field of metabolic disease. We additionally plan to rely on data exclusivity, market exclusivity and patent term extensions when available, and if appropriate, may seek and rely on regulatory protection afforded through orphan drug designations. Our commercial success may depend in part on our ability to obtain and maintain patent and other proprietary protection for our technology, inventions and improvements; to preserve the confidentiality of our trade secrets; to maintain our licenses to use intellectual property owned by third parties; and to defend and enforce our proprietary rights, including our patents.
As of September 30, 2023, our wholly owned patent portfolio consists of two issued U.S. patents, 12 pending U.S. patent applications, seven patents issued in jurisdictions outside of the United States, and 58 patent applications pending in jurisdictions outside of the United States (including three pending international applications submitted under the Patent Cooperation Treaty), that, in many cases, are counterparts to the foregoing U.S. patents and patent applications.
For our product candidates, we will, in general, initially pursue patent protection covering compositions of matter and methods of use. Throughout the development of our product candidates, we seek to identify additional means of obtaining patent protection that would potentially enhance commercial success, including through additional methods of use, process of making, dosing and formulation claims.
With respect to CT-388, as of September 30, 2023, we wholly own three pending US patent applications, one pending international applications submitted under the Patent Cooperation Treaty, and one pending foreign patent applications in Taiwan, directed to composition of matter and methods of use. Any patents that issue from such patent applications (or in the case of priority applications, if issued from future non-provisional applications that we file) are expected to expire between 2042 and 2044, without taking into account any possible patent term adjustment or extensions and assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees.
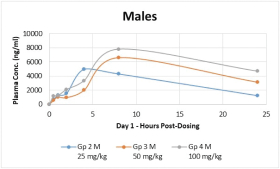
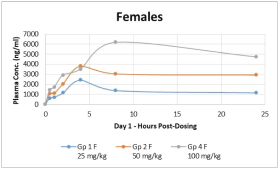
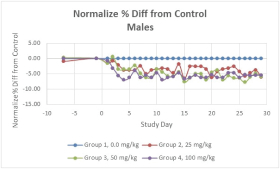
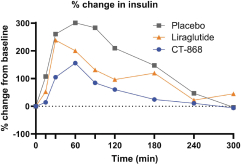
With respect to CT-868, as of September 30, 2023, we wholly own one issued US patent, five pending US patent applications, and 23 pending foreign patent applications in ARIPO, Argentina, Australia, Brazil, Canada, China, Eurasian Patent Office, European Patent Office, Hong Kong, Israel, India, Japan, Korea, Mexico, Malaysia, New Zealand, Philippines, Saudi Arabia, Singapore, South Africa, Taiwan, Ukraine, and United Arab Emirates, directed to composition of matter, methods of use, and methods of making. The patent and any patents that issue from such patent applications (or in the case of priority applications, if issued from future non-provisional applications that we file) are expected to expire between 2039 and 2044, without taking into account any possible patent term adjustment or extensions and assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees.
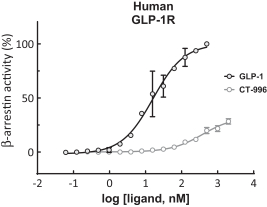
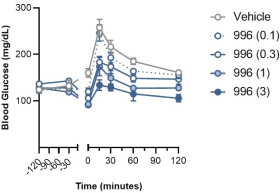
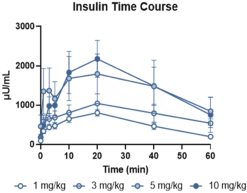
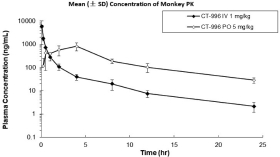
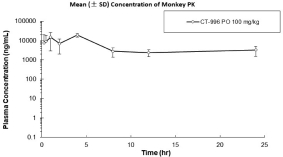
With respect to GLP-1R agonists, as of September 30, 2023, we wholly own four pending US patent applications and 31 pending foreign patent applications filed in various jurisdictions including United Arab Emirates, Australia, Brazil, Canada, Chile, China, Columbia, Costa Rica, the Eurasian Patent Office, Ecuador, Egypt, European Patent Office, Georgia, Israel, India, Jordan, Japan, South Korea, Mexico, Malaysia, Nicaragua, New Zealand, Panama, Peru, Philippines, Saudi Arabia, Singapore, Taiwan, Thailand, Ukraine, and South Africa. We also have two pending international applications submitted under the Patent Cooperation Treaty. Our pending applications are directed to composition of matter GLP-1R agonists, including CT-996, and methods of use for treating a metabolic disease or condition. Any patents that issue from such patent applications (or in the case of priority applications, if issued from future non-provisional applications that we file) are expected to expire between 2042 and 2044, without taking into account any possible patent term adjustment or extensions and assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees.
Individual patents have terms of varying periods of time, depending upon the date of filing of the patent application, the date of patent issuance and the legal term of patents in the countries in which they are obtained. Generally, patents issued for applications filed in the United States are effective for 20 years from the
148