
Exhibit 99.2

Forward-Looking Statements Safe Harbor / Market Data This presentation contains forward-looking statements, including forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These statements relate to: DaxibotulinumtoxinA potential for the treatment of cervical dystonia; its therapeutic and commercial potential; the process and timing of, and ability to complete, current and anticipated future clinical development of our investigational product candidates; the timing and results of the ASPEN Phase 3 clinical program, including results from the ASPEN-OLS trial; timing and outcome of regulatory determinations regarding potential approval of DaxibotulinumtoxinA and expected PDUFA date and commercial potential of our drug candidates; market size and market for our anticipated products; our business strategy, timeline and other goals and market for our anticipated products. These forward-looking statements are subject to the safe harbor provisions under the Private Securities Litigation Reform Act of 1995. Our expectations and beliefs regarding these matters may not materialize. Actual outcomes and results may differ materially from those contemplated by these forward-looking statements as a result of uncertainties, risks and changes in circumstances, including but not limited to risks and uncertainties related to: the outcome, cost, and timing of our product development activities and clinical trials; the uncertain clinical development process, including the risk that the top-line results from ASPEN-1 trial are based on our preliminary analysis of key efficacy and safety data, and the fact that such data may change following a more comprehensive review of the data related to the clinical trial and such top-line data may not accurately reflect the complete results of the trial, and the FDA may not agree with our interpretation of such results; whether the clinical trials have an effective design or generate positive results; our ability to obtain and maintain regulatory approval of our drug product candidates, including our ability to receive timely approval of DaxibotulinumtoxinA for Injection; our ability to obtain funding for our operations; our plans to research, develop, and commercialize our drug product candidates; unanticipated costs or delays in research, development, and commercialization efforts; the size and growth potential of the markets for our drug product candidates; and the impact of the COVID-19 pandemic on our manufacturing operations, supply chain, business operations, commercialization efforts, end user demand for our products, clinical trials and other aspects of our business. Additional risks and uncertainties that could cause actual outcomes and results to differ materially from those contemplated by the forward-looking statements are included under the caption “Risk Factors” and elsewhere in our most recent filings with the SEC, including our Quarterly Report on Form 10-Q for the quarter ended June 30, 2020 and any subsequent reports on Form 10-K, Form 10-Q or Form 8-K filed with the Securities and Exchange Commission from time to time and available at www.sec.gov. These documents can be accessed on our Investor Relations page at https://investors.revance.com/ by clicking on the link titled “Financials and Filings.” The risks and uncertainties may be amplified by the COVID-19 pandemic, which has caused significant economic uncertainty.

First Phase 3 readout for DaxibotulinumtoxinA for Injection in a therapeutic indication. DaxibotulinumtoxinA for Injection could represent an important advancement in cervical dystonia care for suffering patients. DaxibotulinumtoxinA for Injection treatment effect shows consistent long duration across aesthetic and therapeutic Phase 3 studies. Positive Top-Line Results from Phase 3 Clinical Trial in Cervical Dystonia For Investigational Drug Candidate DaxibotulinumtoxinA for Injection
Cervical Dystonia Landscape Cervical dystonia - (also referred to as spasmodic torticollis) – is a painful chronic condition in which the neck muscles contract involuntarily, causing abnormal movements and awkward posture of the head and neck.1 Botulinum neurotoxins (BoNT) are the standard of care for treatment of cervical dystonia. 2 Treatment approach: dosing varies based on severity and clinical presentation. Physicians initiate treatment with a low dose and titrate up over time based on response and tolerability. 1. Dystonia Medical Research Foundation. Web Site. https://dystonia-foundation.org/what-is-dystonia/types-dystonia/cervical-dystonia/. Accessed 8/11/20 2. Simpson M et al. Practice guideline update summary: Botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: Report of the Guideline Development Subcommittee of the American Academy of Neurology, Neurology 2016 3. NORD Web Site https://rarediseases.org/rare-diseases/cervical-dystonia/. Accessed 8/11/20 4. Botulinum Toxin Global Market Trajectory & Analytics September 2020 pages 37, 64 & 77 MARKET Market of BoNT use in cervical dystonia estimated $340M worldwide.4 Cervical dystonia is an orphan disease. Affects women approximately twice as often as men and typically affects people between 40 and 60 years of age. There are an estimated 60,000 Americans who experience cervical dystonia.3 $1B muscle movement disorder segment for BoNTs.2 $ DEFINITION
In a recent survey of 209 respondents with cervical dystonia, 88% reported the reappearance of pre-existing symptoms between their botulinum toxin injections.6 Significant impact on professional and personal lives. 71% of patients would like longer-lasting benefits from neuromodulator treatments.6 DURATION IS A KEY UNMET NEED Challenges Exist With Currently Available BoNTs BOTULINUM TOXINS ARE STANDARD OF CARE BoNTs are the primary and first-line treatment for cervical dystonia, with ~85% of patients receiving injections.1 Patients receiving currently approved BoNT products typically require retreatment between 12 and 14 weeks.2,3,4s) Symptom reemergence not aligned to payer reimbursed treatment intervals 5 Dysphagia (swallowing difficulty) rates between 13-19% are common after treatment with conventional BoNT products. 2,3,4refs 1. Revance® Market Research 2019: Understanding the Value of DaxibotulinumtoxinA for Injections’ Therapeutic Franchise 2. Botox ® Prescribing Information, 2020 3. Dysport ® Prescribing Information, 2020 4. Xeomin ® Prescribing Information, 2010 5. Botulinum Toxin Global Market Trajectory & Analytics September 2020 pages 37, 64 & 77 6. Comella C, et al. Patient perspectives on the therapeutic profile of botulinum neurotoxin type A in cervical dystonia. Neurology, September 2020

Phase 3 Results of DaxibotulinumtoxinA for Injection in CERVICAL DYSTONIA Presented By Roman G. Rubio, MD Senior Vice President Of Clinical Development

A Phase 3, Randomized, Double-Blind, Placebo-Controlled, Parallel Group, Multi-Center Trial to Evaluate the Efficacy and Safety of a Single Treatment of DaxibotulinumtoxinA for Injection in Adults with Cervical Dystonia
Phase 3 Top-Line Results Demonstrated that DaxibotulinumtoxinA for Injection, at either 125U or 250U, was an effective and generally well-tolerated treatment for reducing the signs and symptoms for cervical dystonia with up to a median duration of 24 weeks. DaxibotulinumtoxinA appeared to be generally safe and well-tolerated with adverse events rates similar to or lower than other BoNT products for the treatment of cervical dystonia Incidence of dysphagia and muscular weakness was encouragingly low The positive results reinforce the findings from the previous studies with DaxibotulinumtoxinA for Injection as a highly differentiated neuromodulator The ASPEN-1 pivotal trial demonstrates the scientific and clinical validity of a long-acting neuromodulator DaxibotulinumtoxinA met Primary and all Secondary Endpoints Highly statistically significant results achieved on TWSTRS Total Score primary endpoint at weeks 4 and 6 p<0.0001 (125 U vs. Placebo) p=0.0006 (250 U vs. Placebo) Median duration for time to reach Target TWSTRS Total Score was 24 weeks for 125U dose and 20 weeks for 250U dose Superior improvement observed on CGIC and PGIC compared with placebo at Weeks 4 and 6
Phase 3 Study Endpoints PRIMARY ENDPOINT: * Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) SECONDARY ENDPOINTS Duration of effect, defined as time from treatment to loss of ≥ 80% of peak treatment effect achieved at Weeks 4 and 6 (i.e. target TWSTRS Total Score) Change from baseline in TWSTRS Total Score over time Percentage of subjects with at least “moderate” (a 2-point) improvement on CGIC at Week 4 or 6 Percentage of subjects with at least “moderate” (a 2-point) improvement on PGIC at Week 4 or 6 Safety - incidence of adverse events EXPLORATORY EFFICACY ENDPOINTS Change from baseline in TWSTRS subscale scores (Severity, Disability, and Pain) Average change from baseline in TWSTRS* Total Score at Weeks 4 and 6 PRIMARY ENDPOINT
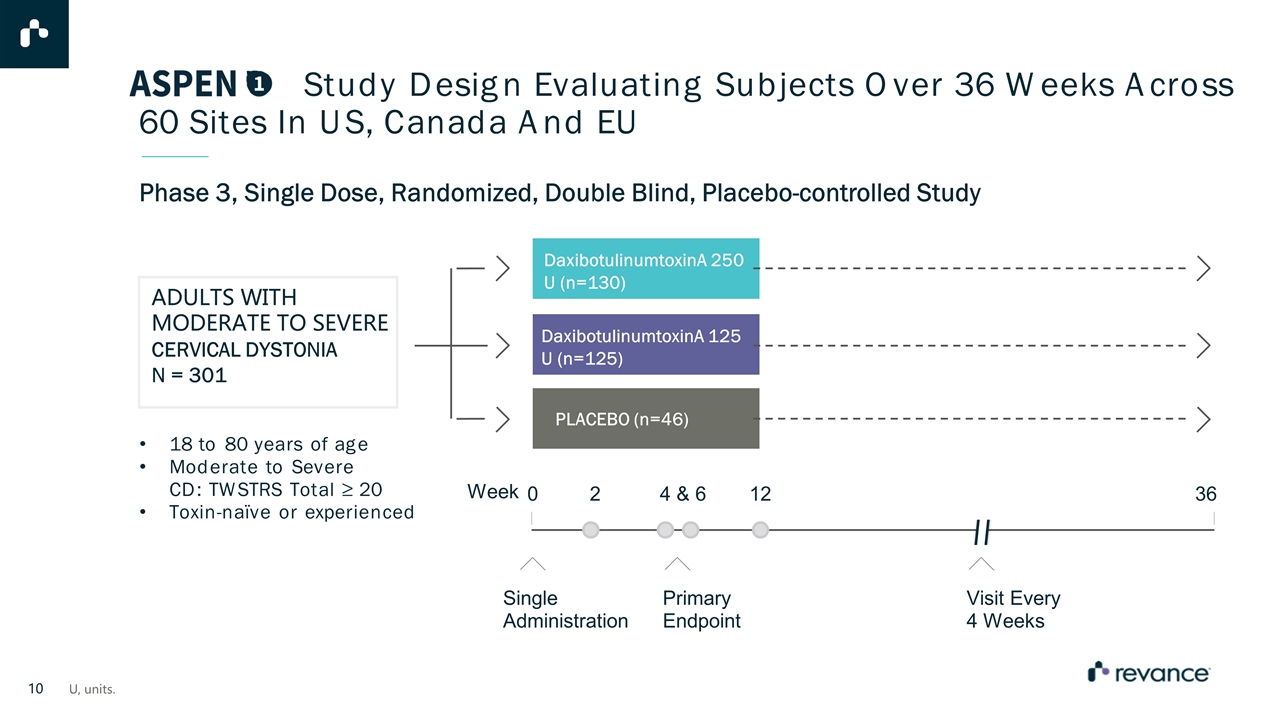
Study Design Evaluating Subjects Over 36 Weeks Across 60 Sites In US, Canada And EU Phase 3, Single Dose, Randomized, Double Blind, Placebo-controlled Study ADULTS WITH MODERATE TO SEVERE CERVICAL DYSTONIA N = 301 18 to 80 years of age Moderate to Severe CD: TWSTRS Total ≥ 20 Toxin-naïve or experienced DaxibotulinumtoxinA 125 U (n=125) DaxibotulinumtoxinA 250 U (n=130) PLACEBO (n=46) Week 0 2 4 & 6 12 36 Single Administration Primary Endpoint Visit Every 4 Weeks U, units.
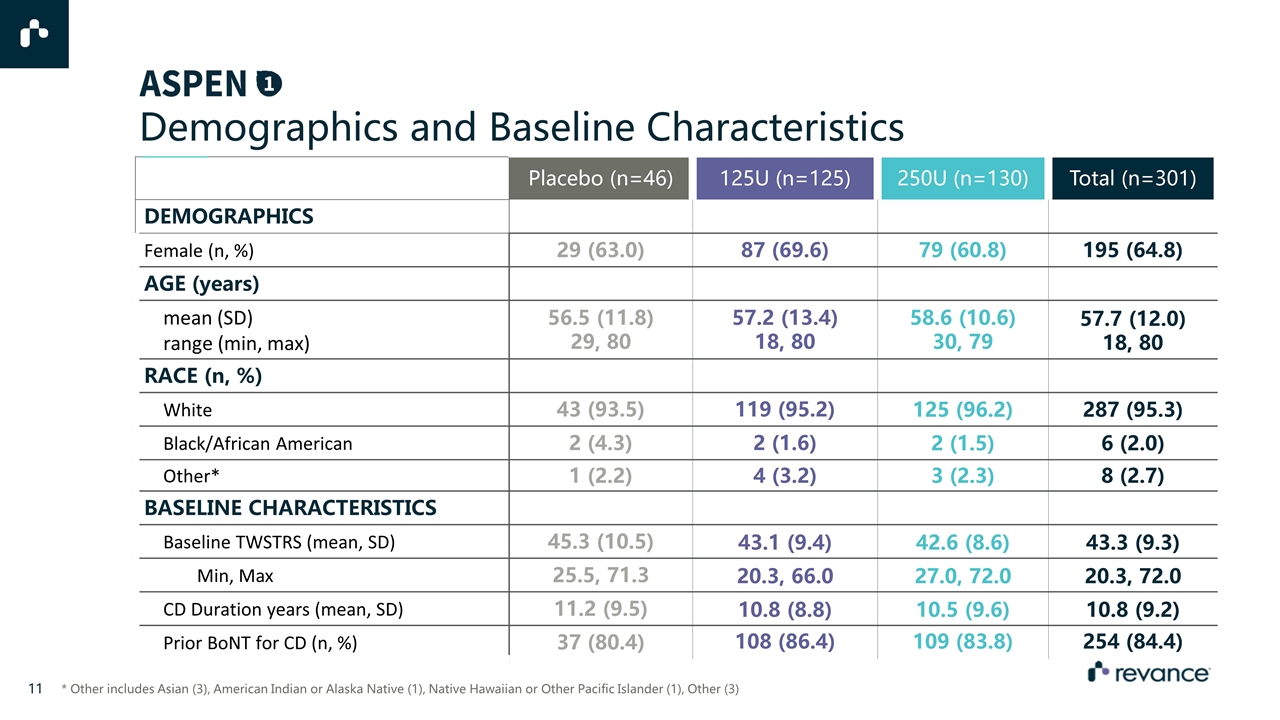
Placebo (n=46) 125U (n=125) 250U (n=130) Total (n=301) DEMOGRAPHICS Female (n, %) 29 (63.0) 87 (69.6) 79 (60.8) 195 (64.8) AGE (years) mean (SD) range (min, max) 56.5 (11.8) 29, 80 57.2 (13.4) 18, 80 58.6 (10.6) 30, 79 57.7 (12.0) 18, 80 RACE (n, %) White 43 (93.5) 119 (95.2) 125 (96.2) 287 (95.3) Black/African American 2 (4.3) 2 (1.6) 2 (1.5) 6 (2.0) Other* 1 (2.2) 4 (3.2) 3 (2.3) 8 (2.7) BASELINE CHARACTERISTICS Baseline TWSTRS (mean, SD) 45.3 (10.5) 43.1 (9.4) 42.6 (8.6) 43.3 (9.3) Min, Max 25.5, 71.3 20.3, 66.0 27.0, 72.0 20.3, 72.0 CD Duration years (mean, SD) 11.2 (9.5) 10.8 (8.8) 10.5 (9.6) 10.8 (9.2) Prior BoNT for CD (n, %) 37 (80.4) 108 (86.4) 109 (83.8) 254 (84.4) Demographics and Baseline Characteristics * Other includes Asian (3), American Indian or Alaska Native (1), Native Hawaiian or Other Pacific Islander (1), Other (3)
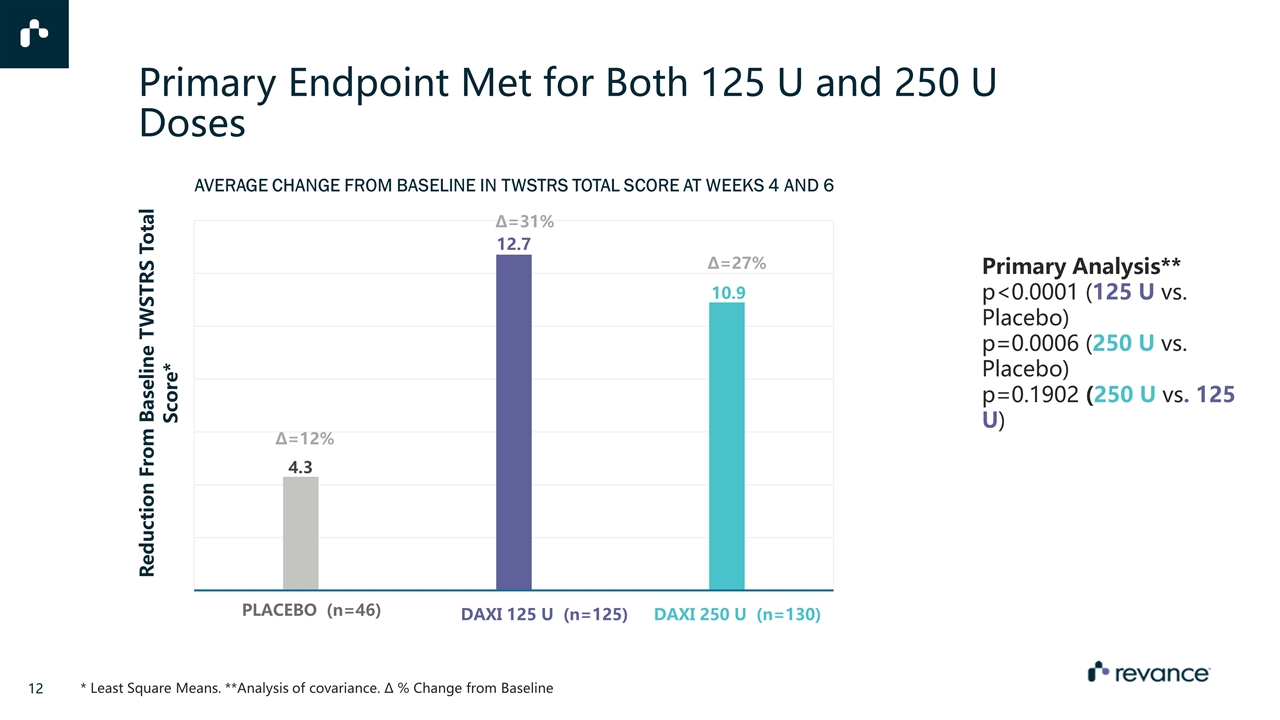
Primary Endpoint Met for Both 125 U and 250 U Doses AVERAGE CHANGE FROM BASELINE IN TWSTRS TOTAL SCORE AT WEEKS 4 AND 6 ∆=12% ∆=31% ∆=27% Placebo (n=46) DAXI 125 U (n=125) DAXI 250 U (n=130) Primary Analysis** p<0.0001 (125 U vs. Placebo) p=0.0006 (250 U vs. Placebo) p=0.1902 (250 U vs. 125 U) * Least Square Means. **Analysis of covariance. ∆ % Change from Baseline
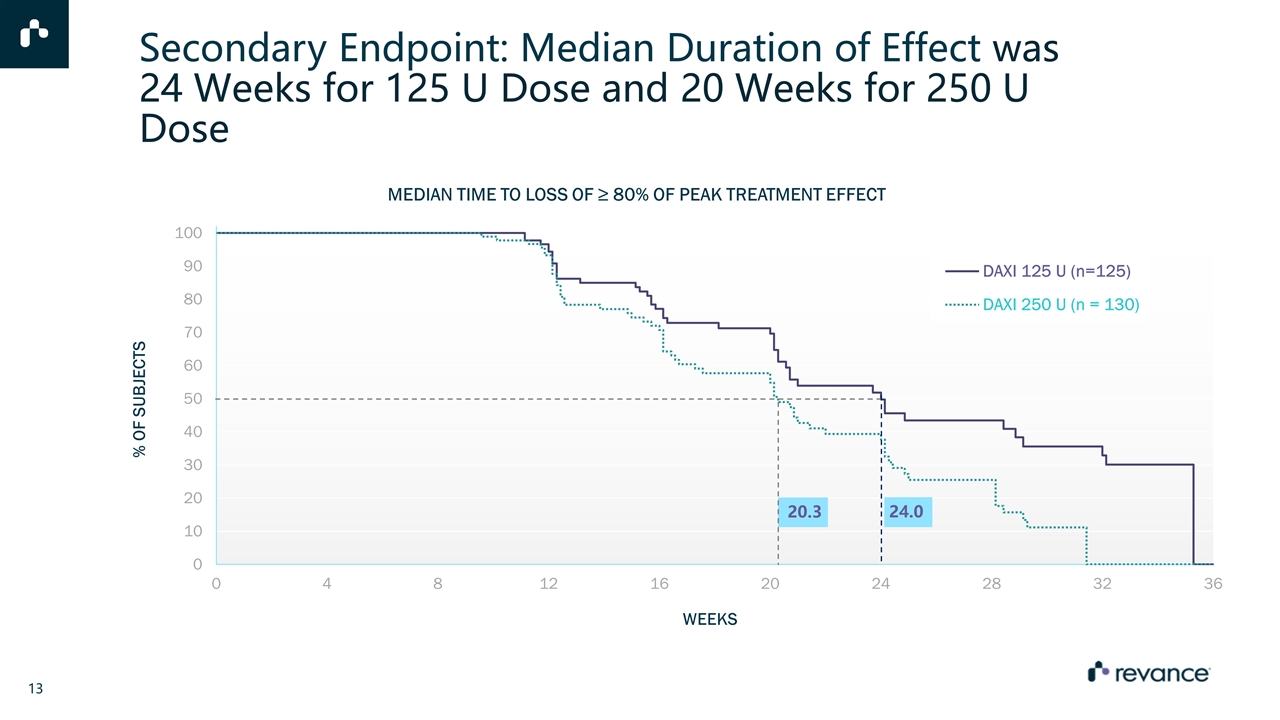
Secondary Endpoint: Median Duration of Effect was 24 Weeks for 125 U Dose and 20 Weeks for 250 U Dose MEDIAN TIME TO LOSS OF ≥ 80% OF PEAK TREATMENT EFFECT
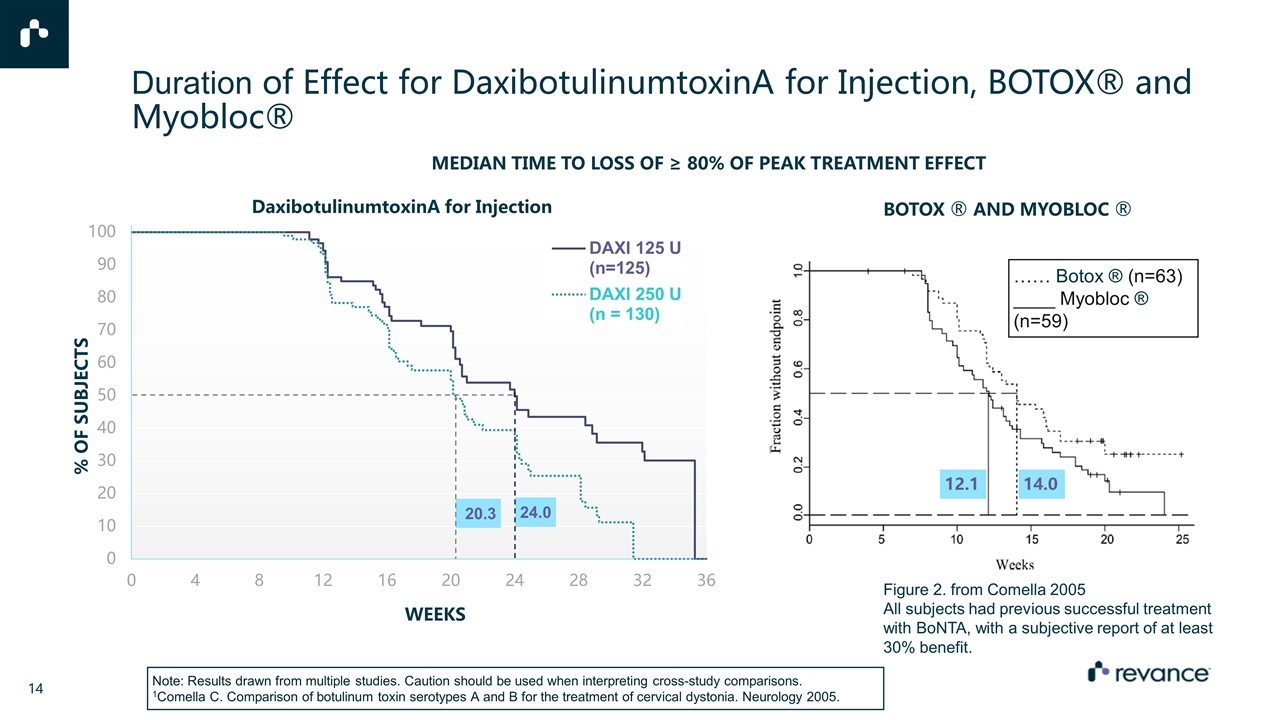
Duration of Effect for DaxibotulinumtoxinA for Injection, BOTOX® and Myobloc® DaxibotulinumtoxinA for Injection Figure 2. from Comella 2005 All subjects had previous successful treatment with BoNTA, with a subjective report of at least 30% benefit. BOTOX ® AND MYOBLOC ® Note: Results drawn from multiple studies. Caution should be used when interpreting cross-study comparisons. 1Comella C. Comparison of botulinum toxin serotypes A and B for the treatment of cervical dystonia. Neurology 2005. MEDIAN TIME TO LOSS OF ≥ 80% OF PEAK TREATMENT EFFECT …… Botox ® (n=63) ____ Myobloc ® (n=59) 12.1 14.0 Opie updated ®
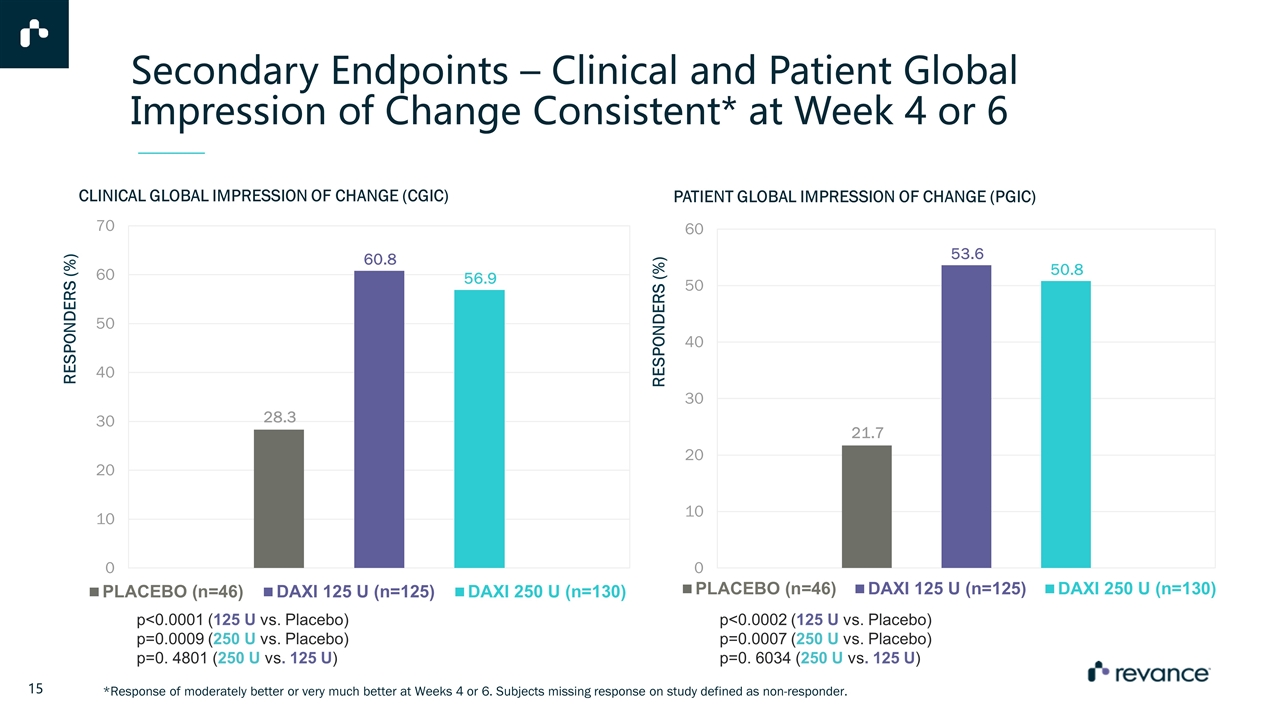
Secondary Endpoints – Clinical and Patient Global Impression of Change Consistent* at Week 4 or 6 *Response of moderately better or very much better at Weeks 4 or 6. Subjects missing response on study defined as non-responder. PATIENT GLOBAL IMPRESSION OF CHANGE (PGIC) p<0.0001 (125 U vs. Placebo) p=0.0009 (250 U vs. Placebo) p=0. 4801 (250 U vs. 125 U) CLINICAL GLOBAL IMPRESSION OF CHANGE (CGIC) p<0.0002 (125 U vs. Placebo) p=0.0007 (250 U vs. Placebo) p=0. 6034 (250 U vs. 125 U)
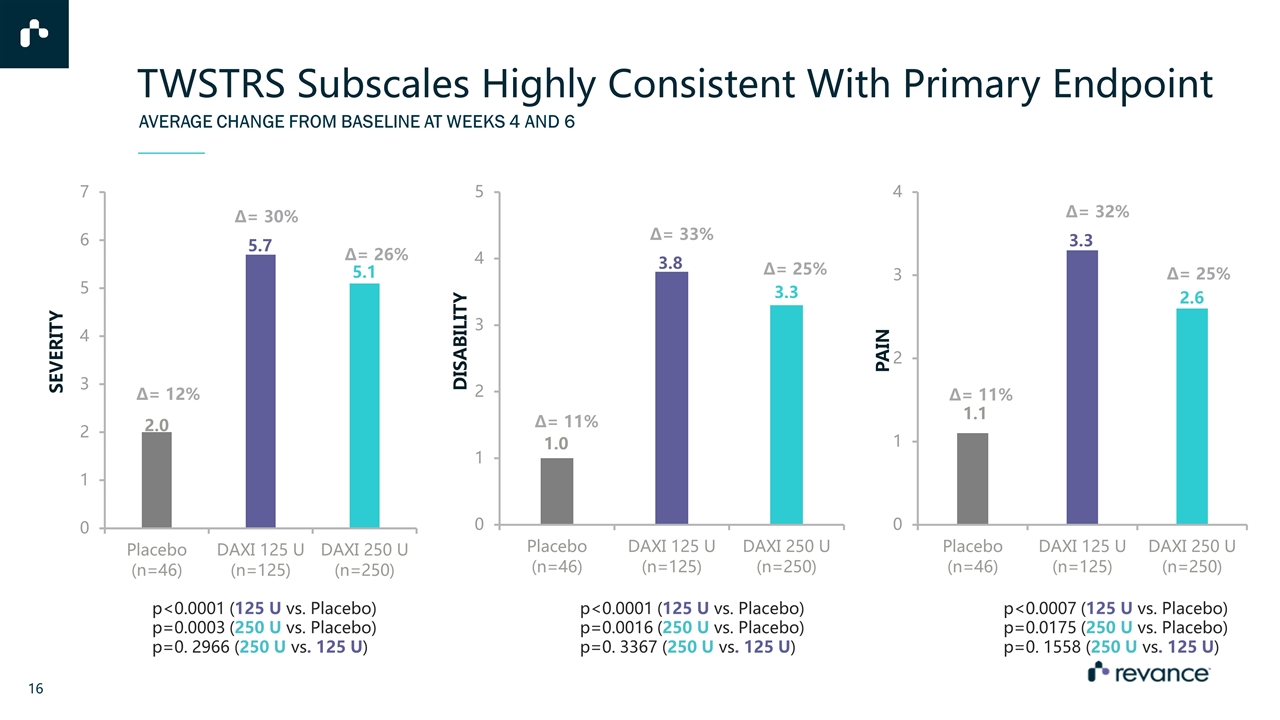
AVERAGE CHANGE FROM BASELINE AT WEEKS 4 AND 6 TWSTRS Subscales Highly Consistent With Primary Endpoint p<0.0001 (125 U vs. Placebo) p=0.0003 (250 U vs. Placebo) p=0. 2966 (250 U vs. 125 U) p<0.0001 (125 U vs. Placebo) p=0.0016 (250 U vs. Placebo) p=0. 3367 (250 U vs. 125 U) p<0.0007 (125 U vs. Placebo) p=0.0175 (250 U vs. Placebo) p=0. 1558 (250 U vs. 125 U)
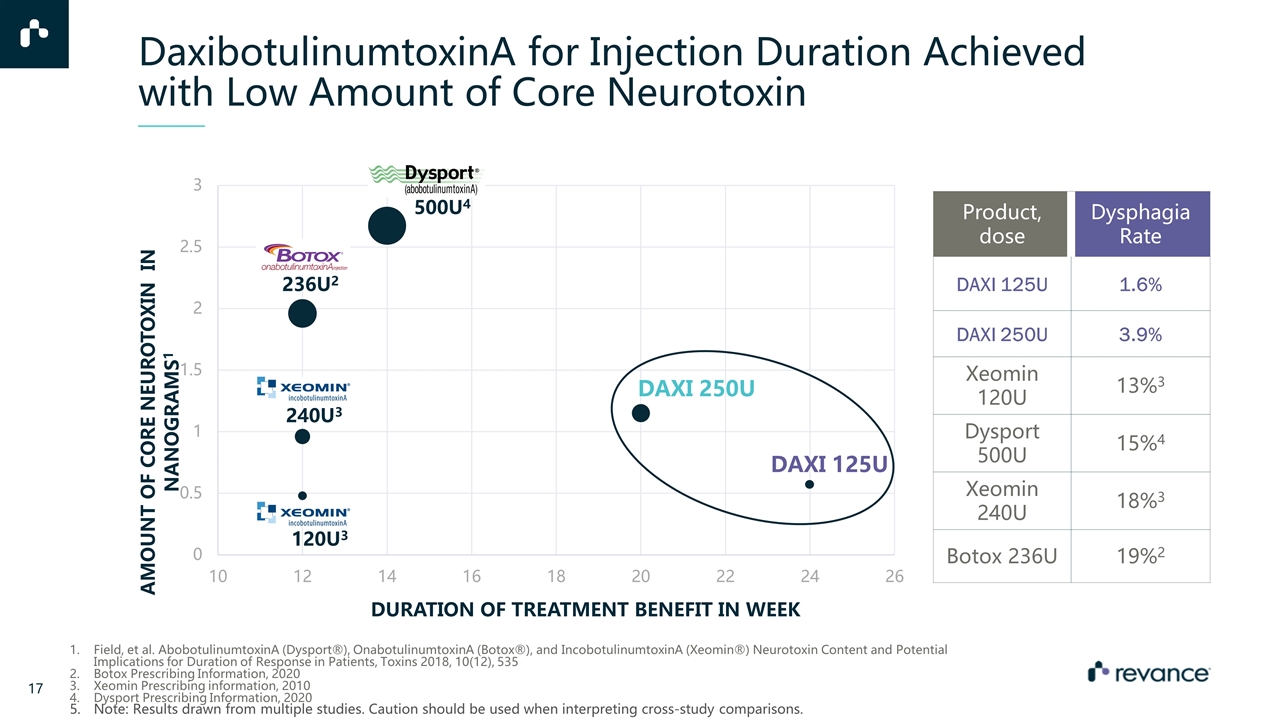
DaxibotulinumtoxinA for Injection Duration Achieved with Low Amount of Core Neurotoxin Product, dose Dysphagia Rate DAXI 125U 1.6% DAXI 250U 3.9% Xeomin 120U 13%3 Dysport 500U 15%4 Xeomin 240U 18%3 Botox 236U 19%2 Field, et al. AbobotulinumtoxinA (Dysport®), OnabotulinumtoxinA (Botox®), and IncobotulinumtoxinA (Xeomin®) Neurotoxin Content and Potential Implications for Duration of Response in Patients, Toxins 2018, 10(12), 535 Botox Prescribing Information, 2020 Xeomin Prescribing information, 2010 Dysport Prescribing Information, 2020 Note: Results drawn from multiple studies. Caution should be used when interpreting cross-study comparisons. DAXI 125U DAXI 250U 240U3 236U2 500U4 120U3
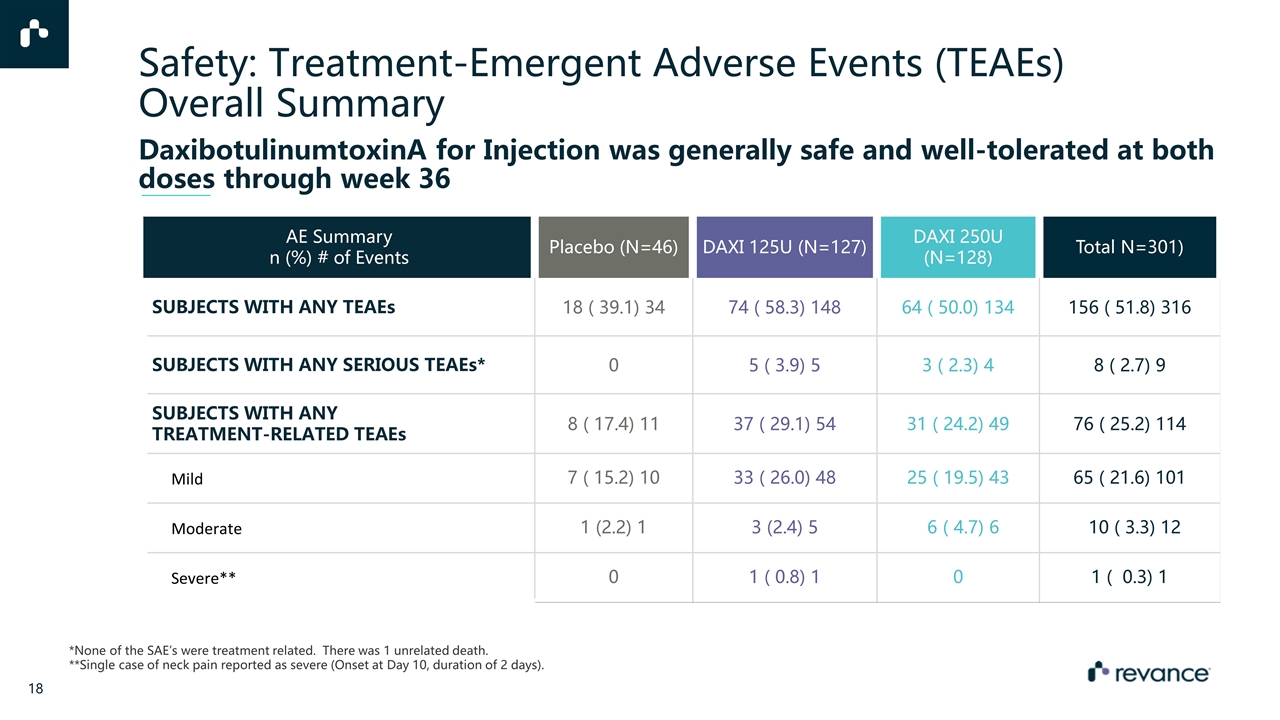
AE Summary n (%) # of Events Placebo (N=46) DAXI 125U (N=127) DAXI 250U (N=128) Total N=301) SUBJECTS WITH ANY TEAEs 18 ( 39.1) 34 74 ( 58.3) 148 64 ( 50.0) 134 156 ( 51.8) 316 SUBJECTS WITH ANY SERIOUS TEAEs* 0 5 ( 3.9) 5 3 ( 2.3) 4 8 ( 2.7) 9 SUBJECTS WITH ANY TREATMENT-RELATED TEAEs 8 ( 17.4) 11 37 ( 29.1) 54 31 ( 24.2) 49 76 ( 25.2) 114 Mild 7 ( 15.2) 10 33 ( 26.0) 48 25 ( 19.5) 43 65 ( 21.6) 101 Moderate 1 (2.2) 1 3 (2.4) 5 6 ( 4.7) 6 10 ( 3.3) 12 Severe** 0 1 ( 0.8) 1 0 1 ( 0.3) 1 *None of the SAE’s were treatment related. There was 1 unrelated death. **Single case of neck pain reported as severe (Onset at Day 10, duration of 2 days). Safety: Treatment-Emergent Adverse Events (TEAEs) Overall Summary DaxibotulinumtoxinA for Injection was generally safe and well-tolerated at both doses through week 36
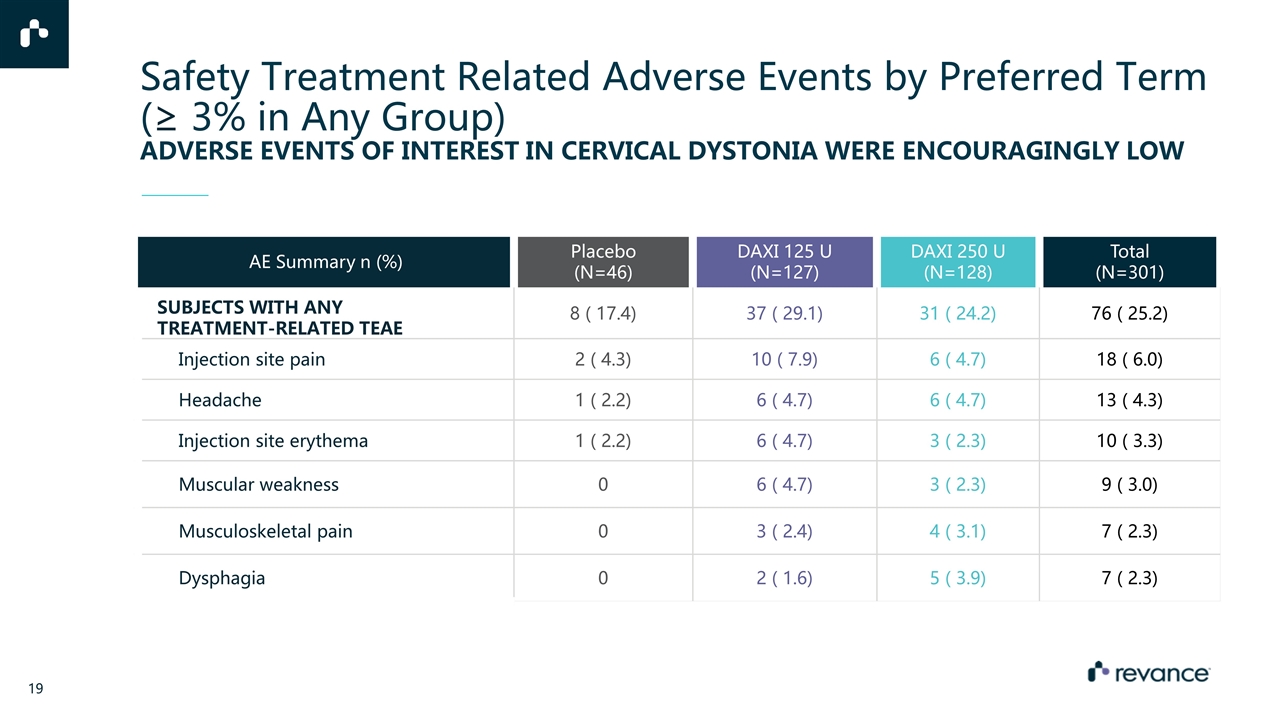
AE Summary n (%) Placebo (N=46) DAXI 125 U (N=127) DAXI 250 U (N=128) Total (N=301) SUBJECTS WITH ANY TREATMENT-RELATED TEAE 8 ( 17.4) 37 ( 29.1) 31 ( 24.2) 76 ( 25.2) Injection site pain 2 ( 4.3) 10 ( 7.9) 6 ( 4.7) 18 ( 6.0) Headache 1 ( 2.2) 6 ( 4.7) 6 ( 4.7) 13 ( 4.3) Injection site erythema 1 ( 2.2) 6 ( 4.7) 3 ( 2.3) 10 ( 3.3) Muscular weakness 0 6 ( 4.7) 3 ( 2.3) 9 ( 3.0) Musculoskeletal pain 0 3 ( 2.4) 4 ( 3.1) 7 ( 2.3) Dysphagia 0 2 ( 1.6) 5 ( 3.9) 7 ( 2.3) Safety Treatment Related Adverse Events by Preferred Term (≥ 3% in Any Group) ADVERSE EVENTS OF INTEREST IN CERVICAL DYSTONIA WERE ENCOURAGINGLY LOW
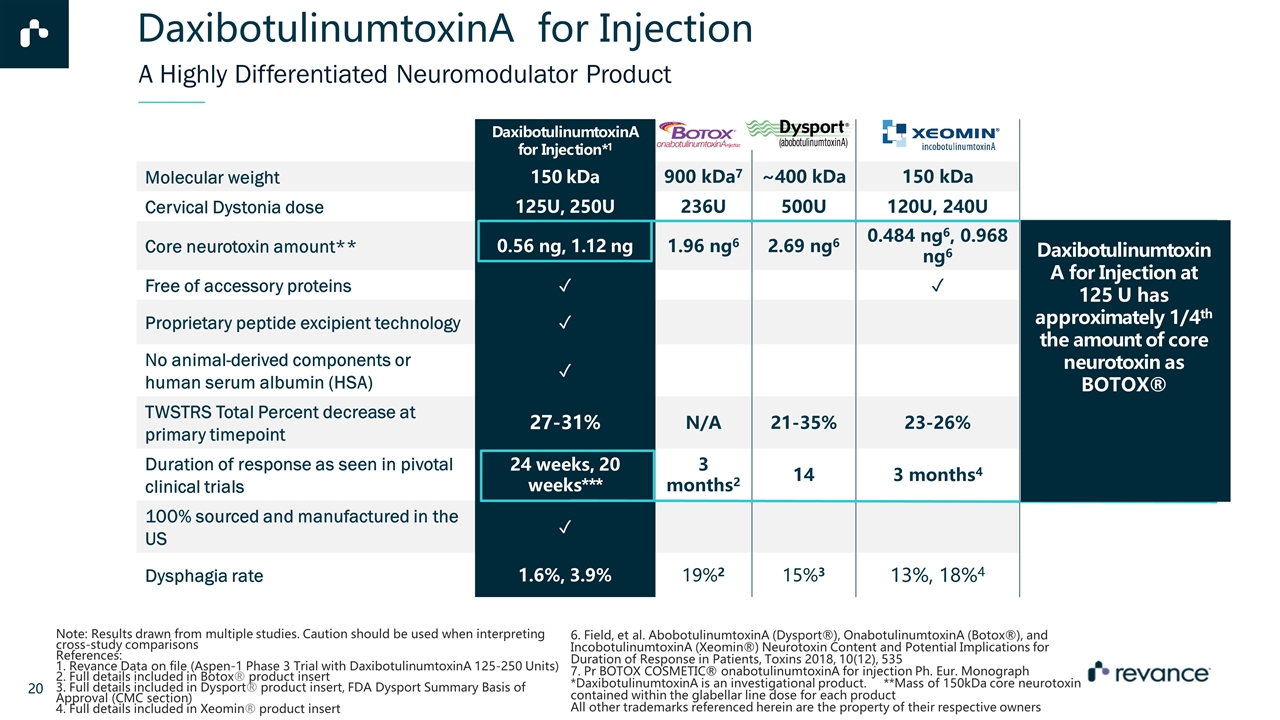
DaxibotulinumtoxinA for Injection*1 Molecular weight 150 kDa 900 kDa7 ~400 kDa 150 kDa Cervical Dystonia dose 125U, 250U 236U 500U 120U, 240U Core neurotoxin amount** 0.56 ng, 1.12 ng 1.96 ng6 2.69 ng6 0.484 ng6, 0.968 ng6 Free of accessory proteins ✓ ✓ Proprietary peptide excipient technology ✓ No animal-derived components or human serum albumin (HSA) ✓ TWSTRS Total Percent decrease at primary timepoint 27-31% N/A 21-35% 23-26% Duration of response as seen in pivotal clinical trials 24 weeks, 20 weeks*** 3 months2 14 weeks3 3 months4 100% sourced and manufactured in the US ✓ Dysphagia rate 1.6%, 3.9% 19%2 15%3 13%, 18%4 A Highly Differentiated Neuromodulator Product Note: Results drawn from multiple studies. Caution should be used when interpreting cross-study comparisons References: 1. Revance Data on file (Aspen-1 Phase 3 Trial with DaxibotulinumtoxinA 125-250 Units) 2. Full details included in Botox® product insert 3. Full details included in Dysport® product insert, FDA Dysport Summary Basis of Approval (CMC section) 4. Full details included in Xeomin® product insert 6. Field, et al. AbobotulinumtoxinA (Dysport®), OnabotulinumtoxinA (Botox®), and IncobotulinumtoxinA (Xeomin®) Neurotoxin Content and Potential Implications for Duration of Response in Patients, Toxins 2018, 10(12), 535 7. Pr BOTOX COSMETIC® onabotulinumtoxinA for injection Ph. Eur. Monograph *DaxibotulinumtoxinA is an investigational product. **Mass of 150kDa core neurotoxin contained within the glabellar line dose for each product All other trademarks referenced herein are the property of their respective owners DaxibotulinumtoxinA for Injection at 125 U has approximately 1/4th the amount of core neurotoxin as BOTOX® DaxibotulinumtoxinA for Injection
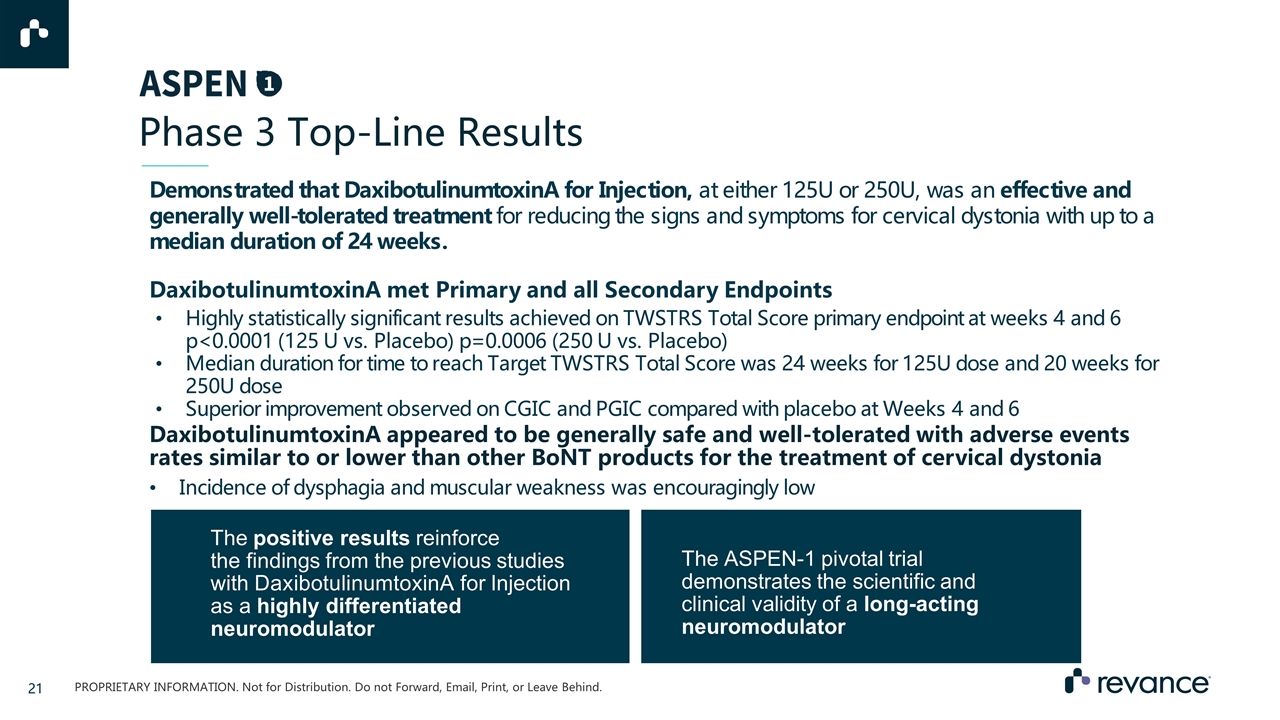
Phase 3 Top-Line Results Demonstrated that DaxibotulinumtoxinA for Injection, at either 125U or 250U, was an effective and generally well-tolerated treatment for reducing the signs and symptoms for cervical dystonia with up to a median duration of 24 weeks. DaxibotulinumtoxinA appeared to be generally safe and well-tolerated with adverse events rates similar to or lower than other BoNT products for the treatment of cervical dystonia Incidence of dysphagia and muscular weakness was encouragingly low The positive results reinforce the findings from the previous studies with DaxibotulinumtoxinA for Injection as a highly differentiated neuromodulator The ASPEN-1 pivotal trial demonstrates the scientific and clinical validity of a long-acting neuromodulator DaxibotulinumtoxinA met Primary and all Secondary Endpoints Highly statistically significant results achieved on TWSTRS Total Score primary endpoint at weeks 4 and 6 p<0.0001 (125 U vs. Placebo) p=0.0006 (250 U vs. Placebo) Median duration for time to reach Target TWSTRS Total Score was 24 weeks for 125U dose and 20 weeks for 250U dose Superior improvement observed on CGIC and PGIC compared with placebo at Weeks 4 and 6

Thank You to Patients, Investigators, CROs & Revance Team

REVANCE THERAPEUTICS

Both physicians and patients consider duration a critically important factor with botulinum toxin treatment.1 1. Timothy Corcoran Flynn , Examining Duration of Effect in Facial Aesthetic Applications , Am J Clin Dermatol 2010; . DaxibotulinumtoxinA For Injection Has Demonstrated Long Duration in Thousands of Patients Globally Including two Phase 3 Aesthetic and Therapeutic Programs Long duration of effect could reduce payer and patient burden of care and provide favorable pharmacoeconomics First true innovation in the neuromodulator market in over 30 years
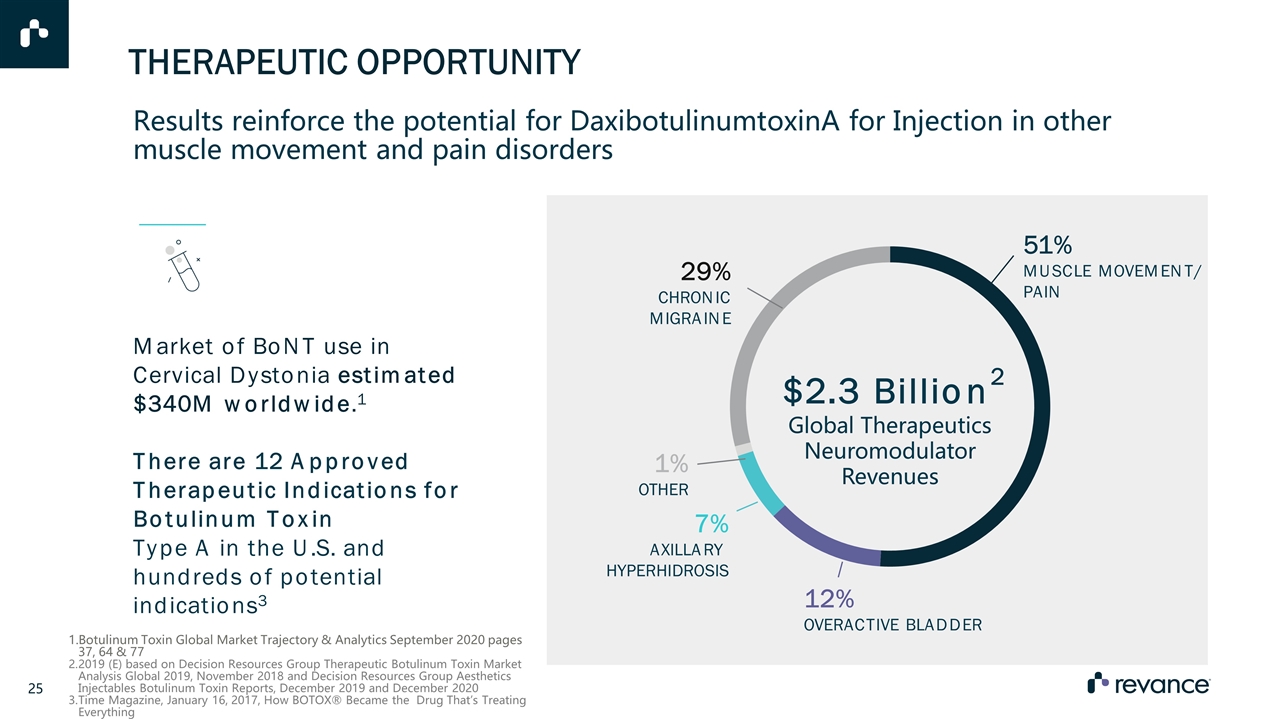
Market of BoNT use in Cervical Dystonia estimated $340M worldwide.1 There are 12 Approved Therapeutic Indications for Botulinum Toxin Type A in the U.S. and hundreds of potential indications3 $2.3 Billion2 Global Therapeutics Neuromodulator Revenues 29% CHRONIC MIGRAINE 51% MUSCLE MOVEMENT/ PAIN 1% OTHER 7% AXILLARY HYPERHIDROSIS 12% OVERACTIVE BLADDER Results reinforce the potential for DaxibotulinumtoxinA for Injection in other muscle movement and pain disorders Botulinum Toxin Global Market Trajectory & Analytics September 2020 pages 37, 64 & 77 2019 (E) based on Decision Resources Group Therapeutic Botulinum Toxin Market Analysis Global 2019, November 2018 and Decision Resources Group Aesthetics Injectables Botulinum Toxin Reports, December 2019 and December 2020 Time Magazine, January 16, 2017, How BOTOX® Became the Drug That’s Treating Everything THERAPEUTIC OPPORTUNITY
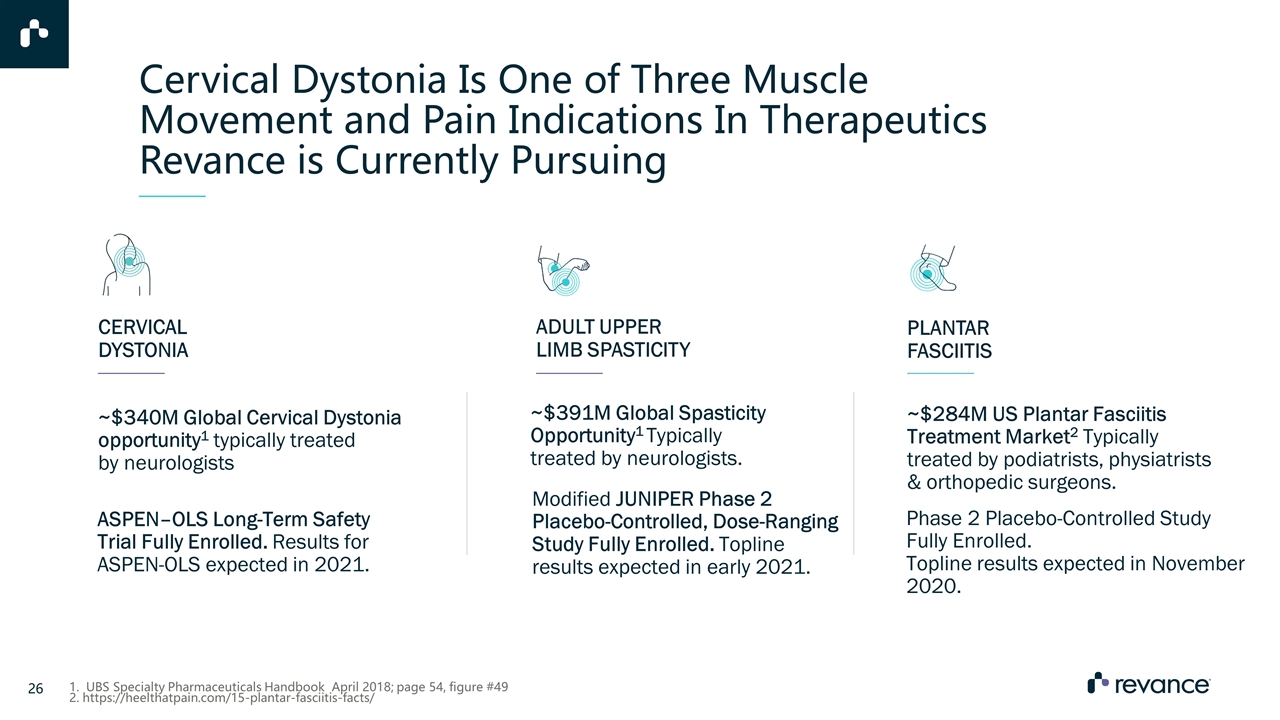
Cervical Dystonia Is One of Three Muscle Movement and Pain Indications In Therapeutics Revance is Currently Pursuing ADULT UPPER LIMB SPASTICITY PLANTAR FASCIITIS ~$391M Global Spasticity Opportunity1 Typically treated by neurologists. Phase 2 Placebo-Controlled Study Fully Enrolled. Topline results expected in November 2020. ~$284M US Plantar Fasciitis Treatment Market2 Typically treated by podiatrists, physiatrists & orthopedic surgeons. Modified JUNIPER Phase 2 Placebo-Controlled, Dose-Ranging Study Fully Enrolled. Topline results expected in early 2021. 1. UBS Specialty Pharmaceuticals Handbook April 2018; page 54, figure #49 2. https://heelthatpain.com/15-plantar-fasciitis-facts/ ASPEN–OLS Long-Term Safety Trial Fully Enrolled. Results for ASPEN-OLS expected in 2021. CERVICAL DYSTONIA ~$340M Global Cervical Dystonia opportunity1 typically treated by neurologists
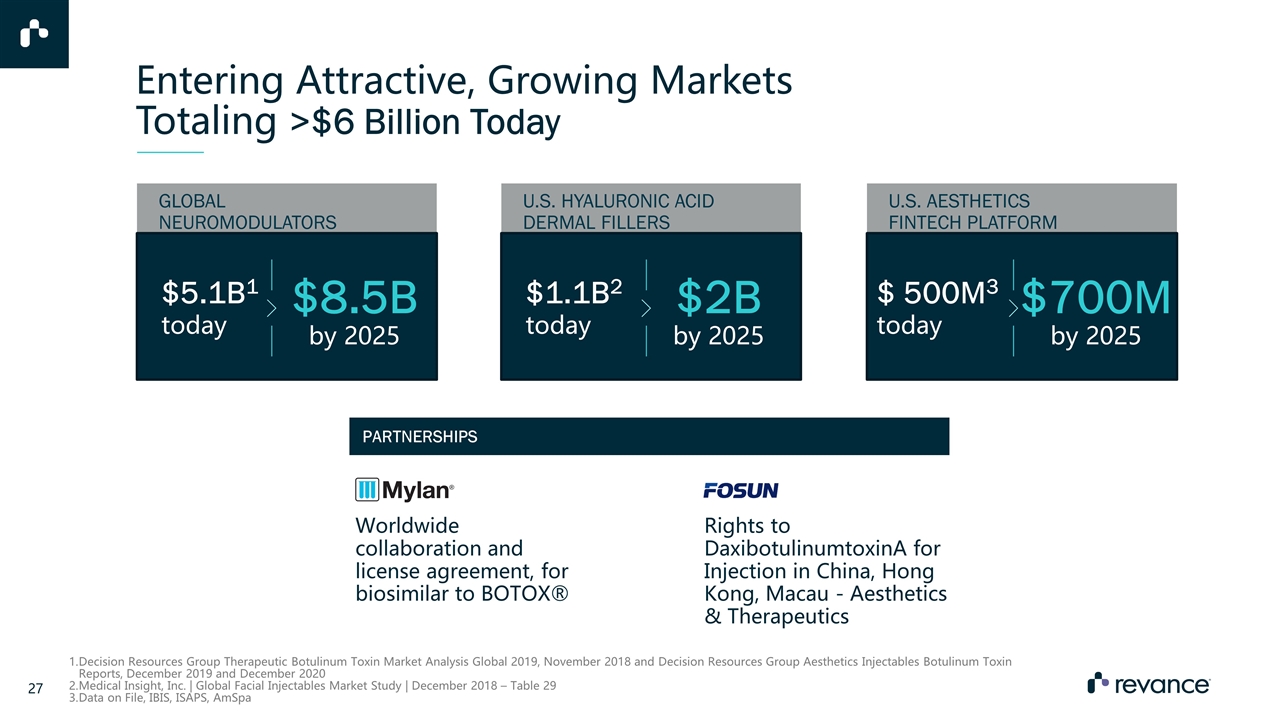
Entering Attractive, Growing Markets Totaling >$6 Billion Today $8.5B by 2025 $5.1B1 today GLOBAL NEUROMODULATORS $2B by 2025 $1.1B2 today U.S. HYALURONIC ACID DERMAL FILLERS $700M by 2025 $ 500M3 today U.S. AESTHETICS FINTECH PLATFORM Decision Resources Group Therapeutic Botulinum Toxin Market Analysis Global 2019, November 2018 and Decision Resources Group Aesthetics Injectables Botulinum Toxin Reports, December 2019 and December 2020 Medical Insight, Inc. | Global Facial Injectables Market Study | December 2018 – Table 29 Data on File, IBIS, ISAPS, AmSpa Worldwide collaboration and license agreement, for biosimilar to BOTOX® Rights to DaxibotulinumtoxinA for Injection in China, Hong Kong, Macau - Aesthetics & Therapeutics PARTNERSHIPS
7555 Gateway Boulevard Newark, California 94560 +1 (510) 742-3400 Thank You.



























