Table of Contents
As filed with the Securities and Exchange Commission on July 16, 2010
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-4
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
APRIA HEALTHCARE GROUP INC.
(Exact name of registrant as specified in its charter)
SEE TABLE OF ADDITIONAL REGISTRANTS
Delaware (State or other jurisdiction of | 8082 (Primary Standard Industrial | 33-0488566 (I.R.S. Employer |
26220 Enterprise Court,
Lake Forest, CA 92630
(949) 639-2000
(Address, including zip code, and telephone number, including area code, of registrants’ principal executive offices)
Robert S. Holcombe, Esq.
Executive Vice President, General Counsel and Secretary
Apria Healthcare Group Inc.
26220 Enterprise Court
Lake Forest, California 92630
(949) 639-2000
(Name, address, including zip code, and telephone number, including area code, of agent for service)
With a copy to:
Edward P. Tolley III, Esq.
Simpson Thacher & Bartlett LLP
425 Lexington Avenue
New York, New York 10017-3954
(212) 455-2000
Approximate date of commencement of proposed exchange offers:As soon as practicable after this Registration Statement is declared effective.
If the securities being registered on this Form are being offered in connection with the formation of a holding company and there is compliance with General Instruction G, check the following box. ¨
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a small reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “small reporting company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ¨ | Accelerated filer ¨ | Non-accelerated filer x (Do not check if a smaller reporting company) | Small reporting company ¨ |
If applicable, place an X in the box to designate the appropriate rule provision relied upon in conducting this transaction:
Exchange Act Rule 13e-4(i) (Cross-Border Issue Tender Offer) ¨
Exchange Act Rule 14d-1(d) (Cross-Border Third-Party Tender Offer) ¨
CALCULATION OF REGISTRATION FEE
Title of Each Class of Securities to be Registered | Amount to be | Proposed Maximum Offering Price Per Note | Proposed Offering Price(1) | Amount of Registration Fee | ||||
11.25% Senior Secured Notes due 2014 (Series A-1 Notes) | $700,000,000 | 100% | $700,000,000 | $49,910.00 | ||||
Guarantees of 11.25% Senior Secured Notes due 2014 (Series A-1 Notes)(2) | (3) | (3) | (3) | (3) | ||||
12.375% Senior Secured Notes due 2014 (Series A-2 Notes) | $317,500,000 | 100% | $317,500,000 | $22,637.75 | ||||
Guarantees of 12.375 Senior Secured Notes due 2014 (Series A-2 Notes)(2) | (3) | (3) | (3) | (3) | ||||
| (1) | Estimated solely for the purpose of calculating the registration fee under Rule 457(f) of the Securities Act of 1933, as amended (the “Securities Act”). |
| (2) | See inside facing page for additional registrant guarantors. |
| (3) | Pursuant to Rule 457(n) under the Securities Act, no separate filing fee is required for the guarantees. |
The Registrants hereby amend this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrants shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the Registration Statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
Table of Contents
Table of Additional Registrant Guarantors
Exact Name of Registrant Guarantor as Specified in its Charter | State or Other Jurisdiction of Incorporation or Organization | I.R.S. Employer Identification Number | Address, Including Zip Code and Telephone Number, Including Area Code, of Registrant Guarantor’s Principal Executive Offices | |||
Apria Healthcare of New York State, Inc. | New York | 16-1031905 | 26220 Enterprise Court Lake Forest, CA 92630 (949) 639-2000 | |||
Apria Healthcare, Inc. | Delaware | 33-0057155 | 26220 Enterprise Court Lake Forest, CA 92630 (949) 639-2000 | |||
ApriaCare Management Systems, Inc. | Delaware | 33-0675340 | 26220 Enterprise Court Lake Forest, CA 92630 (949) 639-2000 | |||
ApriaDirect.Com, Inc. | Delaware | 27-2820256 | 26220 Enterprise Court Lake Forest, CA 92630 (949) 639-2000 | |||
Coram Alternate Site Services, Inc. | Delaware | 76-0215922 | 555 17th Street, Suite 1500 Denver, CO 80202 (303) 292-4973 | |||
Coram Clinical Trials, Inc. | Delaware | 58-2160656 | 555 17th Street, Suite 1500 Denver, CO 80202 (303) 292-4973 | |||
Coram Healthcare Corporation of Alabama | Delaware | 58-1813484 | 555 17th Street, Suite 1500 Denver, CO 80202 (303) 292-4973 | |||
Coram Healthcare Corporation of Florida | Delaware | 58-1949695 | 555 17th Street, Suite 1500 Denver, CO 80202 (303) 292-4973 | |||
Coram Healthcare Corporation of Greater D.C. | Delaware | 58-2035129 | 555 17th Street, Suite 1500 Denver, CO 80202 (303) 292-4973 | |||
Coram Healthcare Corporation of Greater New York | New York | 58-1844719 | 555 17th Street, Suite 1500 Denver, CO 80202 (303) 292-4973 | |||
Coram Healthcare Corporation of Indiana | Delaware | 58-1813491 | 555 17th Street, Suite 1500 Denver, CO 80202 (303) 292-4973 | |||
Coram Healthcare Corporation of Massachusetts | Delaware | 33-0532814 | 555 17th Street, Suite 1500 Denver, CO 80202 (303) 292-4973 | |||
Coram Healthcare Corporation of Mississippi | Delaware | 58-1813479 | 555 17th Street, Suite 1500 Denver, CO 80202 (303) 292-4973 | |||
Coram Healthcare Corporation of Nevada | Delaware | 58-1972771 | 555 17th Street, Suite 1500 Denver, CO 80202 (303) 292-4973 | |||
Coram Healthcare Corporation of New York | New York | 33-0458457 | 555 17th Street, Suite 1500 Denver, CO 80202 (303) 292-4973 | |||
Coram Healthcare Corporation of North Texas | Delaware | 33-0556959 | 555 17th Street, Suite 1500 Denver, CO 80202 (303) 292-4973 | |||
Coram Healthcare Corporation of Northern California | Delaware | 58-1972773 | 555 17th Street, Suite 1500 Denver, CO 80202 (303) 292-4973 | |||
Table of Contents
Exact Name of Registrant Guarantor as Specified in its Charter | State or Other Jurisdiction of Incorporation or Organization | I.R.S. Employer Identification Number | Address, Including Zip Code and Telephone Number, Including Area Code, of Registrant Guarantor’s Principal Executive Offices | |||
Coram Healthcare Corporation of South Carolina | Delaware | 58-1813494 | 555 17th Street, Suite 1500 Denver, CO 80202 (303) 292-4973 | |||
Coram Healthcare Corporation of Southern California | Delaware | 58-2006708 | 555 17th Street, Suite 1500 Denver, CO 80202 (303) 292-4973 | |||
Coram Healthcare Corporation of Southern Florida | Delaware | 58-1949686 | 555 17th Street, Suite 1500 Denver, CO 80202 (303) 292-4973 | |||
Coram Healthcare Corporation of Utah | Delaware | 95-4446209 | 555 17th Street, Suite 1500 Denver, CO 80202 (303) 292-4973 | |||
Coram Healthcare of Wyoming, L.L.C. | Delaware | 84-1463833 | 555 17th Street, Suite 1500 Denver, CO 80202 (303) 292-4973 | |||
Coram Homecare of Minnesota, Inc. | Delaware | 58-1874630 | 555 17th Street, Suite 1500 Denver, CO 80202 (303) 292-4973 | |||
Coram Service Corporation | Delaware | 58-1910054 | 555 17th Street, Suite 1500 Denver, CO 80202 (303) 292-4973 | |||
Coram Specialty Infusion Services, Inc. | Delaware | 58-1813486 | 555 17th Street, Suite 1500 Denver, CO 80202 (303) 292-4973 | |||
Coram, Inc. | Delaware | 84-1300129 | 555 17th Street, Suite 1500 Denver, CO 80202 (303) 292-4973 | |||
CoramRx, LLC | Delaware | 25-1923172 | 555 17th Street, Suite 1500 Denver, CO 80202 (303) 292-4973 | |||
H.M.S.S., Inc. | Delaware | 76-0005650 | 555 17th Street, Suite 1500 Denver, CO 80202 (303) 292-4973 | |||
T2Medical, Inc. | Delaware | 59-2405366 | 555 17th Street, Suite 1500 Denver, CO 80202 (303) 292-4973 | |||
HealthInfusion, Inc. | Florida | 65-0163627 | 555 17th Street, Suite 1500 Denver, CO 80202 (303) 292-4973 | |||
AHNY-DME LLC | New York | 27-2955068 | 26220 Enterprise Court Lake Forest, CA 92630 (949) 639-2000 | |||
AHNY-IV LLC | New York | 27-2954951 | 26220 Enterprise Court Lake Forest, CA 92630 (949) 639-2000 | |||
Table of Contents
The information in this prospectus is not complete and may be changed. We may not issue the exchange notes in the exchange offers until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state or jurisdiction where such offer or sale is not permitted.
Subject to Completion, dated July 16, 2010
| PRELIMINARY PROSPECTUS |

Apria Healthcare Group Inc.
Offers to Exchange
$700,000,000 aggregate principal amount of 11.25% Senior Secured Notes due 2014 (Series A-1) (the “exchange Series A-1 Notes”), which have been registered under the Securities Act of 1933, as amended (the “Securities Act”), for any and all outstanding 11.25% Senior Secured Notes due 2014 (Series A-1) (the “outstanding Series A-1 Notes”).
$317,500,000 aggregate principal amount of 12.375% Senior Secured Notes due 2014 (Series A-2) (the “exchange Series A-2 Notes” and, together with the exchange Series A-1 Notes, the “exchange notes”), which have been registered under the Securities Act, for any and all outstanding 12.375% Senior Secured Notes due 2014 (Series A-2) (the “outstanding Series A-2 Notes” and, together with the outstanding Series A-1 Notes, the “outstanding notes”).
The exchange notes will be fully and unconditionally guaranteed on a senior secured basis by our existing and future wholly-owned domestic subsidiaries that guarantee our existing senior secured asset-based revolving credit facility and the outstanding notes.
We are conducting the exchange offers in order to provide you with an opportunity to exchange your unregistered outstanding notes for freely tradeable exchange notes that have been registered under the Securities Act.
The Exchange Offers
| • | We will exchange all outstanding notes that are validly tendered and not validly withdrawn for an equal principal amount of exchange notes that are freely tradeable. |
| • | You may withdraw tenders of outstanding notes at any time prior to the expiration date of the applicable exchange offer. |
| • | The exchange offers expire at 5:00 p.m., New York City time, on , 2010 which is the 21st business day after the date of this prospectus. |
| • | The exchange of outstanding notes for exchange notes in the exchange offers will not be a taxable event for U.S. federal income tax purposes. |
| • | The terms of the exchange notes to be issued in the exchange offers are substantially identical to the outstanding notes, except that the exchange notes will be freely tradeable. |
Results of the Exchange Offers:
| • | The exchange notes may be sold in the over-the-counter-market, in negotiated transactions or through a combination of such methods. We do not plan to list the exchange notes on a national market. |
All untendered outstanding notes will continue to be subject to the restrictions on transfer set forth in the outstanding notes and in the indenture. In general, the outstanding notes may not be offered or sold, unless registered under the Securities Act, except pursuant to an exemption from, or in a transaction not subject to, the Securities Act and applicable state securities laws. Other than in connection with the exchange offers, we do not currently anticipate that we will register the outstanding notes under the Securities Act.
You should carefully consider the “Risk Factors” beginning on page 24 of this prospectus before participating in the exchange offers.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of the exchange notes to be distributed in the exchange offers or passed upon the accuracy or adequacy of this prospectus. Any representation to the contrary is a criminal offense.
The date of this prospectus is , 2010.
Table of Contents
You should rely only on the information contained in this prospectus. We have not authorized anyone to provide you with different information. This prospectus may be used only for the purposes for which it has been published and no person has been authorized to give any information not contained herein. If you receive any other information, you should not rely on it. We are not making an offer of these securities in any state where the offer is not permitted.
| Page | ||
| ii | ||
| ii | ||
| ii | ||
| iii | ||
| 1 | ||
| 24 | ||
| 47 | ||
| 49 | ||
| 50 | ||
| 51 | ||
Management’s Discussion and Analysis of Financial Condition and Results of Operations | 53 | |
| 92 | ||
| 120 | ||
| 147 | ||
| 150 | ||
| 153 | ||
| 163 | ||
| 239 | ||
| 242 | ||
| 249 | ||
| 251 | ||
| 252 | ||
| 252 | ||
| 252 | ||
| F-1 |
i
Table of Contents
This prospectus includes forward-looking statements regarding, among other things, our plans, strategies and prospects, both business and financial. These statements are based on the beliefs and assumptions of our management. Although we believe that our plans, intentions and expectations reflected in or suggested by these forward-looking statements are reasonable, we cannot assure you that we will achieve or realize these plans, intentions or expectations. Forward-looking statements are inherently subject to risks, uncertainties and assumptions. Generally, statements that are not historical facts, including statements concerning our possible or assumed future actions, business strategies, events or results of operations, are forward-looking statements. These statements may be preceded by, followed by or include the words “believes”, “expects”, “anticipates”, “intends”, “plans”, “estimates” or similar expressions.
Forward-looking statements are not guarantees of performance. You should not put undue reliance on these statements. You should understand that the following important factors, in addition to those discussed in “Risk Factors” and elsewhere in this prospectus, could affect our future results and could cause those results or other outcomes to differ materially from those expressed or implied in our forward-looking statements:
| • | trends and developments affecting the collectibility of accounts receivable; |
| • | government legislative and budget developments that could continue to affect reimbursement levels; |
| • | potential reductions in reimbursement rates by government and third-party payors; |
| • | the effectiveness of our operating systems and controls; |
| • | healthcare reform and the effect of federal and state healthcare regulations; |
| • | economic and political events, international conflicts and natural disasters; |
| • | our ability to implement our outsourcing and other cost savings initiatives and to realize the projected benefits of these initiatives; |
| • | acquisition-related risks; and |
| • | the items discussed under “Risk Factors” in this prospectus. |
All forward-looking statements attributable to us or persons acting on our behalf are expressly qualified in their entirety by the foregoing cautionary statements. We undertake no obligations to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise.
Information included in this prospectus about the healthcare industry, including our general expectations concerning this industry, is based on estimates prepared using data from various sources and on assumptions made by us. We believe data regarding this industry are inherently imprecise, but based on our understanding of the market in which we compete, we believe that such data are generally indicative of this industry. Our estimates, in particular as they relate to our general expectations concerning this industry, involve risks and uncertainties and are subject to change based on various factors, including those discussed under the caption “Risk Factors.” Accordingly, you should not place undue reliance on the market and industry data included in this prospectus.
This prospectus contains some of our trademarks, trade names and service marks. Each one of these trademarks, trade names or service marks is either (i) our registered trademark, (ii) a trademark for which we have a pending application, (iii) a trade name or service mark for which we claim common law rights or (iv) a registered trademark or application for registration which we have been licensed by a third party to use. All other trademarks, trade names or service marks of any other company appearing in this prospectus belong to their respective owners.
ii
Table of Contents
As used in this prospectus, unless otherwise noted or the context otherwise requires, references to “Company,” “we,” “us,” and “our” are to Apria Healthcare Group Inc., a Delaware corporation, and its subsidiaries; references to “Apria” and the “Issuer” are to Apria Healthcare Group Inc., exclusive of its subsidiaries; references to “Merger Sub” are to Sky Merger Sub Corporation, a Delaware corporation; references to “Holdings” are to Apria Holdings LLC, a Delaware limited liability company, exclusive of its subsidiaries; references to “Sky Acquisition” are to “Sky Acquisition LLC”, a Delaware limited liability company, exclusive of its subsidiaries; references to “Blackstone” and the “Sponsor” are to Blackstone Capital Partners V L.P.; references to the “Investor Group” are, collectively, to Blackstone and certain funds affiliated with Blackstone, Dr. Norman C. Payson and certain other members of our management; and references to “home medical equipment,” “durable medical equipment” and “DME” are used synonymously.
The term “outstanding notes” refers to the outstanding 11.25% Senior Secured Notes due 2014 (Series A-1) and 12.375% Senior Secured Notes due 2014 (Series A-2). The term “exchange notes” refers to the 11.25% Senior Secured Notes due 2014 (Series A-1) and 12.375% Senior Secured Notes due 2014 (Series A-2), as registered under the Securities Act. The term “Series A-1 Notes” refers collectively to the outstanding Series A-1 Notes and the exchange Series A-1 Notes; the term “Series A-2 Notes” refers collectively to the outstanding Series A-2 Notes and the exchange Series A-2 Notes; and the term “Notes” refers collectively to the outstanding notes and the exchange notes.
We completed the acquisition of Coram, Inc. (“Coram”) on December 3, 2007. Historical financial information of the Company presented herein includes the results of Coram from the date of the acquisition and does not include the results of Coram prior to the date of the acquisition.
On June 18, 2008, Sky Acquisition and Merger Sub entered into an agreement and plan of merger with Apria (the “Merger Agreement”). Pursuant to the Merger Agreement, on October 28, 2008, Merger Sub merged with and into Apria, with Apria being the surviving corporation following the merger (the “Merger”). As a result of the Merger, the Investor Group beneficially owns all of Apria’s issued and outstanding capital stock.
The initial borrowings under Apria’s senior secured bridge credit agreement dated October 28, 2008 (the “senior secured bridge credit agreement”) and the credit agreement governing our senior secured asset-based revolving credit facility, dated October 28, 2008 (as amended from time to time, the “ABL Facility”), the equity investment by the Investor Group and the repayment of all outstanding indebtedness under our senior secured revolving credit facility, dated November 23, 2004, as amended (the “2004 senior secured revolving credit facility”), and Apria’s credit facility, dated June 18, 2008 (the “Interim Facility”), are collectively referred to in this prospectus as the “Original Financing.” The offerings of outstanding notes, the repayment of approximately $1,010.0 million of borrowings under Apria’s senior secured bridge credit agreement, plus accrued and unpaid interest, and the payment of related fees and expenses with the proceeds of the offerings of the outstanding notes, together with cash on hand, are collectively referred to in this prospectus as the “Refinancing.” The Merger, the Original Financing and the Refinancing are collectively referred to in this prospectus as the “Transactions.” For a more complete description of the Transactions, see “The Transactions” and “Description of Other Indebtedness.”
The term “Successor” refers to the Company following the Merger and the term “Predecessor” refers to the Company prior to the Merger.
Unless the context otherwise requires, the financial information presented herein is the financial information of Apria on a consolidated basis together with its subsidiaries.
iii
Table of Contents
This summary highlights information about us and the exchange offers contained in greater detail elsewhere in this prospectus. This summary is not complete and may not contain all of the information that may be important to you. You should carefully read the entire prospectus, especially the information presented under the headings “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” before participating in the exchange offers.
Our Company
General
We are a quality, cost-efficient provider of home healthcare products and services in the United States, offering a comprehensive range of home respiratory therapy, home infusion therapy and home medical equipment services to over two million patients annually in all 50 states through approximately 500 locations. We hold market-leading positions across all of our major service lines—making us a leader in the homecare market. By targeting the managed care segment of the population, we are better positioned than many of our competitors to minimize risks associated with changes in Medicare/Medicaid reimbursement rates. We are focused on being the industry’s highest-quality provider of homecare services, while maintaining our commitment to being a low-cost operator. Our integrated product and service offerings, combined with our national scale and strong reputation, provide us with a strategic advantage in attracting clients, which include almost all of the national and regional managed care and government payors in the United States, and in retaining our referral base of more than 70,000 physicians, discharge planners, hospitals and third-party payors. For the year ended December 31, 2009 and for the three months ended March 31, 2010 our net revenues were $2,094.6 million and $508.9 million, respectively.
We have two operating segments, (1) home respiratory therapy and home medical equipment and (2) home infusion therapy. Within the two operating segments there are three core service lines: home respiratory therapy, home medical equipment and home infusion therapy. Through these service lines we provide patients with a variety of clinical and administrative support services and related products and supplies, most of which are prescribed by a physician as part of a care plan. We provide substantial benefits to both patients and payors by allowing patients to receive necessary care and services in the comfort of their own home while reducing the cost of treatment. Our services include:
| • | providing in-home clinical respiratory care, infusion nursing and pharmaceutical management services; |
| • | educating patients and caregivers about health conditions or illnesses and providing written instructions about home safety, self-care and the proper use of equipment; |
| • | monitoring patients’ individualized treatment plans; |
| • | reporting patient progress and status to the physician and/or managed care organization; |
| • | providing in-home delivery, set-up and maintenance of equipment and/or supplies; and |
| • | processing claims to third-party payors and billing/collecting patient co-pays and deductibles. |
Home Respiratory Therapy and Home Medical Equipment($1,169.6 million and $278.3 million, or 55.8% and 54.7%, of our net revenues for the year ended December 31, 2009 and the three months ended March 31, 2010, respectively)
Home Respiratory Therapy
We are the largest provider of home respiratory therapies in the United States to the managed care market serving approximately 1.5 million patients annually through our nationwide distribution platform that includes
1
Table of Contents
approximately 400 locations. We offer a full range of home respiratory therapy products and services, from the simplest nebulizer and oxygen concentrator to the most complex ventilator. Our services offer a compelling relative cost advantage to our patients and payors. For example, in-home oxygen treatment costs for a Medicare patient are on average less than $7 per day. Patients utilize our products to treat a variety of conditions, including:
| • | chronic obstructive pulmonary diseases (“COPD”), such as emphysema and chronic bronchitis (the fourth leading cause of death in the U.S.); |
| • | respiratory conditions associated with nervous system disorders or injuries, such as Lou Gehrig’s disease and quadriplegia; |
| • | congestive heart failure; and |
| • | lung cancer. |
By focusing our efforts primarily on the managed care population, we limit our exposure to the highly-regulated Medicare respiratory business, which is subject to changes in coverage, payment and pricing guidelines. As an example, Medicare oxygen accounted for less than 10% and 11% of our total net revenues for the year ended December 31, 2009 and three months ended March 31, 2010, respectively.
We employ a nationwide clinical staff of more than 800 respiratory care professionals, including home respiratory therapists who provide direct patient care, monitoring and 24-hour support services under physician-directed treatment plans and in accordance with our proprietary acuity program. We derive revenues from the provision of oxygen systems, ventilators, respiratory assist devices, and Continuous Positive Airway Pressure (“CPAP”) and bi-level devices, as well as from the provision of infant apnea monitors, nebulizers, home-delivered respiratory medications and related services.
We are also the largest provider of sleep apnea devices, including CPAP/bi-level devices, and patient support services in the United States. The incidence and diagnosis of Obstructive Sleep Apnea (“OSA”) continues to increase in the United States. We believe that the strength of our position in this market is partly due to our significant presence in the managed care market, since OSA largely affects adults between the ages of 35 and 55 rather than the population served by Medicare. To manage our significant new and recurring patient volumes in a cost-effective, clinically sound manner, we developed an innovative care model called the “CPAP Center at Apria Healthcare.” This branch-based model allows Apria’s respiratory care practitioners to educate, on a timely and efficient basis, newly-diagnosed patients about their condition, the equipment and accessories their physician has prescribed for them, and the long-term importance of complying with the physician’s order. The model includes both one-on-one patient education and teaching performed in group settings, depending on the geographic area of the country and the patient’s payor’s contractual preferences.
Home Medical Equipment
As the leading provider of home medical equipment in the United States, we supply a wide range of products to help improve the quality of life for patients with special needs. Our integrated service approach allows patients, hospital and physician referral sources and managed care organizations accessing either our home respiratory or home infusion therapy services to also access needed home medical equipment through a single source. The use of home medical equipment provides a significant relative cost advantage to our patients and payors. For example, on average, it costs $50 per day to create an in-home hospital room versus approximately $1,500 per day for in-patient hospital care, according to the Centers for Medicare and Medicaid Services (“CMS”). Basic categories of equipment are:
| • | manual wheelchairs and ambulatory equipment, such as canes, crutches and walkers; |
| • | hospital room equipment, such as hospital beds and bedside commodes; |
2
Table of Contents
| • | bathroom equipment, such as bath and shower benches, elevated toilet seats and toilet, tub or wall grab bars; |
| • | phototherapy systems, such as blankets, wraps or treatment beds for babies with jaundice; and |
| • | support surfaces, such as pressure pads and mattresses, for patients at risk for developing pressure sores or decubitus ulcers. |
In May 2008, we announced a preferred provider agreement with Smith & Nephew plc, a leading provider of negative pressure wound therapy (“NPWT”), to provide NPWT services and products in the United States. NPWT is a topical treatment intended to promote healing in acute and chronic wounds affected by conditions including diabetes, arterial insufficiency and venous insufficiency.
Home Infusion Therapy ($925.0 million and $230.6 million, or 44.2% and 45.3%, of our net revenues for the year ended December 31, 2009 and three months ended March 31, 2010, respectively)
Through our acquisition of Coram in December 2007, we are the leading provider of home infusion therapy services in the United States—serving approximately 100,000 patients annually through 72 infusion pharmacy locations nationwide. We provide patients with intravenous and injectable medications and clinical services at home or in one of our 62 ambulatory infusion suites nationwide. We employ nursing clinicians who assess patients before their discharge from the hospital whenever possible, and then develop, in conjunction with the physician, a plan of care. Our home infusion products offer a compelling relative cost advantage to our patients and payors. For example, we believe that a home intravenous antibiotic program in a Medicare managed care plan costs significantly less than the cost to provide that service in a hospital setting.
Home infusion therapy is used to administer drugs and other therapeutic agents directly into the body through various types of catheters or tubing. Our services are frequently used to treat patients with infectious diseases, cancer, gastrointestinal diseases, chronic or acute pain syndromes, immune deficiencies, cardiovascular disease or chronic genetic diseases, and those who require therapies associated with bone marrow or solid organ transplantation. We employ licensed pharmacists and registered nurses who specialize in the delivery of home infusion therapy. They are able to respond to emergencies and questions regarding therapy 24 hours a day, seven days a week and provide initial and ongoing training and education to the patient and caregiver. Other support services include supply replenishment, pump management, preventive maintenance, assistance with insurance questions and outcome reporting.
We believe we are also a leading provider of enteral nutrition in the United States. Enteral nutrition, or “tube feeding,” is prescribed to patients whose gastrointestinal system is malfunctioning or who suffer from neurological conditions, swallowing disorders or malnutrition attributable to stroke, cancer or other conditions. In recent years, advances in enteral nutrition have enabled more adults and children to have their nutritional and caloric needs met by tube feeding, as opposed to more invasive and expensive therapies.
Recent Developments
Announcement of Single Payment Amounts (“SPAs”) and Initiation of Contract Offer Process Related to Round 1 Rebid of the Medicare DMEPOS Competitive Bidding Program
In early July 2010, CMS announced the new SPAs for each of the product categories included in the Round 1 Rebid. CMS then began the contracting process with suppliers by issuing contract offer letters to qualified providers. We received contract offers for a substantial majority of the bids we submitted. We did not receive contract offers for certain product categories in certain competitive bidding areas (“CBAs”), but the process will
3
Table of Contents
not be completed until September 2010. Approximately $21 million of our net revenues for the fiscal year ended December 31, 2009 was generated by the products and CBAs included in the Round 1 Rebid. We estimate that the initial results of the Round 1 Rebid would reduce our net revenues in the fiscal year ending December 31, 2011 by approximately $8.0 million, assuming the current contract offers and no changes in volume. Assuming that Round 2 would include the same product categories and bidding rules and the markets currently being proposed by CMS, we estimate that approximately $110 million of our net revenues for the fiscal year ending December 31, 2011 would be subject to competitive bidding. Although the bidding process for Round 2 is currently scheduled to commence in 2011, the new Round 2 rates and guidelines are not scheduled to take effect until January 2013. Therefore, we cannot estimate the impact of potential Round 2 rate reductions on our business until more specific information is published by CMS and its contractors.
Enactment of a Comprehensive Healthcare Reform Package
In March 2010, the Federal government enacted a comprehensive healthcare reform package (the “Reform Package”) which consists of the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010. See “Risks Relating to Our Business—Continued Reductions in Medicare and Medicaid Reimbursement Rates and the Comprehensive Healthcare Reform Package Could Have a Material Adverse Effect on Our Results of Operations and Financial Condition.”
Reorganization of the Senior Leadership Structure of our Home Respiratory Therapy and Home Medical Equipment Segment
In February 2010, we finalized a decision to reorganize the senior leadership structure of the home respiratory therapy and home medical equipment segment. As part of this reorganization, Lawrence A. Mastrovich, President, Home Respiratory Therapy and Home Medical Equipment segment, resigned and his responsibilities were assumed by other senior executives.
Industry Overview
The home healthcare market, which is projected to generate revenues of approximately $75 billion in the United States in 2010, comprises a broad range of products and services—including respiratory therapy, home infusion therapy, home medical equipment, home healthcare nursing, orthotics and prosthetics and general medical supplies—and is expected to grow at a compounded annual growth rate of 7.4% from 2008 through 2013 according to CMS. The markets that we serve—home respiratory therapy (14% of the home healthcare market), infusion therapy (9%) and home medical equipment (5%)—accounted for approximately $15 billion in revenues in 2008 according to the 2008 IBISWorld Industry Report. Our industry is highly-fragmented and is served by approximately 12,000 competitors.
We benefit from the following trends within the home healthcare market:
| • | Favorable industry dynamics. Favorable demographic trends and the continued shift to in-home healthcare have resulted in patient volume growth across our core service lines and are expected to continue to drive growth. As the baby boomer population ages and life expectancy increases, the elderly—who comprise the vast majority of our patients—will represent a higher percentage of the overall population. According to a 2008 U.S. Census Bureau projection, the U.S. population aged 55 and over is expected to grow at approximately twice the average rate of population growth from 76.5 million, or 25% of the population, in 2010 to 112 million, or 30% of the population, by 2030. An aging population, the continued prevalence of smoking, increasing obesity rates and higher diagnosis rates have |
4
Table of Contents
collectively driven growth across our service lines (including Coram) from 2005 to 2008—home respiratory therapy (patient growth of 14%), sleep apnea (patient growth of 21%) and infusion (patient growth of 14%, excluding enteral). |
| • | Compelling in-home economics. By 2017, the nation’s healthcare spending is projected to increase to $4.3 trillion, growing at an average annual rate of 6.7%, according to CMS. The rising cost of healthcare has caused many payors to look for ways to contain costs and home healthcare is increasingly sought out as an attractive, cost-effective, clinically appropriate alternative to expensive facility-based care. For example, in-home oxygen treatment costs for a Medicare patient are on average less than $7 per day. |
| • | Increased prevalence of in-home treatments.Improved technology has resulted in a wider variety of treatments being administered in patients’ homes. These improvements have allowed for earlier patient discharge and have lengthened the portion of the recuperation period spent outside of an institutional setting. In addition, medical advancements have also made medical equipment more simple, adaptable and cost-effective for use in the home. |
| • | Preference for in-home care.Many patients prefer the convenience and typical cost advantages of home healthcare over institutional care as it provides patients with greater independence, increased responsibility and improved responsiveness to treatment. A December 2007 national telephone survey conducted by Harris Interactive found that over 82% of the respondents expressed a preference for homecare over institutional care, and that preference is even more prevalent among the age 55+ population (91%). The same poll found that 74% of adults surveyed agreed that homecare is part of the solution to the problem of rapidly increasing Medicare spending for seniors in the United States. |
| • | Development of new infused and injectable drugs.There is a significant number of new infusion or injectable drugs in the development pipeline. We believe this proliferation of medications, many of which are for chronic conditions that require long-term treatment, will drive further increases in home infusion therapy utilization and referrals to our ambulatory infusion suites. |
Our Competitive Strengths
Leading Market Positions with a Compelling Value Proposition
With approximately 11,400 employees and a national distribution footprint of approximately 500 locations that serve patients in all 50 states, we are the largest provider of home healthcare services in the United States. We are the market leader in infusion therapy and sleep apnea devices, the leading respiratory provider to the managed care market and the leading provider of home medical equipment. We believe that our national platform, comprehensive product line and leading reputation provide us with a greater opportunity than our competitors to attract more customers as our industry continues to grow. Our national presence and scale enables us to frequently obtain preferred provider status from other national and regional managed care payors, negotiate better terms with vendors and leverage our fixed overhead costs. For example, we are a preferred provider for a comprehensive list of home respiratory and medical equipment products and services to many managed care organizations and, for some of these payors, we are the exclusive provider. We believe we are better suited to service large managed care accounts due to our extensive branch network, state of the art logistics systems, national coverage of payors’ members, competitive pricing, comprehensive product line, accreditation from The Joint Commission and the Accreditation Commission for Health Care (the “ACHC” and, together with The Joint Commission, the “Commissions”), and our ability to connect electronically with payors’ systems. We have leveraged this competitive advantage to gain share in the managed care market.
Our acquisition of Coram in December 2007 has allowed us to further penetrate the specialty infusion market. With a significant number new infusion drugs in the pipeline and an increasing use of specialty infusion treatments, this market is expected to grow over the next few years. We are well-positioned in specialty infusion
5
Table of Contents
services, and have aggressively established relationships with pharmaceutical and biotech companies to obtain early access to drugs in various stages of clinical trials. We believe there are other cross-selling opportunities and synergies to be achieved by offering a diverse mix of services. We also believe that an integrated approach allows us to offer patients, hospital and physician referral sources and managed care organizations a highly-valued single source for respiratory therapy, specialty home infusion and home medical equipment.
Diversified Product and Customer Mix
We have one of the most comprehensive product lines and diversified customer mixes among our peers. Our broad product offering has affirmed our status as a leading provider in each market and has made us a more attractive partner to referral sources and payors, as we provide a one-stop solution for homecare products and services.
We contract with a substantial majority of the national managed care organizations���including United HealthCare Services, Aetna Health Management, Humana Health Plans and Kaiser Foundation Health Plan, as well as a large number of regional and local payors. All of our contracted managed care organizations combined service over 217 millionpeople.
The Coram acquisition enabled us to both expand our product offering in specialty infused drugs and rebalance our payor mix by reducing reliance on government payors such as Medicare and Medicaid while expanding relationships with managed care organizations. Managed care payors contributed approximately 72% and 70% of our net revenues for the year ended December 31, 2009 and the three months ended March 31, 2010 respectively, with no single contract accounting for more than 8% of net revenues during the same periods.
Proven Ability to Execute Cost Savings
We have successfully implemented a number of operational efficiency initiatives historically, which have helped to reduce our costs and significantly offset ongoing Medicare reimbursement changes. We launched a substantial cost reduction plan in late 2007 across a number of identified initiatives targeting approximately $198 million in expected annual savings, of which we have realized approximately $127 million through March 31, 2010.
Scalable and Diversified Platform for Home Healthcare Delivery
We currently provide service to more than 2 million patients through a national infrastructure that enables us to deliver services to patients in their homes. Through approximately 500 locations, we are able to deliver a wide variety of cost-effective products and services to various patient groups. We have successfully leveraged this distribution platform across a number of product and service offerings including CPAP/bi-level, enteral nutrition and NPWT devices, and we are using our nursing capacity to provide infusion services through our growing network of ambulatory infusion suites.
We historically supplied CPAP/bi-level devices to a large number of patients, but provided related accessories and supplies primarily on an as-needed basis. Patients who rely on CPAP and bi-level devices periodically require replacement accessories to ensure that they remain compliant to the therapy prescribed by their physician. These accessories include masks, tubing and supplies. Now in operation for over five years, a centralized customer care center for CPAP and bi-level patients provides support and information to patients so that they know what their payors cover in terms of replacement accessories and understand the health value of remaining compliant to their therapy over the long-term. Accessory net revenues were $155.9 million and $34.5 million and represented 47% and 46% of our total CPAP/bi-level net revenues for the year ended December 31, 2009 and the three months ended March 31, 2010, respectively.
6
Table of Contents
In May 2008, we announced a preferred provider agreement with Smith & Nephew plc to provide NPWT products, thus leveraging our existing branch delivery infrastructure and clinical expertise. Centralized patient intake and coordination of care is provided using the same service and systems platform as is used for the CPAP/bi-level direct marketing service program. Although the program is still in its developing stage, interest has been strong from managed care customers who would like to add the NPWT service to our existing contracts with them.
Experienced Management Team
We have a strong and experienced senior management team with over 200 years of combined experience spanning nearly every segment of the healthcare industry, including managed care, manufacturing, supply chain, procurement, home healthcare, acute care, skilled nursing and long-term care. With an average tenure of 20 years within the healthcare industry, this team possesses in-depth knowledge of our industry and the regulatory environment in which we operate, as well as our portfolio of home healthcare services.
Our Business Strategy
Our strategy is to position ourselves in the marketplace as a high-quality provider of a broad range of healthcare services and patient care management programs to our customers. The specific elements of our strategy are to:
| • | Grow profitable revenue and market share. We are focused on growing profitable revenues and increasing market share in our core home infusion therapy and home respiratory therapy service lines. We have undertaken a series of steps towards this end. Through our acquisition of Coram in December 2007, we considerably increased our home infusion capabilities and expanded our platform for further cross- selling opportunities. Since January 1, 2007, we have expanded our home respiratory therapy and home medical equipment sales force by 18%. This focus has allowed us to more effectively market our products and services to physicians, hospital discharge planners and managed care organizations. |
| • | Continue to participate in the managed care market. We participate in the managed care market as a long-term strategic customer group because we believe that our scale, expertise, nationwide presence and array of home healthcare products and services will enable us to sign preferred provider agreements with managed care organizations. Managed care represented approximately 70% of our total net revenues for the three months ended March 31, 2010. |
| • | Leverage our national distribution infrastructure.With approximately 500 locations and a robust platform supporting shared national services, we believe that we can efficiently add products, services and patients to our systems to grow our revenues and leverage our cost structure. For example, we have successfully leveraged this distribution platform across a number of product and service offerings, including a CPAP/bi-level supply replenishment program, enteral nutrition and NPWT services, and we are using our nursing capacity to provide infusion services through our growing network of ambulatory infusion suites. We seek to achieve margin improvements through operational initiatives focused on the continual reduction of costs and delivery of incremental efficiencies. At the same time, we believe that it is essential to consistently deliver superior customer service in order to increase referrals and retain existing patients. Performance improvement initiatives are underway in all aspects of our operations including customer service, patient satisfaction, logistics, supply chain, clinical services and billing/collections. We believe that by being responsive to the needs of our patients and payors we can provide ourselves with opportunities to take market share from our competitors. |
| • | Continue to lead the industry in accreditation. The Medicare Improvement for Patients Act of 2008 (“MIPPA”) made accreditation mandatory for Medicare providers of durable medical equipment, prosthetics, orthotics and supplies (“DMEPOS”), effective October 1, 2009, per CMS regulation. We |
7
Table of Contents
were the first durable medical equipment provider to seek and obtain voluntary accreditation from The Joint Commission. All of our locations are currently accredited by The Joint Commission and our home infusion therapy service line is also accredited by the ACHC. In 2007, we completed a nationwide independent triennial accreditation renewal process conducted by The Joint Commission and we have more than 15 years of continuous accreditation by The Joint Commission—longer than any other homecare provider. In late June 2010, The Joint Commission completed its most recent triennial survey of our respiratory/home medical equipment and infusion locations and is expected to renew our accreditation for another three years. |
| • | Execute our strategic initiatives to drive profitability.For the past several years, we have successfully engaged in a range of cost savings initiatives to ease pressure on our revenue that has been and continues to be caused by Medicare and Medicaid reimbursement changes. These initiatives are designed to improve customer service, delivery and vehicle routing services, streamline the billing and payment process, effectively manage purchasing costs and improve the overall experience of the patients we serve. We launched a substantial cost reduction plan in late 2007. To date, we have made significant progress across a number of the identified initiatives targeting expected annual savings of approximately $198 million, of which we realized approximately $127 million through March 31, 2010. |
Regulatory Overview
We are subject to extensive government regulation, including numerous laws that regulate reimbursement of products and services under various government programs. There are a number of legislative and regulatory activities in Congress, including the March 2010 passage of the Reform Package, and at the Department of Health and Human Services (“HHS”) and CMS, the federal government agency responsible for administering the Medicare and Medicaid programs, that affect or may affect government reimbursement policies for our products and services.
Healthcare Reform Package.In March 2010, the Federal government enacted the comprehensive healthcare Reform Package. Among many other provisions, the Reform Package expands the Medicaid program, mandates extensive insurance market reforms, creates new health insurance access points (e.g., insurance exchanges), provides certain insurance subsidies (e.g., premiums and cost sharing), imposes individual and employer health insurance requirements and makes a number of changes to the U.S. Internal Revenue Code of 1986, as amended (the “Code”).
There are a number of provisions in the Reform Package that may affect us. For example, the Reform Package requires certain pharmaceutical and medical device manufacturers to pay an excise tax to the government, which may, in turn, increase our costs for these products. The Reform Package also provides for cuts in some Medicare payments made to certain providers and to Medicare Part C (“Medicare Advantage”) plans, through which we contract to provide services to Medicare beneficiaries. Also included in the Reform Package is (i) an expansion of the Recovery Audit Contractor Program, (ii) certain fraud and abuse prevention measures and (iii) expanded regulatory authority concerning the types of conduct that can result in additional fines and penalties for those healthcare providers who do not comply with applicable laws and regulations. Furthermore, the Reform Package grants the Secretary of HHS authority to set a date by which certain providers and suppliers will be required to establish a compliance program.
The Reform Package makes a number of changes to how certain of our products and services will be reimbursed by Medicare. The Reform Package also makes changes to the Medicare durable medical equipment consumer price index adjustment for 2011 and each subsequent year based upon the Consumer Price Index (the “CPI”) reduced by a new productivity adjustment which may result in negative updates and includes changes to the Medicare DMEPOS competitive bidding program. Significantly, Round 2 of the competitive bidding program
8
Table of Contents
has been expanded from 70 to 91 of the largest metropolitan statistical areas (“MSAs”). The Reform Package also gives the Secretary of HHS the authority to apply competitive bid pricing to non-bid areas, but details of that process are unlikely to be understood until after CMS issues guidance or completes a related rulemaking process.
In an effort to further strengthen the integrity of the Medicare program, the Reform Package includes additional requirements concerning physician enrollment and certain mandatory face-to-face patient/physician visits in conjunction with the ordering of durable medical equipment. These provisions are likely to be the subject of rulemaking and are a high priority for the American Association for Homecare and other industry representative organizations. We expect the Administration to continue to enhance its oversight efforts, and we strive to incorporate any necessary changes into our overall corporate compliance and internal audit programs on a regular basis.
The effective dates of the various provisions within the Reform Package are staggered over the next several years, with some changes occurring immediately. Much of the interpretation of what the Reform Package requires will be subject to administrative rulemaking, the development of agency guidance and court interpretations.
Capped Rentals and Oxygen Equipment. The Deficit Reduction Act of 2005 (“DRA”) converted Medicare reimbursement for oxygen equipment from an ongoing rental method to a capped rental and rent-to-purchase methodology and limited reimbursement for rental of oxygen equipment to the current 36-month maximum. The DRA mandated that, after the 36-month rental period, the ownership of the equipment would transfer to the Medicare beneficiary.
MIPPA, which became law on July 15, 2008, while maintaining the 36-month rental cap, eliminated the mandatory title transfer for oxygen equipment. As a result, the equipment will continue to be owned by the oxygen provider for as long as the patient’s medical need exists, after which time it will be returned to the oxygen provider. Accordingly, because the 36-month rental period was retroactively applied to January 1, 2006, Medicare services provided on or after January 1, 2009 were the first Medicare claims for which the rental cap impacted Apria. In November 2008, CMS revised its regulations in order to be consistent with the provisions of the DRA and MIPPA. These regulations also set forth that CMS will not pay for any non-routine maintenance, oxygen tubing, cannulas and supplies after the 36-month rental period and that CMS will pay for certain routine maintenance and servicing activities after the 36-month rental cap. There may be future initiatives to implement a reduction to the capped rentals and/or monthly payment rate, but it is uncertain if such initiatives would ultimately be approved.
Competitive Bidding. The Medicare Prescription Drug, Improvement and Modernization Act of 2003 (“MMA”) mandated implementation of a competitive bidding program for certain DMEPOS. By statute, CMS was required to implement the competitive bidding program over time, with the first phase (“Round 1”) of competition occurring in 10 of the MSAs in 2007, launch of the program in 2008 and in 70 additional markets in 2009, and in additional markets after 2009. In 2007 and 2008, CMS accepted and reviewed bids to begin Round 1 of the competitive bidding program. Winning contract suppliers began providing services under Round 1 on July 1, 2008.
With the enactment of MIPPA in July 2008, the competitive bidding program was delayed and all contracts awarded under Round 1 were immediately terminated. The contracting process was restarted through the re-bidding process in October 2009 (the “Round 1 Rebid”). CMS also announced in June 2009 that the new payment rates resulting from the bid process will go into effect in the CBAs in January 2011. Currently, all beneficiaries in the fee-for-service Medicare program may use any provider in any geographic area to obtain durable medical equipment and oxygen therapy services and products. In addition to the delay of the competitive bidding program, MIPPA requires a number of programmatic reforms prior to re-launching the program. To offset the cost of, or “pay for,” the delay in the roll-out of the competitive bidding program, Congress adopted an
9
Table of Contents
average nationwide 9.5% payment reduction in the durable medical equipment fee schedule for product categories included in Round 1, effective January 1, 2009.
The Reform Package makes changes to the competitive bidding program. Significantly, Round 2 of the competitive bidding program has been expanded from 70 to 91 of the largest MSAs. CMS has announced that the effective date of the Round 2 pricing will be January 1, 2013; additional details concerning products to be included and other aspects of implementing Round 2 will not be fully known until after CMS completes a rulemaking process, which is currently scheduled for the summer or fall of 2010. The Reform Package also gives the Secretary of HHS the authority to apply competitive bid pricing to non-bid areas, but details of that process are unlikely to be understood until after CMS issues guidance or completes a related rulemaking process.
At a March 2010 Program Advisory and Oversight Committee (“PAOC”) meeting, CMS briefed the PAOC regarding the next round of the DMEPOS competitive bidding program. With review of Round 1 Rebid bids underway in March 2010, the briefing focused on certain aspects of Round 2, which is mandated by MIPPA to begin in 2011. In late June 2010, CMS published a Proposed Rule containing several provisions related to the competitive bidding program. The Proposed Rule included the proposed list of 21 additional MSAs to be included in Round 2, as well as provisions relating to the diabetic supply category. Those provisions include a proposed definition of “mail order” and “non-mail order” items and a proposal for providers to supply a minimum level of product choices to patients. The public comment period on the Proposed Rule will close in August and a Final Rule is expected in the fall of 2010. CMS expects to make additional changes to the program through the rulemaking process and anticipates that another proposed rule will be published in the summer of 2010, with a final rule to be published in the fall of 2010. Also, during the fall of 2010, CMS plans to announce the Round 2 product categories and begin pre-bidding supplier education. CMS anticipates that it will announce the Round 2 bidding schedule and begin the bidding process, with bidder registration, in the winter of 2011. CMS plans to complete the bid evaluation process, announce the SPAs and begin the contract process for Round 2 in the spring of 2012. In addition, CMS plans to announce the Round 2 contract suppliers in the summer of 2012. The new SPAs for Round 2 markets will take effect in January 2013.
In early July 2010, CMS announced the new SPAs for each of the product categories and each of the CBAs included in the Round 1 Rebid. CMS then began the contracting process with suppliers by issuing contract offer letters to qualified providers. This process is expected to take approximately two months, and CMS expects to announce the list of winners publicly in September 2010.
Reimbursement for Inhalation and Infusion Therapy Drugs. Beginning January 2005, Medicare Part B reimbursement for most drugs, including inhalation drugs, became based upon the manufacturer-reported average sales price (“ASP”) (subject to adjustment each quarter), plus 6%, plus a separate dispensing fee per patient episode. The Medicare reimbursement methodology for non-compounded, infused drugs administered through durable medical equipment, such as infusion pumps, was not affected by this MMA change and remains based upon either 95% of the October 1, 2003 Average Wholesale Price (“AWP”) or, for those drugs whose AWPs were not published in the applicable 2003 compendia, at 95% of the first published AWP.
In 2007 and 2008, changes were made by CMS to the reimbursement methodology for certain inhalation drugs. Beginning in the third quarter of 2007, CMS reimbursement for Xopenex® and albuterol was based on a blended ASP for these two products. Additionally, the Medicare, Medicaid, and State Children’s Health Insurance Program Extension Act of 2007 partially reversed the CMS regulatory decision regarding Xopenex and albuterol. As a result, beginning on April 1, 2008, Medicare began to reimburse providers for Xopenex by blending the ASP of Xopenex and albuterol, but it no longer reimbursed providers for albuterol at the blended price. Rather, albuterol is reimbursed using an albuterol-only ASP.
Since the financial impact of changes in Medicare reimbursement that have been enacted to date began on or before January 1, 2009, our results of operations for the year ended December 31, 2009 and for the three months ended March 31, 2010 include operations after the Medicare reimbursement changes took effect.
10
Table of Contents
The Transactions
On June 18, 2008, Apria, Sky Acquisition and Merger Sub entered into the Merger Agreement, pursuant to which, on October 28, 2008, Merger Sub merged with and into Apria, with Apria being the surviving corporation following the Merger. The Investor Group beneficially owns all of Apria’s issued and outstanding capital stock.
The initial borrowings under our senior secured bridge credit agreement and our ABL Facility, the equity investment by the Investor Group and the repayment of all outstanding indebtedness under our 2004 senior secured revolving credit facility and the Interim Facility are collectively referred to in this prospectus as the “Original Financing.” The offerings of the outstanding Series A-1 Notes and the outstanding Series A-2 Notes in May 2009 and August 2009, respectively, the repayment of approximately $1,010.0 million of borrowings under our senior secured bridge credit agreement, plus accrued and unpaid interest, and the payment of related fees and expenses with the proceeds of the offerings of the outstanding Series A-1 Notes and the outstanding Series A-2 Notes, together with cash on hand, are collectively referred to in this prospectus as the “Refinancing.” The Merger, the Original Financing and the Refinancing are collectively referred to in this prospectus as the “Transactions.” For a more complete description of the Transactions, see “The Transactions” and “Description of Other Indebtedness.”
11
Table of Contents
The following chart summarizes our organizational structure, equity ownership and our principal indebtedness as of the date of this prospectus. This chart is provided for illustrative purposes only and does not represent all legal entities of Apria and its consolidated subsidiaries or all obligations of such entities.
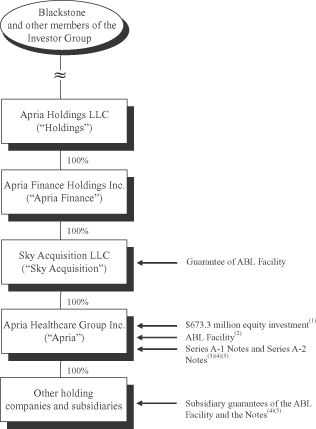
| (1) | Consists of a $673.3 million cash equity investment by the Investor Group in membership interests of our parent entities, which investment includes Dr. Payson’s co-investment. The proceeds of such investment were contributed to Merger Sub, which used such proceeds, together with other sources of funds, to fund the Merger and the related transactions. |
| (2) | Our ABL Facility is secured, subject to certain exceptions and permitted liens, (i) on a first-priority lien basis, by substantially all of our personal property consisting of accounts receivable, inventory, intercompany notes and intangible assets to the extent attached to the foregoing, and certain related assets and proceeds of the foregoing and (ii) on a second-priority lien basis, by all tangible and intangible assets that secure the Notes on a first-priority basis. See “Description of Other Indebtedness—Senior Secured Asset-Based Revolving Credit Facility.” |
| (3) | At the closing of the Merger, we borrowed an aggregate of $1,010.0 million under our senior secured bridge credit agreement. Apria used the proceeds of the offerings of the outstanding Series A-1 Notes and the outstanding Series A-2 Notes in May 2009 and August 2009, respectively, together with cash on hand, to repay all borrowings under the senior secured bridge credit agreement, plus accrued and unpaid interest, and to pay related fees and expenses. Our senior secured bridge credit agreement was terminated concurrently with the closing of the outstanding Series A-2 Notes offering. |
| (4) | The Notes and the related guarantees are secured by our assets, including (i) on a first-priority lien basis (subject to certain exceptions and permitted liens) by substantially all the tangible and intangible assets of Apria and its subsidiaries that are guarantors of the Series A-2 Notes (other than the collateral which secures our ABL Facility on a first-priority lien basis) and (ii) on a second-priority lien basis (subject to certain exceptions and permitted liens) by the collateral securing our ABL Facility on a first-priority lien basis. The Series A-1 Notes are entitled to a priority of payment over the Series A-2 Notes in certain circumstances. |
| (5) | The Notes are guaranteed on a senior secured basis by all of our existing wholly-owned domestic subsidiaries that guarantee the obligations under the ABL Facility and will be guaranteed by our future wholly-owned domestic subsidiaries, subject to certain exceptions. See “Description of Notes—Guarantees.” |
12
Table of Contents
We are incorporated in the State of Delaware. Our principal executive offices are located at 26220 Enterprise Court, Lake Forest, California 92630 and our telephone number is (949) 639-2000.
The Blackstone Group
The Blackstone Group, one of the world’s leading global investment and advisory firms, was founded in 1985. Through its different businesses, as of March 31, 2010, Blackstone had total fee-earning assets under management of approximately $98.1 billion. Blackstone’s alternative asset management businesses include the management of corporate private equity funds, real estate funds, funds of hedge funds, credit-oriented funds, collateralized loan obligation vehicles (CLOs) and closed-end mutual funds. Blackstone also provides various financial advisory services, including mergers and acquisition advisory, restructuring and reorganization advisory, and fund placement services.
13
Table of Contents
The Exchange Offers
General | On May 27, 2009 and August 13, 2009, respectively, the Issuer issued an aggregate of $700.0 million principal amount of 11.25% Senior Secured Notes due 2014 (Series A-1) and $317.5 million principal amount of 12.375% Senior Secured Notes due 2014 (Series A-2) in private offerings. In connection with the private offerings, the Issuer and the guarantors entered into registration rights agreements with the initial purchasers in which they agreed, among other things, to deliver this prospectus to you and to complete the exchange offers within 450 days after the date of issuance and sale of the outstanding Series A-2 Notes. |
You are entitled to exchange in the exchange offers your outstanding notes for exchange notes which are identical in all material respects to the outstanding notes except: |
| • | the exchange notes have been registered under the Securities Act; |
| • | the exchange notes are not entitled to any registration rights which are applicable to the outstanding notes under the registration rights agreements; and |
| • | certain additional interest rate provisions are no longer applicable. |
The Exchange Offers | The Issuer is offering to exchange: |
| • | $700.0 million principal amount of 11.25% Senior Secured Notes due 2014 (Series A-1), which have been registered under the Securities Act, for any and all of its outstanding 11.25% Senior Secured Notes due 2014 (Series A-1); and |
| • | $317.5 million principal amount of 12.375% Senior Secured Notes due 2014 (Series A-2), which have been registered under the Securities Act, for any and all of its outstanding 12.375% Senior Secured Notes due 2014 (Series A-2). |
| You may only exchange outstanding notes in a principal amount of $2,000 or in integral multiples of $1,000 in excess thereof. |
Resale | Based on an interpretation by the staff of the Securities and Exchange Commission (the “SEC”) set forth in no-action letters issued to third parties, the Issuer believes that the exchange notes issued pursuant to the exchange offers in exchange for outstanding notes may be offered for resale, resold and otherwise transferred by you (unless you are our “affiliate” within the meaning of Rule 405 under the Securities Act) without compliance with the registration and prospectus delivery provisions of the Securities Act, provided that: |
| • | you are acquiring the exchange notes in the ordinary course of your business; and |
14
Table of Contents
| • | you have not engaged in, do not intend to engage in, and have no arrangement or understanding with any person to participate in, a distribution of the exchange notes. |
| If you are a broker-dealer and receive exchange notes for your own account in exchange for outstanding notes that you acquired as a result of market-making activities or other trading activities, you must acknowledge that you will deliver this prospectus in connection with any resale of the exchange notes. See “Plan of Distribution.” |
| Any holder of outstanding notes who: |
| • | is our affiliate; |
| • | does not acquire exchange notes in the ordinary course of its business; or |
| • | tenders its outstanding notes in the exchange offers with the intention to participate, or for the purpose of participating, in a distribution of exchange notes; |
| cannot rely on the position of the staff of the SEC enunciated inMorgan Stanley & Co. Incorporated(available June 5, 1991) andExxon Capital Holdings Corporation(available May 13, 1988), as interpreted in the SEC’s letter toShearman & Sterling(available July 2, 1993), or similar no-action letters and, in the absence of an exemption therefrom, must comply with the registration and prospectus delivery requirements of the Securities Act in connection with any resale of the exchange notes. |
Expiration Date | The exchange offers will expire at 5:00 p.m., New York City time, on , 2010, which is the 21st business day after the date of this prospectus, unless extended by us. The Issuer does not currently intend to extend the expiration date. |
Withdrawal | You may withdraw the tender of your outstanding notes at any time prior to the expiration of the applicable exchange offer. The Issuer will return to you any of your outstanding notes that are not accepted for any reason for exchange, without expense to you, promptly after the expiration or termination of the exchange offers. |
Interest on the exchange notes and the outstanding notes | Each exchange note will bear interest at their respective rate per annum set forth on the cover page of this prospectus from the most recent date to which interest has been paid on the outstanding notes. The interest will be payable semi-annually on May 1 and November 1. No interest will be paid on outstanding notes following their acceptance for exchange. |
Conditions to the Exchange Offers | The exchange offers are subject to customary conditions, which the Issuer may waive. See “The Exchange Offers—Conditions to the Exchange Offers.” |
15
Table of Contents
Procedures for Tendering Outstanding Notes | If you wish to participate in the exchange offers, you must complete, sign and date the accompanying letter of transmittal, or a facsimile of such letter of transmittal, according to the instructions contained in this prospectus and the letter of transmittal. You must then mail or otherwise deliver the letter of transmittal, or a facsimile of such letter of transmittal, together with the outstanding notes and any other required documents, to the exchange agent at the address set forth on the cover page of the letter of transmittal. |
| If you hold outstanding notes through The Depository Trust Company (“DTC”) and wish to participate in the exchange offers, you must comply with the Automated Tender Offer Program procedures of DTC, by which you will agree to be bound by the letter of transmittal. By signing, or agreeing to be bound by, the letter of transmittal, you will represent to us that, among other things: |
| • | you are not our “affiliate” within the meaning of Rule 405 under the Securities Act or, if you are our affiliate, that you will comply with any applicable registration and prospectus delivery requirements of the Securities Act; |
| • | you do not have an arrangement or understanding with any person or entity to participate in the distribution of the exchange notes; |
| • | you are acquiring the exchange notes in the ordinary course of your business; and |
| • | if you are a broker-dealer that will receive exchange notes for your own account in exchange for outstanding notes that were acquired as a result of market-making activities, that you will deliver a prospectus, as required by law, in connection with any resale of such exchange notes. |
Special Procedures for Beneficial Owners | If you are a beneficial owner of outstanding notes that are registered in the name of a broker, dealer, commercial bank, trust company or other nominee, and you wish to tender those outstanding notes in the exchange offers, you should contact the registered holder promptly and instruct the registered holder to tender those outstanding notes on your behalf. If you wish to tender on your own behalf, you must, prior to completing and executing the letter of transmittal and delivering your outstanding notes, either make appropriate arrangements to register ownership of the outstanding notes in your name or obtain a properly completed bond power from the registered holder. The transfer of registered ownership may take considerable time and may not be able to be completed prior to the expiration date. |
16
Table of Contents
Guaranteed Delivery Procedures | If you wish to tender your outstanding notes and your outstanding notes are not immediately available or you cannot deliver your outstanding notes, the letter of transmittal or any other required documents, or you cannot comply with the applicable procedures under DTC’s Automated Tender Offer Program for transfer of book-entry interests, prior to the expiration date, you must tender your outstanding notes according to the guaranteed delivery procedures set forth in this prospectus under “The Exchange Offer—Guaranteed Delivery Procedures.” |
Effect on Holders of Outstanding Notes | As a result of the making of, and upon acceptance for exchange of all validly tendered outstanding notes pursuant to the terms of the exchange offers, the Issuer and the guarantors will have fulfilled a covenant under the applicable registration rights agreement. Accordingly, there will be no increase in the interest rate on the outstanding notes under the circumstances described in the registration rights agreements. If you do not tender your outstanding notes in the exchange offers, you will continue to be entitled to all the rights and limitations applicable to the outstanding notes as set forth in the indenture, except the Issuer and the guarantors will not have any further obligation to you to provide for the exchange and registration of the outstanding notes under the applicable registration rights agreement. To the extent that outstanding notes are tendered and accepted in the exchange offers, the trading market for remaining outstanding notes that are not so tendered and exchanged could be adversely affected. |
Consequences of Failure to Exchange | All untendered outstanding notes will continue to be subject to the restrictions on transfer set forth in the outstanding notes and in the indenture. In general, the outstanding notes may not be offered or sold unless registered under the Securities Act, except pursuant to an exemption from, or in a transaction not subject to, the Securities Act and applicable state securities laws. Other than in connection with the exchange offers, the Issuer and the guarantors do not currently anticipate that they will register the outstanding notes under the Securities Act. |
Certain U.S. Federal Income Tax Considerations | The exchange of outstanding notes in the exchange offers will not be a taxable event for United States federal income tax purposes. See “Certain U.S. Federal Income Tax Considerations.” |
Use of Proceeds | The Issuer will not receive any cash proceeds from the issuance of exchange notes in the exchange offers. See “Use of Proceeds.” |
Exchange Agent | U.S. Bank National Association is the exchange agent for the exchange offers. The addresses and telephone numbers of the exchange agent are set forth in the section captioned “The Exchange Offers—Exchange Agent” of this prospectus. |
17
Table of Contents
The Exchange Notes
The terms of the exchange notes are identical in all material respects to the terms of the outstanding notes, except that the exchange notes will not contain terms with respect to transfer restrictions or additional interest upon a failure to fulfill certain of our obligations under the registration rights agreement. The exchange notes will evidence the same debt as the outstanding notes. The exchange notes will be governed by the same indenture under which the outstanding notes were issued. The following summary is not intended to be a complete description of the terms of the exchange notes. For a more detailed description of the Notes, see “Description of Notes.”
Issuer | Apria Healthcare Group Inc. |
Notes Offered | $700.0 million aggregate principal amount of 11.25% Senior Secured Notes due 2014 (Series A-1) and $317.5 million aggregate principal amount of 12.375% Senior Secured Notes due 2014 (Series A-2). |
Maturity Date | The exchange notes will mature on November 1, 2014. |
Interest | The exchange Series A-1 Notes and the exchange Series A-2 Notes will bear interest at a rate of 11.25% and 12.375% per annum, respectively, payable on May 1 and November 1 of each year. |
Guarantees | The exchange notes will be fully and unconditionally guaranteed on a senior secured basis, by all of our existing wholly-owned subsidiaries organized in the United States that guarantee the obligations under the ABL Facility and the outstanding notes, subject to certain limitations described herein. |
| If any of our wholly-owned restricted subsidiaries guarantee certain of our other debt, such subsidiaries shall also guarantee the exchange notes. |
| Under certain circumstances, subsidiaries may be released from these guarantees without the consent of the holders of the exchange notes. |
| See “Description of Notes—Guarantees.” |
Collateral | The exchange notes and the related guarantees will be secured by (1) a first-priority lien (subject to certain exceptions and permitted liens) on substantially all the tangible and intangible assets of the Issuer and the guarantors, including all of the capital stock of the Issuer, of each guarantor and of any material subsidiary of the Issuer (which, in the case of foreign subsidiaries, will be limited to 65% of the stock of each first-tier foreign subsidiary), other than the collateral securing the ABL Facility on a first-priority lien basis and (2) a second-priority lien on accounts receivable arising from the sale of inventory and other goods and services (including related contracts and contract rights, inventory, cash, deposit accounts, other bank accounts and securities accounts), inventory, intercompany notes and intangible assets to the extent attached to the foregoing, and the proceeds thereof, which secures the ABL Facility on a first-priority lien basis. |
18
Table of Contents
| The collateral securing the exchange notes on a first-priority lien basis will not include (i) the collateral securing the ABL Facility on a first priority lien basis, (ii) certain excluded assets and (iii) those assets as to which the collateral agent representing the holders of the exchange notes reasonably determines that the costs of obtaining such a security interest are excessive in relation to the value of the security to be afforded thereby. |
See “Description of Notes—Security for the Notes.”
Ranking | The exchange notes and the related guarantees will be our senior secured obligations. The indebtedness evidenced by the exchange notes and the guarantees will rank: |
| • | equal with all of the Issuer’s and the guarantors’ existing and future senior indebtedness, including any indebtedness under the ABL Facility and the outstanding notes; |
| • | senior to all of the Issuer’s and the guarantors’ existing and future subordinated indebtedness; |
| • | junior in priority as to collateral that secures the ABL Facility on a first-priority lien basis with respect to the Issuer’s and the guarantors’ obligations under the ABL Facility, any other debt incurred after the issue date that has a priority security interest relative to the Notes in the collateral that secures the ABL Facility and all cash management and hedging obligations incurred with any lender under the ABL Facility or any affiliate of any such lender; and |
| • | equal in priority as to collateral that secures the Notes and the guarantees on a first-priority lien basis with respect to the Issuer’s and the guarantors’ obligations under our notes and any other pari passu lien obligations incurred after the issue date;provided that the Series A-1 Notes are entitled to a priority of payment over the Series A-2 Notes in certain circumstances, including upon any acceleration of the obligations under the Notes or any bankruptcy or insolvency event of default. |
| The Notes will also be effectively junior to the liabilities of any non-guarantor subsidiaries. |
| As of March 31, 2010: |
| • | we had $1,017.5 million in aggregate principal amount of senior secured indebtedness outstanding under the outstanding notes; |
| • | we had $16.1 million of drawn letters of credit under the ABL Facility and availability of $133.9 million under the ABL Facility, which are secured on a first-priority lien basis by substantially all of our personal property consisting of accounts receivable, inventory, intercompany notes and intangible assets to the extent attached to the foregoing, and certain related assets and proceeds of the foregoing; and |
19
Table of Contents
| • | we had $3.2 million in aggregate principal amount of other senior indebtedness outstanding (excluding the ABL Facility, the outstanding notes and guarantees of the foregoing). |
| See “Description of Notes—Ranking” and “Description of Notes—Priorities of Payment as between Series A-2 Debt (including the Series A-2 Notes) and Series A-1 Notes.” |
Optional Redemption | We may, at our option, redeem some or all of the exchange notes at 100% of the principal amount thereof plus a “make-whole premium” at any time and from time to time prior to November 1, 2011. |
We may, at our option, redeem some or all of the exchange notes, at any time and from time to time after November 1, 2011, at the redemption prices listed under “Description of Notes—Optional Redemption.”
In addition, prior to November 1, 2011, we may at our option redeem up to 35% of the exchange Series A-1 Notes at a redemption price of 111.25% of their principal amount, and up to 35% of the exchange Series A-2 Notes at a redemption price of 112.375% of their principal amount, plus accrued and unpaid interest, if any, to the date of redemption, from the proceeds of certain equity offerings. See “Description of the Notes—Optional Redemption.”
Mandatory Repurchase Offer | If we experience specific types of changes of control, we must offer to repurchase the exchange notes at a price equal to 101% of the principal amount thereof, plus accrued and unpaid interest to the date of purchase, subject to the rights of holders of exchange notes on the relevant record date to receive interest due on the relevant payment date. |
Certain Covenants | The exchange notes will be governed by the same indenture under which the outstanding notes were issued. The indenture governing the exchange notes contains covenants that, among other things, will limit our ability and the ability of our restricted subsidiaries to: |
| • | incur additional debt; |
| • | pay dividends and make other distributions; |
| • | make certain investments; |
| • | repurchase our stock; |
| • | incur certain liens; |
| • | enter into transactions with affiliates; |
| • | merge or consolidate; |
| • | enter into agreements that restrict the ability of our subsidiaries to make dividends or other payments to us; and |
| • | transfer or sell assets. |
20
Table of Contents
| These covenants are subject to important exceptions and qualifications. See “Description of Notes—Certain Covenants.” |
Use of Proceeds | We will not receive any proceeds from the exchange offers. See “Use of Proceeds.” |
No Prior Market | The exchange notes will generally be freely transferable (subject to certain restrictions discussed in “The Exchange Offers”) but will be a new issue of securities for which there will not initially be a market. Accordingly, there can be no assurance as to the development or liquidity of any market for the exchange notes. The initial purchasers in the private offering of the outstanding notes have advised us that they currently intend to make a market for the exchange notes, as permitted by applicable laws and regulations. However, they are not obligated to do so and may discontinue any such market making activities at any time without notice. We do not intend to apply for a listing of the exchange notes on any securities exchange or automated dealer quotation system. |
Risk Factors
You should carefully consider the information set forth under the caption “Risk Factors” beginning on page 24 of this prospectus before participating in the exchange offers.
21
Table of Contents
Summary Historical Consolidated Financial Data
Our summary historical consolidated financial data set forth below should be read in conjunction with “The Transactions,” “Selected Historical Consolidated Financial Data” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and accompanying notes contained herein. We derived the summary historical consolidated financial data for the year ended December 31, 2007, the periods from January 1, 2008 to October 28, 2008 and October 29, 2008 to December 31, 2008, the year ended December 31, 2009 and as of December 31, 2008 and 2009 from our audited consolidated financial statements which are included in this prospectus. We derived the summary historical consolidated financial data for the year ended December 31, 2006 and as of December 31, 2006 and 2007 from our audited consolidated financial statements which are not included in this prospectus. The historical results presented are not necessarily indicative of future results. Our audited consolidated financial statements for each of the years in the two-year period ended December 31, 2007, the periods from January 1, 2008 to October 28, 2008 and October 29, 2008 to December 31, 2008 and the year ended December 31, 2009 have been audited by Deloitte & Touche LLP, an independent registered public accounting firm.
Our summary historical consolidated financial data for the three months ended March 31, 2009 and as
of and for the three months ended March 31, 2010 have been derived from our unaudited condensed consolidated
financial statements, which are included in this prospectus. We derived the summary historical consolidated financial data as of March 31, 2009 from our unaudited condensed consolidated financial statements, which are not included in this prospectus. The unaudited condensed consolidated financial statements were prepared on a basis consistent with our audited consolidated financial statements. In our opinion, the unaudited condensed consolidated financial statements include all adjustments, consisting of normal recurring accruals, necessary for the fair presentation of those statements. Our results for the three months ended March 31, 2010 should not be considered indicative of the results for the full fiscal year ending December 31, 2010.
Year Ended | Period January 1, 2008 to October 28, 2008 | Period October 29, 2008 to December 31, 2008 | Year Ended December 31, 2009 | Three Months Ended March 31, 2009 | Three Months Ended March 31, 2010 | |||||||||||||||||||
| 2007 | ||||||||||||||||||||||||
| (Predecessor) | (Predecessor) | (Successor) | (Successor) | (Successor) | (Successor) | |||||||||||||||||||
| (dollars in thousands) | ||||||||||||||||||||||||
Statement of Operations Data: | ||||||||||||||||||||||||
Net revenues | $ | 1,631,801 | $ | 1,773,289 | $ | 356,665 | $ | 2,094,561 | $ | 516,431 | $ | 508,876 | ||||||||||||
Cost and expenses: | ||||||||||||||||||||||||
Total cost of net revenues | 564,992 | 691,337 | 137,760 | 867,459 | 207,044 | 202,792 | ||||||||||||||||||
Provision for doubtful accounts | 43,138 | 33,626 | 14,329 | 57,919 | 11,356 | 15,887 | ||||||||||||||||||
Selling, distribution and administrative | 862,062 | 924,536 | 179,362 | 1,050,134 | 263,784 | 257,738 | ||||||||||||||||||
Amortization of intangible assets | 3,079 | 3,461 | 1,008 | 3,716 | 1,277 | 1,657 | ||||||||||||||||||
Total costs and expenses | 1,473,271 | 1,652,960 | 332,459 | 1,979,228 | 483,461 | 478,074 | ||||||||||||||||||
Operating income | 158,530 | 120,329 | 24,206 | 115,333 | 32,970 | 30,802 | ||||||||||||||||||
Interest expense and other, net | 20,493 | 29,684 | 25,441 | 127,591 | 32,267 | 32,489 | ||||||||||||||||||
Income (loss) from continuing operations before taxes | 138,037 | 90,645 | (1,235 | ) | (12,258 | ) | 703 | (1,687 | ) | |||||||||||||||
Income tax expense (benefit) | 51,998 | 34,192 | 659 | (8,438 | ) | 1,180 | (884 | ) | ||||||||||||||||
Net income (loss) from continuing operations | $ | 86,039 | $ | 56,453 | $ | (1,894 | ) | $ | (3,820 | ) | $ | (477 | ) | $ | (803 | ) | ||||||||
22
Table of Contents
| Year Ended December 31, 2007 | Period January 1, 2008 to October 28, 2008 | Period October 29, 2008 to December 31, 2008 | Year Ended December 31, 2009 | Three Months Ended March 31, 2009 | Three Months Ended March 31, 2010 | |||||||||||||||||||||
| (Predecessor) | (Predecessor) | (Successor) | (Successor) | (Successor) | (Successor) | |||||||||||||||||||||
| (dollars in thousands) | ||||||||||||||||||||||||||
Cash Flow Data: | ||||||||||||||||||||||||||
Net cash provided by operating activities | $ | 294,006 | $ | 297,937 | $ | 63,337 | $ | 169,426 | $ | 29,672 | $ | 15,105 | ||||||||||||||
Net cash used in investing activities | (483,235 | ) | (154,493 | ) | (75,466 | ) | (168,656 | ) | (39,592 | ) | (24,013 | ) | ||||||||||||||
Net cash (used in) provided by financing activities | 203,023 | (123,682 | ) | 131,934 | (10,625 | ) | (7,505 | ) | (15,866 | ) | ||||||||||||||||
Capital expenditures | 128,759 | 157,183 | 26,217 | 150,597 | 39,843 | 27,319 | ||||||||||||||||||||
| As of December 31, | As of March 31, 2010 | |||||||||||||
| 2007 | 2008 | 2009 | ||||||||||||
| (Predecessor) | (Successor) | (Successor) | (Successor) | |||||||||||
| (dollars in thousands) | ||||||||||||||
Balance Sheet Data: | ||||||||||||||
Cash and cash equivalents | $ | 28,451 | $ | 168,018 | $ | 158,163 | $ | 133,389 | ||||||
Short-term investments | — | — | 23,673 | 19,183 | ||||||||||
Goodwill and intangible assets | 822,992 | 1,292,403 | 1,341,456 | 1,341,299 | ||||||||||
Total assets | 1,597,802 | 2,210,813 | 2,309,047 | 2,284,270 | ||||||||||
Total debt | 687,283 | 1,022,233 | 1,021,146 | 1,020,704 | ||||||||||
Stockholders’ equity | 512,025 | 672,820 | 678,731 | 678,978 | ||||||||||
23
Table of Contents
You should carefully consider the following risks, in addition to the other information contained in this prospectus, before participating in the exchange offers. The risks described below are not the only risks we face. Any of the following risks, as well as other risks and uncertainties not currently known to us or that we currently deem to be immaterial, could materially adversely affect our business, financial condition or results of operations.
Risks Associated with the Exchange Offers
If you choose not to exchange your outstanding notes in the exchange offers, the transfer restrictions currently applicable to your outstanding notes will remain in force and the market price of your outstanding notes could decline.
If you do not exchange your outstanding notes for exchange notes in the exchange offers, then you will continue to be subject to the transfer restrictions on the outstanding notes as set forth in the offering memorandum distributed in connection with the private offering of the outstanding notes. In general, the outstanding notes may not be offered or sold unless they are registered or exempt from registration under the Securities Act and applicable state securities laws. Except as required by the registration rights agreement, we do not intend to register resales of the outstanding notes under the Securities Act.
The tender of outstanding notes under the exchange offers will reduce the remaining principal amount of the outstanding notes, which may have an adverse effect upon and increase the volatility of, the market price of the outstanding notes due to reduction in liquidity.
Your ability to transfer the notes may be limited by the absence of an active trading market, and an active trading market may not develop for the notes.
The exchange notes are a new issue of securities for which there is no established trading market. We do not intend to have the exchange notes listed on a national securities exchange or to arrange for quotation on any automated quotation system. The initial purchasers have advised us that they intend to make a market in the exchange notes, as permitted by applicable laws and regulations; however, the initial purchasers are not obligated to make a market in the exchange notes, and they may discontinue their market-making activities at any time without notice. Therefore, we cannot assure you as to the development or liquidity of any trading market for the exchange notes. The liquidity of any market for the exchange notes will depend on a number of factors, including:
| • | the number of holders of exchange notes; |
| • | our operating performance and financial condition; |
| • | the market for similar securities; |
| • | the interest of securities dealers in making a market in the exchange notes; and |
| • | prevailing interest rates. |
Historically, the market for non-investment grade debt has been subject to disruptions that have caused substantial volatility in the prices of securities similar to the exchange notes. The market, if any, for the exchange notes may face similar disruptions that may adversely affect the prices at which you may sell your exchange notes. Therefore, you may not be able to sell your exchange notes at a particular time and the price that you receive when you sell may not be favorable.
24
Table of Contents
Risks Relating to Our Business
Continued Reductions in Medicare and Medicaid Reimbursement Rates and the Comprehensive Healthcare Reform Package Could Have a Material Adverse Effect on Our Results of Operations and Financial Condition.
There are ongoing legislative and regulatory efforts to reduce or otherwise adversely affect Medicare reimbursement rates for products and services we provide. For example, the regulations implementing the mandates under the MMA, the DRA and MIPPA reduced the reimbursement for a number of products and services we provide and established a competitive bidding program for certain durable medical equipment under Medicare Part B. The Medicare DMEPOS competitive bidding program is intended to further reduce reimbursement for certain products as well as decrease the number of companies permitted to serve Medicare beneficiaries. In July 2008, MIPPA was passed and included a delay to the competitive bidding program. In order to ensure that the delay would achieve the same level of savings projected for the DMEPOS competitive bidding program, Congress adopted a nationwide average payment reduction of 9.5% in the DMEPOS fee schedule for those product categories included in Round 1, effective January 1, 2009. MIPPA called for those DMEPOS items that were not subject to Round 1 competitive bidding to receive a CPI update each year from 2009 to 2013. The CPI for 2009 for these items was 5%. For 2010, the CPI was -1.4%, however, the annual DMEPOS updates cannot be negative. The Reform Package made changes to the CPI adjustment for 2011 and each subsequent year based upon the CPI reduced by a new productivity adjustment which may result in negative updates.
In January 2009, CMS released an interim final rule implementing certain MIPPA provisions requiring CMS to conduct a second Round 1 competition in 2009 (the “Round 1 Rebid”) and mandated certain changes for both the Round 1 Rebid and subsequent rounds of the program. Despite industry advocacy and Congressional efforts to further delay CMS’ implementation of the program, the final rule took effect on April 18, 2009. In early July 2010, CMS announced the new SPAs for each of the product categories and each of the CBAs included in the Round 1 Rebid. CMS then began the contracting process with suppliers by issuing contract offer letters to qualified providers. We received contract offers for a substantial majority of the bids we submitted. We did not receive contract offers for certain product categories in certain CBAs, but the process will not be completed until September 2010. Approximately $21 million of our net revenues for the fiscal year ended December 31, 2009 was generated by the products and CBAs included in the Round 1 Rebid. We estimate that the initial results of the Round 1 Rebid would impact our net revenues in the fiscal year ending December 31, 2011 by approximately $8.0 million, assuming the current contract offers and no changes in volume. CMS expects to announce the winners in the fall of 2010 and the new rates will take effect in January 2011. The Reform Package also made changes to the competitive bidding program. Significantly, Round 2 of the competitive bidding program has been expanded from 70 to 91 of the largest MSAs. CMS has announced that the effective date of the Round 2 pricing will be January 1, 2013; additional details concerning products to be included and other aspects of implementing Round 2 will not be fully known until after CMS completes a rulemaking process, which is currently scheduled for the summer or fall of 2010. Assuming that Round 2 would include the same product categories, bidding rules and markets currently being proposed by CMS, we estimate that approximately $110 million of our net revenues for the fiscal year ending December 31, 2011 would be subject to competitive bidding. Although the bidding process for Round 2 is currently scheduled to commence in 2011, the effective date of new Round 2 rates and guidelines would take effect in January 2013 at the earliest. Therefore, we cannot estimate the impact of potential Round 2 rate reductions on our business until more specific information is published by CMS and its contractors. The Reform Package also gives the Secretary of Health and Human Services the authority to apply competitive bid pricing to non-bid areas, but details of that process are unlikely to be understood until after CMS issues guidance or completes a related rulemaking process. At this time, we cannot quantify what negative impact, if any, the revised program will have upon our revenue or operations when the program is reinitiated, but such impact could be material.
Further, the DRA resulted in reduced reimbursement rates for certain durable medical equipment, including the home oxygen equipment and services we provide, a reduced period for rental revenue, and potential increased
25
Table of Contents
costs to us associated with replacement of certain patient-owned equipment. There have been various administrative and legislative proposals to further reduce the maximum capped rental period for oxygen equipment below the 36-month level mandated by the DRA to 13 and 18 months, respectively, and/or to reduce the monthly payment rates for oxygen equipment. In 2009, the “Medicare Home Oxygen Therapy Act of 2009” was introduced in the House of Representatives and subsequently offered and withdrawn as an amendment to healthcare reform legislation being debated in the House Energy and Commerce Committee. This bill would, among other things, have amended the current statutory language surrounding the provision of home oxygen therapy. It defined covered services to be provided by all oxygen providers, established criteria for a “qualified home oxygen provider” to meet, aligned the oxygen equipment provided more directly with patient need, eliminated the 36-month cap, provided for certain cost transparency and would be implemented in a budget neutral manner. While these proposals have not been enacted, the home oxygen industry continues to work with Congress on oxygen benefit reform measures, and similar proposals may be raised in the future.
In addition to these activities, certain other proposed legislative and regulatory activities may affect reimbursement policies and rates for other items and services we provide. For example, in March 2010, Congress enacted the Reform Package which includes comprehensive healthcare reform. Among many other provisions, the Reform Package expands the Medicaid program, mandates extensive insurance market reforms, creates new health insurance access points (e.g., insurance exchanges), provides certain insurance subsidies (e.g., premiums and cost sharing), imposes individual and employer health insurance requirements and makes a number of changes to the Code.
There are various provisions in the Reform Package that impact our business. For example, the Reform Package requires certain pharmaceutical and medical device manufacturers to pay an excise tax to the government, which may, in turn, increase our costs for these products. The Reform Package also provides for cuts in some Medicare payments made to certain providers and substantial cuts to Medicare Advantage plans, through which we contract to provide services to Medicare beneficiaries. Also included in the Reform Package are (i) an expansion of the Recovery Audit Contractor Program, (ii) certain fraud and abuse prevention measures and (iii) expanded regulatory authority concerning the types of conduct that can result in additional fines and penalties for those healthcare providers who do not comply with applicable laws and regulations. Furthermore, the Reform Package grants the Secretary of Health and Human Services authority to set a date by which certain providers and suppliers will be required to establish a compliance program.
The Reform Package makes a number of changes to how certain of the Company’s products will be reimbursed by Medicare. The Reform Package also makes changes to the Medicare durable medical equipment CPI adjustment for 2011 and each subsequent year based upon the CPI reduced by a new productivity adjustment which may result in negative updates and, as noted above, includes changes to the Medicare DMEPOS competitive bidding program.
In an effort to further strengthen the integrity of the Medicare program, the Reform Package includes additional requirements concerning physician enrollment and certain mandatory face-to-face patient/physician visits in conjunction with the ordering of durable medical equipment. These provisions are likely to be the subject of rulemaking and are a high priority for the American Association for Homecare and other industry representative organizations. We expect the Administration to continue to enhance its oversight efforts and the Company strives to incorporate any necessary changes into its overall corporate compliance and internal audit programs on a regular basis.
The effective dates of the various provisions within the Reform Package are staggered over the next several years, with some changes occurring immediately. Much of the interpretation of what the Reform Package requires will be subject to administrative rulemaking, the development of agency guidance and court interpretations. We cannot currently predict the adverse impact, if any, that these and other Reform Package changes might have on our operations, cash flow and capital resources, but such impact could be material. In addition, other legislative and regulatory changes could have a material adverse effect on our business, financial condition, results of operations, cash flow, capital resources and liquidity.
26
Table of Contents
There are also ongoing state and federal legislative and regulatory efforts to reduce or otherwise adversely affect Medicaid reimbursement rates for products and services we provide. For a number of years, some states have adopted alternative pricing methodologies for certain drugs, biologicals and home medical equipment reimbursed under the Medicaid program. In a number of states, the changes reduced the level of reimbursement we received for these items without a corresponding offset or increase to compensate for the service costs we incurred. For example, California’s Medicaid program (“Medi-Cal”) adopted a regulation that limits the amounts a provider can bill for certain durable medical equipment and medical supplies. In March 2009, the California Association of Medical Product Suppliers (“CAMPS”) initiated a lawsuit to invalidate this regulation as having been adopted in violation of California’s Administrative Procedure Act. On August 3, 2009, the court entered a decision denying CAMPS’ petition. CAMPS has appealed the court’s decision. If the regulation is ultimately upheld, it could result in our making refunds and other payments to Medi-Cal and our future revenues from Medi-Cal may be reduced. In addition to this Medi-Cal regulation, we currently are examining other similar Medicaid program rules to confirm whether we have complied with the particular states’ Medicaid reimbursement methodologies. The review could result in our making refunds and other payments to these state Medicaid programs and our future revenues may be reduced. Historically, when we have learned that states have adopted such alternative reimbursement methodologies, we have sometimes elected to stop accepting new Medicaid patient referrals for the affected drugs, biologicals and home medical equipment. We are currently evaluating the possibility of stopping or reducing our Medicaid business in a number of states with reimbursement policies that make it difficult for us to conduct operations profitably. Moreover, the Reform Package increases Medicaid enrollment over a number of years and imposes additional requirements on states, which could further strain state budgets and therefore result in additional policy changes or rate reductions. In addition, changes to the federal regulations pertaining to prescription drug pricing may also impact the Medicaid reimbursement available to us. We cannot currently predict the adverse impact, if any, that any such changes to or reduction in our Medicaid business might have on our operations, cash flow and capital resources, but such impact could be material. In addition, we cannot predict whether other states will consider similar or other reimbursement reductions or whether any such changes could have a material adverse effect on our results of operations, cash flow and capital resources.
We cannot estimate the ultimate impact of all legislated and contemplated Medicare and Medicaid reimbursement changes or provide assurance to investors that additional reimbursement reductions will not be made or will not have a material adverse effect on our business, financial condition, results of operations, cash flow, capital resources and liquidity.
For further information, see “Business—Government Regulation—Medicare and Medicaid Revenues” and “Business—Government Regulation—Medicare Reimbursement.”
We Believe That Continued Pressure to Reduce Healthcare Costs Could Have a Material Adverse Effect on Us.
As the result of continuing reductions in payor reimbursement, we, like many other healthcare companies, are making substantial efforts to reduce our costs in providing healthcare services and products. Certain managed care organizations and larger insurers also regularly attempt to seek reductions in the prices at which we provide services to them and their patients. We have a large number of contractual arrangements with managed care organizations and other parties, which represented approximately 70% and 72% of our total net revenues for the three months ended March 31, 2010 and for the year ended December 31, 2009, respectively, and we expect that we will continue to enter into more of these contractual arrangements. Also, the Reform Package significantly reduces the government’s payment rates to Medicare Advantage plans. Other provisions impose minimum medical-loss ratios, state and federal premium review procedures and benefit requirements on insurers. There can be no assurance that we will retain or obtain Medicare Advantage or other such managed care contracts or that such plans will not attempt to further reduce the rates they pay to providers. In addition, if we are unable to successfully reduce our costs, we may be unable to continue to provide services directly to patients of certain payors or through these contractual arrangements. This would have a material adverse effect on our business, financial condition, results of operations, cash flow, capital resources and liquidity.
27
Table of Contents
The segment of the healthcare market in which we operate is highly competitive. In each of our service lines, there are a number of national providers and numerous regional and local providers. Other types of healthcare providers, including industrial gas manufacturers, individual hospitals and hospital systems, home health agencies and health maintenance organizations, have entered and may continue to enter the market to compete with our various service lines. With access to significantly greater financial and market resources than what is available to us, some of these competitors may be better positioned to compete in the market. This may increase pricing pressure and limit our ability to maintain or increase our market share and may have a material adverse effect on our business, financial condition, results of operations, cash flow, capital resources and liquidity.
Non-Compliance With Laws and Regulations Applicable to Our Business and Future Changes in Those Laws and Regulations Could Have a Material Adverse Effect on Us.
We are subject to many stringent and frequently changing laws and regulations, and interpretations thereof, at both the federal and state levels, requiring compliance with burdensome and complex billing and payment, substantiation and record-keeping requirements. On an ongoing basis, we have implemented policies and procedures designed to meet the various documentation requirements of government payors as they have been interpreted and applied. Examples of such documentation requirements are contained in the Durable Medical Equipment Medicare Administrative Contractor (“DMEMAC”) Supplier Manuals which provide that clinical information from the “patient’s medical record” is required to justify the medical necessity for the provision of DME. An auditor for one of the DMEMACs has recently taken the position, among other things, that the “patient’s medical record” refers not to documentation maintained by the DME supplier but instead to documentation maintained by the patient’s physician, healthcare facility, or other clinician, and that clinical information created by the DME supplier’s personnel and confirmed by the patient’s physician is not sufficient to establish medical necessity. Other government auditors have recently taken the same or a similar position. It may be difficult, and sometimes impossible, for us to obtain such documentation from other healthcare providers. If these or other burdensome positions continue to be adopted by auditors, DMEMACs, other contractors or CMS in administering the Medicare program, we have the right to challenge these positions as being contrary to law. If these interpretations of the documentation requirements are ultimately upheld, however, it could result in our making significant refunds and other payments to Medicare and our future revenues from Medicare would likely be substantially reduced. We cannot currently predict the adverse impact, if any, that these new, more burdensome interpretations of the Medicare documentation requirements might have on our operations, cash flow and capital resources, but such impact could be material.
The federal False Claims Act imposes civil and criminal liability on individuals or entities that submit false or fraudulent claims for payment to the government. The federal government and a number of courts also have taken the position that claims presented in violation of certain other statutes, including the federal anti-kickback statute or the Omnibus Budget Reconciliation Act of 1993 (the “Stark Law”), can be considered a violation of the federal False Claims Act. Violations of the federal civil False Claims Act may result in treble damages, civil monetary penalties and exclusion from the Medicare, Medicaid and other federally funded healthcare programs. If certain criteria are satisfied, the federal civil False Claims Act allows a private individual to bring a qui tam suit on behalf of the government and, if the case is successful, to share in any recovery. Federal False Claims Act suits brought directly by the government or private individuals against healthcare providers, like us, are increasingly common and are expected to continue to increase.
The Reform Package also includes certain fraud and abuse prevention measures and expands regulatory authorities concerning the types of conduct that can result in additional fines and penalties for those healthcare providers who do not comply with applicable laws and regulations.
Financial relationships between us and physicians and other referral sources are also subject to strict limitations under laws such as the Stark Law and anti-kickback laws. In addition, strict licensure, accreditation, safety and marketing requirements apply to the provision of services, pharmaceuticals and medical equipment.
28
Table of Contents
Violations of these laws and regulations could subject us to civil and criminal enforcement actions; licensure revocation, suspension or non-renewal; severe fines; facility shutdowns; repayment of amounts received from third party payors and possible exclusion from participation in federal healthcare programs such as Medicare and Medicaid. We cannot assure you that we are in compliance with all applicable existing laws and regulations or that we will be able to comply with any new laws or regulations that may be enacted in the future. In addition, from time to time, we may be the subject of investigations or audits or be a party to additional litigation which alleges violations of law. If any of those matters were successfully asserted against us, there could be a material adverse effect on our business, financial condition, results of operations, cash flow, capital resources, liquidity or prospects.
Changes in public policy, healthcare law, new interpretations of existing laws, or changes in payment methodology may have a significant effect on our business, financial condition, results of operations, cash flow, capital resources and liquidity.
Our Business and Financial Performance May Be Adversely Affected By Our Inability to Effectively Execute and Implement Cost Savings Initiatives.
We launched a substantial cost reduction plan in late 2007 across a number of identified initiatives targeting pre-tax savings of approximately $198 million on an annualized basis, of which we have realized approximately $127 million through March 31, 2010. The programs related to the remaining targeted annualized savings of $71 million to be realized include customer service and billing center centralization (including consolidation and outsourcing or offshoring of certain operations), purchasing initiative synergies, contract cost reductions, outsourcing certain functions of our information technology department, a branch optimization program, outsourced equipment pickups and exchanges, document imaging, and insurance. Projected costs and savings associated with these initiatives are subject to a variety of risks, including:
| • | the contemplated costs to effect these initiatives may exceed estimates; |
| • | the initiatives we are contemplating may require consultation with various customers, employees, labor representatives or regulators, and such consultations may influence the timing, costs and extent of expected savings; |
| • | the loss of skilled employees in connection with the initiatives; and |
| • | the projected savings contemplated under these programs may fall short of targets. |
While we have begun and expect to continue to implement these cost savings initiatives, there can be no assurance that we will be able to do so successfully or that we will realize the projected benefits of these and other restructuring and cost savings initiatives. If we are unable to realize these anticipated cost savings initiatives, our business may be adversely affected. Moreover, our continued implementation of cost savings initiatives may have a material adverse effect on our business, results of operations and financial condition, including but not limited to the loss of revenue, increases in accounts receivable and reserves and/or write-offs of accounts receivable. Also, in response to changing business conditions, we may discontinue or significantly adjust our cost savings initiatives which would affect our ability to achieve future cost savings.
Our Failure to Successfully Design, Modify and Implement Computer and Other Process Changes to Maximize Productivity and Ensure Compliance Could Ultimately Have a Significant Negative Impact on Our Results of Operations and Financial Condition.
We have identified a number of areas throughout our operations where we intend to modify the current processes or systems in order to attain a higher level of productivity or ensure compliance. The ultimate cost savings expected from the successful design and implementation of such initiatives will be necessary to help offset the impact of Medicare and Medicaid reimbursement reductions and continued downward pressure on pricing. Additionally, Medicare and Medicaid often amend their documentation requirements. The DMEPOS competitive bidding program, once fully implemented, will also impose new reporting requirements on
29
Table of Contents
contracted providers. Perot Systems Corporation, our outsourcing partner for certain information systems functions, was acquired by Dell Inc. in late 2009; new management could make operational, leadership or other changes that could impact our plans and cost-savings goals. Our failure to successfully design and implement system or process modifications could have a significant impact on our operations and financial condition. Further, the implementation of these system or process changes could have a disruptive effect on related transaction processing and operations.
Our Failure to Maintain Controls and Processes Over Billing and Collections or to Execute the Outsourcing Effectively, the Deterioration of the Financial Condition of Our Payors or Disputes With Third Parties Could Have a Significant Negative Impact on Our Results of Operations and Financial Condition.
The collection of accounts receivable is one of our most significant challenges and requires constant focus and involvement by management and ongoing enhancements to information systems and billing center operating procedures. For example, we have recently experienced an increase in accounts receivable attributable, among other things, to transitioning of some of our billing and collection functions to our outsourcing partner and to changes in payment practices by some of our payors and their intermediaries. While we believe that a portion of this increase is temporary, there can be no assurance that we will be able to return to or maintain our current levels of collectibility and days sales outstanding in future periods. Further, some of our payors and/or patients may experience financial difficulties, or may otherwise not pay accounts receivable when due, resulting in increased write-offs. If we are unable to properly bill and collect our accounts receivable, our results will be adversely affected. In addition, from time to time we are involved in disputes with various parties, including our payors and their intermediaries regarding their performance of various contractual or regulatory obligations. These disputes sometimes lead to legal and other proceedings and cause us to incur costs or experience delays in collections or loss of revenue. In addition, in the event such disputes are not resolved in our favor or cause us to terminate our relationships such parties, there may be an adverse impact on our results of operations or financial condition.
Our Outsourcing and Offshoring Activities Subject Us to Risks That Could Have a Significant Negative Impact on Our Results of Operations and Financial Condition.
We are pursuing an outsourcing strategy with respect to certain business functions. We are in the process of outsourcing certain billing, collections and other administrative and clerical services to Intelenet Global Services Private Limited (“Intelenet”) and certain information systems functions to Dell Services (formerly Perot Systems Corporation). See “Business—Outsourcing Activities” and “Certain Relationships and Related Party Transactions—Intelenet Agreement”. There is intense competition around the world for skilled business process and technical professionals and we expect that competition to increase, which could result in our outsourcing strategy not having the favorable economic impact currently projected. Operations in other parts of the world involve certain regional geopolitical risks that are different than operating in the United States, including the possibility of civil unrest, terrorism and substantial regulation by the individual governments. In addition, federal and state regulators have expressed concerns regarding the impact of offshoring on American business in general, including, for example, job loss, security and privacy concerns. These factors may cause disruptions in our business processes which could have a material adverse impact on our operations. We also may experience negative reactions from federal and state regulators, payors, patients and referral sources as a result of the actual or perceived concerns caused by the outsourcing of portions of our business operations including increases in accounts receivables or reserves, write-offs of accounts receivables and/or loss of revenues.
Non-Compliance With the Requirements of Coram’s Certification of Compliance Agreement Could Result in the Imposition of Significant Penalties and Sanctions.
As part of our acquisition of Coram, we assumed Coram’s obligations including those imposed on Coram as part of its three-year Certification of Compliance Agreement with the U.S. Department of Health and Human Services’ (“HHS”) Office of Inspector General (“OIG”), which requires Coram to maintain a compliance
30
Table of Contents
program, monitor and ensure compliance with federal healthcare program requirements and submit timely reports to the government regarding the same. The year 2009 marked the second year of the three-year agreement; in both 2008 and 2009, the OIG accepted our annual certification documents as filed. Violation of the terms of the Compliance Agreement could result in the imposition of significant penalties and sanctions, including disqualification from Medicare and other reimbursement programs.
Our Failure to Maintain Required Licenses Could Impact Our Operations.
We are required to maintain a significant number of state and/or federal licenses for our operations and facilities. Certain employees—primarily those with clinical expertise in pharmacy, nursing, respiratory therapy and nutrition—are required to maintain licenses in the states in which they practice. We manage the facility licensing function centrally. In addition, individual clinical employees are responsible for obtaining, maintaining and renewing their professional licenses and we also have processes in place designed to notify branch or pharmacy managers of renewal dates for the clinical employees under their supervision. State and federal licensing requirements are complex and often open to subjective interpretation by various regulatory agencies. In addition, from time to time, we may become subject to new or different licensing requirements due to legislative or regulatory requirements developments or changes in our business, and such developments may cause us to make further changes in our business, the results of which may be material. Although we believe we have the right systems in place to monitor licensure, violations of licensing requirements may occur and our failure to acquire or maintain appropriate licensure for our operations, facilities and clinicians could result in interruptions in our operations, refunds to state and/or federal payors, sanctions or fines, which could have an adverse material impact on our business, financial condition, results of operation, cash flow, capital resources and liquidity.
Our Failure to Maintain Accreditation Could Impact Our Operations.
Accreditation is required by most of our managed care payors and is a mandatory requirement for all Medicare DMEPOS providers effective October 1, 2009. In late June 2010, The Joint Commission completed its triennial survey cycle and we expect it to renew our three-year accreditation. If we or any of our branches lose accreditation, or if any of our new branches are unable to become accredited, our failure to maintain accreditation or become accredited could have a material adverse effect on our business, financial condition, results of operations, cash flow, capital resources and liquidity.
Political and Economic Conditions and the Recent Financial Turmoil in the United States and Global Capital and Credit Markets As Well As Significant Global or Regional Developments Such As Economic and Political Events, International Conflicts and Natural Disasters That are Out of Our Control Could Adversely Affect Our Revenue and Results of Operations and Overall Financial Growth and Could Have a Material Adverse Effect on Us.
Our business can be affected by a number of factors that are beyond our control such as general geopolitical, economic and business conditions, conditions in the financial services markets, and general political and economic developments. For example, the costs of military and security activities, government expenditures to support or bail out financial institutions or the U.S. credit markets in light of recent significant declines and volatility in the financial markets, or prolonged relief efforts in response to a natural disaster could increase pressure to reduce government expenditures for other purposes, including government-funded programs such as Medicare and Medicaid. In addition, reductions in reimbursement from Medicare and Medicaid programs could result if there is a significant change in government spending priorities. Any such reimbursement reductions could have a material adverse effect on our business, financial condition, results of operations, cash flow, capital resources and liquidity.
Recent turmoil in the financial markets, including in the capital and credit markets, the present economic slowdown and the uncertainty over its breadth, depth and duration may continue to put pressure on the global economy and could have a negative effect on our business. Further, the recent worldwide financial and credit
31
Table of Contents
turmoil has reduced the availability of liquidity and credit to fund the continuation and expansion of business operations worldwide. The shortage of liquidity and credit combined with recent substantial losses in worldwide equity markets could extend the economic recession in the United States or worldwide. As widely reported, financial markets in the United States, Europe and Asia have experienced extreme disruption, including, among other things, extreme volatility in security prices, severely diminished liquidity and credit availability, rating downgrades of certain investments and declining valuations of others. Governments have taken unprecedented actions intended to address extreme market conditions that include severely restricted credit and declines in real estate values. There can be no assurance that the deterioration in financial markets will not impair our ability to obtain financing in the future, including, but not limited to, our ability to draw on funds under our ABL Facility and our ability to incur additional indebtedness. If conditions in the global economy, U.S. economy or other key vertical or geographic markets remain uncertain or weaken further, we could experience material adverse impacts on our business, financial condition, results of operations, cash flow, capital resources and liquidity.
Our Strategic Growth Plan, Which Involves the Acquisition of Other Companies, May Not Succeed.
Our strategic growth plan involves, in part, the acquisition of other companies such as our 2007 acquisition of Coram. Such growth involves a number of risks, including:
| • | difficulties related to combining previously separate businesses into a single unit, including product and service offerings, distribution and operational capabilities and business cultures; |
| • | availability of financing to the extent needed to fund acquisitions; |
| • | customer loss and other general business disruption; |
| • | managing the integration process while completing other independent acquisitions or dispositions; |
| • | diversion of management’s attention from day-to-day operations; |
| • | assumption of liabilities of an acquired business, including unforeseen or contingent liabilities or liabilities in excess of the amounts estimated; |
| • | failure to realize anticipated benefits and synergies, such as cost savings and revenue enhancements; |
| • | potentially substantial costs and expenses associated with acquisitions and dispositions; |
| • | failure to retain and motivate key employees; |
| • | coordinating research and development activities to enhance the introduction of new products and services; and |
| • | difficulties in applying our internal control over financial reporting and disclosure controls and procedures to an acquired business. |
We May Not Be Able to Realize Anticipated Cost Savings, Revenue Enhancements or Synergies From the Transactions or From Our Acquisitions.
We may not be able to realize the potential cost savings, synergies and revenue enhancements that we anticipate from the Transactions or from our acquisitions, either in the amount or within the time frame that we expect, and the costs of achieving these benefits may be higher than, and the timing may differ from, what we expect. Our ability to realize anticipated cost savings, synergies and revenue enhancements may be affected by a number of factors, including, but not limited to, the following:
| • | the use of more cash or other financial resources on integration and implementation activities than we expect; |
| • | increases in other expenses unrelated to the Transactions or our acquisitions, which may offset the cost savings and other synergies from those transactions; |
32
Table of Contents
| • | our ability to eliminate effectively duplicative back office overhead and overlapping and redundant selling, general and administrative functions; and |
| • | our ability to avoid labor disruptions in connection with any integration, particularly in connection with any headcount reduction. |
In addition, estimated cost savings are only estimates and may not actually be achieved in the timeframe anticipated or at all. If we fail to realize anticipated cost savings, synergies or revenue enhancements, our financial results will be adversely affected, and we may not generate the cash flow from operations that we anticipated, or that is sufficient to repay our indebtedness.
There is an Inherent Risk of Liability in the Provision of Healthcare Services; Damage to Our Reputation or Our Failure to Adequately Insure Against Losses Could Have a Material Adverse Effect on Our Operations, Financial Condition or Prospects.
There is an inherent risk of liability in the provision of healthcare services. As participants in the healthcare industry, we expect to periodically be subject to lawsuits, some of which may involve large claims and significant costs to defend. In that case, the coverage limits under our insurance programs may not be adequate to protect us. We also cannot be assured that we will be able to maintain this insurance on acceptable terms in the future. A successful claim in excess of our coverage could have a material adverse effect upon our business, financial condition, results of operations, cash flow, capital resources and liquidity. Even where our insurance is adequate to cover claims against us, damage to our reputation in the event of a judgment against us could have an adverse effect on our business, financial condition, results of operations, cash flow, capital resources, liquidity or prospects.
We Experience Competition From Numerous Other Home Respiratory/Home Medical Equipment and Home Infusion Therapy Service Providers, and This Competition Could Adversely Affect Our Revenues and Our Business.
The home respiratory/home medical equipment and home infusion therapy markets are highly competitive and include a large number of providers, some of which are national providers, but most of which are either regional or local providers. We believe that the primary competitive factors are quality considerations such as responsiveness, the technical ability of the professional staff and the ability to provide comprehensive services. These markets are very fragmented. Some of our competitors may now or in the future have greater financial or marketing resources than we do. In addition, in certain markets, competitors may have more effective sales and marketing activities. Our largest national home respiratory/home medical equipment provider competitors are American HomePatient, Inc., Lincare Holdings, Inc. and Rotech Healthcare Inc. Our largest competitors in the home infusion therapy service market are Walgreens/OptionCare and Accredo/Critical Care Systems. The rest of the market in the United States consists of several medium-size competitors, as well as numerous small (under $3.5 million in revenues) local operations. There are relatively few barriers to entry in local home healthcare markets. We cannot assure you that the competitive nature of the homecare environment will not adversely affect our revenues and our business.
Our Business Operations are Labor Intensive. Difficulty Hiring Enough Additional Management and Other Employees, Increasing Costs of Compensation or Employee Benefits, and the Potential Impact of Unionization and Organizing Activities Could Have an Adverse Effect on Our Costs and Results of Operations.
The success of our business depends upon our ability to attract and retain highly motivated, well-qualified management and other employees. One of our largest costs is in the payment of salaries and benefits to our approximately 11,400 employees. We face significant competition in the recruitment of qualified employees, which has caused increased salary and wage rates among certain employee groups. If we are unable to recruit or
33
Table of Contents
retain a sufficient number of qualified employees, or if the costs of compensation or employee benefits increase substantially, our ability to deliver services effectively could suffer and our profitability would likely be adversely affected. The Reform Package may materially increase our cost of providing health benefits to our employees and their dependents. In addition, union organizing activities have occurred in the past and may occur in the future, and the adverse impact of unionization and organizing activities on our costs and operating results could be substantial.
We are Highly Dependent Upon Senior Management; Our Failure to Attract and Retain Key Members of Senior Management Could Have a Material Adverse Effect on Us.
We are highly dependent on the performance and continued efforts of our senior management team. Our future success is dependent on our ability to continue to attract and retain qualified executive officers and senior management. Any inability to manage our operations effectively could have a material adverse effect on our business, financial condition, results of operations, cash flow, capital resources and liquidity.
Our Reliance on Relatively Few Suppliers for the Majority of Our Patient Service Equipment, Pharmaceuticals and Supplies and New Excise Taxes Which Are To Be Imposed on Certain Manufacturers of Such Items Could Adversely Affect Our Ability to Operate.
We currently rely on a relatively small number of suppliers to provide us with the majority of our patient service equipment, pharmaceuticals and supplies. Significant price increases, or disruptions in the ability to obtain such equipment, pharmaceuticals and supplies from existing suppliers, may force us to use alternative suppliers. Additionally, the Reform Package calls for significant new excise taxes to be imposed on manufacturers of certain medical equipment and pharmaceuticals—taxes which they could attempt to pass on to customers such as us. Such manufacturers may be forced to make other changes to their products or manufacturing processes that are unacceptable to us, resulting in our desire to change suppliers. Any change in suppliers we use could cause delays in the delivery of such products and possible losses in revenue, which could adversely affect our results of operations. In addition, alternative suppliers may not be available, or may not provide their products and services at similar or favorable prices. If we cannot obtain the patient service equipment, pharmaceuticals and supplies we currently use, or alternatives at similar or favorable prices, our ability to provide such products may be severely impacted, which could have an adverse effect on our business, financial condition, results of operations, cash flow, capital resources and liquidity.
Our Failure to Establish and Maintain Relationships With Hospital and Physician Referral Sources May Cause Our Revenue to Decline.
Our success is partly dependent on referrals from hospital and physician sources. If we are unable to successfully establish new referral sources and maintain strong relationships with our current referral sources, or if efforts to increase the skill level and effectiveness of our sales force fail, our revenues may decline.
Changes in Medical Equipment Technology and Development of New Treatments May Cause Our Current Equipment or Services to Become Obsolete.
We evaluate changes in home medical equipment technology and treatments on an ongoing basis for purposes of determining the feasibility of replacing or supplementing items currently included in the patient service equipment inventory and services that we offer our customers. The selection of medical equipment and services we offer is formulated on the basis of a variety of factors, including overall quality, functional reliability, availability of supply, payor reimbursement policies, product features, labor costs associated with the technology, acquisition, repair and ownershipcosts and overallpatient and referral source demand, as well as patient therapeutic and lifestyle benefits. Manufacturers continue to invest in research and development to introduce new products to the marketplace. It is possible that major changes in available technology, payor benefit or coverage policies related to those changes, or the preferences of patients and referral sources may cause our current
34
Table of Contents
product offerings to become less competitive or obsolete, and it will be necessary for us to adapt to those changes. We endeavor to anticipate industry trends and initiate new product offerings in a way that minimizes the financial impact of increased cost of goods sold, equipment replacement costs and other expenses associated with changes in technology and demand. For example, Medicare DMEPOS competitive bidding regulations contain policies relating to the provision of products covered by the program. However, unanticipated changes could cause us to incur increased capital expenditures and accelerated equipment write-offs, and could force us to alter our sales, operations and marketing strategies.
Our Operations Involve the Transport of Compressed and Liquid Oxygen, Which Carries an Inherent Risk of Rupture or Other Accidents With the Potential to Cause Substantial Loss.
Our operations are subject to the many hazards inherent in the transportation of medical gas products and compressed and liquid oxygen, including ruptures, leaks and fires. These risks could result in substantial losses due to personal injury or loss of life, severe damage to and destruction of property and equipment and pollution or other environmental damage and may result in curtailment or suspension of our related operations. If a significant accident or event occurs, it could adversely affect our financial position and results of operations.
Our Medical Gas Facilities and Operations are Subject to Extensive Regulation by Federal and State Authorities and There Can Be No Assurance That Our Medical Gas Facilities Will Achieve and Maintain Compliance With Such Regulations.
We have a number of medical gas facilities in several states subject to federal and state regulatory requirements. Our medical gas facilities and operations are subject to extensive regulation by the Food and Drug Administration (“FDA”) and other federal and state authorities. The FDA regulates medical gases, including medical oxygen, pursuant to its authority under the federal Food, Drug and Cosmetic Act (“FFDCA”). Among other requirements, the FDA’s current Good Manufacturing Practice (“cGMP”) regulations impose certain quality control, documentation and recordkeeping requirements on the receipt, processing and distribution of medical gas. Further, in each state in which we do business, our medical gas facilities are subject to regulation under state health and safety laws, which vary from state to state. The FDA and state authorities conduct periodic, unannounced inspections at medical gas facilities to assess compliance with the cGMP and other regulations, and we expend significant time, money and resources in an effort to achieve substantial compliance with the cGMP regulations and other federal and state law requirements at each of our medical gas facilities. In the fourth quarter of 2009, the FDA changed its methodology for medical gas providers to register their sites with the agency; we complied with the regulation. There can be no assurance, however, that these efforts will be successful and that our medical gas facilities will achieve and maintain compliance with federal and state law regulations. Our failure to achieve and maintain regulatory compliance at our medical gas facilities could result in enforcement action, including warning letters, fines, product recalls or seizures, temporary or permanent injunctions, or suspensions in operations at one or more locations, and civil or criminal penalties which would materially harm our business, financial condition, results of operations, cash flow, capital resources and liquidity.
We have in the Past Identified a Material Weakness in Our Internal Controls Over Financial Reporting as it Relates to the Calculation of Accounts Receivable Reserves. If We Do Not Maintain Effective Internal Controls Over Financial Reporting, We Could Fail to Accurately Report Our Financial Results.
We have in the past identified a material weakness in our internal control over financial reporting. In light of this material weakness in internal control over financial reporting, we also concluded that our disclosure controls and procedures were not effective as of certain dates in 2007 and 2008.
A material weakness is defined by the standards issued by the Public Company Accounting Oversight Board as a reasonable possibility that a material misstatement of the annual or interim financial statements will not be prevented or detected. We did not effectively design and perform control activities to prevent or detect material misstatements that might exist in our reserve for uncollectible accounts receivable. Specifically, we did not
35
Table of Contents
perform an analysis with a sufficient level of detail to support management’s estimate of the reserve for uncollectible accounts receivable.
During 2008, we implemented a remediation program designed to address such material weakness. In the fourth quarter of 2008, we concluded that our remediation program was operating effectively and as of December 31, 2008, management concluded that the material weakness was remediated and did not exist as of that date. If our remediation efforts are insufficient to address the material weaknesses, or if additional material weaknesses in our internal controls are discovered in the future, they may adversely affect our ability to record, process, summarize and report financial information timely and accurately and, as a result, our financial statements may contain material misstatements or omissions.
We have completed a number of acquisitions in the past several years, and may continue to pursue growth through strategic acquisitions. Among the risks associated with acquisitions are the risks of control deficiencies that result from the integration of the acquired business.
It is possible that control deficiencies could be identified by our management or by our independent auditing firm in the future or may occur without being identified. Such a failure could result in regulatory scrutiny, cause investors to lose confidence in our reported financial condition, lead to a default under our indebtedness and otherwise materially adversely affect our business and financial condition.
Affiliates of the Sponsor Own Substantially All of the Equity Interests in Us and May Have Conflicts of Interest With Us or the Holders of the Notes in the Future.
As a result of the Merger, investment funds affiliated with the Sponsor collectively own a substantial majority of our capital stock, and the Sponsor designees hold a majority of the seats on our Board of Directors. As a result, affiliates of the Sponsor have control over our decisions to enter into any corporate transaction and have the ability to prevent any transaction that requires the approval of stockholders regardless of whether holders of our Notes believe that any such transactions are in their own best interests. For example, affiliates of the Sponsor could collectively cause us to make acquisitions that increase the amount of our indebtedness or to sell assets, or could cause us to issue additional capital stock or declare dividends. So long as investment funds affiliated with the Sponsor continue to indirectly own a significant amount of the outstanding shares of our common stock, affiliates of the Sponsor will continue to be able to strongly influence or effectively control our decisions. The indenture governing the Notes and the credit agreement governing our ABL Facility permit us to pay advisory and other fees, dividends and make other restricted payments to the Sponsor under certain circumstances and the Sponsor or its affiliates may have an interest in our doing so. In addition, the Sponsor has no obligation to provide us with any additional debt or equity financing.
Additionally, the Sponsor is in the business of making investments in companies and may from time to time acquire and hold interests in businesses that compete directly or indirectly with us or that supply us with goods and services. For example, the Sponsor controls Intelenet, an Indian company with which we contracted in 2009 to assist us with the outsourcing of certain revenue management functions. The Sponsor may also pursue acquisition opportunities that may be complementary to our business and, as a result, those acquisition opportunities may not be available to us. The holders of the Notes should consider that the interests of the Sponsor and other members of the Investor Group may differ from their interests in material respects. See “Security Ownership of Principal Shareholders and Management,” “Certain Relationships and Related Party Transactions,” “Description of Notes,” and “Description of Other Indebtedness.”
Proposed Federal Legislation, If Passed, Would Encourage Greater Unionization and Could Materially Impact Our Labor Costs and Customer Service Provided to Patients.
It is possible that the U.S. Congress will pass the Employee Free Choice Act, legislation which would change existing laws concerning union representation. The proposed Employee Free Choice Act would in certain circumstances eliminate the secret ballot voting process, shorten the time window in which a contract negotiation
36
Table of Contents
between an employee and a labor union must take place and mandate arbitration of contract terms if a negotiated contract is not met within certain timeframes. While the ultimate outcome of this legislation is still unclear, any increased union representation within the homecare industry or mandatory arbitration of contract terms would potentially increase labor and other operating expenses. Additional unionization could also negatively impact our ability to provide high quality service to patients in the event of a strike or other work stoppage.
Our Ability to Retain Certain Hospital-Based Referral Revenue is Contingent on the Quality of Our Referral Process and Patient Service.
For over a decade, we have implemented a contractual business model with a number of hospitals which facilitates continuity of care and quality for patients who are being discharged from those hospitals to the homecare setting. We discontinued most of these arrangements in 2009. In these cases, we continue to work closely with the hospitals to accept discharges for their patients who require our services. However, the dissolution of the contractual relationship may result in the decision by hospitals to refer patients to our competitors in lieu of or in addition to us. We are not able to predict whether the discontinuance of these hospital arrangements will have a material impact on our overall operational and financial results.
Our Payor Contracts are Subject to Renegotiation or Termination Which Could Result in a Decrease in Our Revenue and Profits.
From time to time, our payor contracts are amended, renegotiated or terminated altogether. Sometimes in the renegotiation process, certain lines of business may not be renewed or a payor may enlarge its provider network or otherwise adversely change the way it conducts its business with us. In other cases, a payor may reduce its provider network in exchange for lower payment rates. Our revenue from a payor may also be adversely affected if the payor alters its administrative procedures for payments and audits or changes its order of preference among the providers to which it refers business. We cannot assure you that our payor contracts will not be terminated or altered in ways that are unfavorable to us as a result of renegotiation or such administrative changes.
Risks Relating to the Notes
Our Substantial Indebtedness Could Adversely Affect Our Financial Condition and Prevent Us From Fulfilling Our Obligations Under the Notes.
We have a substantial amount of debt, which requires significant interest and principal payments. As of March 31, 2010, we had approximately $1,020.7 million of total debt outstanding. Subject to the limits contained in the credit agreement governing our ABL Facility, the indenture governing the Notes and our other debt instruments, we may be able to incur substantial additional debt from time to time to finance working capital, capital expenditures, investments or acquisitions, or for other purposes. If we do so, the risks related to our high level of debt could intensify. Specifically, our high level of debt could have important consequences to the holders of the Notes, including the following:
| • | making it more difficult for us to satisfy our obligations with respect to the Notes and our other debt; |
| • | limiting our ability to obtain additional financing to fund future working capital, capital expenditures, acquisitions or other general corporate requirements; |
| • | requiring a substantial portion of our cash flows to be dedicated to debt service payments instead of other purposes, thereby reducing the amount of cash flows available for working capital, capital expenditures, acquisitions and other general corporate purposes; |
| • | increasing our vulnerability to general adverse economic and industry conditions; |
| • | exposing us to the risk of increased interest rates as certain of our borrowings may be at variable rates of interest; |
37
Table of Contents
| • | limiting our flexibility in planning for and reacting to changes in the industry in which we compete; |
| • | placing us at a disadvantage compared to other, less leveraged competitors; and |
| • | increasing our cost of borrowing. |
Our Variable Rate Indebtedness Subjects Us to Interest Rate Risk, Which Could Cause Our Indebtedness Service Obligations to Increase Significantly.
Borrowings under our ABL Facility are at variable rates of interest and expose us to interest rate risk. If interest rates increase, our debt service obligations on the variable rate indebtedness would increase even though the amount borrowed remained the same, and our net income and cash flows, including cash available for servicing our indebtedness, would correspondingly decrease.
We May Be Unable to Service Our Indebtedness, Including the Notes.
Our ability to make scheduled payments on and to refinance our indebtedness, including the Notes, depends on and is subject to our financial and operating performance, which in turn is affected by general and regional economic, financial, competitive, business and other factors beyond our control, including the availability of financing in the international banking and capital markets. We cannot assure you that our business will generate sufficient cash flow from operations or that future borrowings will be available to us in an amount sufficient to enable us to service our debt, including the Notes, to refinance our debt or to fund our other liquidity needs. If we are unable to meet our debt service obligations or to fund our other liquidity needs, we will need to restructure or refinance all or a portion of our debt, including the Notes, which could cause us to default on our debt obligations and impair our liquidity. Any refinancing of our indebtedness could be at higher interest rates and may require us to comply with more onerous covenants that could further restrict our business operations.
The Indenture Governing the Notes and the Credit Agreement Governing Our ABL Facility Impose Significant Operating and Financial Restrictions on Our Company and Our Subsidiaries, Which May Prevent Us From Capitalizing on Business Opportunities.
The indenture governing the Notes and the credit agreement governing our ABL Facility impose significant operating and financial restrictions on us. These restrictions will limit our ability, among other things, to:
| • | incur additional indebtedness or enter into sale and leaseback obligations; |
| • | pay certain dividends or make certain distributions on our capital stock or repurchase or redeem our capital stock; |
| • | make certain capital expenditures; |
| • | make certain loans, investments or other restricted payments; |
| • | place restrictions on the ability of our subsidiaries to pay dividends or make other payments to us; |
| • | engage in transactions with stockholders or affiliates; |
| • | sell certain assets or engage in mergers, acquisitions and other business combinations; |
| • | amend or otherwise alter the terms of our indebtedness; |
| • | alter the business that we conduct; |
| • | guarantee indebtedness or incur other contingent obligations; and |
| • | create liens. |
Our ABL Facility also includes financial covenants. Our ability to comply with these covenants is dependent on our future performance, which will be subject to many factors, some of which are beyond our control.
38
Table of Contents
As a result of these covenants and restrictions, we are limited as to how we conduct our business and we may be unable to raise additional debt or equity financing to compete effectively or to take advantage of new business opportunities. The terms of any future indebtedness we may incur could include more restrictive covenants. We cannot assure you that we will be able to maintain compliance with these covenants in the future and, if we fail to do so, that we will be able to obtain waivers from the lenders and/or amend the covenants.
Our failure to comply with the restrictive covenants described above as well as other terms of our existing indebtedness and/or the terms of any future indebtedness from time to time could result in an event of default, which, if not cured or waived, could result in our being required to repay these borrowings before their due date. If we are forced to refinance these borrowings on less favorable terms, our results of operations and financial condition could be adversely affected.
Claims of Holders of the Series A-2 Notes Will Be Effectively Subordinated to Claims of Lenders Under the ABL Facility to the Extent of the Value of the Collateral Securing the ABL Facility on a First-Priority Lien Basis.
The Series A-2 Notes are secured on a first-priority lien basis by the Notes Collateral (as defined below) and on a second-priority lien basis by the ABL Collateral (as defined below). The Series A-2 Notes and the related guarantees will be effectively subordinated in right of payment to all of our and our subsidiary guarantors’ secured indebtedness under the ABL Facility to the extent of the value of the collateral securing the ABL Facility on a first-priority lien basis. In the event of a bankruptcy, liquidation, dissolution, reorganization or similar proceeding against us, the assets that are securing indebtedness under the ABL Facility on a first-priority lien basis must first be used to pay the first-priority claims under the ABL Facility in full before these assets may be used to make any payments on the Series A-2 Notes. After claims of the lenders under the ABL Facility have been satisfied in full, to the extent of the value of the collateral securing the ABL Facility on a first-priority lien basis, there may be no assets remaining under the ABL Collateral that may be applied to satisfy the claims of holders of the Series A-2 Notes.
Claims of Holders of the Notes Will Be Structurally Subordinated to Claims of Creditors of Certain of Our Subsidiaries That Will Not Guarantee the Notes.
The Notes may not be guaranteed by certain of our future subsidiaries, including all of our non-U.S. subsidiaries. Accordingly, claims of holders of the Notes will be structurally subordinated to the claims of creditors of these non-guarantor subsidiaries, including trade creditors. All obligations of our non-guarantor subsidiaries will have to be satisfied before any of the assets of such subsidiaries would be available for distribution, upon a liquidation or otherwise, to us or a guarantor of the Notes. The indenture governing the Notes will permit these subsidiaries to incur certain additional debt and will not limit their ability to incur other liabilities that are not considered indebtedness under the indenture.
Our Failure to Comply With the Agreements Relating to Our Outstanding Indebtedness, Including as a Result of Events Beyond Our Control, Could Result in an Event of Default That Could Materially and Adversely Affect Our Results of Operations and Our Financial Condition.
If there were an event of default under any of the agreements relating to our outstanding indebtedness, the holders of the defaulted debt could cause all amounts outstanding with respect to that debt to be due and payable immediately. We cannot assure you that our assets or cash flow would be sufficient to fully repay borrowings under our outstanding debt instruments if accelerated upon an event of default. Further, if we are unable to repay, refinance or restructure our indebtedness under our secured debt, the holders of such debt could proceed against the collateral securing that indebtedness. In addition, any event of default or declaration of acceleration under one debt instrument could also result in an event of default under one or more of our other debt instruments.
39
Table of Contents
Federal and State Statutes May Allow Courts, Under Specific Circumstances, to Void the Notes and the Guarantees, Subordinate Claims in Respect of the Notes and the Guarantees and/or Require Holders of the Notes to Return Payments Received From Us.
Under the federal bankruptcy laws and comparable provisions of state fraudulent transfer laws, the Notes and the guarantees could be voided, or claims in respect of the Notes and the guarantees could be subordinated to all of our other debt, if the issuance of the Notes or a guarantee was found to have been made for less than their reasonable equivalent value and we, at the time we incurred the indebtedness evidenced by the Notes:
| • | were insolvent or rendered insolvent by reason of such indebtedness; |
| • | were engaged in, or about to engage in, a business or transaction for which our remaining assets constituted unreasonably small capital; or |
| • | intended to incur, or believed that we would incur, debts beyond our ability to pay such debts as they mature. |
A court might also void the issuance of Notes or a guarantee, without regard to the above factors, if the court found that we issued the Notes or the guarantors entered into their respective guarantees with actual intent to hinder, delay or defraud our or their respective creditors.
A court would likely find that we or a guarantor did not receive reasonably equivalent value or fair consideration for the Notes or the guarantees, respectively, if we or a guarantor did not substantially benefit directly or indirectly from the issuance of the Notes. If a court were to void the issuance of the Notes or the guarantees, you would no longer have a claim against us or the guarantors. Sufficient funds to repay the Notes may not be available from other sources, including the remaining guarantees, if any. In addition, the court might direct you to repay any amounts that you already received from us or the guarantors with respect to the Notes.
In addition, any payment by us pursuant to the Notes made at a time we were found to be insolvent could be voided and required to be returned to us or to a fund for the benefit of our creditors if such payment is made to an insider within a one-year period prior to a bankruptcy filing or within 90 days for any outside party and such payment would give the creditors more than such creditors would have received in a distribution under Title 11 of the United States Code, as amended (the “Bankruptcy Code”).
The measures of insolvency for purposes of these fraudulent transfer laws will vary depending upon the law applied in any proceeding to determine whether a fraudulent transfer has occurred. Generally, however, we would be considered insolvent if:
| • | the sum of our debts, including contingent liabilities, were greater than the fair saleable value of all our assets; |
| • | the present fair saleable value of our assets were less than the amount that would be required to pay our probable liability on existing debts, including contingent liabilities, as they become absolute and mature; or |
| • | we could not pay our debts as they become due. |
In addition, although each guarantee will contain a provision intended to limit that guarantor’s liability to the maximum amount that it could incur without causing the incurrence of obligations under its guarantee to be a fraudulent transfer, this provision may not be effective to protect those guarantees from being voided under fraudulent transfer law, or may reduce that guarantor’s obligation to an amount that effectively makes its guarantee worthless.
Finally, as a court of equity, the bankruptcy court may subordinate the claims in respect of the Notes to other claims against us under the principle of equitable subordination, if the court determines that: (i) the holder of the Notes engaged in some type of inequitable conduct; (ii) such inequitable conduct resulted in injury to our
40
Table of Contents
other creditors or conferred an unfair advantage upon the holder of the Notes; and (iii) equitable subordination is not inconsistent with the provisions of the Bankruptcy Code.
Holders of the Notes May Not Be Able to Fully Realize the Value of Their Liens.
The security interests and liens for the benefit of holders of the Notes may be released without such holders’ consent in specified circumstances. In particular, the security documents governing the Notes and our ABL Facility generally provide for an automatic release of all liens on any asset securing our ABL Facility on a first-priority basis and that is disposed of in compliance with the provisions of our ABL Facility and the indenture governing the Notes. As a result, we cannot assure holders of the Notes that the Notes will continue to be secured by a substantial portion of our assets. In addition, the capital stock of our subsidiaries will be excluded from the collateral to the extent liens thereon would trigger reporting obligations under Rule 3-16 of Regulation S-X, which requires financial statements from any company whose securities are collateral if its book value or market value, whichever is greater, would exceed 20% of the principal amount of the Notes secured thereby.
The Collateral May Not Be Valuable Enough to Satisfy All the Obligations Secured by Such Collateral.
The Notes are secured on a first-priority lien basis (subject to certain exceptions) by substantially all of our and the guarantors’ assets (other than accounts receivable, inventory and cash and proceeds and products of the foregoing and certain assets related thereto) (the “Notes Collateral”) and such collateral may be shared in certain circumstances with our future creditors; provided that the Series A-1 Notes are entitled to a priority of payment over our Series A-2 Notes in certain circumstances, including upon any acceleration of the obligations under the Notes or any bankruptcy or insolvency event of default with respect to the Issuer or any guarantor. The actual value of the Notes Collateral at any time will depend upon market and other economic conditions. The Notes are also secured on a second-priority lien basis (subject to certain exceptions) by our and each guarantor’s accounts receivable, inventory and cash and proceeds and products of the foregoing and certain assets related thereto (the “ABL Collateral”). The ABL Collateral will be subject to a first-priority security interest for the benefit of the lenders under our ABL Facility, and may be shared with our future creditors. Although the holders of obligations secured by first-priority liens on the ABL Collateral and the holders of obligations secured by second-priority liens on the ABL Collateral, including the Notes, will share in the proceeds of the ABL Collateral, the holders of obligations secured by first-priority liens in the ABL Collateral will be entitled to receive proceeds from any realization of the ABL Collateral to repay the obligations held by them in full before the holders of the Notes and the holders of other obligations secured by second-priority liens in the ABL Collateral receive any such proceeds.
In addition, the asset sale covenant and the definition of asset sale in the indenture governing the Notes have a number of significant exceptions pursuant to which we will be able to sell Notes Collateral without being required to reinvest the proceeds of such sale into assets that will comprise Notes Collateral or to make an offer to the holders of the Notes to repurchase the Notes.
All indebtedness under our ABL Facility will be secured by first-priority liens on the ABL Collateral (subject to certain exceptions). In addition, under the terms of the indenture governing the Notes, we may grant certain additional liens on any property or asset that constitutes ABL Collateral. Any grant of additional liens on the ABL Collateral would further dilute the value of the second-priority lien on the ABL Collateral securing the Notes. Further, as discussed above, we will be permitted under the terms of the indenture governing the Notes to sell all assets that constitute ABL Collateral and not apply the proceeds to invest in additional assets that will secure the Notes or repay outstanding indebtedness. The value of the pledged assets in the event of a liquidation will depend upon market and economic conditions, the availability of buyers and similar factors. No independent appraisals of any of the pledged property have been prepared by or on behalf of us in connection with the Notes. Accordingly, we cannot assure holders of the Notes that the proceeds of any sale of the pledged assets following an acceleration to maturity with respect to the Notes would be sufficient to satisfy, or would not be substantially less than, amounts due on the Notes and the other debt secured thereby. If the proceeds of any sale of the pledged assets were not sufficient to repay all amounts due on the Notes, the holders of the Notes (to the extent their Notes were not repaid from the proceeds of the sale of the
41
Table of Contents
pledged assets) would have only an unsecured claim against our remaining assets. By their nature, some or all of the pledged assets may be illiquid and may have no readily ascertainable market value. Likewise, we cannot assure holders of the Notes that the pledged assets will be saleable or, if saleable, that there will not be substantial delays in their liquidation. To the extent that liens, rights and easements granted to third parties encumber assets located on property owned by us or constitute subordinate liens on the pledged assets, those third parties may have or may exercise rights and remedies with respect to the property subject to such encumbrances (including rights to require marshalling of assets) that could adversely affect the value of the pledged assets located at that site and the ability of the collateral agent to realize or foreclose on the pledged assets at that site.
In addition, the indenture governing the Notes permits us to issue additional secured debt, including debt secured equally and ratably by the same assets pledged for the benefit of the holders of the Notes and entitled to a priority of payment over the Series A-2 Notes to the same extent as the Series A-1 Notes. For example, we are permitted to issue additional Series A-1 Notes ($150.0 million at any time and an additional $150.0 million for acquisitions under certain circumstances), and we are permitted to incur up to an additional $200.0 million of debt which ranks equally and ratably with the Series A-1 Notes. This could reduce amounts payable to holders of the Series A-2 Notes from the proceeds of any sale of the collateral.
The Rights of Holders of the Notes With Respect to the ABL Collateral Will Be Substantially Limited by the Terms of the Intercreditor Agreement.
Under the terms of the intercreditor agreement which was entered into in connection with our ABL Facility, at any time that obligations that have the benefit of the first-priority liens on the ABL Collateral are outstanding, any actions that may be taken in respect of the ABL Collateral, including the ability to cause the commencement of enforcement proceedings against the ABL Collateral and to control the conduct of such proceedings, and the approval of amendments to, releases of ABL Collateral from the lien of, and waivers of past defaults under, the security documents, will be at the direction of the holders of the obligations secured by the first-priority liens. Neither the trustee nor the collateral agent, on behalf of the holders of the Notes, will have the ability to control or direct such actions, even if the rights of the holders of the Notes are adversely affected, subject to certain exceptions. See “Description of Notes—Security for the Notes” and “Description of Notes—Amendment, Supplement and Waiver.” Under the terms of the intercreditor agreement, at any time that obligations that have the benefit of the first-priority liens on the ABL Collateral are outstanding, if the holders of such indebtedness release the ABL Collateral for any reason whatsoever, including, without limitation, in connection with any sale of assets, the second-priority security interest in such ABL Collateral securing the Notes will be automatically and simultaneously released without any consent or action by the holders of the Notes, subject to certain exceptions. The ABL Collateral so released will no longer secure our and the guarantors’ obligations under the Notes. In addition, because the holders of the indebtedness secured by first-priority liens in the ABL Collateral control the disposition of the ABL Collateral, such holders could decide not to proceed against the ABL Collateral, regardless of whether there is a default under the documents governing such indebtedness or under the indenture governing the Notes. In such event, the only remedy available to the holders of the Notes would be to sue for payment on the Notes and the related guarantees under the indenture. In addition, the intercreditor agreement gives the holders of first-priority liens on the ABL Collateral the right to access and use the collateral that secures the Notes to allow those holders to protect the ABL Collateral and to process, store and dispose of the ABL Collateral.
The Rights of the Holders of the Notes With Respect to the Notes Collateral Will Be Substantially Limited by the Terms of the Intercreditor and Collateral Agency Agreement.
The relationship among the Series A-1 Notes and the Series A-2 Notes will be governed by an intercreditor and collateral agency agreement that was entered into in connection with the issuance of the Series A-1 Notes. This agreement describes, among other things, the obligations, powers and duties of the Notes Collateral Agent, actions and voting by the Series A-1 Notes and the Series A-2 Notes, the exercise of remedies, and the application of collateral proceeds. Pursuant to this agreement, the holders of the Series A-1 Notes (and certain
42
Table of Contents
other secured indebtedness we are permitted under the indenture to incur) will be entitled to a priority of payment over the holders of the Series A-2 Notes in respect of any amounts received from the Company or any guarantor (or from the proceeds of any Notes Collateral or ABL Collateral) following any acceleration of the obligations under the Notes or any bankruptcy or insolvency event of default with respect to the Company or any “significant subsidiary” under the Notes, whether received from the proceeds of an asset sale, reorganization, liquidation, sale pursuant to section 363 of the bankruptcy code, any adequate protection payments, or otherwise. Pursuant to this priority waterfall, the holders of the Series A-1 Notes must receive an amount equal to all obligations, including accrued and unpaid interest outstanding on or prior to the acceleration or filing date, owing to them in respect of the Series A-1 Notes on the date of any payment or other distribution or other receipt of proceeds (other than amounts calculated in respect of postpetition interest, including amounts payable as “adequate protection”) before the holders of the Series A-2 Notes are entitled to receive any distribution on account of the obligations owing to them in respect of the Series A-2 Notes. In a situation in which the priority waterfall is in effect, if the Notes Collateral was fully liquidated and the proceeds of collateral distributed to the holders of the Series A-1 Notes were not sufficient to repay in full all obligations owing to them in respect of the Series A-1 Notes, then there would not be sufficient collateral proceeds to provide a recovery to holders of the Series A-2 Notes. In such event, holders of the Series A-2 Notes would have only an unsecured claim against our remaining assets.
The Value of the Collateral Securing the Notes May Not Be Sufficient to Secure Post-Petition Interest.
In the event of a bankruptcy, liquidation, dissolution, reorganization or similar proceeding against us, holders of the Notes will only be entitled to post-petition interest under the Bankruptcy Code to the extent that the value of their security interest in the collateral is greater than their pre-bankruptcy claim. Holders of the Notes that have a security interest in collateral with a value equal or less than their pre-bankruptcy claim will not be entitled to post-petition interest under the Bankruptcy Code. No appraisal of the fair market value of the collateral has been prepared in connection with the Notes and we therefore cannot assure you that the value of the noteholders’ interest in the collateral equals or exceeds the principal amount of the Notes. In addition, the ability of the holders of the Notes to receive post-petition interest may be further limited by the intercreditor agreement that was entered into in connection with the offering of the Series A-1 Notes that defines the relative rights of the Notes. In particular, the provisions relating to the priority of payments of the Series A-1 Notes over the Series A-2 Notes in certain circumstances also provide that the holders of the Series A-2 Notes are entitled to postpetition interest only after all obligations, including any accrued and unpaid pre-petition interest, in respect of the Series A-1 Notes are satisfied in full. See “—The Collateral May Not Be Valuable Enough to Satisfy All the Obligations Secured by Such Collateral” and “Description of Notes—Priorities of Payment as between Series A-2 Debt (including the Series A-2 Notes) and Series A-1 Notes—Waterfall of Payment Following Acceleration or in Bankruptcy.”
The Waiver in the Intercreditor Agreement of Rights of Marshaling May Adversely Affect the Recovery Rates of Holders of the Notes in a Bankruptcy or Foreclosure Scenario.
The Notes and the guarantees are secured on a second-priority lien basis by the ABL Collateral. The intercreditor agreement provides that, at any time that obligations that have the benefit of the first-priority liens on the ABL Collateral are outstanding, the holders of the Notes, the trustee under the indenture governing the Notes and the collateral agent for the Notes may not assert or enforce any right of marshaling accorded to a junior lienholder, as against the holders of such indebtedness secured by first-priority liens in the ABL Collateral. Without this waiver of the right of marshaling, holders of such indebtedness secured by first-priority liens in the ABL Collateral would likely be required to liquidate collateral on which the Notes did not have a lien, if any, prior to liquidating the ABL Collateral, thereby maximizing the proceeds of the ABL Collateral that would be available to repay our obligations under the Notes. As a result of this waiver, the proceeds of sales of the ABL Collateral could be applied to repay any indebtedness secured by first-priority liens in the ABL Collateral before applying proceeds of other collateral securing indebtedness, and the holders of the Notes may recover less than they would have if such proceeds were applied in the order most favorable to the holders of the Notes.
43
Table of Contents
In the Event of a Bankruptcy of Us or Any of the Guarantors, Holders of the Notes May Be Deemed to Have an Unsecured Claim to the Extent That Our Obligations in Respect of the Notes Exceed the Fair Market Value of the Collateral Securing the Notes.
In any bankruptcy proceeding with respect to us or any of the guarantors, it is possible that the bankruptcy trustee, the debtor-in-possession or competing creditors will assert that the fair market value of the collateral with respect to the Notes on the date of the bankruptcy filing was less than the then-current principal amount of the Notes. Upon a finding by the bankruptcy court that the Notes are under-collateralized, the claims in the bankruptcy proceeding with respect to the Notes would be bifurcated between a secured claim and an unsecured claim, and the unsecured claim would not be entitled to the benefits of security in the collateral. Other consequences of a finding of under-collateralization would be, among other things, a lack of entitlement on the part of the Notes to receive post-petition interest and a lack of entitlement on the part of the unsecured portion of the Notes to receive other “adequate protection” under federal bankruptcy laws. In addition, if any payments of post-petition interest had been made at the time of such a finding of under-collateralization, those payments could be recharacterized by the bankruptcy court as a reduction of the principal amount of the secured claim with respect to the Notes.
Because Each Guarantor’s Liability Under its Guarantees May Be Reduced to Zero, Avoided or Released Under Certain Circumstances, You May Not Receive Any Payments From Some or All of the Guarantors.
You have the benefit of the guarantees of the guarantors. However, the guarantees by the guarantors are limited to the maximum amount that the guarantors are permitted to guarantee under applicable law. As a result, a guarantor’s liability under its guarantee could be reduced to zero, depending upon the amount of other obligations of such guarantor. Further, under the circumstances discussed more fully above, a court under federal and state fraudulent conveyance and transfer statutes could void the obligations under a guarantee or further subordinate it to all other obligations of the guarantor. See“—Federal and State Statutes May Allow Courts, Under Specific Circumstances, to Void the Notes and the Guarantees, Subordinate Claims in Respect of the Notes and the Guarantees and/or Require Holders of the Notes to Return Payments Received From Us.” In addition, you will lose the benefit of a particular guarantee if it is released under certain circumstances described under “Description of Notes—Guarantees.”
Bankruptcy Laws May Limit the Ability of Holders of the Notes to Realize Value From the Collateral.
The right of the collateral agent to repossess and dispose of the pledged assets upon the occurrence of an event of default under the indenture governing the Notes is likely to be significantly impaired by applicable bankruptcy law if a bankruptcy case were to be commenced by or against us before the collateral agent repossessed and disposed of the pledged assets. For example, under the Bankruptcy Code, pursuant to the automatic stay imposed upon the bankruptcy filing, a secured creditor is prohibited from repossessing its security from a debtor in a bankruptcy case, or from disposing of security repossessed from such debtor, or taking other actions to levy against a debtor, without bankruptcy court approval. Moreover, the Bankruptcy Code permits the debtor to continue to retain and to use collateral even though the debtor is in default under the applicable debt instruments, provided that the secured creditor is given “adequate protection.” The meaning of the term “adequate protection” may vary according to circumstances (and is within the discretion of the bankruptcy court), but it is intended in general to protect the value of the secured creditor’s interest in the collateral and may include cash payments or the granting of additional security, if and at such times as the court in its discretion determines, for any diminution in the value of the collateral as a result of the automatic stay of repossession or disposition or any use of the collateral by the debtor during the pendency of the bankruptcy case. Generally, adequate protection payments, in the form of interest or otherwise, are not required to be paid by a debtor to a secured creditor unless the bankruptcy court determines that the value of the secured creditor’s interest in the collateral is declining during the pendency of the bankruptcy case. In addition, the bankruptcy court may determine not to provide cash payments as adequate protection to the holders of the Notes if, among other possible reasons, the bankruptcy court determines that the fair market value of the collateral with respect to the Notes on the date of
44
Table of Contents
the bankruptcy filing was less than the then-current principal amount of the Notes. Furthermore, due to the imposition of the automatic stay, the lack of a precise definition of the term “adequate protection” and the broad discretionary powers of a bankruptcy court, it is impossible to predict (1) how long payments under the Notes could be delayed following commencement of a bankruptcy case, (2) whether or when the collateral agent could repossess or dispose of the pledged assets or (3) whether or to what extent holders of the Notes would be compensated for any delay in payment or loss of value of the pledged assets through the requirement of “adequate protection.”
The Collateral is Subject to Casualty Risks.
We are obligated under our ABL Facility to at all times cause all the pledged assets to be properly insured and kept insured against loss or damage by fire or other hazards to the extent that such properties are usually insured by corporations operating in the same or similar business. There are, however, some losses, including losses resulting from terrorist acts, that may be either uninsurable or not economically insurable, in whole or in part. As a result, we cannot assure holders of the Notes that the insurance proceeds will compensate us fully for our losses. If there is a total or partial loss of any of the pledged assets, we cannot assure holders of the Notes that the proceeds received by us in respect thereof will be sufficient to satisfy all the secured obligations, including the Notes. In the event of a total or partial loss to any of the mortgaged facilities, certain items of equipment and inventory may not be easily replaced. Accordingly, even though there may be insurance coverage, the extended period needed to manufacture replacement units or inventory could cause significant delays.
Rights of Holders of the Notes in the Collateral May Be Adversely Affected by the Failure to Perfect Security Interests in the Collateral.
Applicable law requires that a security interest in certain tangible and intangible assets can only be properly perfected and its priority retained through certain actions undertaken by the secured party. The liens in the collateral securing the Notes may not be perfected with respect to the claims of the Notes if the collateral agent is not able to take the actions necessary to perfect any of these liens on or prior to the date of the issuance of the Notes.
In addition, applicable law requires that certain property and rights acquired after the grant of a general security interest, such as real property, equipment subject to a certificate of title and certain proceeds, can only be perfected at the time such property and rights are acquired and identified. We and the guarantors have limited obligations to perfect the security interest of the holders of the Notes in specified collateral. There can be no assurance that the trustee or the collateral agent for the Notes will monitor, or that we will inform such trustee or collateral agent of, the future acquisition of property and rights that constitute collateral, and that the necessary action will be taken to properly perfect the security interest in such after-acquired collateral. Neither the trustee nor the collateral agent for the Series A-2 Notes has an obligation to monitor the acquisition of additional property or rights that constitute collateral or the perfection of any security interest. Such failure may result in the loss of the security interest in the collateral or the priority of the security interest in favor of the Notes against third parties.
Any future pledge of collateral in favor of the holders of the Notes might be voidable in bankruptcy. Any future pledge of collateral in favor of the holders of the Notes, including pursuant to security documents delivered after the date of the indenture governing the Notes, might be voidable by the pledgor (as debtor in possession) or by its trustee in bankruptcy if certain events or circumstances exist or occur, including, under the Bankruptcy Code, if the pledgor is insolvent at the time of the pledge, the pledge permits the holders of the Notes to receive a greater recovery than if the pledge had not been given and a bankruptcy proceeding in respect of the pledgor is commenced with 90 days following the pledge, or, in certain circumstances, a longer period.
45
Table of Contents
The Indenture Provides That the Notes Will Be Treated Under the Indenture as a Single Class for Purposes of Most Amendments and Waivers. Moreover, the Series A-1 Notes Are Entitled to a Priority in Payment Over the Series A-2 Notes Following Acceleration or a Bankruptcy Event of Default With Respect to Then Payable Principal and Interest.
The indenture provides that the Notes will be treated under the indenture as a single class for purposes of most amendments and waivers, other than certain matters that only affect the Series A-1 Notes or the Series A-2 Notes. Moreover, most accelerations of the Series A-1 Notes or Series A-2 Notes following an event of default will require action by the holders of 25% of the Notes combined. This may make it more difficult for the holders of the Series A-1 Notes or Series A-2 Notes to accelerate the Notes absent the concurrence of some of the other holders. In addition, for amendments or waivers that require a majority consent, even if a majority of the holders of the Series A-1 Notes or Series A-2 Notes voted in favor of an amendment or waiver, the amendment or waiver may not become effective unless a majority of both the series of Notes collectively vote in favor of the amendment or waiver. The indenture also provides that the Series A-1 Notes are entitled to a priority in payment over the Series A-2 Notes being issued in this offering following acceleration or a bankruptcy event of default with respect to then payable principal and interest, as further described in “Description of Notes—Priorities of Payment as between Series A-2 Debt (including the Series A-2 Notes) and Series A-1 Notes.” This priority applies only following acceleration or a bankruptcy event of default. Further, this priority applies to principal and interest already due, but does not apply to post-petition interest. Post-petition interest, if any, would be paid to the holders of Notes only after the holders of Notes have received their principal and pre-petition interest. Finally, although bankruptcy courts in general honor intercreditor agreements entered into prior to the bankruptcy filing, there can be no assurance that the intercreditor arrangements between the Notes would not be modified, changed or rejected during the course of a bankruptcy proceeding, including a determination that the Notes should be considered separate classes for purposes of the bankruptcy proceeding.
The Series A-1 Notes Were Issued With Original Issue Discount (“OID”) for U.S. Federal Income Tax Purposes.
Since the “stated redemption price at maturity” of the Series A-1 Notes exceeds their “issue price” (both as described below under “Certain U.S. Federal Income Tax Considerations”) by more than a statutory de minimis threshold, the Series A-1 Notes are considered to have been issued with OID for U.S. federal income tax purposes in an amount equal to such excess. U.S. holders (as defined in “Certain U.S. Federal Income Tax Considerations”) of Series A-1 Notes will be required to include such OID in income as it accrues, in advance of the receipt of cash attributable to such income. For a discussion of the tax consequences of an investment in the Series A-1 Notes, see “Certain U.S. Federal Income Tax Considerations.”
We May Not Be Able to Finance a Change of Control Offer Required by the Indenture.
Upon a change of control, as defined under the indenture governing the Notes, you will have the right to require us to offer to purchase all of the Notes then outstanding at a price equal to 101% of the principal amount of the Notes, plus accrued interest. In order to obtain sufficient funds to pay the purchase price of the outstanding Notes, we expect that we would have to refinance the Notes. We cannot assure you that we would be able to refinance the Notes on reasonable terms, if at all. Our failure to offer to purchase all outstanding Notes or to purchase all validly tendered Notes would be an event of default under the indenture. Such an event of default may cause the acceleration of our other debt. Our future debt also may contain restrictions on repayment requirements with respect to specified events or transactions that constitute a change of control under the indenture.
46
Table of Contents
As used in this prospectus, the term “Transactions” means, collectively, the Merger, the Original Financing and the Refinancing described below.
The Merger and the Original Financing
On June 18, 2008, Apria, Sky Acquisition and Merger Sub entered into the Merger Agreement, pursuant to which, on October 28, 2008, Merger Sub merged with and into Apria, with Apria being the surviving corporation following the Merger. The Investor Group beneficially owns all of Apria’s issued and outstanding capital stock.
The Investor Group made a $673.3 million cash equity investment in membership interests of Sky Acquisition and its parent entity, which investment includes Dr. Payson’s co-investment. The proceeds of such investment were contributed to Merger Sub, which used such proceeds, together with other sources of funds, to fund the Merger and the related transactions.
Following the closing of the Merger, Sky Acquisition and its parent entity adopted equity incentive arrangements for directors, executives and other senior management employees. Consistent with these arrangements, certain members of our management team have purchased and/or received, and may, from time to time, purchase and/or receive, equity interests or profit interests in one of our direct or indirect parent entities. Such purchases or awards of equity interests or profit interests may represent a substantial portion of the equity or profits of such parent entity.
In addition to the Merger Agreement, the parties entered into various ancillary agreements governing relationships between the parties after the Merger. See “Certain Relationships and Related Party Transactions.”
Concurrently with the signing of the Merger Agreement, Apria entered into a $280.0 million credit facility pursuant to a credit agreement with Banc of America Bridge LLC, Barclays Capital, the investment banking division of Barclays Bank PLC, Wells Fargo Securities, LLC (formerly known as Wachovia Capital Markets, LLC) and the lenders named therein (the “Interim Facility”). The loans under the Interim Facility bore interest at a rate of 11% per year with a maturity date of March 1, 2009. Proceeds of the Interim Facility were used to fund repurchases of $249.8 million of Apria’s 3.375% Convertible Senior Notes due 2033 (the “convertible senior notes”) in September 2008. We repaid all amounts outstanding under the Interim Facility and terminated it at the closing of the Merger.
The following financing transactions occurred in connection with the closing of the Merger:
| • | a cash investment made by the Investor Group totaling $673.3 million in membership interests of a parent entity of Sky Acquisition (which, in turn, contributed the proceeds of such investment to Sky Acquisition, which, in turn, contributed the proceeds of such investment to Merger Sub); |
| • | borrowings by Apria of $30.0 million under its $150.0 million ABL Facility; and |
| • | borrowings by Apria of $1,010.0 million under its senior secured bridge credit agreement. |
In addition, on the closing date of the Merger, we terminated all commitments and repaid all outstanding borrowings under the 2004 senior secured revolving credit facility and the Interim Facility, totaling $553.8 million as of October 28, 2008, and paid all accrued and unpaid interest thereon.
47
Table of Contents
The sources and uses of the funds used in connection with the Merger and the Original Financing are shown in the table below.
Sources | Uses | |||||||
| (dollars in millions) | ||||||||
ABL Facility(1) | $ | 30.0 | Purchase of equity(4) | $ | 946.4 | |||
Senior secured bridge credit agreement(2) | 1,010.0 | Repayment of existing indebtedness(5): 2004 senior secured revolving credit facility | 305.1 | |||||
Equity investment(3) | 673.3 | Interim facility | 251.9 | |||||
| Increase in cash(6) | 110.8 | |||||||
| Fees and expenses(7) | 99.1 | |||||||
Total sources | $ | 1,713.3 | Total uses | $ | 1,713.3 | |||
| (1) | Upon the closing of the Merger, we entered into a $150.0 million senior secured asset-based revolving credit facility, which has a five-year maturity. We borrowed $30.0 million under the ABL Facility on the closing date of the Merger. See “Description of Other Indebtedness—Senior Secured Asset-Based Revolving Credit Facility” for more information. |
| (2) | Upon the closing of the Merger, we entered into a senior secured bridge credit agreement, which has a six-year maturity. We borrowed an aggregate of $1,010.0 million under the senior secured bridge credit agreement on the closing date of the Merger. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources—Indebtedness—Post-Transactions—Senior Secured Bridge Credit Agreement” for more information. |
| (3) | Represents the cash equity contributed by the Investor Group. |
| (4) | Represents the amount of total consideration paid to holders of approximately 43.9 million of outstanding shares of Apria’s common stock, approximately 0.9 million of outstanding shares of restricted stock and approximately 0.8 million of outstanding options to acquire Apria’s common stock with a net option value of approximately $5.1 million, which is based upon an average exercise price of $14.46 per share. At the effective time of the Merger, each share of Apria’s common stock issued and outstanding immediately prior to the effective time (other than dissenting shares, shares held in treasury or shares held by Sky Acquisition, Merger Sub or any wholly-owned subsidiary of Apria) was converted into a right to receive $21.00 in cash, without interest and less any applicable withholding taxes. |
| (5) | Represents (i) $304.0 million of outstanding borrowings under our 2004 senior secured revolving credit facility and $1.1 million of accrued and unpaid interest related thereto and (ii) $249.8 million of outstanding borrowings under the Interim Facility and $2.1 million of accrued and unpaid interest related thereto, repaid in connection with the Merger. In September 2008, we were required pursuant to the terms of the indenture governing our convertible senior notes to repurchase $249.8 million of the convertible senior notes at a redemption price equal to 100% of the principal amount of the convertible senior notes properly tendered and not withdrawn plus accrued and unpaid interest to the redemption date. We funded the repurchase by borrowing $249.8 million under the Interim Facility. In addition, in December 2008, holders of $151,000 of the remaining outstanding $0.2 million of the convertible senior notes exercised their right to require us to repurchase their notes following the Merger. Approximately $77,000 of the convertible senior notes remain outstanding. |
| (6) | Represents an increase of $110.8 million in cash on hand upon the completion of the Merger and the Original Financing, which is expected to be used for general corporate purposes. |
| (7) | Includes commitment, accounting, legal and other professional fees, including the lenders’ fees and transaction fees paid to affiliates of the Sponsor. |
The Refinancing
We used the proceeds from the offerings of our outstanding Series A-1 Notes and the outstanding Series A-2 Notes in May 2009 and August 2009, respectively, together with cash on hand, to repay all of the outstanding borrowings under our senior secured bridge credit agreement and to pay related fees and expenses. The senior secured bridge credit agreement was terminated concurrently with the closing of the outstanding Series A-2 Notes offering. The sources and uses of the funds in connection with the Refinancing are shown in the table below.
Sources | Uses | |||||||
| (dollars in millions) | ||||||||
| Repayment of the senior secured bridge | ||||||||
Series A-1 Notes | $ | 700.0 | credit agreement(1) | $ | 1,027.2 | |||
Series A-2 Notes | 317.5 | Fees and expenses | 19.1 | |||||
Cash required for the Refinancing | 28.8 | |||||||
Total Sources | $ | 1,046.3 | Total Uses | $ | 1,046.3 | |||
| (1) | Represents $1,010.0 million outstanding under the senior secured bridge credit agreement prior to the offering of the outstanding Series A-1 Notes and $17.2 million of accrued and unpaid interest on the $1,010.0 million outstanding under the senior secured bridge credit agreement from May 1, 2009 until May 26, 2009 and $310.0 million outstanding under the senior secured bridge credit agreement from May 27, 2009 to August 12, 2009. |
48
Table of Contents
We will not receive any proceeds from the issuance of the exchange notes in the exchange offers. The exchange offers are intended to satisfy our obligations under the registration rights agreements that we entered into in connection with the private offerings of the outstanding notes. As consideration for issuing the exchange notes as contemplated in this prospectus, we will receive in exchange a like principal amount of outstanding notes, the terms of which are identical in all material respects to the exchange notes, except that the exchange notes will not contain terms with respect to transfer restrictions or additional interest upon a failure to fulfill certain of our obligations under the registration rights agreements. The outstanding notes that are surrendered in exchange for the exchange notes will be retired and cancelled and cannot be reissued. As a result, the issuance of the exchange notes will not result in any change in our capitalization.
49
Table of Contents
The following table sets forth our consolidated cash and cash equivalents and capitalization as of March 31, 2010.
You should read this table in conjunction with “Prospectus Summary—Summary Historical Consolidated Financial Data,” “Use of Proceeds,” “Selected Historical Consolidated Financial Data,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our historical consolidated financial statements and the related notes thereto included elsewhere in this prospectus.
The outstanding notes that are surrendered in exchange for the exchange notes will be retired and cancelled and cannot be reissued. As a result, the issuance of the exchange notes will not result in any change in our capitalization.
| As of March 31, 2010 | |||
| (dollars in thousands) | |||
Cash and cash equivalents | $ | 133,389 | |
Total debt: | |||
ABL Facility(1) | — | ||
Series A-1 Notes | 700,000 | ||
Series A-2 Notes | 317,500 | ||
Other debt | 3,204 | ||
Total debt | 1,020,704 | ||
Total stockholders’ equity | 678,978 | ||
Total capitalization | $ | 1,699,682 | |
| (1) | Upon the closing of the Merger, we entered into a $150.0 million senior secured asset-based revolving credit facility, which has a five-year maturity. We had no outstanding borrowings and approximately $16.1 million of drawn letters of credit under the ABL Facility as of March 31, 2010. See “Description of Other Indebtedness—Senior Secured Asset-Based Revolving Credit Facility” for more information. |
50
Table of Contents
SELECTED HISTORICAL CONSOLIDATED FINANCIAL DATA
The selected consolidated financial data set forth below should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our historical financial statements and accompanying notes contained herein. We derived the selected consolidated financial data for the year ended December 31, 2007, the periods from January 1, 2008 to October 28, 2008 and October 29, 2008 to December 31, 2008 and the year ended December 31, 2009 and as of December 31, 2008 and 2009 from our consolidated financial statements, which have been audited and are included in this prospectus. We derived the selected consolidated financial data for the years ended December 31, 2005 and 2006 and as of December 31, 2005, 2006 and 2007 from our consolidated financial statements that have been audited and are not included in this prospectus. The historical results presented are not necessarily indicative of future results. The audited consolidated financial statements for each of the years in the three-year period ended December 31, 2007, the periods from January 1, 2008 to October 28, 2008 and October 29, 2008 to December 31, 2008 and the year ended December 31, 2009 have been audited by Deloitte & Touche LLP, an independent registered public accounting firm.
Our selected historical condensed consolidated financial data for the three months ended March 31, 2009 and as of and for the three months ended March 31, 2010 have been derived from our condensed consolidated financial statements, which are included in this prospectus. We derived the selected historical consolidated financial data as of March 31, 2009 from our unaudited condensed consolidated financial statements, which are not included in this prospectus. The unaudited condensed consolidated financial statements were prepared on a basis consistent with our audited consolidated financial statements. In our opinion, the unaudited condensed consolidated financial statements include all adjustments, consisting of normal recurring accruals, necessary for the fair presentation of those statements. Our results for the three months ended March 31, 2010 should not be considered indicative of the results for the full fiscal year ending December 31, 2010.
| Year Ended December 31, | Period January 1, 2008 to October 28, 2008 | Period October 29, 2008 to December 31, 2008 | Year Ended December 31, 2009 | Three Months Ended March 31, 2009 | Three Months Ended March 31, 2010 | |||||||||||||||||||||||||||||
| 2005 | 2006 | 2007 | ||||||||||||||||||||||||||||||||
| (Predecessor) | (Predecessor) | (Predecessor) | (Predecessor) | (Successor) | (Successor) | (Successor) | (Successor) | |||||||||||||||||||||||||||
(dollars in thousands) | ||||||||||||||||||||||||||||||||||
Statement of Operations Data: | ||||||||||||||||||||||||||||||||||
Net revenues | $ | 1,475,670 | $ | 1,516,691 | $ | 1,631,801 | $ | 1,773,289 | $ | 356,665 | $ | 2,094,561 | $ | 516,431 | $ | 508,876 | ||||||||||||||||||
Cost and expenses: | ||||||||||||||||||||||||||||||||||
Total cost of net revenues | 479,213 | 521,580 | 564,992 | 691,337 | 137,760 | 867,459 | 207,044 | 202,792 | ||||||||||||||||||||||||||
Provision for doubtful accounts | 46,948 | 38,723 | 43,138 | 33,626 | 14,329 | 57,919 | 11,356 | 15,887 | ||||||||||||||||||||||||||
Selling, distribution and administrative | 792,031 | 804,285 | 862,062 | 924,536 | 179,362 | 1,050,134 | 263,784 | 257,738 | ||||||||||||||||||||||||||
Qui tamsettlement and related costs | 19,258 | — | — | — | — | — | — | — | ||||||||||||||||||||||||||
Amortization of intangible assets | 6,941 | 5,080 | 3,079 | 3,461 | 1,008 | 3,716 | 1,277 | 1,657 | ||||||||||||||||||||||||||
Total costs and expenses | 1,344,391 | 1,369,668 | 1,473,271 | 1,652,960 | 332,459 | 1,979,228 | 483,461 | 478,074 | ||||||||||||||||||||||||||
Operating income | 131,279 | 147,023 | 158,530 | 120,329 | 24,206 | 115,333 | 32,970 | 30,802 | ||||||||||||||||||||||||||
Interest expense and other, net | 22,119 | 29,463 | 20,493 | 29,684 | 25,441 | 127,591 | 32,267 | 32,489 | ||||||||||||||||||||||||||
Write-off of deferred debt issuance costs | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||
Income (loss) from continuing operations before taxes | 109,160 | 117,560 | 138,037 | 90,645 | (1,235 | ) | (12,258 | ) | 703 | (1,687 | ) | |||||||||||||||||||||||
Income tax expense (benefit) | 40,677 | 43,297 | 51,998 | 34,192 | 659 | (8,438 | ) | 1,180 | (884 | ) | ||||||||||||||||||||||||
Net income (loss) from continuing operations | $ | 68,483 | $ | 74,263 | $ | 86,039 | $ | 56,453 | $ | (1,894 | ) | $ | (3,820 | ) | $ | (477 | ) | $ | (803 | ) | ||||||||||||||
Cash Flow Data: | ||||||||||||||||||||||||||||||||||
Net cash provided by operating activities | $ | 206,299 | $ | 280,914 | $ | 294,006 | $ | 297,937 | $ | 63,337 | $ | 169,426 | $ | 29,672 | $ | 15,105 | ||||||||||||||||||
Net cash used in investing activities | (223,571 | ) | (132,932 | ) | (483,235 | ) | (154,493 | ) | (75,466 | ) | (168,656 | ) | (39,592 | ) | (24,013 | ) | ||||||||||||||||||
Net cash provided by (used in) financing activities | 1,177 | (156,629 | ) | 203,023 | (123,682 | ) | 131,934 | (10,625 | ) | | (7,505 | ) | (15,866 | ) | ||||||||||||||||||||
Capital expenditures | 118,867 | 125,628 | 128,759 | 157,183 | 26,217 | 150,597 | 39,843 | 27,319 | ||||||||||||||||||||||||||
51
Table of Contents
| As of December 31, | As of March 31, 2010 | |||||||||||||||||||
| 2005 | 2006 | 2007 | 2008 | 2009 | ||||||||||||||||
| (Predecessor) | (Predecessor) | (Predecessor) | (Successor) | (Successor) | (Successor) | |||||||||||||||
(dollars in thousands) | ||||||||||||||||||||
Balance Sheet Data: | ||||||||||||||||||||
Total assets | $ | 1,198,461 | $ | 1,154,636 | $ | 1,597,802 | $ | 2,210,813 | $ | 2,309,047 | $ | 2,284,270 | ||||||||
Goodwill and intangible assets | 551,565 | 545,738 | 822,992 | 1,292,403 | 1,341,456 | 1,341,299 | ||||||||||||||
Long-term debt, including current maturities | 645,320 | 487,145 | 687,283 | 1,022,233 | 1,021,146 | 1,020,704 | ||||||||||||||
Stockholders’ equity | 311,651 | 399,693 | 512,025 | 672,820 | 678,731 | 678,978 | ||||||||||||||
| Year Ended December 31, | Period January 1, 2008 to October 28, 2008 | Period October 29, 2008 to December 31, 2008 | Year Ended December 31, 2009 | Three Months Ended March 31, | |||||||||||||||||||||||||
| 2005 | 2006 | 2007 | 2009 | 2010 | |||||||||||||||||||||||||
| (Predecessor) | (Predecessor) | (Predecessor) | (Predecessor) | (Successor) | (Successor) | (Successor) | (Successor) | ||||||||||||||||||||||
Other Data: | |||||||||||||||||||||||||||||
Ratio of earnings to fixed charges(1) | 2.5x | 2.5x | 2.9x | 2.2x | .97x | 0.9x | 1.0x | .96x | |||||||||||||||||||||
Earnings deficiency to cover fixed charges | $ | — | $ | — | $ | — | $ | — | $ | (1,235 | ) | $ | (12,258 | ) | $ | — | $ | (1,687 | ) | ||||||||||
| (1) | Computed by dividing pre-tax net income before fixed charges by fixed charges. Fixed charges means the sum of the following: net interest expense, amortized premiums, discounts and capitalized expenses related to indebtedness, and an estimate of the interest within rental expense. Net interest expense excludes any interest related to tax liabilities, which is recorded as income tax expense. |
52
Table of Contents
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION
AND RESULTS OF OPERATIONS
This Management’s Discussion and Analysis of Financial Condition and Results of Operations is intended to assist in understanding and assessing the trends and significant changes in our results of operations and financial condition. Historical results may not be indicative of future performance. Our forward-looking statements reflect our current views about future events, are based on assumptions and are subject to known and unknown risks and uncertainties such as the current global economic uncertainty, including the tightening of the credit markets and the recent significant declines and volatility in our global financial markets, that could cause actual results to differ materially from those contemplated by these statements. Factors that may cause differences between actual results and those contemplated by forward-looking statements include, but are not limited to, those discussed in the “Risk Factors” and “Forward-Looking Statements” sections of this prospectus. This Management’s Discussion and Analysis of Financial Condition and Results of Operations should be read in conjunction with our consolidated financial statements and the related notes and other information included in this prospectus.
Overview
We have three core service lines: home respiratory therapy, home medical equipment and home infusion therapy. In these core service lines, we offer a variety of patient care management programs, including clinical and administrative support services, products and supplies, most of which are prescribed by a physician as part of a care plan. We provide these services to patients through approximately 500 locations throughout the United States. We have two reportable operating segments:
| • | home respiratory therapy and home medical equipment; and |
| • | home infusion therapy. |
Strategy
Our strategy is to position ourselves in the marketplace as a high-quality provider of a broad range of healthcare services and patient care management programs to our customers. The specific elements of our strategy are to:
| • | Grow profitable revenue and market share. We are focused on growing profitable revenues and increasing market share in our core home respiratory therapy and home infusion therapy service lines. We have undertaken a series of steps towards this end. Since January 1, 2007, we have expanded our home respiratory therapy and home medical equipment sales force by 18%. This focus has allowed us to more effectively market our products and services to physicians, hospital discharge planners and managed care organizations. Through our acquisition of Coram in December 2007, we considerably increased our home infusion capabilities and expanded our platform for further cross-selling opportunities. |
| • | Continue to participate in the managed care market. We participate in the managed care market as a long-term strategic customer group because we believe that our scale, expertise, nationwide presence and array of home healthcare products and services will enable us to sign preferred provider agreements with managed care organizations. Managed care represented approximately 70% of our total net revenues for the three months ended March 31, 2010. |
| • | Leverage our national distribution infrastructure.With approximately 500 locations and a robust platform supporting shared national services, we believe that we can efficiently add products, services and patients to our systems to grow our revenues and leverage our cost structure. For example, we have successfully leveraged this distribution platform across a number of product and service offerings, including a CPAP/bi-level supply replenishment program, enteral nutrition and NPWT services, and we are using our nursing capacity to provide infusion services through our growing network of ambulatory infusion suites. We seek to achieve margin improvements through operational initiatives focused on the |
53
Table of Contents
continual reduction of costs and delivery of incremental efficiencies. At the same time, we believe that it is essential to consistently deliver superior customer service in order to increase referrals and retain existing patients. Performance improvement initiatives are underway in all aspects of our operations including customer service, patient satisfaction, logistics, supply chain, clinical services and billing/collections. We believe that by being responsive to the needs of our patients and payors we can provide ourselves with opportunities to take market share from our competitors. |
| • | Continue to lead the industry in accreditation. MIPPA made accreditation mandatory for Medicare providers of DMEPOS, effective October 1, 2009, per CMS regulation. We were the first durable medical equipment provider to seek and obtain voluntary accreditation from The Joint Commission. All of our locations are currently accredited by The Joint Commission and our home infusion therapy service line is also accredited by the ACHC. In 2007, we completed a nationwide independent triennial accreditation renewal process conducted by The Joint Commission and we have more than 15 years of continuous accreditation by The Joint Commission—longer than any other homecare provider. In late June 2010, The Joint Commission completed its most recent triennial survey of our respiratory/home medical equipment and infusion locations and is expected to renew our accreditation for another three years. |
| • | Execute our strategic initiatives to drive profitability.For the past several years, we have successfully engaged in a range of cost savings initiatives to ease pressure on our revenue that has been and continues to be caused by Medicare and Medicaid reimbursement changes. These initiatives are designed to improve customer service, delivery and vehicle routing services, streamline the payment process, effectively manage procurement costs and improve the overall experience of the patients we serve. We launched a substantial cost reduction plan in late 2007. To date, we have made significant progress across a number of identified initiatives targeting expected annual savings of approximately $198 million, of which we have realized approximately $127 million through March 31, 2010. |
Recent Developments
Enactment of a Comprehensive Healthcare Reform Package. In March 2010, the Federal government enacted a comprehensive healthcare Reform Package which consists of the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010. See “Risk Factors—Risks Relating to Our Business—Continued Reductions in Medicare and Medicaid Reimbursement Rates and the Comprehensive Healthcare Reform Package Could Have a Material Adverse Effect on Our Results of Operations and Financial Condition.”
Reorganization of the Senior Leadership Structure of our Home Respiratory Therapy and Home Medical Equipment Segment. On February 12, 2010, we finalized a decision to reorganize the senior leadership structure of the home respiratory therapy and home medical equipment segment. As part of this reorganization, Lawrence A. Mastrovich, President, Home Respiratory Therapy and Home Medical Equipment segment, resigned and his responsibilities were assumed by other senior executives.
Critical Accounting Policies
We consider the accounting policies that govern revenue recognition and the determination of the net realizable value of accounts receivable to be the most critical in relation to our consolidated financial statements. These policies require the most complex and subjective judgments of management. Additionally, the accounting policies related to goodwill, long-lived assets, share-based compensation and income taxes require significant judgment.
Revenue and Accounts Receivable. Revenues are recognized under fee for service/product arrangements for equipment we rent to patients, sales of equipment, supplies, pharmaceuticals and other items we sell to patients and under capitation arrangements with third party payors for services and equipment we provide to the patients of these payors. Revenue generated from equipment that we rent to patients is recognized over the rental period, typically one month, and commences on delivery of the equipment to the patients. Revenue related to sales of equipment, supplies and pharmaceuticals is recognized on the date of delivery to the patients. Revenues derived
54
Table of Contents
from capitation arrangements were approximately 8%, 8%, 8%, 8%, and 10% of total net revenues for the three months period ended March 31, 2010, the year ended December 31, 2009, the period October 29, 2008 to December 31, 2008, the period January 1, 2008 to October 28, 2008, and the year ended December 31, 2007, respectively. Capitation revenue is earned as a result of entering into a contract with a third party to provide its members certain services without regard to the actual services provided, therefore revenue is recognized in the period that the beneficiaries are entitled to health care services. All revenues are recorded at amounts estimated to be received under reimbursement arrangements with third-party payors, including private insurers, prepaid health plans, Medicare and Medicaid.
In our business, there are multiple services and products delivered to patients. These arrangements involve equipment that is rented and related supplies that may be sold that cannot be returned. In arrangements with multiple deliverables, revenue is recognized when each deliverable is provided to the patient. For example, revenues from equipment rental supplies sales are recognized upon confirmation of delivery of the products, as the supplies sold are considered a separate unit of accounting.
Included in accounts receivable are earned but unbilled receivables of $49.5 million, $44.6 million and $48.2 million at March 31, 2010, December 31, 2009 and 2008, respectively. Delays ranging from a day up to several weeks between the date of service and billing can occur due to delays in obtaining certain required payor-specific documentation from internal and external sources. Earned but unbilled receivables are aged from date of service and are considered in the analysis of historical performance and collectibility.
Due to the nature of the industry and the reimbursement environment in which we operate, certain estimates are required to record total net revenues and accounts receivable at their net realizable values. Inherent in these estimates is the risk that they will have to be revised or updated as additional information becomes available. Specifically, the complexity of many third-party billing arrangements and the uncertainty of reimbursement amounts for certain services from certain payors may result in adjustments to amounts originally recorded. Such adjustments are typically identified and recorded at the point of cash application, claim denial or account review.
Management performs periodic analyses to evaluate accounts receivable balances to ensure that recorded amounts reflect estimated net realizable value. Specifically, management considers historical realization data, accounts receivable aging trends, other operating trends, the extent of contracted business and business combinations. Also considered are relevant business conditions such as governmental and managed care payor claims processing procedures and system changes. Additionally, focused reviews of certain large and/or problematic payors are performed. Due to continuing changes in the healthcare industry and third-party reimbursement, it is possible that management’s estimates could change in the near term, which could have an impact on operations and cash flows.
Accounts receivable are reduced by an allowance for doubtful accounts which provides for those accounts from which payment is not expected to be received, although services were provided and revenue was earned. Upon determination that an account is uncollectible, it is written-off and charged to the allowance.
Goodwill and Long-Lived Assets. Goodwill arising from business combinations represents the excess of the purchase price over the estimated fair value of the net assets of the acquired business. Goodwill and indefinite-lived intangible assets are tested annually for impairment or more frequently if circumstances indicate the potential for impairment. We have selected October 1 to perform our annual impairment test. Also, we test for impairment of our intangible assets and long-lived assets on an ongoing basis, whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Our goodwill impairment test is conducted at a “reporting unit” level and compares each reporting unit’s fair value to its carrying value. We have determined that our two operating segments are reporting units. As such, we have two reporting units, home respiratory therapy/home medical equipment and home infusion therapy. The measurement of fair value for each reporting unit is based on an evaluation of future discounted cash flows. In projecting our reporting units’ cash flows, we consider industry growth rates and trends, known and potential reimbursement reductions,
55
Table of Contents
cost structure changes and local circumstances specific to a service line. Goodwill and indefinite-lived intangible assets were tested for impairment on October 1, 2009 and resulted in no impairment. If an asset had been deemed impaired, an impairment loss would have been recognized to the extent the carrying value of the asset exceeded its estimated fair market value.
Long-lived assets, including property and equipment and purchased intangible assets, are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset or asset group may not be recoverable. Significant judgment is required in determining whether a potential indicator of impairment of long-lived assets exists and in estimating future cash flows for any necessary impairment tests. Recoverability of assets to be held and used is measured by the comparison of the carrying amount of an asset to future undiscounted net cash flows expected to be generated by the asset. If such an asset is considered to be impaired, the impairment to be recognized is measured as the amount by which the carrying amount of the asset exceeds the fair value of the asset. Assets to be disposed of are reported at the lower of the carrying amount or fair value less costs to sell. As of March 31, 2010, December 31, 2009 and 2008, there was no impairment.
Share-Based Compensation. We measure and recognize compensation expense for all share-based payment awards made to employees and non-employee directors based on estimated fair values on the date of grant. The value of the portion of the award that is ultimately expected to vest is recognized as expense over the requisite service period in our consolidated financial statements. Forfeitures are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. We recognize share-based compensation expense on a straight-line basis over the requisite service period. Prior to the Merger, we estimated fair value of our share-based payment awards using the Black-Scholes valuation model. Subsequent to the Merger, the estimate of fair value of share-based awards on the date of grant is determined through the allocation of all outstanding securities to a business enterprise valuation. The enterprise valuation is based upon the income approach methodology of valuation that includes the discounted cash flow method. This determination of fair value is affected by assumptions regarding a number of highly complex and subjective variables. Changes in the subjective assumptions can materially affect the estimate of their fair value.
Income Taxes.Deferred income tax assets and liabilities are computed for differences between the carrying amounts of assets and liabilities for financial statement and tax purposes. Deferred income tax assets are required to be reduced by a valuation allowance when it is determined that it is more likely than not that all or a portion of a deferred tax asset will not be realized. In determining the necessity and amount of a valuation allowance, management considers our current and past performance, the market environment in which we operate, tax planning strategies and the length of tax benefit carryforward periods.
Our provision for income taxes is based on expected income, statutory tax rates and tax planning opportunities available to us in the various jurisdictions in which we operate. Significant management estimates and judgments are required in determining the provision for income taxes. We are routinely under audit by federal, state or local authorities regarding the timing and amount of deductions, allocation of income among various tax jurisdictions and compliance with federal, state and local tax laws. Tax assessments related to these audits may not arise until several years after tax returns have been filed. Although predicting the outcome of such tax assessments involves uncertainty, we believe that the recorded tax liabilities appropriately reflect our potential obligations.
Capitalized Software.Internally developed and purchased software are capitalized and amortized over periods that the assets are expected to provide benefit. Capitalized costs include direct costs of materials and services incurred in developing or obtaining internal-use software and payroll and benefit costs for employees directly involved in the development of internal-use software. In connection with the Merger in 2008, we evaluated our information technology strategy and concluded it was no longer advisable to implement a new enterprise-wide information system. As a result of this change in strategy, we wrote off approximately $65.0 million of capitalized information systems assets as part of our purchase accounting adjustments that were made in connection with the Merger in 2008.
56
Table of Contents
Government Regulation
We are subject to extensive government regulation, including numerous laws directed at regulating reimbursement of our products and services under various government programs and preventing fraud and abuse. We maintain certain safeguards intended to reduce the likelihood that we will engage in conduct or enter into arrangements in violation of these restrictions. Corporate contract services and legal department personnel review and approve written contracts subject to these laws. We also maintain various educational and audit programs designed to keep our managers updated and informed regarding developments on these topics and to reinforce to employees our policy of strict compliance in this area. Federal and state laws require that we obtain regulatory licenses and that we enroll as a supplier with federal and state health programs. Under various federal and state laws, we are required to make filings or submit notices in connection with transactions that might be defined as a change of control of the Company. We are aware of these requirements and routinely make such filings with, and seek such approvals from, the applicable regulatory agencies. Notwithstanding these measures, violations of these laws and regulations may still occur, which could subject us to civil and criminal enforcement actions; licensure revocation, suspension or non-renewal; severe fines and penalties; the repayment of amounts previously paid to us and even the termination of our ability to provide services under certain government programs such as Medicare and Medicaid. See “Risk Factors—Risks Relating to Our Business—Continued Reductions in Medicare and Medicaid Reimbursement Rates and the Comprehensive Healthcare Reform Package Could Have a Material Adverse Effect on Our Results of Operations and Financial Condition.”
For additional information about government regulation of our business and industry, see “Business—Government Regulation.”
Results of Operations
Three Months Ended March 31, 2010 Compared to the Three Months Ended March 31, 2009
Net Revenues. Net revenues decreased $7.5 million, or 1.5%, to $508.9 million in the three months ended March 31, 2010 from $516.4 million in the three months ended March 31, 2009. The decrease in revenue was due primarily to the non-renewal or termination of, or changes to, certain payor contracts. We or the payor terminated or did not renew certain contracts during 2009 and we expect to continue to strategically evaluate the profitability of payor contracts. The revenue decrease was partially offset by increases in home infusion therapy revenue. In addition, revenue in the three months ended March 31, 2009 was positively impacted by the recognition of monthly rental revenue previously deferred for services that were initiated prior to certain 2009 Medicare reimbursement reductions.
We also expect to continue to face pricing pressures from Medicare and Medicaid as well as from our managed care customers as these payors seek to lower costs by obtaining more favorable pricing from providers such as us. In addition to the pricing reductions, such changes could cause us to provide reduced levels of certain products and services in the future, resulting in a corresponding reduction in revenue. See “Business—Government Regulation.”
Gross Profit.Gross profit margin is defined as total net revenues less total costs of total net revenues divided by total net revenues. The gross profit margin in the three months ended March 31, 2010 was 60.1%, compared to 59.9% in the three months ended March 31, 2009. This improvement was primarily the result of our ability to obtain favorable pricing on the purchase of products and the termination of certain low margin or unprofitable payor contracts during 2009. This improvement is partially offset by an increase in infusion therapy drug revenue as a percent of total company revenue. Our infusion therapy drug products have a lower gross profit margin as a percentage of net revenues.
Provision for Doubtful Accounts.The provision for doubtful accounts is based on management’s estimate of the net realizable value of accounts receivable after considering actual write-offs of specific receivables. Accounts receivable estimated to be uncollectible are provided for by computing a required reserve using estimated future cash receipts based on historical cash receipts collections as a percentage of revenue. In
57
Table of Contents
addition, management may adjust for changes in billing practices, cash collection protocols or practices, or changes in general economic conditions, contractual issues with specific payors, new markets or products. The provision for doubtful accounts, expressed as a percentage of total net revenues, was 3.1% and 2.2% in the three months ended March 31, 2010 and the three months ended March 31, 2009, respectively. The increased provision for doubtful accounts in 2010 is primarily the result of unfavorable collections experience occurring in 2010 compared to 2009.
Selling, Distribution and Administrative Expenses.Selling, distribution and administrative expenses are comprised of expenses incurred in direct support of operations and those associated with administrative functions. Expenses incurred by the operating locations include salaries and other expenses in the following functional areas: selling, distribution, clinical services, warehousing and repair. Many of these operating costs are directly variable with revenue growth patterns. Some are also very sensitive to market-driven price fluctuations such as facility lease and fuel costs. The administrative expenses include overhead costs incurred by the operating locations, regional and corporate support functions. These expenses are generally less sensitive to fluctuations in revenue growth than operating costs.
Selling, distribution and administrative expenses, expressed as a percentage of total net revenues was 50.6% for the three months ended March 31, 2010 compared to 51.1% for the three months ended March 31, 2009.
Selling, distribution and administrative expenses decreased by $6.0 million for the three months ended March 31, 2010 over the three months ended March 31, 2009. The $6.0 million decrease was comprised of a $16.1 million decrease in labor and other related expenses offset by an increase in other operating expenses of $10.1 million. The decrease in labor and other related expenses was due to reduced salaries and wages as a result of headcount reductions in 2009 due primarily to the outsourcing of certain functions relating to documentation, billing, reimbursements and other services, outsourcing of the information technology function and branch closure/consolidation as part of the branch optimization program, lower management incentive compensation program expense as a result of not meeting the targets in 2010, and the reduction in expenses related to equity incentive awards. The increases in other operating expenses of $10.1 million were primarily due to professional fees and expenses related to outsourcing certain billing and collection functions that did not exist in 2009, professional fees incurred in connection with the outsourcing of the information technology function, professional fees incurred in connection with a terminated debt offering and increase in our sponsor management fee.
Amortization of Intangible Assets.Amortization of intangible assets was $1.7 million in the three months ended March 31, 2010 and $1.3 million in the three months ended March 31, 2009. The amortization expense primarily results from the revaluation of intangible assets as a result of the Merger, including the finalization of the intangible asset valuation in 2009.
Interest Expense.Interest expense increased $0.2 million, or 0.5%, to $32.6 million in the three months ended March 31, 2010 from $32.4 million in the three months ended March 31, 2009. This increase is primarily due to higher amortization of deferred debt costs related to the $700.0 million of our outstanding 11.25% Senior Secured Notes due 2014 (Series A-1) and $317.5 million of our outstanding 12.375% Senior Secured Notes due 2014 (Series A-2) offset by a lower interest rate of 11.6% in the three months ended March 31, 2010 as compared to 12.0% for the three months ended March 31, 2009.
Interest Income and Other.Interest income and other decreased $0.1 million, or 46.5%, to $0.1 million in the three months ended March 31, 2010 from $0.2 million in the three months ended March 31, 2009.
Income Tax (Benefit) Expense.Our effective tax rate was a benefit of 52.4% for the three months ended March 31, 2010 compared with an expense of 167.8% for the three months ended March 31, 2009.
The 52.4% effective tax rate for the three months ended March 31, 2010 was due to the recognition of the tax benefits for certain state tax refunds.
58
Table of Contents
The 167.8% effective tax rate for the three months ended March 31, 2009 was primarily due to recognition of items related to accrued interest for tax contingencies and adjustments to our 2009 net deferred tax liabilities to reflect changes in state tax law. Additionally, our effective tax rate for the three months ended March 31, 2009 was higher due to the relationship between non-deductible equity compensation as a percentage of our pre-tax income.
Our provision for income taxes is based on expected income, statutory tax rates and tax planning opportunities available to us in the various jurisdictions in which we operate. Significant management estimates and judgments are required in determining the provision for income taxes. We are routinely under audit by federal, state or local authorities regarding the timing and amount of deductions, allocation of income among various tax jurisdictions and compliance with federal, state and local tax laws. Tax assessments related to these audits may not arise until several years after tax returns have been filed. Although predicting the outcome of such tax assessments involves uncertainty, we believe that the recorded tax liabilities appropriately reflect our potential obligations under generally accepted accounting principles.
Segment Net Revenues and Earnings Before Interest and Taxes (“EBIT”)
The following table sets forth a summary of results of operations by segment:
| (dollars in thousands) | Three Months Ended March 31, 2010 | Percentage of Net Revenues | Three Months Ended March 31, 2009 | Percentage of Net Revenues | ||||||||
Net revenues: | ||||||||||||
Home respiratory therapy and home medical equipment | $ | 278,316 | 54.7 | % | $ | 294,651 | 57.1 | % | ||||
Home infusion therapy | 230,560 | 45.3 | 221,780 | 42.9 | ||||||||
Total net revenues | $ | 508,876 | 100.0 | % | $ | 516,431 | 100.0 | % | ||||
EBIT is a measure used by our management to measure operating performance. EBIT is defined as net income (loss) plus interest expense and income taxes. EBIT is not a recognized term under Generally Accepted Accounting Principles (“GAAP”) and does not purport to be an alternative to net income as a measure of operating performance or to cash flows from operating activities as a measure of liquidity.
| Three Months Ended March 31, 2010 | |||||||||||||||
| (dollars in thousands) | Home Respiratory Therapy and Home Medical Equipment | Percentage of Segment Net Revenues | Home Infusion Therapy | Percentage of Segment Net Revenues | Total | ||||||||||
EBIT | $ | 9,866 | 3.5 | % | $ | 20,823 | 9.0 | % | $ | 30,689 | |||||
| Three Months Ended March 31, 2009 | |||||||||||||||
| (dollars in thousands) | Home Respiratory Therapy and Home Medical Equipment | Percentage of Segment Net Revenues | Home Infusion Therapy | Percentage of Segment Net Revenues | Total | ||||||||||
EBIT | $ | 23,523 | 8.0 | % | $ | 9,396 | 4.2 | % | $ | 32,919 | |||||
We allocate certain expenses that are not directly attributable to a product line based upon segment headcount. For a reconciliation of net income (loss) to EBIT, see the table under “Results of Operations—EBIT” at the end of this section.
Home Respiratory Therapy and Home Medical Equipment Segment. For the home respiratory therapy and home medical equipment segment total net revenues decreased $16.4 million, or 5.5%, to $278.3 million in the three months ended March 31, 2010 from $294.7 million in the three months ended March 31, 2009. Revenues for the home respiratory therapy and home medical equipment segment decreased to 54.7% of total revenue in the three months ended March 31, 2010 from 57.1% in the three months ended March 31, 2009.
59
Table of Contents
Home respiratory therapy revenues are derived primarily from the provision of oxygen systems, home ventilators, obstructive sleep apnea equipment, nebulizers, respiratory medications and related services. Revenues from the home respiratory therapy service line decreased by 5.2% in the three months ended March 31, 2010 compared to the three months ended March 31, 2009. The decrease in revenue resulted primarily from decreases in oxygen, sleep apnea and other respiratory revenue, due to the termination of or changes to certain payor contracts, as well as the impact of revenue recognized in the three months ended March 31, 2009 that was previously deferred for services performed prior to certain Medicare reimbursement reductions.
Home medical equipment revenues are derived from the rental and sale of equipment to assist patients with ambulation, safety and general care in and around the home. Home medical equipment revenues decreased by 7.6% in the three months ended March 31, 2010 compared to the three months ended March 31, 2009. The decrease was primarily due to the termination of or changes to certain payor contracts.
EBIT for the home respiratory and home medical equipment segment in the three months ended March 31, 2010 was $9.9 million compared to $23.5 million in the three months ended March 31, 2009. EBIT was 3.5% of segment net revenues in the three months ended March 31, 2010 compared to the 8.0% of segment net revenues in the three months ended March 31, 2009. This decrease in EBIT as a percentage of segment net revenues was primarily due to the unfavorable collection experience in the three months ended March 31, 2010 compared to the three months ended March 31, 2009 and an increase in sales, distribution and administrative costs as a percentage of net segment revenues due to a reduction in net segment revenues in the three months ended March 31, 2010 compared to the three months ended March 31, 2009.
Home Infusion Therapy Segment.For the home infusion therapy segment total net revenues increased $8.8 million, or 4.0%, to $230.6 million in the three months ended March 31, 2010 from $221.8 million in the three months ended March 31, 2009. Revenues for the home infusion therapy segment increased to 45.3% of total revenue in the three months ended March 31, 2010 from 42.9% in the three months ended March 31, 2009.
The home infusion therapy segment involves the administration of drugs or nutrients directly into the body intravenously through a needle or catheter. Infusion therapy services also include administering enteral nutrients directly into the gastrointestinal tract through a feeding tube. Home infusion therapy revenues increased by 4.0% in the three months ended March 31, 2010. The growth in revenue resulted primarily from an increase in the overall volume of specialty drugs, core drugs, non-core revenue and enteral nutrients. These increases were partially offset by a decrease in revenue due to the termination of a payor contract.
EBIT for the home infusion therapy segment in the three months ended March 31, 2010 was $20.8 million compared to $9.4 million in the three months ended March 31, 2009. EBIT was 9.0% of segment net revenues in the three months ended March 31, 2010 compared to 4.2% of segment net revenues in the three months ended March 31, 2009. This increase in EBIT as a percentage of segment net revenues was primarily due to an improvement in the gross profit margin in the three months ended March 31, 2010 compared to the three months ended March 31, 2009, and an improvement in sales, distribution and administrative costs as a percentage of net segment revenues in the three months ended March 31, 2010 compared to the three months ended March 31, 2009.
60
Table of Contents
Year Ended December 31, 2009 Results Compared to the Period October 29, 2008 to December 31, 2008 Results
The following table compares year ended December 31, 2009 results with the period October 29, 2008 to December 31, 2008.
(in thousands) | Year Ended December 31, 2009 | Percentage of Net Revenues | Period October 29, 2008 to December 31, 2008 | Percentage of Net Revenues | ||||||||||
Net revenues: | ||||||||||||||
Fee for service arrangements | $ | 1,930,464 | 92.2 | % | $ | 328,005 | 92.0 | % | ||||||
Capitation | 164,097 | 7.8 | 28,660 | 8.0 | ||||||||||
TOTAL NET REVENUES | 2,094,561 | 100.0 | 356,665 | 100.0 | ||||||||||
Costs and expenses: | ||||||||||||||
Cost of net revenues: | ||||||||||||||
Product and supply costs | 638,452 | 30.5 | 105,120 | 29.5 | ||||||||||
Patient service equipment depreciation | 101,681 | 4.9 | 17,539 | 4.9 | ||||||||||
Amortization of intangible assets | 44,000 | 2.1 | — | — | ||||||||||
Home respiratory therapy services | 34,700 | 1.7 | 6,270 | 1.8 | ||||||||||
Nursing services | 36,345 | 1.7 | 6,276 | 1.8 | ||||||||||
Other | 12,281 | 0.6 | 2,555 | 0.7 | ||||||||||
TOTAL COST OF NET REVENUES | 867,459 | 41.4 | 137,760 | 38.6 | ||||||||||
Provision for doubtful accounts | 57,919 | 2.8 | 14,329 | 4.0 | ||||||||||
Selling, distribution and administrative | 1,050,134 | 50.1 | 179,362 | 50.3 | ||||||||||
Amortization of intangible assets | 3,716 | 0.2 | 1,008 | 0.3 | ||||||||||
TOTAL COSTS AND EXPENSES | 1,979,228 | 94.5 | 332,459 | 93.2 | ||||||||||
OPERATING INCOME | 115,333 | 5.5 | 24,206 | 6.8 | ||||||||||
Interest expense | 129,200 | 6.2 | 26,167 | 7.3 | ||||||||||
Interest income and other | (1,609 | ) | (0.1 | ) | (726 | ) | (0.2 | ) | ||||||
LOSS BEFORE TAXES | (12,258 | ) | (0.6 | ) | (1,235 | ) | (0.3 | ) | ||||||
Income tax (benefit) expense | (8,438 | ) | (0.4 | ) | 659 | 0.2 | ||||||||
NET LOSS | $ | (3,820 | ) | (0.2 | )% | $ | (1,894 | ) | (0.5 | )% | ||||
Net Revenues. Net revenues in the year ended December 31, 2009 were $2,094.6 million compared to $356.7 million in the period October 29, 2008 to December 31, 2008. Revenue for the year ended December 31, 2009 increased due to more operating days in the year ended December 31, 2009 compared to the period October 29, 2008 to December 31, 2008. In the year ended December 31, 2009 revenue was impacted by Medicare reimbursement reductions of $108.7 million that did not occur in the period October 29, 2008 to December 31, 2008. The Medicare reimbursement reductions primarily related to:
| • | respiratory drug reimbursement reductions effective April 1, 2008; |
| • | changing the maximum rental period of oxygen equipment from an unlimited rental period to 36 months (the regulation effective date was January 2006; revenue was therefore impacted beginning in January 2009); and |
| • | MIPPA legislation authorized an average of 9.5% payment reduction in the DMEPOS fee schedule effective January 2009. |
We expect to continue to face pricing pressures from Medicare and Medicaid as well as from our managed care customers as these payers seek to lower costs by obtaining more favorable pricing from providers such as us. In addition to the pricing reductions, such changes could cause us to provide reduced levels of certain
61
Table of Contents
products and services in the future, resulting in a corresponding reduction in revenue. See “Business— Government Regulation.” For the year ended December 31, 2009 and the period October 29, 2008 to December 31, 2008, revenues reimbursed under arrangements with Medicare and Medicaid were approximately 28% and 31%, respectively, as a percentage of total revenues. In the year ended December 31, 2009 and the period October 29, 2008 to December 31, 2008, no other third-party payor group represented more than 8% of our revenues. In fee for service arrangement revenue, rental and sale revenues comprise approximately $654.6 million or 33.9% and $1,275.9 million or 66.1% and $125.7 million or 38.3% and $202.3 or 61.7% in the year ended December 31, 2009 and the period October 29, 2008 to December 31, 2008.
Gross Profit. Gross profit margin is defined as total net revenues less total costs of total net revenues divided by total net revenues. The gross profit margin for the year ended December 31, 2009 was 58.6%, compared to 61.4% in the period October 29, 2008 to December 31, 2008. Included in cost of revenue for the year ended December 31, 2009 is $44.0 million of amortization related to our patient backlog intangible asset which was provisional in the period October 29, 2008 to December 31, 2008. In addition, this decrease in gross profit margin was primarily due to the negative impact of the Medicare reimbursement reductions. Excluding the intangible asset amortization and the impact of Medicare reimbursement reductions, the overall gross profit margin percentage for the year ended December 31, 2009 would have been 62.4%, compared to 61.4% in the period October 29, 2008 to December 31, 2008. This improvement in the gross profit margin was due to a shift in product mix to a higher volume of products with lower costs as a percentage of revenue in our home respiratory therapy service line and our ability to obtain favorable pricing on the purchase of products and supplies in our all three of our service lines. These improvements in the gross profit margin were partially offset by a decrease in the gross profit margin in our home infusion therapy service line as a result of a shift in product mix to more specialty drugs, which have a higher product cost as a percentage of revenue.
Provision for Doubtful Accounts. The provision for doubtful accounts is based on management’s estimate of the net realizable value of accounts receivable after considering actual write-offs of specific receivables. Accounts receivable estimated to be uncollectible are provided for by computing a required reserve using estimated future cash receipts based on historical cash receipts collections as a percentage of revenue. In addition, management may adjust for changes in billing practices, cash collection protocols or practices, or changes in general economic conditions, contractual issues with specific payors, new markets or products. The provision for doubtful accounts, expressed as a percentage of total net revenues, was 2.8% and 4.0% in the year ended December 31, 2009 and the period October 29, 2008 to December 31, 2008, respectively. The decrease in the provision for doubtful accounts in 2009 is primarily the result of favorable collections experience occurring in the year ended December 31, 2009 as a percentage compared to the period October 29, 2008 to December 31, 2008.
Selling, Distribution and Administrative Expenses. Selling, distribution and administrative expenses are comprised of expenses incurred in direct support of operations and those associated with administrative functions. Expenses incurred by the operating locations include salaries and other expenses in the following functional areas: selling, distribution, clinical services, warehousing and repair. Many of these operating costs are directly variable with revenue growth patterns. Some are also very sensitive to market-driven price fluctuations such as facility lease and fuel costs. The administrative expenses include overhead costs incurred by the operating locations and regional and corporate support functions. These expenses are generally less sensitive to fluctuations in revenue growth than operating costs.
Selling, distribution and administrative expenses, expressed as a percentage of total net revenues were 50.1% for the year ended December 31, 2009 compared to 50.3% for the period October 29, 2008 to December 31, 2008. Adjusted for Medicare reimbursement reductions of $108.7 million in the year ended December 31, 2009, the selling, distribution and administrative expense percentage for 2009 would have been 47.7% of revenue in the year ended December 31, 2009.
In the year ended December 31, 2009 and the period October 29, 2008 to December 31, 2008 labor and other related expenses as a percentage of total net revenues were 33.0% and 32.9%, respectively. For the year
62
Table of Contents
ended December 31, 2009 when adjusting for the impact of Medicare reimbursement reductions, labor and related costs were 31.4% of total revenue. The decrease in labor and other related expenses was primarily due to reduced salaries and wages as a result of headcount reductions in 2009 and lower outside labor. These decreases were partially offset by increases due to higher management incentive compensation program expense, as a result of meeting the targets in 2009 and not in 2008, and higher termination and retention expense due to the projects to outsource certain billing, collection and information technology functions.
In the year ended December 31, 2009 and the period October 29, 2008 to December 31, 2008 other operating expenses as a percentage of total net revenues were 17.1% and 17.4%, respectively. For the year ended December 31, 2009 when adjusting for the impact of Medicare reimbursement reductions, other operating expenses were 16.3% of total revenue. The decreases in other operating expenses were primarily due to delivery costs, principally lower fuel prices and lower freight costs due to renegotiation of a contract with a vendor. These decreases were partially offset by expenses incurred in 2009 associated with our projects to outsource certain billing, collection and information technology functions that did not exist in 2008, professional fees incurred in connection with the sale of our outstanding Series A-1 Notes and Series A-2 Notes and expenses associated with other corporate initiative projects in 2009.
Amortization of Intangible Assets. Amortization of intangible assets was 0.2% and 0.3% of total net revenues in the year ended December 31, 2009 and the period October 29, 2008 to December 31, 2008, respectively. The amortization expense primarily results from the revaluation of intangible assets as a result of the Merger, including the finalization of the intangible asset valuation in 2009.
Interest Expense. Interest expense as a percentage of total net revenues was 6.2% for the year ended December 31, 2009. Adjusted for the impact of Medicare reimbursement reductions interest expense was 5.9% of total net revenues in the year ended December 31, 2009. Interest expense for the period October 29, 2008 to December 31, 2008 was 7.3% of revenue. This decrease is primarily due to higher interest expense in the two month period due to higher borrowing on the ABL facility and higher deferred debt costs in 2008 related to the Predecessor Revolving Credit Facility and Senior Secured Bridge Credit Agreement.
Interest Income and Other. Interest income and other was 0.1% as a percentage of total net revenues for the year ended December 31, 2009 compared to 0.2% as a percentage of total net revenues for the period October 29, 2008 to December 31, 2008. This decrease is due to a decrease in interest rates earned on invested cash.
Income Tax Benefit.The income tax benefit as a percentage of total net revenues for the year ended December 31, 2009 was 0.4% compared to a tax expense as a percentage of total net revenues of 0.2% for the period October 29, 2008 to December 31, 2008. The effective tax rate was 68.8% at December 31, 2009 compared to 53.4% for the period October 29, 2008 to December 31, 2008. The income tax benefit in the year ended December 31, 2009 resulted from (1) a reduction in our valuation allowances related to state net operating losses and other state deferred tax assets and (2) a decrease in our tax reserves due to settlements with state tax agencies and the expiration of statute of limitations.
Segment Net Revenues and EBIT
The following table sets forth a summary of results of operations by segment:
(in thousands) | Year Ended December 31, 2009 | Percentage of Net Revenues | Period October 29, 2008 to December 31, 2008 | Percentage of Net Revenues | ||||||||
Net revenues: | ||||||||||||
Home respiratory therapy and home medical equipment | $ | 1,169,609 | 55.8 | % | $ | 209,567 | 58.8 | % | ||||
Home infusion therapy | 924,952 | 44.2 | 147,098 | 41.2 | ||||||||
Total net revenues | $ | 2,094,561 | 100.0 | % | $ | 356,665 | 100.0 | % | ||||
63
Table of Contents
(in thousands) | Year Ended December 31, 2009 | ||||||||||||||
| Home Respiratory Therapy and Home Medical Equipment | Percentage of Segment Net Revenues | Home Infusion Therapy | Percentage of Segment Net Revenues | Total | |||||||||||
EBIT | $ | 50,167 | 4.3 | % | $ | 65,667 | 7.1 | % | $ | 115,834 | |||||
(in thousands) | Period October 29, 2008 to December 31, 2008 | |||||||||||||||
| Home Respiratory Therapy and Home Medical Equipment | Percentage of Segment Net Revenues | Home Infusion Therapy | Percentage of Segment Net Revenues | Total | ||||||||||||
EBIT | $ | 26,255 | 12.5 | % | $ | (1,740 | ) | (1.2 | )% | $ | 24,515 | |||||
We allocate certain expenses that are not directly attributable to a product line based upon segment headcount. For a reconciliation of net income (loss) to EBIT, see the table under “Results of Operations—EBIT” at the end of this section.
Home Respiratory Therapy and Home Medical Equipment Segment. Net revenues for the home respiratory therapy and home medical equipment segment in the year ended December 31, 2009 was $1,169.6 million compared to $209.6 million in the period October 29, 2008 to December 31, 2008. Revenues for the home respiratory therapy and home medical equipment segment decreased to 55.8% of total revenue in the year ended December 31, 2009 from 58.8% in the period October 29, 2008 to December 31, 2008.
Home respiratory therapy revenues are derived primarily from the provision of oxygen systems, home ventilators, obstructive sleep apnea equipment, nebulizers, respiratory medications and related services. Revenues from the respiratory therapy service line decreased to 48.4% of total revenue in the year ended December 31, 2009 compared to 51.5% in the period October 29, 2008 to December 31, 2008. The majority of Medicare reimbursement reductions discussed above impacted the home respiratory therapy services line. Such reductions were $104.0 million for the year ended December 31, 2009. Adjusted for the Medicare reimbursement reductions, respiratory therapy revenues for 2009 would have been 50.7%. Home respiratory therapy revenues as a percentage of total revenue decreased as a result of increased sales as a percentage of total revenue in our home infusion therapy service line.
Home medical equipment revenues are derived from the rental and sale of equipment to assist patients with ambulation, safety and general care in and around the home. Home medical equipment revenues were 7.5% of total revenue in the year ended December 31, 2009 compared to 7.3% of total revenue in the period October 29, 2008 to December 31, 2008. During the year ended December 31, 2009, $1.9 million of the Medicare reimbursement reductions impacted this service line. Excluding the impact of the Medicare reimbursement reductions, home medical equipment revenue would have been 7.2% of total revenues in the year ended December 31, 2009.
EBIT for the home respiratory therapy and home medical equipment segment in the year ended December 31, 2009 was $50.2 million compared to $26.3 million in the period October 29, 2008 to December 31, 2008. EBIT was 4.3% of segment net revenues in the year ended December 31, 2009 compared to 12.5% of segment net revenues in the period October 28, 2008 to December 31, 2008. This decrease in EBIT as a percentage of segment net revenues was primarily due to the negative impact of the Medicare reimbursement reductions. This decrease in the EBIT was partially offset by a shift in product mix to a higher volume of products with lower costs as a percentage of segment net revenues in our home respiratory therapy service line and our ability to obtain favorable pricing on the purchase of products and supplies.
Home Infusion Therapy Segment.For the home infusion therapy segment total net revenues increased $777.9 million, or 528.8%, to $925.0 million in the year ended December 31, 2009 from $147.1 million in the period October 29, 2008 to December 31, 2008. Revenues for the home infusion therapy segment increased to 44.2% of total revenue in the year ended December 31, 2009 from 41.2% in the period October 29, 2008 to December 31, 2008.
64
Table of Contents
Home infusion therapy involves the administration of drugs or nutrients directly into the body intravenously through a needle or catheter. Infusion therapy services also include administering enteral nutrients directly into the gastrointestinal tract through a feeding tube. Home infusion therapy revenues as a percentage of total revenue increased to 44.2% in the year ended December 31, 2009 compared to 41.2% in the period October 29, 2008 to December 31, 2008. The growth in revenue in the year ended December 31, 2009 resulted primarily from an increase in volume of specialty drugs, core drugs and enteral nutrients. The increase in these drugs was offset by a decrease in revenue due to the termination of a payor contract, and a decrease in volume of non-core revenue. As a result of increasing volume, specialty drug revenue also increased as a percentage of total home infusion therapy revenue. During the year ended December 31, 2009, Medicare reimbursement reductions impacted this service line by $2.8 million. Excluding the impact of Medicare reimbursement reductions, home infusion therapy revenue would have been 42.1% as a percentage of total revenue for the year ended December 31, 2009.
EBIT for the home infusion therapy segment in the year ended December 31, 2009 was $65.7 million compared to $(1.7) million in the period October 28, 2008 to December 31, 2008. EBIT was 7.1% of segment net revenues in the year ended December 31, 2009 compared to (1.2)% of segment net revenues in the period October 29, 2008 to December 31, 2008. This increase in EBIT was primarily due to our ability to obtain favorable pricing on the purchase of products and supplies and our ability to reduce our selling distribution and administrative costs as a percentage of segment net revenues partially offset by a decrease in the gross profit margin as a result of a shift in product mix to more specialty drugs, which have a higher product cost as a percentage of segment net revenues.
65
Table of Contents
Year Ended December 31, 2009 Results Compared to Pro forma Year Ended December 31, 2008 Results
The following table compares the year ended December 31, 2009 results to the pro forma results for the year ended December 31, 2008 to reflect the Merger if it had occurred on January 1, 2008. We are providing a comparison of the year ended December 31, 2009 to the pro forma year ended December 31, 2008 for illustrative purposes only to facilitate the comparison of the results of the full year 2008 with the full year 2009.
| Year Ended December 31, 2009 | Period October 29, 2008 to December 31, 2008 | Period January 1, 2008 to October 28, 2008 | Adjustments for the Transactions | Year Ended December 31, 2008 Pro forma | Year Ended December 31, 2009 Compared to Pro forma Year Ended December 31, 2008 | ||||||||||||||||||||||||
(in thousands) | Increase/ Decrease | Percentage Change | |||||||||||||||||||||||||||
| (Successor) | (Successor) | (Predecessor) | |||||||||||||||||||||||||||
Net revenues: | |||||||||||||||||||||||||||||
Fee for service arrangements | $ | 1,930,464 | $ | 328,005 | $ | 1,630,767 | $ | — | $ | 1,958,772 | $ | (28,308 | ) | (1.4 | )% | ||||||||||||||
Capitation | 164,097 | 28,660 | 142,522 | — | 171,182 | (7,085 | ) | (4.1 | ) | ||||||||||||||||||||
TOTAL NET REVENUES | 2,094,561 | 356,665 | 1,773,289 | — | 2,129,954 | (35,393 | ) | (1.7 | ) | ||||||||||||||||||||
Costs and expenses: | |||||||||||||||||||||||||||||
Cost of net revenues: | |||||||||||||||||||||||||||||
Product and supply costs | 638,452 | 105,120 | 526,610 | — | 631,730 | 6,722 | 1.1 | ||||||||||||||||||||||
Patient service equipment depreciation | 101,681 | 17,539 | 89,246 | — | 106,785 | (5,104 | ) | (4.8 | ) | ||||||||||||||||||||
Amortization of intangible assets | 44,000 | — | — | 42,920 | (a) | 42,920 | 1,080 | 2.5 | |||||||||||||||||||||
Home respiratory therapy services | 34,700 | 6,270 | 31,893 | — | 38,163 | (3,463 | ) | (9.1 | ) | ||||||||||||||||||||
Nursing services | 36,345 | 6,276 | 29,773 | — | 36,049 | 296 | 0.8 | ||||||||||||||||||||||
Other | 12,281 | 2,555 | 13,815 | — | 16,370 | (4,089 | ) | (25.0 | ) | ||||||||||||||||||||
TOTAL COST OF NET REVENUES | 867,459 | 137,760 | 691,337 | 42,920 | 872,017 | (4,558 | ) | (0.5 | ) | ||||||||||||||||||||
Provision for doubtful accounts | 57,919 | 14,329 | 33,626 | — | 47,955 | 9,964 | 20.8 | ||||||||||||||||||||||
Selling, distribution and administrative | 1,050,134 | 179,362 | 924,536 | (22,613 | )(b) | 1,081,285 | (31,151 | ) | (2.9 | ) | |||||||||||||||||||
Amortization of intangible assets | 3,716 | 1,008 | 3,461 | (796 | )(c) | 3,673 | 43 | 1.2 | |||||||||||||||||||||
TOTAL COSTS AND EXPENSES | 1,979,228 | 332,459 | 1,652,960 | 19,511 | 2,004,930 | (25,702 | ) | (1.3 | ) | ||||||||||||||||||||
OPERATING INCOME | 115,333 | 24,206 | 120,329 | (19,511 | ) | 125,024 | (9,691 | ) | (7.8 | ) | |||||||||||||||||||
Interest expense | 129,200 | 26,167 | 31,838 | 74,270 | (d) | 132,275 | (3,075 | ) | (2.3 | ) | |||||||||||||||||||
Interest income and other | (1,609 | ) | (726 | ) | (2,154 | ) | — | (2,880 | ) | 1,271 | (44.1 | ) | |||||||||||||||||
(LOSS) INCOME BEFORE TAXES | (12,258 | ) | (1,235 | ) | 90,645 | (93,781 | ) | (4,371 | ) | (7,887 | ) | 180.4 | |||||||||||||||||
Income tax (benefit) expense | (8,438 | ) | 659 | 34,192 | (36,829 | )(e) | (1,978 | ) | (6,460 | ) | 326.6 | ||||||||||||||||||
(LOSS) INCOME | $ | (3,820 | ) | $ | (1,894 | ) | $ | 56,453 | $ | (56,952 | ) | $ | (2,393 | ) | $ | (1,427 | ) | 59.6 | % | ||||||||||
| (a) | Reflects amortization expense of patient backlog intangible assets with finite lives that were identified as a result of the Merger, as set forth below: |
(in thousands) | Gross Carrying Amount at December 31, 2009 | Life | Annual Amortization Expense | |||||
Patient backlog | $ | 44,000 | 1.1 | $ | 42,920 | |||
66
Table of Contents
The adjustment was calculated as follows:
(in thousands) | Year Ended December 31, 2008 | ||
Amortization expense as a results of the Merger | $ | 42,920 | |
Less: historical amortization expense | — | ||
Total adjustment | $ | 42,920 | |
| (b) | Represents adjustments to expenses as set forth below. |
(in thousands) | Year Ended December 31, 2008 | |||
Legal, accounting and other expenses directly attributable to the Merger | $ | (4,458 | ) | |
Management fee(i) | 5,773 | |||
Accelerated stock option vesting (ii) | (22,328 | ) | ||
Executive bonus payments (iii) | (1,600 | ) | ||
Total adjustments to expenses | $ | (22,613 | ) | |
| (i) | Reflects an annual management fee of $7.0 million payable to an affiliate of the Sponsor under the transaction and management fee agreement. |
The adjustments for the management fee were calculated as follows:
(in thousands) | Year Ended December 31, 2008 | |||
Management fee | $ | 7,000 | ||
Less: management fee recorded for the periods shown | (1,227 | ) | ||
Total adjustment | $ | 5,773 | ||
| (ii) | Reflects accelerated compensation expense due to the vesting and payout of all stock-based awards as a result of the Merger. |
| (iii) | Represents incremental bonuses paid as a result of the Merger to certain executive officers pursuant to change in control provisions in their employment agreements. |
| (c) | Reflects the amortization expense of intangible assets with finite lives that were identified as a result of the Merger, as set forth below. |
(in thousands) | Gross Carrying Amount at December 31, 2009 | Life | Annual Amortization Expense | |||||
Capitated relationships | $ | 40,000 | 20 | $ | 2,000 | |||
Payor relationships | 11,000 | 20 | 550 | |||||
Net favorable leasehold interest | 3,553 | 3.2 | 1,123 | |||||
Total intangible assets with finite lives | $ | 54,553 | $ | 3,673 | ||||
The adjustments were calculated as follows:
(in thousands) | Year Ended December 31, 2008 | |||
Amortization expense as a results of the Merger | $ | 3,673 | ||
Less: historical amortization expense | (4,469 | ) | ||
Total adjustment | $ | (796 | ) | |
67
Table of Contents
| (d) | Reflects interest expense resulting from our new capital structure (using current applicable LIBOR rates) as set forth below. |
(in thousands) | Balance | Assumed Rate | Interest Expense Year Ended December 31, 2008 | |||||||
Senior Secured Bridge Credit Agreement(1) | $ | 1,010,000 | 12.00 | % | $ | 121,200 | ||||
ABL Facility(2) | 150,000 | 1.20 | % | 1,803 | ||||||
Ongoing interest on other existing debt to be retained(3) | 4,733 | 1,869 | ||||||||
Total cash interest expense | 124,872 | |||||||||
Amortization of capitalized debt issuance costs related to the Merger(4) | 57,678 | 7,403 | ||||||||
Total pro forma interest expense related to the Merger | 132,275 | |||||||||
Less: historical interest expense | (58,005 | ) | ||||||||
Total adjustment to pro forma interest expense related to the Merger | 74,270 | |||||||||
Total pro forma interest expense | $ | 132,275 | ||||||||
| (1) | Represents $1,010.0 million borrowed under the Senior Secured Bridge Credit Agreement entered into in October 2008, which bears interest at the rate of 12.00% per annum. |
| (2) | Reflects $16.1 million of drawn letters of credit, no borrowings and the undrawn portion in the amount of $133.9 million under the ABL Facility. The interest rate on the ABL Facility used to compute pro format interest expense is an assumed blended interest rate of the letters of credit and the undrawn portion of the ABL Facility. The interest rate on borrowings under the ABL Facility is variable and had there been borrowings under the ABL Facility, the interest rate on the borrowed portion would have been determined using a three-month LIBOR rate of 0.25% plus an assumed applicable margin of 2.75%, based upon an assumed average excess availability of $133.9 million. The pro forma fee expense for letters of credit is assumed at 2.75% to 3.25%, depending on the average excess availability, with the lowest average excess availability resulting in the highest letters of credit fees and applicable margin. The ABL Facility also requires a fee for undrawn amounts ranging from 0.50% to 1.00% depending on the utilization percentage, with the lowest utilization rate resulting in the highest fee rate. The pro forma interest expense assumes a utilization fee of 1.00%. See “—Liquidity and Capital Resources” for more information. A 0.125% variance in the assumed blended interest rate for the ABL Facility would amount to a change in total annual pro forma interest expense of $0.2 million. |
| (3) | Represents $4.7 million of capital leases, deferred compensation and certain other indebtedness that we retained following the Refinancing. On going interest amounts for debt that were retained after the Transactions are based upon actual interest amounts recorded during each of the pro forma periods. Includes interest expense recorded in relation to accretion of the Company’s deferred compensation plan of approximately $0.1 million and $1.0 million for the historical period of October 29, 2008 to December 31, 2008 and the historical period of January 1, 2008 to October 28, 2008. |
| (4) | Debt issuance costs related to the Merger. Amortization is calculated using the effective interest method over the terms of the related debt. |
68
Table of Contents
| (e) | Reflects the estimated tax impact relating to the adjustments for the Merger, calculated at estimated statutory rates as set forth below. |
(in thousands) | Year Ended December 31, 2008 | |||
Total adjustments for the Merger | $ | (93,781 | ) | |
Adjustments without tax benefit | 2,377 | |||
Adjustments resulting in tax benefit | (96,158 | ) | ||
Statutory tax rate | 38.3 | % | ||
Tax effect of the adjustments for the Merger | $ | (36,829 | ) | |
Net Revenues. Net revenues decreased $35.4 million, or 1.7%, to $2,094.6 million in the year ended December 31, 2009 from $2,130.0 million in the pro forma year ended December 31, 2008. The revenue decline for the year ended December 31, 2009 was impacted by Medicare reimbursement reductions of $108.7 million. Had those reductions not been implemented, revenues for 2009 would have increased by 3.4%. The Medicare reimbursement reductions primarily related to:
| • | respiratory drug reimbursement reductions effective April 1, 2008; |
| • | changing the maximum rental period of oxygen equipment from an unlimited rental period to 36 months (regulation effective date of January 2006); and |
| • | MIPPA legislation authorized an average of 9.5% payment reduction in the DMEPOS fee schedule effective January 2009. |
We expect to continue to face pricing pressures from Medicare and Medicaid as well as from our managed care customers as these payers seek to lower costs by obtaining more favorable pricing from providers such as us. In addition to the pricing reductions, such changes could cause us to provide reduced levels of certain products and services in the future, resulting in a corresponding reduction in revenue. See “Business—Government Regulation.”
Gross Profit. Gross profit margin is defined as total net revenues less total costs of total net revenues divided by total net revenues. The gross profit margin in the year ended December 31, 2009 was 58.6%, compared to 59.1% in the pro forma year ended December 31, 2008. Included in cost of revenue is amortization related to our patient backlog intangible asset of $44.0 million during the year ended December 31, 2009 and $42.9 million during the pro forma year ended December 31, 2008. As of December 31, 2009, the patient backlog intangible asset was fully amortized. Excluding the impact of the intangible asset amortization discussed above, the overall gross profit margin percentage for the year ended December, 31, 2009 would have been 60.7%, compared to 61.1% in the pro forma year ended December 31, 2008. This decrease of 0.4% in gross profit margin was primarily due to the negative impact of the Medicare reimbursement reductions. Excluding the intangible asset amortization and the impact of Medicare reimbursement reductions, the overall gross profit margin percentage for the year ended December 31, 2009 would have been 62.4%, compared to 61.1% in the pro forma year ended December 31, 2008. This improvement was primarily the result of a shift in product mix to a higher volume of products with lower cost as a percentage of revenue in our home respiratory service line; our ability to obtain favorable pricing on the purchase of products and supplies in all three of our service lines and the sale of the rehabilitation product line in June 2008. These favorable items were partially offset by a decrease in rental revenue with no corresponding decrease in fixed costs in our home medical equipment service line and a decrease in gross profit margin, as the result of the shift in product mix in our infusion therapy drugs to more specialty drugs, which have a higher product cost as a percentage of net revenues.
Provision for Doubtful Accounts. The provision for doubtful accounts is based on management’s estimate of the net realizable value of accounts receivable after considering actual write-offs of specific receivables.
69
Table of Contents
Accounts receivable estimated to be uncollectible are provided for by computing a required reserve using estimated future cash receipts based on historical cash receipts collections as a percentage of revenue. In addition, management may adjust for changes in billing practices, cash collection protocols or practices, or changes in general economic conditions, contractual issues with specific payors, new markets or products. The provision for doubtful accounts, expressed as a percentage of total net revenues, was 2.8% and 2.3% in the year ended December 31, 2009 and the pro forma year ended December 31, 2008, respectively. The increased provision for doubtful accounts in 2009 is primarily the result of favorable collections experience occurring in 2008 compared to 2009.
Selling, Distribution and Administrative Expenses. Selling, distribution and administrative expenses are comprised of expenses incurred in direct support of operations and those associated with administrative functions. Expenses incurred by the operating locations include salaries and other expenses in the following functional areas: selling, distribution, clinical services, warehousing and repair. Many of these operating costs are directly variable with revenue growth patterns. Some are also very sensitive to market-driven price fluctuations such as facility lease and fuel costs. The administrative expenses include overhead costs incurred by the operating locations, regional and corporate support functions. These expenses are generally less sensitive to fluctuations in revenue growth than operating costs.
Selling, distribution and administrative expenses, expressed as a percentage of total net revenues was 50.1% for the year ended December 31, 2009 compared to 50.8% for the pro forma year ended December 31, 2008. Adjusted for Medicare reimbursement reductions of $108.7 million, the selling, distribution and administrative expense percentage for 2009 would have been 47.7% of revenue.
Selling, distribution and administrative expenses decreased by $31.2 million for the year ended December 31, 2009 over the pro forma year ended December 31, 2008. The $31.2 million decrease was composed of a $12.9 million decrease in labor and other related expenses and by a decrease in other operating expenses of $18.3 million. The decrease in labor and other related expenses was primarily due to reduced salaries and wages as a result of headcount reductions in 2009, the sale of our rehabilitation product line in July 2008, the reduction in expenses related to equity incentive awards, and lower outside labor. These decreases were partially offset by increases due to higher management incentive compensation program expense as a result of meeting the targets in 2009 and not in 2008, and higher termination and retention expense due to the projects to outsource certain billing, collection and information technology functions, and the Merger. The decreases in other operating expenses of $18.3 million were primarily due to delivery costs, principally lower fuel prices, lower freight costs due to renegotiation of a contract with a vendor, reduction in expenses due to sale of our rehabilitation product line in July 2008 and lower depreciation on information technology assets. These decreases were partially offset by expenses incurred in 2009 associated with our projects to outsource certain billing, collection and information technology functions that did not exist in 2008, professional fees incurred in connection with the sale of our outstanding Series A-1 Notes and Series A-2 Notes and expenses associated with other corporate initiative projects in 2009.
Amortization of Intangible Assets. Amortization of intangible assets was $3.7 million in the year ended December 31, 2009 and in the pro forma year ended December 31, 2008. The amortization expense primarily results from the revaluation of intangible assets as a result of the Merger, including the finalization of the intangible asset valuation in 2009.
Interest Expense. Interest expense decreased $3.1 million, or 2.3%, to $129.2 million in the year ended December 31, 2009 from $132.3 million in the pro forma year ended December 31, 2008. This decrease is primarily due to a lower interest rate of 11.8% in the year ended December 31, 2009 as compared to 12.0% for the pro forma year ended December 31, 2008.
Interest Income and Other. Interest income and other decreased $1.3 million, or 44.1%, to $1.6 million in the year ended December 31, 2009 from $2.9 million in the pro forma year ended December 31, 2008.
70
Table of Contents
Income Tax Benefit. Income tax benefit increased $6.5 million to $(8.4) million in the year ended December 31, 2009 from $(1.9) million in the pro forma year ended December 31, 2008. The increase in income tax benefit in the year ended December 31, 2009 from the pro forma year ended December 31, 2008 resulted from (1) a reduction in our valuation allowances related to state net operating losses and other state deferred tax assets and (2) a decrease in our tax reserves due to settlements with state tax agencies and the expiration of statute of limitations. The reductions in our valuation allowances and tax reserves resulted in a 68.8% effective tax benefit for the year ended December 31, 2009 compared with 45.3% for the pro forma year ended December 31, 2008.
Segment Net Revenues and EBIT
The following table sets forth a summary of results of operations by segment:
(in thousands) | Year Ended December 31, 2009 | Percentage of Net Revenues | Pro Forma Year Ended December 31, 2008 | Percentage of Net Revenues | ||||||||
Net revenues: | ||||||||||||
Home respiratory therapy and home medical equipment | $ | 1,169,609 | 55.8 | % | $ | 1,284,278 | 60.3 | % | ||||
Home infusion therapy | 924,952 | 44.2 | 845,676 | 39.7 | ||||||||
Total net revenues | $ | 2,094,561 | 100.0 | % | $ | 2,129,954 | 100.0 | % | ||||
| Year Ended December 31, 2009 | |||||||||||||||
(in thousands) | Home Respiratory Therapy and Home Medical Equipment | Percentage of Segment Net Revenues | Home Infusion Therapy | Percentage of Segment Net Revenues | Total | ||||||||||
EBIT | $ | 50,167 | 4.3 | % | $ | 65,667 | 7.1 | % | $ | 115,834 | |||||
| Pro Forma Year Ended December 31, 2008 | |||||||||||||||
(in thousands) | Home Respiratory Therapy and Home Medical Equipment | Percentage of Segment Net Revenues | Home Infusion Therapy | Percentage of Segment Net Revenues | Total | ||||||||||
EBIT | $ | 111,708 | 8.7 | % | $ | 14,188 | 1.7 | % | $ | 125,896 | |||||
We allocate certain expenses that are not directly attributable to a product line based upon segment headcount. For a reconciliation of net income (loss) to EBIT, see the table under “Results of Operations—EBIT” at the end of this section.
Home Respiratory Therapy and Home Medical Equipment Segment: For the home respiratory therapy and home medical equipment segment total net revenues decreased $114.7 million, or 8.9%, to $1,169.6 million in the year ended December 31, 2009 from $1,284.3 million in the pro forma year ended December 31, 2008. Revenues for the home respiratory therapy and home medical equipment segment decreased to 55.8% of total revenue in the year ended December 31, 2009 from 60.3% in the pro forma year ended December 31, 2008.
Home respiratory therapy revenues are derived primarily from the provision of oxygen systems, home ventilators, obstructive sleep apnea equipment, nebulizers, respiratory medications and related services. Revenues from the home respiratory therapy service line decreased by 7.6% in the year ended December 31, 2009 compared to the pro forma year ended December 31, 2008. The majority of Medicare reimbursement reductions discussed above impacted the home respiratory therapy services line. Such reductions were $104.0 million for year ended December 31, 2009. Adjusted for the Medicare reimbursement reductions, respiratory therapy revenues for 2009 would have increased by 1.9%. The increase in revenue, excluding the Medicare reimbursement reductions, resulted primarily from increases in oxygen, sleep apnea, and ventilator revenue, partially offset by decreases in respiratory medication revenue and nebulizer revenue.
71
Table of Contents
Home medical equipment revenues are derived from the rental and sale of equipment to assist patients with ambulation, safety and general care in and around the home. Home medical equipment revenues decreased by 16.6% in the year ended December 31, 2009 compared to the pro forma year ended December 31, 2008. The decrease was primarily due to the sale of our rehabilitation product line in July 2008. In 2009, $1.9 million of the Medicare reimbursement reductions impacted this service line. Excluding the impact of the Medicare reimbursement reductions, home medical equipment revenues would have decreased 15.6% for the year ended December 31, 2009.
EBIT for the home respiratory and home medical equipment segment in the year ended December 31, 2009 was $50.2 million compared to $111.7 million in the pro forma year ended December 31, 2008. EBIT was 4.3% of segment net revenues in the year ended December 31, 2009 compared to the 8.7% of segment net revenues in the pro forma year ended December 31, 2008. This decrease in EBIT as a percentage of segment net revenues was primarily due to the negative impact of the Medicare reimbursement reductions and a decrease in rental revenue with no corresponding decrease in fixed costs in our home medical equipment service line. These decreases were partially offset by an improvement in gross profit margin as a result of a shift in product mix to a higher volume of products with lower cost as a percentage of segment net revenues in our home respiratory therapy service line; our ability to obtain favorable pricing on the purchase of products and supplies; and the sale of the rehabilitation product line in June 2008.
Home Infusion Therapy Segment. For the home infusion therapy segment total net revenues increased $79.3 million, or 9.4%, to $925.0 million in the year ended December 31, 2009 from $845.7 million in the pro forma year ended December 31, 2008. Revenues for the home infusion therapy segment increased to 44.2% of total revenue in the year ended December 31, 2009 from 39.7% in the pro forma year ended December 31, 2008.
The home infusion therapy segment involves the administration of drugs or nutrients directly into the body intravenously through a needle or catheter. Infusion therapy services also include administering enteral nutrients directly into the gastrointestinal tract through a feeding tube. Home infusion therapy revenues increased by 9.4% in the year ended December 31, 2009. The growth in revenue in the year ended December 31, 2009 resulted primarily from an increase in volume of specialty drugs, core drugs and enteral nutrients. The increase in these drugs was offset by a decrease in revenue due to the termination of a payor contract, and a decrease in volume of non-core revenue. As a result of increasing volume, specialty drug revenue also increased as a percentage of total home infusion therapy revenue. During the year ended December 31, 2009, Medicare reimbursement reductions impacted this service line by $2.8 million. Excluding the impact of Medicare reimbursement reductions, home infusion therapy revenue would have increased by 9.7%.
EBIT for the home infusion therapy segment in the year ended December 31, 2009 was $65.7 million compared to $14.2 million in the pro forma year ended December 31, 2008. EBIT was 7.1% of segment net revenues in the year ended December 31, 2009 compared to 1.7% of segment net revenues in the pro forma year ended December 31, 2008. This increase in EBIT was due to the increase in segment net revenues and the result of favorable product costs on our enteral nutrients and our ability to reduce our selling distribution and administrative costs as a percentage of segment net revenues. This favorability was partially offset by a decrease in gross profit margin, as the result of shift in product mix in our infusion therapy drugs to more specialty drugs, which have a higher product cost as a percentage of segment net revenues.
72
Table of Contents
The Period January 1, 2008 to October 28, 2008 Results Compared to Year Ended December 31, 2007 Results
(in thousands) | Period January 1, 2008 to October 28, 2008 | Percentage of Net Revenues | Year Ended December 31, 2007 | Percentage of Net Revenues | ||||||||||
Net revenues: | ||||||||||||||
Fee for service arrangements | $ | 1,630,767 | 92.0 | % | $ | 1,465,303 | 89.8 | % | ||||||
Capitation | 142,522 | 8.0 | 166,498 | 10.2 | ||||||||||
TOTAL NET REVENUES | 1,773,289 | 100.0 | 1,631,801 | 100.0 | ||||||||||
Costs and expenses: | ||||||||||||||
Cost of net revenues: | ||||||||||||||
Product and supply costs | 526,610 | 29.7 | 386,496 | 23.7 | ||||||||||
Patient service equipment depreciation | 89,246 | 5.0 | 110,775 | 6.8 | ||||||||||
Amortization of intangible assets | — | — | — | — | ||||||||||
Home respiratory therapy services | 31,893 | 1.8 | 38,886 | 2.4 | ||||||||||
Nursing services | 29,773 | 1.7 | 11,353 | 0.7 | ||||||||||
Other | 13,815 | 0.8 | 17,482 | 1.1 | ||||||||||
TOTAL COST OF NET REVENUES | 691,337 | 39.0 | 564,992 | 34.6 | ||||||||||
Provision for doubtful accounts | 33,626 | 1.9 | 43,138 | 2.6 | ||||||||||
Selling, distribution and administrative | 924,536 | 52.1 | 862,062 | 52.8 | ||||||||||
Amortization of intangible assets | 3,461 | 0.2 | 3,079 | 0.2 | ||||||||||
TOTAL COSTS AND EXPENSES | 1,652,960 | 93.2 | 1,473,271 | 90.3 | ||||||||||
OPERATING INCOME | 120,329 | 6.8 | 158,530 | 9.7 | ||||||||||
Interest expense | 31,838 | 1.8 | 22,447 | 1.4 | ||||||||||
Interest income and other | (2,154 | ) | (0.1 | ) | (1,954 | ) | (0.1 | ) | ||||||
INCOME BEFORE TAXES | 90,645 | 5.1 | 138,037 | 8.5 | ||||||||||
Income tax expense | 34,192 | 1.9 | 51,998 | 3.2 | ||||||||||
NET INCOME | $ | 56,453 | 3.2 | % | $ | 86,039 | 5.3 | % | ||||||
Net Revenues. Net revenues increased $141.5 million, or 8.7%, to $1,773.3 million in the period January 1, 2008 to October 28, 2008 from $1,631.8 million in the year ended December 31, 2007. The increase in revenue was primarily in our infusion service line, due to service line growth subsequent to our acquisition of Coram in December 2007, offset by the fact that the period January 1, 2008 to October 28, 2008 had approximately two months less than the year ended December 31, 2007. The revenue growth rate for the period January 1, 2008 to October 28, 2008 was impacted by incremental Medicare reimbursement reductions of $18.6 million due to reimbursement reductions imposed in 2007 and 2008. The Medicare reimbursement reductions in 2008 related to respiratory drug reimbursement reductions, which were effective April 1, 2008.
We expect to continue to face pricing pressures from Medicare and Medicaid as well as from our managed care customers as these payers seek to lower costs by obtaining more favorable pricing from providers such as us. In addition to the pricing reductions, such changes could cause us to provide reduced levels of certain products and services in the future, resulting in a corresponding reduction in revenue. However, given our high volume of managed care business, we are well-positioned among our competitors with respect to serving the managed care market with a diversified array of services. See “Business—Government Regulation.”
Gross Profit.Gross profit margin is defined as total net revenues less total costs of total net revenues divided by total net revenues. The gross profit margin for the period January 1, 2008 to October 28, 2008 was 61.0% compared to 65.4% for the year ended December 31, 2007. The decrease in gross margin is due to the acquisition of the Coram home infusion therapy business being included in the entire period of January 1, 2008 to October 31, 2008 and only in one month in the year end December 31, 2007. The Coram home infusion therapy business has a lower gross profit margin due to the nature of the infusion business compared to our home respiratory therapy and home medical equipment service lines. Excluding the Coram acquisition, the gross profit
73
Table of Contents
margin was 67.6% for the period January 1, 2008 to October 28, 2008 compared to 65.4% for the year ended December 31, 2007. This increase in the gross profit margin percentage resulted from our ability to secure favorable pricing on the purchases of products and supplies and lower depreciation expense.
Provision for Doubtful Accounts.The provision for doubtful accounts is based on management’s estimate of the net realizable value of accounts receivable after considering actual write-offs of specific receivables. Management considers historical realization data, accounts receivable aging trends, other operating trends, the extent of contracted business and business combinations. Also considered are relevant business conditions such as governmental and managed care payor claims processing procedures and system changes. Additionally, focused reviews of certain large and/or problematic payors are performed. The provision for doubtful accounts, expressed as a percentage of net revenues, was 1.9% in the period January 1, 2008 to October 28, 2008 and 2.6% for the year ended December 31, 2007. The decrease in the period January 1, 2008 to October 28, 2008 from the 2007 levels resulted primarily from a decrease in the aging of our accounts receivable.
Selling, Distribution and Administrative Expenses.Selling, distribution and administrative expenses are comprised of expenses incurred in direct support of operations and those associated with administrative functions. Expenses incurred by the operating locations include salaries and other expenses in the following functional areas: selling, distribution, clinical services, warehousing and repair. Many of these operating costs are directly variable with revenue growth patterns. Some are also very sensitive to market-driven price fluctuations such as facility lease and fuel costs. The administrative expenses include overhead costs incurred by the operating locations, regional and corporate support functions. These expenses do not fluctuate with revenue growth as closely as do operating costs.
Selling, distribution and administrative expenses, expressed as a percentage of total net revenues, was 52.1% for the period January 1, 2008 to October 28, 2008 compared to 52.8% for the year ended December 31, 2007. Selling, distribution and administrative expenses increased by $62.5 million for the period January 1, 2008 to October 28, 2008 compared to the year ended December 31, 2007. For period January 1, 2008 to October 28, 2008 labor and related expenses were 34.4% of net revenues compared to 35.1% for the year ended December 31, 2007. The decrease in labor and related expenses were due to the realization of certain synergies associated with the Coram transaction, lower management incentive compensation programs, lower salaries due to the sale of our rehab business and decreases due to changes in estimates related to health benefits. These decreases were partially offset by increases in labor and related expenses due to stock compensation expense from the change in control vesting due to the Merger and higher bonus and commission expenses, due to the revenue management team’s higher collection efforts and sale incentives being met above targeted amounts. Other operating expenses for the period January 1, 2008 to October 28, 2008 were 17.7% of net revenues compared to 17.8% for the year ended December 31, 2007. The decrease in other operating expenses was due to cost savings programs and lower delivery costs (primarily fuel and vehicle lease cost). These decreases were partially offset by the increases in expenses incurred related to the sale of the Company, which resulted in the Merger Agreement with Blackstone, costs incurred in support of our enterprise wide information system project, expenses incurred related to the sale of the rehabilitation product line in July 2008, and professional fees related to our accounts receivable reserve work.
Amortization of Intangible Assets.Amortization of intangible assets increased $0.4 million, or 12.4%, to $3.5 million in the period January 1, 2008 to October 28, 2008 from $3.1 million in year ended December 31, 2007. The increase in amortization expense in the period January 1, 2008 to October 28, 2008, when compared to the year ended December 31, 2007, resulted from our acquisition of Coram intangible assets in December 2007, offset by customer lists and covenants not to compete that became fully amortized in 2008.
Interest Expense.Interest expense increased $9.4 million, or 41.8%, to $31.8 million in the period January 1, 2008 to October 28, 2008 from $22.4 million in the year ended December 31, 2007. The increase in interest expense is partially offset by the fact that the period January 1, 2008 to October 28, 2008 had approximately two months less than the year ended December 31, 2007. The increase in interest expense in the
74
Table of Contents
period January 1, 2008 to October 28, 2008 is due to the funding fee of $5.6 million related to the $280 million credit facility, entered into on June 18, 2008 (the “Interim Facility”), increased costs due to the amortization of debt issuance costs related to the Interim Facility and the corresponding increase in our interest rate on the Interim Facility. These increases were in addition to the impact of the $359.0 million we borrowed to purchase Coram in December 2007 and the write-off of the remaining deferred debt issuance costs related to the prior credit agreement. See “—Liquidity and Capital Resources—Indebtedness—Pre-Transactions” below.
Interest Income.Interest income increased $0.2 million, or 10.2%, to $2.1 million in the period January 1, 2008 to October 28, 2008 from $1.9 million in the year ended December 31, 2007. The increase in interest income is partially offset by the fact that the period January 1, 2008 to October 28, 2008 had approximately two months less than the year ended December 31, 2007.
Income Tax Expense.Income tax expense decreased $17.8 million, or 34.2%, to $34.2 million in the period January 1, 2008 to October 28, 2008 from $52.0 million in the year ended December 31, 2007. The decrease in the period January 1, 2008 to October 28, 2008 from the year ended December 31, 2007 resulted from lower pre-tax earnings; thus, decreasing our income tax expense. This income tax expense decrease was partially off-set by various unfavorable tax expense increases including higher non-deductible expenses in the period January 1, 2008 to October 28, 2008 as compared to the year ended December 31, 2007.
Segment Net Revenues and EBIT
The following table sets forth a summary of results of operations by segment:
(in thousands) | Period January 1, 2008 to October 28, 2008 | Percentage of Net Revenues | Year Ended December 31, 2007 | Percentage of Net Revenues | |||||||||||
Net revenues: | |||||||||||||||
Home respiratory therapy and home medical equipment | $ | 1,074,711 | 60.6 | % | $ | 1,297,619 | 79.5 | % | |||||||
Home infusion therapy | 698,578 | 39.4 | 334,182 | 20.5 | |||||||||||
Total net revenues | $ | 1,773,289 | 100.0 | % | $ | 1,631,801 | 100.0 | % | |||||||
| Period January 1, 2008 to October 28, 2008 | |||||||||||||||
(in thousands) | Home Respiratory Therapy and Home Medical Equipment | Percentage of Segment Net Revenues | Home Infusion Therapy | Percentage of Segment Net Revenues | Total | ||||||||||
EBIT | $ | 102,927 | 9.6 | % | $ | 17,965 | 2.6 | % | $ | 120,892 | |||||
| Year Ended December 31, 2007 | |||||||||||||||
(in thousands) | Home Respiratory Therapy and Home Medical Equipment | Percentage of Segment Net Revenues | Home Infusion Therapy | Percentage of Segment Net Revenues | Total | ||||||||||
EBIT | $ | 118,242 | 9.1 | % | $ | 40,288 | 12.1 | % | $ | 158,530 | |||||
We allocate certain expenses that are not directly attributable to a product line based upon segment headcount. For a reconciliation of net income (loss) to EBIT, see the table under “Results of Operations—EBIT” at the end of this section.
Home Respiratory Therapy and Home Medical Equipment Segment: Net revenues for the home respiratory therapy and home medical equipment segment decreased $222.9 million, or 17.2%, to $1,074.7 million in the period January 1, 2008 to October 28, 2008 from the $1,297.6 million in the year ended December 31, 2007.
75
Table of Contents
Home respiratory therapy revenues are derived primarily from the provision of oxygen systems, home ventilators, sleep apnea equipment, nebulizers, respiratory medications and related services. Revenues from the respiratory therapy service line decreased by 16.0% in the period January 1, 2008 to October 28, 2008 compared to the year ended December 31, 2007. The decrease is primarily due to the period January 1, 2008 to October 28, 2008 having approximately two months less than the year ended December 31, 2007. The majority of the Medicare reimbursement reductions discussed above impacted the respiratory therapy line. Such reductions were $17.3 million in the period January 1, 2008 to October 28, 2008. The growth in revenue dollars for the period January 1, 2008 to October 28, 2008 resulted primarily from an increase in revenue from oxygen equipment rental revenue and an increase in the sale of Bi-level devices and related supplies. These increases were offset by a decrease in respiratory drug revenue, primarily as a result of the Medicare reimbursement reductions in this area. This was offset by the fact that there were approximately two less months in the period January 1, 2008 to October 28, 2008 compared to the year ended December 31, 2007.
Home medical equipment revenues are derived from the rental and sale of equipment to assist patients with ambulation, safety and general care in and around the home. Home medical equipment revenues decreased by 23.1% in the period January 1, 2008 to October 28, 2008 compared to the year ended December 31, 2007. The decrease is primarily due to the period January 1, 2008 to October 28, 2008 having approximately two months less than the year ended December 31, 2007. In the period January 1, 2008 to October 28, 2008, $1.3 million of the Medicare reimbursement reductions impacted this service line. Excluding the impact of the Medicare reimbursement reductions home medical equipment revenue would have decreased by 6.5%. The decrease in revenue dollars for the period January 1, 2008 to October 31, 2008 primarily resulted from the sale of our rehabilitation product line in July 2008.
EBIT for the home respiratory therapy and home medical equipment segment in the period January 1, 2008 to October 28, 2008 was $102.9 million compared to $118.2 million in the year ended December 31, 2007. EBIT was 9.6% of segment net revenues for the period January 1, 2008 to October 28, 2008 compared to 9.1% of segment net revenues in the year ended December 31, 2007. The increase in EBIT as a percentage of segment net revenues was due to the sale of our rehabilitation product line in July 2008 and securing favorable pricing on the purchases of products and supplies.
Home Infusion Therapy Segment. For the home infusion therapy segment total net revenues increased $364.4 million, or 109.0%, to $698.6 million in the period January 1, 2008 to October 28, 2008 from $334.2 million in the year ended December 31, 2007. Revenues for the home infusion therapy segment increased to 39.4% of total revenue in the period January 1, 2008 to October 28, 2008 from 20.5% in the year ended December 31, 2007.
Home infusion therapy involves the administration of drugs or nutrients directly into the body intravenously through a needle or catheter. Infusion therapy services also include administering enteral nutrients directly into the gastrointestinal tract through a feeding tube. Home infusion therapy revenues increased by 109.0% in the period January 1, 2008 to October 28, 2008 compared to the year ended December 31, 2007. The increase in infusion revenue was due to our acquisition of Coram in December 2007.
EBIT for the home infusion therapy segment in the period January 1, 2008 to October 28, 2008 was $18.0 million compared to $40.3 million in the year ended December 31, 2007. EBIT was 2.6% of segment net revenues in the period January 1, 2008 to October 28, 2008 compared to 12.1% of segment net revenues in the year ended December 31, 2007. The decrease in EBIT is due to the inclusion of the results of our acquisition of Coram in December 2007, as it was in all periods from January 1, 2008 to October 28, 2008 and only in one month in the year ended December 31, 2007. The decrease in the EBIT as a percentage of segment net revenues that occurred in the period January 1, 2008 to October 28, 2008 as compared to the year ended December 31, 2007 was also due to the Coram acquisition. The Coram infusion business has a lower margin compared to the infusion business that existed in the first eleven months of the year ended December 31, 2007.
76
Table of Contents
EBIT
EBIT is a measure used by our management to measure operating performance. EBIT is defined as net income (loss) plus interest expense and income taxes. EBIT is not a recognized term under GAAP and does not purport to be an alternative to net income as a measure of operating performance or to cash flows from operating activities as a measure of liquidity.
The following table provides a reconciliation from net income (loss) to EBIT:
| (in thousands) | Year Ended December 31, 2007 | Pro Forma Year Ended December 31, 2008 | |||||||||||||||||||
| Home Respiratory Therapy and Home Medical Equipment | Home Respiratory Therapy and Home Medical Equipment | Home Respiratory Therapy and Home Medical Equipment | Home Respiratory Therapy and Home Medical Equipment | Home Infusion Therapy | Total | ||||||||||||||||
Net income (loss) | $ | 86,039 | $ | (2,393 | ) | ||||||||||||||||
Interest expense, | 20,493 | 130,267 | |||||||||||||||||||
Income tax (benefit) expense | 51,998 | (1,978 | ) | ||||||||||||||||||
EBIT | $ | 118,242 | $ | 40,288 | $ | 158,530 | $ | 111,708 | $ | 14,188 | $ | 125,896 | |||||||||
| (in thousands) | Period January 1, 2008 to October 28, 2008 | Period October 29, 2008 to December 31, 2008 | |||||||||||||||||||
| Home Respiratory Therapy and Home Medical Equipment | Home Infusion Therapy | Total | Home Respiratory Therapy and Home Medical Equipment | Home Infusion Therapy | Total | ||||||||||||||||
Net income (loss) | $ | 56,453 | $ | (1,894 | ) | ||||||||||||||||
Interest expense, | 30,247 | 25,750 | |||||||||||||||||||
Income tax (benefit) expense | 34,192 | 659 | |||||||||||||||||||
EBIT | $ | 102,927 | $ | 17,965 | $ | 120,892 | $ | 26,255 | $ | (1,740 | ) | $ | 24,515 | ||||||||
| (in thousands) | Year Ended December 31, 2009 | ||||||||||||||||||||
| Home Respiratory Therapy and Home Medical Equipment | Home Infusion Therapy | Total | |||||||||||||||||||
Net loss | $ | (3,820 | ) | ||||||||||||||||||
Interest expense, | 128,092 | ||||||||||||||||||||
Income tax (benefit) expense | (8,438 | ) | |||||||||||||||||||
EBIT | $ | 50,167 | $ | 65,667 | $ | 115,834 | |||||||||||||||
77
Table of Contents
| (in thousands) | Three Months Ended March 31, 2009 | Three Months Ended March 31, 2010 | ||||||||||||||||||
| Home Respiratory Therapy and Home Medical Equipment | Home Infusion Therapy | Total | Home Respiratory Therapy and Home Medical Equipment | Home Infusion Therapy | Total | |||||||||||||||
Net loss | $ | (477 | ) | $ | (803 | ) | ||||||||||||||
Interest expense, net (a) | 32,216 | 32,376 | ||||||||||||||||||
Income tax (benefit) expense | 1,180 | (884 | ) | |||||||||||||||||
EBIT | $ | 23,523 | $ | 9,396 | $ | 32,919 | $ | 9,866 | $ | 20,823 | $ | 30,689 | ||||||||
| (a) | Reflects $22.4 million of interest expense, net of $1.9 million in interest income for 2007. Reflects $132.3 million of interest expense, net of $2.0 million in interest income for the pro forma year ended December 31, 2008. Reflects $31.8 million of interest expense, net of $1.6 million of interest income for the period January 1, 2008 to October 28, 2008. Reflects $26.2 million of interest expense, net of $0.4 million of interest income for the period October 29, 2008 to December 31, 2008. Reflects $129.2 million of interest expense, net of $1.1 million of interest income for 2009. Reflects $32.4 million of interest expense, net of $0.2 million of interest income for the three months ended March 31, 2009. Reflects $32.6 million of interest expense, net of $0.2 million of interest income for the three months ended March 31, 2010. |
We allocate certain expenses that are not directly attributable to a product line based upon segment headcount. For a reconciliation of net income (loss) to EBIT, see the table under “Results of Operations—EBIT” at the end of this section.
Impact of Inflation and Changing Prices
We experience pricing pressures in the form of continued reductions in reimbursement rates, particularly from managed care organizations and from governmental payors such as Medicare and Medicaid. We are also impacted by rising costs for certain inflation-sensitive operating expenses such as labor and employee benefits, facility and equipment leases, and vehicle fuel. However, we generally do not believe these impacts are material to our revenues or net income.
Liquidity and Capital Resources
Our principal source of liquidity is our operating cash flow, which is supplemented by our ABL Facility, which provides for revolving credit of up to $150.0 million, subject to borrowing base availability. See “Description of Other Indebtedness—Senior Secured Asset-Based Revolving Credit Facility.” In recent years, we have generated operating cash flows in excess of our operating needs, which has afforded us the ability to pursue acquisitions and fund patient service equipment purchases to support revenue growth. We believe that our operating cash flow, together with our existing cash, cash equivalents, investments and ABL Facility, will continue to be sufficient to fund our operations and growth strategies for at least the next 12 months.
Our short-term investments consist of certificates of deposit with maturities greater than three months from our purchase date. As of March 31, 2010, the carrying value of our short-term investments approximates fair value and such investments do not individually exceed FDIC insurance limits.
Prior to the Merger we had initiated a project to implement a new enterprise-wide information system. The overall objective of the project was to deliver the necessary technology and automation across the organization to enable improvements in service, productivity and access to information. Development on certain modules commenced in 2006 and continued in 2007 and 2008. In connection with the Merger, we evaluated our information technology strategy and concluded that it was no longer advisable to implement a new enterprise- wide information system. As a result of this change in strategy, we wrote off approximately $65.0 million of capitalized information systems assets as part of our purchase accounting adjustments that were made in connection with the Merger.
78
Table of Contents
In the three months ended March 31, 2010, our free cash flow was $(12.2) million. For the three months ended March 31, 2009 our free cash flow was $(10.2) million. We use free cash flow as a performance metric which is not calculated in accordance with GAAP. Free cash flow is defined as cash provided by operating activities less purchases of patient service equipment and property, equipment and improvements, exclusive of effects of acquisitions. It is presented as a supplemental performance measure and is not intended as an alternative to any other cash flow measure calculated in accordance with GAAP. Further, free cash flow may not be comparable to similarly titled measures used by other companies. A table reconciling free cash flow to net cash provided by operating activities is presented below.
(in thousands) | Three Month Ended March 31, 2010 | Three Month Ended March 31, 2009 | ||||||
| Reconciliation — Free Cash Flow: | ||||||||
Net (loss) income | $ | (803 | ) | $ | (477 | ) | ||
Non-cash items | 57,248 | 55,954 | ||||||
Change in operating assets and liabilities | (41,340 | ) | (25,805 | ) | ||||
Net cash provided by operating activities | 15,105 | 29,672 | ||||||
Less: Purchases of patient service equipment and property, equipment and improvements | (27,319 | ) | (39,843 | ) | ||||
Free cash flow | $ | (12,214 | ) | $ | (10,171 | ) | ||
Cash Flow.The following table presents selected data from our consolidated statement of cash flows:
(in thousands) | Three Month Ended March 31, 2010 | Three Month Ended March 31, 2009 | ||||||
Net cash provided by operating activities | $ | 15,105 | $ | 29,672 | ||||
Net cash used in investing activities | (24,013 | ) | (39,592 | ) | ||||
Net cash used in financing activities | (15,866 | ) | (7,505 | ) | ||||
Net decrease in cash and equivalents | (24,774 | ) | (17,425 | ) | ||||
Cash and equivalents at beginning of period | 158,163 | 168,018 | ||||||
Cash and equivalents at end of period | $ | 133,389 | $ | 150,593 | ||||
The Three Months Ended March 31, 2010 Results Compared to the Three Months Ended March 31, 2009
Net cash provided by operating activities in the three months ended March 31, 2010 was $15.1 million compared to $29.7 million in the three months ended March 31, 2009, a decrease of $14.6 million. The decrease in net cash provided by operating activities resulted from a $0.9 million increase in income before non-cash items to $56.4 million in 2010 from $55.5 million in 2009, offset by a $15.5 million increase in the cash used related to the change in operating assets and liabilities to a $41.3 million use of cash in 2010 from a $25.8 million use of cash in 2009.
The $15.5 million increase in cash used related to the change in operating assets and liabilities consisted primarily of the following:
| • | $6.8 million increase in cash provided by inventories to a $8.3 million provision of cash in the three months ended March 31, 2010 from a $1.5 million provision of cash in the three months ended March 31, 2009. The increase is primarily due to a decrease in our infusion and respiratory therapy and home medical equipment inventories. |
79
Table of Contents
| • | $2.9 million decrease in cash used by deferred revenue, net of expenses, to a $0.4 million use of cash in the three months ended March 31, 2010 from a $3.3 million use of cash in the three months ended March 31, 2009. The decrease is due primarily to the recognition of revenue in the three months ended March 31, 2009 that was previously deferred for services initiated prior to certain 2009 Medicare reimbursement reductions. |
| • | $2.9 million decrease in cash used by prepaid expenses and other assets to a $5.8 million use of cash in the three months ended March 31, 2010 from a $8.7 million use of cash in the three months ended March 31, 2009. The decrease is due primarily to the accrual of tax benefits costs related to the Merger and payment of estimated taxes for the full year of 2009 in the three months ended March 31, 2009. |
Offset by:
| • | $22.2 million increase in cash used by accounts receivable to a $47.1 million use of cash in the three months ended March 31, 2010 from a $24.9 million use of cash in the three months ended March 31, 2009. The increase is primarily related to delays in collections due to the initial outsourcing of our billing and collections process. |
| • | $5.9 million increase in cash used by accounts payable to a $13.2 million use of cash in the three months ended March 31, 2010 from a $7.3 million use of cash in the three months ended March 31, 2009. The increase is primarily due to the timing of payment of invoices. |
Net cash used in investing activities in the three months ended March 31, 2010 was $24.0 million, compared to $39.6 million in the three months ended March 31, 2009. The primary use of funds in 2010 was $27.3 million to purchase patient service equipment and property equipment and improvements; $21.4 million related to patient service equipment and $5.9 million related to property, equipment and improvements, primarily due to additions to our information systems hardware and software. Additionally, $12.7 million related to short-term investments that matured during the period which was partially offset by purchases of $8.2 million during this same period. The primary use of funds in 2009 was approximately $39.8 million to purchase patient service equipment and property equipment and improvements; $31.3 million related to patient service equipment and $8.5 million related to property, equipment and improvements, primarily due to additions to our information systems hardware and software and leaseshold improvements on new facilities.
Net cash used in financing activities in the three months ended March 31, 2010 was $15.9 million compared to $7.5 million in the three months ended March 31, 2009. Net cash used in financing activities in the three months ended March 31, 2010 primarily reflected the use of $14.1 million to pay down the book cash overdraft reported in accounts payable and debt issuance costs of $1.2 million incurred during the period. Net cash used in financing activities in the three months ended March 31, 2009 primarily reflected repayment of $5.7 million on the ABL Facility (net of additional borrowings during the period), in addition to $2.0 million used to pay down the book cash overdraft reported in accounts payable.
The Year Ended December 31, 2009 Results
Our cash and equivalents were $158.2 million at December 31, 2009. Net cash from operating activities was $169.4 million for the year ended December 31, 2009. Cash provided by operating activities was primarily the result of our net loss for the year ended December 31, 2009 adjusted for non-cash items, principally our provision for doubtful accounts, depreciation and amortization of intangible assets. These amounts were offset by changes in operating assets, primarily decreases in accounts receivable and accounts payable. Net cash used in investing activities was $168.7 million primarily due to $150.6 million of capital expenditures for normal course of business purchases primarily related to patient service and net, short-term investment purchases of $23.7 million. Our net cash used in financing activities were $10.6 million in the year ended December 31, 2009. This was primarily due to incurring debt issuance costs in connection with the sale of our outstanding Series A-1 Notes and Series A-2 Notes. As a result of the Merger on October 28, 2008, the Company has concluded that a comparison of the year ended December 31, 2009 to the period October 29, 2008 to December 31, 2008 does not provide a meaningful comparison and has chosen to discuss only the year ended December 31, 2009.
80
Table of Contents
The Period October 29, 2008 to December 31, 2008 Results
Our cash increased to $168.0 million at December 31, 2008 from $48.2 million at October 29, 2008. Net cash from operating activities was $63.3 million for the period October 29, 2008 to December 31, 2008. Net cash used in investing activities was $75.5 million primarily due to $49.3 million of Merger-related costs and capital expenditures of $26.2 million for normal course of business purchases primarily related to patient service equipment. Our net cash provided by financing activities increased by $131.9 million in the period due to Merger related activities. Overall, the net increase in cash was $119.8 million.
The Period January 1, 2008 to October 28, 2008 Results Compared to the Year Ended December 31, 2007 Results
Net cash provided by operations in the period January 1, 2008 to October 28, 2008 was $297.9 million compared to $294.0 million in the year ended December 31, 2007, an increase of $3.9 million. Changes in cash provided by operations from the period January 1, 2008 to October 28, 2008 from the year ended December 31, 2007 is partially due to the fact that the period January 1, 2008 to October 28, 2008 had approximately two less months. Net cash provided by operations for the ten months ended October 31, 2007 was $237.7 million. The increase in net cash provided by operations for the period January 1, 2008 to October 28, 2008 compared to the ten months ended October 31, 2007, resulted from a $29.8 million increase in the cash used by the change in operating assets and liabilities to a $17.6 million use of cash in the period January 1, 2008 to October 28, 2008 from a $12.2 million provision of cash in the ten months ended October 31, 2007, offset by a $90.0 million increase in income before non-cash items to $315.5 million in 2008 from $225.5 million in 2007.
The $29.8 million increase in cash used by the change in operating assets and liabilities consisted primarily of the following:
| • | $23.8 million increase in cash used by income taxes payable to a $3.5 million use of cash in the period January 1, 2008 to October 28, 2008 from a $20.3 million provision of cash in the ten months ended October 31, 2007. The net increase in cash used was primarily due to a reduction in taxable income for the period of January 1, 2008 to October 28, 2008 as compared to the ten months ended October 31, 2007. The reduction in taxable income was caused by lower comparative pre-tax earnings, usage of Coram’s net operating loss carryforwards during 2008 and certain favorable tax deductions in the period of January 1, 2008 to October 28, 2008. This increase in cash used was off-set by a net increase in cash provided by income taxes payable which was primarily due to the receipt of significant tax refunds in 2008 resulting from certain favorable 2007 tax return to tax provision adjustments. |
| • | $16.1 million increase in cash used by accrued payroll and related taxes and benefits to a $13.5 use of cash in the period January 1, 2008 to October 28, 2008 from a $2.6 million source in the ten months ended October 31, 2007. The increase was primarily due to an $11.7 million increase for management incentive compensation programs. |
| • | $4.7 million was due to an increase in cash used by the change in inventories, to a $1.2 million use of cash in the period January 1, 2008 to October 28, 2008 from a $3.5 million provision of cash in the ten months ended October 31, 2007, primarily due to a purchase of inventory to be utilized over a ten-month period. |
Offset by:
| • | $13.7 million decrease in cash used in accounts receivable, to a $8.9 million use of cash in the period January 1, 2008 to October 28, 2008 from a $22.6 million use of cash in the ten months ended October 31, 2007. This decrease in use of cash was primarily due to a net increase in accounts receivable primarily related to the Coram acquisition. |
| • | $3.0 million increase in cash provided by prepaid expenses and other current assets to a $4.5 million provision of cash in the period January 1, 2008 to October 31, 2008 from a $1.5 million provision of cash in the ten months ended October 31, 2007. The increase was primarily due to prepaid inventory of $6.2 million due to a change in payment terms, offset by a decrease in prepaid insurance of $3.6 million. |
81
Table of Contents
Investing activities used $154.5 million in the period January 1, 2008 to October 28, 2008 compared to $483.2 million in the year ended December 31, 2007. Investing activities used $90.5 million in the ten months ended October 31, 2007. The primary use of funds in 2008 was approximately $157.2 million to purchase patient service equipment and property equipment and improvements; $104.6 million related to patient service equipment and $52.6 million related to property, plant and equipment, primarily due to additions to our information systems hardware and software, which was subsequently adjusted due to a change in strategy. The primary use of funds in the year ended December 31, 2007 was $350.0 million to purchase Coram in December 2007 and $128.8 million to purchase patient service equipment and property, equipment and improvements. The primary use of funds in the ten months ended October 31, 2007 was approximately $90.8 million to purchase patient service equipment for $62.5 million and property, equipment and improvements of $28.3 million.
Net cash used in financing activities in the period January 1, 2008 to October 28, 2008 was $123.7 million compared to net cash provided by financing activities of $203.0 million in the year ended December 31, 2007. Net cash used in financing activities in the ten months ended October 31, 2007 was $131.6 million. In the period January 1, 2008 to October 28, 2008, net cash used in financing activities primarily reflected our borrowing of $250.0 million in proceeds from the Interim Facility and $18.3 million under the Predecessor Revolving Credit Facility, offset by $138.3 million in repayments on the Predecessor Revolving Credit Facility, defined below, and $249.8 million in cash used for the redemption of our convertible senior notes. Net cash used in financing activities for the ten months ended October 31, 2007 reflected our repayment of $150.0 million on the Predecessor Revolving Credit Facility, offset by the issuance of common stock for $17.0 million in connection with the granting of equity awards and the exercises of stock options.
Contractual Cash Obligations.The following table summarizes the long-term cash payment obligations as of December 31, 2009 to which we are contractually bound. The years presented below represent 12-month periods ending December 31.
| Less than 1 Year | 1-3 Years | 3-5 Years | More than 5 Years | Totals | |||||||||||
| (in millions) | |||||||||||||||
Series A-1 Notes | $ | — | $ | — | $ | 700 | $ | — | $ | 700 | |||||
Series A-2 Notes | — | — | 318 | — | 318 | ||||||||||
ABL Facility(1) | — | — | — | — | — | ||||||||||
Interest Payments on Series A-1 Notes(2) | 79 | 158 | 157 | — | 394 | ||||||||||
Interest Payments on Series A-2 Notes(3) | 39 | 79 | 78 | — | 196 | ||||||||||
Fees on ABL Facility(1)(4) | 2 | 3 | 1 | — | 6 | ||||||||||
Operating Leases | 62 | 93 | 32 | 10 | 197 | ||||||||||
Capitalized Leases | 2 | 1 | — | — | 3 | ||||||||||
Purchase Obligations(5) | 49 | 112 | 113 | 128 | 402 | ||||||||||
Unrecognized Tax Benefits(6) | — | — | — | — | — | ||||||||||
Total Contractual Cash Obligations | $ | 233 | $ | 446 | $ | 1,399 | $ | 138 | $ | 2,216 | |||||
| (1) | Borrowings under the ABL Facility bear interest at a rate per annum equal to, at our option, either (a) a base rate determined by reference to the higher of (1) the prime rate of Bank of America, N.A. and (2) the federal funds effective rate plus 1/2 of 1%, plus an applicable margin of 2.00% or (b) a LIBOR rate determined by reference to LIBOR, adjusted for statutory reserve requirements, plus an applicable margin of 3.00%. The applicable margin for borrowings under our ABL Facility is subject to step ups and step downs based on average excess availability under the ABL Facility. The actual amounts of interest and fee payments under the ABL Facility will ultimately depend on the amount of debt and letters of credit outstanding and the interest rates in effect during each period. We are also required to pay customary letter of credit fees equal to the applicable margin on LIBOR loans and certain agency fees. |
| (2) | Represents aggregate interest payments on $700.0 million of the Series A-1 Notes that are paid semi-annually in May and November. Interest payments on the Series A-1 Notes will total approximately $78 million annually until the Series A-1 Notes mature on November 1, 2014. The Series A-1 Notes bear interest at 11.25% per annum. |
| (3) | Represents aggregate interest payments on $317.5 million of the Series A-2 Notes that are paid semi-annually in May and November. Interest payments on the Series A-2 Notes will total approximately $39 million annually until the Series A-2 Notes mature on November 1, 2014. The Series A-2 Notes bear interest at 12.375% per annum. |
82
Table of Contents
| (4) | The fees payable on the ABL Facility are based on an assumed fee for undrawn amounts of 1.00%, which represents the fees payable under the ABL Facility assuming no borrowings or drawn letters of credit. We are required to pay a commitment fee on the ABL Facility, in respect of the unutilized commitments there under, ranging from 0.50% to 1.00% per annum, which fee is determined based on the utilization of our ABL Facility (increasing when utilization is low and decreasing when utilization is high). The fees also include an administrative fee of $31,250 which is paid quarterly. |
| (5) | The purchase obligations primarily relate to: (i) approximately $197 million due under the Intelenet Agreement (as described under “Business—Outsourcing Activities” below), pursuant to which we outsource to Intelenet certain functions relating to billing, collections and other administrative and clerical services and (ii) approximately $204 million due under an agreement with Dell Services (formerly Perot Systems Corporation), pursuant to which we outsource to Dell Services (formerly Perot Systems Corporation) certain information technology functions. As of March 31, 2010 the purchase obligation related to the Intelenet Agreement was approximately $172 million. See “Business—Outsourcing Activities.” |
| (6) | Gross unrecognized tax benefits of $23.6 million are included within “Income Taxes Payable and Other Non-current Liabilities” in the total liabilities section of our March 31, 2010 consolidated balance sheet. The entire $24.0 million amount is not reflected in the contractual cash obligations table above since we cannot make a reliable estimate of the period in which cash payments will occur. |
Accounts Receivable.Accounts receivable before allowance for doubtful accounts increased to $327.6 million as of March 31, 2010 from $292.1 million at December 31, 2009. Days sales outstanding (calculated as of each period-end by dividing accounts receivable, less allowance for doubtful accounts, by the rolling average of total net revenues) were 50 days at March 31, 2010, compared to 43 days at December 31, 2009. The increase in accounts receivable and days sales outstanding is a direct result of the delays in cash collections due to the outsourcing of our billing and collections process.
Accounts aged in excess of 180 days expressed as percentages of total receivables for certain major payor categories, and in total, are as follows:
| March 31, 2010 | December 31, 2009 | |||||
Total | 17.8 | % | 18.3 | % | ||
Medicare | 17.8 | % | 18.0 | % | ||
Medicaid | 22.3 | % | 19.5 | % | ||
Patient Self pay | 24.9 | % | 31.3 | % | ||
Managed care/other | 16.0 | % | 16.6 | % |
Included in accounts receivable are earned but unbilled receivables of $49.5 million and $44.6 million at March 31, 2010 and December 31, 2009, respectively. Delays, ranging from a day up to several weeks, between the date of service and billing can occur due to delays in obtaining certain required payor-specific documentation from internal and external sources. Earned but unbilled receivables are aged from date of service and are considered in our analysis of historical performance and collectibility.
Inventories and Patient Service Equipment.Inventories consist primarily of pharmaceuticals and disposable products used in conjunction with patient service equipment. Patient service equipment consists of respiratory and home medical equipment that is provided to in-home patients for the course of their care plan, normally on a rental basis, and subsequently returned to us for redistribution after cleaning and maintenance is performed.
The branch locations serve as the primary point from which inventories and patient service equipment are delivered to patients. Certain products and services, such as infusion therapy and respiratory medications, bypass the respiratory/home medical equipment branches and are provided directly to patients from pharmacies or other central locations. The branches are supplied with inventory and equipment from central warehouses that service specific areas of the country. Such warehouses are also responsible for repairs and scheduled maintenance of patient service equipment, which adds to the frequent movement of equipment between locations. Further, the majority of our patient service equipment is located in patients’ homes. While utilization varies widely between equipment types, on the average, approximately 86% of equipment is on rent at any given time. Inherent in this asset flow is the fact that losses will occur. Depending on the product type, we perform physical inventories on
83
Table of Contents
an annual or quarterly basis. Inventory and patient service equipment balances in the financial records are adjusted to reflect the results of these physical inventories. Inventory and patient service equipment gains and losses for the three months ended March 31, 2010 and 2009 were gains of $0.1 million and losses of $0.7 million, respectively.
Indebtedness—Pre-Transactions
2004 Senior Secured Revolving Credit Facility.On November 23, 2004, we entered into a senior secured revolving credit facility with Bank of America and a syndicate of lenders that was amended effective June 23, 2006. The amendment extended the maturity date from November 23, 2009 to June 23, 2011 and lowered the applicable interest rate margins and commitment fees. The 2004 senior secured credit agreement was structured as a $500 million revolving credit facility. In connection with the Merger on October 28, 2008, we repaid all outstanding indebtedness under our 2004 senior secured revolving credit facility.
Convertible Senior Notes. In August 2003, we issued 3.375% Convertible Senior Notes due 2033 in the aggregate principal amount of $250 million under an indenture between us and U.S. Bank National Association in a private placement. Holders of our convertible senior notes had the right to require us to redeem on September 1, 2008 some or all of their notes at a price equal to 100% of the principal amount thereof plus accrued and unpaid interest. The holders of substantially all of the convertible senior notes exercised this right. On September 2, 2008, proceeds of the Interim Facility (described below) were used to fund repurchases of $249.8 million of our convertible senior notes. In addition, holders of the remaining $0.2 million of the convertible senior notes had the right, as a result of the change of control resulting from the Merger, to cause us to repurchase the convertible senior notes at a price equal to 100% of the principal amount thereof plus accrued and unpaid interest. Pursuant to this right, holders of $151,000 of convertible senior notes exercised their option in December 2008. Approximately $77,000 of convertible senior notes remain outstanding in accordance with the terms of the indenture governing the convertible senior notes.
Interim Facility. On June 18, 2008, we entered into a $280 million Interim Facility pursuant to the credit agreement with Banc of America Bridge LLC, Barclays Capital, the investment banking division of Barclays Bank PLC, Wells Fargo Securities, LLC (formerly known as Wachovia Capital Markets, LLC) and the lenders named therein. On September 2, 2008, proceeds of the Interim Facility were used to fund repurchases of our convertible senior notes as described above. The loans under the Interim Facility bore interest at a rate of 11% per year with a maturity date of March 1, 2009. In addition, we paid usual and customary bank fees in connection with entering into the Interim Facility. In connection with the Merger, all borrowings under the Interim Facility were paid off on October 28, 2008 using a portion of the proceeds from the Original Financing.
Indebtedness—Post-Transactions
After the consummation of the Merger we became, and we continue to be, highly leveraged. As of March 31, 2010, our total indebtedness was $1,020.7 million, and we would have had an additional $133.9 million of available borrowings under our ABL Facility, less any limitations on borrowing resulting from actual collateral availability. As of March 31, 2010, the additional availability under our ABL Facility based on the borrowing base as of such date was $133.9 million.
Our liquidity requirements are and will be significant, primarily due to debt service requirements. Our net cash interest expense for the three months ended March 31, 2010 and the year ended December 31, 2009 was $29.8 million and $120.1 million, respectively.
We believe that our existing cash, plus the amounts we expect to generate from operations and amounts available through our ABL Facility, will be sufficient to meet our operating needs for the next twelve months, including working capital requirements, capital expenditures, debt repayment obligations and potential new acquisitions.
84
Table of Contents
While we currently believe that we are in compliance with all of the financial covenants included in our ABL Facility, there is no assurance that we will continue to be able to do so in the future or that, if we do not, we will be able to obtain from our lenders waivers of default or amendments to the credit agreement governing our ABL Facility in the future.
As market conditions warrant, we and our major equityholders, including the Sponsor and its affiliates, may from time to time, depending upon market conditions, seek to repurchase debt securities that we have issued or loans that we have borrowed, including the Notes and the ABL Facility, in privately negotiated or open market transactions, by tender offer or otherwise.
ABL Facility. In connection with the Merger on October 28, 2008, we entered into the ABL Facility with Bank of America, N.A., as administrative agent and collateral agent, Wachovia Bank, National Association and Barclays Capital, the investment banking division of Barclays Bank PLC, as syndication agents, and The Bank of Nova Scotia, as documentation agent, and a syndicate of financial institutions and institutional lenders. Banc of America Securities LLC and Wells Fargo Securities, LLC (formerly known as Wachovia Capital Markets, LLC) acted as joint lead arrangers and Banc of America Securities LLC, Wells Fargo Securities, LLC (formerly known as Wachovia Capital Markets, LLC) and Barclays Capital, the investment banking division of Barclays Bank PLC, acted as joint bookrunners. Set forth below is a summary of the terms of our ABL Facility.
Our ABL Facility provides for revolving credit financing of up to $150.0 million, subject to borrowing base availability, with a maturity of five years, including both a letter of credit and swingline loan sub-facility. The borrowing base at any time is equal to the sum (subject to certain reserves and other adjustments) of 85% of eligible receivables and the lesser of (a) 85% of the net orderly liquidation value of eligible inventory and (b) $20.0 million.
Our ABL Facility includes borrowing capacity available for letters of credit and for borrowings on same-day notice, referred to as swingline loans.
Borrowings under our ABL Facility are subject to the satisfaction of customary conditions, including absence of a default and accuracy of representations and warranties.
Provided that no default or event of default is then existing or would arise therefrom, at our option, we may request that our ABL Facility be increased by an amount not to exceed $25.0 million, subject to certain consent rights of the administrative agent, swingline lender and issuing banks with respect to the lenders providing commitments for such increase. The terms of such incremental revolving facility shall be identical to our ABL Facility.
Interest Rate and Fees. Borrowings under our ABL Facility bear interest at a rate per annum equal to, at our option, either (a) a base rate determined by reference to the higher of (1) the prime rate of Bank of America, N.A. and (2) the federal funds effective rate plus 1/2 of 1% (“Base Rate”), plus an applicable margin of 2.00% or (b) a LIBOR rate determined by reference to the London Interbank Offered Rate, adjusted for statutory reserve requirements (“LIBOR”), plus an applicable margin of 3.00%. Beginning on April 1, 2009, the applicable margin for borrowings under our ABL Facility will be subject to step ups and step downs based on average excess availability under the ABL Facility. In addition to paying interest on outstanding amounts under our ABL Facility, we are required to pay a commitment fee, in respect of the unutilized commitments thereunder, ranging from 0.50% to 1.00% per annum, which fee will be determined based on utilization of our ABL Facility (increasing when utilization is low and decreasing when utilization is high). We must also pay customary letter of credit fees equal to the applicable margin on LIBOR loans and agency fees.
Repayments. If at any time the aggregate amount of outstandings under our ABL Facility, including letter of credit outstandings and swingline loans, exceeds the lesser of (i) the aggregate commitments under our ABL Facility and (ii) the borrowing base (except as a result of overadvance loans or protective advances), we will be
85
Table of Contents
required to repay outstanding loans (including swingline loans) and repay or cash collateralize letters of credit in an aggregate amount equal to such excess. If the amount available under our ABL Facility is less than 12.5% of the lesser of the aggregate commitments or the borrowing base for five consecutive business days or certain events of default have occurred, we will be required, upon the occurrence and during the continuance of such cash dominion event, to deposit cash from our material deposit accounts daily in a core concentration account maintained with the administrative agent under our ABL Facility, which will be used to repay outstanding loans and cash collateralize letters of credit.
We may voluntarily reduce the unutilized portion of the commitment amount and repay outstanding loans at any time (subject to minimum repayment amounts and customary notice periods) without premium or penalty other than customary “breakage” costs with respect to LIBOR loans.
Amortization and Final Maturity. There is no scheduled amortization under our ABL Facility. All outstanding loans under the facility are due and payable in full on the fifth anniversary of the closing date.
Guarantees and Security. All obligations under our ABL Facility, any interest rate protection or other hedging arrangements entered into with any lender in the syndicate or any of its affiliates, and cash management obligations owing to any lender in the syndicate or any of its affiliates are unconditionally guaranteed by our parent and substantially all of our existing and future, direct and indirect, wholly-owned domestic restricted subsidiaries. All obligations under our ABL Facility, any interest rate protection or other hedging arrangements entered into with any lender in the syndicate or any of its affiliates, and cash management obligations owing to any lender in the syndicate or any of its affiliates, and the guarantees of those obligations, are secured, subject to certain exceptions, by substantially all of our assets and the assets of the guarantors, including:
| • | a first-priority security interest in substantially all personal property consisting of accounts receivable arising from the sale of inventory and other goods and services, inventory, intercompany notes and intangible assets to the extent attached to the foregoing, and certain related assets and proceeds of the foregoing; and |
| • | a second-priority security interest in all tangible and intangible assets that secure the Notes on a first-priority basis. |
Restrictive Covenants and Other Matters. Our ABL Facility requires that if excess availability is less than 12.5% of the lesser of the aggregate commitments and the borrowing base, we comply with a minimum fixed charge coverage ratio test. In addition, our ABL Facility includes negative covenants that, subject to significant exceptions, limit our ability and the ability of our parent and subsidiaries to, among other things:
| • | incur, assume or permit to exist additional indebtedness or guarantees; |
| • | incur liens; |
| • | make investments and loans; |
| • | pay dividends, make payments or redeem or repurchase capital stock; |
| • | engage in mergers, liquidations, dissolutions, asset sales and other dispositions (including sale leaseback transactions); |
| • | prepay, redeem or purchase certain indebtedness; |
| • | amend or otherwise alter terms of certain indebtedness; |
| • | enter into agreements limiting subsidiary distributions; |
| • | engage in certain transactions with affiliates; |
| • | alter the business that we conduct; |
| • | change our fiscal year; and |
| • | with respect to our parent, engage in any activities not permitted. |
86
Table of Contents
Our ABL Facility contains certain customary representations and warranties, affirmative covenants and events of default, including among other things payment defaults, breach of representations and warranties, covenant defaults, cross-defaults to certain indebtedness, certain events of bankruptcy, certain events under ERISA, material judgments, failure of any guaranty or security document supporting our ABL Facility to be in full force and effect, and change of control. If such an event of default occurs, the lenders under our ABL Facility would be entitled to take various actions, including the acceleration of amounts due under our ABL Facility and all actions permitted to be taken by a secured creditor.
As of March 31, 2010, there were no borrowings under the ABL Facility, outstanding letters of credit totaled $16.1 million and additional availability under our ABL Facility subject to the borrowing base was $133.9 million. As of March 31, 2010, the additional availability under our ABL Facility based on the borrowing base as of such date was $133.9 million. At March 31, 2010, we were in compliance with all of the financial covenants required by the credit agreement governing our ABL Facility.
Senior Secured Bridge Credit Agreement. In connection with the Merger on October 28, 2008, we entered into a $1,010.0 million senior secured bridge credit agreement with Banc of America Bridge LLC, as administrative agent, Bank of America, N.A., as collateral agent, Wachovia Bank, National Association and Barclays Capital, the investment banking division of Barclays Bank PLC, as syndication agents, and The Bank of Nova Scotia, as documentation agent and a syndicate of financial institutions and institutional lenders. Banc of America Securities LLC and Wells Fargo Securities, LLC (formerly known as Wachovia Capital Markets, LLC) acted as joint lead arrangers and Banc of America Securities LLC, Wells Fargo Securities, LLC (formerly known as Wachovia Capital Markets, LLC) and Barclays Capital, the investment banking division of Barclays Bank PLC, acted as joint bookrunners.
We used the proceeds from the offerings of the Series A-1 Notes in May 2009 and Series A-2 Notes in August 2009, together with cash on hand, to repay all borrowings under the senior secured bridge credit agreement and to pay related fees and expenses. Our senior secured bridge credit agreement was terminated concurrently with the closing of the Series A-2 offering.
Notes. In May 2009, we issued $700.0 million of our outstanding 11.25% Senior Secured Notes due 2014 (Series A-1) and on August 2009, we issued $317.5 million of our outstanding 12.375% Senior Secured Notes due 2014 (Series A-2). The Series A-1 Notes and the Series A-2 Notes bear interest at a rate equal to 11.25% per annum and 12.375% per annum, respectively. The indenture governing the Notes, among other restrictions, limits our ability and the ability of our restricted subsidiaries to:
| • | incur additional debt; |
| • | pay dividends and make other distributions; |
| • | make certain investments; |
| • | repurchase our stock; |
| • | incur certain liens; |
| • | enter into transactions with affiliates; |
| • | merge or consolidate; |
| • | enter into agreements that restrict the ability of our subsidiaries to make dividends or other payments to us; and |
| • | transfer or sell assets. |
Subject to certain exceptions, the indenture governing the Notes permits us and our restricted subsidiaries to incur additional indebtedness, including senior indebtedness and secured indebtedness. The indenture also does
87
Table of Contents
not limit the amount of additional indebtedness that Sky Acquisition may incur. The Series A-1 Notes are entitled to a priority of payment over the Series A-2 Notes in certain circumstances, including upon any acceleration of the obligations under the Series A-1 Notes, the Series A-2 Notes or any bankruptcy or insolvency event of default with respect to Apria or any guarantor of the Notes. For more details, see “Description of Notes.”
Covenant Compliance. Under the indenture governing the Notes and under the credit agreement governing our ABL Facility, our ability to engage in activities such as incurring additional indebtedness, making investments, refinancing certain indebtedness, paying dividends and entering into certain merger transactions is governed, in part, by our ability to satisfy tests based on Adjusted EBITDA.
“Adjusted EBITDA” is defined as net income (loss), plus interest expense, net, provision (benefit) for income taxes and depreciation and amortization, further adjusted for certain other non-cash items, costs incurred related to initiatives, cost reduction and other adjustment items that are permitted by the covenants included in the indenture governing the Notes and the credit agreement governing our ABL Facility.
We believe that the presentation of Adjusted EBITDA is appropriate to provide additional information to investors about the calculation of, and compliance with, certain financial covenants in the indenture governing the Notes and in our ABL Facility. Adjusted EBITDA is a material component of these covenants. We caution investors that amounts presented in accordance with our definition of Adjusted EBITDA may not be comparable to similar measures disclosed by other issuers, because not all issuers and analysts calculate Adjusted EBITDA in the same manner.
Adjusted EBITDA is not a measurement of our financial performance under GAAP and should not be considered as alternatives to net income or any other performance measures derived in accordance with GAAP or as alternatives to cash flows from operating activities as a measure of our liquidity.
The following table provides a reconciliation from our net loss to Adjusted EBITDA:
(in thousands) | Three Months Ended March 31, 2010 | Twelve Months Ended March 31, 2010 | ||||||
Net loss | $ | (803 | ) | $ | (4,146 | ) | ||
Interest expense, net(a) | 32,376 | 128,252 | ||||||
Income tax expense | (884 | ) | (10,502 | ) | ||||
Depreciation and amortization | 33,237 | 172,323 | ||||||
Non-cash items(b) | 5,747 | 23,182 | ||||||
Costs incurred related to initiatives(c) | 10,774 | 58,577 | ||||||
Other adjustment items(d) | 3,787 | 9,119 | ||||||
Projected cost savings and synergies(e) | 12,884 | 71,370 | ||||||
Adjusted EBITDA | $ | 97,118 | $ | 448,175 | ||||
| (a) | Reflects $32.6 million of interest expense, net of $0.2 million of interest income for the three months ended March 31, 2010. Reflects $129.4 million of interest expense, net of $1.1 million of interest income for the twelve months ended March 31, 2010. |
| (b) | Non-cash items are comprised of the following: |
(in thousands) | Three Months Ended March 31, 2010 | Twelve Months Ended March 31, 2010 | ||||
Profit interest units compensation expense | $ | 1,128 | $ | 6,477 | ||
Loss on patient service equipment, disposition of assets and other(i) | 4,619 | 16,705 | ||||
Total non-cash items | $ | 5,747 | $ | 23,182 | ||
| (i) | Primarily represents non-cash losses related to the title transfer of equipment to Medicare patients at the end of the 13-month maximum rental period under the DRA and other disposals or write-offs of capital equipment. Equipment classified as 13-month rental equipment would include hospital beds, wheelchairs, nebulizers, patient lifts and CPAP devices. |
88
Table of Contents
| (c) | Costs incurred related to initiatives are comprised of the following: |
(in thousands) | Three Months Ended March 31, 2010 | Twelve Months Ended March 31, 2010 | ||||
Employee severance(i) | $ | 387 | $ | 2,625 | ||
Employee relocation costs(ii) | 119 | 874 | ||||
Executive severance and retention and other bonuses(iii) | 3,136 | 7,388 | ||||
Coram integration expense(iv) | 80 | 973 | ||||
Consulting fees(v) | 3,074 | 22,705 | ||||
Expenses relating to information technology outsourcing(vi) | 29 | 9,077 | ||||
Expenses relating to branch optimization program(vii) | 29 | 3,062 | ||||
Other(viii) | 3,920 | 11,873 | ||||
Total costs incurred related to initiatives | $ | 10,774 | $ | 58,577 | ||
| (i) | Represents employee severance expenses with respect to a reduction in workforce of approximately 59 employees for the three months ended March 31, 2010 and approximately 381 employees for the twelve months ended March 31, 2010. |
| (ii) | Represents moving expenses with respect to approximately 9 employees who were relocated during the three months ended March 31, 2010 and approximately 61 employees who were relocated during the twelve months ended March 31, 2010. |
| (iii) | Represents $3.2 million related to executive severance as a result of the Merger for the three months ended March 31, 2010 and $4.9 million related to executive severance and $2.4 million related to executive retention and other bonuses as a result of the Merger for the twelve months ended March 31, 2010. |
| (iv) | Represents expenses related to the integration of Coram and retention bonuses for certain Coram employees for the three and twelve months ended March 31, 2010. |
| (v) | Represents consulting fees paid for the three and twelve months ended March 31, 2010, primarily related to three projects: (1) an initiative related to locating certain cost centers offshore; (2) consolidation of purchasing in order to gain cost reductions through volume discounts and renegotiations with supply vendors; and (3) a new cash management system designed to electronically post and match payments in order to enhance and improve workflow productivity and reporting. |
| (vi) | Primarily represents professional fees and other costs associated with our initiative related to the outsourcing of selected information technology functions. |
| (vii) | Represents lease termination and other costs associated with our initiative to close or consolidate approximately 48 branches as part of a branch optimization program. |
| (viii) | For the three months ended March 31, 2010, primarily represents: (1) $2.8 million in fees and expenses related to a terminated debt offering in the first quarter of 2010; (2) $0.6 million in costs and fees related to the conversion to a new billing and collections system for our home infusion therapy business; (3) $0.3 million in costs and expenses related to the centralization of our admissions process for our home infusion therapy business; (4) $0.2 million in costs and fees related to the planned conversion to a new payroll processing system; and (5) $0.1 million in costs related to a new internal costs tracking system. For the twelve months ended March 31, 2010, primarily represents: (1) $6.1 million in fees and expenses related to the issuance of our outstanding Series A-1 Notes and Series A-2 Notes; (2) $2.1 million in costs and fees related to the planned conversion to a new payroll processing system; (3) $2.0 million in costs and fees related to the conversion to a new billing and collections system for our home infusion therapy business; (4) $1.0 million in costs and expenses related to the centralization of our admissions process for our home infusion therapy business; and (5) $0.4 million in costs related to a new internal costs tracking system. |
| (d) | Other adjustment items primarily related to the sponsor management fee of $3.8 million for the three months ended March 31, 2010 and $9.1 million for the twelve months ended March 31, 2010. |
| (e) | Projected net cost saving and synergies to be realized in connection with cost saving, restructuring and other similar initiatives. |
Hedging Activities
We are exposed to interest rate fluctuations on any variable rate long-term debt. However, we currently do not have any variable rate debt outstanding or interest rate swap agreements in effect. Our policy for managing interest rate risk is to evaluate and monitor all available relevant information, including but not limited to, the structure of our interest-bearing assets and liabilities, historical interest rate trends and interest rate forecasts published by major financial institutions. The tools we may utilize to moderate our exposure to fluctuations in the relevant interest rate indices include, but are not limited to: (1) strategic determination of repricing periods and related principal amounts, and (2) derivative financial instruments such as interest rate swap agreements, caps or collars. We do not use derivative financial instruments for trading or other speculative purposes.
At September 30, 2008, we terminated our one remaining interest rate swap agreement which would have expired in January 2009. The impact of the termination was immaterial. During 2008 and during 2007, we had one interest rate swap agreement in effect to fix our LIBOR-based variable rate debt in connection with the Predecessor Revolving Credit Facility. The agreement, a forward-starting contract with a three-year term,
89
Table of Contents
became effective in January 2006, and had a notional amount of $25.0 million that fixed an equivalent amount of our variable rate debt at 4.44%. For the three months ended March 31, 2010, the year ended December 31, 2009 and the periods October 29, 2008 to December 31, 2008 and January 1, 2008 to October 28, 2008, and the year ended December 31, 2007, we (paid) received net settlement amounts of $0, $0, $0, $(0.2) million, and $0.2 million, respectively.
Unrealized gains and losses on the fair value of the swap agreements are reflected, net of taxes, in operating income, as the transactions no longer qualify for hedge accounting treatment. Our exposure to credit loss under the swap agreement was limited to the interest rate spread in the event of counterparty nonperformance. At March 31, 2010, December 31, 2009 and 2008, we did not have any swap agreements in effect.
Treasury Stock.All repurchased shares of common stock were held as treasury shares. All treasury shares were retired as part of the Merger on October 28, 2008.
Business Combinations and Asset Purchases.We periodically acquire complementary businesses. These transactions are accounted for as purchases and the results of operations of the acquired companies are included in the accompanying statements of operations from the dates of acquisition. Covenants not to compete are being amortized over the life of the respective agreements. Customer lists, favorable lease arrangements and patient referral sources are being amortized over the period of their expected benefit.
During the three months ended March 31, 2010, the Company purchased certain assets of a business for $1.5 million. No acquisitions were made during the three months ended March 31, 2009.
During 2009, we purchased the accounts receivable and/or active payment lists from four discount agreement customers.
During 2008, we did not make any acquisitions. However, as discussed above, on October 28, 2008, we were acquired by Sky Acquisition LLC, a company controlled by private investment funds affiliated with the Sponsor.
In December 2007, we acquired Coram for aggregate consideration of approximately $350.0 million. Allocation of this amount includes $78.4 million in net assets, $34.3 million in patient-referral sources, $69.4 million in trade names, $0.6 million in favorable lease arrangements and $176.0 million in goodwill. In 2006, we closed three small acquisitions for an aggregate consideration of $3.6 million. Cash paid for acquisitions, which includes amounts deferred from prior year acquisitions, totaled $354.6 million in 2007.
Inflation.We experience pricing pressures in the form of continued reductions in reimbursement rates, particularly from managed care organizations and from governmental payors such as Medicare and Medicaid. We are also impacted by rising costs for certain inflation-sensitive operating expenses such as labor and employee benefits, facility and equipment leases, and vehicle fuel. However, we generally do not believe these impacts are material to our revenues or net income.
Off-Balance Sheet Arrangements
We are not a party to off-balance sheet arrangements as defined by the SEC. However, from time to time we enter into certain types of contracts that contingently require us to indemnify parties against third-party claims. The contracts primarily relate to: (i) certain asset purchase agreements, under which we may provide customary indemnification to the seller of the business being acquired; (ii) certain real estate leases, under which we may be required to indemnify property owners for environmental and other liabilities, and other claims arising from our use of the applicable premises; and (iii) certain agreements with our officers, directors and employees, under which we may be required to indemnify such persons for liabilities arising out of their relationship with us.
90
Table of Contents
The terms of such obligations vary by contract and in most instances a specific or maximum dollar amount is not explicitly stated therein. Generally, amounts under these contracts cannot be reasonably estimated until a specific claim is asserted. Consequently, no liabilities have been recorded for these obligations on our balance sheets for any of the periods presented.
Quantitative and Qualitative Disclosure About Market Risk
At March 31, 2010, there were no borrowings under our ABL Facility. The credit agreement governing the ABL Facility provides interest rate options based on the following indices: Federal Funds Rate, the Bank of America prime rate or LIBOR. All such interest rate options are subject to the application of an interest margin as specified in the bank credit agreement. At March 31, 2010, all of our outstanding asset-based debt was tied to the Bank of America prime rate. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources—Indebtedness—Post-Transactions.”
During 2008 and 2007, we had one interest rate swap agreement in effect to fix our LIBOR-based variable rate debt in connection with the Predecessor Revolving Credit Facility. The swap agreement was terminated at September 30, 2008. The agreement, a forward-starting contract with a three-year term, became effective in January 2006, and had a notional amount of $25.0 million that fixed an equivalent amount of our variable rate debt at 4.44%. The impact of the termination was deemed immaterial. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources—Hedging Activities.”
Assuming we drew down the entire $150.0 million under the ABL Facility at March 31, 2010, a 1.0% change in the applicable interest rates would increase or decrease our annual cash flow and pretax earnings by approximately $1.5 million. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources—Indebtedness—Post-Transactions.”
91
Table of Contents
Industry Overview
The home healthcare market, which is projected to generate revenues of approximately $75 billion in the United States in 2010, comprises a broad range of products and services—including respiratory therapy, home infusion therapy, home medical equipment, home healthcare nursing, orthotics and prosthetics and general medical supplies—and is expected to grow at a compounded annual growth rate of 7.4% from 2008 through 2013 according to CMS. The markets that we serve—home respiratory therapy (14% of the home healthcare market), infusion therapy (9%) and home medical equipment (5%)—accounted for approximately $15 billion in revenues in 2008 according to the 2008 IBISWorld Industry Report. Our industry is highly-fragmented and is served by approximately 12,000 competitors.
We benefit from the following trends within the home healthcare market:
| • | Favorable industry dynamics. Favorable demographic trends and the continued shift to in-home healthcare have resulted in patient volume growth across our core service lines and are expected to continue to drive growth. As the baby boomer population ages and life expectancy increases, the elderly—who comprise the vast majority of our patients—will represent a higher percentage of the overall population. According to a 2008 U.S. Census Bureau projection, the U.S. population aged 55 and over is expected to grow at approximately twice the average rate of population growth from 76.5 million, or 25% of the population, in 2010 to 112 million, or 30% of the population, by 2030. An aging population, the continued prevalence of smoking, increasing obesity rates and higher diagnosis rates have collectively driven growth across our service lines (including Coram) from 2005 to 2008—home respiratory therapy (patient growth of 14%), sleep apnea (patient growth of 21%) and infusion (patient growth of 14%, excluding enteral). |
| • | Compelling in-home economics. By 2017, the nation’s healthcare spending is projected to increase to $4.3 trillion, growing at an average annual rate of 6.7%, according to CMS. The rising cost of healthcare has caused many payors to look for ways to contain costs and home healthcare is increasingly sought out as an attractive, cost-effective, clinically appropriate alternative to expensive facility-based care. For example, in-home oxygen treatment costs for a Medicare patient are on average less than $7 per day. |
| • | Increased prevalence of in-home treatments.Improved technology has resulted in a wider variety of treatments being administered in patients’ homes. These improvements have allowed for earlier patient discharge and have lengthened the portion of the recuperation period spent outside of an institutional setting. In addition, medical advancements have also made medical equipment more simple, adaptable and cost-effective for use in the home. |
| • | Preference for in-home care.Many patients prefer the convenience and typical cost advantages of home healthcare over institutional care as it provides patients with greater independence, increased responsibility and improved responsiveness to treatment. A December 2007 national telephone survey conducted by Harris Interactive found that over 82% of the respondents expressed a preference for homecare over institutional care, and that preference is even more prevalent among the age 55+ population (91%). The same poll found that 74% of adults surveyed agreed that homecare is part of the solution to the problem of rapidly increasing Medicare spending for seniors in the United States. |
| • | Development of new infused and injectable drugs.There is a significant number of new infusion or injectable drugs in the development pipeline. We believe this proliferation of medications, many of which are for chronic conditions that require long-term treatment, will drive further increases in home infusion therapy utilization and referrals to our ambulatory infusion suites. |
92
Table of Contents
Our Company
General
We are a quality, cost-efficient provider of home healthcare products and services in the United States, offering a comprehensive range of home respiratory therapy, home infusion therapy and home medical equipment services to over two million patients annually in all 50 states through approximately 500 locations. We hold market-leading positions across all of our major service lines—making us a leader in the homecare market. By targeting the managed care segment of the population, we are better positioned than many of our competitors to minimize risks associated with changes in Medicare/Medicaid reimbursement rates. We are focused on being the industry’s highest-quality provider of homecare services, while maintaining our commitment to being a low-cost operator. Our integrated product and service offerings, combined with our national scale and strong reputation, provide us with a strategic advantage in attracting clients, which include almost all of the national and regional managed care and government payors in the United States, and in retaining our referral base of more than 70,000 physicians, discharge planners, hospitals and third-party payors. For the year ended December 31, 2009 and for the three months ended March 31, 2010 our net revenues were $2,094.6 million and 508.9 million, respectively.
History
A predecessor of the Company has been in existence since 1984. In 1995, it was acquired through a merger by Abbey Healthcare Group Incorporated, which changed its name to Apria Healthcare Group Inc. In 2007, we acquired Coram, Inc. On June 18, 2008, Apria, Sky Acquisition and Merger Sub entered into the Merger Agreement. As a result of the Merger, the Investor Group beneficially owns all of Apria’s issued and outstanding capital stock.
Operating Segments
We have two operating segments, (1) home respiratory therapy and home medical equipment and (2) home infusion therapy. Within the two operating segments there are three core service lines: home respiratory therapy, home medical equipment and home infusion therapy. Through these service lines we provide patients with a variety of clinical and administrative support services and related products and supplies, most of which are prescribed by a physician as part of a care plan. We provide substantial benefits to both patients and payors by allowing patients to receive necessary care and services in the comfort of their own home while reducing the cost of treatment. Our services include:
| • | providing in-home clinical respiratory care, infusion nursing and pharmaceutical management services; |
| • | educating patients and caregivers about health conditions or illnesses and providing written instructions about home safety, self-care and the proper use of equipment; |
| • | monitoring patients’ individualized treatment plans; |
| • | reporting patient progress and status to the physician and/or managed care organization; |
| • | providing in-home delivery, set-up and maintenance of equipment and/or supplies; and |
| • | processing claims to third-party payors and billing/collecting patient co-pays and deductibles. |
Home Respiratory Therapy and Home Medical Equipment($1,169.6 million and $278.3 million, or 55.8% and 54.7%, of our net revenues for the year ended December 31, 2009 and the three months ended March 31, 2010, respectively)
Home Respiratory Therapy
We are the largest provider of home respiratory therapies in the United States to the managed care market serving approximately 1.5 million patients annually through our nationwide distribution platform that includes approximately 400 locations. We offer a full range of home respiratory therapy products and services, from the
93
Table of Contents
simplest nebulizer and oxygen concentrator to the most complex ventilator. Our services offer a compelling relative cost advantage to our patients and payors. For example, in-home oxygen treatment costs for a Medicare patient are on average less than $7 per day. Patients utilize our products to treat a variety of conditions, including:
| • | COPD, such as emphysema and chronic bronchitis (the fourth leading cause of death in the U.S.); |
| • | respiratory conditions associated with nervous system disorders or injuries, such as Lou Gehrig’s disease and quadriplegia; |
| • | congestive heart failure; and |
| • | lung cancer. |
By focusing our efforts on the managed care population, we limit our exposure to the highly-regulated Medicare respiratory business, which is subject to changes in coverage, payment and pricing guidelines. As an example, Medicare oxygen accounted for less than 10% and 11% of our total net revenues for the year ended December 31, 2009 and three months ended March 31, 2010, respectively.
We employ a nationwide clinical staff of more than 800 respiratory care professionals, including home respiratory therapists who provide direct patient care, monitoring and 24-hour support services under physician-directed treatment plans and in accordance with our proprietary acuity program. We derive revenues from the provision of oxygen systems, ventilators, respiratory assist devices, and CPAP/bi-level devices, as well as from the provision of infant apnea monitors, nebulizers, home-delivered respiratory medications and related services.
We are also the largest provider of sleep apnea devices, including CPAP/bi-level devices, and patient support services in the United States. The incidence and diagnosis of OSA continues to increase in the United States. We believe that the strength of our position in this market is partly due to our significant presence in the managed care market, since OSA largely affects adults between the ages of 35 and 55 rather than the population served by Medicare. To manage our significant new and recurring patient volumes in a cost-effective, clinically sound manner, we developed an innovative care model called the “CPAP Center at Apria Healthcare.” This branch-based model allows Apria’s respiratory care practitioners to educate, on a timely and efficient basis, newly-diagnosed patients about their condition, the equipment and accessories their physician has prescribed for them, and the long-term importance of complying with the physician’s order. The model includes both one-on-one patient education and teaching performed in group settings, depending on the geographic area of the country and the patient’s payor’s contractual preferences.
Home Medical Equipment
As the leading provider of home medical equipment in the United States, we supply a wide range of products to help improve the quality of life for patients with special needs. Our integrated service approach allows patients, hospital and physician referral sources and managed care organizations accessing either our home respiratory or home infusion therapy services to also access needed home medical equipment through a single source. The use of home medical equipment provides a significant relative cost advantage to our patients and payors. For example, on average, it costs $50 per day to create an in-home hospital room versus approximately $1,500 per day for in-patient hospital care, according to CMS. Basic categories of equipment are:
| • | manual wheelchairs and ambulatory equipment, such as canes, crutches and walkers; |
| • | hospital room equipment, such as hospital beds and bedside commodes; |
| • | bathroom equipment, such as bath and shower benches, elevated toilet seats and toilet, tub or wall grab bars; |
| • | phototherapy systems, such as blankets, wraps or treatment beds for babies with jaundice; and |
| • | support surfaces, such as pressure pads and mattresses, for patients at risk for developing pressure sores or decubitus ulcers. |
94
Table of Contents
In May 2008, we announced a preferred provider agreement with Smith & Nephew plc, a leading provider of NPWT, to provide NPWT services and products in the United States. NPWT is a topical treatment intended to promote healing in acute and chronic wounds affected by conditions including diabetes, arterial insufficiency and venous insufficiency.
Home Infusion Therapy ($925.0 million and $230.6 million, or 44.2% and 45.3%, of our net revenues for the year ended December 31, 2009 and three months ended March 31, 2010, respectively)
Through our acquisition of Coram in December 2007, we are the leading provider of home infusion therapy services in the United States—serving approximately 100,000 patients annually through 72 infusion pharmacy locations nationwide. We provide patients with intravenous and injectable medications and clinical services at home or in one of our 62 ambulatory infusion suites nationwide. We employ nursing clinicians who assess patients before their discharge from the hospital whenever possible, and then develop, in conjunction with the physician, a plan of care. Our home infusion products offer a compelling relative cost advantage to our patients and payors. For example, we believe that a home intravenous antibiotic program in a Medicare managed care plan costs significantly less than the cost to provide that service in a hospital setting.
Home infusion therapy is used to administer drugs and other therapeutic agents directly into the body through various types of catheters or tubing. Our services are frequently used to treat patients with infectious diseases, cancer, gastrointestinal diseases, chronic or acute pain syndromes, immune deficiencies, cardiovascular disease or chronic genetic diseases, and those who require therapies associated with bone marrow or solid organ transplantation. We employ licensed pharmacists and registered nurses who specialize in the delivery of home infusion therapy. They are able to respond to emergencies and questions regarding therapy 24 hours a day, seven days a week and provide initial and ongoing training and education to the patient and caregiver. Other support services include supply replenishment, pump management, preventive maintenance, assistance with insurance questions and outcome reporting.
We believe we are also a leading provider of enteral nutrition in the United States. Enteral nutrition, or “tube feeding,” is prescribed to patients whose gastrointestinal system is malfunctioning or who suffer from neurological conditions, swallowing disorders or malnutrition attributable to stroke, cancer or other conditions. In recent years, advances in enteral nutrition have enabled more adults and children to have their nutritional and caloric needs met by tube feeding, as opposed to more invasive and expensive therapies.
Recent Developments
Announcement of SPAs and Initiation of Contract Offer Process Related to Round 1 Rebid of the Medicare DMEPOS Competitive Bidding Program
In early July 2010, CMS announced the new SPAs for each of the product categories included in the Round 1 Rebid. CMS then began the contracting process with suppliers by issuing contract offer letters to qualified providers. We received contract offers for a substantial majority of the bids we submitted. We did not receive contract offers for certain product categories in CBAs, but the process will not be completed until September 2010. Approximately $21 million of our net revenues for the fiscal year ended December 31, 2009 was generated by the products and CBAs included in the Round 1 Rebid. We estimate that the initial results of the Round 1 Rebid would reduce our net revenues in the fiscal year ending December 31, 2011 by approximately $8.0 million, assuming the current contract offers and no changes in volume. Assuming that Round 2 would include the same product categories and bidding rules and the markets currently being proposed by CMS, we estimate that approximately $110 million of our net revenues for the fiscal year ending December 31, 2011 would be subject to competitive bidding. Although the bidding process for Round 2 is currently scheduled to commence in 2011, the new Round 2 rates and guidelines are not scheduled to take effect until January 2013. Therefore, we cannot estimate the impact of potential Round 2 rate reductions on our business until more specific information is published by CMS and its contractors.
95
Table of Contents
Enactment of a Comprehensive Healthcare Reform Package
In March 2010, the Federal government enacted a comprehensive healthcare Reform Package which consists of the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010. See “Risk Factors—Risks Relating to Our Business—Continued Reductions in Medicare and Medicaid Reimbursement Rates and the Comprehensive Healthcare Reform Package Could Have a Material Adverse Effect on Our Results of Operations and Financial Condition.”
Reorganization of the Senior Leadership Structure of our Home Respiratory Therapy and Home Medical Equipment Segment
In February 2010, we finalized a decision to reorganize the senior leadership structure of the home respiratory therapy and home medical equipment segment. As part of this reorganization, Lawrence A. Mastrovich, President, Home Respiratory Therapy and Home Medical Equipment segment, resigned and his responsibilities were assumed by other senior executives.
Our Competitive Strengths
We believe that we have the following competitive strengths:
Leading Market Positions with a Compelling Value Proposition
With approximately 11,400 employees and a national distribution footprint of approximately 500 locations that serve patients in all 50 states, we are the largest provider of home healthcare services in the United States. We are the market leader in infusion therapy and sleep apnea devices, the leading respiratory provider to the managed care market and the leading provider of home medical equipment. We believe that our national platform, comprehensive product line and leading reputation provide us with a greater opportunity than our competitors to attract more customers as our industry continues to grow. Our national presence and scale enables us to frequently obtain preferred provider status from other national and regional managed care payors, negotiate better terms with vendors and leverage our fixed overhead costs. For example, we are a preferred provider for a comprehensive list of home respiratory and medical equipment products and services to many managed care organizations and, for some of these payors, we are the exclusive provider. We believe we are better suited to service large managed care accounts due to our extensive branch network, state of the art logistic systems, national coverage of payors’ members, competitive pricing, comprehensive product line, accreditation from The Joint Commission and the ACHC, and our ability to connect electronically with payors’ systems. We have leveraged this competitive advantage to gain share in the managed care market.
Our acquisition of Coram in December 2007 has allowed us to further penetrate the specialty infusion market. With a significant number of new infusion drugs in the pipeline and an increasing use of specialty infusion treatments, this market is expected to grow over the next few years. We are well-positioned in specialty infusion services, and have aggressively established relationships with pharmaceutical and biotech companies to obtain early access to drugs in various stages of clinical trials. We believe there are other cross-selling opportunities and synergies to be achieved by offering a diverse mix of services. We also believe that an integrated approach allows us to offer patients, hospital and physician referral sources and managed care organizations a highly-valued single source for respiratory therapy, specialty home infusion and home medical equipment.
Diversified Product and Customer Mix
We have one of the most comprehensive product lines and diversified customer mixes among our peers. Our broad product offering has affirmed our status as a leading provider in each market and has made us a more attractive partner to referral sources and payors, as we provide a one-stop solution for homecare products and services.
96
Table of Contents
We contract with a substantial majority of the national managed care organizations—including United HealthCare Services, Aetna Health Management, Humana Health Plans and Kaiser Foundation Health Plan, as well as a large number of regional and local payors. All of our contracted managed care organizations combined service over 217 million people.
The Coram acquisition enabled us to both expand our product offering in specialty infused drugs and rebalance our payor mix by reducing reliance on government payors such as Medicare and Medicaid while expanding relationships with managed care organizations. Managed care payors contributed approximately 72% and 70% of our net revenues for the year ended December 31, 2009 and the three months ended March 31, 2010, respectively, with no single contract accounting for more than 8% of net revenues during the same periods.
Proven Ability to Execute Cost Savings
We have successfully implemented a number of operational efficiency initiatives historically, which have helped to reduce our costs and significantly offset ongoing Medicare reimbursement changes. We launched a substantial cost reduction plan in late 2007 across a number of identified initiatives targeting approximately $198 million in expected annual savings, of which we have realized approximately $127 million through March 31, 2010.
Scalable and Diversified Platform for Home Healthcare Delivery
We currently provide service to more than 2 million patients through a national infrastructure that enables us to deliver services to patients in their homes. Through approximately 500 locations, we are able to deliver a wide variety of cost-effective products and services to various patient groups. We have successfully leveraged this distribution platform across a number of product and service offerings including CPAP/bi-level, enteral nutrition and NPWT devices, and we are using our nursing capacity to provide infusion services through our growing network of ambulatory infusion suites.
We historically supplied CPAP/bi-level devices to a large number of patients, but provided related accessories and supplies primarily on an as-needed basis. Patients who rely on CPAP and bi-level devices periodically require replacement accessories to ensure that they remain compliant to the therapy prescribed by their physician. These accessories include masks, tubing and supplies. Now in operation for over five years, a centralized customer care center for CPAP and bi-level patients provides support and information to patients so that they know what their payors cover in terms of replacement accessories and understand the health value of remaining compliant to their therapy over the long-term. Accessory net revenues were $155.9 million and $34.5 million and represented 47% and 46% of our total CPAP/bi-level net revenues for the year ended December 31, 2009 and the three months ended March 31, 2010, respectively.
In May 2008, we announced a preferred provider agreement with Smith & Nephew plc to provide NPWT products, thus leveraging our existing branch delivery infrastructure and clinical expertise. Centralized patient intake and coordination of care is provided using the same service and systems platform as is used for the CPAP/bi-level direct marketing service program. Although the program is still in its developing stage, interest has been strong from managed care customers who would like to add the NPWT service to our existing contracts with them.
Experienced Management Team
We have a strong and experienced senior management team with over 200 years of combined experience spanning nearly every segment of the healthcare industry, including managed care, manufacturing, supply chain, procurement, home healthcare, acute care, skilled nursing and long-term care. With an average tenure of 20 years within the healthcare industry, this team possesses in-depth knowledge of our industry and the regulatory environment in which we operate, as well as our portfolio of home healthcare services.
97
Table of Contents
Our Business Strategy
Our strategy is to position ourselves in the marketplace as a high-quality provider of a broad range of healthcare services and patient care management programs to our customers. The specific elements of our strategy are to:
| • | Grow profitable revenue and market share. We are focused on growing profitable revenues and increasing market share in our core home infusion therapy and home respiratory therapy service lines. We have undertaken a series of steps towards this end. Through our acquisition of Coram in December 2007, we considerably increased our home infusion capabilities and expanded our platform for further cross-selling opportunities. Since January 1, 2007, we have expanded our home respiratory therapy and home medical equipment sales force by 18%. This focus has allowed us to more effectively market our products and services to physicians, hospital discharge planners and managed care organizations. |
| • | Continue to participate in the managed care market. We participate in the managed care market as a long-term strategic customer group because we believe that our scale, expertise, nationwide presence and array of home healthcare products and services will enable us to sign preferred provider agreements with managed care organizations. Managed care represented approximately 70% of our total net revenues for the three months ended March 31, 2010. |
| • | Leverage our national distribution infrastructure.With approximately 500 locations and a robust platform supporting shared national services, we believe that we can efficiently add products, services and patients to our systems to grow our revenues and leverage our cost structure. For example, we have successfully leveraged this distribution platform across a number of product and service offerings, including a CPAP/bi-level supply replenishment program, enteral nutrition and NPWT services, and we are using our nursing capacity to provide infusion services through our growing network of ambulatory infusion suites. We seek to achieve margin improvements through operational initiatives focused on the continual reduction of costs and delivery of incremental efficiencies. At the same time, we believe that it is essential to consistently deliver superior customer service in order to increase referrals and retain existing patients. Performance improvement initiatives are underway in all aspects of our operations including customer service, patient satisfaction, logistics, supply chain, clinical services and billing/collections. We believe that by being responsive to the needs of our patients and payors we can provide ourselves with opportunities to take market share from our competitors. |
| • | Continue to lead the industry in accreditation. MIPPA made accreditation mandatory for Medicare providers of DMEPOS, effective October 1, 2009, per CMS regulation. We were the first durable medical equipment provider to seek and obtain voluntary accreditation from The Joint Commission. All of our locations are currently accredited by The Joint Commission and our home infusion therapy service line is also accredited by the ACHC. In 2007, we completed a nationwide independent triennial accreditation renewal process conducted by The Joint Commission and we have more than 15 years of continuous accreditation by The Joint Commission—longer than any other homecare provider. In late June 2010, The Joint Commission completed its most recent triennial survey of our respiratory/home medical equipment and infusion locations and is expected to renew our accreditation for another three years. |
| • | Execute our strategic initiatives to drive profitability.For the past several years, we have successfully engaged in a range of cost savings initiatives to ease pressure on our revenue that has been and continues to be caused by Medicare and Medicaid reimbursement changes. These initiatives are designed to improve customer service, delivery and vehicle routing services, streamline the billing and payment process, effectively manage purchasing costs and improve the overall experience of the patients we serve. We launched a substantial cost reduction plan in late 2007. To date, we have made significant progress across a number of the identified initiatives targeting expected annual savings of approximately $198 million, of which we realized approximately $127 million through March 31, 2010. |
98
Table of Contents
Service Lines
We are a quality, cost-efficient provider of a broad range of home healthcare services through approximately 500 locations that serve patients throughout the United States. We have two operating segments: (1) home respiratory therapy and home medical equipment and (2) home infusion therapy. Within these two segments, there are three core service lines: home respiratory therapy, home medical equipment and home infusion therapy. The following table provides examples of the services and products we provide in each service line:
Service Line | Examples of Services and Products | |
Home respiratory therapy | Provision of oxygen systems, stationary and portable ventilators, obstructive sleep apnea equipment, nebulizers, respiratory medications and related clinical/administrative support services | |
Home medical equipment | Provision of patient safety items, ambulatory aids and in-home equipment, such as wheelchairs, hospital beds and Negative Pressure Wound Therapy | |
Home infusion therapy | Intravenous or injectable administration of anti-infectives, pain management, chemotherapy, nutrients (also administered through a feeding tube), immune globulin (“IVIG”), coagulant and blood clotting factors, antitrypsin deficiency medication, other medications and related clinical/administrative support services | |
The following table sets forth a summary of net revenues by service line, expressed as percentages of total net revenues:
| Year Ended December 31, | Period January 1, 2008 to October 28, 2008 | Period October 29, 2008 to December 31, 2008 | Year Ended December 31, 2009 | Three months ended March 31, 2009 | Three months ended March 31, 2010 | ||||||||||||||||||
| 2006 | 2007 | ||||||||||||||||||||||
| (Predecessor) | (Predecessor) | (Predecessor) | (Successor) | (Successor) | (Successor) | (Successor) | |||||||||||||||||
Home respiratory therapy | 68 | % | 67 | % | 52 | % | 52 | % | 48 | % | 49 | % | 48 | % | |||||||||
Home medical equipment/other | 14 | % | 13 | % | 9 | % | 7 | % | 8 | % | 8 | % | 7 | % | |||||||||
Total home respiratory and home medical equipment segment | 82 | % | 80 | % | 61 | % | 59 | % | 56 | % | 57 | % | 55 | % | |||||||||
Home infusion therapy segment | 18 | % | 20 | % | 39 | % | 41 | % | 44 | % | 43 | % | 45 | % | |||||||||
Total net revenues | 100 | % | 100 | % | 100 | % | 100 | % | 100 | % | 100 | % | 100 | % | |||||||||
Home Respiratory Therapy and Home Medical Equipment($1,169.6 million and $278.3 million, or 55.8% and 54.7%, of our net revenues for the year ended December 31, 2009 and the three months ended March 31, 2010, respectively)
Home Respiratory Therapy
We are the largest provider of home respiratory therapies in the United States to the managed care market serving approximately 1.5 million patients annually through our nationwide distribution platform that includes approximately 400 locations. We offer a full range of home respiratory therapy products and services, from the
99
Table of Contents
simplest nebulizer and oxygen concentrator to the most complex ventilator. Our services offer a compelling relative cost advantage to our patients and payors. For example, in-home oxygen treatment costs for a Medicare patient are on average less than $7 per day. Patients utilize our products to treat a variety of conditions, including:
| • | COPD, such as emphysema and chronic bronchitis (the fourth leading cause of death in the U.S.); |
| • | respiratory conditions associated with nervous system disorders or injuries, such as Lou Gehrig’s disease and quadriplegia; |
| • | congestive heart failure; and |
| • | lung cancer. |
By focusing our efforts primarily on the managed care population, we limit our exposure to the highly-regulated Medicare respiratory business, which is subject to changes in coverage, payment and pricing guidelines. As an example, Medicare oxygen accounted for less than 10% and 11% of our total net revenues for the year ended December 31, 2009 and three months ended March 31, 2010, respectively.
We employ a nationwide clinical staff of more than 800 respiratory care professionals, including home respiratory therapists who provide direct patient care, monitoring and 24-hour support services under physician-directed treatment plans and in accordance with our proprietary acuity program. We derive revenues from the provision of oxygen systems, ventilators, respiratory assist devices, and CPAP/bi-level devices, as well as from the provision of infant apnea monitors, nebulizers, home-delivered respiratory medications and related services.
We are also the largest provider of sleep apnea devices, including CPAP/bi-level devices, and patient support services in the United States. The incidence and diagnosis of OSA continues to increase in the United States. We believe that the strength of our position in this market is partly due to our significant presence in the managed care market, since OSA largely affects adults between the ages of 35 and 55 rather than the population served by Medicare. To manage our significant new and recurring patient volumes in a cost-effective, clinically sound manner, we developed an innovative care model called the “CPAP Center at Apria Healthcare.” This branch-based model allows Apria’s respiratory care practitioners to educate, on a timely and efficient basis, newly-diagnosed patients about their condition, the equipment and accessories their physician has prescribed for them, and the long-term importance of complying with the physician’s order. The model includes both one-on-one patient education and teaching performed in group settings, depending on the geographic area of the country and the patient’s payor’s contractual preferences.
Home Medical Equipment
As the leading provider of home medical equipment in the United States, we supply a wide range of products to help improve the quality of life for patients with special needs. Our integrated service approach allows patients, hospital and physician referral sources and managed care organizations accessing either our home respiratory or home infusion therapy services to also access needed home medical equipment through a single source. The use of home medical equipment provides a significant relative cost advantage to our patients and payors. For example, on average, it costs $50 per day to create an in-home hospital room versus approximately $1,500 per day for in-patient hospital care, according to CMS. Basic categories of equipment are:
| • | manual wheelchairs and ambulatory equipment, such as canes, crutches and walkers; |
| • | hospital room equipment, such as hospital beds and bedside commodes; |
| • | bathroom equipment, such as bath and shower benches, elevated toilet seats and toilet, tub or wall grab bars; |
| • | phototherapy systems, such as blankets, wraps or treatment beds for babies with jaundice; and |
100
Table of Contents
| • | support surfaces, such as pressure pads and mattresses, for patients at risk for developing pressure sores or decubitus ulcers. |
In May 2008, we announced a preferred provider agreement with Smith & Nephew plc, a leading provider of NPWT, to provide NPWT services and products in the United States. NPWT is a topical treatment intended to promote healing in acute and chronic wounds affected by conditions including diabetes, arterial insufficiency and venous insufficiency.
Home Infusion Therapy ($925.0 million and $230.6 million, or 44.2% and 45.3%, of our net revenues for the year ended December 31, 2009 and three months ended March 31, 2010, respectively)
Through our acquisition of Coram in December 2007, we are the leading provider of home infusion therapy services in the United States—serving approximately 100,000 patients annually through 72 infusion pharmacy locations nationwide. We provide patients with intravenous or injectable medications and clinical services at home or in one of our 62 ambulatory infusion suites nationwide. We employ nursing clinicians who assess patients before their discharge from the hospital whenever possible, and then develop, in conjunction with the physician, a plan of care. Our home infusion products offer a compelling relative cost advantage to our patients and payors. For example, we believe that a home intravenous antibiotic program in a Medicare managed care plan costs significantly less than the cost to provide that service in a hospital setting.
Home infusion therapy is used to administer drugs and other therapeutic agents directly into the body through various types of catheters or tubing. Our services are frequently used to treat patients with infectious diseases, cancer, gastrointestinal diseases, chronic or acute pain syndromes, immune deficiencies, cardiovascular disease or chronic genetic diseases, and those who require therapies associated with bone marrow or solid organ transplantation. We employ licensed pharmacists and registered nurses who specialize in the delivery of home infusion therapy. They are able to respond to emergencies and questions regarding therapy 24 hours a day, seven days a week and provide initial and ongoing training and education to the patient and caregiver. Other support services include supply replenishment, pump management, preventive maintenance, assistance with insurance questions and outcome reporting.
We believe we are also a leading provider of enteral nutrition in the United States. Enteral nutrition, or “tube feeding,” is prescribed to patients whose gastrointestinal system is malfunctioning or who suffer from neurological conditions, swallowing disorders or malnutrition attributable to stroke, cancer or other conditions. In recent years, advances in enteral nutrition have enabled more adults and children to have their nutritional and caloric needs met by tube feeding, as opposed to more invasive and expensive therapies. Our patient intake and care model for enteral patients is unique in that it includes centralized customer service and monthly refill operations. Rather than performing those functions at all branches, they are centralized in four regional centers, with first-dose support provided by the branches.
Organization and Operations
Organization. Our approximately 500 locations deliver home healthcare products and services to patients in their homes and to other care sites through our delivery fleet and our qualified delivery professionals and clinical employees. Our home respiratory therapy, home medical equipment and home infusion therapy service lines are organized into geographic divisions that provide management oversight.
Corporate Compliance. As a leader in the home healthcare industry, we have implemented a compliance program to further our commitment to providing quality home healthcare services and products while maintaining high standards of ethical and legal conduct. We believe that it is essential to operate our business with integrity and in full compliance with applicable regulations. Our Corporate Compliance Program includes a written Code of Ethical Business Conduct that employees receive as part of their initial orientation process and which is reinforced on an ongoing basis via training, communications and the application of disciplinary
101
Table of Contents
guidelines. Additionally, managers and above are required to complete an annual regulatory certification process during which they attest that they have reviewed the Code with their direct reports. The compliance program is designed to accomplish the goals described above through employee education, a confidential disclosure program, written policy guidelines, periodic reviews, frequent reinforcement, compliance audits, a formal disciplinary component and other programs. Compliance oversight is provided by the Corporate Compliance Committee, which meets quarterly, consisting of senior and mid-level management personnel from various functional disciplines. In addition to updates provided to the Board of Directors during its regular meetings, a written Compliance Program Report is submitted annually to the Board for review and discussion.
Pursuant to the merger agreement to acquire Coram, we assumed Coram’s obligations under its Certification of Compliance Agreement with the OIG of the HHS (the “Compliance Agreement”). The Compliance Agreement, which became effective on August 22, 2007, obligates Coram to maintain a compliance program to monitor and ensure compliance with federal healthcare program requirements. Under the Compliance Agreement, Coram’s compliance program must include maintenance of specified funding levels for the compliance program for at least three years following the date of the Compliance Agreement, prompt refunding of any overpayments, and implementation of various compliance program elements such as training, auditing and disclosure programs, development of a code of conduct and appointment of a compliance officer and compliance committee. Additionally, the Compliance Agreement requires notification to the OIG of certain events and imposes an annual certification requirement on Coram. The Compliance Agreement provides for stipulated penalties for failure to comply with its provisions. The year 2009 marked the second year of the three-year Compliance Agreement; in both 2008 and 2009, the OIG accepted our annual certification documents as filed.
Internal Audit. Our internal audit function reports directly to the Audit Committee of the Board of Directors and provides ongoing assessments of our system of disclosure controls and procedures, and internal control over financial reporting. Our internal audit function is responsible for both operational and financial reviews of our operations, for monitoring compliance with policies and procedures and for the identification and development of best practices within the organization.
Operating Systems and Controls. Our business is dependent, to a substantial degree, upon the quality of our operating and field information policies and procedures for proper contract administration, accurate order entry and pricing, billing and collections, and inventory and patient service equipment management. These policies and procedures also provide reporting that enables us to monitor and evaluate contract profitability. Our information services department works closely with all of the operating areas of our business to ensure that our policies and procedures are compliant with government regulations and payor requirements and to support their business improvement initiatives with technological solutions. See “Risk Factors—Risks Relating to Our Business—Our Failure to Successfully Design, Modify and Implement Computer and Other Process Changes to Maximize Productivity and Ensure Compliance Could Ultimately Have a Significant Negative Impact on Our Results of Operations and Financial Condition.”
We have established performance indicators which measure operating results against expected thresholds for the purpose of allowing all levels of management to identify and modify areas requiring improvement and to monitor the resulting progress. We have also developed mechanisms for measuring and reporting patient and customer satisfaction. Operating models with strategic targets have been developed to move us toward more effective management of the sales, customer service, accounts receivable, clinical and distribution areas of our business. Our management team is compensated using performance-based incentives focused on certain specified criteria such as Adjusted EBITDA and adjusted free cash flow. See “Executive Compensation—Compensation Discussion and Analysis—Compensation Elements.”
Payors. We derive substantially all our revenues from third-party payors, including private insurers, managed care organizations, Medicare and Medicaid. For the year ended December 31, 2009, approximately 22% of our total net revenues were derived from Medicare and 6% from Medicaid. Generally, each third-party payor has specific requirements which must be met before claim submission will result in payment. Certain
102
Table of Contents
payor-related functions are now being administered by Intelenet. We have policies and procedures in place to manage the claims submission process, including verification procedures to facilitate complete and accurate documentation. Notwithstanding these measures, violations of these requirements may still occur and could result in the termination of a contract with a payor, the repayment of amounts previously received or other potentially significant liability. When the third party payor is a governmental entity, violations of these requirements could subject us to civil, administrative and criminal enforcement actions. From time to time, we engage in renegotiation, sometimes precipitated by a written or verbal termination notice, with payors with which we are contracted to provide our various products and services. See “Risk Factors—Risks Relating to Our Business—Continued Reductions in Medicare and Medicaid Reimbursement Rates and the Comprehensive Healthcare Reform Package Could Have a Material Adverse Effect on Our Results of Operations and Financial Condition,” “Risk Factors—Risks Relating to Our Business—Non-Compliance With Laws and Regulations Applicable to Our Business and Future Changes in Those Laws and Regulations Could Have a Material Adverse Effect on Us,” “Risk Factors—Risks Relating to Our Business—Our Outsourcing and Offshoring Activities Subject Us to Risks That Could Have a Significant Negative Impact on Our Results of Operations and Financial Condition,” “Risk Factors—Risks Relating to Our Business—Our Payor Contracts are Subject to Renegotiation or Termination Which Could Result in a Decrease in Our Revenue and Profits” and “Certain Relationships and Related Party Transactions—Intelenet Agreement.”
Receivables Management. We operate in an environment with complex requirements governing billing and reimbursement for our products and services. Initiatives focused specifically on receivables management such as system enhancements, process refinements and organizational changes have resulted in improvement and consistency in key accounts receivable indicators.
We are expanding our use of technology in areas such as electronic claims submission and electronic funds transfer with managed care organizations to more efficiently process business transactions. This use of technology can expedite claims processing and reduce the administrative cost associated with this activity for both us and our customers/payors. We now submit approximately 93% of our home respiratory and home medical equipment claims and approximately 80% of our home infusion therapy claims electronically. We are also focusing our resources on developing internal expertise with the unique reimbursement requirements of certain large third-party payors, which should help reduce subsequent denials and shorten related collection periods. Our policy is to collect co-payments from the patient or applicable secondary payor. In the absence of a secondary payor, we generally require the co-payment at the time the patient is initially established with the product/service. Subsequent months’ co-payments are billed to the patient. We are also seeking to streamline related processes in order to maximize the co-payment collection rate. Certain patient pay management functions are now being administered by Intelenet. We have established policies and procedures for Intelenet to perform effectively on our behalf. See “Risk Factors—Risks Relating to Our Business—Our Outsourcing and Offshoring Activities Subject Us to Risks That Could Have a Significant Negative Impact on Our Results of Operations and Financial Condition,” “Risk Factors—Risks Relating to Our Business—Our Failure to Maintain Controls and Processes Over Billing and Collections or the Deterioration of the Financial Condition of Our Payors Could Have a Significant Negative Impact on Our Results of Operations and Financial Condition” and “Certain Relationships and Related Party Transactions—Intelenet Agreement.”
Marketing
Through our field sales force, we market our services primarily to physicians, managed care organizations, hospitals, medical groups, home health agencies and case managers. We have developed and put into practice several marketing initiatives, including but not limited to:
Automated Call Routing Through Toll-Free Numbers.This allows select managed care organizations to reach any of our locations and to access the full range of our services through toll free telephone numbers.
103
Table of Contents
Nationwide Accreditation.All of our branch locations are accredited by the Joint Commission and our home infusion therapy service line is also accredited by the ACHC. Each of the two Commissions is a nationally recognized organization that develops standards for various healthcare industry segments and monitors compliance with those standards through voluntary surveys of participating providers. As the home healthcare industry has grown, and accreditation has become a mandatory requirement for Medicare DMEPOS providers, the need for objective quality measurements has increased. Accreditation by either Commission entails a lengthy voluntary review process that is conducted every three years. Accreditation is also widely considered a prerequisite for entering into contracts with managed care organizations at every level and is required for Medicare competitive bidding. Because accreditation is expensive and time consuming, not all providers choose to undergo the process.
Essential Care Model. We have developed the Essential Care Model, a proprietary model that defines the services, supplies and products delivered in conjunction with prescribed homecare equipment and therapies. The Essential Care Model is used to establish consistent and clear expectations for referral sources, payors and patients.
Patient Satisfaction and Complaint Resolution Process. We have a centralized patient satisfaction survey function that periodically conducts targeted member satisfaction studies for key managed care organizations as specified by the various contractual arrangements. The same centralized group manages a complaint resolution process through which service improvements are identified and implemented at the field level. We believe that both centralized processes afford us visibility to centralized performance improvement data and trends that enable us to amend policies and procedures as necessary to meet the needs of patients and referral sources.
Apria Great Escapes® Travel Program. Our more than 500 location network facilitates travel for patients who require oxygen, alternate site infusion or other products, services and therapies. We coordinate equipment and service needs for thousands of traveling patients annually, which enhances their mobility and quality of life.
Comprehensive, Patient-Centric Clinical and Therapy Management Programs.We offer a number of clinical management programs designed to help physicians and managed care customers better manage patients through the use of homecare and ambulatory infusion suites to achieve substantial healthcare savings through the careful and appropriate oversight and management of medical equipment services and biotherapies. Our COPD Care Management, Sleep Management, RespiratoryAssist™, SatAssist™, Nourish™ and Tube Feeding Programs provide feedback to physicians regarding changes in patients’ clinical status, thus preventing unnecessary hospital or emergency admissions. Our proprietary EyeOn® infusion therapy management programs for Hemophilia and IVIG support thousands of patients each year. Our extensive experience and clinical expertise have enabled our development of proprietary, proven therapy management programs designed specifically for these high cost and highly complex biotherapies. The EyeOn® program creates proven cost savings through careful risk assessment, management, and appropriate utilization management techniques.
Sales
As of March 31, 2010, we employed approximately 1,000 sales professionals whose primary responsibility is to generate new referrals and to maintain existing relationships for all of our service lines. Key customers include physicians and their staffs, hospital-based healthcare professionals and managed care organizations, among others. We provide our sales professionals with the necessary clinical and technical training to represent our major service offerings. As larger segments of the marketplace become involved with managed care, specially trained members of our sales force provide us with a competitive advantage based on their working knowledge of pricing, contracting and negotiating with payors.
An integral component of our overall sales strategy is to increase volume through managed care referral sources and traditional physician referral channels. Specific growth initiatives designed to increase customer awareness of our clinical and operational programs are in place with the goal of securing a greater share of the
104
Table of Contents
traditional market. The ultimate decision makers for healthcare services vary greatly, from closed model managed care organizations to preferred provider networks, which are controlled by more traditional means. Our selling structure and strategies are designed to adapt to changing market factors and will continue to adjust as further changes in the industry occur. Managed care organizations continue to represent a significant portion of our business in several of our primary metropolitan markets. No third-party managed care payor group, however, represented more than 8% of our total net revenues for the three months ended March 31, 2010. Among our more significant managed care customers during 2009 and three months ended March 31, 2010 were Aetna Health Management, CIGNA Health Corporation, Kaiser Foundation Health Plan and United HealthCare Services. We also offer various fee-for-service arrangements to hospitals or hospital systems whose patients have home healthcare needs. See “Risk Factors—Risks Relating to Our Business—We Believe That Continued Pressure to Reduce Healthcare Costs Could Have a Material Adverse Effect on Us” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
Competition
The segment of the healthcare market in which we operate is highly competitive. In each of our service lines there are a limited number of national providers and numerous regional and local providers. The competitive factors most important in the regional and local markets are:
| • | reputation with referral sources, including local physicians and hospital-based professionals; |
| • | accessibility and responsiveness; |
| • | price of services; |
| • | overall ease of doing business; |
| • | quality of patient care and associated services; and |
| • | range of home healthcare services and products. |
In addition to the foregoing, the most important competitive factors in the larger, national markets are:
| • | ability to service a wide geographic area; |
| • | ability to develop and maintain contractual relationships with managed care organizations; |
| • | access to capital; |
| • | information systems capabilities; and |
| • | accreditation by the Commissions or a similar accrediting body. |
We believe that we compete effectively in each of our service lines with respect to all of the above factors and that we have an established record as a quality provider, as reflected by the accreditation of all of our branches.
In each of our service lines there are a number of national providers and numerous regional and local providers with which we directly compete. Among the national providers are, American HomePatient, Inc., Medco/Critical Care Systems, Lincare Holdings, Inc., Walgreen’s Option Care and Rotech Healthcare Inc. Other types of healthcare providers, including industrial gas manufacturers, individual hospitals and hospital systems, home health agencies and health maintenance organizations, have entered and may continue to enter the market to compete with our various service lines. Depending on their business strategies and financial position, it is possible that our competitors may have access to significantly greater financial and marketing resources than we do. This may increase pricing pressure and limit our ability to maintain or increase our market share. See “Risk Factors—Risks Relating to Our Business—We Believe That Continued Pressure to Reduce Healthcare Costs Could Have a Material Adverse Effect on Us” and “Risk Factors—Risks Relating to Our Business—We Experience Competition From Numerous Other Home Respiratory/Home Medical Equipment and Home Infusion Therapy Service Providers, and This Competition Could Adversely Affect Our Revenues and Our Business.”
105
Table of Contents
Acquisition and Development Activities
In order to take advantage of our core competencies, expand our service offerings and enhance our value proposition for our customers, we may elect to make selective acquisitions of businesses with complementary products and services, or with operations in additional markets. We expect to carefully evaluate each acquisition opportunity through an extensive due diligence process to determine those that have the greatest potential for growth and increased profitability under our operating structure.
Outsourcing Activities
We are pursuing an outsourcing strategy with respect to certain billing, collections, administrative and information systems functions and have selected two business process outsourcing firms, Intelenet and Dell Services (formerly Perot Systems Corporation), to assist with implementing a portion of this strategy.
Intelenet Agreement
In May 2009, we entered into the Master Service Agreement (“Intelenet Agreement”) with Intelenet, an Indian company affiliated with the Sponsor, regarding the outsourcing of certain functions relating to billing, collections and other administrative and clerical services. A portion of such services to be outsourced were transitioned to Intelenet during 2009. A portion of such services to be outsourced have been transitioned to Intelenet and additional services are expected to be transitioned over the next 18 months. Only certain services are currently subject to the Intelenet Agreement. However, if all services currently planned for outsourcing to Intelenet are in fact handled by Intelenet, we expect to make payments to Intelenet of approximately $200 million over a seven-year period that began in the second quarter of 2009. One of the members of our Board of Directors, Mr. Patrick Bourke, is an employee of the Sponsor and also serves on the Board of Directors of Intelenet. During the year ended December 31, 2009 and the three months ended March 31, 2010, we paid approximately $3.7 million and $4.0 million, respectively, under the Intelenet Agreement.
Perot Systems Agreement
In April 2009, we entered into an Information Technology Services Agreement (the “Perot Agreement”) with Perot Systems Corporation (“Perot Systems”) to outsource certain information technology functions to Perot Systems. Dell Inc. acquired Perot Systems in November 2009 and created a new business unit called Dell Services, which provides the services covered by the Perot Agreement. We expect to pay approximately $216.0 million to Dell Services over the ten-year term of the Perot Agreement. During the year ended December 31, 2009 and the three months ended March 31, 2010, we paid approximately $11.4 million and $7.4 million, respectively, under the Perot Agreement. In addition to amounts under the ten-year term of the agreement, we expect to pay approximately $15.0 million over the first 60 months of the contract for services rendered primarily in support of the cost savings initiatives described earlier relating to operations and revenue management functions.
Government Regulation
We are subject to extensive government regulation, including numerous laws directed at regulating reimbursement of our products and services under various government programs and preventing fraud and abuse, as more fully described below. We maintain certain safeguards intended to reduce the likelihood that we will engage in conduct or enter into arrangements in violation of these restrictions. Corporate contract services and legal department personnel review and approve written contracts subject to these laws. We also maintain various educational and audit programs designed to keep our managers updated and informed regarding developments on these topics and to reinforce to employees our policy of strict compliance in this area. Federal and state laws require that we obtain facility and other regulatory licenses and that we enroll as a supplier with federal and state health programs. Under various federal and state laws, we are required to make filings or submit notices in connection with transactions that might be defined as a change of control of the Company. We are aware of these requirements and routinely make such filings with, and seek such approvals from, the applicable regulatory
106
Table of Contents
agencies. Notwithstanding these measures, due to changes in and new interpretations of such laws and regulations, and changes in our business, violations of these laws and regulations may still occur, which could subject us to civil and criminal enforcement actions; licensure revocation, suspension or non-renewal; severe fines and penalties; the repayment of amounts previously paid to us and even the termination of our ability to provide services, including those provided under certain government programs such as Medicare and Medicaid. See “Risk Factors—Risks Relating to Our Business—Continued Reductions in Medicare and Medicaid Reimbursement Rates and the Comprehensive Healthcare Reform Package Could Have a Material Adverse Effect on Our Results of Operations and Financial Condition” and “Risk Factors—Risks Relating to Our Business—Our Failure To Maintain Required Licenses Could Impact Our Operations.”
Medicare and Medicaid Revenues.In the three months ended March 31, 2010 approximately 24% and 6% of our net revenues were reimbursed by the Medicare and state Medicaid programs, respectively. No other third-party payor represented more than 8% of our total net revenues for the three months ended March 31, 2010. The majority of our revenues are derived from rental income on equipment rented and related services provided to patients, sales of equipment, supplies and pharmaceuticals and other items we sell to patients for patient care under fee-for-service arrangements. Revenues derived from capitation arrangements represented 8% of total net revenues for the three months ended March 31, 2010.
Medicare Reimbursement.There are a number of legislative and regulatory activities in Congress and at CMS that affect or may affect Medicare reimbursement policies for products and services we provide. Specifically, a number of important legislative changes that affect our business were included in the Medicare Prescription Drug, Improvement and Modernization Act of 2003 (“MMA”), which was signed into law in December 2003, the Deficit Reduction Act of 2005 (“DRA”), which was signed into law in February 2006, MIPPA, which became law on July 15, 2008, and the Reform Package. These Acts and their implementing regulations and guidelines contain numerous provisions that were significant to us and continue to have an impact on our operations today, as described below.
DMEPOS Competitive Bidding. The MMA required implementation of a competitive bidding program for certain DMEPOS items. By statute, CMS was required to implement the DMEPOS competitive bidding program over time, with Round 1 of competition occurring in portions of 10 of the largest MSAs, in 2007, launch of the program in 2008 and in 70 additional markets in 2009, and in additional markets after 2009.
In 2007 and 2008, CMS sought and reviewed bids and developed a plan to implement Round 1 on July 1, 2008. CMS offered us contracts in several CBAs in Round 1; we accepted the contracts for certain product categories and declined others due to unacceptably low competitively determined SPAs in certain markets, which would not adequately cover the cost of providing quality service to our patients in those areas. We, along with other winning contract suppliers, began providing services under Round 1 on July 1, 2008.
The bidding process for Round 1 was controversial and complex, which resulted in deadline extensions. Moreover, CMS was subject to numerous lawsuits seeking a delay of Round 1. Then on July 15, 2008, MIPPA was enacted which, among other provisions, delayed the DMEPOS competitive bidding program by requiring that Round 1 competition commence in 2009, and required a number of program reforms prior to CMS’ re-launching the program. As a result, contracts that were awarded under Round 1 were terminated. The new “Round 1 Rebid” contracting process opened on October 21, 2009 and bids had to be submitted by December 21, 2009. We submitted our bids and related documentation by the stated deadlines and CMS informed us of their receipt. In late June 2010, CMS published a Proposed Rule containing several provisions related to the competitive bidding program. The Proposed Rule included the proposed list of 21 additional MSAs to be included in Round 2, as well as provisions relating to the diabetic supply category. Those provisions include a proposed definition of “mail order” and “non-mail order” items and a proposal for providers to supply a minimum level of product choices to patients. The public comment period on the Proposed Rule will close in August and a Final Rule is expected in the fall of 2010. In early July 2010, CMS announced the new SPAs for each of the product categories and each of the CBAs included in the Round 1 Rebid. CMS then began the
107
Table of Contents
contracting process with suppliers by issuing contract offer letters to qualified providers. This process is expected to take approximately two months, and CMS expects to announce the list of winners publicly in September 2010. The new rates will take effect in January 2011. Until then, all Medicare beneficiaries in the fee-for-service Medicare program may use any provider in any geographic area, including the CBAs, to obtain home medical equipment and oxygen therapy services and products.
Under MIPPA, the initial CBAs and product categories subject to rebidding are very similar to those of Round 1. MIPPA also excludes Negative Pressure Wound Therapy Pumps and Related Supplies and Accessories as a competitive bidding product category in Round 1 and permanently excludes Group 3 Complex Rehabilitative Power Wheelchairs and Related Accessories as a competitive bidding product category. MIPPA also includes a new provision requiring bids for mail order diabetes testing supplies after Round 1 to include a certain percentage of all types of available diabetic testing strips.
The estimated annual total net revenues associated with the items that would have been subject to competitive bidding in Round 1 of what was to be the initial year of the program represented approximately 1.4% of our annual total net revenues. Although Medicare has not yet published its 2009 expenditures by CBA or product category, based on 2008 data provided during the Round 1 Rebid process, we estimate that after the DRA and MIPPA reimbursement reductions of 2009 and a change in our business mix since 2007 are allocated for, the estimated annual total net revenues associated with items subject to competitive bidding in the Round 1 Rebid is approximately 1.0% of our annual total net revenues. In early July 2010, CMS announced the new SPAs for each of the product categories and each of the CBAs included in the Round 1 Rebid. CMS then began the contracting process with suppliers by issuing contract offer letters to qualified providers. We received contract offers for a substantial majority of the bids we submitted. We did not receive contract offers for certain product categories in certain CBAs, but the process will not be completed until September 2010. Approximately $21 million of our net revenues for the fiscal year ended December 31, 2009 was generated by the products and CBAs included in the Round 1 Rebid. We estimate that the initial results of the Round 1 Rebid would impact our net revenues in the fiscal year ending December 31, 2011 by approximately $8.0 million, assuming the current contract offers and no changes in volume.
Notwithstanding the changes MIPPA requires, competitive bidding imposes a significant risk to DMEPOS suppliers. Under the rules governing Round 1 and the Round 1 Rebid which proceeded after the delay, if a DMEPOS supplier operating in a CBA is not awarded a contract for that CBA, the supplier generally will not be able to bill and be reimbursed by Medicare for DMEPOS items supplied in that CBA for the time period covered by the competitive bidding program unless the supplier meets certain exceptions or acquires a winning bidder. Because the applicable statutes mandate financial savings from the competitive bidding program, a winning contract supplier will receive lower Medicare payment rates under competitive bidding than the otherwise applicable DMEPOS fee schedule rates. As competitive bidding is phased in across the country under the revised MIPPA implementation schedule, we will likely experience a reduction in reimbursement, as will most if not all other DMEPOS suppliers in the impacted areas. In addition, there is a risk that the new competitive bidding prices will become a benchmark for reimbursement from other payors. MIPPA does not prevent CMS from adjusting prices for DMEPOS items in non-bid areas; however, before using its authority to adjust prices in non-bid areas, MIPPA requires that CMS issue a regulation that specifies the methodology to be used and consider how prices through competitive bidding compare to costs for those items and services in the non-bid areas. In January 2009, CMS released an interim final rule on the DMEPOS competitive bidding program implementing certain MIPPA provisions requiring CMS to conduct the Round 1 Rebid in 2009 and mandating certain changes for both the Round 1 Rebid and subsequent rounds of the program, including (i) a decreased financial documentation burden, (ii) a process for providing feedback to suppliers regarding missing hardcopy documentation (primarily financial in nature, not the electronic bid form itself), and (iii) requiring bidders and, ultimately, contracted providers, to disclose to CMS information regarding accreditation status and subcontracting relationships. This interim final rule also formally exempted certain DMEPOS items (including crutches, canes, walkers, folding manual wheelchairs, blood glucose monitors and infusion pumps that are durable medical equipment) when furnished by hospitals to the hospitals’ own patients
108
Table of Contents
during an admission or on the date of discharge. In addition, the regulations permanently exclude Group 3 Complex Rehabilitative Power Wheelchairs and Related Accessories as a competitive bidding product category. The competitive bidding interim final rule became effective on April 18, 2009.
The Reform Package includes changes to the Medicare DMEPOS competitive bidding program. Significantly, Round 2 of the competitive bidding program has been expanded from 70 to 91 of the largest MSAs. CMS has announced that the effective date of the Round 2 pricing will be January 1, 2013; additional details concerning products to be included and other aspects of implementing Round 2 will not be fully known until after CMS completes a rulemaking process, which is currently scheduled for the summer or fall of 2010. Assuming that Round 2 would include the same product categories, bidding rules and markets currently being proposed by CMS, we estimate that approximately $110 million of our net revenues for the fiscal year ending December 31, 2011 would be subject to competitive bidding. Although the bidding process for Round 2 is currently scheduled to commence in 2011, the new Round 2 rates and guidelines are not scheduled to take effect until January 2013. Therefore, we cannot estimate the impact of potential Round 2 rate reductions on our business until more specific information is published by CMS and its contractors. The Reform Package also gives the Secretary of Health and Human Services the authority to apply competitive bid pricing to non-bid areas, but details of that process are unlikely to be understood until after CMS issues guidance or completes a related rulemaking process.
With respect to the competitive bidding program generally, at a March 2010 PAOC meeting, CMS briefed the PAOC regarding the next round of the DMEPOS competitive bidding program. With review of Round 1 Rebid bids then underway, the briefing focused on certain aspects of Round 2, which is mandated by MIPPA to begin in 2011. CMS expects to make changes to the program through the rulemaking process and anticipates that another proposed rule will be published in the summer of 2010, with a final rule to be published in the fall of 2010 relating to the Round 2 product categories, and then begin pre-bidding supplier education. CMS anticipates that it will announce the Round 2 bidding schedule and begin the bidding process, with bidder registration, in the winter of 2011. CMS plans to complete the bid evaluation process, announce the SPAs and begin the contract process in the spring of 2012. In addition, CMS plans to announce the Round 2 contract suppliers in the summer of 2012. We cannot quantify what negative impact, if any, the revised program, including the expansion of Round 2, will have upon our revenue or operations once the program is reinitiated, but it could be material.
Nevertheless, we believe that our geographic coverage, clinical marketing programs and purchasing strength provide competitive advantages to maintain and enhance market share under Medicare competitive bidding. However, there is no guarantee that we will be selected as a winning contract supplier in any phase of the program and be awarded competitive bidding contracts by CMS. Under the current competitive bidding regulations, if we are not selected as a winning contract supplier for a particular CBA, we will generally not be allowed to supply Medicare beneficiaries in the CBA with products subject to competitive bidding, unless we elect to continue to service existing patients under the “grandfathering provision” of the most recent final rule or we acquire a winning supplier. Because of our combination of both managed care and traditional business, we believe we can nevertheless maintain a favorable overall market position in a particular CBA even if we are not selected as a contract supplier.
Medicare Fee Schedule for DMEPOS and CPI Adjustments. In addition to the adoption of the DMEPOS competitive bidding program, the MMA implemented a five-year freeze on annual CPI payment increases for most durable medical equipment from 2004 to 2008. In MIPPA, in order to offset the cost of, or “pay for,” the delay in the implementation of the DMEPOS competitive bidding program, Congress approved a nationwide average payment reduction of 9.5% in the DMEPOS fee schedule for those product categories included in Round 1, effective January 1, 2009. These product categories could be subjected to a legislated CPI update in 2014, except if the item is still subject to competitive bidding or CMS has otherwise adjusted the payment rate.
The MIPPA legislation also authorized nationwide CPI increases from 2009 to 2013 for those DMEPOS items that were not subject to competitive bidding in July 2008. The CPI update for 2009 for non-competitively
109
Table of Contents
bid items was 5%. For 2010, the CPI was -1.4%, however, the annual DMEPOS updates cannot be negative. Accordingly, the CPI update in 2010 is 0%. The Reform Package makes changes to the Medicare durable medical equipment consumer price index adjustment for 2011 and each subsequent year based upon the CPI reduced by a new productivity adjustment which may result in negative updates.
Capped Rentals and Oxygen Equipment. Under the DRA, beginning with Medicare beneficiaries who received DMEPOS products and services as of January 2006, ownership of certain durable medical equipment categorized by CMS in the “capped rental” category (e.g., hospital beds, wheelchairs, nebulizers, patient lifts and CPAP devices) automatically transfers to the Medicare beneficiary at the end of a maximum rental period. As of January 1, 2006, the maximum rental period for this category became 13 months. Therefore, the first month in which the new policy had an impact on our revenue was February 2007. In addition, the service and maintenance fee, which had been paid to suppliers twice yearly after the rental period ended in order to cover various non-equipment service costs for patients who require use of the equipment, was eliminated for those patients who commenced service on or after January 1, 2006. However, the DRA provides for additional payments for maintenance and service of the item for repair parts and labor not covered by a supplier’s or manufacturer’s warranty. Implementing regulations also imposed other repair and replacement obligations on suppliers with respect to equipment that does not last the useful lifetime of the equipment, which CMS has generally defined as being five years.
With respect to oxygen equipment, the DRA converted Medicare reimbursement for oxygen equipment from an ongoing rental method to a capped rental and rent-to-purchase methodology and limited reimbursement for rental of oxygen equipment to the current 36-month maximum. The DRA mandated that, after the 36-month rental period, the ownership of the equipment would transfer to the Medicare beneficiary, who would assume primary responsibility for identifying when repairs or preventive maintenance are needed. However, MIPPA repealed the mandatory title transfer for oxygen equipment. The existing implementing regulations to the DRA and MIPPA provisions also limit supplier replacement of oxygen equipment during the rental period, and require suppliers to replace beneficiary-owned equipment that does not last the useful lifetime of the equipment, which CMS has generally defined as being five years. As a result, the equipment will continue to be owned by the home oxygen provider for as long as the patient’s medical need exists, after which time it will be returned to the home oxygen provider.
The 36-month rental period was retroactively applied to January 1, 2006 for all beneficiaries requiring oxygen as of December 31, 2005. Accordingly, Medicare services provided on or after January 1, 2009 were the first Medicare claims in which the rental cap impacted us. DRA regulations, which remain intact despite the repeal of mandatory title transfer, established new payment classes for oxygen equipment, including transfilling and portable equipment, new monthly rental reimbursement rates, and new reimbursement rates for the delivery of oxygen contents, if applicable. On November 19, 2008, CMS published revised regulations implementing DRA and MIPPA. Under the revised regulations, suppliers must continue furnishing oxygen equipment after the 36-month rental cap period during any period of medical need for the remainder of the reasonable useful lifetime of the equipment, with certain limited exceptions. CMS also specified that a new period of continuous use will not begin following the 36-month rental cap period until the end of the equipment’s reasonable useful lifetime, unless the equipment is replaced because it is lost, stolen, irreparably damaged, or is replaced after the reasonable useful life expires. CMS has provided that the reasonable useful lifetime of oxygen equipment is five years (60 months). Therefore, a new oxygen capped rental period (36 months) may begin after the five year (60 months) useful lifetime of the oxygen equipment. However, at least one DMEMAC has provided that a patient must request that the supplier provide the new oxygen equipment and the supplier may not arbitrarily issue new equipment. Among other provisions, CMS also stated that it would not reimburse suppliers for oxygen tubing, cannulas and supplies patients may need between the 37th and 60th months of oxygen therapy and requires that the initial supplier of oxygen therapy make arrangements with another supplier if a patient relocates temporarily or permanently outside of the initial supplier’s service area. In addition, CMS stated that it would not establish any reimbursement rates for non-routine services patients may require after the 36-month rental period. Our policies and procedures conform to the pertinent implementing regulations of the DRA and MIPPA.
110
Table of Contents
Regarding repairs and maintenance of oxygen equipment, CMS revised its regulations so that for services provided on or after January 1, 2009, the implementing regulations permitted payment in calendar year 2009 only to suppliers for general maintenance and servicing of certain oxygen equipment every six months, beginning after the first six-month period elapsed after the initial 36-month rental period. The final rule governing repairs and maintenance of oxygen equipment limits payment for general maintenance and servicing visits to 30 minutes of labor based on rates the Medicare contractors establish. With respect to equipment parts, CMS has stated that payments will not be made for equipment parts and that the supplier is responsible for replacing the parts on equipment from the supplier’s inventory in order to meet the patient’s medical need for oxygen. CMS issued guidance in November 2009 continuing the general maintenance and servicing payments for certain oxygen equipment. We cannot speculate on any future changes CMS may make to its repair, maintenance and service, supply or other fee schedules related to oxygen. We may or may not continue to provide repair and maintenance service on oxygen equipment that has met the cap. We routinely evaluate the impact of the changes caused by both the DRA and MIPPA and adjust our operating policies accordingly.
In January 2006, CMS published a final regulation that shifted payment for certain respiratory assist devices from the “frequent and substantial” payment category to the “capped rental” category. Under “frequent and substantial” payment, Medicare payment continues for the duration of the beneficiary’s medical need for the device, while “capped rental” payment is limited to 13 months despite the length of medical need. The change in the payment category became effective April 1, 2006. Although the change impacted company revenues and operating income from April 2006 through partial year 2008, there was no residual impact in 2009. We estimate that this change in payment categories resulted in a reduction in revenues of approximately $0.9 million in the period January 1, 2008 to October 28, 2008 from the same period in 2007. There was no impact in the year ended December 31, 2009 or the period October 29, 2008 to December 31, 2008.
In recent years, there have been several legislative and executive branch efforts to further reduce the maximum rental period for oxygen therapy, equipment and related services. Former President Bush’s 2007, 2008 and 2009 healthcare budget proposals sought to reduce the maximum rental period for oxygen equipment from the DRA-mandated 36 months to 13 months, which was recommended by the HHS OIG in a limited study of the oxygen benefit published in 2006 entitled “Medicare Home Oxygen: Equipment Cost and Servicing.” Neither President Obama’s 2010 budget proposal nor the Reform Package included a reduction in the oxygen rental period. However, it is premature to know whether future budgets or proposals will contain such a provision.
Over the course of 2008, CMS and the DMEMACs issued coverage determinations for positive airway pressure (“PAP”) devices, including CPAP and bi-level devices. Among other changes, the new Medicare DMEMAC local coverage determinations (“LCDs”) require additional documentation of clinical benefit of the PAP devices for continued coverage of the device beyond the first three months of therapy. Specifically, for PAP devices with initial dates of service on or after November 1, 2008, documentation of clinical benefit must be demonstrated by: (1) a face-to-face clinical re-evaluation by the treating physician (between the 31st and 90th day) with documentation that symptoms of obstructive sleep apnea are improved; and (2) objective evidence of adherence to use of the PAP device, reviewed by the treating physician. The LCDs define adherence to therapy as the use of the PAP device greater than or equal to 4 hours per night on 70% of nights during a consecutive thirty (30) day period anytime during the first three months of initial usage. If the clinical benefit requirements are not met, then continued coverage of the PAP device and related accessories are denied by Medicare as not medically necessary. We believe these requirements effectively require suppliers to supply PAP devices that monitor patient compliance and record hours of use, which adds to our expense structure without a corresponding increase in payments from Medicare. In late 2008 and throughout 2009, we have adjusted our operational model, patient care and payment policies to comply with these new Medicare requirements. These requirements only apply to Medicare Part B fee-for-service patients, not to those patients enrolled in Medicare Advantage or commercial health plans, and Medicare Part B fee-for-service represents a smaller portion of the overall PAP patient market. Despite our intensive efforts to educate patients about the importance of complying with their physician-prescribed therapy, some of our Medicare Part B patients did not meet the threshold for compliance in
111
Table of Contents
2009. In order to reduce the impact of the LCDs, we are continuing to educate patients and referral sources concerning the importance of compliance with the patient’s prescribed therapy. However, these LCDs are likely to continue to impact the PAP industry.
Reimbursement for Inhalation and Infusion Therapy Drugs. As a result of MMA, beginning January 2005, Medicare Part B reimbursement for most drugs, including inhalation drugs, became based upon the manufacturer-reported ASP (subject to adjustment each quarter), plus 6%, plus a separate dispensing fee per patient episode. CMS publishes the ASP plus 6% payment levels in the month that precedes the first day of each quarter, and we have no way of knowing if the quarterly ASPs will increase or decrease since manufacturers report applicable ASP information directly to CMS. Since 2006, dispensing fees have remained at $57.00 for a 30-day supply for a new patient, $33.00 for each 30-day supply thereafter, and $66.00 for each 90-day supply.
The Medicare reimbursement methodology for non-compounded, infused drugs administered through durable medical equipment, such as infusion pumps, was not affected by this MMA change. It remains based upon either 95% of the October 1, 2003 AWP or, for those drugs whose AWPs were not published in the applicable 2003 compendia, at 95% of the first published AWP. Also, coding and reimbursement changes pertaining to compounded medications, issued in 2007, did not have a material impact on us due to the extremely low volume of patient-specific, physician-prescribed compounding that was performed by our inhalation pharmacies.
Although CMS had considered issuing a National Coverage Decision (“NCD”) for certain inhalation drug therapies, in the third quarter of 2007, CMS issued a NCD that stated that no national coverage policy was appropriate at that time. Rather, CMS stated that it would continue to defer decisions about the medical necessity of individual respiratory drugs to the local contractors. Thereafter, in April of 2008, the DMEMACs finalized proposed LCD policies for several respiratory drugs, including Xopenex® and DuoNeb®1. Each of these two drugs was subjected to a separate “least costly alternative” (“LCA”) policy which would have changed the reimbursement methodology in a way that would effectively have eliminated Medicare beneficiary access to these drugs which are frequently used to treat COPD. After complaints were filed by Medicare beneficiaries in the Federal District Court for the District of Columbia, CMS announced that it planned to withdraw the LCA for Xopenex. On November 5, 2008, the plaintiffs in the Xopenex case filed a Motion to Voluntarily Dismiss all claims in the litigation. Subsequent to the filing of the complaint in the DuoNeb case, CMS postponed the LCA for DuoNeb until November 1, 2008. In November 2008, the Federal District Court for the District of Columbia enjoined CMS’s LCA for DuoNeb, saying that the Medicare program’s policy of paying for only “the least costly alternative” was not permitted under the Medicare law and finding that Medicare and some of its contractors had unlawfully limited payments for DuoNeb. The court made two distinct findings on the merits, in summary: (1) with limited exceptions (e.g., a public health emergency) CMS does not have the authority to deviate from the 106% ASP calculation for a covered Part B drug, and (2) the “reasonable and necessary” language in Section 1862 refers to coverage only and cannot be applied to reimbursement determinations. The court reasoned that CMS’s position would have given the Secretary of HHS significant discretion to determine the amount paid for every item and service covered by Medicare, without reference to the detailed formulas established in the laws enacted by Congress. This decision was appealed by the government in the U.S. Court of Appeals for the District of Columbia. In December 2009, the U.S. Court of Appeals for the District of Columbia upheld the District Court’s ruling in all regards and confirmed the distinction between Medicare coverage and reimbursement by ruling that the Medicare statute precludes the Secretary from issuing a coverage determination that sets the reimbursement rate for a covered drug based on the “least costly alternative”. This decision has national implications for the coverage of inhalation drugs. The time period for the government to file an appeal of the Court of Appeals’ decision has expired, and the government did not appeal.
In 2007 and 2008, there were other changes to the reimbursement methodology for the inhalation drugs Xopenex and albuterol. Beginning in the third quarter of 2007, CMS began reimbursing providers of Xopenex and albuterol a blended ASP for these two inhalation drugs. On December 29, 2007, the President signed into law
| 1 | Xopenex is a registered trademark of Sepracor, Inc., and DuoNeb is a registered trademark of Dey Labs, LLC. |
112
Table of Contents
the Medicare, Medicaid, and State Children’s Health Insurance Program Extension Act of 2007, which partially reversed the CMS regulatory decision regarding Xopenex and albuterol. Beginning on April 1, 2008, Medicare began to reimburse providers for Xopenex by blending the average sales prices of Xopenex and albuterol, but it no longer reimbursed providers for albuterol at the blended price. Rather, albuterol is reimbursed using an albuterol-only ASP.
We estimate that the combined effect of these changes to inhalation drug reimbursement resulted in a $7.9 million decline in revenue for the year ended December 31, 2009 from the same period in 2008. However, we have undertaken strategies intended to partially mitigate these negative impacts, including the discontinuation of the inhalation drug Xopenex from our inhalation pharmacies’ drug formulary and other formulary changes.
A limited number of infusion therapies, supplies and equipment are covered by Medicare Part B. The MMA, through the new Medicare Part D program, provided expanded coverage for certain home infusion therapy drugs, but excluded coverage for the corresponding supplies and clinical services needed to safely and effectively administer these drugs. We have contracted with a limited number of Medicare Part D prescription drug plans in order to provide continuity of care for certain patients.
Due to ongoing Part D and Part B coverage and payment issues associated with home infusion therapy, the industry is continuing to work with CMS and Congress to rectify the Medicare coverage and payment limitations that restrict Medicare beneficiary and referral source access to quality home infusion therapy services. Bills were introduced in the 111th Congress in January 2009 (as they were in both the 109th and 110th Congress) to consolidate home infusion therapy coverage under Part B. The Medicare Home Infusion Coverage Act of 2009 would provide for Medicare infusion benefit coverage in a more comprehensive manner that is analogous to how the therapy is covered by the managed care sector, including Medicare Advantage plans. Industry representatives continue to present the cost-saving and patient care advantages of home infusion therapy to CMS, members of Congress and the Obama Administration in an effort to, at a minimum, include a formal demonstration project in future legislation. Testimony before the Senate Finance Committee in September 2009 acknowledged the current gap in coverage and potential benefits of home infusion therapy to the Medicare program and beneficiaries. At this time, we cannot predict whether the legislation will be introduced in the 112th Congress or become law.
Enrollment and Accreditation of Durable Medical Equipment Suppliers; Surety Bond Requirements. While we support the elimination of fraudulent suppliers and are working with CMS to support these initiatives, we also note that a number of initiatives and developments with respect to the enrollment and accreditation of providers could impact our operations in the future. For example, MIPPA mandated a September 30, 2009 deadline for accreditation of all durable medical equipment providers who bill the Medicare program for DMEPOS services and products. We and all of our branches currently are accredited. If we or any of our branches lose accreditation, or if any of our new branches are unable to become accredited, that could have a material adverse effect on our results of operations, cash flow and capital resources.
In July 2007, CMS issued a proposed rule requiring all DMEPOS suppliers to provide CMS with a surety bond of at least $65,000 for each National Provider Identifier (“NPI”) number the supplier holds. On January 2, 2009, CMS issued the final rule, requiring a surety bond of $50,000 by October 2, 2009 per NPI number which Medicare has approved for billing privileges. Apria obtained the required surety bonds for all of its applicable locations before the deadline and received confirmation from the National Supplier Clearinghouse (“NSC”) that the NSC has recorded the bonds properly in its records. In addition, the NSC prescribes an elevated bond amount of $50,000 per occurrence of an adverse legal action within the 10 years preceding enrollment, reenrollment or revalidation. The rule, effective March 3, 2009, is designed to ensure that Medicare can recover any erroneous payment amounts or civil money penalties up to $50,000 that result from fraudulent or abusive supplier billing practices.
In October 2008, CMS announced enhancements to its program integrity initiatives designed to identify and prevent waste, fraud and abuse. The initiatives include: (i) conducting more stringent reviews of DMEPOS
113
Table of Contents
suppliers’ applications, including background checks of new DMEPOS suppliers’ principals and owners to ensure they have not been suspended by Medicare; (ii) making unannounced site visits to suppliers and home health agencies to ensure they are active, legitimate businesses; (iii) implementing extensive pre- and post-payment claims review; (iv) verifying the relationship between physicians who order a large volume of DMEPOS equipment and the beneficiaries for whom they ordered these services; and (v) identifying and visiting beneficiaries to ensure appropriate receipt of Medicare-reimbursable items and services. We work cooperatively with CMS and its contractors in response to these initiatives but cannot predict whether CMS’ various program integrity efforts will or will not negatively impact our operations.
Following the implementation of a 3-year demonstration program using Recovery Audit Contractors (“RACs”) to detect and correct improper payments in the Medicare fee-for-service program, the Tax Relief and Health Care Act of 2006 required HHS to establish the RAC initiative as a permanent, nationwide program by no later than January 1, 2010. CMS selected the four RAC contractors for the permanent RAC program and the permanent RAC program is currently underway. Prior to initiating any audits, RACs are required to obtain CMS’ pre-approval of the issue that will be subject to audit, and then post the approved audit issue on their websites. All RACs have now posted CMS-approved audit issues on their websites. The currently posted approved audit issues include those which apply to Durable Medical Equipment (“DME”) suppliers. States have also implemented similar state Medicaid audit programs, often know as Medicaid Integrity Contractors (“MICs”). The Reform Package expands the RAC program to include Medicare Parts C and D in the program. In addition, the Reform Package requires states to establish contracts with RACs to identify underpayments and overpayments and to recoup overpayments made for services provided under State Medicaid programs. In addition, in March of 2010, President Obama issued a presidential memorandum announcing a government-wide program expanding the use “payment recapture audits” in order to reclaim improper payments. We cannot at this time quantify any negative impact that the expansion of the RAC program or other similar programs may have on us.
In October 2008, CMS announced the establishment of new Zone Program Integrity Contractors (“ZPICs”), who are responsible for ensuring the integrity of all Medicare-related claims. The ZPICs assumed the responsibilities previously held by Medicare’s Program Safeguard Contractors (“PSCs”).
Other Issues
| • | Medical Necessity & Other Documentation Requirements. In order to ensure that Medicare beneficiaries only receive medically necessary and appropriate items and services, the Medicare program has adopted a number of documentation requirements. For example, the DMEMAC Supplier Manuals provide that clinical information from the “patient’s medical record” is required to justify the medical necessity for the provision of DME. We have implemented policies and procedures to meet Medicare’s documentation requirements. However, an auditor for one of the DMEMACs has taken the position, among other things, that the “patient’s medical record” refers not to documentation maintained by the DME supplier but instead to documentation maintained by the patient’s physician, healthcare facility or other clinician, and that clinical information created by the DME supplier’s personnel and confirmed by the patient’s physician is not sufficient to establish medical necessity. It may be difficult, and sometimes impossible, for us to obtain documentation from other healthcare providers. If these or other burdensome positions are generally adopted by auditors, DMEMACs, other contractors or CMS in administering the Medicare program, we would have the right to challenge these positions as being contrary to law. If these interpretations of the documentation requirements are ultimately upheld, however, it could result in our making refunds and other payments to Medicare and our future revenues from Medicare may be reduced. We cannot currently predict the adverse impact, if any, these interpretations of the Medicare documentation requirements might have on our operations, cash flow and capital resources, but such impact could be material. |
| • | Inherent Reasonableness. The Balanced Budget Act of 1997 granted authority to HHS to increase or reduce Medicare Part B reimbursement for home medical equipment, including oxygen, by up to 15% each year under |
114
Table of Contents
an “inherent reasonableness” authority. Pursuant to that authority, CMS published a final rule that established a process by which such adjustments may be made. The rule applies to all Medicare Part B services except those paid under a physician fee schedule, a prospective payment system, or a competitive bidding program. Neither HHS nor CMS has issued any subsequent communication or information for several years and therefore, we cannot predict whether or when HHS would exercise its authority in this area or predict any negative impact of any such change. |
The impact of changes in Medicare reimbursement that have been enacted to date are reflected in our results of operations for the year ended December 31, 2009. We cannot estimate the combined possible impact of all legislative, regulatory and contemplated reimbursement changes that could have a material adverse effect on our results of operations, cash flow, and capital resources. Moreover, our estimates of the impact of certain of these changes appearing in this “Government Regulation” section are based on a number of assumptions and are subject to uncertainties and there can be no assurance that the actual impact was not or will not be different from our estimates.
Medicaid Reimbursement.State Medicaid programs implement reimbursement policies for the items and services we provide that may or may not be similar to those of the Medicare program. Budget pressures on these state programs often result in pricing and coverage changes that may have a detrimental impact on our operations and/or financial performance. States sometimes have adopted alternative pricing methodologies for certain drugs, biologicals, and home medical equipment under their Medicaid programs that reduce the level of reimbursement received by us without a corresponding offset or increase to compensate for the service costs incurred. For example, Medi-Cal adopted a regulation that limits the amounts a provider can bill for certain durable medical equipment and medical supplies. In March 2009, CAMPS initiated a lawsuit to invalidate this regulation as having been adopted in violation of California’s Administrative Procedure Act. On August 3, 2009, the court entered a decision denying CAMPS’ petition. CAMPS has appealed the court’s decision. If the regulation is ultimately upheld, it would most likely result in our making refunds and other payments to Medi-Cal and our future revenues from Medi-Cal may be reduced. Historically, when such alternative pricing methodologies were adopted, we have sometimes elected to stop accepting new Medicaid patient referrals for the affected drugs, biologicals, and home medical equipment. We are currently evaluating the possibility of stopping or reducing our Medicaid business in a number of states with reimbursement policies that make it difficult for us to safely care for patients or conduct operations profitably. Moreover, the Reform Package increases Medicaid enrollment over a number of years and imposes additional requirements on states which, combined with the current economic recession and state deficits, could further strain state budgets and therefore result in additional policy changes or rate reductions. We cannot currently predict the adverse impact, if any, that any such change to or reduction in our Medicaid business might have on our operations, cash flow and capital resources, but such impact could be material. In addition, we cannot predict whether other states will consider similar or other reimbursement reductions, whether healthcare reform provisions pertaining to Medicaid will ultimately be passed into law or whether any such changes would have a material adverse effect on our results of operations, cash flow and capital resources.
HIPAA.The Health Insurance Portability and Accountability Act of 1996 (“HIPAA”) is comprised of a number of components pertaining to the privacy and security of certain protected health information (“PHI”), as well as the standard formatting of certain electronic health transactions. Many states have similar, but not identical, restrictions. Existing and any new laws or regulations have a significant effect on the manner in which we handle healthcare related data and communicate with payors. Among other provisions, the HITECH Act of the American Recovery and Reinvestment Act of 2009 includes additional requirements related to the privacy and security of PHI, clarifies and increases penalties of HIPAA and provides State Attorneys General with HIPAA enforcement authority. We have adopted a number of policies and procedures to conform to HIPAA requirements, as modified by the HITECH Act of the American Recovery and Reinvestment Act of 2009, throughout our operations and educated our workforce about HIPAA provisions. We face potential administrative, civil and criminal sanctions if we do not comply with the existing or new laws and regulations. Imposition of these sanctions could have a material adverse effect on our operations.
115
Table of Contents
Enforcement of Healthcare Fraud and Abuse Laws.In recent years, the federal government has made a policy decision to significantly increase the financial resources allocated to enforcing the healthcare fraud and abuse laws. Moreover, Congress adopted a number of additional provisions in the Reform Package that are designed to reduce healthcare fraud and abuse. In addition, private insurers and various state enforcement agencies have increased their level of scrutiny of healthcare claims in an effort to identify and prosecute fraudulent and abusive practices in the healthcare area. From time to time, we may be the subject of investigations or a party to additional litigation which alleges violations of law. If any of those matters were successfully asserted against us, there could be a material adverse effect on our business, financial position, results of operations or prospects.
Anti-Kickback Statutes. As a provider of services under the Medicare and Medicaid programs, we must comply with a provision of the federal Social Security Act, commonly known as the “federal anti-kickback statute.” The federal anti-kickback statute prohibits the offer or receipt of any bribe, kickback or rebate in return for the referral or arranging for the referral of patients, products or services covered by federal healthcare programs. Federal healthcare programs have been defined to include plans and programs that provide health benefits funded by the United States Government, including Medicare, Medicaid and TRICARE (formerly known as the Civilian Health and Medical Program of the Uniformed Services or CHAMPUS), among others. Some courts and the OIG interpret the statute to cover any arrangement where even one purpose of the remuneration is to influence referrals. Violations of the federal anti-kickback statute may result in civil and criminal penalties and exclusion from participation in federal healthcare programs.
Due to the breadth of the federal anti-kickback statute’s broad prohibition, there are a few statutory exceptions that protect various common business transactions and arrangements from prosecution. In addition, the OIG has published safe harbor regulations that outline other arrangements that also are deemed protected from prosecution under the federal anti-kickback statute, provided all applicable criteria are met. The failure of an activity to meet all of the applicable safe harbor criteria does not necessarily mean that the particular arrangement violates the federal anti-kickback law, but these arrangements will be subject to greater scrutiny by enforcement agencies.
Some states have enacted statutes and regulations similar to the federal anti-kickback statute, but which apply not only to the federal healthcare programs, but also to any payor source of the patient. These state laws may contain exceptions and safe harbors that are different from those of the federal law and that may vary from state to state. A number of states in which we operate have laws that prohibit fee-splitting arrangements between healthcare providers, if such arrangements are designed to induce or encourage the referral of patients to a particular provider. Additionally, several states have passed laws further regulating interactions between healthcare providers and physician referral sources. In late 2009, the state of New York enacted a requirement for certain healthcare providers to file a formal statement in which they attest that they have adopted a formal corporate compliance program which meets the state’s specific requirements; we complied with that requirement. Possible sanctions for violations of these restrictions include exclusion from state-funded healthcare programs, loss of licensure, and civil and criminal penalties. Such statutes vary from state to state, are often vague and often have been subject to only limited court or regulatory agency interpretation.
Marketing Laws.Because of our drug compounding and oxygen services, we may be subject to new and increasingly common state laws and regulations regarding our marketing activities and the nature of our interactions with physicians and other healthcare entity customers. These laws may require us to comply with certain codes of conduct, limit or report certain marketing expenses, disclose certain physician and customer arrangements, and ensure the appropriate licensure of certain sales personnel. There have also been similar federal legislative and regulatory initiatives. Violations of these laws and regulations, to the extent applicable, could subject us to civil and criminal fines and penalties, as well as possible exclusion from participation in federal healthcare programs, such as Medicare and Medicaid. From time to time, we may be the subject of investigations or audits or be a party to litigation which alleges violations of these laws. If any of those matters were successfully asserted against us, there could be a material adverse effect on our business, financial position, results of operations or prospects.
116
Table of Contents
Physician Self-Referral. Certain provisions of the Omnibus Budget Reconciliation Act of 1993 (the “Stark Law”) prohibit healthcare providers such as us, subject to certain exceptions, from submitting claims to the Medicare and Medicaid programs for designated health services if we have a financial relationship with the physician making the referral for such services or with a member of such physician’s immediate family. The term “designated health services” includes several services commonly performed or supplied by us, including durable medical equipment and home health services. In addition, “financial relationship” is broadly defined to include any ownership or investment interest or compensation arrangement pursuant to which a physician receives remuneration from the provider at issue. The Stark Law prohibition applies regardless of the reasons for the financial relationship and the referral; and therefore, unlike the federal anti-kickback statute, an intent to violate the law is not required. Like the federal anti-kickback statute, the Stark Law contains a number of statutory and regulatory exceptions intended to protect certain types of transactions and business arrangements from penalty. In order to qualify an arrangement under a Stark Law exception, compliance with all of the exception’s requirements is necessary. Violations of the Stark Law may result in loss of Medicare and Medicaid reimbursement, civil penalties and exclusion from participation in the Medicare and Medicaid programs.
In addition, a number of the states in which we operate have similar prohibitions against physician self-referrals, which may not necessarily be limited to Medicare or Medicaid services and may not include the same statutory and regulatory exceptions found in the Stark Law.
False Claims. The federal False Claims Acts impose civil and criminal liability on individuals or entities that submit false or fraudulent claims for payment to the government. Violations of the federal civil False Claims Act may result in treble damages, civil monetary penalties and exclusion from the Medicare, Medicaid and other federally funded healthcare programs. If certain criteria are satisfied, the federal civil False Claims Act allows a private individual to bring a qui tam suit on behalf of the government and, if the case is successful, to share in any recovery. Federal False Claims Act suits brought directly by the government or private individuals against healthcare providers, like us, are increasingly common and are expected to continue to increase.
The federal government has used the federal False Claims Act to prosecute a wide variety of alleged false claims and fraud allegedly perpetrated against Medicare and state healthcare programs. The government and a number of courts also have taken the position that claims presented in violation of certain other statutes, including the federal anti-kickback statute or the Stark Law, can be considered a violation of the federal False Claims Act, based on the theory that a provider impliedly certifies compliance with all applicable laws, regulations and other rules when submitting claims for reimbursement.
On May 20, 2009, President Obama signed into law the Fraud Enforcement and Recovery Act of 2009 (“FERA”). Among other things, FERA modifies the federal False Claims Act by expanding liability to contractors and subcontractors who do not directly present claims to the federal government. FERA also expanded the False Claims Act liability for what is referred to as a “reverse false claim” by explicitly making it unlawful to knowingly conceal or knowingly and improperly avoid or decrease an obligation owed to the federal government.
A number of states have enacted false claims acts that are similar to the federal False Claims Act. Even more states are expected to do so in the future because Section 6031 of the DRA amended the federal law to encourage these types of changes in law at the state level. In addition, there is a corresponding increase in state-initiated false claims enforcement efforts.
Other Fraud and Abuse Laws. HIPAA created, in part, two new federal crimes: “Healthcare Fraud” and “False Statements Relating to Healthcare Matters.” The Healthcare Fraud statute prohibits executing a knowing and willful scheme or artifice to defraud any healthcare benefit program. A violation of this statute is a felony and may result in fines and/or imprisonment. The False Statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact by any trick, scheme or device or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. A violation of this statute is a felony and may result in fines and/or imprisonment.
117
Table of Contents
The increased public focus on waste, fraud and abuse and their related cost to society will likely result in additional Congressional hearings, CMS regulatory changes or new laws. The Reform Package also provides for new regulatory authority, additional fines and penalties. At this time, we cannot predict whether these or other reforms will ultimately become law, or the impact of such reforms on our business operations and financial performance.
Certification of Compliance Agreement. Pursuant to the merger agreement to acquire Coram, we assumed Coram’s obligations. On August 22, 2007, Coram entered into a three-year Compliance Agreement with the OIG which obligates Coram to maintain a compliance program to monitor, ensure compliance with federal healthcare program requirements and submit timely reports to the government regarding the same. Violation of the terms of the Compliance Agreement could result in the imposition of penalties and sanctions, including disqualification from Medicare and other reimbursement programs. The year 2009 marked the second year of the three-year agreement; in both 2008 and 2009, the OIG accepted our annual certification documents as filed. See “—Organization and Operations—Corporate Compliance” and “Risk Factors—Risks Relating to Our Business— Non-Compliance With the Requirements of Coram’s Certification of Compliance Agreement Could Result in the Imposition of Significant Penalties and Sanctions” for additional information regarding the Compliance Agreement.
Facility and Clinician Licensure.Various federal and state authorities and clinical practice boards regulate the licensure of our facilities and clinical specialists working for us, either directly as employees or on a per diem or contractual basis. Regulations and requirements vary from state to state, and in some states, we are required to make filings in connection with transactions that may be defined as a change of control. Moreover, several states are currently contemplating the establishment or expansion of facility licensure related to the home healthcare industry. We are committed to complying with all applicable licensing requirements and maintain centralized functions to manage over 4,500 facility licenses and/or permits that are required to operate our business.
Healthcare Reform Legislation.Economic, political and regulatory influences are causing fundamental changes in the healthcare industry in the United States. Various healthcare reform proposals are formulated and proposed by the legislative and administrative branches of the federal government on a regular basis. In addition, some of the states in which we operate periodically consider various healthcare reform proposals. Even with the passage of the Reform Package, we anticipate that federal and state governments will continue to review and assess alternative healthcare delivery systems and payment methodologies and public debate of these issues will continue in the future. A number of parties, including some State governments, have expressed their intentions to challenge the Reform Package, and we cannot predict the outcome of such challenges, if any. Changes in the law or new interpretations of existing laws can have a substantial effect on permissible activities, the relative costs associated with doing business in the healthcare industry and the amount of reimbursement by governmental and other third-party payors. Due to uncertainties regarding the ultimate features of reform initiatives and their enactment and implementation, we cannot predict which, if any, of such reform proposals will be adopted, or when they may be adopted, or that any such reforms will not have a material adverse effect on our results of operations, cash flow, capital resources and liquidity.
Employees
As of March 31, 2010, we had 11,419 employees, of which 10,011 were full-time and 1,408 were part-time and per-diem. As of March 31, 2010, none of our employees were represented by a labor union or other labor organization.
Properties
We lease our headquarters, located in Lake Forest, California, which consists of approximately 100,000 square feet of office space. The lease expires in January 2012.
118
Table of Contents
We have approximately 500 locations that serve patients in all 50 states, including branches, billing centers, pharmacies, warehouse and storage facilities. The regional facilities usually house a branch and various regional support functions such as repair, billing and distribution. The regional facilities are typically located in light industrial areas and generally range from 16,000 to 133,000 square feet. The typical branch facility, other than those that share a building with a region, is a combination warehouse and office and can range from 650 to 50,000 square feet. We lease substantially all of our facilities with lease terms of ten years or less.
Legal Proceedings
We are also engaged in the defense of certain claims and lawsuits arising out of the ordinary course and conduct of our business, the outcomes of which are not determinable at this time. Insurance policies covering such potential losses, where such coverage is cost effective, are maintained. In the opinion of management, any liability that might be incurred upon the resolution of these claims and lawsuits will not, in the aggregate, have a material adverse effect on our financial condition or results of operations.
119
Table of Contents
Directors and Executive Officers
The following table sets forth information with respect to the individuals who have been serving as the members of our Board of Directors since the consummation of the Merger as well as information relating to the executive officers of Apria (ages are as of July 1, 2010).
Name | Age | Position | ||
Norman C. Payson, M.D. | 62 | Executive Chairman of the Board of Directors; Chief Executive Officer | ||
Chris A. Karkenny | 42 | Executive Vice President and Chief Financial Officer | ||
James G. Gallas | 47 | Executive Vice President and Chief Administrative Officer | ||
Daniel E. Greenleaf | 45 | President, Home Infusion Therapy Segment | ||
Neil P. Simpkins | 44 | Director | ||
Michael Dal Bello | 39 | Director | ||
Patrick J. Bourke III | 51 | Director |
Norman C. Payson, M.D. was appointed Executive Chairman of the Board of Directors and Chief Executive Officer effective as of October 2008. He currently serves as Vice Chairman of the Board of Directors and Chairman of the Board Finance Committee of City of Hope, a not-for-profit tertiary cancer hospital and research center. He was Chief Executive Officer of Oxford Health Plans from 1998 through 2002. Dr. Payson co-founded Healthsource, Inc., a large health plan operating in 15 states, in 1985 and served as its Chief Executive Officer from 1985 through 1997.
On October 26, 2004, the SEC issued an order finding that Dr. Payson violated Section 13(d) of the Exchange Act in connection with the submission of certain Section 13D filings relating to Dr. Payson’s holdings in Oxford Health Plans, Inc. that were not filed on a timely basis and that contained certain inaccurate and incomplete disclosures. At the time of his initial election to our Board of Directors in 2008, our Corporate Governance and Nominating Committee and the Board of Directors reviewed the circumstances in detail and determined that such violations were not an adverse reflection on Dr. Payson’s ability to serve on the Board of Directors and that such violations are not material to the evaluation of his qualifications or integrity.
Chris A. Karkennyjoined us as Executive Vice President and Chief Financial Officer in November 2006. From January 2003 to February 2006, Mr. Karkenny served as Senior Vice President of Corporate Development and Treasury Operations of PacifiCare Health Systems, Inc., a Fortune 500 company. From August 1999 to December 2002, Mr. Karkenny served as Chief Executive Officer of NetCatalyst, a California investment banking firm. From July 1998 to August 1999, Mr. Karkenny served as a partner in Technologz, a California-based business incubator, and was a founder, Board member and initial Chief Financial Officer of CardioNow, a healthcare application service provider. From 1995 to March 1998, he served as Treasurer of Quarterdeck Corporation.
James G. Gallas joined us as Executive Vice President and Chief Administrative Officer in April 2009. Mr. Gallas most recently served as Principal in charge of KPMG’s Healthcare Revenue Cycle Management Practice from January 2009 to March 2009. Prior to that, from September 2005 to December 2008, Mr. Gallas served as Senior Vice President in charge of the Healthcare Segment of BearingPoint, Inc., formerly KPMG Consulting, Inc., a global management and technology consulting company. From January 2000 to August 2005, Mr. Gallas served as Vice President in charge of BearingPoint, Inc.’s Healthcare Provider Practice. Prior to that time, from August 1997 to December 1999, Mr. Gallas served as a Partner at KPMG, LLP until the time KPMG Consulting, Inc. separated from KPMG LLP, at which point Mr. Gallas served as a Managing Director for KPMG Consulting, Inc. From July 1985 to July 1997, Mr. Gallas served as an accountant, Manager and Senior Manager at KPMG, LLP.
120
Table of Contents
Daniel E. Greenleaf was appointed President of the Home Infusion Therapy Segment in April 2008. Mr. Greenleaf most recently served as President and Chief Executive Officer of VioQuest Pharmaceuticals, Inc., a New Jersey-based biopharmaceutical company focused on the acquisition, development and commercialization of oncology drug therapies, from 2005 to 2007. Prior to his role at VioQuest Pharmaceuticals, Inc., Mr. Greenleaf was President of U.S. Operations for Celltech Biopharmaceuticals, a NYSE listed company prior to its sale to UCB in 2004. Celltech is a global biopharmaceutical leader focused on severe diseases such as central nervous system disorders, rheumatoid arthritis, Crohn’s disease and multiple sclerosis. Additional experience includes serving as Senior Vice President of Operations for Nabi Pharmaceuticals, a research company focused on the development of products targeting nicotine addiction and serious infections, as well as progressively more responsible management and executive-level positions with Schering-Plough Corporation, a global healthcare company, from 1992 to 2002. Mr. Greenleaf was a captain and navigator in the United States Air Force and served in Operation Desert Storm.
Neil P. Simpkins became one of our directors immediately after the completion of the Merger in October 2008. Mr. Simpkins has served as a Senior Managing Director in the Private Equity Group of Blackstone since December 1999. From 1993 until the time he joined Blackstone, Mr. Simpkins was a principal at Bain Capital. Prior to joining Bain Capital, Mr. Simpkins was a consultant at Bain & Company in London and the Asia Pacific region. He currently serves as Chairman of the Board of Directors of TRW Automotive Inc. and as a director of Vanguard Health Systems, Inc., Team Health, Inc. and Summit Materials, LLC.
Michael Dal Bello became one of our directors immediately after the completion of the Merger in October 2008. Mr. Dal Bello has been a Managing Director in the Private Equity Group of The Blackstone Group since December 2008 and was a Principal in this group from 2005 until 2008, and an Associate from 2002 until 2005. Prior to joining Blackstone, Mr. Dal Bello received an M.B.A. from Harvard Business School in 2002. Mr. Dal Bello serves on the Board of Directors of Biomet, Inc., Catalent Pharma Solutions, Inc., Sithe Global Power, LLC, Team Health, Inc. and Vanguard Health Systems, Inc.
Patrick J. Bourke III became one of our directors immediately after the completion of the Merger in October 2008. Mr. Bourke is an Executive Director in the Private Equity Group of Blackstone. Before joining Blackstone in 2008, Mr. Bourke was the Executive Vice President of Business Operations and Chief Re-engineering Officer of Travelport Limited. Prior to Travelport Limited, Mr. Bourke was a key executive at Perot Systems, Inc. where he held numerous management roles. Mr. Bourke started his career at Electronic Data Systems Corporation in their Systems Engineering Development program. Mr. Bourke serves on the Board of Directors of Intelenet Global Services Private Limited.
On February 12, 2010, we finalized a decision to reorganize the senior leadership structure of the home respiratory therapy and home medical equipment segment. As part of this reorganization, Lawrence A. Mastrovich, President, Home Respiratory Therapy and Home Medical Equipment service lines, resigned and his responsibilities were assumed by the other senior executives.
Corporate Governance Matters
Background and Experience of Directors. When considering whether directors and nominees have the experience, qualifications, attributes or skills, taken as a whole, to enable our Board of Directors to satisfy its oversight responsibilities effectively in light of our business and structure, our Board of Directors focused primarily on each person’s background and experience as reflected in the information discussed in each of the directors’ individual biographies set forth immediately above. In particular, the members of our Board of Directors considered the following important characteristics: (i) Messrs. Neil P. Simpkins, Michael Dal Bello and Patrick J. Bourke III are representatives appointed by The Blackstone Group, our principal stockholder, and have significant financial and investment experience from their involvement in Blackstone’s investment in numerous portfolio companies, particularly those in the healthcare industry, and have played active roles in overseeing those businesses and (ii) our Chief Executive Officer has extensive experience in the healthcare industry and in executive management.
121
Table of Contents
Executive Compensation
Compensation Discussion and Analysis
Introduction
Our executive compensation plan is designed to attract and retain individuals qualified to manage and lead the Company and to also motivate them to contribute to achievement of our financial goals and ultimately create and grow our equity value.
In connection with the Merger, we recruited some of our senior management employees, including our Chief Executive Officer and our Executive Vice President and Chief Administrative Officer, and entered into new employment arrangements or amendments to existing employment agreements with other senior executives. We continued to compensate our senior management on a basis similar to immediately prior to the Merger, except that, in light of our status as a private company, we adopted less broad-based equity incentive arrangements for our senior executives and did not continue a practice of granting equity awards on an annual basis.
Our named executive officers for 2009 are:
| • | Norman C. Payson, M.D., our Executive Chairman of the Board of Directors and Chief Executive Officer; |
| • | Chris A. Karkenny, our Executive Vice President and Chief Financial Officer; and |
| • | our three other most highly compensated executive officers who served in such capacities at December 31, 2009, namely, |
| • | James G. Gallas, our Executive Vice President and Chief Administrative Officer, |
| • | Lawrence A. Mastrovich, our former President, Home Respiratory Therapy and Home Medical Equipment segment and |
| • | Daniel E. Greenleaf, our President, Home Infusion Therapy segment. |
Dr. Payson and Mr. Gallas joined us subsequent to the Merger, and Mr. Mastrovich resigned from the Company, effective as of March 15, 2010. See “Business—Our Company—Recent Developments.”
Executive Compensation Objectives and Philosophy
Our primary executive compensation objective is to:
| • | attract, retain and motivate leaders who are capable of advancing our mission and strategy and ultimately, create and maintain our long-term equity value. Such leaders must engage in a collaborative approach and possess the ability to execute our strategy in an industry characterized by competitiveness, growth and a challenging business environment; |
| • | reward senior management in a manner aligned with our financial performance; and |
| • | align senior management’s interests with our equity owners’ long-term interests through equity participation and ownership. |
To achieve our objective, we deliver executive compensation through a combination of the following components:
| • | Base salary; |
| • | Bonuses (annual cash incentive compensation and discretionary bonus); |
| • | Long-term incentive compensation; |
122
Table of Contents
| • | Broad-based employee benefits; |
| • | Supplemental executive benefits and perquisites; and |
| • | Severance benefits. |
We provide competitive base salaries and other benefits and perquisites, including severance benefits, to attract and retain senior management talent. We also use annual cash incentive compensation and long-term equity incentives to ensure a performance-based delivery of pay that aligns as closely as possible the rewards of our named executive officers with the long-term interests of our equity-owners while enhancing executive retention.
Compensation Determination Process
Following the Merger, our Board of Directors makes all decisions about our executive compensation. Presently, our Board of Directors does not have a compensation committee.
In making initial compensation determinations with respect to our named executive officers following the Merger, our Board of Directors considered a number of variables, consistent with our executive compensation objective, including individual circumstances related to each executive’s recruitment or retention. For example, compensation for each of Dr. Payson and Mr. Karkenny was determined as part of the negotiation of each executive’s employment agreement. In connection with the negotiation of Mr. Karkenny’s employment agreement, our Board of Directors considered the fact that he would be entitled to severance if he terminated his employment following the Merger pursuant to the terms of his then existing employment agreement and provided him with an overall compensation package, that included an increase in base salary and a grant of restricted equity units, intended to induce him to stay with us. In addition, our Board of Directors decided to grant to Dr. Payson substantially greater equity incentive awards with vesting terms that differ from the terms applicable to other senior executives in light of his role as our Executive Chairman of the Board of Directors and Chief Executive Officer and his substantially greater equity investment in our ultimate parent, BP Healthcare Holdings LLC. The specific terms of each of Dr. Payson’s employment agreement and Mr. Karkenny’s employment agreement are discussed below under “Narrative Disclosure to Summary Compensation Table and Grants of Plan-Based Awards—Employment Agreements.” Subsequent to the determination of initial compensation packages following the Merger, our Board of Directors has not made any compensation determinations with respect to our named executive officers other than determinations of annual cash incentive awards described below under “—Compensation Elements—Bonuses—Annual Cash Incentive Compensation.” Our Board of Directors did not use any compensation consultants in making its compensation determinations.
Dr. Payson and Mr. Karkenny generally participate in discussions and deliberations of our Board of Directors regarding the determinations of annual cash incentive awards for our executives. Specifically, they make recommendations to our Board of Directors regarding the performance targets to be used under our annual Executive Bonus Plan and the amounts of annual cash incentive awards and any discretionary bonus amounts to be made to our senior executives. In the future, we expect that Dr. Payson will also make recommendations to our Board of Directors regarding base salary adjustments for other executives based on his annual review of each officer’s performance. We expect that our Board of Directors will consider Dr. Payson’s recommendations and may exercise discretion in modifying his recommendations.
Compensation Elements
The following is a discussion and analysis of each component of our executive compensation program.
Base Salary.
Base salary compensates executives for performing requirements of their position and provides executives with a level of cash income predictability and stability with respect to a portion of their total compensation. Our
123
Table of Contents
Board of Directors believes that the level of an executive officer’s base salary should reflect that executive officer’s performance, experience and breadth of responsibilities, salaries for similar positions within the community and in our industry generally and any other factors relevant to that particular job. In determining applicable base salaries with respect to those named executive officers that were hired subsequent to the Merger, namely, Dr. Payson and Mr. Gallas, our Board of Directors also consulted with outside recruiters to ensure that we could recruit the candidate of our choice by offering a competitive base salary. In connection with the negotiation of Mr. Karkenny’s employment agreement, our Board of Directors increased his then annual base salary in order to provide an overall compensation package that would incentivize him to stay with us notwithstanding his entitlement to severance upon his resignation. In approving amended employment agreements for Messrs. Mastrovich and Greenleaf, our Board of Directors did not make any adjustments to their respective base salaries previously approved by the former Board’s compensation committee because they believed that the amounts of their base salaries were appropriate in light of their duties, performance and similar positions within our community and industry.
Base salaries may be adjusted annually and, in certain circumstances, adjusted mid-year to deal with competitive pressures or changes in job responsibilities, in the sole discretion of our Board of Directors. Our Board of Directors did not make any adjustments to any of our named executive officers’ base salaries in 2009.
Bonuses.
Annual Cash Incentive Compensation.Annual cash incentive awards are available to our named executive officers under our annual Executive Bonus Plan in order to motivate our executive officers to achieve short-term performance goals and tie a portion of their cash compensation to performance.
Under our Executive Bonus Plan for 2009 (the “2009 Bonus Plan”), each named executive officer was eligible to earn an annual cash incentive award based on achievement of performance targets for 2009. These performance targets were determined by our Board of Directors early in the year, after taking into consideration Dr. Payson and Mr. Karkenny’s recommendations and our budget for the year. The potential amount of the annual cash incentive award was based on a percentage of the executive officer’s base salary actually paid during 2009, except with respect to Mr. Gallas, whose potential award was based on a percentage of his annualized base salary rate at date of hire pursuant to the terms of his offer letter. The following table illustrates our named executive officers’ potential cash incentive awards as a percentage of their base salary.
| Threshold Bonus Opportunity for achieving threshold performance | Target Bonus Opportunity for achieving target performance | Maximum Bonus Opportunity for achieving maximum performance | ||||
Norman C. Payson, M.D. | 0% | 100% | 200% | |||
Chris A. Karkenny | 0% | 100% | 200% | |||
James G. Gallas(1) | — | 100% | 150% | |||
Lawrence A. Mastrovich | 0% | 100% | 150% | |||
Daniel E. Greenleaf | 0% | 100% | 150% |
| (1) | Pursuant to Mr. Gallas’ offer letter dated March 10, 2009, for the performance year 2009, he was guaranteed a minimum bonus of $262,500. Therefore, there was no threshold bonus opportunity for achieving threshold performance that applied to Mr. Gallas. |
124
Table of Contents
In 2009, the cash incentive awards were based on Company-wide Adjusted EBITDA target, EBITDA target of the particular business for which the executive has primary responsibilities, Adjusted Free Cash Flow target or a combination of one or more of these targets. The following table lists the performance metrics and relative weightings given thereto with respect of each of our named executive officers under the 2009 Bonus Plan.
| Company-wide Adjusted EBITDA | EBITDA(1) | Adjusted Free Cash Flow | ||||
Norman C. Payson, M.D. | 100% | — | — | |||
Chris A. Karkenny | 100% | — | — | |||
James G. Gallas | 80% | — | 20% | |||
Lawrence A. Mastrovich | 24% | 56% | 20% | |||
Daniel E. Greenleaf | 24% | 56% | 20% |
| (1) | Represents EBITDA of the particular business for which the executive has primary responsibilities. For Mr. Mastrovich, represents EBITDA of Home Respiratory Therapy and Home Medical Equipment segment plus Enteral business, and for Mr. Greenleaf, represents EBITDA of Home Infusion Therapy segment less Enteral business. |
Company-wide Adjusted EBITDA, as used under the 2009 Bonus Plan, is calculated as Adjusted EBITDA (as calculated under the indenture governing the Notes), as further adjusted for certain items approved by our Board of Directors, such as loss on disposition of assets and other, employee severance costs, employee relocation costs and other income including joint ventures. Adjusted Free Cash Flow, as used under the 2009 Bonus Plan, is calculated as Free Cash Flow (as defined under “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources”), as further adjusted for certain items approved by our Board of Directors, such as equity compensation expense, costs incurred related to initiatives (other than employee severance costs and employee relocation costs), the Sponsor monitoring fee and unbudgeted initiative capital expenditures. EBITDA of Home Respiratory Therapy and Home Medical Equipment segment plus Enteral business is calculated as earnings before interest, taxes, depreciation and amortization of Home Respiratory Therapy and Home Medical Equipment segment plus Enteral business, and EBITDA of Home Infusion Therapy segment less Enteral business is calculated as earnings before interest, taxes, depreciation and amortization of Home Infusion Therapy segment less Enteral business.
Under our 2009 Bonus Plan, no cash incentive award was to be made with respect to any performance metric unless our actual Company-wide Adjusted EBITDA for 2009 was above 90% of the target Company-wide Adjusted EBITDA, and no cash incentive award was to be made with respect to a performance metric unless actual achievement of that metric was above 90% of the target. The target cash incentive award amount with respect to a performance metric was to be paid if the actual performance was 100% of the target, and the maximum cash incentive award amount with respect to a performance metric was to be paid if the actual performance was 120% of the target or above. Straight-line interpolation determined the cash payout for performance which falls between the threshold and target or between target and maximum.
Notwithstanding the establishment of the performance goals and the formula for determining the cash incentive award payment amounts as illustrated in the tables above, our Board of Directors could award lesser amounts under our annual Executive Bonus Plan to one or more named executive officers, if, in the exercise of its business judgment, our Board of Directors determined that they were warranted under the circumstances and in our best interest. In addition, our Board of Directors could award a discretionary bonus in addition to the amount the executive would be eligible to receive under our annual Executive Bonus Plan for exceptional performance or achievement.
For 2009, our Board of Directors set a Company-wide Adjusted EBITDA target of $352.9 million, Adjusted Free Cash Flow target of $92.5 million, Home Respiratory Therapy and Home Medical Equipment segment plus Enteral business EBITDA target of $426.0 million and Home Infusion Therapy segment less Enteral business EBITDA target of $67.2 million.
125
Table of Contents
In 2009, we achieved 101.0% of the Company-wide Adjusted EBITDA target (or $356.4 million), 104.5% of the Adjusted Free Cash Flow target (or $96.6 million), 102.4% of the Home Respiratory Therapy and Home Medical Equipment segment plus Enteral business EBITDA target (or $436.3 million) and 115.9% of Home Infusion Therapy segment less Enteral business EBITDA target (or $77.8 million).
The following table illustrates the calculation of the annual cash incentive awards paid to each of our named executive officers under our 2009 Bonus Plan in light of these performance results. The total amount awarded to each of the named executive officers shown in the table below is reported under the Non-Equity Incentive Plan Compensation column of the Summary Compensation Table, except that for Mr. Gallas, the guaranteed minimum amount is reported under the Bonus column.
| Actual Amount Awarded for Company-wide Adjusted EBITDA Achievement | Actual Amount Awarded for EBITDA Achievement(1) | Actual Amount Awarded for Adjusted Free Cash Flow Achievement | Total Amount Awarded | ||||||||
Norman C. Payson, M.D. | $825,175 | — | — | $ | 825,175 | ||||||
Chris A. Karkenny | $514,173 | — | — | $ | 514,173 | ||||||
James G. Gallas | $430,324 | — | $ | 116,730 | $ | 547,055 | |||||
Lawrence A. Mastrovich | $147,561 | $ | 356,305 | $ | 133,425 | $ | 637,291 | ||||
Daniel E. Greenleaf | $121,297 | $ | 386,045 | $ | 109,677 | $ | 617,019 | ||||
| (1) | Represents actual amount awarded for EBITDA achievement of the particular business for which the executive has primary responsibilities. For Mr. Mastrovich, represents the amount awarded for Home Respiratory Therapy and Home Medical Equipment segment plus Enteral business EBITDA achievement, and for Mr. Greenleaf, represents the amount awarded for Home Infusion Therapy segment less Enteral business EBITDA achievement. |
The specific bonus awards under our 2009 Bonus Plan were reviewed and approved by our Board of Directors in February, 2010 and were paid to our named executive officers in March, 2010.
In March 2010, our Board of Directors approved our Executive Bonus Plan for 2010 (the “2010 Bonus Plan”). The performance metrics and the formula for determining the payout amounts under the 2010 Bonus Plan for each named executive officer (although not the financial performance target levels) remained the same as the 2009 Bonus Plan except that for Dr. Payson and Mr. Karkenny the potential payouts are based 80% on the Company-wide Adjusted EBITDA and 20% on the Adjusted Free Cash Flow, the same as for Mr. Gallas, rather than being 100% based on the Company-wide Adjusted EBITDA. The financial performance target levels under the 2010 Bonus Plan were approved by our Board of Directors after consultation with Dr. Payson and Mr. Karkenny.
Discretionary Bonus.
In addition, our Chief Financial Officer, Mr. Karkenny, was paid a retention bonus of $2,100,000 in January 2009, subject to a claw-back obligation under specified termination scenarios, pursuant to the terms of his employment agreement. Mr. Gallas was also paid a sign-on bonus of $150,000 in March 2009, subject to a claw-back obligation under specified termination scenarios, in connection with the commencement of his employment with us. See “Narrative Disclosure to Summary Compensation Table and Grants of Plan-Based Awards—Employment Agreements” for descriptions of Mr. Karkenny’s employment agreement and Mr. Gallas’ offer letter.
Long-Term Incentive Compensation.
Following the Merger, our Board of Directors determined to grant to our management employees, including our named executive officers, long-term incentive awards that are designed to promote our interests by providing
126
Table of Contents
our management employees with the opportunity to acquire an equity interest in the Company as an incentive for the person to remain in our service. Our Board of Directors granted these long-term incentive awards to our Chief Executive Officer, Dr. Payson, in the form of Class B Units of BP Healthcare Holdings LLC, our ultimate parent, and to our other named executive officers in the form of Class B Units and Class C Units of Sky Acquisition LLC, our direct parent, and, in the case of Mr. Karkenny, Class A-2 Units of Sky Acquisition LLC as well, as further described below. Pursuant to a reorganization we conducted in March 2010, the Class B Units and Class C Units of Sky Acquisition LLC were converted or exchanged into Class B Units and Class C Units of Holdings. See “Prospectus Summary—The Transactions.” For purposes of the discussion of equity award grants under this “—Long-Term Incentive Compensation” section, the Class B Units and Class C Units of Holdings mean the Class B Units and Class C Units of Sky Acquisition LLC granted prior to the reorganization, and our Board of Directors means our Board of Directors acting in its capacity as the board of directors of the applicable parent entity, unless the context indicates otherwise.
Class A-2 Units of Holdings are equity interests in Holdings and have economic characteristics that are similar to those of shares of common stock in a corporation. Certain of our executives have made equity investments in Holdings by purchasing Class A-2 Units of Holdings. In addition, our Board of Directors granted to Mr. Karkenny Class A-2 Units of Holdings, subject to vesting terms, similar to restricted common stock in a corporation. Mr. Karkenny’s Class A-2 Units vest if an initial public offering or change of control occurs and the valuation of Class A-1 Units of Holdings implied by the transaction exceeds 110% of the aggregate capital contributions of affiliates of the Sponsor for the Class A-1 Units. Mr. Karkenny does not need to be employed at the time of the initial public offering or change in control to vest.
Class B and Class C Units of Holdings are limited liability company profits interests having economic characteristics similar to stock appreciation rights and representing the right to share in any increase in the equity value of Holdings that exceeds specified thresholds. For a Class B Unit, the threshold is the value of a Class A Unit on grant date which generally was $1.00, so a Class B Unit has a value at any given time equal to the value of a Class A Unit minus $1.00). For a Class C unit, the threshold was $2.00, so a Class C Unit has a value at any given time equal to the value of a Class A Unit minus $2.00. Some Class B Units have a higher threshold representing the estimated fair value of a Class A unit on the applicable grant date.
The Class B Units are divided into a time-vesting portion (2/3 of the Class B Units granted) and a performance-vesting portion (1/3 of the Class B Units granted). All Class C Units are performance-vesting. See “Narrative Disclosure to Summary Compensation Table and Grants of Plan-Based Awards Table—Terms of Equity Award Grants—Equity Units of Holdings Granted to Our Named Executive Officers (Other than Our Chief Executive Officer)” below for a discussion of the vesting and other terms of these equity units.
Our Board of Directors granted to Dr. Payson 38,697,318 Class B units of BP Healthcare Holdings LLC in November 2008 with a grant date fair value of $13.9 million. These Class B Units are economically equivalent to the Class B Units of Holdings. These Class B Units are 80% time-vesting and 20% performance-vesting. See “Narrative Disclosure to Summary Compensation Table and Grants of Plan-Based Awards Table—Terms of Equity Award Grants—Equity Units of BP Healthcare Holdings LLC Granted to Our Chief Executive Officer.” The Class B Units granted to Dr. Payson are designed to incentivize him to remain in our service and motivate him to focus on efforts that will increase the value of equity in BP Healthcare Holdings over the long term.
Our Board of Directors granted to Mr. Karkenny 500,000 Class A-2 Units, 6,675,287 Class B Units and 2,225,096 Class C Units of Holdings in December 2008 with an aggregate grant date fair value of $3.0 million. The Class A-2 Units granted to Mr. Karkenny are designed to motivate him to focus on efforts that will deliver our financial success and also enable him to participate in our long-term growth. Our Board of Directors granted each of our other named executive officers a combination of Class B Units and Class C Units of Holdings in March 2009 and, in the case of Mr. Gallas, in April 2009 in connection with the commencement of his employment. The aggregate grant date fair values of all such Class B and Class C Units of Holdings were $3.4 million. The Class B Units and Class C Units of Holdings granted to our named executive officers are designed
127
Table of Contents
to motivate them to focus on efforts that will increase the value of our equity while enhancing their retention. The specific sizes of the equity grants made to our named executive officers were determined in light of the Sponsor’s practices with respect to management equity programs at other private companies in its portfolio and the executive officer’s position and level of responsibilities within the Company. In addition, in the case of Dr. Payson, consideration was also given to his substantial equity investment in BP Healthcare Holdings, and in the case of Mr. Karkenny, consideration was also given to enhancing his retention following the Merger. Subsequent to these initial grants of equity awards, our Board of Directors has not made additional grants of equity awards to our named executive officers.
Broad-based Employee Benefits.
We provide to all our employees, including our named executive officers, broad-based benefits that are intended to attract and retain employees while providing them with retirement and health and welfare security. Broad-based employee benefits include:
| • | a 401(k) savings plan; |
| • | medical, dental, vision, life and accident insurance, disability coverage, dependent care and healthcare flexible spending accounts; and |
| • | employee assistance program benefits. |
Under our 401(k) savings plan, we match a portion of the funds set aside by the employee. At no cost to the employee, we provide $10,000 in basic life and accident insurance coverage and $100,000 in business travel accident insurance. The employee may also select supplemental life and accident insurance, for a premium to be paid by the employee.
Supplemental Executive Benefits and Perquisites.
We provide a nonqualified deferred compensation plan and modest perquisites which are also intended to attract and retain executives. The deferred compensation plan is intended to promote retention by providing to participants a long-term savings opportunity on a tax-efficient basis and is accomplished with only a modest administrative cost to the Company as the employees’ deferrals are not matched by the Company. Under the deferred compensation plan, participants may defer certain portions of their salary, annual bonus and annual 401(k) savings plan refund offset amount, as more fully explained in the narrative following the “Nonqualified Deferred Compensation” table below.
Our named executive officers also receive modest perquisites provided or reimbursed by us. These perquisites include supplemental long-term disability coverage, executive medical and dental benefits and transportation-related benefits. In addition, Messrs. Gallas and Greenleaf have received relocation-related expense reimbursements as part of the terms of their offer letter and employment agreement, respectively. We provide these perquisites because they are cost-effective and promote retention and recruitment. These perquisites are reflected in the All Other Compensation column of the Summary Compensation Table and the accompanying footnote.
Severance Arrangements and Noncompetition Agreements
Our Board of Directors believes that severance arrangements are necessary to attract and retain the talent necessary for our long-term success. Our Board of Directors views our severance arrangements as recruitment and retention devices that help secure the continued employment and dedication of our named executive officers, including when we are considering strategic alternatives.
Each of our named executive officers (other than Mr. Mastrovich, who has resigned from the Company) has a severance arrangement with us either as part of his employment agreement or as a separate severance
128
Table of Contents
agreement. Under the terms of these severance arrangements, each named executive officer is entitled to severance benefits if he is terminated by us without cause or by him as a result of constructive termination or for good reason, as applicable. In connection with Mr. Mastrovich’s resignation from the Company, we entered into a general release of claims agreement with Mr. Mastrovich pursuant to which we agreed to pay him severance compensation. In addition, Messrs. Karkenny and Greenleaf have separate noncompetition agreements with us. These agreements provide for additional payments upon a termination of the executive’s employment by us without cause or by the executive for good reason, in each case, during a specified period.
The severance payments under these agreements are contingent upon the affected executive’s compliance with the post-termination restrictive covenants contained therein. See “Potential Payments to Named Executive Officers Upon Termination of Employment or Change in Control—Severance Arrangements” for descriptions of these agreements.
129
Table of Contents
Summary Compensation Table
The following table provides summary information concerning compensation paid or accrued by us to or on behalf of our named executive officers for services rendered to us during 2009.
Name and Principal Position | Salary ($) | Bonus (1) ($) | Stock Awards(2) ($) | Non-Equity Incentive Plan Compensation(3) ($) | All Other Compensation (4)($) | Total ($) | ||||||
Norman C. Payson, M.D. Executive Chairman of the Board of Directors and Chief Executive Officer | 752,060 | — | — | 825,175 | 13,737 | 1,590,972 | ||||||
Chris A. Karkenny Executive Vice President and Chief Financial Officer | 476,305 | 2,100,000 | — | 514,173 | 13,273 | 3,103,751 | ||||||
James G. Gallas (5) Executive Vice President and Chief Administrative Officer | 396,635 | 412,500 | 685,039 | 284,555 | 67,452 | 1,846,181 | ||||||
Lawrence A. Mastrovich (6) Former President, Home Respiratory Therapy and Home Medical Equipment Segment | 581,593 | — | 1,501,456 | 637,291 | 23,730 | 2,744,070 | ||||||
Daniel E. Greenleaf President, Home Infusion Therapy Segment | 476,305 | — | 1,219,933 | 617,019 | 69,633 | 2,382,890 | ||||||
| (1) | Amounts included in this column reflect: |
| • | a retention bonus paid during 2009 for Mr. Karkenny, which bonus was subject to a clawback obligation under specified employment termination scenarios and |
| • | a signing bonus of $150,000, which bonus was subject to a clawback obligation under specified employment termination scenarios, and a guaranteed minimum bonus for 2009 of $262,500 paid pursuant to the terms of the offer letter for Mr. Gallas. |
See the discussion under “Compensation Discussion and Analysis—Bonuses.”
| (2) | Amounts included in this column reflect the aggregate grant date fair value of Class B Units and Class C Units of Holdings granted during 2009, calculated in accordance with FASB ASC Topic 718, excluding the effect of estimated forfeitures, utilizing the assumptions discussed in Note 9 to our financial statements for the year ended December 31, 2009. With respect to the performance-vesting Class B Units and Class C Units, the estimate of the grant date fair value determined in accordance with FASB ASC Topic 718 assumes the vesting of 100% of the units awarded. The values of the performance-vesting Class B and Class C Units at the grant date assuming achievement of the highest performance conditions are not determinable because there are no maximum performance conditions under the terms of Class B and Class C Units. See “Narrative Disclosure to Summary Compensation Table and Grants of Plan-Based Awards—Terms of Equity Award Grants—Equity Units of Holdings Granted to Our Named Executive Officers (Other than Our Chief Executive Officer).” |
| (3) | Amounts included in this column reflect the cash incentive awards paid under the 2009 Bonus Plan, except that for Mr. Gallas, the guaranteed minimum bonus of $262,500 is included in the Bonus column. See “Compensation Discussion and Analysis — Bonuses —Annual Cash Incentive Compensation.” |
| (4) | Amounts reported under All Other Compensation for each of the named executive officers include the premiums paid by us on behalf of the executive officer for executive medical insurance and executive dental insurance coverages. In addition, they include: |
| • | For Mr. Karkenny – premiums paid by us on behalf of Mr. Karkenny for the executive long-term disability insurance coverage and gas card/toll road fees paid by us and the related amount of tax gross-up; |
| • | For Mr. Gallas – gas card fees paid by us and the related amount of tax gross-up; relocation expenses reimbursed by us and the related amount of tax gross-up; and corporate housing expenses reimbursed by us; |
| • | For Mr. Mastrovich – premiums paid by us on behalf of Mr. Mastrovich for the executive long-term disability insurance coverage and gas card/toll road fees paid by us and the related amount of tax gross-up; and |
| • | For Mr. Greenleaf – premiums paid by us on behalf of Mr. Greenleaf for the executive long-term disability insurance coverage; gas card fees paid by us and the related amount of tax gross-up; and $38,000 of expenses reimbursed by us in connection with his relocation to Denver, Colorado pursuant to the terms of his employment agreement and $19,899 of the related amount of tax gross-up. |
Each perquisite was valued at the actual amount paid to the provider by us on behalf of the named executive officer.
130
Table of Contents
| (5) | Mr. Gallas joined the Company on April 1, 2009. Therefore, the amount reflected under the Salary column for Mr. Gallas reflects the base salary earned by him for the partial year period from the commencement of his employment to December 31, 2009. |
| (6) | Mr. Mastrovich resigned from the Company on March 15, 2010. The severance compensation we agreed to pay him is reflected in the table under the “Potential Payments Upon Termination or Change-in-Control” section. |
Grants of Plan-Based Awards
The following table provides supplemental information relating to grants of plan-based awards made to our named executive officers during 2009.
Name | Grant Date | Type of Award | Estimated Future Payouts Under Non-Equity Incentive Plan Awards (1) | Estimated Future Payouts Under Equity Incentive Plan Awards | All Other Stock Awards: Number of Shares of Stock or Units (#)(4) | Grant Date Fair Value of Stock and Option Awards($)(5) | ||||||||||||||
| Threshold ($) | Target ($) | Maximum ($) | Threshold (#) | Target (#)(3) | Maximum (#) | |||||||||||||||
Norman C. Payson, M.D. | 0 | 786,507 | 1,573,014 | |||||||||||||||||
Chris A. Karkenny | 0 | 490,079 | 980,158 | |||||||||||||||||
James G. Gallas (2) | — | 525,000 | 787,500 | |||||||||||||||||
| 4/29/09 | Class B | 706,226 | 1,412,452 | 593,230 | ||||||||||||||||
| 4/29/09 | Class C | 706,226 | 91,809 | |||||||||||||||||
Lawrence A. Mastrovich | 0 | 600,085 | 900,128 | |||||||||||||||||
| 3/11/09 | Class B | 1,547,893 | 3,095,785 | 1,300,230 | ||||||||||||||||
| 3/11/09 | Class C | 1,547,893 | 201,226 | |||||||||||||||||
Daniel E. Greenleaf | 0 | 493,279 | 739,919 | |||||||||||||||||
| 3/11/09 | Class B | 1,257,663 | 2,515,326 | 1,056,437 | ||||||||||||||||
| 3/11/09 | Class C | 1,257,663 | 163,496 | |||||||||||||||||
| (1) | Reflects possible payouts under our 2009 Bonus Plan. See “Compensation Discussion and Analysis—Bonus—Annual Cash Incentive Compensation” for a discussion of threshold, target and maximum cash incentive compensation payouts. The actual amounts of cash incentive compensation paid to our named executive officers under our 2009 Bonus Plan are disclosed in the Summary Compensation Table under the Non-Equity Incentive Plan Compensation column. |
| (2) | Mr. Gallas was guaranteed a minimum bonus of $262,500 for 2009 pursuant to the terms of his offer letter. This guaranteed minimum bonus is reflected in the Bonus column of the Summary Compensation Table. In addition, pursuant to the terms of his offer letter, Mr. Gallas’ target cash incentive compensation payout amount reflected in the table above is based on his annualized base salary rate at date of hire instead of his base salary paid during 2009. |
| (3) | Reflects the number of performance-vesting Class B Units and Class C Units of Holdings granted during 2009. |
| (4) | Reflects the number of time-vesting Class B Units of Holdings granted during 2009. |
| (5) | Represents the aggregate grant date fair value of Class B Units or Class C Units, as applicable, calculated in accordance with the guidance in FASB ASC Topic 718, utilizing the assumptions discussed in Note 9 to our financial statements for the year ended December 31, 2009. With respect to the performance-vesting Class B Units and Class C Units which are reflected under the “Estimated Future Payouts Under Equity Incentive Plan Awards”, the estimate of the grant date fair value determined in accordance with FASB ASC Topic 718 assumes the vesting of 100% of the units awarded. |
Narrative Disclosure to Summary Compensation Table and Grants of Plan-Based Awards
Employment Agreements
Norman C. Payson
Apria and its indirect parent entity, BP Healthcare Holdings LLC, entered into an employment agreement, effective October 28, 2008, with Dr. Payson pursuant to which Dr. Payson serves as our Chief Executive Officer and Executive Chairman of our Board of Directors. His employment agreement has a four-year term with automatic annual renewals and also contains the terms summarized below.
131
Table of Contents
Compensation Arrangements
| • | base salary at the annual rate of $750,000, subject to increases as determined by the Board of Directors; |
| • | target annual bonus award of 100% of base salary (and a maximum bonus award of 200% of base salary), based upon certain performance goals established by our Board of Directors; |
| • | eligibility for equity award grants as determined by our Board of Directors; |
| • | participation in our employee benefit plans; and |
| • | reimbursement for reasonable and customary business expenses, as well as reimbursement for his private airplane operating expenses relating to business travel up to a maximum of $1.55 million per year. |
Termination Provisions
Generally, either party may terminate Dr. Payson’s employment agreement at any time, but Dr. Payson must provide 60 days advance written notice to us of his resignation. See “Potential Payments upon Termination or Change-in-Control—Severance Arrangements” for a description of the severance provisions related to Dr. Payson’s termination of employment.
In addition, if Dr. Payson resigns, or we remove him, from the position of Chief Executive Officer of Apria and/or Sky Acquisition LLC but the party initiating such action notifies the other party that it desires Mr. Payson to continue to serve as the Executive Chairman of the Board of Directors of Apria or the Chairman of the Board of Directors of Sky Acquisition, then we must terminate his employment agreement and enter into a services agreement with Dr. Payson, pursuant to which he would continue to serve as the Executive Chairman of the Board of Directors of Apria and the Chairman of the Board of Directors of Sky Acquisition and serve as a senior advisor to BP Healthcare Holdings, and we would pay him $500,000 per year for his services to Apria. The services agreement would terminate on October 28, 2012, unless earlier terminated by either party or extended by mutual agreement of the parties thereto.
Chris A. Karkenny
Apria entered into an employment agreement, effective October 28, 2008, with Mr. Karkenny pursuant to which Mr. Karkenny continues to serve as our Chief Financial Officer. His employment agreement has a five-year term with automatic annual renewals and also contains the terms summarized below.
Compensation Arrangements
| • | base salary at the annual rate of $475,000 after December 31, 2008, subject to increases as determined by our Board of Directors; |
| • | target annual bonus award of 100% of base salary (and a maximum bonus award of 200% of base salary), based upon certain performance goals established by our Board of Directors; |
| • | eligibility for equity award grants as determined by our Board of Directors; |
| • | retention bonus payment of $2,100,000, payable on January 5, 2009, subject to a claw-back obligation described below; |
| • | participation in our employee benefit plans; and |
| • | reimbursement of reasonable and customary business expenses. |
Notwithstanding the foregoing, the agreement provides that in the event Mr. Karkenny terminates his employment with Apria, other than his resignation as a result of a “constructive termination”, or upon his death or disability, in each case prior to March 31, 2010, he is required to pay to Apria the amount by which $2,100,000 exceeds the product of (x) $140,000 and (y) the number of full months following January 1, 2009 that he was continuously employed by Apria.
132
Table of Contents
Termination Provisions
Generally, either party may terminate Mr. Karkenny’s employment agreement at any time, but Mr. Karkenny must provide 30 days advance written notice to Apria of his resignation. See “Potential Payments Upon Termination or Change-in-Control—Severance Arrangements” for a description of the severance provisions related to Mr. Karkenny’s termination of employment.
James G. Gallas
Apria entered into an offer letter, dated March 10, 2009, with Mr. Gallas pursuant to which Mr. Gallas serves as our Chief Administrative Officer. Mr. Gallas’ employment with Apria is on an “at-will” basis. His offer letter contains the terms summarized below.
Compensation Arrangements
| • | base salary at the annual rate of $525,000, subject to merit based wage adjustments consistent with other senior executives under our wage administration policy; |
| • | target annual bonus award of 100% of base salary (and a maximum bonus award of 150% of base salary), with a guaranteed minimum bonus of $262,500 for the plan year 2009; |
| • | a sign-on bonus of $150,000, a portion of which is required to be refunded to Apria on a ratable basis if he voluntarily terminates his employment or is terminated for misconduct before completing 24 months of employment; |
| • | eligibility for equity award grants equal to 0.365% of the fully diluted ownership of Holdings; |
| • | participation in our employee benefit plans; and |
| • | relocation benefits, including reasonable air, hotel, meal and transportation expenses associated with his temporary commute from Ohio to Southern California up to the time of completion of relocation and six months of temporary housing expenses in the Southern California area up to $5,000 per month. |
Termination Provisions
We also entered into a severance agreement, dated March 10, 2009, with Mr. Gallas. See “Potential Payments Upon Termination or Change-in-Control—Severance Arrangements” for a description of the severance provisions contained in his severance agreement.
Daniel E. Greenleaf
Apria entered into an amended and restated employment agreement with Mr. Greenleaf on October 24, 2008, which was effective as of April 7, 2008 and pursuant to which Mr. Greenleaf serves as President of Coram, Inc., our Home Infusion Therapy segment. Mr. Greenleaf’s employment with Apria is on an “at-will” basis. His employment agreement contains the terms summarized below.
Compensation Arrangements
| • | base salary at the annual rate of $475,000, subject to increases from time to time by Apria; |
| • | target annual bonus award of 100% of base salary (and a maximum award of 150% of base salary); |
| • | eligibility for equity award grants; |
| • | participation in our employee benefit plans; and |
| • | reimbursement of certain relocation expenses and reasonable and customary business expenses. Relocation expenses include an amount equal to the greater of $7,000 gross or $4,500 net per month, for up to six months, to cover the carrying costs of Mr. Greenleaf’s old residence if his residence becomes |
133
Table of Contents
vacant and remains unsold and up to a total of $30,000 of the interest costs associated with indebtedness incurred by Mr. Greenleaf to fund the equity portion of the purchase price for his new residence in Denver, Colorado during the period prior to the sale of his old residence. |
Termination Provisions
Generally, either party may terminate Mr. Greenleaf’s employment agreement at any time, by giving the other party at least 30 days advance written notice. See “Potential Payments Upon Termination or Change-in-Control—Severance Arrangements” for a description of the severance provisions related to Mr. Greenleaf’s termination of employment.
Lawrence A. Mastrovich
Apria entered into an employment agreement, effective October 24, 2008 and amended as of April 3, 2009, with Mr. Mastrovich pursuant to which Mr. Mastrovich served as our President, Home Respiratory Therapy and Home Medical Equipment segment until March 15, 2010. His employment agreement contained the terms summarized below.
Compensation Arrangements
| • | base salary at the annual rate of $580,000, subject to increases as determined by Apria; |
| • | participation in all annual bonus, incentive, savings and retirement plans, practices, policies and programs applicable generally to our Chief Executive Officer, including our Executive Bonus Plan and 401(k) savings plan; |
| • | participation in our welfare, savings and retirement benefit plans; and |
| • | reimbursement for reasonable employment expenses. |
Termination Provisions
In connection with our reorganization of the senior leadership structure of the home respiratory therapy and home medical equipment service lines, Mr. Mastrovich resigned as President, Home Respiratory Therapy and Home Medical Equipment segment, effective as of March 15, 2010. See “Potential Payments Upon Termination or Change-in-Control—Severance Arrangements” for a description of the termination provisions of his employment agreement that were triggered upon his resignation.
General Release of Claims Agreement
In connection with Mr. Mastrovich’s resignation from the Company, Apria entered into a general release of claims agreement with him, effective March 15, 2010, pursuant to which Mr. Mastrovich agreed to release us and our affiliates from all claims, subject to certain conditions and qualifications therein, and we agreed to pay him a total of $2,568,849 in severance compensation and $77,395 to repurchase his vested equity units. See “Potential Payments upon Termination or Change-in-Control—Severance Arrangements” for a description of the severance payments we agreed to make to Mr. Mastrovich pursuant to this agreement.
Terms of Equity Award Grants
Equity Units of BP Healthcare Holdings LLC Granted to Our Chief Executive Officer
Vesting Terms
The Class B Units of BP Healthcare Holdings granted to our Chief Executive Officer are 80% time-vesting and 20% performance-vesting. The time-vesting units vest over 4 years starting on October 28, 2008 in quarterly tranches but will become fully vested on an accelerated basis either (x) upon a change in control while he
134
Table of Contents
continues to provide services to us or (y) if affiliates of the Sponsor receive cash proceeds in respect of 50% of their units in BP Healthcare Holdings equal to at least 200% of their aggregate capital contributions in respect of such units while he continues to provide services to us. In addition, if his services are terminated (a) by us without “cause” or (b) by him as a result of “constructive termination,” an additional number of these time-vesting Class B Units will vest equal to the number that would have vested over the 24-month period following the applicable termination date. Any of these time-vesting Class B Units that are unvested on termination of his services will be forfeited.
One-half of the performance-vesting Class B Units granted to our Chief Executive Officer vest only if affiliates of the Sponsor receive cash proceeds equal to at least 200% of their aggregate capital contributions in respect of all of their units in BP Healthcare Holdings and the other half vest only if they receive cash proceeds equal to at least 300% of their aggregate capital contributions in respect of all of their units in BP Healthcare Holdings. Any of these performance-vesting units that are unvested upon a termination of his services (x) by us without “cause,” (y) by him as a result of “constructive termination” or (z) by him for any reason on or following October 28, 2012 will remain outstanding until the second anniversary of the applicable termination date (unless they vest prior to that date). If the performance-vesting units do not vest by such anniversary, then they will be immediately forfeited.
Put and Call Rights
Prior to our initial public offering, if the Chief Executive Officer’s employment is terminated due to death or disability, he has the right, subject to certain limitations, for a specified period following the termination date, to cause us to purchase on one occasion all, but not less than all, of his vested Class B Units at the fair market value of such units. Our Chief Executive Officer does not have the right to require us to repurchase the Class A-2 units of BP Healthcare Holdings LLC that he purchased.
If the Chief Executive Officer’s employment is terminated by us “for cause,” due to his death or disability or, prior to the fourth anniversary of the vesting date, by him (other than as a result of constructive termination), then we have the right for a specified period following the termination to cause the Chief Executive Officer (or his permitted transferees) to sell to us all vested Class B units held by him at (i) the lesser of fair market value thereof and cost, in the event of termination for cause, which means that such vested units will be effectively forfeited or (ii) the fair market value thereof, in the event of termination due to death or disability or voluntary termination (other than as a result of constructive termination) prior to the fourth anniversary of the vesting date.
Equity Units of Holdings Granted to Our Named Executive Officers Other than Our Chief Executive Officer
Vesting Terms
Class A-2 Units.Class A-2 Units of Holdings granted to Mr. Karkenny vest if an initial public offering or change of control occurs and the valuation of Class A-1 Units of Holdings implied by the transaction exceeds 110% of the aggregate capital contributions of affiliates of the Sponsor for the Class A-1 Units. Mr. Karkenny does not need to be employed at the time of our initial public offering or change in control to vest. The Class A-2 Units will be forfeited if an initial public offering or change of control occurs at a valuation that does not result in vesting.
Class B Units.The Class B Units of Holdings granted to all of our named executive officers other than our Chief Executive Officer are divided into a time-vesting portion (2/3 of the Class B Units granted) and a performance-vesting portion (1/3 of the Class B Units granted). The time-vesting portion of these Class B Units vest over 5 years, with 20% vesting on the 12-month anniversary of the later of (x) October 28, 2008 and (y) the date of the executive’s commencement of employment with us and the remainder in quarterly tranches thereafter, except that in the case of Mr. Karkenny, they vest over 57 months, with 25% vesting on October 28, 2009 and the remainder in quarterly tranches thereafter, in each case subject to the executive officer’s continued employment
135
Table of Contents
through each vesting date. Notwithstanding the foregoing, the time-vesting Class B units of Holdings will become fully vested on an accelerated basis upon a change in control while the executive continues to provide services to us.
The performance-vesting portion of these Class B Units vest only if affiliates of the Sponsor receive cash proceeds (not subject to any clawback, indemnity or similar contractual obligation) in respect of 25% of its units equal to 200% of its aggregate capital contributions for such units.
Class C Units.All Class C Units are performance-vesting and have the same vesting terms as the performance-vesting Class B Units.
Any of the Class B Units or Class C Units that are unvested on termination of the executive’s services will be forfeited.
Put and Call Rights
Prior to our initial public offering, if the executive’s employment is terminated due to death or disability, he has the right, subject to certain limitations, for a specified period following the termination date, to cause us to purchase on one occasion all, but not less than all, of his vested Class A-2, Class B or Class C Units at the fair market value thereof.
If the executive’s employment is terminated due to (i) death or disability, (ii) by us without cause, (iii) voluntarily as a result of constructive termination or (iv) voluntarily (other than as a result of a constructive termination) after the later of (x) October 28, 2010 and (y) the second anniversary of the date the executive commenced his employment with us or, in the case of Mr. Karkenny, after January 28, 2010, or the executive engages in any conduct that would be a violation of a restrictive covenant set forth in the management unit subscription agreement but for the fact that the conduct occurred outside the relevant periods, then we have the right to purchase all of the executive’s vested Class B or Class C Units at the fair market value thereof.
If the executive’s employment is terminated (i) by us for “cause” or (ii) voluntarily (other than as a result of a constructive termination) on or before the later of (x) October 28, 2010 and (y) the second anniversary of the date the executive commenced employment with us or, in the case of Mr. Karkenny, on or before January 28, 2010, or the executive breaches any of the restrictive covenants set forth in the management unit subscription agreement, then we have the right to purchase all of the executive’s vested Class B or Class C Units at the lesser of fair market value thereof and cost, which means that such vested Class B or Class C Units will be effectively forfeited.
Restrictive Covenants
As a condition of receiving the units, our named executive officers have agreed to certain restrictive covenants, including confidentiality of information, non-competition, non-solicitation and non-disparagement covenants in the management unit subscription agreements. As described above, we have the right to purchase our named executive officers’ vested units in the event of breach of these restrictive covenants either within or outside the periods covered by the restrictive covenants.
136
Table of Contents
Outstanding Equity Awards at Fiscal-Year End
The following table provides information regarding outstanding equity awards made to our named executive officers as of December 31, 2009. The equity awards held by Dr. Payson are Class B Units of BP Healthcare Holdings, and the equity awards held by the other named executive officers are Class B and Class C Units of Holdings.
| Stock Awards | ||||||||||||
Name | Grant Date | Number of Shares or Units of Stock That Have Not Vested (1) (#) | Market Value of Shares or Units of Stock That Have Not Vested (1) ($) | Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested (2) (#) | Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested (2) ($) | |||||||
Norman C. Payson M.D. | 11/24/2008 | 23,218,391 | (3 | ) | 7,739,463 | (3 | ) | |||||
Chris A. Karkenny | 12/19/2008 | 3,337,310 | (3 | ) | 4,950,637 | (3 | ) | |||||
James G. Gallas | 4/29/2009 | 1,412,452 | (3 | ) | 1,412,452 | (3 | ) | |||||
Lawrence A. Mastrovich(4) | 3/11/2009 | 2,476,628 | (3 | ) | 3,095,786 | (3 | ) | |||||
Daniel A. Greenleaf | 3/11/2009 | 2,012,261 | (3 | ) | 2,515,326 | (3 | ) | |||||
| (1) | Reflects time-vesting Class B Units that have not vested. The following provides information with respect to the vesting schedule of the time-vesting Class B Units that have not vested as of December 31, 2009: |
| • | Dr. Payson – 1,934,866 units vest every three months starting on January 28, 2010 until all of them vest on October 28, 2012. |
| • | Mr. Karkenny – 222,487 units vest every three months starting on January 28, 2010 until all of them vest on July 28, 2013. |
| • | Mr. Gallas – 282,490 units vested on April 1, 2010 and 70,623 units vest every three months starting on July 1, 2010 until all of them vest on April 1, 2014. |
| • | Mr. Greenleaf – 125,766 units vest every three months starting on January 28, 2010 until all of them vest on October 28, 2013. |
Vesting will be accelerated under specified events while the executive continues to provide services to us, as described under “Narrative Disclosure to Summary Compensation Table and Grants of Plan-Based Awards-Terms of Equity Award Grants.”
| (2) | Reflects performance-vesting Class B Units and Class C Units and, with respect to Mr. Karkenny, Class A-2 Units. |
| (3) | There was no public market for the units as of December 31, 2009, and thus the market value as of that date is not determinable. For purposes of FASB ASC Topic 718, the grant date fair value of Dr. Payson’s Class B Units was $0.36 per unit, the grant date fair value of Mr. Karkenny’s Class A-2, Class B and Class C Units were $0.75 per unit, 0.35 per unit and 0.15 per unit, respectively, and the grant date fair values of the other named executive officers’s Class B and Class C Units were $0.28 per unit and $0.13 per unit, respectively. |
| (4) | Mr. Mastrovich forfeited all of his unvested Class B and Class C Units upon his resignation from the Company on March 15, 2010. |
137
Table of Contents
Option Exercises and Stock Vested
The following table provides information regarding the number of equity units that vested for our named executive officers during 2009.
| Stock Awards | |||||
Name | Number of Shares Acquired on Vesting (#) | Value Realized on Vesting ($) | |||
Norman C. Payson, M.D. | 7,739,464 | (1 | ) | ||
Chris A. Karkenny | 1,112,437 | (1 | ) | ||
James G. Gallas | — | — | |||
Lawrence A. Mastrovich | 619,157 | (1 | ) | ||
Daniel A. Greenleaf | 503,065 | (1 | ) | ||
| (1) | There was no public market for the units as of the vesting dates, and thus the market values as of the vesting dates are not determinable. For purposes of FASB ASC Topic 718, the grant date fair value of Dr. Payson’s Class B Units was $0.36 per unit, the grant date fair value of Mr. Karkenny’s Class B Unit was $0.35 per unit and the grant date fair value of the other named executive officers’s Class B Units were $0.28 per unit. |
Nonqualified Deferred Compensation
The following table provides information regarding activity in our nonqualified deferred compensation plan for the named executive officers during 2009. Mr. Mastrovich was the only named executive officer who participated in our nonqualified deferred compensation plan during 2009.
Name | Executive Contributions in Last FY ($) | Registrant Contributions in Last FY ($) | Aggregate Earnings in Last FY ($) | Aggregate Withdrawals/ Distributions ($) | Aggregate Balance at Last FYE ($) | |||||
Norman C. Payson, M.D. | — | — | — | — | — | |||||
Chris A. Karkenny | — | — | — | — | — | |||||
James G. Gallas | — | — | — | — | — | |||||
Lawrence A. Mastrovich (1) | — | — | 307 | 1,534,279 | 388,145 | |||||
Daniel A. Greenleaf | — | — | — | — | — |
| (1) | In connection with Mr. Mastrovich’s resignation from the Company, he received the remaining balance in his account of $396,314 on April 12, 2010. |
Under our nonqualified deferred compensation plan, the participants may defer up to 50% of their salary, up to 100% of their annual bonus, and 100% of their annual 401(k) savings plan refund offset amount, the latter of which is an amount equal to their refund (if any) from our 401(k) savings plan. Returns on deferrals in an individual’s account under the nonqualified deferred compensation plan are credited or debited based on the performance of hypothetical measurement funds selected by the individual, which selection can be changed as often as daily, from a menu of options offered in connection with the plan. We do not match amounts that are deferred by employees pursuant to the nonqualified deferred compensation plan.
An individual may choose to receive distributions in either a lump sum or in annual installments at death, retirement, or termination of employment with us or, in the event of an in-service distribution, at a date specified by the individual at least three years after the end of the year in which the deferral is made. An individual may also receive a distribution if he or she experiences an unforeseeable financial emergency, as defined in the nonqualified deferred compensation plan.
138
Table of Contents
Potential Payments Upon Termination or Change-in-Control
The following table describes the potential payments and benefits that would have been payable to our named executive officers under existing plans and contractual arrangements assuming (i) a termination of employment or (ii) a change of control occurred on December 31, 2009, except that the amounts shown in the following table for Mr. Mastrovich reflect the amounts we agreed to pay to him pursuant to the general release of claims agreement, dated as of March 15, 2010.
The amounts shown in the table do not include payments and benefits to the extent they are provided generally to all salaried employees upon termination of employment and do not discriminate in scope, terms or operation in favor of the named executive officers. These include accrued salary, distributions of plan balances under our 401(k) savings plan and distributions of plan balances under the non-qualified deferred compensation plan. Furthermore, the amounts shown in the table do not include amounts that may be payable to a named executive officer upon the sale or purchase of his vested equity units pursuant to the exercise of the put or call rights described under “Narrative Disclosure to Summary Compensation Table and Grants of Plan-Based Awards—Terms of Equity Award Grants.”
Name | Cash Severance Payment ($)(1) | Continuation of Group Health Plans ($)(2) | Total Termination Benefits (Excluding Acceleration of Equity Awards)(3) ($) | |||
Norman C. Payson, M.D. | ||||||
Without Cause or as a result of Constructive Termination | 825,175 | 17,981 | 843,156 | |||
Chris A. Karkenny | ||||||
Without Cause or as a result of Constructive Termination | 3,194,331 | 35,977 | 3,230,308 | |||
James G. Gallas | ||||||
Without Cause or with Good Reason | 1,050,000 | 30,570 | 1,080,570 | |||
Daniel A. Greenleaf | ||||||
Without Cause or with Good Reason | 2,792,020 | 40,247 | 2,832,267 | |||
Lawrence A. Mastrovich | ||||||
Without Cause or with Good Reason | 2,512,841 | 56,008 | 2,568,849 | |||
| (1) | Cash severance payment includes the following: |
| • | Dr. Payson — his annual bonus earned under the 2009 Bonus Plan of $825,175; |
| • | Mr. Karkenny — (i) his annual bonus earned under the 2009 Bonus Plan of $514,173; (ii) two times the sum of (x) his annual base salary rate of $475,000 and (y) his target annual bonus of $490,079 and (iii) $750,000 payable under the noncompetition agreement we entered into with him prior to the Merger; |
| • | Mr. Gallas — two times his annual base salary rate of $525,000; and |
| • | Mr. Greenleaf — (i) two times the sum of (x) his annual base salary rate of $475,000 and (y) the average of his earned bonus for 2009 and his annual base salary rate for 2009, which equals $546,010 and (ii) $750,000 payable under the noncompetition agreement we entered into with him prior to the Merger |
Cash severance payment for Mr. Mastrovich is described below under “Severance Arrangements—Lawrence A. Mastrovich—General Release of Claims Agreement.”
| (2) | Reflects the cost of providing the executive officer with a continuation of medical, dental and vision insurance under COBRA, including the cost of his participation in the senior executive medical and dental programs, for one year after termination in the case of Dr. Payson and Mr. Gallas and for two years after termination in the case of Messrs. Karkenny and Greenleaf. We have included such costs because |
139
Table of Contents
notwithstanding the specific contractual provisions in the applicable named executive officer’s employment agreement or severance agreement, we would pay out such amounts in the event the named executive officers were terminated under the scenarios illustrated in the table above. |
| (3) | If Dr. Payson were terminated by us without cause or by him as a result of constructive termination, an additional number of his time-vesting Class B Units equal to the number that would have vested over the 24-month period following the termination date would become immediately vested. Total termination benefits for Dr. Payson excludes the market value of 15,478,927 such unvested time-vesting Class B Units that would become immediately vested. Because there was no public market for the Class B Units of BP Healthcare Holdings as of December 31, 2009, the market value as of that date is not determinable. For purposes of FASB ASC Topic 718, the grant date fair value of Dr. Payson’s Class B Units was $0.36 per unit. |
In addition, upon a change of control, our other named executive officers’ unvested time-vesting Class B Units would become immediately vested. As of December 31, 2009, the number of Class B units that were subject to acceleration of vesting upon a change of control was as follows: Mr. Karkenny — 3,337,310; Mr. Gallas — 1,412,452 and Mr. Greenleaf — 2,012,261. Because there was no public market for Holdings’ Class B Units as of December 31, 2009, the market value as of that date is not determinable. For purposes of FASB ASC Topic 718, the grant date fair value of each Class B Unit of Holdings was $0.35 for Mr. Karkenny and $0.28 for Messrs. Gallas and Greenleaf.
Total termination benefits for Mr. Mastrovich does not include the $77,395 we agreed to pay him to repurchase his vested equity units.
Severance Arrangements
Norman C. Payson
Pursuant to the terms of Dr. Payson’s employment agreement, if Dr. Payson’s employment is terminated without “cause” by Apria or by him as a result of a “constructive termination,” Dr. Payson will be entitled to receive the following severance benefits:
| • | a pro rata portion of an annual bonus in respect of the year of termination and |
| • | continued coverage under our group health plans until the earlier of (x) twelve months from the date of Dr. Payson’s termination of employment with Apria and (y) the date Dr. Payson is or becomes eligible for comparable coverage under health plans of another employer. |
The amounts payable to Dr. Payson upon a termination of employment described above are subject to Dr. Payson providing a release of all claims to us. Following termination without cause or as a result of a constructive termination, except as set forth above, Dr. Payson will have no further rights to any compensation or any other benefits under his employment agreement.
Furthermore, if Dr. Payson fails to comply with the non-competition, non-solicitation and confidentiality covenants contained in his employment agreement, we have the right to terminate the payments described above. The confidentiality covenant has an indefinite term, whereas the non-competition and non-solicitation covenants have terms of twelve and twenty-four months, respectively. The term of a particular covenant will be extended by the length of any period that Dr. Payson is in breach of such covenant; however, we have the right to waive a breach of any covenant by Dr. Payson.
In addition, pursuant to the terms of Dr. Payson’s employment agreement, if Dr. Payson resigns, or we remove him, from the position of Chief Executive Officer of Apria and/or Sky Acquisition, and we terminate his employment agreement and enter into a services agreement with him, then we would pay him $500,000 per year for his services to Apria under the services agreement. Any such resignation by Dr. Payson does not constitute “cause” and any such removal by us does not constitute “constructive termination” under the terms of his
140
Table of Contents
employment agreement. See “Narrative Disclosure to Summary Compensation Table and Grants of Plan-Based Awards—Employment Agreements—Norman C. Payson.”
For purposes of Dr. Payson’s employment agreement, “cause” means (i) Dr. Payson’s willful and continued failure to substantially perform his duties to us or our affiliates (other than as a result of total or partial incapacity due to physical or mental illness or as a result of him resigning as our Chief Executive Officer); (ii) his engagement in fraud or willful dishonesty (other than dishonesty that has no material detrimental impact on our reputation or business and our affiliates); (iii) any act on the part of Dr. Payson that constitutes a felony (other than traffic offenses) or its equivalent under applicable non-U.S. law or (iv) his material breach of the restrictive covenant provisions of the agreement; provided, further, that “cause” ceases to exist for an event on the ninetieth (90th) day following the later of its occurrence or the knowledge thereof by a majority of our Board of Directors, unless we or our affiliates have given him written notice thereof prior to such date.
For purposes of Dr. Payson’s employment agreement, “constructive termination” means the occurrence of one of the following: (i) our failure to pay or cause to be paid Dr. Payson’s base salary, annual bonus (if any) or reimbursable expenses when due; (ii) except in certain circumstances, a reduction in his base salary or target annual bonus; (iii) any substantial and sustained diminution in his authority or responsibilities; (iv) any material breach by us of any material agreement with him; provided that none of these events shall constitute constructive termination unless we fail to cure such event within 30 days after receipt from him of written notice specifying in reasonable detail the event which constitutes constructive termination; provided, further, that “constructive termination” ceases to exist for an event on the ninetieth (90th) day following the later of its occurrence or his knowledge thereof, unless he has given Apria written notice thereof prior to such date.
Chris A. Karkenny
Pursuant to the terms of Mr. Karkenny’s employment agreement, if Mr. Karkenny’s employment is terminated without cause by Apria or by him as a result of a constructive termination, Mr. Karkenny will be entitled to receive the following severance benefits:
| • | a pro rata portion of an annual bonus in respect of the year of termination; |
| • | subject to his compliance with the restrictive covenants described below, two times the sum of (x) his annual base salary and (y) his target annual bonus payable over twenty-four months; and |
| • | continued coverage under our group health plans until the earlier of (x) twenty-four months from the executive’s date of termination of employment with Apria and (y) the date the executive is or becomes eligible for comparable coverage under health plans of another employer. |
The amounts payable to Mr. Karkenny upon a termination of employment described above are subject to Mr. Karkenny providing a release of all claims to us. Following termination without cause or as a result of a constructive termination, except as set forth above, Mr. Karkenny will have no further rights to any compensation or any other benefits under his employment agreement.
Furthermore, the payment of severance compensation equal to two times the sum of (x) his annual base salary and (y) his target annual bonus described above is contingent upon Mr. Karkenny’s continued compliance with the non-competition, non-solicitation, non-disparagement and confidentiality covenants contained in his employment agreement. The confidentiality covenant has an indefinite term, and the non-competition, non-disparagement and non-solicitation covenants each have a term of eighteen months. The term of a particular covenant will be extended by the length of any period that Mr. Karkenny is in breach of such covenant.
In addition, pursuant to the terms of Mr. Karkenny’s employment agreement, if Mr. Karkenny had terminated his employment prior to March 31, 2010, other than as a result of “constructive termination” or upon his death or disability, he would have been required to pay to us a portion of his $2,100,000 retention bonus equal to the amount by which $2,100,000 exceeds the product of (x) $140,000 and (y) the number of full months following January 1, 2009 that he was continuously employed by us.
141
Table of Contents
For purposes of Mr. Karkenny’s employment agreement, “cause” generally means that our Board of directors has determined that Mr. Karkenny has (i) engaged in or committed willful misconduct; (ii) engaged in or committed theft, fraud or other illegal conduct; (iii) refused or demonstrated an unwillingness to substantially perform his duties for a 30-day period after written notice from Apria; (iv) refused or demonstrated an unwillingness to reasonably cooperate in good faith with certain investigations; (v) engaged in or committed insubordination; (vi) engaged in or committed any willful act that is injurious to our reputation or business; (vii) willfully violated his fiduciary duty or his duty of loyalty to us or our Code of Ethical Business Conduct in any material respect; (viii) used alcohol or drugs (other than prescribed drugs for their intended purpose) in a manner which materially and repeatedly interferes with the performance of his duties; or (ix) engaged in or committed a material breach of his employment agreement for a 30-day period after written notification is delivered by us.
For purposes of Mr. Karkenny’s employment agreement, “constructive termination” means the occurrence of one of the following: (i) our failure to pay or cause to be paid Mr. Karkenny’s base salary, annual bonus (if any) or retention payments when due; (ii) a reduction in Mr. Karkenny’s retention payments, base salary or target annual bonus; (iii) any substantial and sustained diminution in his authority or responsibilities; (iv) a material reduction in, or the failure of Apria to provide in all material respects, the employee benefits to which he is entitled to or receiving as of the date hereof (excluding any reductions generally applicable to all senior executives); (iv) a relocation of his principal place of business which will result in an increase by more than 30 miles in his one-way commute; (v) a reduction in his title or a material reduction in the nature, status or scope of his authorities, duties and/or responsibilities; (vi) the failure of a successor employer to Apria to assume Mr. Karkenny’s employment agreement in writing; (vii) our delivery of a written notice to not extend the employment term; or (viii) his not being the Executive Vice President and Chief Financial Officer of the operating entity following the occurrence of a change of control; provided that none of these events shall constitute constructive termination unless we fail to cure such event within 30 days after receipt from him of written notice specifying in reasonable detail the event which constitutes constructive termination; provided, further, that “constructive termination” shall cease to exist for an event on the ninetieth (90th) day following the later of its occurrence or his knowledge thereof, unless he has given Apria written notice thereof prior to such date.
James G. Gallas
Pursuant to the terms of Mr. Gallas’ severance agreement, either party may terminate Mr. Gallas’s employment at any time. If Mr. Gallas’ employment is terminated without cause by Apria or with good reason by Mr. Gallas, then the terminating party must give the other party at least 30 days advance written notice.
If Mr. Gallas’ employment is terminated without cause by us or terminated with good reason by Mr. Gallas, he will be entitled to receive the following severance benefits:
| • | annual base salary at the rate in effect at the time of termination and |
| • | the sum of (x) the average of his two most recent annual bonuses, if any, received prior to termination, and (y) the annual cost of providing him with a continuation of medical, dental and vision insurance under COBRA, including the cost of his participation in the senior executive medical and dental programs; provided however, that in the event he has been employed for less than one year and for that reason has not yet received an annual bonus, then his average annual bonus shall be deemed to be 100% of his annual base salary rate as of the date of his employment with Apria. |
The amounts payable to Mr. Gallas upon a termination of employment described above shall be paid in periodic installments over a period of twelve months provided that Mr. Gallas executes a release of all claims to us. Such payments are also contingent upon Mr. Gallas’s continued compliance with certain non-competition, non-solicitation and confidentiality covenants contained in his employment agreement. The confidentiality covenant has an indefinite term, whereas the non-competition and non-solicitation covenants each have a term of twelve months. If we believe that Mr. Gallas is in violation of the non-competition or non-solicitation covenant
142
Table of Contents
after his termination, we may suspend all of the payments described above until he establishes that he is not in violation of such covenants. However, we also have the right to waive a breach of any covenant by Mr. Gallas.
Pursuant to the terms of Mr. Gallas’ offer letter, he is required to refund a portion of the $150,000 sign-on bonus paid to him if he voluntarily terminates his employment with us (other than as a result of constructive termination) or is terminated for misconduct before completing 24 months of employment, in an amount equal to 1/24th of the total for each month less than 24 completed.
For purposes of Mr. Gallas’ executive severance agreement, “cause” generally means that we have determined that Mr. Gallas has (i) engaged in or committed willful misconduct; (ii) engaged in or committed theft, fraud or other illegal conduct; (iii) refused or demonstrated an unwillingness to substantially perform his duties after written notice from Apria; (iv) refused or demonstrated an unwillingness to reasonably cooperate in good faith with certain investigations; (v) engaged in or committed insubordination; (vi) engaged in or committed any willful act that is injurious to our reputation or business; (vii) violated his fiduciary duty or his duty of loyalty to us or our Code of Ethical Business Conduct in any material respect; (viii) used alcohol or drugs (other than prescribed drugs for their intended purpose) in a manner which materially and repeatedly interferes with the performance of his duties; or (ix) engaged in or committed a material breach of his severance agreement.
Under his severance agreement, “good reason” generally means (i) the reduction of Mr. Gallas’s annual base salary (except in the case of certain reductions affecting all executive officers equally); (ii) the addition of greater than 30 miles to Mr. Gallas’s one-way commute (except in connection with a relocation of our principal executive offices); or (iii) our failure to require our successor to expressly assume and perform Mr. Gallas’ severance agreement. However, “good reason” will cease to exist under Mr. Gallas’ severance agreement if Mr. Gallas agrees to any of the above conditions in writing, fails to terminate his employment within 120 days after the occurrence of such conditions, or if we remedy such conditions within 30 days after receipt of written notice (within 60 days of the initial existence of the condition from Mr. Gallas).
Daniel E. Greenleaf
Pursuant to the terms of Mr. Greenleaf’s employment agreement, if Mr. Greenleaf’s employment is terminated without cause by Apria or by him with good reason, Mr. Greenleaf will be entitled to receive the following severance benefits:
| • | a payment equal to two times the sum of (x) his annual base salary at the rate in effect at the time of termination, (y) the average of his annual bonuses with respect to our two most recently completed fiscal years, if any, and (z) the annual cost for Mr. Greenleaf to obtain medical, dental and vision insurance under COBRA; provided however, that in the event he has been employed for a period which includes one (but not two) full annual bonus cycles prior to the termination of employment, the average of his annual bonuses described above shall be deemed to be equal to the average of the earned annual bonus for the full bonus cycle during his employment plus an amount equal to his base salary for the year of termination. |
The amounts payable to Mr. Greenleaf upon a termination of employment described above shall be paid in periodic installments over a period of twenty-four months provided that Mr. Greenleaf executes a release of all claims to us. Such payments are also contingent upon Mr. Greenleaf’s continued compliance with certain non-competition, non-solicitation, non-disparagement and confidentiality covenants contained in his employment agreement. The confidentiality covenant has an indefinite term, whereas the non-competition and non-solicitation covenants each have a term of twenty-four months and the non-disparagement covenant has a term of twelve months. If we believe that Mr. Greenleaf is in violation of the non-competition, non-solicitation, non-disparagement or confidentiality covenant after his termination, we may suspend all of the payments described above until he establishes that he is not in violation of such covenant. However, we also have the right to waive a breach of any covenant by Mr. Greenleaf.
143
Table of Contents
For purposes of Mr. Greenleaf’s employment agreement, “cause” generally means that our Board of Directors determines that the executive has done any of the following: (i) engaged in or committed willful misconduct; (ii) engaged in or committed theft, fraud or other conduct constituting a felony (other than traffic related offenses or as a result of vicarious liability); (iii) refused or demonstrated an unwillingness to substantially perform his duties for a 30-day period after written demand from Apria; (iv) refused or demonstrated an unwillingness to reasonably cooperate in good faith with certain investigations; (v) engaged in or committed any willful act that is injurious to our business or reputation; (vi) willfully violated his fiduciary duty or his duty of loyalty to us or our Code of Ethical Business Conduct in any material respect; (vii) used alcohol or drugs (other than prescribed drugs for their intended purposes) in a manner which materially and repeatedly interferes with the performance of his duties or which has the effect of materially injuring our business or reputation; or (viii) engaged in or committed a material breach of his amended and restated employment agreement for a 30-day period after written notice from Apria.
For the purposes of Mr. Greenleaf’s employment agreement during a period that (i) begins with the first to occur of (1) the initial public announcement of a change of control or (2) the 90th day preceding a change of control and (ii) ends two years following such change of control, “cause” generally means only the occurrence of either or both of the following: (A) Mr. Greenleaf’s conviction for committing an act of fraud, embezzlement, theft, or other act constituting a felony (other than traffic related offenses or as a result of vicarious liability); or (B) the willful engaging by Mr. Greenleaf in misconduct that is significantly injurious to us. For purposes of the above clause (B) no act, or failure to act, on Mr. Greenleaf’s part shall be considered willful unless done or omitted to be done, by him not in good faith or without reasonable belief that his action or omission was in the best interest of the Company.
For purposes of Mr. Greenleaf’s employment agreement during the period that begins with the first to occur of (i) the initial public announcement of a change of control or (ii) the ninetieth (90th) day preceding a change of control and ends two years following such change of control, “good reason” means, without the executive’s written consent, the occurrence of any of the following: (i) a material reduction in the nature, status or scope of Mr. Greenleaf’s authorities, duties, and/or responsibilities from their level in effect on the day immediately prior to the change of control; (ii) a reduction in Mr. Greenleaf’s base salary from its highest level in effect at any point in the three months preceding the change of control or a significant reduction in Mr. Greenleaf’s aggregate incentive opportunities under our short and/or long-term incentive programs, as such opportunities exist immediately prior to the change of control; (iii) our failure to maintain Mr. Greenleaf’s relative level of coverage and accruals under our employee benefit and/or retirement plans, policies, practices or arrangements in which he participates immediately prior to the change of control; (iv) Mr. Greenleaf is informed by us that his principal place of employment will be relocated to a location that will result in an increase of more than 30 miles in his one-way commute; and (v) for purposes of his employment agreement, we are not permitting him to continue to serve in a mutually acceptable senior executive position.
For purposes of Mr. Greenleaf’s employment agreement, in circumstances unrelated to a change of control or subsequent to the expiration of the two-year period following a change of control, “good reason” generally means the occurrence of any one of the following events without Mr. Greenleaf’s written consent: (i) his annual base salary is reduced, except for a one-time “across-the-board” salary reduction not exceeding ten percent which is imposed simultaneously on all executive officers; (ii) we require Mr. Greenleaf to be based at an office location which will result in an increase of more than 30 miles in his one-way commute; (iii) we do not permit him to serve in a mutually acceptable senior executive position; or (iv) Mr. Greenleaf ceases to continue to serve in his current position or another mutually acceptable senior executive position.
Lawrence A. Mastrovich
Pursuant to the terms of Mr. Mastrovich’s employment agreement, if Mr. Mastrovich had been terminated by us without cause or by him for good reason, Mr. Mastrovich would have been entitled to receive the following severance benefits:
| • | 200% of his base salary; |
144
Table of Contents
| • | 200% of the average of his annual bonuses for the two most recently completed fiscal years; and |
| • | 200% of the annual cost of obtaining medical, dental and vision insurance under COBRA. |
Under Mr. Mastrovich’s employment agreement, “cause” generally meant that we had determined that Mr. Mastrovich had (i) engaged in or committed willful misconduct; (ii) engaged in or committed theft, fraud or other illegal conduct; (iii) refused or demonstrated an unwillingness to substantially perform his duties after a written notice from Apria; (iv) refused or demonstrated an unwillingness to reasonably cooperate in good faith with certain investigations; (v) engaged in or committed insubordination; (vi) engaged in or committed any willful act injurious to our business or reputation; (vii) willfully violated his fiduciary duty or his duty of loyalty to us or our Code of Ethical Business Conduct in any material respect; (viii) used alcohol or drugs in a manner which materially and repeatedly interfered with the performance of his duties; or (ix) engaged in or committed a material breach of his employment agreement.
For the purposes of Mr. Mastrovich’s employment agreement, “good reason” meant (i) the reduction of Mr. Mastrovich’s annual base salary (except in the case of certain reductions affecting all executive officer’s equally); (ii) the addition of greater than 30 miles to Mr. Mastrovich’s one-way commute (except in connection with a relocation of our principal executive offices); or (iii) our failure to permit Mr. Mastrovich to continue to serve as the President, Home Respiratory Therapy and Home Medical Equipment segment or another mutually acceptable senior executive position.
General Release of Claims Agreement
In connection with Mr. Mastrovich’s resignation from the Company, we entered into a general release of claims agreement with Mr. Mastrovich, effective March 15, 2010, pursuant to which we agreed to pay Mr. Mastrovich:
| • | severance compensation in the gross amount of $2,568,849, to be paid over the one-year period of his non-competition agreement, consisting of: |
| • | 200% of his base salary, totaling $1,160,000; |
| • | 200% of the average of his annual bonuses for the two most recently completed fiscal years, totaling $1,102,841; |
| • | 200% of the annual cost of obtaining medical, dental and vision insurance under COBRA, totaling $56,008; and |
| • | $250,000 in consideration for his continued compliance with certain non-competition and anti-solicitation provisions in his employment agreement and |
| • | $77,395 to repurchase his vested equity units. |
The payments described above are subject to Mr. Mastrovich’s continued compliance with restrictive covenants contained in his employment agreement, including non-competition, non-solicitation and non-disparagement covenants for a period of two years, as well as non-competition and non-solicitation provisions contained in the separate noncompetition agreement he had entered into with us. Furthermore, Mr. Mastrovich must abide by the confidentiality provision of his employment agreement and refrain from seeking to revoke his various releases of claims under the general release of claims agreement. A provision of the general release of claims agreement may be waived in writing by the party adversely affected by such waiver. Furthermore, the general release of claims agreement does not affect Mr. Mastrovich’s rights under the deferred compensation, 401(k) savings plan or similar benefit plans.
145
Table of Contents
Noncompetition Agreements
We have noncompetition agreements with Mr. Karkenny and Mr. Greenleaf that they entered into prior to the Merger. These noncompetition agreements provide for a payment of $750,000 upon a termination of the executive’s employment with Apria either by Apria without cause or by the executive for good reason, in each case, during the period that begins with the first to occur of (i) the initial public announcement of a change of control or (ii) the ninetieth (90th) day preceding a change of control and ends two years following such change of control. The payments under these noncompetition agreements are contingent upon the affected executive’s compliance with the post-termination noncompetition covenant contained therein. Currently, the two-year period specified in these noncompetition agreements expires on October 28, 2010.
For purposes of the noncompetition agreements, during the period that begins with the first to occur of (x) the initial public announcement of a change of control or (y) the ninetieth (90th) day preceding a change of control, and ends two years following such change of control, “cause” means only the occurrence of either or both of the following: (i) the executive’s conviction for committing an act of fraud, embezzlement, theft, or other act constituting a felony; or (ii) the willful engaging by the executive in misconduct that is significantly injurious to us.
“Good reason” during the same period means the occurrence of any of the following: (i) a material reduction in the nature, status or scope of the executive’s authorities, duties, and/or responsibilities from their level in effect on the day immediately prior to the change of control; (ii) a reduction in the executive’s base salary from its highest level in effect at any point in the three months preceding the change of control or a significant reduction in the executive’s aggregate incentive opportunities under our short and/or long-term incentive programs, as such opportunities exist immediately prior to the change of control; (iii) our failure to maintain the executive’s relative level of coverage and accruals under our employee benefit and/or retirement plans, policies, practices or arrangements in which the executive participates immediately prior to the change of control; (iv) the executive is informed by us that his principal place of employment will be relocated to a location that will result in an increase of more than thirty miles in the executive’s one-way commute; and (v) for purposes of the employment agreements, we are not permitting the executive to continue to serve in a mutually acceptable senior executive position.
Director Compensation
Directors who are also our employees receive no separate compensation for service on our Board of Directors or committees of our Board of Directors. Non-employee directors also currently receive no separate compensation for service on our Board of Directors or committees of our Board of Directors.
146
Table of Contents
SECURITY OWNERSHIP OF PRINCIPAL SHAREHOLDERS AND MANAGEMENT
Holdings owns 100% of the issued and outstanding common stock of Apria Finance Holdings Inc., which owns 100% of the limited liability company interests of Sky Acquisition LLC, which owns 100% of issued and outstanding common stock of the Issuer. The limited liability company interests of Holdings consist of Class A-1 Units, Class A-2 Units, Class B Units and Class C Units. Class A-1 and Class A-2 Units are equity interests in Holdings and have economic characteristics that are similar to those of shares of common stock in a corporation. The Class A-2 Units generally have the same voting and economic rights as Class A-1 Units, subject to certain restrictions and put and call rights applicable to units held by employees. Class B and Class C units are limited liability company profits interests having economic characteristics similar to stock appreciation rights and representing the right to share in any increase in the equity value of Holdings that exceeds specified thresholds. Class B Units and Class C Units are subject to different vesting schedules and other conditions including certain transfer restrictions and put and call rights applicable only to employees. For additional information, see “Management—Executive Compensation—Compensation Discussion and Analysis—Compensation Elements—Long-Term Incentive Compensation”, “Management—Executive Compensation—Narrative Disclosure to Summary Compensation Table and Grants of Plan-Based Awards—Terms of Equity Award Grants” and “Certain Relationships and Related Party Transactions.”
The following table sets forth information with respect to the beneficial ownership of the Class A-1 Units and Class A-2 Units of Holdings taken together as a single class, the Class B Units of Holdings, the Class C Units of Holdings, and the aggregate Class A-1 Units, Class A-2 Units, Class B Units and Class C Units taken together as a single class, in each case, as of March 31, 2010 for (i) each individual or entity known by us to own beneficially more than 5% of the aggregate Units, (ii) each of our named executive officers, (iii) each of our directors and (iv) all of our directors and our executive officers as a group.
The amounts and percentages of Units beneficially owned are reported on the basis of SEC regulations governing the determination of beneficial ownership of securities. Under SEC rules, a person is deemed to be a “beneficial owner” of a security if that person has or shares voting power or investment power, which includes the power to dispose of or to direct the disposition of such security. A person is also deemed to be a beneficial owner of any securities of which that person has a right to acquire beneficial ownership within 60 days. Securities that can be so acquired are deemed to be outstanding for purposes of computing such person’s ownership percentage, but not for purposes of computing any other person’s percentage. Under these rules, more than one person may be deemed to be a beneficial owner of the same securities and a person may be deemed to be a beneficial owner of securities as to which such person has no economic interest.
Except as otherwise indicated in the footnotes below, each of the beneficial owners has, to our knowledge, sole voting and investment power with respect to the indicated Class A-1 Units, Class A-2 Units, Class B Units and Class C Units. Unless otherwise noted, the address of each beneficial owner of is c/o Apria Healthcare Group LLC, 26220 Enterprise Court, Lake Forest, CA 92630.
| Class A Units | Class B Units | Class C Units | Aggregate | |||||||||||||||||||||
Name and Address of | Amount and Nature of Beneficial Ownership | Percent | Amount and Nature of Beneficial Ownership | Percent | Amount and Nature of Beneficial Ownership | Percent | Amount and Nature of Beneficial Ownership | Percent | ||||||||||||||||
Blackstone Funds | 673,333,333 | (1) | 99.62 | % | 41,178,785 | (1) | 48.40 | % | 827,155 | (1) | 5.34 | % | 715,339,273 | (2) | 92.12 | % | ||||||||
Norman C. Payson, M.D. | (3) | (3) | (3) | (3) | (3) | (3) | (3) | (3) | ||||||||||||||||
Chris A. Karkenny | 500,000 | (4) | * | 6,675,287 | 7.84 | % | 2,225,096 | 14.38 | % | 9,400,383 | 1.21 | % | ||||||||||||
James G. Gallas | — | — | 2,118,678 | 2.49 | % | 706,226 | 4.56 | % | 2,824,904 | 0.36 | % | |||||||||||||
Daniel E. Greenleaf | — | — | 3,772,989 | 4.43 | % | 1,257,663 | 8.13 | % | 5,030,652 | 0.65 | % | |||||||||||||
Neil P. Simpkins (5) | — | — | — | — | — | — | — | — | ||||||||||||||||
Michael Dal Bello (6) | — | — | — | — | — | — | — | — | ||||||||||||||||
Patrick J. Bourke (7) | — | — | — | — | — | — | — | — | ||||||||||||||||
All Directors and Executive Officers as a Group (7 persons) | 500,000 | (4) | * | 12,566,954 | 14.76 | % | 4,188,985 | 27.07 | % | 17,255,939 | 2.22 | % | ||||||||||||
| * | Less than 1%. |
147
Table of Contents
| (1) | Units of Holdings shown as beneficially owned by the Blackstone Funds (as hereinafter defined) are held directly by BP Healthcare Holdings LLC (“BP Holdings”). Through BP Holdings, the Blackstone Funds beneficially own all of the Class A-1 Units of Holdings, including (i) 388,296,685 Class A-1 Units in which Blackstone Capital Partners V L.P. (“BCP V”) has an economic interest, representing 57.45% of the Class A Units of Holdings, (ii) 101,814,701 Class A-1 Units in which Blackstone Capital Partners V-AC L.P. (“BCP V-AC”) has an economic interest, representing 15.06% of the Class A Units of Holdings, (iii) 2,051,801 Class A-1 Units in which Blackstone Family Investment Partnership V L.P (“Family”) has an economic interest, representing 0.30% of the Class A Units of Holdings, (iv) 895,435 Class A-1 Units in which Blackstone Participation Partnership V L.P. (“Participation”) has an economic interest, representing 0.13% of the Class A Units of Holdings, (v) 96,388,572 Class A-1 Units in which BCP V-S L.P. (“BCP V-S”) has an economic interest, representing 14.26% of the Class A Units of Holdings, (vi) 73,886,139 Class A-1 Units held by BCP V Co-Investors L.P. (“BCP V Co-Investors, and collectively, the “Blackstone Funds”), representing 10.93% of the Class A Units of Holdings. Dr. Payson owns 10,000,000 Class A-2 Units and 38,697,318 Class B Units of BP Holdings. By virtue of the limited liability company agreement of BP Holdings, which provides that BCP V has the sole authority to appoint members of the board of directors of BP Holdings, BCP V may also be deemed to beneficially own 10,000,000 Class A-2 Units of Holdings, representing 1.48% of the Class A Units of Holdings, and 38,697,318 Class B Units of Holdings, representing 45.45% of the Class B Units of Holdings, held by BP Holdings in which Dr. Payson has an economic interest. The general partner of BCP V, BCP V-AC, BCP V-S and BCP V Co-Investors is Blackstone Management Associates V L.L.C. BMA V L.L.C. is the sole member of Blackstone Management Associates V L.L.C. The general partner of Family and Participation is BCP V Side-By-Side GP L.L.C. Blackstone Holdings III L.P. is the managing member and majority in interest owner of BMA V L.L.C. and the sole member of BCP V Side-By-Side GP L.L.C. Blackstone Holdings III L.P. is indirectly controlled by The Blackstone Group L.P. and is owned, directly or indirectly, by Blackstone professionals and The Blackstone Group L.P. The Blackstone Group L.P. is controlled by its general partner, Blackstone Group Management L.L.C., which is in turn wholly owned by Blackstone’s senior managing directors and controlled by its founder, Stephen A. Schwarzman. Each of such Blackstone entities and Mr. Schwarzman may be deemed to beneficially own the securities beneficially owned by the Blackstone Funds directly or indirectly controlled by it or him, but each disclaims beneficial ownership of such securities except to the extent of its or his indirect pecuniary interest therein. The address of each of the entities listed in this note is c/o The Blackstone Group, L.P., 345 Park Avenue, New York, New York 10154. |
| (2) | The securityholders agreement of Holdings provides (i) that each unit of Holdings owned by an employee will vote in the same proportion as the units held by BP Holdings LLC, (ii) that BP Holdings has the right to require each unit owned by an employee to participate in any transaction constituting a change of control or any other transaction involving a transfer of units owned by BP Holding to a third-party and (iii) generally restricts the transfer of each unit owned by an employee until the earliest of (x) six months following an initial public offering, (y) a change of control or (z) October 28, 2015. As a result, BP Holdings and BCP V may be deemed to beneficially own 100% of outstanding Units of Holdings. The units of Holdings held by employees that may be so deemed beneficially owned by BP Holdings and BCP V are not reported in the table above. For additional information, see “Management—Executive Compensation—Compensation Discussion and Analysis—Compensation Elements—Long-Term Incentive Compensation” and “Certain Relationships and Related Party Transactions.” |
| (3) | Dr. Payson owns 10,000,000 Class A-2 Units and 38,697,318 Class B Units of BP Holdings. For additional information, see Note 9 to our financial statements for the year ending December 31, 2009. |
| (4) | Represents Class A-2 Units. |
| (5) | Mr. Simpkins is a Senior Managing Director of The Blackstone Group. Mr. Simpkins disclaims beneficial ownership of any shares owned directly or indirectly by the Blackstone Funds, except to the extent of his indirect pecuniary interest therein. Mr. Simpkins’ address is c/o The Blackstone Group, L.P., 345 Park Avenue, New York, New York 10017. |
148
Table of Contents
| (6) | Mr. Dal Bello is a Managing Director of The Blackstone Group. Mr. Dal Bello disclaims beneficial ownership of any shares owned directly or indirectly by the Blackstone Funds, except to the extent of his indirect pecuniary interest therein. Mr. Dal Bello’s address is c/o The Blackstone Group, L.P., 345 Park Avenue, New York, New York 10017. |
| (7) | Mr. Bourke is an Executive Director of The Blackstone Group. Mr. Bourke disclaims beneficial ownership of any shares owned directly or indirectly by the Blackstone Funds, except to the extent of his indirect pecuniary interest therein. Mr. Bourke’s address is c/o The Blackstone Group, L.P., 345 Park Avenue, New York, New York 10017. |
149
Table of Contents
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Transaction and Management Fee Agreement
In connection with the Merger, Merger Sub entered into a transaction and management fee agreement with Blackstone Management Partners V L.L.C. (“BMP”). The Company succeeded to and assumed the rights and obligations of Merger Sub pursuant to the transaction and management fee agreement upon the closing of the Merger. Under the transaction and management fee agreement, Merger Sub agreed to pay BMP, at the closing of the Merger, a $18.7 million transaction fee as consideration for BMP undertaking financial and structural analysis, due diligence and other assistance in connection with the Merger. In addition, the Company agreed to reimburse BMP for any out-of-pocket expenses incurred by BMP and its affiliates in connection with the Merger and the provision of services under the transaction and management fee agreement.
In addition, under this agreement, BMP (including through its affiliates) agreed to provide services, including without limitation, (a) advice regarding the structure, distribution and timing of debt and equity offerings and advice regarding relationships with the Company’s lenders and bankers, (b) advice regarding the business and strategy of the Company, including compensation arrangements, (c) advice regarding dispositions and/or acquisitions and (d) such advice directly related or ancillary to the above financial advisory services as may be reasonably requested by the Company. In consideration for the services, the Company will pay BMP at the beginning of each fiscal year a management fee equal to the greater of $7.0 million or 2.0% of the Company’s consolidated EBITDA for the immediately preceding fiscal year. BMP shall have no obligation to provide any other services to the Company absent express agreement. In addition, in the absence of an express agreement to provide investment banking or other financial advisory services to the Company, and without regard to whether such services were provided, BMP is entitled to receive a fee equal to 1.0% of the aggregate transaction value upon the consummation of any acquisition, divestiture, disposition, merger, consolidation, restructuring, refinancing, recapitalization, issuance of private or public debt or equity securities (including an initial public offering of equity securities), financing or similar transaction by the Company.
At any time in connection with or in anticipation of a change of control of the Company, a sale of all or substantially all of the Company’s assets or an initial public offering of common equity of the Company or its successor, BMP may elect to receive, in consideration of BMP’s role in facilitating such transaction and in settlement of the termination of the services, a single lump sum cash payment equal to the then-present value of all then-current and future annual management fees payable under the transaction and management fee agreement, assuming a hypothetical termination date of the agreement to be the twelfth anniversary of such election. The transaction and management fee agreement will continue until the earlier of the twelfth anniversary of the date of the agreement or such date as the Company and BMP may mutually determine. The Company has agreed to indemnify BMP and its affiliates, directors, officers, employees, agents and representatives from and against all liabilities relating to the services contemplated by the transaction and management fee agreement and the engagement of BMP pursuant to, and the performance of BMP and its affiliates of the services contemplated by, the transaction and management fee agreement.
Limited Liability Company Agreements, Securityholders Agreements and Subscription Agreements of Sky Acquisition and BP Holdings
In connection with the Transactions, certain funds affiliated with the Sponsor and Dr. Payson entered into a limited liability company agreement with BP Holdings setting forth the economic and governance rights of the holders of units representing limited liability company membership interests of BP Holdings. In addition, BP Holdings, Mr. Karkenny and other members of our management entered into a limited liability company agreement with Sky Acquisition setting forth the economic and governance rights of the holders of units representing limited liability company membership interests of Sky Acquisition. The limited liability company agreements of BP Holdings and Sky Acquisition include provisions governing the appointment of the directors and officers of BP Holdings and Sky Acquisition, the distributions to the members of BP Holdings and Sky Acquisition, and other corporate governance provisions and indemnification provisions.
150
Table of Contents
In connection with their subscription for equity units of BP Holdings, certain funds affiliated with the Sponsor and Dr. Payson entered into securityholders and subscription agreements with BP Holdings. In addition, BP Holdings, Mr. Karkenny and other members of our management entered into securityholders and subscription agreements with Sky Acquisition in connection with their subscription for equity units of Sky Acquisition. The securityholders and subscription agreements contain certain rights and obligations of the parties thereto with respect to voting, transfer restrictions and rights, including tag-along rights, drag-along rights, registration rights and rights of first refusal, and certain other matters.
In March 2010, we conducted a reorganization pursuant to which we added two new companies, Holdings and Apria Finance, to our corporate structure. Upon the completion of the reorganization, Holdings became a company beneficially owned by the Sponsor and other members of the Investor Group and all of the equity securities of Sky Acquisition held by such holders were converted or exchanged into equivalent securities of Holdings. See “Prospectus Summary—The Transactions.”
In connection with the reorganization, the security holders and subscription agreements with Sky Acquisition were assigned to Holdings.
Intelenet Agreement
In May 2009, we entered into the Intelenet Agreement with Intelenet, an Indian company affiliated with the Sponsor, regarding the outsourcing of certain functions relating to billing, collections and other administrative and clerical services. See “Business—Outsourcing Activities.” If all services currently planned for outsourcing to Intelenet are in fact handled by Intelenet, we expect to make payments to Intelenet of approximately $200 million over a seven-year period that began in the second quarter of 2009. One of the members of our Board of Directors, Mr. Patrick Bourke, is an employee of the Sponsor and also serves on the Board of Directors of Intelenet. During the year ended December 31, 2009 and the three months ended March 31, 2010, we paid approximately $3.7 million and $4.0 million, respectively, under the Intelenet Agreement.
Equity Healthcare Agreement
We have entered into an employer health program agreement with Equity Healthcare LLC (“Equity Healthcare”), effective as of January 1, 2010. Equity Healthcare negotiates with providers of standard administrative services for health benefit plans as well as other related services for cost discounts and quality of service monitoring capability by Equity Healthcare. Because of the combined purchasing power of its client participants, Equity Healthcare is able to negotiate pricing terms for providers that are believed to be more favorable than the companies could obtain for themselves on an individual basis. In consideration for Equity Healthcare’s provision of access to these favorable arrangements and its monitoring of the contracted third parties’ delivery of contracted services to us, we pay Equity Healthcare a fee of $2 per participating employee per month (“PEPM Fee”). As of March 31, 2010, we had approximately 6,600 employees enrolled in our Equity Healthcare health benefit plans in the United States.
Equity Healthcare may also receive a fee (“Health Plan Fees”) from one or more of the health plans with whom Equity Healthcare has contractual arrangements if the total number of employees joining such health plans from participating companies exceeds specified thresholds. If and when Equity Healthcare reaches the point at which the aggregate of its receipts from the PEPM Fee and the Health Plan Fees have covered all of its allocated costs, it will apply the incremental revenues derived from all such fees to (a) reduce the PEPM Fee otherwise payable by us; (b) avoid or reduce an increase in the PEPM Fee that might otherwise have occurred on contract renewal; or (c) arrange for additional services to us at no cost or reduced cost.
Equity Healthcare is an affiliate of Blackstone, with whom Neil P. Simpkins, Michael Del Bello and Patrick Bourke, members of our Board, are affiliated and in which they may have an indirect pecuniary interest.
151
Table of Contents
Expense Reimbursement
Pursuant to the terms of Dr. Payson’s employment agreement, we reimbursed Dr. Payson for his private airplane operating expenses relating to business travel in the amount of $580,500 during 2009. See “Management—Executive Compensation—Narrative Disclosure to Summary Compensation Table and Grants of Plan-Based Awards—Employment Agreements—Norman C. Payson” for a description of Dr. Payson’s employment agreement.
Procedures with Respect to Review and Approval of Related Person Transactions
The Board of Directors has not adopted a formal written policy for the review and approval of transactions with related persons. However, the Board of Directors reviews and approves transactions with related persons as appropriate.
152
Table of Contents
Purpose and Effect of the Exchange Offers
The Issuer and the guarantors of the outstanding notes have entered into registration rights agreements with the initial purchasers of the outstanding notes in which the Issuer and the guarantors agreed, under certain circumstances, to use their reasonable best efforts to file a registration statement relating to offers to exchange the outstanding notes for exchange notes, to cause the registration statement to become effective under the Securities Act and to consummate the exchange offers no later than 450 days after the date of issuance and sale of the Series A-2 Notes. As of the date of this prospectus, $700.0 million aggregate principal amount of the 11.25% Senior Secured Notes due 2014 (Series A-1) and $317.5 million aggregate principal amount of the 12.375% Senior Secured Notes due 2014 (Series A-2) are outstanding. The outstanding Series A-1 Notes and the outstanding Series A-2 Notes were issued in May 2009 and August 2009, respectively.
Under the circumstances set forth below, the Issuer and the guarantors will use their reasonable best efforts to cause the SEC to declare effective a shelf registration statement with respect to the resale of the outstanding notes within the time periods specified in the registration rights agreements and keep such registration statement effective for up to two years after the effective date of the shelf registration statement. These circumstances include:
| • | if any changes in law, SEC rules or regulations or applicable interpretations thereof by the SEC do not permit the Issuer and the guarantors to effect the exchange offers as contemplated by the registration rights agreements; |
| • | if the exchange offers are not consummated within 450 days after the date of issuance of the Series A-2 Notes; |
| • | if any initial purchaser so requests with respect to the outstanding notes not eligible to be exchanged for the exchange notes and held by it within 30 days after the consummation of the exchange offers; or |
| • | if any holder that participates in the exchange offers does not receive freely transferable exchange notes in exchange for tendered outstanding notes and notifies the Issuer within 30 days after such holder becomes aware of it. |
Under each registration rights agreement, if we fail to complete the exchange offers (other than in the event we file a shelf registration statement) or the shelf registration statement, if required thereby, is not declared effective, in either case on or prior to 450 days after the date of issuance and sale of the Series A-2 Notes (the “target registration date”), the interest rate on the outstanding notes will be increased by (x) 0.25% per annum for the first 90-day period immediately following the target registration date and (y) an additional 0.25% per annum with respect to each subsequent 90-day period, in each case, until the exchange offers are completed or the shelf registration statement, if required, is declared effective by the SEC, up to a maximum of 1.00% per annum of additional interest. Copies of the registration rights agreements have filed as exhibits to the registration statement of which this prospectus is a part.
If you wish to exchange your outstanding notes for exchange notes in the exchange offers, you will be required to make the following written representations:
| • | you are not an affiliate of the Issuer or any guarantor within the meaning of Rule 405 of the Securities Act; |
| • | you have no arrangement or understanding with any person to participate in a distribution (within the meaning of the Securities Act) of the exchange notes in violation of the provisions of the Securities Act; |
| • | you are not engaged in, and do not intend to engage in, a distribution of the exchange notes; and |
| • | you are acquiring the exchange notes in the ordinary course of your business. |
153
Table of Contents
Each broker-dealer that receives exchange notes for its own account in exchange for outstanding notes, where the broker-dealer acquired the outstanding notes as a result of market-making activities or other trading activities, must acknowledge that it will deliver a prospectus in connection with any resale of such exchange notes. Please see “Plan of Distribution.”
Resale of Exchange Notes
Based on interpretations by the SEC set forth in no-action letters issued to third parties, we believe that you may resell or otherwise transfer exchange notes issued in the exchange offers without complying with the registration and prospectus delivery provisions of the Securities Act, if:
| • | you are not an affiliate of the Issuer or any guarantor within the meaning of Rule 405 under the Securities Act; |
| • | you do not have an arrangement or understanding with any person to participate in a distribution of the exchange notes; |
| • | you are not engaged in, and do not intend to engage in, a distribution of the exchange notes; and |
| • | you are acquiring the exchange notes in the ordinary course of your business. |
If you are an affiliate of the Issuer or any guarantor, or are engaging in, or intend to engage in, or have any arrangement or understanding with any person to participate in, a distribution of the exchange notes, or are not acquiring the exchange notes in the ordinary course of your business:
| • | you cannot rely on the position of the SEC set forth in Morgan Stanley & Co. Incorporated (available June 5, 1991) and Exxon Capital Holdings Corporation (available May 13, 1988), as interpreted in the SEC’s letter to Shearman & Sterling, dated July 2, 1993, or similar no-action letters; and |
| • | in the absence of an exception from the position stated immediately above, you must comply with the registration and prospectus delivery requirements of the Securities Act in connection with any resale of the exchange notes. |
This prospectus may be used for an offer to resell, resale or other transfer of exchange notes only as specifically set forth in this prospectus. With regard to broker-dealers, only broker-dealers that acquired the outstanding notes as a result of market-making activities or other trading activities may participate in the exchange offers. Each broker-dealer that receives exchange notes for its own account in exchange for outstanding notes, where such outstanding notes were acquired by such broker-dealer as a result of market-making activities or other trading activities, must acknowledge that it will deliver a prospectus in connection with any resale of the exchange notes. Please read “Plan of Distribution” for more details regarding the transfer of exchange notes.
Terms of the Exchange Offers
On the terms and subject to the conditions set forth in this prospectus and in the accompanying letters of transmittal, the Issuer will accept for exchange in the exchange offers any outstanding notes that are validly tendered and not validly withdrawn prior to the expiration date. Outstanding notes may only be tendered in a principal amount of $2,000 and in integral multiples of $1,000 in excess thereof. The Issuer will issue $1,000 principal amount of exchange notes in exchange for each $1,000 principal amount of outstanding notes surrendered in the exchange offers.
The form and terms of the exchange notes will be identical in all material respects to the form and terms of the outstanding notes except the exchange notes will be registered under the Securities Act, will not bear legends restricting their transfer and will not provide for any additional interest upon failure by the Issuer and the guarantors to fulfill their obligations under the applicable registration rights agreement to complete the exchange offers, or file, and cause to be effective, a shelf registration statement, if required thereby, within the specified
154
Table of Contents
time period. The exchange notes will evidence the same debt as the outstanding notes. The exchange notes will be issued under and entitled to the benefits of the same indenture that governs the terms of the outstanding notes. For a description of the indenture, see “Description of Notes.”
The exchange offers are not conditioned upon any minimum aggregate principal amount of outstanding notes being tendered for exchange.
This prospectus and the letters of transmittal are being sent to all registered holders of outstanding notes. There will be no fixed record date for determining registered holders of outstanding notes entitled to participate in the exchange offers. The Issuer and the guarantors intend to conduct the exchange offers in accordance with the provisions of the registration rights agreements, the applicable requirements of the Securities Act and the Securities Exchange Act of 1934, as amended (the “Exchange Act”) and the rules and regulations of the SEC. Outstanding notes that are not tendered for exchange in the exchange offers will remain outstanding and continue to accrue interest and will be entitled to the rights and benefits such holders have under the indenture and the applicable registration rights agreement except the Issuer and the guarantors will not have any further obligation to you to provide for the registration of the outstanding notes under the registration rights agreements.
The Issuer will be deemed to have accepted for exchange properly tendered outstanding notes when the Issuer has given written notice of the acceptance to the exchange agent. The exchange agent will act as agent for the tendering holders for the purposes of receiving the exchange notes from the Issuer and delivering exchange notes to holders. Subject to the terms of the applicable registration rights agreement, the Issuer expressly reserves the right to amend or terminate the exchange offers and to refuse to accept the occurrence of any of the conditions specified below under “—Conditions to the Exchange Offers.”
If you tender your outstanding notes in the exchange offers, you will not be required to pay brokerage commissions or fees or, subject to the instructions in the letter of transmittal, transfer taxes with respect to the exchange of outstanding notes. We will pay all charges and expenses, other than certain applicable taxes described below in connection with the exchange offers. It is important that you read “—Fees and Expenses” below for more details regarding fees and expenses incurred in the exchange offers.
Expiration Date; Extensions, Amendments
As used in this prospectus, the term “expiration date” means 5:00 p.m., New York City time, on , 2010, which is the 21st business day after the date of this prospectus. However, if the Issuer, in its sole discretion, extends the period of time for which the applicable exchange offer is open, the term “expiration date” will mean the latest time and date to which the Issuer shall have extended the expiration of the applicable exchange offer.
To extend the period of time during which an exchange offer is open, the Issuer will notify the exchange agent of any extension by written notice, followed by notification by press release or other public announcement to the registered holders of the outstanding notes no later than 9:00 a.m., New York City time, on the next business day after the previously scheduled expiration date.
The Issuer reserves the right, in its sole discretion:
| • | to delay accepting for exchange any outstanding notes (if the Issuer amends or extends the exchange offers); |
| • | to extend the exchange offers or to terminate the exchange offers if any of the conditions set forth below under “—Conditions to the Exchange Offers” have not been satisfied, by giving written notice of such delay, extension or termination to the exchange agent; and |
| • | subject to the terms of the applicable registration rights agreement, to amend the terms of the exchange offers in any manner. |
155
Table of Contents
Any delay in acceptance, extension, termination or amendment will be followed as promptly as practicable by notice to the registered holders of the outstanding notes. If the Issuer amends the exchange offers in a manner that it determines to constitute a material change, the Issuer will promptly disclose the amendment in a manner reasonably calculated to inform the holders of applicable outstanding notes of that amendment.
Conditions to the Exchange Offers
Despite any other term of the exchange offers, the Issuer will not be required to accept for exchange, or to issue exchange notes in exchange for, any outstanding notes and the Issuer may terminate or amend the exchange offers as provided in this prospectus prior to the expiration date if in their reasonable judgment:
| • | the exchange offers or the making of any exchange by a holder violates any applicable law or interpretation of the SEC; or |
| • | any action or proceeding has been instituted or threatened in any court or by or before any governmental agency with respect to the exchange offers that, in their judgment, would reasonably be expected to impair their ability to proceed with the exchange offers. |
In addition, the Issuer will not be obligated to accept for exchange the outstanding notes of any holder that has not made to the Issuer:
| • | the representations described under “—Purpose and Effect of the Exchange Offers,” “—Procedures for Tendering Outstanding Notes” and “Plan of Distribution;” or |
| • | any other representations as may be reasonably necessary under applicable SEC rules, regulations, or interpretations to make available to the Issuer an appropriate form for registration of the exchange notes under the Securities Act. |
The Issuer expressly reserves the right at any time or at various times to extend the period of time during which the exchange offers are open. Consequently, the Issuer may delay acceptance of any outstanding notes by giving written notice of such extension to their holders. The Issuer will return any outstanding notes that the Issuer does not accept for exchange for any reason without expense to their tendering holder promptly after the expiration or termination of the exchange offers.
The Issuer expressly reserves the right to amend or terminate the exchange offers and to reject for exchange any outstanding notes not previously accepted for exchange, upon the occurrence of any of the conditions of the exchange offers specified above. In addition, the Issuer is generally required to extend the offering period for any material change, including the waiver of a material condition, so that at least five business days remain in the exchange offers after the change. The Issuer will give written notice of any extension, amendment, non-acceptance or termination to the holders of the outstanding notes as promptly as practicable. In the case of any extension, such notice will be issued no later than 9:00 a.m. New York City time, on the next business day after the previously scheduled expiration date.
These conditions are for sole benefit of the Issuer and the Issuer may assert them regardless of the circumstances that may give rise to them or waive them in whole or in part at any or at various times prior to the expiration date in its sole discretion. If the Issuer fails at any time to exercise any of the foregoing rights, this failure will not constitute a waiver of such right. Each such right will be deemed an ongoing right that the Issuer may assert at any time or at various times prior to the expiration date.
In addition, the Issuer will not accept for exchange any outstanding notes tendered, and will not issue exchange notes in exchange for any such outstanding notes, if at such time any stop order is threatened or in effect with respect to the registration statement of which this prospectus constitutes a part or the qualification of the indenture under the Trust Indenture Act of 1939 (the “TIA”).
156
Table of Contents
Procedures for Tendering Outstanding Notes
To tender your outstanding notes in the exchange offers, you must comply with either of the following:
| • | complete, sign and date the letter of transmittal, or a facsimile of the letter of transmittal, have the signature(s) on the letter of transmittal guaranteed if required by the letter of transmittal and mail or deliver such letter of transmittal or facsimile thereof to the exchange agent at the address set forth below under “—Exchange Agent” prior to the expiration date; or |
| • | comply with DTC’s Automated Tender Offer Program procedures described below. |
In addition, either:
| • | the exchange agent must receive certificates for outstanding notes along with the letter of transmittal prior to the expiration date; |
| • | the exchange agent must receive a timely confirmation of book-entry transfer of outstanding notes into the exchange agent’s account at DTC according to the procedures for book-entry transfer described below or a properly transmitted agent’s message prior to the expiration date; or |
| • | you must comply with the guaranteed delivery procedures described below. |
Your tender, if not withdrawn prior to the expiration date, constitutes an agreement between the Issuer and you upon the terms and subject to the conditions described in this prospectus and in the letter of transmittal.
The method of delivery of outstanding notes, letters of transmittal, and all other required documents to the exchange agent is at your election and risk. We recommend that instead of delivery by mail, you use an overnight or hand delivery service, properly insured. In all cases, you should allow sufficient time to assure timely delivery to the exchange agent before the expiration date. You should not send letters of transmittal or certificates representing outstanding notes to us. You may request that your broker, dealer, commercial bank, trust company or nominee effect the above transactions for you.
If you are a beneficial owner whose outstanding notes are registered in the name of a broker, dealer, commercial bank, trust company, or other nominee and you wish to tender your notes, you should promptly contact the registered holder and instruct the registered holder to tender on your behalf. If you wish to tender the outstanding notes yourself, you must, prior to completing and executing the letter of transmittal and delivering your outstanding notes, either:
| • | make appropriate arrangements to register ownership of the outstanding notes in your name; or |
| • | obtain a properly completed bond power from the registered holder of outstanding notes. |
The transfer of registered ownership may take considerable time and may not be able to be completed prior to the expiration date.
Signatures on the letter of transmittal or a notice of withdrawal, as the case may be, must be guaranteed by a member firm of a registered national securities exchange or of the Financial Industry Regulatory Authority, Inc., a commercial bank or trust company having an office or correspondent in the United States or another “eligible guarantor institution” within the meaning of Rule 17A(d)-15 under the Exchange Act unless the outstanding notes surrendered for exchange are tendered:
| • | by a registered holder of the outstanding notes who has not completed the box entitled “Special Issuance Instructions” or “Special Delivery Instructions” in the letter of transmittal; or |
| • | for the account of an eligible guarantor institution. |
157
Table of Contents
If the letter of transmittal is signed by a person other than the registered holder of any outstanding notes listed on the outstanding notes, such outstanding notes must be endorsed or accompanied by a properly completed bond power. The bond power must be signed by the registered holder as the registered holder’s name appears on the outstanding notes and an eligible guarantor institution must guarantee the signature on the bond power.
If the letter of transmittal or any certificates representing outstanding notes, or bond powers are signed by trustees, executors, administrators, guardians, attorneys-in-fact, officers of corporations, or others acting in a fiduciary or representative capacity, those persons should also indicate when signing and, unless waived by the Issuer, they should also submit evidence satisfactory to the Issuer of their authority to so act.
The exchange agent and DTC have confirmed that any financial institution that is a participant in DTC’s system may use DTC’s Automated Tender Offer Program to tender. Participants in the program may, instead of physically completing and signing the letter of transmittal and delivering it to the exchange agent, electronically transmit their acceptance of the exchange by causing DTC to transfer the outstanding notes to the exchange agent in accordance with DTC’s Automated Tender Offer Program procedures for transfer. DTC will then send an agent’s message to the exchange agent. The term “agent’s message” means a message transmitted by DTC, received by the exchange agent and forming part of the book-entry confirmation, which states that:
| • | DTC has received an express acknowledgment from a participant in its Automated Tender Offer Program that is tendering outstanding notes that are the subject of the book-entry confirmation; |
| • | the participant has received and agrees to be bound by the terms of the letter of transmittal, or in the case of an agent’s message relating to guaranteed delivery, that such participant has received and agrees to be bound by the applicable notice of guaranteed delivery; and |
| • | the Issuer may enforce that agreement against such participant. |
Acceptance of Exchange Notes
In all cases, the Issuer will promptly issue exchange notes for outstanding notes that it has accepted for exchange under the exchange offers only after the exchange agent timely receives:
| • | outstanding notes or a timely book-entry confirmation of such outstanding notes into the exchange agent’s account at the book-entry transfer facility; and |
| • | a properly completed and duly executed letter of transmittal and all other required documents or a properly transmitted agent’s message. |
By tendering outstanding notes pursuant to the exchange offers, you will represent to the Issuer that, among other things:
| • | you are not an affiliate of the Issuer or the guarantors within the meaning of Rule 405 under the Securities Act; |
| • | you do not have an arrangement or understanding with any person or entity to participate in a distribution of the exchange notes; and |
| • | you are acquiring the exchange notes in the ordinary course of your business. |
In addition, each broker-dealer that is to receive exchange notes for its own account in exchange for outstanding notes must represent that such outstanding notes were acquired by that broker-dealer as a result of market-making activities or other trading activities and must acknowledge that it will deliver a prospectus that meets the requirements of the Securities Act in connection with any resale of the exchange notes. The letter of transmittal states that by so acknowledging and by delivering a prospectus, a broker-dealer will not be deemed to admit that it is an “underwriter” within the meaning of the Securities Act. See “Plan of Distribution.”
158
Table of Contents
The Issuer will interpret the terms and conditions of the exchange offers, including the letters of transmittal and the instructions to the letters of transmittal, and will resolve all questions as to the validity, form, eligibility, including time of receipt, and acceptance of outstanding notes tendered for exchange. Determinations of the Issuer in this regard will be final and binding on all parties. The Issuer reserves the absolute right to reject any and all tenders of any particular outstanding notes not properly tendered or to not accept any particular outstanding notes if the acceptance might, in their or their counsel’s judgment, be unlawful. The Issuer also reserves the absolute right to waive any defects or irregularities as to any particular outstanding notes prior to the expiration date.
Unless waived, any defects or irregularities in connection with tenders of outstanding notes for exchange must be cured within such reasonable period of time as the Issuer determine. Neither the Issuer, the exchange agent, nor any other person will be under any duty to give notification of any defect or irregularity with respect to any tender of outstanding notes for exchange, nor will any of them incur any liability for any failure to give notification. Any outstanding notes received by the exchange agent that are not properly tendered and as to which the irregularities have not been cured or waived will be returned by the exchange agent to the tendering holder, unless otherwise provided in the letter of transmittal, promptly after the expiration date.
Book-Entry Delivery Procedures
Promptly after the date of this prospectus, the exchange agent will establish an account with respect to the outstanding notes at DTC, as book-entry transfer facilities, for purposes of the exchange offers. Any financial institution that is a participant in the book-entry transfer facility’s system may make book-entry delivery of the outstanding notes by causing the book-entry transfer facility to transfer those outstanding notes into the exchange agent’s account at the facility in accordance with the facility’s procedures for such transfer. To be timely, book-entry delivery of outstanding notes requires receipt of a confirmation of a book-entry transfer, a “book-entry confirmation,” prior to the expiration date. In addition, although delivery of outstanding notes may be effected through book-entry transfer into the exchange agent’s account at the book-entry transfer facility, the letter of transmittal or a manually signed facsimile thereof, together with any required signature guarantees and any other required documents, or an “agent’s message,” as defined below, in connection with a book-entry transfer, must, in any case, be delivered or transmitted to and received by the exchange agent at its address set forth on the cover page of the letter of transmittal prior to the expiration date to receive exchange notes for tendered outstanding notes, or the guaranteed delivery procedure described below must be complied with. Tender will not be deemed made until such documents are received by the exchange agent. Delivery of documents to the book-entry transfer facility does not constitute delivery to the exchange agent.
Holders of outstanding notes who are unable to deliver confirmation of the book-entry tender of their outstanding notes into the exchange agent’s account at the book-entry transfer facility or all other documents required by the letter of transmittal to the exchange agent on or prior to the expiration date must tender their outstanding notes according to the guaranteed delivery procedures described below.
Guaranteed Delivery Procedures
If you wish to tender your outstanding notes but your outstanding notes are not immediately available or you cannot deliver your outstanding notes, the letter of transmittal or any other required documents to the exchange agent or comply with the applicable procedures under DTC’s Automatic Tender Offer Program, prior to the expiration date, you may still tender if:
| • | the tender is made through an eligible guarantor institution; |
| • | prior to the expiration date, the exchange agent receives from such eligible guarantor institution either a properly completed and duly executed notice of guaranteed delivery, by facsimile transmission, mail, or hand delivery or a properly transmitted agent’s message and notice of guaranteed delivery, that (1) sets forth your name and address, the certificate number(s) of such outstanding notes and the principal amount |
159
Table of Contents
of outstanding notes tendered; (2) states that the tender is being made thereby; and (3) guarantees that, within three New York Stock Exchange trading days after the expiration date, the letter of transmittal, or facsimile thereof, together with the outstanding notes or a book-entry confirmation, and any other documents required by the letter of transmittal, will be deposited by the eligible guarantor institution with the exchange agent; and |
| • | the exchange agent receives the properly completed and executed letter of transmittal or facsimile thereof, as well as certificate(s) representing all tendered outstanding notes in proper form for transfer or a book-entry confirmation of transfer of the outstanding notes into the exchange agent’s account at DTC, and all other documents required by the letter of transmittal within three New York Stock Exchange trading days after the expiration date. |
Upon request, the exchange agent will send to you a notice of guaranteed delivery if you wish to tender your outstanding notes according to the guaranteed delivery procedures.
Withdrawal Rights
Except as otherwise provided in this prospectus, you may withdraw your tender of outstanding notes at any time prior to 5:00 p.m., New York City time, on the expiration date.
For a withdrawal to be effective:
| • | the exchange agent must receive a written notice, which may be by telegram, telex, facsimile or letter, of withdrawal at its address set forth below under “—Exchange Agent;” or |
| • | you must comply with the appropriate procedures of DTC’s Automated Tender Offer Program system. |
Any notice of withdrawal must:
| • | specify the name of the person who tendered the outstanding notes to be withdrawn; |
| • | identify the outstanding notes to be withdrawn, including the certificate numbers and principal amount of the outstanding notes; and |
| • | where certificates for outstanding notes have been transmitted, specify the name in which such outstanding notes were registered, if different from that of the withdrawing holder. |
If certificates for outstanding notes have been delivered or otherwise identified to the exchange agent, then, prior to the release of such certificates, you must also submit:
| • | the serial numbers of the particular certificates to be withdrawn; and |
| • | a signed notice of withdrawal with signatures guaranteed by an eligible institution unless you are an eligible guarantor institution. |
If outstanding notes have been tendered pursuant to the procedures for book-entry transfer described above, any notice of withdrawal must specify the name and number of the account at the book-entry transfer facility to be credited with the withdrawn outstanding notes and otherwise comply with the procedures of the facility. The Issuer will determine all questions as to the validity, form, and eligibility, including time of receipt of notices of withdrawal and its determination will be final and binding on all parties. Any outstanding notes so withdrawn will be deemed not to have been validly tendered for exchange for purposes of the exchange offers. Any outstanding notes that have been tendered for exchange but that are not exchanged for any reason will be returned to their holder, without cost to the holder, or, in the case of book-entry transfer, the outstanding notes will be credited to an account at the book-entry transfer facility, promptly after withdrawal, rejection of tender or termination of the exchange offers. Properly withdrawn outstanding notes may be retendered by following the procedures described under “—Procedures for Tendering Outstanding Notes” above at any time on or prior to the expiration date.
160
Table of Contents
Exchange Agent
U.S. Bank National Association has been appointed as the exchange agent for the exchange offers. The U.S. Bank National Association also acts as trustee under the indenture governing the notes. You should direct all executed letters of transmittal and all questions and requests for assistance, requests for additional copies of this prospectus or of the letters of transmittal, and requests for notices of guaranteed delivery to the exchange agent addressed as follows:
| By Mail or Overnight Courier: | By Facsimile: | By Hand Delivery: | ||
U. S. Bank National Association 60 Livingston Ave. St. Paul, Minnesota 55107 Attn: Specialized Finance | 651/495- 8158 | U. S. Bank National Association 60 Livingston Ave. 1st Floor—Bond Drop Window St. Paul, Minnesota 55107 | ||
To Confirm by Telephone: 1-800-934-6802 | ||||
If you deliver the letter of transmittal to an address other than the one set forth above or transmit instructions via facsimile other than the one set forth above, that delivery or those instructions will not be effective.
Fees and Expenses
The registration rights agreements provide that we will bear all expenses in connection with the performance of our obligations relating to the registration of the exchange notes and the conduct of the exchange offers. These expenses include registration and filing fees, accounting and legal fees and printing costs, among others. We will pay the exchange agent reasonable and customary fees for its services and reasonable out-of-pocket expenses. We will also reimburse brokerage houses and other custodians, nominees and fiduciaries for customary mailing and handling expenses incurred by them in forwarding this prospectus and related documents to their clients that are holders of outstanding notes and for handling or tendering for such clients.
We have not retained any dealer-manager in connection with the exchange offers and will not pay any fee or commission to any broker, dealer, nominee or other person, other than the exchange agent, for soliciting tenders of outstanding unregistered notes pursuant to the exchange offers.
Accounting Treatment
We will record the exchange notes in our accounting records at the same carrying value as the outstanding notes, which is the aggregate principal amount as reflected in our accounting records on the date of exchanges, as the terms of the exchange notes are substantially identical to the terms of the outstanding notes. Accordingly, we will not recognize any gain or loss for accounting purposes upon the consummation of the exchange offers. We will capitalize the expenses relating to the exchange offers.
Transfer Taxes
The Issuer and the guarantors will pay all transfer taxes, if any, applicable to the exchanges of outstanding notes under the exchange offers. The tendering holder, however, will be required to pay any transfer taxes, whether imposed on the registered holder or any other person, if:
| • | certificates representing outstanding notes for principal amounts not tendered or accepted for exchange are to be delivered to, or are to be issued in the name of, any person other than the registered holder of outstanding notes tendered; |
| • | tendered outstanding notes are registered in the name of any person other than the person signing the letter of transmittal; or |
| • | a transfer tax is imposed for any reason other than the exchange of outstanding notes under the exchange offers. |
161
Table of Contents
If satisfactory evidence of payment of such taxes is not submitted with the letter of transmittal, the amount of such transfer taxes will be billed to that tendering holder.
Holders who tender their outstanding notes for exchange will not be required to pay any transfer taxes. However, holders who instruct the Issuer to register exchange notes in the name of, or request that outstanding notes not tendered or not accepted in the exchange offers be returned to, a person other than the registered tendering holder will be required to pay any applicable transfer tax.
Consequences of Failure to Exchange
If you do not exchange your outstanding notes for exchange notes under the exchange offers, your outstanding notes will remain subject to the restrictions on transfer of such outstanding notes:
| • | as set forth in the legend printed on the outstanding notes as a consequence of the issuances of the outstanding notes pursuant to the exemptions from, or in transactions not subject to, the registration requirements of the Securities Act and applicable state securities laws; and |
| • | as otherwise set forth in the applicable offering memorandum distributed in connection with the private offerings of the outstanding notes. |
In general, you may not offer or sell your outstanding notes unless they are registered under the Securities Act or if the offer or sale is exempt from registration under the Securities Act and applicable state securities laws. Except as required by the applicable registration rights agreement, we do not intend to register resales of the outstanding notes under the Securities Act.
Other
Participating in the exchange offers are voluntary, and you should carefully consider whether to accept. You are urged to consult your financial and tax advisors in making your own decision on what action to take.
We may in the future seek to acquire untendered outstanding notes in open market or privately negotiated transactions, through subsequent exchange offers or otherwise. We have no present plans to acquire any outstanding notes that are not tendered in the exchange offers or to file a registration statement to permit resales of any untendered outstanding notes.
162
Table of Contents
General
Certain terms used in this description are defined under the subheading “—Certain Definitions.” In this description, (1) the term “Company” refers only to Apria Healthcare Group Inc. and not any of its Affiliates and (2) the terms “we,” “our” and “us” each refer to the Company and its consolidated Subsidiaries.
The Company has previously issued $700 million of 11.25% Senior Secured Notes due 2014 (Series A-1) (the “Series A-1 Notes”) and $317.5 million aggregate principal amount of its 12.375% senior secured notes due 2014 (Series A-2) (the “Series A-2 Notes”) under an indenture dated May 27, 2009 (the “Indenture”) among the Company, the Guarantors and U.S. Bank National Association, as trustee (the “Trustee”). The Series A-2 Notes are identical in all respects to the Series A-1 Notes, except that (1) the holders of the Series A-1 Notes are entitled to a priority of payment over the holders of the Series A-2 Debt in the circumstances described below under “—Security for the Notes—Priorities of Payment as between Series A-2 Debt (including the Series A-2 Notes) and Series A-1 Notes” and (2) the Series A-2 Notes have different redemption terms than the Series A-1 Notes. References herein to “Series A-2 Debt” include the Series A-2 Notes other obligations. As of the date of this prospectus, Series A-2 Debt consisted entirely of the outstanding Series A-2 Notes. Unless the context requires otherwise, references to the “Series A-1 Notes” include the outstanding Series A-1 Notes and the exchange Series A-1 Notes; references to the “Series A-2 Notes” include the outstanding Series A-2 Notes and the exchange Series A-2 Notes; and references herein to the “Notes” include the Series A-1 Notes and the Series A-2 Notes. In this description, we sometimes generically refer to each of the Series A-1 Notes and the Series A-2 Notes, separately, as a “Series of Notes.” We also sometimes generically refer to each of the Series A-1 Notes, the Series A-2 Notes and the Term Loans, separately, as a “Series of Debt.”
The following description is only a summary of the material provisions of the Indenture and the Collateral Documents and does not purport to be complete and is qualified in its entirety by reference to the provisions of the Indenture and the Collateral Documents, including the definitions therein of certain terms used below. We urge you to read the Indenture because it, not this description, defines your rights as Holders of the Series A-2 Notes. You may request copies of the Indenture and the Collateral Documents at our address set forth under the heading “Summary.”
Brief Description of the Notes
The Notes:
| • | are general secured senior obligations of the Company; |
| • | are secured, together with the Series A-1 Notes and any additional Series A-2 Debt, on a first priority lien basis by the Notes/Term Collateral and on a second priority lien basis by the ABL Collateral, in each case subject to certain liens permitted by the Indenture; |
| • | are effectively senior to all unsecured Indebtedness of the Company to the extent of the value of the collateral securing the Series A-2 Notes; |
| • | are effectively subordinated to the ABL Facility to the extent of the value of the ABL Collateral; |
| • | the Series A-2 Notes rank equally with the Series A-1 Notes and any additional Series A-2 Debt with respect to the Notes/Term Collateral and the ABL Collateral, subject to the Series A-1 Notes’ priority of payment described below; |
| • | without giving effect to security interests, rank equally in right of payment with all existing and future Senior Indebtedness of the Company; |
| • | are structurally subordinated to all existing and future Indebtedness, claims of holders of Preferred Stock and other liabilities of the Company’s Subsidiaries that do not guarantee the Notes; |
| • | are senior in right of payment to any Subordinated Indebtedness of the Company; |
163
Table of Contents
| • | are guaranteed on a senior secured basis by the Guarantors, as described under “—Guarantees”; and |
| • | are subject to registration with the SEC pursuant to a Registration Rights Agreement. |
The Series A-1 Notes are entitled to a priority of payment over the Series A-2 Debt in the circumstances described below under “—Security for the Notes—Priorities of Payment as between Series A-2 Debt (including the Series A-2 Notes) and Series A-1 Notes.”
As of the date of this prospectus, all of the Company’s wholly-owned domestic subsidiaries are “Restricted Subsidiaries.” However, under certain circumstances, we will be permitted to designate certain of our subsidiaries as “Unrestricted Subsidiaries.” Any Unrestricted Subsidiaries will not be subject to any of the restrictive covenants in the Indenture and will not guarantee the Series A-2 Notes.
Guarantees
The Guarantors, as primary obligors and not merely as sureties, have initially jointly and severally, fully and unconditionally guaranteed, on a senior secured basis, the performance and full and punctual payment when due, whether at maturity, by acceleration or otherwise, of all obligations of the Company under the Indenture and the Notes, whether for payment of principal of, any premium or interest on or Additional Interest in respect of the Notes, expenses, indemnification or otherwise, on the terms set forth in the Indenture by executing the Indenture.
All of the Restricted Subsidiaries of the Company (other than as detailed below) have initially guaranteed the Notes. None of our Foreign Subsidiaries have guaranteed or will guarantee the Notes. Each of the Guarantees of the Notes is a general senior secured obligation of each Guarantor, is secured on a first priority lien basis by the assets of such Guarantor constituting Notes/Term Collateral and on a second priority basis by the assets of such Guarantor constituting ABL Collateral, in each case subject to certain liens permitted by the Indenture, is effectively senior to all unsecured Indebtedness of such Guarantor to the extent of the value of the collateral securing such Guarantee, is effectively subordinated to such Guarantor’s Guarantee of the ABL Facility to the extent of the value of the ABL Collateral owned by such Guarantor and, without giving effect to security interests, rankspari passu in right of payment with all existing and future Senior Indebtedness of each such Guarantor, and is senior in right of payment to all existing and future Subordinated Indebtedness of each such entity. Each of the Guarantees is structurally subordinated to Indebtedness, claims of holders of Preferred Stock and other liabilities of Subsidiaries of each Guarantor that does not Guarantee the Notes.
Although all of the Company’s existing wholly-owned domestic subsidiaries Guarantee the Notes, not all of the Company’s future Subsidiaries will be required to Guarantee the Notes. In the event of a bankruptcy, liquidation or reorganization of any of these non-guarantor Subsidiaries, the non-guarantor Subsidiaries will pay the holders of their debt and their trade creditors before they will be able to distribute any of their assets to the Company. As a result, all of the existing and future liabilities of these non-guarantor Subsidiaries, including any claims of trade creditors, will be effectively senior to the Notes.
The obligations of each Guarantor under its Guarantee will be limited as necessary to prevent the Guarantee from constituting a fraudulent conveyance under applicable law. This provision may not, however, be effective to protect a Guarantee from being voided under fraudulent transfer law, or may reduce the applicable Guarantor’s obligation to an amount that effectively makes its Guarantee worthless.
Any entity that makes a payment under its Guarantee will be entitled upon payment in full of all guaranteed obligations under the Indenture to a contribution from each other Guarantor in an amount equal to such other Guarantor’s pro rata portion of such payment based on the respective net assets of all the Guarantors at the time of such payment determined in accordance with GAAP.
If a Guarantee were rendered voidable, it could be subordinated by a court to all other indebtedness (including guarantees and other contingent liabilities) of the Guarantor, and, depending on the amount of such
164
Table of Contents
indebtedness, a Guarantor’s liability on its Guarantee could be reduced to zero. See “Risk Factors—Risks Relating to the Notes—Federal and State Statutes May Allow Courts, Under Specific Circumstances, to Void the Notes and the Guarantees, Subordinate Claims in Respect of the Notes and the Guarantees and/or Require Holders of the Notes to Return Payments Received From Us.”
A Guarantee by a Guarantor shall provide by its terms that it shall be automatically and unconditionally released and discharged with respect to the Notes upon:
(1)(a) any sale, exchange or transfer (by merger or otherwise) of the Capital Stock of such Guarantor, after which the applicable Guarantor is no longer a Restricted Subsidiary, if such sale, exchange or transfer is made in compliance with the applicable provisions of the Indenture;
(b) the release or discharge of the guarantee by such Guarantor of the ABL Facility or the guarantee which resulted in the creation of such Guarantee, except a discharge or release by or as a result of payment under such guarantee;
(c) the proper designation of any Restricted Subsidiary that is a Guarantor as an Unrestricted Subsidiary in accordance with the provisions set forth under “—Certain Covenants—Limitation on Restricted Payments” and the definition of “Unrestricted Subsidiary”; or
(d) the Company exercising its legal defeasance option or covenant defeasance option with respect to the Notes as described under “—Legal Defeasance and Covenant Defeasance” or the Company’s obligations under the Indenture being discharged with respect to the Notes in accordance with the terms of the Indenture; and
(2) such Guarantor delivering to the Trustee an Officer’s Certificate and an Opinion of Counsel, each stating that all conditions precedent provided for in the Indenture relating to such transaction have been complied with.
Ranking
The payment of the principal of, premium, if any, and interest on the Notes and the payment of any Guarantee will bepari passu in right of payment with all Senior Indebtedness of the Company or the relevant Guarantor, as the case may be, including the obligations of the Company and such Guarantor under the ABL Facility, subject to the collateral and intercreditor arrangements described below.
The Notes and the Guarantees will be effectively senior in right of payment to all of the Company’s and the Guarantors’ existing and future unsecured Indebtedness to the extent of the value of the collateral securing the Notes. The Series A-2 Notes and the Guarantees rank equally with, and are secured equally with, the $700.0 million aggregate principal amount of Series A-1 Notes and any additional Series A-2 Debt, subject to the Series A-1 Notes’ priority of payment described below. The Series A-2 Notes will be effectively subordinated to all of the Company’s and each Guarantor’s existing and future Secured Indebtedness other than the Series A-1 Notes (including Indebtedness under the ABL Facility) to the extent of the value of the assets that do not constitute the Notes/Term Collateral securing such Indebtedness on a first priority lien basis. As of March 31, 2010, the Company and the Guarantors had $3.1 million principal amount of Secured Indebtedness outstanding other than the Notes, consisting of $3.1 million of existing capital leases. In addition, as of March 31, 2010, the Company had $133.9 million of available borrowing capacity under the ABL Facility and the option to raise incremental borrowing capacity under the ABL Facility of up to $25.0 million, all of which would constitute Secured Indebtedness.
Although the Indenture contains limitations on the amount of additional Indebtedness that the Company and the Guarantors may incur, under certain circumstances the amount of such Indebtedness could be substantial and, in any case, such Indebtedness may be Senior Indebtedness. The Indenture also does not limit the amount of additional Indebtedness that Holdings may incur. See “—Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock.”
165
Table of Contents
The $700.0 million of Series A-1 Notes are entitled to a priority of payment over the Series A-2 Notes and any additional Series A-2 Debt in the circumstances described below under “—Priorities of Payment as between Series A-2 Debt (including the Series A-2 Notes) and Series A-1 Notes.” The Company may issue up to an additional $300.0 million of Series A-1 Notes under the Indenture which would also be entitled to this priority of payment over the Series A-2 Notes. In addition, the Company may issue up to an additional $200.0 million of indebtedness (which may be in the form of notes or loans) which would also be entitled to this priority of payment over the Series A-2 Notes.
Paying Agent and Registrar for the Notes
The Company will maintain one or more paying agents for the Notes in the Borough of Manhattan, City of New York. The initial paying agent for the Notes is the Trustee.
The Company will also maintain a registrar with offices in the Borough of Manhattan, City of New York. The initial registrar is the Trustee. The registrar will maintain a register reflecting ownership of the Notes outstanding from time to time and will make payments on and facilitate transfers of Notes on behalf of the Company.
The Company may change the paying agents or the registrars without prior notice to the Holders. The Company or any of its Subsidiaries may act as a paying agent or registrar.
Transfer and Exchange
A Holder may transfer or exchange the Notes in accordance with the Indenture. The registrar and the Trustee may require a Holder to furnish appropriate endorsements and transfer documents in connection with a transfer of the Notes. Holders will be required to pay all taxes due on transfer. The Company will not be required to transfer or exchange any Note selected for redemption. Also, the Company will not be required to transfer or exchange any Note for a period of 15 days before a selection of the Notes to be redeemed.
Principal, Maturity and Interest
The Notes will mature on November 1, 2014. Subject to compliance with the covenant described below under the caption “—Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock,” the Company may issue (1) up to $150.0 million of additional Notes, plus (2) up to $150.0 million of additional Notes the proceeds of which will be used solely to finance acquisitions (solely to the extent the Fixed Charge Coverage Ratio following any such acquisition and debt incurrence would have been at least 3.50 to 1.00, determined on apro forma basis), in each case from time to time after this offering under the Indenture (any such additional Notes, “Additional Notes”). All Notes, including any additional Notes subsequently issued under the Indenture, will be treated as a single class for all purposes under the Indenture, including waivers, amendments, redemptions and offers to purchase. Holders of Additional Notes will share equally and ratably in the Collateral;provided that the Series A-1 Notes will be entitled to a priority of payment over the Series A-2 Debt in the circumstances described below under “—Priorities of Payment as between Series A-2 Debt (including the Series A-2 Notes) and Series A-1 Notes.” Unless the context requires otherwise, references to “Notes” for all purposes of the Indenture and this “Description of Notes” include any additional Notes that are actually issued. The Company will issue Notes in denominations of $2,000 and integral multiples of $1,000 in excess thereof.
Interest on the Series A-1 Notes will accrue at the rate of 11.25% per annum and will be payable semiannually in arrears on November 1 and May 1, commencing on November 1, 2009 to the Holders of Series A-1 Notes of record on the immediately preceding October 15 and April 15. Interest on the Series A-2 Notes will accrue at the rate of 12.375% per annum and will be payable semiannually in arrears on November 1 and May 1, commencing on November 1, 2009 to the Holders of Series A-2 Notes of record on the immediately preceding
166
Table of Contents
October 15 and April 15. Interest on the Notes will accrue from the most recent date to which interest has been paid or, if no interest has been paid, from and including the issue date of the Notes. Interest on the Notes will be computed on the basis of a 360-day year comprised of twelve 30-day months.
Additional Interest may accrue on the outstanding notes in certain circumstances pursuant to the Registration Rights Agreement. All references in the Indenture, in any context, to any interest or other amount payable on or with respect to the Notes shall be deemed to include any Additional Interest pursuant to the Registration Rights Agreement.
Principal of, premium, if any, and interest on the Notes will be payable at the office or agency of the Company maintained for such purpose within the City and State of New York or, at the option of the Company, payment of interest may be made by check mailed to the Holders of the Notes at their respective addresses set forth in the register of Holders;provided that all payments of principal, premium, if any, and interest with respect to the Notes represented by one or more global notes registered in the name of or held by DTC or its nominee will be made by wire transfer of immediately available funds to the accounts specified by the Holder or Holders thereof. Until otherwise designated by the Company, the Company’s office or agency in New York will be the office of the Trustee maintained for such purpose.
Security for the Notes
The Notes and the Guarantees will have the benefit of the Collateral, which will consist of (i) the Notes/Term Collateral (defined below) as to which the Holders of the Notes, the holders of the Additional Notes (if any) and the holders of any additional Series A-2 Debt will have a first-priority security interest (subject to Permitted Liens) and the holders of ABL Lenders Debt will have a second-priority security interest and (ii) the ABL Collateral as to which the holders of ABL Lenders Debt will have a first-priority security interest and the Holders of the Notes, the holders of Additional Notes (if any) and the holders of any additional Series A-2 Debt will have a second-priority security interest (subject to Permitted Liens). The Company and the Guarantors will be able to Incur additional Indebtedness in the future which could share in the Collateral, including up to $300 million of additional Series A-1 Notes and up to $200 million of additional indebtedness (which may be in the form of notes or loans) which will have a priority of payment over the Series A-2 Notes. The amount of all such additional Indebtedness will be limited by the covenants disclosed under “—Certain Covenants—Liens” and “—Limitation on Incurrence of Indebtedness and Issuances of Disqualified Stock.” Under certain circumstances the amount of such additional Secured Indebtedness could be significant.
Notes/Term Collateral
The Notes/Term Collateral will be pledged as collateral to the Notes/Term Collateral Agent for the benefit of the Trustee, the Notes/Term Collateral Agent, the Holders of the Notes and the holders of any additional Series A-2 Debt. The Notes and Guarantees will be secured, together with any additional Series A-2 Debt, by first-priority security interests in the Notes/Term Collateral, subject to Permitted Liens. The Notes/Term Collateral consists of (i) substantially all of the present and future tangible and intangible assets of the Company and the Guarantors, including without limitation equipment, contracts, intellectual property, real property, general intangibles, material intercompany notes and proceeds of the foregoing, and (ii) all of the Capital Stock of the Company, each Guarantor and each Restricted Subsidiary of the Company (limited, in the case of foreign subsidiaries, to 65% of the Capital Stock of each first-tier Foreign Subsidiary), in each case other than the ABL Collateral, Excluded Assets and subject to the limitations and exclusions described under “—Limitations on Stock Collateral” (collectively, the “Notes/Term Collateral”).
Initially, subject to Permitted Liens, only the Notes and any additional Series A-2 Debt will have the benefit of the first-priority security interest in the Notes/Term Collateral. Except for Indebtedness secured by Permitted Liens, no other Indebtedness incurred by the Company may share in the first-priority security interest in the Notes/Term Collateral other than any Additional Notes.
167
Table of Contents
The Company initially will grant a second-priority lien on and security interest in the Notes/Term Collateral for the benefit of the ABL Lenders Debt, which initially will consist of the loans outstanding under the ABL Facility, obligations with respect to letters of credit issued under the ABL Facility, certain hedging and cash management obligations incurred with the lenders under the ABL Facility or their affiliates and any other obligations under the ABL Facility. Any additional Indebtedness that is incurred by the Company in compliance with the terms of the Indenture may also be given a lien on and security interest in the Notes/Term Collateral (to the extent such lien constitutes a Permitted Lien) that ranks junior to the lien of the Notes and any additional Series A-2 Debt in the Notes/Term Collateral. Except as provided in the Intercreditor Agreement, holders of such junior liens will not be able to take any enforcement action with respect to the Notes/Term Collateral so long as any Notes or any additional Series A-2 Debt are outstanding.
ABL Collateral
The Notes and any additional Series A-2 Debt, will also be secured by a second-priority lien on and security interest in the ABL Collateral (subject to Permitted Liens). The ABL Collateral will consist of substantially all accounts receivable arising from the sale of inventory and other goods and services (including related contracts and contract rights, inventory, cash (other than certain cash proceeds of the Notes/Term Collateral), deposit accounts, other bank accounts and securities accounts), inventory, intercompany notes and intangible assets to the extent attached to the foregoing and the proceeds thereof, in each case held by the Company, the Guarantors and Holdings (collectively, the “ABL Collateral”). Generally, the Notes’ and any additional Series A-2 Debt’s second-priority lien on and security interest in the ABL Collateral will be terminated and automatically released if the lien on such ABL Collateral is released.
From and after the Issue Date, subject to the limitations contained under “—Certain Covenants—Liens” and the definition of “Permitted Liens,” the Company or any Guarantor may grant an additional lien on any property or asset that constitutes ABL Collateral in order to secure any obligation permitted to be incurred pursuant to the Indenture. In general, any such additional liens (other than Permitted Liens) must rank junior to the second-priority lien securing the Notes and any additional Series A-2 Debt.
Excluded Assets
Notwithstanding the foregoing, the Notes and any additional Series A-2 Debt will not be secured by a lien on Excluded Assets and will be subject to Permitted Liens.
The Notes/Term Collateral does not and will not include the following (collectively, the “Excluded Assets”):
(1) any property or assets owned by any Foreign Subsidiary,
(2) Excluded Contracts;
(3) Excluded Equipment;
(4) any interest in fee-owned real property of the Company and the Guarantors if the greater of its cost and book value is less than $5.0 million;
(5) any interest in leased real property of the Company and the Guarantors;
(6) motor vehicles and other assets subject to certificates of title;
(7) letter of credit rights;
(8) commercial tort claims of less than $20.0 million;
(9) assets requiring perfection through control agreements;
(10) pledges and security interests prohibited by law;
168
Table of Contents
(11) assets to the extent a security interest in such assets would result in material adverse tax consequences as reasonably determined by the Company; and
(12) proceeds and products from any and all of the foregoing excluded collateral described in clauses (1) through (11), unless such proceeds or products would otherwise constitute Notes/Term Collateral;
provided, however, that Excluded Assets will not include any asset which secures obligations with respect to ABL Lenders Debt. In addition, the Company and its Subsidiaries shall not be required to obtain any landlord waivers, estoppels or collateral access letters.
Limitations on Stock Collateral
The Capital Stock and other securities of a Subsidiary of the Company that are owned by the Company or any Guarantor will constitute Notes/Term Collateral only to the extent that such Capital Stock and other securities can secure the Notes and any additional Series A-2 Debt without Rule 3-16 of Regulation S-X under the Securities Act (or any other law, rule or regulation) requiring separate financial statements of such Subsidiary to be filed with the SEC (or any other governmental agency). In the event that Rule 3-16 of Regulation S-X under the Securities Act requires or is amended, modified or interpreted by the SEC to require (or is replaced with another rule or regulation, or any other law, rule or regulation is adopted, which would require) the filing with the SEC (or any other governmental agency) of separate financial statements of any Subsidiary (other than the Company) due to the fact that such Subsidiary’s Capital Stock and other securities secure the Notes or any additional Series A-2 Debt, then the Capital Stock and other securities of such Subsidiary shall automatically be deemed not to be part of the Notes/Term Collateral (but only to the extent necessary to not be subject to such requirement). In such event, the Collateral Documents may be amended or modified, without the consent of any Holder of Notes, to the extent necessary to release the security interests in the shares of Capital Stock and other securities that are so deemed to no longer constitute part of the Notes/Term Collateral.
In the event that Rule 3-16 of Regulation S-X under the Securities Act is amended, modified or interpreted by the SEC to permit (or is replaced with another rule or regulation, or any other law, rule or regulation is adopted, which would permit) such Subsidiary’s Capital Stock and other securities to secure the Notes and any additional Series A-2 Debt in excess of the amount then pledged without the filing with the SEC (or any other governmental agency) of separate financial statements of such Subsidiary, then the Capital Stock and other securities of such Subsidiary shall automatically be deemed to be a part of the Notes/ Term Collateral (but only to the extent necessary to not be subject to any such financial statement requirement). In such event, the Collateral Documents may be amended or modified, without the consent of any Holder of Notes, to the extent necessary to subject to the Liens under the Collateral Documents such additional Capital Stock and other securities.
In accordance with the limitations set forth in the two immediately preceding paragraphs, the Notes/Term Collateral includes shares of Capital Stock of Subsidiaries of the Company only to the extent that the applicable value of such Capital Stock (on a Subsidiary-by-Subsidiary basis) is less than 20% of the aggregate principal amount of the Notes outstanding. Following the Issue Date, however, the portion of the Capital Stock of Subsidiaries constituting Notes/Term Collateral may decrease or increase as described above.
Permitted Liens
The Company and the Guarantors are permitted by the Indenture to create or incur Permitted Liens. The Notes and any additional Series A-2 Debt will be effectively subordinated to existing and future secured Indebtedness and other liabilities to the extent of the Company’s or the Guarantor’s assets serving as collateral for such Permitted Liens.
In particular, the Notes will be effectively subordinated to security interests on acquired property or assets of acquired companies which are secured prior to (and not in connection with) such acquisition; such security interests generally constitute Permitted Liens. Indebtedness of Foreign Subsidiaries permitted by the Indenture
169
Table of Contents
may also be secured by security interests on the property and assets of such Foreign Subsidiaries. The Indenture also permits the Company and the Guarantors to create other Permitted Liens, including up to $200 million of liens which are senior to the liens securing the Series A-2 Notes. See “Risk Factors—Risks Relating to the Notes—Holders of the Notes May Not Be Able to Fully Realize the Value of Their Liens” and “Risk Factors—Risks Relating to the Notes—The Collateral May Not Be Valuable Enough to Satisfy All the Obligations Secured by Such Collateral.”
Collateral Documents and Certain Related Intercreditor Provisions
The Company, the Guarantors and the Notes/Term Collateral Agent (on behalf of the Trustee and the Holders of the Notes, the Series A-1 Notes and the holders of any additional Series A-2 Debt) have entered into the Collateral Documents creating and establishing the terms of the security interests that secure the Notes and the guarantees thereof and any additional Series A-2 Debt. These security interests will secure the payment and performance when due of all of the obligations of the Company and the Guarantors under the Notes, the Indenture, the Guarantees, any additional Series A-2 Debt, and the Collateral Documents, as provided in the Collateral Documents. The Trustee became the Notes/Term Collateral Agent, replacing Bank of America, N.A., upon the consummation of the offering of Series A-2 Notes in August 2009. The Trustee, the Notes/Term Collateral Agent, each Holder of the Notes and each holder of any additional Series A-2 Debt and each other holder of, or obligee in respect of, any Obligations in respect of the Notes and any additional Series A-2 Debt outstanding at such time are referred to collectively as the “Notes/Term Secured Parties.”
Intercreditor Agreement
The Company, the Guarantors, the Notes/Term Collateral Agent and the ABL Collateral Agent have entered into the Intercreditor Agreement. Although the Holders of the Notes are not party to the Intercreditor Agreement, by their acceptance of the Notes they will agree to be bound thereby. Pursuant to the terms of the Intercreditor Agreement, the Notes/Term Collateral Agent will determine the time and method by which the security interests in the Notes/Term Collateral will be enforced and the ABL Collateral Agent will determine the time and method by which the security interests in the ABL Collateral will be enforced.
The aggregate amount of the obligations secured by the ABL Collateral may, subject to the limitations set forth in the Indenture, be increased.
A portion of the obligations secured by the ABL Collateral consists or may consist of Indebtedness that is revolving in nature, and the amount thereof that may be outstanding at any time or from time to time may be increased or reduced and subsequently reborrowed and such obligations may, subject to the limitations set forth in the Indenture, be increased, extended, renewed, replaced, restated, supplemented, restructured, repaid, refunded, refinanced or otherwise amended or modified from time to time, all without affecting the subordination of the liens held by the Holders or the provisions of the Intercreditor Agreement defining the relative rights of the parties thereto. The lien priorities provided for in the Intercreditor Agreement shall not be altered or otherwise affected by any amendment, modification, supplement, extension, increase, replacement, renewal, restatement or refinancing of either the obligations secured by the ABL Collateral or the obligations secured by the Notes/Term Collateral, by the release of any Collateral or of any guarantees securing any secured obligations or by any action that any representative or secured party may take or fail to take in respect of any Collateral.
No Action With Respect to the ABL Collateral
The Intercreditor Agreement provides that none of the Notes/Term Secured Parties may commence any judicial or nonjudicial foreclosure proceedings with respect to, seek to have a trustee, receiver, liquidator or similar official appointed for or over, attempt any action to take possession of, exercise any right, remedy or power with respect to, or otherwise take any action to enforce its interest in or realize upon, or take any other action available to it in respect of, the ABL Collateral under any Collateral Document, applicable law or
170
Table of Contents
otherwise, at any time when the ABL Collateral is subject to any first-priority security interest and any ABL Lenders Debt secured by such ABL Collateral remains outstanding or any commitment to extend credit that would constitute such ABL Lenders Debt remains in effect. Only the ABL Collateral Agent shall be entitled to take any such actions or exercise any such remedies. Notwithstanding the foregoing, the Notes/Term Collateral Agent may, but shall have no obligation to, take all such actions it deems necessary to perfect or continue the perfection of the second-priority security interest in the ABL Collateral of the Holders of the Notes and any additional Series A-2 Debt. The ABL Collateral Agent will be subject to similar restrictions with respect to its ability to enforce the second-priority security interest in the Notes/Term Collateral held by holders of ABL Lenders Debt.
No Duties of ABL Collateral Agent
The Intercreditor Agreement provides that neither the ABL Collateral Agent nor any holder of any ABL Lenders Debt secured by any ABL Collateral will have any duties or other obligations to any Notes/Term Secured Party with respect to the ABL Collateral, other than to transfer to the Notes/Term Collateral Agent any proceeds of any such ABL Collateral in which the Notes/Term Collateral Agent continues to hold a security interest remaining following any sale, transfer or other disposition of such ABL Collateral (in each case, unless the lien on all such ABL Collateral of the Holders of the Notes and any additional Series A-2 Debt is terminated and released prior to or concurrently with such sale, transfer, disposition, payment or satisfaction), the payment and satisfaction in full of such ABL Lenders Debt and the termination of any commitment to extend credit that would constitute such ABL Lenders Debt, or, if the ABL Collateral Agent is in possession of all or any part of such ABL Collateral after such payment and satisfaction in full and termination, such ABL Collateral or any part thereof remaining, in each case without representation or warranty on the part of the ABL Collateral Agent or any such holder of ABL Lenders Debt. In addition, the Intercreditor Agreement will further provide that, until the ABL Lenders Debt secured by any ABL Collateral shall have been paid and satisfied in full and any commitment to extend credit that would constitute ABL Lenders Debt secured thereby shall have been terminated, the ABL Collateral Agent will be entitled, for the benefit of the holders of such ABL Lenders Debt, to sell, transfer or otherwise dispose of or deal with such ABL Collateral without regard to any second-priority security interest therein or any rights to which any Notes/Term Secured Party would otherwise be entitled as a result of such second-priority security interest. Without limiting the foregoing, the Notes/Term Collateral Agent has agreed in the Intercreditor Agreement and each Holder of the Notes and any additional Series A-2 Debt has or will agree by its acceptance of the Notes and any additional Series A-2 Debt, as applicable, that neither the ABL Collateral Agent nor any holder of any ABL Lenders Debt secured by any ABL Collateral will have any duty or obligation first to marshal or realize upon the ABL Collateral, or to sell, dispose of or otherwise liquidate all or any portion of the ABL Collateral, in any manner that would maximize the return to the Notes/Term Secured Parties, notwithstanding that the order and timing of any such realization, sale, disposition or liquidation may affect the amount of proceeds actually received by the Notes/Term Secured Parties from such realization, sale, disposition or liquidation. The Intercreditor Agreement has similar provisions regarding the duties owed to the ABL Collateral Agent and the holders of any ABL Lenders Debt by the Notes/ Term Secured Parties with respect to the Notes/Term Collateral.
The Intercreditor Agreement additionally provides that the Notes/Term Collateral Agent waives, and each Holder of the Notes and any additional Series A-2 Debt has or will waive, by its acceptance of the Notes and any additional Series A-2 Debt, any claim that may be had against the ABL Collateral Agent or any holder of any ABL Lenders Debt arising out of (i) any actions which the ABL Collateral Agent or such holder of ABL Lenders Debt take or omit to take (including, actions with respect to the creation, perfection or continuation of Liens on any Collateral, actions with respect to the foreclosure upon, sale, release or depreciation of, or failure to realize upon, any of the Collateral and actions with respect to the collection of any claim for all or any part of the ABL Lenders Debt from any account debtor, guarantor or any other party) in accordance with the documents governing any such ABL Lenders Debt or any other agreement related thereto or to the collection of such ABL Lenders Debt or the valuation, use, protection or release of any security for such ABL Lenders Debt, (ii) any election by the ABL Collateral Agent or such holder of ABL Lenders Debt, in any proceeding instituted under
171
Table of Contents
Title 11 of the United States Code of the application of Section 111 1 (b) of Title 11 of the United States Code or (iii) any borrowing of, or grant of a security interest or administrative expense priority under Section 364 of Title 11 of the United States Code to, the Company or any of its Subsidiaries as debtor-in-possession. The ABL Collateral Agent and holders of ABL Lenders Debt has agreed to waive similar claims with respect to the actions of any of the Notes/Term Secured Parties.
No Interference; Payment Over; Reinstatement
The Notes/Term Collateral Agent has agreed in the Intercreditor Agreement and each Holder of the Notes and each holder of any additional Series A-2 Debt will agree by its acceptance of the Notes and any additional Series A-2 Debt, as applicable, that:
| • | it will not take or cause to be taken any action the purpose or effect of which is, or could be, to make any Lien that the Notes/Term Collateral Agent has (on behalf of the Holders of the Notes and any additional Series A-2 Debt) on the ABL Collateralpari passu with, or to give the Notes/Term Collateral Agent, the Trustee, the Holders of the Notes and any additional Series A-2 Debt any preference or priority relative to, any Lien that the holders of any ABL Lenders Debt secured by any ABL Collateral have with respect to such ABL Collateral; |
| • | it will not challenge or question in any proceeding the validity or enforceability of any first-priority security interest in the ABL Collateral, the validity, attachment, perfection or priority of any lien held by the holders of any ABL Lenders Debt secured by any ABL Collateral, or the validity or enforceability of the priorities, rights or duties established by or other provisions of the Intercreditor Agreement; |
| • | it will not take or cause to be taken any action the purpose or intent of which is, or could be, to interfere, hinder or delay, in any manner, whether by judicial proceedings or otherwise, any sale, transfer or other disposition of the ABL Collateral by the ABL Collateral Agent or the holders of any ABL Lenders Debt secured by such ABL Collateral; |
| • | it will have no right to (A) direct the ABL Collateral Agent or any holder of any ABL Lenders Debt secured by any ABL Collateral to exercise any right, remedy or power with respect to such ABL Collateral or (B) consent to the exercise by the ABL Collateral Agent or any holder of any ABL Lenders Debt secured by the ABL Collateral of any right, remedy or power with respect to such ABL Collateral; |
| • | it will not institute any suit or assert in any suit, bankruptcy, insolvency or other proceeding any claim against the ABL Collateral Agent or any holder of any ABL Lenders Debt secured by any ABL Collateral seeking damages from or other relief by way of specific performance, instructions or otherwise with respect to, and neither the ABL Collateral Agent nor any holders of any ABL Lenders Debt secured by any ABL Collateral will be liable for, any action taken or omitted to be taken by the ABL Collateral Agent or such lenders with respect to such ABL Collateral; |
| • | it will not seek, and will waive any right, to have any ABL Collateral or any part thereof marshaled upon any foreclosure or other disposition of such ABL Collateral; and |
| • | it will not attempt, directly or indirectly, whether by judicial proceedings or otherwise, to challenge the enforceability of any provision of the Intercreditor Agreement. |
The ABL Collateral Agent and the holders of ABL Lenders Debt have agreed to similar limitations with respect to their rights in the Notes/Term Collateral and their ability to bring a suit against the Notes/Term Collateral Agent, the Holders of the Notes or any additional Series A-2 Debt.
The Notes/Term Collateral Agent has agreed in the Intercreditor Agreement and each Holder of the Notes and any additional Series A-2 Debt will agree by its acceptance of the Notes and any additional Series A-2 Debt, as applicable, that if it obtains possession of the ABL Collateral or realizes any proceeds or payment in respect of the ABL Collateral, pursuant to any Collateral Document or by the exercise of any rights available to it under
172
Table of Contents
applicable law or in any bankruptcy, insolvency or similar proceeding or through any other exercise of remedies, at any time when any ABL Lenders Debt secured or intended to be secured by such ABL Collateral remains outstanding or any commitment to extend credit that would constitute ABL Lenders Debt secured or intended to be secured by such ABL Collateral remains in effect, then it will hold such ABL Collateral, proceeds or payment in trust for the ABL Collateral Agent and the holders of any ABL Lenders Debt secured by such ABL Collateral and transfer such ABL Collateral, proceeds or payment, as the case may be, to the ABL Collateral Agent. The Notes/Term Collateral Agent, each Holder of the Notes and any additional Series A-2 Debt will further agree that if, at any time, all or part of any payment with respect to any ABL Lenders Debt secured by any ABL Collateral previously made shall be rescinded for any reason whatsoever, it will promptly pay over to the ABL Collateral Agent any payment received by it in respect of any such ABL Collateral and shall promptly turn any such ABL Collateral then held by it over to the ABL Collateral Agent, and the provisions set forth in the Intercreditor Agreement will be reinstated as if such payment had not been made, until the payment and satisfaction in full of such ABL Lenders Debt. The ABL Collateral Agent and the holders of ABL Lenders Debt will be subject to similar limitations with respect to the Notes/Term Collateral and any proceeds or payments in respect of any Notes/Term Collateral.
Entry Upon Premises by ABL Collateral Agent and Holders of ABL Lenders Debt
The Intercreditor Agreement provides that if the ABL Collateral Agent takes any enforcement action with respect to the ABL Collateral, the Notes/Term Secured Parties (i) will cooperate with the ABL Collateral Agent in its efforts to enforce its security interest in the ABL Collateral and to finish any work-in-process and assemble the ABL Collateral, (ii) will not hinder or restrict in any respect the ABL Collateral Agent from enforcing its security interest in the ABL Collateral or from finishing any work-in-process or assembling the ABL Collateral, and (iii) will, subject to the rights of any landlords under real estate leases, permit the ABL Collateral Agent, its employees, agents, advisers and representatives, at the sole cost and expense of the ABL Collateral Agent and the holders of ABL Lenders Debt, to enter upon and use the Notes/Term Collateral (including (x) equipment, processors, computers and other machinery related to the storage or processing of records, documents or files and (y) intellectual property), for a period not to exceed 180 days after the taking of such enforcement action, for purposes of(A) assembling and storing the ABL Collateral and completing the processing of and turning into finished goods of any ABL Collateral consisting of work-in-process, (B) selling any or all of the ABL Collateral located on such Notes/Term Collateral, whether in bulk, in lots or to customers in the ordinary course of business or otherwise, (C) removing any or all of the ABL Collateral located on such Notes/Term Collateral, or (D) taking reasonable actions to protect, secure, and otherwise enforce the rights of the ABL Collateral Agent and the holders of ABL Lenders Debt in and to the ABL Collateral;provided, however, that nothing contained in the Intercreditor Agreement will restrict the rights of the Notes/Term Collateral Agent from selling, assigning or otherwise transferring any Notes/Term Collateral prior to the expiration of such 180-day period if the purchaser, assignee or transferee thereof agrees to be bound by the provisions of the Intercreditor Agreement. If any stay or other order prohibiting the exercise of remedies with respect to the ABL Collateral has been entered by a court of competent jurisdiction, such 180-day period shall be tolled during the tendency of any such stay or other order. If the ABL Collateral Agent conducts a public auction or private sale of the ABL Collateral at any of the real property included within the Notes/Term Collateral, the ABL Collateral Agent shall provide the Notes/Term Collateral Agent with reasonable notice and use reasonable efforts to hold such auction or sale in a manner which would not unduly disrupt the Notes/Term Collateral Agent’s use of such real property.
During the period of actual occupation, use or control by the ABL Collateral Agent or the holders of ABL Lenders Debt or their agents or representatives of any Notes/Term Collateral, the ABL Collateral Agent and the holders of ABL Lenders Debt will (i) be responsible for the ordinary course third-party expenses related thereto, including costs with respect to heat, light, electricity, water and real property taxes with respect to that portion of any premises so used or occupied, and (ii) be obligated to repair at their expense any physical damage to such Notes/Term Collateral or other assets or property resulting from such occupancy, use or control, and to leave such Notes/Term Collateral or other assets or property in substantially the same condition as it was at the commencement of such occupancy, use or control, ordinary wear and tear excepted. The ABL Collateral Agent
173
Table of Contents
and the holders of ABL Lenders Debt agree to pay, indemnify and hold the Notes/Term Collateral Agent harmless from and against any third-party liability resulting from the gross negligence or willful misconduct of the ABL Collateral Agent or any of its agents, representatives or invitees in its or their operation of such facilities. In the event, and only in the event, that in connection with its use of some or all of the premises constituting Notes/Term Collateral, the ABL Collateral Agent requires the services of any employees of the Company or any of its Subsidiaries, the ABL Collateral Agent shall pay directly to any such employees the appropriate, allocated wages of such employees, if any, during the time periods that the ABL Collateral Agent requires their services. Notwithstanding the foregoing, in no event shall the ABL Collateral Agent or the holders of ABL Lenders Debt have any liability to the Notes/Term Secured Parties pursuant to the Intercreditor Agreement as a result of any condition (including any environmental condition, claim or liability) on or with respect to the Notes/Term Collateral existing prior to the date of the exercise by the ABL Collateral Agent or the holders of ABL Lenders Debt of their rights under the Intercreditor Agreement and the ABL Collateral Agent and the holders of ABL Lenders Debt will not have any duty or liability to maintain the Notes/Term Collateral in a condition or manner better than that in which it was maintained prior to the use thereof by them, or for any diminution in the value of the Notes/Term Collateral that results solely from ordinary wear and tear resulting from the use of the Notes/Term Collateral by such persons in the manner and for the time periods specified under the Intercreditor Agreement. Without limiting the rights granted under the Intercreditor Agreement, the ABL Collateral Agent and the holders of ABL Lenders Debt will cooperate with the Notes/Term Secured Parties in connection with any efforts made by the Notes/Term Secured Parties to sell the Notes/Term Collateral.
Agreements With Respect to Bankruptcy or Insolvency Proceedings
If the Company or any of its Subsidiaries becomes subject to a case under Title 11 of the United States Code, as amended (the “Bankruptcy Code”) and, as debtor(s)-in-possession, moves for approval of financing (“DIP Financing”) to be provided by one or more lenders (the “DIP Lenders”) under Section 364 of the Bankruptcy Code or the use of cash collateral with the consent of the DIP Lenders under Section 363 of the Bankruptcy Code, the Notes/Term Collateral Agent has agreed in the Intercreditor Agreement and each Holder of the Notes and each holder of any additional Series A-2 Debt has or will agree by its acceptance of the Notes and additional Series A-2 Debt, as applicable, that it will raise no objection to any such financing or to the Liens on the ABL Collateral securing the same (“DIP Financing Liens”) or to any use of cash collateral that constitutes ABL Collateral, unless the ABL Collateral Agent or the holders of any ABL Lenders Debt secured by such ABL Collateral oppose or object to such DIP Financing or such DIP Financing Liens or use of such cash collateral (and, to the extent that such DIP Financing Liens are senior to, or rankpari passu with, the Liens of such ABL Lenders Debt in such ABL Collateral, the Notes/Term Collateral Agent will, for themselves and on behalf of the Holders of the Notes and the holders of any additional Series A-2 Debt, subordinate the liens of the Notes/Term Secured Parties in such ABL Collateral to the liens of the ABL Lenders Debt in such ABL Collateral and the DIP Financing Liens), so long as the Notes/Term Secured Parties retain liens on all the Notes/Term Collateral, including proceeds thereof arising after the commencement of such proceeding, with the same priority as existed prior to the commencement of the case under the Bankruptcy Code. The ABL Collateral Agent and the holders of ABL Lenders Debt will agree to similar provisions with respect to any DIP Financing.
The Notes/Term Collateral Agent has agreed in the Intercreditor Agreement and each Holder of the Notes and each holder of any additional Series A-2 Debt will agree by its acceptance of the Notes and additional Series A-2 Debt, as applicable, that it will not object to or oppose a sale or other disposition of any ABL Collateral (or any portion thereof) under Section 363 of the Bankruptcy Code or any other provision of the Bankruptcy Code if the ABL Collateral Agent and the holders of ABL Lenders Debt shall have consented to such sale or disposition of such ABL Collateral. The ABL Collateral Agent and the holders of ABL Lenders Debt have agreed to similar limitations with respect to their right to object to a sale of Notes/Term Collateral.
Insurance
Unless and until written notice by the ABL Collateral Agent to the Notes/Term Collateral Agent that the obligations under the ABL Facility have been paid in full and all commitments to extend credit under the ABL
174
Table of Contents
Facility shall have been terminated, as between the ABL Collateral Agent, on the one hand, and the Notes/Term Collateral Agent, as the case may be, on the other hand, only the ABL Collateral Agent will have the right (subject to the rights of the Grantors under the security documents related to the ABL Facility, the Term Loan Facility and the Indenture and the Collateral Documents) to adjust or settle any insurance policy or claim covering or constituting ABL Collateral in the event of any loss thereunder and to approve any award granted in any condemnation or similar proceeding affecting the ABL Collateral. Unless and until written notice by the Trustee and the Notes/Term Collateral Agent to the ABL Collateral Agent that the obligations under the Indenture, the Notes and any additional Series A-2 Debt have been paid in full, as between the ABL Collateral Agent, on the one hand, and the Notes/Term Collateral Agent, as the case may be, on the other hand, only the Notes/Term Collateral Agent will have the right (subject to the rights of the Grantors under the security documents related to the ABL Facility, the Term Loan Facility and the Indenture and the Collateral Documents) to adjust or settle any insurance policy covering or constituting Notes/Term Collateral in the event of any loss thereunder and to approve any award granted in any condemnation or similar proceeding solely affecting the Notes/Term Collateral. To the extent that an insured loss covers or constitutes both ABL Collateral and Notes/ Term Collateral, then the ABL Collateral Agent and the Notes/Term Collateral Agent will work jointly and in good faith to collect, adjust or settle (subject to the rights of the Grantors under the security documents related to the ABL Facility, the Term Loan Facility and the Indenture and the Collateral Documents) under the relevant insurance policy.
Refinancings of the ABL Facility, the Notes and any additional Series A-2 Debt
The obligations under the ABL Facility, the obligations under the Indenture and the Notes and the obligations under any additional Series A-2 Debt may be refinanced or replaced, in whole or in part, in each case, without notice to, or the consent (except to the extent a consent is otherwise required to permit the refinancing transaction under the ABL Facility or any security document related thereto, the Term Loan Facility, the Indenture or the Collateral Documents) of the ABL Collateral Agent or any holder of ABL Lenders Debt or any Notes/Term Secured Party, all without affecting the Lien priorities provided for in the Intercreditor Agreement;provided, however, that the holders of any such refinancing or replacement indebtedness (or an authorized agent or trustee on their behalf) bind themselves in writing to the terms of the Intercreditor Agreement pursuant to such documents or agreements (including amendments or supplements to the Intercreditor Agreement) as the ABL Collateral Agent or the Notes/Term Collateral Agent, as the case may be, shall reasonably request and in form and substance reasonably acceptable to the ABL Collateral Agent or the Notes/Term Collateral Agent, as the case may be.
In connection with any refinancing or replacement contemplated by the foregoing paragraph, the Intercreditor Agreement may be amended at the request and sole expense of the Company, and without the consent of either the ABL Collateral Agent or the Notes/Term Collateral Agent, (a) to add parties (or any authorized agent or trustee therefor) providing any such refinancing or replacement indebtedness, (b) to establish that Liens on any Notes/Term Collateral securing such refinancing or replacement Indebtedness shall have the same priority as the Liens on any Notes/Term Collateral securing the Indebtedness being refinanced or replaced and (c) to establish that the Liens on any ABL Collateral securing such refinancing or replacement indebtedness shall have the same priority as the Liens on any ABL Collateral securing the Indebtedness being refinanced or replaced, all on the terms provided for herein immediately prior to such refinancing or replacement.
Use of Proceeds of ABL Collateral
After the satisfaction of all obligations under any ABL Lenders Debt secured by ABL Collateral and the termination of all commitments to extend credit that would constitute ABL Lenders Debt secured or intended to be secured by any ABL Collateral, the Trustee and the Notes/Term Collateral Agent, in accordance with the terms of the Indenture and the Collateral Documents, will distribute all cash proceeds (after payment of the costs of enforcement and collateral administration, including any amounts owed to the Trustee in its capacity as Trustee or Notes/Term Collateral Agent) of the ABL Collateral received by it under the Collateral Documents for the ratable benefit of the Holders of the Notes and any Additional Notes and any additional Series A-2 Debt.
175
Table of Contents
Subject to the terms of the Collateral Documents, the Company and the Guarantors will have the right to remain in possession and retain exclusive control of the Collateral securing the Notes and any additional Series A-2 Debt (other than any cash, securities, obligations and Cash Equivalents constituting part of the Collateral and deposited with the Notes/Term Collateral Agent or the ABL Collateral Agent in accordance with the provisions of the Collateral Documents and other than as set forth in the Collateral Documents), to freely operate the Collateral and to collect, invest and dispose of any income there from.
Release of Collateral
The Company and the Guarantors will be entitled to the releases of property and other assets included in the Collateral from the Liens securing the Notes under any one or more of the following circumstances:
| • | to enable the disposition of such property or assets to the extent not prohibited under the covenant described under “—Repurchase at the Option of Holders—Asset Sales”; |
| • | in the case of a Guarantor that is released from its Guarantee, the release of the property and assets of such Guarantor; or |
| • | as described under “—Amendment, Supplement and Waiver” below. |
Subject to the provisions contained in the Intercreditor Agreement, in general the second-priority lien on the ABL Collateral securing the Notes and any additional Series A-2 Debt will remain in full force and effect notwithstanding the termination and repayment in full of the ABL Facility, subject to certain exceptions.
The second-priority lien on the ABL Collateral securing the Notes and any additional Series A-2 Debt will terminate and be released automatically if the first-priority liens on the ABL Collateral are released by the ABL Collateral Agent (unless, at the time of such release of such first-priority liens, an Event of Default shall have occurred and be continuing under the Indenture). Notwithstanding the existence of an Event of Default, the second-priority lien on the ABL Collateral securing the Notes and any additional Series A-2 Debt shall also terminate and be released automatically to the extent the first-priority liens on the ABL Collateral are released by the ABL Collateral Agent in connection with a sale, transfer or disposition of ABL Collateral that is either not prohibited under the Indenture and the Series A-2 Debt or occurs in connection with the foreclosure of, or other exercise of remedies with respect to, such ABL Collateral by the ABL Collateral Agent (except with respect to any proceeds of such sale, transfer or disposition that remain after satisfaction in full of the ABL Lenders Debt). The liens on the Collateral securing the Notes and any additional Series A-2 Debt that otherwise would have been released pursuant to the first sentence of this paragraph but for the occurrence and continuation of an Event of Default will be released when such Event of Default and all other Events of Default under the Indenture and the Series A-2 Debt cease to exist.
The security interests in all Collateral securing the Notes also will be released upon (i) payment in full of the principal of, together with accrued and unpaid interest (including additional interest, if any) on, the Notes and all other obligations related thereto under the Indenture, the Guarantees under the Indenture and the Collateral Documents that are due and payable at or prior to the time such principal, together with accrued and unpaid interest (including additional interest, if any), are paid or (ii) a legal defeasance or covenant defeasance under the Indenture as described below under “—Legal Defeasance and Covenant Defeasance” or a discharge of the Indenture as described under “—Satisfaction and Discharge.”
No Impairment of Security Interests
Subject to the rights of the holders of Permitted Liens, neither the Company nor any of its Restricted Subsidiaries is permitted to take any action, or knowingly or negligently omit to take any action, which action or omission would or could reasonably be expected to have the result of materially impairing the security interest with respect to the Collateral for the benefit of the Trustee and Holders.
176
Table of Contents
The Indenture governing the Notes provides that any release of Collateral in accordance with the provisions of the Indenture governing the Notes and the Collateral Documents will not be deemed to impair the security under the Indenture governing the Notes and that any Person may rely on such provision in delivering a certificate requesting release so long as all other provisions of the Indenture governing the Series A-2 Notes with respect to such release have been complied with.
In addition, the Company will not amend, modify or supplement, or permit or consent to any amendment, modification or supplement of, the Collateral Documents in any manner that would be adverse to the Holders of the Notes in any material respect, except as permitted under “—Amendment, Supplement and Waiver.”
Sufficiency of Notes/Term Collateral
In the event of foreclosure on the Notes/Term Collateral, the proceeds from the sale of the Collateral may not be sufficient to satisfy in full the Company’s obligations under the Notes, any additional Series A-2 Debt and the ABL Lenders Debt. The amount to be received upon such a sale would be dependent on numerous factors, including but not limited to the timing and the manner of the sale. In addition, the book value of the Notes/Term Collateral should not be relied on as a measure of realizable value for such assets. By its nature, the book value of certain portions of the Notes/Term Collateral may have to be greatly discounted when ascertaining its marketable value and portions of the Notes/Term Collateral may be illiquid and may have no readily ascertainable market value at all. In particular, the Notes/Term Collateral (including intellectual property) is generally more illiquid than the ABL Collateral (receivables and inventory). Accordingly, there can be no assurance that the Notes/Term Collateral can be sold in a short period of time in an orderly manner. A significant portion of the Notes/Term Collateral includes assets that may only be usable, and thus retain value, as part of the existing operating business of the Company and its subsidiaries. Accordingly, any such sale of the Notes/Term Collateral separate from the sale of certain of the operating businesses of the Company and its subsidiaries may not be feasible or of significant value.
Certain Bankruptcy Limitations
The right of the Notes/Term Collateral Agent to repossess and dispose of the Notes/Term Collateral upon the occurrence of an Event of Default would be significantly impaired by applicable bankruptcy law in the event that a bankruptcy case were to be commenced by or against the Company or any of the Guarantors prior to the Notes/Term Collateral Agent having repossessed and disposed of the Notes/Term Collateral. Upon the commencement of a case for relief under the Bankruptcy Code, a secured creditor such as the Notes/Term Collateral Agent is prohibited from repossessing its security from a debtor in a bankruptcy case, or from disposing of security repossessed from the debtor, without bankruptcy court approval. Moreover, the Bankruptcy Code permits the debtor to continue to retain and use Notes/Term Collateral even though the debtor is in default under the applicable debt instrumentsprovided that the secured creditor is given adequate protection. The meaning of the term “adequate protection” may vary according to the circumstances, but it is intended in general to protect the value of the secured creditor’s interest in the Notes/Term Collateral and may include cash payments or the granting of additional security, if and at such times as the court in its discretion determines, for any diminution in the value of the Notes/Term Collateral as a result of the stay of repossession or disposition as a result of the automatic stay under the Bankruptcy Code or any use of the Notes/Term Collateral by the debtor during the pendency of the bankruptcy case. A bankruptcy court may determine that a secured creditor may not require compensation for a diminution in the value of the Notes/Term Collateral if the value of the Notes/Term Collateral exceeds the debt it secures. In addition, a bankruptcy court may determine not to provide cash payments as adequate protection to a secured creditor if (among other reasons) the bankruptcy court determines that the amount due under the Notes exceeds the value of the Notes/Term Collateral.
In view of the broad equitable powers of a bankruptcy court, it is impossible to predict how long payments under the Notes could be delayed following commencement of a bankruptcy case (to the extent such payments are made during the pendency of the bankruptcy case), whether or when the Notes/Term Collateral Agent could repossess or dispose of the Collateral, the value of the Notes/Term Collateral at the time of the bankruptcy
177
Table of Contents
petition or whether or to what extent Holders of the Notes would be compensated for any delay in payment or loss of value of the Notes/Term Collateral through the requirement of “adequate protection.” Any disposition of the Notes/Term Collateral during a bankruptcy case would also require permission from the bankruptcy court. Furthermore, in the event a bankruptcy court determines the value of the Collateral is not sufficient to repay all amounts due on the Notes, the claims of the Holders of the Notes in the bankruptcy case would be bifurcated into secured and unsecured components: they would hold secured claims to the extent of the value of the Notes/Term Collateral to which the Holders of the Notes are entitled, and unsecured claims with respect to such shortfall. The Bankruptcy Code only permits the payment and/or accrual of post-petition interest, costs and attorney’s fees to a secured creditor during a debtor’s bankruptcy case to the extent the value of the Notes/Term Collateral is determined by the bankruptcy court to exceed the aggregate outstanding principal amount of the obligations secured by the Notes/Term Collateral. To the extent the Holders of the Notes are determined to be undersecured, interest accrual under the Notes would cease as of the date of the bankruptcy filing.
In addition, the Notes/Term Collateral Agent may need to evaluate the impact of the potential liabilities before determining to foreclose on the secured property because lenders that hold a security interest in real property may be held liable under environmental laws for the costs of remediating or preventing release or threatened releases of hazardous substances at the secured property. In this regard, the Notes/Term Collateral Agent may decline to foreclose on the Notes/Term Collateral or exercise remedies available if it does not receive indemnification to its satisfaction from the Holders of the Notes and the holders of any additional Series A-2 Debt. Finally, the Notes/Term Collateral Agent’s ability to foreclose on the Notes/Term Collateral on behalf of Holders of the Notes and holders of any additional Series A-2 Debt may be subject to lack of perfection, the consent of third parties, prior liens and practical problems associated with the realization of the Notes/Term Collateral Agent’s security interest in the Notes/Term Collateral.
Compliance with Trust Indenture Act
The Trust Indenture Act will become applicable to the Indenture upon the qualification of the Indenture under the Trust Indenture Act, which will occur at such time as the Notes have been registered under the Securities Act. The Indenture provides that the Company will comply with the provisions of §314 of the Trust Indenture Act to the extent applicable. To the extent applicable, the Company will cause §313(b) of the Trust Indenture Act, relating to reports, and §314(d) of the Trust Indenture Act, relating to the release of property or securities subject to the Lien of the Collateral Documents, to be complied with. Any certificate or opinion required by §314(d) of the Trust Indenture Act may be made by an officer or legal counsel, as applicable, of the Company except in cases where §314(d) of the Trust Indenture Act requires that such certificate or opinion be made by an independent Person, which Person will be an independent engineer, appraiser or other expert selected by or reasonably satisfactory to the Trustee. Notwithstanding anything to the contrary in this paragraph, the Company will not be required to comply with all or any portion of §314(d) of the Trust Indenture Act if it determines, in good faith based on the written advice of counsel, a copy of which written advice shall be provided to the Trustee, that under the terms of §314(d) of the Trust Indenture Act or any interpretation or guidance as to the meaning thereof of the SEC and its staff, including “no action” letters or exemptive orders, all or any portion of §314(d) of the Trust Indenture Act is inapplicable to any release or series of releases of Collateral.
Priorities of Payment as between Series A-2 Debt (including the Series A-2 Notes) and Series A-1 Notes
The priorities of payment between the Series A-2 Debt (including the Series A-2 Notes) and the Series A-1 Notes is governed by an Intercreditor and Collateral Agency Agreement, dated as of May 27, 2009, among the Company, Banc of America Bridge LLC, as bridge loan agent (the “Bridge Loan Agent”), U.S. Bank National Association, as indenture trustee, and Bank of America, N.A., as Notes/Term Collateral Agent. This agreement describes, among other things, the obligations, powers and duties of the Notes/Term Collateral Agent, actions and voting by the Series A-2 Debt and Series A-1 Notes, the exercise of remedies, and the application of collateral proceeds.
178
Table of Contents
Voting as Single Class
The Series A-2 Notes, the Series A-1 Notes and any Term Loans then outstanding will generally vote together as a single class for all purposes to the extent described below under “Events of Default” and “Amendment, Modification and Waiver.”
Waterfall of Payment Following Acceleration or in Bankruptcy
The Series A-1 Notes shall be entitled to a priority of payment over the Series A-2 Debt in the circumstances and to the extent described below. Any amount received by the Trustee or the Notes/Term Collateral Agent from the Company or any Guarantor (or from proceeds of any Notes/Term Collateral or ABL Collateral) following any acceleration of the obligations under the Series A-1 Notes or Series A-2 Debt or any bankruptcy or insolvency Event of Default with respect to the Company or any Significant Subsidiary under the Series A-1 Notes or Series A-2 Debt, whether received from the proceeds of an asset sale, reorganization, liquidation, sale pursuant to Section 363 of the Bankruptcy Code, adequate protection payments, or otherwise, shall be applied:
(i)first, to the payment of all reasonable and documented costs and expenses incurred by the Trustee, the Bridge Loan Agent or the Notes/Term Collateral Agent in connection with any collection or sale or otherwise in connection with the Indenture, the Term Loan Facility or any Collateral Document or arrangement in connection therewith, including all court costs and the reasonable fees and expenses of its agents and legal counsel and any other reasonable and documented costs or expenses incurred in connection with the exercise of any right or remedy under the Indenture or Term Loan Facility or any Collateral Document or arrangement in connection therewith (and, if there shall be a shortfall in the amount available pursuant to this clause, to pay all amounts due under this clause on a pro rata basis taking into account all amounts due under this clause (including on account of fees, expenses or otherwise, as applicable));
(ii) second, to the holders of the Series A-1 Notes, an amount equal to all obligations, including accrued and unpaid interest outstanding on or prior to the acceleration or filing date, owing to them in respect of the Series A-1 Notes on the date of any payment or other distribution or other receipt of proceeds (other than any amounts calculated in respect of post-petition interest, including amounts payable as “adequate protection”) (and, if there shall be a shortfall in the amount available pursuant to this clause, to pay all amounts due under this clause on a pro rata basis taking into account all amounts due under this clause (including on account of principal, interest, fees, expenses or otherwise, as applicable));
(iii)third, to the holders of the Series A-2 Debt, an amount equal to all obligations, including accrued and unpaid interest outstanding on or prior to the acceleration or filing date, owing to them in respect of the Series A-2 Debt on the date of any payment or other distribution or other receipt of proceeds (other than any amounts calculated in respect of post-petition interest, including amounts payable as “adequate protection”) (and, if there shall be a shortfall in the amount available pursuant to this clause, to pay all amounts due under this clause on a pro rata basis taking into account all amounts due under this clause (including on account of principal, interest, fees, expenses or otherwise, as applicable));
(iv)fourth, to the holders of the Series A-1 Notes, an amount equal to the amount calculated as owing to them in respect of post-petition interest, calculated at the contract rate of interest (regardless of whether such amount is allowed or allowable as a claim) (and, if there shall be a shortfall in the amount available pursuant to this clause, to pay all amounts due under this clause on a pro rata basis taking into account all amounts due under this clause);
(v)fifth, to the holders of the Series A-2 Debt, an amount equal to the amount calculated as owing to them in respect of post-petition interest, calculated at the contract rate of interest (regardless of whether such amount is allowed or allowable as a claim) (and, if there shall be a shortfall in the amount available pursuant to this clause, to pay all amounts due under this clause on a pro rata basis taking into account all amounts due under this clause); and
179
Table of Contents
(vi)sixth, any surplus then remaining shall be paid to the Company or the applicable Guarantor or their successors or assigns or to whomsoever may be lawfully entitled to receive the same or as a court of competent jurisdiction may direct;provided that any amount received constituting ABL Collateral shall be applied in accordance with the provisions set forth in the Intercreditor Agreement.
The Bridge Loan Agent has agreed and each holder of Series A-2 Debt will be deemed to agree to turn over to the Trustee or the Notes/Term Collateral Agent, on behalf of the holders of the Series A-1 Notes, amounts otherwise received or receivable by them to the extent necessary to effectuate the priority of payments set forth above, even if such turnover has the effect of reducing the claim or recovery of the holders of the Series A-2 Debt.
The waterfall of payment provisions described above relate only to the relationship between the Series A-1 Notes and the Series A-2 Debt. The Company may issue up to $300.0 million of Additional Notes which may be Series A-1 Notes and would therefore be entitled to be treated as Series A-1 Notes for purposes of the waterfall. In addition, the Company may incur up to $200.0 million of additional Permitted Liens which may be secured equally and ratably with the Series A-1 Notes; the debt secured by these additional Permitted Liens would also be entitled to be treated as Series A-1 Notes for purposes of the waterfall of payment provisions described above.
Certain Other Intercreditor Arrangements
The Holders of Series A-2 Notes, the holders of Series A-1 Notes, the Bridge Loan Agent, the holders of any additional Series A-2 Debt, the Trustee and the Notes/Term Collateral Agent have agreed or will agree that (a) the grant of Liens pursuant to the Collateral Documents constitutes a single grant of Liens for the ratable benefit of the Holders of Series A-2 Notes, the holders of Series A-1 Notes and holders of any additional Series A-2 Debt and (b) the Series A-1 Notes and the Series A-2 Debt shall be classified as a single class of secured claims (or, if relevant, a single class of secured claims and a single class of unsecured claims) in any liquidation or plan of reorganization proposed or adopted in a bankruptcy, insolvency or liquidation case. To further effectuate the intent of the immediately preceding sentence, the Holders of Series A-2 Notes, the holders of Series A-1 Notes, the Bridge Loan Agent, the holders of any additional Series A-2 Debt, the Trustee and the Notes/Term Collateral Agent have agreed or will agree that, if it is held that the claims of the Series A-1 Notes and the Series A-2 Debt in respect of the Collateral constitute two classes of claims (rather than one class of secured claims or, if relevant, a single class of secured claims and a single class of unsecured claims), any distributions in respect of Collateral in any bankruptcy, insolvency or liquidation case that are made to any of them will be reallocated among the holders of Series A-1 Notes and the holders of Series A-2 Debt as if there were a single class of secured claims (or, if relevant, a single class of secured claims and a single class of unsecured claims) against the Company and the Guarantors in respect of the Collateral in compliance with the provisions described under “—Waterfall of Payment Following Acceleration or in Bankruptcy” above. Moreover, the Holders of Series A-2 Notes, the holders of Series A-1 Notes, the Bridge Loan Agent, the holders of any additional Series A-2 Debt, the Trustee and the Notes/Term Collateral Agent have agreed or will agree not to take actions, and not to initiate or prosecute or encourage any other Person to initiate or prosecute any claim, action, objection or other proceeding or otherwise assert any position inconsistent with the intent of the first sentence of this paragraph.
If, in any bankruptcy, insolvency or liquidation case, any equity securities, debt securities or other non-cash consideration from the reorganized debtor is distributed pursuant to a plan of reorganization or similar dispositive restructuring plan, the amount of such non-cash consideration to be distributed to each of the holders of the Series A-1 Notes and the holders of the Series A-2 Debt, respectively, shall be determined in accordance with the provisions described under “—Waterfall of Payment Following Acceleration or in Bankruptcy” above. In addition, if, in any bankruptcy, insolvency or liquidation case, debt obligations of the reorganized debtor secured by Liens upon any property of the reorganized debtor are distributed pursuant to a plan of reorganization or similar dispositive restructuring plan, both on account of the Series A-1 Notes and on account of the Series A-2 Debt, then, to the extent the debt obligations distributed on account of the Series A-1 Notes and on account of the
180
Table of Contents
Series A-2 Debt are secured by Liens upon the same property, the provisions described under “—Waterfall of Payment Following Acceleration or in Bankruptcy” above will survive the distribution of such debt obligations pursuant to such plan and will apply with like effect to such debt obligations.
For purposes of distribution of non-cash consideration, including any equity securities or debt securities, the value of such non-cash consideration shall be equal to either the current market price or the fair market value thereof and will be determined as follows: (i) if such non-cash consideration is a marketable security, the average daily closing price thereof on a principal national securities exchange or NASDAQ for a specified preceding period or (ii) if such non-cash consideration is not a marketable security or the average daily closing price thereof cannot be determined, by one or more nationally recognized investment banks with experience in similar transactions according to procedures customary for similar transactions.
For purposes of these intercreditor arrangements, all references to the Company or any Guarantor shall include such Person as a debtor-in-possession and any receiver or trustee for such Person in any bankruptcy, insolvency or liquidation case.
Mandatory Redemption; Offers to Purchase; Open Market Purchases
The Company is not required to make any mandatory redemption or sinking fund payments with respect to the Notes. However, under certain circumstances, the Company may be required to make an offer to purchase Notes as described under the caption “—Repurchase at the Option of Holders.” In addition, we may, at our discretion, at any time and from time to time purchase the Notes or any additional Series A-2 Debt in the open market or otherwise.
Optional Redemption
Except as set forth below, the Company will not be entitled to redeem the Notes at its option prior to November 1, 2011.
At any time prior to November 1, 2011, the Company may redeem all or a part of the Notes, upon not less than 30 nor more than 60 days prior notice mailed by first-class mail to the registered address of each Holder or otherwise delivered in accordance with the procedures of DTC, at a redemption price equal to 100% of the principal amount of the Notes redeemed plus the Applicable Premium as of, and accrued and unpaid interest and Additional Interest, if any, to the date of redemption (the “Redemption Date”), subject to the rights of Holders on the relevant record date to receive interest due on the relevant interest payment date.
On and after November 1, 2011, the Company may redeem the Notes, in whole or in part, upon notice as described under the heading “—Repurchase at the Option of Holders—Selection and Notice” at the redemption prices (expressed as percentages of principal amount of the Notes to be redeemed) set forth below, plus accrued and unpaid interest thereon and Additional Interest, if any, to the applicable Redemption Date, subject to the right of Holders of record on the relevant record date to receive interest due on the relevant interest payment date, if redeemed during the twelve-month period beginning on November 1 of each of the years indicated below:
Series A-1 Notes
Year | Percentage | ||
2011 | 105.625 | % | |
2012 | 102.813 | % | |
2013 and thereafter | 100.000 | % |
Series A-2 Notes
Year | Percentage | ||
2011 | 106.188 | % | |
2012 | 103.094 | % | |
2013 and thereafter | 100.000 | % |
181
Table of Contents
Until November 1, 2011, the Company may, at its option, redeem up to 35% of the aggregate principal amount of Series A-1 Notes issued by it at a redemption price equal to 111.25% of the aggregate principal amount thereof, plus accrued and unpaid interest thereon and Additional Interest, if any, to the applicable Redemption Date, subject to the right of Holders of Series A-1 Notes of record on the relevant record date to receive interest due on the relevant interest payment date, with the net cash proceeds of one or more Equity Offerings;provided that at least 65% of the aggregate principal amount of Series A-1 Notes originally issued under the Indenture and any Additional Notes that are Series A-1 Notes issued under the Indenture after the issue date of the Series A-1 Notes (excluding Series A-1 Notes and Additional Notes that are Series A-1 Notes held by the Company or Subsidiaries or Affiliates of the Company) remains outstanding immediately after the occurrence of each such redemption;provided further that each such redemption occurs within 180 days of the date of closing of each such Equity Offering.
In addition, until November 1, 2011, the Company may, at its option, redeem up to 35% of the aggregate principal amount of Series A-2 Notes issued by it at a redemption price equal to 112.375% of the aggregate principal amount thereof, plus accrued and unpaid interest thereon and Additional Interest, if any, to the applicable Redemption Date, subject to the right of Holders of Series A-2 Notes of record on the relevant record date to receive interest due on the relevant interest payment date, with the net cash proceeds of one or more Equity Offerings;provided that at least 65% of the aggregate principal amount of Series A-2 Notes originally issued under the Indenture and any Additional Notes that are Series A-2 Notes issued under the Indenture after the issue date of the Series A-2 Notes (excluding Series A-2 Notes and Additional Notes that are Series A-2 Notes held by the Company or Subsidiaries or Affiliates of the Company) remains outstanding immediately after the occurrence of each such redemption;provided further that each such redemption occurs within 180 days of the date of closing of each such Equity Offering.
Notice of any redemption upon any Equity Offering may be given prior to the completion thereof, and any such redemption or notice may, at the Company’s discretion, be subject to one or more conditions precedent, including, but not limited to, the completion of the related Equity Offering.
The Trustee shall select the Notes to be purchased in the manner described under “—Repurchase at the Option of Holders—Selection and Notice.”
Repurchase at the Option of Holders
Change of Control
The Indenture provides that if a Change of Control occurs, unless the Company has previously or concurrently mailed a redemption notice with respect to all the outstanding Notes as described under “—Optional Redemption,” the Company will make an offer to purchase all of the Notes pursuant to the offer described below (the “Change of Control Offer”) at a price in cash (the “Change of Control Payment”) equal to 101% of the aggregate principal amount thereof plus accrued and unpaid interest and Additional Interest, if any, to the date of purchase, subject to the right of Holders of the Notes of record on the relevant record date to receive interest due on the relevant interest payment date. Within 30 days following any Change of Control, the Company will send notice of such Change of Control Offer by first-class mail, with a copy to the Trustee, to each Holder of Notes to the address of such Holder appearing in the security register or otherwise in accordance with the procedures of DTC with a copy to the Trustee, with the following information:
(1) that a Change of Control Offer is being made pursuant to the covenant entitled “Change of Control” under the Indenture and that all Notes properly tendered pursuant to such Change of Control Offer will be accepted for payment by the Company;
(2) the purchase price and the purchase date, which will be no earlier than 30 days nor later than 60 days from the date such notice is mailed (the “Change of Control Payment Date”);
(3) that any Note not properly tendered will remain outstanding and continue to accrue interest;
182
Table of Contents
(4) that unless the Company defaults in the payment of the Change of Control Payment, all Notes accepted for payment pursuant to the Change of Control Offer will cease to accrue interest on the Change of Control Payment Date;
(5) that Holders electing to have any Notes purchased pursuant to a Change of Control Offer will be required to surrender such Notes, with the form entitled “Option of Holder to Elect Purchase” on the reverse of such Notes completed, to the paying agent specified in the notice at the address specified in the notice prior to the close of business on the third Business Day preceding the Change of Control Payment Date;
(6) that Holders will be entitled to withdraw their tendered Notes and their election to require the Company to purchase such Notes;provided that the paying agent receives, not later than the close of business on the expiration date of the Change of Control Offer, a telegram, telex, facsimile transmission or letter setting forth the name of the Holder of the Notes, the principal amount of Notes tendered for purchase, and a statement that such Holder is withdrawing its tendered Notes and its election to have such Notes purchased;
(7) that if the Company is repurchasing less than all of the Notes, the remaining Notes will be equal in principal amount to the unpurchased portion of the Notes surrendered. The unpurchased portion of the Notes must be equal to $2,000 or an integral multiple of $1,000 in excess thereof;
(8) the other instructions, as determined by the Company, consistent with the covenant described hereunder, that a Holder must follow; and
(9) if such notice is mailed prior to the occurrence of a Change of Control, stating that the Change of Control Offer is conditional upon the occurrence of such Change of Control.
The Company will comply with the requirements of Rule 14e-1 under the Exchange Act and any other securities laws and regulations thereunder to the extent such laws or regulations are applicable in connection with the repurchase of Notes pursuant to a Change of Control Offer. To the extent that the provisions of any securities laws or regulations conflict with the provisions of the Indenture, the Company will comply with the applicable securities laws and regulations and shall not be deemed to have breached its obligations described in the Indenture by virtue thereof.
On the Change of Control Payment Date, the Company will, to the extent permitted by law,
(1) accept for payment all Notes issued by it or portions thereof properly tendered pursuant to the Change of Control Offer,
(2) deposit with the paying agent an amount equal to the aggregate Change of Control Payment in respect of all Notes or portions thereof so tendered, and
(3) deliver, or cause to be delivered, to the Trustee for cancellation the Notes so accepted together with an Officer’s Certificate to the Trustee stating that such Notes or portions thereof have been tendered to, and purchased by, the Company.
The ABL Facility provides, and future credit agreements or other agreements relating to Senior Indebtedness to which the Company becomes a party may provide, that certain change of control events with respect to the Company would constitute a default thereunder (including events that would constitute a Change of Control under the Indenture). If we experience a change of control event that triggers a default under the ABL Facility or any such future Indebtedness, we could seek a waiver of such default or repurchase or prepayment provision or seek to refinance the ABL Facility or such future Indebtedness. In the event we do not obtain such a waiver or refinance the ABL Facility or such future Indebtedness, such default could result in amounts outstanding under the ABL Facility or such future Indebtedness being declared due and payable.
Our ability to pay cash to the Holders of Notes following the occurrence of a Change of Control may be limited by our then-existing financial resources. Therefore, sufficient funds may not be available when necessary to make any required repurchases.
183
Table of Contents
The Change of Control purchase feature of the Notes may in certain circumstances make more difficult or discourage a sale or takeover of us and, thus, the removal of incumbent management. The Change of Control purchase feature is a result of negotiations between the Initial Purchasers and us. We have no present intention to engage in a transaction involving a Change of Control, although it is possible that we could decide to do so in the future. Subject to the limitations discussed below, we could, in the future, enter into certain transactions, including acquisitions, refinancings or other recapitalizations, that would not constitute a Change of Control under the Indenture, but that could increase the amount of indebtedness outstanding at such time or otherwise affect our capital structure or credit ratings. Restrictions on our ability to incur additional Indebtedness are contained in the covenants described under “—Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” and “—Certain Covenants—Liens.” Such restrictions in the Indenture can be waived only with the consent of the holders of a majority in principal amount of the Notes and any additional Series A-2 Debt then outstanding. Except for the limitations contained in such covenants, however, the Indenture does not contain any covenants or provisions that may afford Holders of the Notes protection in the event of a highly leveraged transaction.
We will not be required to make a Change of Control Offer following a Change of Control if a third party makes the Change of Control Offer in the manner, at the times and otherwise in compliance with the requirements set forth in the Indenture applicable to a Change of Control Offer made by us and purchases all Notes validly tendered and not withdrawn under such Change of Control Offer. Notwithstanding anything to the contrary herein, a Change of Control Offer may be made in advance of a Change of Control, conditional upon such Change of Control, if a definitive agreement is in place for the Change of Control at the time of making of the Change of Control Offer.
The definition of “Change of Control” includes a disposition of all or substantially all of the assets of the Company to any Person. Although there is a limited body of case law interpreting the phrase “substantially all,” there is no precise established definition of the phrase under applicable law. Accordingly, in certain circumstances there may be a degree of uncertainty as to whether a particular transaction would involve a disposition of “all or substantially all” of the assets of the Company. As a result, it may be unclear as to whether a Change of Control has occurred and whether a Holder of Notes may require the Company to make an offer to repurchase the Notes as described above.
The provisions under the Indenture relating to the Company’s obligation to make an offer to repurchase the Notes as a result of a Change of Control may be waived or modified with the written consent of the Holders of a majority in principal amount of the Notes.
Asset Sales
The Indenture provides that the Company will not, and will not permit any of its Restricted Subsidiaries to consummate an Asset Sale, unless:
(1) the Company or such Restricted Subsidiary, as the case may be, receives consideration at the time of such Asset Sale at least equal to the fair market value (as determined in good faith by the Company or, if $40.0 million or more, the board of directors of the Company) of the assets sold or otherwise disposed of;
(2) except in the case of a Permitted Asset Swap, at least 75% of the consideration therefor received by the Company or such Restricted Subsidiary, as the case may be, is in the form of cash or Cash Equivalents;provided that the following shall be deemed to be cash for purposes of this provision and for no other purpose:
(a) any liabilities (as reflected in the Company’s or such Restricted Subsidiary’s most recent balance sheet or in the footnotes thereto) of the Company or such Restricted Subsidiary (other than liabilities that are by their terms subordinated to the Notes or liabilities to the extent owed to the Company or any Affiliate of the Company) that are assumed by the transferee of any such assets and for which the Company and all of its Restricted Subsidiaries have been validly released by all applicable creditors in writing;
184
Table of Contents
(b) any securities, notes or other similar obligations received by the Company or such Restricted Subsidiary from such transferee that are converted by the Company or such Restricted Subsidiary into cash (to the extent of the cash received) within 180 days following the closing of such Asset Sale;
(c) any Designated Non-cash Consideration received by the Company or such Restricted Subsidiary in such Asset Sale having an aggregate fair market value, taken together with all other Designated Non-cash Consideration received pursuant to this clause (c) that is at that time outstanding, not to exceed the greater of (i) $50.0 million and (ii) 2.5% of Total Assets at the time of the receipt of such Designated Non-cash Consideration, with the fair market value of each item of Designated Non-cash Consideration being measured at the time received and without giving effect to subsequent changes in value; and
(3) to the extent that any assets received by the Company and its Restricted Subsidiaries in such Asset Sale constitute securities or may be used or useful in a Similar Business, such assets are concurrently with their acquisition added to the Notes/Term Collateral securing the Notes, other than Excluded Assets and subject to the limitations and exclusions described under “—Security for the Notes—Limitations on Stock Collateral.”
Within 365 days after the receipt of any Net Proceeds of any Asset Sale, the Company or such Restricted Subsidiary, at its option, may apply the Net Proceeds from such Asset Sale,
(1) to permanently reduce Indebtedness as follows:
(a) if the assets subject of such Asset Sale constitute Notes/Term Collateral, to permanently reduce (or offer to reduce, as applicable) Obligations under the Series A-1 Notes and the Series A-2 Debt on a pro rata basis;provided further that all reductions of obligations under the Series A-2 Notes shall be made as provided under “Optional Redemption,” through open-market purchases (to the extent such purchases are at or above 100% of the principal amount thereof plus accrued unpaid interest) or by making an offer (in accordance with the procedures set forth below for an Asset Sale Offer) to all Holders of Notes to purchase their Notes at 100% of the principal amount thereof, plus the amount of accrued but unpaid interest, if any, on the amount of Notes that would otherwise be prepaid;
(b) if the assets subject of such Asset Sale do not constitute Collateral, but constitute collateral for other Senior Indebtedness, which Lien is permitted by the Indenture, to permanently reduce Obligations under such other Senior Indebtedness that is secured by a Lien, which Lien is permitted by the Indenture, and to correspondingly reduce commitments with respect thereto;
(c) if the assets subject of such Asset Sale do not constitute Collateral, to permanently reduce Obligations under other Senior Indebtedness (and to correspondingly reduce commitments with respect thereto), provided that the Company shall equally and ratably reduce (or offer to reduce, as applicable) Obligations under the Series A-1 Notes and Series A-2 Debt on a pro rata basis;provided further that all reductions of obligations under the Notes shall be made as provided under “Optional Redemption,” through open-market purchases (to the extent such purchases are at or above 100% of the principal amount thereof plus accrued and unpaid interest) or by making an offer (in accordance with the procedures set forth below for an Asset Sale Offer) to all Holders of Notes to purchase their Notes at 100% of the principal amount thereof, plus the amount of accrued but unpaid interest, if any, on the amount of Notes that would otherwise be prepaid; or
(d) if the assets subject of such Asset Sale do not constitute Collateral, to permanently reduce Indebtedness of a Restricted Subsidiary that is not a Guarantor, other than Indebtedness owed to the Company or any Restricted Subsidiary,
(2) to make (a) an Investment in any one or more businesses;provided that such Investment in any business is in the form of the acquisition of Capital Stock and results in the Company or another of its Restricted Subsidiaries, as the case may be, owning an amount of the Capital Stock of such business such that it constitutes a Restricted Subsidiary, (b) capital expenditures or (c) acquisitions of other assets, in each
185
Table of Contents
of (a), (b) and (c), used or useful in a Similar Business,provided that the assets (including Capital Stock) acquired with the Net Proceeds of a disposition of Collateral are pledged as Collateral to the extent required under the Collateral Documents; or
(3) to make an investment in (a) any one or more businesses;provided that such Investment in any business is in the form of the acquisition of Capital Stock and results in the Company or another of its Restricted Subsidiaries, as the case may be, owning an amount of the Capital Stock of such business such that it constitutes a Restricted Subsidiary, (b) properties or (c) acquisitions of other assets that, in each of (a), (b) and (c), replace the businesses, properties and/or assets that are the subject of such Asset Sale,provided that the assets (including Capital Stock) acquired with the Net Proceeds of a disposition of Collateral are pledged as Collateral to the extent required under the Collateral Documents;
provided that, in the case of clauses (2) and (3) above, a binding commitment entered into not later than such 365th day shall be treated as a permitted application of the Net Proceeds from the date of such commitment so long as the Company, or such other Restricted Subsidiary enters into such commitment with the good faith expectation that such Net Proceeds will be applied to satisfy such commitment within 120 days of such commitment (an “Acceptable Commitment”) and, in the event any Acceptable Commitment is later cancelled or terminated for any reason before the Net Proceeds are applied in connection therewith, or such Net Proceeds are not actually so invested or paid in accordance with clauses (2) or (3) above by the end of such 120-day period, then such Net Proceeds shall constitute Excess Proceeds.
Any Net Proceeds from the Asset Sale that are not invested or applied as provided and within the time period set forth in the first sentence of the preceding paragraph will be deemed to constitute “Excess Proceeds.” When the aggregate amount of Excess Proceeds exceeds $30.0 million, the Company shall make an offer to all Holders of the Notes and, if required by the terms of any Indebtedness that ispari passu with the Notes or any Guarantee (“Pari passu Indebtedness”), to the holders of suchPari passu Indebtedness (an “Asset Sale Offer”), to purchase the maximum aggregate principal amount of the Notes and suchPari passu Indebtedness that is an integral multiple of $1,000 (but in minimum amounts of $2,000) that may be purchased out of the Excess Proceeds at an offer price in cash in an amount equal to 100% of the principal amount thereof, plus accrued and unpaid interest and Additional Interest, if any, to the date fixed for the closing of such offer, in accordance with the procedures set forth in the Indenture. The Company will commence an Asset Sale Offer with respect to Excess Proceeds within ten Business Days after the date that Excess Proceeds exceed $30.0 million by mailing the notice required pursuant to the terms of the Indenture, with a copy to the Trustee. The Company may satisfy the foregoing obligations with respect to any Net Proceeds from an Asset Sale by making an Asset Sale Offer with respect to such Net Proceeds prior to the expiration of the relevant 365 days or with respect to Excess Proceeds of $30.0 million or less.
To the extent that the aggregate amount of Notes and suchPari passu Indebtedness tendered pursuant to an Asset Sale Offer is less than the Excess Proceeds, the Company may use any remaining Excess Proceeds for general corporate purposes, subject to other covenants contained in the Indenture. If the aggregate principal amount of Notes or thePari passu Indebtedness surrendered by such holders thereof exceeds the amount of Excess Proceeds, the Trustee shall select the Notes and suchPari passu Indebtedness to be purchased on a pro rata basis based on the accreted value or principal amount of the Notes or suchPari passu Indebtedness tendered. Additionally, the Company may, at its option, make an Asset Sale Offer using proceeds from any Asset Sale at any time after consummation of such Asset Sale. Upon consummation of any Asset Sale Offer, any Net Proceeds not used to purchase Notes in such Asset Sale Offer shall not be deemed Excess Proceeds and the Company may use any Net Proceeds not required to be used for general corporate purposes, subject to other covenants contained in the Indenture. To the extent the Asset Sale giving rise to the Asset Sale Offer involves Notes/ Term Collateral, the Asset Sale Offer will be made first to Holders of Series A-1 Notes and holders of Series A-2 Debt and, if any Excess Proceeds remain, second to holders of otherPari passu Indebtedness.
Pending the final application of any Net Proceeds pursuant to this covenant, the holder of such Net Proceeds may apply such Net Proceeds temporarily to reduce Indebtedness outstanding under a revolving credit facility or otherwise invest such Net Proceeds in any manner not prohibited by the Indenture.
186
Table of Contents
The Company will comply with the requirements of Rule 14e-1 under the Exchange Act and any other securities laws and regulations thereunder to the extent such laws or regulations are applicable in connection with the repurchase of the Notes pursuant to an Asset Sale Offer. To the extent that the provisions of any securities laws or regulations conflict with the provisions of the Indenture, the Company will comply with the applicable securities laws and regulations and shall not be deemed to have breached their obligations described in the Indenture by virtue thereof.
Selection and Notice
If the Company is redeeming less than all of the Notes issued by it at any time, the Trustee will select the Notes to be redeemed (a) if the Notes are listed on any national securities exchange, in compliance with the requirements of the principal national securities exchange on which the Notes are listed or (b) on a pro rata basis to the extent practicable or, to the extent that selection on a pro rata basis is not practicable, by lot or such other similar method in accordance with the procedures of DTC.
Notices of purchase or redemption shall be mailed by first-class mail, postage prepaid, at least 30 but not more than 60 days before the purchase or redemption date to each Holder of Notes at such Holder’s registered address or otherwise in accordance with the procedures of DTC, except that redemption notices may be mailed more than 60 days prior to a redemption date if the notice is issued in connection with a defeasance of the Notes or a satisfaction and discharge of the Indenture. If any Note is to be purchased or redeemed in part only, any notice of purchase or redemption that relates to such Note shall state the portion of the principal amount thereof that has been or is to be purchased or redeemed.
The Company will issue a new Note in a principal amount equal to the unredeemed portion of the original Note in the name of the Holder upon cancellation of the original Note. Notes called for redemption become due on the date fixed for redemption. On and after the redemption date, interest ceases to accrue on Notes or portions thereof called for redemption.
Certain Covenants
Set forth below are summaries of certain covenants contained in the Indenture.
Limitation on Restricted Payments
The Company will not, and will not permit any of its Restricted Subsidiaries to, directly or indirectly:
(I) declare or pay any dividend or make any payment or distribution on account of the Company’s, or any of its Restricted Subsidiaries’ Equity Interests, including, without limitation, payable in connection with any merger or consolidation other than:
(a) dividends or distributions by the Company payable solely in Equity Interests (other than Disqualified Stock) of the Company; or
(b) dividends, payments or distributions by a Restricted Subsidiary so long as, in the case of any dividend, payment or distribution payable on or in respect of any class or series of securities issued by a Restricted Subsidiary other than a Wholly-Owned Subsidiary, the Company or a Restricted Subsidiary receives at least its pro rata share of such dividend or distribution in accordance with its Equity Interests in such class or series of securities;
(II) purchase, redeem, defease or otherwise acquire or retire for value any Equity Interests of the Company, or any direct or indirect parent of the Company, including, without limitation, in connection with any merger or consolidation;
187
Table of Contents
(III) make any principal payment on, or redeem, repurchase, defease or otherwise acquire or retire for value in each case, prior to any scheduled repayment, sinking fund payment or maturity, any Subordinated Indebtedness, other than (a) Indebtedness permitted under clauses (7) and (8) of the covenant described under “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” or (a) the purchase, repurchase or other acquisition of Subordinated Indebtedness purchased in anticipation of satisfying a sinking fund obligation, principal installment or final maturity, in each case due within one year of the date of purchase, repurchase or acquisition; or
(IV) make any Restricted Investment
(all such payments and other actions set forth in clauses (I) through (IV) above (other than any exceptions thereof) being collectively referred to as “Restricted Payments”), unless, at the time of such Restricted Payment:
(1) no Default or Event of Default shall have occurred and be continuing or would occur as a consequence thereof;
(2) immediately after giving effect to such transaction on apro forma basis, the Company could incur $1.00 of additional Indebtedness under the provisions of the first paragraph of the covenant described under “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”; and
(3) such Restricted Payment, together with the aggregate amount of all other Restricted Payments made by the Company and its Restricted Subsidiaries after the Issue Date (including Restricted Payments permitted by clauses (1), (2) (with respect to the payment of dividends on Refunding Capital Stock (as defined below) pursuant to clause (b) thereof only), (6)(c), (7), (9) and (14) (to the extent not deducted in calculating Consolidated Net Income) of the next succeeding paragraph, but excluding all other Restricted Payments permitted by the next succeeding paragraph), is less than the sum of (without duplication):
(a) 50% of the Consolidated Net Income of the Company for the period (taken as one accounting period) beginning October 1, 2008 to the end of the Company’s most recently ended fiscal quarter for which internal financial statements are available at the time of such Restricted Payment, or, in the case such Consolidated Net Income for such period is a deficit, minus 100% of such deficit; plus
(b) 100% of the aggregate net cash proceeds and the fair market value, as determined in good faith by the Company, of marketable securities or other property (other than assets or Equity Interests constituting entire portfolio companies owned by the Permitted Holders) received by the Company since immediately after the Issue Date (other than net cash proceeds to the extent such net cash proceeds have been used to incur Indebtedness, Disqualified Stock or Preferred Stock pursuant to clause (12)(a) of the second paragraph of “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”) from the issue or sale of:
(i) (A) Equity Interests of the Company, including Treasury Capital Stock (as defined below), but excluding cash proceeds and the fair market value, as determined in good faith by the Company, of marketable securities or other property received from the sale of:
(x) Equity Interests to employees, directors or consultants of the Company, any direct or indirect parent company of the Company and the Company’s Subsidiaries after the Issue Date to the extent such amounts have been applied to Restricted Payments made in accordance with clause (4) of the next succeeding paragraph; and
(y) Designated Preferred Stock; and
(B) to the extent such net cash proceeds are actually contributed to the Company as equity (other than Disqualified Stock), Equity Interests of any of the Company’s direct or
188
Table of Contents
indirect parent companies (excluding contributions of the proceeds from the sale of Designated Preferred Stock of any such companies or contributions to the extent such amounts have been applied to Restricted Payments made in accordance with clause (4) of the next succeeding paragraph); or
(ii) debt securities of the Company that have been converted into or exchanged for such Equity Interests of the Company;
provided, however, that this clause (b) shall not include the proceeds from (W) Refunding Capital Stock (as defined below), (X) Equity Interests or debt securities of the Company sold to a Restricted Subsidiary, as the case may be, (Y) Disqualified Stock or debt securities that have been converted into Disqualified Stock or (Z) Excluded Contributions;plus
(c) 100% of the aggregate amount of cash and the fair market value, as determined in good faith by the Company, of marketable securities or other property (other than assets or Equity Interests constituting entire portfolio companies owned by the Permitted Holders) contributed to the capital of the Company (other than as Disqualified Stock) following the Issue Date (other than (i) net cash proceeds to the extent such net cash proceeds have been used to incur Indebtedness, Disqualified Stock or Preferred Stock pursuant to clause (12)(a) of the second paragraph of “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”, (ii) contributions from a Restricted Subsidiary or (iii) any Excluded Contribution); plus
(d) 100% of the aggregate amount received in cash and the fair market value, as determined in good faith by the Company, of marketable securities or other property (other than assets or Equity Interests constituting entire portfolio companies owned by the Permitted Holders) received by the Company or any Restricted Subsidiary by means of:
(i) the sale or other disposition (other than to the Company or a Restricted Subsidiary) of Restricted Investments made by the Company or its Restricted Subsidiaries and repurchases and redemptions of such Restricted Investments from the Company or its Restricted Subsidiaries and repayments of loans or advances, and releases of guarantees, which constitute Restricted Investments by the Company or its Restricted Subsidiaries, in each case after the Issue Date; or
(ii) the sale (other than to the Company or a Restricted Subsidiary) of the stock of an Unrestricted Subsidiary (other than to the extent the Investment in such Unrestricted Subsidiary was made by the Company or a Restricted Subsidiary pursuant to clause (7) of the next succeeding paragraph or to the extent such Investment constituted a Permitted Investment) or a distribution or dividend from an Unrestricted Subsidiary, in each case, after the Issue Date; plus
(e) in the case of the redesignation of an Unrestricted Subsidiary as a Restricted Subsidiary after the Issue Date, the fair market value of the Investment in such Unrestricted Subsidiary, as determined by the Company in good faith or if, in the case of an Unrestricted Subsidiary, such fair market value may exceed $35.0 million, in writing by an Independent Financial Advisor, at the time of the redesignation of such Unrestricted Subsidiary as a Restricted Subsidiary other than an Unrestricted Subsidiary to the extent the Investment in such Unrestricted Subsidiary was made by the Company or a Restricted Subsidiary pursuant to clause (7) of the next succeeding paragraph or to the extent such Investment constituted a Permitted Investment;
provided, however, that for purposes of determining the fair market value of such other property received by the Company or any Restricted Subsidiary or contributed to the capital of the Company, as the case may be, pursuant to clauses (3)(b), (c) and (d) above, the Company shall deliver to the Trustee an Officer’s Certificate signed by the Chief Financial Officer of the Company certifying as to the fair market value of such other property and, if the fair market value is at least $75.0 million, a written opinion of a recognized independent expert stating the fair market value of such other property.
189
Table of Contents
The foregoing provisions will not prohibit:
(1) the payment of any dividend or distribution or the consummation of any irrevocable redemption within 60 days after the date of declaration thereof or the giving of the irrevocable redemption notice, as applicable, if at the date of declaration or notice such payment would have complied with the provisions of the Indenture;
(2)(a) the redemption, repurchase, defeasance, retirement or other acquisition of any Equity Interests (“Treasury Capital Stock”) or Subordinated Indebtedness of the Company or any Equity Interests of any direct or indirect parent company of the Company, in exchange for, or out of the proceeds of the substantially concurrent sale (other than to a Restricted Subsidiary) of, Equity Interests of the Company or any direct or indirect parent company of the Company to the extent contributed to the Company (in each case, other than any Disqualified Stock or Designated Preferred Stock) (“Refunding Capital Stock”) and (b) if immediately prior to the retirement of Treasury Capital Stock, the declaration and payment of dividends thereon was permitted under clause (6) of this paragraph, the declaration and payment of dividends on the Refunding Capital Stock (other than Refunding Capital Stock the proceeds of which were used to redeem, repurchase, retire or otherwise acquire any Equity Interests of any direct or indirect parent company of the Company) in an aggregate amount no greater than the aggregate amount per year of dividends per annum that were declarable and payable on such Treasury Capital Stock immediately prior to such retirement;
(3) the redemption, repurchase, defeasance or other acquisition or retirement of Subordinated Indebtedness of the Company or a Guarantor made in exchange for, or out of the proceeds of the substantially concurrent sale of, new Indebtedness of the Company or a Guarantor, as the case may be, which is incurred in compliance with “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” so long as:
(a) the principal amount (or accreted value, if applicable) of such new Indebtedness does not exceed the principal amount of (or accreted value, if applicable), plus any accrued and unpaid interest on, the Subordinated Indebtedness being so redeemed, repurchased, acquired or retired for value,plus the amount of any reasonable premium to be paid (including reasonable premiums) and any reasonable fees and expenses incurred in connection with the issuance of such new Indebtedness;
(b) such new Indebtedness is subordinated to the Series A-2 Notes or the applicable Guarantee at least to the same extent as such Subordinated Indebtedness so purchased, exchanged, redeemed, repurchased, defeased, acquired or retired for value;
(c) such new Indebtedness has a final scheduled maturity date equal to or later than the final scheduled maturity date of the Subordinated Indebtedness being so redeemed, repurchased, defeased, acquired or retired; and
(d) such new Indebtedness has a Weighted Average Life to Maturity equal to or greater than the remaining Weighted Average Life to Maturity of the Subordinated Indebtedness being so redeemed, repurchased, defeased, acquired or retired;
(4) a Restricted Payment to pay for the repurchase, redemption or other acquisition or retirement for value of Equity Interests (other than Disqualified Stock) of the Company or any of its direct or indirect parent companies held by any future, present or former employee, director or consultant of the Company, any of its Restricted Subsidiaries or any of its direct or indirect parent companies pursuant to any management equity plan or stock option plan or any other management or employee benefit plan or agreement, including any Equity Interests rolled over by management of the Company in connection with the Transaction, (x) upon the death or disability of such employee, director or consultant or (y) upon the resignation or other termination of employment of such employee, director or consultant;provided,however, that the aggregate Restricted Payments made under this clause (4) do not exceed in any calendar year $15.0 million (which shall increase to $25.0 million subsequent to the consummation of an underwritten public Equity Offering by the Company or any direct or indirect parent corporation of the
190
Table of Contents
Company) (with unused amounts in any calendar year being carried over to succeeding calendar years subject to a maximum (without giving effect to the following proviso) of $25.0 million in any calendar year (which shall increase to $40.0 million subsequent to the consummation of an underwritten public Equity Offering by the Company or any direct or indirect parent of the Company));provided further that such amount in any calendar year may be increased by an amount not to exceed:
(a) the cash proceeds from the sale of Equity Interests (other than Disqualified Stock) of the Company and, to the extent contributed to the Company, Equity Interests of any of the Company’s direct or indirect parent companies, in each case to members of management, directors or consultants of the Company, any of its Subsidiaries or any of its direct or indirect parent companies that occurs after the Issue Date, to the extent the cash proceeds from the sale of such Equity Interests have not otherwise been applied to the payment of Restricted Payments by virtue of clause (3) of the preceding paragraph; plus
(b) the cash proceeds of key man life insurance policies received by the Company or its Restricted Subsidiaries after the Issue Date; less
(c) the amount of any Restricted Payments previously made with the cash proceeds described in clauses (a) and (b) of this clause (4);
andprovided further that cancellation of Indebtedness owing to the Company or any of its Restricted Subsidiaries from members of management of the Company, any of the Company’s direct or indirect parent companies or any of the Company’s Subsidiaries in connection with a repurchase of Equity Interests of the Company or any of its direct or indirect parent companies will not be deemed to constitute a Restricted Payment for purposes of this covenant or any other provision of the Indenture;
(5) the declaration and payment of dividends to holders of any class or series of Disqualified Stock of the Company or any of its Restricted Subsidiaries and of Preferred Stock of any Restricted Subsidiary issued in accordance with the covenant described under “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” to the extent such dividends are included in the definition of “Fixed Charges”;
(6)(a) the declaration and payment of dividends to holders of any class or series of Designated Preferred Stock (other than Disqualified Stock) issued by the Company after the Issue Date;
(b) the declaration and payment of dividends to a direct or indirect parent company of the Company, the proceeds of which will be used to fund the payment of dividends to holders of any class or series of Designated Preferred Stock (other than Disqualified Stock) of such parent corporation issued after the Issue Date, provided that the amount of dividends paid pursuant to this clause (b) shall not exceed the aggregate amount of cash actually contributed to the Company from the sale of such Designated Preferred Stock; or
(c) the declaration and payment of dividends on Refunding Capital Stock that is Preferred Stock in excess of the dividends declarable and payable thereon pursuant to clause (2) of this paragraph;
provided, however, in the case of each of (a), (b) and (c) of this clause (6), that for the most recently ended four full fiscal quarters for which internal financial statements are available immediately preceding the date of issuance of such Designated Preferred Stock or the declaration of such dividends on Refunding Capital Stock that is Preferred Stock, after giving effect to such issuance or declaration on apro forma basis, the Company and its Restricted Subsidiaries on a consolidated basis would have had a Fixed Charge Coverage Ratio of at least 2.00 to 1.00;
(7) Investments in Unrestricted Subsidiaries having an aggregate fair market value, taken together with all other Investments made pursuant to this clause (7) that are at the time outstanding, without giving effect to the sale of an Unrestricted Subsidiary to the extent the proceeds of such sale do not consist of cash or marketable securities, not to exceed the greater of (x) $30.0 million and (y) 1.0% of Total Assets at the time of such Investment (with the fair market value of each Investment being measured at the time made and without giving effect to subsequent changes in value);
191
Table of Contents
(8) repurchases of Equity Interests deemed to occur upon exercise of stock options or warrants if such Equity Interests represent a portion of the exercise price of such options or warrants;
(9) the declaration and payment of dividends on the Company’s common stock (or payments of dividends to any direct or indirect parent entity to fund payments of dividends on such entity’s common stock), following the consummation of an underwritten public offering of the Company’s common stock or the common stock of any of its direct or indirect parent companies after the Issue Date, of up to 6% per annum of the net cash proceeds received by or contributed to the Company in or from any such public offering, other than public offerings with respect to common stock registered on Form S-4 or Form S-8 and other than any public sale constituting an Excluded Contribution;
(10) Restricted Payments in an aggregate amount equal to the amount of Excluded Contributions previously received by the Company and its Restricted Subsidiaries;
(11) other Restricted Payments in an aggregate amount taken together with all other Restricted Payments made pursuant to this clause (11) not to exceed the greater of (x) $30.0 million and (y) 1.0% of Total Assets at the time made;
(12) distributions or payments of Receivables Fees;
(13) any Restricted Payment made as part of the Transaction (including payments made after the Issue Date in respect of long-term incentive plans or tax gross-ups or other deferred compensation), and the fees and expenses related thereto, or used to fund amounts owed to Affiliates (including dividends to any direct or indirect parent of the Company to permit payment by such parent of such amounts), in each case to the extent permitted by (or, in the case of a dividend to fund such payment, to the extent such payment, if made by the Company, would be permitted by) the covenant described under “—Transactions with Affiliates”;
(14) the repurchase, redemption or other acquisition or retirement for value of any Subordinated Indebtedness in accordance with the provisions similar to those described under the captions “—Repurchase at the Option of Holders—Change of Control” and “—Repurchase at the Option of Holders—Asset Sales”;provided that all Notes tendered in connection with a Change of Control Offer or Asset Sale Offer, as applicable, have first been repurchased, redeemed or acquired for value;
(15) the declaration and payment of dividends by the Company to, or the making of loans to, any direct or indirect parent in amounts required for any direct or indirect parent companies to pay, in each case without duplication:
(a) franchise and excise taxes and other fees, taxes and expenses, in each case to the extent required to maintain their corporate existence;
(b) federal, state and local income taxes, to the extent such income taxes are attributable to the income of the Company and its Restricted Subsidiaries and, to the extent of the amount actually received from its Unrestricted Subsidiaries, in amounts required to pay such taxes to the extent attributable to the income of such Unrestricted Subsidiaries;provided that in each case the amount of such payments in any fiscal year does not exceed the amount that the Company and its Restricted Subsidiaries would be required to pay in respect of federal, state and local taxes for such fiscal year were the Company, its Restricted Subsidiaries and its Unrestricted Subsidiaries (to the extent described above) to pay such taxes separately from any such parent entity;
(c) customary salary, bonus and other benefits payable to officers and employees of any direct or indirect parent company of the Company to the extent such salaries, bonuses and other benefits are attributable to the ownership or operation of the Company and its Restricted Subsidiaries;
(d) general corporate operating and overhead costs and expenses of any direct or indirect parent company of the Company to the extent such costs and expenses are attributable to the ownership or operation of the Company and its Restricted Subsidiaries; and
(e) fees and expenses other than to Affiliates of the Company related to any unsuccessful equity or debt offering of such parent entity; and
192
Table of Contents
(16) the distribution, by dividend or otherwise, of shares of Capital Stock of, or Indebtedness owed to the Company or a Restricted Subsidiary by Unrestricted Subsidiaries (other than Unrestricted Subsidiaries, the primary assets of which are cash and/or Cash Equivalents or were contributed to such Unrestricted Subsidiary in anticipation of such distribution, dividend or other payment);
provided, however, that at the time of, and after giving effect to, any Restricted Payment permitted under clauses (7), (11) and (16), no Default shall have occurred and be continuing or would occur as a consequence thereof.
As of the date of this prospectus, all of the Company’s wholly-owned domestic subsidiaries will be Restricted Subsidiaries. The Company will not permit any Unrestricted Subsidiary to become a Restricted Subsidiary except pursuant to the last sentence of the definition of “Unrestricted Subsidiary.” For purposes of designating any Restricted Subsidiary as an Unrestricted Subsidiary, all outstanding Investments by the Company and its Restricted Subsidiaries (except to the extent repaid) in the Subsidiary so designated will be deemed to be Restricted Payments in an amount determined as set forth in the last sentence of the definition of “Investments.” Such designation will be permitted only if a Restricted Payment in such amount would be permitted at such time, whether pursuant to the first paragraph of this covenant or under clause (7), (10) or (11) of the second paragraph of this covenant, or pursuant to the definition of “Permitted Investment,” and if such Subsidiary otherwise meets the definition of an Unrestricted Subsidiary. Unrestricted Subsidiaries will not be subject to any of the restrictive covenants set forth in the Indenture.
Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock
The Company will not, and will not permit any of its Restricted Subsidiaries to, directly or indirectly, create, incur, issue, assume, guarantee or otherwise become directly or indirectly liable, contingently or otherwise (collectively, “incur” and collectively, an “incurrence”) with respect to any Indebtedness (including Acquired Indebtedness) and the Company will not issue any shares of Disqualified Stock and will not permit any Restricted Subsidiary to issue any shares of Disqualified Stock or Preferred Stock;provided, however, that the Company may incur Indebtedness (including Acquired Indebtedness) or issue shares of Disqualified Stock, and subject to the second proviso in this paragraph any of its Restricted Subsidiaries may incur Indebtedness (including Acquired Indebtedness), issue shares of Disqualified Stock and issue shares of Preferred Stock, if the Fixed Charge Coverage Ratio on a consolidated basis for the Company and its Restricted Subsidiaries’ most recently ended four fiscal quarters for which internal financial statements are available immediately preceding the date on which such additional Indebtedness is incurred or such Disqualified Stock or Preferred Stock is issued would have been at least 2.00 to 1.00, determined on apro forma basis (including apro forma application of the net proceeds therefrom), as if the additional Indebtedness had been incurred, or the Disqualified Stock or Preferred Stock had been issued, as the case may be, and the application of proceeds therefrom had occurred at the beginning of such four-quarter period;provided further, that Restricted Subsidiaries that are not Guarantors may not incur Indebtedness or issue Disqualified Stock or Preferred Stock pursuant to this paragraph if, after givingpro forma effect to such incurrence or issuance (including apro forma application of the net proceeds therefrom), the aggregate amount of Indebtedness, Disqualified Stock and Preferred Stock of Restricted Subsidiaries that are not Guarantors incurred or issued pursuant to this paragraph would exceed $25.0 million.
The foregoing limitations will not apply to:
(1) the incurrence of Indebtedness under Credit Facilities by the Company or any of its Restricted Subsidiaries and the issuance and creation of letters of credit and bankers’ acceptances thereunder (with letters of credit and bankers’ acceptances being deemed to have a principal amount equal to the face amount thereof), up to an aggregate principal amount at any one time outstanding not to exceed the greater of (x) $175.0 million and (y) the Borrowing Base;
(2) the incurrence by the Company and any Guarantor of (a) Indebtedness represented by the Series A-1 Notes (including any Guarantee) (other than any Additional Notes) and any notes (including Guarantees thereof) issued in exchange for the Series A-1 Notes pursuant to the Registration Rights Agreement or
193
Table of Contents
similar agreement and (b) Indebtedness under the Term Loan Facility and/or Series A-2 Notes (including Guarantees thereof) (other than any Additional Notes) and any notes (including Guarantees thereof) issued in exchange for the Series A-2 Notes pursuant to a registration rights agreement, up to an aggregate principal amount of $310.0 million (plus up to an additional $15.0 million of Series A-2 Notes which the Company has the right to issue);
(3) Indebtedness of the Company and its Restricted Subsidiaries in existence on the Issue Date (other than Indebtedness described in clauses (1) and (2));
(4) Indebtedness (including Capitalized Lease Obligations) and Disqualified Stock incurred or issued by the Company or any of its Restricted Subsidiaries, and Preferred Stock issued by any of the Company’s Restricted Subsidiaries, to finance the purchase, lease or improvement of property (real or personal) or equipment (other than software) that is used or useful in a Similar Business, whether through the direct purchase of assets or the Capital Stock of any Person owning such assets, in an aggregate principal amount at the date of such incurrence (including all Refinancing Indebtedness Incurred to refinance any other Indebtedness incurred pursuant to this clause (4)) not to exceed 4.0% of Total Assets;provided, however, that such Indebtedness exists at the date of such purchase or transaction or is created within 270 days thereafter (it being understood that any Indebtedness, Disqualified Stock or Preferred Stock incurred pursuant to this clause (4) shall cease to be deemed incurred or outstanding for purposes of this clause (4) but shall be deemed incurred for the purposes of the first paragraph of this covenant from and after the first date on which the Company or such Restricted Subsidiary could have incurred such Indebtedness, Disqualified Stock or Preferred Stock under the first paragraph of this covenant without reliance on this clause (4));
(5) Indebtedness incurred by the Company or any of its Restricted Subsidiaries constituting reimbursement obligations with respect to letters of credit issued in the ordinary course of business, including letters of credit in respect of workers’ compensation claims, or other Indebtedness with respect to reimbursement type obligations regarding workers’ compensation claims;provided, however, that upon the drawing of such letters of credit or the incurrence of such Indebtedness, such obligations are reimbursed within 30 days following such drawing or incurrence;
(6) Indebtedness arising from agreements of the Company or its Restricted Subsidiaries providing for indemnification, adjustment of purchase price or similar obligations, in each case, incurred or assumed in connection with the disposition of any business, assets or a Subsidiary, other than guarantees of Indebtedness incurred by any Person acquiring all or any portion of such business, assets or a Subsidiary for the purpose of financing such acquisition;provided, however, that
(a) such Indebtedness is not reflected on the balance sheet of the Company, or any of its Restricted Subsidiaries (contingent obligations referred to in a footnote to financial statements and not otherwise reflected on the balance sheet will not be deemed to be reflected on such balance sheet for purposes of this subclause (a)); and
(b) the maximum assumable liability in respect of all such Indebtedness shall at no time exceed the gross proceeds including non-cash proceeds (the fair market value of such non-cash proceeds being measured at the time received and without giving effect to any subsequent changes in value) actually received by the Company and its Restricted Subsidiaries in connection with such disposition;
(7) Indebtedness of the Company to a Restricted Subsidiary;provided that any such Indebtedness owing to a Restricted Subsidiary that is not a Guarantor is expressly subordinated in right of payment to the Notes;provided further that any subsequent issuance or transfer of any Capital Stock or any other event which results in the Restricted Subsidiary holding such Indebtedness ceasing to be a Restricted Subsidiary or any other subsequent transfer of any such Indebtedness (except to the Company or another Restricted Subsidiary) shall be deemed, in each case, to be an incurrence of such Indebtedness not permitted by this clause;
(8) Indebtedness of a Restricted Subsidiary to the Company or another Restricted Subsidiary;provided that if a Guarantor incurs such Indebtedness to a Restricted Subsidiary that is not a Guarantor, such Indebtedness is expressly subordinated in right of payment to the Guarantee of the Notes of such Guarantor;
194
Table of Contents
provided further that any subsequent issuance or transfer of any Capital Stock or any other event which results in any such Indebtedness being held by a person other than the Company or a Restricted Subsidiary or any subsequent transfer of any such Indebtedness (except to the Company or another Restricted Subsidiary) shall be deemed, in each case, to be an incurrence of such Indebtedness not permitted by this clause;
(9) shares of Preferred Stock of a Restricted Subsidiary issued to the Company or another Restricted Subsidiary,provided that any subsequent issuance or transfer of any Capital Stock or any other event which results in any such Restricted Subsidiary ceasing to be a Restricted Subsidiary or any other subsequent transfer of any such shares of Preferred Stock (except to the Company or another Restricted Subsidiary) shall be deemed in each case to be an issuance of such shares of Preferred Stock not permitted by this clause;
(10) Hedging Obligations (excluding Hedging Obligations entered into for speculative purposes) for the purpose of limiting interest rate risk with respect to any Indebtedness of the Company or any Restricted Subsidiary permitted to be incurred pursuant to “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock,” exchange rate risk or commodity pricing risk;
(11) obligations in respect of performance, bid, appeal and surety bonds and completion guarantees provided by the Company or any of its Restricted Subsidiaries in the ordinary course of business;
(12)(a) Indebtedness or Disqualified Stock of the Company and Indebtedness, Disqualified Stock or Preferred Stock of any Restricted Subsidiary equal to 100.0% of the net cash proceeds received by the Company since immediately after the Issue Date from the issue or sale of Equity Interests of the Company or cash contributed to the capital of the Company (in each case, other than proceeds of Disqualified Stock or sales of Equity Interests to the Company or any of its Subsidiaries) as determined in accordance with clauses (3)(b) and (3)(c) of the first paragraph of “—Limitation on Restricted Payments” to the extent such net cash proceeds or cash have not been applied pursuant to such clauses to make Restricted Payments or to make other Investments, payments or exchanges pursuant to the second paragraph of “—Limitation on Restricted Payments” or to make Permitted Investments (other than Permitted Investments specified in clauses (1), (2) and (3) of the definition thereof) and (b) Indebtedness or Disqualified Stock of the Company and Indebtedness, Disqualified Stock or Preferred Stock of any Restricted Subsidiary not otherwise permitted hereunder in an aggregate principal amount or liquidation preference, which when aggregated with the principal amount and liquidation preference of all other Indebtedness, Disqualified Stock and Preferred Stock then outstanding and incurred pursuant to this clause (12)(b), does not at any one time outstanding exceed the greater of (x) $150.0 million and (y) 5.0% of Total Assets (it being understood that any Indebtedness, Disqualified Stock or Preferred Stock incurred pursuant to this clause (12)(b) shall cease to be deemed incurred or outstanding for purposes of this clause (12)(b) but shall be deemed incurred for the purposes of the first paragraph of this covenant from and after the first date on which the Company or such Restricted Subsidiary could have incurred such Indebtedness, Disqualified Stock or Preferred Stock under the first paragraph of this covenant without reliance on this clause (12)(b));
(13) the incurrence or issuance by the Company or any Restricted Subsidiary of Indebtedness or Disqualified Stock, and the issuance by any Restricted Subsidiary of Preferred Stock, in each case which serves to refund, refinance, replace, renew, extend or defease any Indebtedness, Disqualified Stock or Preferred Stock of the Company or any Restricted Subsidiary incurred as permitted under the first paragraph of this covenant and clauses (2), (3), (4) and (12)(a) above, this clause (13) and clause (14) below or any Indebtedness, Disqualified Stock or Preferred Stock issued to so refund, refinance, replace, renew, extend or defease such Indebtedness, Disqualified Stock or Preferred Stock including additional Indebtedness, Disqualified Stock or Preferred Stock incurred to pay premiums (including reasonable tender premiums), defeasance costs and fees in connection therewith (the “Refinancing Indebtedness”) prior to its respective maturity;provided, however, that such Refinancing Indebtedness:
(a) has a Weighted Average Life to Maturity at the time such Refinancing Indebtedness is incurred which is not less than the remaining Weighted Average Life to Maturity of the Indebtedness, Disqualified Stock or Preferred Stock being refunded, refinanced, replaced, renewed, extended or defeased,
195
Table of Contents
(b) to the extent such Refinancing Indebtedness refinances (i) Indebtedness subordinated orpari passu to the Notes or any Guarantee thereof, such Refinancing Indebtedness is subordinated orpari passu to the Notes or the Guarantee at least to the same extent as the Indebtedness being refinanced or refunded or (ii) Disqualified Stock or Preferred Stock, such Refinancing Indebtedness must be Disqualified Stock or Preferred Stock, respectively, and
(c) shall not include (i) Indebtedness, Disqualified Stock or Preferred Stock of a Subsidiary of the Company that is not a Guarantor that refinances Indebtedness or Disqualified Stock of the Company, (ii) Indebtedness, Disqualified Stock or Preferred Stock of a Subsidiary of the Company that is not a Guarantor that refinances Indebtedness, Disqualified Stock or Preferred Stock of a Guarantor, or (iii) Indebtedness or Disqualified Stock of the Company or Indebtedness, Disqualified Stock or Preferred Stock of a Restricted Subsidiary that refinances Indebtedness, Disqualified Stock or Preferred Stock of an Unrestricted Subsidiary;
provided further that subclause (a) of this clause (13) will not apply to any refunding or refinancing of any Secured Indebtedness;
(14)(x) Indebtedness or Disqualified Stock of the Company and Indebtedness, Disqualified Stock or Preferred Stock of a Restricted Subsidiary, incurred or issued to finance an acquisition or (y) Indebtedness, Disqualified Stock or Preferred Stock of Persons that are acquired by the Company or any Restricted Subsidiary or merged into the Company or a Restricted Subsidiary in accordance with the terms of the Indenture;provided that in the case of (x) and (y) after giving effect to such acquisition or merger, either (a) the Company would be permitted to incur at least $1.00 of additional Indebtedness pursuant to the Fixed Charge Coverage Ratio test set forth in the first sentence of this covenant or (b) the Fixed Charge Coverage Ratio of the Company and the Restricted Subsidiaries is greater than immediately prior to such acquisition or merger;
(15) Indebtedness arising from the honoring by a bank or other financial institution of a check, draft or similar instrument drawn against insufficient funds in the ordinary course of business, provided that such Indebtedness is extinguished within two Business Days of its incurrence;
(16) Indebtedness of the Company or any of its Restricted Subsidiaries supported by a letter of credit issued pursuant to the Credit Facilities, in a principal amount not in excess of the stated amount of such letter of credit;
(17)(a) any guarantee by the Company or a Restricted Subsidiary of Indebtedness or other obligations of any Restricted Subsidiary so long as the incurrence of such Indebtedness incurred by such Restricted Subsidiary is permitted under the terms of the Indenture, or
(b) any guarantee by a Restricted Subsidiary of Indebtedness of the Company provided that such guarantee is incurred in accordance with the covenant described below under “—Limitation on Guarantees of Indebtedness by Restricted Subsidiaries”;
(18) Indebtedness of Foreign Subsidiaries of the Company in an amount not to exceed, at any one time outstanding and together with any other Indebtedness incurred under this clause (18), the greater of (x) $50.0 million and (y) 10.0% of the total assets of the Foreign Subsidiaries on a consolidated basis as shown on the Company’s most recent balance sheet (it being understood that any Indebtedness incurred pursuant to this clause (18) shall cease to be deemed incurred or outstanding for purposes of this clause (18) but shall be deemed incurred for the purposes of the first paragraph of this covenant from and after the first date on which the Company or its Restricted Subsidiaries could have incurred such Indebtedness under the first paragraph of this covenant without reliance on this clause (18));
(19) Indebtedness of the Company or any of its Restricted Subsidiaries consisting of (i) the financing of insurance premiums or (ii) take-or-pay obligations contained in supply arrangements in each case, incurred in the ordinary course of business; and
196
Table of Contents
(20) Indebtedness consisting of Indebtedness issued by the Company or any of its Restricted Subsidiaries to current or former officers, directors and employees thereof, their respective estates, spouses or former spouses, in each case to finance the purchase or redemption of Equity Interests of the Company or any direct or indirect parent company of the Company to the extent described in clause (4) of the second paragraph under the caption “—Limitation on Restricted Payments.”
For purposes of determining compliance with this covenant:
(1) in the event that an item of Indebtedness, Disqualified Stock or Preferred Stock (or any portion thereof) meets the criteria of more than one of the categories of permitted Indebtedness, Disqualified Stock or Preferred Stock described in clauses (1) through (20) above or is entitled to be incurred pursuant to the first paragraph of this covenant, the Company, in its sole discretion, will classify or reclassify such item of Indebtedness, Disqualified Stock or Preferred Stock (or any portion thereof) and will only be required to include the amount and type of such Indebtedness, Disqualified Stock or Preferred Stock in one of the above clauses; and
(2) at the time of incurrence, the Company will be entitled to divide and classify an item of Indebtedness in more than one of the types of Indebtedness described in the first and second paragraphs above;provided that all Indebtedness outstanding under the Credit Facilities on the Issue Date will be treated as incurred on the Issue Date under clause (1) of the preceding paragraph.
Accrual of interest or dividends, the accretion of accreted value, the accretion or amortization of original issue discount, the payment of interest in the form of additional Indebtedness and the payment of dividends in the form of additional Disqualified Stock or Preferred Stock, as applicable, will in each case not be deemed to be an incurrence of Indebtedness, Disqualified Stock or Preferred Stock for purposes of this covenant.
For purposes of determining compliance with any U.S. dollar-denominated restriction on the incurrence of Indebtedness, the U.S. dollar-equivalent principal amount of Indebtedness denominated in a foreign currency shall be calculated based on the relevant currency exchange rate in effect on the date such Indebtedness was incurred, in the case of term debt, or first committed, in the case of revolving credit debt;provided that if such Indebtedness is incurred to refinance other Indebtedness denominated in a foreign currency, and such refinancing would cause the applicable U.S. dollar denominated restriction to be exceeded if calculated at the relevant currency exchange rate in effect on the date of such refinancing, such U.S. dollar-denominated restriction shall be deemed not to have been exceeded so long as the principal amount of such refinancing Indebtedness does not exceed the principal amount of such Indebtedness being refinanced, plus the amount of any reasonable premium (including reasonable tender premiums), defeasance costs and any reasonable fees and expenses incurred in connection with the issuance of such new Indebtedness.
The principal amount of any Indebtedness incurred to refinance other Indebtedness, if incurred in a different currency from the Indebtedness being refinanced, shall be calculated based on the currency exchange rate applicable to the currencies in which such respective Indebtedness is denominated that is in effect on the date of such refinancing.
The Indenture provides that the Company will not, and will not permit any Guarantor to, directly or indirectly, incur any Indebtedness (including Acquired Indebtedness) that is subordinated or junior in right of payment to any Indebtedness of the Company or such Guarantor, as the case may be, unless such Indebtedness is expressly subordinated in right of payment to the Notes or such Guarantor’s Guarantee to the extent and in the same manner as such Indebtedness is subordinated to other Indebtedness of the Company or such Guarantor, as the case may be.
The Indenture does not treat (1) unsecured Indebtedness as subordinated or junior to Secured Indebtedness merely because it is unsecured or (2) Senior Indebtedness as subordinated or junior to any other Senior Indebtedness merely because it has a junior priority with respect to the same collateral.
197
Table of Contents
Liens
The Company will not, and will not permit any Guarantor to, directly or indirectly, create, incur, assume or otherwise cause or suffer to exist any Lien (except Permitted Liens) that secures obligations under any Indebtedness or any related Guarantee of the Company or any Guarantor (any such Lien, the “Initial Lien”), on any asset or property of the Company or any Guarantor, or any income or profits therefrom, or assign or convey any right to receive income therefrom except, in the case of any asset or property that does not constitute Collateral, any Initial Lien if the Notes are equally and ratably secured with (or on a senior basis to, in the case such Initial Lien secures any Subordinated Indebtedness) the obligations secured by such Initial Lien.
Any Lien created for the benefit of the Holders of the Notes pursuant to the last clause of the preceding paragraph shall provide by its terms that such Lien shall be automatically and unconditionally released and discharged upon the release and discharge of the Initial Lien which release and discharge in the case of any sale of any such asset or property shall not affect any Lien that the Notes/Term Collateral Agent may have on the proceeds from such sale.
Merger, Consolidation or Sale of All or Substantially All Assets
Company. The Company may not, directly or indirectly, consolidate or merge with or into or wind up into (whether or not the Company is the surviving corporation), or sell, assign, transfer, lease, convey or otherwise dispose of all or substantially all of the Company’s properties or assets, in one or more related transactions, to any Person unless:
(1) the Company is the surviving corporation or the Person formed by or surviving any such consolidation or merger (if other than the Company) or to which such sale, assignment, transfer, lease, conveyance or other disposition will have been made is a corporation, partnership (including a limited partnership), trust or limited liability company organized or existing under the laws of the jurisdiction of organization of the Company or the laws of the United States, any state thereof, the District of Columbia or any territory thereof (such Person, as the case may be, being herein called the “Successor Company”);provided that in the case where the Successor Company is not a corporation, a co-obligor of the Notes is a corporation;
(2) the Successor Company, if other than the Company, expressly assumes all the obligations of the Company under the Notes and the Collateral Documents, pursuant to supplemental indentures or other documents or instruments in form reasonably satisfactory to the Trustee, and the Registration Rights Agreement if the exchange offer contemplated therein has not been consummated or if the Company continues to have an obligation to file or maintain the effectiveness of a shelf registration statement as provided under such agreement;
(3) immediately after such transaction, no Default or Event of Default exists;
(4) immediately after givingpro forma effect to such transaction and any related financing transactions, as if such transactions had occurred at the beginning of the applicable four-quarter period,
(a) the Company or the Successor Company, as applicable, would be permitted to incur at least $1.00 of additional Indebtedness pursuant to the Fixed Charge Coverage Ratio test set forth in the first sentence of the covenant described under “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock,” or
(b) the Fixed Charge Coverage Ratio for the Company (or, if applicable, the Successor Company) and its Restricted Subsidiaries would be greater than such Ratio for the Company and its Restricted Subsidiaries immediately prior to such transaction;
(5) each Guarantor, unless it is the other party to the transactions described above, in which case subclause (b) of the second succeeding paragraph shall apply, shall have by supplemental indenture confirmed that its Guarantee shall apply to such Person’s obligations under the Indenture, the Notes, the
198
Table of Contents
Collateral Documents and the Registration Rights Agreement if the exchange offer contemplated therein has not been consummated or if the Company continues to have an obligation to file or maintain the effectiveness of a shelf registration statement as provided under such agreement;
(6) the Company (or, if applicable, the Successor Company) shall have delivered to the Trustee an Officer’s Certificate and an Opinion of Counsel, each stating that such consolidation, merger or transfer and such supplemental indentures, if any, comply with the Indenture;
(7) the Collateral transferred to the Successor Company will (a) continue to constitute Collateral under the Indenture and the Collateral Documents, (b) be subject to the Lien in favor of the Trustee for the benefit of the holders of the Notes, and (c) not be subject to any Lien, other than Liens permitted by the terms of the Indenture; and
(8) to the extent that the assets of the Person which is merged or consolidated with or into the Successor Company are assets of the type which would constitute Collateral under the Collateral Documents, the Successor Company will take such actions as may be reasonably necessary to cause such property and assets to be made subject to the Lien of the Collateral Documents in the manner and to the extent required in the Indenture.
The Successor Company will succeed to, and be substituted for the Company, as the case may be, under the Indenture, the Guarantees and the Notes, as applicable. Notwithstanding the foregoing clauses (3) and (4),
(1) any Restricted Subsidiary may consolidate with or merge into or transfer all or part of its properties and assets to the Company, and
(2) the Company may merge with an Affiliate of the Company, as the case may be, solely for the purpose of reincorporating the Company in the United States, any state thereof, the District of Columbia or any territory thereof so long as the amount of Indebtedness of the Company and its Restricted Subsidiaries is not increased thereby.
Guarantors. Subject to certain limitations described in the Indenture governing release of a Guarantee upon the sale, disposition or transfer of a guarantor, no Guarantor will, and the Company will not permit any Guarantor to, consolidate or merge with or into or wind up into (whether or not the Company or Guarantor is the surviving corporation), or sell, assign, transfer, lease, convey or otherwise dispose of all or substantially all of its properties or assets, in one or more related transactions, to any Person unless:
(1)(a) such Guarantor is the surviving corporation or the Person formed by or surviving any such consolidation or merger (if other than such Guarantor) or to which such sale, assignment, transfer, lease, conveyance or other disposition will have been made is a corporation, partnership, trust or limited liability company organized or existing under the laws of the jurisdiction of organization of such Guarantor, as the case may be, or the laws of the United States, any state thereof, the District of Columbia or any territory thereof (such Guarantor or such Person, as the case may be, being herein called the “Successor Person”);
(b) the Successor Person, if other than such Guarantor, expressly assumes all the obligations of such Guarantor under the Indenture, such Guarantor’s related Guarantee and the Collateral Documents pursuant to supplemental indentures or other documents or instruments in form reasonably satisfactory to the Trustee;
(c) immediately after such transaction, no Default or Event of Default exists;
(d) the Company shall have delivered to the Trustee an Officer’s Certificate and an Opinion of Counsel, each stating that such consolidation, merger or transfer and such supplemental indentures, if any, comply with the Indenture;
(e) the Collateral transferred to the Successor Person will (a) continue to constitute Collateral under the Indenture and the Collateral Documents, (b) be subject to the Lien in favor of the trustee for the benefit of the holders of the Notes, and (c) not be subject to any Lien, other than Liens permitted by the terms of the Indenture; and
199
Table of Contents
(f) to the extent that the assets of the Person which is merged or consolidated with or into the Successor Person are assets of the type which would constitute Collateral under the Collateral Documents, the Successor Person will take such action as may be reasonably necessary to cause such property and assets to be made subject to the Lien of the Collateral Documents in the manner and to the extent required in the Indenture; or
(2) the transaction is made in compliance with the covenant described under “—Repurchase at the Option of Holders—Asset Sales.”
Subject to certain limitations described in the Indenture, the Successor Person will succeed to, and be substituted for, such Guarantor under the Indenture and such Guarantor’s Guarantee. Notwithstanding the foregoing, any Guarantor may (i) merge into or transfer all or part of its properties and assets to another Guarantor or the Company, (ii) merge with an Affiliate of the Company solely for the purpose of reincorporating the Guarantor in the United States, any state thereof, the District of Columbia or any territory thereof or (iii) convert into a corporation, partnership, limited partnership, limited liability company or trust organized under the laws of the jurisdiction of organization of such Guarantor, in each case without regard to the requirements set forth in the preceding paragraph.
Transactions with Affiliates
The Company will not, and will not permit any of its Restricted Subsidiaries to, make any payment to, or sell, lease, transfer or otherwise dispose of any of its properties or assets to, or purchase any property or assets from, or enter into or make or amend, any transaction, contract, agreement, understanding, loan, advance or guarantee with, or for the benefit of any Affiliate of the Company (each of the foregoing, an “Affiliate Transaction”) involving aggregate payments or consideration in excess of $12.5 million, unless:
(1) such Affiliate Transaction is on terms that are not materially less favorable to the Company or its relevant Restricted Subsidiary than those that would have been obtained in a comparable transaction by the Company or such Restricted Subsidiary with an unrelated Person on an arm’s-length basis; and
(2) the Company delivers to the Trustee, with respect to any Affiliate Transaction or series of related Affiliate Transactions involving aggregate payments or consideration in excess of $25.0 million, a resolution adopted by the majority of the board of directors of the Company approving such Affiliate Transaction and set forth in an Officer’s Certificate certifying that such Affiliate Transaction complies with clause (1) above.
The foregoing provisions will not apply to the following:
(1) transactions between or among the Company or any of its Restricted Subsidiaries;
(2) Restricted Payments permitted by the provisions of the Indenture described above under the covenant “—Limitation on Restricted Payments” and the definition of “Permitted Investment”;
(3) the payment of management, consulting, monitoring and advisory fees and related expenses to the Investors pursuant to the Sponsor Management Agreement in an aggregate amount in any fiscal year not to exceed the greater of $7.0 million and 2.0% of EBITDA for such fiscal year (calculated, solely for the purpose of this clause (3), assuming (a) that such fees and related expenses had not been paid, when calculating Net Income, and (b) without giving effect to clause (g) of the definition of EBITDA) (plus any unpaid management, consulting, monitoring and advisory fees and related expenses within such amount accrued in any prior year) and the termination fees pursuant to the Sponsor Management Agreement not to exceed the amount set forth in the Sponsor Management Agreement as in effect on the Issue Date or any amendment thereto (so long as any such amendment is not disadvantageous to the Holders when taken as a whole as compared to the Sponsor Management Agreement in effect on the Issue Date);
(4) the payment of reasonable and customary fees paid to, and indemnities provided for the benefit of, former, current or future officers, directors, employees or consultants of the Company, any of its direct or indirect parent companies or any of its Restricted Subsidiaries;
200
Table of Contents
(5) transactions in which the Company or any of its Restricted Subsidiaries, as the case may be, delivers to the Trustee a letter from an Independent Financial Advisor stating that such transaction is fair to the Company or such Restricted Subsidiary from a financial point of view or stating that such terms are not materially less favorable to the Company or its relevant Restricted Subsidiary than those that would have been obtained in a comparable transaction by the Company or such Restricted Subsidiary with an unrelated Person on an arm’s-length basis;
(6) any agreement as in effect as of the Issue Date, or any amendment thereto (so long as any such amendment is not disadvantageous to the Holders when taken as a whole as compared to the applicable agreement as in effect on the Issue Date);
(7) the existence of, or the performance by the Company or any of its Restricted Subsidiaries of its obligations under the terms of, any stockholders agreement (including any registration rights agreement or purchase agreement related thereto) to which it is a party as of the Issue Date and any similar agreements which it may enter into thereafter;provided, however, that the existence of, or the performance by the Company or any of its Restricted Subsidiaries of obligations under any future amendment to any such existing agreement or under any similar agreement entered into after the Issue Date shall only be permitted by this clause (7) to the extent that the terms of any such amendment or new agreement are not otherwise disadvantageous to the Holders when taken as a whole;
(8) the Transaction and the payment of all fees and expenses related to the Transaction;
(9) transactions with customers, clients, suppliers, or purchasers or sellers of goods or services, in each case in the ordinary course of business and otherwise in compliance with the terms of the Indenture which are fair to the Company and its Restricted Subsidiaries, in the reasonable determination of the board of directors of the Company or the senior management thereof, or are on terms at least as favorable as might reasonably have been obtained at such time from an unaffiliated party;
(10) the issuance of Equity Interests (other than Disqualified Stock) of the Company to any Permitted Holder or to any director, officer, employee or consultant;
(11) sales of accounts receivable, or participations therein, in connection with any Receivables Facility;
(12) payments by the Company or any of its Restricted Subsidiaries to any of the Investors made for any financial advisory, financing, underwriting or placement services or in respect of other investment banking activities, including, without limitation, in connection with acquisitions or divestitures which payments are approved by a majority of the board of directors of the Company in good faith;
(13) payments or loans (or cancellation of loans) to employees or consultants of the Company, any of its direct or indirect parent companies or any of its Restricted Subsidiaries and employment agreements, stock option plans and other similar arrangements with such employees or consultants which, in each case, are approved by the Company in good faith;
(14) investments by the Investors in securities of the Company or any of its Restricted Subsidiaries so long as (i) the investment is being offered generally to other investors on the same or more favorable terms and (ii) the investment constitutes less than 5.0% of the proposed or outstanding issue amount of such class of securities; and
(15) the pledge of Equity Interests of any Unrestricted Subsidiary to lenders to support the Indebtedness of such Unrestricted Subsidiary owed to such lenders.
201
Table of Contents
Dividend and Other Payment Restrictions Affecting Restricted Subsidiaries
The Company will not, and will not permit any of its Restricted Subsidiaries that are not Guarantors to, directly or indirectly, create or otherwise cause or suffer to exist or become effective any consensual encumbrance or consensual restriction on the ability of any Restricted Subsidiary that is not a Guarantor to:
(1)(a) pay dividends or make any other distributions to the Company or any of its Restricted Subsidiaries on its Capital Stock or with respect to any other interest or participation in, or measured by, its profits, or
(b) pay any liabilities owed to the Company or any of its Restricted Subsidiaries;
(2) make loans or advances to the Company or any of its Restricted Subsidiaries; or
(3) sell, lease or transfer any of its properties or assets to the Company or any of its Restricted Subsidiaries,
except (in each case) for such encumbrances or restrictions existing under or by reason of:
(a) contractual encumbrances or restrictions in effect on the Issue Date, including pursuant to the ABL Facility and the Term Loan Facility and the related documentation;
(b) the Indenture and the Notes;
(c) purchase money obligations for property acquired in the ordinary course of business that impose restrictions of the nature discussed in clause (3) above on the property so acquired;
(d) applicable law or any applicable rule, regulation or order;
(e) any agreement or other instrument of a Person acquired by the Company or any Restricted Subsidiaries in existence at the time of such acquisition (but not created in contemplation thereof), which encumbrance or restriction is not applicable to any Person, or the properties or assets of any Person, other than the Person and its Subsidiaries, or the property or assets of the Person and its Subsidiaries, so acquired;
(f) contracts for the sale of assets, including customary restrictions with respect to a Subsidiary of the Company pursuant to an agreement that has been entered into for the sale or disposition of all or substantially all of the Capital Stock or assets of such Subsidiary;
(g) Secured Indebtedness otherwise permitted to be incurred pursuant to the covenants described under “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” and “—Liens” that limit the right of the debtor to dispose of the assets securing such Indebtedness;
(h) restrictions on cash or other deposits or net worth imposed by customers under contracts entered into in the ordinary course of business;
(i) other Indebtedness, Disqualified Stock or Preferred Stock of Foreign Subsidiaries permitted to be incurred subsequent to the Issue Date pursuant to the provisions of the covenant described under “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”;
(j) customary provisions in joint venture agreements and other similar agreements relating solely to such joint venture;
(k) customary provisions contained in leases or licenses of intellectual property and other agreements, in each case, entered into in the ordinary course of business;
(l) any encumbrances or restrictions of the type referred to in clauses (1), (2) and (3) above imposed by any amendments, modifications, restatements, renewals, increases, supplements, refundings, replacements or refinancings of the contracts, instruments or obligations referred to in clauses (a) through (k) above;provided that such amendments, modifications, restatements, renewals,
202
Table of Contents
increases, supplements, refundings, replacements or refinancings are, in the good faith judgment of the Company, no more restrictive with respect to such encumbrance and other restrictions taken as a whole than those prior to such amendment, modification, restatement, renewal, increase, supplement, refunding, replacement or refinancing; and
(m) restrictions created in connection with any Receivables Facility that, in the good faith determination of the Company are necessary or advisable to effect the transactions contemplated under such Receivables Facility.
Limitation on Guarantees of Indebtedness by Restricted Subsidiaries
The Company will not permit any of its Wholly-Owned Subsidiaries that are Restricted Subsidiaries (and non-Wholly Owned Subsidiaries if such non-Wholly Owned Subsidiaries guarantee other capital markets debt securities), other than a Guarantor or a Foreign Subsidiary guaranteeing Indebtedness of another Foreign Subsidiary, to guarantee the payment of any Indebtedness of the Company or any other Guarantor unless:
(1) such Restricted Subsidiary within 30 days executes and delivers a supplemental indenture to the Indenture providing for a Guarantee by such Restricted Subsidiary, except that with respect to a guarantee of Indebtedness of the Company or any Guarantor:
(a) if such Indebtedness is by its express terms subordinated in right of payment to the Notes or such Guarantor’s Guarantee, any such guarantee by such Restricted Subsidiary with respect to such Indebtedness shall be subordinated in right of payment to such Guarantee substantially to the same extent as such Indebtedness is subordinated to the Notes; and
(b) such Restricted Subsidiary waives and will not in any manner whatsoever claim or take the benefit or advantage of, any rights of reimbursement, indemnity or subrogation or any other rights against the Company or any other Restricted Subsidiary as a result of any payment by such Restricted Subsidiary under its Guarantee; and
(2) such Restricted Subsidiary within 30 days executes and delivers a joinder agreement to the Collateral Documents providing for a pledge of its assets as Collateral for the Notes to the same extent as set forth in the Indenture and the Collateral Documents;
provided that this covenant shall not be applicable to any guarantee of any Restricted Subsidiary that existed at the time such Person became a Restricted Subsidiary and was not incurred in connection with, or in contemplation of, such Person becoming a Restricted Subsidiary.
Events of Loss
Subject to the Intercreditor Agreement and the other Collateral Documents, in the case of an Event of Loss with respect to any Notes/Term Collateral, the Company or the affected Restricted Subsidiary, as the case may be, will apply the Net Loss Proceeds from such Event of Loss, within 365 days after receipt, at its option to:
(1) permanently reduce Obligations under the Notes and any additional Series A-2 Debt in accordance with paragraph (1)(a) of the second paragraph under “—Repurchase at the Option of Holders—Asset Sales”;
(2) rebuild, repair, replace or construct improvements to the affected property or facility (or enter into a binding agreement to do so,provided that (x) such rebuilding, repair, replacement or construction has been completed within six months after the date of such binding agreement and (y) if such rebuilding, repair, replacement or construction is not consummated within the period set forth in subclause (x), the Net Loss Proceeds not so applied will be deemed to be Excess Loss Proceeds (as defined below)); or
(3) invest in assets and properties as described in clauses (2) and (3) of the second paragraph under the caption “—Repurchase at the Option of Holders—Asset Sales,” substituting the term “Event of Loss” for the term “Asset Sale,” the term “Net Loss Proceeds” for the term “Net Proceeds” and the term “Excess Loss Proceeds” for the term “Excess Proceeds.”
203
Table of Contents
In case of clause (2) or (3) above, any replacement assets or property shall be pledged as Notes/Term Collateral, in accordance with the Collateral Documents and otherwise in compliance with the provisions in the Indenture governing After-Acquired Property.
Any Net Loss Proceeds from an Event of Loss that are not applied or invested as provided in the prior paragraph will be deemed to constitute “Excess Loss Proceeds.” When the aggregate amount of Excess Loss Proceeds exceeds $30.0 million, the Company will make an offer (a “Loss Proceeds Offer”) to all holders of Notes and holders of Series A-2 Debt to purchase the maximum principal amount of Notes and any additional Series A-2 Debt that may be purchased out of such Excess Loss Proceeds, at an offer price in cash in an amount equal to 100% of the principal amount of the Notes and any additional Series A-2 Debt plus accrued and unpaid interest thereon, if any, to the date of purchase. If any Excess Loss Proceeds remain after consummation of a Loss Proceeds Offer, such Excess Loss Proceeds may be used for any purpose not otherwise prohibited by the Indenture. If the aggregate principal amount of the Notes and any additional Series A-2 Debt tendered into such Loss Proceeds Offer exceeds the amount of Excess Loss Proceeds, then the Notes and such additional Series A-2 Debt will be purchased on a pro rata basis based on the principal amount of the Notes and such additional Series A-2 Debt tendered. The Company may satisfy the foregoing obligations with respect to any Net Loss Proceeds from an Event of Loss by making a Loss Proceeds Offer with respect to such Net Loss Proceeds prior to the expiration of the relevant 365 days or with respect to Net Loss Proceeds of $30.0 million or less.
The Indenture provides that the Company will comply with the applicable tender offer rules, including Rule 14e-1 under the Exchange Act, and any other applicable securities laws or regulations in connection with a Loss Proceeds Offer.
“Casualty” means any casualty, loss, damage, destruction or other similar loss with respect to real or personal property or improvements.
“Condemnation” means any taking by a Governmental Authority of property or assets, or any part thereof or interest therein, for public or quasi-public use under the power of eminent domain, by reason of any public improvement or condemnation or in any other manner.
“Condemnation Award” means all proceeds of any Condemnation or transfer in lieu thereof.
“Event of Loss” means, with respect to any Collateral, any (1) Casualty of such Collateral, (2) Condemnation or seizure (other than pursuant to foreclosure or confiscation or requisition of the use of such Collateral) or (3) settlement in lieu of clause (2) above, in each case having a fair market value in excess of $10.0 million.
“Governmental Authority” means the government of the United States or any other nation, or of any political subdivision thereof, whether state or local, and any agency, authority, instrumentality, regulatory body, court, central bank or other entity exercising executive, legislative, judicial, taxing, regulatory or administrative powers or functions of or pertaining to government (including any supra-national bodies such as the European Union or the European Central bank).
“Net Loss Proceeds” means, with respect to any Event of Loss, the proceeds in the form of (a) cash or Cash Equivalents, (b) insurance proceeds, (c) Condemnation Awards or (d) damages awarded by any judgment, in each case received by the Company or any of its Restricted Subsidiaries from such Event of Loss, net of:
(1) reasonable out-of-pocket expenses and fees relating to such Event of Loss (including without limitation legal, accounting and appraisal or insurance adjuster fees);
(2) taxes paid or payable after taking into account any reduction in consolidated tax liability due to available tax credits or deductions and any tax sharing arrangements;
204
Table of Contents
(3) any repayment of Indebtedness that is secured by, or directly related to, the property or assets that are the subject of such Event of Loss;
(4) amounts required to be paid to any Person (other than the Company or any Restricted Subsidiary) owning a beneficial interest in the assets subject to the Event of Loss or having a Lien thereon; and
(5) appropriate amounts to be provided by the Company or any Restricted Subsidiary, as the case may be, as a reserve, in accordance with GAAP, against any liabilities associated with such Event of Loss and retained by the Company or any Restricted Subsidiary, as the case may be, after such Event of Loss, including, without limitation, liabilities related to environmental matters and liabilities under any indemnification obligations associated with such Event of Loss.
After-Acquired Property
Promptly following the acquisition by the Company or any Guarantor of any After-Acquired Property (but subject to the limitations, if applicable, described under “—Security for the Notes—Notes/Term Collateral” and “—Security for the Notes—Limitations on Stock Collateral”) the Company or such Guarantor shall execute and deliver such mortgages, deeds of trust, security instruments, financing statements and certificates and opinions of counsel as shall be reasonably necessary to vest in the Notes/Term Collateral Agent a perfected security interest in such After-Acquired Property and to have such After-Acquired Property added to the Notes/Term Collateral or the ABL Collateral, as applicable, and thereupon all provisions of the Indenture relating to the Notes/Term Collateral or the ABL Collateral, as applicable, shall be deemed to relate to such After-Acquired Property to the same extent and with the same force and effect.
Reports and Other Information
Notwithstanding that the Company may not be subject to the reporting requirements of Section 13 or 15(d) of the Exchange Act or otherwise report on an annual and quarterly basis on forms provided for such annual and quarterly reporting pursuant to rules and regulations promulgated by the SEC, the Indenture will require the Company to file with the SEC (and make available to the Trustee and Holders of the Notes (without exhibits), without cost to any Holder, within 15 days after it files them with the SEC) from and after the Issue Date,
(1) within 90 days (or any other time period then in effect under the rules and regulations of the Exchange Act with respect to the filing of a Form 10-K by a non-accelerated filer) after the end of each fiscal year, annual reports on Form 10-K, or any successor or comparable form, containing the information required to be contained therein, or required in such successor or comparable form;
(2) within 45 days after the end of each of the first three fiscal quarters of each fiscal year, reports on Form 10-Q containing all quarterly information that would be required to be contained in Form 10-Q, or any successor or comparable form;
(3) promptly from time to time after the occurrence of an event required to be therein reported, such other reports on Form 8-K, or any successor or comparable form; and
(4) any other information, documents and other reports which the Company would be required to file with the SEC if it were subject to Section 13 or 15(d) of the Exchange Act;
in each case, in a manner that complies in all material respects with the requirements specified in such form;provided that the Company shall not be so obligated to file such reports with the SEC if the SEC does not permit such filing, in which event the Company will make available such information to prospective purchasers of the Notes, in addition to providing such information to the Trustee and the Holders of the Notes, in each case within 15 days after the time the Company would be required to file such information with the SEC, if it were subject to Section 13 or 15(d) of the Exchange Act. In addition, to the extent not satisfied by the foregoing, the Company will agree that, for so long as any Notes are outstanding, it will furnish to Holders and to securities analysts and prospective investors, upon their request, the information required to be delivered pursuant to Rule 144A(d)(4) under the Securities Act.
205
Table of Contents
In the event that any direct or indirect parent company of the Company becomes a guarantor of the Notes, the Indenture will permit the Company to satisfy its obligations in this covenant with respect to financial information relating to the Company by furnishing financial information relating to such parent company;provided that the same is accompanied by consolidating information that explains in reasonable detail the differences between the information relating to such parent, on the one hand, and the information relating to the Company and its Restricted Subsidiaries on a standalone basis, on the other hand.
Notwithstanding the foregoing, such requirements shall be deemed satisfied prior to the commencement of the exchange offer or the effectiveness of the shelf registration statement (but in no event later than the date specified in the applicable registration rights agreement by which the exchange offer for the Notes must be consummated) (1) by the filing with the SEC of the exchange offer registration statement or shelf registration statement (or any other registration statement), and any amendments thereto, with such financial information that satisfies Regulation S-X of the Securities Act or (2) by posting reports that would be required to be filed substantially in the form required by the SEC on the Company’s website (or on the website of any of its parent companies) or providing such reports to the Trustee, with financial information that satisfied Regulation S-X of the Securities Act, subject to exceptions consistent with the presentation of financial information in the offering memoranda distributed in connection with the private offerings of the outstanding notes, to the extent filed within the times specified above.
Notwithstanding anything herein to the contrary, the Company will not be deemed to have failed to comply with any of its obligations hereunder for purposes of clause (3) under “—Events of Default and Remedies” until 90 days after the date any report hereunder is due.
Further Assurances
The Company and the Guarantors shall execute any and all further documents, financing statements, agreements and instruments, and take all further action that may be required under applicable law, or that the Trustee may reasonably request, in order to grant, preserve, protect and perfect the validity and priority of the security interests created or intended to be created by the Collateral Documents in the Collateral. In addition, from time to time, the Company will reasonably promptly secure the obligations under the Indenture and the Collateral Documents by pledging or creating, or causing to be pledged or created, perfected security interests with respect to the Collateral. Such security interests and Liens will be created under the Collateral Documents and other security agreements, mortgages, deeds of trust and other instruments and documents in form and substance reasonably satisfactory to the Trustee.
Events of Default and Remedies
The Indenture provides that each of the following is an Event of Default:
(1) default in payment when due and payable (whether at maturity, upon redemption, acceleration or otherwise), of principal of, or premium, if any, on (A) with respect to holders of the Series A-1 Notes, the Series A-1 Notes and (B) with respect to holders of the Series A-2 Notes, the Series A-2 Notes;
(2) default for 30 days or more in the payment when due of interest or Additional Interest on or with respect to on (A) with respect to holders of the Series A-1 Notes, the Series A-1 Notes and (B) with respect to holders of the Series A-2 Notes, the Series A-2 Notes;
(3) failure by the Company or any Guarantor for 60 days after receipt of written notice given by the Trustee or the holders of not less than 25% of the aggregate principal amount of all then outstanding Series of Debt (unless such failure affects some but not all Series of Debt, in which case the notice of such failure may be given by the Trustee or the holders of not less than 25% of the aggregate principal amount of the then outstanding Series of Debt that are affected by such failure) to comply with any of its obligations, covenants or agreements (other than a default referred to in clauses (1) and (2) above) contained in the Indenture, the Notes or the Collateral Documents;
206
Table of Contents
(4) default under any mortgage, indenture or instrument under which there is issued or by which there is secured or evidenced any Indebtedness for money borrowed by the Company or any of its Restricted Subsidiaries or the payment of which is guaranteed by the Company or any of its Restricted Subsidiaries, other than Indebtedness owed to the Company or a Restricted Subsidiary, whether such Indebtedness or guarantee now exists or is created after the issuance of the Notes, if both:
(a) such default either results from the failure to pay any principal of such Indebtedness at its stated final maturity (after giving effect to any applicable grace periods) or relates to an obligation other than the obligation to pay principal of any such Indebtedness at its stated final maturity and results in the holder or holders of such Indebtedness causing such Indebtedness to become due prior to its stated maturity; and
(b) the principal amount of such Indebtedness, together with the principal amount of any other such Indebtedness in default for failure to pay principal at stated final maturity (after giving effect to any applicable grace periods), or the maturity of which has been so accelerated, aggregate $50.0 million or more at any one time outstanding;
(5) failure by the Company or any Significant Subsidiary to pay final judgments aggregating in excess of $50.0 million, which final judgments remain unpaid, undischarged and unstayed for a period of more than 60 days after such judgment becomes final, and in the event such judgment is covered by insurance, an enforcement proceeding has been commenced by any creditor upon such judgment or decree which is not promptly stayed;
(6) certain events of bankruptcy or insolvency with respect to the Company or any Significant Subsidiary; the Guarantee of any Significant Subsidiary shall for any reason cease to be in full force and effect or be declared null and void or any responsible officer of any Guarantor that is a Significant Subsidiary, as
(7) the case may be, denies that it has any further liability under its Guarantee or gives notice to such effect, other than by reason of the termination of the Indenture or the release of any such Guarantee in accordance with the Indenture; or
(8) any of the Collateral Documents ceases to be in full force and effect, or any of the Collateral Documents ceases to give the Holders of the Notes the Liens purported to be created thereby, or any of the Collateral Documents is declared null and void or the Company or any Restricted Subsidiary denies in writing that it has any further liability under any Collateral Document or gives written notice to such effect (in each case, other than in accordance with the terms of the Indenture or the terms of the Collateral Documents), provided that if a failure of the sort described in this clause (8) is susceptible of cure, no Event of Default shall arise under this clause (8) with respect thereto until 30 days after notice of such failure shall have been given to the Company by the Trustee or the holders of not less than 25% of the aggregate principal amount of all then outstanding Series of Debt (unless such failure affects some but not all Series of Debt, in which case the notice of such failure may be given by the holders of not less than 25% of the aggregate principal amount of the then outstanding Series of Debt that are affected by such failure).
If any Event of Default (other than of a type specified in clause (6) above) occurs and is continuing under the Indenture, the Trustee or the holders of not less than 25% of the aggregate principal amount of all then outstanding Series of Debt (unless such Event of Default affects some but not all Series of Debt, in which case the holders of not less than 25% of the aggregate principal amount of the then outstanding Series of Debt that are affected by such Event of Default) may (subject to the terms and conditions of the Notes/Term Intercreditor Agreement) declare the principal, premium, if any, interest and any other monetary obligations on all the then outstanding Notes to be due and payable immediately;provided, however, that (i) with respect to any payment Event of Default with respect to the Series A-1 Notes but not the Series A-2 Notes or the Term Loans (if any), the Trustee or the holders of at least 25% in principal amount of the then outstanding Series A-1 Notes may declare the principal, premium, if any, interest and any other monetary obligations on all the then outstanding Series A-1 Notes to be due and payable immediately and (ii) with respect to any payment Event of Default with
207
Table of Contents
respect to the Series A-2 Notes, but not the Series A-1 Notes or the Term Loans (if any), the Trustee or the Holders of at least 25% in principal amount of the then outstanding Series A-2 Notes may declare the principal, premium, if any, interest and any other monetary obligations on all the then outstanding Series A-2 Notes to be due and payable immediately.
Upon the effectiveness of such declaration, such principal and interest will be due and payable immediately. Notwithstanding the foregoing, in the case of an Event of Default arising under clause (6) of the first paragraph of this section, all outstanding Notes will become due and payable without further action or notice. The Indenture provides that the Trustee may withhold from the Holders notice of any continuing Default, except a Default relating to the payment of principal, premium, if any, or interest, if it determines that withholding notice is in their interest. In addition, the Trustee shall have no obligation to accelerate the Notes if in the best judgment of the Trustee acceleration is not in the best interest of the holders of the Notes.
The Indenture provides that the holders of a majority of the aggregate principal amount of all then outstanding Series of Debt (unless such Event of Default affects some but not all Series of Debt, in which case the holders of a majority of the aggregate principal amount of the then outstanding Series of Debt that are affected by such Event of Default), by notice to the Trustee, may waive any existing Default and its consequences under the Indenture or the Collateral Documents except a continuing Default in the payment of interest on, premium, if any, or the principal of any Note held by a non-consenting holder and rescind any acceleration with respect to the Notes and its consequences (provided such rescission would not conflict with any judgment of a court of competent jurisdiction),provided that any rescission of acceleration of the Series A-1 Notes, which acceleration resulted from a payment Event of Default, must be approved by the holders of more than 50% in principal amount of the then outstanding Series A-1 Notes, and any rescission of acceleration of the Series A-2 Notes, which acceleration resulted from a payment Event of Default, must be approved by the Holders of more than 50% in principal amount of the then outstanding Series A-2 Notes.
In the event of any Event of Default specified in clause (4) above, such Event of Default and all consequences thereof (excluding any resulting payment default, other than as a result of acceleration of the Notes) shall be annulled, waived and rescinded, automatically and without any action by the Trustee or the Holders, if within 20 days after such Event of Default arose:
(1) the Indebtedness or guarantee that is the basis for such Event of Default has been discharged;
(2) holders thereof have rescinded or waived the acceleration, notice or action (as the case may be) giving rise to such Event of Default; or
(3) the default that is the basis for such Event of Default has been cured.
Subject to the provisions of the Indenture relating to the duties of the Trustee thereunder, in case an Event of Default occurs and is continuing, the Trustee will be under no obligation to exercise any of the rights or powers under the Indenture at the request or direction of any of the Holders of the Notes unless the Holders have offered to the Trustee reasonable indemnity or security against any loss, liability or expense. Except to enforce the right to receive payment of principal, premium (if any) or interest when due, no Holder of a Note may pursue any remedy with respect to the Indenture or the Notes unless, subject to the provisions of the Notes/Term Intercreditor Agreement and the Intercreditor Agreement:
(1) such Holder has previously given the Trustee notice that an Event of Default is continuing;
(2) holders of at least 25% in the aggregate principal amount of all then outstanding Series of Notes (or, in the case of an Event of Default that affects some but not all Series of Notes, holders of at least 25% in the aggregate principal amount of the then outstanding Series of Notes that are affected by such Event of Default) have requested the Trustee to pursue the remedy;
(3) Holders of the Notes have offered the Trustee reasonable security or indemnity against any loss, liability or expense;
208
Table of Contents
(4) the Trustee has not complied with such request within 60 days after the receipt thereof and the offer of security or indemnity; and
(5) holders of a majority in principal amount of all then outstanding Series of Notes (or, in the case of an Event of Default that affects some but not all Series of Notes, holders of a majority in principal amount of the then outstanding Series of Notes that are affected by such Event of Default) have not given the Trustee a direction inconsistent with such request within such 60-day period.
Subject to certain restrictions contained in the Indenture, the Notes/Term Intercreditor Agreement and the Intercreditor Agreement, the holders of a majority in principal amount of the total outstanding Notes are given the right to direct the time, method and place of conducting any proceeding for any remedy available to the Trustee or of exercising any trust or power conferred on the Trustee. The Trustee, however, may refuse to follow any direction that conflicts with law or the Indenture or that the Trustee determines is unduly prejudicial to the rights of any other holder of a Note or that would involve the Trustee in personal liability.
The Indenture provides that the Company is required to deliver to the Trustee annually a statement regarding compliance with the Indenture, and the Company is required, within 30 days, upon becoming aware of any Default, to deliver to the Trustee a statement specifying such Default.
In addition to acceleration of maturity of the Notes, if an Event of Default occurs and is continuing, the Trustee or the Notes/Term Collateral Agent, as applicable, subject to the provisions contained in the Notes/Term Intercreditor Agreement and the Intercreditor Agreement, will have the right to exercise remedies with respect to the Collateral, such as foreclosure, as are available under the Indenture, the Collateral Documents and at law.
No Personal Liability of Directors, Officers, Employees and Stockholders
No director, officer, employee, incorporator or stockholder of the Company or any Guarantor or any of their parent companies shall have any liability for any obligations of the Company or the Guarantors under the Notes, the Guarantees, the Indenture or the Collateral Documents or for any claim based on, in respect of, or by reason of such obligations or their creation. Each Holder by accepting the Notes waives and releases all such liability. The waiver and release are part of the consideration for issuance of the Notes. Such waiver may not be effective to waive liabilities under the federal securities laws and it is the view of the SEC that such waiver is against public policy.
Legal Defeasance and Covenant Defeasance
The obligations of the Company and the Guarantors with respect to the Notes under the Indenture, the Notes, the Guarantees and the Collateral Documents, as the case may be, will terminate (other than certain obligations) and will be released upon payment in full of all of the Notes. The Company may, at its option and at any time, elect to have all of its obligations discharged with respect to the Notes and have each Guarantor’s obligation discharged with respect to its Guarantee (“Legal Defeasance”) and cure all then existing Events of Default except for:
(1) the rights of Holders of Notes to receive payments in respect of the principal of, premium, if any, and interest on the Notes when such payments are due solely out of the trust created pursuant to the Indenture;
(2) the Company’s obligations with respect to Notes concerning issuing temporary Notes, registration of such Notes, mutilated, destroyed, lost or stolen Notes and the maintenance of an office or agency for payment and money for security payments held in trust;
(3) the rights, powers, trusts, duties and immunities of the Trustee, and the Company’s obligations in connection therewith;
(4) the Legal Defeasance provisions of the Indenture; and
(5) the priority of payment provisions between the Series A-1 Notes and the Series A-2 Debt.
209
Table of Contents
In addition, the Company may, at its option and at any time, elect to have its obligations and those of each Guarantor released with respect to substantially all the restrictive covenants that are described in the Indenture (“Covenant Defeasance”) and thereafter any omission to comply with such obligations shall not constitute a Default with respect to the Notes. In the event Covenant Defeasance occurs, certain events (not including bankruptcy, receivership, rehabilitation and insolvency events pertaining to the Company) described under “—Events of Default and Remedies” will no longer constitute an Event of Default with respect to the Notes.
In order to exercise either Legal Defeasance or Covenant Defeasance with respect to the Notes:
(1) the Company must irrevocably deposit with the Trustee, in trust, for the benefit of the Holders of the Notes, cash in U.S. dollars, Government Securities, or a combination thereof, in such amounts as will be sufficient, in the opinion of a nationally recognized firm of independent public accountants, to pay the principal of, premium, if any, and interest due on the Notes on the stated maturity date or on the redemption date, as the case may be, of such principal, premium, if any, or interest on such Notes and the Company must specify whether such Notes are being defeased to maturity or to a particular redemption date;
(2) in the case of Legal Defeasance, the Company shall have delivered to the Trustee an Opinion of Counsel reasonably acceptable to the Trustee confirming that, subject to customary assumptions and exclusions,
(a) the Company has received from, or there has been published by, the United States Internal Revenue Service a ruling, or
(b) since the issuance of the Notes, there has been a change in the applicable U.S. federal income tax law,
in either case to the effect that, and based thereon such Opinion of Counsel shall confirm that, subject to customary assumptions and exclusions, the Holders of the Notes will not recognize income, gain or loss for U.S. federal income tax purposes, as applicable, as a result of such Legal Defeasance and will be subject to U.S. federal income tax on the same amounts, in the same manner and at the same times as would have been the case if such Legal Defeasance had not occurred;
(3) in the case of Covenant Defeasance, the Company shall have delivered to the Trustee an Opinion of Counsel reasonably acceptable to the Trustee confirming that, subject to customary assumptions and exclusions, the Holders of the Notes will not recognize income, gain or loss for U.S. federal income tax purposes as a result of such Covenant Defeasance and will be subject to such tax on the same amounts, in the same manner and at the same times as would have been the case if such Covenant Defeasance had not occurred;
(4) no Default (other than that resulting from borrowing funds to be applied to make the deposit required to effect such Legal Defeasance or Covenant Defeasance and any similar and simultaneous deposit relating to other Indebtedness and, in each case, the granting of Liens in connection therewith) shall have occurred and be continuing on the date of such deposit;
(5) such Legal Defeasance or Covenant Defeasance shall not result in a breach or violation of, or constitute a default under, the ABL Facility, the Term Loan Facility or any other material agreement or instrument (other than the Indenture) to which the Company or any Guarantor is a party or by which the Company or any Guarantor is bound (other than that resulting with respect to any Indebtedness being defeased from any borrowing of funds to be applied to make the deposit required to effect such Legal Defeasance or Covenant Defeasance and any similar and simultaneous deposit relating to such Indebtedness, and the granting of Liens in connection therewith);
(6) the Company shall have delivered to the Trustee an Opinion of Counsel to the effect that, as of the date of such opinion and subject to customary assumptions and exclusions following the deposit, the trust funds will not be subject to the effect of Section 547 of Title 11 of the United States Code;
210
Table of Contents
(7) the Company shall have delivered to the Trustee an Officer’s Certificate stating that the deposit was not made by the Company with the intent of defeating, hindering, delaying or defrauding any creditors of the Company or any Guarantor or others; and
(8) the Company shall have delivered to the Trustee an Officer’s Certificate and an Opinion of Counsel (which Opinion of Counsel may be subject to customary assumptions and exclusions) each stating that all conditions precedent provided for or relating to the Legal Defeasance or the Covenant Defeasance, as the case may be, have been complied with.
Any Legal Defeasance or Covenant Defeasance may be made by the Company with respect to the Series A-2 Notes, the Series A-1 Notes, or both Series of Notes.
Satisfaction and Discharge
The Indenture will be discharged and will cease to be of further effect as to all Notes, when:
(1) either
(a) all Notes theretofore authenticated and delivered, except lost, stolen or destroyed Notes which have been replaced or paid and Notes for whose payment money has theretofore been deposited in trust, have been delivered to the Trustee for cancellation; or
(b) all Notes not theretofore delivered to the Trustee for cancellation have become due and payable by reason of the making of a notice of redemption or otherwise, will become due and payable within one year or are to be called for redemption within one year under arrangements satisfactory to the Trustee for the giving of notice of redemption by the Trustee in the name, and at the expense, of the Company and the Company or any Guarantor has irrevocably deposited or caused to be deposited with the Trustee as trust funds in trust solely for the benefit of the Holders of the Notes, cash in U.S. dollars, Government Securities, or a combination thereof, in such amounts as will be sufficient without consideration of any reinvestment of interest to pay and discharge the entire indebtedness on the Notes not theretofore delivered to the Trustee for cancellation for principal, premium, if any, and accrued interest to the date of maturity or redemption;
(2) no Default (other than that resulting from borrowing funds to be applied to make such deposit or any similar or simultaneous deposit relating to other Indebtedness) with respect to the Indenture or the Notes shall have occurred and be continuing on the date of such deposit or shall occur as a result of such deposit and such deposit will not result in a breach or violation of, or constitute a default under the ABL Facility, the Term Loan Facility or any other material agreement or instrument (other than the Indenture) to which the Company or any Guarantor is a party or by which the Company or any Guarantor is bound (other than resulting from any borrowing of funds to be applied to make such deposit and any similar deposit relating to other Indebtedness and the granting of liens in connection therewith);
(3) the Company has paid or caused to be paid all sums payable by it under the Indenture; and
(4) the Company has delivered irrevocable instructions to the Trustee to apply the deposited money toward the payment of the Notes at maturity or the redemption date, as the case may be.
In addition, the Company must deliver an Officer’s Certificate and an Opinion of Counsel to the Trustee stating that all conditions precedent to satisfaction and discharge have been satisfied.
The Company may satisfy and discharge its obligations under the Indenture with respect to the Series A-2 Notes, the Series A-1 Notes or both Series of Notes.
211
Table of Contents
Amendment, Supplement and Waiver
Except as provided below, the Indenture, any Guarantee, the Notes and the Collateral Documents may be amended or supplemented with the consent of the holders of at least a majority in aggregate principal amount of the Series A-1 Notes and the Series A-2 Notes then outstanding, collectively, including consents obtained in connection with a purchase of, or tender offer or exchange offer for, Notes, and any existing Default or compliance with any provision of the Indenture, the Notes issued thereunder or any Collateral Document may be waived with the consent of the holders of a majority in aggregate principal amount of the Series A-1 Notes and the Series A-2 Notes and the Term Loans (if any) then outstanding, collectively, in each case, other than Notes beneficially owned by the Company or its Affiliates (including consents obtained in connection with a purchase of or tender offer or exchange offer for the Notes);provided that any provision or Default that affects some but not all Series of Debt may be amended, supplemented or waived, as the case may be, with the consent of the holders of a majority in aggregate principal amount of the then outstanding Series of Debt that are affected by such provision or Default.
The Indenture provides that, without the consent of each affected Holder of Notes, an amendment or waiver may not, with respect to any Notes held by a non-consenting Holder:
(1) reduce the principal amount of such Notes whose Holders must consent to an amendment, supplement or waiver;
(2) reduce the principal of or change the fixed final maturity of any such Note or alter or waive the provisions with respect to the redemption of such Series A-2 Notes (other than provisions relating to the covenants described above under the caption “Repurchase at the Option of Holders”);
(3) reduce the rate of or change the time for payment of interest on any Note;
(4)(A) waive a Default in the payment of principal of or premium, if any, or interest on the Notes, except a rescission of acceleration of the Notes by the holders of a majority in aggregate principal amount of all then outstanding Series of Debt (or, in the case of a Default that affects some but not all Series of Debt, by the holders of a majority in aggregate principal amount of the then outstanding Series of Debt that are affected by such Default), and a waiver of the payment default that resulted from such acceleration, or (B) waive a Default in respect of a covenant or provision contained in the Indenture or any Guarantee which cannot be amended or modified without the consent of all Holders;
(5) make any Note payable in money other than U.S. dollars;
(6) make any change in the provisions of the Indenture relating to waivers of past Defaults or the rights of Holders to receive payments of principal of or premium, if any, or interest or Additional Interest on the Notes;
(7) make any change in these amendment and waiver provisions;
(8) impair the right of any Holder to receive payment of principal of, premium, if any, or interest on such Holder’s Notes on or after the due dates therefor or to institute suit for the enforcement of any payment on or with respect to such Holder’s Notes or the Guarantees;
(9) make any change to or modify the ranking of the Notes that would adversely affect the Holders;
(10) except as expressly permitted by the Indenture, modify the Guarantee of any Significant Subsidiary in any manner adverse to the Holders of the Notes;
(11) release the Liens created by the Collateral Documents on all or substantially all the Collateral (other than in accordance with the terms of the ABL Facility, the Term Loan Facility and the Collateral Documents); or
(12) make any change in the provisions of the Indenture or any Collateral Document dealing with the application of proceeds of the Collateral (including, without limitation, the provisions under “—Security for the Notes—Priorities of Payment as between Series A-2 Debt (including the Series A-2 Notes) and Series A-1 Notes—Waterfall of Payment Following Acceleration or in Bankruptcy”) that would adversely affect the Holders.
212
Table of Contents
Notwithstanding the foregoing, the Company, any Guarantor (with respect to a Guarantee or the Indenture to which it is a party) and the Trustee may amend or supplement the Indenture, the Collateral Documents and any Guarantee or Notes without the consent of any Holder:
(1) to cure any ambiguity, omission, mistake, defect or inconsistency;
(2) to provide for uncertificated Notes of such series in addition to or in place of certificated Notes;
(3) to comply with the covenant relating to mergers, consolidations and sales of assets;
(4) to provide for the assumption of the Company’s or any Guarantor’s obligations to the Holders;
(5) to make any change that would provide any additional rights or benefits to the Holders or that does not adversely affect the legal rights under the Indenture of any such Holder;
(6) to add covenants for the benefit of the Holders or to surrender any right or power conferred upon the Company or any Guarantor;
(7) to comply with requirements of the SEC in order to effect or maintain the qualification of the Indenture under the Trust Indenture Act;
(8) to evidence and provide for the acceptance and appointment under the Indenture of a successor Trustee thereunder pursuant to the requirements thereof;
(9) to provide for the issuance of exchange notes or private exchange notes, which are identical to exchange notes except that they are not freely transferable;
(10) to provide for the issuance of Additional Notes in accordance with the Indenture and to secure Additional Note Obligations, if any;
(11) to add a Guarantor under the Indenture or to release a Guarantor in accordance with the terms of the Indenture;
(12) to conform the text of the Indenture, Guarantees or the Notes to any provisions of this “Description of Notes” (or any similar description with respect to the Notes in the offering memoranda relating to the original issuances of the outstanding notes) to the extent that such provision in this “Description of Notes” (or any description of the Notes in the offering memoranda relating to the original issuances of the outstanding notes) was intended to be a verbatim recitation of a provision of the Indenture, Guarantee or Notes;
(13) to make any amendment to the provisions of the Indenture relating to the transfer and legending of Notes as permitted by the Indenture, including, without limitation to facilitate the issuance and administration of the Notes;provided, however, that (i) compliance with the Indenture as so amended would not result in Notes being transferred in violation of the Securities Act or any applicable securities law and (ii) such amendment does not materially and adversely affect the rights of holders to transfer Notes;
(14) to provide for the succession of any parties to the Collateral Documents or the Intercreditor Agreement (and other amendments that are administrative or ministerial in nature) in connection with an amendment, renewal, extension, substitution, refinancing, restructuring, replacement, supplementing or other modification from time to time of the ABL Facility, the Term Loan Facility or any other agreement that is not prohibited by the Indenture;
(15) to provide for the release or addition of Collateral or Guarantees in accordance with the terms of the Indenture and the Collateral Documents;
(16) to provide for the issuance of the Notes in a manner consistent with the terms of the Indenture; or
(17) to provide for the succession of the Trustee as collateral agent under the Indenture, the Intercreditor Agreement and the Collateral Documents.
213
Table of Contents
Holders of the Series A-2 Notes and holders of the Series A-1 Notes then outstanding will vote as a single class for all purposes under the Indenture and the Collateral Documents, including waivers, supplements and amendments, except that the holders of each Series of Debt then outstanding which is affected thereby will each vote separately with respect to:
(a) matters that propose to amend or otherwise modify the priority of payment provisions and other intercreditor provisions described above under “—Security for the Notes—Priorities of Payment as between Series A-2 Debt (including the Series A-2 Notes) and Series A-1 Notes” or otherwise contained in the Notes/Term Intercreditor Agreement; and
(b) any matter that adversely and disproportionately affects the rights or obligations of one Series of Debt as compared to any other Series of Debt.
In addition, the Intercreditor Agreement provides that, subject to certain exceptions, any amendment, waiver or consent to any of the collateral documents securing the obligations under the ABL Facility, to the extent applicable to the ABL Collateral, will also apply automatically to the comparable Collateral Documents with respect to the Holders’ interest in the ABL Collateral. The Intercreditor Agreement has a similar provision regarding the effect of any amendment, waiver or consent to any of the Collateral Documents, to the extent applicable to the Notes/Term Collateral, on the corresponding collateral documents with respect to any obligations under the ABL Facility.
The Company has elected to exclude the provisions of Section 315(d)(3) and 316(a)(1) of the Trust Indenture Act from the Indenture. When the Indenture becomes subject to the Trust Indenture Act, to the extent any Term Loans remain outstanding at that time, and to the extent any of the voting and/or remedial provisions contained in the Indenture are deemed by any court or governmental authority to be inconsistent with the Trust Indenture Act, any action under the Indenture that requires the vote or participation of then outstanding Term Loans shall be taken without the participation of the then outstanding Term Loans.
The consent of the Holders is not necessary under the Indenture to approve the particular form of any proposed amendment. It is sufficient if such consent approves the substance of the proposed amendment.
Notices
Notices given by publication will be deemed given on the first date on which publication is made and notices given by first-class mail, postage prepaid, will be deemed given five calendar days after mailing.
Concerning the Trustee
The Indenture contains certain limitations on the rights of the Trustee thereunder, should it become a creditor of the Company, to obtain payment of claims in certain cases, or to realize on certain property received in respect of any such claim as security or otherwise. The Trustee is permitted to engage in other transactions;however, if it acquires any conflicting interest, it must eliminate such conflict within 90 days, apply to the SEC for permission to continue or resign.
The Indenture provides that the holders of a majority in principal amount of all then outstanding Series of Notes will have the right to direct the time, method and place of conducting any proceeding for exercising any remedy available to the Trustee, subject to certain exceptions. The Indenture provides that in case an Event of Default shall occur (which shall not be cured), the Trustee will be required, in the exercise of its power, to use the degree of care of a prudent person in the conduct of his own affairs. Subject to such provisions, the Trustee is under no obligation to exercise any of its rights or powers under the Indenture at the request of any Holder of the Notes, unless such Holder shall have offered to the Trustee security and indemnity satisfactory to it against any loss, liability or expense.
214
Table of Contents
Governing Law
The Indenture, the Notes and any Guarantee are or will be governed by and construed in accordance with the laws of the State of New York.
Certain Definitions
Set forth below are certain defined terms used in the Indenture. For purposes of the Indenture, unless otherwise specifically indicated, the term “consolidated” with respect to any Person refers to such Person consolidated with its Restricted Subsidiaries, and excludes from such consolidation any Unrestricted Subsidiary as if such Unrestricted Subsidiary were not an Affiliate of such Person.
“ABL Facility” means the Credit Facility, dated as of October 28, 2008, by and among the Company, the Guarantors, the lenders party thereto in their capacities as lenders thereunder and Bank of America, N.A., as Administrative Agent, including any guarantees, collateral documents, instruments and agreements executed in connection therewith, and any amendments, supplements, modifications, extensions, renewals, restatements, refundings or refinancings thereof and any indentures or credit facilities or commercial paper facilities with banks or other institutional lenders or investors that replace, refund or refinance any part of the loans, notes, other credit facilities or commitments thereunder, including any such replacement, refunding or refinancing facility or indenture that increases the amount borrowable thereunder or alters the maturity thereof (provided that such increase in borrowings is permitted under “—Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” above).
“ABL Collateral Agent” means Bank of America, N.A. and any successor under the ABL Facility, or if there is no ABL Facility, the “ABL Collateral Agent” designated pursuant to the terms of the ABL Lenders Debt.
“ABL Lenders Debt” means (i) any Indebtedness outstanding from time to time under the ABL Facility, (ii) any Indebtedness which has a senior priority security interest relative to the Series A-1 Notes and Series A-2 Debt in the ABL Collateral, (iii) all obligations with respect to such Indebtedness and any Hedging Obligations directly related to any ABL Lenders Debt entered into with any lender (or its affiliates) under the ABL Facility and (iv) all Bank Products entered into with any lender (or its affiliates) under the ABL Facility.
“Acquired Indebtedness” means, with respect to any specified Person,
(1) Indebtedness of any other Person existing at the time such other Person is merged with or into or became a Restricted Subsidiary of such specified Person, including Indebtedness incurred in connection with, or in contemplation of, such other Person merging with or into or becoming a Restricted Subsidiary of such specified Person, and
(2) Indebtedness secured by a Lien encumbering any asset acquired by such specified Person.
“Acquisition” means the acquisition of the Company by the Investors on October 28, 2008 and the related transactions contemplated by the Transaction Agreement.
“Additional Interest” means all additional interest then owing pursuant to the Registration Rights Agreement.
“Additional Note Obligations” means Obligations of the Company with respect to Additional Notes permitted to be incurred under the Indenture which are secured by a Lien on the Notes/Term Collateral equally and ratably with the Series A-1 Notes issued on the Issue Date, provided that such Lien is permitted to be incurred under the Indenture.
“Affiliate” of any specified Person means any other Person directly or indirectly controlling or controlled by or under direct or indirect common control with such specified Person. For purposes of this definition, “control”
215
Table of Contents
(including, with correlative meanings, the terms “controlling,” “controlled by” and “under common control with”), as used with respect to any Person, shall mean the possession, directly or indirectly, of the power to direct or cause the direction of the management or policies of such Person, whether through the ownership of voting securities, by agreement or otherwise.
“After-Acquired Property” means any and all assets or property (other than Excluded Assets) acquired after the Issue Date, including any property or assets acquired by the Company or a Guarantor from another Guarantor, which in each case constitutes Collateral as defined in the Indenture.
“Applicable Premium” means, with respect to any Note on any Redemption Date, the greater of:
(1) 1.0% of the principal amount of such Note; and
(2) the excess, if any, of (a) the present value at such Redemption Date of (i) the redemption price of such Note at November 1, 2011 (each such redemption price being set forth in the table appearing above under the caption “Optional Redemption”), plus (ii) all required interest payments due on such Note through November 1, 2011 (excluding accrued but unpaid interest to the Redemption Date), computed using a discount rate equal to the Treasury Rate as of such Redemption Date plus 50 basis points; over (b) the principal amount of such Note.
“Asset Sale” means:
(1) the sale, conveyance, transfer or other disposition, whether in a single transaction or a series of related transactions, of property or assets (including by way of a Sale and Lease-Back Transaction) of the Company or any of its Restricted Subsidiaries (each referred to in this definition as a “disposition”); or
(2) the issuance or sale of Equity Interests of any Restricted Subsidiary (other than Preferred Stock of Restricted Subsidiaries issued in compliance with the covenant described under “—Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”), whether in a single transaction or a series of related transactions;
in each case, other than:
(a) any disposition of Cash Equivalents or Investment Grade Securities or surplus, obsolete or worn-out equipment in the ordinary course of business or any disposition of inventory or goods (or other assets) held for sale in the ordinary course of business or any disposition of ABL Collateral;
(b) the disposition of all or substantially all of the assets of the Company governed by, and in a manner permitted pursuant to, the provisions described above under “—Certain Covenants—Merger, Consolidation or Sale of All or Substantially All Assets” or any disposition that constitutes a Change of Control pursuant to the Indenture;
(c) the making of any Restricted Payment or Permitted Investment that is permitted to be made, and is made, under the covenant described above under “—Certain Covenants—Limitation on Restricted Payments”;
(d) any disposition of assets or issuance or sale of Equity Interests of any Restricted Subsidiary in any transaction or series of transactions with an aggregate fair market value of less than $ 15.0 million;
(e) any disposition of property or assets or issuance of securities by a Restricted Subsidiary of the Company to the Company or by the Company or a Restricted Subsidiary of the Company to another Restricted Subsidiary of the Company;
(f) to the extent allowable under Section 1031 of the Internal Revenue Code of 1986 or comparable law or regulation, any exchange of like property (excluding any boot thereon) for use in a Similar Business;
216
Table of Contents
(g) the lease, assignment or sub-lease of any real or personal property in the ordinary course of business;
(h) any issuance or sale of Equity Interests in, or Indebtedness or other securities of, an Unrestricted Subsidiary;
(i) foreclosures, condemnations or any similar action on assets;
(j) sales of accounts receivable, or participations therein, in connection with any Receivables Facility;
(k) any financing transaction with respect to the acquisition or construction of property by the Company or any Restricted Subsidiary after the Issue Date, including Sale and Lease-Back Transactions and asset securitizations permitted by the Indenture; and
(l) the licensing and sub-licensing of intellectual property or other general intangibles in the ordinary course of business or consistent with past practice.
“Bank Products” means any facilities or services related to cash management, including treasury, depository, overdraft, credit or debit card, purchase card, electronic funds transfer and other cash management arrangements.
“Borrowing Base” means, as of any date, an amount equal to:
(1) 50% of the value of all net accounts receivable owned by the Company and its Restricted Subsidiaries as of the end of the most recent fiscal quarter preceding such date; plus
(2) 50% of the value of all net inventory owned by the Company and its Restricted Subsidiaries as of the end of the most recent fiscal quarter preceding such date;
all calculated on a consolidated basis and in accordance with GAAP.
“Business Day” means each day which is not a Legal Holiday.
“Capital Stock” means:
(1) in the case of a corporation, corporate stock;
(2) in the case of an association or business entity, any and all shares, interests, participations, rights or other equivalents (however designated) of corporate stock;
(3) in the case of a partnership or limited liability company, partnership or membership interests (whether general or limited); and
(4) any other interest or participation that confers on a Person the right to receive a share of the profits and losses of, or distributions of assets of, the issuing Person.
“Capitalized Lease Obligation” means, at the time any determination thereof is to be made, the amount of the liability in respect of a capital lease that would at such time be required to be capitalized and reflected as a liability on a balance sheet (excluding the footnotes thereto) in accordance with GAAP.
“Cash Equivalents” means:
(1) United States dollars;
(2)(a) €, or any national currency of any participating member state of the EMU; or
(b) such local currencies held by the Company or any Restricted Subsidiary from time to time in the ordinary course of business;
217
Table of Contents
(3) securities issued or directly and fully and unconditionally guaranteed or insured by the U.S. government (or any agency or instrumentality thereof the securities of which are unconditionally guaranteed as a full faith and credit obligation of the U.S. government), with maturities of 24 months or less from the date of acquisition;
(4) certificates of deposit, time deposits and Eurodollar time deposits with maturities of one year or less from the date of acquisition, bankers’ acceptances with maturities not exceeding one year and overnight bank deposits, in each case with any commercial bank having capital and surplus of not less than $500.0 million in the case of U.S. banks and $100.0 million (or the U.S. dollar equivalent as of the date of determination) in the case of non-U.S. banks;
(5) repurchase obligations for underlying securities of the types described in clauses (3) and (4) entered into with any financial institution meeting the qualifications specified in clause (4) above;
(6) commercial paper rated at least P-1 by Moody’s or at least A-1 by S&P and in each case maturing within 24 months after the date of creation thereof;
(7) marketable short-term money market and similar securities having a rating of at least P-2 or A-2 from either Moody’s or S&P, respectively (or, if at any time neither Moody’s nor S&P shall be rating such obligations, an equivalent rating from another Rating Agency) and in each case maturing within 24 months after the date of creation thereof;
(8) investment funds investing 95% of their assets in securities of the types described in clauses (1) through (7) above;
(9) readily marketable direct obligations issued by any state, commonwealth or territory of the United States or any political subdivision or taxing authority thereof having an Investment Grade Rating from either Moody’s or S&P with maturities of 24 months or less from the date of acquisition;
(10) Indebtedness or Preferred Stock issued by Persons with a rating of “A” or higher from S&P or “A2” or higher from Moody’s with maturities of 24 months or less from the date of acquisition; and
(11) Investments with average maturities of 24 months or less from the date of acquisition in money market funds rated AAA- (or the equivalent thereof) or better by S&P or Aaa3 (or the equivalent thereof) or better by Moody’s.
Notwithstanding the foregoing, Cash Equivalents shall include amounts denominated in currencies other than those set forth in clauses (1) and (2) above, provided that such amounts are converted into any currency listed in clauses (1) and (2) as promptly as practicable and in any event within ten Business Days following the receipt of such amounts.
“Change of Control” means the occurrence of any of the following:
(1) the sale, lease or transfer, in one or a series of related transactions, of all or substantially all of the assets of the Company and its Subsidiaries, taken as a whole, to any Person other than a Permitted Holder; or
(2) the Company becomes aware (by way of a report or any other filing pursuant to Section 13(d) of the Exchange Act, proxy, vote, written notice or otherwise) of the acquisition by any Person or group (within the meaning of Section 13(d)(3) or Section 14(d)(2) of the Exchange Act, or any successor provision), including any group acting for the purpose of acquiring, holding or disposing of securities (within the meaning of Rule 13d-5(b)(1) under the Exchange Act), other than the Permitted Holders, in a single transaction or in a related series of transactions, by way of merger, consolidation or other business combination or purchase of beneficial ownership (within the meaning of Rule 13d-3 under the Exchange Act, or any successor provision) of 50% or more of the total voting power of the Voting Stock of the Company or any of its direct or indirect parent companies.
218
Table of Contents
“Collateral” means all of the assets and properties subject to the Liens created by the Collateral Documents.
“Collateral Documents” means, collectively, the security agreements, pledge agreements, mortgages, collateral assignments, deeds of trust and all other pledges, agreements, financing statements, patent, trademark or copyright filings, mortgages or other filings or documents that create or purport to create a Lien in the Collateral in favor of the Notes/Term Collateral Agent and/or the Trustee (for the benefit of the holders of Series A-1 Notes and Series A-2 Notes), the Intercreditor Agreement and the Notes/Term Intercreditor Agreement, in each case as they may be amended from time to time, and any instruments of assignment, control agreements, lockbox letters or other instruments or agreements executed pursuant to the foregoing.
“Consolidated Depreciation and Amortization Expense” means with respect to any Person for any period, the total amount of depreciation and amortization expense, including the amortization of deferred financing fees of such Person and its Restricted Subsidiaries for such period on a consolidated basis and otherwise determined in accordance with GAAP.
“Consolidated Interest Expense” means, with respect to any Person for any period, without duplication, the sum of:
(1) consolidated interest expense of such Person and its Restricted Subsidiaries for such period, to the extent such expense was deducted (and not added back) in computing Consolidated Net Income (including (a) amortization of original issue discount resulting from the issuance of Indebtedness at less than par, (b) all commissions, discounts and other fees and charges owed with respect to letters of credit or bankers acceptances, (c) non-cash interest payments (but excluding any non-cash interest expense attributable to the movement in the mark to market valuation of Hedging Obligations or other derivative instruments pursuant to GAAP), (d) the interest component of Capitalized Lease Obligations and (e) net payments, if any, pursuant to interest rate Hedging Obligations with respect to Indebtedness and excluding (u) accretion or accrual of discounted liabilities not constituting Indebtedness, (v) any expense resulting from the discounting of any outstanding Indebtedness in connection with the application of purchase accounting in connection with any acquisition, (w) any Additional Interest, (x) amortization of deferred financing fees, debt issuance costs, commissions, fees and expenses, (y) any expensing of bridge, commitment and other financing fees and (z) commissions, discounts, yield and other fees and charges (including any interest expense) related to any Receivables Facility); plus
(2) consolidated capitalized interest of such Person and its Restricted Subsidiaries for such period, whether paid or accrued; less
(3) interest income for such period.
For purposes of this definition, interest on a Capitalized Lease Obligation shall be deemed to accrue at an interest rate reasonably determined by such Person to be the rate of interest implicit in such Capitalized Lease Obligation in accordance with GAAP.
“Consolidated Net Income” means, with respect to any Person for any period, the aggregate of the Net Income, of such Person and its Restricted Subsidiaries for such period, on a consolidated basis, and otherwise determined in accordance with GAAP;provided, however, that, without duplication,
(1) any after-tax effect of extraordinary, non-recurring or unusual gains or losses (less all fees and expenses relating thereto) or expenses (including relating to the Transaction), severance, relocation costs and curtailments or modifications to pension and post-retirement employee benefit plans and other restructuring costs shall be excluded,
(2) the cumulative effect of a change in accounting principles during such period shall be excluded,
(3) any after-tax effect of income (loss) from disposed, abandoned, transferred, closed or discontinued operations and any net after-tax gains or losses on disposal of disposed, abandoned, transferred, closed or discontinued operations shall be excluded,
219
Table of Contents
(4) any after-tax effect of gains or losses (less all fees and expenses relating thereto) attributable to asset dispositions other than in the ordinary course of business, as determined in good faith by the Company, shall be excluded,
(5) the Net Income for such period of any Person that is not a Subsidiary or is an Unrestricted Subsidiary or that is accounted for by the equity method of accounting, shall be excluded;provided that Consolidated Net Income of the Company shall be increased by the amount of dividends or distributions or other payments that are actually paid in cash (or to the extent converted into cash) to the referent Person or a Restricted Subsidiary thereof in respect of such period,
(6) solely for the purpose of determining the amount available for Restricted Payments under clause (3)(a) of the first paragraph of “—Certain Covenants—Limitation on Restricted Payments,” the Net Income for such period of any Restricted Subsidiary (other than any Guarantor) shall be excluded to the extent that the declaration or payment of dividends or similar distributions by that Restricted Subsidiary of its Net Income is not at the date of determination permitted without any prior governmental approval (which has not been obtained) or, directly or indirectly, by the operation of the terms of its charter or any agreement, instrument, judgment, decree, order, statute, rule, or governmental regulation applicable to that Restricted Subsidiary or its stockholders, unless such restriction with respect to the payment of dividends or similar distributions has been legally waived,provided that Consolidated Net Income of the Company will be increased by the amount of dividends or other distributions or other payments actually paid in cash (or to the extent converted into cash) or Cash Equivalents to the Company or a Restricted Subsidiary thereof in respect of such period, to the extent not already included therein,
(7) effects of adjustments (including the effects of such adjustments pushed down to the Company and its Restricted Subsidiaries) in the property and equipment, inventory and other intangible assets, deferred revenue and debt line items in such Person’s consolidated financial statements pursuant to GAAP resulting from the application of purchase accounting in relation to the Transaction or any consummated acquisition or the amortization or write-off of any amounts thereof, net of taxes, shall be excluded,
(8) any after-tax effect of income (loss) from the early extinguishment of Indebtedness or Hedging Obligations or other derivative instruments shall be excluded,
(9) any impairment charge or asset write-off, in each case, pursuant to GAAP and the amortization of intangibles arising pursuant to GAAP shall be excluded,
(10) any non-cash compensation expense recorded from grants of stock appreciation or similar rights, stock options, restricted stock or other rights shall be excluded,
(11) any fees and expenses incurred during such period, or any amortization thereof for such period, in connection with any acquisition, disposition, recapitalization, Investment, Asset Sale, issuance or repayment of Indebtedness, issuance of Equity Interests, refinancing transaction or amendment or modification of any debt instrument (in each case, including any such transaction consummated prior to the Issue Date and any such transaction undertaken but not completed) and any charges or non-recurring merger costs incurred during such period as a result of any such transaction shall be excluded, and
(12) accruals and reserves that are established or adjusted within twelve months of the Issue Date that are so required to be established or adjusted as a result of the Transaction in accordance with GAAP or changes as a result of a modification of accounting policies shall be excluded.
Notwithstanding the foregoing, for the purpose of the covenant described under “—Certain Covenants—Limitation on Restricted Payments” only (other than clause (3)(d) thereof), there shall be excluded from Consolidated Net Income any income arising from any sale or other disposition of Restricted Investments made by the Company and its Restricted Subsidiaries, any repurchases and redemptions of Restricted Investments from the Company and its Restricted Subsidiaries, any repayments of loans and advances which constitute Restricted Investments by the Company or any of its Restricted Subsidiaries, any sale of the stock of an Unrestricted Subsidiary or any distribution or dividend from an Unrestricted Subsidiary, in each case only to the extent such amounts increase the amount of Restricted Payments permitted under such covenant pursuant to clause (3)(d) thereof.
220
Table of Contents
“Contingent Obligations” means, with respect to any Person, any obligation of such Person guaranteeing any leases, dividends or other obligations that do not constitute Indebtedness (“primary obligations”) of any other Person (the “primary obligor”) in any manner, whether directly or indirectly, including, without limitation, any obligation of such Person, whether or not contingent,
(1) to purchase any such primary obligation or any property constituting direct or indirect security therefor,
(2) to advance or supply funds
(a) for the purchase or payment of any such primary obligation, or
(b) to maintain working capital or equity capital of the primary obligor or otherwise to maintain the net worth or solvency of the primary obligor, or
(3) to purchase property, securities or services primarily for the purpose of assuring the owner of any such primary obligation of the ability of the primary obligor to make payment of such primary obligation against loss in respect thereof.
“Coram Acquisition” means the acquisition by the Company of Coram, Inc. completed on December 3, 2007.
“Credit Facilities” means, with respect to the Company or any of its Restricted Subsidiaries, one or more debt facilities, including the ABL Facility and the Term Loan Facility, or other financing arrangements (including, without limitation, commercial paper facilities or indentures), providing for revolving credit loans, term loans, letters of credit or other long-term indebtedness, including any notes, mortgages, guarantees, collateral documents, instruments and agreements executed in connection therewith, and any amendments, supplements, modifications, extensions, renewals, restatements or refundings thereof and any indentures or credit facilities or commercial paper facilities that replace, refund or refinance any part of the loans, notes, other credit facilities or commitments thereunder, including any such replacement, refunding or refinancing facility or indenture that increases the amount permitted to be borrowed thereunder or alters the maturity thereof (provided that such increase in borrowings is permitted under “—Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”) or adds Restricted Subsidiaries as additional borrowers or guarantors thereunder and whether by the same or any other agent, lender or group of lenders.
“Default” means any event that is, or with the passage of time or the giving of notice or both would be, an Event of Default.
“Designated Non-cash Consideration” means the fair market value of non-cash consideration received by the Company or a Restricted Subsidiary in connection with an Asset Sale that is so designated as Designated Non-cash Consideration pursuant to an Officer’s Certificate, setting forth the basis of such valuation, executed by the principal financial officer of the Company, less the amount of cash or Cash Equivalents received in connection with a subsequent sale of or collection on such Designated Non-cash Consideration.
“Designated Preferred Stock” means Preferred Stock of the Company or any parent corporation thereof (in each case other than Disqualified Stock) that is issued for cash (other than to a Restricted Subsidiary or an employee stock ownership plan or trust established by the Company or any of its Subsidiaries) and is so designated as Designated Preferred Stock, pursuant to an Officer’s Certificate executed by the principal financial officer of the Company or the applicable parent corporation thereof, as the case may be, on the issuance date thereof, the cash proceeds of which are excluded from the calculation set forth in clause (3) of the first paragraph of the “—Certain Covenants—Limitation on Restricted Payments” covenant.
“Disqualified Stock” means, with respect to any Person, any Capital Stock of such Person which, by its terms, or by the terms of any security into which it is convertible or for which it is putable or exchangeable, or
221
Table of Contents
upon the happening of any event, matures or is mandatorily redeemable (other than solely as a result of a change of control or asset sale) pursuant to a sinking fund obligation or otherwise, or is redeemable at the option of the holder thereof (other than solely as a result of a change of control or asset sale), in whole or in part, in each case prior to the date 91 days after the maturity date of the Notes;provided, however, that if such Capital Stock is issued to any plan for the benefit of employees of the Company or its Subsidiaries or by any such plan to such employees, such Capital Stock shall not constitute Disqualified Stock solely because it may be required to be repurchased by the Company or its Subsidiaries in order to satisfy applicable statutory or regulatory obligations.
“EBITDA” means, with respect to any Person for any period, the Consolidated Net Income of such Person for such period
(1) increased (without duplication) by:
(a) provision for taxes based on income or profits or capital gains, including, without limitation, state, franchise and similar taxes and foreign withholding taxes of such Person paid or accrued during such period to the extent the same was deducted (and not added back) in computing Consolidated Net Income;plus
(b) Fixed Charges of such Person for such period (including (x) net losses on Hedging Obligations or other derivative instruments entered into for the purpose of hedging interest rate risk and (y) costs of surety bonds in connection with financing activities, in each case, to the extent included in Fixed Charges) to the extent the same was deducted (and not added back) in calculating such Consolidated Net Income;plus
(c) Consolidated Depreciation and Amortization Expense of such Person for such period to the extent the same were deducted (and not added back) in computing Consolidated Net Income;plus
(d) the amount of any integration costs or other business optimization expenses or reserves deducted (and not added back) in such period in computing Consolidated Net Income, including any one-time costs incurred in connection with acquisitions after the Issue Date and costs related to the closure and/or consolidation of facilities;plus
(e) any other non-cash charges, including any write-offs or write-downs, reducing Consolidated Net Income for such period (provided that if any such non-cash charges represent an accrual or reserve for potential cash items in any future period, the cash payment in respect thereof in such future period shall be subtracted from EBITDA to such extent, and excluding amortization of a prepaid cash item that was paid in a prior period);plus
(f) the amount of any minority interest expense consisting of Subsidiary income attributable to minority equity interests of third parties in any non-Wholly-Owned Subsidiary deducted (and not added back) in such period in calculating Consolidated Net Income;plus
(g) the amount of management, monitoring, consulting and advisory fees and related expenses paid in such period to the Investors to the extent otherwise permitted under “—Certain Covenants— Transactions with Affiliates”; plus
(h) the amount of net cost savings and synergies projected by the Company in good faith to be realized as a result of specified actions taken or with respect to which substantial steps have been taken (in the good faith determination of the Company) and which are expected to be realized within 12 months of the date thereof in connection with the Acquisition, future acquisitions and cost saving, restructuring and other similar initiatives (which cost savings shall be added to EBITDA until fully realized and calculated on apro forma basis as though such cost savings had been realized on the first day of such period), net of the amount of actual benefits realized during such period from such actions;provided that such cost savings are reasonably identifiable and factually supportable (which adjustments may be incremental topro forma adjustments made pursuant to the second paragraph of the definition of “Fixed Charge Coverage Ratio”) (notwithstanding the foregoing, all net cost savings and synergies described in the offering memorandum for the Series A-1 Notes may be added back to EBITDA);plus
222
Table of Contents
(i) the amount of loss on sale of receivables and related assets to the Receivables Subsidiary in connection with a Receivables Facility; plus
(j) any costs or expense incurred by the Company or a Restricted Subsidiary pursuant to any management equity plan or stock option plan or any other management or employee benefit plan or agreement or any stock subscription or shareholder agreement, to the extent that such cost or expenses are funded with cash proceeds contributed to the capital of the Company or net cash proceeds of an issuance of Equity Interests of the Company (other than Disqualified Stock) solely to the extent that such net cash proceeds are excluded from the calculation set forth in clause (3) of the first paragraph under “—Certain Covenants—Limitation on Restricted Payments;”
(2) decreased by (without duplication) non-cash gains increasing Consolidated Net Income of such Person for such period, excluding any non-cash gains to the extent they represent the reversal of an accrual or reserve for a potential cash item that reduced EBITDA in any prior period; and
(3) increased or decreased by (without duplication):
(a) any net gain or loss resulting in such period from Hedging Obligations and the application of Statement of Financial Accounting Standards No. 133;plus orminus, as applicable,
(b) any net gain or loss resulting in such period from currency translation gains or losses related to currency remeasurements of Indebtedness (including any net loss or gain resulting from hedge agreements for currency exchange risk).
“EMU” means economic and monetary union as contemplated in the Treaty on European Union.
“Equity Interests” means Capital Stock and all warrants, options or other rights to acquire Capital Stock, but excluding any debt security that is convertible into, or exchangeable for, Capital Stock.
“Equity Offering” means any public or private sale of common stock or Preferred Stock of the Company (excluding Disqualified Stock) or any of its direct or indirect parent companies to the extent contributed to the Company as Equity (other than Disqualified Stock), other than:
(1) public offerings with respect to the Company’s or any direct or indirect parent company’s common stock registered on Form S-8;
(2) issuances to any Subsidiary of the Company; and
(3) any such public or private sale that constitutes an Excluded Contribution.
“€” means the single currency of participating member states of the EMU.
“Exchange Act” means the Securities Exchange Act of 1934, as amended, and the rules and regulations of the SEC promulgated thereunder.
“Excluded Contract” means at any date any rights or interest of the Company or any Guarantor in any assets or under any agreement, contract, license, instrument, document or other general intangible (referred to solely for purposes of this definition as a “Contract”) to the extent that such Contract by the terms of a restriction in favor of a Person who is not the Company or any Guarantor, or any requirement of law, prohibits, or requires any consent or establishes any other condition for or would terminate because of an assignment thereof or a grant of a security interest therein by the Company or a Guarantor;provided that: (i) rights to payment under any such Contract otherwise constituting an Excluded Contract by virtue of this definition shall be included in the Collateral to the extent permitted thereby or by Section 9-406 or Section 9-408 of the Uniform Commercial Code and (ii) all proceeds paid or payable to any of the Company or any Guarantor from any sale, transfer or assignment of such Contract and all rights to receive such proceeds shall be included in the Collateral.
223
Table of Contents
“Excluded Contribution” means net cash proceeds, marketable securities or Qualified Proceeds received by the Company from
(1) contributions to its common equity capital, and
(2) the sale (other than to a Subsidiary of the Company or to any management equity plan or stock option plan or any other management or employee benefit plan or agreement of the Company) of Capital Stock (other than Disqualified Stock and Designated Preferred Stock) of the Company,
in each case after the Issue Date and in each case designated as Excluded Contributions pursuant to an officer’s certificate executed by the principal financial officer of the Company on the date such capital contributions are made or the date such Equity Interests are sold, as the case may be, which are excluded from the calculation set forth in clause (3) of the first paragraph under “—Certain Covenants—Limitation on Restricted Payments.”
“Excluded Equipment” means at any date any equipment or other assets of the Company or any Guarantor which is subject to, or secured by, a Capitalized Lease Obligation or a purchase money obligation if and to the extent that (i) a restriction in favor of a Person who is not the Company or a Restricted Subsidiary contained in the agreements or documents granting or governing such Capitalized Lease Obligation or purchase money obligation prohibits, or requires any consent or establishes any other conditions for or would result in the termination of such agreement or document because of an assignment thereof, or a grant of a security interest therein, by the Company or any Guarantor and (ii) such restriction relates only to the asset or assets acquired by the Company or any Guarantor with the proceeds of such Capitalized Lease Obligation or purchase money obligation and attachments thereto, improvements thereof or substitutions therefor;provided that all proceeds paid or payable to any of the Company or any Guarantor from any sale, transfer or assignment or other voluntary or involuntary disposition of such assets and all rights to receive such proceeds shall be included in the Collateral to the extent not otherwise required to be paid to the holder of any Capitalized Lease Obligations or purchase money obligations secured by such assets.
“Fixed Charge Coverage Ratio” means, with respect to any Person for any period, the ratio of EBITDA of such Person for such period to the Fixed Charges of such Person for such period. In the event that the Company or any Restricted Subsidiary incurs, assumes, guarantees, redeems, retires or extinguishes any Indebtedness (other than Indebtedness incurred under any revolving credit facility unless such Indebtedness has been permanently repaid and has not been replaced) or issues or redeems Disqualified Stock or Preferred Stock subsequent to the commencement of the period for which the Fixed Charge Coverage Ratio is being calculated but prior to or simultaneously with the event for which the calculation of the Fixed Charge Coverage Ratio is made (the “Fixed Charge Coverage Ratio Calculation Date”), then the Fixed Charge Coverage Ratio shall be calculated givingpro forma effect to such incurrence, assumption, guarantee, redemption, retirement or extinguishment of Indebtedness, or such issuance or redemption of Disqualified Stock or Preferred Stock, as if the same had occurred at the beginning of the applicable four-quarter period.
For purposes of making the computation referred to above, Investments, acquisitions, dispositions, mergers, consolidations and disposed operations (as determined in accordance with GAAP) that have been made by the Company or any of its Restricted Subsidiaries during the four-quarter reference period or subsequent to such reference period and on or prior to or simultaneously with the Fixed Charge Coverage Ratio Calculation Date shall be calculated on apro forma basis, assuming that all such Investments, acquisitions, dispositions, mergers, consolidations and disposed operations (and the change in any associated fixed charge obligations and the change in EBITDA resulting therefrom) had occurred on the first day of the four-quarter reference period. If since the beginning of such period any Person that subsequently became a Restricted Subsidiary or was merged with or into the Company or any of its Restricted Subsidiaries since the beginning of such period shall have made any Investment, acquisition, disposition, merger, consolidation or disposed operation that would have required adjustment pursuant to this definition, then the Fixed Charge Coverage Ratio shall be calculated givingpro forma effect thereto for such period as if such Investment, acquisition, disposition, merger, consolidation or disposed operation had occurred at the beginning of the applicable four-quarter period.
224
Table of Contents
For purposes of this definition, wheneverpro forma effect is to be given to an Investment, acquisition, disposition, merger or consolidation (including the Transaction and the Coram Acquisition) or any other transaction, thepro forma calculations shall be made in good faith by a responsible financial or accounting officer of the Company (and may include, for the avoidance of doubt and without duplication, cost savings, synergies and operating expense resulting from such Investment, acquisition, disposition, merger or consolidation (including the Transaction and the Coram Acquisition) or other transaction, in each case calculated in the manner described in the definition of “EBITDA” herein). If any Indebtedness bears a floating rate of interest and is being givenpro forma effect, the interest on such Indebtedness shall be calculated as if the rate in effect on the Fixed Charge Coverage Ratio Calculation Date had been the applicable rate for the entire period (taking into account any Hedging Obligations applicable to such Indebtedness). Interest on a Capitalized Lease Obligation shall be deemed to accrue at an interest rate reasonably determined by a responsible financial or accounting officer of the Company to be the rate of interest implicit in such Capitalized Lease Obligation in accordance with GAAP. For purposes of making the computation referred to above, interest on any Indebtedness under a revolving credit facility computed on apro forma basis shall be computed based upon the average daily balance of such Indebtedness during the applicable period except as set forth in the first paragraph of this definition. Interest on Indebtedness that may optionally be determined at an interest rate based upon a factor of a prime or similar rate, a Eurocurrency interbank offered rate, or other rate, shall be deemed to have been based upon the rate actually chosen, or, if none, then based upon such optional rate chosen as the Company may designate.
“Fixed Charges” means, with respect to any Person for any period, the sum of:
(1) Consolidated Interest Expense of such Person for such period;
(2) all cash dividends or other distributions paid (excluding items eliminated in consolidation) on any series of Preferred Stock of any Restricted Subsidiary during such period; and
(3) all dividends or other distributions accrued (excluding items eliminated in consolidation) on any series of Disqualified Stock during such period.
“Foreign Subsidiary” means, with respect to any Person, any Restricted Subsidiary of such Person that is not organized or existing under the laws of the United States, any state thereof, or the District of Columbia and any Restricted Subsidiary of such Foreign Subsidiary.
“GAAP” means generally accepted accounting principles in the United States which are in effect on the Issue Date.
“Government Securities” means securities that are:
(1) direct obligations of the United States of America for the timely payment of which its full faith and credit is pledged; or
(2) obligations of a Person controlled or supervised by and acting as an agency or instrumentality of the United States of America the timely payment of which is unconditionally guaranteed as a full faith and credit obligation by the United States of America, which, in either case, are not callable or redeemable at the option of the issuers thereof, and shall also include a depository receipt issued by a bank (as defined in Section 3(a)(2) of the Securities Act), as custodian with respect to any such Government Securities or a specific payment of principal of or interest on any such Government Securities held by such custodian for the account of the holder of such depository receipt;provided that (except as required by law) such custodian is not authorized to make any deduction from the amount payable to the holder of such depository receipt from any amount received by the custodian in respect of the Government Securities or the specific payment of principal of or interest on the Government Securities evidenced by such depository receipt.
“guarantee” means a guarantee (other than by endorsement of negotiable instruments for collection in the ordinary course of business), direct or indirect, in any manner (including letters of credit and reimbursement agreements in respect thereof), of all or any part of any Indebtedness or other obligations.
225
Table of Contents
“Guarantee” means the guarantee by any Guarantor of the Company’s Obligations under the Indenture.
“Guarantor” means each Restricted Subsidiary that Guarantees the Notes in accordance with the terms of the Indenture and its successors and assigns, until released from its obligations under its Guarantee in accordance with the terms of the Indenture.
“Hedging Obligations” means, with respect to any Person, the obligations of such Person under any interest rate swap agreement, interest rate cap agreement, interest rate collar agreement, commodity swap agreement, commodity cap agreement, commodity collar agreement, foreign exchange contract, currency swap agreement or similar agreement providing for the transfer, modification or mitigation of interest rate, commodity or currency risks either generally or under specific contingencies.
“Holder” means the Person in whose name a Note is registered on the registrar’s books.
“Holdings” means Sky Acquisition LLC, a Delaware limited liability company.
“Indebtedness” means, with respect to any Person, without duplication:
(1) any indebtedness of such Person, whether or not contingent:
(a) in respect of borrowed money;
(b) evidenced by bonds, notes, debentures or similar instruments or letters of credit or bankers’ acceptances (or, without duplication, reimbursement agreements in respect thereof);
(c) representing the balance deferred and unpaid of the purchase price of any property (including Capitalized Lease Obligations), except (i) any such balance that constitutes a trade payable or similar obligation to a trade creditor, in each case accrued in the ordinary course of business and (ii) any earn-out obligations until such obligation becomes a liability on the balance sheet of such Person in accordance with GAAP; or
(d) representing net obligations under any Hedging Obligations;
if and to the extent that any of the foregoing Indebtedness (other than letters of credit and Hedging Obligations) would appear as a liability upon a balance sheet (excluding the footnotes thereto) of such Person prepared in accordance with GAAP;
(2) to the extent not otherwise included, any obligation by such Person to be liable for, or to pay, as obligor, guarantor or otherwise on, the obligations of the type referred to in clause (1) of a third Person (whether or not such items would appear upon the balance sheet of the such obligor or guarantor), other than by endorsement of negotiable instruments for collection in the ordinary course of business; and
(3) to the extent not otherwise included, the obligations of the type referred to in clause (1) of a third Person secured by a Lien on any asset owned by such first Person, whether or not such Indebtedness is assumed by such first Person;
provided, however, that notwithstanding the foregoing, Indebtedness shall be deemed not to include (a) Contingent Obligations incurred in the ordinary course of business or (b) obligations under or in respect of Receivables Facilities.
“Independent Financial Advisor” means an accounting, appraisal, investment banking firm or consultant to Persons engaged in Similar Businesses of nationally recognized standing that is, in the good faith judgment of the Company, qualified to perform the task for which it has been engaged.
“Initial Purchasers” means Banc of America Securities LLC, Wells Fargo Securities, LLC (formerly known as Wachovia Capital Markets, LLC), Barclays Capital Inc. and Scotia Capital (USA) Inc.
226
Table of Contents
“Intercreditor Agreement” means the Lien Subordination and Intercreditor Agreement, dated as of October 28, 2008, among the ABL Collateral Agent, the Notes/Term Collateral Agent, Sky Acquisition LLC, Sky Merger Sub Corporation, the Company and each Guarantor, as it may be amended from time to time in accordance with the Indenture.
“Investment Grade Rating” means a rating equal to or higher than Baa3 (or the equivalent) by Moody’s and BBB- (or the equivalent) by S&P, or an equivalent rating by any other Rating Agency.
“Investment Grade Securities” means:
(1) securities issued or directly and fully guaranteed or insured by the United States government or any agency or instrumentality thereof (other than Cash Equivalents);
(2) debt securities or debt instruments with an Investment Grade Rating, but excluding any debt securities or instruments constituting loans or advances among the Company and its Subsidiaries;
(3) investments in any fund that invests exclusively in investments of the type described in clauses (1) and (2) which fund may also hold immaterial amounts of cash pending investment or distribution; and
(4) corresponding instruments in countries other than the United States customarily utilized for high quality investments.
“Investments” means, with respect to any Person, all investments by such Person in other Persons (including Affiliates) in the form of loans (including guarantees), advances or capital contributions (excluding accounts receivable, trade credit, advances to customers, commission, travel and similar advances to officers and employees, in each case made in the ordinary course of business), purchases or other acquisitions for consideration of Indebtedness, Equity Interests or other securities issued by any other Person and investments that are required by GAAP to be classified on the balance sheet (excluding the footnotes) of the Company in the same manner as the other investments included in this definition to the extent such transactions involve the transfer of cash or other property. For purposes of the definition of “Unrestricted Subsidiary” and the covenant described under “—Certain Covenants—Limitation on Restricted Payments”:
(1) “Investments” shall include the portion (proportionate to the Company’s equity interest in such Subsidiary) of the fair market value of the net assets of a Subsidiary of the Company at the time that such Subsidiary is designated an Unrestricted Subsidiary;provided, however, that upon a redesignation of such Subsidiary as a Restricted Subsidiary, the Company shall be deemed to continue to have a permanent “Investment” in an Unrestricted Subsidiary in an amount (if positive) equal to:
(a) the Company’s “Investment” in such Subsidiary at the time of such redesignation; less
(b) the portion (proportionate to the Company’s equity interest in such Subsidiary) of the fair market value of the net assets of such Subsidiary at the time of such redesignation; and
(2) any property transferred to or from an Unrestricted Subsidiary shall be valued at its fair market value at the time of such transfer, in each case as determined in good faith by the Company.
“Investors” means The Blackstone Group and each of its Affiliates, but not including any of its portfolio companies.
“Issue Date” means May 27, 2009.
“Legal Holiday” means a Saturday, a Sunday or a day on which commercial banking institutions are not required to be open in the State of New York.
“Lien” means, with respect to any asset, any mortgage, lien (statutory or otherwise), pledge, hypothecation, charge, security interest, preference, priority or encumbrance of any kind in respect of such asset, whether or not filed, recorded or otherwise perfected under applicable law, including any conditional sale or other title retention
227
Table of Contents
agreement, any lease in the nature thereof, any option or other agreement to sell or give a security interest in and any filing of or agreement to give any financing statement under the Uniform Commercial Code (or equivalent statutes) of any jurisdiction;provided that in no event shall an operating lease be deemed to constitute a Lien.
“Moody’s” means Moody’s Investors Service, Inc. and any successor to its rating agency business.
“Net Income” means, with respect to any Person, the net income (loss) of such Person, determined in accordance with GAAP and before any reduction in respect of Preferred Stock dividends.
“Net Proceeds” means the aggregate cash proceeds and Cash Equivalents received by the Company or any of its Restricted Subsidiaries in respect of any Asset Sale, including any cash and Cash Equivalents received upon the sale or other disposition of any Designated Non-cash Consideration received in any Asset Sale, net of the direct costs relating to such Asset Sale and the sale or disposition of such Designated Non-cash Consideration, including legal, accounting and investment banking fees, and brokerage and sales commissions, any relocation expenses incurred as a result thereof, taxes paid or payable as a result thereof (after taking into account any available tax credits or deductions and any tax sharing arrangements), amounts required to be applied to the repayment of Indebtedness secured by a Lien on such assets (other than as required by clause (1) of the second paragraph of “—Repurchase at the Option of Holders—Asset Sales”) and any deduction of appropriate amounts to be provided by the Company or any of its Restricted Subsidiaries as a reserve in accordance with GAAP against any liabilities associated with the asset disposed of in such transaction and retained by the Company or any of its Restricted Subsidiaries after such sale or other disposition thereof, including pension and other post-employment benefit liabilities and liabilities related to environmental matters or against any indemnification obligations associated with such transaction.
“Notes/Term Collateral Agent” means Bank of America, N.A., in its capacity as “Collateral Agent” under the Intercreditor Agreement, the Notes/Term Intercreditor Agreement, and the other Collateral Documents, and any successor thereto in such capacity.
“Notes/Term Intercreditor Agreement” means the Intercreditor and Collateral Agency Agreement, dated as of the Issue Date, among the Company, each Guarantor, Banc of America Bridge LLC, as bridge loan agent, Bank of America, N.A., as Notes/Term Collateral Agent, and U.S. Bank National Association, as Trustee, and as it may be amended from time to time in accordance with the Indenture.
“Obligations” means any principal, interest (including any interest accruing subsequent to the filing of a petition in bankruptcy, reorganization or similar proceeding at the rate provided for in the documentation with respect thereto, whether or not such interest is an allowed claim under applicable state, federal or foreign law), penalties, fees, indemnifications, reimbursements (including reimbursement obligations with respect to letters of credit and banker’s acceptances), damages and other liabilities, and guarantees of payment of such principal, interest, penalties, fees, indemnifications, reimbursements, damages and other liabilities, payable under the documentation governing any Indebtedness.
“Officer” means the Chairman of the Board, the Chief Executive Officer, Chief Financial Officer, Chief Operating Officer, the President, any Executive Vice President, Senior Vice President or Vice President, the Treasurer or the Secretary of the Company or a Guarantor.
“Officer’s Certificate” means a certificate signed on behalf of the Company by an Officer of the Company or on behalf of a Guarantor by an Officer of such Guarantor, who must be the principal executive officer, the principal financial officer, the treasurer or the principal accounting officer of the Company or any officer of such Guarantor that meets the requirements set forth in the Indenture.
“Opinion of Counsel” means a written opinion from legal counsel who is acceptable to the Trustee. The counsel may be an employee of or counsel to the Company, a Subsidiary of the Company or the Trustee.
228
Table of Contents
“Permitted Asset Swap” means the concurrent purchase and sale or exchange of Related Business Assets or a combination of Related Business Assets and cash or Cash Equivalents between the Company or any of its Restricted Subsidiaries and another Person; provided, that any cash or Cash Equivalents received must be applied in accordance with the “Repurchase at the Option of Holders—Asset Sales” covenant;provided further that the assets received are pledged as Collateral to the extent required by the Collateral Documents to the extent that the assets disposed of constituted Collateral.
“Permitted Holders” means each of the Investors and members of management of the Company (or its direct or indirect parent or Subsidiary) on the Issue Date who are holders of Equity Interests of the Company (or any of its direct or indirect parent companies) and any group (within the meaning of Section 13(d)(3) or Section 14(d)(2) of the Exchange Act or any successor provision) of which any of the foregoing are members;provided that, in the case of such group and without giving effect to the existence of such group or any other group, such Investors and members of management, collectively, have beneficial ownership of more than 50% of the total voting power of the Voting Stock of the Company or any of its direct or indirect parent companies.
“Permitted Investment” means:
(1) any Investment in the Company or any of its Restricted Subsidiaries;
(2) any Investment in cash and Cash Equivalents or Investment Grade Securities;
(3) any Investment by the Company or any of its Restricted Subsidiaries in a Person that is engaged in a Similar Business if as a result of such Investment:
(a) such Person becomes a Restricted Subsidiary; or
(b) such Person, in one transaction or a series of related transactions, is merged or consolidated with or into, or transfers or conveys substantially all of its assets to, or is liquidated into, the Company or a Restricted Subsidiary,
and, in each case, any Investment held by such Person; provided, that such Investment was not acquired by such Person in contemplation of such acquisition, merger, consolidation or transfer;
(4) any Investment in securities or other assets, including earnouts, not constituting cash and Cash Equivalents and received in connection with an Asset Sale made pursuant to the provisions of “—Repurchase at the Option of Holders—Asset Sales” or any other disposition of assets not constituting an Asset Sale;
(5) any Investment existing on the Issue Date;
(6) any Investment acquired by the Company or any of its Restricted Subsidiaries:
(a) in exchange for any other Investment or accounts receivable held by the Company or any such Restricted Subsidiary in connection with or as a result of a bankruptcy, workout, reorganization or recapitalization of the issuer of such other Investment or accounts receivable; or
(b) as a result of a foreclosure by the Company or any of its Restricted Subsidiaries with respect to any secured Investment or other transfer of title with respect to any secured Investment in default;
(7) Hedging Obligations permitted under clause (10) of the second paragraph under the covenant described in “—Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”;
(8) [intentionally omitted];
(9) Investments the payment for which consists of Equity Interests (exclusive of Disqualified Stock) of the Company, or any of its direct or indirect parent companies;provided, however, that such Equity Interests will not increase the amount available for Restricted Payments under clause (3) of the first paragraph under the covenant described in “—Certain Covenants—Limitations on Restricted Payments”;
229
Table of Contents
(10) guarantees of Indebtedness of the Company and any Restricted Subsidiary permitted under the covenant described in “—Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”;
(11) any transaction to the extent it constitutes an Investment that is permitted and made in accordance with the provisions of the second paragraph of the covenant described under “—Certain Covenants— Transactions with Affiliates” (except transactions described in clauses (2), (5) and (9) of such paragraph);
(12) Investments consisting of purchases and acquisitions of inventory, supplies, material or equipment;
(13) additional Investments having an aggregate fair market value, taken together with all other Investments made pursuant to this clause (13) that are at that time outstanding (without giving effect to the sale of an Unrestricted Subsidiary to the extent the proceeds of such sale do not consist of cash or marketable securities), not to exceed the greater of (x) $95.0 million and (y) 3.5% of Total Assets at the time of such Investment (with the fair market value of each Investment being measured at the time made and without giving effect to subsequent changes in value);
(14) Investments relating to a Receivables Subsidiary that, in the good faith determination of the Company are necessary or advisable to effect any Receivables Facility;
(15) loans and advances to officers, directors and employees, in each case incurred in the ordinary course of business or consistent with past practices or to fund such Person’s purchase of Equity Interests of the Company or any direct or indirect parent company thereof;
(16) Investments (including debt obligations and Equity Interests) received in connection with the bankruptcy or reorganization of suppliers and customers or in settlement of delinquent obligations of, or other disputes with, customers and suppliers arising in the ordinary course of business or upon the foreclosure with respect to any secured Investment or other transfer of title with respect to any secured Investment; and
(17) Investments in joint ventures of the Company or any of its Restricted Subsidiaries existing on the Issue Date or created after the Issue Date in an aggregate amount not to exceed $20.0 million.
“Permitted Liens” means, with respect to any Person:
(1) pledges or deposits by such Person under workmen’s compensation laws, unemployment insurance laws or similar legislation, or good faith deposits in connection with bids, tenders, contracts (other than for the payment of Indebtedness) or leases to which such Person is a party, or deposits to secure public or statutory obligations of such Person or deposits of cash or U.S. government bonds to secure surety or appeal bonds to which such Person is a party, or deposits as security for contested taxes or import duties or for the payment of rent, in each case incurred in the ordinary course of business;
(2) Liens imposed by law, such as carriers’, warehousemen’s and mechanics’ Liens, in each case for sums not yet overdue for a period of more than 30 days or being contested in good faith by appropriate proceedings or other Liens arising out of judgments or awards against such Person with respect to which such Person shall then be proceeding with an appeal or other proceedings for review if adequate reserves with respect thereto are maintained on the books of such Person in accordance with GAAP;
(3) Liens for taxes, assessments or other governmental charges not yet overdue for a period of more than 30 days or payable or subject to penalties for nonpayment or which are being contested in good faith by appropriate proceedings diligently conducted, if adequate reserves with respect thereto are maintained on the books of such Person in accordance with GAAP;
(4) Liens in favor of issuers of performance and surety bonds or bid bonds or with respect to other regulatory requirements or letters of credit issued pursuant to the request of and for the account of such Person in the ordinary course of its business;
230
Table of Contents
(5) minor survey exceptions, minor encumbrances, easements or reservations of, or rights of others for, licenses, rights-of-way, sewers, electric lines, telegraph and telephone lines and other similar purposes, or zoning or other restrictions as to the use of real properties or Liens incidental, to the conduct of the business of such Person or to the ownership of its properties which were not incurred in connection with Indebtedness and which do not in the aggregate materially adversely affect the value of said properties or materially impair their use in the operation of the business of such Person;
(6) Liens (including Liens on Collateral) securing Indebtedness permitted to be incurred pursuant to clauses (4), (10) and (12)(b) of the second paragraph under “—Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”;provided, however, that Liens securing Indebtedness permitted to be incurred pursuant to such clause (12)(b) shall not secure such Indebtedness in excess of $100.0 million at any one time outstanding;
(7) Liens existing on the Issue Date;
(8) Liens on property or shares of stock of a Person at the time such Person becomes a Subsidiary;provided, however, such Liens are not created or incurred in connection with, or in contemplation of, such other Person becoming such a Subsidiary;provided further,however, that such Liens may not extend to any other property owned by the Company or any of its Restricted Subsidiaries;
(9) Liens on property at the time the Company or a Restricted Subsidiary acquired the property, including any acquisition by means of a merger or consolidation with or into the Company or any of its Restricted Subsidiaries;provided, however, that such Liens are not created or incurred in connection with, or in contemplation of, such acquisition;provided further,however, that the Liens may not extend to any other property owned by the Company or any of its Restricted Subsidiaries;
(10) Liens securing Indebtedness or other obligations of a Restricted Subsidiary owing to the Company or another Restricted Subsidiary permitted to be incurred in accordance with the covenant described under “—Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”;
(11) Liens securing Hedging Obligations so long as related Indebtedness is, and is permitted to be under the Indenture, secured by a Lien on the same property securing such Hedging Obligations;
(12) Liens on specific items of inventory of other goods and proceeds of any Person securing such Person’s obligations in respect of bankers’ acceptances issued or created for the account of such Person to facilitate the purchase, shipment or storage of such inventory or other goods;
(13) leases, subleases, licenses or sublicenses granted to others in the ordinary course of business which do not materially interfere with the ordinary conduct of the business of the Company or any of its Restricted Subsidiaries and do not secure any Indebtedness;
(14) Liens arising from Uniform Commercial Code financing statement filings regarding operating leases entered into by the Company and its Restricted Subsidiaries in the ordinary course of business;
(15) Liens in favor of the Company or any Guarantor;
(16) Liens on equipment of the Company or any of its Restricted Subsidiaries granted in the ordinary course of business to the Company’s clients;
(17) Liens on accounts receivable and related assets incurred in connection with a Receivables Facility;
(18) Liens to secure any refinancing, refunding, extension, renewal or replacement (or successive refinancing, refunding, extensions, renewals or replacements) as a whole, or in part, of any Indebtedness secured by any Lien referred to in the foregoing clauses (7), (8) and (9);provided, however, that (a) such new Lien shall be limited to all or part of the same property that secured the original Lien (plus improvements on such property), and (b) the Indebtedness secured by such Lien at such time is not increased to any amount greater than the sum of (i) the outstanding principal amount or, if greater,
231
Table of Contents
committed amount of the Indebtedness described under clauses (7), (8) and (9) at the time the original Lien became a Permitted Lien under the Indenture, and (ii) an amount necessary to pay any fees and expenses, including premiums, related to such refinancing, refunding, extension, renewal or replacement;
(19) deposits made in the ordinary course of business to secure liability to insurance carriers;
(20) other Liens (including Liens on Collateral) securing obligations not to exceed $100.0 million at any one time outstanding;
(21) Liens securing Indebtedness of any Foreign Subsidiary permitted to be incurred under the Indenture, to the extent such Liens relate only to the assets and properties of such Foreign Subsidiary;
(22) Liens securing judgments for the payment of money not constituting an Event of Default under clause (5) under the caption “Events of Default and Remedies” so long as such Liens are adequately bonded and any appropriate legal proceedings that may have been duly initiated for the review of such judgment have not been finally terminated or the period within which such proceedings may be initiated has not expired;
(23) Liens in favor of customs and revenue authorities arising as a matter of law to secure payment of customs duties in connection with the importation of goods in the ordinary course of business;
(24) Liens (i) of a collection bank arising under Section 4-210 of the Uniform Commercial Code or any comparable or successor provision on items in the course of collection, (ii) attaching to commodity trading accounts or other commodity brokerage accounts incurred in the ordinary course of business, and (iii) in favor of banking institutions arising as a matter of law encumbering deposits (including the right of set-off) and which are within the general parameters customary in the banking industry;
(25) Liens deemed to exist in connection with Investments in repurchase agreements permitted under “—Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”;provided that such Liens do not extend to any assets other than those that are the subject of such repurchase agreement;
(26) Liens encumbering reasonable customary initial deposits and margin deposits and similar Liens attaching to commodity trading accounts or other brokerage accounts incurred in the ordinary course of business and not for speculative purposes;
(27) Liens that are contractual rights of set-off (i) relating to the establishment of depository relations with banks not given in connection with the issuance of Indebtedness, (ii) relating to pooled deposit or sweep accounts of the Company or any of its Restricted Subsidiaries to permit satisfaction of overdraft or similar obligations incurred in the ordinary course of business of the Company and its Restricted Subsidiaries or (iii) relating to purchase orders and other agreements entered into with customers of the Company or any of its Restricted Subsidiaries in the ordinary course of business;
(28)(a) Liens securing the Series A-1 Notes and the related Guarantees of the Series A-1 Notes (and exchange notes in respect thereof), up to $150.0 million of Additional Notes or Term Loans or a combination thereof and up to $150.0 million of Additional Notes or Term Loans or a combination thereof the proceeds of which will be used solely to finance acquisitions (solely to the extent the Fixed Charge Coverage Ratio following any such acquisition and debt incurrence would have been at least 3.50 to 1.00, determined on apro forma basis) and (b) Liens securing the Series A-2 Debt, in an amount no greater than $310.0 million (plus up to an additional $15.0 million of Series A-2 Notes which the Company has the right to issue), so long as the Series A-1 Notes are equally and ratably secured and such Series A-2 Debt is subject to the priority of payment provisions contained in the Notes/Term Intercreditor Agreement and the Indenture;
(29) Liens securing (x) Indebtedness and other obligations permitted to be incurred under Credit Facilities, including any letter of credit facility relating thereto, that was incurred pursuant to clause (1) of the second paragraph under “—Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance
232
Table of Contents
of Disqualified Stock and Preferred Stock” in an amount up to the greater of (A) $175.0 million and (B) the Borrowing Base, and (y) obligations of the Company or any Subsidiary in respect of any Bank Products provided by any lender party to the ABL Facility or any Affiliate of such lender (or any Person that was a lender or an Affiliate of a lender at the time the applicable agreements pursuant to which such Bank Products are provided were entered into);provided, however, that any Liens on Notes/Term Collateral granted pursuant to this clause (29) must be junior in priority to the Liens on such Collateral granted in favor of the Notes/Term Collateral Agent for the benefit of the Trustee and the holders of the Series A-1 Notes and the Series A-2 Notes and the benefit of the Term Loan Lenders and the holders of the Term Loans pursuant to the Collateral Documents and the terms of such junior interest may be no more favorable to the beneficiaries thereof than the terms contained in the Intercreditor Agreement; and provided, further, that no Liens may be granted pursuant to this clause (29) unless the Series A-1 Notes, the Series A-2 Notes and the Term Loans are secured by a second-priority Lien that is junior in priority to the Liens on such Collateral but senior in priority to any other Liens granted on such Collateral;
(30) Liens on the Collateral to secure obligations in respect of any Indebtedness permitted to be incurred pursuant to the first paragraph under “—Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”;provided, however, that such Liens on the Collateral are junior in priority to the Liens granted to holders of the Series A-1 Notes, the Series A-2 Notes and the Term Loans;
(31) any encumbrance or restriction (including put and call arrangements) with respect to capital stock of any joint venture or similar arrangement pursuant to any joint venture or similar agreement;
(32) Liens arising out of conditional sale, title retention, consignment or similar arrangements for the sale or purchase of goods entered into by the Company or any of its Restricted Subsidiaries in the ordinary course of business; and
(33) Liens solely on any cash earnest money deposits made by the Company or any of its Restricted Subsidiaries in connection with any letter of intent or purchase agreement permitted hereunder.
For purposes of this definition, the term “Indebtedness” shall be deemed to include interest on such Indebtedness.
“Person” means any individual, corporation, limited liability company, partnership, joint venture, association, joint stock company, trust, unincorporated organization, government or any agency or political subdivision thereof or any other entity.
“Preferred Stock” means any Equity Interest with preferential rights of payment of dividends or upon liquidation, dissolution, or winding up.
“Qualified Proceeds” means assets that are used or useful in, or Capital Stock of any Person engaged in, a Similar Business;provided that the fair market value of any such assets or Capital Stock shall be determined by the Company in good faith.
“Rating Agencies” means Moody’s and S&P or if Moody’s or S&P or both shall not make a rating on the Series A-1 Notes publicly available, a nationally recognized statistical rating agency or agencies, as the case may be, selected by the Company which shall be substituted for Moody’s or S&P or both, as the case may be.
“Receivables Facility” means any of one or more receivables financing facilities as amended, supplemented, modified, extended, renewed, restated or refunded from time to time, the Obligations of which are non-recourse (except for customary representations, warranties, covenants and indemnities made in connection with such facilities) to the Company or any of its Restricted Subsidiaries (other than a Receivables Subsidiary) pursuant to which the Company or any of its Restricted Subsidiaries sells its accounts receivable to either (a) a Person that is not a Restricted Subsidiary or (b) a Receivables Subsidiary that in turn sells its accounts receivable to a Person that is not a Restricted Subsidiary.
233
Table of Contents
“Receivables Fees” means distributions or payments made directly or by means of discounts with respect to any accounts receivable or participation interest therein issued or sold in connection with, and other fees paid to a Person that is not a Restricted Subsidiary in connection with, any Receivables Facility.
“Receivables Subsidiary” means any Subsidiary formed for the purpose of, and that solely engages only in one or more Receivables Facilities and other activities reasonably related thereto.
“Registration Rights Agreement” means the Registration Rights Agreement with respect to the applicable Series of Notes, among the Company, the Guarantors and the Initial Purchasers, as such agreement may be amended, modified or supplemented from time to time and, with respect to any Additional Notes, one or more registration rights agreements among the Company and the other parties thereto, as such agreement(s) may be amended, modified or supplemented from time to time, relating to rights given by the Company to the purchasers of Additional Notes to register such Additional Notes under the Securities Act.
“Related Business Assets” means assets (other than cash or Cash Equivalents) used or useful in a Similar Business, provided that any assets received by the Company or a Restricted Subsidiary in exchange for assets transferred by the Company or a Restricted Subsidiary shall not be deemed to be Related Business Assets if they consist of securities of a Person, unless upon receipt of the securities of such Person, such Person would become a Restricted Subsidiary.
“Restricted Investment” means an Investment other than a Permitted Investment.
“Restricted Subsidiary” means, at any time, any direct or indirect Subsidiary of the Company (including any Foreign Subsidiary) that is not then an Unrestricted Subsidiary;provided, however, that upon the occurrence of an Unrestricted Subsidiary ceasing to be an Unrestricted Subsidiary, such Subsidiary shall be included in the definition of “Restricted Subsidiary.”
“S&P” means Standard & Poor’s, a division of The McGraw-Hill Companies, Inc., and any successor to its rating agency business.
“Sale and Lease-Back Transaction” means any arrangement providing for the leasing by the Company or any of its Restricted Subsidiaries of any real or tangible personal property, which property has been or is to be sold or transferred by the Company or such Restricted Subsidiary to a third Person in contemplation of such leasing.
“SEC” means the U.S. Securities and Exchange Commission.
“Secured Indebtedness” means any Indebtedness of the Company or any of its Restricted Subsidiaries secured by a Lien.
“Securities Act” means the Securities Act of 1933, as amended, and the rules and regulations of the SEC promulgated thereunder.
“Senior Indebtedness” means:
(1) all Indebtedness of the Company or any Guarantor outstanding under the ABL Facility (including interest accruing on or after the filing of any petition in bankruptcy or similar proceeding or for reorganization of the Company or any Guarantor (at the rate provided for in the documentation with respect thereto, regardless of whether or not a claim for post-filing interest is allowed in such proceedings)), and any and all other fees, expense reimbursement obligations, indemnification amounts, penalties, and other amounts (whether existing on the Issue Date or thereafter created or incurred) and all obligations of the Company or any Guarantor to reimburse any bank or other Person in respect of amounts paid under letters of credit, acceptances or other similar instruments;
234
Table of Contents
(2) all Hedging Obligations (and guarantees thereof) owing to a Lender (as defined in the ABL Facility) or any Affiliate of such Lender (or any Person that was a Lender or an Affiliate of such Lender at the time the applicable agreement giving rise to such Hedging Obligation was entered into), provided that such Hedging Obligations are permitted to be incurred under the terms of the Indenture;
(3) all Series A-2 Debt;
(4) any other Indebtedness of the Company or any Guarantor permitted to be incurred under the terms of the Indenture, unless the instrument under which such Indebtedness is incurred expressly provides that it is subordinate in right of payment to the Notes or any related Guarantee; and
(5) all Obligations with respect to the items listed in the preceding clauses (1), (2), (3) and (4);provided, however, that Senior Indebtedness shall not include:
(a) any obligation of such Person to the Company or any of its Subsidiaries;
(b) any liability for federal, state, local or other taxes owed or owing by such Person;
(c) any accounts payable or other liability to trade creditors arising in the ordinary course of business;
(d) any Indebtedness or other Obligation of such Person which is subordinate or junior in any respect to any other Indebtedness or other Obligation of such Person; or
(e) that portion of any Indebtedness which at the time of incurrence is incurred in violation of the Indenture;provided, however, that such Indebtedness shall be deemed not to have been incurred in violation of the Indenture for purposes of this clause if the holder(s) of such Indebtedness or their agent or representative (i) had no actual knowledge at the time of incurrence that the incurrence of such Indebtedness violated the Indenture and (ii) shall have received a certificate from an officer of the Company to the effect that the incurrence of such Indebtedness does not violate the provisions of the Indenture.
“Series A-2 Debt” means (i) any Indebtedness outstanding from time to time under the Term Loan Facility, including without limitation any “Rollover Loans” as defined therein, (ii) any Series A-2 Notes, and (iii) all obligations with respect to such Indebtedness and any Hedging Obligations permitted under any Series A-2 Debt owed or owing to any lender (or its affiliates) under the Term Loan Facility or the ABL Facility which qualifies as a “Secured Hedge Agreement” under the Term Loan Facility.
“Significant Subsidiary” means any Restricted Subsidiary that would be a “significant subsidiary” as defined in Article 1, Rule 1-02 of Regulation S-X, promulgated pursuant to the Securities Act, as such regulation is in effect on the Issue Date.
“Similar Business” means any business conducted or proposed to be conducted by the Company and its Restricted Subsidiaries on the Issue Date or any business that is similar, reasonably related, incidental or ancillary thereto.
“Sponsor Management Agreement” means the management agreement between certain of the management companies associated with the Investors and the Company and/or one of its direct or indirect parent companies as in effect on the Issue Date.
“Subordinated Indebtedness” means, with respect to the Notes,
(1) any Indebtedness of the Company which is by its terms subordinated in right of payment to the Notes, and
(2) any Indebtedness of any Guarantor which is by its terms subordinated in right of payment to the Guarantee of such entity of the Notes.
235
Table of Contents
The Series A-2 Debt shall not be deemed “Subordinated Indebtedness” hereunder by virtue of the provisions described under “—Security for the Notes—Priorities of Payment as between Series A-2 Debt (including the Series A-2 Notes) and Series A-1 Notes.”
“Subsidiary” means, with respect to any Person:
(1) any corporation, association, or other business entity (other than a partnership, joint venture, limited liability company or similar entity) of which more than 50% of the total voting power of shares of Capital Stock entitled (without regard to the occurrence of any contingency) to vote in the election of directors, managers or trustees thereof is at the time of determination owned or controlled, directly or indirectly, by such Person or one or more of the other Subsidiaries of that Person or a combination thereof or is consolidated under GAAP with such Person at such time; and
(2) any partnership, joint venture, limited liability company or similar entity of which
(a) more than 50% of the capital accounts, distribution rights, total equity and voting interests or general or limited partnership interests, as applicable, are owned or controlled, directly or indirectly, by such Person or one or more of the other Subsidiaries of that Person or a combination thereof whether in the form of membership, general, special or limited partnership or otherwise, and
(b) such Person or any Restricted Subsidiary of such Person is a general partner or otherwise controls such entity.
“Term Loans” means loans made pursuant to and in accordance with the Term Loan Facility, including the Rollover Loans as defined therein.
“Term Loan Lenders” means the lenders under the Term Loan Facility.
“Term Loan Facility” means the Senior Secured Bridge Credit Agreement, dated as of October 28, 2008, by and among the Company, the Guarantors, the lenders party thereto in their capacities as lenders thereunder and Bank of America Bridge LLC, as Administrative Agent, including any guarantees, collateral documents, instruments and agreements executed in connection therewith, and any amendments, supplements, modifications, extensions, renewals, restatements, refundings or refinancings thereof and any indentures or credit facilities or commercial paper facilities with banks or other institutional lenders or investors that replace, refund or refinance any part of the loans, notes, other credit facilities or commitments thereunder, including any such replacement, refunding or refinancing facility or indenture that increases the amount borrowable thereunder or alters the maturity thereof (provided that such increase in borrowings is permitted under “—Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” above).
“Total Assets” means the total assets of the Company, except where expressly provided otherwise, and its Restricted Subsidiaries on a consolidated basis, as shown on the most recent balance sheet of the Company;provided, however, that in no event at anytime shall Total Assets be deemed to equal an amount less than the amount of total assets of the Company and its Restricted Subsidiaries on a consolidated basis as of the Issue Date.
“Transaction” means the merger contemplated by the Transaction Agreement, the issuances of the Series A-1 Notes and the Series A-2 Notes and borrowings under the ABL Facility and the Term Loan Facility on October 28, 2008 in order to finance the merger and repay certain debt as described in the offering memorandum relating to the sale of the Series A-1 Notes.
“Transaction Agreement” means the Agreement and Plan of Merger, dated as of June 18, 2008, by and among Apria Healthcare Group Inc., Sky Acquisition LLC and Sky Merger Sub Corporation, as the same may be amended prior to the Issue Date.
236
Table of Contents
“Treasury Rate” means, as of any Redemption Date, the yield to maturity as of such Redemption Date of United States Treasury securities with a constant maturity (as compiled and published in the most recent Federal Reserve Statistical Release H. 15 (519) that has become publicly available at least two Business Days prior to the Redemption Date (or, if such Statistical Release is no longer published, any publicly available source of similar market data)) most nearly equal to the period from the Redemption Date to November 1, 2011;provided, however, that if the period from the Redemption Date to November 1, 2011 is less than one year, the weekly average yield on actually traded United States Treasury securities adjusted to a constant maturity of one year will be used.
“Trust Indenture Act” means the Trust Indenture Act of 1939, as amended (15 U.S.C. §§ 77aaa-777bbbb).
“Unrestricted Subsidiary” means:
(1) any Subsidiary of the Company which at the time of determination is an Unrestricted Subsidiary (as designated by the Company, as provided below); and
(2) any Subsidiary of an Unrestricted Subsidiary.
The Company may designate any Subsidiary of the Company (including any existing Subsidiary and any newly acquired or newly formed Subsidiary) to be an Unrestricted Subsidiary unless such Subsidiary or any of its Subsidiaries owns any Equity Interests or Indebtedness of, or owns or holds any Lien on, any property of, the Company or any Subsidiary of the Company (other than solely any Subsidiary of the Subsidiary to be so designated);provided that
(1) any Unrestricted Subsidiary must be an entity of which the Equity Interests entitled to cast at least a majority of the votes that may be cast by all Equity Interests having ordinary voting power for the election of directors or Persons performing a similar function are owned, directly or indirectly, by the Company;
(2) such designation complies with the covenant described under “Certain Covenants—Limitation on Restricted Payments”; and
(3) each of:
(a) the Subsidiary to be so designated; and
(b) its Subsidiaries has not at the time of designation, and does not thereafter, create, incur, issue, assume, guarantee or otherwise become directly or indirectly liable with respect to any Indebtedness pursuant to which the lender has recourse to any of the assets of the Company or any Restricted Subsidiary.
The Company may designate any Unrestricted Subsidiary to be a Restricted Subsidiary;provided that, immediately after giving effect to such designation, no Default shall have occurred and be continuing and either:
(1) the Company could incur at least $1.00 of additional Indebtedness pursuant to the Fixed Charge Coverage Ratio test described in the first paragraph under “—Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”; or
(2) the Fixed Charge Coverage Ratio for the Company and its Restricted Subsidiaries would be greater than such ratio for the Company and its Restricted Subsidiaries immediately prior to such designation, in each case on apro forma basis taking into account such designation.
Any such designation by the Company shall be notified by the Company to the Trustee by promptly filing with the Trustee a copy of the resolution of the board of directors of the Company or any committee thereof giving effect to such designation and an Officer’s Certificate certifying that such designation complied with the foregoing provisions.
237
Table of Contents
“Voting Stock” of any Person as of any date means the Capital Stock of such Person that is at the time entitled to vote in the election of the board of directors of such Person.
“Weighted Average Life to Maturity” means, when applied to any Indebtedness, Disqualified Stock or Preferred Stock, as the case may be, at any date, the quotient obtained by dividing:
(1) the sum of the products of the number of years from the date of determination to the date of each successive scheduled principal payment of such Indebtedness or redemption or similar payment with respect to such Disqualified Stock or Preferred Stock multiplied by the amount of such payment; by
(2) the sum of all such payments.
“Wholly-Owned Subsidiary” of any Person means a Subsidiary of such Person, 100% of the outstanding Equity Interests of which (other than directors’ qualifying shares) shall at the time be owned by such Person or by one or more Wholly-Owned Subsidiaries of such Person.
238
Table of Contents
DESCRIPTION OF OTHER INDEBTEDNESS
Senior Secured Asset-Based Revolving Credit Facility
We summarize below the principal terms of the agreements that govern our senior secured asset-based revolving credit facility. This summary is not a complete description of all the terms of such agreements.
General
In connection with the Merger, on October 28, 2008, we entered into a senior secured asset-based revolving credit facility, or ABL Facility, with Bank of America, N.A., as administrative agent and collateral agent, Wachovia Bank, National Association and Barclays Capital, the investment banking division of Barclays Bank PLC, as syndication agents, and The Bank of Nova Scotia, as documentation agent, and a syndicate of financial institutions and institutional lenders. Banc of America Securities LLC and Wells Fargo Securities, LLC (formerly known as Wachovia Capital Markets, LLC) acted as joint lead arrangers and Banc of America Securities LLC, Wells Fargo Securities, LLC (formerly known as Wachovia Capital Markets, LLC) and Barclays Capital, the investment banking division of Barclays Bank PLC, acted as joint bookrunners.
Our ABL Facility provides for revolving credit financing of up to $150.0 million, subject to borrowing base availability, with a maturity of five years, including both a letter of credit and swingline loan sub-facility.
The borrowing base at any time is equal to the sum (subject to certain reserves and other adjustments) of:
| • | 85% of eligible receivables; and |
| • | the lesser of (a) 85% of the net orderly liquidation value of eligible inventory and (b) $20.0 million. |
Our ABL Facility includes borrowing capacity available for letters of credit and for borrowings on same-day notice, referred to as swingline loans.
Borrowings under our ABL Facility are subject to the satisfaction of customary conditions, including absence of a default and accuracy of representations and warranties.
Provided that no default or event of default is then existing or would arise therefrom, at our option, we may request that our ABL Facility be increased by an amount not to exceed $25.0 million, subject to certain consent rights of the administrative agent, swingline lender and issuing banks with respect to the lenders providing commitments for such increase. The terms of such incremental revolving facility shall be identical to our ABL Facility.
Interest Rate and Fees
Borrowings under our ABL Facility bear interest at a rate per annum equal to, at our option, either (a) a base rate determined by reference to the higher of (1) the prime rate of Bank of America, N.A. and (2) the federal funds effective rate plus 1/2 of 1% (“Base Rate”), plus an applicable margin of 2.00% or (b) a LIBOR rate determined by reference to LIBOR, adjusted for statutory reserve requirements, plus an applicable margin of 3.00%. The applicable margin for borrowings under our ABL Facility is subject to step ups and step downs based on average excess availability under the ABL Facility. In addition to paying interest on outstanding amounts under our ABL Facility, we are required to pay a commitment fee, in respect of the unutilized commitments thereunder, ranging from 0.50% to 1.00% per annum, which fee will be determined based on utilization of our ABL Facility (increasing when utilization is low and decreasing when utilization is high). We must also pay customary letter of credit fees equal to the applicable margin on LIBOR loans and agency fees.
Mandatory Repayments
If at any time the aggregate amount of outstandings under our ABL Facility, including letter of credit outstandings and swingline loans, exceeds the lesser of (i) the aggregate commitments under our ABL Facility
239
Table of Contents
and (ii) the borrowing base (except as a result of overadvance loans or protective advances), we will be required to repay outstanding loans (including swingline loans) and repay or cash collateralize letters of credit in an aggregate amount equal to such excess. If the amount available under our ABL Facility is less than 12.5% of the lesser of the aggregate commitments or the borrowing base for five consecutive business days or certain events of default have occurred, we will be required, upon the occurrence and during the continuance of such cash dominion event, to deposit cash from our material deposit accounts daily in a core concentration account maintained with the administrative agent under our ABL Facility, which will be used to repay outstanding loans and cash collateralize letters of credit.
Voluntary Repayments
We may voluntarily reduce the unutilized portion of the commitment amount and repay outstanding loans at any time (subject to minimum repayment amounts and customary notice periods) without premium or penalty other than customary “breakage” costs with respect to LIBOR loans.
Amortization and Final Maturity
There is no scheduled amortization under our ABL Facility. All outstanding loans under the facility are due and payable in full on the fifth anniversary of the closing date.
Guarantees and Security
All obligations under our ABL Facility, any interest rate protection or other hedging arrangements entered into with any lender in the syndicate or any of its affiliates, and cash management obligations owing to any lender in the syndicate or any of its affiliates are unconditionally guaranteed by our parent and substantially all of our existing and future, direct and indirect, wholly-owned domestic restricted subsidiaries. All obligations under our ABL Facility, any interest rate protection or other hedging arrangements entered into with any lender in the syndicate or any of its affiliates, and cash management obligations owing to any lender in the syndicate or any of its affiliates, and the guarantees of those obligations, are secured, subject to certain exceptions, by substantially all of our assets and the assets of the guarantors, including:
| • | a first-priority security interest in substantially all personal property consisting of accounts receivable arising from the sale of inventory and other goods and services, inventory, intercompany notes and intangible assets to the extent attached to the foregoing, and certain related assets and proceeds of the foregoing; and |
| • | a second-priority security interest in all tangible and intangible assets that secure the Notes on a first-priority basis. |
Restrictive Covenants and Other Matters
Our ABL Facility requires that if excess availability is less than 12.5% of the lesser of the aggregate commitments and the borrowing base, we comply with a minimum fixed charge coverage ratio test. In addition, our ABL Facility includes negative covenants that, subject to significant exceptions, limit our ability and the ability of our parent and subsidiaries to, among other things:
| • | incur, assume or permit to exist additional indebtedness or guarantees; |
| • | incur liens; |
| • | make investments and loans; |
| • | pay dividends, make payments or redeem or repurchase capital stock; |
| • | engage in mergers, liquidations, dissolutions, asset sales and other dispositions (including sale leaseback transactions); |
240
Table of Contents
| • | prepay, redeem or purchase certain indebtedness; |
| • | amend or otherwise alter terms of certain indebtedness; |
| • | enter into agreements limiting subsidiary distributions; |
| • | engage in certain transactions with affiliates; |
| • | alter the business that we conduct; |
| • | change our fiscal year; and |
| • | with respect to our parent, engage in any activities not permitted. |
Our ABL Facility contains certain customary representations and warranties, affirmative covenants and events of default, including among other things payment defaults, breach of representations and warranties, covenant defaults, cross-defaults to certain indebtedness, certain events of bankruptcy, certain events under ERISA, material judgments, failure of any guaranty or security document supporting our ABL Facility to be in full force and effect, and change of control. If such an event of default occurs, the lenders under our ABL Facility would be entitled to take various actions, including the acceleration of amounts due under our ABL Facility and all actions permitted to be taken by a secured creditor.
As of March 31, 2010, there were no outstanding borrowings under the ABL Facility, outstanding letters of credit totaled $16.1 million and additional availability under our ABL Facility subject to the borrowing base was $133.9 million. As of March 31, 2010, our availability under the borrowing base certificate was $133.9 million. At March 31, 2010, we were in compliance with all of the financial covenants required by the credit agreement governing our ABL Facility.
241
Table of Contents
CERTAIN U.S. FEDERAL INCOME TAX CONSIDERATIONS
Exchange Offers
The exchange of outstanding notes for exchange notes in the exchange offers will not constitute a taxable event to holders for U.S. federal income tax purposes. Consequently, you will not recognize gain or loss upon receipt of an exchange note, the holding period of the exchange note will include the holding period of the outstanding note exchanged therefor and the basis of the exchange note will be the same as the basis of the outstanding note immediately before the exchange.
In any event, persons considering the exchange of outstanding notes for exchange notes should consult their own tax advisors concerning the U.S. federal income tax consequences in light of their particular situations as well as any consequences arising under the laws of any other taxing jurisdiction.
Ownership of the Notes
The following is a summary of certain U.S. federal income tax consequences of the acquisition, ownership, and disposition of the Notes as of the date hereof. It is based on provisions of the U.S. Internal Revenue Code of 1986, as amended (the “Code”), existing and proposed Treasury regulations promulgated thereunder (the “Treasury Regulations”), and administrative and judicial interpretations thereof, all as of the date hereof and all of which are subject to change, possibly on a retroactive basis. No ruling from the Internal Revenue Service (the “IRS”) has been or is expected to be sought with respect to any aspect of the transactions described herein. Accordingly, no assurance can be given that the IRS will agree with the views expressed in this summary, or that a court will not sustain any challenge by the IRS in the event of litigation. The following relates only to Notes that are held as capital assets (i.e., generally, property held for investment). This summary does not address all of the U.S. federal income tax consequences that may be relevant to particular holders in light of their personal circumstances, or to certain types of holders that may be subject to special tax treatment (such as banks and other financial institutions, employee stock ownership plans, partnerships or other pass-through entities for U.S. federal income tax purposes, certain former citizens or residents of the United States, controlled foreign corporations, corporations that accumulate earnings to avoid U.S. federal income tax, regulated investment companies, real estate investment trusts, passive foreign investment companies, traders in securities that have elected the mark-to-market method of accounting, insurance companies, tax-exempt organizations, dealers in securities and foreign currencies, brokers, persons who hold the Notes as part of a straddle, hedge or other integrated transaction or who hedge the interest rate on the Notes, persons deemed to sell Notes under the constructive sale provisions of the Code, “U.S. holders” (as defined below) whose functional currency is not the U.S. dollar, or persons subject to the alternative minimum tax). In addition, this summary does not include any description of the tax laws of any state, local, or non-U.S. jurisdiction that may be applicable to a particular holder and does not consider any aspects of U.S. federal tax law other than income taxation (such as estate and gift taxation).
For purposes of this discussion, a “U.S. holder” is a beneficial owner of the Notes that is, for U.S. federal income tax purposes:
| • | an individual who is a citizen or resident of the United States; |
| • | a corporation (or other business entity treated as a corporation) created or organized in or under the laws of the United States or any state thereof or the District of Columbia; |
| • | an estate the income of which is subject to U.S. federal income taxation regardless of its source; or |
| • | a trust if a court within the United States can exercise primary supervision over its administration, and one or more United States persons have the authority to control all of the substantial decisions of that trust (or a trust that was in existence on August 20, 1996, and validly elected to continue to be treated as a U.S. trust). |
242
Table of Contents
A “non-U.S. holder” is an individual, corporation, estate, or trust that is a beneficial owner of the Notes that is not a U.S. holder.
The U.S. federal income tax treatment of a partner in a partnership (or other entity or arrangement classified as a partnership for U.S. federal income tax purposes) that holds the Notes generally will depend on such partner’s particular circumstances and on the activities of the partnership. Partners in such partnerships should consult their own tax advisors.
U.S. Federal Income Tax Consequences to U.S. Holders
Additional Interest
Our obligation to pay you additional interest in the event that we fail to comply with specified obligations under the registration rights agreements and the indenture governing the Notes may implicate the provisions of the Treasury Regulations relating to “contingent payment debt instruments.” We believe and are taking the position that the likelihood that we will make payments of additional interest is remote. Therefore, we are taking the position that the Notes should not be treated as contingent payment debt instruments and this discussion generally assumes that the Treasury Regulations relating to contingent payment debt instruments are not applicable. However, the determination of whether such a contingency is remote or not is inherently factual. Therefore, we can give you no assurance that our position would be sustained if challenged by the IRS. A successful challenge of this position by the IRS could affect the timing and amount of a U.S. holder’s income and could cause the gain from the sale or other disposition of a Note to be treated as ordinary income, rather than capital gain. Our position for purposes of the contingent payment debt regulations as to the likelihood of these additional payments being remote is binding on a U.S. holder, unless the U.S. holder discloses in the proper manner to the IRS that it is taking a different position. If, contrary to our expectations, we pay additional interest, such additional interest should be taxable to a U.S. holder as ordinary interest income at the time it is paid or accrues in accordance with the U.S. holder’s method of accounting for U.S. federal income tax purposes.
Treatment of Interest
Stated interest on the Notes will generally be taxable to U.S. holders as ordinary interest income as the interest accrues or is paid in accordance with the holder’s regular method of accounting for U.S. federal income tax purposes.
Original Issue Discount
The Series A-1 Notes will be treated as having been issued with original issue discount (“OID”) for U.S. federal income tax purposes in an amount equal to the difference between their “stated redemption price at maturity” (the sum of all payments to be made on the Series A-1 Notes other than payments of “qualified stated interest”) and their “issue price”. You should be aware that you generally must include OID in gross income in advance of the receipt of cash attributable to that income. The Series A-2 Notes were not issued with OID for U.S. federal income tax purposes.
We are taking the position that the “issue price” of the Series A-1 Notes is the first price at which a substantial amount of the Series A-1 Notes was sold for cash to the public (not including bond houses, brokers or similar persons or organizations acting in the capacity of underwriters, placement agents or wholesalers). There can be no assurance, however, that the IRS will not assert a contrary position, and U.S. holders should consult their own tax advisors in this regard.
The term “qualified stated interest” means stated interest that is unconditionally payable in cash or in property (other than debt instruments of the issuer) at least annually at a single fixed rate (or, subject to certain conditions, at a rate based on one or more interest indices). The stated interest payments on the Notes are qualified stated interest, and are treated as described above under “—Treatment of Interest.”
243
Table of Contents
The amount of OID that you must include in income is the sum of the “daily portions” of OID with respect to the Series A-1 Note for each day during the taxable year or portion of the taxable year in which you held such Series A-1 Note (“accrued OID”). The daily portion is determined by allocating to each day in any “accrual period” a pro rata portion of the OID allocable to that accrual period. The “accrual period” for a Series A-1 Note may be of any length and may vary in length over the term of the Series A-1 Note, provided that each accrual period is no longer than one year and each scheduled payment of principal or interest occurs on the first day or the final day of an accrual period. The amount of OID allocable to any accrual period other than the final accrual period is an amount equal to the excess, if any, of:
| • | the product of the Series A-1 Note’s adjusted issue price at the beginning of such accrual period (as described below) and its yield to maturity (determined on the basis of compounding at the close of each accrual period and properly adjusted for the length of the accrual period), over |
| • | the aggregate of all qualified stated interest allocable to the accrual period. |
OID allocable to a final accrual period is the difference between the amount payable at maturity (other than a payment of qualified stated interest) and the adjusted issue price at the beginning of the final accrual period. The “adjusted issue price” of a Series A-1 Note at the beginning of any accrual period is equal to its issue price increased by the accrued OID for each prior accrual period, determined without regard to the amortization of any acquisition premium or amortizable bond premium, as described below under “—Acquisition Premium, Amortizable Bond Premium.” Under these rules, you will have to include in income increasingly greater amounts of OID in successive accrual periods. We are required to provide information returns stating the amount of OID accrued on Series A-1 Notes held of record by persons other than corporations and other exempt holders.
You may elect to treat all interest on a Series A-1 Note as OID and calculate the amount includible in gross income under the constant yield method described above. The election is to be made for the taxable year in which you acquired the Series A-1 Note, and may not be revoked without the consent of the IRS. You should consult with your own tax advisors about this election.
Market Discount
If you purchase a Note for an amount that is less than its stated principal amount (in the case of the Series A-2 Notes) or adjusted issue price (in the case of the Series A-1 Notes), the amount of the difference will be treated as market discount for U.S. federal income tax purposes, unless that difference is less than a specified de minimis amount. Under the market discount rules, you will be required to treat any principal payment on, or any gain on the sale, exchange, retirement or other disposition of, a Note as ordinary income to the extent of the market discount that you have not previously included in income and are treated as having accrued on the Note at the time of the payment or disposition.
In addition, you may be required to defer, until the maturity of the Note or its earlier disposition in a taxable transaction, the deduction of all or a portion of the interest expense on any indebtedness attributable to the Note. You may elect, on a Note-by- Note basis, to deduct the deferred interest expense in a tax year prior to the year of disposition. You should consult your own tax advisors before making this election.
Any market discount will be considered to accrue ratably during the period from the date of acquisition to the maturity date of the Note, unless you elect to accrue on a constant interest method. You may elect to include market discount in income currently as it accrues, on either a ratable or constant interest method, in which case the rule described above regarding deferral of interest deductions will not apply.
Acquisition Premium, Amortizable Bond Premium
If you purchase a Series A-1 Note for an amount that is greater than its adjusted issue price but equal to or less than its stated principal amount, you will be considered to have purchased that Series A-1 Note at an
244
Table of Contents
“acquisition premium.” Under the acquisition premium rules, the amount of OID that you must include in gross income with respect to a Series A-1 Note for any taxable year will be reduced by the portion of the acquisition premium properly allocable to that year.
If you purchase a Note for an amount in excess of its stated principal amount, you will be considered to have purchased the Note at a premium and, in the case of the Series A-1 Notes, you will not be required to include any OID in income. You generally may elect to amortize the premium over the remaining term of the Note on a constant yield method as an offset to interest when includible in income under your regular accounting method. If you do not elect to amortize bond premium, that premium will decrease the gain or increase the loss you would otherwise recognize on disposition of the Note.
Sale, Exchange, or Other Disposition of the Notes
Your adjusted tax basis in a Note will, in general, be your cost for that Note, increased by any OID or market discount previously included in income, and reduced by any amortized premium. In general, upon the sale, exchange, redemption, retirement at maturity, or other taxable disposition of a Note, a U.S. holder will recognize taxable gain or loss equal to the difference between (1) the amount of the cash and the fair market value of any property received (less any portion allocable to any accrued and unpaid qualified stated interest, which will be taxable as interest to the extent not previously included in income) and (2) the U.S. holder’s adjusted tax basis in the Note (as described above). Except as described above with respect to market discount, gain or loss realized on the sale, exchange, redemption, retirement at maturity, or other taxable disposition of a Note will generally be capital gain or loss. Capital gains of non-corporate taxpayers derived in respect of capital assets held for more than one year are eligible for reduced rates of taxation. The deductibility of capital losses is subject to limitations.
Backup Withholding and Information Reporting
In general, a U.S. holder of the Notes will be subject to backup withholding with respect to interest on the Notes (including OID, if any), and the proceeds of a sale or disposition of the Notes (including a redemption or retirement), at the applicable tax rate (currently 28%), unless such holder (a) is an entity that is exempt from withholding and, when required, demonstrates this fact, or (b) timely provides the payor with its taxpayer identification number (“TIN”), certifies under penalty of perjury that the TIN provided to the payor is correct and that the holder has not been notified by the IRS that such holder is subject to backup withholding due to underreporting of interest or dividends, and otherwise complies with applicable requirements of the backup withholding rules. In addition, such payments to U.S. holders that are not exempt entities will generally be subject to information reporting requirements. A U.S. holder who does not timely provide the payor with its correct TIN may be subject to penalties imposed by the IRS. The amount of any backup withholding from a payment to a U.S. holder will be allowed as a credit against such holder’s U.S. federal income tax liability and may entitle such holder to a refund, provided that the required information is timely furnished to the IRS.
U.S. Federal Income Tax Consequences to Non-U.S. Holders
Additional Interest
The interest rate on the Notes is subject to increase if we fail to comply with specified obligations under the registration rights agreements and the indenture governing the Notes. We believe that the likelihood that we will make payments of additional interest is remote. However, if we do make such payments, it is possible that the payments might be subject to U.S. federal withholding tax at a rate of 30% or lower treaty rate, if applicable. Non-U.S. holders should consult their own tax advisors as to the tax considerations that relate to the potential additional interest payments.
245
Table of Contents
Treatment of Interest
Subject to the discussion of backup withholding below, under the “portfolio interest exemption,” a non-U.S. holder will generally not be subject to U.S. federal income tax (or any withholding tax) on payments of interest on the Notes (which for purposes of this discussion includes any OID), provided that:
| • | interest paid on the Notes is not effectively connected with a non-U.S. holder’s conduct of a trade or business in the United States; |
| • | the non-U.S. holder does not actually or constructively own 10% or more of the total combined voting power of all classes of our stock entitled to vote; |
| • | the non-U.S. holder is not, and is not treated as, a bank receiving interest on an extension of credit pursuant to a loan agreement entered into in the ordinary course of its trade or business; |
| • | the non-U.S. holder is not a “controlled foreign corporation” that is related (directly or indirectly) to us; and |
| • | certain certification requirements are met. |
Under current law, the certification requirements will be satisfied in any of the following circumstances:
| • | if a non-U.S. holder provides to us or our paying agent a statement on IRS Form W-8BEN (or suitable successor or substitute form), together with all appropriate attachments, signed under penalty of perjury, identifying the non-U.S. holder by name and address and stating, among other things, that the non-U.S. holder is not a United States person; |
| • | if (i) a Note is held through a securities clearing organization, bank or another financial institution that holds customers’ securities in the ordinary course of its trade or business, (ii) the non-U.S. holder provides the form described above to such organization or institution, and (iii) such organization or institution, under penalty of perjury, certifies to us that it has received such statement from the beneficial owner or another intermediary and furnishes us or our paying agent with a copy thereof; or |
| • | if a financial institution or other intermediary that holds the Note on behalf of the non-U.S. holder has entered into a withholding agreement with the IRS and submits an IRS Form W-8IMY (or suitable successor form) and certain other required documentation to us or our paying agent. |
If the requirements of the portfolio interest exemption described above are not satisfied, a 30% withholding tax will apply to the gross amount of interest on the Notes (including OID) that is paid to a non-U.S. holder, unless either: (a) an applicable income tax treaty reduces or eliminates such tax, and the non-U.S. holder claims the benefit of that treaty by providing a properly completed and duly executed IRS Form W-8BEN (or suitable successor or substitute form) establishing qualification for benefits under the treaty, or (b) the interest is effectively connected with the non-U.S. holder’s conduct of a trade or business in the United States and the non-U.S. holder provides an appropriate statement to that effect on a properly completed and duly executed IRS Form W-8ECI (or suitable successor form).
If a non-U.S. holder is engaged in a trade or business in the United States and interest (including OID) on a Note is effectively connected with the conduct of that trade or business, the non-U.S. holder will be required to pay U.S. federal income tax on that interest on a net income basis (and the 30% withholding tax described above will not apply provided the duly executed IRS Form W-8ECI is provided to us or our paying agent) generally in the same manner as a U.S. person unless an applicable income tax treaty provides otherwise. If a non-U.S. holder is eligible for the benefits of an income tax treaty between the United States and its country of residence, and the non-U.S. holder claims the benefit of the treaty by properly submitting an IRS Form W-8BEN, any interest income that is effectively connected with a U.S. trade or business will be subject to U.S. federal income tax in the manner specified by the treaty and generally will only be subject to such tax if such income is attributable to a permanent establishment (or a fixed base in the case of an individual) maintained by the non-U.S. holder in the
246
Table of Contents
United States. In addition, a non-U.S. holder that is treated as a foreign corporation for U.S. federal income tax purposes may be subject to an additional branch profits tax equal to 30% (or lower applicable treaty rate) of its earnings and profits for the taxable year that are effectively connected with its conduct of a trade or business in the United States, subject to adjustments.
Sale, Exchange, or Other Disposition of the Notes
Subject to the discussion of backup withholding below, a non-U.S. holder generally will not be subject to U.S. federal income tax (or any withholding thereof) on any gain realized by such holder upon a sale, exchange, redemption, retirement at maturity, or other taxable disposition of the Notes, unless:
| • | the non-U.S. holder is an individual present in the United States for 183 days or more during the taxable year of disposition and who has a “tax home” in the United States and certain other conditions are met; |
| • | the gain is effectively connected with the conduct of a U.S. trade or business of the non-U.S. holder (and, if an applicable income tax treaty so provides, the gain is attributable to a U.S. permanent establishment of the non-U.S. holder or a fixed base in the case of an individual); or |
| • | the disposition proceeds represent accrued and unpaid interest and the non-U.S. holder cannot satisfy the requirements of the “portfolio interest exemption” described above. |
If the first exception applies, the non-U.S. holder generally will be subject to U.S. federal income tax at a rate of 30% on the amount by which its U.S.-source capital gains exceed its U.S.-source capital losses. If the second exception applies, the non-U.S. holder will generally be subject to U.S. federal income tax on the net gain derived from the sale, exchange, redemption, retirement at maturity or other taxable disposition of the Notes in the same manner as a U.S. holder. In addition, corporate non-U.S. holders may be subject to an additional 30% branch profits tax (or lower applicable treaty rate) on any such effectively connected gain. If the third exception applies, the non-U.S. holder generally will be subject to U.S. federal income tax at a rate of 30% on the amount of accrued and unpaid interest. If a non-U.S. holder is eligible for the benefits of an income tax treaty between the United States and its country of residence, the U.S. federal income tax treatment of any such gain may be modified in the manner specified by the treaty.
Information Reporting and Backup Withholding
When required, we or our paying agent will report to the IRS and to each non-U.S. holder the amount of any interest paid on the Notes (including any OID) in each calendar year, and the amount of U.S. federal income tax withheld, if any, with respect to these payments. Copies of the information returns reporting such interest payments and any withholding may also be made available to the tax authorities in the country in which the non-U.S. holder resides under the provisions of a treaty or agreement.
Non-U.S. holders who have provided certification as to their non-U.S. status or who have otherwise established an exemption will generally not be subject to backup withholding tax on payments of principal or interest (including any OID) if neither we nor our agent have actual knowledge or reason to know that such certification is unreliable or that the conditions of the exemption are in fact not satisfied.
Payments of the proceeds from the sale or other disposition of a Note (including a redemption or retirement) outside the U.S. to or through a foreign office of a broker generally will not be subject to information reporting or backup withholding. However, additional information reporting, but generally not backup withholding, may apply to those payments if the broker is one of the following: (a) a United States person, (b) a “controlled foreign corporation” for U.S. federal income tax purposes, (c) a foreign person 50 percent or more of whose gross income from all sources for the three-year period ending with the close of its taxable year preceding the payment was effectively connected with a U.S. trade or business, or (d) a foreign partnership with specified connections to the United States.
247
Table of Contents
Payment of the proceeds from a sale or other disposition of a Note (including a redemption or retirement) to or through the U.S. office of a broker will be subject to information reporting and backup withholding unless the non-U.S. holder certifies as to its non-U.S. status or otherwise establishes an exemption from information reporting and backup withholding.
The amount of any backup withholding from a payment to a non-U.S. holder will be allowed as a credit against such holder’s U.S. federal income tax liability, if any, and may entitle such holder to a refund, provided that the required information is timely furnished to the IRS.
248
Table of Contents
The following is a summary of certain considerations associated with the acquisition of the Notes by employee benefit plans that are subject to the Employee Retirement Income Security Act of 1974, as amended (“ERISA”), plans, individual retirement accounts and other arrangements that are subject to Section 4975 of the Internal Revenue Code of 1986, as amended (the “Code”) or provisions under any other federal, state, local, non-U.S. or other laws, or rules or regulations that are similar to such provisions of ERISA or the Code (collectively, “Similar Laws”), and entities whose underlying assets are considered to include “plan assets” of any such employee benefit plan, plan, account or arrangement (each, a “Plan”).
General Fiduciary Matters
ERISA and the Code impose certain duties on persons who are fiduciaries of a Plan subject to Title I of ERISA or Section 4975 of the Code (an “ERISA Plan”) and prohibit certain transactions involving the assets of an ERISA Plan and its fiduciaries or other interested parties.
In considering an investment in the Notes with a portion of the assets of any Plan, a fiduciary should determine whether the investment is in accordance with the documents and instruments governing the Plan and the applicable provisions of ERISA, the Code or any Similar Law relating to a fiduciary’s duties to the Plan including, without limitation, the prudence, diversification, delegation of control and prohibited transaction provisions of ERISA, the Code and any other applicable Similar Laws. Under ERISA and the Code, any person who exercises any discretionary authority or control over the administration of an ERISA Plan or the management or disposition of the assets of an ERISA Plan, or who renders investment advice for a fee or other compensation to an ERISA Plan, is generally considered to be a fiduciary of the ERISA Plan.
Prohibited Transaction Issues
Section 406 of ERISA and Section 4975 of the Code prohibit ERISA Plans from engaging in specified transactions involving plan assets with persons or entities who are “parties in interest,” within the meaning of ERISA, or “disqualified persons,” within the meaning of Section 4975 of the Code, unless an exemption is available. A party in interest or disqualified person who engaged in a non-exempt prohibited transaction may be subject to excise taxes and other penalties and liabilities under Title I of ERISA and/or Section 4975 of the Code. In addition, the fiduciary of the ERISA Plan that engaged in such a non-exempt prohibited transaction may be subject to penalties and liabilities under ERISA and/or the Code. The acquisition and/or holding of Notes by an ERISA Plan with respect to which we or any of the guarantors are considered a party in interest or disqualified person may constitute or result in a direct or indirect prohibited transaction under Section 406 of ERISA and/or Section 4975 of the Code, unless the investment is acquired and held in accordance with an applicable statutory, class or individual prohibited transaction exemption. Included among the exemptions that may apply to the acquisition and holding of the Notes are U.S. Department of Labor prohibited transaction class exemption (“PTCE”) 84-14, respecting transactions determined by independent qualified professional asset managers, PTCE 90-1, respecting insurance company pooled separate accounts, PTCE 91-38, respecting bank collective investment funds, PTCE 95-60, respecting life insurance company general accounts and PTCE 96-23, respecting transactions determined by in-house asset managers. In addition, Section 408(b)(17) of ERISA and Section 4975(d)(20) of the Code provide limited relief from the prohibited transaction provisions of ERISA and Section 4975 of the Code for certain transactions, provided that neither the issuer of the securities nor any of its affiliates (directly or indirectly) have or exercise any discretionary authority or control or render any investment advice with respect to the assets of any ERISA Plan involved in the transaction and provided further that the ERISA Plan pays no more than adequate consideration in connection with the transaction. There can be no assurance that all of the conditions of any such exemptions will be satisfied.
Because of the foregoing, the Notes should not be acquired or held by any person investing “plan assets” of any Plan, unless such acquisition and holding (and the exchange of outstanding notes for exchange notes) will not constitute a non-exempt prohibited transaction under ERISA or Section 4975 of the Code or a similar violation under any applicable Similar Laws.
249
Table of Contents
Representation
By acceptance of a Note (including an exchange note), each holder and subsequent transferee will be deemed to have represented and warranted that either (i) no portion of the assets used by such holder or transferee to acquire or hold the Notes or any interest therein constitutes assets of any Plan or (ii) the acquisition and holding of the Notes (and the exchange of outstanding notes for exchange notes) or any interest therein by such holder or transferee will not constitute a non-exempt prohibited transaction under Section 406 of ERISA or Section 4975 of the Code or a similar violation under any applicable Similar Laws.
The foregoing discussion is general in nature and is not intended to be all-inclusive. Due to the complexity of these rules and the penalties that may be imposed upon persons involved in non-exempt prohibited transactions, it is particularly important that fiduciaries or other persons considering acquiring or holding the Notes on behalf of, or with the assets of, any Plan, consult with their counsel regarding the potential applicability of ERISA, Section 4975 of the Code and any Similar Laws to such transactions and whether an exemption would be applicable to the acquisition and holding of the Notes.
250
Table of Contents
Each broker-dealer that receives exchange notes for its own account pursuant to the exchange offers must acknowledge that it will deliver a prospectus in connection with any resale of the exchange notes. This prospectus, as it may be amended or supplemented from time to time, may be used by a broker-dealer in connection with resales of exchange notes received in exchange for outstanding notes where the outstanding notes were acquired as a result of market-making activities or other trading activities. To the extent any such broker-dealer participates in the exchange offers, we have agreed that for a period of up to 90 days, we will use our reasonable best efforts to make this prospectus, as amended or supplemented, available to such broker-dealer for use in connection with any such resale, and will deliver as many additional copies of this prospectus and each amendment or supplement to this prospectus and any documents incorporated by reference in this prospectus as such broker-dealer may reasonably request.
We will not receive any proceeds from any sale of exchange notes by broker-dealers. Exchange notes received by broker-dealers for their own accounts pursuant to the exchange offers may be sold from time to time in one or more transactions in the over-the-counter market, in negotiated transactions, through the writing of options on the exchange notes or a combination of these methods of resale, at market prices prevailing at the time of resale, at prices related to the prevailing market prices or negotiated prices. Any resale may be made directly to purchasers or to or through brokers or dealers who may receive compensation in the form of commissions or concessions from any broker-dealer or the purchasers of any exchange notes. Any broker-dealer that resells exchange notes that were received by it for its own account pursuant to the exchange offers and any broker or dealer that participates in a distribution of the exchange notes may be deemed to be an “underwriter” within the meaning of the Securities Act and any profit on any resale of exchange notes and any commissions or concessions received by these persons may be deemed to be underwriting compensation under the Securities Act. The letter of transmittal states that by acknowledging that it will deliver and by delivering a prospectus, a broker-dealer will not be deemed to admit that it is an “underwriter” within the meaning of the Securities Act.
We have agreed to pay all expenses incident to the exchange offers and will indemnify the holders of outstanding notes, including any broker-dealers, against certain liabilities, including liabilities under the Securities Act.
251
Table of Contents
The validity and enforceability of the exchange notes and certain exchange guarantees will be passed upon for us by Simpson Thacher & Bartlett LLP, New York, New York. An investment vehicle comprised of several partners of Simpson Thacher & Bartlett LLP, members of their families, related persons and others own interests representing less than 1% of the capital commitments of funds affiliated with Blackstone. Certain matters under Florida law will be passed upon by Holland & Knight LLP.
The consolidated financial statements of Apria Healthcare Group, Inc. and subsidiaries as of December 31, 2009 (Successor) and December 31, 2008 (Successor) and the year ended December 31, 2009 and for the period from October 29, 2008 through December 31, 2008 for the Successor and for the period from January 1, 2008 through October 28, 2008 (Predecessor) and for the year ended December 31, 2007 for the Predecessor included in this prospectus and the related financial statement schedule included elsewhere in the prospectus, have been audited by Deloitte & Touche LLP, an independent registered public accounting firm, as stated in their report appearing herein and elsewhere in the prospectus (which report expresses an unqualified opinion on the financial statements and financial statement schedule and includes an explanatory paragraph referring to the merger of the Company with and into Sky Merger Sub Corporation, a Delaware corporation, a wholly-owned subsidiary of Sky Acquisition LLC, a Delaware limited liability company on October 28, 2008). Such financial statements and financial statement schedule have been so included in reliance upon the report of Deloitte & Touche LLP given upon their authority as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We and our guarantors have filed with the SEC a registration statement on Form S-4 under the Securities Act with respect to the exchange notes. This prospectus, which forms a part of the registration statement, does not contain all of the information set forth in the registration statement. For further information with respect to us, our guarantors and the exchange notes, reference is made to the registration statement. Statements contained in this prospectus as to the contents of any contract or other document are not necessarily complete, and, where such contract or other document is an exhibit to the registration statement, each such statement is qualified by the provisions in such exhibit, to which reference is hereby made. The registration statement and other information can be inspected and copied at the Public Reference Room of the SEC located at Room 1580, 100 F Street, N.E., Washington D.C. 20549. Copies of such materials, including copies of all or any portion of the registration statement, can be obtained from the Public Reference Room of the SEC at prescribed rates. You can call the SEC at 1-800-SEC-0330 to obtain information on the operation of the Public Reference Room. Such materials may also be accessed electronically by means of the SEC’s home page on the Internet (http://www.sec.gov). However, any such information filed with the SEC does not constitute a part of this prospectus.
So long as we are subject to the periodic reporting requirements of the Exchange Act, we are required to furnish the information required to be filed with the SEC to the trustee and the holders of the outstanding unregistered notes. We have agreed that, even if we are not required under the Exchange Act to furnish such information to the SEC, we will nonetheless continue to furnish information that would be required to be furnished by us by Section 13 or 15(d) of the Exchange Act.
252
Table of Contents
| Page | ||
Audited Financial Statements of Apria Healthcare Group Inc. | ||
| F-2 | ||
| F-3 | ||
| F-4 | ||
| F-5 | ||
| F-6 | ||
| F-8 | ||
| F-51 | ||
Unaudited Financial Statements of Apria Healthcare Group Inc. | ||
Condensed Consolidated Balance Sheets—March 31, 2010 and December 31, 2009 | F-52 | |
Condensed Consolidated Statement of Operations—Three months ended March 31, 2010 and 2009 | F-53 | |
Condensed Consolidated Statements of Cash Flows—Three months ended March 31, 2010 and 2009 | F-54 | |
Notes to Unaudited Condensed Consolidated Financial Statements | F-55 |
F-1
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
Apria Healthcare Group Inc., a wholly-owned subsidiary of Sky Acquisition LLC
Lake Forest, California
We have audited the accompanying consolidated balance sheets of Apria Healthcare Group Inc. and subsidiaries (the “Company”) as of December 31, 2009 (“Successor”) and December 31, 2008 (“Successor”), and the related consolidated statements of operations, stockholders’ equity, and cash flows for the year ended December 31, 2009 and for the period from October 29, 2008 through December 31, 2008 for the Successor. We have also audited the related consolidated statements of operations, stockholders’ equity, and cash flows for the period from January 1, 2008 to October 28, 2008 (“Predecessor”) and the year ended December 31, 2007 for the Predecessor. Our audits also included the financial statement schedule listed in the Index at Page F-1. These financial statements and financial statement schedule are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements and financial statement schedule based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
As discussed in Note 2 to the consolidated financial statements, on October 28, 2008, Sky Merger Sub Corporation, a Delaware corporation, a wholly-owned subsidiary of Sky Acquisition LLC, a Delaware limited liability company, merged with and into the Company with the Company continuing as the surviving corporation and wholly-owned subsidiary of Sky Acquisition LLC.
In our opinion, such consolidated financial statements present fairly, in all material respects, the financial position of Apria Healthcare Group Inc. and subsidiaries as of December 31, 2009 (“Successor”) and December 31, 2008 (“Successor”), and the results of their operations and cash flows for the year ended December 31, 2009 and for the period from October 29, 2008 through December 31, 2008 for the Successor, and the results of their operations and cash flows for the period from January 1, 2008 to October 28, 2008 (“Predecessor”) and the year ended December 31, 2007 for the Predecessor, in conformity with accounting principles generally accepted in the United States of America. Also, in our opinion, such financial statement schedule, when considered in relation to the basic consolidated financial statements taken as a whole, presents fairly, in all material respects, the information set forth therein.
DELOITTE & TOUCHE LLP
Costa Mesa, California
March 2, 2010,
(July 16, 2010 as to Note 15 and Note 17)
F-2
Table of Contents
APRIA HEALTHCARE GROUP INC.
| December 31, | ||||||||
| 2009 | 2008 | |||||||
| (Successor) | (Successor) | |||||||
| (in thousands, except share data) | ||||||||
ASSETS | ||||||||
CURRENT ASSETS | ||||||||
Cash and cash equivalents | $ | 158,163 | $ | 168,018 | ||||
Short-term investments | 23,673 | — | ||||||
Accounts receivable, less allowance for doubtful accounts of $39,927 and $25,271 at December 31, 2009 and 2008, respectively | 252,133 | 273,205 | ||||||
Inventories | 68,267 | 59,219 | ||||||
Deferred income taxes | 87,016 | 58,444 | ||||||
Deferred expenses | 3,049 | 3,181 | ||||||
Prepaid expenses and other current assets | 24,362 | 18,131 | ||||||
TOTAL CURRENT ASSETS | 616,663 | 580,198 | ||||||
PATIENT SERVICE EQUIPMENT, less accumulated depreciation of $97,874 and $17,410 at December 31, 2009 and 2008, respectively | 198,808 | 209,740 | ||||||
PROPERTY, EQUIPMENT AND IMPROVEMENTS, NET | 80,227 | 67,755 | ||||||
GOODWILL | 759,197 | 895,069 | ||||||
INTANGIBLE ASSETS, NET | 582,259 | 397,334 | ||||||
DEFERRED DEBT ISSUANCE COSTS, NET | 63,110 | 49,648 | ||||||
OTHER ASSETS | 8,783 | 11,069 | ||||||
| $ | 2,309,047 | $ | 2,210,813 | |||||
LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
CURRENT LIABILITIES | ||||||||
Accounts payable | $ | 123,856 | $ | 141,382 | ||||
Accrued payroll and related taxes and benefits | 66,842 | 74,238 | ||||||
Other accrued liabilities | 100,768 | 100,989 | ||||||
Deferred revenue | 27,253 | 30,515 | ||||||
Current portion of long-term debt | 1,690 | 8,750 | ||||||
TOTAL CURRENT LIABILITIES | 320,409 | 355,874 | ||||||
LONG-TERM DEBT, net of current portion | 1,019,456 | 1,013,483 | ||||||
DEFERRED INCOME TAXES | 259,030 | 127,707 | ||||||
INCOME TAXES PAYABLE AND OTHER NON-CURRENT LIABILITIES | 31,421 | 40,929 | ||||||
TOTAL LIABILITIES | 1,630,316 | 1,537,993 | ||||||
COMMITMENTS AND CONTINGENCIES (Note 13) | ||||||||
STOCKHOLDERS’ EQUITY | ||||||||
Common stock, $0.01 par value: 1,000 shares authorized; 100 shares issued | — | — | ||||||
Additional paid-in capital | 684,445 | 674,714 | ||||||
Accumulated deficit | (5,714 | ) | (1,894 | ) | ||||
TOTAL STOCKHOLDERS’ EQUITY | 678,731 | 672,820 | ||||||
| $ | 2,309,047 | $ | 2,210,813 | |||||
See notes to consolidated financial statements.
F-3
Table of Contents
APRIA HEALTHCARE GROUP INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
| Year Ended December 31, 2009 | Period October 29, 2008 to December 31, 2008 | Period January 1, 2008 to October 28, 2008 | Year Ended December 31, 2007 | |||||||||||||||
| (Successor) | (Successor) | (Predecessor) | (Predecessor) | |||||||||||||||
| (in thousands) | ||||||||||||||||||
Net revenues: | ||||||||||||||||||
Fee for service arrangements | $ | 1,930,464 | $ | 328,005 | $ | 1,630,767 | $ | 1,465,303 | ||||||||||
Capitation | 164,097 | 28,660 | 142,522 | 166,498 | ||||||||||||||
TOTAL NET REVENUES | 2,094,561 | 356,665 | 1,773,289 | 1,631,801 | ||||||||||||||
Costs and expenses: | ||||||||||||||||||
Cost of net revenues: | ||||||||||||||||||
Product and supply costs | 638,452 | 105,120 | 526,610 | 386,496 | ||||||||||||||
Patient service equipment depreciation | 101,681 | 17,539 | 89,246 | 110,775 | ||||||||||||||
Amortization of intangible assets | 44,000 | — | — | — | ||||||||||||||
Home respiratory therapy services | 34,700 | 6,270 | 31,893 | 38,886 | ||||||||||||||
Nursing services | 36,345 | 6,276 | 29,773 | 11,353 | ||||||||||||||
Other | 12,281 | 2,555 | 13,815 | 17,482 | ||||||||||||||
TOTAL COST OF NET REVENUES | 867,459 | 137,760 | 691,337 | 564,992 | ||||||||||||||
Provision for doubtful accounts | 57,919 | 14,329 | 33,626 | 43,138 | ||||||||||||||
Selling, distribution and administrative | 1,050,134 | 179,362 | 924,536 | 862,062 | ||||||||||||||
Amortization of intangible assets | 3,716 | 1,008 | 3,461 | 3,079 | ||||||||||||||
TOTAL COSTS AND EXPENSES | 1,979,228 | 332,459 | 1,652,960 | 1,473,271 | ||||||||||||||
OPERATING INCOME | 115,333 | 24,206 | 120,329 | 158,530 | ||||||||||||||
Interest expense | 129,200 | 26,167 | 31,838 | 22,447 | ||||||||||||||
Interest income and other | (1,609 | ) | (726 | ) | (2,154 | ) | (1,954 | ) | ||||||||||
(LOSS) INCOME BEFORE TAXES | (12,258 | ) | (1,235 | ) | 90,645 | 138,037 | ||||||||||||
Income tax (benefit) expense | (8,438 | ) | 659 | 34,192 | 51,998 | |||||||||||||
NET (LOSS) INCOME | $ | (3,820 | ) | $ | (1,894 | ) | $ | 56,453 | $ | 86,039 | ||||||||
See notes to consolidated financial statements.
F-4
Table of Contents
APRIA HEALTHCARE GROUP INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
| Common Stock | Additional Paid-In Capital | Treasury Stock | Retained Earnings (Accumulated Deficit) | Accumulated Other Comprehensive (Loss) Income | Total Stockholders Equity | ||||||||||||||||||||||
| Shares | Par Value | Shares | Cost | ||||||||||||||||||||||||
| (in thousands) | |||||||||||||||||||||||||||
(Predecessor) | |||||||||||||||||||||||||||
Balance at January 1, 2007 | 59,762 | $ | 60 | $ | 482,123 | 16,973 | $ | (429,573 | ) | $ | 346,732 | $ | 351 | $ | 399,693 | ||||||||||||
Cumulative effect adjustment for uncertain tax positions | (4,233 | ) | (4,233 | ) | |||||||||||||||||||||||
Exercise of stock options | 887 | 1 | 17,617 | 17,618 | |||||||||||||||||||||||
Tax benefits related to stock options | 4,057 | 4,057 | |||||||||||||||||||||||||
Tax shortfalls on share-based compensation | (99 | ) | (99 | ) | |||||||||||||||||||||||
Compensatory stock options and awards | 196 | 11,150 | 11,150 | ||||||||||||||||||||||||
Restricted stock retained in treasury upon vesting | 77 | (2,078 | ) | (2,078 | ) | ||||||||||||||||||||||
Unrealized loss on interest rate swap agreements, net of taxes | (122 | ) | (122 | ) | |||||||||||||||||||||||
Net income | 86,039 | 86,039 | |||||||||||||||||||||||||
Total comprehensive income | 86,039 | (122 | ) | 85,917 | |||||||||||||||||||||||
Balance at December 31, 2007 | 60,845 | 61 | 514,848 | 17,050 | (431,651 | ) | 428,538 | 229 | 512,025 | ||||||||||||||||||
Exercise of stock options | 73 | 509 | 509 | ||||||||||||||||||||||||
Tax expense related to stock options | (7,719 | ) | (7,719 | ) | |||||||||||||||||||||||
Tax benefits on share-based compensation | 497 | 497 | |||||||||||||||||||||||||
Compensatory stock options and awards | 144 | 10,626 | 10,626 | ||||||||||||||||||||||||
Restricted stock retained in treasury upon vesting | 62 | (1,360 | ) | (1,360 | ) | ||||||||||||||||||||||
Unrealized loss on interest rate swap agreements, net of taxes | (229 | ) | (229 | ) | |||||||||||||||||||||||
Net income | 56,453 | 56,453 | |||||||||||||||||||||||||
Total comprehensive income | 56,453 | (229 | ) | 56,224 | |||||||||||||||||||||||
Balance at October 28, 2008 | 61,062 | 61 | 518,761 | 17,112 | (433,011 | ) | 484,991 | — | 570,802 | ||||||||||||||||||
(Successor) | |||||||||||||||||||||||||||
Issuance of common stock | 673,334 | 673,334 | |||||||||||||||||||||||||
Profit interest | 1,380 | 1,380 | |||||||||||||||||||||||||
Net loss | (1,894 | ) | (1,894 | ) | |||||||||||||||||||||||
Balance at December 31, 2008 | — | $ | — | $ | 674,714 | — | $ | — | $ | (1,894 | ) | $ | — | $ | 672,820 | ||||||||||||
Equity contributions | 2,075 | 2,075 | |||||||||||||||||||||||||
Profit interest | 7,656 | 7,656 | |||||||||||||||||||||||||
Net loss | (3,820 | ) | (3,820 | ) | |||||||||||||||||||||||
Balance at December 31, 2009 | — | $ | — | $ | 684,445 | — | $ | — | $ | (5,714 | ) | $ | — | $ | 678,731 | ||||||||||||
See notes to consolidated financial statements.
F-5
Table of Contents
APRIA HEALTHCARE GROUP INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
| Year Ended December 31, 2009 | Period October 29, 2008 to December 31, 2008 | Period January 1, 2008 to October 28, 2008 | Year Ended December 31, 2007 | |||||||||||||||
| (Successor) | (Successor) | (Predecessor) | (Predecessor) | |||||||||||||||
| (in thousands) | ||||||||||||||||||
OPERATING ACTIVITIES | ||||||||||||||||||
Net (loss) income | $ | (3,820 | ) | $ | (1,894 | ) | $ | 56,453 | $ | 86,039 | ||||||||
Items included in net income not requiring cash: | ||||||||||||||||||
Provision for doubtful accounts | 57,919 | 14,329 | 33,626 | 43,138 | ||||||||||||||
Depreciation | 125,327 | 22,049 | 112,759 | 131,645 | ||||||||||||||
Amortization of intangible assets | 47,716 | 1,008 | 3,461 | 3,079 | ||||||||||||||
Amortization of deferred debt issuance costs | 8,023 | 4,237 | 9,064 | 1,778 | ||||||||||||||
Deferred income taxes | (3,224 | ) | 8,016 | 54,162 | 4,605 | |||||||||||||
Expense on profit interest units | 7,656 | 1,380 | — | — | ||||||||||||||
Share-based compensation | — | — | 34,162 | 11,150 | ||||||||||||||
Excess tax benefits from shared-based compensation | — | — | (497 | ) | (4,057 | ) | ||||||||||||
Loss (gain) on disposition of assets and other | 15,899 | 2,849 | 12,324 | (536 | ) | |||||||||||||
Other | (4,944 | ) | — | — | — | |||||||||||||
Changes in operating assets and liabilities, exclusive of effects of acquisitions: | ||||||||||||||||||
Accounts receivable | (39,599 | ) | (6,995 | ) | (8,926 | ) | (33,724 | ) | ||||||||||
Inventories | (9,048 | ) | (10,233 | ) | (1,164 | ) | 2,589 | |||||||||||
Prepaid expenses and other assets | (7,198 | ) | 1,169 | 4,513 | (48 | ) | ||||||||||||
Accounts payable, exclusive of book cash overdraft | (14,077 | ) | 14,103 | 1,133 | 7,043 | |||||||||||||
Accrued payroll and related taxes and benefits | (8,596 | ) | (120 | ) | (13,526 | ) | 6,873 | |||||||||||
Income taxes payable | (2,263 | ) | (6,235 | ) | (3,506 | ) | 20,380 | |||||||||||
Deferred revenue, net of deferred expenses | (3,130 | ) | (1,103 | ) | 1,834 | 289 | ||||||||||||
Accrued expenses | 2,785 | 20,777 | 2,065 | 13,763 | ||||||||||||||
NET CASH PROVIDED BY OPERATING ACTIVITIES | 169,426 | 63,337 | 297,937 | 294,006 | ||||||||||||||
INVESTING ACTIVITIES | ||||||||||||||||||
Purchases of patient service equipment and property, equipment and improvements, exclusive of effects of acquisitions | (150,597 | ) | (26,217 | ) | (157,183 | ) | (128,759 | ) | ||||||||||
Purchases of short-term investments | (37,554 | ) | — | — | — | |||||||||||||
Maturities of short-term investments | 13,881 | — | — | — | ||||||||||||||
Merger related costs | — | (49,269 | ) | — | — | |||||||||||||
Proceeds from disposition of assets | 6,893 | 20 | 5,295 | 102 | ||||||||||||||
Cash paid for acquisitions | (1,279 | ) | — | (2,605 | ) | (354,578 | ) | |||||||||||
NET CASH USED IN INVESTING ACTIVITIES | (168,656 | ) | (75,466 | ) | (154,493 | ) | (483,235 | ) | ||||||||||
FINANCING ACTIVITIES | ||||||||||||||||||
Proceeds from Predecessor Revolving Credit Facility | — | — | 18,300 | 359,000 | ||||||||||||||
Payments on Predecessor Revolving Credit Facility | — | (304,000 | ) | (138,300 | ) | (170,000 | ) | |||||||||||
Proceeds from Senior Secured Bridge Credit Agreement | — | 1,010,000 | 250,000 | — | ||||||||||||||
Payments on Senior Secured Bridge Credit Agreement | (1,010,000 | ) | — | — | — | |||||||||||||
Payments on Interim Facility | — | (249,772 | ) | (228 | ) | — | ||||||||||||
Redemption of convertible senior notes | — | (151 | ) | (249,772 | ) | — | ||||||||||||
Proceeds from ABL Facility | 630 | 36,000 | — | — | ||||||||||||||
Payments on ABL Facility | (6,630 | ) | (30,000 | ) | — | — | ||||||||||||
Payments on other long-term debt | (2,856 | ) | (5,245 | ) | (3,159 | ) | (3,264 | ) | ||||||||||
Proceeds from issuance of Series A-1 Notes | 700,000 | — | — | — | ||||||||||||||
Proceeds from issuance of Series A-2 Notes | 317,500 | — | — | — | ||||||||||||||
Change in book cash overdraft included in accounts payable | 7,902 | (850 | ) | 7,771 | (4,291 | ) | ||||||||||||
Debt issuance costs related to the Interim Facility | — | (45,661 | ) | (9,204 | ) | — | ||||||||||||
Debt issuance costs related to Series A-1 and Series A-2 Notes | (19,039 | ) | — | — | — | |||||||||||||
Debt issuance costs related to the ABL Facility | (207 | ) | (5,250 | ) | — | — | ||||||||||||
Equity contribution | 2,075 | 673,334 | — | — | ||||||||||||||
Repurchases of common stock | — | (922,935 | ) | — | — | |||||||||||||
Change in control payment for options and awards | — | (23,536 | ) | — | — | |||||||||||||
Excess tax benefits from share-based compensation | — | — | 497 | 4,057 | ||||||||||||||
Issuances of common stock | — | — | 413 | 17,521 | ||||||||||||||
NET CASH (USED IN) PROVIDED BY FINANCING ACTIVITIES | (10,625 | ) | 131,934 | (123,682 | ) | 203,023 | ||||||||||||
NET (DECREASE) INCREASE IN CASH AND CASH EQUIVALENTS | (9,855 | ) | 119,805 | 19,762 | 13,794 | |||||||||||||
Cash and cash equivalents at beginning of period | 168,018 | 48,213 | 28,451 | 14,657 | ||||||||||||||
CASH AND CASH EQUIVALENTS AT END OF PERIOD | $ | 158,163 | $ | 168,018 | $ | 48,213 | $ | 28,451 | ||||||||||
F-6
Table of Contents
APRIA HEALTHCARE GROUP INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS—(Continued)
SUPPLEMENTAL DISCLOSURES—See Note 8—Long-term Debt and Note 10—Income Taxes for cash paid for interest and income taxes, respectively.
NON-CASH TRANSACTIONS—See Statements of Stockholders’ Equity, Note 6—Business Combinations and Note 11—Leases for tax benefit from stock option exercises, non-cash treasury stock transactions, liabilities assumed in acquisitions and purchase of property and equipment under capital leases, respectively.
Purchases of patient service equipment and property, equipment and improvements exclude purchases that remain unpaid at the end of the respective year. Such amounts are then included in the following year’s purchases. Unpaid purchases were $11,011, $17,292 and $10,994 at December 31, 2009, 2008 and 2007, respectively.
See notes to consolidated financial statements.
F-7
Table of Contents
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1—Summary of Significant Accounting Policies
Basis of Presentation: The accompanying consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America. These statements include the accounts of Apria Healthcare Group Inc. (“Apria” or “the Company”) and its subsidiaries. Intercompany transactions and accounts have been eliminated in consolidation.
On October 28, 2008, the Company completed its merger (the “Merger”) with Sky Merger Sub Corporation (“Merger Sub”), a Delaware corporation and wholly-owned subsidiary of Sky Acquisition LLC, a Delaware limited liability company (“Sky LLC”). Sky LLC is controlled by private investment funds affiliated with The Blackstone Group (“Blackstone” or “Sponsor”). Pursuant to the terms of the Merger Agreement, holders of our common stock received $21.00 in cash for each share of common stock that they owned immediately prior to the effective time of the Merger. As a result of the Merger, our common stock was delisted from the New York Stock Exchange and we ceased to be a publicly held corporation. (See Note 2—The Merger.)
The historical financial results for the periods prior to the completion of the Merger on October 28, 2008 relate to our accounting predecessor (the “Predecessor”). The period subsequent to the completion of the Merger relates to our accounting successor (“the Successor”). The terms “us,” “we,” “our,” “Apria,” and the “Company” as used in these consolidated financial statements refer to the Successor and its subsidiaries subsequent to the Merger on October 28, 2008 and the Predecessor and its subsidiaries prior to that date. The consolidated financial statements for all predecessor periods have been prepared using the historical basis of accounting for the Company. As a result of the Merger and the associated purchase accounting, our consolidated financial statements for periods subsequent to the Merger are not comparable to periods preceding the Merger.
Company Background: Apria operates in the home healthcare segment of the healthcare industry, providing a variety of high-quality clinical patient care management programs, related products and supplies as prescribed by a physician and/or authorized by a case manager as part of a care plan. Essentially all products and services offered by the Company are provided through the Company’s network of approximately 500 locations, which are located throughout the United States. We provide services and products in two operating segments and within these two operating segments there are three core service lines: home respiratory therapy, home medical equipment and home infusion therapy. Both segments provide products and services in the home setting to patients and are primarily paid for by a third-party payor, such as Medicare, Medicaid, managed care or other third-party insurer. Sales for both segments are primarily derived from referral sources such as hospital discharge planners, medical groups or independent physicians.
Use of Accounting Estimates: The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. Actual results could differ from those estimates. Among the significant estimates affecting the consolidated financial statements are those related to revenue recognition and the resulting accounts receivable, share-based compensation, income taxes, goodwill and long-lived assets.
Revenue Recognition and Concentration of Credit Risk: Revenues are recognized under fee for service/product arrangements for equipment the Company rents to patients, sales of equipment, supplies, pharmaceuticals and other items the Company sells to patients and under capitation arrangements with third party payors for services and equipment the Company provides to the patients of these payors. Revenue generated from equipment that the Company rents to patients is recognized over the rental period, typically one month, and commences on delivery of the equipment to the patients. Revenue related to sales of equipment, supplies and pharmaceuticals is recognized on the date of delivery to the patients. Revenues derived from capitation arrangements were approximately 8%, 8%, 8% and 10% of total net revenues for the year ended December 31,
F-8
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
2009, the periods October 29, 2008 to December 31, 2008, January 1, 2008 to October 28, 2008, and the year ended December 31, 2007, respectively. Capitation revenue is earned as a result of entering into a contract with a third party to provide its members certain services without regard to the actual services provided, therefore revenue is recognized in the period that the beneficiaries are entitled to health care services. All revenues are recorded at amounts estimated to be received under reimbursement arrangements with third-party payors, including private insurers, prepaid health plans, Medicare and Medicaid. For the year ended December 31, 2009, the period October 29, 2008 to December 31, 2008, the period January 1, 2008 to October 28, 2008, and the year ended December 31, 2007, revenues reimbursed under arrangements with Medicare and Medicaid were approximately 28%, 31%, 32% and 35%, respectively, as a percentage of total net revenues. In the year ended December 31, 2009, the periods January 1, 2008 to October 28, 2008 and October 29, 2008 to December 31, 2008, no other third-party payor group represented more than 8% of the Company’s revenues. In the year ended December 31, 2007, no other third-party payor group represented more than 9% of the Company’s revenue. In fee for service arrangement revenue, rental and sale revenues comprise approximately $654.6 million or 33.9% and $1,275.9 million or 66.1%; $125.7 million or 38.3% and $202.3 million or 61.7%; $622.4 million or 38.2% and $1,008.3 million or 61.8%; and $730.5 million or 49.9% and $734.8 million or 50.1% in the year ended December 31, 2009, the periods October 29, 2008 to December 31, 2008, January 1, 2008 to October 28, 2008, and the year ended December 31, 2007, respectively.
In the Company’s business, there are multiple services and products delivered to patients. These arrangements involve equipment that is rented and related supplies that may be sold that cannot be returned. In arrangements with multiple deliverables, revenue is recognized when each deliverable is provided to the patient. For example, revenues from equipment rental supplies sales are recognized upon of delivery of the products, as the supplies sold are considered a separate unit of accounting.
Cash and Cash Equivalents: Cash is maintained with various financial institutions. These financial institutions are located throughout the United States and the Company’s cash management practices limit exposure to any one institution. Book cash overdrafts, which are reported as a component of accounts payable, were $32.5 million and $21.1 million at December 31, 2009 and 2008, respectively. Management considers all highly liquid instruments purchased with a maturity of less than three months to be cash equivalents.
Short-Term Investments: Certificates of deposit with maturities greater than three months from our purchase date are classified as short-term investments. As of December 31, 2009, the carrying value of the Company’s short-term investments approximates fair value and such investments do not individually exceed the $0.25 million FDIC insurance.
Accounts Receivable: Included in accounts receivable are earned but unbilled receivables of $44.6 million and $48.2 million at December 31, 2009 and 2008, respectively. Delays ranging from a day up to several weeks between the date of service and billing can occur due to delays in obtaining certain required payor-specific documentation from internal and external sources. Earned but unbilled receivables are aged from date of service and are considered in the analysis of historical performance and collectibility.
Due to the nature of the industry and the reimbursement environment in which the Company operates, certain estimates are required to record total net revenues and accounts receivable at their net realizable values. Inherent in these estimates is the risk that they will have to be revised or updated as additional information becomes available. Specifically, the complexity of many third-party billing arrangements and the uncertainty of reimbursement amounts for certain services from certain payors may result in adjustments to amounts originally recorded. Such adjustments are typically identified and recorded at the point of cash application, claim denial or account review.
F-9
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Management performs periodic analyses to evaluate accounts receivable balances to ensure that recorded amounts reflect estimated net realizable value. Specifically, management considers historical realization data, accounts receivable aging trends, other operating trends, the extent of contracted business and business combinations. Also considered are relevant business conditions such as governmental and managed care payor claims processing procedures and system changes. Additionally, focused reviews of certain large and/or problematic payors are performed. Due to continuing changes in the healthcare industry and third-party reimbursement, it is possible that management’s estimates could change in the near term, which could have an impact on operations and cash flows.
Accounts receivable are reduced by an allowance for doubtful accounts which provides for those accounts from which payment is not expected to be received, although services were provided and revenue was earned. Upon determination that an account is uncollectible, it is written-off and charged to the allowance.
Deferred Revenue and Deferred Expense: A lessor is required to recognize rental income over the lease term. Rental of patient equipment is billed on a monthly basis beginning on the date the equipment is delivered. Since deliveries can occur on any day during a month, the amount of billings that apply to the next month are deferred. Only the direct costs associated with the initial rental period are deferred.
Inventories: Inventories are stated at the lower of cost (first-in, first-out method) or market and consist primarily of pharmaceuticals and items used in conjunction with patient service equipment. Inventories are reduced by a reserve for slow moving or obsolete inventory.
Patient Service Equipment: Patient service equipment is stated at cost and consists of medical equipment rented to patients on a month-to-month basis. Depreciation is provided using the straight-line method over the estimated useful lives of the equipment, which range from one to ten years.
Property, Equipment and Improvements: Property, equipment and improvements are stated at cost. Included in property and equipment are assets under capitalized leases which consist of information systems hardware and software. Depreciation is provided using the straight-line method over the estimated useful lives of the assets. Estimated useful lives for each of the categories presented in Note 5 are as follows: leasehold improvements—the shorter of the remaining lease term or seven years; equipment and furnishings—three to fifteen years; and information systems—three to six years.
Capitalized Software: Included in property, equipment and improvements are costs related to internally developed and purchased software that are capitalized and amortized over periods that the assets are expected to provide benefit. Capitalized costs include direct costs of materials and services incurred in developing or obtaining internal-use software and payroll and benefit costs for employees directly involved in the development of internal-use software. In connection with the Merger, we evaluated our information technology strategy and concluded it was no longer advisable to implement a new enterprise-wide information system. As a result of this change in strategy, we wrote off approximately $65.0 million of capitalized information systems assets as part of our purchase accounting adjustments that were made in connection with the Merger. Additions to capitalized internally developed software totaled $9.5 million for the year ended December 31, 2009.
Goodwill and long-lived assets: Goodwill is recorded as the difference, if any, between the aggregate consideration paid for an acquisition and the fair value of the net tangible and intangible assets acquired. The amounts and useful lives assigned to intangible assets acquired, other than goodwill, impact the amount and timing of future amortization.
F-10
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Goodwill and indefinite-lived intangible assets are not amortized but instead tested at least annually for impairment, or more frequently when events or changes in circumstances indicate that the assets might be impaired. Goodwill is tested for impairment by comparing the carrying value to the fair value of the reporting unit to which the goodwill is assigned. A two-step test is used to identify the potential impairment and to measure the amount of impairment, if any. The first step is to compare the fair value of the reporting unit with its carrying amount, including goodwill. If the fair value of the reporting unit exceeds its carrying amount, goodwill is considered not impaired; otherwise, goodwill is impaired and the loss is measured by performing step two. Under step two, the impairment loss is measured by comparing the implied fair value of the reporting unit with the carrying amount of goodwill. Management has determined that our two operating segments are reporting units. As such, the Company has two reporting units, home respiratory therapy/home medical equipment and home infusion therapy. The Company performs the annual test for impairment as of the first day of its fourth quarter and determines fair value using the income approach methodology of valuation that includes the discounted cash flow method. During the annual goodwill impairment test in 2009, the Company completed step one and determined that there was no impairment of goodwill since the fair value of the reporting units exceeded the carrying value. Our annual indefinite-lived intangible assets impairment test in 2009 also resulted in no impairment as the fair value of the assets excluded the carrying value.
Long-lived assets, including property and equipment and purchased intangible assets, are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset or asset group may not be recoverable. Significant judgment is required in determining whether a potential indicator of impairment of long-lived assets exists and in estimating future cash flows for any necessary impairment tests. Recoverability of assets to be held and used is measured by the comparison of the carrying amount of an asset to future undiscounted net cash flows expected to be generated by the asset. If such an asset is considered to be impaired, the impairment to be recognized is measured as the amount by which the carrying amount of the asset exceeds the fair value of the asset. Assets to be disposed of are reported at the lower of the carrying amount or fair value less costs to sell.
Purchased intangible assets consist primarily of trade names, patient backlog, capitated relationships and payor relationships resulting from the Merger. Purchased intangible assets that have definite lives are amortized on a straight-line basis over the estimated useful lives of the related assets, generally ranging from one to twenty years.
Deferred Debt Issuance Costs: Capitalized debt issuance costs include those associated with the Company’s Series A-1 Notes, Series A-2 Notes and Asset Based Revolving Credit Facility (“ABL Facility”). Such costs are classified as non-current assets. Costs relating to the ABL Facility are being amortized through the maturity date of October 2013. Costs relating to the Series A-1 Notes and Series A-2 Notes are amortized from the issuance date through October 2014. See Note 8—Long-term Debt.
Fair Value of Financial Instruments: The carrying value of Apria’s bank debt approximates fair value because the underlying instruments are variable notes that reprice frequently. The fair value of the Series A-1 Notes and Series A-2 Notes, as determined by reference to quoted prices for private transactions of Apria’s issuances, is $768.3 million and $349.3 million at December 31, 2009. The carrying amounts of cash and cash equivalents, accounts receivable, trade payables and accrued expenses approximate fair value due to their short maturity.
Product and Supply Costs: Product and supply costs presented within cost of total net revenues are comprised primarily of the cost of supplies and equipment provided to patients, infusion drug costs and enteral product costs.
F-11
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Home Respiratory Therapy Expenses: Home respiratory therapy expenses presented within cost of total net revenues are comprised primarily of employee salary and benefit costs or contract fees paid to respiratory therapists and other related professionals who are deployed to service a patient. Home respiratory therapy personnel are also engaged in a number of administrative and marketing tasks, and accordingly, these costs are classified within selling, distribution and administrative expenses and amounted to $23.5 million, $3.9 million, $18.0 million and $19.8 million in the year ended December 31, 2009, the periods October 29, 2008 to December 31, 2008, January 1, 2008 to October 28, 2008 and the year ended December 31, 2007, respectively.
Distribution Expenses: Distribution expenses are included in selling, distribution and administrative expenses and totaled $159.4 million, $27.8 million, $158.2 million and $175.9 million in the year ended December 31, 2009, the periods October 29, 2008 to December 31, 2008, January 1, 2008 to October 28, 2008, and the year ended December 31, 2007, respectively. Such expense represents the cost incurred to coordinate and deliver products and services to the patients. Included in distribution expenses are leasing, maintenance, licensing and fuel costs for the vehicle fleet; salaries and other costs related to drivers and dispatch personnel; and amounts paid to courier and other outside shipping vendors. Such expenses fall within the definition of “shipping and handling” costs and are classified within selling and administrative expenses and may not be comparable to other companies.
Self-Insurance: Coverage for certain employee medical claims and benefits, as well as workers’ compensation, vehicle liability, professional and general liability are self-insured. Accruals for medical claims at December 31, 2009 and 2008 were $7.0 million and $6.3 million, respectively. Amounts accrued for costs of the other liability coverages totaled $27.8 million and $23.4 million at December 31, 2009 and 2008, respectively. All such amounts are classified in other accrued liabilities.
Advertising: Advertising costs are expensed as incurred. Such expenses are included in selling, distribution and administrative expenses and amounted to $2.9 million, $0.7 million, $2.7 million and $3.8 million for the year ended December 31, 2009, the period October 29, 2008 to December 31, 2008, the period January 1, 2008 to October 28, 2008, and the year ended December 31, 2007, respectively.
Income Taxes: Deferred income tax assets and liabilities are computed for differences between the carrying amounts of assets and liabilities for financial statement and tax purposes. Deferred income tax assets are required to be reduced by a valuation allowance when it is determined that it is more likely than not that all or a portion of a deferred tax asset will not be realized. In determining the necessity and amount of a valuation allowance, management considers our current and past performance, the market environment in which we operate, tax planning strategies and the length of tax benefit carryforward periods.
Derivative Instruments and Hedging Activities: From time to time derivative financial instruments were used to limit exposure to interest rate fluctuations on the Company’s variable rate long-term debt. Unrealized gains and losses on the fair value of the swap agreements were reflected, net of taxes, in operating income, as the transactions no longer qualify for hedge accounting treatment. Exposure to credit loss under the swap agreement is limited to the interest rate spread in the event of counterparty nonperformance. At December 31, 2009 and 2008, we did not have any swap agreements in effect.
Share-Based Compensation/Profit Interest Units: We measure and recognize compensation expense for all share-based payment awards made to employees and non-employee directors based on estimated fair values on the date of grant. The value of the portion of the award that is ultimately expected to vest is recognized as expense over the requisite service period in our consolidated financial statements. Forfeitures are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. We recognize share-based compensation expense on a straight-line basis over the requisite service period. Prior to the
F-12
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Merger, we estimated fair value of our share-based payment awards using the Black-Scholes valuation model. Subsequent to the Merger, the estimate of fair value of share-based awards on the date of grant is determined through the allocation of all outstanding securities to a business enterprise valuation. The enterprise valuation is based upon a combination of the income approach and the market approach. The income approach is based on discounted cash flows. The market approach uses a selection of comparable companies in determining value. This determination of fair value is affected by assumptions regarding a number of highly complex and subjective variables. Changes in the subjective assumptions can materially affect the estimate of their fair value.
Comprehensive Income: For the year ended December 31, 2009, the periods October 29, 2008 to December 31, 2008 and January 1, 2008 to October 28, 2008, and the year ended December 31, 2007, the difference between net income and comprehensive income is $0, $0, $0 and $0.1 million, respectively, net of taxes, which is attributable to unrealized (losses) and gains on various interest rate swap agreements.
Reclassification: Certain amounts from the prior year have been reclassified on the balance sheet to conform with the current year presentation. For the year ended December 31, 2008, $6.0 million was reclassified from other accrued liabilities to accrued payroll and related taxes and benefits related to management incentive compensation programs.
NOTE 2—The Merger
On June 18, 2008, the Company entered into an Agreement and Plan of Merger (the “Merger Agreement”) with Sky LLC and Merger Sub. Sky LLC is controlled by private investment funds affiliated with Blackstone.
Pursuant to the terms of the Merger Agreement, on October 28, 2008, Merger Sub merged with and into the Company (the “Merger”) with the Company continuing as the surviving corporation and a wholly-owned subsidiary of Sky LLC. The total purchase price of the Merger was $1,713.3 million, inclusive of retired debt and associated transaction costs. As a result of the Merger, Apria’s common stock, par value $0.001 per share, was delisted from the New York Stock Exchange and the Company ceased to be publicly traded. At the effective time of the Merger, each share of Apria’s common stock then outstanding was converted into the right to receive $21.00 in cash.
In order to finance the Merger, debt repayment and other transaction expenses, we incurred new indebtedness totaling approximately $1,040.0 million (see Note 8—Long-term Debt). This new debt, together with approximately $673.3 million of equity invested by affiliates of Blackstone, was used to fund the acquisition of our previously outstanding common stock, pay transaction costs, establish certain cash reserves and finance the retirement of debt.
Allocation of Purchase Price
The total purchase price was allocated to the assets acquired and liabilities assumed based on their fair values at the acquisition date. In valuing acquired assets and assumed liabilities, fair values are based on, but are not limited to, quoted market prices, expected future cash flows, current replacement costs, market rate assumptions and appropriate discount and growth rates.
Under the purchase method of accounting, the assets and liabilities of our Predecessor were recorded at their respective fair values as of the date of the acquisition. In September 2009, we finalized the valuation of the intangible assets related to the Merger, resulting in adjustments to the value of our capitated relationships, patient backlogs, trade names and goodwill. The adjustments to our capitated relationships and patient backlogs also resulted in additional amortization expense. If we had finalized the valuation of the intangible assets as of the
F-13
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
date of the Merger, $11.9 million of the amortization expense recorded during the three months ended September 30, 2009 would have been recorded in 2008. The following table summarizes the fair values of the assets acquired and liabilities assumed.
| Fair Value | ||||
| (in millions) | ||||
Current assets, including $158.2 in cash and cash equivalents | $ | 567.5 | ||
Property and equipment | 268.8 | |||
Amortizable intangible assets | 98.6 | |||
Non-amortizable intangible assets | 532.0 | |||
Goodwill | 759.2 | |||
Other assets | 71.3 | |||
Current liabilities | (304.9 | ) | ||
Long-term debt | (7.2 | ) | ||
Deferred income taxes | (230.5 | ) | ||
Other non-current liabilities | (41.5 | ) | ||
| $ | 1,713.3 | |||
We do not expect any of the goodwill resulting from this transaction to be tax deductible. Goodwill and trade names are considered to have an indefinite life and are not amortized, but rather are reviewed annually for impairment or more frequently if indicators of impairment exist.
NOTE 3—Segments
The Company has two operating segments and within these two operating segments there are three core service lines: home respiratory therapy, home medical equipment and home infusion therapy. We have two reportable operating segments (1) home respiratory therapy and home medical equipment and (2) home infusion therapy. The home respiratory therapy and home medical equipment segment provides services and equipment to assist patients with oxygen systems, sleep apnea, ambulation and general care around the home, as well as to provide respiratory medications and related services. The home infusion therapy segment primarily provides patients with pharmaceuticals and services prescribed in conjunction with the administration of nutrients or medication intravenously or through a gastrointestinal tube.
Amounts for prior periods have been recast to the current management view.
| Net Revenues | ||||||||||||
Operating Segment | Year Ended December 31, 2009 | Period October 29, 2008 to December 31, 2008 (Successor) | Period January 1, 2008 to October 28, 2008 (Predecessor) | Year Ended December 31, 2007 | ||||||||
Home Respiratory Therapy and Home Medical Equipment | $ | 1,169,609 | $ | 209,567 | $ | 1,074,711 | $ | 1,297,619 | ||||
Home Infusion Therapy | 924,952 | 147,098 | 698,578 | 334,182 | ||||||||
Total | $ | 2,094,561 | $ | 356,665 | $ | 1,773,289 | $ | 1,631,801 | ||||
F-14
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
| EBIT | |||||||||||||
Operating Segment | Year Ended December 31, 2009 | Period October 29, 2008 to December 31, 2008 (Successor) | Period January 1, 2008 to October 28, 2008 (Predecessor) | Year Ended December 31, 2007 | |||||||||
Home Respiratory Therapy and Home Medical Equipment | $ | 50,167 | $ | 26,255 | $ | 102,927 | $ | 118,242 | |||||
Home Infusion Therapy | 65,667 | (1,740 | ) | 17,965 | 40,288 | ||||||||
Total | $ | 115,834 | $ | 24,515 | $ | 120,892 | $ | 158,530 | |||||
| Depreciation and Amortization | |||||||||||||
Operating Segment | Year Ended December 31, 2009 | Period October 29, 2008 to December 31, 2008 (Successor) | Period January 1, 2008 to October 28, 2008 (Predecessor) | Year Ended December 31, 2007 | |||||||||
Home Respiratory Therapy and Home Medical Equipment | $ | 151,287 | $ | 20,041 | $ | 101,479 | $ | 128,640 | |||||
Home Infusion Therapy | 21,756 | 3,015 | 14,742 | 6,084 | |||||||||
Total | $ | 173,043 | $ | 23,056 | $ | 116,221 | $ | 134,724 | |||||
Our Chief Operating Decision Maker (“CODM”) does not review assets assigned to segments. Therefore, such items are not reported in the table above.
Earnings before interest and taxes (“EBIT”). EBIT is a measure used by our management to measure operating performance. EBIT is defined as net income (loss) plus interest expense and income taxes. EBIT is not a recognized term under Generally Accepted Accounting Principles (“GAAP”) and does not purport to be an alternative to net income as a measure of operating performance or to cash flows from operating activities as a measure of liquidity.
The following table provides a reconciliation from net (loss) income to EBIT:
| Year Ended December 31, 2009 | Period October 29, 2008 to December 31, 2008 | ||||||||||||||||||||
| Home Respiratory Therapy and Home Medical Equipment | Home Infusion Therapy | Total | Home Respiratory Therapy and Home Medical Equipment | Home Infusion Therapy | Total | ||||||||||||||||
| (in thousands) | |||||||||||||||||||||
Net (loss) income | $ | (3,820 | ) | $ | (1,894 | ) | |||||||||||||||
Interest expense, net (a) | 128,092 | 25,750 | |||||||||||||||||||
Income tax (benefit) expense | (8,438 | ) | 659 | ||||||||||||||||||
EBIT | $ | 50,167 | $ | 65,667 | $ | 115,834 | $ | 26,255 | $ | (1,740 | ) | $ | 24,515 | ||||||||
F-15
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
| Period January 1, 2008 to October 28, 2008 | Year Ended December 31, 2007 | |||||||||||||||||
| Home Respiratory Therapy and Home Medical Equipment | Home Infusion Therapy | Total | Home Respiratory Therapy and Home Medical Equipment | Home Infusion Therapy | Total | |||||||||||||
| (in thousands) | ||||||||||||||||||
Net (loss) income | $ | 56,453 | $ | 86,039 | ||||||||||||||
Interest expense, net (a) | 30,247 | 20,493 | ||||||||||||||||
Income tax (benefit) expense | 34,192 | 51,998 | ||||||||||||||||
EBIT | $ | 102,927 | $ | 17,965 | $ | 120,892 | $ | 118,242 | $ | 40,288 | $ | 158,530 | ||||||
| (a) | Reflects $129.2 million of interest expense, net of $1.1 million of interest income for 2009. Reflects $26.2 million of interest expense, net of $0.4 million of interest income for the period October 29, 2008 to December 31, 2008. Reflects $31.8 million of interest expense, net of $1.6 million of interest income for the period January 1, 2008 to October 28, 2008. Reflects $22.4 million of interest expense, net of $1.9 million in interest income for 2007. |
We allocate certain expenses that are not directly attributable to a product line based upon segment headcount.
NOTE 4—Recent Accounting Pronouncements
In June 2009, the FASB issued Accounting Standards Codification (“ASC”) 105Generally Accepted Accounting Principles (the “Codification”) as the single source of authoritative nongovernmental U.S. Generally Accepted Accounting Principles (“GAAP”) to be launched on July 1, 2009. The Codification does not change current U.S. GAAP, but is intended to simplify user access to all authoritative U.S. GAAP by providing all the authoritative literature related to a particular topic in one place. The Codification is effective for interim and annual periods ending after September 15, 2009. The Codification was adopted by the Company in the interim period ending September 30, 2009. All references made to U.S GAAP use the new Codification numbering system prescribed by the FASB. However, as the Codification is not intended to change existing GAAP, it did not have any impact on the Company’s consolidated financial statements.
In June 2009, the FASB issued ASC 810, “Consolidation” (“ASC 810”) (originally issued as Statement of Financial Accounting Standard No. 167, “Amendments to FASB Interpretation No. 46(R)”). Among other items, ASC 810 responds to concerns about the application of certain key provisions of FIN 46(R), including those regarding the transparency of the involvement with variable interest entities. ASC 810 is effective for calendar year companies beginning on January 1, 2010. The Company does not believe the adoption of ASC 810 will have a significant impact on its financial position, results of operations, cash flows, or disclosures.
In August 2009, the FASB issued Accounting Standards Update (ASU) No. 2009-05, “Measuring Liabilities at Fair Value” (“ASU 2009-05”). ASU 2009-05 provides additional guidance clarifying the measurement of liabilities at fair value. ASU 2009-05 is effective in fourth quarter 2009 for a calendar-year entity. The Company adopted the provisions of ASU 2009-05 in the fourth quarter of 2009.
In October 2009, the FASB issued ASU 2009-13, “Multiple-Deliverable Revenue Arrangements” which amends ASC Topic 605, Revenue Recognition (“ASU 2009-13”) Under this standard, management is no longer required to obtain vendor-specific objective evidence or third party evidence of fair value for each deliverable in
F-16
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
an arrangement with multiple elements, and where evidence is not available we may now estimate the proportion of the selling price attributable to each deliverable. ASU 2009-13 will be effective prospectively for revenue arrangements entered into or materially modified in the fiscal years beginning on or after June 15, 2010. The Company is currently evaluating the impact of ASU 2009-13 on its financial position, results of operations, cash flows and disclosures.
In January 2010, the FASB issued ASU 2010-6, “Improving Disclosures About Fair Value Measurements” (“ASU 2010-6”) which requires reporting entities to make new disclosures about recurring or nonrecurring fair-value measurements including significant transfers into and out of Level 1 and Level 2 fair-value measurements and information on purchases, sales, issuances, and settlements on a gross basis in the reconciliation of Level 3 fair- value measurements. ASU 2010-6 is effective for annual reporting periods beginning after December 15, 2009, except for Level 3 reconciliation disclosures which are effective for annual periods beginning after December 15, 2010. We do not expect the adoption of ASU 2010-6 to have a material impact on our consolidated financial statements.
NOTE 5—Property, Equipment and Improvements
Property, equipment and improvements consist of the following:
| December 31, | ||||||||
| 2009 | 2008 | |||||||
| (in thousands) | ||||||||
Leasehold improvements | $ | 34,720 | $ | 27,978 | ||||
Equipment and furnishings | 20,471 | 16,547 | ||||||
Information systems—hardware | 31,285 | 18,352 | ||||||
Information systems—software | 24,796 | 9,723 | ||||||
| 111,272 | 72,600 | |||||||
Less accumulated depreciation | (31,045 | ) | (4,845 | ) | ||||
| $ | 80,227 | $ | 67,755 | |||||
The Company has corrected the classification of certain of its assets as of December 31, 2008. Depreciation expense for property, equipment and improvements was $23.7 million, $4.9 million, $23.5 million and $20.9 million for the year ended December 31, 2009, the periods October 29, 2008 to December 31, 2008 and January 1, 2008 to October 28, 2008, and for the year ended December 31, 2007, respectively.
NOTE 6—Business Combinations and Asset Purchases
During 2009, the Company purchased the accounts receivable and/or active patient lists from four discount agreement customers. During 2008 we did not make any acquisitions. However, as discussed above, on October 28, 2008, we were acquired by Sky Acquisition LLC, a company controlled by private investment funds affiliated with The Blackstone Group. See Note 2—The Merger.
In connection with the Merger, we recorded amounts in 2008 related to intangible assets based upon a preliminary valuation. During 2009, we completed the valuation of the intangible assets related to the Merger, resulting in adjustments to the value of our capitated relationships, patient backlogs, trade names and goodwill. If we had finalized the valuation of the intangible assets as of the date of the Merger, $11.9 million of the amortization expense recorded during the three months ended September 30, 2009 would have been recorded in 2008.
F-17
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Changes in goodwill related to the Merger during 2009 were as follows:
| (in thousands) | ||||
Balance at December 31, 2008 | $ | 895,069 | ||
Additions: | ||||
Tax adjustments relating to establishing deferred tax liabilities for intercompany payables for subsidiaries | 15,123 | |||
Professional fees related to the Merger | 1,912 | |||
Change in estimated value of favorable leasehold interests | 1,790 | |||
Executive severance | 1,200 | |||
Other | 582 | |||
Reductions: | ||||
Adjustments resulting from completion of intangible asset valuations net of related tax adjustments | (145,846 | ) | ||
Tax adjustments relating to Merger costs | (3,772 | ) | ||
Change in estimated value of deferred debt issuance costs | (6,180 | ) | ||
Tax benefit from true-up of pre-acquisition tax returns | (681 | ) | ||
Balance at December 31, 2009 | $ | 759,197 | ||
None of the amounts recorded as goodwill in connection with the Merger are expected to be deductible for tax purposes.
On December 3, 2007, the acquisition of Coram, Inc. (“Coram”) was completed. Coram, which was privately-held and a national provider of home infusion and specialty pharmaceutical services. The Coram acquisition has strategically provided the Company the opportunity to diversify our product and payor mix and lessen our reliance on Medicare-reimbursed respiratory and HME services. Additionally it has positioned the Company as a dominant player in the growing clinical and specialty pharmaceutical and infusion market. The integration of Coram’s and Apria’s infusion businesses was largely complete by mid-2009. This transaction was accounted for as purchases and, accordingly, the results of operations are included in the consolidated income statements from the date of acquisition.
F-18
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
The following table summarizes the allocation of the purchase price of all 2007 acquisitions. Payments for prior years’ acquisitions totaled $0, $0, $2.6 million and $0.1 million for the year ended December 31, 2009, the periods October 29, 2008 to December 31, 2008 and January 1, 2008 to October 28, 2008, and the year ended December 31, 2007, respectively. At December 31, 2009 and 2008 there was no remaining unpaid consideration.
| Year Ended December 31, 2007 | ||||
| (in thousands) | ||||
Fair value of patient service equipment acquired | $ | 7,014 | ||
Fair value of property and equipment acquired | 24,916 | |||
Fair value of other assets acquired | 132,070 | (1) | ||
Intangible assets | 104,284 | |||
Goodwill | 176,048 | |||
Total assets acquired | 444,332 | |||
Liabilities assumed and accrued, net of payments from prior years’ acquisitions | (89,754 | )(2) | ||
Net assets acquired | $ | 354,578 | ||
| (1) | Consists primarily of $82.5 million in net accounts receivable, $14.0 million in net inventory and $28.3 million in deferred tax assets. |
| (2) | Consists primarily of $44.9 million in accounts payable, $17.7 million in accrued compensation and $9.4 million in other accrued liabilities. |
The following supplemental unaudited pro forma information presents the combined operating results of Apria and Coram, as if the acquisitions had occurred at the beginning of the period presented. The pro forma information is based on the historical financial statements of Apria and Coram. Amounts are not necessarily indicative of the results that may have been attained had the combinations been in effect at the beginning of the periods presented or that may be achieved in the future.
| Year Ended December 31, 2007 | |||
| (in thousands) | |||
Net revenues | $ | 2,103,791 | |
Net income | 86,275 | ||
F-19
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
NOTE 7—Goodwill and Intangible Assets
Changes in goodwill by segment are as follows:
| Home Infusion Therapy | Home Respiratory Therapy and Home Medical Equipment | Total | ||||||||||
| (in thousands) | ||||||||||||
Balance, December 31, 2007 (Predecessor) | $ | 256,455 | $ | 458,780 | $ | 715,235 | ||||||
Purchase accounting adjustments (Predecessor) | (4,058 | ) | — | (4,058 | ) | |||||||
Balance October 28, 2008 (Predecessor) | 252,397 | 458,780 | 711,177 | |||||||||
Purchase accounting adjustments as a result of the Merger, net | 51,568 | 132,324 | 183,892 | |||||||||
Balance, December 31, 2008 (Successor) | 303,965 | 591,104 | 895,069 | |||||||||
Purchase accounting adjustments related to the Merger | (46,142 | ) | (89,730 | ) | (135,872 | ) | ||||||
| �� | ||||||||||||
Balance, December 31, 2009 (Successor) | $ | 257,823 | $ | 501,374 | $ | 759,197 | ||||||
Intangible assets consist of the following:
| December 31, 2009 | December 31, 2008 | |||||||||||||||||||||
| Average Life in Years | Gross Carrying Amount | Accumulated Amortization | Net Book Value | Gross Carrying Amount | Accumulated Amortization | Net Book Value | ||||||||||||||||
| (dollars in thousands) | ||||||||||||||||||||||
Intangible assets subject to amortization: | ||||||||||||||||||||||
Patient backlog | 1.1 | $ | 44,000 | $ | (44,000 | ) | $ | — | $ | — | $ | — | $ | — | ||||||||
Capitated relationships | 20.0 | 40,000 | (2,333 | ) | 37,667 | 75,000 | (625 | ) | 74,375 | |||||||||||||
Payor relationships | 20.0 | 11,000 | (642 | ) | 10,358 | 11,000 | (92 | ) | 10,908 | |||||||||||||
Net favorable leasehold interest | 3.2 | 3,553 | (1,319 | ) | 2,234 | 5,343 | (292 | ) | 5,051 | |||||||||||||
Subtotal | 2.4 | 98,553 | (48,294 | ) | 50,259 | 91,343 | (1,008 | ) | 90,334 | |||||||||||||
Intangible assets not subject to amortization: | ||||||||||||||||||||||
Trade names | — | 525,000 | — | 525,000 | 300,000 | — | 300,000 | |||||||||||||||
Accreditations with commissions | — | 7,000 | — | 7,000 | 7,000 | — | 7,000 | |||||||||||||||
Subtotal | — | 532,000 | — | 532,000 | 307,000 | — | 307,000 | |||||||||||||||
Total | $ | 630,553 | $ | (48,294 | ) | $ | 582,259 | $ | 398,343 | $ | (1,008 | ) | $ | 397,334 | ||||||||
Amortization expense was $47.7 million, $1.0 million, $3.5 million and $3.1 million for the year ended December 31, 2009, the period October 29, 2008 to December 31, 2008, the period January 1, 2008 to October 28, 2008 and the year ended December 31, 2007, respectively. Estimated amortization expense for each of the fiscal years ending December 31, is presented below:
Year Ending December 31, | (in thousands) | ||
2010 | $ | 3,556 | |
2011 | 3,474 | ||
2012 | 2,854 | ||
2013 | 2,550 | ||
2014 | 2,550 | ||
F-20
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
NOTE 8—Long-Term Debt
Long-term debt consists of the following:
| December 31, | ||||||||
| 2009 | 2008 | |||||||
| (in thousands) | ||||||||
Senior Secured Bridge Credit Agreement | $ | — | $ | 1,010,000 | ||||
Series A-1 Notes | 700,000 | — | ||||||
Series A-2 Notes | 317,500 | — | ||||||
Notes payable relating to ABL Facility | — | 6,000 | ||||||
Convertible senior notes | 77 | 77 | ||||||
Capital lease obligations (see Note 11) | 3,569 | 5,778 | ||||||
Other | 378 | |||||||
| 1,021,146 | 1,022,233 | |||||||
Less: current maturities | (1,690 | ) | (8,750 | ) | ||||
| $ | 1,019,456 | $ | 1,013,483 | |||||
Series A-1 Notes and Series A-2 Notes. In May 2009, we issued $700.0 million of its 11.25% Senior Secured Notes due 2014 (Series A-1) and on August 2009, we issued $317.5 million of its 12.375% Senior Secured Notes due 2014 (Series A-2). The Series A-1 Notes and the Series A-2 Notes bear interest at a rate equal to 11.25% per annum and 12.375% per annum, respectively. The indenture governing the Series A-1 Notes and the Series A-2 Notes, among other restrictions, limits our ability and the ability of our restricted subsidiaries to:
| • | incur additional debt; |
| • | pay dividends and make other distributions; |
| • | make certain investments; |
| • | repurchase our stock; |
| • | incur certain liens; |
| • | enter into transactions with affiliates; |
| • | merge or consolidate; |
| • | enter into agreements that restrict the ability of our subsidiaries to make dividends or other payments to us; and |
| • | transfer or sell assets. |
Subject to certain exceptions, the indenture governing the Series A-1 Notes and the Series A-2 Notes permits Apria and its restricted subsidiaries to incur additional indebtedness, including senior indebtedness and secured indebtedness. The indenture also does not limit the amount of additional indebtedness that Sky Acquisition may incur. The Series A-1 Notes are entitled to a priority of payment over the Series A-2 notes in certain circumstances, including upon any acceleration of the obligations under the Series A-1 Notes, the Series A-2 Notes or any bankruptcy or insolvency event or default with respect to Apria or any guarantor of the Series A-1 Notes and the Series A-2 Notes.
F-21
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Senior Secured Bridge Credit Agreement. In connection with the Merger on October 28, 2008, we entered into a $1,010.0 million Senior Secured Bridge Credit Agreement with Banc of America Securities LLC and Wachovia Capital Markets, LLC, as joint lead arrangers, Banc of America Securities LLC, Wachovia Capital Markets, LLC and Barclays Capital, the investment banking division of Barclays Bank PLC, as joint bookrunners and Banc of America Bridge LLC, as administrative agent and Bank of America, N.A., as collateral agent, and a syndicate of financial institutions and institutional lenders. In connection with the issuance of the Series A-1 and Series A-2 Notes, we repaid all borrowings and terminated the Senior Secured Bridge Credit Agreement.
ABL Facility. In connection with the Merger on October 28, 2008, we entered into the ABL Facility with Banc of America Securities LLC and Wachovia Capital Markets, LLC, as joint lead arrangers, Banc of America Securities LLC, Wachovia Capital Markets, LLC and Barclays Capital, the investment banking division of Barclays Bank PLC, as joint bookrunners and Bank of America, N.A., as administrative agent and collateral agent, and a syndicate of financial institutions and institutional lenders.
Our ABL Facility provides for revolving credit financing of up to $150.0 million, subject to borrowing base availability, with a maturity of five years, including both a letter of credit and swingline loan sub-facility. The borrowing base at any time is equal to the sum (subject to certain reserves and other adjustments) of 85% of eligible receivables and the lesser of (a) 85% of the net orderly liquidation value of eligible inventory and (b) $20.0 million.
Our ABL Facility includes borrowing capacity available for letters of credit and for borrowings on same-day notice, referred to as swingline loans.
Borrowings under our ABL Facility are subject to the satisfaction of customary conditions, including absence of a default and accuracy of representations and warranties.
Provided that no default or event of default is then existing or would arise therefrom, at our option, we may request that our ABL Facility be increased by an amount not to exceed $25.0 million, subject to certain consent rights of the administrative agent, swingline lender and issuing banks with respect to the lenders providing commitments for such increase. The terms of such incremental revolving facility shall be identical to our ABL Facility.
Interest Rate and Fees. Borrowings under our ABL Facility bear interest at a rate per annum equal to, at our option, either (a) a base rate determined by reference to the higher of (1) the prime rate of Bank of America, N.A. and (2) the federal funds effective rate plus 1/2 of 1% (“Base Rate”), plus an applicable margin of 2.00% or (b) a LIBOR rate determined by reference to the London Interbank Offered Rate, adjusted for statutory reserve requirements (“LIBOR”), plus an applicable margin of 3.00%. Beginning on April 1, 2009, the applicable margin for borrowings under our ABL Facility will be subject to step ups and step downs based on average excess availability under that facility. In addition to paying interest on outstanding amounts under our ABL Facility, we are required to pay a commitment fee, in respect of the unutilized commitments thereunder, ranging from 0.50% to 1.00% per annum, which fee will be determined based on utilization of our ABL Facility (increasing when utilization is low and decreasing when utilization is high). We must also pay customary letter of credit fees equal to the applicable margin on LIBOR loans and agency fees.
Repayments. If at any time the aggregate amount of outstanding borrowings under our ABL Facility, including letters of credit and swingline loans, exceeds the lesser of (i) the aggregate commitments under our ABL Facility and (ii) the borrowing base (except as a result of overadvance loans or protective advances), we will be required to repay outstanding loans (including swingline loans) and repay or cash collateralize letters of credit in an aggregate amount equal to such excess. If the amount available under our ABL Facility is less than
F-22
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
12.5% of the lesser of the aggregate commitments or the borrowing base for five consecutive business days or certain events of default have occurred, we will be required, upon the occurrence and during the continuance of such cash dominion event, to deposit cash from our material deposit accounts daily in a core concentration account maintained with the administrative agent under our ABL Facility, which will be used to repay outstanding loans and cash collateralize letters of credit.
We may voluntarily reduce the unutilized portion of the commitment amount and repay outstanding loans at any time (subject to minimum repayment amounts and customary notice periods) without premium or penalty other than customary “breakage” costs with respect to LIBOR loans.
Amortization and Final Maturity. There is no scheduled amortization under our ABL Facility. All outstanding loans under the facility are due and payable in full on the fifth anniversary of the closing date.
Guarantees and Security. All obligations under our ABL Facility, any interest rate protection or other hedging arrangements entered into with any lender in the syndicate or any of its affiliates, and cash management obligations owing to any lender in the syndicate or any of its affiliates are unconditionally guaranteed by our parent and substantially all of our existing and future, direct and indirect, wholly-owned domestic restricted subsidiaries. All obligations under our ABL Facility, any interest rate protection or other hedging arrangements entered into with any lender in the syndicate or any of its affiliates, and cash management obligations owing to any lender in the syndicate or any of its affiliates, and the guarantees of those obligations, are secured, subject to certain exceptions, by substantially all of our assets and the assets of the guarantors, including:
| • | a first-priority security interest in substantially all personal property consisting of accounts receivable arising from the sale of inventory and other goods and services, inventory, intercompany notes and intangible assets to the extent attached to the foregoing, and certain related assets and proceeds of the foregoing; and |
| • | a second-priority security interest in all tangible and intangible assets that secure the Senior Secured Bridge Credit Agreement on a first-priority basis. |
Restrictive Covenants and Other Matters. Our ABL Facility requires that if excess availability is less than 12.5% of the lesser of the aggregate commitments and the borrowing base, we comply with a minimum fixed charge coverage ratio test. In addition, our ABL Facility includes negative covenants that, subject to significant exceptions, limit our ability and the ability of our parent and subsidiaries to, among other things:
| • | incur, assume or permit to existing additional indebtedness or guarantees; |
| • | incur liens; |
| • | make investments and loans; |
| • | pay dividends, make payments or redeem or repurchase capital stock; |
| • | engage in mergers, liquidations, dissolutions, asset sales and other dispositions (including sale leaseback transactions); |
| • | prepay, redeem or purchase certain indebtedness; |
| • | amend or otherwise alter terms of certain indebtedness; |
| • | enter into agreements limiting subsidiary distributions; |
| • | engage in certain transactions with affiliates; |
| • | alter the business that we conduct; |
F-23
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
| • | change our fiscal year; and |
| • | with respect to our parent, engage in any activities not permitted. |
Our ABL Facility contains certain customary representations and warranties, affirmative covenants and events of default, including among other things payment defaults, breach of representations and warranties, covenant defaults, cross-defaults to certain indebtedness, certain events of bankruptcy, certain events under ERISA, material judgments, failure of any guaranty or security document supporting our ABL Facility to be in full force and effect, and change of control. If such an event of default occurs, the lenders under our ABL Facility would be entitled to take various actions, including the acceleration of amounts due under our ABL Facility and all actions permitted to be taken by a secured creditor.
As of December 31, 2009 there were no borrowings on the ABL Facility, outstanding letters of credit totaled $16.1 million and additional availability under the ABL Facility subject to the borrowing base was $133.9 million. As of December 31, 2009, the available borrowing base of the ABL Facility was approximately $123.9 million. At December 31, 2009, we were in compliance with all of the financial covenants required by the credit agreement governing the ABL Facility.
Interim Facility. On June 18, 2008, we entered into the Interim Facility pursuant to the credit agreement with Banc of America Bridge LLC, Barclays Capital, the investment banking division of Barclays Bank PLC, Wachovia Capital Markets, LLC and the lenders named therein. On September 2, 2008, proceeds of the Interim Facility were used to fund repurchases of our 3 3/8% Convertible Senior Notes due 2033 (the “convertible senior notes”) as described below. The loans under the Interim Facility bore interest at a rate of eleven per cent (11%) per year with a maturity date of March 1, 2009. In addition, we paid usual and customary bank fees in connection with entering into the Interim Facility. The credit agreement governing the Interim Facility included restrictions on additional indebtedness, business operations, liens, transfers and sales of assets, and transactions with affiliates. The credit agreement governing the Interim Facility also contained customary events of default which would permit the lenders to accelerate payments under the Interim Facility if not cured within applicable grace periods, including the failure to make timely payments under the Interim Facility and the failure to follow certain covenants. All borrowings under the Interim Facility were paid off on October 28, 2008 using a portion of the proceeds from the Merger financings.
Convertible Senior Notes. In August 2003, we issued 3.375% Convertible Senior Notes due 2033 in the aggregate principal amount of $250.0 million under an indenture between us and U.S. Bank National Association in a private placement. Holders of our convertible senior notes had the right to require us to redeem on September 1, 2008 some or all of their notes at a price equal to 100% of the principal amount thereof plus accrued and unpaid interest. The holders of substantially all of the convertible senior notes exercised this right. On September 2, 2008, proceeds of the Interim Facility were used to fund repurchases of $249.8 million of our convertible senior notes. In addition, holders of the remaining $0.2 million of the convertible senior notes had the right, as a result of the change of control resulting from the Merger, to cause us to repurchase the convertible senior notes at a price equal to 100% of the principal amount thereof plus accrued and unpaid interest. Pursuant to this right, holders of $0.2 million of convertible senior notes exercised their option in December 2008. Approximately $0.1 million of convertible senior notes remain outstanding at December 31, 2009 in accordance with the terms of the indenture governing the convertible senior notes.
Predecessor Revolving Credit Facility. On November 23, 2004, we entered into a senior secured revolving credit facility (“Predecessor Revolving Credit Facility”) with Bank of America and a syndicate of lenders that was amended effective June 23, 2006. The amendment extended the maturity date from November 23, 2009 to June 23, 2011 and lowered the applicable interest rate margins and commitment fees. The credit agreement was
F-24
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
structured as a $500.0 million revolving credit facility. In connection with the Merger on October 28, 2008, we repaid all outstanding indebtedness under our Predecessor Revolving Credit Facility.
As market conditions warrant, we and our major equity holders, including the Sponsor and its affiliates, may from time to time, depending upon market conditions, seek to repurchase our debt securities or loans in privately negotiated or open market transactions, by tender offer or otherwise.
Maturities of long-term debt are as follows:
Year Ending December 31, | (in thousands) | ||
2010 | $ | 1,690 | |
2011 | 1,358 | ||
2012 | 343 | ||
2013 | 240 | ||
2014 | 1,017,515 | ||
Thereafter | — | ||
| $ | 1,021,146 | ||
Total interest paid on debt in the year ended December 31, 2009, the period October 29, 2008 to December 31, 2008, the period January 1, 2008 to October 28, 2008, and the year ended December 31, 2007 amounted to $122.7 million, $3.2 million, $23.4 million and $18.1 million, respectively. Amounts accrued for interest totaled $19.8 million and $21.9 million at December 31, 2009 and 2008, respectively. All such amounts are classified in other accrued liabilities.
Hedging Activities. We are exposed to interest rate fluctuations on our variable rate long-term debt. However, we currently do not have any interest rate swap agreements in effect. Our policy for managing interest rate risk is to evaluate and monitor all available relevant information, including but not limited to, the structure of our interest-bearing assets and liabilities, historical interest rate trends and interest rate forecasts published by major financial institutions. The tools we may utilize to moderate our exposure to fluctuations in the relevant interest rate indices include, but are not limited to: (1) strategic determination of repricing periods and related principal amounts, and (2) derivative financial instruments such as interest rate swap agreements, caps or collars. We do not use derivative financial instruments for trading or other speculative purposes.
At September 30, 2008, we terminated our one remaining interest rate swap agreement which would have expired in January 2009. The impact of the termination was immaterial. During 2008 and during 2007, we had one interest rate swap agreement in effect to fix our LIBOR-based variable rate debt in connection with the Predecessor Revolving Credit Facility. The agreement, a forward-starting contract with a three-year term, became effective in January 2006, and had a notional amount of $25.0 million that fixed an equivalent amount of our variable rate debt at 4.44%. For the year ended December 31, 2009, the periods October 29, 2008 to December 31, 2008 and January 1, 2008 to October 28, 2008, and the year ended December 31, 2007, we (paid) received net settlement amounts of $0, $0, ($0.2) million and $0.2 million, respectively.
Unrealized gains and losses on the fair value of the swap agreements are reflected, net of taxes, in operating income, as the transactions no longer qualify for hedge accounting treatment. Our exposure to credit loss under the swap agreement was limited to the interest rate spread in the event of counterparty nonperformance. At December 31, 2009 and 2008, we did not have any swap agreements in effect.
F-25
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
NOTE 9—Profit Interest Units, Share-Based Compensation and Stockholders’ Equity
Profit Interest Units: In November and December of 2008, BP Healthcare Holdings LLC (“BP Holdings”) and Sky LLC, parent entities of the Company affiliated with the Sponsor, granted equity units to the Company’s Chief Executive Officer and the Company’s Chief Financial Officer for purposes of retaining them and enabling such individuals to participate in the long-term growth and financial success of the Company. In addition, in 2009, Sky LLC granted equity units to certain management employees for purposes of retaining them and enabling such individuals to participate in the long-term growth and financial success of the Company. Profit interest units are measured at the grant date, based on the calculated fair value of the award, and are recognized as an expense over the employee’s requisite service provided. These equity awards were issued in exchange for services to be performed.
BP Holdings granted the Company’s Chief Executive Officer 38,697,318 Class B units, all of which are subject to vesting terms based on either (i) continued service to BP Holdings or its subsidiaries and/or (ii) performance/market conditions.
| • | Time-Vesting Units. The portion of the Class B units that vest based on continued service represent 80% of the total Class B units. These units vest ratably over 48 months starting on October 28, 2008 based on continued service, but will become fully vested on an accelerated basis either (x) upon a change in control while the Company’s Chief Executive Officer continues to provide services to BP Holdings or its subsidiaries or (y) if affiliates of the Sponsor receive cash proceeds in respect to 50% of their units in BP Holdings equal to at least 200% of their aggregate capital contributions in respect of such units while the Company’s Chief Executive Officer continues to provide services to BP Holdings or its subsidiaries. In addition, if the Company’s Chief Executive Officer’s services are terminated (a) by the Company without “cause” or (b) by the Chief Executive Officer as a result of “constructive termination,” an additional number of these time-vesting Class B units will vest equal to the number that would have vested over the 24-month period following the applicable termination date. Any of these time-vested Class B units that are unvested on termination of the executive’s services will be forfeited. |
| • | Performance-Vesting Units. The remaining portion of the Class B units that vest based on performance/market conditions represent 20% of the total Class B units. One-half of these units will vest if affiliates of the Sponsor receive cash proceeds equal to at least 200% of their aggregate capital contributions in respect of all of their units in BP Holdings, with the other half eligible to vest if they receive cash proceeds equal to at least 300% of their aggregate capital contributions in respect of all of their units in BP Holdings. Any of these performance-vesting units that are unvested upon a termination of the Company’s Chief Executive Officer’s services (x) by the Company without “cause,” (y) by the executive as a result of “constructive termination” or (z) by the executive for any reason on or following October 28, 2012, will remain outstanding until the second anniversary of the applicable termination date (unless they vest prior to that date). If the units do not vest by such anniversary, then any unvested performance-vesting units shall be immediately forfeited. |
Assumptions used were as follows:
Expected volatility(1) | 23.0 | % | |
Risk free interest rate(2) | 2.24 | % | |
Expected life(3) | 5.0 years |
| (1) | The expected volatility is derived from the volatilities of comparable publicly traded companies. |
| (2) | The risk free interest rate is interpolated from the constant maturity treasury rate (“CMT Rate”) as of the valuation date with the maturity matching the expected life. |
| (3) | The expected life is based on management’s estimates. |
F-26
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
The following table summarizes activity for profit interest units of BP Holdings for the period October 29, 2008 to December 31, 2009:
| Class B Units | Weighted Average Grant Date Fair Value | Weighted Average Remaining Contractual Life | |||||
Balance at October 29, 2008 | — | $ | — | ||||
Granted on November 24, 2008 | 38,697,318 | 0.36 | |||||
Balance at December 31, 2008 | 38,697,318 | ||||||
Granted | — | ||||||
Exercised | — | ||||||
Forfeited | — | ||||||
Balance at December 31, 2009 | 38,697,318 | 3.8 | |||||
Vested units at December 31, 2009 | 7,739,464 | 3.8 | |||||
The fair value of the B units is $13.9 million.
Sky LLC granted the Company’s Chief Financial Officer 500,000 Class A-2 units, 6,675,287 Class B units and 2,225,096 Class C units, all of which are subject to vesting terms based on either (i) continued service to Sky LLC or its subsidiaries or (ii) performance/market conditions.
| • | Class A-2 Units. The Class A-2 units vest if an initial public offering (“IPO”) or change of control occurs and the valuation of Class A-1 units of Sky LLC implied by the transaction exceeds 110% of the aggregate capital contributions of affiliates of the Sponsor for the Class A-1 units. The Company’s Chief Financial Officer does not need to be employed at the time of the IPO or change in control to vest. The Class A-2 Units will be forfeited if an IPO or change of control occurs at a valuation that does not result in vesting. |
| • | Time-Vesting Units. The portion of the Class B units that vest based on continued service represent 66 2/3% of the total Class B units. These units vest ratably over 57 months starting on October 28, 2008 based on continued service, but will become fully vested on an accelerated basis upon a change in control while the Company’s Chief Financial Officer continues to provide services to Sky LLC or its subsidiaries. Any of these time-vested Class B units that are unvested on termination of the executive’s services will be forfeited. |
| • | Performance-Vesting Units. The remaining portion of the Class B units and all of the Class C units vest based on performance/market conditions. These units will vest if affiliates of the Sponsor receive cash proceeds equal to at least 200% of their aggregate capital contributions in respect of 25% of their units in Sky LLC while the Company’s Chief Financial Officer continues to provide services to Sky LLC or its subsidiaries. |
Notwithstanding the vesting terms described above, if the Company’s Chief Financial Officer voluntarily resigns (in the absence of “constructive termination”) before January 28, 2010, then Sky LLC may require the forfeiture of any vested Class B or C units.
F-27
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Assumptions used were as follows:
Expected volatility(1) | 23.0 | % | |
Risk free interest rate(2) | 1.35 | % | |
Estimated life(3) | 5.0 years |
| (1) | The expected volatility is derived from the volatilities of comparable publicly traded companies. |
| (2) | The risk free interest rate is interpolated from the CMT Rate as of the valuation date with the maturity matching the estimated life. |
| (3) | The estimated life is based on management’s estimates. |
The following table summarizes activity for profit interest units of Sky LLC granted to the Company’s Chief Financial Officer for the period October 29, 2008 to December 31, 2009:
| Granted A-2 Units | Weighted Average Grant Date Fair Value | Granted B Units | Weighted Average Grant Date Fair Value | Granted C Units | Weighted Average Grant Date Fair Value | ||||||||||
Balance at October 29, 2008 | — | $ | — | — | $ | — | — | $ | — | ||||||
Granted on December 19, 2008 | 500,000 | 0.75 | 6,675,287 | 0.35 | 2,225,096 | 0.15 | |||||||||
Balance at December 31, 2008 | 500,000 | 6,675,287 | 2,225,096 | ||||||||||||
Exercised | — | — | — | — | — | — | |||||||||
Forfeited | — | — | — | — | — | — | |||||||||
Balance at December 31, 2009 | 500,000 | 6,675,287 | 2,225,096 | ||||||||||||
Vested units at December 31, 2009 | — | 1,112,437 | — | ||||||||||||
The fair value of the A-2 units is $0.4 million, the fair value of the B units is $2.3 million and the fair value of the C units is $0.3 million.
During 2009, Sky LLC granted certain management employees 38,542,529 Class B units and 12,847,509 Class C units, all of which are subject to vesting terms based on either (i) continued service to Sky LLC or its subsidiaries or (ii) performance/market conditions.
| • | Time-Vesting Units. The portion of the Class B units that vest based on continued service represent 66 2/3% of the total Class B units. These units vest ratably over 60 months starting on October 28, 2008 based on continued service, but will become fully vested on an accelerated basis upon a change in control while the employee continues to provide services to Sky LLC or its subsidiaries. Any of these time-vested Class B units that are unvested on termination of the employee’s services will be forfeited. |
| • | Performance-Vesting Units. The remaining portion of the Class B units and all of the Class C units vest based on performance/market conditions. These units will vest if affiliates of the Sponsor receive cash proceeds equal to at least 200% of their aggregate capital contributions in respect of 25% of their units in Sky LLC while the employee continues to provide services to Sky LLC or its subsidiaries. |
Notwithstanding the vesting terms described above, if the employee voluntarily resigns (in the absence of “constructive termination”) then Sky LLC may require the forfeiture of any vested Class B or C units.
F-28
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Assumptions used were as follows:
Expected volatility(1) | 25.0 | % | |
Risk free interest rate(2) | 1.96 | % | |
Estimated life(3) | 5.0 years |
| (1) | The expected volatility is derived from the volatilities of comparable publicly traded companies. |
| (2) | The risk free interest rate is interpolated from the CMT Rate as of the valuation date with the maturity matching the estimated life. |
| (3) | The estimated life is based on management’s estimates. |
The following table summarizes activity for profit interest units of Sky LLC granted to or purchased by certain management employees for the period January 1, 2009 to December 31, 2009:
| Purchased A-2 Units | Weighted Average Grant Date Fair Value | Granted B Units | Weighted Average Grant Date Fair Value | Granted C Units | Weighted Average Grant Date Fair Value | ||||||||||||
Balance at December 31, 2008 | — | $ | — | — | $ | — | — | $ | — | ||||||||
Granted in 2009 | 2,075,000 | 0.59 | 38,542,529 | 0.28 | 12,847,509 | 0.13 | |||||||||||
Forfeited | — | — | (1,262,500 | ) | — | (420,833 | ) | — | |||||||||
Exercised | — | — | — | — | — | — | |||||||||||
Balance at December 31, 2009 | 2,075,000 | 37,280,029 | 12,426,676 | ||||||||||||||
Vested units at December 31, 2009 | — | 4,219,521 | — | ||||||||||||||
The fair value of the A-2 units is $1.2 million, the fair value of the B units is $10.4 million and the fair value of the C units is $1.6 million.
Expense recorded related to profit interest units was $7.7 million and $1.4 million in the year ended December 31, 2009 and the period October 29, 2008 to December 31, 2008, respectively. As of December 31, 2009, total unrecognized profit interest compensation cost related to unvested profit interest units was $9.7 million, which is expected to be expensed over a weighted average period of 3.5 years.
Share-based Compensation: In connection with the Merger on October 28, 2008, all of our then existing share-based awards became fully vested. Each share of common stock was converted into the right to receive $21.00 in cash. The Predecessor recognized approximately $22.3 million of additional compensation expense due to the vesting and payout of all stock-based awards. In total, the Company paid cash of approximately $23.5 million to settle all of the Predecessor’s stock-based awards outstanding at October 28, 2008.
Share-based compensation cost is measured at the grant date, based on the calculated fair value of the award, and is recognized as an expense over the employee’s requisite service period (generally the vesting period of the equity grant).
For the period January 1, 2008 to October 28, 2008, share-based compensation expense was $34.7 million, of which $3.6 million was related to awards issued prior to the adoption of ASC 718Compensation – Stock Compensation. For the year ended December 31, 2007, share-based compensation expense was $11.2 million. All such compensation is reflected in the accompanying condensed consolidated income statement within the selling, distribution and administrative expense line item. The related awards were granted to administrative
F-29
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
personnel or members of the Board of Directors and therefore no portion of the share-based compensation has been classified within cost of total net revenues.
For the period January 1, 2008 to October 28, 2008, cash received from the exercise of options totaled $0.4 million. Income tax expense related to stock-based compensation arrangements amounted to $7.2 million.
Estimates of the fair value of stock options are determined using the Black-Scholes valuation model. Key input assumptions used to estimate the fair value of stock options include the exercise price of the award, the expected option term, the expected volatility of the Company’s stock over the option’s expected term, the risk-free interest rate over the option’s term, and the expected annual dividend yield. Management believes that the valuation technique and the approach utilized to develop the underlying assumptions are appropriate in calculating the fair values of stock options granted in 2008. Estimates of fair value are not intended to predict actual future events or the value ultimately realized by persons who receive equity awards.
The key input assumptions that were utilized in the valuation of the stock options granted are summarized in the table below.
| Period January 1, 2008 to October 28, 2008 | Year Ended December 31, 2007 | |||||
Expected option term in years(1) | 6.2 | 4.6 | ||||
Expected volatility(2) | 37.3 | % | 29.9 | % | ||
Risk-free interest rate(3) | 3.1 | % | 4.5 | % | ||
Expected annual dividend yield | 0 | % | 0 | % |
| (1) | The expected option term is based on historical exercise and post-vesting termination patterns. |
| (2) | Expected volatility represents a combination of historical stock price volatility and implied volatility from publicly-traded options on Apria’s common stock. |
| (3) | The risk-free interest rate is based on the implied yield on a U.S. Treasury zero coupon issue with a remaining term equal to the expected term of the option. As a result of the Merger, profit interest units were granted to certain members of management as a new form of incentive compensation. |
2003 Performance Incentive Plan: In July 2003, stockholders approved the 2003 Performance Incentive Plan (“2003 Plan”), which permitted the grant of stock options, stock appreciation rights (“SARs”), stock bonuses, restricted stock, performance stock, stock units, phantom stock, dividend equivalents, or similar rights to purchase or acquire shares, and cash awards. The 2003 Plan was terminated at the Merger date in October 2008.
Stock Options: The 2003 Plan provided for the granting of stock options to employees and non-employee directors. Such grants to employees included non-qualified and incentive stock options. The exercise price of an option was established at the fair market value of a share of common stock on the date of grant. Vesting of stock options was time-based, generally over a three-year period. All stock options were terminated at the Merger date in October 2008.
F-30
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
The following table summarizes the activity for stock options for the year ended December 31, 2007 and the period January 1, 2008 to October 28, 2008:
| Options | Weighted Average Exercise Price | |||||
Outstanding at January 1, 2007 | 4,056,314 | $ | 24.59 | |||
Granted | 759,830 | 30.84 | ||||
Exercised | (863,693 | ) | 20.23 | |||
Forfeited | (219,068 | ) | 29.26 | |||
Outstanding at December 31, 2007 | 3,733,383 | 26.60 | ||||
Granted | 15,000 | 21.02 | ||||
Exercised | (273,249 | ) | 15.50 | |||
Forfeited | (3,475,134 | ) | 27.44 | |||
Outstanding at October 28, 2008 | — | — | ||||
The weighted-average fair value of stock options granted during the period ended January 1, 2008 to October 28, 2008, and the year ended December 31, 2007 were $6.55 and $10.20, respectively. There were 759,830 stock options granted in the year 2007. The total intrinsic value of options exercised was $1.2 million and $9.6 million for the period January 1, 2008 to October 28, 2008 and the year ended December 31, 2007, respectively.
Restricted Stock Purchase Rights: In 2003 and 2004, restricted stock purchase rights were granted under the 2003 Plan to certain members of executive management. The awards represented the right to purchase a certain number of shares of common stock at a future date at a specified exercise price. The exercise price was established at 25% of the fair market value of a share of common stock on the date of grant. Such awards generally required that certain performance conditions and service conditions be met before the awards would vest. All restricted stock purchase rights were terminated at the Merger date in October 2008.
The following table summarizes the activity for restricted stock purchase rights for the year ended December 31, 2007 and the period January 1, 2008 to October 28, 2008:
| Restricted Stock Purchase Rights | Weighted Average Exercise Price | |||||
Outstanding at January 1, 2007 | 300,000 | $ | 6.79 | |||
Granted | — | — | ||||
Exercised | (23,000 | ) | 6.46 | |||
Forfeited | — | — | ||||
Outstanding at December 31, 2007 | 277,000 | 6.82 | ||||
Granted | — | — | ||||
Exercised | (277,000 | ) | 6.82 | |||
Forfeited | — | — | ||||
Outstanding at October 28, 2008 | — | — | ||||
The total intrinsic value of restricted stock purchase rights exercised was $3.9 million and $0.5 million for the period ended January 1, 2008 to October 28, 2008 and the year ended December 31, 2007, respectively. No such awards were granted during these three periods.
F-31
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Stock Appreciation Rights: On February 29, 2008, Apria granted stock appreciation rights to certain members of executive management under the 2003 Performance Incentive Plan. The awards represented the right to receive a payment in stock, equal to the excess of the fair market value of a specified number of shares of Apria common stock on the date the stock appreciation right was exercised over the fair market value of a share of Apria common stock on the date the stock appreciation right was granted (the “base price”). Generally, the base price would not be less than the per share fair market value on the date of grant. Vesting of stock appreciation rights was time-based over a four-year period. All stock appreciation rights were terminated at the Merger date in October 2008.
The following table summarizes the activity for stock appreciation rights for the period January 1, 2008 to October 28, 2008:
| Stock Appreciation Rights | Weighted-Average Exercise Price | |||||
Outstanding at January 1, 2008 | — | $ | — | |||
Granted | 773,850 | 20.51 | ||||
Exercised | (305,960 | ) | 18.67 | |||
Forfeited | (467,890 | ) | 21.71 | |||
Outstanding at October 28, 2008 | — | — | ||||
The weighted-average fair value of stock appreciation rights granted during the period January 1, 2008 to October 28, 2008 was $8.59. There were no stock appreciation rights granted in the prior period. The total intrinsic value of stock appreciation rights exercised was $0.7 million for the period January 1, 2008 to October 28, 2008.
Restricted Stock Awards and Units: The 2003 Plan provided for the granting of restricted stock and restricted stock units to its non-employee directors and employees. Such awards generally required that certain performance conditions and service conditions be met before the awards would vest. All restricted stock awards and units were terminated at the Merger date in October 2008.
The following table summarizes the activity for restricted stock awards and units for the year ended December 31, 2007 and the period January 1, 2008 to October 28, 2008:
| Shares or Share Units | Weighted-Average Grant-Date Fair Value | |||||
Nonvested restricted stock awards and units at January 1, 2007 | 486,922 | $ | 27.16 | |||
Granted | 405,310 | 29.82 | ||||
Vested and released | (186,901 | ) | 24.09 | |||
Forfeited | (64,460 | ) | 27.32 | |||
Nonvested restricted stock awards and units at December 31, 2007 | 640,871 | 29.73 | ||||
Granted | 423,890 | 21.35 | ||||
Vested and released | (1,045,070 | ) | 26.41 | |||
Forfeited | (19,691 | ) | 25.69 | |||
Nonvested restricted stock awards and units at October 28, 2008 | — | |||||
F-32
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
The total intrinsic value of restricted stock awards or units released was $22.0 million and $5.1 million for the period January 1, 2008 to October 28, 2008, and the year ended December 31, 2007, respectively.
The following activity occurred under the 2003 Plan:
| Period January 1, 2008 to October 28, 2008 | Year Ended December 31, 2007 | |||||
| (in thousands) | ||||||
Total fair value of stock awards vested | $ | 21,999 | $ | 5,081 | ||
Treasury Stock: All repurchased shares of common stock were held as treasury shares. All treasury shares were retired as part of the Merger on October 28, 2008.
In 2007, 68,863 shares of employee restricted stock, valued at $1.8 million, were retained upon vesting to satisfy the related tax obligations and 8,689 shares of employee restricted stock purchase rights valued at $0.3 million, were retained upon vesting to satisfy the related exercise price and tax obligations.
NOTE 10—Income Taxes
Income tax (benefit) expense consists of the following:
| Year Ended December 31, 2009 | Period October 29, 2008 to December 31, 2008 | Period January 1, 2008 to October 28, 2008 | Year Ended December 31, 2007 | ||||||||||||||
| (Successor) | (Successor) | (Predecessor) | (Predecessor) | ||||||||||||||
| (in thousands) | |||||||||||||||||
Current | |||||||||||||||||
Federal | $ | (5,912 | ) | $ | (7,826 | ) | $ | (9,561 | ) | $ | 28,710 | ||||||
State | 168 | 180 | 2,468 | 3,954 | |||||||||||||
| (5,744 | ) | (7,646 | ) | (7,093 | ) | 32,664 | |||||||||||
Deferred | |||||||||||||||||
Federal | (1,575 | ) | 8,318 | 39,682 | 16,388 | ||||||||||||
State | (1,119 | ) | (13 | ) | 1,603 | 2,946 | |||||||||||
| (2,694 | ) | 8,305 | 41,285 | 19,334 | |||||||||||||
| $ | (8,438 | ) | $ | 659 | $ | 34,192 | $ | 51,998 | |||||||||
The current tax expense for the year ended December 31, 2009 includes a net tax benefit of $5.7 million relating to the release of accruals for tax uncertainties. The current tax expense for the period of October 29, 2008 to December 31, 2008 includes a net tax expense of $0.2 million relating to tax uncertainty accruals. Income tax benefits of $1.3 million and $1.4 million relating to tax uncertainty accruals are included in the current tax expense for the period of January 1, 2008 to October 29, 2008 and for the year ended December 31, 2007, respectively.
Tax shortfalls from share-based payments of $0 and $7.2 million were debited to additional paid-in capital for the periods of October 29, 2008 to December 31, 2008 and January 1, 2008 to October 29, 2008, respectively. Excess tax benefits from share-based payments of $4.0 million were credited to additional paid-in capital for the year ended December 31, 2007. See Consolidated Statements of Stockholders’ Equity.
F-33
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
A reconciliation of the differences between income tax expense and an amount calculated utilizing the federal statutory rate is as follows:
| Year Ended December 31, 2009 | Period October 29, 2008 to December 31, 2008 | Period January 1, 2008 to October 28, 2008 | Year Ended December 31, 2007 | |||||||||||||||
| (Successor) | (Successor) | (Predecessor) | (Predecessor) | |||||||||||||||
| (in thousands) | ||||||||||||||||||
Income tax expense at statutory rate | $ | (4,290 | ) | $ | (432 | ) | $ | 31,726 | $ | 48,294 | ||||||||
Non-deductible expenses | 778 | 207 | 1,345 | 533 | ||||||||||||||
State taxes, net of federal benefit and state loss carryforwards | 3,342 | 95 | 2,205 | 6,033 | ||||||||||||||
Share-based compensation | 2,679 | 483 | 650 | 469 | ||||||||||||||
Change in valuation allowance | (4,646 | ) | 32 | 237 | (336 | ) | ||||||||||||
Change in liability for unrecognized tax benefits | (5,660 | ) | 243 | (1,339 | ) | (1,405 | ) | |||||||||||
Other | (641 | ) | 31 | (632 | ) | (1,590 | ) | |||||||||||
| $ | (8,438 | ) | $ | 659 | $ | 34,192 | $ | 51,998 | ||||||||||
Significant components of deferred tax assets and liabilities are as follows:
| December 31, | ||||||||
| 2009 | 2008 | |||||||
| (in thousands) | ||||||||
Deferred tax assets: | ||||||||
Allowance for doubtful accounts | $ | 21,521 | $ | 18,081 | ||||
Overpayment reserve | 4,880 | — | ||||||
Accruals | 16,938 | 17,152 | ||||||
Accrued vacation | 7,320 | 9,937 | ||||||
Asset valuation reserves | 2,159 | 5,501 | ||||||
Net operating loss carryforward and tax credits | 65,663 | 43,352 | ||||||
Intangible assets | 7,859 | 8,973 | ||||||
Tax benefits related to unrecognized state tax benefits and interest accrued | 3,097 | 4,957 | ||||||
Other, net | 9,290 | 12,125 | ||||||
| 138,727 | 120,078 | |||||||
Less: valuation allowance | (4,373 | ) | (10,702 | ) | ||||
Total deferred tax assets | 134,354 | 109,376 | ||||||
Deferred tax liabilities: | ||||||||
Tax over book depreciation | (32,983 | ) | (22,291 | ) | ||||
Tax over book goodwill amortization | (21,135 | ) | (3,093 | ) | ||||
Separately identifiable intangibles | (220,110 | ) | (149,084 | ) | ||||
Contingent debt interest | — | — | ||||||
Subsidiary basis difference | (14,407 | ) | — | |||||
Deferred expenses | (1,319 | ) | (1,372 | ) | ||||
Debt issuance costs and related amounts | (14,275 | ) | — | |||||
Other, net | (2,139 | ) | (2,799 | ) | ||||
Total deferred tax liabilities | (306,368 | ) | (178,639 | ) | ||||
Net deferred tax liabilities | $ | (172,014 | ) | $ | (69,263 | ) | ||
F-34
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Deferred income taxes are recognized for the difference between the carrying amounts of assets and liabilities for financial statement and tax purposes. Deferred income tax assets are required to be reduced by a valuation allowance when it is determined that it is more likely than not that all or a portion of a deferred tax asset will not be realized. During 2009, the valuation allowance was decreased by $6.3 million based on the Company’s projection of future taxable income with respect to certain separate state tax filings made by certain subsidiaries of the Company.
As of December 31, 2009, federal NOLs of approximately $138.4 million are available to offset future federal taxable income. Such NOLs will expire at various times and in varying amounts during our calendar 2010 through 2029 tax years. A significant portion of these NOLs are subject to an annual utilization limitation as required by Section 382 of the Internal Revenue Code of 1986, as amended.
During 2009, the Company reclassified $30.8 million of federal NOLs from a non-current asset to a current asset on the consolidated balance sheet. The reclassification was based on the amount of federal NOL the Company expects to utilize in calendar 2010.
The Company’s non-current deferred tax liabilities increased $131.3 million to $259.0 million at December 31, 2009 from $127.7 million at December 31, 2008. The $131.3 million increase was primarily due to adjustments resulting from the finalization of the Company’s intangible asset valuation related to the merger that resulted in adjustments to the value of capitated relationships, patient backlogs, and trade names.
A reconciliation of the beginning and ending balances of the gross liability for unrecognized tax benefits at December 31, 2009, 2008 and 2007 is as follows:
| 2009 | 2008 | 2007 | ||||||||||
| (in thousands) | ||||||||||||
Balance included in Income Taxes Payable and Other Non-Current Liabilities at January 1 | $ | 26,895 | $ | 27,000 | $ | 17,687 | ||||||
Balance included in Deferred Income Taxes at January 1 | 84,644 | 88,960 | 10,029 | |||||||||
Total gross unrecognized tax benefits at January 1 | 111,539 | 115,960 | 27,716 | |||||||||
Additions for tax positions related to the current year | 1,238 | 2,461 | 3,630 | |||||||||
Additions for tax positions related to prior years | 3,763 | 12,477 | 6,661 | |||||||||
Additions due to acquisitions during the year | — | — | 83,203 | |||||||||
Reductions for tax positions related to prior years | (13,557 | ) | (14,214 | ) | (941 | ) | ||||||
Settlements | (424 | ) | (1,158 | ) | (1,231 | ) | ||||||
Reductions due to lapse in statute of limitations | (2,137 | ) | (3,956 | ) | (3,078 | ) | ||||||
Other | — | (31 | ) | — | ||||||||
Total gross unrecognized tax benefits at December 31 | $ | 100,422 | $ | 111,539 | $ | 115,960 | ||||||
Total gross unrecognized tax benefits of $100.4 million is reflected on the Company’s December 31, 2009 balance sheet as follows: (a) $20.3 million included in Income Taxes Payable and Other Non-Current Liabilities and (b) $80.2 million included in Deferred Income Taxes.
Total gross unrecognized tax benefits of $111.5 million is reflected on the Company’s December 31, 2008 balance sheet as follows: (a) $26.9 million included in Income Taxes Payable and Other Non-Current Liabilities and (b) $84.6 million included in Deferred Income Taxes.
F-35
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
As of December 31, 2009, the amount of unrecognized tax benefits which, if ultimately recognized, could affect the effective tax rate in a future period is $87.2 million. The $87.2 million amount is net of federal and/or state tax benefits and is inclusive of $2.2 million of penalties and interest (net of tax benefit).
As of December 31, 2009, the Company does not expect material increases or decreases to its unrecognized tax benefits for the 12-month rolling period ending December 31, 2010.
Interest expense and penalties related to unrecognized tax benefits are recognized as part of the provision for income taxes. Gross interest and penalties of $3.3 million and $6.4 million are provided for within the liability for unrecognized tax benefits as December 31, 2009 and December 31, 2008, respectively.
The Company files federal and state income tax returns in jurisdictions with varying statutes of limitations expiration dates. The calendar 2006 through 2009 tax years generally remains subject to examination by tax authorities. Certain state tax agencies are currently examining the tax years 2002 and forward.
Net income taxes paid in 2009 amounted to $0.6 million. Net income taxes paid in the period of October 29, 2008 through December 31, 2008 amounted to $0.1 million. Net income tax refunds for the period of January 1, 2008 through October 28, 2008 amounted to $16.5 million. Net income taxes paid during 2007 amounted to $26.9 million.
NOTE 11—Leases
The Company leases all of its facilities. Lease terms are generally ten years or less with renewal options for additional periods. The occasionally unused facility space is subleased when a lease buyout is not a viable option. Sublease income is recognized monthly and is offset against facility lease expense. Sublease income in the year ended December 31, 2009, the periods October 29, 2008 to December 31, 2008, January 1, 2008 to October 28, 2008, and the year ended December 31, 2007, amounted to $0.9 million, $0, $0.1 million and $0.8 million, respectively. In addition, delivery vehicles and office equipment are leased under operating leases. Many leases provide that taxes, maintenance, insurance and other expenses are the responsibility of the Company. Rentals are generally increased annually by the Consumer Price Index, subject to certain maximum amounts defined within individual agreements. Net rent expense in the year ended December 31, 2009, the period October 29, 2008 to December 31, 2008, the period January 1, 2008 to October 28, 2008, and the year ended December 31, 2007 amounted to $82.1 million, $13.8 million, $68.9 million and $76.2 million, respectively.
For the year ended December 31, 2009, infusion pumps totaling $0.3 million were acquired under a capital lease arrangement, with a lease term of 60 months. During the period January 1, 2008 to October 28, 2008, infusion pumps totaling $1.1 million were acquired under a capital lease arrangement, with a lease term of 60 months. In December 2007, infusion pumps and vehicles were acquired, as a result of the acquisition of Coram, totaling $6.9 million under capital lease arrangements with lease terms ranging from 36 to 60 months. Related amortization amounted to $2.0 million, $0.3 million, $1.9 million and $0.2 million for the year ended December 31, 2009, the periods October 29, 2008 to December 31, 2008, January 1, 2008 to October 28, 2008 and the one-month period in 2007, respectively.
F-36
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
The following amounts for assets under capital lease obligations are included in property, equipment and improvements:
| December 31, | ||||||||
| 2009 | 2008 | |||||||
| (in thousands) | ||||||||
Infusion pumps | $ | 5,901 | $ | 5,821 | ||||
Vehicles | 428 | 443 | ||||||
Less accumulated depreciation | (2,362 | ) | (344 | ) | ||||
| $ | 3,967 | $ | 5,920 | |||||
Future minimum payments, by year and in the aggregate, required under capital lease obligations and noncancelable operating leases consist of the following at December 31, 2009:
| Capital Leases | Operating Leases | ||||||
| (in thousands) | |||||||
2010 | $ | 1,765 | $ | 61,694 | |||
2011 | 1,430 | 52,180 | |||||
2012 | 368 | 41,175 | |||||
2013 | 247 | 21,882 | |||||
2014 | 15 | 9,312 | |||||
Thereafter | — | 10,568 | |||||
| 3,825 | $ | 196,811 | |||||
Less interest included in minimum lease payments | (256 | ) | |||||
Present value of minimum lease payments | 3,569 | ||||||
Less current portion | (1,613 | ) | |||||
| $ | 1,956 | ||||||
NOTE 12—Employee Benefit Plans
401(k) Savings Plan: The Company has a 401(k) defined contribution plan, whereby eligible employees may contribute up to 35% of their annual base earnings. The Company matches 25% of the first 8% of employee contributions. Total expenses related to the defined contribution plan were $3.2 million, $0.5 million, $2.5 million and $2.0 million in the year ended December 31, 2009, the period October 29, 2008 to December 31, 2008, the period January 1, 2008 to October 28, 2008, and the year ended December 31, 2007, respectively.
Deferred Compensation Plan: A non-qualified deferred compensation plan is available for approximately 235 employees. The plan provides participants with the advantages of pre-tax contributions and tax deferred compounding of interest. Plan assets, which represent the fair market value of the investments, were $4.9 million and $4.6 million, and plan liabilities were $2.8 million and $3.8 million at December 31, 2009 and 2008, respectively.
NOTE 13—Commitments and Contingencies
Litigation: During 2009, the Company was the defendant in a purported California class action lawsuit asserting blanket claims of liability under various California employee protection statutes and regulations relating to payment of regular and overtime wages(Venegas v. Apria Healthcare,Inc. Case No. CHC 06 449669). In July
F-37
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
2009, the parties agreed on a settlement of this case which was finally approved by means of a final order and judgment entered by the Court on February 8, 2010. The case is now fully resolved and the Company has paid a total of $3.0 million pursuant to the settlement.
The Company is also engaged in the defense of certain claims and lawsuits arising out of the ordinary course and conduct of its business, the outcomes of which are not determinable at this time. Insurance policies covering such potential losses, where such coverage is cost effective, are maintained. In the opinion of management, any liability that might be incurred upon the resolution of these claims and lawsuits will not, in the aggregate, have a material adverse effect on the Company’s financial condition or results of operations.
Medicare and Medicaid Reimbursement: There are a number of provisions contained within recent legislation or proposed legislation that affect or may affect Medicare and Medicaid reimbursement polices for items and services provided. In addition, recent bills introduced in Congress to finance national healthcare reform could change the Company’s tax rates in the future. The Company cannot be certain of the ultimate impact of all legislated and contemplated changes, and therefore, cannot provide assurance that these changes will not have a material adverse effect on the Company’s financial condition or results of operations.
Supplier Concentration: Currently, approximately 58.2% of purchases for patient service equipment and supplies are from five vendors. Although there are a limited number of suppliers, management believes that other vendors could provide similar products on comparable terms. However, a change in suppliers could cause delays in service delivery and possible losses in revenue, which could adversely affect the Company’s financial condition or results of operations.
Guarantees and Indemnities: From time to time, certain types of contracts are entered into that contingently require indemnification of parties against third party claims. These contracts primarily relate to (i) certain asset purchase agreements, under which indemnification may be provided to the seller of the business being acquired; (ii) certain real estate leases, which may require indemnification to property owners for environmental or other liabilities and other claims arising from use of the applicable premises; and (iii) certain agreements with officers, directors and employees, which may require indemnification of such persons for liabilities arising out of their relationship with the Company.
The terms of such obligations vary by contract and in most instances a specific or maximum dollar amount is not explicitly stated therein. Generally, amounts under these contracts cannot be reasonably estimated until a specific claim is asserted. Consequently, no liabilities have been recorded for these obligations on the balance sheet for any of the periods presented.
NOTE 14—Certain Relationships and Related Party Transactions
Transaction and Management Fee Agreement: In connection with the Merger, Merger Sub entered into a transaction and management fee agreement with Blackstone Management Partners V L.L.C. (“BMP”). The Company succeeded to and assumed the rights and obligations of Merger Sub pursuant to the transaction and management fee agreement upon the closing of the Merger. Under the transaction and management fee agreement, Merger Sub agreed to pay BMP undertaking financial and structural analysis, due diligence and other assistance in connection with the Merger. In addition the Company agreed to reimburse BMP for any out-of-pocket expenses incurred by BMP and its affiliates in connection with the Merger and the provision of services under the transaction and management fee agreement.
In addition, under this agreement, BMP (including through its affiliates) agree to provide services, including without limitation, (a) advice regarding the structure, distribution and timing of debt and equity offerings and
F-38
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
advice regarding relationships with the Company’s lenders and bankers, (b) advice regarding the business and strategy of the Company, including compensation arrangements, (c) advice regarding dispositions and/or acquisitions and (d) such advice directly related or ancillary to the above financial advisory services as may be reasonably requested by the Company. In consideration for the services, Merger Sub will pay BMP at the beginning of each fiscal year a management fee equal to the greater of $7.0 million or 2.0% of the Company’s consolidated EBITDA for the immediately preceding fiscal year. BMP shall have no obligation to provide any other services to the Company absent express agreement. In addition, in the absence of an express agreement to provide investment banking or other financial advisory services to the Company, and without regard to whether such services were provided, BMP is entitled to receive a fee equal to 1.0% of the aggregate transaction value upon the consummation of any acquisition, divestiture, disposition, merger, consolidation, restructuring, refinancing, recapitalization, issuance of private or public debt of equity securities (including an initial public offering of equity securities), financing or similar transaction by the Company.
At any time in connection with or in anticipation of a change of control of the Company, a sale of all or substantially all of the Company’s assets or an initial public offering of common equity of the Company or its successor, BMP may elect to receive, in consideration of BMP’s role in facilitating such transaction and in settlement of the termination of the services, a single lump sum cash payment equal to the then-present value of all then-current and future annual management fees payable under the transaction and management fee agreement, assuming a hypothetical termination date of the agreement to be the twelfth anniversary of such election. The transaction and management fee agreement will continue until the earlier of the twelfth anniversary of the date of the agreement or such date as the Company and BMP may mutually determine. The Company has agreed to indemnify BMP and its affiliates, directors, officers, employees, agents and representatives from and against all liabilities relating to the services contemplated by the transaction and management fee agreement and the engagement of BMP pursuant to, and the performance of BMP and its affiliates of the services contemplated by, the transaction and management fee agreement.
Intelenet Agreement: In May 2009, we entered into the Intelenet Agreement with Intelenet, an Indian company affiliated with the Sponsor, regarding the outsourcing of certain functions relating to billing, collections and other administrative and clerical services. A portion of such services to be outsourced were transitioned to Intelenet during 2009. A portion of such services to be outsourced have been transitioned to Intelenet and additional services are expected to be transitioned over the next 18 months. Only certain services are currently subject to the Intelenet Agreement. However, if all services currently planned for outsourcing to Intelenet are in fact handled by Intelenet, we expect to make payments to Intelenet of approximately $200.0 million over a seven-year period that began in the second quarter of 2009. One of the members of our Board of Directors, Mr. Patrick Bourke, is an employee of the Sponsor and also services on the Board of Directors of Intelenet. During the year ended December 31, 2009, the Company paid approximately $3.7 million to Intelenet.
NOTE 15—Financial Information for Guarantor Subsidiaries and Non-Guarantor Subsidiaries
The Company conducts substantially all of its business through its subsidiaries. Substantially all of the Company’s wholly-owned domestic subsidiaries (the “Guarantors”) fully and unconditionally guarantee the Series A-1 Notes and Series A-2 Notes on a senior secured basis. The Guarantors also guarantee our ABL Facility.
See alsoNote 8—Long-Term Debt.
The following condensed consolidated financial statements quantify the financial position as of December 31, 2009 and December 31, 2008 and the operations and cash flows for the year ended December 31, 2009, the period October 29, 2008 to December 31, 2008, the period January 1, 2008 to October 28, 2008 and the year ended December 31, 2007 present financial information for the parent Company issuer, the guarantor subsidiaries, the non-guarantor subsidiaries and consolidating adjustments, consisting of the entries that eliminate the investment in subsidiaries and intercompany balances and transactions.
F-39
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
CONDENSED CONSOLIDATED BALANCE SHEETS
December 31, 2009
(Successor)
| Parent Issuer | Guarantor Subsidiaries | Non- Guarantor Subsidiaries | Consolidating Adjustments | Condensed Consolidated | |||||||||||||||
| (in thousands) | |||||||||||||||||||
ASSETS | |||||||||||||||||||
CURRENT ASSETS | |||||||||||||||||||
Cash and cash equivalents | $ | 150,364 | $ | 7,208 | $ | 591 | $ | — | $ | 158,163 | |||||||||
Short-term investments | 23,673 | — | — | — | 23,673 | ||||||||||||||
Accounts receivable less allowance for doubtful accounts | 7 | 251,149 | 977 | — | 252,133 | ||||||||||||||
Inventories, net | — | 68,007 | 260 | — | 68,267 | ||||||||||||||
Deferred income taxes | 17,497 | 69,519 | — | — | 87,016 | ||||||||||||||
Deferred expenses | — | 3,049 | — | — | 3,049 | ||||||||||||||
Intercompany | 606,473 | 381,071 | — | (987,544 | ) | — | |||||||||||||
Prepaid expenses and other current assets | 5,213 | 19,149 | — | — | 24,362 | ||||||||||||||
TOTAL CURRENT ASSETS | 803,227 | 799,152 | 1,828 | (987,544 | ) | 616,663 | |||||||||||||
PATIENT SERVICE EQUIPMENT, less accumulated depreciation | — | 198,770 | 38 | — | 198,808 | ||||||||||||||
PROPERTY, EQUIPMENT AND IMPROVEMENTS, NET | 28,393 | 51,805 | 29 | — | 80,227 | ||||||||||||||
GOODWILL | — | 759,197 | — | — | 759,197 | ||||||||||||||
INTANGIBLE ASSETS, NET | 460,000 | 122,259 | — | — | 582,259 | ||||||||||||||
DEFERRED DEBT ISSUANCE COSTS, NET | 63,110 | — | — | — | 63,110 | ||||||||||||||
INTERCOMPANY RECEIVABLE | 350,000 | 2,902 | — | (352,902 | ) | — | |||||||||||||
INVESTMENT IN SUBSIDIARIES | 380,455 | 538 | — | (380,993 | ) | — | |||||||||||||
OTHER ASSETS | 4,911 | 3,872 | — | — | 8,783 | ||||||||||||||
| $ | 2,090,096 | $ | 1,938,495 | $ | 1,895 | $ | (1,721,439 | ) | $ | 2,309,047 | |||||||||
LIABILITIES AND STOCKHOLDERS’ EQUITY | |||||||||||||||||||
CURRENT LIABILITIES | |||||||||||||||||||
Accounts payable | $ | — | $ | 123,597 | $ | 259 | $ | — | $ | 123,856 | |||||||||
Accrued payroll and related taxes and benefits | 20,893 | 45,843 | 106 | — | 66,842 | ||||||||||||||
Other accrued liabilities | 20,799 | 78,977 | 992 | — | 100,768 | ||||||||||||||
Deferred revenue | — | 27,253 | — | — | 27,253 | ||||||||||||||
Intercompany | 157,140 | 470,404 | — | (627,544 | ) | — | |||||||||||||
Current portion of long-term debt | 77 | 361,613 | — | (360,000 | ) | 1,690 | |||||||||||||
TOTAL CURRENT LIABILITIES | 198,909 | 1,107,687 | 1,357 | (987,544 | ) | 320,409 | |||||||||||||
LONG-TERM DEBT, net of current portion | 1,020,402 | 351,956 | — | (352,902 | ) | 1,019,456 | |||||||||||||
DEFERRED INCOME TAXES | 183,105 | 75,925 | — | — | 259,030 | ||||||||||||||
INCOME TAXES PAYABLE AND OTHER NON-CURRENT LIABILITIES | 8,949 | 22,472 | — | — | 31,421 | ||||||||||||||
TOTAL LIABILITIES | 1,411,365 | 1,558,040 | 1,357 | (1,340,446 | ) | 1,630,316 | |||||||||||||
STOCKHOLDERS’ EQUITY | |||||||||||||||||||
Common stock | — | 1 | — | (1 | ) | — | |||||||||||||
Additional paid-in capital | 684,445 | 396,766 | — | (396,766 | ) | 684,445 | |||||||||||||
(Accumulated deficit) retained earnings | (5,714 | ) | (16,312 | ) | 538 | 15,774 | (5,714 | ) | |||||||||||
TOTAL STOCKHOLDERS’ EQUITY | 678,731 | 380,455 | 538 | (380,993 | ) | 678,731 | |||||||||||||
| $ | 2,090,096 | $ | 1,938,495 | $ | 1,895 | $ | (1,721,439 | ) | $ | 2,309,047 | |||||||||
F-40
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
CONDENSED CONSOLIDATED BALANCE SHEETS
December 31, 2008
(Successor)
| Parent Issuer | Guarantor Subsidiaries | Non- Guarantor Subsidiaries | Consolidating Adjustments | Condensed Consolidated | |||||||||||||||
| (in thousands) | |||||||||||||||||||
ASSETS | |||||||||||||||||||
CURRENT ASSETS | |||||||||||||||||||
Cash and cash equivalents | $ | 159,393 | $ | 8,260 | $ | 365 | $ | — | $ | 168,018 | |||||||||
Accounts receivable less allowance for doubtful accounts | 59 | 272,144 | 1,002 | — | 273,205 | ||||||||||||||
Inventories, net | — | 59,061 | 158 | — | 59,219 | ||||||||||||||
Deferred income taxes | 7,156 | 51,288 | — | — | 58,444 | ||||||||||||||
Deferred expenses | — | 3,181 | — | — | 3,181 | ||||||||||||||
Intercompany | 761,639 | 255,928 | — | (1,017,567 | ) | — | |||||||||||||
Prepaid expenses and other current assets | 1,240 | 16,891 | — | — | 18,131 | ||||||||||||||
TOTAL CURRENT ASSETS | 929,487 | 666,753 | 1,525 | (1,017,567 | ) | 580,198 | |||||||||||||
PATIENT SERVICE EQUIPMENT, less accumulated depreciation | — | 209,683 | 57 | — | 209,740 | ||||||||||||||
PROPERTY, EQUIPMENT AND IMPROVEMENTS, NET | 12,884 | 54,805 | 66 | — | 67,755 | ||||||||||||||
GOODWILL | — | 895,069 | — | — | 895,069 | ||||||||||||||
INTANGIBLE ASSETS, NET | 235,000 | 162,334 | — | — | 397,334 | ||||||||||||||
DEFERRED DEBT ISSUANCE COSTS, NET | 49,648 | — | — | — | 49,648 | ||||||||||||||
INTERCOMPANY RECEIVABLE | 350,000 | 2,939 | — | (352,939 | ) | — | |||||||||||||
INVESTMENT IN SUBSIDIARIES | 395,474 | 666 | — | (396,140 | ) | — | |||||||||||||
OTHER ASSETS | 4,612 | 6,457 | — | — | 11,069 | ||||||||||||||
| $ | 1,977,105 | $ | 1,998,706 | $ | 1,648 | $ | (1,766,646 | ) | $ | 2,210,813 | |||||||||
LIABILITIES AND STOCKHOLDERS’ EQUITY | |||||||||||||||||||
CURRENT LIABILITIES | |||||||||||||||||||
Accounts payable | $ | — | $ | 141,215 | $ | 167 | $ | — | $ | 141,382 | |||||||||
Accrued payroll and related taxes and benefits | 10,063 | 64,060 | 115 | — | 74,238 | ||||||||||||||
Other accrued liabilities | 22,203 | 78,086 | 700 | — | 100,989 | ||||||||||||||
Deferred revenue | — | 30,515 | — | — | 30,515 | ||||||||||||||
Intercompany | 153,377 | 504,190 | — | (657,567 | ) | — | |||||||||||||
Current portion of long-term debt | 6,000 | 362,750 | — | (360,000 | ) | 8,750 | |||||||||||||
TOTAL CURRENT LIABILITIES | 191,643 | 1,180,816 | 982 | (1,017,567 | ) | 355,874 | |||||||||||||
LONG-TERM DEBT, net of current portion | 1,013,016 | 353,406 | — | (352,939 | ) | 1,013,483 | |||||||||||||
DEFERRED INCOME TAXES | 81,307 | 46,400 | — | — | 127,707 | ||||||||||||||
INCOME TAXES PAYABLE AND OTHER NON-CURRENT LIABILITIES | 18,319 | 22,610 | — | — | 40,929 | ||||||||||||||
TOTAL LIABILITIES | 1,304,285 | 1,603,232 | 982 | (1,370,506 | ) | 1,537,993 | |||||||||||||
STOCKHOLDERS’ EQUITY | |||||||||||||||||||
Common stock | — | 1 | — | (1 | ) | — | |||||||||||||
Additional paid-in capital | 674,714 | 396,766 | — | (396,766 | ) | 674,714 | |||||||||||||
(Accumulated deficit) retained earnings | (1,894 | ) | (1,293 | ) | 666 | 627 | (1,894 | ) | |||||||||||
TOTAL STOCKHOLDERS’ EQUITY | 672,820 | 395,474 | 666 | (396,140 | ) | 672,820 | |||||||||||||
| $ | 1,977,105 | $ | 1,998,706 | $ | 1,648 | $ | (1,766,646 | ) | $ | 2,210,813 | |||||||||
F-41
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
Year Ended December 31, 2009
(Successor)
| Parent Issuer | Guarantor Subsidiaries | Non- Guarantor Subsidiaries | Consolidating Adjustments | Condensed Consolidated | ||||||||||||||
| (in thousands) | ||||||||||||||||||
Operating net revenues | $ | — | $ | 2,087,326 | $ | 7,235 | $ | — | $ | 2,094,561 | ||||||||
Net revenues from subsidiaries | 220,449 | — | — | (220,449 | ) | — | ||||||||||||
TOTAL NET REVENUES | 220,449 | 2,087,326 | 7,235 | (220,449 | ) | 2,094,561 | ||||||||||||
TOTAL COST OF NET REVENUES | — | 863,305 | 4,154 | — | 867,459 | |||||||||||||
Provision for doubtful accounts | — | 57,811 | 108 | — | 57,919 | |||||||||||||
Selling, distribution and administrative | 197,666 | 1,070,641 | 2,276 | (220,449 | ) | 1,050,134 | ||||||||||||
Amortization of intangible assets | 832 | 2,884 | — | — | 3,716 | |||||||||||||
TOTAL COSTS AND EXPENSES | 198,498 | 1,994,641 | 6,538 | (220,449 | ) | 1,979,228 | ||||||||||||
OPERATING INCOME | 21,951 | 92,685 | 697 | — | 115,333 | |||||||||||||
Interest expense | 128,363 | 837 | — | — | 129,200 | |||||||||||||
Interest income and other | (71,820 | ) | 69,871 | 340 | — | (1,609 | ) | |||||||||||
(LOSS) INCOME BEFORE TAXES | (34,592 | ) | 21,977 | 357 | — | (12,258 | ) | |||||||||||
Income tax (benefit) expense | (15,132 | ) | 6,694 | — | — | (8,438 | ) | |||||||||||
NET (LOSS) INCOME | (19,460 | ) | 15,283 | 357 | — | (3,820 | ) | |||||||||||
Equity in income of subsidiaries, net of tax | 15,640 | 357 | — | (15,997 | ) | — | ||||||||||||
NET (LOSS) INCOME ATTRIBUTABLE TO PARENT ISSUER | $ | (3,820 | ) | $ | 15,640 | $ | 357 | $ | (15,997 | ) | $ | (3,820 | ) | |||||
F-42
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
Period October 29, 2008 to December 31, 2008
(Successor)
| Parent Issuer | Guarantor Subsidiaries | Non- Guarantor Subsidiaries | Consolidating Adjustments | Condensed Consolidated | |||||||||||||||
| (in thousands) | |||||||||||||||||||
Operating net revenues | $ | — | $ | 355,371 | $ | 1,294 | $ | — | $ | 356,665 | |||||||||
Net revenues from subsidiaries | 51,447 | — | — | (51,447 | ) | — | |||||||||||||
TOTAL NET REVENUES | 51,447 | 355,371 | 1,294 | (51,447 | ) | 356,665 | |||||||||||||
TOTAL COST OF NET REVENUES | — | 137,084 | 676 | — | 137,760 | ||||||||||||||
Provision for doubtful accounts | — | 14,292 | 37 | — | 14,329 | ||||||||||||||
Selling, distribution and administrative | 28,141 | 202,287 | 381 | (51,447 | ) | 179,362 | |||||||||||||
Amortization of intangible assets | 238 | 770 | — | — | 1,008 | ||||||||||||||
TOTAL COSTS AND EXPENSES | 28,379 | 354,433 | 1,094 | (51,447 | ) | 332,459 | |||||||||||||
OPERATING INCOME | 23,068 | 938 | 200 | — | 24,206 | ||||||||||||||
Interest expense | 25,872 | 295 | — | — | 26,167 | ||||||||||||||
Interest income and other | (5,665 | ) | 4,836 | 103 | — | (726 | ) | ||||||||||||
INCOME (LOSS) BEFORE TAXES | 2,861 | (4,193 | ) | 97 | — | (1,235 | ) | ||||||||||||
Income tax expense (benefit) | 1,867 | (1,208 | ) | — | — | 659 | |||||||||||||
NET INCOME (LOSS) | 994 | (2,985 | ) | 97 | — | (1,894 | ) | ||||||||||||
Equity in loss of subsidiaries, net of tax | (2,888 | ) | 97 | — | 2,791 | — | |||||||||||||
NET (LOSS) INCOME ATTRIBUTABLE TO PARENT ISSUER | $ | (1,894 | ) | $ | (2,888 | ) | $ | 97 | $ | 2,791 | $ | (1,894 | ) | ||||||
F-43
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
Period January 1, 2008 to October 28, 2008
(Predecessor)
| Parent Issuer | Guarantor Subsidiaries | Non- Guarantor Subsidiaries | Consolidating Adjustments | Condensed Consolidated | ||||||||||||||
| (in thousands) | ||||||||||||||||||
Operating net revenues | $ | — | $ | 1,766,882 | $ | 6,407 | $ | — | $ | 1,773,289 | ||||||||
Net revenues from subsidiaries | 179,186 | — | — | (179,186 | ) | — | ||||||||||||
TOTAL NET REVENUES | 179,186 | 1,766,882 | 6,407 | (179,186 | ) | 1,773,289 | ||||||||||||
TOTAL COST OF NET REVENUES | — | 688,123 | 3,214 | — | 691,337 | |||||||||||||
Provision for doubtful accounts | — | 33,525 | 101 | — | 33,626 | |||||||||||||
Selling, distribution and administrative | 131,387 | 970,086 | 2,249 | (179,186 | ) | 924,536 | ||||||||||||
Amortization of intangible assets | — | 3,461 | — | — | 3,461 | |||||||||||||
TOTAL COSTS AND EXPENSES | 131,387 | 1,695,195 | 5,564 | (179,186 | ) | 1,652,960 | ||||||||||||
OPERATING INCOME | 47,799 | 71,687 | 843 | — | 120,329 | |||||||||||||
Interest expense | 30,294 | 1,544 | — | — | 31,838 | |||||||||||||
Interest income and other | (32,634 | ) | 30,068 | 412 | — | (2,154 | ) | |||||||||||
INCOME BEFORE TAXES | 50,139 | 40,075 | 431 | — | 90,645 | |||||||||||||
Income tax expense | 17,506 | 16,686 | — | — | 34,192 | |||||||||||||
NET INCOME | 32,633 | 23,389 | 431 | — | 56,453 | |||||||||||||
Equity in income of subsidiaries, net of tax | 23,820 | 431 | — | (24,251 | ) | — | ||||||||||||
NET INCOME (LOSS) ATTRIBUTABLE TO PARENT ISSUER | $ | 56,453 | $ | 23,820 | $ | 431 | $ | (24,251 | ) | $ | 56,453 | |||||||
F-44
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
Year Ended December 31, 2007
(Predecessor)
| Parent Issuer | Guarantor Subsidiaries | Non- Guarantor Subsidiaries | Consolidating Adjustments | Condensed Consolidated | ||||||||||||||||
| (in thousands) | ||||||||||||||||||||
Operating net revenues | $ | — | $ | 1,631,172 | $ | 629 | $ | — | $ | 1,631,801 | ||||||||||
Net revenues from subsidiaries | 172,959 | — | — | (172,959 | ) | — | ||||||||||||||
TOTAL NET REVENUES | 172,959 | 1,631,172 | 629 | (172,959 | ) | 1,631,801 | ||||||||||||||
TOTAL COST OF NET REVENUES | — | 564,658 | 334 | — | 564,992 | |||||||||||||||
Provision for doubtful accounts | — | 43,011 | 127 | — | 43,138 | |||||||||||||||
Selling, distribution and administrative | 119,106 | 915,706 | 209 | (172,959 | ) | 862,062 | ||||||||||||||
Amortization of intangible assets | — | 3,079 | — | — | 3,079 | |||||||||||||||
TOTAL COSTS AND EXPENSES | 119,106 | 1,526,454 | 670 | (172,959 | ) | 1,473,271 | ||||||||||||||
OPERATING INCOME (LOSS) | 53,853 | 104,718 | (41 | ) | — | 158,530 | ||||||||||||||
Interest expense | 20,698 | 1,769 | (20 | ) | — | 22,447 | ||||||||||||||
Interest income and other | (22,607 | ) | 20,653 | — | — | (1,954 | ) | |||||||||||||
INCOME (LOSS) BEFORE TAXES | 55,762 | 82,296 | (21 | ) | — | 138,037 | ||||||||||||||
Income tax expense (benefit) | 22,077 | 29,921 | — | — | 51,998 | |||||||||||||||
NET INCOME (LOSS) | 33,685 | 52,375 | (21 | ) | — | 86,039 | ||||||||||||||
Equity in income of subsidiaries, net of tax | 52,354 | (21 | ) | — | (52,333 | ) | — | |||||||||||||
NET INCOME (LOSS) ATTRIBUTABLE TO PARENT ISSUER | $ | 86,039 | $ | 52,354 | $ | (21 | ) | $ | (52,333 | ) | $ | 86,039 | ||||||||
F-45
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
Year Ended December 31, 2009
(Successor)
| Parent Issuer | Guarantor Subsidiaries | Non-Guarantor Subsidiaries | Consolidating Adjustments | Condensed Consolidated | |||||||||||||||
| (in thousands) | |||||||||||||||||||
OPERATING ACTIVITIES | |||||||||||||||||||
NET CASH PROVIDED BY OPERATING ACTIVITIES | $ | 49,741 | $ | 119,426 | $ | 259 | $ | — | $ | 169,426 | |||||||||
INVESTING ACTIVITIES | |||||||||||||||||||
Purchases of patient service equipment and property, equipment and improvements, exclusive of effects of acquisitions | (19,426 | ) | (131,138 | ) | (33 | ) | — | (150,597 | ) | ||||||||||
Purchases of short-term investments | (37,554 | ) | — | — | — | (37,554 | ) | ||||||||||||
Maturities of short-term investments | 13,881 | — | — | — | 13,881 | ||||||||||||||
Proceeds from disposition of assets | — | 6,893 | — | — | 6,893 | ||||||||||||||
Cash paid for acquisitions | — | (1,279 | ) | — | — | (1,279 | ) | ||||||||||||
NET CASH USED IN INVESTING ACTIVITIES | (43,099 | ) | (125,524 | ) | (33 | ) | — | (168,656 | ) | ||||||||||
FINANCING ACTIVITIES | |||||||||||||||||||
Payments on Senior Secured Bridge Credit Agreement | (1,010,000 | ) | — | — | — | (1,010,000 | ) | ||||||||||||
Proceeds from ABL Facility | 630 | — | — | — | 630 | ||||||||||||||
Payments on ABL Facility | (6,630 | ) | — | — | — | (6,630 | ) | ||||||||||||
Payments on other long term debt | — | (2,856 | ) | — | — | (2,856 | ) | ||||||||||||
Proceeds from issuance of Series A-1 Notes | 700,000 | — | — | — | 700,000 | ||||||||||||||
Proceeds from issuance of Series A-2 Notes | 317,500 | �� | — | — | 317,500 | ||||||||||||||
Change in book cash overdraft included in accounts payable | — | 7,902 | — | — | 7,902 | ||||||||||||||
Debt issuance costs related to Series A-1 and Series A-2 Notes | (19,039 | ) | — | — | — | (19,039 | ) | ||||||||||||
Debt issuance costs related to the ABL Facility | (207 | ) | — | — | — | (207 | ) | ||||||||||||
Equity contribution | 2,075 | — | — | — | 2,075 | ||||||||||||||
NET CASH (USED IN) PROVIDED BY FINANCING ACTIVITIES | (15,671 | ) | 5,046 | — | — | (10,625 | ) | ||||||||||||
NET (DECREASE) INCREASE IN CASH AND CASH EQUIVALENTS | (9,029 | ) | (1,052 | ) | 226 | — | (9,855 | ) | |||||||||||
CASH AND CASH EQUIVALENTS AT BEGINNING OF PERIOD | 159,393 | 8,260 | 365 | — | 168,018 | ||||||||||||||
CASH AND CASH EQUIVALENTS AT END OF PERIOD | $ | 150,364 | $ | 7,208 | $ | 591 | $ | — | $ | 158,163 | |||||||||
F-46
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
Period October 29, 2009 to December 31, 2008
(Successor)
| Parent Issuer | Guarantor Subsidiaries | Non-Guarantor Subsidiaries | Consolidating Adjustments | Condensed Consolidated | |||||||||||||||
| (in thousands) | |||||||||||||||||||
OPERATING ACTIVITIES | |||||||||||||||||||
NET CASH PROVIDED BY (USED IN) OPERATING ACTIVITIES | $ | 33,146 | $ | 30,276 | $ | (85 | ) | $ | — | $ | 63,337 | ||||||||
INVESTING ACTIVITIES | |||||||||||||||||||
Purchases of patient service equipment and property, equipment and improvements, exclusive of effects of acquisitions | (739 | ) | (25,440 | ) | (38 | ) | — | (26,217 | ) | ||||||||||
Merger related costs | (49,269 | ) | — | — | — | (49,269 | ) | ||||||||||||
Proceeds from disposition of assets | — | 20 | — | — | 20 | ||||||||||||||
NET CASH USED IN INVESTING ACTIVITIES | (50,008 | ) | (25,420 | ) | (38 | ) | — | (75,466 | ) | ||||||||||
FINANCING ACTIVITIES | |||||||||||||||||||
Payments on Predecessor Revolving Credit Facility | (304,000 | ) | — | — | — | (304,000 | ) | ||||||||||||
Proceeds from Senior Secured Bridge Credit Agreement | 1,010,000 | — | — | — | 1,010,000 | ||||||||||||||
Payments on Interim Facility | (249,772 | ) | — | — | — | (249,772 | ) | ||||||||||||
Redemption of convertible senior notes | (151 | ) | — | — | — | (151 | ) | ||||||||||||
Proceeds from ABL Facility | 36,000 | — | — | — | 36,000 | ||||||||||||||
Payments on ABL Facility | (30,000 | ) | — | — | — | (30,000 | ) | ||||||||||||
Payments on other long term debt | (4,717 | ) | (528 | ) | — | — | (5,245 | ) | |||||||||||
Change in book cash overdraft included in accounts payable | — | (850 | ) | — | — | (850 | ) | ||||||||||||
Debt issuance costs related to the Interim Facility | (45,661 | ) | — | — | — | (45,661 | ) | ||||||||||||
Debt issuance costs related to the ABL Facility | (5,250 | ) | — | — | — | (5,250 | ) | ||||||||||||
Equity contribution | 673,334 | — | — | — | 673,334 | ||||||||||||||
Repurchases of common stock | (922,935 | ) | — | — | — | (922,935 | ) | ||||||||||||
Change in control payment for options and awards | (23,536 | ) | — | — | — | (23,536 | ) | ||||||||||||
NET CASH PROVIDED BY (USED IN) FINANCING ACTIVITIES | 133,312 | (1,378 | ) | — | — | 131,934 | |||||||||||||
NET INCREASE (DECREASE) IN CASH AND CASH EQUIVALENTS | 116,450 | 3,478 | (123 | ) | — | 119,805 | |||||||||||||
CASH AND CASH EQUIVALENTS AT BEGINNING OF PERIOD | 42,943 | 4,782 | 488 | 48,213 | |||||||||||||||
CASH AND CASH EQUIVALENTS AT END OF PERIOD | $ | 159,393 | $ | 8,260 | $ | 365 | $ | — | $ | 168,018 | |||||||||
F-47
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
Period January 1, 2008 to October 28, 2008
(Predecessor)
| Parent Issuer | Guarantor Subsidiaries | Non- Guarantor Subsidiaries | Consolidating Adjustments | Condensed Consolidated | |||||||||||||||
| (in thousands) | |||||||||||||||||||
OPERATING ACTIVITIES | |||||||||||||||||||
NET CASH PROVIDED BY OPERATING ACTIVITIES | $ | 193,096 | $ | 104,443 | $ | 398 | $ | — | $ | 297,937 | |||||||||
INVESTING ACTIVITIES | |||||||||||||||||||
Purchases of patient service equipment and property, equipment and improvements, exclusive of effects of acquisitions | (35,682 | ) | (121,482 | ) | (19 | ) | — | (157,183 | ) | ||||||||||
Proceeds from disposition of assets | — | 5,295 | — | — | 5,295 | ||||||||||||||
Cash paid for acquisitions | — | (2,605 | ) | — | — | (2,605 | ) | ||||||||||||
NET CASH USED IN INVESTING ACTIVITIES | (35,682 | ) | (118,792 | ) | (19 | ) | — | (154,493 | ) | ||||||||||
FINANCING ACTIVITIES | |||||||||||||||||||
Proceeds from Predecessor Revolving Credit Facility | 18,300 | — | — | — | 18,300 | ||||||||||||||
Payments on Predecessor Revolving Credit Facility | (138,300 | ) | — | — | — | (138,300 | ) | ||||||||||||
Proceeds from Senior Secured Bridge Credit Agreement | 250,000 | — | — | — | 250,000 | ||||||||||||||
Payments on Interim Facility | (228 | ) | — | — | — | (228 | ) | ||||||||||||
Redemption of convertible senior notes | (249,772 | ) | — | — | — | (249,772 | ) | ||||||||||||
Payments on other long term debt | (551 | ) | (2,608 | ) | — | — | (3,159 | ) | |||||||||||
Change in book cash overdraft included in accounts payable | — | 7,771 | — | — | 7,771 | ||||||||||||||
Debt issuance costs related to the Interim Facility | (9,204 | ) | — | — | — | (9,204 | ) | ||||||||||||
Excess tax benefits from share-based compensation | 497 | — | — | — | 497 | ||||||||||||||
Issuances of common stock | 413 | — | — | — | 413 | ||||||||||||||
NET CASH (USED IN) PROVIDED BY FINANCING ACTIVITIES | (128,845 | ) | 5,163 | — | — | (123,682 | ) | ||||||||||||
NET INCREASE (DECREASE) IN CASH AND CASH EQUIVALENTS | 28,569 | (9,186 | ) | 379 | — | 19,762 | |||||||||||||
CASH AND CASH EQUIVALENTS AT BEGINNING OF PERIOD | 14,374 | 13,968 | 109 | — | 28,451 | ||||||||||||||
CASH AND CASH EQUIVALENTS AT END OF PERIOD | $ | 42,943 | $ | 4,782 | $ | 488 | $ | — | $ | 48,213 | |||||||||
F-48
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
Year Ended December 31, 2007
(Predecessor)
| Parent Issuer | Guarantor Subsidiaries | Non-Guarantor Subsidiaries | Consolidating Adjustments | Condensed Consolidated | |||||||||||||||
| (in thousands) | |||||||||||||||||||
OPERATING ACTIVITIES | |||||||||||||||||||
NET CASH PROVIDED BY (USED IN) OPERATING ACTIVITIES | $ | 180,799 | $ | 113,228 | $ | (21 | ) | $ | — | $ | 294,006 | ||||||||
INVESTING ACTIVITIES | |||||||||||||||||||
Purchases of patient service equipment and property, equipment and improvements, exclusive of effects of acquisitions | (32,083 | ) | (96,676 | ) | — | — | (128,759 | ) | |||||||||||
Proceeds from disposition of assets | — | 102 | — | — | 102 | ||||||||||||||
Cash paid for acquisitions | — | (354,708 | ) | 130 | — | (354,578 | ) | ||||||||||||
NET CASH (USED IN) PROVIDED BY INVESTING ACTIVITIES | (32,083 | ) | (451,282 | ) | 130 | — | (483,235 | ) | |||||||||||
FINANCING ACTIVITIES | |||||||||||||||||||
Intercompany loan | (350,000 | ) | 350,000 | — | — | — | |||||||||||||
Proceeds from Predecessor Revolving Credit Facility | 359,000 | — | — | — | 359,000 | ||||||||||||||
Payments on Predecessor Revolving Credit Facility | (170,000 | ) | — | — | — | (170,000 | ) | ||||||||||||
Payments on other long term debt | (2,716 | ) | (548 | ) | — | — | (3,264 | ) | |||||||||||
Change in book cash overdraft included in accounts payable | — | (4,291 | ) | — | — | (4,291 | ) | ||||||||||||
Excess tax benefits from share-based compensation | 4,057 | — | — | — | 4,057 | ||||||||||||||
Issuances of common stock | 17,521 | — | — | — | 17,521 | ||||||||||||||
NET CASH (USED IN) PROVIDED BY FINANCING ACTIVITIES | (142,138 | ) | 345,161 | — | — | 203,023 | |||||||||||||
NET INCREASE IN CASH AND CASH EQUIVALENTS | 6,578 | 7,107 | 109 | — | 13,794 | ||||||||||||||
CASH AND CASH EQUIVALENTS AT BEGINNING OF PERIOD | 7,796 | 6,861 | — | — | 14,657 | ||||||||||||||
CASH AND CASH EQUIVALENTS AT END OF PERIOD | $ | 14,374 | $ | 13,968 | $ | 109 | $ | — | $ | 28,451 | |||||||||
F-49
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
NOTE 16—Selected Quarterly Financial Data (unaudited)
| First | Second | Third | Fourth | |||||||||||
| (Successor) | (Successor) | (Successor) | (Successor) | |||||||||||
| (in thousands) | ||||||||||||||
2009 | ||||||||||||||
Net Revenues | $ | 516,431 | $ | 522,528 | $ | 519,515 | $ | 536,087 | ||||||
Gross Profit | 309,387 | 319,062 | 273,860 | 324,793 | ||||||||||
Operating Income (Loss) | 32,970 | 40,628 | (3,748 | ) | 45,483 | |||||||||
Net (Loss) Income | $ | (477 | ) | $ | 4,015 | $ | (22,981 | ) | $ | 15,623 | ||||
| First | Second | Third | Period October 1, 2008 to October 28, 2008 | Period October 29, 2008 to December 31, 2008 | |||||||||||||
| (Predecessor) | (Predecessor) | (Predecessor) | (Predecessor) | (Successor) | |||||||||||||
| (in thousands) | |||||||||||||||||
2008 | |||||||||||||||||
Net Revenues | $ | 527,978 | $ | 531,248 | $ | 530,828 | $ | 183,235 | $ | 356,665 | |||||||
Gross Profit | 322,708 | 321,788 | 327,541 | 109,915 | 218,905 | ||||||||||||
Operating Income (Loss) | 42,298 | 43,649 | 47,264 | (12,882 | ) | 24,206 | |||||||||||
Net Income (Loss) | $ | 20,772 | $ | 23,155 | $ | 22,100 | $ | (9,574 | ) | $ | (1,894 | ) | |||||
During the third quarter of 2009, we finalized the valuation of the intangible assets related to the Merger, resulting in adjustments to the value of our capitated relationships, patient backlogs, trade names and goodwill. The adjustments to our capitated relationships and patient backlogs also resulted in additional amortization expense of $35.7 million during the quarter.
NOTE 17—Subsequent Events
We evaluated all subsequent events that occurred after the balance sheet date through July 16, 2010.
F-50
Table of Contents
SCHEDULE II—VALUATION AND QUALIFYING ACCOUNTS
| Balance at Beginning of Period | Increase Due to Coram Acquisition | Charged to Costs and Expenses | Deductions | Balance at End of Period | |||||||||||
| (in thousands) | |||||||||||||||
Year ended December 31, 2009 (Successor) Deducted from asset accounts: | |||||||||||||||
Allowance for doubtful accounts | $ | 25,271 | $ | — | $ | 57,919 | $ | 43,263 | $ | 39,927 | |||||
Reserve for inventory and patient service equipment shortages | $ | 6,091 | $ | — | $ | 2,327 | $ | 6,241 | $ | 2,177 | |||||
Period October 29, 2008 to Deducted from asset accounts: | |||||||||||||||
Allowance for doubtful accounts | $ | 35,286 | $ | — | $ | 14,329 | $ | 24,344 | $ | 25,271 | |||||
Reserve for inventory and patient service equipment shortages | $ | 5,914 | $ | — | $ | 1,554 | $ | 1,377 | $ | 6,091 | |||||
Period January 1, 2008 to Deducted from asset accounts: | |||||||||||||||
Allowance for doubtful accounts | $ | 47,823 | $ | — | $ | 33,626 | $ | 46,163 | $ | 35,286 | |||||
Reserve for inventory and patient service equipment shortages | $ | 5,366 | $ | — | $ | 2,583 | $ | 2,035 | $ | 5,914 | |||||
Year ended December 31, 2007 (Predecessor) Deducted from asset accounts: | |||||||||||||||
Allowance for doubtful accounts | $ | 27,324 | $ | 21,557 | $ | 43,138 | $ | 44,196 | $ | 47,823 | |||||
Reserve for inventory and patient service equipment shortages | $ | 4,420 | $ | 195 | $ | 3,351 | $ | 2,600 | $ | 5,366 | |||||
F-51
Table of Contents
APRIA HEALTHCARE GROUP INC.
CONDENSED CONSOLIDATED BALANCE SHEETS
(Unaudited)
| March 31, 2010 | December 31, 2009 | |||||||
| (in thousands, except share data) | ||||||||
ASSETS | ||||||||
CURRENT ASSETS | ||||||||
Cash and cash equivalents | $ | 133,389 | $ | 158,163 | ||||
Short-term investments | 19,183 | 23,673 | ||||||
Accounts receivable, less allowance for doubtful accounts of $45,036 and $39,927 at March 31, 2010 and December 31, 2009, respectively | 282,608 | 252,133 | ||||||
Inventories | 60,013 | 68,267 | ||||||
Deferred income taxes | 80,407 | 87,016 | ||||||
Deferred expenses | 3,017 | 3,049 | ||||||
Prepaid expenses and other current assets | 30,898 | 24,362 | ||||||
TOTAL CURRENT ASSETS | 609,515 | 616,663 | ||||||
PATIENT SERVICE EQUIPMENT, less accumulated depreciation of $109,322 and $97,874 at March 31, 2010 and December 31, 2009, respectively | 186,366 | 198,808 | ||||||
PROPERTY, EQUIPMENT AND IMPROVEMENTS, NET | 79,408 | 80,227 | ||||||
GOODWILL | 759,947 | 759,197 | ||||||
INTANGIBLE ASSETS, NET | 581,352 | 582,259 | ||||||
DEFERRED DEBT ISSUANCE COSTS, NET | 60,558 | 63,110 | ||||||
OTHER ASSETS | 7,124 | 8,783 | ||||||
TOTAL ASSETS | $ | 2,284,270 | $ | 2,309,047 | ||||
LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
CURRENT LIABILITIES | ||||||||
Accounts payable | $ | 90,344 | $ | 123,856 | ||||
Accrued payroll and related taxes and benefits | 54,734 | 66,842 | ||||||
Other accrued liabilities | 126,777 | 100,768 | ||||||
Deferred revenue | 26,806 | 27,253 | ||||||
Current portion of long-term debt | 1,643 | 1,690 | ||||||
TOTAL CURRENT LIABILITIES | 300,304 | 320,409 | ||||||
LONG-TERM DEBT, net of current portion | 1,019,061 | 1,019,456 | ||||||
DEFERRED INCOME TAXES | 252,246 | 259,030 | ||||||
INCOME TAXES PAYABLE AND OTHER NON-CURRENT LIABILITIES | 33,681 | 31,421 | ||||||
TOTAL LIABILITIES | 1,605,292 | 1,630,316 | ||||||
COMMITMENTS AND CONTINGENCIES (Note 10) | ||||||||
STOCKHOLDERS’ EQUITY | ||||||||
Common stock, $0.01 par value: 1,000 shares authorized; 100 shares issued at March 31, 2010 and December 31, 2009 | — | — | ||||||
Additional paid-in capital | 685,495 | 684,445 | ||||||
Accumulated deficit | (6,517 | ) | (5,714 | ) | ||||
TOTAL STOCKHOLDERS’ EQUITY | 678,978 | 678,731 | ||||||
| $ | 2,284,270 | $ | 2,309,047 | |||||
See notes to unaudited condensed consolidated financial statements.
F-52
Table of Contents
APRIA HEALTHCARE GROUP INC.
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
(Unaudited)
| Three Months Ended March 31, | ||||||||
| 2010 | 2009 | |||||||
| (in thousands) | ||||||||
Net revenues: | ||||||||
Fee for service/product arrangements | $ | 469,836 | $ | 473,822 | ||||
Capitation arrangements | 39,040 | 42,609 | ||||||
TOTAL NET REVENUES | 508,876 | 516,431 | ||||||
Costs and expenses: | ||||||||
Cost of net revenues: | ||||||||
Product and supply costs | 156,833 | 160,612 | ||||||
Patient service equipment depreciation | 25,056 | 26,400 | ||||||
Home respiratory therapy services | 8,193 | 8,463 | ||||||
Nursing services | 9,088 | 8,844 | ||||||
Other | 3,622 | 2,725 | ||||||
TOTAL COST OF NET REVENUES | 202,792 | 207,044 | ||||||
Provision for doubtful accounts | 15,887 | 11,356 | ||||||
Selling, distribution and administrative | 257,738 | 263,784 | ||||||
Amortization of intangible assets | 1,657 | 1,277 | ||||||
TOTAL COSTS AND EXPENSES | 478,074 | 483,461 | ||||||
OPERATING INCOME | 30,802 | 32,970 | ||||||
Interest expense | 32,572 | 32,422 | ||||||
Interest income and other | (83 | ) | (155 | ) | ||||
(LOSS) INCOME BEFORE TAXES | (1,687 | ) | 703 | |||||
Income tax (benefit) expense | (884 | ) | 1,180 | |||||
NET LOSS | $ | (803 | ) | $ | (477 | ) | ||
See notes to unaudited condensed consolidated financial statements.
F-53
Table of Contents
APRIA HEALTHCARE GROUP INC.
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(Unaudited)
| Three Months Ended March 31, | ||||||||
| 2010 | 2009 | |||||||
| (in thousands) | ||||||||
OPERATING ACTIVITIES | ||||||||
Net loss | $ | (803 | ) | $ | (477 | ) | ||
Items included in net income not requiring cash: | ||||||||
Provision for doubtful accounts | 15,887 | 11,356 | ||||||
Depreciation | 31,580 | 32,681 | ||||||
Amortization of intangible assets | 1,657 | 1,277 | ||||||
Amortization of deferred debt issuance costs | 2,552 | 1,460 | ||||||
Deferred income taxes | (175 | ) | 3,059 | |||||
Profit interest compensation | 1,128 | 2,308 | ||||||
Loss on disposition of assets and other | 4,619 | 3,813 | ||||||
Changes in operating assets and liabilities: | ||||||||
Accounts receivable | (47,062 | ) | (24,889 | ) | ||||
Inventories | 8,254 | 1,476 | ||||||
Prepaid expenses and other assets | (5,797 | ) | (8,681 | ) | ||||
Accounts payable, exclusive of book-cash overdraft | (13,179 | ) | (7,319 | ) | ||||
Accrued payroll and related taxes and benefits | (12,108 | ) | (13,164 | ) | ||||
Income taxes payable | 381 | 1,016 | ||||||
Deferred revenue, net of related expenses | (415 | ) | (3,339 | ) | ||||
Accrued expenses | 28,586 | 29,095 | ||||||
NET CASH PROVIDED BY OPERATING ACTIVITIES | 15,105 | 29,672 | ||||||
INVESTING ACTIVITIES | ||||||||
Purchases of patient service equipment and property, equipment and improvements | (27,319 | ) | (39,843 | ) | ||||
Purchases of short-term investments | (8,189 | ) | — | |||||
Maturities of short-term investments | 12,680 | — | ||||||
Proceeds from disposition of assets | 15 | 251 | ||||||
Cash paid for acquisition | (1,200 | ) | — | |||||
NET CASH USED IN INVESTING ACTIVITIES | (24,013 | ) | (39,592 | ) | ||||
FINANCING ACTIVITIES | ||||||||
Proceeds from ABL Facility | — | 378 | ||||||
Payments on ABL Facility | — | (6,035 | ) | |||||
Payments on other long-term debt | (441 | ) | (688 | ) | ||||
Change in book-cash overdraft included in accounts payable | (14,137 | ) | (2,029 | ) | ||||
Debt issuance costs | (1,210 | ) | (206 | ) | ||||
Equity contributions | — | 1,075 | ||||||
Cash paid on profit interest units | (78 | ) | — | |||||
NET CASH USED IN FINANCING ACTIVITIES | (15,866 | ) | (7,505 | ) | ||||
NET DECREASE IN CASH AND CASH EQUIVALENTS | (24,774 | ) | (17,425 | ) | ||||
CASH AND CASH EQUIVALENTS AT BEGINNING OF PERIOD | 158,163 | 168,018 | ||||||
CASH AND CASH EQUIVALENTS AT END OF PERIOD | $ | 133,389 | $ | 150,593 | ||||
SUPPLEMENTAL DISCLOSURES—See Note 6 and Note 9 for a discussion of cash paid for interest and income taxes, respectively.
Purchases of patient service equipment and property, equipment and improvements exclude purchases that remain unpaid at the end of the respective quarter. Such amounts are then included in the following period’s purchases when paid. Unpaid purchases were $5,729 and $11,011 at March 31, 2010 and December 31, 2009, respectively.
See notes to unaudited condensed consolidated financial statements.
F-54
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1—Summary of Significant Accounting Policies
Basis of Presentation: The accompanying consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America. These statements include the accounts of Apria Healthcare Group Inc. (“Apria” or “the Company”) and its subsidiaries. Intercompany transactions and accounts have been eliminated in consolidation.
In the opinion of management, all adjustments, consisting of normal recurring accruals necessary for a fair presentation of the results of operations for the interim periods presented, have been reflected herein. The unaudited results of operations for interim periods are not necessarily indicative of the results to be expected for the entire year. For further information, refer to the consolidated financial statements and notes thereto included in the Company’s Annual Report for the fiscal year ended December 31, 2009.
On October 28, 2008, the Company completed its merger (the “Merger”) with Sky Merger Sub Corporation (“Merger Sub”), a Delaware corporation and wholly-owned subsidiary of Sky Acquisition LLC, a Delaware limited liability company (“Buyer” or “Sky LLC”). Buyer is controlled by a private investment fund affiliated with The Blackstone Group (the “Sponsor”).
Company Background: Apria operates in the home healthcare segment of the healthcare industry, providing a variety of high-quality clinical patient care management programs, related products and supplies as prescribed by a physician and/or authorized by a case manager as part of a care plan. Essentially all products and services offered by the Company are provided through the Company’s network of approximately 500 locations, which are located throughout the United States. The Company provides services and products in two reportable operating segments: (1) home respiratory therapy and home medical equipment and (2) home infusion therapy. Within these two operating segments there are three core service lines: home respiratory therapy, home medical equipment and home infusion therapy. Both segments provide products and services in the home setting to patients and are primarily paid for by a third-party payor, such as Medicare, Medicaid, managed care or other third-party insurer. Sales for both segments are primarily derived from referral sources such as hospital discharge planners, medical groups or independent physicians.
Use of Accounting Estimates: The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. Actual results could differ from those estimates. Among the significant estimates affecting the consolidated financial statements are those related to revenue recognition and the resulting accounts receivable, share-based compensation, income taxes, goodwill and long-lived assets.
Revenue Recognition and Concentration of Credit Risk: Revenues are recognized under fee-for-service/product arrangements for equipment the Company rents to patients, sales of equipment, supplies, pharmaceuticals and other items the Company sells to patients and for capitation payments received from third party payors for services and equipment the Company provides to the patients of these payors. Revenue generated from equipment that the Company rents to patients is recognized over the rental period, typically one month, and commences on delivery of the equipment to the patients. Revenue related to sales of equipment, supplies and pharmaceuticals is recognized on the date of delivery to the patients. Revenues derived from capitation arrangements were approximately 8% of total net revenues for the three months ended March 31, 2010 and the three months ended March 31, 2009. Capitation revenue is earned as a result of entering into a contract with a third party to provide its members certain services without regard to the actual services provided, therefore revenue is recognized in the period that the beneficiaries are entitled to health care services. All revenues are recorded at amounts estimated to be received under reimbursement arrangements with third-party payors,
F-55
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
including private insurers, prepaid health plans, Medicare and Medicaid. For the three months ended March 31, 2010 and 2009, revenues reimbursed under arrangements with Medicare and Medicaid were approximately 30% and 28%, respectively, as a percentage of total revenues. No other third-party payor group represented more than 8% of the Company’s revenues in the three months ended March 31, 2010 or 2009. Rental and sale revenues in the fee-for-service/product arrangement revenue line item were approximately $158.2 million or 33.7% and $311.6 million or 66.3%, respectively, for the three months ended March 31, 2010 and $165.5 million or 34.9% and $308.3 million or 65.1%, respectively, for the three months ended March 31, 2009.
In the Company’s business, there are multiple products that are delivered to patients. These arrangements involve equipment that is rented and related supplies that may be sold that cannot be returned. In arrangements with multiple deliverables, revenue is recognized when each deliverable is provided to the patient. For example, revenues from equipment rental supplies sales are recognized upon delivery of the products, as the supplies sold are considered a separate unit of accounting.
Cash and Cash Equivalents: Cash is maintained with several financial institutions. These financial institutions are located throughout the United States and the Company’s cash management practices limit exposure to any one institution. Book cash overdrafts, which are reported as a component of accounts payable, were $18.4 million and $32.5 million at March 31, 2010 and December 31, 2009, respectively. Management considers all highly liquid instruments purchased with a maturity of less than three months to be cash equivalents.
Short-Term Investments: Certificates of deposit with maturities greater than three months from our purchase date are classified as short-term investments. As of March 31, 2010, the carrying value of the Company’s short-term investments approximates fair value and such investments do not individually exceed FDIC insurance limits.
Accounts Receivable: Included in accounts receivable are earned but unbilled receivables of $49.5 million and $44.6 million at March 31, 2010 and December 31, 2009, respectively. Delays ranging from a day up to several weeks between the date of service and billing can occur due to delays in obtaining certain required payor-specific documentation from internal and external sources. Earned but unbilled receivables are aged from date of service and are considered in the analysis of historical performance and collectibility.
Due to the nature of the industry and the reimbursement environment in which the Company operates, certain estimates are required to record total net revenues and accounts receivable at their net realizable values. Inherent in these estimates is the risk that they will have to be revised or updated as additional information becomes available. Specifically, the complexity of many third-party billing arrangements and the uncertainty of reimbursement amounts for certain services from certain payors may result in adjustments to amounts originally recorded. Such adjustments are typically identified and recorded at the point of cash application, claim denial or account review.
Management performs periodic analyses to evaluate accounts receivable balances to ensure that recorded amounts reflect estimated net realizable value. Specifically, management considers historical realization data, accounts receivable aging trends, other operating trends, the extent of contracted business and business combinations. Also considered are relevant business conditions such as governmental and managed care payor claims processing procedures and system changes. Additionally, focused reviews of certain large or problematic payors are performed. Due to continuing changes in the healthcare industry and third-party reimbursement, it is possible that management’s estimates could change in the near term, which could have an adverse impact on operations and cash flows.
F-56
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Accounts receivable are reduced by an allowance for doubtful accounts which provides for those accounts from which payment is not expected to be received, although services were provided and revenue was earned. Upon determination that an account is uncollectible, it is written-off and charged to the allowance.
Deferred Revenue and Deferred Expense: A lessor is required to recognize rental income over the lease term. Rental of patient equipment is billed on a monthly basis beginning on the date the equipment is delivered. Since deliveries can occur on any day during a month, the amount of billings that apply to the next month are deferred. Only the direct costs associated with the initial rental period are deferred.
Inventories: Inventories are stated at the lower of cost (first-in, first-out method) or market and consist primarily of pharmaceuticals and items used in conjunction with patient service equipment. Inventories are written down to fair value for slow moving or obsolete inventory.
Patient Service Equipment: Patient service equipment is stated at cost and consists of medical equipment rented to patients on a month-to-month basis. Depreciation is provided using the straight-line method over the estimated useful lives of the equipment, which range from one to ten years.
Property, Equipment and Improvements: Property, equipment and improvements are stated at cost. Included in property and equipment are assets under capitalized leases which consist of information systems hardware and software. Depreciation is calculated using the straight-line method over the estimated useful lives of the assets.
Capitalized Software: Included in property, equipment and improvements are costs related to internally developed and purchased software that are capitalized and amortized over periods that the assets are expected to provide benefit. Capitalized costs include direct costs of materials and services incurred in developing or obtaining internal-use software and payroll and benefit costs for employees directly involved in the development of internal-use software. Additions to capitalized software totaled $2.9 million for the three months ended March 31, 2010.
Goodwill and long-lived assets: Goodwill is recorded as the difference, if any, between the aggregate consideration paid for an acquisition and the fair value of the net tangible and intangible assets acquired. The amounts and useful lives assigned to intangible assets acquired, other than goodwill, impact the amount and timing of future amortization.
Goodwill and indefinite-lived intangible assets are not amortized but instead tested at least annually for impairment, or more frequently when events or changes in circumstances indicate that the assets might be impaired. Goodwill is tested for impairment by comparing the carrying value to the fair value of the reporting unit to which the goodwill is assigned. A two-step test is used to identify the potential impairment and to measure the amount of impairment, if any. The first step is to compare the fair value of the reporting unit with its carrying amount, including goodwill. If the fair value of the reporting unit exceeds its carrying amount, goodwill is considered not impaired; otherwise, goodwill is impaired and the loss is measured by performing step two. Under step two, the impairment loss is measured by comparing the implied fair value of the reporting unit with the carrying amount of goodwill. Management has determined that our two operating segments, home respiratory therapy/home medical equipment and home infusion therapy, are reporting units. The Company performs the annual test for impairment as of the first day of its fourth quarter and determines fair value using the income approach methodology of valuation that includes the discounted cash flow method. During the annual goodwill impairment test in 2009, the Company completed step one and determined that there was no impairment of goodwill since the fair value of the reporting units exceeded the carrying value. Our annual indefinite-lived intangible assets impairment test in 2009 also resulted in no impairment as the fair value of the assets exceeded the carrying value.
F-57
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Long-lived assets, including property and equipment and purchased intangible assets, are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset or asset group may not be recoverable. Significant judgment is required in determining whether a potential indicator of impairment of long-lived assets exists and in estimating future cash flows for any necessary impairment tests. Recoverability of assets to be held and used is measured by the comparison of the carrying amount of an asset to future undiscounted net cash flows expected to be generated by the asset. If such an asset is considered to be impaired, the impairment to be recognized is measured as the amount by which the carrying amount of the asset exceeds the fair value of the asset. Assets to be disposed of are reported at the lower of the carrying amount or fair value less costs to sell.
Purchased intangible assets consist primarily of trade names, patient backlog, capitated relationships and payor relationships resulting from the Merger. Purchased intangible assets that have definite lives are amortized over the estimated useful lives of the related assets, generally ranging from one to twenty years.
Deferred Debt Issuance Costs: Capitalized debt issuance costs include those associated with $700.0 million of the Company’s 11.25% Senior Secured Notes due 2014 (Series A-1) (the “Series A-1 Notes”), $317.5 million of the Company’s 12.375% Senior Secured Notes due 2014 (Series A-2) (the “Series A-2 Notes”) and the Company’s senior secured asset-based revolving credit facility (“ABL Facility”). Such costs are classified as non-current assets. Costs relating to the ABL Facility are amortized through the maturity date in October 2013. Costs relating to the Series A-1 Notes and Series A-2 Notes are amortized from the issuance date through October 2014. See Note 6—Long-term Debt.
Fair Value of Financial Instruments: The carrying value of Apria’s bank debt approximates fair value because the underlying instruments are variable notes that reprice frequently. The fair value of the Series A-1 Notes and Series A-2 Notes, as determined by reference to quoted prices for private transactions of Apria’s issuances, are $758.6 million and $346.5 million at March 31, 2010, respectively. The carrying amounts of cash and cash equivalents, accounts receivable, trade payables and accrued expenses approximate fair value due to their short maturity.
Product and Supply Costs: Product and supply costs presented within cost of total net revenues are comprised primarily of the cost of supplies and equipment provided to patients, infusion drug costs and enteral product costs.
Home Respiratory Therapy Expenses: Home respiratory therapy expenses presented within cost of net revenues are comprised primarily of employee salary and benefit costs or contract fees paid to respiratory therapists and other related professionals who are deployed to service a patient. Home respiratory therapy personnel are also engaged in a number of administrative and marketing tasks, and, accordingly, these costs are included within selling, distribution and administrative expenses and amounted to $6.4 million and $6.1 million for the three-month periods ended March 31, 2010 and 2009, respectively.
Distribution Expenses: Distribution expenses are included in selling, distribution and administrative expenses and totaled $38.8 million and $42.6 million for the three months ended March 31, 2010 and 2009, respectively. Such expenses represent the cost incurred to coordinate and deliver products and services to patients. Included in distribution expenses are leasing, maintenance, licensing and fuel costs for the vehicle fleet; salaries and other costs related to drivers and dispatch personnel; and amounts paid to courier and other outside shipping vendors. Such expenses fall within the definition of “shipping and handling” costs and are classified within selling and administrative expenses and as a result, cost of net revenues may not be comparable to other companies.
Self-Insurance: Coverage for certain employee medical claims and benefits, as well as workers’ compensation, vehicle liability, professional and general liability are self-insured. Accruals for medical claims at
F-58
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
March 31, 2010 and December 31, 2009 were $7.5 million and $7.0 million, respectively. Amounts accrued for costs of the other liability coverages totaled $28.1 million and $27.8 million at March 31, 2010 and December 31, 2009, respectively. All such amounts are classified in other accrued liabilities.
Advertising: Advertising costs are expensed as incurred. Such expenses are included in selling, distribution and administrative expenses and amounted to $1.0 million and $0.7 million for the three months ended March 31, 2010 and 2009, respectively.
Income Taxes: Deferred income tax assets and liabilities are computed for differences between the carrying amounts of assets and liabilities for financial statement and tax purposes. Deferred income tax assets are required to be reduced by a valuation allowance when it is determined that it is more likely than not that all or a portion of a deferred tax asset will not be realized. In determining the necessity and amount of a valuation allowance, management considers our current and past performance, the market environment in which the Company operates, tax planning strategies and the length of tax benefit carryforward periods.
Profit Interest Units: Compensation expense for all profit interest unit awards made to employees is measured and recognized based on estimated fair values on the date of grant. The value of the portion of the award that is ultimately expected to vest is recognized as expense over the requisite service period in our consolidated financial statements. Forfeitures are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. Profit interest units expense is recognized on a straight-line basis over the requisite service period. The estimate of fair value of profit interest unit awards on the date of grant is determined through the allocation of all outstanding securities to a business enterprise valuation. The enterprise valuation is based upon a combination of the income approach and the market approach. The income approach is based on discounted cash flows. The market approach uses a selection of comparable companies in determining value. This determination of fair value is affected by assumptions regarding a number of highly complex and subjective variables. Changes in these assumptions can materially affect the estimate of the fair value.
Reclassification: Certain amounts from the prior year have been reclassified on the statements of cash flows to conform with the current year presentation. For the three months ended March 31, 2009, $1.7 million was reclassified from accrued expenses to accrued payroll and related taxes and benefits.
NOTE 2—Recent Developments
Enactment of a Comprehensive Healthcare Reform Package: In March 2010, the Federal government enacted the comprehensive healthcare Reform Package which consists of the Patient Protection and Affordable Care Act, as amended by the Health Care Education Reconciliation Act of 2010. Among many other provisions, the Reform Package expands the Medicaid program, mandates extensive insurance market reforms, creates new health insurance access points (e.g., insurance exchanges), provides certain insurance subsidies (e.g., premiums and cost sharing), imposes individual and employer health insurance requirements and makes a number of changes to the U.S. Internal Revenue Code of 1986, as amended (the “Code”).
There are a number of provisions in the Reform Package that may affect the Company. For example, the Reform Package requires certain pharmaceutical and medical device manufacturers to pay an excise tax to the government, which may, in turn, increase costs for these products. The Reform Package also provides for cuts in some Medicare payments made to certain providers and to Medicare Advantage plans, through which the Company contracts to provide services to Medicare beneficiaries. Also included in the Reform Package are (i) an expansion of the Recovery Audit Contractor Program, (ii) certain fraud and abuse prevention measures and (iii) expanded regulatory authority concerning the types of conduct that can result in additional fines and
F-59
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
penalties for those healthcare providers who do not comply with applicable laws and regulations. Furthermore, the Reform Package grants the Secretary of HHS authority to set a date by which certain providers and suppliers will be required to establish a compliance program.
The Reform Package makes a number of changes to how certain of our products and services will be reimbursed by Medicare. The Reform Package also makes changes to the Medicare durable medical equipment consumer price index adjustment for 2011 and each subsequent year based upon the CPI reduced by a new productivity adjustment which may result in negative updates and includes changes to the Medicare DMEPOS competitive bidding program. Significantly, Round 2 of the competitive bidding program has been expanded from 70 to 91 of the largest MSAs. The Reform Package also gives the Secretary of HHS the authority to apply competitive bid pricing to non-bid areas, but details of that process are unlikely to be understood until after CMS issues guidance or completes a related rulemaking process.
In an effort to further strengthen the integrity of the Medicare program, the Reform Package includes additional requirements concerning physician enrollment and certain mandatory face-to-face patient/physician visits in conjunction with the ordering of durable medical equipment. These provisions are likely to be the subject of rulemaking and are a high priority for the American Association for Homecare and other industry representative organizations. The Company expects the Administration to continue to enhance its oversight efforts, and we strive to incorporate any necessary changes into our overall corporate compliance and internal audit programs on a regular basis.
The effective dates of the various provisions within the Reform Package are staggered over the next several years, with some changes occurring immediately. Much of the interpretation of what the Reform Package requires will be subject to administrative rulemaking, the development of agency guidance and court interpretations. The Company will continue to assess the impact of the Reform Package on our products and services.
Reorganization of the Senior Leadership Structure of our Home Respiratory Therapy and Home Medical Equipment Segment: On February 12, 2010, the Company finalized a decision to reorganize the senior leadership structure of the home respiratory therapy and home medical equipment segment. As part of this reorganization, Lawrence A. Mastrovich, President, Home Respiratory Therapy and Home Medical Equipment segment, resigned and his responsibilities were assumed by other senior executives.
NOTE 3—Recent Accounting Pronouncements
In June 2009, the Financial Accounting Standards Board (“FASB”) issued ASC 810, “Consolidation” (“ASC 810”) (originally issued as Statement of Financial Accounting Standard No. 167, “Amendments to FASB Interpretation No. 46(R)”). Among other items, ASC 810 responds to concerns about the application of certain key provisions of FIN 46(R), including those regarding the transparency of the involvement with variable interest entities. ASC 810 is effective for calendar year companies beginning on January 1, 2010. The Company adopted the Standard for the interim period ended March 31, 2010. There was no impact on its financial position, results of operations, cash flows or disclosures.
In October 2009, the FASB issued ASU 2009-13, “Multiple-Deliverable Revenue Arrangements” which amends ASC Topic 605, Revenue Recognition (“ASU 2009-13”) Under this standard, management is no longer required to obtain vendor-specific objective evidence or third party evidence of fair value for each deliverable in an arrangement with multiple elements, and where evidence is not available we may now estimate the proportion of the selling price attributable to each deliverable. ASU 2009-13 will be effective prospectively for revenue arrangements entered into or materially modified in the fiscal years beginning on or after June 15, 2010. The Company is currently evaluating the impact of ASU 2009-13 on its financial position, results of operations, cash flows and disclosures.
F-60
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
In January 2010, the FASB issued ASU 2010-6, “Improving Disclosures About Fair Value Measurements” (“ASU 2010-6”) which requires reporting entities to make new disclosures about recurring or nonrecurring fair-value measurements including significant transfers into and out of Level 1 and Level 2 fair-value measurements and information on purchases, sales, issuances, and settlements on a gross basis in the reconciliation of Level 3 fair- value measurements. ASU 2010-6 is effective for annual reporting periods beginning after December 15, 2009, except for Level 3 reconciliation disclosures which are effective for annual periods beginning after December 15, 2010. We do not expect the adoption of ASU 2010-6 to have a material impact on our consolidated financial statements.
NOTE 4—Business Combinations
The Company periodically acquires complementary businesses in specific geographic markets. The results of operations of the acquired companies are included in the accompanying condensed consolidated statements of operations from the dates of acquisition. During the three months ended March 31, 2010, the Company purchased certain assets of a business for $1.5 million. No acquisitions were made during the three months ended March 31, 2009.
NOTE 5—Goodwill and Intangible Assets
Goodwill activity during the three months ended March 31, 2010 was as follows:
| Home Infusion Therapy | Home Respiratory Therapy and Home Medical Equipment | Total | |||||||
| (in thousands) | |||||||||
Balance, December 31, 2009 | $ | 257,823 | $ | 501,374 | $ | 759,197 | |||
Acquisition | — | 750 | 750 | ||||||
Balance, March 31, 2010 | $ | 257,823 | $ | 502,124 | $ | 759,947 | |||
Intangible assets consist of the following:
| March 31, 2010 | December 31, 2009 | |||||||||||||||||||||
| Average Life in Years | Gross Carrying Amount | Accumulated Amortization | Net Book Value | Gross Carrying Amount | Accumulated Amortization | Net Book Value | ||||||||||||||||
| (dollars in thousands) | ||||||||||||||||||||||
Intangible assets subject to amortization: | ||||||||||||||||||||||
Patient backlog | — | $ | — | $ | — | $ | — | $ | 44,000 | $ | (44,000 | ) | $ | — | ||||||||
Capitated relationships | 20.0 | 40,000 | (2,833 | ) | 37,167 | 40,000 | (2,333 | ) | 37,667 | |||||||||||||
Payor relationships | 20.0 | 11,000 | (779 | ) | 10,221 | 11,000 | (642 | ) | 10,358 | |||||||||||||
Net favorable leasehold interest | 3.2 | 3,552 | (1,588 | ) | 1,964 | 3,553 | (1,319 | ) | 2,234 | |||||||||||||
Subtotal | 54,552 | (5,200 | ) | 49,352 | 98,553 | (48,294 | ) | 50,259 | ||||||||||||||
Intangible assets not subject to amortization: | ||||||||||||||||||||||
Trade names | — | 525,000 | — | 525,000 | 525,000 | — | 525,000 | |||||||||||||||
Accreditations with commissions | — | 7,000 | — | 7,000 | 7,000 | — | 7,000 | |||||||||||||||
Subtotal | — | 532,000 | — | 532,000 | 532,000 | — | 532,000 | |||||||||||||||
Total | $ | 586,552 | $ | (5,200 | ) | $ | 581,352 | $ | 630,553 | $ | (48,294 | ) | $ | 582,259 | ||||||||
F-61
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Amortization expense amounted to $1.7 million and $1.3 million for the three months ended March 31, 2010 and 2009, respectively. Estimated amortization expense for each of the fiscal years ending December 31 is presented below:
Year Ending December 31, | (in thousands) | ||
2009 | $ | 4,306 | |
2010 | 3,474 | ||
2011 | 2,854 | ||
2012 | 2,550 | ||
2013 | 2,550 | ||
NOTE 6—Long-Term Debt
Series A-1 Notes and Series A-2 Notes. Series A-1 Notes and Series A-2 Notes were issued by the Company in May 2009 and August 2009, respectively. The Series A-1 Notes and the Series A-2 Notes bear interest at a rate equal to 11.25% per annum and 12.375% per annum, respectively. The indenture governing the Series A-1 Notes and the Series A-2 Notes, among other restrictions, limits the Company’s ability and the ability of its restricted subsidiaries to:
| • | incur additional debt; |
| • | pay dividends and make other distributions; |
| • | make certain investments; |
| • | repurchase our stock; |
| • | incur certain liens; |
| • | enter into transactions with affiliates; |
| • | merge or consolidate; |
| • | enter into agreements that restrict the ability of our subsidiaries to make dividends or other payments to us; and |
| • | transfer or sell assets. |
Subject to certain exceptions, the indenture governing the Series A-1 Notes and the Series A-2 Notes permits Apria and its restricted subsidiaries to incur additional indebtedness, including senior indebtedness and secured indebtedness. The Series A-1 Notes are entitled to a priority of payment over the Series A-2 notes in certain circumstances, including upon any acceleration of the obligations under the Series A-1 Notes, the Series A-2 Notes or any bankruptcy or insolvency event or default with respect to Apria or any guarantor of the Series A-1 Notes and the Series A-2 Notes.
Senior Secured Bridge Credit Agreement: In connection with the Merger on October 28, 2008, the Company entered into a $1,010.0 million senior secured bridge credit agreement (the “senior secured bridge credit agreement”) with Banc of America Securities LLC and Wachovia Capital Markets, LLC, as joint lead arrangers, Banc of America Securities LLC, Wachovia Capital Markets, LLC and Barclays Capital, the investment banking division of Barclays Bank PLC, as joint bookrunners and Banc of America Bridge LLC, as administrative agent and Bank of America, N.A., as collateral agent, and a syndicate of financial institutions and institutional lenders. Proceeds from the issuance of the Series A-1 and Series A-2 Notes, together with cash on hand, were used to repay all borrowings under the senior secured bridge credit agreement and the senior secured bridge credit agreement was terminated.
F-62
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
ABL Facility: In connection with the Merger on October 28, 2008, the Company entered into the ABL Facility with Banc of America Securities LLC and Wachovia Capital Markets, LLC, as joint lead arrangers, Banc of America Securities LLC, Wachovia Capital Markets, LLC and Barclays Capital, the investment banking division of Barclays Bank PLC, as joint bookrunners and Bank of America, N.A., as administrative agent and collateral agent, and a syndicate of financial institutions and institutional lenders.
The ABL Facility provides for revolving credit financing of up to $150.0 million, subject to borrowing base availability, with a maturity of five years, including both a letter of credit and swingline loan sub-facility. The borrowing base at any time is equal to the sum (subject to certain reserves and other adjustments) of 85% of eligible receivables and the lesser of (a) 85% of the net orderly liquidation value of eligible inventory and (b) $20.0 million.
As of March 31, 2010, there were no outstanding borrowings under the ABL Facility, outstanding letters of credit totaled $16.1 million and additional availability under the ABL Facility, subject to the borrowing base, was $133.9 million. As of March 31, 2010, the available borrowing base did not constrain ability to borrow the entire $133.9 million of available borrowings under the ABL Facility. At March 31, 2010, the Company was in compliance with all of the financial covenants required by the credit agreement governing the ABL Facility.
Interest paid on debt totaled $0.5 million and $0.4 million for the three months ended March 31, 2010 and 2009, respectively. Interest expense for the three months ended March 31, 2010 and 2009 was $32.6 million and $32.4 million, respectively.
NOTE 7—Stockholders’ Equity
For the three months ended March 31, 2010, changes to stockholders’ equity were comprised of the following amounts:
Net loss | $ | (803 | ) | |
Cash paid on profit interest units | (78 | ) | ||
Profit interest compensation | 1,128 | |||
| $ | 247 | |||
NOTE 8—Equity-Based Compensation
Profit Interest Units: In November and December of 2008, BP Healthcare Holdings LLC (“BP Holdings”) and Sky LLC, parent entities of the Company affiliated with the Sponsor, granted equity units to the Company’s Chief Executive Officer and the Company’s Chief Financial Officer for purposes of retaining them and enabling such individuals to participate in the long-term growth and financial success of the Company. In addition, in 2009, Sky LLC granted equity units to certain management employees for purposes of retaining them and enabling such individuals to participate in the long-term growth and financial success of the Company. Profit interest units are measured at the grant date, based on the calculated fair value of the award, and are recognized as an expense over the employee’s requisite service period. These equity awards were issued in exchange for services to be performed.
BP Holdings granted the Company’s Chief Executive Officer 38,697,318 Class B units, all of which are subject to vesting terms based on either (i) continued service to BP Holdings or its subsidiaries and/or (ii) performance/market conditions.
F-63
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
| • | Time-Vesting Units. The portion of the Class B units that vest based on continued service represent 80% of the total Class B units. These units vest ratably over 48 months starting on October 28, 2008 based on continued service, but will become fully vested on an accelerated basis either (x) upon a change in control while the Company’s Chief Executive Officer continues to provide services to BP Holdings or its subsidiaries or (y) if affiliates of the Sponsor receive cash proceeds in respect to 50% of their units in BP Holdings equal to at least 200% of their aggregate capital contributions in respect of such units while the Company’s Chief Executive Officer continues to provide services to BP Holdings or its subsidiaries. In addition, if the Company’s Chief Executive Officer’s services are terminated (a) by the Company without “cause” or (b) by the Chief Executive Officer as a result of “constructive termination,” an additional number of these time-vesting Class B units will vest equal to the number that would have vested over the 24-month period following the applicable termination date. Any of these time-vested Class B units that are unvested on termination of the executive’s services will be forfeited. |
| • | Performance-Vesting Units. The remaining portion of the Class B units that vest based on performance/market conditions represent 20% of the total Class B units. One-half of these units will vest if affiliates of the Sponsor receive cash proceeds equal to at least 200% of their aggregate capital contributions in respect of all of their units in BP Holdings, with the other half eligible to vest if they receive cash proceeds equal to at least 300% of their aggregate capital contributions in respect of all of their units in BP Holdings. Any of these performance-vesting units that are unvested upon a termination of the Company’s Chief Executive Officer’s services (x) by the Company without “cause,” (y) by the executive as a result of “constructive termination” or (z) by the executive for any reason on or following October 28, 2012, will remain outstanding until the second anniversary of the applicable termination date (unless they vest prior to that date). If the units do not vest by such anniversary, then any unvested performance-vesting units shall be immediately forfeited. |
Assumptions used were as follows:
Expected Asset Volatility(1) | 23.0 | % | |
Risk Free Interest Rate(2) | 2.24 | % | |
Expected Life(3) | 5.0 years |
| (1) | The expected asset volatility is derived from the asset volatilities of comparable publicly traded companies. |
| (2) | The risk free interest rate is interpolated from the constant maturity treasury rate (“CMT Rate”) as of the valuation date with the maturity matching the contractual life. |
| (3) | The expected life is based on management’s estimate. |
The following table summarizes activity for profit interest units for the period October 29, 2008 to March 31, 2009:
| Class B Units | Weighted Average Grant Date Fair Value | Weighted Average Remaining Contractual Life | |||||
Balance at December 31, 2009 | 38,697,318 | $ | 0.36 | ||||
Granted | — | ||||||
Exercised | — | ||||||
Forfeited | — | ||||||
Balance at March 31, 2010 | 38,697,318 | 2.6 | |||||
Vested units at March 31, 2010 | 9,674,330 | 2.6 | |||||
F-64
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
The grant date fair value of the B units was $13.9 million.
Sky LLC granted the Company’s Chief Financial Officer 500,000 Class A-2 units, 6,675,287 Class B units and 2,225,096 Class C units, all of which are subject to vesting terms based on either (i) continued service to Sky LLC or its subsidiaries or (ii) performance/market conditions.
| • | Class A-2 Units. The Class A-2 units vest if an initial public offering (“IPO”) or change of control occurs and the valuation of Class A-1 units of Sky LLC implied by the transaction exceeds 110% of the aggregate capital contributions of affiliates of the Sponsor for the Class A-1 units. The Company’s Chief Financial Officer does not need to be employed at the time of the IPO or change in control to vest. The Class A-2 Units will be forfeited if an IPO or change of control occurs at a valuation that does not result in vesting. |
| • | Time-Vesting Units. The portion of the Class B units that vest based on continued service represent 66 2/3% of the total Class B units. These units vest ratably over 57 months starting on October 28, 2008 based on continued service, but will become fully vested on an accelerated basis upon a change in control while the Company’s Chief Financial Officer continues to provide services to Sky LLC or its subsidiaries. Any of these time-vested Class B units that are unvested on termination of the executive’s services will be forfeited. |
| • | Performance-Vesting Units. The remaining portion of the Class B units and all of the Class C units vest based on performance/market conditions. These units will vest if affiliates of the Sponsor receive cash proceeds equal to at least 200% of their aggregate capital contributions in respect of 25% of their units in Sky LLC while the Company’s Chief Financial Officer continues to provide services to Sky LLC or its subsidiaries. |
Assumptions used were as follows:
Expected Asset Volatility(1) | 23.0 | % | |
Risk Free Interest Rate(2) | 1.35 | % | |
Estimated Life(3) | 5.0 years |
| (1) | The expected asset volatility is derived from the asset volatilities of comparable publicly traded companies. |
| (2) | The risk free interest rate is interpolated from the CMT Rate as of the valuation date with the maturity matching the contractual life. |
| (3) | The estimated life is based on management’s estimates. |
The following table summarizes activity for profit interest units for the period October 29, 2008 to March 31, 2009:
| Class A-2 Units | Weighted Average Grant Date Fair Value | Class B Units | Weighted Average Grant Date Fair Value | Class C Units | Weighted Average Grant Date Fair Value | ||||||||||
Balance at December 31, 2009 | 500,000 | $ | 0.75 | 6,675,287 | $ | 0.35 | 2,225,096 | $ | 0.15 | ||||||
Exercised | — | — | — | — | — | — | |||||||||
Forfeited | — | — | — | — | — | — | |||||||||
Balance at March 31, 2009 | 500,000 | 6,675,287 | 2,225,096 | ||||||||||||
Vested units at March 31, 2010 | 1,334,924 | ||||||||||||||
F-65
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
The grant date fair value of the 500,000 A-2 units is $0.4 million, the grant date fair value of the B units is $2.3 million and grant date the fair value of the C units is $0.3 million.
Sky LLC granted certain management employees 38,542,529 Class B units and 12,847,509 Class C units, all of which are subject to vesting terms based on either (i) continued service to Sky LLC or its subsidiaries or (ii) performance/market conditions.
| • | Time-Vesting Units. The portion of the Class B units that vest based on continued service represent 66 2/3% of the total Class B units. These units vest ratably over 60 months starting on the later of (x) October 28, 2008 and (y) the date the employee commenced employment based on continued service, but will become fully vested on an accelerated basis upon a change in control while the employee continues to provide services to Sky LLC or its subsidiaries. Any of these time-vested Class B units that are unvested on termination of the employee’s services will be forfeited. |
| • | Performance-Vesting Units. The remaining portion of the Class B units and all of the Class C units vest based on performance/market conditions. These units will vest if affiliates of the Sponsor receive cash proceeds equal to at least 200% of their aggregate capital contributions in respect of 25% of their units in Sky LLC while the employee continues to provide services to Sky LLC or its subsidiaries. |
Notwithstanding the vesting terms described above, if the employee voluntarily resigns (in the absence of “constructive termination”) then Sky LLC may require the forfeiture of any vested Class B or C units.
Assumptions used were as follows:
Expected Asset Volatility(1) | 25.0 | % | |
Risk Free Interest Rate(2) | 1.96 | % | |
Estimated Life(3) | 5.0 years |
| (1) | The expected asset volatility is derived from the asset volatilities of comparable publicly traded companies. |
| (2) | The risk free interest rate is interpolated from the CMT Rate as of the valuation date with the maturity matching the contractual life. |
| (3) | The estimated life is based on management’s estimate. |
The following table summarizes activity for profit interest units for the period January 1, 2009 to March 31, 2009:
| Class A-2 Units | Weighted Average Grant Date Fair Value | Class B Units | Weighted Average Grant Date Fair Value | Class C Units | Weighted Average Grant Date Fair Value | ||||||||||
Balance at December 31, 2009 | 2,075,000 | $ | 0.59 | 37,280,029 | $ | 0.28 | 12,426,676 | $ | 0.13 | ||||||
Granted | — | — | — | — | — | — | |||||||||
Exercised | — | — | — | — | — | — | |||||||||
Forfeited | — | — | 4,643,678 | — | 1,547,893 | — | |||||||||
Balance at March 31, 2010 | 2,075,000 | 32,636,351 | 10,878,783 | ||||||||||||
Vested units at March 31, 2010 | 4,500,532 | ||||||||||||||
The grant date fair value of the A-2 units is $1.2 million, the grant date fair value of the outstanding B units is $9.1 million and the grant date fair value of the outstanding C units is $1.4 million.
F-66
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Expense recorded related to profit interest units was $1.1 million and $2.3 million in the three months ended March 31, 2010 and 2009, respectively. As of March 31, 2010, total unrecognized profit interest compensation cost related to unvested profit interest units was $6.5 million, which is expected to be expensed over a weighted average period of 3.3 years.
In March 2010, all of the equity securities of Sky LLC held by employee holders were converted or exchanged into equivalent securities of Sky LLC’s indirect parent entity.
NOTE 9—Income Taxes
The Company’s effective tax rate was 52.4% for the three months ended March 31, 2010 compared with 167.8% for the three months ended March 31, 2009.
The Company accounts for its tax uncertainties under generally accepted accounting principles. Accordingly, the Company is required to disclose, within its interim financial statements, material changes to: (a) the gross amounts of unrecognized tax benefits recorded on its balance sheet; (b) the total amount of unrecognized tax benefits that, if recognized, would affect the effective tax rate; (c) interest and penalties related to tax uncertainties; (d) amounts and relevant information concerning unrecognized tax benefits for which it is reasonably possible that an increase or decrease would occur during the 12-month rolling period ending March 31, 2010 and (e) disclosure of tax years and major tax jurisdictions which remain subject to examination by taxing authorities. For the three months ended March 31, 2010, no material changes occurred with respect to the Company’s unrecognized tax benefits and the five disclosure categories discussed above.
As of March 31, 2010, federal net operating loss (“NOLs”) carryforwards of approximately $151.2 million were available to offset future federal taxable income. Such NOLs will expire at various times and in varying amounts during our calendar 2010 through 2030 tax years. A significant portion of these NOLS are subject to an annual utilization limitation as required by Section 382 of the Internal Revenue Code of 1986, as amended.
Net income tax refunds received for the three-month period ended March 31, 2010 was $0.7 million. Net income taxes paid for the three-month period ended March 31, 2009 was $0.6 million.
NOTE 10—Commitments and Contingencies
Enactment of a Comprehensive Healthcare Reform Package: In March 2010, the Federal government enacted a comprehensive healthcare Reform Package which consists of the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010. See Note 2—Recent Developments—Enactment of a Comprehensive Healthcare Reform Package.
Litigation: The Company is engaged in the defense of certain claims and lawsuits arising out of the ordinary course and conduct of its business, the outcomes of which are not determinable at this time. Insurance policies covering such potential losses, where such coverage is cost effective, are maintained. In the opinion of management, any liability that might be incurred upon the resolution of these claims and lawsuits will not, in the aggregate, have a material adverse effect on the Company’s financial condition or results of operations.
Medicare and Medicaid Reimbursement: There are a number of provisions contained within recent legislation or proposed legislation that affect or may affect Medicare and Medicaid reimbursement polices for items and services provided. The Company cannot be certain of the ultimate impact of all legislated and contemplated changes, and therefore, cannot provide assurance that these changes will not have a material adverse effect on the Company’s financial condition or results of operations.
F-67
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Supplier Concentration:Currently, approximately 63.3% of purchases for patient service equipment and supplies are from five vendors. Although there are a limited number of suppliers, management believes that other vendors could provide similar products on comparable terms. However, a change in suppliers could cause delays in service delivery and possible losses in revenue, which could adversely affect the Company’s financial condition or operating results.
Guarantees and Indemnities: From time to time, certain types of contracts are entered into that contingently require indemnification of parties against third party claims. These contracts primarily relate to (i) certain asset purchase agreements, under which indemnification may be provided to the seller of the business being acquired; (ii) certain real estate leases, which may require indemnification to property owners for environmental or other liabilities and other claims arising from use of the applicable premises; and (iii) certain agreements with officers, directors and employees, which may require indemnification of such persons for liabilities arising out of their relationship with the Company.
The terms of such obligations vary by contract and in most instances a specific or maximum dollar amount is not explicitly stated therein. Generally, amounts under these contracts cannot be reasonably estimated until a specific claim is asserted. Consequently, no liabilities have been recorded for these obligations on the balance sheets for any of the periods presented.
NOTE 11—Segments
The Company has two reportable operating segments (1) home respiratory therapy and home medical equipment and (2) home infusion therapy. Within these two operating segments there are three core service lines: home respiratory therapy, home medical equipment and home infusion therapy. The home respiratory therapy and home medical equipment segment provides services and equipment to assist patients with oxygen systems, sleep apnea, ambulation and general care around the home, as well as to provide respiratory medications and related services. The home infusion therapy segment primarily provides patients with pharmaceuticals and services prescribed in conjunction with the administration of nutrients or medication intravenously or through a gastrointestinal tube.
| Net Revenues | ||||||
Operating Segment | Three Months Ended March 31, 2010 | Three Months Ended March 31, 2009 | ||||
| (in thousands) | ||||||
Home Respiratory Therapy and Home Medical Equipment | $ | 278,316 | $ | 294,651 | ||
Home Infusion Therapy | 230,560 | 221,780 | ||||
Total | $ | 508,876 | $ | 516,431 | ||
| EBIT | ||||||
Operating Segment | Three Months Ended March 31, 2010 | Three Months Ended March 31, 2009 | ||||
| (in thousands) | ||||||
Home Respiratory Therapy and Home Medical Equipment | $ | 9,866 | $ | 23,523 | ||
Home Infusion Therapy | 20,823 | 9,396 | ||||
Total | $ | 30,689 | $ | 32,919 | ||
F-68
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
| Depreciation and Amortization | ||||||
Operating Segment | Three Months Ended March 31, 2010 | Three Months Ended March 31, 2009 | ||||
| (in thousands) | ||||||
Home Respiratory Therapy and Home Medical Equipment | $ | 29,713 | $ | 30,351 | ||
Home Infusion Therapy | 3,524 | 3,607 | ||||
Total | $ | 33,237 | $ | 33,958 | ||
Our Chief Operating Decision Maker (“CODM”) does not review assets assigned to segments. Therefore, such items are not reflected in the table above.
Earnings before interest and taxes (“EBIT”). EBIT is a measure used by our management to measure operating performance. EBIT is defined as net income (loss) plus interest expense and income taxes. EBIT is not a recognized term under Generally Accepted Accounting Principles (“GAAP”) and does not purport to be an alternative to net income as a measure of operating performance or to cash flows from operating activities as a measure of liquidity.
The following table provides a reconciliation from net loss to EBIT:
| Three Months Ended March 31, 2010 | Three Months Ended March 31, 2009 | |||||||||||||||||||
| Home Respiratory Therapy and Home Medical Equipment | Home Infusion Therapy | Total | Home Respiratory Therapy and Home Medical Equipment | Home Infusion Therapy | Total | |||||||||||||||
| (in thousands) | ||||||||||||||||||||
Net loss | $ | (803 | ) | $ | (477 | ) | ||||||||||||||
Interest expense, net (a) | 32,376 | 32,216 | ||||||||||||||||||
Income tax (benefit) expense | (884 | ) | 1,180 | |||||||||||||||||
EBIT | $ | 9,866 | $ | 20,823 | $ | 30,689 | $ | 23,523 | $ | 9,396 | $ | 32,919 | ||||||||
| (a) | Reflects $32.6 million of interest expense, net of $0.2 million of interest income for the three months ended March 31, 2010. Reflects $32.4 million of interest expense, net of $0.2 million of interest income for the three months ended March 31, 2009. |
The Company allocates certain expenses that are not directly attributable to a product line based upon segment headcount.
NOTE 12—Certain Relationships and Related Party Transactions
Transaction and Management Fee Agreement: In connection with the Merger, Merger Sub entered into a transaction and management fee agreement with Blackstone Management Partners V L.L.C. (“BMP”). The Company succeeded to and assumed the rights and obligations of Merger Sub pursuant to the transaction and management fee agreement upon the closing of the Merger. Under the transaction and management fee agreement, Merger Sub agreed to pay BMP, at the closing of the Merger, a $18.7 million transaction fee in consideration for BMP undertaking financial and structural analysis, due diligence and other assistance in
F-69
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
connection with the Merger. In addition the Company agreed to reimburse BMP for any out-of-pocket expenses incurred by BMP and its affiliates in connection with the Merger and the provision of services under the transaction and management fee agreement.
In addition, under this agreement, BMP (including through its affiliates) agreed to provide services, including without limitation, (a) advice regarding the structure, distribution and timing of debt and equity offerings and advice regarding relationships with the Company’s lenders and bankers, (b) advice regarding the business and strategy of the Company, including compensation arrangements, (c) advice regarding dispositions and/or acquisitions and (d) such advice directly related or ancillary to the above financial advisory services as may be reasonably requested by the Company. In consideration for the services, the Company pays BMP at the beginning of each fiscal year a management fee equal to the greater of $7.0 million or 2.0% of the Company’s consolidated EBITDA, as defined in the agreement, for the immediately preceding fiscal year. BMP shall have no obligation to provide any other services to the Company absent express agreement. In addition, in the absence of an express agreement to provide investment banking or other financial advisory services to the Company, and without regard to whether such services were provided, BMP is entitled to receive a fee equal to 1.0% of the aggregate transaction value upon the consummation of any acquisition, divestiture, disposition, merger, consolidation, restructuring, refinancing, recapitalization, issuance of private or public debt of equity securities (including an initial public offering of equity securities), financing or similar transaction by the Company.
At any time in connection with or in anticipation of a change of control of the Company, a sale of all or substantially all of the Company’s assets or an initial public offering of common equity of the Company or its successor, BMP may elect to receive, in consideration of BMP’s role in facilitating such transaction and in settlement of the termination of the services, a single lump sum cash payment equal to the then-present value of all then-current and future annual management fees payable under the transaction and management fee agreement, assuming a hypothetical termination date of the agreement to be the twelfth anniversary of such election. The transaction and management fee agreement will continue until the earlier of the twelfth anniversary of the date of the agreement or such date as the Company and BMP may mutually determine. The Company has agreed to indemnify BMP and its affiliates, directors, officers, employees, agents and representatives from and against all liabilities relating to the services contemplated by the transaction and management fee agreement and the engagement of BMP pursuant to, and the performance of BMP and its affiliates of the services contemplated by, the transaction and management fee agreement.
Intelenet Agreement: In May 2009, the Company entered into the Intelenet Agreement with Intelenet, an Indian company affiliated with the Sponsor, regarding the outsourcing of certain functions relating to billing, collections and other administrative and clerical services. A portion of such services to be outsourced were transitioned to Intelenet during 2009. A portion of such services to be outsourced have been transitioned to Intelenet and additional services are expected to be transitioned over the next 18 months. Only certain services are currently subject to the Intelenet Agreement. However, if all services currently planned for outsourcing to Intelenet are in fact handled by Intelenet, the Company expects to make payments to Intelenet of approximately $200.0 million over a seven-year period that began in the second quarter of 2009. One of the members of its board of directors, Mr. Patrick Bourke, is an employee of the Sponsor and also serves on the board of directors of Intelenet. During the three months ended March 31, 2010, the Company paid approximately $4.0 million to Intelenet.
Equity Healthcare Agreement: Effective as of January 1, 2010, the Company entered into an employer health program agreement with Equity Healthcare LLC (“Equity Healthcare”), an affiliate of the Sponsor, pursuant to which Equity Healthcare will provide to the Company certain negotiating, monitoring and other services in connection with our health benefit plans. In consideration for Equity Healthcare’s services, the
F-70
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Company will pay Equity Healthcare a fee of $2 per participating employee per month. As of March 31, 2010, the Company had approximately 6,600 employees enrolled in Equity Healthcare health benefit plans.
NOTE 13—Financial Information for Guarantor Subsidiaries and Non-Guarantor Subsidiaries
The Company conducts substantially all of its business through its subsidiaries. Substantially all of the Company’s wholly-owned domestic subsidiaries (the “Guarantors”) fully and unconditionally guarantee the Series A-1 Notes and Series A-2 Notes on a senior secured basis. The Guarantors also guarantee our ABL Facility.
See alsoNote 6—Long-Term Debt.
The following unaudited condensed consolidated financial statements quantify the financial position as of March 31, 2010 and December 31, 2009 and the operations and cash flows for the three months ended March 31, 2010 and March 31, 2009 present financial information for the parent Company issuer, the guarantor subsidiaries, the non-guarantor subsidiaries and consolidating adjustments, consisting of the entries that eliminate the investment in subsidiaries and intercompany balances and transactions.
F-71
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
CONDENSED CONSOLIDATED BALANCE SHEETS
(Unaudited)
March 31, 2010
| Parent Issuer | Guarantor Subsidiaries | Non- Guarantor Subsidiaries | Consolidating Adjustments | Condensed Consolidated | |||||||||||||||
| (in thousands) | |||||||||||||||||||
ASSETS | |||||||||||||||||||
CURRENT ASSETS | |||||||||||||||||||
Cash and cash equivalents | $ | 125,277 | $ | 7,391 | $ | 721 | $ | — | $ | 133,389 | |||||||||
Short-term investments | 19,183 | — | — | — | 19,183 | ||||||||||||||
Accounts receivable less allowance for doubtful accounts | — | 281,873 | 735 | — | 282,608 | ||||||||||||||
Inventories, net | — | 59,775 | 238 | — | 60,013 | ||||||||||||||
Deferred income taxes | 11,358 | 69,049 | — | — | 80,407 | ||||||||||||||
Deferred expenses | — | 3,017 | — | — | 3,017 | ||||||||||||||
Intercompany | 515,215 | 250,177 | — | (765,392 | ) | — | |||||||||||||
Prepaid expenses and other current assets | 5,162 | 25,725 | 11 | — | 30,898 | ||||||||||||||
TOTAL CURRENT ASSETS | 676,195 | 697,007 | 1,705 | (765,392 | ) | 609,515 | |||||||||||||
PATIENT SERVICE EQUIPMENT, less accumulated depreciation | — | 186,332 | 34 | — | 186,366 | ||||||||||||||
PROPERTY, EQUIPMENT AND IMPROVEMENTS, NET | 30,984 | 48,402 | 22 | — | 79,408 | ||||||||||||||
GOOD WILL | — | 759,947 | — | — | 759,947 | ||||||||||||||
INTANGIBLE ASSETS, NET | 460,000 | 121,352 | — | — | 581,352 | ||||||||||||||
DEFERRED DEBT ISSUANCE COSTS, NET | 60,558 | — | — | — | 60,558 | ||||||||||||||
INTERCOMPANY RECEIVABLE | 350,000 | 2,892 | — | (352,892 | ) | — | |||||||||||||
INVESTMENT IN SUBSIDIARIES | 380,253 | 619 | — | (380,872 | ) | — | |||||||||||||
OTHER ASSETS | 3,114 | 4,010 | — | — | 7,124 | ||||||||||||||
| $ | 1,961,104 | $ | 1,820,561 | $ | 1,761 | $ | (1,499,156 | ) | $ | 2,284,270 | |||||||||
LIABILITIES AND STOCKHOLDERS’ EQUITY | |||||||||||||||||||
CURRENT LIABILITIES | |||||||||||||||||||
Accounts payable | $ | — | $ | 90,125 | $ | 219 | $ | — | $ | 90,344 | |||||||||
Accrued payroll and related taxes and benefits | 4,514 | 50,121 | 99 | — | 54,734 | ||||||||||||||
Other accrued liabilities | 49,563 | 76,390 | 824 | — | 126,777 | ||||||||||||||
Deferred revenue | — | 26,806 | — | — | 26,806 | ||||||||||||||
Intercompany | 19,777 | 385,615 | — | (405,392 | ) | — | |||||||||||||
Current portion of long-term debt | 77 | 361,566 | — | (360,000 | ) | 1,643 | |||||||||||||
TOTAL CURRENT LIABILITIES | 73,931 | 990,623 | 1,142 | (765,392 | ) | 300,304 | |||||||||||||
LONG-TERM DEBT, net of current portion | 1,020,392 | 351,561 | — | (352,892 | ) | 1,019,061 | |||||||||||||
DEFERRED INCOME TAXES | 178,356 | 73,890 | — | — | 252,246 | ||||||||||||||
INCOME TAXES PAYABLE AND OTHER NON-CURRENT LIABILITIES | 9,447 | 24,234 | — | — | 33,681 | ||||||||||||||
TOTAL LIABILITIES | 1,282,126 | 1,440,308 | 1,142 | (1,118,284 | ) | 1,605,292 | |||||||||||||
STOCKHOLDERS’ EQUITY | |||||||||||||||||||
Common stock | — | 1 | — | (1 | ) | — | |||||||||||||
Additional paid-in capital | 685,495 | 396,766 | — | (396,766 | ) | 685,495 | |||||||||||||
(Accumulated deficit) retained earnings | (6,517 | ) | (16,514 | ) | 619 | 15,895 | (6,517 | ) | |||||||||||
TOTAL STOCKHOLDERS’ EQUITY | 678,978 | 380,253 | 619 | (380,872 | ) | 678,978 | |||||||||||||
| $ | 1,961,104 | $ | 1,820,561 | $ | 1,761 | $ | (1,499,156 | ) | $ | 2,284,270 | |||||||||
F-72
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
CONDENSED CONSOLIDATED BALANCE SHEETS
December 31, 2009
| Parent Issuer | Guarantor Subsidiaries | Non- Guarantor Subsidiaries | Consolidating Adjustments | Condensed Consolidated | |||||||||||||||
| (in thousands) | |||||||||||||||||||
ASSETS | |||||||||||||||||||
CURRENT ASSETS | |||||||||||||||||||
Cash and cash equivalents | $ | 150,364 | $ | 7,208 | $ | 591 | $ | — | $ | 158,163 | |||||||||
Short-term investments | 23,673 | — | — | — | 23,673 | ||||||||||||||
Accounts receivable less allowance for doubtful accounts | 7 | 251,149 | 977 | — | 252,133 | ||||||||||||||
Inventories, net | — | 68,007 | 260 | — | 68,267 | ||||||||||||||
Deferred income taxes | 17,497 | 69,519 | — | — | 87,016 | ||||||||||||||
Deferred expenses | — | 3,049 | — | — | 3,049 | ||||||||||||||
Intercompany | 606,473 | 381,071 | — | (987,544 | ) | — | |||||||||||||
Prepaid expenses and other current assets | 5,213 | 19,149 | — | — | 24,362 | ||||||||||||||
TOTAL CURRENT ASSETS | 803,227 | 799,152 | 1,828 | (987,544 | ) | 616,663 | |||||||||||||
PATIENT SERVICE EQUIPMENT, less accumulated depreciation | — | 198,770 | 38 | — | 198,808 | ||||||||||||||
PROPERTY, EQUIPMENT AND IMPROVEMENTS, NET | 28,393 | 51,805 | 29 | — | 80,227 | ||||||||||||||
GOODWILL | — | 759,197 | — | — | 759,197 | ||||||||||||||
INTANGIBLE ASSETS, NET | 460,000 | 122,259 | — | — | 582,259 | ||||||||||||||
DEFERRED DEBT ISSUANCE COSTS, NET | 63,110 | — | — | — | 63,110 | ||||||||||||||
INTERCOMPANY RECEIVABLE | 350,000 | 2,902 | — | (352,902 | ) | — | |||||||||||||
INVESTMENT IN SUBSIDIARIES | 380,455 | 538 | — | (380,993 | ) | — | |||||||||||||
OTHER ASSETS | 4,911 | 3,872 | — | — | 8,783 | ||||||||||||||
| $ | 2,090,096 | $ | 1,938,495 | $ | 1,895 | $ | (1,721,439 | ) | $ | 2,309,047 | |||||||||
LIABILITIES AND STOCKHOLDERS’ EQUITY | |||||||||||||||||||
CURRENT LIABILITIES | |||||||||||||||||||
Account payable | $ | — | $ | 123,597 | $ | 259 | $ | — | $ | 123,856 | |||||||||
Accrued payroll and related taxes and benefits | 20,893 | 45,843 | 106 | — | 66,842 | ||||||||||||||
Other accrued liabilities | 20,799 | 78,977 | 992 | — | 100,768 | ||||||||||||||
Deferred revenue | — | 27,253 | — | — | 27,253 | ||||||||||||||
Intercompany | 157,140 | 470,404 | — | (627,544 | ) | — | |||||||||||||
Current portion of long-term debt | 77 | 361,613 | — | (360,000 | ) | 1,690 | |||||||||||||
TOTAL CURRENT LIABILITIES | 198,909 | 1,107,687 | 1,357 | (987,544 | ) | 320,409 | |||||||||||||
LONG-TERM DEBT, net of current portion | 1,020,402 | 351,956 | — | (352,902 | ) | 1,019,456 | |||||||||||||
DEFERRED INCOME TAXES | 183,105 | 75,925 | — | — | 259,030 | ||||||||||||||
INCOME TAXES PAYABLE AND OTHER NON-CURRENT LIABILITIES | 8,949 | 22,472 | — | — | 31,421 | ||||||||||||||
TOTAL LIABILITIES | 1,411,365 | 1,558,040 | 1,357 | (1,340,446 | ) | 1,630,316 | |||||||||||||
STOCKHOLDERS’ EQUITY | |||||||||||||||||||
Common stock | — | 1 | — | (1 | ) | — | |||||||||||||
Additional paid-in-capital | 684,445 | 396,766 | — | (396,766 | ) | 684,445 | |||||||||||||
(Accumulated deficit) retained earnings | (5,714 | ) | (16,312 | ) | 538 | 15,774 | (5,714 | ) | |||||||||||
TOTAL STOCKHOLDERS’ EQUITY | 678,731 | 380,455 | 538 | (380,993 | ) | 678,731 | |||||||||||||
| $ | 2,090,096 | $ | 1,938,495 | $ | 1,895 | $ | (1,721,439 | ) | $ | 2,309,047 | |||||||||
F-73
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
(Unaudited)
Three Months Ended March 31, 2010
| Parent Issuer | Guarantor Subsidiaries | Non- Guarantor Subsidiaries | Consolidating Adjustments | Condensed Consolidated | ||||||||||||||
| (in thousands) | ||||||||||||||||||
Operating net revenues | $ | — | $ | 507,155 | $ | 1,721 | $ | — | $ | 508,876 | ||||||||
Net revenues from subsidiaries | 57,215 | — | — | (57,215 | ) | — | ||||||||||||
TOTAL NET REVENUES | 57,215 | 507,155 | 1,721 | (57,215 | ) | 508,876 | ||||||||||||
TOTAL COST OF NET REVENUES | — | 201,889 | 903 | — | 202,792 | |||||||||||||
Provision for doubtful accounts | — | 15,845 | 42 | — | 15,887 | |||||||||||||
Selling, distribution and administrative | 51,187 | 263,148 | 618 | (57,215 | ) | 257,738 | ||||||||||||
Amortization of intangible assets | 229 | 1,428 | — | — | 1,657 | |||||||||||||
TOTAL COSTS AND EXPENSES | 51,416 | 482,310 | 1,563 | (57,215 | ) | 478,074 | ||||||||||||
OPERATING INCOME | 5,799 | 24,845 | 158 | — | 30,802 | |||||||||||||
Interest expense | 32,459 | 113 | — | — | 32,572 | |||||||||||||
Interest income and other | (15,820 | ) | 15,660 | 77 | — | (83 | ) | |||||||||||
(LOSS) INCOME BEFORE TAXES | (10,840 | ) | 9,072 | 81 | — | (1,687 | ) | |||||||||||
Income tax expense (benefit) | (5,095 | ) | 4,211 | — | — | (884 | ) | |||||||||||
NET (LOSS) INCOME | (5,745 | ) | 4,861 | 81 | — | (803 | ) | |||||||||||
Equity in income of subsidiaries, net of tax | 4,942 | 81 | — | (5,023 | ) | — | ||||||||||||
NET (LOSS) INCOME ATTRIBUTABLE TO PARENT ISSUER | $ | (803 | ) | $ | 4,942 | $ | 81 | $ | (5,023 | ) | $ | (803 | ) | |||||
F-74
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
CONDENSED CONSOLIDATED STATEMENT OF OPERATIONS
(Unaudited)
Three Months Ended March 31, 2009
| Parent Issuer | Guarantor Subsidiaries | Non- Guarantor Subsidiaries | Consolidating Adjustments | Condensed Consolidated | |||||||||||||||
| (in thousands) | |||||||||||||||||||
Operating net revenues | $ | — | $ | 514,384 | $ | 2,047 | $ | — | $ | 516,431 | |||||||||
Net revenues from subsidiaries | 55,059 | — | — | (55,059 | ) | — | |||||||||||||
TOTAL NET REVENUES | 55,059 | 514,384 | 2,047 | (55,059 | ) | 516,431 | |||||||||||||
TOTAL COST OF NET REVENUES | — | 205,917 | 1,127 | — | 207,044 | ||||||||||||||
Provision for doubtful accounts | — | 11,351 | 5 | — | 11,356 | ||||||||||||||
Selling, distribution and administrative | 39,846 | 278,378 | 619 | (55,059 | ) | 263,784 | |||||||||||||
Amortization of intangible assets | 144 | 1,133 | — | — | 1,277 | ||||||||||||||
TOTAL COSTS AND EXPENSES | 39,990 | 496,779 | 1,751 | (55,059 | ) | 483,461 | |||||||||||||
OPERATING INCOME | 15,069 | 17,605 | 296 | — | 32,970 | ||||||||||||||
Interest expense | 32,255 | 167 | — | — | 32,422 | ||||||||||||||
Interest income and other | (23,787 | ) | 23,487 | 145 | — | (155 | ) | ||||||||||||
INCOME (LOSS) BEFORE TAXES | 6,601 | (6,049 | ) | 151 | — | 703 | |||||||||||||
Income tax expense (benefit) | 5,204 | (4,024 | ) | — | — | 1,180 | |||||||||||||
NET INCOME (LOSS) | 1,397 | (2,025 | ) | 151 | — | (477 | ) | ||||||||||||
Equity in loss of subsidiaries, net of tax | (1,874 | ) | 151 | — | 1,723 | — | |||||||||||||
NET (LOSS) INCOME ATTRIBUTABLE TO PARENT ISSUER | $ | (477 | ) | $ | (1,874 | ) | $ | 151 | $ | 1,723 | $ | (477 | ) | ||||||
F-75
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(Unaudited)
Three Months Ended March 31, 2010
| Parent Issuer | Guarantor Subsidiaries | Non- Guarantor Subsidiaries | Consolidating Adjustments | Condensed Consolidated | |||||||||||||||
| (in thousands) | |||||||||||||||||||
OPERATING ACTIVITIES | |||||||||||||||||||
NET CASH (USED IN) PROVIDED BY OPERATING ACTIVITIES | $ | (23,760 | ) | $ | 38,733 | $ | 132 | $ | — | $ | 15,105 | ||||||||
INVESTING ACTIVITIES | |||||||||||||||||||
Purchases of patient service equipment and property, equipment and improvements, exclusive of effects of acquisitions | (4,530 | ) | (22,787 | ) | (2 | ) | — | (27,319 | ) | ||||||||||
Purchases of short-term investments | (8,189 | ) | — | — | — | (8,189 | ) | ||||||||||||
Maturities of short-term investments | 12,680 | — | — | — | 12,680 | ||||||||||||||
Proceeds from disposition of assets | — | 15 | — | — | 15 | ||||||||||||||
Cash paid for acquisitions | — | (1,200 | ) | — | — | (1,200 | ) | ||||||||||||
NET CASH USED IN INVESTING ACTIVITIES | (39 | ) | (23,972 | ) | (2 | ) | — | (24,013 | ) | ||||||||||
FINANCING ACTIVITIES | |||||||||||||||||||
Payments on other long term debt | — | (441 | ) | — | — | (441 | ) | ||||||||||||
Change in book cash overdraft included in accounts payable | — | (14,137 | ) | — | — | (14,137 | ) | ||||||||||||
Debt issuance costs | (1,210 | ) | — | — | — | (1,210 | ) | ||||||||||||
Cash paid on profit interest units | (78 | ) | — | — | — | (78 | ) | ||||||||||||
NET CASH USED IN FINANCING ACTIVITIES | (1,288 | ) | (14,578 | ) | — | — | (15,866 | ) | |||||||||||
NET (DECREASE) INCREASE IN CASH AND CASH EQUIVALENTS | (25,087 | ) | 183 | 130 | — | (24,774 | ) | ||||||||||||
CASH AND CASH EQUIVALENTS AT BEGINNING OF PERIOD | 150,364 | 7,208 | 591 | — | 158,163 | ||||||||||||||
CASH AND CASH EQUIVALENTS AT END OF PERIOD | $ | 125,277 | $ | 7,391 | $ | 721 | $ | — | $ | 133,389 | |||||||||
F-76
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(Unaudited)
Three Months Ended March 31,2009
| Parent Issuer | Guarantor Subsidiaries | Non- Guarantor Subsidiaries | Consolidating Adjustments | Condensed Consolidated | |||||||||||||||
| (in thousands) | |||||||||||||||||||
OPERATING ACTIVITIES | |||||||||||||||||||
NET CASH (USED IN) PROVIDED BY OPERATING ACTIVITIES | $ | (4,766 | ) | $ | 34,219 | $ | 219 | $ | — | $ | 29,672 | ||||||||
INVESTING ACTIVITIES | |||||||||||||||||||
Purchases of patient service equipment and property, equipment and improvements | (4,163 | ) | (35,666 | ) | (14 | ) | — | (39,843 | ) | ||||||||||
Proceeds from disposition of assets | — | 251 | — | — | 251 | ||||||||||||||
NET CASH USED IN INVESTING ACTIVITIES | (4,163 | ) | (35,415 | ) | (14 | ) | — | (39,592 | ) | ||||||||||
FINANCING ACTIVITIES | |||||||||||||||||||
Proceeds from ABL Facility | 378 | — | — | — | 378 | ||||||||||||||
Payments on ABL Facility | (6,035 | ) | — | — | — | (6,035 | ) | ||||||||||||
Payments on other long term debt | — | (688 | ) | — | — | (688 | ) | ||||||||||||
Change in book cash overdraft included in accounts payable | — | (2,029 | ) | — | — | (2,029 | ) | ||||||||||||
Debt issuance costs | (206 | ) | — | — | — | (206 | ) | ||||||||||||
Equity contributions | 1,075 | — | — | — | 1,075 | ||||||||||||||
NET CASH USED IN FINANCING ACTIVITIES | (4,788 | ) | (2,717 | ) | — | — | (7,505 | ) | |||||||||||
NET (DECREASE) INCREASE IN CASH AND CASH EQUIVALENTS | (13,717 | ) | (3,913 | ) | 205 | — | (17,425 | ) | |||||||||||
CASH AND CASH EQUIVALENTS AT BEGINNING OF PERIOD | 159,393 | 8,260 | 365 | — | 168,018 | ||||||||||||||
CASH AND CASH EQUIVALENTS AT END OF PERIOD | $ | 145,676 | $ | 4,347 | $ | 570 | $ | — | $ | 150,593 | |||||||||
NOTE 14—Subsequent Events
The Company evaluated all subsequent events that occurred after the balance sheet date through July 16, 2010.
F-77
Table of Contents

Table of Contents
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
| Item 20. | Indemnification of Directors and Officers. |
(a) The following entities are incorporated under the laws of Delaware: Apria Healthcare Group Inc., Apria Care Management Systems, Inc., ApriaDirect.Com, Inc., Apria Healthcare, Inc., Coram Healthcare Corporation of Greater D.C., Coram Healthcare Corporation of Utah, Coram Clinical Trials, Inc., Coram Healthcare Corporation of North Texas, Coram Healthcare Corporation of Northern California, Coram Healthcare Corporation of South Carolina, Coram Healthcare Corporation of Southern California, Coram Healthcare Corporation of Southern Florida, Coram Healthcare Corporation of Mississippi, Coram Healthcare Corporation of Nevada, Coram Healthcare Corporation of Indiana, Coram Healthcare Corporation of Alabama, Coram Healthcare Corporation of Florida, Coram Homecare of Minnesota, Inc., Coram Service Corporation, Coram Specialty Infusion Services, Inc., Coram Alternate Site Services, Inc., H.M.S.S., Inc., T2 Medical, inc., Coram, Inc. and Coram Healthcare Corporation of Massachusetts (collectively, the “Delaware Corporations”).
Delaware General Corporation Law
Section 145(a) of the Delaware General Corporation Law provides that a corporation may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative (other than an action by or in the right of the corporation) by reason of the fact that such person is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise, against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by such person in connection with such action, suit or proceeding if such person acted in good faith and in a manner such person reasonably believed to be in or not opposed to the best interests of the corporation, and, with respect to any criminal action or proceeding, had no reasonable cause to believe such person’s conduct was unlawful.
Section 145(b) of the Delaware General Corporation Law provides that a corporation may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action or suit by or in the right of the corporation to procure a judgment in its favor by reason of the fact that such person is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise against expenses (including attorneys’ fees) actually and reasonably incurred by such person in connection with the defense or settlement of such action or suit if such person acted in good faith and in a manner such person reasonably believed to be in or not opposed to the best interests of the corporation and except that no indemnification shall be made in respect of any claim, issue or matter as to which such person shall have been adjudged to be liable to the corporation unless and only to the extent that the Delaware Court of Chancery or the court in which such action or suit was brought shall determine upon application that, despite the adjudication of liability but in view of all the circumstances of the case, such person is fairly and reasonably entitled to indemnity for such expenses which the Delaware Court of Chancery or such other court shall deem proper.
Section 145(c) of the Delaware General Corporation Law provides that to the extent that a director, officer, employee or agent of a corporation has been successful on the merits or otherwise in defense of any action, suit or proceeding referred to in Section 145(a) and (b), or in defense of any claim, issue or matter therein, such person shall be indemnified against expenses (including attorneys’ fees) actually and reasonably incurred by such person in connection therewith.
Section 145(d) of the Delaware General Corporation Law provides that any indemnification under Section 145(a) and (b) (unless ordered by a court) shall be made by the corporation only as authorized in the
II-1
Table of Contents
specific case upon a determination that indemnification of the director, officer, employee or agent is proper in the circumstances because such person has met the applicable standard of conduct set forth in Section 145(a) and (b). Such determination shall be made (1) by a majority vote of the directors who were not parties to such action, suit or proceeding, even though less than a quorum, or (2) if there are no such directors, or if such directors so direct, by independent legal counsel in a written opinion, or (3) by the stockholders.
Section 145(e) of the Delaware General Corporation Law provides that expenses (including attorneys’ fees) incurred by an officer or director in defending any civil, criminal, administrative or investigative action, suit or proceeding may be paid by the corporation in advance of the final disposition of such action, suit or proceeding upon receipt of an undertaking by or on behalf of such director or officer to repay such amount if it shall ultimately be determined that such person is not entitled to be indemnified by the corporation as authorized in Section 145. Such expenses (including attorneys’ fees) incurred by other employees and agents may be so paid upon such terms and conditions, if any, as the board of directors deems appropriate.
Section 145(f) of the Delaware General Corporation Law provides that the indemnification and advancement of expenses provided by, or granted pursuant to, Section 145 shall not be deemed exclusive of any other rights to which those seeking indemnification or advancement of expenses may be entitled under any bylaw, agreement, vote of stockholders or disinterested directors or otherwise.
Section 145(g) of the Delaware General Corporation Law provides that a corporation shall have the power to purchase and maintain insurance on behalf of any person who is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise against any liability asserted against such person and incurred by such person in any such capacity, or arising out of such person’s capacity as such, whether or not the corporation would have the power to indemnify such person against such liability under Section 145.
Section 174 of the Delaware General Corporation Law provides, among other things, that a director, who willfully or negligently approves of an unlawful payment of dividends or an unlawful stock purchase or redemption, may be held liable for such actions. A director who was either absent when the unlawful actions were approved or dissented at the time, may avoid liability by causing his or her dissent to such actions to be entered in the books containing the minutes of the meetings of the board of directors at the time such action occurred or immediately after such absent director receives notice of the unlawful acts.
Organizational Documents of Delaware Registrants
The articles of incorporation and/or bylaws of each of the Delaware Corporations provide that, to the fullest extent permitted by the Delaware General Corporation Law, the corporation shall indemnify any current or former Director or officer of the corporation and may, at the discretion of the Board of Directors, indemnify any current or former employee or agent of the corporation against all expenses, judgments, fines and amounts paid in settlement actually and reasonably incurred by him or her in connection with any threatened, pending or completed action, suit or proceeding brought by or in the right of the corporation or otherwise, to which he or she was or is a party or is threatened to be made a party by reason of his or her current or former position with the corporation or by reason of the fact that he or she is or was serving, at the request of the corporation, as a director, officer, partner, member, employee or agent of another corporation, partnership, limited liability company, joint venture, trust or other enterprise (including the heirs, executor, administrators or estate of such person).
(b) Coram Healthcare of Wyoming L.L.C and CoramRX, LLC are limited liability companies organized under the laws of Delaware.
Section 18-108 of the Delaware Limited Liability Company Act empowers a Delaware limited liability company to indemnify and hold harmless any member or manager of the limited liability company from and against any and all claims and demands whatsoever.
II-2
Table of Contents
The limited liability company agreements of each of Coram Healthcare of Wyoming L.L.C. and CoramRX, LLC provide that neither the sole member nor any director, officer or employee, or other agent or representative (each a “Covered Person”) shall be liable to the company, the sole member or any other person or entity who or that has an interest in the company for any loss, damage or claim incurred by reason of any act or omission performed or omitted by such Covered Person in good faith on behalf of the company and in a manner reasonably believed to be within the scope of the authority conferred on such Covered Person, except that a Covered Person shall be liable for any such loss, damage or claim incurred by reason of such Covered Person’s gross negligence or willful misconduct. To the full extent permitted by applicable law, each Covered Person shall be entitled to indemnification from the company for any loss, damage or claim incurred by such Covered Person by reason of any act or omission performed or omitted by such Covered Person in good faith on behalf of the Company and in a manner reasonably believed to be within the scope of the authority conferred on such Covered Person, except that no Covered Person shall be entitled to be indemnified in respect of any loss, damage or claim incurred by such Covered Person by reason of gross negligence or willful misconduct with respect to such acts or omissions; provided, however, that any indemnity shall be provided out of and to the extent of company assets only, and the sole member shall not have personal liability on account thereof.
(c) Apria Healthcare of New York State, Inc., Coram Healthcare Corporation of Greater New York and Coram Healthcare Corporation of New York (collectively, the “New York Corporations”) are incorporated under the laws of New York.
New York Business Corporation Law
Section 722(a) of the New York Business Corporation Law (“NYBCL”) provides that a corporation may indemnify any officer or director made, or threatened to be made, a party to an action or proceeding (other than one by or in the right of the corporation to procure judgment in its favor), whether civil or criminal, including an action by or in the right of any other corporation, or other enterprise, which any director or officer of the corporation served in any capacity at the request of the corporation, by reason of the fact that he was a director or officer of the corporation, or served such other corporation or other enterprise in any capacity, against judgments, fines, amounts paid in settlement and reasonable expenses, including attorneys’ fees actually and necessarily incurred as a result of such action or proceeding, or any appeal therein, if such director or officer acted, in good faith, for a purpose which he reasonably believed to be in, or, in the case of service for any other corporation or other enterprise, not opposed to, the best interests of the corporation and, in criminal actions or proceedings, had no reasonable cause to believe that his conduct was unlawful.
Section 722(c) of the NYBCL provides that a corporation may indemnify any officer or director made, or threatened to be made, a party to an action by or in the right of the corporation to procure judgment in its favor by reason of the fact that he is or was a director or officer of the corporation, or is or was serving at the request of the corporation as a director or officer of any other corporation of any type or kind, or other enterprise, against amounts paid in settlement and reasonable expenses, including attorneys’ fees, actually and necessarily incurred by him in connection with the defense or settlement of such action, or in connection with an appeal therein, if such director or officer acted, in good faith, for a purpose which he reasonably believed to be in, or, in the case of service for another corporation or other enterprise, not opposed to, the best interests of the corporation. The corporation may not, however, indemnify any officer or director pursuant to Section 722(c) in respect of (1) a threatened action, or a pending action which is settled or otherwise disposed of, or (2) any claim, issue or matter as to which such person shall have been adjudged to be liable to the corporation, unless and only to the extent that the court in which the action was brought or, if no action was brought, any court of competent jurisdiction, determines upon application, that the person is fairly and reasonably entitled to indemnity for such portion of the settlement and expenses as the court deems proper.
Section 723 of the NYBCL provides that an officer or director who has been successful, on the merits or otherwise, in the defense of a civil or criminal action or proceeding of the character set forth in Section 722 is entitled to indemnification as permitted in such section. Section 724 of the NYBCL permits a court to award the indemnification required by Section 722.
II-3
Table of Contents
Section 721 of the NYBCL provides that, in addition to indemnification provided in Article 7 of the NYBCL, a corporation may indemnify a director or officer by a provision contained in the certificate of incorporation or by-laws or by a duly authorized resolution of its shareholders or directors or by agreement, provided that no indemnification may be made to or on behalf of any director or officer if a judgment or other final adjudication adverse to the director or officer establishes that his acts were committed in bad faith or were the result of active and deliberate dishonesty and were material to the cause of action so adjudicated, or that such director or officer personally gained in fact a financial profit or other advantage to which he was not legally entitled.
Section 402(b) of the NYBCL provides that a corporation’s certificate of incorporation may include a provision eliminating or limiting the personal liability of its directors to the corporation or its shareholders for damages for any breach of duty in such capacity, except (i) liability of a director if a judgment or other final adjudication adverse to such director establishes that the director’s acts or omissions were in bad faith or involved intentional misconduct or a knowing violation of law or that he personally gained in fact a financial profit or other advantage to which he was not legally entitled or that his acts violated Section 719 of the NYBCL or (ii) liability of any director for any act or omission prior to the adoption of a provision authorized by Section 402(b) of the NYBCL
Organizational Documents of New York Registrants
The articles of incorporation and/or bylaws of each of the New York Corporations provide that, to the fullest extent permitted by the NYBCL, the corporation shall indemnify any current or former Director or officer of the corporation and may, at the discretion of the Board of Directors, indemnify any current or former employee or agent of the corporation against all expenses, judgments, fines and amounts paid in settlement actually and reasonably incurred by him or her in connection with any threatened, pending or completed action, suit or proceeding brought by or in the right of the corporation or otherwise, to which he or she was or is a party or is threatened to be made a party by reason of his or her current or former position with the corporation or by reason of the fact that he or she is or was serving, at the request of the corporation, as a director, officer, partner, member, employee or agent of another corporation, partnership, limited liability company, joint venture, trust or other enterprise (including the heirs, executor, administrators or estate of such person).
(d) AHNY-DME LLC and AHNY-IV LLC are limited liability companies organized under the laws of New York.
Section 420 of the New York Limited Liability Company Law provides that a limited liability company may indemnify and hold harmless, and advance expenses to, any member, manager or other person, or any testator or intestate of such member, manager or other person, from and against any and all claims and demands whatsoever; provided, however, that no indemnification may be made to or on behalf of any member, manager or other person if a judgment or other final adjudication adverse to such member, manager or other person establishes (a) that his or her acts were committed in bad faith or were the result of active and deliberate dishonesty and were material to the cause of action so adjudicated or (b) that he or she personally gained in fact a financial profit or other advantage to which he or she was not legally entitled.
The limited liability company agreements of each of AHNY-DME LLC and AHNY-IV LLC provide that each Covered Person shall not be liable to the company, the sole member or any other person or entity who or that has an interest in the company for any loss, damage or claim incurred by reason of any act or omission performed or omitted by such Covered Person in good faith on behalf of the company and in a manner reasonably believed to be within the scope of the authority conferred on such Covered Person, except that a Covered Person shall be liable for any such loss, damage or claim incurred by reason of such Covered Person’s gross negligence or willful misconduct. To the full extent permitted by applicable law, each Covered Person shall be entitled to indemnification from the company for any loss, damage or claim incurred by such Covered Person by reason of any act or omission performed or omitted by such Covered Person in good faith on behalf of the Company and in a manner reasonably believed to be within the scope of the authority conferred on such Covered Person, except that no Covered Person shall be entitled to be indemnified in respect of any loss, damage or claim
II-4
Table of Contents
incurred by such Covered Person by reason of gross negligence or willful misconduct with respect to such acts or omissions; provided, however, that any indemnity shall be provided out of and to the extent of company assets only, and the sole member shall not have personal liability on account thereof.
(e) HealthInfusion, Inc. is a corporation incorporated under the laws of Florida.
Florida Business Corporation Act
Under Section 607.0850, Florida Statutes, the Company is entitled to indemnify its directors and officers against reasonable expenses incurred by such persons in certain proceedings by reason of the fact that the person is or was a director or officer of the Company, subject to certain limitations.
Organizational Documents of Florida Registrant
The articles of incorporation and the bylaws of Healthinfusion, Inc. provide that, to the fullest extent permitted by the Florida Business Corporation Act, or otherwise pursuant to applicable Florida law, the corporation shall indemnify any current or former Director or officer of the corporation and may, at the discretion of the Board of Directors, indemnify any current or former employee or agent of the corporation against all expenses, judgments, fines and amounts paid in settlement actually and reasonably incurred by him or her in connection with any threatened, pending or completed action, suit or proceeding brought by or in the right of the corporation or otherwise, to which he or she was or is a party or is threatened to be made a party by reason of his or her current or former position with the corporation or by reason of the fact that he or she is or was serving, at the request of the corporation, as a director, officer, partner, member, employee or agent of another corporation, partnership, limited liability company, joint venture, trust or other enterprise (including the heirs, executor, administrators or estate of such person).
II-5
Table of Contents
| Item 21. | Exhibits and Financial Statement Schedules. |
(a) Exhibits
| Exhibit No. | Description | |
| 2.1* | Agreement and Plan of Merger, dated as of June 18, 2008, by and among Apria Healthcare Group Inc., Sky Acquisition LLC and Sky Merger Sub Corporation | |
| 3.1 | Second Amended and Restated Certificate of Incorporation of Apria Healthcare Group Inc. | |
| 3.2 | Amended and Restated Bylaws of Apria Healthcare Group Inc. | |
| 3.3 | Amended and Restated Certificate of Incorporation of Apria Healthcare, Inc. | |
| 3.4 | Amended and Restated Bylaws of Apria Healthcare, Inc. | |
| 3.5 | Amended and Restated Certificate of Incorporation of ApriaCare Management Systems, Inc. | |
| 3.6 | Amended and Restated Bylaws of ApriaCare Management Systems, Inc. | |
| 3.7 | Certificate of Incorporation of ApriaDirect.Com, Inc. | |
| 3.8 | Amended and Restated Bylaws of ApriaDirect.Com, Inc. | |
| 3.9 | Restated Certificate of Incorporation of Apria Healthcare of New York State, Inc. | |
| 3.10 | Amended and Restated Bylaws of Apria Healthcare of New York State, Inc. | |
| 3.11 | Restated Certificate of Incorporation of Coram Alternate Site Services, Inc. | |
| 3.12 | Amended and Restated Bylaws of Coram Alternate Site Services, Inc. | |
| 3.13 | Restated Certificate of Incorporation of Coram Clinical Trials, Inc. | |
| 3.14 | Amended and Restated Bylaws of Coram Clinical Trials, Inc. | |
| 3.15 | Restated Certificate of Incorporation of Coram Healthcare Corporation of Alabama | |
| 3.16 | Amended and Restated Bylaws of Coram Healthcare Corporation of Alabama | |
| 3.17 | Restated Certificate of Incorporation of Coram Healthcare Corporation of Florida | |
| 3.18 | Amended and Restated Bylaws of Coram Healthcare Corporation of Florida | |
| 3.19 | Restated Certificate of Incorporation of Coram Healthcare Corporation of Greater D.C. | |
| 3.20 | Amended and Restated Bylaws of Coram Healthcare Corporation of Greater D.C. | |
| 3.21 | Restated Certificate of Incorporation of Coram Healthcare Corporation of Greater New York | |
| 3.22 | Amended and Restated Bylaws of Coram Healthcare Corporation of Greater New York | |
| 3.23 | Restated Certificate of Incorporation of Coram Healthcare Corporation of Indiana | |
| 3.24 | Amended and Restated Bylaws of Coram Healthcare Corporation of Indiana | |
| 3.25 | Restated Certificate of Incorporation of Coram Healthcare Corporation of Massachusetts | |
| 3.26 | Amended and Restated Bylaws of Coram Healthcare Corporation of Massachusetts | |
| 3.27 | Restated Certificate of Incorporation of Coram Healthcare Corporation of Mississippi | |
| 3.28 | Amended and Restated Bylaws of Coram Healthcare Corporation of Mississippi | |
| 3.29 | Restated Certificate of Incorporation of Coram Healthcare Corporation of Nevada | |
| 3.30 | Amended and Restated Bylaws of Coram Healthcare Corporation of Nevada | |
| 3.31 | Restated Certificate of Incorporation of Coram Healthcare Corporation of New York | |
| 3.32 | Amended and Restated Bylaws of Coram Healthcare Corporation of New York | |
| 3.33 | Restated Certificate of Incorporation of Coram Healthcare Corporation of North Texas | |
II-6
Table of Contents
| Exhibit No. | Description | |
| 3.34 | Amended and Restated Bylaws of Coram Healthcare Corporation of North Texas | |
| 3.35 | Restated Certificate of Incorporation of Coram Healthcare Corporation of Northern California | |
| 3.36 | Amended and Restated Bylaws of Coram Healthcare Corporation of Northern California | |
| 3.37 | Restated Certificate of Incorporation of Coram Healthcare Corporation of South Carolina | |
| 3.38 | Amended and Restated Bylaws of Coram Healthcare Corporation of South Carolina | |
| 3.39 | Restated Certificate of Incorporation of Coram Healthcare Corporation of Southern California | |
| 3.40 | Amended and Restated Bylaws of Coram Healthcare Corporation of Southern California | |
| 3.41 | Restated Certificate of Incorporation of Coram Healthcare Corporation of Southern Florida | |
| 3.42 | Amended and Restated Bylaws of Coram Healthcare Corporation of Southern Florida | |
| 3.43 | Restated Certificate of Incorporation of Coram Healthcare Corporation of Utah | |
| 3.44 | Amended and Restated Bylaws of Coram Healthcare Corporation of Utah | |
| 3.45 | Certificate of Formation of Coram Healthcare of Wyoming, L.L.C. | |
| 3.46 | Amended and Restated Limited Liability Company Agreement of Coram Healthcare of Wyoming, L.L.C. | |
| 3.47 | Restated Certificate of Incorporation of Coram Homecare of Minnesota, Inc. | |
| 3.48 | Amended and Restated Bylaws of Coram Homecare of Minnesota, Inc. | |
| 3.49 | Restated Certificate of Incorporation of Coram Specialty Infusion Services, Inc. | |
| 3.50 | Amended and Restated Bylaws of Coram Specialty Infusion Services, Inc. | |
| 3.51 | Fourth Amended and Restated Certificate of Incorporation of Coram, Inc. | |
| 3.52 | Amended and Restated Bylaws of Coram, Inc. | |
| 3.53 | Certificate of Formation of CoramRx, LLC | |
| 3.54 | Amended and Restated Limited Liability Company Agreement of CoramRx, LLC | |
| 3.55 | Restated Certificate of Incorporation of Coram Service Corporation | |
| 3.56 | Amended and Restated Bylaws of Coram Service Corporation | |
| 3.57 | Second Restated Certificate of Incorporation of H.M.S.S., Inc. | |
| 3.58 | Amended and Restated Bylaws of H.M.S.S., Inc. | |
| 3.59 | Second Amended and Restated Articles of Incorporation of HealthInfusion, Inc. and Corporation Reinstatement | |
| 3.60 | Amended and Restated Bylaws of HealthInfusion, Inc. | |
| 3.61 | Second Restated Certificate of Incorporation of T2 Medical, Inc. | |
| 3.62 | Amended and Restated Bylaws of T2 Medical, Inc. | |
| 3.63 | Articles of Organization of AHNY-DME LLC | |
| 3.64 | Limited Liability Company Agreement of AHNY-DME LLC | |
| 3.65 | Articles of Organization of AHNY-IV LLC | |
| 3.66 | Limited Liability Company Agreement of AHNY-IV LLC | |
| 4.1 | Indenture, dated as of May 27, 2009 (the “Indenture”), among Apria Healthcare Group Inc., the guarantors thereto and U.S. Bank National Association, as trustee. | |
| 4.2 | First Supplemental Indenture, dated as of August 13, 2009, among Apria Healthcare Group Inc., the guarantors thereto and U.S. Bank National Association, as trustee | |
| 4.3 | Second Supplemental Indenture, dated as of July 13, 2010, among Apria Healthcare Group Inc., the guarantors thereto and U.S. bank National Association |
II-7
Table of Contents
| Exhibit No. | Description | |
| 4.4 | Registration Rights Agreement, dated as of May 27, 2009, among Apria Healthcare Group Inc., the guarantors named therein and Banc of America Securities LLC, Wells Fargo Securities, LLC (formerly known as Wachovia Capital Markets, LLC), Barclays Capital Inc. and Scotia Capital (USA) Inc. | |
| 4.5 | Registration Rights Agreement, dated as of August 13, 2009, among Apria Healthcare Group Inc., the guarantors named therein and Banc of America Securities LLC, Wells Fargo Securities, LLC, Barclays Capital Inc. and Scotia Capital (USA) Inc. | |
| 4.6 | Form of Note (attached as exhibit to Exhibit 4.1) | |
| 5.1 | Opinion of Simpson Thacher & Bartlett LLP | |
| 5.2 | Opinion of Holland & Knight LLP | |
| 10.1 | Transaction and Management Fee Agreement, dated as of October 28, 2008, among Apria Healthcare Group Inc. (as successor to Sky Merger Sub Corporation) and Blackstone Management Partners V L.L.C. | |
| 10.2** | Master Service Agreement (the “Master Service Agreement”), dated as of May 14, 2009, between Apria Healthcare Group Inc. and Intelenet Global Services Private Limited, and amendments thereto | |
| 10.3** | Amendment No. 1 to the Master Service Agreement, dated as of September 18, 2009 | |
| 10.4 | Employment Agreement, dated November 21, 2008, among Norman C. Payson, Apria Healthcare Group Inc. and BP Healthcare Holdings LLC | |
| 10.5 | Employment Agreement, dated December 19, 2008, between Chris A. Karkenny and Apria Healthcare Group Inc. | |
| 10.6 | Amended and Restated Employment Agreement, effective as of October 24, 2008, between Lawrence A. Mastrovich and Apria Healthcare Group Inc. | |
| 10.7 | Amendment to Employment Agreement, dated April 3, 2009, between Lawrence A. Mastrovich and Apria Healthcare Group Inc. | |
| 10.8 | Offer Letter, dated as of March 10, 2009, from Apria Healthcare Group Inc. to James Gallas | |
| 10.9 | Amended and Restated Executive Severance Agreement, dated as of March 10, 2009, between James Gallas and Apria Healthcare Group Inc. | |
| 10.10 | Amended and Restated Employment Agreement, dated as of October 24, 2008 between Daniel E. Greenleaf and Apria Healthcare Group Inc. | |
| 10.11 | Amended and Restated Noncompetition Agreement, dated as of March 7, 2007, between Apria Healthcare Group Inc. and Chris A. Karkenny | |
| 10.12 | Amended and Restated Noncompetition and Nonsolicitation Agreement, dated as of October 24, 2008, between Apria Healthcare Group Inc. and Daniel E. Greenleaf | |
| 10.13 | Management Unit Subscription Agreement (Class B Units), dated as of November 21, 2008, between Norman C. Payson and BP Healthcare Holdings LLC | |
| 10.14 | Management Unit Subscription Agreement (Class A-2 Units, Class B Units and Class C Units), dated as of December 19, 2008, between and Chris A. Karkenny and Sky Acquisition LLC | |
| 10.15 | Form of Management Unit Subscription Agreement for Class B Units and Class C Units of Apria Holdings LLC | |
| 10.16 | Assignment and Assumption Agreement, dated as of March 25, 2010, between Sky Acquisition LLC and Apria Holdings LLC | |
II-8
Table of Contents
| Exhibit No. | Description | |
| 10.17 | Form of Annual Executive Bonus Plan of Apria Healthcare Group Inc. | |
| 10.18 | Credit Agreement, dated as of October 28, 2008 (the “ABL Credit Agreement”), among Apria Healthcare Group Inc., Sky Acquisition LLC, the other borrowers party thereto, Bank of America, N.A. as administrative agent and collateral agent thereunder (the “ABL Collateral Agent”), and the other agents and lenders parties thereto | |
| 10.19 | Amendment No. 1 to the ABL Credit Agreement, dated as of May 27, 2009 | |
| 10.20 | Supplement No. 1 to the ABL Credit Agreement, dated as of July 13, 2010 | |
| 10.21 | Guaranty, dated as of October 28, 2008 (the “ABL Guaranty”), among Sky Acquisition LLC, certain subsidiaries of Sky Acquisition LLC from time to time party hereto and the ABL Collateral Agent | |
| 10.22 | Supplement No. 1 to the ABL Guaranty, dated as of July 13, 2010 | |
| 10.23 | Security Agreement, dated as of October 28, 2008 (the “ABL Security Agreement”), among Sky Acquisition LLC, Sky Merger Sub Corporation, Apria Healthcare Group Inc., the other grantors party thereto and the ABL Collateral Agent | |
| 10.24 | Supplement No. 1 to the ABL Security Agreement, dated as of July 13, 2010 | |
| 10.25 | Security Agreement, dated as of October 28, 2008 (the “Notes Security Agreement”) among Sky Acquisition LLC, Sky Merger Sub Corporation, Apria Healthcare Group Inc., the other grantors party thereto and Bank of America, N.A. Collateral Agent† | |
| 10.26 | Supplement No. 1 to the Notes Security Agreement, dated as of July 13, 2010, among U.S. Bank National Association, as collateral agent, and the grantors party thereto | |
| 10.27 | Lien Subordination and Intercreditor Agreement, dated as of October 28, 2008, among the ABL Collateral Agent, the Term Debt Collateral Agent, Sky Acquisition LLC, Sky Merger Sub Corporation, Apria Healthcare Group Inc., and the Guarantors party thereto | |
| 10.28*** | Certification of Compliance Agreement, dated August 22, 2007, between the Office of Inspector General of the U.S. Department of Health and Human Services and Coram Inc. | |
| 12.1 | Computation of Ratio of Earnings to Fixed Charges | |
| 21.1 | Subsidiaries of Apria Healthcare Group Inc. | |
| 23.1 | Consent of Simpson Thacher & Bartlett LLP (included as part of its opinion filed as Exhibit 5.1 hereto). | |
| 23.2 | Consent of Holland & Knight LLP (included as part of its opinion filed as Exhibit 5.2 hereto). | |
| 23.4 | Consent of Deloitte & Touche LLP | |
| 24.1 | Powers of Attorney (included in signature pages of the initial filing of this Registration Statement). | |
| 25.1 | Form T-1 Statement of Eligibility under the Trust Indenture Act of 1939 of U.S. Bank National Association with respect to the Indenture governing the 11.25% Senior Secured Notes due 2014 (Series A-1) and 12.375% Senior Secured Notes due 2014 (Series A-2) | |
| 99.1 | Form of Letter of Transmittal | |
| 99.2 | Form of Letter to Brokers, Dealers, Commercial Banks, Trust Companies and Other Nominees | |
| 99.3 | Form of Letter to Clients | |
| 99.4 | Form of Notice of Guaranteed Delivery | |
| * | Incorporated by reference to Current Report on Form 8-K, dated June 18, 2008, filed on June 20, 2008. |
| ** | Portions of this exhibit have been omitted pursuant to a request for confidential treatment. |
| *** | Incorporated by reference to Annual Report on Form 10-K for the fiscal year ended December 31, 2007, filed by the Company on February 29, 2008. |
| † | On August 13, 2009, U.S. Bank National Association succeeded Bank of America, N.A. as the Notes collateral agent. |
II-9
Table of Contents
(b) Financial Statement Schedules
SCHEDULE II—VALUATION AND QUALIFYING ACCOUNTS
| Balance at Beginning of Period | Increase Due to Coram Acquisition | Charged to Costs and Expenses | Deductions | Balance at End of Period | |||||||||||
| (in thousands) | |||||||||||||||
Year ended December 31, 2009 (Successor) Deducted from asset accounts: | |||||||||||||||
Allowance for doubtful accounts | $ | 25,271 | $ | — | $ | 57,919 | $ | 43,263 | $ | 39,927 | |||||
Reserve for inventory and patient service equipment shortages | $ | 6,091 | $ | — | $ | 2,327 | $ | 6,241 | $ | 2,177 | |||||
Period October 29, 2008 to Deducted from asset accounts: | |||||||||||||||
Allowance for doubtful accounts | $ | 35,286 | $ | — | $ | 14,329 | $ | 24,344 | $ | 25,271 | |||||
Reserve for inventory and patient service equipment shortages | $ | 5,914 | $ | — | $ | 1,554 | $ | 1,377 | $ | 6,091 | |||||
Period January 1, 2008 to Deducted from asset accounts: | |||||||||||||||
Allowance for doubtful accounts | $ | 47,823 | $ | — | $ | 33,626 | $ | 46,163 | $ | 35,286 | |||||
Reserve for inventory and patient service equipment shortages | $ | 5,366 | $ | — | $ | 2,583 | $ | 2,035 | $ | 5,914 | |||||
Year ended December 31, 2007 (Predecessor) Deducted from asset accounts: | |||||||||||||||
Allowance for doubtful accounts | $ | 27,324 | $ | 21,557 | $ | 43,138 | $ | 44,196 | $ | 47,823 | |||||
Reserve for inventory and patient service equipment shortages | $ | 4,420 | $ | 195 | $ | 3,351 | $ | 2,600 | $ | 5,366 | |||||
| Item 22. | Undertakings. |
(a) Each of the undersigned registrants hereby undertakes:
(1) to file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i) to include any prospectus required by Section 10(a)(3) of the Securities Act;
(ii) to reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in the volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Securities and Exchange Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20 percent change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and
(iii) to include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement;
II-10
Table of Contents
(2) that, for the purpose of determining any liability under the Securities Act, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof;
(3) to remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering;
(4) that, for the purpose of determining liability under the Securities Act to any purchaser, if the registrants are subject to Rule 430C, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use; and
(5) that, for the purpose of determining liability of the registrants under the Securities Act to any purchaser in the initial distribution of the securities, each of the undersigned registrants undertakes that in a primary offering of securities of the undersigned registrants pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrants will be sellers to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i) Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424;
(ii) Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
(iii) The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
(iv) Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
(b) Each of the undersigned registrants hereby undertakes to respond to requests for information that is incorporated by reference into the prospectus pursuant to Items 4, 10(b), 11 or 13 of this form, within one business day of receipt of such request, and to send the incorporated documents by first class mail or equally prompt means. This includes information contained in documents filed subsequent to the effective date of the registration statement through the date of responding to the request.
(c) Each of the undersigned registrants hereby undertakes to supply by means of a post-effective amendment all information concerning a transaction, and the company being acquired involved therein, that was not the subject of and included in the registration statement when it became effective.
(d) Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers, or controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrants of expenses incurred or paid by a director, officer or controlling person of the registrants in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrants will, unless in the opinion of it counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
II-11
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Lake Forest, State of California, on July 16, 2010.
APRIA HEALTHCARE GROUP INC. | ||
By: | /s/ Norman C. Payson | |
Name: Norman C. Payson, M.D. | ||
Title: Executive Chairman of the Board of Directors and Chief Executive Officer | ||
SIGNATURES AND POWERS OF ATTORNEY
Each person whose signature appears below hereby constitutes and appoints Robert S. Holcombe and Peter A. Reynolds and each of them, the true and lawful attorneys-in-fact and agents of the undersigned, with full power of substitution and resubstitution, for and in the name, place and stead of the undersigned, in any and all capacities, to sign this Registration Statement and any and all amendments (including post-effective amendments) to this Registration Statement, including any filings pursuant to Rule 462(b) under the Securities Act of 1933, as amended, and to file the same, with all exhibits thereto, and all other documents in connection therewith, with the Securities and Exchange Commission, and hereby grants to such attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and anything necessary to be done, as fully to all intents and purposes as the undersigned might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them, or their or his substitute, or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Title | Date | ||
/s/ Norman C. Payson Norman C. Payson, M.D. | Executive Chairman of the Board of Directors and Chief Executive Officer (Principal Executive Officer) | July 16, 2010 | ||
/s/ Chris A. Karkenny Chris A. Karkenny | Executive Vice-President and Chief Financial Officer (Principal Financial Officer) | July 16, 2010 | ||
/s/ Peter A. Reynolds Peter A. Reynolds | Chief Accounting Officer and Controller (Principal Accounting Officer) | July 16, 2010 | ||
/s/ Neil P. Simpkins Neil P. Simpkins | Director | July 16, 2010 | ||
/s/ Michael Dal Bello Michael Dal Bello | Director | July 16, 2010 | ||
/s/ Patrick J. Bourke III Patrick J. Bourke III | Director | July 16, 2010 | ||
II-12
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Lake Forest, State of California, on July 16, 2010.
| APRIA HEALTHCARE OF NEW YORK STATE, INC. | ||
By: | /s/ NORMAN C. PAYSON, M.D. | |
Name: Norman C. Payson, M.D. | ||
Title: Chief Executive Officer | ||
SIGNATURES AND POWERS OF ATTORNEY
Each person whose signature appears below hereby constitutes and appoints Robert S. Holcombe and Peter A. Reynolds and each of them, the true and lawful attorneys-in-fact and agents of the undersigned, with full power of substitution and resubstitution, for and in the name, place and stead of the undersigned, in any and all capacities, to sign this Registration Statement and any and all amendments (including post-effective amendments) to this Registration Statement, including any filings pursuant to Rule 462(b) under the Securities Act of 1933, as amended, and to file the same, with all exhibits thereto, and all other documents in connection therewith, with the Securities and Exchange Commission, and hereby grants to such attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and anything necessary to be done, as fully to all intents and purposes as the undersigned might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them, or their or his substitute, or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Title | Date | ||
/s/ NORMAN C. PAYSON, M.D. Norman C. Payson, M.D. | Chief Executive Officer (Principal Executive Officer) | July 16, 2010 | ||
/s/ CHRIS A. KARKENNY Chris A. Karkenny | Executive Vice-President and Chief Financial Officer (Principal Financial Officer) | July 16, 2010 | ||
/s/ PETER A. REYNOLDS Peter A. Reynolds | Chief Accounting Officer and Controller (Principal Accounting Officer) | July 16, 2010 | ||
/s/ NORMAN C. PAYSON, M.D. Norman C. Payson, M.D. | Director | July 16, 2010 | ||
II-13
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Lake Forest, State of California, on July 16, 2010.
| APRIA HEALTHCARE, INC. | ||
By: | /s/ NORMAN C. PAYSON, M.D. | |
Name: Norman C. Payson, M.D. | ||
Title: Chief Executive Officer | ||
SIGNATURES AND POWERS OF ATTORNEY
Each person whose signature appears below hereby constitutes and appoints Robert S. Holcombe and Peter A. Reynolds and each of them, the true and lawful attorneys-in-fact and agents of the undersigned, with full power of substitution and resubstitution, for and in the name, place and stead of the undersigned, in any and all capacities, to sign this Registration Statement and any and all amendments (including post-effective amendments) to this Registration Statement, including any filings pursuant to Rule 462(b) under the Securities Act of 1933, as amended, and to file the same, with all exhibits thereto, and all other documents in connection therewith, with the Securities and Exchange Commission, and hereby grants to such attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and anything necessary to be done, as fully to all intents and purposes as the undersigned might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them, or their or his substitute, or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Title | Date | ||
/s/ NORMAN C. PAYSON, M.D. Norman C. Payson, M.D. | Chief Executive Officer (Principal Executive Officer) | July 16, 2010 | ||
/s/ CHRIS A. KARKENNY Chris A. Karkenny | Executive Vice-President and Chief Financial Officer (Principal Financial Officer) | July 16, 2010 | ||
/s/ PETER A. REYNOLDS Peter A. Reynolds | Chief Accounting Officer and Controller (Principal Accounting Officer) | July 16, 2010 | ||
/s/ NORMAN C. PAYSON, M.D. Norman C. Payson, M.D. | Director | July 16, 2010 | ||
II-14
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Lake Forest, State of California, on July 16, 2010.
APRIACARE MANAGEMENT SYSTEMS, INC. | ||
By: | /s/ NORMAN C. PAYSON, M.D. | |
Name: Norman C. Payson, M.D. | ||
Title: Chief Executive Officer | ||
SIGNATURES AND POWERS OF ATTORNEY
Each person whose signature appears below hereby constitutes and appoints Robert S. Holcombe and Peter A. Reynolds and each of them, the true and lawful attorneys-in-fact and agents of the undersigned, with full power of substitution and resubstitution, for and in the name, place and stead of the undersigned, in any and all capacities, to sign this Registration Statement and any and all amendments (including post-effective amendments) to this Registration Statement, including any filings pursuant to Rule 462(b) under the Securities Act of 1933, as amended, and to file the same, with all exhibits thereto, and all other documents in connection therewith, with the Securities and Exchange Commission, and hereby grants to such attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and anything necessary to be done, as fully to all intents and purposes as the undersigned might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them, or their or his substitute, or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Title | Date | ||
/s/ NORMAN C. PAYSON, M.D. Norman C. Payson, M.D. | Chief Executive Officer (Principal Executive Officer) | July 16, 2010 | ||
/s/ CHRIS A. KARKENNY Chris A. Karkenny | Executive Vice-President and Chief Financial Officer (Principal Financial Officer) | July 16, 2010 | ||
/s/ PETER A. REYNOLDS Peter A. Reynolds | Chief Accounting Officer and Controller (Principal Accounting Officer) | July 16, 2010 | ||
/s/ NORMAN C. PAYSON, M.D. Norman C. Payson, M.D. | Director | July 16, 2010 | ||
II-15
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Lake Forest, State of California, on July 16, 2010.
APRIADIRECT.COM, INC. | ||
By: | /s/ NORMAN C. PAYSON, M.D. | |
Name: Norman C. Payson, M.D. | ||
Title: Chief Executive Officer | ||
SIGNATURES AND POWERS OF ATTORNEY
Each person whose signature appears below hereby constitutes and appoints Robert S. Holcombe and Peter A. Reynolds and each of them, the true and lawful attorneys-in-fact and agents of the undersigned, with full power of substitution and resubstitution, for and in the name, place and stead of the undersigned, in any and all capacities, to sign this Registration Statement and any and all amendments (including post-effective amendments) to this Registration Statement, including any filings pursuant to Rule 462(b) under the Securities Act of 1933, as amended, and to file the same, with all exhibits thereto, and all other documents in connection therewith, with the Securities and Exchange Commission, and hereby grants to such attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and anything necessary to be done, as fully to all intents and purposes as the undersigned might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them, or their or his substitute, or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Title | Date | ||
/s/ NORMAN C. PAYSON, M.D. Norman C. Payson, M.D. | Chief Executive Officer (Principal Executive Officer) | July 16, 2010 | ||
/s/ CHRIS A. KARKENNY Chris A. Karkenny | Executive Vice-President and Chief Financial Officer (Principal Financial Officer) | July 16, 2010 | ||
/s/ PETER A. REYNOLDS Peter A. Reynolds | Chief Accounting Officer and Controller (Principal Accounting Officer) | July 16, 2010 | ||
/s/ NORMAN C. PAYSON, M.D. Norman C. Payson, M.D. | Director | July 16, 2010 | ||
II-16
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Denver, State of Colorado, on July 16, 2010.
CORAM ALTERNATE SITE SERVICES, INC. | ||
By: | /S/ ROBERT T. ALLEN | |
Name: Robert T. Allen | ||
Title: President, Chief Financial Officer and Treasurer | ||
SIGNATURES AND POWERS OF ATTORNEY
Each person whose signature appears below hereby constitutes and appoints Robert S. Holcombe and Peter A. Reynolds and each of them, the true and lawful attorneys-in-fact and agents of the undersigned, with full power of substitution and resubstitution, for and in the name, place and stead of the undersigned, in any and all capacities, to sign this Registration Statement and any and all amendments (including post-effective amendments) to this Registration Statement, including any filings pursuant to Rule 462(b) under the Securities Act of 1933, as amended, and to file the same, with all exhibits thereto, and all other documents in connection therewith, with the Securities and Exchange Commission, and hereby grants to such attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and anything necessary to be done, as fully to all intents and purposes as the undersigned might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them, or their or his substitute, or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Title | Date | ||
/s/ ROBERT T. ALLEN Robert T. Allen | President, Chief Financial Officer and Treasurer (Principal Executive Officer, Principal Financial Officer and Principal Accounting Officer) | July 16, 2010 | ||
/s/ ROBERT T. ALLEN Robert T. Allen | Director | July 16, 2010 | ||
II-17
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Denver, State of Colorado, on July 16, 2010.
CORAM CLINICAL TRIALS, INC. | ||
By: | /s/ ROBERT T. ALLEN | |
Name: Robert T. Allen | ||
Title: President, Chief Financial Officer and Treasurer | ||
SIGNATURES AND POWERS OF ATTORNEY
Each person whose signature appears below hereby constitutes and appoints Robert S. Holcombe and Peter A. Reynolds and each of them, the true and lawful attorneys-in-fact and agents of the undersigned, with full power of substitution and resubstitution, for and in the name, place and stead of the undersigned, in any and all capacities, to sign this Registration Statement and any and all amendments (including post-effective amendments) to this Registration Statement, including any filings pursuant to Rule 462(b) under the Securities Act of 1933, as amended, and to file the same, with all exhibits thereto, and all other documents in connection therewith, with the Securities and Exchange Commission, and hereby grants to such attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and anything necessary to be done, as fully to all intents and purposes as the undersigned might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them, or their or his substitute, or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Title | Date | ||
/s/ ROBERT T. ALLEN Robert T. Allen | President, Chief Financial Officer and Treasurer (Principal Executive Officer, Principal Financial Officer and Principal Accounting Officer) | July 16, 2010 | ||
/s/ ROBERT T. ALLEN Robert T. Allen | Director | July 16, 2010 | ||
II-18
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Denver, State of Colorado, on July 16, 2010.
| CORAM HEALTHCARE CORPORATION OF ALABAMA | ||
By: | /s/ ROBERT T. ALLEN | |
Name: Robert T. Allen | ||
Title: President, Chief Financial Officer and Treasurer | ||
SIGNATURES AND POWERS OF ATTORNEY
Each person whose signature appears below hereby constitutes and appoints Robert S. Holcombe and Peter A. Reynolds and each of them, the true and lawful attorneys-in-fact and agents of the undersigned, with full power of substitution and resubstitution, for and in the name, place and stead of the undersigned, in any and all capacities, to sign this Registration Statement and any and all amendments (including post-effective amendments) to this Registration Statement, including any filings pursuant to Rule 462(b) under the Securities Act of 1933, as amended, and to file the same, with all exhibits thereto, and all other documents in connection therewith, with the Securities and Exchange Commission, and hereby grants to such attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and anything necessary to be done, as fully to all intents and purposes as the undersigned might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them, or their or his substitute, or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Title | Date | ||
/s/ ROBERT T. ALLEN Robert T. Allen | President, Chief Financial Officer and Treasurer (Principal Executive Officer, Principal Financial Officer and Principal Accounting Officer) | July 16, 2010 | ||
/s/ ROBERT T. ALLEN Robert T. Allen | Director | July 16, 2010 | ||
II-19
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Denver, State of Colorado, on July 16, 2010.
| CORAM HEALTHCARE CORPORATION OF FLORIDA | ||
By: | /s/ ROBERT T. ALLEN | |
Name: Robert T. Allen | ||
Title: President, Chief Financial Officer and Treasurer | ||
SIGNATURES AND POWERS OF ATTORNEY
Each person whose signature appears below hereby constitutes and appoints Robert S. Holcombe and Peter A. Reynolds and each of them, the true and lawful attorneys-in-fact and agents of the undersigned, with full power of substitution and resubstitution, for and in the name, place and stead of the undersigned, in any and all capacities, to sign this Registration Statement and any and all amendments (including post-effective amendments) to this Registration Statement, including any filings pursuant to Rule 462(b) under the Securities Act of 1933, as amended, and to file the same, with all exhibits thereto, and all other documents in connection therewith, with the Securities and Exchange Commission, and hereby grants to such attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and anything necessary to be done, as fully to all intents and purposes as the undersigned might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them, or their or his substitute, or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Title | Date | ||
/s/ ROBERT T. ALLEN Robert T. Allen | President, Chief Financial Officer and Treasurer (Principal Executive Officer, Principal Financial Officer and Principal Accounting Officer) | July 16, 2010 | ||
/s/ ROBERT T. ALLEN Robert T. Allen | Director | July 16, 2010 | ||
II-20
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Denver, State of Colorado, on July 16, 2010.
| CORAM HEALTHCARE CORPORATION OF GREATER D.C. | ||
By: | /s/ ROBERT T. ALLEN | |
Name: Robert T. Allen | ||
Title: President, Chief Financial Officer and Treasurer | ||
SIGNATURES AND POWERS OF ATTORNEY
Each person whose signature appears below hereby constitutes and appoints Robert S. Holcombe and Peter A. Reynolds and each of them, the true and lawful attorneys-in-fact and agents of the undersigned, with full power of substitution and resubstitution, for and in the name, place and stead of the undersigned, in any and all capacities, to sign this Registration Statement and any and all amendments (including post-effective amendments) to this Registration Statement, including any filings pursuant to Rule 462(b) under the Securities Act of 1933, as amended, and to file the same, with all exhibits thereto, and all other documents in connection therewith, with the Securities and Exchange Commission, and hereby grants to such attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and anything necessary to be done, as fully to all intents and purposes as the undersigned might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them, or their or his substitute, or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Title | Date | ||
/s/ ROBERT T. ALLEN Robert T. Allen | President, Chief Financial Officer and Treasurer (Principal Executive Officer, Principal Financial Officer and Principal Accounting Officer) | July 16, 2010 | ||
/s/ ROBERT T. ALLEN Robert T. Allen | Director | July 16, 2010 | ||
II-21
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Denver, State of Colorado, on July 16, 2010.
| CORAM HEALTHCARE CORPORATION OF GREATER NEW YORK | ||
By: | /s/ ROBERT T. ALLEN | |
Name: Robert T. Allen | ||
Title: President, Chief Financial Officer and Treasurer | ||
SIGNATURES AND POWERS OF ATTORNEY
Each person whose signature appears below hereby constitutes and appoints Robert S. Holcombe and Peter A. Reynolds and each of them, the true and lawful attorneys-in-fact and agents of the undersigned, with full power of substitution and resubstitution, for and in the name, place and stead of the undersigned, in any and all capacities, to sign this Registration Statement and any and all amendments (including post-effective amendments) to this Registration Statement, including any filings pursuant to Rule 462(b) under the Securities Act of 1933, as amended, and to file the same, with all exhibits thereto, and all other documents in connection therewith, with the Securities and Exchange Commission, and hereby grants to such attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and anything necessary to be done, as fully to all intents and purposes as the undersigned might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them, or their or his substitute, or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Title | Date | ||
/s/ ROBERT T. ALLEN Robert T. Allen | President, Chief Financial Officer and Treasurer (Principal Executive Officer, Principal Financial Officer and Principal Accounting Officer) | July 16, 2010 | ||
/s/ ROBERT T. ALLEN Robert T. Allen | Director | July 16, 2010 | ||
II-22
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Denver, State of Colorado, on July 16, 2010.
| CORAM HEALTHCARE CORPORATION OF INDIANA | ||
By: | /s/ ROBERT T. ALLEN | |
Name: Robert T. Allen | ||
Title: President, Chief Financial Officer and Treasurer | ||
SIGNATURES AND POWERS OF ATTORNEY
Each person whose signature appears below hereby constitutes and appoints Robert S. Holcombe and Peter A. Reynolds and each of them, the true and lawful attorneys-in-fact and agents of the undersigned, with full power of substitution and resubstitution, for and in the name, place and stead of the undersigned, in any and all capacities, to sign this Registration Statement and any and all amendments (including post-effective amendments) to this Registration Statement, including any filings pursuant to Rule 462(b) under the Securities Act of 1933, as amended, and to file the same, with all exhibits thereto, and all other documents in connection therewith, with the Securities and Exchange Commission, and hereby grants to such attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and anything necessary to be done, as fully to all intents and purposes as the undersigned might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them, or their or his substitute, or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Title | Date | ||
/s/ ROBERT T. ALLEN Robert T. Allen | President, Chief Financial Officer and Treasurer (Principal Executive Officer, Principal Financial Officer and Principal Accounting Officer) | July 16, 2010 | ||
/s/ ROBERT T. ALLEN Robert T. Allen | Director | July 16, 2010 | ||
II-23
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Denver, State of Colorado, on July 16, 2010.
| CORAM HEALTHCARE CORPORATION OF MASSACHUSETTS | ||
By: | /s/ ROBERT T. ALLEN | |
Name: Robert T. Allen | ||
Title: President, Chief Financial Officer and Treasurer | ||
SIGNATURES AND POWERS OF ATTORNEY
Each person whose signature appears below hereby constitutes and appoints Robert S. Holcombe and Peter A. Reynolds and each of them, the true and lawful attorneys-in-fact and agents of the undersigned, with full power of substitution and resubstitution, for and in the name, place and stead of the undersigned, in any and all capacities, to sign this Registration Statement and any and all amendments (including post-effective amendments) to this Registration Statement, including any filings pursuant to Rule 462(b) under the Securities Act of 1933, as amended, and to file the same, with all exhibits thereto, and all other documents in connection therewith, with the Securities and Exchange Commission, and hereby grants to such attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and anything necessary to be done, as fully to all intents and purposes as the undersigned might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them, or their or his substitute, or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Title | Date | ||
/s/ ROBERT T. ALLEN Robert T. Allen | President, Chief Financial Officer and Treasurer (Principal Executive Officer, Principal Financial Officer and Principal Accounting Officer) | July 16, 2010 | ||
/s/ ROBERT T. ALLEN Robert T. Allen | Director | July 16, 2010 | ||
II-24
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Denver, State of Colorado, on July 16, 2010.
| CORAM HEALTHCARE CORPORATION OF MISSISSIPPI | ||
By: | /s/ ROBERT T. ALLEN | |
Name: Robert T. Allen | ||
Title: President, Chief Financial Officer and Treasurer | ||
SIGNATURES AND POWERS OF ATTORNEY
Each person whose signature appears below hereby constitutes and appoints Robert S. Holcombe and Peter A. Reynolds and each of them, the true and lawful attorneys-in-fact and agents of the undersigned, with full power of substitution and resubstitution, for and in the name, place and stead of the undersigned, in any and all capacities, to sign this Registration Statement and any and all amendments (including post-effective amendments) to this Registration Statement, including any filings pursuant to Rule 462(b) under the Securities Act of 1933, as amended, and to file the same, with all exhibits thereto, and all other documents in connection therewith, with the Securities and Exchange Commission, and hereby grants to such attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and anything necessary to be done, as fully to all intents and purposes as the undersigned might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them, or their or his substitute, or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Title | Date | ||
/s/ ROBERT T. ALLEN Robert T. Allen | President, Chief Financial Officer and Treasurer (Principal Executive Officer, Principal Financial Officer and Principal Accounting Officer) | July 16, 2010 | ||
/s/ ROBERT T. ALLEN Robert T. Allen | Director | July 16, 2010 | ||
II-25
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Denver, State of Colorado, on July 16, 2010.
| CORAM HEALTHCARE CORPORATION OF NEVADA | ||
By: | /s/ ROBERT T. ALLEN | |
Name: Robert T. Allen | ||
Title: President, Chief Financial Officer and Treasurer | ||
SIGNATURES AND POWERS OF ATTORNEY
Each person whose signature appears below hereby constitutes and appoints Robert S. Holcombe and Peter A. Reynolds and each of them, the true and lawful attorneys-in-fact and agents of the undersigned, with full power of substitution and resubstitution, for and in the name, place and stead of the undersigned, in any and all capacities, to sign this Registration Statement and any and all amendments (including post-effective amendments) to this Registration Statement, including any filings pursuant to Rule 462(b) under the Securities Act of 1933, as amended, and to file the same, with all exhibits thereto, and all other documents in connection therewith, with the Securities and Exchange Commission, and hereby grants to such attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and anything necessary to be done, as fully to all intents and purposes as the undersigned might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them, or their or his substitute, or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Title | Date | ||
/s/ ROBERT T. ALLEN Robert T. Allen | President, Chief Financial Officer and Treasurer (Principal Executive Officer, Principal Financial Officer and Principal Accounting Officer) | July 16, 2010 | ||
/s/ ROBERT T. ALLEN Robert T. Allen | Director | July 16, 2010 | ||
II-26
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Denver, State of Colorado, on July 16, 2010.
| CORAM HEALTHCARE CORPORATION OF NEW YORK | ||
By: | /s/ ROBERT T. ALLEN | |
Name: Robert T. Allen | ||
Title: President, Chief Financial Officer and Treasurer | ||
SIGNATURES AND POWERS OF ATTORNEY
Each person whose signature appears below hereby constitutes and appoints Robert S. Holcombe and Peter A. Reynolds and each of them, the true and lawful attorneys-in-fact and agents of the undersigned, with full power of substitution and resubstitution, for and in the name, place and stead of the undersigned, in any and all capacities, to sign this Registration Statement and any and all amendments (including post-effective amendments) to this Registration Statement, including any filings pursuant to Rule 462(b) under the Securities Act of 1933, as amended, and to file the same, with all exhibits thereto, and all other documents in connection therewith, with the Securities and Exchange Commission, and hereby grants to such attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and anything necessary to be done, as fully to all intents and purposes as the undersigned might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them, or their or his substitute, or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Title | Date | ||
/s/ ROBERT T. ALLEN Robert T. Allen | President, Chief Financial Officer and Treasurer (Principal Executive Officer, Principal Financial Officer and Principal Accounting Officer) | July 16, 2010 | ||
/s/ ROBERT T. ALLEN Robert T. Allen | Director | July 16, 2010 | ||
II-27
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Denver, State of Colorado, on July 16, 2010.
| CORAM HEALTHCARE CORPORATION OF NORTH TEXAS | ||
By: | /s/ ROBERT T. ALLEN | |
Name: Robert T. Allen | ||
Title: President, Chief Financial Officer and Treasurer | ||
SIGNATURES AND POWERS OF ATTORNEY
Each person whose signature appears below hereby constitutes and appoints Robert S. Holcombe and Peter A. Reynolds and each of them, the true and lawful attorneys-in-fact and agents of the undersigned, with full power of substitution and resubstitution, for and in the name, place and stead of the undersigned, in any and all capacities, to sign this Registration Statement and any and all amendments (including post-effective amendments) to this Registration Statement, including any filings pursuant to Rule 462(b) under the Securities Act of 1933, as amended, and to file the same, with all exhibits thereto, and all other documents in connection therewith, with the Securities and Exchange Commission, and hereby grants to such attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and anything necessary to be done, as fully to all intents and purposes as the undersigned might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them, or their or his substitute, or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Title | Date | ||
/s/ ROBERT T. ALLEN Robert T. Allen | President, Chief Financial Officer and Treasurer (Principal Executive Officer, Principal Financial Officer and Principal Accounting Officer) | July 16, 2010 | ||
/s/ ROBERT T. ALLEN Robert T. Allen | Director | July 16, 2010 | ||
II-28
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Denver, State of Colorado, on July 16, 2010.
| CORAM HEALTHCARE CORPORATION OF NORTHERN CALIFORNIA | ||
By: | /s/ ROBERT T. ALLEN | |
Name: Robert T. Allen | ||
Title: President, Chief Financial Officer and Treasurer | ||
SIGNATURES AND POWERS OF ATTORNEY
Each person whose signature appears below hereby constitutes and appoints Robert S. Holcombe and Peter A. Reynolds and each of them, the true and lawful attorneys-in-fact and agents of the undersigned, with full power of substitution and resubstitution, for and in the name, place and stead of the undersigned, in any and all capacities, to sign this Registration Statement and any and all amendments (including post-effective amendments) to this Registration Statement, including any filings pursuant to Rule 462(b) under the Securities Act of 1933, as amended, and to file the same, with all exhibits thereto, and all other documents in connection therewith, with the Securities and Exchange Commission, and hereby grants to such attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and anything necessary to be done, as fully to all intents and purposes as the undersigned might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them, or their or his substitute, or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Title | Date | ||
/s/ ROBERT T. ALLEN Robert T. Allen | President, Chief Financial Officer and Treasurer (Principal Executive Officer, Principal Financial Officer and Principal Accounting Officer) | July 16, 2010 | ||
/s/ ROBERT T. ALLEN Robert T. Allen | Director | July 16, 2010 | ||
II-29
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Denver, State of Colorado, on July 16, 2010.
| CORAM HEALTHCARE CORPORATION OF SOUTH CAROLINA | ||
By: | /s/ ROBERT T. ALLEN | |
Name: Robert T. Allen | ||
Title: President, Chief Financial Officer and Treasurer | ||
SIGNATURES AND POWERS OF ATTORNEY
Each person whose signature appears below hereby constitutes and appoints Robert S. Holcombe and Peter A. Reynolds and each of them, the true and lawful attorneys-in-fact and agents of the undersigned, with full power of substitution and resubstitution, for and in the name, place and stead of the undersigned, in any and all capacities, to sign this Registration Statement and any and all amendments (including post-effective amendments) to this Registration Statement, including any filings pursuant to Rule 462(b) under the Securities Act of 1933, as amended, and to file the same, with all exhibits thereto, and all other documents in connection therewith, with the Securities and Exchange Commission, and hereby grants to such attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and anything necessary to be done, as fully to all intents and purposes as the undersigned might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them, or their or his substitute, or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Title | Date | ||
/s/ ROBERT T. ALLEN Robert T. Allen | President, Chief Financial Officer and Treasurer (Principal Executive Officer, Principal Financial Officer and Principal Accounting Officer) | July 16, 2010 | ||
/s/ ROBERT T. ALLEN Robert T. Allen | Director | July 16, 2010 | ||
II-30
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Denver, State of Colorado, on July 16, 2010.
| CORAM HEALTHCARE CORPORATION OF SOUTHERN CALIFORNIA | ||
By: | /s/ ROBERT T. ALLEN | |
Name: Robert T. Allen | ||
Title: President, Chief Financial Officer and Treasurer | ||
SIGNATURES AND POWERS OF ATTORNEY
Each person whose signature appears below hereby constitutes and appoints Robert S. Holcombe and Peter A. Reynolds and each of them, the true and lawful attorneys-in-fact and agents of the undersigned, with full power of substitution and resubstitution, for and in the name, place and stead of the undersigned, in any and all capacities, to sign this Registration Statement and any and all amendments (including post-effective amendments) to this Registration Statement, including any filings pursuant to Rule 462(b) under the Securities Act of 1933, as amended, and to file the same, with all exhibits thereto, and all other documents in connection therewith, with the Securities and Exchange Commission, and hereby grants to such attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and anything necessary to be done, as fully to all intents and purposes as the undersigned might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them, or their or his substitute, or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Title | Date | ||
/s/ ROBERT T. ALLEN Robert T. Allen | President, Chief Financial Officer and Treasurer (Principal Executive Officer, Principal Financial Officer and Principal Accounting Officer) | July 16, 2010 | ||
/s/ ROBERT T. ALLEN Robert T. Allen | Director | July 16, 2010 | ||
II-31
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Denver, State of Colorado, on July 16, 2010.
| CORAM HEALTHCARE CORPORATION OF SOUTHERN FLORIDA | ||
By: | /s/ ROBERT T. ALLEN | |
Name: Robert T. Allen | ||
Title: President, Chief Financial Officer and Treasurer | ||
SIGNATURES AND POWERS OF ATTORNEY
Each person whose signature appears below hereby constitutes and appoints Robert S. Holcombe and Peter A. Reynolds and each of them, the true and lawful attorneys-in-fact and agents of the undersigned, with full power of substitution and resubstitution, for and in the name, place and stead of the undersigned, in any and all capacities, to sign this Registration Statement and any and all amendments (including post-effective amendments) to this Registration Statement, including any filings pursuant to Rule 462(b) under the Securities Act of 1933, as amended, and to file the same, with all exhibits thereto, and all other documents in connection therewith, with the Securities and Exchange Commission, and hereby grants to such attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and anything necessary to be done, as fully to all intents and purposes as the undersigned might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them, or their or his substitute, or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Title | Date | ||
/s/ ROBERT T. ALLEN Robert T. Allen | President, Chief Financial Officer and Treasurer (Principal Executive Officer, Principal Financial Officer and Principal Accounting Officer) | July 16, 2010 | ||
/s/ ROBERT T. ALLEN Robert T. Allen | Director | July 16, 2010 | ||
II-32
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Denver, State of Colorado, on July 16, 2010.
| CORAM HEALTHCARE CORPORATION OF UTAH | ||
By: | /S/ ROBERT T. ALLEN | |
Name: Robert T. Allen | ||
Title: President, Chief Financial Officer and Treasurer | ||
SIGNATURES AND POWERS OF ATTORNEY
Each person whose signature appears below hereby constitutes and appoints Robert S. Holcombe and Peter A. Reynolds and each of them, the true and lawful attorneys-in-fact and agents of the undersigned, with full power of substitution and resubstitution, for and in the name, place and stead of the undersigned, in any and all capacities, to sign this Registration Statement and any and all amendments (including post-effective amendments) to this Registration Statement, including any filings pursuant to Rule 462(b) under the Securities Act of 1933, as amended, and to file the same, with all exhibits thereto, and all other documents in connection therewith, with the Securities and Exchange Commission, and hereby grants to such attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and anything necessary to be done, as fully to all intents and purposes as the undersigned might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them, or their or his substitute, or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Title | Date | ||
/s/ ROBERT T. ALLEN Robert T. Allen | President, Chief Financial Officer and Treasurer (Principal Executive Officer, Principal Financial Officer and Principal Accounting Officer) | July 16, 2010 | ||
/s/ ROBERT T. ALLEN Robert T. Allen | Director | July 16, 2010 | ||
II-33
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Denver, State of Colorado, on July 16, 2010.
| CORAM HEALTHCARE OF WYOMING, L.L.C. | ||
By: | /S/ ROBERT T. ALLEN | |
Name: Robert T. Allen | ||
Title: President, Chief Financial Officer and Treasurer | ||
SIGNATURES AND POWERS OF ATTORNEY
Each person whose signature appears below hereby constitutes and appoints Robert S. Holcombe and Peter A. Reynolds and each of them, the true and lawful attorneys-in-fact and agents of the undersigned, with full power of substitution and resubstitution, for and in the name, place and stead of the undersigned, in any and all capacities, to sign this Registration Statement and any and all amendments (including post-effective amendments) to this Registration Statement, including any filings pursuant to Rule 462(b) under the Securities Act of 1933, as amended, and to file the same, with all exhibits thereto, and all other documents in connection therewith, with the Securities and Exchange Commission, and hereby grants to such attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and anything necessary to be done, as fully to all intents and purposes as the undersigned might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them, or their or his substitute, or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Title | Date | ||
/s/ ROBERT T. ALLEN Robert T. Allen | President, Chief Financial Officer and Treasurer (Principal Executive Officer, Principal Financial Officer and Principal Accounting Officer) | July 16, 2010 | ||
/s/ ROBERT T. ALLEN Robert T. Allen | Manager | July 16, 2010 | ||
/S/ MICHAEL E. DELL Michael E. Dell | Manager | July 16, 2010 | ||
II-34
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Denver, State of Colorado, on July 16, 2010.
| CORAM HOMECARE OF MINNESOTA, INC. | ||
By: | /S/ ROBERT T. ALLEN | |
Name: Robert T. Allen | ||
Title: President, Chief Financial Officer and Treasurer | ||
SIGNATURES AND POWERS OF ATTORNEY
Each person whose signature appears below hereby constitutes and appoints Robert S. Holcombe and Peter A. Reynolds and each of them, the true and lawful attorneys-in-fact and agents of the undersigned, with full power of substitution and resubstitution, for and in the name, place and stead of the undersigned, in any and all capacities, to sign this Registration Statement and any and all amendments (including post-effective amendments) to this Registration Statement, including any filings pursuant to Rule 462(b) under the Securities Act of 1933, as amended, and to file the same, with all exhibits thereto, and all other documents in connection therewith, with the Securities and Exchange Commission, and hereby grants to such attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and anything necessary to be done, as fully to all intents and purposes as the undersigned might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them, or their or his substitute, or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Title | Date | ||
/s/ ROBERT T. ALLEN Robert T. Allen | President, Chief Financial Officer and Treasurer (Principal Executive Officer, Principal Financial Officer and Principal Accounting Officer) | July 16, 2010 | ||
/s/ ROBERT T. ALLEN Robert T. Allen | Director | July 16, 2010 | ||
II-35
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Denver, State of Colorado, on July 16, 2010.
| CORAM SERVICE CORPORATION | ||
By: | /S/ ROBERT T. ALLEN | |
Name: Robert T. Allen | ||
Title: President, Chief Financial Officer and Treasurer | ||
SIGNATURES AND POWERS OF ATTORNEY
Each person whose signature appears below hereby constitutes and appoints Robert S. Holcombe and Peter A. Reynolds and each of them, the true and lawful attorneys-in-fact and agents of the undersigned, with full power of substitution and resubstitution, for and in the name, place and stead of the undersigned, in any and all capacities, to sign this Registration Statement and any and all amendments (including post-effective amendments) to this Registration Statement, including any filings pursuant to Rule 462(b) under the Securities Act of 1933, as amended, and to file the same, with all exhibits thereto, and all other documents in connection therewith, with the Securities and Exchange Commission, and hereby grants to such attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and anything necessary to be done, as fully to all intents and purposes as the undersigned might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them, or their or his substitute, or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Title | Date | ||
/s/ ROBERT T. ALLEN Robert T. Allen | President, Chief Financial Officer and Treasurer (Principal Executive Officer, Principal Financial Officer and Principal Accounting Officer) | July 16, 2010 | ||
/s/ ROBERT T. ALLEN Robert T. Allen | Director | July 16, 2010 | ||
II-36
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Denver, State of Colorado, on July 16, 2010.
| CORAM SPECIALTY INFUSION SERVICES, INC. | ||
By: | /s/ ROBERT T. ALLEN | |
Name: Robert T. Allen | ||
Title: President, Chief Financial Officer and Treasurer | ||
SIGNATURES AND POWERS OF ATTORNEY
Each person whose signature appears below hereby constitutes and appoints Robert S. Holcombe and Peter A. Reynolds and each of them, the true and lawful attorneys-in-fact and agents of the undersigned, with full power of substitution and resubstitution, for and in the name, place and stead of the undersigned, in any and all capacities, to sign this Registration Statement and any and all amendments (including post-effective amendments) to this Registration Statement, including any filings pursuant to Rule 462(b) under the Securities Act of 1933, as amended, and to file the same, with all exhibits thereto, and all other documents in connection therewith, with the Securities and Exchange Commission, and hereby grants to such attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and anything necessary to be done, as fully to all intents and purposes as the undersigned might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them, or their or his substitute, or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Title | Date | ||
/s/ ROBERT T. ALLEN Robert T. Allen | President, Chief Financial Officer and Treasurer (Principal Executive Officer, Principal Financial Officer and Principal Accounting Officer) | July 16, 2010 | ||
/s/ ROBERT T. ALLEN Robert T. Allen | Director | July 16, 2010 | ||
II-37
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Denver, State of Colorado, on July 16, 2010.
| CORAM, INC. | ||
By: | /s/ DANIEL E. GREENLEAF | |
Name: Daniel E. Greenleaf | ||
Title: President | ||
SIGNATURES AND POWERS OF ATTORNEY
Each person whose signature appears below hereby constitutes and appoints Robert S. Holcombe and Peter A. Reynolds and each of them, the true and lawful attorneys-in-fact and agents of the undersigned, with full power of substitution and resubstitution, for and in the name, place and stead of the undersigned, in any and all capacities, to sign this Registration Statement and any and all amendments (including post-effective amendments) to this Registration Statement, including any filings pursuant to Rule 462(b) under the Securities Act of 1933, as amended, and to file the same, with all exhibits thereto, and all other documents in connection therewith, with the Securities and Exchange Commission, and hereby grants to such attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and anything necessary to be done, as fully to all intents and purposes as the undersigned might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them, or their or his substitute, or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Title | Date | ||
/s/ DANIEL E. GREENLEAF Daniel E. Greenleaf | President (Principal Executive Officer) | July 16, 2010 | ||
/s/ CHRIS A. KARKENNY Chris A. Karkenny | Executive Vice-President and Chief Financial Officer (Principal Financial Officer) | July 16, 2010 | ||
/s/ PETER A. REYNOLDS Peter A. Reynolds | Chief Accounting Officer and Controller (Principal Accounting Officer) | July 16, 2010 | ||
/s/ NORMAN C. PAYSON, M.D. Norman C. Payson, M.D. | Director | July 16, 2010 | ||
II-38
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Denver, State of Colorado, on July 16, 2010.
CORAMRX, LLC | ||
By: | /s/ ROBERT T. ALLEN | |
Name: Robert T. Allen | ||
Title: President, Chief Financial Officer and Treasurer | ||
SIGNATURES AND POWERS OF ATTORNEY
Each person whose signature appears below hereby constitutes and appoints Robert S. Holcombe and Peter A. Reynolds and each of them, the true and lawful attorneys-in-fact and agents of the undersigned, with full power of substitution and resubstitution, for and in the name, place and stead of the undersigned, in any and all capacities, to sign this Registration Statement and any and all amendments (including post-effective amendments) to this Registration Statement, including any filings pursuant to Rule 462(b) under the Securities Act of 1933, as amended, and to file the same, with all exhibits thereto, and all other documents in connection therewith, with the Securities and Exchange Commission, and hereby grants to such attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and anything necessary to be done, as fully to all intents and purposes as the undersigned might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them, or their or his substitute, or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Title | Date | ||
/s/ ROBERT T. ALLEN Robert T. Allen | President, Chief Financial Officer and Treasurer (Principal Executive Officer, Principal Financial Officer and Principal Accounting Officer) | July 16, 2010 | ||
/s/ ROBERT T. ALLEN Robert T. Allen | Manager | July 16, 2010 | ||
/s/ MICHAEL E. DELL Michael E. Dell | Manager | July 16, 2010 | ||
II-39
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Denver, State of Colorado, on July 16, 2010.
H.M.S.S., INC. | ||
By: | /s/ ROBERT T. ALLEN | |
Name: Robert T. Allen | ||
Title: President, Chief Financial Officer and Treasurer | ||
SIGNATURES AND POWERS OF ATTORNEY
Each person whose signature appears below hereby constitutes and appoints Robert S. Holcombe and Peter A. Reynolds and each of them, the true and lawful attorneys-in-fact and agents of the undersigned, with full power of substitution and resubstitution, for and in the name, place and stead of the undersigned, in any and all capacities, to sign this Registration Statement and any and all amendments (including post-effective amendments) to this Registration Statement, including any filings pursuant to Rule 462(b) under the Securities Act of 1933, as amended, and to file the same, with all exhibits thereto, and all other documents in connection therewith, with the Securities and Exchange Commission, and hereby grants to such attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and anything necessary to be done, as fully to all intents and purposes as the undersigned might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them, or their or his substitute, or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Title | Date | ||
/s/ ROBERT T. ALLEN Robert T. Allen | President, Chief Financial Officer and Treasurer (Principal Executive Officer, Principal Financial Officer and Principal Accounting Officer) | July 16, 2010 | ||
/s/ ROBERT T. ALLEN Robert T. Allen | Director | July 16, 2010 | ||
II-40
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Denver, State of Colorado, on July 16, 2010.
T2MEDICAL, INC. | ||
By: | /s/ ROBERT T. ALLEN | |
Name: Robert T. Allen | ||
Title: President, Chief Financial Officer and Treasurer | ||
SIGNATURES AND POWERS OF ATTORNEY
Each person whose signature appears below hereby constitutes and appoints Robert S. Holcombe and Peter A. Reynolds and each of them, the true and lawful attorneys-in-fact and agents of the undersigned, with full power of substitution and resubstitution, for and in the name, place and stead of the undersigned, in any and all capacities, to sign this Registration Statement and any and all amendments (including post-effective amendments) to this Registration Statement, including any filings pursuant to Rule 462(b) under the Securities Act of 1933, as amended, and to file the same, with all exhibits thereto, and all other documents in connection therewith, with the Securities and Exchange Commission, and hereby grants to such attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and anything necessary to be done, as fully to all intents and purposes as the undersigned might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them, or their or his substitute, or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Title | Date | ||
/s/ ROBERT T. ALLEN Robert T. Allen | President, Chief Financial Officer and Treasurer (Principal Executive Officer, Principal Financial Officer and Principal Accounting Officer) | July 16, 2010 | ||
/s/ ROBERT T. ALLEN Robert T. Allen | Director | July 16, 2010 | ||
II-41
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Denver, State of Colorado, on July 16, 2010.
| HEALTHINFUSION, INC. | ||
By: | /s/ ROBERT T. ALLEN | |
Name: Robert T. Allen | ||
Title: President, Chief Financial Officer and Treasurer | ||
SIGNATURES AND POWERS OF ATTORNEY
Each person whose signature appears below hereby constitutes and appoints Robert S. Holcombe and Peter A. Reynolds and each of them, the true and lawful attorneys-in-fact and agents of the undersigned, with full power of substitution and resubstitution, for and in the name, place and stead of the undersigned, in any and all capacities, to sign this Registration Statement and any and all amendments (including post-effective amendments) to this Registration Statement, including any filings pursuant to Rule 462(b) under the Securities Act of 1933, as amended, and to file the same, with all exhibits thereto, and all other documents in connection therewith, with the Securities and Exchange Commission, and hereby grants to such attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and anything necessary to be done, as fully to all intents and purposes as the undersigned might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them, or their or his substitute, or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Title | Date | ||
/s/ ROBERT T. ALLEN Robert T. Allen | President, Chief Financial Officer and Treasurer (Principal Executive Officer, Principal Financial Officer and Principal Accounting Officer) | July 16, 2010 | ||
/s/ ROBERT T. ALLEN Robert T. Allen | Director | July 16, 2010 | ||
II-42
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Lake Forest, State of California, on July 16, 2010.
AHNY-DME LLC | ||
By: | /S/ NORMAN C. PAYSON, M.D. | |
Name: Norman C. Payson, M.D. | ||
Title: Executive Chairman and Chief Executive Officer | ||
SIGNATURES AND POWERS OF ATTORNEY
Each person whose signature appears below hereby constitutes and appoints Robert S. Holcombe and Peter A. Reynolds and each of them, the true and lawful attorneys-in-fact and agents of the undersigned, with full power of substitution and resubstitution, for and in the name, place and stead of the undersigned, in any and all capacities, to sign this Registration Statement and any and all amendments (including post-effective amendments) to this Registration Statement, including any filings pursuant to Rule 462(b) under the Securities Act of 1933, as amended, and to file the same, with all exhibits thereto, and all other documents in connection therewith, with the Securities and Exchange Commission, and hereby grants to such attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and anything necessary to be done, as fully to all intents and purposes as the undersigned might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them, or their or his substitute, or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Title | Date | ||
/S/ NORMAN C. PAYSON, M.D. Norman C. Payson, M.D. | Executive Chairman and Chief Executive Officer (Principal Executive Officer) | July 16, 2010 | ||
/S/ CHRIS A. KARKENNY Chris A. Karkenny | Executive Vice President and Chief Financial Officer (Principal Financial Officer) | July 16, 2010 | ||
/S/ PETER A. REYNOLDS Peter A. Reynolds | Chief Accounting Officer and Controller (Principal Accounting Officer) | July 16, 2010 | ||
II-43
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Lake Forest, State of California, on July 16, 2010.
| AHNY-IV LLC | ||
By: | /S/ NORMAN C. PAYSON, M.D | |
| Name: Norman C. Payson, M.D. | ||
| Title: Executive Chairman and Chief Executive Officer | ||
SIGNATURES AND POWERS OF ATTORNEY
Each person whose signature appears below hereby constitutes and appoints Robert S. Holcombe and Peter A. Reynolds and each of them, the true and lawful attorneys-in-fact and agents of the undersigned, with full power of substitution and resubstitution, for and in the name, place and stead of the undersigned, in any and all capacities, to sign this Registration Statement and any and all amendments (including post-effective amendments) to this Registration Statement, including any filings pursuant to Rule 462(b) under the Securities Act of 1933, as amended, and to file the same, with all exhibits thereto, and all other documents in connection therewith, with the Securities and Exchange Commission, and hereby grants to such attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and anything necessary to be done, as fully to all intents and purposes as the undersigned might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them, or their or his substitute, or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Title | Date | ||
/S/ NORMAN C. PAYSON, M.D. Norman C. Payson, M.D. | Executive Chairman and Chief Executive Officer (Principal Executive Officer) | July 16, 2010 | ||
/S/ CHRIS A. KARKENNY Chris A. Karkenny | Executive Vice President and Chief Financial Officer (Principal Financial Officer) | July 16, 2010 | ||
/S/ PETER A. REYNOLDS Peter A. Reynolds | Chief Accounting Officer and Controller (Principal Accounting Officer) | July 16, 2010 | ||
II-44