
January 14, 2016 J.P. Morgan 2016 Healthcare Conference Exhibit 99.1

Important Notice Not an Offer for Securities This presentation does not constitute an offer, invitation, solicitation or recommendation with respect to the purchase or sale of any security in the Company nor does it constitute financial product advice nor take into account your investment objectives, taxation situation, financial situation or needs. An investor must not act on the basis of any matter contained in this presentation but must make its own assessment of the Company and conduct its own investigations and analysis. Information is a Synopsis Only This presentation only contains a synopsis of information on the Company and, accordingly, no reliance may be placed for any purpose whatsoever on the sufficiency or completeness of such information. Information presented in this presentation is subject to change without notice and REVA does not have any responsibility or obligation to inform you of any matter arising or coming to their notice after the date of this presentation, which may affect any matter in the presentation. Forward-Looking Statements This presentation contains or may contain forward-looking statements that are based on management's beliefs, assumptions and expectations and on information currently available to management. All statements that are not statements of historical fact, including those statements that address future operating performance and events or developments that we expect or anticipate will occur in the future, are forward-looking statements, such as those statements regarding our ability to obtain regulatory approvals, timely and successfully complete our clinical trials, protect our intellectual property position, commercialize our products if and when approved, develop and commercialize new products, recruit and retain our key personnel, and estimates regarding our capital requirements and financial performance, including profitability. You should not place undue reliance on these forward-looking statements. Although management believes these forward-looking statements are reasonable as and when made, forward-looking statements are subject to a number of risks and uncertainties that may cause our actual results to vary materially from those expressed in the forward-looking statements, including the risks and uncertainties that are described in the "Risk Factors" section of our Annual Report on Form 10-K filed with the United States Securities and Exchange Commission (the “SEC”) on March 30, 2015, and as may be updated in our periodic reports thereafter. Any forward-looking statements in this presentation speak only as of the date when made. REVA does not assume any obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events, or otherwise. Disclaimer This presentation and any supplemental materials have been prepared by the Company based on available information. The information contained in this presentation is an overview and does not contain all information necessary to make an investment decision. Although reasonable care has been taken to ensure the facts stated in this presentation are accurate and that the opinions expressed are fair and reasonable, no representation or warranty, express or implied, is made as to the fairness, accuracy, completeness, or correctness of such information and opinions and no reliance should be placed on such information or opinions. To the maximum extent permitted by law, none of the Company, or any of its members, directors, officers, employees, or agents or advisers, nor any other person accepts any liability whatsoever for any loss, however arising, from the use of the presentation or its contents or otherwise arising in connection with it, including, without limitation, any liability arising from fault or negligence on the part of the Company or any of its directors, officers, employees, or agents.
Mission To restore patient health for life through the application of biomaterials in the development and sale of implantable medical devices Focused on commercialization of an advanced drug-eluting bioresorbable scaffold for the treatment of cardiovascular disease Fantom® and Focus

Corporate Overview Located in San Diego, CA ASX listed (RVA.AX); SEC registered ISO 13485:2012 certified 58 employees

Reggie Groves Joined REVA as CEO September 2015 Medtronic VP & GM: Built Atrial Fibrillation business into market leading position VP Quality & Regulatory: Led largest recall in the industry iXL: Global Managing Partner Kaiser Permanente: CFO, Southeast Division McKinsey & Company: Senior Engagement Manager Egleston Childrens’ Hospital: CFO
The Potential of Bioresorbable Scaffolds Preserves future treatment options May reduce long-term adverse events Restoration of vasomotion No lifetime anti-coagulation

Fantom® Sirolimus-Eluting Bioresorbable Scaffold The Product Solution
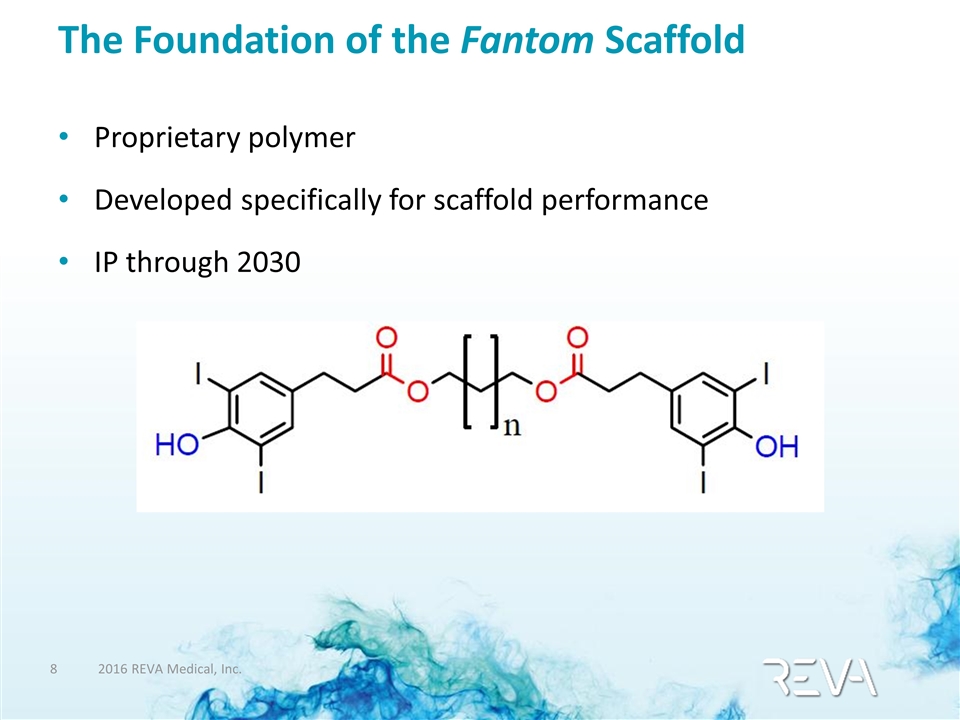
The Foundation of the Fantom Scaffold Proprietary polymer Developed specifically for scaffold performance IP through 2030

Fantom’s Key Advantages Driven by REVA’s Proprietary Polymer Visible Precision during procedure Thin Low profile Deliverable Rapid healing Metal-like Behavior Straightforward implant
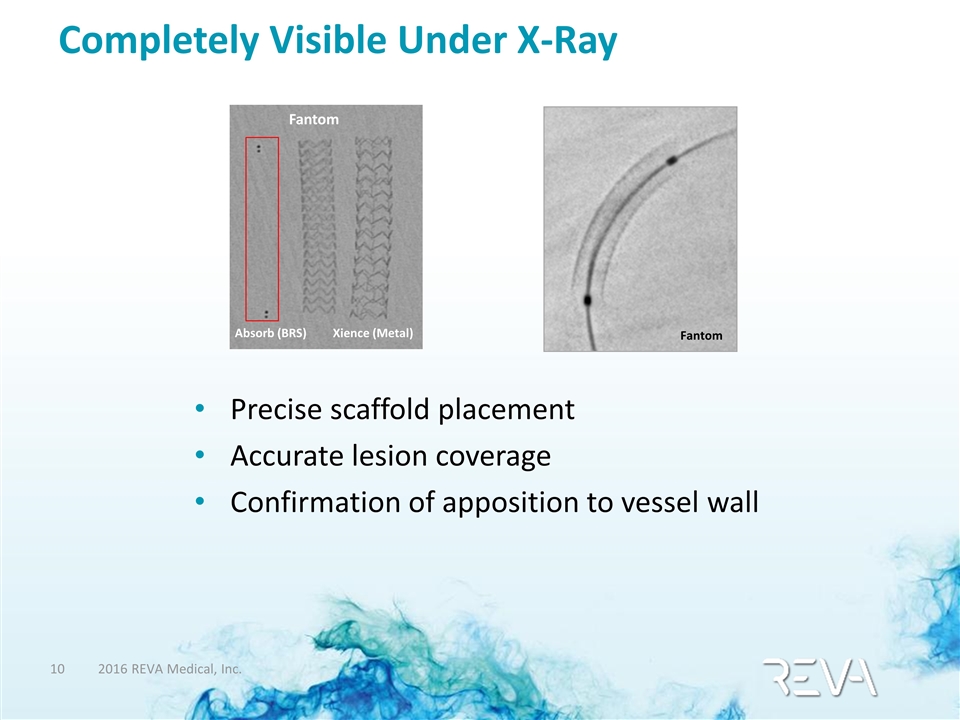
Completely Visible Under X-Ray Precise scaffold placement Accurate lesion coverage Confirmation of apposition to vessel wall Fantom Absorb (BRS) Fantom Xience (Metal)
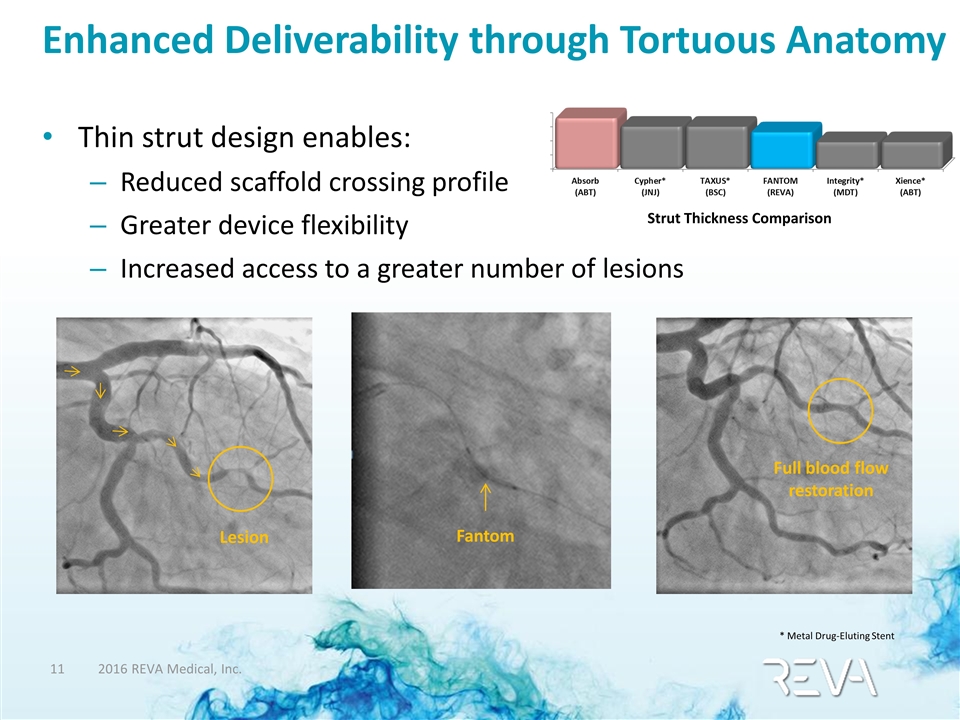
Enhanced Deliverability through Tortuous Anatomy Thin strut design enables: Reduced scaffold crossing profile Greater device flexibility Increased access to a greater number of lesions Full blood flow restoration Fantom Lesion Strut Thickness Comparison * Metal Drug-Eluting Stent

Rapid Healing with Complete Tissue Coverage Thin strut design & polymer biocompatibility enable: Rapid strut coverage 99+% of struts covered within 4 months in FANTOM I Minimizes risk of adverse events Complete Strut Coverage

Straightforward Implant Single-step inflation Reduced procedure time Room temperature storage Implant immediately without temperature concerns No procedural limitations Broad expansion range with sustained strength

FANTOM: The Clinical Studies

FANTOM I Pilot Study 7 patients, 2 clinical sites Device Safety All patients through 9-month follow-up 4 patients through 12-month follow-up No MACE events 100% procedural success Excellent healing response MACE: Major Adverse Cardiac Events
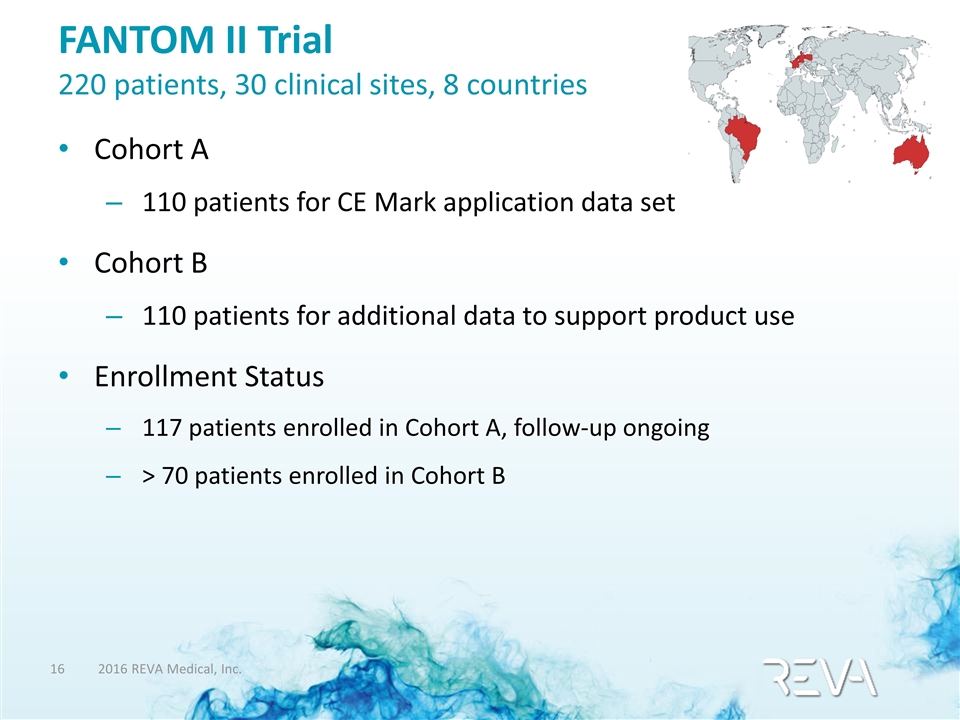
FANTOM II Trial 220 patients, 30 clinical sites, 8 countries Cohort A 110 patients for CE Mark application data set Cohort B 110 patients for additional data to support product use Enrollment Status 117 patients enrolled in Cohort A, follow-up ongoing > 70 patients enrolled in Cohort B
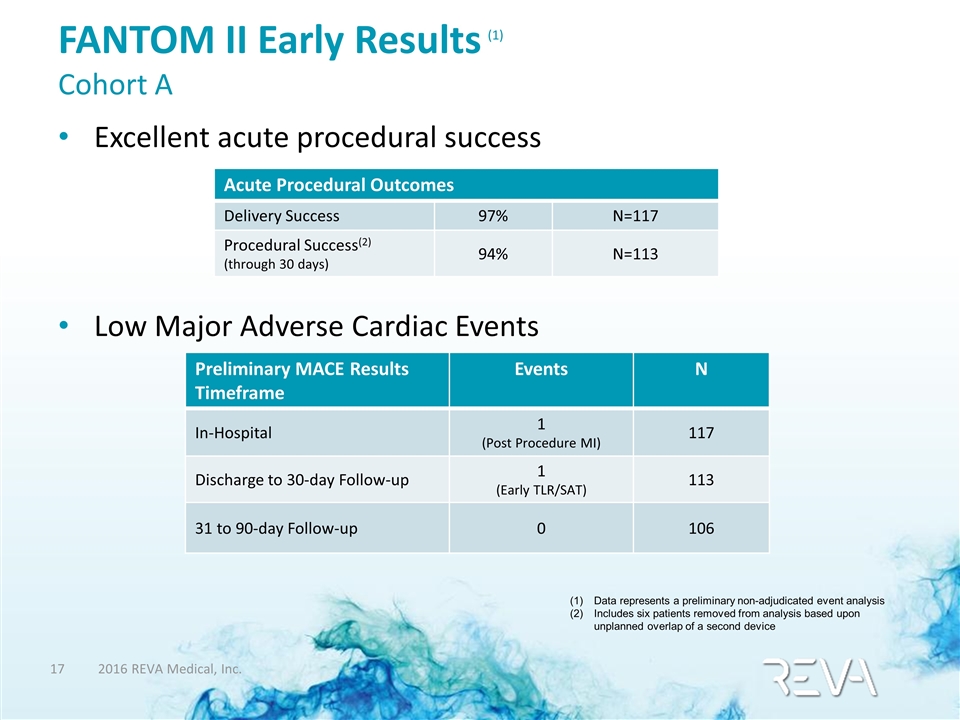
Excellent acute procedural success Low Major Adverse Cardiac Events FANTOM II Early Results (1) Cohort A Acute Procedural Outcomes Delivery Success 97% N=117 Procedural Success(2) (through 30 days) 94% N=113 Preliminary MACE Results Timeframe Events N In-Hospital 1 (Post Procedure MI) 117 Discharge to 30-day Follow-up 1 (Early TLR/SAT) 113 31 to 90-day Follow-up 0 106 Data represents a preliminary non-adjudicated event analysis Includes six patients removed from analysis based upon unplanned overlap of a second device
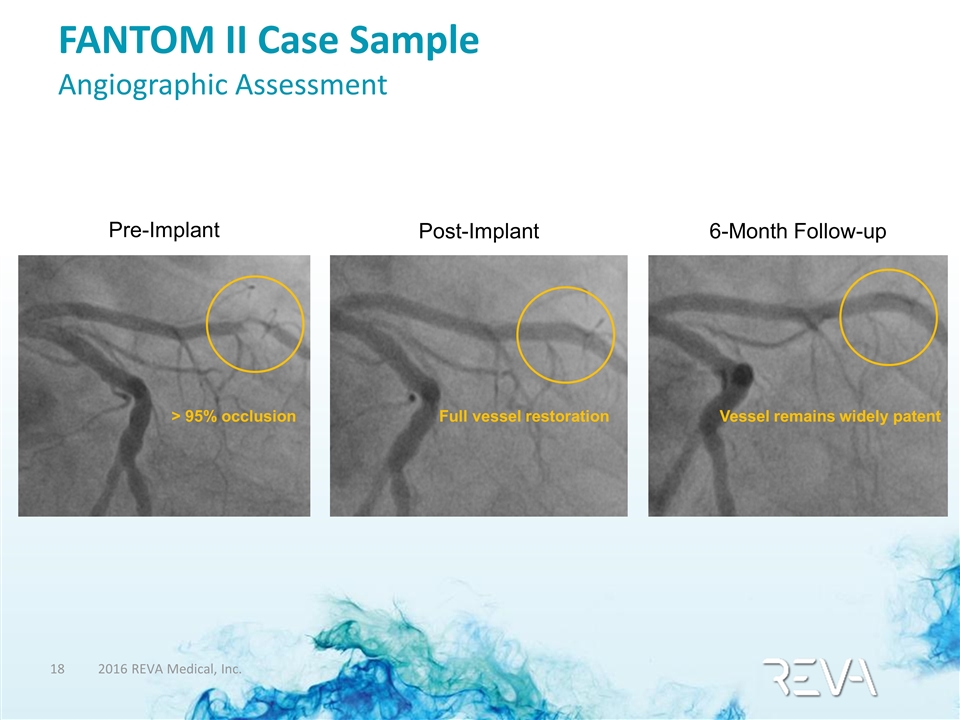
FANTOM II Case Sample Angiographic Assessment > 95% occlusion Pre-Implant Post-Implant Full vessel restoration Vessel remains widely patent 6-Month Follow-up
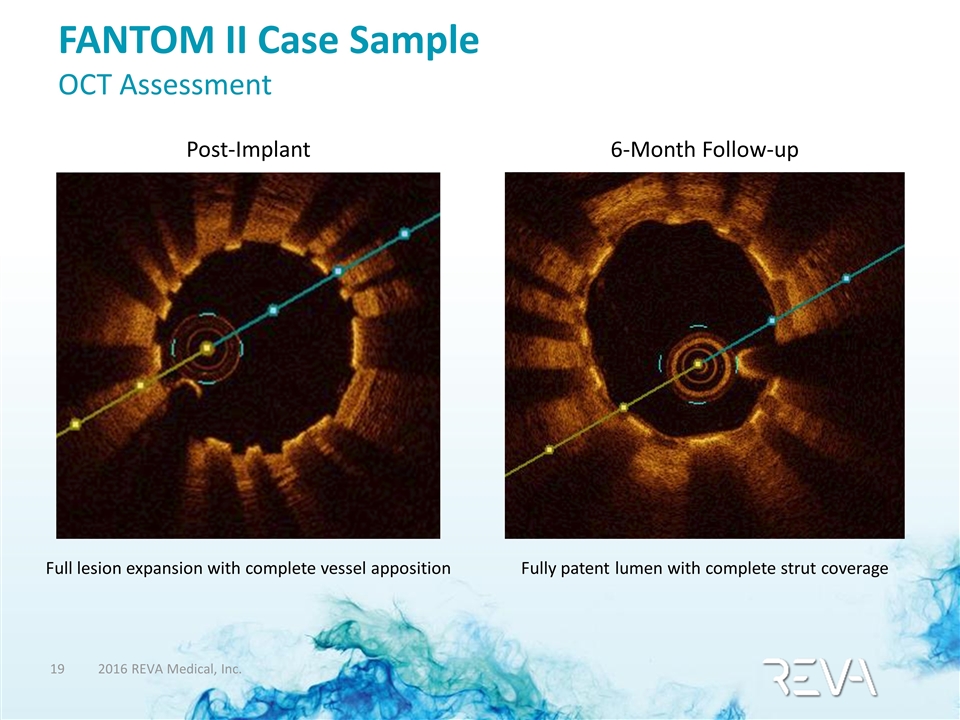
FANTOM II Case Sample OCT Assessment 6-Month Follow-up Fully patent lumen with complete strut coverage Post-Implant Full lesion expansion with complete vessel apposition

Commercialization

Commercialization Timeline CE Mark submission planned Q3’16 CE Mark approval targeted Q4’16 Commercialization anticipated H1’17

Manufacturing Readiness ~20,000 sq. ft. available for manufacturing State-of-the-art equipment ISO 13485:2012 certified Tube Fabrication cGMP Polymer Manufacturing Scaffold Assembly

Summary Established public company ~$4b market opportunity Product advantages well received by physicians Fantom demonstrating clinical success CE Mark on the horizon Commercially capable out of current facility

Thank you























