
Advantages of Desaminotyrosine Polycarbonate Polymers Gregg W. Stone MD Columbia University Medical Center The Cardiovascular Research Foundation EXHIBIT 99.2

Potential conflicts of interest Gregg W. Stone Consultant to Reva
Fantom BRS Polymer Polymer structure Material and scaffold characteristics Biologic safety Clinical advantages
Fantom Polymer Polymer Structure
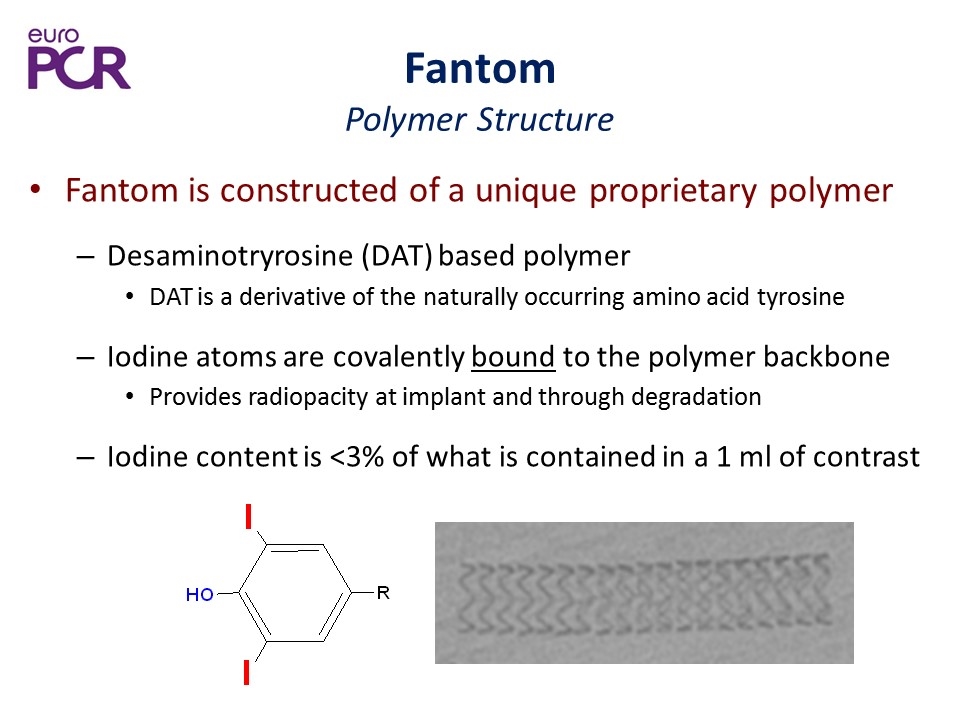
Fantom Polymer Structure Fantom is constructed of a unique proprietary polymer Desaminotryrosine (DAT) based polymer DAT is a derivative of the naturally occurring amino acid tyrosine Iodine atoms are covalently bound to the polymer backbone Provides radiopacity at implant and through degradation Iodine content is <3% of what is contained in a 1 ml of contrast
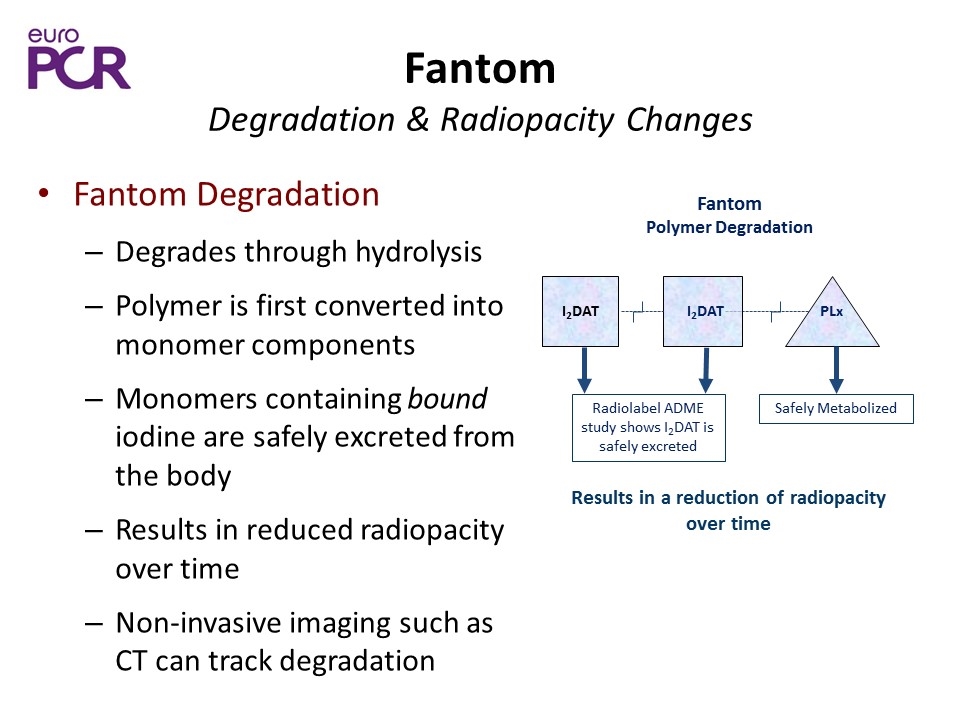
Fantom Degradation & Radiopacity Changes Fantom Degradation Degrades through hydrolysis Polymer is first converted into monomer components Monomers containing bound iodine are safely excreted from the body Results in reduced radiopacity over time Non-invasive imaging such as CT can track degradation Results in a reduction of radiopacity over time Fantom Polymer Degradation I2DAT I2DAT PLx Radiolabel ADME study shows I2DAT is safely excreted Safely Metabolized
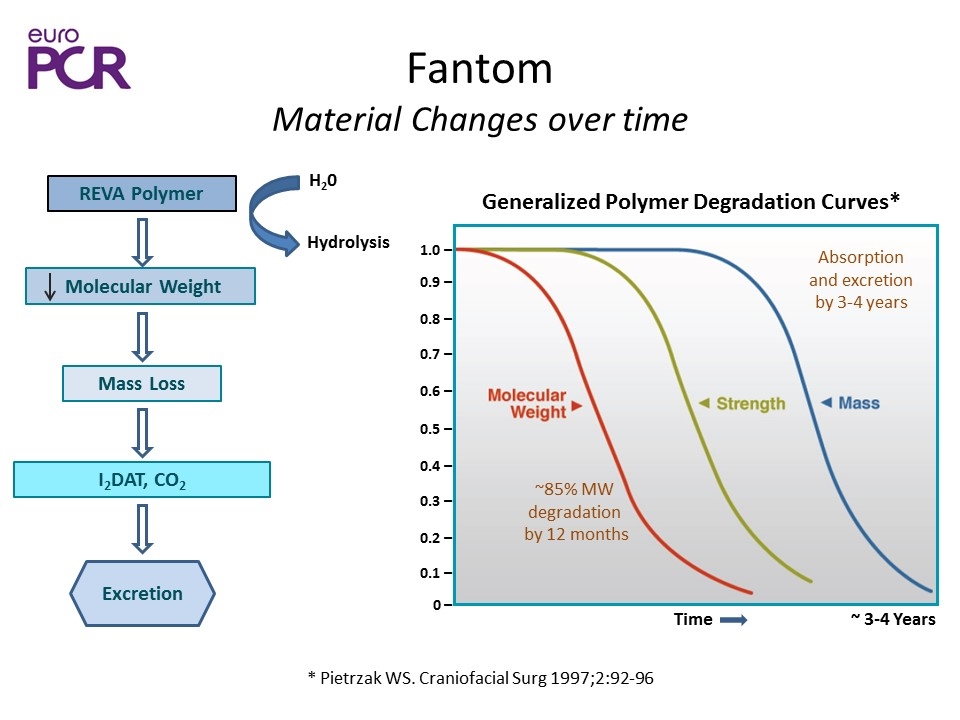
Fantom Material Changes over time * Pietrzak WS. Craniofacial Surg 1997;2:92-96 1.0 ̶ 0.9 ̶ 0.4 ̶ 0.8 ̶ 0.3 ̶ 0.7 ̶ 0.2 ̶ 0.6 ̶ 0.1 ̶ 0.5 ̶ 0 ̶ Time ~ 3-4 Years Generalized Polymer Degradation Curves* Hydrolysis REVA Polymer Molecular Weight Mass Loss I2DAT, CO2 Excretion H20 ~85% MW degradation by 12 months Absorption and excretion by 3-4 years

Fantom Polymer Material Characteristics
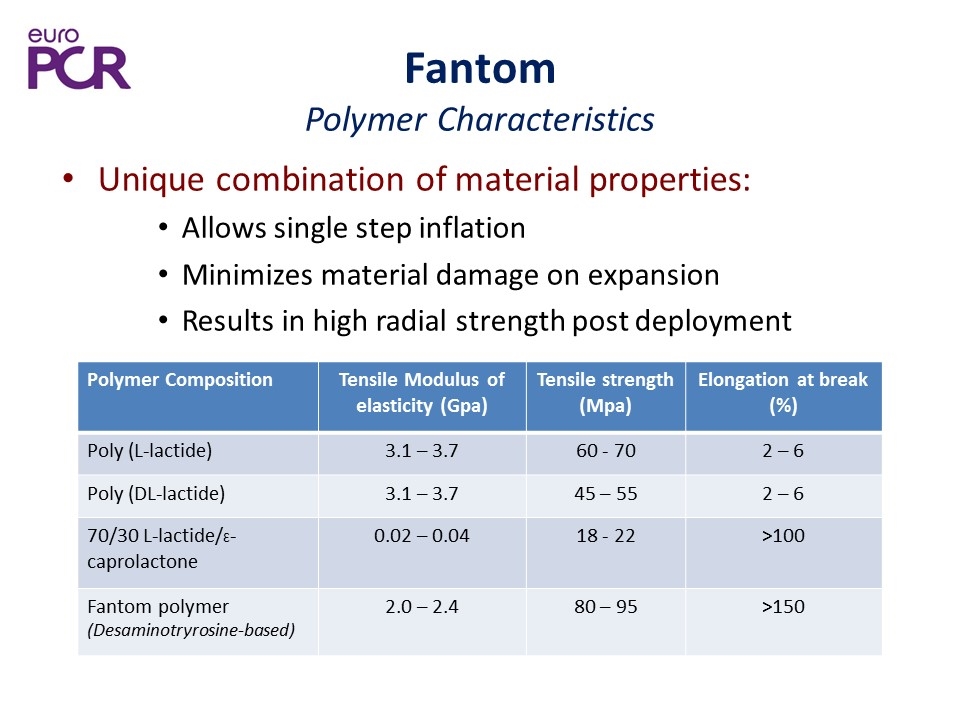
Fantom Polymer Characteristics Unique combination of material properties: Allows single step inflation Minimizes material damage on expansion Results in high radial strength post deployment Polymer Composition Tensile Modulus of elasticity (Gpa) Tensile strength (Mpa) Elongation at break (%) Poly (L-lactide) 3.1 – 3.7 60 - 70 2 – 6 Poly (DL-lactide) 3.1 – 3.7 45 – 55 2 – 6 70/30 L-lactide/Ɛ-caprolactone 0.02 – 0.04 18 - 22 >100 Fantom polymer (Desaminotryrosine-based) 2.0 – 2.4 80 – 95 >150
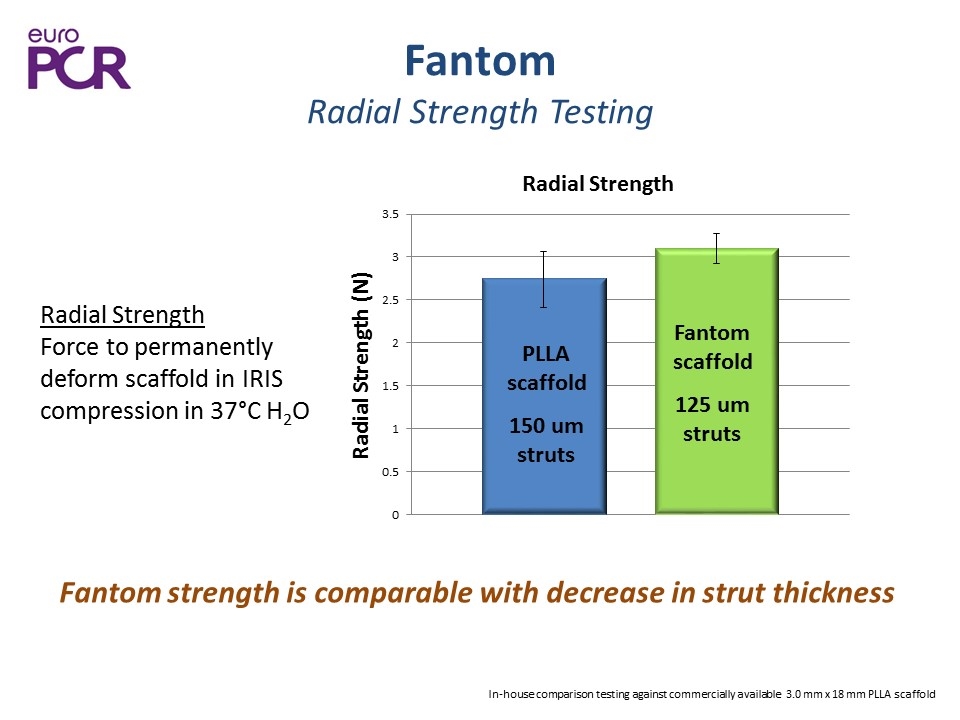
Fantom Radial Strength Testing Fantom strength is comparable with decrease in strut thickness In-house comparison testing against commercially available 3.0 mm x 18 mm PLLA scaffold Radial Strength Force to permanently deform scaffold in IRIS compression in 37°C H2O PLLA scaffold 150 um struts Fantom scaffold 125 um struts
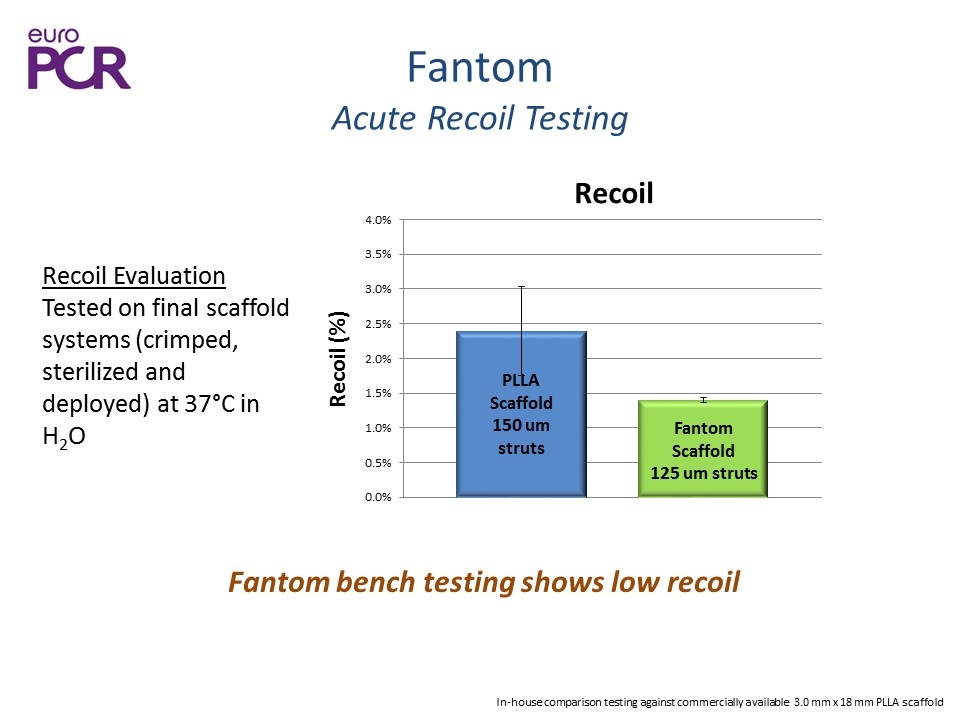
Fantom Acute Recoil Testing Fantom bench testing shows low recoil Recoil Evaluation Tested on final scaffold systems (crimped, sterilized and deployed) at 37°C in H2O In-house comparison testing against commercially available 3.0 mm x 18 mm PLLA scaffold
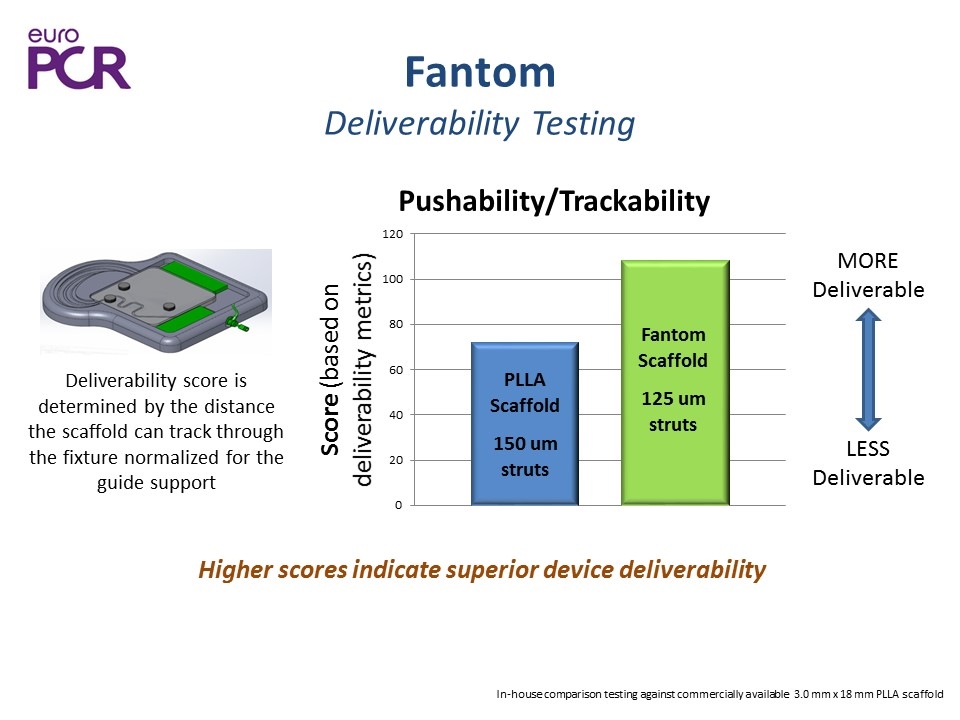
Fantom Deliverability Testing Higher scores indicate superior device deliverability Deliverability score is determined by the distance the scaffold can track through the fixture normalized for the guide support MORE Deliverable LESS Deliverable In-house comparison testing against commercially available 3.0 mm x 18 mm PLLA scaffold 125 um struts PLLA Scaffold 150 um struts

Fantom Polymer Biological Safety

Fantom Biocompatibility Evaluated in full series of biocompatibility assessments Test Name Result Genotoxicity Bacterial Reverse Mutation Study Mouse Peripheral Blood Micronucleus Study In Vitro Chromosomal Aberration Study in Passed Non-Mutagenic and Non-Genotoxic Interactions with blood ASTM Hemolysis Study C3a Complement Activation Assay SC5b-9 Complement Activation Assay ASTM Partial Thromboplastin Time Assay Passed Non-Hemolytic & Non to Minimal Activator Cytotoxicity ISO MEM Elution Assay Passed Non-Cytotoxic Sensitization ISO Maximization Sensitization Study Passed Non-Sensitizing Irritation ISO Intracutaneous Study Passed Non-Irritating Systemic toxicity ISO Systemic Toxicity Study Passed Non-Toxic
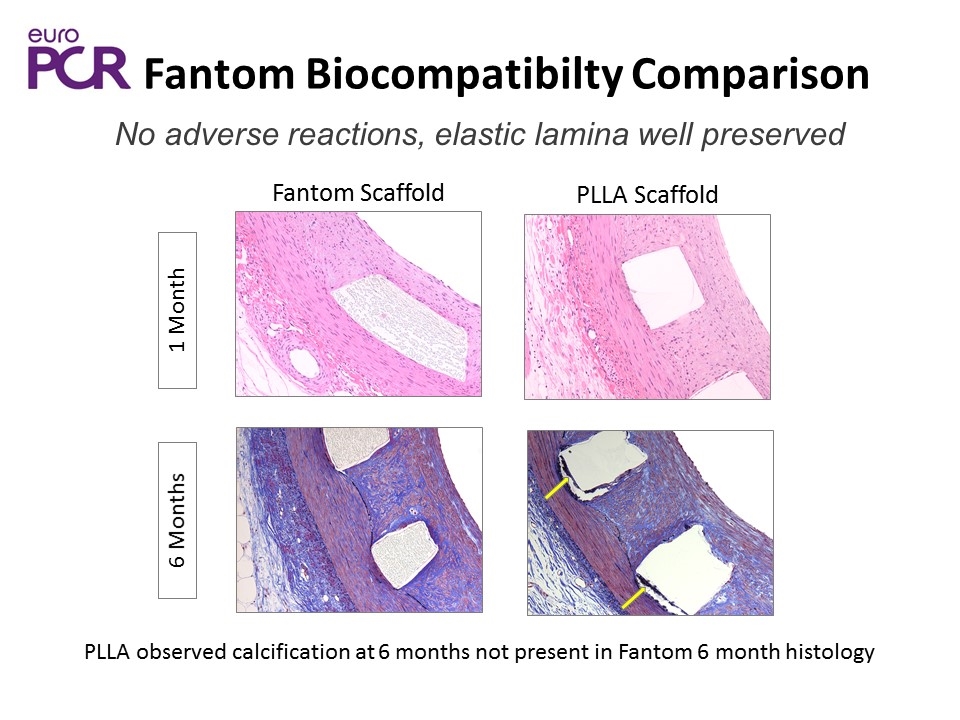
Fantom Biocompatibilty Comparison PLLA observed calcification at 6 months not present in Fantom 6 month histology No adverse reactions, elastic lamina well preserved 1 Month Fantom Scaffold PLLA Scaffold 6 Months
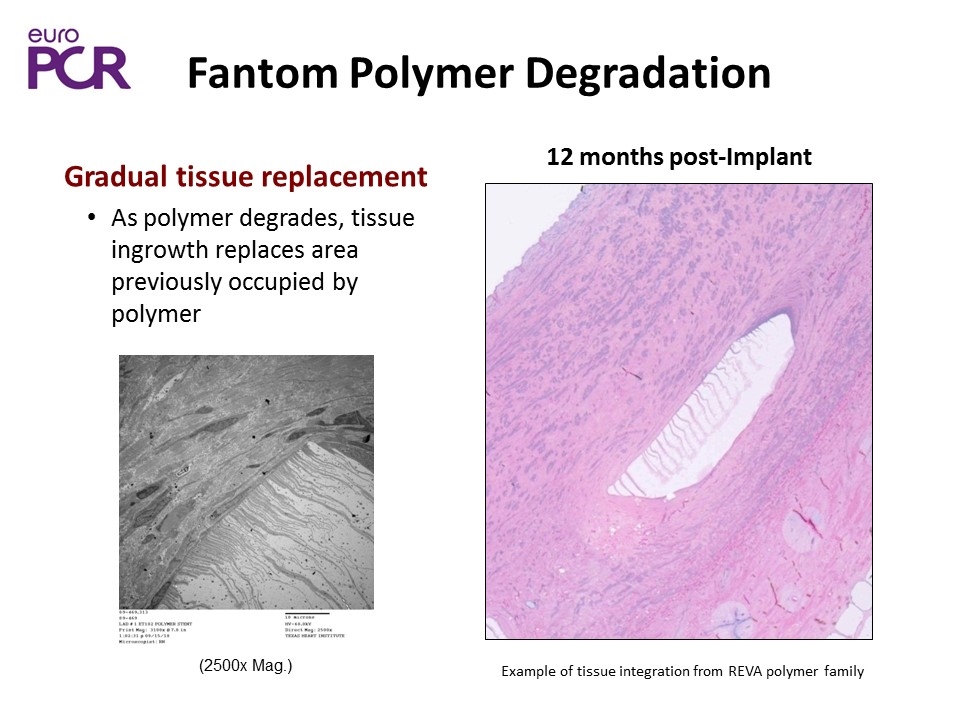
Fantom Polymer Degradation (2500x Mag.) 12 months post-Implant Gradual tissue replacement As polymer degrades, tissue ingrowth replaces area previously occupied by polymer Example of tissue integration from REVA polymer family
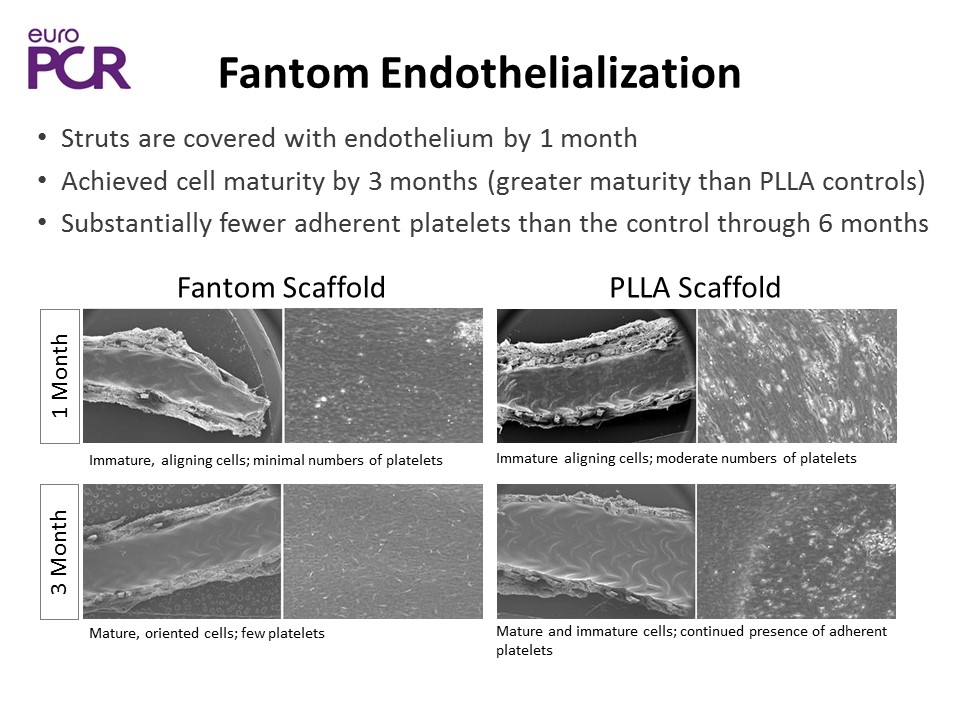
Fantom Scaffold PLLA Scaffold 3 Month 1 Month Struts are covered with endothelium by 1 month Achieved cell maturity by 3 months (greater maturity than PLLA controls) Substantially fewer adherent platelets than the control through 6 months Immature, aligning cells; minimal numbers of platelets Mature, oriented cells; few platelets Immature aligning cells; moderate numbers of platelets Mature and immature cells; continued presence of adherent platelets Fantom Endothelialization
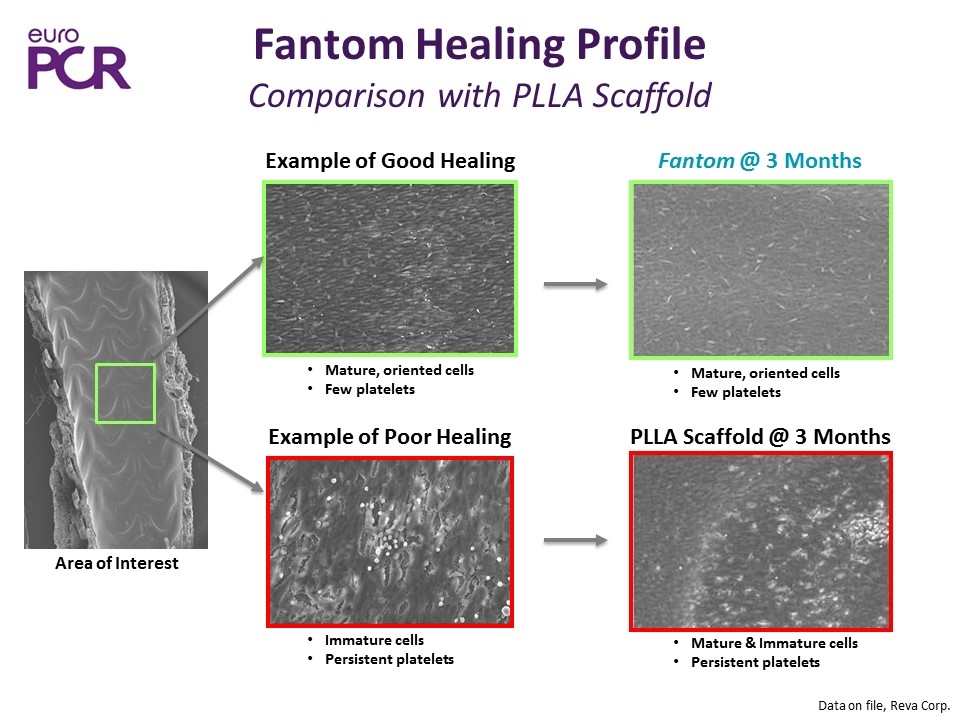
Fantom Healing Profile Comparison with PLLA Scaffold Data on file, Reva Corp. Example of Good Healing Mature, oriented cells Few platelets Immature cells Persistent platelets Area of Interest Fantom @ 3 Months Mature, oriented cells Few platelets Mature & Immature cells Persistent platelets PLLA Scaffold @ 3 Months Example of Poor Healing
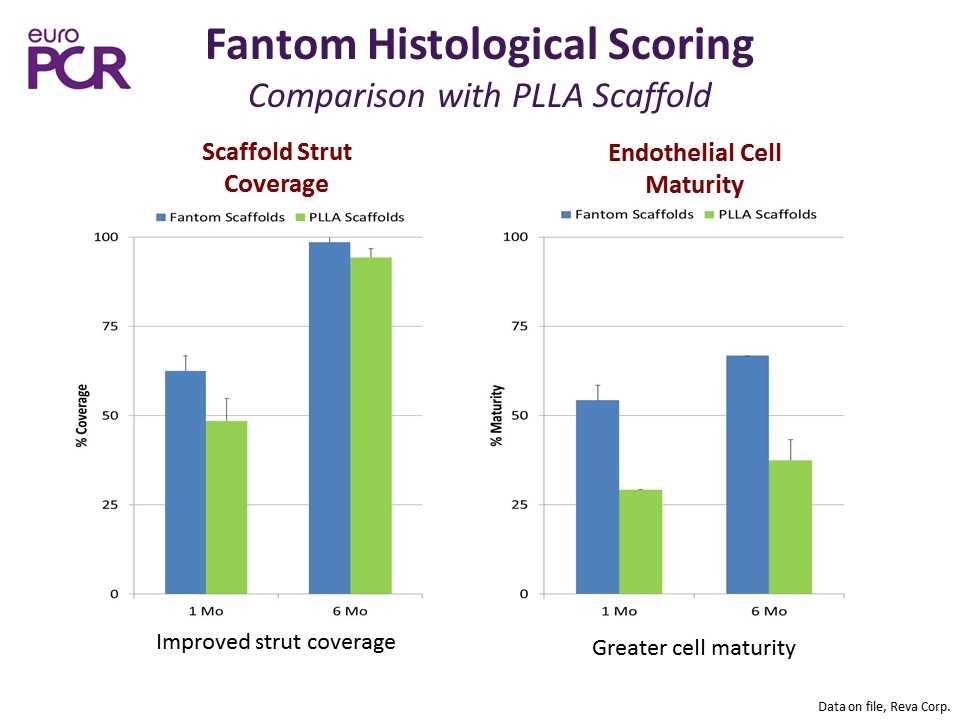
Fantom Histological Scoring Comparison with PLLA Scaffold Data on file, Reva Corp. Scaffold Strut Coverage Endothelial Cell Maturity Improved strut coverage Greater cell maturity

Fantom Polymer Potential Clinical Advantages
Fantom Potential Clinical Advantages Fantom’s novel polymer material provides several unique advantages Radiographic visibility Improved deliverability Continuous non-interrupted inflation Greater post-dilation (expansion) capability No special storage or handling requirements Return of vasomotion within 12 months
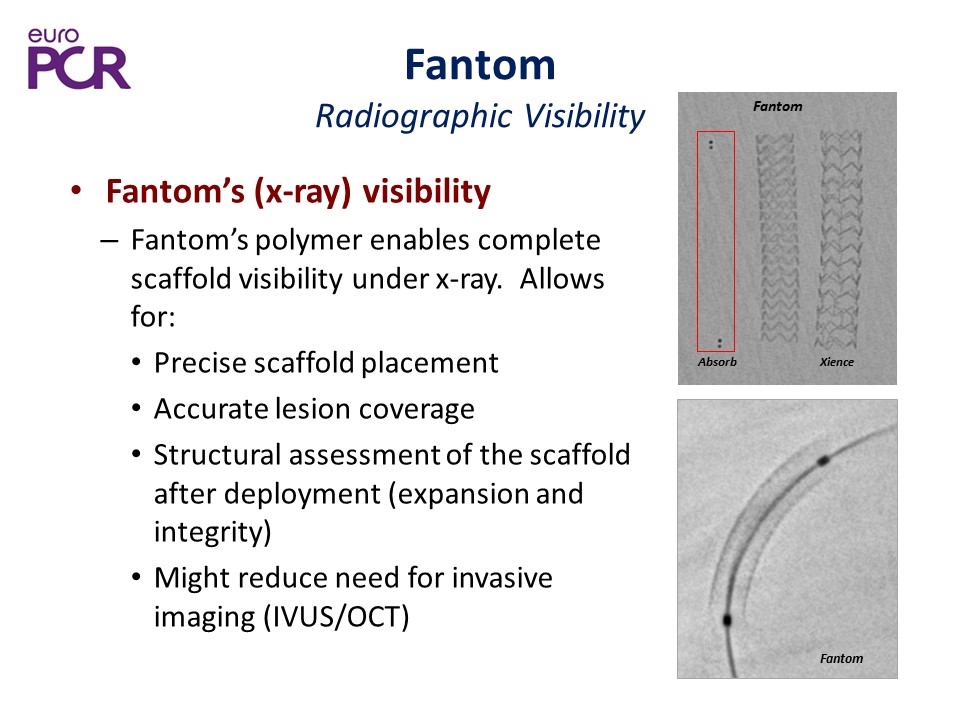
Fantom Radiographic Visibility Fantom’s (x-ray) visibility Fantom’s polymer enables complete scaffold visibility under x-ray. Allows for: Precise scaffold placement Accurate lesion coverage Structural assessment of the scaffold after deployment (expansion and integrity) Might reduce need for invasive imaging (IVUS/OCT) Absorb Fantom Xience Fantom
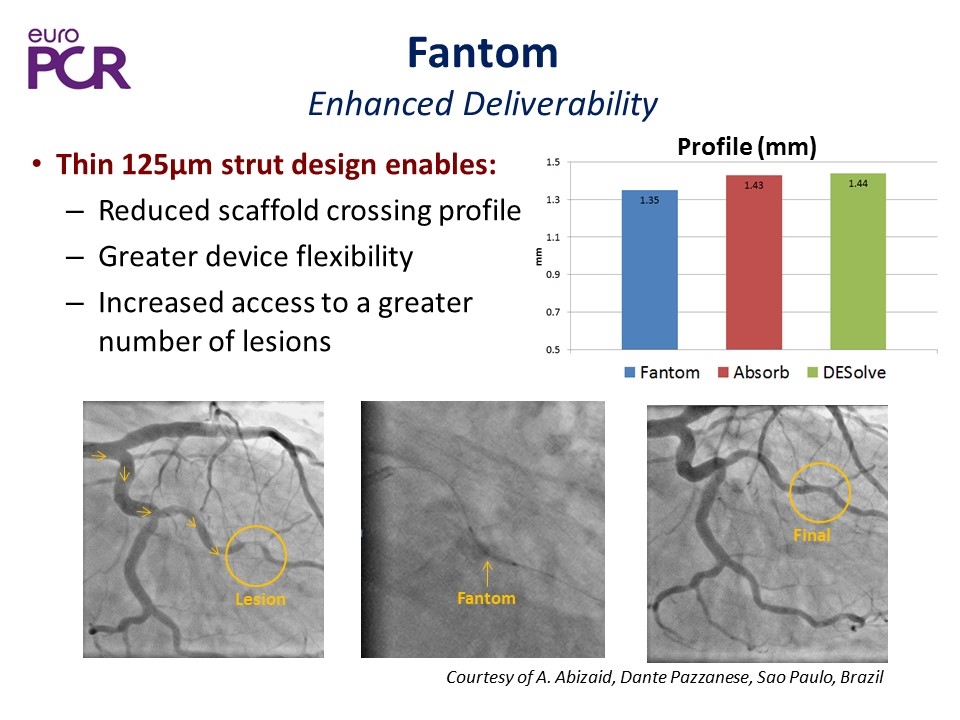
Fantom Enhanced Deliverability Thin 125µm strut design enables: Reduced scaffold crossing profile Greater device flexibility Increased access to a greater number of lesions Final Fantom Lesion Courtesy of A. Abizaid, Dante Pazzanese, Sao Paulo, Brazil Profile (mm)
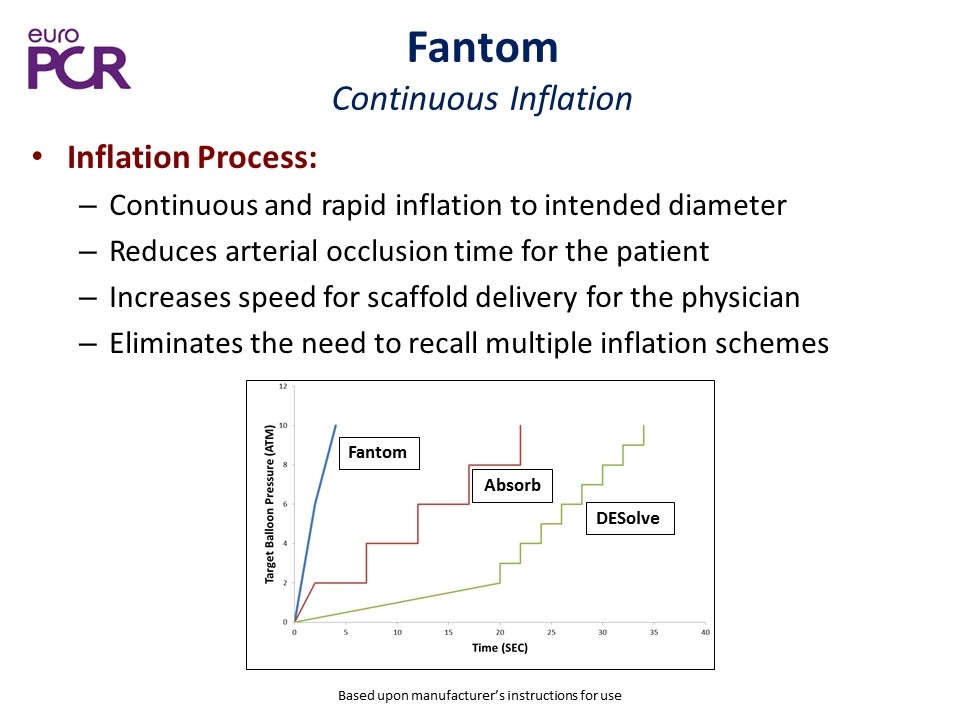
Fantom Continuous Inflation Inflation Process: Continuous and rapid inflation to intended diameter Reduces arterial occlusion time for the patient Increases speed for scaffold delivery for the physician Eliminates the need to recall multiple inflation schemes Fantom Absorb DESolve Based upon manufacturer’s instructions for use
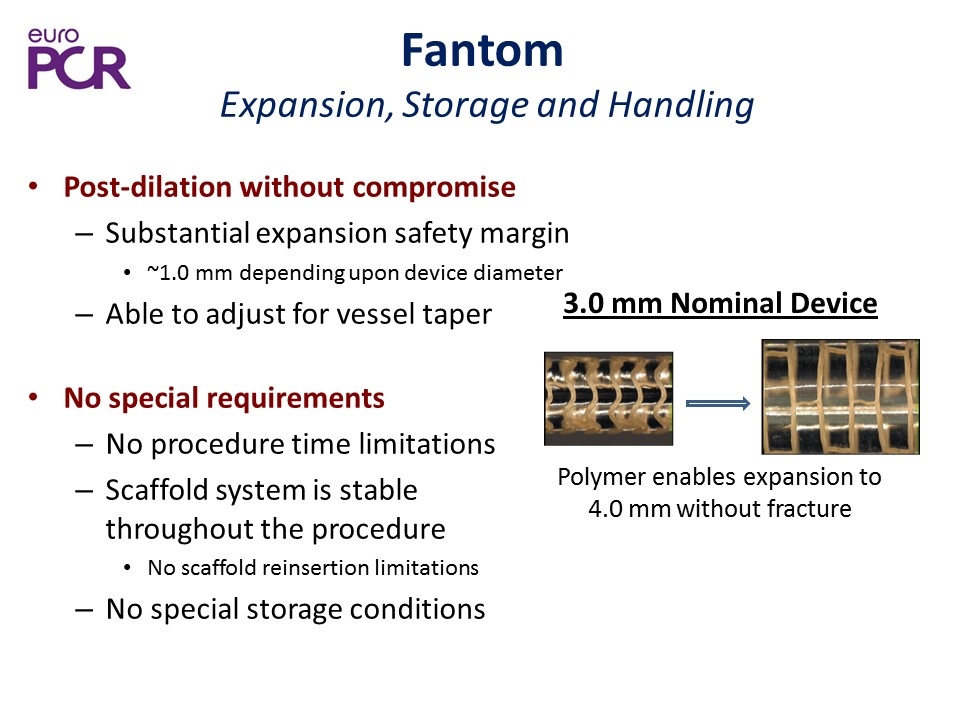
Post-dilation without compromise Substantial expansion safety margin ~1.0 mm depending upon device diameter Able to adjust for vessel taper No special requirements No procedure time limitations Scaffold system is stable throughout the procedure No scaffold reinsertion limitations No special storage conditions Polymer enables expansion to 4.0 mm without fracture 3.0 mm Nominal Device Fantom Expansion, Storage and Handling
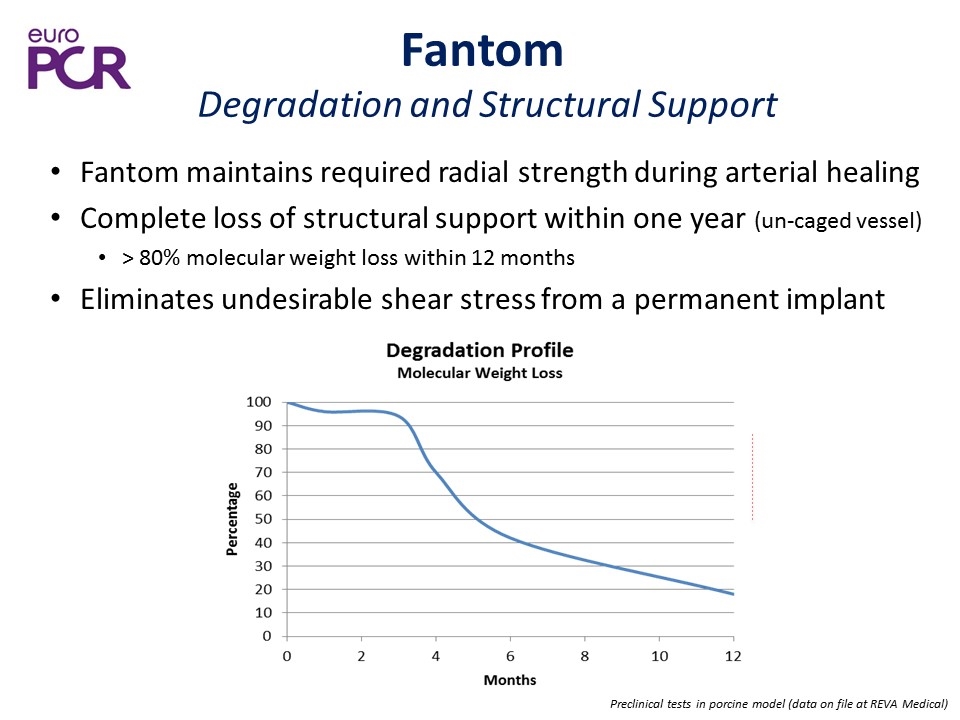
Fantom maintains required radial strength during arterial healing Complete loss of structural support within one year (un-caged vessel) > 80% molecular weight loss within 12 months Eliminates undesirable shear stress from a permanent implant Preclinical tests in porcine model (data on file at REVA Medical) Fantom Degradation and Structural Support
Fantom Iodinated Desaminotryrosine (DAT)-based polymer provides: Superior ease of use features, including scaffold visibility and metallic DES-like storage, handling and expansion characteristics Rapid endothelialization and vascular healing Complete scaffold bioabsorption and tissue integration within 3-4 years Fantom Materials Summary


























