
HA RNESSING THE POWER OF ENGINEERED MACROPHAGES Carisma Therapeutics December 2022

212/8/2022 Cautionary Note Regarding Forward-Looking Statements Regarding Carisma Statements in this presentation regarding Carisma about future expectations, plans, and prospects, as well as any other statements regarding matters that are not historical facts, may constitute "forward-looking statements." These statements include, but are not limited to, statements relating to the timing and expectations of Carisma’s ongoing and planned clinical trials, research and development programs and collaborations, approval of pending patent applications, the availability of data from clinical trials, the potential benefits of CT-0508, including in combination with other drugs, and the expansion of Carisma’s ex-vivo autologous approach into other modalities. The words "anticipate," "believe," "continue," "could," "estimate," "expect," "intend," "may," "plan," "potential," "predict," "project," "should," "target," "will," "would," and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Any forward-looking statements are based on management's current expectations of future events and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in, or implied by, such forward-looking statements. These risks and uncertainties include, but are not limited to, risks associated with Carisma’s ability to conduct its ongoing Phase 1 clinical trial of CT-0508 and related combination therapy programs; realize the anticipated benefits of its research and development programs, strategic partnerships, research and licensing programs and academic and other collaborations; obtain and maintain necessary approvals from the FDA and other regulatory authorities; continue to advance its product candidates in preclinical studies and clinical trials; replicate in later clinical trials positive results found in preclinical studies and early-stage clinical trials of its product candidates; advance the development of its product candidates under the timelines it anticipates in planned and future clinical trials; obtain, maintain, and protect intellectual property rights related to its product candidates; manage expenses; raise the substantial additional capital needed to achieve its business objectives; consummate the proposed merger between Carisma, Seahawk Merger Sub, Inc. and Sesen Bio, Inc. (“Sesen”), including completing the pre-closing financing transaction; and realize the anticipated benefits of the proposed merger, including with respect to future financial and operating results. For a discussion of other risks and uncertainties, and other important factors, any of which could cause Carisma’s actual results to differ from those contained in the forward-looking statements, see the "Risk Factors" section of Exhibit 99.2 furnished with Sesen’s Current Report on Form 8-K filed with the Securities and Exchange Commission on September 21, 2022. In addition, the forward-looking statements included in this presentation represent Carisma’s views as of the date hereof and should not be relied upon as representing Carisma’s views as of any date subsequent to the date hereof. Carisma anticipates that subsequent events and developments will cause its views to change. However, while Carisma may elect to update these forward-looking statements at some point in the future, Carisma specifically disclaims any obligation to do so.

3 Important Additional Information In connection with the proposed transaction between Carisma Therapeutics and Sesen Bio, Sesen Bio filed with the SEC a registration statement on Form S-4 on October 14, 2022 and Amendment No. 1 to the Form S-4 on November 21, 2022 (as amended, the Form S-4). The Form S-4 includes a preliminary proxy statement of Sesen Bio and also constitutes a prospectus of Sesen Bio with respect to shares of Sesen Bio’s common stock to be issued in the proposed transaction (Preliminary Proxy Statement/Prospectus). The Preliminary Proxy Statement/Prospectus is not final and may be further amended. The definitive proxy statement/prospectus (if and when available) will be delivered to Sesen Bio’s stockholders. Sesen Bio may also file other relevant documents regarding the proposed transaction with the SEC. INVESTORS AND SECURITY HOLDERS ARE URGED TO READ THESE MATERIALS, INCLUDING THE REGISTRATION STATEMENT, THE DEFINITIVE PROXY STATEMENT/PROSPECTUS, AND ALL OTHER RELEVANT DOCUMENTS THAT ARE OR WILL BE FILED WITH THE SEC IN CONNECTION WITH THE PROPOSED TRANSACTION, INCLUDING ANY AMENDMENTS OR SUPPLEMENTS TO THESE MATERIALS, BECAUSE THEY CONTAIN OR WILL CONTAIN IMPORTANT INFORMATION ABOUT THE PROPOSED TRANSACTION AND THE PARTIES TO THE PROPOSED TRANSACTION. Investors and security holders are able to obtain the Preliminary Proxy Statement/Prospectus, the definitive proxy statement/prospectus (when it becomes available) and other documents that are filed or will be filed by Sesen Bio with the SEC free of charge from the SEC’s website at www.sec.gov or from Sesen Bio at the SEC Filings section of www.sesenbio.com. No Offer or Solicitation This presentation shall not constitute an offer to sell or the solicitation of an offer to buy any securities, nor shall there be any sale of securities in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction. No offering of securities shall be made except by means of a prospectus meeting the requirements of Section 10 of the Securities Act of 1933, as amended. Subject to certain exceptions to be approved by the relevant regulators or certain facts to be ascertained, a public offer will not be made directly or indirectly, in or into any jurisdiction where to do so would constitute a violation of the laws of such jurisdiction, or by use of the mails or by any means or instrumentality (including without limitation, facsimile transmission, telephone or internet) of interstate or foreign commerce, or any facility of a national securities exchange, of any such jurisdiction. Participants in the Solicitation Sesen Bio and Carisma Therapeutics and their respective directors, executive officers and other members of management may be deemed to be participants in the solicitation of proxies in respect of the proposed transaction. Information about Sesen Bio’s directors and executive officers is available in Sesen Bio’s Annual Report on Form 10-K for the fiscal year ended December 31, 2021, its definitive proxy statement dated April 28, 2022 for its 2022 Annual Meeting of Stockholders and its Current Report on Form 8-K filed with the SEC on August 31, 2022. Other information regarding the participants in the proxy solicitation and a description of their interests in the proposed transaction, by security holdings or otherwise, is included in the Preliminary Proxy Statement/Prospectus and other relevant materials that are or will be filed with the SEC regarding the proposed transaction. Investors should read the definitive proxy statement/prospectus carefully (when it becomes available) before making any voting or investment decisions. You may obtain free copies of these documents from Sesen Bio or the SEC’s website as indicated above. 12/8/2022

4 • Cutting edge research and bioengineering: o Proprietary platform for macrophage targeted therapies o Autologous/ allogeneic/ in-vivo modalities o Broad potential therapeutic applications, in oncology & beyond • Strong patent position covering all CAR-M therapies • Early clinical data for lead program demonstrating feasibility, tolerability, and MoA in HER2+ solid tumors • Validating partnership with Moderna to develop up to 12 in-vivo cancer therapies with $80M upfront ($45M cash plus $35M equity in a convertible note), full R&D funding, and potential significant milestones and royalties • Multiple potential value inflection points over the next 18 months Carisma is Positioned for Success Rapid progress with significant opportunity to become a breakthrough therapeutics company Our Mission is to Develop Transformative Macrophage Targeted Therapies for Patients with Devastating Diseases C O M PA N Y H I G H L I G H T S : CAR-M = Chimeric Antigen Receptor Macrophage 12/8/2022
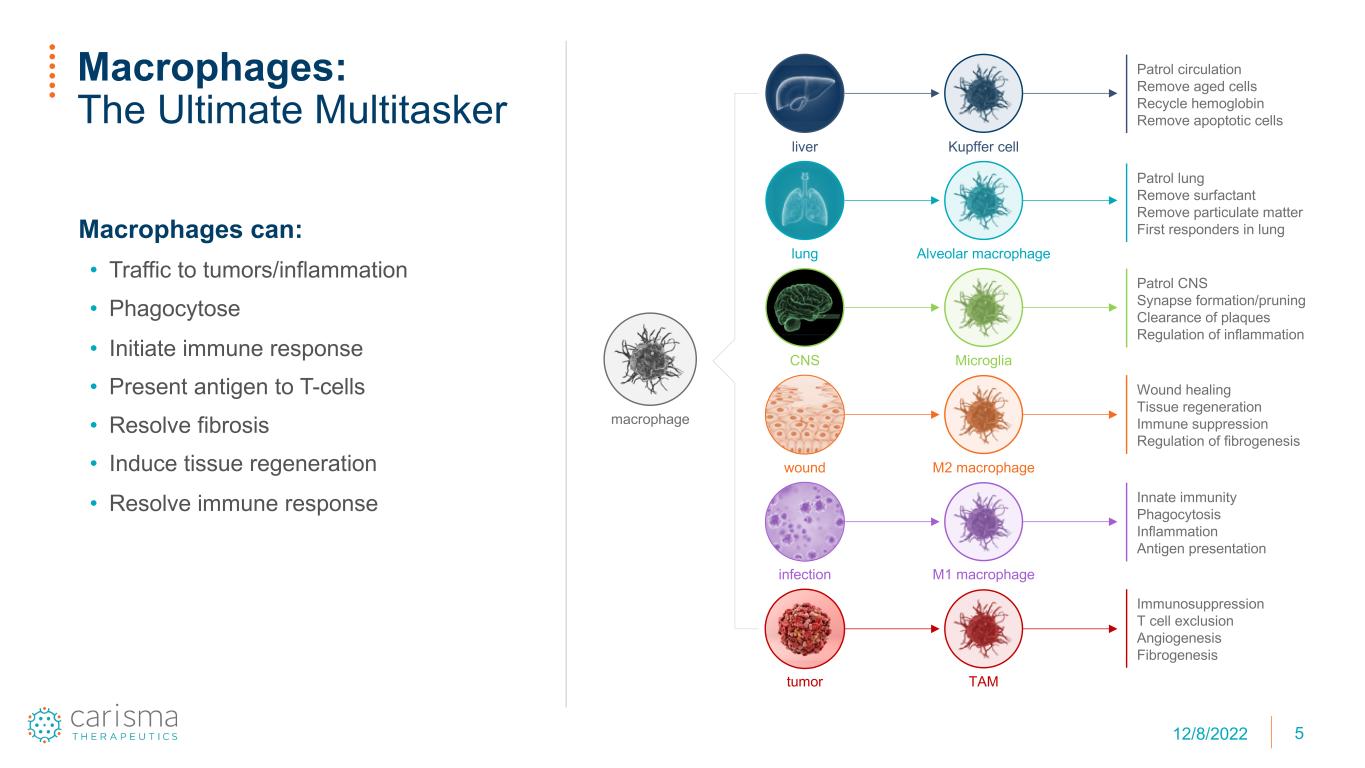
5 Macrophages can: • Traffic to tumors/inflammation • Phagocytose • Initiate immune response • Present antigen to T-cells • Resolve fibrosis • Induce tissue regeneration • Resolve immune response Macrophages: The Ultimate Multitasker liver lung CNS wound infection tumor TAM M1 macrophage M2 macrophage Microglia Alveolar macrophage Kupffer cell macrophage Patrol circulation Remove aged cells Recycle hemoglobin Remove apoptotic cells Patrol lung Remove surfactant Remove particulate matter First responders in lung Patrol CNS Synapse formation/pruning Clearance of plaques Regulation of inflammation Wound healing Tissue regeneration Immune suppression Regulation of fibrogenesis Innate immunity Phagocytosis Inflammation Antigen presentation Immunosuppression T cell exclusion Angiogenesis Fibrogenesis 1st time only or for platform pitch 12/8/2022
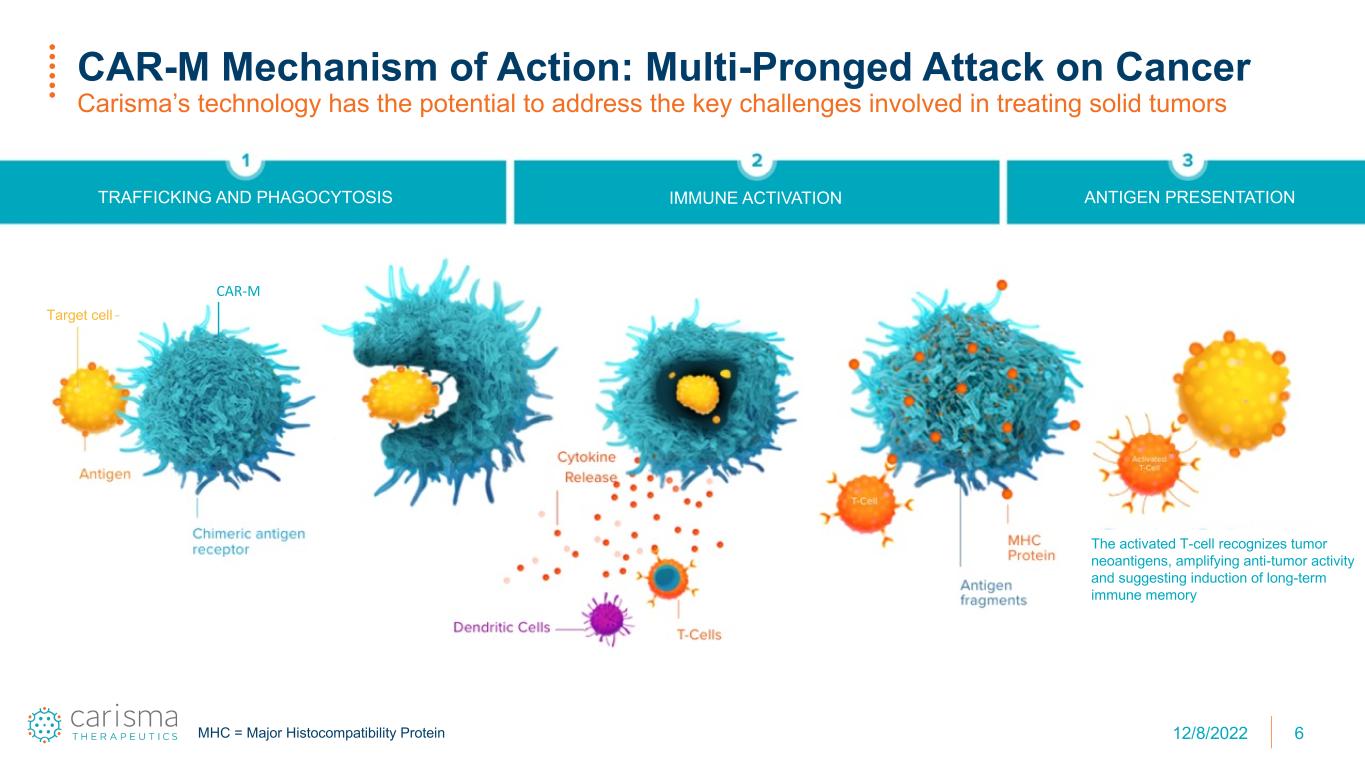
6 CAR-M Mechanism of Action: Multi-Pronged Attack on Cancer Carisma’s technology has the potential to address the key challenges involved in treating solid tumors TRAFFICKING AND PHAGOCYTOSIS IMMUNE ACTIVATION ANTIGEN PRESENTATION Target cell 1st time only CAR-M The activated T-cell recognizes tumor neoantigens, amplifying anti-tumor activity and suggesting induction of long-term immune memory MHC = Major Histocompatibility Protein 12/8/2022
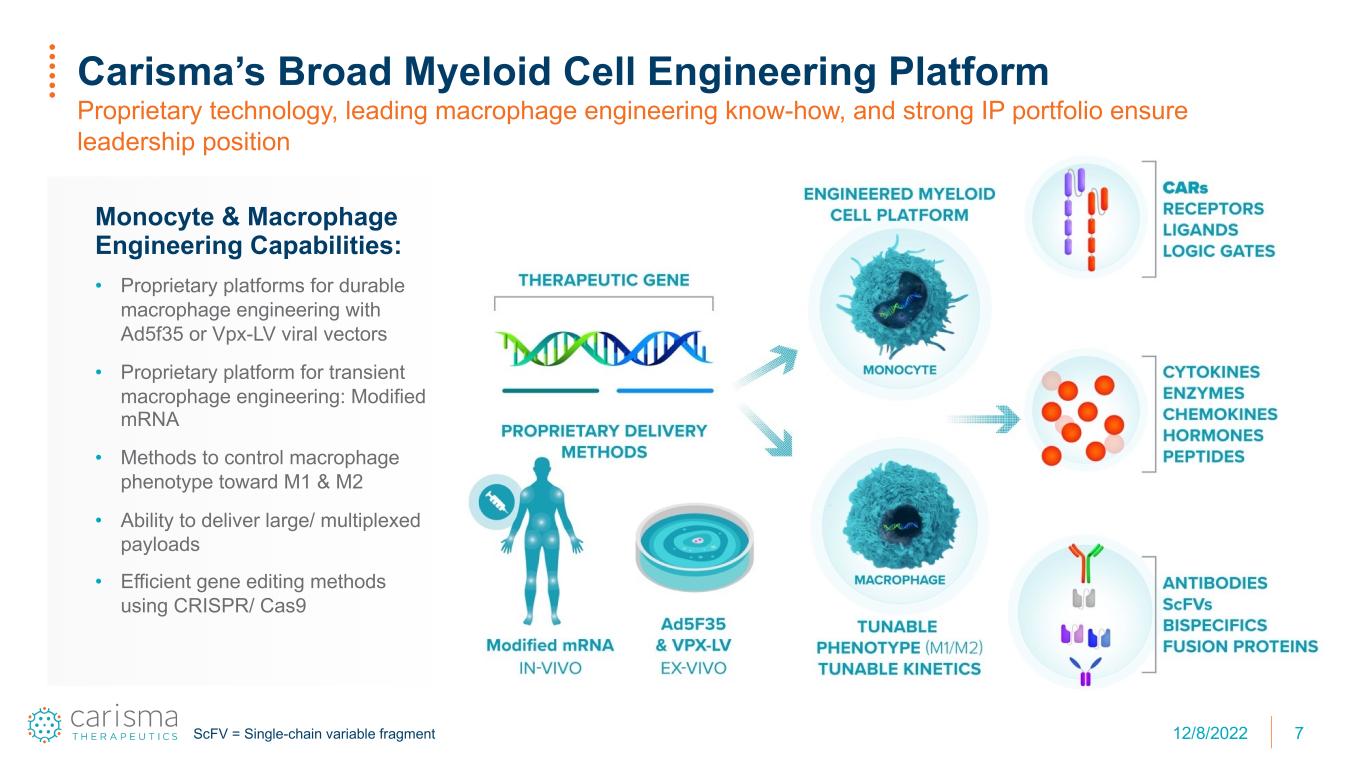
7 Carisma’s Broad Myeloid Cell Engineering Platform Proprietary technology, leading macrophage engineering know-how, and strong IP portfolio ensure leadership position Monocyte & Macrophage Engineering Capabilities: • Proprietary platforms for durable macrophage engineering with Ad5f35 or Vpx-LV viral vectors • Proprietary platform for transient macrophage engineering: Modified mRNA • Methods to control macrophage phenotype toward M1 & M2 • Ability to deliver large/ multiplexed payloads • Efficient gene editing methods using CRISPR/ Cas9 ScFV = Single-chain variable fragment 12/8/2022
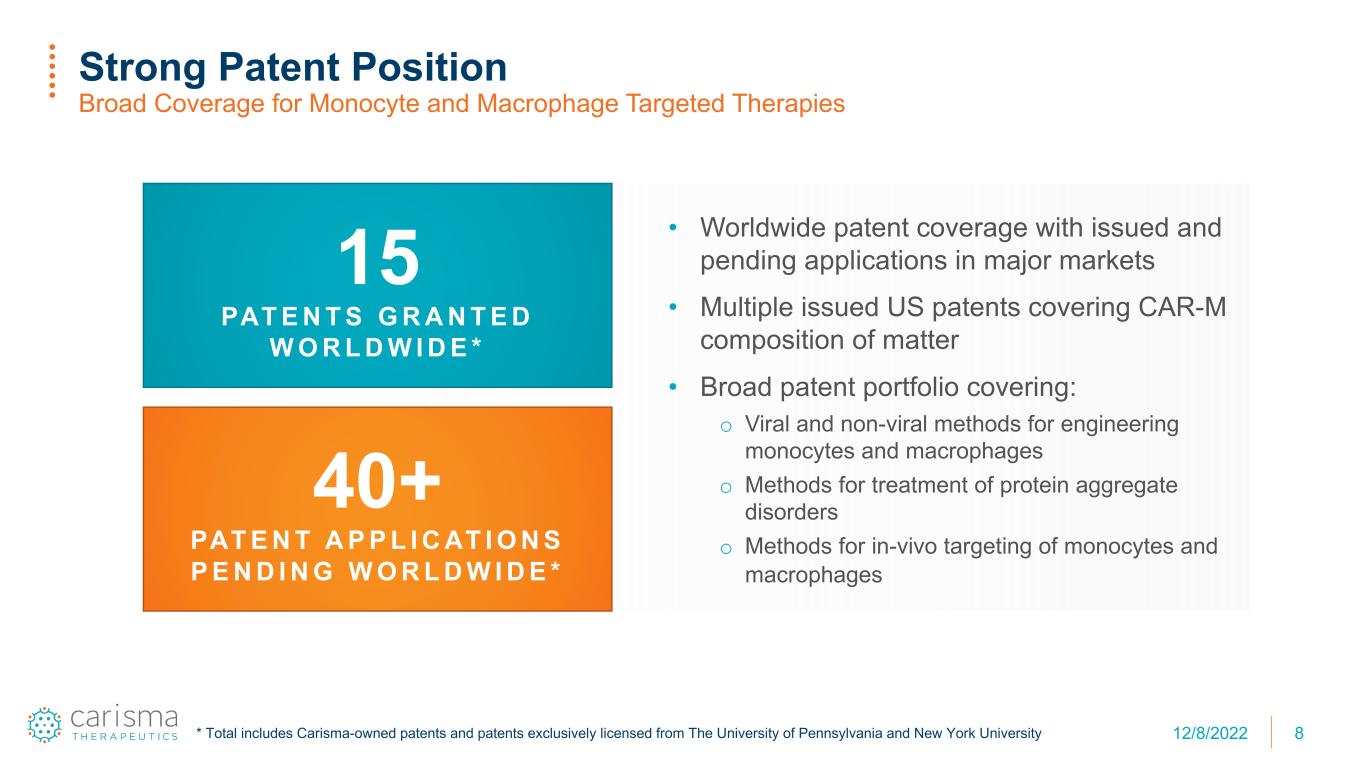
8 Strong Patent Position Broad Coverage for Monocyte and Macrophage Targeted Therapies • Worldwide patent coverage with issued and pending applications in major markets • Multiple issued US patents covering CAR-M composition of matter • Broad patent portfolio covering: o Viral and non-viral methods for engineering monocytes and macrophages o Methods for treatment of protein aggregate disorders o Methods for in-vivo targeting of monocytes and macrophages 15 PAT E N T S G R A N T E D W O R L D W I D E * 40+ PAT E N T A P P L I C AT I O N S P E N D I N G W O R L D W I D E * * Total includes Carisma-owned patents and patents exclusively licensed from The University of Pennsylvania and New York University 12/8/2022
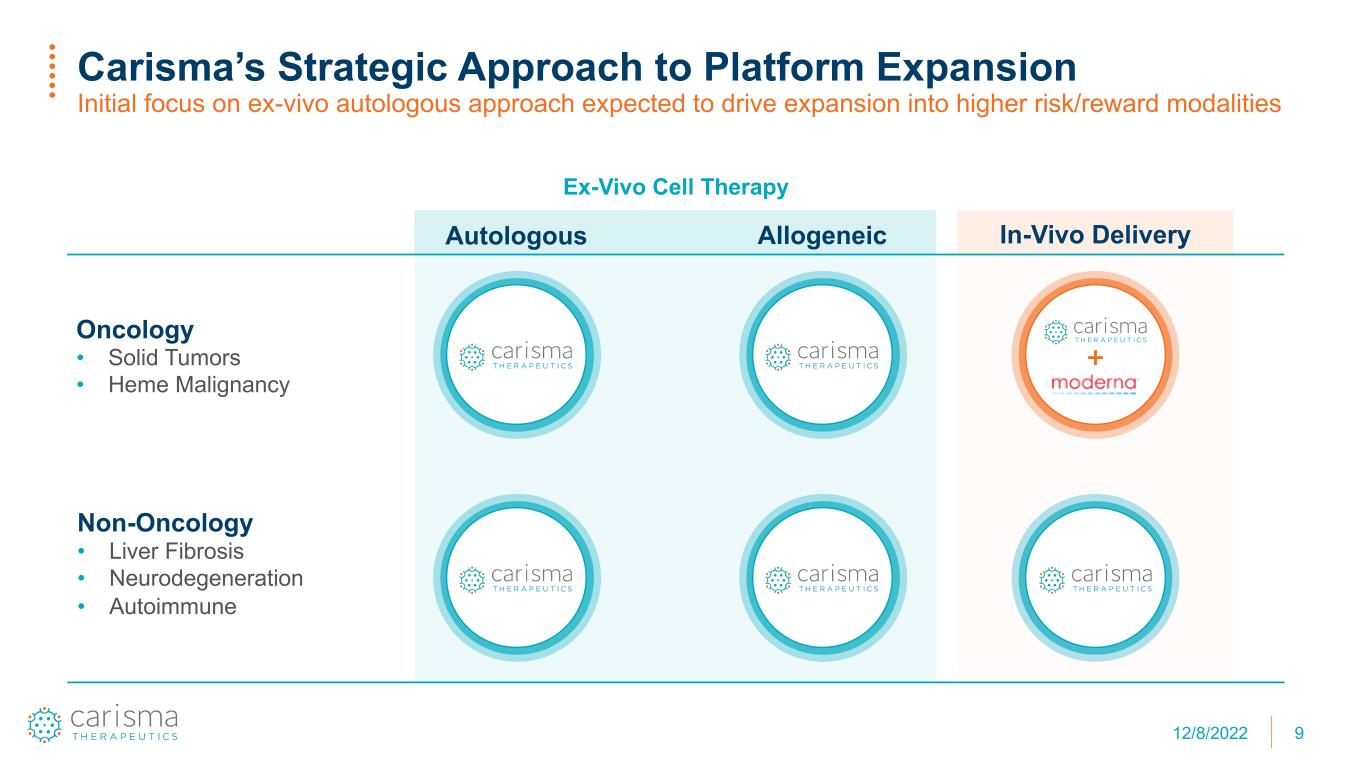
9 Carisma’s Strategic Approach to Platform Expansion Initial focus on ex-vivo autologous approach expected to drive expansion into higher risk/reward modalities In-Vivo Delivery Oncology • Solid Tumors • Heme Malignancy Autologous Allogeneic Non-Oncology • Liver Fibrosis • Neurodegeneration • Autoimmune Ex-Vivo Cell Therapy 12/8/2022

10 Moderna Partnership Validates Approach and Provides Significant Potential Value Inflection Points Promising data emerging with rapid execution on lead programs • Multiple development programs initiated, with goal of adding 2-3 new programs/year • LNP delivery demonstrating high specificity to myeloid cells and ability to re-dose • High CAR expression, viability, and CAR-M function • Animal studies initiated with PoC data expected in Q1 2023 Broad partnership to develop mRNA based in vivo CAR-M for oncology • Multi-year collaboration with options for up to 12 oncology targets • Carisma receives $45 million up- front cash and $35 million equity in a convertible note • Moderna provides full research funding, technology & expertise • Carisma eligible for significant milestone and royalty payments IV = Intra-venous LNP = Lipid nanoparticle PoC = Proof of concept 12/8/2022
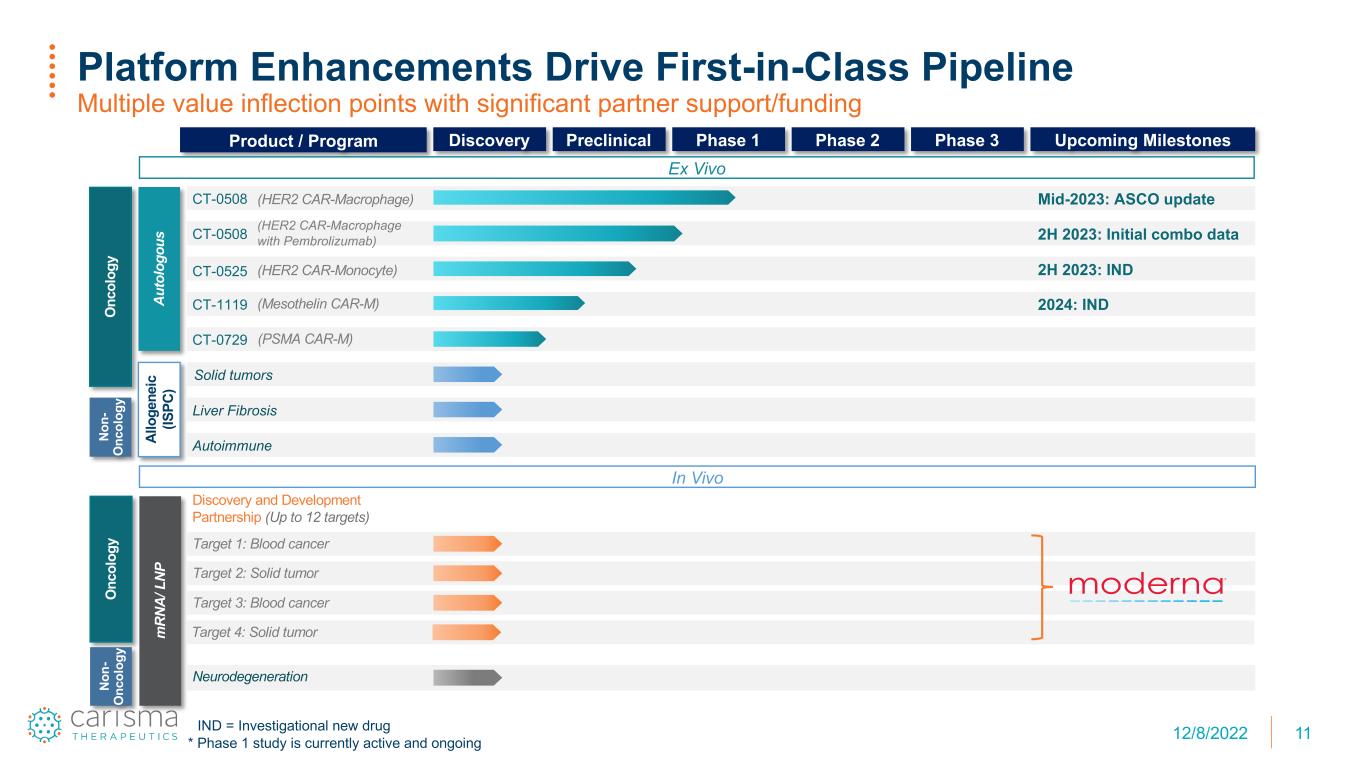
11 Platform Enhancements Drive First-in-Class Pipeline Multiple value inflection points with significant partner support/funding IND = Investigational new drug * Phase 1 study is currently active and ongoing O nc ol og y Au to lo go us m RN A/ L NP No n- O nc ol og y Al lo ge ne ic (IS PC ) Product / Program Discovery Preclinical Phase 1 Phase 2 Phase 3 Upcoming Milestones Target 4: Solid tumor Discovery and Development Partnership (Up to 12 targets) Target 3: Blood cancer Target 2: Solid tumor Target 1: Blood cancer (PSMA CAR-M)CT-0729 (Mesothelin CAR-M) 2024: INDCT-1119 (HER2 CAR-Macrophage with Pembrolizumab) 2H 2023: Initial combo dataCT-0508 Mid-2023: ASCO update(HER2 CAR-Macrophage)CT-0508 (HER2 CAR-Monocyte) 2H 2023: INDCT-0525 Liver Fibrosis Autoimmune Neurodegeneration Ex Vivo In Vivo Solid tumors O nc ol og y No n- O nc ol og y 12/8/2022
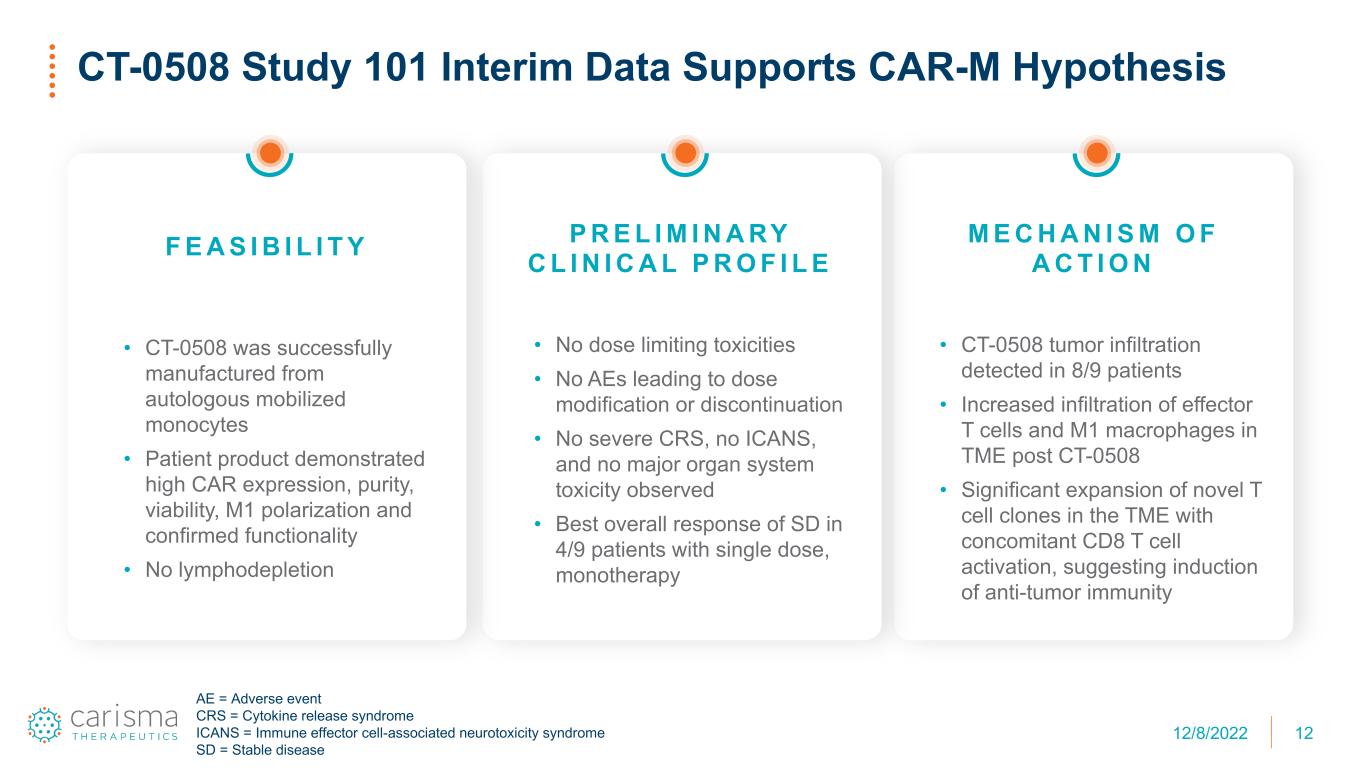
F E A S I B I L I T Y • CT-0508 was successfully manufactured from autologous mobilized monocytes • Patient product demonstrated high CAR expression, purity, viability, M1 polarization and confirmed functionality • No lymphodepletion 12 CT-0508 Study 101 Interim Data Supports CAR-M Hypothesis M E C H A N I S M O F A C T I O N • CT-0508 tumor infiltration detected in 8/9 patients • Increased infiltration of effector T cells and M1 macrophages in TME post CT-0508 • Significant expansion of novel T cell clones in the TME with concomitant CD8 T cell activation, suggesting induction of anti-tumor immunity P R E L I M I N A RY C L I N I C A L P R O F I L E • No dose limiting toxicities • No AEs leading to dose modification or discontinuation • No severe CRS, no ICANS, and no major organ system toxicity observed • Best overall response of SD in 4/9 patients with single dose, monotherapy AE = Adverse event CRS = Cytokine release syndrome ICANS = Immune effector cell-associated neurotoxicity syndrome SD = Stable disease 12/8/2022
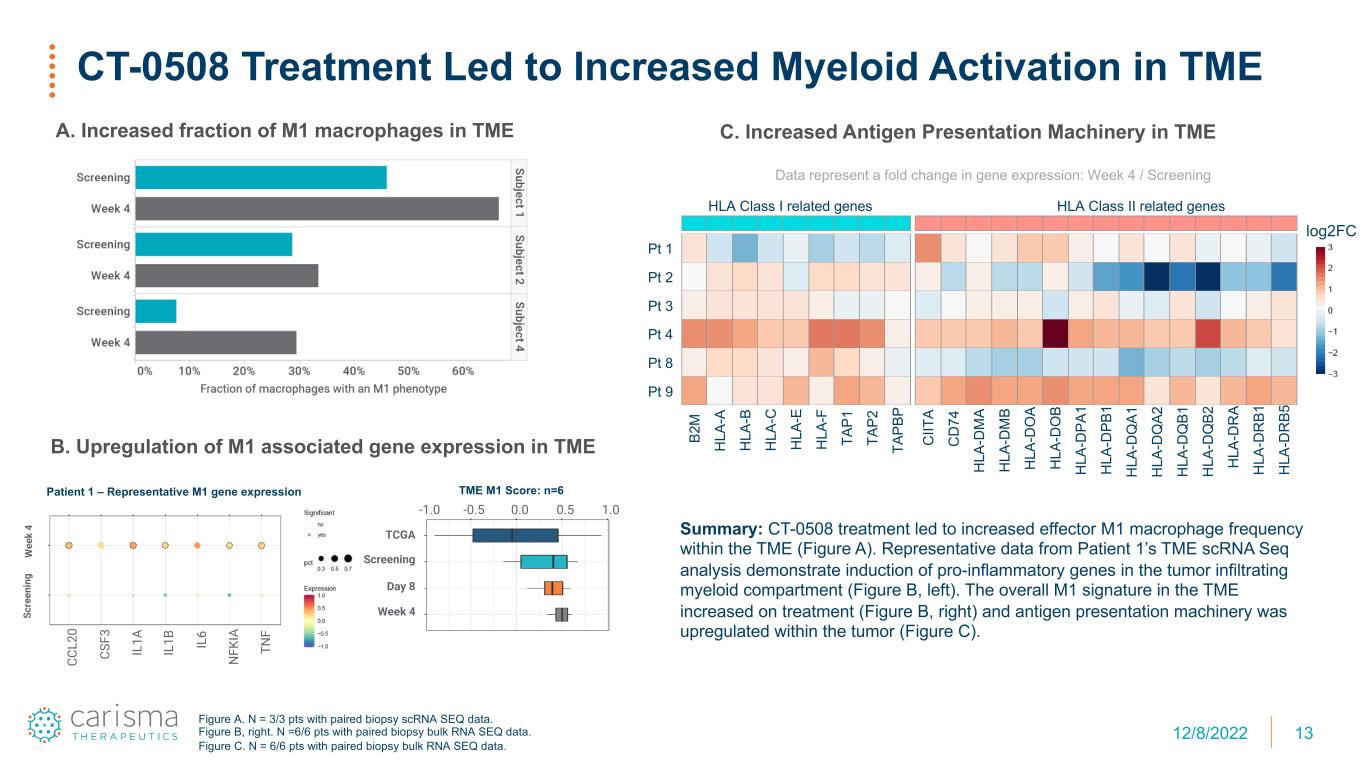
13 CT-0508 Treatment Led to Increased Myeloid Activation in TME A. Increased fraction of M1 macrophages in TME Figure A. N = 3/3 pts with paired biopsy scRNA SEQ data. Figure B, right. N =6/6 pts with paired biopsy bulk RNA SEQ data. Figure C. N = 6/6 pts with paired biopsy bulk RNA SEQ data. CS F3 CC L2 0 IL 1B IL 1A N FK IAIL 6 TN F Sc re en in g W ee k 4 B. Upregulation of M1 associated gene expression in TME C. Increased Antigen Presentation Machinery in TME HLA Class I related genes HLA Class II related genes B2 M H LA -A H LA -B H LA -C H LA -E H LA -F TA P1 TA P2 TA PB P C IIT A C D 74 H LA -D M A H LA -D M B H LA -D O A H LA -D O B H LA -D PA 1 H LA -D PB 1 H LA -D Q A1 H LA -D Q A2 H LA -D Q B1 H LA -D Q B2 H LA -D R A H LA -D R B1 H LA -D R B5 Pt 1 Pt 2 Pt 3 Pt 4 Pt 8 Pt 9 log2FC Data represent a fold change in gene expression: Week 4 / Screening Summary: CT-0508 treatment led to increased effector M1 macrophage frequency within the TME (Figure A). Representative data from Patient 1’s TME scRNA Seq analysis demonstrate induction of pro-inflammatory genes in the tumor infiltrating myeloid compartment (Figure B, left). The overall M1 signature in the TME increased on treatment (Figure B, right) and antigen presentation machinery was upregulated within the tumor (Figure C). 0.0 0.5 1.0-0.5-1.0 TCGA Screening Day 8 Week 4 Patient 1 – Representative M1 gene expression TME M1 Score: n=6 12/8/2022
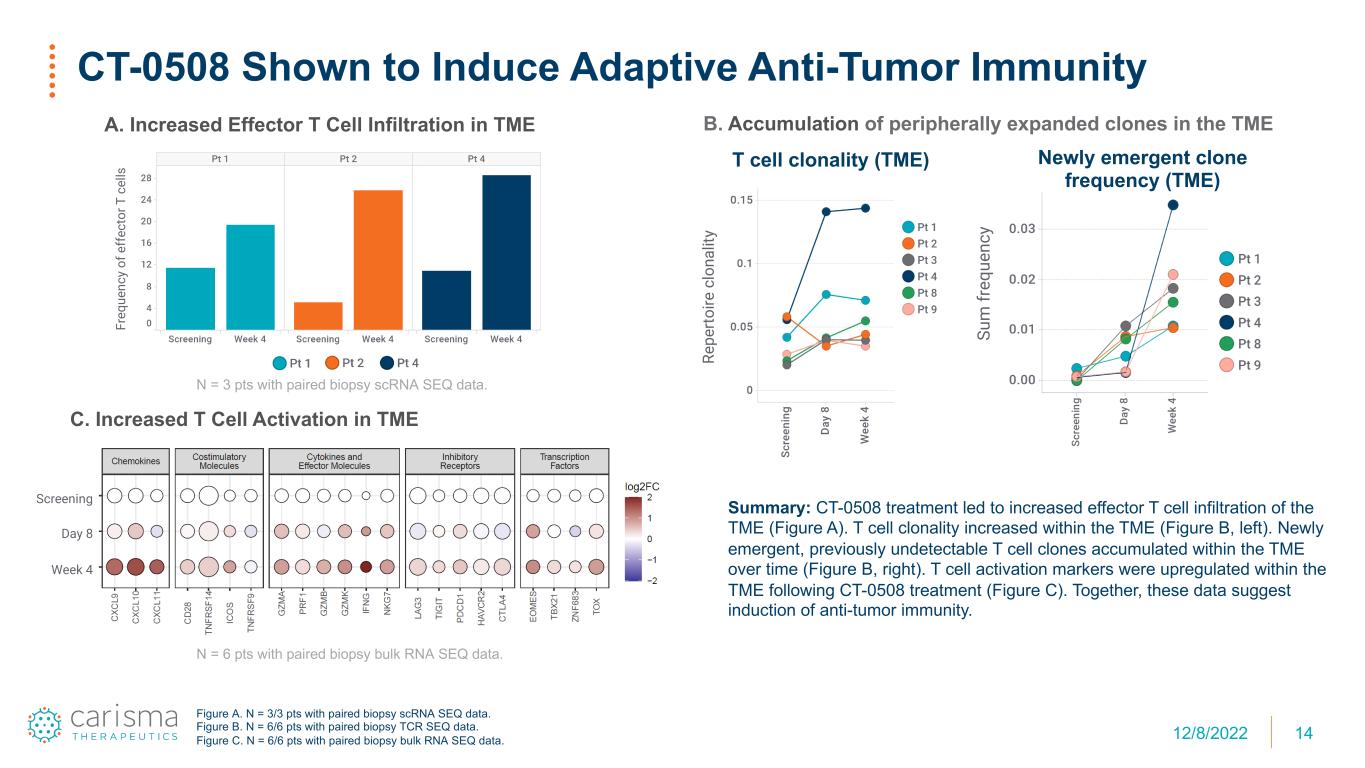
14 CT-0508 Shown to Induce Adaptive Anti-Tumor Immunity Summary: CT-0508 treatment led to increased effector T cell infiltration of the TME (Figure A). T cell clonality increased within the TME (Figure B, left). Newly emergent, previously undetectable T cell clones accumulated within the TME over time (Figure B, right). T cell activation markers were upregulated within the TME following CT-0508 treatment (Figure C). Together, these data suggest induction of anti-tumor immunity. B. Accumulation of peripherally expanded clones in the TME Su m fr eq ue nc y T cell clonality (TME) Newly emergent clone frequency (TME) A. Increased Effector T Cell Infiltration in TME Fr eq ue nc y of e ff ec to r T c el ls C. Increased T Cell Activation in TME Screening Day 8 Week 4 N = 3 pts with paired biopsy scRNA SEQ data. N = 6 pts with paired biopsy bulk RNA SEQ data. Figure A. N = 3/3 pts with paired biopsy scRNA SEQ data. Figure B. N = 6/6 pts with paired biopsy TCR SEQ data. Figure C. N = 6/6 pts with paired biopsy bulk RNA SEQ data. 12/8/2022
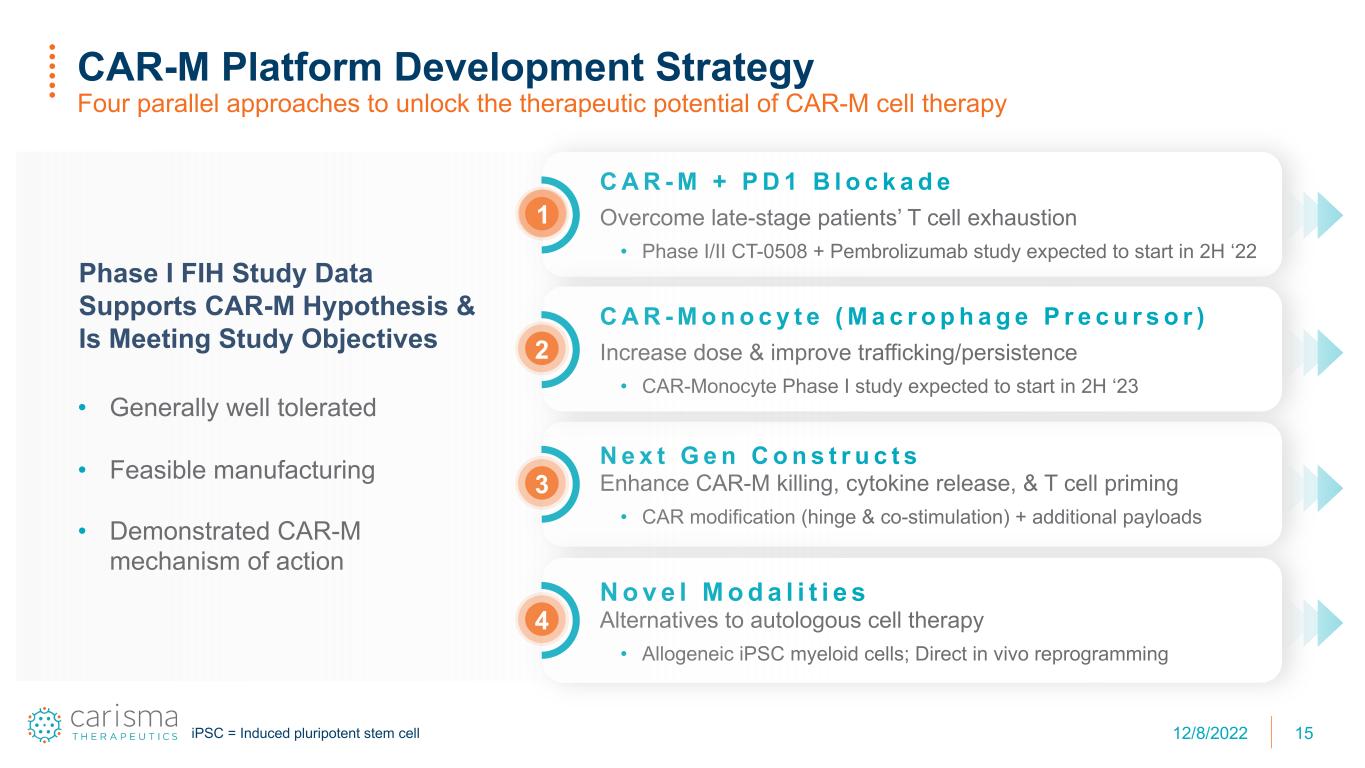
N e x t G e n C o n s t r u c t s Enhance CAR-M killing, cytokine release, & T cell priming • CAR modification (hinge & co-stimulation) + additional payloads C A R - M + P D 1 B l o c k a d e Overcome late-stage patients’ T cell exhaustion • Phase I/II CT-0508 + Pembrolizumab study expected to start in 2H ‘22 15 CAR-M Platform Development Strategy Four parallel approaches to unlock the therapeutic potential of CAR-M cell therapy C A R - M o n o c y t e ( M a c r o p h a g e P r e c u r s o r ) Increase dose & improve trafficking/persistence • CAR-Monocyte Phase I study expected to start in 2H ‘23 2 3 N o v e l M o d a l i t i e s Alternatives to autologous cell therapy • Allogeneic iPSC myeloid cells; Direct in vivo reprogramming 4 1 Phase I FIH Study Data Supports CAR-M Hypothesis & Is Meeting Study Objectives • Generally well tolerated • Feasible manufacturing • Demonstrated CAR-M mechanism of action iPSC = Induced pluripotent stem cell 12/8/2022
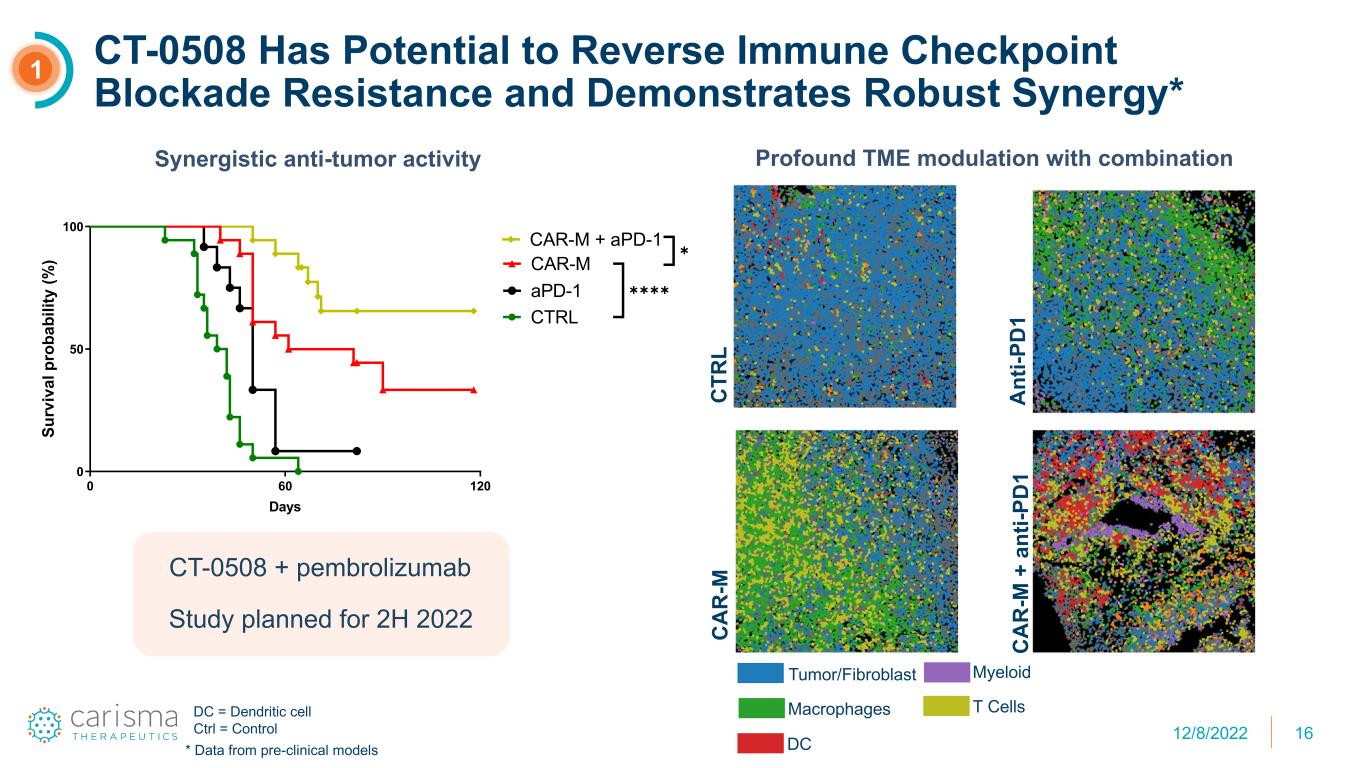
16 CT-0508 Has Potential to Reverse Immune Checkpoint Blockade Resistance and Demonstrates Robust Synergy* 0 60 120 0 50 100 Days Su rv iv al p ro ba bi lit y (% ) CTRL CAR-M CAR-M + aPD-1 aPD-1 ✱✱✱✱ ✱ CT-0508 + pembrolizumab Study planned for 2H 2022 1 Synergistic anti-tumor activity Profound TME modulation with combination C TR L A nt i-P D 1 C A R -M C A R -M + a nt i-P D 1 Tumor/Fibroblast Macrophages DC Myeloid T CellsDC = Dendritic cell Ctrl = Control * Data from pre-clinical models 12/8/2022
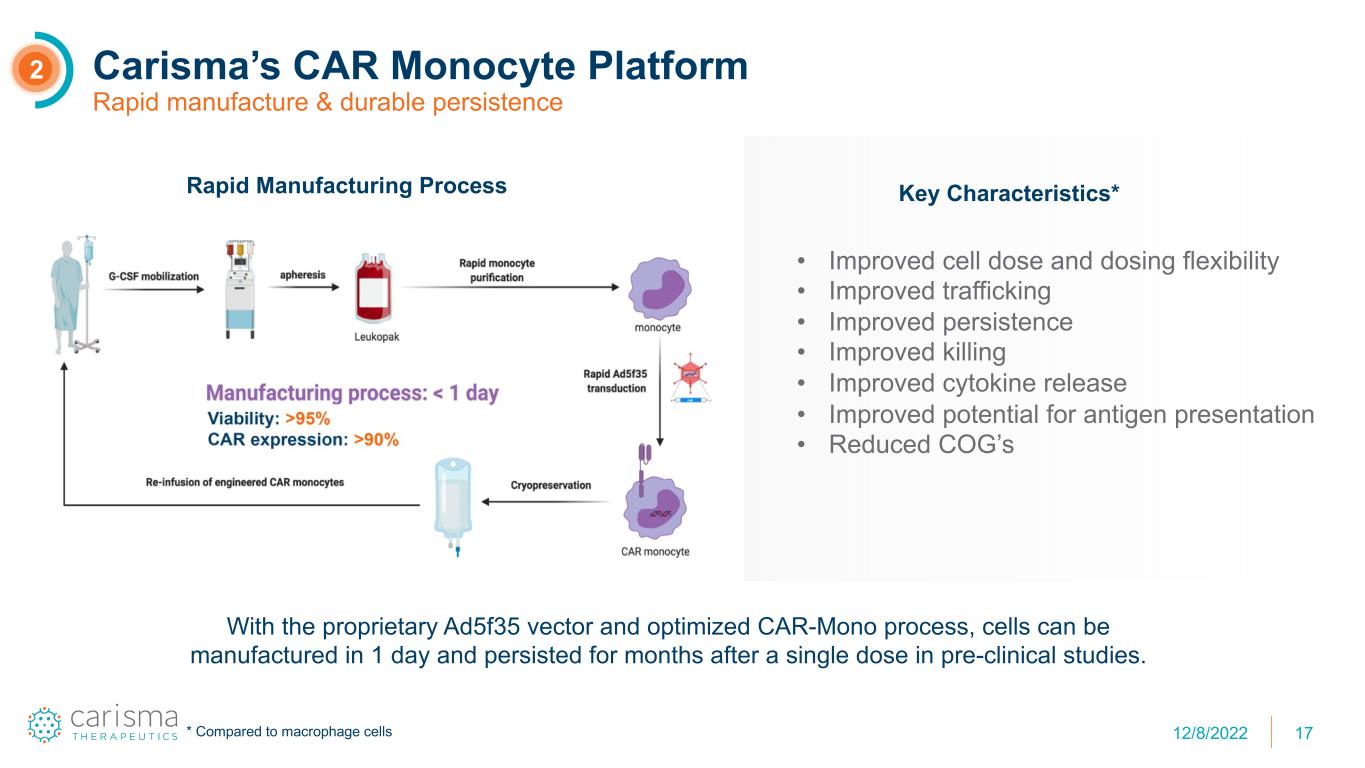
17 Carisma’s CAR Monocyte Platform Rapid manufacture & durable persistence 2 Rapid Manufacturing Process Key Characteristics* • Improved cell dose and dosing flexibility • Improved trafficking • Improved persistence • Improved killing • Improved cytokine release • Improved potential for antigen presentation • Reduced COG’s With the proprietary Ad5f35 vector and optimized CAR-Mono process, cells can be manufactured in 1 day and persisted for months after a single dose in pre-clinical studies. * Compared to macrophage cells 12/8/2022
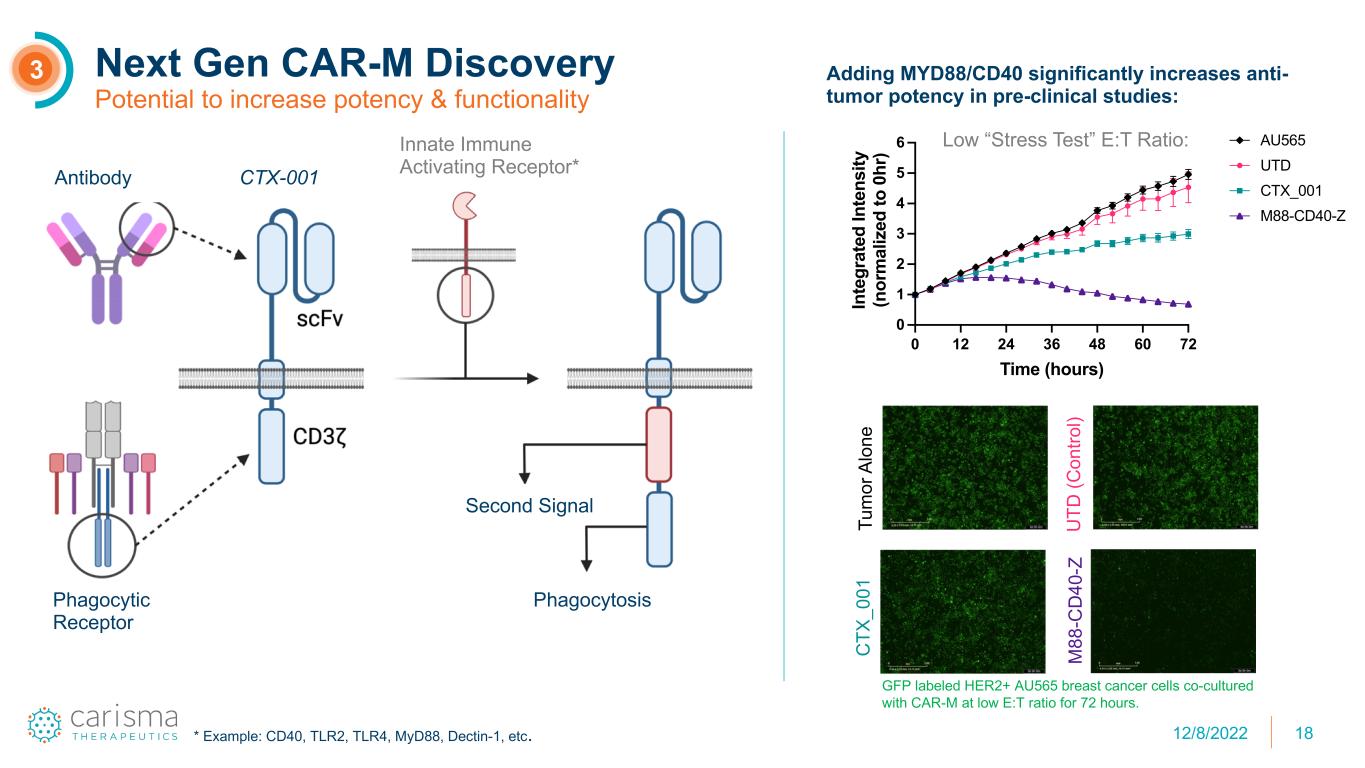
18 Next Gen CAR-M Discovery Potential to increase potency & functionality 3 Antibody Phagocytic Receptor CTX-001 Innate Immune Activating Receptor* Phagocytosis Second Signal * Example: CD40, TLR2, TLR4, MyD88, Dectin-1, etc. Low “Stress Test” E:T Ratio: Adding MYD88/CD40 significantly increases anti- tumor potency in pre-clinical studies: 0 12 24 36 48 60 72 0 1 2 3 4 5 6 Time (hours) In te gr at ed In te ns ity (n or m al iz ed to 0 hr ) 1:2 E:T (5k:10k) AU565 UTD CTX_001 M88-CD40-Z Tu m or A lo ne U TD (C on tro l) C TX _0 01 M 88 -C D 40 -Z GFP labeled HER2+ AU565 breast cancer cells co-cultured with CAR-M at low E:T ratio for 72 hours. 12/8/2022
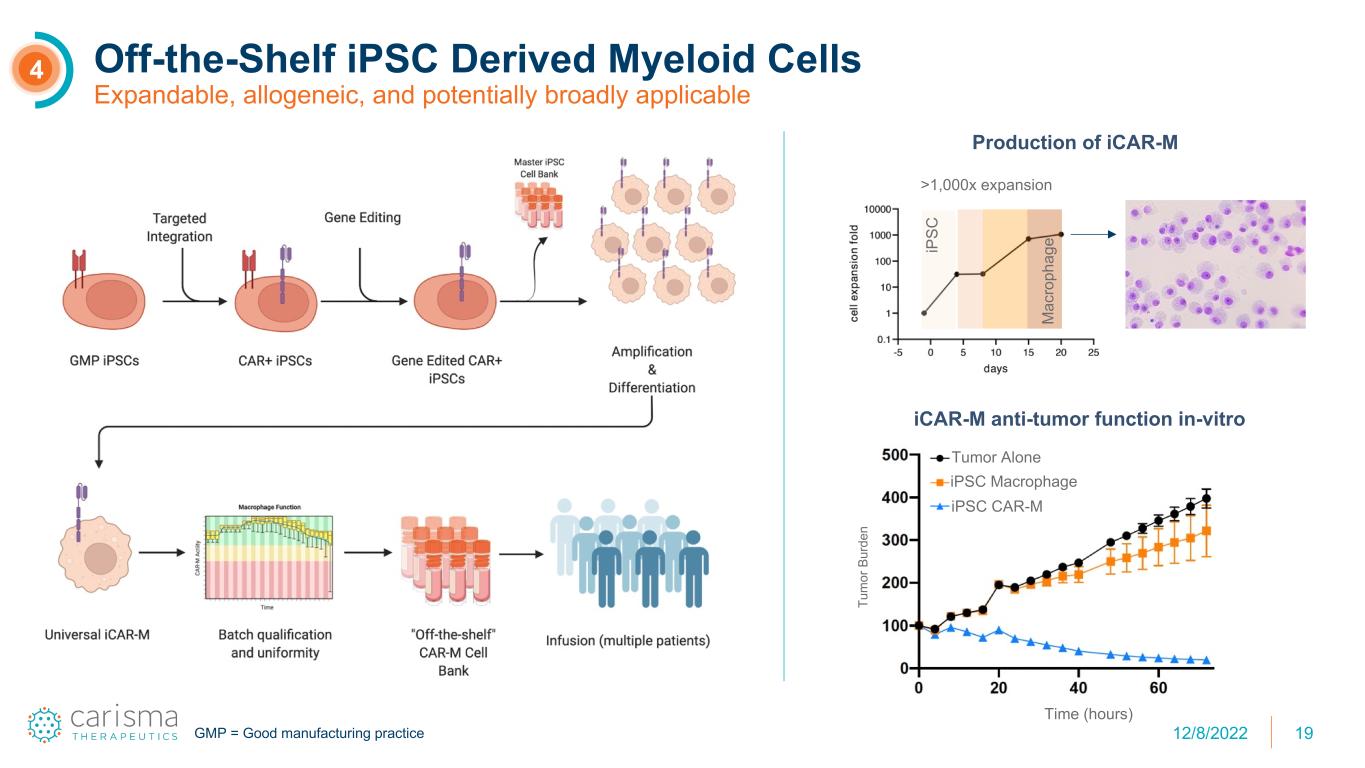
19 Off-the-Shelf iPSC Derived Myeloid Cells Expandable, allogeneic, and potentially broadly applicable 4 Tumor Alone iPSC Macrophage iPSC CAR-M Tu m or B ur de n Time (hours) iCAR-M anti-tumor function in-vitro Production of iCAR-M >1,000x expansion iP SC M ac ro ph ag e GMP = Good manufacturing practice 12/8/2022

20 Directly Reprogramming Myeloid Cells In Vivo with mRNA/LNP • Myeloid tropic LNP have demonstrated specificity and efficient transfection in vivo • LNP have proven safety profile in previous clinical studies; repeat dosing well tolerated • High CAR expression and function in vitro. • In vivo studies ongoing w/ POC expected in Q1 2023. • Lead identified for first target, early in-vitro POC demonstrated. 4 Tumor spheroids containing HER2+ breast cancer cells and TAMs. LNPs are added to spheroids, and TAMs are directly reprogrammed to CAR-M to kill tumors cells. 12/8/2022

21 Platform Enables Potential Non-Oncology Applications Significant opportunity for strategic partnerships 1 Anti-inflammatory, anti-fibrotic macrophages: • Modality: Auto/Allo Cell Therapy • Potential indication: Liver Fibrosis • Payload: Immunosuppressive cytokine + anti-fibrotic enzyme 2 In vivo reprogrammed microglia: • Modality: In vivo reprogramming (LNP) • Potential indication: Alzheimer’s, Parkinson’s • Payload: Anti-Aβ CAR, Anti-αSyn CAR, Anti-Tau CAR 3 Switch receptors for inflammatory disease: • Modality: Auto/Allo Cell Therapy • Potential field: Immunologic/Transplant • Payload: Proprietary M1 à M2 switch receptors 12/8/2022

22 Strong Leadership Team and Advisors Deep research, clinical and operational expertise in cell and gene therapy and oncology Board of Directors • Sanford Zweifach – Chairperson • Briggs Morrison – Independent Director • Margarita Chavez – AbbVie Ventures • Bjorn Odlander – HealthCap • Regina Hodits – Wellington Partners • Chidozie Ugwumba – SymBiosis Key Advisors • Saar Gill, MD, PhD – Penn (Co-Founder, Co-Inventor) • Carl June, MD – Penn (Co-Inventor) • Hy Levitsky, MD – Century Tx • Lisa Coussens, PhD – OHSU • Prasad S. Adusamilli, MD FACS – MSKCC • Nina Bhardwaj, MD, PhD – Mt Sinai • Nabil Ahmed, MD – Baylor College of Medicine Management D A N IE L C U S H IN G , P H D Chief Technology & Development Officer S T E V E N K E L L Y President & CEO M IC H A E L K L IC H IN S K Y , P H D Co-Founder & CSO R IC H A R D M O R R IS Chief Financial Officer T O M W IL T O N Chief Business Officer 12/8/2022

23 Operating Plan and Corporate Milestones Capital efficient R&D program designed to reach significant value inflection points over next 18 months Complete expanded CT-0508 Phase I study • Cohort 2: Bolus dosing • IP Administration • Anti-PD1 combination Advance our engineered macrophage platform • Progress next gen CAR-M design to candidate selection • Develop CAR-Mono and Allo to expand the performance and utility of the platform • Expand internal in-vivo capability Advance CAR-M pipeline • Demonstrate in-vivo CAR-Mono POC with Moderna and select clinical candidate • File IND for Mesothelin targeted CAR-M (CT-1119) • Progress neurodegeneration and liver fibrosis programs to candidate selection 2H1H 2023 1H 2024 CT-0508 + aPD1 data In-Vivo pre- clinical PoC data Allo pre-clinical PoC data CAR-monocyte IND CT-1119 IND Key Expected Milestones Clinical milestone Pre-clinical milestone CS for Moderna programs CT-0508 IP data 12/8/2022
24 Corporate Summary Significant opportunity to become a breakthrough therapeutics company Proprietary engineered macrophage platform Emerging pipeline of oncology CAR-Ms Validating partnership and clinical data Experienced leadership team and advisors Multiple potential value catalysts over next 18 months Carisma is the leader in engineered macrophage technology with broad potential therapeutic applications in cancer and beyond 12/8/2022























