
Exhibit 99.1 Corporate Presentation December 2022 NASDAQ: CFRX

Forward Looking Statements/Disclaimer This presentation contains, and our officers and representatives may from time to time make, “forward-looking statements” within the meaning of the U.S. federal securities laws. Forward-looking statements can be identified by words such as “projects,” “may,” “will,” “could,” “would,” “should,” “believe,” “expect,” “target”, “anticipate,” “estimate,” “intend,” “plan,” “proposed”, “potential” or similar references to future periods. Examples of forward-looking statements in this presentation include, without limitation, statements made regarding ContraFect Corporation’s (“ContraFect”) ability to develop DLAs as new medical modalities for the treatment of life-threatening, antibiotic- resistant infections, cited information, clinical trial and pre-clinical study results, data analyses and comparisons, health economic data, safety and efficacy of exebacase, exebacase’s value proposition, patent protection, commercial assessments, in vitro and in vivo study results, ContraFect’s plans regarding its next IND and extrapolated data. Forward-looking statements are statements that are not historical facts, nor assurances of future performance. Instead, they are based on ContraFect’s current beliefs, expectations and assumptions regarding the future of its business, future plans, proposals, strategies, projections, anticipated events and trends, the economy and other future conditions. Because forward-looking statements relate to the future, they are subject to inherent risks, uncertainties and changes in circumstances that are difficult to predict and many of which are beyond ContraFect’s control, including ContraFect's need for substantial additional funding, the risks related to the COVID-19 pandemic, the occurrence of any adverse events related to the discovery, development and commercialization of the Company’s product candidates such as unfavorable clinical trial results, changes in management, insufficient supplies of drug products, or lack of regulatory approval, risks related to ContraFect's BARDA contract, the unsuccessful attainment or maintenance of patent protection, ContraFect's ability to remain listed on the Nasdaq Stock Exchange or another exchange, litigation risks and other important risks detailed in ContraFect’s Quarterly Report on Form 10-Q for the period ended September 30, 2022, and other filings with the Securities and Exchange Commission (“SEC”). Actual results may differ from those set forth in the forward-looking statements. Any forward-looking statement made by ContraFect in this presentation is based only on information currently available and speaks only as of the date on which it is made. No representation or warranty is made as to the completeness or accuracy of the information provided in this presentation. Except as required by applicable law, ContraFect expressly disclaims any obligations to publicly update any forward-looking statements, whether written or oral, that may be made from time to time, whether as a result of new information, future developments or otherwise. Audiences are cautioned that forward-looking statements or similar information are not guarantees of future performance and, accordingly, are expressly cautioned not to put undue reliance on forward-looking statements or similar information due to the inherent uncertainty therein. ©2022 ContraFect Corporation

The Burden of Bacterial Antimicrobial Resistance Deaths by Resistant Pathogen There remains an urgent Deaths by need for alternatives that will Infection Type circumvent bacterial resistance to current medicines Antibacterial innovation stems from small, emerging biotechnology companies Sources:(1) The State of Innovation in Antibacterial Therapeutics, Bio, February 2022, excluding agents for C. difficile (2) Global burden of bacterial antimicrobial resistance in 2019:a systematic analysis, Lancet, January 2022

About ContraFect ContraFect is a clinical-stage biotechnology company leading the development of direct lytic agents (DLAs), which include lysins and amurin peptides, as new medical modalities for the treatment of life-threatening and antibiotic-resistant infections

Senior Leadership Roger Pomerantz, MD, FACP – CEO Michael Messinger– CFO Garrett Nichols – Interim CMO Natalie Bogdanos – General Counsel Ray Schuch– VP of Research Gary Woodnutt – SVP of Translational 5

Board of Directors Roger Pomerantz, MD, FACP – Chair Lishan Aklog, MD David Low Former Chairman and CEO, Investment Banker at MTS CEO of Seres PAVmed Formerly at Lazard, JP Morgan, Lehman Sol Barer, PhD – Lead Independent Jane F. Barlow, MD, MPH Michael Otto, PhD EVP & CCO, Real Endpoints Former Chairman and Former CSO, Former Associate CMO, CVS Health CEO of Celgene Pharmasset Former VP, Clinical Innovation, Medco Cary Sucoff Steven C. Gilman, PhD – Vice Chair President, Former CEO, ContraFect Equity Source Partners Former EVP, R&D and CSO, Cubist 6

Medical Paradigm Shifts in Standard-of-Care PRIOR TREATMENT NEW TREATMENT Cancer Immunotherapies Chemotherapy No treatments Gene therapies Congenital Disorders available Potential Therapies Superiority Mortality benefits Microbiome Anti-Infectives Positive health Direct lytic agents economics Antibiotics 7

Portfolio LEADING THE DEVELOPMENT OF DLAs AS NEW MEDICAL MODALITIES Program Discovery Preclinical Phase 1 Phase 2 Phase 3 Prosthetic joint infections (PJI) CTA approved* Staphylococcus aureus Exebacase Staphylococcus epidermidis Staph aureus bacteremia, including endocarditis Stopped Gram-negative pathogens Pseudomonas aeruginosa CF-370 Klebsiella pneumoniae Acinetobacter baumannii Burkholderia cepacia Yersinia pestis BioDefense Serratia marcescens 8 *CTA approved by ANSM (France)

Novel, First-In-Class, Direct Lytic Agent for Invasive Staphylococcal Infections, Exebacase (CF-301) Including S. aureus and S. epidermidis

Exebacase: Potential First-in-Class Lysin Candidate Key attributes Clinical-stage program FDA designations for Fast Track and Breakthrough 26 kDa bacterial cell wall hydrolase enzyme (1)(2) Therapy Highly potent in vitro and in vivo against S. aureus Eligible for Streamlined Development for antibacterial and S. epidermidis, unique lysis and eradication of therapies Staph biofilms Expect to initiate dosing in Q1 2023 of Phase 1b/2 Potent synergy with broad range of anti- study of intra-articular exebacase in patients with staphylococcal antibiotics in pre-clinical studies chronic PJI, due to S. aureus or S. epidermidis Grounded in positive data from 15 compassionate use Broad patent protection cases Growing incidence in aging population Patent with composition of matter claims expiring in 2032 Disease of high economic burden and unmet need Patents with method claims for killing all Staph strains expiring in 2032 and method claims for Notes: (1) Fast Track designation received for the treatment of bacteremia, including endocarditis, due to S. aureus treatment of Staph strain biofilms expiring in 2033 (2) Breakthrough Therapy designation received for the treatment of S. aureus bloodstream infections, including right-sided infective endocarditis, when used in addition to SOC anti-staphylococcal antibiotics in adult patients. 10

Exebacase: Interim Analysis of the Phase 3 DISRUPT Study Superiority design study Preliminary findings of internal analysis Randomized, double-blind, placebo-controlled, conducted Unexpectedly high SOCA alone response rate in the US only, in patients with S. aureus bacteremia, Exebacase Phase 2 SOCA alone: 31.3% including right-sided endocarditis Historical Phase 3 SOCA alone comparisons: Comparison of efficacy of single IV dose of exebacase plus Fosfomycin Phase 3 (daptomycin arm), 2020: 42.0% standard-of-care antibiotics (SOCA) to SOCA alone Daptomycin Phase 3 (vancomycin arm), 2006: 31.8% Imbalance in baseline disease severity (APACHE II score) Interim futility analysis favoring SOCA alone arm DSMB recommended trial to be stopped for futility after Impact of trial conducted during COVID pandemic? interim futility analysis of 84 MRSA patients, or ~60% of Findings to date show trial to be UNINTERPRETABLE the primary efficacy endpoint population, was conducted in July 2022 Operational aspects functioned as designed No SAEs were determined to be related to exebacase Randomization code and IXRS performed correctly Primary efficacy endpoint: Clinical response at Day Exebacase was present in patients randomized to receive it, 14 in MRSA patients and not present in placebo recipients Exebacase + SOCA: 52.7% vs. SOCA alone: 58.6% Exebacase drug product testing met all specifications

DISRUPT: Baseline Characteristics from Adjudicated Futility Dataset Exebacase + SOCA SOCA alone Patient characteristics generally well balanced (n=55) (n=29) Final diagnosis by Adjudication Committee Complicated S. aureus bloodstream infection 42 (76.4%) 20 (69.0%) Uncomplicated S. aureus bloodstream infection 3 (5.5%) 4 (13.8%) Right-sided S. aureus infective endocarditis 8 (14.5%) 4 (13.8%) Left-sided S. aureus infective endocarditis 1 (1.8%) 1 (3.4%) Both right- and left-sided S. aureus infective endocarditis 1 (1.8%) 0 Primary source of infection by Adjudication Committee Catheter 10 (18.2%) 1 (3.5%) Hemodialysis access 5 (9.1%) 1 (3.5%) Orthopedic hardware/prosthetic joint 1 (1.8%) 2 (6.9%) Septic joint/osteomyelitis 12 (21.8%) 8 (27.6%) Skin/soft tissue 11 (20.0%) 10 (34.5%) IVDU 5 (9.1%) 3 (10.3%) Other or unknown** 11 (20.0%) 4 (13.8%)

DISRUPT: Baseline Characteristics from Adjudicated Futility Dataset Exebacase + SOCA SOCA alone Patient characteristics generally well balanced (n=55) (n=29) Risk factors for complicated S. aureus bloodstream infection/infective endocarditis Poorly controlled diabetes 10 (18.2%) 6 (20.7%) Injection drug use 7 (12.7%) 3 (10.3%) Preexisting valvular heart disease 1 (1.8%) 2 (6.9%) Surgery within previous 30 days 6 (10.9%) 4 (13.8%) Hemodialysis 6 (10.9%) 2 (6.9%) Extravascular foreign material 10 (18.2%) 6 (20.7%) Systemic inflammatory response syndrome 33 (60.0%) 15 (51.7%) Background SOCA therapy Vancomycin 34 (61.8%) 18 (62.1%) Daptomycin 12 (21.8%) 6 (20.7%) Beta lactam 6 (10.9%) 3 (10.3%) Van/Dap + beta lactam 3 (5.4%) 2 (7.0%) 13
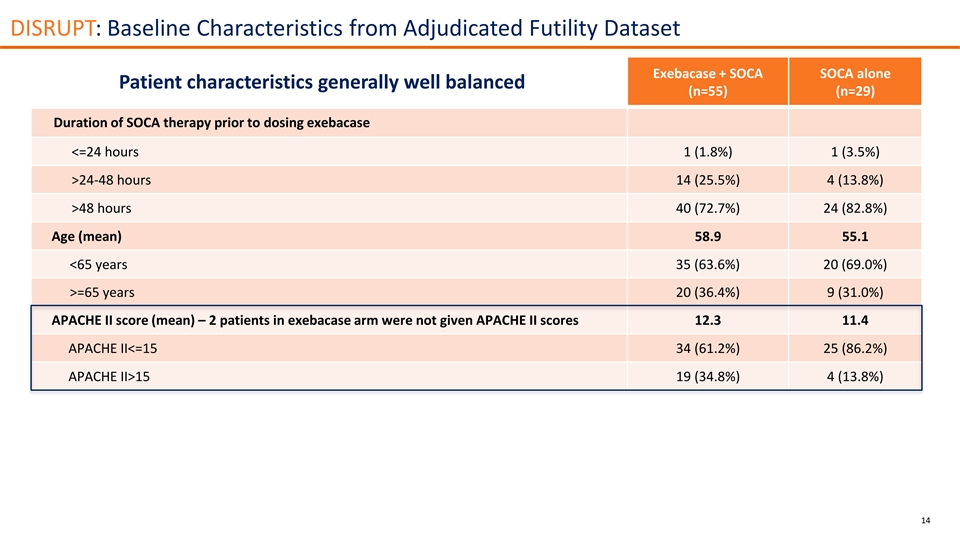
DISRUPT: Baseline Characteristics from Adjudicated Futility Dataset Exebacase + SOCA SOCA alone Patient characteristics generally well balanced (n=55) (n=29) Duration of SOCA therapy prior to dosing exebacase <=24 hours 1 (1.8%) 1 (3.5%) >24-48 hours 14 (25.5%) 4 (13.8%) >48 hours 40 (72.7%) 24 (82.8%) Age (mean) 58.9 55.1 <65 years 35 (63.6%) 20 (69.0%) >=65 years 20 (36.4%) 9 (31.0%) APACHE II score (mean) – 2 patients in exebacase arm were not given APACHE II scores 12.3 11.4 APACHE II<=15 34 (61.2%) 25 (86.2%) APACHE II>15 19 (34.8%) 4 (13.8%) 14

Exebacase: Preliminary Findings from Internal Analysis Extensive analysis of the preliminary data set from July 2022 All subgroup analyses, other than APACHE II score were as expected based on results of interim futility analysis Exebacase arm had imbalance of MRSA patients with extremely MRSA MSSA poor prognosis (APACHE II>15) Exebacase + Exebacase + No SAEs were determined to be related to exebacase SOCA SOCA alone SOCA SOCA alone 30-day all-cause mortality (Ns=61,31,95,49) 13 (21.3%) 3 (9.7%) 4 (4.2%) 3 (6.1%) (7 patients, 6 exebacase:1 SOCA, were not given APACHE II scores) APACHE II<=15, N 36 (59.0%) 26 (83.9%) 70 (73.7%) 32 (65.3%) 30-day all-cause mortality 4 (11.1%) 3 (11.5%) 2 (2.9%) 1 (3.1%) Predicted mortality of patient with APACHE II score of 10 and infection/sepsis* 11.3%-12.5% APACHE II>15-25, N 17 (27.9%) 5 (16.1%) 23 (24.2%) 16 (32.7%) 30-day all-cause mortality 6 (35.3%) 0 2 (8.7%) 2 (12.5%) Predicted mortality of patient with APACHE II score of 20 and infection/sepsis* 35.5%-38.1% APACHE II>25, N 4 (6.6%) 0 0 0 30-day all-cause mortality 2 (50.0%) 0 0 0 Predicted mortality of patient with APACHE II score of 25 and infection/sepsis* 53.3%-56.1% *APACHE II score can be directly converted to a percent risk of mortality with the patient's indication for ICU admission. Calculator at clincalc.com.

Exebacase for Chronic Prosthetic Joint Infections (PJI) S. aureus or Coagulase-Negative Staphylococci (CoNS) ©2022 ContraFect Corporation
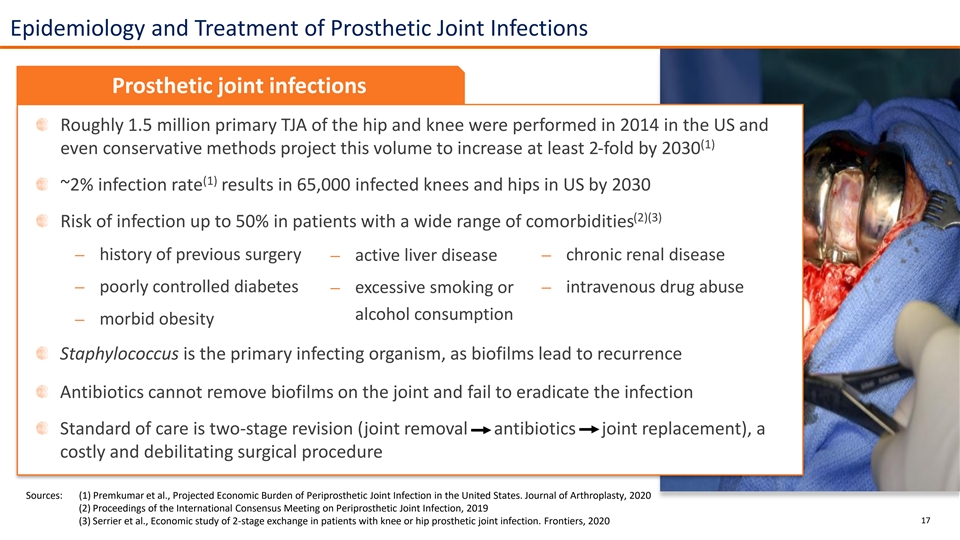
Epidemiology and Treatment of Prosthetic Joint Infections Prosthetic joint infections Roughly 1.5 million primary TJA of the hip and knee were performed in 2014 in the US and (1) even conservative methods project this volume to increase at least 2-fold by 2030 (1) ~2% infection rate results in 65,000 infected knees and hips in US by 2030 (2)(3) Risk of infection up to 50% in patients with a wide range of comorbidities ⎼ history of previous surgery⎼ chronic renal disease ⎼ active liver disease ⎼ poorly controlled diabetes ⎼ excessive smoking or ⎼ intravenous drug abuse alcohol consumption ⎼ morbid obesity Staphylococcus is the primary infecting organism, as biofilms lead to recurrence Antibiotics cannot remove biofilms on the joint and fail to eradicate the infection Standard of care is two-stage revision (joint removal antibiotics joint replacement), a costly and debilitating surgical procedure Sources: (1) Premkumar et al., Projected Economic Burden of Periprosthetic Joint Infection in the United States. Journal of Arthroplasty, 2020 (2) Proceedings of the International Consensus Meeting on Periprosthetic Joint Infection, 2019 (3) Serrier et al., Economic study of 2-stage exchange in patients with knee or hip prosthetic joint infection. Frontiers, 2020 17
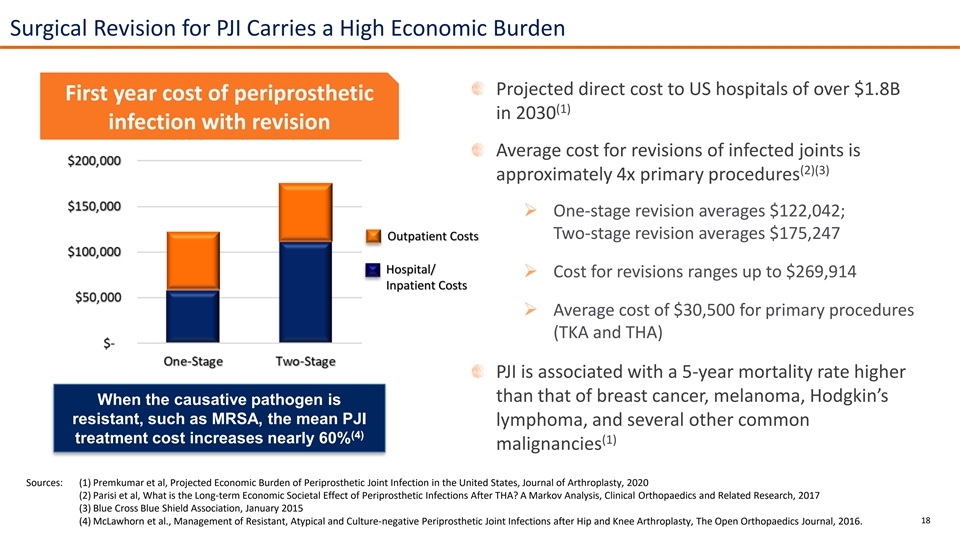
Surgical Revision for PJI Carries a High Economic Burden Projected direct cost to US hospitals of over $1.8B First year cost of periprosthetic (1) in 2030 infection with revision Average cost for revisions of infected joints is (2)(3) approximately 4x primary procedures ➢ One-stage revision averages $122,042; Two-stage revision averages $175,247 Outpatient Costs Hospital/ ➢ Cost for revisions ranges up to $269,914 Inpatient Costs ➢ Average cost of $30,500 for primary procedures (TKA and THA) PJI is associated with a 5-year mortality rate higher than that of breast cancer, melanoma, Hodgkin’s When the causative pathogen is resistant, such as MRSA, the mean PJI lymphoma, and several other common (4) treatment cost increases nearly 60% (1) malignancies Sources: (1) Premkumar et al, Projected Economic Burden of Periprosthetic Joint Infection in the United States, Journal of Arthroplasty, 2020 (2) Parisi et al, What is the Long-term Economic Societal Effect of Periprosthetic Infections After THA? A Markov Analysis, Clinical Orthopaedics and Related Research, 2017 (3) Blue Cross Blue Shield Association, January 2015 18 (4) McLawhorn et al., Management of Resistant, Atypical and Culture-negative Periprosthetic Joint Infections after Hip and Knee Arthroplasty, The Open Orthopaedics Journal, 2016.
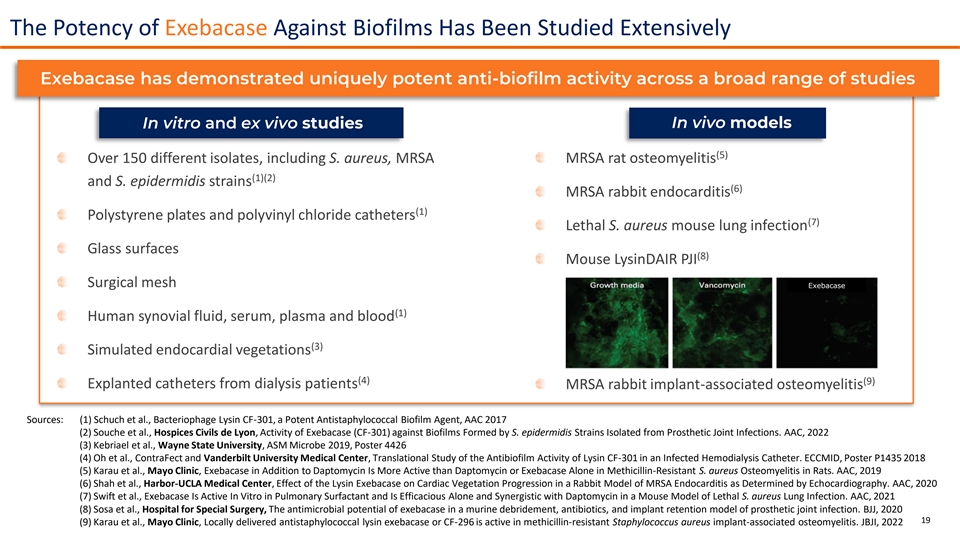
The Potency of Exebacase Against Biofilms Has Been Studied Extensively Exebacase has demonstrated uniquely potent anti-biofilm activity across a broad range of studies In vivo models In vitro and ex vivo studies (5) Over 150 different isolates, including S. aureus, MRSA MRSA rat osteomyelitis (1)(2) and S. epidermidis strains (6) MRSA rabbit endocarditis (1) Polystyrene plates and polyvinyl chloride catheters (7) Lethal S. aureus mouse lung infection Glass surfaces (8) Mouse LysinDAIR PJI Surgical mesh Exebacase (1) Human synovial fluid, serum, plasma and blood (3) Simulated endocardial vegetations (4) (9) Explanted catheters from dialysis patients MRSA rabbit implant-associated osteomyelitis Sources: (1) Schuch et al., Bacteriophage Lysin CF-301, a Potent Antistaphylococcal Biofilm Agent, AAC 2017 (2) Souche et al., Hospices Civils de Lyon, Activity of Exebacase (CF-301) against Biofilms Formed by S. epidermidis Strains Isolated from Prosthetic Joint Infections. AAC, 2022 (3) Kebriael et al., Wayne State University, ASM Microbe 2019, Poster 4426 (4) Oh et al., ContraFect and Vanderbilt University Medical Center, Translational Study of the Antibiofilm Activity of Lysin CF-301 in an Infected Hemodialysis Catheter. ECCMID, Poster P1435 2018 (5) Karau et al., Mayo Clinic, Exebacase in Addition to Daptomycin Is More Active than Daptomycin or Exebacase Alone in Methicillin-Resistant S. aureus Osteomyelitis in Rats. AAC, 2019 (6) Shah et al., Harbor-UCLA Medical Center, Effect of the Lysin Exebacase on Cardiac Vegetation Progression in a Rabbit Model of MRSA Endocarditis as Determined by Echocardiography. AAC, 2020 (7) Swift et al., Exebacase Is Active In Vitro in Pulmonary Surfactant and Is Efficacious Alone and Synergistic with Daptomycin in a Mouse Model of Lethal S. aureus Lung Infection. AAC, 2021 (8) Sosa et al., Hospital for Special Surgery, The antimicrobial potential of exebacase in a murine debridement, antibiotics, and implant retention model of prosthetic joint infection. BJJ, 2020 19 (9) Karau et al., Mayo Clinic, Locally delivered antistaphylococcal lysin exebacase or CF-296 is active in methicillin-resistant Staphylococcus aureus implant-associated osteomyelitis. JBJI, 2022

Exebacase Demonstrated Potency In Vitro Against CoNS Biofilms Isolated from PJIs Biomass reduction of exebacase alone and in synergy with antibiotics 20 Source: Souche et al., Hospices Civils de Lyon, Activity of Exebacase (CF-301) against Biofilms Formed by Staphylococcus epidermidis Strains Isolated from Prosthetic Joint Infections. AAC, 2022

Exebacase Demonstrated In Vivo Potency in MRSA Implant-Associated Osteomyelitis Model Rabbit implant-associated osteomyelitis model Bacterial burden on implant Bacterial burden of the bone *p<0.0025 vs vehicle control **p<0.0098 vs daptomycin Exebacase as a monotherapy, or in addition to daptomycin, demonstrated significant reduction in bacterial densities on implants and bone 21 Source: Karau et al., Mayo Clinic, Locally delivered antistaphylococcal lysin exebacase or CF-296 is active in methicillin-resistant Staphylococcus aureus implant-associated osteomyelitis. JBJI, 2022

Exebacase for PJI: Compassionate Use Summary — Hôpital de la Croix Rousse 16 patients treated to-date with intra-articular exebacase Minimally invasive arthroscopic debridement with joint retention No adverse events have occurred during arthroscopy (2) Four patients treated in patients similar to population for clinical study Favorable clinical outcomes observed in all four patients treated without prior explantation Four patients have been evaluated after 12-months showing durable positive outcomes Two patients have been evaluated after 24-months with continuing positive outcomes (3) Published on long-term data from initial patients treated Favorable clinical outcomes observed in patients with septic arthritis, demonstrating disappearance of clinical signs for over two years (4) Long-term data from initial three hip patients presented at IDWeek Favorable clinical outcomes observed in all patients, including continued resolution of fistula, for over two years Notes: (1) additional patients treated: two patients presented with chronic knee infection - one failed due to infection persistence and one patient was not evaluable due to death from COVID infection (2) presented by Dr. Tristan Ferry at the 40th Annual Meeting of the European Bone and Joint Infection Society (3) Ferry et al., Arthroscopic Debridement And Implant Retention (DAIR) with local administration of Exebacase (Lysin CF-301), Frontiers, 2021 22 (4) Ferry et al., Single center, exploratory, open-label prospective study in patients with suspected relapsing chronic CoNS prosthetic hip infection. IDWeek, Poster 919, 2022
Exebacase for PJI: Expected to Initiate Dosing in Patients in Q1 2023 Phase 1b/2 Study Design Approved by ANSM Randomized, placebo controlled study of intra-articular exebacase vs. placebo after arthroscopic debridement and joint retention followed by systemic antibiotics In patients with chronic prosthetic joint infections of the knee due to S. aureus or Coagulase-Negative Staphylococci • Cohort 1 (n=8 patients): 6:2 exebacase:placebo • Cohort 2 (n=16 patients): 12:4 exebacase:placebo Endpoints: • Clinical outcome at day 42 (longer-term follow-ups at day 90, 180, 360 and 720) • Pharmacokinetics in plasma and synovial fluid • Bacterial clearance in synovial fluid • Safety and immunogenicity • Health resource utilization and quality of life (QoL) 23

Novel, Engineered Direct Lytic Agent Combating Gram-negative Pathogens, CF-370 Including MDR and XDR Strains

CF-370 is Advancing to IND Submission in 2023 Discovered using proprietary lysin platform First lysin engineered to bypass the outer membrane of Gram-negative (GN) bacteria and show potent activity in human blood Pre-clinical data In vitro data demonstrated potent activity against Gram-negative pathogens In vivo activity observed across multiple animal studies Completion of GLP toxicology studies in two species Potential for administration of multiple-dose regimen in human clinical trials Patent applications filed for GN lysin candidates U.S. Patent No. 10,988,520 issued for composition of matter and methods of treating Gram-negative bacterial infections 25
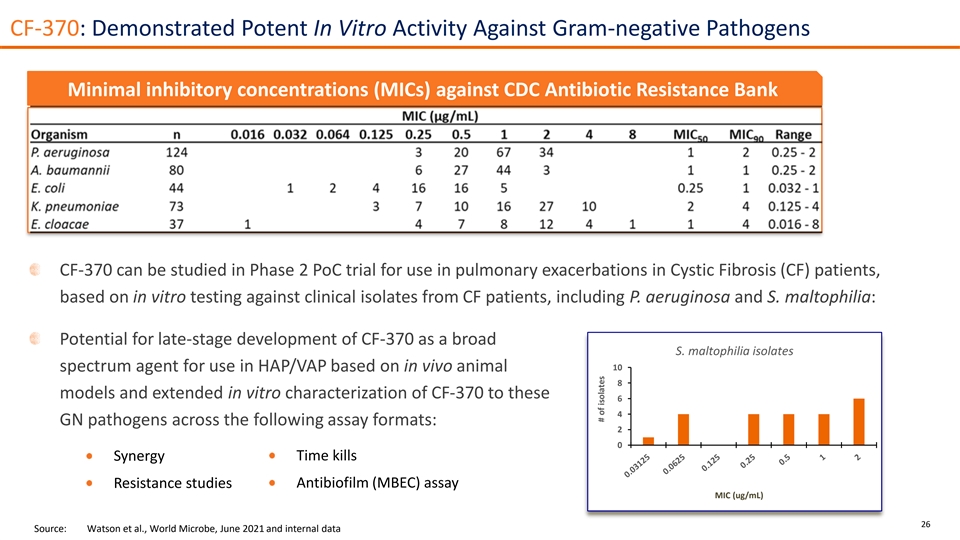
CF-370: Demonstrated Potent In Vitro Activity Against Gram-negative Pathogens Minimal inhibitory concentrations (MICs) against CDC Antibiotic Resistance Bank CF-370 can be studied in Phase 2 PoC trial for use in pulmonary exacerbations in Cystic Fibrosis (CF) patients, based on in vitro testing against clinical isolates from CF patients, including P. aeruginosa and S. maltophilia: Potential for late-stage development of CF-370 as a broad S. maltophilia isolates 10 spectrum agent for use in HAP/VAP based on in vivo animal 8 models and extended in vitro characterization of CF-370 to these 6 4 GN pathogens across the following assay formats: 2 0 Time kills Synergy Resistance studies Antibiofilm (MBEC) assay MIC (ug/mL) 26 Source: Watson et al., World Microbe, June 2021 and internal data # of isolates
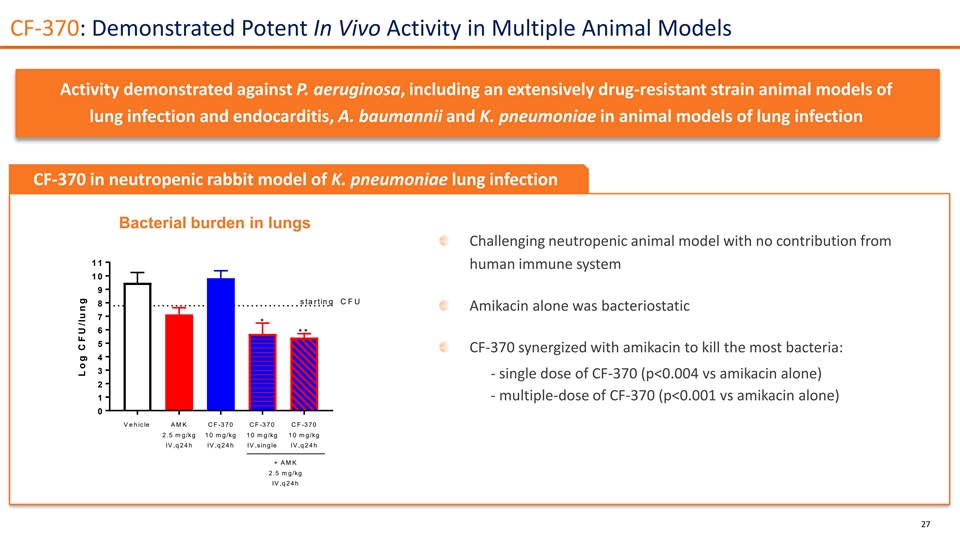
CF-370: Demonstrated Potent In Vivo Activity in Multiple Animal Models Activity demonstrated against P. aeruginosa, including an extensively drug-resistant strain animal models of lung infection and endocarditis, A. baumannii and K. pneumoniae in animal models of lung infection CF-370 in neutropenic rabbit model of K. pneumoniae lung infection Bacterial burden in lungs Challenging neutropenic animal model with no contribution from 1 1 human immune system 1 0 9 s ta rtin g C F U 8 Amikacin alone was bacteriostatic 7 * 6 * * 5 CF-370 synergized with amikacin to kill the most bacteria: 4 3 - single dose of CF-370 (p<0.004 vs amikacin alone) 2 - multiple-dose of CF-370 (p<0.001 vs amikacin alone) 1 0 V e h ic le A M K C F -3 70 C F -3 70 C F -3 70 2 .5 m g/kg 1 0 m g/kg 1 0 m g/kg 1 0 m g/kg IV ,q 2 4 h IV ,q 2 4 h IV ,sin g le IV ,q 2 4 h + A M K 2 .5 m g/kg IV ,q 2 4 h 27 L o g C F U /lu n g

CF-370: Early-Stage Clinical Development Plan Phase 1 Clinical Study Anticipated in Q2 2023 First-in-human Phase 1 SAD/MAD with BAL in healthy volunteers SAD: approximately 32 subjects, randomized 6:2 (CF-370:placebo) across four single ascending dose groups MAD: approximately 32 subjects, randomized 6:2 (CF-370:placebo) across four ascending groups over multiple days of dosing Anticipated Phase 2 Clinical PoC Study Phase 2 study of CF-370 in addition to standard-of-care antibiotics for the treatment of pulmonary exacerbations in patients with Cystic Fibrosis Broad spectrum activity suggests potential for late-stage development of CF-370 for the treatment of HAP/VAP patients as well as potential line extensions to cUTIs, cIAIs, burn/wound infections, catheter-related infections and bacteremia with susceptible organisms 28

Direct Lytic Agents as Potential Countermeasures BioDefense Agents for Biological Weapons
BioDefense Agents Initial pathogenic targets Potential non-dilutive funding Several lead compounds with potent Funding may be available from a variety of activity against: government agencies (BARDA, NIH, USAMRDC and others) Burkholderia cepacia ContraFect has ongoing relationships with BARDA Yersinia pestis and USAMRDC Serratia marcescens BioDefense agents are critical for the protection of public health and national security Animal rule: 21 CFR 601.91 for biologics allows for the licensure of biological products when human efficacy studies are not ethical and field trials to study the effectiveness of biological products are not feasible Efficacy can be established based on adequate and well-controlled studies in animal models of the human disease Intended for biological products developed to reduce or prevent serious or life-threatening conditions caused by exposure to lethal or permanently disabling toxic chemical, biological, radiological, or nuclear substances. Approved agents commonly garner significant stockpiling contracts 30

Corporate Information

Financial Information Key Metrics September 30, 2022* Cash, cash equivalents and $17.6 million* marketable securities Total debt ―― Common shares outstanding 39.3 million *Cash, cash equivalents and marketable securities does not include approximately $6.2 million in net proceeds from our registered direct offering 32 closed on December 15, 2022.

Developing differentiated, first-in-class direct lytic agents (DLAs) for life-threatening, drug-resistant infections NASDAQ: CFRX
































