MANAGEMENT’S DISCUSSION AND ANALYSIS
For the three and nine months ended September 30, 2012
Tables are expressed in USD $000’s except share and per share amounts
The following provides management’s discussion and analysis (‘‘MD&A’’) of IMRIS Inc.’s consolidated results of operations and financial condition for the three and nine months ended September 30, 2012. In this MD&A, “IMRIS”, the “Company”, “we”, “our” and “us” are used to refer to IMRIS Inc.
This MD&A is dated as at November 8, 2012 and should be read in conjunction with the interim unaudited consolidated financial statements and the notes thereto for the three and nine months ended September 30, 2012 and with the audited consolidated financial statements and notes thereto for the year ending December 31, 2011.
Effective January 1, 2011, the Company has adopted United States generally accepted accounting principles (“U.S. GAAP”) as its basis of accounting and the US dollar as its reporting currency. Unless otherwise indicated, all currency amounts referenced in this MD&A are denominated in US dollars.
This MD&A contains forward-looking statements about future events or future performance and reflects management’s expectations and assumptions regarding our growth, results of operations, performance and business prospects and opportunities. Such forward-looking statements reflect management’s current beliefs and are based on information currently available to us. In some cases, forward-looking statements can be identified by terminology such as “may”, “would”, “could”, “will”, “should”, “expect”, “plan”, “intend”, “anticipate”, “believe”, “estimate”, “predict”, “potential”, “continue” or the negative of these terms or other similar expressions concerning matters that are not historical facts. In particular, statements regarding our future operating results, economic performance and product development efforts are or involve forward-looking statements.
A number of factors could cause actual events, performance or results, including those in respect of the foregoing items, to differ materially from the events, performance and results discussed in the forward-looking statements. Factors which could cause future outcomes to differ materially from those set forth in the forward-looking statements include, but are not limited to: [i] timing and amount of revenue recognition of order backlog and the Company’s expectation of sales and margin growth [ii] obtaining sufficient and suitable financing to support operations and commercialization of products, [iii] adequately protecting proprietary information and technology from competitors, [iv] obtaining regulatory approvals and successfully completing new product launches, [v] successfully competing in the targeted markets, and [vi] maintaining third party relationships, including key personnel, and key suppliers. In evaluating these forward-looking statements, readers should specifically consider various factors, including the risks outlined under “Risks and Uncertainties”, which may cause actual events, performance or results to differ materially from any forward-looking statement.
Readers are cautioned that our expectation, beliefs, projections and assumptions used in preparation of such information, although considered reasonable at the time of preparation, may prove to be wrong, and as such, undue reliance should not be placed on forward-looking statements. By their nature, forward-looking statements are subject to numerous known and unknown risks and uncertainties so as a result, we can give no assurance that any of the actual events, performance, results, or expectations will occur or be realized. These forward-looking statements are expressly qualified by this cautionary statement as of the date of this MD&A and we do not intend, and do not assume any obligation, to update or revise them to reflect new or future events or circumstances.
IMRIS Inc.
Management's Discussion and Analysis – November 8, 2012
Page 1 of 26 |
OVERVIEW
IMRIS designs, manufactures and marketsImage Guided Therapy Systems that enhance the effectiveness of therapy delivery. Our Image Guided Therapy Systems are a combination of real time visualization products and therapy delivery products that are designed to improve patient outcomes and reduce the cost of patient care. We accomplish this by combining our visualization technology products with therapy delivery products in a single integrated system that has the ability to provide timely information to clinicians to properly assess the treatment plan at the point of therapy delivery. We believe this approach to patient care not only improves patient outcomes, but also contributes to reduced cost of care for those patients. Our goal is too continuously deliver products that improve therapy delivery for an increasing number of medical procedures while at the same time are supported by peer reviewed published measurement of improved outcomes and reduced cost of care.
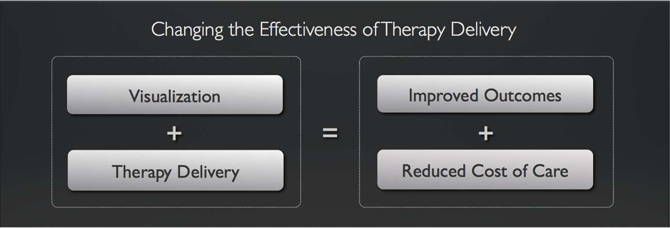
Visualization and Therapy Delivery
In 2005 at the founding of the company, we created a visualization platform based on a single Magnetic Resonance (MR) Imaging product. Since then we have introduced a variety of next generation imaging capabilities into our visualization products. These include multiple field strength MR systems, X-Ray Fluoroscopy (AX) systems, and Computed Tomography (CT) systems, all designed to provide imaging capabilities for different therapy delivery products. All of these imaging capabilities are marketed as theVISIUS Surgical Theatre.
Our goal is to design visualization products that have the ability to be used in a large number of surgical and interventional procedures and to become a foundational investment in every hospital. To do this the system must be flexible enough to meet current and evolving procedural requirements while at the same time improving patient care and reducing costs to the hospital. The VISIUS Surgical Theatre can incorporate multiple configurations and imaging modalities while reducing patient risk and delivering real-time information to clinicians while preserving optimal surgical access and techniques.
Our visualization product, the VISIUS Surgical Theatre, is used in combination with multiple therapy delivery systems including traditional surgery, Surgeon directed robotic surgery, and Radio-surgery. It is our goal to provide a means for clinicians to improve therapy delivery by moving towards a minimally invasive surgical (MIS) procedure whenever possible. The transition to an MIS procedure is expected to contribute to improved outcomes and reduced costs of care versus traditional surgical methods.
We sell our VISIUS Surgical Theatre globally to hospitals that deliver clinical services to patients in the neurosurgical, cerebrovascular, and cardiovascular markets. Historically our products have enabled therapy delivery through traditional surgical techniques, primarily for neurosurgical applications. We believe that the VISIUS Surgical Theatre, in combination with therapy delivery, has the ability to continue to expand across a large number of clinical procedures. As we continue to work with clinicians to promote and identify potential new areas of clinical application, new high value procedures are expected, resulting in increased utilization and further adoption of the products.
IMRIS Inc.
Management's Discussion and Analysis – November 8, 2012
Page 2 of 26 |
Value Proposition
We believe that the combination of the VISIUS Surgical Theatre with therapy delivery benefits patients, physicians and hospitals:
Patients
| · | Improved Outcomes: Peer reviewed published research has shown significant improvements in patient outcomes when the intraoperative MRI available in the VISIUS Surgical Theatre is used in a procedure. |
| · | Risk Reduction: The risk of requiring a repeat operation because of incomplete procedures is significantly reduced due to improved levels of complete resection in the case of brain tumors as a result of the intraoperative visualization. |
Clinicians
| · | Enhanced Efficiency and Effectiveness for Clinicians: High resolution imaging information is captured rapidly and presented in a manner designed to enhance clinician efficiency and effectiveness. |
| · | Enhanced Workflow for Clinicians: The patient can be maintained in the optimal surgical position throughout the procedure and the MRI or CT imaging system is removed from the surgical or interventional theatre when not required resulting in unrestricted access to the patient by the surgical team. |
Hospitals
| · | Greater Utilization of the VISIUS Surgical Theatre:The VISIUS Surgical Theatre permits greater utilization of the imaging equipment as the MR or CT scanner can be shared by multiple operating rooms and a diagnostic imaging suite allowing for a single asset to be used by numerous clinicians. |
| · | Increased Patient Volumes: Improved patient outcomes may result in higher patient volumes and revenue for hospitals. |
| · | Technology Attracts Clinicians: Access to technologies such as the VISIUS Surgical Theatre can assist in both the recruitment and retention of clinicians. |
PRODUCT PORTFOLIO
The VISIUS Surgical Theatre is the foundational technology of our Company and continues to evolve in its utilization across numerous surgical and interventional applications. We have invested in research and development to further broaden our product portfolio by introducing new procedures into the VISIUS Surgical Theatre as well as combining it with new therapy products.
Our product portfolio consists of three therapy delivery systems made up of the VISIUS Surgical Theatre in combination with:
| 1: | Traditional surgical techniques, |
| 2: | The SYMBIS Surgical System, a surgeon controlled surgical robot, and |
| 3: | The TrueBeamTM radiation therapy product from Varian Medical Systems, Inc. (Varian). |
IMRIS Inc.
Management's Discussion and Analysis – November 8, 2012
Page 3 of 26 |
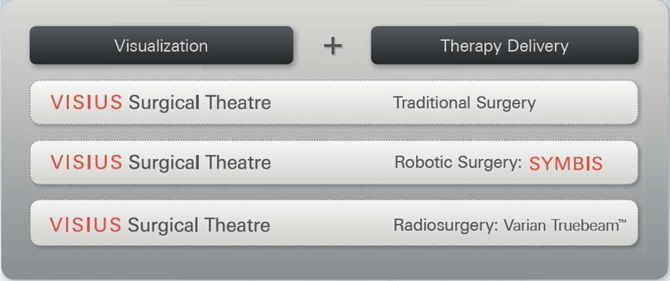
(The SYMBIS surgical system and the Radiosurgery product with the TrueBeamTM system are both works in progress and not available for commercial sale)
All of these Image Guided Therapy systems include our proprietary VISIUS Surgical Theatre in combination with therapy delivery systems that are integrated with multiple proprietary supporting products and technologies. These include patient handling systems, data management and information presentation systems, surgical devices, imaging and system control software platforms, and safety and remote management products. These are proprietary products that underlay the VISIUS Surgical Theatre’s ability to be integrated with each therapy delivery product.
| I. | VISIUS Surgical Theatre and Traditional Surgical Procedures |
The VISIUS Surgical Theatrecan be configured to support the delivery of a wide rangeof neurosurgical, cardiovascular and cerebrovascular procedures using traditional surgical techniques. The VISIUS Surgical Theatre can be equipped with intraoperative imaging utilizing MRI, x-ray angiography and computed tomography, alone or in multimodality combinations.
The VISIUS Surgical Theatre provides a fully integrated surgical environment with the availability of high-resolution images for use in a number of surgical procedures. These procedures include neurological tumor resection, epilepsy foci resection, arteriovenous malformation, aneurysm, upper C-spine and frame-based stereotaxy. Due to the invasive nature of brain surgery and the importance of minimizing disturbance to healthy brain tissue, neurosurgical procedures may particularly benefit from an MRI's unique ability to distinguish between diseased and healthy brain tissue. The VISIUS Surgical Theatre provides visualization information to allow clinicians to make adjustments to the procedure while the procedure is in progress, which may lead to improved patient outcomes and reduce the likelihood that repeat surgeries will be needed.
When equipped with an MR scanner and integrated x-ray angiography system, the VISIUS Surgical Theatre provides clinicians with timely and accurate images for visualizing the patient anatomy before, during and after interventions for the treatment of a wide variety of cardiovascular and cerebrovascular conditions, such as atrial fibrillation, certain structural heart disorders and stroke. With seamless transitions between MR and x-ray angiography systems, the VISIUS Surgical Theatre enables surgical and catheter-based treatments and real-time assessment of therapy in a single integrated suite. The single integrated system in a VISIUS Surgical Theatre eliminates patient transport between imaging modalities and streamlines workflow. After MR scanning, the patient can be transitioned to image-guided intervention on the angiography system without moving the patient from the table. During and immediately after the procedure, new MR images can be taken to assess treatment and to determine if further intervention is required.
IMRIS Inc.
Management's Discussion and Analysis – November 8, 2012
Page 4 of 26 |
In certain cranial and spinal procedures, the VISIUS Surgical Theatre can also be equipped with a multi-slice floor mounted CT which moves over top of a stationary patient, to provide intraoperative images of diagnostic quality, without the additional risk of moving the patient. The VISIUS Surgical Theatre with CT imaging delivers real-time information to clinicians while preserving optimal surgical access and techniques.
We are currently developing a ceiling mounted CT based version of the VISIUS Surgical Theatre leveraging our know-how from our existing VISIUS Surgical Theatre offering in MR systems. This system will have the ability to move between multiple operating rooms and offer significant benefits over the current floor mounted system. This product is expected to be submitted to the FDA with an anticipated regulatory clearance in the first half of 2013.
| II. | VISIUS Surgical Theatre and the SYMBIS Surgical System |
In February 2010, we acquired NeuroArm Surgical Ltd and all of its intellectual property. Since then we have been developing the SYMBIS Surgical System, a surgeon controlled surgical robot designed to enable minimally invasive procedures that are currently performed in a more invasive manner. This system consists of a MR compatible robot and surgical control console integrated together with the VISIUS Surgical Theatre. We believe that the combination of optical and MR imaging integrated with a surgical robot may have the ability to transform a number of surgical procedures to MIS procedures. The robot has been designed to operate in the bore of a high field MR system that can provide unprecedented visualization of the surgical site by providing both optical and MR views of the surgical target. The SYMBIS Surgical System is a micro-surgical system that has all of the traditional attributes of a robotic system such as accuracy, repeatability, and control, but also has integrated MR and optical imaging, along with haptic feedback to the clinician. The haptic feedback or “sense of touch” may enable surgeons to complete procedures in a way never before possible. The SYMBIS Surgical System is designed to be installed in both existing VISIUS Surgical Theatre systems, and in new installations.
We are developing surgical instruments for the SYMBIS that are procedure specific and are designed to enable greater precision and flexibility for the surgeon. We believe that the SYMBIS Surgical System will be applicable for a large number of high volume surgical procedures with the potential to be clinically meaningful and thereby further adoption of the system.
Our SYMBIS Surgical System is currently in a single site clinical trial with a planned cohort of 120 neurosurgical patients. It is expected that the initial outcomes will be published in peer reviewed journals over the next 6 months.
The SYMBIS Surgical System was submitted to the FDA in August 2012 and is pending clearance.
| III. | VISIUS Surgical Theatre and the TrueBeamTMSystem for Radiosurgery |
On October 5, 2011 we announced our agreement with Varian to integrate the capabilities of the VISIUS Surgical Theatre together with the therapy capability of Varian’s TrueBeamTM radiotherapy system. This product has the potential to provide a number of high value capabilities to radiation oncology centres that are hospital based or standalone clinics. This system is designed to provide a radiation oncology centre with the ability to deliver MR guided radiation therapy, MR simulation, and MR guided brachytherapy from a single integrated system. The system consists of three connected rooms that provide radiosurgery, simulation, and brachytherapy all with a common MR imaging platform.
MR simulation is a planning and imaging procedure that is done in conjunction with a patient’s preparation for radiation therapy delivery. Our system allows for a high field MR to be used in a diagnostic simulation suite and then, on demand, be available for use in MR guided radiosurgery, or MR guided brachy therapy. This may provide a significant economic advantage over other means of completing the same procedure.
For MR guided radiation therapy, the patient is located in the radiation therapy bunker and a high field MR moves into the bunker over top of a stationary patient. The MR image is acquired, the MR moves out of the room, and the therapy treatment plan is developed and delivered to the TrueBeamTMradiosurgery system which executes the treatment. The ability to image soft tissue lesions with MR, immediately before the application of radiation therapy may allow for more accurate targeting of the lesion, resulting in a reduction in the radiation delivered to adjacent healthy tissue. This improved targeting may also result in the ability to increase the energy delivered at a treatment session, which may result in fewer treatment sessions for the patient. This new approach to treatment delivery is expected to provide improved patient outcomes versus existing radiosurgery technology systems and have the opportunity to reduce the cost of care.
IMRIS Inc.
Management's Discussion and Analysis – November 8, 2012
Page 5 of 26 |
We believe that the ability to deliver MR guided brachytherapy in a single suite may have compelling advantages over other means of delivering brachytherapy to patents. Brachytherapy is the deposition of high dose radiation seeds into target tissue for the delivery of radiation. It requires the ability to image, target, and deliver the seeds with precision and confidence. Our system is designed to enhance the workflow and provide improved procedural outcomes.
This product is currently under development with regulatory clearance anticipated in 2013.
Technology and Product Development
Underlying all of our image guided therapy solutions is advanced proprietary technology and intellectual property that we have developed as part of our unique solutions. The protection of these products, our processes and know-how is integral to our business. We currently have 45 patents either issued or pending. As we develop our technologies, we will continue to seek patent protection to contribute to our competitive advantage. We have patents in place in the United States, Canada and other countries, where available, to protect our core patent family and we have filed a number of additional patent applications that are directed to specific aspects of our technology.
Innovation and the creation of high value novel products is a cornerstone of IMRIS’s development activities. To grow the Company and remain competitive, we are continuously engaged in new product development and enhancement and each year we invest significantly in research and development to drive continuing innovations that support our competitive position.
As we move forward our product development efforts will be focused on enhancing the capabilities of the VISIUS Surgical Theatre so that we are increasing the number and quantity of traditional procedures that can be completed in our theatres. Following commercialization of our products that combine robotics and radiosurgery with the VISIUS Surgical Theatre, we expect to continue to expand the capabilities of these systems to continue to grow their value proposition.
Regulatory
IMRIS is a global company serving global markets. We have registered our core MR VISIUS Surgical Theatre in the United States, Canada, European Union, Australia, Japan, China, Singapore and South Korea. We continue to maintain our facility and product registrations to serve these global markets. We successfully completed re-certification to ISO-13485 in September 2012, which is a requirement for many of our global markets. During this period, IMRIS cleared the Oncology Package for the VISIUS Surgical Theatre with the FDA, providing a suite of tools to enable radiation therapy planning using the VISIUS Surgical Theatre MR system.
IMRIS currently has two 510(k)s pending with the United States FDA involving innovative products and industry firsts: the SYMBIS Surgical Robot (the first microsurgical robot for neurosurgery) and the VISIUS Wireless Coil (the first inductive wireless coil for MRI).
IMRIS maintains a proven network of global regulatory partners and will seek product registrations based on market demand and product launch strategy as the new planned therapy products and technologies are developed.
Market and Sales Cycle
We sell our VISIUS Surgical Theatres globally to hospitals that deliver clinical services to patients in the neurosurgical, cerebrovascular and cardiovascular markets. We believe that the primary market for our current product portfolio is comprised of those hospitals having relatively large neurosurgical, cerebrovascular or cardiovascular practices. Clinical appreciation for the benefits of VISIUS Surgical Theatres for neurosurgical applications is growing supported by repeat purchases from hospitals and market penetration within regions in which our product is being sold.
We have a direct sales force in the United States, Canada, China, Japan and Europe, excluding Italy and Eastern European countries. We utilize distributors in all other markets that we serve.
IMRIS Inc.
Management's Discussion and Analysis – November 8, 2012
Page 6 of 26 |
Our sales force is focusing its efforts on hospitals with the greatest ability to benefit from neurosurgical applications. They are aggressively working our sales pipeline and we are increasing our marketing efforts. As a result, we expect that market interest will develop for new applications of the product and the new products being developed, which can be utilized within our Theatres.
The purchase and installation of a VISIUS Surgical Theatre for traditional surgical procedures represents a significant capital project for our customers that can range in price from approximately $4 million to $12 million depending on the product solution, the configuration of the VISIUS Surgical Theatre layout and system options selected. In addition to the capital equipment sale, most of our customers enter into equipment service contracts that are generally 4-5 years in duration. These contracts begin after the typical one-year warranty period and are on average equal to approximately 5% of the original equipment purchase price per year in revenues. In addition to our equipment and services, customers may require further capital expenditures for construction and ancillary equipment. The sales cycle for our VISIUS Surgical Theatres is both complex and lengthy and can be more than 12 months from initial customer engagement to receipt of a purchase order.
Following the receipt of a customer purchase order, the delivery and installation cycle for one of our VISIUS Surgical Theatres typically ranges from 8 months to 18 months or more depending on the configuration of our system and the amount of additional construction work that may be required to be completed by the customer. We invoice customers for a VISIUS Surgical Theatre in installments spread over a number of milestones, which typically include a deposit at the time of order and a percentage of the remaining total price upon delivery of the equipment, completion of installation and final acceptance. Due to the project nature of our VISIUS Surgical Theatre sales, we recognize revenues and related cost of sales on a percentage-of-completion basis as the VISIUS Surgical Theatre is installed.
As our newer products are commercialized, we believe we can leverage our significant customer relationships to accelerate new product introductions. Moreover, our VISIUS Surgical Theatres equipped with CT or the SYMBIS Surgical System are being designed to have shorter installation timeframes. These factors together are expected to result in significantly shorter sales and installation cycles for our Company.
IMRIS Inc.
Management's Discussion and Analysis – November 8, 2012
Page 7 of 26 |
2012 Highlights
Highlights from the third quarter of 2012 include:
| Ø | Third quarter 2012 orders of $18.2 million contribute to total order bookings of$64.3million in the first nine months of the year |
| Ø | Backlog increases 34% in first nine months to $127.5 million at September 30, 2012 |
| Ø | Two VISIUS Surgical Theatres booked in the third quarter with one in the U.S and one in China |
| Ø | Appointment of Jay D. Miller as IMRIS President & COO strengthens operational leadership |
| Ø | Oncology package for planned MR guided radiation therapy system receives FDA approval |
| Ø | Clearance application for Image Guided Surgical Robotics system filed with the FDA in August 2012 |
| Ø | U.S. patent allowed for movable magnet in combination with radiation therapy |
SUMMARY OF SELECTED FINANCIAL INFORMATION
Results of Operations
The following table sets forth selected financial information for the dates and periods indicated:
Selected Financial Information
(Thousands of US dollars, except per share amounts)
(Unaudited)
| | | Three months ended | | | | | | Nine months ended | | | | |
| | | September 30 | | | % | | | September 30 | | | % | |
| | | 2012 | | | 2011 | | | Change | | | 2012 | | | 2011 | | | Change | |
| | | | | | | | | | | | | | | | | | | |
| Sales | | $ | 11,569 | | | $ | 7,182 | | | | 61 | % | | $ | 32,297 | | | $ | 37,120 | | | | -13 | % |
| Gross profit | | | 2,751 | | | $ | 2,078 | | | | 32 | % | | | 10,803 | | | | 12,852 | | | | -16 | % |
| Gross profit % | | | 23.8 | % | | | 28.9 | % | | | | | | | 33.4 | % | | | 34.6 | % | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Operating expenses | | | 11,341 | | | | 9,235 | | | | 23 | % | | | 32,011 | | | | 28,116 | | | | 14 | % |
| Operating loss | | | (8,590 | ) | | | (7,157 | ) | | | 20 | % | | | (21,208 | ) | | | (15,264 | ) | | | 39 | % |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Income taxes | | | 48 | | | | - | | | | | | | | 66 | | | | - | | | | - | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Net loss | | $ | (8,520 | ) | | $ | (8,505 | ) | | | 0 | % | | $ | (21,152 | ) | | $ | (15,966 | ) | | | 32 | % |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Basic and diluted loss per share | | $ | (0.19 | ) | | $ | (0.19 | ) | | | 0 | % | | $ | (0.46 | ) | | $ | (0.36 | ) | | | 28 | % |
| Balance Sheet Data | | As at
September 30,
2012 | | | As at
December 31,
2011 | |
| | | | | | | |
| Cash | | | 28,383 | | | $ | 40,425 | |
| Total assets | | | 85,957 | | | | 94,290 | |
| Deferred revenue | | | 14,251 | | | | 7,147 | |
| Total liabilities | | | 29,361 | | | | 19,731 | |
| Shareholders' equity | | | 56,596 | | | | 74,559 | |
IMRIS Inc.
Management's Discussion and Analysis – November 8, 2012
Page 8 of 26 |
Revenues
Revenues by sales classification
| (Thousands of US dollars) | | Three months ended | | | | | | Nine months ended | | | | |
| | | September 30 | | | % | | | September 30 | | | % | |
| | | 2012 | | | 2011 | | | Change | | | 2012 | | | 2011 | | | Change | |
| | | | | | | | | | | | | | | | | | | |
| VISIUS Surgical Theatres | | $ | 10,247 | | | $ | 6,250 | | | | 64 | % | | $ | 28,742 | | | $ | 34,759 | | | | -17 | % |
| Extended maintenance contracts | | | 1,322 | | | | 932 | | | | 42 | % | | | 3,555 | | | | 2,361 | | | | 51 | % |
| Total revenues | | $ | 11,569 | | | $ | 7,182 | | | | 61 | % | | $ | 32,297 | | | $ | 37,120 | | | | -13 | % |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| VISIUS Surgical Theatres as a percentage of total revenues | | | 89 | % | | | 87 | % | | | | | | | 89 | % | | | 94 | % | | | | |
| Extended maintenance contracts as a percentage of total revenues | | | 11 | % | | | 13 | % | | | | | | | 11 | % | | | 6 | % | | | | |
Revenues by region
| (Thousands of US dollars) | | Three months ended | | | | | | Nine months ended | | | | |
| | | September 30 | | | % | | | September 30 | | | % | |
| | | 2012 | | | 2011 | | | Change | | | 2012 | | | 2011 | | | Change | |
| North America | | $ | 7,939 | | | $ | 2,528 | | | | 214 | % | | $ | 23,270 | | | $ | 17,116 | | | | 36 | % |
| Europe and Middle East | | | 65 | | | | 4,075 | | | | -98 | % | | | 306 | | | | 8,661 | | | | -96 | % |
| Asia Pacfic | | | 3,565 | | | | 579 | | | | 516 | % | | | 8,721 | | | | 11,343 | | | | -23 | % |
| | | $ | 11,569 | | | $ | 7,182 | | | | | | | $ | 32,297 | | | $ | 37,120 | | | | | |
Revenues increased $4.4 million or 61% to $11.6 million for the three months ended September 30, 2012. Additional customer delivery activities resulted in a $4.0 million increase in product revenues compared to the prior year quarter. Extended maintenance contract revenue increased approximately $0.4 million from the same period in 2011 as a result of an increase in the install base of VISIUS Surgical Theatres, which have transitioned off warranty to chargeable service programs.
Revenues decreased by approximately $4.8 million or 13% to $32.3 million for the nine months ended September 30, 2012. Year to date VISIUS system revenues decreased $6.1 million from the previous period due to lower conversion of product backlog into deliveries and changes in product mix between the two periods. Extended maintenance contract revenue increased $1.2 million from the same period in 2011 as a result of a larger installation base of VISIUS Surgical Theatres, which have transitioned to chargeable service programs.
Third quarter revenues were higher in North America and Asia Pacific due to increased VISIUS Surgical Theatre system deliveries and increased service revenue in the United States compared to the same period in 2011. There were no significant delivery activities in Europe in the current period.
Year to date revenues were higher in North America as a result of increased VISIUS system delivery activity and service revenues resulting from an increase of the install base coming off warranty. The increase in North America was partially offset by a significant reduction in Europe and Middle East activity as there was limited product deliveries in 2012. Asia Pacific revenues were also lower as compared to 2011 because the prior year included the delivery of VISIUS a cardiovascular system, which has a higher average sales price than systems delivered in 2012.
IMRIS Inc.
Management's Discussion and Analysis – November 8, 2012
Page 9 of 26 |
Gross Profit
| (Thousands of US dollars) | | Three months ended | | | | | | Nine months ended | | | | |
| | | September 30 | | | % | | | September 30 | | | % | |
| | | 2012 | | | 2011 | | | Change | | | 2012 | | | 2011 | | | Change | |
| | | | | | | | | | | | | | | | | | | |
| Gross profit | | $ | 2,751 | | | $ | 2,078 | | | | 32 | % | | $ | 10,803 | | | $ | 12,852 | | | | -16 | % |
| As a percentage of sales | | | 23.8 | % | | | 28.9 | % | | | | | | | 33.4 | % | | | 34.6 | % | | | | |
Gross profit in the third quarter of 2012 increased from $2.1 million to $2.8 million. Gross profit as a percentage of sales decreased from 28.9% in the third quarter of 2011 to 23.8% in third quarter 2012. Gross margin and gross margin as a percentage of sales was negatively impacted by an expected low margin project on our first installation in Japan. This project carried a lower margin as it was converted to a VISIUS Surgical Theatre sale from a competitor’s offering late in the sales cycle at the hospital. The project also experienced additional margin erosion as unplanned costs have arisen during the delivery phase in the period. The establishment of our first installation in the Japanese market is an important milestone in our efforts to extend the benefits of our systems in this market and expect to see future margins more consistent with established Company markets. Margins in the current period were also reduced as a result of the planned delivery of equipment as part of a collaborative arrangement the Company has entered into in the area of interventional cerebrovascular science and imaging, with specific focus on the integration of angiography and MR imaging modalities during interventional stroke procedures.
Gross profit for the nine months ended September 30, 2012 decreased from $12.9 million to $10.8 million. Gross profit as a percentage of sales decreased from 34.6% in 2011 to 33.4% in 2012. Overall margins are lower in 2011 and 2012 because of equipment deliveries related to collaborative arrangements and the low margin project in Japan.
Operating Expenses
Operating expenses for the three months ended September 30, 2012 were $11.3 million, an increase of approximately $2.1 million or 23% over the same period in 2011. On a year to date basis, total operating expenses increased to $32.0 million from $28.1 in 2011, an increase of $3.9 million or 14%. The increase for both the quarter and year to date is primarily related to the planned increase in research and development costs for robotics, MR-guided radiation therapy and other ancillary research projects.
Operating expenses in the third quarter of 2012 also included increased costs for corporate travel and recruitment costs as a result of the hiring of the President and Chief Operating Officer and other senior positions in the quarter. Professional fees have decreased in the quarter because initial consulting costs related to our 2011 US listing and our conversion of the financial systems to US dollars and US accounting standards were not required in 2012.
On a year to date basis, operating expenses also included higher amortization expense and rent charges offset by lower travel and marketing costs. Rent charges mainly increased as a result of the establishment of an office in Minneapolis during the year. Travel between the offices did increase during the period; however travel expense was lower overall because administrative travel overall was lower compared to 2011. Marketing and promotion costs were also lower because the Company did not host a Global User meeting in 2012.
IMRIS Inc.
Management's Discussion and Analysis – November 8, 2012
Page 10 of 26 |
Administrative
| (Thousands of US dollars) | | Three months ended | | | | | | Nine months ended | | | | |
| | | September 30 | | | % | | | September 30 | | | % | |
| | | 2012 | | | 2011 | | | Change | | | 2012 | | | 2011 | | | Change | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Administrative | | $ | 2,063 | | | $ | 2,086 | | | | -1 | % | | $ | 5,647 | | | $ | 6,258 | | | | -10 | % |
Administrative expense for the three months ended September 30, 2012 was consistent with the same period in 2011 as a result of offsetting changes in various expense categories. Employee costs were higher as a result of placement fees for several senior management positions, including the appointment of the President and Chief Executive Officer ($0.1 million). Travel costs were higher ($0.1 million) as a result of additional employee recruitment activities. Professional fees were lower ($0.2 million) as a result of non-recurring costs from 2011 related to the US listing and our conversion of the financial systems to US dollars.
Administration expenses for the nine months ended September 30, 2012 were $0.7 million lower compared to the same period in 2011. Employee costs were lower ($0.2 million) because the President and Chief Operating Officer was not appointed until the end of the third quarter and stock option expense was lower because a number of stock option tranches became fully vested. Professional fees are lower ($0.3) because of a planned reduction in consulting and legal costs related to the 2011 US listing and conversion of the financial systems to US dollars. Travel costs were down as administrative travel in the period was significantly lower ($0.2 million).
Sales and marketing
| (Thousands of US dollars) | | Three months ended | | | | | | Nine months ended | | | | |
| | | September 30 | | | % | | | September 30 | | | % | |
| | | 2012 | | | 2011 | | | Change | | | 2012 | | | 2011 | | | Change | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Sales and marketing | | $ | 2,419 | | | $ | 2,177 | | | | 11 | % | | $ | 7,033 | | | $ | 7,032 | | | | 0 | % |
Sales and marketing expense for the three months ended September 30, 2012 was $0.2 million higher compared to the same period in 2011. Commission costs were higher as a result of increased VISIUS system deliveries in the period and recruiting fees increased for new hires resulting in higher ($0.1 million) employee costs. Marketing and promotion costs were higher ($0.1 million) because the Company attended several new tradeshows and conventions this quarter as compared to 2011.
Sales and marketing expense for the nine months ended September 30, 2012 was consistent compared to the same period in 2011. Recruiting costs increased ($0.3 million) as a result of placement fees for new staff and consulting costs increased ($0.2 million) to develop the Company’s corporate marketing campaign. The Global User meeting was not held in 2012 reducing travel ($0.2 million) and marketing costs ($0.3 million) in the period.
Customer support and operations
| (Thousands of US dollars) | | Three months ended | | | | | | Nine months ended | | | | |
| | | September 30 | | | % | | | September 30 | | | % | |
| | | 2012 | | | 2011 | | | Change | | | 2012 | | | 2011 | | | Change | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Customer support and operations | | $ | 1,973 | | | $ | 1,561 | | | | 26 | % | | $ | 5,519 | | | $ | 5,064 | | | | 9 | % |
Customer support and operations expense for the three months ended September 30, 2012 was $0.4 million higher compared to the same period in 2011. Recruiting costs ($0.2 million) increased as a result of placement charges for new staff. Business travel increased ($0.2 million) as a result of additional travel for customer support activities.
IMRIS Inc.
Management's Discussion and Analysis – November 8, 2012
Page 11 of 26 |
Customer support and operations expense for the nine months ended September 30, 2012 increased $0.4 million compared to the same period in 2011. Business travel is higher ($0.2 million) as a result of additional customer support activities. Office costs are higher ($0.2 million) because of an increase in the percentage of costs being allocated as a result of additional manufacturing space.
Research and development
| (Thousands of US dollars) | | Three months ended | | | | | | Nine months ended | | | | |
| | | September 30 | | | % | | | September 30 | | | % | |
| | | 2012 | | | 2011 | | | Change | | | 2012 | | | 2011 | | | Change | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Research and development | | $ | 3,819 | | | $ | 2,527 | | | | 51 | % | | $ | 10,786 | | | $ | 7,153 | | | | 51 | % |
Research and development expenses for the three and nine months ended September 30, 2012 increased $1.3 million and $3.6 million, respectively over the same period in 2011. The change in both periods is mainly due to planned increases in technical development spending relating to the SYMBIS Surgical System, MR guided radiation therapy development, and other ongoing development projects.
Amortization
| (Thousands of US dollars) | | Three months ended | | | | | | Nine months ended | | | | |
| | | September 30 | | | % | | | September 30 | | | % | |
| | | 2012 | | | 2011 | | | Change | | | 2012 | | | 2011 | | | Change | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Amortization | | $ | 1,067 | | | $ | 884 | | | | 21 | % | | $ | 3,026 | | | $ | 2,609 | | | | 16 | % |
The increase in amortization expense for the three and nine months ended September 30, 2012 is due to additional amortization on fixed asset additions.
Foreign exchange
| (Thousands of US dollars) | | Three months ended | | | | | | Nine months ended | | | | |
| | | September 30 | | | % | | | September 30 | | | % | |
| | | 2012 | | | 2011 | | | Change | | | 2012 | | | 2011 | | | Change | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Foreign exchange gain (loss) | | $ | 118 | | | $ | (1,351 | ) | | | nm | | | $ | 107 | | | $ | (736 | ) | | | nm | |
nm – not meaningful
Foreign exchange gains for the three and nine months ended September 30, 2012 are mainly the result of a weakening of the US dollar resulting in gains on translation of non-USD net monetary assets and non-USD transactions compared to previous periods. The previous period losses resulted from the translation of a large non-USD receivable at period end.
IMRIS Inc.
Management's Discussion and Analysis – November 8, 2012
Page 12 of 26 |
Income tax
| (Thousands of US dollars) | | Three months ended | | | | | | Nine months ended | | | | |
| | | September 30 | | | % | | | September 30 | | | % | |
| | | 2012 | | | 2011 | | | Change | | | 2012 | | | 2011 | | | Change | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Income tax expense | | $ | 48 | | | $ | - | | | | nm | | | $ | 66 | | | $ | - | | | | nm | |
nm – not meaningful
The Company generates taxable income in several of its foreign subsidiaries due to transfer pricing policies used in those foreign jurisdictions. As a result of activities in these foreign subsidiaries, the Company has recognized tax expense during the three and nine months ended September 30, 2012.
Operating Loss and Net Loss
| (Thousands of US dollars) | | Three months ended | | | | | | Nine months ended | | | | |
| | | September 30 | | | % | | | September 30 | | | % | |
| | | 2012 | | | 2011 | | | Change | | | 2012 | | | 2011 | | | Change | |
| | | | | | | | | | | | | | | | | | | |
| Operating loss | | $ | (8,590 | ) | | $ | (7,157 | ) | | | 20 | % | | $ | (21,208 | ) | | $ | (15,264 | ) | | | 39 | % |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Net loss | | $ | (8,520 | ) | | $ | (8,505 | ) | | | 0 | % | | $ | (21,152 | ) | | $ | (15,966 | ) | | | 32 | % |
The $1.4 million increase in operating loss in the quarter was mainly the result of reduced margins in the quarter and increased research and development costs related to the robotics and radiation therapy programs. For the nine months ended September 30, 2012, the $5.9 million increase in operating loss was mainly a result of reduced margins in the period and increased research and development costs related to the robotics and radiation therapy programs.
The net loss for the three months ended September 30, 2012 was consistent with the same period in 2011 because the $1.3 million increase in research and development costs over the previous quarter was offset by a foreign exchange loss in 2011. On a year to date basis, the $5.2 million increase in net loss compared to 2011 was due to reduced margins and increased research and development partially offset by foreign exchange gains in 2012 versus foreign exchange losses in in the prior year.
Adjusted EBITDA
| (Thousands of US dollars) | | Three months ended | | | | | | Nine months ended | | | | |
| | | September 30 | | | % | | | September 30 | | | % | |
| | | 2012 | | | 2011 | | | Change | | | 2012 | | | 2011 | | | Change | |
| | | | | | | | | | | | | | | | | | | |
| Adjusted EBITDA | | $ | (7,128 | ) | | $ | (5,885 | ) | | | 21 | % | | $ | (17,078 | ) | | $ | (11,585 | ) | | | 47 | % |
We use the non-GAAP measure Adjusted EBITDA to measure aspects of our financial performance (see “Non-GAAP Financial Measures” for a reconciliation of adjusted EBITDA to GAAP measures). We define Adjusted EBITDA as earnings (loss) before stock based compensation, interest income (expense), foreign exchange gain (loss), embedded derivatives gain (loss), income taxes and amortization.
For the three months ended September 30, 2012, Adjusted EBITDA was negative $7.1 million compared with negative $5.9 million for the same period in 2011. The decrease in Adjusted EBITDA during the quarter was primarily due to reduced margins and higher research and development costs.
For the nine months ended September 30, 2012, Adjusted EBITDA was negative $17.1 million compared with negative $11.6 million for the same period in 2011. The decrease in Adjusted EBITDA during the quarter was primarily due to reduced margins and higher research and development costs.
IMRIS Inc.
Management's Discussion and Analysis – November 8, 2012
Page 13 of 26 |
SUMMARY OF QUARTERLY RESULTS
The following table is a summary of our financial results for the past eight quarters:
| (Thousands of US dollars) | | Q3 | | | Q2 | | | Q1 | | | Q4 | | | Q3 | | | Q2 | | | Q1 | | | Q4 | |
| | | 2012 | | | 2012 | | | 2012 | | | 2011 | | | 2011 | | | 2011 | | | 2011 | | | 2010 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Sales | | $ | 11,569 | | | $ | 17,235 | | | $ | 3,493 | | | $ | 14,677 | | | $ | 7,182 | | | $ | 18,881 | | | $ | 11,057 | | | $ | 24,840 | |
| Cost of sales | | | 8,818 | | | | 10,577 | | | | 2,099 | | | | 9,855 | | | | 5,104 | | | | 12,560 | | | | 6,604 | | | | 13,314 | |
| Gross profit | | | 2,751 | | | | 6,658 | | | | 1,394 | | | | 4,822 | | | | 2,078 | | | | 6,321 | | | | 4,453 | | | | 11,526 | |
| As a percentage of sales | | | 23.8 | % | | | 38.6 | % | | | 39.9 | % | | | 32.9 | % | | | 28.9 | % | | | 33.5 | % | | | 40.3 | % | | | 46.4 | % |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Operating expenses | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Administration | | | 2,063 | | | | 1,899 | | | | 1,685 | | | | 2,071 | | | | 2,086 | | | | 2,155 | | | | 2,017 | | | | 1,600 | |
| Sales and marketing | | | 2,419 | | | | 2,549 | | | | 2,065 | | | | 2,937 | | | | 2,177 | | | | 2,520 | | | | 2,335 | | | | 3,110 | |
| Customer support and operations | | | 1,973 | | | | 1,892 | | | | 1,654 | | | | 1,787 | | | | 1,561 | | | | 1,740 | | | | 1,763 | | | | 1,705 | |
| Research and development | | | 3,819 | | | | 3,401 | | | | 3,566 | | | | 2,421 | | | | 2,527 | | | | 2,127 | | | | 2,499 | | | | 222 | |
| Amortization | | | 1,067 | | | | 990 | | | | 969 | | | | 908 | | | | 884 | | | | 871 | | | | 854 | | | | 912 | |
| | | | 11,341 | | | | 10,731 | | | | 9,939 | | | | 10,124 | | | | 9,235 | | | | 9,413 | | | | 9,468 | | | | 7,549 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Operating income (loss) before the following: | | | (8,590 | ) | | | (4,073 | ) | | | (8,545 | ) | | | (5,302 | ) | | | (7,157 | ) | | | (3,092 | ) | | | (5,015 | ) | | | 3,977 | |
| Foreign exchange | | | 118 | | | | (207 | ) | | | 196 | | | | 710 | | | | (1,351 | ) | | | 210 | | | | 405 | | | | (1,021 | ) |
| Interest | | | - | | | | (2 | ) | | | 17 | | | | (1 | ) | | | 3 | | | | 9 | | | | 22 | | | | 14 | |
| Income (loss) before taxes | | $ | (8,472 | ) | | $ | (4,282 | ) | | $ | (8,332 | ) | | $ | (4,593 | ) | | $ | (8,505 | ) | | $ | (2,873 | ) | | $ | (4,588 | ) | | $ | 2,970 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Income taxes | | | 48 | | | | - | | | | 18 | | | | 366 | | | | - | | | | - | | | | - | | | | - | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net income (loss) for the quarter | | $ | (8,520 | ) | | $ | (4,282 | ) | | $ | (8,350 | ) | | $ | (4,959 | ) | | $ | (8,505 | ) | | $ | (2,873 | ) | | $ | (4,588 | ) | | $ | 2,970 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Earning (loss) per share | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Basic | | $ | (0.19 | ) | | $ | (0.09 | ) | | $ | (0.18 | ) | | $ | (0.11 | ) | | $ | (0.19 | ) | | $ | (0.06 | ) | | $ | (0.10 | ) | | $ | 0.03 | |
| Diluted | | $ | (0.19 | ) | | $ | (0.09 | ) | | $ | (0.18 | ) | | $ | (0.11 | ) | | $ | (0.19 | ) | | $ | (0.06 | ) | | $ | (0.10 | ) | | $ | 0.02 | |
The financial results for the eight most recent quarters reflect the progression of an early stage Company with a limited operating history. Factors that have caused our results to vary are described below.
| Ø | As a result of the limited number of VISIUS Surgical Theatres sold and installed to date and the high dollar value associated with each sale, our revenues recorded from quarter to quarter have varied depending on the number and stage of active projects in any given quarter. |
| Ø | Gross margins for the VISIUS Surgical Theatre are largely dependent on whether a particular product application has achieved acceptance amongst clinical thought leaders. Given the maturity and clinical data surrounding the neurosurgical application of the VISIUS Surgical Theatre, margins for these products have generally been stronger than other newer clinical applications. |
| Ø | The decrease in gross profit percentage in the latter stages of 2011 is primarily tied to market penetration pricing for the introduction of the VISIUS Surgical Theatre for cerebrovascular and cardiovascular applications, as well as the provision of certain equipment for research purposes to a third party customer. The decrease in gross margins in 2012 is mainly due to higher installation costs with third quarter margins being further impacted by the provision of certain equipment for research purposes and lower margins for the Company’s first customer installation in Japan. |
IMRIS Inc.
Management's Discussion and Analysis – November 8, 2012
Page 14 of 26 |
| Ø | Net losses generally vary depending on the timing of when specific projects were installed and the pricing associated with the respective projects. Net losses in 2011 in 2012 have largely been the result of lower product installations and planned increases in research and development activity for the Company’s MR guided radiation therapy program and the image-guided robotics program. |
| Ø | Operating expenses began to increase in 2011 because of planned research and development activities for robotics, MR-guided radiation therapy and other ancillary research projects, increased employee costs, and professional fees as a result of additional reporting requirements as a NASDAQ registrant. Operating expenses during the first part of 2012, in areas outside of research and development, are lower as a result of cost management measures undertaken by management. Increases in the third quarter reflect additional efforts to recruit qualified personnel and expansion of office locations to the US. |
| Ø | Although the majority of the Company’s sales are denominated in US dollars, the Company sells its VISIUS Surgical Theatres in a variety of foreign currencies. As well, a significant portion of the Company’s operating costs are in Canadian dollars. This gives rise to foreign exchange gains or losses each quarter depending on the change in value of the US dollar versus the Canadian dollar and other currencies in each quarter. |
| Ø | The Company generated taxable income in several of its foreign subsidiaries in 2011 because of our transfer pricing methodology. As a result, the Company began recognizing tax expense in the 4th quarter of 2011 related to these subsidiaries. |
Backlog
In the third quarter of 2012, total order bookings were $18.2 million, consisting of $15.6 million of product bookings including two new VISIUS Surgical Theatres order and $2.6 million of new service contracts. We converted $11.6 million of backlog into revenues and changes in foreign exchange increased backlog by $1.0 million for a total backlog at September 30, 2012 of $127.5 million. We evaluate our backlog and individual order conversion on a regular basis and our experience is that orders typically convert into revenues over 12 to 18 months on average. We have one order in backlog valued at approximately $9.6 million, which has not proceeded at our expectations. The order continues to meet our defined criteria for inclusion in order backlog and the customer has not informed us of any change in their plans, but we are continuing to monitor the order for inclusion in our backlog.
The table below provides the Company’s backlog on a segmented basis, as at September 30, 2012 and its comparable periods for each of the last three years as of December 31:
| | | December 31 | | | March 31, | | | June 30, | | | September 30, | |
| | | 2009 | | | 2010 | | | 2011 | | | 2012 | | | 2012 | | | 2012 | |
| VISIUS Surgical Theatres | | $ | 85,045 | | | $ | 86,505 | | | $ | 58,583 | | | $ | 78,068 | | | $ | 78,765 | | | $ | 84,687 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Service contracts | | | 24,035 | | | | 30,619 | | | | 36,430 | | | | 37,615 | | | | 41,154 | | | | 42,854 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Total backlog | | $ | 109,080 | | | $ | 117,124 | | | $ | 95,013 | | | $ | 115,683 | | | $ | 119,919 | | | $ | 127,541 | |
To September 30, 2012, we have sold 57 systems, of which 43 are installed and 14 are in the delivery phase. Of the 57 sold, 36 are in the United States, 8 are in Canada, 9 are in Asia Pacific and 4 are in Europe and the Middle East.
We use the non-GAAP measure “backlog” to measure aspects of our financial performance. Backlog is defined as the unrecognized portion of (i) revenues anticipated to be recorded from VISIUS Surgical Theatre orders, including confirmed orders and orders subject to the completion of formal documentation and (ii) service contracts with a term of four to five years and which commence at the conclusion of the warranty period on our VISIUS Surgical Theatres. The term of our service contracts generally ranges from 4 to 5 years commencing at the conclusion of the warranty period on our VISIUS Surgical Theatres, which are typically 1 year in length. Service contract revenue is recognized ratably over the term of the contract.
IMRIS Inc.
Management's Discussion and Analysis – November 8, 2012
Page 15 of 26 |
OUTLOOK
We have experienced improving demand for our products through the first three quarter of 2012 and continue to see strong signs of customer engagement for new orders. We continue to execute against the 2012 business plans with a focus to support two fundamental priorities:
Improved Bookings Growth – We have a large base of qualified customers who are at advanced stages of the sales cycle and we have aggressively worked to convert these opportunities into new sales orders. This has been evidenced in our growth in order bookings in the first nine months of the year of $64.3 million, up $34.8 million from the annual total recorded in 2011. We believe the establishment of a broad customer pull marketing program that includes market specific public relations campaigns, expansion of our peer reviewed publications program, and a series of customer education initiatives has had a positive impact on our results to date. We believe the clinical demand created will continue to contribute significant strengthening of our bookings performance.
New Product Development and Commercialization – We have employed a disciplined approach to product development ensuring resources are appropriately focused on projects and programs with the greatest potential to create long term value. We have advanced our product development and commercialization activities in MR guided radiation therapy and image-guided surgical robotics and as well continue to improve the capabilities of the VISIUS Surgical Theatre. We continue to focus on driving development activities of key 2012 development programs including:
| Ø | In August 2012, our image-guided surgical robotic system, the SYMBIS Surgical System clearance application was submitted to the FDA. We have received questions on our 510(k) application which we are reviewing and responding to. |
| Ø | In September, 2012 the US patent and trademark office allowed the patent for MR Guided Radiation Therapy involving the combination of radiation therapy and a moveable magnet system. |
| Ø | In September 2012, the U.S. FDA cleared the oncology package for our planned MR Guided Radiation Therapy system. Our second filing of three clearance applications for this system is on track for completion in the fourth quarter of 2012. |
| Ø | Our VISIUS Surgical Theatre utilizing computed tomography is expected to be submitted to the FDA for clearance application in the fourth quarter of 2012. This new product offering will directly address the very large market for image-guided spinal procedures. |
These new products, together with our existing VISIUS Surgical Theatre will further broaden our clinical applications and introduce new price point products that can drive rapid customer adoption. Currently, our sales resources are being focused on those hospitals that have the greatest opportunity to utilize the VISIUS Surgical Theatre for neurosurgical applications. From that base we intend to build future sales opportunities for cardiovascular and cerebrovascular applications as the recognition of the benefits of the VISIUS Surgical Theatre expands and leverage existing customer relationships when the new products are commercialized.
Financial Outlook
Revenues
Our ability to complete installations and recognize revenue on a timely basis is directly influenced by the circumstances of each hospital and schedules can shift because of unique customer specific requirements. The delivery cycle and installation process for a VISIUS Surgical Theatre is lengthy and installation times can be further lengthened depending on additional site-specific construction work that may be required to be completed by the customer. Our conversion of backlog in revenue has largely tracked against our 2012 revenue plan. In the fourth quarter of 2012, we anticipate revenues to be in the range of $21 - $22 million for a total of $53 - 54 million for the year.
IMRIS Inc.
Management's Discussion and Analysis – November 8, 2012
Page 16 of 26 |
In 2012, we have seen a significant increase in order bookings from the prior year. This was in-line with expectations against our sales funnel and against a backdrop of an improving demand in North America for our products. We continue to see an improving order bookings profile from the prior year and expect we will eclipse our previous high in annual order bookings.
Gross Profit
Over the course of our Company‘s history, we have seen strong improvement in gross profit for VISIUS Surgical Theatres for neurosurgical applications, reflecting the shift from market penetration-based pricing in new markets to value-based pricing. Our latest neurosurgical installation in the Japanese market has reflected the same penetration pricing approach, but we anticipate we will quickly convert to consistently higher margins in the region with the market acceptance of the technology.
A similar pricing approach has been used for VISIUS Surgical Theatre more recently introduced applications for cerebrovascular, cardiovascular and spine configurations, which has translated into lower margins. In Q3 2012, the delivery of certain equipment as part of a collaborative research arrangement for cerebrovascular applications commenced as planned which has had the effect of reducing our overall gross margin for 2012.
In 2012, we continue to expect our overall annual gross margin to be comparable to 2011 levels as each of these systems is completed. In Q4 2012, overall margins are expected to increase to approximately 37% of revenues as the Company a large portion of revenue associated with the lower margin projects noted will have been recognized. Quarterly gross margin levels are expected to continue to vary depending on the underlying system installations in the respective quarters. The Company’s project margins are anticipated to normalize in the coming year increasing into the 40% range.
Operating Expenses
Our priorities for 2012 include a focused sales and marketing drive to maximize bookings together with increased investment to complete development of our image guided surgical robot, image guided radiation therapy product, and to further improve the capabilities of the VISIUS Surgical Theatre. Research & development expenses in 2012 are expected to be approximately $13 million to $15 million with the dollar value of operating expenses, excluding research and development, forecast to be unchanged compared with 2011 levels.
Liquidity and Capital Resources
Our Company is well positioned to continue to build the business. With cash and accounts receivable at September 30, 2012 of $37.6 million and order backlog of $127.5 million, we have a strong base from which to continue to fund our operations and product development projects.
Our cash requirements in 2012 include funding for operations, capital investments related to robotics, iCT and MRgRT test labs and prepaid development costs associated with our collaborative arrangements. Our total capital expenditures for the remainder of the year are expected to be in the range of $0.5 million to $1.0 million.
The prepaid development costs are part of the collaborative arrangement we entered into in October 2011 with the University Health Network at Princess Margaret Hospital in Toronto. Our image guided radiation therapy platform will be used to conduct research at the hospital in order to clinically validate the system and develop a commercially viable version of the platform. These costs will be deferred until the installation is complete and then expensed as research and development when clinical validation can begin. We expect to incur approximately $3.0 to $3.5 million in cash costs in addition to the $3.5 million incurred up to September 30, 2012. We currently anticipate that we will expense approximately $1.5 to $2.5 million of these deferred costs in the first half of 2013, with the remainder being expensed in the second half of 2013 as the various components of the installation are completed.
IMRIS Inc.
Management's Discussion and Analysis – November 8, 2012
Page 17 of 26 |
LIQUIDITY AND CAPITAL RESOURCES
Our principal capital needs are for funding scientific research and development programs, supporting our sales and marketing activities and funding capital expenditures and working capital. The Company has financed its cash requirements primarily through issuances of securities and advanced customer deposits from new orders.
We had cash of $28.4 million as at September 30, 2012, a decrease of $12.0 million from December 31, 2011. The $12.0 million decrease from December 31, 2011 primarily resulted from operating and financing activities. Operating activities included an operating loss (excluding non-cash related items) of $17.9 million and cash from working capital of $9.3 million. Financing activities included capital spending of $5.9 million. These uses of cash were offset by $2.4 million raised through employee stock options being exercised and a foreign exchange translation adjustment of $0.1 million.
The following table sets forth the summary statement of cash flows for the dates and periods indicated:
Statements of Cash Flows
(Thousands of US dollars)
(Unaudited)
| | | Three months ended | | | Nine months ended | |
| | | September 30, | | | September 30, | |
| | | 2012 | | | 2011 | | | Change | | | 2012 | | | 2011 | | | Change | |
| Cash flows: | | | | | | | | | | | | | | | | | | | | | | | | |
| Used in Operating Activities | | $ | (3,622 | ) | | $ | (5,031 | ) | | $ | 1,409 | | | $ | (8,605 | ) | | $ | (19,070 | ) | | $ | 10,465 | |
| From Financing Activities | | | 515 | | | | 284 | | | | 231 | | | | 2,346 | | | | 984 | | | | 1,362 | |
| Used in Investing Activities | | | (890 | ) | | | (426 | ) | | | (464 | ) | | | (5,866 | ) | | | (1,990 | ) | | | (3,876 | ) |
| Foreign exchange translation adjustment | | | (325 | ) | | | 239 | | | | (564 | ) | | | 83 | | | | 185 | | | | (102 | ) |
| Net decrease | | | (4,322 | ) | | | (4,934 | ) | | | 612 | | | | (12,042 | ) | | | (19,891 | ) | | | 7,849 | |
| Cash and cash equivalents, opening | | | 32,705 | | | | 45,816 | | | | | | | | 40,425 | | | | 60,773 | | | | | |
| Cash and cash equivalents, closing | | $ | 28,383 | | | $ | 40,882 | | | $ | (12,499 | ) | | $ | 28,383 | | | $ | 40,882 | | | $ | (12,499 | ) |
Operating Activities
The cash used from operating activities for the three months ended September 30, 2012 was $3.6 million. The cash from operating activities was comprised of an operating loss of approximately $8.1 million (excluding non-cash related items) and $4.5 million cash from working capital. The $4.5 million in cash from working capital consists of a decrease in accounts receivable ($2.0 million), a decrease in inventory ($0.2 million), an increase in accounts payable and accrued liabilities ($1.4 million) and an increase in deferred revenue ($2.0 million) offset by an increase in prepaid ($0.2 million) and an increase in unbilled receivables ($0.9 million).
Financing Activities
Financing activities for the three months ended September 30, 2012 was $0.5 million compared to $0.3 million in the same period in 2011. The cash generated in financing activities for both 2011 and 2012 was a result of employee share options being exercised.
Investing Activities
The cash used from investing activities for the current quarter was approximately $0.9 million compared to approximately $0.4 million in the same period in 2011. Investing activities in 2012 include the release of restricted cash ($1.5 million) offset by the acquisition of tangible and intangible capital assets ($2.1 million) and the increases in other assets ($0.3 million). The $2.1 million in capital asset consists mainly of additions to our test labs, including the new labs for robotics and iCT. The $0.3 million increase in other assets consists mainly of additional research and development costs related to collaborative arrangements.
IMRIS Inc.
Management's Discussion and Analysis – November 8, 2012
Page 18 of 26 |
Liquidity and Capital Resources Summary
Our cash as at September 30, 2012 totaled $28.4 million which includes cash equivalents of $1.6 million. This cash position is expected to provide sufficient liquidity to meet the anticipated needs of current operations and existing projects and budgeted capital asset expenditures. There have been no material changes in operating lease payments the Company expects to make over the next five years.
OUTSTANDING SHARE DATA
The following table sets forth our outstanding share data as at the dates given:
| | | Authorized | | November 8, 2012 | | December 31, 2011 |
| | | | | | | |
| Common shares | | unlimited | | $147,710,031 (46,030,367 common shares) | | $144,410,000 (44,975,109 common shares) |
| Preferred shares | | unlimited | | Nil | | Nil |
| | | | | | | |
| Additional paid-in capital | | | | $4,441,295 | | $4,291,000 |
As at November 8, 2012, a total of 4,369,795 stock options were outstanding under the Company’s stock option plan.
NON-GAAP FINANCIAL MEASURES
In this MD&A, we use the non-GAAP measure “Backlog” and "Adjusted EBITDA". We define backlog as the unrecognized portion of the revenues anticipated to be recorded from VISIUS Surgical Theatre orders, including confirmed orders and orders subject to completion of formal documentation and the unrecognized portion of service contracts which have a term of 4-5 years commencing at the conclusion of the warranty period on our theatres, which is typically one year in length. In view of the long sales cycle, high unit price and limited quarterly installations that are characteristic of our business, we believe that our backlog provides a better measure at any particular point in time of the long-term performance prospects of our business than our quarterly operating results. Backlog does not have any standardized meaning prescribed by U.S GAAP and is, therefore, unlikely to be comparable to similar measures presented by other companies.
We define Adjusted EBITDA as earnings before stock based compensation, interest income (expense), foreign exchange gain (loss), embedded derivative gain (loss), income taxes, and amortization. We have begun reporting Adjusted EBITDA because we believe investors use it as another measure of our operating performance. Adjusted EBITDA does not have a standardized meaning as prescribed by U.S. GAAP and it is not necessarily comparable to similarly titled measures used by other companies. Reconciliation to the most comparable U.S. GAAP measure for Adjusted EBITDA for the three and nine months ended September 30, 2012 is as follows:
| (Thousands of US dollars) | | Three months ended | | | Nine months ended | |
| (Unaudited) | | September 30, | | | September 30, | | | September 30, | | | September 30, | |
| | | 2012 | | | 2011 | | | 2012 | | | 2011 | |
| Net loss for the period | | $ | (8,520 | ) | | $ | (8,505 | ) | | $ | (21,152 | ) | | $ | (15,966 | ) |
| Stock based compensation | | | 395 | | | | 388 | | | | 1,104 | | | | 1,070 | |
| Foreign exchange (gain) loss | | | (118 | ) | | | 1,351 | | | | (107 | ) | | | 736 | |
| Interest (income) expense | | | - | | | | (3 | ) | | | (15 | ) | | | (34 | ) |
| Amortization | | | 1,067 | | | | 884 | | | | 3,026 | | | | 2,609 | |
| Income taxes | | | 48 | | | | - | | | | 66 | | | | - | |
| Adjusted EBITDA | | $ | (7,128 | ) | | $ | (5,885 | ) | | $ | (17,078 | ) | | $ | (11,585 | ) |
IMRIS Inc.
Management's Discussion and Analysis – November 8, 2012
Page 19 of 26 |
FINANCIAL INSTRUMENTS
Our financial instruments consist of cash, cash equivalents, accounts receivables, unbilled receivables, accounts payable and accrued liabilities.
We are subject to credit risk with respect to our accounts receivable and unbilled receivables to the extent debtors do not meet their obligations and we are subject to foreign exchange risk with respect to financial instruments denominated in a currency other than the US dollar.
Our accounts receivable at September 30, 2012 were $9.2 million, of which $7.5 million is considered current (less than 60 days old). Accounts receivable includes $1.3 million denominated in a currency other than the US dollar.
RELATED PARTY TRANSACTIONS
The Company leases air travel time from a company, which is controlled by the Chairman of IMRIS Inc. The amount charged to travel expenses with respect to transactions conducted on an estimated third party comparable cost basis with this related party during the three and nine months ended September 30, 2012 was $106,000 and $267,000 (Three and nine months ended September 30, 2011 - $58,000 and $267,000).
At September 30, 2012, there was a payable to related parties of $46,000 (December 31, 2011 - $Nil).
CRITICAL ACCOUNTING POLICIES AND ESTIMATES
In our 2011 annual audited consolidated financial statements and notes, as well as in our 2011 Annual MD&A, we have identified the accounting policies and estimates that are critical to understanding our business operations and our results of operations. Other than the following change, our critical accounting estimates and assumptions remain substantially unchanged from those disclosed in these documents.
CHANGES IN ACCOUNTING POLICY
In June 2011, the ASC guidance on presentation of comprehensive income was updated eliminating the option to present the components of other comprehensive income as part of the statement of equity but not changing the items that must be reported in other comprehensive income. The updated guidance requires an entity to present the components of net income and other comprehensive income in either a single continuous statement of comprehensive income or in two separate but consecutive statements. This guidance is effective for interim and annual periods beginning after December 15, 2011 and was adopted in the quarter commencing January 1, 2012. This guidance affected presentation only and did not have a material impact on the results of operations or financial condition reflected in the consolidated financial statements. The following table presents the retrospective application of the updated ASC guidance on presentation of comprehensive income for the years ended December 31, 2011 and 2010.
IMRIS INC.
Consolidated Statements of Comprehensive Loss
Expressed in US $000’s
| | | 2011 | | | 2010 | |
| | | | | | | |
| Net loss for the year | | $ | (20,925 | ) | | $ | (1,766 | ) |
| Other comprehensive income | | | | | | | | |
| Foreign currency translation adjustment | | | 44 | | | | 3,617 | |
| Other comprehensive income | | $ | 44 | | | $ | 3,617 | |
| | | | | | | | | |
| Comprehensive income (loss) for the year | | $ | (20,881 | ) | | $ | 1,851 | |
IMRIS Inc.
Management's Discussion and Analysis – November 8, 2012
Page 20 of 26 |
DISCLOSURE AND INTERNAL CONTROLS
We have established and maintain disclosure controls and procedures in order to provide reasonable assurance that material information relating to IMRIS is made known in a timely manner. We have evaluated the effectiveness of our disclosure controls and procedures as at the date of our 2011 Financial Statements and are not aware of any material changes that are required to be made to these controls and procedures; we believe them to be effective in providing such reasonable assurance.
We are also responsible for the design of our internal controls over financial reporting (ICFR) in order to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with U.S. GAAP. Management used the framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) to evaluate the effectiveness of internal controls in fiscal 2011, based on this evaluation management concluded that our internal control over financial reporting was effective as at December 31, 2011. The Company’s independent registered public accounting firm, Deloitte and Touche LLP, has issued an unqualified opinion on the Company’s internal controls over financial reporting as at December 31, 2011.
As of the date of this report, there have been no changes to the Company’s internal controls over financial reporting that materially affect, or are reasonably likely to materially affect, its internal controls over financial reporting.
In compliance with rules of the Canadian Securities Administration and the US Securities and Exchange Commission, we have filed certificates signed by our Chief Executive Officer (‘‘CEO’’) and Chief Financial Officer (‘‘CFO’’) regarding the effectiveness of our disclosure controls and our internal controls over financial reporting. It should be noted however, that while the Company’s CEO and CFO believe the organizations disclosure controls and internal controls over financial reporting are effective any system of internal control has inherent limitations and cannot prevent all errors or fraud. Even systems determined to be effective can provide only reasonable assurance of the reliability of financial statement preparation and presentation.
RISKS AND UNCERTAINTIES
The operating results, business prospects and financial position of the Company are subject to a number of risks and uncertainties.Risks relating to our business include: our long sales cycle; high unit price and limited quarterly installations; our limited operating history and accumulated deficit; our lack of product diversity; our dependence on our suppliers; the development of VISIUS Surgical Theatres for cardiovascular and cerebrovascular procedures; our reliance on key personnel; the lack of supporting clinical data; market competition and technological advances; patent protection and trade secrets; intellectual property litigation; our ability to shift from research and development to commercialization; our ability to manage growth; foreign exchange fluctuations; additional financing requirements; and regulatory matters.If any of the events described as risks or uncertainties actually occurs, our business, prospects, financial condition and operating results would likely suffer, possibly materially. We have discussed several of the more significant risks and uncertainties that may affect the business below; however, for a more comprehensive list of the risks and uncertainties affecting the business, readers are advised to refer to our 2011 Annual Information Form (AIF) and our Base Shelf Prospectus (Base Shelf) filed October 2, 2012. Both the AIF and Base Shelf are available atwww.sedar.com and on theUnited States Securities and Exchange Commission (SEC) website atwww.sec.gov. The AIF is locatedin the Company’s Annual Report on Form 40-F.
Financial Risks
The Company is exposed to a variety of financial risks by virtue of its activities. These risks include market risk (including currency risk; fair value interest rate risk; cash flow interest rate risk); credit risk and liquidity risk. The Company’s overall risk management efforts focus on the unpredictability of financial markets and seek to minimize potential adverse effects on financial performance. Management identifies and evaluates financial risks in close cooperation and direction from the Board of Directors. Management is charged with the responsibility of establishing controls and procedures to ensure that financial risks are mitigated.
IMRIS Inc.
Management's Discussion and Analysis – November 8, 2012
Page 21 of 26 |
The following is a brief overview of the Company’s financial risk management for each of the risks identified above:
Market Risks
Currency risk
The Company operates internationally and is exposed to foreign exchange risk from various currencies. Foreign exchange risk arises from future sales and purchase transactions as well as recognized financial assets and liabilities denominated in foreign currencies. The Company’s main objective in managing its foreign exchange risk is to preserve gross margins and reduce variations in performance. The Company prices a significant portion of its VISIUS Surgical Theatre sales in USD. To offset these revenues, the Company sources a major portion of the components it delivers in US dollars. In addition, the Company incurs nearly all of its sales expenses in US dollars.
Cash flow and fair value interest rate risk
Interest rate risk is the risk that the fair value or future cash flows of a financial instrument will fluctuate because of changes in market interest rates. Financial assets and financial liabilities with variable interest rates expose the Company to cash flow interest rate risk. The Company’s restricted cash includes short-term highly liquid investments that earn interest at market rates. Financial assets and financial liabilities that bear interest at fixed rates are subject to fair value interest rate risk. The Company’s restricted cash is the only financial asset bearing fixed interest rates. The Company manages its interest rate risk by minimizing financing costs on its borrowings and maximizing the interest income earned on excess funds while maintaining the liquidity necessary to conduct operations on a day-to-day basis. The Company’s investment policy limits the investing of excess funds to Bankers Acceptances, Canadian Chartered bank term deposits, and short-term highly liquid money market mutual funds sponsored by Canadian Chartered banks.
Credit Risk
Credit risk is the risk of financial loss to the Company if a customer or counterparty to a financial instrument fails to meet its contractual obligation. The maximum exposure to credit risk of the Company at quarter end is the carrying value of its financial assets. The Company manages its credit risk on financial assets by dealing solely with reputable banks and financial institutions. The Company’s North American customers are large credit worthy medical hospitals and thus there is very little exposure to credit risk. When selling internationally, the Company uses irrevocable letters of credit to reduce its exposure to credit risk. The Company reviews the collectability of its accounts receivable and would record an allowance for doubtful accounts receivable if accounts were determined to be uncollectible. The loss would be recognized in the income statement within ‘Administrative expense’. Significant financial difficulties of the debtor, probability that the debtor will enter bankruptcy or financial reorganization, and default or delinquency in payments are considered indicators that the account receivable is uncollectible.
Liquidity Risk
Liquidity risk is the risk that the Company will not be able to meet its obligations as they fall due. The Company manages its liquidity risk by forecasting cash flows from operations and anticipated investing and financing activities. The Board of Directors reviews and approves the Company’s operating and capital budgets as well as any material transactions that are not in the ordinary course of business.
IMRIS Inc.
Management's Discussion and Analysis – November 8, 2012
Page 22 of 26 |
Long Sales Cycle, High Unit Price and Limited Installations
The long sales cycle, as well as the high unit price of the VISIUS Surgical Theatre, among other factors, may contribute to substantial fluctuations in our quarterly operating results. Because of the high unit price of VISIUS Surgical Theatres and the fact that we have installed only 43 units over the Company’s history, each installation currently represents a significant component of our revenue for a particular quarter. VISIUS Surgical Theatres represent a significant capital expenditure for our customers and adverse global economic and business conditions could result in a loss of consumer confidence, which could decrease capital spending or increase the length of time and effort our customers require to gain approval for capital spending. If we lose a single customer order or if customers defer installation of a VISIUS Surgical Theatre for even a short period of time, recognition of a significant amount of revenue may be lost or deferred to a subsequent period. Given that our operating costs are relatively fixed, our inability to recognize revenue in a particular quarter may adversely affect our profitability in that quarter.
We expect that revenues from a limited number of new customers will account for a large percentage of total revenues in future quarters. Our ability to attract new customers will depend on a variety of internal factors, including the capability, safety, efficacy, ease of use, price, quality and reliability of our products and effective sales support, training and service. In addition, if we are unable to fulfill our current purchase orders and other commitments on a timely basis or at all, market acceptance of our products could be adversely affected and hospitals may instead purchase our competitors’ products. The loss or delay of individual orders, failure to add new customers or worsening macroeconomic conditions could have a significant impact on future revenues and operating results.
Limited Operating History and Accumulated Deficit
We have a limited operating history from which investors can evaluate our business and prospects.We have a large accumulated deficit and we may not achieve profitability. We have incurred substantial losses since inception and may incur additional operating losses in the near term. If the time required to generate significant revenues and consistently achieve profitability is longer than anticipated, we may not be able to continue our operations without additional capital.Our prospects must be considered in light of the risks and uncertainties encountered by an early-stage company in the continuously evolving surgical imaging market. If we cannot successfully address these risks, our business and financial condition would suffer.
Actual Bookings May Vary from Forecast
We periodically forecast information about the rate we will be able to convert potential customers in our order pipeline into actual bookings we expect to achieve in future periods. The actual bookings we achieve in a period may vary from the bookings we forecast in public disclosure documents, presentations or other public information and these variations could be material and adverse. Our forecasts reflect numerous assumptions concerning our expected performance, as well as other factors, which may not be accurate. Actual bookings rely on the occurrence of numerous factors beyond our control such as a customer’s capital budget, their patient requirements and timing. Although we believe that the assumptions underlying our guidance and other forward looking statements were reasonable at the time such statements were made, actual results could be materially different. If actual bookings vary from our announced guidance, the price of our Common Shares may decline, and such a decline could be substantial. Except as required under applicable securities legislation, we do not undertake to update any guidance or other forward-looking information we may provide, whether as a result of new information, future events or otherwise.
IMRIS Inc.
Management's Discussion and Analysis – November 8, 2012
Page 23 of 26 |
Backlog may not Result in Future Net Sales
Our backlog at any particular date may not be indicative of recognized revenues in our financial statements in subsequent periods. We evaluate each customer order to determine qualification for inclusion in backlog; however, there can be no assurance that amounts included in backlog will ultimately result in recognition of revenue or occur in a financial reporting period. In addition, in any given period management can reduce backlog due to factors such as a cancellation of an order, lack of confidence in the customer proceeding with the order, changes in the financial condition at a customer, changes in government or third party funding for customers, and changes in installation schedules. Changes to backlog during any particular period, or the failure of our backlog to result in future sales, could harm our business, financial condition, results of operations and cash flows.
Lack of Product Diversity
Currently, our commercially available products include the VISIUS Surgical Theatre for neurosurgical, cardiovascular and cerebrovascular applications. Although we expect sales of our VISIUS Surgical Theatres to increase with market acceptance of the theatres for cardiovascular and cerebrovascular applications, we currently generate substantially all of our revenue from sales of VISIUS Surgical Theatres for neurosurgical applications and multiyear service plans for the theatres. If we are unable to sustain or grow sales of the VISIUS Surgical Theatre for use in multiple applications beyond neurosurgery, we may not generate sufficient revenue to support our business. Accordingly, we are currently dependent on our ability to market and sell the VISIUS Surgical Theatre for neurosurgical applications. Any factor materially and/or adversely affecting our ability to market and sell the VISIUS Surgical Theatre for neurosurgical applications or pricing and demand for the theatre may have a material and adverse effect on our financial condition and results of operations.
Foreign Exchange Fluctuations
We currently generate a significant portion of our sales in US dollars but many of our expenses are denominated in Canadian dollars. To date, we have not used forward exchange contracts to hedge exposures denominated in foreign currencies or any other derivative instrument for trading, hedging or speculative purposes. As such, we are exposed to fluctuations in the exchange rate between certain foreign currencies, including Canadian, Euros and Australian dollars, versus the US dollar as a result of the translation into US dollars of our balance sheet and income statement items denominated in those foreign currencies.
Regulatory Matters
Products intended for diagnostic and therapeutic use for humans are governed by a wide array of regulatory authorities in various jurisdictions throughout the world. For most of these products in most jurisdictions, applicable statutes and regulations require testing and government review and approval prior to marketing the product. This procedure can take a number of years and involves the expenditure of substantial resources. Any failure or delay by us to obtain regulatory approvals or clearances could adversely affect the marketing of any products developed by us and our ability to receive product revenue. There is no assurance that any of our planned products will be approved by any regulatory authority on a timely basis, or at all. Also, in the event that a regulatory authority revokes any approvals granted in respect of our products, or a recall of our products is required in the event of material deficiencies or defects, our business, financial condition and results of operations could be adversely affected.
IMRIS Inc.
Management's Discussion and Analysis – November 8, 2012
Page 24 of 26 |
Dependence on Suppliers
We depend on Siemens to supply the MR scanner, CT scanner and angiography systems for our VISIUS Surgical Theatres. Our current agreement with Siemens was entered into as of November 2009 for a five-year term with automatic renewal provisions thereafter, subject to six months' advance written notice of termination by either party. The agreement may be terminated earlier in the event of default or in the event of insolvency or equivalent proceedings against either party or in the event of a change of control or similar sale transaction affecting IMRIS where the buyer or controlling shareholder is a direct competitor to Siemens. If for any reason, we could not obtain MR scanners, CT scanners and angiography systems from Siemens there is no certainty that we could find another vendor willing to supply this equipment for the VISIUS Surgical Theatre and a change would require a redesign of the VISIUS Surgical Theatre, which could take a year or more to implement. We are dependent on Siemens to provide support and maintenance services to our customers under contract to IMRIS; if Siemens' services became unavailable, any resulting service issues could disrupt our customer relationships and cause damage to our reputation.
We purchase certain other components of our VISIUS Surgical Theatre from outside vendors, including radio-frequency shielding systems, certain hardware components for our surgical information management system and operating room booms and lights. For the majority of our theatre components, we do not have long-term supply contracts with the suppliers; however, we attempt to establish dual sourcing for most of these other components of our theatre and we believe that we would be able to establish alternative sources for these components, subject to any regulatory qualifications, as may be required. It is possible that a disruption of the supply of these components could result in increased costs and delays in deliveries of VISIUS Surgical Theatre, which could adversely affect our reputation and results of operations.Additionally,any transition to alternate manufacturers or suppliers would likely result in operational problems and increased expenses and could delay the shipment of, or limit our ability to provide our products.
We purchase all significant components for development of our image guided surgical robotics program from MacDonald, Dettwiler and Associates Ltd. (MDA). We do not have a formal long-term supply contracts with MDA; however, IMRIS and MDA have signed a collaborative development agreement for development of the surgical robotics program. It is possible that a disruption of the supply of these components could result in increased costs and delays in the development of our image guided surgical robotics program, which could adversely affect our reputation and results of operations. Additionally, any transition to alternate manufacturers or suppliers would likely result in operational problems and increased expenses, which could also delay the development of our image guided surgical robotics program.
Competition and Technological Advances
The surgical imaging industry is subject to intense and increasing competition and rapidly evolving technologies. Many government, academic and business entities are investing substantial resources in research and development of treatments and new products that may render surgical imaging obsolete, including radiation treatment, new drug treatments and gene therapy. Successful developments that result in new approaches for treatments could reduce the attractiveness of our products or render them obsolete. MRI competes with other surgical imaging technologies such as CT, fluoroscopy and ultrasound for market share in the overall surgical imaging market.
The market for surgical imaging is highly competitive, with a number of companies providing competing surgical imaging systems. Many of these competitors are large medical system suppliers which have considerably greater resources at their disposal to advance the development of their systems. These competitors or other companies may at any time develop new or improved surgical imaging solutions. Alternatively, these competitors may choose to increase their respective market share by changing their pricing model or by lowering the price of their surgical imaging solutions or ancillary supplies. If we are unable to address these competitor tactics by either continuing to enhance and improve our current product(s) or we are unable to maintain or increase our selling price in the face of competition, there can be no assurance that the Company will be able to maintain its desired market share or achieve its financial objectives.
IMRIS Inc.
Management's Discussion and Analysis – November 8, 2012
Page 25 of 26 |
Changes to Financial Accounting Standards
A change in accounting standards or practices can have a significant effect on our reported results and may even affect our reporting of transactions completed before the change is effective. New accounting pronouncements and varying interpretations of accounting pronouncements have occurred and may occur in the future. Changes to existing standards or the questioning of current practices may adversely affect our reported financial results or the way we conduct our business.
ADDITIONAL INFORMATION
Additional information about IMRIS, including our annual information form, can be found on the SEDAR website at www.sedar.com or the United States Securities and Exchange Commission (SEC) website at www.sec.gov.
IMRIS Inc.
Management's Discussion and Analysis – November 8, 2012
Page 26 of 26 |

