Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2014
or
o | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission file number: 000-54192
REVA MEDICAL, INC.
(Exact name of registrant as specified in its charter)
Delaware | | 33-0810505 |
(State or other jurisdiction of
incorporation or organization) | | (I.R.S. Employer
Identification No.) |
| | |
5751 Copley Drive, San Diego, CA 92111 | | (858) 966-3000 |
(Address of principal executive offices
including zip code) | | (Registrant’s telephone number,
including area code) |
Securities registered pursuant to Section 12(b) of the Act: None
Securities registered pursuant to Section 12(g) of the Act: Common Stock, $0.0001 par value per share
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No x
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§ 229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
Large accelerated filer o | | Accelerated filer o |
| | |
Non-accelerated filer o | | Smaller reporting company x |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes o No x
The aggregate market value of the common equity held by non-affiliates of the registrant as of June 30, 2014 totaled approximately $35,163,000 based on the closing price for the registrant’s Common Stock trading in the form of CHESS Depositary Interests, or CDIs, as reported by the Australian Securities Exchange and based on the closing currency exchange rate in effect that day. Such value excludes Common Stock and CDIs held by directors, executive officers, and 10% or greater stockholders as of June 30, 2014. The identification of 10% or greater stockholders as of June 30, 2014 is based on Schedule 13G and amended Schedule 13G reports publicly filed before June 30, 2014. This calculation does not reflect a determination that such parties are affiliates for any other purposes.
As of March 15, 2015, there were 33,579,778 shares of the registrant’s Common Stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
| Document Description |
| |
| Portions of the registrant’s definitive proxy statement for its 2015 annual meeting of stockholders to be filed pursuant to Regulation 14A within 120 days after the registrant’s fiscal year end of December 31, 2014 are incorporated by reference into Part III (items 10, 11, 12, 13, and 14) of this report. |
Table of Contents
PART I
Forward-Looking Statements
This Annual Report on Form 10-K for the year ended December 31, 2014, or “Form 10-K,” contains forward-looking statements concerning our business, operations, and financial performance and condition, as well as our plans, objectives, and expectations for business operations and financial performance and condition. Any statements contained herein other than statements of historical facts may be deemed to be forward-looking statements. You can identify these statements by words such as “aim,” “anticipate,” “assume,” “believe,” “could,” “due,” “estimate,” “expect,” “forecast,” “goal,” “intend,” “may,” “objective,” “plan,” “predict,” “project,” “potential,” “positioned,” “should,” “target,” “will,” “would,” and other similar expressions that are predictions of or indicate future events and future trends. These forward-looking statements are based on current expectations, estimates, forecasts, and projections about our business and the industry in which we operate and management’s beliefs and assumptions and are not guarantees of future performance or development and involve known and unknown risks, uncertainties, and other factors that are in some cases beyond our control. We caution readers that forward-looking statements are not guarantees of future performance and our actual results may differ materially from those anticipated, projected, or assumed in the forward-looking statements in this Form 10-K. Factors that can cause our actual results to differ materially from those anticipated in the forward-looking statements include, but are not limited to, the risks described under “Risk Factors,” including:
· our history of net losses and our expectation of significant operating losses for the foreseeable future;
· our ability to continue as a going concern;
· our ability to repay our convertible notes when, and if, required or otherwise comply with the requirements under the convertible notes;
· expectations as to the timing and amount of cash proceeds from the exercise, if any, of outstanding warrants to purchase common stock;
· changes in the fair value of our convertible notes and warrants to purchase common stock and the gains or losses that may arise upon the changes in those fair values each reporting period;
· failure to raise additional financing to fund our operations when needed or on terms favorable to us;
· failure of our FantomTM scaffold, or any future product, to meet our required clinical specifications;
· our inability to obtain regulatory clearance or approval for any of our products;
· our inability to attract or retain skilled personnel for our product development and commercialization efforts;
· increases in our projected expenditures on research and development and administrative activities;
· failure of our products to gain market acceptance domestically or internationally;
· less than anticipated growth in the market for bioresorbable scaffolds generally;
· changes in the regulatory environment which may adversely impact the commercialization of our products and result in significant additional capital expenditures;
· our inability to protect our intellectual property and operate our business without infringing upon the intellectual rights of others, which could result in litigation and significant expenditures; and,
· refusal of third-party payors to reimburse our customers for use of our products.
Stockholders, potential investors, and other readers are urged to consider these factors carefully in evaluating the forward-looking statements and are cautioned not to place undue reliance on the forward-looking statements. These forward-looking statements speak only as of the date of this Form 10-K. Unless required by law, we do not intend to publicly update or revise any forward-looking statements to reflect new information or future events or otherwise. You should, however, review the factors and risks we describe in the reports we will file from time to time with the Securities and Exchange Commission, or SEC, after the date of this Form 10-K.
2
Table of Contents
General Information
Unless the context implies otherwise, references in this report and the information incorporated herein by reference to “REVA Medical,” “REVA,” the “Company,” “we,” “us,” and “our” refer to REVA Medical, Inc.
We have applied for trademark registrations of the trademark FantomTM in the United States, European Union, Brazil, and Australia. Our product name ReZolve® has received trademark approval in the United States, the European Union, Australia and Brazil. All other trademarks, trade names, and service marks appearing in this report are the property of their respective owners. Use or display by us of other parties’ trademarks, trade dress, or products is not intended to and does not imply a relationship with, or endorsement or sponsorship of, us by the trademark or trade dress owner.
Unless indicated otherwise in this Form 10-K, all references to “$” or “dollars” refer to United States dollars, the lawful currency of the United States of America. References to “A$” refer to Australian dollars, the lawful currency of the Commonwealth of Australia.
Item 1. Business
Overview
We are a development stage medical device company working toward commercialization of our proprietary technologies to provide minimally invasive medical devices for the treatment of coronary artery disease in the human heart. Since our inception in 1998, our efforts have been concentrated on the development of a stent for use in coronary applications. We currently are in the later stages of developing and clinically testing bioresorbable drug-eluting coronary stents. We refer to bioresorbable stents as “scaffolds” because they are not permanent devices like metal stents. In clinical use, a scaffold is implanted by an interventional cardiologist utilizing x-ray imaging during a minimally invasive surgery. The scaffold is delivered to the site of a lesion, or blockage, in a coronary artery with a delivery catheter system, whereupon it is deployed to restore blood flow to the artery. It additionally medicates the artery to prevent further tissue growth from the stenting procedure, which is also called “restenosis.” Our products are designed to offer full x-ray visibility, clinically relevant sizing, and safe resorption. We believe that, due to certain risks associated with commercially available metal stents, the coronary stent market is beginning to convert from metal drug-eluting stents to fully bioresorbable polymer scaffolds, and, if we receive approval for commercialization from the relevant regulatory authorities, we believe our products would enable us to compete effectively in the stent market, which had approximately $4.2 billion in worldwide revenues during 2014.
Our scaffolds are still in a testing phase and will require extensive clinical results and regulatory approval before they can be sold and generate any revenue. In 2007, we enrolled patients in a small clinical study that proved the viability of our stent technology while confirming the areas needing further development. We have been developing and advancing our technology in both its design and polymer composition since that study and have undertaken significant laboratory and preclinical testing that has shown the technology to be safe and effective across various models. Our scaffolds combine our proprietary bioresorbable polymer with various designs, including unibody deformable designs and our proprietary “slide and lock” non-deformable designs.
We are currently testing our Fantom scaffold, which was introduced in humans during December 2014. Prior to our development of Fantom, we had enrolled a total of 165 patients in three clinical trials between June 2007 and January 2014 with predecessor scaffolds that were developed utilizing our proprietary x-ray visible polymer in combination with our “slide and lock” stent design. While these predecessor scaffolds demonstrated viability of the technology, we believe the enhanced characteristics of Fantom better position it for commercial success. The enhanced features include a unibody design, lower strut thickness, smaller crossing profile, optimized polymer properties, and streamlined manufacturing processes, while still maintaining all the beneficial features of our prior scaffolds such as radiopacity (visibility under x-ray) and drug-elution and resorption properties. In March 2014 we announced Fantom as our sole focus for development and testing and concurrently reduced headcount by approximately 45 percent and reduced other overhead costs to a lesser extent. We intend to enroll up to 110 patients in a clinical trial with Fantom during 2015, obtain follow-up data at a six-month time point, and if this data has acceptable safety and efficacy results, apply for regulatory approval in mid-2016. We may also enroll supplemental patients in the Fantom clinical trial during 2015 to provide data to support Boston Scientific Corporation’s distribution option (see “— Distribution and License Agreements” below for additional information).
3
Table of Contents
Our current plan is to apply for European CE Marking, the regulatory approval that would allow us to sell our scaffolds in Europe and other countries that recognize the CE Mark. When, and if, we receive CE Mark approval, we will evaluate how best to implement our sales and marketing strategies for commercialization. While our Fantom scaffold could be approved for sale in late 2016, our efforts to generate substantial revenue and achieve positive cash flow from our operations may take several years, even if our clinical results are favorable.
Concurrently with developing and testing Fantom, we have been performing feasibility tests on additional technologies in our patent portfolio and are in the early stages of developing a follow-on product pipeline.
We perform all of our research and development activities from our location in San Diego, California. We have three clean rooms and multiple engineering and chemistry labs at our facility, which is also our corporate and administrative office. We are ISO certified to the medical device standard 13485:2012 and intend to maintain the certification to support our commercialization plans. We have invented, co-invented, and in-licensed a portfolio of proprietary technologies. Our design-related technologies have been invented by our employees and consultants and our materials-related technologies have been either invented by our employees or licensed from, or co-invented with, Rutgers, The State University of New Jersey. We consider our patent portfolio to be significant and have invested considerable time and funds to develop and maintain it. We intend to continue to maintain and add to our patent portfolio.
We had 46 employees as of December 31, 2014, a significant number of whom are degreed professionals and six of whom are PhDs. We leverage our internal expertise with contract research and preclinical laboratories, catheter manufacturing, and other outside services as needed.
We have funded our research and development with the proceeds from our Initial Public Offering (“IPO”) completed in December 2010 and, prior to the IPO, from investments from health care venture capital funds and global medical device manufacturers including Medtronic, Inc. (“Medtronic”) and Boston Scientific Corporation (“BSC”). During 2014, we negotiated and completed a financing to provide the ongoing capital for our operations, including the Fantom clinical trial and application for CE Mark. This financing was completed in November 2014 with the issuance of 250 senior unsecured convertible notes, each with a face value of $100,000, and warrants to purchase 8,750,000 shares of our common stock. We received proceeds of $25.0 million from the notes and have the potential to receive up to an additional $22.8 million when, and if, the warrants are exercised. Both the notes and the warrants have five-year lives.
We expect our losses to continue for the next several years as we continue our development work, clinical studies, and preparations for commercialization and, if these efforts are successful and we are able to obtain approval to sell our products, we expect to commence product sales thereafter. In order to successfully transition to profitable operations, we will need to achieve a level of revenues and product margins to support the Company’s cost structure. Until such time as we generate positive cash flow, we plan to continue to fund our operating and capital needs by utilizing our current cash and investments and by raising additional capital through equity or debt financings or strategic or other transactions.
Our company was founded in California in June 1998 as MD3, Inc. We changed our name to REVA Medical, Inc. in March 2002. We reincorporated from the State of California to the State of Delaware in October 2010; as a result, the rights of our stockholders are governed by the Delaware General Corporation Law. We formed a wholly owned subsidiary in Germany in 2007 to facilitate our clinical trials and our planned commercialization of products; we have not used this subsidiary yet for any operating activities.
Market Opportunity
Coronary Artery Disease
Cardiovascular disease (“CVD”) is a term used to describe all diseases and conditions that relate to the heart and blood vessels. Coronary arteries, which supply blood to heart muscle, are susceptible to the buildup of plaque and formation of lesions, which can inhibit or block blood flow, a condition known as coronary artery disease. If arteries become too narrow as a result of plaque buildup, cardiac tissue may become starved of nutrients and oxygen, resulting in severe chest pain known as angina. As artery narrowing becomes more severe, death of cardiac muscle downstream from the blockage can occur due to a lack of oxygen. The sudden death of cardiac muscle can result in a life threatening condition that is commonly known as a heart attack, or “myocardial infarction.”
4
Table of Contents
Coronary artery disease is a leading cause of death. In a January 2015 report published by the World Health Organization, CVD was the number one cause of death globally with an estimated 17.5 million deaths in 2012, representing 31 percent of all global deaths. Of these, an estimated 7.4 million deaths were due to coronary heart disease. The American Heart Association (“AHA”) reported that coronary artery disease accounted for 375,295 deaths in the United States during 2011, or approximately one in every seven deaths, and that coronary artery disease will cost an estimated $129.6 billion in direct and indirect costs in 2015. According to the AHA, approximately 1.1 million people in the United States will have a new or recurrent coronary heart attack annually.
The European Heart Network reported in 2012 that coronary artery disease is the most common cause of death in Europe, accounting for approximately 1.8 million deaths per year, or approximately 20 percent of all male and 22 percent of all female deaths. In addition, the Australia Institute of Health and Welfare reported that coronary artery disease kills more Australians than any other disease, accounting for 21,500 deaths in 2011, or 15 percent of all deaths in Australia. In 2011, an estimated 69,900 people in Australia over the age of 25 had a heart attack.
Current Interventional Treatments for Coronary Artery Disease
The treatment options available to patients with coronary artery disease vary between invasive and non-invasive techniques and, within these groups, there are a variety of interventions that have varying degrees of benefits and side effects. Since lifestyle factors are associated with coronary artery disease, interventions that can reverse these factors, such as living a healthy and active lifestyle, are used for prevention and treatment. Lifestyle changes include regular exercise, smoking cessation, and a healthy diet. Evidence shows that the healthy lifestyle alternative is not being universally adopted. Medication therapy using cholesterol-lowering medications, beta blockers, diuretics, aspirin, nitroglycerin, calcium channel blockers, and others aim to reduce blood pressure and cholesterol levels and aid in the treatment of coronary artery disease. Although drug therapy can improve quality of life and also prolong survival, many drug therapies must be combined with stenting to achieve satisfactory long-term solutions to coronary artery disease for a large number of patients.
When lifestyle changes and medications fail to prevent the development of coronary artery disease, open heart and other surgical procedures are usually required. These procedures, developed and used over the past four decades, quickly and safely restore blood flow by either rerouting the flow around a plaque buildup with a surgical procedure or by reopening the artery with an interventional procedure. The procedures have evolved from invasive surgeries to minimally invasive catheter-based therapies. These advancements have generally resulted in less severe procedure-related complications, as well as reduced costs due to shorter procedure and recovery times. Physicians have rapidly adopted each new therapy. The main treatment options typically used by physicians and available to patients are:
· Coronary Artery Bypass Surgery: Bypass surgery is an extremely invasive technique whereby open heart surgery is required. The bypass is achieved by removing a vein or artery from somewhere else in a patient’s body and connecting it to the blocked artery, bypassing the blockage. This allows oxygen-rich blood to reach the heart muscle. Surgeons can bypass multiple blocked coronary arteries during one surgery.
· Balloon Angioplasty: Developed in the late 1970s, balloon angioplasty was a significant advancement. This minimally invasive therapy allows a physician to insert a slender balloon-tipped catheter into the femoral artery in the groin or the radial artery in the wrist to access a blockage in the heart. At the blockage site, the balloon is inflated to compress plaque and widen the narrowed artery so that blood can flow more easily. This therapy was rapidly adopted because it is minimally invasive and results in shorter hospital and recovery times compared to bypass surgery. However, the long-term effectiveness of balloon angioplasty is limited by restenosis, a re-narrowing of the artery caused by the elastic recoil of the artery wall and/or formation of scar tissue within the artery. Restenosis typically requires a repeat of the balloon angioplasty procedure or a bypass surgery to overcome. Additionally, some patients experience abrupt vessel closure after angioplasty, which leads to complications that include heart attack, emergency bypass surgery, or death.
· Bare Metal Stents: To address the issues of abrupt vessel closure and high rates of restenosis following balloon angioplasty, coronary stents were introduced in the 1990s. Stents are small tube-like devices that are inserted into an artery following balloon angioplasty; they stabilize the artery by propping it open to facilitate blood flow. Bare metal stents are flexible metal wire mesh tubes that are permanently implanted; they are typically mounted on a balloon and expanded, stretching open to the desired diameter during implantation. While bare metal stents minimized the issues and complications of abrupt vessel closure, restenosis continued to be a significant problem.
5
Table of Contents
· Drug-Eluting Metal Stents: After coronary stents were introduced, physicians determined that restenosis was caused by the body’s inflammatory response to the trauma caused by the angioplasty procedure and the stent, rather than by coronary artery disease. A number of methods were designed to overcome restenosis, the most common being the use of pharmacological agents to prevent restenosis at the implant site. Drug-eluting stents, which deliver a therapeutic drug usually contained within a thin polymer coating on the outside of a metal stent, help to minimize the buildup of scar tissue during healing. Drug-eluting stents contain drugs that range from cytotoxic types (for example, paclitaxel) to immunosuppressants (sirolimus, zotarolimus, and everolimus). Patients usually also undergo treatment with aspirin and anti-clotting or anti-platelet drugs, such as clopidogrel (sold as Plavix) or ticlopidine (Ticlid) after stenting, to reduce the incidence of blood clots, or “thrombosis.” In coronary stenting, we believe the key measures of success or failure of the therapy are:
· Target Lesion Revascularization, or “TLR,” which measures the incidence of required re-stenting or bypass surgery due to a failure of the initial coronary angioplasty and stenting; and
· Major Adverse Cardiac Events, or “MACE,” which are events of death, ischemia, TLR, or heart attack.
· Bioresorbable Stents: After studies showed that drug-eluting metal stents succeeded in lowering the rates of restenosis, safety concerns were raised when other studies suggested risks arose from late-stent thrombosis and the failure to restore natural movement of the artery. While coronary stents were originally conceived to be temporary devices and initial work was undertaken to develop them from biodegradable polymers so they would dissolve over time, most stents used currently are metal and remain in place permanently. In recent years there has been increasing interest in the development of a coronary stent that provides all of the proven benefits of a drug-eluting metal stent, but then dissolves or resorbs from the body and allows the artery to return to its natural function. The first bioresorbable stent was developed by researchers at Duke University in the early 1980s. While there have been a number of researchers developing bioresorbable stents intended to be resorbed by the body over time, there are many technical challenges, and to date, only two coronary bioresorbable stents are available for sale, and only in locations outside the United States. Due to their temporary nature as compared to metal stents and their intended design to hold the artery open while it is healing following angioplasty, bioresorbable stents are often described as bioresorbable scaffolds or “scaffolds.”
Coronary Stent Market
In 2014, annual worldwide revenues from coronary stent sales approximated $4.2 billion, of which drug-eluting stents accounted for approximately $3.9 billion in revenues and 90 percent of units sold. We believe there are three companies with significant market share which have received both FDA and CE Mark approval for five drug-eluting metal stents. According to analyst reports, approximate 2014 annual coronary stent revenues were:
· $1.4 billion in the United States from approximately 1.1 million stent implants;
· $2.3 billion in Europe and Asia (excluding Japan) and other countries that rely on CE Mark approval from approximately 3.1 million stent implants; and,
· $0.5 billion in Japan from approximately 239,000 stent implants.
Sales of bioresorbable scaffolds began in 2012 in locations outside the United States. Of the worldwide stent sales, bioresorbable scaffold revenues were estimated to be $86.0 million in 2013 and $111.0 million in 2014. Scaffold revenues are anticipated to continue to grow, and become a larger percentage of all stent sales, as adoption of the technology increases and as the scaffolds are further approved for sale in additional countries, including the anticipated approval for sale in the United States by the end of 2016.
Stent sales in Europe represent the majority of all stent revenues. Our plan is to initially sell in Europe after, and if, we receive CE Marking, the European regulatory approval required for commercial sales. We would then expand into additional territories as we apply for, and receive, in-country and other regulatory approvals. Due to the extensive clinical data and regulatory approvals needed to commercialize in the United States, we do not anticipate selling in the United States until several years after we’ve achieved initial sales in Europe.
6
Table of Contents
Our Products
The products we currently are developing and testing are drug-eluting fully bioresorbable polymer stents. We refer to bioresorbable stents as “scaffolds” because they are not permanent devices like the metal stents that are commonly used today. After being implanted, our scaffolds are designed to become fully captured inside the artery wall and maintain their strength for at least three months, a period of time that allows for sufficient healing of the artery following the implant procedure. Following artery healing, our scaffolds are designed to gradually degrade and benignly clear from the body, a process called “resorption.” As a scaffold resorbs, there is an integration of artery tissue into the space previously occupied by the scaffold and the artery returns to its natural state, allowing for return of its natural “vasomotion,” or the ability to contract and expand with blood flow and exertion.
We believe the features of bioresorbable scaffolds, combined with their temporary nature, provide advantages over permanent metal stents. We believe the primary advantage is the ability of the artery to return to its natural state and vasomotion, thereby allowing better quality of life in the near term and better retreatment options if future cardiovascular disease occurs in the long-term.
We have designed our bioresorbable scaffolds to overcome many of the limitations associated with bare metal and drug-eluting metal stents. Our extensive preclinical testing, including bench and animal tests, provides data and results that indicate our scaffolds have the potential to provide the following benefits:
· Restoration of Vessel Movement: We believe there is significant benefit to allowing an artery’s natural movement, which could be possible if the artery were not restricted with a permanent metal stent. Our bioresorbable scaffolds dissolve after an artery has healed, allowing restoration of the natural expansion and contraction, or “vasomotion,” of the artery. We also believe that because the artery returns to its natural state and blood flow is restored, disease progression downstream in the artery may be reduced.
· Minimization of Thrombosis Risk and Reduction of Long-Term Drug Therapy: We believe the potential for late-stent thrombosis is reduced because our bioresorbable scaffolds become fully encapsulated into the artery and they safely dissolve over time. We believe these characteristics will help in reducing the incidence of blood clots, potentially decreasing the need for prolonged anti-platelet drug therapy.
· Enhanced Applications for Future Medical Treatment: Since our bioresorbable scaffolds dissolve, we believe that potential complications of subsequent medical treatments are reduced. A patient could likely undergo re-stenting, receive treatment for lesions located downstream from the original stent, or undergo surgical procedures to an artery because there is no metal obstruction. In addition, we believe our products have potential to be used as a delivery vehicle for agents such as drugs and genes in coronary arteries to treat a number of different indications, including the treatment and reduction of vulnerable plaque. If our products are used for these purposes, we believe our bioresorbable scaffolds will be able to treat a broader range of lesions more safely than today’s stent alternatives.
We have been developing and advancing our bioresorbable technology in both its design and polymer composition since approximately 2003. We have developed the following key specifications we believe our scaffold products should possess to be commercially viable and competitive:
· Intended Use: Implanted using minimally invasive techniques; resorbs leaving no permanent device;
· Efficacy: Restores blood flow through the artery; the artery’s natural movement is restored as the scaffold begins to resorb;
· Drug Eluting: Delivers standard anti-restenotic drug to the stented artery;
· Standard Deployment: Catheter mounted scaffold that does not require presoaking and that deploys in a one-step continuous inflation the same as current clinical practice, including deliverability through the radial artery;
· Storage and Handling: Clinical handling and storage the same as current practice with no refrigeration requirements;
· Size: Treats arteries with diameters of 2.75mm and larger, the diameters most commonly treated;
· Expansion Range: Allows expansion within a clinically relevant range of the sized artery, to allow for taper of the artery and other implant procedure needs and variations;
· Recoil: Limited stent recoil, which we believe decreases the risk of restenosis;
7
Table of Contents
· Radiopaque: Visible by x-ray during and after implant, allowing verification of placement in the artery;
· Strength: Maintains adequate “hoop” strength for at least three months during the artery’s healing period; and,
· Manufacturing: Manufactured with standard repeatable processes in compliance with applicable manufacturing standards.
We believe that due to risks associated with bare metal and drug-eluting metal stents, the coronary stent market will continue to convert from metal stents to fully bioresorbable polymer scaffolds. To help ensure our bioresorbable scaffolds are commercially competitive, we have designed them with the following features:
· Proprietary Strong and Resilient Polymer: Our proprietary polymer, and the manner in which we process it, allows our scaffold to maintain its strength during the critical 90-day healing period following implant, offers standard clinical deliverability, and is less prone to breaking than other polymers that have been tested for this application.
· No Change to Clinical Practice: Our bioresorbable scaffolds are implanted using a standard balloon catheter and the profile of the device is compatible with a standard 6-french delivery catheter. Our bioresorbable scaffolds do not require any change to traditional storage or handling.
· Visible Using Standard Imaging Techniques: Our bioresorbable scaffolds are visible under x-ray, thereby allowing physicians to see the scaffold during implant and at early patient follow-up. It is also compatible with magnetic resonance imaging (“MRI”) and computed tomography (“CT”) imaging technologies, both of which may become more widely used in the diagnosis and treatment of coronary artery disease.
· Controlled Resorption Rate: Our polymer is designed to degrade and clear from the body in a predictable and safe manner. We have the ability to, and may, adjust the degradation profile of future polymer formulations if it is determined that a shorter or longer degradation period could lead to improved patient outcomes.
· Biocompatible and Safe: We use a combination of desaminotyrosine polycarbonate with polylactic acid as the base polymer in our bioresorbable scaffolds. Polylactic acid is widely used for medical implant purposes. Desaminotyrosine polycarbonate has been demonstrated to be biocompatible in preclinical testing; in a 12-month study during which the scaffold was degrading, it showed no indication of adverse biological reactions, consistent with the other tests of the polymer.
We have extensively tested our bioresorbable scaffolds during their development, a period spanning over ten years, in multiple types of preclinical tests. Our laboratory tests show the technology to be safe and effective in animals, with over 1,000 scaffolds tested across various models. Bench tests confirm the intended product features, with over 10,000 scaffolds tested in various manners. Our preclinical tests generally comprise the following:
· Comparative Testing: We have extensively compared our technology to commercially available metal stents and, to a lesser extent, to bioresorbable scaffolds. Our tests show that our scaffolds maintain the opening of the artery in the 90 days following implant, and then as the lumen size (the inside area of the artery) increases during the time the scaffold begins and continues to resorb, our technology leaves a more normal lumen area. Comparatively, the lumen size of arteries implanted with metal stents was almost unchanged.
· Strength and Fatigue Testing: We have conducted engineering and life cycle testing with machines that are designed to replicate both the physiological conditions in the coronary artery as well as measure the maximum stress levels that our technology can withstand. These tests have demonstrated satisfactory scaffold design and polymer strength, low levels of embrittlement of the polymer, and resistance to fatigue prior to significant degradation of the scaffold.
· Biocompatibility Testing: The biological response to our scaffold has been evaluated by assessing healing in animal coronary arteries using standard microscopy for stented arteries. These tests have demonstrated that the polymer is safe and no adverse response occurs in the artery, including while the polymer degrades.
· Rate of Degradation Testing: Our degradation rate tests demonstrated that our scaffolds maintained their structural integrity and strength for at least three months, a period of time that allows an artery to heal following the implant procedure. By design, at 12 months the scaffold no longer has significant mechanical strength and the polymer continues to resorb and be eliminated from the body for approximately four years. A study of the byproducts resulting from the resorption of our scaffold showed no accumulation in key organs or tissues of the animal’s body and a substantial portion of the byproducts were cleared from the body.
8
Table of Contents
· Toxicity Testing: As required by ISO-10993-1 regulations, our technology has undergone testing for genotoxicity. Our preclinical studies have shown that there is no change to the DNA or chromosomes of cells tested and no other genetic effect showing our polymer is genotoxic. We have conducted preclinical tests for several other types of toxicity that have demonstrated the polymer is safe.
· Drug Testing: The implant of a stent can injure an artery and the body’s wound-healing process can cause excessive scar tissue to form inside the stent, referred to as “in-stent restenosis.” The drug sirolimus minimizes the overgrowth of tissue, thereby minimizing the incidence of in-stent restenosis; it was used in the first commercial drug-eluting stents, has a demonstrated safety profile, and has proven effective at reducing restenosis. We have tested the effects of sirolimus, which is applied to the surface of our scaffold as a drug coating. Our studies demonstrated no major drug toxicity.
In addition to the significant laboratory and preclinical testing that have shown the technology to be safe and effective, we have conducted human clinical trials. We enrolled a total of 165 patients in three clinical studies between 2007 and 2014 with our predecessor scaffolds, which combined our proprietary “slide and lock” designs with our proprietary polymer formulations. While these predecessor scaffolds demonstrated viability of the technology, we believed enhanced characteristics were needed and, therefore, developed our Fantom scaffold during 2014.
Our Fantom scaffold, which is drug-eluting and made from our proprietary polymer, is implanted in a coronary artery using a balloon-mounted angioplasty catheter during a minimally invasive procedure. Fantom’s features include full x-ray visibility both during and after implant, a unibody deformable design, low strut thickness, crossing profile to accommodate a 6-french delivery catheter, optimized polymer properties, and streamlined manufacturing processes. Our production process involves manufacture of the scaffold device, application of a drug coating, assembly onto the balloon catheter system, sterilization, and packaging. The handling and storage requirements of Fantom, as well as the clinical procedure for implant, do not vary from those commonly used in clinical practice with metal stents. Because of its unique full x-ray visibility and other polymer properties, it is currently the only bioresorbable scaffold that allows for single-step inflation during implant and visual confirmation of correct placement over a lesion and successful expansion of the scaffold against the artery wall. While Fantom contains features that overcome a number of limitations of other bioresorbable scaffolds, it is not designed for smallest diameter vessel applications or highly calcified lesions. As a result, it will not be able to initially address the needs of all patients requiring a coronary stent.
Following bench and laboratory testing, we implanted Fantom in humans for the first time in December 2014. We intend to continue clinical studies of Fantom in 2015. We plan to enroll up to 110 patients in a trial between the second and third quarters of 2015 and have identified approximately 30 sites in eight countries, including Australia, Brazil, and Europe, to participate in the trial. Follow-up patient data is scheduled to be obtained at a six-month time point and, if this data has acceptable safety and efficacy results, we intend to use it to apply for regulatory approval of the scaffold in mid-2016. Following completion of enrollment of this trial, including any supplemental patients we may enroll to provide data to support the BSC distribution option (see “— Distribution and License Agreements” below for additional information), we intend to design and conduct additional trials outside the United States for regulatory and marketing purposes.
Concurrently with developing and testing Fantom, we have been performing feasibility tests on additional technologies in our patent portfolio and are in the early stages of developing a follow-on product pipeline.
Our Product Strategy
Our goal is to become a world leader in the development and commercialization of bioresorbable stent products for use in humans. To achieve this goal, we are pursuing the following business strategies:
· Demonstrate Clinical Safety and Efficacy and Gain Regulatory Approval for our Products: We intend to demonstrate the clinical safety and efficacy of our products through human clinical trials and have developed a clinical and regulatory strategy covering these trials and the pathway to application for commercial sales. We enrolled a total of 165 patients in three clinical studies between 2007 and 2014 with our predecessor scaffolds. Additionally, we began implanting our Fantom scaffolds in humans in December 2014 and we plan to use the data and results from a larger clinical study of Fantom, if they are acceptable, to apply for European CE Marking, which we expect by mid-2016. When, and if, we receive CE Mark approval we will evaluate how best to implement our sales and marketing strategies for commercialization.
9
Table of Contents
· Develop Follow-on Products: We intend to perform feasibility tests on additional technologies in our patent portfolio at the same time we are conducting clinical studies of our Fantom scaffolds. If feasibility is proven, we will determine a course of development for potential products and seek to provide follow-on products.
· Commercialize and Drive Adoption of our Products: Concurrent with our clinical studies, we will be focusing on commercialization readiness. As part of our plan, and in order to meet supply demands for anticipated sales should we receive regulatory approval in the European Union and other markets, such as Australia, we intend to expand our manufacturing capabilities to required levels. We have also granted BSC an option for a worldwide, exclusive right to market, distribute, and sell our products, subject to certain requirements. See “— Distribution and License Agreements” below for additional information.
· Build Awareness and Support among Leading Physicians: Our clinical strategy includes an aspect of collaboration with key opinion leaders in the field of interventional cardiology. We believe these key opinion leaders can be valuable advocates of our technology and important in the market adoption of our products once our products are approved and commercialized. In addition, we intend to look to these physicians to generate and publish scientific data that further supports the benefits of our scaffold technology.
· Leverage Our Technology Platform into Other Therapeutic Areas: We believe our technology is applicable to therapies beyond coronary artery disease. For example, we may pursue the use of our technology to treat peripheral artery disease, which is an expanding market. We believe current treatments for peripheral artery disease, particularly in the superficial femoral artery, have demonstrated only marginal benefit. We believe the application of our technology to the development of a bioresorbable peripheral scaffold could be significant.
· Provide the Highest Quality Products for Our Customers and Patients: We have assembled a team of employees and consultants who are experienced professionals in the medical device industry and who are focused on patient safety and product quality. We incorporate these principles in every aspect of our products, including development, manufacturing, quality assurance, and clinical research. We intend to offer only the highest quality products to patients and physician customers.
· Expand and Strengthen Our Intellectual Property Portfolio: We plan to continue to expand our current intellectual property portfolio. While we believe that our current portfolio will allow us to effectively market our products for the treatment of coronary artery disease, we plan to originate, license, and acquire additional intellectual property to enhance our existing position and enable us to more effectively protect our technology.
· Explore Licensing Opportunities: We intend to explore opportunities to leverage our intellectual property portfolio by licensing our technology to third parties or through the establishment of partnerships. For example, we may seek a partner to license our polymer for use as embolic beads or as a drug-delivery device for other pharmaceutical applications.
Our Technology
Our Fantom scaffolds are drug-eluting fully bioresorbable polymer scaffolds that are implanted using a balloon catheter. The underlying technology primarily consists of a proprietary polymer, a drug coating, and a unibody deformable stent design.
Our patented polymer is based on an iodinated, tyrosine-derived polycarbonate. We license the polymer and all improvements on the polymer from Rutgers, The State University of New Jersey, or “Rutgers.” See “— Distribution and License Agreements” for additional information. We work in collaboration with Rutgers to continually develop and enhance the polymer. The polymer formulation used in Fantom is a combination of our desaminotyrosine polycarbonate polymer and other polymeric components.
We believe our polymer offers the following advantages as compared to other polymer-based stents and scaffolds:
· Strength: We have developed our polymer so that, in conjunction with our design and our method of processing, it maintains the strength and structural integrity necessary to support an artery during the critical 90-day healing period after implant. We believe our specific polymer formulation is inherently less prone to cracking and breakage than other polymers.
10
Table of Contents
· Biocompatibility: Between 2007 and 2014, we performed human clinical trials with earlier versions of our polymer; none of those earlier versions has shown any adverse biological reactions. The current polymer formulation has been designed to enhance mechanical properties and reduce scaffold profile. Our current and previous polymer formulations demonstrate equal biocompatibility in preclinical testing. A 12-month study of our desaminotyrosine polycarbonate polymer showed no indication of adverse biological reactions while the scaffold material was degrading, consistent with other testing of the polymer.
· Predictable Degradation and Resorption: Our polymer degrades into benign metabolites (consisting of monomers and carbon dioxide) that are cleared from the body. Our polymer also allows us to change the formulation to allow for a more rapid degradation process to occur that will facilitate, for example, the short-term treatment of vulnerable plaque with drugs.
· Visibility: The use of iodine in our polymer enables our entire scaffold to be visible under x-ray, including standard fluoroscopy, providing visibility approximating that of metal stents. Other commercially available bioresorbable scaffolds utilize metal “markers” at each end of a scaffold; under x-ray, these metal markers are the only visible portion of those scaffolds and they remain in the vessel wall permanently. Improved visibility allows interventional cardiologists to more accurately assess the implant quality and position.
Our bioresorbable scaffolds are drug-eluting so that they may help to inhibit restenosis of the artery in the location of the scaffold. For our commercial devices, we intend to use the drug sirolimus, an anti-restenotic drug that has been used in commercial drug-eluting stents. This drug is available from a number of different sources and has been approved by both European and U.S. regulatory bodies. We coat the outside surface of our scaffold using a polymer solution containing a target dose of sirolimus. The polymer used for the coating solution is the same polymer used in the scaffold structure. Through our preclinical studies, we have demonstrated a controlled release of the drug over 30 days; most of the drug is released within 90 days. We believe this early and slow release characteristic optimizes the efficacy of the drug and that delivery of the drug within 90 days may help with the healing process.
The unibody design of our Fantom scaffold allows for delivery to, and deployment in, the artery utilizing a standard balloon-mounted angioplasty catheter and procedure through a standard 6-french guide catheter. Facilitated by our polymer’s properties, our scaffold is designed to maintain its strength during the critical 90-day healing period following implant, exhibit minimal recoil, and allow expansion within a clinically relevant range of the sized artery in order to allow for taper of the artery and other implant procedure needs and variation. With this design, we believe our Fantom scaffold provides standard clinical use features.
Preclinical Testing
We have undertaken significant laboratory and preclinical testing during the development of our technology, with tests of more than 1,000 scaffolds in various animal models and more than 10,000 scaffolds in various bench tests. This testing has shown that our technology was sufficiently safe and effective in animals to support continued product development. Our preclinical tests have included strength, embrittlement, and fatigue tests; biocompatibility and toxicity tests; drug release tests; deployment and degradation tests; and, tests of comparability to commercial metal stents and bioresorbable scaffolds. We used the data from our preclinical tests in our submissions to the relevant regulatory bodies, for which we received approval to proceed with clinical trials.
Clinical Studies and Regulatory Strategy
We have targeted Europe as our initial commercial market. Accordingly, we have developed a regulatory strategy that concentrates on our clinical trials and pathway to commercialization in Europe and other countries that recognize the European CE Mark regulatory approval for commercial sales. Our strategy contemplates additional markets, including the United States, after we’ve achieved initial sales in Europe.
The European Medical Devices Directive (“MDD”) 93/42/EEC sets out the general requirements for clinical trials in the European Union (the “EU”) and other essential requirements for approval and CE Marking; there are numerous other directives and standards regulating the design, manufacture, clinical trials, and labeling for medical devices. For our products to bear the CE Mark and be sold commercially throughout the EU, we will need human clinical trial data and to comply with the other requirements of the MDD.
11
Table of Contents
In Australia, the Therapeutic Goods Administration (“TGA”) is responsible for administering the Therapeutic Goods Act and maintaining the Australian Register of Therapeutic Goods. Unless exempt, all therapeutic goods for human use, including medical devices, must be included on the register before they may be imported, supplied in, or exported from Australia. Any unapproved medical devices used in humans in Australia, even in pilot trials, require an exemption from the requirement for inclusion on the register. In addition to agreeing to trial protocols and obtaining ethics committee approvals at these centers, we have obtained an exemption from the Australian Register of Therapeutic Goods for human clinical trials of our scaffolds in Australia. In order to sell commercially in Australia, we would need TGA approval.
In the United States, medical devices are subject to approval by the Food and Drug Administration (“FDA”). The FDA has classified approximately 1,700 different generic types of devices, organized within 16 medical specialties. A device’s classification reflects the FDA’s determination of the level of regulatory control necessary to assure the safety and effectiveness of that type of device, and determines the process the manufacturer must complete in order to market the device. The two basic types of marketing authorizations are the 510(k) premarket notification and the premarket approval (“PMA”). The three general classes of medical devices are as follows:
· Class I (lowest risk): “General controls” are sufficient to provide reasonable assurance of safety and effectiveness.
· Class II (moderate risk): In conjunction with general controls, there is sufficient information to establish “special controls” to provide reasonable assurance of safety and effectiveness.
· Class III (highest risk): I and II above are not sufficient to provide reasonable assurance of safety and effectiveness, the device is for use in supporting or sustaining life, or of substantial importance in preventing impairment to human life.
Devices such as our bioresorbable scaffolds are designated as Class III and require the FDA’s PMA prior to commercialization. PMA is the most stringent type of device marketing application and it requires sufficient valid scientific evidence, including clinical trial data, to assure the device is safe and effective for its intended use.
We enrolled a total of 165 patients in three clinical studies between 2007 and 2014 with our predecessor scaffolds, which combined our proprietary “slide and lock” designs with our proprietary polymer formulations. While these predecessor scaffolds demonstrated the viability of the technology, we believed enhanced characteristics were needed and, therefore, developed our Fantom scaffold during 2014 and initiated a pilot human clinical study of it in December 2014. We do not intend to commercialize any of the predecessor scaffolds.
We intend to continue clinical studies of Fantom in 2015. We plan to enroll up to 110 patients in a non-randomized, prospective, trial between the second and third quarters of 2015 and have identified approximately 30 sites in eight countries, including Australia, Brazil, and Europe, to participate in the trial. Follow-up patient data is scheduled to be obtained at a six-month time point and, if this data has acceptable safety and efficacy results, we intend to use it to apply for regulatory approval of the scaffold in mid-2016. We may also enroll supplemental patients in the Fantom clinical trial during 2015 to provide data to support the BSC distribution option (see “— Distribution and License Agreements” below for additional information). Following completion of enrollment of this trial, we intend to design and conduct additional trials of Fantom outside the United States for regulatory and marketing purposes.
Our clinical trials utilize standard industry measures of safety, including patient monitoring on a prescribed and regular basis, and are designed to evaluate both safety and performance and provide the data necessary to apply for CE Marking. For our Fantom trial, we have set forth the following:
· Product Performance: The primary endpoints we will evaluate are late lumen loss (reduction of internal artery diameter) and MACE (death, heart attack, and target lesion revascularization) as compared to historical values for marketed drug-eluting metal stents.
· Patient Safety: Clinical follow-up will be performed with all patients on a regular basis between the date of implant and six months, at six months, and annually thereafter for a period of up to five years following implant.
· Product Performance, Patient Safety, and Study Data: Patients will return for an interventional follow-up at six months in order to evaluate the healing process and obtain images of the artery and scaffold.
12
Table of Contents
While we have commenced clinical trials of our Fantom scaffold and expect to be in a position to apply for CE Marking by mid-2016, no guarantee can be given that we will achieve our expected results from the clinical trials or that CE Marking will be attained in a timely fashion, or at all.
Based on the outcome of our previous human clinical trials and the outcome of our upcoming Fantom trials, we plan to conduct a clinical trial in the United States, which is expected to be a randomized trial of 2,000 or more patients. Pursuant to our clinical and regulatory strategy, the timing of this trial will be determined after evaluating the CE Marking results, our capacity to manage multiple trials concurrently, and the availability of future funding.
Manufacturing
Manufacturing of medical devices is subject to strict quality requirements imposed by regulators, referred to as Good Manufacturing Practices (“GMPs”). We intend to follow GMPs for production of our scaffolds as we believe we are responsible for the quality and compliance of products we introduce in the clinic and, if we successfully commercialize our product, to the market. Accordingly, we utilize a quality management system that is designed to comply with the ISO standards and FDA regulations that govern medical device products in areas such as design, manufacture, testing, product and product component release, and raw material receipt and control. We have developed controlled methods and processes for the consistent manufacture of our products. All key outsourcing partners are ISO-certified to help ensure a continual supply of high quality components.
Our operations take place at our facility in San Diego, California, an approximately 37,000 square foot building dedicated to development and manufacturing under a lease that expires in January 2018. The facility includes laboratories for polymer development and synthesis, chemistry, engineering, and product assembly, including clean rooms and quality assurance laboratories. Our San Diego facility has the capacity to produce quantities of Fantom that will be needed for our planned clinical trials and initial commercial sales; the facility is currently certified to ISO 13485:2012, with such certification made by an independent third-party.
In order to produce commercial quantities of our scaffold, if and when that time arrives, we will need to scale-up our manufacturing processes and expand our capabilities to allow for such things as increased production volumes and additional scaffold sizes. Our plans are to begin implementing the methods and processes for scale-up, including work on the product’s size offerings, in 2015 and to continue manufacturing preparedness in 2016 as we approach commercialization. We may expand our manufacturing beyond our current facility to allow for continued sales growth when, and if, our sales volumes grow following our planned initial product introduction in Europe.
Although certain portions of our scaffold manufacturing process are completed by external parties, we have not entered into any material agreements with any third parties regarding our supply chain or manufacturing process. Our suppliers have no contractual obligation to supply, and we are not obligated to purchase, any components used in our bioresorbable scaffolds, which may result in supply interruptions. The strategy of outsourcing selected manufacturing processes is intended to minimize capital and operating costs while at the same time maintaining required quality standards.
The process to manufacture our bioresorbable scaffolds involves seven main components, some of which currently involve a degree of manual intervention. We plan to continue to improve our manufacturing process with the objectives of improving capacity, yield, and automation. These seven manufacturing steps are as follows:
· Polymer Manufacture: Performed at our facility.
· Polymer Tube Fabrication: Performed at our facility.
· Lasing of Polymer Tubes: Currently outsourced to third parties.
· Drug Coating: Drug currently purchased from foreign supplier; coating prepared and applied at our facility.
· Catheter System: Finished system currently purchased from domestic supplier.
· Assembly, Mounting on the Catheter, Quality Assurance, and Packaging: Performed at our facility.
· Sterilization: Currently outsourced to a domestic lab.
Currently, our catheter supply and lasing process are outsourced to third parties. We believe we have a number of qualified suppliers readily available; however, any interruption or delay in obtaining products from third-party suppliers, or our inability to obtain products from sources at acceptable prices in a timely manner, could impair our ability to meet the demand for our planned clinical trials or could delay commercialization of our products.
13
Table of Contents
Competition
The coronary stent industry is highly competitive. Many of our competitors have significantly greater financial resources, human resources, and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals, and marketing approved products than we do. Many of these competitors also have developed worldwide distribution channels and have more established reputations with our target customers. These competitors include Abbott Vascular, Boston Scientific, and Medtronic. Smaller or early-stage companies may also prove to be significant competitors to us, particularly if they enter into collaborative arrangements with the large and established companies. These companies compete with us in recruiting and retaining qualified scientific, production, and management personnel. They also compete with us in establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies and technology licenses complementary to our programs or advantageous to our business. As a result, we cannot provide assurances that we will be able to compete effectively against these competitors or their products.
Although the field of interventional cardiology is extremely competitive with requirements for high-performance products, we believe interventional cardiologists have historically been rapid adopters of new technology. While physicians may recommend alternative treatments such as drug therapy, bypass surgery, angioplasty, or bare metal stenting, we expect the primary competition for our products to be drug-eluting stents and other bioresorbable scaffolds. The market leaders for metal stents (bare and drug-eluting) are Abbott, Boston Scientific, and Medtronic. Of the three, only Abbott currently offers a bioresorbable scaffold.
Abbott began selling its Absorb bioresorbable scaffold during 2012; it is currently available in more than 60 countries outside the United States. Sales of Absorb during 2014 were estimated to be approximately $111.0 million. In January 2013, Abbott initiated a U.S. randomized clinical trial, ABSORB III, which enrolled 2,250 patients with Absorb or Abbott’s market-leading drug-eluting stent, XienceTM. A non-inferiority finding will allow Abbott to obtain U.S. PMA approval of Absorb, which we expect could occur in late 2016. Abbott is enrolling an additional 3,000 patients in the ABSORB IV trial, which compares rates of angina at one year and target lesion failure rates between one and five years. From the ABSORB IV trial, a finding of superiority against Xience would demonstrate added clinical benefits of bioresorbable scaffolds and potentially lead to differentiated product claims and perhaps further differentiated reimbursement rates from those of metal stents. Abbott’s ability or inability to obtain reimbursement for, and secure adoption of, the Absorb scaffold may play a significant role in expanding the market for bioresorbable scaffolds over the next several years.
In October 2012, Elixir Medical completed enrollment in a 120-patient clinical trial. In May 2013, they announced they had received CE Mark approval for their DESolve bioresorbable scaffold, and their first commercial implant was performed in January 2014. Additionally, during 2014, Elixir received CE Mark approval for a second, thinner strut bioresorbable scaffold; they are currently conducting a clinical trial of this thinner device prior to commercializing it. In addition to Abbott’s activities, Elixir’s ability or inability to obtain reimbursement for, and secure adoption of, its scaffolds may further define the marketing potential for bioresorbable scaffolds.
A number of other companies are developing bioresorbable scaffolds; they have not yet obtained regulatory approval to sell their products. These include Biotronik SE & Co. KG, Arterial Remodeling Technologies, and Amaranth Medical. Biotronik is developing an absorbable metal scaffold called Dreams, which is deformable, balloon-expandable, and manufactured from a magnesium alloy. Unlike polymer bioresorbable scaffolds that take multiple years to degrade, the magnesium scaffold is designed to absorb within the first year. In 2010, Biotronik initiated a 50-patient clinical trial of Dreams and reported six- and 12-month data. In February 2015, Biotronik completed a 120-patient clinical trial of a newer version of Dreams, which we believe will provide the data necessary to apply for a CE Mark. Arterial Remodeling Technologies and Amaranth Medical have initiated first-in-man clinical trials of their bioresorbable polymer scaffolds and have released early data at industry conferences.
Because of the prevalence of coronary artery disease and the resulting market opportunities, competitors will most likely continue to dedicate significant resources to aggressively promote their products. New product developments that could compete with us more effectively are likely because the coronary artery disease treatment market is characterized by extensive research efforts and technological progress. Accordingly, competitors may develop technologies and products that are safer, more effective, easier to use, or less expensive than our products.
14
Table of Contents
We believe our success is likely to be driven by, and depends on, our ability to innovate, manufacture in commercial quantities, obtain regulatory approvals and reimbursement, and successfully market and sell our products. We expect to encounter potential customers who, due to existing relationships with our competitors, are committed to or prefer the products offered by these competitors. To compete effectively, we must demonstrate that our products are attractive alternatives to other devices and treatments by differentiating our products on the basis of safety, efficacy, performance, ease of use, brand and name recognition, reputation, service, and cost-effectiveness.
Research and Development
Since inception, we have devoted a significant amount of resources to develop our technology. Our research and development expenses, which include the costs to conduct our human clinical trials, were $15.8 million in 2012, $19.2 million in 2013, and $14.3 million in 2014. We expect our research and development expenditures to increase in 2015 as we devote significant resources to the Fantom clinical trials, the continuing testing and preparation of Fantom for commercialization, and the feasibility assessments and possible development of products in our contemplated product pipeline.
Sales and Marketing
As a pre-revenue company, we have a limited sales and marketing focus and currently have no experience in the sale, marketing, or distribution of products. To achieve commercial success for any approved product, we will need to develop a sales and marketing organization or enter into arrangements with others to market and sell our products. During 2013, we engaged a managing director to plan our commercialization activities, including the development of a sales and marketing launch plan for our products, so we could be in a position to sell our products following regulatory approval. We continue to revise and update that sales and marketing launch plan based on market changes, competitor activities, and our product progress. The plan contemplates initial sales of our Fantom scaffold in Europe in late 2016.
We have considered many aspects of commercial sales, including product pricing and collection. In most countries, a significant portion of patient medical expenses is covered by third-party payors. In the United States, hospitals and physicians generally rely on payors such as Medicare, private health insurance plans, and health maintenance organizations to reimburse all or part of the cost of medical devices and the related surgical procedures; however, for a new device there will often be significant uncertainty as to reimbursement status and rates that will apply. Reimbursement in the EU varies by country and often by hospital. We believe that numerous hospitals have established budgets to purchase coronary stents and the purchase decision is often driven by the interventional cardiologists. In the United States, third-party payor requirements and government regulations impose substantial program requirements, ongoing compliance requirements, and limits on the manner in which medical device companies may market products and interact with healthcare professionals.
Currently, coronary stents are sold directly or through distribution channels, primarily targeting interventional cardiologists who treat patients likely to require stenting. We believe the costs and barriers are large to develop a sales and distribution channel focused around one group of products. We may, therefore, consider partnering with an established distributor. For example, we have entered into a Distribution Option Agreement with BSC that would cover the sale and distribution of our scaffolds in markets in which the technology is approved for sale. The terms of this agreement are described under “— Distribution and License Agreements” below.
If BSC does not exercise its option to negotiate to market and distribute our products, or if we are unable to reach an agreement on the terms of distribution, we may elect to sell our products through a combination of direct sales and independent distributors. Our sales strategy will be solidified in early 2016 and will depend on the types and timing of our product roll-out, assuming receipt of the necessary regulatory approvals and clearances. Generally, our planned targeted roll-out will occur as follows:
· Initial Market: The EU and other countries outside the United States that recognize the CE Mark will be our initial target commercial market since the CE Marking is our first targeted regulatory approval.
· Follow-on Markets: Australia, China, India, Japan and the Middle East will comprise our follow-on commercial markets because we believe regulatory approval in these countries will require additional clinical trials and/or approvals beyond the CE Mark.
· United States: The United States will be a later commercial market since completion of U.S. FDA trials and PMA approval requires extensive, and expensive, clinical trial results.
15
Table of Contents
Intellectual Property
We rely on a combination of patents, trade secrets, and copyright, together with non-disclosure and confidentiality agreements, to establish and protect our proprietary rights in our technologies. Our patents and patent applications covering the fundamental technology underlying our “slide and lock” design have been developed internally, while the technology underlying our polymer has been either licensed or developed by us.
As of March 15, 2015, on a worldwide basis, our patent portfolio comprises 307 issued and pending U.S. and foreign patents that we own directly or for which we are the licensee. Our latest patent expiration date with respect to these patents is 2034. We have been issued 49 U.S. patents and have 15 U.S. patent applications that are pending examination or have been allowed in the United States Patent and Trademark Office. For these 64 technology patents and applications, we have sought intellectual property protection outside the United States and have been granted 198 foreign patents and have 45 pending foreign applications. We do not know if any of our pending patent applications will be issued, nor do we know whether our patents, if issued, will adequately cover our technology or will be able to be successfully enforced. Even if valid and enforceable, our patents may not be sufficiently broad to prevent others from inventing a scaffold like ours, despite our patent rights. We have received no communications from third parties concerning the patentability, validity, or enforceability of our patents or patent applications. We believe that the remaining lives of our patents provide adequate time to generate revenues from sales, subject to timing of the clinical pathway and regulatory approvals.
We actively monitor our intellectual property position and review new developments periodically to identify prudent extensions to our patent portfolio to ensure that we protect our key technology, as well as to maximize our defensive strategy through the coverage of similar technology developments. We have an in-house patent attorney and also employ external patent counsel to assist us in managing our intellectual property portfolio. The stent industry has been subject to numerous patent filing and patent infringement lawsuits. Whether we would, upon commercialization, infringe any patent claim will not be known with certainty unless and until a court interprets a patent claim in the context of litigation. If an infringement allegation is made against us, we may seek to invalidate the asserted patent claim and may allege non-infringement of the asserted patent claim. In order for us to invalidate a U.S. patent claim, we would need to rebut the presumption of validity afforded to patents issued in the United States with clear and convincing evidence of invalidity, which is a high burden of proof. To date, none of our patents or patent applications has been subject to reexamination, interference, or other legal challenge.
We require all employees to sign confidentiality and invention assignment agreements under which they are bound to assign to us inventions made during the term of their employment. These agreements prohibit our employees from using, disclosing, or bringing onto the premises any proprietary information belonging to a third party. In addition, our consultants are required to sign agreements under which they must assign to us any inventions that relate to our business. These agreements also prohibit our consultants from incorporating into any inventions the proprietary rights of third parties without informing us. It is our policy to require all employees to document potential inventions and other intellectual property in laboratory notebooks and to disclose inventions to patent counsel in written form.
We also rely on confidentiality restrictions and trade secrets to protect our technology. We generally require our consultants and other parties who may be exposed to our proprietary technology to sign non-disclosure agreements which prohibit such parties from disclosing or using our proprietary information except as may be authorized by us.
Distribution and License Agreements
BSC Agreement
In 2007, we entered into a Distribution Option Agreement with Boston Scientific Corporation ( “BSC”) in which we granted BSC an option to negotiate for a worldwide exclusive right to sell, market, and distribute our scaffolds. If BSC exercises its option, we will negotiate to enter into a mutually acceptable distribution agreement that will include the following provisions: (i) the distribution agreement shall last at least five years; (ii) the transfer price for our products shall be equal to 50 percent of BSC’s average selling price for such products; (iii) other than the transfer price, BSC shall not be required to pay us for the sale, marketing, or distribution of such products; (iv) we shall meet all legal and regulatory requirements, as well as BSC quality standards, with respect to the design, development, and manufacturing of products; (v) BSC shall have sole discretion over all marketing and sales decisions relating to the products; and, (vi) BSC shall be the exclusive distributor of such products so long as BSC does not commence to sell, market, or distribute a directly competitive stent product (distribution becomes non-exclusive in locations where BSC sells, markets, or distributes a directly competitive stent product).
16
Table of Contents
If we are unable to agree on the terms of a distribution arrangement within 90 days after BSC exercises its option, then we may sell, market, and distribute our products to a third party, provided that the terms of an offer to, and any definitive agreement with, a third party shall not be on terms more favorable than the terms offered to BSC.
BSC’s distribution option, if not otherwise exercised, terminates 90 days after we deliver clinical data to BSC that contains the following: (i) imaging, death, acute myocardial infarction, stent thrombosis, and target lesion revascu-larization data from one year follow-up of at least 200 patients implanted with our resorbable drug-coated stents; (ii) core lab acute gain, late loss, and binary angiographic restenosis data from eight- to nine-month angiographic follow-up of at least 100 of the implanted stents; and, (iii) eight- to nine-month optical coherence tomography of at least 40 of the implanted stents.
Under the Distribution Option Agreement, we have also agreed not to take certain actions that would prevent BSC from exercising its distribution option; however, we may market, sell, or distribute any product on a non-exclusive basis in any country or territory where BSC directly competes with such product. In addition, if we receive regulatory approval for any product in any country or territory outside the United States prior to submission to the FDA, and BSC does not exercise its distribution option within 90 days following written notice of the approval, then we may sell, market, and distribute such product in any foreign country or territory where the product has received approval, directly or through any third party that is not a direct competitor of BSC, provided however, that any such arrangement must be terminable without cost to BSC on no more than 90 days’ written notice.
Rutgers License
In July 2010, we entered into an Exclusive License Agreement, or the License, with Rutgers, The State University of New Jersey, or “Rutgers,” that superseded our 2004 Exclusive License Agreement with Rutgers. Under the 2010 License, Rutgers granted us an exclusive, worldwide right, including sublicensing rights, to develop and commercialize products that utilize certain polymers in the vascular field. Terms of the License require us to pay annual license fees until a product is commercially sold in a major market. In order to maintain our rights under the Rutgers License, we have to satisfy certain development and commercialization obligations specified in the agreement. The term of the Rutgers License continues until the expiration of the last to expire of the patents licensed to us, which we believe is 2034. The License allows Rutgers to sublicense certain technology that Rutgers invented, we jointly invented with Rutgers, or that we solely invented, outside the field of use specified in the License. If Rutgers sublicenses inventions and improvements solely owned by us, Rutgers shall pay us a percentage of all income and consideration Rutgers receives from such sublicenses.
The royalties due under the Rutgers License vary depending upon type of product, use of product, stage of product, location of sale, and ultimate sales volume and price. We believe the royalties due under the Rutgers License will range from a minimum of approximately $25 to a maximum of approximately $100 per product sale, with license provisions for escalating minimum royalties that could be as high as $2.2 million per year. Additionally, in the event we receive certain milestone payments related to this technology, the licenses require that up to 40 percent of the milestone amount be paid to the licensors. The Rutgers License requires annual licensing payments of $175,000 until the underlying technology has been commercialized and royalties would be due. The Rutgers License also requires other payments to occur during commercialization that could total $950,000, payment of $350,000 upon a change in control of ownership, payments of up to $300,000 annually to extend regulatory filing periods related to certain technology, and payment of patent filing, maintenance, and defense fees.
Third-Party Reimbursement
In most countries throughout the world, a significant portion of patient medical expense is covered by third-party reimbursement, consisting of both government-funded and private insurance programs. While each payor develops and maintains its own coverage and reimbursement policies, the vast majority of payors have established policies for stents. We believe that our products generally will fall within existing reimbursement guidelines, or within new reimbursement guidelines that are being established by competing bioresorbable scaffold companies, although some refinement in policies may be needed for our products. Before we can obtain reimbursement for our products in Europe, Australia, or the United States, we will need to obtain appropriate regulatory approvals for product sales.
17
Table of Contents
The Center for Medicare and Medicaid Services (“CMS”) is the U.S. government entity that administers the Medicare program, which is considered a reimbursement benchmark. CMS establishes, reviews, and updates Medicare coverage and reimbursement policies for medical products and procedures. Both CMS and commercial payors have established coverage and reimbursement policies for stents currently being sold; however, we have no assurances these existing reimbursement codes would apply to the bioresorbable scaffolds that we are developing. We also have no assurance that existing payment rates under these reimbursement codes will continue.
Outside of the United States, there are many reimbursement programs through private payors as well as government programs. In some countries, government reimbursement is the predominant program available to patients and hospitals. While the vast majority of countries have existing reimbursement for stents, a small number of countries may require us to gather additional clinical data before agreeing to coverage and reimbursement for our products. We intend to complete the requisite clinical studies and obtain coverage and reimbursement approval in countries where it makes economic sense to do so.
In certain regions, such as Europe, innovative pricing and reimbursement agreements are being used to balance the interests and objectives of medical technology manufacturers, payors, parties assessing health technology, clinicians, and patients. Manufacturers and health technology assessors/assessments, or “HTAs,” are increasingly using risk sharing and value-based schemes as a way to obtain HTA approval. HTAs typically have two elements, clinical effectiveness and cost effectiveness. Some countries in Europe have national HTA (for example, France, Germany, and Sweden) and others have regional ones (such as, Italy, Spain, and the United Kingdom). Some manufacturers who proactively propose such schemes to HTAs may gain competitive advantage. Each country within Europe has its own system of pricing and reimbursement for medical devices and products.
In Australia, the Department of Health and Ageing is the government department and Medicare is the government entity responsible for administering the Medicare Benefits Scheme and the Medicare Benefits Schedule (“MBS”). Medicare establishes coverage and reimbursement policies for medical products and procedures and such policies are periodically reviewed and updated. Medicare and MBS have established coverage and reimbursement policies for stents that are currently being sold. However, similar to the United States, there are no assurances that existing policies or reimbursement codes will be used for the bioresorbable scaffolds that we are developing or that existing payment rates under the reimbursement codes will continue.
In addition, U.S. governmental and private sector payors have instituted initiatives to limit the growth of health care costs, using, for example, price regulation or controls and competitive pricing programs. Some third-party payors also require pre-approval of coverage for new or innovative devices or therapies before they will reimburse health care providers who use such devices or therapies. Providers also have sought ways to manage costs, such as through the use of group purchasing organizations. While we believe the clinical performance of our scaffolds will be sufficient to secure reimbursement, there remains uncertainty as to whether our products will be viewed as sufficiently cost-effective to warrant adequate coverage and reimbursement levels.
Government Regulation
United States
Our products are considered combination products because they comprise two regulated components in a single product: a drug and a medical device. In the United States, the FDA assigns the review of a combination product, based on the product’s “primary mode of action,” to one of its centers, such as the Center for Drug Evaluation and Research (“CDER”) or the Center for Devices and Radiological Health (“CDRH”). The center to which the product is assigned will have primary jurisdiction over the PMA of the product.
Because the primary mode of action for our products is that of a medical device, we anticipate that when, and if, we apply for approval in the United States, our products will be reviewed by the FDA under the Federal Food, Drug, and Cosmetic Act with CDRH having primary responsibility for review and regulation of our products. As a result, we expect our clinical trial of drug-eluting scaffolds to be conducted under an IDE application in accordance with 21 CFR Part 812. However, it is possible the FDA may assign our products to CDER. Based on FDA precedent and jurisdictional statements to date, we believe that the drug component of our products will not require separate FDA approval and that it will be reviewed in the context of our PMA, with CDRH consulting with CDER as needed. If the FDA does assign our products to be regulated by CDER, the drug component of the product will in all likelihood not require separate CDER approval, but will be evaluated in the context of our PMA as a whole, with application of drug standards as deemed appropriate by FDA based on the circumstances.
18
Table of Contents
FDA regulations govern the following activities that we and our suppliers, licensors, and partners perform and will continue to perform to ensure that the products we distribute domestically or export internationally are safe and effective for their intended uses:
· product design and development;
· product testing;
· product manufacturing and production;
· product safety;
· product labeling;
· product storage;
· record keeping;
· premarket approval;
· advertising and promotion;
· product sales and distribution; and,
· postmarketing requirements including monitoring for and reporting of adverse events and malfunctions.
Premarket Clearance and Approval Requirements: The FDA classifies medical devices into one of three classes. Devices deemed by the FDA to pose the greatest risk, such as life-sustaining, life-supporting, or implantable devices or devices not substantially equivalent to a previously cleared 510(k) device, are placed in Class III, requiring PMA. Our scaffolds are Class III devices and will require FDA approval. A PMA must be supported by extensive data, including but not limited to, technical, preclinical, clinical, manufacturing, and labeling to demonstrate to the FDA’s satisfaction the safety and efficacy of the device. A PMA must also contain a full description of the device and its components and a full description of the methods, facilities, and controls used for manufacturing of the device.
Product Modifications: New PMAs or PMA supplements are required for all significant modifications to a manufacturing process, labeling, use, or design of a device that is approved through the PMA process. PMA supplements often require submission of the same type of information as an initial application, except the supplement is limited to information needed to support the device changes. Certain modifications may not require as extensive clinical data or the convening of an advisory panel.
Clinical Trials: Clinical trial data is almost always required to support a PMA application. Clinical trials for our product candidates require the submission of an IDE application and FDA approval. The IDE application must be supported by appropriate data, such as animal and laboratory data showing that it is safe to test the device in humans and that the testing protocol is scientifically sound, and the application must be for a specified number of patients. Clinical trials may begin once the application is cleared by the FDA, as well as the appropriate institutional review boards at the clinical trial sites. Clinical trials must be conducted in accordance with applicable regulations including Good Clinical Practices and policies, procedures, and trial conduct must adhere to extensive record keeping and reporting requirements. Our clinical trials must be conducted under the oversight of an institutional review board at the relevant clinical trial site and in accordance with applicable regulations and policies. We, the FDA, or the institutional review board at a clinical site may suspend a clinical trial at any time for any reason, including a belief that the risks to the patients in a clinical trial outweigh the anticipated benefits. Clinical trials of the scope we anticipate for our products can typically take years to complete and may encounter challenges at any stage that require the trial to be halted.
Pervasive and Continuing Regulation: After a device is placed on the market, numerous regulatory requirements apply. These include:
· Good Manufacturing Practices (“GMP”) and Quality System Regulations (“QSR”) that require manufacturers, including third-party suppliers, to follow stringent design, testing, control, documentation, and other quality assurance procedures during all aspects of the manufacturing process;
· labeling and promotion regulations, which limit the manner in which companies can market their products and impose requirements for content and format of labeling and promotional materials, and FDA prohibitions against promotion of products for unapproved or “off-label” uses;
19
Table of Contents
· medical device reporting regulations, which require manufacturers to report to the FDA if their device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if the malfunction were to recur;
· post-market surveillance regulations, which will apply when necessary to protect the public health or to provide additional safety and efficacy data for the device; and,
· specific conditions of approval that may be imposed on a specific PMA.
The FDA has broad post-market and regulatory enforcement powers. We will be subject to unannounced inspections by the FDA and the Food and Drug Branch of the California Department of Health Services to determine our compliance with the QSR and other regulations. The manufacturing facilities of our suppliers and subcontractors may also be inspected by the FDA or other regulatory authorities to determine their compliance with GMP regulations. The FDA monitors marketing and promotional activities for matters of concern, and may receive complaints from competitors or other third parties if they have concerns regarding our promotional activities.
In addition, discovery of previously unknown problems with a medical device, manufacturer, or facility may result in restrictions on the manufacturing or marketing of an approved device, including costly recalls or withdrawal of the device from the market. Failure to comply with applicable regulatory requirements may result in enforcement action being taken by the FDA, which may include any of the following sanctions:
· inspectional observations or warning letters, identifying concerns that must be corrected;
· fines, injunctions, consent decrees, and civil penalties;
· recall or seizure of our products;
· operating restrictions, partial suspension, or total shutdown of production;
· refusing our requests for PMA or new intended uses;
· withdrawing PMA that are already granted; and/or,
· criminal prosecution.
The FDA also has the authority to require us to repair, replace, or refund the cost of any medical device that we have manufactured or distributed. If any of these events were to occur, they could have a material adverse effect on our business. We are also subject to a wide range of federal, state, and local laws and regulations, including those related to the environment, health and safety, and land use.
Fraud and Abuse: Our operations will be directly, or indirectly through our customers, subject to various state and federal fraud and abuse laws, including, without limitation, the federal Anti-Kickback Statute and the False Claims Act. These laws may impact, among other things, our proposed sales and marketing programs.
The federal Anti-Kickback Statute prohibits persons from knowingly and willfully soliciting, offering, receiving, or providing payments, directly or indirectly, in exchange for, or to induce, either the referral of an individual, or the furnishing or arranging for a good or service, for which payment is made under a federal program such as Medicare or Medicaid. This statute is broad and prohibits many arrangements and practices that are lawful outside the health care industry. Recognizing that this statute is broad and may technically prohibit many innocuous or beneficial arrangements, Congress authorized a series of safe harbor regulations. The safe harbors set forth provisions that give some assurance to health care providers and other parties that they will not be prosecuted. The failure of a transaction or arrangement to fit precisely within a safe harbor does not necessarily mean that it is illegal or that prosecution will be pursued; however, conduct and arrangements that do not fully satisfy each applicable safe harbor may result in increased scrutiny by enforcement authorities. All parties to a prohibited transaction may be prosecuted, whether any party sought or received payment from any federally funded program. Penalties for violations of the Anti-Kickback Statute include criminal and civil sanctions such as fines, imprisonment, and possible exclusion from Medicare, Medicaid, and other federal health care programs. Many states have adopted laws similar to the federal statute.
20
Table of Contents
The U.S. False Claims Act prohibits persons from knowingly filing, or causing to be filed, a false claim or using false statements to obtain payment from the federal government. Suits filed under the False Claims Act, known as “qui tam” actions, can be brought by any individual on behalf of the government and such individuals, commonly referred to as “whistleblowers,” may share in any amounts paid to the government in fines or settlement. The frequency of filing of qui tam actions has increased significantly in recent years, causing more health care companies to defend False Claims. When an entity is determined to have violated the False Claims Act, it may be required to pay up to three times the actual damages sustained by the government, plus civil penalties ranging from $5,500 to $11,000 for each separate False Claim. Various states have also enacted laws modeled after the federal False Claims Act. Similarly, the federal Civil Monetary Penalty statute imposes penalties of up to $50,000 per violation for filing certain types of proscribed claims or engaging in prohibited acts.
In addition to the laws described above, the Health Insurance Portability and Accountability Act of 1996 created two new federal crimes: health care fraud and false statements relating to health care matters. The health care fraud statute prohibits knowingly and willfully executing a scheme to defraud any health care benefit program, including those of private payors. A violation of this statute is a felony and may result in fines, imprisonment, or exclusion from government sponsored programs. The false statements statute prohibits knowingly and willfully falsifying, concealing, or covering up a material fact or making any materially false, fictitious, or fraudulent statement in connection with the delivery of, or payment for, health care benefits, items, or services. A violation of this statute is a felony and may result in fines or imprisonment.
If our operations are found to be in violation of any of the laws described above or other applicable state and federal fraud and abuse laws, we may be subject to penalties, including civil and criminal penalties, damages, fines, exclusion from government health care programs, and the curtailment or restructuring of our operations.
Patient Protection and Affordable Care Act: Our operations may be impacted by the federal Patient Protection and Affordable Care Act of 2010, as modified by the Health Care and Education Reconciliation Act of 2010, which we refer to as the Affordable Care Act (“ACA”). Among other things, the ACA imposes a 2.3 percent excise tax on sales of medical devices that are sold in the United States and that are intended for use by humans, with limited exclusions. There is no exemption for small companies; we believe the tax will apply to our scaffolds and we expect to begin paying the tax when we begin commercial sales of our products in the U.S. The ACA also requires (under what are referred to as “Sunshine” or “Open Payments” requirements) manufacturers of covered devices to report details regarding certain payments and other financial arrangements with physicians and teaching hospitals. These reporting provisions preempt state laws that require reporting of the same information, but not those that require reports of different or additional information. Failure to comply subjects the manufacturer to significant civil monetary penalties. We expect compliance with the ACA to impose significant administrative and financial burdens on us.
Environmental Regulation: We are subject to numerous federal, state, and local laws relating to such matters as safe working conditions, manufacturing practices, environmental protection, fire hazard control, and disposal of hazardous substances. Some of these laws require us to obtain licenses or permits to conduct our operations. We have numerous policies and procedures in place to ensure compliance with these laws and to minimize the risk of occupational exposure to hazardous materials. We do not expect our operations to produce significant quantities of hazardous or toxic waste or radiation that would require the use of extraordinary disposal practices. Although the costs to comply with these laws and regulations have not been material, we cannot predict the impact of new or amended laws or regulations or any changes in the way existing and future laws and regulations are interpreted or enforced, nor can we ensure we will be able to obtain or maintain any required licenses or permits.
International
International sales of medical devices are subject to foreign governmental regulations, which vary substantially from country to country. The time required to obtain clearance or approval by a particular country may be longer or shorter than that required for FDA clearance or approval, and the requirements may be different. We expect to be subject to foreign regulations prior to the time we would be subject to the United States regulations.
The primary regulatory environment in Europe is the EU, which consists of 28 countries. Three members of the European Free Trade Association, Iceland, Norway, and Liechtenstein have voluntarily adopted medical device laws and regulations that mirror those of the EU. Other countries, such as Switzerland, have entered into Mutual Recognition Agreements (“MRA”) and allow the sale of medical devices that meet EU requirements.
21
Table of Contents
The EU has three core directives concerning medical devices: Medical Devices Directive (“MDD”), In-Vitro Diagnostic Medical Devices Directive, and Active Implantable Medical Devices Directive. Also, the European Committees for Standardization have set forth voluntary standards regulating the design, manufacture, clinical trials, labeling, and adverse event reporting for medical devices. Before a medical device can be marketed or used in the EU, it must undergo a conformity assessment process as set forth in the relevant medical devices directives (frequently known as Conformité Européenne, or “CE”). Once a medical device is approved for CE Marking, it can be commercially distributed in the EU, the member states of the European Free Trade Association, and countries with MRAs. The method of assessing conformity varies depending on the type and class of product, but normally involves a self-assessment by the manufacturer and an assessment by a third-party notified body, an independent and neutral institution appointed in an EU country. The assessment may also include an audit of the manufacturer’s quality system and specific testing of the device for compliance with ISO 13485, which are voluntary harmonized standards. Each EU member country implements the MDD into national laws that are enforced by a competent authority in that country. For example, the authority in the United Kingdom is the Medicines and Healthcare Products Regulatory Agency. In addition to obtaining CE Marking, many EU countries require completion of a formal registration process before products can be commercially sold. This in-country process may delay our ability to commercialize after obtaining CE Marking.
Before any medical device can be supplied within Australia, it must be included on the Australian Register of Therapeutic Goods and comply with the provisions of the Australian Therapeutic Goods Act. Compliance generally requires, among other things:
· full technical documentation demonstrating compliance to all relevant standards and regulations;
· full quality assurance certification to the key international standard; and,
· the ability of the manufacturer to undertake post market surveillance processes.
However, much of the documentation produced for obtaining the CE Marking in Europe can be used to obtain registration in Australia and the regulatory requirements with respect to the approval of medical devices are similar to European regulations.
Employees
As of December 31, 2014, we had 46 employees, all on whom were full-time A total of 39 were in research and development and 7 were in general and administrative functions. We have never had a work stoppage and none of our employees are covered by collective bargaining agreements or are represented by a labor union.
Executive Officers
Our executive officers and their ages and backgrounds as of March 15, 2015, are as follows:
Robert B. Stockman, age 61, our co-founder, has served as our Chairman of the Board and director since 1999 and as our Chief Executive Officer since August 2010. He serves as a director of HeartWare Limited/HeartWare International, Inc., an ASX and NASDAQ listed medical device company, since December 2006. Mr. Stockman also serves as a board member for MuseAmi, Inc., a privately held advanced music software company that he co-founded. He previously served on the board of ZELTIQ Aesthetics, Inc., a medical technology company listed on NASDAQ, from July 2010 until April 2012. Since 1999, Mr. Stockman has been the President and Chief Executive Officer of Group Outcome LLC, a U.S.-based merchant banking firm that deploys its capital and that of its financial partners in private equity and venture capital investments in medical technology companies. Mr. Stockman also co-founded Centrimed, Inc., an internet-based software company, that was acquired by the Global Healthcare Exchange, LLC, and led the buyouts of Ioptex, an intraocular lens manufacturer, and two Johnson & Johnson divestitures, “A” Company Orthodontics, Inc. and Critikon Company, LLC, each of which was subsequently acquired. Prior to establishing Group Outcome LLC, Mr. Stockman spent 18 years with Johnston Associates, Inc. and Narragansett Capital Corporation, where he focused on venture capital investments and merger advisory work in health care. Mr. Stockman holds a Bachelor’s Degree from Harvard College and a Master in Business Administration from The Tuck School at Dartmouth College, where he serves on Tuck’s Board of Overseers.
22
Table of Contents
Robert K. Schultz, Ph.D., age 58, has served as our President and Chief Operating Officer since 2003. His background comprises over 30 years in pharmaceutical, medical device and combination products. Prior to joining REVA, Dr. Schultz held positions of Vice President of Research and Development and Vice President of Technology Strategy and Licensing for Dura Pharmaceuticals, a specialty respiratory pharmaceutical and pulmonary drug delivery company, and Research Specialist for 3M Pharmaceuticals, a diversified international technology company. He obtained his Ph.D. in Pharmaceutics and his B.S. degree in Pharmacy from the University of Minnesota.
Katrina L. Thompson, age 56, has served as our Chief Financial Officer and Corporate Secretary since 2003. Her experience encompasses over 30 years in accounting, finance, and corporate administration. Prior to joining REVA in 2003, Ms. Thompson held senior financial positions in the telecommunications, commercial real estate development, commercial nursery, and high technology industries. She spent the early part of her career as an auditor with Price Waterhouse, a provider of tax, audit and advisory services. Ms. Thompson received her B.S. in Business Administration from San Diego State University.
Jeffrey A. Anderson, age 48, has served as our Senior Vice President of Clinical and Regulatory affairs since December 2013 and as our Vice President of Clinical and Regulatory affairs since February 2011, a position he previously held at REVA from 2004 to 2008. He has over 20 years of experience in the medical device industry, including his positions of Vice President of Clinical & Regulatory Affairs and Vice President of Research & Development for Neomend, a biomedical device company engaged in the development and commercialization of surgical wound healing products, where he served from October 2008 through February 2011. Additionally, Mr. Anderson has held senior positions at Abbott Vascular, Jomed, CRS Clinical Research, and Medtronic. He received his B.S. in Physics from California State University at Fullerton.
Donald K. Brandom, Ph.D., age 55, has served as our Senior Vice President of Product Development since December 2013, our Vice President of Product Development since December 2010, and our Vice President of Biomaterial Product Development since January 2008. He has directed all biomaterial development activities since 2003 and the scaffold development program since 2010. In his over 25 years of industry experience, he has held technical, senior, and executive management product development positions in the aerospace, microelectronics, and medical device industries. Dr. Brandom earned his Ph.D. in Materials Engineering Science at Virginia Tech and has a B.S. in Chemistry from the University of California, Davis.
Joan Zeltinger, Ph.D., age 52, has served as our Vice President of Scientific Affairs since June 2004 and has directed our biological activities since 2000. Dr. Zeltinger has over 20 years of industry research and business experience that includes several publications and patents. Dr. Zeltinger previously directed the bioresorbable coronary graft and tissue engineered heart valve programs at Advanced Tissue Sciences and chaired the American Society for Testing and Materials, or ASTM, standard development for combination medical products. She received her Ph.D. in Biology from the University of South Carolina with post-doctoral work conducted at the University of Washington, School of Medicine, and has a B.S. in Biology from the University of North Dakota.
Corporate Information
Our company was founded in California in June 1998 as MD3, Inc. We changed our name to REVA Medical, Inc. in March 2002. In October 2010, we reincorporated from the State of California to the State of Delaware. Our principal executive offices are located at 5751 Copley Drive, San Diego, California 92111, and our telephone number is (858) 966-3000. Our website address is www.revamedical.com. The information on, or accessible through, our website is not part of this report.
We make available on our website, free of charge, our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and any amendments to those reports, as soon as reasonably practicable after we electronically file such material with, or furnish it to, the Australian Securities Exchange (the “ASX”) and the U.S. Securities and Exchange Commission (the “SEC”). Our SEC reports can be accessed in the Investor Relations section of our website. The information found on our website is not part of this or any other report we file with or furnish to the SEC.
23
Table of Contents
Item 1A. Risk Factors
You should carefully consider the risks described below and all of the other information set forth elsewhere in this Form 10-K, including our consolidated financial statements and the related notes and “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” in evaluating our business and our prospects. If any of the events or developments described below occurs, our business, financial condition, or results of operations could be negatively affected. In that case, the market price of our CDIs or common stock could decline.
Risks Related to Our Business
We have a history of net losses and negative cash flows and we may never achieve or maintain profitability.
We are a development stage medical device company. We have incurred net losses since our inception, including net losses of approximately $23.8 million, $27.9 million, and $51.0 million for the fiscal years ended December 31, 2012, 2013, and 2014 respectively. As of December 31, 2014, our accumulated deficit was approximately $252.5 million. Also as of December 31, 2014, we had $26.8 million in cash and investments and we had outstanding long-term convertible notes with a redemption value of $25.0 million. Currently, we have no products approved for sale in any jurisdiction. We expect to continue to incur significant operating losses for the foreseeable future as we incur costs associated with:
· conducting our CE clinical trials to obtain human data on our Fantom scaffold;
· seeking regulatory approvals in the EU, Australia, and possibly the United States for Fantom;
· additional product research and development efforts;
· growing, maintaining, and protecting our intellectual property;
· expanding our manufacturing capabilities and developing our sales and marketing capabilities;
· broadening our infrastructure and systems in order to meet the needs of our operations; and,
· complying with the requirements of being a public company in the United States, listed on the ASX.
We cannot predict the extent of our future operating losses and accumulated deficit, we may never generate sufficient revenues to achieve or sustain profitability, and we may be unable to repay our notes payable or debt, if they were to become due and payable before their maturity date. To become and remain profitable, we must succeed in developing and obtaining required regulatory approvals and commercializing products with significant market potential. This will require us to succeed in a range of challenging activities, including all of the activities listed above. We may never succeed in these activities, and we may never obtain regulatory approvals in the markets in which we expect to operate or otherwise generate revenues sufficient to achieve profitability. If we do achieve profitability, we may not be able to sustain it.
We may need substantial additional funding to meet our future operating, capital, and debt service needs and may be unable to raise capital when needed.
Our future operating and capital requirements will depend on many factors, including achievement of regulatory approval of our products, the growth of revenue, the amount of intellectual property and technology expenditures, the number and size of our clinical trials, the extent of new product development, and the timing of repayment of our convertible notes, should they become due and payable. Until we generate a level of revenue to support our cost structure, we expect to continue to incur substantial operating losses and net cash outflows. We had cash and investments totaling $26.8 million as of December 31, 2014 and warrants for the purchase of common stock, which, if exercised could provide us with additional cash proceeds to be used for operations of between $19.0 million and $22.8 million. If the warrants are not exercised in time to accommodate our cash flow needs, we may need to raise additional capital. Failure to raise capital when needed would force us to delay, reduce, or eliminate our product development programs or commercialization efforts.
Any equity or debt financing, if available at all, may be on terms that are not favorable to us or may be substantially dilutive to our current security holders. Equity financings could result in dilution to our existing security holders, and the securities issued in future financings may have rights, preferences, and privileges that are senior to those of our existing security holders. Because our need for capital arises as a result of significant losses, the occurrence of these losses may make it more difficult for us to raise necessary capital when needed, which would force us to delay, reduce, or eliminate our product development programs or commercialization efforts.
24
Table of Contents
Holders of our convertible notes can elect to be repaid in cash, rather than converting the notes into common stock. Until such time as we can generate positive cash flows, we may not have the cash resources to repay the notes. If we cannot repay the notes when, and if, required, we would be in default of the note provisions, which could have a significant adverse effect on our Company, including its ability to remain in business.
We issued $25.0 million in convertible notes in November 2014, bearing interest at 7.54 percent per annum, with no interest required to be paid until redemption. While the notes have a five-year maturity, their terms allow the noteholders a one-time election to be repaid in full in January 2017, or at any time we become in default of the notes. The notes are convertible into common stock at any time. The conversion rate of $2.17275 per share as of December 31, 2014 was favorable for the noteholders, as it was below the $3.32 market price of our common stock. However, in a decision to either convert or redeem the notes, many factors may influence the noteholders; some of these factors may be out of our control and, even if in our control, we may fail to perform, which may cause the noteholders to consider redemption options over conversion options. For example, a noteholder may consider global economic trends in making their decision, or they may evaluate the progress we have made, or not made, and the results achieved in our human clinical trials.
If the noteholders collectively, or individually, elect redemption prior to converting the notes into common stock or prior to the maturity date of the notes, we most likely would not have the cash resources to repay the notes and would then be in default of the note provisions. If we were unable to cure the default by raising additional capital, which might not be available on favorable terms, if at all, the noteholders could cause the Company to take extreme measures, including reduction of operations and personnel, sale of assets, including intellectual property assets, and/or declaring bankruptcy. Any of these actions would have a material adverse effect on the Company.
Our ability to generate revenue depends upon our successfully completing clinical trials, obtaining regulatory approval, and commercializing our products, which we may be unable to accomplish.
Our products will require extensive clinical testing, regulatory approval, and significant marketing and distribution efforts before they can be sold and generate any revenue. Our efforts to generate revenue may not succeed for a number of reasons including:
· we may experience delays with our bioresorbable scaffold program, including the enrollment and successful completion of our clinical trials and our planned CE applications;
· our Fantom scaffold may not demonstrate safety and efficacy in our clinical trials;
· we may not be able to obtain regulatory approvals for Fantom in the markets in which we expect to operate, or the approved indications for Fantom may be narrower than we currently anticipate;
· our products may not be accepted in the marketplace by physicians and patients;
· physicians may not receive adequate coverage and reimbursement for procedures using our products;
· new product introductions by our competitors or any rapid technological change may make our technology and product candidates obsolete;
· we may not be able to manufacture or distribute our products in commercial quantities or at an acceptable cost;
· we depend on suppliers of critical components for our products, including lasing of the scaffold and the balloon catheter system it deploys from, and we may be significantly impacted by any regulatory delays or barriers that our suppliers may encounter; and,
· we may be sued for infringement of intellectual property rights which could prevent us from manufacturing or selling our products.
We cannot market our products in the EU until we receive a CE Mark or in the United States until we receive a PMA. We cannot guarantee that we will receive regulatory approval on a timely basis, or at all. Our operating plan is based in part on our expectations regarding the timing for receipt of regulatory approvals and if we experience significant delays in the approval process, we may be unable to reduce our expenditures in a timely manner to compensate for such delays and we may not have adequate financial or other resources to complete the approval process. Accordingly, a significant delay in the regulatory approval process would have a material adverse effect on our ability to successfully sell our products and on our financial condition. In addition, a delay may require us to raise additional financing, including equity or debt financing, to fund our operations, which could be dilutive to existing stockholders or require us to relinquish important rights to our technology or products.
25
Table of Contents
We will depend heavily on the success of our Fantom scaffold and any factors that negatively impact its sales potential will adversely affect our business, financial condition, and results of operations.
Since Fantom will be our first commercial product, our ability to successfully generate revenues and to consider additional products for commercialization will depend on our abilities to market and sell Fantom. The degree of market acceptance for this scaffold will depend on a number of factors, including:
· its perceived advantages and disadvantages compared to existing stents and other treatments and technologies;
· its safety and efficacy and the prevalence and severity of any adverse events or side effects;
· its ease of use compared to existing products and competitive treatments and technologies;
· our ability to provide additional preclinical and clinical data regarding its potential long-term benefits;
· the strength of our sales and marketing initiatives; and,
· the selling price and the third-party coverage and reimbursement for procedures using Fantom.
If our Fantom scaffold does not achieve an adequate level of acceptance by physicians, patients, and health care payors, we may not generate or maintain positive gross margins and we may not become profitable, or be able to sustain profitability, and we may not commercialize additional products. Even if Fantom does achieve market acceptance, we may not be able to sustain it or otherwise achieve it to a degree that would support the ongoing viability of our operations.
Physicians may not widely adopt our products unless they determine that the use of our products provides a safe and effective alternative to other existing treatments for coronary artery disease.
We believe that physicians will not widely adopt our products unless they determine, based on experience, long-term clinical data, and published peer-reviewed journal articles, that the use of our products provides a safe and effective alternative to other existing treatments. We cannot provide any assurance that the data collected from our clinical trials will be sufficient to demonstrate that our bioresorbable scaffolds are an attractive alternative to other procedures. If we fail to demonstrate safety and efficacy that is at least comparable to other stents and scaffolds that are available for sale, our ability to successfully market our scaffolds will be limited. Even if data collected from clinical studies or clinical experience indicate positive results, each physician’s actual experience with our scaffolds will vary. We also believe that published peer-reviewed journal articles and recommendations and support by influential physicians regarding our scaffolds will be important for market acceptance and adoption of our products; we may be unable to receive these recommendations and support.
We compete against companies that have longer operating histories, more established or approved products, and greater resources, which may prevent us from achieving market penetration or improving operating results.
Competition in the stent industry is intense. Our products will compete against products offered by substantial, global, public companies, as well as smaller and private companies. Global stent sales are dominated by Abbott, BSC, and Medtronic, who together recorded an estimated 94 percent of the $4.2 billion worldwide stent sales in 2014. All three companies have significantly greater technical, regulatory, financial, manufacturing, and human resources than we do. They also have established reputations, approved metal stents and bioresorbable scaffolds, significantly greater name recognition, and distribution channels and sales and marketing capabilities that are large and established. Our ability to compete effectively depends upon our ability to distinguish our Company and our products from our competitors and their products. We believe the factors affecting our competitive position include:
· name and brand recognition;
· relationships with physicians and patients;
· the availability of other products and procedures, including bundled product offerings;
· product performance and design;
· product safety and the availability of supporting clinical data;
· sales, marketing and distribution capabilities;
· success and timing of new product development and introductions; and,
· intellectual property protection.
26
Table of Contents
The stent industry has undergone, and is expected to continue to undergo, rapid and significant technological change. We expect competition to intensify as technical advances are made. Our competitors may develop and commercialize stents or other medical device or pharmaceutical products that are safer or more effective, have fewer side effects, or are less expensive than products we may develop. For example, we are aware of companies that are developing less-invasive technologies for treating cardiovascular disease, which could limit the market potential for our scaffolds. We also compete to recruit and retain qualified scientific and management personnel, establish clinical trial sites and patient registrations, and acquire technologies complementary to our programs or advantageous to our business. For all these reasons, we may not be able to compete successfully against current and future competitors.
Failed attempts by our competitors to market and sell bioresorbable scaffolds could discredit or produce a bias against bioresorbable technology and adversely impact our ability to commercialize our bioresorbable scaffolds.
Abbott initiated sales of bioresorbable scaffolds in 2012 in limited markets outside the United States; a second company, Elixir, began sales of its bioresorbable scaffold in Germany in January 2014. We believe that Abbott, Elixir, and perhaps other competitors will obtain regulatory approval to market bioresorbable scaffolds in many countries prior to the time we begin sales of our product. If these competitors, including Abbott, who may have significant resources and established reputations, launch bioresorbable scaffolds and are unable, for any reason, to capture a significant portion of the stent market, this failure could discredit or produce a bias against bioresorbable scaffold technology. If physicians and other key influencers perceive a competitor’s failure to capture a significant portion of the stent market with scaffolds as an indication of inadequacies with the bioresorbable technology, our ability to later launch and successfully commercialize our bioresorbable scaffolds could be adversely impacted.
Product liability claims could damage our reputation or adversely affect our business.
The design, manufacture, and sale of human medical devices, particularly implantable life-sustaining devices like our scaffolds, carry inherent risks of product liability and other damage claims. A product liability or other damages claim against our product, a product recall, or a product misuse, regardless of the ultimate outcome, could require us to spend significant time and financial resources in litigation or to pay significant damages and could seriously harm our business. We maintain clinical trial insurance and limited product liability insurance; we cannot be certain that such insurance will be sufficient to cover all claims that may be made against us. Our insurance policies generally must be renewed on an annual basis; we may not be able to maintain or increase such insurance on acceptable terms or at reasonable costs. A successful claim brought against us in excess, or outside, of our insurance coverage could seriously harm our financial condition and results of operations. Such claims, regardless of their merit, could result in significant awards against us that could materially adversely harm our business, financial condition, results of operations, and prospects. A product liability or other damages claim, product recall, or product misuse involving any type of coronary stent, but especially involving one of ours, could also materially and adversely damage our reputation and affect our ability to attract and retain customers, whether or not such claim had merit.
We have limited manufacturing capabilities and personnel, and if we are unable to provide an adequate supply of our scaffolds, we may not be able to support our clinical trials, which would delay our regulatory approval process, or following such approval, we may not be able to meet our commercial demands.
We currently manufacture our scaffolds at our facility in San Diego, California. If we encounter a disruption to the facility or the surrounding area, for example, due to a natural disaster, we would have no means to manufacture scaffolds until we were able to restore our facility or procure alternative manufacturing facilities. If we are unable to produce sufficient quantities of our products for use in our planned clinical trials, or if our manufacturing process yields substandard product, our regulatory approval process may be delayed.
Assuming we receive regulatory approval for our scaffolds, we currently have limited resources and facilities and no prior history of commercially manufacturing products. In order to produce commercial quantities of our products, we will need to substantially enhance our production processes and the efficiency of our manufacturing operations. There are significant technical and regulatory challenges to increasing manufacturing capacity and efficiency, and developing commercial-scale manufacturing facilities will require substantial capital investment and the addition of managing and technical personnel who have relevant manufacturing experience. We may not successfully complete increases in our manufacturing in a timely or economically viable manner, or at all. In addition, we may not be able to receive the necessary regulatory approvals for our manufacturing facilities on a timely basis, or at all. If we are unable to manufacture a sufficient or consistent supply of our scaffolds, or if we cannot do so efficiently, our revenues, business, and financial prospects would be adversely affected.
27
Table of Contents
We rely on specialized suppliers for certain components and processes for our bioresorbable scaffolds and any disruption from these suppliers could adversely affect our ability to produce scaffolds.
We rely on suppliers for critical components of our bioresorbable scaffolds, including lasing of the scaffold and the balloon catheter system it deploys from and we outsource sterilization of the finished product. Our reliance on third-party suppliers subjects us to risks that could interrupt our supply chain in a variety of manners, including:
· we are not a major customer of many of our suppliers, and therefore, these suppliers may give other customers’ needs higher priority than ours;
· our suppliers have no contractual obligation to supply, and we are not obligated to purchase from them, which may result in supply interruptions;
· our suppliers source raw materials through supply chains that periodically are unable to provide needed materials in a timely manner, which may cause delays in providing critical components to us;
· the availability of second-source suppliers may be extremely limited or their implementation as a supplier may be lengthy due to the tight tolerances and specifications that we require for our scaffolds; and,
· changing suppliers may require product redesign and submission to the regulatory authorities to whom we are seeking approval for our scaffolds.
Our current clinical trial and commercialization plans are predicated on a steady supply of scaffolds, which we believe is dependent on maintaining strong relationships for our supply chain and avoiding problems or delays in our own manufacturing and assembly processes. If we are unsuccessful in this regard, or are unable to secure or maintain agreements with these manufacturers on favorable terms, or at all, or if we are required to source or develop new suppliers, we may experience delays in our regulatory approval and commercialization timelines, which would have a material adverse effect on our business and financial prospects.
If we are unable to retain or hire key personnel, we may not be able to sustain or grow our business.
Our ability to operate successfully and manage our potential future growth depends significantly upon our ability to attract, retain, and motivate highly skilled and qualified research, technical, clinical, regulatory, sales, marketing, managerial, and financial personnel. We compete for talent with numerous companies, as well as universities and non-profit research organizations. Our future success also depends on the personal efforts and abilities of the principal members of our senior management and scientific staff to provide strategic direction, manage our operations, and maintain a cohesive and stable environment. Except with respect to our agreements with Robert B. Stockman, our Chief Executive Officer, Robert K. Schultz, our President and Chief Operating Officer, Katrina L. Thompson, our Chief Financial Officer, and Jeffrey A. Anderson, our Senior Vice President of Clinical and Regulatory Affairs, we have not entered into any employment agreements with our employees, nor do we maintain key person life insurance on any of our senior team. Although we have a stock option plan pursuant to which we provide our key personnel with various economic incentives to remain employed with us, these incentives may not be sufficient to retain them. The loss of key personnel for any reason or our inability to hire, retain, and motivate additional qualified personnel in the future could prevent us from sustaining or growing our business.
BSC has an option to distribute our products, which may limit our ability to negotiate more favorable terms with other potential distributors.
In December 2007, we entered into a Distribution Option Agreement with BSC under which we granted BSC an option to negotiate the right to be the worldwide, exclusive distributor of our scaffold products. If BSC exercises its option, we are required to negotiate with BSC to enter into a mutually acceptable definitive distribution agreement. If we are unable to agree on the terms of a definitive distribution agreement with BSC, the restrictions in the Distribution Option Agreement may limit our ability to negotiate more favorable terms with other potential distribution partners.
28
Table of Contents
If we do not enter into a distribution arrangement with BSC, we will need to find another distribution partner for the sale of our products or develop our own sales network. Any delay or problems associated with a distribution partner or our own sales network could have a serious impact on our sales and our financial performance.
We do not have any experience in marketing, selling, or distributing products. We have developed a preliminary sales and marketing launch strategy, which focuses on selecting a distribution partner to assist in the marketing and sale of our products in jurisdictions where it is approved for commercial sale. While BSC has an option to negotiate a distribution agreement with us, there is no guarantee they will exercise the option, or that we will be able to reach a definitive distribution agreement with BSC even if they do exercise the option. If we do not enter into a distribution arrangement with BSC, we will need to find another distribution partner for the sale of our products or develop our own sales and marketing network. There can be no assurance that we will be able to identify and enter into a distribution arrangement with a third party distributor on acceptable terms, or at all. The development of our own sales, distribution, and marketing network would require significant amounts of financial and management resources and we will face a number of risks, including:
· our ability to attract and build a significant, successful, or qualified marketing or sales force;
· the cost of establishing, training, and providing regulatory oversight for a marketing or sales force may be substantial; and,
· any failure to comply with legal and regulatory requirements for sales, marketing, and distribution could result in enforcement actions by the FDA or other authorities and could jeopardize our ability to market our products or could subject us to substantial liability.
Any delay or problems associated with a distribution partner or our own sales network could have a material adverse impact on our sales and our financial performance.
If we commercialize outside the United States, we may be subject to the risks associated with operating in foreign markets.
Our operations are currently located in the United States. We currently intend to seek regulatory approvals for our products in the EU, Australia, and elsewhere prior to seeking a PMA in the United States. If we expand into these foreign markets, or even commercialize in the United States, we will be subject to new business risks, including:
· failure to fulfill foreign regulatory requirements on a timely basis, or at all, to market our products;
· availability of, and changes in, reimbursement within prevailing foreign health care payment systems;
· differing laws and regulations, business and clinical practices, and patient preferences in foreign countries;
· difficulties in managing foreign relationships and operations, including any relationships that we establish with foreign partners, distributors, or sales or marketing agents;
· limited protection for intellectual property rights in some countries;
· difficulty in collecting accounts receivable and longer collection periods;
· costs of enforcing contractual obligations in foreign jurisdictions;
· recessions, political instability, and changes in diplomatic and trade relationships in foreign countries;
· currency exchange rate fluctuations; and,
· potentially adverse tax consequences.
If we are successful in introducing Fantom or future products into foreign markets, we will be affected by these additional business risks, which may adversely impact our business, financial condition, and results of operations. In addition, expansion into foreign markets imposes additional burdens on our executive and administrative personnel, research and sales departments, and general managerial resources. Our efforts to introduce our current or future products into foreign markets may not be successful, in which case we may have expended significant resources without realizing the expected benefit. Ultimately, the investment required for expansion into foreign markets could exceed the results of operations generated from this expansion.
29
Table of Contents
Risk Factors Related to Regulation
In order to conduct clinical trials we need to obtain regulatory and other approvals. Any delay in receiving such approvals, or any denial of approval, could have a significant adverse effect on our timeline and ability to commercialize our technology.
Through December 31, 2014, we had received approvals of all our applications to conduct clinical trials with our predecessor scaffolds; we had also received approval to conduct the pilot trial of Fantom. We plan to conduct a larger clinical trial of Fantom in 2015 at multiple centers in eight countries outside the United States and we plan to continue to conduct additional clinical trials with Fantom and other products that may be developed from our technology. We will need to submit applications and receive regulatory approvals for each clinical trial. There is no guarantee that we will be able to obtain regulatory approval for any future clinical trial or that additional work and preclinical testing will not be required before regulatory approval is granted. If we are unable to obtain approvals, or obtain them timely, our ability to commercialize on our timeline, if at all, could be significantly adversely affected. Before we can commence any clinical trial, we require approvals from:
· relevant Ethics Committees (Investigational Review Boards) in each of our chosen clinical trial centers; and
· relevant regulatory bodies.
In the United States, prior to conducting human clinical trials, we will need to obtain approval of an IDE application from the FDA. Before we can sell our products in the United States, PMA approval is required from the FDA, which is a lengthy and uncertain process. The procedure for submitting an application for PMA is expensive and typically requires extensive preclinical and clinical trial data as well as considerable technical data. Submitted data will need to be obtained in accordance with the FDA’s QSR. We are planning to use the data obtained from our CE Marking clinical trials to evaluate whether to proceed with the U.S. approval process. There is a risk that the FDA may not allow the data to be used in the PMA application, which would result in a delay and increase in costs of U.S. approvals.
We cannot predict the outcome of our human clinical trials. Our Fantom scaffold may not meet the intended clinical results or may cause adverse or unexpected events. There is no guarantee that we will be able to address issues arising from clinical trials, which could negatively impact our future prospects.
The outcome of human clinical trials cannot be predicted, even when preclinical results are favorable. If our Fantom scaffold demonstrates adverse issues such as restenosis, stroke, thrombosis, and/or death, it is likely the clinical trial will need to be halted. In such case, we may need to modify our technology to address the issues. Our clinical trials may be suspended or terminated at any time by regulatory authorities, the U.S. Data Safety and Monitoring Board, or by us, including during the enrollment period or during the subsequent patient follow-up period. There is no guarantee that we will be able to successfully address and overcome any adverse events arising in the clinical trials. If we are unable to address these issues, we will not be able to commercialize our technology, and it will likely have a nominal value, if any at all.
The completion of our clinical trials could also be substantially delayed or prevented by several factors, including:
· slower than expected rates of patient recruitment and enrollment, including as a result of our competitors’ undertaking similar clinical trials or having functionally comparable products that are approved for sale;
· patient preference to use approved devices or other experimental treatments or devices other than ours;
· unforeseen safety issues;
· inability or unwillingness of patients or medical investigators to follow our clinical trial protocols;
· failure of patients to complete the clinical trial or our inability to monitor patients adequately during or after treatment;
· risks associated with trial design, which may result in a failure of the trial to show statistically significant results even if the product is effective;
· governmental and regulatory delays or changes in regulatory requirements, policies, or guidelines;
· varying interpretation of data by regulatory agencies; and,
· perceived lack of product efficacy during clinical trials.
30
Table of Contents
There is no guarantee that, even with successful data from our clinical trials, we will be able to receive regulatory approval to market and sell our products, which could negatively impact our future prospects.
The process of obtaining marketing approval or clearance from regulatory authorities to market and sell our Fantom scaffold or any future products or enhancements or modifications to any products, could:
· take a significant period of time;
· require the expenditure of substantial resources;
· involve rigorous preclinical and clinical testing;
· require changes to our products; and,
· result in limitations on the indicated uses of the products.
There can be no assurance that we will receive the required approvals from the regulatory authorities or, if we do receive the required approvals, that we will receive them on a timely basis or that we will otherwise be able to satisfy the conditions of such approval, if any. The failure to receive product approval by the regulatory authorities will have a material adverse effect on our business, financial condition, and results of operations.
We must generate long-term human data on the safety and efficacy of our scaffolds. Any long-term data that is generated may not be consistent with our limited short-term data, which could affect the regulatory approval of our products or the rate at which our products are adopted.
An important factor in our clinical trials, upon which the safety and efficacy of our scaffolds may be measured, is the rate of restenosis, or the re-narrowing of the treated artery over time, and the rate of re-intervention, or retreatment, following scaffold implant. We believe that physicians and regulators will compare the rates of long-term restenosis and re-intervention for Fantom against other bioresorbable or bare metal and drug-eluting metal stent procedures and other alternative procedures.
If we fail to demonstrate reasonable restenosis and re-intervention rates, as well as other clinical trial endpoints, and product performance comparable to other stents or scaffolds that have been approved by the FDA and other regulatory authorities, our ability to successfully market our Fantom scaffold may be significantly limited. If Fantom’s long-term rates of restenosis and re-intervention do not meet regulators’ or physicians’ expectations, it may not receive regulatory approval or, if approved, it may not be widely adopted and physicians may recommend alternative treatments for patients. Another performance measurement of Fantom will be the incidence of late-stent thrombosis. We cannot assure that our long-term data, once obtained, will prove a lower incidence of late-stent thrombosis as compared to drug-eluting metal stents. If the results obtained from our clinical trials indicate that our scaffolds are not as safe or effective as other treatment options or as effective as current short-term data would suggest, our products may not be approved, adoption of our products may suffer, and our business would be harmed.
We plan to operate in multiple regulatory environments that require costly and time consuming approvals.
We will need to obtain regulatory approval in each jurisdiction in which we intend to commercialize our products. The regulatory requirements will vary from country to country. In addition, the laws and regulations regarding the manufacture and sale of our products will be subject to future changes, as are administrative interpretations and policies of regulatory agencies. If we fail to comply with applicable laws or regulations, we could be subject to enforcement actions. Enforcement actions could include product seizures, recalls, withdrawal of clearances or approvals, and civil and criminal penalties, which, in each case, would harm our business.
Our manufacturing facilities and those of our suppliers must comply with applicable regulatory requirements. If these facilities do not achieve regulatory approval, our business and our results of operations would be harmed.
Completion of our clinical trials and commercialization of our products require access to, or the development of, facilities that meet applicable regulatory standards to manufacture a sufficient supply of our products. Approvals are required to achieve CE Marking in Europe; similar facility approvals must be obtained from the FDA to manufacture products for U.S. purposes. Suppliers of components and products used to manufacture our products must also comply with applicable regulatory requirements, which often require significant time, money, resources, record-keeping, and quality assurance efforts and subject us and our suppliers to potential regulatory inspections and stoppages. If we or our suppliers fail to comply with the regulatory requirements for our manufacturing operations, our commercialization efforts could be delayed, which would harm our business and our results of operations.
31
Table of Contents
We may not meet regulatory quality standards applicable to our manufacturing and quality processes, which could have an adverse effect on our business, financial condition, or results of operations.
Even after products receive marketing approval, they can be withdrawn due to failure to comply with regulatory standards or the occurrence of problems following initial approval. As a device manufacturer, we will be required to demonstrate and maintain compliance with a variety of regulatory requirements. In the EU, we are required to maintain certain ISO certifications in order to sell our products and must undergo periodic inspections by notified bodies to obtain and maintain these certifications.
We have received a Certificate of Registration certifying that our Quality Management System complies with the requirements of ISO 13485:2012. In the future, if we fail to continue to comply with ISO regulations, or any other regulation that we may be subject to, the relevant regulatory authorities may withdraw our approval to market, require a product recall, or take other enforcement action. Compliance is subject to continual review and is rigorously monitored through periodic inspections. If we fail to comply with the requirements or to take satisfactory corrective action in response to an adverse inspection, we could be subject to enforcement actions, including a public warning letter, a shutdown of or restrictions on our manufacturing operations, delays in approving a product, refusal to permit the import or export of our products, a recall or seizure of our products, fines, injunctions, civil or criminal penalties, or other sanctions, any of which could cause our business and operating results to materially suffer.
Our operations involve hazardous materials and we must comply with environmental laws and regulations, which can be expensive.
Our research, development, and manufacturing activities involve the controlled use of hazardous chemicals. Our operations also produce hazardous waste products. We are subject to a variety of federal, state, and local regulations relating to the use, handling, storage, and disposal of these materials. We generally contract with third parties for the disposal of such substances. We cannot eliminate the risk of accidental contamination or injury from these materials. We may be required to incur substantial costs to comply with current or future environmental and safety regulations. If an accident or contamination occurred, we would likely incur significant costs to remedy the situation and also may be subject to civil penalties or criminal fines. Current or future environmental regulation may impair our research, development, or production efforts.
If we fail to obtain and maintain adequate reimbursement for our products by third-party payors, there may be no commercially viable markets for our products or the markets may be much smaller than expected.
The availability and levels of reimbursement by governmental and other third-party payors affect the market for our products. Reimbursement and health care payment systems vary significantly by country, and include both government-sponsored health care and private insurance. Payors may attempt to limit coverage and the level of reimbursement of new therapeutic products. Government and other third-party payors also continually attempt to contain or reduce the costs of health care by challenging prices charged for health care products and services.
To obtain reimbursement or pricing approval in some countries, we may be required to produce clinical data, which may involve one or more clinical trials, that compares the cost-effectiveness of our products to other available therapies. In addition, the efficacy, safety, performance, and cost-effectiveness of our products in comparison to any competing products may determine the availability and level of reimbursement for our products.
We believe that future reimbursement may be subject to increased restrictions both in the United States and in international markets. Future legislation, regulation, or reimbursement policies of third-party payors may adversely affect the demand for our products and limit our ability to sell our products on a profitable basis. We cannot predict how pending or future legislative and regulatory proposals would influence the manner in which medical devices, including ours, are purchased or covered and reimbursed. For example, the American Recovery and Reinvestment Act of 2009 provided funding to study the comparative effectiveness of health care treatments and strategies. This funding is used to, among other things, conduct, support, or synthesize research that compares and evaluates the risks and benefits, clinical outcomes, effectiveness, and appropriateness of medical products; it remains unclear how the research will impact coverage, reimbursement, or other third-party payor policies.
If reimbursement for our products is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, sales of our products would be impaired and our future revenues would be materially adversely affected.
32
Table of Contents
Health care reform legislation could adversely affect our future revenue and financial condition.
In recent years in the United States and other countries, there have been numerous initiatives for reforms affecting the availability of, and reimbursement for, health care services. These initiatives have ranged from proposals that would fundamentally change health care reimbursement programs to minor modifications of existing programs. In addition, recent U.S. legislation and proposed bills provide funding to assess the comparative effectiveness of medical devices. It is unclear what impact the comparative effectiveness analysis would have on our products or our financial results. The ultimate content or timing of any future health care reform legislation, and its impact on medical device companies such as ours, is impossible to predict. If significant reforms are made to the United States or other health care systems, they may have a material adverse effect on our financial condition and results of operations.
Our operations may also be impacted by the U.S. Affordable Care Act (“ACA”). Among other things, the ACA imposes a 2.3 percent excise tax on sales of medical devices intended for use by humans, with limited exclusions. There is no exemption for small companies; we believe the tax will apply to our scaffolds and we expect to begin paying the tax when we begin commercial sales of our products in the U.S. The ACA also requires (under what are referred to as “Sunshine” or “Open Payments” requirements) manufacturers of covered devices to report details regarding certain payments and other financial arrangements with physicians and teaching hospitals. These reporting provisions preempt state laws that require reporting of the same information, but not those that require reports of different or additional information. Failure to comply subjects the manufacturer to significant civil monetary penalties. We expect compliance with the ACA to impose significant administrative and financial burdens on us.
We are subject to various federal and state laws pertaining to health care fraud and abuse, including anti-kickback, self-referral, false claims, civil monetary penalties, and fraud laws. Any violations by us of such laws could result in fines, penalties, or other criminal prosecution. In addition, compliance with these laws may result in significant additional expense to us and limit our ability to commercialize our products.
Our commercial, research, and other financial relationships with health care providers and institutions are subject to various federal and state laws intended to prevent health care fraud and abuse. We are also subject to regulation by other regional, national, state, and local agencies, including the Department of Justice, the Office of Inspector General of the U.S. Department of Health and Human Services and other regulatory bodies. Violations of any of these laws and regulations could result in penalties or fines being assessed against us, significant additional compliance expense, or even a limitation on our ability to commercialize our products.
The federal Anti-Kickback Statute prohibits the knowing offer, receipt, or payment of remuneration in exchange for or to induce the referral of patients or the use of products or services that would be paid for in whole or part by Medicare, Medicaid, or other federal health care programs. Remuneration has been broadly defined to include anything of value, including cash, improper discounts, and free or reduced price items and services. Many states have similar laws that apply to their state health care programs as well as private payors. Violations of the anti-kickback laws can result in exclusion from federal health care programs and substantial civil and criminal penalties.
The U.S. False Claims Act (“FCA”) imposes liability on persons who, among other things, present false or fraudulent claims for payment by a federal health care program. The FCA has been used to prosecute persons submitting claims for payment that are inaccurate or fraudulent, that are for services not provided as claimed, or for services that are not medically necessary. The FCA includes a whistleblower provision that allows individuals to bring actions on behalf of the federal government and share a portion of the recovery of successful claims. If our marketing or other arrangements were determined to violate anti-kickback or related laws, including the FCA, then our revenues could be adversely affected, which would likely have a material adverse effect on our business, financial conditions, and results of operations. Similarly, the federal Civil Monetary Penalty statute imposes significant penalties for filing certain types of improper claims or engaging in prohibited acts related to federal program integrity.
State and federal authorities have aggressively targeted medical device companies for alleged violations of these anti-fraud statutes, based on improper research or consulting contracts with doctors, certain marketing arrangements that rely on volume-based pricing, off-label marketing schemes, and other improper promotional practices. Compliance with various federal and state laws is difficult and time consuming, and companies that violate them may face substantial penalties. Because of the breadth of these laws and the lack of extensive legal guidance in the form of regulations or court decisions, it is possible that some of our business activities or those of our commercial partners could be subject to challenge under one or more of these laws. Such a challenge could have a material adverse effect on our business and financial condition and growth prospects.
33
Table of Contents
Companies targeted in such prosecutions have paid substantial fines in the hundreds of millions of dollars or more, have been forced to implement extensive corrective action plans, and have often become subject to consent decrees severely restricting the manner in which they conduct their business. If we become the target of such an investigation or prosecution based on our contractual relationships with providers or institutions, or our marketing and promotional practices, we could face similar sanctions which would materially negatively affect our business.
If we are found to have violated laws protecting the privacy and security of patient information, we could be subject to civil suits and civil or criminal penalties, which could increase our liabilities and harm our reputation or our business.
We are subject to privacy laws in the countries in which we do business. We have in place a specific Australian Privacy Policy and plan to expand our privacy policies to cover the privacy laws we are, or will be, subject to. These laws, including the federal and state privacy laws in the United States, are designed to protect the privacy and security of personally identifiable information, including patient health information and patient records, by, among other things, limiting its use and disclosure, establishing patient rights, requiring security safeguards, and mandating notice to the government and individuals if information is compromised (i.e., a breach). Many local jurisdictions also have similar laws protecting the privacy and security of personally identifiable information, including breach notification requirements. If we violate applicable privacy laws, we could be subject to civil lawsuits based on state law or tort (including class actions) and civil or criminal penalties, which could increase our liabilities, harm our reputation, and have a material adverse effect on our business, financial condition, and results of operations.
Risk Factors Related to Intellectual Property
We rely on certain licenses for patents and other technology related to our products. The termination of these license agreements could delay or prevent us from being able to commercialize our products.
We have licensed certain patent rights and other technology that we use for our scaffolds. For example, we have licensed a majority of the polymer technology that we use from Rutgers University. In order to maintain our rights under the Rutgers License Agreement, we must satisfy certain development and commercialization obligations. If we fail to satisfy these obligations, Rutgers might license some or all of this technology to one or more of our competitors and our ability to compete may be diminished. Furthermore, if we fail to comply with material obligations under the license agreement or if the license if terminated for any reason, we could lose license rights that are important to our business. The license agreement expires on the expiration date of the last patent to expire under this agreement, which we believe is currently approximately 2034; if we need to renew the license, there is no guarantee we will be able to renew it on commercially reasonable terms, if at all.
In addition, we expect that we may need to license other technology or patents to commercialize our scaffolds or future products. These licenses may not be available to us on commercially reasonable terms, or at all, which could adversely affect our results of operations and growth prospects.
If we are unable to obtain, maintain, and enforce intellectual property protection covering our products, others may be able to make, use, or sell products similar to ours, which could adversely affect our ability to compete.
Our commercial success depends in part on obtaining, maintaining, and enforcing intellectual property rights, including patents, covering our scaffolds and future product candidates. If we are unable to obtain, maintain, or enforce intellectual property protection covering our products, others may be able to make, use, or sell products that are substantially the same as ours without incurring the sizeable development and licensing costs we have incurred, which would adversely affect our ability to compete in the market.
Currently, our patent portfolio is comprised, on a worldwide basis, of 307 issued and pending U.S. and foreign patents that we own directly or for which we are the licensee and that expire as late as 2034. Pending patent applications could further extend our patent portfolio life. However, patents may not be issued based on any pending or future patent applications owned by or licensed to us and, moreover, issued patents owned or licensed to us now or in the future may be found by a court to be invalid or otherwise unenforceable. Even if our patents are determined by a court to be valid and enforceable, they may not be sufficiently broad to prevent others from marketing products similar to ours or designing around our patents, despite our patent rights, nor do they provide us with freedom to operate unimpeded by the patent rights of others.
34
Table of Contents
We have licensed certain intellectual property from third parties related to our products, and we rely on them to file and prosecute patent applications, maintain patents, and otherwise protect that intellectual property. We cannot be certain that such activities by third parties have been or will be conducted in compliance with applicable laws and regulations or will result in valid and enforceable patents and other intellectual property rights. In addition, we cannot be certain that our licensors will allocate sufficient resources or prioritize their or our enforcement of such patents or defense of such claims to protect our interests in the licensed patents.
The patent positions of medical device companies can be highly uncertain and involve complex legal and factual questions for which important legal principles remain unresolved. No consistent policy regarding the breadth of claims allowed in patents in these fields has emerged to date in the United States or in many foreign jurisdictions. Both the U.S. Supreme Court and the Court of Appeals for the Federal Circuit have made, and will likely continue to make, changes in how the U.S. patent laws are interpreted. In addition, Congress is currently considering legislation that would change provisions of the patent law. We cannot predict future changes in the interpretation of patent laws or changes to patent laws that might be enacted into law. Those changes may materially affect our patents, our ability to obtain patents, or the patents and applications of our collaborators and licensors. The patent situation in the medical device and disease diagnostic fields outside the United States is even more uncertain.
We have numerous foreign patents and applications. The laws of some foreign jurisdictions do not protect intellectual property rights to the same extent as laws in the United States and many companies have encountered significant difficulties in obtaining, protecting, and defending such rights in foreign jurisdictions. If we encounter such difficulties or we are otherwise precluded from effectively protecting our intellectual property rights in foreign jurisdictions, our business prospects could be substantially harmed.
We rely on trade-secret protection for certain of our proprietary know-how and for processes for which patents are difficult to obtain or enforce. We may not be able to protect our trade secrets adequately as we have limited control over our licensors, collaborators, and suppliers. Although we use reasonable efforts to protect our trade secrets, our employees, consultants, contractors, outside scientific collaborators, and other advisors may unintentionally or willfully disclose our information to competitors. Enforcing a claim that a third party illegally obtained and used any of our trade secrets is expensive and time consuming, and the outcome is unpredictable. In addition, courts outside the United States are sometimes less willing to protect trade secrets. We rely, in part, on non-disclosure and confidentiality agreements with our employees, consultants, and other parties to protect our trade secrets and other proprietary technology. These agreements may be breached and we may not have adequate remedies for any breach. Moreover, others may independently develop equivalent proprietary information or third parties may otherwise gain access to our trade secrets and proprietary knowledge. Any disclosure of confidential data into the public domain or to third parties could allow our competitors to use the information against us.
Claims that our current or future products infringe or misappropriate the proprietary rights of others could adversely affect our ability to sell those products and cause us to incur additional costs.
Intellectual property rights, including patent rights, play a critical role in stents and stent delivery systems. We face significant risks relating to our patents, as well as to patents held by third parties. If any third-party intellectual property claim against us is successful, we could be prevented from commercializing our scaffolds or other future product candidates.
There are numerous U.S. and foreign issued patents and pending patent applications owned by third parties with patent claims in areas that relate to our scaffolds. Also, because patent applications can take many years to issue, there may be other pending applications, unknown to us, that may result in future patents that pose a material risk to us. We are aware of patents owned by third parties, to which we do not have licenses, that relate to, among other things:
· stent structures, materials, and designs;
· catheters used to deliver stents; and,
· polymer and stent manufacturing and coating processes.
We expect that we could be increasingly subject to third-party infringement claims as we receive regulatory approval to sell products, our revenues increase, we are faced with more competitors, or the functionality of products and technology in different industry segments overlaps. Third parties may currently have, or may eventually be issued, patents on which our current or future products or technologies may infringe. Any of these third parties might make a claim of infringement against us.
35
Table of Contents
All of the major companies in the stent and related markets, including BSC, Abbott, and Medtronic have been involved in patent litigation relating to stents since at least 1997. The stent and related markets have experienced rapid technological change and obsolescence in the past, and our competitors have strong incentives to stop or delay the introduction of new products and technologies. We may pose a competitive threat to many companies in the stent and related markets. Accordingly, many of these companies will have a strong incentive to take steps, through patent litigation or otherwise, to prevent us from commercializing our products.
Any litigation, regardless of its outcome, would likely result in significant expenses and the diversion of our management’s time and resources. In addition, litigation in which we are accused of infringement may cause negative publicity, adversely impact prospective customers, cause product shipment delays, prohibit us from manufacturing, marketing, or selling our products, require us to develop non-infringing technology, make substantial payments to third parties, or enter into royalty or license agreements, which may not be available on acceptable terms, or at all. If a successful claim of infringement were made against us and we could not develop non-infringing technology, invalidate the claim, or license the infringed or similar technology on a timely and cost-effective basis, our revenues may decrease substantially and we could be exposed to significant liability. A court could enter orders that temporarily, preliminarily, or permanently prevent us or our customers from making, using, selling, offering to sell, or importing our current or future products, or could enter an order mandating that we undertake certain remedial activities. Claims that we have misappropriated the confidential information or trade secrets of third parties can have a similar negative impact on our reputation, business, financial condition, or results of operations.
We may need to initiate lawsuits to protect our patents or other intellectual property rights, which could be expensive and which, if lost, could result in loss of intellectual property rights, which would harm our business.
We rely on patents to protect a portion of our intellectual property and competitive position. Patent law relating to the technology fields in which we operate is still evolving and, consequently, patent positions in the medical device industry are generally uncertain. In order to protect or enforce our patent rights, we may initiate patent litigation against third parties, such as infringement suits or interference proceedings. Litigation may be necessary to:
· assert claims of infringement;
· enforce our patents;
· protect our trade secrets or know-how; or,
· determine the enforceability, scope, and validity of the proprietary rights of others.
Any lawsuits that we initiate could be expensive, take significant time, and divert management’s attention from other business concerns. Litigation also puts our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing. Additionally, we may provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially valuable. The occurrence of any of these events may have a material adverse effect on our business, financial condition, and results of operations.
Risks Related to Our CDIs and Common Stock
The market price of our CDIs and common stock may be volatile and fluctuate significantly, which could result in substantial losses for investors.
We are a development stage company without a product available for sale; consequently, we have no revenues and continue to generate operating losses. Additionally, we issued $25.0 million in convertible notes in November 2014 and, while the notes do not mature until 2019, the noteholders have an option for full repayment in January 2017; if this option is elected, we may not have sufficient cash at that time to redeem the notes. Because we are still developing our products, our progress is partly measured by the achievement of technological milestones, such as initiating or successfully completing clinical trials; these milestones do not occur on a regular or predictable schedule. Our securities are listed for sale only on the Australian Securities Exchange (the “ASX”) in the form of CHESS Depositary Interests (“CDIs”). Until we achieve commercialization, start generating revenues and cash receipts, have the ability to service our notes payable or debt, demonstrate regular measureable performance, or list our securities for sale on an additional stock exchange, the market for our CDIs may continue to be illiquid and the market price of our CDIs may continue to be volatile. In addition to the matters described in this “Risk Factors” section, the market price of our CDIs may fluctuate due to other risks and factors, including:
36
Table of Contents
· announcements of our development progress, including delays or advancements in our timelines;
· announcements regarding the regulatory status of our scaffolds and future product candidates;
· any reported adverse events in our human clinical trials;
· failure to service our debt or raise additional financing to fund our operations when needed or on terms favorable to us or on terms that are not overly dilutive to our current security holders;
· announcements of technological innovations or new products by us or our competitors;
· announcements of contracts, acquisitions, or strategic alliances by us or our competitors;
· changes in the estimates of the future size and growth rate of our markets;
· changes in market valuations or earnings of our competitors;
· changes in legislation or regulatory policies, practices, or actions;
· the commencement or outcome of litigation involving our company, our general industry, or both;
· recruitment or departure of one or more members of our executive management team;
· changes in our capital structure, such as future issuances of securities or the incurrence of additional debt;
· actual or expected sales of our CDIs or common stock by existing holders;
· the trading volume of our CDIs; and,
· changes in general economic, industry, and market conditions.
Stock markets in general, and submarkets for medical technology companies in particular, have experienced volatility that has often been unrelated to the operating performance of companies. These broad market and industry factors may materially affect the market price of our CDIs. Litigation has often been brought against companies whose securities have experienced volatility in market price. Class-action litigation, even if unsuccessful, could be costly to defend and divert management’s attention and resources, which could materially harm our financial condition and results of operations.
Investors may experience difficulty in trading our CDIs due to their relatively limited liquidity on the ASX.
Although our CDIs are listed on the ASX, there can be no guarantee of a ready liquid market for them, particularly since a small number of security holders own a majority of our outstanding capital. It may be more difficult for an investor to realize an investment on the ASX than it would be to realize an investment in a company whose shares or other securities are quoted on the New York Stock Exchange, the NASDAQ Stock Market, or any other exchange.
We may not retain our ASX listing and we may not qualify for listing on another securities exchange.
We cannot assure investors that we will always retain a listing on ASX and our common stock is not currently listed for trading on a U.S. or any other securities exchange. The provisions of the Note Deed we signed on September 25, 2014 call for us to use reasonable efforts to list on NASDAQ or another securities exchange as soon as practicable after September 2015. If we fail to retain our ASX listing or if we do not list on another securities exchange, certain investors may decide to sell their securities and/or there may not be a market for the securities, which could have an adverse impact on the price of the securities. There is no assurance that we can qualify in the future for listing any of our securities on the New York Stock Exchange, the NASDAQ Stock Market, or any other exchange.
Some of our existing stockholders can exert control over us and may not make decisions that are in the best interests of all stockholders.
As of March 15, 2015, officers, directors, and stockholders holding more than five percent of our outstanding shares collectively controlled approximately two-thirds of our outstanding common stock. As a result, these stockholders, if they act together, would be able to exert a significant degree of influence over our management and affairs and over matters requiring stockholder approval, including the election of directors and approval of significant corporate transactions. Accordingly, this concentration of ownership may harm the market price of our shares by delaying or preventing a change in control, even if a change is in the best interests of our other stockholders. In addition, the interests of this concentration of ownership may not always coincide with the interests of other stockholders and, accordingly, they could cause us to enter into transactions or agreements that we would not otherwise consider.
37
Table of Contents
Future sales of our common stock may depress the market price of our CDIs.
The holders of an aggregate of approximately 19.2 million shares of our outstanding common stock, as well as the holders of our convertible notes, if such notes are converted into common stock, have certain rights to cause us to file a registration statement on their behalf and to include their shares in registration statements that we may file on behalf of other stockholders. In addition, shares of common stock reserved for issuance under our 2010 Equity Incentive Plan, as amended (the “Plan”), have been registered and, accordingly, any vested and exercised shares of stock issued in accordance with the Plan may be freely sold under the federal securities laws and may be tradable under state securities laws if a holder satisfies such laws or is exempt from them. Additionally, the Plan provides for annual increases in the number of shares available for issuance under the Plan, which we intend to register annually. From time to time, we also may sell additional common stock in subsequent public offerings or private placements. Sales of a substantial number of common shares or CDIs in the public market, whether by us or by our stockholders, or the perception that these sales may occur, could cause the market price of our CDIs to decline and make it more difficult for holders to sell CDIs or shares of common stock in the Company.
We have broad discretion in the use of our assets and our investment of these assets may not yield a favorable return, which could harm our business and depress the market price of our securities.
Our management has discretion in the application of our assets and other resources and may use them for a broad range of purposes. Accordingly, security holders will have to rely upon our management’s judgment with respect to the use of the Company’s assets. Management may spend a portion or all of our assets in ways that holders of our securities may not desire or that may not yield a significant return, or any return at all. The failure by our management to apply these funds effectively could harm our business and depress the market price of our securities. Pending their use, we may also invest our assets in a manner that does not produce income or that loses value.
We do not currently intend to pay dividends on our CDIs or common stock; consequently, the return on an investment in our securities will depend on appreciation in the market price of our CDIs.
We currently intend to invest our future earnings, if any, to fund the development and growth of our business. The payment of dividends will be at the discretion of our Board and will depend on our operating results, capital needs, financial condition, future prospects, debt covenants, contractual arrangements, restrictions imposed by applicable law, and other factors our Board may deem relevant. If we do not pay dividends, the ability to achieve a return on an investment in REVA will depend on any future appreciation in the market price of our CDIs or other securities. There is no guarantee that our CDIs will appreciate or even maintain the price at which they were purchased.
We incur exchange rate risks relating to our listing on the ASX.
Our securities, in the form of CDIs, are listed on the ASX and priced in Australian dollars. However, we report in U.S. dollars. As a result, movements in foreign exchange rates may cause the price of our securities to fluctuate for reasons unrelated to our financial condition or performance and may result in a discrepancy between our actual results of operations and investors’ expectations of returns on our securities expressed in Australian dollars.
We expend substantial costs and management resources to comply with the laws and regulations affecting public companies in the United States as well as listing requirements of the ASX, which may adversely affect our operating results. Failure to maintain effective internal control over financial reporting in accordance with the Sarbanes-Oxley Act could cause investors to lose confidence in our operating results and in the accuracy of our financial reports and could have a material adverse effect on our business and on the market price of our CDIs.
As an SEC-registered U.S. public company with securities listed on the ASX, we incur substantial legal, accounting, and other reporting and shareholder expenses. In addition, changing laws, regulations, and standards relating to corporate governance and public disclosure, including SEC regulations, may increase legal and financial compliance costs and make some corporate activities more time consuming. Since our securities are traded on the ASX, we must comply with ASX Listing Rules. We believe our policies and procedures are designed to provide reasonable assurance of ASX Listing Rules compliance; however, if we do not follow those procedures and policies, or they are not sufficient to prevent non-compliance, we could be subject to liability, fines, and lawsuits. These laws, regulations, and standards are subject to varying interpretations and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. We expend significant management resources to comply with securities regulations, which may divert attention from revenue-generating activities. If our efforts to comply with new laws, regulations, and standards are unsuccessful, regulatory authorities may initiate legal proceedings against us and our business may be harmed.
38
Table of Contents
Additionally, we are required, pursuant to Section 404 of the Sarbanes-Oxley Act of 2002 (“Section 404”) to furnish reports by management to the SEC on, among other things, the effectiveness of our internal controls over financial reporting. The controls and other procedures are designed to ensure that information required to be disclosed by us in reports that we file with the SEC is accurate and recorded, processed, summarized, and reported within the time periods specified in SEC rules and forms. Although we have developed effective controls, these controls may become inadequate because of changes in conditions, our degree of compliance may deteriorate, or weaknesses in our internal controls may be discovered. If we, or our auditors, are unable to certify that our internal controls over financial reporting are effective and in compliance with Section 404, or we are unable to produce timely or accurate financial reports, we may be subject to sanctions or investigations, and investors may lose confidence in the accuracy and completeness of our financial reports, which would have a material adverse effect on our business, the market price of our CDIs, and our ability to access the capital markets.
Failure to comply with the SEC and ASX rules and regulations might also make it more difficult for us to obtain certain types of insurance, including director and officer liability insurance, and we might be forced to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. The impact of these events could also make it more difficult for us to attract and retain qualified persons to serve on our board of directors, on committees of our board of directors, or as members of senior management.
Provisions of our certificate of incorporation, our bylaws, and Delaware corporation law could make an acquisition of us, which may be beneficial to our stockholders, more difficult and may prevent attempts by our stockholders to replace or remove the current members of our board and management.
We are a Delaware corporation, subject to the provisions of Delaware General Corporation Law. Those laws, in addition to certain provisions of our certificate of incorporation and our bylaws could discourage, delay, or prevent a merger, acquisition, or other change of control that stockholders may consider favorable, including transactions involving a premium over market price for our CDIs. Furthermore, these provisions could prevent or frustrate attempts by our stockholders to replace or remove members of our board of directors. These provisions also could limit the price that investors might be willing to pay in the future for our CDIs, thereby depressing the market price of our CDIs. Stockholders who wish to participate in these transactions may not have the opportunity to do so. These provisions:
· allow the authorized number of directors to be changed only by resolution of our board of directors ( “Board”);
· provide that our stockholders may only remove our directors for cause;
· establish a classified Board so that not all members of the Board may be elected at one time;
· authorize our Board to issue, without stockholder approval but subject to ASX listing rules, up to 100,000,000 shares of common stock or up to 5,000,000 shares of preferred stock, that, if issued, would dilute ownership and operate as a “poison pill” to help prevent an acquisition that is not approved by the Board;
· require that stockholder actions occur at a duly called stockholder meeting or by unanimous written consent;
· establish advance notice requirements for stockholder nominations to our Board or for stockholder proposals that can be voted at stockholder meetings;
· limit who may call stockholder meetings; and,
· require approval from 80 percent of the outstanding shares of our capital stock in order to amend certain provisions of our certificate of incorporation and bylaws.
In addition, provisions of Section 203 of the Delaware General Corporation Law may, unless certain criteria are met, prohibit large stockholders, in particular those owning 15 percent or more of the voting rights on our common stock, from merging or combining with us for a prescribed period of time.
Item 1B. Unresolved Staff Comments
We do not have any unresolved staff comments relating to our periodic or current reports.
39
Table of Contents
Item 2. Properties
Our primary facility is located at 5751 Copley Drive, San Diego, California, where we lease and occupy approximately 37,000 square feet of research, lab, and office space. We lease an entire building and are the only tenant in the building. The lease on this facility expires in January 2018.
We do not own any real property. We believe that our leased facility is adequate to meet our current needs, as well as our future office, lab, and manufacturing needs through at least application to CE Marking and initial commercial sales. We may consider additional or different facilities and locations for manufacturing after we have commenced commercial sales.
Item 3. Legal Proceedings
We may from time to time become subject to various claims and legal actions during the ordinary course of our business. We are not party to any legal proceedings at the date of filing of this Annual Report on Form 10-K.
Item 4. Mine Safety Disclosures
Not applicable.
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters, and Issuer Purchases of Equity Securities
Market Information
Shares of our common stock began trading in the form of CHESS Depositary Interests (“CDIs”), each CDI representing one-tenth of a share of our common stock, on the Australian Securities Exchange (“ASX”) under the symbol “RVA” on December 23, 2010. Prior to such time, there was no public market for our securities.
Between January 1, 2014 and December 31, 2014, the sales price of our CDIs ranged from a low sales price of A$0.11 to a high sales price of A$0.57, or a low sales price per share of common stock of $0.98 and a high sales price of $4.74 after giving effect to the ten-for-one CDI-to-common stock exchange ratio and after converting to U.S. dollars using the closing exchange rate applicable on the relevant date as reported by the Reserve Bank of Australia.
The high and low sales prices for our CDIs during each quarter, and on an equivalent basis as converted to common stock and U.S. dollars, were as follows:
| | CDI Price Range | | Stock Price Range | |
| | Low | | High | | Low | | High | |
| | | | | | | | | |
Year Ended December 31, 2013: | | | | | | | | | |
First quarter | | $ | 0.39 | | $ | 0.59 | | $ | 4.09 | | $ | 6.07 | |
Second quarter | | 0.49 | | 0.65 | | 4.94 | | 6.23 | |
Third quarter | | 0.52 | | 0.65 | | 4.74 | | 6.17 | |
Fourth quarter | | 0.43 | | 0.61 | | 3.85 | | 5.87 | |
| | | | | | | | | |
Year Ended December 31, 2014: | | | | | | | | | |
First quarter | | $ | 0.14 | | $ | 0.48 | | $ | 1.29 | | $ | 4.31 | |
Second quarter | | 0.12 | | 0.21 | | 1.13 | | 1.97 | |
Third quarter | | 0.11 | | 0.33 | | 0.98 | | 2.83 | |
Fourth quarter | | 0.17 | | 0.57 | | 1.48 | | 4.74 | |
40
Table of Contents
As of March 15, 2015 we had 33,479,778 shares of common stock issued and outstanding with approximately 909 holders of record. The holders included CHESS Depositary Nominee Pty Limited, which held 18,726,290 shares of our common stock in the form of CDIs on behalf of the CDI holders; there were approximately 854 registered owners of our CDIs on March 15, 2015.
Stock Price Performance Graph
The following graph compares our total common stock return, after giving effect to the ten-for-one CDI-to-common stock exchange ratio and after converting to U.S. dollars using the spot rate applicable on the relevant date, with the total return for (i) the NASDAQ Composite Index and (ii) the NASDAQ Biotechnology Index for the period from December 23, 2010 (the date our common stock commenced trading on the ASX) through December 31, 2014. The figures represented below assume an investment of $100 in our common stock at the closing price of $12.52 per share of common stock on December 23, 2010, and in the NASDAQ Composite Index and the NASDAQ Biotechnology Index on December 23, 2010, and the reinvestment of dividends, if any, into shares of common stock. The comparisons in the table are disclosures in accordance with SEC requirements and are not intended to forecast or be indicative of possible future performance of our common stock. This graph shall not be deemed “soliciting material” or to be “filed” for purposes of Section 18 of the Exchange Act or otherwise subject to the liabilities under that Section, and shall not be deemed to be incorporated by reference into any of our filings under the Securities Act or the Exchange Act.
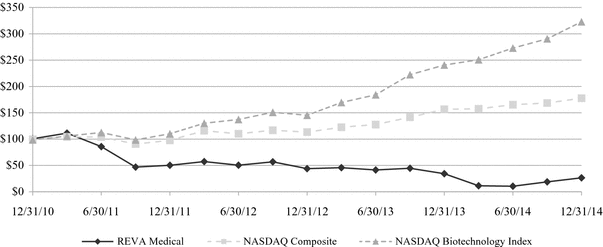
Dividend Policy
We have never declared or paid any cash dividends on our common stock. We currently intend to retain all available funds and any future earnings to support operations and finance the growth and development of our business and do not intend to pay cash dividends on our common stock or CDIs for the foreseeable future. Any future determination related to our dividend policy will be made at the discretion of our board of directors.
Recent Sales of Unregistered Securities
On November 14, 2014, we issued 250 senior unsecured convertible notes (the “Notes”), each with a face value of $100,000, and warrants to purchase 8,750,000 shares of our common stock. We received proceeds of $25.0 million from the Notes and have the potential to receive an additional $22.8 million when, and if, the warrants are exercised. None of the Notes or the warrants are registered securities. The Notes are convertible into, and the warrants are exercisable for, common stock at the rate of $2.17275 per share. The exercise price for the warrants increases to $2.6073 per share when, and if, we complete enrollment of 110 patients in a clinical trial with our Fantom scaffold.
41
Table of Contents
Both the notes and the warrants have five-year lives. The holders of the Notes and the warrants have registration rights related to the underlying common stock; such registration rights are on the same terms as the registration rights previously existing and held by other stockholders in the Company.
Use of Proceeds from Public Offering of Common Stock
In December 2010, we completed an initial public offering (the “IPO”), of our common stock, in which we sold 77,272,730 CDIs, representing 7,727,273 shares of common stock, at a price of A$1.10 per CDI or A$11.00 per share. The aggregate proceeds from the offering were A$85.0 million (which was approximately US$84.3 million). The CDIs issued in the IPO were registered under the Securities Act pursuant to a registration statement on Form S-1 (File No. 333-168852), which was declared effective by the SEC on November 15, 2010. We also lodged a Prospectus with the Australian Securities and Investments Commission prior to the allotment and issuance of the CDIs. Our net IPO proceeds, after deducting placement agent fees and other offering expenses, were approximately US$76.2 million. We had used approximately 96 percent of the proceeds through December 31, 2014, in a manner generally consistent with our projections at the time of the IPO, as follows:
· $40.0 million was projected to be used for research and development activities, including advancement of our scaffolds and development of pipeline products, if any. Approximately $40.0 million has been used for R&D.
· $10.0 million was projected to be used for clinical trials and approximately $6.0 million has been used for that purpose.
· $4.0 million was projected to be used for commercial infrastructure, including manufacturing capacity expansion. Approximately $4.0 million has been used for R&D equipment, laboratory improvements, and related infrastructure.
· The balance of the IPO proceeds were expected to be used for working capital and other general corporate purposes and they have been used for those purposes.
While we expect to utilize the remaining IPO proceeds, as well as the proceeds from the Notes issued in November 2014, for further development and testing of our scaffolds in our effort to prepare a product for commercialization, as well as to meet the general corporate requirements of a public company, the amounts and timing of our actual expenditures may vary significantly and will depend upon numerous factors, including the timing and success of our clinical trials. We plan to evaluate whether to conduct clinical trials in the United States after we receive acceptable data from the European clinical trials. Due to the regulatory requirements in the United States for a study with a large number of patients, we anticipate needing additional funding in order to carry out any U.S. clinical trials.
Pending the use of proceeds to the Company, we invest excess cash in accordance with our investment policy, which allows for short-term and long-term interest-bearing obligations, investment grade instruments, certificates of deposit, or guaranteed obligations of the U.S. government.
Item 6. Selected Financial Data
We have derived our statements of operations data for the years ended December 31, 2010 and 2011 and our balance sheet data as of December 31, 2010, 2011, and 2012 from our audited financial statements which are not included in this Form 10-K. We have derived our statements of operations data for the years ended December 31, 2012, 2013, and 2014 and our balance sheet data as of December 31, 2013 and 2014 from our audited financial statements appearing elsewhere in this Form 10-K. Our audited financial information is prepared and presented in accordance with generally accepted accounting principles in the United States, or U.S. GAAP.
Our selected consolidated financial data should be read together with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and related notes included elsewhere in this Annual Report on Form 10-K.
42
Table of Contents
The following tables present our selected financial data for the five-year period ending December 31, 2014.
| | Year Ended December 31, | |
| | 2010 | | 2011 | | 2012 | | 2013 | | 2014 | |
| | (in thousands, except per share data) | |
Statements of Operations Data: | | | | | | | | | | | |
| | | | | | | | | | | |
Operating Expense: | | | | | | | | | | | |
Research and development | | $ | 6,826 | | $ | 13,401 | | $ | 15,822 | | $ | 19,212 | | $ | 14,318 | |
General and administrative | | 3,292 | | 7,695 | | 8,043 | | 8,731 | | 7,645 | |
| | | | | | | | | | | |
Loss from operations | | (10,118 | ) | (21,096 | ) | (23,865 | ) | (27,943 | ) | (21,963 | ) |
| | | | | | | | | | | |
Other Income (Expense): | | | | | | | | | | | |
Interest income | | 116 | | 188 | | 92 | | 30 | | 8 | |
Interest expense | | (1,549 | )(1) | — | | — | | — | | (986 | ) |
Interest from amortization | | 2,283 | | — | | — | | — | | — | |
Loss on issuance of convertible notes payable and warrants | | — | | — | | — | | — | | (15,627 | ) |
Loss on change in fair value of convertible notes payable and preferred and common stock warrant liabilities | | (990 | ) | — | | — | | — | | (12,542 | ) |
Loss on extinguishment of notes payable | | (13,285 | ) | — | | — | | — | | — | |
Other income (expense) | | 36 | | — | | (3 | ) | (9 | ) | 73 | |
| | | | | | | | | | | |
Net Loss | | (23,507 | ) | (20,908 | ) | (23,776 | ) | (27,922 | ) | (51,037 | ) |
| | | | | | | | | | | |
Cumulative dividends and deemed dividends on Series H convertible preferred stock | | (7,200 | ) | — | | — | | — | | — | |
| | | | | | | | | | | |
Net Loss Attributable to Common Stockholders | | $ | (30,707 | ) | $ | (20,908 | ) | $ | (23,776 | ) | $ | (27,922 | ) | $ | (51,037 | ) |
| | | | | | | | | | | |
Net Loss Per Share: (2) | | | | | | | | | | | |
Net loss per share, basic and diluted | | $ | (7.72 | ) | $ | (0.64 | ) | $ | (0.72 | ) | $ | (0.84 | ) | $ | (1.53 | ) |
Shares used to compute net loss per share,basic and diluted | | 3,975,144 | | 32,777,509 | | 33,072,058 | | 33,124,655 | | 33,382,381 | |
(1) | Includes $1,510 to related parties. |
| |
(2) | See Note 3 to our consolidated financial statements for an explanation of the method used to compute the net loss per share and the number of shares used in the computation of the per share amounts. |
| | Year Ended December 31, | |
| | 2010 | | 2011 | | 2012 | | 2013 | | 2014 | |
| | (in thousands) | |
Balance Sheet Data: | | | | | | | | | | | |
Cash and cash equivalents | | $ | 81,747 | | $ | 59,161 | | $ | 38,876 | | $ | 19,229 | | $ | 25,814 | |
Short- and long-term investments | | — | | 5,226 | | 5,223 | | 1,492 | | 995 | |
Working capital | | 80,984 | | 59,847 | | 42,323 | | 17,656 | | 24,351 | |
Total assets | | 83,475 | | 67,320 | | 47,397 | | 24,785 | | 30,195 | |
Convertible notes payable | | — | | — | | — | | — | | 37,780 | |
Common stock warrant liability | | — | | — | | — | | — | | 15,389 | |
Total liabilities | | 1,528 | | 2,737 | | 2,771 | | 3,960 | | 56,644 | |
Accumulated deficit | | (128,903 | ) | (149,811 | ) | (173,587 | ) | (201,509 | ) | (252,546 | ) |
Total stockholders’ equity (deficit) | | 81,947 | | 64,583 | | 44,626 | | 20,825 | | (26,449 | ) |
| | | | | | | | | | | | | | | | |
43
Table of Contents
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
You should read the following discussion and analysis of our financial condition and results of operations in conjunction with the “Selected Consolidated Financial Data” and our consolidated financial statements and the related notes thereto that appear elsewhere in this Annual Report on Form 10-K. In addition to historical information, the following discussion and analysis includes forward-looking information that involves risks, uncertainties, and assumptions. Actual results and the timing of events could differ materially from those anticipated by these forward-looking statements as a result of many factors, including those discussed under “Risk Factors” elsewhere in this Annual Report on Form 10-K. See also “Forward-Looking Statements” included elsewhere in this Annual Report on Form 10-K.
Overview
We are a development stage medical device company working toward commercialization of our proprietary technologies to provide minimally invasive medical devices for the treatment of conditions in the human body. We are in the later stages of developing and clinically testing bioresorbable drug-eluting coronary stents. We refer to bioresorbable stents as “scaffolds” because they are not permanent devices like metal stents that are commonly used today. In clinical use, a scaffold is guided by x-ray by an interventional cardiologist during a minimally invasive surgery to a coronary artery location with a delivery catheter system, whereupon it is deployed to restore blood flow to the artery and medicate the artery to prevent further blocking, or restenosis. Our scaffolds combine our proprietary bioresorbable polymer with various designs, including deformable designs and our proprietary “slide and lock” non-deformable designs. Our scaffolds are designed to offer full x-ray visibility, clinically relevant sizing, and a controlled and safe resorption rate.
We believe that due to the risks and limitations associated with commercially available metal stents, bioresorbable scaffolds will be the next major advance in coronary stent technology. Because we have designed our scaffolds to provide the same benefits as traditional metal stents, but with the additional benefit of eliminating the presence of a permanently implanted device, we believe that our products would enable us to compete effectively in the stent market, providing we are able to complete development and clinical testing, successfully implement manufacturing processes and procedures, receive regulatory approval to sell commercially, and execute our sales and marketing strategies effectively. Worldwide revenues from coronary stent sales approximated $4.2 billion in 2014.
Our stent products have not yet been approved for sale and will require successful clinical trial results and regulatory approval before they can generate sales. We have invested significant time and funds in development, having performed scientific research, engineering development, and testing in laboratory and preclinical studies. We have developed, tested, and selected the polymer formulation, tested and selected the anti-restenotic drug and coating process, created and iterated the device design, and identified and implemented methods and processes to produce and test the scaffold. We designed and performed extensive preclinical tests that ranged from bench and engineering studies to in vitro and in vivo laboratory studies. In 2007, we enrolled patients in a small human clinical study that proved the viability of the technology while confirming the areas needing further development. We have been developing and advancing our scaffolds in both design and polymer composition since that study and have undertaken significant testing that has shown the viability of the technology across various models.
We are currently testing our Fantom scaffold, which was introduced in humans during December 2014. Prior to developing Fantom, we had enrolled a total of 165 patients in three clinical trials between June 2007 and January 2014 with predecessor scaffolds that were developed utilizing our proprietary x-ray visible polymer in combination with our “slide and lock” stent design. While these predecessor scaffolds demonstrated viability of the technology, we believe the enhanced characteristics of Fantom better position it for commercial success. The enhanced features include a unibody design, lower strut thickness, smaller crossing profile, optimized polymer properties, and streamlined manufacturing processes, while still maintaining all the beneficial features of our prior scaffolds. In March 2014 we announced Fantom as our sole focus for development and testing and concurrently reduced headcount by approximately 45 percent and reduced other overhead costs to a lesser extent. We intend to enroll up to 110 patients in a clinical trial with Fantom during 2015, obtain follow-up data at a six-month time point, and if this data has acceptable safety and efficacy results, apply for regulatory approval in mid-2016. We may also enroll supplemental patients in the Fantom clinical trial during 2015 to provide data to support the BSC distribution option (see “— Distribution and License Agreements” above for additional information).
44
Table of Contents
Our current plan is to apply for European CE Marking, the regulatory approval that would allow us to sell our scaffolds in Europe and other countries that recognize the CE Mark. When, and if, we receive CE Mark approval, we will evaluate how best to implement our sales and marketing strategies for commercialization. While our Fantom scaffold could be approved for sale in late 2016, our efforts to generate substantial revenue and achieve positive cash flows from our operations may take several years, even if our clinical results are favorable.
In order to produce quantities of our scaffold large enough to accommodate commercial needs, when that time arrives, we will need to scale up our manufacturing processes and expand our capabilities to allow for such things as additional scaffold sizes. We developed plans and began implementation of the methods and processes for such manufacturing scale-up, including work on the product sizes, in 2014. We will continue implementation of manufacturing preparedness as we approach commercialization.
During the course of our product development and testing, we have invented, co-invented, and licensed a portfolio of proprietary technologies. Our design-related technologies have been invented by our employees and consultants and our materials-related technologies have been either invented by our employees or licensed from, or co-invented with, Rutgers, The State University of New Jersey. We consider our patent portfolio to be significant and have invested considerable time and funds to develop and maintain it. Our goal is to continue to perform feasibility tests on additional technologies in our patent portfolio as our resources allow and, if feasibility is proven, determine a course of development for additional products.
During our development efforts, we have also pursued, tested, and abandoned development programs that we determined would not lead to feasible products or for which a product could not be developed in a timeframe that would allow for reasonable commercialization. The largest of these abandoned programs centered on development of a thin metal stent technology for use in small blood vessels. Although abandoned in 2002 after approximately $13.0 million had been invested and used, this technology became the basis for the “slide and lock” mechanism we maintain in our patent portfolio. Additionally, we licensed a potential anti-restenotic drug in 2001 with the intent to develop it for use as a stand-alone drug or as a complement to our scaffold. Although the drug’s development was abandoned in 2004 after we had invested approximately $6.0 million, the knowledge we gained from that program was used in our development of the drug coating for our current scaffolds. We also formed a wholly owned subsidiary in Germany in 2007 to facilitate our clinical trials and our planned commercialization of products; we have not used this subsidiary yet for any operating activities.
We have performed all of our research and development activities from one location in San Diego, California. As of December 31, 2014, we had 46 employees, a majority of which are degreed professionals and six of whom are PhDs. We leverage our internal expertise with contract research and preclinical laboratories, outside catheter manufacturing, and other outside services as needed. We have three clean rooms and multiple engineering and chemistry labs at our facility, in addition to our corporate and administrative office. We are ISO certified to the medical device standard 13485:2012 and intend to maintain the certification to support our commercialization plans.
During 2014, we negotiated and completed a financing to provide the ongoing capital for our operations, including the Fantom clinical trial and application for CE Mark. This financing was completed in November 2014 with the issuance of 250 senior unsecured convertible notes, each with a face value of $100,000, and warrants to purchase 8,750,000 shares of our common stock. We received cash proceeds of $25.0 million from the notes and have the potential to receive up to an additional $22.8 million cash proceeds when, and if, the warrants are exercised. Both the notes and the warrants have a five-year maturity or expiration.
We have not yet developed a product to a saleable stage and we have not, therefore, generated any product or other revenues. Our development efforts have been funded with a variety of capital received from angel investors, venture capitalists, strategic partners, hedge funds, the proceeds from our IPO in 2010, and the issuance of convertible notes in November 2014. Since our inception, we have received approximately $153.9 million in equity proceeds and $53.5 million from issuances of notes payable ($28.5 million of the notes payable were converted to common stock upon our IPO in 2010). As of December 31, 2014, we had approximately $26.8 million in cash and investments available for operations. We have incurred substantial losses since our inception; as of December 31, 2014, we had accumulated a deficit of approximately $252.5 million.
45
Table of Contents
We expect our losses to continue for the next several years as we continue our development work, clinical studies, and preparations for commercialization and, if these efforts are successful and we are able to obtain approval to sell our products, we expect to commence product sales thereafter. In order to successfully transition to profitable operations, we will need to achieve a level of revenues and product margins to support the Company’s cost structure. Until such time as we generate positive cash flow, we plan to continue to fund our operating and capital needs by utilizing our current cash and investments and by raising additional capital through equity or debt financings or strategic or other transactions, if the warrants issued in November 2014 are not exercised, or not exercised on a timeframe that coincides with our cash needs.
Key Components of our Results of Operations
Since we are still in a pre-revenue stage and our activities are focused on further developing and testing our bioresorbable coronary scaffold with the goal of commercially selling it, as well as performing minimal research and tests to determine the feasibility of other product possibilities, our operating results primarily consist of research and development expenses, including costs to perform clinical trials, general and administrative expenses, and other expenses that are primarily the costs of the convertible notes and warrants that we issued in November 2014 to provide the funds to continue our developments efforts.
Research and Development Expenses: Our research and development expenses arise from a combination of internal and external costs. Our internal costs primarily consist of employee salaries and benefits, facility and other overhead expenses, and engineering and other supplies that we use in our labs for prototyping, testing, and producing our scaffolds and other product possibilities. Our external costs primarily consist of contract research, engineering consulting, polymer consulting and certain production costs, polymer lasing costs, catheter system and anti-restenotic drug purchases, preclinical and clinical study expenses, and license fees paid for the technology underlying our polymer materials. All research and development costs are expensed when incurred.
Through December 31, 2014, we have incurred approximately $137.1 million in research and development expenses since our inception, which represents approximately 72 percent of our cumulative operating expenses. In March 2014 we announced a change in scaffold programs, moving to Fantom as our sole focus for development and testing. This change resulted in decreased research and development expenses in 2014, primarily due to a concurrent reduction in headcount by approximately 46 percent upon the change and a reduction of other development and clinical trial expenses due to the earlier stage of development for Fantom compared to the predecessor program. Research and development expenses as a percentage of our total operating expenses were 65 percent in 2014. We expect to increase our research and development expense in 2015 as we continue the testing of Fantom. We intend to enroll up to 110 patients in a clinical trial with Fantom during 2015, obtain follow-up data at a six-month time point, and if this data has acceptable safety and efficacy results, apply for regulatory approval in mid-2016. We may also enroll supplemental patients in the Fantom clinical trial during 2015 to provide data for other business purposes. We also expect to incur increasing expenses in the future for development of final manufacturing processes and equipment as we prepare for commercialization and as we research the feasibility of developing additional products from technology in our intellectual property portfolio.
General and Administrative Expenses: Our general and administrative expenses consist primarily of salaries and benefits for our executive officers and administrative staff, corporate office and other overhead expenses, legal expenses including patent filing and maintenance costs, audit and tax fees, investor relations and other public company costs, and travel expenses. Although our patent portfolio is one of our most valuable assets, we record legal costs related to patent development, filing, and maintenance as expense when the costs are incurred since the underlying technology associated with these assets is purchased or incurred in connection with our research and development efforts and the future realizable value cannot be determined.
Through December 31, 2014, we have incurred approximately $52.3 million in general and administrative expenses since our inception, which represents approximately 28 percent of our cumulative operating expenses. In March 2014, upon the change to the Fantom scaffold program, we made a concurrent reduction in general and administrative personnel by approximately 44 percent and we took additional steps to reduce other overhead expenses. We anticipate that we will continue to invest in patents at similar levels as we have in the past. Additionally, we anticipate that we will expand our corporate infrastructure in 2015 and 2016 to continue to support the needs of being a public company and to prepare for commercial sales of our products, which will increase our general and administrative expenses accordingly. We also expect to begin to incur sales and marketing expenses toward the end of 2015 or early 2016 as we prepare for product sales in the event we receive CE Marking.
46
Table of Contents
Other Income and Expense: Since our IPO in 2010 and through September 30, 2014, our other income and expense was relatively immaterial and primarily comprised interest income on investments and gains and losses from foreign currency fluctuations. Following our issuance of convertible notes and warrants in November 2014, the components of other income and expense also include interest expense on the notes and losses related to the changes in fair values of both the notes and warrants. We recorded the notes and warrants at fair value upon issuance, which resulted in a non-recurring loss on issuance because their values exceeded the cash proceeds from issuance. We will remeasure the fair values of the notes and warrants at each future reporting date, and if those fair values change, we will record a corresponding gain (upon decrease in fair value) or loss (upon an increase in fair value). Accordingly, we expect our other income and expense to fluctuate, and possibly fluctuate by a significant amount, in future periods by the gains or losses on changes in fair value until such time as the notes are either converted into common stock or repaid and the warrants are either exercised or expire. Also, we will accrue and record interest expense on the notes at the rate of 7.54 percent per annum until they are either converted or repaid. We do not expect any material changes in interest income or foreign currency gains or losses during 2015.
Critical Accounting Policies and Significant Estimates
Our consolidated financial statements are prepared in accordance with accounting principles generally accepted in the United States. The preparation of our financial statements and related disclosures requires us to make estimates, assumptions, and judgments that affect the reported amounts of assets, liabilities, stockholders’ equity, expenses, and the presentation and disclosures related to those items. We base our estimates and assumptions on historical experience and other factors that we believe to be reasonable under the circumstances. We evaluate our estimates and assumptions on an ongoing basis; changes in our estimates and assumptions are reasonably likely to occur from period to period. Additionally, actual results could differ significantly from the estimates we make. To the extent there are material changes in our estimates or material differences between our estimates and our actual results, our future financial statement presentation, financial condition, results of operations, and cash flows will be affected.
While our significant accounting policies are described in more detail in Note 3 to our consolidated financial statements included elsewhere in this Annual Report on Form 10-K, we believe the following accounting policies involve a greater degree of judgment and complexity than our other accounting policies and, therefore, are the most critical to understanding and evaluating our consolidated financial condition and results of operations.
Research and Development Costs: We expense research and development costs as incurred. Our preclinical and clinical study costs are incurred on a contract basis and generally span a period from a few months to longer than a year. We record costs incurred under these contracts as the work occurs and make payments according to contractual terms. Until a contract is completed, we estimate the amount of work performed and accrue for estimated costs that have been incurred but not paid. As actual costs become known, we adjust our accruals. We expect our clinical expense accruals to increase as we continue to initiate and enroll patients in clinical trials. We expect to make estimates of work performed throughout the term of these trials, each of which is expected to be five years or longer. As these costs increase, if our estimates are inaccurate, possible material changes to our accruals could be required, which could materially affect our results of operations within any fiscal period. To date, there have been no material changes in our research and development expense estimates, including our estimates for accrued clinical costs.
Stock-Based Compensation: We have granted stock options to employees and consultants for the purchase of common stock. These options generally have a ten-year life during which the option holder can exercise at any time, they generally vest over a four-year service period and their exercise price equals the fair market value of our common stock on the date they are granted. Since our IPO in 2010, for purposes of reward, recruitment, and retention, we have granted options to purchase common stock to our employees, members of our board of directors, and outside consultants and have awarded a relatively small number of shares of restricted stock to our employees.
For options granted to employees, we determine the amount of compensation expense by estimating the fair value of each option on its date of grant and then we amortize that fair value on a straight-line basis over the employee’s service period, generally four years, and record the expense as either research and development or general and administrative based on the employee’s work classification. We estimate the fair value by using the Black-Scholes option pricing model, which requires use of assumptions. The assumptions used represent our best estimates, but these estimates involve inherent uncertainties. For the model inputs, we use the market value of the underlying common stock, a risk-free interest rate that corresponds to the vesting period of the option, an expected life of the option ranging from 5.5 to 6.25 years, and an estimate of volatility based on the market trading prices of comparative peer companies. Additionally, we reduce the amount of recorded compensation expense to allow for potential forfeitures of the options; the forfeiture rate is based on our actual historical forfeitures and has ranged from approximately 2.3 percent to 3.4 percent.
47
Table of Contents
For options granted to consultants, we estimate the fair value at each vesting and reporting date and record compensation expense in our consolidated statement of operations based on the fair value during the service period of the consultant, which is generally a four-year vesting period. We estimate the fair value by using the Black-Scholes option pricing model with the same approach to inputs and assumptions as we use to estimate the fair value of options granted to employees. For the model inputs, we use the market value of the underlying common stock, a risk-free interest rate that corresponds to the option life, an expected option life equal to the remaining life of the option, and an estimate of volatility based on the market trading prices of comparative peer companies.
We expect to increase the levels of option grants and restricted stock awards starting in 2015 as we expand our workforce and prepare for commercialization. Accordingly, we expect our stock-based compensation to increase in the future and as a result of our use of estimates, if factors change and we use different assumptions, the amount of our stock-based compensation expense could be materially different in the future.
Notes Payable: We analyze notes payable as of their issue date to determine their classification, issue discounts or premiums, and embedded or derivative features, if any. If embedded or derivative features exist, such as a right to convert notes into common stock, we evaluate the features in accordance with accounting guidance for derivative securities, determine whether such features would give rise to separate accounting, and, if they do, make an election to account for the notes at cost or at fair value. As of December 31, 2014, we had elected to account for our convertible notes payable at fair value, which does not require separate accounting for derivative features. On the issue date, we record the difference, if any, between the issue price of the notes and their fair value as a gain or loss in the consolidated statement of operations. Until such time as the notes are converted into common stock or repaid, we accrue interest on the notes at the stated interest rate. We additionally remeasure the fair value of the notes at each reporting date and record a gain (upon decrease in fair value) or loss (upon an increase in fair value) for any change in fair value. Fair value is determined using a binomial valuation model, which requires the use of subjective assumptions, including unobservable inputs that are supported by little or no market activity. The assumptions represent our best estimates, but involve certain inherent uncertainties. Inputs to the model include the market value of the underlying stock, a life equal to the contractual life of the notes, incremental borrowing rates that correspond to debt with similar credit worthiness, estimated volatility based on the historical prices of our trading securities, and we make assumptions as to our abilities to test and commercialize our product(s), to obtain future financings when and if needed, and to comply with the terms and conditions of the notes. Since the determination of fair value is complex and involves the use of subjective assumptions, if our assumptions, estimates, or modeling approaches change and we use different assumptions or methods, our fair values could be materially different in the future.
Common Stock Warrants: We record the fair value of the warrants we have issued for the purchase of common stock as a liability since they call for issuance of registered shares upon exercise, a condition that we may not be able to accommodate and which would then result in a net settlement of the warrants. Until the time the warrants are exercised or expire, the fair value is assessed at each reporting date utilizing a binomial valuation model with the same approach to inputs and assumptions as we use to estimate the fair value of our notes payable. Any change in fair value is recorded as a gain (upon decrease in fair value) or loss (upon an increase in fair value) in the consolidated statement of operations. Since the determination of fair value of the warrants is complex and involves the use of subjective assumptions, if our assumptions, estimates, or modeling approaches change and we use different assumptions or methods, our fair values could be materially different in the future.
Results of Operations
During 2013 and the first quarter of 2014, our operating activities focused on testing, preparing, and enrolling patients with a predecessor version scaffold. In March 2014 we announced our Fantom scaffold as our sole focus for development and testing and concurrently reduced headcount by approximately 45 percent and reduced other overhead costs to a lesser extent. In addition to the development and testing of Fantom in 2014, we began the initial human clinical trial of Fantom in December of 2014.
Also during 2014, we negotiated and completed a financing to provide the ongoing capital for our operations. This financing was completed in November 2014 with the issuance of 250 senior unsecured convertible notes payable, each with a face value of $100,000, and warrants to purchase 8,750,000 shares of our common stock. We received cash proceeds of $25.0 million from the notes; we recorded the notes and warrant liability at their fair values, recognizing losses both at issue date and at December 31, 2014 related to these fair values.
48
Table of Contents
Comparison of the Years Ended December 31, 2013 and 2014
| | Year Ended December 31, | | Change | |
| | 2013 | | 2014 | | $ | | % | |
| | (dollars in thousands) | |
| | | | | | | | | |
Research and development expense | | $ | 19,212 | | $ | 14,318 | | $ | (4,894 | ) | (25 | )% |
General and administrative expense | | $ | 8,731 | | $ | 7,645 | | $ | (1,086 | ) | (12 | )% |
Interest expense | | $ | — | | $ | 986 | | $ | 986 | | 100 | % |
Loss on issuance of convertible notes payable and warrants | | $ | — | | $ | 15,627 | | $ | 15,627 | | 100 | % |
Loss on change in fair values of convertible notes payable and warrant liability | | $ | — | | $ | 12,542 | | $ | 12,542 | | 100 | % |
Interest and other income | | $ | 21 | | $ | 81 | | $ | 60 | | >100 | % |
Research and development expense decreased $4,894,000, or 25 percent, for the year ended December 31, 2014 compared to the year ended December 31, 2013. This decrease was primarily a result of our change in product development and testing focus to Fantom in late March 2014, the related reduction in headcount on March 26, 2014, and the completion of patient enrollment in a predecessor scaffold clinical study in January 2014. Direct materials, including purchased catheters and polymer lasing costs, decreased $1,708,000 and preclinical and consulting costs decreased $1,446,000 because of the type and timing of Fantom development work in 2014 compared to the predecessor scaffold testing and scale-up work performed in 2013. Personnel costs, including benefits, bonuses, and stock-based compensation, decreased $1,227,000 primarily due to the approximate 46 percent decrease in headcount during March 2014; these reductions were offset by $237,000 in severance benefits and payroll taxes recorded as a result of the headcount reduction. Clinical costs decreased $726,000 between years due to the timing of patient enrollments in our clinical trials; 111 patients were enrolled during 2013 compared to 5 patients in 2014. We paid a one-time licensing fee of $100,000 for certain polymer technology in 2013 for which we had no corresponding expense in 2014. Offsetting these decreases, depreciation increased $143,000 in 2014 primarily due to the addition of lab space, production equipment, and a back-up generator in 2013. The remainder of the change in research and development expenses between years resulted from individually immaterial changes in lab supplies, quality control, and facilities expenses.
General and administrative expense decreased $1,086,000, or 12 percent, for the year ended December 31, 2014 compared to the year ended December 31, 2013. A combination of items contributed to this decrease. Personnel costs, including benefits, bonuses, and stock-based compensation expense, decreased $872,000 primarily due to a decrease of $707,000 in stock compensation upon final vesting of stock option grants made in 2010 for which there were not corresponding grants in 2014. Other personnel costs decreased $165,000 due to an approximate 44 percent decrease in headcount during March 2014; these reductions were offset by $178,000 in severance benefits and payroll taxes recorded as a result of the headcount reduction. Travel and entertainment costs decreased $334,000 in 2014 compared to 2013 as a result of the headcount reduction combined with reduced clinical travel following completion of patient enrollments in the predecessor scaffold clinical study. The remainder of the change in general and administrative expenses between periods was due to individually immaterial changes in investor relations costs, office supplies, depreciation, insurance, franchise taxes, and other overhead expenses.
Our other non-operating expenses during 2014 primarily arose from the issuance on November 14, 2014 of convertible notes payable (“Notes”) and warrants to purchase common stock. Interest expense of $986,000 related to the Notes and comprised $248,000 in interest accruing on the notes during the period from the issuance date through December 31, 2014 and $738,000 in costs to complete the financing. The $15,627,000 loss on issuance of convertible notes payable and warrants was a non-recurring charge that resulted because the fair value of the Notes and warrants on the issuance date exceeded the cash proceeds received. We additionally recorded a $12,542,000 loss on the change in fair value of the Notes and warrants for the period from November 14, 2014 through December 31, 2014.
Interest income was $8,000 and other income was $73,000 for the year ended December 31, 2014, each of which are considered immaterial.
49
Table of Contents
Comparison of the Years Ended December 31, 2012 and 2013
| | Year Ended December 31, | | Change | |
| | 2012 | | 2013 | | $ | | % | |
| | (dollars in thousands) | |
| | | | | | | | | |
Research and development expense | | $ | 15,822 | | $ | 19,212 | | $ | 3,390 | | 21 | % |
General and administrative expense | | $ | 8,043 | | $ | 8,731 | | $ | 688 | | 9 | % |
Interest and other expense, net | | $ | 89 | | $ | 21 | | $ | (68 | ) | (76 | )% |
Research and development expense increased $3,390,000, or 21 percent, for the year ended December 31, 2013 compared to the year ended December 31, 2012. The increase was due to several factors. Personnel costs, including benefits, bonuses, and stock-based compensation, increased $934,000 primarily due to an approximate 11 percent increase in headcount for engineering, operations, and quality assurance employees, combined with increases of $228,000 for stock-based compensation and $65,000 for bonuses. Clinical costs increased $1,453,000 as we enrolled 111 patients in our ReZolve2 clinical trial during the year ended December 31, 2013 and monitored patients in our prior clinical study. Material costs, including polymer, lasing, and catheter delivery systems, increased $684,000 and outside engineering costs increased $606,000 as we produced supplies for clinical enrollment, refined our manufacturing processes and equipment in advance of commercialization, and performed feasibility work on our next generation scaffold. Depreciation increased $205,000 due to the addition of lab equipment and significant leasehold improvements completed in 2012. During 2013, we also paid a one-time licensing fee of $100,000 for technology in our product pipeline. Offsetting these increases, preclinical study costs decreased $620,000 due to the timing of such work; numerous studies undertaken to test and validate the ReZolve2 device in 2012 were not repeated in 2013. The remainder of the change in research and development expenses between years resulted from individually immaterial changes in lab supplies, quality control, facilities, and outside research expenses.
General and administrative expense increased $688,000, or nine percent, for the year ended December 31, 2013 compared to the year ended December 31, 2012. A combination of items contributed to this increase. Personnel costs, including benefits, bonuses, and stock-based compensation expense, increased $450,000 primarily due to an increase of $365,000 in stock compensation from ongoing option grants and restricted stock awards and $57,000 in year-end bonuses to officers under our bonus program. We incurred $268,000 in compensation to our European-based sales and marketing consultant in 2013 following his engagement in May 2013. Our audit and tax fees increased $155,000 in 2013 primarily as a result of a non-recurring tax analysis related to our tax losses. Travel costs increased $101,000 primarily due to our clinical activities. Offsetting these increases, legal fees decreased $156,000 in 2013 primarily due to the timing of intellectual property filings and office actions. The remainder of the change in general and administrative expenses between periods was due to individually immaterial changes in investor relations costs, office supplies, depreciation, insurance, franchise taxes, and other overhead expenses.
Interest income was $30,000 for the year ended December 31, 2013, a decrease of $62,000 from the year ended December 31, 2012, as a result of lower cash and investment balances. Other expenses were immaterial both years.
Liquidity and Capital Resources
Sources of Liquidity
We are in the clinical testing phase of product development, but have not commercialized or generated revenue from the sale of any products and have incurred losses since our inception in June 1998. We do not anticipate having a product available for sale unless, and until, we successfully receive CE Marking or other regulatory approval for, and begin selling, or licensing, one of our products, which we do not anticipate will occur until late 2016 at the earliest. We anticipate that we will continue to incur substantial net losses and cash outflows through at least 2016 as we continue our development work, conduct and complete preclinical and clinical trials, expand our corporate infrastructure, and prepare for the potential commercial launch of our products.
Our development efforts have been funded with a variety of capital received from angel investors, venture capitalists, strategic partners, hedge funds, the proceeds from our IPO in 2010, and the issuance of convertible notes in November 2014. Since our inception, we have received approximately $153.9 million in equity proceeds and $53.5 million from issuance of notes payable ($28.5 million of the notes payable were converted to common stock upon our IPO in 2010). As of December 31, 2014, we had approximately $26.8 million in cash and investments available for operations. We have incurred substantial losses since our inception; as of December 31, 2014, we had accumulated a deficit of approximately $252.5 million.
50
Table of Contents
The financing that we negotiated and completed in November 2014 is intended to provide the ongoing capital for our operations, including the Fantom clinical trial and application for CE Mark. This financing was completed with the issuance of 250 senior unsecured convertible notes payable, each with a face value of $100,000, and warrants to purchase 8,750,000 shares of our common stock. We received gross cash proceeds of $25.0 million from the notes and have the potential to receive up to an additional $22.8 million cash proceeds when, and if, the warrants are exercised. Both the notes and the warrants have five-year lives.
We expect our losses to continue for the next several years as we continue our development work, clinical studies, and preparations for commercialization. In order to successfully transition to profitable operations, we will need to achieve a level of revenues and product margins to support the Company’s cost structure. Until such time as we generate positive cash flow, we plan to continue to fund our operating and capital needs by utilizing our current cash and investments and by raising additional capital through equity or debt financings or strategic or other transactions, if the warrants issued in November 2014 are not exercised, or not exercised on a timeframe that coincides with our cash needs. We currently have no set plans for raising additional capital.
Cash Flows
Below is a summary of our cash flows from operating activities, investing activities, and financing activities for the periods indicated.
| | Year Ended December 31, | |
| | 2012 | | 2013 | | 2014 | |
| | (in thousands) | |
| | | | | | | |
Net cash used for operating activities | | $ | (18,661 | ) | $ | (21,943 | ) | $ | (17,930 | ) |
Net cash provided by (used for) investing activities | | $ | (1,946 | ) | $ | 2,265 | | $ | (44 | ) |
Net cash provided by financing activities | | $ | 322 | | $ | 31 | | $ | 24,559 | |
| | | | | | | |
Net increase (decrease) in cash and cash equivalents | | $ | (20,285 | ) | $ | (19,647 | ) | $ | 6,585 | |
Net Cash Flow from Operating Activities
Net cash used for operating activities during 2012 primarily reflects the net loss of $23,776,000, offset by non-cash expenses of $3,497,000 for stock-based compensation, $860,000 from changes in operating assets and liabilities, $677,000 of depreciation and amortization, and $81,000 of other non-cash expense.
Net cash used for operating activities during 2013 primarily reflects the net loss of $27,922,000, offset by non-cash expenses of $4,090,000 for stock-based compensation, $978,000 from changes in operating assets and liabilities, $892,000 of depreciation and amortization, and $19,000 of other non-cash expense.
Net cash used for operating activities during 2014 primarily reflects the loss from operations of $21,963,000 and the changes in operating assets and liabilities of $610,000. These items were offset by non-cash expenses of $3,516,000 for stock-based compensation, $1,027,000 of depreciation and amortization, interest and other income of $81,000, and $19,000 of other non-cash expense. The loss from issuance of convertible notes payable and warrants and the change in fair value of convertible notes payable and warrant liability that were recorded during 2014 were non-cash items that had no effect on cash flows.
Net Cash Flow from Investing Activities
Net cash used for investing activities during 2012 primarily consisted of the purchase of property and equipment.
Net cash was provided by investing activities during 2013, which consisted of $3,731,000 in net maturities of investments offset by $1,466,000 in purchases of property and equipment.
Net cash used for investing activities during 2014 consisted of property and equipment and equipment purchases of $541,000, offset by $497,000 in net maturities of investments.
51
Table of Contents
Net Cash Flow from Financing Activities
Net cash provided by financing activities in 2012 and 2013 consists of proceeds from the issuance of common stock upon exercise of employee stock options.
Net cash provided by financing activities in 2014 consisted of $247,000 in proceeds from the issuance of common stock upon exercise of employee stock options and $25,000,000 in proceeds from the issuance of convertible notes payable, offset by payment of $688,000 in issuance costs.
Operating Capital and Capital Expenditure Requirements
To date, we have not commercialized any products. We do not anticipate generating any revenue unless and until we successfully obtain CE Mark or other regulatory approval to sell, or license for sale, our scaffolds or one of our other product possibilities. We anticipate that we will continue to incur substantial net losses and cash outflows for the next several years as we continue our development work, conduct and complete preclinical and clinical trials, apply for regulatory approval to sell our products, expand our corporate infrastructure, prepare to commercially manufacture and sell our products, and collect cash from sales of our product(s).
We had cash and investments totaling $26.8 million as of December 31, 2014; we believe that these resources will be sufficient to meet our anticipated cash requirements during the next year, and beyond. Additionally, the terms of the warrants that we issued in November 2014, which have a five-year life, call for the warrants to be exercised in cash, if they are exercised at all. We issued a total of 8,750,000 warrants; their exercise price is set at $2.17275 per share until such time as we complete enrollment of the CE Mark clinical trial of our Fantom scaffold; the exercise price increases to $2.6073 per share upon completion of enrollment, which we expect could occur in mid-2015. If the warrants are exercised, we have the potential to receive up to $22.8 million cash proceeds.
If the warrants are not exercised, or if they are not exercised in time to accommodate our cash flow needs, and if our available cash resources are insufficient to satisfy our liquidity requirements before we are able to maintain our operations from our cash inflows, or if we develop additional products or pursue new uses for our products, we may need to raise additional capital. Any such needed additional capital may not be available on reasonable terms, if at all. Additionally, we may be limited under the terms of the convertible notes as to the type, quantity, timing, or other aspects of a financing, unless the noteholders agree. Any financing, even one to which the noteholders agree, may result in additional dilution to our current securityholders, could have rights senior to those of our common stock, and/or could contain provisions that would restrict our operations. If we are unable to raise additional capital, if needed, we may be compelled to sell certain assets, including intellectual property assets. Even if we are able to commercialize our products and raise additional capital if and when needed, we may never become profitable, or if we do attain profitable operations, we may not be able to sustain profitability and cash flows on a recurring basis.
Because of the numerous risks and uncertainties associated with the development of medical devices, such as our bioresorbable scaffolds, we are unable to estimate the exact amounts of, or timing of, capital outlays and operating expenditures necessary to complete development, continue ongoing preclinical studies, conduct human clinical trials, successfully deliver a commercial product to market, and collect on our trade receivables. Our future funding requirements will depend on many factors, including, but not limited to:
· the time and effort it will take to successfully complete development and testing of our products;
· the scope, enrollment rate, and costs of our human clinical trials;
· the time and effort it will take to identify, develop, and scale-up manufacturing processes;
· the cost of establishing clinical and commercial supplies of our products;
· the scope of research and development for any of our other product opportunities;
· the cost of filing and prosecuting patentable technologies and defending and enforcing our patent and other intellectual property rights;
· the terms and timing of any collaborative, licensing, or other arrangements that we may establish;
· the requirements, cost, and timing of regulatory approvals;
· the cost and timing of establishing sales, marketing, and distribution capabilities; and,
· the effect of competing technological and market developments.
52
Table of Contents
Future capital requirements will also depend on the extent to which we acquire or invest in businesses, products, and technologies; we currently have no commitments or agreements relating to any of these types of transactions.
Contractual Obligations, Commitments, and Contingencies
The following table summarizes our outstanding contractual obligations as of December 31, 2014:
| | Payments Due by Period | |
| | Less than | | | | | | | |
| | 1 Year | | 1-3 Years | | 3-5 Years | | Total | |
| | (in thousands) | |
| | | | | | | | | |
Operating lease obligations | | $ | 644 | | $ | 1,401 | | $ | 60 | | $ | 2,105 | |
Purchase obligations | | 90 | | 18 | | — | | 108 | |
| | | | | | | | | |
Total contractual obligations | | $ | 734 | | $ | 1,419 | | $ | 60 | | $ | 2,213 | |
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements.
Recent Accounting Pronouncements
None
Item 7A. Quantitative and Qualitative Disclosures about Market Risk
Not applicable.
Item 8. Financial Statements and Supplementary Data
Our consolidated financial statements, and accompanying notes, and the Reports of Grant Thornton LLP and Ernst & Young LLP, our Independent Registered Public Accounting Firms, are included in this Annual Report on Form 10-K on pages F-1 through F-21.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
Not applicable.
Item 9A. Controls and Procedures
Management’s Report on Internal Control Over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is defined in Rule 13a-15(f) or 15d-15(f) under the Securities Exchange Act of 1934 as a process designed by, or under the supervision of, the Company’s principal executive and principal financial officers and effected by the Company’s board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles and includes those policies and procedures that:
· pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the Company’s assets;
53
Table of Contents
· provide reasonable assurance that transactions are recorded as necessary to permit the preparation of financial statements in accordance with generally accepted accounting principles, and that the receipts and expenditures of the Company are being made only in accordance with authorizations of management and directors of the Company; and,
· provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the Company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Our management, including our Chief Executive Officer and Chief Financial Officer, assessed the effectiveness of our internal control over financial reporting as of the end of the fiscal year covered by this Annual Report on Form 10-K. In making this assessment, our management used the criteria set forth in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 Framework), or “COSO.” Based on their assessment, management has concluded that, as of December 31, 2014, our Company’s internal control over financial reporting is effective based on the COSO criteria.
Evaluation of Disclosure Controls and Procedures
Our management, including our Chief Executive Officer and Chief Financial Officer, have evaluated the effectiveness of our “disclosure controls and procedures” (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934) as of the end of the period covered by this Annual Report on Form 10-K. Based on their evaluation, they have concluded that our disclosure controls and procedures are effective as of the end of the period covered by this report.
Changes in Internal Control Over Financial Reporting
There was no change in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act of 1934) that occurred during the quarterly period ended December 31, 2014 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Item 9B. Other Information
None.
PART III
Item 10. Directors, Executive Officers, and Corporate Governance
The information required by this item related to our directors is incorporated by reference to our Definitive Proxy Statement for our 2015 Annual Meeting of Stockholders, to be filed with the Securities and Exchange Commission within 120 days of December 31, 2014 (the “2015 Proxy Statement”), under the heading “Election of Directors.”
Information concerning our executive officers is set forth under “Executive Officers” in Item 1 of Part I of this Annual Report on Form 10-K and is incorporated herein by reference.
Code of Business Conduct and Ethics
We have adopted a Code of Business Conduct and Ethics that applies to all of our officers, directors and employees. We have posted a copy of our Code of Business Conduct and Ethics, and intend to post amendments to this code, or any waivers of its requirements, in the Corporate Governance section on our website at www.revamedical.com.
54
Table of Contents
Australian Disclosure Requirements
Because we are listed on the ASX, we are required to comply with various disclosure requirements as set out in the ASX Listing Rules. The following information is provided to comply with the ASX Listing Rules and is not intended to fulfill SEC information required by Part III of this Annual Report on Form 10-K.
Substantial Holders at March 15, 2015
The number of our equity securities held by our substantial securityholders (i.e., those securityholders, who together with their affiliates, have an interest in at least five percent of our voting securities), assuming the conversion of common stock held by those securityholders into CHESS Depositary Interests, or “CDIs” (ten CDIs are equivalent to one share of common stock) as of March 15, 2015 are as follows:
| | Number of | | % of Total | | | | | | Total Holdings | |
Security Holder | | Common
Shares
Held | | Common
Shares
Outstanding | | Number of
CDIs
Held | | % of Total
CDIs
Outstanding | | Equivalent
Number of
CDIs Held | | % of Total
Securities
Outstanding | |
Brookside Capital and affiliates | | — | | — | | 29,650,222 | | 15.8 | % | 29,650,222 | | 8.8 | % |
Cerberus and affiliates | | — | | — | | 28,844,260 | | 15.4 | % | 28,844,260 | | 8.6 | % |
Citicorp Nominees Pty Limited | | — | | — | | 21,558,411 | | 11.5 | % | 21,558,411 | | 6.4 | % |
Domain Partners and affiliates | | 3,691,188 | | 24.9 | % | — | | — | | 36,911,880 | | 11.0 | % |
Elliott Associates, L.P. | | 3,227,031 | | 21.7 | % | — | | — | | 32,270,310 | | 9.6 | % |
Group Outcome Investors/Robert B. Stockman | | 1,694,906 | | 11.4 | % | 870,500 | | 0.5 | % | 17,819,560 | | 5.3 | % |
HSBC Custody Nominees (Australia) Limited | | — | | — | | 15,800,483 | | 8.4 | % | 15,800,483 | | 4.7 | % |
Kenneth Rainin Trust and affiliates | | — | | — | | 13,470,695 | | 7.2 | % | 13,470,695 | | 4.0 | % |
Medtronic, Inc. | | 379,651 | | 2.6 | % | 22,558,280 | | 12.0 | % | 26,354,790 | | 7.9 | % |
Gordon E. Nye | | 823,531 | | 5.5 | % | 96,000 | | 0.1 | % | 8,331,310 | | 2.5 | % |
Saints Capital Everest, L.P. | | 3,223,513 | | 21.7 | % | — | | — | | 32,235,130 | | 9.6 | % |
| | | | | | | | | | | | | |
Total securities held by > 5% holders | | 13,039,820 | | 87.8 | % | 132,848,851 | | 70.9 | % | 263,247,051 | | 78.4 | % |
Total securities held by all other holders | | 1,813,668 | | 12.2 | % | 54,414,049 | | 29.1 | % | 72,550,729 | | 21.6 | % |
Distribution of Security Holders as of March 15, 2015
As of March 15, 2015, we had a total of 33,579,778 shares of common stock issued and outstanding, a portion of which were held as CDIs (ten CDIs are equivalent to one share of common stock). The following table presents the number of shares of common stock (including restricted stock) and CDIs held, as well as the number of shares underlying outstanding stock options and warrants to purchase common stock, convertible notes, and restricted stock units.
| | Common Stock
(includes Restricted Stock) | | CDIs | | Options
(unlisted) | | Warrants
(unlisted) | | Convertible Notes
(unlisted) | | Restricted Stock
Units (unlisted) | |
| | # of
Holders | | # of
Shares | | # of
Holders | | # of
CDIs | | # of
Holders | | # of
Shares | | # of
Holders | | # of
Shares | | # of
Holders | | # of
Shares | | # of
Holders | | # of
Shares | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
1 – 1,000 | | 11 | | 4,614 | | 95 | | 40,505 | | 2 | | 2,000 | | — | | — | | — | | — | | — | | — | |
1,001 – 5,000 | | 10 | | 37,000 | | 193 | | 623,265 | | 3 | | 7,000 | | — | | — | | — | | — | | — | | — | |
5,001 – 10,000 | | 4 | | 28,963 | | 138 | | 1,188,709 | | — | | — | | — | | — | | — | | — | | — | | — | |
10,001 – 100,000 | | 16 | | 584,166 | | 359 | | 12,793,169 | | 16 | | 632,000 | | — | | — | | — | | — | | 8 | | 278,000 | |
100,001 and over | | 14 | | 14,198,745 | | 69 | | 172,617,252 | | 13 | | 3,904,925 | | 2 | | 8,750,000 | | 2 | | 11,506,155 | | 4 | | 546,200 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Total holders and securities | | 55 | | 14,853,488 | | 854 | | 187,262,900 | | 34 | | 4,545,925 | | 2 | | 8,750,000 | | 2 | | 11,506,155 | | 12 | | 824,200 | |
The number of shareholders holding less than a marketable parcel of CDIs (being a parcel of securities not less than A$500) as of March 15, 2015 was 76.
55
Table of Contents
Top 20 CDI Holders as of March 15, 2015
Following are the top 20 holders of our CDIs as of March 15, 2015 (does not include holdings in common stock):
| | | Number of
CDIs Held | | % of CDIs
Outstanding | |
| | | | | | |
1. | Citicorp Nominees Pty Limited | | 50,402,671 | | 26.9 | % |
2. | Brookside Capital Partners Fund LP | | 27,832,040 | | 14.9 | % |
3. | Merrill Lynch (Australia) Nominees Pty Limited | | 25,558,336 | | 13.7 | % |
4. | HSBC Custody Nominees (Australia) Limited — GSCO ECA | | 15,800,483 | | 8.4 | % |
5. | Robert B Stockman + Jennifer Rainin <Kenneth Rainin Admin A/C> | | 11,016,150 | | 5.9 | % |
6. | Mr Timothy J Barberich | | 4,600,930 | | 2.5 | % |
7. | JP Morgan Nominees Australia Limited | | 4,547,340 | | 2.4 | % |
8. | Frederic H. Moll | | 3,345,610 | | 1.8 | % |
9. | Trienos Group LLC | | 3,000,000 | | 1.6 | % |
10. | UBS Nominees Pty Ltd | | 2,618,182 | | 1.4 | % |
11. | Mr. Jon Benjamin Platt | | 2,000,000 | | 1.1 | % |
12. | Moore Family Nominee Pty Ltd <Moore Family A/C> | | 1,500,000 | | 0.8 | % |
13. | Warman Investments Pty Ltd | | 1,451,771 | | 0.8 | % |
14. | Asia Union Investments Pty Ltd | | 1,350,000 | | 0.7 | % |
15. | Viking Management Services Pty Ltd <VHK Superannuation Fund A/C> | | 1,159,121 | | 0.6 | % |
16. | Mrs Danielle Susan Borgas | | 1,006,000 | | 0.5 | % |
17. | Lightstorm Pty Ltd <Hotspice A/C> | | 962,000 | | 0.5 | % |
18. | Mr. Antony Richard Kerr + Mr. Peter Michael Clerk <AR Kerr Family A/C> | | 900,000 | | 0.5 | % |
19. | Little Blue Porsche Pty Ltd <G C Steele Super Fund A/C> | | 650,000 | | 0.3 | % |
20. | Mr. Robert Thomas + Mrs. Kyrenia Thomas <Rob Thomas Super Fund A/C> | | 650,000 | | 0.3 | % |
| | | | | | |
| Total CDIs held by top 20 CDI holders | | 160,350,634 | | 85.6 | % |
| Total CDIs held by all other CDI holders | | 26,912,266 | | 14.4 | % |
| | | | | | |
| Total CDIs outstanding | | 187,262,900 | | 100 | % |
The table below provides a list of the top 20 holders of our securities as of March 15, 2015, taking into account securities held in the form of both common stock and CDIs and prepared on the assumption that all CDIs are held as common stock. Related but separate legal entities are not aggregated for the purposes of the table below.
Security Holder | | Shares of
Common
Stock Held | | CDIs Held
(common stock
equivalent) | | Total Number
of Securities
Held | | % of
Outstanding
Capital | |
| | | | | | | | | | |
1. | Domain Partners V, L.P. | | 3,606,002 | | — | | 3,606,002 | | 10.7 | % |
2. | Elliott Associates, L.P. | | 3,227,031 | | — | | 3,227,031 | | 9.6 | % |
3. | Saints Capital Everest, L.P. | | 3,223,513 | | — | | 3,223,513 | | 9.6 | % |
4. | Brookside Capital Partners Fund, LP | | — | | 2,783,204 | | 2,783,204 | | 8.3 | % |
5. | Medtronic, Inc. | | 379,651 | | 2,255,828 | | 2,635,479 | | 7.8 | % |
6. | Citicorp Nominees Pty Limited | | — | | 2,477,587 | | 2,477,587 | | 7.4 | % |
7. | HSBC Custody Nominees (Australia) Limited — GSCO ECA | | — | | 1,580,048 | | 1,580,048 | | 4.7 | % |
8. | Group Outcome Investors I, LLC | | 1,341,175 | | — | | 1,341,175 | | 4.0 | % |
9. | Robert B Stockman+Jennifer Rainin <Kenneth Rainin Admin A/C> | | — | | 1,101,615 | | 1,101,615 | | 3.3 | % |
10. | Cerberus Series Four Holdings, LLC | | — | | 1,046,486 | | 1,046,486 | | 3.1 | % |
11. | Cerberus International, Ltd | | — | | 995,553 | | 995,553 | | 3.0 | % |
12. | Gordon E. Nye | | 823,531 | | 9,600 | | 833,131 | | 2.5 | % |
13. | Cerberus Partners, L.P. | | — | | 520,641 | | 520,641 | | 1.6 | % |
14. | Timothy J. Barberich | | — | | 460,093 | | 460,093 | | 1.4 | % |
15. | JP Morgan Nominees Australia Limited | | — | | 454,734 | | 454,734 | | 1.3 | % |
16. | C. Raymond Larkin Jr. | | 351,749 | | — | | 351,749 | | 1.0 | % |
17. | Frederic H. Moll | | — | | 334,561 | | 334,561 | | 1.0 | % |
18. | Merrill Lynch (Australia) Nominees Pty Limited | | — | | 300,006 | | 300,006 | | 0.9 | % |
19. | Trienos Group LLC | | — | | 300,000 | | 300,000 | | 0.9 | % |
20. | UBS Nominees Pty Ltd | | — | | 261,818 | | 261,818 | | 0.8 | % |
| | | | | | | | | | |
| Total securities held by top 20 holders (stated as common stock) | | 12,952,652 | | 14,881,774 | | 27,834,426 | | 82.9 | % |
| Total securities held by all other holders (stated as common stock) | | 1,900,836 | | 3,844,516 | | 5,745,352 | | 17.1 | % |
56
Table of Contents
Unlisted Options, Unlisted Warrants, Unlisted Convertible Notes, and Unlisted Restricted Stock Units
As of March 15, 2015, we had 4,545,925 options to purchase shares of common stock on issue under the 2010 Equity Incentive Plan and the 2001 Stock Option/Stock Issuance Plan. These options are held by 34 individuals, with no single person holding 20 percent or more.
As of March 15, 2015, we had 8,750,000 warrants to purchase shares of common stock on issue. These warrants are held equally by two entities, Goldman Sachs International and Senrigan Master Fund.
As of March 15, 2015, we had issued 250 convertible notes, each with a face value of $100,000, and each of which is convertible into 46,024.623 shares of common stock. The convertible notes are held equally by two entities, Goldman Sachs International and Senrigan Master Fund.
As of March 15, 2015, we had issued 824,200 restricted stock units; each restricted stock unit entitles the holder to one share of common stock upon vesting. These restricted stock units are held by 12 individuals, with no single person holding 20 percent or more.
Restricted Stock
As of March 15, 2015, we had 72,125 shares of restricted stock on issue under our 2010 Equity Incentive Plan, held by three individuals.
Voting Rights
Our amended and restated certificate of incorporation and by-laws provide that each stockholder has one vote for every share of common stock entitled to vote and held by such stockholder on a record date. In addition, although holders of restricted stock are subject to restrictions on transfer until vesting, holders of restricted stock have the same voting rights as holders of shares of common stock.
If holders of CDIs wish to attend our general meetings, they will be able to do so. Under the ASX Listing Rules, REVA Medical, Inc., as an issuer of CDIs, must allow CDI holders to attend any meeting of the holders of the underlying securities unless relevant U.S. law at the time of the meeting prevents CDI holders from attending those meetings. In order to vote at such meetings, CDI holders have the following options:
· instructing CHESS Depositary Nominee or “CDN,” as the legal owner, to vote the shares of REVA Medical common stock underlying their CDIs in a particular manner. The instruction form must be completed and returned to our share registry prior to the meeting;
· informing REVA Medical that they wish to nominate themselves or another person to be appointed as CDN’s proxy for the purposes of attending and voting at the general meeting; and,
· converting their CDIs into a holding of shares of REVA Medical common stock and voting these at the meeting (however, if thereafter the former CDI holder wishes to sell their investment on ASX, it would be necessary to convert shares of common stock back to CDIs). This must be done prior to the record date for the meeting.
Because holders of CDIs do not appear on REVA Medical’s share register as the legal holders of the common stock, they will not be entitled to vote at our stockholder meetings unless one of the above steps is undertaken.
Proxy forms and details of these alternatives will be included in each notice of meeting sent to CDI holders by REVA Medical.
Holders of options and warrants to purchase stock, convertible notes, and restricted stock units are not entitled to vote.
Required Statements
REVA Medical makes the following disclosures:
· There is no current on-market buy-back of the Company’s securities.
· REVA Medical, Inc. is incorporated in the state of Delaware in the United States of America.
57
Table of Contents
· REVA Medical, Inc. is not subject to Chapters 6, 6A, 6B, or 6C of the Corporations Act dealing with the acquisitions of shares (including substantial shareholdings and takeovers).
· Under the Delaware General Corporation Law, shares are generally freely transferable subject to restrictions imposed by U.S. federal or state securities laws, by our certificate of incorporation or by-laws, or by an agreement signed with the holders of the shares at issue. Our amended and restated certificate of incorporation and by-laws do not impose any specific restrictions on transfer. Delaware General Corporation Law prohibits a publicly held Delaware Corporation from engaging in a “business combination” with an “interested shareholder” for a period of three years following the time the person became an interested shareholder, unless the business combination or acquisition of shares that resulted in a shareholder’s becoming an interested shareholder is approved in a prescribed manner. A “business combination” can include a merger, asset or share sale, or other transaction resulting in a financial benefit to an interested shareholder. Generally, an interested shareholder is a person who, together with its affiliates and associates, owns (or within three years prior to the determination of interested shareholder status did own) 15 percent or more of a corporation’s voting shares. The existence of this provision would be expected to have an anti-takeover effect with respect to transactions not approved in advance by the Board, including discouraging attempts that might result in a premium over the market price for the shares of common stock held by shareholders.
· The securities of REVA Medical, Inc. are not quoted on any other exchange.
General Information
The name of the Company Secretary is Katrina Thompson.
The address of our registered office in Australia is c/o Buchan Pty Ltd, Suite 4, Level 14, 6 O’Connell Street, Sydney NSW 2000, telephone +61 2 9237 2800.
The address of our office in the United States, which is our principal administrative office, is REVA Medical, Inc., 5751 Copley Dr., San Diego, California 92111, telephone +1 (858) 966-3000.
Registers of CDI securities are held at Computershare Investor Services Pty Limited, Level 3, 60 Carrington Street, Sydney NSW 2000, Australia, Investor Enquiries 1300 855 080. Registers of common stock securities are held at Computershare Trust Company, N.A., 250 Royall Street, Canton, MA 02021, USA, Investor Inquiries (800) 962-4284.
Quotation has been granted for CDIs (and the underlying shares of common stock) on the ASX Limited.
Australian Corporate Governance Statement
Our Board of Directors (the “Board”) is committed to promoting and strengthening good corporate governance practices and a culture of good corporate governance and ethical conduct throughout the Company. The Board has evaluated the Company’s corporate governance policies and practices in light of the ASX Corporate Governance Council’s Corporate Governance Principles and Recommendations 2nd Edition (“ASX Recommendations”) in force for the Company’s financial year ended December 31, 2014 and is pleased to confirm that the Company’s corporate governance framework is generally consistent with the ASX Recommendations, other than as set forth below. Following is a summary of the approach adopted and used by the Company during the year ended December 31, 2014, using the same numbering sequence as contained in the ASX Recommendations.
Principle 1 — Lay solid foundations for management and oversight
Recommendation 1.1 — Establish the functions reserved to the board and those delegated to senior executives and disclose those functions
The Board’s responsibilities are defined by the Company’s Corporate Governance Guidelines, a copy of which is available in the Corporate Governance section on the Company’s website at www.revamedical.com, and there is a clear delineation between the Board’s responsibility for the Company’s strategy and activities and the day-to-day management of operations conferred upon the Company’s senior executives.
58
Table of Contents
Recommendation 1.2 — Disclose the process for evaluating the performance of senior executives
In accordance with its charter, the Compensation Committee reviews and approves corporate and personal performance goals and objectives relevant to the compensation of all executive officers, evaluates the performance of each executive officer in light of those goals and objectives, and sets each executive officer’s compensation, including but not limited to salary, bonus, incentive compensation, and equity awards based on such an evaluation. In addition, the Compensation Committee is responsible for regularly reviewing the Company’s compensation, recruitment, retention, and termination policies for senior executives.
In setting the compensation for our executive officers, our Board places significant emphasis on the recommendation of our Chief Executive Officer (other than with respect to determining his own compensation), considering our overall performance during the prior fiscal year, the executive’s individual contributions during the prior fiscal year, the individual’s annual performance reviews based on achievement of annual goals, and relevant market data, including benchmarking to equivalent positions in peer group companies. With respect to new hires, our Board considers an executive’s background and historical compensation in lieu of prior year performance. For the year ended December 31, 2014, our Board evaluated the performance of senior executives in accordance with the process set for such evaluation and disclosed to the relevant executive. We retain an independent compensation consultant to assist us with our benchmarking process. Further information regarding executive compensation for the year ended December 31, 2014, as required by Item 11 of this Annual Report on Form 10-K, is incorporated by reference to the applicable information in our Definitive Proxy Statement for our 2015 Annual Meeting of Stockholders, to be filed pursuant to Registration 14A with the Securities and Exchange Commission (the “SEC”) and the ASX within 120 days of December 31, 2014 (the “2015 Proxy Statement”). Such information is incorporated herein by reference.
Recommendation 1.3 — Provide the information required to be disclosed under Principle 1
Reporting Requirement
The Company fully complied with ASX Recommendations 1.1 through 1.3 during the year ended December 31, 2014.
Principle 2 — Structure the board to add value
Recommendation 2.1 — A majority of the board should be independent directors
During the fiscal year ended December 31, 2014, the Company’s Board comprised six director seats; for the period from July 23, 2014 through December 31 2014, one vacancy existed and the Company had five directors during that period. For ASX purposes, during the period January 1, 2014 through July 22, 2014, the directors comprised four independent non-executive directors, one executive director (being the Chief Executive Officer & Chairman) and one non-independent, non-executive director. On July 23, 2014, one of the non-executive directors, James Schiro, resigned from the Board for personal health reasons.
The Board held nine meetings during the year ended December 31, 2014. Three Board members attended all nine meetings, two members attended eight meetings, and the member who resigned on July 23, 2014 attended three meetings prior to his resignation.
The composition of the Board as of December 31, 2014 and length of tenure of each member was as follows:
| | | | | | | | Committees |
Director | | Director Position | | Year Appointed | | Independent | | Audit | | Compensation | | Nominating
and Corp.
Governance |
| | | | | | | | | | | | |
Robert B. Stockman | | Chairman & CEO | | 1999 | | No | | — | | — | | — |
Brian H. Dovey* | | Non-Executive | | 2001 | | * | | X | | X | | — |
Anne Keating | | Non-Executive | | 2010 | | Yes | | X | | — | | Chair |
Gordon E. Nye | | Non-Executive | | 1999 | | Yes | | — | | Chair | | X |
Robert Thomas | | Non-Executive | | 2010 | | Yes | | Chair | | X | | — |
* Independent Director under the rules of NASDAQ and the SEC, but not considered independent under the ASX
59
Table of Contents
Effective January 28, 2015, Dr. Ross Breckenridge was appointed to the Board to fill the vacant seat. Additionally, on March 25, 2015, the Board expanded the number of director seats to seven and appointed Scott Huennekens as a Board member.
The Company considers that a director to be independent when that director is free from any interest and any business or other relationship that could, or could reasonably be perceived to, materially interfere with the director’s decisions relating to the Company or with the director’s ability to act in the best interests of the Company. The Company also assesses the independence of its directors regarding the requirements for independence set out under ASX Recommendation 2.2.
In January 2015, the Board approved a one-time cash payment to Gordon Nye in the amount of $87,500 as compensation for consulting services provided to the Company during the period August 2014 through January 2015. Although the compensation Mr. Nye received was in excess of his director fees for 2014, the Board does not believe the compensation was material in amount or created a material relationship with the Company, and as such, the Board continues to believe that Mr. Nye qualifies as an independent director.
At the Company’s expense, the Board collectively or the directors acting as individuals are entitled to seek advice from independent external advisors in relation to any matter that is considered necessary to fulfill their relevant duties and responsibilities. Individual directors seeking such advice must obtain approval of the Chairman (which may not be unreasonably withheld). Any advice so obtained will be made available to all Board members.
Recommendation 2.2 — The chair should be an independent director
Recommendation 2.3 — The roles of chair and chief executive officer should not be exercised by the same individual
While the Company has complied with ASX Recommendation 2.1, it is not compliant with ASX Recommendations 2.2 and 2.3. While the majority of the Board is comprised of independent directors for ASX purposes, the Chairman is not an independent director and he also serves as the Company’s Chief Executive Officer, contrary to ASX Recommendations.
The Board believes that Mr. Stockman is not able to exert undue influence on the decision-making process or the governance functions of the Board, despite Mr. Stockman’s not being independent. In addition, while the Chairman and Chief Executive Officer roles have not been separated, the Company has also appointed Dr. Schultz as President and Chief Operating Officer with responsibility for the Company’s day-to-day operations and Ms. Thompson as Chief Financial Officer and Corporate Secretary with responsibility for the Company’s day-to-day financial and corporate administrative functions. Dr. Schultz and Ms. Thompson attend board meetings by invitation but not as Directors. The Board believes that this creates a collaborative management style approach between the Chairman and management with appropriate checks and balances.
Recommendation 2.4 — The board should establish a nomination committee
The Board has established a Nominating and Corporate Governance Committee to oversee the selection and appointment practices of the Company. The members of the Committee are Anne Keating (Chair) and Gordon Nye. All members of the Committee are non-executives and are considered independent directors for both ASX and SEC purposes. The Committee held one formal and numerous informal meetings during the year ended December 31, 2014, all of which both members attended. A copy of the Nominating and Corporate Governance Committee Charter is available in the Corporate Governance section on the Company’s website at www.revamedical.com.
Recommendation 2.5 — Disclose the process for evaluating the performance of the board, its committees, and individual directors
The Company’s Corporate Governance Guidelines provide for annual assessments of the performance of the Board and each committee of the Board, to be provided to the Nominating and Corporate Governance Committee. The performance assessments include evaluations of numerous items, including each Board and committee member’s independence and skills levels, process and effectiveness in addressing Company, Board, and committee matters, interactions with management and outside service providers, meeting attendance, and governance items, including annual charter reviews. The assessments are to be completed by individual Board members, aggregated by the Nominating and Corporate Governance Committee, and evaluated and discussed by the Board and the individual committees of the Board. Such Board and committee assessments were not performed and evaluated for the year ended December 31, 2014, but are planned to be completed as soon as reasonably practicable in 2015.
60
Table of Contents
The Company’s Corporate Governance Guidelines do not call for evaluation of each individual director. The size of the Board and each committee is relatively small, Board and committee meetings are held frequently throughout the year, and the process to assess the Board and each committee considers the involvement and effectiveness of the individual directors. These factors allow for continuous self-assessment, as well as Board level assessments and feedback, of individual performance and contribution.
Recommendation 2.6 — Provide the information required to be disclosed under Principle 2
The Company believes that the backgrounds and qualifications of its directors, considered as a group, should provide a significant mix of experience, knowledge, and abilities that allow the Board to fulfill its responsibilities. As set forth in the Company’s Corporate Governance Guidelines, these criteria generally include, among other things, an individual’s business experience and skills (including skills in core areas such as operations, management, technology, accounting and finance, strategic planning, and international markets), as well as independence, judgment, knowledge of REVA’s business and industry, professional reputation, leadership, integrity, and the ability to represent the best interests of the Company’s shareholders.
The skills, experience, expertise, diversity, independence, and related information for each of our directors holding office as of March 30, 2015 are set forth below:
Ross Breckenridge, MD, MRCP, PhD, age 45, was appointed as a director in January 2015. Dr. Breckenridge is a senior clinical lecturer and Programme Director for the Masters Programme in Clinical and Experimental Medicine at University College London, a Fellow of the Royal College of Physicians (London), and a Consultant Physician at University College London Hospital. His research focuses on the heart’s response to low levels of oxygen, with an overall aim to identify novel therapeutic targets for cardiac disease. Dr. Breckenridge provides consultation services to investors in the biotech and healthcare sector. He is a board member of Senrigan Capital, Empower India, and the Cornelia de Lange Society of Great Britain. He obtained his medical degree from Oxford University, followed by his PhD in Developmental Biology at the University of Cambridge. He then completed his training in Clinical Pharmacology at University College London. Dr. Breckenridge is qualified to sit on our Board due to his extensive medical background, particularly as it relates to research of cardiac disease, his experience serving on multiple other boards of directors, and his general business proficiency.
Brian H. Dovey, age 73, has served as a director since June 2001. Since 1988, Mr. Dovey has been a partner of Domain Associates, LLC, a private venture capital management firm focused on life sciences, where he has led innovative investments and has established and directed new initiatives such as the collaboration between Domain and Rusnano. Since joining Domain, he has served on the board of directors of over 35 private and public companies and has been Chairman of five. He currently sits on the board of three public companies: REVA, Otonomy, and Orexigen Therapeutics. Prior to joining Domain, Mr. Dovey spent six years at Rorer Group, Inc. (now part of Sanofi-Aventis), a pharmaceutical and medical device company listed on the NYSE. As president of Rorer from 1986 to 1988, he was the primary architect of the company’s strategic shift to pharmaceuticals. Previous to that, he was President of Survival Technology, Inc., a start-up medical products company. He also held management positions with Howmedica, Inc., Howmet Corporation, and New York Telephone Company. Mr. Dovey has served as both President and Chairman of the National Venture Capital Association. He is former Chair and currently serves on the Board of Trustees of the Wistar Institute, a non-profit preclinical biomedical research company. Mr. Dovey serves on the board of directors and is also Chairman at the Center for Venture Education (Kauffman Fellows Program) and on the La Jolla Playhouse Board of Trustees. He was also a former board member of the industry association representing the medical device industry, as well as the association representing consumer pharmaceuticals. His is a trustee emeritus of Germantown Academy and is a former trustee of the University of Pennsylvania School of Nursing and the Burnham Institute for Medical Research. Mr. Dovey received his B.A. in mathematics from Colgate University and his MBA from the Harvard Business School. We believe Mr. Dovey is qualified to sit on our Board due to his strong financial expertise, his experience in corporate governance and risk management, his service as a director on over 35 private and public companies, his broad executive experience with medical device companies, and his extensive experience at a health care venture capital firm.
Scott Huennekens, age 50, was appointed as a director on March 25, 2015. From April 2002 to February 2015, Mr. Huennekens was President and Chief Executive Officer of Volcano Corporation, a manufacturer of intravascular imaging equipment for coronary and peripheral applications. He previously was President and Chief Executive Officer of Digirad Corporation, a diagnostic imaging solutions provider, and also held senior positions at Baxter International, Inc. in the Edwards Cardiovascular Division and the Novacor division. Mr. Huennekens currently serves on the boards of EndoChoice, Sonendo, Scripps Translational Science Institute, and the Medical Device Manufacturers Association (“MDMA”). He received his B.S. in Business Administration from the University of Southern California and an MBA from Harvard Business School. We believe Mr. Huennekens is qualified to sit on our Board due to his vast experience in executive positions with medical equipment manufacturers, his broad business background, and his experience serving on multiple other boards of directors.
61
Table of Contents
Anne Keating, age 61, has served as a director since October 2010. Ms. Keating is currently a director of a number of ASX-listed companies in a range of different industries, including GI Dynamics, Inc., a U.S.-based medical device company, and Goodman Group Limited, a global property development and management company. Ms. Keating is also a Director for the Garvan Institute of Medical Research and an Inaugural Governor for the Cerebral Palsy Foundation. From 1993 to 2001, Ms. Keating held the position of General Manager, Australia for United Airlines. She was also a Delegate to the Australian/American Leadership Dialogue for 14 years. Ms. Keating previously served on the board of ClearviewWealth Ltd., a fully diversified life insurance and wealth management company listed on the ASX, from December 2010 until December 2012, was an inaugural board member of the Victor Chang Cardiac Research Institute for ten years and also previously served on the board of NRMA/Insurance/IAG Ltd. for nine years. She has also held former directorships with Spencer Street Station Redevelopment Holdings Limited, Easy FM China Pty Ltd, Radio 2CH Pty Ltd, and Workcover Authority of New South Wales. We believe Ms. Keating is qualified to sit on our Board due to her extensive business, management, and governance experience, including her positions on a number of boards of ASX-listed companies. Ms. Keating also brings Australian medical research and cardiac experience from her years of service with the Garvan Institute of Medical Research and the Victor Chang Cardiac Research Institute.
Gordon E. Nye, age 60, has served as a director since 1999. He is currently Chief Executive Officer of R2 Dermatology, a development stage medical device company. He served as Chief Executive Officer of ZELTIQ Aesthetics, Inc., a medical device company, from September 2009 to April 2012. From August 2003 to July 2009, Mr. Nye served as general partner of Prism Venture Partners, a venture capital firm, where he was a member of the life sciences investment team. Prior to that time, he served as our Chief Executive Officer from 2001 to 2003 and President and Chief Executive Officer of two former Johnson & Johnson divisions (“A” Company Orthodontics, Inc. and Critikon Company, LLC) after they were acquired in management buyouts. He has also held a variety of marketing, sales, and general management roles for L.A. Gear, Inc., Olin Ski Company, Inc., Reebok, Ltd., and The Gillette Company. Mr. Nye received his MBA from the Amos Tuck School of Business at Dartmouth College where he also received his undergraduate degree. We believe Mr. Nye’s qualifications to sit on our Board include his knowledge of the medical device business, his broad operating experience as a senior executive of R2 Dermatology, ZELTIQ Aesthetics, Inc. and two former Johnson & Johnson divisions, his extensive consumer marketing background, and his other board service.
Robert B. Stockman, age 61, our co-founder, has served as Chairman of the Board since 1999 and Chief Executive Officer since August 2010. He is a director of HeartWare Limited/HeartWare International, Inc., an ASX and NASDAQ listed medical device company, since December 2006. Mr. Stockman also serves as a board member for MuseAmi, Inc., a privately held advanced music software company that he co-founded. He previously served on the board of ZELTIQ Aesthetics, Inc., a medical technology company listed on NASDAQ, from July 2010 until April 2012. Since 1999, Mr. Stockman has been the President and Chief Executive Officer of Group Outcome LLC, a U.S.-based merchant banking firm that deploys its capital and that of its financial partners in private equity and venture capital investments in medical technology companies. Mr. Stockman also co-founded Centrimed, Inc., an internet-based software company, that was acquired by the Global Healthcare Exchange, LLC, and led the buyouts of Ioptex, an intraocular lens manufacturer, and two Johnson & Johnson divestitures, “A” Company Orthodontics, Inc. and Critikon Company, LLC, each of which was subsequently acquired. Prior to establishing Group Outcome LLC, Mr. Stockman spent 18 years with Johnston Associates, Inc. and Narragansett Capital Corporation, where he focused on venture capital investments and merger advisory work in health care. Mr. Stockman holds a Bachelor’s Degree from Harvard College and an MBA from The Tuck School at Dartmouth College, where he serves on Tuck’s Board of Overseers. We believe Mr. Stockman is qualified to sit on our Board due to his extensive experience as an entrepreneur driving the growth of five medical products companies, his experience as an executive of medical device companies, and his experience as an executive in the investment banking industry. Mr. Stockman’s qualifications also include his strong financial background, including his work early in his career at Price Waterhouse and his ability to provide financial expertise to the Board.
62
Table of Contents
Robert Thomas, age 69, has served as a director since July 2010. He has also been a director and non-executive Chairman of the Board of HeartWare Limited/HeartWare International, Inc., a U.S. medical device company listed on ASX and NASDAQ, since November 2004. He is currently a director of a number of Australian public companies, including Virgin Australia Limited, Biotron Limited, and Starpharma Limited. Between October 2004 and September 2008, Mr. Thomas was a consultant to Citigroup Corporate and Investment Bank. Between March 2003 and September 2004, he was Chairman of Global Corporate and Investment Bank, Citigroup Global Markets, Australia and New Zealand. Prior to that time, Mr. Thomas was Chief Executive Officer of Citigroup’s Corporate and Investment Bank (formerly known as Salomon Smith Barney), Australia and New Zealand from October 1999 until February 2003. Mr. Thomas is Chairman of Aus Bio Limited, a director of O’Connell Street Associates and Grahger Capital Securities. He also is a member of the advisory board of Inteq Limited. Mr. Thomas holds a Bachelor of Economics from Monash University, Australia. He is a member of the Stockbrokers Association of Australia and is a Master Stockbroker. Mr. Thomas is also a Fellow of the Financial Services Institute of Australia and the Australian Institute of Company Directors. He is on the board of the NSW State Library Foundation and serves on NSW State Library’s Audit and Risk Committee. We believe Mr. Thomas is qualified to sit on our Board due to his extensive investment banking experience, including his leadership of finance and strategic transactions, his involvement with medical device companies, and his experience in governance and risk management across a wide range of industries. Mr. Thomas also brings capital market and economics expertise to the Board from his years of service as a securities analyst and experience as a director of ASX-listed companies.
Reporting Requirement
Except as discussed above, the Company fully complied with ASX Recommendations 2.1 to 2.6 during the year ended December 31, 2014.
Principle 3 — Promote ethical and responsible decision-making
Recommendation 3.1 — Establish a code of conduct and disclose the code or a summary of the code as to (a) the practices necessary to maintain confidence in the company’s integrity, (b) the practices necessary to take into account their legal obligations and the reasonable expectations of their stakeholders, and (c) the responsibility and accountability of individuals for reporting and investigating reports of unethical practices
The Company has adopted a Code of Business Conduct and Ethics, an Insider Trading Policy, and a Related Party Transaction Policy. A copy of each policy is available in the Corporate Governance section on the Company’s website at www.revamedical.com.
Recommendation 3.2 — Establish a policy concerning diversity and disclose the policy or a summary of that policy. The policy should include requirements for the board to establish measurable objectives for achieving gender diversity for the board to assess annually both the objectives and progress in achieving them
The Company has adopted a Diversity Policy, which includes measurable objectives for achieving gender diversity and provisions for the Board to annually assess both the objectives and the Company’s progress in achieving them. A copy of the Diversity Policy is available in the Corporate Governance section on the Company’s website at www.revamedical.com.
Recommendation 3.3 — Disclose in each annual report the measurable objectives for achieving gender diversity set by the board in accordance with the diversity policy and progress towards achieving them
The Board continued to evaluate the gender diversity of the Company’s employees, its senior management, and its Board during 2014 and determined that the gender diversity continued at levels generally consistent with the prior year and in line with expectations. The Board endorsed the Company’s objective for diversity to remain at the same relative proportions, if not higher, of females in each category measured. The base level expectations for females are a minimum 15 percent of board members, 30 percent of senior management, and 40 percent of employees.
Recommendation 3.4 — Disclose in each annual report the proportion of women employees in the whole organization, women in senior executive positions and women on the board
As of December 31, 2014, a total of 39 percent of the Company’s employees was female, 33 percent of its senior management was female, and 20 percent of its Board members was female.
63
Table of Contents
Recommendation 3.5 — Provide the information required to be disclosed under Principle 3
Reporting Requirement
The Company fully complied with ASX Recommendations 3.1 through 3.5 for the year ended December 31, 2014.
Principle 4 — Safeguard integrity in financial reporting
Recommendation 4.1 — The board should establish an audit committee
The Board has established an Audit Committee to oversee the management of the Company’s financial and internal risks and reporting.
Recommendation 4.2 — The audit committee should be structured so that it (a) consists only of non-executive directors, (b) consists of a majority of independent directors, (c) is chaired by an independent chair, who is not chair of the board, and (d) has at least three members
Members of the Audit Committee are Robert Thomas (Chair), Brian Dovey, and Anne Keating who are all non-executive directors. Robert Thomas and Anne Keating are both considered independent directors for ASX purposes; however, Brian Dovey is not considered to be independent for ASX purposes but is considered to be independent under SEC rules. The Committee held four meetings during 2014; two of the Committee members attended all four meetings and one of the Committee members attended one of the meetings.
All three members of the Audit Committee are considered to be financially literate and familiar with financial and accounting matters and qualified to adequately understand the financial and accounting matters that relate to the Company. Brian Dovey is considered to be a financial professional with appropriate financial and accounting expertise.
Recommendation 4.3 — The audit committee should have a formal charter
The Audit Committee has adopted and is governed by a formal charter, a copy of which is available in the Corporate Governance section on the Company’s website at www.revamedical.com. The Audit Committee regularly reports to the Board about committee activities, issues, and related recommendations.
Recommendation 4.4 — Provide the information required to be disclosed under Principle 4
In its Audit Committee charter, the Company has disclosed its procedures for the selection and appointment of the independent auditor and provides for the rotation of independent audit engagement partners.
Reporting Requirement
Except as disclosed above, the Company fully complied with ASX Recommendations 4.1 through 4.4 during the year ended December 31, 2014.
Principle 5 — Make timely and balanced disclosure
Recommendation 5.1 — Establish written policies designed to ensure compliance with ASX Listing Rule disclosure requirements and to ensure accountability at a senior executive level for that compliance and disclose those policies or a summary of those policies
The Company is committed to providing timely and balanced disclosure to the market in accordance with its continuous disclosure obligations. In accordance with its commitment to fully comply with these obligations and to ensure accountability at a senior management level for that compliance, the Company has adopted a Continuous Disclosure Policy, together with other internal mechanisms and reporting requirements. A copy of the Company’s Continuous Disclosure Policy is available in the Corporate Governance section on its website at www.revamedical.com. In addition, a copy of all the Company’s ASX announcements, financial reports, and related public information are also available on the Company’s website.
64
Table of Contents
Recommendation 5.2 — Provide the information required to be disclosed under Principle 5
Reporting Requirement
The Company fully complied with ASX Recommendations 5.1 and 5.2 during the year ended December 31, 2014.
Principle 6 — Respect the rights of shareholders
Recommendation 6.1 — Design a communications policy for promoting effective communication with shareholders and encouraging their participation at general meetings and disclose their policy or a summary of that policy
The Company has adopted a Shareholder Communication Policy that supports effective two-way communication with its shareholders. The Shareholder Communication Policy is included in the Company’s Corporate Governance Guidelines, a copy which is available in the Corporate Governance section on the Company’s website at www.revamedical.com. The Company seeks to utilize numerous modes of communication, including electronic communication, to ensure that its communication with Shareholders is frequent, clear, and accessible. Additionally, the Company announces briefing calls in advance of such calls, provides relevant information on its website, and maintains internal records of matters discussed with shareholders.
All shareholders are invited to attend the Company’s annual meeting either in person or by proxy. The Board regards the annual meeting as an excellent forum in which to discuss issues relevant to the Company and accordingly encourages full participation by shareholders. Shareholders have an opportunity to submit questions to the Board and auditors. The meeting may also be audio cast and/or webcast to provide access to those shareholders who are unable to attend the annual general meeting in person.
Recommendation 6.2 — Provide the information required to be disclosed under Principle 6
Reporting Requirement
The Company has fully complied with ASX Recommendations 6.1 and 6.2 during the year ended December 31, 2014.
Principle 7 — Recognize and manage risk
Recommendation 7.1 — Establish policies for the oversight and management of material business risks and disclose a summary of those policies
The Company has adopted a Risk Management Policy that sets forth the process to identify, assess, and manage risk in the Company’s business operations. A copy of the Risk Management Policy is available in the Corporate Governance section on the Company’s website at www.revamedical.com.
Recommendation 7.2 — The board should require management to design and implement the risk management and internal control system to manage the company’s material business risk and report to it on whether those risks are being managed effectively. The board should disclose that management has reported to it as to the effectiveness of the company’s management of its material business risks
The Board’s role in risk oversight includes receiving reports from members of management on a regular basis regarding material risks faced by the Company and applicable mitigation strategies and activities, at least on a quarterly basis. The reports cover the critical areas of operations, research and development, regulatory and quality affairs, intellectual property, clinical developments, and legal and financial affairs, as well as management’s assessment of risks facing the Company. The Board and its committees consider these reports, discuss matters with management, and identify and evaluate any potential strategic or operational risks and appropriate activity to address those risks, thereby ensuring the effectiveness of REVA’s management in identifying material business risks.
During the year ended December 31, 2014, the Board received and considered reports from management for each Board meeting held, with such reports addressing the Company’s activities, material risks, and mitigation strategies.
Recommendation 7.3 — The board should disclose whether it has received assurance from the chief executive officer (or equivalent) and the chief financial officer (or equivalent) that the declaration provided in accordance with section 295A of the Corporations Act is founded on a sound system of risk management and internal control and that the system is operating effectively in all material respects in relation to financial reporting risks
65
Table of Contents
As the Company prepares and files its financial statements under United States accounting practices and laws, management is required to provide representations to the Board on a wide range of issues, including the effectiveness of the Company’s disclosure controls and procedures as well as the design or operation of internal control over financial reporting. However, as the Company is incorporated in the United States and is not bound by the financial reporting provisions under the Australian Corporations Act 2001 (Cth), no declaration is required under section 295A of the Corporations Act. To this end, shareholders’ attention is drawn to Item 9A of this Annual Report on Form 10-K and the certifications provided by the Chief Executive Officer and the Chief Financial Officer at the end of the Form 10-K. As stated above, Item 9A discloses information regarding the Company’s controls and procedures and management’s evaluation of the effectiveness of our internal control over financial reporting.
Recommendation 7.4 — Provide the information required to be disclosed under Principle 7
Reporting Requirement
Except as disclosed above, the Company fully complied with ASX Recommendations 7.1 through 7.4 during the year ended December 31, 2014.
Principle 8 — Remunerate fairly and responsibly
Recommendation 8.1 — The board should establish a remuneration committee
The Board has established a Compensation Committee to review and assess executive and director compensation. The Compensation Committee has adopted and is governed by a formal charter, a copy of which is available in the Corporate Governance section on the Company’s website at www.revamedical.com. The Compensation Committee regularly reports to the Board about committee activities, issues, and related recommendations.
Recommendation 8.2 — The remuneration committee should be structured so that it (a) consists of a majority of independent directors, (b) is chaired by an independent chair, and (c) has at least three members
The members of the Compensation Committee are Gordon Nye (Chair), Brian Dovey, and Robert Thomas. Gordon Nye and Robert Thomas are both considered to be independent for ASX purposes; however, Brian Dovey is not considered to be independent for ASX purposes but is considered to be independent under the SEC rules. The Compensation Committee, therefore, consists of a majority of independent directors and is also chaired by an independent director. The Committee held one formal and numerous informal meetings during 2014, which all Committee members attended.
Recommendation 8.3 — Clearly distinguish the structure of non-executive directors’ remuneration from that of executive directors and senior executives
In accordance with its charter, the Compensation Committee is responsible for ensuring that the structure of non-executive and executive directors’ compensation is clearly distinguished. The Company has adopted a non-executive director compensation policy pursuant to which non-executive directors are compensated for their services to the Board. Non-executive director compensation comprises a base salary as well as the ability to receive annual grants of options at the Board’s discretion (subject to shareholders’ approval being obtained as required under the ASX Listing Rules). The Company has adopted a separate executive compensation program that consists of base salary, equity-based incentives, performance-based cash bonuses, severance benefits, and other customary benefits such as health insurance on the same basis as provided to all other employees. The Company’s Chairman, who is also currently serving as our Chief Executive Officer, is eligible to receive a cash bonus at the discretion of the Board of up to 60 percent of his salary each year. None of the Company’s non-executive directors will be entitled to any retirement benefits.
66
Table of Contents
While the Compensation Committee reviews and reports compensation items to the Board for both non-executive directors and executive management, including each individual’s skills, knowledge, and contributions to the Company, the Committee does not provide a separate report of compensation by gender. The Company’s Insider Trading Policy, a copy of which is available in the Corporate Governance section on the Company’s website at www.revamedical.com, sets out the Company’s policy that prohibits Directors, certain officers, and key management personnel from entering into hedging transactions involving REVA’s securities.
Recommendation 8.4 — Provide the information required to be disclosed under Principle 8
Except as disclosed above, the Company fully complied with ASX Recommendations 8.1 through 8.4 during the year ended December 31, 2014.
Further information regarding the Compensation Committee, as required by Item 10 of this Annual Report on Form 10-K, will be contained in our 2015 Proxy Statement. Such information is incorporated herein by reference.
Item 11. Executive Compensation
The information required by this item is incorporated by reference to our 2015 Proxy Statement under the headings “Non-Employee Director Compensation” and “Executive Compensation.”
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The Information required by this item is incorporated by reference to our 2015 Proxy Statement under the heading “Security Ownership of Certain Beneficial Owners and Management.”
The following table sets forth information regarding outstanding options and shares reserved for future issuance as of December 31, 2014 under equity compensation plans approved by our stockholders. We do not have any equity compensation plans that have not been approved by stockholders.
Plan Category | | Number of Shares
to be Issued
upon Exercise
or Vesting
of Outstanding
Awards | | Weighted
Average
Exercise
Price
of Outstanding
Options | | Number of Shares Remaining
Available for
Future Issuance (1) | |
| | | | | | | |
Equity compensation plans approved by stockholders (2) | | 4,315,550 | | $ | 7.01 | | 3,012,835 | |
| | | | | | | | |
(1) Our 2010 Equity Incentive Plan, as amended, contains a provision for an automatic increase each January 1st of the number of shares available for grant. The automatic increase shall be the lessor of (i) 3% of the number of shares of our common stock issued and outstanding on January 1st, or (ii) a number of shares set by our Board.
(2) Consists of grants and awards from our 2001 Stock Option/Stock Issuance Plan and our 2010 Equity Incentive Plan, as amended, including 4,243,425 outstanding options to purchase common stock and 72,125 shares of restricted stock subject to future vesting.
Item 13. Certain Relationships and Related Transactions, and Director Independence
The information required by this item is incorporated by reference to our 2015 Proxy Statement under the heading “Related Party Transactions.”
Item 14. Principal Accounting Fees and Services
The information required by this item is incorporated by reference to our 2015 Proxy Statement under the heading “Audit and Non-Audit Fees.”
67
Table of Contents
PART IV
Item 15. Exhibits and Financial Statement Schedules
The following documents are filed as part of this Annual Report on Form 10-K:
1. Financial Statements — The following financial statements are included in this report:
Report of Independent Registered Public Accounting Firm (current and predecessor)
Consolidated Balance Sheets
Consolidated Statements of Operations and Comprehensive Loss
Consolidated Statements of Cash Flows
Consolidated Statements of Stockholders’ Equity (Deficit)
Notes to Consolidated Financial Statements
2. List of Financial Statement Schedules — All schedules are omitted because they are not applicable or the required information is shown in the financial statements or notes thereto.
Exhibits — The exhibits listed in the accompanying Index to Exhibits are filed or incorporated by reference as part of this Annual Report on Form 10-K.
68
Table of Contents
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this Annual Report on Form 10-K to be signed on its behalf by the undersigned, thereunto duly authorized.
| REVA Medical, Inc. |
| |
Dated: March 30, 2015 | By: | /s/ Robert B. Stockman |
| Name: | Robert B. Stockman |
| Title: | Chairman of the Board and |
| | Chief Executive Officer |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons in the capacities and on the dates indicated.
Signature | | Title | | Date |
| | | | |
/s/ Robert B. Stockman | | Chairman of the Board and | | March 30, 2015 |
Robert B. Stockman | | Chief Executive Officer (Principal Executive Officer) | | |
| | | | |
/s/ Katrina L. Thompson | | Chief Financial Officer | | March 30, 2015 |
Katrina L. Thompson | | (Principal Financial Officer and Principal Accounting Officer) | | |
| | | | |
/s/ Ross Breckenridge | | Director | | March 30, 2015 |
Ross Breckenridge | | | | |
| | | | |
/s/ Brian H. Dovey | | Director | | March 30, 2015 |
Brian H. Dovey | | | | |
| | | | |
/s/ Scott Huennekens | | Director | | March 30, 2015 |
Scott Huennekens | | | | |
| | | | |
/s/ Anne Keating | | Director | | March 30, 2015 |
Anne Keating | | | | |
| | | | |
/s/ Gordon E. Nye | | Director | | March 30, 2015 |
Gordon E. Nye | | | | |
| | | | |
/s/ Robert Thomas | | Director | | March 30, 2015 |
Robert Thomas | | | | |
69
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Board of Directors and Stockholders
REVA Medical, Inc.
We have audited the accompanying consolidated balance sheet of REVA Medical, Inc., a Delaware corporation, (the “Company”) as of December 31, 2014, and the related consolidated statements of operations and comprehensive loss, cash flows, and changes in stockholders’ equity (deficit) for the year ended December 31, 2014. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. We were not engaged to perform an audit of the Company’s internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of REVA Medical, Inc. as of December 31, 2014, and the results of its operations and its cash flows for the year ended December 31, 2014, in conformity with accounting principles generally accepted in the United States of America.
As discussed in Note 3 to the consolidated financial statements, the Company adopted new accounting guidance in 2014 related to the elimination of certain financial reporting requirements for development stage entities.
San Diego, California
March 30, 2015
F-2
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders of
REVA Medical, Inc.
We have audited the accompanying consolidated balance sheet of REVA Medical, Inc. (a development stage company) (the Company) as of December 31, 2013 and the related consolidated statements of operations and comprehensive loss, cash flows and stockholders’ equity (deficit) for each of the two years in the period ended December 31, 2013. These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the consolidated financial position of REVA Medical, Inc. at December 31, 2013 and the consolidated results of its operations and its cash flows for each of the two years in the period ended December 31, 2013, in conformity with U.S. generally accepted accounting principles.
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. The Company has recurring losses and negative cash flows from operations that raise substantial doubt about its ability to continue as a going concern. The consolidated financial statements do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities that may result from the outcome of this uncertainty.
San Diego, California
March 17, 2014
F-3
Table of Contents
REVA Medical, Inc.
Consolidated Balance Sheets
(in thousands, except share and per share amounts)
| | December 31, | |
| | 2013 | | 2014 | |
Assets | |
| | | | | |
Current Assets: | | | | | |
Cash and cash equivalents | | $ | 19,229 | | $ | 25,814 | |
Short-term investments | | 1,492 | | 995 | |
Prepaid expenses and other current assets | | 415 | | 406 | |
| | | | | |
Total current assets | | 21,136 | | 27,215 | |
| | | | | |
Non-Current Assets: | | | | | |
Property and equipment, net | | 3,589 | | 2,920 | |
Other non-current assets | | 60 | | 60 | |
| | | | | |
Total non-current assets | | 3,649 | | 2,980 | |
| | | | | |
Total Assets | | $ | 24,785 | | $ | 30,195 | |
| | | | | |
Liabilities and Stockholders’ Equity (Deficit) | |
| | | | | |
Current Liabilities: | | | | | |
Accounts payable | | $ | 1,400 | | $ | 651 | |
Accrued expenses and other current liabilities | | 2,080 | | 2,213 | |
| | | | | |
Total current liabilities | | 3,480 | | 2,864 | |
| | | | | |
Long-Term Liabilities: | | | | | |
Convertible notes payable | | — | | 37,780 | |
Common stock warrant liability | | — | | 15,389 | |
Other long-term liabilities | | 480 | | 611 | |
| | | | | |
Total long-term liabilities | | 480 | | 53,780 | |
| | | | | |
Total Liabilities | | 3,960 | | 56,644 | |
| | | | | |
Commitments and contingencies (Note 9) | | | | | |
| | | | | |
Stockholders’ Equity (Deficit): | | | | | |
Common stock — $0.0001 par value; 100,000,000 shares authorized; 33,270,053 and 33,529,778 shares issued and outstanding at December 31, 2013 and 2014, respectively | | 3 | | 3 | |
Class B common stock — $0.0001 par value; 25,000,000 shares authorized; no shares issued or outstanding | | — | | — | |
Undesignated preferred stock — $0.0001 par value; 5,000,000 shares authorized; no shares issued or outstanding | | — | | — | |
Additional paid-in capital | | 222,331 | | 226,094 | |
Accumulated deficit | | (201,509 | ) | (252,546 | ) |
| | | | | |
Total Stockholders’ Equity (Deficit) | | 20,825 | | (26,449 | ) |
| | | | | |
Total Liabilities and Stockholders’ Equity (Deficit) | | $ | 24,785 | | $ | 30,195 | |
The accompanying notes are an integral part of these financial statements.
F-4
Table of Contents
REVA Medical, Inc.
Consolidated Statements of Operations and Comprehensive Loss
(in thousands, except share and per share amounts)
| | Year Ended December 31, | |
| | 2012 | | 2013 | | 2014 | |
| | | | | | | |
Operating Expense: | | | | | | | |
Research and development | | $ | 15,822 | | $ | 19,212 | | $ | 14,318 | |
General and administrative | | 8,043 | | 8,731 | | 7,645 | |
| | | | | | | |
Loss from operations | | (23,865 | ) | (27,943 | ) | (21,963 | ) |
| | | | | | | |
Other Income (Expense): | | | | | | | |
Interest income | | 92 | | 30 | | 8 | |
Interest expense | | — | | — | | (986 | ) |
Loss on issuance of convertible notes payable and warrants | | — | | — | | (15,627 | ) |
Loss on change in fair value of convertible notes payable and warrant liability | | — | | — | | (12,542 | ) |
Other income (expense) | | (3 | ) | (9 | ) | 73 | |
| | | | | | | |
Other income (expense) | | 89 | | 21 | | (29,074 | ) |
| | | | | | | |
Net Loss and Comprehensive Loss | | $ | (23,776 | ) | $ | (27,922 | ) | $ | (51,037 | ) |
| | | | | | | |
Net Loss Per Common Share: | | | | | | | |
| | | | | | | |
Net loss per share, basic and diluted | | $ | (0.72 | ) | $ | (0.84 | ) | $ | (1.53 | ) |
| | | | | | | |
Shares used to compute net loss per share, basic and diluted | | 33,072,058 | | 33,124,655 | | 33,382,381 | |
The accompanying notes are an integral part of these financial statements.
F-5
Table of Contents
REVA Medical, Inc.
Consolidated Statements of Cash Flows
(in thousands)
| | Year Ended December 31, | |
| | 2012 | | 2013 | | 2014 | |
| | | | | | | |
Cash Flows from Operating Activities: | | | | | | | |
Net loss | | $ | (23,776 | ) | $ | (27,922 | ) | $ | (51,037 | ) |
Non-cash adjustments to reconcile net loss to net cash used for operating activities: | | | | | | | |
Depreciation and amortization | | 677 | | 892 | | 1,027 | |
Loss on property and equipment disposal | | 1 | | 1 | | — | |
Stock-based compensation | | 3,497 | | 4,090 | | 3,516 | |
Interest on convertible notes payable | | — | | — | | 986 | |
Loss on issuance of convertible notes payable and warrants | | — | | — | | 15,627 | |
Loss on change in fair value of convertible notes payable and warrant liability | | — | | — | | 12,542 | |
Other non-cash expenses | | 80 | | 18 | | 19 | |
Changes in operating assets and liabilities: | | | | | | | |
Prepaid expenses and other current assets | | 496 | | 2 | | 9 | |
Accounts payable | | (218 | ) | 549 | | (566 | ) |
Accrued expenses and other current liabilities | | 522 | | 525 | | 64 | |
Other non-current liabilities | | 60 | | (98 | ) | (117 | ) |
| | | | | | | |
Net cash used for operating activities | | (18,661 | ) | (21,943 | ) | (17,930 | ) |
| | | | | | | |
Cash Flows from Investing Activities: | | | | | | | |
Purchases of property and equipment | | (1,949 | ) | (1,466 | ) | (541 | ) |
Purchases of investments | | (1,989 | ) | (1,492 | ) | (995 | ) |
Maturities of investments | | 1,992 | | 5,223 | | 1,492 | |
| | | | | | | |
Net cash provided by (used for) investing activities | | (1,946 | ) | 2,265 | | (44 | ) |
| | | | | | | |
Cash Flows from Financing Activities: | | | | | | | |
Proceeds from issuances of common stock | | 322 | | 31 | | 247 | |
Proceeds from issuances of convertible notes payable and warrants, net | | — | | — | | 24,312 | |
| | | | | | | |
Net cash provided by financing activities | | 322 | | 31 | | 24,559 | |
| | | | | | | |
Net increase (decrease) in cash and cash equivalents | | (20,285 | ) | (19,647 | ) | 6,585 | |
Cash and cash equivalents at beginning of period | | 59,161 | | 38,876 | | 19,229 | |
| | | | | | | |
Cash and Cash Equivalents at End of Period | | $ | 38,876 | | $ | 19,229 | | $ | 25,814 | |
| | | | | | | |
Supplemental Non-Cash Information: | | | | | | | |
Property and equipment in accounts payable | | $ | 99 | | $ | 195 | | $ | 12 | |
The accompanying notes are an integral part of these financial statements.
F-6
Table of Contents
REVA Medical, Inc.
Consolidated Statements of Stockholders’ Equity (Deficit)
(in thousands, except share and per share amounts)
| | | | | | | | | | Total | |
| | Common Stock | | Additional | | Accumulated | | Stockholders’ | |
| | Shares | | Amount | | Paid-In Capital | | Deficit | | Equity (Deficit) | |
| | | | | | | | | | | |
Balance at December 31, 2011 | | 32,810,503 | | $ | 3 | | $ | 214,391 | | $ | (149,811 | ) | $ | 64,583 | |
| | | | | | | | | | | |
Net loss and comprehensive loss | | — | | — | | — | | (23,776 | ) | (23,776 | ) |
Common stock issued January through August upon exercise of stock options for cash at $0.61 to $1.40 per share | | 288,700 | | — | | 322 | | — | | 322 | |
Restricted common stock issued in July under equity incentive plan | | 33,000 | | — | | — | | — | | — | |
Stock-based compensation expense | | — | | — | | 3,497 | | — | | 3,497 | |
| | | | | | | | | | | |
Balance at December 31, 2012 | | 33,132,203 | | $ | 3 | | $ | 218,210 | | $ | (173,587 | ) | $ | 44,626 | |
| | | | | | | | | | | |
Net loss and comprehensive loss | | — | | — | | — | | (27,922 | ) | (27,922 | ) |
Common stock issued May and November upon exercise of stock options for cash at $0.61 per share | | 50,350 | | — | | 31 | | — | | 31 | |
Restricted common stock issued January and May under equity incentive plan | | 87,500 | | — | | — | | — | | — | |
Stock-based compensation expense | | — | | — | | 4,090 | | — | | 4,090 | |
| | | | | | | | | | | |
Balance at December 31, 2013 | | 33,270,053 | | 3 | | 222,331 | | (201,509 | ) | 20,825 | |
| | | | | | | | | | | |
Net loss and comprehensive loss | | — | | — | | — | | (51,037 | ) | (51,037 | ) |
Common stock issued January through October upon exercise of stock options for cash at $0.61 to $1.40 per share | | 259,725 | | — | | 247 | | — | | 247 | |
Stock-based compensation expense | | — | | — | | 3,516 | | — | | 3,516 | |
| | | | | | | | | | | |
Balance at December 31, 2014 | | 33,529,778 | | $ | 3 | | $ | 226,094 | | $ | (252,546 | ) | $ | (26,449 | ) |
The accompanying notes are an integral part of these financial statements.
F-7
Table of Contents
REVA Medical, Inc.
Notes to Consolidated Financial Statements
1. Description of Business
REVA Medical, Inc. (“REVA” or the “Company”) was incorporated in California in 1998 under the name MD3, Inc. In March 2002 we changed our name to REVA Medical, Inc. In October 2010 we reincorporated in Delaware. We established a non-operating wholly owned subsidiary, REVA Germany GmbH, in 2007. In these notes the terms “us,” “we,” or “our” refer to REVA and our consolidated subsidiary unless context dictates otherwise.
We do not yet have a product available for sale; our product(s) will become available following completion of required clinical studies with acceptable data and when, and if, we receive regulatory approval. We are currently developing and testing a bioresorbable stent to treat vascular disease in humans. This stent, which we have named Fantom, was introduced in humans during December 2014. We intend to enroll up to 110 patients in a clinical trial with Fantom, obtain follow-up data at a six-month time point, and if this data has acceptable safety and efficacy results, apply for a European CE Marking, the regulatory approval that would allow us to commercialize Fantom in Europe.
In December 2010 we completed an initial public offering (the “IPO”) of our common stock in Australia and registered with the U.S. Securities and Exchange Commission (“SEC”) and, consequently, became an SEC filer. Our stock is traded in the form of CHESS Depository Interests (“CDIs”) on the Australian Securities Exchange (“ASX”); each share of our common stock is equivalent to ten CDIs. Our trading symbol is “RVA.AX.”
2. Capital Resources and Basis of Presentation
Capital Resources: We had cash and investments totaling $26,809,000 as of December 31, 2014, which reflect the receipt of $25,000,000 cash proceeds from the issuance of convertible notes payable and warrants to purchase common stock on November 14, 2014. We believe these resources are sufficient to meet our operating and capital needs through at least 2015. We have experienced recurring losses and negative cash flows from operating activities since our inception and, as of December 31, 2014, we had an accumulated deficit of $252,546,000. Until we generate revenue, and at a level to support our cost structure, we expect to continue to incur substantial operating losses and net cash outflows. Even if we do attain revenue, we may never become profitable and even if we do attain profitable operations, we may not be able to sustain that profitability or positive cash flows on a recurring basis.
Basis of Presentation: We have prepared the accompanying consolidated financial statements in accordance with U.S. generally accepted accounting principles (“GAAP”) and the rules and regulations of the SEC. The consolidated financial statements include the accounts of REVA and our wholly owned subsidiary, REVA Germany GmbH. All intercompany transactions and balances, if any, have been eliminated in consolidation.
Use of Estimates: In order to prepare our financial statements in conformity with accounting principles generally accepted in the United States, we are required to make estimates and assumptions that affect the reported amounts in the financial statements and accompanying notes. Our most significant estimates relate to the fair value of our convertible notes payable, the fair value of our warrant liability, our expense accruals, including clinical study expenses, and our stock-based compensation expense. Actual results could differ from our estimates.
3. Significant Accounting Policies
Cash and Cash Equivalents: All highly liquid investments with original maturities of three months or less are classified as cash equivalents.
Investments: Excess cash is invested in high-quality marketable securities. Our investments are classified as either short- or long-term based on their maturity dates. Investments with a maturity of less than one year are classified as short-term; all others are classified as long-term. We have categorized the investments as “held-to-maturity” based on our intent and ability to hold to maturity. Our investments are stated at cost; their fair value is determined each reporting period through quoted market prices of similar instruments in active markets. During the reporting period there were no declines in fair value that were deemed to be other than temporary.
F-8
Table of Contents
REVA Medical, Inc.
Notes to Consolidated Financial Statements
3. Significant Accounting Policies (continued)
Property and Equipment: Property and equipment is stated at cost, less accumulated depreciation and amortization. Depreciation is determined using the straight-line method over the estimated useful lives of the related assets, generally three to five years. Amortization of leasehold improvements is determined using the straight-line method over the lesser of the useful life of the asset or the term of the underlying lease. Upon disposition or retirement of an asset, its cost and related accumulated depreciation or amortization are removed from the accounts and any gain or loss is recognized in the consolidated statement of operations.
Patents: Costs related to patent development, filing, and maintenance are expensed as incurred since the underlying technology associated with these assets is purchased or incurred in connection with our research and development efforts and the future realizable value cannot be determined.
Impairment of Long-Lived Assets: We review our long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable and exceeds its undiscounted future cash flows. The amount of impairment, if any, is determined by comparing an asset’s estimated fair value to the asset’s respective carrying amount. During the years ended December 31, 2012, 2013, and 2014 we determined there were no indications of asset impairment.
Concentrations of Credit Risk: Our financial instruments, which potentially subject us to concentration of credit risk, comprise cash, cash equivalents, and investments. We maintain our cash and cash equivalents in bank accounts, the balances of which generally exceed limits that are insured by the Federal Deposit Insurance Corporation. Our investments are held in custody by a large financial asset manager in the United States. Management believes the Company is not exposed to significant credit risk due to the financial position of the depository institutions in which the assets are held. Additionally, we maintain our cash and investments in accordance with our investment policy, which is designed to maintain safety and liquidity. We have not realized any losses in our investments and believe we are not exposed to significant credit risk related to our cash and cash equivalents.
Convertible Notes Payable: Convertible notes payable are analyzed at issue date to determine balance sheet classification, issue discounts or premiums, and embedded or derivative features. Embedded or derivative features are evaluated in accordance with accounting guidance for derivative securities and, if the features give rise to separate accounting, we make an election to account for the notes at cost or at fair value. If fair value accounting is elected, on the issue date we record the difference between the issue price of the notes and their fair value as a gain or loss in the consolidated statement of operations. We remeasure the fair value at each reporting date and record a gain (upon a decrease in fair value) or loss (upon an increase in fair value) for the change in fair value. Fair value is determined using a binomial valuation model with; inputs to the model include the market value of the underlying stock, a life equal to the contractual life of the notes, incremental borrowing rates that correspond to debt with similar credit worthiness, estimated volatility based on the historical prices of our trading securities, and we make assumptions as to our abilities to test and commercialize our product(s), to obtain future financings when and if needed, and to comply with the terms and conditions of the notes. Following an analysis of their embedded and derivative features and a projection of the volatility of their effective interest rates under the cost method, we elected to utilize fair value accounting for the convertible notes payable we issued on November 14, 2014. Management believes the fair value method of accounting provides a more appropriate presentation of these liabilities than would be provided under the cost method.
Common Stock Warrants: In accordance with ASC 480 “Distinguishing Liabilities from Equity,” we record the fair value of warrants issued for the purchase of common stock as a liability since the warrants call for issuance of registered shares upon exercise, a condition that we may not be able to accommodate and which would then result in a net settlement of the warrants. Until the time the warrants are exercised or expire, the fair value is assessed at each reporting date utilizing a binomial valuation model and any change in value is recorded as a gain or loss component of other income (expense) in our consolidated statement of operations. Inputs to the valuation model are of the same nature as those used for our convertible notes payable.
F-9
Table of Contents
REVA Medical, Inc.
Notes to Consolidated Financial Statements
3. Significant Accounting Policies (continued)
Research and Development: Research and development costs are expensed as incurred. These costs include salaries, employee benefits, laboratory supplies, consulting services, manufacturing products and services, preclinical and clinical costs, technology license fees, laboratory equipment depreciation, facility costs, and certain indirect costs.
Segment Information: We operate in one business segment, which is the development and commercialization of medical devices.
Foreign Currency: The functional currency of our subsidiary REVA Germany GmbH is the euro. Balance sheet accounts of our subsidiary are translated into United States dollars using the exchange rate in effect at the balance sheet date while expenses are translated using the average exchange rate in effect during the period. Gains and losses arising from translation of our subsidiary’s financial statements are recorded to other comprehensive income (loss). These gains and losses, in the aggregate, were insignificant through December 31, 2014.
Income Taxes: We account for income taxes using the asset and liability method, under which the current income tax expense or benefit is the amount of income tax expected to be payable or refundable in the current year. Deferred tax assets and liabilities are recorded for the estimated future tax consequences of temporary differences between the financial statement carrying amounts of assets and liabilities and their respective tax bases, and for operating loss and tax credit carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the year in which the temporary differences are expected to be recovered or settled.
We evaluate the realizability of our deferred tax assets and establish a valuation allowance when it is more likely than not that all or a portion of our deferred tax assets will not be realized. In making such a determination, we consider all available positive and negative evidence, including future reversals of existing taxable temporary differences, projected future taxable income, tax planning strategies, and results of recent operations. If we determine that we would be able to realize our deferred tax assets in the future in excess of their net recorded amount, we would make an adjustment to the deferred tax asset valuation allowance, which would reduce the provision for income taxes.
We account for the uncertainty in income tax components based on tax positions taken or expected to be taken in a tax return. To recognize a benefit, a tax position must be more likely than not to be sustained upon examination by taxing authorities. We do not recognize tax benefits that have a less than 50 percent likelihood of being sustained. Our policy is to recognize interest and tax penalties related to unrecognized tax benefits in income tax expense; no interest or tax penalties on uncertain tax benefits have been recorded through December 31, 2014.
We are subject to taxation in U.S. and California jurisdictions. As of December 31, 2014, our tax years beginning December 1, 1999 remain subject to examination by taxing authorities. We are not currently under Internal Revenue Service (“IRS”), state, or local tax examination.
Stock-Based Compensation: We account for stock-based compensation by measuring and recognizing expense for all stock-based payments made to employees and directors based on estimated grant date fair values. We use the straight-line method to allocate compensation expense to reporting periods over each optionee’s requisite service period, which is generally the vesting period, and estimate the fair value of stock-based awards to employees and directors using the Black-Scholes option valuation model. The Black-Scholes model requires the input of assumptions, including volatility, the expected term, and the fair value of the underlying common stock on the date of grant, among other inputs. We record the option value to compensation expense based on the financial statement category for which an optionee’s services are rendered and cash compensation is recorded. We adjust stock-based compensation expense for estimated option forfeitures based on our five-year historical average of actual forfeitures.
We account for stock options issued to consultants as expense at their fair value over the related service period, as determined in accordance with authoritative guidance. We revalue the consultants’ stock options as they vest.
F-10
Table of Contents
REVA Medical, Inc.
Notes to Consolidated Financial Statements
3. Significant Accounting Policies (continued)
Net Loss Per Common Share: Basic net loss per common share is calculated by dividing the net loss attributable to common stockholders by the weighted average number of common shares outstanding for the period, without consideration for common stock equivalents. Diluted net loss per share is computed by dividing the net loss attributable to common stockholders by the weighted average number of common share equivalents outstanding for the period determined using the treasury-stock method and the if-converted method, as applicable. For purpose of this calculation, unvested restricted stock and stock options are considered to be common stock equivalents and are included in the calculation of diluted net loss per share only when their effect is dilutive.
During the years ended December 31, 2012, 2013, and 2014 we excluded options to purchase common stock of 3,300,039, 3,901,316, and 4,355,536 weighted average shares, respectively, and excluded 17,648, 96,347, and 91,750 weighted average shares, respectively, of restricted common stock from the computation of diluted net loss per share because including them would have been antidilutive. During the year ended December 31, 2014, we additionally excluded weighted average common share equivalents attributable to the convertible notes payable and warrants issued on November 14, 2014, of 1,513,138 and 1,150,685 shares, respectively, from the computation of diluted net loss per share as they would have been antidilutive.
Fair Value Measurements: We measure the fair value of our financial and non-financial assets and liabilities at each reporting date. Fair value is defined as the exchange price at which an asset or liability would be transferred in the principal or most advantageous market in an orderly transaction between market participants as of a measurement date. Accounting guidance provides an established hierarchy to be used in measuring fair value that maximizes the use of observable inputs and minimizes the use of unobservable inputs; observable inputs are required to be used when available. Observable inputs are those used by market participants to value an asset or liability and are developed based on market data obtained from sources independent of us. Unobservable inputs are those that reflect our assumptions about factors that market participants would use to value an asset or liability. Fair value measurements are classified and disclosed in one of the following three categories:
Level 1 — Quoted market prices for identical assets or liabilities in active markets at the measurement date;
Level 2 — Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities in active or non-active markets, or other inputs that can be corroborated by observable market data for substantially the full term of an asset or liability; and,
Level 3 — Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of an asset or liability, including management’s best estimate of the factors that market participants would use in pricing an asset or liability at the measurement date.
We carry our convertible notes payable and common stock warrant liability at fair value. We carry our other financial instruments at amortized cost; these items include cash, investments, accounts payable, and accrued expenses. The carrying amounts of our cash and cash equivalents, accounts payable, and accrued expenses are considered to be reasonable estimates of their respective fair values due to their short-term nature and, therefore, fair value information is not provided in the following table.
F-11
Table of Contents
REVA Medical, Inc.
Notes to Consolidated Financial Statements
3. Significant Accounting Policies (continued)
Fair Value Measurements (continued): Utilizing the lowest level inputs available under the measurement hierarchy, the fair values of our measured financial instruments comprise the following (we had no Level 1 financial instruments):
| | Level 2 | | Level 3 | |
| | (in thousands) | |
Fair Value at December 31, 2013: | | | | | |
Certificates of deposit due in one year or less | | $ | 1,488 | | $ | — | |
| | | | | |
Fair Value at December 31, 2014: | | | | | |
Assets: | | | | | |
Certificates of deposit due in one year or less | | $ | 991 | | $ | — | |
| | | | | |
Liabilities: | | | | | |
Convertible notes payable | | $ | — | | $ | 37,780 | |
Common stock warrant liability | | — | | 15,389 | |
| | | | | |
| | $ | — | | $ | 53,169 | |
Our Level 2 financial assets consist of certificates of deposit (“CDs”) that are held to maturity and carried at cost; their fair value is determined each reporting period through quoted market prices of similar instruments in active markets. Unrealized losses on these CDs as of December 31, 2013 and 2014 were $4,000 each year.
Our Level 3 financial liabilities consist of convertible notes payable (the “Notes”) and warrants for the purchase of common stock, all of which were issued on November 14, 2014. The fair values of these liabilities as of their issuance date and the subsequent measurement date of December 31, 2014 were determined utilizing a binomial valuation model, which requires use of unobservable inputs. The inputs are determined by management, with the assistance of independent experts; they represent our best estimates, but involve certain inherent uncertainties. We used the market value of the underlying stock, a life equal to the contractual life of the financial instrument, incremental borrowing rates and bond yields that correspond to instruments of similar credit worthiness and the instrument’s remaining life, an estimate of volatility based on the historical prices of our trading securities, and we made assumptions as to our abilities to test and commercialize our product(s), to obtain future financings when and if needed, and to comply with the terms and conditions of our Notes. A summary of the assumptions used to value the Notes and warrants at each valuation date in 2014 is as follows:
| | November 14, | | December 31, | |
| | 2014 | | 2014 | |
| | | | | |
Market price per share of common stock | | $ | 2.71 | | $ | 3.35 | |
Risk-free interest rate | | 2.89 | % | 2.30 | % |
Expected volatility of common stock | | 88.1 | % | 87.2 | % |
Expected life — years | | 5.00 | | 4.87 | |
Bond yield of equivalent securities | | 29.9 | % | 28.4 | % |
| | | | | | | |
A significant change in the market price per share, expected volatility, or bond yield of equivalent securities, in isolation, would result in significantly higher or lower fair value measurements. In combination, changes in these inputs could result in a significantly higher or lower fair value measurement if the input changes were to be aligned, or could result in a minimally higher or lower fair value measurement if the input changes were of a compensating nature.
F-12
Table of Contents
REVA Medical, Inc.
Notes to Consolidated Financial Statements
3. Significant Accounting Policies (continued)
Fair Value Measurements (continued): The fair value activity of our Level 3 financial liabilities, which were issued and arose on November 14, 2014, is as follows:
| | Level 3 | |
| | (in thousands) | |
| | | |
Balance at December 31, 2013 | | $ | — | |
| | | |
Fair Value on Issuance Date: | | | |
Convertible notes payable | | 29,689 | |
Warrants to purchase common stock | | 10,938 | |
| | | |
Balance at November 14, 2014 | | 40,627 | |
| | | |
Change in Fair Value: | | | |
Convertible notes payable | | 8,091 | |
Warrants to purchase common stock | | 4,451 | |
| | | |
Balance at December 31, 2014 | | $ | 53,169 | |
Recent Accounting Pronouncements: Effective January 1, 2014, we adopted Accounting Standards Update No. 2013-11 (“ASU 2013-11”), Presentation of an Unrecognized Tax Benefit When a Net Operating Loss Carryforward, a Similar Tax Loss, or a Tax Credit Carryforward Exists. ASU 2013-11 provides explicit guidance on the financial statement presentation of an unrecognized tax benefit when a net operating loss carryforward, a similar tax loss, or a tax credit carryforward exists. The adoption of ASU 2013-11 did not have an effect on our financial position, results of operations, or related financial statement disclosures.
In April 2014, ASU 2014-08, Reporting Discontinued Operations and Disclosures of Disposals of Components of an Entity, was issued. ASU 2014-08 raises the threshold for a disposal to qualify as a discontinued operation and requires new disclosures for certain other disposals that do not meet the definition of a discontinued operation. This ASU is effective for REVA beginning January 1, 2015; we do not expect the implementation to have an effect on our financial position or results of operations.
In June 2014, ASU 2014-10, Development Stage Entities: Elimination of Certain Financial Reporting Requirements, was issued. ASU 2014-10 removes financial reporting distinction between development stage entities and other reporting entities. Although ASU 2014-10 is effective beginning January 1, 2015, we elected early adoption for the year ended December 31, 2014. The adoption of ASU 2014-10 did not have an effect on our financial position or results of operations. Upon adoption, we conformed our financial statement presentation and disclosures and, accordingly, eliminated all references to “development stage” and discontinued presentation of “inception to date” information.
In August 2014, ASU 2014-15, Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern, was issued. ASU 2014-15 requires management to assess an entity’s ability to continue as a going concern and to provide related footnote disclosure in certain circumstances. This ASU is effective for annual and interim periods ending after December 15, 2016. We are currently evaluating the provisions of ASU 2014-15 and assessing the impact, if any, it may have on our financial disclosures.
F-13
Table of Contents
REVA Medical, Inc.
Notes to Consolidated Financial Statements
4. Convertible Notes Payable and Warrants to Purchase Common Stock
On November 14, 2014, we issued 250 convertible notes payable (the “Notes”), each with a face value of $100,000, for total cash proceeds of $25,000,000. The Notes are convertible into 11,506,155 shares of common stock, which is a conversion rate of $2.17275 per share. The Notes are convertible at any time at the holders’ election, except the Notes will automatically convert in the case where the Company has received a CE Mark approval for its Fantom product and has sustained a market trading price of A$0.60 per CDI for 20 consecutive trading days. The Notes mature on November 14, 2019, if not converted or redeemed earlier. Interest accrues on the Notes at the rate of 7.54 percent per annum, compounded annually, and is payable upon redemption or maturity; accrued interest is not payable or convertible upon conversion of the Notes. Interest expense of $248,000 for the year ended December 31, 2014 was recorded in the consolidated statement of operations. The Notes provide the holders a one-time option for cash redemption in January 2017, if not previously converted or redeemed, for the face value plus accrued interest.
On the issue date, we evaluated the embedded conversion feature of the Notes and certain other rights provided to the noteholders and determined that they qualified as embedded derivatives that required bifurcation from the Notes and separate accounting. Following this evaluation, we made an irrevocable election to account for the Notes at fair value. The fair value of the Notes on the date of issue was calculated to be $29,689,000. This fair value exceeded the stated value of the Notes by $4,689,000; we recorded the excess as a loss on issuance. The fair value of the Notes as of December 31, 2014 was calculated to be $37,780,000, which was $12,780,000 more than the unpaid principal balance of the Notes. The change in fair value of the Notes between November 14, 2014 and December 31, 2014 of $8,091,000 was recorded as a loss in the consolidated statement of operations. As of December 31, 2014, the fair value of the 11,506,155 shares into which the Notes are convertible was calculated to be $38,200,000.
In connection with issuing the Notes, we issued warrants to the noteholders to purchase up to 8,750,000 shares of common stock at $2.17275 per share. The warrants are exercisable immediately and expire in November 2019. The exercise price of the warrants will increase to $2.6073 per share if, and when, we achieve full enrollment in our clinical trial of Fantom with the required number of patients that would provide data for a CE Mark application. The fair value of the warrants on the date of issue of $10,938,000 was recorded as a loss on issuance since we elected fair value accounting for the Notes. The fair value of the warrants as of December 31, 2014 was calculated to be $15,389,000; the change in fair value of the warrant liability between November 14, 2014 and December 31, 2014 of $4,451,000 was recorded as a loss in the consolidated statement of operations.
5. Balance Sheet Details
| | December 31, | |
| | 2013 | | 2014 | |
| | (in thousands) | |
Property and equipment: | | | | | |
Furniture, office equipment, and software | | $ | 656 | | $ | 648 | |
Laboratory equipment | | 4,896 | | 5,187 | |
Leasehold improvements | | 2,305 | | 2,361 | |
| | | | | |
| | 7,857 | | 8,196 | |
Accumulated depreciation and amortization | | (4,268 | ) | (5,276 | ) |
| | | | | |
| | $ | 3,589 | | $ | 2,920 | |
| | | | | |
Accrued expenses and other current liabilities: | | | | | |
Accrued salaries and other employee costs | | $ | 1,371 | | $ | 1,315 | |
Accrued operating expenses | | 560 | | 769 | |
Accrued use taxes and other | | 149 | | 129 | |
| | | | | |
| | $ | 2,080 | | $ | 2,213 | |
F-14
Table of Contents
REVA Medical, Inc.
Notes to Consolidated Financial Statements
6. Income Taxes
We have reported net losses for all periods through December 31, 2014; therefore, no provision for income taxes has been recorded. The following table provides the reconciliation between income taxes computed at the federal statutory rate and our provision for income taxes:
| | Year Ended December 31, | |
| | 2012 | | 2013 | | 2014 | |
| | (in thousands) | |
| | | | | | | |
Federal income taxes at 34% | | $ | (8,084 | ) | $ | (9,493 | ) | $ | (17,352 | ) |
State income taxes, net of federal benefit | | (1,363 | ) | (1,553 | ) | (1,243 | ) |
Research and development credits | | (240 | ) | (1,425 | ) | (660 | ) |
Fair value adjustments on convertible notes payable and common stock warrant liability | | — | | — | | 9,577 | |
Interest on convertible notes payable | | — | | — | | 152 | |
Stock-based compensation expense | | 131 | | 191 | | 358 | |
Increase in valuation allowance | | 59,186 | | 11,622 | | 8,716 | |
Reinstatement of deferred tax assets for net operating loss and tax credit carryover deferred tax assets | | (50,311 | ) | — | | — | |
Expiration of state net operating losses | | 673 | | 677 | | 450 | |
Other | | 8 | | (19 | ) | 2 | |
| | | | | | | |
Provision for income taxes | | $ | — | | $ | — | | $ | — | |
Our deferred income taxes reflect the net tax effects of temporary differences between the carrying amount of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of our deferred tax assets and liabilities are as follows:
| | December 31, | |
| | 2013 | | 2014 | |
| | (in thousands) | |
Deferred Tax Assets: | | | | | |
Net operating loss carryforwards | | $ | 61,629 | | $ | 62,738 | |
Research and development credits | | 6,277 | | 6,891 | |
Amortization | | 2 | | 5,737 | |
Stock-based compensation expense | | 4,182 | | 5,085 | |
Depreciation | | 230 | | 366 | |
Accrued operating expenses | | 12 | | 64 | |
Other | | 291 | | 459 | |
| | | | | |
| | 72,623 | | 81,340 | |
Valuation Allowance | | (72,623 | ) | (81,340 | ) |
| | | | | |
Net Deferred Income Taxes | | $ | — | | $ | — | |
As of December 31, 2014 we had aggregate federal and California state net operating loss carryforwards of approximately $162,797,000 and $128,411,000, respectively, which may be available to offset future taxable income for income tax purposes. The federal net operating loss carryforwards begin to expire in 2019 and the California carryforwards begin to expire in 2015, with $11,869,000 expiring in 2015.
F-15
Table of Contents
REVA Medical, Inc.
Notes to Consolidated Financial Statements
As of December 31, 2014, we also had federal and California state research tax credit carryforwards of approximately $5,797,000 and $5,137,000, respectively. The federal carryforwards begin to expire in 2020 and the California carryforwards have no expiration.
6. Income Taxes (continued)
A total of $267,000 of the federal and California net operating loss relates to excess tax benefits generated from stock compensation that will be recorded as an increase to additional paid-in capital if, and when, realized.
Under Internal Revenue Code (“IRC”) Sections 382 and 383, annual use of our net operating loss and research tax credit carryforwards to offset taxable income may be limited based on cumulative changes in ownership. An analysis of the impact of this provision from December 1, 1999 through December 31, 2014 has been performed and it was determined that, although ownership changes had occurred, the carryovers should be available for utilization by the Company before they expire, provided we generate sufficient future taxable income. Based on the results of this analysis in 2012, we reinstated the deferred tax assets arising from the net operating loss and tax credit carryforwards, with a corresponding increase to the valuation allowance for the year ended December 31, 2012. Future ownership changes could result in further limitations and may impact the realizability of these loss and credit carryforwards in future periods.
As of December 31, 2014, we had deferred tax assets of $81,340,000 primarily comprising net operating loss and research tax credit carryforwards. We have established a valuation allowance against our deferred tax assets due to the uncertainty surrounding the Company’s ability to generate future taxable income to realize those assets. The change in the valuation allowance for the years ended December 31, 2013 and 2014 was $11,622,000 and $8,717,000, respectively.
We recognize a tax benefit from an uncertain tax position when it is more likely than not that the position will be sustained upon examination, including resolutions of any related appeals or litigation processes, based on the technical merits. Income tax positions must meet a more-likely-than-not recognition at the effective date to be recognized. As of December 31, 2014, the unrecognized tax benefits recorded were approximately $2,734,000. We do not anticipate a significant change in the unrecognized tax benefits within the next 12 months.
A reconciliation of the beginning and ending amount of gross unrecognized tax benefits for 2013 and 2014, excluding interest and penalties, is as follows:
| | December 31, | |
| | 2013 | | 2014 | |
| | (in thousands) | |
| | | | | |
Balance at Beginning of Year | | $ | 1,954 | | $ | 2,490 | |
Additions (reductions) for prior year tax positions | | 167 | | (15 | ) |
Additions for current year tax positions | | 369 | | 259 | |
| | | | | |
Balance at End of Year | | $ | 2,490 | | $ | 2,734 | |
Due to our valuation allowance position, none of the unrecognized tax benefits, if recognized, will impact the Company’s effective tax rate.
F-16
Table of Contents
REVA Medical, Inc.
Notes to Consolidated Financial Statements
7. Stock-Based Compensation
Our 2010 Equity Incentive Award Plan was a follow-on to our 2001 Stock Option/Stock Issuance Plan and the two plans are collectively referred to as the “Plan.” The Plan provides for restricted stock awards as well as for grants of incentive and non-qualified stock options to purchase our common stock at a price per share equal to the closing market price of our stock on the date of option grant. All stock issuances under the plan are made with new shares. The number of shares reserved under the Plan may be increased annually by up to three percent of the outstanding stock of the Company. On January 1, 2014, an additional 998,101 shares were added, resulting in a total of 7,256,260 shares reserved under the Plan as of December 31, 2014. The term of the options granted under the Plan may not exceed ten years. Vesting periods of stock awards and option grants are determined by the Company’s board of directors and are generally four- or five-year periods. All options are immediately exercisable upon grant and are subject to repurchase by us at the exercise price in the event an optionee terminates service prior to being fully vested.
Option activity under the Plan is as follows:
| | | | | | Weighted | | | |
| | | | Weighted | | Average | | | |
| | | | Average | | Remaining | | Aggregate | |
| | Options | | Exercise | | Contractual | | Intrinsic | |
| | Outstanding | | Price | | Term (years) | | Value | |
| | | | | | | | | |
Balance at December 31, 2011 | | 3,304,000 | | $ | 6.99 | | | | | |
Granted | | 544,000 | | $ | 5.95 | | | | | |
Cancelled | | (9,300 | ) | $ | 12.64 | | | | | |
Exercised | | (288,700 | ) | $ | 1.11 | | | | | |
| | | | | | | | | |
Balance at December 31, 2012 | | 3,550,000 | | $ | 7.30 | | | | | |
Granted | | 589,500 | | $ | 5.36 | | | | | |
Cancelled | | (42,500 | ) | $ | 2.00 | | | | | |
Exercised | | (50,350 | ) | $ | 0.61 | | | | | |
| | | | | | | | | |
Balance at December 31, 2013 | | 4,046,650 | | $ | 7.15 | | | | | |
Granted | | 637,000 | | $ | 3.53 | | | | | |
Cancelled | | (180,500 | ) | $ | 6.61 | | | | | |
Exercised | | (259,725 | ) | $ | 0.95 | | | | | |
| | | | | | | | | |
Balance at December 31, 2014 | | 4,243,425 | | $ | 7.01 | | 6.43 | | $ | 1,659,000 | |
| | | | | | | | | |
Vested at December 31, 2014 | | 3,164,746 | | $ | 7.78 | | 5.48 | | $ | 1,604,000 | |
| | | | | | | | | |
Vested and Expected to Vest at December 31, 2014 | | 4,218,131 | | $ | 7.02 | | 6.19 | | $ | 1,657,000 | |
The unvested portion of outstanding options as of December 31, 2014 has vesting dates scheduled through 2018. Following is the vesting activity under the Plan for the year ended December 31, 2014:
| | | | Weighted | |
| | | | Average | |
| | Options | | Grant Date | |
| | Outstanding | | Fair Value | |
| | | | | |
Unvested Options at December 31, 2013 | | 1,308,149 | | $ | 4.20 | |
Granted | | 637,000 | | $ | 1.89 | |
Vested | | (795,709 | ) | $ | 4.46 | |
Forfeited | | (70,761 | ) | $ | 2.82 | |
| | | | | |
Unvested Options at December 31, 2014 | | 1,078,679 | | $ | 2.64 | |
F-17
Table of Contents
REVA Medical, Inc.
Notes to Consolidated Financial Statements
7. Stock-Based Compensation (continued)
We awarded 33,000 and 87,500 shares of restricted stock during the years ended December 31, 2012 and 2013, respectively, all of which vest at the rate of 25 percent annually on each award anniversary date. No restricted stock was awarded during the year ended December 31, 2014.
No tax benefits arising from stock-based compensation have been recognized in the consolidated statements of operations through December 31, 2014.
Stock Options and Restricted Stock to Employees: We account for option grants and restricted stock awards to employees based on the estimated fair values on the date of grant or award, with the resulting stock-based compensation recorded over the vesting period on a straight-line basis. We include non-employee directors as employees for this purpose.
Expense recorded for employee options and awards under the Plan is as follows:
| | Year Ended December 31, | |
| | 2012 | | 2013 | | 2014 | |
| | (in thousands) | |
| | | | | | | |
Research and development | | $ | 832 | | $ | 1,069 | | $ | 1,142 | |
General and administrative | | 2,647 | | 2,965 | | 2,284 | |
| | | | | | | |
Total stock-based compensation | | $ | 3,479 | | $ | 4,034 | | $ | 3,426 | |
As of December 31, 2014, we had approximately $2,234,000 of total unrecognized compensation costs related to unvested employee options that are expected to be recognized over a weighted average period of 1.63 years.
The fair value of options granted was estimated using the following weighted-average assumptions:
| | Year Ended December 31, | |
| | 2012 | | 2013 | | 2014 | |
| | | | | | | |
Risk-free interest rate | | 1.03 | % | 1.38 | % | 2.18 | % |
Expected volatility of common stock | | 62.1 | % | 60.1 | % | 59.3 | % |
Expected life in years | | 6.25 | | 6.25 | | 6.14 | |
Dividend yield | | 0 | % | 0 | % | 0 | % |
The assumed risk-free interest rate was based on the implied yield on a U.S. Treasury zero-coupon issue with a remaining term equal to the expected life of the option. The assumed volatility was calculated from the historical market prices of a selected group of publicly traded companies considered to be our peers. We used peer group data due to the fact that we have limited historical trading data. The expected option life was calculated using the simplified method under the accounting standard for stock compensation and a ten-year option expiration. The simplified method is used since we believe our future option activity as a public company will differ from that of our own historical experience. The expected dividend yield of zero reflects that we have not paid cash dividends since inception and do not intend to pay cash dividends in the foreseeable future.
F-18
Table of Contents
REVA Medical, Inc.
Notes to Consolidated Financial Statements
7. Stock-Based Compensation (continued)
A summary of grant date fair value and intrinsic value for options granted to employees is as follows:
| | Year Ended December 31, | |
| | 2012 | | 2013 | | 2014 | |
| | (in thousands, except per share data) | |
| | | | | | | |
Weighted average grant date fair value per share | | $ | 3.43 | | $ | 2.95 | | $ | 1.98 | |
Intrinsic value of options exercised | | $ | 1,392 | | $ | 231 | | $ | 553 | |
Total fair value of options vested during period | | $ | 3,802 | | $ | 3,809 | | $ | 3,546 | |
Stock Options to Consultants: We account for stock options granted to consultants at their fair value. Under this method, the fair value is estimated at each reporting date during the vesting period using the Black-Scholes option-pricing model. The resulting stock-based compensation is recorded over the consultant’s service period. No options were issued to consultants during 2012; options to purchase 100,000 and 110,000 shares of common stock were granted to consultants during the years ended December 31, 2013 and 2014, respectively. The fair value of these awards was determined with the following assumptions: Assumed risk-free interest rate of 1.7 to 2.8 percent; assumed volatility of 57 to 59 percent; expected option life of 5.0 to 10.0 years; and, expected dividend yield of zero percent. The total fair value of consultant options vested during 2012, 2013 and 2014 was $42,000, $40,000, and $116,000, respectively. The weighted average fair value of unvested consultant options at December 31, 2012, 2013, and 2014 was estimated to be $4.37, $2.84, and $1.92 per share, respectively, based on the following assumptions:
| | Year Ended December 31, | |
| | 2012 | | 2013 | | 2014 | |
| | | | | | | |
Risk-free interest rate | | 1.18 | % | 2.96 | % | 2.17 | % |
Expected volatility of common stock | | 62.1 | % | 59.4 | % | 57.2 | % |
Expected life — years | | 6.71 | | 9.45 | | 8.94 | |
Dividend yield | | 0.0 | % | 0.0 | % | 0.0 | % |
Consultant stock-based compensation expense, or income if the fair value declined in a reporting period, is recorded to the financial statement line item for which the optionee’s services are rendered. Expense recorded for consultant stock options under the Plan is as follows:
| | Year Ended December 31, | |
| | 2012 | | 2013 | | 2014 | |
| | (in thousands) | |
| | | | | | | |
Research and development | | $ | 18 | | $ | 9 | | $ | 69 | |
General and administrative | | — | | 47 | | 21 | |
| | | | | | | |
Total stock-based compensation | | $ | 18 | | $ | 56 | | $ | 90 | |
8. Retirement Plan
In 2003 we adopted a qualified 401(k) profit sharing plan (the “401(k) Plan”) for the benefit of our employees. Employees are eligible to participate in the 401(k) Plan the month following hire and may defer up to the maximum allowed under IRS regulations, on an annual basis. We match 25 percent of an employee’s deferral amount, up to a maximum of four percent of qualified compensation. We may, at our discretion, make additional contributions. Employees are immediately vested in the employer matching contributions. Our contributions to the 401(k) Plan were $46,000, $52,000, and $46,000 for the years ended December 31, 2012, 2013, and 2014, respectively.
F-19
Table of Contents
REVA Medical, Inc.
Notes to Consolidated Financial Statements
9. Commitments and Contingencies
We have licensed certain patents and other intellectual property rights related to the composition and coating of our bioresorbable stent and our other biomaterial products. Terms of these licenses include provisions for royalty payments on any future sales of products, if any, utilizing this technology, with provisions for minimum royalties once product sales begin. The amount of royalties varies depending upon type of product, use of product, stage of product, location of sale, and ultimate sales volume, and ranges from a minimum of approximately $25 per unit to a maximum of approximately $100 per unit sold, with license provisions for escalating minimum royalties that could be as high as $2,200,000 per year. Additionally, in the event we sublicense the technology and receive certain milestone payments, the licenses require that up to 40 percent of the milestone amount be paid to the licensors. Additional terms of the technology licenses include annual licensing payments of $175,000 until the underlying technology has been commercialized. Terms of the licenses also include other payments to occur during commercialization that could total $950,000, payment of $350,000 upon a change in control of ownership, payments of up to $300,000 annually to extend filing periods related to certain technology, and payment of patent filing, maintenance, and defense fees. The license terms remain in effect until the last patent expires.
In connection with our development activities, we periodically enter into contracts with consultants and vendors. These contracts are generally cancelable with 30 days’ written notice. As of December 31, 2014, the minimum future payments on these contracts totaled approximately $108,000.
We currently lease our office and lab facilities under a non-cancelable operating lease that expires in January 2018. The lease contains fixed annual escalations, an option for a five-year extension, leasehold improvement allowances and credits of $523,000, and rent abatements of $136,000. We record rent expense on a straight-line basis over the life of the lease; the difference between average rent expense and cash payments for rent is recorded as a deferred liability. As of December 31, 2014, our deferred rent totaled $480,000, of which $117,000 was classified as a current liability. We recorded rent expense of $636,000, $666,000, and $683,000 for the years ended December 31, 2012, 2013, and 2014, respectively.
Future minimum payments under the lease as of December 31, 2014 are as follows:
| | Minimum | |
| | Payment | |
| | (in thousands) | |
| | | |
2015 | | $ | 644 | |
2016 | | 690 | |
2017 | | 711 | |
2018 | | 60 | |
| | | |
Total minimum lease payments | | $ | 2,105 | |
10. Related Parties
Our related parties include the members of our board of directors and investors with five percent or more of our outstanding securities. We had no related party transactions during the years ended December 31, 2012, 2013, or 2014.
F-20
Table of Contents
REVA Medical, Inc.
Notes to Consolidated Financial Statements
11. Selected Quarterly Financial Information (unaudited)
The following table presents selected quarterly financial information that has been derived from our unaudited quarterly consolidated financial statements, which, in the opinion of management, include all adjustments (consisting only of normal recurring items) necessary for a fair presentation. The quarterly per share data presented below was calculated separately and may not sum to the annual figures presented in the consolidated financial statements. These operating results are also not necessarily indicative of results for any future period.
| | Quarter Ended | | Year Ended | |
| | March 31, | | June 30, | | September 30, | | December 31, | | December 31, | |
| | (in thousands, except per share amounts) | |
| | | | | | | | | | | |
2013: | | | | | | | | | | | |
Loss from operations | | $ | (6,343 | ) | $ | (6,666 | ) | $ | (7,182 | ) | $ | (7,752 | ) | $ | (27,943 | ) |
Net loss | | (6,331 | ) | (6,647 | ) | (7,191 | ) | (7,753 | ) | (27,922 | ) |
Net loss per common share, basic and diluted | | $ | (0.19 | ) | $ | (0.20 | ) | $ | (0.22 | ) | $ | (0.23 | ) | $ | (0.84 | ) |
| | | | | | | | | | | |
2014: | | | | | | | | | | | |
Loss from operations | | $ | (7,272 | ) | $ | (4,832 | ) | $ | (4,446 | ) | $ | (5,413 | ) | $ | (21,963 | ) |
Net loss | | (7,276 | ) | (4,833 | ) | (4,397 | ) | (34,531 | ) | (51,037 | ) |
Net loss per common share, basic and diluted | | $ | (0.22 | ) | $ | (0.14 | ) | $ | (0.13 | ) | $ | (1.03 | ) | $ | (1.53 | ) |
F-21
Table of Contents
INDEX TO EXHIBITS
| | | | Filed | | | | | | |
Exhibit | | | | with this
Form | | Incorporated by Reference |
Number | | Description of Exhibits | | 10-K | | Form | | File No. | | Date Filed |
3.1 | | Amended and Restated Certificate of Incorporation | | | | S-1/A | | 333-168852 | | 10/22/2010 |
3.2 | | Amended and Restated Bylaws | | | | S-1/A | | 333-168852 | | 10/22/2010 |
4.1 | | Form of Stock Certificate | | | | S-1/A | | 333-168852 | | 11/12/2010 |
4.2 | | Form of Amended and Restated Investors’ Rights Agreement, by and among REVA Medical, Inc. and holders of our common stock and convertible notes payable set forth therein | | | | DEF14A | | 000-54192 | | 10/14/2014 |
10.1 | | Telecom Business Center Business Lease between FSP Telecom Business Center Limited Partnership and REVA Medical, Inc. dated December 18, 2001 | | | | S-1 | | 333-168852 | | 8/13/2010 |
10.2 | | First Amendment to Telecom Business Center Business Lease between FSP Telecom Business Center Limited Partnership and REVA Medical, Inc. dated January 3, 2005 | | | | S-1 | | 333-168852 | | 8/13/2010 |
10.3 | | Second Amendment to Telecom Business Center Business Lease between ARI Commercial Properties, Inc. and REVA Medical, Inc. dated February 18, 2006 | | | | S-1 | | 333-168852 | | 8/13/2010 |
10.4 | | Third Amendment to Telecom Business Center Business Lease between ARI Commercial Properties, Inc. and REVA Medical, Inc. dated December 14, 2006 | | | | S-1 | | 333-168852 | | 8/13/2010 |
10.5 | | Fourth Amendment to Telecom Business Center Business Lease between ARI Commercial Properties, Inc. and REVA Medical, Inc. dated May 7, 2008 | | | | S-1 | | 333-168852 | | 8/13/2010 |
10.6 | | Fifth Amendment to Telecom Business Center Business Lease between ARI Commercial Properties, Inc. and REVA Medical, Inc. dated for reference purposes only as of August 28, 2011, executed and delivered on November 21, 2011 | | | | 8-K | | 000-54192 | | 11/23/2011 |
10.7 | | Distribution Option Agreement, dated December 7, 2007, by and between REVA Medical, Inc. and Boston Scientific Corporation | | | | S-1/A | | 333-168852 | | 10/22/2010 |
10.8 | | First Amendment to Distribution Option Agreement, dated February 12, 2014, by and between REVA Medical, Inc. and Boston Scientific Corporation | | | | 10-K | | 000-54192 | | 3/17/2014 |
10.9 | | Exclusive License Agreement Number 2 between Rutgers, The State University of New Jersey and REVA Medical, Inc. dated July 1, 2010** | | | | S-1/A | | 333-168852 | | 9/21/2010 |
10.10 | | Amendment #2 to Exclusive License Agreement Number 2 between Rutgers, The State University of New Jersey and REVA Medical, Inc. effective July 1, 2010** | | | | 10-Q | | 000-54192 | | 11/6/2014 |
10.11 | | Royalty and License Agreement between Integra/LifeSciences Corporation and REVA Medical, Inc. dated February 2, 2004** | | | | S-1/A | | 333-168852 | | 9/21/2010 |
10.12 | | 2001 Stock Option/Stock Issuance Plan* | | | | S-1 | | 333-168852 | | 8/13/2010 |
10.13 | | Form of Stock Option Agreement* | | | | S-1 | | 333-168852 | | 8/13/2010 |
10.14 | | Form of Addendum to Stock Option Agreement* | | | | S-1 | | 333-168852 | | 8/13/2010 |
10.15 | | 2010 Equity Incentive Plan* | | | | S-1/A | | 333-168852 | | 10/22/2010 |
10.16 | | Form of Stock Option Agreement* | | | | S-1/A | | 333-168852 | | 11/12/2010 |
10.17 | | Form of Stock Option Agreement entered into with Robert Thomas and Anne Keating* | | | | S-1/A | | 333-168852 | | 11/12/2010 |
10.18 | | Form of Director and Officer Indemnification Agreement* | | | | S-1 | | 333-168852 | | 8/13/2010 |
10.19 | | Director Compensation policy | | | | 10-K | | 000-54192 | | 3/17/2014 |
10.20 | | Employment Agreement, dated July 1, 2010, by and between REVA Medical, Inc. and Robert B. Stockman* | | | | S-1/A | | 333-168852 | | 10/22/2010 |
Table of Contents
| | | | Filed | | | | | | |
Exhibit | | | | with this
Form | | Incorporated by Reference |
Number | | Description of Exhibits | | 10-K | | Form | | File No. | | Date Filed |
10.21 | | Employment Agreement, dated October 21, 2010, by and between REVA Medical, Inc. and Robert Schultz* | | | | S-1/A | | 333-168852 | | 11/12/2010 |
10.22 | | Employment Agreement, dated October 21, 2010, by and between REVA Medical, Inc. and Katrina Thompson* | | | | S-1/A | | 333-168852 | | 11/12/2010 |
10.23 | | Employment Agreement, dated February 22, 2011, by and between REVA Medical, Inc. and Jeffrey Anderson* | | | | 10-K | | 000-54192 | | 3/17/2014 |
10.24 | | Convertible Note Deed dated September 25, 2014 by and between REVA Medical, Inc. and the holders of our convertible notes payable set forth therein | | | | DEF14A | | 000-54192 | | 10/14/2014 |
21.1 | | List of Subsidiaries | | | | S-1 | | 333-168852 | | 8/13/2010 |
23.1 | | Consent of Grant Thornton LLP Independent Registered Public Accounting Firm | | X | | | | | | |
23.2 | | Consent of Ernst & Young LLP Independent Registered Public Accounting Firm | | X | | | | | | |
31.1 | | Certification of Principal Executive Officer Required Under Rule 13a-14(a) and 15d-14(a) of the Securities Exchange Act of 1934, as amended | | X | | | | | | |
31.2 | | Certification of Principal Financial Officer Required Under Rule 13a-14(a) and 15d-14(a) of the Securities Exchange Act of 1934, as amended | | X | | | | | | |
32.1 (1) | | Certification of Principal Executive Officer and Principal Financial Officer Required Under Rule 13a-14(b) of the Securities Exchange Act of 1934, as amended, and 18 U.S.C. §1350 | | X | | | | | | |
99.1 | | Section 13 of the ASX Settlement Rules | | | | S-1/A | | 333-168852 | | 10/22/2010 |
101.INS | | XBRL Instance Document | | X | | | | | | |
101.SCH | | XBRL Taxonomy Extension Schema Document | | X | | | | | | |
101.CAL | | XBRL Calculation Linkbase Document | | X | | | | | | |
101.DEF | | XBRL Taxonomy Definition Linkbase Document | | X | | | | | | |
101.LAB | | XBRL Taxonomy Label Linkbase Document | | X | | | | | | |
101.PRE | | XBRL Taxonomy Presentation Linkbase Document | | X | | | | | | |
* Management Compensatory Plan or Arrangement
** Confidential treatment has been granted with respect to certain portions of this exhibit.
(1) These certifications are being furnished solely to accompany this annual report pursuant to 18 U.S.C. Section 1350, and are not being filed for purposes of Section 18 of the Securities Exchange Act of 1934 and are not to be incorporated by reference into any filing of REVA Medical, Inc., whether made before or after the date hereof, regardless of any general incorporation language in such filing.
