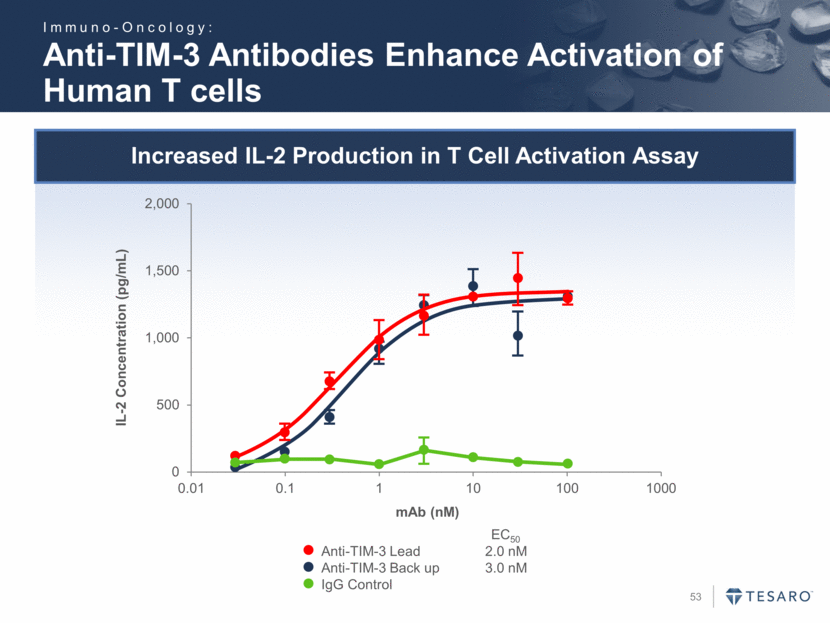
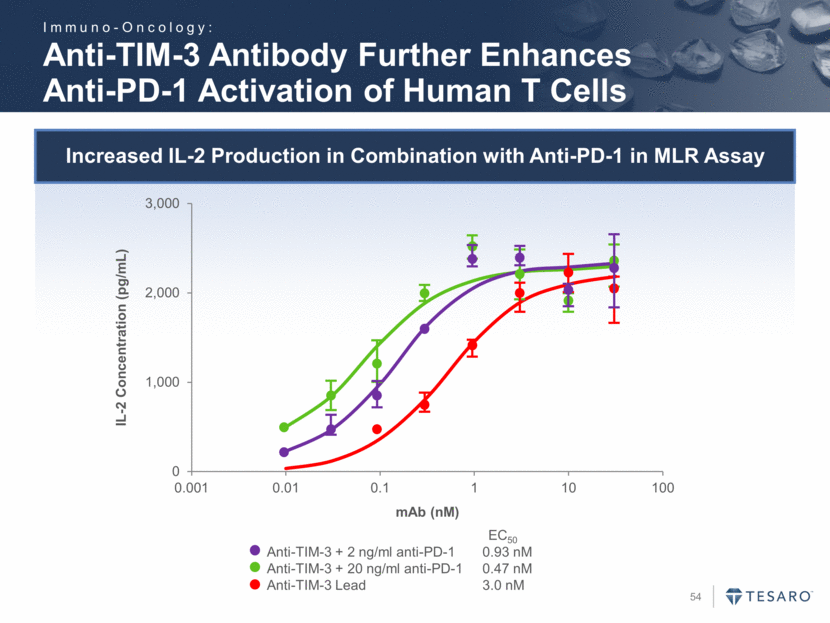
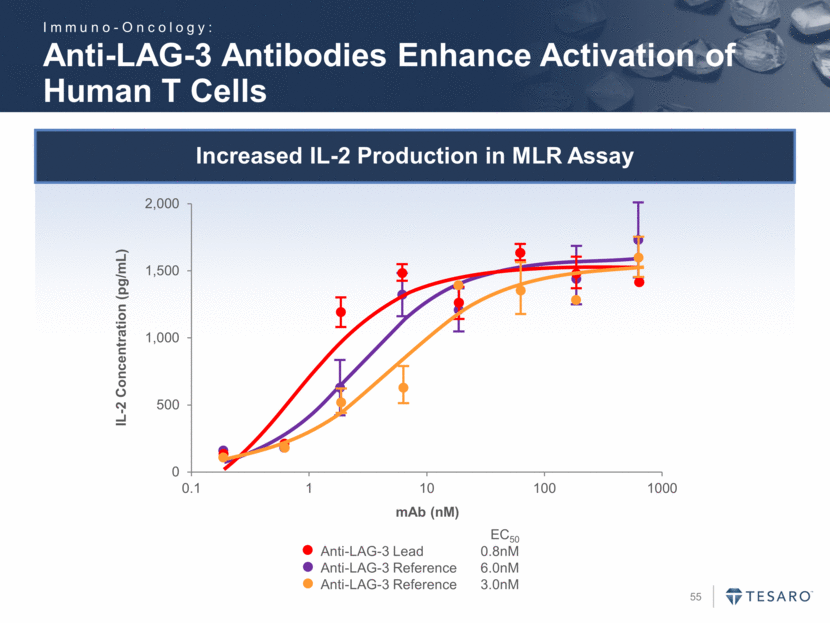
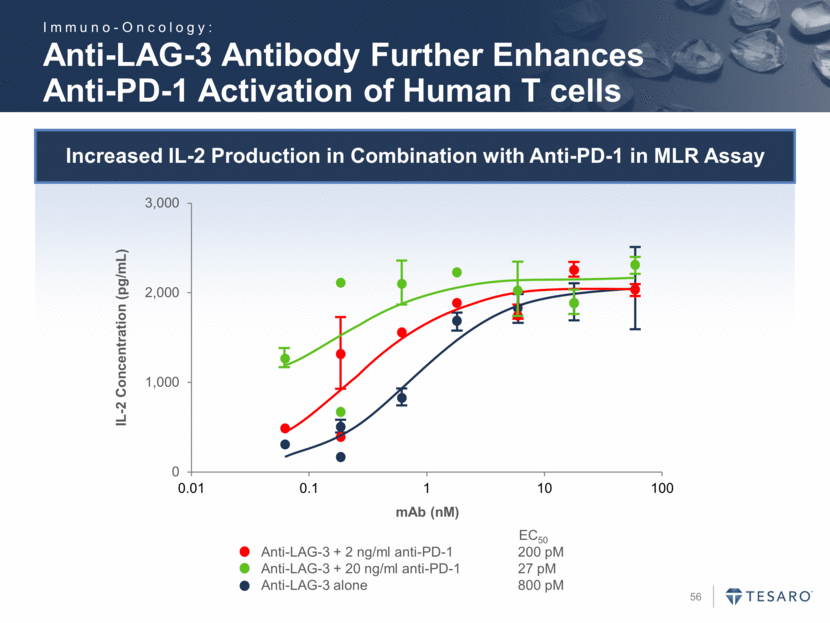
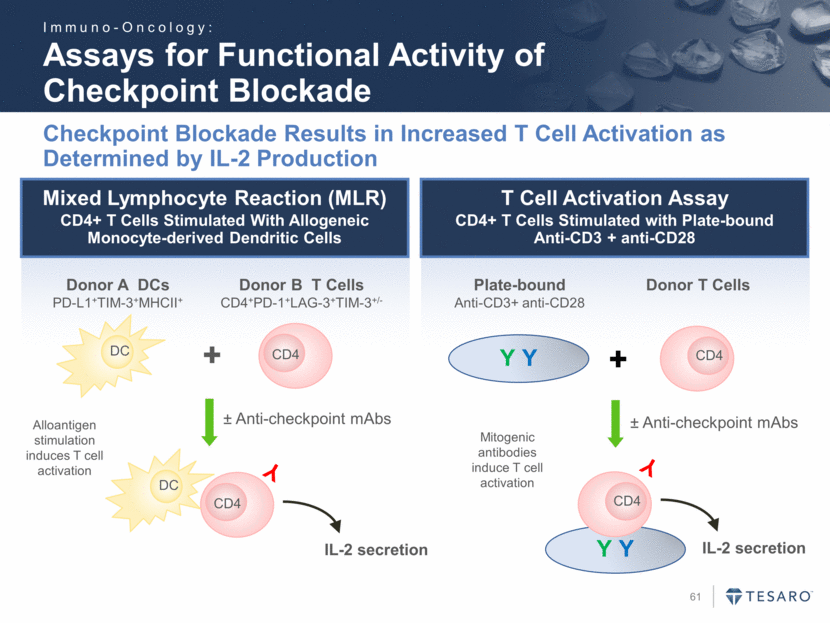
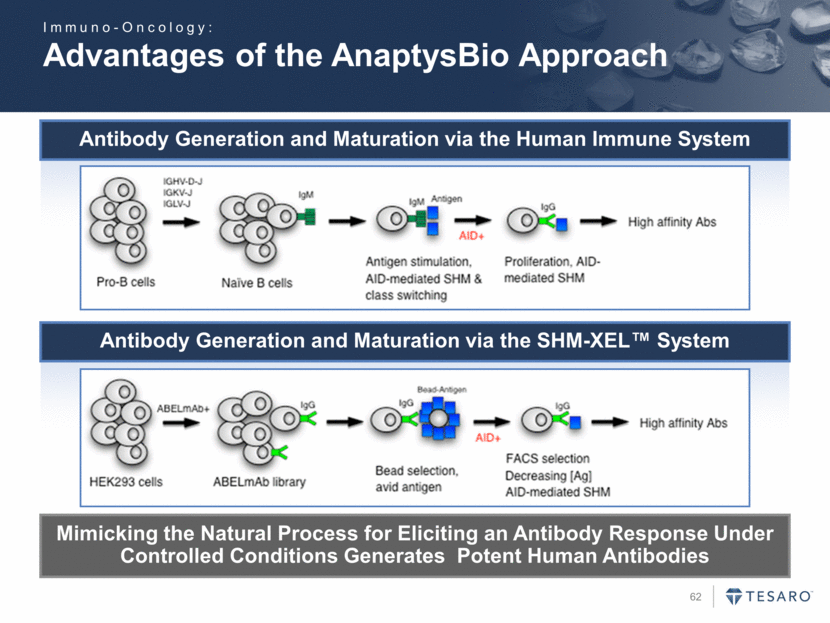
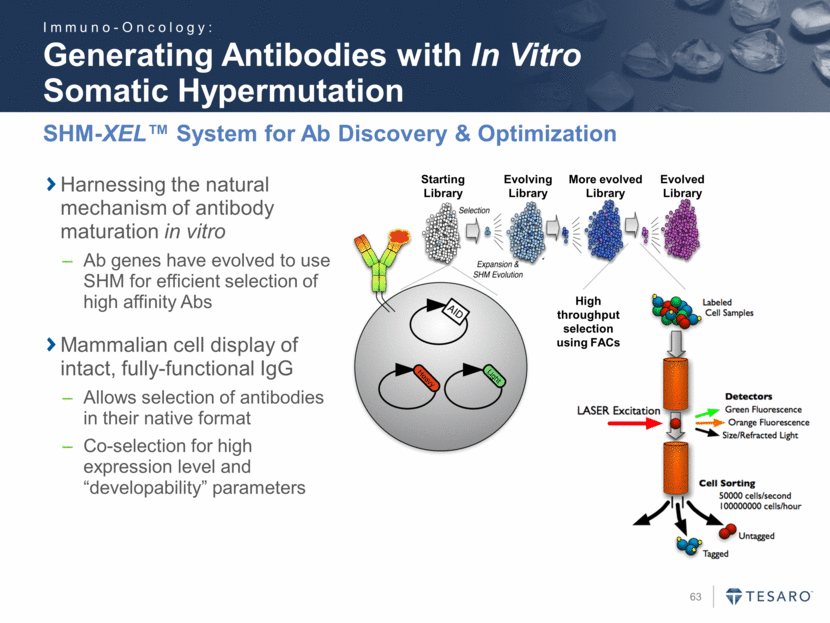
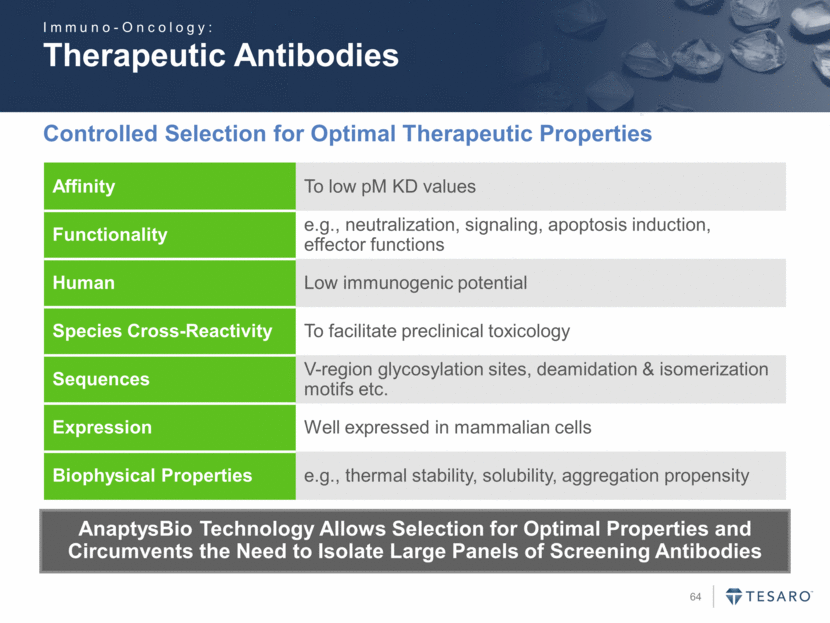
Safe Harbor Statement To the extent that statements contained in this presentation are not descriptions of historical facts regarding TESARO, they are forward-looking statements reflecting the current beliefs and expectations of management made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Words such as "may," "will," "expect," "anticipate," "estimate," "intend," and similar expressions (as well as other words or expressions referencing future events, conditions, or circumstances) are intended to identify forward-looking statements. Examples of forward-looking statements contained in this press release include, among others, statements regarding our projected VARUBI P&L break-even revenue run rate, our vision of becoming a leading oncology-focused biopharmaceutical company, our plans to expand niraparib development into additional therapeutic areas, our expectation to have NOVA and QUADRA data in the second quarter of 2016, the expected timing of the oral rolapitant MAA submission and other regulatory filings with respect to our product candidates, the expected timing of data from our various clinical trials, our plans regarding future clinical trials with niraparib, statements regarding our various 2016 corporate goals, the estimated time periods when we expect clinical trials to commence or be completed, statements regarding our expectations about the timing of both the selection of clinical candidates from our immuno-oncology programs and the commencement of clinical testing for those candidates, and statements regarding the potential market opportunity for our products or product candidates. Forward-looking statements in this release involve substantial risks and uncertainties that could cause our research and pre-clinical development programs, clinical development programs, future results, performance, or achievements to differ significantly from those expressed or implied by the forward-looking statements. Such risks and uncertainties include, among others, the uncertainties inherent in the execution and completion of clinical trials, uncertainties surrounding the timing of availability of data from our clinical trials, risks regarding ongoing discussions with and actions by regulatory authorities, patient accrual rates for clinical trials, risks from competitors, and other matters that could affect the timing of availability of data from or initiation of our clinical trials, uncertainties regarding regulatory approvals, uncertainties regarding certain expenditures, and other matters that could affect the availability or commercial potential of our drug candidates. TESARO undertakes no obligation to update or revise any forward-looking statements. For a further description of the risks and uncertainties that could cause actual results to differ from those expressed in these forward-looking statements, as well as risks relating to the business of the Company in general, see TESARO's Annual Report on Form 10-K for the year ended December 31, 2015.