Exhibit 99.1

AGM Presentation
Sydney, Australia and San Diego, California (Thursday 30 May 2013 AEST)– REVA Medical, Inc. (ASX: RVA) (“REVA” or the “Company”) is pleased to provide the attached presentation that will be made at the Company’s 2013 Annual General Meeting of Stockholders (the “AGM”) following the conclusion of the formal business of the meeting.
The AGM is being held on Thursday 30 May 2013 at 10:30 a.m. AEST (which is 5:30 p.m. on Wednesday, May 29, 2013 U.S. PDT) at the AGL Theatre in the Museum of Sydney at the corner of Phillip and Bridge Streets in Sydney, Australia.
The AGM will be audiocast and may be accessed within Australia by calling (02) 8223 9876 five minutes prior to the start time. Callers within the United States and Canada may access the audiocast by dialing 1-877-312-5413. If you are asked to provide an access code, please spell out the word “REVA” to the operator and you will be connected promptly.
The call-in information and the presentation to be made at the meeting are available on our website atwww.revamedical.com. A replay of the audiocast will be available on our website after the meeting.
About REVA
REVA is a development stage medical device company incorporated in Delaware, USA, that is focused on the development and eventual commercialization of its proprietary bioresorbable stent products. TheReZolve® product family, which is in a clinical study phase, combines REVA’s proprietary stent design with a proprietary polymer that is metabolized and cleared from the body. REVA’s anticipated commercial product, theReZolve2 scaffold, is designed to offer full x-ray visibility, clinically relevant sizing, and a controlled and safe resorption rate. In addition, by early encapsulation of the stent in the artery tissue coupled with the loss of scaffold structure over time, theReZolve2 scaffold may reduce the incidence of late forming blood clots or otherwise reduce long-term disease progression, potential benefits of bioresorbable scaffolds that have yet to be proven. REVA will require clinical results and regulatory approval before it can begin selling theReZolve2 scaffold.
Forward-Looking Statements
This announcement contains or may contain forward-looking statements that are based on management’s beliefs, assumptions and expectations and on information currently available to management. All statements that are not
HEAD OFFICE: 5751 Copley Drive, San Diego, CA 92111 • +1 (858) 966-3000 • +1 (858) 966-3099 (FAX) • www.revamedical.com
AUSTRALIAN OFFICE: Level 6, 175 Macquarie Street, Sydney, NSW 2000 • +61 2 9231 3322 • +61 9229 2727 (FAX) • ARBN 146 505 777
REVA Medical, Inc., is a foreign company incorporated in Delaware, USA, whose stockholders have limited liability.
| | |
| REVA Medical, Inc. – ASX Announcement | | Page 2 |
statements of historical fact, including those statements that address future operating performance and events or developments that we expect or anticipate will occur in the future, are forward-looking statements, such as those statements regarding our ability to obtain the regulatory approvals required to market our ReZolve scaffold, our ability to timely and successfully complete our clinical trials, our ability to protect our intellectual property position, our ability to commercialize our products if and when approved, our ability to develop and commercialize new products, and our estimates regarding our capital requirements and financial performance, including profitability. You should not place undue reliance on these forward-looking statements. Although management believes these forward-looking statements are reasonable as and when made, forward-looking statements are subject to a number of risks and uncertainties that may cause our actual results to vary materially from those expressed in the forward-looking statements, which risks and uncertainties are described in the “Risk Factors” section of our Annual Report on Form 10-K filed with the United States Securities and Exchange Commission (the “SEC”) on 28 February 2013. Any forward-looking statements in this announcement speak only as of the date when made. REVA does not assume any obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events, or otherwise.
| | |
United States Investor and Media Enquiries: Cheryl Liberatore Director, Investor Relations and Marketing REVA Medical, Inc. +1 858 966-3045 | | Australia Investor Enquiries: Kim Jacobs Inteq Limited +61 2 9231 3322 Media Enquiries: Haley Price or Rebecca Wilson Buchan Consulting +61 3 9866 4722 |
HEAD OFFICE: 5751 Copley Drive, San Diego, CA 92111 • +1 (858) 966-3000 • +1 (858) 966-3099 (FAX) • www.revamedical.com
AUSTRALIAN OFFICE: Level 6, 175 Macquarie Street, Sydney, NSW 2000 • +61 2 9231 3322 • +61 9229 2727 (FAX) • ARBN 146 505 777
REVA Medical, Inc., is a foreign company incorporated in Delaware, USA, whose stockholders have limited liability.

REVA
2013 Annual General Meeting
10:30 am, 30 May 2013
Sydney, Australia

Important Notice
Not an Offer for Securities
This presentation has been prepared by REVA Medical, Inc. (“REVA” or the “Company”) solely for its use. This presentation does not constitute an offer, invitation, solicitation, or recommendation with respect to the purchase or sale of any security in the Company nor does it constitute financial product advice nor take into account your investment objectives, taxation situation, financial situation, or needs. Investors must not act on the basis of any matter contained in this presentation but must make their own assessment of the Company and conduct their own investigations and analysis.
Information is a Synopsis Only
This presentation only contains a synopsis of information on the Company and, accordingly, no reliance may be placed for any purpose whatsoever on the sufficiency or completeness of such information. Information presented in this presentation is subject to change without notice and REVA does not have any responsibility or obligation to inform you of any matter arising or coming to their notice after the date of this presentation, which may affect any matter in the presentation.
Currency References
Financial amounts in this presentation are expressed in US Dollars, except where specifically noted.
Forward-Looking Statements
This presentation contains or may contain forward-looking statements that are based on management’s beliefs, assumptions, and expectations and on information currently available to management. All statements that are not statements of historical fact, including those statements that address future operating performance and events or developments that we expect or anticipate will occur in the future, are forward-looking statements, such as those statements regarding our ability to obtain the regulatory approvals required to market our products, our ability to timely and successfully complete our clinical trials, our ability to protect our intellectual property position, our ability to commercialize our products if and when approved, our ability to develop and commercialize new products, and our estimates regarding our capital requirements and financial performance, including profitability. You should not place undue reliance on these forward-looking statements. Although management believes these forward-looking statements are reasonable as and when made, forward-looking statements are subject to a number of risks and uncertainties that may cause actual results to vary materially from those expressed in the forward-looking statements, which risks and uncertainties are described in the “Risk Factors” section of our Annual Report on Form 10-K filed with the US Securities and Exchange Commission (the “SEC”) on 28 February 2013. Any forward-looking statement in this presentation speak only as of the date when made. REVA does not assume any obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events, or otherwise.
Disclaimer
This presentation has been prepared by the Company based on available information. The information contained in this presentation is an overview and does not contain all information necessary to make an investment decision. Although reasonable care has been taken to ensure the facts stated in this presentation are accurate and that the opinions expressed are fair and reasonable, no representation or warranty, express or implied, is made as to the fairness, accuracy, completeness, or correctness of such information and opinions and no reliance should be placed on such information or opinions. To the maximum extent permitted by law, none of the Company, or any of its members, directors, officers, employees, or agents or advisers, nor any other person accepts any liability whatsoever for any loss, however arising, from the use of the presentation or its contents or otherwise arising in connection with it, including, without limitation, any liability arising from fault or negligence on the part of the Company or any of its directors, officers, employees, or agents.
1 2013 REVA Medical, Inc.
REVA

Introductions
• Board of Directors
– Robert B. Stockman, Chairman/CEO
– Brian H. Dovey
– Anne Keating
– Gordon E. Nye
– James J. Schiro
– Robert Thomas
• Officers
– Robert K. Schultz, PhD, COO
– Katrina L. Thompson, CFO
– Jeffrey A. Anderson, VP
• Legal Counsel
– Michael Kagnoff, DLA Piper
– Catherine Merity, DLA Piper
• Auditors
– Gamini Martinus, Ernst & Young
• Australian Advisors
– Kim Jacobs, Inteq Limited
– David Collins, Inteq Limited
2 2013 REVA Medical, Inc.
REVA

Establish Quorum
Call to Order
3 2013 REVA Medical, Inc.
REVA

Proxy Proposals
• Elect Class III directors – James J. Schiro and Robert B. Stockman
• Ratify appointment of independent registered accounting firm
• Approve grant of 15,000 options to each of the non-executive directors Brian H. Dovey, Anne Keating, Gordon E. Nye, James J. Schiro, Robert Thomas
• Approve grant of 150,000 options and 47,500 shares of restricted stock to executive director Robert B. Stockman
• Approve the issuance of equity securities up to 10% of the issued capital of the Company
• Approve the issuance and transfer of securities under the Company’s 2010 Equity Incentive Plan
• Approve, on an advisory basis, executive compensation
4 2013 REVA Medical, Inc.
REVA

Results of Voting
• Collection of ballots/voting
• Inspector report on voting
• Other business
• Adjournment
5 2013 REVA Medical, Inc.
REVA

Company Update
6 2013 REVA Medical, Inc.
REVA

Accomplishments since 2012 AGM
• Enrolled 26 patients in the Pilot Clinical Trial
• Developed commercial 6 Fr. device 12 mos. ahead of plan
• Identified and engaging up to 30 top clinical sites for CE Mark
Trial (AUS, BR, GE, POL, SVN, NZ)
• Resourced manufacturing capacity (added cleanroom, equipment and personnel)
• Brought polymer development capability in-house
• Engaged Managing Director, International
Initiated CE Mark RESTORE trial in March 2013
7 2013 REVA Medical, Inc.
REVA

REVA Technology is Highly Differentiated
• Findings from Pilot Study encouraging as to ReZolve platform
• REVA’s core technologies set up ReZolve device well for commercial success:
– Low recoil to support tough lesions (“work horse”)
– X-ray visible
– Robust design during clinical sizing (“work horse”)
– Traditional inflation to desired diameter
– No special handling/storage
These Features Differentiate REVA from BRS Competition
8 2013 REVA Medical, Inc.
REVA

Market is Adopting Bioresorbable Scaffolds (BRS)
In EU and ROW markets
• Clinicians “eager to use” now
– EuroPCR Panel: “80% of cases in next decade” will be BRS
• Abbott and Elixir Medical CE marked; REVA next up in market that is converting from DES to BRS
• Wall Street pegs BRS to grow to multi-billion category
Adoption to BRS being driven by product appeal, perceived clinical benefits, and equivalency to DES
9 2013 REVA Medical, Inc.
REVA
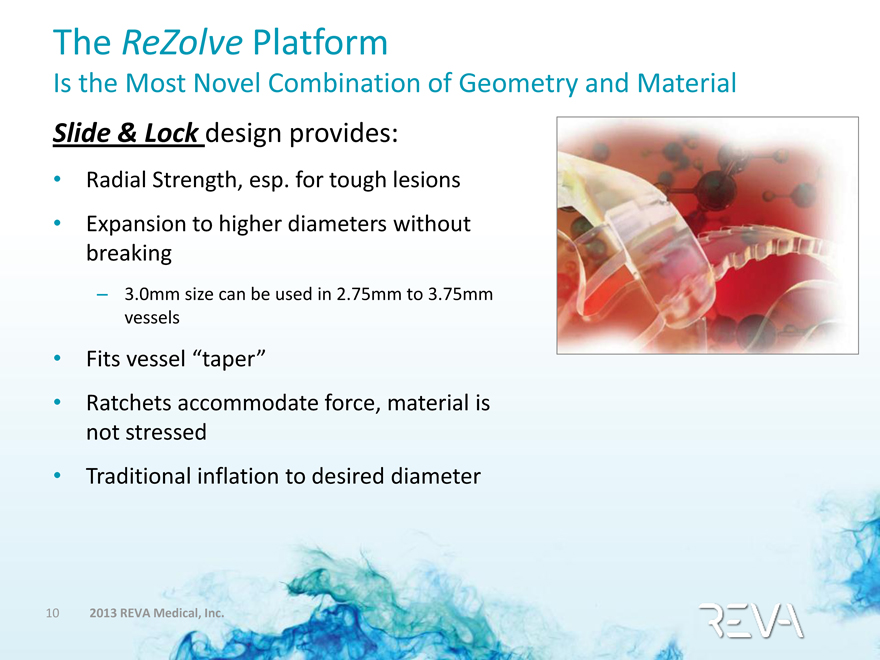
The ReZolve Platform
Is the Most Novel Combination of Geometry and Material
Slide & Lock design provides:
• Radial Strength, esp. for tough lesions
• Expansion to higher diameters without breaking
– 3.0mm size can be used in 2.75mm to 3.75mm vessels
• Fits vessel “taper”
• Ratchets accommodate force, material is not stressed
• Traditional inflation to desired diameter
10 2013 REVA Medical, Inc.
REVA
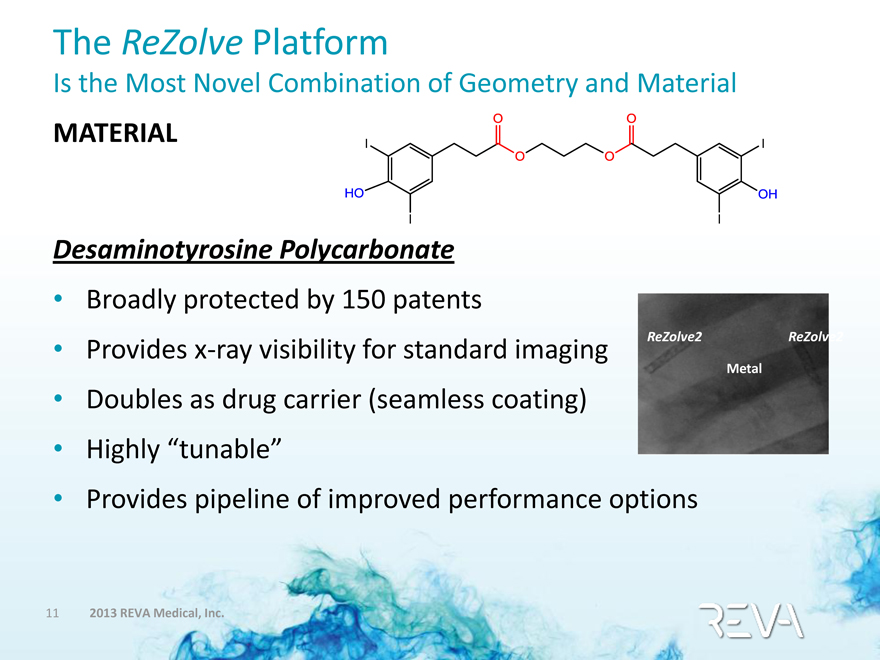
The ReZolve Platform
Is the Most Novel Combination of Geometry and Material
MATERIAL
HO I I O O O O I I OH
Desaminotyrosine Polycarbonate
• Broadly protected by 150 patents
• Provides x-ray visibility for standard imaging
• Doubles as drug carrier (seamless coating)
• Highly “tunable”
• Provides pipeline of improved performance options
ReZolve2
ReZolve2
Metal
11 2013 REVA Medical, Inc.
REVA

RESTORE Pilot Clinical Trial
• December 2011-July 2012
– 22 patients implanted
– BR, GE, POL, and AU
– Dr. Alexandre Abizaid was P.I.
• Primary endpoints:
– Freedom from ischemic-driven target lesion revascularization (“TLR”) at 6 months
– Quantitative measurements at 12 months (QCA/IVUS) (late lumen loss, “LLL”)
12 2013 REVA Medical, Inc.
REVA
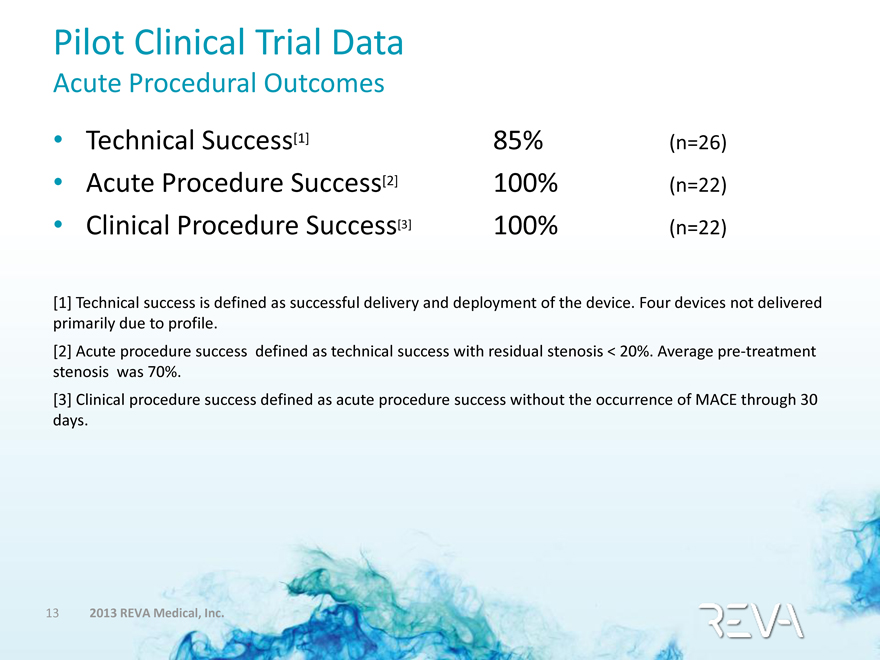
Pilot Clinical Trial Data
Acute Procedural Outcomes
• Technical Success[1] 85% (n=26)
• Acute Procedure Success[2] 100% (n=22)
• Clinical Procedure Success[3] 100% (n=22)
[1] Technical success is defined as successful delivery and deployment of the device. Four devices not delivered primarily due to profile.
[2] Acute procedure success defined as technical success with residual stenosis < 20%. Average pre-treatment stenosis was 70%.
[3] Clinical procedure success defined as acute procedure success without the occurrence of MACE through 30 days.
13 2013 REVA Medical, Inc.
REVA
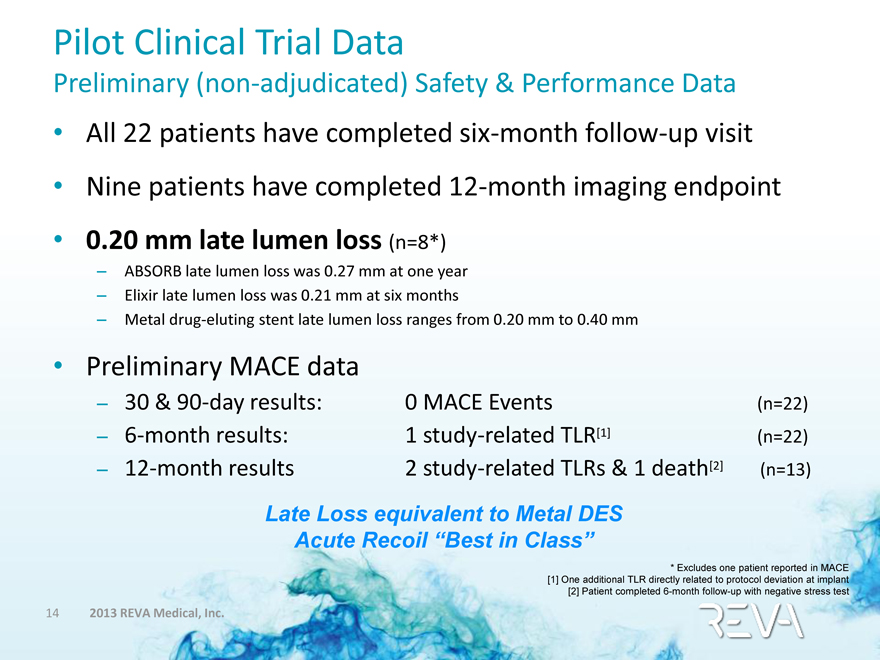
Pilot Clinical Trial Data
Preliminary (non-adjudicated) Safety & Performance Data
• All 22 patients have completed six-month follow-up visit
• Nine patients have completed 12-month imaging endpoint
• 0.20 mm late lumen loss (n=8*)
– ABSORB late lumen loss was 0.27 mm at one year
– Elixir late lumen loss was 0.21 mm at six months
– Metal drug-eluting stent late lumen loss ranges from 0.20 mm to 0.40 mm
• Preliminary MACE data
– 30 & 90-day results: 0 MACE Events (n=22)
– 6-month results: 1 study-related TLR[1] (n=22)
– 12-month results 2 study-related TLRs & 1 death[2] (n=13)
Late Loss equivalent to Metal DES
Acute Recoil “Best in Class”
* Excludes one patient reported in MACE
[1] One additional TLR directly related to protocol deviation at implant
[2] Patient completed 6-month follow-up with negative stress test
14 2013 REVA Medical, Inc.
REVA
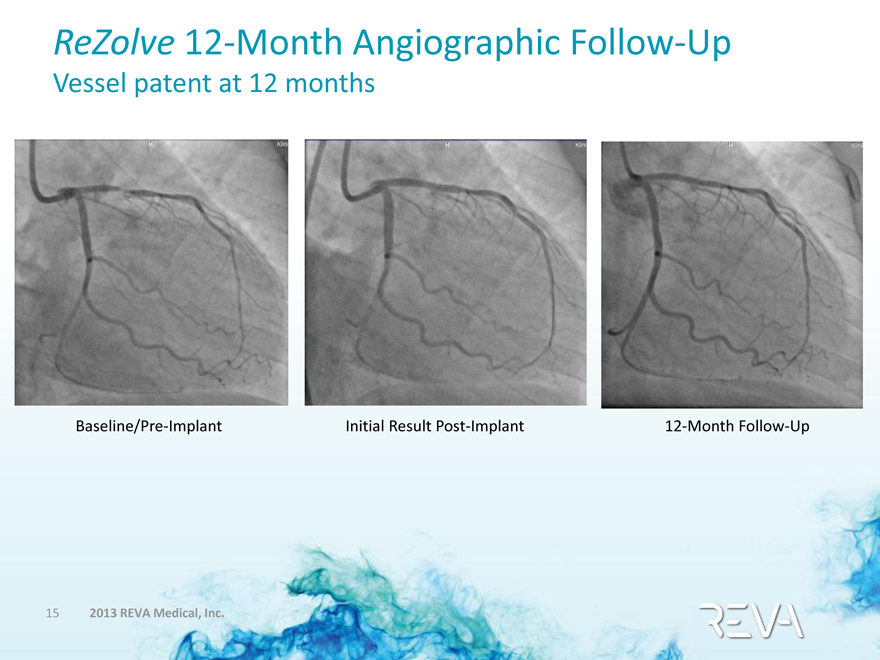
ReZolve 12-Month Angiographic Follow-Up
Vessel patent at 12 months
Baseline/Pre-Implant Initial Result Post-Implant 12-Month Follow-Up
15 2013 REVA Medical, Inc.
REVA
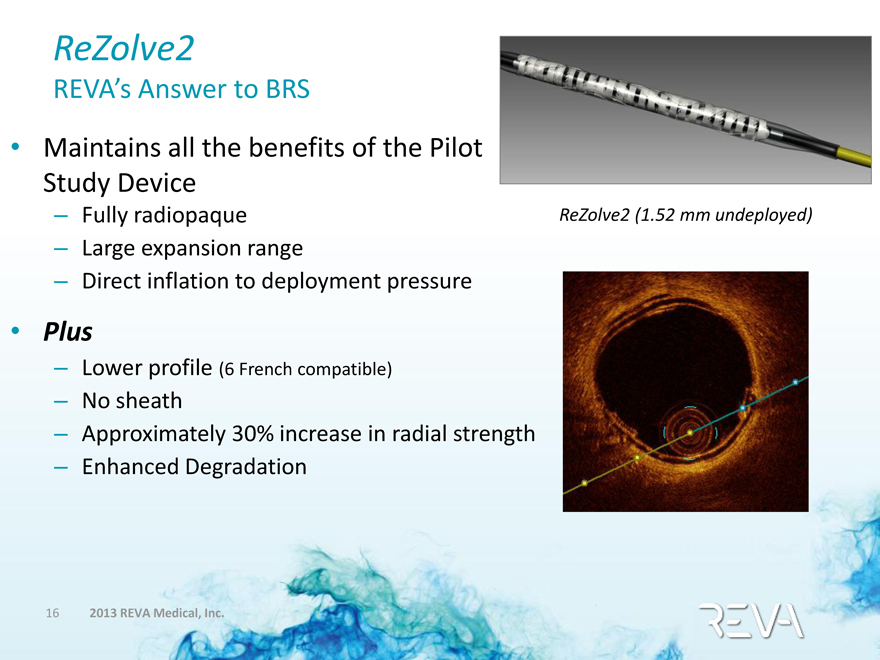
ReZolve2
REVA’s Answer to BRS
• Maintains all the benefits of the Pilot Study Device
– Fully radiopaque
– Large expansion range
– Direct inflation to deployment pressure
ReZolve2 (1.52 mm undeployed)
• Plus
– Lower profile (6 French compatible)
– No sheath
– Approximately 30% increase in radial strength
– Enhanced Degradation
16 2013 REVA Medical, Inc.
REVA
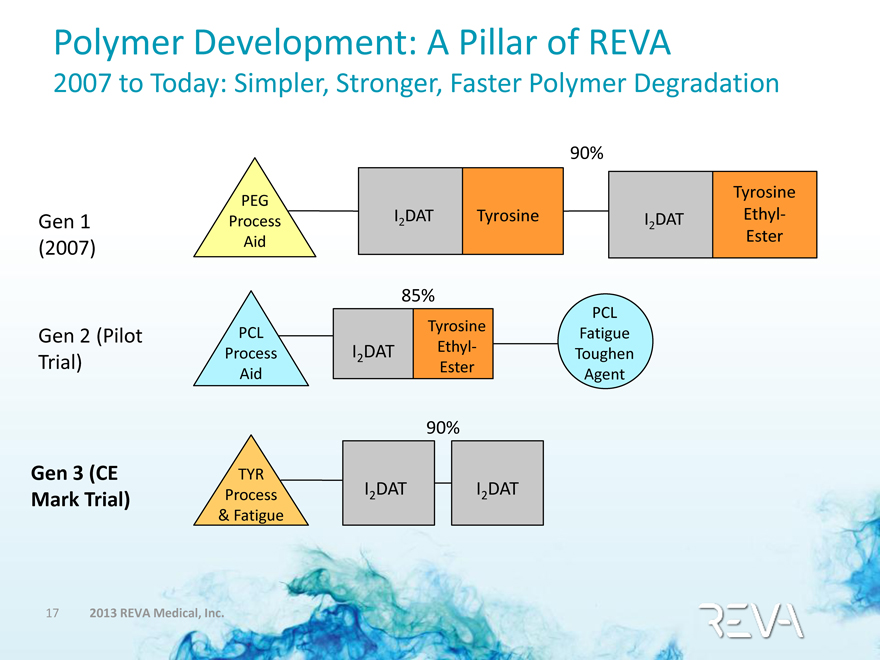
Polymer Development: A Pillar of REVA
2007 to Today: Simpler, Stronger, Faster Polymer Degradation
90%
Gen 1 (2007) PEG Process Aid I2 DAT Tyrosine I2 DAT Tyrosine Ethyl-Ester
85%
Gen 2 (Pilot Trial) PCL Process Aid I2 DAT Tyrosine Ethyl-Ester PCL Fatigue Toughen Agent
90%
Gen 3 (CE Mark Trial) TYR Process & Fatigue I2 DAT I2 DAT
17 2013 REVA Medical, Inc.
REVA
Latest Polymer Enhancements: 3rd Gen
Enables thinner design without compromising performance
• 30% Increased tensile strength
– Holds back large plaque loads inside artery
• Maintains sufficient elongation (“toughness”)
– Withstands structural integrity under larger forces
• Maintains strength, yet maintains resorption time
– molecular weight maintained for 4 to 6 mos.
– Mass loss accelerated (resorbs faster)
18 2013 REVA Medical, Inc.
REVA

RESTORE CE Mark Trial Initiated March 2013
• Prospective single arm study
• Principal Investigators
• Drs. Alexandre Abizaid and David Muller
• 125 total patients
• Up to 30 Clinical Sites
– GE, POL, SVN, BR, AUS, NZ
Enrollment completion anticipated end of 3Q 2013
19 2013 REVA Medical, Inc.
REVA
ReZolve2 Clinical Summary
• 8 patients implanted in first month (3 clinical sites)
• 100% acute technical procedure success
• Femoral and radial delivery
• Enrollment expanding up to 30 sites
– All approved sites are screening patients vigorously
20 2013 REVA Medical, Inc.
REVA
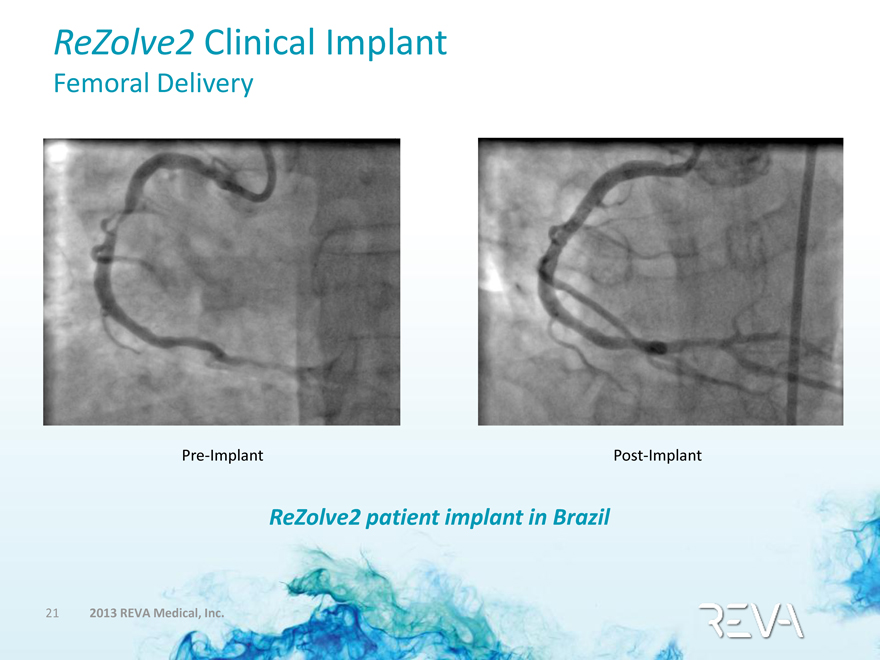
ReZolve2 Clinical Implant
Femoral Delivery
Pre-Implant
Post-Implant
ReZolve2 patient implant in Brazil
21 2013 REVA Medical, Inc.
REVA
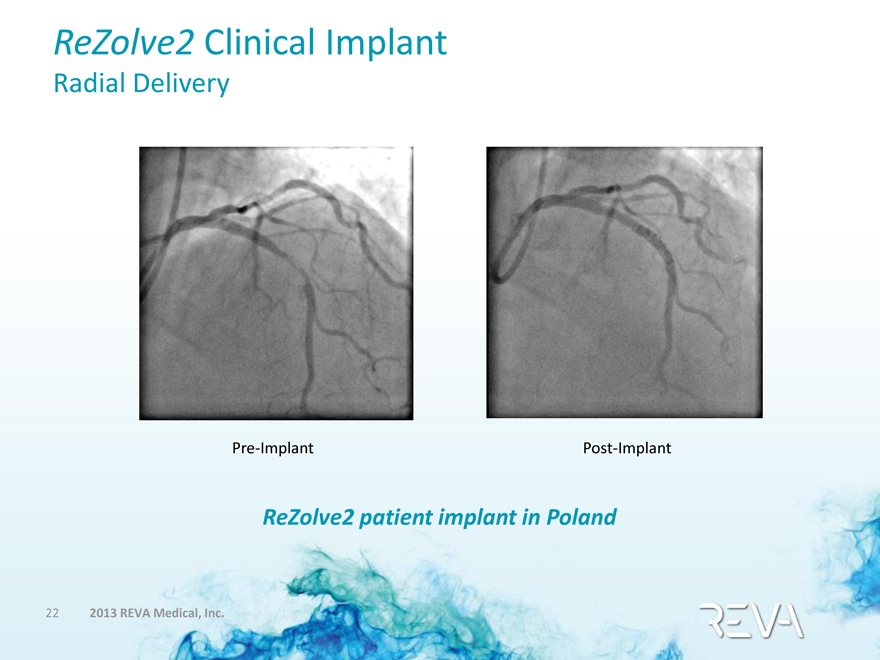
ReZolve2 Clinical Implant
Radial Delivery
Pre-Implant Post-Implant
ReZolve2 patient implant in Poland
22 2013 REVA Medical, Inc.
REVA
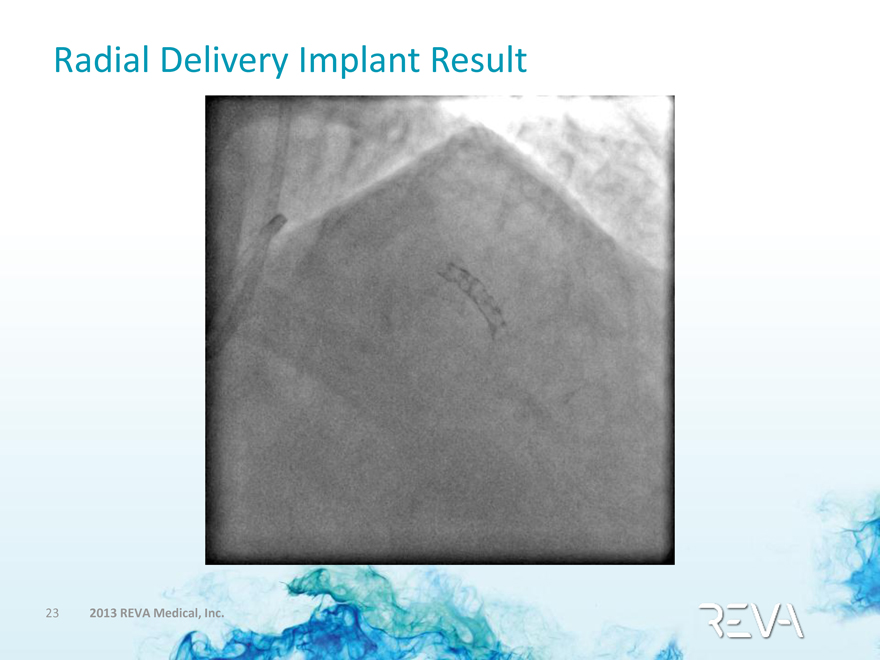
Radial Delivery Implant Result
23 2013 REVA Medical, Inc.
REVA
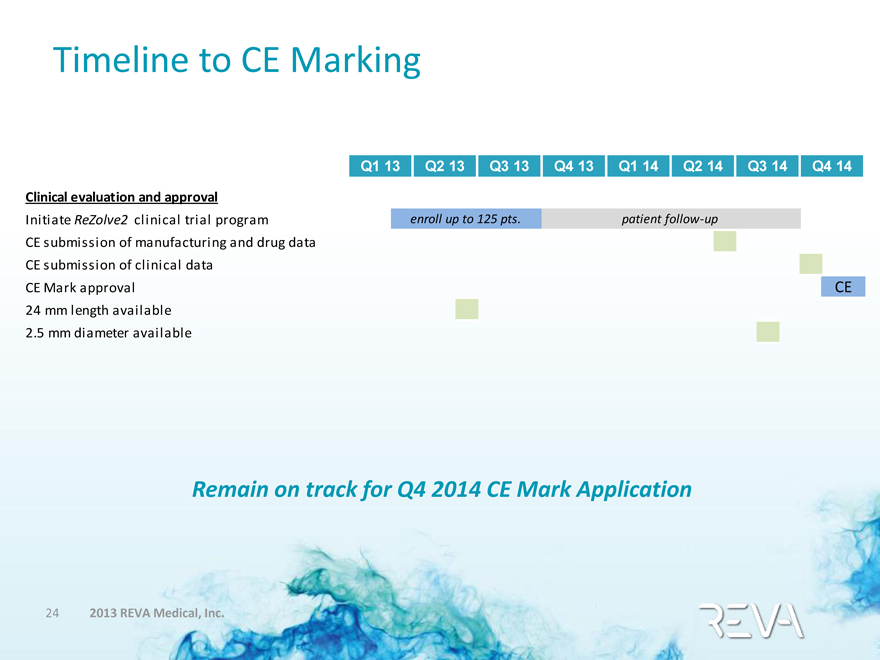
Timeline to CE Marking
Q1 13 Q2 13 Q3 13 Q4 13 Q1 14 Q2 14 Q3 14 Q4 14
Clinical evaluation and approval
Initiate ReZolve2 clinical trial program enroll up to 125 pts. patient follow-up
CE submission of manufacturing and drug data
CE submission of clinical data
CE Mark approval CE
24 mm length available
2.5 mm diameter available
Remain on track for Q4 2014 CE Mark Application
24 2013 REVA Medical, Inc.
REVA

Coronary Stent Market
• 2013 world-wide revenues projected to be $4.7B*
– 80% drug-eluting stents (“DES”) and 20% bare metal
– DES market leaders are ABT, BSC, and MDT
– DES selling prices projected to decline 7% annually
• Bioresorbable coronary scaffolds commercially available
– CE Marking granted to two products
• Abbott’s ABSORB™ available in multiple markets
• Elixir’s DESolve® not yet launched
* JP Morgan Interventional Cardiology Market Model, Jan. 2013
25 2013 REVA Medical, Inc.
REVA

Bioresorbable Scaffold Sales
• 2013 will be the first complete year for ABSORB sales
• Current use estimated at 7% (Credit Suisse)
• Future use projected to increase
– 16% to 22% of cases with non-inferior clinical results
– 28% to 42% of cases with superior clinical results
• Physicians predict significant adoption
– EuroPCR physician panel projected 80% usage within 10 years
26 2013 REVA Medical, Inc.
REVA

Bioresorbable Competition
Abbott’s ABSORB™ bioresorbable scaffold
• Available in Europe, the Middle East, and select markets in Latin America and Asia Pacific
• Abbott’s U.S. trial underway
– 2,250 patients; randomized against Xience
– Primary endpoint target lesion failure at 12 months
– Enrollment slow due to FDA limitation of a single size
• Trial in Japan initiated
– 400 patients; randomized against Xience
– Primary endpoint target lesion failure at 12 months
27 2013 REVA Medical, Inc.
REVA

Bioresorbable Competition
Elixir Medical’s DESolve® bioresorbable scaffold
• CE Mark approval for DESolve scaffold announced May 15
• Data used for CE mark submission included:
– Non-randomized six-month data on 126 patients
– 0.21 ± 0.34 mm late lumen loss
– 3.25% MACE rate
• Validates REVA’s CE Mark application strategy
– 125 non-randomized patients at 9-month endpoint
– Evaluating possibility of earlier submission endpoint
• Elixir to launch DESolve later this year
– Will assess how Abbott responds to first commercial competitor
28 2013 REVA Medical, Inc.
REVA

Commercial Planning
29 2013 REVA Medical, Inc.
REVA

Commercial Planning
• Company Strategy
– Maximize revenue and GP margin in target countries
• Near-Term Activities:
– Scale up manufacturing
– Identify target markets with expedited regulatory approvals and clear reimbursement methods
– Direct vs. independent distributors
– Brand strategy
– Build out EU team
Goal is to position REVA as “Best in Class” BRS option
30 2013 REVA Medical, Inc.
REVA

Managing Director, International
Michel Vanbrabant
• PneumRx
– VP EMEAC
• St. Jude Medical
Marketing Director Neuromod/EU
• Guidant
– Marketing Director EU
– Country Manager – France
– Sales rep interventional cardiology
31 2013 REVA Medical, Inc.
REVA
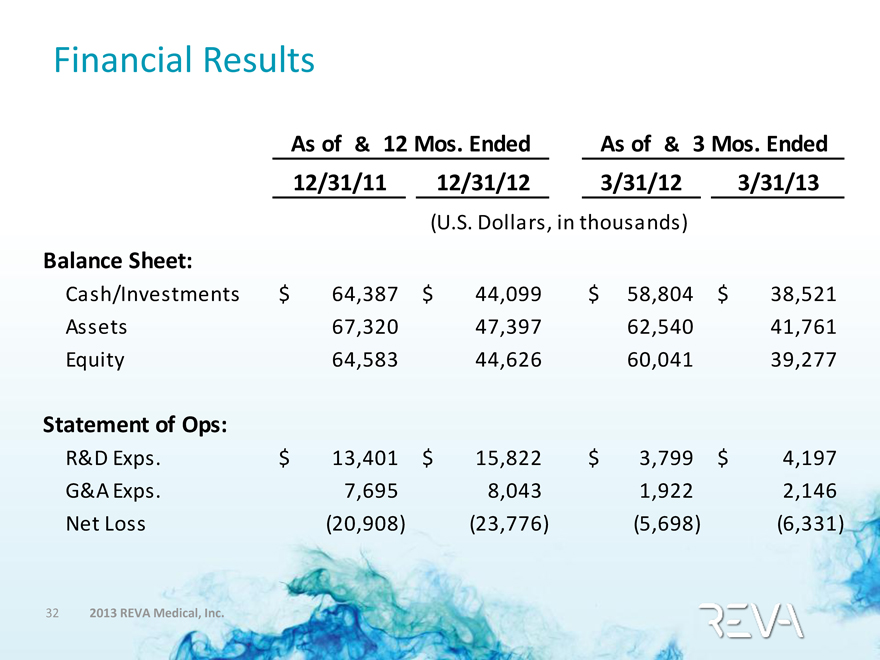
Financial Results
As of & 12 Mos. Ended As of & 3 Mos. Ended
12/31/11 12/31/12 3/31/12 3/31/13
(U.S. Dollars, in thousands)
Balance Sheet:
Cash/Investments $64,387 $44,099 $58,804 $38,521
Assets 67,320 47,397 62,540 41,761
Equity 64,583 44,626 60,041 39,277
Statement of Ops:
R&D Exps. $13,401 $15,822 $3,799 $4,197
G&A Exps. 7,695 8,043 1,922 2,146
Net Loss (20,908) (23,776) (5,698) (6,331)
32 2013 REVA Medical, Inc.
REVA

Leveraging REVA Technology
33 2013 REVA Medical, Inc.
REVA

2013 Goals and Summary
• Complete 12-mo follow up on Pilot Clinical Study patients
• Complete enrollment of CE Mark trial of up to 125 patients
• Achieve initial scale-up capacity
• Develop additional sizes to support product launch
• Develop Commercialization Plan for Launch for 4Q14
Establish REVA as Best In Class BRS Solution
34 2013 REVA Medical, Inc.
REVA

Thank you
36 2013 REVA Medical, Inc.
REVA




































