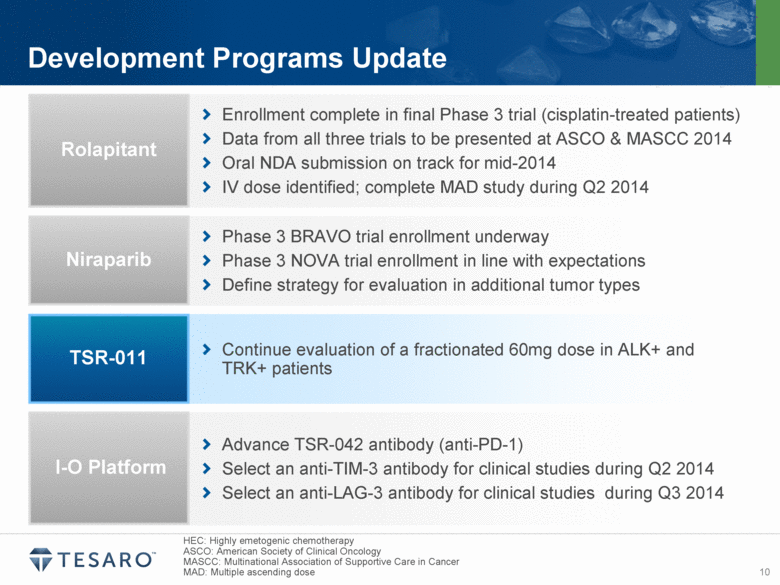
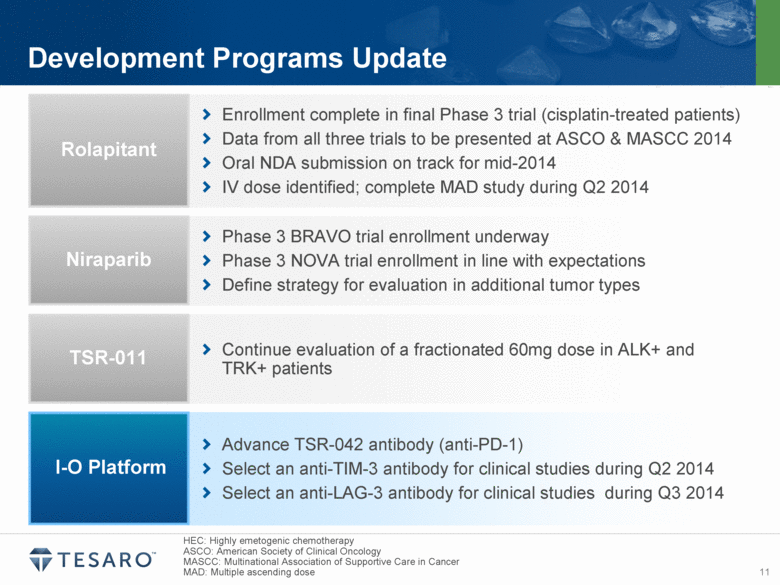
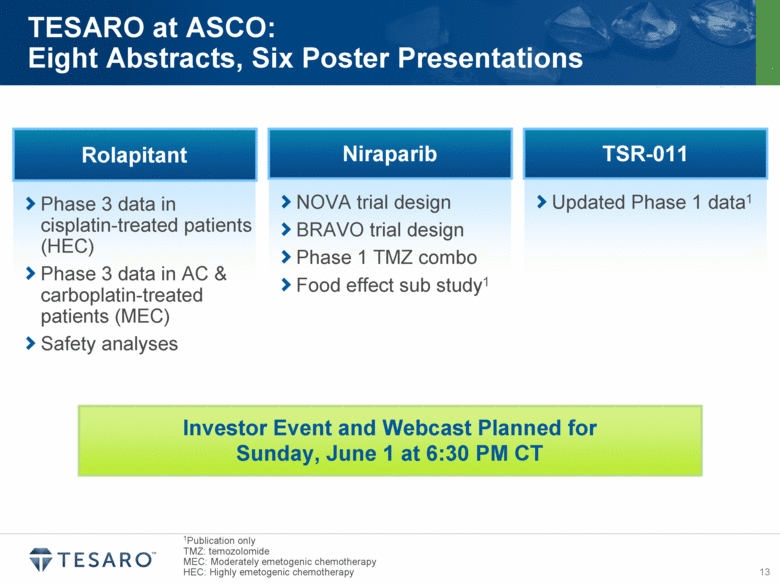
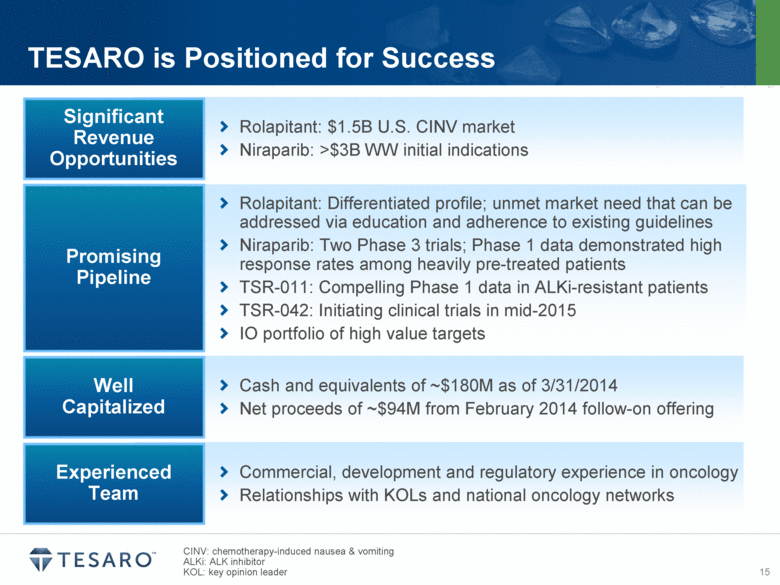
| Safe Harbor Statement Statements made in this presentation about TESARO, Inc. that are not descriptions of historical facts are forward-looking statements reflecting the current beliefs and expectations of management. Forward-looking statements are sometimes identified by words such as “plan,” “may,” “will,” “expect,” and similar expressions referencing future events, conditions or circumstances. These forward-looking statements involve substantial risks and uncertainties that could cause our clinical development programs, future results, performance or achievements to differ significantly from those expressed or implied by the forward-looking statements. Actual results could differ materially from those expressed or implied by these forward-looking statements as a result of various factors, including, without limitation, the various risks described in our Annual Report on Form 10-K for the year ended December 31, 2013 and our subsequent filings with the SEC. TESARO, Inc. undertakes no obligation to update or revise any forward-looking statement for any reason. Rolapitant, niraparib and TSR-011 are investigational products that have not been approved by any regulatory agency. The most frequently observed adverse events in the two completed rolapitant Phase 3 studies were balanced across treatment arms and included fatigue, alopecia and loss of appetite. Phase 1 data indicate that the most frequently observed adverse events for niraparib at a 300 milligram dose included grade 1/2 anemia, fatigue and nausea. Phase 1 data indicate that TSR-011 was well tolerated at therapeutic dose levels, and the most frequently occurring dose limiting toxicities included ECG changes and dysaethesia, both of which were reversible. |