U. S. Securities and Exchange Commission
Washington, D. C. 20549
FORM 10-Q
☒ QUARTERLY REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the quarterly period ended May 31, 2017
☐ TRANSITION REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from _____ to _____
Commission File No. 0-54451
CHINA GEWANG BIOTECHNOLOGY, INC. |
| (Exact Name of Registrant in its Charter) |
| Nevada | | 42-1769584 |
(State or Other Jurisdiction of
incorporation or organization) | | (I.R.S. Employer I.D. No.) |
| | | |
Floor 29, No. 334, Huanshi East Road, Yuexiu District, Guangzhou City Guangdong Province, P.R. China 510623 |
| (Address of Principal Executive Offices) |
| Issuer’s Telephone Number: 86-024-2397-4663 |
Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Sections 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files.) Yes ☒ No ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act) Yes ☐ No ☒
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check One)
| Large accelerated filer | ☐ | Accelerated filer | ☒ | Non-accelerated filer | ☐ | Smaller reporting company | ☐ |
Emerging growth company x
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.☐
APPLICABLE ONLY TO CORPORATE ISSUERS: Indicate the number of shares outstanding of each of the Registrant's classes of common stock, as of the latest practicable date:
July 17, 2017
Common Voting Stock: 75,000,000
CHINA GEWANG BIOTECHNOLOGY, INC.
QUARTERLY report on Form 10-Q
for the fiscal QUARTER ended MARCH 31, 2017
Table of Contents
| | | Page No |
| Part I | Financial Information | |
| | | |
| Item 1. | Financial Statements (unaudited): | |
| | Consolidated Balance Sheets – May 31, 2017 and November 30, 2016 | 1 |
| | Consolidated Statements of Income and Comprehensive Income - for the Three and Six Month Periods Ended May 31, 2017 and May 31, 2016 | 3 |
| | Consolidated Statements of Changes in Stockholders’ Equity – for the Six Months Ended May 31, 2017 | 5 |
| | Consolidated Statements of Cash Flows - for the Six Months Ended May 31, 2017 and May 31, 2016 | 6 |
| | Notes to Consolidated Financial Statements | 8 |
| Item 2. | Management’s Discussion and Analysis of Financial Condition and Results of Operations | 31 |
| Item 3 | Quantitative and Qualitative Disclosures about Market Risk | 36 |
| Item 4. | Controls and Procedures | 36 |
| | | |
| Part II | Other Information | |
| | | |
| Item 1. | Legal Proceedings | 37 |
| Items 1A. | Risk Factors | 37 |
| Item 2. | Unregistered Sales of Equity Securities and Use of Proceeds | 37 |
| Item 3. | Defaults upon Senior Securities | 37 |
| Item 4. | Mine Safety Disclosures | 37 |
| Item 5. | Other Information | 37 |
| Item 6. | Exhibits | 37 |
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS(IN U.S. $)
| | | May 31, | | | November 30, | |
| ASSETS | | 2017 | | | 2016 | |
| | | (Unaudited) | | | | |
| | | | | | | |
| Current assets: | | | | | | |
| Cash | | $ | 22,415,103 | | | $ | 13,108,340 | |
| Accounts receivable | | | 14,336,885 | | | | 11,205,011 | |
| Inventory | | | 168,267 | | | | 107,830 | |
| Prepaid expenses | | | 2,852,914 | | | | 5,540,051 | |
| | | | | | | | | |
| Total current assets | | | 39,773,169 | | | | 29,961,232 | |
| | | | | | | | | |
| Property, plant and equipment, net | | | 104,289 | | | | 128,767 | |
| | | | | | | | | |
| Other assets: | | | | | | | | |
| Deferred registration costs | | | 180,495 | | | | 110,086 | |
| Equity investment | | | 10,669,359 | | | | 6,003,412 | |
| | | | | | | | | |
| Total Assets | | $ | 50,727,312 | | | $ | 36,203,497 | |
See accompanying notes to the consolidated financial statements.
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS (CONTINUED)(IN U.S. $)
| | | May 31, | | | November 30, | |
| LIABILITIES AND STOCKHOLDERS’ EQUITY | | 2017 | | | 2016 | |
| | | (Unaudited) | | | | |
| | | | | | | |
| Current liabilities: | | | | | | |
| Accounts payable | | $ | 4,525,711 | | | $ | 3,367,174 | |
| Taxes payable | | | 1,155,282 | | | | 789,370 | |
| Accrued expenses and other payables | | | 312,325 | | | | 193,090 | |
| Loans from third party | | | 228,359 | | | | 228,238 | |
| Loans from stockholder | | | 407,677 | | | | 237,639 | |
| | | | | | | | | |
| Total current liabilities | | | 6,629,354 | | | | 4,815,511 | |
| | | | | | | | | |
| Commitments and contingencies | | | | | | | | |
| | | | | | | | | |
| Stockholders’ equity: | | | | | | | | |
| Common stock - $0.001 par value, 100,000,000 and 100,000,000 shares authorized, 75,000,000 and 75,000,000 shares issued and outstanding as of May 31, 2017 and November 30, 2016, respectively | | | 75,000 | | | | 75,000 | |
| Additional paid-in capital | | | 16,980,102 | | | | 16,980,102 | |
| Retained earnings | | | 27,082,001 | | | | 15,026,053 | |
| Statutory reserve fund | | | 759,094 | | | | 759,094 | |
| Other comprehensive (loss) | | | (1,211,862 | ) | | | (1,707,064 | ) |
| | | | | | | | | |
| Stockholders’ equity before noncontrolling interests | | | 43,684,335 | | | | 31,133,185 | |
| | | | | | | | | |
| Noncontrolling interests | | | 413,623 | | | | 254,801 | |
| | | | | | | | | |
| Total stockholders’ equity | | | 44,097,958 | | | | 31,387,986 | |
| | | | | | | | | |
TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY | | $ | 50,727,312 | | | $ | 36,203,497 | |
See accompanying notes to the consolidated financial statements.
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF INCOME AND COMPREHENSIVE INCOME
FOR THE THREE AND SIX MONTHS ENDED MAY 31, 2017 AND 2016
(UNAUDITED) (IN U.S. $)
| | | Three Months ended May 31, | | | Six Months ended May 31, | |
| | | 2017 | | | 2016 | | | 2017 | | | 2016 | |
| | | | | | | | | | | | | |
| Revenue | | $ | 22,264,734 | | | $ | 5,437,183 | | | $ | 43,406,866 | | | $ | 6,655,281 | |
| Cost of goods sold | | | (14,645,390 | ) | | | (3,025,001 | ) | | | (28,599,695 | ) | | | (3,367,609 | ) |
| | | | | | | | | | | | | | | | | |
| Gross profit | | | 7,619,344 | | | | 2,412,182 | | | | 14,847,171 | | | | 3,287,672 | |
| | | | | | | | | | | | | | | | | |
| Operating expenses: | | | | | | | | | | | | | | | | |
| R&D expenses | | | 583,868 | | | | - | | | | 1,163,280 | | | | - | |
| Selling and marketing | | | 1,407,424 | | | | 649,302 | | | | 2,852,337 | | | | 919,973 | |
| General and administrative | | | 382,422 | | | | 122,978 | | | | 763,777 | | | | 259,939 | |
| | | | | | | | | | | | | | | | | |
| Total operating expenses | | | 2,373,714 | | | | 772,280 | | | | 4,779,394 | | | | 1,179,912 | |
| | | | | | | | | | | | | | | | | |
| Operating income | | | 5,245,630 | | | | 1,639,902 | | | | 10,067,777 | | | | 2,107,760 | |
| | | | | | | | | | | | | | | | | |
| Other income: | | | | | | | | | | | | | | | | |
| Interest income | | | 20,880 | | | | 6,327 | | | | 19,822 | | | | 11,095 | |
| Other non-operating income | | | - | | | | - | | | | 1,335 | | | | 1,490 | |
| | | | | | | | | | | | | | | | | |
| Total other income | | | 20,880 | | | | 6,327 | | | | 21,157 | | | | 12,585 | |
| | | | | | | | | | | | | | | | | |
| Income before provision for income taxes | | | 5,266,510 | | | | 1,646,229 | | | | 10,088,934 | | | | 2,120,345 | |
| Provision for income taxes | | | 1,319,811 | | | | 416,553 | | | | 2,546,163 | | | | 539,969 | |
See accompanying notes to the consolidated financial statements.
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF INCOME AND COMPREHENSIVE INCOME (CONTINUED)
FOR THE THREE AND SIX MONTHS ENDED MAY 31, 2017 AND 2016
(UNAUDITED) (IN U.S. $)
| | | Three Months Ended May 31, | | | Six Months Ended May 31, | |
| | | 2017 | | | 2016 | | | 2017 | | | 2016 | |
| | | | | | | | | | | | | |
| Equity in income of investee | | | 2,512,935 | | | | 408,275 | | | | 4,665,947 | | | | 408,275 | |
| | | | | | | | | | | | | | | | | |
| Net income before noncontrolling interests | | | 6,459,634 | | | | 1,637,951 | | | | 12,208,718 | | | | 1,988,651 | |
| Noncontrolling interests | | | (79,622 | ) | | | (62,483 | ) | | | (152,770 | ) | | | (80,995 | ) |
| | | | | | | | | | | | | | | | | |
| Net income attributable to common stockholders | | $ | 6,380,012 | | | $ | 1,575,468 | | | $ | 12,055,948 | | | $ | 1,907,656 | |
| | | | | | | | | | | | | | | | | |
| Earnings per common share-basic and diluted | | $ | 0.09 | | | $ | 0.03 | | | $ | 0.16 | | | $ | 0.03 | |
| | | | | | | | | | | | | | | | | |
| Weighted average shares outstanding-basic and diluted | | | 75,000,000 | | | | 60,543,478 | | | | 75,000,000 | | | | 55,882,514 | |
| | | | | | | | | | | | | | | | | |
| Comprehensive income | | | | | | | | | | | | | | | | |
| Net income before noncontrolling interests | | $ | 6,459,634 | | | $ | 1,637,951 | | | $ | 12,208,718 | | | $ | 1,988,651 | |
| Foreign currency translation adjustment | | | 442,410 | | | | (122,371 | ) | | | 501,254 | | | | (308,784 | ) |
| | | | | | | | | | | | | | | | | |
| Total comprehensive income | | | 6,902,044 | | | | 1,515,580 | | | | 12,709,972 | | | | 1,679,867 | |
| Comprehensive income attributable to noncontrolling interests | | | (70,774 | ) | | | (72,978 | ) | | | (146,718 | ) | | | (82,171 | ) |
| | | | | | | | | | | | | | | | | |
Net comprehensive income attributable to common stockholders | | $ | 6,831,270 | | | $ | 1,442,602 | | | $ | 12,563,254 | | | $ | 1,597,696 | |
See accompanying notes to the consolidated financial statements.
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENT OF CHANGES IN STOCKHOLDERS’ EQUITY
FOR THE SIX MONTHS ENDED MAY 31, 2017 (UNAUDITED) (IN U.S. $)
| | | Common Stock | | | Additional Paid-in Capital | | | Retained Earnings | | | Non controlling Interests | | | Statutory Reserve Fund | | | Other Comprehensive Income (Loss) | | | Total | |
| Balance, November 30, 2016 | | $ | 75,000 | | | $ | 16,980,102 | | | $ | 15,026,053 | | | $ | 254,801 | | | $ | 759,094 | | | $ | (1,707,064 | ) | | $ | 31,387,986 | |
| Net income | | | - | | | | - | | | | 12,055,948 | | | | 152,770 | | | | - | | | | - | | | | 12,208,718 | |
| Other comprehensive income | | | - | | | | - | | | | - | | | | 6,052 | | | | - | | | | 495,202 | | | | 501,254 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance, May 31, 2017 (Unaudited) | | $ | 75,000 | | | $ | 16,980,102 | | | $ | 27,082,001 | | | $ | 413,623 | | | $ | 759,094 | | | $ | (1,211,862 | ) | | $ | 44,097,958 | |
See accompanying notes to theconsolidatedfinancial statements.
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
FOR THE SIX MONTHS ENDED MAY 31, 2017 AND 2016
(UNAUDITED) (IN U.S. $)
| | | 2017 | | | 2016 | |
| | | | | | | |
| Cash flows from operating activities: | | | | | | |
| Net income | | $ | 12,208,718 | | | $ | 1,988,651 | |
| Adjustments to reconcile net income to net cash provided by (used in) operating activities: | | | | | | | | |
| Depreciation | | | 26,050 | | | | 24,761 | |
| (Income) from equity investment | | | (4,665,947 | ) | | | (408,275 | ) |
| Changes in operating assets and liabilities: | | | | | | | | |
| (Increase) in accounts receivable | | | (3,131,874 | ) | | | (3,330,508 | ) |
| (Increase) decrease in inventory | | | (60,437 | ) | | | 134,534 | |
| Decrease (increase) in prepaid expenses | | | 2,687,137 | | | | (1,346,283 | ) |
| Increase in accounts payable | | | 1,158,537 | | | | 1,235,947 | |
| Increase in taxes payable | | | 365,912 | | | | 342,454 | |
| Increase (decrease) in accrued expenses and other liabilities | | | 218,984 | | | | (82,198 | ) |
| | | | | | | | | |
| Net cash provided by (used in) operating activities | | | 8,807,080 | | | | (1,440,917 | ) |
| | | | | | | | | |
| Cash flows from investing activities: | | | | | | | | |
| Purchase of equipment | | | - | | | | (78,567 | ) |
| | | | | | | | | |
| Net cash (used in) investing activities | | | - | | | | (78,567 | ) |
| | | | | | | | | |
| Cash flows from financing activities: | | | | | | | | |
| Proceeds from sale of common stock | | | - | | | | 9,848,200 | |
| | | | | | | | | |
| Net cash provided by financing activities | | | - | | | | 9,848,200 | |
| | | | | | | | | |
| Effect of exchange rate changes on cash | | | 499,683 | | | | (306,441 | ) |
| | | | | | | | | |
| Net change in cash | | | 9,306,763 | | | | 8,022,165 | |
| Cash, beginning | | | 13,108,340 | | | | 8,669,034 | |
| | | | | | | | | |
| Cash, end | | $ | 22,415,103 | | | $ | 16,691,199 | |
See accompanying notes to theconsolidatedfinancial statements.
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
FOR THE SIX MONTHS ENDED MAY 31, 2017 AND 2016
(UNAUDITED) (IN U.S. $)
| | | 2017 | | | 2016 | |
| | | | | | | |
| Supplemental disclosure of cash flow information: | | | | | | |
| Cash paid for interest | | $ | - | | | $ | - | |
| Cash paid for income taxes | | $ | 2,234,942 | | | $ | 264,032 | |
| | | | | | | | | |
| Noncash investing activities: | | | | | | | | |
| Payable for purchase of equity investment | | $ | - | | | $ | 1,000,000 | |
| Additional paid-in capital – equity in excess of purchase price of investee under common control | | $ | - | | | $ | 466,652 | |
| | | | | | | | | |
| Noncash financing activities: | | | | | | | | |
| Payment of accrued expenses and other payables by shareholder | | $ | 170,016 | | | $ | 52,190 | |
See accompanying notes to theconsolidatedfinancial statements.
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE THREE AND SIX MONTHS ENDED MAY 31, 2017 AND 2016
(UNAUDITED) (IN U.S. $)
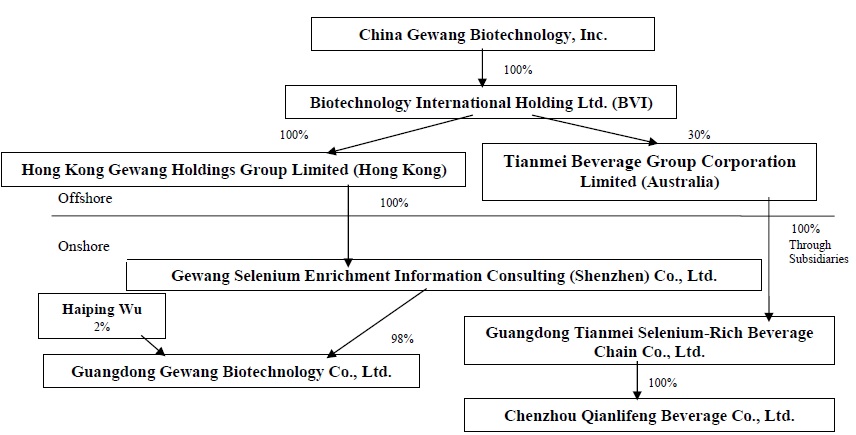
China Gewang Biotechnology, Inc. (the “Company”), formerly known as Rich Star Development, was incorporated under the laws of the State of Nevada on May 29, 2009. From its inception until the closing of the reverse merger described below, the Company was a development-stage company in the business of sourcing and distributing food products, paper products, janitorial products, restaurant utensils and equipment to the food service industry in the PRC.
On April 20, 2015, the Company completed a reverse merger transaction through a share exchange with the stockholders of Biotechnology International Holding Ltd. (“Biotechnology International”), whereby the Company acquired 100% of the outstanding shares of Biotechnology International in exchange for 32,000,000 shares of its common stock, representing 90.14% of the issued and outstanding shares of common stock. As a result of the reverse merger, Biotechnology International became the Company’s wholly-owned subsidiary and the former Biotechnology International stockholders became our controlling stockholders. The share exchange transaction was treated as a reverse acquisition, with Biotechnology International as the acquirer and the Company as the acquired party for accounting purposes.
On January 8, 2015, the Company filed a certificate of amendment to its articles of incorporation to change its name from “Rich Star Development” to “China Gewang Biotechnology, Inc.”
On July 20, 2016, the Company filed with the Nevada Secretary of State a Certificate of Amendment to its Articles of Incorporation. The Certificate of Amendment increased the number of authorized shares of common stock from 75 million to 100 million.
Majority-owned subsidiary: Guangdong Gewang
As a result of the transaction with Biotechnology International, the Company owns all of the issued and outstanding common stock of Hong Kong Gewang Holdings Group Limited (“Hong Kong Gewang”), a wholly-owned subsidiary of Biotechnology International, which in turn owns all of the issued and outstanding common stock of Gewang Selenium Enrichment Information Consulting (Shenzhen) Co., Ltd. (“Gewang Selenium”). Before August 8, 2016, the Company effectively and substantially controlled Guangdong Gewang Biotechnology Co., Ltd. (“Guangdong Gewang”) through a series of captive agreements between Guangdong Gewang and Gewang Selenium. Guangdong Gewang, incorporated under the laws of the People’s Republic of China (“PRC”) on June 2010, is primarily engaged in the sale of selenium supplements within the PRC. It is a member of the Chinese Selenium Supplements Association.
On July 13, 2016, Gewang Selenium exercised its option to purchase all of the registered equity of Guangdong Gewang. The purchase price paid for the equity was RMB10,000 (approximately US$1,500). The equity was purchased from Shili Zhang, Yun Zeng and Wei Xu. Shili Zhang was the Company’s CEO until April 8, 2016 and is the father of Mengdi Zhang, who was the beneficial owner of 22.7% of the Company's outstanding common stock at the time of the sale on July 13, 2016. The other two sellers are not affiliated with the Company.
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE THREE AND SIX MONTHS ENDED MAY 31, 2017 AND 2016
(UNAUDITED) (IN U.S. $)
| 1. | ORGANIZATION (continued) |
Upon application to the provincial government for registration of the transfer of equity, the Company was informed that Gewang Selenium would not be permitted to own 100% of Guangdong Gewang. Therefore, the parties modified the exercise of the option to provide that Gewang Selenium would purchase only 98% of the registered equity of Guangdong Gewang. The purchase price paid for the equity was RMB 9,800 (approximately US$1,500). The remaining 2% of the registered equity was then sold by Yun Zeng to Haiping Wu for a price of RMB 200,000 (approximately US$30,400), which equaled 2% of the registered equity of Guangdong Gewang. Haiping Wu is a Director of Guangdong Gewang. The acquisition, as modified, was then approved by the provincial government on August 8, 2016.
Prior to the acquisition, Gewang Selenium controlled Guangdong Gewang through a series of contractual agreements, which made Guangdong Gewang a variable interest entity, the effect of which was to cause the balance sheet and operating results of Guangdong Gewang to be consolidated with those of Gewang Selenium in the Company's financial statements. As a result of the acquisition by Gewang Selenium of the registered ownership of Guangdong Gewang, the balance sheet and operating results of Guangdong Gewang will hereafter continue to be consolidated with those of Gewang Selenium as its majority-owned subsidiary. The previous non-controlling interest was reclassified to additional paid-in-capital.
The Company’s business, through its operating entity in China (Guangdong Gewang), consists of:
Sale of selenium supplements
Through a partnership with the Academy of Agricultural Sciences of Shangdong Province (the “Academy”), a highly regarded research institute in China, the Company has licensed the exclusive rights to contract for the manufacture and marketing of selenium products, comprised of two selenium capsules and one selenium powder, using three selenium formulas developed and owned by the Academy.
Sale of selenium products
Since March 2016, the Company has signed agreements with distributors to distribute 89 distinct selenium products to hundreds of retail stores. The products include processed foods such as selenium enriched porridge, ready to eat foods such as selenium-enriched peanuts, and ingredients such as selenium enriched flour. The Company is actively engaged in marketing healthy selenium rich foods including Selenium-Rich Maize Residue, Selenium-Rich Brown Rice. Selenium Enriched Black Beans, Selenium-Enriched Buckwheat Kernel and Selenium-Enriched Ormosia. These foods compliment the Company’s selenium supplements by raising awareness of the need for selenium in the diets of our target consumer market.
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE THREE AND SIX MONTHS ENDED MAY 31, 2017 AND 2016
(UNAUDITED) (IN U.S. $)
| 1. | ORGANIZATION (continued) |
Equity investment: Guangdong Tianmei
On April 28, 2016, the Company's wholly-owned subsidiary, Biotechnology International, entered into an investment agreement with Guangdong Tianmei Selenium-Rich Beverage Chain Co., Ltd. (“Guangdong Tianmei”). Guangdong Tianmei was organized in May 2015, and is engaged in the business of distributing selenium-rich bottled water and also functions as a placement agent for a variety of products from various manufacturers, all within the PRC. The investment agreement provided that Biotechnology International would pay US$1,000,000 to acquire a 30% interest in an Australian corporation to be formed, which would indirectly own all of the equity in Guangdong Tianmei.
The foregoing acquisition by Biotechnology International of 30% of Tianmei Beverage Group Corporation Limited, an Australian corporation ("Tianmei Australia"), was completed in May 2016. The $1,000,000 purchase price was paid in full on June 17, 2016. Concurrently. Tianmei Australia acquired ownership, through its wholly owned subsidiaries, of Guangdong Tianmei.
On February 23, 2017, Guangdong Tianmei entered into an acquisition agreement with Chenzhou Qianlifeng Beverage Co., Ltd. (“Chenzhou Qianlifeng”). Chenzhou Qianlifeng specializes in the bottling and packaging of selenium-rich water. Chenzhou Qianlifeng had a contract with Guangdong Tianmei, to bottle the selenium-rich water under Guangdong Tianmei’s label. The acquisition agreement provided that Guangdong Tianmei would pay RMB 5,000,000 (approximately US$724,600) to acquire a 100% interest in Chenzhou Qianlifeng. The total purchase price was fully paid on February 23, 2017.
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE THREE AND SIX MONTHS ENDED MAY 31, 2017 AND 2016
(UNAUDITED) (IN U.S. $)
| 1. | ORGANIZATION (continued) |
Equity investment: Guangdong Tianmei
As a result of the entry into the foregoing agreements, the Company has a corporate structure which is as follows:
| 2. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES |
Basis of accounting and presentation
The accompanying consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America. The consolidated financial statements include those of the Company and its subsidiaries. All significant intercompany accounts and transactions have been eliminated in consolidation.
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE THREE AND SIX MONTHS ENDED MAY 31, 2017 AND 2016
(UNAUDITED) (IN U.S. $)
| 2. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued) |
Basis of accounting and presentation (continued)
The unaudited interim consolidated financial statements of the Company as of May 31, 2017, and for the three and six months ended May 31, 2017 and 2016 have been prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”) and the rules and regulations of the Securities and Exchange Commission (the “SEC”) which apply to interim financial statements. Accordingly, they do not include all of the information and footnotes normally required by accounting principles generally accepted in the United States of America for annual financial statements. In the opinion of management, such information contains all adjustments, consisting only of normal recurring adjustments, necessary for a fair presentation of the results for the periods presented. The results of operations for the three and six months ended May 31, 2017 are not necessarily indicative of the results to be expected for future quarters or for the year ending November 30, 2017. The interim consolidated financial information should be read in conjunction with the consolidated financial statements and the notes thereto, included in the Company’s Form 10-K filed with the SEC.
The Company uses the equity method of accounting for its 30% equity investment in Tianmei Australia. Under the equity method, investments are carried at cost and increased or decreased by the Company’s pro-rata share of earnings or losses. The carrying cost of this investment is also increased or decreased to reflect additional contributions or withdrawals of capital. Any difference in the book equity and the Company’s pro-rata share of the net assets of the investment will be reported as gain or loss at the liquidation of the investment. Losses in excess of the investment are recorded when the Company is committed to provide additional financial support. The Company uses the equity method for its investment because the Company has the ability to exercise significant influence over Tianmei Australia and its subsidiaries.
The consolidated financial statements and notes thereto are presented in United States dollars (“US Dollar” or “US$” or “$”).
Use of estimates
The preparation of consolidated financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect certain reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting periods. Actual results could differ from those estimates.
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE THREE AND SIX MONTHS ENDED MAY 31, 2017 AND 2016
(UNAUDITED) (IN U.S. $)
| 2. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued) |
Foreign currency translations
Almost all of the Company assets are located in the PRC. The functional currency for the Company’s operations is the Renminbi (“RMB”). The Company uses the United States Dollar (“US Dollar” or “US$” or “$”) for financial reporting purposes. The financial statements of the Company have been translated into US Dollars in accordance with Financial Accounting Standards Board ("FASB”) Accounting Standards Codification ("ASC") Section 830, “Foreign Currency Matters.”
All asset and liability accounts have been translated using the exchange rate in effect at the balance sheet date. Equity accounts have been translated at their historical exchange rates when the capital transactions occurred. Statements of income and comprehensive income, changes in stockholders’ equity and cash flows have been translated using the average exchange rate for the periods presented. Adjustments resulting from the translation of the Company’s financial statements are recorded as other comprehensive income (loss).
The exchange rates used to translate amounts in RMB into US Dollars for the purposes of preparing the financial statements are as follows:
| | | | May 31, 2017 | | | November 30, 2016 | |
| | | | (Unaudited) | | | | |
| | Balance sheet items, except for stockholders’ equity, as of periods end | | | 0.1474 | | | | 0.1452 | |
| | | | Three Months Ended | |
| | | | May 31, 2017 | | | May 31, 2016 | |
| | | | (Unaudited) | | | (Unaudited) | |
| | Amounts included in the statements of income and comprehensive income, changes in stockholders’ equity and cash flows for the periods presented | | | 0.1451 | | | | 0.1537 | |
| | | | Six Months Ended | |
| | | | May 31, 2017 | | | May 31, 2016 | |
| | | | (Unaudited) | | | (Unaudited) | |
| | Amounts included in the statements of income and comprehensive income, changes in stockholders’ equity and cash flows for the periods presented | | | 0.1454 | | | | 0.1535 | |
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE THREE AND SIX MONTHS ENDED MAY 31, 2017 AND 2016
(UNAUDITED) (IN U.S. $)
| 2. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued) |
Foreign currency translations (continued)
The exchange rate used to translate amounts in Australian Dollars into US Dollars for the purpose of preparing the financial statements are as follows:
| | | | May 31, 2017 | | | November 30, 2016 | |
| | | | (Unaudited) | | | | |
| | Balance sheet items, except for stockholders’ equity, as of periods end | | | 0.7435 | | | | 0.7388 | |
| | | | Three Months Ended | |
| | | | May 31, 2017 | | | May 31, 2016 | |
| | | | (Unaudited) | | | (Unaudited) | |
| | Amounts included in the statements of income and comprehensive income, changes in stockholders’ equity and cash flows for the periods presented | | | 0.7560 | | | | 0.7492 | |
| | | | Six Months Ended | |
| | | | May 31, 2017 | | | May 31, 2016 | |
| | | | (Unaudited) | | | (Unaudited) | |
| | Amounts included in the statements of income and comprehensive income, changes in stockholders’ equity and cash flows for the periods presented | | | 0.7505 | | | | 0.7314 | |
For the three months ended May 31, 2017 and 2016, foreign currency translation adjustments of $442,410and $(122,371), respectively, and for the six months ended May 31, 2017 and 2016, $501,254 and $(308,784), respectively, have been reported as other comprehensive income (loss). Other comprehensive income (loss) of the Company consists entirely of foreign currency translation adjustments.
Although the PRC government regulations now allow convertibility of the RMB for current account transactions, significant restrictions still remain. Hence, such translations should not be construed as representations that the RMB could be converted into US Dollars at that rate or any other rate.
The value of the RMB against the US Dollar and other currencies may fluctuate and is affected by, among other things, changes in the PRC’s political and economic conditions. Any significant revaluation of the RMB may materially affect the Company’s financial condition in terms of US Dollar reporting. During the year ended December 31, 2016, the PRC devalued its currency by approximately 8%. Further devaluations of its currency could occur in the future.
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE THREE AND SIX MONTHS ENDED MAY 31, 2017 AND 2016
(UNAUDITED) (IN U.S. $)
| 2. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued) |
Revenue recognition
Revenues are primarily derived from selling selenium supplements and selenium products to wholesale customers, contract distributors, and from our retail stores. The Company’s revenue recognition policies comply with FASB ASC 605 “Revenue Recognition.” The Company recognizes revenue when the following fundamental criteria are met: (i) persuasive evidence of an arrangement exists, (ii) delivery has occurred, (iii) the price paid by the customer is fixed or determinable and (iv) collection of the resulting accounts receivable is reasonably assured. The Company recognizes revenue for sales upon transfer of title to the customers. Customer purchase orders and/or contracts are generally used to determine the existence of an arrangement. Shipping documents and the completion of any customer acceptance requirements, when applicable, are used to verify product delivery. The Company assesses whether a price is fixed or determinable based upon the payment terms associated with the transaction and whether the sales price is subject to refund or adjustment. For manufacturing defects identified by customers at acceptance, the Company will return the goods to the manufacturer and receive replacements. The Company has had no product returns or sales discounts and allowances because goods delivered and accepted by customers are not returnable.
Wholesale Revenue
Wholesale revenue is recognized when title to the product is transferred to the distributors. Title is transferred upon receipt at the distributors’ locations, as determined by the specific sales terms.
The Company pays distributors certain incentives for promoting and placing its products, which allows the Company to quickly expand its distribution network and sales volume. The costs associated with these incentives is deducted from gross revenue in the consolidated statements of income and comprehensive income
Retail Revenue
Company-operated retail store revenues are recognized when payment is tendered at the point of sale.
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE THREE AND SIX MONTHS ENDED MAY 31, 2017 AND 2016
(UNAUDITED) (IN U.S. $)
| 2. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued) |
Revenue recognition (continued)
The Company’s net revenues for the three and six months ended May 31, 2017 and 2016 were comprised as follows:
| | | | Three months ended May 31, 2017 | | | Three months ended May 31, 2016 | |
| | | | | | | | |
| | Wholesale gross revenue-selenium supplements | | $ | 3,145,551 | | | $ | 3,660,259 | |
| | Wholesale gross revenue-selenium products | | | 19,018,207 | | | | 1,295,138 | |
| | Less: promotion fees-selenium products, | | | (2,090,364 | ) | | | - | |
| | | | | | | | | | |
| | Wholesale revenues, net | | | 20,073,394 | | | | 4,955,397 | |
| | Retail revenue-selenium supplements | | | 1,254,615 | | | | 481,786 | |
| | Retail revenue-selenium products | | | 936,725 | | | | - | |
| | | | | | | | | | |
| | | | $ | 22,264,734 | | | $ | 5,437,183 | |
| | | | Six months ended
May 31,
2017 | | | Six months ended
May 31,
2016 | |
| | | | | | | | |
| | Wholesale gross revenue-selenium supplements | | $ | 6,220,654 | | | $ | 3,660,259 | |
| | Wholesale gross revenue-selenium products | | | 37,031,232 | | | | 2,124,223 | |
| | Less: promotion fees-selenium products, | | | (4,164,776 | ) | | | - | |
| | | | | | | | | | |
| | Wholesale revenues, net | | | 39,087,110 | | | | 5,784,482 | |
| | Retail revenue-selenium supplements | | | 2,462,150 | | | | 870,799 | |
| | Retail revenue-selenium products | | | 1,857,606 | | | | - | |
| | | | | | | | | | |
| | | | $ | 43,406,866 | | | $ | 6,655,281 | |
Shipping costs
Shipping costs incurred by the Company are recorded as selling expenses. Shipping costs for the three and six months ended May 31, 2017 and 2016 were $20,045 and $24,427, respectively, $45,638 and $39,453, respectively.
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE THREE AND SIX MONTHS ENDED MAY 31, 2017 AND 2016
(UNAUDITED) (IN U.S. $)
| 2. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued) |
Advertising costs
Advertising costs are charged to operations when incurred. For the three and months ended May 31, 2017 and 2016, advertising expenses were $437,957 and $23,057, respectively, $887,001 and $44,519, respectively.
Cash and cash equivalents
The Company considers all demand and time deposits and all highly liquid investments with an original maturity of three months or less to be cash equivalents.
Accounts receivable
Accounts receivable are recorded at the contract amount after deduction of trade discounts and, allowances, if any, and do not bear interest. The allowance for doubtful accounts, when necessary, is the Company’s best estimate of the amount of probable credit losses from accounts receivable. The Company determines the allowance based on historical write-off experience, the level of past-due accounts based on the contractual terms of the receivable, the relationship with the customer and current economic conditions.
Account balances are charged off against the allowance after all means of collection have been exhausted and the potential for recovery is considered remote. The Company does not have any off-balance-sheet credit exposure related to its customers.
As of May 31, 2017 and November 30, 2016, accounts receivable was $14,336,885 and $11,205,011, respectively. The increase is primarily due to the recent sales with new wholesale distributors which has been subsequently fully collected. Therefore, the Company determined that an allowance for doubtful accounts was not necessary. Historically, the Company has not had any uncollectable accounts receivable.
Inventory
Inventory, comprised principally of boxed selenium capsules, selenium-glossy ganoderma capsules and selenium powder, is valued at the lower of cost or market. The value of inventory is determined using the first-in, first-out method.
The Company periodically estimates an inventory allowance for estimated unmarketable inventories when necessary. Inventory amounts are reported net of such allowances, if any. There were no allowances for inventory as of May 31, 2017 and November 30, 2016.
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE THREE AND SIX MONTHS ENDED MAY 31, 2017 AND 2016
(UNAUDITED) (IN U.S. $)
| 2. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued) |
Fair value of financial instruments
FASB ASC 820,“Fair Value Measurement” specifies a hierarchy of valuation techniques based upon whether the inputs to those valuation techniques reflect assumptions other market participants would use based upon market data obtained from independent sources (observable inputs). In accordance with ASC 820, the following summarizes the fair value hierarchy:
Level 1 Inputs – Unadjusted quoted market prices for identical assets and liabilities in an active market that the Company has the ability to access.
Level 2 Inputs – Inputs other than the quoted prices in active markets that are observable either directly or indirectly.
Level 3 Inputs – Inputs based on valuation techniques that are both unobservable and significant to the overall fair value measurements.
ASC 820 requires the use of observable market data, when available, in making fair value measurements. When inputs used to measure fair value fall within different levels of the hierarchy, the level within which the fair value measurement is categorized is based on the lowest level input that is significant to the fair value measurement. Valuation techniques used need to maximize the use of observable inputs and minimize the use of unobservable inputs.
The Company did not identify any assets or liabilities that are required to be presented at fair value on a recurring basis. Carrying values of non-derivative financial instruments, including cash and cash equivalents, accounts receivable, inventory, prepaid expenses, accounts payable, taxes payable, accrued liabilities and other payables, and loan from stockholder, approximated their fair values due to the short nature of these financial instruments. There were no changes in methods or assumptions during the periods presented.
Property and Equipment
Fixed assets are recorded at cost, less accumulated depreciation. Cost includes the prices paid to acquire the assets, and any expenditures that substantially increase an asset’s value or extends the useful life of an existing asset. Depreciation is computed using the straight-line method over the estimated useful lives of the assets. Major repairs and betterments that significantly extend original useful lives or improve productivity are capitalized and depreciated over the periods benefited. Maintenance and repairs are generally expensed as incurred. The estimated useful lives for fixed asset categories are as follows:
| | Furniture and equipment | 3-5years |
| | Motor vehicles | 4 years |
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE THREE AND SIX MONTHS ENDED MAY 31, 2017 AND 2016
(UNAUDITED) (IN U.S. $)
| 2. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued) |
Prepaid expenses
On January 5, 2011, the Company entered into a license agreement for the technology utilized for the manufacture of its selenium capsules and powder products from an unrelated third party for five years from January 2011 to December 2015. On December 30, 2015, the Company renewed the license agreement for another five years to December 2020 for $86,912 (RMB 600,000) each year. The related prepaid licensing fees as of May 31, 2017, and November 30, 2016 were $51,594 and $7,259, respectively. The license provides for renewal options. Since this agreement requires the advance payment of the annual licensing fee, there were no minimum payments remaining under this agreement as of May 31, 2017 and November 30, 2016.
On September 30, 2016, the Company entered into a six-month agreement with an advertising company for $908,725 (RMB 6,000,000). OnApril 1, 2017, the Company renewed the advertising agreement for another six months which started on April 1, 2017 and ends on September 30, 2017. As of May 31, 2017, and November 30, 2016, the unamortized balance of $589,640 and $605,816, respectively, was included in prepaid expenses on the balance sheet.
Impairment of long-lived assets
The Company applies FASB ASC 360, “Property, Plant and Equipment,” which addresses the financial accounting and reporting for the recognition and measurement of impairment losses for long-lived assets. In accordance with ASC 360, long-lived assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. The Company will recognize the impairment of long-lived assets in the event the net book value of such assets exceeds the future undiscounted cash flows attributable to those assets. No impairment of long-lived assets was recognized for the three and six months ended May 31, 2017 and 2016.
Statutory reserve fund
Pursuant to corporate law of the PRC, the Company is required to transfer 10% of its net income, as determined under PRC accounting rules and regulations, to a statutory reserve fund until such reserve balance reaches 50% of the Company’s registered capital. The statutory reserve fund is non-distributable other than during liquidation and can be used to fund previous years’ losses, if any, and may be utilized for business expansion or used to increase registered capital, provided that the remaining reserve balance after use is not less than 25% of registered capital. The statutory reserve fund was $759,094 as of May 31, 2017 and November 30, 2016, respectively. As of November 30, 2016, the required statutory reserve fund has been fully funded.
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE THREE AND SIX MONTHS ENDED MAY 31, 2017 AND 2016
(UNAUDITED) (IN U.S. $)
| 2. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued) |
Income taxes
The Company accounts for income taxes in accordance with FASB ASC 740, “Income Taxes” (“ASC 740”), which requires the recognition of deferred income taxes for differences between the basis of assets and liabilities for financial statement and income tax purposes. Deferred tax assets and liabilities represent the future tax consequences for those differences, which will either be taxable or deductible when the assets and liabilities are recovered or settled. Deferred taxes are also recognized for operating losses that are available to offset future taxable income. A valuation allowance is established when necessary to reduce deferred tax assets to the amount expected to be realized.
ASC 740 addresses the determination of whether tax benefits claimed or expected to be claimed on a tax return should be recorded in the financial statements. Under ASC 740, the Company may recognize the tax benefit from an uncertain tax position only if it is more likely than not that the tax position will be sustained on examination by the taxing authorities, based on the technical merits of the position. The tax benefits recognized in the financial statements from such a position would be measured based on the largest benefit that has a greater than 50% likelihood of being realized upon ultimate settlement. ASC 740 also provides guidance on de-recognition of income tax assets and liabilities, classification of current and deferred income tax assets and liabilities, and accounting for interest and penalties associated with tax positions. As of May 31, 2017 and November 30, 2016, the Company does not have a liability for any unrecognized tax benefits. The Company’s tax filings are subject to examination by the tax authorities. The tax years of 2013, 2014 and 2015 remain open to examination by tax authorities in the PRC.
The income tax laws of various jurisdictions in which the Company and its subsidiaries operate are summarized as follows:
United States
The Company is subject to United States tax at graduated rates from 15% to 35%. No provisions for income tax in the United States have been made as the Company had no U.S. taxable income for the three and six months ended May 31, 2017 and 2016.
British Virgin Islands (“BVI”)
Biotechnology International is incorporated in the BVI and is governed by the income tax laws of the BVI. According to current BVI income tax law, the applicable income tax rate for the Company is 0%.
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE THREE AND SIX MONTHS ENDED MAY 31, 2017 AND 2016
(UNAUDITED) (IN U.S. $)
| 2. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued) |
Income taxes (continued)
Hong Kong
Hong Kong Gewang is incorporated in Hong Kong. Pursuant to the income tax laws of Hong Kong, the Company is not subject to tax on non-Hong Kong source income.
The People's Republic of China (“PRC”)
Gewang Selenium and Guangdong Gewang are subject to an Enterprise Income Tax at 25% and file their own tax returns.
| 3. | RECENTLY ISSUED ACCOUNTING STANDARDS |
In May 2014, the FASB issued ASU No. 2014-09,"Revenue from Contracts with Customers (Topic 606).'' This guidance supersedes current guidance on revenue recognition in Topic 605, "Revenue Recognition.'' In addition, there are disclosure requirements related to the nature, amount, timing,and uncertainty of revenue recognition. In August 2015, the FASB issued ASU No.2015-14 to defer the effective date of ASU No. 2014-09 for all entities by one year. For public business entities that follow U.S. GAAP, the deferral results in the new revenue standard being effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2017, with early adoption permitted for interim and annual periods beginning after December 15, 2016. In March 2016, the FASB issued Accounting Standards Update No. 2016-12, Revenue from Contracts with Customers, with respect to Principal versus Agent Considerations. In April 2016, the FASB issued Accounting Standards Update No. 2016-12, Revenue from Contracts with Customers, with respect to Identifying Performance Obligations and Licensing. In April 2016, the FASB issued Accounting Standards Update No. 2016-12, Revenue from Contracts with Customers, with respect to Narrow-Scope Improvements and Practical Expedients. In December 2016, the FASB issued Accounting Standards Update No. 2016-20, Revenue from Contracts with Customers, with respect to Technical Corrections and Improvements. We do not believe this standard will have a material impact on our consolidated financial statements.
In February 2016, the FASB issued ASU 2016-02, “Leases.” The new standard establishes a right-of-use (“ROU”) model that requires a lessee to record a ROU asset and a lease liability on the balance sheet for all leases with terms longer than 12 months. Leases will be classified as either finance or operating, with classification affecting the pattern of expense recognition in the income statement. The new standard is effective for fiscal years beginning after December 15, 2018, including interim periods within those fiscal years. A modified retrospective transition approach is required for lessees for capital and operating leases existing at, or entered into after, the beginning of the earliest comparative period presented in the financial statements, with certain practical expedients available. We do not believe this standard will have a material impact on our consolidated financial statements as currently all leases are prepaid.
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE THREE AND SIX MONTHS ENDED MAY 31, 2017 AND 2016
(UNAUDITED) (IN U.S. $)
| 3. | RECENTLY ISSUED ACCOUNTING STANDARDS (continued) |
In July 2015, the FASB issued ASU 2015-11 (Subtopic 330), “Simplifying the Measurement of Inventory,” which provides guidance to companies who account for inventory using either the first-in, first-out (“FIFO”) or average cost methods. The guidance states that companies should measure inventory at the lower of cost or net realizable value. Net realizable value is defined as the estimated selling prices in the ordinary course of business, less reasonably predictable costs of completion, disposal and transportation. ASU 2015-11 is effective for fiscal years beginning after December 15, 2016. Early adoption is permitted. This accounting standard update is not expected to have a material impact on the Company’s consolidated financial statements.
In August 2014, the FASB issued authoritative guidance that requires an entity’s management to evaluate whether there are conditions or events, considered in the aggregate, that raise substantial doubt about the entity’s ability to continue as a going concern and requires additional disclosures if certain criteria are met. This guidance is effective for fiscal periods ending after December 15, 2016, with early adoption permitted. This accounting standard update is not expected to have a material impact on the Company’s consolidated financial statements.
| 4. | RELATED PARTY TRANSACTIONS |
The Company entered into a one-year promotion agreement that expired on March 31, 2017 with Guangdong Tianmei, in which it holds an indirect 30% ownership interest and renewed the promotion agreement on March 31, 2017 for one year expiring on March 31, 2018. Under the agreement, Guangdong Tianmei introduced the Company to Guangzhou Huayuda Commerce and Trade Co., Ltd. (“Guangzhou Huayuda”), which initially had 14 supermarket stores and in August 2016 expanded to 200 stores. The Company sells selenium enriched products to these supermarkets and pays a monthly promotion fee to Guangdong Tianmei for each product sold in each store of RMB 108 (US$16.60). Sales to Guangzhou Huayuda were approximately RMB 5,366,000 (US$780,000) and RMB 1,154,000 (US$177,000), respectively, RMB 14,747,000 (US$2,144,000) and RMB 1,154,000 (US$177,000), respectively, for the three and six months ended May 31, 2017 and 2016. Promotion expenses incurred during the three and six months ended May 31, 2017 and 2016 were $357,373 and $20,421, respectively, $716,115 and $20,421, respectively.
The Company entered into an agreement with Guangdong Tianmei on June 10, 2015 to license the use of the Company’s trademark for 10 years. Trademark revenue recorded for the three and six months ended May 31, 2017 and 2016 were $0 and $0, respectively, $1,412 and $1,490, respectively. The future commitment is approximately $1,400 each year.
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE THREE AND SIX MONTHS ENDED MAY 31, 2017 AND 2016
(UNAUDITED) (IN U.S. $)
| 4. | RELATED PARTY TRANSACTIONS (continued) |
Equity investment
On April 28, 2016, the Company's wholly-owned subsidiary, Biotechnology International, entered into an investment agreement with Guangdong Tianmei. At that time, 88% of the equity in Guangdong Tianmei was owned by two individuals who together directly or indirectly owned approximately 57% of the Company's outstanding shares. The investment agreement required that Biotechnology International pay US$1,000,000 to acquire a 30% interest in an Australian corporation, which would indirectly own 100% of the equity in Guangdong Tianmei.
The acquisition by Biotechnology International of 30% of Tianmei Australia was completed in May 2016, at which time Tianmei Australia acquired ownership, through its wholly owned subsidiaries, of Guangdong Tianmei.
The net worth of Guangdong Tianmei at the time of the acquisition was $4,888,840, 30% of which was $1,466,652. As the Company and Guangdong Tianmei were under common control at the time of the acquisition, the $466,652 by which the Company's share of the net book value of Guangdong Tianmei exceeded the purchase price had been recorded as an increase to additional paid-in capital.
The changes in the equity investment are summarized as follows:
| | Initial investment-April 28, 2016 | | $ | 1,466,652 | |
| | Pro rata share of net income for the year ended November 30, 2016 | | | 4,536,760 | |
| | | | | | |
| | Investment, November 30, 2016 | | | 6,003,412 | |
| | Pro rata share of net income for the six months ended May 31, 2017 | | | 4,665,947 | |
| | | | | | |
| | Investment, May 31, 2017 | | $ | 10,669,359 | |
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE THREE AND SIX MONTHS ENDED MAY 31, 2017 AND 2016
(UNAUDITED) (IN U.S. $)
| 4. | RELATED PARTY TRANSACTIONS (continued) |
The following is summarized balance sheet information of the investeeas ofended May 31, 2017:
| | Current assets | | $ | 51,625,008 | |
| | | | | | |
| | Noncurrent assets | | $ | 1,825,790 | |
| | | | | | |
| | Current liabilities | | $ | 3,123,635 | |
| | | | | | |
| | Noncurrent liabilities | | $ | - | |
| | | | | | |
| | Equity | | $ | 50,327,163 | |
The following is a summary of results of operations of the investee for the six months ended May 31, 2017:
| | Revenue | | $ | 39,446,904 | |
| | Cost of revenue | | | (13,228,818 | ) |
| | Expenses | | | (12,383,619 | ) |
| | | | | | |
| | Net income | | $ | 13,834,467 | |
The Company leases its warehouse and office space from an unrelated third party under a one-year operating lease. The lease required the Company to prepay the total rent of US$86,912 (RMB 600,000) in advance for one year. On June 29, 2016, the Company renewed the lease with total rent of approximately US$86,900 (RMB 600,000), which commenced on July 2, 2016 and expires on July 1, 2017.
The following additional leases were in effect at May 31, 2017:
| ● | The Company leases its flagship store in Guangzhou from an unrelated third party. The lease commenced on June 1, 2016 and was to expire on May 31, 2017, and was renewed for another year. The lease required the Company to prepay the rent of $154,514(RMB 960,000) in advance for one year. The Company paid the rent in June 2017. |
| ● | The Company leases its Foshan store, Longyan store and Zhuzhou store from three unrelated third parties. All three leases commenced on June 1, 2016 and were to expire on May 31, 2017 and were renewed for another year and expire on May 31, 2018. These leases each required the Company to prepay the rent of $61,912 (RMB 420,000) in advance for one year. The Company fully paid the rent in June 2017. Since these leases require the advance payment of the annual rent, there are no minimum payments remaining under these leases. |
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE THREE AND SIX MONTHS ENDED MAY 31, 2017 AND 2016
(UNAUDITED) (IN U.S. $)
Prepaid lease payments were $7,371 and $ 221,123 at May 31, 2017 and November 30, 2016, respectively. Rent expense for the three and six months ended May 31, 2017 and 2016 was $102,907 and $66,872, respectively, $205,028 and $133,557, respectively.
Fixed assets as of May 31, 2017 and November 30, 2016 are summarized as follows:
| | | | May 31, 2017 | | | November 30,
2016 | |
| | | | | | | | |
| | Electronic equipment | | $ | 127,785 | | | $ | 125,850 | |
| | Motor vehicles | | | 123,014 | | | | 121,151 | |
| | Office equipment | | | 12,216 | | | | 12,031 | |
| | | | | | | | | | |
| | | | | 263,015 | | | | 259,032 | |
| | Less: accumulated depreciation | | | (158,726 | ) | | | (130,265 | ) |
| | | | | | | | | | |
| | Fixed assets - net | | $ | 104,289 | | | $ | 128,767 | |
For the three and six months ended May 31, 2017 and 2016, depreciation expense was $13,050 and $14,653, respectively, $26,050 and $24,761, respectively.
The Company obtained non-interest bearing demand loans from a former officer and stockholder, who resigned on April 8, 2016. The loans of $228,359 and $228,238 at May 31, 2017 and November 30, 2016, respectively, are reflected as loans from third party.
The Company obtainednon-interest bearingdemand loans from one of its stockholders. The loans of $407,677 and $237,639 atMay 31, 2017 and November 30, 2016, respectively, are reflected as loans from stockholder.
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE THREE AND SIX MONTHS ENDED MAY 31, 2017 AND 2016
(UNAUDITED) (IN U.S. $)
The provision for income taxes for the three and six months ended May 31, 2017 and 2016 consisted of the following:
| | | | Three Months Ended May 31, | | | Six Months Ended May 31, | |
| | | | 2017 | | | 2016 | | | 2017 | | | 2016 | |
| | | | (Unaudited) | | | (Unaudited) | | | (Unaudited) | | | (Unaudited) | |
| | | | | | | | | | | | | | |
| | Current | | $ | 1,319,811 | | | $ | 416,553 | | | $ | 2,546,163 | | | $ | 539,969 | |
| | Deferred | | | - | | | | - | | | | - | | | | - | |
| | | | | | | | | | | | | | | | | | |
| | | | $ | 1,319,811 | | | $ | 416,553 | | | $ | 2,546,163 | | | $ | 539,969 | |
No provisions for income taxes in the United States have been made. The Company did not generate any income in the United States or otherwise have any U.S. taxable income. The Company does not believe that it has any U.S. Federal income tax liabilities with respect to any transactions that the Company or any of its subsidiaries may have engaged in through May 31, 2017. However, there can be no assurance that the Internal Revenue Service (“IRS”) will agree with this position, and therefore the Company ultimately could be liable for U.S. Federal income taxes, interest and penalties. The tax years ended November 30, 2016 and December 31, 2015 and 2014 remain open to examination by the IRS.
The Company has not established deferred income taxes on accumulated undistributed earnings of Tianmei Australia. Such earnings are expected to remain reinvested indefinitely. Further, repatriation of all accumulated earnings would be impracticable to the extent that such earnings represents capital needed to support normal business operations.
The Company did not file on time its U.S. federal income tax returns, including, without limitation, information returns on IRS Form 5471, “Information Return of U.S. Persons with Respect to Certain Foreign Corporations” for the short year tax return ended November 30, 2015 filed as a result of the change in fiscal year. Failure to furnish any income tax returns and information returns with respect to any foreign business entity required, within the time prescribed by the IRS, subjects the Company to certain civil penalties. Management is of the opinion that penalties, if any, that may be assessed would not be material to the consolidated financial statements.
| 9. | CONCENTRATION OF CREDIT AND BUSINESS RISKS |
Cash and cash equivalents
Substantially all of the Company’s assets and bank accounts are in banks located in the PRC and are not covered by protection similar to that provided by the FDIC on funds held in United States banks.
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE THREE AND SIX MONTHS ENDED MAY 31, 2017 AND 2016
(UNAUDITED) (IN U.S. $)
| 9. | CONCENTRATION OF CREDIT AND BUSINESS RISKS (continued) |
Major customers
For the three and six months ended May 31, 2017, three customers accounted for 66% and 64% of total sales, respectively, the largest of which accounted for 25% and 25%, respectively. For the three and six months ended May 31, 2016, three customers accounted for 64% and 64% of total sales, respectively, the largest of which accounted for 29% and 27%, respectively. As of May 31, 2017, three customers accounted for 71% of trade accounts receivable, the largest of which accounted for 27%. As of November 30, 2016, three customers accounted for 64% of trade accounts receivable, the largest of which accounted for 24%.
Vulnerability Due to Operations in the PRC
The Company’s operations may be adversely affected by significant political, economic and social uncertainties in the PRC. Although the PRC government has been pursuing economic reform policies for more than twenty years, no assurance can be given that the PRC government will continue to pursue such policies or that such policies may not be significantly altered, especially in the event of a change in leadership, social or political disruption or unforeseen circumstances affecting the PRC’s political, economic and social conditions. There is also no guarantee that the PRC government’s pursuit of economic reforms will be consistent or effective. The economy in the PRC has recently started to narrow.
| 10. | COMMITMENTS AND CONTINGENCIES |
On December 30, 2015, the Company entered into a technology usage agreement with the Academy for the right to use a non-patented selenium-enrichment technology for its supplements manufacturing. The agreement commenced on December 30, 2015 and expires on December 29, 2020. The annual fee of RMB 600,000 (approximately US$87,000) is required to be paid in advance before December 30 each year.
As of September 1, 2016, the Company has a long-term agreement with theAcademy. This agreement entitles the Company to the exclusive right of first refusal to use the research related to advanced selenium-enrichment techniques and technology that the Academy develops. The agreement calls for quarterly payments of approximately $579,700 (RMB 4,000,000). For the use of the techniques and/or technology developed, additional charges will be negotiated. The agreement expires on August 31, 2026.
On April 8, 2016, Guangdong Gewang entered into a Performance Salary Assessment Agreement with the Company’s Chief Executive Officer (“CEO”). The agreement states that the CEO would receive additional monthly compensation of RMB 50,000 (approximately US$7,000), only when the monthly net income of Guangdong Gewang exceeds RMB 2,500,000 (approximately US$363,000). The agreement was to expire on April 7, 2017 and was renewed for an additional year. For the three and six months ended May 31, 2017, the CEO received $21,000 and $42,000, respectively, as additional monthly compensation. For the three and six months ended May 31, 2016, the CEO received $7,000 and $7,000, respectively, as additional monthly compensation.
On July 1, 2016, the Company entered into three year agreements with four of its directors for total compensation of approximately $15,000 (RMB 110,000) per month.
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE THREE AND SIX MONTHS ENDED MAY 31, 2017 AND 2016
(UNAUDITED) (IN U.S. $)
| 11. | PARENT COMPANY ONLY CONDENSED FINANCIAL INFORMATION |
The following is the condensed financial information of China Gewang Biotechnology, Inc., the US parent, consisting of the balance sheet as of November 30, 2016, and statements of income and cash flows for the year ended November 30, 2016.
Condensed Balance Sheet
| | ASSETS | | November 30, 2016 | |
| | | | | |
| | Other receivable from Guangdong Gewang | | $ | 14,848,200 | |
| | Investment in subsidiaries | | | 10,516,759 | |
| | Equity investment | | | 6,003,412 | |
| | | | | | |
| | TOTAL ASSETS | | $ | 31,368,371 | |
| | | | | | |
| | LIABILITIES AND stockholders’ EQUITY | | | | |
| | | | | | |
| | Current liabilities: | | | | |
| | Accrued expenses | | $ | 33,375 | |
| | Loans from third party | | | 220,396 | |
| | Loans from stockholder | | | 236,216 | |
| | | | | | |
| | Total current liabilities | | | 489,987 | |
| | | | | | |
| | Stockholders’ equity: | | | | |
| | Common stock, $0.001 par value, 100,000,000 authorized, 75,000,000 shares issued and outstanding as of November 30, 2016 | | | 75,000 | |
| | Additional paid-in capital | | | 16,980,102 | |
| | Retained earnings | | | 14,771,252 | |
| | Statutory reserve fund | | | 759,094 | |
| | Other comprehensive (loss) | | | (1,707,064 | ) |
| | | | | | |
| | Total stockholder’s equity | | | 30,878,384 | |
| | | | | | |
| | TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY | | $ | 31,368,371 | |
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE THREE AND SIX MONTHS ENDED MAY 31, 2017 AND 2016
(UNAUDITED) (IN U.S. $)
| 11. | PARENT COMPANY ONLY CONDENSED FINANCIAL INFORMATION (continued) |
Condensed Statement of Income
| | | | For the year ended November 30, 2016 | |
| | | | | |
| | Revenues: | | | |
| | Share of earnings from investment in subsidiaries | | $ | 8,821,783 | |
| | Equity investment | | | 4,536,760 | |
| | | | | | |
| | Operating expenses: | | | | |
| | General and administrative | | | 188,852 | |
| | | | | | |
| | Net income | | $ | 13,169,691 | |
Condensed Statement of Cash Flows
| | | | For the year ended November 30, 2016 | |
| | | | | |
| | Cash flows from operating activities: | | | |
| | Net income | | $ | 13,169,691 | |
| | Adjustments to reconcile net income to net cash provided by (used in) operating activities | | | | |
| | Share of earnings from investment in subsidiaries | | | (8,821,783 | ) |
| | Share of earnings from equity investment | | | (4,536,760 | ) |
| | Increase in accrued expenses and other liabilities | | | 188,852 | |
| | | | | | |
| | Net cash provided by (used in) operating activities | | | - | |
| | | | | | |
| | Net change in cash | | | - | |
| | Cash, beginning of period | | | - | |
| | | | | | |
| | Cash, end of period | | $ | - | |
| | | | | | |
| | Noncash financing activities: | | | | |
| | Payment of accrued expenses and other payables by shareholder | | $ | 298,938 | |
CHINA GEWANG BIOTECHNOLOGY, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE THREE AND SIX MONTHS ENDED MAY 31, 2017 AND 2016
(UNAUDITED) (IN U.S. $)
| 11. | PARENT COMPANY ONLY CONDENSED FINANCIAL INFORMATION (continued) |
Basis of Presentation
The Company records its investment in its subsidiaries and its equity investment under the equity method of accounting. Such investments are presented as “Investment in subsidiaries” and “Equity investment” on the condensed balance sheet and the subsidiaries profits are presented as “Share of earnings from investment in subsidiaries” and “Share of earnings from equity investment” on the condensed statement of income.
Certain information and footnote disclosures normally included in financial statements prepared in conformity with accounting principles generally accepted in the United States of America have been condensed or omitted. The parent only financial information has been derived from the Company’s consolidated financial statements and should be read in conjunction with the Company’s consolidated financial statements.
There were no cash transactions in the US parent company during the year ended November 30, 2016.
Restricted Net Assets
Under the PRC laws and regulations, the Company’s PRC subsidiaries are restricted in their ability to transfer certain of their net assets to the Company in the form of dividend payments, loans or advances. The restricted net assets of the Company’s PRC subsidiaries were approximately $31,368,000 as of November 30, 2016.
The Company’s operations and revenues are conducted and generated in the PRC, and all of the Company’s revenues being earned and currency received are denominated in RMB. RMB is subject to the foreign exchange control regulations in China, and, as a result, the Company may be unable to distribute any dividends outside of China due to the PRC foreign exchange control regulations that restrict the Company’s ability to convert RMB into US Dollars.
Schedule I of Article 5-04 of Regulation S-X requires the condensed financial information of the parent company to be filed when the restricted net assets of consolidated subsidiaries exceed 25 percent of consolidated net assets as of the end of the most recently completed fiscal year. For purposes of the above test, restricted net assets of consolidated subsidiaries shall mean that amount of the Company’s proportionate share of net assets of consolidated subsidiaries (after intercompany eliminations) which as of the end of the most recent fiscal year may not be transferred to the parent company by its subsidiaries in the form of loans, advances or cash dividends without the consent of a third party. The condensed parent company only financial statements have been prepared in accordance with Rule 12-04, Schedule I of Regulation S-X as the restricted net assets of the Company’s PRC subsidiaries exceed 25% of the consolidated net assets of the Company.
The Company’s management has performed subsequent events procedures through July 12, 2017, which is the date the consolidated financial statements were available to be issued. There were no subsequent events requiring adjustment to or disclosure in the consolidated financial statements.
| ITEM 2. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
Results of Operations
The following table sets forth key components of our results of operations during the three and six months ended May 31, 2017 and 2016, and the percentage changes between those two periods.
Three months ended May 31, 2017 and 2016:
| | | May 31, | | | May 31, | | | | |
| | | 2017 | | | 2016 | | | Change | |
| | | (US $) | | | (US $) | | | % | |
| Revenue | | $ | 22,264,734 | | | $ | 5,437,183 | | | $ | 309 | % |
| Cost of Sales | | | (14,645,390 | ) | | | (3,025,001 | ) | | | 384 | % |
| Gross profit | | | 7,619,344 | | | | 2,412,182 | | | | 216 | % |
| Selling and marketing expenses | | | 1,407,424 | | | | 649,302 | | | | 117 | % |
| General and administrative expenses | | | 382,422 | | | | 122,978 | | | | 211 | % |
| Research and development expenses | | | 583,868 | | | | - | | | | 100 | % |
| Total operating expenses | | | 2,373,714 | | | | 772,280 | | | | 207 | % |
| Income from operations | | | 5,245,630 | | | | 1,639,902 | | | | 220 | % |
| Other income | | | 20,880 | | | | 6,327 | | | | 230 | % |
| Income before provision for income taxes | | | 5,266,510 | | | | 1,649,229 | | | | 219 | % |
| Provision for income taxes | | | 1,319,811 | | | | 416,553 | | | | 217 | % |
| Equity in income of investee | | | 2,512,935 | | | | 408,275 | | | | 516 | % |
| Net income before noncontrolling interests | | | 6,459,634 | | | | 1,637,951 | | | | 294 | % |
| Noncontrolling interests | | | (79,622 | ) | | | (62,483 | ) | | | 27 | % |
| Net income attributable to common stockholders | | $ | 6,380,012 | | | $ | 1,575,468 | | | | 305 | % |
| Earnings per common share-basic and diluted | | | 0.09 | | | | 0.03 | | | | 200 | % |
Six months ended May 31, 2017 and 2016:
| | | May 31, | | | May 31, | | | | |
| | | 2017 | | | 2016 | | | Change | |
| | | (US $) | | | (US $) | | | % | |
| Revenue | | $ | 43,406,866 | | | $ | 6,655,281 | | | $ | 552 | % |
| Cost of Sales | | | (28,599,695 | ) | | | (3,367,609 | ) | | | 749 | % |
| Gross profit | | | 14,847,171 | | | | 3,287,672 | | | | 352 | % |
| Selling and marketing expenses | | | 2,852,337 | | | | 919,973 | | | | 210 | % |
| General and administrative expenses | | | 763,777 | | | | 259,939 | | | | 194 | % |
| Research and development expenses | | | 1,163,280 | | | | - | | | | 100 | % |
| Total operating expenses | | | 4,779,394 | | | | 1,179,912 | | | | 305 | % |
| Income from operations | | | 10,067,777 | | | | 2,107,760 | | | | 378 | % |
| Other income | | | 21,157 | | | | 12,585 | | | | 68 | % |
| Income before provision for income taxes | | | 10,088,934 | | | | 2,120,345 | | | | 376 | % |
| Provision for income taxes | | | 2,546,163 | | | | 539,969 | | | | 372 | % |
| Equity in income of investee | | | 4,665,947 | | | | 408,275 | | | | 1,043 | % |
| Net income before noncontrolling interests | | | 12,208,718 | | | | 1,988,651 | | | | 514 | % |
| Noncontrolling interests | | | (152,770 | ) | | | (80,995 | ) | | | 89 | % |
| Net income attributable to common stockholders | | $ | 12,055,948 | | | $ | 1,907,656 | | | | 532 | % |
| Earnings per common share-basic and diluted | | | 0.16 | | | | 0.03 | | | | 433 | % |
Sales
Our sales increased to $22,264,734 and $43,406,866 for the three and six months ended May 31, 2017, respectively, from $5,437,183 and $6,655,281 for the three and six months ended May 31, 2016, respectively. Since the end of the prior year's quarter, we have begun to wholesale products manufactured by other companies through our distribution network and have also increased the volume of sales of our Jindanli branded products. Since March 2016, we have signed agreements with seven distributors to distribute selenium products to chain stores encompassing hundreds of retail stores, and we made net sales of selenium-related products totaling $16,927,842 and $32,866,456 to these distributors during the three and six months ended May 31, 2017, respectively. The following factors had the greatest impact on our increase in sales:
| | ● | Total wholesale net sales, including our Jindanli branded products and products manufactured by other companies to our distribution network of 15 wholesale customers by our headquarters marketing personnel increased by $15,117,997 and $33,302,628 for the three and six months ended May 31, 2017, respectively, in comparison to prior periods, primarily due to an increase in the number of sales personnel during the latter portion of fiscal year 2016; |
| | | |
| | ● | Of the total increase in wholesale sales for three and six months ended May 31, 2017, $16,927,842 and $32,866,456 was attributable to the wholesale of 89 selenium product types manufactured by other companies. In the first quarter of fiscal year 2016, we did not wholesale any products other than our Jindanli branded products and in the second quarter total wholesale sales of non-Jindanli products were only $1,295,138. Since March 2016, we have signed agreements with six manufacturers and with seven distributors to create a network for our distribution of selenium products to chain stores, and this expansion of our customer base has greatly increased our volume of sales. |
| | | |
| | ● | Retail sales increased by $1,709,554 and $3,448,957, or 355% and 396% during the three and six months ended May 31, 2017, respectively. Due to increased rent and unsatisfactory pedestrian flow, we closed our Changcheng, Xiamen and Changsha stores on May 31, 2016 and opened stores in Foshan, Longyan and Zhuzhou and a flagship store in Guangzhou on June 1, 2016. The new stores are located in areas with better pedestrian flow, resulting in a sharp increase in retail sales. In addition, the new stores stock an inventory of over 90 selenium products, whereas the previous stores stocked only our three selenium supplements. |
Gross Profit
For the past three years we have sold our three selenium supplements, and only in March 2016 began to resell a wide variety of selenium-related products that we purchase at wholesale. After increasing during the 2014 and 2015 fiscal years, the unit prices that we pay to our manufacturers for the selenium supplements stabilized in the second quarter of fiscal 2016. As a result, our gross margin for selenium supplements was not significantly changed: 72.1% and 72.1% in the three and six months ended May 31, 2017, respectively, compared to 71.6% and 72.7% in the three and six months ended May 31, 2016, respectively. However, the selenium-related products that we resold during three and six months ended May 31, 2017 yielded a gross margin of only 28% and 25%, respectively. As a result, our overall gross margin during the three and six months ended May 31, 2017 declined to 34.2% from 44.4% and 34.2% from 49.4%, respectively.
Selling expenses
Our selling and marketing expenses increased by 117% to $1,407,424 and 210% to $2,852,337, respectively, for the three and six months ended May 31, 2017 from $649,302 and $919,973 for the three and six months ended May 31, 2016, respectively. Our selling expenses include rent, transportation expenses, advertising expenses and salaries incurred for the sales function, all of which will tend to increase as our sales increase. In the three and six months ended May 31, 2017, we incurred $437,957 and $887,001, respectively, in advertising expense, which is significantly higher than we incurred in the past. We are committed to paying our current rate of advertising costs until September 30, 2017.
General and administrative expenses
Our general and administrative (“G&A”) expenses increased by 211% to $382,422 and 194% to $763,777 for the three and six months ended May 31, 2017, respectively, from $122,978 and $259,939 for the three and six months ended May 31, 2016, respectively. The largest components of our G&A expenses are the salaries of administrative personnel and fringe benefits provided to all of our staff. The change in G&A expenses occurred primarily as a result of the expansion of our business over the prior year, as reflected in the growth of our sales, which led to increases in travel and entertainment expenses. In addition, to enable us to manage our expanding operations, we added administrative personnel.
Income from operations
Because the sharp increase in our sales generated a material increase in our gross profit, income from operations increased by 220% to $5,245,630 and 378% to $10,067,777 for the three and six months ended May 31, 2017, respectively, from $1,639,902 and $2,107,760 for the three and six months ended May 31, 2016, respectively.
As we have very little debt, our interest expense was substantially offset by interest income earned on our bank balances, resulting in other income of $20,880 and $6,327, respectively, for the three months ended May 31, 2017 and 2016, and $21,157 and $12,585, respectively, for the six months ended May 31, 2017 and 2016. Our pre-tax income, therefore, was $5,266,510 and $10,088,934 for the three and six months ended May 31, 2017, respectively, from $1,646,229 and $2,120,345 for the three and six months ended May 31, 2016, respectively.
Net income
Due to the increase in our pre-tax income, our provision for income taxes increased by 217% to $1,319,811 and 372% to $2,546,163 for the three and six months ended May 31, 2017, respectively, from $416,553 and $539,969 for the three and six months ended May 31, 2016, respectively. In this period, our effective tax rate was the same as the statutory rate of 25%.
We also recorded equity investment income of $2,512,935 and $4,665,947, representing our 30% interest in the net income reported by Tianmei Australia for the three and six months ended May 31, 2017, respectively. After deducting the provision for income taxes and adding equity in income of investee, China Gewang reported net income before noncontrolling interests of $6,459,634 and $12,208,718, respectively, $1,637,951 and $1,988,651, respectively, for the three and six months ended May 31, 2017 and 2016.
Before August 8, 2016, the VIE Agreements assigned to Gewang Selenium 95% of the net income of Guangdong Gewang. On August 8, 2016, Gewang Selenium purchased 98% of the registered equity of Guangdong Gewang. Our non-controlling interest, therefore, was reduced from 5% to 2%. We recorded a deduction for noncontrolling interests of $79,622 and $152,770, respectively, $62,483 and $80,995, respectively, for the three and six months ended May 31, 2017 and 2016, after which our net income attributable to common stockholders was $6,380,012 ($0.09 per share) and $12,055,948 ($0.16 per share), respectively, for the three and six months ended May 31, 2017, compared to $1,575,468 ($0.03 per share) and $1,907,656 ($0.03 per share), respectively, for the three and six months ended May 31, 2016.
Foreign Currency Translation Adjustment
Our reporting currency is the U.S. dollar. Our local currency, the Renminbi (RMB), is our functional currency. Results of operations and cash flow are translated at average exchange rates during the period being reported upon, and assets and liabilities are translated at the unified exchange rate as quoted by the People’s Bank of China on the balance sheet date. Translation adjustments resulting from this process are included in other comprehensive income. For the three and six months ended May 31, 2017 and 2016, foreign currency translation adjustments of $442,410 and $501,254, respectively, $(122,371) and $(308,784), respectively, have been reported as other comprehensive income (loss) in the consolidated statement of income and comprehensive income (loss). The loss during the three and six months ended May 31, 2016 was primarily due to devaluation of the PRC currency of approximately 3.5% in three months ended February 29, 2016. Additional devaluations could occur in the future and affect our results.
RMB is not freely convertible into the currency of other nations. All such exchange transactions must take place through authorized institutions. There is, therefore, no guarantee the RMB amounts could have been, or could be, converted into US dollars at the rates used in translation. Substantially all of our assets are located in the PRC, which makes it difficult for any funds to be utilized outside the PRC.
Liquidity and Capital Resources
We have financed our operations primarily through cash flow from operations and sale of our common stock. As a result, at May 31, 2017, our only debt was $636,036 in demand loans, which consisted of US Dollars loaned to pay our administrative expenses in the U.S.. The debt at May 31, 2017 represented an increase of $170,159 during the six months.
During the six months ended May 31, 2017, our working capital of $33,143,815 represented an increase of $7,998,094, approximately equal to our net income of $7,542,771 before adding our portion of Guangdong Tianmei's income. The approximation of our net income from the operations of Guangdong Gewang to the increase in our working capital occurs because our operations, which involve no manufacturing and limited real estate, require very modest capital investments and, as a result, almost all of our assets (other than our investment in Guangdong Tianmei) and all of our liabilities are current. Until we further implement our plan to open a series of dedicated retail stores, which will add depreciable capital assets to our balance sheet, net income should continue to increase our working capital.
During the six months ended May 31, 2017, we used cash for neither investing nor financing. During the six months ended May 31, 2016 we used $78,567 to purchase equipment, and we obtained $9,848,200 from the sale of shares of our common stock. As we develop our physical presence by investing in retail stores, cash used in investing activities will increase, and may require expansion of our cash flows from financing activities.
During the six months ended May 31, 2017, although we recorded net income of $12,208,718, our operations provided only $8,807,080 in net cash. The primary reasons for the difference were:
| | ● | Our net income included $4,665,947 attributable to our investment in Guangdong Tianmei, which represents our 21.2% equity interest in the net income of Guangdong Tianmei for the quarter. |
| | | |
| | ● | The marked increase in our sales volume during the six months caused our accounts receivable balance at May 31, 2017 to exceed the balance at November 30, 2016 by $3,131,874. All of these receivables have been collected since May 31, 2017. |
These uses of cash were partially offset by a $2,687,137 reduction in our prepaid expenses balance. This primarily represents the amortization of promotion fees prepaid during fiscal year 2016 to certain or our distributors, which are being amortized over the terms of our distribution agreements. In addition, we recorded a $1,158,537 increase in our accounts payable and a $365,912 increase in our tax payable, both of which reflect the growth in our operations.
We have no long-term debt or any debt other than the loans of U.S. dollars discussed above. Our most significant capital commitments for the next year are:
| | ● | Our quarterly payments to the Academy for a selenium enrichment technology and research related to advanced selenium-enrichment techniques. Currently, we have committed to paying the Academy RMB 600,000 (US$87,000) per year for the next four years for selenium-enrichment technology and RMB 4,000,000 (US$579,700) quarterly for research related to advanced selenium-enrichment techniques for the next ten years. We expect to fund these fees from operations. |
| | | |
| | ● | In the short term, over the next year we expect to renew all of our current leases, which will require prepayment of RMB 2,820,000 (US$410,056), which we expect to pay from operations. We also hope to open up to 26 new retail stores, which will require a capital commitment of US$2,309,000. |
| | | |
| | ● | During fiscal year 2016 we expended $3,745,884 on promotion fees to distributors. These fees are generally paid up front on an annual basis. Because of the increase in our sales, which we believe has resulted in part from our sales team seeking out new wholesale customers and paying these promotion fees, we plan to spend more on promotion fees in the upcoming fiscal year. We have projected that we could spend up to RMB 57,283,200 (US$8,279,367) in promotion fees in the 2017 fiscal year. |
We have ample cash and our operations are profitable. In addition, we expect our recent success in collecting our entire accounts receivable balance within 60 days to be replicated, due to our close relationship with our customers. For these reasons, we do not anticipate incurring significant additional debt. Therefore, our liquidity should be adequate to sustain the full implementation of our business plan for at least the next twelve months and the foreseeable future. We intend to apply a portion of our cash resources to an increase in the number of distributors with which we have relationships. Since the settlement period for payment of invoices by the distributors is, in some cases, as long as 90 days, our cash resources will be needed to offset the gap between the cash collections and cash payments.
Impact of Recent Accounting Pronouncements
New accounting rules and disclosure requirements can significantly impact the comparability of our financial statements. Please refer to Note 3 to our consolidated financial statements included in this Form 10-Q.
In February 2016, the Financial Accounting Standards Board ("FASB”) issued Accounting Standards Update ("ASU") ASU 2016-02 – Leases. The new standard establishes a right-of-use (“ROU”) model that requires a lessee to record a ROU asset and a lease liability on the balance sheet for all leases with terms longer than 12 months. Leases will be classified as either finance or operating, with classification affecting the pattern of expense recognition in the income statement. The new standard is effective for fiscal years beginning after December 15, 2018, including interim periods within those fiscal years. A modified retrospective transition approach is required for lessees for capital and operating leases existing at, or entered into after, the beginning of the earliest comparative period presented in the financial statements, with certain practical expedients available. We believe the adoption of the new standard will have no effect on our financial statements since all of our leases are prepaid.
Except for the foregoing ASU, there were no recent accounting pronouncements that we expect to have a material effect on the Registrant’s financial position or results of operations.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition or results of operations.
| ITEM 3 | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
Foreign Exchange Risk
Substantially all of our revenues and our expenses are denominated in RMB. We believe the impact of foreign currency risk is not material and we have not used any forward contracts, currency borrowings or derivative instruments to hedge our exposure to foreign currency exchange risk. Although in general our exposure to foreign exchange risks should be limited, the value of your investment in our shares will be affected by the foreign exchange rate between U.S. dollars and RMB because the value of our business is effectively denominated in RMB, while we use the U.S. dollar as our reporting currency and our common stock is traded in U.S. dollars. The value of the RMB against the U.S. dollar and other currencies is affected by, among other things, changes in China’s political and economic conditions and China’s foreign exchange policies.
To the extent that we need to convert U.S. dollars into RMB for our operations, acquisitions or other uses within China, appreciation of the RMB against the U.S. dollar would have an adverse effect on the RMB amount we receive from the conversion. To the extent that we seek to convert RMB into U.S. dollars, depreciation of the RMB against the U.S. dollar would have an adverse effect on the U.S. dollar amount we receive from the conversion. As of May 31, 2017, we had RMB-denominated cash, cash equivalents, and short-term investments of RMB152.1 million, which we recorded as US$22,415,103 on our balance sheet. A 10% change in the exchange rate between the RMB and the U.S. dollar would result in an increase or decrease of $2,241,510 in the U.S. dollar-denominated value of this cash, cash equivalents and short-term investments.
Interest Rate Risk
Our exposure to interest rate risk primarily relates to the interest income generated by our cash resources, which is mostly held in interest-bearing bank deposits. We have not used any derivative financial instruments to manage our interest risk exposure. Interest-earning instruments carry a degree of interest rate risk. We have not been exposed, nor do we anticipate being exposed, to material risks due to changes in interest rates. However, our future interest income may be lower than expected due to changes in market interest rates.
| ITEM 4 | CONTROLS AND PROCEDURES |
Evaluation of Disclosure Controls and Procedures. Our Chief Executive Officer and Chief Financial Officer carried out an evaluation of the effectiveness of the Company’s disclosure controls and procedures (as defined in Rule13a-15(e) promulgated by the Securities and Exchange Commission) as of May 31, 2017. The evaluation revealed that there are material weaknesses in our disclosure controls, specifically:
| | ● | The relatively small number of employees who are responsible for accounting functions prevents us from segregating duties within our internal control system. |
| | | |
| | ● | Our internal financial staff lack expertise in identifying and addressing complex accounting issued under U.S. Generally Accepted Accounting Principles. |
| | | |
| | ● | Our executive officers are not familiar with the accounting and reporting requirements of a U.S. public company. |
| | | |
| | ● | We have not developed sufficient documentation concerning our existing financial processes, risk assessment and internal controls. |
Based on their evaluation, our Chief Executive Officer and Chief Financial Officer concluded that the Company’s system of disclosure controls and procedures was not effective as of May 31, 2017.
Changes in Internal Controls. There was no change in internal controls over financial reporting (as defined in Rule 13a-15(f) promulgated under the Securities Exchange Act of 1934) identified in connection with the evaluation described in the preceding paragraph that occurred during the Company’s first fiscal quarter that has materially affected or is reasonably likely to materially affect the Company’s internal control over financial reporting.
PART II - OTHER INFORMATION
None.
There have been no material changes from the risk factors included in our Annual Report on Form 10-K for the year ended November 30, 2016.
| Item 2. | Unregistered Sale of Securities and Use of Proceeds |
(a) Unregistered sales of equity securities
None during the 2nd quarter of fiscal 2017.
(c) Purchases of equity securities
The Company did not repurchase any of its equity securities that were registered under Section 12 of the Securities Exchange Act during the 2nd quarter of fiscal 2017.
| Item 3. | Defaults Upon Senior Securities. |
None.
| Item 4. | Mine Safety Disclosures |
None.
| Item 5. | Other Information. |
None.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this Report to be signed on its behalf by the undersigned thereunto duly authorized.
| | CHINA GEWANG BIOTECHNOLOGY, INC. |
| | | |
| Date: July 17, 2017 | By: | /s/ Li Wang |
| | | Li Wang,
Chief Executive Officer |
| | | |
| | By: | /s/ Ming Cheng |
| | | Ming Cheng,
Chief Financial and Accounting Officer |
* * * * *
38
