
Building a Leading ENT / Allergy Specialty Company C o r p o r a t e P r e s e n t a t i o n A u g u s t 1 1 , 2 0 2 2

2 Forward-Looking Statements This presentation and our accompanying remarks contain “forward-looking statements” within the meaning of the U.S. Private Securities Litigation Reform Act of 1995. All statements that are not historical facts are hereby identified as forward-looking statements for this purpose and include, among others, statements relating to: potential for continued XHANCE prescription and net revenue growth and factors supporting such growth; prescription, refill and market share trends; potential effects of INS market seasonality on XHANCE prescriptions; the effects of changes made to the XHANCE co-pay assistance program in January 2022 and the potential benefits of such changes; projected Company GAAP operating expenses and stock-based compensation for 2022; projected XHANCE net revenues for full year 2022; projected XHANCE average net revenue per prescription for full year 2022; the potential benefits of XHANCE for the treatment of chronic sinusitis; the Company's plans to seek approval for a follow-on indication for XHANCE for the treatment of chronic sinusitis and its plan to submit an sNDA by the end of 2022; the potential for XHANCE to be the first FDA-approved drug treatment for chronic sinusitis and the potential market expansion opportunities and other benefits of obtaining such indication; the Company's plan to secure a partnership to promote XHANCE in primary care and the prospects for, and potential benefits of, such potential partnership; and other statements regarding the Company’s future operations, financial performance, prospects, intentions, objectives and other future events. Forward-looking statements are based upon management’s current expectations and assumptions and are subject to a number of risks, uncertainties and other factors that could cause actual results and events to differ materially and adversely from those indicated by such forward-looking statements including, among others: impact of, and the uncertainties caused by, the COVID-19 pandemic; physician and patient acceptance of XHANCE for its current and any potential future indication; the Company’s ability to maintain adequate third party reimbursement for XHANCE (market access); the Company’s ability to grow XHANCE prescriptions and net revenues; the prevalence of chronic sinusitis and market opportunities for XHANCE may be smaller than expected; unexpected costs and expenses; potential for varying interpretation of the results from the ReOpen program; uncertainties related to the clinical development program and regulatory approval of XHANCE for the treatment of chronic sinusitis; the Company’s ability to comply with the covenants and other terms of the Pharmakon note purchase agreement; risks and uncertainties relating to intellectual property; and the risks, uncertainties and other factors discussed in the “Risk Factors” section and elsewhere in our most recent Form 10-K and Form 10-Q filings with the Securities and Exchange Commission – which are available at http://www.sec.gov. As a result, you are cautioned not to place undue reliance on any forward-looking statements. Any forward-looking statements made in this presentation speak only as of the date of this presentation, and we undertake no obligation to update such forward-looking statements, whether as a result of new information, future developments or otherwise.

Q2 2022 Performance
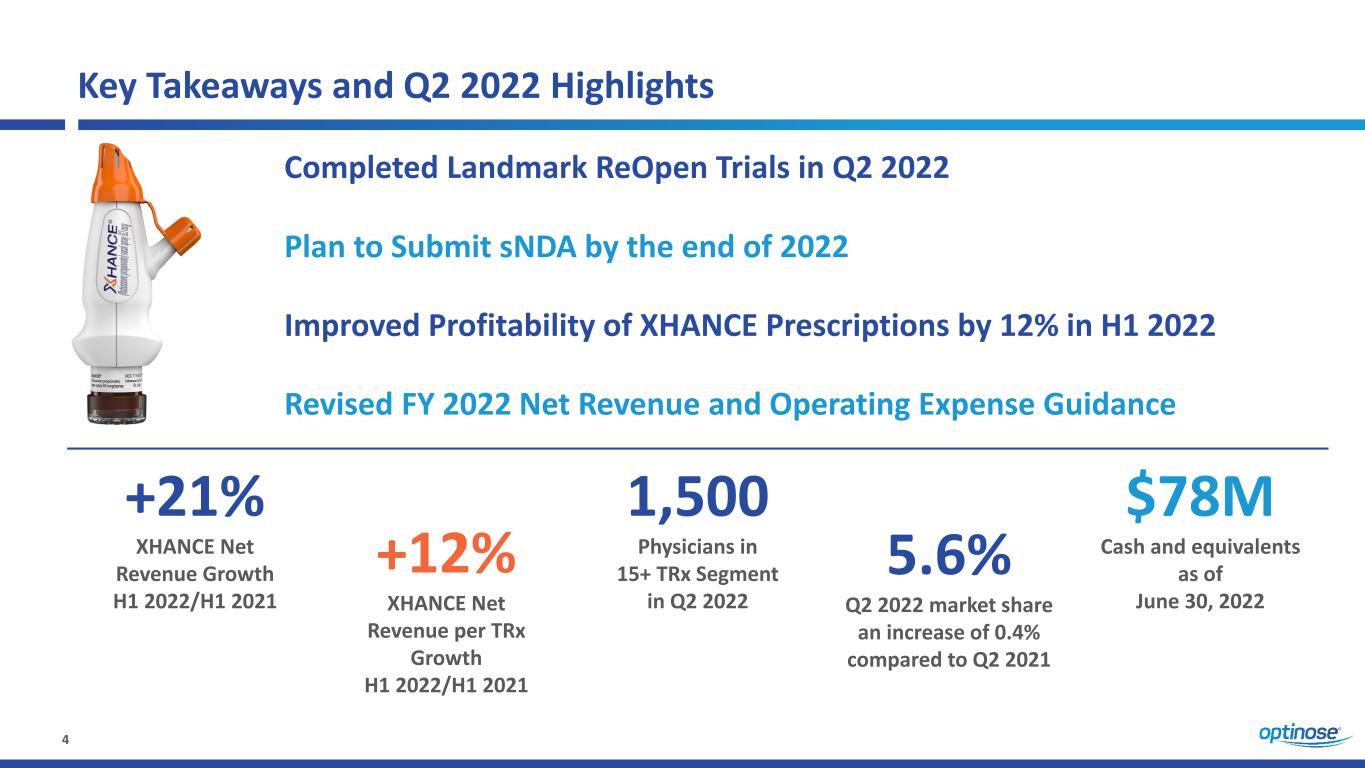
4 Key Takeaways and Q2 2022 Highlights Completed Landmark ReOpen Trials in Q2 2022 Plan to Submit sNDA by the end of 2022 Improved Profitability of XHANCE Prescriptions by 12% in H1 2022 +21% XHANCE Net Revenue Growth H1 2022/H1 2021 +12% XHANCE Net Revenue per TRx Growth H1 2022/H1 2021 1,500 Physicians in 15+ TRx Segment in Q2 2022 Revised FY 2022 Net Revenue and Operating Expense Guidance $78M Cash and equivalents as of June 30, 2022 5.6% Q2 2022 market share an increase of 0.4% compared to Q2 2021

5 Successful Development of XHANCE as the First FDA-approved Drug Treatment for Chronic Sinusitis Creates Multiple New Opportunities for Growth Up to ~1 Million patients with nasal polyps (NP) are treated by specialty physicians in our current sales deployment $1B TAM $3B TAM Up to ~10 Million patients with either NP (~3M) or CS (~7M) are treated by a specialty or PCP physician annually $10B TAM Today With a CS Indication Up to ~3 Million patients with either NP or CS are treated by specialty physicians in our current sales deployment For a Partner, there are 6 to 7 million NP+CS patients currently treated by a Primary Care Physician plus 20 million lapsed patients that could be activated into care

ReOpen Program Update

7 Combined Symptom Score (Co-Primary Endpoint) Improvement in combined symptoms with XHANCE; Consistent with NAVIGATE I and II 1 -0.81 -1.54* -1.74‡ -2.0 -1.8 -1.6 -1.4 -1.2 -1.0 -0.8 -0.6 -0.4 -0.2 0.0 CS S Po in t R ed uc tio n LS Mean Change in CSS from Baseline to Week 4 EDS-placebo BID OPN-375 186 μg BID OPN-375 372 μg BID OPN-375 is XHANCE; BID, twice daily; CSNS, composite symptom nasal score. N = 75 N = 72 N = 73 *P ≤ .05 vs EDS placebo. ‡P ≤ .001 vs EDS-placebo. 1 -0.62 -1.58‡ -1.60‡ -2.0 -1.8 -1.6 -1.4 -1.2 -1.0 -0.8 -0.6 -0.4 -0.2 0.0 CS S Po in t R ed uc tio n LS Mean Change in CSS from Baseline to Week 4 EDS-placebo BID OPN-375 186 μg BID OPN-375 372 μg BID N = 110 N = 110 N = 107

8 Average of Percentages of Opacified Volume (Ethmoid and Maxillary) Objective Evidence of Effect in Sinus Cavities by CT Scan; Co-Primary Endpoint 1.19 -7.00‡ -5.14♰ -8 -7 -6 -5 -4 -3 -2 -1 0 1 2 Pe rc en t R ed uc tio n LS Mean Change in APOV of the Ethmoid and Maxillary Sinuses from Baseline to Week 24 EDS-placebo BID OPN-375 186 μg BID OPN-375 372 μg BID N = 75 N = 72 N = 73 *P ≤ .05 vs EDS placebo. ♰P ≤ .01 vs EDS-placebo. ‡P ≤ .001 vs EDS-placebo. -1.60 -5.58* -6.20* -7 -6 -5 -4 -3 -2 -1 0 Pe rc en t R ed uc tio n LS Mean Change in APOV of the Ethmoid and Maxillary Sinuses from Baseline to Week 24 EDS-placebo BID OPN-375 186 μg BID OPN-375 372 μg BID N = 110 N = 110 N = 107 OPN-375 is XHANCE; BID, twice daily; CSNS, composite symptom nasal score.

9 Pooled Analysis Exacerbations (Key Secondary Endpoint) XHANCE is the first and only nasal medication ever shown in Phase 3 controlled trials to reduce exacerbations for patients with chronic sinusitis 1 0.441 0.343 0.0 0.2 0.4 0.6 0.8 1.0 1.2 In ci de nc e Ra te R at io LS Mean Frequency of Exacerbations through 24 Weeks relative to EDS- Placebo EDS-placebo BID OPN-375 186 μg BID OPN-375 372 μg BID BID, twice daily; CSNS, composite symptom nasal score. N = 73 Summary Acute exacerbations are a major source of disability for the roughly 30 million patients in the United States who suffer from chronic sinusitis. There are approximately 10 million office visits for chronic rhinosinusitis every year, and approximately 70% of patients who visit a doctor for chronic rhinosinusitis receive an antibiotic. Reducing widespread use of antibiotics is important because frequent use of antibiotics poses risks to the individuals using them, and because of societal risks, such as emergence of drug-resistant organisms. *P ≤ .05 vs EDS placebo. This is a nominal p-value. ♰P ≤ .01 vs EDS-placebo. This is a type-1 error controlled p-value. ♰ * N = 185 N = 182 N = 180

10 Anticipated Next Steps Meeting with FDA prior to data submission scheduled for the end of September – Meeting minutes available in fourth quarter Plan to submit sNDA by the end of 2022

Q2 2022 Commercial Update
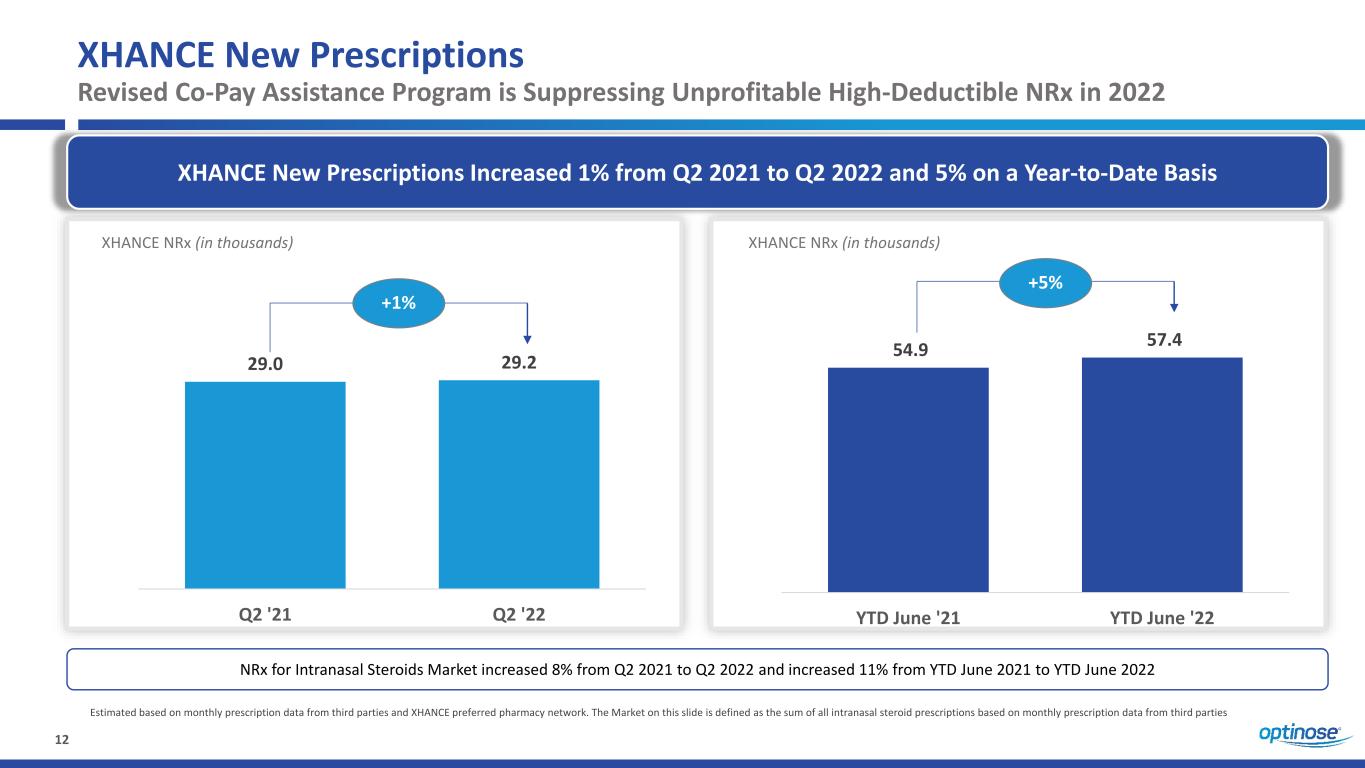
12 XHANCE New Prescriptions Revised Co-Pay Assistance Program is Suppressing Unprofitable High-Deductible NRx in 2022 XHANCE New Prescriptions Increased 1% from Q2 2021 to Q2 2022 and 5% on a Year-to-Date Basis Estimated based on monthly prescription data from third parties and XHANCE preferred pharmacy network. The Market on this slide is defined as the sum of all intranasal steroid prescriptions based on monthly prescription data from third parties 29.0 29.2 Q2 '21 Q2 '22 XHANCE NRx (in thousands) +1% NRx for Intranasal Steroids Market increased 8% from Q2 2021 to Q2 2022 and increased 11% from YTD June 2021 to YTD June 2022 54.9 57.4 YTD June '21 YTD June '22 XHANCE NRx (in thousands) +5%

13 XHANCE Total Prescriptions Revised Co-Pay Assistance Program is Suppressing Unprofitable High-Deductible TRx in 2022 XHANCE Total Prescriptions Increased 6% from Q2 2021 to Q2 2022 and 8% on a Year-to-Date Basis Estimated based on monthly prescription data from third parties and XHANCE preferred pharmacy network. The Market on this slide is defined as the sum of all intranasal steroid prescriptions based on monthly prescription data from third parties 82.9 87.6 Q2 '21 Q2 '22 TRx for Intranasal Steroids Market increased 4% from Q2 2021 to Q2 2022 and increased 6% from YTD June 2021 to YTD June 2022 155.5 168.2 YTD June '21 YTD June '22 XHANCE Prescriptions (in thousands) +8%+6% XHANCE Prescriptions (in thousands)

14 XHANCE Market Share & Prescribers by Prescribing Frequency The Market on this slide is defined as the sum of all intranasal steroid prescriptions written by physicians in the XHANCE target physician audience of approximately 21,000 physicians. Estimated based on monthly prescription data from third parties and XHANCE preferred pharmacy network. 5.2% 5.6% Q2 '21 Q2 '22 XHANCE Share of INS TRx Within Target Physician Audience 4,015 4,424 1,759 1,927 1,414 1,500 Q2 '21 Q2 '22 <5 TRx per Qtr 5 to 15 TRx per Qtr >15 TRx per Qtr XHANCE Prescribers 7,851 7,188 XHANCE market share increased from 5.2% to 5.6% and HCPs who had more than 15 XHANCE prescriptions filled by their patients in a quarter increased by 6% (1,500 versus 1,414) from Q2 2021 to Q2 2022

Q2 2022 Financial Update

16 Financial Review – XHANCE Net Revenue XHANCE Net Revenue Increased 12% from Q2 2021 to Q2 2022 and 21% on a Year-to-Date Basis $18.4 $20.6 Q2 '21 Q2 '22 ($M) +12% Net Revenue Net Revenue $29.3 $35.3 YTD June '21 YTD June '22 ($M) +21%
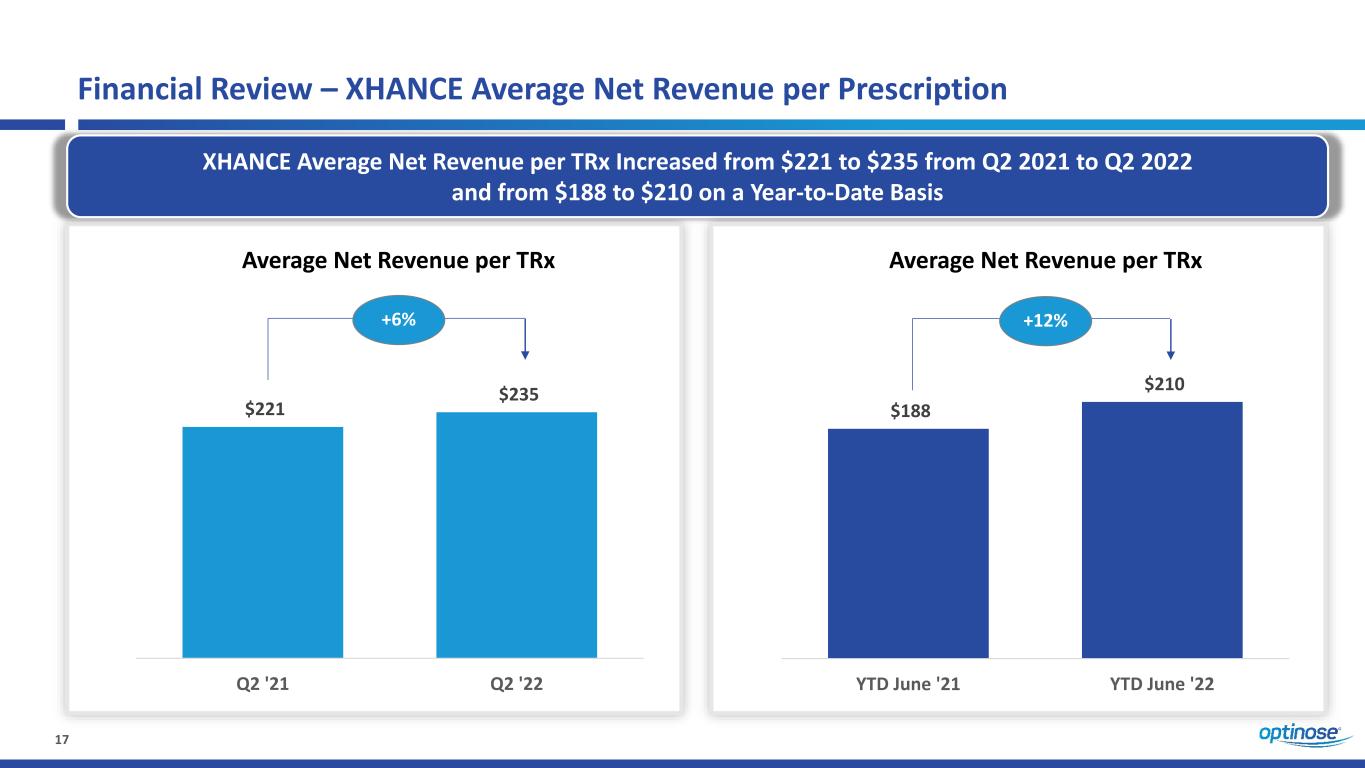
17 $221 $235 Q2 '21 Q2 '22 Financial Review – XHANCE Average Net Revenue per Prescription XHANCE Average Net Revenue per TRx Increased from $221 to $235 from Q2 2021 to Q2 2022 and from $188 to $210 on a Year-to-Date Basis $188 $210 YTD June '21 YTD June '22 +12% Average Net Revenue per TRx Average Net Revenue per TRx +6%

18 Full Year 2022 Financial Guidance and Pharmakon Agreement XHANCE Net Revenue ‒ Expected to be between $85 - $92 million; Previously expected to be at least $90 million XHANCE Average Net Revenue per Prescription ‒ FY 2022 expected to exceed $220 Operating Expense (GAAP) ‒ Expected to be between $129 – $134 million; approximately $9 million of which represents stock-based compensation; ‒ Previously expected to be between $135 – $140 million; approximately $10 million of which represents stock-based compensation Pharmakon Amendment ‒ Entered into the Third Amendment to the Note Purchase Agreement with Pharmakon (the Third Amendment). The Third Amendment reduced the Company's December 31, 2022, trailing twelve-month net revenue covenant from $90 million to $85 million

Closing Remarks

20 Key Takeaways and Q2 2022 Highlights Completed Landmark ReOpen Trials in Q2 2022 Plan to Submit sNDA by the end of 2022 Improved Profitability of XHANCE Prescriptions by 12% in H1 2022 +21% XHANCE Net Revenue Growth H1 2022/H1 2021 +12% XHANCE Net Revenue per TRx Growth H1 2022/H1 2021 1,500 Physicians in 15+ TRx Segment in Q2 2022 Revised FY 2022 Net Revenue and Operating Expense Guidance $78M Cash and equivalents as of June 30, 2022 5.6% Q2 2022 market share an increase of 0.4% compared to Q2 2021
21 Investor Relations – NASDAQ: OPTN Optinose Investor Contact Jonathan Neely, VP, Investor Relations and Business Development 267-521-0531 Investors@optinose.com As of June 30, 2022: – $78.3 million in cash – Long-term debt: $130 million – 83.0 million common shares o/s – 15.0 million options, warrants & RSUs o/s 1 - Optinose is followed by the analysts listed above. Please note that any opinions, estimates or forecasts regarding the Company’s performance made by these analysts are theirs alone and do not represent opinions, forecasts or predictions of Optinose or its management. Optinose does not by its reference above or distribution imply its endorsement of or concurrence with such information, conclusions or recommendations. investors@optinose.com www.optinose.com @optinose Analyst Coverage 1 BMO: Gary Nachman Cantor Fitzgerald: Brandon Folkes Cowen: Ken Cacciatore Piper Sandler: David Amsellem

Building a Leading ENT / Allergy Specialty Company C o r p o r a t e P r e s e n t a t i o n A u g u s t 1 1 , 2 0 2 2





















