been granted to the employees and directors at an exercise price of US$0.7250 (RMB4.9551) per share. All options granted to employees vest over the related requisite service period of four years using a graded vesting schedule of 25% of the options vesting on each of the first, second, third and fourth anniversaries of the grant date. For consultants, a performance condition exists as the services are directly related to, and the options are vested upon, a qualified IPO. The former employee options vest upon the date when a qualified IPO is completed. All options granted to employees expire four years after their respective vest dates and options granted to non-employees expire after 7 days from the date of a qualified IPO.
On October 6, 2008, the Company adopted a share option scheme (the “2008 Option Plan”) which allows the Company’s board of directors, at its discretion, to offer options to officers (including directors), employees and consultants of the Group to purchase up to 1,800,000 ordinary shares of the Company. As of December, 31, 2009, no options under 2008 Option Plan have been granted.
The binomial option pricing model was applied in determining the fair value of the options granted to employees and Black-Scholes pricing model in determining the fair value of the options granted to non-employees. These models require the input of highly subjective assumptions, including the expected stock price volatility and the expected price multiple at which employees are likely to exercise share options. For expected volatilities, the Company has made reference to historical volatilities of several comparable companies. The risk-free rate for periods within the contractual life of the option is based on the U.S. Treasury Bills yield in effect at the time of grant. The fair value of the ordinary shares, at the option grant dates, was determined by Sallmanns. The Group’s management is ultimately responsible for the determination of the fair value of the ordinary shares.
F-51
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(CONT’D)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
22. SHARE OPTION PLAN (CONT’D)
(a) Options Granted to Employees
The following table summarized the Company’s employee share option activity under the 2007 Option Plan:
| | | | | | | | |
| | | Number of
options | | Weighted
average
exercise
price | | Weighted
average
remaining
contractual
life | | Aggregate
intrinsic value |
| | | | | (US$) | | (Years) | | (US$’000) |
| | | | |
Outstanding, January 1, 2007 | | — | | — | | | | |
Granted | | 5,500,000 | | 0.4613 | | | | |
Exercised | | — | | — | | | | |
Forfeited | | — | | — | | | | |
| | | | | | | | |
| | | | |
Outstanding, December 31, 2007 | | 5,500,000 | | 0.4613 | | 6.47 | | 1,038 |
| | | | | | | | |
| | | | |
Expected to vest at December 31, 2007 | | 5,421,900 | | 0.4613 | | 6.47 | | 1,023 |
| | | | | | | | |
| | | | |
Exercisable at December 31, 2007 | | — | | | | | | |
| | | | | | | | |
| | | | |
Granted | | 1,200,000 | | 0.7250 | | | | |
Exercised | | — | | — | | | | |
Forfeited | | 220,000 | | 0.5812 | | | | |
| | | | | | | | |
| | | | |
Outstanding, December 31, 2008 | | 6,480,000 | | 0.5061 | | 5.61 | | 1,896 |
| | | | | | | | |
| | | | |
Expected to vest at December 31, 2008 | | 6,164,235 | | 0.5061 | | 5.61 | | 1,803 |
| | | | | | | | |
| | | | |
Exercisable at December 31, 2008 | | 1,345,000 | | | | | | |
| | | | | | | | |
| | | | |
Granted | | — | | — | | | | |
Exercised | | — | | — | | | | |
Forfeited | | 190,000 | | 0.4613 | | | | |
| | | | | | | | |
| | | | |
Outstanding, December 31, 2009 | | 6,290,000 | | 0.5074 | | 4.61 | | 3,853 |
| | | | | | | | |
| | | | |
Expected to vest at December 31, 2009 | | 6,127,500 | | 0.5074 | | 4.61 | | 3,754 |
| | | | | | | | |
| | | | |
Exercisable at December 31, 2009 | | 2,870,000 | | | | | | |
| | | | | | | | |
The aggregate intrinsic value is calculated as the difference between the exercise price of the underlying awards and the estimated fair value of the underlying stock at each reporting date, for those awards that have an exercise price currently below the fair value of the Company’s shares. As of December 31, 2009, the Company has options outstanding to purchase an aggregate of 6,290,000 shares with an exercise price below the fair value of the Company’s shares, resulting in an aggregate intrinsic value of RMB26,301,237 (US$ 3,853,153).
F-52
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(CONT’D)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
22. SHARE OPTION PLAN (CONT’D)
(a) Options Granted to Employees (Cont’d)
Prior to the IPO, the Company has accounted for its options granted to employees as liability awards as the options are indexed to the U.S. dollar to RMB exchange rate in addition to the fair value of the underlying shares, as the exercise price was dominated in US$ while the functional currency of the Company is RMB. The share-based compensation liability is initially recognized at fair value on the date of grant and is subsequently remeasured at the end of each reporting period and IPO date with an adjustment for fair value recorded to the current period expense in order to properly reflect the cumulative expense based on the current fair value of the vested options over the vesting period. The Company has recognized compensation expenses using the straight-line method for liability classified share options granted with service conditions that have a graded vesting schedule. Upon the IPO, the Company started to account for its options granted to employees as equity awards, given that the Company’s shares are publicly traded in U.S. and therefore, the options are no longer considered dual-indexed. The fair value of the options granted to employees was remeasured on the IPO date, with the fair value of the options reclassified to equity and the fair value of the unvested options to be recognized over the remaining requisite service period as compensation expenses using the straight-line method.
The fair values of the options granted to employees at the grant dates was determined to be RMB12,881,757 (US$1,887,188) and RMB2,920,819 (US$427,902) for the 2007 and 2008 grants, respectively. As of December 9, 2009, the IPO date, the fair values were RMB24,716,866 (US$3,621,041) and RMB4,300,494 (US$630,026) for the 2007 and 2008 grants, respectively; whereas the fair values were RMB15,680,664 and RMB2,800,788 for the 2007 and 2008 grants as of December 31, 2008.
The Company calculated the estimated fair value of the options on the grant date using the binomial option pricing model with the following assumptions:
| | | | | | | | |
| | | December 19, 2007 | | | October 11, 2008 | |
| | |
Suboptimal exercise factor | | | 1.5 | | | | 1.5 | |
Risk-free interest rates | | | 3.43% - 3.98 | % | | | 2.83% - 3.86 | % |
Expected volatility | | | 46.34% - 60.04 | % | | | 48.27% - 57.94 | % |
Expected dividend yield | | | 0 | % | | | 0 | % |
Fair value of share options | | US$ | 0.343 | | | US$ | 0.356 | |
The Company calculated the estimated fair value of the options at each reporting date and IPO date using the binomial option pricing model with the following assumptions:
| | | | | | | | | |
| | | December 31, 2007 | | | December 31, 2008 | | | December 9, 2009 | |
| | | |
Suboptimal exercise factor | | 1.5 | | | 1.5 | | | 1.5 | |
Risk-free interest rates | | 3.43% - 3.98 | % | | 1% - 1.62 | % | | 1.28% - 2.67 | % |
Expected volatility | | 46.34% - 60.04 | % | | 57.63% - 59.91 | % | | 63.10% - 78.15 | % |
Expected dividend yield | | 0 | % | | 0 | % | | 0 | % |
F-53
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(CONT’D)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
22. SHARE OPTION PLAN (CONT’D)
(a) Options Granted to Employees (Cont’d)
Share-based compensation expense is recorded net of estimated forfeitures such that expense was recorded only for those share-based awards that are expected to vest. The estimation of the forfeiture rate was based primarily upon historical experience of employee turnover. To the extent the Company revises this estimate in the future, the share-based payments could be materially impacted in the year of revision, as well as in following years. During the years ended December 31, 2008 and 2009, the Company increased the forfeiture rate for both the management group and the non-management group primarily due to changes in historical employee turnover rates.
Total compensation expenses relating to employee share options recognized for the years ended December 31, 2007, 2008 and 2009, respectively, and were included in the following captions:
| | | | | | | | |
| | | 2007 | | 2008 | | 2009 |
| | | (RMB’000) | | (RMB’000) | | (RMB’000) | | (US$’000) |
| | | | |
Cost of revenue | | — | | 99 | | 222 | | 33 |
Research and development costs | | — | | 142 | | 317 | | 46 |
Selling, marketing and distribution expenses | | — | | 1,215 | | 2,556 | | 374 |
General and administrative expenses | | 105 | | 2,523 | | 6,300 | | 923 |
| | | | | | | | |
| | | | |
| | 105 | | 3,979 | | 9,395 | | 1,376 |
| | | | | | | | |
As of December 31, 2009, there was RMB15,520,684 (US$2,273,793) of unrecognized share-based compensation cost related to share options issued to employees. RMB12,538,446 (US$1,836,893) and RMB2,982,238 (US$436,900) are expected to be recognized following the straight-line method over a period of 1.97 years and 2.78 years, respectively.
(b) Share options issued to non-employees
The following table summarized the Company’s non-employee share option activities under the 2007 Option Plan:
| | | | | | |
| | | Number of
options | | Weighted average
exercise price | | Aggregate
intrinsic value |
| | | | | (US$) | | (US$’000) |
| | | |
Outstanding, January 1, 2008 | | 960,000 | | 0.4613 | | |
Granted | | — | | — | | |
Exercised | | — | | — | | |
Forfeited/Cancelled | | — | | — | | |
| | | | | | |
| | | |
Outstanding, December 31, 2008 | | 960,000 | | 0.4613 | | 181 |
| | | | | | |
| | | |
Expected to vest at December 31, 2008 | | 960,000 | | 0.4613 | | 181 |
| | | | | | |
| | | |
Exercisable at December 31, 2008 | | — | | | | |
| | | | | | |
| | | |
Exercised | | 960,000 | | 0.4613 | | |
Forfeited/Cancelled | | — | | | | |
| | | | | | |
| | | |
Outstanding, December 31, 2009 | | — | | | | |
| | | | | | |
F-54
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(CONT’D)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
22. SHARE OPTION PLAN (CONT’D)
(b) Share options issued to non-employees (Cont’d)
The aggregate intrinsic value is calculated as the difference between the exercise price of the underlying awards and the estimated fair value of the underlying stock at each reporting date, for those awards that have an exercise price currently below the fair value of the Company’s shares.
The Group recorded compensation expenses as equal to the fair value of the share options at the measurement date. The measurement date was set upon a qualified IPO, which occurred in December 9, 2009. All non-employee options were exercised shortly after the IPO, resulting in RMB4,317,525 (US$632,521) of compensation expenses recorded in general and administrative expenses, with a corresponding credit to additional paid-in capital during the year ended December 31, 2009.
The Company calculated the estimated fair value of the options on the measurement date using the Black-Scholes pricing model with the following assumptions:
| | | | |
| | | December 9, 2009 | |
| |
Suboptimal exercise factor | | | 1.5 | |
Risk-free interest rate | | | 0.03 | % |
Expected volatility | | | 78.15 | % |
Expected dividend yield | | | 0 | % |
Fair value of share options | | US$ | 0.659 | |
F-55
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(CONT’D)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
23. RELATED PARTY TRANSACTIONS
| | | | |
Name of related party | | | | Relationship with the Group |
| | |
Mr. Xue | | | | Ultimate shareholder of the Company |
The movements of the related party balances are as follows:
| | | |
| | | Mr. Xue | |
| | | (RMB’000) | |
| |
Balance as of January 1, 2008 | | (3,302 | ) |
US$ loan from shareholder | | (1,470 | ) |
Repayment of loan from shareholder | | 3,166 | |
Foreign currency translation gain for the US$ loan from shareholder | | 371 | |
Expenses paid by related party on behalf of the Group | | (13,700 | ) |
Expenses paid by the Group on behalf of related party | | 12,909 | |
| | | |
| |
Balance as of December 31, 2008 | | (2,026 | ) |
| | | |
| |
Foreign currency translation gain for the US$ loan from shareholder | | 40 | |
Repayment of loan from shareholder | | 5,809 | |
Expenses paid by related party on behalf of the Group | | 752 | |
Expenses paid by the Group on behalf of related party | | (4,575 | ) |
| | | |
| |
Balance as of December 31, 2009 | | — | |
| | | |
Balance as of December 31, 2009 (US$’000) | | — | |
| | | |
As of December 31, 2008 and 2009, bank loans of the Group totaling, RMB52,000,000 and RMB78,000,000 (US$11,427,065), respectively, were secured by certain of the Group’s property, plant and equipment, land use rights and guaranteed by Mr. Xue (Note 14).
As of December 31, 2008 and 2009, balances with related party were unsecured, non-interest-bearing and repayable on demand.
F-56
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(CONT’D)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
24. EMPLOYEE DEFINED CONTRIBUTION PLAN
Pursuant to the relevant PRC regulations, the Group is required to make contributions for each employee at a rate of 13% to 20% on a standard salary as determined by the local Social Security Bureau, to a defined contribution retirement scheme organized by the local Social Security Bureau in respect of the retirement benefits for the Group’s employees in the PRC. The total amounts of contributions of RMB1,248,986, RMB2,124,157 and RMB2,506,043 (US$367,137) for the years ended December 31, 2007, 2008 and 2009, respectively, were charged to expenses in the consolidated statements of income. The Group has no other obligation to make payments in respect of retirement benefits of the employees.
25. SUBSCRIPTION OF EQUITY SHARES OF VHK
On October 19, 2009, Nuokang Distribution paid US$5,000,000 (RMB34,137,500) in subscription of 133,333 ordinary shares of VHK, which represents a 93% ownership interest in VHK. On the acquisition date, there were no other assets and liabilities carried by VHK other than intellectual property rights, and the transaction was accounted for as an asset acquisition. The cost of the intellectual property rights was RMB2,571,000 (US$376,653), which approximated the fair value. The intellectual property rights will be amortized under straight line method over their useful lives of 9 to 13 years.
26. COMMITMENTS AND CONTINGENCIES
(a) Variable Interest Entity Structure
Penglai Nuokang has entered into a series of contractual arrangements with Nuokang Distribution, pursuant to which Penglai Nuokang provides Nuokang Distribution with technical and consultancy services in exchange for fees, and Penglai Nuokang undertakes to provide financial support to Nuokang Distribution to the extent necessary for its operations. In addition, through the contractual agreements, Penglai Nuokang has the ability to control Nuokang Distribution. In the opinion of management, (i) the ownership structure of the Company, Penglai Nuokang and Nuokang Distribution are in compliance with existing PRC laws and regulations; (ii) the contractual arrangements with Penglai Nuokang, Nuokang Distribution and its shareholder are valid and binding, and will not result in any violation of PRC laws or regulations currently in effect; and (iii) the Group’s business operations are in compliance with existing PRC laws and regulations in all material respects.
F-57
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(CONT’D)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
26. COMMITMENTS AND CONTINGENCIES (CONT’D)
(a) Variable Interest Entity Structure (Cont’d)
However, there are substantial uncertainties regarding the interpretation and application of current and future PRC laws and regulations. Accordingly, the Company cannot be assured that PRC regulatory authorities will not ultimately take a contrary view to its opinion. If the current ownership structure of the Group and its contractual arrangements with Nuokang Distribution are found to be in violation of any existing or future PRC laws and regulations, the Group may be required to restructure its ownership structure and operations in the PRC to comply with the changing and new PRC laws and regulations. In the opinion of management, the likelihood of loss in respect of the Group’s current ownership structure or the contractual arrangements with Nuokang Distribution is remote based on current facts and circumstances.
(b) Pending Arbitration
On November 15, 2007, the Company received an objection raised by Beijing Shunxin Agriculture Co., Ltd. (“Beijing Shunxin”), a company established in the PRC, in connection with one of the Group’s trademark under registration process with the PRC Trademark Office. Beijing Shunxin alleged that the Group was attempting to register the trademark for its products which imitated one of Beijing Shunxin’s trademarks used in a similar product of Beijing Shunxin. The Group has filed its response to the Trademark Office. On May 5, 2010, the Group received a letter from the PRC Trademark Office indicating that there was insufficient evidence supporting Beijing Shunxin’s claims and that if Beijing Shunxin does not request a second review of its objection, the Group will receive registration for “Shunxin” Trademark.
Litigation is subject to uncertainties and the outcome of individual litigated matters is not predictable with assurance. The directors of the Company do not believe a loss arising from the arbitration is probable. The Group believes that the plaintiff’s positions in the litigation are without merit and intends to vigorously defend itself in the litigation.
(c) Purchase Commitments
As of December 31, 2008 and 2009, the Group had outstanding purchase commitments in relation to construction-in-progress, office equipment and exclusive distribution right (Note 9) of RMB6,054,000 and RMB1,251,113 (US$183,289), respectively.
On August 1, 2008, Liaoning Nuokang entered into a supply contract of snake venom, the primary raw material of the Group’s final product, with a foreign supplier. The unit prices are specified in the contract for snake venom purchase during the period from January 1, 2009 to August, 2011, but subject to mutual written confirmation between the supplier and the Company on an annual basis for adjustments taking into consideration currency rate fluctuations, changes in production costs, changes in environmental law, changes in government taxes and availability of the product. Minimum annual purchase volumes are also specified in the contract, totaling US$940,000 over the contract term. This amount is subject to change depending on annual adjustments to the unit price. Penalty of failure to meet the minimum quantity by either party will be 50% of the total missing deliveries, determined using the average unit price of the 12 latest invoices. As of December 31, 2009, the Group was not expected to incur any penalties in relation to failure of meeting minimum quantity for the year ended December 31, 2009. The term of this supply contract is three years and subject to automatic renewal. As of December 31, 2009, the aggregate amounts of required minimum payment were as follows:
| | | | |
| | | December 31, 2009 |
| | | (RMB’000) | | (US$’000) |
| | |
2010 | | 2,417 | | 354 |
2011 | | 1,611 | | 236 |
| | | | |
| | |
| | 4,028 | | 590 |
| | | | |
F-58
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(CONT’D)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
26. COMMITMENTS AND CONTINGENCIES (CONT’D)
(c) Purchase Commitments (Cont’d)
On October 30, 2008, Liaoning Nuokang entered into a product distribution agreement with a third party effective until December 31, 2014, which allows the Group to expand into other product sales. Under this agreement, the Group has minimum inventory purchase commitments of RMB7,188,000 (US$1,053,048) for each year thereafter until December 31, 2014. Penalty of failure to meet the minimum quantity by either party will be RMB500,000. As of December 31, 2009, the Group was not expected to incur any penalties in relation to failure of meeting minimum quantity for the year ended December 31, 2009.
(d) Operating Leases
As of December 31, 2009, the Group had minimum lease payments under non-cancellable operating leases in relation to office premises as follows:
| | | | |
| | | December 31, 2009 |
| | | (RMB’000) | | (US$’000) |
| | |
2010 | | 826 | | 121 |
2011 | | 57 | | 8 |
2012 | | 23 | | 3 |
| | | | |
| | |
| | 906 | | 132 |
| | | | |
The table above excludes the payment for the right to use the Manufacturing Assets (Note 26 (e)).
Total rental expenses for the operating lease were RMB353,529, RMB1,473,376, RMB1,625,361 (US$238,117) for the years ended December 31, 2007, 2008 and 2009, respectively. The terms of the leases do not contain rent escalation or contingent rents.
(e) Manufacturing Assets Use Right Payment
As of December 31, 2009, the Group had contractual future payments of RMB8,000,000 (US$1,172,007) to SDPP for which RMB1,600,000 (US$234,401) per annum will be made until December 31, 2014 (Note 4) in connection with the acquisition of non-controlling interest in Penglai Nuokang.
F-59
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(CONT’D)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
26. COMMITMENTS AND CONTINGENCIES (CONT’D)
(f) Income Tax
Effective from January 1, 2007, the Group adopted FASB ASC 740 to account for uncertainties in income taxes, which prescribes a more-likely-than-not threshold for financial statement recognition and measurement of a tax position taken in the tax return. This interpretation also provides guidance on de-recognition of income tax assets and liabilities, classification current and deferred income tax assets and liabilities, accounting for interest and penalties associated with tax positions, accounting for income taxes in interim periods and income tax disclosures. The Group has determined that there was no financial impact of adoption of FASB ASC 740 by the end of 2008. Accordingly, the Company has not recorded any liabilities for adoption of FASB ASC 740 for any of the periods presented. For the year ended December 31, 2009, the Group recorded a liability of RMB808,765 (US$118,485) for unrecognized tax benefits, all of which related to an unreported consulting income.
27. SUBSEQUENT EVENTS
The Group has evaluated all events or transactions that occurred after December 31, 2009 as of the date the consolidated financial statements were issued. There were no significant subsequent events occurred.
F-60
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(CONT’D)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
28. CONDENSED FINANCIAL INFORMATION OF THE COMPANY
Under PRC laws and regulations, the Company’s PRC subsidiaries and VIE are restricted in their ability to transfer certain of their net assets to the Company in the form of dividend payments, loans, or advances. The amount restricted include share capital and statutory reserve of PRC subsidiaries and net assets of VIE, as determined pursuant to PRC generally accepted accounting principles, totaling RMB176,155,983 (US$25,806,997) as of December 31, 2009.
Balance sheets
| | | | | | |
| | | 2008 | | 2009 |
| | | (RMB’000) | | (RMB’000) | | (US$’000) |
| | |
ASSETS | | | | |
Current assets: | | | | | | |
Cash and cash equivalents | | 1,071 | | 256,639 | | 37,598 |
Other receivables due from a subsidiary | | 92,999 | | 91,740 | | 13,440 |
| | | | | | |
| | | |
Total current assets | | 94,070 | | 348,379 | | 51,038 |
| | | | | | |
| | | |
Non-current assets: | | | | | | |
Investment in subsidiaries | | 166,680 | | 246,026 | | 36,043 |
| | | | | | |
| | | |
TOTAL ASSETS | | 260,750 | | 594,405 | | 87,081 |
| | | | | | |
| | | |
LIABILITIES, PREFERENCE SHARES AND SHAREHOLDERS’ EQUITY | | | | | | |
Current liabilities: | | | | | | |
Accrued expenses and other payables | | 1,700 | | 14,021 | | 2,054 |
Share-based compensation liability | | 4,084 | | — | | — |
| | | | | | |
| | | |
Total current liabilities | | 5,784 | | 14,021 | | 2,054 |
| | | | | | |
| | | |
Convertible redeemable preference shares | | | | | | |
Series A preference shares (25,800,000 and nil shares authorized and 25,796,662 and nil shares issued and outstanding with par value of US$0.0005, each as of December 31, 2008 and 2009) | | 127,245 | | — | | — |
| | | | | | |
| | | |
Shareholders’ equity: | | | | | | |
Ordinary shares (par value US$0.0005 per share, 474,200,000 shares authorized and 95,447,648 shares issued and outstanding as of December 31, 2008; 474,200,000 shares authorized and 158,420,142 share issued and outstanding as of December 31, 2009) | | 382 | | 597 | | 87 |
Additional paid-in capital | | 50,477 | | 451,716 | | 66,177 |
Retained earnings | | 76,862 | | 128,071 | | 18,763 |
| | | | | | |
| | | |
Total shareholders’ equity | | 127,721 | | 580,384 | | 85,027 |
| | | | | | |
| | | |
TOTAL LIABILITIES, PREFERENCE SHARES AND SHAREHOLDERS’ EQUITY | | 260,750 | | 594,405 | | 87,081 |
| | | | | | |
F-61
CHINA NUOKANG BIO-PHARMACEUTICAL INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(CONT’D)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
28. CONDENSED FINANCIAL INFORMATION OF THE COMPANY (CONT’D)
Statements of income
| | | | | | | | | |
| | | 2008 | | | 2009 | |
| | | (RMB’000) | | | (RMB’000) | | | (US$’000) | |
| | | |
General and administrative expenses | | (1,214 | ) | | (2,940 | ) | | (431 | ) |
Other income, net | | 1,637 | | | 90 | | | 13 | |
Equity in profit of subsidiaries | | 63,197 | | | 67,945 | | | 9,954 | |
| | | | | | | | | |
Income before tax | | 63,620 | | | 65,095 | | | 9,536 | |
Income tax | | — | | | — | | | — | |
| | | | | | | | | |
Net income | | 63,620 | | | 65,095 | | | 9,536 | |
| | | | | | | | | |
Statements of cash flows
| | | | | | | | | |
| | | 2008 | | | 2009 | |
| | | (RMB’000) | | | (RMB’000) | | | (US$’000) | |
| | | |
Net cash used in operating activities | | (1,475 | ) | | (1,079 | ) | | (158 | ) |
| | | |
Net cash used in from investing activities | | — | | | — | | | — | |
| | | |
Net cash generated from financing activities | | 347 | | | 256,647 | | | 37,599 | |
| | | | | | | | | |
| | | |
Cash at the beginning of year | | 2,199 | | | 1,071 | | | 157 | |
| | | | | | | | | |
| | | |
Cash at the end of year | | 1,071 | | | 256,639 | | | 37,598 | |
| | | | | | | | | |
(a) Basis of presentation
In the Company financial statements, the Company’s investment in subsidiary is stated at cost plus equity in undistributed earnings of subsidiary since inception. The Company financial statements should be read in conjunction with the Company’s consolidated financial statements.
The Company records its investment in its subsidiary under the equity method of accounting as prescribed in FASB ASC 323, “Investment-Equity Method and Joint Venture”. Such investment is presented on the balance sheet as “Investments in subsidiaries” and share of the subsidiaries’ profit or loss as “Equity in profit of subsidiaries” on the statements of income.
The subsidiary did not pay any dividend to the Company for the periods presented.
Certain information and footnote disclosures normally included in financial statements prepared in accordance with US GAAP have been condensed or omitted by reference to the consolidated financial statements.
(b) Commitments
The Company did not have any significant commitments or long-term obligations as of any of the periods presented.
F-62
EXHIBIT INDEX
| | |
Exhibit
Number | | Description of Document |
| |
1.1 | | Amended and Restated Memorandum and Articles of Association of the Registrant, as currently in effect (incorporated by reference to Exhibit 3.2 of our Registration Statement on Form F-1 (file No. 333-163250), as amended, initially filed with the Securities and Exchange Commission on November 20, 2009) |
| |
2.1 | | Form of Registrant’s American Depositary Receipt (included in Exhibit 2.3) |
| |
2.2 | | Registrant’s Specimen Certificate for Ordinary Shares (incorporated by reference to Exhibit 4.2 of our Registration Statement on Form F-1 (file No. 333-163250), as amended, initially filed with the Securities and Exchange Commission on November 20, 2009) |
| |
2.3 | | Form of Deposit Agreement among the Registrant, the depositary and Owners and Beneficial Owners of the American Depositary Shares issued thereunder (incorporated by reference to Exhibit 4.3 of our Registration Statement on Form F-1 (file No. 333-163250), as amended, initially filed with the Securities and Exchange Commission on November 20, 2009) |
| |
4.1 | | 2007 Share Incentive Plan, as amended (incorporated by reference to Exhibit 10.1 of our Registration Statement on Form F-1 (file No. 333-163250), as amended, initially filed with the Securities and Exchange Commission on November 20, 2009) |
| |
4.2 | | 2008 Share Incentive Plan (incorporated by reference to Exhibit 10.2 of our Registration Statement on Form F-1 (file No. 333-163250), as amended, initially filed with the Securities and Exchange Commission on November 20, 2009) |
| |
4.3 | | Form of Indemnification Agreement with the Registrant’s directors and officers (incorporated by reference to Exhibit 10.3 of our Registration Statement on Form F-1 (file No. 333-163250), as amended, initially filed with the Securities and Exchange Commission on November 20, 2009) |
| |
4.4 | | Form of Employment Agreement between the Registrant and an Executive Officer of the Registrant (incorporated by reference to Exhibit 10.4 of our Registration Statement on Form F-1 (file No. 333-163250), as amended, initially filed with the Securities and Exchange Commission on November 20, 2009) |
| |
4.5 | | Preference A Shares Purchase Agreement dated December 20, 2007 among the Registrant, the Management Shareholder and the Purchasers, each as defined therein (incorporated by reference to Exhibit 10.5 of our Registration Statement on Form F-1 (file No. 333-163250), as amended, initially filed with the Securities and Exchange Commission on November 20, 2009) |
| |
4.6 | | Investors’ Rights Agreement dated December 20, 2007 among the Registrant, Anglo China Bio-Technology Investment Holdings Limited and Britain Ukan Technology Investment Holdings (Group) Limited, the Management Shareholders and the Preference A Holders, each as defined therein (incorporated by reference to Exhibit 10.6 of our Registration Statement on Form F-1 (file No. 333-163250), as amended, initially filed with the Securities and Exchange Commission on November 20, 2009) |
| | |
| |
4.7 | | Side Letter dated June 2, 2008 to Preference A Shares Purchase Agreement dated December 20, 2007 among the Registrant, the Management Shareholder and the Purchasers, each as defined therein (incorporated by reference to Exhibit 10.7 of our Registration Statement on Form F-1 (file No. 333-163250), as amended, initially filed with the Securities and Exchange Commission on November 20, 2009) |
| |
4.8 | | Voting Agreement dated December 20, 2007 among the Registrant, Anglo China Bio-Technology Investment Holdings Limited and Britain Ukan Technology Investment Holdings (Group) Limited, the Management Shareholders and the Preference A Holders, each as defined therein (incorporated by reference to Exhibit 10.8 of our Registration Statement on Form F-1 (file No. 333-163250), as amended, initially filed with the Securities and Exchange Commission on November 20, 2009) |
| |
4.9 | | Right of First Refusal and Co-Sale Agreement dated December 20, 2007 among the Registrant, Anglo China Bio-Technology Investment Holdings Limited and Britain Ukan Technology Investment Holdings (Group) Limited, the Management Shareholders and the Preference A Holders, each as defined therein (incorporated by reference to Exhibit 10.9 of our Registration Statement on Form F-1 (file No. 333-163250), as amended, initially filed with the Securities and Exchange Commission on November 20, 2009) |
| |
4.10 | | Repurchase Agreement dated December 20, 2007 between the Registrant and Anglo China Bio-Technology Investment Holdings Limited (incorporated by reference to Exhibit 10.10 of our Registration Statement on Form F-1 (file No. 333-163250), as amended, initially filed with the Securities and Exchange Commission on November 20, 2009) |
| |
4.11 | | English translation of Equity Pledge Agreement dated December 14, 2007 among Penglai Nuokang Pharmaceutical Co., Ltd., Liaoning Nuokang Medicines Co., Ltd. and the shareholder of Liaoning Nuokang Medicines Co., Ltd. (incorporated by reference to Exhibit 10.11 of our Registration Statement on Form F-1 (file No. 333-163250), as amended, initially filed with the Securities and Exchange Commission on November 20, 2009) |
| |
4.12 | | English translation of Exclusive Call Option Agreement dated December 14, 2007 among Penglai Nuokang Pharmaceutical Co., Ltd., Liaoning Nuokang Medicines Co., Ltd. and the shareholder of Liaoning Nuokang Medicines Co., Ltd. (incorporated by reference to Exhibit 10.12 of our Registration Statement on Form F-1 (file No. 333-163250), as amended, initially filed with the Securities and Exchange Commission on November 20, 2009) |
| |
4.13 | | English translation of Business Operation Agreement dated December 14, 2007 among Penglai Nuokang Pharmaceutical Co., Ltd., Liaoning Nuokang Medicines Co., Ltd. and the shareholder of Liaoning Nuokang Medicines Co., Ltd. (incorporated by reference to Exhibit 10.13 of our Registration Statement on Form F-1 (file No. 333-163250), as amended, initially filed with the Securities and Exchange Commission on November 20, 2009) |
| | |
| |
4.14 | | English translation of Power of Attorney dated December 14, 2007 from the shareholder of Liaoning Nuokang Medicines Co., Ltd. (incorporated by reference to Exhibit 10.14 of our Registration Statement on Form F-1 (file No. 333-163250), as amended, initially filed with the Securities and Exchange Commission on November 20, 2009) |
| |
4.15 | | English translation of Exclusive Technology Support and Management Consulting Service Agreement dated December 14, 2007 among Penglai Nuokang Pharmaceutical Co., Ltd. and Liaoning Nuokang Medicines Co., Ltd. (incorporated by reference to Exhibit 10.15 of our Registration Statement on Form F-1 (file No. 333-163250), as amended, initially filed with the Securities and Exchange Commission on November 20, 2009) |
| |
4.16 | | English translation of Real Estate Lease Contract dated August 22, 2006 between Penglai Nuokang Pharmaceutical Co., Ltd. and Shandong Penglai Pharmaceutical Plant (incorporated by reference to Exhibit 10.16 of our Registration Statement on Form F-1 (file No. 333-163250), as amended, initially filed with the Securities and Exchange Commission on November 20, 2009) |
| |
4.17 | | English translation of Land Use Right Grant Contract dated September 7, 2005 between the Hunnan New District branch bureau of Shenyang City Planning and National Land Resources Bureau and Liaoning Nuokang Bio-pharmaceutical Co., Ltd. (incorporated by reference to Exhibit 10.17 of our Registration Statement on Form F-1 (file No. 333-163250), as amended, initially filed with the Securities and Exchange Commission on November 20, 2009) |
| |
4.18** | | Contract dated August 1, 2008 among Shenyang Nanhu Import and Export Co., Ltd., Liaoning Nuokang Bio-pharmaceutical Co., Ltd., Procamp Coml Import Export Ltda. and Ceta-Centro de Extraçåo de Toxinas Animais Ltda. (incorporated by reference to Exhibit 10.18 of our Registration Statement on Form F-1 (file No. 333-163250), as amended, initially filed with the Securities and Exchange Commission on November 20, 2009) |
| |
4.19** | | English translation of Exclusive Regional Distribution Agreement dated December 28, 2008 between Liaoning Nuokang Medicines Co., Ltd. and Jilin Yuhua Pharmaceutical Co., Ltd., and its Supplemental Agreement dated June 28, 2009 (incorporated by reference to Exhibit 10.19 of our Registration Statement on Form F-1 (file No. 333-163250), as amended, initially filed with the Securities and Exchange Commission on November 20, 2009) |
| |
4.20 | | Subscription Agreement dated July 10, 2009 between Venomics Hong Kong Limited, Liaoning Nuokang Medicines Co., Ltd., Venomics Pty Limited and QRxPharma Limited (incorporated by reference to Exhibit 10.20 of our Registration Statement on Form F-1 (file No. 333-163250), as amended, initially filed with the Securities and Exchange Commission on November 20, 2009) |
| | |
| |
4.21* | | English translation of Second Supplemental Agreement dated December 28, 2009 to Exclusive Regional Distribution Agreement dated December 28, 2008 between Liaoning Nuokang Medicines Co., Ltd. and Jilin Yuhua Pharmaceutical Co., Ltd. |
| |
4.22* | | English translation of Third Supplemental Agreement dated April 30, 2010 to Exclusive Regional Distribution Agreement dated December 28, 2008 between Liaoning Nuokang Medicines Co., Ltd. and Jilin Yuhua Pharmaceutical Co., Ltd. |
| |
8.1 | | Subsidiaries of the Registrant (incorporated by reference to Exhibit 21.1 of our Registration Statement on Form F-1 (file No. 333-163250), as amended, initially filed with the Securities and Exchange Commission on November 20, 2009) |
| |
11.1 | | Code of Business Conduct and Ethics of the Registrant (incorporated by reference to Exhibit 99.1 of our Registration Statement on Form F-1 (file No. 333-163250), as amended, initially filed with the Securities and Exchange Commission on November 20, 2009) |
| |
12.1* | | CEO Certification Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| |
12.2* | | CFO Certification Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| |
13.1* | | CEO Certification Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
| |
13.2* | | CFO Certification Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
| |
15.1* | | Consent of Tian Yuan Law Firm |
| |
15.2* | | Consent of Ernst & Young Hua Ming, Independent Registered Public Accounting Firm |
| * | Filed with this annual report on Form 20-F. |
| ** | Confidential treatment has been granted with respect to the redacted portions of this exhibit. |
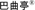 ” (Baquting) and “
” (Baquting) and “ ” (Danya) that are owned by Penglai Nuokang, all other trademarks we are currently using are owned by Nuokang Distribution. We received long term licenses from Nuokang Distribution to use these trademarks. We may need additional licenses from Nuokang Distribution in the future if we plan to use other trademarks it owns. As a result of the conflict of interest between Nuokang Distribution and us, the terms of such licenses may not be favorable to us.
” (Danya) that are owned by Penglai Nuokang, all other trademarks we are currently using are owned by Nuokang Distribution. We received long term licenses from Nuokang Distribution to use these trademarks. We may need additional licenses from Nuokang Distribution in the future if we plan to use other trademarks it owns. As a result of the conflict of interest between Nuokang Distribution and us, the terms of such licenses may not be favorable to us. , our flagship bleeding control product, (ii) Aiduo
, our flagship bleeding control product, (ii) Aiduo , our cardiovascular stress imaging agent and (iii) Aiwen
, our cardiovascular stress imaging agent and (iii) Aiwen , our anti-arrhythmic agent. We have also entered into an exclusive agreement for the marketing and distribution in China of Kaitong
, our anti-arrhythmic agent. We have also entered into an exclusive agreement for the marketing and distribution in China of Kaitong , an intravenous injectable lipid emulsion of vasodilator alprostadil for the treatment of peripheral vascular diseases, cardiocerebral microcirculation disorders and post-surgery thrombosis. Our product pipeline includes four product candidates under development to address growing market and medical needs for bleeding control and hematological, cardiovascular and cerebrovascular disease diagnosis, treatment and prevention.
, an intravenous injectable lipid emulsion of vasodilator alprostadil for the treatment of peripheral vascular diseases, cardiocerebral microcirculation disorders and post-surgery thrombosis. Our product pipeline includes four product candidates under development to address growing market and medical needs for bleeding control and hematological, cardiovascular and cerebrovascular disease diagnosis, treatment and prevention. brand name and our well-known branded pharmaceutical, Baquting, enable our sales and marketing team to effectively promote our principal products and help to stimulate the sales of our other pharmaceuticals. We employ a physician-targeted marketing model that is focused on promoting our products by providing physicians and hospitals with information on the benefits and differentiating clinical aspects of our products. Our sales and marketing activities receive strong academic support from a specialist network comprising approximately 100 national key opinion leaders and physicians in various clinical areas, and over 100 peer-reviewed academic articles have been published in recognized academic magazines or periodicals regarding the use of our principal products. As of March 31, 2010, our distribution network included approximately 200 distributors.
brand name and our well-known branded pharmaceutical, Baquting, enable our sales and marketing team to effectively promote our principal products and help to stimulate the sales of our other pharmaceuticals. We employ a physician-targeted marketing model that is focused on promoting our products by providing physicians and hospitals with information on the benefits and differentiating clinical aspects of our products. Our sales and marketing activities receive strong academic support from a specialist network comprising approximately 100 national key opinion leaders and physicians in various clinical areas, and over 100 peer-reviewed academic articles have been published in recognized academic magazines or periodicals regarding the use of our principal products. As of March 31, 2010, our distribution network included approximately 200 distributors.
 , our cardiovascular stress imaging agent, is administered at 30ml/90mg per dose, and Aiwen
, our cardiovascular stress imaging agent, is administered at 30ml/90mg per dose, and Aiwen , our product used in the diagnosis and treatment of PSVT, is administered at 2ml/6mg per dose. We are also developing an adenosine product for myocardial protection.
, our product used in the diagnosis and treatment of PSVT, is administered at 2ml/6mg per dose. We are also developing an adenosine product for myocardial protection.
 (Baquting),
(Baquting), (Aiduo) and
(Aiduo) and (Aiwen) in connection with our marketed products with expiration dates ranging from 2012 to 2019. On November 15, 2007, we received an objection raised during the publication period of our “Shunxin” trademark from Beijing Shunxin Agriculture Co., Ltd., or Beijing Shunxin, a PRC company, claiming that our “Shunxin” trademark under registration with the PRC Trademark Office imitated one of Beijing Shunxin’s trademarks used in its products. We filed our response with the PRC Trademark Office on December 13, 2007. On May 5, 2010, we received a letter from the PRC Trademark Office indicating that there was insufficient evidence supporting Beijing Shunxin’s claims and that if Beijing Shunxun does not request a second review of its objection, we will receive registration for our “Shunxin” trademark.
(Aiwen) in connection with our marketed products with expiration dates ranging from 2012 to 2019. On November 15, 2007, we received an objection raised during the publication period of our “Shunxin” trademark from Beijing Shunxin Agriculture Co., Ltd., or Beijing Shunxin, a PRC company, claiming that our “Shunxin” trademark under registration with the PRC Trademark Office imitated one of Beijing Shunxin’s trademarks used in its products. We filed our response with the PRC Trademark Office on December 13, 2007. On May 5, 2010, we received a letter from the PRC Trademark Office indicating that there was insufficient evidence supporting Beijing Shunxin’s claims and that if Beijing Shunxun does not request a second review of its objection, we will receive registration for our “Shunxin” trademark.
 , Aiduo
, Aiduo and Aiwen
and Aiwen . Our product pipeline includes four product candidates under development that seek to better address the growing market and medical needs for bleeding control and hematological, cardiovascular and cerebrovascular disease diagnosis, treatment and prevention.
. Our product pipeline includes four product candidates under development that seek to better address the growing market and medical needs for bleeding control and hematological, cardiovascular and cerebrovascular disease diagnosis, treatment and prevention. , a lipid emulsion alprostadil product candidate for the treatment of peripheral vascular diseases, cardiocerebral microcirculation disorders and post-surgery thrombosis, subject to the ability of Jilin Yuhua, the manufacturer of Kaitong, to receive a production permit from SFDA. We also plan to launch dipyridamole aspirin sustained release capsules once we receive SFDA manufacturing and marketing approval.
, a lipid emulsion alprostadil product candidate for the treatment of peripheral vascular diseases, cardiocerebral microcirculation disorders and post-surgery thrombosis, subject to the ability of Jilin Yuhua, the manufacturer of Kaitong, to receive a production permit from SFDA. We also plan to launch dipyridamole aspirin sustained release capsules once we receive SFDA manufacturing and marketing approval.