UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934.
For the fiscal year ended November 30, 2016.
or
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934.
Commission File Number 0-54744
CHINA GEWANG BIOTECHNOLOGY, INC.
(Name of Registrant as Specified in its Charter)
| Nevada | | 42-1769584 |
(State of Other Jurisdiction of incorporation or organization) | | (I.R.S.) Employer
I.D. No.) |
Floor 29 No. 334, Huanshi East Road, Yuexiu District
Guangzhou City, Guangdong Province, P.R. China 510623
(Address of Principal Executive Offices)
Registrant's Telephone Number: 86-024-2397-4663
Securities Registered Pursuant to Section 12(b) of the Act: None
Securities Registered Pursuant to Section 12(g) of the Act:
Common Stock, $.001 par value per share
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 406 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Sections 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files.) Yes ☒ No ☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§ 229.405) is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. Yes ☐ No ☒
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check One)
Large accelerated filer ☐ Accelerated filer ☒ Non-accelerated filer ☐ Smaller reporting company ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
As of May 31, 2016 (the last business day of the most recently completed second fiscal quarter) the aggregate market value of the common stock held by non-affiliates was approximately $129,148,425, based on the closing market price on that date.
As of February 10, 2017, there were 75,000,000 shares of common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE: None
CHINA GEWANG BIOTECHNOLOGY, INC.
ANNUAL REPORT ON FORM 10-K
FOR THE FISCAL YEAR ENDED NOVEMBER 30, 2016
TABLE OF CONTENTS
| Part I | | Page No |
| | | |
| Item 1 | Business | 1 |
| Item 1A | Risk Factors | 12 |
| Item 1B | Unresolved Staff Comments | 24 |
| Item 2 | Description of Properties | 24 |
| Item 3 | Legal Proceedings | 24 |
| Item 4 | Mine Safety Disclosure | 24 |
| | | |
| Part II | | |
| | | |
| Item 5 | Market for Registrant’s Common Equity, Related Stockholder Matters, and Issuer Purchase of Equity Securities | 25 |
| Item 6 | Selected Financial Data | 27 |
| Item 7 | Management’s Discussion and Analysis | 28 |
| Item 7A | Quantitative and Qualitative Disclosure about Market Risk | 35 |
| Item 8 | Financial Statements and Supplementary Schedules | 36 |
| Item 9 | Changes and Disagreements with Accountants on Accounting and Financial Disclosure | 37 |
| Item 9A | Controls and Procedures | 37 |
| Item 9B | Other Information | 38 |
| | | |
| Part III | | |
| | | |
| Item 10 | Directors, Executive Officers and Corporate Governance | 39 |
| Item 11 | Executive Compensation | 41 |
| Item 12 | Security Ownership of Certain Beneficial Owners, and Management and Related Stockholder Matters | 42 |
| Item 13 | Certain Relationships and Related Transactions and Director Independence | 44 |
| Item 14 | Principal Accountant Fees and Services | 45 |
| | | |
| Part IV | | |
| | | |
| Item 15 | Exhibits and Financial Statement Schedules | 46 |
| | | |
| | Signatures | 47 |
FORWARD-LOOKING STATEMENTS: NO ASSURANCES INTENDED
In addition to historical information, this Annual Report contains forward-looking statements, which are generally identifiable by use of the words “believes,” “expects,” “intends,” “anticipates,” “plans to,” “estimates,” “projects,” or similar expressions. These forward-looking statements represent Management’s belief as to the future of China Gewang Biotechnology, Inc. Whether those beliefs become reality will depend on many factors that are not under Management’s control. Many risks and uncertainties exist that could cause actual results to differ materially from those reflected in these forward-looking statements. Factors that might cause such a difference include, but are not limited to, those discussed in Section 1A of this Report, entitled “Risk Factors.” Readers are cautioned not to place undue reliance on these forward-looking statements. We undertake no obligation to revise or publicly release the results of any revision to these forward-looking statements.
PART I
Item 1. Business
Guangdong Gewang was founded in June 2010 in Guangzhou City, with registered capital of RMB 10 million (US$1,561,000). Guangdong Gewang is engaged in the sale of selenium supplements within the PRC. It is a standing member of the Chinese Selenium Supplements Association.
Guangdong Gewang initiated its business by cooperating with the Academy of Agricultural Sciences of Shandong Province (the Academy) in the development of formulas for three selenium supplements: a selenium capsule, a capsule combining selenium with glossy ganoderma (a therapeutic mushroom), and a selenium powder. The Academy has given Guangdong Gewang an exclusive license to manufacture and market the three products. As a result of our relationship with the Academy, Guangdong Gewang has required no investment in research and development within the past three years other than payments directly to the Academy.
The Academy also assisted Guangdong Gewang in identifying manufacturing companies possessing the advanced nanometer processing technology and production processes needed to produce the products. Guangdong Gewang now outsources the manufacturing of the products, then sells them under the trademarked brand “Jindanli”. We currently have three separate trademarks in China on aspects of the Jindanli brand, which expire in 2022, 2023 and 2025, at which time Guangdong Gewang can apply for renewal. The Jindanli brand is important to our efforts to distinguish our products from other manufacturers.
Guangdong Gewang entered into a Licensing Agreement and a License Agreement Supplement Agreement (together, the “Licensing Agreements”) with the Academy which have been in effect since December 30, 2015. The Licensing Agreements have a term of five years and allow Guangdong Gewang the exclusive right to use the Academy-developed selenium formulas in its trademarked Jindanli branded products. The selenium formulas belong to the Academy but Guangdong Gewang has a license to use the formulas as it sees fit, including by contracting with third-party manufacturers to produce the products. The formulas are trade secrets, and Guangdong Gewang is contractually obligated to ensure the confidentiality of the formulas pursuant to the Licensing Agreements. The Academy must notify Guangdong Gewang of any improvements or upgrades to the selenium formulas, whereupon Guangdong Gewang has the option to choose to use the new formulas in its products. The Licensing Agreements require Guangdong Gewang to pay an annual fee of 600,000 RMB ($87,000).
In September 2016 Guangdong Gewang entered into an additional agreement with the Academy, under which the Academy granted Guangdong Gewang an exclusive right of first refusal to use any research related to advanced selenium enrichment techniques and technology developed by the Academy relating to selenium research techniques. The agreement calls for annual payments of 4,000,000 RMB ($580,000) payable quarterly. Additional payments will be required for use of any techniques or technology developed by the Academy.
Initially, for the convenience of management, Guangdong Gewang marketed exclusively on a wholesale basis to chain stores that retail health care products. Early in 2014, as the Registrant developed the necessary infrastructure, Guangdong Gewang commenced direct marketing to consumers from its executive “home office.” Subsequently we have opened three more physical stores dedicated to the sale of the three Jindanli products.
In March 2016, Guangdong Gewang entered into cooperation agreements with 6 selenium enriched food product manufacturers for Guangdong Gewang to distribute their selenium enriched food products to chain stores and to sell these products in Guangdong Gewang’s retail stores. Guangdong Gewang pays promotion fees to chain stores to ensure optimal product placement and widespread distribution of these products. Guangdong Gewang has worked with the Academy to ensure that each of these selenium enriched food product manufacturers meets its standards. Guangdong Gewang has also begun to sell these selenium enriched food products in its flagship retail store alongside its Jindanli branded selenium products. The wholesale and retail sales of selenium enriched food products has broadened Guangdong Gewang’s reach in China and helped to further expand the Registrant’s market share in selenium products beyond selenium supplements.
Selenium
Background on Selenium
Selenium is one of the “essential” nutrients for humans, meaning that our bodies cannot produce it, and so we have to get it from our diet. Selenium deficiency can cause health problems including Keshan’s disease, and the World Health Organization has found that between 50 and 250 micrograms of selenium constitute a healthy daily intake.
Selenium was discovered as an element in 1817 by the Swedish chemist Jöns Jacob Berzelius, who determined the atomic weights of many elements and developed a system of chemical symbols. It was first thought to be a toxin, but scientists determined that selenium was an essential mineral in the 1950s. By the 1960s doctors began researching selenium’s possible tumor fighting properties in animals, according to the American Cancer Society.
Scientists now know selenium is necessary in the body’s production of selenoproteins, a family of proteins that contain selenium in the form of an amino acid. So far, 25 different selenoproteins in the body have been isolated, but only half of their functions have been identified. Selenium is one of several nutrients known to have antioxidant properties, meaning selenium plays a part in chemical reactions that stop free radicals from damaging cells and DNA. Free radicals are unstable molecules from environmental toxins, or from byproducts of the human body’s metabolism. In 1973, a paper published by JT Rotruk et al. showed that selenium is the basic component of erythrocyte glutathione peroxidase (GSH-PX), an enzyme that removes harmful substances produced by cell respiration. Further research into the antioxidant functions of selenium have shown that six other enzymes (glycine reductase, formate dehydrogenase, nicotinic acid hydroxylase, sulfur dehydrogenase and xanthine solution enzyme) are activated only in the presence of selenium.
Human and animal research has found selenoproteins are involved in embryo development, thyroid hormone metabolism, antioxidant defense, sperm production, muscle function and the immune system’s response to vaccinations. Antioxidant supplements, including selenium, are often touted to help prevent heart disease, cancer and vision loss.
According to the Chinese Selenium Supplements Association, selenium is purported to help people with asthma, and reduce the risk of rheumatoid arthritis and cardiovascular disease. Selenium levels drop with age, so some have claimed selenium can slow the aging process, cognitive decline and dementia. Low selenium levels are also implicated in depression, male infertility, weak immune systems and thyroid problems.
Plants grown in soil containing selenium convert it into a form that is usable by humans or animals. Soil around the world varies in its selenium concentration. The higher the concentration of selenium is in soil, the higher the concentration of selenium is in crops. Soil in Nebraska, South and North Dakota, for example, is especially rich in selenium, and people living in these areas typically have the highest dietary intake of selenium in the United States.
Soil in some areas of China and Russia is naturally low in selenium. Selenium deficiencies in the Keshan region in northeast China were severe enough to spur a form of heart disease called cardiomyopathy, now called Keshan’s disease. Chinese government programs to supplement people’s diets with selenium in the 1970s greatly reduced cases of Keshan disease, according to the National Institutes of Health’s Office of Dietary Supplements. Low selenium levels in China, Tibet and Siberia may also play a role in a type of osteoarthritis called Kashin-Beck disease.
Seventy-two percent (72%) of the land in China is selenium-poor. In the area from the three provinces in Northeast China to the Yunnan Guizhou plateau, two-thirds of the arable land is recognized as having a selenium deficiency, where the selenium content of the principal crops is less than 0.05ppm.
Selenium Toxicity
The human body only needs a trace amount of selenium, so it is possible to overdose with selenium supplements. For example, in 2008, a liquid dietary supplement that was 200 times more concentrated than advertised led to selenium poisoning in more than 200 people in the U.S. The most common effects were diarrhea, fatigue, hair loss, joint pain, brittle nails and nausea. A third of the people affected continued to experience symptoms 90 days after taking the mislabeled supplements.
Taking too much selenium over time can lead to selenosis, which can cause hair loss, nail loss, nausea, irritability, fatigue and some nerve damage. Other symptoms of chronic selenium overdose are a metallic taste in the mouth, and a garlic scent on the breath. A selenium overdose can cause skin lesions and nervous system abnormalities. In severe cases, selenium toxicity can cause tremors, kidney failure, cardiac failure, respiratory distress or even death.
The Institute of Medicine’s Food and Nutrition Board caps the safe daily intake of selenium at 45 micrograms for infants, 60 to 90 micrograms in toddlers, 150 to 280 micrograms in prepubescent children and 400 micrograms in adults.
Selenium may increase the risk of bleeding if it is combined with blood thinners such as clopidogrel (Plavix), coumadin, heparin or aspirin, according to the University of Maryland Medical Center. Animal studies show selenium supplements may also extend the effects of sedatives. Antioxidant supplements that included selenium have been shown to interfere with cholesterol-lowering treatments.
For these reasons, our marketing program combines both information about the need for adequate selenium intake with advice and warnings about the risk of excess ingestion of selenium.
The Opportunity in China
We believe that the importance of selenium to human health and the fact of selenium deficiency in large parts of China create a vast market potential for development. Selenium has been studied extensively in China. These efforts have resulted in confirming that selenium is an important element for human health and that there are areas within China that are significantly deficient in the soil and water. In the past decade, Chinese government policy has helped to enhance the potential of the selenium market.
Since 2008, the government has assisted Ankang City in developing selenium-rich products as a pillar industry. Government officials reviewed the experience of selenium supplements at home and abroad, and solicited the opinions of experts on selenium supplement research. Based on that review, the government developed the first provincial “selenium content standards” for Shaanxi Province, completed a census of selenium-rich resources in Ankang prefecture, drew an atlas of soil rich in selenium, established a selenium-rich food label system, selenium research and development institutions, cultivated selenium-rich food enterprises, organized a food industry association, and established a sound selenium-rich food industry development framework and system. These activities evidence the government’s support of the selenium industry.
The selenium industry is still in its infancy. It is currently fragmented with no dominant players. According to the Chinese Selenium Supplements Association, over three hundred, mostly small, enterprises are engaged in producing selenium products. Production costs are high because the products have not yet achieved economic scale. In addition, because the industry is new, the quality of selenium-rich health products is uneven, in part due to the lack of a nationwide standard for selenium products in China. In spite of these challenges, the selenium industry as a whole grew to 26.6 billion RMB (US$4.6 billion) in 2015 with a historical annual growth rate of 9.3% to 13.1%, and we believe the industry will experience rapid growth in the future.
Our Products
Through a partnership with the Academy of Agricultural Sciences of Shangdong Province, a highly regarded research institute in China, we have licensed the exclusive right to contract for the manufacture and marketing of products using three selenium formulas developed and owned by the Academy. In compensation for the license, we pay the Academy a fee of 50,000 RMB ($8,104) per month.
Currently Guangong Gewang distributes 89 distinct products which include processed foods such as selenium enriched porridge, ready to eat foods such as selenium-enriched peanuts and ingredients such as selenium enriched flower. Guangong Gewang is actively engaged in marketing healthy selenium rich foods including Selenium-Rich Maize Residue, Selenium-Rich Brown Rice. Selenium Enriched Black Beans, Selenium-Enriched Buckwheat Kernel and Selenium-Enriched Ormosia. These foods compliment the Registrant’s Jindanli branded products by raising awareness of the need for selenium in the diets of our target consumer market.
We currently offer the following products for sale under the brand “Jindanli”:
| ● | Selenium Capsules. 12% of our sales in fiscal year 2016 were sales of our selenium capsules. The selenium capsules are sold in a box of 300 capsules, with a recommended dosage of one capsule twice daily. A box of selenium capsules (i.e. a five month supply) retails for 1,380 RMB ($215) with a wholesale price of 650 RMB ($101). |
| ● | Selenium - Glossy Ganoderma Capsules. 8% of our sales in fiscal year 2016 were sales of our selenium - glossy ganoderma capsules. Glossy ganoderma is an edible fungus, known as the “magical oriental mushroom” in traditional Chinese medicine. Glossy ganoderma is believed to boost energy, control blood pressure, and strengthen the immune system. Guangdong Gewang offers glossy ganoderma combined with selenium because the anti-oxidant effects of the selenium help prevent oxidation of the glossy ganoderma spore powder, thereby maximizing the effectiveness of the glossy ganoderma. A box of 300 selenium - glossy ganoderma capsules (i.e. a five month supply) retails for 1,380 RMB ($215) with a wholesale price of 650 RMB ($101). |
| ● | Organic Selenium Powder. 6% of our sales in fiscal year 2016 were sales of our selenium powder. We offer the selenium powder in a box of 90 bags, with a recommended dosage of one or two bags daily. A box (i.e. a 1½ to three month supply) retails for 1,380 RMB ($215) with a wholesale price of 650 RMB ($101). |
The only significant raw material needed for our selenium capsules and selenium powder is selenium. Selenium is readily available, as it has numerous industrial uses. For our selenium - glossy ganoderma capsules, we also need glossy ganoderma. Historically, the reisha mushroom, which is the source of glossy ganoderma, was rare in the wild. Recently, however, farmers have been successful in domesticating the reisha mushroom, which has resulted in an abundance of the reisha mushroom and of its derivative glossy ganoderma. As a result, sourcing our raw materials has not been a matter of concern, nor are we subject to significant effects from changes in the prices of our raw materials.
Our Manufacturing
Currently, we outsource our manufacturing to three production companies:
| ● | Yantai Yisheng Pharmaceutical Co., Ltd., which produces our selenium capsules; |
| ● | Taian Zhishengtang Ganoderma Lucidum Co., Ltd., which produces our selenium - glossy ganoderma capsules; and |
| ● | Beijing Technology Development Company of CAAS, which produces our organic selenium powder. |
The inventory held by Guangdong Gewang consists only of inventory in our retail stores. Generally, upon receipt of an order from our customer, we place a corresponding order with the appropriate manufacturer. Guangdong Gewang and our colleagues at the Academy strictly supervise the production process, and we inspect and accept the finished product. When the products are ready for shipment, either our logistics team or the manufacturer (depending on our agreement with the manufacturer) engages a delivery service to pick up the product from the manufacturer’s site and deliver it to the customer.
We have signed with each manufacturer, as well as with the Academy, a Product Manufacture and Purchase Agreement. Each agreement has a five year term, and mandates that the products be manufactured to our specification, with the manufacturer bearing responsibility for any product defects. The agreement gives Guangdong Gewang the authority to supervise the manufacturing process. We have also signed with each manufacturer a Non-Disclosure Agreement, which requires the manufacturer to protect the product formula and trade secrets, and prevents the manufacturer from entering into any venture in competition with us.
Our Marketing
Selenium deficiency is harmful to all humans. It is of particular concern, however, to the elderly and to lactating women. As we deliver information to the populations of areas with selenium-poor soil, those two groups are our target market. Nevertheless, our staff is committed to raising awareness of selenium issues throughout China, particularly in the eastern regions with large populations and selenium-poor soil. Our marketing staff makes personal appearances throughout our prime markets, both to raise awareness of the problem of selenium deficiency and to educate consumers about the proper use of selenium supplements and the risks of excess selenium ingestion. Additionally, we plan to develop a media advertising program in the future.
At present, Guangdong Gewang has distribution agreements with sixteen major wholesale customers, including eight well-established customers that retail health care products and eight distributors that sell selenium-enriched food in China. Each wholesale customer is assigned an exclusive market and barred by contract from selling in other markets. The sixteen wholesale customers, their designated markets and the expiration of the respective contracts are:
| Customers and Distributors | | | Market | | | | Expiration | |
| Dongguan Renzheng Pharmaceuticals and Trading Co., Ltd. | | | Dongguan | | | | March 5, 2017 | |
| Shenzhen Youmeikang Biotechnology Co., Ltd. | | | Shenzhen | | | | March 7, 2017 | |
| Foshan Kangchen Biotechnology Co., Ltd. | | | Foshan | | | | March 25, 2017 | |
| Fujian Beier Pharmaceuticals Co., Ltd. | | | Fujian | | | | April 14, 2017 | |
| Guangdong Sinopharm Pharmaceuticals Co., Ltd. | | | Guangdong | | | | May 9, 2017 | |
| Huizhou Liqi Pharmaceuticals Co., Ltd. | | | Huizhou | | | | May 9, 2017 | |
| Heyuan City Corning Pharmaceutical Biotechnology Co., Ltd | | | Heyuan | | | | October 23, 2018 | |
| Anhui Huishanghongfu Chain Supermarket LLC | | | Anhui | | | | February 28, 2017 | |
| Zhejiang Supply &Sales Supermarket Co., Ltd. | | | Zhejiang | | | | March 1, 2017 | |
| Tianjin Quanbao Supermarket Limited Liability Company | | | Tianjin | | | | March 29, 2017 | |
| Shanghai Liangyou Jinban Convenience Chain Co., Ltd. | | | Shanghai | | | | June 2, 2017 | |
| Shanxi Taiyuan Tangjiu Supermarket Co., Ltd. | | | Shanxi | | | | July 26, 2017 | |
| Guangzhou Huayuda Commerce and Trade Co., Ltd. | | | Guangzhou | | | | March 30, 2017 | |
| Shanwei City Urban Area Dongsheng Health Products Co., Ltd. | | | Shanwei | | | | August 16, 2019 | |
| Hubei Fudi Industrial Inc. | | | Hubei | | | | August 31, 2017 | |
| Guangdong Tianmei Selenium Enrichment Beverage Chain Corporation | | | Guangdong | | | | March 31, 2017 | |
Our distribution agreements fix the payment terms, which vary among customers. In each distribution agreement, Guangdong Gewang warrants that the products will conform to all the requirements set forth in the New National Pharmacopeia of the National Food and Drug Supervision and Management Bureau, and that all products will have a remaining viable period of no less than 12 months when delivered.
Our distribution agreements with the wholesale customers do not prevent them from selling competitor’s selenium products. Rather we assure ourselves of their loyalty by providing focused advertising of our brand in the chain stores’ markets, thereby making sale of the Jindanli products an attractive, low-effort proposition for our wholesale customers.
Our Competition
There are a limited number of name brands of selenium supplements in China, as the industry is in its early development period. In the markets where we do face competition, we emphasize the high quality of the Jindanli products such as the following:
| | ● | The Jindanli Selenium Capsule is designed using carageenan capsules to offer rapid absorption and high quality, yielding a product useful for all persons in need of added selenium in their diet. |
| | | |
| | ● | The Jindanli Selenium - Glossy Ganoderma Capsules offer an attractive combination of contemporary biotechnology with traditional healing. We source pure ganoderma spore powder from Mount Taishan wood, combine it with Jindanli selenium and package it in a carageenan capsule. |
| | | |
| | ● | The Jindanli Organic Selenium Powder provides the benefits of selenium to infants and the infirm, for whom swallowing capsules is difficult. |
While the industry has grown significantly, we believe the market is still in its early stages which is why we believe the market is fragmented and lacking a nationally recognized brand of selenium supplements.
Due to the fragmented market comprised of small players, many potential customers’ initial experiences with selenium supplements are with what we believe to be inferior products, many of which make unsupportable health claims. According to the Chinese Selenium Supplements Association, this has led to a lack of confidence in the selenium supplements among the Chinese people. Therefore, our marketing strategy must include education and strong prudent promotion, coupled with publicity to build confidence in the industry and our brands.
We believe that the quality of our products, along with our association with top quality manufacturers and chain stores, will enable us to compete effectively and gain market share, as the selenium supplement industry grows.
Our Growth Strategy
Through our wholesale and retail operations, we are currently selling selenium products in Guangdong Province, Fujian Province, Zhejiang Province, Anhui Province, Hunan Province, Shanxi Province, Hubei Province and the cities of Tianjin, Shanghai and Shenzhen. We also have current plans to expand our sales through a combination of retail stores and wholesale chain stores to Jiangsu Province, Jiangxi Province, Chongqing Province and Sichuan Province. We have begun our efforts to establish a nationwide reach through the wholesale and retail operations carried on at our home office, and our immediate plan is to bring intense marketing to regions where the need for selenium supplements is most pronounced, by locating dedicated corporate stores in these key markets. The stores will feature the Jindanli products, allowing us, by our pronounced presence, to bring attention to the issue of selenium deficiency, attract new customers, and provide customers with the information about the proper use of selenium supplements. The stores will also function as promoters of the Jindanli brand, and we believe this will enable us to build our brand as a high quality choice and become a dominant player in the market.
We currently have four stores, strategically located near our large chain customers. We opened our first store, in Chancheng, in September 2014, and opened two more stores, in Xiamen and Changsha, during fiscal year 2015. In June 2016, we opened our flagship store in Guangzhou. Also in June 2016, our Chancheng store moved to Foshan, our Xianmen store moved to Longyan and our Changshai store moved to Zhuzhou. Our goal is to open up to 26 new stores in the fiscal year 2017. Each store requires an investment of 300,000 to 400,000 RMB ($47,000 to $62,000) along with working capital of 100,000 to 200,000 RMB ($16,000 to $31,000).
The stores do not carry any products that compete against the Jindanli brand products. However, the stores do carry other quality selenium products and related health food and other products allowing us to bring attention to the issue of selenium deficiency, attract new customers, provide them the information about the proper use of selenium supplements and offering a full array of quality selenium products and other health food and related products to allow customers to increase their understanding of selenium and one location for their health food and related products. The stores will also function as a training center for the employees of our large chain customers to support our education and marketing strategy throughout their respective stores with a focus on the Jindanli brand, allowing us to fill the void in the selenium industry that lacks any well-known brand.
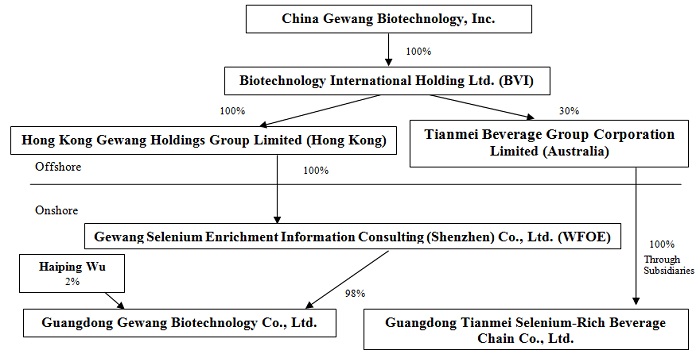
Corporate Structure
China Gewang Biotechnology, Inc., our U.S. parent company (the “Registrant” or the “U.S. Company”) was incorporated on May 29, 2009 in the State of Nevada under the name Rich Star Development Corporation. On January 8, 2015 the U.S. Company’s corporate name was changed to “China Gewang Biotechnology, Inc.” The name change was effected as an amendment to its Articles of Incorporation pursuant to Nevada Revised Statutes Section 92A.200.
The U.S. Company was originally formed for the purpose of sourcing and distributing food products, paper products, janitorial products, restaurant utensils and equipment to the food service industry in the PRC. Due to lack of financing, the U.S. Company never initiated operations. On December 1, 2014, control of the U.S. Company was transferred by its management and its primary shareholders to Mr. Shili Zhang. Mr. Zhang immediately abandoned the U.S. Company’s prior business plan and set about causing the U.S. Company to acquire control of Guangdong Gewang.
Organization and Acquisition of Biotechnology International and Guangdong Gewang
The corporate structure of the U.S. Company and its subsidiaries and affiliates was developed through the following steps:
| ● | In June 2010 three individuals (Shili Zhang, Yun Zeng, Wei Xu) organized Guangdong Gewang as a limited liability company in China under the name “Guangzhou Qinxiyuan Food Co., Ltd.” The registered equity was allocated among the founders thus: Shili Zhang - 54%, Yun Zeng - 28%, Wei Xu - 18%. On December 27, 2011, the limited liability company changed its name to Guangzhou Qinxiyuan Biotechnology Co., Ltd. Subsequently on July 17, 2013, the name was changed to Guangdong Gewang Biotechnology Co., Ltd. Since the time of its organization, Guangdong Gewang has been engaged in the wholesale marketing of selenium supplements. Since January 2014 it has also engaged in retail marketing of selenium supplements. |
| ● | On June 3, 2014, Bing Hua Wu, a resident of the PRC, organized Hong Kong Gewang in Hong Kong. Bing Hua Wu organized Hong Kong Gewang at the behest of Shili Zhang, as the first step in Mr. Zhang’s plan to place Guangdong Gewang under foreign control. The registered capital of Hong Kong Gewang was 10,000 Hong Kong Dollars. Mr. Zhang established Hong Kong Gewang to take advantage of tax advantages offered by the government of China to WFOEs whose equity-owners are Hong Kong residents. On August 29, 2014, Bing Hua Wu transferred the capital stock of Hong Kong Gewang to Shili Zhang, who in turn transferred it to Bin Wang on October 17, 2014. On January 20, 2015, Bin Wang transferred the shares to Gao Yishi Yang, a resident of Beijing. |
| ● | On March 10, 2015, Hong Kong Gewang received a Certificate of Approval from the local government of the PRC approving the establishment of Gewang Selenium as a WFOE wholly owned by Hong Kong Gewang. |
| ● | On March 17, 2015, Shili Zhang organized Biotechnology International under the BVI Business Companies Act, 2004 in the British Virgin Islands. Mr. Zhang was appointed Director of Biotechnology International, and held 25% of the outstanding shares. The remaining 50,000 shares were purchased for US$1.00 each by nineteen of Mr. Zhang’s business associates, none of whom acquired more than 4.9% of the outstanding shares. Mr. Zhang organized a British Virgin Islands holding company because the tax policies as well as the flexible corporation laws of the British Virgin Islands are advantageous to residents of the PRC requiring an offshore holding company. |
| ● | On April 2, 2015, Gao Yishi Yang transferred to Biotechnology International all of the outstanding shares of Hong Kong Gewang in exchange for 10,000 Hong Kong Dollars. |
| ● | On April 6, 2015, Gewang Selenium, Guangdong Gewang and the equity owners in Guangdong Gewang entered into the Variable Interest Entity Agreements (the “VIE Agreements”), as a result of which Guangdong Gewang became a controlled affiliate (variable interest entity) of Gewang Selenium. |
| ● | On April 20, 2015, the U.S. Company acquired all of the capital stock of Biotechnology International through the Share Exchange with the shareholders of Biotechnology International. |
| ● | On July 13, 2016, the U.S. Company's wholly owned subsidiary, Gewang Selenium, exercised its option to purchase all of the registered equity of Guangdong Gewang. The purchase price paid for the equity was RMB10,000 (approximately $1,500). The equity was purchased from Shili Zhang, Yun Zeng and Wei Xu. Shili Zhang was the U.S. Company's CEO until April 8, 2016 and is the father of Mengdi Zhang, who owned 12.7% of the U.S. Company’s outstanding common stock at the time of the sale on July 13, 2016. The other two sellers were not affiliated with the U.S. Company. Upon application to the provincial government for registration of the transfer of equity, the U.S. Company was informed that Gewang Selenium would not be permitted to own 100% of Guangdong Gewang. Therefore the parties modified the exercise of the option to provide that Gewang Selenium would purchase 98% of the registered equity of Guangdong Gewang. The remaining 2% of the registered equity was then sold by Yun Zeng to Haiping Wu for a price of RMB200,000 (approximately $30,000), which equaled 2% of the registered equity of Guangdong Gewang. The acquisition, as modified, was then approved by the provincial government on August 8, 2016. Prior to the acquisition, Gewang Selenium controlled Guangdong Gewang through a series of contractual agreements, which made Guangdong Gewang a variable interest entity, the effect of which was to cause the balance sheet and operating results of Guangdong Gewang to be consolidated with those of Gewang Selenium in the U.S. Company's financial statements. As a result of the acquisition by Gewang Selenium of registered ownership of Guangdong Gewang, the balance sheet and operating results of Guangdong Gewang will hereafter continue to be consolidated with those of Gewang Selenium as its majority owned subsidiary and the VIE Agreements are no longer in effect. The previous non-controlling interest has been reclassified to additional paid-in-capital. |
Acquisition of Tianmei Australia and Guangdong Tianmei
| | ● | On April 28, 2016, the Registrant's wholly-owned subsidiary, Biotechnology International, entered into an investment agreement with Guangdong Tianmei. The investment agreement provided that Biotechnology International would pay US$1,000,000 to acquire a 30% interest in an Australian corporation to be formed, which would indirectly own all of the equity in Guangdong Tianmei. On May 6, 2016, Biotechnology International participated in the organization of Tianmei Beverage Group Corporation Limited ("Tianmei Australia"). |
| ● | On May 16, 2016, Tianmei Australia purchased all of the outstanding shares of Tianmei BVI. The purchase price paid by Tianmei Australia was US$50,000. The seller was not affiliated with the Registrant or its subsidiaries. Tianmei BVI, through its wholly owned subsidiary, a Hong Kong holding company, owns all of the equity of a wholly foreign-owned subsidiary organized in the PRC, which wholly owns Guangdong Tianmei. Guangdong Tianmei was organized in May 2015, and is engaged in the business of distributing selenium-rich bottled water and also functions as a placement agent for a variety of products from various manufacturers, all within the PRC. |
Corporate Structure Chart
Our organizational structure is as follows:
Employees
Guangdong Gewang has 130 full time employees: 5 in human resources, 6 in administration, 7 in accounting, 11 in the purchase department, 4 in logistics, 6 in technology quality control, 10 in brand management, 8 in the customer center, 7 in the Chairman’s office and 66 in external collaboration, which includes our store sales, training and regional management employees.
PRC Government Regulations
Because our operating subsidiary, Guangdong Gewang, is located in the PRC, our business is regulated by the national and local laws of the PRC. We believe our conduct of business complies with existing PRC laws, rules and regulations.
General Regulation of Businesses
We believe we are in material compliance with all applicable labor and safety laws and regulations in the PRC, including the PRC Labor Contract Law, the PRC Production Safety Law, the PRC Regulation for Insurance for Labor Injury, the PRC Unemployment Insurance Law, the PRC Provisional Insurance Measures for Maternity of Employees, PRC Interim Provisions on Registration of Social Insurance, PRC Interim Regulation on the Collection and Payment of Social Insurance Premiums and other related regulations, rules and provisions issued by the relevant governmental authorities from time to time.
According to the PRC Labor Contract Law, we are required to enter into labor contracts with our employees. We are required to pay no less than local minimum wages to our employees. We are also required to provide employees with labor safety and sanitation conditions meeting PRC government laws and regulations and carry out regular health examinations of our employees engaged in hazardous occupations.
Foreign Currency Exchange
The principal regulation governing foreign currency exchange in China is the Foreign Currency Administration Rules (1996), as amended (2008). Under these Rules, the RMB is freely convertible for current account items, such as trade and service-related foreign exchange transactions, but not for capital account items, such as direct investment, loan or investment in securities outside China unless the prior approval of, and/or registration with, the State Administration of Foreign Exchange of the People’s Republic of China, or SAFE, or its local counterparts (as the case may be) is obtained.
Pursuant to the Foreign Currency Administration Rules, foreign invested enterprises, or FIEs, in China may purchase foreign currency without the approval of SAFE for trade and service-related foreign exchange transactions by providing commercial documents evidencing these transactions. They may also retain foreign exchange (subject to a cap approved by SAFE) to satisfy foreign exchange liabilities or to pay dividends. In addition, if a foreign company acquires a subsidiary in China, the acquired company will also become an FIE. However, the relevant PRC government authorities may limit or eliminate the ability of FIEs to purchase and retain foreign currencies in the future. In addition, foreign exchange transactions for direct investment, loans and investment in securities outside China are still subject to limitations and require approvals from, and/or registration with, SAFE.
Dividend Distributions
Under applicable PRC regulations, FIEs in China may pay dividends only out of their accumulated profits, if any, determined in accordance with PRC accounting standards and regulations. In addition, a FIE in China is required to set aside at least 10% of its after-tax profit based on PRC accounting standards each year as as general reserve until the cumulative amount of such reserves reach 50% of its registered capital. These reserves are not distributable as cash dividends.
The Enterprise Income Tax Law and its implementing rules generally provide that a 10% withholding tax applies to China-sourced income derived by non-resident enterprises for PRC enterprise income tax purposes unless the jurisdiction of incorporation of such enterprises’ shareholder has a tax treaty with China that provides for a different withholding arrangement. Gewang Selenium is considered a FIE and is directly held by our subsidiary in Hong Kong, Hong Kong Gewang. According to a 2006 tax treaty between the Mainland and Hong Kong, dividends payable by an FIE in China to a company in Hong Kong that directly holds at least 25% of the equity interest in the FIE will be subject to a withholding tax of no more than 5%. We expect that such 5% withholding tax will apply to dividends paid to Hong Kong Gewang by Gewang Selenium, but this treatment will depend on our status as a non-resident enterprise.
Item 1A. Risk Factors
Investing in our common stock involves risk. You should carefully consider the risks described below together with all of the other information contained in this Report, including the financial statements and the related notes, before deciding whether to purchase any shares of our common stock. If any of the following risks is realized, our business, financial condition or operating results could materially suffer. In that event, the trading price of our common stock could decline and you may lose all or part of your investment.
RISKS RELATED TO OUR BUSINESS
Our management has limited experience in managing and operating a public company. Any failure to comply with federal securities laws, rules or regulations could subject us to fines or regulatory actions, which may materially adversely affect our business, results of operations, financial condition and the market price of our stock.
Our management personnel have no prior experience managing and operating a public company. They will rely in many instances on the professional experience and advice of third parties, including our attorneys and accountants. None of the members of our management staff were educated and trained in U.S. business systems, and we may have difficulty hiring new employees in the PRC with such training. As a result, we may experience difficulty in establishing management, legal and financial controls, collecting financial data and preparing financial statements, books of account and corporate records and instituting business practices that meet U.S. standards. This may result in significant deficiencies or material weaknesses in our internal controls, which could impact the reliability of our financial statements and prevent us from complying with the SEC rules and regulations. Failure to comply with any laws, rules, or regulations applicable to our business may result in fines or regulatory actions, which may materially adversely affect our business, results of operation, or financial condition and could result in delays in development of an active and liquid trading market for our common stock. To the extent that the market place perceives that we do not have a strong financial staff and financial controls, the market for, and price of, our stock may be impaired.
During the past two fiscal years, management has identified material weaknesses in our internal controls, including:
| ● | The relatively small number of employees who are responsible for accounting functions prevents us from segregating duties within our internal control system; |
| | | |
| ● | Our internal financial staff lack expertise in identifying and addressing complex accounting issues under U.S. Generally Accepted Accounting Principles; |
| | | |
| ● | Our executive officers are not familiar with the accounting and reporting requirements of a U.S. public company; and |
| | | |
| ● | We have not developed sufficient documentation concerning our existing financial processes, risk assessment and internal controls. |
We plan to continue to address these deficiencies by nominating independent directors including a “financial expert” highly experienced in internal control systems and creating an Audit Committee to address these material weaknesses. Pursuant to an Audit Committee Charter, which we plan to adopt, the members of the Audit Committee will recommend to management concrete actions to address each material weakness and review the impact of such actions to remedy the deficiencies in our internal controls.
We depend on our partnership with the Academy for the development and conduct of our business. Any interference with that partnership could jeopardize our ability to conduct our business.
The formulas for our proprietary selenium products have been developed by the Academy of Agricultural Sciences of Shandong Province, which licenses to us the right to market and sell these products. The Academy also identifies the manufacturers of our products and provides technical expertise to those manufacturers. If we are unable to maintain the current arrangement with the Academy, we could lose the right to sell our products and our relationships with our contract manufacturers as well as our general reputation could suffer. If the Academy provides the formula or otherwise cooperates with any of our competitors, we could lose the competitive advantage inherent in our current arrangements with the Academy. Also, if the Academy decides to discontinue its work further developing selenium formulas, our potential for further growth could suffer.
We rely on a single manufacturer to manufacture each of our three products. Events that interfere with any manufacturer’s ability to fill our orders could damage our business.
We currently depend on three contracted manufacturers, one to manufacture each of the three products that we sell. If any significant problems occur at the production facility of one of our third-party manufacturers, our ability to deliver that manufacturer’s products could be adversely affected. If any of our contract manufacturers are unable to maintain adequate manufacturing and shipping capacity, timely delivery of products of acceptable quality could become problematic. Our inability to meet our customers’ demand for our products could have a material adverse impact on our business, financial condition and results of operations. Additionally, if the prices charged by any of our contractors increase for reasons such as increases in labor costs, our cost of manufacturing would increase, adversely affecting our operations. We require our contract manufacturers to meet our standards in terms of product quality and other matters. A failure by any of our contract manufacturers to meet these standards, to adhere to labor or other laws or to diverge from our mandated practices, and the potential negative publicity relating to any of these events, could harm our business and reputation.
The lack of expertise in U.S. GAAP among the staff of our finance department could result in errors in our filings.
The books and records of Guangdong Gewang, our operating entity, are maintained in accordance with bookkeeping practices that are customary in China. The financial statements of Guangdong Gewang and Gewang Selenium are prepared in accordance with accounting principles generally accepted in China. The staff of our finance department, which prepares those financial statements, has experience with Chinese GAAP, but very limited experience with U.S. GAAP. Therefore, in order to file with the SEC consolidated financial statements prepared in accordance with U.S. GAAP, we have engaged an independent consultant who makes the adjustments to the financial statements of Guangdong Gewang and Gewang Selenium necessary to achieve compliance with U.S. GAAP, and performs the consolidation required to produce the consolidated financial statements of China Gewang. Because that consultant, who is not present in our executive offices, is the only participant in the preparation of our financial statements possessing a familiarity with U.S. GAAP, there is a risk that the persons responsible for the initial classifications of the elements of our financial results will err in making those classifications, which will cause our reported financial statements to be erroneous. Any such errors, besides being misleading to investors, could result in subsequent restatements, which could have an adverse effect on the perception of the U.S. Company among investors.
We may not be able to meet the internal control reporting requirements imposed by the SEC resulting in a possible decline in the price of our common stock and our inability to obtain future equity or debt financing.
As directed by Section 404 of the Sarbanes-Oxley Act, the SEC adopted rules requiring each public company to include a report of management on its internal controls over financial reporting in its annual reports. Although the Dodd-Frank Wall Street Reform and Consumer Protection Act exempts emerging growth companies from the requirement that our independent registered public accounting firm attest to our financial controls, this exemption does not affect the requirement that we include a report of management on our internal control over financial reporting and does not affect the requirement to include the independent registered public accounting firm’s attestation when we cease to be an emerging growth company. For the fiscal year ending November 30, 2016, we were not subject to the requirement that we include an attestation report, as we are an emerging growth company.
While we expect to expend significant resources in developing the necessary documentation and testing procedures required by Section 404 of the Sarbanes-Oxley Act, there is a risk that we may not be able to comply timely with all of the requirements imposed by this rule. Regardless of whether we are required to receive a positive attestation from our independent registered public accounting firm with respect to our internal controls, if we are unable to do so, investors and others may lose confidence in the reliability of our financial statements and our stock price and ability to obtain equity or debt financing as needed could suffer.
In addition, in the event that our independent registered public accounting firm is unable to rely on our internal controls in connection with its audit of our financial statements, and in the further event that it is unable to devise alternative procedures in order to satisfy itself as to the material accuracy of our financial statements and related disclosures, it is possible that we would be unable to file our Annual Report on Form 10-K with the SEC, which could also adversely affect the market for and the market price of our common stock and our ability to secure additional equity or debt financing as needed.
The residents of China have only recently begun to use supplements to offset selenium deficiency in their diets. We cannot, therefore, predict the potential market for our products. If the market fails to develop adequately, our financial results will be insufficient to produce a profitable return for our investors.
Selenium deficiency has been a problem in eastern China for centuries, and the relationship of selenium deficiencies to Keshan Disease has long been known. Until recently, efforts to alleviate selenium deficiency have been limited to changes in diet, the introduction of selenium-rich foods, where available. The use of selenium supplements, such as those sold by Guangdong Gewang is relatively recent. For that reason, we cannot know the extent to which we will be able to develop a sizeable market for our supplements. As food production and transportation rapidly increases in China, selenium-rich foods will become available to more of the residents of eastern China, where the problem of selenium deficiency is most acute. If Chinese people prefer to alter their diets to include imported selenium-rich foods, the demand for our selenium supplements will be reduced. In addition, concerns among the population about the possibility of harm from ingestion of excessive selenium could reduce demand for our products. If we are not able to persuade a sizeable market that use of selenium supplements is a safe, cost-effective method of avoiding selenium deficiency, our company will not grow.
The loss of the services of our key employees, particularly the services rendered by Li Wang, our Chief Executive Officer, could harm our business.
Our success depends to a significant degree on the services rendered to us by our key employees. If we fail to attract, train and retain sufficient numbers of these qualified people, our prospects, business, financial condition and results of operations will be materially and adversely affected. In particular, we are heavily dependent on the continued services of Li Wang, our chief executive officer. We currently do not have key employee insurance for our officers and directors. The loss of any these key employees, including members of our senior management team, and our inability to attract highly skilled personnel with sufficient experience in our industry could harm our business.
We require highly qualified personnel and, if we are unable to hire or retain qualified personnel, we may not be able to grow effectively.
Our future success also depends upon our ability to attract and retain highly qualified personnel. Expansion of our business and the proposed growth of our business will require additional managers and employees with industry experience, and our success will be highly dependent on our ability to attract and retain skilled management personnel and other employees. We may not be able to attract or retain highly qualified personnel. Competition for skilled marketing and administrative personnel is significant. This competition may make it more difficult and expensive to attract, hire and retain qualified managers and employees.
Product liability claims could materially impact operating results and profitability.
Excessive ingestion of selenium can have serious harmful effects on an individual. We intend to use our best efforts to educate our customers regarding the proper amount of selenium to add to their diets. If, however, an individual intentionally or inadvertently ingests too much selenium and is injured, we may be subject to a lawsuit for damages. Such lawsuits could drain our financial resources, particularly as we do not presently carry any product liability insurance or business interruption insurance. Lawsuits by customers may also distract the time and attention of our management. In addition, a product liability claim, regardless of merit or eventual outcome, could result in damage to our reputation, decreased demand for our products, product recalls and loss of revenue.
Government regulation or other influences may cause us to disclose the formulas for our products, which could assist our competitors in producing copies of our products.
We have a significant competitive advantage in the Jindanli brand, which represents a group of products available only from Guangdong Gewang. The exclusive quality of our products, which is comprised of the formula for each, is known only to Guangdong Gewang, our manufacturers, and our colleagues at the Academy of Agricultural Sciences of Shandong Province. However, because excess ingestion of selenium is known to be harmful, it may occur that one or more government bodies will mandate that the selenium content of our products must be disclosed. In addition, disclosure of the selenium content of our products may occur as a result of malfeasance by employees, accidental disclosure, or litigation. If the formula for our products becomes known in our industry, we will lose the competitive advantage that comes with being the exclusive source for the Jindanli products.
Our inability to protect our trademarks and license rights may prevent us from successfully marketing our products and competing effectively.
Failure to protect our intellectual property could harm our brands and our reputation, and adversely affect our ability to compete effectively. Further, enforcing or defending our intellectual property rights, including our trademarks, trade secrets, and our exclusive rights with the Academy to our product formulas could result in the expenditure of significant financial and managerial resources. We produce, market and sell our products under the brand “Jindanli”. We regard our intellectual property, particularly our trademarks and license rights, to be of considerable value and importance to our business and our success. There can be no assurance that the steps taken by us to protect these proprietary rights will be adequate or that third parties will not infringe or misappropriate our trademarks or the formulas for our products.
RISKS RELATED TO DOING BUSINESS IN CHINA
Uncertainties with respect to the PRC legal system could limit the legal protections available to you and us.
We conduct substantially all of our business through our operating subsidiary and affiliate in the PRC. Our operating subsidiary is generally subject to laws and regulations applicable to foreign investments in China and, in particular, laws applicable to foreign-invested enterprises. The PRC legal system is based on written statutes, and prior court decisions may be cited for reference but have limited precedential value. Since 1979, a series of new PRC laws and regulations have significantly enhanced the protections afforded to various forms of foreign investments in China. However, since the PRC legal system continues to rapidly evolve, the interpretations of many laws, regulations and rules are not always uniform and enforcement of these laws, regulations and rules involve uncertainties, which may limit legal protections available to you and us. In addition, any litigation in China may be protracted and result in substantial costs and diversion of resources and management attention.
The Registrant is a Nevada holding company and most of our assets are located outside of the United States. All of our current business operations are conducted in the PRC through Guangdong Gewang. In addition, all of our directors and officers are nationals and residents of the PRC, and the assets of these persons are located outside the United States. As a result, it may be difficult for you to effect service of process within the United States upon these persons. It may also be difficult for you to enforce in U.S. courts judgments on the civil liability provisions of the U.S. federal securities laws against us and our officers and directors, most of whom are not residents in the United States and the substantial majority of whose assets are located outside of the United States. In addition, there is uncertainty as to whether the courts of the PRC would recognize or enforce judgments of U.S. courts. China does not have any treaties or other arrangements that provide for the reciprocal recognition and enforcement of foreign judgments with the United States. In addition, according to the PRC Civil Procedures Law, courts in the PRC will not enforce a foreign judgment against us or our directors and officers if they decide that the judgment violates basic principles of PRC law or national sovereignty, security or the public interest. So it is uncertain whether a PRC court would enforce a judgment rendered by a court in the United States
Restrictions on currency exchange may limit our ability to receive and use our funds effectively.
All our sales revenue and expenses are denominated in RMB. Under PRC law, the RMB is currently convertible under the “current account,” which includes dividends and trade and service-related foreign exchange transactions, but not under the “capital account,” which includes foreign direct investment and loans. Currently, our PRC operating subsidiary may purchase foreign currencies for settlement of current account transactions, including payments of dividends to us, without the approval of the State Administration of Foreign Exchange, or SAFE, by complying with certain procedural requirements. However, the relevant PRC government authorities may limit or eliminate our ability to purchase foreign currencies in the future. Since a significant amount of our future revenue will be denominated in RMB, any existing and future restrictions on currency exchange may limit our ability to utilize funds denominated in RMB to fund any future business activities outside China or to utilize foreign currencies should the need to do so arise.
Foreign exchange transactions by our PRC operating subsidiary under the capital account continue to be subject to significant foreign exchange controls and require the approval of or need to register with PRC government authorities, including SAFE. In particular, if our PRC operating subsidiary borrows foreign currency through loans from us or other foreign lenders, these loans must be registered with SAFE, and if we finance the subsidiary by means of additional capital contributions, these capital contributions must be approved by certain government authorities, including the Ministry of Commerce, or MOFCOM, or their respective local counterparts. These limitations could affect their ability to obtain foreign exchange through debt or equity financing.
Fluctuations in exchange rates could adversely affect our business and the value of our securities.
The value of our common stock will be indirectly affected by the foreign exchange rate between U.S. dollars and RMB and between those currencies and other currencies in which our sales may be denominated. Appreciation or depreciation in the value of the RMB relative to the U.S. dollar would affect our financial results reported in U.S. dollar terms without there being any underlying change in our business or results of operations. Fluctuations in the exchange rate will also affect the relative value of any dividend we issue that will be exchanged into U.S. dollars as well as earnings from, and the value of, any U.S. dollar-denominated investments we make in the future.
In August 2015, the PRC government devaluated the RMB by approximately 3.5%, and from January 2016 through November 2016 the PRC government devalued its currency by an additional 7.5%. Additional devaluation could occur in the future and affect our results.
Since July 2005, the RMB is no longer pegged to the U.S. dollar. Although the People’s Bank of China regularly intervenes in the foreign exchange market to prevent significant short-term fluctuations in the exchange rate, the RMB may appreciate or depreciate significantly in value against the U.S. dollar in the medium to long term. Moreover, it is possible that in the future PRC authorities may lift restrictions on fluctuations in the RMB exchange rate and lessen intervention in the foreign exchange market.
Very limited hedging transactions are available in China to reduce our exposure to exchange rate fluctuations. To date, we have not entered into any hedging transactions. While we may enter into hedging transactions in the future, the availability and effectiveness of these transactions may be limited, and we may not be able to successfully hedge our exposure at all. In addition, our foreign currency exchange losses may be magnified by PRC exchange control regulations that restrict our ability to convert RMB into foreign currencies.
Restrictions under PRC law on our PRC subsidiary’s ability to make dividend and other distributions could materially and adversely affect our ability to grow, make investments or complete acquisitions that could benefit our business, pay dividends to you, and otherwise fund and conduct our businesses.
Substantially all of our revenues are earned by our PRC subsidiary. However, PRC regulations restrict the ability of our PRC subsidiary to make dividend and other payments to its offshore parent company. PRC legal restrictions permit payments of dividend by our PRC subsidiary only out of its accumulated after-tax profits, if any, determined in accordance with PRC accounting standards and regulations. Our PRC subsidiary is also required under PRC laws and regulations to allocate at least 10% of our annual after-tax profits determined in accordance with PRC GAAP to a statutory general reserve fund until the amounts in said fund reaches 50% of our registered capital. Allocations to the statutory reserve funds can only be used for specific purposes and are not transferable to us in the form of loans, advances or cash dividends. Any limitations on the ability of our PRC subsidiary to transfer funds to us could materially and adversely limit our ability to grow, make investments or acquisitions that could be beneficial to our business, pay dividends and otherwise fund and conduct our business.
Under the Enterprise Income Tax (the “EIT”) Law, we may be classified as a “resident enterprise” of China. Such classification will likely result in unfavorable tax consequences to us and our non-PRC stockholders.
Under the New Income Tax Law, enterprises established outside the PRC whose “de facto management bodies” are located in the PRC are considered “resident enterprises” and their global income will generally be subject to the uniform 25% enterprise income tax rate. On December 6, 2007, the PRC State Council promulgated the Implementation Regulations on the New Income Tax Law, which define “de facto management bodies” as bodies that have material and overall management control over the business, personnel, accounts and properties of an enterprise. In addition, a circular issued by the State Administration of Taxation on April 22, 2009 provides that a foreign enterprise controlled by a PRC company or a PRC company group will be classified as a “resident enterprise” with its “de facto management bodies” located within the PRC if the following requirements are satisfied:
| i. | the senior management and core management departments in charge of its daily operations function mainly in the PRC; |
| ii. | its financial and human resources decisions are subject to determination or approval by persons or bodies in the PRC; |
| iii. | its major assets, accounting books, company seals, and minutes and files of its board and shareholders’ meetings are located or kept in the PRC; and |
| iv. | more than half of the enterprise’s directors or senior management with voting rights reside in the PRC. |
Because the EIT Law, its implementing rules and the recent circular are relatively new, no official interpretation or application of this new “resident enterprise” classification is available. Therefore, it is unclear how tax authorities will determine tax residency based on the facts of each case.
If the PRC tax authorities determine that we are a “resident enterprise” for PRC enterprise income tax purposes, a number of potentially unfavorable PRC tax consequences could follow. First, we may be subject to the enterprise income tax at a rate of 25% on any worldwide taxable income as well as PRC enterprise income tax reporting obligations. In our case, this would mean that non-China source income would be subject to PRC enterprise income tax at a rate of 25%. Second, although under the EIT Law and its implementing rules dividends paid to us from our PRC subsidiary would qualify as “tax-exempt income,” we cannot guarantee that such dividends will not be subject to a 5% withholding tax, as the PRC foreign exchange control authorities, which enforce the withholding tax, have not yet issued guidance with respect to the processing of outbound remittances to entities that are treated as resident enterprises for PRC enterprise income tax purposes. Finally, it is possible that future guidance issued with respect to the new “resident enterprise” classification could result in a situation in which a 10% withholding tax is imposed on dividends we pay to our non-PRC stockholders and with respect to gains derived by our non-PRC stockholders from transferring our shares.
If we were treated as a “resident enterprise” by PRC tax authorities, we would be subject to taxation in both the U.S. and China, and our PRC tax may not be creditable against our U.S. tax.
If the China Securities Regulatory Commission (“CSRC”) or another PRC regulatory agency determines that CSRC approval was required in connection with the reverse acquisition of Biotechnology International, the reverse acquisition may be unwound, or we may become subject to penalties.
On August 8, 2006, six PRC regulatory agencies, including the CSRC, promulgated the Provisions Regarding Mergers and Acquisitions of Domestic Enterprises by Foreign Investors (the “M&A Rule”), which became effective on September 8, 2006. The M&A Rule, among other things, requires that an offshore company controlled by PRC companies or individuals that have acquired a PRC domestic company for the purpose of listing the PRC domestic company’s equity interest on an overseas stock exchange must obtain the approval of the CSRC prior to the listing and trading of such offshore company’s securities on an overseas stock exchange. In addition, when an offshore company acquires a PRC domestic company, the offshore company is generally required to pay the acquisition consideration within three months after the issuance of the foreign-invested company license unless certain ratification from the relevant PRC regulatory agency is obtained. On September 21, 2006, the CSRC, pursuant to the M&A Rule, published on its official web site procedures specifying documents and materials required to be submitted to it by offshore companies seeking CSRC approval of their overseas listings.
We believe the M&A Rule mandating CSRC approval for acquisition of a PRC domestic company by an offshore company controlled by PRC companies or individuals should not apply to our reverse acquisition of Biotechnology International because none of Biotechnology International, Hong Kong Gewang or Gewang Selenium was a “Special Purpose Vehicle” or an “offshore company controlled by PRC companies or individuals” at the moment of acquisition. Because we believe the M&A Rule does not apply, we have not sought approval from the CSRC or any other agency, including MOFCOM for the reverse acquisition of Biotechnology International. However, if the PRC regulatory authorities take the view that the reverse acquisition of Biotechnology International constituted a “round-trip investment” without MOFCOM approval, they could invalidate our acquisition and ownership of Biotechnology International. We cannot make any assurance in such a case that we would be able to obtain the approval required from MOFCOM.
Failure to comply with PRC regulations relating to the establishment of offshore special purpose companies by PRC residents may subject our PRC resident shareholders to personal liability, limit our ability to acquire PRC companies or to inject capital into our PRC subsidiary, limit our PRC subsidiary’s and affiliate’s ability to distribute profits to us or otherwise materially adversely affect us.
On July 4, 2014, SAFE issued the Notice on Issues Relating to the Administration of Foreign Exchange for Overseas Investment and Financing and Reverse Investment by Domestic Residents via Special Purpose Vehicles, or Circular 37, which replaced the Notice on Issues Relating to the Administration of Foreign Exchange for the Financing and Reverse Investment by Domestic Residents via Offshore Special Purpose Vehicles issued by SAFE in October 2005, or Circular 75. Pursuant to Circular 37, any PRC residents, including both PRC institutions and individual residents, are required to register with the local SAFE branch before making any contribution to a company set up or controlled by the PRC residents outside of the PRC for the purpose of overseas investment or financing with their legally owned domestic or offshore assets or interests, referred to in this circular as a "special purpose vehicle." Under Circular 37, the term "PRC institutions" refers to entities with legal person status or other economic organizations established within the territory of the PRC. The term "PRC individual residents" includes all PRC citizens (also including PRC citizens abroad) and foreigners who habitually reside in the PRC for economic benefits. A registered special purpose vehicle is required to amend its SAFE registration in the event of any change of basic information including PRC individual resident shareholder, name, term of operation, or PRC individual resident's increase or decrease of capital, transfer or exchange of shares, merger, division or other material changes. In addition, if a non-listed special purpose vehicle grants any equity incentives to directors, supervisors or employees of domestic companies under its direct or indirect control, the relevant PRC individual residents could register with the local SAFE branch before exercising such options. The SAFE simultaneously issued a series of guidances to its local branches with respect to the implementation of Circular 37. Circular 37 modified certain defined terms under Circular 75 to clarify the SAFE registration scope. For example, Circular 37 broadened the definition of special purpose vehicle to offshore entities that were (i) established for the purpose of overseas investments by PRC residents (in addition to for the purpose of financing as defined under Circular 75) and (ii) established by PRC residents with their legally owned offshore assets or interests (in addition to domestic assets or interests as defined under Circular 75); and it also broadened the definition of reverse investment to include establishing new foreign invested entities or projects as a way of domestic direct investment by PRC residents, directly or indirectly, through a special purpose vehicle, which was excluded by Circular 75. Furthermore, Circular 37 modified certain SAFE registration procedures and requirements for special purpose vehicles and clarified the SAFE registration procedures for equity incentive awards granted by non-listed special purpose vehicles to directors, supervisors or employees of their controlled domestic companies.
We have advised our shareholders who are PRC residents, as defined in Circular 37 , to register with the relevant branch of SAFE, as currently required, in connection with their equity interests in us and our acquisitions of equity interests in our PRC subsidiary and affiliate. However, as SAFE registration is a personal obligation of each shareholder, we cannot provide any assurances that their existing registrations have fully complied with, and they have made all necessary amendments to their registration to fully comply with, all applicable registrations or approvals required by Circular 37. Moreover, because of uncertainty over how Circular 37 will be interpreted and implemented, and how or whether SAFE will apply it to us, we cannot predict how it will affect our business operations or future strategies. For example, our present and prospective PRC subsidiaries’ ability to conduct foreign exchange activities, such as the remittance of dividends and foreign currency-denominated borrowings, may be subject to compliance with Circular 37 by our PRC resident beneficial holders. In addition, such PRC residents may not always be able to complete the necessary registration procedures required by Circular 37. We also have little control over either our present or prospective direct or indirect shareholders or the outcome of such registration procedures. A failure by our PRC resident beneficial holders or future PRC resident shareholders to comply with Circular 37, if SAFE requires it, could subject these PRC resident beneficial holders to fines or legal sanctions, restrict our overseas or cross-border investment activities, limit our subsidiaries’ ability to make distributions or pay dividends or affect our ownership structure, which could adversely affect our business and prospects.
Additionally in October of 2016, the Interim Measures for the Administration of the Establishment and Record Alteration of Foreign Investment Enterprises (“Interim Measures”) took effect and now mandates that WFOEs, among other types of PRC domiciled companies must register with MOFCOM and request MOFCOM’s approval for any change in ownership by foreign investors. Neither our U.S. parent company nor our operating subsidiary, Guangdong Gewang, are affected by the Interim Measures, but our subsidiary Gewang Selenium is subject to the Interim Measures as a WFOE. As such, any change in ownership of Gewang Selenium would require the approval of MOFCOM, and such approval cannot be guaranteed. Any failure to seek approval of any change in ownership of Gewang Selenium could create liability affecting our U.S. parent company, and the potential barrier in changing the ownership structure of the U.S. parent and its subsidiaries owned by Gewang Selenium could limit opportunities for restructuring. Gewang Selenium has already registered its current ownership with MOFCOM prior to the effective date of the Interim Measures, so under the U.S. Company’s current corporate structure, the Interim Measures will not affect our business. Gewang Selenium does not have any material operations, and we plan to retain the current ownership structure under Gewang Selenium’s existing registration with MOFCOM in order to avoid any risk.
We may be exposed to liabilities under the Foreign Corrupt Practices Act and Chinese anti-corruption law, and any determination that we violated these laws could have a material adverse effect on our business.
We are subject to the U.S. Foreign Corrupt Practices Act, (“FCPA”) and other laws that prohibit improper payments or offers of payments to foreign governments and their officials and political parties by U.S. persons and issuers for the purpose of obtaining or retaining business. We are also subject to Chinese anti-corruption laws, which strictly prohibit the payment of bribes to government officials.
We principally have operations, agreements with third parties and make sales in China, which may experience corruption. Our activities in China create the risk of unauthorized payments or offers of payments by one of the employees or consultants of our company, because these parties are not always subject to our control. We believe that to date we have complied in all material respects with the provisions of the FCPA and Chinese anti-corruption law. However, our existing safeguards and any future improvements may prove to be less than effective, and the employees, consultants or distributors of our Company may engage in conduct for which we might be held responsible. Violations of the FCPA or Chinese anti-corruption law may result in severe criminal or civil sanctions, and we may be subject to other liabilities, which could negatively affect our business, operating results and financial condition. In addition, the government may seek to hold our Company liable for successor liability FCPA violations committed by companies in which we invest or that we acquire.
Lack of bank deposit insurance in the PRC puts our cash balances at risk of loss, the loss of which could have a material adverse effect on our business.
We maintain bank accounts in China the balances of which are not insured and are not protected by U.S. FDIC insurance or other insurance. As of January 9, 2017 we held the equivalent of approximately $14,921,000 in US Dollars in bank accounts in China. If a Chinese bank holding our funds were to experience insolvency or closure, it may not permit us to withdraw our funds, which would result in a loss of such funds, which could have a material adverse effect on our business.
RISKS RELATED TO THE MARKET FOR OUR STOCK
There is only a very limited market for our Common Stock.
While our common stock is listing for quotation on the OTCQB, there is currently little trading in our common stock. We cannot provide any assurances as to if or when an active market will develop for our common stock.
Registrant is an “emerging growth company” and we cannot be certain if the reduced disclosure requirements applicable to emerging growth companies will make our common stock less attractive to investors.
We are an “emerging growth company” or “”EGC” as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. We will remain an EGC until the earlier of: the last day of the fiscal year in which we have total annual gross revenues of $1.0 billion or more; the date on which we have issued more than $1.0 billion in nonconvertible debt during the previous three years; the date on which we are deemed to be a large accelerated filer under the rules of the SEC; or November 30, 2019 (i.e., five years from the closing of our initial public offering).
An emerging growth company may take advantage of reduced reporting requirements that are otherwise applicable to public companies. These provisions include, but are not limited to:
| ● | not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, as amended; |
| | | |
| ● | reduced disclosure obligations regarding executive compensation in our periodic reports , proxy statements and registration statements; and |
| | | |
| ● | exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. |
We have elected to take advantage of certain of the reduced disclosure obligations in this Report and may elect to take advantage of other reduced reporting requirements in our future filings with SEC.
The Jobs Act also provides that we can take advantage of the extended transition period for complying with new or revised accounting standards. Thus, an EGC can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have elected to avail ourselves of this extended transition period and, as a result, we may not adopt new or revised accounting standards until those standards would otherwise apply to private companies.
We cannot predict if investors will find our common stock less attractive because we may rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile when trading occurs.
For so long as we are an emerging growth company, we may rely on certain exemptions provided in the JOBS Act, including reduced disclosure regarding executive compensation, not seeking an advisory vote with respect to executive compensation and not requiring our independent registered public accounting firm to attest to the effectiveness of our internal control over financial reporting, which could make our common stock less attractive to investors due to the nature of the reduced disclosure.
We are an “emerging growth company,” as defined in the JOBS Act. For so long as we remain an emerging growth company, we are permitted and plan to rely on exemptions from certain disclosure requirements that are applicable to other public companies that are not emerging growth companies. These exemptions include not being required to comply with the auditor attestation requirements of Section 404(b) of the Sarbanes-Oxley Act of 2002, or SOX Section 404, not being required to comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements, reduced disclosure obligations regarding executive compensation and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. In this Report, we have not included all of the executive compensation related information that would be required if we were not an emerging growth company, nor have we included an audit report on our internal controls. We cannot predict whether investors will find our common stock less attractive if we rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile. In addition, the JOBS Act provides that an emerging growth company can take advantage of an extended transition period for complying with new or revised accounting standards. This allows an emerging growth company to delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have elected to avail ourselves of this extended transition period and, as a result, we may not adopt new or revised accounting standards until those standards would otherwise apply to private companies.
If securities or industry analysts do not publish research or reports or publish unfavorable research about our business, the price and trading volume of our common stock could decline.
The trading market for our common stock will depend in part on the research and reports that securities or industry analysts publish about us or our business. We do not currently have and may never obtain research coverage by securities and industry analysts. If no securities or industry analysts commence coverage of us, the trading price for our common stock and other securities would be negatively affected. In the event we obtain securities or industry analyst coverage, if one or more of the analysts who covers us downgrades our securities, the price of our securities would likely decline. If one or more of these analysts ceases to cover us or fails to publish regular reports on us, interest in the purchase of our securities could decrease, which could cause the price of our common stock and other securities and their trading volume to decline.
If our common stock becomes a “penny stock,” you may have greater difficulty selling your shares.
Penny stocks are generally equity securities with a price of less than $5.00, other than securities registered on certain national securities exchanges or quoted on the NASDAQ system, provided that current price and volume information with respect to transactions in such securities is provided by the exchange or quotation system. Our common stock may become a “penny stock” within the meaning of the rules, as the rules apply to us and to our securities if we are not listed on a national securities exchange. These rules may further affect the ability of owners of shares to sell our securities in any market that might develop for them. As long as the trading price of our common stock is less than $5.00 per share, even if our common stock is quoted on either the OTCQX or OTCQB market place operated by the OTC Markets, our common stock will be subject to Rule 15g-9 under the Exchange Act (the “Penny Stock Rules”). The Penny Stock Rules require a broker-dealer, prior to a transaction in a penny stock, to deliver a standardized risk disclosure document prepared by the SEC, that:
| ● | contains a description of the nature and level of risk in the market for penny stocks in both public offerings and secondary trading; |
| | | |
| ● | contains a description of the broker’s or dealer’s duties to the customer and of the rights and remedies available to the customer with respect to a violation to such duties or other requirements of securities laws; |
| | | |
| ● | contains a brief, clear, narrative description of a dealer market, including bid and ask prices for penny stocks and the significance of the spread between the bid and ask price; |
| | | |
| ● | contains a toll-free telephone number for inquiries on disciplinary actions; |
| | | |
| ● | defines significant terms in the disclosure document or in the conduct of trading in penny stocks; and |
| | | |
| ● | contains such other information and is in such form, including language, type, size and format, as the SEC shall require by rule or regulation. |
The broker-dealer also must provide, prior to effecting any transaction in a penny stock, the customer with: (a) bid and offer quotations for the penny stock; (b) the compensation of the broker-dealer and its salesperson in the transaction; (c) the number of shares to which such bid and ask prices apply, or other comparable information relating to the depth and liquidity of the market for such stock; and (d) a monthly account statements showing the market value of each penny stock held in the customer’s account. In addition, the penny stock rules require that prior to a transaction in a penny stock not otherwise exempt from those rules; the broker-dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser’s written acknowledgment of the receipt of a risk disclosure statement, a written agreement to transactions involving penny stocks, and a signed and dated copy of a written suitably statement. These disclosure requirements may have the effect of reducing the trading activity in the secondary market for our stock it becomes designated as a Penny Stock.
Because we do not intend to pay any cash dividends on our common stock, our stockholders will not be able to receive a return on their shares unless they sell them.
We currently intend to retain any future earnings to finance the development and expansion of our business. We do not anticipate paying any cash dividends on our common stock in the foreseeable future. Unless we pay dividends, our stockholders will not be able to receive a return on their shares unless they sell them. There is no assurance that stockholders will be able to sell shares when desired.
Item 1B. Unresolved Staff Comments
Not applicable.
Item 2. Properties
Because our business is exclusively sales, we require only modest real property to operate. Our home office in Guangzhou houses both our administrative functions and our marketing operations. We lease that property for an annual fee of 600,000 RMB ($93,660), with a lease termination date of July 1, 2017. Our flagship store is leased for an annual fee of 960,000 RMB ($143,701), with a lease termination date of May 31, 2017.
We lease the properties that house our home office, flagship store and three other stores under the following terms:
| Location | | Annual Rent | | | Terminates | |
| Guangzhou - office | | 600,000 RMB
($91,000) | | | July 1, 2017 | |
| Guangzhou - flagship store | | 960,000 RMB
($143,000) | | | May 31, 2017 | |
| Foshan | | 420,000 RMB
($62,000) | | | May 31, 2017 | |
| Longyan | | 420,000 RMB
($62,000) | | | May 31, 2017 | |
| Zhuzhou | | 420,000 RMB
($62,000) | | | May 31, 2017 | |
Item 3. Legal Proceedings
None.
Item 4. Mine Safety Disclosures.
Not Applicable.
PART II
Item 5. Market For Registrant’s Common Equity, Related Stockholder Matters And Issuer Purchases Of Equity Securities.
(a) Market Information
The Registrant’s common stock was first quoted on the OTC Pink Market on April 24, 2015. Since September 26, 2016 it has been quoted on the OTCQB under the symbol “CGWB.” The following table sets forth for the periods indicated the prices of the common stock, as reported by the OTC Markets. Such prices are inter-dealer bid and asked prices, without markup, markdown, commissions, or adjustments, and may not represent actual transactions.
| | | Bid | |
| Quarter Ending | | High | | | Low | |
| May 31, 2015 | | $ | 2.83 | | | $ | 2.20 | |
| August 31, 2015 | | $ | 2.86 | | | $ | 2.80 | |
| November 30, 2015 | | $ | 3.59 | | | $ | 2.84 | |
| | | | | | | | | |
| February 29, 2016 | | $ | 3.89 | | | $ | 3.59 | |
| May 31, 2016 | | $ | 6.00 | | | $ | 3.88 | |
| August 31, 2016 | | $ | 6.00 | | | $ | 4.48 | |
| November 30, 2016 | | $ | 5.39 | | | $ | 5.34 | |
(b) Shareholders
Our shareholders list contains the names of 2,074 registered stockholders of record of the Registrant’s Common Stock.
Our stock transfer agent is Island Stock Transfer: 15500 Roosevelt Blvd., Suite 301, Clearwater, Florida 33760; (727) 289-0010.
(c) Dividends
The Registrant has never paid or declared any cash dividends on its Common Stock and does not foresee doing so in the foreseeable future. The Registrant intends to retain any future earnings for the operation and expansion of the business. Any decision as to future payment of dividends will depend on the available earnings, the capital requirements of the Registrant, its general financial condition, legal restrictions and other factors deemed pertinent by the Board of Directors.
(d) Securities Authorized for Issuance Under Equity Compensation Plans
The Registrant had no securities authorized for issuance under equity compensation plans as of November 30, 2016.
(e) Performance Graph
The following Performance Graph and related information shall not be deemed "soliciting material" or deemed to be "filed" with the Securities and Exchange Commission, nor shall such information be incorporated by reference into any future filing under the Securities Act of 1933 or the Securities Exchange Act of 1934, except to the extent that we specifically incorporate such information by reference into such filing.
The graph below presents a comparison of the annual (or partial-year) change in the cumulative total return on our common stock, over the period from April 24, 2015 (when our common stock was first traded publicly) to November 30, 2016, with the cumulative total return of the NASDAQ Composite Index and the Russell Microcap Index over the same period. The graph assumes an investment of $100 in our common stock and in each of the indices at the closing price on April 24, 2015 and assumes all dividends were reinvested. The figures set forth in the graph have been calculated based on the closing prices on the last trading day for each period indicated.
Comparison of Cumulative Total Return
Assumes Initial Investment of $100
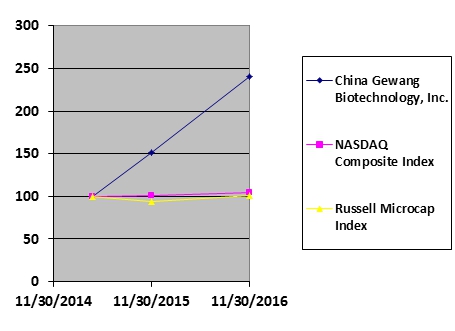
(f) Sale of Unregistered Securities
The Registrant did not issue any unregistered equity securities during the 4th quarter of fiscal 2016.
(g) Repurchase of Equity Securities
The Registrant did not repurchase any shares of its common stock during the 4th quarter of fiscal 2016.
Item 6. Selected Financial Data
The consolidated selected balance sheet data as of November 30, 2016 and 2015 and the consolidated selected statements of operations data for the years ended November 30, 2016, 2015 and 2014 set forth below have been derived from our audited consolidated financial statements included in this Report, and should be read in conjunction with those financial statements (including the notes thereto). The consolidated selected balance sheet data as of November 30, 2014 and 2013 and the consolidated selected statements of operations data for the year ended November 30, 2013 have been derived from audited consolidated financial statements not included herein, but which were previously filed with the SEC.
Consolidated Statements of Operations Data
(in thousands, except per share data)
| | | For the Years Ended November 30, | |
| | | 2016 | | | 2015 | | | 2014(1) | | | 2013(1) | |
| Revenue | | $ | 45,519 | | | $ | 4,184 | | | $ | 2,368 | | | $ | 1,468 | |
| Gross profit | | | 16,754 | | | | 2,998 | | | | 1,643 | | | | 960 | |
| Operating income | | | 12,098 | | | | 1,633 | | | | 1,019 | | | | 600 | |
| Income before income taxes | | | 12,113 | | | | 1,647 | | | | 1,022 | | | | 605 | |
| Net income including noncontrolling interest in operating subsidiary | | | 13,574 | | | | 1,189 | | | | 757 | | | | 453 | |
| Net income per common share | | $ | 0.20 | | | $ | 0.02 | | | $ | 0.02 | | | | N.A. | |
| Weighted average shares outstanding | | | 65,441 | | | | 40,944 | | | | 35,500 | | | | N.A. | |
| Cash dividends declared | | | - | | | | - | | | | - | | | | - | |
Consolidated Balance Sheet Data
(in thousands, except per share data)
| | | At November 30, | |
| | | 2016 | | | 2015 | | | 2014(1) | | | 2013(1) | |
| Cash | | $ | 13,108 | | | $ | 8,669 | | | $ | 3,013 | | | $ | 2,289 | |
| Total assets | | | 36,203 | | | | 9,361 | | | | 3,220 | | | | 2,371 | |
| Long-term obligations | | | - | | | | - | | | | - | | | | - | |
| Total liabilities | | | 4,815 | | | | 405 | | | | 122 | | | | 61 | |
| Book value per share | | $ | 0.48 | | | $ | 0.20 | | | $ | 0.09 | | | | N.A. | |
| (1) | Financial information for the years ended and as of November 30, 2014 and 2013 are derived from the financial statements of Guangdong Gewang Biotechnology Co., Ltd. included in the Registrant's Current Report on Form 8-K filed on April 21, 2015. The Registrant acquired control of Guangdong Gewang Biotechnology Co, Ltd. on April 20, 2015. |
Item 7. Management’s Discussion and Analysis
Results of Operations for the Year Ended November 30, 2016 Compared with the Year Ended November 30, 2015
The following table sets forth key components of our results of operations during the years ended November 30, 2016 and 2015, and the percentage changes between 2016 and 2015.
| | | Fiscal Year
2016
(US $) | | | Fiscal Year
2015
(US $) | | | Change
% | |
| Net revenue | | $ | 45,519,999 | | | $ | 4,184,255 | | | | 988 | % |
| Cost of Sales | | | (28,765,888 | ) | | | (1,186,461 | ) | | | 2,325 | % |
| Gross profit | | | 16,754,111 | | | | 2,997,794 | | | | 459 | % |
| Selling and marketing expenses | | | 3,044,135 | | | | 836,040 | | | | 264 | % |
| General and administrative expenses | | | 1,005,385 | | | | 528,627 | | | | 90 | % |
| Research and development | | | 605,816 | | | | - | | | | - | |
| Total operating expenses | | | 4,655,336 | | | | 1,364,667 | | | | 241 | % |
| Operating income | | | 12,098,775 | | | | 1,633,127 | | | | 640 | % |
| Other income | | | 14,868 | | | | 13,508 | | | | 10 | % |
| Income before provision for income taxes | | | 12,113,643 | | | | 1,646,635 | | | | 635 | % |
| Provision for income taxes | | | 3,075,733 | | | | 457,922 | | | | 571 | % |
| Equity in income of investee | | | 4,536,760 | | | | - | | | | - | |
| Net income before noncontrolling interests | | | 13,574,670 | | | | 1,188,713 | | | | 1,042 | % |
| Noncontrolling interests | | | (326,213 | ) | | | (61,790 | ) | | | 427 | % |
| Net income attributable to common stockholders | | $ | 13,348,457 | | | $ | 1,126,923 | | | | 1,075 | % |
Sales
Our sales increased to $45,519,999 for the fiscal year ended November 30, 2016 from $4,184,255 for the year ended November 30, 2015, an increase of $41,335,744 or 988%. We did not increase the prices of any of our products. However, we have begun to wholesale products manufactured by other companies through our distribution network and have also increased the volume of sales of our Jindanli branded products. Since March 2016, we have signed agreements with eight distributors to distribute selenium products to hundreds of retail stores, and we made net sales of selenium and selenium-related products totaling $41,160,605 to these distributors during fiscal year 2016. The following factors had the greatest impact on our increase in sales:
| | ● | Total wholesale sales, including of our Jindanli branded products and products manufactured by other companies, to our distribution network of 16 wholesale customers and distributors by our headquarters marketing personnel increased by $38,215,165, primarily due to an increase in the number of sales personnel during fiscal year 2016. |
| ● | Of the total increase in wholesale sales during the year ended November 30 2016, $32,559,461 was attributable to the wholesale of 89 selenium product types manufactured by other companies. In fiscal year 2015, we did not wholesale any products other than our Jindanli branded products. Of the total increase in wholesale sales during fiscal year 2016, $32,559,461 is attributable to new wholesale customers. Since March 2016, we have signed agreements with eight distributors to distribute selenium products to hundreds of retail stores, and this expansion of our customer base has greatly increased our volume of sales. |
| | | |
| ● | Of the total increase in wholesale sales during the year ended November 30, 2016, $5,654,374 is attributable to an increase in the volume of our three Jindanli branded selenium supplements, from $2,945,440 during fiscal year 2015, an increase of 192%. The increase is related to an expansion of our sales staff, which has increased the volume of sales of our existing products. |
| ● | Retail sales increased by $3,085,289, or 249% during the year ended November 30, 2016. Due to increased rent and unsatisfactory pedestrian flow, we closed our Changcheng, Xiamen and Changsha stores on May 31, 2016 and opened stores in Foshan, Longyan and Zhuzhou and a flagship store in Guangzhou on June 1, 2016. The new stores are located in areas with better pedestrian flow, resulting in a sharp increase in retail sales. In addition, the new stores stock an inventory of over 90 selenium products, whereas the previous stores stocked only our three selenium supplements. |
During the summer of 2016, we commenced franchising the use of our trademark, name identification and other business resources. The franchisees were required to pay us a franchise fee and monthly management fees. During the year ended November 30, 2016, two franchisees signed with us. However, we terminated the franchises in the fall of 2016 and do not plan to carry on franchise operations in the near future.
The following table shows the source of our revenue in the comparable periods:
| | | Fiscal Year 2016 | | | Fiscal Year 2015 | |
| | | Sales | | | % of total | | | Sales | | | % of total | |
| Headquarters wholesale-selenium supplements | | $ | 8,599,814 | | | | 18.89 | % | | $ | 2,945,440 | | | | 70.39 | % |
| Headquarters wholesale-selenium products | | | 32,559,461 | | | | 71.53 | % | | | - | | | | - | |
| Headquarters retail store | | | 241,466 | | | | 0.53 | % | | | 505,406 | | | | 12.08 | % |
| Changcheng retail store | | | 265,612 | | | | 0.58 | % | | | 621,025 | | | | 14.84 | % |
| Xiamen retail store | | | 196,419 | | | | 0.43 | % | | | 93,618 | | | | 2.23 | % |
| Changsha retail store | | | 161,315 | | | | 0.35 | % | | | 18,766 | | | | 0.45 | % |
| Foshan retail store | | | 485,773 | | | | 1.07 | % | | | - | | | | - | |
| Longyan retail store | | | 431,392 | | | | 0.95 | % | | | - | | | | - | |
| Zhuzhou retail store | | | 411,304 | | | | 0.90 | % | | | - | | | | - | |
| Flagship retail store | | | 2,130,683 | | | | 4.68 | % | | | - | | | | - | |
| Franchise and management fees | | | 35,290 | | | | 0.08 | % | | | - | | | | - | |
| | | $ | 45,519,999 | | | | 100 | % | | $ | 4,184,255 | | | | 100 | % |
Gross Profit
For the past three years we have sold our three selenium supplements, and only in March 2016 began to resell a wide variety of selenium-related products that we purchase at wholesale. After increasing over the past two fiscal years, the unit prices that we pay to our manufacturers for the selenium supplements stabilized in the second quarter of fiscal 2016. As a result, our gross margin for selenium supplements was not significantly changed: 71.4% in fiscal year 2016 compared to 71.6% in fiscal year 2015. However, the selenium-related products that we resold during fiscal year 2016 yielded a gross margin of only 25%. As a result, our overall gross margin during fiscal year 2016 declined to 36.8% from 71.6% recorded on the sale of selenium supplements alone during fiscal year 2015.
Selling expenses
Our selling and marketing expenses increased by 459% to $3,044,135 for the year ended November 30, 2016 from $836,040 for the year ended November 30, 2015. Our selling expenses include rent, transportation expenses, advertising expenses and salaries incurred for the sales function, all of which will tend to increase as our sales increase. In fiscal 2016, we incurred RMB 3,790,000 (US$574,000) in advertising expense, which is significantly higher than we incurred in the past. We are committed to paying our current rate of advertising costs until March 31, 2017.
General and administrative expenses
Our general and administrative (“G&A”) expenses increased by 90% to $1,005,385 for the year ended November 30, 2016 from $528,627 for the year ended November 30, 2015. The largest components of our G&A expenses are the salaries of administrative personnel and fringe benefits provided to all of our staff. The change in G&A expenses occurred primarily as a result of the expansion of our business over the prior year, as reflected in the growth of our sales, which led to increases in travel and entertainment expenses. In addition, to enable us to manage our expanding operations, we added administrative personnel.
Income from operations
Because the sharp increase in our sales generated a material increase in our gross profit, our operating income increased by 640% to $12,098,775 for the year ended November 30, 2016, from $1,633,127 for the year ended November 30, 2015.
As we have very little debt, our other income for the years ended November 30, 2016 and 2015 consisted of interest income earned on our bank balances of $14,868 and $13,508. Our pre-tax income, therefore, was $12,113,643 and $1,646,635 for the years ended November 30, 2016 and 2015, respectively.
Net income
Due to the increase in our pre-tax income, our provision for income taxes increased by 639% to $3,075,733 for the year ended November 30, 2016 from $457,922 for the year ended November 30, 2015. In this period, our effective tax rate was the same as the statutory rate of 25%. We also recorded equity investment income of $4,536,760, representing our 30% interest in the net income reported by Tianmei Australia for the year ended November 30, 2016. After deducting the provision for income taxes and adding equity in income of investee, China Gewang reported net income before noncontrolling interests of $13,574,670 and $1,188,713 for the year ended November 30, 2016 and 2015, respectively.
Before August 8, 2016, the VIE Agreements assigned to Gewang Selenium 95% of the net income of Guangdong Gewang. On August 8, 2016, Gewang Selenium purchased 98% of the registered equity of Guangdong Gewang. Our non-controlling interest, therefore, was reduced from 5% to 2%. We recorded a deduction for noncontrolling interests of $326,213 and $61,790 for the years ended November 30, 2016 and 2015, respectively, after which our net income attributable to common stockholders was $13,248,457 ($0.20 per share) and $1,126,923 ($0.03 per share) for the years ended November 30, 2016 and 2015, respectively.
Foreign Currency Translation Adjustment
Our reporting currency is the U.S. dollar. Our local currency, the Renminbi (RMB), is our functional currency. Results of operations and cash flow are translated at average exchange rates during the period being reported upon, and assets and liabilities are translated at the unified exchange rate as quoted by the People’s Bank of China on the balance sheet date. Translation adjustments resulting from this process are included in other comprehensive income. For the years ended November 30, 2016 and 2015, foreign currency translation adjustments of $(1,455,633) and $(315,020), respectively, have been reported as other comprehensive loss in the consolidated statement of income and comprehensive income (loss). The loss during year ended November 30, 2016 was primarily due to devaluation of the PRC currency of approximately 3.5% in August 2015 and further devaluations aggregating 7.5% through November 2016. Additional devaluations could occur in the future and affect our results.
RMB is not freely convertible into the currency of other nations. All such exchange transactions must take place through authorized institutions. There is, therefore, no guarantee the RMB amounts could have been, or could be, converted into US dollars at the rates used in translation. Substantially all of our assets are located in the PRC which makes it difficult for any funds to be utilized outside the PRC.
Results of Operations for the Year Ended November 30, 2015 Compared with the Year Ended November 30, 2014
The following table sets forth key components of our results of operations during the years ended November 30, 2015 and 2014, and the percentage changes between 2015 and 2014.
| | | November 30, | | | November 30, | | | | |
| | | 2015 | | | 2014 | | | Change | |
| | | (US $) | | | (US $) | | | % | |
| Revenue | | $ | 4,184,255 | | | $ | 2,368,016 | | | | 77 | % |
| Cost of Sales | | | (1,186,461 | ) | | | (725,449 | ) | | | 64 | % |
| Gross profit | | | 2,997,794 | | | | 1,642,567 | | | | 83 | % |
| Selling and marketing expenses | | | 836,040 | | | | 473,670 | | | | 77 | % |
| General and administrative expenses | | | 528,627 | | | | 150,154 | | | | 252 | % |
| Total operating expenses | | | 1,364,667 | | | | 623,824 | | | | 119 | % |
| Income from operations | | | 1,633,127 | | | | 1,018,743 | | | | 60 | % |
| Other income | | | 13,508 | | | | 2,900 | | | | 366 | % |
Income before provision for income taxes | | | 1,646,635 | | | | 1,021,643 | | | | 61 | % |
| Provision for income taxes | | | 457,922 | | | | 264,553 | | | | 73 | % |
| Net income before noncontrolling interests | | | 1,188,713 | | | | 757,090 | | | | 57 | % |
| Noncontrolling interests | | | (61,790 | ) | | | (38,952 | ) | | | 59 | % |
| Net income attributable to common stockholders | | $ | 1,126,923 | | | $ | 718,138 | | | | 57 | % |
Sales. Our sales increased to $4,184,255 for the year ended November 30, 2015 from $2,368,016 for the year ended November 30, 2014, an increase of $1,816,239 or 77%. In addition to the beneficial effects of our marketing efforts, the primary causes of the increase were:
| ● | Increased demand for our products allowed us to implement a 30% increase in our wholesale prices. Our average unit price in and before March 2014 was $81 (RMB 500) and increased to $105 (RMB 650) from April 2014. |
| | | |
| ● | During the first half of fiscal 2014, our sales were made exclusively from the head office, mostly to wholesale customers but with modest retail sales. During the latter part of fiscal 2014, we increased retail sales from our head office and opened our first physical store in Chancheng. In June 2015, we opened a second store in Xiamen. In October 2015, we opened a third store in Changsha. Retail sales were 30% and 18% of total sales for the years ended November 30, 2015 and 2014. The retail average unit selling price during fiscal 2015 was $222 (RMB 1,380). |
The following table shows the source of our revenue in the comparable periods:
| | | Year ended Nov. 30, 2015 | | | Year Ended Nov. 30, 2014 | |
| | | Sales | | | % of total | | | Sales | | | % of total | |
| Office wholesale | | $ | 2,945,440 | | | | 70 | % | | $ | 1,930,057 | | | | 82 | % |
| Office retail | | | 505,406 | | | | 12 | % | | | 380,368 | | | | 16 | % |
| Changcheng store retail | | | 621,025 | | | | 15 | % | | | 57,591 | | | | 2 | % |
| Xiamen store retail | | | 93,618 | | | | 2 | % | | | - | | | | 0 | % |
| Changsha store retail | | | 18,766 | | | | <1 | % | | | - | | | | 0 | % |
| | | $ | 4,184,255 | | | | 100 | % | | $ | 2,368,016 | | | | 100 | % |
Gross profit. Our cost of sales increased during the two years ended November 30, 2015 due to increased unit prices paid to our manufacturers. Although our cost for "Organic Selenium Powder” increased by 44% in April 2014, the increase in cost for our two best selling products was less than the 30% overall increase in our sales price: our unit purchase price for the “Xikang Capsule” increased by 22% to $35; the unit purchase price of our “Xizhi Capsule” increased by 25% to $32. Therefore, our gross profit margin increased by 2% to 71.6% for the year ended November 30, 2015.
Selling expenses. Our selling expenses increased by 77% to $836,040 for the year ended November 30, 2015 from $473,670 for the year ended November 30, 2014. Our selling expenses will not increase in strict proportion to an increase in sales, because a large component of our selling expenses is a fixed license fee that we pay to the Shandong Academy of Agriculture for use of the selenium formulae developed by the Academy. On the other hand, our selling expenses include rent, transportation expenses, advertising expenses and salaries incurred for the sales function, all of which will tend to increase as our sales increase. In addition, one specific reason for the increase in our selling expenses was the opening of our retail stores in Foshan, Xiamen and Changsha since September 2014.
General and administrative expenses. Our general and administrative (“G&A”) expenses increased by 252% to $528,627 for the year ended November 30, 2015 from $150,154 for the year ended November 30, 2014. The largest components of our G&A expenses are the salaries of administrative personnel and government-mandated benefits provided to all of our staff. The change in G&A expenses from year to year occurred primarily as a result of the expansion of our business in the past year, as reflected in the growth of our sales.
Pre-tax income. Because our operating expenses increased at a faster rate than our gross profit during the year ended November 30, 2015, our operating income increased by only 60%, to $1,633,127 in the year ended November 30, 2015 from $1,018,743 in the year ended November 30, 2014, respectively, less than the 83% increase in our gross profit.
As we have very little debt, our only other income consisted entirely of interest income earned on our bank balances: $13,508 and $2,900 during the years ended November 30, 2015 and 2014, respectively. Our pre-tax income, therefore, was $1,646,635 and $ 1,021,643 for the years ended November 30, 2015 and 2014 respectively.
Net income. Due to the 61% increase in our pre-tax income for year ended November 30, 2015, our provision for income taxes increased to $457,922 for the year ended November 30, 2015 from $264,553 for the year ended November 30, 2014. Our effective tax rate was the same as the statutory rate of 25% for the years ended November 30, 2015 and 2014. After deducting the provision for income taxes and noncontrolling interests, China Gewang reported net income before noncontrolling interests of $1,188,713 and $757,090 for the years ended November 30, 2015 and 2014, respectively. Because the VIE Agreements assigned to Gewang Selenium only 95% of the net income of Guangdong Gewang, we recorded a deduction for noncontrolling interests, after which our net income attributable to common stockholders was $1,126,923 ($0.02 per share) for the year ended November 30, 2015 and $718,138 ($0.02 per share) for the year ended November 30, 2014.
Foreign Currency Translation Adjustment.For the years ended November 30, 2015 and 2014, foreign currency translation adjustments of $(315,020) and $1,333, respectively, have been reported as other comprehensive income (loss) in the consolidated statement of income and comprehensive income (loss). The loss during year ended November 30, 2015 was primarily due to devaluation of the PRC currency of approximately 3.5% in August 2015. Additional devaluations could occur in the future and affect our results.
RMB is not freely convertible into the currency of other nations. All such exchange transactions must take place through authorized institutions. There is, therefore, no guarantee the RMB amounts could have been, or could be, converted into US dollars at the rates used in translation. Substantially all of our assets are located in the PRC which makes it difficult for any funds to be utilized outside the PRC.
Liquidity and Capital Resources
We have financed our operations primarily through cash flows from operations and sale of our common stock. As a result, at November 30, 2016, our only debt was $237,639 in loans from a stockholder and $228,238 in loans from a third party, all of which consisted of US Dollars loaned to pay our administrative expenses in the U.S.
During the year ended November 30, 2016, the increase in our working capital was approximately equal to the sum of our net income for the period less net income attributable to the increase in value of our investment in Guangdong Tianmei, supplemented by the proceeds of two private placements of common stock (together generating net proceeds of $9,848,200). Our working capital as of November 30, 2016 was $25,145,721, which represented an increase of $16,256,017 during fiscal year 2016. The approximation of our net income from the operations of Guangdong Gewang (plus capital paid-in during the period) to the increase in our working capital occurs because our operations, which involve no manufacturing and limited real estate, require very modest capital investments and, as a result, almost all of our assets (other than our investment in Guangdong Tianmei) and all of our liabilities are current. Until we further implement our plan to open a series of dedicated retail stores, which will add depreciable capital assets to our balance sheet, net income should continue to increase our working capital.
For the same reasons, during the year ended November 30, 2015, the increase in working capital was approximately equal to the sum of our net income for the year and the proceeds of a private placement of common stock for $5,000,000. Our working capital as of November 30, 2015 was $8,889,704, which represented an increase of $5,839,436 during the fiscal year then ended.
For the year ended November 30, 2016, our principal investing activity was the purchase of a 30% interest in Tianmei Australia for $995,811. In addition, we purchased equipment for $123,352. Our investing cash flows for the years ended November 30, 2015 and 2014 consisted of the purchase of fixed assets for $52,485 and $6,676, respectively. As we develop our physical presence by investing in retail stores, cash used in investing activities will increase, and may require expansion of our cash flows from financing activities.
For the years ended November 30, 2016 and 2015, our financing activities provided $9,848,200 and $5,000,000, respectively, which represented the proceeds of two private placements of our common stock during fiscal year 2016 and one during fiscal year 2015. We had no financing activities during the year ended November 30, 2014.
During the year ended November 30, 2016, although we recorded net income of $13,574,670, our operations used $2,835,968 in net cash. The primary reasons for the difference were:
| ● | Our net income included $4,536,760 attributable to our investment in Guangdong Tianmei, which represents our 30% equity interest in the net income of Guangdong Tianmei for the year. |
| | | |
| ● | The marked increase in our sales volume during the fourth quarter of fiscal 2016 caused our accounts receivable balance at November 30, 2016 to significantly increase by approximately $10,937,143. All of these receivables have been collected since the fiscal year-end. |
| | | |
| ● | In anticipation of further growth, we increased the balance of our prepaid expenses by $5,338,682, to assure delivery of inventories as needed. Included in that amount was $4,929,890 in promotion fees prepaid to certain of our distributors. Those prepaid fees will be amortized over the terms of our distribution agreements. |
These uses of cash were partially offset by a $3,367,174 increase in our accounts payable and a $725,217 increase in our tax payable, both of which reflect the growth in our operations.
In contrast, during the year ended November 30, 2015, when the level of our operations was modest, our operations yielded $1,188,713 in net income and provided $1,037,620 in cash, as the increase in our accounts receivable during that year was modest. Likewise, our operations for the year ended November 30, 2014 yielded $793,656 in net income and provided $728,904 in cash.
We have no long-term debt or any debt other than to our shareholder. Our most significant capital commitments for the next year are:
| ● | Our greatest capital commitments in the long term are our quarterly payments to the Academy for a selenium enrichment technology and research research related to advanced selenium-enrichment techniques. Currently, we have committed to paying the Academy RMB 600,000 (US$86,724) per year for the next four years for selenium-enrichment technology and RMB 4,000,000 (US$578,160) quarterly for research related to advanced selenium-enrichment techniques for the next ten years. We expect to fund these fees from operations. |
| | | |
| ● | In the short term, over the next year we expect to renew all of our current leases, which will require prepayment of RMB 2,820,000 (US$407,603), which we expect to pay from operations. We also hope to open up to 26 new retail stores, which will require a capital commitment of US$2,309,000. |
| | | |
| ● | During fiscal year 2016 we expended $4,607,301 on promotion fees to distributors. These fees are generally paid up front on an annual basis. Because of the increase in our sales, which we believe has resulted in part from our sales team seeking out new wholesale customers and paying these promotion fees, we plan to spend more on promotion fees in the upcoming fiscal year. We have projected that we could spend up to RMB 58,104,000 (US$8,398,000) in promotion fees in the 2017 fiscal year. |
We have ample cash and our operations are profitable. In addition, we expect our recent success in collecting our entire accounts receivable balance within 60 days to be replicated, due to our close relationship with our customers. For these reasons, we do not anticipate incurring significant additional debt. Therefore, our liquidity should be adequate to sustain the full implementation of our business plan for at least the next twelve months and the foreseeable future. We intend to apply a portion of our cash resources to an increase in the number of distributors with which we have relationships. Since the settlement period for payment of invoices by the distributors is, in some cases, as long as 90 days, our cash resources will be needed to offset the gap between the cash collections and cash payments.
Application of Critical Accounting Policies
In preparing our financial statements, we are required to formulate working policies regarding valuation of our assets and liabilities and to develop estimates of those values. In our preparation of the financial statements for fiscal 2016, there was one estimate made which was (a) subject to a high degree of uncertainty and (b) material to our results:
| | ● | The determination, described in Note 2 to our Consolidated Financial Statements, to record as an asset the full value of prepaid promotional fees totaling $4,275,602 included in our prepaid expenses at November 30, 2016. The determination was based on our prior experience with the distributors to whom the fees were paid and our expectation of the continuation of those relationships. |
We made no material changes to our critical accounting policies in connection with the preparation of financial statements for fiscal 2016.
Operating Segments
We have performed an internal analysis to identify the our reportable operating segments. Various factors were assessed and we concluded that all streams of revenue use the same resources (management, HR, fixed assets, advertising, etc.). The chief operating decision maker is only provided with a breakdown of wholesale and retail revenues. Due to the operations and management being the same for both revenue streams, we believe that segmental reporting is not required.
Impact of Recent Accounting Pronouncements
New accounting rules and disclosure requirements can significantly impact the comparability of our financial statements. Please refer to Note 2 of our consolidated financial statements included in Item 8 of this Form 10-K.
In May 2016, the FASB issued ASU 2016-12 (Subtopic 606), “Revenue from Contracts with Customers.” The new standards address that an entity should recognize revenue to depict the transfer of goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. The new standard is effective for fiscal years beginning after December 15, 2017 and interim and annual reporting periods thereafter (which equates to January 1, 2018 for public entities with a December 31 year-end). Revenue is recognized when a company satisfies a performance obligation by transferring a promised good or service to a customer, which is when the customer obtains control of that good or service. We are currently evaluating the impact of our pending adoption of the new standard on our financial statements.
Except for the foregoing ASU, there were no recent accounting pronouncements that we expect to have a material effect on the Registrant’s financial position or results of operations.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition or results of operations.
Item 7A Quantitative And Qualitative Disclosures About Market Risk
Foreign Exchange Risk
Substantially all of our revenues and our expenses are denominated in RMB. We believe the impact of foreign currency risk is not material and we have not used any forward contracts, currency borrowings or derivative instruments to hedge our exposure to foreign currency exchange risk. Although in general our exposure to foreign exchange risks should be limited, the value of your investment in our shares will be affected by the foreign exchange rate between U.S. dollars and RMB because the value of our business is effectively denominated in RMB, while we use the U.S. dollar as our reporting currency and our common stock is traded in U.S. dollars. The value of the RMB against the U.S. dollar and other currencies is affected by, among other things, changes in China’s political and economic conditions and China’s foreign exchange policies.
To the extent that we need to convert U.S. dollars into RMB for our operations, acquisitions or other uses within China, appreciation of the RMB against the U.S. dollar would have an adverse effect on the RMB amount we receive from the conversion. To the extent that we seek to convert RMB into U.S. dollars, depreciation of the RMB against the U.S. dollar would have an adverse effect on the U.S. dollar amount we receive from the conversion. As of November 30, 2016, we had RMB-denominated cash, cash equivalents, and short-term investments of RMB90.3 million, which we recorded as US$13,108,340 on our balance sheet. A 10% change in the exchange rate between the RMB and the U.S. dollar would result in an increase or decrease of $1,310,834 in the U.S. dollar-denominated value of this cash, cash equivalents and short-term investments.
Interest Rate Risk
Our exposure to interest rate risk primarily relates to the interest income generated by our cash resources, which is mostly held in interest-bearing bank deposits. We have not used any derivative financial instruments to manage our interest risk exposure. Interest-earning instruments carry a degree of interest rate risk. We have not been exposed, nor do we anticipate being exposed, to material risks due to changes in interest rates. However, our future interest income may be lower than expected due to changes in market interest rates.
Item 8. Financial Statements
INDEX TO FINANCIAL STATEMENTS
Page
| F-1 | Report of Independent Registered Public Accounting Firm. |
| F-2 | Consolidated Balance Sheets as of November 30, 2016 and 2015. |
| F-4 | Consolidated Statements of Operations and Comprehensive Income for the Years Ended November 30, 2016, 2015 and 2014. |
| F-6 | Consolidated Statement of Changes in Stockholders’ Equity for the Years Ended November 30, 2016, 2015 and 2014. |
| F-8 | Consolidated Statements of Cash Flows for the Years Ended November 30, 2016, 2015 and 2014. |
| F-10 to F-32 | Notes to Consolidated Financial Statements. |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and
Stockholders of China Gewang Biotechnology, Inc. and Subsidiaries
We have audited the accompanying consolidated balance sheets of China Gewang Biotechnology, Inc. and Subsidiaries (the “Company”) as of November 30, 2016 and 2015, and the related consolidated statements of income and comprehensive income, changes in stockholders’ equity, and cash flows for each of the years in the three-year period ended November 30, 2016. China Gewang Biotechnology, Inc. and Subsidiaries’ management is responsible for these financial statements. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of China Gewang Biotechnology, Inc. and Subsidiaries as of November 30, 2016 and 2015, and the results of its operations and its cash flows for each of the years in the three-year period ended November 30, 2016, in conformity with accounting principles generally accepted in the United States of America.
/s/ Wei, Wei & Co., LLP
Flushing, New York
February 10, 2017
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS(IN U.S. $)
| | | November 30, | | | November 30, | |
| ASSETS | | 2016 | | | 2015 | |
| | | | | | | |
| Current assets: | | | | | | |
| Cash | | $ | 13,108,340 | | | $ | 8,669,034 | |
| Accounts receivable | | | 11,205,011 | | | | 267,868 | |
| Inventory | | | 107,830 | | | | 156,778 | |
| Prepaid expenses | | | 5,540,051 | | | | 201,369 | |
| | | | | | | | | |
| Total current assets | | | 29,961,232 | | | | 9,295,049 | |
| | | | | | | | | |
| Property, plant and equipment, net | | | 128,767 | | | | 65,860 | |
| | | | | | | | | |
| Other assets: | | | | | | | | |
| Deferred registration cost | | | 110,086 | | | | - | |
| Equity investment | | | 6,003,412 | | | | - | |
| | | | | | | | | |
| Total Assets | | $ | 36,203,497 | | | $ | 9,360,909 | |
See accompanying notes to the consolidated financial statements.
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS (CONTINUED)(IN U.S. $)
| | | November 30, | | | November 30, | |
| LIABILITIES AND STOCKHOLDERS’ EQUITY | | 2016 | | | 2015 | |
| | | | | | | |
| Current liabilities: | | | | | | |
| Accounts payable | | $ | 3,367,174 | | | $ | - | |
| Taxes payable | | | 789,370 | | | | 64,153 | |
| Accrued expenses and other payables | | | 193,090 | | | | 175,086 | |
| Loans from third party | | | 228,238 | | | | - | |
| Loans from stockholder | | | 237,639 | | | | 166,106 | |
| | | | | | | | | |
| Total current liabilities | | | 4,815,511 | | | | 405,345 | |
| | | | | | | | | |
| Commitments and contingencies | | | | | | | | |
| | | | | | | | | |
| Stockholders’ equity: | | | | | | | | |
| Common stock - $0.001 par value, 100,000,000 and 75,000,000 shares authorized, 75,000,000 and 45,500,000 shares issued and outstanding as of November 30, 2016 and November 30, 2015, respectively | | | 75,000 | | | | 45,500 | |
| Additional paid-in capital | | | 16,980,102 | | | | 6,525,743 | |
| Retained earnings | | | 15,026,053 | | | | 2,270,416 | |
| Statutory reserve fund | | | 759,094 | | | | 281,766 | |
| Other comprehensive (loss) | | | (1,707,064 | ) | | | (252,022 | ) |
| | | | | | | | | |
| Stockholders’ equity before noncontrolling interests | | | 31,133,185 | | | | 8,871,403 | |
| | | | | | | | | |
| Noncontrolling interests | | | 254,801 | | | | 84,161 | |
| | | | | | | | | |
| Total stockholders’ equity | | | 31,387,986 | | | | 8,955,564 | |
| | | | | | | | | |
TOTAL LIABILITIES AND STOCKHOLDERS’
EQUITY | | $ | 36,203,497 | | | $ | 9,360,909 | |
See accompanying notes to the consolidated financial statements.
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF INCOME AND COMPREHENSIVE INCOME
FOR THE YEARS ENDED NOVEMBER 30, 2016, 2015 and 2014(IN U.S. $)
| | | 2016 | | | 2015 | | | 2014 | |
| | | | | | | | | | |
| Net revenues | | $ | 45,519,999 | | | $ | 4,184,255 | | | $ | 2,368,016 | |
| Cost of goods sold | | | (28,765,888) | | | | (1,186,461) | | | | (725,449 | ) |
| | | | | | | | | | | | | |
| Gross profit | | | 16,754,111 | | | | 2,997,794 | | | | 1,642,567 | |
| | | | | | | | | | | | | |
| Operating expenses: | | | | | | | | | | | | |
| Selling and marketing | | | 3,044,135 | | | | 836,040 | | | | 473,670 | |
| General and administrative | | | 1,005,385 | | | | 528,627 | | | | 150,154 | |
| Research and development | | | 605,816 | | | | - | | | | - | |
| | | | | | | | | | | | | |
| Total operating expenses | | | 4,655,336 | | | | 1,364,667 | | | | 623,824 | |
| | | | | | | | | | | | | |
| Operating income | | | 12,098,775 | | | | 1,633,127 | | | | 1,018,743 | |
| | | | | | | | | | | | | |
| Other income | | | | | | | | | | | | |
| Interest income | | | 14,868 | | | | 13,508 | | | | 2,900 | |
| | | | | | | | | | | | | |
| Income before provision for income taxes | | | 12,113,643 | | | | 1,646,635 | | | | 1,021,643 | |
| Provision for income taxes | | | 3,075,733 | | | | 457,922 | | | | 264,553 | |
| | | | | | | | | | | | | |
| Equity in income of investee | | | 4,536,760 | | | | - | | | | - | |
| | | | | | | | | | | | | |
| Net income before noncontrolling interests | | | 13,574,670 | | | | 1,188,713 | | | | 757,090 | |
| Noncontrolling interests | | | (326,213 | ) | | | (61,790 | ) | | | (38,952 | ) |
| | | | | | | | | | | | | |
| Net income attributable to common stockholders | | $ | 13,248,457 | | | $ | 1,126,923 | | | $ | 718,138 | |
| | | | | | | | | | | | | |
| Earnings per common share | | $ | 0.20 | | | $ | 0.03 | | | $ | 0.02 | |
| | | | | | | | | | | | | |
| Weighted average shares outstanding | | | 65,441,257 | | | | 40,944,444 | | | | 35,500,000 | |
See accompanying notes to the consolidated financial statements.
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF INCOME AND COMPREHENSIVE INCOME (CONTINUED)
FOR THE YEARS ENDED NOVEMBER 30, 2016, 2015 and 2014(IN U.S. $)
| | | 2016 | | | 2015 | | | 2014 | |
| | | | | | | | | | |
| Comprehensive income | | | | | | | | | |
| Net income before noncontrolling interests | | $ | 13,574,670 | | | $ | 1,188,713 | | | $ | 757,090 | |
| Foreign currency translation adjustment | | | (1,455,633 | ) | | | (315,020 | ) | | | 1,333 | |
| | | | | | | | | | | | | |
| Total comprehensive income | | | 12,119,037 | | | | 873,693 | | | | 758,423 | |
| Comprehensive (loss) income attributable to noncontrolling interests | | | (170,640 | ) | | | (46,039 | ) | | | (39,019 | ) |
| | | | | | | | | | | | | |
| Net comprehensive income attributable to common stockholders | | $ | 11,948,397 | | | $ | 827,654 | | | $ | 719,404 | |
See accompanying notes to the consolidated financial statements.
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENT OF CHANGES IN STOCKHOLDERS’ EQUITY
FOR THE YEARS ENDED NOVEMBER 30, 2016, 2015 and 2014(IN U.S. $)
| | | Common Stock | | | Additional Paid-in Capital | | | Retained Earnings | | | Noncon- trolling Interests | | | Statutory Reserve Fund | | | Other Comprehensive Income (Loss) | | | Total | |
Balance, November 30, 2013 | | $ | 35,500 | | | $ | 1,539,275 | | | $ | 592,024 | | | $ | 16,113 | | | $ | 65,089 | | | $ | 61,665 | | | $ | 2,309,666 | |
| Net income | | | - | | | | - | | | | 761,313 | | | | 22,839 | | | | - | | | | - | | | | 784,152 | |
| Statutory reserve | | | - | | | | - | | | | (76,131 | ) | | | - | | | | 76,131 | | | | - | | | | - | |
| Other comprehensive income | | | - | | | | - | | | | 67 | | | | - | | | | | | | | 1,226 | | | | 1,333 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance, November 30, 2014 | | | 35,500 | | | | 1,539,275 | | | | 1,277,273 | | | | 38,952 | | | | 144,454 | | | | 62,998 | | | | 3,098,452 | |
| Reverse merger adjustment | | | - | | | | (3,532 | ) | | | 3,532 | | | | - | | | | - | | | | - | | | | - | |
| Issuance of Common Stock | | | 10,000 | | | | 4,990,000 | | | | - | | | | - | | | | - | | | | - | | | | 5,000,000 | |
| Net income | | | - | | | | - | | | | 1,126,923 | | | | 61,790 | | | | - | | | | - | | | | 1,188,713 | |
| Statutory reserve | | | - | | | | - | | | | (137,312 | ) | | | - | | | | 137,312 | | | | - | | | | - | |
| Other comprehensive income | | | - | | | | - | | | | - | | | | (16,581 | ) | | | - | | | | (315,020 | ) | | | (331,601 | ) |
See accompanying notes to theconsolidated financial statements.
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENT OF CHANGES IN STOCKHOLDERS’ EQUITY
FOR THE YEARS ENDED NOVEMBER 30, 2016, 2015 and 2014(IN U.S. $)
| | | Common Stock | | | Additional Paid-in Capital | | | Retained Earnings | | | Noncon- trolling Interests | | | Statutory Reserve Fund | | | Other Comprehensive Income (Loss) | | | Total | |
Balance, November 30, 2015 | | $ | 45,500 | | | $ | 6,525,743 | | | $ | 2,270,416 | | | $ | 84,161 | | | $ | 281,766 | | | $ | (252,022 | ) | | $ | 8,955,564 | |
| Issuance of common stock | | | 29,500 | | | | 9,818,700 | | | | - | | | | - | | | | - | | | | - | | | | 9,848,200 | |
| Equity in excess of purchase price of investee under common control | | | - | | | | 466,652 | | | | - | | | | - | | | | - | | | | - | | | | 466,652 | |
| Acquisition of VIE non-controlling interest | | | - | | | | 169,007 | | | | 135,147 | | | | (264,372 | ) | | | (15,492 | ) | | | (25,757 | ) | | | (1,467 | ) |
| Net income | | | - | | | | - | | | | 13,113,310 | | | | 461,360 | | | | - | | | | - | | | | 13,574,670 | |
| Statutory reserve | | | - | | | | - | | | | (492,820 | ) | | | - | | | | 492,820 | | | | - | | | | - | |
| Other comprehensive income (loss) | | | - | | | | - | | | | - | | | | (26,348 | ) | | | - | | | | (1,429,285 | ) | | | (1,455,633 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance, November 30, 2016 | | $ | 75,000 | | | $ | 16,980,102 | | | $ | 15,026,053 | | | $ | 254,801 | | | $ | 759,094 | | | $ | (1,707,064 | ) | | $ | 31,387,986 | |
See accompanying notes to theconsolidated financial statements.
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
FOR THE YEARS ENDED NOVEMBER 30, 2016, 2015 and 2014(IN U.S. $)
| | | 2016 | | | 2015 | | | 2014 | |
| Cash flows from operating activities: | | | | | | | | | |
| Net income | | $ | 13,574,670 | | | $ | 1,188,713 | | | $ | 793,656 | |
| Adjustments to reconcile net income to net cash provided by operating activities: | | | | | | | | | | | | |
| Depreciation | | | 52,919 | | | | 32,121 | | | | 25,962 | |
| (Income) from equity investment | | | (4,536,760 | ) | | | - | | | | - | |
| Changes in operating assets and liabilities: | | | | | | | | | | | | |
| (Increase) in accounts receivable | | | (10,937,143 | ) | | | (267,868 | ) | | | | |
| Decrease (increase) in inventory | | | 48,948 | | | | (102,782 | ) | | | (53,996 | ) |
| (Increase) in prepaid expenses | | | (5,338,682 | ) | | | (96,169 | ) | | | (90,470 | ) |
| Increase (decrease) in accounts payable | | | 3,367,174 | | | | (7,225 | ) | | | - | |
| (Decrease) in advances from customers | | | - | | | | (56,930 | ) | | | (14,997 | ) |
| Increase in taxes payable | | | 725,217 | | | | 16,824 | | | | 29,345 | |
| Increase in accrued expenses and other payables | | | 207,689 | | | | 330,936 | | | | 9,410 | |
| | | | | | | | | | | | | |
| Net cash (used in) provided by operating activities | | | (2,835,968 | ) | | | 1,037,620 | | | | 728,904 | |
| | | | | | | | | | | | | |
| Cash flows from investing activities: | | | | | | | | | | | | |
| Purchase of equipment | | | (123,352 | ) | | | (52,485 | ) | | | (6,676 | ) |
| Payment for equity in investee | | | (995,811 | ) | | | - | | | | - | |
| | | | | | | | | | | | | |
| Net cash (used in) investing activities | | | (1,119,163 | ) | | | (52,485 | ) | | | (6,676 | ) |
| | | | | | | | | | | | | |
| Cash flows from financing activities: | | | | | | | | | | | | |
| Proceeds from sale of common stock | | | 9,848,200 | | | | 5,000,000 | | | | - | |
| | | | | | | | | | | | | |
| Net cash provided by financing activities | | | 9,848,200 | | | | 5,000,000 | | | | - | |
| | | | | | | | | | | | | |
| Effect of exchange rate changes on cash | | | (1,453,763 | ) | | | (328,913 | ) | | | 1,366 | |
| | | | | | | | | | | | | |
| Net change in cash | | | 4,439,306 | | | | 5,656,222 | | | | 723,594 | |
| Cash, beginning | | | 8,669,034 | | | | 3,012,812 | | | | 2,289,218 | |
| | | | | | | | | | | | | |
| Cash, end | | $ | 13,108,340 | | | $ | 8,669,034 | | | $ | 3,012,812 | |
See accompanying notes to theconsolidated financial statements.
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
FOR THE YEARS ENDED NOVEMBER 30, 2016, 2015 and 2014(IN U.S. $)
| | | 2016 | | | 2015 | | | 2014 | |
| | | | | | | | | | |
| Supplemental disclosure of cash flow information: | | | | | | | | | |
| Cash paid for interest | | $ | - | | | $ | - | | | $ | - | |
| | | | | | | | | | | | | |
| Cash paid for income taxes | | $ | 2,543,744 | | | $ | 446,012 | | | $ | 238,026 | |
| | | | | | | | | | | | | |
| Additional paid-in capital – equity in excess of purchase price of investee under common control | | $ | 466,652 | | | $ | - | | | $ | - | |
| | | | | | | | | | | | | |
| Noncash financing activities: | | | | | | | | | | | | |
| Payment of accrued expenses and other payables by shareholder | | $ | 298,938 | | | $ | 159,689 | | | $ | 6,417 | |
See accompanying notes to theconsolidated financial statements.
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED NOVEMBER 30, 2016, 2015 and 2014(IN U.S. $)
China Gewang Biotechnology, Inc. (the “Company”), formerly known as Rich Star Development, was incorporated under the laws of the State of Nevada on May 29, 2009. From its inception until the closing of the reverse merger described below, the Company was a development-stage company in the business ofsourcing and distributing food products, paper products, janitorial products, restaurant utensils and equipment to the food service industry in the PRC.
On April 20, 2015, the Company completed a reverse merger transaction through a share exchange with the stockholders of Biotechnology International Holding Ltd. (“Biotechnology International”), whereby the Company acquired 100% of the outstanding shares of Biotechnology International in exchange for 32,000,000 shares of its common stock, representing 90.14% of the issued and outstanding shares of common stock. As a result of the reverse merger, Biotechnology International became the Company’s wholly-owned subsidiary and the former Biotechnology International stockholders became our controlling stockholders. The share exchange transaction was treated as a reverse acquisition, with Biotechnology International as the acquirer and the Company as the acquired party for accounting purposes.
On January 8, 2015, the Company filed a certificate of amendment to its articles of incorporation to change its name from “Rich Star Development” to “China Gewang Biotechnology, Inc.”
On July 20, 2016 the Company filed with the Nevada Secretary of State a Certificate of Amendment to Articles of Incorporation. The Certificate of Amendment increased the number of authorized shares of common stock from 75 million to 100 million.
Majority-owned subsidiary: Guangdong Gewang
As a result of the transaction with Biotechnology International, the Company owns all of the issued and outstanding common stock of Hong Kong Gewang Holdings Group Limited (“Hong Kong Gewang”), a wholly-owned subsidiary of Biotechnology International, which in turn owns all of the issued and outstanding common stock of Gewang Selenium Enrichment Information Consulting (Shenzhen) Co., Ltd. (“Gewang Selenium”). Before August 8, 2016, the Company effectively and substantially controlled Guangdong Gewang Biotechnology Co., Ltd. (“Guangdong Gewang”) through a series of captive agreements between Guangdong Gewang and Gewang Selenium. Guangdong Gewang, incorporated under the laws of the People’s Republic of China (“PRC”) on June 2010, is primarily engaged in the sale of selenium supplements within the PRC. It is a member of the Chinese Selenium Supplements Association.
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED NOVEMBER 30, 2016, 2015 and 2014(IN U.S. $)
| 1. | ORGANIZATION (continued) |
On July 13, 2016, Gewang Selenium exercised its option to purchase all of the registered equity of Guangdong Gewang. The purchase price paid for the equity was RMB10,000 (approximately $1,500). The equity was purchased from Shili Zhang, Yun Zeng and Wei Xu. Shili Zhang was the Company’s CEO until April 8, 2016 and is the father of Mengdi Zhang, who was the beneficial owner of 22.7% of the Company's outstanding common stock at the time of the sale on July 13, 2016. The other two sellers are not affiliated with the Company.
Upon application to the provincial government for registration of the transfer of equity, the Company was informed that Gewang Selenium would not be permitted to own 100% of Guangdong Gewang. Therefore the parties modified the exercise of the option to provide that Gewang Selenium would purchase only 98% of the registered equity of Guangdong Gewang. The purchase price paid for the equity was RMB 9,800 (approximately $1,500). The remaining 2% of the registered equity was then sold by Yun Zeng to Haiping Wu for a price of RMB 200,000 (approximately $30,400), which equaled 2% of the registered equity of Guangdong Gewang. Haiping Wu is a Director of Guangdong Gewang. The acquisition, as modified, was then approved by the provincial government on August 8, 2016.
Prior to the acquisition, Gewang Selenium controlled Guangdong Gewang through a series of contractual agreements, which made Guangdong Gewang a variable interest entity, the effect of which was to cause the balance sheet and operating results of Guangdong Gewang to be consolidated with those of Gewang Selenium in the Company's financial statements. As a result of the acquisition by Gewang Selenium of registered ownership of Guangdong Gewang, the balance sheet and operating results of Guangdong Gewang will hereafter continue to be consolidated with those of Gewang Selenium as its majority-owned subsidiary. The previous non-controlling interest was reclassified to additional paid-in-capital.
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED NOVEMBER 30, 2016, 2015 and 2014(IN U.S. $)
| 1. | ORGANIZATION (continued) |
Equity investment: Guangdong Tianmei
On April 28, 2016, the Company's wholly-owned subsidiary, Biotechnology International, entered into an investment agreement with Guangdong Tianmei Selenium-Rich Beverage Chain Co., Ltd. (“Guangdong Tianmei”). Guangdong Tianmei was organized in May 2015, and is engaged in the business of distributing selenium-rich bottled water and also functions as a placement agent for a variety of products from various manufacturers, all within the PRC. The investment agreement provided that Biotechnology International would pay US$1,000,000 to acquire a 30% interest in an Australian corporation to be formed, which would indirectly own all of the equity in Guangdong Tianmei.
The foregoing acquisition by Biotechnology International of 30% of Tianmei Beverage Group Corporation Limited, an Australian corporation ("Tianmei Australia"), was completed in May 2016, at which time Tianmei Australia acquired ownership, through subsidiaries, of Guangdong Tianmei. The $1,000,000 purchase price was paid in full on June 17, 2016.
As a result of the entry into the foregoing agreements, the Company has a corporate structure which is as follows:

CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED NOVEMBER 30, 2016, 2015 and 2014(IN U.S. $)
| 2. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES |
Basis of accounting and presentation
The accompanying consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America. The consolidated financial statements include those of the Company and its subsidiaries. All significant intercompany accounts and transactions have been eliminated in consolidation.
The Company uses the equity method of accounting for its equity investments in Tianmei Australia. The investment was under common control and can be significantly influenced. Under the equity method, investments are carried at cost and increased or decreased by the Company’s pro-rata share of earnings or losses. The carrying costs of these investments is also increased or decreased to reflect additional contributions or withdrawals of capital. Any difference in the book equity and the Company’s pro-rata share of the net assets of the investment will be reported as gain or loss at the liquidation of the investment. Losses in excess of the investments are recorded when the Company is committed to provide additional financial support. The Company uses the equity method for investment of 30% because the Company has the ability to exercise significant influence over these entities.
All consolidated financial statements and notes to the consolidated financial statements are presented in United States dollars (“US Dollar” or “US$” or “$”).
Use of estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect certain reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting periods. Actual results could differ from those estimates.
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED NOVEMBER 30, 2016, 2015 and 2014(IN U.S. $)
| 1. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued) |
Foreign currency translations
Almost all of the Company assets are located in the PRC. The functional currency for the Company’s operations is the Renminbi (“RMB”). The Company uses the United States Dollar (“US Dollar” or “US$” or “$”) for financial reporting purposes. The financial statements of the Company have been translated into US Dollars in accordance with Financial Accounting Standards Board ("FASB”) Accounting Standards Update ("ASU") Section 830, “Foreign Currency Matters.”
All asset and liability accounts have been translated using the exchange rate in effect at the balance sheet date. Equity accounts have been translated at their historical exchange rates when the capital transactions occurred. Statements of income (loss) and comprehensive income (loss), changes in stockholders’ equity and cash flows have been translated using the average exchange rate for the periods presented. Adjustments resulting from the translation of the Company’s financial statements are recorded as other comprehensive income (loss).
The exchange rates used to translate amounts in RMB into US Dollars for the purposes of preparing the financial statements are as follows:
| | | | November 30, 2016 | | | November 30, 2015 | | | November 30, 2014 | |
| | | | | | | | | | | | | | |
| | Balance sheet items, except for stockholders’ equity, as of year end | | | 0.1452 | | | | 0.1561 | | | | 0.1631 | |
| | | | Year Ended November 30, | |
| | | | 2016 | | | 2015 | | | 2014 | |
| | | | | | | | | | | | | | |
| | Amounts included in the statements of income and comprehensive income, changes in stockholders’ equity and cash flows for the year presented | | | 0.1515 | | | | 0.1610 | | | | 0.1628 | |
For the years ended November 30, 2016, 2015 and 2014, foreign currency translation adjustments of $(1,455,633), $(315,020), and $1,333, respectively, have been reported as other comprehensive income (loss). Other comprehensive income (loss) of the Company consists entirely of foreign currency translation adjustments.
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED NOVEMBER 30, 2016, 2015 and 2014(IN U.S. $)
| 2. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued) |
Foreign currency translations (continued)
Although PRC government regulations now allow convertibility of the RMB for current account transactions, significant restrictions still remain. Hence, such translations should not be construed as representations that the RMB could be converted into US Dollars at that rate or any other rate.
The value of the RMB against the US Dollar and other currencies may fluctuate and is affected by, among other things, changes in the PRC’s political and economic conditions. Any significant revaluation of the RMB may materially affect the Company’s financial condition in terms of US Dollar reporting. In August 2015, the PRC devalued its currency by approximately 3.5%; in January 2016 the PRC devalued its currency by an additional 0.5%; from February 2016 to November 2016, the PRC devalued its currency by an additional 7%. Further devaluations of its currency could occur.
Revenue recognition
Revenues are primarily derived from selling selenium supplements and selenium products to wholesale customers, contract distributors, and from our retail stores. The Company’s revenue recognition policies comply with FASB ASC 605 “Revenue Recognition.” The Company recognizes revenue when the following fundamental criteria are met: (i) persuasive evidence of an arrangement exists, (ii) delivery has occurred, (iii) the price paid by the customer is fixed or determinable and (iv) collection of the resulting account receivable is reasonably assured. The Company recognizes revenue for sales upon transfer of title to the customers. Customer purchase orders and/or contracts are generally used to determine the existence of an arrangement. Shipping documents and the completion of any customer acceptance requirements, when applicable, are used to verify product delivery. The Company assesses whether a price is fixed or determinable based upon the payment terms associated with the transaction and whether the sales price is subject to refund or adjustment. For manufacturing defects identified by customers at acceptance, the Company will return the goods to the manufacturer and receive replacements. The Company has no product returns or sales discounts and allowances because goods delivered and accepted by customers are not returnable.
Wholesale Revenue
Wholesale revenue is recognized when title to the product is transferred to the distributors. Title is transferred upon receipt at the distributors’ locations, as determined by the specific sales terms.
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED NOVEMBER 30, 2016, 2015 and 2014(IN U.S. $)
| 2. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued) |
Revenue recognition (continued)
Wholesale Revenue (continued)
The Company pays distributors certain incentives for promoting and placing its products, which allows the Company to quickly expand its distribution network and sales volume. The costs associated with these incentives is deducted from gross revenue in the consolidated statements of income and comprehensive income
Retail Revenue
Company-operated retail store revenues are recognized when payment is tendered at the point of sale.
Franchise Revenue
In June 2016, the Company commenced franchising the use of the Company's trademark, name identification and other business resources. The franchisee is required to pay franchise fees and management fees to the Company. Franchise fees are recognized only when all material services or conditions relating to the sale have been substantially performed or satisfied by the Company. In September 2016, the Company terminated its two franchise agreements for marketing reasons. The Company returned the total franchise fees to the franchisees and kept the management fees of $35,290 as per the termination agreements.
The Company’s net revenues for the years ended November 30, 2016, 2015 and 2014 were comprised as follows:
| | | | 2016 | | | 2015 | | | 2014 | |
| | | | | | | | | | | |
| | Wholesale gross revenue-selenium supplements | | $ | 8,600,092 | | | $ | 2,945,440 | | | $ | 1,930,057 | |
| | Wholesale gross revenue-selenium products | | | 36,306,396 | | | | - | | | | - | |
| | Less: promotion fees-selenium products, | | | (3,745,883 | ) | | | - | | | | - | |
| | | | | | | | | | | | | | |
| | Wholesale revenues, net | | | 41,160,605 | | | | 2,945,440 | | | | 1,930,057 | |
| | Retail revenue-selenium supplements | | | 2,946,182 | | | | - | | | | - | |
| | Retail revenues-selenium products | | | 1,377,922 | | | | 1,238,815 | | | | 437,959 | |
| | Management fee revenues | | | 35,290 | | | | - | | | | - | |
| | | | | | | | | | | | | | |
| | | | $ | 45,519,999 | | | $ | 4,184,255 | | | $ | 2,368,016 | |
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED NOVEMBER 30, 2016, 2015 and 2014(IN U.S. $)
| 2. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued) |
Shipping costs
Shipping costs incurred by the Company are recorded as selling expenses. Shipping costs for the years ended November 30, 2016, 2015 and 2014 were $96,969, $44,326 and $27,545, respectively.
Advertising costs
Advertising costs are charged to operations when incurred. For the years ended November 30, 2016, 2015 and 2014, advertising expenses were $619,447, $82,110 and $70,013, respectively.
Cash and cash equivalents
The Company considers all demand and time deposits and all highly liquid investments with an original maturity of three months or less to be cash equivalents.
Accounts receivable
Accounts receivable are recorded at the contract amount after deduction of trade discounts and, allowances, if any, and do not bear interest. The allowance for doubtful accounts, when necessary, is the Company’s best estimate of the amount of probable credit losses from accounts receivable. The Company determines the allowance based on historical write-off experience, the level of past-due accounts based on the contractual terms of the receivable, the relationship with the customer and current economic conditions.
Account balances are charged off against the allowance after all means of collection have been exhausted and the potential for recovery is considered remote. The Company does not have any off-balance-sheet credit exposure related to its customers.
As of November 30, 2016, 2015 and 2014, accounts receivable were $11,205,011, $267,868 and $0, respectively. The increase is primarily due to the recent sales with new wholesale distributors which has been subsequently fully collected. Therefore, the Company determined that an allowance for doubtful accounts was not necessary. Historically, the Company did not have any uncollectable accounts receivable.
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED NOVEMBER 30, 2016, 2015 and 2014(IN U.S. $)
| 2. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued) |
Inventory
Inventory, comprised principally of boxed selenium capsules, selenium-glossy ganoderma capsules and selenium powder, is valued at the lower of cost or market. The value of inventory is determined using the first-in, first-out method.
The Company periodically estimates an inventory allowance for estimated unmarketable inventories when necessary. Inventory amounts are reported net of such allowances, if any. There were no allowances for inventory as of November 30, 2016, 2015 and 2014.
Fair value of financial instruments
FASB ASC 820,“Fair Value Measurement” specifies a hierarchy of valuation techniques based upon whether the inputs to those valuation techniques reflect assumptions other market participants would use based upon market data obtained from independent sources (observable inputs). In accordance with ASC 820, the following summarizes the fair value hierarchy:
| | Level 1 Inputs – | Unadjusted quoted market prices for identical assets and liabilities in an active market that the Company has the ability to access. |
| | Level 2 Inputs – | Inputs other than the quoted prices in active markets that are observable either directly or indirectly. |
| | Level 3 Inputs – | Inputs based on valuation techniques that are both unobservable and significant to the overall fair value measurements. |
ASC 820 requires the use of observable market data, when available, in making fair value measurements. When inputs used to measure fair value fall within different levels of the hierarchy, the level within which the fair value measurement is categorized is based on the lowest level input that is significant to the fair value measurement. Valuation techniques used need to maximize the use of observable inputs and minimize the use of unobservable inputs.
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED NOVEMBER 30, 2016, 2015 and 2014(IN U.S. $)
| 2. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued) |
Fair value of financial instruments (continued)
The Company did not identify any assets or liabilities that are required to be presented at fair value on a recurring basis. Carrying values of non-derivative financial instruments, including cash and cash equivalents, accounts receivable, inventory, prepaid expenses, equity investment, accounts payable, taxes payable, accrued liabilities and other payables, and loan from stockholder, approximated their fair values due to the short nature of these financial instruments. There were no changes in methods or assumptions during the periods presented.
Prepaid expenses
Prepaid expenses primarily consist of promotion expenses, rent, advertising expenses and licensing fees.
Prepaid promotion expenses represent prepayments made to resellers for distributing products to retail stores. In March 2016, the Company entered into agreements with four distributors. In June, July and September 2016, the Company entered into agreements with another four distributors. Prepaid promotion expenses as of November 30, 2016, 2015 and 2014 were $4,275,602, $0 and $0, respectively.
On January 5, 2011, the Company entered into a license agreement for the technology utilized for the manufacture of its products from an unrelated third party for five years from January 2011 to December 2015. On December 30, 2015, the Company renewed the license agreement for another five years to December 2020 for $90,872 (RMB 600,000) each year. The related prepaid licensing fees as of November 30, 2016, 2015 and 2014 were $7,259, $7,805 and $8,155, respectively. The license provides for renewal options. Since this agreement requires the advance payment of the annual licensing fee, there were no minimum payments remaining under this agreement as of November 30, 2016, 2015 and 2014.
On September 30, 2016, the Company entered into a six-month agreement with an advertising company for $908,725 (RMB 6,000,000). As of November 30, 2016, the unamortized balance of $605,816 was included in prepaid expenses on the balance sheet.
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED NOVEMBER 30, 2016, 2015 and 2014(IN U.S. $)
| 2. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued) |
Impairment of long-lived assets
The Company applies FASB ASC 360, “Property, Plant and Equipment,” which addresses the financial accounting and reporting for the recognition and measurement of impairment losses for long-lived assets. In accordance with ASC 360, long-lived assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. The Company will recognize the impairment of long-lived assets in the event the net book value of such assets exceeds the future undiscounted cash flows attributable to those assets. No impairment of long-lived assets was recognized for the years ended November 30, 2016, 2015 and 2014.
Statutory reserve fund
Pursuant to corporate law of the PRC, the Company is required to transfer 10% of its net income, as determined under PRC accounting rules and regulations, to a statutory reserve fund until such reserve balance reaches 50% of the Company’s registered capital. The statutory reserve fund is non-distributable other than during liquidation and can be used to fund previous years’ losses, if any, and may be utilized for business expansion or used to increase registered capital, provided that the remaining reserve balance after use is not less than 25% of registered capital. The statutory reserve fund was $759,094, $281,766, and $144,454 as of November 30, 2016, 2015 and 2014, respectively. As of November 30, 2016, the required statutory reserve fund has been fully funded.
Income taxes
The Company accounts for income taxes in accordance with FASB ASC 740, “Income Taxes” (“ASC 740”), which requires the recognition of deferred income taxes for differences between the basis of assets and liabilities for financial statement and income tax purposes. Deferred tax assets and liabilities represent the future tax consequences for those differences, which will either be taxable or deductible when the assets and liabilities are recovered or settled. Deferred taxes are also recognized for operating losses that are available to offset future taxable income. A valuation allowance is established when necessary to reduce deferred tax assets to the amount expected to be realized.
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED NOVEMBER 30, 2016, 2015 and 2014(IN U.S. $)
| 2. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued) |
Income taxes (continued)
ASC 740 addresses the determination of whether tax benefits claimed or expected to be claimed on a tax return should be recorded in the financial statements. Under ASC 740, the Company may recognize the tax benefit from an uncertain tax position only if it is more likely than not that the tax position will be sustained on examination by the taxing authorities, based on the technical merits of the position. The tax benefits recognized in the financial statements from such a position would be measured based on the largest benefit that has a greater than 50% likelihood of being realized upon ultimate settlement. ASC 740 also provides guidance on de-recognition of income tax assets and liabilities, classification of current and deferred income tax assets and liabilities, and accounting for interest and penalties associated with tax positions. As of November 30, 2016, 2015 and 2014, the Company does not have a liability for any unrecognized tax benefits. The Company’s tax filings are subject to examination by the tax authorities. The tax years of 2103, 2014 and 2015 remain open to examination by tax authorities in the PRC.
The income tax laws of various jurisdictions in which the Company and its subsidiaries operate are summarized as follows:
United States
The Company is subject to United States tax at graduated rates from 15% to 35%. No provisions for income tax in the United States have been made as the Company had no U.S. taxable income for the years ended November 30, 2016, 2015 and 2014.
British Virgin Islands (“BVI”)
Biotechnology International is incorporated in the BVI and is governed by the income tax laws of the BVI. According to current BVI income tax law, the applicable income tax rate for the Company is 0%.
Hong Kong
Hong Kong Gewang is incorporated in Hong Kong. Pursuant to the income tax laws of Hong Kong, the Company is not subject to tax on non-Hong Kong source income.
The People's Republic of China (“PRC”)
Gewang Selenium and Guangdong Gewang are subject to an Enterprise Income Tax at 25% and file their own tax returns.
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED NOVEMBER 30, 2016, 2015 and 2014(IN U.S. $)
| 3. | RECENTLY ISSUED ACCOUNTING STANDARDS |
In May 2016, the FASB issued ASU 2016-12 (Subtopic 606), “Revenue from Contracts with Customers.” The new standards address that an entity should recognize revenue to depict the transfer of goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. The new standard is effective for fiscal years beginning after December 15, 2017 and interim and annual reporting periods thereafter (which equates to January 1, 2018 for public entities with a December 31 year-end). Revenue is recognized when a company satisfies a performance obligation by transferring a promised good or service to a customer, which is when the customer obtains control of that good or service. We are currently evaluating the impact of our pending adoption of the new standard on our financial statements.
In February 2016, the FASB issued ASU 2016-02, “Leases.” The new standard establishes a right-of-use (“ROU”) model that requires a lessee to record a ROU asset and a lease liability on the balance sheet for all leases with terms longer than 12 months. Leases will be classified as either finance or operating, with classification affecting the pattern of expense recognition in the income statement. The new standard is effective for fiscal years beginning after December 15, 2018, including interim periods within those fiscal years. A modified retrospective transition approach is required for lessees for capital and operating leases existing at, or entered into after, the beginning of the earliest comparative period presented in the financial statements, with certain practical expedients available. We do not believe this will have a material impact on our consolidated financial statements as currently all leases are prepaid.
In July 2015, the FASB issued ASU 2015-11 (Subtopic 330), “Simplifying the Measurement of Inventory,” which provides guidance to companies who account for inventory using either the first-in, first-out (“FIFO”) or average cost methods. The guidance states that companies should measure inventory at the lower of cost or net realizable value. Net realizable value is defined as the estimated selling prices in the ordinary course of business, less reasonably predictable costs of completion, disposal and transportation. ASU 2015-11 is effective for fiscal years beginning after December 15, 2016. Early adoption is permitted. This accounting standard update is not expected to have a material impact on the Company’s consolidated financial statements.
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED NOVEMBER 30, 2016, 2015 and 2014(IN U.S. $)
| 3. | RECENTLY ISSUED ACCOUNTING STANDARDS (continued) |
In January 2015, the FASB issued ASU 2015-01 (Subtopic 225-20), “Income Statement – Extraordinary and Unusual Items.” This ASU addressed the simplification of income statement presentation by eliminating the concept of extraordinary items. The amendments in this update are effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2015. A reporting entity may apply the amendments prospectively. A reporting entity also may apply the amendments retrospectively to all prior periods presented in the financial statements. Early adoption is permitted provided that the guidance is applied from the beginning of the fiscal year of adoption. This accounting standard update is not expected to have a material impact on the Company’s consolidated financial statements.
In August 2014, the FASB issued authoritative guidance that requires an entity’s management to evaluate whether there are conditions or events, considered in the aggregate, that raise substantial doubt about the entity’s ability to continue as a going concern and requires additional disclosures if certain criteria are met. This guidance is effective for fiscal periods ending after December 15, 2016, with early adoption permitted. This accounting standard update is not expected to have a material impact on the Company’s consolidated financial statements.
| 4. | RELATED PARTY TRANSACTIONS |
The Company entered into a promotion agreement with Guangdong Tianmei, in which it holds an indirect 30% ownership interest. Promotions expenses incurred during the year ended November 30, 2016, 2015 and 2014 in connection with this relationship were $626,147, $0, and $0, respectively.
The Company entered into an agreement with Guangdong Tianmei on June 10, 2015 to license the use of the Company’s trademark for 10 years. Trademark revenue recorded for the years ended November 30, 2016, 2015 and 2014 were $1,470, $0 and $0, respectively. The future commitment is approximately $1,500 each year.
Equity investment
On April 28, 2016, the Company's wholly-owned subsidiary, Biotechnology International, entered into an investment agreement with Guangdong Tianmei. At that time, 88% of the equity in Guangdong Tianmei was owned by two individuals who together directly or indirectly owned over 60% of the Company's outstanding shares. The investment agreement provided that Biotechnology International would pay US$1,000,000 to acquire a 30% interest in an Australian corporation to be formed, which would indirectly own all of the equity in Guangdong Tianmei.
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED NOVEMBER 30, 2016, 2015 and 2014(IN U.S. $)
| 4. | RELATED PARTY TRANSACTIONS (continued) |
Equity investment (continued)
The acquisition by Biotechnology International of 30% of Tianmei Australia was completed in May 2016, at which time Tianmei Australia acquired ownership, through subsidiaries, of Guangdong Tianmei. The investment agreement provided that payment of the $1,000,000 purchase price was due on June 20, 2016, which was paid in full on June 17, 2016.
The net worth of Guangdong Tianmei at the time of the acquisition was $4,888,840, 30% of which was $1,466,652. Because the Company and Guangdong Tianmei were under common control at the time of the acquisition, the $466,652 by which the Company's share of the net book value of Guangdong Tianmei exceeded the purchase price has been recorded as an increase to additional paid-in capital.
The changes in the equity investment are summarized as follows:
| | | | November 30, 2016 | | | November 30, 2015 | | | November 30, 2014 | |
| | | | | | | | | | | |
| | Initial investment | | $ | 1,466,652 | | | $ | - | | | $ | - | |
| | Pro rata share of net income | | | 4,536,760 | | | | - | | | | - | |
| | | | | | | | | | | | | | |
| | Investment, end of year | | $ | 6,003,412 | | | $ | - | | | $ | - | |
The following is a summary of balance sheet of the investee for the year ended November 30, 2016:
| | Current assets | | $ | 41,948,599 | |
| | | | | | |
| | Noncurrent assets | | $ | 1,321,713 | |
| | | | | | |
| | Current liabilities | | $ | 14,760,307 | |
| | | | | | |
| | Noncurrent liabilities | | $ | - | |
| | | | | | |
| | Equity | | $ | 28,510,005 | |
The following is a summary of results of operations of the investee for the period from the acquisition date to November 30, 2016:
| | Revenue | | $ | 41,285,348 | |
| | Cost of revenue | | | (15,168,370 | ) |
| | Expenses | | | (10,994,445 | ) |
| | | | | | |
| | Net income | | $ | 15,122,533 | |
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED NOVEMBER 30, 2016, 2015 and 2014(IN U.S. $)
The Company leased its warehouse and office space from an unrelated third party under a one-year operating lease, which expired on July 1, 2016. The lease required the Company to prepay the total rent of $90,872 (RMB 600,000) in advance for one year. On June 29, 2016, the Company renewed the lease, which commenced on July 2, 2016 and expires on July 1, 2017.
The following leases terminated during the year:
| ● | The Company leased its Chancheng store from an unrelated third party. The lease, which expired on August 31, 2015, required the Company to prepay the rent of $41,801 (RMB 276,000) in advance for one year. The Company renewed this lease to August 31, 2016 and prepaid the rent of $54,523 (RMB 360,000) in advance for one year. On May 31, 2016, the Company terminated the lease with a $4,576 settlement fee. |
| ● | The Company leased its Xiamen store from an unrelated third party. The lease expired on June 1, 2016 and the Company decided not to renew the lease. |
| ● | The Company also leased its Changsha store from an unrelated third party. The lease, which was to expire on October 7, 2018, required the Company to prepay the rent of $63,611 (RMB 420,000) in advance for one year. On May 31, 2016, the Company terminated the lease without any settlement fee. |
The following leases remained in effect at November 30, 2016:
| ● | The Company leases its flagship store in Guangzhou from an unrelated third party. The lease commenced on June 1, 2016 and expires on May 31, 2017. The lease required the Company to prepay the rent of $145,396 (RMB 960,000) in advance for one year. The Company paid the rent in June 2016. |
| ● | The Company leases its Foshan store, Longyan store and Zhuzhou store from three unrelated third parties. All three leases commenced on June 1, 2016 and expire on May 31, 2017. These leases each required the Company to prepay the rent of $63,611 (RMB 420,000) in advance for one year. The Company fully paid the rent in June 2016. Since these leases require the advance payment of the annual rent, there are no minimum payments remaining under these leases. |
Prepaid lease payments were $221,123 and $179,515 at November 30, 2016 and 2015, respectively. Rent expense for the years ended November 30, 2016, 2015 and 2014 was $345,315, $181,125 and $44,501, respectively.
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED NOVEMBER 30, 2016, 2015 and 2014(IN U.S. $)
Fixed assets as of November 30, 2016 and 2015 are summarized as follows:
| | | | 2016 | | | 2015 | |
| | | | | | | | |
| | Electronic equipment | | $ | 125,850 | | | $ | 68,733 | |
| | Motor vehicles | | | 121,151 | | | | 69,714 | |
| | Office equipment | | | 12,031 | | | | 12,936 | |
| | | | | | | | | | |
| | | | | 259,032 | | | | 151,383 | |
| | Less: accumulated depreciation | | | (130,265 | ) | | | (85,523 | ) |
| | | | | | | | | | |
| | Fixed assets - net | | $ | 128,767 | | | $ | 65,860 | |
For the years ended November 30, 2016, 2015 and 2014, depreciation expense was $52,919 $32,121 and $25,962, respectively.
The Company obtained demand and non-interest bearing loans from a former officer and stockholder, who resigned from office on April 8, 2016. The loans of $228,238 and $0 at November 30, 2016 and 2015, respectively, are reflected as loans from third party.
The Company obtained demand and non-interest bearing loans from one of its stockholders. The loans of $237,639 and $166,106 at November 30, 2016 and 2015, respectively, are reflected as loans from stockholder.
The provision for income taxes for the years ended November 30, 2016, 2015 and 2014 consisted of the following:
| | | | 2016 | | | 2015 | | | 2014 | |
| | | | | | | | | | | |
| | Current | | $ | 3,075,733 | | | $ | 457,922 | | | $ | 264,553 | |
| | Deferred | | | - | | | | - | | | | - | |
| | | | | | | | | | | | | | |
| | | | $ | 3,075,733 | | | $ | 457,922 | | | $ | 264,553 | |
No provisions for income taxes in the United States have been made. The Company did not generate any income in the United States or otherwise have any U.S. taxable income. The Company does not believe that it has any U.S. Federal income tax liabilities with respect to any transactions that the Company or any of its subsidiaries may have engaged in through November 30, 2016. However, there can be no assurance that the Internal Revenue Service (“IRS”) will agree with this position, and therefore the Company ultimately could be liable for U.S. Federal income taxes, interest and penalties. The tax years ended November 30, 2015, December 31, 2014, 2013 and 2012 remain open to examination by the IRS.
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED NOVEMBER 30, 2016, 2015 and 2014(IN U.S. $)
| 8. | INCOME TAXES (continued) |
The Company did not file on time its U.S. federal income tax returns, including, without limitation, information returns on IRS Form 5471, “Information Return of U.S. Persons with Respect to Certain Foreign Corporations” for the short year tax return ended November 30, 2015 required to be filed as a result of the change in fiscal year. Failure to furnish any income tax returns and information returns with respect to any foreign business entity required, within the time prescribed by the IRS, subjects the Company to certain civil penalties. Management is of the opinion that penalties, if any, that may be assessed would not be material to the consolidated financial statements.
| 9. | CONCENTRATION OF CREDIT AND BUSINESS RISKS |
Cash and cash equivalents
Substantially all of the Company’s assets and bank accounts are in banks located in the PRC and are not covered by protection similar to that provided by the FDIC on funds held in United States banks.
Major customers
For the year ended November 30, 2016, three customers accounted for 48% of total sales. For the year ended November 30, 2015, no customers accounted for over 10% of total sales. For the year ended November 30, 2014, three customers accounted for 35% of revenue. As of November 30, 2016, three customers accounted for 64% of trade accounts receivable. As of November 30, 2015, seven customers accounted for 90% of accounts receivable. There was no accounts receivable as of November 30,2014.
Vulnerability Due to Operations in the PRC
The Company’s operations may be adversely affected by significant political, economic and social uncertainties in the PRC. Although the PRC government has been pursuing economic reform policies for more than twenty years, no assurance can be given that the PRC government will continue to pursue such policies or that such policies may not be significantly altered, especially in the event of a change in leadership, social or political disruption or unforeseen circumstances affecting the PRC’s political, economic and social conditions. There is also no guarantee that the PRC government’s pursuit of economic reforms will be consistent or effective. The economy in the PRC has recently started to narrow.
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED NOVEMBER 30, 2016, 2015 and 2014(IN U.S. $)
| 10. | ISSUANCE OF COMMON STOCK |
On January 18, 2016 the Company sold an aggregate of 12,000,000 shares of common stock to four individuals in a private offering. None of the purchasers were affiliated with the Company. The purchase price for the shares was three RMB (approximately US$0.4561) per share, or a total of 36 million RMB (approximately US$5,473,200). The purchase price was paid by the investors to Guangdong Gewang, which at that time was managed by the Company’s wholly-owned subsidiary and accounted for as a variable interest entity.
On May 16, 2016 the Company sold an aggregate of 17,500,000 shares of common stock to two entities in a private offering. Neither of the purchasers were affiliated with the Company. The purchase price for the shares was US$0.25 per share, or a total of US$4,375,000. The purchase price was paid by the investors to Guangdong Gewang, which at that time was managed by a wholly-owned subsidiary of the Company and accounted for as a variable interest entity.
| 11. | COMMITMENTS AND CONTINGENCIES |
On December 30, 2015, the Company entered into a technology usage agreement with the Academy of Agricultural Sciences of Shandong Province (the "Academy") for the right of using a non-patented selenium-enrichment technology for its supplements manufacturing. The agreement commenced on December 30, 2015 and expires on December 29, 2020. Annual fee of RMB 600,000, approximately $87,000, is required to be paid in advance by the agreement before December 30 each year.
As of September 1, 2016, the Company entered into a long-term agreement with the Academy. This agreement entitles the Company to the exclusive right of first refusal to use the research related to advanced selenium-enrichment techniques and technology that the Academy develops. The agreement calls for annual payments of approximately $2,324,000 (RMB 4,000,000) to be paid on a quarterly basis. For the use of the techniques and/or technology developed, there will be additional charges to be negotiated.
On July 1, 2016, the Company entered into three year agreements with four of its directors for a total of approximately $15,000 (RMB 110,000) per month.
On April 8, 2016, Guangdong Gewang entered into a Performance Salary Assessment Agreement with the Company’s Chief Executive Officer (“CEO”). The agreement states that the CEO would receive additional monthly compensation of RMB 50,000 (approximately $7,000), only when the monthly net income of Guangdong Gewang exceeds RMB 2,500,000 (approximately $363,000). The agreement commenced on April 8, 2016 and expires on April 7, 2017.
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED NOVEMBER 30, 2016, 2015 and 2014(IN U.S. $)
| 12. | PARENT COMPANY ONLY CONDENSED FINANCIAL INFORMATION |
The following is the condensed financial information of China Gewang Biotechnology, Inc., the US parent, consisting of balance sheets as of November 30, 2016 and 2015, and statements of income and cash flows for the years ended November 30, 2016, 2015 and 2014.
Condensed Balance Sheets
| | ASSETS | | November 30, 2016 | | | November 30,
2015 | |
| | | | | | | | |
| | Other receivable from Guangdong Gewang | | $ | 14,848,200 | | | $ | 5,000,000 | |
| | Investments in subsidiaries and VIE | | | 16,520,171 | | | | 3,977,483 | |
| | | | | | | | | | |
| | TOTAL ASSETS | | $ | 31,368,371 | | | $ | 8,977,483 | |
| | | | | | | | | | |
| | LIABILITIES AND stockholders’ EQUITY | | | | | | | | |
| | | | | | | | | | |
| | Current liabilities: | | | | | | | | |
| | Accrued expenses | | $ | 33,375 | | | $ | 33,375 | |
| | Loans from third party | | | 220,396 | | | | | - |
| | Loan from stockholder | | | 236,216 | | | | 156,866 | |
| | | | | | | | | | |
| | Total current liabilities | | | 489,987 | | | | 190,241 | |
| | | | | | | | | | |
| | Stockholders’ equity: | | | | | | | | |
| | Common stock, $0.001 par value, 100,000,000 and 75,000,000 shares authorized, 75,000,000 and 45,500,000 shares issued and outstanding as of November 30, 2016, 2015 and 2014, respectively | | | 75,000 | | | | 45,500 | |
| | Additional paid-in capital | | | 16,980,102 | | | | 6,525,743 | |
| | Retained earnings | | | 14,771,252 | | | | 2,270,416 | |
| | Statutory reserve fund | | | 759,094 | | | | 281,766 | |
| | Other comprehensive (loss) income | | | (1,707,064 | ) | | | (252,022 | ) |
| | | | | | | | | | |
| | Total stockholder’s equity | | | 30,878,384 | | | | 8,787,242 | |
| | | | | | | | | | |
| | TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY | | $ | 31,368,371 | | | $ | 8,977,483 | |
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED NOVEMBER 30, 2016, 2015 and 2014(IN U.S. $)
| 12. | PARENT COMPANY ONLY CONDENSED FINANCIAL INFORMATION (continued) |
Condensed Statements of Income
| | | | Year Ended November 30, | |
| | | | 2016 | | | 2015 | | | 2014 | |
| | | | | | | | | | | |
| | Revenues: | | | | | | | | | |
| | Share of earnings from investments in subsidiaries and VIE | | $ | 13,358,543 | | | $ | 1,310,747 | | | $ | 724,555 | |
| | | | | | | | | | | | | | |
| | Operating expenses: | | | | | | | | | | | | |
| | General and administrative | | | 188,852 | | | | 183,824 | | | | 6,417 | |
| | | | | | | | | | | | | | |
| | Net income | | $ | 13,169,691 | | | $ | 1,126,923 | | | $ | 718,138 | |
Condensed Statements of Cash Flows
| | | | Year Ended November 30, | |
| | | | 2016 | | | 2015 | | | 2014 | |
| | | | | | | | | | | |
| | Cash flows from operating activities: | | | | | | | | | |
| | Net income | | $ | 13,169,691 | | | $ | 1,126,923 | | | $ | 718,138 | |
| | Adjustments to reconcile net income to net cash provided by (used in) operating activities | | | | | | | | | | | | |
| | Share of earnings from investment in subsidiaries and VIE | | | (13,358,543 | ) | | | (1,310,747 | ) | | | (724,555 | ) |
| | Increase in accrued expenses and other liabilities | | | 188,852 | | | | 183,824 | | | | 6,417 | |
| | | | | | | | | | | | | | |
| | Net cash provided by (used in) operating activities | | | - | | | | - | | | | - | |
| | | | | | | | | | | | | | |
| | Net change in cash | | | - | | | | - | | | | - | |
| | Cash, beginning of year | | | - | | | | - | | | | - | |
| | | | | | | | | | | | | | |
| | Cash, end of year | | $ | - | | | $ | - | | | $ | - | |
| | | | | | | | | | | | | | |
| | Noncash financing activities: | | | | | | | | | | | | |
| | Payment of accrued expenses and other payables by shareholder | | $ | 298,938 | | | $ | 159,689 | | | $ | 6,417 | |
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED NOVEMBER 30, 2016, 2015 and 2014(IN U.S. $)
| 12. | PARENT COMPANY ONLY CONDENSED FINANCIAL INFORMATION (continued) |
Basis of Presentation
The Company records its investment in its subsidiaries and VIE under the equity method of accounting. Such investments are presented as “Investments in subsidiaries and VIE” on the condensed balance sheets and the subsidiaries and VIE profits are presented as “Share of earnings from investments in subsidiaries and VIE” on the condensed statements of income.
Certain information and footnote disclosures normally included in financial statements prepared in conformity with accounting principles generally accepted in the United States of America have been condensed or omitted. The parent only financial information has been derived from the Company’s consolidated financial statements and should be read in conjunction with the Company’s consolidated financial statements.
There were no cash transactions in the US parent company during the years ended November 30, 2016, 2015 and 2014.
Restricted Net Assets
Under the PRC laws and regulations, the Company’s PRC subsidiaries and VIE are restricted in their ability to transfer certain of their net assets to the Company in the form of dividend payments, loans or advances. The restricted net assets of the Company’s PRC subsidiaries and the VIE were approximately $31,368,000 and $8,977,000 as of November 30, 2016 and 2015, respectively.
The Company’s operations and revenues are conducted and generated in the PRC, and all of the Company’s revenues being earned and currency received are denominated in RMB. RMB is subject to the foreign exchange control regulations in China, and, as a result, the Company may be unable to distribute any dividends outside of China due to the PRC foreign exchange control regulations that restrict the Company’s ability to convert RMB into US Dollars.
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED NOVEMBER 30, 2016, 2015 and 2014(IN U.S. $)
| 12. | PARENT COMPANY ONLY CONDENSED FINANCIAL INFORMATION (continued) |
Restricted Net Assets (continued)
Schedule I of Article 5-04 of Regulation S-X requires the condensed financial information of the parent company to be filed when the restricted net assets of consolidated subsidiaries exceed 25 percent of consolidated net assets as of the end of the most recently completed fiscal year. For purposes of the above test, restricted net assets of consolidated subsidiaries shall mean that amount of the Company’s proportionate share of net assets of consolidated subsidiaries (after intercompany eliminations) which as of the end of the most recent fiscal year may not be transferred to the parent company by its subsidiaries in the form of loans, advances or cash dividends without the consent of a third party. The condensed parent company only financial statements have been prepared in accordance with Rule 12-04, Schedule I of Regulation S-X as the restricted net assets of the Company’s PRC subsidiaries exceed 25% of the consolidated net assets of the Company.
The Company’s management has performed subsequent events procedures through February 10, 2017, which is the date the consolidated financial statements were available to be issued. There were no subsequent events requiring adjustment to or disclosure in the consolidated financial statements
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
Not Applicable.
Item 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures. Our Chief Executive Officer and Chief Financial Officer carried out an evaluation of the effectiveness of the Registrant’s disclosure controls and procedures (as defined in Rule13a-15(e) promulgated by the Securities and Exchange Commission) as of November 30, 2016. The evaluation revealed that there are material weaknesses in our disclosure controls, specifically:
| | ● | The relatively small number of employees who are responsible for accounting functions prevents us from segregating duties within our internal control system. |
| | | |
| | ● | Our internal financial staff lack expertise in identifying and addressing complex accounting issued under U.S. Generally Accepted Accounting Principles. |
| | | |
| | ● | Our executive officers are not familiar with the accounting and reporting requirements of a U.S. public company. |
| | | |
| | ● | We have not developed sufficient documentation concerning our existing financial processes, risk assessment and internal controls. |
Based on their evaluation, our Chief Executive Officer and Chief Financial Officer concluded that the Registrant’s system of disclosure controls and procedures was not effective as of November 30, 2016.
We plan to continue to address these deficiencies by nominating independent directors including a “financial expert” highly experienced in internal control systems and creating an Audit Committee to address these material weaknesses. Pursuant to an Audit Committee Charter, which we plan to adopt, the members of the Audit Committee will recommend to management concrete actions to address each material weakness and review the impact of such actions to remedy the deficiencies in our internal controls.
Changes in Internal Controls. There was no change in internal controls over financial reporting (as defined in Rule 13a-15(f) promulgated under the Securities Exchange Act of 1934) identified in connection with the evaluation described in the preceding paragraph that occurred during the Registrant’s fourth fiscal quarter that has materially affected or is reasonably likely to materially affect the Registrant’s internal control over financial reporting.
Management’s Report on Internal Control over Financial Reporting
Management of the Registrant is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rule 13a-15(f) under the Securities Exchange Act of 1934. We have assessed the effectiveness of those internal controls as of November 30, 2016, using the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”) Internal Control – Integrated Framework (2013) as a basis for our assessment.
Because of inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies and procedures may deteriorate. All internal control systems, no matter how well designed, have inherent limitations. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation.
A material weakness in internal controls is a deficiency in internal control, or combination of control deficiencies, that adversely affects the Registrant’s ability to initiate, authorize, record, process, or report external financial data reliably in accordance with accounting principles generally accepted in the United States of America such that there is more than a remote likelihood that a material misstatement of the Registrant’s annual or interim financial statements that is more than inconsequential will not be prevented or detected. In the course of making our assessment of the effectiveness of internal controls over financial reporting, we identified three material weaknesses in our internal control over financial reporting. These material weaknesses consisted of:
| | ● | The relatively small number of employees who are responsible for accounting functions prevents us from segregating duties within our internal control system. |
| | | |
| | ● | Our internal financial staff lack expertise in identifying and addressing complex accounting issued under U.S. Generally Accepted Accounting Principles. |
| | | |
| | ● | Our executive officers are not familiar with the accounting and reporting requirements of a U.S. public company. |
| | | |
| | ● | We have not developed sufficient documentation concerning our existing financial processes, risk assessment and internal controls. |
We plan to continue to address these deficiencies by nominating independent directors including a “financial expert” highly experienced in internal control systems and creating an Audit Committee to address these material weaknesses. Pursuant to an Audit Committee Charter, which we plan to adopt, the members of the Audit Committee will recommend to management concrete actions to address each material weakness and review the impact of such actions to remedy the deficiencies in our internal controls.
Because of the above conditions, management’s assessment is that the Registrant’s internal controls over financial reporting were not effective as of November 30, 2016.
This annual report does not include an attestation report from the Registrant’s independent registered public accounting firm regarding internal control over financial reporting.
Item 9B Other Information
None.
PART III
Item 10. Directors, Executive Officers and Corporate Governance
The names of our current officers and directors, as well as certain information about them, are set forth below:
Name | | Age | | Position(s) | | Director Since |
| Jun Wen | | 50 | | Chairman of the Board | | 2016 |
| Li Wang | | 46 | | Chief Executive Officer, Director | | 2016 |
| Yingping Zhang | | 46 | | President, Chief Operating Officer and Director | | 2016 |
| Hai Lin | | 49 | | Director | | 2016 |
| Ruigang Guo | | 42 | | Director | | 2016 |
| Ming Cheng | | 36 | | Chief Financial Officer and Treasurer | | - |
| Lingjing Zhao | | 28 | | Secretary | | - |
Biographies of Officers and Directors
Jun Wen. Mr. Wen has been employed since 2013 as Chairman of the China Xin Fu Group. From 2012 to 2013 Mr. Wen was employed as General Manager of Nami Kente Group in Hong Kong, a water processing manufacturer, specializing in the manufacture of Nanometer high-energy water-fluctuating machines. From 2009 to 2012, Mr. Wen served as Researcher and Assistant to the Dean of Beijing Huixia Scientific Medical School. In 2008 Mr. Wen was employed as Marketing Director of Shanxi Sanbafule Corporation. From 2006 to 2008 he was employed as Marketing Director of Jiutong Market in Hefei, Anhui Province. In 1988 Mr. Wen was awarded an Executive Master of Business Administration degree by Shanxi University. We believe Mr. Wen is qualified to serve as a member of our board of directors because of his marketing, business and research experience as well as his perspective as the Chairman of our largest shareholder.
Li Wang. Since 2016 Ms. Wang has been employed as General Manager of Guangdong Gewang Biotechnology Co., Ltd., our operating subsidiary. She has served as the Registrant's Chief Executive Officer since April 2016. From January 2015 to December 2015, she served as Sales Director of By-Health Co., Ltd., a company specializing in the research and development and production of health products as well as business services. From 2004 through 2014 Ms. Wang was employed as Chairman of Beijing Tianrun Gewei Trading Co., Ltd., a company specializing in the import and export of medical and firefighting rescue products, where she was responsible for development strategy, management policy and budgeting, as well as operations management. From 1991 through 2003, Ms. Wang was employed as Sales Manager for Beijing China Textiles Import and Export (Group) Corporation. In 1991 Ms. Wang was awarded a Bachelor’s degree in economics with a major in International Business Administration by the University of International Business and Economics in Beijing. From 1998 through 2000, Ms. Wang did postgraduate study in International Trade at that same institution. We believe Ms. Wang is qualified to serve as a member of our board of directors because of the experience and perspective she brings as our Chief Executive Officer and as General Manager of our operating subsidiary as well as her decades of experience in senior management of Chinese companies.
Yingping Zhang. Since 2016 Dr. Zhang has served as our President and Chief Operating Officer, as well as a member of the Board. After receiving his degree from Bethune Medical University in 1990, Dr. Zhang was employed as Chief Physician by the Bethune International Peace Hospital (1991 to 2002), Beijing 252 Military Hospital (2003 to 2009) and Chinese People’s Liberation Army 153 Hospital (2010 to 2015). Dr. Zhang currently serves as the Executive Director of the China Selenium Association, Director of the China Health Water Association, Researcher at China’s Difficult Disease Research Center, Secretary General of the Chinese Anti-Cancer Academy, Member of the China Trace Element Association, and Director of the Three High Four Disease Prevention Office. He is also the designated Successor for the Chief Team Leader and Selenium Application Experts of the China Selenium Supplement Project. We believe Dr. Zhang is qualified to serve as a member of our board of directors because of his experience in the healthcare field and because of his extensive knowledge of selenium and its medicinal value.
Hai Lin. Since 2015 Mr. Lin has been employed as General Manager of Red 13 Financial Holdings (Hong Kong) Co., Ltd. From 2013 to 2014 he was employed as General Manager of Shenzhen Qianhai Chengtai Investment Fund Management Co., Ltd. From 2010 to 2013, Mr. Lin was employed as Vice President of Gaoneng Tiancheng Investment Co., Ltd. In 1992 Mr. Lin was awarded a Master’s Degree in Finance by Fudan University. We believe Mr. Lin is qualified to serve as a member of our board of directors because of his experience in finance and his strategic insight as an investment professional.
Ruigang Guo. In October 2016 Mr. Guo was engaged by Guangdong gewang to serve as Vice General Manager. Since 2012 Mr. Guo has been employed as General Manager of Hangzhou Chunlu Network Technology Co., Ltd., which supports a members-only trading platform for rare curios and antiques. From 2007 to 2010, Mr. Guo served as Senior Partner of the Beijing Alliance PKU Management Consultants Group. From 2005 to 2007, Mr. Guo was employed as Senior Consultant by the Beijing Redetac Management and Consulting Limited Liability Company. Mr. Guo was awarded a Masters Degree in Business Administration by the University of International Business and Economics in 2005, and a Bachelor’s Degree with a concentration in Economics by the Central University of Finance and Economics in 1997. We believe Mr. Guo is qualified to serve as a member of our board of directors because of more than 20 years experience in business management and as an expert in professional management practices.
Ming Cheng. Since 2016 Mr. Cheng has served as our Chief Financial Officer and Treasurer. From 2013 to 2015 Mr. Cheng was employed as Financial Manager of the Harbin Power Plant Equipment Corporation. From 2010 to 2013 he was employed as Financial Manager by Harbin Shanglin Electronics Co., Ltd. In 2002 Mr. Cheng was awarded a Bachelor’s Degree with a major in financial accounting by the Central University of Finance and Economics.
Lingjing Zhao. Ms. Zhao has been employed since 2013 as Director of Marketing by Guangdong Gewang, our operating subsidiary. In 2015 she was elected to serve as a member of the Registrant’s Board of Directors, a position she resigned on June 27, 2016. Ms. Zhao has served as Secretary of the Registrant since June 2016. From 2009 to 2012 Ms. Zhao was employed as a Brand Director by the Guangdong Fangyuan Group, which marketed cosmetics to the health profession. From 2008 to 2009 Ms. Zhao was employed as an English translator at the Yuyuan Gardens in Shanghai. In 2008 Ms. Zhao was awarded a Bachelor’s Degree by the Central South University.
Code of Ethics
The Company has not adopted a formal code of ethics applicable to its executive officers. The Company expects to adopt a formal code of ethics during fiscal year 2017.
Director Independence
Hai Lin is the Registrant's only independent directors, as the term “independent” is defined by the rules of NASDAQ .
Family Relationships
There are no family relationships among any of our officers or directors.
Board Committees
We presently do not have an audit committee, compensation committee or nominating committee or committee performing similar functions, as our management believes that until this point it has been premature at the early stage of our management and business development to form an audit, compensation or nominating committee. Until these committees are established, these decisions will continue to be made by our Board of Directors. We also, for the same reason, do not have an audit committee financial expert. We intend to recruit an audit committee financial expert and organize the aforementioned committees during fiscal 2017.
Section 16(a) Compliance
Section 16(a) of the U.S. Securities and Exchange Act of 1934 requires that directors and executive officers, and persons who own beneficially more than ten percent (10%) of the Registrant's Common Stock, to file reports of ownership and changes of ownership with the U.S. Securities and Exchange Commission. Copies of all filed reports are required to be furnished to the Registrant pursuant to Section 16(a). Based solely on the reports received and on written representations from the reporting persons, we believe that, during the 2016 fiscal year, our CEO, Li Wang, our CFO and Treasurer, Ming Cheng, our Secretary, Lingjing Zhao, our directors Jun Wen, Hai Lin and Ruigang Guo, and our 10% stockholders Hong Kong Nuoxin Investment Management Co, Limited, Hong Kong Quansheng Holding Management Co., Ltd and China Xin Fu Group Limited filed Form 3s later than is required under Section 16(a).
Item 11. Executive Compensation
The following table sets forth all compensation awarded to, earned by, or paid by China Gewang Biotechnology, Inc. and its subsidiaries to its Chief Executive Officer and Chief Financial Officer during the past three fiscal years. There was no other officer or employee whose compensation for fiscal year 2016 exceeded $100,000.
| | | Fiscal Year | | | Salary | | | Bonus | | | Stock Awards | | | Option Awards | | | Other Compensation | | | Total | |
| Li Wang(1) | | | 2016 | | | $ | 75,218 | | | | - | | | | - | | | | - | | | | - | | | $ | 75,218 | |
| Shili Zhang(1)(3) | | | 2016 | | | $ | 40,523 | | | | - | | | | - | | | | - | | | | - | | | $ | 40,523 | |
| | | | 2015 | | | $ | 116,455 | (2) | | | - | | | | - | | | | - | | | | - | | | $ | 116,455 | |
| Siu Mun Kung(1) | | | 2014 | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | |
| Ming Cheng(3) | | | 2016 | | | $ | 7,072 | | | | - | | | | - | | | | - | | | | - | | | $ | 7,071 | |
| Fengxia Wu(3) | | | 2016 | | | $ | 8,960 | | | | - | | | | - | | | | - | | | | - | | | $ | 8,960 | |
| | | | 2015 | | | $ | 13,901 | | | | - | | | | - | | | | - | | | | - | | | $ | 13,901 | |
| (1) | Siu Mun Kung served as Chief Executive Officer and Chief Financial Officer during the fiscal year ended November 30, 2014, and resigned from those positions on December 1, 2014. Shili Zhang was appointed Chief Executive Officer on December 1, 2014 in connection with the closing of his acquisition of controlling interest in the Registrant at that time. Mr. Zhang resigned from office on April 8, 2016, and was replaced by Li Wang on that date. Mr. Zhang also served as Chief Financial Officer from December 1, 2014 to April 20, 2015. |
| (2) | Includes $17,999 paid to Mr. Zhang during the year ended November 30, 2015 and $98,456 accrued as of November 30, 2015 and subsequently paid. |
| (3) | Shili Zhang served as Chief Financial Officer from December 1, 2014 until April 20, 2015. Fengxia Wu was appointed Chief Financial Officer on April 20, 2015 and served until June 29, 2016, when Ming Cheng was appointed to that position. |
Employment Agreements
Our operating subsidiary, Guangdong Gewang, is party to a Labor Contract dated April 8, 2016 with our Chief Executive Officer, Li Wang. The contract, whose form is mandated by the PRC Labor Contract Law, provides that Ms. Wang will serve as General Manager of Guangdong Gewang for a term ending on April 7, 2019. In compensation, Guangdong Gewang will pay Ms. Wang a salary of 12,000 RMB ($1,875) per month and afford her the benefits required by PRC Labor Laws. In addition, Guangdong Gewang and Li Wang signed a Performance Salary Assessment Agreement, also dated April 8, 2016. The Performance Agreement provides that Guangdong Gewang will pay Ms. Wang a bonus of 50,000 RMB ($7,812) per month after the monthly net income of Guangdong Gewang exceeds 2,500,000 RMB ($390,625). The Performance Agreement terminates on April 7, 2017.
Equity Grants
The following tables set forth certain information regarding the stock options acquired by the Registrant’s Chief Executive Officer and Chief Financial Officer during the year ended November 30, 2016 and those options held by either of them on November 30, 2016.
Option Grants in the Last Fiscal Year
| | | Number of securities underlying option | | | Percent of total options granted to employees in | | | Exercise Price | | | Expiration | | | Potential realizable
value at assumed
annual rates
of appreciation
for option term | |
| | | granted | | | fiscal year | | | ($/share) | | | Date | | | 5% | | | 10% | |
| Li Wang | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | |
| Ming Cheng | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | |
The following table sets forth certain information regarding the stock grants received by the executive officers named in the table above during the year ended November 30, 2016 and held by them unvested at November 30, 2016.
Unvested Stock Awards in the Last Fiscal Year
| | | | Number of Shares That Have Not Vested | | | | Market Value of Shares That Have Not Vested | |
| Li Wang | | | - | | | | - | |
| Ming Cheng | | | - | | | | - | |
Compensation of Directors
On July 1, 2016, the Company entered into three year agreements with four of its directors (Jun Wen, Li Wang, Yingping Zhang and Hai Lin) for a total of approximately $15,000 (RMB 110,000) per month.
Item 12. Security Ownership of Certain Beneficial Owners and Management
The following table sets forth information known to us with respect to the beneficial ownership of our common stock as of the date of this report by the following:
| ● | each shareholder known by us to own beneficially more than 5% of our common stock; |
| | | |
| ● | Li Wang, our Chief Executive Officer |
| | | |
| ● | each of our directors; and |
| | | |
| ● | all directors and executive officers as a group. |
There are 75,000,000 shares of our common stock outstanding on the date of this report. Except as otherwise indicated, we believe that the beneficial owners of the common stock listed below have sole voting power and investment power with respect to their shares, subject to community property laws where applicable. Beneficial ownership is determined in accordance with the rules of the Securities and Exchange Commission.
In computing the number of shares beneficially owned by a person and the percent ownership of that person, we include shares of common stock subject to options or warrants held by that person that are currently exercisable or will become exercisable within 60 days. We do not, however, include these “issuable” shares in the outstanding shares when we compute the percent ownership of any other person.
| Name and Address of Beneficial Owner(1) | | Amount and Nature of Beneficial Ownership | | | Percentage of Class | |
| Li Wang | | | - | | | | - | |
| Jun Wen(2) | | | 5,000,000 | (3) | | | 6.7 | % |
| Zhang Yingping | | | 17,000 | | | | 0.1 | % |
| Hai Lin | | | - | | | | - | |
| Ruigang Guo | | | - | | | | - | |
All officers and
directors as a group (7 persons) | | | 5,028,500 | (3) | | | 6.7 | % |
| Han Xu(4) | | | 25,071,500 | (5) | | | 33.4 | % |
| Xiuqin Jiang(6) | | | 15,746,500 | (7) | | | 22.7 | % |
| China Xin Fu Group Limited(8) | | | 12,775,000 | | | | 17.0 | % |
| Hong Kong Quansheng Holding Management Co., Limited(9) | | | 10,000,000 | | | | 13.3 | % |
| Hong Kong Nuoxin Investment Management Co, Limited(10) | | | 7,500,000 | | | | 10.0 | % |
| (1) | Unless otherwise noted, the address for an owner is c/o China Gewang Biotechnology, Inc. |
| (2) | The address for Jun Wen is No. 1, Row 3, Zhuping District, Tongkuan, Yuanqu County, Shanxi Province, P.R. China. |
| (3) | The shares attributed to Jun Wen do not include 12,775,000 shares owned by China Xin Fu Group Limited. Jun Wen is a director of China Xin Fu Group Limited, but denies that he controls the voting or disposition of shares owned by that entity. |
| (4) | The address for Han Xu is No. 438-501-2 Binjiang East Road, Haizhu District, Guangzhou, Guangdong, P.R. China. |
| (5) | The shares attributed to Han Xu include 19,000,000 shares with respect to which she exercises voting control only, including 10,000,000 shares owned by Hong Kong Quansheng Holding Management Co., Limited, 5,000,000 shares owned by Jun Wen, and 2,971,500 shares owned by Xiuqin Jiang. |
| (6) | The address for Xiuqin Jiang is No. 27 Dongjin Road, Jingcheng Town, Jingjiang City, Jiangsu Province, P.R. China. |
| (7) | The shares attributed to Xiuqin Jiang include 12,775,000 shares owned by China Xin Fu Group Limited, which Xiuqin Jiang controls as director of that company. |
| (8) | The address for China Xin Fu Group Limited is Unit 04, 7/F Bright Way Tower No. 33, Mong Kok RD KL, Hong Kong. Xiuqin Jiang has voting and dispositive control over the shares owned by China Xin Fu Group Limited. |
| (9) | The address for Hong Kong Quansheng Holding Management Co., Limited is Unit A1, 7/F, Cheuk Nang Plaza 250 Hennessy Road, Wanchai, Hong Kong. Xin Zhao has voting and dispositive control, and Han Xu has voting control, over the shares owned by Hong Kong Quansheng Holding Management Co., Limited. |
| (10) | The address for Hong Kong Nuoxin Investment Management Co., Limited is Unit A1, 7/F, Cheuk Nang Plaza 250 Hennessy Road, Wanchai, Hong Kong. Fanfei Guan has voting and dispositive control, and Mengdi Zhang has voting control, over the shares owned by Hong Kong Nuoxin Investment Management Co., Limited. |
Item 13. Certain Relationships and Related Transactions and Director Independence Related Party TransactionsEquity Investment
On April 28, 2016 the Registrant’s wholly owned subsidiary, Biotechnology International, entered into an investment agreement with Guangdong Tianmei. At that time, 88% of the equity in Guangdong Tianmei was owned by two individuals who together, directly and indirectly, owned 57.5% of the Registrant’s outstanding shares. The investment agreement provided that Biotechnology International would pay US$1,000,000 to acquire a 30% interest in an Australian corporation to be formed, which would indirectly own all of the equity in Guangdong Tianmei.
The acquisition by Biotechnology International of 30% Tianmei Australia was completed in May 2016, at which time Tianmei Australia acquired ownership, through subsidiaries, of Guangdong Tianmei. The investment agreement provided that payment of the $1,000,000 purchase price was due on June 20, 2016, which was paid in full on June 17, 2016.
The net worth of Guangdong Tianmei at the time of the acquisition was $4,888,840, 30% of which is $1,466,652. Because the Registrant and Guangdong Tianmei were considered to be under common control at the time of the acquisition by reason of the commonality of majority shareholders as quantified above, the $466,652 by which the Registrant’s share of the net worth of Guangdong Tianmei exceeded the purchase price has been recorded as an increase to additional paid-in capital.
The changes in the equity investment are summarized as follows:
| | | August 31,
2016 | | | November 30, 2015 | |
| Initial investment | | $ | 1,000,000 | | | $ | - | |
| Equity in excess of purchase price of investee under common control | | | 466,652 | | | | - | |
| Pro rata share of net income | | | 4,536,760 | | | | - | |
| Investment, end of period/year | | $ | 6,003,412 | | | $ | - | |
Other Related Party Transactions
The Registrant obtained demand and non-interest bearing loans from a former officer and stockholder, who resigned from office on April 8, 2016. The loans of $228,238 and $0 at November 30, 2016 and 2015, respectively, are reflected as loans from third party.
The Registrant obtained demand and non-interest bearing loans from one of its stockholders. The loans of $ 237,639 and $166,106 at November 30, 2016 and 2015, respectively, are reflected as loans from stockholder.
Guangdong Gewang entered into a promotion agreement with Guangdong Tianmei, in which the Registrant holds an indirect 30% ownership interest. Promotion expenses incurred during the year ended November 30, 2016, 2015 and 2014 were $626,147, $0 and $0, respectively.
Guangdong Gewang entered into an agreement with Guandong Tianmei on June 10, 2015 to license the usage of the Registrant’s trademark for 10 years. Trademark revenue recorded for the years ended November 30, 2016 and 2015 were $1,470 and $0, respectively. The future commitment is approximately $1,500 each year.
Director Independence
The Board of Directors has determined that among its members only Hai Lin is independent, as “independence” is defined in the Rules of NASDAQ.
Item 14. Principal Accountant Fees and Services
Audit Fees
Wei, Wei & Co., LLP billed $70,000 in connection with the audit and reviews of the Registrant’s financial statements for the year ended November 30, 2016. Wei, Wei & Co., LLP billed $72,000 in connection with the audit and reviews of the Registrant’s financial statements for the year ended November 30, 2015. Also included are those services normally provided by the accountant in connection with the Registrant’s statutory and regulatory filings.
Audit-Related Fees
Wei, Wei & Co., LLP did not bill the Registrant for any Audit-Related fees in fiscal 2016 or fiscal 2015.
Tax Fees
Wei, Wei & Co., LLP did not bill the Registrant for any tax compliance, tax advice or tax planning in fiscal 2016. Wei, Wei & Co., LLP billed $2,900 for tax-related services in fiscal 2015.
All Other Fees
Wei, Wei & Co., LLP did not bill the Registrant for any other fees in fiscal 2016 or fiscal 2015.
It is the policy of the Registrant that all services, other than audit, review or attest services, must be pre-approved by the Board of Directors.
PART IV
Item 15. Exhibits and Financial Statement Schedules
(b) Exhibit List
| 3.1 | Articles of Incorporation, as amended.(1) |
| 3.2 | Articles of Merger of China Gewang Biotechnology, Inc. into Rich Star Development - filed as an exhibit to the Registrant’s Current Report on Form 8-K filed on January 12, 2015, and incorporated herein by reference. |
| 3.3 | By-laws - filed as an exhibit to the Registrant’s Registration Statement on Form S-1 (File No. 333-166454) and incorporated herein by reference. |
| 10.1 | Exclusive Technical Service and Business Consulting Agreement dated April 6, 2015 between Guangdong Gewang Biotechnology Co., Ltd. and Gewang Selenium Enrichment Information Consulting (Shenzhen) Co., Ltd.(2) |
| 10.2 | Call Option Agreement dated April 6, 2015 among Gewang Selenium Enrichment Information Consulting (Shenzhen) Co., Ltd., Zhang Shili, Zeng Yun and Xu Wei.(2) |
| 10.3 | Proxy Agreement dated April 6, 2015 among Guangdong Gewang Biotechnology Co., Ltd., Gewang Selenium Enrichment Information Consulting (Shenzhen) Co., Ltd., Zhang Shili, Zeng Yun and Xu Wei.(2) |
| 10.4 | Share Pledge Agreement dated April 6, 2015 among Guangdong Gewang Biotechnology Co., Ltd., Gewang Selenium Enrichment Information Consulting (Shenzhen) Co., Ltd., Zhang Shili, Zeng Yun and Xu Wei.(2) |
| 10.5 | Product Manufacture and Purchase Agreement among Guangzhou Qinxiyuan Food Co., Ltd., Yantai Yisheng Pharmaceutical Co., Ltd. and Shandong Academy of Agricultural Sciences.(2) |
| 10.6 | Product Manufacture and Purchase Agreement, dated June 30, 2016, by and among Guangdong Gewang Biotechnology Co., Ltd., Beijing Technology Development Company of CAAS and the Shandong Academy of Agricultural Sciences.(3) |
| 10.7 | Licensing Agreement, dated December 30, 2015, between the Shandong Academy of Agriculture and Guangdong Gewang Biotechnology Co., Ltd.(3) |
| 10.8 | License Agreement Supplement Agreement dated January 14, 2015 between the Shandong Academy of Agriculture and Guangdong Gewang Biotechnology Co., Ltd.(3) |
| 10.9 | Product Manufacture and Purchase Agreement dated January 27, 2016 by and among Guangdong Gewang Biotechnology Co., Ltd., Taian Zhishengtang Ganoderma Co., Ltd. and the Shandong Academy of Agricultural Sciences.(1) |
| 10.10 | Labor Contract between Guangdong Gewang Biotechnology Co. Ltd. and Li Wang dated April 8, 2016.(1) |
| 10.11 | Performance Salary Assessment Agreement between Guangdong Gewang Biotechnology Co. Ltd. and Li Wang dated April 8, 2016.(1) |
| 21 | Subsidiaries |
| 31.1 | Rule 13a-14(a) Certification - CEO |
| 31.2 | Rule 13a-14(a) Certification - CFO |
| 32 | Rule 13a-14(b) Certifications |
| 101.INS | XBRL Instance |
| 101.SCH | XBRLSchema |
| 101.CAL | XBRLCalculation |
| 101.DEF | XBRLDefinition |
| 101.LAB | XBRLLabel |
| 101.PRE | XBRLPresentation |
|
(1) | Filed as an exhibit to the Registrant's Registration Statement on Form S-1/A (Amendment No. 1), filed on January 12, 2017 (File No. 333-214597), and incorporated herein by reference. |
| (2) | Filed as an exhibit to the Registrant's Current Report on Form 8-K filed on April 21, 2015 and incorporated herein by reference. |
| (3) | Filed as an exhibit to the Registrant's Registration Statement on Form S-1, filed on November 14, 2016 (File No. 333-214597), and incorporated herein by reference. |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized, on February 10, 2017.
| | China Gewang Biotechnology, Inc. |
| | | |
| | By: | /s/ Li Wang |
| | | Li Wang, |
| | | Chief Executive Officer |
Pursuant to the requirements of the Securities Exchange Act of 1934, this Report has been signed below on February 10, 2017 by the following persons, on behalf of the Registrant and in the capacities indicated.
| /s/ Li Wang | |
| Li Wang, Director | |
| Chief Executive Officer | |
| | |
| /s/ Ming Cheng | |
| Ming Cheng | |
| Chief Financial and Accounting Officer | |
| | |
| /s/ Jun Wen | |
| Jun Wen, Director | |
| | |
| /s/ Yingping Zhang | |
| Yingping Zhang, Director | |
| | |
| /s/ Hai Lin | |
| Hai Lin, Director | |
| | |
| /s/ Ruigang Guo | |
| Ruigang Guo, Director | |
* * * * *
47

