EXHIBIT 99.1

Heat Biologics NASDAQ: HTBX CORPORATE PRESENTATION May 2019

Forward Looking Statements This presentation includes statements that are, or may be deemed, ‘‘forward - looking statements’’ within the meaning of the Private Securities Litigation Reform Act of 1995 , as amended . In some cases, these forward - looking statements can be identified by the use of forward - looking terminology, including the terms “believes,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should,” “approximately” or, in each case, their negative or other variations thereon or comparable terminology, although not all forward - looking statements contain these words . They appear in a number of places throughout this presentation and include statements regarding our intentions, beliefs, projections, outlook, analyses or current expectations concerning, among other things, our ongoing and planned discovery and development of drugs targeting cancer, the strength and breadth of our intellectual property, our ongoing and planned preclinical studies and clinical trials, the timing of and our ability to complete clinical trials and make regulatory filings and obtain and maintain regulatory approvals for our product candidates, our ability to partner our product development, the degree of clinical utility of our products, particularly in specific patient populations, expectations regarding clinical trial data, our results of operations, financial condition, liquidity, prospects, growth and strategies, the length of time that we will be able to continue to fund our operating expenses and capital expenditures, our expected financing needs and sources of financing, the industry in which we operate and the trends that may affect the industry or us . By their nature, forward - looking statements involve risks and uncertainties because they relate to events, competitive dynamics, and healthcare, regulatory and scientific developments and depend on the economic circumstances that may or may not occur in the future or may occur on longer or shorter timelines than anticipated . Although we believe that we have a reasonable basis for each forward - looking statement contained in this presentation, we caution you that forward - looking statements are not guarantees of future performance and that our actual results of operations, financial condition and liquidity, and the development of the industry in which we operate may differ materially from the forward - looking statements contained in this presentation as a result of, among other factors, the factors referenced in the “Risk Factors” section of our Annual Report on Form 10 - K/A for the year ended December 31 , 2018 , our quarterly reports on Form 10 - Q for the subsequent quarters and our other subsequent filings with the Securities and Exchange Commission (collectively, our “SEC Filings”) . In addition, even if our results of operations, financial condition and liquidity, and the development of the industry in which we operate are consistent with the forward - looking statements contained in this presentation, they may not be predictive of results or developments in future periods . Any forward - looking statements that we make in this presentation speak only as of the date of such statement, and we undertake no obligation to update such statements to reflect events or circumstances after the date of this presentation, except as required by law . 2
Snapshot of Heat Biologics (Nasdaq: HTBX) HS - 110, an “off the shelf” cell - based immunotherapy product that has the potential to improve PD(L) - 1 based therapy ◦ Planning for registrational trial ◦ Ongoing Phase 2 program demonstrates signals of efficacy in two treatment settings ◦ Broad market potential in multiple oncology indications ◦ Fully allogeneic product with low COGs Promising pipeline based on T - cell activation and co - stimulation ◦ Two additional candidates with targeted IND filing in 2019 ◦ Product pipeline offers additive clinical benefit when combined with anti - PD - (L)1 and other immunotherapies Experienced management team with proven track record advancing oncology drugs to the market Seeking commercial partners for HS - 110 to realize platform potential US - based biopharmaceutical company developing a suite of immunotherapy products designed to “Turn Cold Tumors Hot” 3
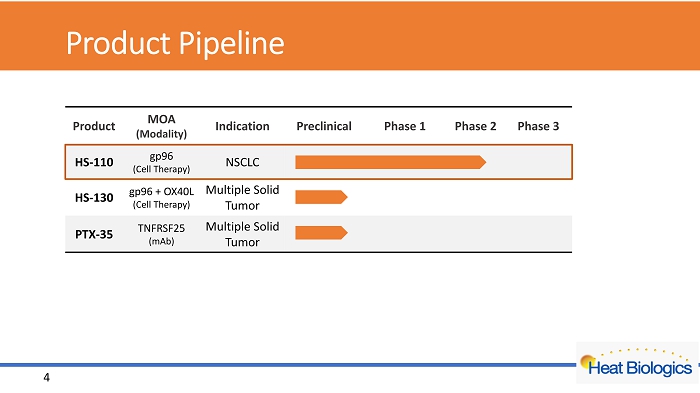
Product Pipeline Product MOA (Modality) Indication Preclinical Phase 1 Phase 2 Phase 3 HS - 110 gp96 (Cell Therapy) NSCLC HS - 130 gp96 + OX40L (Cell Therapy) Multiple Solid Tumor PTX - 35 TNFRSF25 (mAb) Multiple Solid Tumor 4
HS - 110 Overview • HS - 110 is a Phase 2 cell - based immunotherapy administered in combination with PD - (L)1 therapies to improve clinical outcomes for NSCLC patients ◦ Allogeneic cells with engineered gp96 to present 70+ different cancer testis antigens ◦ Selectively activate CD8+ “killer” T cells ◦ Enable infiltration of CD8+ T cells into the tumor microenvironment • PD - (L)1 is approved for multiple cancers but clinical benefit is not observed in majority of biomarker - unselected patients ◦ In unselected NSCLC patients treated in second line or greater, the response rate to anti - PD(L)1 antibodies is 13.6% to 23% ‡ The combination of HS - 110 and PD - (L)1 therapy may benefit patients that have progressed on prior PD - (L)1 therapy ‡ Shukuya & Carbone 2016. Journal of Thoracic Oncology, Vol.11 No.7: 976 - 988 5
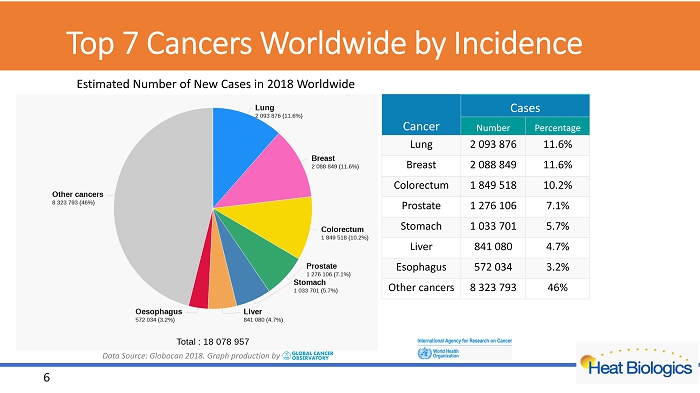
Top 7 Cancers W orldwide by Incidence 6 Data Source: Globocan 2018. Graph production by E stimated Number of New Cases in 2018 Worldwide Cancer Cases Number Percentage Lung 2 093 876 11.6% Breast 2 088 849 11.6% Colorectum 1 849 518 10.2% Prostate 1 276 106 7.1% Stomach 1 033 701 5.7% Liver 841 080 4.7% Esophagus 572 034 3.2% Other cancers 8 323 793 46%
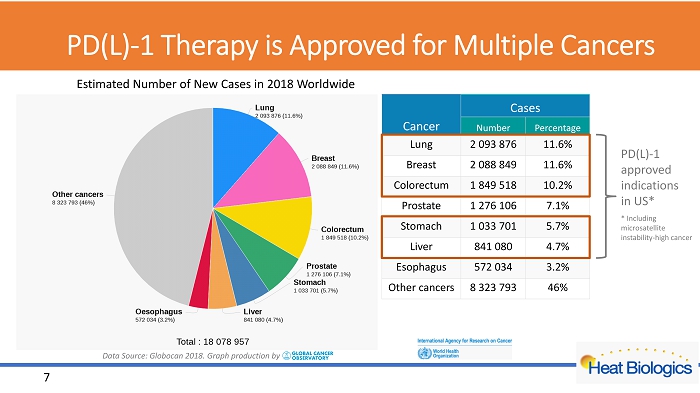
PD(L) - 1 Therapy is Approved for Multiple Cancers 7 Data Source: Globocan 2018. Graph production by Cancer Cases Number Percentage Lung 2 093 876 11.6% Breast 2 088 849 11.6% Colorectum 1 849 518 10.2% Prostate 1 276 106 7.1% Stomach 1 033 701 5.7% Liver 841 080 4.7% Esophagus 572 034 3.2% Other cancers 8 323 793 46% PD(L) - 1 approved indications in US* * Including microsatellite instability - high cancer E stimated Number of New Cases in 2018 Worldwide
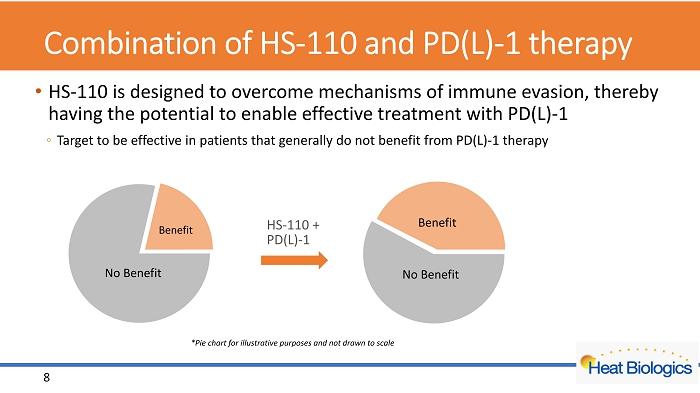
Combination of HS - 110 and PD(L) - 1 therapy • HS - 110 is designed to overcome mechanisms of immune evasion, thereby having the potential to enable effective treatment with PD(L) - 1 ◦ Target to be effective in patients that generally do not benefit from PD(L) - 1 therapy Cold Tumor HS - 110 + PD(L) - 1 No Benefit Benefit No Benefit Benefit 8 *Pie chart for illustrative purposes and not drawn to scale
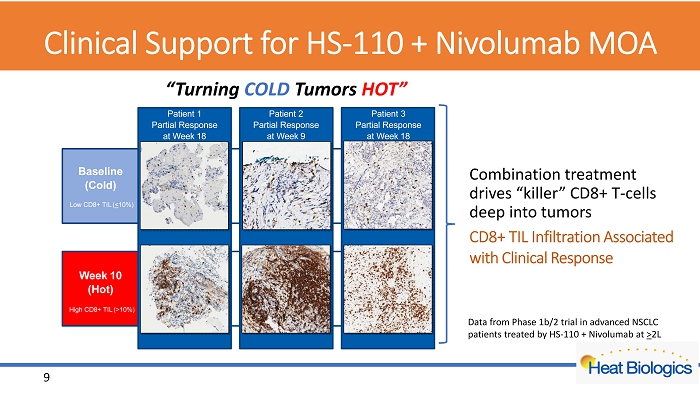
Clinical Support for HS - 110 + Nivolumab MOA CD8+ TIL Infiltration Associated with Clinical Response Combination treatment drives “killer” CD8+ T - cells deep into tumors Baseline (Cold) Week 10 (Hot) Patient 1 Partial Response at Week 18 High CD8+ TIL ( > 10%) Patient 2 Partial Response at Week 9 Patient 3 Partial Response at Week 18 Low CD8+ TIL ( < 10%) “Turning COLD Tumors HOT” 9 Data from Phase 1b/2 trial in advanced NSCLC patients treated by HS - 110 + Nivolumab at > 2L
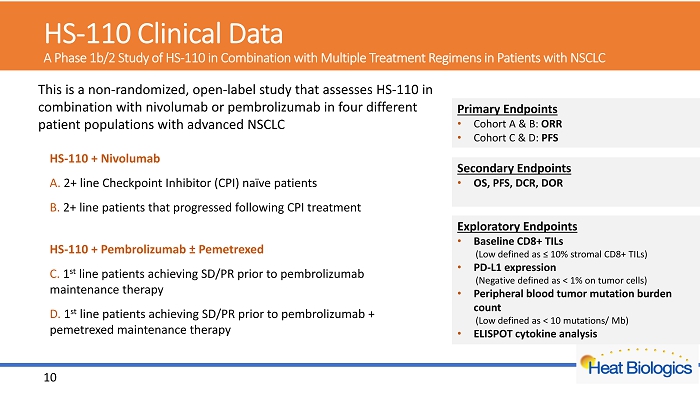
HS - 110 Clinical Data A Phase 1b/2 Study of HS - 110 in Combination with Multiple Treatment Regimens in Patients with NSCLC Exploratory Endpoints • Baseline CD8+ TILs (Low defined as ≤ 10% stromal CD8+ TILs) • PD - L1 expression (Negative defined as < 1% on tumor cells) • Peripheral blood tumor mutation burden count (Low defined as < 10 mutations/ Mb) • ELISPOT cytokine analysis Primary Endpoints • Cohort A & B: ORR • Cohort C & D: PFS Secondary Endpoints • OS, PFS, DCR, DOR 10 This is a non - randomized, open - label study that assesses HS - 110 in combination with nivolumab or pembrolizumab in four different patient populations with advanced NSCLC HS - 110 + Nivolumab A. 2+ line Checkpoint Inhibitor (CPI) naïve patients B. 2+ line patients that progressed following CPI treatment HS - 110 + Pembrolizumab ± Pemetrexed C. 1 st line patients achieving SD/PR prior to pembrolizumab maintenance therapy D. 1 st line patients achieving SD/PR prior to pembrolizumab + pemetrexed maintenance therapy
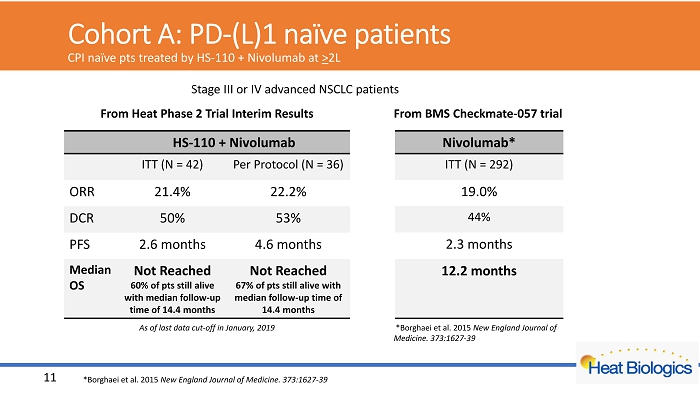
Cohort A: PD - (L)1 naïve patients CPI naïve pts treated by HS - 110 + Nivolumab at > 2L HS - 110 + Nivolumab ITT (N = 42) Per Protocol (N = 36) ORR 21.4% 22.2% DCR 50% 53% PFS 2.6 months 4.6 months Median OS Not Reached 60% of pts still alive with median follow - up time of 14.4 months Not Reached 67% of pts still alive with median follow - up time of 14.4 months *Borghaei et al. 2015 New England Journal of Medicine. 373:1627 - 39 11 Nivolumab* ITT (N = 292) 19.0% 44% 2.3 months 12.2 months From Heat Phase 2 Trial Interim Results From BMS Checkmate - 057 trial Stage III or IV advanced NSCLC patients *Borghaei et al. 2015 New England Journal of Medicine. 373:1627 - 39 As of last data cut - off in January, 2019
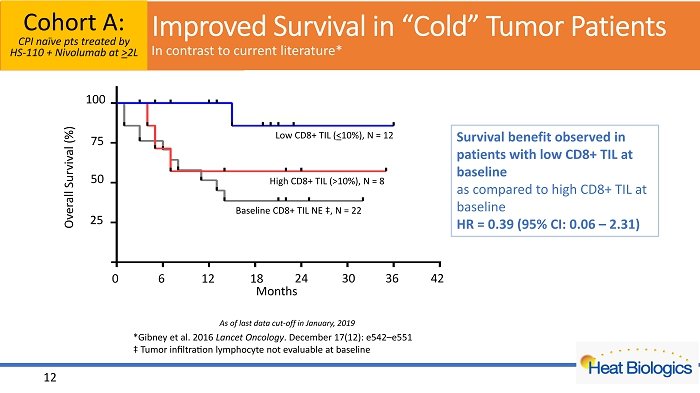
0 6 12 18 24 30 36 42 0 25 50 75 100 Months S u r v i v a l ( % ) CD8+ TIL (10%), n = 12 CD8+ TIL (>10%), n = 8 CD8+ TIL NE, n = 22 0 6 12 18 24 30 36 42 25 50 75 Overall Survival (%) 100 Months Low CD8+ TIL ( < 10%), N = 12 High CD8+ TIL (>10%), N = 8 Baseline CD8+ TIL NE ‡, N = 22 *Gibney et al. 2016 Lancet Oncology . December 17(12): e542 – e551 ‡ Tumor infiltration lymphocyte not evaluable at baseline 12 Cohort A: CPI naïve pts treated by HS - 110 + Nivolumab at > 2L As of last data cut - off in January, 2019 Survival benefit observed in patients with low CD8+ TIL at baseline as compared to high CD8+ TIL at baseline HR = 0.39 (95% CI: 0.06 – 2.31) Improved Survival in “Cold” Tumor Patients In contrast to current literature*
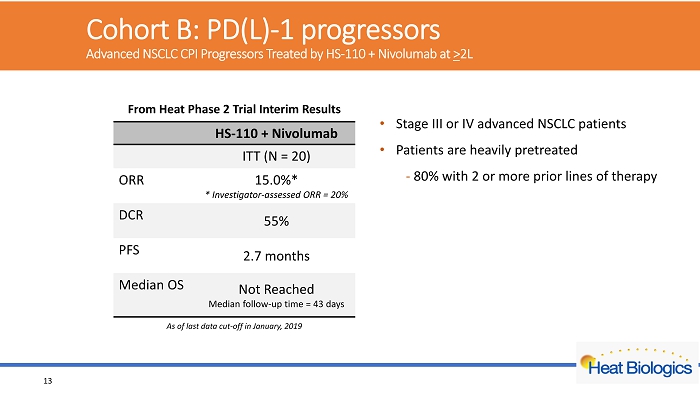
Cohort B: PD(L) - 1 progressors Advanced NSCLC CPI Progressors Treated by HS - 110 + Nivolumab at > 2L 13 HS - 110 + Nivolumab ITT (N = 20) ORR 15.0%* * Investigator - assessed ORR = 20% DCR 55% PFS 2.7 months Median OS Not Reached Median follow - up time = 43 days From Heat Phase 2 Trial Interim Results • Stage III or IV advanced NSCLC patients • Patients are heavily pretreated - 80% with 2 or more prior lines of therapy As of last data cut - off in January, 2019
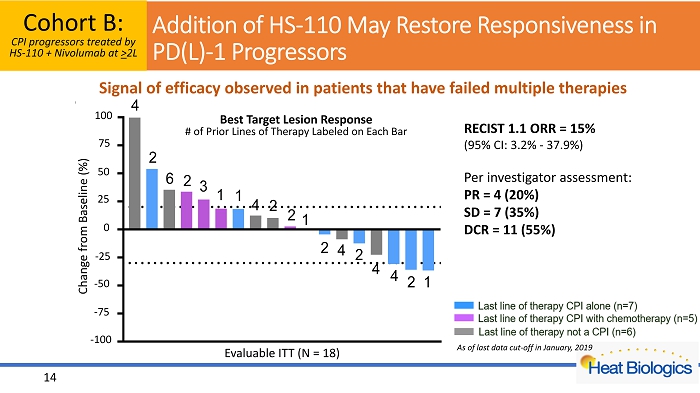
14 Addition of HS - 110 May Restore Responsiveness in PD(L) - 1 Progressors -100 -75 -50 -25 0 25 50 75 100 C h a n g e f r o m B a s e l i n e ( % ) 4 2 6 2 3 1 1 4 2 2 1 2 4 2 4 4 2 1 Best Target Lesion Response # of Prior Lines of Therapy Labeled on Each Bar 25 50 100 75 0 - 25 - 50 - 75 Change from Baseline (%) - 100 Evaluable ITT (N = 18) Signal of efficacy observed in patients that have failed multiple therapies Cohort B: CPI progressors treated by HS - 110 + Nivolumab at > 2L RECIST 1.1 ORR = 15% (95% CI: 3.2% - 37.9%) Per investigator assessment: PR = 4 (20%) SD = 7 (35%) DCR = 11 (55%) As of last data cut - off in January, 2019
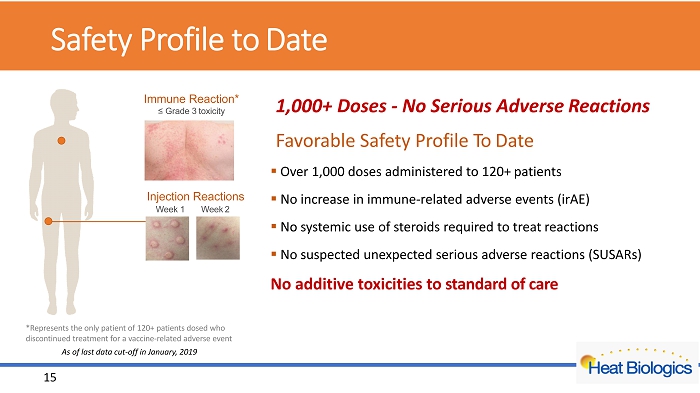
Safety Profile to Date 1,000+ Doses - No Serious Adverse Reactions Favorable Safety Profile To Date ▪ Over 1,000 doses administered to 120+ patients ▪ No increase in immune - related adverse events ( irAE ) ▪ No systemic use of steroids required to treat reactions ▪ No suspected unexpected serious adverse reactions (SUSARs) No additive toxicities to standard of care Immune Reaction* ≤ Grade 3 toxicity Injection Reactions Week 1 Week 2 15 *Represents the only patient of 12 0 + patients dosed who discontinued treatment for a vaccine - related adverse event As of last data cut - off in January, 2019
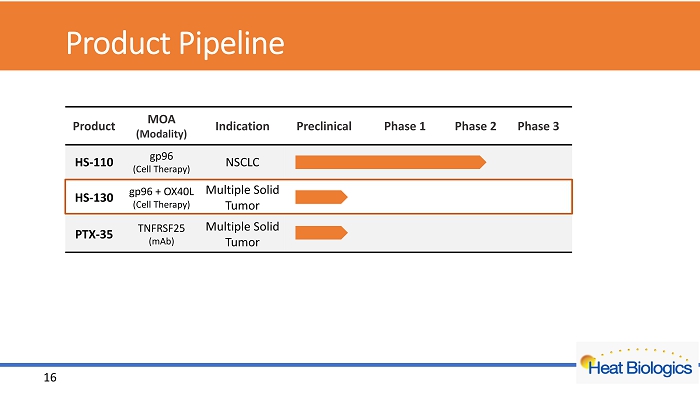
Product Pipeline Product MOA (Modality) Indication Preclinical Phase 1 Phase 2 Phase 3 HS - 110 gp96 (Cell Therapy) NSCLC HS - 130 gp96 + OX40L (Cell Therapy) Multiple Solid Tumor PTX - 35 TNFRSF25 (mAb) Multiple Solid Tumor 16

HS - 130 Overview HS - 130 is a “off - the - shelf” cell - based immunotherapy product ◦ Leverage HS - 110 clinical experience and manufacturing know - how ◦ Addition of OX40L to extend and expand T cell memory ◦ IND filing expected in 2019 Mechanism of Action offers broad market potential Heat Biologics has worldwide rights Partnership opportunities available 17

Product Pipeline Product MOA (Modality) Indication Preclinical Phase 1 Phase 2 Phase 3 HS - 110 gp96 (Cell Therapy) NSCLC HS - 130 gp96 + OX40L (Cell Therapy) Multiple Solid Tumor PTX - 35 TNFRSF25 (mAb) Multiple Solid Tumor 18
PTX - 35 Overview Potential first - in - class T cell co - stimulator targeting TNFRSF25, with preferential specificity to generate “memory” CD8+ T cells ◦ IND filing expected in 2019 Broad market potential ◦ Efficacy demonstrated in multiple preclinical in vivo colon, lung and breast cancer models Synergistic combination with immunotherapies including HS - 110 and CPIs Awarded a $15.2M grant to fund 70 pt. clinical trial Heat Biologics has worldwide rights Partnership opportunities available 19
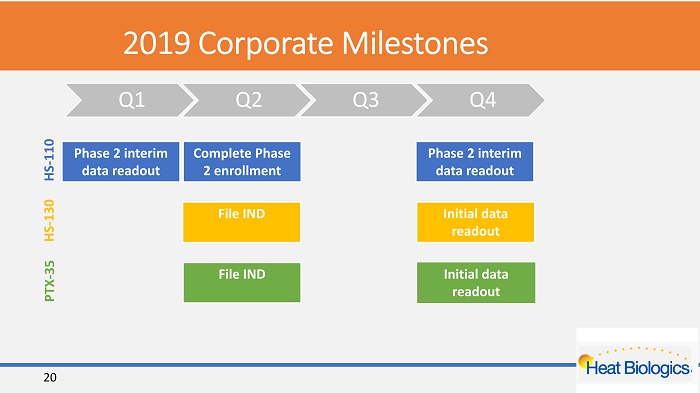
2019 Corporate Milestones Q1 Q2 Q3 Q4 Phase 2 interim data readout Complete Phase 2 enrollment Phase 2 interim data readout HS - 110 File IND Initial data readout PTX - 35 File IND Initial data readout HS - 130 20
Heat Biologics’ Value Proposition 21 Unique platform based on harnessing natural innate immunity with transformative capacity of “Turning Cold Tumors Hot” and restoring responsiveness to patients failing CPI therapy HS - 110, an “off the shelf” cell - based immunotherapy product that has the potential to improve PD(L) - 1 based therapy • Planning for registrational trial • Ongoing Phase 2 trials demonstrate signals of efficacy in two treatment settings • Broad market potential in multiple oncology indications • Fully allogeneic product with low COGs Experienced management team with proven track record in advancing multiple cancer drugs to the market Promising pipeline based on T - cell activation and co - stimulation • Two additional candidates with targeted IND filing in 2019 • Product pipeline offers additive clinical benefit when combined with anti - PD - (L)1 and other immunotherapies Seeking commercial partners for HS - 110 and HS - 130 to realize platform potential

Heat Biologics NASDAQ: HTBX APPENDIX
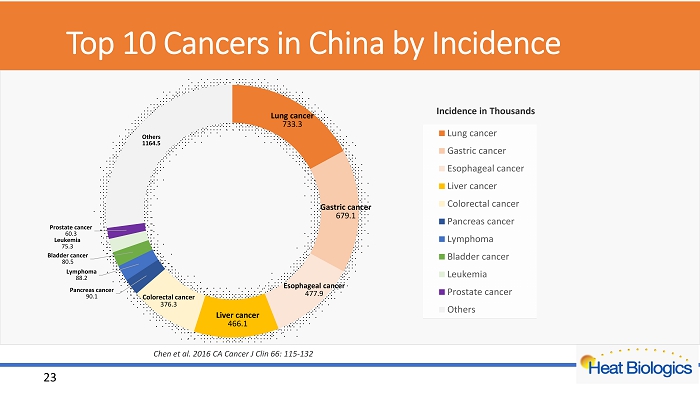
Top 10 Cancers in China by Incidence Lung cancer 733.3 Gastric cancer 679.1 Esophageal cancer 477.9 Liver cancer 466.1 Colorectal cancer 376.3 Pancreas cancer 90.1 Lymphoma 88.2 Bladder cancer 80.5 Leukemia 75.3 Prostate cancer 60.3 Others 1164.5 Lung cancer Gastric cancer Esophageal cancer Liver cancer Colorectal cancer Pancreas cancer Lymphoma Bladder cancer Leukemia Prostate cancer Others Incidence in Thousands 23 Chen et al. 2016 CA Cancer J Clin 66: 115 - 132
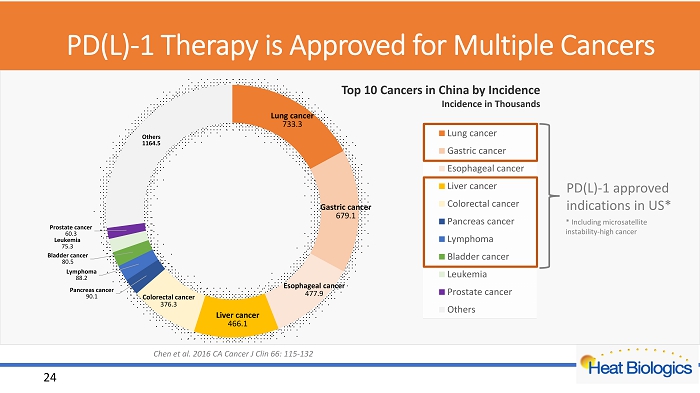
PD(L) - 1 Therapy is Approved for Multiple Cancers Lung cancer 733.3 Gastric cancer 679.1 Esophageal cancer 477.9 Liver cancer 466.1 Colorectal cancer 376.3 Pancreas cancer 90.1 Lymphoma 88.2 Bladder cancer 80.5 Leukemia 75.3 Prostate cancer 60.3 Others 1164.5 Lung cancer Gastric cancer Esophageal cancer Liver cancer Colorectal cancer Pancreas cancer Lymphoma Bladder cancer Leukemia Prostate cancer Others PD(L) - 1 approved indications in US* 24 Chen et al. 2016 CA Cancer J Clin 66: 115 - 132 Top 10 Cancers in China by Incidence Incidence in Thousands * Including microsatellite instability - high cancer
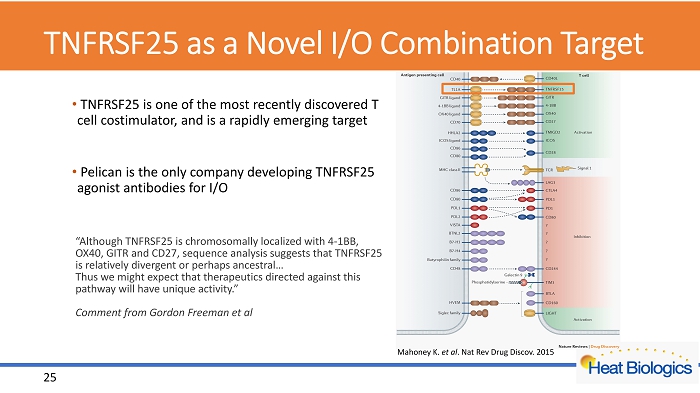
TNFRSF25 as a Novel I/O Combination Target • TNFRSF25 is one of the most recently discovered T cell costimulator , and is a rapidly emerging target • Pelican is the only company developing TNFRSF25 agonist antibodies for I/O Mahoney K. et al . Nat Rev Drug Discov . 2015 “Although TNFRSF25 is chromosomally localized with 4 - 1BB, OX40, GITR and CD27, sequence analysis suggests that TNFRSF25 is relatively divergent or perhaps ancestral… Thus we might expect that therapeutics directed against this pathway will have unique activity.” Comment from Gordon Freeman et al 25
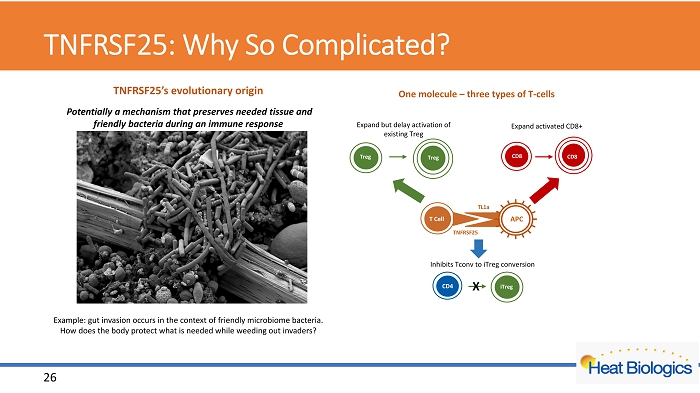
TNFRSF25: Why So Complicated? TNFRSF25’s evolutionary origin Potentially a mechanism that preserves needed tissue and friendly bacteria during an immune response Example: gut invasion occurs in the context of friendly microbiome bacteria. How does the body protect what is needed while weeding out invaders? T Cell APC TL1a TNFRSF25 CD8 CD8 Treg Treg Inhibits Tconv to iTreg conversion CD4 iTreg X One molecule – three types of T - cells Expand activated CD8+ Expand but delay activation of existing Treg 26
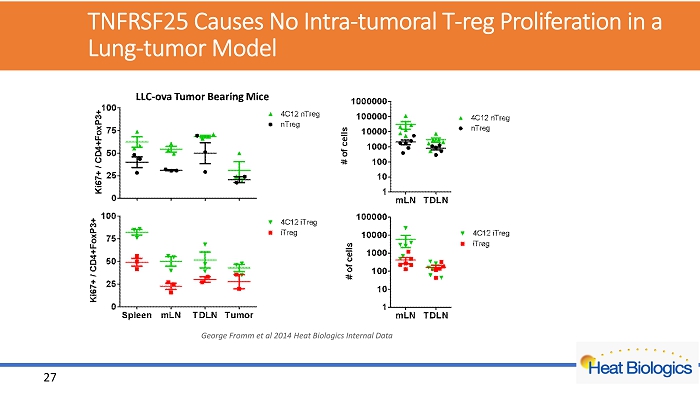
TNFRSF25 Causes No Intra - tumoral T - reg Proliferation in a Lung - tumor Model George Fromm et al 2014 Heat Biologics Internal Data 27
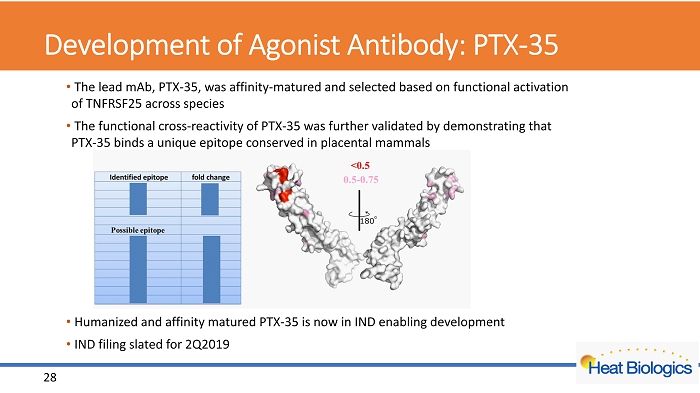
Development of Agonist Antibody: PTX - 35 • The lead mAb , PTX - 35, was affinity - matured and selected based on functional activation of TNFRSF25 across species • The functional cross - reactivity of PTX - 35 was further validated by demonstrating that PTX - 35 binds a unique epitope conserved in placental mammals • Humanized and affinity matured PTX - 35 is now in IND enabling development • IND filing slated for 2Q2019 28