EXHIBIT 99.3

Heat Biologics N asdaq : HTBX CORPORATE PRESENTATION MAY 2021 1

Forward Looking Statements This presentation includes statements that are, or may be deemed, ‘‘forward - looking statements’’ within the meaning of the Private Securities Litigation Reform Act of 1995 , as amended . In some cases, these forward - looking statements can be identified by the use of forward - looking terminology, including the terms “believes,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should,” “approximately” or, in each case, their negative or other variations thereon or comparable terminology, although not all forward - looking statements contain these words . They appear in a number of places throughout this presentation and include statements regarding our intentions, beliefs, projections, outlook, analyses or current expectations concerning, among other things, our ongoing and planned discovery and development of drugs targeting cancer, autoimmune diseases and infectious diseases , our planned discovery and development of a COVID - 19 vaccine, the strength and breadth of our intellectual property, our ongoing and planned preclinical studies and clinical trials, the timing of and our ability to complete clinical trials and make regulatory filings and obtain and maintain regulatory approvals for our product candidates, our ability to partner our product development, the degree of clinical utility of our products, particularly in specific patient populations, expectations regarding clinical trial data, our results of operations, financial condition, liquidity, prospects, growth and strategies, the length of time that we will be able to continue to fund our operating expenses and capital expenditures, our expected financing needs and sources of financing, the industry in which we operate and the trends that may affect the industry or us . By their nature, forward - looking statements involve risks and uncertainties because they relate to events, competitive dynamics, and healthcare, regulatory and scientific developments and depend on the economic circumstances that may or may not occur in the future or may occur on longer or shorter timelines than anticipated . Although we believe that we have a reasonable basis for each forward - looking statement contained in this presentation, we caution you that forward - looking statements are not guarantees of future performance and that our actual results of operations, financial condition and liquidity, and the development of the industry in which we operate may differ materially from the forward - looking statements contained in this presentation as a result of, among other factors, the factors referenced in the “Risk Factors” section of our Annual Report on Form 10 - K for the year ended December 31 , 2020 , our quarterly reports on Form 10 - Q for the subsequent quarters and our other subsequent filings with the Securities and Exchange Commission (collectively, our “SEC Filings”) . In addition, even if our results of operations, financial condition and liquidity, and the development of the industry in which we operate are consistent with the forward - looking statements contained in this presentation, they may not be predictive of results or developments in future periods . Any forward - looking statements that we make in this presentation speak only as of the date of such statement, and we undertake no obligation to update such statements to reflect events or circumstances after the date of this presentation, except as required by law . 2
Snapshot of Heat Biologics (Nasdaq: HTBX) Headquarter s : Morrisville, NC • F ocus on developing first - in - class immunotherapies • Diversified and differentiated clinical - stage product portfolio - Cell therapies using proprietary gp96 platform - Antibody - based therapeutics • Lead product HS - 110 has the potential to improve PD - (L)1 therapy - Clinical proof - of - concept achieved: positive survival data in two distinct treatment settings of NSCLC • Two additional oncology programs in Phase 1 trials • Solid balance sheet with $132M in cash and cash equivalents* 3 *as of March 31, 2021

P roduct Pipeline 4 CTA = cancer testis antigen NSCLC = Non - small cell lung cancer

H eat Biologics’ gp96 Platform Activating the Immune System • Function of heat shock protein gp96: - Potent mucosal adaptive memory inducer - Chaperones antigens (pathogens or tumor) to the immune system - Activates B cell response and drives antigen - specific CD4+ and CD8+ T cel l activation • platform • Leverages gp96’s role as a natural molecular warning system • Engineered to secrete viral antigens bound to gp96 • Off - the - shelf allogeneic cell vaccine • Feasible for large scale manufacturing • Amenable to stockpiling • Broad applications in infectious diseases and cancer • Lead product in Phase 2 trial for NSCLC 5

H eat Biologics’ gp96 Platform Activating the Immune System • Function of heat shock protein gp96: - Potent mucosal adaptive memory inducer - Chaperones antigens (pathogens or tumor) to the immune system - Activates B cell response and drives antigen - specific CD4+ and CD8+ T cel l activation • Key features of Heat’s gp96 platform - Leverages gp96’s role as a natural molecular warning system - Engineered to secrete antigens bound to gp96 - Off - the - shelf allogeneic cell vaccine - Feasible for large scale manufacturing - Amenable to stockpiling - Broad applications in infectious diseases and cancer • Lead product in Phase 2 trial for NSCLC 6

HS - 110 Overview • HS - 110 in Phase 2 has broad potential for providing multiple treatment options to NSCLC patients in combination with a PD - 1 inhibitor - HS - 110 is a first - in - class, off - the - shelf, allogeneic cell therapy designed to utilize gp96 for immune activation against multiple tumor testis antigens - Positive interim survival data has been demonstrated in two distinct treatment settings in previously treated PD - (L)1 naïve and PD - (L)1 progressor NSCLC patients - Plan to discuss Phase 3 registrational pathways with FDA as well as potential partners • Combination of HS - 110 and PD - (L)1 therapies may confer additional survival benefit • Heat Biologics has worldwide rights 7 References: Strbo et al 2013. Immunologic research; Strbo et al 2013. Journal of immunology; Yifei Wang et al. 2018. J Immunol; Heat Biologics Internal data; ‡ Shukuya & Carbone 2016. Journal of Thoracic Oncology, Vol.11 No.7: 976 - 988
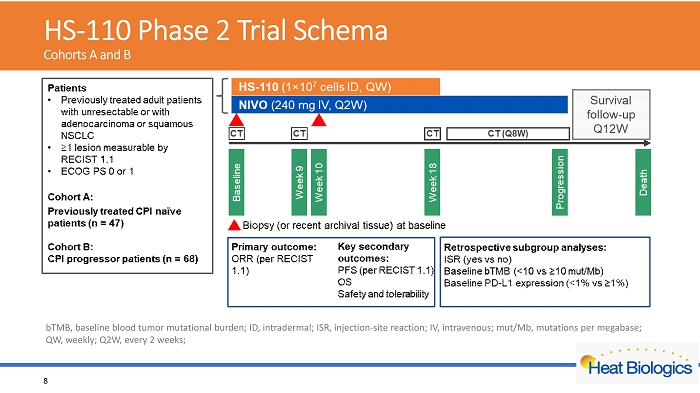
HS - 110 Phase 2 Trial Schema Cohorts A and B 8 bTMB , baseline blood tumor mutational burden; ID, intradermal; ISR, injection - site reaction; IV, intravenous; mut/Mb, mutations per megabase ; QW, weekly; Q2W, every 2 weeks;
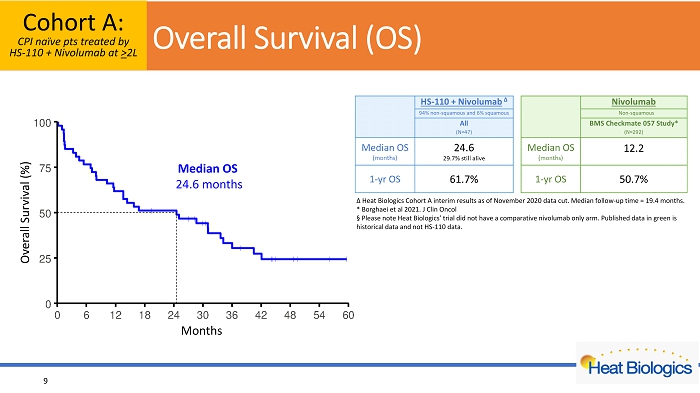
Overall Survival (%) Months Cohort A: CPI naïve pts treated by HS - 110 + Nivolumab at > 2L 9 Δ Heat Biologics Cohort A interim results as of November 2020 data cut. Median follow - up time = 19.4 months. * Borghaei et al 2021. J Clin Oncol Α Please note Heat Biologics’ trial did not have a comparative nivolumab only arm. Published data in green is historical data and not HS - 110 data. O verall Survival (OS) Median OS 24.6 months
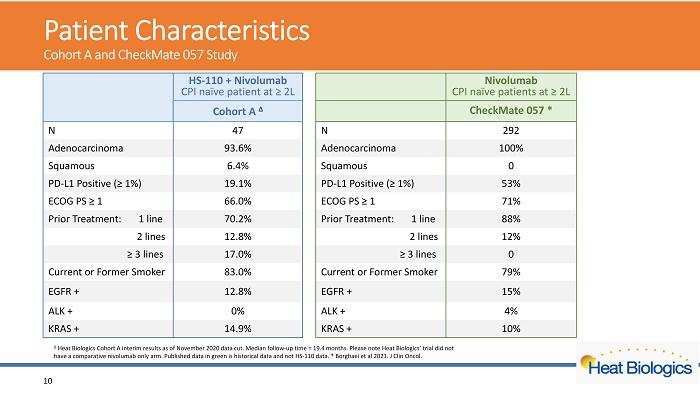
Patient Characteristics C ohort A and CheckMate 057 Study 10 HS - 110 + Nivolumab CPI naïve patient at ≥ 2L Nivolumab CPI naïve patients at ≥ 2L Cohort A Δ CheckMate 057 * N 47 N 292 Adenocarcinoma 93.6% Adenocarcinoma 100% Squamous 6.4% Squamous 0 PD - L1 Positive (≥ 1%) 19.1% PD - L1 Positive (≥ 1%) 53% ECOG PS ≥ 1 66.0% ECOG PS ≥ 1 71% Prior Treatment: 1 line 70.2% Prior Treatment: 1 line 88% 2 lines 12.8% 2 lines 12% ≥ 3 lines 17.0% ≥ 3 lines 0 Current or Former Smoker 83.0% Current or Former Smoker 79% EGFR + 12.8% EGFR + 15% ALK + 0% ALK + 4% KRAS + 14.9% KRAS + 10% Δ Heat Biologics Cohort A interim results as of November 2020 data cut. Median follow - up time = 19.4 months. Please note Heat Bio logics’ trial did not have a comparative nivolumab only arm. Published data in green is historical data and not HS - 110 data. * Borghaei et al 2021. J Clin Oncol.
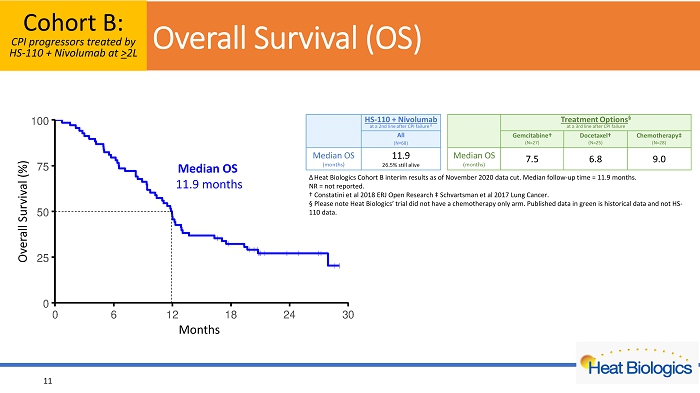
O verall Survival (OS) Cohort B: CPI progressors treated by HS - 110 + Nivolumab at > 2L 11 Overall Survival (%) Months Δ Heat Biologics Cohort B interim results as of November 2020 data cut . Median follow - up time = 11.9 months. NR = not reported. † C onstatini et al 2018 ERJ Open Research ‡ Schvartsman et al 2017 Lung Cancer. Α Please note Heat Biologics’ trial did not have a chemotherapy only arm. Published data in green is historical data and not HS - 110 data. Median OS 11.9 months
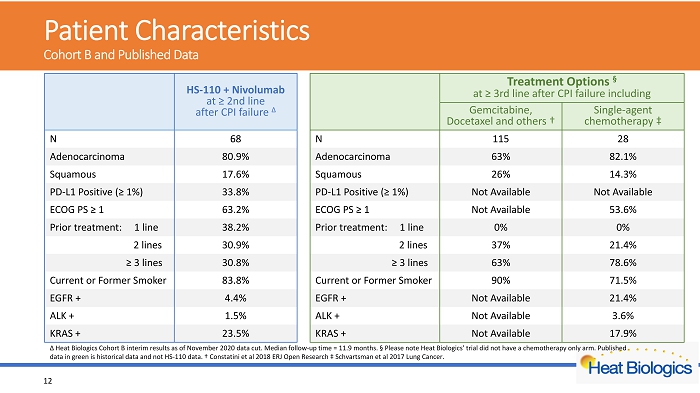
Patient Characteristics C ohort B and Published Data 12 Δ Heat Biologics Cohort B interim results as of November 2020 data cut. Median follow - up time = 11.9 months. § Please note Heat Biologics’ trial did not have a chemotherapy only arm. Published data in green is historical data and not HS - 110 data. † Constatini et al 2018 ERJ Open Research ‡ Schvartsman et al 2017 Lung Cancer. HS - 110 + Nivolumab at ≥ 2nd line after CPI failure Δ Treatment Options § at ≥ 3rd line after CPI failure including Gemcitabine, Docetaxel and others † Single - agent chemotherapy ‡ N 68 N 115 28 Adenocarcinoma 80.9% Adenocarcinoma 63% 82.1% Squamous 17.6% Squamous 26% 14.3% PD - L1 Positive (≥ 1%) 33.8% PD - L1 Positive (≥ 1%) Not Available Not Available ECOG PS ≥ 1 63.2% ECOG PS ≥ 1 Not Available 53.6% Prior treatment: 1 line 38.2% Prior treatment: 1 line 0% 0% 2 lines 30.9% 2 lines 37% 21.4% ≥ 3 lines 30.8% ≥ 3 lines 63% 78.6% Current or Former Smoker 83.8% Current or Former Smoker 90% 71.5% EGFR + 4.4% EGFR + Not Available 21.4% ALK + 1.5% ALK + Not Available 3.6% KRAS + 23.5% KRAS + Not Available 17.9%
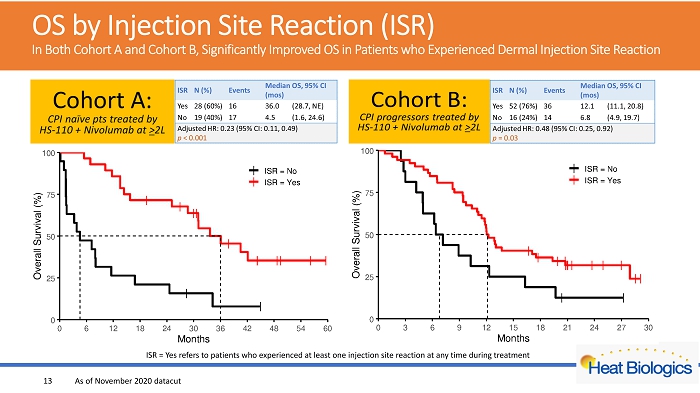
OS by Injection Site Reaction (ISR) In Both Cohort A and Cohort B, Significantly Improved OS in Patients who Experienced Dermal Injection Site Reaction ISR = Yes refers to patients who experienced at least one injection site reaction at any time during treatment 13 As of November 2020 datacut
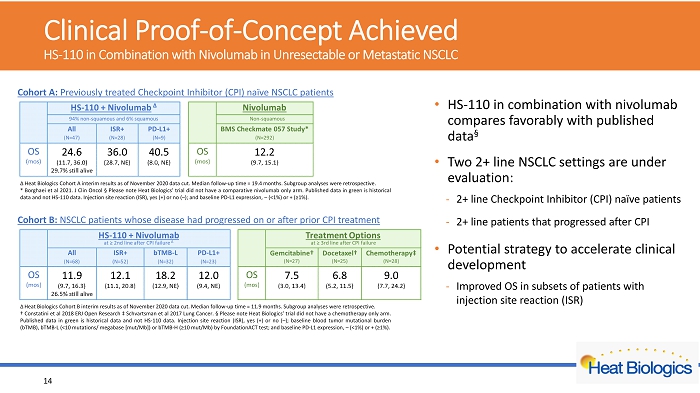
Cohort A: Previously treated Checkpoint Inhibitor (CPI) naïve NSCLC patients Cohort B: NSCLC p atients whose disease had progressed on or after prior CPI treatment Δ Heat Biologics Cohort A interim results as of November 2020 data cut . Median follow - up time = 19 . 4 months . Subgroup analyses were retrospective . * Borghaei et al 2021 . J Clin Oncol Α Please note Heat Biologics’ trial did not have a comparative nivolumab only arm . Published data in green is historical data and not HS - 110 data . Injection site reaction (ISR), yes (+) or no ( – ) ; and baseline PD - L 1 expression, – (< 1 % ) or + (≥ 1 % ) . Δ Heat Biologics Cohort B interim results as of November 2020 data cut . Median follow - up time = 11 . 9 months . Subgroup analyses were retrospective . † C onstatini et al 2018 ERJ Open Research ‡ Schvartsman et al 2017 Lung Cancer . Α Please note Heat Biologics’ trial did not have a chemotherapy only arm . Published data in green is historical data and not HS - 110 data . Injection site reaction (ISR), yes (+) or no ( – ) ; baseline blood tumor mutational burden ( bTMB ), bTMB - L (< 10 mutations/ megabase [mut/Mb]) or bTMB - H (≥ 10 mut/Mb) by FoundationACT test ; and baseline PD - L 1 expression, – (< 1 % ) or + (≥ 1 % ) . Clinical Proof - of - Concept Achieved HS - 110 in Combination with Nivolumab in Unresectable or Metastatic NSCLC • HS - 110 in combination with nivolumab compares favorably with published data Α • Two 2+ line NSCLC settings are under evaluation: - 2+ line Checkpoint Inhibitor (CPI) naïve patients - 2+ line patients that progressed after CPI • Potential strategy to accelerate clinical development - Improved OS in subsets of patients with injection site reaction (ISR) 14

HS - 130 Overview • HS - 130 is the first allogeneic, off - the - shelf, cell therapy approach utilizing OX40 - mediated co - stimulation to enhance activation of dormant immune signal - Leverage HS - 110 clinical experience and manufacturing know - how - Addition of OX40L fusion protein to extend and expand T cell memory • Mechanism of action offers broad market potential • Phase 1 in oncology currently enrolling • Heat Biologics has worldwide rights 15
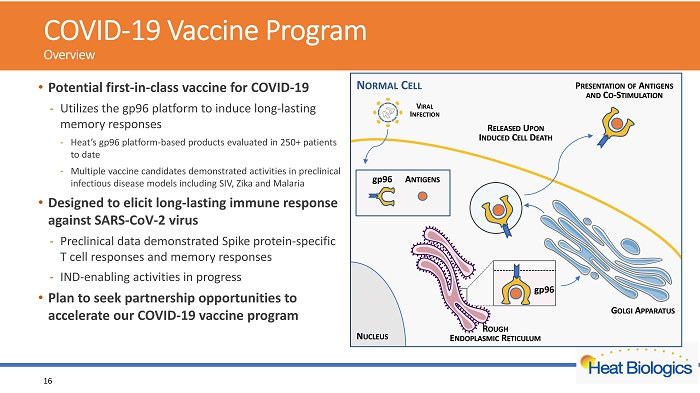
COVID - 19 Vaccine Program Overview • Potential first - in - class vaccine for COVID - 19 - Utilizes the gp96 platform to induce long - lasting memory responses - Heat’s gp96 platform - based products evaluated in 250+ patients to date - Multiple vaccine candidates demonstrated activities in preclinical infectious disease models including SIV, Zika and Malaria • Designed to elicit long - lasting immune response against SARS - CoV - 2 virus - Preclinical data demonstrated Spike protein - specific T cell responses and memory responses - IND - enabling activities in progress • Plan to seek partnership opportunities to accelerate our COVID - 19 vaccine program 16
PTX - 35 Overview • PTX - 35 offers a unique opportunity to modulate conventional or regulatory T - cells - Context driven depending on specific disease settings - Broad applications in cancer and autoimmunity • Potential first - in - class antibody targeting TNFRSF25 for oncology - Phase 1 trial in solid tumors currently enrolling - Anti - tumor activity demonstrated in multiple preclinical in vivo colon, lung and breast cancer models - Preclinical data demonstrated anti - tumor activities, expansion of antigen - specific CD8+ T cells and decreased Treg suppression in presence of tumor antigen - Awarded a $15.2M grant to fund Phase 1 clinical development • Worldwide rights licensed by Heat Biologics 17
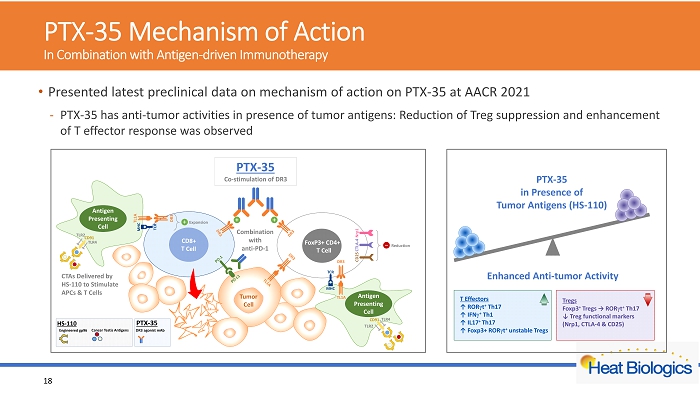
PTX - 35 Mechanism of Action In Combination with Antigen - driven Immunotherapy 18 • Presented latest preclinical data on mechanism of action on PTX - 35 at AACR 2021 - PTX - 35 has anti - tumor activities in presence of tumor antigens: Reduction of Treg suppression and enhancement of T effector response was observed

Management Team of Heat Biologics Successful Track Record in Advancing Multiple Products to the Market 19 Jeff Wolf, JD, MBA Founder & CEO George Peoples, MD Chief Medical Advisor Gary Vinson VP of CMC & Quality Cheni Kwok, PhD, CLP Senior Advisor of Business Development William Ostrander C hief Financial Officer Bill Arana, MS Exec Dir, Clinical Dev Anthony Manning, PhD Chief Scientific Advisor
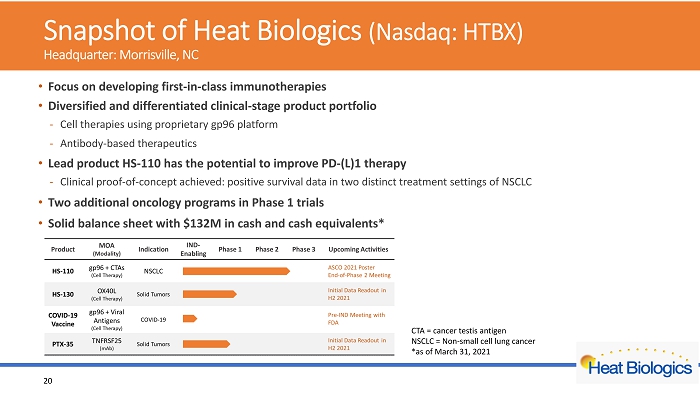
Snapshot of Heat Biologics (Nasdaq: HTBX) Headquarter: Morrisville, NC • F ocus on developing first - in - class immunotherapies • Diversified and differentiated clinical - stage product portfolio - Cell therapies using proprietary gp96 platform - Antibody - based therapeutics • Lead product HS - 110 has the potential to improve PD - (L)1 therapy - Clinical proof - of - concept achieved: positive survival data in two distinct treatment settings of NSCLC • Two additional oncology programs in Phase 1 trials • Solid balance sheet with $132M in cash and cash equivalents* 20 CTA = cancer testis antigen NSCLC = Non - small cell lung cancer *as of March 31, 2021



















