Exhibit 99.1

1 CT - 0508 Study 101 Monotherapy Patient Demographics (n=14) Heavily pre - treated pts with HER2 2+/3+ solid tumors Summary of Participant and Tumor Characteristics Characteristic N = 14 Characteristic N = 14 Median age (range), years 58 (45, 81) Tumor Type, n (%) Breast Cancer Esophageal Cancer Salivary Carcinoma Cholangiocarcinoma Ovarian Cancer 8 (57.1) 2 (14.3) 2 (14.3) 1 (7.1) 1 (7.1) Gender, n (%) Male Female 4 (28.6) 10 (71.4) Race, n (%) White 14 (100) Median Number of Prior Cancer Therapies, n (range) 5 (2, 12) ECOG PS, n (%) 0 1 9 (64.3) 4 (28.6) Median Number of Prior Anti - HER2 Therapies, n (range) Subjects with Prior Anti - HER2 Therapy 2 (0, 9) 13 (92.9) HER2 Overexpression, n (%) IHC 3+ IHC 2+/FISH+ 9 (64.3) 5 (35.7) Prior Radiotherapy, n (%) Yes 9 (64.3) Microsatellite Instability (MSI)* MSS/MSI - Low MSI - High Unknown 13 (92.9) 0 (0) 1 (7.1) Tumor Mutational Burden (TMB)* Low (<10 mut/Mb) High (≥10 mut/Mb) Unknown 11 (78.6) 2 (14.3) † 1 (7.1) * MSI - high and TMB high are known biomarkers associated with improved response to immune checkpoint † 1 patient had received 11 lines of prior therapy and 1 patient has demonstrated HLA - A and HLA - C loss of Heterozygosity

2 4/9/2024 40.7% of all target lesions had reduced in size on at least 1 scan Anatomic Location Frequency of tumor lesions that reduced on treatment on at least 1 scan Breast 1/1 (100%) Liver 4/5 (80%) Lung 1/7 (14.3%) Lymph Node 4/8 (50%) Other 1/4 (25%) Skin/Subcutaneous 0/2 (0%) All Lesions 11/27 (40.7%) Best changes in individual target lesions by anatomic site: Target lesion reduction by anatomic site: Each column represents a single target tumor lesion, not a patient.
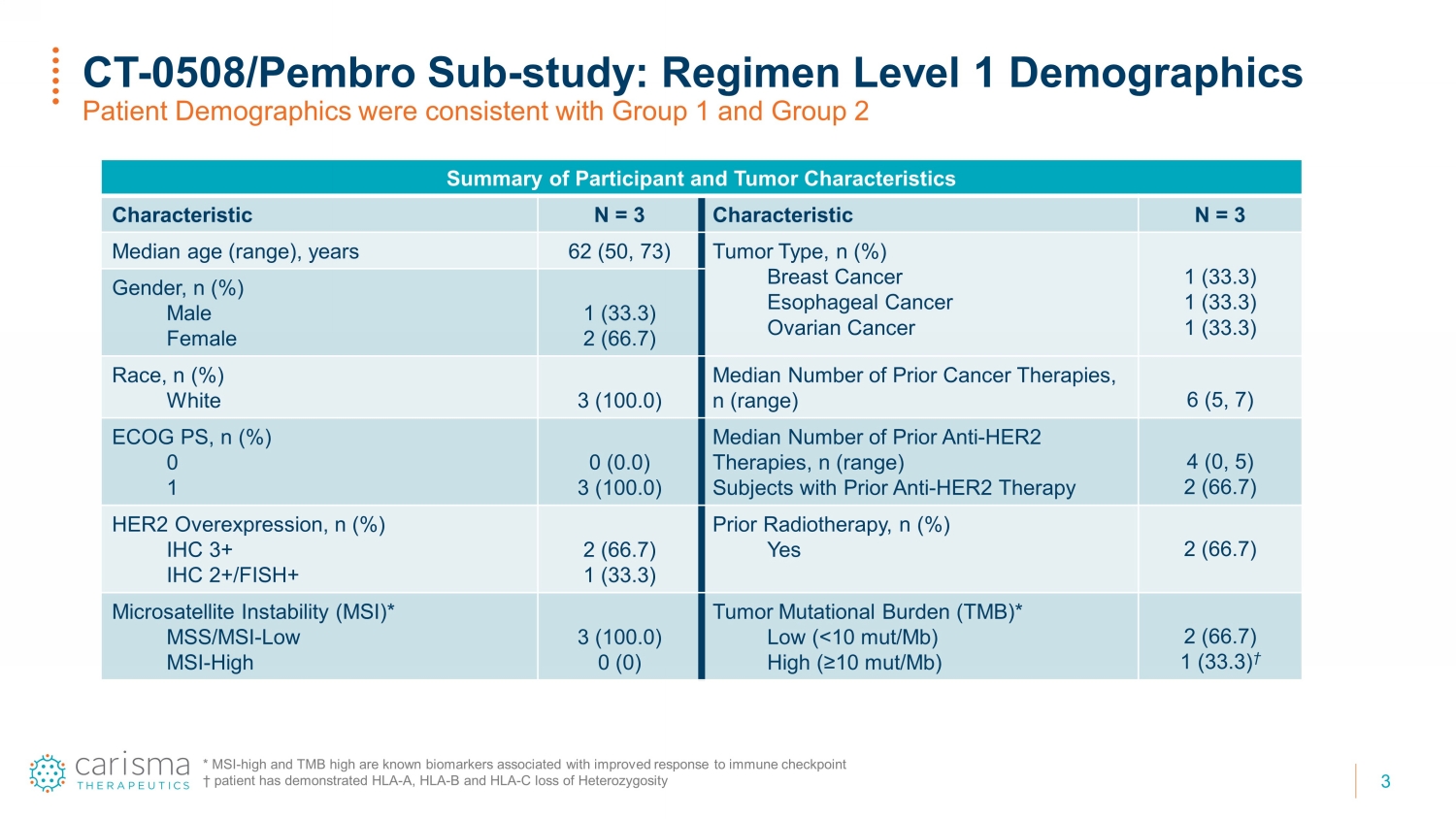
3 CT - 0508/ Pembro Sub - study: Regimen Level 1 Demographics Patient Demographics were consistent with Group 1 and Group 2 Summary of Participant and Tumor Characteristics Characteristic N = 3 Characteristic N = 3 Median age (range), years 62 (50, 73) Tumor Type, n (%) Breast Cancer Esophageal Cancer Ovarian Cancer 1 (33.3) 1 (33.3) 1 (33.3) Gender, n (%) Male Female 1 (33.3) 2 (66.7) Race, n (%) White 3 (100.0) Median Number of Prior Cancer Therapies, n (range) 6 (5, 7) ECOG PS, n (%) 0 1 0 (0.0) 3 (100.0) Median Number of Prior Anti - HER2 Therapies, n (range) Subjects with Prior Anti - HER2 Therapy 4 (0, 5) 2 (66.7) HER2 Overexpression, n (%) IHC 3+ IHC 2+/FISH+ 2 (66.7) 1 (33.3) Prior Radiotherapy, n (%) Yes 2 (66.7) Microsatellite Instability (MSI)* MSS/MSI - Low MSI - High 3 (100.0) 0 (0) Tumor Mutational Burden (TMB)* Low (<10 mut/Mb) High (≥10 mut/Mb) 2 (66.7) 1 (33.3) † * MSI - high and TMB high are known biomarkers associated with improved response to immune checkpoint † patient has demonstrated HLA - A, HLA - B and HLA - C loss of Heterozygosity

4 CT - 0508/ Pembro Sub - study: Well Tolerated, No Dose Limiting Toxicities, Similar Safety Profile to CT - 0508 Monotherapy CT - 0508 Monotherapy Group 1: Fractionated Dosing CT - 0508 Monotherapy Group 2: Bolus Dosing CT - 0508 + Pembrolizumab Regimen 1 Patients Treated N=9 (%) N=5 (%) N=3 (%) 1 Any treatment - emergent AEs (TEAE) 9 (100) 5 (100) 3 (100) Grade 1 - 2 4 (44) 2 (40) 1 (33) Grade 3 - 4 5 (56) 3 (60) 2 (66) Any TEAEs related to CT - 0508 8 (89) 4 (80%) 3 (100) Any TEAEs related to pembrolizumab N/A N/A 1 (33%) Any treatment - emergent SAEs (TESAE) 4 (44) 3 (60) 3 (100) Any TESAEs related to CT - 0508 2 2 (22) 2 (40) 3 (100) Any TESAEs related to pembrolizumab N/A N/A 0 (0) Cytokine release syndrome (CRS) 6 (67) 3 (60) 2 (67) Grade 1 - 2 6 (67) 3 (60) 2 (67) Grade 3 - 4 0 (0) 0 (0) 0 (0) Immune effector cell - associated neurotoxicity syndrome (ICANS) 0 (0) 0 (0) 0 (0) 1. 2 of the 3 patients in the combination study were treated with corticosteroids post CT - 0508, prior to pembrolizumab 2. All TESAEs related to CT - 0508 were due to hospitalization for monitoring of either Grade 2 CRS or Grade 2 infusion reaction. Similar safety profile between CT - 0508 as monotherapy & in combination with pembrolizumab No severe CRS or ICANS

5 CT - 0508/ Pembro Sub - study : Regimen Level 1 (n=3) Summary First two patients received corticosteroids prior to pembrolizumab Patient Steroids Given Prior to Pembro Best Overall Response Disease HER2 Status Additional Treatment Details Patient 1 Yes PD Stage IV Breast Cancer HER2 2+ • Treated with dexamethasone due to G2 CRS post CT - 0508 infusion, prior to pembrolizumab administration Patient 2 Yes PD Stage IV Ovarian Cancer HER2 3+ • Treated with methylpredinosolone due to G3 Infusion reaction post CT - 0508 infusion, prior to pembrolizumab administration • Triple HLA Class I loss of heterozygosity (HLA - A, B and C deletion in tumor genome). Patient 3 No SD (One out of two target lesions reduced by ~46%) Stage IV Esophageal Cancer HER2 3+ • Missed an early cycle (2nd infusion) of pembrolizumab due to medical issues unrelated to therapy • Patient had brain metastasis and progressed per RECIST 1.1 week 14 due to new brain met Additional Information on Corticosteroids and CT - 0508 • Systemic corticosteroids have the potential to reverse the activity of CT - 0508. • Based on in vitro studies, corticosteroids lead to CT - 0508 cell death. • Steroids were given post CT - 0508, pre - pembrolizumab.

6 CT - 0508/ Pembro Sub - study: Patient Case Study Patient #3: HER2+ Esophageal Adenocarcinoma w/ 6 prior lines of therapy and refractory to Enhertu Patient 3 - Prior Line Prior Therapy Start Time End Time Best Overall Response 1 Neoadjuvant carboplatin/paclitaxel Feb 2019 April 2019 CR 2 Adjuvant Capacitabine , oxaliplatin, trastuzumab Nov 2020 Nov 2020 Unknown 3 Fluorouracil, folinic acid, oxaliplatin, trastuzumab Dec 2020 April 2021 PR 4 Fluorouracil, trastuzumab May 2021 March 2022 SD 5 Paclitaxel, ramucirumab, trastuzumab, tucatinib May 2022 Jan 2023 SD 6 Enhertu Feb 2023 April 2023 PD * BOR: Best Overall Response CR: Complete Response; PR: Partial Response; SD: Stable Disease; PD: Progressive disease Cancer type: Stage IV Esophageal adenocarcinoma (EAC), HER2 3+ Prior history: 6 Prior lines of therapy; Most recent prior line: achieved BOR* of PD and DC’d Enhertu in 2 months Pembrolizumab clinical studies in EAC: • EAC is resistant to pembrolizumab monotherapy (KEYNOTE 180) • ORR 5% • PFS 1.5 months • Pembrolizumab did not show a survival benefit over SOC chemotherapy in PDL1+ EAC (KEYNOTE 181)

7 CT - 0508/ Pembro Sub - study: Patient Case Study Patient #3: 46% reduction in 1 of 2 target lesions Paratracheal LN Target Lesion: 46% reduction by week 13 Baseline Week 8 Week 13 Dosing • Patient received 3.10E+ 09 cells • Patient missed the 2nd cycle of pembrolizumab Tumor assessments • Paratracheal target lesion reduction of 46% by week 13; 21.9mm to 11.8mm • Mediastinal mass target lesion grew 31% by week 13; 26.9 to 35.3mm Outcome Comparators PFS Patient 3 – Regimen 1 CT - 0508 / Pembro 3.25 months Patient 3 – 6 th Line of Therapy on Enhertu 2.0 months Pembrolizumab monotherapy in KEYNOTE 180* 1.5 months *KEYNOTE 180: Efficacy and Safety of Pembrolizumab for Heavily Pretreated Patients With Advanced, Metastatic Adenocarcinoma o r S quamous Cell Carcinoma of the Esophagus. JAMA Oncology. 2019. Clinical assessments • Achieved a BOR of SD per RECIST 1.1 • PD per RECIST at week 13 due to new CNS metastasis • PFS of 3.25 months (13.3 weeks)

8 CT - 0508/ Pembro Sub - study: Pt 3 had high baseline peripheral CD8 T cell exhaustion

9 CT - 0508/ Pembro Sub - study: Individual Case Study Patient 3: Greatest increase in peripheral blood T cell clonality seen to - date across all 17 patients treated with CT - 0508 Increased T cell clonality in the peripheral blood T cell clonality (Fold change over screening) Monotherapy Pt #3 - SD Pembrolizumab combo – cohort 1