
Pioneering the Development of Engineered IgM Antibodies Corporate Presentation December 2020 Exhibit 99.2
Forward-Looking Statements This presentation contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 that reflect the current views of IGM Biosciences, Inc. (the “Company,” “we” or “our”) with respect to the Company’s future financial condition, results of operations, business strategy, expectations, milestones and plans. All statements other than statements of historical fact could be deemed forward-looking, including but not limited to statements with words such as “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potentially” “predict,” “should,” “target,” “will” or the negative of these terms or other similar expressions. These forward-looking statements are subject to a number of risks, uncertainties and assumptions, including, among other things: market conditions; the timing of the initiation, progress and results of our preclinical studies, clinical trials and our discovery programs; potential delays and disruption resulting from the COVID-19 pandemic and governmental responses to the pandemic, including any future impacts to our operations, the manufacturing of our product candidates, the progression of our current clinical trials, and enrollment and maintaining patients in our current and future clinical trials; our early stages of clinical drug development; our ability to achieve clinical goals; risks related to the use of engineered IgM antibodies, which is a novel and unproven therapeutic approach; our ability to utilize our lgM antibody platform to generate and advance additional product candidates; our ability to advance product candidates into, and successfully complete, clinical trials; our ability to adequately demonstrate sufficient safety and efficacy of our product candidates; the timing or likelihood of regulatory filings and approvals; our estimates of the number of patients who suffer from the diseases we are targeting and the number of patients that may enroll in our clinical trials; the commercializing of our product candidates, if approved; our ability and the potential to successfully manufacture and supply our product candidates for clinical trials and for commercial use, if approved; our ability to accurately forecast future financial results and timelines; future strategic arrangements and/or collaborations and the potential benefits of such arrangements; our anticipated use of our existing resources, our estimates regarding expenses, future revenue, capital requirements and needs for additional financing and our ability to obtain additional capital; the sufficiency of our existing cash and investments to fund our future operating expenses and capital expenditure requirements; our ability to retain the continued service of our key personnel and to identify, hire and retain additional qualified professionals; the implementation of our business model, strategic plans for our business and product candidates; the scope of protection we are able to establish and maintain for intellectual property rights, including our lgM platform, product candidates and discovery programs; our ability to contract with third-party suppliers and manufacturers and their ability to perform adequately; the pricing, coverage and reimbursement of our product candidates, if approved; developments relating to our competitors and our industry, including competing product candidates and therapies; and other risks described in the “Risk Factors” section included in our public filings that we have made and will make with the Securities and Exchange Commission (“SEC”). New risk factors emerge from time to time and it is not possible for our management to predict all risk factors, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in, or implied by, any forward-looking statements. You should not rely upon forward-looking statements as predictions of future events. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance or achievements. Except as required by law, we undertake no obligation to update publicly any forward-looking statements for any reason after the date of this presentation. We have filed and will file Current Reports on Form 8-K, Quarterly Reports on Form 10-Q and Annual Reports on Form 10-K, and other documents with the SEC. You should read these documents for more complete information about us. You may obtain these documents for free by visiting EDGAR on the SEC website at www.sec.gov. This presentation concerns products that are under clinical investigation and which have not yet been approved for marketing by the U.S. Food and Drug Administration. It is currently limited by federal law to investigational use, and no representation is made as to its safety or efficacy for the purposes for which it is being investigated.
IGM Overview Global leaders in the development of engineered IgM antibodies for therapeutic use Lead Programs Proprietary IGM antibody technology: 27 patent families Strategy: extend our global leadership in the development of engineered IgM antibodies $180.2M Cash and Investments Balance, September 30, 2020 CD20 x CD3 | Non-Hodgkin’s Lymphoma | Phase 1 in R/R B cell NHL underway DR5 | Solid and Heme Malignancies | Phase 1 in solid tumors & NHL underway IL-15 x PD-L1 | Solid and Heme Malignancies | IND filing: 2021 (anticipated) Advance product candidates and increase research and development efforts Build and control manufacturing capabilities Participate in commercialization if approved Expand intellectual property portfolio
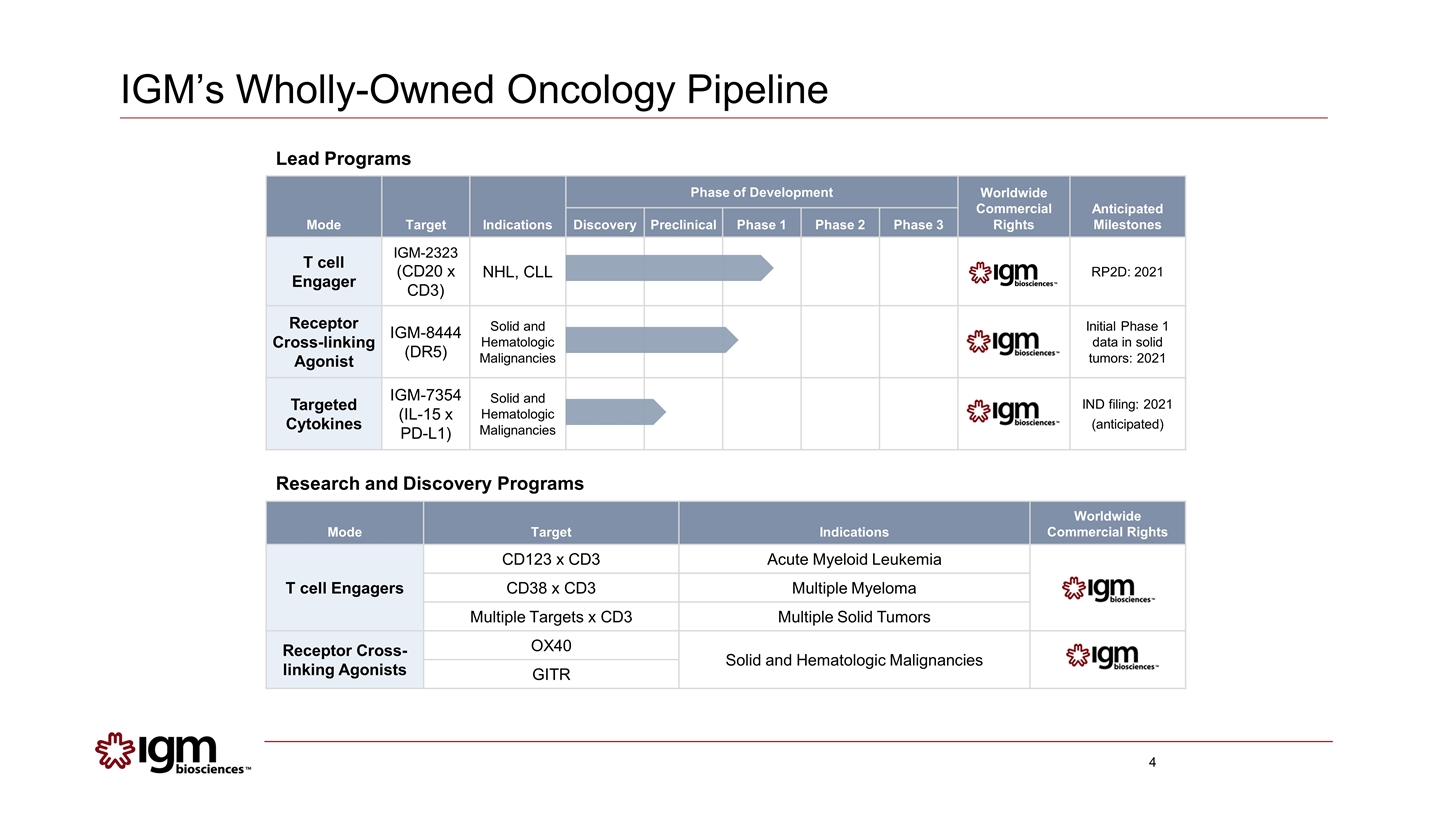
Mode Target Indications Worldwide Commercial Rights T cell Engagers CD123 x CD3 Acute Myeloid Leukemia CD38 x CD3 Multiple Myeloma Multiple Targets x CD3 Multiple Solid Tumors Receptor Cross- linking Agonists OX40 Solid and Hematologic Malignancies GITR Mode Target Indications Phase of Development Worldwide Commercial Rights Anticipated Milestones Discovery Preclinical Phase 1 Phase 2 Phase 3 T cell Engager IGM-2323 (CD20 x CD3) NHL, CLL RP2D: 2021 Receptor Cross-linking Agonist IGM-8444 (DR5) Solid and Hematologic Malignancies Initial Phase 1 data in solid tumors: 2021 Targeted Cytokines IGM-7354 (IL-15 x PD-L1) Solid and Hematologic Malignancies IND filing: 2021 (anticipated) Lead Programs Research and Discovery Programs IGM’s Wholly-Owned Oncology Pipeline
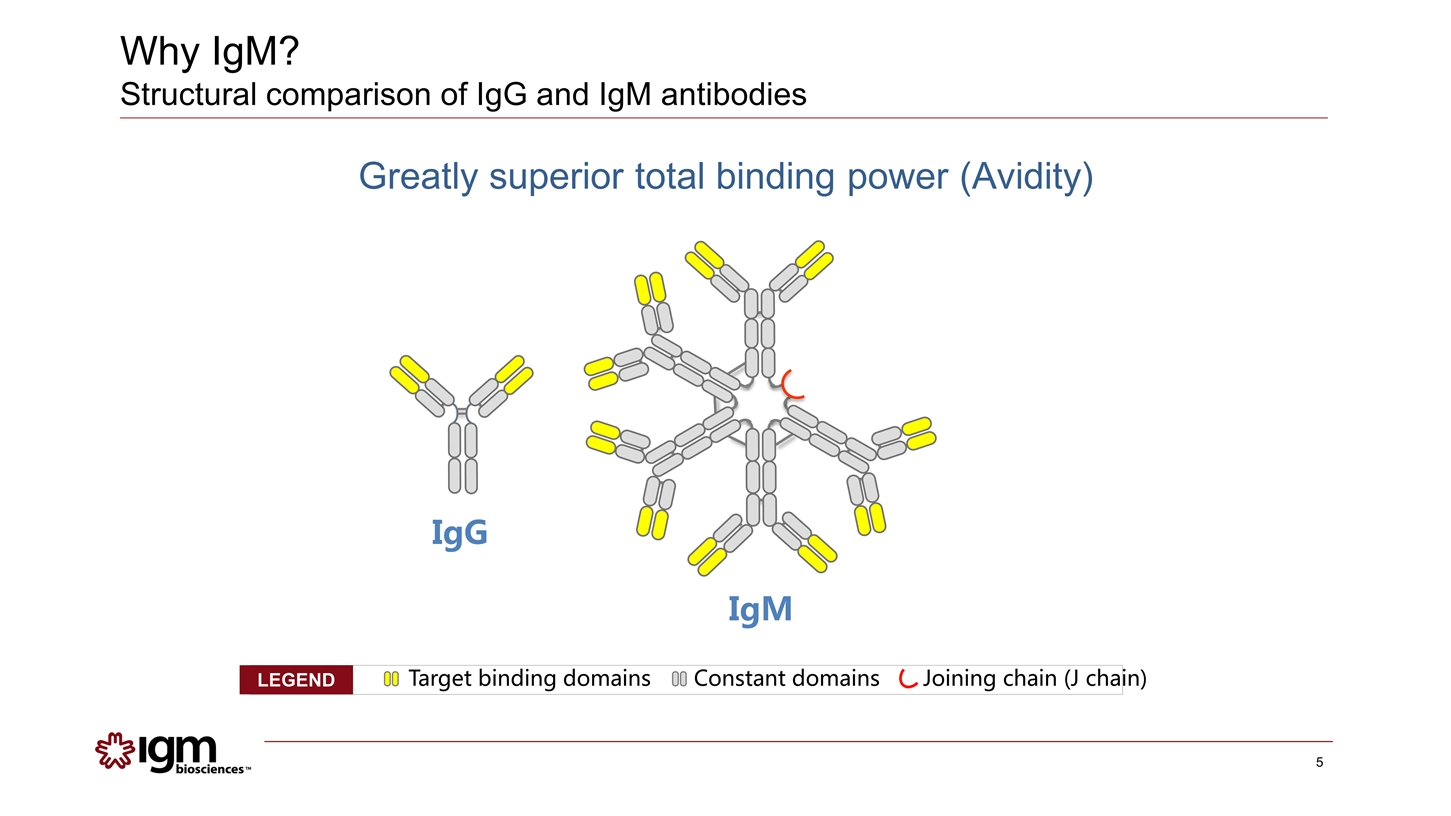
Why IgM? Structural comparison of IgG and IgM antibodies Greatly superior total binding power (Avidity) Target binding domains Constant domains Joining chain (J chain) LEGEND IgG IgM
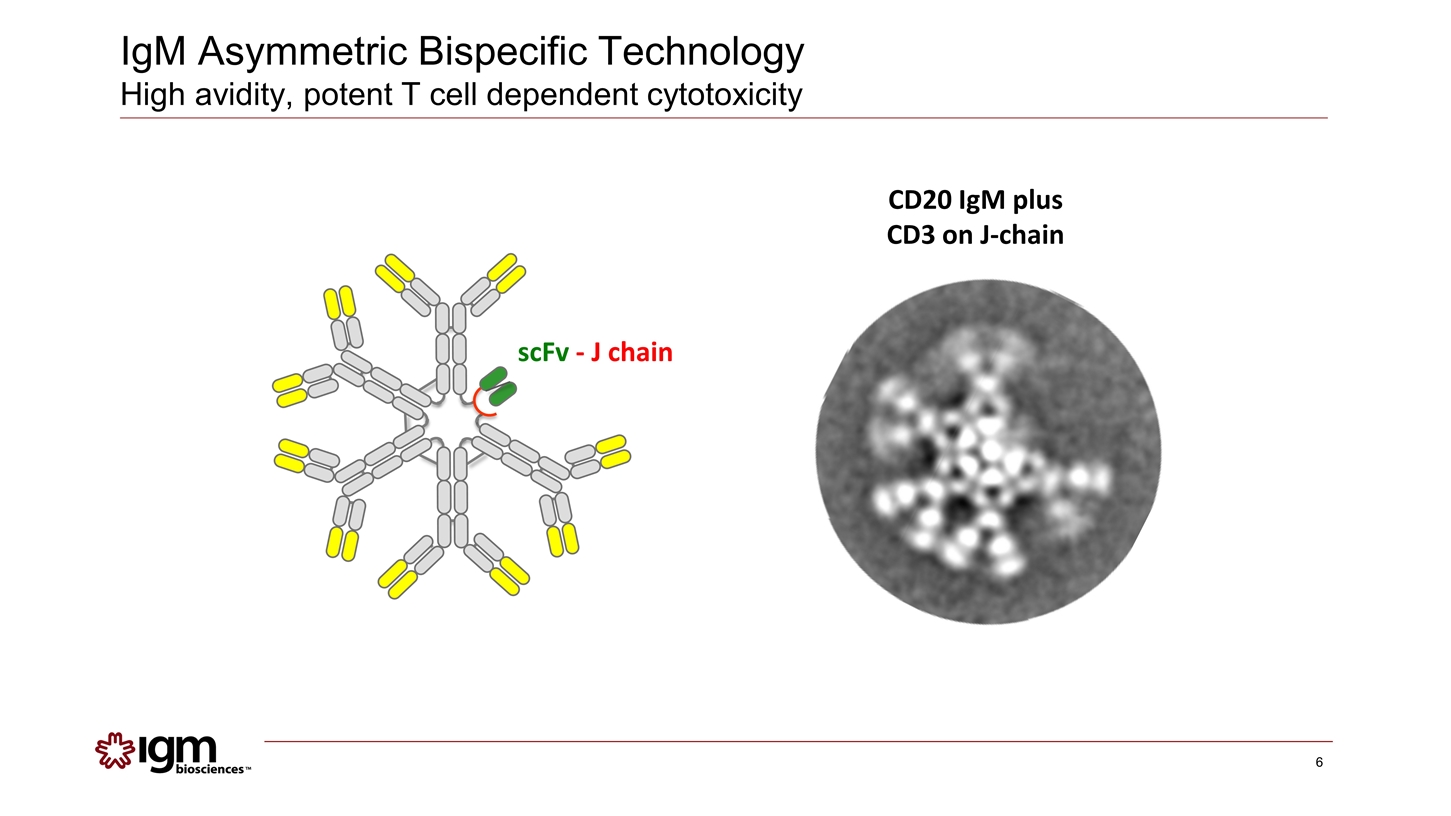
CD20 IgM plus CD3 on J-chain scFv - J chain IgM Asymmetric Bispecific Technology High avidity, potent T cell dependent cytotoxicity
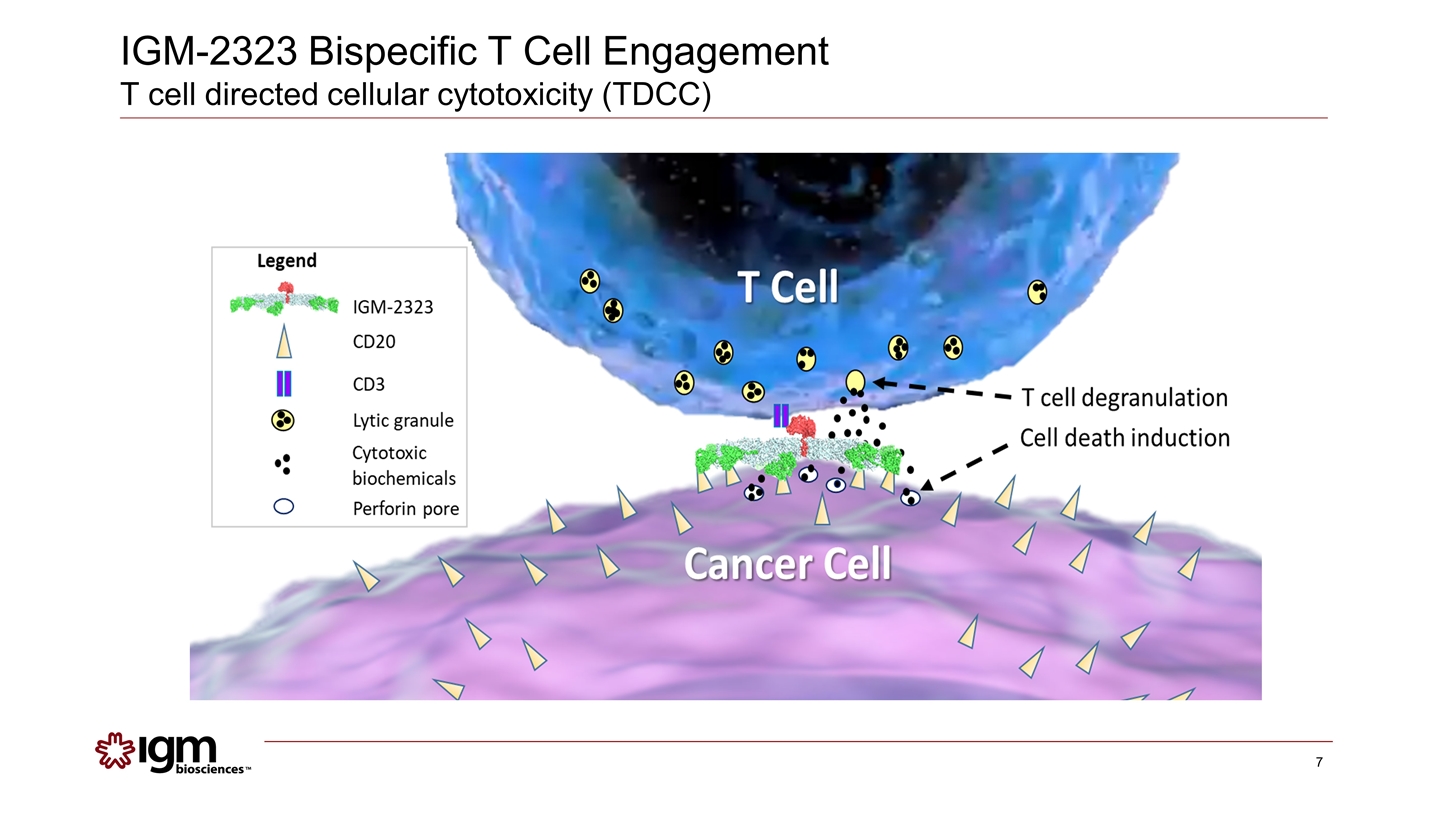
IGM-2323 Bispecific T Cell Engagement T cell directed cellular cytotoxicity (TDCC)
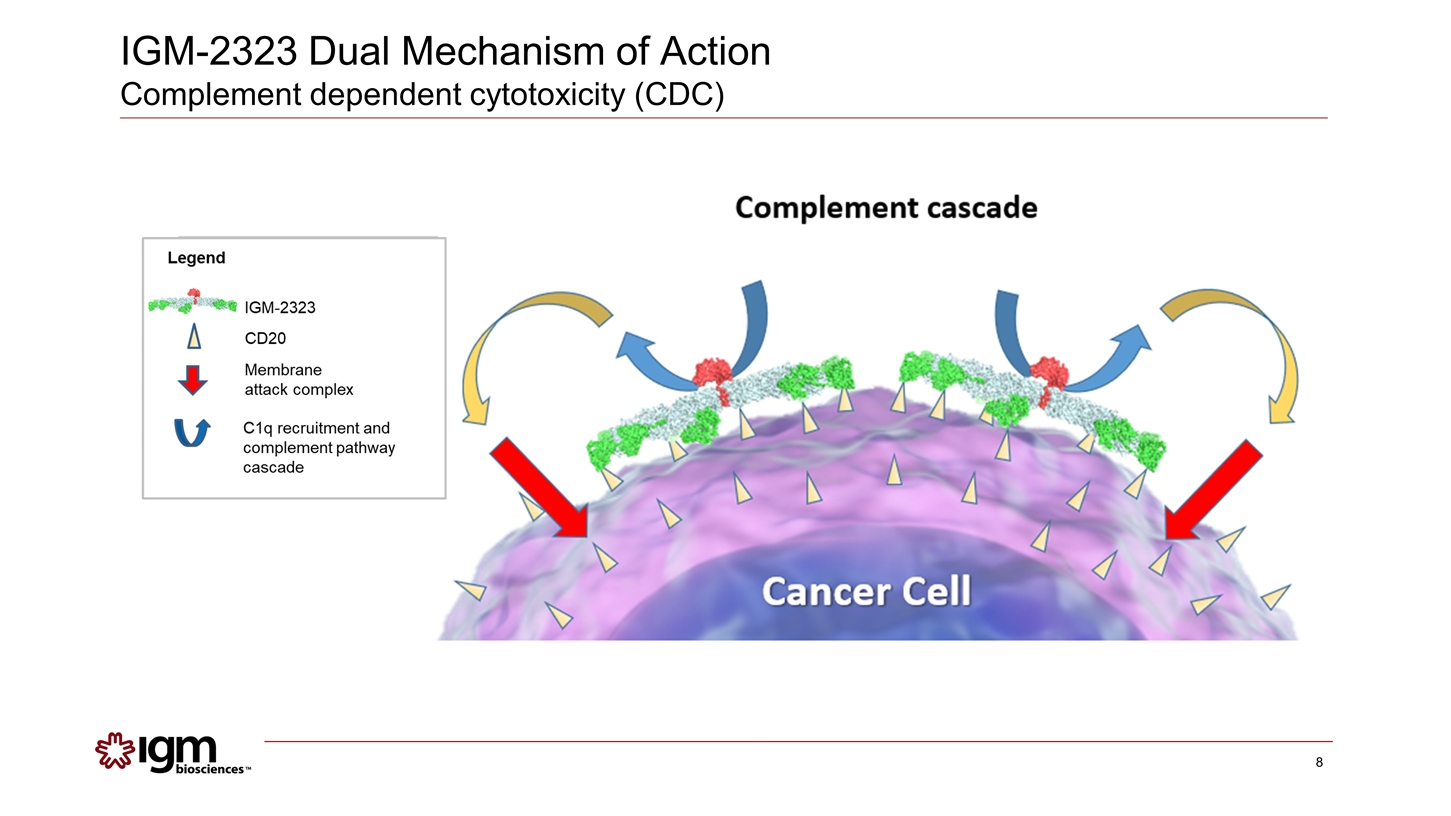
IGM-2323 Dual Mechanism of Action Complement dependent cytotoxicity (CDC)
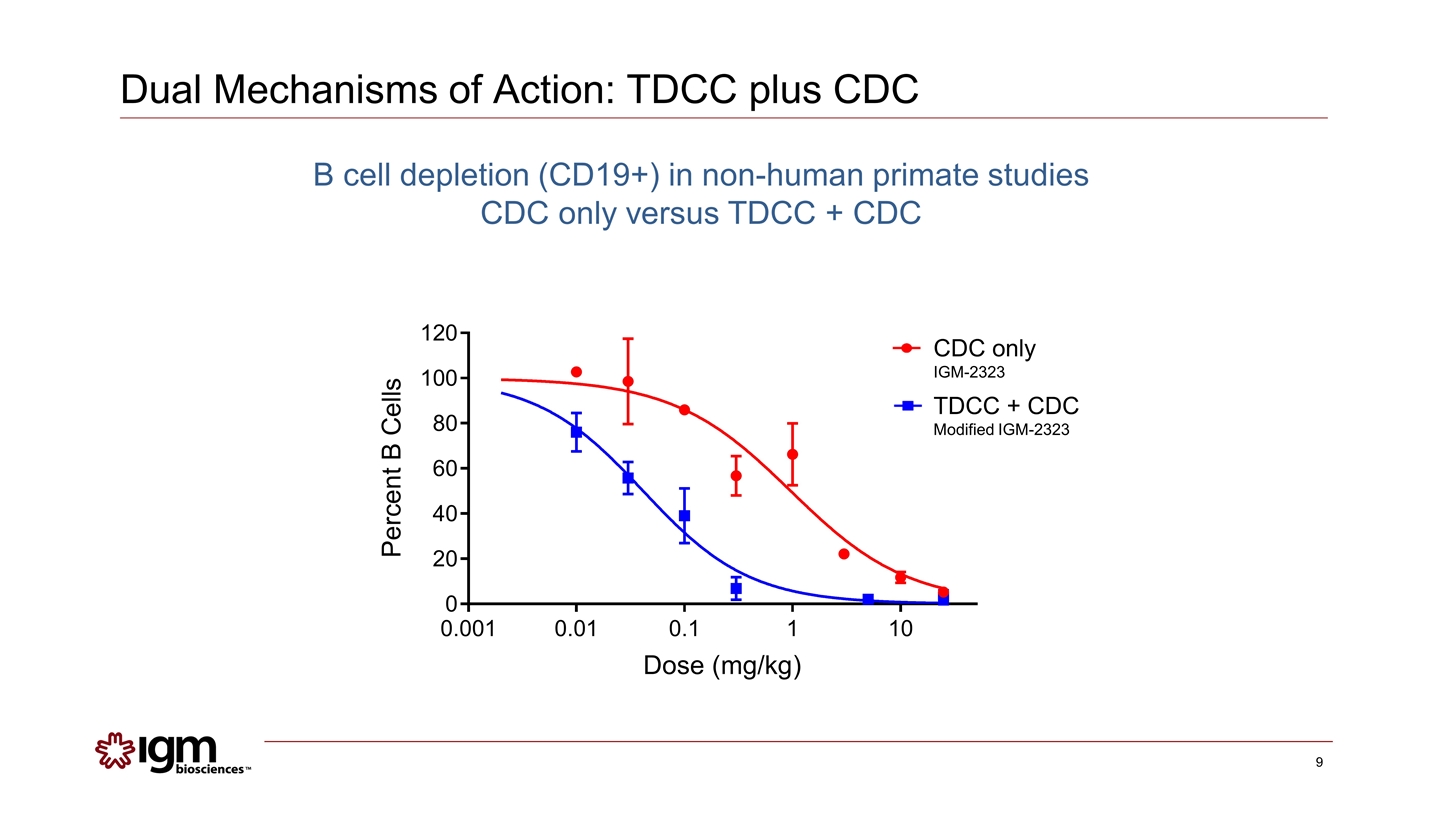
Dual Mechanisms of Action: TDCC plus CDC CDC only IGM-2323 TDCC + CDC Modified IGM-2323 B cell depletion (CD19+) in non-human primate studies CDC only versus TDCC + CDC
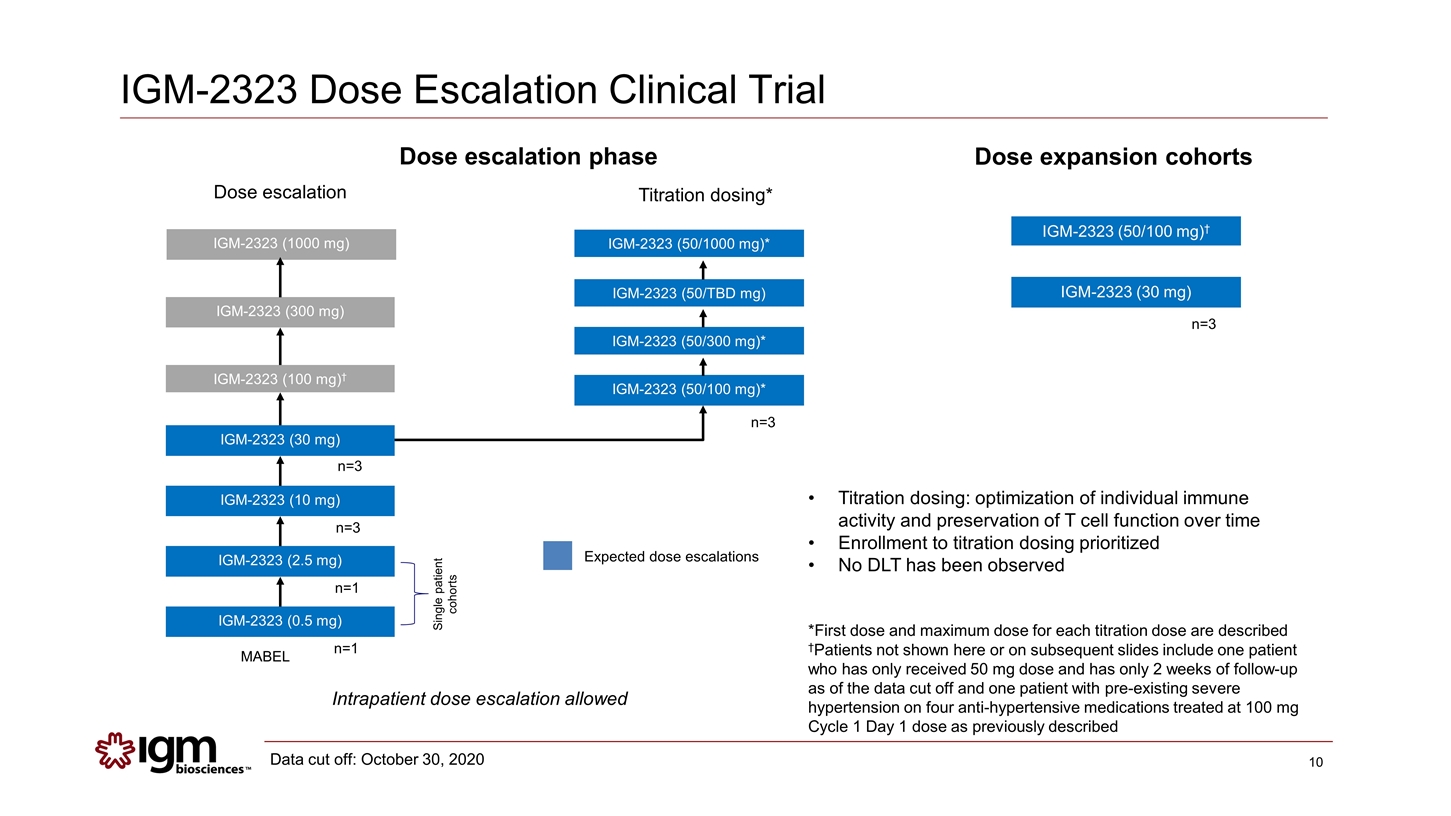
IGM-2323 Dose Escalation Clinical Trial Dose escalation phase IGM-2323 (0.5 mg) IGM-2323 (2.5 mg) IGM-2323 (10 mg) IGM-2323 (30 mg) IGM-2323 (100 mg)† IGM-2323 (300 mg) IGM-2323 (1000 mg) IGM-2323 (50/300 mg)* IGM-2323 (50/100 mg)* Single patient cohorts Dose escalation Titration dosing* MABEL n=1 n=1 n=3 n=3 n=3 IGM-2323 (50/100 mg)† Dose expansion cohorts n=3 IGM-2323 (30 mg) IGM-2323 (50/1000 mg)* Expected dose escalations Titration dosing: optimization of individual immune activity and preservation of T cell function over time Enrollment to titration dosing prioritized No DLT has been observed *First dose and maximum dose for each titration dose are described †Patients not shown here or on subsequent slides include one patient who has only received 50 mg dose and has only 2 weeks of follow-up as of the data cut off and one patient with pre-existing severe hypertension on four anti-hypertensive medications treated at 100 mg Cycle 1 Day 1 dose as previously described IGM-2323 (50/TBD mg) Intrapatient dose escalation allowed Data cut off: October 30, 2020
Initial Insights from 14 Patients: Efficacy Efficacy Headlines 9 of 14 patients showed reduction in tumor size, despite relatively low doses and short follow-up at higher doses Evidence of activity across all initial dose cohorts, starting at 0.5 mg Intra-patient dose escalation allowed after higher dose cohort cleared Top dose cohort completed 50/100; currently enrolling 50/300 Two partial responses, both near complete radiologic responses Target lesion sizes returned to normal Minor residual PET signature One response in post-CAR-T DLBCL, post stem cell transplant Extended Duration of Activity demonstrated Data cut off: October 30, 2020

Initial Insights from 14 Patients: Safety Safety Headlines Minimal pre-treatment: 10 mg of dexamethasone prior to first dose; optional on subsequent doses No neurotoxicity No Dose Limiting Toxicities Dosing cohorts: 0.5, 2.5, 10, 30 and 50/100 (N=14) 3 patients had CRS (21%) All Grade 1, transient, chills/fever No CRS in 50/100 in dose cohort to date Data cut off: October 30, 2020
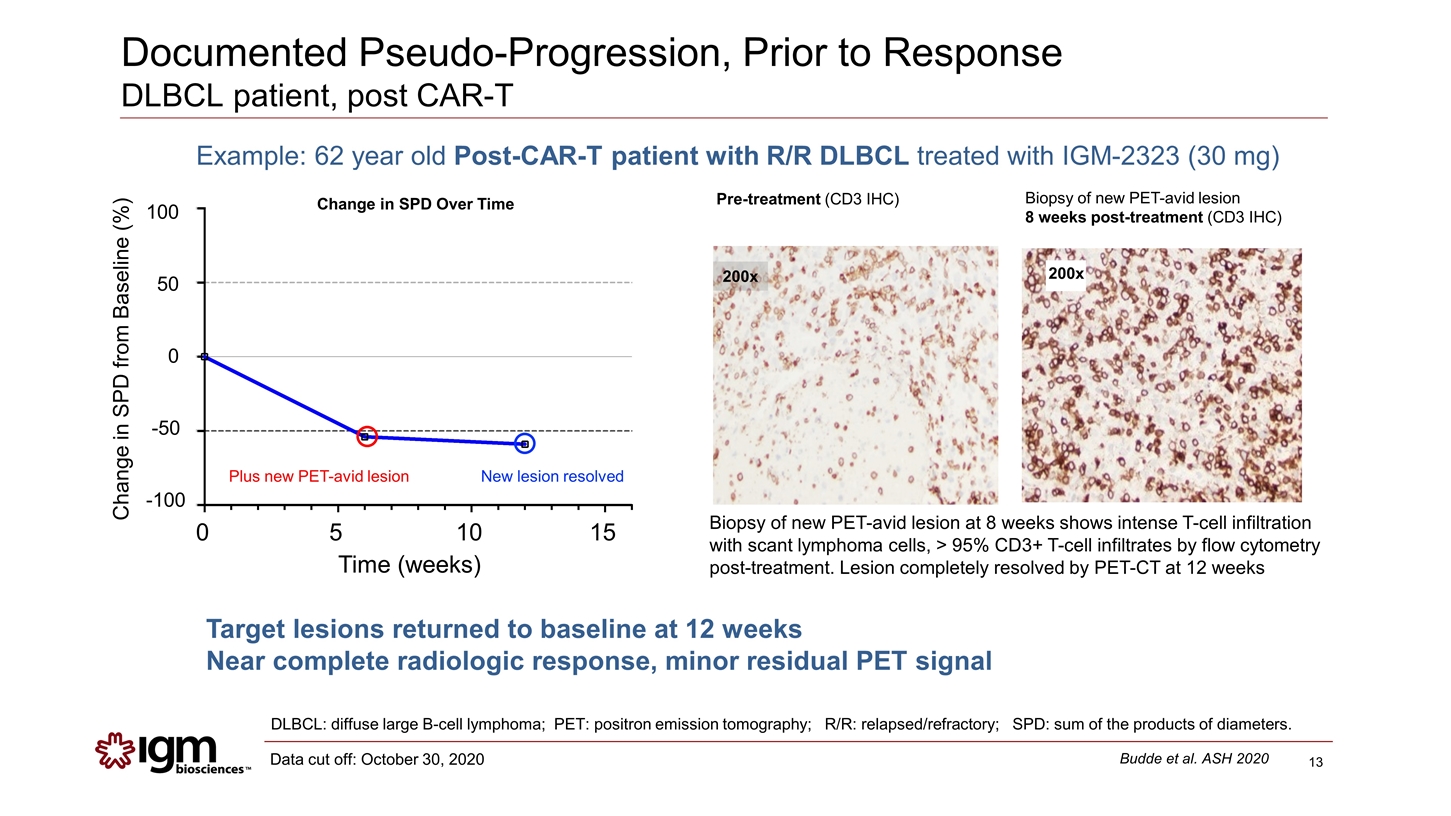
Documented Pseudo-Progression, Prior to Response DLBCL patient, post CAR-T DLBCL: diffuse large B-cell lymphoma; PET: positron emission tomography; R/R: relapsed/refractory; SPD: sum of the products of diameters. Biopsy of new PET-avid lesion at 8 weeks shows intense T-cell infiltration with scant lymphoma cells, > 95% CD3+ T-cell infiltrates by flow cytometry post-treatment. Lesion completely resolved by PET-CT at 12 weeks 200x Pre-treatment (CD3 IHC) Biopsy of new PET-avid lesion 8 weeks post-treatment (CD3 IHC) Budde et al. ASH 2020 Data cut off: October 30, 2020 Example: 62 year old Post-CAR-T patient with R/R DLBCL treated with IGM-2323 (30 mg) 200x Plus new PET-avid lesion New lesion resolved 100 50 0 -50 -100 Change in SPD from Baseline (%) Change in SPD Over Time 0 5 10 15 Time (weeks) Target lesions returned to baseline at 12 weeks Near complete radiologic response, minor residual PET signal
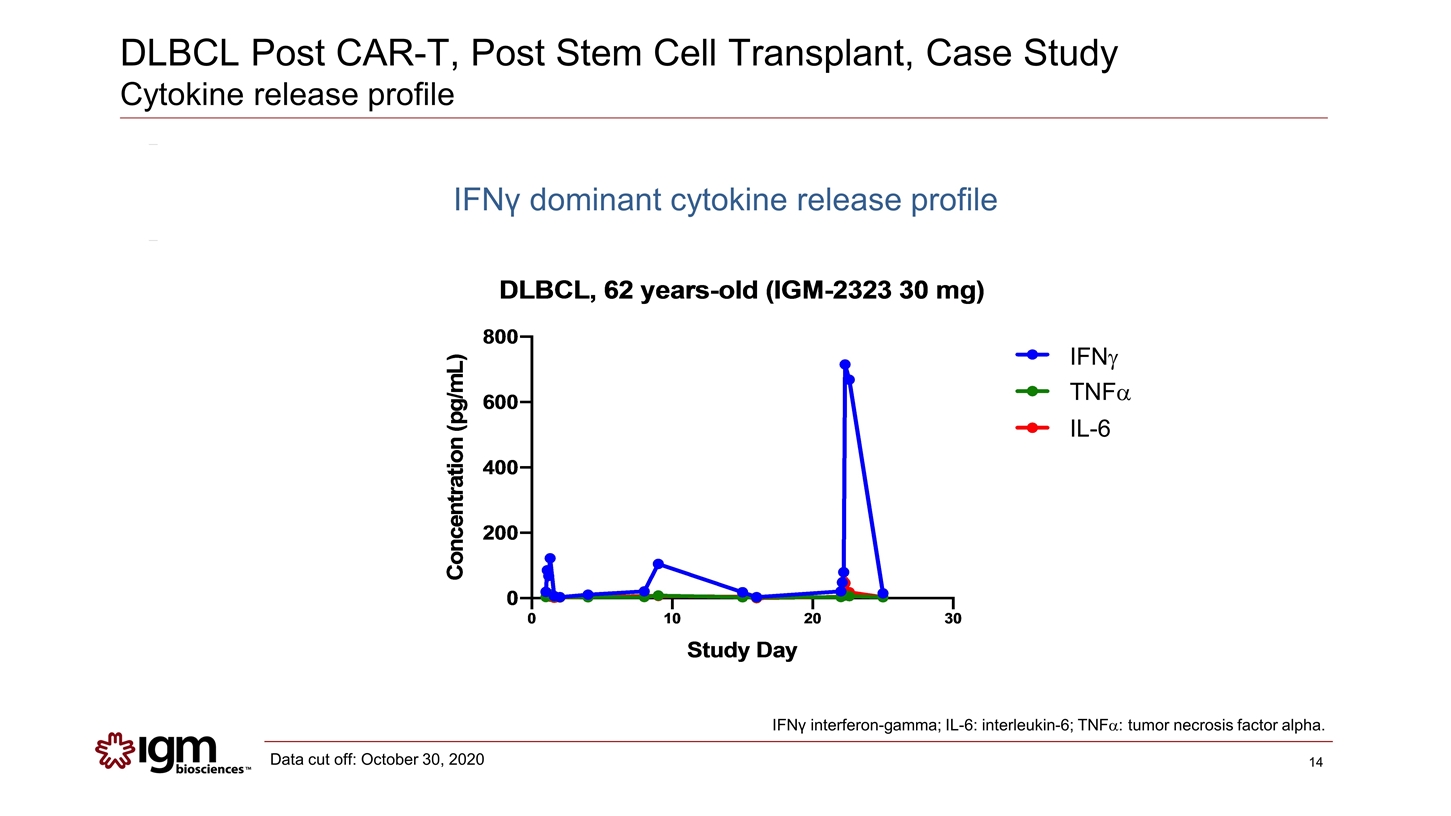
DLBCL Post CAR-T, Post Stem Cell Transplant, Case Study Cytokine release profile IFNg TNFa IL-6 IFNγ interferon-gamma; IL-6: interleukin-6; TNFa: tumor necrosis factor alpha. Data cut off: October 30, 2020 IFNγ dominant cytokine release profile
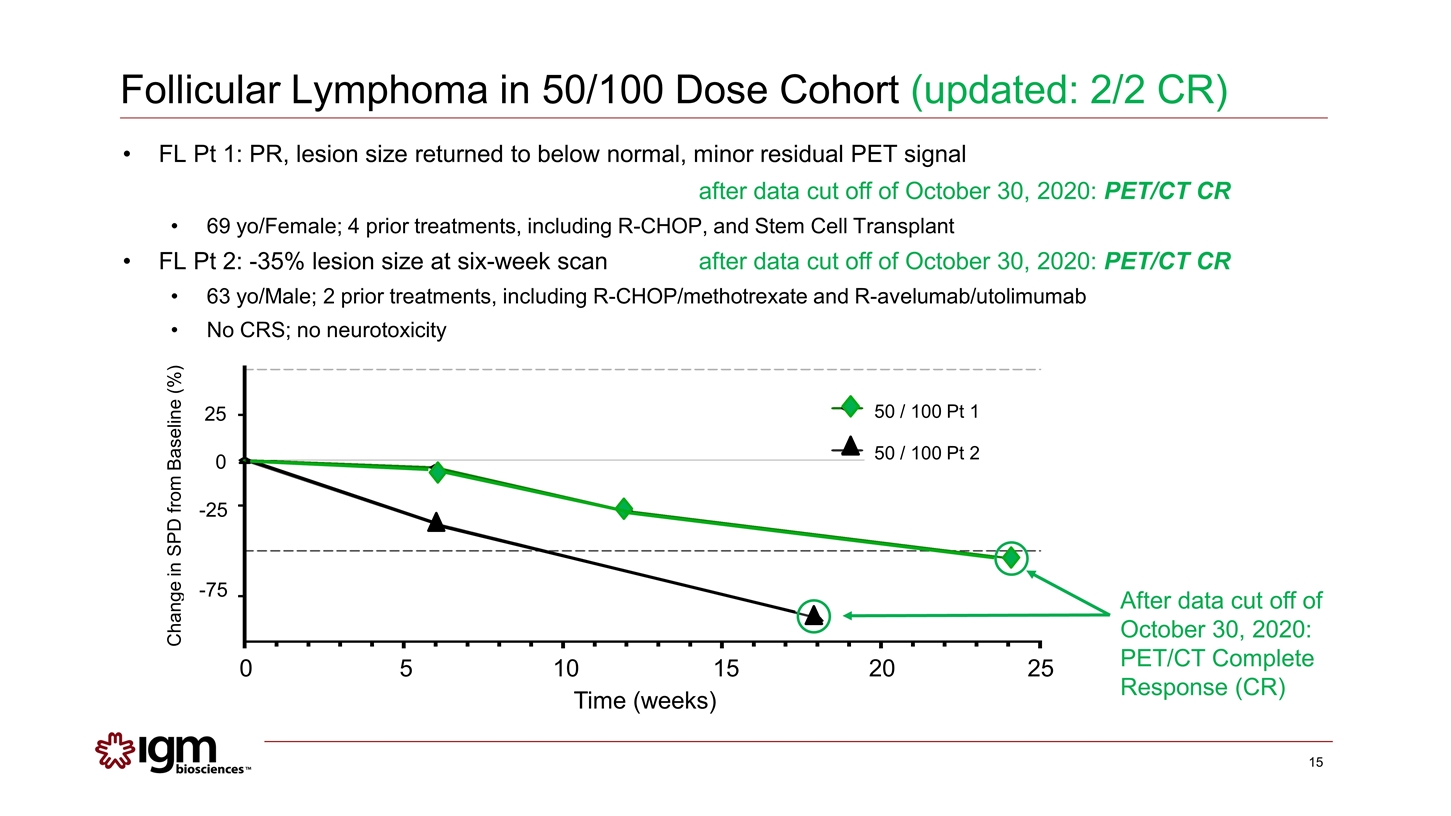
Follicular Lymphoma in 50/100 Dose Cohort (updated: 2/2 CR) After data cut off of October 30, 2020: PET/CT Complete Response (CR) Change in SPD from Baseline (%) 25 0 -25 -75 FL Pt 1: PR, lesion size returned to below normal, minor residual PET signal after data cut off of October 30, 2020: PET/CT CR 69 yo/Female; 4 prior treatments, including R-CHOP, and Stem Cell Transplant FL Pt 2: -35% lesion size at six-week scanafter data cut off of October 30, 2020: PET/CT CR 63 yo/Male; 2 prior treatments, including R-CHOP/methotrexate and R-avelumab/utolimumab No CRS; no neurotoxicity 0 5 10 15 20 25 Time (weeks) 50 / 100 Pt 1 50 / 100 Pt 2
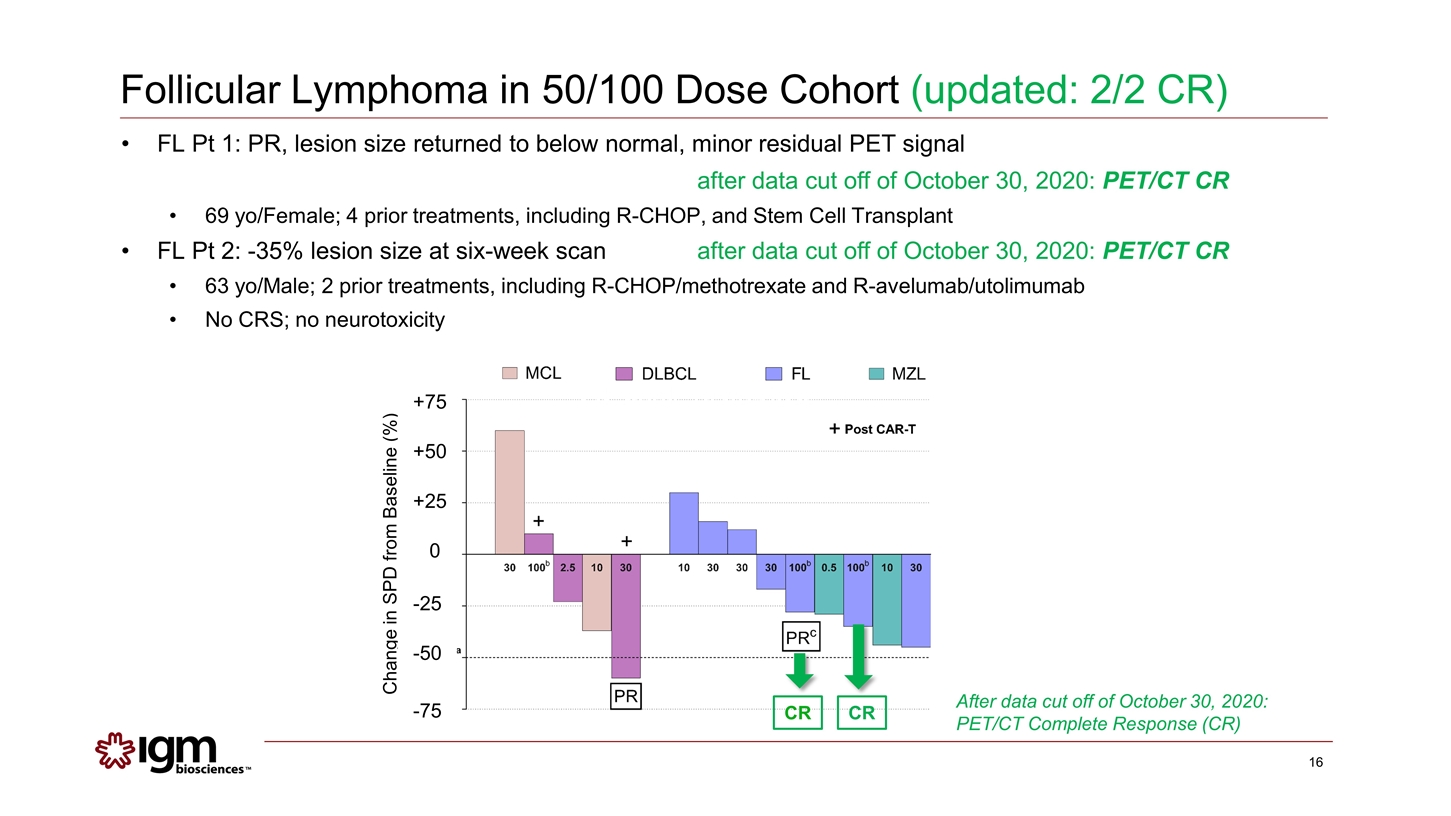
Follicular Lymphoma in 50/100 Dose Cohort (updated: 2/2 CR) After data cut off of October 30, 2020: PET/CT Complete Response (CR) CR FL Pt 1: PR, lesion size returned to below normal, minor residual PET signal after data cut off of October 30, 2020: PET/CT CR 69 yo/Female; 4 prior treatments, including R-CHOP, and Stem Cell Transplant FL Pt 2: -35% lesion size at six-week scanafter data cut off of October 30, 2020: PET/CT CR 63 yo/Male; 2 prior treatments, including R-CHOP/methotrexate and R-avelumab/utolimumab No CRS; no neurotoxicity CR Change in SPD from Baseline (%) +75 +50 +25 0 -25 -50 -75
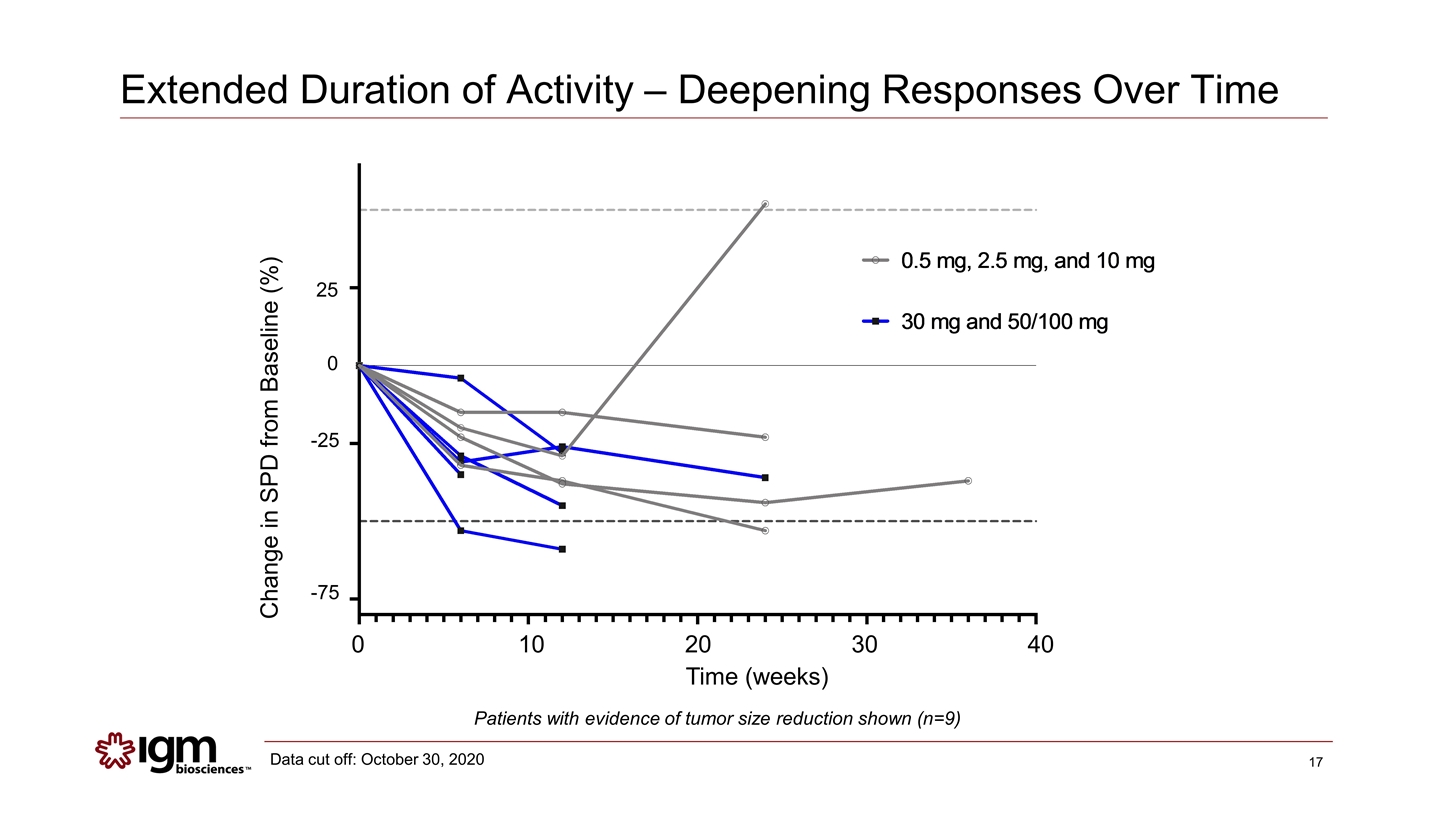
Extended Duration of Activity – Deepening Responses Over Time Data cut off: October 30, 2020 Patients with evidence of tumor size reduction shown (n=9) 0 10 20 30 40 Time (weeks) 25 0 -25 -75 Change in SPD from Baseline (%)
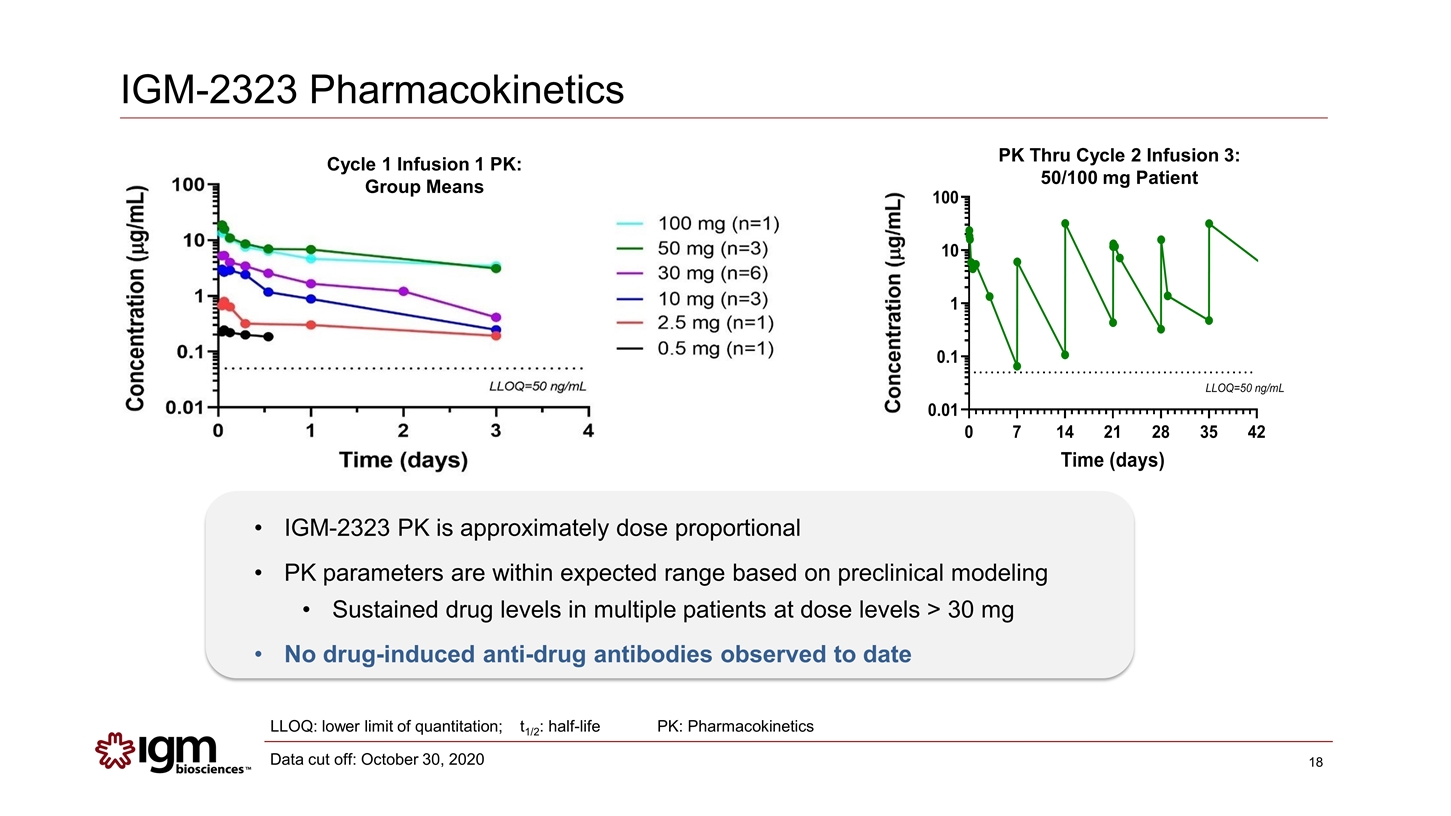
IGM-2323 Pharmacokinetics IGM-2323 PK is approximately dose proportional PK parameters are within expected range based on preclinical modeling Sustained drug levels in multiple patients at dose levels > 30 mg No drug-induced anti-drug antibodies observed to date LLOQ: lower limit of quantitation; t1/2: half-life Cycle 1 Infusion 1 PK: Group Means PK Thru Cycle 2 Infusion 3: 50/100 mg Patient PK: Pharmacokinetics Data cut off: October 30, 2020
Potential Best-in-Class Safety Profile Minimal pre-treatment to date 10 mg dexamethasone prior to first dose Optional on subsequent doses No neurotoxicity at any dose to date 14 patients dosed at 0.5, 2.5, 10, 30 and 50/100 mg 3 patients had CRS (21%) All Grade 1, transient, chills/fever May be related to IFNg instead of IL-6 and other cytokines 3 patients dosed at highest completed cohort (50/100 mg) No CRS Well tolerated Data cut off: October 30, 2020
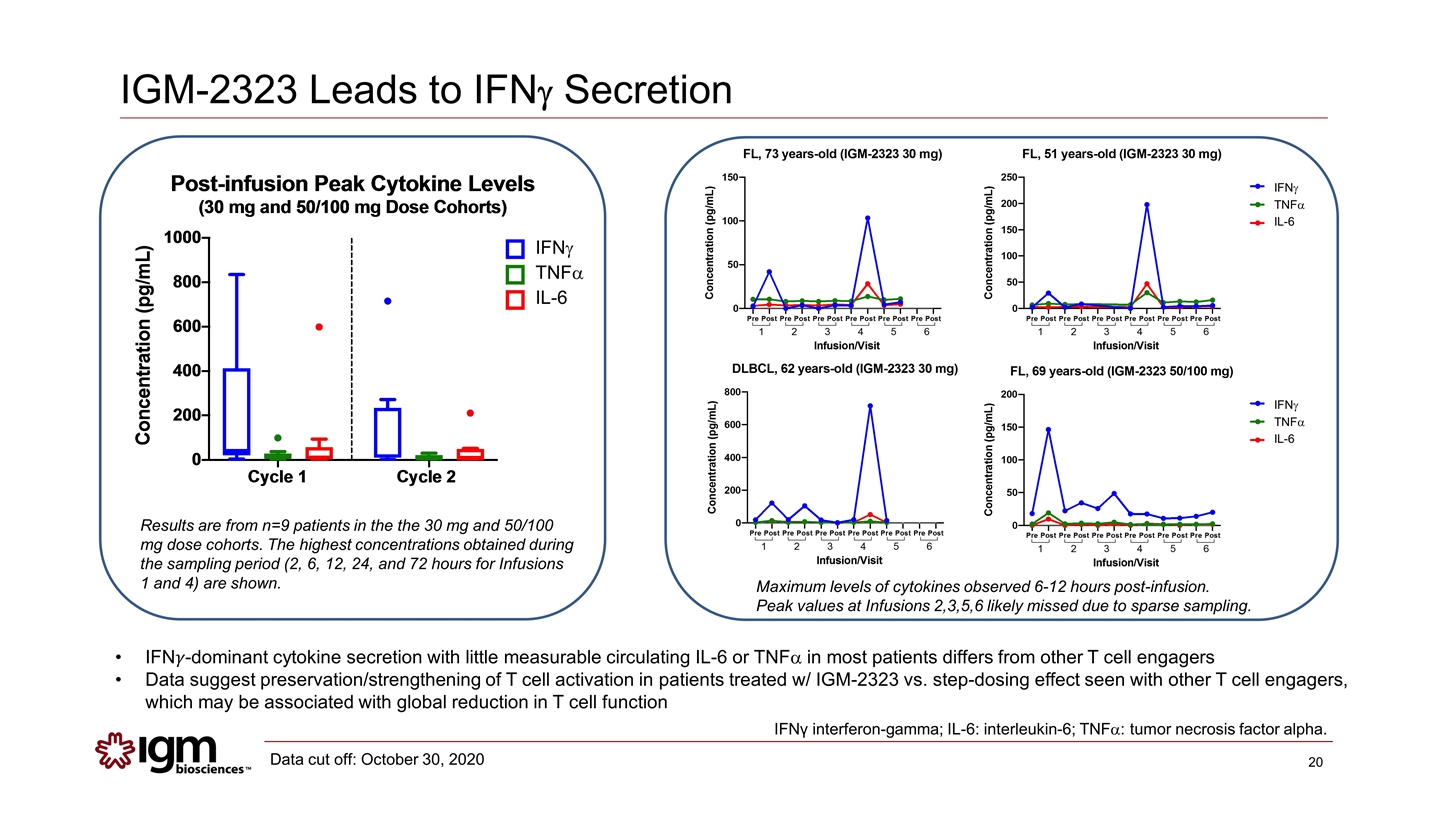
IGM-2323 Leads to IFNg Secretion IFN��-dominant cytokine secretion with little measurable circulating IL-6 or TNFa in most patients differs from other T cell engagers Data suggest preservation/strengthening of T cell activation in patients treated w/ IGM-2323 vs. step-dosing effect seen with other T cell engagers, which may be associated with global reduction in T cell function IFNγ interferon-gamma; IL-6: interleukin-6; TNFa: tumor necrosis factor alpha. Maximum levels of cytokines observed 6-12 hours post-infusion. Peak values at Infusions 2,3,5,6 likely missed due to sparse sampling. IFNg TNFa IL-6 Results are from n=9 patients in the the 30 mg and 50/100 mg dose cohorts. The highest concentrations obtained during the sampling period (2, 6, 12, 24, and 72 hours for Infusions 1 and 4) are shown. IFNg TNFa IL-6 IFNg TNFa IL-6 Data cut off: October 30, 2020
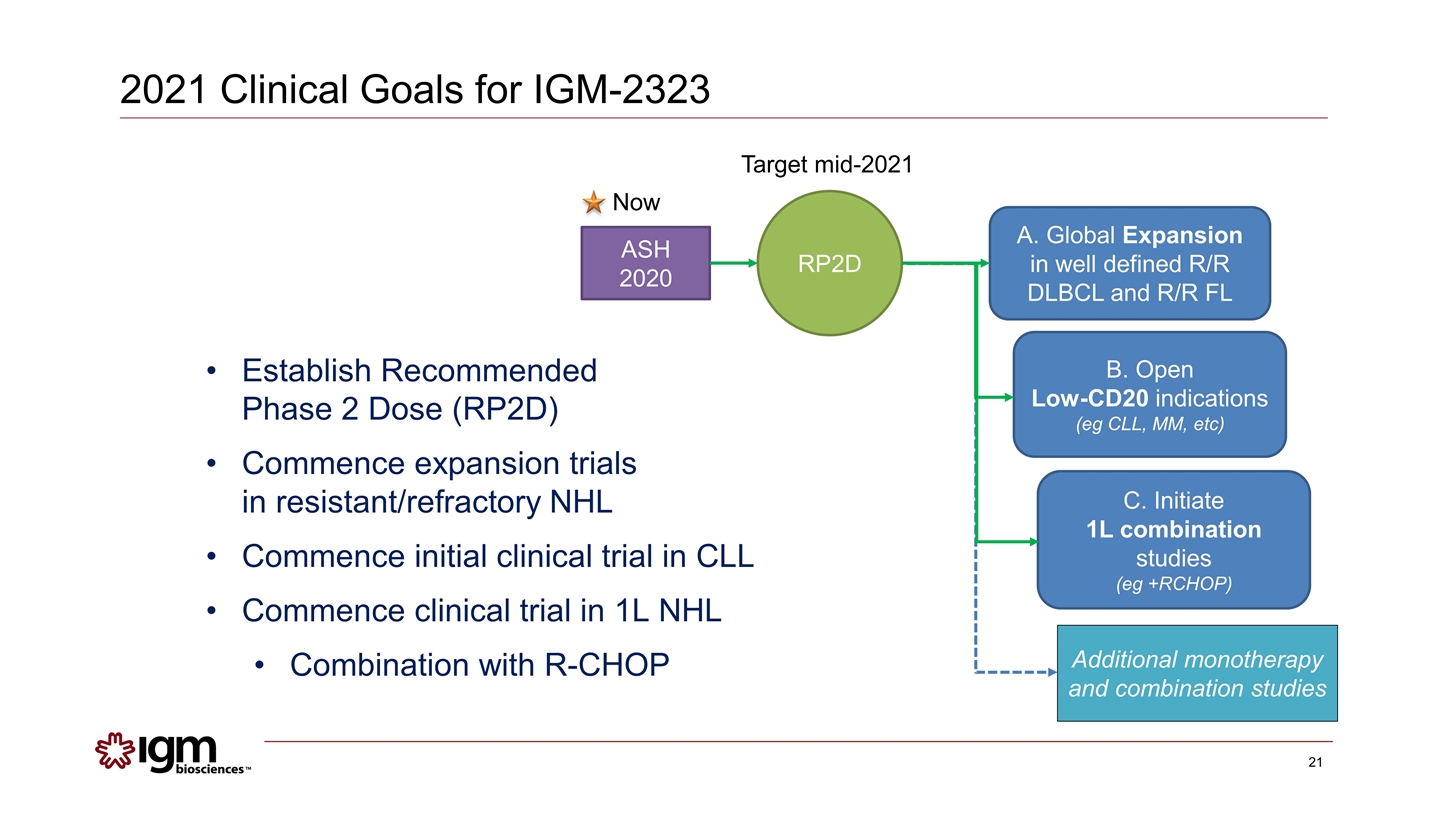
2021 Clinical Goals for IGM-2323 Establish Recommended Phase 2 Dose (RP2D) Commence expansion trials in resistant/refractory NHL Commence initial clinical trial in CLL Commence clinical trial in 1L NHL Combination with R-CHOP ASH 2020 RP2D A. Global Expansion in well defined R/R DLBCL and R/R FL C. Initiate 1L combination studies (eg +RCHOP) B. Open Low-CD20 indications (eg CLL, MM, etc) Target mid-2021 Now Additional monotherapy and combination studies
IGM-2323 Key Takeaways Strong activity seen at low doses 9 of 14 patients in early-stage dose escalation showed lesion size reduction Activity across dose cohorts from 0.5 mg initial dose to 50/100 mg Stronger activity shown in higher dose cohorts On track to complete 50/300 dose cohort enrollment by end 2020 Expect Recommended Phase 2 Dose (RP2D) between 50/100 mg and 50/1000 mg Response in post-CAR-T DLBCL in 30 mg dose cohort Target lesions reduced to normal size, minor residual PET signal In 50/100 dose cohort: 2 of 2 FL patients showed complete responses (updated post-October 30th, 2020 data cut off) One complete response at 18 weeks One complete response at 24 weeks Potential best in class safety profile Minimal pre-treatment of safety evaluable patients No neurotoxicity to date at any dose 14 patients at 0.5, 2.5, 10, 30 and 50/100 mg 3 patients had CRS (21%) All Grade 1, transient, chills/fever No CRS in 50/100 dose cohort (3 patients)
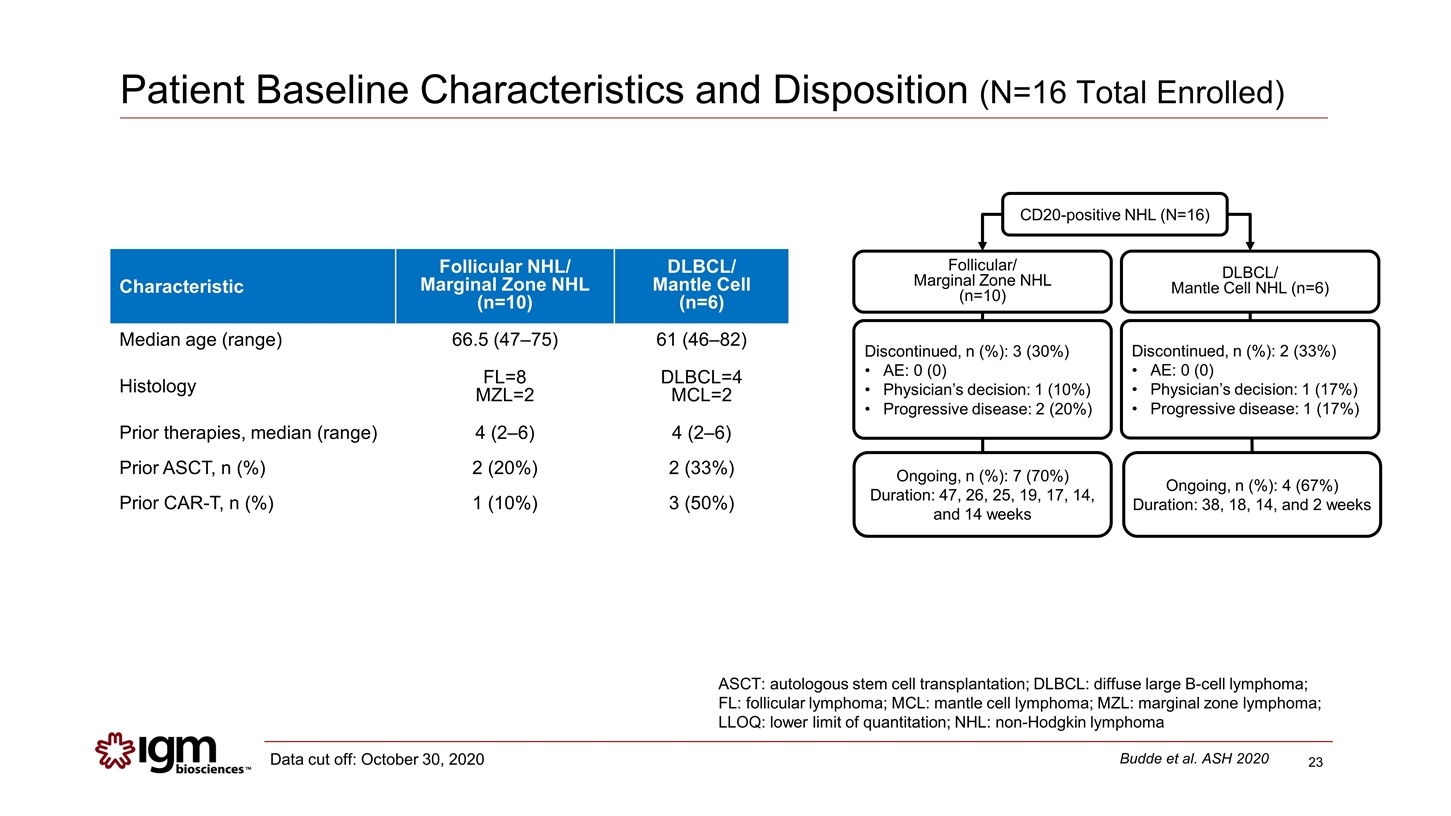
Patient Baseline Characteristics and Disposition (N=16 Total Enrolled) Characteristic Follicular NHL/ Marginal Zone NHL (n=10) DLBCL/ Mantle Cell (n=6) Median age (range) 66.5 (47–75) 61 (46–82) Histology FL=8 MZL=2 DLBCL=4 MCL=2 Prior therapies, median (range) 4 (2–6) 4 (2–6) Prior ASCT, n (%) 2 (20%) 2 (33%) Prior CAR-T, n (%) 1 (10%) 3 (50%) CD20-positive NHL (N=16) Discontinued, n (%): 3 (30%) AE: 0 (0) Physician’s decision: 1 (10%) Progressive disease: 2 (20%) Ongoing, n (%): 7 (70%) Duration: 47, 26, 25, 19, 17, 14, and 14 weeks Follicular/ Marginal Zone NHL (n=10) Discontinued, n (%): 2 (33%) AE: 0 (0) Physician’s decision: 1 (17%) Progressive disease: 1 (17%) Ongoing, n (%): 4 (67%) Duration: 38, 18, 14, and 2 weeks DLBCL/ Mantle Cell NHL (n=6) ASCT: autologous stem cell transplantation; DLBCL: diffuse large B-cell lymphoma; FL: follicular lymphoma; MCL: mantle cell lymphoma; MZL: marginal zone lymphoma; LLOQ: lower limit of quantitation; NHL: non-Hodgkin lymphoma Budde et al. ASH 2020 Data cut off: October 30, 2020
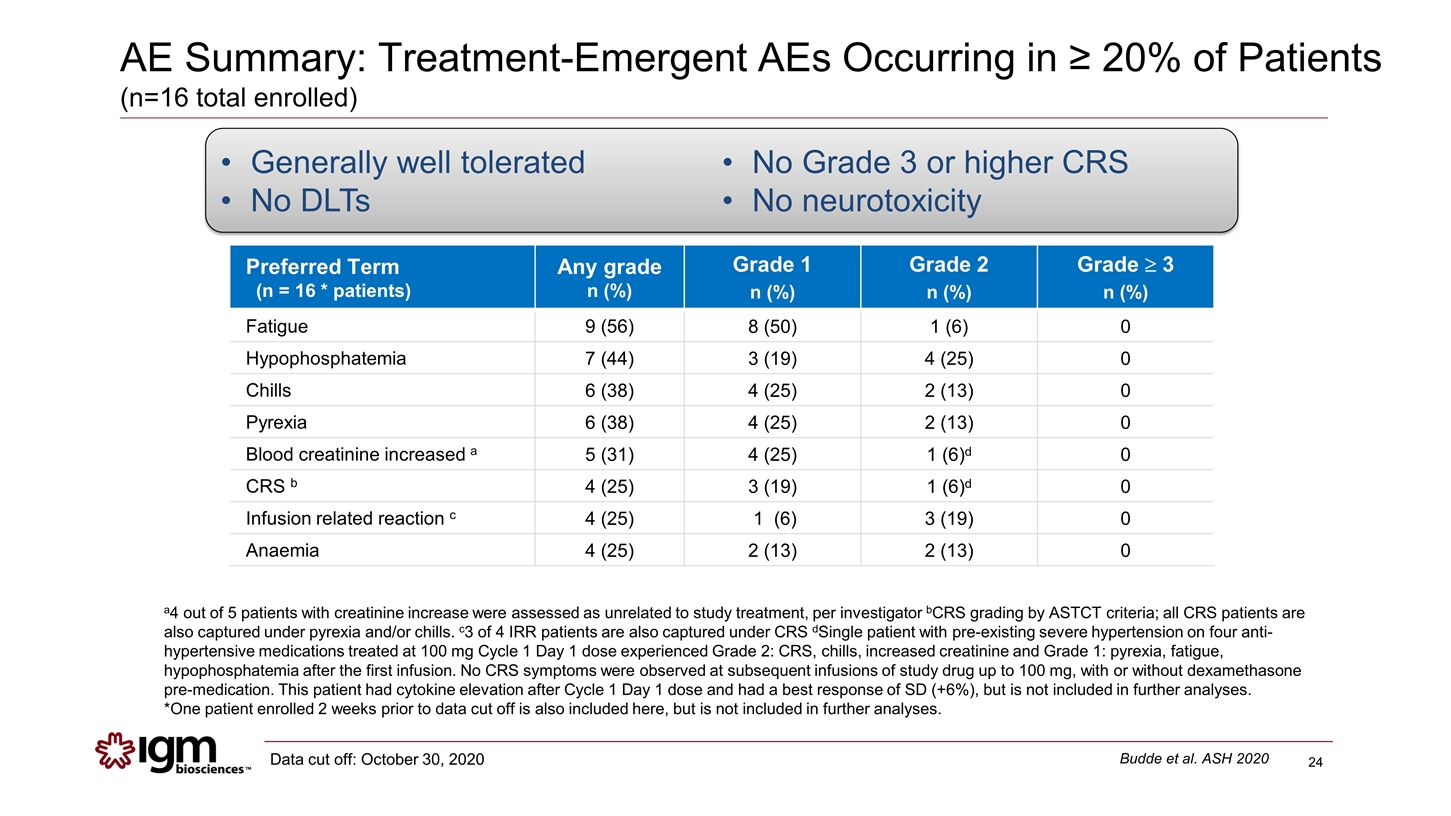
AE Summary: Treatment-Emergent AEs Occurring in ≥ 20% of Patients (n=16 total enrolled) a4 out of 5 patients with creatinine increase were assessed as unrelated to study treatment, per investigator bCRS grading by ASTCT criteria; all CRS patients are also captured under pyrexia and/or chills. c3 of 4 IRR patients are also captured under CRS dSingle patient with pre-existing severe hypertension on four anti-hypertensive medications treated at 100 mg Cycle 1 Day 1 dose experienced Grade 2: CRS, chills, increased creatinine and Grade 1: pyrexia, fatigue, hypophosphatemia after the first infusion. No CRS symptoms were observed at subsequent infusions of study drug up to 100 mg, with or without dexamethasone pre-medication. This patient had cytokine elevation after Cycle 1 Day 1 dose and had a best response of SD (+6%), but is not included in further analyses. *One patient enrolled 2 weeks prior to data cut off is also included here, but is not included in further analyses. Generally well tolerated No DLTs No Grade 3 or higher CRS No neurotoxicity Preferred Term (n = 16 * patients) Any grade n (%) Grade 1 n (%) Grade 2 n (%) Grade ³ 3 n (%) Fatigue 9 (56) 8 (50) 1 (6) 0 Hypophosphatemia 7 (44) 3 (19) 4 (25) 0 Chills 6 (38) 4 (25) 2 (13) 0 Pyrexia 6 (38) 4 (25) 2 (13) 0 Blood creatinine increased a 5 (31) 4 (25) 1 (6)d 0 CRS b 4 (25) 3 (19) 1 (6)d 0 Infusion related reaction c 4 (25) 1 (6) 3 (19) 0 Anaemia 4 (25) 2 (13) 2 (13) 0 Budde et al. ASH 2020 Data cut off: October 30, 2020
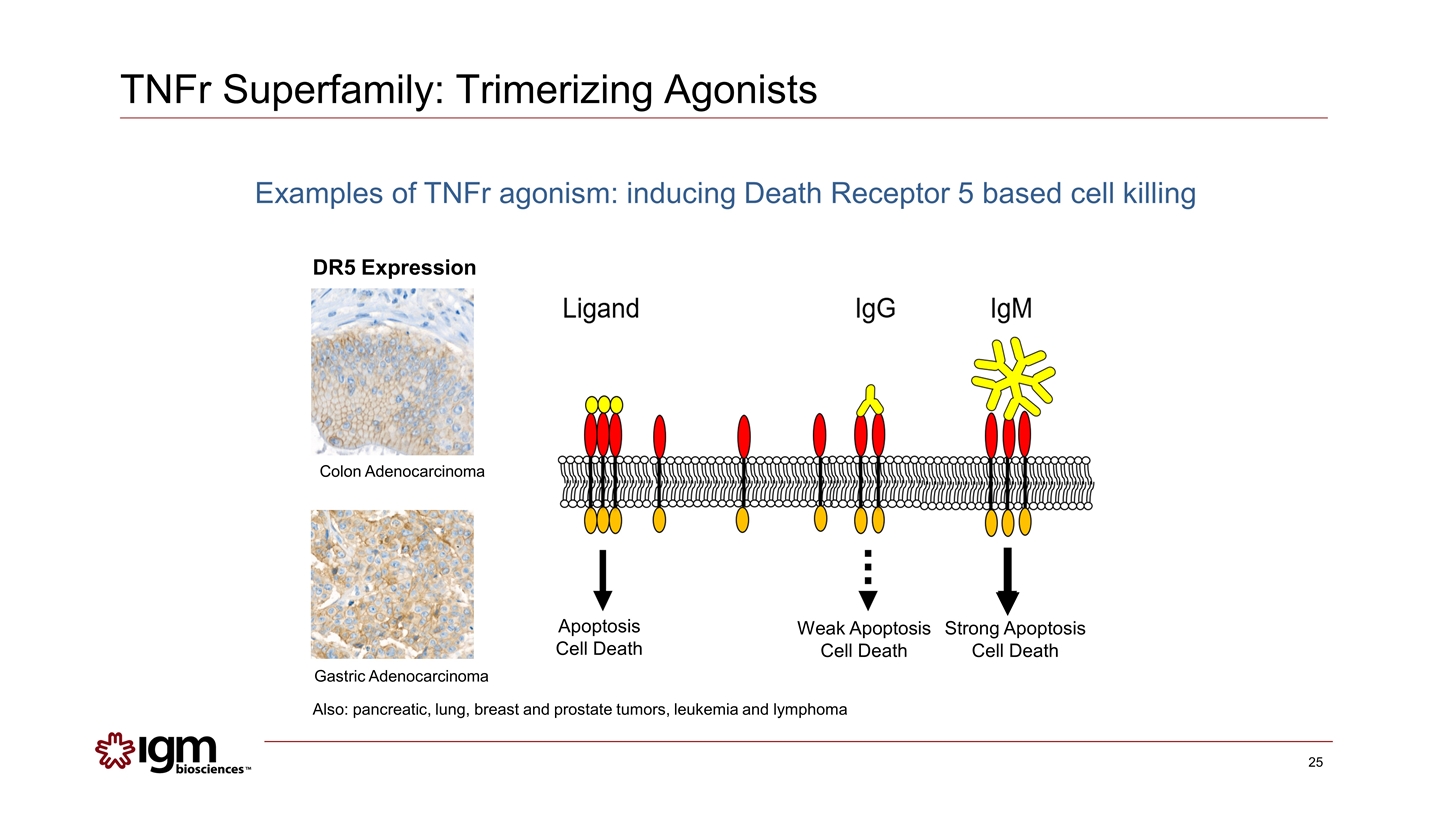
TNFr Superfamily: Trimerizing Agonists Apoptosis Cell Death Strong Apoptosis Cell Death Weak Apoptosis Cell Death Gastric Adenocarcinoma Colon Adenocarcinoma DR5 Expression Also: pancreatic, lung, breast and prostate tumors, leukemia and lymphoma Examples of TNFr agonism: inducing Death Receptor 5 based cell killing
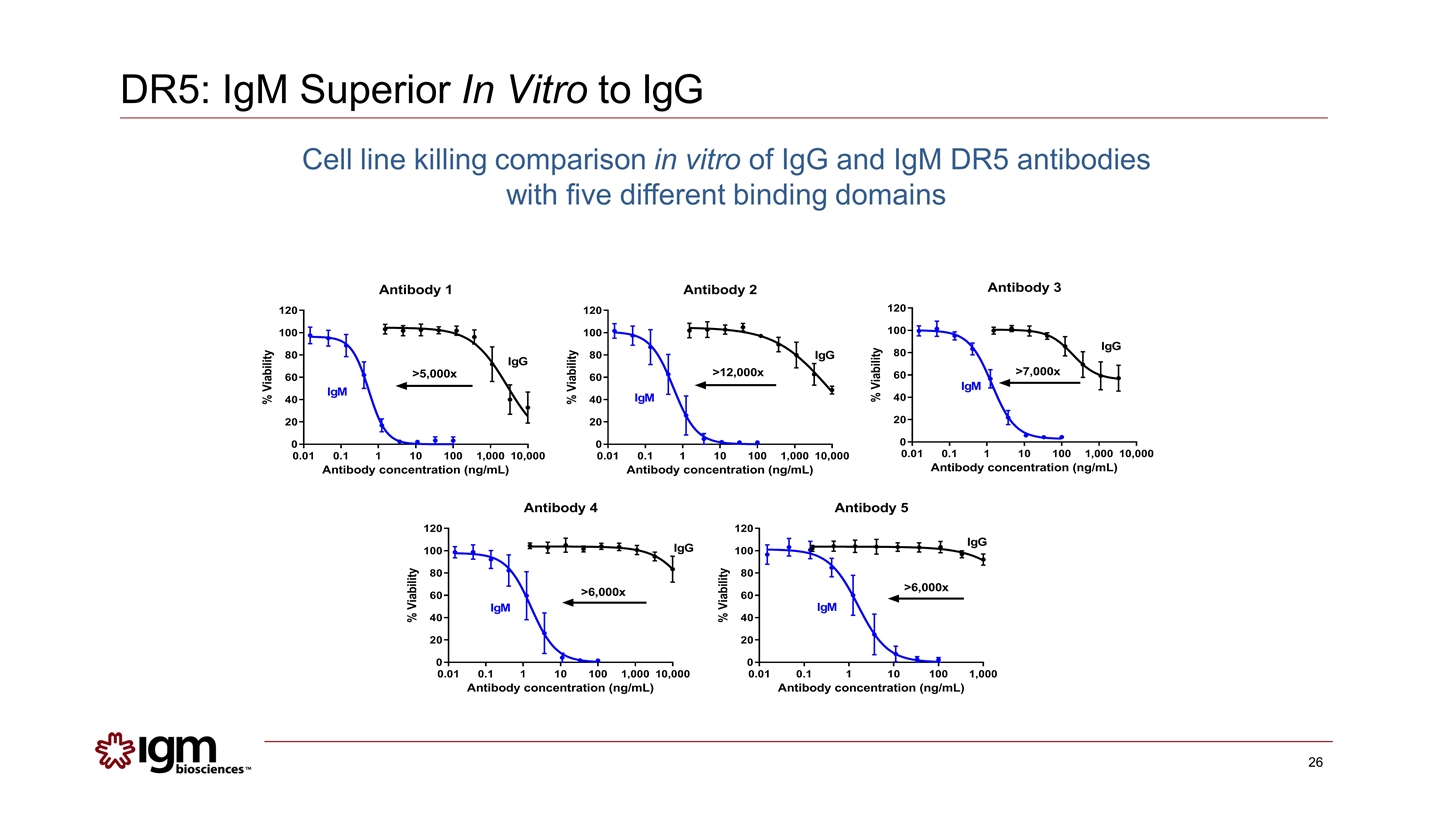
DR5: IgM Superior In Vitro to IgG Cell line killing comparison in vitro of IgG and IgM DR5 antibodies with five different binding domains
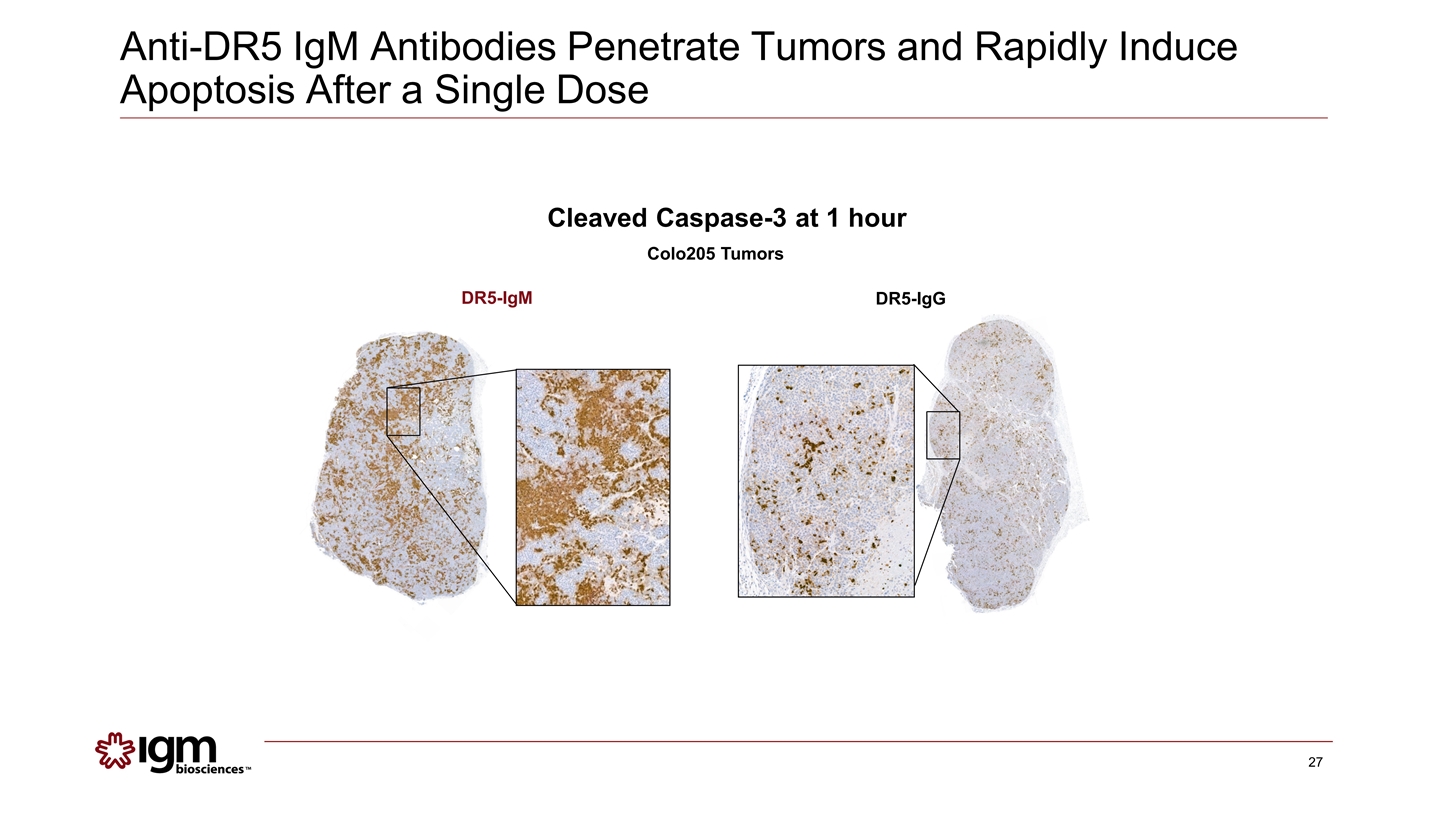
DR5-IgM DR5-IgG Cleaved Caspase-3 at 1 hour Colo205 Tumors Anti-DR5 IgM Antibodies Penetrate Tumors and Rapidly Induce Apoptosis After a Single Dose
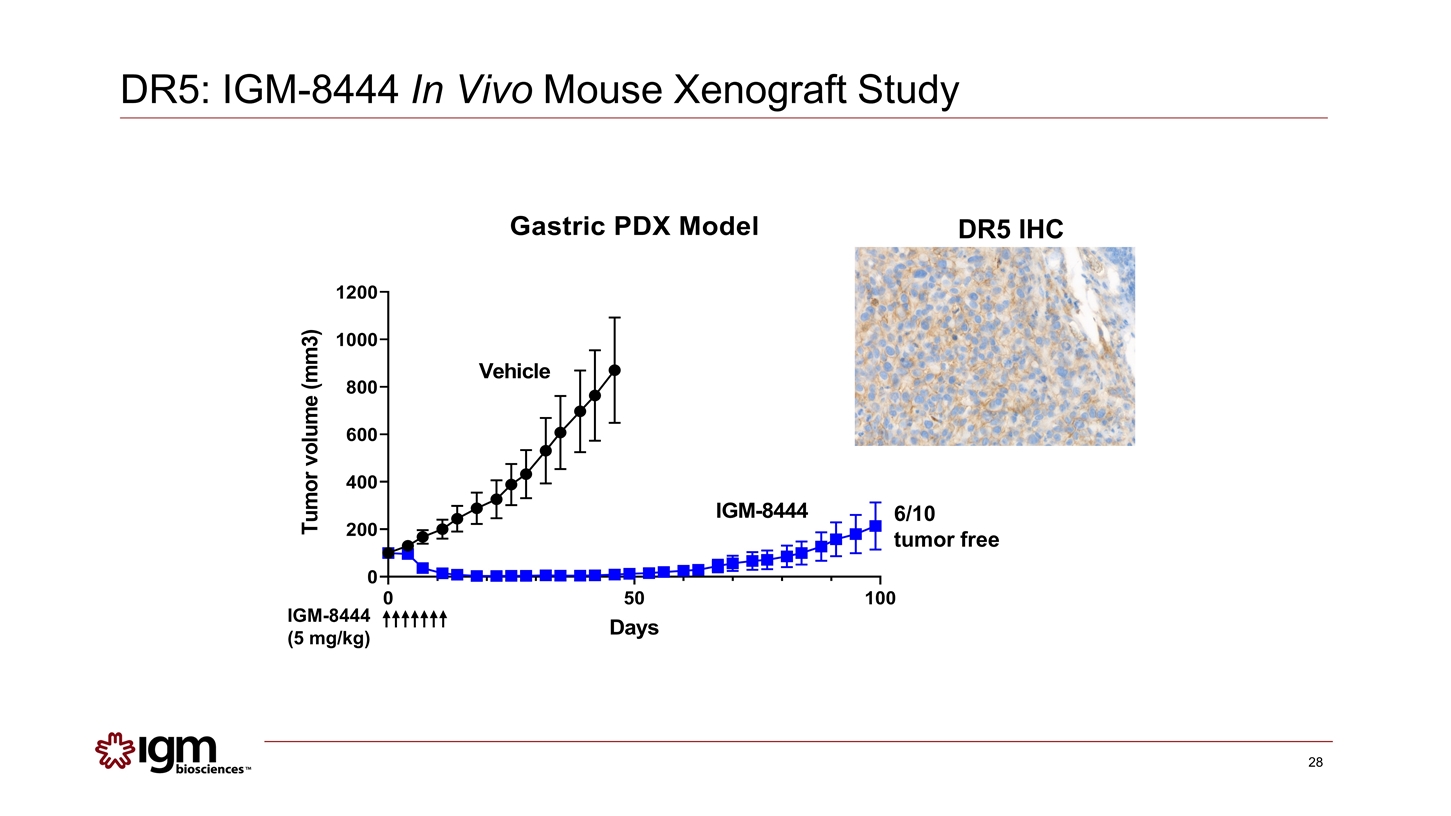
6/10 tumor free IGM-8444 (5 mg/kg) DR5 IHC DR5: IGM-8444 In Vivo Mouse Xenograft Study
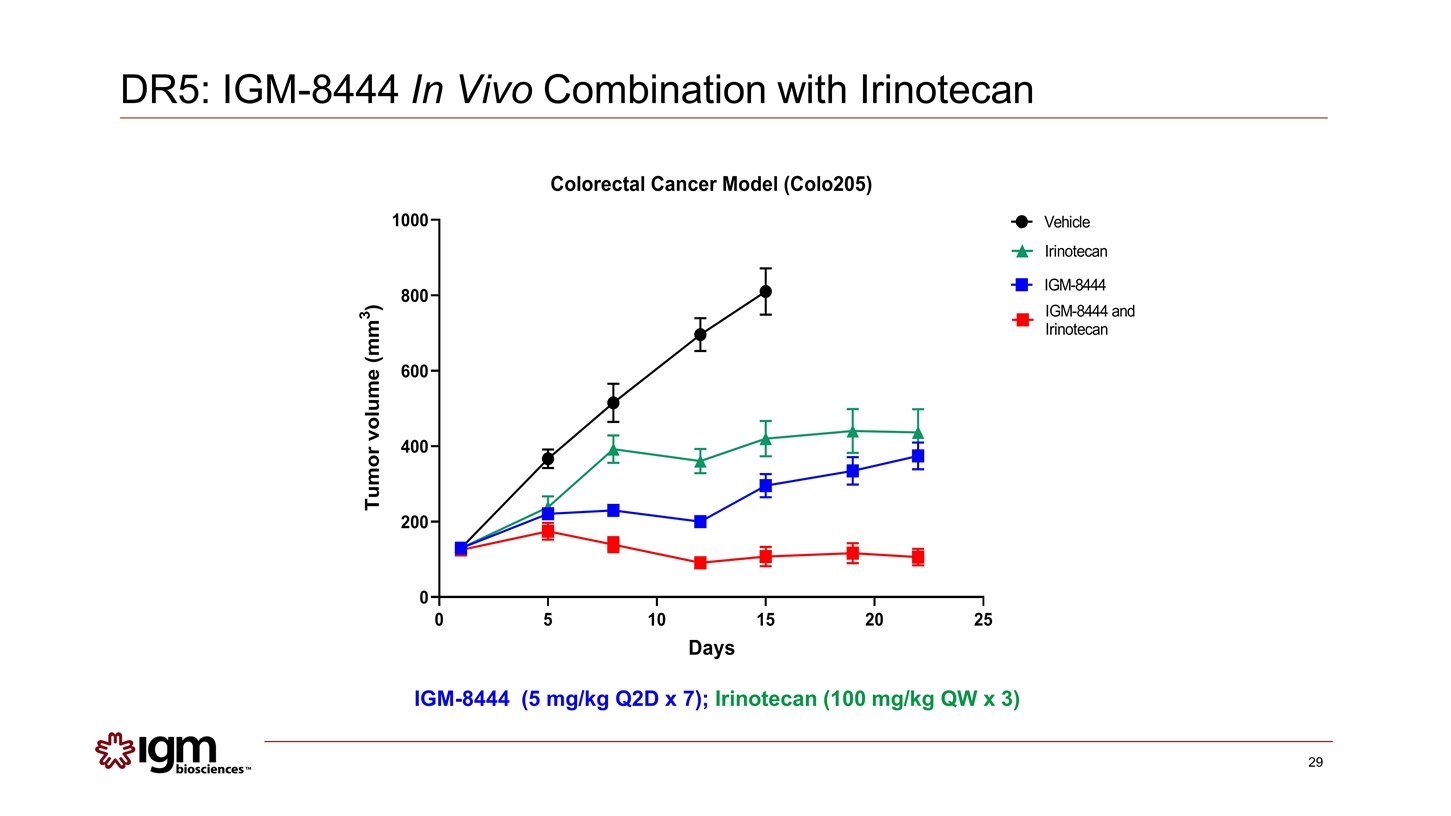
IGM-8444 (5 mg/kg Q2D x 7); Irinotecan (100 mg/kg QW x 3) DR5: IGM-8444 In Vivo Combination with Irinotecan
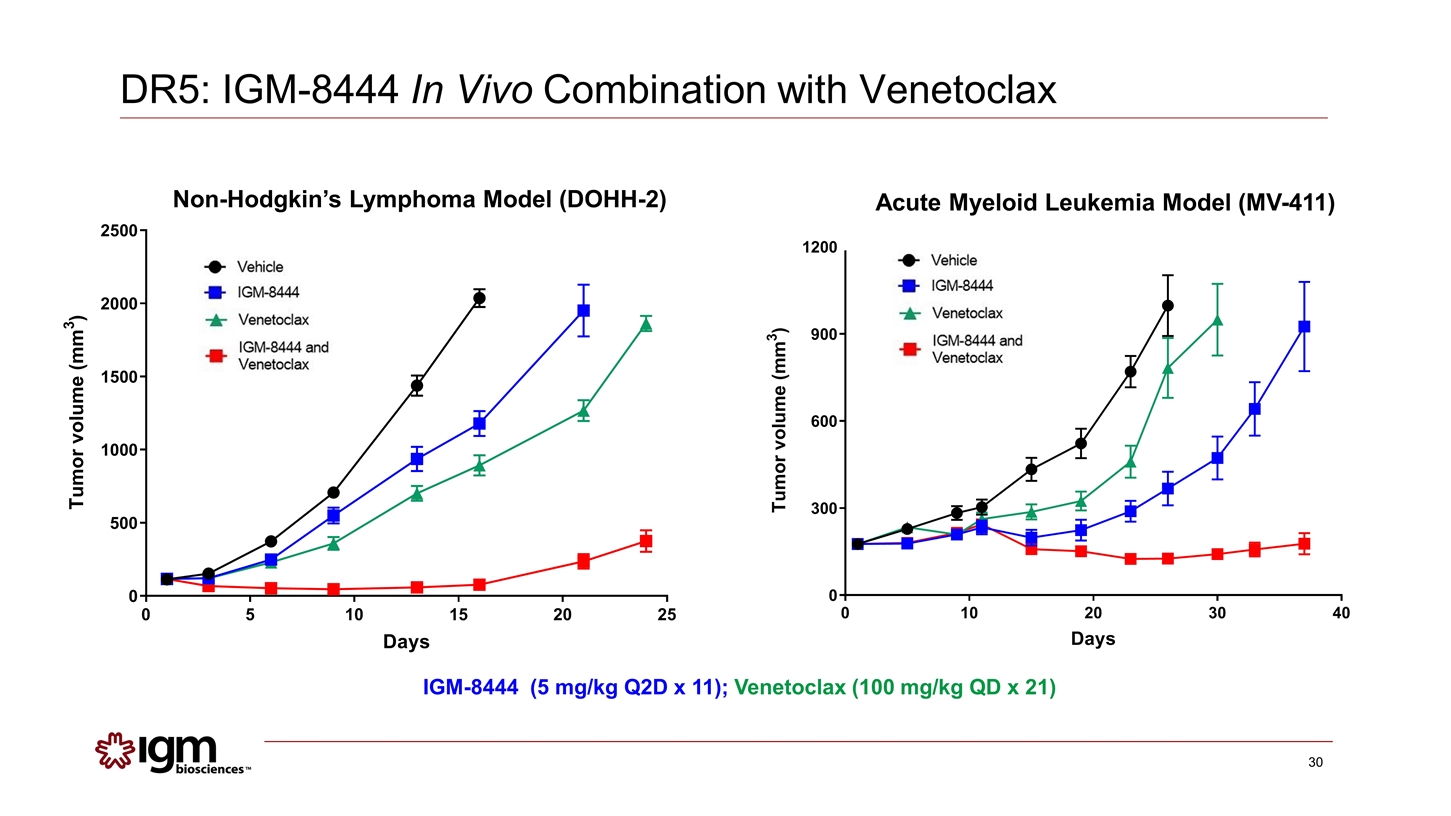
Non-Hodgkin’s Lymphoma Model (DOHH-2) DR5: IGM-8444 In Vivo Combination with Venetoclax Acute Myeloid Leukemia Model (MV-411) IGM-8444 (5 mg/kg Q2D x 11); Venetoclax (100 mg/kg QD x 21)
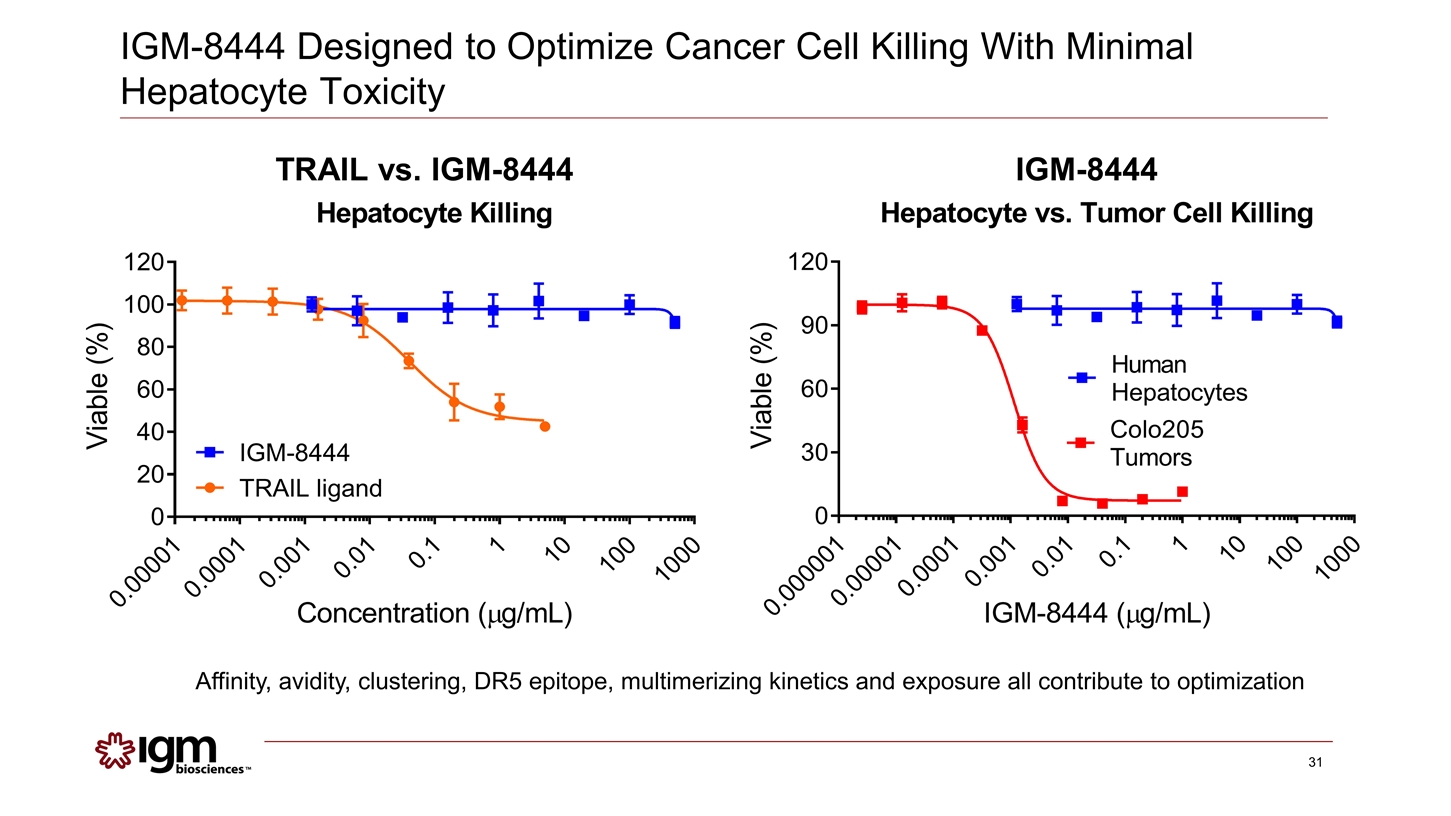
IGM-8444 Designed to Optimize Cancer Cell Killing With Minimal Hepatocyte Toxicity TRAIL vs. IGM-8444 IGM-8444 Affinity, avidity, clustering, DR5 epitope, multimerizing kinetics and exposure all contribute to optimization
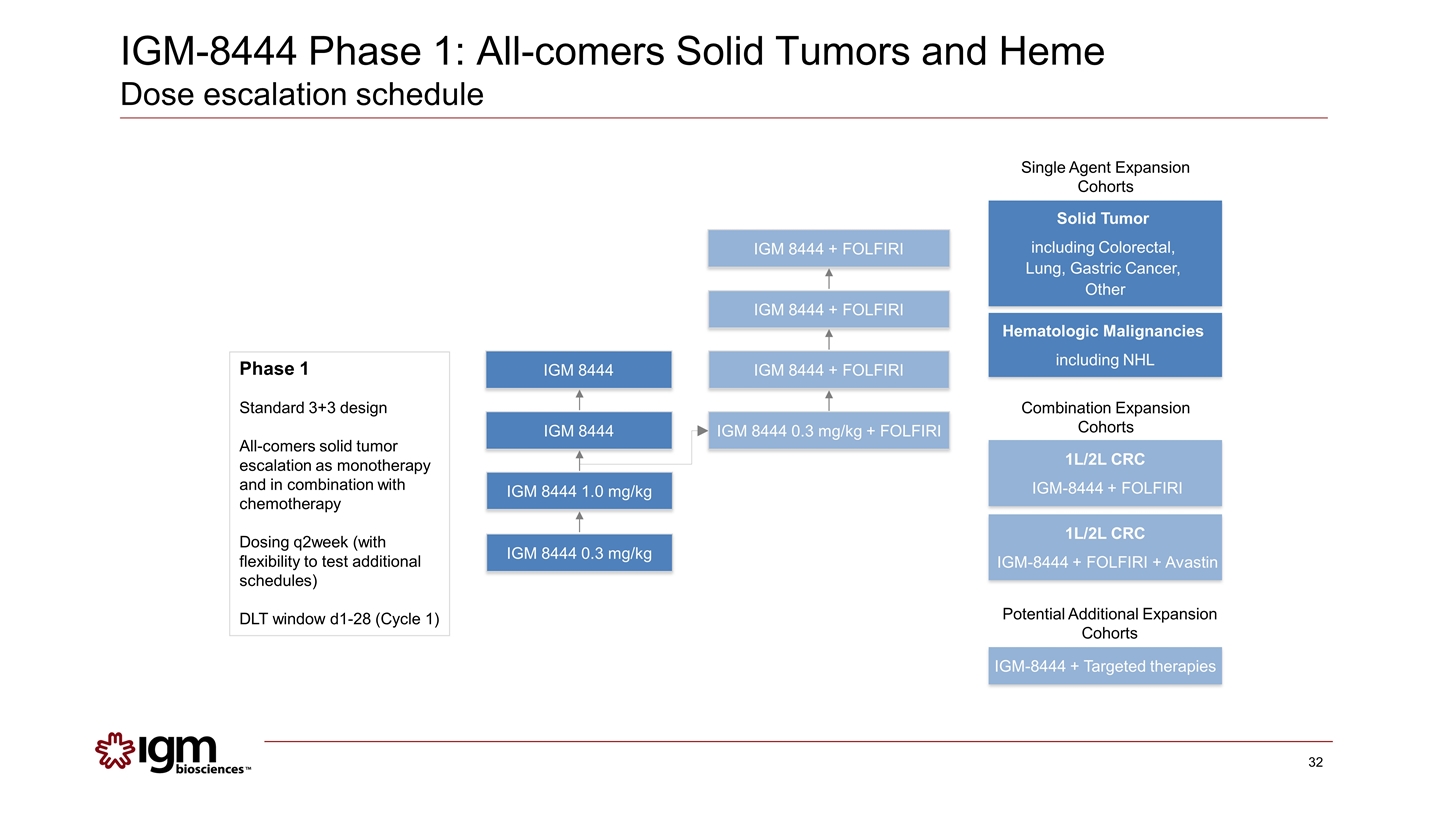
Phase 1 Standard 3+3 design All-comers solid tumor escalation as monotherapy and in combination with chemotherapy Dosing q2week (with flexibility to test additional schedules) DLT window d1-28 (Cycle 1) IGM 8444 0.3 mg/kg IGM 8444 1.0 mg/kg IGM 8444 IGM 8444 IGM 8444 0.3 mg/kg + FOLFIRI IGM 8444 + FOLFIRI IGM 8444 + FOLFIRI IGM 8444 + FOLFIRI Solid Tumor including Colorectal, Lung, Gastric Cancer, Other 1L/2L CRC IGM-8444 + FOLFIRI Combination Expansion Cohorts Single Agent Expansion Cohorts IGM-8444 + Targeted therapies Potential Additional Expansion Cohorts Hematologic Malignancies including NHL 1L/2L CRC IGM-8444 + FOLFIRI + Avastin IGM-8444 Phase 1: All-comers Solid Tumors and Heme Dose escalation schedule
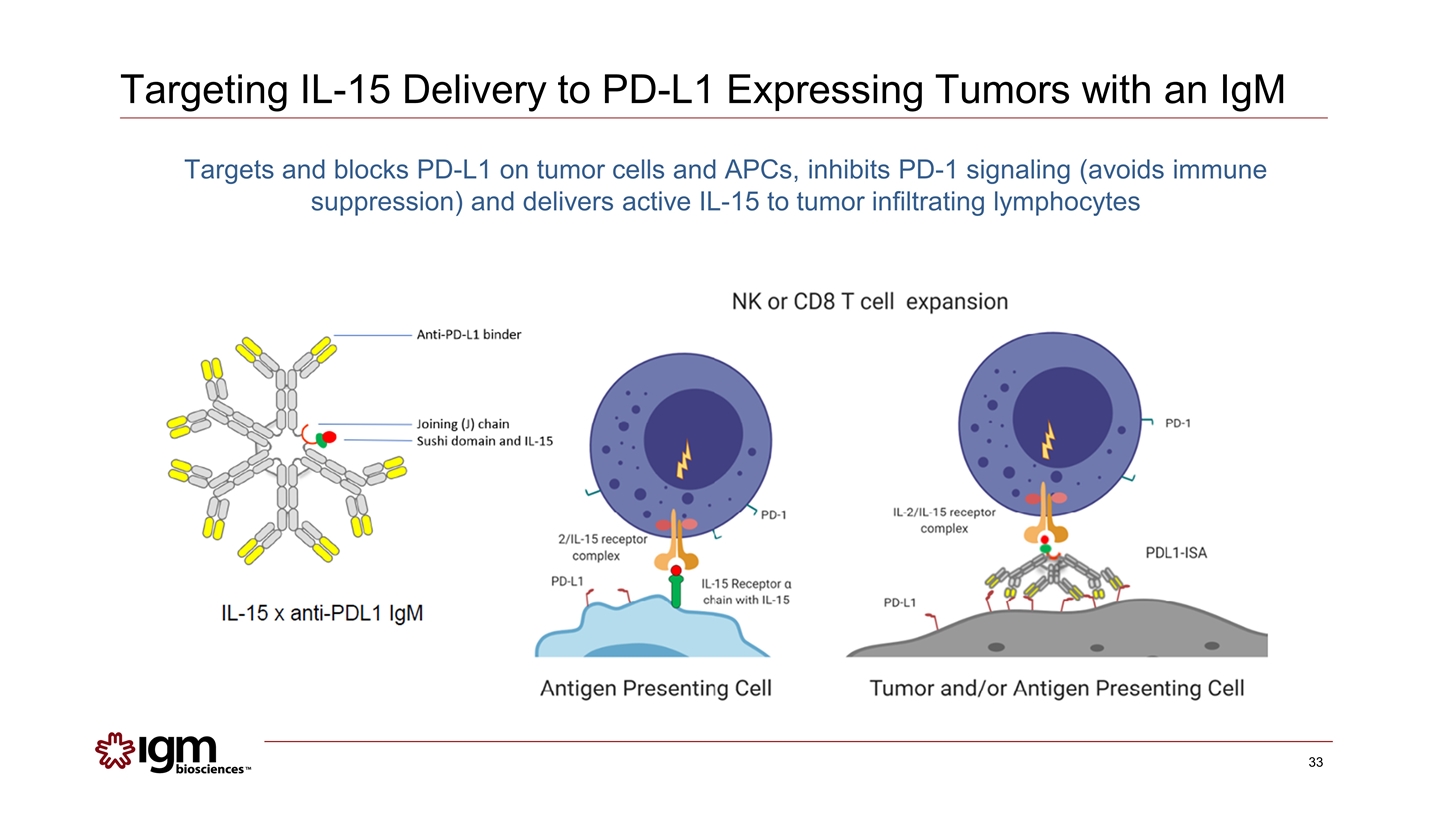
Targeting IL-15 Delivery to PD-L1 Expressing Tumors with an IgM Targets and blocks PD-L1 on tumor cells and APCs, inhibits PD-1 signaling (avoids immune suppression) and delivers active IL-15 to tumor infiltrating lymphocytes
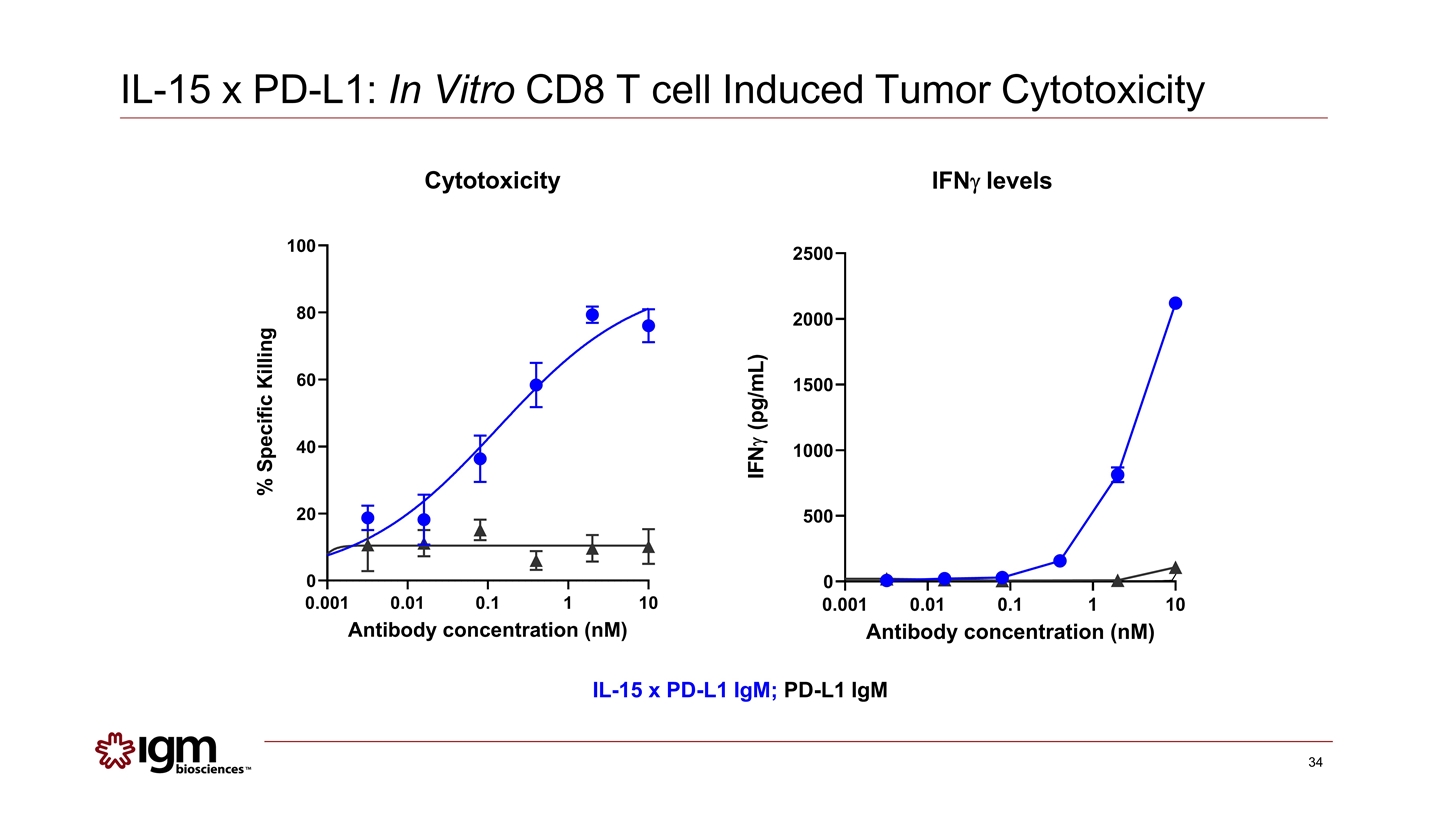
IL-15 x PD-L1 IgM; PD-L1 IgM Cytotoxicity IFNg levels IL-15 x PD-L1: In Vitro CD8 T cell Induced Tumor Cytotoxicity
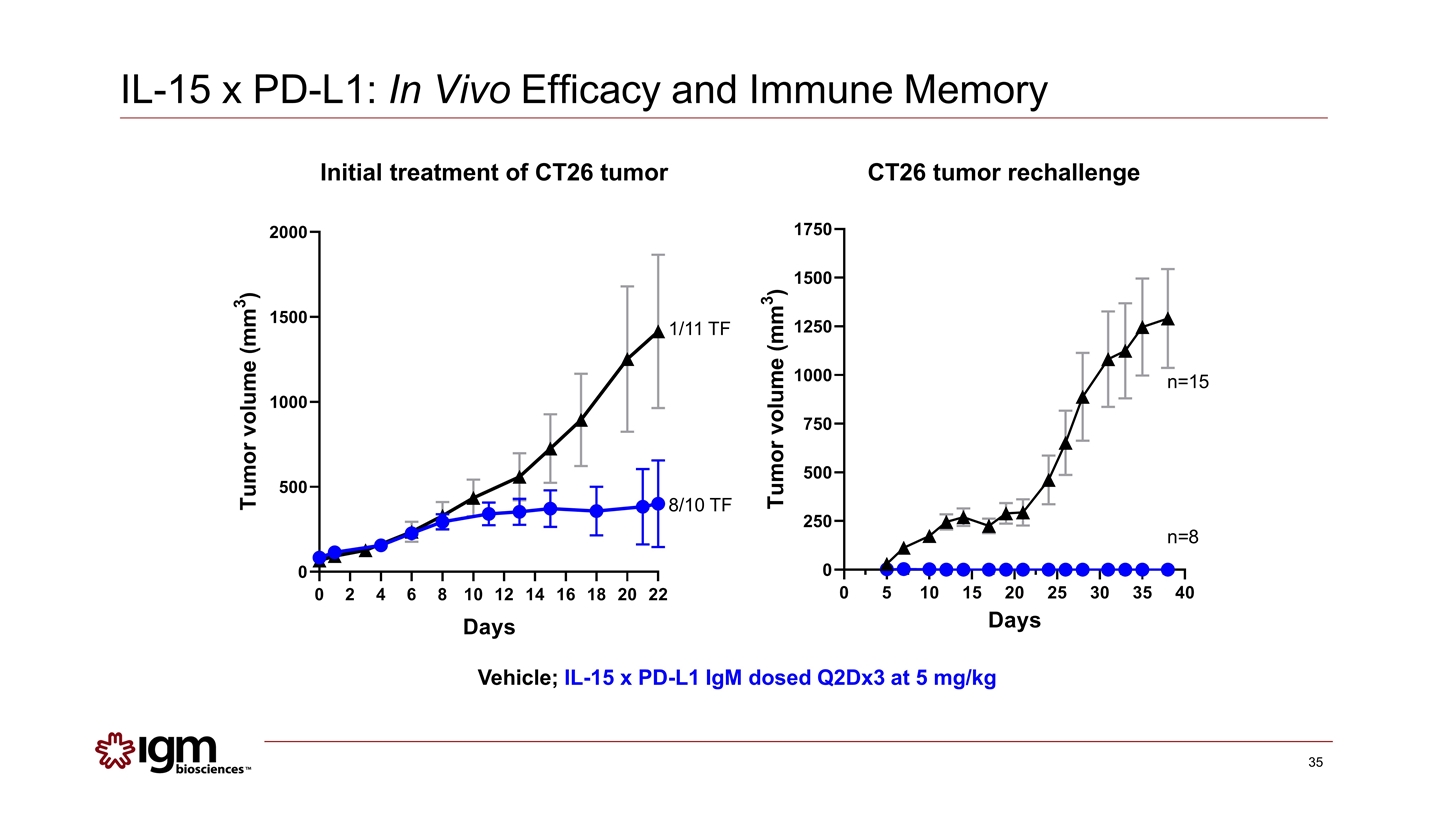
Vehicle; IL-15 x PD-L1 IgM dosed Q2Dx3 at 5 mg/kg Initial treatment of CT26 tumor CT26 tumor rechallenge 8/10 TF 1/11 TF IL-15 x PD-L1: In Vivo Efficacy and Immune Memory
Potential Applications of IgM Antibody Technology Platform T cell Engager Receptor Cross-linking Agonist Targeted Cytokines Antibody Drug Conjugates Therapeutic Areas Oncology Infectious Disease Autoimmune Disease
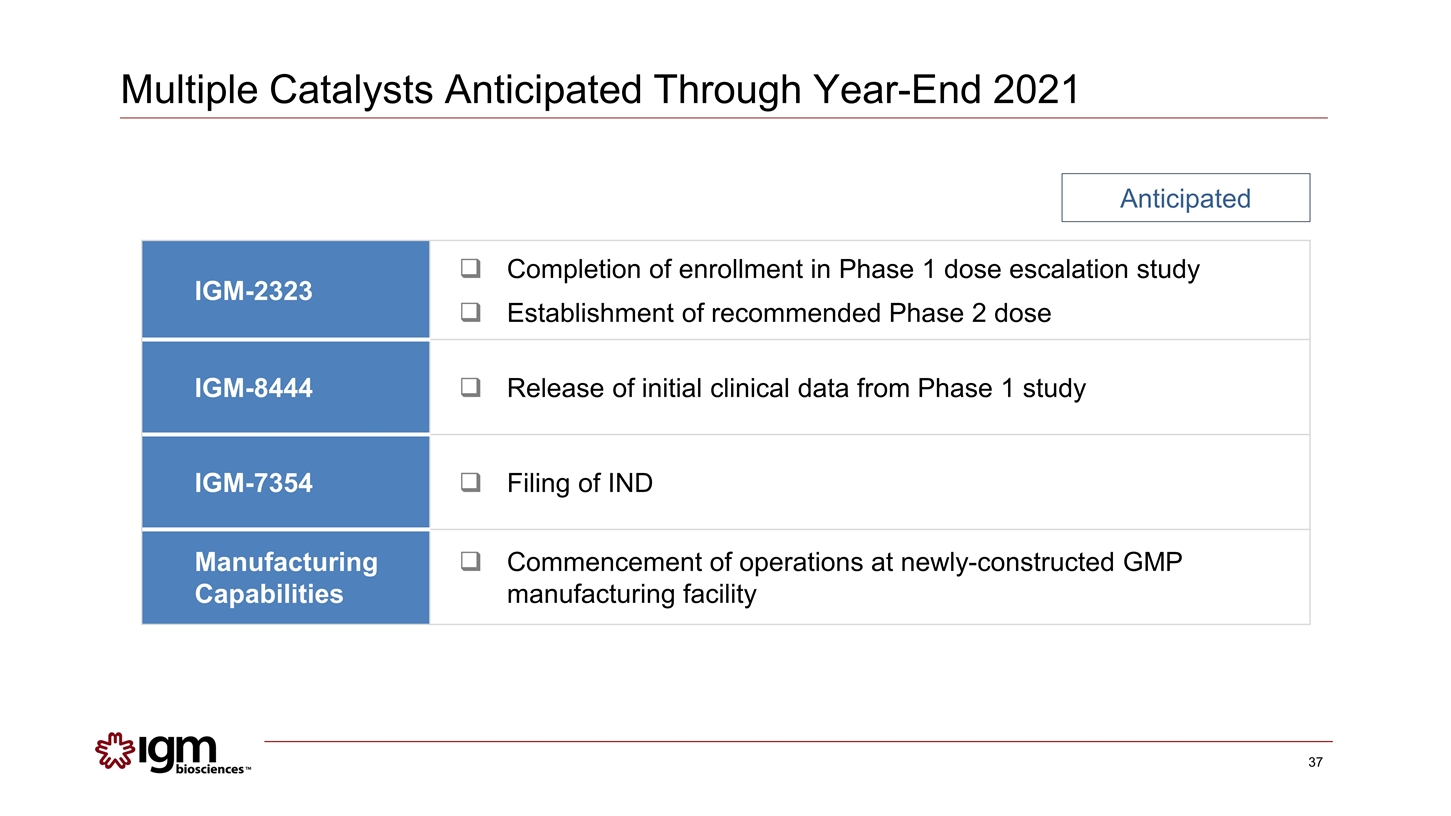
Multiple Catalysts Anticipated Through Year-End 2021 IGM-2323 Completion of enrollment in Phase 1 dose escalation study Establishment of recommended Phase 2 dose IGM-8444 Release of initial clinical data from Phase 1 study IGM-7354 Filing of IND Manufacturing Capabilities Commencement of operations at newly-constructed GMP manufacturing facility Anticipated

DANIEL S. CHEN, MD, PhD Chief Medical Officer MISBAH TAHIR Chief Financial Officer ELIZABETH HAANES, PhD VP, Intellectual Property FRED M. SCHWARZER Chief Executive Officer BRUCE KEYT, PhD Chief Scientific Officer WAYNE GODFREY, MD VP, Clinical Development SUZETTE TAUBER VP, Human Resources ANGUS SINCLAIR, PhD VP, Immuno-Oncology ERIC HUMKE, MD, PhD VP, Clinical Development MARVIN PETERSON, PhD VP, Process Sciences & Manufacturing STEVE CARROLL, PhD VP, Preclinical Sciences KATHY MILLER, PhD VP, Antibody Discovery Leadership Team

Pioneering the Development of Engineered IgM Antibodies Corporate Presentation December 2020






































