
FANTOM II: Six-Month Clinical and Angiographic Results With a Radiopaque Desaminotyrosine Polycarbonate-Based Sirolimus-Eluting Bioresorbable Vascular Scaffold in Patients With Coronary Artery Disease Dr. Alexandre Abizaid Instituto Dante Pazzanese de Cardiologia Sao Paulo, Brazil On behalf of the FANTOM II investigators FANTOM II Exhibit 99.2

Disclosure Statement of Financial Interest Research Grants Consulting Fees/Honoraria Abbott Vascular Boston Scientific Elixir Medical Medtronic REVA Medical Within the past 12 months, I or my spouse/partner have had a financial interest/arrangement or affiliation with the organization(s) listed below. Affiliation/Financial Relationship Company
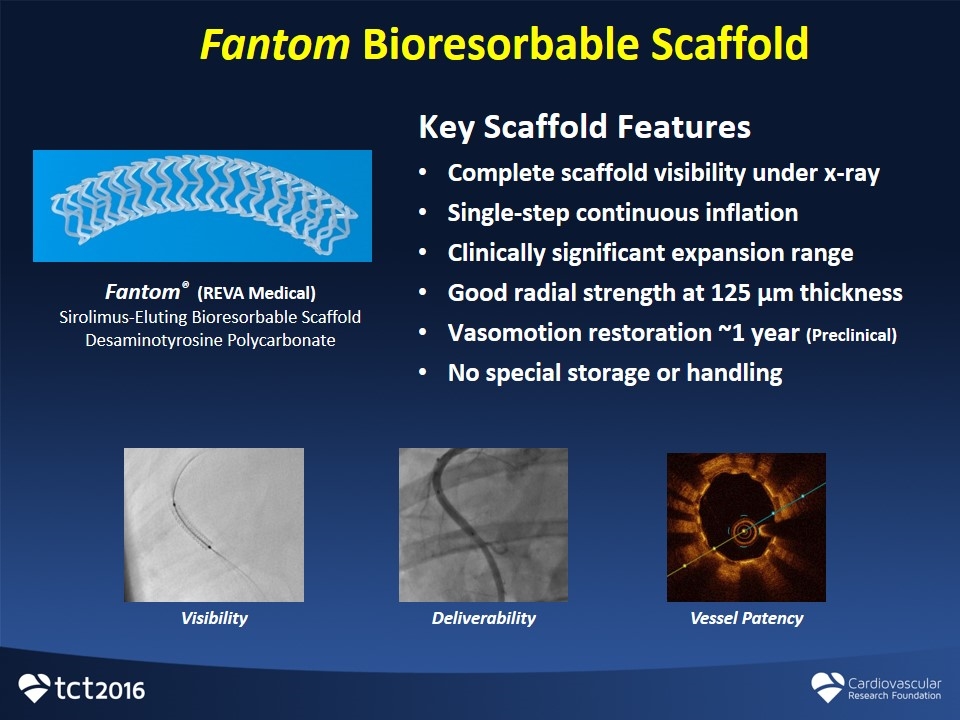
Fantom Bioresorbable Scaffold Fantom® (REVA Medical) Sirolimus-Eluting Bioresorbable Scaffold Desaminotyrosine Polycarbonate Key Scaffold Features Complete scaffold visibility under x-ray Single-step continuous inflation Clinically significant expansion range Good radial strength at 125 µm thickness Vasomotion restoration ~1 year (Preclinical) No special storage or handling Deliverability Vessel Patency Visibility
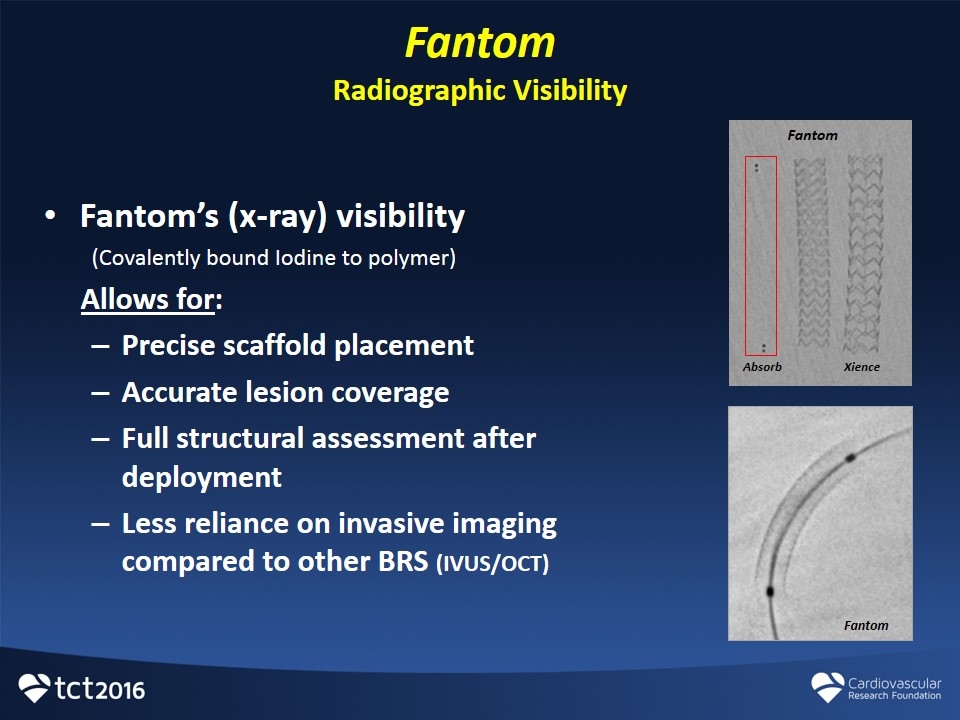
Fantom Radiographic Visibility Fantom’s (x-ray) visibility (Covalently bound Iodine to polymer) Allows for: Precise scaffold placement Accurate lesion coverage Full structural assessment after deployment Less reliance on invasive imaging compared to other BRS (IVUS/OCT) Absorb Fantom Xience Fantom
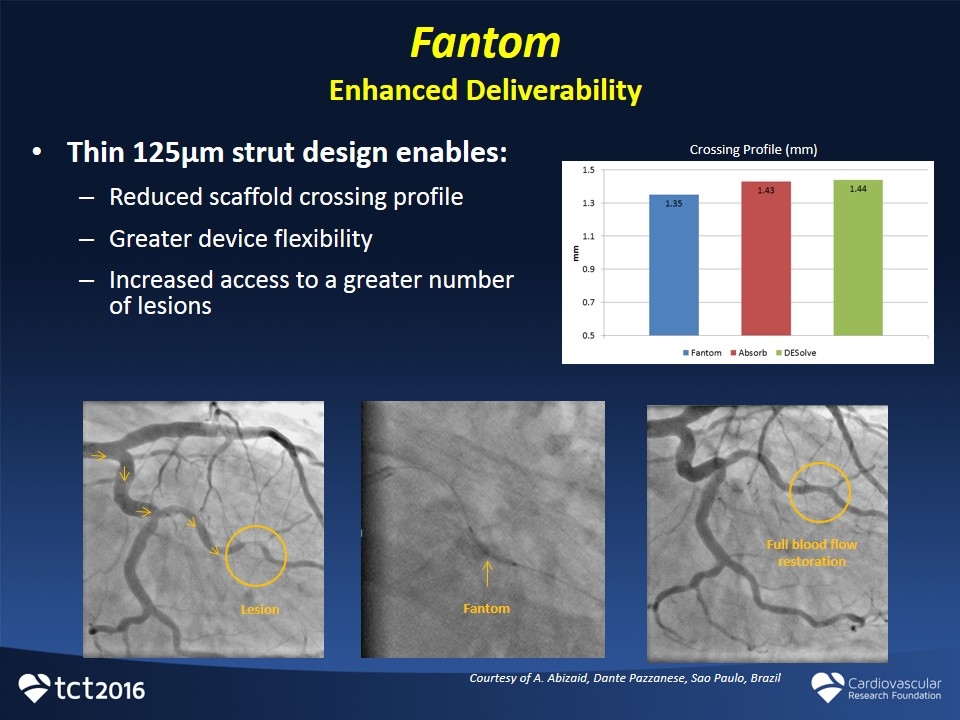
Fantom Enhanced Deliverability Thin 125µm strut design enables: Reduced scaffold crossing profile Greater device flexibility Increased access to a greater number of lesions Full blood flow restoration Fantom Lesion Courtesy of A. Abizaid, Dante Pazzanese, Sao Paulo, Brazil Crossing Profile (mm)

Inflation process: Continuous inflation to intended diameter Reduces arterial occlusion time for the patient Increases speed for scaffold delivery for the physician Eliminates the need to recall multiple inflation schemes Fantom Bioresorbable Scaffold Single Step Inflation REVAs Advanced Polymer enables single step inflation
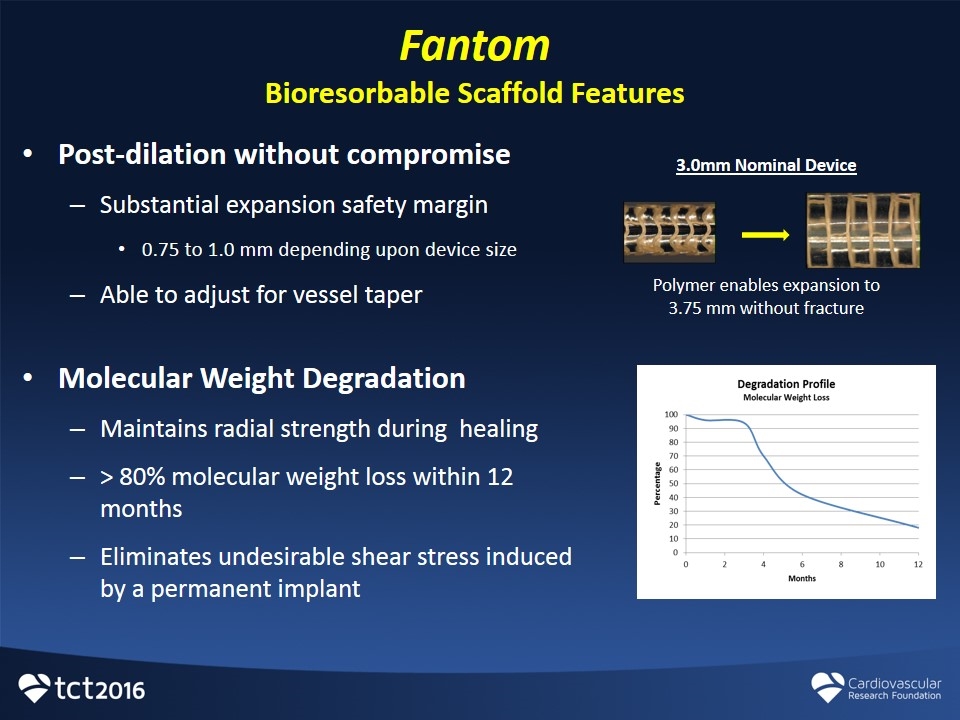
Fantom Bioresorbable Scaffold Features Post-dilation without compromise Substantial expansion safety margin 0.75 to 1.0 mm depending upon device size Able to adjust for vessel taper Molecular Weight Degradation Maintains radial strength during healing > 80% molecular weight loss within 12 months Eliminates undesirable shear stress induced by a permanent implant Polymer enables expansion to 3.75 mm without fracture 3.0mm Nominal Device
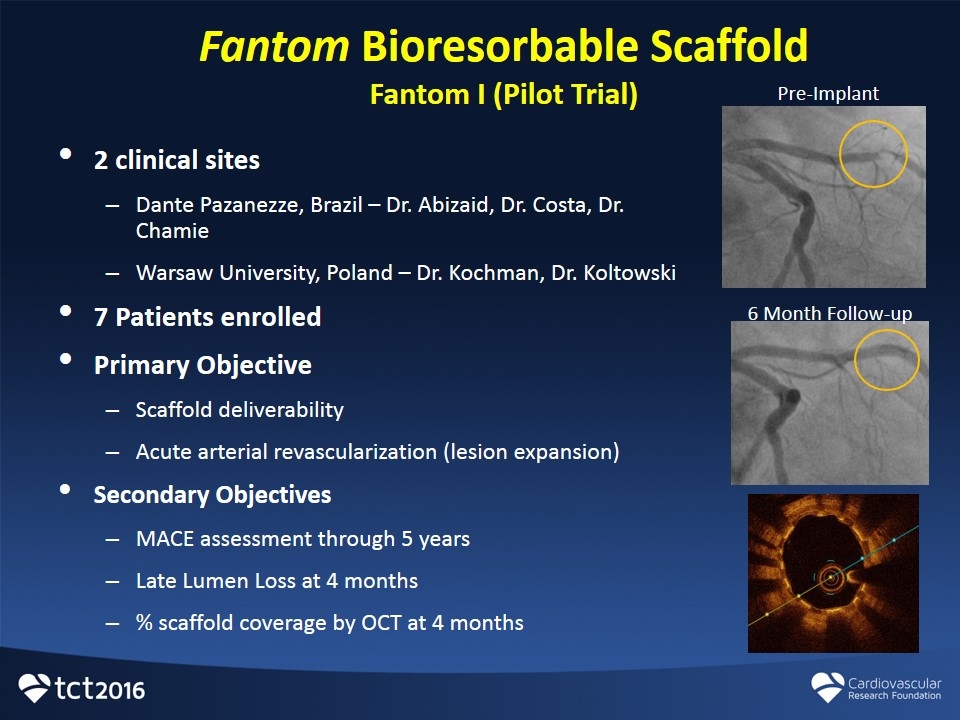
Fantom Bioresorbable Scaffold Fantom I (Pilot Trial) 2 clinical sites Dante Pazanezze, Brazil – Dr. Abizaid, Dr. Costa, Dr. Chamie Warsaw University, Poland – Dr. Kochman, Dr. Koltowski 7 Patients enrolled Primary Objective Scaffold deliverability Acute arterial revascularization (lesion expansion) Secondary Objectives MACE assessment through 5 years Late Lumen Loss at 4 months % scaffold coverage by OCT at 4 months Pre-Implant 6 Month Follow-up

FANTOM II Trial Safety & Performance Study for the Fantom Sirolimus-Eluting Bioresorbable Coronary Scaffold

FANTOM II Study Investigators Australia Dr. Muller, Dr. Jepson, Dr. Walters Belgium Dr. De Bruyne Brazil Dr. Abizaid, Dr. Costa, Dr. Chamie, Dr. Perin Denmark Dr. Christiansen, Dr. Lassen, Dr. Okkels-Jensen France Dr. Carrié, Dr. Chevalier, Dr. Fajadet, Dr. Collet Germany Dr. Weber-Albers, Dr. Naber, Dr. Achenbach, Dr. Frey, Dr. Lutz, Dr. Kische, Dr. Ince, Dr. Brachman Netherlands Dr. Amoroso, Dr. Wykrzykowska, Dr. Daemen Poland Dr. Dudek, Dr. Kochman, Dr. Koltowski, Dr. Lesiak, Dr. Wojdyla
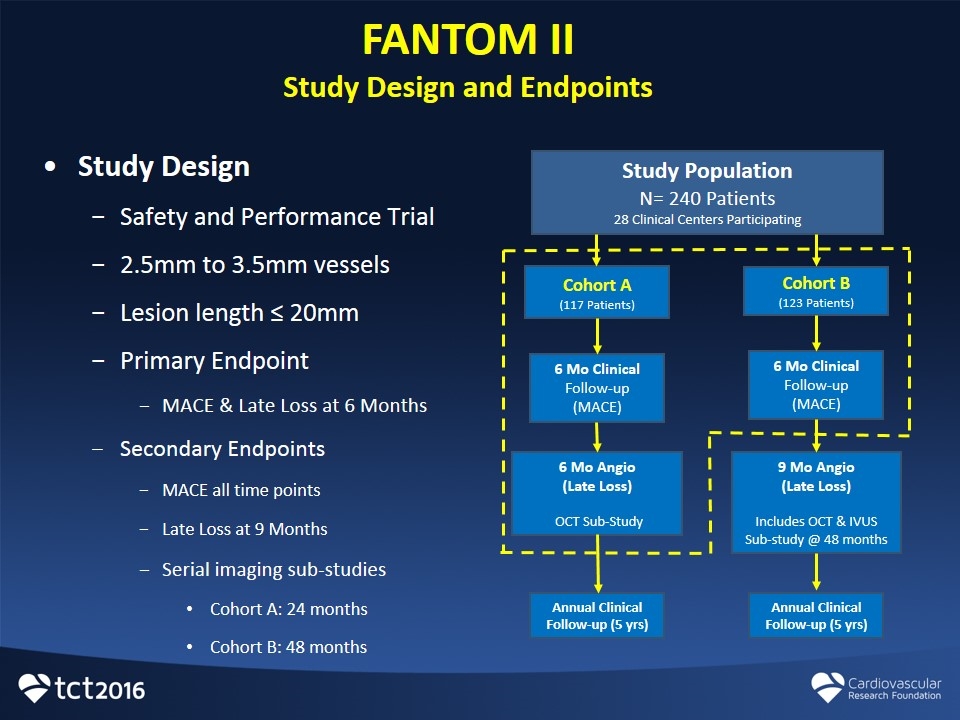
FANTOM II Study Design and Endpoints Study Design Safety and Performance Trial 2.5mm to 3.5mm vessels Lesion length ≤ 20mm Primary Endpoint MACE & Late Loss at 6 Months Secondary Endpoints MACE all time points Late Loss at 9 Months Serial imaging sub-studies Cohort A: 24 months Cohort B: 48 months Study Population N= 240 Patients 28 Clinical Centers Participating Cohort A (117 Patients) 6 Mo Clinical Follow-up (MACE) Cohort B (123 Patients) 6 Mo Clinical Follow-up (MACE) 6 Mo Angio (Late Loss) OCT Sub-Study 9 Mo Angio (Late Loss) Includes OCT & IVUS Sub-study @ 48 months Annual Clinical Follow-up (5 yrs) Annual Clinical Follow-up (5 yrs)
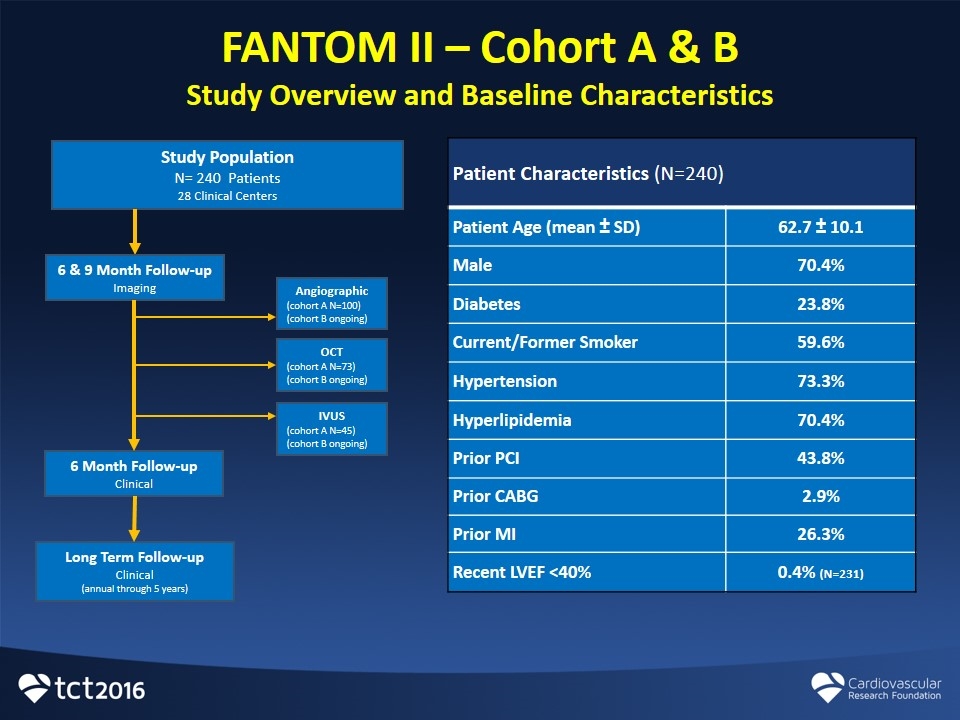
FANTOM II – Cohort A & B Study Overview and Baseline Characteristics Patient Characteristics (N=240) Patient Age (mean ± SD) 62.7 ± 10.1 Male 70.4% Diabetes 23.8% Current/Former Smoker 59.6% Hypertension 73.3% Hyperlipidemia 70.4% Prior PCI 43.8% Prior CABG 2.9% Prior MI 26.3% Recent LVEF <40% 0.4% (N=231) Study Population N= 240 Patients 28 Clinical Centers 6 & 9 Month Follow-up Imaging 6 Month Follow-up Clinical Angiographic (cohort A N=100) (cohort B ongoing) OCT (cohort A N=73) (cohort B ongoing) IVUS (cohort A N=45) (cohort B ongoing) Long Term Follow-up Clinical (annual through 5 years)
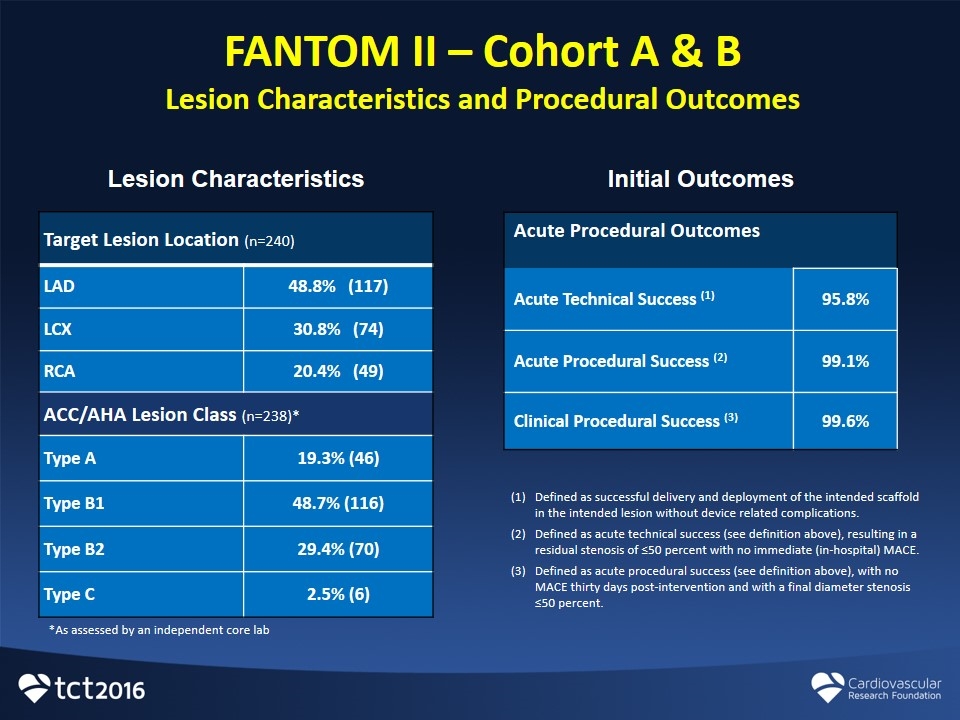
FANTOM II – Cohort A & B Lesion Characteristics and Procedural Outcomes Target Lesion Location (n=240) LAD 48.8% (117) LCX 30.8% (74) RCA 20.4% (49) ACC/AHA Lesion Class (n=238)* Type A 19.3% (46) Type B1 48.7% (116) Type B2 29.4% (70) Type C 2.5% (6) Lesion Characteristics Initial Outcomes Acute Procedural Outcomes Acute Technical Success (1) 95.8% Acute Procedural Success (2) 99.1% Clinical Procedural Success (3) 99.6% Defined as successful delivery and deployment of the intended scaffold in the intended lesion without device related complications. Defined as acute technical success (see definition above), resulting in a residual stenosis of ≤50 percent with no immediate (in-hospital) MACE. Defined as acute procedural success (see definition above), with no MACE thirty days post-intervention and with a final diameter stenosis ≤50 percent. *As assessed by an independent core lab
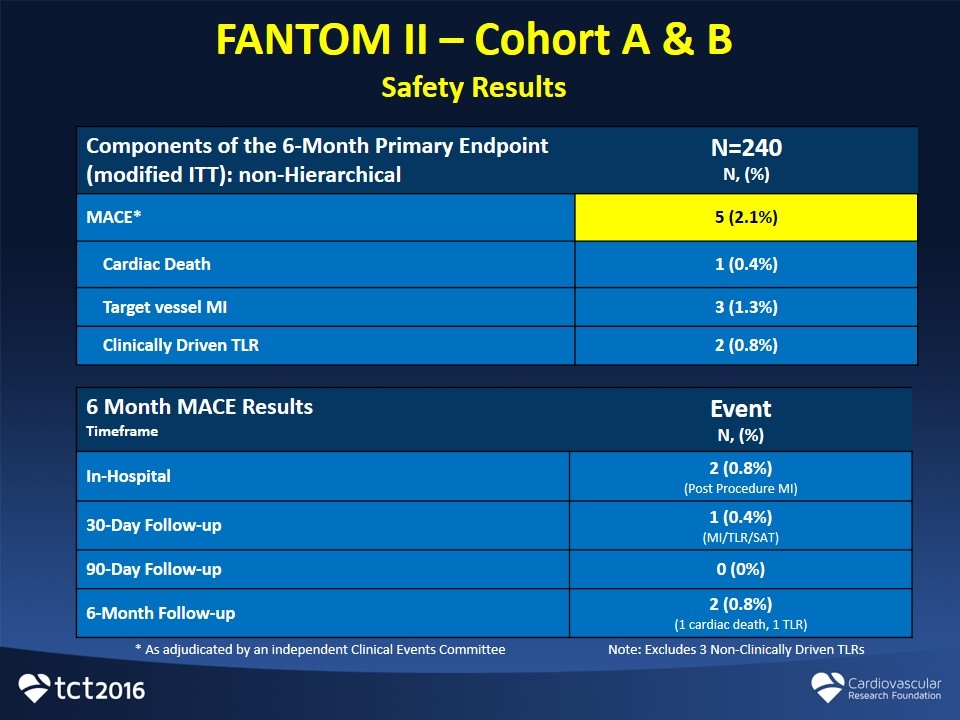
FANTOM II – Cohort A & B Safety Results 6 Month MACE Results Timeframe Event N, (%) In-Hospital 2 (0.8%) (Post Procedure MI) 30-Day Follow-up 1 (0.4%) (MI/TLR/SAT) 90-Day Follow-up 0 (0%) 6-Month Follow-up 2 (0.8%) (1 cardiac death, 1 TLR) Components of the 6-Month Primary Endpoint (modified ITT): non-Hierarchical N=240 N, (%) MACE* 5 (2.1%) Cardiac Death 1 (0.4%) Target vessel MI 3 (1.3%) Clinically Driven TLR 2 (0.8%) * As adjudicated by an independent Clinical Events Committee Note: Excludes 3 Non-Clinically Driven TLRs
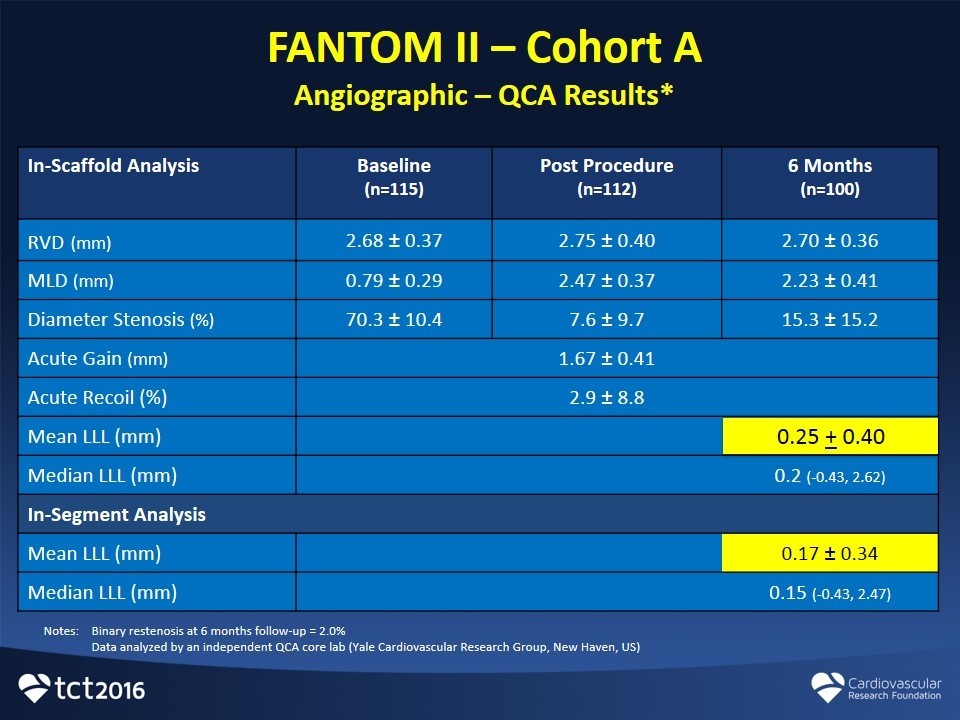
FANTOM II – Cohort A Angiographic – QCA Results* In-Scaffold Analysis Baseline (n=115) Post Procedure (n=112) 6 Months (n=100) RVD (mm) 2.68 ± 0.37 2.75 ± 0.40 2.70 ± 0.36 MLD (mm) 0.79 ± 0.29 2.47 ± 0.37 2.23 ± 0.41 Diameter Stenosis (%) 70.3 ± 10.4 7.6 ± 9.7 15.3 ± 15.2 Acute Gain (mm) 1.67 ± 0.41 Acute Recoil (%) 2.9 ± 8.8 Mean LLL (mm) 0.5 ± 0.38 Median LLL (mm) 0.2 (-0.43, 2.62) In-Segment Analysis Mean LLL (mm) 0.17 ± 0.34 Median LLL (mm) 0.15 (-0.43, 2.47) Notes:Binary restenosis at 6 months follow-up = 2.0% Data analyzed by an independent QCA core lab (Yale Cardiovascular Research Group, New Haven, US) 0.25 + 0.40
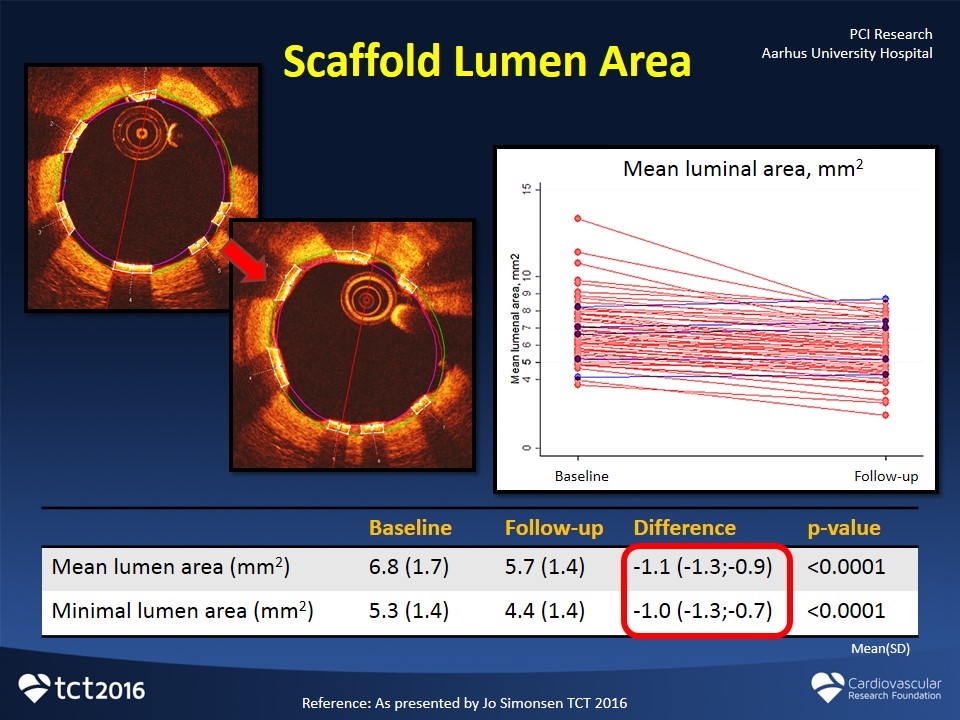
Scaffold Lumen Area Baseline Follow-up Difference p-value Mean lumen area (mm2) 6.8 (1.7) 5.7 (1.4) -1.1 (-1.3;-0.9) <0.0001 Minimal lumen area (mm2) 5.3 (1.4) 4.4 (1.4) -1.0 (-1.3;-0.7) <0.0001 Mean luminal area, mm2 Baseline Follow-up Mean(SD) Reference: As presented by Jo Simonsen TCT 2016 PCI Research Aarhus University Hospital

Stent Area Baseline Follow-up Difference p-value Mean stent area (mm2) 7.1 (1.5) 7.2 (1.4) 0.1 (-0.02;0.24) 0.12 Minimal stent area (mm2) 5.9 (1.3) 6.0 (1.3) 0.1 (-0.02;0.25) 0.08 Mean stent area, mm2 Baseline Follow-up Mean(SD) Reference: As presented by Jo Simonsen TCT 2016 PCI Research Aarhus University Hospital

Strut Coverage Follow-up Covered struts 98.1% (95.9;99.4) Median(IQR) Reference: As presented by Jo Simonsen TCT 2016 PCI Research Aarhus University Hospital

FANTOM II Case Review
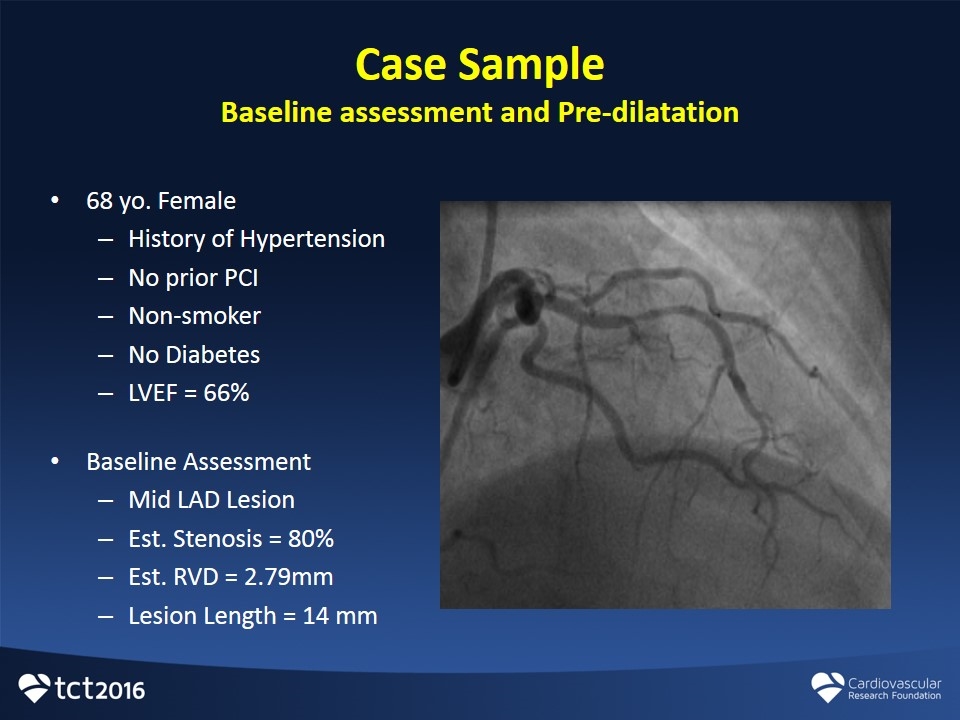
68 yo. Female History of Hypertension No prior PCI Non-smoker No Diabetes LVEF = 66% Baseline Assessment Mid LAD Lesion Est. Stenosis = 80% Est. RVD = 2.79mm Lesion Length = 14 mm Case Sample Baseline assessment and Pre-dilatation
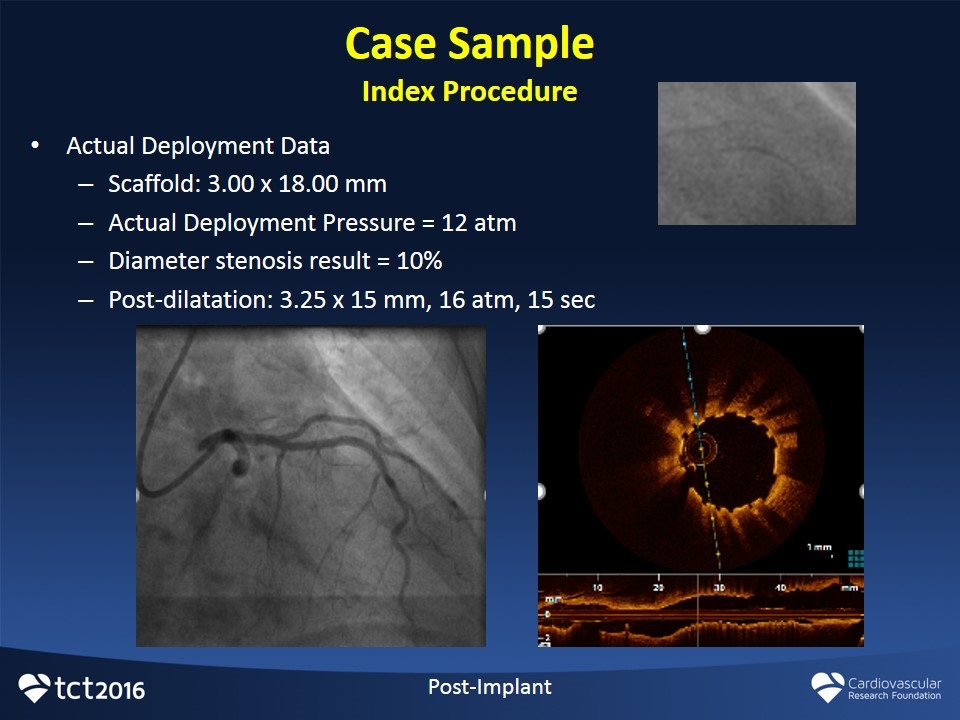
Case Sample Index Procedure Post-Implant Actual Deployment Data Scaffold: 3.00 x 18.00 mm Actual Deployment Pressure = 12 atm Diameter stenosis result = 10% Post-dilatation: 3.25 x 15 mm, 16 atm, 15 sec
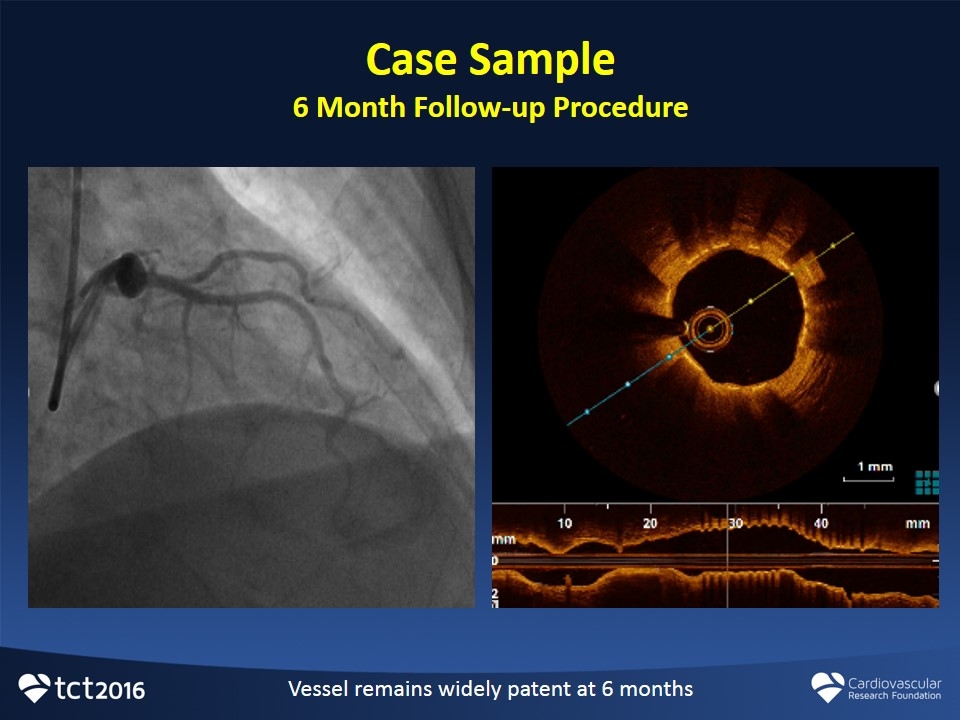
Case Sample 6 Month Follow-up Procedure Vessel remains widely patent at 6 months

FANTOM Program Clinical Summary Fantom offers new and clinically important features Radiopacity Deliverability Single-step inflation No special handling requirements Initial clinical data demonstrates Good acute performance Enhanced device deliverability Minimal residual stenosis and acute recoil (3%) Sustained performance and safety through 6 months Low MACE Rate (2.1%) Low late lumen loss (0.25mm)

Thanks!























