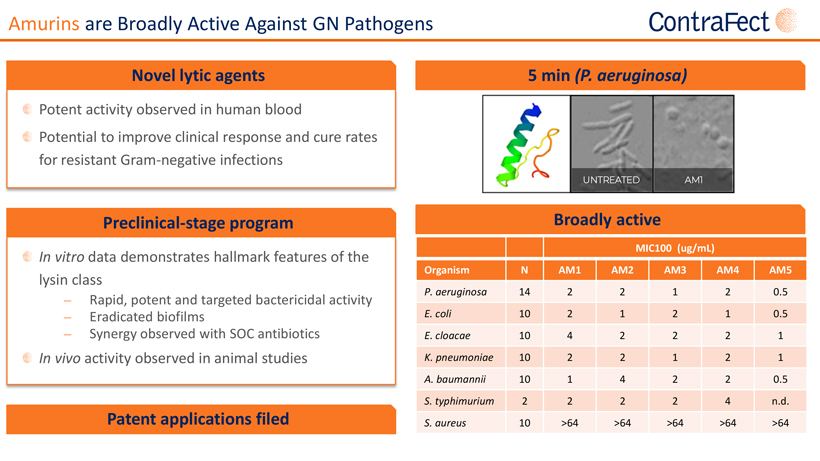
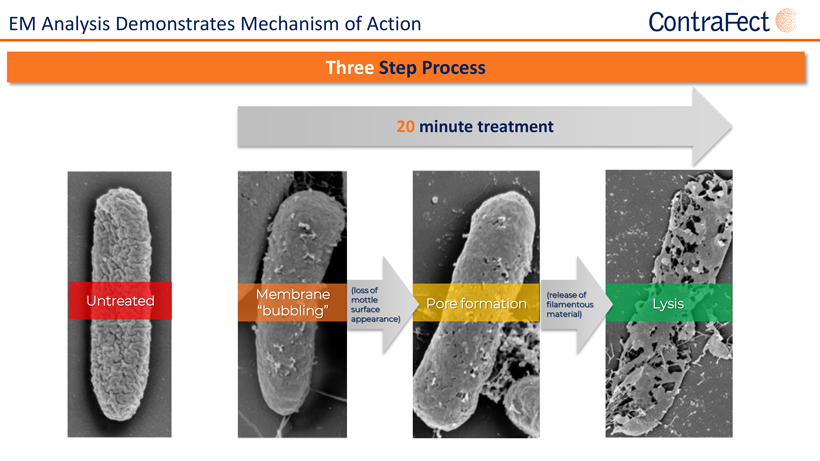
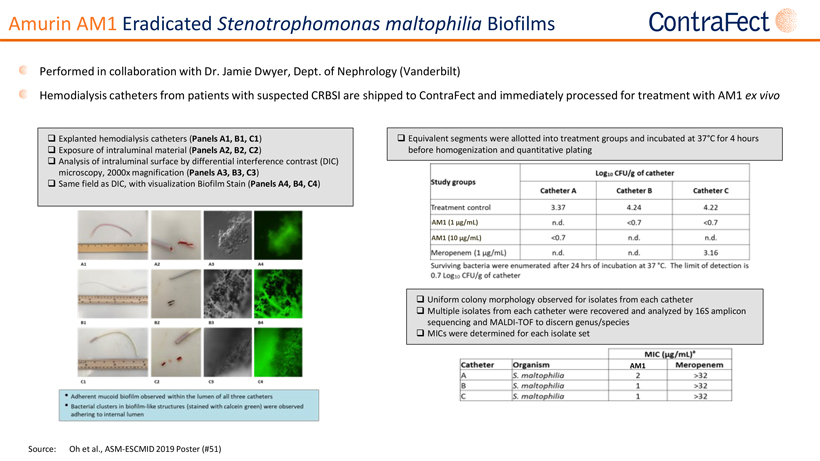
Forward Looking Statements This presentationcontains,andourofficers andrepresentatives mayfrom time to timemake,“forward-lookingstatements”withinthe meaning of the U.S. federal securities laws. Forward-looking statements can be identified by words such as “projects,” “may,” “will,” “could,” “would,” “should,” “believe,” “expect,” “target”, “anticipate,” “estimate,” “intend,” “plan,” “proposed”, “potential” or similar references to future periods. Examples of forward-looking statements in this presentation include, without limitation, statements made regarding ContraFect Corporation’s (“ContraFect”) ability to develop DLAs as new medical modalities for the treatment of life-threatening, antibiotic-resistant infections, cited information, ContraFect’s End-of Phase 2 meeting with the FDA, Phase 3 plans, designs and timing, Phase 2 study results, data analyses and comparisons, health economic data, safety and efficacy of exebacase, exebacase’s value proposition, patent protection, commercial assessments, in vitro and in vivo study results, ContraFect’s plans regarding its next IND and extrapolated data. Forward-looking statements are statements that are not historical facts, nor assurances of future performance. Instead, they are based on ContraFect’s current beliefs, expectations and assumptions regarding the future of its business, future plans, proposals, strategies, projections, anticipated events and trends, the economy and other future conditions. Because forward-looking statements relate to the future, they are subject to inherent risks,uncertaintiesandchangesincircumstancesthataredifficulttopredictandmanyofwhicharebeyondContraFect’scontrol,includingthose detailedinContraFect’sQuarterlyReportonForm 10-Q forthe periodendedMarch31,2022,andotherfilings withthe Securities andExchange Commission(“SEC”). Actualresultsmaydifferfromthosesetforthintheforward-lookingstatements. Importantfactorsthatcouldcauseactual results to differ include, among others, the occurrence of any adverse events related to the discovery, development and commercialization of ContraFect’s product candidates such as unfavorable clinical trial results, insufficient supplies of drug products, the lack of regulatory approval, or the unsuccessful attainment or maintenance of patent protection. Any forward-looking statement made by ContraFect in this presentation is based only on information currently available and speaks only as of the date on which it is made. No representation or warranty is made as to the completeness or accuracy of the information provided in this presentation. Except as required by applicable law, ContraFect expressly disclaims any obligations to publicly update any forward-looking statements, whether written or oral, that may be made from time to time, whether as a result of new information, future developments or otherwise. Audiences are cautioned that forward-looking statements or similar information are not guarantees of future performance and, accordingly, are expressly cautioned not to put undue reliance on forward-looking statementsorsimilarinformationduetotheinherentuncertaintytherein. ©2022 ContraFect Corporation