Exhibit 99.1

Transforming Diagnostics, Patient Care & Economics September 2017 (NASDAQ: TTOO)

Forward-Looking Statements
This presentation contains forward-looking statements. Such statements reflect the current views of senior management of T2 Biosystems, Inc. (“we”, “us”, “our”, “T2”, “T2 Biosystems” or the “Company”) and include those about T2’s goals, strategies, plans, objectives, prospects, milestones, future operations, business and industry, anticipated product benefits, future events and conditions and potential scenarios. Such statements and those that include the words “expect,” “intend,” “plan,” “believe,” “project,” “forecast,” “estimate,” “may,” “should,” “anticipate” and similar statements of a future or forward-looking nature identify forward-looking statements for purposes of the federal securities laws or otherwise. Forward-looking statements address matters that involve risks and uncertainties. Each forward-looking statement is subject to risks and uncertainties that could cause actual results to differ materially from those expressed or implied in such statement, including, for example: (i) our status as an early commercial-stage company and expectation to incur losses in the future; (ii) our ability to obtain marketing authorization from the FDA or regulatory clearance for additional product candidates in the United States or abroad; (iii) the market acceptance of our technology; (iv) our ability to timely and successfully develop and commercialize existing and future product candidates; (v) our lengthy and variable sales cycle and lack of sales history; (vi) our ability to successfully manage growth; (vii) federal, state and foreign regulatory requirements; (viii) our uncertain future capital needs and ability to raise future capital; (ix) dependence on third parties; (x) recruiting, training and retaining key personnel; (xi) competitive factors; (xii) manufacturing and other product risks; (xii) risks related to intellectual property; and (xiii) other risk factors included in our annual report on form10-K filed with the Securities and Exchange Commission (SEC) on March 15, 2017 and other documents we file with the SEC from time to time. Accordingly, there are or will be important factors that could cause our actual results to differ materially from those indicated in these statements. The statements made herein speak only as of the date of this presentation. We do not undertake, and specifically disclaim, any obligation to update any forward-looking statements contained in this presentation.
Transforming Diagnostics, Patient Care & Economics | 2
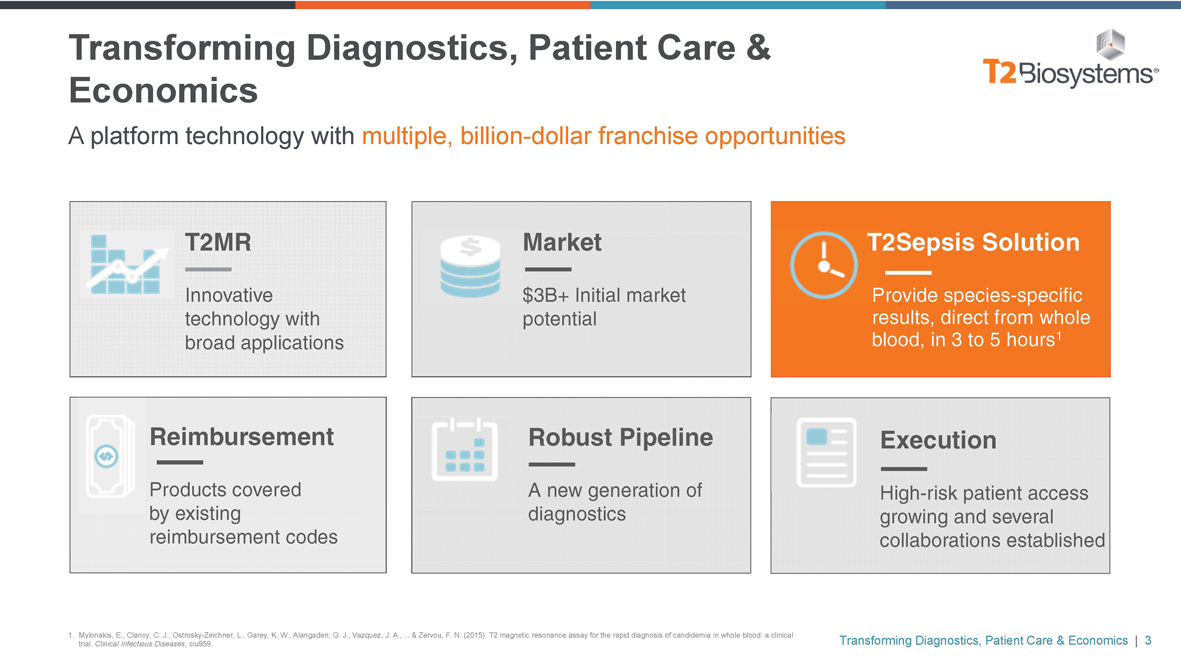
Transforming Diagnostics, Patient Care & Economics
A platform technology with multiple, billion-dollar franchise opportunities
T2MR Market
Innovative $3B+ Initial market technology with potential broad applications
T2Sepsis Solution
Provide species-specific results, direct from whole blood, in 3 to 5 hours1
Reimbursement
Products covered by existing reimbursement codes
Robust Pipeline
A new generation of diagnostics
Execution
High-risk patient access growing and several collaborations established
1. Mylonakis, E., Clancy, C. J., Ostrosky-Zeichner, L., Garey, K. W., Alangaden, G. J., Vazquez, J. A., ... & Zervou, F. N. (2015). T2 magnetic resonance assay for the rapid diagnosis of candidemia in whole blood: a clinical trial. Clinical Infectious Diseases, ciu959.
Transforming Diagnostics, Patient Care & Economics | 3
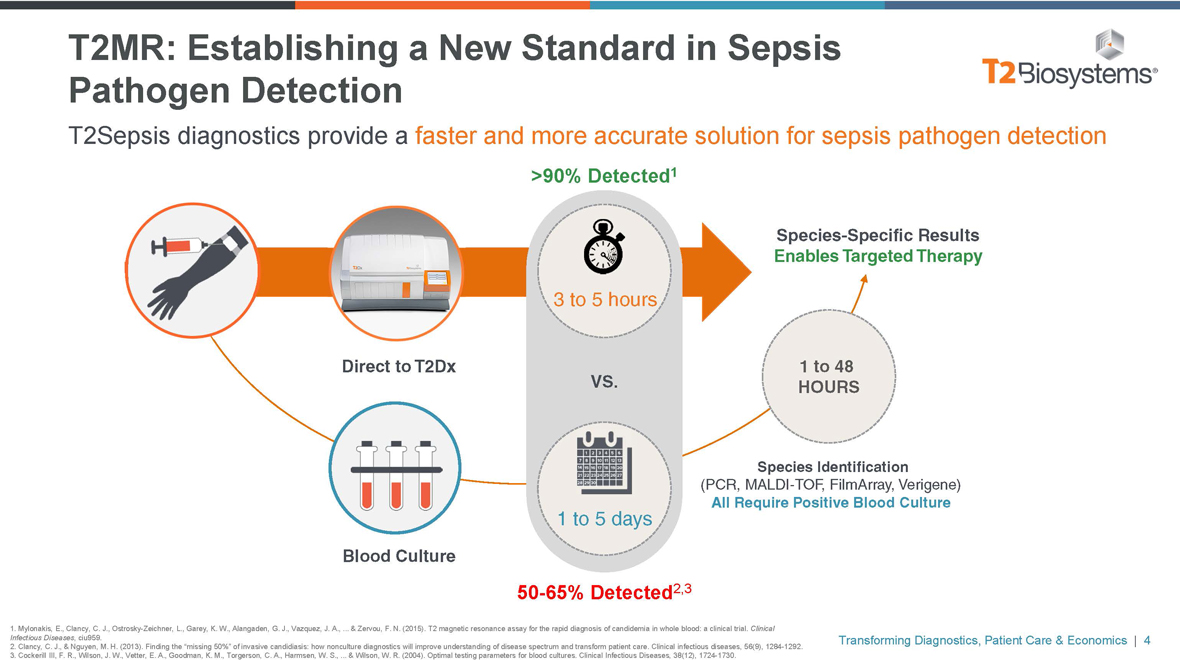
T2MR: Establishing a New Standard in Sepsis Pathogen Detection T2Sepsis diagnostics provide a faster and more accurate solution for sepsis pathogen detection >90% Detected1 Species-Specific Results Enables Targeted Therapy 3 to 5 hours Direct to T2Dx 1 to 48 VS. HOURS Species Identification (PCR,MALDI-TOF, FilmArray, Verigene) All Require Positive Blood Culture 1 to 5 days Blood Culture50-65% Detected2,3 1. Mylonakis, E., Clancy, C. J., Ostrosky-Zeichner, L., Garey, K. W., Alangaden, G. J., Vazquez, J. A., ... & Zervou, F. N. (2015). T2 magnetic resonance assay for the rapid diagnosis of candidemia in whole blood: a clinical trial. Clinical Infectious Diseases, ciu959. 2. Clancy, C. J., & Nguyen, M. H. (2013). Finding the “missing 50%” of invasive candidiasis: how nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clinical infectious diseases, 56(9), 1284-1292. 3. Cockerill III, F. R., Wilson, J. W., Vetter, E. A., Goodman, K. M., Torgerson, C. A., Harmsen, W. S., ... & Wilson, W. R. (2004). Optimal testing parameters for blood cultures. Clinical Infectious Diseases, 38(12), 1724-1730. Transforming Diagnostics, Patient Care & Economics | 4

Product Pipeline Highlights – Enabled by Highly-Sensitive Detection Directly from whole blood – no requirement for blood culture 2016 2017 2018 and beyond1 T2Candida Panel T2Candida Panel FUNGAL CE & IVD Including pan-Candida T2Bacteria Panel T2Bacteria Panel BACTERIAL Including additional bacteria CE SEPSIS targets T2GNR1 BACTERIAL Gram Neg Resistance RESISTANCE Markers TICK-BORNE 1. This slide contains T2’s future goals and aspirations, which constitute forward-looking statement that are subject to risks and uncertainties that could cause actual results to differ materially from those expressed or implied by such statement. See “Forward-Looking Statements” on slide 2. Transforming Diagnostics, Patient Care & Economics | 5

T2MR Featured at Tradeshows and in Over 200 Publications Worldwide T2Candida – 91.1% Sensitivity 99.4% Specificity LoD1-3 CFU/mL T2Candida – 96.4% Sensitivity 99.4% Specificity T2Candida featured as sensitive and specific diagnostic test for invasive candidiasis T2Candida projected to reduce deaths 60% and to save each site $5.8M/yr T2MR detected Lyme Disease-causing bacteria in blood T2Candida – Customers present cost savings and superiority to blood culture T2Candida – Customers present cost savings, LOS savings, and decreased time to appropriate antifungal therapy T2Candida – Customers present antifungal savings, improved care, and preliminary study on predicting patient outcomes. T2Bacteria—Early experience with performance evaluations in Europe. T2Sepsis Solution –Customers present stewardship benefits of T2Candida and early experience with T2Bacteria RUO Transforming Diagnostics, Patient Care & Economics | 6

Shortcomings of Sepsis Management Transforming Diagnostics, Patient Care & Economics | 7
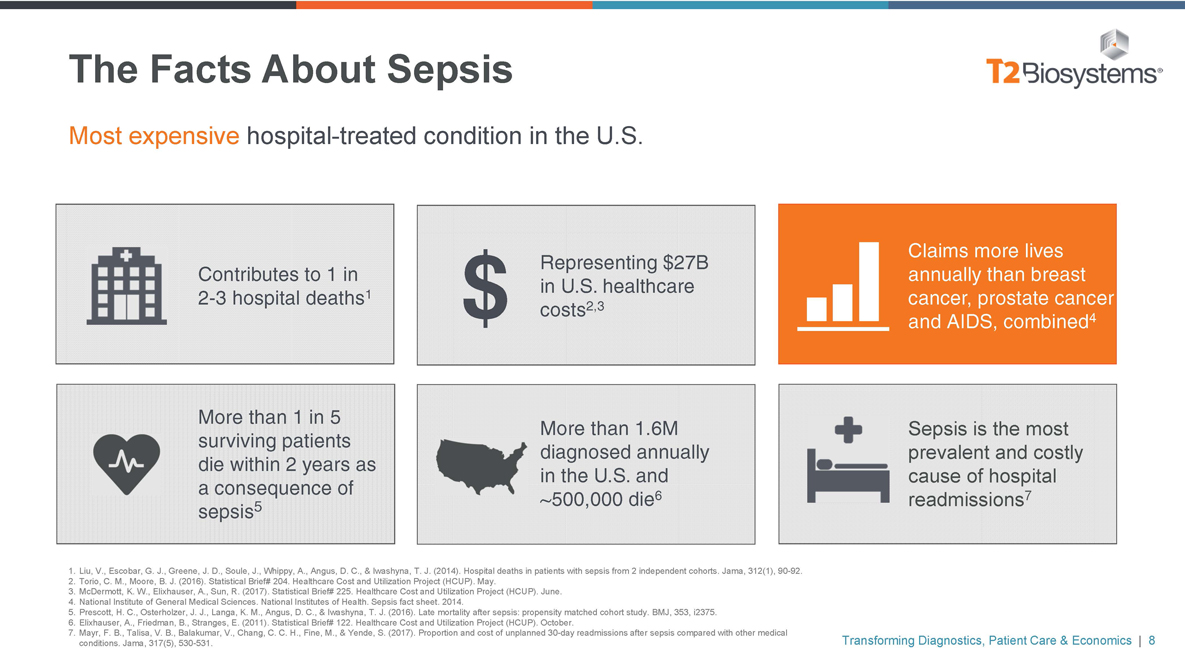
The Facts About Sepsis Most expensive hospital-treated condition in the U.S. Contributes to 1 in2-3 hospital deaths1 Representing $27B in U.S. healthcare $ costs2,3 Claims more lives annually than breast cancer, prostate cancer and AIDS, combined4 More than 1 in 5 surviving patients die within 2 years as a consequence of sepsis5 More than 1.6M diagnosed annually in the U.S. and ~500,000 die6 Sepsis is the most prevalent and costly cause of hospital readmissions7 1. Liu, V., Escobar, G. J., Greene, J. D., Soule, J., Whippy, A., Angus, D. C., & Iwashyna, T. J. (2014). Hospital deaths in patients with sepsis from 2 independent cohorts. Jama, 312(1),90-92. 2. Torio, C. M., Moore, B. J. (2016). Statistical Brief# 204. Healthcare Cost and Utilization Project (HCUP). May. 3. McDermott, K. W., Elixhauser, A., Sun, R. (2017). Statistical Brief# 225. Healthcare Cost and Utilization Project (HCUP). June. 4. National Institute of General Medical Sciences. National Institutes of Health. Sepsis fact sheet. 2014. 5. Prescott, H. C., Osterholzer, J. J., Langa, K. M., Angus, D. C., & Iwashyna, T. J. (2016). Late mortality after sepsis: propensity matched cohort study. BMJ, 353, i2375. 6. Elixhauser, A., Friedman, B., Stranges, E. (2011). Statistical Brief# 122. Healthcare Cost and Utilization Project (HCUP). October. 7. Mayr, F. B., Talisa, V. B., Balakumar, V., Chang, C. C. H., Fine, M., & Yende, S. (2017). Proportion and cost of unplanned30-day readmissions after sepsis compared with other medical conditions. Jama, 317(5),530-531. Transforming Diagnostics, Patient Care & Economics | 8
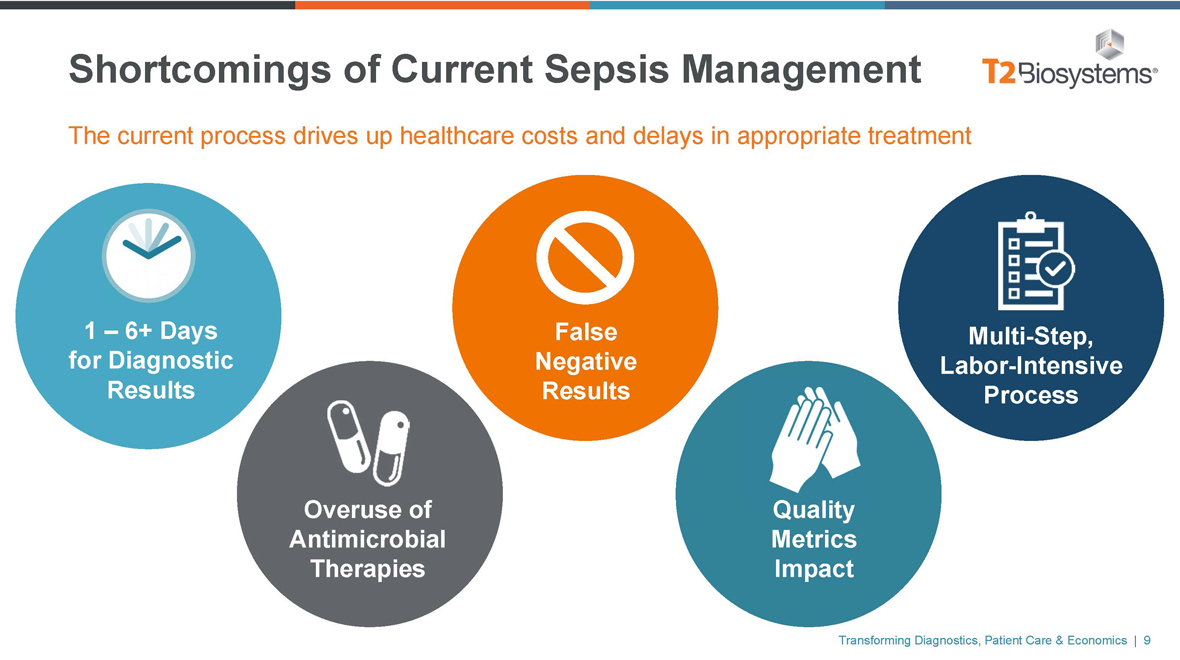
Shortcomings of Current Sepsis Management The current process drives up healthcare costs and delays in appropriate treatment 1 – 6+ Days for Diagnostic Results Overuse of Antimicrobial Therapies False Negative Results Quality Metrics Impact Multi-Step, Labor-Intensive Process Transforming Diagnostics, Patient Care & Economics | 9
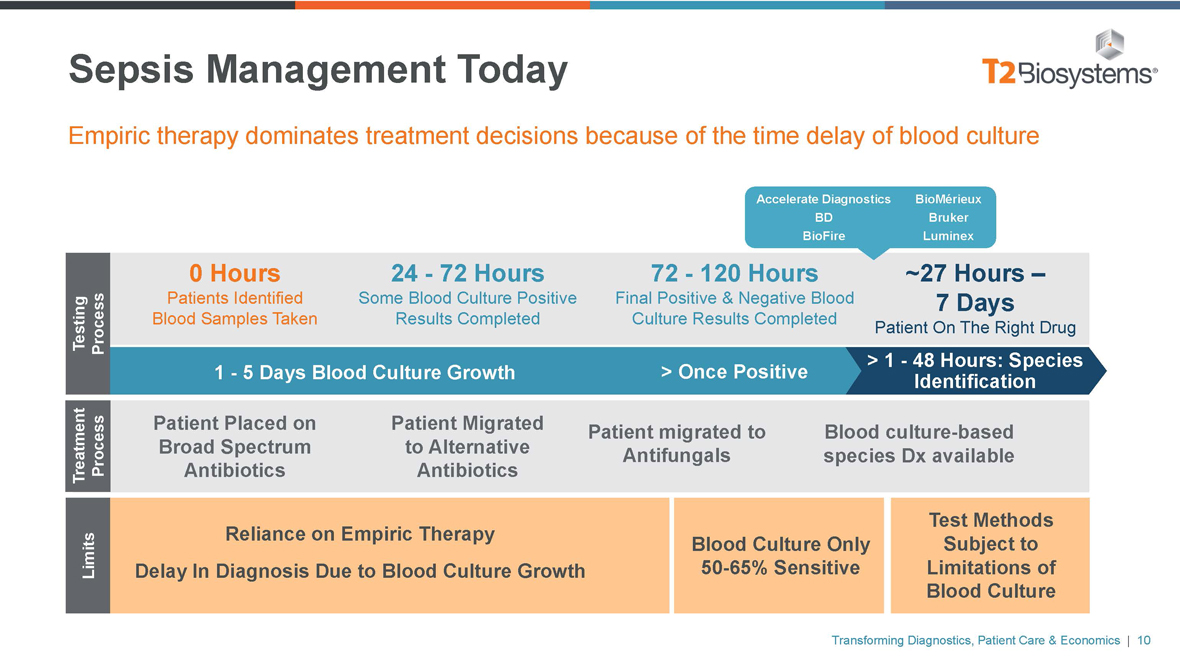
Sepsis Management Today Empiric therapy dominates treatment decisions because of the time delay of blood culture Accelerate Diagnostics BioMérieux BD Bruker BioFire Luminex 0 Hours 24—72 Hours 72—120 Hours ~27 Hours – Patients Identified Some Blood Culture Positive Final Positive & Negative Blood7 Days Testing Process Blood Samples Taken Results CompletedCulture Results CompletedPatient On The Right Drug > 1—48 Hours: Species 1—5 Days Blood Culture Growth > Once Positive Identification Patient Placed on Patient Migrated Patient migrated to Blood culture-based Broad Spectrum to Alternative Antifungals species Dx available Treatment Process Antibiotics Antibiotics Test Methods Reliance on Empiric Therapy Blood Culture Only Subject to Limits Delay In Diagnosis Due to Blood Culture Growth50-65% Sensitive Limitations of Blood Culture Transforming Diagnostics, Patient Care & Economics | 10

T2Sepsis Solution Transforming Diagnostics, Patient Care & Economics | 11
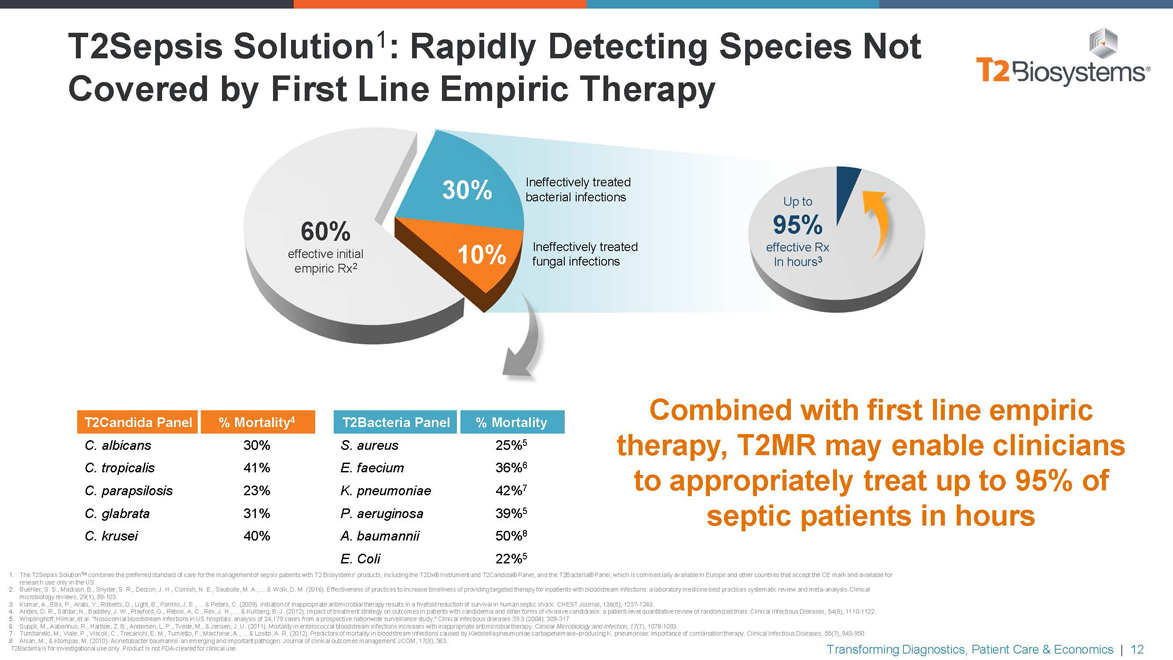
T2Sepsis Solution1: Rapidly Detecting Species Not Covered by First Line Empiric Therapy 30% Ineffectively treated bacterial infections Up to 60% 95% Ineffectively treated effective Rx effective initial 10% fungal infections In hours3 empiric Rx2 T2Candida Panel % Mortality4 T2Bacteria Panel % Mortality C. albicans 30% S. aureus 25%5 C. tropicalis 41% E. faecium 36%6 C. parapsilosis 23% K. pneumoniae 42%7 C. glabrata 31% P. aeruginosa 39%5 C. krusei 40% A. baumannii 50%8 E. Coli 22%5 Combined with first line empiric therapy, T2MR may enable clinicians to appropriately treat up to 95% of septic patients in hours 1. The T2Sepsis SolutionTM combines the preferred standard of care for the management of sepsis patients with T2 Biosystems’ products, including the T2Dx® Instrument and T2Candida® Panel, and the T2Bacteria® Panel, which is commercially available in Europe and other countries that accept the CE mark and available for research use only in the US 2. Buehler, S. S., Madison, B., Snyder, S. R., Derzon, J. H., Cornish, N. E., Saubolle, M. A., ... & Wolk, D. M. (2016). Effectiveness of practices to increase timeliness of providing targeted therapy for inpatients with bloodstream infections: a laboratory medicine best practices systematic review and meta-analysis. Clinical microbiology reviews, 29(1),59-103. 3. Kumar, A., Ellis, P., Arabi, Y., Roberts, D., Light, B., Parrillo, J. E., ... & Peters, C. (2009). Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. CHEST Journal, 136(5), 1237-1248. 4. Andes, D. R., Safdar, N., Baddley, J. W., Playford, G., Reboli, A. C., Rex, J. H., ... & Kullberg, B. J. (2012). Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: a patient-level quantitative review of randomized trials. Clinical Infectious Diseases, 54(8), 1110-1122. 5. Wisplinghoff, Hilmar, et al. “Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study.” Clinical infectious diseases 39.3 (2004):309-317. 6. Suppli, M., Aabenhus, R., Harboe, Z. B., Andersen, L. P., Tvede, M., & Jensen, J. U. (2011). Mortality in enterococcal bloodstream infections increases with inappropriate antimicrobial therapy. Clinical Microbiology and Infection, 17(7), 1078-1083. 7. Tumbarello, M., Viale, P., Viscoli, C., Trecarichi, E. M., Tumietto, F., Marchese, A., ... & Losito, A. R. (2012). Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase–producing K. pneumoniae: importance of combination therapy. Clinical Infectious Diseases, 55(7), Transforming 943-950. Diagnostics, Patient Care & Economics | 12 8. Alsan, M., & Klompas, M. (2010). Acinetobacter baumannii: an emerging and important pathogen. Journal of clinical outcomes management: JCOM, 17(8), 363. T2Bacteria is for investigational use only. Product is notFDA-cleared for clinical use.
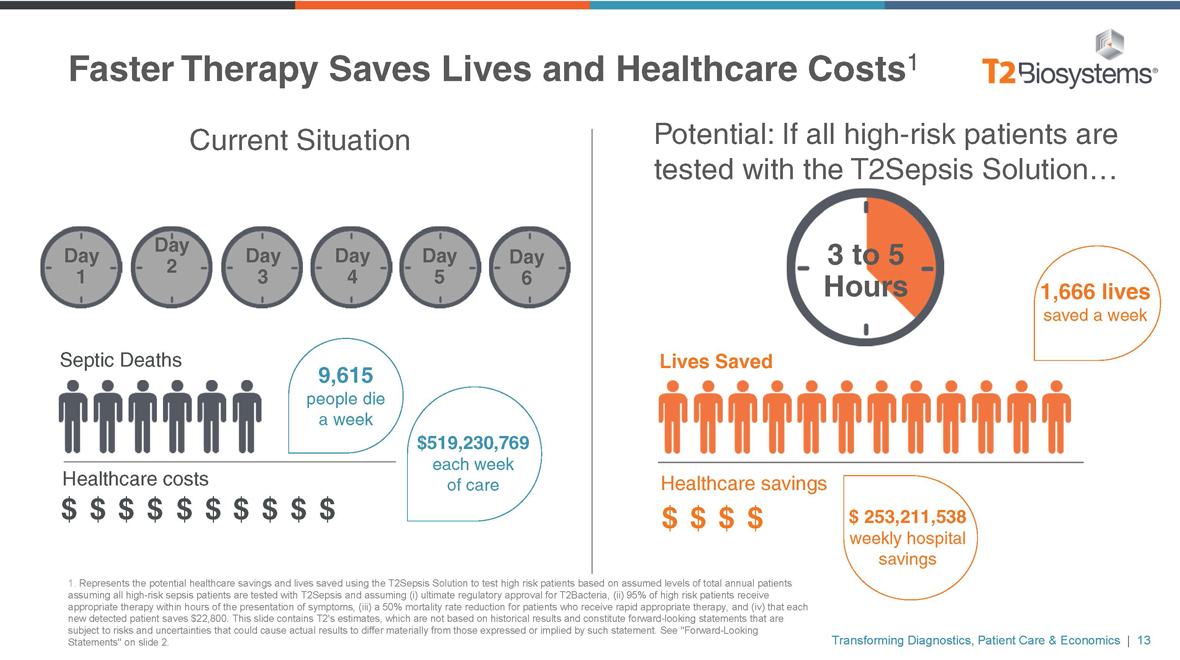
Faster Therapy Saves Lives and Healthcare Costs1 Current Situation Day Day Day Day Day Day 2 1 3 4 5 6 Septic Deaths 9,615 people die a week $519,230,769 each week Healthcare costs of care $ $ $ $ $ $ $ $ $ $ 1. Represents the potential healthcare savings and lives saved using the T2Sepsis Solution to test high risk patients based on assumed levels of total annual patients assuming all high-risk sepsis patients are tested with T2Sepsis and assuming (i) ultimate regulatory approval for T2Bacteria, (ii) 95% of high risk patients receive appropriate therapy within hours of the presentation of symptoms, (iii) a 50% mortality rate reduction for patients who receive rapid appropriate therapy, and (iv) that each new detected patient saves $22,800. This slide contains T2’s estimates, which are not based on historical results and constitute forward-looking statements that are subject to risks and uncertainties that could cause actual results to differ materially from those expressed or implied by such statement. See “Forward-Looking Statements” on slide 2. Potential: If all high-risk patients are tested with the T2Sepsis Solution… 3 to 5 Hours 1,666 lives saved a week Lives Saved Healthcare savings $ $ $ $ $ 253,211,538 weekly hospital savings Transforming Diagnostics, Patient Care & Economics | 13
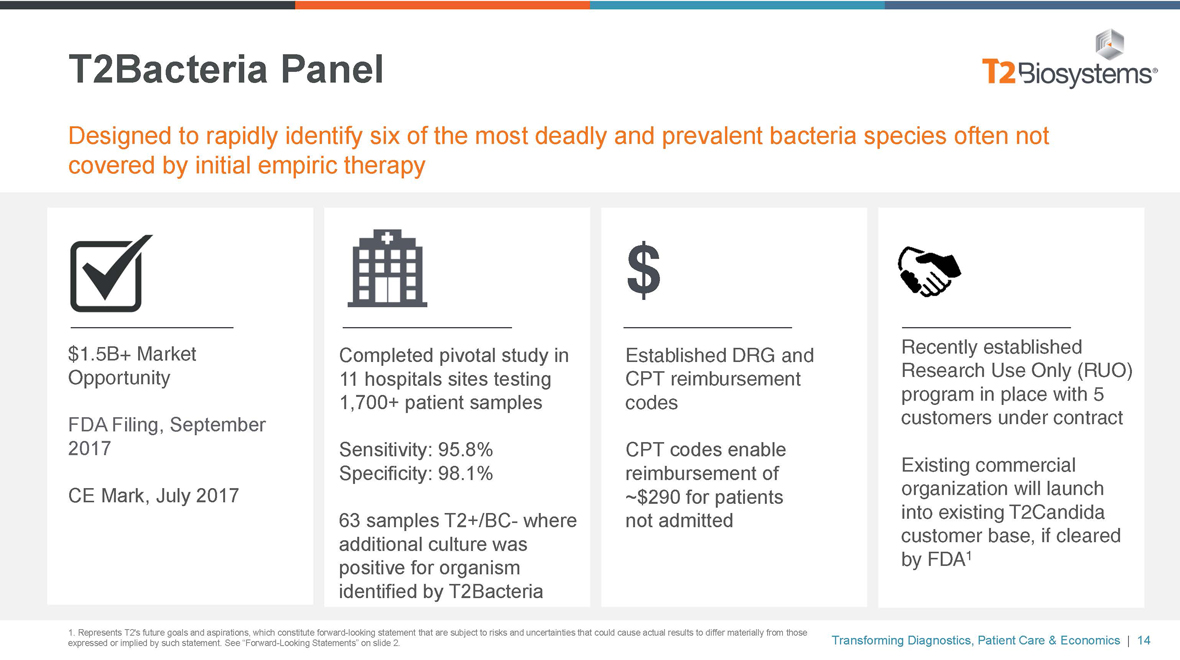
T2Bacteria Panel Designed to rapidly identify six of the most deadly and prevalent bacteria species often not covered by initial empiric therapy $1.5B+ Market Opportunity FDA Filing, September 2017 CE Mark, July 2017 Completed pivotal study in 11 hospitals sites testing 1,700+ patient samples Sensitivity: 95.8% Specificity: 98.1% 63 samplesT2+/BC- where additional culture was positive for organism identified by T2Bacteria Established DRG and CPT reimbursement codes CPT codes enable reimbursement of ~$290 for patients not admitted Recently established Research Use Only (RUO) program in place with 5 customers under contract Existing commercial organization will launch into existing T2Candida customer base, if cleared by FDA1 1. Represents T2’s future goals and aspirations, which constitute forward-looking statement that are subject to risks and uncertainties that could cause actual results to differ materially from those expressed or implied by such statement. See “Forward-Looking Statements” on slide 2. Transforming Diagnostics, Patient Care & Economics | 14
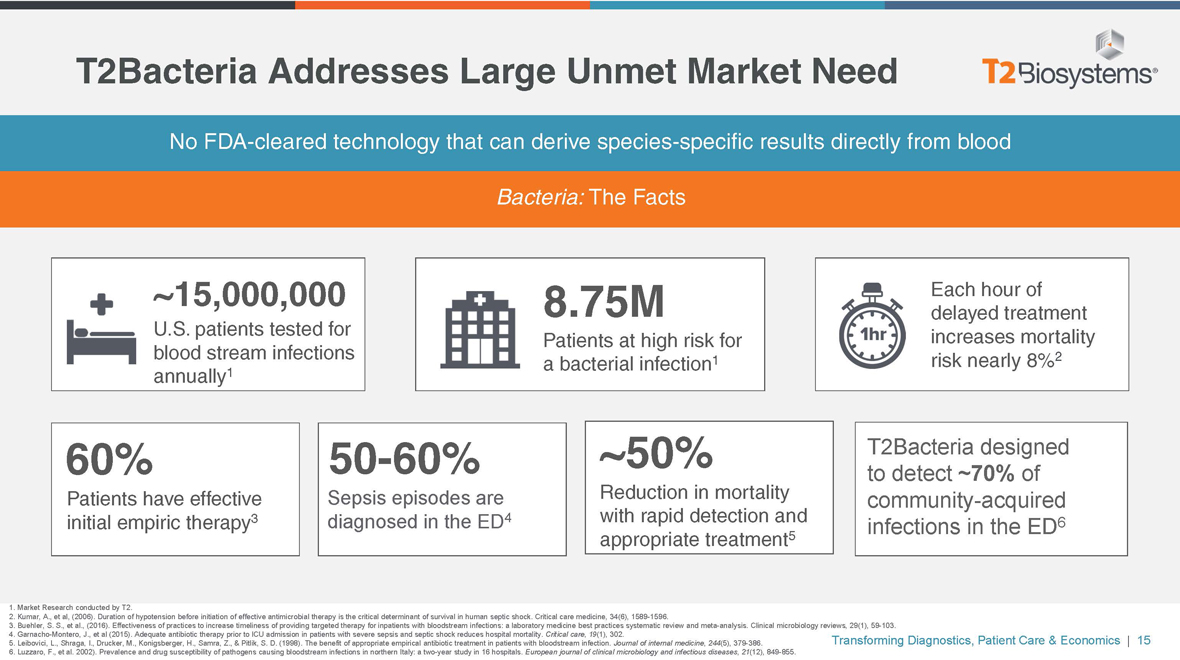
T2Bacteria Addresses Large Unmet Market Need NoFDA-cleared technology that can derive species-specific results directly from blood Bacteria: The Facts ~15,000,000 U.S. patients tested for blood stream infections annually1 8.75M Patients at high risk for a bacterial infection1 Each hour of delayed treatment increases mortality risk nearly 8%2 60% Patients have effective initial empiric therapy350-60% Sepsis episodes are diagnosed in the ED4 ~50% Reduction in mortality with rapid detection and appropriate treatment5 T2Bacteria designed to detect ~70% of community-acquired infections in the ED6 1. Market Research conducted by T2. 2. Kumar, A., et al, (2006). Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Critical care medicine, 34(6), 1589-1596. 3. Buehler, S. S., et al., (2016). Effectiveness of practices to increase timeliness of providing targeted therapy for inpatients with bloodstream infections: a laboratory medicine best practices systematic review and meta-analysis. Clinical microbiology reviews, 29(1),59-103. 4. Garnacho-Montero, J., et al (2015). Adequate antibiotic therapy prior to ICU admission in patients with severe sepsis and septic shock reduces hospital mortality. Critical care, 19(1), 302. 5. Leibovici, L., Shraga, I., Drucker, M., Konigsberger, H., Samra, Z., & Pitlik, S. D. (1998). The benefit of appropriate empirical antibiotic treatment in patients with bloodstream infection. Journal of internal medicine, 244(5),379-386. 6. Luzzaro, F., et al. 2002). Prevalence and drug susceptibility of pathogens causing bloodstream infections in northern Italy: atwo-year study in 16 hospitals. European journal of clinical microbiology and infectious diseases, 21(12),849-855. Transforming Diagnostics, Patient Care & Economics | 15
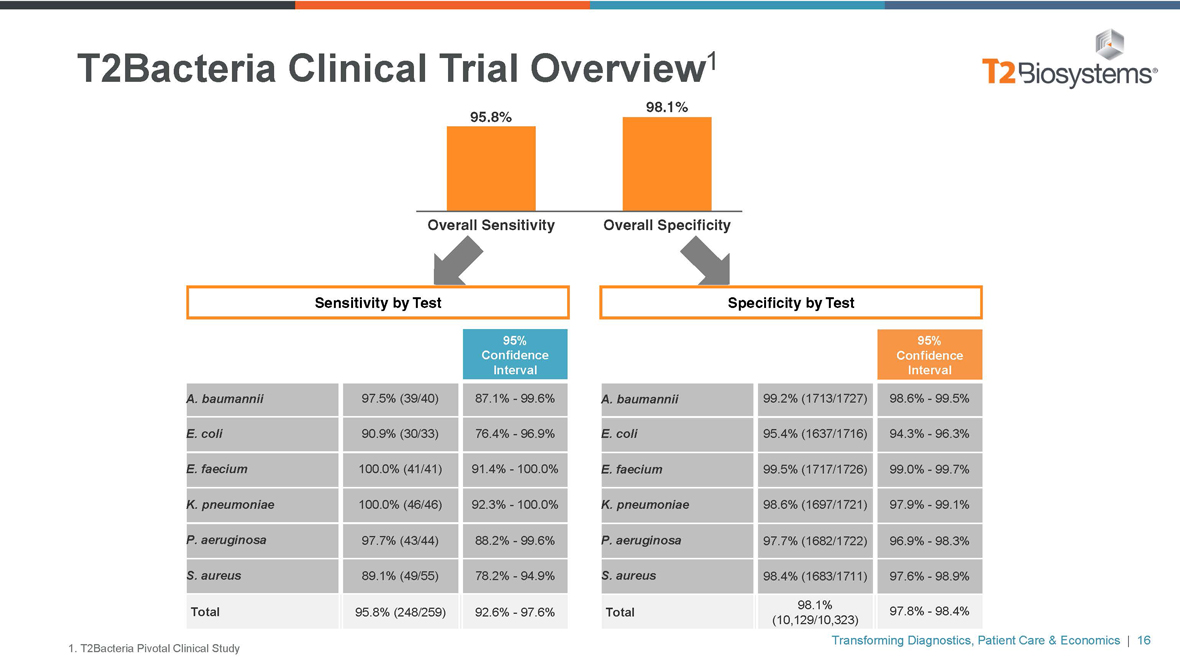
T2Bacteria Clinical Trial Overview1 98.1% 95.8%
Overall Sensitivity Overall Specificity
Sensitivity by Test Specificity by Test
95%
Confidence
Interval
A. baumannii 97.5% (39/40)87.1%-99.6%
E. coli 90.9% (30/33)76.4%-96.9%
E. faecium 100.0% (41/41) 91.4%—100.0%
K. pneumoniae 100.0% (46/46) 92.3%—100.0%
P. aeruginosa 97.7% (43/44)88.2%-99.6%
S. aureus 89.1% (49/55)78.2%-94.9%
Total 95.8% (248/259) 92.6%-97.6%
95%
Confidence
Interval
A. baumannii 99.2% (1713/1727)98.6%-99.5%
E. coli 95.4% (1637/1716)94.3%-96.3%
E. faecium 99.5% (1717/1726)99.0%-99.7%
K. pneumoniae 98.6% (1697/1721)97.9%-99.1%
P. aeruginosa 97.7% (1682/1722)96.9%-98.3%
S. aureus 98.4% (1683/1711)97.6%-98.9%
98.1%
Total 97.8%-98.4%
(10,129/10,323)
1. T2Bacteria Pivotal Clinical Study
Transforming Diagnostics, Patient Care & Economics | 16
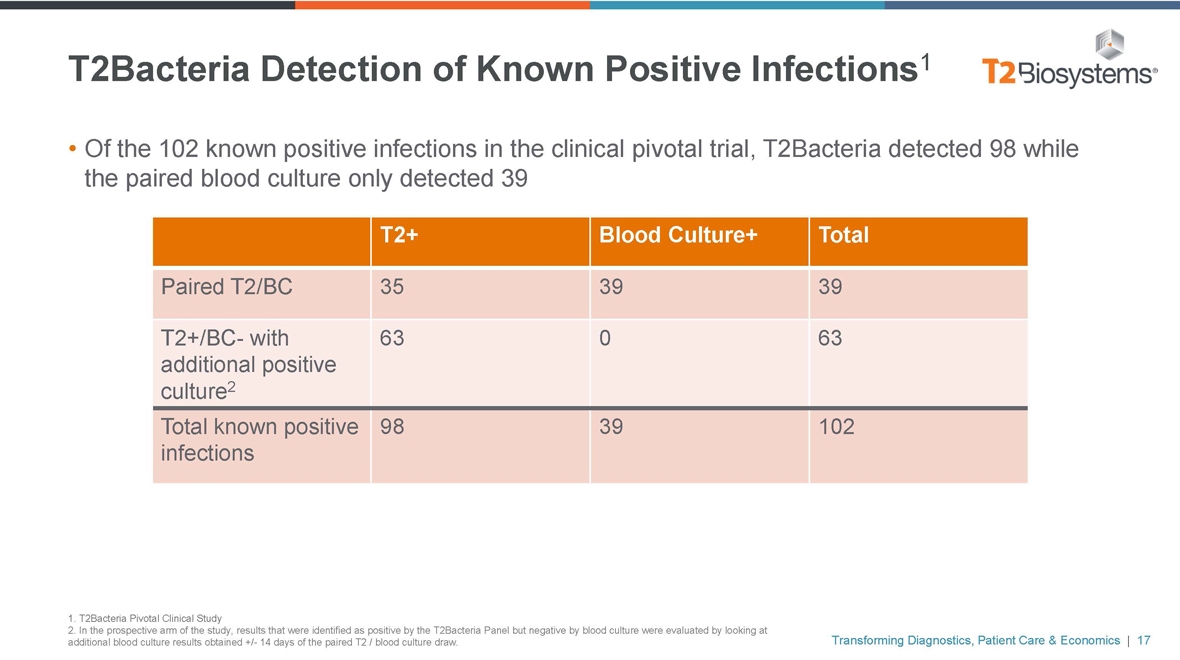
T2Bacteria Detection of Known Positive Infections1
• Of the 102 known positive infections in the clinical pivotal trial, T2Bacteria detected 98 while
the paired blood culture only detected 39
T2+ Blood Culture+ Total
Paired T2/BC 35 3939
T2+/BC- with 63 063
additional positive
culture2
Total known positive 98 39102
infections
1. T2Bacteria Pivotal Clinical Study
2. In the prospective arm of the study, results that were identified as positive by the T2Bacteria Panel but negative by blood culture were evaluated by looking at
additional blood culture results obtained +/- 14 days of the paired T2 / blood culture draw. Transforming Diagnostics, Patient Care & Economics |17
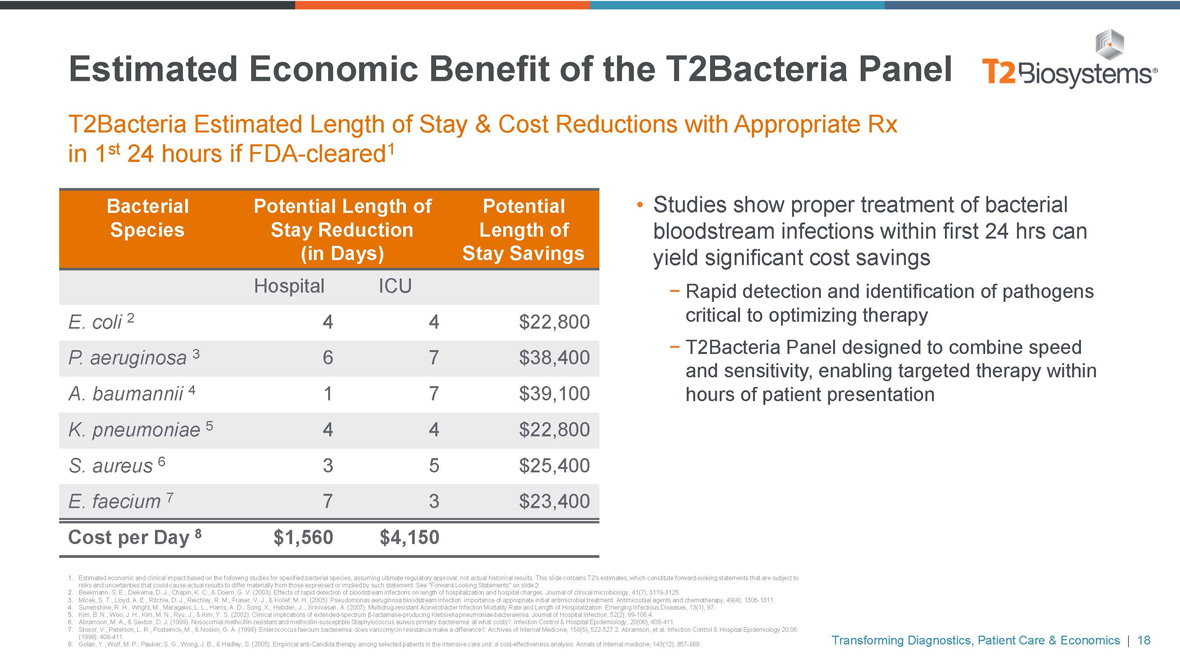
Estimated Economic Benefit of the T2Bacteria Panel
T2Bacteria Estimated Length of Stay & Cost Reductions with Appropriate Rx in 1st 24 hours ifFDA-cleared1
Bacterial Potential Length of Potential
Species Stay Reduction Length of
(in Days) Stay Savings
Hospital ICU
E. coli 2 44$22,800
P. aeruginosa 3 67$38,400
A. baumannii 4 17$39,100
K. pneumoniae 5 44$22,800
S. aureus 6 35$25,400
E. faecium 7 73$23,400
Cost per Day 8 $1,560 $4,150
1. Estimated economic and clinical impact based on the following studies for specified bacterial species, assuming ultimate regulatory approval, not actual historical results. This slide contains T2’s estimates, which constitute forward-looking statements that are subject to risks and uncertainties that could cause actual results to differ materially from those expressed or implied by such statement. See “Forward-Looking Statements” on slide 2.
2. Beekmann, S. E., Diekema, D. J., Chapin, K. C., & Doern, G. V. (2003). Effects of rapid detection of bloodstream infections on length of hospitalization and hospital charges. Journal of clinical microbiology, 41(7), 3119-3125.
3. Micek, S. T., Lloyd, A. E., Ritchie, D. J., Reichley, R. M., Fraser, V. J., & Kollef, M. H. (2005). Pseudomonas aeruginosa bloodstream infection: importance of appropriate initial antimicrobial treatment. Antimicrobial agents and chemotherapy, 49(4), 1306-1311.
4. Sunenshine, R. H., Wright, M., Maragakis, L. L., Harris, A. D., Song, X., Hebden, J....Srinivasan, A. (2007). Multidrug-resistant Acinetobacter Infection Mortality Rate and Length of Hospitalization. Emerging Infectious Diseases, 13(1), 97.
5. Kim, B. N., Woo, J. H., Kim, M. N., Ryu, J., & Kim, Y. S. (2002). Clinical implications of extended-spectrum â-lactamase-producing Klebsiella pneumoniae bacteraemia. Journal of Hospital Infection, 52(2),99-106.4.
6. Abramson, M. A., & Sexton, D. J. (1999). Nosocomial methicillin-resistant and methicillin-susceptible Staphylococcus aureus primary bacteremia: at what costs?. Infection Control & Hospital Epidemiology, 20(06),408-411.
7. Stosor, V., Peterson, L. R., Postelnick, M., & Noskin, G. A. (1998). Enterococcus faecium bacteremia: does vancomycin resistance make a difference?. Archives of Internal Medicine, 158(5),522-527.2. Abramson, et al. Infection Control & Hospital Epidemiology 20.06 (1999):408-411.
8. Golan, Y., Wolf, M. P., Pauker, S. G., Wong, J. B., & Hadley, S. (2005). Empirical anti-Candida therapy among selected patients in the intensive care unit: a cost-effectiveness analysis. Annals of internal medicine, 143(12),857-869.
• Studies show proper treatment of bacterial bloodstream infections within first 24 hrs can yield significant cost savings
- Rapid detection and identification of pathogens critical to optimizing therapy
- T2Bacteria Panel designed to combine speed and sensitivity, enabling targeted therapy within hours of patient presentation
Transforming Diagnostics, Patient Care & Economics | 18
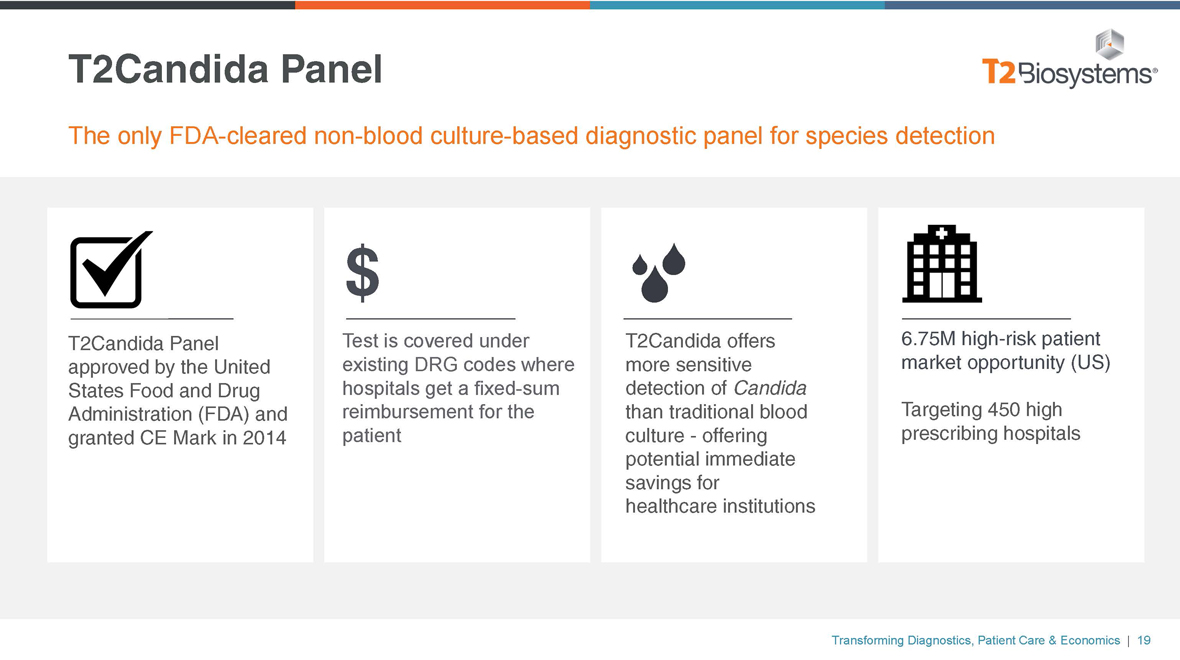
T2Candida Panel
The onlyFDA-clearednon-blood culture-based diagnostic panel for species detection
T2Candida Panel Test is covered under T2Candida offers 6.75M high-risk patient approved by the United existing DRG codes where more sensitive market opportunity (US) States Food and Drug hospitals get afixed-sum detection of Candida Administration (FDA) and reimbursement for the than traditional blood Targeting 450 high granted CE Mark in 2014 patient culture—offering prescribing hospitals potential immediate savings for healthcare institutions
Transforming Diagnostics, Patient Care & Economics | 19
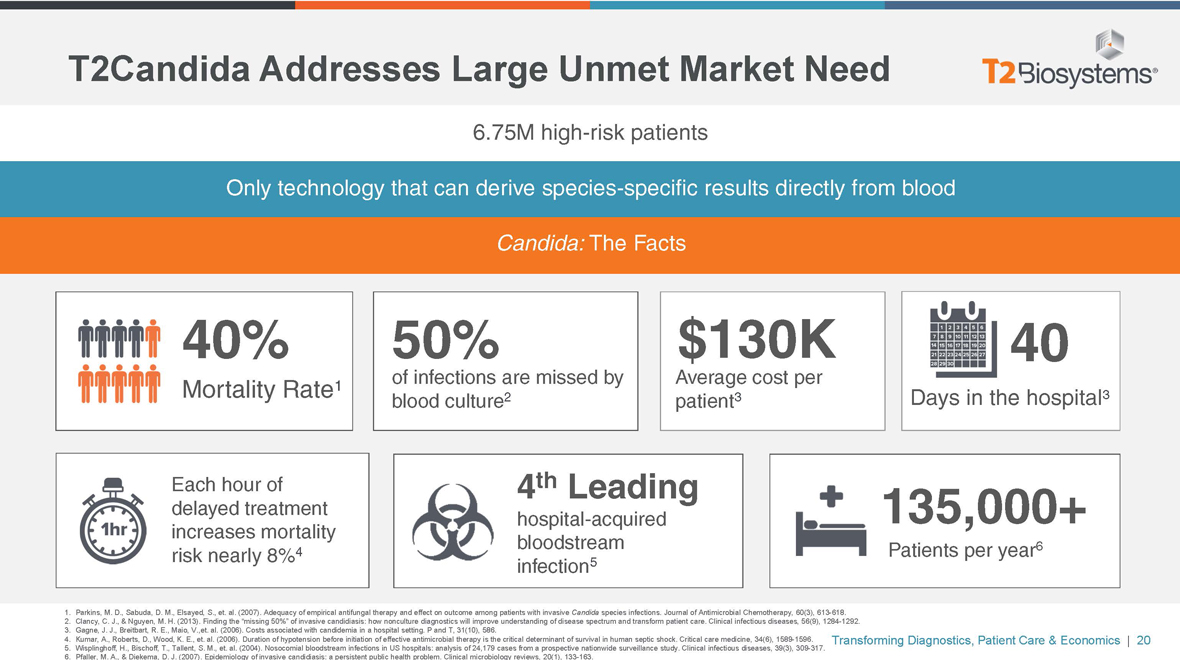
T2Candida Addresses Large Unmet Market Need
6.75M high-risk patients
Only technology that can derive species-specific results directly from blood
Candida: The Facts
40%
Mortality Rate1
Each hour of delayed treatment increases mortality risk nearly 8%4
50% $130K 40
of infections are missed by Average cost per blood culture2 patient3 Days in the hospital3
4th Leading 135,000+
hospital-acquired bloodstream 6 Patients per year infection5
1. Parkins, M. D., Sabuda, D. M., Elsayed, S., et. al. (2007). Adequacy of empirical antifungal therapy and effect on outcome among patients with invasive Candida species infections. Journal of Antimicrobial Chemotherapy, 60(3),613-618.
2. Clancy, C. J., & Nguyen, M. H. (2013). Finding the “missing 50%” of invasive candidiasis: how nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clinical infectious diseases, 56(9), 1284-1292.
3. Gagne, J. J., Breitbart, R. E., Maio, V.,et. al. (2006). Costs associated with candidemia in a hospital setting. P and T, 31(10), 586.
4. Kumar, A., Roberts, D., Wood, K. E., et. al. (2006). Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Critical care medicine, 34(6), 1589-1596.
5. Wisplinghoff, H., Bischoff, T., Tallent, S. M., et. al. (2004). Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clinical infectious diseases, 39(3),309-317.
6. Pfaller, M. A., & Diekema, D. J. (2007). Epidemiology of invasive candidiasis: a persistent public health problem. Clinical microbiology reviews, 20(1),133-163.
Transforming Diagnostics, Patient Care & Economics | 20
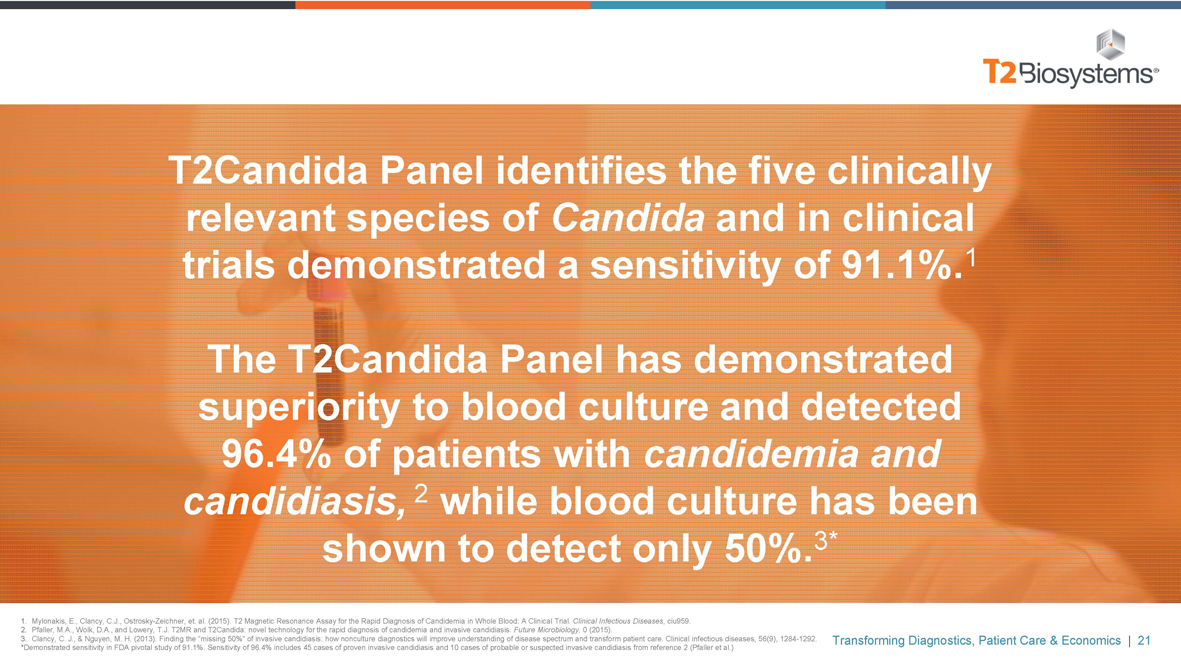
T2Candida Panel identifies the five clinically relevant species of Candida and in clinical trials demonstrated a sensitivity of 91.1%.1
The T2Candida Panel has demonstrated superiority to blood culture and detected 96.4% of patients with candidemia and candidiasis, 2 while blood culture has been shown to detect only 50%.3*
1. Mylonakis, E., Clancy, C.J., Ostrosky-Zeichner, et. al. (2015). T2 Magnetic Resonance Assay for the Rapid Diagnosis of Candidemia in Whole Blood: A Clinical Trial. Clinical Infectious Diseases, ciu959.
2. Pfaller, M.A., Wolk, D.A., and Lowery, T.J. T2MR and T2Candida: novel technology for the rapid diagnosis of candidemia and invasive candidiasis. Future Microbiology, 0 (2015).
3. Clancy, C. J., & Nguyen, M. H. (2013). Finding the “missing 50%” of invasive candidiasis: how nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clinical infectious diseases, 56(9), 1284-1292.
*Demonstrated sensitivity in FDA pivotal study of 91.1%. Sensitivity of 96.4% includes 45 cases of proven invasive candidiasis and 10 cases of probable or suspected invasive candidiasis from reference 2 (Pfaller et al.)
Transforming Diagnostics, Patient Care & Economics | 21
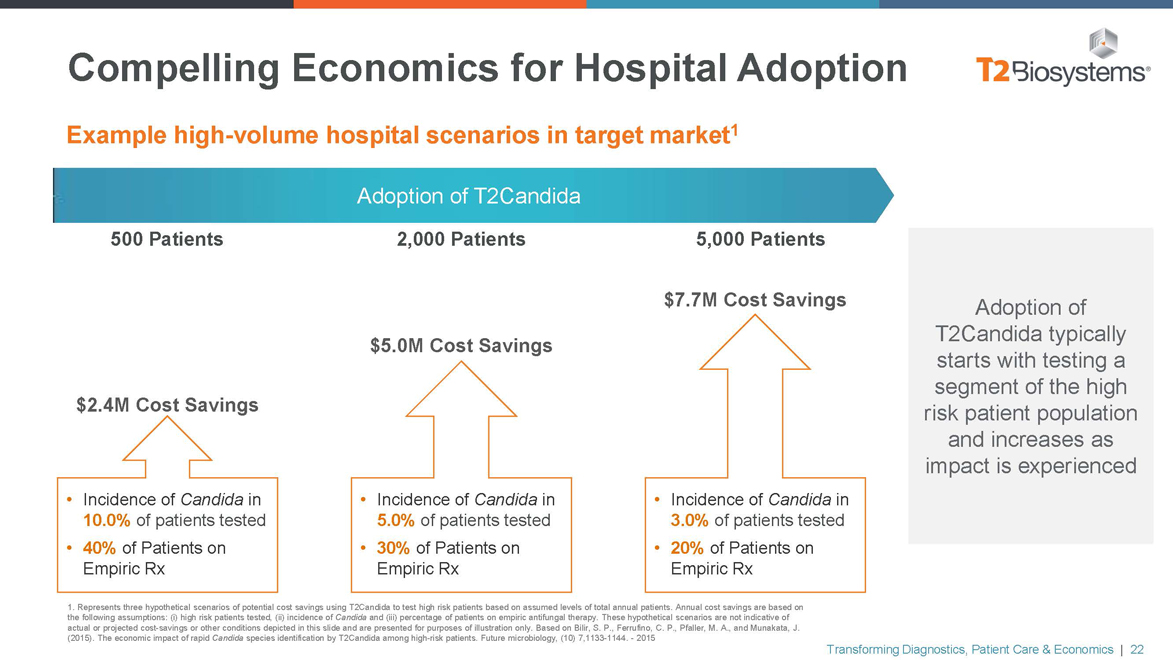
Compelling Economics for Hospital Adoption
Example high-volume hospital scenarios in target market1
Adoption of T2Candida
500 Patients 2,000 Patients 5,000 Patients
$7.7M Cost Savings
$5.0M Cost Savings
$2.4M Cost Savings
• Incidence of Candida in • Incidence of Candida in • Incidence of Candida in
10.0% of patients tested 5.0% of patients tested 3.0% of patients tested
40% of Patients on •30% of Patients on • 20% of Patients on
Empiric Rx Empiric RxEmpiric Rx
1. Represents three hypothetical scenarios of potential cost savings using T2Candida to test high risk patients based on assumed levels of total annual patients. Annual cost savings are based on the following assumptions: (i) high risk patients tested, (ii) incidence of Candida and (iii) percentage of patients on empiric antifungal therapy. These hypothetical scenarios are not indicative of actual or projected cost-savings or other conditions depicted in this slide and are presented for purposes of illustration only. Based on Bilir, S. P., Ferrufino, C. P., Pfaller, M. A., and Munakata, J. (2015). The economic impact of rapid Candida species identification by T2Candida among high-risk patients. Future microbiology, (10) 7,1133-1144.—2015
Adoption of T2Candida typically starts with testing a segment of the high risk patient population and increases as impact is experienced
Transforming Diagnostics, Patient Care & Economics | 22
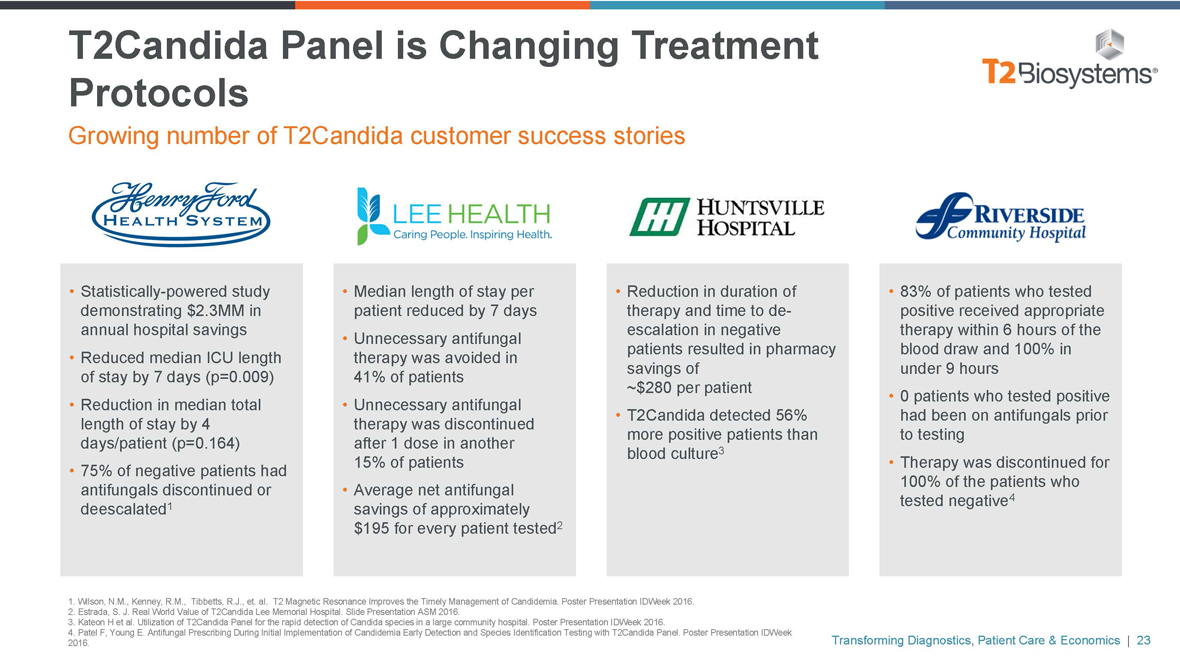
T2Candida Panel is Changing Treatment Protocols
Growing number of T2Candida customer success stories
• Statistically-powered study demonstrating $2.3MM in annual hospital savings
• Reduced median ICU length of stay by 7 days (p=0.009)
• Reduction in median total length of stay by 4 days/patient (p=0.164)
• 75% of negative patients had antifungals discontinued or deescalated1
• Median length of stay per patient reduced by 7 days
• Unnecessary antifungal therapy was avoided in 41% of patients
• Unnecessary antifungal therapy was discontinued after 1 dose in another 15% of patients
• Average net antifungal savings of approximately $195 for every patient tested2
• Reduction in duration of therapy and time tode-escalation in negative patients resulted in pharmacy savings of
~$280 per patient
• T2Candida detected 56% more positive patients than blood culture3
• 83% of patients who tested positive received appropriate therapy within 6 hours of the blood draw and 100% in under 9 hours
• 0 patients who tested positive had been on antifungals prior to testing
• Therapy was discontinued for 100% of the patients who tested negative4
1. Wilson, N.M., Kenney, R.M., Tibbetts, R.J., et. al. T2 Magnetic Resonance Improves the Timely Management of Candidemia. Poster Presentation IDWeek 2016.
2. Estrada, S. J. Real World Value of T2Candida Lee Memorial Hospital. Slide Presentation ASM 2016.
3. Kateon H et al. Utilization of T2Candida Panel for the rapid detection of Candida species in a large community hospital. Poster Presentation IDWeek 2016.
4. Patel F, Young E. Antifungal Prescribing During Initial Implementation of Candidemia Early Detection and Species Identification Testing with T2Candida Panel. Poster Presentation IDWeek 2016.
Transforming Diagnostics, Patient Care & Economics | 23
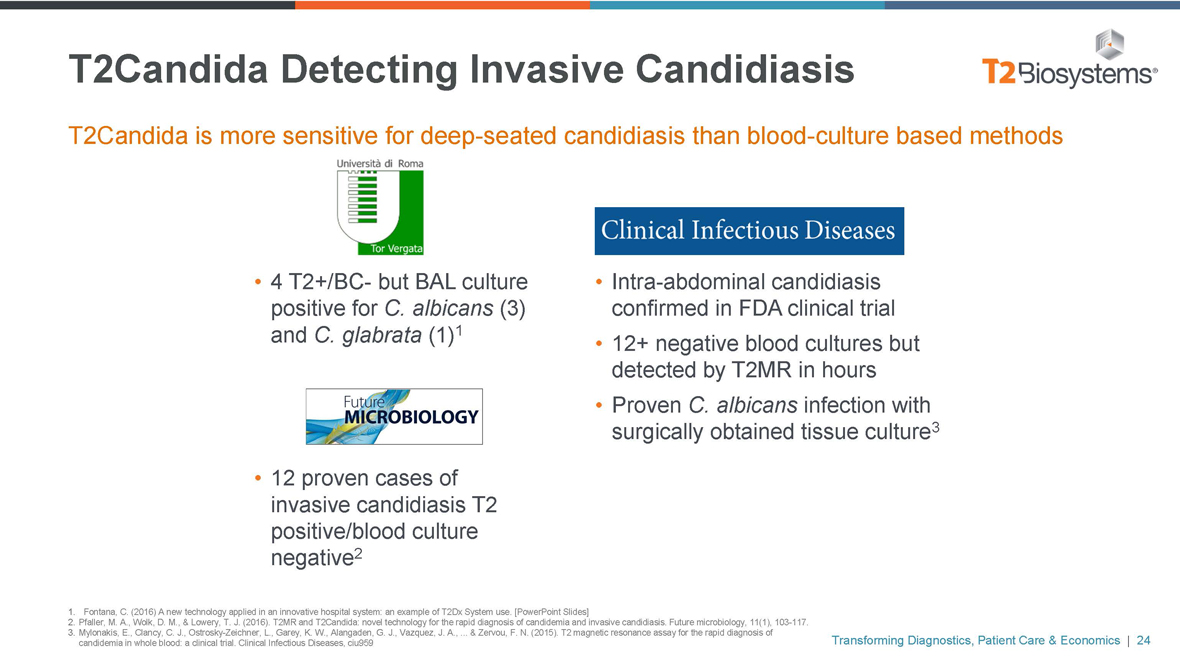
T2Candida Detecting Invasive Candidiasis
T2Candida is more sensitive for deep-seated candidiasis than blood-culture based methods
• 4T2+/BC- but BAL culture positive for C. albicans (3) and C. glabrata (1)1
• 12 proven cases of invasive candidiasis T2 positive/blood culture negative2
• Intra-abdominal candidiasis confirmed in FDA clinical trial
• 12+ negative blood cultures but detected by T2MR in hours
• Proven C. albicans infection with surgically obtained tissue culture3
1. Fontana, C. (2016) A new technology applied in an innovative hospital system: an example of T2Dx System use. [PowerPoint Slides]
2. Pfaller, M. A., Wolk, D. M., & Lowery, T. J. (2016). T2MR and T2Candida: novel technology for the rapid diagnosis of candidemia and invasive candidiasis. Future microbiology, 11(1),103-117.
3. Mylonakis, E., Clancy, C. J., Ostrosky-Zeichner, L., Garey, K. W., Alangaden, G. J., Vazquez, J. A., ... & Zervou, F. N. (2015). T2 magnetic resonance assay for the rapid diagnosis of candidemia in whole blood: a clinical trial. Clinical Infectious Diseases, ciu959
Transforming Diagnostics, Patient Care & Economics | 24

Commercial Strategy
Transforming Diagnostics, Patient Care & Economics | 25
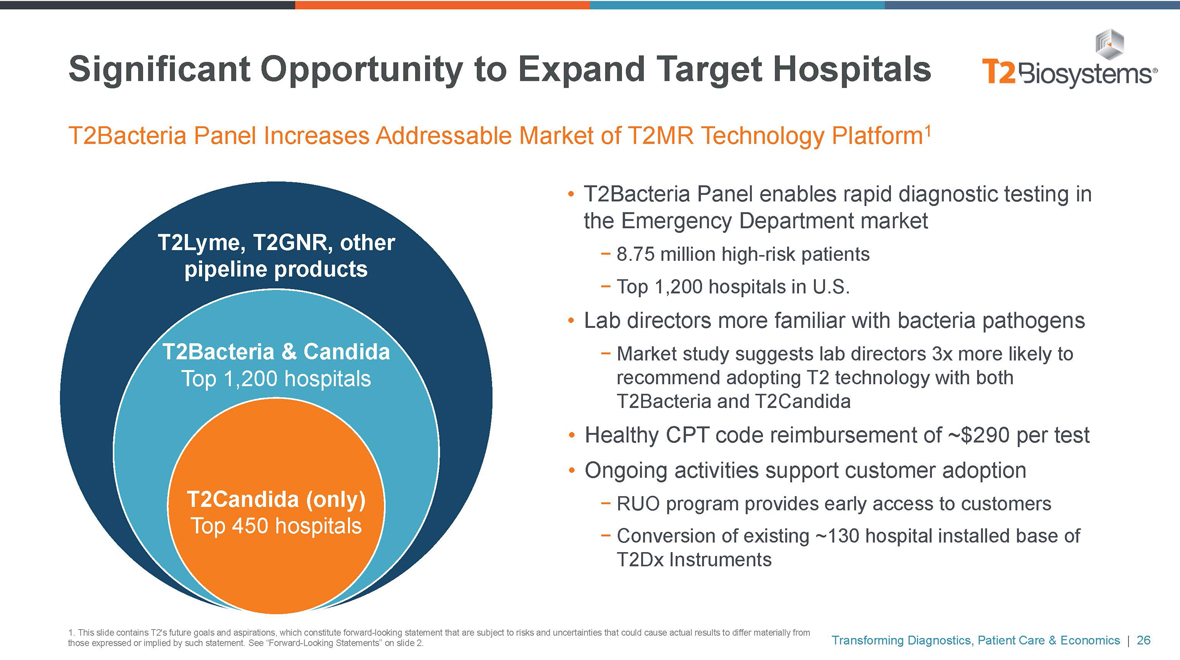
Significant Opportunity to Expand Target Hospitals
T2Bacteria Panel Increases Addressable Market of T2MR Technology Platform1
T2Lyme, T2GNR, other pipeline products
T2Bacteria & Candida
Top 1,200 hospitals
T2Candida (only)
Top 450 hospitals
• T2Bacteria Panel enables rapid diagnostic testing in the Emergency Department market
- 8.75 million high-risk patients
- Top 1,200 hospitals in U.S.
• Lab directors more familiar with bacteria pathogens
- Market study suggests lab directors 3x more likely to recommend adopting T2 technology with both T2Bacteria and T2Candida
• Healthy CPT code reimbursement of ~$290 per test
• Ongoing activities support customer adoption
- RUO program provides early access to customers
- Conversion of existing ~130 hospital installed base of T2Dx Instruments
1. This slide contains T2’s future goals and aspirations, which constitute forward-looking statement that are subject to risks and uncertainties that could cause actual results to differ materially from those expressed or implied by such statement. See “Forward-Looking Statements” on slide 2.
Transforming Diagnostics, Patient Care & Economics | 26
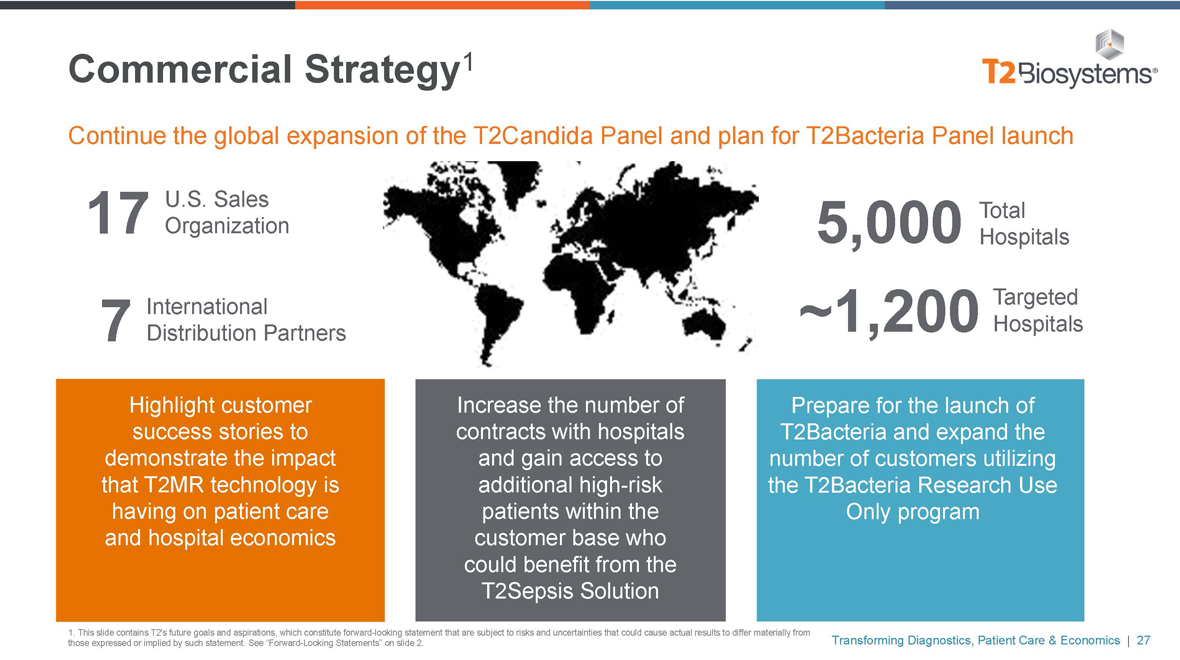
Commercial Strategy1
Continue the global expansion of the T2Candida Panel and plan for T2Bacteria Panel launch
U.S. Sales
17 Organization International
7 Distribution Partners
5,000 Total
Hospitals
Targeted ~1,200 Hospitals
Highlight customer success stories to demonstrate the impact that T2MR technology is having on patient care and hospital economics
Increase the number of contracts with hospitals and gain access to additional high-risk patients within the customer base who could benefit from the T2Sepsis Solution
Prepare for the launch of T2Bacteria and expand the number of customers utilizing the T2Bacteria Research Use Only program
1. This slide contains T2’s future goals and aspirations, which constitute forward-looking statement that are subject to risks and uncertainties that could cause actual results to differ materially from those expressed or implied by such statement. See “Forward-Looking Statements” on slide 2.
Transforming Diagnostics, Patient Care & Economics | 27
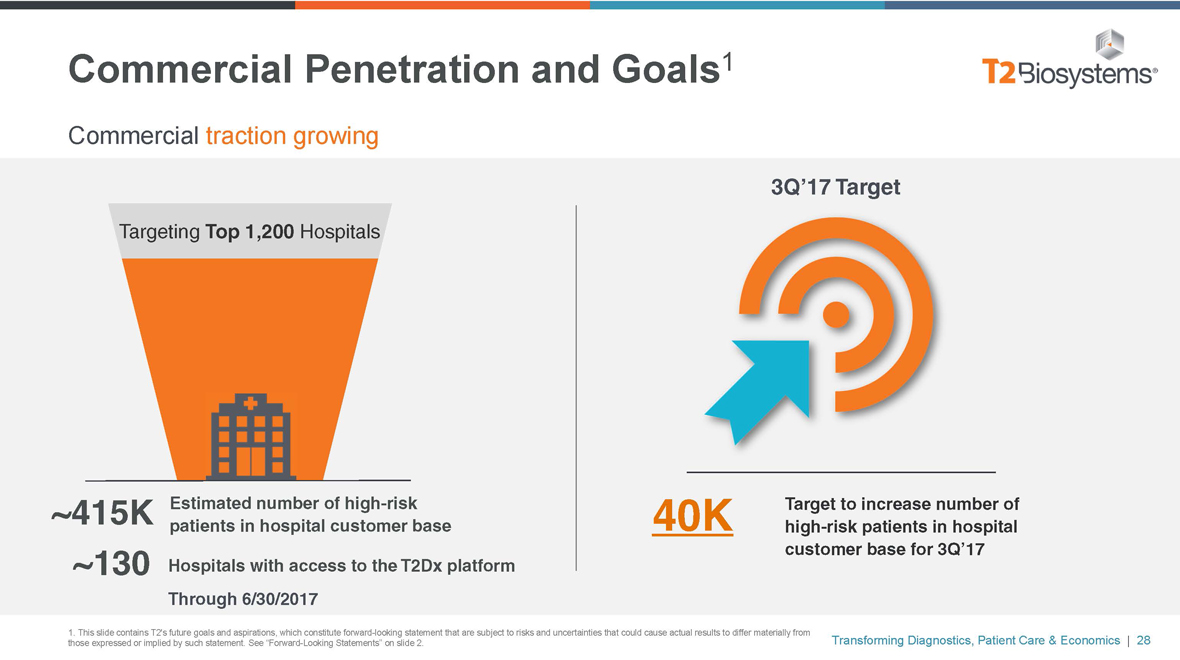
Commercial Penetration and Goals1
Commercial traction growing
Targeting Top 1,200 Hospitals
~415K Estimated number of high-risk patients in hospital customer base
~130 Hospitals with access to the T2Dx platform Through 6/30/2017
3Q’17 Target
40K Target to increase number of high-risk patients in hospital customer base for 3Q’17
1. This slide contains T2’s future goals and aspirations, which constitute forward-looking statement that are subject to risks and uncertainties that could cause actual results to differ materially from those expressed or implied by such statement. See “Forward-Looking Statements” on slide 2.
Transforming Diagnostics, Patient Care & Economics | 28
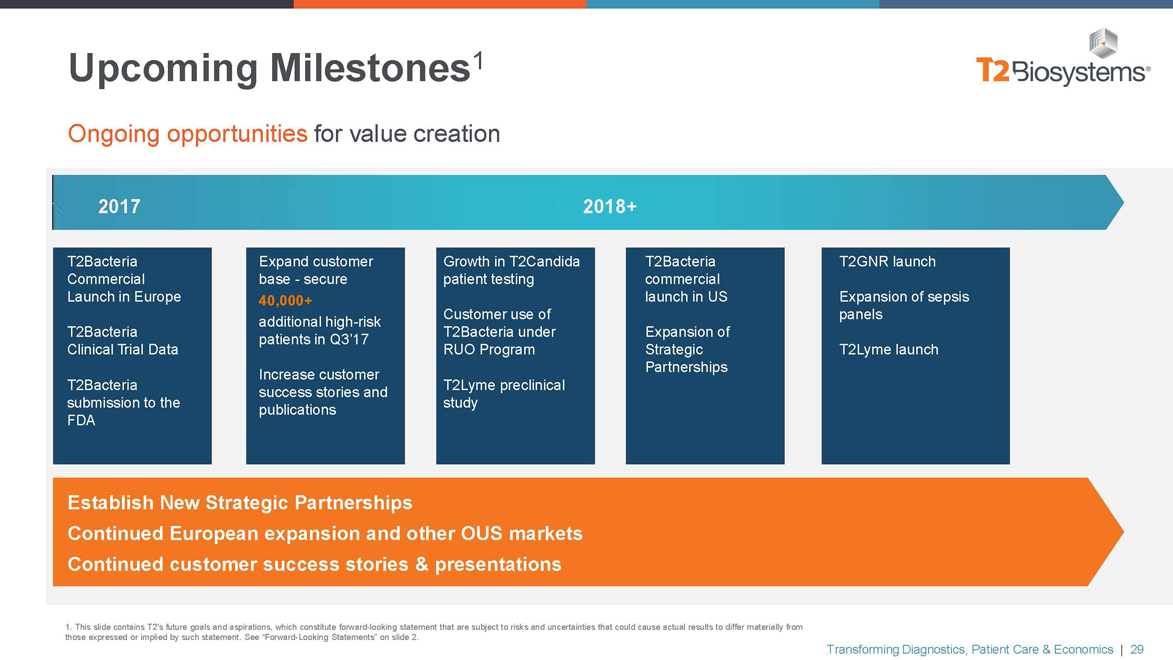
Upcoming Milestones1
Ongoing opportunities for value creation
2017 2018+
T2Bacteria Commercial Launch in Europe
T2Bacteria Clinical Trial Data
T2Bacteria submission to the FDA
Expand customer base—secure
40,000+ additional high-risk patients in Q3’17
Increase customer success stories and publications
Growth in T2Candida patient testing
Customer use of T2Bacteria under RUO Program
T2Lyme preclinical study
T2Bacteria commercial launch in US
Expansion of Strategic Partnerships
T2GNR launch
Expansion of sepsis panels
T2Lyme launch
Establish New Strategic Partnerships
Continued European expansion and other OUS markets Continued customer success stories & presentations
1. This slide contains T2’s future goals and aspirations, which constitute forward-looking statement that are subject to risks and uncertainties that could cause actual results to differ materially from those expressed or implied by such statement. See “Forward-Looking Statements” on slide 2.
Transforming Diagnostics, Patient Care & Economics | 29

Strategic Collaboration
Endorsement of T2MR detection capabilities from pharma industry leader
Overview
Agreement with Allergan to create an additional panel on the T2Dx Instrument. The panel will be designed to identify drug resistance to enable physicians to provide targeted therapy faster.
Economics
Allergan will pay T2 Biosystems up to $4 million in development milestone payments.
Commercial
T2 Biosystems retains exclusive worldwide distribution rights. Allergan may cooperatively market T2 Biosystems’ T2Candida, T2Bacteria and similar future products to targeted hospitals through Allergan’s physician-facing institutional sales force.
Transforming Diagnostics, Patient Care & Economics | 30
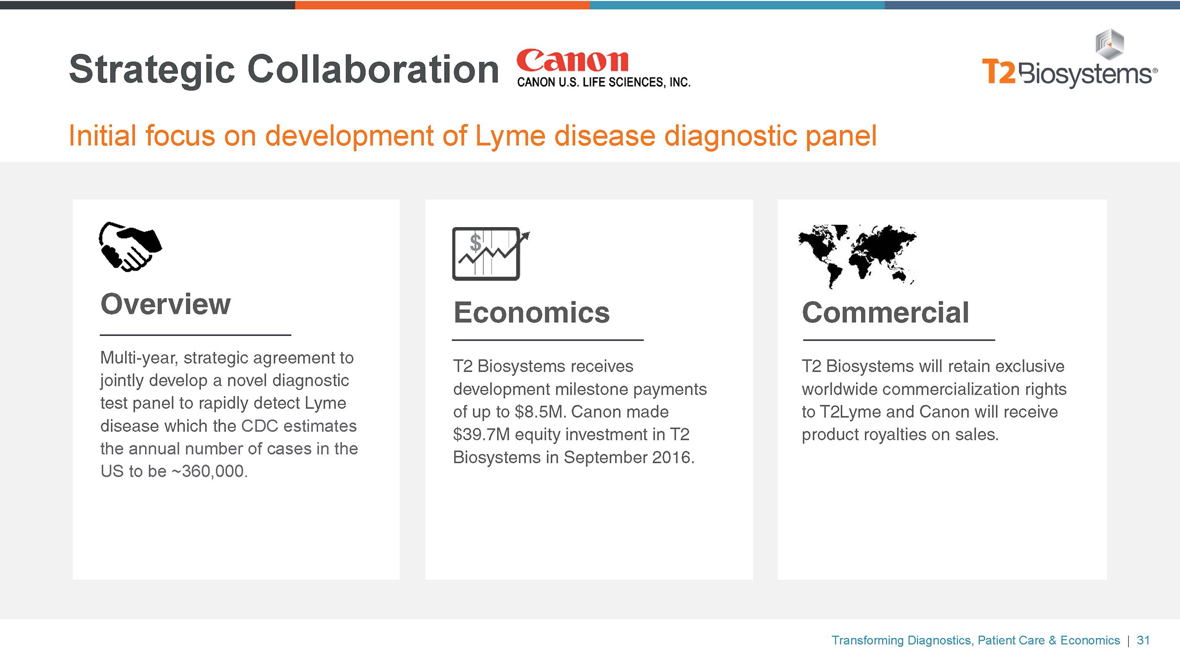
Strategic Collaboration
Initial focus on development of Lyme disease diagnostic panel
Overview
Multi-year, strategic agreement to jointly develop a novel diagnostic test panel to rapidly detect Lyme disease which the CDC estimates the annual number of cases in the US to be ~360,000.
Economics
T2 Biosystems receives development milestone payments of up to $8.5M. Canon made $39.7M equity investment in T2 Biosystems in September 2016.
Commercial
T2 Biosystems will retain exclusive worldwide commercialization rights to T2Lyme and Canon will receive product royalties on sales.
Transforming Diagnostics, Patient Care & Economics | 31
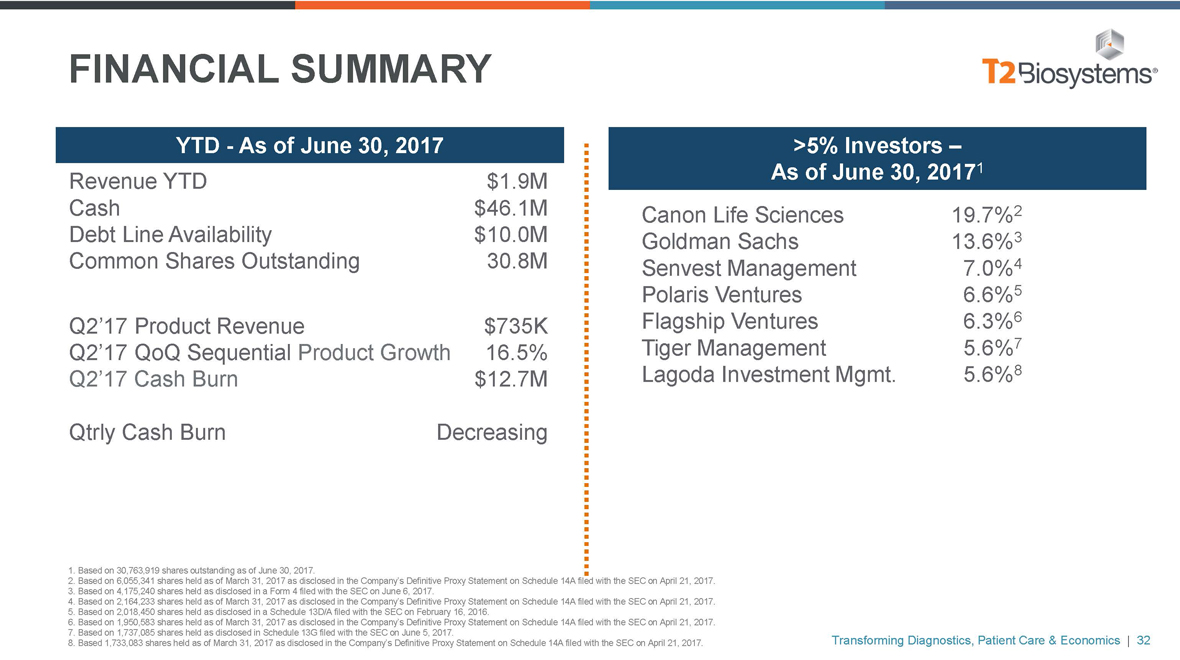
FINANCIAL SUMMARY
YTD—As of June 30, 2017
Revenue YTD $1.9M
Cash $46.1M
Debt Line Availability $10.0M
Common Shares Outstanding 30.8M
Q2’17 Product Revenue $735K
Q2’17 QoQ Sequential Product Growth 16.5%
Q2’17 Cash Burn $12.7M
Qtrly Cash Burn Decreasing
>5% Investors –
As of June 30, 20171
Canon Life Sciences 19.7%2
Goldman Sachs 13.6%3
Senvest Management 7.0%4
Polaris Ventures 6.6%5
Flagship Ventures 6.3%6
Tiger Management 5.6%7
Lagoda Investment Mgmt. 5.6%8
1. Based on 30,763,919 shares outstanding as of June 30, 2017.
2. Based on 6,055,341 shares held as of March 31, 2017 as disclosed in the Company’s Definitive Proxy Statement on Schedule 14A filed with the SEC on April 21, 2017.
3. Based on 4,175,240 shares held as disclosed in a Form 4 filed with the SEC on June 6, 2017.
4. Based on 2,164,233 shares held as of March 31, 2017 as disclosed in the Company’s Definitive Proxy Statement on Schedule 14A filed with the SEC on April 21, 2017.
5. Based on 2,018,450 shares held as disclosed in a Schedule 13D/A filed with the SEC on February 16, 2016.
6. Based on 1,950,583 shares held as of March 31, 2017 as disclosed in the Company’s Definitive Proxy Statement on Schedule 14A filed with the SEC on April 21, 2017.
7. Based on 1,737,085 shares held as disclosed in Schedule 13G filed with the SEC on June 5, 2017.
8. Based 1,733,083 shares held as of March 31, 2017 as disclosed in the Company’s Definitive Proxy Statement on Schedule 14A filed with the SEC on April 21, 2017.
Transforming Diagnostics, Patient Care & Economics | 32
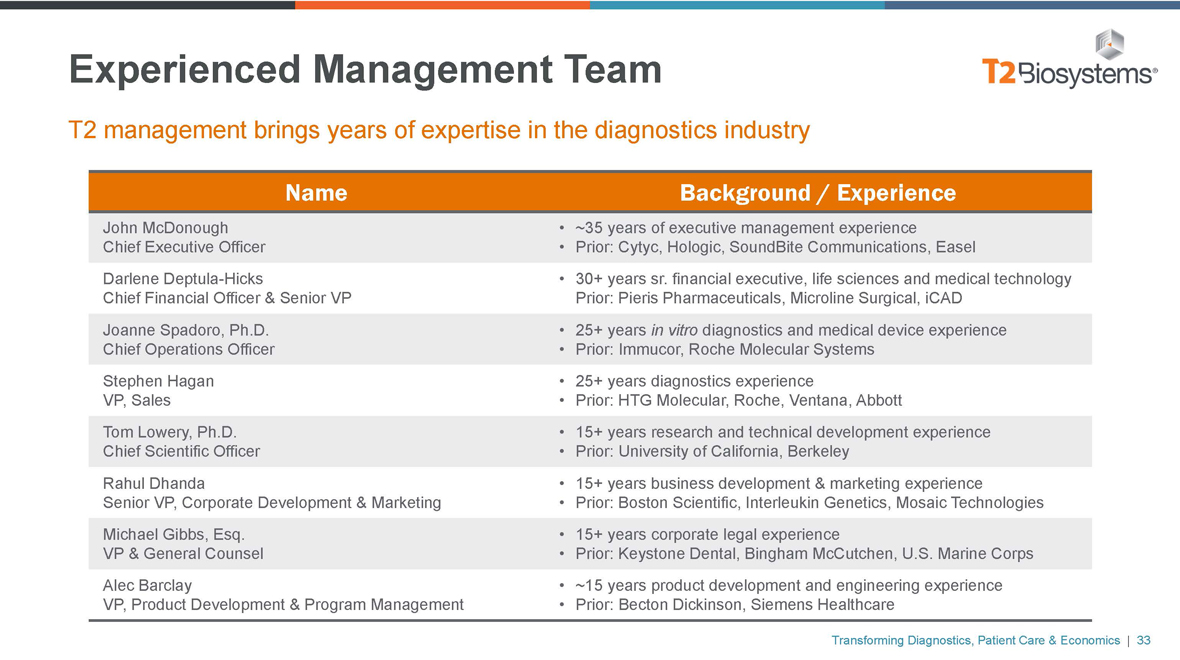
Experienced Management Team
T2 management brings years of expertise in the diagnostics industry
Name
John McDonough Chief Executive Officer Darlene Deptula-Hicks
Chief Financial Officer & Senior VP Joanne Spadoro, Ph.D.
Chief Operations Officer Stephen Hagan VP, Sales Tom Lowery, Ph.D. Chief Scientific Officer Rahul Dhanda
Senior VP, Corporate Development & Marketing Michael Gibbs, Esq.
VP & General Counsel Alec Barclay
VP, Product Development & Program Management
Background / Experience
• ~35 years of executive management experience
• Prior: Cytyc, Hologic, SoundBite Communications, Easel
• 30+ years sr. financial executive, life sciences and medical technology Prior: Pieris Pharmaceuticals, Microline Surgical, iCAD
• 25+ years in vitro diagnostics and medical device experience
• Prior: Immucor, Roche Molecular Systems
• 25+ years diagnostics experience
• Prior: HTG Molecular, Roche, Ventana, Abbott
• 15+ years research and technical development experience
• Prior: University of California, Berkeley
• 15+ years business development & marketing experience
• Prior: Boston Scientific, Interleukin Genetics, Mosaic Technologies
• 15+ years corporate legal experience
• Prior: Keystone Dental, Bingham McCutchen, U.S. Marine Corps
• ~15 years product development and engineering experience
• Prior: Becton Dickinson, Siemens Healthcare
Transforming Diagnostics, Patient Care & Economics | 33

Investment Highlights
A platform technology with multiple, billion-dollar franchise opportunities
T2MR Market T2Sepsis Solution
Innovative $3B+ Initial market Provide species-specific technology with potential results, direct from whole broad applications blood, in 3 to 5 hours1
Reimbursement Robust Pipeline Execution
Products covered A new generation of High-risk patient access by existing diagnostics growing and several reimbursement codes collaborations established
1. Mylonakis, E., Clancy, C. J., Ostrosky-Zeichner, L., Garey, K. W., Alangaden, G. J., Vazquez, J. A., ... & Zervou, F. N. (2015). T2 magnetic resonance assay for the rapid diagnosis of candidemia in whole blood: a clinical trial. Clinical Infectious Diseases, ciu959.
Transforming Diagnostics, Patient Care & Economics | 34

THANK YOU
Transforming Diagnostics, Patient Care & Economics | 35


































