
Topline Results 5 December 2017 SAKURA 1 and 2 Phase 3 Pivotal Studies DaxibotulinumtoxinA for Injection (RT002) for the Treatment of Moderate to Severe Glabellar Lines Exhibit 99.2
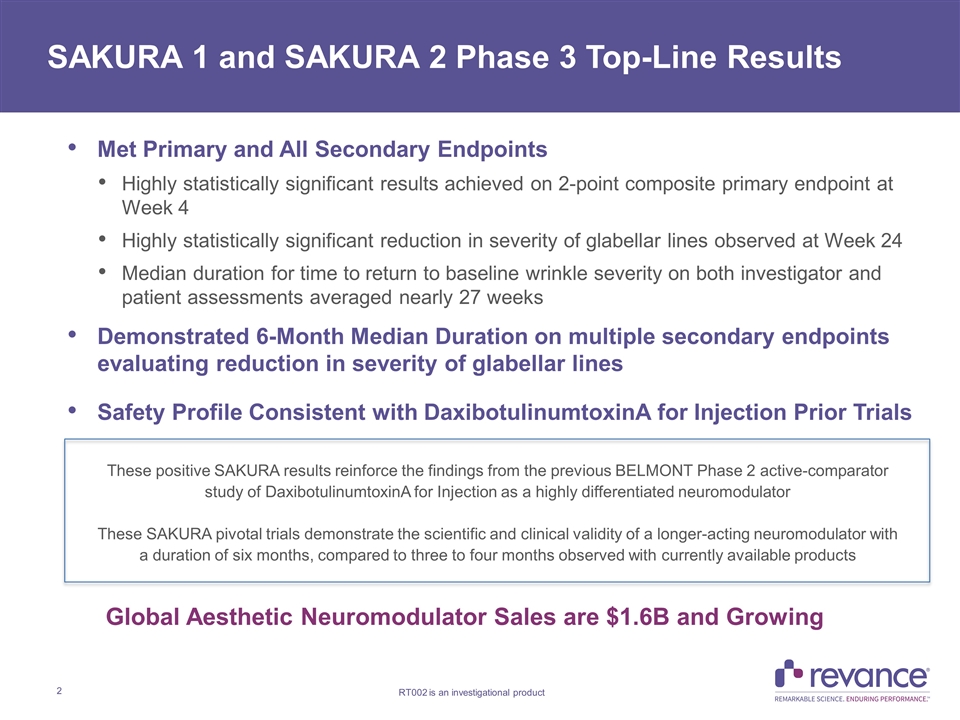
Met Primary and All Secondary Endpoints Highly statistically significant results achieved on 2-point composite primary endpoint at Week 4 Highly statistically significant reduction in severity of glabellar lines observed at Week 24 Median duration for time to return to baseline wrinkle severity on both investigator and patient assessments averaged nearly 27 weeks Demonstrated 6-Month Median Duration on multiple secondary endpoints evaluating reduction in severity of glabellar lines Safety Profile Consistent with DaxibotulinumtoxinA for Injection Prior Trials SAKURA 1 and SAKURA 2 Phase 3 Top-Line Results These positive SAKURA results reinforce the findings from the previous BELMONT Phase 2 active-comparator study of DaxibotulinumtoxinA for Injection as a highly differentiated neuromodulator These SAKURA pivotal trials demonstrate the scientific and clinical validity of a longer-acting neuromodulator with a duration of six months, compared to three to four months observed with currently available products Global Aesthetic Neuromodulator Sales are $1.6B and Growing
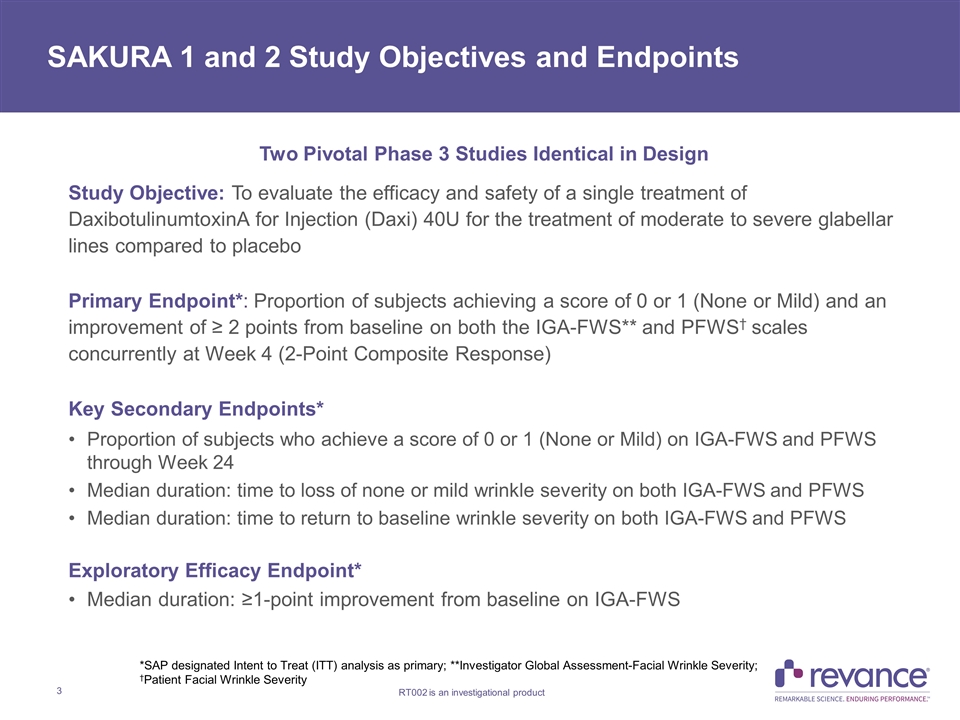
Two Pivotal Phase 3 Studies Identical in Design Study Objective: To evaluate the efficacy and safety of a single treatment of DaxibotulinumtoxinA for Injection (Daxi) 40U for the treatment of moderate to severe glabellar lines compared to placebo Primary Endpoint*: Proportion of subjects achieving a score of 0 or 1 (None or Mild) and an improvement of ≥ 2 points from baseline on both the IGA-FWS** and PFWS† scales concurrently at Week 4 (2-Point Composite Response) Key Secondary Endpoints* Proportion of subjects who achieve a score of 0 or 1 (None or Mild) on IGA-FWS and PFWS through Week 24 Median duration: time to loss of none or mild wrinkle severity on both IGA-FWS and PFWS Median duration: time to return to baseline wrinkle severity on both IGA-FWS and PFWS Exploratory Efficacy Endpoint* Median duration: ≥1-point improvement from baseline on IGA-FWS SAKURA 1 and 2 Study Objectives and Endpoints *SAP designated Intent to Treat (ITT) analysis as primary; **Investigator Global Assessment-Facial Wrinkle Severity; †Patient Facial Wrinkle Severity
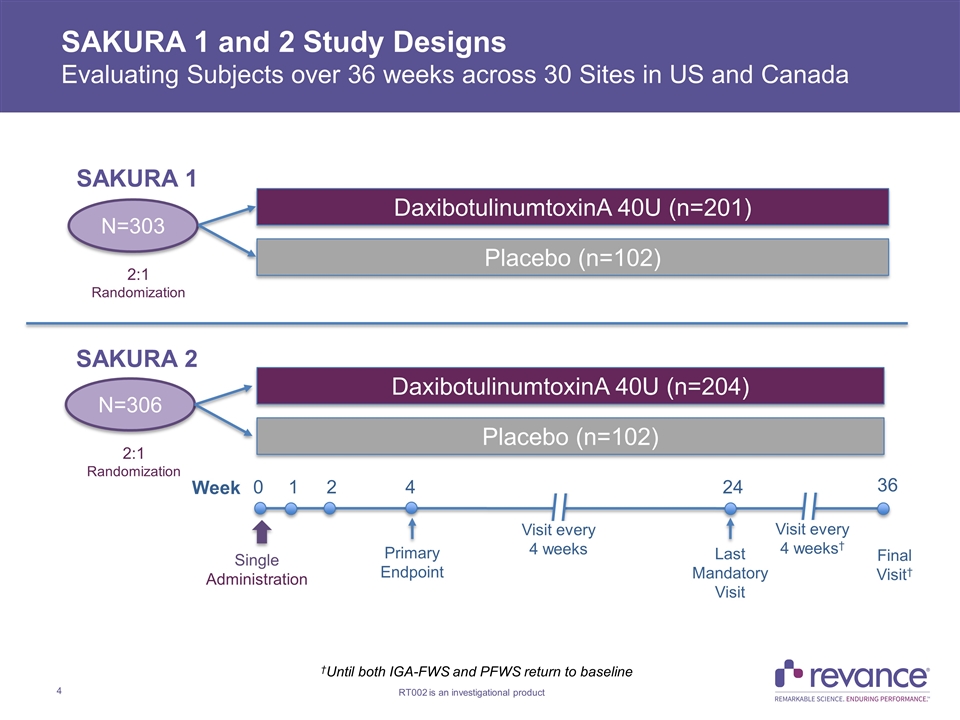
SAKURA 1 and 2 Study Designs Evaluating Subjects over 36 weeks across 30 Sites in US and Canada N=303 2:1 Randomization Week 0 1 2 4 24 36 Primary Endpoint Single Administration Last Mandatory Visit †Until both IGA-FWS and PFWS return to baseline Final Visit† Visit every 4 weeks Visit every 4 weeks† DaxibotulinumtoxinA 40U (n=201) Placebo (n=102) N=306 2:1 Randomization DaxibotulinumtoxinA 40U (n=204) Placebo (n=102) SAKURA 1 SAKURA 2
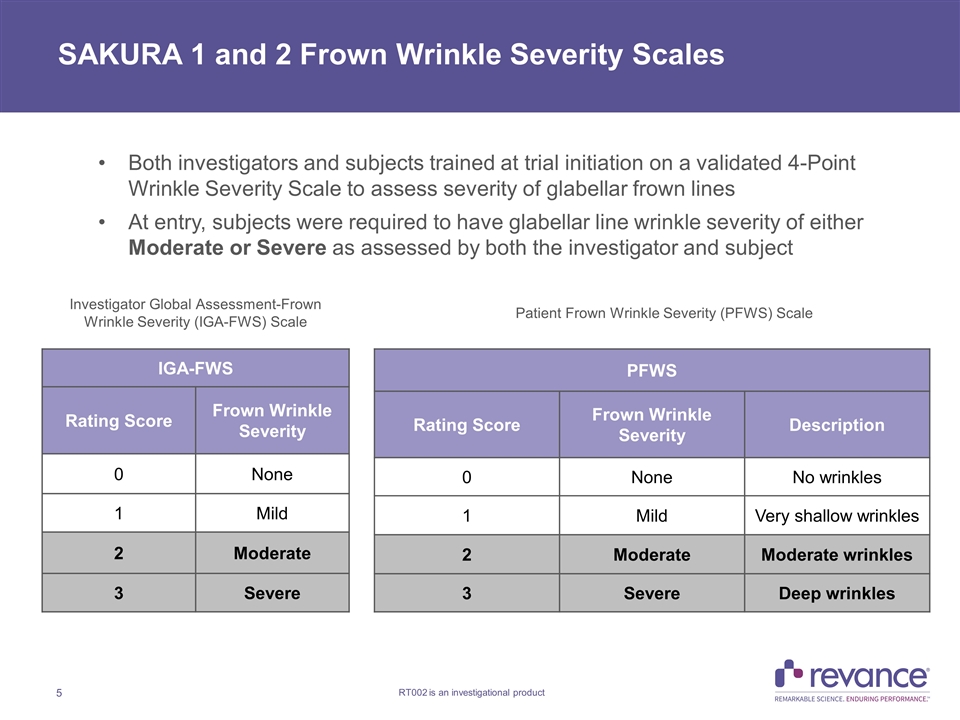
SAKURA 1 and 2 Frown Wrinkle Severity Scales Both investigators and subjects trained at trial initiation on a validated 4-Point Wrinkle Severity Scale to assess severity of glabellar frown lines At entry, subjects were required to have glabellar line wrinkle severity of either Moderate or Severe as assessed by both the investigator and subject IGA-FWS Rating Score Frown Wrinkle Severity 0 None 1 Mild 2 Moderate 3 Severe PFWS Rating Score Frown Wrinkle Severity Description 0 None No wrinkles 1 Mild Very shallow wrinkles 2 Moderate Moderate wrinkles 3 Severe Deep wrinkles Investigator Global Assessment-Frown Wrinkle Severity (IGA-FWS) Scale Patient Frown Wrinkle Severity (PFWS) Scale
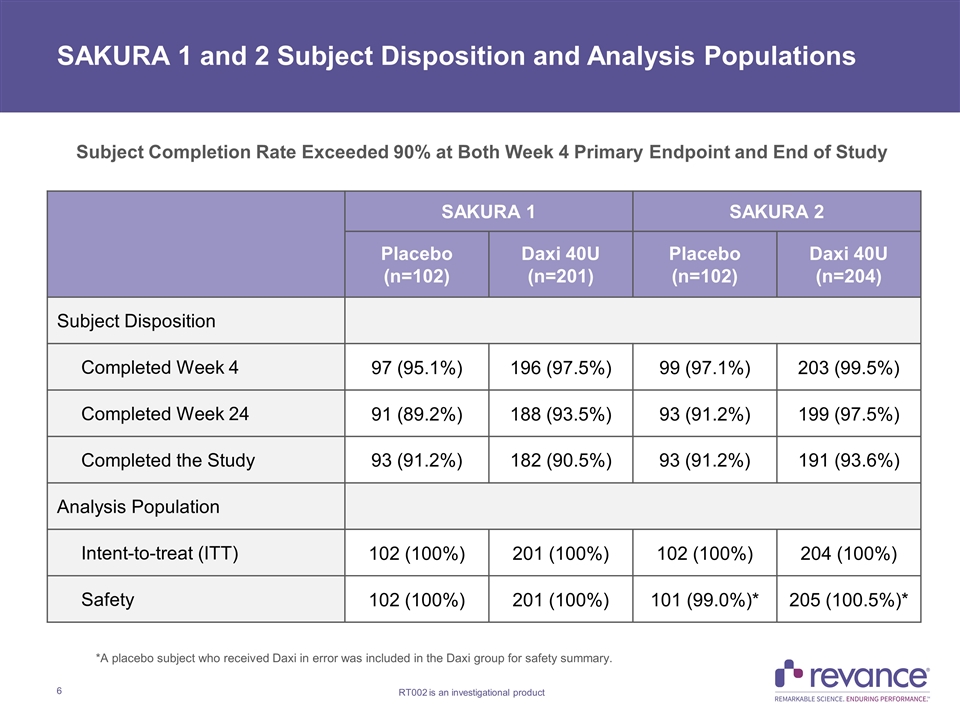
SAKURA 1 SAKURA 2 Placebo (n=102) Daxi 40U (n=201) Placebo (n=102) Daxi 40U (n=204) Subject Disposition Completed Week 4 97 (95.1%) 196 (97.5%) 99 (97.1%) 203 (99.5%) Completed Week 24 91 (89.2%) 188 (93.5%) 93 (91.2%) 199 (97.5%) Completed the Study 93 (91.2%) 182 (90.5%) 93 (91.2%) 191 (93.6%) Analysis Population Intent-to-treat (ITT) 102 (100%) 201 (100%) 102 (100%) 204 (100%) Safety 102 (100%) 201 (100%) 101 (99.0%)* 205 (100.5%)* SAKURA 1 and 2 Subject Disposition and Analysis Populations *A placebo subject who received Daxi in error was included in the Daxi group for safety summary. Subject Completion Rate Exceeded 90% at Both Week 4 Primary Endpoint and End of Study
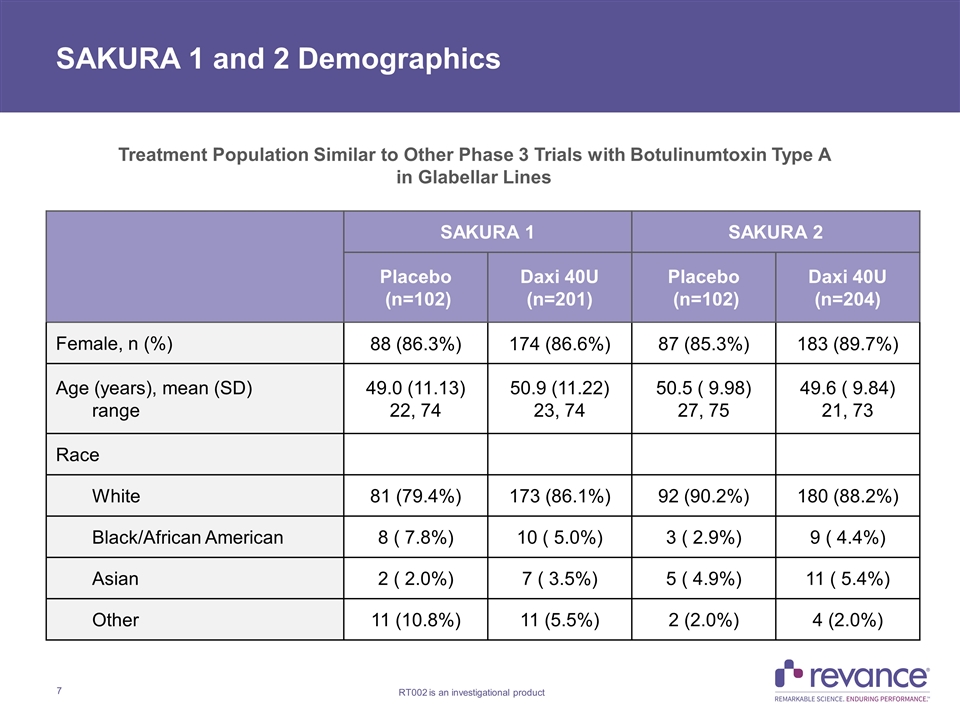
SAKURA 1 and 2 Demographics SAKURA 1 SAKURA 2 Placebo (n=102) Daxi 40U (n=201) Placebo (n=102) Daxi 40U (n=204) Female, n (%) 88 (86.3%) 174 (86.6%) 87 (85.3%) 183 (89.7%) Age (years), mean (SD) range 49.0 (11.13) 22, 74 50.9 (11.22) 23, 74 50.5 ( 9.98) 27, 75 49.6 ( 9.84) 21, 73 Race White 81 (79.4%) 173 (86.1%) 92 (90.2%) 180 (88.2%) Black/African American 8 ( 7.8%) 10 ( 5.0%) 3 ( 2.9%) 9 ( 4.4%) Asian 2 ( 2.0%) 7 ( 3.5%) 5 ( 4.9%) 11 ( 5.4%) Other 11 (10.8%) 11 (5.5%) 2 (2.0%) 4 (2.0%) Treatment Population Similar to Other Phase 3 Trials with Botulinumtoxin Type A in Glabellar Lines
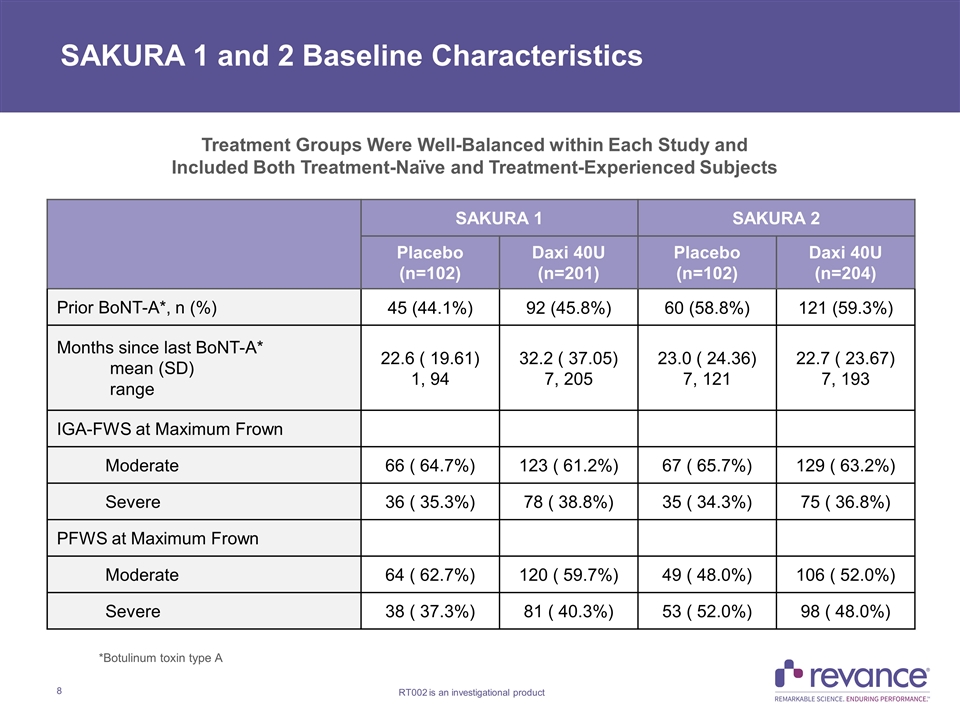
SAKURA 1 and 2 Baseline Characteristics SAKURA 1 SAKURA 2 Placebo (n=102) Daxi 40U (n=201) Placebo (n=102) Daxi 40U (n=204) Prior BoNT-A*, n (%) 45 (44.1%) 92 (45.8%) 60 (58.8%) 121 (59.3%) Months since last BoNT-A* mean (SD) range 22.6 ( 19.61) 1, 94 32.2 ( 37.05) 7, 205 23.0 ( 24.36) 7, 121 22.7 ( 23.67) 7, 193 IGA-FWS at Maximum Frown Moderate 66 ( 64.7%) 123 ( 61.2%) 67 ( 65.7%) 129 ( 63.2%) Severe 36 ( 35.3%) 78 ( 38.8%) 35 ( 34.3%) 75 ( 36.8%) PFWS at Maximum Frown Moderate 64 ( 62.7%) 120 ( 59.7%) 49 ( 48.0%) 106 ( 52.0%) Severe 38 ( 37.3%) 81 ( 40.3%) 53 ( 52.0%) 98 ( 48.0%) *Botulinum toxin type A Treatment Groups Were Well-Balanced within Each Study and Included Both Treatment-Naïve and Treatment-Experienced Subjects
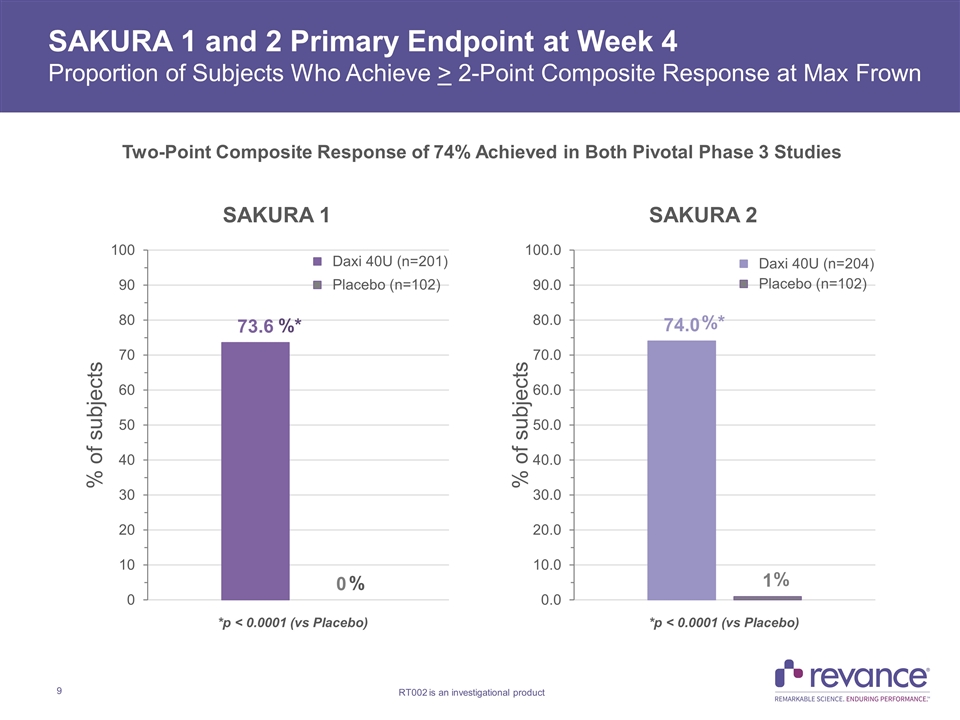
SAKURA 1 and 2 Primary Endpoint at Week 4 Proportion of Subjects Who Achieve > 2-Point Composite Response at Max Frown Two-Point Composite Response of 74% Achieved in Both Pivotal Phase 3 Studies %* %* % *p < 0.0001 (vs Placebo) *p < 0.0001 (vs Placebo)
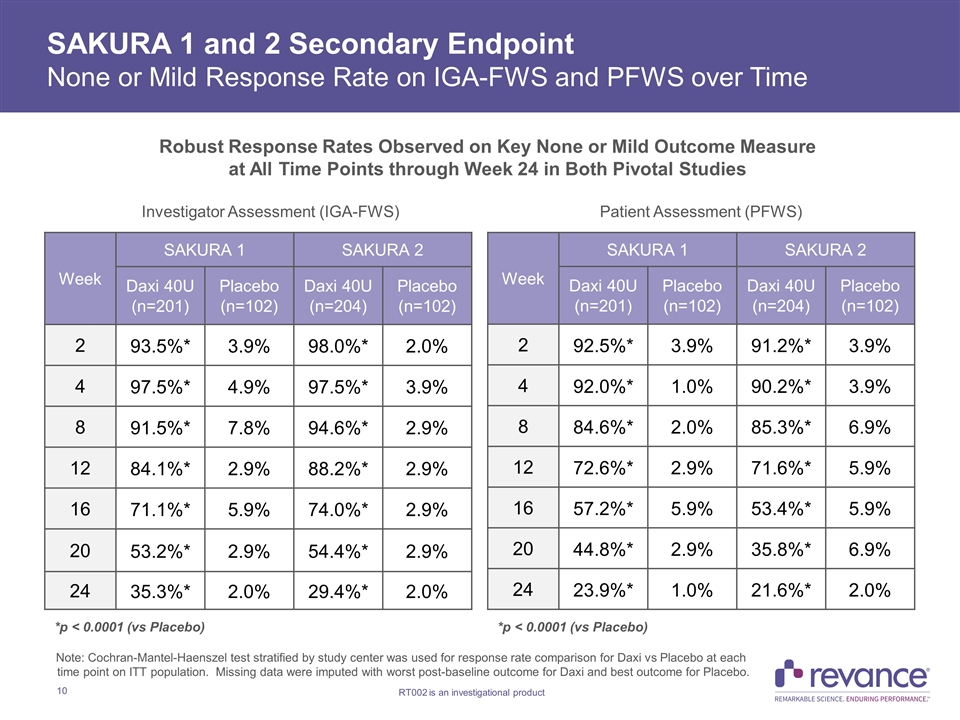
SAKURA 1 and 2 Secondary Endpoint None or Mild Response Rate on IGA-FWS and PFWS over Time Week SAKURA 1 SAKURA 2 Daxi 40U (n=201) Placebo (n=102) Daxi 40U (n=204) Placebo (n=102) 2 93.5%* 3.9% 98.0%* 2.0% 4 97.5%* 4.9% 97.5%* 3.9% 8 91.5%* 7.8% 94.6%* 2.9% 12 84.1%* 2.9% 88.2%* 2.9% 16 71.1%* 5.9% 74.0%* 2.9% 20 53.2%* 2.9% 54.4%* 2.9% 24 35.3%* 2.0% 29.4%* 2.0% Week SAKURA 1 SAKURA 2 Daxi 40U (n=201) Placebo (n=102) Daxi 40U (n=204) Placebo (n=102) 2 92.5%* 3.9% 91.2%* 3.9% 4 92.0%* 1.0% 90.2%* 3.9% 8 84.6%* 2.0% 85.3%* 6.9% 12 72.6%* 2.9% 71.6%* 5.9% 16 57.2%* 5.9% 53.4%* 5.9% 20 44.8%* 2.9% 35.8%* 6.9% 24 23.9%* 1.0% 21.6%* 2.0% Investigator Assessment (IGA-FWS) Patient Assessment (PFWS) Note: Cochran-Mantel-Haenszel test stratified by study center was used for response rate comparison for Daxi vs Placebo at each time point on ITT population. Missing data were imputed with worst post-baseline outcome for Daxi and best outcome for Placebo. *p < 0.0001 (vs Placebo) *p < 0.0001 (vs Placebo) Robust Response Rates Observed on Key None or Mild Outcome Measure at All Time Points through Week 24 in Both Pivotal Studies
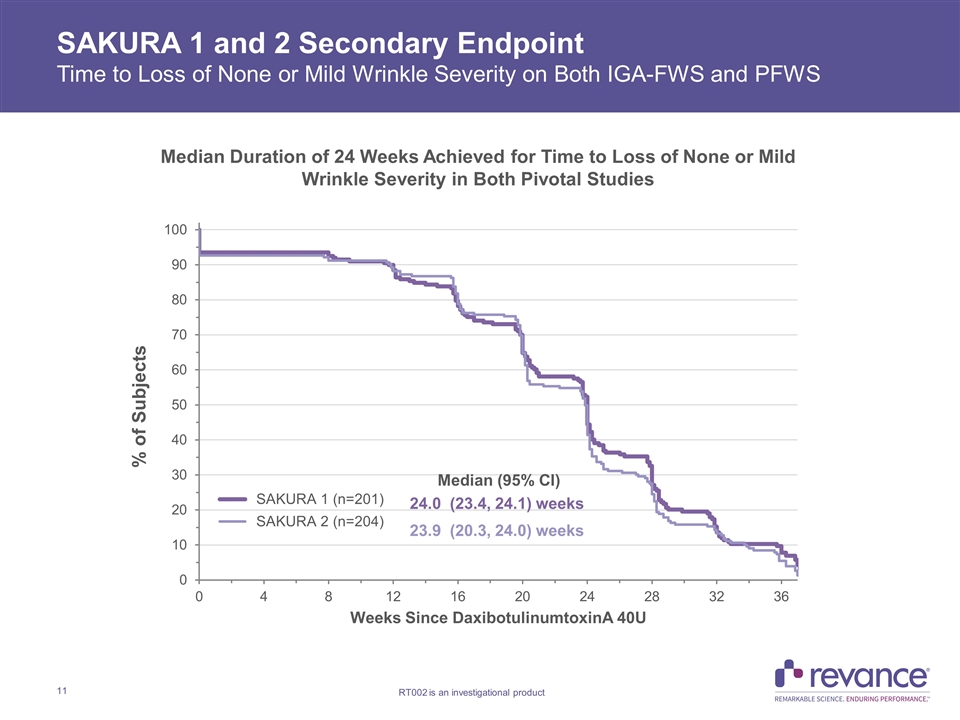
SAKURA 1 and 2 Secondary Endpoint Time to Loss of None or Mild Wrinkle Severity on Both IGA-FWS and PFWS Median Duration of 24 Weeks Achieved for Time to Loss of None or Mild Wrinkle Severity in Both Pivotal Studies
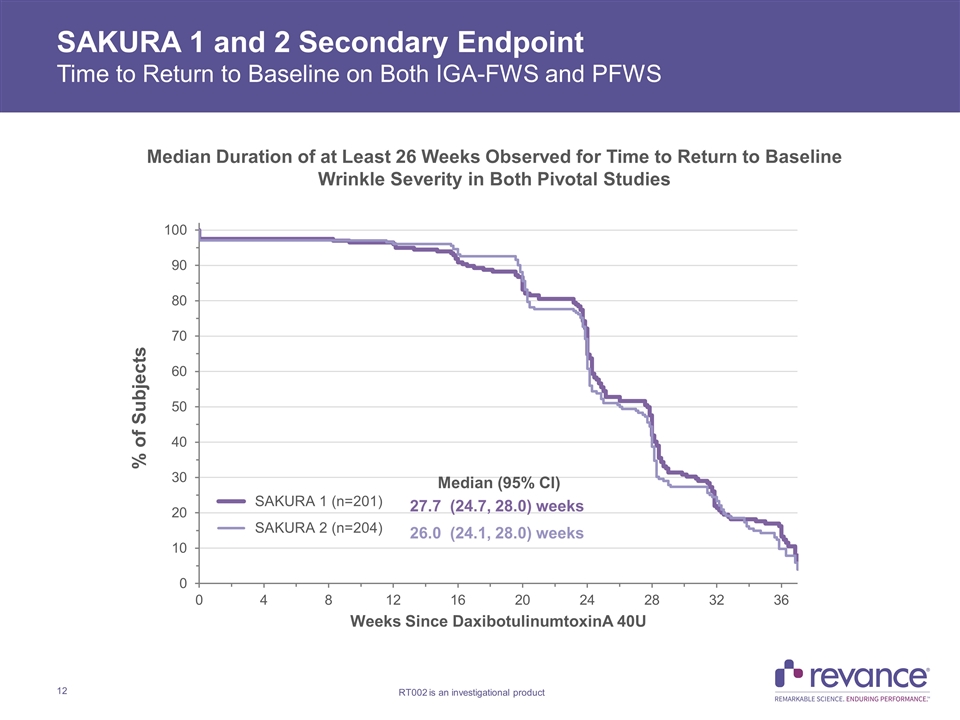
SAKURA 1 and 2 Secondary Endpoint Time to Return to Baseline on Both IGA-FWS and PFWS Median Duration of at Least 26 Weeks Observed for Time to Return to Baseline Wrinkle Severity in Both Pivotal Studies
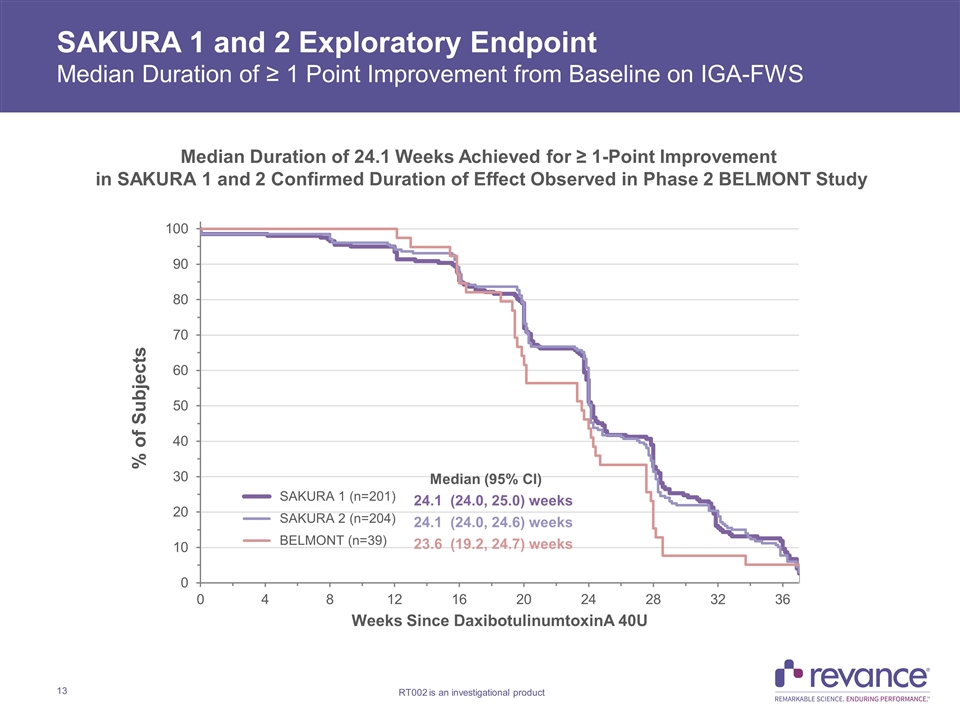
SAKURA 1 and 2 Exploratory Endpoint Median Duration of ≥ 1 Point Improvement from Baseline on IGA-FWS Median Duration of 24.1 Weeks Achieved for ≥ 1-Point Improvement in SAKURA 1 and 2 Confirmed Duration of Effect Observed in Phase 2 BELMONT Study
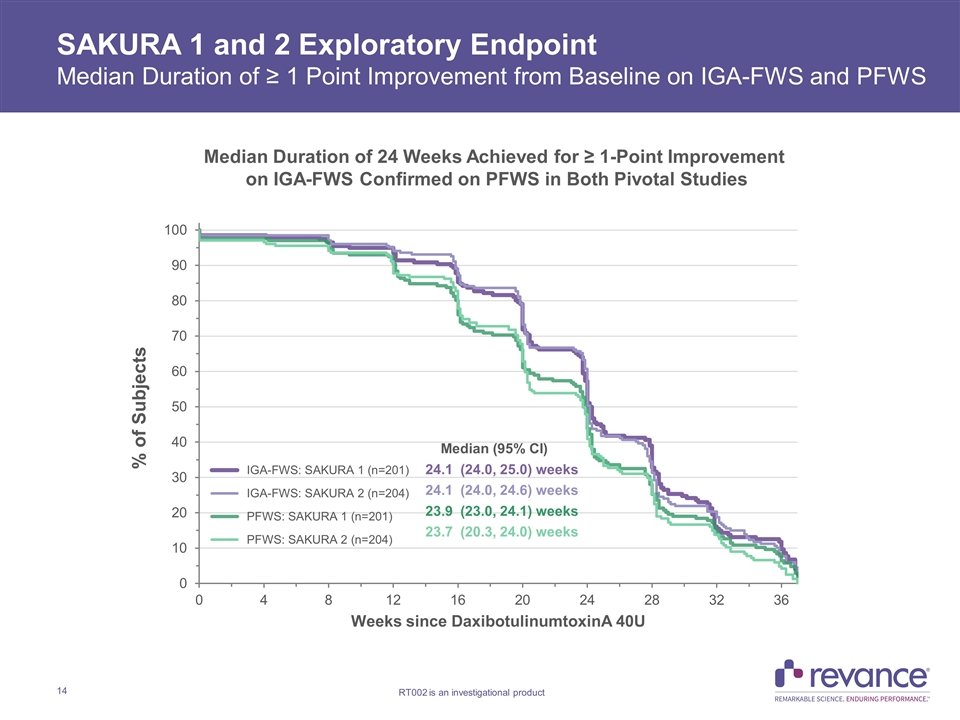
SAKURA 1 and 2 Exploratory Endpoint Median Duration of ≥ 1 Point Improvement from Baseline on IGA-FWS and PFWS Median Duration of 24 Weeks Achieved for ≥ 1-Point Improvement on IGA-FWS Confirmed on PFWS in Both Pivotal Studies
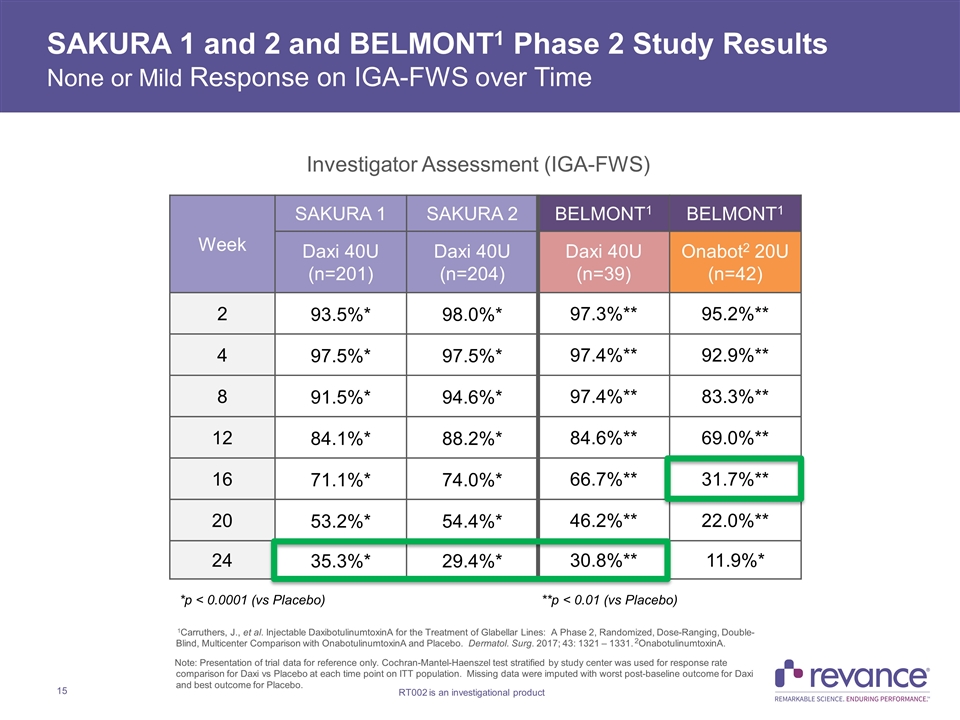
SAKURA 1 and 2 and BELMONT1 Phase 2 Study Results None or Mild Response on IGA-FWS over Time Week SAKURA 1 SAKURA 2 BELMONT1 BELMONT1 Daxi 40U (n=201) Daxi 40U (n=204) Daxi 40U (n=39) Onabot2 20U (n=42) 2 93.5%* 98.0%* 97.3%** 95.2%** 4 97.5%* 97.5%* 97.4%** 92.9%** 8 91.5%* 94.6%* 97.4%** 83.3%** 12 84.1%* 88.2%* 84.6%** 69.0%** 16 71.1%* 74.0%* 66.7%** 31.7%** 20 53.2%* 54.4%* 46.2%** 22.0%** 24 35.3%* 29.4%* 30.8%** 11.9%* Investigator Assessment (IGA-FWS) 1Carruthers, J., et al. Injectable DaxibotulinumtoxinA for the Treatment of Glabellar Lines: A Phase 2, Randomized, Dose-Ranging, Double-Blind, Multicenter Comparison with OnabotulinumtoxinA and Placebo. Dermatol. Surg. 2017; 43: 1321 – 1331. 2OnabotulinumtoxinA. Note: Presentation of trial data for reference only. Cochran-Mantel-Haenszel test stratified by study center was used for response rate comparison for Daxi vs Placebo at each time point on ITT population. Missing data were imputed with worst post-baseline outcome for Daxi and best outcome for Placebo. *p < 0.0001 (vs Placebo) **p < 0.01 (vs Placebo)
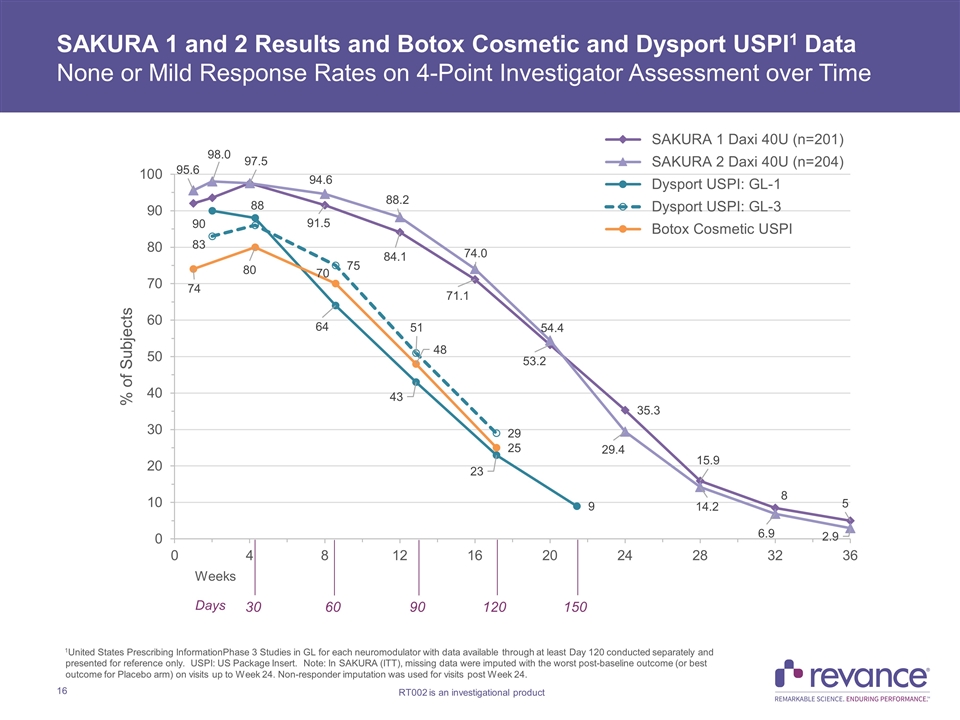
30 60 90 120 150 Weeks Days SAKURA 1 and 2 Results and Botox Cosmetic and Dysport USPI1 Data None or Mild Response Rates on 4-Point Investigator Assessment over Time 1United States Prescribing InformationPhase 3 Studies in GL for each neuromodulator with data available through at least Day 120 conducted separately and presented for reference only. USPI: US Package Insert. Note: In SAKURA (ITT), missing data were imputed with the worst post-baseline outcome (or best outcome for Placebo arm) on visits up to Week 24. Non-responder imputation was used for visits post Week 24.

Baseline Scores: IGA-FWS: 3 PFWS: 3 DaxibotulinumtoxinA 40U MAXIMUM FROWN Week 4 Week 24 Week 4 Scores: IGA-FWS: 0 PFWS: 0 Week 24 Scores: IGA-FWS: 0 PFWS: 1 SAKURA 1 and 2 Pre-treatment Three Point Improvement from Baseline on Investigator and Subject Assessment Three Point Improvement from Baseline on Investigator Assessment and Two Point Improvement on Subject Assessment Example 3-Point Improvement by IGA-FWS & PFWS at Week 4 Three-Point Sustained Duration of Effect by IGA-FWS over 24 Weeks
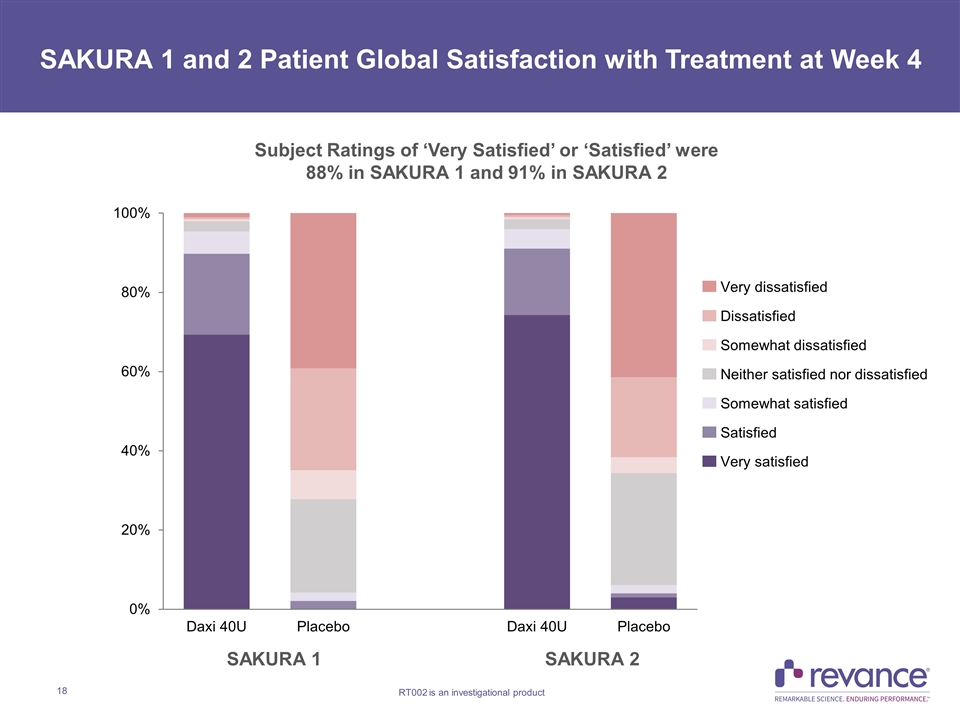
SAKURA 1 and 2 Patient Global Satisfaction with Treatment at Week 4 SAKURA 1 SAKURA 2 Very dissatisfied Dissatisfied Somewhat dissatisfied Neither satisfied nor dissatisfied Somewhat satisfied Satisfied Very satisfied Subject Ratings of ‘Very Satisfied’ or ‘Satisfied’ were 88% in SAKURA 1 and 91% in SAKURA 2
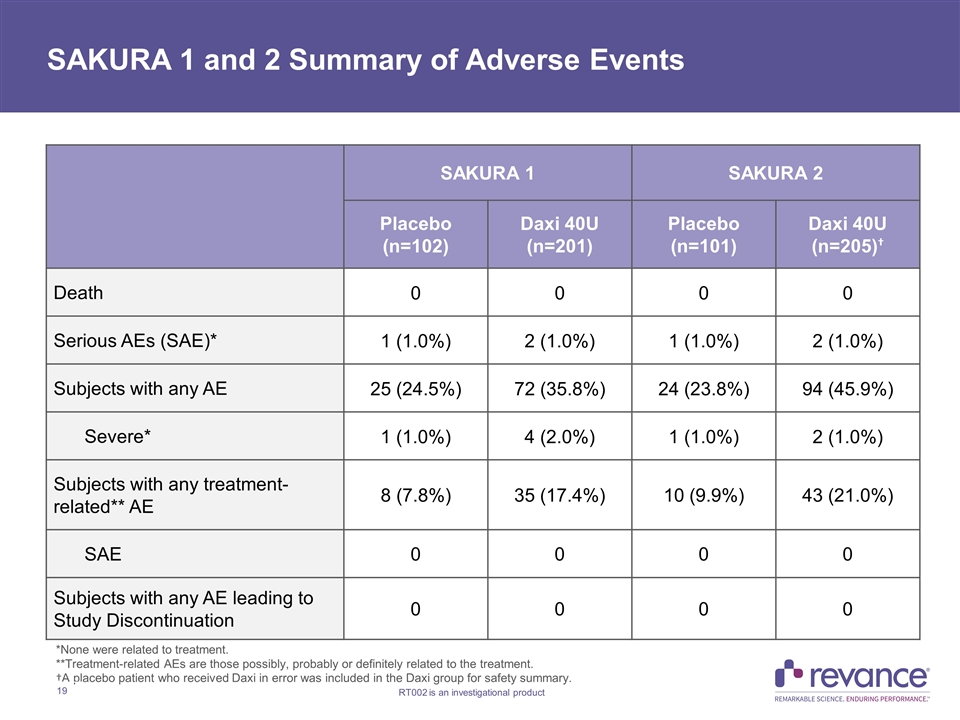
SAKURA 1 and 2 Summary of Adverse Events SAKURA 1 SAKURA 2 Placebo (n=102) Daxi 40U (n=201) Placebo (n=101) Daxi 40U (n=205)† Death 0 0 0 0 Serious AEs (SAE)* 1 (1.0%) 2 (1.0%) 1 (1.0%) 2 (1.0%) Subjects with any AE 25 (24.5%) 72 (35.8%) 24 (23.8%) 94 (45.9%) Severe* 1 (1.0%) 4 (2.0%) 1 (1.0%) 2 (1.0%) Subjects with any treatment-related** AE 8 (7.8%) 35 (17.4%) 10 (9.9%) 43 (21.0%) SAE 0 0 0 0 Subjects with any AE leading to Study Discontinuation 0 0 0 0 *None were related to treatment. **Treatment-related AEs are those possibly, probably or definitely related to the treatment. †A placebo patient who received Daxi in error was included in the Daxi group for safety summary.
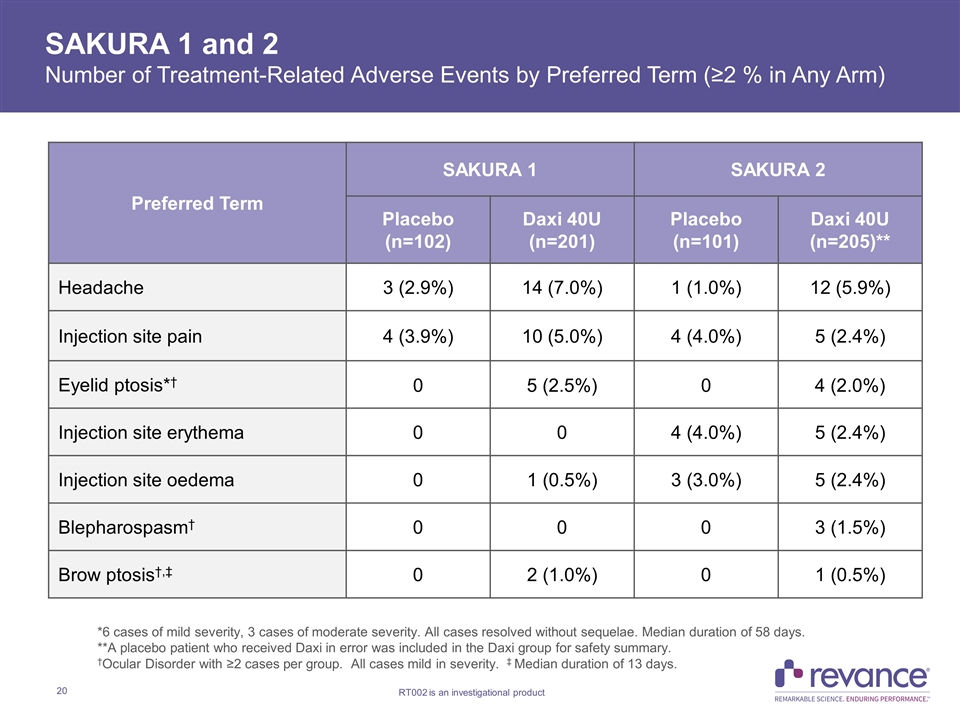
SAKURA 1 and 2 Number of Treatment-Related Adverse Events by Preferred Term (≥2 % in Any Arm) Preferred Term SAKURA 1 SAKURA 2 Placebo (n=102) Daxi 40U (n=201) Placebo (n=101) Daxi 40U (n=205)** Headache 3 (2.9%) 14 (7.0%) 1 (1.0%) 12 (5.9%) Injection site pain 4 (3.9%) 10 (5.0%) 4 (4.0%) 5 (2.4%) Eyelid ptosis*† 0 5 (2.5%) 0 4 (2.0%) Injection site erythema 0 0 4 (4.0%) 5 (2.4%) Injection site oedema 0 1 (0.5%) 3 (3.0%) 5 (2.4%) Blepharospasm† 0 0 0 3 (1.5%) Brow ptosis†,‡ 0 2 (1.0%) 0 1 (0.5%) *6 cases of mild severity, 3 cases of moderate severity. All cases resolved without sequelae. Median duration of 58 days. **A placebo patient who received Daxi in error was included in the Daxi group for safety summary. †Ocular Disorder with ≥2 cases per group. All cases mild in severity. ‡ Median duration of 13 days.
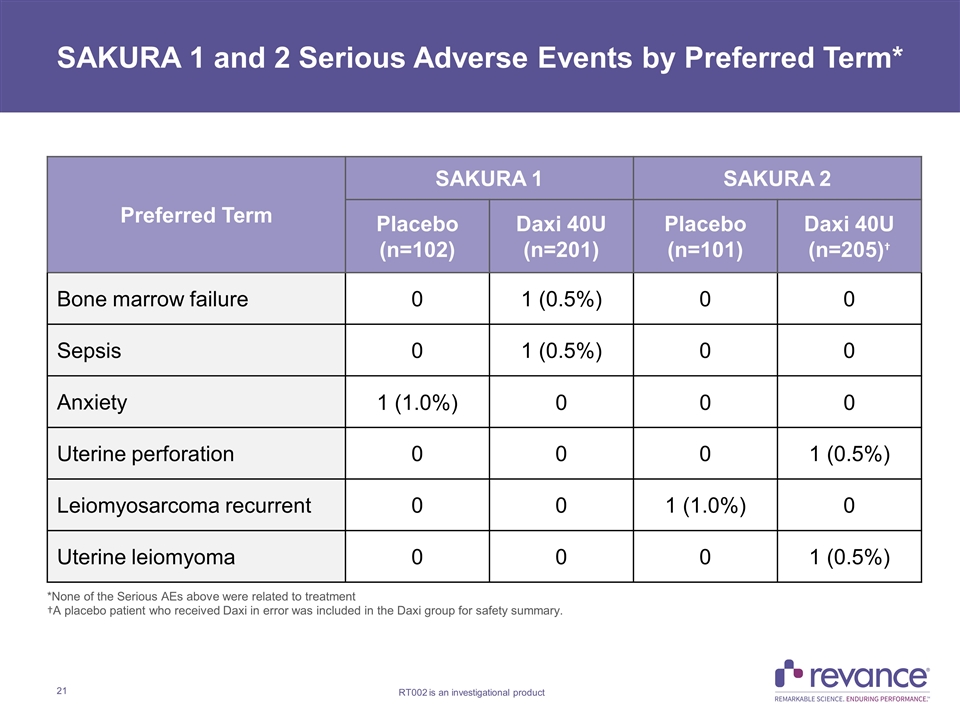
SAKURA 1 and 2 Serious Adverse Events by Preferred Term* Preferred Term SAKURA 1 SAKURA 2 Placebo (n=102) Daxi 40U (n=201) Placebo (n=101) Daxi 40U (n=205)† Bone marrow failure 0 1 (0.5%) 0 0 Sepsis 0 1 (0.5%) 0 0 Anxiety 1 (1.0%) 0 0 0 Uterine perforation 0 0 0 1 (0.5%) Leiomyosarcoma recurrent 0 0 1 (1.0%) 0 Uterine leiomyoma 0 0 0 1 (0.5%) *None of the Serious AEs above were related to treatment †A placebo patient who received Daxi in error was included in the Daxi group for safety summary.
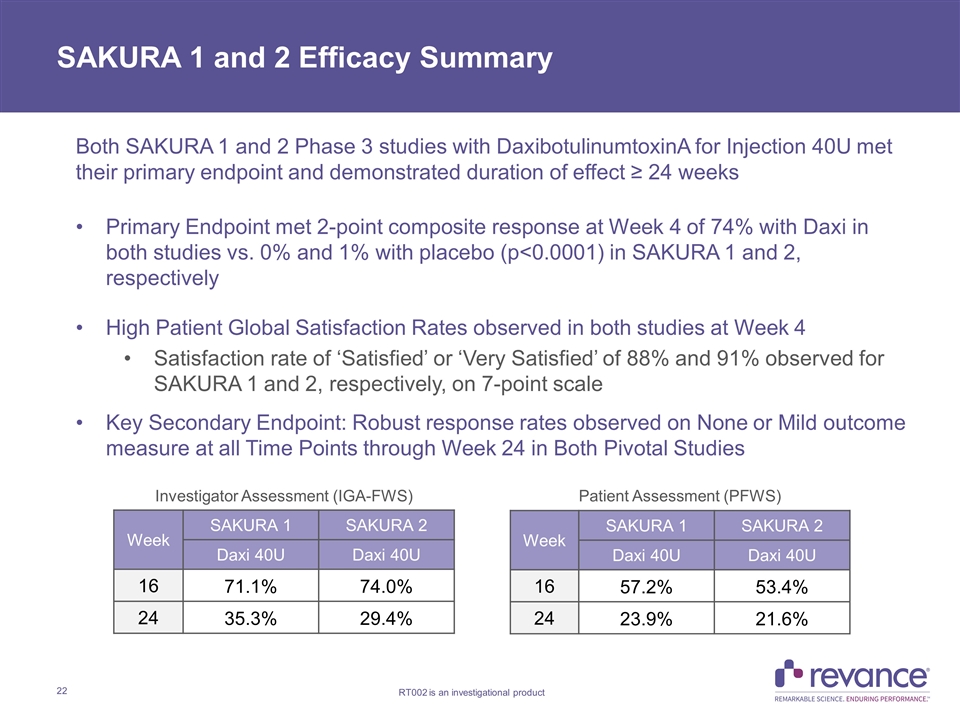
SAKURA 1 and 2 Efficacy Summary Both SAKURA 1 and 2 Phase 3 studies with DaxibotulinumtoxinA for Injection 40U met their primary endpoint and demonstrated duration of effect ≥ 24 weeks Primary Endpoint met 2-point composite response at Week 4 of 74% with Daxi in both studies vs. 0% and 1% with placebo (p<0.0001) in SAKURA 1 and 2, respectively High Patient Global Satisfaction Rates observed in both studies at Week 4 Satisfaction rate of ‘Satisfied’ or ‘Very Satisfied’ of 88% and 91% observed for SAKURA 1 and 2, respectively, on 7-point scale Key Secondary Endpoint: Robust response rates observed on None or Mild outcome measure at all Time Points through Week 24 in Both Pivotal Studies Week SAKURA 1 SAKURA 2 Daxi 40U Daxi 40U 16 71.1% 74.0% 24 35.3% 29.4% Week SAKURA 1 SAKURA 2 Daxi 40U Daxi 40U 16 57.2% 53.4% 24 23.9% 21.6% Investigator Assessment (IGA-FWS) Patient Assessment (PFWS)
SAKURA 1 and 2 Duration of Effect Summary 1Carruthers, J., et al. Injectable DaxibotulinumtoxinA for the Treatment of Glabellar Lines: A Phase 2, Randomized, Dose-Ranging, Double-Blind, Multicenter Comparison with OnabotulinumtoxinA and Placebo. Dermatol. Surg. 2017; 43: 1321 – 1331. SAKURA 1 and SAKURA 2 are the first and only Phase 3 confirmatory studies in patients with moderate to severe glabellar lines that demonstrated median duration of ≥ 24 weeks on multiple clinically meaningful outcome measures Time to loss of None or Mild wrinkle severity (Score of 0 or 1) with Daxi 40U on both IGA-FWS and PFWS replicated duration observed with ≥1 point improvement results Median duration of 24.0 Weeks and 23.9 weeks in SAKURA 1 and 2, respectively Time to return to baseline wrinkle severity with Daxi on both IGA-FWS and PFWS exceeds six months Median duration of 27.7 weeks and 26 weeks in SAKURA 1 and 2, respectively Median duration of ≥1-point improvement from baseline of 24 Weeks observed on IGA-FWS confirmed on patient PFWS assessment measure IGA-FWS: 24.1 Weeks in both SAKURA 1 and SAKURA 2 PFWS: 23.9 Weeks in SAKURA 1 and 23.7 Weeks in SAKURA 2 BELMONT ≥1 point improvement on IGA-FWS: 23.6 weeks with Daxi 40U vs. 18.8 weeks with OnabotulinumtoxinA 20U (p<0.03)1
SAKURA 1 and 2 Studies Safety Summary DaxibotulinumtoxinA 40U was observed to be generally safe and well-tolerated through Week 36 in both pivotal studies Percentage of subjects in SAKURA 1 and 2 with adverse events in the Daxi group were 36% and 46%, respectively, and 25% and 24% in the placebo group, respectively Majority of events in the Daxi group were mild in severity and considered to be unrelated to study drug No subjects in the Daxi group discontinued secondary to AEs Two subjects in the Daxi group in each study experienced a Serious AE, none of which were treatment related Treatment-related AEs in SAKURA 1 and 2 occurred in 17% and 21% of subjects in the Daxi group, respectively, and 8% and 10% in the placebo group, respectively. In the Daxi group across both studies: Most common events were headache (5.9% - 7.0%) and injection site pain (2.4% - 5.0%) Rates of injection-related edema and erythema were < 2.4% and all cases were mild in severity Eyelid ptosis rate of 2.2% observed
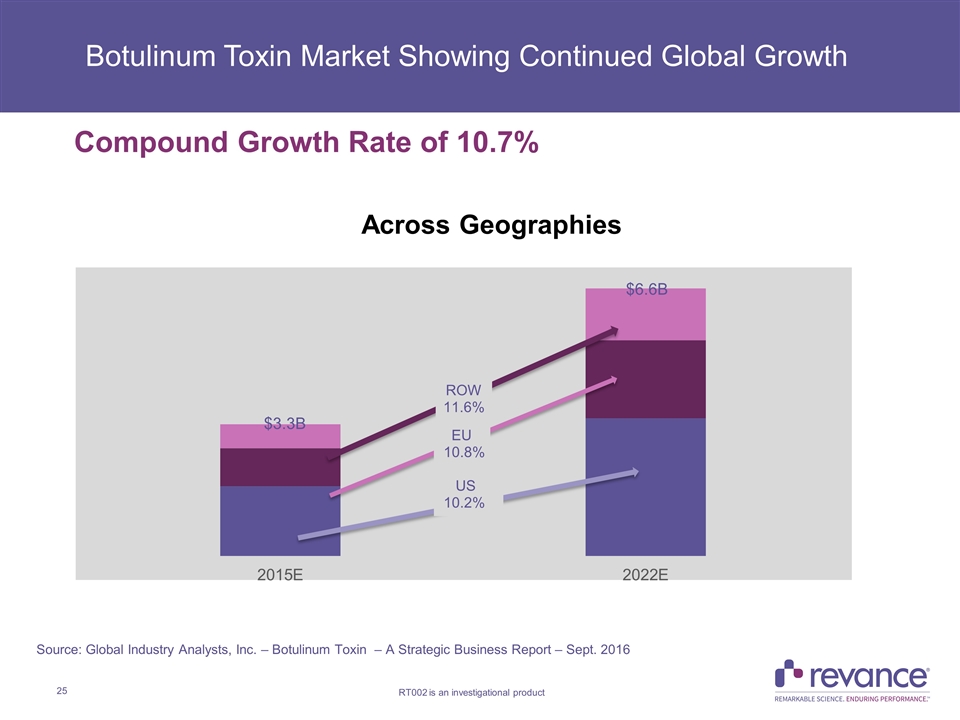
Botulinum Toxin Market Showing Continued Global Growth Compound Growth Rate of 10.7% Across Geographies Source: Global Industry Analysts, Inc. – Botulinum Toxin – A Strategic Business Report – Sept. 2016
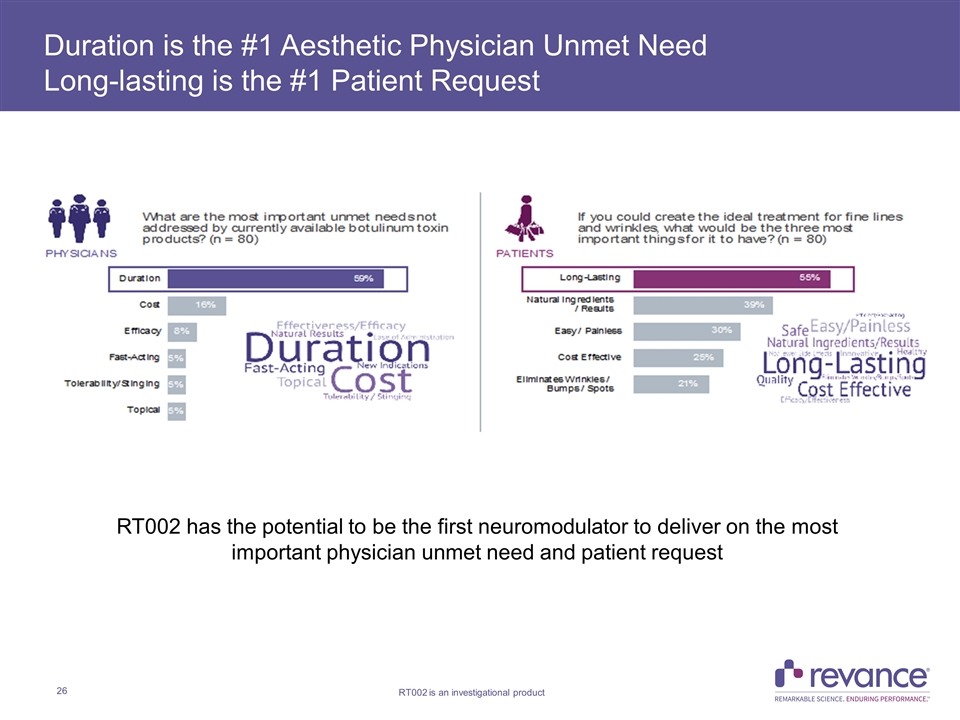
Duration is the #1 Aesthetic Physician Unmet Need Long-lasting is the #1 Patient Request RT002 has the potential to be the first neuromodulator to deliver on the most important physician unmet need and patient request
Building Momentum for Commercial Success BRANDING AND MARKET LAUNCH PREPARATIONS UNDERWAY NURTURING RELATIONSHIPS & FOSTERING ADVOCACY ASSEMBLING COMMERCIAL OPERATION To Drive MARKET ADOPTION REVANCE PRODUCT VELOCITY LAUNCH PLAN
Met Primary and All Secondary Endpoints Highly statistically significant results achieved on 2-point composite primary endpoint at Week 4 Highly statistically significant reduction in severity of glabellar lines observed at Week 24 Median duration for time to return to baseline wrinkle severity on both investigator and patient assessments averaged nearly 27 weeks Demonstrated 6-Month Median Duration on multiple secondary endpoints evaluating reduction in severity of glabellar lines Safety Profile Consistent with DaxibotulinumtoxinA for Injection Prior Trials SAKURA 1 and SAKURA 2 Phase 3 Top-Line Results These positive SAKURA results reinforce the findings from the previous BELMONT Phase 2 active-comparator study of DaxibotulinumtoxinA for Injection as a highly differentiated neuromodulator These SAKURA pivotal trials demonstrate the scientific and clinical validity of a longer-acting neuromodulator with a duration of six months, compared to three to four months observed with currently available products Global Aesthetic Neuromodulator Sales are $1.6B and Growing



























