Filed Pursuant to Rule 424(b)(4)
Registration No. 333-169699
5,800,000 American Depositary Shares

ShangPharma Corporation
Representing 104,400,000 Ordinary Shares
This is our initial public offering. We are offering 3,200,000 American depositary shares, or ADSs, and certain of our shareholders identified in this prospectus are offering an additional 2,600,000 ADSs. Each ADS represents 18 of our ordinary shares, par value $0.001 per share. We will not receive any proceeds from the ADSs sold by the selling shareholders. No public market currently exists for our shares or ADSs.
The initial public offering price of our ADSs is $15.00 per ADS. Our ADSs have been approved for listing on the New York Stock Exchange under the symbol “SHP.”
Investing in our ADSs involves a high degree of risk. See “Risk Factors” beginning on page 11.
| | | | | | | | |
| | | Per ADS | | | Total | |
Public offering price | | $ | 15.00 | | | $ | 87,000,000 | |
Underwriting discounts and commissions | | $ | 1.05 | | | $ | 6,090,000 | |
Proceeds, before expenses, to ShangPharma Corporation | | $ | 13.95 | | | $ | 44,640,000 | |
Proceeds, before expenses, to the selling shareholders | | $ | 13.95 | | | $ | 36,270,000 | |
The selling shareholders have granted the underwriters a 30-day option to purchase up to 870,000 additional ADSs at the initial public offering price less underwriting discounts and commissions.
Delivery of our ADSs will be made on or about October 22, 2010.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
| | |
| Citi | | J.P. Morgan |
| |
| William Blair & Company | | Oppenheimer & Co. |
The date of this prospectus is October 18, 2010.

TABLE OF CONTENTS
You should rely only on the information contained in this prospectus or in any related free writing prospectus that we have filed with the Securities and Exchange Commission, or the SEC. We have not authorized anyone to provide you with information that is different. This prospectus may only be used where it is legal to offer and sell these securities. Unless otherwise indicated, the information in this document may only be accurate as of the date of this document.
We have not taken any action to permit a public offering of the ADSs outside the United States. Persons outside the United States who come into possession of this prospectus must inform themselves about and observe any restrictions relating to the offering of the ADSs and the distribution of the prospectus outside the United States.
Through and including November 12, 2010, all dealers that effect transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers’ obligation to deliver a prospectus when acting as an underwriter and with respect to their unsold allotments or subscriptions.
PROSPECTUS SUMMARY
You should read the following summary together with the entire prospectus, including the more detailed information regarding us, the ADSs being sold in this offering, and our consolidated financial statements and related notes appearing elsewhere in this prospectus. You should consider carefully, among other things, the matters discussed in the section entitled “Risk Factors.” This summary and other sections of this prospectus contain information from a report, referred to in this prospectus as the Frost & Sullivan Report, which we commissioned Frost & Sullivan, an independent market research firm, to provide information on the industry in which we operate, including our market position in that industry.
Our Company
We are a leading China-based pharmaceutical and biotechnology research and development, or R&D, outsourcing company. We provide a broad range of high-quality, integrated services across the drug discovery and development process to international and Chinese pharmaceutical and biotechnology companies. Our services consist of discovery chemistry, discovery biology and preclinical development, pharmaceutical development and biologics services. We offer our services on a full-time-equivalent, or FTE, basis or a fee-for-service basis. Capitalizing on our broad service offerings, the experience and expertise of our skilled scientists and our state-of-the-art facilities and equipment, we help our customers discover and develop novel drug candidates efficiently. We have a diversified and loyal global customer base. We provide services to over 100 customers, including all of the top ten pharmaceutical and biotechnology companies in the world in terms of 2009 revenues ranked by Frost & Sullivan and a number of fast-growing biotechnology and specialty pharmaceutical companies, as well as renowned academic and research institutions in the U.S. and China. Most of our customers return to us for additional and often larger projects, and all of our top ten customers in each of 2008 and 2009 remain our customers in 2010.
Our experienced and strong R&D team contributes significantly to our market leadership. We have successfully grown our scientific research staff from 988 as of December 31, 2007 to 1,525 as of June 30, 2010. Over 60% of our scientific research staff have post-graduate degrees, including many native Chinese scientists and managers who have returned to China after studying or working overseas with experience in global pharmaceutical and biotechnology companies and knowledge of Western business practices. These Western-educated native Chinese who have returned to China are commonly referred to as returnees. Based in China and headquartered in Shanghai, we are well-positioned to attract a large number of returnees and benefit from an abundant supply of highly skilled, domestically-trained scientific talent in China. We attract, retain and motivate our employees through a comprehensive training and career development program and equity incentive plans.
We conduct our laboratory activities in four primary facilities in China: (i) an approximately 450,000 square-foot laboratory and headquarters in Shanghai Zhangjiang Hi-Tech Park; (ii) an approximately 13,000 square-foot AAALAC-accredited animal facility in Shanghai Zhangjiang Hi-Tech Park; (iii) an approximately 36,000 square-foot R&D center in Chengdu; and (iv) an approximately 28,000 square-foot manufacturing facility in Nanhui, Shanghai. In addition, we are constructing an approximately 460,000 square-foot cGMP-quality multi-purpose manufacturing facility in Fengxian, Shanghai, designed to manufacture advanced intermediates and active pharmaceutical ingredients, or APIs, for preclinical testing and clinical trials. We are also constructing an approximately 150,000 square-foot laboratory services building in Fengxian, Shanghai primarily for pharmaceutical development services. Our strategic location in China provides us many advantages, including relatively low-cost labor, developed infrastructure and favorable government incentives as well as a large talent pool.
1
We have experienced significant growth in recent years. Our net revenues increased from $34.7 million in 2007 to $60.5 million in 2008 and $72.3 million in 2009, representing a compound annual growth rate, or CAGR, of 44.4%, and increased by 27.2% from $32.7 million in the six months ended June 30, 2009 to $41.6 million in the six months ended June 30, 2010. The number of our customers increased from 71 in 2007 to 99 in 2008, 134 in 2009 and 151 in the six months ended June 30, 2010. Our net income increased from $7.5 million in 2007 to $9.1 million in 2008 and $9.8 million in 2009, and increased from $4.6 million in the six months ended June 30, 2009 to $6.9 million in the six months ended June 30, 2010.
In 2008, 2009 and the nine months ended September 30, 2010, our founders granted restricted share units, or RSUs, convertible to 7,450,000, 2,100,000 and 2,200,000 ordinary shares, respectively, to certain members of our senior management and other employees under the Founder’s 2008 Equity and Performance Incentive Plan, or the Founder’s Plan. We have not recorded any share-based compensation expense in connection with these RSU grants because one of the vesting conditions for the RSUs is the completion of our initial public offering. As a result, immediately upon completion of this offering, we expect to incur a significant share-based compensation charge of approximately $2.5 million, which will materially and adversely affect our results of operations for the quarter in which this offering is completed.
Our Services
We provide a broad range of high-quality, integrated services across the drug discovery and development process to help international and Chinese pharmaceutical and biotechnology companies discover and develop novel drug candidates efficiently. Our service offerings include the following:
| | • | | discovery chemistry, consisting primarily of medicinal chemistry, synthetic chemistry, library generation and analytical chemistry; |
| | • | | discovery biology and preclinical development, consisting primarily of assay development and high throughput screening, pharmacology, reagent generation, DMPK, ADME profiling, metabolite identification, bioanalytical services and non-GLP toxicology; |
| | • | | pharmaceutical development, consisting primarily of preformulation and formulation, process R&D, analytical development and research manufacturing; and |
| | • | | biologics, consisting primarily of therapeutic antibody generation, antibody optimizing and engineering, and analytical services for large molecules. |
We believe our customers value our ability to offer a wide range of quality services to meet their drug R&D needs, and we expect to expand our service offerings along the drug discovery and development value chain.
Our Industry
The global contract research organization, or CRO, industry has grown significantly in recent years and is expected to continue to expand, driven primarily by the ability of CROs to contain costs, provide increased flexibility, deliver a broad service platform and reduce time to market.
Outsourcing to emerging countries such as China has increased in recent years. According to Frost & Sullivan, the Chinese CRO market grew at a CAGR of 27.2% from $380 million in 2007 to $615 million in 2009, and is projected to grow at a CAGR of 20.7% to $1.3 billion in 2013. The principal growth drivers include lower-cost and high-quality services, a large talent pool, the increasing breath of R&D service offerings, a large supply of treatment naive patients, a growing domestic pharmaceutical market and strong government support.
2
Our Strengths and Strategies
We believe that the following strengths contribute to our success and differentiate us from our competitors:
| | • | | leading market position with proven track record; |
| | • | | seasoned management and experienced R&D team; |
| | • | | integrated service platform with comprehensive service offerings; |
| | • | | loyal and diversified global customer base; |
| | • | | efficient service provider with competitive cost structure; and |
| | • | | state-of-the-art facilities with flexible and scalable capacities. |
We aim to become one of the world’s leading pharmaceutical and biotechnology R&D outsourcing companies. To achieve this, we intend to pursue the following strategies:
| | • | | broaden our service offerings and enhance our service capabilities; |
| | • | | maintain and expand our customer base; |
| | • | | attract, train and retain scientific talent; |
| | • | | enhance our operating efficiency; and |
| | • | | selectively evaluate and pursue strategic alliance and acquisition opportunities. |
Our Challenges
We expect to face risks and uncertainties related to our ability to:
| | • | | attract, train, motivate and retain skilled scientists; |
| | • | | diversify our customer base and adapt to potential loss of sales to, or significant reduction in orders from, any of our major customers; |
| | • | | adapt our business to industry trends, such as fluctuations in the R&D budgets of pharmaceutical and biotechnology industry participants; |
| | • | | protect the intellectual property rights of our customers; |
| | • | | comply with applicable regulations and industry standards; |
| | • | | compete effectively in our industry, which may subject us to increasing pricing pressure and reduce the demand for our services; |
| | • | | expand and market our services and manage our growth; and |
| | • | | develop and maintain effective internal control over financial reporting. |
See “Risk Factors” for a detailed discussion of these and other risks that we face.
Corporate History and Structure
We are a Cayman Islands holding company and conduct substantially all of our business through our operating subsidiaries in China. We commenced operations in China in 2002 and established ShangPharma
3
Corporation as an offshore holding company in the Cayman Islands on August 30, 2007. We own 100% of all of our operating subsidiaries in China through two wholly-owned companies incorporated in Hong Kong, China Gateway Life Science (Holdings) Limited and ChemExplorer Company Limited.
The following diagram illustrates our corporate structure and the place of formation of our principal subsidiaries as of the date of this prospectus.
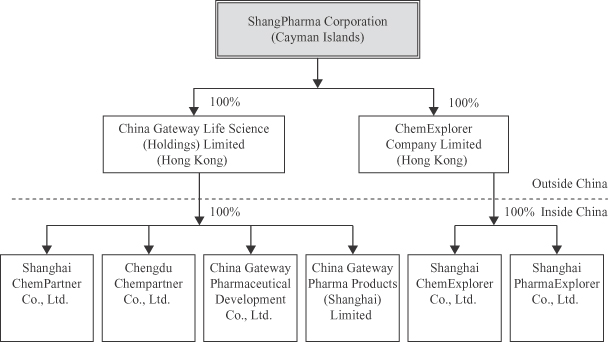
Our Corporate Information
Our principal executive offices are located at No. 5 Building, 998 Halei Road, Zhangjiang Hi-Tech Park, Pudong New Area, Shanghai, 201203, People’s Republic of China. Our telephone number at this address is (86-21) 5132-0088. Our registered office in the Cayman Islands is located at Cricket Square, Hutchins Drive, P.O. Box 2681, Grand Cayman, KY1-1111, Cayman Islands. Our agent for service of process in the United States is Law Debenture Corporate Services Inc. located at 400 Madison Avenue, 4th Floor, New York, New York 10017.
Investors should submit any inquiries to the address and telephone number of our principal executive offices. Our main website iswww.shangpharma.com. The information contained on our website is not a part of this prospectus.
4
Conventions That Apply to This Prospectus
Unless we indicate otherwise, all information in this prospectus assumes no exercise by the underwriters of their option to purchase up to 870,000 additional ADSs representing 15,660,000 ordinary shares from the selling shareholders.
Except where the context otherwise requires and for purposes of this prospectus only:
| | • | | “we,” “us,” “our company,” “our” and “ShangPharma” refer to ShangPharma Corporation, a Cayman Islands company, and its subsidiaries; |
| | • | | “ADSs” refers to our American depositary shares, each of which represents 18 ordinary shares, and “ADRs” refers to American depositary receipts, which, if issued, evidence our ADSs; |
| | • | | “China” or the “PRC” refer to the People’s Republic of China excluding, for the purpose of this prospectus only, Hong Kong, Macau and Taiwan; |
| | • | | “RMB” or “Renminbi” refers to the legal currency of China and “$,” “dollar,” “US$” or “U.S. dollar” refers to the legal currency of the United States; and |
| | • | | “shares” or “ordinary shares” refers to our ordinary shares, par value $0.001 per share, and “preferred shares” refers to our Series A convertible preferred shares, par value $0.001 per share. |
5
The Offering
The following assumes that the underwriters will not exercise their option to purchase additional ADSs in the offering, unless otherwise indicated.
ADSs offered by us | 3,200,000 ADSs |
ADSs offered by the selling shareholders | 2,600,000 ADSs |
Offering price | $15.00 per ADS. |
ADSs outstanding immediately after this offering | 5,800,000 ADSs |
Ordinary shares outstanding immediately after this offering | 335,600,000 shares |
The ADSs | Each ADS represents 18 ordinary shares, par value $0.001 per share. |
The depositary will be the holder of the ordinary shares underlying your ADSs and you will have rights as provided in the deposit agreement.
Although we do not expect to pay dividends in the foreseeable future, if we declare dividends on our ordinary shares, the depositary will pay you the cash dividends and other distributions it receives on our ordinary shares, after deducting fees of up to $0.05 per ADS per distribution and expenses.
In addition, the depositary may charge a fee of up to $0.05 per ADS per calendar year (or portion thereof) for services it performs in administering the ADSs (which may be charged on a periodic basis during each calendar year and shall be assessed against holders of ADSs as of the record date or record dates set by the depositary during each calendar year).
| | You may surrender your ADSs to the depositary along with the ADS cancellation fees of $0.05 per ADS and receive the ordinary shares underlying your ADSs. |
We may amend or terminate the deposit agreement without your consent, and if you continue to hold your ADSs, you agree to be bound by the deposit agreement as amended.
You should carefully read the section in this prospectus entitled “Description of American Depositary Shares” to better understand the terms of the ADSs. You should also read the deposit agreement,
6
| | which is an exhibit to the registration statement that includes this prospectus. |
Listing | Our ADSs have been approved for listing on the New York Stock Exchange under the symbol “SHP.” Our ADSs and shares will not be listed on any other exchange or quoted for trading on any other automated quotation system. |
Option to purchase additional ADSs | The selling shareholders have granted to the underwriters an option, exercisable within 30 days from the date of this prospectus, to purchase up to an aggregate of 870,000 additional ADSs. |
Use of proceeds | Our net proceeds from this offering are expected to be approximately $41.0 million, after deducting underwriting discounts and commissions and the estimated offering expenses payable by us. We will not receive any proceeds from the ADSs sold by the selling shareholders. We anticipate using the net proceeds as follows: |
| | • | | approximately $15.0 million for the construction of our manufacturing facility and laboratory services building in Fengxian, Shanghai; |
| | • | | approximately $15.0 million for the purchase of equipment; |
| | • | | approximately $5.0 million for the expansion of our service offerings; |
| | • | | approximately $5.0 million for working capital needs; and |
| | • | | the balance for other general corporate purposes, including potential acquisitions (although we are not currently negotiating any such acquisitions). |
Depositary | JPMorgan Chase Bank, N.A. |
Risk factors | See “Risk Factors��� and other information included in this prospectus for a discussion of risks you should carefully consider before investing in the ADSs. |
Lock-up | We, all of our directors, executive officers and existing shareholders, and certain holders of options and RSUs, have agreed with the underwriters not to sell, transfer or otherwise dispose of any of our ordinary shares or ADSs representing our ordinary shares for 180 days after the date of this prospectus. See “Underwriting.” |
The number of ordinary shares that will be outstanding immediately after this offering:
| | • | | assumes the conversion of all outstanding preferred shares into 69,994,014 ordinary shares immediately prior to the completion of this offering; |
7
| | • | | excludes 36,046,200 ordinary shares issuable upon the exercise of options outstanding as of September 30, 2010 under our 2008 equity and performance incentive plan, as amended and restated on February 24, 2010, or the Plan, at a weighted average exercise price of approximately $0.55 per share; and |
| | • | | excludes 516,158 ordinary shares reserved for future issuance under the Plan. |
8
SUMMARY CONSOLIDATED FINANCIAL DATA
The following summary consolidated statements of operations data for the years ended December 31, 2007, 2008 and 2009 and the summary consolidated balance sheet data as of December 31, 2008 and 2009 have been derived from our audited consolidated financial statements included elsewhere in this prospectus. The following summary consolidated statement of operations data for the six months ended June 30, 2009 and 2010 and the summary consolidated balance sheet data as of June 30, 2010 have been derived from our unaudited condensed consolidated financial statements included elsewhere in this prospectus and have been prepared on the same basis as our audited consolidated financial statements. Our historical results do not necessarily indicate our expected results for any future periods. You should read this Summary Consolidated Financial Data together with our consolidated financial statements and the related notes and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included elsewhere in this prospectus. Our consolidated financial statements are prepared and presented in accordance with accounting principles generally accepted in the United States of America, or U.S. GAAP.
| | | | | | | | | | | | | | | | | | | | |
| | | Year Ended December 31, | | | Six Months Ended
June 30, | |
| | | 2007 | | | 2008 | | | 2009 | | | 2009 | | | 2010 | |
| | | (in millions of $, except for share, per share and per ADS data) | |
Consolidated Statements of Operations Data: | | | | | | | | | | | | | | | | | | | | |
Net revenues | | | 34.7 | | | | 60.5 | | | | 72.3 | | | | 32.7 | | | | 41.6 | |
Cost of revenues | | | (22.8 | ) | | | (40.5 | ) | | | (48.4 | ) | | | (21.8 | ) | | | (27.2 | ) |
| | | | | | | | | | | | | | | | | | | | |
Gross profit | | | 11.9 | | | | 20.0 | | | | 23.9 | | | | 10.9 | | | | 14.4 | |
| | | | | | | | | | | | | | | | | | | | |
Operating expenses: | | | | | | | | | | | | | | | | | | | | |
Selling and marketing | | | (0.5 | ) | | | (0.8 | ) | | | (1.7 | ) | | | (0.7 | ) | | | (1.1 | ) |
General and administrative | | | (4.0 | ) | | | (9.3 | ) | | | (12.0 | ) | | | (5.6 | ) | | | (6.9 | ) |
| | | | | | | | | | | | | | | | | | | | |
Total operating expenses | | | (4.5 | ) | | | (10.1 | ) | | | (13.7 | ) | | | (6.3 | ) | | | (8.0 | ) |
| | | | | | | | | | | | | | | | | | | | |
Profit from operations | | | 7.4 | | | | 9.9 | | | | 10.2 | | | | 4.6 | | | | 6.4 | |
Interest income | | | 0.2 | | | | 0.2 | | | | * | | | | * | | | | * | |
Interest expense | | | — | | | | — | | | | — | | | | — | | | | * | |
Other income | | | 0.2 | | | | 0.9 | | | | 1.3 | | | | 0.8 | | | | 1.6 | |
Other expenses | | | (0.1 | ) | | | (1.6 | ) | | | (0.2 | ) | | | (0.1 | ) | | | (0.2 | ) |
| | | | | | | | | | | | | | | | | | | | |
Income from operations before income taxes | | | 7.7 | | | | 9.4 | | | | 11.4 | | | | 5.3 | | | | 7.8 | |
Income taxes | | | (0.2 | ) | | | (0.3 | ) | | | (1.6 | ) | | | (0.7 | ) | | | (0.9 | ) |
| | | | | | | | | | | | | | | | | | | | |
Net income attributable to ShangPharma Corporation | | | 7.5 | | | | 9.1 | | | | 9.8 | | | | 4.6 | | | | 6.9 | |
Allocation to preferred shareholders | | | (0.7 | ) | | | (2.3 | ) | | | (2.5 | ) | | | (1.2 | ) | | | (1.8 | ) |
| | | | | | | | | | | | | | | | | | | | |
Net income attributable to ShangPharma Corporation’s ordinary shareholders | | | 6.8 | | | | 6.8 | | | | 7.3 | | | | 3.4 | | | | 5.1 | |
| | | | | | | | | | | | | | | | | | | | |
Net income attributable to ShangPharma Corporation’s ordinary shareholders per share | | | | | | | | | | | | | | | | | | | | |
Basic | | | 0.03 | | | | 0.03 | | | | 0.04 | | | | 0.02 | | | | 0.02 | |
| | | | | | | | | | | | | | | | | | | | |
Diluted | | | 0.03 | | | | 0.03 | | | | 0.04 | | | | 0.02 | | | | 0.02 | |
| | | | | | | | | | | | | | | | | | | | |
Net income attributable to ShangPharma Corporation’s ordinary shareholders per ADS(1) | | | | | | | | | | | | | | | | | | | | |
Basic | | | 0.59 | | | | 0.59 | | | | 0.63 | | | | 0.30 | | | | 0.44 | |
| | | | | | | | | | | | | | | | | | | | |
Diluted | | | 0.59 | | | | 0.59 | | | | 0.63 | | | | 0.30 | | | | 0.44 | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | |
Weighted average ordinary shares outstanding(2) | �� | | | | | | | | | | | | | | | | | | | |
Basic | | | 208,005,986 | | | | 208,005,986 | | | | 208,005,986 | | | | 208,005,986 | | | | 208,005,986 | |
| | | | | | | | | | | | | | | | | | | | |
Diluted | | | 230,381,122 | | | | 278,000,000 | | | | 278,051,299 | | | | 278,000,000 | | | | 210,362,840 | |
| | | | | | | | | | | | | | | | | | | | |
Pro forma earnings per share attributable to ShangPharma Corporation’s ordinary shareholders (unaudited)(3) | | | | | | | | | | | | | | | | | | | | |
Basic | | | | | | | | | | | 0.04 | | | | | | | | 0.02 | |
| | | | | | | | | | | | | | | | | | | | |
Diluted | | | | | | | | | | | 0.04 | | | | | | | | 0.02 | |
| | | | | | | | | | | | | | | | | | | | |
Pro forma weighted average ordinary shares outstanding (unaudited)(2) (3) (4) | | | | | | | | | | | | | | | | | | | | |
Basic | | | | | | | | | | | 278,000,000 | | | | | | | | 278,000,000 | |
| | | | | | | | | | | | | | | | | | | | |
Diluted | | | | | | | | | | | 278,051,299 | | | | | | | | 280,356,854 | |
| | | | | | | | | | | | | | | | | | | | |
9
| (1) | Each ADS represents 18 ordinary shares. |
| (2) | In May 2008, we effected a bonus share issuance in the form a share split by issuing an additional approximately 20,800 ordinary shares for each ordinary share outstanding and an additional approximately 20,800 preferred shares for each preferred share outstanding. As a result of the bonus share issuance, the number of outstanding ordinary shares and preferred shares increased to 208,005,986 and 69,994,014, respectively. See “Related Party Transactions—Share Issuances and Splits.” The effect of the bonus share issuance is retroactively reflected for all periods presented herein. |
| (3) | For a description of the pro forma adjustments used to calculate pro forma earnings per share for the year ended December 31, 2009, see “Note 19—Unaudited Pro Forma Balance Sheet and Earnings Per Share for Conversion of Preferred Shares” to our consolidated financial statements included elsewhere in this prospectus. |
| (4) | For a description of the pro forma adjustments used to calculate pro forma earnings per share for the six months ended June 30, 2010, see “Note 15—Unaudited Pro Forma Balance Sheet and Earnings Per Share for Conversion of Preferred Shares” to our unaudited condensed consolidated financial statements included elsewhere in this prospectus. |
| | | | | | | | | | | | | | | | |
| | | As of December 31, | | | As of June 30, | |
| | | 2008 | | | 2009 | | | 2010 | | | 2010 | |
| | | (in millions of $) | |
| | | | | | | | | (Pro forma)(1) | |
Consolidated Balance Sheet Data: | | | | | | | | | | | | | | | | |
Cash | | | 15.8 | | | | 12.2 | | | | 10.0 | | | | 10.0 | |
Total current assets | | | 28.7 | | | | 30.1 | | | | 31.9 | | | | 31.9 | |
Total assets | | | 61.7 | | | | 70.0 | | | | 78.8 | | | | 78.8 | |
Total current liabilities | | | 17.3 | | | | 15.5 | | | | 16.9 | | | | 16.9 | |
Total liabilities | | | 17.3 | | | | 15.5 | | | | 16.9 | | | | 16.9 | |
Series A convertible preferred shares | | | 34.4 | | | | 34.4 | | | | 34.4 | | | | — | |
Total equity | | | 10.0 | | | | 20.1 | | | | 27.5 | | | | 61.9 | |
| (1) | Pro forma basis reflects the conversion of all outstanding preferred shares on a 1-for-1 basis into an aggregate of 69,994,014 ordinary shares upon the completion of this offering. |
10
RISK FACTORS
You should carefully consider the risks described below and all other information contained in this prospectus before making an investment decision. If any of the following risks actually occurs, our business, financial condition and results of operations could be materially and adversely affected. In that event, the trading price of our ADSs could decline, and you may lose part or all of your investment.
Risks Related to Our Business and Industry
Our ability to execute projects, maintain, expand or renew existing customer engagements and obtain new customers depends largely on our ability to attract, train, motivate and retain skilled scientists.
Our success largely depends on the R&D efforts of our skilled scientists and their ability to keep pace with changes in pharmaceutical and biotechnology R&D technologies and methodologies. In particular, our customers value highly skilled Western-trained scientists, preferably with large pharmaceutical and/or biotechnology company experience. Our success also depends on the depth and quantity of our scientific personnel. We face challenges in attracting new employees and maintaining consistent quality standards throughout our employee base at our current growth rate. Our employee base increased from 1,115 as of December 2007 to 1,755 as of June 30, 2010, and is expected to increase in the second half of 2010.
We compete with pharmaceutical, biotechnology and contract research firms and academic and research institutions for qualified and experienced scientists, particularly in chemistry and biology. To effectively compete, we may be required to offer higher compensation and other benefits that could materially and adversely affect our financial condition and results of operations. We may be unable to hire and retain enough skilled and experienced scientists to replace those who leave our company. Additionally, we may be unable to redeploy and retrain our professionals to keep pace with technological changes, evolving standards and changing customer preferences. Any inability to attract, train, motivate or retain skilled scientists may materially and adversely affect our business, financial condition, results of operations and prospects.
A limited number of our customers have accounted for and are expected to account for a high percentage of our revenues. The loss or significant reduction in orders from any of these customers could materially and adversely affect our business, financial condition, results of operations and prospects.
Our two largest customers in 2009 and the six months ended June 30, 2010, Eli Lilly and Company, or Lilly, and GlaxoSmithKline plc, or GSK, respectively accounted for 27% and 10% of our net revenues in 2009 and 20% and 9% of our net revenues in the six months ended June 30, 2010. No other customer accounted for more than 10% of our net revenues in 2009 and the six months ended June 30, 2010. Our top ten customers, which varied in each of the last three years and the six months ended June 30, 2010, accounted for approximately 85%, 77%, 65% and 63% of our net revenues in 2007, 2008, 2009 and the six months ended June 30, 2010, respectively. Furthermore, we generated a significant majority of our net revenues during the same periods from sales to customers located in the United States.
Due to our customer concentration, any of the following events, among others, may cause material revenue fluctuations or declines and materially and adversely affect our business, financial condition, results of operations and prospects:
| | • | | order or contract reduction, delay or cancellation by one or more of our significant customers and our failure to identify and acquire additional or replacement customers; and |
| | • | | a substantial reduction by one or more of our significant customers in the price they are willing to pay for our services and products. |
Any failure to retain our existing customers or expand our customer base may result in our inability to maintain or increase our revenues.
Our existing customers may not continue to generate significant revenues for us once our engagements with them are concluded and our relationships with them may not present further business opportunities.
11
We have entered into master services agreements with some customers, the terms of which typically range from one to five years. If we fail to build new customer relationships or our relationships with existing customers terminate, we may not be able to maintain or increase our revenues.
A reduction in R&D budgets by pharmaceutical and biotechnology companies may result in a reduction or discontinued use of our services, which may adversely affect our business.
Fluctuations in the R&D budgets of pharmaceutical and biotechnology industry participants could significantly affect the demand for our services. R&D budgets fluctuate due to, among other things, pharmaceutical and biotechnology industry downturns, consolidation of pharmaceutical and biotechnology companies, general economic conditions, and changes in available resources, spending priorities and institutional budgetary policies. The global pharmaceutical and biotechnology industry has experienced significant consolidation and, during the global financial crisis, some pharmaceutical and biotechnology companies experienced financial difficulties. We have experienced reductions, delays or cancellations of orders or contracts from certain customers and difficulties in collecting fees from certain customers who experienced financial difficulties or consolidation in the global financial crisis. For example, two of our North America-based biotechnology customers experienced financial difficulties during the global financial crisis and terminated their contracts with us in late 2008. We collected all of the accounts receivable from one of the two customers and part of the accounts receivable from the other one. The amount of uncollected accounts receivable was immaterial and fully reserved for. If pharmaceutical and biotechnology companies discontinue or decrease the use of our services due to factors such as a prolonged slowdown in the overall U.S. or global economy or further industry consolidation, our revenues and earnings could be lower than we expect and our revenues may decrease or fail to grow at historical rates.
The outsourcing trend in the drug R&D industry may decrease, which could slow our growth.
The success of our business depends primarily on the number of contracts and the size of the contracts that we obtain from pharmaceutical and biotechnology companies. Over the past several years, our business has benefited from increased levels of outsourcing of drug R&D by pharmaceutical and biotechnology companies. While the outsourcing trend is expected to continue for the next several years, a reversal or slowing of this trend could result in diminished growth in our expected growth areas and materially adversely affect our business, financial condition, results of operations and prospects.
If we fail to protect the intellectual property rights of our customers, we may be subject to liability for breach of contract and may suffer damage to our reputation.
Protection of intellectual property associated with pharmaceutical and biotechnology R&D services is critical to all our customers. Our customers generally retain ownership of associated intellectual property rights, including those they provide to us and those arising from the services we provide. Our success therefore depends in substantial part on our ability to protect the proprietary rights of our customers. This is particularly important for us because almost all of our operations are based in China, which has not traditionally enforced intellectual property protection to the same extent as in the United States. Despite measures we take to protect our customers’ and our own intellectual property, unauthorized parties may attempt to obtain and use information that we regard as proprietary. Any unauthorized disclosure of our customers’ proprietary information could subject us to liability for breach of contract and significantly damage our reputation, which could materially harm our business, financial condition, results of operations and prospects.
Any failure to comply with applicable regulations and industry standards or obtain various licenses and permits could harm our reputation and our business, results of operations and prospects.
A number of governmental agencies or industry regulatory bodies in China, the United States and Europe, impose strict rules, regulations and industry standards governing pharmaceutical and biotechnology R&D activities, which apply to our customers and us. Our failure to comply with such regulations could result in the termination of ongoing research, administrative penalties imposed by regulatory bodies or the disqualification of data for submission to regulatory authorities. This could harm our reputation, prospects for future work and
12
operating results. For example, if we were to treat research animals inhumanely or in violation of international standards set out by the Association for Assessment and Accreditation of Laboratory Animal Care, or AAALAC, AAALAC could revoke any such accreditation and the accuracy of our animal research data could be questioned.
In addition, our subsidiaries in China need to obtain various licenses and permits to conduct their current operations. If any of our subsidiaries in China is unable to obtain all the requisite licenses and permits or fails to renew any of the licenses or permits timely, it may be subject to fines and other penalties, including suspension of its operations.
Compliance with environmental regulations can be expensive, and noncompliance with these regulations may result in significant monetary damages, fines and other penalties.
As our pharmaceutical and biotechnology R&D processes generate waste water, toxic and hazardous substances and other industrial wastes, we must comply with all national and local environmental laws and regulations in China. We are required to undertake environmental impact assessment procedures and pass the subsequent inspection and approval procedures before commencing our operations. We are also required to register with, or obtain approvals from, relevant environmental protection authorities for various environmental matters such as discharging waste generated in our operations. We did not complete certain environmental assessment or approval procedures for some of our facilities. We are taking remedial measures necessary to obtain the requisite approvals. We have engaged a qualified assessment consultant to conduct environmental impact assessments. We had submitted the application for approval of the environmental impact assessment report for our principal facilities in Shanghai by the end of August 2010. According to relevant PRC regulations, the local environmental protection authorities are required to complete the inspection and approval procedures within several months upon receipt of an application for approval of the environmental impact assessment. However, we cannot assure you that the remedial measures we are taking will be completed timely and successfully. If for any reason the relevant government authorities in China determine that we are not in compliance with environmental laws and regulations, we may be required to pay fines or damages to third parties or suspend our operations. In addition, because the requirements imposed by environmental laws and regulations may change and more stringent regulations may be adopted, we may be unable to accurately predict the cost of complying with these laws and regulations, which could be substantial.
We are subject to safety and health laws and regulations in China, and any failure to comply could adversely affect our operations.
Our operations and facilities are subject to extensive safety and health laws and regulations. We must ensure that our operations and facilities comply with PRC standards and requirements on fire prevention, hazardous and radioactive materials handling, work environment safety and employees’ health conditions. We have not completed certain procedures for some of our facilities or projects required for compliance with these laws and regulations, such as having our relevant work environments inspected for occupational hazards. We are taking remedial measures to rectify such non-compliance. For example, we have had our material work areas inspected for occupational hazards. However, we may be subject to government orders to rectify non-compliance within a specified period, pay fines or suspend our operations. In addition, we cannot eliminate the risk of accidental contamination or injury from hazardous or radioactive materials used in our operations. If we fail to prevent contamination or injury, we could be liable for any resulting damages, which may materially and adversely impact our business, financial condition, results of operations and prospects.
Any failure by us to satisfy our customers’ audits and inspections could harm our reputation and our business, financial condition, results of operations and prospects.
Our customers routinely audit and inspect our facilities, processes and practices to ensure that we meet their standards for drug discovery and development. To date, we are not aware of any material negative findings from our customers’ audits and inspections. However, we may not be able to pass all such audits and inspections to our customers’ satisfaction, and any such failure may significantly harm our reputation and materially and adversely affect our business, financial condition, results of operations and prospects.
13
We face increasingly intense competition. If we do not compete successfully against new and existing competitors, demand for our services and related revenues may decrease and we may be subject to increasing pricing pressure.
The global pharmaceutical and biotechnology R&D outsourcing market is highly competitive, and we expect competition to intensify. We face competition based on several factors, including the quality and scope of services, the ability to protect confidential information and intellectual property, the ability to be responsive and efficient with respect to customers’ requests, depth of customer relationship and pricing. We compete with WuXi PharmaTech (Cayman) Inc., or WuXi PharmaTech, across the breadth of our service offerings. We also compete with industry participants in particular service areas, including Albany Molecular Research, Inc. in discovery chemistry and pharmaceutical development, and Cerep S.A. and Covance Inc. in discovery biology and preclinical development.
We expect to increasingly compete against multinational companies, both domestically and internationally, as we offer more complex and sophisticated services. Additionally, several major pharmaceutical and biotechnology companies have made in-house R&D investments in China, a trend we expect to continue. These in-house investments may result in increased competition for qualified personnel. Some of our larger competitors may have greater financial, research and other resources, broader scope of services, greater pricing flexibility, more extensive technical capabilities and greater name recognition than we do. Furthermore, consolidation within the global pharmaceutical and biotechnology R&D outsourcing markets may create stronger competitors than those we are facing today.
We also expect increased competition as new companies enter into our market and more advanced technologies become available. Our services and expertise may be rendered obsolete or uneconomical by technological advances or new approaches or technologies. Our competitors’ existing or new approaches or technologies they develop may be more effective than those we develop. Furthermore, increased competition may subject us to increasing pricing pressure and reduce demand for our services, which could reduce our margins and profitability.
We may fail to effectively expand and market our service offerings and capabilities, which may harm our growth opportunities and prospects, possibly resulting in related losses.
We intend to expand our existing services and offer new services across the drug discovery value chain. Initially focusing on discovery chemistry services, we introduced pharmaceutical development services in 2005 and discovery biology and preclinical development services in 2007. We began offering biologics services in early 2010. We are constructing an approximately 460,000 square-foot cGMP-quality multi-purpose manufacturing facility in Fengxian, Shanghai designed to manufacture advanced intermediates and APIs for preclinical testing and clinical trials. We are also constructing an approximately 150,000 square-foot laboratory services building in Fengxian, Shanghai primarily for pharmaceutical development services. To successfully develop and market our new services, we must:
| | • | | accurately assess and meet customer needs and market demands; |
| | • | | optimize our drug discovery and development processes to predict and control costs; |
| | • | | hire, train and retain scientists and other personnel; |
| | • | | provide services in a timely manner; |
| | • | | increase customer awareness and acceptance of our services; |
| | • | | obtain required regulatory clearances or approvals; |
| | • | | compete effectively with other R&D outsourcing providers; |
| | • | | price our services competitively; and |
| | • | | effectively integrate customer feedback into our business planning. |
14
If we are unable to expand our service offerings and create demand for those newly expanded services, our business, results of operations, financial condition and prospects may be materially and adversely affected.
We may be unable to expand our capacity and scale up our operations as anticipated, possibly resulting in material delay, increased costs and lost business opportunities.
We are developing an approximately 460,000 square-foot cGMP-quality multi-purpose manufacturing facility in Fengxian, Shanghai designed to manufacture advanced intermediates and APIs for preclinical testing and clinical trials. We are also constructing an approximately 150,000 square-foot laboratory services building in Fengxian, Shanghai primarily for pharmaceutical development services. We may not construct such facility or building in accordance with the anticipated timetable or within budget. We may lease or build additional animal facilities in Shanghai if the demand for our discovery biology and preclinical development services increases. In addition, we may lease additional lab and office facilities in Chengdu if the demand for our discovery chemistry services increases. Any material delay in bringing these facilities online or scaling up operations, or any substantial cost increases to complete them or scale up operations, could materially and adversely affect our financial condition and results of operations, and result in lost business opportunities.
If we fail to effectively manage our anticipated growth and execute our growth strategies, our business, financial condition, results of operations and prospects could suffer.
Pursuing our growth strategies, including integrating and expanding our facilities and service offerings to meet our customers’ needs, has resulted in and will continue to place substantial demands on our management and resources. Managing this growth and executing our growth strategies will require, among other things:
| | • | | enhancement of our pharmaceutical and biotechnology R&D capabilities; |
| | • | | effective coordination and integration of our research facilities and teams, particularly those located in different or newly opened facilities; |
| | • | | successful personnel hiring and training; |
| | • | | effective cost controls and sufficient liquidity; |
| | • | | effective and efficient financial and management controls; |
| | • | | increased marketing and sales support activities; |
| | • | | effective quality control; and |
| | • | | our ability to manage our various vendors and suppliers and leverage our purchasing power. |
Any failure to effectively manage our anticipated growth and execute our growth strategies could materially adversely affect our business, financial condition, results of operations and prospects.
If our company or our founders grant employees share options and other share-based compensation in the future, our net income may be adversely affected. In addition, we will incur non-cash share-based compensation charges in connection with the RSUs granted under the Founder’s Plan upon completion of this offering, which will materially and adversely impact our results of operations.
Our equity incentive plans and other similar types of incentive plans are important in order to attract and retain key personnel. Our company and our founders have granted our employees share options and restricted share units in the past pursuant to the Plan and the Founder’s Plan. As a result of the issuance of options and RSUs under these plans, we have in the past and expect in the future to incur share-based compensation expenses. We account for compensation costs for all share options, including share options granted to our employees, non-employee directors and officers using the fair value determined at the grant date and recognize the expense in our consolidated statement of operations. We also account for compensation costs for the conversion of RSUs into our ordinary shares. These compensation costs may materially and adversely affect our net income. Moreover, the additional expenses associated with share-based compensation may reduce the attractiveness of our equity incentive plans.
15
In 2008, 2009 and the nine months ended September 30, 2010, our founders granted RSUs convertible to 7,450,000, 2,100,000 and 2,200,000 ordinary shares, respectively, to certain members of our senior management and other employees under the Founder’s Plan. We have not recorded any share-based compensation expense in connection with these RSU grants because one of the vesting conditions for the RSUs is the completion of our initial public offering. As a result, immediately upon completion of this offering, we will incur a significant share-based compensation charge of approximately $2.5 million, which will materially and adversely affect our results of operations for the quarter in which this offering is completed.
In providing our pharmaceutical and biotechnology R&D outsourcing services, we face health and safety liability and product liability risks.
In providing our services in connection with drug discovery and development, we face a range of potential liabilities, which include risks that disease models and animals infected with diseases for research interests may be harmful, or even lethal, to themselves and humans despite preventive measures we take for the quarantine and handling of animals. We also face product liability risks should the drugs we assist in developing and manufacturing become subject to product liability claims. We provide services in the development, testing and manufacturing of drugs that may ultimately be used by humans, although we do not commercially market or sell the products to end users. If any of these drugs harm people, we may be subject to litigation and may be required to pay damages to those persons.
We do not have general liability or product liability insurance covering our whole business or all of our products and services. We currently maintain general liability insurance for four contracts covering, among other things, bodily injury and property damage arising out of the products or services provided under these contracts. The aggregate net revenues derived from these contracts in the six months ended June 30, 2010 accounted for 11.0% of our total net revenues in this period. The aggregate maximum coverage amount under the insurance policy for these contracts is $5 million for bodily injury and property damage arising out of our products or services and economic loss sustained by persons or entities due to deficiencies in our products or services. Damages awarded in a product liability action could be substantial and, to the extent not covered by our insurance, could materially and adversely impact our business, financial condition and results of operations.
Because many of our fee-for-service based contracts are contingent on successful completion and are of a fixed price nature, we may bear risk if we do not successfully or timely develop a service or enter contracts with pricing below our estimated cost due to competitive pressures or strategic objectives, or incur overrun costs.
A significant portion of our net revenues is based on fee-for-service contracts, which are contingent on successful completion and are often for a fixed price. Therefore, we bear financial risk if we do not successfully or timely complete services or if we price our fee-for-service based contracts below our estimated cost of completing these contracts or otherwise incur cost overruns. We also face pricing pressures from some of our competitors. To capture market share, we have, in the past, intentionally priced some fee-for-service based contracts below our estimated cost of completing these contracts. Below-cost pricing or significant cost overruns could materially and adversely affect our business, financial condition, results of operations and prospects.
The loss of services of our senior management and key scientific personnel could severely disrupt our business and growth.
Our success significantly depends upon the continued service of our senior management and key scientific personnel. We are highly dependent on Mr. Michael Xin Hui, our co-founder and chief executive officer, who has managed our business, operations and sales and marketing activities and maintained personal and direct relationships with our major customers since our inception, as well as other members of our management and other key scientific personnel. The loss of any one of them, in particular Mr. Hui, would materially and adversely affect our business. Although each member of our senior management and key scientific personnel has signed a noncompete agreement with us, we may be unable to successfully enforce these provisions in the event of a dispute. If we lose the services of any of our senior management members or key scientific personnel, we may be unable to locate suitable or qualified replacements, and may incur additional expenses to recruit and train new personnel, which could severely disrupt our business and growth.
16
A payment failure by any of our large customers could adversely affect our cash flows and profitability.
Historically, we have not experienced any significant bad debt or collection problems, but such problems may arise in the future. The failure of any of our customers to make timely payments could require us to write off accounts receivable or increase provisions made against our accounts receivable, either of which could adversely affect our cash flows and profitability.
Our limited operating history may make it difficult for you to evaluate our business and future prospects.
We commenced operations and began offering our pharmaceutical and biotechnology R&D outsourcing services in 2002. Our business model changes with the evolution of the pharmaceutical and biotechnology R&D outsourcing market in China and around the world. Accordingly, our operating history upon which you can evaluate the viability and sustainability of our business and its acceptance by industry participants is limited. These circumstances may make it difficult for you to evaluate our business and future prospects, and you should not rely on our past results or our historic growth rate as an indication of our future performance.
In preparing our consolidated financial statements, we have identified one material weakness and other deficiencies in our internal control over financial reporting. If we fail to develop and maintain an effective system of internal control over financial reporting, we may be unable to accurately report our financial results or prevent fraud, and investor confidence in our company and the market price of our ADSs may be adversely affected.
Prior to this offering, we have been a private company with limited accounting personnel and other resources with which to address our internal control over financial reporting. In preparing our consolidated financial statements, we and our independent registered public accounting firm identified one material weakness and other deficiencies in our internal control over financial reporting as of December 31, 2009. As defined in the standards established by the U.S. Public Company Accounting Oversight Board, or the PCAOB, a “material weakness” is a deficiency, or combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the annual or interim financial statements will not be prevented or detected on a timely basis.
The material weakness identified related to a lack of sufficient competent personnel with appropriate levels of accounting knowledge and experience to perform period end reporting procedures, address complex U.S. GAAP accounting issues and prepare and review financial statements and related disclosures under U.S. GAAP. Neither we nor our independent registered public accounting firm undertook a comprehensive assessment of our internal control for purposes of identifying and reporting material weaknesses and other control deficiencies in our internal control over financial reporting as we and they will be required to do once we become a public company. Had we performed a formal assessment of our internal control over financial reporting or had our independent registered public accounting firm performed an audit of our internal control over financial reporting, additional deficiencies may have been identified.
Following the identification of the material weakness and other control deficiencies, we have taken measures and plan to continue to take measures to remedy these control deficiencies. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Internal Control over Financial Reporting.” However, the implementation of these measures may not fully address these deficiencies in our internal control over financial reporting, and we cannot conclude that they have been fully remedied. Our failure to correct these control deficiencies or our failure to discover and address any other control deficiencies could result in inaccuracies in our financial statements and impair our ability to comply with applicable financial reporting requirements and related regulatory filings on a timely basis. As a result, our business, financial condition, results of operations and prospects, as well as the trading price of our ADSs, could be materially and adversely affected. Moreover, ineffective internal control over financial reporting significantly hinders our ability to prevent fraud.
17
Upon completion of this offering, we will become subject to the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act. Section 404 of the Sarbanes-Oxley Act requires that we include a report from management on the effectiveness of our internal control over financial reporting in our annual report on Form 20-F beginning with our annual report for the fiscal year ending December 31, 2011. In addition, beginning at the same time, our independent registered public accounting firm must report on the effectiveness of our internal control over financial reporting. If we fail to remedy the problems identified above, our management and our independent registered public accounting firm may conclude that our internal control over financial reporting is not effective. This could adversely impact the market price of our ADSs due to a loss of investor confidence in the reliability of our reporting processes. We will need to incur significant costs and use significant management and other resources in order to comply with Section 404 of the Sarbanes-Oxley Act.
Our business is sensitive to the current global economic crisis. A severe or prolonged downturn in the global or Chinese economy could materially and adversely affect our business, results of operations and financial condition.
The global financial markets have experienced significant disruptions since 2008, and most of the world’s major economies are in or are just emerging from recession. While there has been improvement in some areas, it is still unclear whether the recovery is sustainable. There is considerable uncertainty over the long-term effects of the expansionary monetary and fiscal policies adopted by the central banks and financial authorities of the world’s leading economies, including those of the United States and China. Continued concerns about the systemic impact of potential long-term and wide-spread recession, energy costs, geopolitical tensions, the availability and cost of credit, and the global housing and mortgage markets have contributed to increased market volatility and diminished expectations for economic growth around the world. Any prolonged slowdown in the global or Chinese economy or downturn in the pharmaceutical and biotechnology industry may negatively impact our business, results of operations and financial condition, and continued turbulence in the international markets may adversely affect our ability to access the capital markets to meet liquidity needs.
Our customer agreements contain provisions that are adverse to our interests or expose us to potential liability.
Our agreements with our customers generally provide that customers can terminate the agreements or reduce the scope of services under the agreements upon short notice. In a majority of our customer agreements, our customers have the unilateral right to terminate for convenience upon one to three months’ prior notice. Although we have not been materially and adversely affected by previous contract cancellations or modifications, if a customer terminates a contract with us, we are only entitled under the terms of the contract to receive revenue earned until the date of termination. Therefore, cancellation or modification of a large contract or cancellation or modification of multiple contracts could materially and adversely affect our business, financial condition, results of operations and prospects.
In certain of our customer agreements, we have assumed indemnification obligations for intellectual property infringement resulting from the misuse of confidential information by our employees or from the customer deliverables that we provide to the extent that we create the infringing aspect of the deliverables. As a result, we could be potentially exposed to substantial liability.
In addition, in some of our customer agreements, we agree, either by ourselves or together with third parties, not to compete with the customer. In some cases, we are required to seek the customer’s prior written consent before working for other customers on similar projects. Complying with these noncompete obligations may restrict our ability to expand certain service offerings, and failure to comply could significantly harm our business and reputation, as well as expose us to liability for breach of contract.
18
We use a limited number of suppliers, including a related party, Labpartner (Shanghai) Co., Ltd., for several of our service offerings, which, if interrupted, could disrupt or delay our services, reduce our sales and force us to use more expensive supply sources.
We use a limited number of sources for our supply of certain reagents and other chemicals required in our product and service offerings. In particular, we purchased significant amounts of reagents and other chemicals from Labpartner (Shanghai) Co., Ltd., or Labpartner, a related party, in each of 2007, 2008 and 2009. Disruptions or delays with respect to our suppliers may arise from health problems, export or import restrictions or embargoes, foreign government or economic instability, severe weather conditions, disruptions to the air travel system, contract disputes or other disruptions. If the supply of certain materials were interrupted, our services may be delayed. We also may not be able to secure alternative supply sources in a timely and cost-effective manner. If we are unable to obtain adequate supplies of required materials that meet our standards or at acceptable costs, or at all, our ability to accept and fulfill customer orders in the required quality and quantity and at the required time could be restricted, which in turn may materially and adversely affect our business, financial condition, results of operations and prospects.
We have limited insurance coverage, and any claims beyond our insurance coverage may result in our incurring substantial costs and a diversion of resources.
We maintain property insurance policies covering physical damage or loss of our equipment and office furniture, employer’s liability insurance generally covering death or work injury of employees, public liability insurance covering third party bodily injury and property damage incurred in connection with our business in China, and directors and officers liability insurance. We do not have general liability or product liability insurance covering our whole business or all of our products and services. We currently maintain general liability insurance for four contracts covering, among other things, bodily injury and property damage arising out of the products or services provided under these contracts. The aggregate net revenues derived from these contracts in the six months ended June 30, 2010 accounted for 11.0% of our total net revenues in this period. The aggregate maximum coverage amount under the insurance policy for these contracts is $5 million for bodily injury and property damage arising out of our products or services and economic loss sustained by persons or entities due to deficiencies in our products or services. We do not maintain business disruption insurance or key man life insurance. Any business disruption or litigation, or any liability or damage to, or caused by, our facilities or our personnel beyond our insurance coverage may result in substantial costs and a diversion of resources.
Our quarterly revenues and operating results may be difficult to predict and could fall below investor expectations, which could cause the market price of our ADSs to decline.
Our quarterly revenues and operating results have fluctuated in the past and may continue to fluctuate significantly due to numerous factors, including:
| | • | | the commencement, postponement, completion or cancellation of large contracts; |
| | • | | the progress of ongoing contracts; |
| | • | | the delivery schedule of our customers; |
| | • | | budget cycles of our customers; |
| | • | | changes in the mix of our revenues from our services based on a fee-for-service or FTE basis or the percentage of our fee-for-service based contracts that are contingent upon successful completion; |
| | • | | changes in the industry operating environment; |
| | • | | changes in government policies or regulations or their enforcement; |
| | • | | exchange rate fluctuations; |
19
| | • | | a downturn in general economic conditions in the United States, China or internationally; and |
| | • | | the timing of and charges associated with completed acquisitions or other events. |
Many of these factors are beyond our control, making our quarterly results fluctuate and difficult to predict. For example, revenues may not be recognized during the third quarter of 2010 due to changes in delivery schedules initiated by customers or delays in customer acceptance of deliverables. As a result, our revenues in the third quarter of 2010 may not grow as quickly as they have in previous quarterly periods. Fluctuations in our quarterly revenues and operating results could cause the trading price of our ADSs to decline below investor expectations.
We may need additional capital that we may be unable to obtain in a timely manner or on acceptable terms, or at all.
We may require additional capital in order to grow, remain competitive, develop new services and expand our capacity. Our ability to obtain capital is subject to a variety of uncertainties, including:
| | • | | our financial condition, results of operations and cash flows; |
| | • | | general market conditions for capital raising activities by healthcare and related companies; and |
| | • | | economic, political and other conditions in China, the United States and elsewhere. |
However, financing may not be available in amounts or on terms acceptable to us, if at all.
Our capital needs may require us to sell additional equity or debt securities that may dilute our shareholders or introduce covenants that may restrict our operations or our ability to pay dividends.
Our capital needs and other business reasons could require us to sell additional equity or debt securities or obtain additional credit facilities. The sale of additional equity or equity-linked securities could dilute our shareholders. The incurrence of indebtedness would increase our debt service obligations and could result in operating and financing covenants that would restrict our operations or our ability to pay dividends.
Acquisitions or investments could disrupt our ongoing business, distract our management and employees, increase our expenses and adversely affect our business.
We anticipate that a portion of any future growth of our business might be accomplished by acquiring businesses, products or technologies. The success of any acquisition will depend upon, among other things, our ability to integrate acquired personnel, operations, products and technologies into our organization effectively, to retain and motivate key personnel of the acquired businesses and to retain their clients. In addition, we might not be able to identify suitable acquisition opportunities or obtain any necessary financing on acceptable terms. We might also spend time and money investigating and negotiating with potential acquisition or investment targets, but not complete a transaction. Any acquisition could involve other risks, including the assumption of additional liabilities and expenses, issuances of potentially dilutive securities or interest-bearing debt, transaction costs, reduction in our ADS price as a result of any of these or because of market reaction to a transaction and diversion of management’s attention from other concerns.
Recent healthcare reform creates uncertainty in the need for our services.
In March 2010, the United States Congress passed the Patient Protection and Affordable Care Act. Implementation of this newly enacted act, which is intended to control health care costs, may limit profits from the development of new drugs. Similar reform movements have occurred or may occur in parts of Europe and Asia. It is unclear how these reforms may affect R&D expenditures by pharmaceutical and biotechnology
20
companies, which creates uncertainty in the business opportunities available to us in the United States and other countries.
Negative attention from special interest groups may impair our ability to operate our business efficiently.
Some of the services we provide, or intend to provide, involve the use of large and small animals, as well as non-human primates. Certain special interest groups categorically object to the use of animals for research purposes. Historically, our core research model activities with small animals have not been the subject of animal rights media attention, and as we expand our service biology offerings into non-GLP toxicology, we anticipate that we will work extensively with large animals and non-human primates. However, research activities with animals have been the subject of adverse attention, negatively impacting our industry. Any negative attention or threats directed against our animal research activities could impair our ability to operate our business efficiently. In addition, if regulatory authorities were to mandate a significant reduction in safety testing procedures that utilize laboratory animals, as has been advocated by certain groups, our business could be materially and adversely affected.
Contamination in our animal populations can distort or compromise the quality of our research results, cause human infection, increase costs and decrease revenue.
Our research models must be free of certain infectious agents such as certain viruses and bacteria. The presence of these infectious agents in our animal facilities could distort or compromise the quality of our research results and could adversely impact human or animal health. Removing or preventing contamination typically requires cleaning, renovating, disinfecting, retesting and restarting our animal facilities, which result in clean-up and start-up costs and reduced revenue due to lost customer confidence in the accuracy of our research results. In some cases, we may import animals carrying infectious agents capable of causing diseases in humans. As a result, there could be a possible risk of human exposure and infection. Although we have not had serious contamination in the past, contamination may occur in the future, which could materially and adversely impact our financial results.
For our customers’ future drugs to be marketed in the United States, we may need to obtain clearance from the FDA and our operations will need to comply with FDA standards. Any adverse action by the FDA against us would negatively impact our ability to offer our services and harm our business and prospects.
As we expand our service offerings, we may need to obtain clearance by the U.S. Food and Drug Administration, or FDA, in the event that our customers’ clinical trials reach the stage of filing a New Drug Application, or NDA, with the FDA, to grant permission to market the drug in the United States. All facilities and manufacturing techniques used to manufacture drugs and biologics in the United States must conform to standards established by the FDA. The FDA may conduct scheduled periodic inspections of our facilities to monitor our compliance with regulatory standards. If the FDA finds that we have failed to comply with the appropriate regulatory standards, it may impose fines or take other actions against us or our customers, or we may no longer be able to offer our services to U.S. customers. The resulting corrective measures may be lengthy and costly. As a result, we may be unable to fulfill our contractual obligations. Any adverse action by the FDA would materially and adversely impact our reputation and our business, financial condition, results of operations and prospects. We may or may not obtain clearance from FDA standards in the event that we are inspected, or maintain such clearance over time.
New technologies or methodologies may be developed, validated and increasingly used in the global pharmaceutical and biotechnology R&D outsourcing industry that could reduce demand for our services.
The global pharmaceutical and biotechnology R&D outsourcing industry is evolving, and we must keep pace with new technologies and methodologies in the industry to maintain our competitive position. As a result, we must invest significant human and capital resources in R&D to enhance our technology and our existing
21
services and introduce new services utilizing advanced technologies. However, we may not be successful in adapting to or commercializing these new technologies if developed. New technologies could decrease the need for our existing technologies, and we may not be able to develop new service or technologies effectively or in a timely manner. Our failure to develop, enhance or adapt, to new technologies and methodologies could significantly reduce demand for our services and harm our business and prospects.
Our principal facilities may be vulnerable to natural disasters or other unforeseen catastrophic events.
We conduct our operations primarily at our headquarters in Shanghai Zhangjiang Hi-Tech Park and our R&D center in Chengdu. We also have animal facilities in Shanghai Zhangjiang Hi-Tech Park and a manufacturing facility in Nanhui, Shanghai. In addition, we are constructing a cGMP-quality multiple-purpose manufacturing facility and a laboratory services building in Fengxian, Shanghai. We depend on our facilities for our business. Natural disasters or other unanticipated catastrophic events, including power interruptions, water shortage, storms, fires, earthquakes, terrorist attacks and wars could significantly impair our ability to operate our business in its ordinary course. Our facilities and certain equipment located in these facilities would be difficult to replace in any such event and could require substantial replacement time. The occurrence of any such event may materially and adversely affect our business, financial condition, results of operations and prospects.
We face risks related to health epidemics and other outbreaks, which could significantly disrupt our staffing and may even result in temporary closure of our facilities.
Our business could be materially and adversely affected by the outbreak of avian influenza, severe acute respiratory syndrome, or SARS, or another epidemic. In April 2009, a new strain of influenza A virus subtype H1N1 was discovered in North America and quickly spread to other parts of the world, including China. In early June 2009, the World Health Organization declared the outbreak to be a pandemic, while noting that most of the illnesses were of moderate severity. The PRC Ministry of Health has reported a few hundred deaths caused by the influenza A (H1N1). Any outbreak of avian influenza, SARS, the influenza A (H1N1) or other adverse public health developments in China may materially and adversely affect our business operations. These occurrences could severely disrupt to our daily operations.
Risks Related to Doing Business in China
Fluctuations in exchange rates may materially and adversely affect your investment.
The value of the Renminbi against the U.S. dollar and other currencies is affected by, among other things, changes in China’s political and economic conditions and China’s foreign exchange policies. The conversion of the Renminbi into foreign currencies, including the U.S. dollar, has been based on exchange rates set by the People’s Bank of China. On July 21, 2005, the PRC government changed its decade-old policy of pegging the value of the Renminbi solely to the U.S. dollar. Under this revised policy, the Renminbi is permitted to fluctuate within a narrow and managed band against a basket of certain foreign currencies. Following the removal of the U.S. dollar peg, the Renminbi appreciated approximately 21.5% against the U.S. dollar over the following three years. Since July 2008, however, the Renminbi has traded within a narrow range against the U.S. dollar. As a consequence, the Renminbi has fluctuated significantly since July 2008 against other freely traded currencies, in tandem with the U.S. dollar. On June 20, 2010, the People’s Bank of China announced that the PRC government would further reform the Renminbi exchange rate regime and increase the flexibility of the exchange rate. It is difficult to predict how this new policy may impact the Renminbi exchange rate.
The reporting and functional currency of ShangPharma Corporation is the U.S. dollar. Our offshore subsidiaries’ functional currency is the U.S. dollar. However, the functional currency of our PRC subsidiaries is the Renminbi. A significant majority of our revenues are denominated in U.S. dollars and most of our costs and expenses are denominated in Renminbi. The net proceeds from this offering will be denominated in U.S. dollars.
22
Fluctuations in exchange rates, primarily those involving the U.S. dollar, may affect the relative purchasing power of these proceeds. In addition, appreciation or depreciation in the value of the Renminbi relative to the U.S. dollar would affect our financial results reported in U.S. dollar terms without giving effect to any underlying change in our business or results of operations. Fluctuations in the exchange rate will also affect the relative value of earnings from and the value of any U.S. dollar-denominated investments we make.
Limited hedging options are available in China to reduce our exposure to exchange rate fluctuations. We began hedging our U.S. dollar exposure using forward contracts from the second half of 2008 based in part on our expected monthly revenues. We may continue to enter similar or other types of hedging transactions in the future. However, the availability and effectiveness of these hedges may be limited and we may not be able to adequately hedge our exposure or at all. In addition, our currency exchange losses may be magnified by PRC exchange control regulations that restrict our ability to convert between the Renminbi and foreign currency. As a result, fluctuations in exchange rates may materially and adversely affect your investment.
Adverse changes in the political and economic policies of the PRC government could materially and adversely affect the overall economic growth of China, which could adversely affect our business.
Substantially all of our assets are located in China and all of our revenues are derived from our operations there. Accordingly, economic, political and legal developments in China significantly affect our business, financial condition, results of operations and prospects. The Chinese economy differs from the economies of most developed countries in many respects, including the amount of government involvement, level of development, growth rate, control of foreign exchange and allocation of resources. While the Chinese economy has grown significantly in the past 30 years, the growth has been uneven across different periods, regions and economic sectors of China. We cannot assure you that the Chinese economy will continue to grow, or that any such growth will be steady and uniform, or that if there is a slowdown, such a slowdown will not have a negative effect on our business.
The PRC government also exercises significant control over China’s economic growth through the allocation of resources, controlling payment of foreign currency-denominated obligations, setting monetary policy and providing preferential treatment to particular industries or companies. From late 2003 to mid-2008, the PRC government implemented a number of measures, such as increasing the People’s Bank of China’s statutory deposit reserve ratio and imposing commercial bank lending guidelines that had the effect of slowing the growth of credit, which in turn may have slowed the growth of the Chinese economy. In response to the recent global and Chinese economic downturn, the PRC government has promulgated several measures aimed at expanding credit and stimulating economic growth. Since August 2008, the People’s Bank of China has decreased the statutory deposit reserve ratio and lowered benchmark interest rates several times. It is unclear whether the PRC government will continue such policies, or whether PRC economic policies will be effective in creating stable economic growth. Any further slowdown in the economic growth of China could reduce demand for our solutions and services, which could materially and adversely affect our business, our financial condition and results of operations.
Uncertainties with respect to the PRC legal system could adversely affect us.
We conduct our business primarily through our PRC subsidiaries. Our operations in China are governed by PRC laws and regulations. Our PRC subsidiaries are foreign-invested enterprises and are subject to laws and regulations applicable to foreign investment in China and, in particular, laws applicable to foreign-invested enterprises. The PRC legal system is largely a civil law system based on written statutes. Unlike the common law system, prior court decisions may be cited for reference but have limited precedential value.
In 1979, the PRC government began to promulgate a comprehensive system of laws and regulations governing general economic matters. The overall effect of legislation over the past two decades has significantly enhanced the protections afforded to various foreign investments in China. However, China has not developed a
23
fully integrated legal system, and recently enacted laws and regulations may not sufficiently cover all aspects of economic activities in China. Because these laws and regulations are relatively new, and published court decisions are limited and nonbinding in nature, the interpretation and enforcement of these laws and regulations involve uncertainties. In addition, the PRC legal system is based in part on government policies and internal rules, some of which are not published on a timely basis or at all, which may have a retroactive effect. As a result, we may not be aware of our violation of these policies and rules until after the violation occurs.
Any administrative and court proceedings in China may be protracted, resulting in substantial costs and diversion of resources and management attention. However, since PRC administrative and court authorities have significant discretion in interpreting and implementing statutory and contractual terms, it may be more difficult to evaluate the outcome of administrative and court proceedings and the level of legal protection we enjoy than in more developed legal systems. These uncertainties may also impede our ability to enforce the contracts entered into by our PRC subsidiaries. As a result, these uncertainties could materially and adversely affect our business and results of operations.
SAFE regulations may limit our ability to finance our PRC subsidiaries effectively and affect the value of your investment and may make it more difficult for us to pursue growth through acquisition.
If we finance our PRC subsidiaries through additional capital contributions, the Ministry of Commerce in China or its local counterpart must approve the amount of these capital contributions. On August 29, 2008, the State Administration of Foreign Exchange, or SAFE, promulgated Circular 142, a notice regulating the conversion by a foreign-invested company of foreign currency into Renminbi by restricting how the converted Renminbi may be used. The notice requires that Renminbi converted from the foreign currency-denominated capital of a foreign-invested company may only be used for purposes within the business scope approved by the applicable governmental authority and may not be used for equity investments in the PRC unless otherwise provided by laws and regulations. In addition, SAFE strengthened its oversight of the flow and use of Renminbi funds converted from the foreign currency denominated capital of a foreign-invested company. The use of such Renminbi may not be changed without approval from SAFE, and may not be used to repay Renminbi loans if the proceeds of such loans have not yet been used for purposes within the company’s approved business scope. Violations of Circular 142 may result in severe penalties, including substantial fines as set forth in the Foreign Exchange Administration Regulations. We cannot assure you that we will be able to complete the necessary government registrations or obtain the necessary government approvals on a timely basis, if at all, with respect to future loans by us to our PRC subsidiaries or with respect to future capital contributions by us to our PRC subsidiaries. If we fail to complete such registrations or obtain such approvals, our ability to contribute additional capital to fund our PRC operations may be negatively affected, which could adversely and materially affect our liquidity and our ability to fund and expand our business.
PRC regulations relating to the establishment of offshore special purpose companies by PRC residents may subject our PRC resident beneficial owners or our PRC subsidiaries to liability or penalties, limit our ability to inject capital into our PRC subsidiaries, limit our PRC subsidiaries’ ability to increase their registered capital or distribute profits to us, or may otherwise adversely affect us.
On October 21, 2005, SAFE issued a public circular, or Circular 75, which became effective on November 1, 2005. Circular 75 requires PRC residents to register with the local SAFE branch before establishing or controlling any company, referred to in the circular as an “offshore special purpose vehicle,” outside of the PRC for capital financing and to register again after completing an investment in or acquisition of any operating subsidiaries in the PRC, which is commonly referred to as a round-trip investment. Also, any change of shareholding or any other material capital alteration in such offshore special purpose vehicle shall be filed with the local SAFE branch within 30 days after the shareholding change or capital alteration.
In 2003, our founders, who may be deemed PRC residents for the purpose of Circular 75, established our intermediary holding companies in Hong Kong, which subsequently acquired or established their directly-owned subsidiaries in China. Circular 75 retroactively applies to our initial formation of the corporate structure
24
as a round-trip investment and required our founders to register their respective shareholdings in our company with the local SAFE branch in Shanghai by March 31, 2006. Our founders inadvertently missed this filing deadline. We have urged our founders to, and they have been making efforts in order to, apply for a remedial SAFE registration. Although such a remedial registration is permissible under supplementary rules of Circular 75, our founders’ attempted registration has not been accepted by the local SAFE branch in Shanghai due to the lack of specific administrative procedures for remedial registrations. We will continue to urge our founders to, and our founders will continue making efforts to, apply for a remedial SAFE registration. However, we cannot assure you that our founders will be able to make the remedial registration. If for any reason the relevant government authorities in China determine that our founders are not in compliance with Circular 75, our founders may be subject to administrative measures and penalties, such as orders to rectify and monetary fines, and any proposed foreign exchange transaction between our Hong Kong intermediary holding companies and their directly owned subsidiaries in China may be restricted, which may materially and adversely affect our business operations and liquidity in China.
Failure to comply with PRC regulations regarding the registration requirements for employee stock ownership plans or share option plans may subject the PRC plan participants or us to fines and other legal or administrative sanctions.
Under applicable PRC regulations, all foreign exchange matters relating to employee stock ownership plans, share option plans or similar plans in which Chinese citizens participate require approval from SAFE or its authorized local branch. In addition, Chinese citizens who are granted share options, shares or other equity interests such as RSUs by an offshore listed company are required, through a Chinese agent or Chinese subsidiary of the offshore listed company, to register with SAFE and complete certain other procedures. We will become an offshore listed company upon the completion of this offering and as a result we and our Chinese employees who have been granted share options, RSUs or shares will be subject to, and intend to comply with, these regulations. If we or our Chinese employees fail to comply with these regulations after we become an offshore listed company, we or our Chinese employees may face sanctions imposed by SAFE or other PRC government authorities, including restrictions on foreign currency conversions and additional capital contributions to our PRC subsidiaries.
We rely principally on dividends and other distributions on equity paid by our subsidiaries to fund any cash and financing requirements we may have, and any limitation on the ability of our subsidiaries entities to make payments to us could materially and adversely affect our ability to conduct our business.
We are a holding company and we rely principally on dividends from our subsidiaries in Hong Kong for our cash requirements, including any debt we may incur. We currently receive substantially all of our revenues in U.S. dollars. In future periods, however, we expect to receive an increasing portion of our revenues in RMB. Current PRC regulations permit our subsidiaries to pay dividends to us only out of their accumulated profits, if any, determined in accordance with PRC accounting standards and regulations. In addition, each of our subsidiaries in China is required to set aside a certain amount of its after-tax profits each year, if any, to fund certain statutory reserves. These reserves are not distributable as cash dividends. As of December 31, 2009, our aggregate net assets in the amount of $5.4 million were not distributable in the form of dividends to us due to these PRC regulations. Furthermore, if our subsidiaries in China incur debt, the instruments governing the debt may restrict their ability to pay dividends or make other payments to us. In addition, the PRC tax authorities may require us to adjust our taxable income under the contractual arrangements we have in place in a manner that would materially and adversely affect our subsidiaries’ ability to pay dividends and other distributions to us. Any limitation on the ability of our subsidiaries to distribute dividends or other payments to us could materially and adversely limit our ability to grow, make investments or acquisitions that could be beneficial to our businesses, pay dividends or otherwise fund and conduct our business.
25
The discontinuation, reduction or delay of any of the preferential tax treatment or other government incentives available to us in the PRC or imposition of any additional PRC taxes on us could adversely affect our financial condition and results of operations.
China has passed a new PRC Enterprise Income Tax Law and its implementing rules, both of which became effective on January 1, 2008. The PRC Enterprise Income Tax Law significantly curtails tax incentives granted to foreign-invested enterprises under the Foreign-Invested Enterprise Income Tax Law in effect before January 1, 2008. The PRC Enterprise Income Tax Law, however, (i) reduces the statutory rate of the enterprise income tax from 33% to 25%, (ii) permits companies established before March 16, 2007 to continue to enjoy their existing tax incentives, adjusted by certain transitional phase-out rules promulgated by the State Council on December 26, 2007, and (iii) introduces new tax incentives, subject to various qualification criteria.
The PRC Enterprise Income Tax Law and its implementing rules permit certain “high and new technology enterprises” which hold independent ownership of core intellectual property and simultaneously meet a list of other criteria, to enjoy a reduced 15% enterprise income tax rate subject to certain new qualification criteria. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Income Taxes.”
In addition, local governments have used different incentives to encourage or accelerate the development of pharmaceutical and biotechnology R&D outsourcing industries. We have historically received sales tax exemptions for certain laboratory services upon approval from local authorities. We also benefited from government incentives in the form of cash subsidies in 2008 and 2009.
Preferential tax treatment and other government incentives granted to our subsidiaries by local governmental authorities are subject to review and may be adjusted or revoked at any time. The discontinuation of any preferential tax treatment or other government incentives available to us and our wholly-owned PRC subsidiaries will cause our effective tax rate to increase or our other income to decrease, which could materially and adversely affect our financial condition and results of operations.
Our global income and the dividends that we may receive from our PRC subsidiaries may be subject to PRC taxes under the PRC Enterprise Income Tax Law, which would materially and adversely affect our results of operations.
Under the PRC Enterprise Income Tax Law and its implementing rules, both effective from January 1, 2008, an enterprise established outside of the PRC with “de facto management bodies” within the PRC is considered a resident enterprise and will be subject to the enterprise income tax at the rate of 25% on its global income. The implementation rules define the term “de facto management bodies” as “establishments that carry out substantial and overall management and control over the manufacturing and business operations, personnel, accounting, properties, etc. of an enterprise.” The State Tax Administration issued the Notice Regarding the Determination of Chinese-Controlled Offshore Incorporated Enterprises as PRC Tax Resident Enterprises on the Basis of De Facto Management Bodies, or SAT Circular 82, on April 22, 2009. SAT Circular 82 provides certain criteria for determining whether the “de facto management body” of an offshore-incorporated enterprise controlled by PRC enterprises is located in China.
Although SAT Circular 82 only applies to offshore enterprises controlled by PRC enterprises and not those controlled by PRC or foreign individuals or foreign enterprises, the criteria set forth therein may reflect State Tax Administration’s general position on how the “de facto management body” test should be applied in determining the tax resident status of offshore enterprises, regardless of whether they are controlled by PRC or foreign enterprises or individuals. Accordingly, we may be considered a resident enterprise and may therefore be subject to the enterprise income tax at 25% on our global income other than dividends from our PRC subsidiaries, which could significantly increase our tax burden and materially and adversely affect our cash flow and profitability.
Under the applicable PRC tax laws in effect before January 1, 2008, dividend payments to foreign investors made by foreign-invested enterprises in China, such as our PRC subsidiaries, were exempt from PRC
26
withholding tax. However, our PRC counsel, Fangda Partners, has advised us that pursuant to the PRC Enterprise Income Tax Law, dividends generated after January 1, 2008 and payable by a foreign-invested enterprise in China to its foreign investors that are not PRC resident enterprises will be subject to a 10% withholding tax, unless any such foreign investor’s jurisdiction of incorporation has a tax treaty with China that provides for a different withholding arrangement. We are a Cayman Islands holding company and substantially all of our income may come from dividends we receive from our subsidiaries located in Hong Kong, which are the direct holding companies of our PRC subsidiaries. If our Hong Kong subsidiaries are considered non-PRC resident enterprises, dividends that they receive from our PRC subsidiaries may be subject to withholding tax at a preferential rate of no more than 5% under the Arrangement between the PRC and the Hong Kong Special Administrative Region on the Avoidance of Double Taxation and Prevention of Fiscal Evasion, effective on January 1, 2007, upon receiving approval from the local tax authority. However, if our Hong Kong subsidiaries are not considered to be the beneficial owners of our PRC subsidiaries under a tax notice promulgated on October 27, 2009, such dividends would be subject to withholding tax at a rate of 10%. See “Regulation—Regulations on Tax—Dividend Withholding Tax.”
According to our PRC counsel, Fangda Partners, because there remains uncertainty regarding the interpretation and implementation of the PRC Enterprise Income Tax Law and its implementation rules, it is uncertain whether, if we are regarded as a PRC resident enterprise, any dividends to be distributed by us to our non-PRC shareholders and ADS holders would be subject to any PRC withholding tax. If we are required under the PRC Enterprise Income Tax Law to withhold PRC income tax on our dividends payable to our non-PRC enterprise shareholders and ADS holders, your investment in our ordinary shares or ADSs may be materially and adversely affected.
We face uncertainty with respect to indirect transfers of equity interests in PRC resident enterprises by their non-PRC holding companies.
Pursuant to the Notice on Strengthening Administration of Enterprise Income Tax for Share Transfers by Non-PRC Resident Enterprises, or SAT Circular 698, issued by the State Administration of Taxation on December 10, 2009 with retroactive effect from January 1, 2008, where a non-resident enterprise transfers the equity interests of a PRC resident enterprise indirectly via disposing of the equity interests of an overseas holding company, or an Indirect Transfer, and such overseas holding company is located in a tax jurisdiction that: (i) has an effective tax rate less than 12.5% or (ii) does not tax foreign income of its residents, the foreign investor shall report to the competent tax authority of the PRC resident enterprise this Indirect Transfer. Using a “substance over form” principle, the PRC tax authority may disregard the existence of the overseas holding company if it lacks a reasonable commercial purpose and was established for the purpose of avoiding PRC tax. As a result, gains derived from such Indirect Transfer may be subject to PRC withholding tax at a rate of up to 10%. SAT Circular 698 also provides that, where a non-PRC resident enterprise transfers its equity interests in a PRC resident enterprise to its related parties at a price lower than the fair market value, the relevant tax authority has the power to make a reasonable adjustment to the taxable income of the transaction.
There is uncertainty as to the application of SAT Circular 698. For example, while the term “Indirect Transfer” is not clearly defined, it is understood that the relevant PRC tax authorities have jurisdiction regarding requests for information over a wide range of foreign entities having no direct contact with China. Moreover, the relevant authority has not yet promulgated any formal provisions or formally declared or stated how to calculate the effective tax rates in foreign tax jurisdictions, and the process and format of the reporting of an Indirect Transfer to the competent tax authority of the relevant PRC resident enterprise. In addition, there are not any formal declarations with regard to how to determine whether a foreign investor has adopted an abusive arrangement in order to avoid PRC tax. As a result, we may become at risk of being taxed under SAT Circular 698 and we may be required to expend valuable resources to comply with SAT Circular 698 or to establish that we should not be taxed under SAT Circular 698, which may have a material adverse effect on our financial condition and results of operations.
27
The approval of the China Securities Regulatory Commission may be required in connection with this offering under a regulation adopted in August 2006, and, if required, we cannot predict whether we will be able to obtain such approval.
In 2006, six PRC regulatory agencies jointly adopted the Regulations on Mergers and Acquisitions of Domestic Enterprises by Foreign Investors, or the M&A Rule. This rule requires that, if an overseas company established or controlled by PRC domestic companies or citizens intends to acquire equity interests or assets of any other PRC domestic company affiliated with the PRC domestic companies or citizens, such acquisition must be submitted to the Ministry of Commerce, rather than local regulators, for approval. In addition, this regulation requires that an overseas company controlled directly or indirectly by PRC companies or citizens and holding equity interests of PRC domestic companies must obtain the approval of the China Securities Regulatory Commission, or CSRC, prior to listing its securities on an overseas stock exchange. On September 21, 2006, the CSRC published a notice on its official website specifying the documents and materials required to be submitted by overseas special purpose companies seeking CSRC’s approval of their overseas listings.
While the application of the M&A Rule remains unclear, based on their understanding of current PRC laws, regulations, and the notice published on September 21, 2006, our PRC counsel, Fangda Partners, has advised us that, we are not required to submit an application to the Ministry of Commerce or the CSRC for its approval for the listing and trading of our ADSs on the New York Stock Exchange because our PRC subsidiaries were either converted into wholly foreign owned enterprises prior to September 8, 2006, the effective date of the M&A rule, or incorporated as wholly foreign owned enterprises.
Fangda has further advised us that their opinions summarized above are subject to the timing and content of any new laws, rules and regulations or clearer implementation and interpretations from the Ministry of Commerce and CSRC in any form relating to the M&A Rule.
If the CSRC or another PRC regulatory agency subsequently determines that CSRC approval was required for this offering, we may need to apply for a remedial approval from the CSRC and may be subject to certain administrative punishments or other sanctions from PRC regulatory agencies. The regulatory agencies may impose fines and penalties on our operations in the PRC, limit our operating privileges in the PRC, delay or restrict the repatriation of our foreign currency in our offshore bank accounts into the PRC, or take other actions that could materially and adversely affect our business, financial condition, results of operations, reputation and prospects, as well as the trading price of our ADSs.
Also, if the CSRC later requires that we obtain its approval, we may be unable to obtain a waiver of the CSRC approval requirements, if and when procedures are established to obtain such a waiver. Any uncertainties or negative publicity regarding these CSRC approval requirements could materially and adversely affect the trading price of our ADSs.
The M&A Rule sets forth complex procedures for acquisitions conducted by foreign investors that could make it more difficult to pursue acquisitions.
The M&A Rule sets forth complex procedures and requirements that could make merger and acquisition activities by foreign investors more time-consuming and complex, including requirements in some instances that the Ministry of Commerce be notified in advance of any change-of-control transaction in which a foreign investor takes control of a PRC domestic enterprise. Complying with the requirements of the M&A Rule to complete such transactions could be time-consuming, and any required approval processes, including obtaining approval from the Ministry of Commerce, may delay or inhibit our ability to complete such transactions, which could affect our ability to expand our business or maintain our market share.
28
The failure of the owners of our leased facilities to comply with PRC property related laws and other matters may materially and adversely affect our ability to use our R&D facilities.
We perform various R&D services in leased facilities in Shanghai and Chengdu. The owners of our leased facilities have not met a number of land- and property-related legal requirements. For example, owners of some of our leased facilities have not been able to provide us with relevant building ownership certificates. Certain properties leased by us are used for R&D purposes while they are under zoning restrictions to be used for industrial or other purposes. In addition, certain leased facilities have been mortgaged by their respective owners. As a result, third parties or government authorities may interfere with the use of our leased properties, we may be subject to fines by the government, our leases may be invalidated and our rights under these leases may be adversely affected. We may be forced to relocate any affected facilities. All of these consequences could materially and adversely affect our business, financial condition, results of operations and prospects.
Risks Related to this Offering
There has been no public market for our shares or ADSs prior to this offering, and you may not be able to resell our ADSs at or above the price you paid, or at all.
Prior to this offering, there has been no public market for our shares or ADSs. Our ADSs have been approved for listing on the New York Stock Exchange. Our ordinary shares will not be listed on any exchange or quoted for trading on any over-the-counter trading system. If an active trading market for our ADSs does not develop after this offering, the market price and liquidity of our ADSs will be materially and adversely affected.
Negotiations with the underwriters will determine the initial public offering price for our ADSs which may bear no relationship to their market price after this offering. We cannot assure you that an active trading market for our ADSs will develop or that the market price of our ADSs will remain at or above the initial public offering price.
The market price for our ADSs may be volatile.
The market price for our ADSs is likely to be highly volatile and subject to wide fluctuations in response to factors including the following:
| | • | | regulatory developments in our target markets affecting us, our customers or our competitors; |
| | • | | announcements of studies and reports relating to the quality of our solutions and services or those of our competitors; |
| | • | | changes in the economic performance or market valuations of other companies that provide pharmaceutical and biotechnology R&D outsourcing services; |
| | • | | actual or anticipated fluctuations in our quarterly results of operations and changes or revisions of our expected results; |
| | • | | changes in financial estimates by securities research analysts; |
| | • | | conditions in the pharmaceutical and biotechnology R&D outsourcing services industry; |
| | • | | announcements by us or our competitors of new services, acquisitions, strategic relationships, joint ventures or capital commitments; |
| | • | | additions to or departures of our senior management; |
| | • | | fluctuations of exchange rates between the Renminbi and the U.S. dollar; |
| | • | | release or expiration of lock-up or other transfer restrictions on our outstanding ordinary shares or ADSs; and |
| | • | | sales or perceived potential sales of additional ordinary shares or ADSs. |
29
In addition, the securities market has experienced significant price and volume fluctuations unrelated to the operating performance of any particular companies. These market fluctuations may also materially and adversely affect the market price of our ADSs.
Because our initial public offering price is substantially higher than our net tangible book value per share, you will experience immediate and substantial dilution.
If you purchase ADSs in this offering, you will pay more for your ADSs than the amount paid by our existing shareholders for their ordinary shares on a per ADS basis. As a result, you will experience immediate and substantial dilution of approximately $9.52 per ADS, representing the difference between the initial public offering price of $15.00 per ADS and our net tangible book value per ADS as of June 30, 2010, after giving effect to the automatic conversion of our preferred shares, immediately upon the completion of this offering and net proceeds to us from this offering. In addition, you may experience further dilution to the extent that our ordinary shares are issued upon the exercise of share options.
Because we do not expect to pay dividends in the foreseeable future after this offering, you must rely on price appreciation of our ADSs for return on your investment.
We intend to retain most, if not all, of our available funds and earnings after this offering to fund the development and growth of our business. As a result, we do not expect to pay any cash dividends in the foreseeable future. Therefore, you should not rely on an investment in our ADSs as a source for any future dividend income.
Our board of directors has significant discretion as to whether to distribute dividends. Even if our board of directors decides to declare and pay dividends, the timing, amount and form of future dividends, if any, will depend on, among other things, our future results of operations and cash flow, our capital requirements and surplus, the amount of distributions, if any, received by us from our subsidiaries, our financial condition, contractual restrictions and other factors deemed relevant by our board of directors. Accordingly, the return on your investment in our ADSs will likely depend entirely upon any future price appreciation of our ADSs. There is no guarantee that our ADSs will appreciate in value after this offering or even maintain the price at which you purchased the ADSs. You may not realize a return on your investment in our ADSs and you may even lose your entire investment in our ADSs.
Substantial future sales or perceived potential sales of our ADSs in the public market could cause the price of our ADSs to decline.
Sales of our ADSs or ordinary shares in the public market after this offering, or the perception that these sales could occur, could cause the market price of our ADSs to decline. Upon completion of this offering, we will have 335,600,000 ordinary shares outstanding including 104,400,000 ordinary shares represented by ADSs. All ADSs sold in this offering will be freely transferable without restriction or additional registration under the United States Securities Act of 1933, as amended, or the Securities Act, unless held by our “affiliates” as that term is defined in Rule 144 under the Securities Act. The remaining ordinary shares outstanding after this offering will be available for sale, upon the expiration of the 180-day lock-up period beginning from the date of this prospectus, subject to volume and other restrictions as applicable under Rules 144 and 701 under the Securities Act. Any or all of these shares may be released prior to the expiration of the lock-up period at the discretion of the representatives. To the extent shares are released before the expiration of the lock-up period and sold into the market, the market price of our ADSs could decline.
Upon completion of this offering, certain holders of our ordinary shares will have the right to cause us to register under the Securities Act the sale of their shares, subject to the 180-day lock-up period in connection with
30
this offering. Registration of these shares under the Securities Act would result in ADSs representing these shares becoming freely tradable without restriction under the Securities Act immediately upon the effectiveness of the registration. Sales of these registered shares in the form of ADSs, in the public market could cause the price of our ADSs to decline.
You may not have the same voting rights as the holders of our ordinary shares and may not receive voting materials in time to be able to exercise your right to vote.
Except as described in this prospectus and in the deposit agreement, holders of our ADSs will not be able to exercise voting rights attaching to the shares represented by our ADSs on an individual basis. Holders of our ADSs will appoint the depositary or its nominee as their representative to exercise the voting rights attaching to the shares represented by the ADSs. You may not receive voting materials in time to instruct the depositary to vote, and it is possible that you, or persons who hold their ADSs through brokers, dealers or other third parties, will not have the opportunity to exercise a right to vote.
Your right to participate in any rights offerings may be limited, which may cause dilution to your holdings, and you may not receive cash dividends if it is impractical to make them available to you.
We may from time to time distribute rights to our shareholders, including rights to acquire our securities. However, we cannot make rights available to you in the United States unless we register both the rights and the securities to which the rights relate under the Securities Act or an exemption from the registration requirements is available. Under the deposit agreement, the depositary will not make rights available to you unless both the rights and the underlying securities to be distributed to ADS holders are either registered under the Securities Act or exempt from registration under the Securities Act. We are under no obligation to file a registration statement with respect to any such rights or securities or to endeavor to cause such a registration statement to be declared effective and we may not be able to establish a necessary exemption from registration under the Securities Act. Accordingly, you may be unable to participate in our rights offerings and may experience dilution in your holdings.
The depositary of our ADSs has agreed to pay to you the cash dividends or other distributions it or the custodian receives on our ordinary shares or other deposited securities after deducting its fees and expenses. You will receive these distributions in proportion to the number of ordinary shares your ADSs represent. However, the depositary may, at its discretion, decide that it is inequitable or impractical to make a distribution available to any holders of ADSs. For example, the depositary may determine that it is not practicable to distribute certain property through the mail, or that the value of certain distributions may be less than the cost of mailing them. In these cases, the depositary may decide not to distribute such property to you.
You may be subject to limitations on transfer of your ADSs.
Your ADSs are transferable on the books of the depositary. However, the depositary may close its transfer books at any time or from time to time when it deems expedient in connection with the performance of its duties. In addition, the depositary may refuse to deliver, transfer or register transfers of ADSs generally when our books or the books of the depositary are closed, or at any time if we or the depositary deems it advisable to do so because of any requirement of law or of any government or governmental body, or under any provision of the deposit agreement, or for any other reason.
You may face difficulties in protecting your interests, and your ability to protect your rights through the U.S. federal courts may be limited because we are incorporated under Cayman Islands law, we conduct substantially all of our operations in China and all of our directors and officers reside outside the United States.
We are incorporated in the Cayman Islands and conduct substantially all of our operations in China through our PRC subsidiaries. All of our directors and officers reside outside the United States and a substantial
31
portion of their assets are located outside of the United States. As a result, it may be difficult or impossible for you to bring an action against us or against these individuals in the Cayman Islands or in China in the event that you believe that your rights have been infringed under the securities laws or otherwise. Even if you are successful in bringing an action of this kind, the laws of the Cayman Islands and of China may render you unable to enforce a judgment against our assets or the assets of our directors and officers.
There is no statutory recognition in the Cayman Islands of judgments obtained in the United States, although the courts of the Cayman Islands will recognize as a valid judgment, a final and conclusive judgment in personam obtained in a federal or state court of the United States under which a sum of money is payable (other than a sum of money payable in respect of multiple damages, taxes or other charges of a like nature or in respect of a fine or other penalty) and would give a judgment based thereon; provided that (a) such courts had proper jurisdiction over the parties subject to such judgment; (b) such courts did not contravene the rules of natural justice of the Cayman Islands; (c) such judgment was not obtained by fraud; (d) the enforcement of the judgment would not be contrary to the public policy of the Cayman Islands; (e) no new admissible evidence relevant to the action is submitted prior to the rendering of the judgment by the courts of the Cayman Islands; and (f) there is due compliance with the correct procedures under the laws of the Cayman Islands. For more information regarding the relevant laws of the Cayman Islands and China, see “Enforceability of Civil Liabilities.”
Our corporate affairs are governed by our memorandum and articles of association, as amended and restated from time to time, and by the Companies Law (2010 Revision) and common law of the Cayman Islands. The rights of shareholders to take legal action against us and our directors, actions by minority shareholders and the fiduciary responsibilities of our directors are to a large extent governed by the common law of the Cayman Islands. The common law of the Cayman Islands is derived in part from comparatively limited judicial precedent in the Cayman Islands as well as from English common law, which provides persuasive, but not binding, authority on a court in the Cayman Islands. The rights of our shareholders and the fiduciary responsibilities of our directors under Cayman Islands law are not as clearly established as they would be under statutes or judicial precedents in the United States. In particular, the Cayman Islands has a less developed body of securities laws than the United States and provides significantly less protection. In addition, Cayman Islands companies may not have standing to initiate a shareholder derivative action in U.S. federal courts.
As a result, our public shareholders may have more difficulty in protecting their interests through actions against us, our management, our directors or our major shareholders than would shareholders of a corporation incorporated in a jurisdiction in the United States.
You must rely on the judgment of our management as to the use of the net proceeds from this offering, and such use may not produce income or increase our ADS price.
We have not allocated a significant portion of the net proceeds of this offering to any particular purpose. Rather, our management will have considerable discretion in the application of the net proceeds received by us. You will not have the opportunity, as part of your investment decision, to assess whether proceeds are being used appropriately. You must rely on the judgment of our management regarding the application of the net proceeds of this offering. The net proceeds may be used for corporate purposes that do not improve our efforts to maintain profitability or increase our ADS price. The net proceeds from this offering may be placed in investments that do not produce income or that lose value.
Our memorandum and articles of association will contain anti-takeover provisions that could adversely affect the rights of holders of our ordinary shares and ADSs.
We have adopted a second amended and restated memorandum and articles of association that will become effective immediately upon the completion of this offering. Our new memorandum and articles of association will contain certain provisions that could limit the ability of others to acquire control of our company, including a provision that grants authority to our board of directors to establish from time to time one or more series of preferred shares without action by our shareholders and to determine, with respect to any series of
32
preferred shares, the terms and rights of that series. The provisions could have the effect of depriving our shareholders of the opportunity to sell their shares, including shares represented by ADSs, at a premium over the prevailing market price by discouraging third parties from seeking to obtain control of our company in a tender offer or similar transactions.
Our corporate actions are substantially controlled by our directors, executive officers and other principal shareholders, who can exert significant influence over important corporate matters, which may reduce the price of our ADSs and deprive you of an opportunity to receive a premium for your ADSs.
After this offering, our directors, executive officers and principal shareholders will beneficially own approximately 66.9% of our outstanding ordinary shares. These shareholders, if acting together, could exert substantial influence over matters such as electing directors and approving material mergers, acquisitions or other business combination transactions. This concentration of ownership may also discourage, delay or prevent a change in control of our company, which could have the dual effect of depriving our shareholders of an opportunity to receive a premium for their shares as part of a sale of our company and reducing the price of our ADSs. These actions may be taken even if they are opposed by our other shareholders, including those who purchase ADSs in this offering. In addition, these persons could divert business opportunities away from us to themselves or others.
We may be classified as a passive foreign investment company for United States federal income tax purposes, which could subject United States investors in the ADSs or ordinary shares to significant adverse United States federal income tax consequences.
Depending upon the value of our ordinary shares and ADSs and the nature of our assets and income over time, we could be classified as a “passive foreign investment company,” or PFIC, for U.S. federal income tax purposes. A non-United States corporation will be treated as a PFIC for any taxable year if either (i) 75% or more of its gross income for such year consists of certain types of “passive” income, or (ii) 50% or more of the value of its assets (determined on the basis of a quarterly average) during such year is attributable to assets that produce passive income or are held for the production of passive income, or the asset test. Passive income is any income that would be foreign personal holding company income under the Internal Revenue Code of 1986, as amended, including, without limitation, dividends, interest, royalties, rents, annuities, net gains from the sale or exchange of property producing such income, net gains from commodity transactions, net foreign currency gains and income from notional principal contracts.
Based on our current income and assets and projections as to the value of our ordinary shares and ADSs pursuant to this offering, we do not expect to be classified as a PFIC for the current taxable year or the foreseeable future. While we do not anticipate becoming a PFIC, because the value of our assets for purposes of the asset test will generally be determined by reference to the market price of our ADSs or ordinary shares, fluctuations in the market price of our ADSs or ordinary shares may cause us to become a PFIC for the current or subsequent taxable years. Because there are uncertainties in the application of the relevant rules and PFIC status is a factual determination made annually after the close of each taxable year on the basis of the composition of our income and the value of our active versus passive assets, there can be no assurance that we will not be a PFIC for the taxable year 2010 or any future taxable year.
We have not sought a ruling from the United States Internal Revenue Service, or the IRS, with respect to our PFIC status, and there can be no assurance that the IRS will agree with our determination. The overall level of our passive assets will be affected by (i) future growth in activities that may potentially produce passive income, and (ii) how, and how quickly, we spend our liquid assets and the cash raised in this offering. Under circumstances where revenues from activities that produce passive income significantly increase relative to our revenues from activities that produce non-passive income or where we determine not to deploy significant amounts of cash for active purposes, our risk of becoming classified as a PFIC may substantially increase.
If we were to be or become classified as a PFIC, a U.S. Holder (as defined in “Taxation—Material United States Federal Income Tax Considerations—General”) may incur significantly increased United States income tax on gain recognized on the sale or other disposition of the ADSs or ordinary shares and on the receipt of distributions on the ADSs or ordinary shares to the extent such gain or distribution is treated as an “excess
33
distribution” under the United States federal income tax rules. Further, if we are classified as a PFIC for any year during which a U.S. Holder holds our ADSs or ordinary shares, we generally will continue to be treated as a PFIC for all succeeding years during which such U.S. Holder holds our ADSs or ordinary shares. We urge you to consult your tax advisor concerning the United States federal income tax consequences of acquiring, holding and disposing of ADSs or ordinary shares if we are or become classified as a PFIC. For more information, see “Taxation—Material United States Federal Income Tax Considerations—Passive Foreign Investment Company Considerations.”
We will incur increased costs as a result of being a public company.
As a public company, we will incur significant accounting, legal and other expenses that we did not incur as a private company. The Sarbanes-Oxley Act, as well as rules subsequently implemented by the Securities and Exchange Commission and the New York Stock Exchange, have detailed requirements concerning corporate governance practices of public companies, including Section 404 relating to internal controls over financial reporting. We expect these and other rules and regulations applicable to public companies to increase our accounting, legal and financial compliance costs and to make certain corporate activities more time-consuming and costly. We are evaluating and monitoring developments with respect to these new rules, and we cannot predict or estimate the additional costs we may incur or the timing of such costs.
34
FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements that reflect our current expectations and views of future events. The forward looking statements are contained principally in the sections entitled “Prospectus Summary,” “Risk Factors,” “Use of Proceeds,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “Business.” Known and unknown risks, uncertainties and other factors, including those listed under “Risk Factors,” may cause our actual results, performance or achievements to be materially different from those expressed or implied by the forward-looking statements.
You can identify some of these forward-looking statements by words or phrases such as “may,” “will,” “expect,” “anticipate,” “aim,” “estimate,” “intend,” “plan,” “believe,” “likely to,” “potential,” “continue” or other similar expressions. We have based these forward-looking statements largely on our current expectations and projections about future events that we believe may affect our financial condition, results of operations, business strategy and financial needs. These forward-looking statements include statements relating to:
| | • | | our goals and strategies; |
| | • | | our future development, financial condition and results of operations; |
| | • | | the expected growth of the pharmaceutical and biotechnology outsourcing industry in China and globally; |
| | • | | market acceptance of our services; |
| | • | | our expectations regarding demand for our services; |
| | • | | our ability to stay abreast of market trends and technological advances; |
| | • | | our ability to effectively protect our customers’ intellectual property rights and not infringe on the intellectual property rights of others; |
| | • | | competition in the pharmaceutical and biotechnology outsourcing industry; |
| | • | | PRC and U.S. governmental policies and regulations relating to the pharmaceutical and biotechnology outsourcing industry; |
| | • | | litigation and government proceedings involving our company and industry; and |
| | • | | general economic and business conditions, particularly in the United States and China. |
These forward-looking statements involve various risks and uncertainties. Although we believe that our expectations expressed in these forward-looking statements are reasonable, our expectations may later be found to be incorrect. Our actual results could be materially different from our expectations. Important risks and factors that could cause our actual results to be materially different from our expectations are generally set forth in “Prospectus Summary—Our Challenges,” “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” “Business,” “Regulation” and other sections in this prospectus. You should read this prospectus and the documents that we refer to in this prospectus and have filed as exhibits to the registration statement, of which this prospectus is a part, completely and with the understanding that our actual future results may be materially different from what we expect. We qualify all of our forward-looking statements by these cautionary statements.
The forward-looking statements made in this prospectus relate only to events or information as of the date on which the statements are made in this prospectus. Except as required by law, we undertake no obligation to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise, after the date on which the statements are made or to reflect the occurrence of unanticipated events.
35
Market Data and Forecasts
Unless otherwise indicated, information in this prospectus concerning economic conditions and our industry is based on information from independent industry analysts and publications, as well as our estimates. Except where otherwise noted, our estimates are derived from publicly available information released by third-party sources, as well as data from our internal research, and are based on such data and our knowledge of our industry, which we believe to be reasonable.
This prospectus also contains data related to the global and Chinese pharmaceutical and biotechnology R&D outsourcing industries. These market data include estimates and projections that are based on a number of assumptions. If any one or more of the assumptions underlying the market data turn out to be incorrect, actual results may differ significantly from the projections. For example, the global and Chinese pharmaceutical and biotechnology R&D outsourcing markets may not grow at the rate projected by market data, or at all. In addition, the rapidly changing nature of the pharmaceutical and biotechnology R&D outsourcing industry subjects any projections or estimates relating to the growth prospects or future condition of our market to significant uncertainties.
36
USE OF PROCEEDS
We estimate that we will receive net proceeds from this offering of approximately $41.0 million after deducting underwriting discounts and the estimated offering expenses payable by us. We will not receive any proceeds from the ADSs sold by the selling shareholders.
We plan to use the net proceeds of this offering as follows:
| | • | | approximately $15.0 million for the construction of our manufacturing facility and laboratory services building in Fengxian, Shanghai; |
| | • | | approximately $15.0 million for the purchase of equipment; |
| | • | | approximately $5.0 million for the expansion of our service offerings; |
| | • | | approximately $5.0 million for working capital needs; and |
| | • | | the balance for other general corporate purposes, including potential acquisitions (although we are not currently negotiating any such acquisitions). |
The foregoing represents our current intentions based upon our present plans and business conditions to use and allocate the net proceeds of this offering. Our management, however, will have significant flexibility and discretion to apply the net proceeds of this offering. If an unforeseen event occurs or business conditions change, we may use the proceeds of this offering differently than as described in this prospectus. See “Risk Factors—Risks Related to this Offering—You must rely on the judgment of our management as to the use of the net proceeds from this offering, and such use may not produce income or increase our ADS price.”
Pending any use of the net proceeds, as described above, we plan to invest the net proceeds in short-term, interest-bearing debt instruments or bank deposits. These investments may materially and adversely affect the U.S. federal income tax consequences of your investment in our ADSs. In particular, it is possible that we may become a passive foreign investment company for U.S. federal income tax purposes, which could result in negative tax consequences for you. See “Risk Factors—Risks Relating to this Offering—We may be classified as a passive foreign investment company for United States federal income tax purposes, which could subject United States investors in the ADSs or ordinary shares to significant adverse United States federal income tax consequences” and “Taxation—U.S. Federal Income Taxation—U.S. Holders—Passive Foreign Investment Company.”
In using the proceeds from this offering, as an offshore holding company, we are permitted, under PRC laws and regulations, to provide funding to our PRC subsidiaries only through loans or capital contributions. Subject to satisfaction of applicable government registration and approval requirements, we may extend inter-company loans to our PRC subsidiaries or make additional capital contributions to our PRC subsidiaries to fund their capital expenditures or working capital. We cannot assure you that we will be able to obtain these government registrations or approvals on a timely basis, if at all. See “Risk Factors—Risks Related to Doing Business in China—PRC regulations relating to the establishment of offshore special purpose companies by PRC residents may subject our PRC resident beneficial owners or our PRC subsidiaries to liability or penalties, limit our ability to inject capital into our PRC subsidiaries, limit our PRC subsidiaries’ ability to increase their registered capital or distribute profits to us, or may otherwise adversely affect us.”
37
DIVIDEND POLICY
We do not have any present plan to pay any cash dividends on our ordinary shares in the foreseeable future after this offering. We currently intend to retain most, if not all, of our available funds and any future earnings to operate and expand our business.
Our board of directors has significant discretion on whether to distribute dividends. Even if our board of directors decides to pay dividends, the form, frequency and amount will depend upon our future operations and earnings, capital requirements and surplus, general financial condition, contractual restrictions and other factors that the board of directors may deem relevant. If we pay any dividends, the depositary will distribute such payments to our ADS holders to the same extent as holders of our ordinary shares, subject to the terms of the deposit agreement, including the fees and expenses payable thereunder. See “Description of American Depositary Shares.” Cash dividends on our ordinary shares, if any, will be paid in U.S. dollars.
38
CAPITALIZATION
The following table sets forth our capitalization as of June 30, 2010:
| | • | | on a pro forma basis to reflect the automatic conversion of all of our outstanding preferred shares into 69,994,014 ordinary shares immediately upon the completion of this offering; and |
| | • | | on a pro forma as adjusted basis to reflect the automatic conversion of all of our outstanding preferred shares into 69,994,014 ordinary shares immediately upon the completion of this offering, and the sale of 57,600,000 ordinary shares in the form of ADSs by us in this offering at the initial public offering price of $15.00 per ADS, after deducting the underwriting discounts and commissions and estimated offering expenses payable by us. |
You should read this table together with our consolidated financial statements and the related notes included elsewhere in this prospectus and the information under “Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
| | | | | | | | | | | | |
| | | As of June 30, 2010 | |
| | | Actual | | | Pro Forma | | | Pro Forma
As Adjusted | |
| | | (in millions of $) | |
Series A convertible redeemable preferred shares ($0.001 par value; 70,000,650 shares authorized; 69,994,014 shares issued and outstanding; none outstanding on a pro forma basis and a pro forma as adjusted basis)(1) | | | 34.4 | | | | — | | | | — | |
| | | |
Equity: | | | | | | | | | | | | |
Ordinary shares ($0.001 par value, 429,999,350 shares authorized, 208,005,986 shares issued and outstanding; 278,000,000 shares outstanding on a pro forma basis; 335,600,000 shares outstanding on a pro forma as adjusted basis)(1) | | | 0.2 | | | | 0.3 | | | | 0.3 | |
Additional paid-in capital | | | 0.7 | | | | 35.0 | | | | 76.0 | |
Statutory reserves | | | 5.4 | | | | 5.4 | | | | 5.4 | |
Retained earnings | | | 18.9 | | | | 18.9 | | | | 18.9 | |
Accumulated other comprehensive income | | | 2.3 | | | | 2.3 | | | | 2.3 | |
| | | | | | | | | | | | |
| Total equity | | | 27.5 | | | | 61.9 | | | | 102.9 | |
| | | | | | | | | | | | |
| Total capitalization | | | 61.9 | | | | 61.9 | | | | 102.9 | |
| | | | | | | | | | | | |
| (1) | In May 2008, we effected a bonus share issuance in the form a share split by issuing an additional approximately 20,800 ordinary shares for each ordinary share outstanding and an additional approximately 20,800 preferred shares for each preferred share outstanding. As a result of the bonus share issuance, the number of outstanding ordinary shares and preferred shares increased to 208,005,986 and 69,994,014, respectively. See “Related Party Transactions—Share Issuances and Splits.” The effect of the bonus share issuance is retroactively reflected for all periods presented herein. |
39
DILUTION
Our net tangible book value as of June 30, 2010 was approximately $26.7 million, or $0.13 per ordinary share as of that date, and $2.34 per ADS. Net tangible book value represents the amount of our total consolidated tangible assets, less the amount of our total consolidated liabilities and our preferred shares. Our pro forma net tangible book value as of June 30, 2010 was approximately $61.1 million, or $0.22 per ordinary share and $3.96 per ADS. Pro forma net tangible book value after giving effect to the conversion of our preferred shares represents our total consolidated tangible assets, less the amount of our total consolidated liabilities. Dilution is determined by subtracting pro forma as adjusted net tangible book value per ordinary share, after giving effect to the conversion of all outstanding preferred shares into ordinary shares immediately upon the completion of this offering and the additional proceeds we will receive from this offering, based on the initial public offering price per ordinary share and after deducting underwriting discounts and commissions and estimated offering expenses payable by us.
Without taking into account any other changes in net tangible book value after June 30, 2010, other than to give effect to the conversion of all outstanding preferred shares into ordinary shares immediately upon the completion of this offering and our sale of the ADSs offered in this offering at the initial public offering price of $15.00 per ADS, after deduction of the underwriting discounts and commissions and estimated offering expenses payable by us, our pro forma as adjusted net tangible book value as of June 30, 2010 would have been $102.1 million, or $0.30 per outstanding ordinary share and $5.48 per ADS. This represents an immediate increase in pro forma net tangible book value of $0.08 per ordinary share and $1.52 per ADS to our existing shareholders and an immediate dilution in pro forma net tangible book value of $0.53 per ordinary share and $9.52 per ADS to investors purchasing ADSs in this offering. The following table illustrates such dilution:
| | | | | | | | |
| | | Per Ordinary Share | | | Per ADS | |
| | |
Initial public offering price | | $ | 0.83 | | | $ | 15.00 | |
Net tangible book value as of June 30, 2010 | | $ | 0.13 | | | $ | 2.34 | |
Pro forma net tangible book value after giving effect to the conversion of our preferred shares | | $ | 0.22 | | | $ | 3.96 | |
Pro forma as adjusted net tangible book value after giving effect to the conversion of our preferred shares and this offering | | $ | 0.30 | | | $ | 5.48 | |
Amount of dilution in pro forma net tangible book value per share to new investors in this offering | | $ | 0.53 | | | $ | 9.52 | |
The amount of dilution in pro forma net tangible book value to new investors in this offering set forth above is calculated by deducting (i) the pro forma as adjusted net tangible book value after giving effect to the automatic conversion of our preferred shares and this offering from (ii) the initial public offering price.
40
The following table summarizes, on a pro forma basis as of June 30, 2010, the differences between our existing shareholders, including holders of our preferred shares that will be automatically converted into ordinary shares immediately upon the completion of this offering, and the new investors with respect to the number of ordinary shares (in the form of ADSs or shares) purchased from us, the total consideration paid and the average price per ordinary share/ADS paid before deducting the underwriting discounts and commissions and estimated offering expenses payable by us.
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | Ordinary Shares
Purchased | | | Total Consideration | | | Average
Price Per
Ordinary
Share | | | Average
Price Per
ADS | |
| | | Number | | | % | | | Amount | | | % | | | | | | | |
Existing shareholders | | | 278,000,000 | (1) | | | 82.8 | % | | $ | 35,000,000 | | | | 42.2 | % | | | 0.13 | | | | 2.27 | |
New investors | | | 57,600,000 | | | | 17.2 | % | | $ | 48,000,000 | | | | 57.8 | % | | | 0.83 | | | | 15.00 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Total | | | 335,600,000 | | | | 100.0 | % | | $ | 83,000,000 | | | | 100.0 | % | | | 0.25 | | | | 4.45 | |
| (1) | Assumes automatic conversion of all of our preferred shares into ordinary shares upon completion of this offering. |
The discussion and tables above also assume no exercise of any outstanding share options. As of September 30, 2010, there were 36,046,200 ordinary shares issuable upon exercise of outstanding share options at a weighted average exercise price of $0.55 per share, and there were 516,158 ordinary shares available for future issuance upon the exercise of future grants under the Plan. To the extent that any of these options are exercised, there will be further dilution to new investors.
41
EXCHANGE RATES
We conduct substantially all of our operations in China. A substantial portion of our sales are denominated in U.S. dollars, while a significant portion of our costs and expenses are denominated in Renminbi. We make no representation that any Renminbi or U.S. dollar amounts could have been, or could be, converted into U.S. dollars or Renminbi, as the case may be, at any particular rate, at the rates stated below, or at all. The PRC government imposes control over its foreign currency reserves in part through direct regulation of the conversion of Renminbi into foreign exchange and through restrictions on foreign trade. On October 15, 2010, the noon buying rate was RMB6.6905 to $1.00.
The following table sets forth information concerning exchange rates between the Renminbi and the U.S. dollar for the periods indicated. These rates are provided solely for your convenience and are not necessarily the exchange rates that we used in this prospectus or will use in the preparation of our periodic reports or any other information to be provided to you. For all dates and periods through December 31, 2008, exchange rates of Renminbi into the U.S. dollar are based on the noon buying rate in The City of New York for cable transfers of Renminbi as certified for customs purposes by the Federal Reserve Bank of New York. For January 1, 2009 and all later dates and periods, the exchange rate refers to the exchange rate as set forth in the H.10 statistical release of the Federal Reserve Board. Unless otherwise noted, all translations from Renminbi to U.S. dollars and from U.S. dollars to Renminbi in this prospectus were made at a rate of RMB6.8259 to $1.00, the exchange rate set forth in the H.10 statistical release of the Federal Reserve Board on December 31, 2009.
| | | | | | | | | | | | | | | | |
| | | Noon Buying Rate | |
| Period | | Period End | | | Average(1) | | | Low | | | High | |
| | | (RMB per $1.00) | |
| | | | |
2006 | | | 7.8041 | | | | 7.9579 | | | | 8.0702 | | | | 7.8041 | |
2007 | | | 7.2946 | | | | 7.5806 | | | | 7.8127 | | | | 7.2946 | |
2008 | | | 6.8225 | | | | 6.9193 | | | | 7.2946 | | | | 6.7800 | |
2009 | | | 6.8259 | | | | 6.8295 | | | | 6.8395 | | | | 6.8180 | |
2010 | | | | | | | | | | | | | | | | |
April | | | 6.8247 | | | | 6.8256 | | | | 6.8275 | | | | 6.8229 | |
May | | | 6.8305 | | | | 6.8274 | | | | 6.8310 | | | | 6.8245 | |
June | | | 6.7815 | | | | 6.8184 | | | | 6.8323 | | | | 6.7815 | |
July | | | 6.7735 | | | | 6.7762 | | | | 6.7807 | | | | 6.7709 | |
August | | | 6.8069 | | | | 6.7873 | | | | 6.8069 | | | | 6.7670 | |
September | | | 6.6905 | | | | 6.7396 | | | | 6.8102 | | | | 6.6869 | |
October (through October 15) | | | 6.6397 | | | | 6.6749 | | | | 6.6912 | | | | 6.6397 | |
Source: Federal Reserve Statistical Release
| (1) | Annual averages are calculated using the average of month-end rates of the relevant year. Monthly averages are calculated using the average of the daily rates during the relevant period. |
42
ENFORCEABILITY OF CIVIL LIABILITIES
We were incorporated in the Cayman Islands in order to enjoy certain benefits, such as political and economic stability, an effective judicial system, a favorable tax system, the absence of exchange control or currency restrictions and the availability of professional and support services. However, certain disadvantages accompany incorporation in the Cayman Islands. These disadvantages include a less developed body of Cayman Islands securities laws that provide significantly less protection to investors compared to the laws of the United States, and the potential lack of standing by Cayman Islands companies to sue before the federal courts of the United States.
Our organizational documents do not contain provisions requiring disputes, including those arising under the securities laws of the United States, between us and our officers, directors and shareholders, be arbitrated.
We conduct substantially all of our operations in China, and substantially all of our assets are located in China. Some of our directors and officers are nationals or residents of jurisdictions other than the United States and a substantial portion of their assets are located outside the United States. As a result, it may be difficult for a shareholder to effect service of process within the United States upon us or these persons, or to enforce against us or them judgments obtained in United States courts, including judgments predicated upon the civil liability provisions of the securities laws of the United States or any state in the United States.
We have appointed Law Debenture Corporate Services Inc. located at 400 Madison Avenue, 4th Floor, New York, New York 10017, as our agent upon whom process may be served in any action brought against us under the securities laws of the United States.
Conyers Dill & Pearman, our counsel as to Cayman Islands law, and Fangda Partners, our counsel as to PRC law, have advised us, respectively, that there is uncertainty as to whether the courts of the Cayman Islands and China, respectively, would:
| | • | | recognize or enforce judgments of United States courts obtained against us or our directors or officers predicated upon the civil liability provisions of the securities laws of the United States or any state in the United States; or |
| | • | | entertain original actions brought in each respective jurisdiction against us or our directors or officers predicated upon the securities laws of the United States or any state in the United States. |
Conyers Dill & Pearman has further advised us that the courts of the Cayman Islands would recognize as a valid judgment, a final and conclusive judgment in personam obtained in a federal or state court of the United States under which a sum of money is payable (other than a sum of money payable in respect of multiple damages, taxes or other charges of a like nature or in respect of a fine or other penalty) and would give a judgment based thereon; provided that (a) such courts had proper jurisdiction over the parties subject to such judgment; (b) such courts did not contravene the rules of natural justice of the Cayman Islands; (c) such judgment was not obtained by fraud; (d) the enforcement of the judgment would not be contrary to the public policy of the Cayman Islands; (e) no new admissible evidence relevant to the action is submitted prior to the rendering of the judgment by the courts of the Cayman Islands; and (f) there is due compliance with the correct procedures under the laws of the Cayman Islands.
Fangda Partners has further advised us that the recognition and enforcement of foreign judgments are provided for under the PRC Civil Procedures Law. PRC courts may recognize and enforce foreign judgments in accordance with the requirements of the PRC Civil Procedures Law based either on treaties between China and the country where the judgment is made or on principles of reciprocity between jurisdictions. China does not have any treaties or other form of reciprocity with the United States that provide for the reciprocal recognition and enforcement of foreign judgments. In addition, according to the PRC Civil Procedures Law, courts in the PRC will not enforce a foreign judgment against us or our directors and officers if they decide that the judgment
43
violates the basic principles of PRC law or national sovereignty, security or public interest. As a result, it is uncertain whether and on what basis a PRC court would enforce a judgment rendered by a court in the United States. Under the PRC Civil Procedures Law, there is no mechanism through which a company established in a foreign jurisdiction could be deemed a PRC resident. Whether a company could be deemed a PRC resident under other regulations, such as tax regulations, does not impact the ability or inability of the shareholders of such company to bring actions against the company under PRC laws. Since the legal connections between our shareholders and our company arise from the laws of jurisdictions other than the PRC, normally it would not be feasible for our shareholders to bring actions against us under PRC laws.
44
SELECTED CONSOLIDATED FINANCIAL DATA
The following selected consolidated financial data for the periods and as of the dates indicated should be read in conjunction with our consolidated financial statements and related notes and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included elsewhere in this prospectus.
Our selected consolidated financial data presented below for the years ended December 31, 2007, 2008 and 2009 and our balance sheet data as of December 31, 2008 and 2009 have been derived from our audited consolidated financial statements included elsewhere in this prospectus. Our selected consolidated statement of operations data for the year ended December 31, 2006 and our selected consolidated balance sheet data as of December 2006 and 2007 have been derived from our audited consolidated financial statements not included in this prospectus. Our audited consolidated financial statements are prepared in accordance with U.S. GAAP and have been audited by PricewaterhouseCoopers Zhong Tian CPAs Limited Company, an independent registered public accounting firm. We have not included financial information for the year ended December 31, 2005, as such information is not available on a basis that is consistent with the consolidated financial information for the years ended December 31, 2006, 2007, 2008 and 2009, and cannot be provided on a U.S. GAAP basis without unreasonable effort or expense.
The selected consolidated statement of operations data for the six months ended June 30, 2009 and 2010 and the selected consolidated balance sheet data as of June 30, 2010 have been derived from our unaudited condensed consolidated financial statements included elsewhere in this prospectus and have been prepared on the same basis as our audited consolidated financial statements. The unaudited financial information includes all adjustments, consisting only of normal and recurring adjustments, that we consider necessary for a fair statement of our financial position and operating results for the periods presented. Our historical results do not necessarily indicate results expected for any future periods.
45
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | Year Ended December 31, | | | Six Months Ended June 30, | |
| | | 2006 | | | 2007 | | | 2008 | | | 2009 | | | 2009 | | | 2010 | |
| | | (in millions of $, except for share, per share and per ADS data) | |
Consolidated Statements of Operations Data: | | | | | | | | | | | | | | | | | | | | | | | | |
Net revenues | | | 17.8 | | | | 34.7 | | | | 60.5 | | | | 72.3 | | | | 32.7 | | | | 41.6 | |
Cost of revenues | | | (10.1 | ) | | | (22.8 | ) | | | (40.5 | ) | | | (48.4 | ) | | | (21.8 | ) | | | (27.2 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Gross profit | | | 7.7 | | | | 11.9 | | | | 20.0 | | | | 23.9 | | | | 10.9 | | | | 14.4 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Operating expenses: | | | | | | | | | | | | | | | | | | | | | | | | |
Selling and marketing | | | (0.3 | ) | | | (0.5 | ) | | | (0.8 | ) | | | (1.7 | ) | | | (0.7 | ) | | | (1.1 | ) |
General and administrative | | | (1.9 | ) | | | (4.0 | ) | | | (9.3 | ) | | | (12.0 | ) | | | (5.6 | ) | | | (6.9 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Total operating expenses | | | (2.2 | ) | | | (4.5 | ) | | | (10.1 | ) | | | (13.7 | ) | | | (6.3 | ) | | | (8.0 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Profit from operations | | | 5.5 | | | | 7.4 | | | | 9.9 | | | | 10.2 | | | | 4.6 | | | | 6.4 | |
| | | | | | |
Interest income | | | * | | | | 0.2 | | | | 0.2 | | | | * | | | | * | | | | * | |
Interest expense | | | — | | | | — | | | | — | | | | — | | | | — | | | | * | |
Other income | | | * | | | | 0.2 | | | | 0.9 | | | | 1.3 | | | | 0.8 | | | | 1.6 | |
Other expenses | | | * | | | | (0.1 | ) | | | (1.6 | ) | | | (0.2 | ) | | | (0.1 | ) | | | (0.2 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Income from operations before income taxes | | | 5.4 | | | | 7.7 | | | | 9.4 | | | | 11.4 | | | | 5.3 | | | | 7.8 | |
Income taxes | | | (1.7 | ) | | | (0.2 | ) | | | (0.3 | ) | | | (1.6 | ) | | | (0.7 | ) | | | (0.9 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Net income attributable to ShangPharma Corporation | | | 3.7 | | | | 7.5 | | | | 9.1 | | | | 9.8 | | | | 4.6 | | | | 6.9 | |
Allocation to preferred shareholders | | | — | | | | (0.7 | ) | | | (2.3 | ) | | | (2.5 | ) | | | (1.2 | ) | | | (1.8 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Net income attributable to ShangPharma Corporation’s ordinary shareholders | | | 3.7 | | | | 6.8 | | | | 6.8 | | | | 7.3 | | | | 3.4 | | | | 5.1 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Net income attributable to ShangPharma Corporation’s ordinary shareholders per share | | | | | | | | | | | | | | | | | | | | | | | | |
Basic | | | 0.02 | | | | 0.03 | | | | 0.03 | | | | 0.04 | | | | 0.02 | | | | 0.02 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Diluted | | | 0.02 | | | | 0.03 | | | | 0.03 | | | | 0.04 | | | | 0.02 | | | | 0.02 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Net income attributable to ShangPharma Corporation’s ordinary shareholders per ADS(1) | | | | | | | | | | | | | | | | | | | | | | | | |
Basic | | | 0.32 | | | | 0.59 | | | | 0.59 | | | | 0.63 | | | | 0.30 | | | | 0.44 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Diluted | | | 0.32 | | | | 0.59 | | | | 0.59 | | | | 0.63 | | | | 0.30 | | | | 0.44 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | |
Weighted average ordinary shares outstanding(2) | | | | | | | | | | | | | | | | | | | | | | | | |
Basic | | | 208,005,986 | | | | 208,005,986 | | | | 208,005,986 | | | | 208,005,986 | | | | 208,005,986 | | | | 208,005,986 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Diluted | | | 208,005,986 | | | | 230,381,122 | | | | 278,000,000 | | | | 278,051,299 | | | | 278,000,000 | | | | 210,362,840 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Pro forma earnings per share attributable to ShangPharma Corporation’s ordinary shareholders (unaudited)(3) | | | | | | | | | | | | | | | | | | | | | | | | |
Basic | | | | | | | | | | | | | | | 0.04 | | | | | | | | 0.02 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Diluted | | | | | | | | | | | | | | | 0.04 | | | | | | | | 0.02 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Pro forma weighted average ordinary shares outstanding (unaudited)(2) (3) (4) | | | | | | | | | | | | | | | | | | | | | | | | |
Basic | | | | | | | | | | | | | | | 278,000,000 | | | | | | | | 278,000,000 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Diluted | | | | | | | | | | | | | | | 278,051,299 | | | | | | | | 280,356,854 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
| (1) | Each ADS represents 18 ordinary shares. |
| (2) | In May 2008, we effected a bonus share issuance in the form a share split by issuing an additional approximately 20,800 ordinary shares for each ordinary share outstanding and an additional approximately 20,800 preferred shares for each preferred share outstanding. As a result of the bonus share issuance, the number of outstanding ordinary shares and preferred shares increased to 208,005,986 and |
46
| | 69,994,014, respectively. See “Related Party Transactions—Share Issuances and Splits.” The effect of the bonus share issuance is retroactively reflected for all periods presented herein. |
| (3) | For a description of the pro forma adjustments used to calculate pro forma earnings per share for the year ended December 31, 2009, see “Note 19—Unaudited Pro Forma Balance Sheet and Earnings Per Share for Conversion of Preferred Shares” to our consolidated financial statements included elsewhere in this prospectus. |
| (4) | For a description of the pro forma adjustments used to calculate pro forma earnings per share for the six months ended June 30, 2010, see “Note 15—Unaudited Pro Forma Balance Sheet and Earnings Per Share for Conversion of Preferred Shares” to our unaudited condensed consolidated financial statements included elsewhere in this prospectus. |
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | As of December 31, | | | As of June 30, | |
| | | 2006 | | | 2007 | | | 2008 | | | 2009 | | | 2010 | | | 2010 | |
| | | (in millions of $) | |
| | | | | | | | | (Pro forma)(1) | |
Consolidated Balance Sheet Data: | | | | | | | | | | | | | | | | | | | | | | | | |
Cash | | | 2.3 | | | | 19.6 | | | | 15.8 | | | | 12.2 | | | | 10.0 | | | | 10.0 | |
Total current assets | | | 6.0 | | | | 27.5 | | | | 28.7 | | | | 30.1 | | | | 31.9 | | | | 31.9 | |
Total assets | | | 14.4 | | | | 45.3 | | | | 61.7 | | | | 70.0 | | | | 78.8 | | | | 78.8 | |
Total current liabilities | | | 6.3 | | | | 11.5 | | | | 17.3 | | | | 15.5 | | | | 16.9 | | | | 16.9 | |
Total liabilities | | | 6.3 | | | | 11.5 | | | | 17.3 | | | | 15.5 | | | | 16.9 | | | | 16.9 | |
Series A convertible preferred shares | | | — | | | | 34.4 | | | | 34.4 | | | | 34.4 | | | | 34.4 | | | | — | |
Total equity | | | 8.1 | | | | (0.6 | ) | | | 10.0 | | | | 20.1 | | | | 27.5 | | | | 61.9 | |
Total liabilities and equity | | | 14.4 | | | | 45.3 | | | | 61.7 | | | | 70.0 | | | | 78.8 | | | | 78.8 | |
| (1) | Pro forma basis reflects the conversion of all outstanding preferred shares on a 1-for-1 basis into an aggregate of 69,994,014 ordinary shares upon the completion of this offering. |
47
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of our financial condition and results of operations in conjunction with the section entitled “Selected Consolidated Financial Data” and our consolidated financial statements and the related notes included elsewhere in this prospectus. This discussion contains forward-looking statements that involve risks and uncertainties. Our actual results and the timing of selected events could differ materially from those anticipated in these forward-looking statements as a result of various factors, including those set forth under “Risk Factors” and elsewhere in this prospectus.
Overview
We are a leading China-based pharmaceutical and biotechnology R&D outsourcing company. We provide a broad range of high-quality, integrated services across the drug discovery and development process to international and Chinese pharmaceutical and biotechnology companies. Capitalizing on our broad service offerings, the experience and expertise of our skilled scientists and our state-of-the-art facilities and equipment, we help our customers discover and develop novel drug candidates efficiently. We have a strong and diversified customer base. We provide services to over 100 customers, including all of the top 10 pharmaceutical and biotechnology companies in the world in terms of 2009 revenues ranked by Frost & Sullivan and a number of fast-growing biotechnology and specialty pharmaceutical companies, as well as renowned academic and research institutions in the U.S. and China. We have experienced significant growth in recent years. The number of our customers increased from 71 in 2007 to 134 in 2009 and 151 in the six months ended June 30, 2010, and our scientific research staff increased from 988 as of December 31, 2007 to 1,525 as of June 30, 2010.
We operate our business as a single segment. Our services consist of discovery chemistry, discovery biology and preclinical development, pharmaceutical development and biologics services. Our core service offering is discovery chemistry, which achieved strong revenue growth from 2007 to 2009. Newer services, such as discovery biology and preclinical development and pharmaceutical development, have grown at a higher rate than discovery chemistry. We began offering biologics services in early 2010. Our integrated service platform allows us to cross-sell additional services to our existing customers. We saw increasing demand from our existing and new customers for integrated services, and a significant majority of our customers hired us for more than one service in each of 2007, 2008 and 2009. We offer our services on an FTE basis or a fee-for-service basis. We derive a significant majority of our net revenues from FTE-based services.
Our net revenues increased from $34.7 million in 2007 to $60.5 million in 2008 and $72.3 million in 2009, representing a CAGR of 44.4%, and increased by 27.2% from $32.7 million in the six months ended June 30, 2009 to $41.6 million in the six months ended June 30, 2010. Such increases were primarily attributable to an increase in the number of customers and resulting projects, broadened service offerings and expanded service capabilities. Our average net revenues per top ten customer increased from $2.9 million in 2007 to $4.7 million in each of 2008 and 2009 and were $2.6 million in the six months ended June 30, 2010. Our net income increased from $7.5 million in 2007 to $9.1 million in 2008 and $9.8 million in 2009, and increased from $4.6 million in the six months ended June 30, 2009 to $6.9 million in the six months ended June 30, 2010.
The global pharmaceutical and biotechnology industry has experienced significant consolidation and, during the global financial crisis, some pharmaceutical and biotechnology companies experienced financial difficulties. As a result, some of our customers reduced their R&D budgets in 2009. We believe our strong growth in net revenues from 2007 to 2009 and from the six months ended June 30, 2009 to the same period ended June 30, 2010 despite industry consolidation and global financial crisis reflected our customers’ recognition of our high-quality, integrated services.
We intend to broaden our service offerings and expand our service capabilities to drive our growth, particularly in research manufacturing and biologics. We are constructing an approximately 460,000 square-foot cGMP-quality multi-purpose manufacturing facility in Fengxian, Shanghai, designed to manufacture advanced
48
intermediates and APIs for preclinical testing and clinical trials. Phase A of the Fengxian facility, comprising approximately 200,000 square feet, is scheduled to commence operations in early 2011. Phase B of such facility, comprising approximately 260,000 square feet, is estimated to commence operations in 2011 or later. We are also constructing an approximately 150,000 square-foot laboratory services building in Fengxian, Shanghai primarily for pharmaceutical development services. This building is estimated to commence operations in 2011.
In 2008, 2009 and the nine months ended September 30, 2010, our founders granted RSUs convertible to 7,450,000, 2,100,000 and 2,200,000 ordinary shares, respectively, to certain members of our senior management and other employees under the Founder’s Plan. We have not recorded any share-based compensation expense in connection with these RSU grants because one of the vesting conditions for the RSUs is the completion of our initial public offering. As a result, immediately upon completion of this offering, we expect to incur a significant share-based compensation charge of approximately $2.5 million, which will materially and adversely affect our results of operations for the quarter in which this offering is completed.
Factors Affecting Our Results of Operations
We believe the following factors have had, and will continue to have, a significant effect on our results of operations:
Growth of the Global Pharmaceutical and Biotechnology R&D Outsourcing Industry
The growth of the global pharmaceutical and biotechnology R&D outsourcing industry has significantly affected, and is expected to continue to significantly affect, our results of operations. This growth has been primarily driven by the need to contain the cost of drug R&D, accelerate drug R&D process and meet the rising demand from the biotechnology industry. Many large pharmaceutical and biotechnology companies have been “offshoring” and/or outsourcing R&D activities to regions with significant resource and cost advantages, such as China. Advantages offered by China include a large talent pool in the chemistry, biology and medical sciences and related fields, relatively low-cost labor, developed infrastructure and favorable government incentives. The global pharmaceutical and biotechnology industry has experienced significant consolidation and, during the global financial crisis, some pharmaceutical and biotechnology companies experienced financial difficulties. As a result, some of our customers reduced their R&D budgets in 2009. However, the trend in increasing global pharmaceutical and biotechnology R&D outsourcing activities has continued and is expected to continue in the near future. See “Industry.”
Expansion of Our Service Offerings and Capabilities
Expansion and marketing of our service offerings and capabilities are essential to the growth of our business. We believe our customers value our ability to offer a wide range of quality services to meet their drug R&D needs, and we expect to capitalize on our strong customer base and expand our service offerings along the drug discovery and development value chain. Initially focusing on discovery chemistry services, we introduced pharmaceutical development services in 2005 and discovery biology and preclinical development services in 2007. We began offering biologics services in early 2010. We are constructing an approximately 460,000 square-foot cGMP-quality multi-purpose manufacturing facility and an approximately 150,000 square-foot laboratory services building in Fengxian, Shanghai. Our business and results of operations will depend significantly on our ability to expand our service offerings and capabilities as planned and create demand for those newly expanded services or capabilities.
Labor Costs
Labor costs, which constitute a significant component of our cost of revenues, selling and marketing expenses and general and administrative expenses, significantly affect our profitability. Our employee base increased substantially from 1,115 as of December 31, 2007 to 1,755 as of June 30, 2010 and is expected to increase to meet our anticipated growth needs. In addition, our labor costs increased from 2007 to 2009 as we
49
hired more senior scientists for newer services, such as discovery biology and preclinical development, pharmaceutical development and biologics, as well as more senior management as part of our strategy to grow our business. The increases in labor costs were also attributable to appreciation of the Renminbi relative to the U.S. dollar, as we generally pay our employees in Renminbi, and overall rising labor costs in China. These increases were partially offset by efficiencies gained by our scientists and by our entry into higher margin services. We expect our labor costs as a percentage of total net revenues to decrease or remain relatively stable in the near future as we expect to hire more junior scientists to further expand our R&D team and leverage the experience and expertise of our senior scientists.
Fluctuations in Exchange Rates
Fluctuations in exchange rates affect our cost of revenues and net income and materially impact our operating margins, because our net revenues are primarily generated from sales denominated in U.S. dollars, and a majority of our cost of revenues and operating expenses are denominated in Renminbi. The value of the Renminbi against the U.S. dollar and other currencies may fluctuate and is affected by, among other things, changes in China’s political and economic conditions. The conversion of Renminbi into foreign currencies, including the U.S. dollar, has been based on rates set by the People’s Bank of China. On July 21, 2005, the PRC government changed its policy of pegging the value of the Renminbi to the U.S. dollar and began allowing modest appreciation of the Renminbi versus the U.S. dollar. This change in policy has resulted in an appreciation of approximately 22.0% of the Renminbi against the U.S. dollar from July 21, 2005 to June 30, 2010. The PRC government faces significant international pressure to further liberalize its currency policy, which could result in further appreciation in the value of the Renminbi against the U.S. dollar. Any further appreciation in the value of the Renminbi against the U.S. dollar will increase our cost of revenues and operating expenses and, if not passed on to our customers in the form of higher prices, adversely affect on our margins.
Selected Statements of Operations Items
Net Revenues
Our net revenues comprise our total revenues from operations, less sales taxes. We offer our laboratory services on an FTE basis or a fee-for-service basis. An FTE arrangement differs from fee-for-service arrangements in that generally a team of our scientists is assigned to the needs of an individual customer for a particular period. The customer pays a fixed rate per FTE regardless of the workload or changes in the assignment, as determined in each contract, and the price generally includes some of the material costs incurred. Fees from FTE services are generally not contingent on outcomes or other deliverables or milestones. Revenues from fee-for-service contracts are typically contingent on successful performance of a service or delivery of a product and the price is often fixed in the contract. Fee-for-service contracts have different terms but are generally shorter in duration than FTE contracts. Revenues from these contracts fluctuate from period to period depending on, among other things, the nature of services and the completion schedule.
The increases in our net revenues from 2007 to 2009 and from the six months ended June 30, 2009 to the same period ended June 30, 2010 were attributable primarily to a larger customer base and a broader scope of services we provided to our customers. We began providing pharmaceutical development services in 2005 and discovery biology and preclinical development services in 2007. We introduced biologics services in early 2010. We derive a significant majority of our revenues from FTE contracts. The following table sets forth our FTE-based net revenues and fee-for-service-based net revenues by amount and as a percentage of our total net revenues for the periods indicated:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Year Ended December 31, | | | Six Months Ended June 30, | |
| | | 2007 | | | 2008 | | | 2009 | | | 2009 | | | 2010 | |
| | | Net
Revenues | | | % | | | Net
Revenues | | | % | | | Net
Revenues | | | % | | | Net
Revenues | | | % | | | Net
Revenues | | | % | |
| | | (in millions of $, except for percentages) | |
FTE-based net revenues | | | 29.9 | | | | 86.0 | % | | | 47.8 | | | | 79.1 | % | | | 56.7 | | | | 78.4 | % | | | 26.5 | | | | 81.2 | % | | | 30.9 | | | | 74.4 | % |
Fee-for-service-based net revenues | | | 4.8 | | | | 14.0 | | | | 12.7 | | | | 20.9 | | | | 15.6 | | | | 21.6 | | | | 6.2 | | | | 18.8 | | | | 10.7 | | | | 25.6 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total net revenues | | | 34.7 | | | | 100.0 | % | | | 60.5 | | | | 100.0 | % | | | 72.3 | | | | 100.0 | % | | | 32.7 | | | | 100.0 | % | | | 41.6 | | | | 100.0 | % |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
50
We derive FTE-based revenues primarily from our discovery chemistry services. FTE-based revenues as a percentage of our total net revenues decreased from 86.0% in 2007 to 78.4% in 2009 and 74.4% in the six months ended June 30, 2010 as we expanded our newer service offerings, such as discovery biology and preclinical development and pharmaceutical development, which are more typically covered by fee-for-service contracts.
We generated a significant majority of our net revenues in 2007, 2008, 2009 and the six months ended June 30, 2010 from U.S.-based customers, with most of the remaining revenues from customers based in Europe, Japan and China. A majority of our net revenues came from pharmaceutical companies, while biotechnology and specialty pharmaceutical customers contributed to a significant portion of our net revenues during the same periods.
Cost of Revenues
Cost of revenues consists primarily of (i) labor costs, including salaries, bonuses and other benefits, for employees who provide laboratory services, (ii) raw material costs, including catalysts, reagents, solvents and other laboratory supplies and (iii) overhead costs, which primarily include the depreciation of equipment, property improvement and operating lease expenses for our leased facilities.
Labor is a significant component of our cost of revenues and therefore an important factor affecting our profitability. Our headcount increased substantially from 2007 to the six months ended June 30, 2010 as we expanded our service offerings and is expected to continue increasing to meet our anticipated growth needs. Our labor costs as a percentage of total net revenues increased slightly from 2007 to 2009 and from the six months ended June 30, 2009 to the same period ended June 30, 2010 due to our hiring of more senior scientists for our newer services, appreciation of the Renminbi relative to the U.S. dollar and overall rising labor costs in China. Such increase was partially offset by efficiencies gained by our scientists and by our entry into higher margin services.
Our raw material costs as a percentage of our total net revenues decreased from 2007 to 2009 and from the six months ended June 30, 2009 to the same period ended June 30, 2010 due to our tighter cost control measures, such as improving raw material usage efficiencies, as well as our increased bargaining power resulting from economies of scale.
Our overhead costs increased from 2007 to 2009 and from the six months ended June 30, 2009 to the same period ended June 30, 2010 as we expanded our facilities and purchased new equipment. We expect our overhead costs to increase as a result of depreciation of our manufacturing facility and laboratory services building in Fengxian, Shanghai upon its completion.
Cost of revenues also includes an allocation of our share-based compensation charges. See “—Critical Accounting Policies—Share-Based Compensation Expenses.”
Gross Profit and Margin
Gross profit is equal to net revenues less cost of revenues. Gross margin is equal to gross profit divided by net revenues. Changes in our gross profit and margin from period to period are primarily driven by our service mix and net revenue per FTE as well as exchange rate fluctuations. Our gross margin was 34.2% in 2007, 33.0% in 2008, 33.0% in 2009 and 33.3% and 34.5% in the six months ended June 30, 2009 and 2010. Our gross margin was negatively impacted by the appreciation of the Renminbi relative to the U.S. dollar during these periods. Appreciation of the Renminbi, the currency in which we incur most of our expenses, in relation to the U.S. dollar, the currency in which we earn most of our revenues, puts pressure on our gross margin. Our gross margin was also negatively impacted by our increased labor costs and, from 2007 to 2009, by increased depreciation expenses related to our expanded facilities and equipment. These adverse effects were partially offset by our
51
improved cost control measures, growth in higher margin service offerings, increases in net revenue per FTE, economies of scale and improvements in operational efficiency.
Operating Expenses
Our operating expenses consist of selling and marketing expenses and general and administrative expenses.
Selling and Marketing Expenses. Selling and marketing expenses consist primarily of salaries, bonuses, commissions and other benefits of our sales and business development personnel and promotional activities and tradeshow expenses. Our selling and marketing expenses increased from 2007 to 2009 and from the six months ended June 30, 2009 to the same period ended June 30, 2010 primarily because we built sales and marketing teams in the U.S. and Europe during these periods. We expect that selling and marketing expenses will grow as a result of our efforts to increase market presence and drive revenue growth.
General and Administrative Expenses. General and administrative expenses consist primarily of (i) salaries, bonuses and other benefits for management and employees in our administration departments including finance, human resources, executive office, legal and information technology departments, (ii) depreciation and amortization of non-laboratory property, equipment and software and (iii) professional service fees.
Our general and administrative expenses increased from 2007 to 2009 and from the six months ended June 30, 2009 to the same period ended June 30, 2010 as our business expanded, including the hiring of additional executives and management staff. We expect our general and administrative expenses to increase in future periods as our business grows and we incur increased costs related to complying with our reporting obligations under the U.S. securities laws as a public company. In addition, upon completion of this offering, we expect to incur a significant share-based compensation charge in connection with the vesting of granted RSUs. See “—Critical Accounting Policies—Share-Based Compensation Expenses—Founder’s 2008 Equity and Performance Incentive Plan.”
Our operating expenses include an allocation of our share-based compensation charges. See “—Critical Accounting Policies—Share-Based Compensation Expenses.”
Income Taxes
Cayman Islands
We are incorporated in the Cayman Islands. Under the current laws of the Cayman Islands, we are not subject to income or capital gains tax. In addition, dividend payments are not subject to withholding tax in the Cayman Islands.
Hong Kong
Our subsidiaries did not have assessable profits that were earned in or derived from Hong Kong during the years ended December 31, 2007, 2008 and 2009 and the six months ended June 30, 2010. Accordingly, we did not pay Hong Kong profits tax during these periods.
PRC
Prior to the effectiveness of the PRC Enterprise Income Tax Law on January 1, 2008, enterprises in China were generally subject to an enterprise income tax at a statutory rate of 33% unless they qualified for certain preferential treatment. Starting January 1, 2008, the PRC Enterprise Income Tax Law has imposed a
52
uniform enterprise income tax rate of 25% on all domestic enterprises and foreign-invested enterprises unless they qualify for certain exceptions. Enterprises established before March 16, 2007 that were entitled to the then available preferential tax treatment may continue to enjoy such treatment (i) in the case of preferential tax rates, for a maximum of a five-year period starting from January 1, 2008; during such period, the tax rate will gradually increase to 25%, or (ii) in the case of preferential tax exemption or reduction for a specified term, until the expiration of such term.
Shanghai ChemExplorer Co., Ltd. was entitled to a one-year exemption from enterprise income tax for year 2007, and a 50% reduction on the otherwise applicable enterprise income tax rate for the subsequent three years, and Shanghai PharmaExplorer Co., Ltd. and Shanghai ChemPartner Co., Ltd. are entitled to a two-year exemption from enterprise income tax for 2007 and 2008, and a 50% reduction on the otherwise applicable enterprise income tax rate for the subsequent three years. Accordingly, the enterprise income tax rate applicable to Shanghai ChemExplorer Co., Ltd. will gradually increase from 9% to 11% from 2008 to 2010. The enterprise income tax rate applicable to Shanghai PharmaExplorer Co., Ltd. and Shanghai ChemPartner Co., Ltd. will gradually increase from 10% to 12% from 2009 to 2011. In addition, Shanghai ChemExplorer Co., Ltd. and Shanghai ChemPartner Co., Ltd. have been approved as high and new technology enterprises since 2008 and are entitled to a preferential tax rate of 15%. Chengdu Chempartner Co., Ltd. is located in Chengdu, China and was recognized as a high and new technology enterprise in 2008 and will be entitled to an enterprise income tax rate of 15% if it generates taxable income and continues to qualify as a high and new technology enterprise. The high and new technology enterprise status is subject to approval and renewal every three years.
Prior to 2006, we recorded a majority of our income in our offshore entities, which are exempted from China’s enterprise income tax. In 2007, we concluded that this treatment no longer corresponds to our operations. We subsequently revised the manner in which we record our income. We voluntarily disclosed the revision with the local tax authorities and agreed to pay a total of $2.0 million to the tax authorities in April 2008, which consisted of income tax expense of $1.7 million and a late filing interest charge of $0.3 million. We recorded the $1.7 million as income tax expenses in 2006 and the late interest charge of $0.2 million and $0.1 million for 2007 and 2008 as income tax expenses in 2007 and 2008, respectively.
Our effective enterprise tax rate was 2% for 2007, 3% for 2008, 14% for 2009, and 14% and 12% for the six months ended June 30, 2009 and 2010, respectively. See “Note 11—Taxation” to our consolidated financial statements included elsewhere in this prospectus.
Critical Accounting Policies
We prepare our financial statements in conformity with U.S. GAAP, which requires us to make estimates and assumptions that affect our reporting of, among other things, assets and liabilities, contingent assets and liabilities and net revenues and expenses. We regularly evaluate these estimates and assumptions based on the most recently available information, our historical experience and other factors that we believe to be relevant under the circumstances. Since our financial reporting process inherently relies on the use of estimates and assumptions, our actual results could differ from what we expect. This is especially true with some accounting policies that require higher degrees of judgment than others in their application. We consider the policies discussed below to be critical to an understanding of our audited consolidated financial statements because they involve the greatest reliance on our management’s judgment.
Revenue Recognition
Revenues are recognized when persuasive evidence of an arrangement exists, the sales price is fixed or determinable, delivery of the product or performance of the service has occurred and there is reasonable assurance of collection of the sales proceeds.
53
For laboratory services provided on an FTE basis, the customer pays a fixed rate per laboratory worker on a full time employment basis and we recognize revenue as the services are provided. There is no requirement for fixed deliverables by us or acceptance by our customers under the FTE contracts.
For laboratory services provided on a fee-for-service with one or multiple elements of deliverables, we recognize revenue as each element is earned, provided that the fair value of the undelivered element(s) has been determined, the delivered element(s) has stand-alone value, there is no right of return on delivered element(s) and we are in control of the undelivered element(s). An element is considered earned upon the delivery and acceptance of the deliverables. For arrangements that include service elements, revenue is recognized for the service element using the proportionate performance method.
Under certain arrangements, a customer pays us a bonus if we achieve certain milestones. Such bonus payment is recognized as revenue upon the achievement of the milestones.
Costs incurred prior to the delivery and acceptance of the deliverables are capitalized in inventory as work in progress. Cash received in advance of the delivery and acceptance of the deliverables is recorded as an advance from customers.
Revenues from the sale of manufactured products are recognized upon delivery and acceptance by the customer after title and risk of loss have been transferred.
Impairment of Long-Lived Assets
We review long-lived assets for impairment whenever events or changes in circumstances indicate the carrying amount of an asset may not be recoverable. Recoverability of these assets is measured by comparing their carrying amounts to the future undiscounted cash flows the assets are expected to generate. If the sum of the expected undiscounted cash flow is less than the carrying amount of the assets, we recognize an impairment loss equal to the amount by which the carrying value of the asset exceeds its fair value. We need to make judgments and estimates on expected cash flow and discount rates to derive fair value. No impairment of long-lived assets was recognized for the years ended December 31, 2007, 2008 and 2009 and the six months ended June 30, 2010.
Accounts Receivable
Accounts receivable are presented net of allowance for doubtful accounts. We provide specific allowance for doubtful accounts when facts and circumstances indicate that the collection is doubtful and a loss is probable and estimable. For the years ended December 31, 2007, 2008 and 2009, we recorded allowances for doubtful accounts of nil, $83,435 and $30,000, respectively, and did not write off any doubtful accounts for these periods. For the six months ended June 30, 2009 and 2010, we recorded allowances for doubtful accounts of $30,000 and $34,058, respectively, and wrote off doubtful accounts of nil and $83,435, respectively. Allowance for doubtful accounts was $113,435 as of December 31, 2009 and $64,058 as of June 30, 2010.
Income Taxes
Income taxes are accounted for under the asset and liability method. Deferred taxes are determined based upon differences between the financial reporting and tax bases of assets and liabilities at currently enacted statutory tax rates for the years in which the differences are expected to reverse. The effect on deferred taxes of a change in tax rates is recognized in the consolidated statements of operations in the period of change. A valuation allowance is provided on deferred tax assets to the extent that it is more likely than not that such deferred tax assets will not be realized. In assessing whether such deferred tax assets can be realized in the future, we need to make judgments and estimates on the ability to generate taxable income in future years. The total income tax provision includes current tax expenses under applicable tax regulations and the change in the balance of deferred tax assets and liabilities. We follow the guidance on accounting for uncertain tax positions in income taxes to recognize and measure our uncertain tax positions.
54
Share-Based Compensation Expenses
2008 Equity and Performance Incentive Plan
On May 19, 2008, we adopted the Plan to attract and retain the best available personnel and provide additional incentives to employees, directors and consultants. On February 24, 2010, we amended and restated the Plan to increase the maximum aggregate number of our shares that may be issued under the Plan to 36,562,358 shares. As of September 30, 2010, options to purchase 36,046,200 ordinary shares were outstanding. The following table sets forth the options granted since the adoption of the Plan that were outstanding as of September 30, 2010:
| | | | | | | | | | | | | | | | | | | | |
Date of Grant | | Options
Outstanding | | | Exercise
Price | | | Fair Value of
Ordinary Shares | | | Weighted-
Average Fair
Value of Options | | | Intrinsic Value | |
| | | | | | ($) | | | ($) | | | ($) | | | ($) | |
May 19, 2008 | | | 11,296,000 | | | | 0.50 | | | | 0.20 | | | | 0.04 | | | | — | |
October 27, 2008 | | | 6,177,050 | | | | 0.50 | | | | 0.21 | | | | 0.04 | | | | — | |
May 13, 2009 | | | 3,679,700 | | | | 0.50 | | | | 0.45 | | | | 0.20 | | | | — | |
October 14, 2009 | | | 3,815,700 | | | | 0.50 | | | | 0.55 | | | | 0.27 | | | | 0.05 | |
May 24, 2010 | | | 3,695,650 | | | | 0.65 | | | | 0.65 | | | | 0.30 | | |
| —
|
|
July 30, 2010 | | | 7,382,100 | | | | 0.65 | | | | 0.69 | | | | 0.33 | | | | 0.04 | |
Share-based compensation expense for all share-based awards granted to employees is determined based on the grant date fair value. Share-based compensation is expensed ratably on a straight-line basis for awards with vesting schedules based on service conditions only over the requisite service period, which is generally the vesting term of the share-based awards. A graded vesting method is applied to awards with vesting schedules with performance conditions.
Certain recipients of our share options became our consultants after their respective grant dates. We account for equity instruments issued to external consultants under the fair value method. The measurement date of the fair value of an equity instrument issued is the date on which the consultant’s performance is completed. Prior to the measurement date, the equity instrument is measured at its then-current fair value at each of the reporting dates. Share-based expense will be recognized over the service period using the graded-vesting method, and will be adjusted to reflect changes in the fair value of our ordinary shares between the reporting periods up to the measurement date.
We engaged Jones Lang LaSalle Sallmanns Limited, or Sallmanns, an independent third party appraiser, to assess the fair value of our share options and the ordinary shares underlying the options. We and Sallmanns estimated share-based compensation expense for share options on the grant date based on each option’s fair value as calculated using the Black-Scholes option pricing model and the assumptions set forth in the following table:
| | | | | | | | | | | | |
| | | For the Year Ended December 31, | | | For the Seven Months
Ended July 31,
2010 | |
| | | 2008 | | | 2009 | | |
Risk-free interest rate(1) | | | 4.42-5.34% | | | | 3-3.72% | | | | 2.75-3.37% | |
Expected life (in years)(2) | | | 5-7 years | | | | 5-7 years | | | | 5-7 years | |
Expected dividend yield(3) | | | 0% | | | | 0% | | | | 0% | |
Expected volatility(4) | | | 33-39% | | | | 42-43% | | | | 41-44% | |
Fair value per option at grant date | | | $0.02-0.05 | | | | $0.18-0.28 | | | | $0.28-0.35 | |
| (1) | The risk-free interest rate for the contractual term of the share option is based on the yield of China government bonds denominated in U.S. dollars in effect at the time of grant for a term consistent with the expected term of the awards. |
| (2) | The expected life of the share options granted under the Plan is based on vesting period, contractual term, share price, the employee’s level within our company and the expected volatility of the underlying shares. |
| (3) | We do not expect to pay dividends on our ordinary shares during the term of the options. |
| (4) | Expected volatility is estimated based on the historical volatilities of the comparable companies in a similar industry as of the valuation dates. |
55
As a private company with no quoted market for our ordinary shares, we need to estimate the fair value of our ordinary shares at the relevant grant dates. In determining the fair values of our ordinary shares as of each award grant date, Sallmanns considered three generally accepted valuation approaches: market, cost and income. The market approach requires market transactions of comparable assets as an indication of value. However, current comparable market transactions were not identified. The cost approach considers the cost to reproduce or replace the subject assets for similar assets at current market prices. While useful for certain purposes, the cost approach is generally considered not applicable to the valuation of a company as a going concern, as it does not capture the future earning potential of the business. In view of the above, Sallmanns determined that the income approach is the most appropriate method to derive the fair values of our ordinary shares.
The income approach involved applying appropriate discount rates to projected cash flows using management’s best estimates as of the valuation dates. The projected cash flows reflected, among other things, an analysis of projected revenue growth, profit margins, effective tax rates, capital expenditures and terminal multiples. The major assumptions used in determining the equity value of our company were consistent with our business plan and major milestones that we achieved. Other major assumptions used in determining the equity value of our company included the following:
| | • | | weighted average costs of capital of 13.6%, 13.9%, 14.4%, 13.6%, 14.9% and 15.0% were used on May 19, 2008, October 27, 2008, May 13, 2009, October 14, 2009, May 24, 2010 and July 30, 2010, respectively. They were the combined results of changes in the risk-free rate, equity risk premium, industry average beta, country premium and size premium; and |
| | • | | discounts for lack of marketability of 17.6%, 17.6%, 17.6%, 17.6%, 7.5% and 8.0% were used on May 19, 2008, October 27, 2008, May 13, 2009, October 14, 2009, May 24, 2010 and July 30, 2010, respectively, to reflect that, as of these grant dates, we were a closely-held company and there was no public market for our ordinary shares. This took into consideration our proposed initial public offering in the near future. |
We and Sallmanns also used other general inherently uncertain but best available assumptions, including the following: no material changes in the existing political, legal, fiscal and economic conditions and the CRO industry in China; no significant contingent liabilities, unusual contractual obligations or substantial commitments; our ability to retain competent management and key personnel to support our ongoing operations; and no material deviation in market conditions from economic forecasts.
The valuation model then allocated the equity value between our ordinary shares and our preferred shares. The fair value of the equity interest allocated to the preferred shares was calculated using the option pricing method. Under the option pricing method, the preferred shares were treated as a call option on our equity value, with the exercise price based on the liquidation preference of the preferred shares. Because a call option was used, the option pricing method commonly used is the Black-Scholes model, which takes into account the expected life of the option, a risk-free interest rate, dividend yield and volatility. Because we are a private company, Sallmanns approximated volatility using the historical volatility of selected comparable publicly traded CROs listed in the U.S. In selecting such comparable companies, Sallmanns considered factors including the nature of business, product offerings in the drug R&D process, target markets and customers, financial performance and stage of development.
Based on the foregoing analysis and valuation performed by Sallmanns, we concluded that the fair value of our ordinary shares was $0.20, $0.21, $0.45, $0.55, $0.65 and $0.69 per share as of May 19, 2008, October 27, 2008, May 13, 2009, October 14, 2009, May 24, 2010 and July 30, 2010, respectively.
The fair value of our ordinary shares only increased slightly from May 19, 2008 to October 27, 2008 because there were no significant developments in our business or changes in cash flow projections or other assumptions during this period.
56
The increase in the fair value of our ordinary shares from October 27, 2008 to May 13, 2009 was primarily attributable to the following developments during such period:
| | • | | in response to the global financial crisis and economic downturn, we strengthened our measures to control costs and capital expenditures and improved operational efficiency, which were expected to sustain our gross and net profit margins for the following few years. For example, we established a monitoring and control system to achieve efficient staffing, improve procurement efficiency and maximize utilization of equipment; |
| | • | | we changed the construction schedule of Phase B of the manufacturing facility in Fengxian, Shanghai, so that we would start the construction of Phase B after Phase A achieves a satisfactory utilization rate (instead of shortly after Phase A commences operation), which was expected to reduce our capital expenditures in the near term; and |
| | • | | the Renminbi had appreciated at a slower pace, or approximately 0.4% from October 2008 to May 2009, and was expected to continue appreciating at a relatively slow pace, which benefited our results of operations. |
The increase in the fair value of our ordinary shares from May 13, 2009 to October 14, 2009 was primarily attributable to the following developments during such period:
| | • | | we strengthened our measures to control costs and capital expenditures and improved operational efficiency, which were expected to sustain our gross and net profit margins for the following few years; and |
| | • | | a decrease in weighted average costs of capital from 14.4% to 13.6% as a result of relevant capital market developments, including changes in the risk-free rate, equity risk premium and size premium. |
The increase in the fair value of our ordinary shares from October 14, 2009 to May 24, 2010 was primarily attributable to the following developments during such period:
| | • | | a decrease in the discount for lack of marketability from 17.6% to 7.5% as we prepared for our initial public offering in the first half of 2010; |
| | • | | we experienced increases in net revenues and net income from $19.8 million and $2.5 million, respectively, in the fourth quarter of 2009 to $21.8 million and $4.1 million, respectively, in the second quarter of 2010; and |
| | • | | our gross margin increased from 32.6% in the fourth quarter of 2009 to 33.9% in the second quarter of 2010, due in part to our measures to control costs and capital expenditures and our improved operational efficiency. |
The increase in the fair value of our ordinary shares from May 24, 2010 to July 30, 2010 was primarily attributable to the increased probability that we would undertake our proposed initial public offering.
We have considered the guidance prescribed by the American Institute of Certified Public Accountants (AICPA) Audit and Accounting Practice Aid,Valuation of Privately-Held-Company Equity Securities Issued as Compensation, or the Practice Aid, in determining the fair value of our ordinary shares as of various dates before this offering. A detailed description of the valuation method used and the factors contributing to the changes in the fair value of our ordinary shares through July 30, 2010 is set out above. Paragraph 113 of the Practice Aid states that “the ultimate IPO price itself also is generally not likely to be a reasonable estimate of the fair value for pre-IPO equity transactions of the enterprise.” We therefore believe the ultimate price of this offering is generally not likely to be a reasonable estimate of the fair value of our ordinary shares as of various dates before this offering.
57
Nevertheless, we believe that the implied increase in fair value of our ordinary shares from $0.69 per share as of July 30, 2010 to the initial public offering price (after adjusting for the ADS to ordinary share ratio), is primarily attributable to the following factors:
| | • | | The impending launch of this offering is expected to provide us with additional capital, enhance our ability to access capital markets and raise our profile; and |
| | • | | The completion of this offering is expected to result in increased liquidity and marketability of our ordinary shares. |
Our share-based compensation expense related to the options granted by us under the Plan amounted to $68,752 in 2008, $251,950 in 2009 and $353,370 in the six months ended June 30, 2010. Determining the value of our share-based compensation expenses requires the input of highly subjective assumptions, including the expected life of the share-based awards, estimated forfeitures and the price volatility of the underlying shares. The assumptions used in calculating the fair value of share-based awards represent our best estimates, but these estimates involve inherent uncertainties and the application of our judgment. As a result, if factors change and we use different assumptions, our share-based compensation expenses could be materially different in the future.
Founder’s 2008 Equity and Performance Incentive Plan
In May 2008, our founders and our company established the Founder’s Plan to attract, motivate and retain our employees, non-employee directors and consultants. See “Management—Founder’s 2008 Equity and Performance Incentive Plan” for a summary of the key terms of the Founder’s Plan.
In 2008, 2009 and the nine months ended September 30, 2010, our founders granted RSUs convertible to 7,450,000, 2,100,000 and 2,200,000 ordinary shares, respectively, to certain members of our senior management and other employees under the Founder’s Plan. We estimated the share-based compensation expense for RSUs granted under the Founder’s Plan based on the fair value of the RSUs on the date of grant. We have not recorded any share-based compensation expense in connection with these RSU grants because one of the vesting conditions for the RSUs is the completion of our initial public offering. As a result, immediately upon completion of this offering, we expect to incur a significant share-based compensation charge of approximately $2.5 million, which will materially and adversely affect our results of operations for the quarter in which this offering is completed.
Internal Control over Financial Reporting
In preparing our consolidated financial statements, we and our independent registered public accounting firm identified one material weakness and other deficiencies in our internal control over financial reporting as of December 31, 2009. As defined in standards established by the PCAOB, a “material weakness” is a deficiency, or combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the annual or interim financial statements will not be prevented or detected on a timely basis.
The material weakness identified related to a lack of sufficient competent personnel with appropriate levels of accounting knowledge and experience to perform period end reporting procedures, address complex U.S. GAAP accounting issues and prepare and review financial statements and related disclosures under U.S. GAAP. Neither we nor our independent registered public accounting firm undertook a comprehensive assessment of our internal control for purposes of identifying and reporting material weaknesses and other control deficiencies in our internal control over financial reporting, as we and they will be required to do once we become a public company. Had we performed a formal assessment of our internal control over financial reporting or had our independent registered public accounting firm performed an audit of our internal control over financial reporting, additional control deficiencies may have been identified.
58
Following the identification of this material weakness and other control deficiencies and in connection with preparation of our consolidated financial statements, we performed additional review procedures, including a thorough review of journal entries and reconciliations for key accounts, to ensure the completeness and accuracy of the consolidated financial statements prepared in accordance with U.S. GAAP.
To remedy our control deficiencies, we have adopted several measures to improve our internal control over financial reporting. We recently hired additional finance and accounting personnel, including a finance controller and a finance manager with U.S. GAAP and SEC reporting experience. In addition, we have (i) provided, and intend to continue to provide, on-going training to our accounting and operating personnel across different subsidiaries and departments to improve their U.S. GAAP accounting knowledge; (ii) established and strengthened our IT systems and controls related to the IT systems to ensure proper record keeping; (iii) developed comprehensive quarterly closing procedures that require certain documentation and records to be completed and reviewed; (iv) updated and improved our accounting manual; (v) hired an internal audit director with prior experience at a China-based company listed in the United States; and (vi) engaged an outside consultant to assist us in preparing for compliance with Section 404 of the Sarbanes-Oxley Act. We are also in the process of, among other things, evaluating our financial accounting system for adequacy and potential upgrades. We will continue to implement measures to remedy our internal control deficiencies in order to meet the deadline imposed by Section 404 of the Sarbanes-Oxley Act. However, the implementation of these measures may not fully address the deficiencies in our internal control over financial reporting. We are not able to estimate with reasonable certainty the costs that we will need to incur to implement these and other measures designed to improve our internal control over financial reporting. See “Risk Factors—Risks Related to Our Business and Industry—In preparing our consolidated financial statements, we have identified one material weakness and other deficiencies in our internal control over financial reporting. If we fail to develop and maintain an effective system of internal control over financial reporting, we may be unable to accurately report our financial results or prevent fraud, and investor confidence in our company and the market price of our ADSs may be adversely affected.”
59
Results of Operations
The following table sets forth a summary of our consolidated results of operations by amount and as a percentage of our total net revenues for the periods indicated. This information should be read together with our consolidated financial statements and related notes included elsewhere in this prospectus. The operating results in any period are not necessarily indicative of the results that may be expected for any future period.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Year Ended December 31, | | | Six Months Ended June 30, | |
| | | 2007 | | | 2008 | | | 2009 | | | 2009 | | | 2010 | |
| | | Net
Revenues | | | % | | | Net
Revenues | | | % | | | Net
Revenues | | | % | | | Net
Revenues | | | % | | | Net
Revenues | | | % | |
| | | (in millions of $, except for percentages) | |
| | | | | | | | | | |
Net revenues | | | 34.7 | | | | 100.0 | % | | | 60.5 | | | | 100.0 | % | | | 72.3 | | | | 100.0 | % | | | 32.7 | | | | 100.0 | % | | | 41.6 | | | | 100.0 | % |
Cost of revenues | | | (22.8 | ) | | | (65.8 | ) | | | (40.5 | ) | | | (67.0 | ) | | | (48.4 | ) | | | (67.0 | ) | | | (21.8 | ) | | | (66.7 | ) | | | (27.2 | ) | | | (65.5 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Gross profit | | | 11.9 | | | | 34.2 | | | | 20.0 | | | | 33.0 | | | | 23.9 | | | | 33.0 | | | | 10.9 | | | | 33.3 | | | | 14.4 | | | | 34.5 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Operating expenses: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Selling and marketing | | | (0.5 | ) | | | (1.5 | ) | | | (0.8 | ) | | | (1.4 | ) | | | (1.7 | ) | | | (2.2 | ) | | | (0.7 | ) | | | (1.9 | ) | | | (1.1 | ) | | | (2.6 | ) |
General and administrative | | | (4.0 | ) | | | (11.5 | ) | | | (9.3 | ) | | | (15.3 | ) | | | (12.0 | ) | | | (16.6 | ) | | | (5.6 | ) | | | (17.3 | ) | | | (6.9 | ) | | | (16.6 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total operating expenses | | | (4.5 | ) | | | (13.0 | ) | | | (10.1 | ) | | | (16.7 | ) | | | (13.7 | ) | | | (18.8 | ) | | | (6.3 | ) | | | (19.2 | ) | | | (8.0 | ) | | | (19.2 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Profit from operations | | | 7.4 | | | | 21.2 | | | | 9.9 | | | | 16.3 | | | | 10.2 | | | | 14.2 | | | | 4.6 | | | | 14.1 | | | | 6.4 | | | | 15.3 | |
Interest income | | | 0.2 | | | | 0.6 | | | | 0.2 | | | | 0.4 | | | | * | | | | ** | | | | * | | | | ** | | | | * | | | | ** | |
Interest expense | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | * | | | | ** | |
Other income | | | 0.2 | | | | 0.6 | | | | 0.9 | | | | 1.5 | | | | 1.3 | | | | 1.8 | | | | 0.8 | | | | 2.5 | | | | 1.6 | | | | 3.9 | |
Other expenses | | | (0.1 | ) | | | (0.3 | ) | | | (1.6 | ) | | | (2.7 | ) | | | (0.2 | ) | | | (0.3 | ) | | | (0.1 | ) | | | (0.3 | ) | | | (0.2 | ) | | | (0.4 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Income from operations before income taxes | | | 7.7 | | | | 22.1 | | | | 9.4 | | | | 15.5 | | | | 11.4 | | | | 15.7 | | | | 5.3 | | | | 16.3 | | | | 7.8 | | | | 18.8 | |
Income taxes | | | (0.2 | ) | | | (0.5 | ) | | | (0.3 | ) | | | (0.5 | ) | | | (1.6 | ) | | | (2.1 | ) | | | (0.7 | ) | | | (2.3 | ) | | | (0.9 | ) | | | (2.3 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net income | | | 7.5 | | | | 21.6 | % | | | 9.1 | | | | 15.0 | % | | | 9.8 | | | | 13.6 | % | | | 4.6 | | | | 14.0 | % | | | 6.9 | | | | 16.5 | % |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Six Months Ended June 30, 2010 Compared to Six Months Ended June 30, 2009
Net Revenues
Our net revenues increased by 27.2% from $32.7 million in the six months ended June 30, 2009 to $41.6 million in the six months ended June 30, 2010, primarily due to an increase in the number of customers and our expanded scope of services. We provided services to 151 customers in the six months ended June 30, 2010 compared to 88 customers in the same period ended June 30, 2009. The following table sets forth our FTE-based net revenues and fee-for-service-based net revenues by amount and as a percentage of our total net revenues for the periods indicated:
| | | | | | | | | | | | | | | | |
| | | Six Months Ended June 30, | |
| | | 2009 | | | 2010 | |
| | | Net
Revenues | | | % | | | Net
Revenues | | | % | |
| | | (in millions of $, except for percentages) | |
| | | | |
FTE-based net revenues | | | 26.5 | | | | 81.2 | % | | | 30.9 | | | | 74.4 | % |
Fee-for-service-based net revenues | | | 6.2 | | | | 18.8 | | | | 10.7 | | | | 25.6 | |
| | | | | | | | | | | | | | | | |
Total net revenues | | | 32.7 | | | | 100.0 | % | | | 41.6 | | | | 100.0 | % |
| | | | | | | | | | | | | | | | |
60
Net revenues from FTE-based services increased by 16.3% from $26.5 million in the six months ended June 30, 2009 to $30.9 million in the six months ended June 30, 2010, due to increases in the average number of FTEs and net revenue per FTE. Net revenues from our fee-for-service-based services increased by 74.7% from $6.2 million in the six months ended June 30, 2009 to $10.7 million in the six months ended June 30, 2010. The increase in demand for our FTE-based and fee-for-service-based services was primarily due to an increase in demand for our pharmaceutical development, discovery biology and preclinical development and discovery chemistry services. FTE-based revenues as a percentage of our total net revenues decreased from 81.2% in the six months ended June 30, 2009 to 74.4% in the six months ended June 30, 2010 as we expanded our newer services offerings, such as discovery biology and preclinical development and pharmaceutical development, which are more typically covered by fee-for-service contracts.
Cost of Revenues
Our cost of revenues increased by 24.9% from $21.8 million in the six months ended June 30, 2009 to $27.2 million in the six months ended June 30, 2010. The increase in our cost of revenues was primarily due to increases in labor, raw materials and overhead costs. The increase in our labor costs was primarily due to higher headcount and the hiring of more senior scientists for our newer services, such as biologics, as well as overall rising labor costs in China. Such increase was partially offset by efficiencies gained by our scientists and by our entry into higher margin services. Our raw material costs as a percentage of our total net revenues decreased from the six months ended June 30, 2009 to the same period ended June 30, 2010 due to our cost control measures, such as improving raw materials usage efficiencies, as well as our increased bargaining power resulting from economies of scale. Our overhead costs increased as we expanded our facilities and purchased new equipment.
Gross Profit and Margin
Our gross profit increased by 31.9% from $10.9 million in the six months ended June 30, 2009 to $14.3 million in the six months ended June 30, 2010, primarily due to the increase in our net revenues. Our gross margin increased from 33.3% in the six months ended June 30, 2009 to 34.5% in the six months ended June 30, 2010, mainly attributable to our improved cost control measures, growth in higher margin services, economies of scale and improvements in operational efficiency. Such increase was partially offset by increased labor costs, particularly those of newly hired senior scientists for our newer services still in development.
Operating Expenses
Our total operating expenses increased by 27.2% from $6.3 million in the six months ended June 30, 2009 to $8.0 million in the six months ended June 30, 2010.
Selling and Marketing Expenses. Our selling and marketing expenses increased by 71.4% from $0.7 million in the six months ended June 30, 2009 to $1.1 million in the six months ended June 30, 2010. The increase in selling and marketing expenses was primarily due to our hiring of new sales and business development personnel in the U.S. and Europe in the second half of 2009 and the first half of 2010 to increase market penetration and demand for our services in those regions.
General and Administrative Expenses. Our general and administrative expenses increased by 22.3% from $5.6 million in the six months ended June 30, 2009 to $6.9 million in the six months ended June 30, 2010, primarily due to an increase in the headcount of our senior management and administrative employees.
Other Income
Our other income increased from $0.8 million in the six months ended June 30, 2009 to $1.6 million in the six months ended June 30, 2010, primarily due to a gain of $1.0 million on foreign exchange forward
61
contracts in the six months ended June 30, 2010, compared to a gain of $0.1 million on such contracts in the six months ended June 30, 2009.
Income Tax Expense
Our income tax expense increased from $0.7 million in the six months ended June 30, 2009 to $0.9 million in the six months ended June 30, 2010, reflecting an increase in income before tax, partially offset by a decrease in our effective tax rate from 14% to 12%.
Net Income
As a result of the foregoing, our net income increased from $4.6 million in the six months ended June 30, 2009 to $6.9 million in the six months ended June 30, 2010.
Year Ended December 31, 2009 Compared to Year Ended 31, December 2008
Net Revenues
Our net revenues increased by 19.5% from $60.5 million in 2008 to $72.3 million in 2009, primarily due to an increase in the number of customers and our expanded scope of services. We provided services to 134 customers in 2009 compared to 99 customers in 2008. The following table sets forth our FTE-based net revenues and fee-for-service-based net revenues by amount and as a percentage of our total net revenues for the periods indicated:
| | | | | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2008 | | | 2009 | |
| | | Net
Revenues | | | % | | | Net
Revenues | | | % | |
| | | (in millions of $, except for percentages) | |
| | | | |
FTE-based net revenues | | | 47.8 | | | | 79.1 | % | | | 56.7 | | | | 78.4 | % |
Fee-for-service-based net revenues | | | 12.7 | | | | 20.9 | | | | 15.6 | | | | 21.6 | |
| | | | | | | | | | | | | | | | |
Total net revenues | | | 60.5 | | | | 100.0 | % | | | 72.3 | | | | 100.0 | % |
| | | | | | | | | | | | | | | | |
Net revenues from FTE-based services increased by 18.4% from $47.8 million in 2008 to $56.7 million in 2009 due to increases in the average number of FTEs and net revenue per FTE. Net revenues from our fee-for-service-based services increased by 23.5% from $12.7 million in 2008 to $15.6 million in 2009. The increase in demand for our FTE-based and fee-for-service-based services was primarily due to an increase in demand for our discovery biology and preclinical development and discovery chemistry services. FTE-based revenues as a percentage of our total net revenues decreased from 79.1% in 2008 to 78.4% in 2009 as we expanded our newer service offerings, such as discovery biology and preclinical development and pharmaceutical development, which are more typically covered by fee-for-service contracts.
Cost of Revenues
Our cost of revenues increased by 19.5% from $40.5 million in 2008 to $48.4 million in 2009, in line with the increase in our net revenues. The increase in our cost of revenues was primarily due to increases in labor, raw materials and overhead costs. Our labor costs increased from 2008 to 2009 primarily due to the increase in our headcount and the hiring of more senior scientists for our newer services, such as discovery biology and preclinical development, as well as overall rising labor costs in China. Such increase was partially offset by efficiencies gained by our scientists and by our entry into higher margin services. Our raw material costs as a percentage of our total net revenues decreased from 2008 to 2009 due to our cost control measures,
62
such as improving raw materials usage efficiencies, as well as our increased bargaining power resulting from economies of scale. Our overhead costs increased as we expanded our facilities and purchased new equipment.
Gross Profit and Margin
Our gross profit increased by 19.4% from $20.0 million in 2008 to $23.9 million in 2009, primarily due to the increase in our net revenues. Our gross margin was 33.0% in each of 2008 and 2009. Our gross margin was negatively impacted in 2009 by our increased labor costs, particularly those of newly hired senior scientists for our newer services still in development. These adverse effects were offset by our improved cost control measures, growth in higher margin services, economies of scale and improvements in operational efficiency.
Operating Expenses
Our total operating expenses increased by 34.8% from $10.1 million in 2008 to $13.6 million in 2009.
Selling and Marketing Expenses. Our selling and marketing expenses increased by 93.0% from $0.8 million in 2008 to $1.6 million in 2009. The increase in selling and marketing expenses in 2009 was partially due to our hiring of several sales and business development personnel in the U.S. and Europe in 2009 to increase market penetration and demand for our services in those regions.
General and Administrative Expenses. Our general and administrative expenses increased by 29.5% from $9.3 million in 2008 to $12.0 million in 2009, primarily due to an increase in the headcount of our senior management and administrative employees.
Other Income
Our other income increased from $0.9 million in 2008 to $1.3 million in 2009, primarily due to an increase in government subsidies.
Other Expenses
Our other expenses decreased from $1.6 million in 2008 to $0.2 million in 2009, primarily due to a gain on foreign exchange forward contracts in 2009 compared to a loss on such contracts in 2008.
Income Tax Expense
Our income tax expense increased from $0.3 million in 2008 to $1.6 million in 2009 primarily reflecting (i) an increase in our effective tax rate from 3% in 2008 to 14% in 2009 as a result of the expiration of the full tax exempt status of some of our PRC subsidiaries and (ii) an increase in income before taxes. See “—Selected Statements of Operations Items—Income Taxes.”
Net Income
As a result of the foregoing, our net income increased from $9.1 million in 2008 to $9.8 million in 2009.
Year Ended December 31, 2008 Compared to Year Ended December 31, 2007
Net Revenues
Our net revenues increased by 74.5% from $34.7 million in 2007 to $60.5 million in 2008, primarily due to an increase in the number of customers and our expanded scope of services. We provided services to 99 customers in 2008 compared to 71 customers in 2007. Our average net revenues per top ten customer increased
63
from $2.9 million in 2007 to $4.7 million in 2008. The following table sets forth our FTE-based net revenues and fee-for-service-based net revenues by amount and as a percentage of our total net revenues for the periods indicated:
| | | | | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2007 | | | 2008 | |
| | | Net Revenues | | | % | | | Net Revenues | | | % | |
| | | (in millions of $, except for percentages) | |
| | | | |
FTE-based net revenues | | | 29.9 | | | | 86.0 | % | | | 47.8 | | | | 79.1 | % |
Fee-for-service-based net revenues | | | 4.8 | | | | 14.0 | | | | 12.7 | | | | 20.9 | |
| | | | | | | | | | | | | | | | |
Total net revenues | | | 34.7 | | | | 100.0 | % | | | 60.5 | | | | 100.0 | % |
| | | | | | | | | | | | | | | | |
Net revenues from FTE-based services increased by 60.4% from $29.9 million in 2007 to $47.8 million in 2008 due to increases in the average number of FTEs and net revenue per FTE. Net revenues from our fee-for-service-based services increased by 161.1% from $4.8 million in 2007 to $12.7 million in 2008. The increase in demand for our FTE-based and fee-for-service-based services was primarily due to an increase in demand for our core discovery chemistry services and, to a lesser extent, for our newer services, such as discovery biology and preclinical development and pharmaceutical development, which grew at a higher rate than discovery chemistry. FTE-based revenues as a percentage of our total net revenues decreased from 86.0% in 2007 to 79.1% in 2008 as we expanded our newer service offerings, such as discovery biology and preclinical development and pharmaceutical development, which are more typically covered by fee-for-service contracts.
Cost of Revenues
Our cost of revenues increased by 77.6% from $22.8 million in 2007 to $40.5 million in 2008, in line with the increase in our net revenues. The increase in our cost of revenues was primarily due to increases in labor, raw materials and overhead costs. Our labor costs increased from 2007 to 2008 primarily due to the increase in our headcount, appreciation of Renminbi relative to the U.S. dollar and the hiring of more senior scientists for our newer services, as well as overall rising labor costs in China. Such increase was partially offset by efficiencies gained by our scientists and by our entry into higher margin services. Our raw material costs as a percentage of our total net revenues decreased from 2007 to 2008 due to our cost control measures, such as improving raw materials usage efficiencies, as well as our increased bargaining power resulting from economies of scale. Our overhead costs increased from 2007 to 2008 partially due to depreciation of expanded or improved facilities and equipment.
Gross Profit and Margin
Our gross profit increased by 68.5% from $11.9 million in 2007 to $20.0 million in 2008, primarily due to the increase in our net revenues. Our gross margin decreased from 34.2% in 2007 to 33.0% in 2008. Our gross margin was negatively impacted in 2008 by the appreciation of the Renminbi relative to the U.S. dollar and increased labor costs, particularly those of newly hired senior scientists for our newer services that were still at the development stage. These adverse effects were partially offset by our improved cost control measures, growth in higher margin services, increases in net revenue per FTE, economies of scale and improvements in operational efficiency.
Operating Expenses
Our total operating expenses increased by 124.8% from $4.5 million in 2007 to $10.1 million in 2008.
Selling and Marketing Expenses. Our selling and marketing expenses increased by 61.2% from $0.5 million in 2007 to $0.8 million in 2008. The increase in selling and marketing expenses in 2008 was primarily due to the hiring of more sales and business development personnel.
64
General and Administrative Expenses. Our general and administrative expenses increased by 133.1% from $4.0 million in 2007 to $9.3 million in 2008, primarily due to (i) an increase in the headcount of our senior management and administrative employees, (ii) an increase in professional services expenses, principally auditing and general consulting fees and (iii) an increase in depreciation and amortization of property, equipment and software.
Other Income
Our other income increased from $0.2 million in 2007 to $0.9 million in 2008, primarily due to an increase in rental income from the sublease of excess space at the leased animal facility in Shanghai Zhangjiang Hi-Tech Park and an increase in government subsidies.
Other Expenses
Our other expenses increased from $0.1 million in 2007 to $1.6 million in 2008, primarily due to an increase in foreign exchange loss and a loss on foreign exchange forward contracts in 2008.
Income Tax Expense
Our income tax expense increased from $0.2 million in 2007 to $0.3 million in 2008.
Net Income
As a result of the foregoing, our net income increased from $7.5 million in 2007 to $9.1 million in 2008.
65
Selected Quarterly Results of Operations
The following table sets forth our unaudited condensed consolidated quarterly results of operations for the six quarters ended June 30, 2010. You should read the following table in conjunction with our consolidated financial statements and the related notes included elsewhere in this prospectus. We have prepared the unaudited condensed consolidated quarterly financial information on the same basis as we have prepared our audited consolidated financial statements. The unaudited condensed consolidated financial information includes all adjustments, consisting only of normal and recurring adjustments, that we consider necessary for a fair statement of our financial position and operating results for the quarters presented.
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | Three Months Ended | |
| | | March 31,
2009 | | | June 30,
2009 | | | September 30,
2009 | | | December 31,
2009 | | | March 31,
2010 | | | June 30,
2010 | |
| | | (in millions of $) | |
Net revenues | | | 15.1 | | | | 17.6 | | | | 19.8 | | | | 19.8 | | | | 19.7 | | | | 21.8 | |
Cost of revenues | | | (9.9 | ) | | | (11.9 | ) | | | (13.3 | ) | | | (13.3 | ) | | | (12.8 | ) | | | (14.4 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Gross profit | | | 5.2 | | | | 5.7 | | | | 6.5 | | | | 6.5 | | | | 6.9 | | | | 7.4 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Operating expenses: | | | | | | | | | | | | | | | | | | | | | | | | |
Selling and marketing | | | (0.3 | ) | | | (0.3 | ) | | | (0.4 | ) | | | (0.5 | ) | | | (0.5 | ) | | | (0.6 | ) |
General and administrative | | | (2.7 | ) | | | (3.0 | ) | | | (3.1 | ) | | | (3.3 | ) | | | (3.3 | ) | | | (3.6 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Total operating expenses | | | (3.0 | ) | | | (3.3 | ) | | | (3.5 | ) | | | (3.8 | ) | | | (3.8 | ) | | | (4.2 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Profit from operations | | | 2.2 | | | | 2.4 | | | | 3.0 | | | | 2.7 | | | | 3.1 | | | | 3.2 | |
Interest income | | | * | | | | * | | | | * | | | | * | | | | * | | | | * | |
Interest expense | | | — | | | | — | | | | — | | | | — | | | | * | | | | * | |
Other income | | | 0.2 | | | | 0.6 | | | | 0.1 | | | | 0.3 | | | | 0.2 | | | | 1.4 | |
Other expenses | | | (0.1 | ) | | | * | | | | * | | | | (0.1 | ) | | | (0.2 | ) | | | * | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Income from operations before income taxes | | | 2.3 | | | | 3.0 | | | | 3.1 | | | | 2.9 | | | | 3.1 | | | | 4.6 | |
Income taxes | | | (0.3 | ) | | | (0.4 | ) | | | (0.4 | ) | | | (0.4 | ) | | | (0.4 | ) | | | (0.5 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Net income | | | 2.0 | | | | 2.6 | | | | 2.7 | | | | 2.5 | | | | 2.7 | | | | 4.1 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Our quarterly net revenues generally increased in the six quarters ended June 30, 2010, although our net revenues were flat from the third quarter of 2009 to the first quarter of 2010. The general increases in our quarterly net revenues were attributable primarily to a larger customer base and a broader scope of services we provided to our customers. The global pharmaceutical and biotechnology industry has experienced significant consolidation and, during the global financial crisis, some pharmaceutical and biotechnology companies experienced financial difficulties. As a result, some of our customers reduced their R&D budgets in 2009. We believe our general growth in net revenues in 2009 and the first half of 2010 despite industry consolidation and the global financial crisis reflected our customers’ recognition of our high-quality, integrated services. Our net revenues were flat from the third quarter of 2009 to the first quarter of 2010, primarily due to (i) consolidation in the global pharmaceutical and biotechnology industry and the global financial crisis in late 2009 and (ii) fewer working days in the first quarter in the PRC as a result of traditional Chinese holidays. Our quarterly revenues and operating results may continue to experience fluctuations in the future due to numerous factors. See “Risk Factors—Risks Related to Our Business and Industry.”
Liquidity and Capital Resources
Our primary sources of liquidity have historically been cash generated from operating and financing activities, which consisted of a private placement of preferred shares. As of June 30, 2010, we had approximately
66
$10.0 million in cash. As of September 30, 2010, we had an available short-term revolving bank credit facility for up to an aggregate principal amount of $5.0 million, under which the actual amounts available for drawdown are subject to, among other things, the amounts of approved accounts receivable that we assign to the relevant bank. As of the same date, we had a short-term revolving bank credit facility of $2.0 million and a three-year term loan commitment of $5.0 million from the same bank, both of which will be secured by properties owned by one of our subsidiaries. No amounts had been drawn down under these facilities and term loan commitment as of September 30, 2010. In addition, we had short-term revolving bank credit facilities of RMB35.0 million, or $5.1 million. As of September 30, 2010, no amounts had been drawn down under these facilities. In July 2010, one of our subsidiaries entered into a loan agreement with a bank for a one-year working capital loan of up to RMB10.0 million ($1.5 million). As of September 30, 2010, we had drawn down RMB5.0 million ($0.7 million) under this loan.
We expect to require cash to fund our ongoing business needs, particularly salary and benefits and material costs and expenses. Other cash needs include primarily the working capital for our daily operations, expenditures related to our manufacturing facility and laboratory services building in Fengxian, Shanghai, and purchase of equipment for our laboratory services. We believe that our cash, anticipated cash flow from operations, the net proceeds we expect to receive from this offering and proceeds from our bank credit facilities will be sufficient to meet our anticipated cash needs for the next 12 months, including the construction of our manufacturing facility and laboratory services building in Fengxian, Shanghai. The following table sets forth a summary of our cash flows for the periods indicated:
| | | | | | | | | | | | | | | | | | | | |
| | | For the Year Ended December 31, | | | For the Six Months
ended June 30, | |
| | | 2007 | | | 2008 | | | 2009 | | | 2009 | | | 2010 | |
| | | (in millions of $) | |
| | | | | |
Net cash provided by operating activities | | | 8.5 | | | | 10.2 | | | | 14.7 | | | | 5.0 | | | | 3.8 | |
Net cash used in investing activities | | | (9.0 | ) | | | (14.1 | ) | | | (18.3 | ) | | | (13.2 | ) | | | (8.1 | ) |
Net cash provided in financing activities | | | 17.7 | | | | — | | | | — | | | | — | | | | 2.1 | |
Effect of foreign exchange rate changes on cash | | | 0.1 | | | | 0.1 | | | | * | | | | * | | | | * | |
| | | | | | | | | | | | | | | | | | | | |
Net increase (decrease) in cash | | | 17.3 | | | | (3.7 | ) | | | (3.6 | ) | | | (8.2 | ) | | | (2.2 | ) |
Cash at the beginning of the year | | | 2.3 | | | | 19.6 | | | | 15.8 | | | | 15.8 | | | | 12.2 | |
| | | | | | | | | | | | | | | | | | | | |
Cash at the end of the year | | | 19.6 | | | | 15.8 | | | | 12.2 | | | | 7.6 | | | | 10.0 | |
| | | | | | | | | | | | | | | | | | | | |
Operating Activities
Net cash provided by operating activities consisted primarily of our net income increased by non-cash adjustments, such as depreciation and amortization of property, equipment and software, and adjusted by changes in assets and liabilities, such as accounts receivable, accounts payable and amounts due to related parties. Net cash provided by operating activities increased, year over year, primarily as a result of increases in net income, driven by our expanding revenue base. The increases in our accounts receivable were primarily due to the growth of our business and timing of revenue recognized and collection of accounts receivable. The increases in our accounts payable and amounts due to related parties, which are primarily our suppliers, were largely due to increased purchases related to the growth of our business and the timing of our payables.
Net cash provided by operating activities amounted to $3.8 million in the six months ended June 30, 2010, primarily attributable to net income of $6.9 million and add-back of certain non-cash adjustments such as depreciation and amortization of $3.2 million, partially offset by an increase of $2.8 million in accounts receivable.
Net cash provided by operating activities amounted to $14.7 million in 2009, primarily attributable to net income of $9.8 million, and add-back of certain non-cash adjustments such as depreciation and amortization
67
of $5.9 million and an increase of $1.5 million in accounts payable, partially offset by an increase of $4.8 million in accounts receivable.
Net cash provided by operating activities amounted to $10.2 million in 2008, primarily attributable to net income of $9.1 million and an add-back of certain non-cash adjustments such as depreciation and amortization of $4.3 million, partially offset by an increase of $3.8 million in accounts receivable and a decrease of $1.7 million in income taxes payable related to the payment of income taxes for the years before 2007.
Net cash provided by operating activities amount to $8.5 million in 2007, primarily attributable to net income of $7.5 million, an add-back of certain non-cash adjustments such as depreciation and amortization of $2.1 million and an increase of $1.6 million in amounts due to related parties, partially offset by an increase of $4.1 million in accounts receivable.
Investing Activities
Net cash used in investing activities largely reflects our capital expenditures, which consist of purchases of property, equipment and software made in connection with the expansion and upgrade of our laboratory facilities as well as purchase of land use rights. We expect our net cash used in investing activities in 2010 to increase as we continue constructing our manufacturing facility and laboratory services building in Fengxian, Shanghai.
Net cash used in investing activities amounted to $8.1 million in the six months ended June 30, 2010, primarily attributable to $7.9 million in purchase of property, equipment and software.
Net cash used in investing activities amounted to $18.3 million in 2009, primarily attributable to $14.0 million in purchases of property, equipment and software and $4.2 million in purchase of land use rights for our manufacturing facility in Fengxian, Shanghai.
Net cash used in investing activities amounted to $14.1 million in 2008, primarily attributable to $13.6 million in purchases of property, equipment and software.
Net cash used in investing activities amounted to $9.0 million in 2007, primarily attributable to $8.9 million in purchases of property, equipment and software.
Financing Activities
Net cash provided by financing activities amounted to $2.1 million for the six months ended June 30, 2010, attributable to a $2.1 million borrowing under a short-term revolving bank credit facility.
Net cash provided by financing activities amounted to $17.7 million in 2007, attributable to $20.4 million proceeds from the issuance and sale of our preferred shares, partially offset by $2.6 million dividends paid to our then existing shareholders prior to the issuance and sale of our preferred shares. We did not have any inflows or outflows from financing activities in 2008 and 2009.
Capital Expenditures
We incur capital expenditures primarily for purchases of property, equipment and software and land use rights, the construction of facilities and leasehold improvements. Our capital expenditures were $8.9 million in 2007, $13.6 million in 2008 and $18.2 million in 2009. Our capital expenditures in 2010 are estimated to be approximately $30.0 million, of which we had spent $7.9 million as of June 30, 2010. Our primary planned capital expenditures for rest of 2010 are the construction of our manufacturing facilities and laboratory services building in Fengxian, Shanghai and purchase of equipment for our headquarters in Shanghai Zhangjiang Hi-Tech
68
Park. As of June 30, 2010, our capital commitments for new plant construction and equipment purchases were $7.7 million.
Contractual Obligations
The following table sets forth our contractual obligations as of December 31, 2009:
| | | | | | | | | | | | | | | | | | | | |
| | | Payment Due by Period | |
| | | Total | | | Less
than
1 year | | | 1-3
years | | | 3-5
years | | | more
than
5 years | |
| | | (in millions of $) | |
Operating Lease Obligations(1) | | | 6.4 | | | | 2.9 | | | | 2.9 | | | | 0.6 | | | | — | |
Capital Commitments | | | 6.7 | | | | 5.2 | | | | 1.5 | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | |
Total | | | 13.1 | | | | 8.1 | | | | 4.4 | | | | 0.6 | | | | — | |
| | | | | | | | | | | | | | | | | | | | |
| (1) | Operating lease obligations are primarily related to the lease of office space and vehicles. |
Off-Balance Sheet Commitments and Arrangements
We do not have any outstanding off-balance sheet guarantees, interest rate swap transactions except for certain foreign currency forward contracts as discussed in “—Quantitative and Qualitative Disclosure about Market Risk” below. We do not engage in trading activities involving non-exchange traded contracts. In our ongoing business, we do not enter into transactions involving, or otherwise form relationships with, unconsolidated entities or financials partnerships that are established for the purpose of facilitating off-balance sheet arrangements or other contractually narrow or limited purposes.
Quantitative and Qualitative Disclosure about Market Risk
Foreign Exchange Risk
Fluctuations in exchange rates affect our cost of revenues and net income and significantly impact our operating margins, because our net revenues are primarily generated from sales denominated in U.S. dollars, and a majority of our cost of revenues and operating expenses are denominated in Renminbi. In addition, we are exposed to foreign exchange risk related to cash and cash equivalents dominated in U.S. dollars.
The value of the Renminbi against the U.S. dollar and other currencies may fluctuate and is affected by, among other things, changes in China’s political and economic conditions. The conversion of Renminbi into foreign currencies, including the U.S. dollar, has been based on rates set by the People’s Bank of China. On July 21, 2005, the PRC government changed its policy of pegging the value of the Renminbi to the U.S. dollar and began allowing modest appreciation of the Renminbi versus the U.S. dollar. Under the new policy, the Renminbi is permitted to fluctuate within a narrow and managed band against a basket of certain foreign currencies, and the People’s Bank of China continues to intervene in the foreign-exchange market to prevent significant short-term fluctuations in the Renminbi-dollar exchange rate. This change in policy resulted in an appreciation of approximately 22.0% of the Renminbi against the U.S. dollar from July 21, 2005 to June 30, 2010. On June 20, 2010, the People’s Bank of China announced that the PRC government would further reform the Renminbi exchange rate regime and increase the flexibility of the exchange rate. It is difficult to predict how this new policy may impact the Renminbi exchange rate.
To the extent that we need to convert U.S. dollars into Renminbi for our operations, appreciation of the Renminbi against the U.S. dollar would have an adverse effect on the Renminbi amount that we receive from the conversion. Conversely, if we decide to convert our Renminbi into the U.S. dollar for the purpose of paying dividends on our ordinary shares or ADSs or for other business purposes, appreciation of the U.S. dollar in
69
relation to the Renminbi would negatively affect the corresponding U.S. dollar amount available to us. Considering the amount of our cash denominated in U.S. dollars as of June 30, 2010, a 1.0% change in the exchange rates between the Renminbi and the U.S. dollar would result in an increase or decrease in the amount in the Renminbi of RMB0.50 million to our total cash denominated in U.S. dollars. In addition, if we were to convert the net proceeds received in this offering into the Renminbi, a 1.0% change in the exchange rate between the Renminbi and the U.S. dollar would result in an increase or decrease in the amount we receive in the Renminbi by RMB2.7 million.
We periodically purchase derivative financial instruments, such as foreign-exchange forward contracts, to manage our exposure to Renminbi-U.S. dollar currency-exchange risk. The counterparties for these contracts are generally banks. The maturity periods of these contracts range from one to 18 months. We recorded losses of nil and $0.7 million on foreign-exchange forward contracts in 2007 and 2008, respectively. We recorded a gain of $0.1 million and $1.0 million on foreign-exchange forward contracts in 2009 and the six months ended June 30, 2010, respectively. Our accounting policy requires us to mark to market at the end of each reporting period and to recognize the change in fair value in earnings immediately. We held foreign-exchange forward contracts with an aggregate notional amount of $120.0 million as of June 30, 2010. As of June 30, 2010, the forward contracts had a fair value of $1.0 million.
Interest Rate Risk
Our exposure to interest rate risk primarily relates to the interest income generated by bank deposits with original maturities of three months or less. We have not used any derivative financial instruments to manage our interest risk exposure. Interest-earning instruments carry a degree of interest rate risk. We have not been exposed to material risks due to changes in interest rates. However, our future interest income may be lower than expected due to changes in market interest rates.
Inflation
Inflation in China has not materially impacted our results of operations in recent years. According to the National Bureau of Statistics of China, the change of consumer price index in China was 4.8%, 5.9%, and (0.7)% in 2007, 2008 and 2009, respectively.
Recent Accounting Pronouncements
In October 2009, the Financial Accounting Standards Board, or the FASB, issued an accounting standard update on revenue recognition relating to multiple deliverable revenue arrangements. The fair value requirements of existing accounting guidance are modified by allowing the use of the “best estimate of selling price” in addition to vendor-specific objective evidence, or VSOE, and third-party evidence, or TPE, for determining the selling price of a deliverable. A vendor is now required to use its best estimate of the selling price when VSOE or TPE of the selling price cannot be determined. In addition, the residual method of allocating arrangement consideration is no longer permitted. The FASB also issued an update in the same month that amends the scope of pre-existing software revenue guidance by removing from the existing guidance on software revenue recognition non-software components of tangible products and certain software components of tangible products. These updates require expanded qualitative and quantitative disclosure and they are effective for fiscal years beginning on or after June 15, 2010 although early adoption is permitted. These updates may be applied either prospectively from the beginning of the fiscal year for new or materially modified arrangement or retrospectively. We are currently assessing the impact that the adoption of these updates will have on our financial statements and disclosures.
In January 2010, the FASB issued an accounting standard update on improving disclosures about fair value measurements. The updated guidance amends existing disclosure requirements by adding required disclosures about items transferring into and out of Levels 1 and 2 in the fair value hierarchy; adding separate
70
disclosures about purchase, sales, issuances, and settlements relative to Level 3 measurements; and clarifying, among other things, the existing fair value disclosures about the level of disaggregation. This update is effective for fiscal years beginning after December 15, 2009, except for the requirement to provide Level 3 activity of purchases, sales, issuances, and settlements on a gross basis, which is effective beginning the first quarter of 2011. The adoption of this update beginning on January 1, 2010 did not have a material impact on our condensed consolidated financial statements.
In April 2010, FASB issued an update that provides guidance on the criteria that should be met for determining whether the milestone method of revenue recognition is appropriate. A vendor can recognize consideration that is contingent upon achievement of a milestone in its entirety as revenue in the period in which the milestone is achieved only if the milestone meets all criteria to be considered substantive. Determining whether a milestone is substantive is a matter of judgment made at the inception of the arrangement. A milestone should be considered substantive in its entirety. An individual milestone may not be bifurcated. An arrangement may include more than one milestone, and each milestone should be evaluated separately to determine whether the milestone is substantive. A vendor’s decision to use the milestone method of revenue recognition for transactions within the scope of the amendments in this update is a policy election. A vendor that is affected by the amendments in this update is required to provide certain disclosures. This update is effective on a prospective basis for milestones achieved in fiscal years, and interim periods within those years, beginning on or after June 15, 2010. Early adoption is permitted with certain disclosures required. A vendor may also elect, but is not required, to adopt the amendments in this update retrospectively for all prior periods. We are evaluating the impact on our financial statements of adopting this standard.
71
OUR CORPORATE STRUCTURE
We are a Cayman Islands holding company and conduct substantially all of our business through our seven operating subsidiaries in China. We commenced operations in China in 2002 and established the holding company in the Cayman Islands on August 30, 2007. We own 100% of all of our operating subsidiaries in China through two wholly-owned companies, China Gateway Life Science (Holdings) Limited and ChemExplorer Company Limited, which were incorporated in Hong Kong on June 23, 2003 and January 3, 2003, respectively. Our corporate structure reflects common practice for companies with operations in China where separate legal entities are often required or advisable for purposes of obtaining relevant tax and other incentives in the relevant jurisdictions.
The following diagram illustrates our corporate structure and the place of formation of our principal subsidiaries as of the date of this prospectus.

72
OUR INDUSTRY
The sections entitled “Overview of the Global CRO Market,” “Overview of the Chinese CRO Market” and “Trends and Outlook for the Global CMO Industry” in this “Our Industry” section contain information and statistics relating to the industry in which we operate that areexplicitly stated in the Frost & Sullivan Report, except for CAGR figures. The period CAGR figures are calculated from the respective period’s starting and ending figures, which are explicitly stated in the Frost & Sullivan Report.
Overview of the Global CRO Market
Global R&D spending by pharmaceutical and biotechnology companies grew at a CAGR of approximately 9.1% from $74 billion in 2006 to $96 billion in 2009. R&D spending has been driven primarily by the need to replenish drug pipelines as existing patents for blockbuster drugs expire, the need to develop drugs with significant benefits over existing drugs, and the increased complexity of drug R&D and regulatory processes. Global R&D expenditures are expected to grow at a CAGR of approximately 4.8% from 2009 to 2013 as companies combine their R&D capabilities following industry consolidation and focus their R&D efforts on key therapeutic areas, reaching $116 billion by 2013.
The following chart sets forth the growth of global pharmaceutical and biotechnology R&D expenditures for the years indicated:
Global Pharmaceutical & Biotechnology Companies R&D Expenditures ($ billions)
CROs provide substantial R&D capacity to drug developers and contribute to global pharmaceutical and biotechnology R&D activities. New drug discovery and development is an expensive and lengthy process, and companies seek to increase R&D productivity and accelerate the drug development process. Meanwhile, competition from generic drug manufacturers and government attempts to reduce public expenditures on drugs have placed pricing and profitability pressures on drug developers, resulting in a need to streamline costs. Many biotechnology companies also seek to make efficient use of their financial resources by reducing investments in internal R&D infrastructure and headcount. As such, many drug developers outsource R&D to service providers, including “off-shoring” or outsourcing drug R&D to service providers in developing countries. Over the past several years, the growth of the global CRO industry has outpaced the growth of global pharmaceutical and biotechnology R&D spending.
73
The following chart sets forth the growth of the global CRO market for the years indicated:
Global CRO Market ($ billions)
The following table sets forth the breakdown of the global CRO market by the stages of drug R&D process for the years indicated. See “—Overview of the Drug R&D Process” for more information on the individual stages.
| | | | | | | | | | | | | | | | | | | | |
| R&D Stage | | Market Size ($ billions)/% of Market | | | CAGR | |
| | | 2009 | | | 2013E | | | 2009 – 2013E | |
Discovery | | | 5.7 | | | | 26.8 | % | | | 10.2 | | | | 29.0 | % | | | 15.7 | % |
Preclinical Development | | | 3.9 | | | | 18.0 | | | | 6.6 | | | | 18.9 | | | | 14.1 | |
Clinical Phases I – IV | | | 10.7 | | | | 50.0 | | | | 16.7 | | | | 47.6 | | | | 11.8 | |
Others | | | 1.1 | | | | 5.2 | | | | 1.6 | | | | 4.5 | | | | 9.8 | |
Total | | | 21.4 | | | | 100.0 | | | | 35.1 | | | | 100.0 | | | | 13.2 | |
The global CRO market is projected to grow from $21 billion in revenues in 2009 to $35 billion in 2013, representing a CAGR of 13.2%. This growth is primarily due to increases in the level of R&D outsourced by pharmaceutical and biotechnology companies to CROs. In 2006, drug developers outsourced approximately 18.9% of total R&D expenditures. This percentage increased to approximately 21.9% in 2009 and is expected to reach approximately 30.2% in 2013. The growth rate of the drug discovery and preclinical development sectors is expected to exceed the growth rate of the overall CRO market over the same period. This is due to increased outsourcing of these functions to lower cost emerging markets, where intellectual property protection has been improving and technological advances have enhanced the capabilities of CROs.
The principal growth drivers for the global CRO industry include the following:
Cost Containment and Increased Flexibility
The cost of drug development has increased significantly over the past few decades. According to the Pharmaceutical Research and Manufacturers of America, or PhRMA, the cost of developing an FDA approved drug grew from an average of approximately $100 million in 1979 to approximately $800 million in 2000 and approximately $1.2 billion in 2009. The pharmaceutical industry has responded by streamlining R&D operations, diverting resources that would otherwise have been invested in-house to CROs and reducing the level of fixed costs required to maintain R&D internally.
For example, pharmaceutical companies are increasingly outsourcing their drug discovery and preclinical R&D functions and utilizing CROs on an as-needed basis. Because CROs offer their services to multiple customers, they can conduct R&D on a greater scale and more fully utilize capacity, performing R&D at costs lower than those of pharmaceutical and biotechnology companies.
74
Broad Service Platform As Technical Expertise of CROs Increases
As CROs make advancements in drug R&D, they are offering more sophisticated services. For example, technological advances in target identification and lead generation have enhanced the capabilities of CROs in drug discovery. CROs are also adding new services to create a more integrated R&D platform for their customers. With increased capabilities and broader service offerings, CROs have become an attractive source for pharmaceutical and biotechnology companies in supplementing their R&D functions.
Reduced Time to Market
Reducing time spent on drug discovery and development is crucial as pharmaceutical companies face increasing pressures to bring new drugs to market. Bringing drugs to market more quickly also maximizes the time during which a drug receives patent protection, increasing revenues from the drug. By focusing exclusively on R&D activities, CROs are able to conduct R&D more efficiently. For example, studies have shown that outsourcing could shorten drug discovery cycle time by as much as 30% compared to performing this process in-house at a pharmaceutical or biotechnology company.
Growing Demand from the Biotechnology Sector
Biotechnology companies have been successful in bringing new and innovative therapies to market in areas with significant unmet needs. Studies estimate that one third of all pipeline products are biotechnology-based, a proportion expected to reach approximately 50% within the next three to five years. Biotechnology companies, particularly smaller ones, generally prefer outsourcing R&D services in order to maintain financial flexibility and reduce expenditures required to maintain in-house R&D infrastructure. The strong potential of the biotechnology sector provides significant upside for growth of the overall CRO industry.
Trends and Development of the Global CRO Market
The outlook for the global CRO market remains strong as pharmaceutical and biotechnology companies seek more cost efficient methods to develop new medicines. Drug developers are also expected to increasingly utilize CROs in lower cost regions such as China, India and Eastern Europe to achieve this objective. CROs in these regions are steadily improving their capabilities and offering a range of services that are becoming increasingly comparable to those offered by CROs in developed regions. Meanwhile, international CROs are recognizing the benefits of establishing operations in emerging markets. Because it will take considerable time and effort to build scale in these markets, international CROs are considering alliances or mergers with local companies.
In addition, the CRO industry is expected to evolve towards a more integrated service model to meet the needs of the pharmaceutical and biotechnology industry. Drug developers increasingly prefer using CROs with multiple service capabilities due to shorter turnaround times, elimination of the need to manage multiple CROs and the ability to leverage knowledge obtained at earlier stages of the R&D process within the same organization. The reputation of a CRO is critical in gaining drug developers’ confidence in its capability to deliver quality services in new service areas. As a result, larger CROs with strong track records have a stronger ability in cross-selling new services to customers.
Recent consolidation in the global CRO industry also demonstrates the willingness of CROs to access new capabilities through acquisitions. Examples include Pharmaceutical Product Development Inc.’s acquisitions of BioDuro, LLC and Excel PharmaStudies, Inc. in China. Acquisitions in emerging countries provide international CROs access to a large pool of skilled scientific talent, lower labor costs and proximity to high growth markets.
Overview of the Chinese CRO Market
As outsourcing R&D has become a major trend in the global CRO industry, outsourcing to emerging countries such as China has increased in recent years. The Chinese CRO market grew at a CAGR of 27.2% from
75
$380 million in 2007 to $615 million in 2009, and is projected to grow at a CAGR of 20.7% to $1.3 billion in 2013.
The following chart sets forth the growth of the Chinese CRO market for the years indicated:
Chinese CRO Market ($ millions)
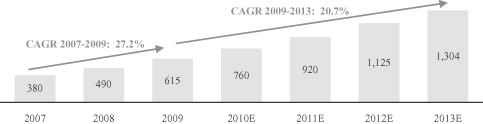
The principal growth drivers for the Chinese CRO market include the following:
Lower-cost, High-quality Services
As Western drug developers face rising R&D costs drug developers need to invest in cost-effective R&D to sustain long-term profitability. Leading CROs in China have performed R&D at costs significantly lower than those of Western-based CROs, while meeting Western drug development and safety standards. For example, the average cost of a full time junior and mid-level pharmaceutical researcher in China is significantly lower than that of a similar researcher in Europe or the United States.
In addition, large CROs in China are performing increasingly complicated and integrated R&D processes. With the ability to provide services at lower costs while maintaining standards comparable to Western-based CROs, a greater number of projects, including those that are complex and integrated, are expected to be outsourced to CROs in China.
Large and High-Quality Talent Pool
China offers a large talent pool in pharmaceutical R&D, including a deep base of local graduates and an increasing number of Western-educated researchers and overseas returnees with R&D experience at global pharmaceutical and biotechnology companies. By capitalizing on this talent pool, CROs in China are expected to expand their service offerings and capabilities, driving the overall development of the industry.
Increasing Breadth of R&D Service Offerings
Historically, CROs in China have focused mainly on performing research on discovery chemistry. However, more established and larger CROs in China have increasingly positioned themselves as integrated service providers by expanding into higher value-added activities such as discovery biology, preclinical development and certain pharmaceutical development services, and are offering a broader range of R&D services along the drug development chain. For a Western drug developer, the combination of lower costs in China, quality service and the benefits of using multiple services from a single service provider increase the attractiveness of outsourcing to China.
Large Supply of Treatment Naive Patients
With increasingly stringent protocols being implemented for new clinical trials, it is becoming more difficult for pharmaceutical companies to recruit a sufficient number of volunteer patients in clinical trials within
76
the specified timeframe of a trial. Approximately 80% of delayed projects are caused by the inability to recruit the required number of patients for clinical trials. As the most populous country in the world, China offers a large patient pool, which facilitates patient recruitment in clinical trials. Conducting clinical trials in China constitutes an attractive strategy for drug developers, benefiting CROs that conduct clinical trials in China.
Growing Domestic Pharmaceutical Market
The Chinese pharmaceutical and biotechnology market provides an additional source of revenue for CROs, as local Chinese and multinational companies increasingly develop innovative products and biologics in China. This contrasts with Chinese companies’ past strategy of focusing on contract manufacturing, API and generic drug production, increasing opportunities for CROs in China. International pharmaceutical companies have also recognized the high growth potential of the pharmaceutical and biotechnology market in China. As a result, they are investing in drugs that target the local population and are leveraging the expertise of local CROs to bring these medicines to market.
Strong Government Support
The Chinese government continues to provide strong support for R&D in China. Tax relief, direct funding opportunities, investment in logistics and infrastructure and other measures have attracted investment in R&D. Increased career opportunities in China have also encouraged scientific personnel to remain in the country or return from abroad. In addition, the government has strengthened intellectual property regulations. These factors have increased the attraction of conducting drug R&D activities in China.
Competitive Landscape of the Chinese CRO Industry
The Chinese CRO market has over 100 domestic and international CROs operating in China. The majority of CROs operating in China are small or medium sized while large CROs, especially domestic companies, remain scarce. The top two CROs, WuXi PharmaTech and our company, in aggregate accounted for approximately 40% of the CRO market in China in 2009.
Despite the large number of CROs in China, companies vary widely in terms of service quality and capabilities. Small and medium sized CROs typically offer low-value added services in a competitive market. Larger CROs differentiate themselves by the quality of their scientific talent, the breadth of the services offered and advanced facilities. In addition, most small to medium sized domestic CROs focus on a limited number of services, with only a few large CROs in China capable of providing integrated services.
In addition, services offered by domestic and international CROs differ considerably. In general, most domestic CROs provide services at the earlier stages of the drug R&D chain, such as drug discovery and preclinical development services. In contrast, international CROs in China generally focus on services at the clinical stages. For example, an international CRO may conduct a clinical trial in China for a global client as part of a multi-country research project for the same drug. However, recent proposed and completed cross-border acquisitions into China reflect the desire of a growing number of international CROs to offer a fuller range of services.
Trends and Outlook for the Chinese CRO Industry
The China CRO industry remains at an early stage of development. With cost advantages and enhanced capabilities, Chinese CROs are expected to become increasingly competitive internationally. The market outlook for drug discovery and preclinical development outsourcing in China remains strong, as Western pharmaceutical companies outsource their early stage R&D to China, driven by competitive costs, improved technologies and intellectual property protection, quality of scientific talent and increasing range of service offerings in China.
77
As the Chinese CRO market grows, large and reputable CROs who provide broad, integrated services are expected to benefit as customers increasingly value the advantages of an integrated service platform. These organizations are expected to expand their service offerings. In addition, the Chinese CRO industry is expected to consolidate due to the large number of small CROs in the industry. Organizations that provide quality and innovative R&D services are attractive acquisition candidates for larger CROs seeking to expand their service offerings and acquire new capabilities instead of developing them internally. Meanwhile, small and inefficient CROs that offer undifferentiated and a narrow range of services are likely to have difficulties competing in an evolving landscape.
Trends and Outlook for the Global CMO Industry
Pharmaceutical companies are increasingly turning to contract manufacturing organizations, or CMOs, to capture efficiencies and minimize costs required to maintain facilities, up-to-date equipment and skilled personnel. Outsourcing also allows drug developers to achieve scalability in capacity as production volumes fluctuate and provides access to the latest manufacturing expertise and technologies. As a result, CMOs are evolving into strategic partners of pharmaceutical and biotechnology companies. In addition, Western drug developers have increased their focus on CMOs in countries such as China and India in the past few years due to significant cost savings, improving manufacturing capabilities and greater adherence to global manufacturing standards. The global CMO market grew by CAGR of 10.0% from $30 billion in 2005 to $44 billion in 2009, and is expected to grow at a CAGR of 7.9% to reach $60 billion in 2013.
Overview of the Drug R&D Process
R&D is a long, challenging process requiring skill and persistence. The R&D process involves multiple stages, disciplines and personnel. From initial laboratory testing to approval by the FDA, the process typically lasts an average of 10 to 15 years. The rate of success is low, with only one out of approximately every 5,000 to 10,000 compounds tested eventually becoming a commercial drug. The small number of products that receive FDA approval may require years of additional research and surveillance. The chart below summarizes the typical stages of the R&D process:
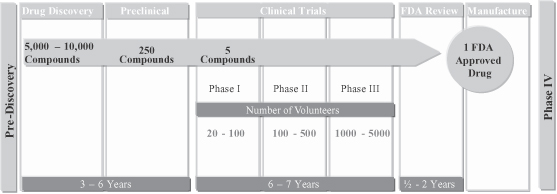
Source:PhRMA
Drug Discovery
Target Discovery
Target discovery represents the first step in the drug discovery process. Scientists identify a “target,” or cellular or genetic material in the body believed to be associated with a disease, as a focus for pharmaceutical development. Scientists attempt to identify a target and investigate how the target functions and why it causes the disease. Scientists then develop drugs to treat these disease-causing mechanisms.
78
Assay Development
After identifying a target, scientists develop evaluation systems to assess the therapeutic values of compounds. These systems, known as “assays,” screen out a large number of compounds based on their therapeutic values. Assays developed for screening compounds are typically in vitro assays ranging from enzyme assays to cell/tissue assays, which evaluate the precision and potency of the reaction between a compound and the target. In vivo assays that involve animals in preclinical development test the physiological effects of the potential drug on animals to evaluate the safety and efficacy of the drug.
Lead Generation
After assays are developed and validated, scientists test large libraries of compounds for their ability to interact with the target and its biologic function. These compounds include small molecules, antibodies, proteins and genes. Scientists identify each compound that reacts with the target as a “hit,” which serves as a starting point for identifying leads. Scientists design new analogs around the hit and develop structure activity relationships, or SAR, to identify leads.
Lead Optimization
In lead optimization, scientists synthesize and screen compounds structurally similar to a lead to improve the qualities of the lead. These qualities include potency, selectivity, physicochemical properties, pharmacokinetic properties, in vivo efficacy and safety profile. Other factors scientists consider in deciding which leads or compounds to develop include ease of synthesis and the number of steps required to produce large quantities of the drug which impact costs and development time. The optimized leads that show desired properties will become potential drug candidates for preclinical development.
Preclinical Development
Preclinical development is a stage of drug research conducted before clinical trials. The purpose of preclinical development is to evaluate the drug metabolism, pharmacokinetics, safety profiles and other properties of a potential drug candidate before testing on humans. During preclinical development, scientists evaluate a compound’s safety profile and biological properties using in vitro and in vivo testing methods.
DMPK
DMPK refers to drug metabolism and pharmacokinetics. DMPK studies attempt to determine the absorption and distribution of an administered drug, the rate at which a drug takes effect, the duration a drug maintains its effects and what happens to the drug after being metabolized by the body. Scientists modify a drug candidate based on the results of these studies.
ADME Profiling
ADME profiling studies the disposition of a drug in the human body with respect to absorption, distribution, metabolism and excretion. When a drug is administered, it must overcome multiple obstacles presented by the body’s physiological and biochemical processes as well as defense mechanisms. For example, the compound may fail to be absorbed into the bloodstream to reach tissues or organs because the compound is insoluble, or the compound may break down after entering the body as metabolism occurs. The human body must also be able to eliminate the drug from the body using natural processes. As a result, scientists evaluate ADME profiles of compounds for their suitability as drug candidates. Typically, scientists perform these studies on animals referred to as PK profiling, or on cells or cellular components such as proteins or enzymes using an in vitro approach, referred as in vitro ADME profiling.
79
Toxicology Studies
The main goals of toxicology studies are to determine a product’s ultimate safety profile for human use. Typically, both in vitro and in vivo animal tests will be performed for the safety assessment. Studies of a drug’s toxicity include which organs are targeted by that drug, as well as assessing whether there are any long-term carcinogenic or toxic effects. The information collected from these studies is vital so that safe human testing can begin.
Metabolite Identification
When a drug is administered into the body, it metabolically modifies into products known as metabolites. In some cases, the metabolite is actually the drug that is active but often unstable. By identifying metabolites in the body during the different phases of metabolism, research scientists can map the metabolite pathway of a drug candidate. Early identification of potentially active or toxic metabolites can help identify more potent and safer drug candidates, helping scientists determine whether a drug candidate warrants further development.
Pharmaceutical Development
Formulation
Scientists must determine an optimal form of dosage before administering a drug to humans to ensure that the drug is stable and will achieve the desired therapeutic effect. This process involves combining the active pharmaceutical ingredient with other ingredients to produce a medicine. Pre-formulation refers to selecting these ingredients by assessing the drug’s physical and chemical properties, and the overall stability of these ingredients when combined under different environmental conditions. Other steps involved in formulation include stabilizing of the active ingredient in bulk form and the formulation of the drug substance into appropriate dosage forms to achieve the desired therapeutic impact.
Process R&D
During drug discovery and the earlier phases of preclinical development, scientists often synthesize leads in relatively small amounts. To meet the demands of later phases of preclinical development and clinical trials, scientists must synthesize larger quantities of the active pharmaceutical ingredient and its intermediates. Process R&D attempts to optimize the synthesis of large quantities of the active pharmaceutical ingredient and its intermediates by evaluating and improving efficiency, supply of raw materials, safety, facility requirement, cost of production, and other conditions during the process.
Manufacturing
Engineers, chemists and other scientists work together to produce high quality drugs on a large scale. Researchers often begin planning mass production prior to regulatory approval to effectively support product market launch. Companies also manufacture smaller batches of APIs and advanced intermediates for clinical research trials.
Clinical Development
Clinical Trials (Phases I – III)
The first phase of clinical trials assesses a drug candidate’s safety and evaluates adverse events to the human body. These studies are usually conducted on small groups of healthy volunteers. The second phase of clinical trials tests the drug candidate in actual patients. Researchers further study the drug candidate’s safety and begin examining its efficacy against the targeted disease. The third phase of clinical trials involves large-scale
80
trials with hundreds to thousands of patients. Researchers further evaluate the efficacy of the drug candidate on a larger scale and examine rare adverse events, if any, not arising in the first two phases of clinical trials due to sample size.
Commercialization
Upon successful completion of clinical trials, the company submits a New Drug Application, or NDA, to the FDA. The NDA consists of an extensive collection of documents, including results from preclinical and clinical studies and details of the manufacturing plans. The FDA can approve a new medicine, request more information or studies, or deny approval.
Post-approval Research (Phase IV)
Studies and monitoring continue during the market life of the medicine. For example, the FDA may require Phase IV studies to obtain more information about the medicine. The company may also expand the current indication or purpose for which a drug is used, or research additional indications to treat other diseases. Finally, the company must monitor and report adverse events to the FDA.
81
BUSINESS
Overview
We are a leading China-based pharmaceutical and biotechnology R&D outsourcing company. We provide a broad range of high-quality, integrated services across the drug discovery and development process to international and Chinese pharmaceutical and biotechnology companies. Our services consist of discovery chemistry, discovery biology and preclinical development, pharmaceutical development and biologics services. We offer our services on an FTE basis or a fee-for-service basis. Capitalizing on our broad service offerings, the experience and expertise of our skilled scientists and our state-of-the-art facilities and equipment, we help our customers discover and develop novel drug candidates efficiently. We have a diversified and loyal global customer base. We provide services to over 100 customers, including all of the top ten pharmaceutical and biotechnology companies in the world in terms of 2009 revenues ranked by Frost & Sullivan and a number of fast-growing biotechnology and specialty pharmaceutical companies, as well as renowned academic and research institutions in the U.S. and China. Most of our customers return to us for additional and often larger projects, and all of our top ten customers in each of 2008 and 2009 remain our customers in 2010.
Our experienced and strong R&D team contributes significantly to our market leadership. We have successfully grown our scientific research staff from 988 as of December 31, 2007 to 1,525 as of June 30, 2010. Over 60% of our scientific research staff have post-graduate degrees, including many native Chinese scientists and managers who have returned to China after studying or working overseas with experience in global pharmaceutical and biotechnology companies and knowledge of Western business practices. Based in China and headquartered in Shanghai, we are well-positioned to attract a large number of returnees and benefit from an abundant supply of highly skilled, domestically-trained scientific talent in China. We attract, retain and motivate our employees through a comprehensive training and career development program and equity incentive plans.
We conduct our laboratory activities in four primary facilities in China: (i) an approximately 450,000 square-foot laboratory and headquarters in Shanghai Zhangjiang Hi-Tech Park; (ii) an approximately 13,000 square-foot AAALAC-accredited animal facility in Shanghai Zhangjiang Hi-Tech Park; (iii) an approximately 36,000 square-foot R&D center in Chengdu; and (iv) an approximately 28,000 square-foot manufacturing facility in Nanhui, Shanghai. In addition, we are constructing an approximately 460,000 square-foot cGMP-quality multi-purpose manufacturing facility in Fengxian, Shanghai, designed to manufacture advanced intermediates and active pharmaceutical ingredients, or APIs, for preclinical testing and clinical trials. We are also constructing an approximately 150,000 square-foot laboratory services building in Fengxian, Shanghai primarily for pharmaceutical development services. Our strategic location in China provides us many advantages, including relatively low-cost labor, developed infrastructure and favorable government incentives as well as a large talent pool.
We have experienced significant growth in recent years. Our net revenues increased from $34.7 million in 2007 to $60.5 million in 2008 and $72.3 million in 2009, representing a CAGR of 44.4%, and increased by 27.2% from $32.7 million in the six months ended June 30, 2009 to $41.6 million in the six months ended June 30, 2010. The number of our customers increased from 71 in 2007 to 99 in 2008, 134 in 2009 and 151 in the six months ended June 30, 2010. Our net income increased from $7.5 million in 2007 to $9.1 million in 2008 and $9.8 million in 2009, and increased from $4.6 million in the six months ended June 30, 2009 to $6.9 million in the six months ended June 30, 2010.
Our Strengths
We believe that the following strengths contribute to our success and differentiate us from our competitors:
Leading Market Position with Proven Track Record
We are the second largest China-based pharmaceutical and biotechnology R&D outsourcing company in terms of 2009 revenues, according to the Frost & Sullivan Report. We have a proven track record of customer satisfaction. Our strong R&D capabilities and ability to help customers plan and structure their projects have
82
enabled us to consistently meet or exceed our customers’ key performance targets. We have established widespread brand recognition among leading global pharmaceutical and biotechnology companies and in the CRO industry. For example, Lilly, our largest customer in 2009 and the six months ended June 30, 2010, has listed us as a “Global Preferred Partner.” We are a preferred provider of outsourced discovery services to GSK. We were also named as the “Most Valuable Partner” by Roche in 2009, and received an “Excellent Performance Award” from Sunovion Pharmaceuticals Inc. (formerly known as Sepracor Inc.) in 2008.
We have experienced significant organic growth in recent years. Our net revenues increased from $34.7 million in 2007 to $72.3 million in 2009, representing a CAGR of 44.4%, and increased by 27.2% from $32.7 million in the six months ended June 30, 2009 to $41.6 million in the six months ended June 30, 2010. The number of our customers increased from 71 in 2007 to 134 in 2009 and 151 in the six months ended June 30, 2010. Most of our customers return to us for additional and often larger projects. Our average net revenues per top ten customer increased from $2.9 million in 2007 to $4.7 million in 2009 and were $2.6 million in the six months ended June 30, 2010.
Seasoned Management and Experienced R&D Team
We have grown from a start-up company focusing solely on discovery chemistry to a provider of broad, integrated drug discovery and development R&D services, with 1,755 employees as of June 30, 2010. Our management team has in-depth industry knowledge and an average of 13 years of business and financial experience, enabling us to respond quickly and effectively to industry changes and capitalize on market opportunities. We have an experienced and strong R&D team consisting of 1,525 dedicated scientific research staff as of June 30, 2010, 18.4% of whom hold Ph.D.s or M.D.s, 44.3% of whom hold master’s degrees and approximately 8.3% of whom are returnees.
Our R&D team is led by over 100 senior scientists and management who have an average of 13 years of relevant industry experience, including work experience in global pharmaceutical and biotechnology companies such as Lilly, GSK, Merck & Co., Inc. and Amgen Inc. They have published approximately 1,500 scientific papers and are the named inventors on approximately 400 patents. We believe their broad and diverse industry experience enables us to understand our customers’ needs and provide solutions and a customer experience that differentiate us from our competitors. We have also established a scientific advisory board consisting of five world-renowned scientists with significant academic achievements at top universities, such as Harvard and Princeton, and extensive industry experience at leading global pharmaceutical and biotechnology companies. They advise our management and scientists with respect to our R&D services and play an active role in strengthening our scientific expertise.
Integrated Service Platform with Comprehensive Service Offerings
We are one of the few China-based CROs offering a broad range of services, including discovery chemistry, discovery biology and preclinical development, pharmaceutical development and biologics services. Our integrated service platform allows us to cross-sell our services and enhances our service offerings. We work closely with customers to identify additional services that complement the existing services we provide them. We assign a project manager to each multi-service customer project to facilitate knowledge sharing and effective communication and collaboration among our service teams. Our integrated service platform shortens turnaround time and eliminates our customers’ need to manage multiple CROs. Our customers have taken advantage of our comprehensive service offerings. The proportion of net revenues derived from customers who used multiple services from us increased from 58% in 2007 to 89% in 2009.
Loyal and Diversified Global Customer Base
Leveraging our reputation and market leadership, we have established a loyal and diversified global customer base. Our strong commitment to providing customers with high-quality services has helped us build trust with our customers and bring in recurring business. Our top five customers in 2009 have been with us for an average of five years and all of our top ten customers in each of 2008 and 2009 remain our customers in 2010. In
83
2009, we had 134 customers, including all of the top ten pharmaceutical and biotechnology companies in the world in terms of 2009 revenues ranked by Frost & Sullivan. We have diversified our customer base in recent years, with our top ten customers contributing 65% and 63% of our total net revenues in 2009 and the six months ended June 30, 2010, respectively, compared to 85% in 2007. We also have a number of fast-growing biotechnology and specialty pharmaceutical customers, which contribute a significant portion of our net revenues. Our customers are geographically diversified. In the six months ended June 30, 2010, net revenues originated from North America, Europe, China and the rest of the world represented 81%, 10%, 4% and 5% of our total net revenues, respectively.
Efficient Service Provider with Competitive Cost Structure
As a China-based CRO, we are strategically positioned to benefit from the advantages of doing business in China, including a large talent pool, relatively low-cost labor, developed infrastructure and favorable government incentives. Through the establishment of our Chengdu R&D center, we benefit from additional cost advantages and favorable government incentives in western China. We have built a streamlined and flat reporting system, which improves communication, reduces bureaucratic inefficiencies and improves our responsiveness and flexibility to industry trends and customers’ needs. We have also established a monitoring and control system to help manage our labor, equipment, raw materials and property related costs. This system has enabled us to achieve efficient staffing and maximize the utilization of our equipment, enhancing operating efficiency and profitability. We provide comprehensive training to local hires to increase their productivity and groom them for higher-level positions that would otherwise require returnees.
State-of-the-art Facilities with Flexible and Scalable Capacities
We have state-of-the-art facilities equipped with advanced technologies and equipment. We maintain flexible and scalable service capacities by leasing all of our laboratory and office space except for the manufacturing facility and laboratory services building in Fengxian, Shanghai, which are under construction. Our headquarters in Shanghai Zhangjiang Hi-Tech Park has approximately 450,000 square feet of laboratory and office space and approximately 13,000 square feet of AAALAC-accredited animal facilities. We have approximately 36,000 square feet of laboratory and office space in Chengdu mainly for discovery chemistry.
We are building an approximately 460,000 square-foot cGMP-quality, multi-purpose manufacturing facility in Fengxian, Shanghai, including kilo-labs, a pilot plant and other manufacturing and formulation facilities, to expand our pharmaceutical development services. We are also constructing an approximately 150,000 square-foot laboratory services building in Fengxian, Shanghai primarily for pharmaceutical development services. We use advanced preparatory and analytical equipment from leading global manufacturers such as Agilent Technologies, Inc., Waters Corporation and Shimadzu Corporation. Our equipment provides high-speed and reliable experimental and analytical results, allowing us to consistently deliver high-quality services.
Our Strategies
We aim to become one of the world’s leading pharmaceutical and biotechnology R&D outsourcing companies. To achieve this, we intend to pursue the following strategies:
Broaden Our Service Offerings and Enhance Our Service Capabilities
We intend to broaden the scope of our services to strengthen our integrated service platform and meet our customers’ evolving service needs. Phase A of our Fengxian facility, expected to commence operations in early 2011, will enable us to manufacture advanced intermediates and APIs for preclinical testing and early-stage clinical trials. We began offering biologics services in early 2010 and intend to expand these services. These new services will allow us to provide more integrated services across the drug discovery and development value chain. In addition, we intend to strengthen our leading position in laboratory services by hiring more qualified professionals, enhancing service capabilities, establishing new facilities and investing in advanced equipment. By
84
focusing on service quality, turnaround time and intellectual property protection, we believe the demand for our services, particularly our higher-value-added services, will increase and drive our growth.
Maintain and Expand Our Customer Base
We seek to maintain and develop long-term and strategic relationships with existing customers by providing high-quality services and consistent project execution. By adding more service offerings to our integrated platform, we will be able to better meet our customers’ evolving needs and strengthen our relationships with them. In addition, we believe the rapid growth of biotechnology and specialty pharmaceutical customers as well as China-based customers represents attractive opportunities. We intend to capitalize on such opportunities through targeted marketing and customized service offerings.
Attract, Train and Retain Scientific Talent
Our skilled scientists constitute our most valuable assets and represent the key to our success. We will continue offering performance incentives to our senior scientists and management, particularly returnees with work experience at leading global pharmaceutical and biotechnology companies. Our performance incentives include performance bonuses and equity awards to reward and motivate our senior scientists and management and provide them the opportunity to share in our success. We will further leverage our senior scientists’ strong expertise and extensive experience by having them provide training in scientific and technical skills to our local hires through programs such as “ShangPharma University.” We also provide training to our employees in compliance, management, leadership and communication skills.
Enhance Our Operating Efficiency
We intend to enhance our operating efficiency through, among other things, the following:
| | • | | hiring more local talent and leveraging our senior scientists and management to optimize the structure of our working teams; |
| | • | | improving employee productivity through effective monitoring of key performance indicators and implementation of a performance-linked compensation structure; |
| | • | | expanding into lower-cost areas, such as leasing additional R&D facilities and increasing hiring in Chengdu to benefit from lower costs and favorable government incentives; and |
| | • | | leveraging our scale to obtain better pricing terms from our suppliers. |
Selectively Evaluate and Pursue Strategic Alliance and Acquisition Opportunities
In addition to developing new services and enhancing service capabilities, we will prudently and selectively evaluate and pursue strategic alliance and acquisition opportunities to complement our existing services when these opportunities arise. Such opportunities will help us rapidly build new capabilities and broaden our service offerings.
Our Services
We provide a broad range of high-quality, integrated services across the drug discovery and development process to international and Chinese pharmaceutical and biotechnology companies. Our services consist of discovery chemistry, discovery biology and preclinical development, pharmaceutical development and
85
biologics services. We believe our customers value our ability to offer a wide range of quality services to meet their drug R&D needs, and we expect to capitalize on our strong customer base and expand our service offerings along the drug discovery and development value chain, especially in research manufacturing and biologics.
We conduct our operations primarily at our headquarters in Shanghai Zhangjiang Hi-Tech Park and our R&D center in Chengdu. As a purely China-based contract research company, we are well positioned to capitalize on the advantages of conducting drug R&D operations in China, while also emphasizing quality, responsiveness, protection of customer intellectual property and reliability.
The following chart sets forth the drug R&D process and our services:
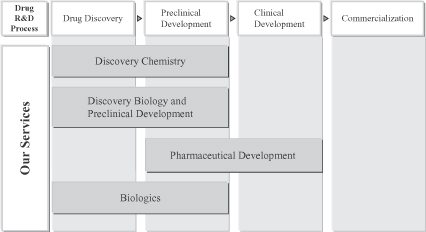
Discovery Chemistry
Our core service offering is discovery chemistry. In the drug discovery process, we seek to identify hits, which are chemical compounds or therapeutic antibodies or proteins that interact with a potential drug target with sufficient potency and selectivity to warrant further testing and refinement as possible drug candidates, or leads. When we identify and assess a lead, we further refine and optimize the chemical structure of the lead to improve its drug characteristics with the goal of producing a preclinical drug candidate. We believe that our scientists’ extensive experience and expertise enable us to provide discovery chemistry services tailored to our customers’ specific needs. As of June 30, 2010, our discovery chemistry team consisted of 1,037 scientific research staff. Our discovery chemistry services include:
Medicinal chemistry. Our medicinal chemistry services include hit generation and assessment, hit-to-lead and lead optimization services. We focus on a traditional medicinal chemistry approach, a process through which a series of compounds are carefully designed with the aid of modern computational chemistry and SAR analysis, followed by chemical synthesis and evaluations of biological activities and DMPK, ADME or toxicology properties. We also design and synthesize focused libraries to support SAR analysis.
Synthetic chemistry. Our synthetic chemistry services include syntheses of custom-designed compounds, as well as complex reference compounds. We have completed the syntheses of a number of complex compounds with more than 20 steps.
Library generation. Our library generation services include designing and synthesizing libraries, as well as building blocks and scaffold for library synthesis, reference compound synthesis and custom synthesis.
86
Analytical chemistry. We provide broad analysis and purification services, including high throughput analysis and purification, large scale compound purification, natural product isolation and structure elucidation, among other things. Our analytical chemistry services internally support synthetic chemistry, library generation and medicinal chemistry services, and offer external services focused on chiral separation, bulk material purification and high-throughput library purification.
Discovery Biology and Preclinical Development
Capitalizing on strong customer demand, we began providing discovery biology and preclinical development offerings in 2007 and we believe we are one of the few China-based companies with the capability to provide integrated chemistry- and biology-based drug R&D services. As of June 30, 2010, our discovery biology and preclinical development team consisted of 245 scientific research staff. With increasing customer demand, we anticipate that discovery biology and preclinical development services will increasingly contribute to our growth and profitability.
Discovery Biology
Our discovery biology team provides biology-based assays, models and other services for the drug discovery process, which primarily include:
| | • | | Assay development and high throughput screening. We develop biochemical and cell-based assays to examine the activity of small molecule compounds at targets such as kinases, G-protein-coupled receptors, nuclear receptors and ion channels. |
| | • | | Pharmacology. We develop rodent and other animal disease models to evaluate compounds in various therapeutic categories including cancer, metabolic and central nervous system diseases, inflammation and infectious diseases. |
| | • | | Reagent generation. We generate and produce proteins, antibodies and cell lines for biochemical and cell-based assays. |
Preclinical Development
Our preclinical development team provides biology-based screening, profiling and other services for the drug preclinical development process, which primarily include:
| | • | | DMPK. Our DMPK services includein vivo rodent screening based on pharmacokinetic characteristics for rapid drug candidate selection and dog and non-human primate PK for prediction of drug properties in humans. We have the expertise to administer drugs to animals orally, intravenously and by infusion. We perform mass balance studies by administering radioactive drug molecules to animals and monitoring metabolites in plasma, urine, bile and tissue samples. |
| | • | | ADME profiling. Ourin vitro ADME profiling services include analyzing (i) the metabolic stability of drug candidates in cell particles and liver cells, (ii) drug-drug interaction, (iii) plasma protein binding and (iv) the manner in which drugs are transported in the body through transporter assays. |
| | • | | Metabolite identification. Our metabolite identification services include metabolite profiling, characterization, and structure elucidation as well as pharmacokinetic evaluation of parent and metabolites in preclinical development. |
| | • | | Bioanalytical services. Our centralized bioanalytical labs provide quantitative and qualitative sample analysis for preclinical studies. In addition to internally supporting DMPK, non-GLP toxicology and biology services, they offer external services such as immunoassay for protein drugs and biomarkers, which are traits, proteins or other substances used to measure or indicate the progress or existence of a disease or condition, and metabolite identification. |
87
| | • | | Non-GLP toxicology. We offer non-GLP toxicology services, including general toxicology in rodents, dogs and non-human primates. We lease an approximately 13,000 square-foot AAALAC-accredited animal facility in Shanghai Zhangjiang Hi-Tech Park. |
Pharmaceutical Development
We provide broad, integrated pharmaceutical development services to our customers in the drug development process. Our pharmaceutical development services focus on the early phases of the drug development process and consist of preformulation and formulation, process R&D, analytical development and research manufacturing services. We began offering pharmaceutical development services in 2005. As of June 30, 2010, our pharmaceutical development services team consisted of 219 scientific research staff. Our pharmaceutical development services include:
| | • | | Preformulation and Formulation.Working in close collaboration with our process R&D, research manufacturing and analytical development groups, our preformulation and formulation groups offer a wide range of services for our customers’ new chemical entities in the preclinical stage. Our preformulation group provides analytical approaches to choose the right form of a drug candidate (e.g. solid or liquid), evaluate its physical properties, and understand the drug candidate’s stability under various conditions. Our formulation group focuses on developing the most conventional solid or liquid dosage form or innovative dosage form and drug delivery system for the drug candidate. |
| | • | | Process R&D. Our process R&D service involves optimizing the chemical synthesis process in order to yield much larger quantities of a drug than those needed in the previous development phases. Processes developed for small scale production of a compound may not be suitable for larger scale production because they may be too expensive, environmentally unacceptable or present safety concerns. Our process R&D team seeks to select and optimize the most effective method of compound synthesis by, among other things, reducing the number of chemicals used in synthesis, improving the yield of the desired compound and reducing the time needed for synthesis. |
| | • | | Analytical Development. Our analytical development group provides analytical services for pharmaceutical development projects. It supports internal process R&D and offers external services focused on analytical method development, method validation, in-process quality control, reference standard qualification, release testing and stability sample storage testing. |
| | • | | Research Manufacturing. We provide manufacturing services for our customers to manufacture drug substances under cGMP regulations in sufficient quantities to conduct early stage drug development. Our cGMP-quality kilo-lab in Shanghai Zhangjiang Hi-Tech Park and pilot plant in Nanhui, Shanghai, have the capacity to manufacture drugs in increasing quantities ranging from lab quantities, which are measured in grams, to scale-up quantities, which are measured in kilograms, to process optimization quantities, which are measured in hundreds of kilograms. |
We are constructing an approximately 460,000 square-foot cGMP-quality multi-purpose manufacturing facility in Fengxian, Shanghai, which is designed to manufacture advanced intermediates and APIs for preclinical testing and clinical trials. Phase A of the Fengxian facility, comprising approximately 200,000 square feet, will include cGMP-quality kilo-labs and a pilot plant. It is planned to primarily manufacture advanced intermediates and APIs for preclinical testing and early-stage clinical trials and is scheduled to commence operations in early 2011. Phase B of the Fengxian facility, comprising approximately 260,000 square feet, will include a cGMP-quality API plant and a formulation pilot plant. We plan for this facility to primarily manufacture APIs for later-stage clinical trials and potential FDA-approved drugs. We estimate it will commence operations in 2011 or later. We are also constructing an approximately 150,000 square-foot laboratory services building in Fengxian, Shanghai primarily for pharmaceutical development services. This building is expected to commence operations in 2011. We may build additional manufacturing facilities if we experience strong demand for commercial manufacturing of FDA- or EMEA-approved drugs.
88
Biologics
Capitalizing on the commercial success of therapeutic monoclonal antibodies and recombinant proteins in the past decade and the increasing demand for biologics contract research, we began providing biologics services in early 2010, with an initial focus on the discovery and preclinical stages, to design and implement strategies for the discovery, engineering and delivery of therapeutic antibody and recombinant protein drug candidates for clinical development. As of June 30, 2010, our biologics team consisted of 24 scientific research staff. Our current biologics services primarily include:
| | • | | Therapeutic antibody generation. We use hybridoma technology, combined with stringent screening funnels, to identify early therapeutic antibody leads with the desired biological and pharmacological properties. As part of our integrated services, we also develop surrogate antibodies forin vivo studies in animal models for target validation and proof-of-concept analysis. |
| | • | | Antibody optimization and engineering.We provide humanization design services for selected therapeutic hybridoma antibody leads, and perform laboratory tests and analysis for humanized lead selection. In addition, we perform affinity maturation to improve the binding affinity and biological potency of the humanized antibodies. These services are supported by our strong protein analytical capabilities. |
| | • | | Analytical services for large molecules. We provide experimental measures to analyze the drug-like properties of therapeutic antibody or recombinant protein drug candidates. Such measures include stability and solubility studies to provide a better understanding of key properties of the drug candidate formulation, as well as pharmacokinetic analysis in rodents and non-human primates. |
We intend to further expand our biologics service offerings and offer a broad spectrum of quality biologics services across the drug discovery and development process, including general molecular biology, protein production and purification, cell line generation, epitope mapping and protein crystallography.
Our Facilities
We are headquartered in Shanghai and conduct our laboratory activities in four primary facilities in China:
| | • | | An approximately 450,000 square-foot headquarters in Shanghai Zhangjiang Hi-Tech Park, primarily used for discovery chemistry and discovery biology services. |
| | • | | An approximately 13,000 square-foot AAALAC-accredited animal facility in Shanghai Zhangjiang Hi-Tech Park, primarily used for discovery biology and preclinical development services. We may build or lease additional animal facilities if the demand for our discovery biology and preclinical development services increases. |
| | • | | An approximately 36,000 square-foot R&D center in Chengdu, primarily used for discovery chemistry services. We have an option to lease additional laboratory and office facilities in Chengdu if the demand for our discovery chemistry services increases. |
| | • | | An approximately 28,000 square-foot manufacturing facility, including a mini-pilot plant, a kilo-lab and a cleaning room unit, in Nanhui, Shanghai, primarily used for pharmaceutical development services. |
Our four primary facilities consist of buildings leased from several property owners, including Shanghai PharmValley Corp., a related party. We have entered into a lease for each building. The leases generally have terms from two to five years and may be renewed by the parties. We generally make rental payments quarterly. Most of our leased buildings are used for office space and research activities.
89
In addition, we are constructing an approximately 460,000 square-foot cGMP-quality multi-purpose manufacturing facility in Fengxian, Shanghai, designed to manufacture advanced intermediates and APIs for preclinical testing and clinical trials. Phase A of the Fengxian facility is scheduled to commence operations in early 2011 and Phase B of such facility is estimated to commence operations in 2011 or later. We are also constructing an approximately 150,000 square-foot laboratory services building in Fengxian, Shanghai primarily for pharmaceutical development services. This building is expected to commence operations in 2011. We will own the manufacturing facility and laboratory services building in Fengxian. We intend to finance the construction of (i) Phase A of the Fengxian manufacturing facility from cash flow from operations and proceeds from unused bank credit facilities, (ii) Phase B of the Fengxian manufacturing facility from the proceeds of this offering, cash flow from operations and bank loans and (iii) the laboratory services building in Fengxian from cash flow from operations, proceeds from this offering and bank loans. We may build additional manufacturing facilities if we experience strong demand for commercial manufacturing of FDA- or EMEA-approved drugs.
Sales and Marketing
We identify potential customers by actively networking with our existing customers, employees and consultants, conducting market research, participating in trade conferences and shows, and publishing marketing materials, among other activities. We also receive a significant amount of business from customer referrals.
Once we identify a potential customer and after making an initial customer contact, we seek to identify the potential customer’s needs for services and align such needs with our service capabilities either through on-site visits to, or teleconferences with, the potential customer. Subsequently, we typically conduct technical due diligence on the potential customer, make specific project proposals and, if the potential customer desires to use our services, negotiate and enter into a service agreement with the customer. We have entered into master services agreements with some customers, the terms of which typically range from one to five years.
With regard to existing customers, we provide quality services in a cost effective manner and seek to renew our contracts on pricing and other terms that are more favorable to us, as well as to sell other services to these customers. We also intend to increase our customer base by broadening the scope of our service offerings, such as introducing biologics and research manufacturing services. We intend to diversify our customer base by targeting more biotechnology companies, which often lack in-house drug discovery and development capabilities, as well as China-based pharmaceutical and biotechnology companies, which have increasingly focused on R&D efforts and outsourced R&D activities, particularly discovery biology, preclinical development and pharmaceutical development activities.
We have significantly expanded our sales and marketing teams in the U.S. and Europe to increase market penetration in our targeted markets and drive revenues growth.
Customers
Our customers consist primarily of large pharmaceutical and biotechnology companies located throughout the world. We provide services to over 100 customers, including all of the top ten pharmaceutical and biotechnology companies in the world in terms of 2009 revenues ranked by Frost & Sullivan and a number of fast-growing biotechnology and specialty pharmaceutical companies, as well as renowned academic and research institutions in the U.S. and China. We generated a significant majority of our total net revenues over the last three years from sales to customers located in the United States. We have received significant recognition and numerous awards from our customers. For example, Lilly, our largest customer in 2009 and the six months ended June 30, 2010, has listed us as a “Global Preferred Partner.” We are a preferred provider of outsourced discovery services to GSK. We were also named as the “Most Valuable Partner” by Roche in 2009, and received an “Excellent Performance Award” from Sunovion Pharmaceuticals Inc. in 2008. Most of our customers return to us for additional and often larger projects, and all of our top ten customers in each of 2008 and 2009 remain our customers in 2010.
90
The following table sets forth our growing and recurring customer base:
| | | | | | | | | | | | | | | | |
| | | For the years ended December 31, | | | For the six
months ended
June 30, 2010 | |
| | | 2007 | | | 2008 | | | 2009 | | |
Top ten customer concentration (% of net revenues) | | | 85 | | | | 77 | | | | 65 | | | | 63 | |
Total number of customers | | | 71 | | | | 99 | | | | 134 | | | | 151 | |
Number of top ten customers that remain as our customers in 2010 | | | 9 | | | | 10 | | | | 10 | | | | 10 | |
Average net revenues per top ten customer (millions
of $) | | | 2.9 | | | | 4.7 | | | | 4.7 | | | | 2.6 | |
For certain major customers, we provide dedicated teams of scientists, laboratory facilities, analytical support and independent information technology and security services. This physical and operational separation of customer projects ensures enhanced security and protection of our customers’ intellectual property. We can tailor our laboratory configuration and setup, research plan, operating procedures, information technology and security protocols to our customers’ specifications.
Our two largest customers in each of 2007, 2008, 2009 and the six months ended June 30, 2010, Lilly and GSK, respectively accounted for 48% and 14% in 2007, 34% and 13% in 2008, 27% and 10% in 2009, and 20% and 9% in the six months ended June 30, 2010 of our net revenues. No other customer accounted for more than 10% of our net revenues in those periods. See “Risk Factors—Risks Relating to Our Business and Industry—A limited number of our customers have accounted and are expected to continue to account for a high percentage of our revenues. The loss of or significant reduction in orders from any of these customers could significantly reduce our revenues and materially and adversely affect our financial condition, results of operations and prospects.”
We entered into two master laboratory service agreements with Lilly in March 2008 and January 2009, respectively. These agreements will expire in June 2011 and December 2011, respectively. Under these agreements, Lilly may place individual work orders with us for various laboratory services. We are entitled to receive payments for services performed. Lilly may terminate these agreements upon 90-days’ notice for any reason.
We also entered into a global research and development outsourced services agreement with GSK in December 2009 with an initial term of three years. GSK has an option to extend the term for up to additional two years. Under this agreement, GSK may execute individual statements of work or research agreements with us for various laboratory services or research programs. We are entitled to receive payments for services performed. GSK may terminate this agreement upon 90-days’ notice for any reason.
Project Management and Customer Support
We believe that we have an established reputation among our customers for high productivity, rapid turnaround time and comprehensive customer support. We generally assume full project management responsibility in our service offerings. We seek to strictly adhere to our internal quality and project management processes. We believe these processes reduce the overall cost to the customer and enhance the quality and speed of delivery. We have developed a project management methodology to ensure timely, consistent and accurate delivery of quality services. To facilitate project management, we developed an online monitoring and reporting system, allowing a customer’s project manager to monitor the progress of its projects through a secure encrypted website. Additionally, our project team interacts with the customer’s project management team via regular conference calls, daily emails and bi-weekly reports. Our project management follows our strategic imperative to protect our customers’ confidentiality and intellectual property. See “—Intellectual Property” below.
91
We conduct frequent external customer satisfaction surveys of key performance indicators to improve our planning, execution, evaluation and support. Internally, we focus on operational improvement and innovation to achieve lower direct costs, better use of assets, faster development time, increased accuracy, greater customization or precision, more added value and simplified processes. Our customer support focuses on sales support and relationship management with our customers, and is dedicated to improving responsiveness to our customers’ needs and inquiries. Less than satisfactory feedback is scrutinized for root causes to improve our operations and services.
Employees
We had 1,115, 1,676 and 1,717 employees as of December 31, 2007, 2008 and 2009, respectively. As of June 30, 2010, we had 1,755 employees, including 1,037 in our discovery chemistry group, 245 in our discovery biology and preclinical development group, 219 in our pharmaceutical development group, 24 in our biologics group and 230 non-scientific personnel. Of the total 1,755 employees as of June 30, 2010, 1,549 worked in our headquarters in Shanghai Zhangjiang Hi-Tech Park, 134 worked in our R&D center in Chengdu, 60 worked in our manufacturing facility in Nanhui, Shanghai, seven worked at the construction site for the planned manufacturing facility and laboratory services building in Fengxian, Shanghai, and five worked at our overseas offices. We consider our relations with our employees to be good. The following chart illustrates the composition of our scientific research staff by educational level as of June 30, 2010:
As of June 30, 2010, we had 1,525 scientific research staff members, over 60% of whom have post-college degrees. We recruit both recent graduates and experienced professionals. Our primary hiring strategy in China is to recruit from colleagues and universities. We also recruit overseas, primarily focusing on returnees and Ph.D.s with significant educational and/or industry background with major pharmaceutical and biotechnology companies, many of whom used to work for our customers, and through referrals, websites, advertisements in trade magazines and job fairs. We expect to hire a significant number of China-based employees during 2010.
Our future growth and profitability depend upon the research and efforts of our experienced and skilled scientists and mid-level managers, and their ability to keep pace with changes in drug discovery and development technologies. We compete vigorously with pharmaceutical firms, biotechnology firms, contract research firms and academic and research institutions to recruit scientists and mid-level managers and employees. See “Risk Factors—Risks Relating to Our Business and Industry—Our ability to execute projects, maintain, expand or renew existing customer engagements and obtain new customers depends largely on our ability to attract, train, motivate and retain skilled scientists.”
We recognize that our employees constitute our most valuable assets and remain focused on training and retention. We are dedicated to cultivating a corporate culture that is customer centric and people oriented through employees who exhibit a can-do attitude, teamwork and innovation. In addition to on-the-job training programs, we have a comprehensive formal training program for employees at different levels, including orientation programs for recent college graduates, management and leadership training for senior employees, and technical skills training, team building and integration training and communication skills training for domestically-trained
92
employees. We have implemented a comprehensive review and incentive system that aligns performance and compensation as well as internal promotions, which also help us motivate and retain our employees.
We pay base salaries and bonuses to all of our employees and pay additional awards to some employees for exceptional performance. As required by PRC regulations, for our China-based employees, we participate in various employee benefit plans organized by municipal and provincial governments, including pension insurance, work-related injury benefits, maternity insurance, medical insurance, unemployment benefit plans and housing funds. We are required under PRC law to make contributions to the employee benefit plans for our China employees at specified percentages of the salaries, bonuses and certain allowances of our employees, up to a maximum amount specified by the local government from time to time.
Intellectual Property
Protection of intellectual property associated with drug R&D is critical to our customers. In our business of providing drug R&D services, our customers generally retain ownership of all associated intellectual property, including intellectual property they provide to us and intellectual property arising from the services we provide. Our success therefore depends in substantial part on our ability to protect the proprietary rights of our customers. This is particularly important because a substantial part of our operations are based in China, and China, as well as Chinese companies, have not traditionally enforced intellectual property protection to the same extent as the United States and U.S. companies.
We take necessary precautions to protect the intellectual property of our customers. We have established standard intellectual property protection procedures to control information access on a need-to know basis. We scan laboratory records into our electronic archives periodically and on an as-needed basis. We enter into agreements with all our employees under which they disown intellectual property they create during their employment, and they waive relevant intellectual property rights or claims. Our employees are also bound by confidentiality obligations and have agreed to disclose and assign to us inventions conceived by them during their respective terms of employment. During their entrance interviews, employees are required to confirm in writing that they will not disclose or use the intellectual property obtained from their previous employers and, during the exit interviews, the departing employees are required to confirm their confidentiality obligations to us and our customers in writing. Furthermore, our service agreements provide that all intellectual property generated during a project is exclusively the property of the customer for whom we are conducting the project.
Although our intellectual property rights are important to our results of operations, we believe that factors such as the technical expertise, knowledge, ability and experience of our employees are more important, and that, overall, these technical capabilities provide significant benefits to our customers.
Despite measures we take to protect intellectual property of our customers or our company, unauthorized parties may attempt to obtain and use information that we regard as proprietary. See “Risk Factors—Risks Relating to Our Business and Industry—If we fail to protect the intellectual property rights of our customers, we may be subject to liability for breach of contract and may suffer damage to our reputation.” To date, we are not aware of any material misuse of our proprietary information.
As of September 30, 2010, we had ten registered trademarks in China, two registered trademarks in Japan and two registered trademarks in the Europe Union. We currently have eight pending trademark applications in China, two pending applications in the United States, and two pending applications in India. In addition, we own 47 Internet domain names as of the date of this prospectus.
Competition
We primarily compete with international and Chinese CROs. We anticipate that we will face increased competition as new companies enter the market and advanced technologies become available. See “Risk
93
Factors—Risks Relating to Our Business and Industry—We face increasingly intense competition. If we do not compete successfully against new and existing competitors, demand for our services and related revenues may decrease and we may be subject to increasing pricing pressure.”
The pharmaceutical and biotechnology R&D outsourcing market remains highly competitive. We compete with WuXi PharmaTech across the breadth of our service offerings. We also compete with industry participants in certain service areas, for example with Albany Molecular Research, Inc. in discovery chemistry and pharmaceutical development, and Cerep S.A. and Covance Inc. in discovery biology and preclinical development.
We compete primarily on the basis of the quality and scope of services, the ability to protect confidential information and intellectual property, the ability to be responsive to and efficient with respect to customers’ requests and pricing. We emphasize high-quality services and place a strategic emphasis on protection of customer intellectual property, both of which result in greater customer acceptance of our services. We also compete on the basis of our relationships with customers. Most of our customers return to us for additional and often larger projects, and all of our top ten customers in each of 2008 and 2009 remain our customers in 2010. We believe our high customer retention rate reflects our customers’ general satisfaction with our services. In addition, we compete on the basis of our turnaround time, resources and geography. We believe we compete favorably on the basis of each of these factors. However, many of our current or future competitors may have longer operating histories, better name recognition, greater levels of consumer trust, stronger management capabilities, better supplier relationships, a larger technical staff and sales force and/or greater financial, technical or marketing resources than we do. In addition, consolidation within the global pharmaceutical and biotechnology R&D outsourcing markets may create stronger competitors than those we currently face.
The successful recruitment and retention of highly qualified scientists is a key element in achieving our strategic goals. We believe that as competitive pressures in the drug R&D industry increase, the recruitment and retention of scientists will become increasingly competitive. To meet this challenge, we actively recruit scientists at colleges and universities and through other means. We believe the sophisticated drug R&D services that we perform and our growth prospects assist us in attracting and retaining highly qualified scientists. We also offer competitive salaries and benefits to recruit and retain highly skilled scientists.
Insurance
We maintain property insurance policies covering physical damage or loss of our equipment, and office furniture, employer’s liability insurance generally covering death or work injury of employees, public liability insurance covering third party bodily injury and property damage incurred in connection with our business within China, and directors and officers liability insurance. We do not have general liability or product liability insurance covering our whole business or all of our products and services. We currently maintain general liability insurance for four contracts covering, among other things, bodily injury and property damages arising out of the products or services provided under these contracts. The aggregate net revenues derived from these contracts in the six months ended June 30, 2010 accounted for 11.0% of our total net revenues in this period. The aggregate maximum coverage amount under the insurance policy for these contracts is $5 million for bodily injury and property damage arising out of our products or services and economic loss sustained by persons or entities due to deficiencies in our products or services. We do not maintain business disruption insurance or key man life insurance. We believe our insurance coverage is customary for companies of comparable size in comparable industries in China. See “Risk Factors—Risks Relating to Our Business and Industry—We have limited insurance coverage, and any claims beyond our insurance coverage may result in our incurring substantial costs and a diversion of resources.”
Environmental Protection and Health and Safety
Our pharmaceutical and biotechnology R&D outsourcing services use highly toxic and hazardous materials. Any failure by us to control the use of, restrict adequately the discharge of, or protect our employees
94
from hazardous substances could subject us to potentially significant monetary damages and fines or suspensions in our business operations. We are subject to national, provincial and local regulations governing the use, manufacture, handling, storage and disposal of hazardous materials.
We have adopted detailed safety procedures in using lab equipment, operating animal facilities and handling chemical, biological and radioactive materials in compliance with applicable laws and regulations. In addition, many of our major pharmaceutical company customers have audited or inspected our facilities for compliance with their own quality assurance standards.
While we are committed to maintaining high safety standards at our work environment, we cannot completely eliminate the risk of contamination or injury resulting from hazardous materials and we may incur liability as a result of any contamination or injury, which may materially and adversely impact our business, financial condition, results of operations and prospects. We have not completed certain environmental assessment or approval procedures or the procedures required to comply with applicable safety and health laws for some of our facilities or projects. We are taking remedial measures to complete all the required procedures and obtain all the necessary approvals. If the relevant government authorities in China determine that we are not in compliance with applicable laws and regulations, we may be required to rectify non-compliance within a specified period of time frame, pay fines or damages to third parties or suspend our operations. See “Risk Factors—Risks Related to Our Business and Industry—Compliance with environmental regulations can be expensive, and noncompliance with these regulations may result in significant monetary damages, fines and other penalties” and “Risk factors—Risks Related to Our Business and Industry—We are subject to safety and health laws and regulations in China, and any failure to comply could adversely affect our operations.”
Legal Proceedings
We may from time to time be subject to various legal or administrative proceedings, either as plaintiff or defendant, arising in the ordinary course of our business. We are not currently a party to, nor are we aware of, any legal proceedings, investigation or claim that, in the view of our management, is likely to materially and adversely affect our business, financial condition or results of operations.
95
REGULATION
Regulation of the Pharmaceutical and Biotechnology R&D Industry in China
This section summarizes the principal laws and regulations relevant to our business activities in China.
State Food and Drug Administration
In the PRC, the State Food and Drug Administration, or SFDA, is the authority that monitors and supervises the administration of pharmaceutical products and medical appliances and equipment as well as food and cosmetics.
The SFDA’s primary responsibilities include:
| | • | | monitoring and supervising the administration of pharmaceutical products, medical appliances and equipment as well as food and cosmetics in the PRC; |
| | • | | formulating administrative rules and policies concerning the supervision and administration of food, dietary supplements, cosmetics and the pharmaceutical industry; |
| | • | | evaluating, registering and approving new drugs, generic drugs, imported drugs and traditional Chinese medicine; and |
| | • | | approving and issuing permits for the manufacture and export/import of pharmaceutical products and medical appliances and equipment and approving the establishment of enterprises for pharmaceutical manufacture and distribution. |
PRC Drug Administration Law and Implementation Rules
The PRC Drug Administration Law promulgated by the Standing Committee of the National People’s Congress in 1984 and the Implementing Measures of the PRC Drug Administration Law promulgated by the MOH in 1989 have established the legal framework for the establishment of pharmaceutical manufacturing enterprises and pharmaceutical trading enterprises and for the administration of pharmaceutical products, which includes the development, research and manufacturing of new drugs and medical preparations by medical institutions. The PRC Drug Administration Law also regulates the packaging, trademark and advertisement of pharmaceutical products in the PRC.
Certain revisions to the PRC Drug Administration Law took effect in December 2001. They were formulated to strengthen the supervision and administration of pharmaceutical products, and to ensure the quality and safety of pharmaceutical products for human use. The revised PRC Drug Administration Law applies to entities and individuals engaged in the development, production, trade, application, supervision and administration of pharmaceutical products. It regulates and prescribes a framework for the administration of pharmaceutical manufacturers, pharmaceutical trading companies, medical preparations of medical institutions and the development, research, manufacturing, distribution, packaging, pricing and advertisement of pharmaceutical products.
The PRC Drug Administration Implementing Rules promulgated by the State Council took effect in September 2002 to provide detailed implementing regulations for the revised PRC Drug Administration Law.
Blood and Reagent Import and Export
According to the Circular on Strengthening Administration on Entry and Exit Inspection and Quarantine of Special Articles for Medical Use, import or export of medical special articles, including without limitation blood and reagents, must be inspected by the relevant inspection and quarantine authorities and be approved by the Ministry of Health or its local counterparts or other authorities, as the case may be.
96
Safety, Health and Environmental Matters
Our operations and properties are subject to extensive environmental protection and health and safety laws and regulations. These laws and regulations govern, among other things, the generation, storage, handling, use and transportation of hazardous or radioactive materials, the handling and disposal of hazardous, biohazardous and radioactive waste generated at our facilities, and the prevention and treatment of occupational diseases.
These laws and regulations generally impose liability regardless of the negligence or fault of a responsible party, unless such party has legally defined immunities. These laws and regulations also require us to obtain verifications, approvals or permits from governmental authorities for certain operations or facilities. If we violate or fail to comply with these laws, regulations or permits, we could be fined or otherwise sanctioned by regulators. Under certain environmental laws and regulations, we could also be held responsible for all of the costs relating to any contamination at our past or present facilities and at third party waste disposal sites.
Although we believe that our costs of complying with current and future environmental and health and safety laws, and our liabilities arising from past or future releases of, or exposure to, hazardous substances will not materially and adversely affect our business, results of operations or financial condition, they may do so. In addition, we cannot eliminate the risk of accidental contamination or injury from these hazardous materials. In that event, we could be liable for any resulting damage, which could exceed our resources and harm our results of operations. We also have not completed certain environmental assessment or approval procedures or certain procedures required for compliance with safety and health laws for some of our facilities or projects. See “Risk Factors—Risks Relating to Our Business and Industry—Compliance with environmental regulations can be expensive, and noncompliance with these regulations may result in significant monetary damages and fines and other penalties” and “Risk Factors—Risks Relating to Our Business and Industry—We are subject to safety and health laws and regulations in China, and any failure to comply could adversely affect our operations.”
Trade Secrets
According to the Anti-unfair Competition Law of the PRC, “trade secrets” refer to technical and business information that is unknown to the public, has utility and may create business interest or profit for its legal owners or holders, and which is maintained as secrets by its legal owners or holders.
Under this law, business persons are prohibited from employing the following methods to infringe trade secrets: (i) to obtain the trade secrets from the legal owners or holders by any unfair methods such as stealing, solicitation or coercion; (ii) to disclose, use or permit others to use the trade secrets obtained illegally under item (i) above; or (iii) to disclose, use or permit others to use the trade secrets in violation of any contractual agreements or any requirements of the legal owners or holders to keep such trade secrets in confidence. If a third party knows or should have known the above-mentioned illegal conduct but nevertheless obtains, uses or discloses such trade secrets of others, it may be deemed to have misappropriated others’ trade secrets. The parties being harmed may petition for administrative corrections, and competent regulatory authorities may stop any illegal activities and may fine infringing parties in the amount of RMB10,000 to RMB200,000. Alternatively, persons being harmed may file lawsuits for damages caused due to the misappropriation of the trade secrets in a competent PRC court.
The measures to protect trade secrets include oral or written agreements or other reasonable measures to require the employees of or persons in business contact with legal owners or holders to keep such trade secrets confidential. As soon as the legal owners or holders request others to keep the trade secrets confidential and adopt reasonable protection measures, the requested persons shall bear the liability to keep the trade secrets confidential.
Animal Test Permit and Other Related Regulatory Requirements
According to the Administrative Measures on Certificate for Animal Experimentation (Trial), performing experimentation on animals requires a Certificate for Use of Laboratory Animals. Applicants must
97
satisfy the conditions as follows: (i) laboratory animals must be qualified and from entities that have Certificates for Production of Laboratory Animals; (ii) environment and facilities for laboratory animals’ living and propagating must meet state requirements; (iii) laboratory animals’ feed must meet state requirements; (iv) workers feeding or experimenting on laboratory animals must have received professional training; (v) management systems must be effective and efficient; and (vi) the applicable entity must follow other requirements as stipulated by PRC laws and regulations.
Pathogenic Microorganisms Lab Classification
According to the Administrative Rules on the Bio-safety of Pathogenic Microorganism Labs promulgated by the State Council on November 12, 2004, the labs conducting pathogenic microorganism-related experiments in China are classified into four levels based on their different bio-safety protection levels as well as in accordance with the national bio-safety standards. Level 1 and Level 2 labs are prohibited from conducting experiments relating to highly pathogenic microorganisms, and the establishment, alteration and extension of such labs are required to be filed with the local administrative authority of health or veterinary.
Radioisotope Use License and Other Related Regulatory Requirements
According to applicable PRC laws and regulations, it is mandatory to obtain a radiation safety license for radioisotope material handling. To apply for such license, applicants are required to meet certain conditions, including having: (i) professionals who have the relevant safety and self-protection knowledge, meet health requirements and are qualified to handle radioisotope materials; (ii) the facilities and equipment that meet national environmental, health and safety standards; (iii) a dedicated safety and monitoring committee or dedicated safety and monitoring personnel equipped with appropriate protective equipment and monitoring instruments; (iv) sound internal safety and monitoring rules and procedures and emergency response measures to prevent and minimize the damages caused by radiation accidents; and (v) the capability to treat any radioactive waste gas, liquid or solid or having a feasible treatment plan to ensure that the radioactive waste is discharged appropriately.
In addition, the purchase of radioisotope materials needs to be approved by relevant environmental protection authorities and registered with relevant environmental protection authorities after the transfer and the disposal of radioisotope material waste should be performed by qualified entities and be filed with relevant environmental protection authorities.
Use of Narcotic Drugs and Psychotropic Drugs
China regulates the production, distribution and use of narcotic drugs and psychotropic drugs pursuant to the Regulations on the Administration of Narcotic Drugs and Psychotropic Drugs, which became effective on November 1, 2005. Under these regulations, only those entities designated by the State Food and Drug Administration or its provincial branches may engage in the production or distribution of narcotic drugs and psychotropic drugs. In case that an entity engaged in scientific researches needs to use narcotic drugs or psychotropic drugs to carry out experiment activities, it may, upon the approval of provincial food and drug administration, purchase such drugs from those designated entities.
Regulations on Tax
PRC Enterprise Income Tax
The PRC enterprise income tax is calculated based on the taxable income determined under the PRC laws and accounting standards. On March 16, 2007, the National People’s Congress of China enacted a new PRC Enterprise Income Tax Law, which became effective on January 1, 2008. On December 6, 2007, the State Council promulgated the Implementing Rules to the PRC Enterprise Income Tax Law, or the Implementing
98
Rules, which also became effective on January 1, 2008. On December 26, 2007, the State Council issued the Notice on Implementation of Enterprise Income Tax Transition Preferential Policy under the PRC Enterprise Income Tax Law, or the Transition Preferential Policy Circular, which became effective simultaneously with the PRC Enterprise Income Tax Law. The PRC Enterprise Income Tax Law imposes an uniform enterprise income tax rate of 25% on all domestic enterprises, including foreign-invested enterprises unless they qualify for certain exceptions, and terminates most of the tax exemptions, reductions and preferential treatments available under previous tax laws and regulations. Under the PRC Enterprise Income Tax Law and the Transition Preferential Policy Circular, enterprises established before March 16, 2007 that already enjoyed preferential tax treatments will continue to enjoy them (i) in the case of preferential tax rates, for a maximum of a five-year period starting from January 1, 2008; during the five-year period, the tax rate will gradually increase from their current preferential tax rate to 25%, or (ii) in the case of preferential tax exemption or reduction for a specified term, until the expiration of such term.
Prior to the effectiveness of the PRC Enterprise Income Tax Law on January 1, 2008, domestic companies were generally subject to an enterprise income tax at a statutory rate of 33%. However, our PRC subsidiaries in China that satisfied certain conditions enjoyed preferential tax treatment.
The new PRC Enterprise Income Tax Law and the Implementing Rules permit certain “high and new technology enterprises strongly supported by the state” that hold independent ownership of core intellectual property and simultaneously meet a list of other criteria, financial or non-financial, as stipulated in the Implementing Rules, to enjoy a reduced 15% enterprise income tax rate subject to certain new qualification criteria. The State Administration of Taxation, the Ministry of Science and Technology and the Ministry of Finance jointly issued the Administrative Rules for the Certification of High and New Technology Enterprises delineating the specific criteria and procedures for the “high and new technology enterprises” certification on April 14, 2008.
Moreover, under the PRC Enterprise Income Tax Law, enterprises organized under the laws of jurisdictions outside China with “de facto management bodies” located within China may be considered PRC resident enterprises and therefore subject to a 25% PRC enterprise income tax on their worldwide income. The Implementing Rules define “de facto management body” as the management body that exercises full and substantial control and overall management over the business, productions, personnel, accounts and properties of an enterprise. In addition, SAT Circular 82 provides that a foreign enterprise controlled by a PRC company or a PRC company group will be classified as a “resident enterprise” with its “de facto management bodies” located within China if the following requirements are satisfied: (i) the senior management and core management departments in charge of its daily operations function mainly in the PRC; (ii) its financial and human resource decisions are subject to determination or approval by persons or bodies in the PRC; (iii) its major assets, accounting books, company seals, and minutes and files of its board and shareholders’ meetings are located or kept in the PRC; and (iv) more than half of the enterprise’s directors or senior management with voting rights reside in the PRC. Although the SAT Circular 82 only applies to offshore enterprises controlled by PRC enterprises and not those controlled by PRC individuals, foreigners or foreign enterprises, the determining criteria set forth in the Identification Circular may reflect the State Administration of Taxation’s general position on how the “de facto management body” test should be applied in determining the tax resident status of offshore enterprises, regardless of whether they are controlled by PRC or foreign enterprises or individuals.
Although we believe we are not a PRC resident enterprise for enterprise income tax purposes, substantial uncertainty exists. In the event that our company or any of our Hong Kong subsidiaries is considered a PRC resident enterprise, (1) we would be subject to the PRC enterprise income tax at the rate of 25% on our worldwide income; and (2) dividend income that our Hong Kong subsidiaries receive from our PRC subsidiaries, however, would be exempt from the PRC withholding tax since such income is exempted under the PRC
99
Enterprise Income Tax Law for PRC resident enterprise recipients. See “Risk Factors—Risks Related to Doing Business in China—Our global income and the dividends that we may receive from our PRC subsidiaries may be subject to PRC taxes under the PRC Enterprise Income Tax Law, which would materially and adversely affect our results of operations.”
Under SAT Circular 698, if a non-resident enterprise transfers the equity interests of a PRC resident enterprise indirectly via disposing of the equity interests of an overseas holding company, or Indirect Transfer, and such overseas holding company is located in a tax jurisdiction that: (i) has an effective tax rate less than 12.5% or (ii) does not tax foreign income of its residents, the foreign investor shall report to the competent tax authority of the PRC resident enterprise this Indirect Transfer. Using a “substance over form” principle, the PRC tax authority may disregard the existence of the overseas holding company if it lacks a reasonable commercial purpose and was established for the purpose of avoiding PRC tax. As a result, gains derived from such Indirect Transfer may be subject to PRC withholding tax at a rate of up to 10%. SAT Circular 698 also provides that, where a non-PRC resident enterprise transfers its equity interests in a PRC resident enterprise to its related parties at a price lower than the fair market value, the relevant tax authority has the power to make a reasonable adjustment to the taxable income of the transaction. SAT Circular 698 is retroactively effective on January 1, 2008.
PRC Business Tax and Value Added Tax
Pursuant to applicable PRC laws and regulations, any entity or individual conducting business in the service industry is generally required to pay a business tax at the rate of 5% on the revenues generated from providing such services. However, if the services provided are related to technology development and transfer, such business tax may be exempted subject to the approval of relevant tax authorities.
Pursuant to the PRC Provisional Regulation on the Value Added Tax, or VAT, and its implementing rules, we are required to pay a VAT on the gross sales proceeds received from compound delivery services or product sales activities, less any creditable input VAT already paid or borne. VAT is levied generally at a rate of 17%, while certain export sales are entitled to certain VAT credits.
Dividend Withholding Tax
Under the PRC tax laws effective prior to January 1, 2008, dividends paid to foreign investors by foreign-invested enterprises, such as dividends paid to us by our PRC subsidiaries, were exempted from PRC withholding tax. We are a Cayman Islands holding company and substantially all of our income may come from dividends we receive from our subsidiaries located in Hong Kong, which are the direct holding companies of our PRC subsidiaries. Pursuant to the PRC Enterprise Income Tax Law and the Implementation Rules, as well as the Arrangement between the Mainland of China and Hong Kong Special Administrative Region on the Avoidance of Double Taxation and Prevention of Fiscal Evasion with Respect to Taxes on Income, or the Arrangement, dividends generated after January 1, 2008 and distributed to our Hong Kong subsidiaries by our PRC subsidiaries may be subject to withholding tax at a preferential rate of no more than 5%, provided that our Hong Kong subsidiaries are determined by the relevant PRC tax authorities to be “non-resident enterprises” and hold at least 25% of the equity interests of our PRC subsidiaries. A non-resident enterprise under the PRC Enterprise Income Tax Law is incorporated in jurisdictions other than PRC but has an establishment or place of business (other than Management Bodies) in China or, despite the non-existence of such establishment or place in China, derives income within China. The State Administration for Taxation promulgated Notice on How to Understand and Determine the Beneficial Owners in Tax Agreement on October 27, 2009, or SAT Circular 601, which provides guidance for determining whether a resident of a contracting state is the “beneficial owner” of an item of income under China’s tax treaties and tax arrangements. According to SAT Circular 601, a beneficial owner generally must be engaged in substantive business activities. An agent or conduit company will not be regarded as a
100
beneficial owner and, therefore, will not qualify for treaty benefits. The conduit company normally refers to a company established for the purpose of avoiding or reducing taxes or transferring or accumulating profits. In addition, as described above, our Hong Kong subsidiaries may be considered PRC resident enterprises for enterprise income tax purposes, in which case dividends received by them from our PRC subsidiaries would be exempt from the PRC withholding tax because such income is exempt under the PRC Enterprise Income Tax Law for PRC resident enterprise recipients.
As there remains uncertainty regarding the interpretation and implementation of the PRC Enterprise Income Tax Law and the Implementation Rules as well as the Arrangement, it is uncertain whether, if we are deemed a PRC resident enterprise, any dividends to be distributed by us to our non-PRC shareholders and ADS holders would be subject to any PRC withholding tax. See “Risk Factors—Risks Related to Doing Business in China—Our global income and the dividends that we may receive from our PRC subsidiaries may be subject to PRC taxes under the PRC Enterprise Income Tax Law, which would materially and adversely affect our results of operations.”
Regulations on Foreign Exchange
Foreign exchange activities in China are primarily governed by the following regulations:
| | • | | Foreign Currency Administration Rules (1996), as amended, or the Exchange Rules; and |
| | • | | Administration Rules of the Settlement, Sale and Payment of Foreign Exchange (1996), or the Administration Rules. |
Under the Exchange Rules, the RMB is convertible for current account items, including the distribution of dividends, interest and royalties payments, trade and service-related foreign exchange transactions. Conversion of RMB for capital account items, such as direct investment, loan, securities investment and repatriation of investment, however, is subject to the approval of SAFE or its local counterpart.
Under the Administration Rules, foreign-invested enterprises may only buy, sell and/or remit foreign currencies at banks authorized to conduct foreign exchange business after providing valid commercial documents and, in the case of capital account item transactions, obtaining approval from SAFE or its local counterpart. Capital investments outside of China, after obtaining the required approvals of the relevant approval authorities, such as Ministry of Commerce and the National Development and Reform Commission or their local counterparts, are also required to register with SAFE or its local counterpart.
Pursuant to Circular 142, the RMB fund from the settlement of foreign currency capital of a foreign-invested enterprise must be used within the business scope as approved by the examination and approval department of the government, and cannot be used for domestic equity investment unless it is otherwise provided for. Documents certifying the purposes of the RMB fund from the settlement of foreign currency capital, including a business contract, must also be submitted for the settlement of the foreign currency. In addition, SAFE strengthened its oversight of the flow and use of the RMB capital converted from foreign currency registered capital of a foreign-invested company. The use of such RMB capital may not be altered without SAFE’s approval, and such RMB capital may not in any case be used to repay RMB loans if the proceeds of such loans have not been used. Violations of the Circular 142 could result in severe monetary fines or penalties.
Regulations on Dividend Distribution
The principal regulations governing dividend distributions of wholly foreign-owned companies include:
| | • | | the Companies Law (2005); |
| | • | | the Wholly Foreign-Owned Enterprise Law (1986), as amended; and |
| | • | | the Wholly Foreign-Owned Enterprise Law Implementing Rules (1990), as amended. |
101
Under these regulations, wholly foreign-owned companies in the PRC may pay dividends only out of their accumulated profits as determined in accordance with PRC accounting standards and regulations. In addition, these wholly foreign-owned companies are required to set aside at least 10% of their respective accumulated profits each year, if any, to fund certain reserve funds, until the accumulative amount of such fund reaches 50% of its registered capital. At the discretion of these wholly foreign-owned companies, they may allocate a portion of their after-tax profits based on PRC accounting standards to staff welfare and bonus funds. These reserve funds and staff welfare and bonus funds are not distributable as cash dividends.
Regulations on Offshore Investment by PRC Residents
Pursuant to SAFE Circular 75, issued on October 21, 2005, (i) a PRC citizen residing in the PRC or non-PRC citizen primarily residing in the PRC due to his or her economic ties to the PRC, who is referred to as a PRC resident in Circular 75, shall register with the local branch of SAFE before it establishes or controls an overseas special purpose company, for the purpose of overseas equity financing; (ii) when a PRC resident contributes the assets of or its equity interests in a domestic enterprise into an overseas special purpose company, or engages in overseas financing after contributing assets or equity interests into a special purpose company, such PRC resident shall register his or her interest in the special purpose company and the change thereof with the local branch of SAFE; and (iii) when the special purpose company undergoes a material event outside of China, such as change in share capital, creation of any security interests on its assets or merger and acquisition, the PRC resident shall, within 30 days from the occurrence of such event, register such change with the local branch of SAFE. PRC residents who are shareholders of special purpose companies established before November 1, 2005 were required to register with the local branch of SAFE before March 31, 2006.
In 2003, our founders, who may be deemed PRC residents for the purpose of Circular 75, established our intermediary holding companies in Hong Kong, which subsequently acquired or established their directly-owned subsidiaries in China. Circular 75 retroactively applies to our initial formation of the corporate structure as a round-trip investment and required our founders to register their respective shareholdings in our company with the local SAFE branch in Shanghai by March 31, 2006. Our founders inadvertently missed this filing deadline. We have urged our founders to, and they have been making efforts in order to, apply for a remedial SAFE registration. Although such a remedial registration is permissible under supplementary rules of Circular 75, the local SAFE branch in Shanghai has not accepted our founders’ respective applications due to the lack of specific administrative procedures for remedial registrations. We will urge our founders to, and our founders will continue making efforts to, apply for a remedial SAFE registration. If for any reason the relevant government authorities in China determine that our founders do not comply with Circular 75, our founders may be subject to administrative measures and penalties, such as orders to rectify and monetary fines, and any proposed foreign exchange transaction between our Hong Kong intermediary holding companies and their directly owned subsidiaries in China may be restricted. See “Risk Factors—Risks Related to Doing Business in China—PRC regulations relating to the establishment of offshore special purpose companies by PRC residents may subject our PRC resident beneficial owners or our PRC subsidiaries to liability or penalties, limit our ability to inject capital into our PRC subsidiaries, limit our PRC subsidiaries’ ability to increase their registered capital or distribute profits to us, or may otherwise adversely affect us.”
Regulations on Employee Stock Options Plan
In December 2006, the People’s Bank of China promulgated the Administrative Measures of Foreign Exchange Matters for Individuals, setting forth the respective requirements for foreign exchange transactions by individuals (both PRC or non-PRC citizens) under either the current account or the capital account. In January 2007, SAFE issued implementing rules for the Administrative Measures of Foreign Exchange Matters for Individuals, which, among other things, specified approval requirements for certain capital account transactions, such as a PRC citizen’s participation in employee stock ownership plans or share option plans of an overseas publicly-listed company. On March 28, 2007, the State Administration of Foreign Exchange promulgated the Application Procedures of Foreign Exchange Administration for Domestic Individuals Participating in Employee
102
Stock Ownership Plan or Stock Option Plan of Overseas Listed Company, or the Stock Option Rules. The purpose of the Stock Option Rules is to regulate the foreign exchange administration of PRC domestic individuals who participate in employee stock holding plans and share option plans of overseas listed companies.
According to the Stock Option Rules, if a PRC domestic individual participates in any employee stock ownership plan or share option plan of an overseas listed company, a PRC domestic qualified agent or the PRC subsidiary of such overseas listed company must, among other things, file, on behalf of such individual, an application with SAFE or its local counterpart to obtain approval for an annual allowance with respect to the purchase of foreign exchange in connection with stock holding or share option exercises as PRC domestic individuals may not directly use overseas funds to purchase shares or exercise share options. Concurrent with the filing of such application with SAFE or its local counterpart, the PRC domestic qualified agent or the PRC subsidiary shall obtain approval from SAFE or its local counterpart to open a special foreign exchange account at a PRC domestic bank to hold the funds required in connection with the stock purchase or option exercise, any returned principal or profits upon sales of shares, any dividends issued on the stock and any other income or expenditures approved by SAFE or its local counterpart. The PRC domestic qualified agent or the PRC subsidiary is also required to obtain approval from SAFE or its local counterpart to open an overseas special foreign exchange account at an overseas trust bank with custody qualifications to hold overseas funds used in connection with any shares purchase. Upon the completion of this offering, we will become an overseas listed company, and we and our Chinese employees who have been granted share options, RSUs or shares will be subject to, and intend to comply with, the foregoing requirements.
Under the Foreign Currency Administration Rules, as amended in 2008, the foreign exchange proceeds of domestic entities and individuals can be remitted into China or deposited abroad, subject to the terms and conditions to be issued by SAFE. However, the implementation rules in respect of depositing the foreign exchange proceeds abroad have not been issued by SAFE. The foreign exchange proceeds from the sales of shares can be converted into RMB or transferred to such individuals’ foreign exchange savings account after the proceeds have been remitted back to the special foreign exchange account opened at the PRC domestic bank. If share options are exercised in a cashless exercise, the PRC domestic individuals are required to remit the proceeds to special foreign exchange accounts.
Many issues with respect to the Stock Option Rules require further interpretation. We and our PRC employees who have participated in an employee stock ownership plan or share option plan will be subject to the Stock Option Rules when our company becomes an overseas listed company. If we or our PRC employees fail to comply with the Stock Option Rules, we and our PRC employees may face sanctions imposed by the PRC foreign exchange authority or any other PRC government authorities, including restriction on foreign currency conversions and additional capital contribution to our PRC subsidiaries.
In addition, the State Administration of Taxation has issued a few circulars concerning employee share options. Under these circulars, our employees working in China who exercise share options will be subject to PRC individual income tax. Our PRC subsidiaries have obligations to file documents related to employee share options with relevant tax authorities and withhold the individual income taxes of employees who exercise their share options. If our employees fail to pay and we fail to withhold their income taxes, we may face sanctions imposed by tax authorities or any other PRC government authorities.
Labor Laws and Social Insurance
Pursuant to the PRC Labor Law and the PRC Labor Contract Law, employers must execute written labor contracts with employees in order to establish an employment relationship. All employers must compensate their employees with wages equal to at least the local minimum wage standards. All employers are required to establish a system for labor safety and sanitation, strictly abide by state rules and standards and provide employees with workplace safety training. Violations of the PRC Labor Contract Law and the PRC Labor Law may result in the imposition of fines and other administrative liabilities. Criminal liability may arise for serious violations.
103
In addition, employers in China are obliged to provide employees with welfare schemes covering pension insurance, unemployment insurance, maternity insurance, work-related injury insurance, medical insurance and housing funds.
Regulations on Overseas Listing
In 2006, six PRC regulatory agencies jointly adopted the Regulations on Mergers and Acquisitions of Domestic Enterprises by Foreign Investors, or the M&A Rule. This rule requires that, if an overseas company established or controlled by PRC domestic companies or citizens intends to acquire equity interests or assets of any other PRC domestic company affiliated with the PRC domestic companies or citizens, such acquisition must be submitted to the Ministry of Commerce, rather than local regulators, for approval. In addition, this regulation requires that an overseas company controlled directly or indirectly by PRC companies or citizens and holding equity interests of PRC domestic companies needs to obtain the approval of the CSRC prior to listing its securities on an overseas stock exchange. On September 21, 2006, the CSRC, published a notice on its official website specifying the documents and materials required to be submitted by overseas special purpose companies seeking the CSRC’s approval of their overseas listings.
While the application of the M&A Rule remains unclear, based on their understanding of current PRC laws, regulations, and the notice published on September 21, 2006, our PRC counsel, Fangda Partners, has advised us that we are not required to submit an application to the CSRC for its approval for the listing and trading of our ADSs on the New York Stock Exchange, because our PRC subsidiaries were either converted into wholly foreign owned enterprises prior to September 8, 2006, the effective date of the M&A rule or were incorporated as wholly foreign owned enterprises.
Fangda has further advised us that their opinions summarized above are subject to the timing and content of any new laws, rules and regulations or clearer implementation and interpretations from the Ministry of Commerce and CSRC in any form relating to the M&A Rule.
See “Risk Factors—Risks Related to Doing Business in China—The approval of the China Securities Regulatory Commission may be required in connection with this offering under a regulation adopted in August 2006, and, if required, we cannot predict whether we will be able to obtain such approval.”
104
MANAGEMENT
Directors and Executive Officers
The following table sets forth information regarding our directors and executive officers as of the date of this prospectus.
| | | | |
Directors and Executive Officers | | Age | | Position/Title |
Michael Xin Hui | | 39 | | Chairman of the Board of Directors and Chief Executive Officer |
Kevin Penghui Chen | | 38 | | Director, Chief Operating Officer and Chief Financial Officer |
Wenjuan Xiao | | 69 | | Director |
Julian Ralph Worley | | 65 | | Independent Director |
Yuk Lam Lo | | 62 | | Independent Director |
Lan Xie | | 37 | | Vice President of Finance and Operations |
Chunyu Zhang | | 44 | | Vice President of Human Resources |
Michael Xin Hui has served as our chairman of the board of directors and chief executive officer since the inception of our company in 2002. Mr. Hui is a co-founder of our company and one of the key managerial personnel of our business, and is responsible for operations, strategic planning and business development. From 2000 to 2002, Mr. Hui was a vice president at Softbank Investment E2-Capital, a company based in Shanghai and Hong Kong, China. From 1999 to 2000, Mr. Hui worked as an investment manager of Phytomedica, Inc., a company in New York. In 1998, Mr. Hui worked as a project manager of China Innovation Center For Life Science in New York. Prior to that, Mr. Hui worked as a research scientist for American Home Products Corporation in New York. Mr. Hui holds a bachelor’s degree in chemistry from Oregon State University, a master’s degree in chemistry from Tulane University and an MBA from New York University.
Kevin Penghui Chenhas served as our director and chief operating officer since 2008 and our chief financial officer since September 2010. Prior to joining our company, Mr. Chen was a vice president at CITIC Capital, a China-focused private equity fund in Shanghai, China. In 2007, Mr. Chen worked for Eaton Corporation in Shanghai, China in charge of corporate development. From 2003 to 2006, Mr. Chen worked for DuPont Corporation in Shanghai as a business development manager. Prior to that, he worked as a scientist at Ligand Pharmaceuticals, Inc. Mr. Chen holds a bachelor’s degree in chemistry from Nanjing University, a master’s degree in chemistry from Tulane University and an MBA from Kellogg School of Management, Northwestern University.
Wenjuan Xiaohas served as our director since the inception of our company in 2002. Ms. Xiao is a co-founder of our company. Ms. Xiao was an associate professor from 1989 to 1997 and an research associate from 1980 to 1989 at Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences. She holds a bachelor’s degree in science from Fudan University. Ms. Xiao is the mother of Michael Xin Hui.
Julian Ralph Worley has served as our independent director since October 18, 2010. Mr. Worley has served as an independent non-executive director of Suntech Power Holdings Co. Ltd, a leading solar energy company based in China and listed on the New York Stock Exchange, since 2006. From 2005 to February 2010, he was an independent non-executive director of Mandra Forestry Finance Limited and its holding company, Mandra Forestry Holdings Limited, an owner and operator of forestry plantations. From 2000 to 2003, he was a consultant at partner level in PricewaterhouseCoopers Philippines. He served as an audit partner in PricewaterhouseCoopers Hong Kong from 1998 to 2000 and an audit partner in Price Waterhouse Hong Kong from 1974 to 1998. He is a fellow of the Hong Kong Institute of Directors, a fellow of the Institute of Chartered Accountants in England and Wales and a fellow of the Hong Kong Institute of Certified Public Accountants.
105
Mr. Worley holds a bachelor’s degree in economics from London School of Economics and Political Science, University of London.
YukLam Lohas served as our independent director since October 18, 2010. Mr. Lo has served as an independent director of Sinovac Biotech Limited, a biopharmaceutical company based in China and listed on NASDAQ, since 2006, an independent non-executive director of South East Group Limited, a property development and investment company listed on the Hong Kong Stock Exchange, since 2006 and as a senior director of Questmark Asia Limited, a private equity firm, since 2009. Mr. Lo is presently the chairman of the Chinese Manufacturers’ Association of Hong Kong – Innovation and Technology Committee and the honorary life chairman of Hong Kong Biotechnology Organization. He was the former chairman of the Innovation and Technology Fund (Biotechnology Projects) Vetting Committee and the Biotechnology Committee of the Industry and Technology Development Council of the Hong Kong government. Mr. Lo is currently an adjunct professor of the Chinese University of Hong Kong, a special advisor to the Hong Kong University of Science and Technology, an honorary court member of the Hong Kong Baptist University and honorary professor in several universities in mainland China. Mr. Lo has been awarded an honorary fellowship by the Hong Kong University of Science and Technology.
Lan Xie has served as our vice president of finance and operations since March 2010 and, prior to that, our financial controller and vice president of finance since November 2007. Prior to November 2007, Ms. Xie was a senior manager at PricewaterhouseCoopers in Shanghai, China from 2005 to 2007. From 2003 to 2005, Ms. Xie was a manager at Deloitte & Touche LLP in New York. From 1998 to 2002, Ms. Xie worked as a finance manager at Reuters Incorporated. Prior to that, Ms. Xie worked as a senior associate at PricewaterhouseCoopers LLP in Boston. Ms. Xie holds a bachelor’s degree in business administration from Boston University and an MBA from INSEAD.
Chunyu Zhang has served as our vice president of human resources since 2009. From 2008 to 2009, Ms. Zhang worked as a human resources consultant for us. From 2007 to 2008, Ms. Zhang worked as chief consultant for Shanghai Ren-link Management Consulting Co. Ltd. In 2007, Ms. Zhang worked at Goodyear Tire Management Company as a human resources business leader of operations responsible for supervising human resources managers in nine countries. From 2005 to 2007, Ms. Zhang worked as a human resources director of the digital film imaging system division at Kodak China (Investment) Co., Ltd. From 2002 to 2005, Ms. Zhang was a human resources business partner at Avaya (China) Communication Co., Ltd. in Shanghai. From 1999 to 2002, Ms. Zhang was a human resources director at Ingram Micro (China) Co., Ltd. Prior to that, Ms. Zhang held various human resources related positions in several other multi-national companies. Ms. Zhang holds a bachelor’s degree in arts from Beijing Foreign Studies University and an EMBA from China Europe International Business School.
Board of Directors
Our board of directors consists of five directors. A director is not required to hold any shares in the company by way of qualification. A director may vote with respect to any contract, proposed contract or arrangement in which he is materially interested provided the nature of the interest is disclosed prior to voting. A director may exercise all the powers of the company to borrow money, mortgage its undertaking, property and uncalled capital, and issue debentures or other securities whenever money is borrowed or as security for any obligation of the company or of any third party. None of our non-executive directors has a service contract with us that provides for benefits upon termination of employment.
106
Committees of the Board of Directors
We have established three committees under the board of directors: the audit committee, the compensation committee and the nominating and corporate governance committee. We have adopted a charter for each of these committees. Each committee’s members and functions are described below.
Audit Committee. Our audit committee consists of Messrs. Julian Ralph Worley, Yuk Lam Lo and Kevin Penghui Chen, and is chaired by Mr. Worley. Messrs. Worley and Lo satisfy the “independence” requirements of Section 303A of the Corporate Governance Rules of the New York Stock Exchange and Rule 10A-3 under the Securities Exchange Act of 1934. The audit committee oversees our accounting and financial reporting processes and the audits of the financial statements of our company. The audit committee is responsible for, among other things:
| | • | | selecting the independent auditors and pre-approving all auditing and non-auditing services permitted to be performed by the independent auditors; |
| | • | | reviewing with the independent auditors any audit problems or difficulties and management’s response; |
| | • | | reviewing and approving past or proposed related party transactions; |
| | • | | reviewing the annual audited financial statements with management and the independent auditors; |
| | • | | reviewing major issues as to the adequacy of our internal controls and any special audit steps adopted in light of material control deficiencies; |
| | • | | meeting separately and periodically with management and the independent auditors; and |
| | • | | monitoring compliance with our code of business conduct and ethics, including reviewing the adequacy and effectiveness of our procedures to ensure proper compliance. |
Compensation Committee. Our compensation committee consists of Messrs. Yuk Lam Lo, Julian Ralph Worley and Michael Xin Hui, and is chaired by Mr. Lo. Messrs. Lo and Worley satisfy the “independence” requirements of Section 303A of the Corporate Governance Rules of the New York Stock Exchange. The compensation committee assists the board in reviewing and approving the compensation structure, including all forms of compensation, relating to our directors and executive officers. Our chief executive officer may not be present at any committee meeting during which his compensation is deliberated. The compensation committee is responsible for, among other things:
| | • | | reviewing and approving the total compensation package for our chief executive officer; |
| | • | | reviewing and recommending to the board the compensation of our other executive officers; |
| | • | | evaluating the compensation of our non-employee directors; and |
| | • | | reviewing periodically our general compensation plans and other employee-related plans, including incentive compensation or equity plans. |
Nominating and Corporate Governance Committee. Our nominating and corporate governance committee consists of Messrs. Michael Xin Hui, Julian Ralph Worley and Yuk Lam Lo, and is chaired by Mr. Hui. Messrs. Lo and Worley satisfy the “independence” requirements of Section 303A of the Corporate Governance Rules of the New York Stock Exchange. The nominating and corporate governance committee assists the board of directors in identifying individuals qualified to become our directors and in determining the composition of the board and its committees. The nominating and corporate governance committee will be responsible for, among other things:
| | • | | identifying and recommending to the board nominees for election or re-election to the board; |
107
| | • | | reviewing annually with the board the composition of the board in light of the characteristics of independence, age, skills, experience and availability of service to us; |
| | • | | identifying and recommending to the board the directors to serve as members of the board’s committees; and |
| | • | | advising the board periodically with respect to significant developments in the law and practice of corporate governance as well as our compliance with applicable laws and regulations, and making recommendations to the board on all matters of corporate governance and on any corrective action to be taken. |
Duties of Directors
Under Cayman Islands law, our directors have a statutory duty of loyalty to act honestly in good faith with a view to our best interests. Our directors also have a duty to exercise the skill they actually possess and such care and diligence that a reasonably prudent person would exercise in comparable circumstances. In fulfilling their duty of care to us, our directors must ensure compliance with our memorandum and articles of association. A shareholder has the right to seek damages if a duty owed by our directors is breached.
Terms of Directors and Officers
Our officers are elected by and serve at the discretion of the board of directors. Our directors are not subject to a term of office and hold office until such time as they resign or are removed from office by ordinary resolution of our shareholders or affirmative votes of at least two thirds of our directors then in office. A director will be removed from office automatically if, among other things, the director (i) becomes bankrupt or makes any arrangement or composition with his creditors; or (ii) dies or is found by our company to be or becomes of unsound mind.
Employment Agreements
We have entered into employment agreements with each of our executive officers. Under these agreements, each of our executive officers is employed for a specified period. We may terminate employment for cause, at any time, without notice or remuneration, for certain acts of the employee, such as causing material losses and damages to our company via serious neglect of duties, serious misconduct for selfish ends and fraudulent behavior and being charged with criminal offense. We may also terminate an executive officer’s employment without cause with severance payments.
Each executive officer has agreed to hold, both during and after the termination or expiration of his or her employment agreement, in strict confidence and not to use, except as required in the performance of his or her duties in connection with the employment, any of our confidential information or trade secrets, any confidential information or trade secretes of our customers or prospective customers, or the confidential or proprietary information of any third party received by us and for which we have confidential obligations. In addition, each executive officer has agreed to be bound by non-competition restrictions during his or her employment and for one year after the termination of his or her employment. Specifically, each executive officer has agreed during the non-competition period (i) not to participate in R&D work at the top ten pharmaceutical and biotechnology R&D outsourcing companies in mainland China; (ii) not to provide services to, own or operate any business that provides products, services or technologies substantially similar to the business currently conducted or proposed to be conducted by us; and (iii) not to induce any of our employees or consultants to terminate his or her employment or engagement with us.
Compensation of Directors and Executive Officers
For the fiscal year ended December 31, 2009, we paid an aggregate of approximately RMB5.3 million ($0.8 million) in cash to our executive officers and directors as a group, and paid an aggregate of approximately
108
RMB25,200 ($3,700) in premiums for commercial medical insurance coverage for some of our executive officers. We have not set aside or accrued any amount to provide pension, retirement or other similar benefits to our executive officers and directors. Our PRC subsidiaries are required by law to make contributions equal to certain percentages of each employee’s salary for his or her pension insurance, medical insurance, housing fund, unemployment and other statutory benefits.
Scientific Advisory Board
We have established a scientific advisory board comprising leading experts in their fields. Our scientific advisors participate in advisory board meetings as well as provide ad hoc individual consulting services to management and our scientists. We regularly seek advice and input from these experienced scientific leaders on matters related to our research and development programs. Our scientific advisory board consists of experts across a range of key disciplines relevant to our programs and science. We intend to leverage the broad expertise of our advisors by seeking their counsel on important topics relating to our drug discovery and development programs.
We pay our advisors a fee for their attendance at scientific advisory board meetings and consulting services, and reimburse them for their expenses. Our current advisors are:
| | |
Name | | Professional Affiliation |
| Gregory Verdine, Ph.D. (chairman) | | Erving Professor of Chemistry and Arthur Cope Scholar, Harvard University |
| |
Robert Booth, Ph.D. | | Operating partner at TPG; former senior vice president at Roche; former chief scientific officer at Celera |
Paul J. Reider, Ph.D. | | Professor at Princeton University; former head of chemistry at Amgen Inc.; former vice president of process chemistry, Merck & Co., Inc. |
| Malcolm MacCoss, Ph.D. | | Former vice president of basic chemistry and drug discovery science at Merck & Co., Inc.; former group vice president of chemistry at Schering-Plough; winner of two Thomas Edison Patent Awards and Arthur C. Cope Scholar Award |
Robert Kaman, Ph.D. | | Former president of Abbott Bioresearch Center and BASF Bioresearch Center |
2008 Equity and Performance Incentive Plan
On May 19, 2008, we adopted the Plan to attract and retain the best available personnel and provide additional incentives to employees, directors and consultants. On February 24, 2010, we amended and restated the Plan to increase the maximum aggregate number of our ordinary shares that may be issued under the Plan to 36,562,358 shares. As of September 30, 2010, options to purchase 36,046,200 ordinary shares were outstanding.
109
The following table summarizes the share options granted under the Plan to certain of our directors and executive officers and to other individuals as a group that were outstanding as of September 30, 2010. These share options will vest in equal amounts on the first, second, third and fourth anniversary of their respective dates of grant.
| | | | | | | | | | | | |
Name | | Options
Outstanding | | Exercise Price
($/Share) | | | Date of Grant | | Date of
Expiration | | Vesting
Schedule |
Michael Xin Hui | | — | | | — | | | — | | — | | — |
Wenjuan Xiao | | — | | | — | | | — | | — | | — |
Kevin Penghui Chen | | * | | | $0.50 | | | Oct 27, 2008 | | Oct 27, 2018 | | 4 years |
| | * | | | $0.65 | | | July 30, 2010 | | July 30, 2020 | | 4 years |
Lan Xie | | * | | | $0.50 | | | May 19, 2008 | | May 19, 2018 | | 4 years |
| | * | | | $0.65 | | | July 30, 2010 | | July 30, 2020 | | 4 years |
Chunyu Zhang | | * | | | $0.50 | | | Oct 14, 2009 | | Oct 14, 2019 | | 4 years |
| | * | | | $0.65 | | | May 24, 2010 | | May 24, 2020 | | 4 years |
| | * | | | $0.65 | | | July 30, 2010 | | July 30, 2020 | | 4 years |
Other individuals as a group | | 11,008,000 | |
| $0.50
|
| | May 19, 2008 | | May 19, 2018 | | 4 years |
| | 4,977,050 | |
| $0.50
|
| | Oct 27, 2008 | | Oct 27, 2018 | | 4 years |
| | 3,679,700 | |
| $0.50
|
| | May 13, 2009 | | May 13, 2019 | | 4 years |
| | 3,544,700 | | | $0.50 | | | Oct 14, 2009 | | Oct 14, 2019 | | 4 years |
| | 3,678,650 | | | $0.65 | | | May 24, 2010 | | May 24, 2020 | | 4 years |
| | 5,817,100 | | | $0.65 | | | July 30, 2010 | | July 30, 2020 | | 4 years |
| | | | | | | | | | | | |
Total | | 36,046,200 | | | | | | | | | | |
| | | | | | | | | | | | |
| * | Less than 1% of our total outstanding shares. |
The following paragraphs describe the principal terms of the Plan.
Plan Administration. Our board of directors, a committee or a sub-committee designated by our board of directors will administer the Plan. The committee or the full board of directors, as appropriate, will determine the provisions and terms and conditions of each award grant.
Award Agreement. Awards granted under the Plan are evidenced by an award agreement that sets forth terms, conditions and limitations for each award. In addition, the award agreement may provide that securities granted are subject to a 180-day lock-up period following the effective date of a registration statement filed by us under the Securities Act, if so requested by us or any representative of the underwriters in connection with any registration of the offering of any of our securities.
Evidence of Award. Awards can be evidenced by an agreement, certificate, resolution or other type of writing or an electronic medium approved by the board of directors that sets forth the terms and conditions of the awards granted. An evidence of award, with the approval of the board of directors, need not be signed by a representative of our company or the recipient.
Eligibility. We may grant awards to any employee, non-employee director, officer or consultant of our company or any one or more of our subsidiaries, or any person who has agreed to commence serving in any of such capacities within 90 days of the date of grant.
Acceleration of Awards upon Change in Control of the Company.The outstanding awards are subject to accelerated vesting upon occurrence of a change-of-control corporate transaction, as determined by our board of directors in its sole discretion.
Exercise Price and Term of Awards. Our board of directors, or a committee designated by our board of directors, determines the exercise price, grant price and expiration date for each award.
110
Vesting Schedule. In general, our board of directors, or a committee designated by our board of directors, determines, or the evidence of award specifies, the vesting schedule.
Transfer Restrictions. Subject to certain exceptions, awards may not be transferred in any manner by the recipient other than by will or the laws of descent and distribution.
Termination of the Plan. Unless terminated earlier, the Plan will terminate automatically in 2018. Our board of directors has the authority to amend or terminate the Plan to the extent necessary to comply with applicable law or the rules of the principal securities exchange upon which our ADSs are traded or quoted.
Founder’s 2008 Equity and Performance Incentive Plan
In May 2008, two companies controlled by our founders, ChemExplorer Investment Holdings Ltd. and ChemPartner Investment Holdings Limited, established the Founder’s Plan to attract, motivate and retain our employees, directors and consultants. The maximum aggregate number of our ordinary shares that may be transferred by these two companies to grantees upon vesting of RSUs granted under the Founder’s Plan is 20,000,000.
Under the Founder’s Plan, 7,450,000, 2,100,000 and 2,200,000 RSUs were granted to certain members of our senior management and other employees in 2008, 2009 and the nine months ended September 30, 2010, respectively. Each RSU represents the right to receive one ordinary share upon vesting. Subject to the conditions described below, fifty-percent of each grantee’s RSUs will vest on the first anniversary of the date of grant and the remaining fifty-percent will vest on the second anniversary of the date of grant. The actual number of RSUs vesting annually is subject to certain annual corporate, business unit and individual performance targets. If the annual performance targets are not met, a portion of the awards may not vest and may be carried over to the third year of vesting. Any granted RSU that has not vested due to a grantee’s failure to meet the specified performance targets will be subject to a modified vesting schedule under which the RSU will vest on the third anniversary of the date of grant if the grantee remains our employee at that time. As of September 30, 2010, the vesting schedules of a total of 873,408 RSUs held by our employees had been modified for the reason stated above. Among these RSUs, 26,250 RSUs are held by Ms. Lan Xie, a member of our senior management, whose RSU vesting schedule is subject to the following performance criteria: the revenue target of our company, the performance of the finance division where Ms. Xie works and her individual performance. Since the revenue target of our company and the performance target of the finance division were not met in 2008 and 2009, the vesting schedule for Ms. Xie has been modified.
In addition, the vesting of RSUs is subject to the occurrence of an initial public offering or a change of control event (as defined in the Founder’s Plan), whichever occurs earlier. Granted RSUs, whether vested or not, may be forfeited if a grantee’s employment with us is terminated for cause or the grantee voluntarily leaves our company prior to an initial public offering or a change of control event, whichever occurs earlier. Pursuant to the Founder’s Plan, ChemExplorer Investment Holdings Ltd. and ChemPartner Investment Holdings Limited will transfer one ordinary share beneficially owned by them to a grantee for each vested RSU as soon as administratively practicable following the earlier of the effective date of an initial public offering or a change of control event described above.
We have not recorded any share-based compensation expense in connection with the RSU grants because the vesting conditions for the RSUs (the occurrence of an initial public offering or a change of control event) were not met. All of the RSUs were accounted for as if they were unvested as of December 31, 2008 and 2009 and September 30, 2010. Upon the completion of this offering, 7,164,092 RSUs will be vested and approximately $2.5 million will be recorded immediately as share-based compensation expense. A grantee has no rights as a shareholder with respect to any RSUs held by the grantee until the date when the RSUs are vested and the corresponding ordinary shares are transferred from our founders to the grantee through ChemExplorer Investment Holdings Ltd. and ChemPartner Investment Holdings Limited. No adjustment will be made for dividends, distributions or other rights, the record date of which is prior to such conversion date.
111
The following table summarizes, as of September 30, 2010, the RSUs granted under the Founder’s Plan to our senior management and other employees.
| | | | | | | | | | | | | | | | | | | | |
Name | | RSU
Granted | | | Date of
Grant | | | Date of Expiration | | | Vesting
Schedule | | | Transferable
Ordinary
Shares(1) | |
Kevin Penghui Chen | | | * | | | | May 19, 2008 | | | | May 19, 2018 | | | | 2 years | | | | * | |
Lan Xie | | | * | | | | May 19, 2008 | | | | May 19, 2018 | | | | 3 years | | | | * | |
Chunyu Zhang | | | * | | | | Oct 14, 2009 | | | | Oct 14, 2019 | | | | 2 years | | | | * | |
Other individuals as a group | | | 3,800,000 | | | | May 19, 2008 | | | | May 19, 2018 | | | | 3 years | | | | 3,237,113 | |
| | | * | | | | Oct 27, 2008 | | | | Oct 27, 2018 | | | | 3 years | | | | * | |
| | | * | | | | May 13, 2009 | | | | May 13, 2019 | | | | 3 years | | | | * | |
| | | * | | | | Oct 14, 2009 | | | | Oct 14, 2019 | | | | 3 years | | | | * | |
| | | * | | | | May 24, 2010 | | | | May 24, 2020 | | | | 2 years | (2) | | | * | |
| | | * | | | | August 16, 2010 | | | | August 16, 2020 | | | | 2 years | (3) | | | * | |
| | | | | | | | | | | | | | | | | | | | |
Total | | | 11,750,000(4) | | | | | | | | | | | | | | | | 7,164,092 | |
| | | | | | | | | | | | | | | | | | | | |
| * | Less than 1% of our total outstanding shares. |
| (1) | Represents the number of our ordinary shares that the RSU grantee will receive upon the completion of this offering. |
| (2) | These RSUs were granted on May 24, 2010 and are scheduled to vest starting on May 24, 2011, the first anniversary of the grant date. |
| (3) | These RSUs were granted on August 16, 2010 and are scheduled to vest starting on August 16, 2011, the first anniversary of the grant date. |
| (4) | Most outstanding RSUs have been transferred to the family members of the grantees or entities established for the grantees’ estate planning purposes, and the vesting of these RSUs are subject to the same terms and conditions as if they were held by the original grantees. |
112
PRINCIPAL AND SELLING SHAREHOLDERS
Except as specifically noted in the table, the following table sets forth information with respect to the beneficial ownership of our ordinary shares as of the date of this prospectus and as adjusted to reflect the sale of ADSs offered in this offering by:
| | • | | each of our directors and executive officers; |
| | • | | each person known to us to own beneficially more than 5% of our ordinary shares; and |
| | • | | each selling shareholder. |
Beneficial ownership is determined in accordance with the rules and regulations of the U.S. Securities and Exchange Commission. In computing the number of shares beneficially owned by a person and the percentage ownership of that person, we have included shares that the person has the right to acquire within 60 days after the date of this prospectus, including through the exercise of any option, warrant or other right or the conversion of RSUs or any other security. These shares, however, are not included in the computation of the percentage ownership of any other person.
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | Ordinary Shares
Beneficially Owned Prior
to This Offering | | | Ordinary Shares
Being Sold in This
Offering | | | Ordinary Shares
Beneficially Owned After
This Offering(1) | |
| | | Number | | | %(2) | | | Number | | | % | | | Number | | | %(2) | |
Directors and Executive Officers: | | | | | | | | | | | | | | | | | | | | | | | | |
Michael Xin Hui(3) | | | 208,005,986 | | | | 74.8 | % | | | 14,040,000 | | | | 5.1 | % | | | 186,801,894 | (8) | | | 55.7 | % |
Wenjuan Xiao(4) | | | 208,005,986 | | | | 74.8 | % | | | 14,040,000 | | | | 5.1 | % | | | 186,801,894 | (8) | | | 55.7 | % |
Kevin Penghui Chen | | | * | | | | * | | | | — | | | | — | | | | * | | | | * | |
Julian Ralph Worley | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
Yuk Lam Lo | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
Lan Xie | | | * | | | | * | | | | — | | | | — | | | | * | | | | * | |
Chunyu Zhang | | | * | | | | * | | | | — | | | | — | | | | * | | | | * | |
All Directors and Executive Officers as a group | | | 208,529,736 | | | | 74.9 | % | | | 14,040,000 | | | | 5.0 | % | | | 187,325,644 | (8) | | | 55.8 | % |
Principal and Selling Shareholders: | | | | | | | | | | | | | | | | | | | | | | | | |
ChemExplorer Investment Holdings Ltd.(5) | | | 104,002,993 | | | | 37.4 | % | | | 14,040,000 | | | | 5.1 | % | | | 89,962,993 | | | | 26.8 | % |
ChemPartner Investment Holdings Limited(6) | | | 104,002,993 | | | | 37.4 | % | | | — | | | | — | | | | 96,838,901 | (8) | | | 28.9 | % |
Investment funds affiliated with TPG, represented by TPG Star Charisma Ltd. and TPG Biotech II Charisma Ltd.(7) | | | 69,994,014 | | | | 25.2 | % | | | 32,760,000 | | | | 11.8 | % | | | 37,234,014 | | | | 11.1 | % |
| * | Less than 1% of our total outstanding shares. |
| (1) | Assumes that the underwriters do not exercise their option to purchase additional ADSs and no other change to the number of ADSs offered by us as set forth on the cover page of this prospectus. |
| (2) | For each person and group included in this table, percentage ownership is calculated by dividing the number of shares beneficially owned by such person or group by the sum of the number of shares outstanding and the number of shares such person or group has the right to acquire within 60 days after the date of this prospectus. The total number of shares outstanding as of the date of this prospectus is 278,000,000. The total number of shares outstanding after the completion of this offering will be 335,600,000. |
| (3) | Includes 104,002,993 ordinary shares owned by ChemExplorer Investment Holdings Ltd. and 104,002,993 ordinary shares owned by ChemPartner Investment Holdings Limited, each a British Virgin Islands company ultimately owned by Michael Xin Hui and the Hui Family Trust, a British Virgin Islands trust, as of the date of this prospectus. Wenjuan Xiao is the settlor and one of the beneficiaries of Hui Family Trust. Wenjuan Xiao is the mother of Michael Xin Hui. Michael Xin Hui and Wenjuan Xiao are directors of ChemExplorer Investment Holdings Limited and ChemPartner Investment Holdings Limited. Michael Xin Hui disclaims beneficial ownership with respect to the above shares except to the extent of his pecuniary interest therein. The business address of Michael Xin Hui is No. 5 Building, 998 Halei Road, Zhangjiang Hi-tech Park, Pudong New Area, Shanghai, China, 201203. |
113
| (4) | Includes 104,002,993 ordinary shares owned by ChemExplorer Investment Holdings Ltd. and 104,002,993 ordinary shares owned by ChemPartner Investment Holdings Limited, each a British Virgin Islands company ultimately owned by the Hui Family Trust, a British Virgin Islands trust and Michael Xin Hui, as of the date of this prospectus. Wenjuan Xiao is the settlor of Hui Family Trust and one of the beneficiaries. Wenjuan Xiao is the mother of Michael Xin Hui. Michael Xin Hui and Wenjuan Xiao are directors of ChemExplorer Investment Holdings Limited. Wenjuan Xiao disclaims beneficial ownership with respect to the above shares except to the extent of her pecuniary interest therein. The business address of Wenjuan Xiao is No. 5 Building, 998 Halei Road, Zhangjiang Hi-tech Park, Pudong New Area, Shanghai, China, 201203. |
| (5) | ChemExplorer Investment Holdings Ltd. is a British Virgin Islands company ultimately owned by Michael Xin Hui and the Hui Family Trust, a British Virgin Islands trust. Wenjuan Xiao is the settlor and one of the beneficiaries of Hui Family Trust. |
| (6) | ChemPartner Investment Holdings Limited is a British Virgin Islands company ultimately owned by Michael Xin Hui and the Hui Family Trust, a British Virgin Islands trust. Wenjuan Xiao is the settlor and one of the beneficiaries of Hui Family Trust. |
| (7) | Includes 46,676,543 ordinary shares convertible from preferred shares held by TPG Star Charisma Limited and 23,317,471 ordinary shares convertible from preferred shares held by TPG Biotech II Charisma Limited. TPG Star Charisma Limited is a company incorporated in Hong Kong, whose sole shareholder is TPG Star, L.P., a Delaware limited partnership, which is managed by its general partner, TPG Star GenPar, L.P., a Delaware limited partnership, which is managed by its general partner, TPG Star Advisors, LLC, a Delaware limited liability company, whose sole member is TPG Ventures Holdings, LLC, whose managing member is TPG Ventures Partners, L.P., which is managed by its general partner, TPG Ventures Professionals, L.P., which is managed by its general partner, Tarrant Advisors, Inc., whose sole shareholder is Tarrant Capital Advisors, Inc., whose shareholders are David Bonderman and James G. Coulter. TPG Biotech II Charisma Limited is a company incorporated in Hong Kong, whose sole shareholder is TPG Biotechnology Partners II, L.P., a Delaware limited partnership, which is managed by its general partner, TPG Biotechnology GenPar II, L.P., a Delaware limited partnership, which is managed by its general partner, TPG Biotech Advisors II, LLC, a Delaware limited liability company, whose sole member is TPG Ventures Holdings, LLC, a Delaware limited liability company, whose managing member is TPG Ventures Partners, L.P., a Delaware limited partnership, which is managed by its general partner, TPG Ventures Professionals, L.P., a Delaware limited partnership, which is managed by its general partner, Tarrant Advisors, Inc., a Texas company, whose sole shareholder is Tarrant Capital Advisors, Inc., a Delaware company, whose shareholders are David Bonderman and James G. Coulter. The registered address for both of these companies is 57th Floor, Two International Finance Centre, 8 Finance Street, Central, Hong Kong. TPG Star Charisma Limited and TPG Biotech II Charisma Limited were granted an option, exercisable no fewer than five business days prior to the completion of this offering, to subscribe for a certain number of our ordinary shares that would result in these two entities having the same shareholding percentage in our company following the completion of this offering on a fully diluted basis as these two entities held immediately after the closing of the issuance and sale of our preferred shares. See “Related Party Transactions—Share Issuance and Splits.” |
| (8) | Gives effect to the transfer of 7,164,092 ordinary shares from ChemPartner Investment Holdings Limited to the holders of vested RSUs upon the completion of this offering. See “Management—Founder’s 2008 Equity and Performance Incentive Plan.” |
As of the date of this prospectus, none of our ordinary shares or preferred shares is held by record holders in the United States. None of our existing shareholders has different voting rights from other shareholders after the completion of this offering. We are not aware of any arrangement that may, at a subsequent date, result in a change of control of our company.
114
RELATED PARTY TRANSACTIONS
Share Issuances and Splits
Ordinary shares. In September 2007, in connection with our corporate restructuring and the incorporation of our Cayman Islands holding company, we issued a total of 5,000 ordinary shares, par value $0.001 per share, to ChemExplorer Investment Holdings Ltd. and a total of 5,000 ordinary shares to ChemPartner Investment Holdings Limited. ChemExplorer Investment Holdings Ltd. and ChemPartner Investment Holdings Limited are British Virgin Islands companies ultimately owned by Mr. Michael Xin Hui and the Hui Family Trust, a British Virgin Islands trust. Ms. Wenjuan Xiao is the settlor and one of the beneficiaries of the Hui Family Trust.
Issuance and sale of preferred shares. In September 2007, we issued 2,019 preferred shares, par value $0.001 per share, in a private placement to TPG Star Charisma Ltd. and TPG Biotech II Charisma Ltd., for a total amount of $21.0 million, and an existing shareholder of our company sold 1,346 preferred shares to TPG Star Charisma Ltd. and TPG Biotech II Charisma Ltd. for a total amount of $14.0 million in the same transaction. Each of TPG Star Charisma Ltd. and TPG Biotech II Charisma Ltd. was an unrelated third party prior to the issuance and sale of the preferred shares. The purchase price per share was determined through an arm’s-length negotiation with these investors and was approved by our board of directors. TPG Star Charisma Ltd. and TPG Biotech II Charisma Ltd. were granted an option, exercisable no fewer than five business days prior to the completion of this offering, to subscribe for a certain number of our ordinary shares that would result in these two entities having the same shareholding percentage in our company following the completion of this offering on a fully diluted basis as these two entities held immediately after the closing of the issuance and sale of our preferred shares. These two shareholders have notified us that they will not exercise this option. Holders of our preferred shares are entitled to vote on an “as converted” basis together with the holders of ordinary shares.
Each preferred share will automatically convert into one ordinary share upon completion of this offering. Pursuant to our current memorandum and articles of association, each preferred share will be automatically converted into one ordinary share upon the closing of a qualified public offering. A qualified public offering means an initial public offering on a qualified exchange that values our company at no less than $250,000,000 immediately after the offering and that results in aggregate proceeds to our company of no less than $62,500,000, net of selling expenses. On September 30, 2010, the definition of a qualified public offering in our memorandum and articles of association was amended to mean an initial public offering on a qualified exchange that values our company at no less than $250,000,000 immediately after the offering and that results in aggregate gross proceeds from the offering of no less than $75,000,000, including aggregate gross proceeds to TPG Star Charisma Ltd. and TPG Biotech II Charisma Ltd. of no less than $25,000,000. We believe that this offering will be a qualified public offering as defined in our memorandum and articles of association, as amended on September 30, 2010.
Bonus share issuance. In May 2008, we effected a bonus share issuance in the form of a share split such that an additional 20,800 ordinary shares were issued for each ordinary share outstanding and additional approximately 20,800 preferred shares for each preferred shares outstanding.
Investors’ Rights Agreement
In connection with the issuance and sale of our preferred shares, we entered into an investors’ rights agreement with holders of our preferred shares, holders of our ordinary shares, our founders and certain other parties. We have granted the holders of our preferred shares certain registration rights, including demand and piggyback registration rights and Form F-3 registration rights. See “Description of Share Capital—Registration Rights.” In addition, the investors’ rights agreement provided that grants of share options pursuant to the Plan shall be subject to the condition that we achieve certain profit targets. On May 19, 2008, this agreement was amended and, among other things, this requirement was waived. On September 30, 2010, the investor’s rights agreement was further amended such that the definition of a qualified public offering has the same meaning as that in our memorandum and articles of association, as amended on September 30, 2010.
115
Loans Extended to Related Parties
Historically, we extended a loan to Shanghai BioExplorer Co., Ltd., or BioSH, a company wholly-owned by ChemExplorer Investment Holdings Limited. On December 27, 2006, we sold our interest in BioSH to BioExplorer Company Limited, which was a related party of us at that time and became an unrelated party of us in April 2007. The loan receivable from BioSH was $273,800 as of December 31, 2007, and was fully paid in 2008.
Transactions with Related Parties
Since April 2007, Labpartner, a company 50%-owned by immediate family members of Mr. Michael Xin Hui, has become our largest supplier for reagents and other raw materials. Labpartner follows the same bidding and selection process as all of our other suppliers. In the first half of 2007, we had four PRC subsidiaries, each of which entered into a separate framework service outsourcing agreement with LabPartner for a five year term, which may be terminated by either party upon six months’ notice. Under the framework agreements, each of these subsidiaries entered into additional agreements with LabPartner, including a purchase and sales agreement and a warehousing and logistics agreement, for the supply of reagents and other raw materials, logistics management, equipment purchase and maintenance and customs clearance. We purchase raw materials from LabPartner at prices determined through a competitive bidding process with other third party vendors. We pay LabPartner purchase prices and service fees on a monthly basis. The purchase prices and service fees charged by Labpartner amounted to $3.0 million, $10.4 million, $6.1 million and $3.1 million in 2007, 2008, 2009 and the six months ended June 30, 2010, respectively.
Shanghai Kehui Catering Management Co., Ltd., a company wholly-owned by immediate family members of Mr. Michael Xin Hui, provides employee lunches, dinners and other catering services in our company cafeteria. The services fee charged by Shanghai Kehui Catering Management Co., Ltd. amounted to $0.5 million, $0.9 million, $0.8 million and $0.4 million in 2007, 2008, 2009 and the six months ended June 30, 2010, respectively.
We lease four buildings at our headquarters in Shanghai Zhangjiang Hi-Tech Park from Shanghai PharmValley Corp., a company 20%-owned by immediate family members of Mr. Michael Xin Hui. These buildings are used for office space and research activities and have an aggregate gross floor area of approximately 200,000 square feet. The office rent charged by Shanghai PharmValley Corp. amounted to $0.9 million, $1.2 million, $2.0 million and $1.1 million in 2007, 2008, 2009 and the six months ended June 30, 2010, respectively.
We believe the foregoing related party transactions were entered into on arm’s length terms.
Employment Agreements
See “Management—Employment Agreements.”
Equity and Performance Incentive Plan
See “Management—2008 Equity and Performance Incentive Plan” and “Management—Founder’s 2008 Equity and Performance Incentive Plan.”
116
DESCRIPTION OF SHARE CAPITAL
We are a Cayman Islands company and our affairs are governed by our amended and restated memorandum and articles of association and the Companies Law (2010 Revision) of the Cayman Islands, referred to as the Companies Law below.
As of the date of this prospectus, our authorized share capital consists of 429,999,350 ordinary shares, with a par value of $0.001 each, and 70,000,650 preferred shares, with a par value of $0.001 each. As of the date of this prospectus, there are 208,005,986 ordinary shares issued and outstanding and 69,994,014 preferred shares issued and outstanding. All of our issued and outstanding preferred shares will automatically convert into 69,994,014 ordinary shares immediately upon the completion of this initial public offering.
Our second amended and restated memorandum and articles of association will become effective upon completion of this offering and will replace the current amended and restated memorandum and articles of association in its entirety. The following are summaries of material provisions of our proposed second amended and restated memorandum and articles of association and the Companies Law insofar as they relate to the material terms of our ordinary shares.
Ordinary Shares
General. All of our outstanding ordinary shares are fully paid and non-assessable. Certificates representing the ordinary shares are issued in registered form. Our shareholders who are non-residents of the Cayman Islands may freely hold and vote their ordinary shares.
Dividends. The holders of our ordinary shares are entitled to such dividends as may be declared by our board of directors subject to the Companies Law and to our second amended and restated memorandum and articles of association.
Voting Rights. Each ordinary share is entitled to one vote on all matters upon which the ordinary shares are entitled to vote. Voting at any shareholders’ meeting is by show of hands unless voting by way of a poll is required by the rules of the stock exchange where our ADSs are traded. A poll may be demanded by our chairman of such meeting or any one shareholder present in person or by proxy, or if the shareholder is a corporation, by its duly authorized representative. Voting cannot be conducted electronically.
An ordinary resolution to be passed by the shareholders requires the affirmative vote of a simple majority of the votes attaching to the ordinary shares cast in a general meeting, while a special resolution requires the affirmative vote of no less than two-thirds of the votes cast attaching to the ordinary shares. A special resolution is required for important matters such as amending our second amended and restated memorandum and articles of association. Holders of the ordinary shares may effect certain changes by ordinary resolution, including increase the amount of our authorized share capital, consolidate and divide all or any of our share capital into shares of larger amount than our existing share capital, and cancel any unissued shares.
Transfer of Shares. Subject to the restrictions contained in our second amended and restated memorandum and articles of association, any of our shareholders may transfer all or any of his or her ordinary shares by an instrument of transfer in the usual or common form or any other form approved by our board of directors.
Our board of directors may, in its sole discretion, decline to register any transfer of any ordinary share. Our directors may also decline to register any transfer of any ordinary share unless (a) the instrument of transfer is lodged with us, accompanied by the certificate for the ordinary shares to which it relates and such other evidence as our board of directors may reasonably require to show the right of the transferor to make the transfer; (b) the instrument of transfer is in respect of only one class of ordinary shares; (c) the instrument of transfer is properly stamped, if required; (d) the ordinary shares transferred are fully paid and free of any lien in favor of us; (e) in the case of a transfer to joint holders, the number of joint holders to whom the ordinary share is to be transferred does not exceed four; or (f) any fee related to the transfer has been paid to us.
117
If our directors refuse to register a transfer they shall, within three months after the date on which the instrument of transfer was lodged, send to each of the transferor and the transferee notice of such refusal. The registration of transfers may, after compliance with any notice requirements of the New York Stock Exchange, be suspended and the register closed at such times and for such periods as our board of directors may from time to time determine, provided, however, that the registration of transfers shall not be suspended nor the register closed for more than 30 days in any year.
Liquidation. On a return of capital on winding up or otherwise (other than on conversion, redemption or purchase of shares), assets available for distribution among the holders of ordinary shares shall be distributed among the holders of the ordinary shares on a pro rata basis. If our assets available for distribution are insufficient to repay all of the paid-up capital, the assets will be distributed so that the losses are borne by our shareholders proportionately.
Redemption of Shares. Subject to the provisions of the Companies Law and other applicable law, we may issue shares on terms that are subject to redemption, at our option or at the option of the holders, on such terms and in such manner, including out of capital, as may be determined by the board of directors.
Variations of Rights of Shares. All or any of the special rights attached to any class of shares may, subject to the provisions of the Companies Law, be varied with the sanction of a special resolution passed at a general meeting of the holders of the shares of that class. The rights conferred upon the holders of the shares of any class issued with preferred or other rights shall not, unless otherwise expressly provided by the terms of issue of the shares of that class, be deemed to be varied by the creation or issue of further shares ranking pari passu with such previously existing class of shares.
Inspection of Books and Records. Holders of our ordinary shares will have no general right under Cayman Islands law to inspect or obtain copies of our list of shareholders or our corporate records. However, we will in our second amended and restated memorandum and articles of association provide our shareholders with the right to inspect our list of shareholders and to receive annual audited financial statements. See “Where You Can Find Additional Information.”
Anti-Takeover Provisions. Some provisions of our second amended and restated memorandum and articles of association may discourage, delay or prevent a change of control of our company or management that shareholders may consider favorable, including provisions that:
| | • | | authorize our board of directors to issue preferred shares in one or more series and to designate the price, rights, preferences, privileges and restrictions of such preferred shares without any further vote or action by our shareholders; and |
| | • | | limit the ability of shareholders to call general meetings of shareholders. |
However, under Cayman Islands law, our directors may only exercise the rights and powers granted to them under our memorandum and articles of association for a proper purpose and for what they believe in good faith to be in the best interests of our company.
General Meetings of Shareholders.Shareholders’ meetings may be convened by a majority of our board of directors or our chairman. Advance notice of at least ten days is required for the convening of our annual general shareholders’ meeting and any other general meeting of our shareholders. A quorum for a meeting of shareholders consists of at least two shareholders present or by proxy, representing not less than one-third in nominal value of the total issued voting shares in our company.
118
History of Securities Issuances
The following is a summary of our securities issuances since the inception of ShangPharma Corporation.
Ordinary Shares.See “Related party transactions—Share Issuances and Splits” for a description of ordinary shares we have issued as of the date of this prospectus.
Series A Preferred Shares.See “Related party transactions—Share Issuances and Splits” for a description of preferred shares we have issued as of the date of this prospectus.
Shares Splits.See “Related party transactions—Share Issuances and Splits” for a description of the bonus issue we have completed as of the date of this prospectus.
Option and Restricted Share Grants. As of September 30, 2010, we had 36,046,200 ordinary shares issuable upon the exercise of outstanding share options under the Plan and 11,750,000 ordinary shares convertible from the RSUs granted under the Founder’s Plan. See “Management—2008 Equity and Performance Incentive Plan” and “Management—Founder’s 2008 Equity and Performance Incentive Plan.”
Differences in Corporate Law
The Companies Law is modeled after that of England and Wales but does not follow recent statutory enactments in England. In addition, the Companies Law differs from laws applicable to United States corporations and their shareholders. Set forth below is a summary of the significant differences between the provisions of the Companies Law applicable to us and the laws applicable to companies incorporated in the United States and their shareholders.
Mergers and Similar Arrangements. Previously, Cayman Islands law did not provide for mergers as that expression is understood under United States corporate law. However, pursuant to the Companies (Amendment) Law, 2009 that came into force on May 11, 2009, in addition to the existing schemes of arrangements described below, it introduced a new mechanism for mergers and consolidations between Cayman Islands companies and between Cayman companies and foreign companies.
The procedure to effect a merger or consolidation is as follows:
| | • | | the directors of each constituent company must approve a written plan of merger or consolidation, or a Merger Plan; |
| | • | | the Merger Plan must be authorized by each constituent company by (a) a shareholder resolution by majority in number representing 75% in value of the shareholders voting together as one class; and (b) if the shares to be issued to each shareholder in the consolidated or surviving company are to have the same rights and economic value as the shares held in the constituent company, a special resolution of the shareholders voting together as one class. A proposed merger between a Cayman parent company and its Cayman subsidiary or subsidiaries will not require authorization by shareholder resolution; |
| | • | | the consent of each holder of a fixed or floating security interest of a constituent company in a proposed merger or consolidation is required unless the court (upon the application of the constituent company that has issued the security) waives the requirement for consent; |
| | • | | the Merger Plan must be signed by a director on behalf of each constituent company and filed with the register of companies together with the required supporting documents; |
| | • | | a certificate of merger or consolidation is issued by the register of companies which isprima facieevidence of compliance with all statutory requirements in respect of the merger or consolidation. |
119
| | All rights and property of each of the constituent companies will then vest in the surviving or consolidated company which will also be liable for all debts, contracts, obligations and liabilities of each constituent company. Similarly, any existing claims, proceedings or rulings of each constituent company will automatically be continued against the surviving or consolidated company; and |
| | • | | provision is made for a dissenting shareholder of a Cayman constituent company to be entitled to payment of the fair value of his shares upon dissenting to the merger or consolidation. Where the parties cannot agree on the price to be paid to the dissenting shareholder, either party may file a petition to the court to determine fair value of the shares. These rights are not available where an open market exists on a recognized stock exchange for the shares of the class held by the dissenting shareholder. |
In addition, the Companies Law contains statutory provisions facilitate the reconstruction and amalgamation of companies, provided that the arrangement is approved by a majority in number of each class of shareholders and creditors with whom the arrangement is to be made, and who must in addition represent three-fourths in value of each such class of shareholders or creditors, as the case may be, that are present and voting either in person or by proxy at a meeting, or meetings, convened for that purpose. The convening of the meetings and subsequently the arrangement must be sanctioned by the Grand Court of the Cayman Islands. While a dissenting shareholder has the right to express to the court the view that the transaction ought not to be approved, the court can be expected to approve the arrangement if it determines that:
| | • | | the company is not proposing to act illegally or beyond the scope of its authority; |
| | • | | the statutory provisions as to the required majority vote have been met; |
| | • | | the shareholders have been fairly represented at the meeting in question and the statutory majority are acting bona fide without coercion of the minority to promote interests adverse to those of the class; |
| | • | | the arrangement is such that may be reasonably approved by an intelligent and honest man of that class acting in respect of his interest; and |
| | • | | the arrangement is not one that would more properly be sanctioned under some other provision of the Companies Law or that would amount to a “fraud on the minority” under the Cayman Islands law. |
When a take-over offer is made and accepted by holders of 90.0% of the shares within four months, the offeror may, within a two-month period commencing on the expiration of such four month period, require the holders of the remaining shares to transfer such shares on the terms of the offer. An objection can be made to the Grand Court of the Cayman Islands but this is unlikely to succeed in the case of an offer which has been so approved unless there is evidence of fraud, bad faith or collusion.
If the arrangement and reconstruction is thus approved, the dissenting shareholder would have no rights comparable to appraisal rights, which would otherwise ordinarily be available to dissenting shareholders of United States corporations, providing rights to receive payment in cash for the judicially determined value of the shares.
Shareholders’ Suits. In principle, we will normally be the proper plaintiff and a derivative action may not be brought by a minority shareholder. However, based on English authority, which would in all likelihood be of persuasive authority in the Cayman Islands, exceptions to the foregoing principle apply in circumstances in which:
| | • | | a company is acting or proposing to act illegally orultra vires; |
| | • | | the act complained of, although notultra vires, could be effected if duly authorized by more than a simple majority vote which has not been obtained; and |
| | • | | those who control the company are perpetrating a “fraud on the minority.” |
120
Transactions with Directors. Under the Delaware General Corporation Law, or the DGCL, transactions with directors must be approved by disinterested directors or by the shareholders, or otherwise proven to be fair to the company as of the time it is approved. Any such transaction will be void or voidable, unless (i) the material facts of any interested directors’ interests are disclosed or are known to the board of directors and the transaction is approved by the affirmative votes of a majority of the disinterested directors, even though the disinterested directors be less than a quorum; (ii) the material facts of any interested directors’ interests are disclosed or are known to the shareholders entitled to vote thereon, and the transaction is specifically approved in good faith by vote of the shareholders; or (iii) the transaction is fair to the company as of the time it is approved.
Cayman Islands laws do not restrict transactions with directors, requiring only that directors exercise a duty of care and owe a fiduciary duty to the companies for which they serve. Under our second amended and restated memorandum and articles of association, subject to any separate requirement for audit committee approval under the applicable rules of the New York Stock Exchange or unless disqualified by the chairman of the relevant board meeting, so long as a director discloses the nature of his interest in any contract or arrangement which he is interested in, such a director may vote in respect of any contract or proposed contract or arrangement in which such director is interested and may be counted in the quorum at such a meeting.
Directors’ Fiduciary Duties. Under Delaware corporate law, a director of a Delaware corporation has a fiduciary duty to the corporation and its shareholders. This duty has two components: the duty of care and the duty of loyalty. The duty of care generally requires that a director act in good faith, with the care that an ordinarily prudent person would exercise under similar circumstances. Under this duty, a director must inform himself of all material information reasonably available regarding a significant transaction. The duty of loyalty requires that a director act in a manner he reasonably believes to be in the best interests of the corporation. He must not use his corporate position for personal gain or advantage. This duty prohibits self-dealing by a director and mandates that the best interest of the corporation and its shareholders take precedence over any interest possessed by a director, officer or controlling shareholder and not shared by the shareholders generally. In general, but subject to certain exceptions, actions of a director are presumed to have been made on an informed basis, in good faith and in the honest belief that the action taken was in the best interests of the corporation. However, this presumption may be rebutted by evidence of a breach of one of the fiduciary duties.
Under Cayman Islands law, a director of a Cayman Islands company is in the position of a fiduciary with respect to the company, and therefore he or she owes the following duties to the company: a duty to act bona fide in the best interests of the company; a duty not to make a profit out of his or her position as director (unless the company permits him or her to do so); and a duty not to put himself or herself in a position where the interests of the company conflict with his or her personal interests or his or her duty to a third party. A director of a Cayman Islands company owes to the company a duty to act with skill and care. It was previously considered that a director need not exhibit in the performance of his or her duties a greater degree of skill than may reasonably be expected from a person of his or her knowledge and experience. However, English and Commonwealth courts have moved towards an objective standard with regard to the required skill and care and these authorities are likely to be followed in the Cayman Islands.
Under our second amended and restated memorandum and articles of association, directors who are in any way, whether directly or indirectly, interested in a contract or proposed contract with our company shall declare the nature of their interest at a meeting of the board of directors. Following such declaration, a director may vote in respect of any contract or proposed contract notwithstanding his interest and be counted in the quorum at such meeting.
Majority Independent Board. A domestic U.S. company listed on the New York Stock Exchange must comply with requirement that a majority of the board of directors must comprise independent directors as defined under Section 303A of the Corporate Governance Rules of the New York Stock Exchange. As a Cayman Islands company, we are allowed to follow home country practices in lieu of certain corporate governance requirements under the rules of the New York Stock Exchange where there is no similar requirement under the laws of the Cayman Islands. However, we have no present intention to rely on home country practice with corporate
121
governance matters, and we intend to comply with the rules of the New York Stock Exchange after the completion of this offering.
Shareholder Action by Written Consent. Under the DGCL, a corporation may eliminate the right of shareholders to act by written consent by inclusion of such a restriction in its certificate of incorporation. Our second amended and restated memorandum and articles of association provide that shareholders may not approve corporate matters by way of a unanimous written resolution signed by or on behalf of each shareholder who would have been entitled to vote on such matter at a general meeting without a meeting being held.
Shareholder Proposals. The DGCL does not provide shareholders an express right to put any proposal before the annual meeting of shareholders, but in keeping with common law, Delaware corporations generally afford shareholders an opportunity to make proposals and nominations provided that they comply with the notice provisions in the certificate of incorporation or bylaws. A special meeting may be called by the board of directors or any other person authorized to do so in the certificate of incorporation or bylaws, but shareholders may be precluded from calling special meetings.
Neither the Companies Law nor our second amended and restated memorandum and articles of association allow our shareholders to requisition a general meeting. As an exempted Cayman Islands company, we are not obliged by law to call shareholders’ annual general meetings. However, our second amended and restated articles of association require us to call such meetings every year. Neither the Companies Law nor our second amended and restated memorandum and articles of association provides shareholders any right to bring business before a general meeting or to nominate directors. Our second amended and restated articles of association only allow a majority of our board of directors or the chairman of our board of directors to call an extraordinary general meeting. Unless otherwise provided by the rules of the New York Stock Exchange, only a person recommended by our board of directors may be eligible for election as a director at a general meeting.
Cumulative Voting. Under the DGCL, cumulative voting for elections of directors is not permitted unless the corporation’s certificate of incorporation specifically provides for it. Cumulative voting potentially facilitates the representation of minority shareholders on a board of directors since it permits the minority shareholder to cast all the votes to which the shareholder is entitled on a single director, which increases the shareholder’s voting power with respect to electing such director.
There are no prohibitions in relation to cumulative voting under the laws of the Cayman Islands, but our second amended and restated memorandum and articles of association do not provide for cumulative voting. As a result, our shareholders are not afforded any fewer protections or rights on this issue than shareholders of a Delaware corporation.
Removal of Directors. Under the DGCL, a director of a corporation with a classified board may be removed only for cause with the approval of a majority of the outstanding shares entitled to vote, unless the certificate of incorporation provides otherwise. Under our second amended and restated memorandum and articles of association, directors can be removed by an ordinary resolution of shareholders.
Transactions with Interested Shareholders. The DGCL contains a business combination statute applicable to Delaware public corporations whereby, unless the corporation has specifically elected not to be governed by such statute by an amendment to its certificate of incorporation or bylaws that is approved by its shareholders, it is prohibited from engaging in certain business combinations with an “interested shareholder” for three years following the date that such person becomes an interested shareholder. An interested shareholder generally is a person or a group who or which owns 15% or more of the corporation’s outstanding voting stock or who or which is an affiliate or associate of the corporation and owned 15% or more of the corporation’s outstanding voting stock within the past three years. This has the effect of limiting the ability of a potential acquirer to make a two-tiered bid for the target in which all shareholders would not be treated equally. The
122
statute does not apply if, among others, prior to the date on which such shareholder becomes an interested shareholder, the board of directors approves either the business combination or the transaction which resulted in the person becoming an interested shareholder. This encourages any potential acquirer of a Delaware public corporation to negotiate the terms of any acquisition transaction with the target’s board of directors.
Cayman Islands law has no comparable statute. As a result, we cannot avail ourselves of the protections afforded by the Delaware business combination statute. However, although Cayman Islands law does not regulate transactions between a company and its significant shareholders, it does provide that such transactions must be entered into bona fide in the best interests of the company and for a proper corporate purpose and not with the effect of constituting a fraud on the minority shareholders.
Amendment of Governing Documents. Under the DGCL, a corporation’s certificate of incorporation may be amended only if adopted and declared advisable by the board of directors and approved by a majority of the outstanding shares entitled to vote, and the bylaws may be amended with the approval of a majority of the outstanding shares entitled to vote and may, if so provided in the certificate of incorporation, also be amended by the board of directors. As permitted by Cayman Islands law, our second amended and restated memorandum and articles of association may be amended by a special resolution of shareholders.
Rights of Non-resident or Foreign Shareholders. There are no limitations imposed by our amended and restated memorandum and articles of association on the rights of non-resident or foreign shareholders to hold or exercise voting rights on our shares. In addition, there are no provisions in our second amended and restated memorandum and articles of association governing the ownership threshold above which shareholder ownership must be disclosed.
Indemnification.Cayman Islands law does not limit the extent to which a company’s articles of association may provide for indemnification of officers and directors, except to the extent any such provision may be held by the Cayman Islands courts to be contrary to public policy, such as to provide indemnification against civil fraud or the consequences of committing a crime.
Our second amended and restated memorandum and articles of association permit indemnification of officers and directors for losses, damages, costs and expenses incurred in their capacities as such unless such losses or damages arise from dishonesty or fraud which may attach to such directors or officers. This standard of conduct is generally the same as permitted under the Delaware General Corporation Law for a Delaware corporation.
We intend to enter into indemnification agreements with our directors and executive officers to indemnify them to the fullest extent permitted by applicable law and our second amended and restated memorandum and articles of association, from and against all costs, charges, expenses, liabilities and losses incurred in connection with any litigation, suit or proceeding to which such director or executive director is or is threatened to be made a party, witness or other participant.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling us under the foregoing provisions, we have been advised that in the opinion of the Securities and Exchange Commission, such indemnification is against public policy as expressed in the Securities Act and therefore is unenforceable.
Registration Rights
We have granted registration rights to holders of our preferred shares in connection with their purchase of the preferred shares in September 2007. Set forth below is a description of the registration rights granted under the investors’ rights agreement.
123
Demand Registration Rights. At any time after six months following an initial public offering, the holders of at least 25% of the ordinary shares issuable or issued upon conversion of our preferred shares, or Registrable Securities, or the Initiating Holders, have the right to request that we file a registration statement covering the offer and sale of part or all of their Registrable Securities, provided that the securities proposed to be registered have an estimated market value of at least $7,500,000. We are not obligated to effect more than three such demand registrations. A demand registration is not considered to have been effected if an underwritten offering is contemplated by such demand registration and the conditions to closing specified in the applicable underwriting agreement are not satisfied for any reason, other than by reason of a failure by the Initiating Holders.
Form F-3 or Form S-3 Registration Rights. At any time after six months following a qualified initial public offering, as defined in the investors’ rights agreement, if we are eligible to use Form F-3 or Form S-3, the Initiating Holders have the right to request that we file a registration statement on Form F-3 or Form S-3 covering part or all of their Registrable Securities provided that the securities proposed to be registered have an estimated market value of at least $3,000,000. There is no limit to the number of Form F-3 or Form S-3 registrations the Initiating Holders may request.
Piggyback Registration Rights.If we propose to file a registration statement with respect to an offering of equity or equity–related securities of our company, we must generally offer each holder of Registrable Securities the opportunity to include its Registrable Securities in the registration statement. We have the right to withdraw or delay any registration whether or not any such holder has elected to participate in the registration. We are not required to register any Registrable Securities in an underwritten offering unless these securities are included in the underwriting and their holders enter into an underwriting agreement in customary form with the underwriters selected by us. The underwriters may exclude some or all of these securities if including them would have a significant adverse effect on the underwritten offering, provided that such exclusion does not reduce the number of Registrable Securities to be included by such holders below 20% of the total number of ordinary shares to be included in such offering.
Expenses of Registration. We will pay all expenses relating to any demand, Form F-3, Form S-3 or piggyback registration, except for underwriting discounts and commissions.
Our Right to Defer Registration.We have the right to defer the registration requested by the Initiating Holders pursuant to their demand registration rights and Form F-3 or Form S-3 registration rights, if within 10 days of the receipt of any registration request from the Initiating Holders we inform the Initiating Holders of our bona fide intention to file our own registration statement within 60 days of the receipt of such request, or if such request is made within six months immediately following the effective date of any registration statement relating to securities of our company. We can also defer such registration for a period of not more than 90 days if, in the good faith judgment of a majority of our board of directors, filing a registration statement at that time would require us to make a public disclosure of material non-public information which, but for the filing of the registration statement, would not be required to be disclosed and if disclosed, would have a materially adverse impact on us. We cannot use the deferral right described in the previous sentence more than once in any twelve month period.
124
DESCRIPTION OF AMERICAN DEPOSITARY SHARES
American Depositary Receipts
JPMorgan Chase Bank, N.A., as depositary will issue the ADSs which you will be entitled to receive in this offering. Each ADS will represent an ownership interest in 18 ordinary shares which we will deposit with the custodian, as agent of the depositary, under the deposit agreement among ourselves, the depositary and yourself as an ADR holder. In the future, each ADS will also represent any securities, cash or other property deposited with the depositary but which they have not distributed directly to you. Unless specifically requested by you, all ADSs will be issued on the books of our depositary in book-entry form and periodic statements will be mailed to you which reflect your ownership interest in such ADSs. In our description, references to American depositary receipts or ADRs shall include the statements you will receive which reflect your ownership of ADSs.
The depositary’s office is located at 1 Chase Manhattan Plaza, Floor 58, New York, NY, 10005-1401.
You may hold ADSs either directly or indirectly through your broker or other financial institution. If you hold ADSs directly, by having an ADS registered in your name on the books of the depositary, you are an ADR holder. This description assumes you hold your ADSs directly. If you hold the ADSs through your broker or financial institution nominee, you must rely on the procedures of such broker or financial institution to assert the rights of an ADR holder described in this section. You should consult with your broker or financial institution to find out what those procedures are.
As an ADR holder, we will not treat you as a shareholder of ours and you will not have any shareholder rights. Cayman Island law governs shareholder rights. Because the depositary or its nominee will be the shareholder of record for the shares represented by all outstanding ADSs, shareholder rights rest with such record holder. Your rights are those of an ADR holder. Such rights derive from the terms of the deposit agreement to be entered into among us, the depositary and all registered holders from time to time of ADSs issued under the deposit agreement. The obligations of the depositary and its agents are also set out in the deposit agreement. Because the depositary or its nominee will actually be the registered owner of the ordinary shares, you must rely on it to exercise the rights of a shareholder on your behalf. The deposit agreement and the ADSs are governed by New York law.
The following is a summary of the material terms of the deposit agreement. Because it is a summary, it does not contain all the information that may be important to you. For more complete information, you should read the entire deposit agreement and the form of ADR which contains the terms of your ADSs. You can read a copy of the deposit agreement which is filed as an exhibit to the registration statement of which this prospectus forms a part. You may also obtain a copy of the deposit agreement at the SEC’s Public Reference Room, which is located at 100 F Street, NE, Washington, DC 20549. You may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-732-0330. You may also find the registration statement and the attached deposit agreement on the SEC’s website at http://www.sec.gov.
Share Dividends and Other Distributions
How will I receive dividends and other distributions on the shares underlying my ADSs?
We may make various types of distributions with respect to our securities. The depositary has agreed that, to the extent practicable, it will pay to you the cash dividends or other distributions it or the custodian receives on shares or other deposited securities, after converting any cash received into U.S. dollars and, in all cases, making any necessary deductions provided for in the deposit agreement. You will receive these distributions in proportion to the number of underlying securities that your ADSs represent.
Except as stated below, the depositary will deliver such distributions to ADR holders in proportion to their interests in the following manner:
| | • | | Cash. The depositary will distribute any U.S. dollars available to it resulting from a cash dividend or other cash distribution or the net proceeds of sales of any other distribution or portion thereof (to |
125
| | the extent applicable), on an averaged or other practicable basis, subject to (i) appropriate adjustments for taxes withheld, (ii) such distribution being impermissible or impracticable with respect to certain registered ADR holders, and (iii) deduction of the depositary’s expenses in (1) converting any foreign currency to U.S. dollars to the extent that it determines that such conversion may be made on a reasonable basis, (2) transferring foreign currency or U.S. dollars to the United States by such means as the depositary may determine to the extent that it determines that such transfer may be made on a reasonable basis, (3) obtaining any approval or license of any governmental authority required for such conversion or transfer, which is obtainable at a reasonable cost and within a reasonable time and (4) making any sale by public or private means in any commercially reasonable manner.If exchange rates fluctuate during a time when the depositary cannot convert a foreign currency, you may lose some or all of the value of the distribution. |
| | • | | Shares. In the case of a distribution in shares, the depositary will issue additional ADRs to evidence the number of ADSs representing such shares. Only whole ADSs will be issued. Any shares which would result in fractional ADSs will be sold and the net proceeds will be distributed in the same manner as cash to the ADR holders entitled thereto. |
| | • | | Rights to receive additional shares. In the case of a distribution of rights to subscribe for additional shares or other rights, if we provide evidence satisfactory to the depositary that it may lawfully distribute such rights, the depositary will distribute warrants or other instruments in the discretion of the depositary representing such rights. However, if we do not furnish such evidence, the depositary may: |
| | • | | sell such rights if practicable and distribute the net proceeds in the same manner as cash to the ADR holders entitled thereto; or |
| | • | | if it is not practicable to sell such rights, do nothing and allow such rights to lapse, in which case ADR holders will receive nothing. |
We have no obligation to file a registration statement under the Securities Act in order to make any rights available to ADR holders.
| | • | | Other Distributions. In the case of a distribution of securities or property other than those described above, the depositary may either (i) distribute such securities or property in any manner it deems equitable and practicable or (ii) to the extent the depositary deems distribution of such securities or property not to be equitable and practicable, sell such securities or property and distribute any net proceeds in the same way it distributes cash. |
If the depositary determines that any distribution described above is not practicable with respect to any specific registered ADR holder, the depositary may choose any method of distribution that it deems practicable for such ADR holder, including the distribution of foreign currency, securities or property, or it may retain such items, without paying interest on or investing them, on behalf of the ADR holder as deposited securities, in which case the ADSs will also represent the retained items.
Any U.S. dollars will be distributed by checks drawn on a bank in the United States for whole dollars and cents. Fractional cents will be withheld without liability and dealt with by the depositary in accordance with its then current practices.
The depositary is not responsible if it decides that it is unlawful or impractical to make a distribution available to any ADR holders.
There can be no assurance that the depositary will be able to convert any currency at a specified exchange rate or sell any property, rights, shares or other securities at a specified price, nor that any of such transactions can be completed within a specified time period.
126
Deposit, Withdrawal and Cancellation
How does the depositary issue ADSs?
The depositary will issue ADSs if you or your broker deposit shares or evidence of rights to receive shares with the custodian and pay the fees and expenses owing to the depositary in connection with such issuance. In the case of the ADSs to be issued under this prospectus, we will arrange with the underwriters named herein to deposit such shares.
Shares deposited in the future with the custodian must be accompanied by certain delivery documentation, including instruments showing that such shares have been properly transferred or endorsed to the person on whose behalf the deposit is being made.
The custodian will hold all deposited shares (including those being deposited by or on our behalf in connection with the offering to which this prospectus relates) for the account of the depositary. ADR holders thus have no direct ownership interest in the shares and only have such rights as are contained in the deposit agreement. The custodian will also hold any additional securities, property and cash received on or in substitution for the deposited shares. The deposited shares and any such additional items are referred to as “deposited securities.”
Upon each deposit of shares, receipt of related delivery documentation and compliance with the other provisions of the deposit agreement, including the payment of the fees and charges of the depositary and any taxes or other fees or charges owing, the depositary will issue an ADR or ADRs in the name or upon the order of the person entitled thereto evidencing the number of ADSs to which such person is entitled. All of the ADSs issued will, unless specifically requested to the contrary, be part of the depositary’s direct registration system, and a registered holder will receive periodic statements from the depositary which will show the number of ADSs registered in such holder’s name. An ADR holder can request that the ADSs not be held through the depositary’s direct registration system and that a certificated ADR be issued.
Except for shares deposited by us in connection with this offering, existing shareholders, option holders and RSU holders of our company are restricted from depositing any shares during a period of 180 days after the date of this prospectus, which period may be extended in line with the extension of the lock-up period in the lock-up agreements agreed to by our executive officers, directors and existing shareholders and certain holders of our options and RSUs.
How do ADR holders cancel an ADS and obtain deposited securities?
When you turn in your ADR certificate at the depositary’s office, or when you provide proper instructions and documentation in the case of direct registration ADSs, the depositary will, upon payment of certain applicable fees, charges and taxes, deliver the underlying shares to you or upon your written order. At your risk, expense and request, the depositary may deliver deposited securities at such other place as you may request.
The depositary may only restrict the withdrawal of deposited securities in connection with:
| | • | | temporary delays caused by closing our transfer books or those of the depositary or the deposit of shares in connection with voting at a shareholders’ meeting, or the payment of dividends; |
| | • | | the payment of fees, taxes and similar charges; or |
| | • | | compliance with any U.S. or foreign laws or governmental regulations relating to the ADRs or to the withdrawal of deposited securities. |
This right of withdrawal may not be limited by any other provision of the deposit agreement.
Record Dates
The depositary may, after consultation with us if practicable, fix record dates for the determination of the registered ADR holders who will be entitled (or obligated, as the case may be):
| | • | | to receive any distribution on or in respect of shares, |
127
| | • | | to give instructions for the exercise of voting rights at a meeting of holders of shares, |
| | • | | to pay the fee assessed by the depositary for administration of the ADR program and for any expenses as provided for in the ADR, or |
| | • | | receive any notice or to act in respect of other matters, |
all subject to the provisions of the deposit agreement.
Voting Rights
How do I vote?
If you are an ADR holder and the depositary asks you to provide it with voting instructions, you may instruct the depositary how to exercise the voting rights for the shares which underlie your ADSs. As soon as practicable after receiving notice of any meeting or solicitation of consents or proxies from us, the depositary will distribute to the registered ADR holders a notice stating such information as is contained in the voting materials received by the depositary and describing how you may instruct or will be deemed to have instructed the depositary to exercise the voting rights for the shares which underlie your ADSs, including instructions for giving a discretionary proxy to a person designated by us. For instructions to be valid, the depositary must receive them in the manner and on or before the date specified. The depositary will try, as far as is practical, subject to the provisions of and governing the underlying shares or other deposited securities, to vote or to have its agents vote the shares or other deposited securities as you instruct. To the extent the depositary has been provided with no less than 30 days prior notice of a meeting of shareholders and voting instructions are not so received by the depositary from any holder, the depositary shall deem such holder to have instructed the depositary to give a discretionary proxy to a person designated by us and the depositary shall endeavor insofar as practicable and permitted under the provisions of or governing shares to give a discretionary proxy to a person designated by us to vote the shares represented by the ADSs evidenced by such holder’s ADRs as to which such deemed instructions are so given, provided that no such instruction shall be deemed given and no discretionary proxy shall be given:
| | • | | with respect to any matter as to which we inform the depositary (and we have agreed to provide such information promptly in writing when and if applicable) that (x) we do not wish such proxy to be given, (y) substantial opposition exists with respect to any agenda item for which the proxy would be given or (z) materially affects the rights of holders of shares, and |
| | • | | unless, with respect to such meeting, the depositary has been provided with an opinion of counsel to the company, in form and substance satisfactory to the depositary, to the effect that (a) the granting of such discretionary proxy does not subject the depositary to any reporting obligations in the Cayman Islands, (b) the granting of such proxy will not result in a violation of Cayman Islands law, rule, regulation or permit, (c) the voting arrangement and deemed instruction as contemplated herein will be given effect under Cayman Islands law upon a discretionary proxy being granted in the manner as aforesaid, and (d) the granting of such discretionary proxy alone will not result in the shares represented by the ADSs being considered subject to attachment or appropriation by creditors of the custodian or the depositary under Cayman Islands law, to the extent Cayman Islands law is relevant. The depositary will only vote or attempt to vote as you instruct. |
The depositary will not itself exercise any voting discretion. Furthermore, neither the depositary nor its agents are responsible for any failure to carry out any voting instructions, for the manner in which any vote is cast or for the effect of any vote.
Under our constituent documents, the depositary would be able to provide us with voting instructions without having to personally attend meetings in person or by proxy. Such voting instructions may be provided to us via facsimile, email, mail, courier or other recognized form of delivery and we agree to accept any such delivery so long as it is timely received prior to the meeting. While we will endeavor to provide the depositary with sufficient prior notice of meetings of shareholders so as to enable it to solicit voting instructions, there is no guarantee that you will receive voting materials in time to instruct the depositary to vote and it is possible that you, or persons who hold their ADSs through brokers, dealers or other third parties, will not have the opportunity to exercise a right to vote.
128
Reports and Other Communications
Will ADR holders be able to view our reports?
The depositary will make available for inspection by ADR holders at the offices of the depositary and the custodian the deposit agreement, the provisions of or governing deposited securities, and any written communications from us which are both received by the custodian or its nominee as a holder of deposited securities and made generally available to the holders of deposited securities.
Additionally, if we make any written communications generally available to holders of our shares, and we furnish copies thereof (or English translations or summaries) to the depositary, it will distribute the same to registered ADR holders.
Fees and Expenses
What fees and expenses will I be responsible for paying?
The depositary may charge each person to whom ADSs are issued, including, without limitation, issuances against deposits of shares, issuances in respect of share distributions, rights and other distributions, issuances pursuant to a stock dividend or stock split declared by us or issuances pursuant to a merger, exchange of securities or any other transaction or event affecting the ADSs or deposited securities, and each person surrendering ADSs for withdrawal of deposited securities or whose ADRs are cancelled or reduced for any other reason, $5.00 for each 100 ADSs (or any portion thereof) issued, delivered, reduced, cancelled or surrendered, as the case may be. The depositary may sell (by public or private sale) sufficient securities and property received in respect of a share distribution, rights and/or other distribution prior to such deposit to pay such charge.
The following additional charges shall be incurred by the ADR holders, by any party depositing or withdrawing shares or by any party surrendering ADSs or to whom ADSs are issued (including, without limitation, issuance pursuant to a stock dividend or stock split declared by us or an exchange of stock regarding the ADRs or the deposited securities or a distribution of ADSs), whichever is applicable:
| | • | | a fee of $1.50 per ADR or ADRs for transfers of certificated or direct registration ADRs; |
| | • | | a fee of up to $0.05 per ADS for any cash distribution made pursuant to the deposit agreement; |
| | • | | a fee of up to $0.05 per ADS per calendar year (or portion thereof) for services performed by the depositary in administering the ADRs (which fee may be charged on a periodic basis during each calendar year and shall be assessed against holders of ADRs as of the record date or record dates set by the depositary during each calendar year and shall be payable in the manner described in the next succeeding provision); |
| | • | | reimbursement of such fees, charges and expenses as are incurred by the depositary and/or any of the depositary’s agents (including, without limitation, the custodian and expenses incurred on behalf of holders in connection with compliance with foreign exchange control regulations or any law or regulation relating to foreign investment) in connection with the servicing of the shares or other deposited securities, the delivery of deposited securities or otherwise in connection with the depositary’s or its custodian’s compliance with applicable law, rule or regulation (which charge shall be assessed on a proportionate basis against holders as of the record date or dates set by the depositary and shall be payable at the sole discretion of the depositary by billing such holders or by deducting such charge from one or more cash dividends or other cash distributions); |
| | • | | a fee for the distribution of securities (or the sale of securities in connection with a distribution), such fee being in an amount equal to the fee for the execution and delivery of ADSs which would have been charged as a result of the deposit of such securities (treating all such securities as if they were shares) but which securities or the net cash proceeds from the sale thereof are instead distributed by the depositary to those holders entitled thereto; |
129
| | • | | stock transfer or other taxes and other governmental charges; |
| | • | | cable, telex and facsimile transmission and delivery charges incurred at your request in connection with the deposit or delivery of shares; |
| | • | | transfer or registration fees for the registration of transfer of deposited securities on any applicable register in connection with the deposit or withdrawal of deposited securities; and |
| | • | | expenses of the depositary in connection with the conversion of foreign currency into U.S. dollars. |
We will pay all other charges and expenses of the depositary and any agent of the depositary (except the custodian) pursuant to agreements from time to time between us and the depositary. The charges described above may be amended from time to time by agreement between us and the depositary.
Our depositary has agreed to reimburse us for certain expenses we incur that are related to establishment and maintenance of the ADR program, including investor relations expenses and exchange application and listing fees. Neither the depositary nor we can determine the exact amount to be made available to us because (i) the number of ADSs that will be issued and outstanding, (ii) the level of fees to be charged to holders of ADSs and (iii) our reimbursable expenses related to the ADR program are not known at this time. The depositary collects its fees for issuance and cancellation of ADSs directly from investors depositing shares or surrendering ADSs for the purpose of withdrawal or from intermediaries acting for them. The depositary collects fees for making distributions to investors by deducting those fees from the amounts distributed or by selling a portion of distributable property to pay the fees. The depositary may collect its annual fee for depositary services by deduction from cash distributions, or by directly billing investors, or by charging the book-entry system accounts of participants acting for them. The depositary will generally set off the fees and expenses owing to it from distributions made to holders of ADSs. If, however, no distribution exists and payment owing to the depositary is not timely received by it, it may refuse to provide any further services to holders that have not paid those fees and expenses owing to it until such fees and expenses have been paid.
Payment of Taxes
ADR holders must pay any tax or other governmental charge payable by the custodian or the depositary on any ADS or ADR, deposited security or distribution. If an ADR holder owes any tax or other governmental charge, the depositary may (i) deduct the amount thereof from any cash distributions, or (ii) sell deposited securities (by public or private sale) and deduct the amount owing from the net proceeds of such sale. In either case the ADR holder remains liable for any shortfall. Additionally, if any tax or governmental charge is unpaid, the depositary may also refuse to effect any registration, registration of transfer, split-up or combination of deposited securities or withdrawal of deposited securities until such payment is made. If any tax or governmental charge is required to be withheld on any cash distribution, the depositary may deduct the amount required to be withheld from any cash distribution or, in the case of a non-cash distribution, sell the distributed property or securities (by public or private sale) to pay such taxes and distribute any remaining net proceeds to the ADR holders entitled thereto.
By holding an ADR or an interest therein, you will be agreeing to indemnify us, the depositary, its custodian and any of our or their respective directors, employees, agents and affiliates against, and hold each of them harmless from, any claims by any governmental authority with respect to taxes, additions to tax, penalties or interest arising out of any refund of taxes, reduced rate of withholding at source or other tax benefit obtained.
130
Reclassifications, Recapitalizations and Mergers
If we take certain actions that affect the deposited securities, including (i) any change in par value, split-up, consolidation, cancellation or other reclassification of deposited securities or (ii) any distributions not made to holders of ADRs or (iii) any recapitalization, reorganization, merger, consolidation, liquidation, receivership, bankruptcy or sale of all or substantially all of our assets, then the depositary may choose to:
| | • | | distribute additional or amended ADRs; |
| | • | | distribute cash, securities or other property it has received in connection with such actions; |
| | • | | sell any securities or property received and distribute the proceeds as cash; or |
If the depositary does not choose any of the above options, any of the cash, securities or other property it receives will constitute part of the deposited securities and each ADS will then represent a proportionate interest in such property.
Amendment and Termination
How may the deposit agreement be amended?
We may agree with the depositary to amend the deposit agreement and the ADSs without your consent for any reason. ADR holders must be given at least 30 days notice of any amendment that imposes or increases any fees or charges (other than stock transfer or other taxes and other governmental charges, transfer or registration fees, cable, telex or facsimile transmission costs, delivery costs or other such expenses), or otherwise prejudices any substantial existing right of ADR holders. Such notice need not describe in detail the specific amendments effectuated thereby, but must give ADR holders a means to access the text of such amendment. If an ADR holder continues to hold an ADR or ADRs after being so notified, such ADR holder is deemed to agree to such amendment and to be bound by the deposit agreement as so amended. Notwithstanding the foregoing, if any governmental body or regulatory body should adopt new laws, rules or regulations which would require amendment or supplement of the deposit agreement or the form of ADR to ensure compliance therewith, we and the depositary may amend or supplement the deposit agreement and the ADR at any time in accordance with such changed laws, rules or regulations, which amendment or supplement may take effect before a notice is given or within any other period of time as required for compliance. No amendment, however, will impair your right to surrender your ADSs and receive the underlying securities, except in order to comply with mandatory provisions of applicable law.
How may the deposit agreement be terminated?
The depositary may, and shall at our written direction, terminate the deposit agreement and the ADRs by mailing notice of such termination to the registered holders of ADRs at least 30 days prior to the date fixed in such notice for such termination; provided, however, if the depositary shall have (i) resigned as depositary under the deposit agreement, notice of such termination by the depositary shall not be provided to registered holders unless a successor depositary shall not be operating under the deposit agreement within 45 days of the date of such resignation, and (ii) been removed as depositary under the deposit agreement, notice of such termination by the depositary shall not be provided to registered holders of ADRs unless a successor depositary shall not be operating under the deposit agreement on the 90th day after our notice of removal was first provided to the depositary. After termination, the depositary’s only responsibility will be (i) to deliver deposited securities to ADR holders who surrender their ADRs, and (ii) to hold or sell distributions received on deposited securities. As soon as practicable after the expiration of six months from the termination date, the depositary will sell the deposited securities which remain and hold the net proceeds of such sales (as long as it may lawfully do so), without liability for interest, in trust for the ADR holders who have not yet surrendered their ADRs. After making such sale, the depositary shall have no obligations except to account for such proceeds and other cash.
131
Limitations on Obligations and Liability to ADR holders
Limits on our obligations and the obligations of the depositary; limits on liability to ADR holders and holders of ADSs
Prior to the issue, registration, registration of transfer, split-up, combination, or cancellation of any ADRs, or the delivery of any distribution in respect thereof, and from time to time, we or the depositary or its custodian may require:
| | • | | payment with respect thereto of (i) any stock transfer or other tax or other governmental charge, (ii) any stock transfer or registration fees in effect for the registration of transfers of shares or other deposited securities upon any applicable register and (iii) any applicable fees and expenses described in the deposit agreement; |
| | • | | the production of proof satisfactory to it of (i) the identity of any signatory and genuineness of any signature and (ii) such other information, including without limitation, information as to citizenship, residence, exchange control approval, beneficial ownership of any securities, compliance with applicable law, regulations, provisions of or governing deposited securities and terms of the deposit agreement and the ADRs, as it may deem necessary or proper; and |
| | • | | compliance with such regulations as the depositary may establish consistent with the deposit agreement. |
The issuance of ADRs, the acceptance of deposits of shares, the registration, registration of transfer, split-up or combination of ADRs or the withdrawal of shares, may be suspended, generally or in particular instances, when the ADR register or any register for deposited securities is closed or when any such action is deemed advisable by the depositary or when reasonably requested by us in order to enable us to comply with applicable law; provided that the ability to withdrawal shares may only be limited under the following circumstances: (i) temporary delays caused by closing transfer books of the depositary or our transfer books or the deposit of shares in connection with voting at a shareholders’ meeting, or the payment of dividends, (ii) the payment of fees, taxes, and similar charges, and (iii) compliance with any laws or governmental regulations relating to ADRs or to the withdrawal of deposited securities.
The deposit agreement expressly limits the obligations and liability of the depositary, ourselves and our respective agents. Neither we nor the depositary nor any such agent will be liable if:
| | • | | any present or future law, rule, regulation, fiat, order or decree of the United States, the Cayman Islands, the People’s Republic of China (including the Hong Kong Special Administrative Region) or any other country, or of any governmental or regulatory authority or securities exchange or market or automated quotation system, the provisions of or governing any deposited securities, any present or future provision of our charter, any act of God, war, terrorism or other circumstance beyond our, the depositary’s or our respective agents’ control shall prevent, delay or subject to any civil or criminal penalty any act which the deposit agreement or the ADRs provide shall be done or performed by us, the depositary or our respective agents (including, without limitation, voting); |
| | • | | it exercises or fails to exercise discretion under the deposit agreement or the ADR; |
| | • | | it performs its obligations under the deposit agreement and ADRs without gross negligence or bad faith; |
| | • | | it takes any action or refrains from taking any action in reliance upon the advice of or information from legal counsel, accountants, any person presenting shares for deposit, any registered holder of ADRs, or any other person believed by it to be competent to give such advice or information; or |
| | • | | it relies upon any written notice, request, direction or other document believed by it to be genuine and to have been signed or presented by the proper party or parties. |
132
Neither the depositary nor its agents have any obligation to appear in, prosecute or defend any action, suit or other proceeding in respect of any deposited securities or the ADRs. We and our agents shall only be obligated to appear in, prosecute or defend any action, suit or other proceeding in respect of any deposited securities or the ADRs, which in our opinion may involve us in expense or liability, if indemnity satisfactory to us against all expense (including fees and disbursements of counsel) and liability is furnished as often as may be required. The depositary and its agents may fully respond to any and all demands or requests for information maintained by or on its behalf in connection with the deposit agreement, any registered holder or holders of ADRs, any ADRs or otherwise related to the deposit agreement or ADRs to the extent such information is requested or required by or pursuant to any lawful authority, including without limitation laws, rules, regulations, administrative or judicial process, banking, securities or other regulators. The depositary shall not be liable for the acts or omissions made by any securities depository, clearing agency or settlement system in connection with or arising out of book-entry settlement of deposited securities or otherwise so long as such acts or omissions are not caused as a direct result of the gross negligence or willful misconduct of the depositary. Furthermore, the depositary shall not be responsible for, and shall incur no liability in connection with or arising from, the insolvency of any custodian that is not a branch or affiliate of JPMorgan Chase Bank, N.A.
Additionally, none of us, the depositary or the custodian shall be liable for the failure by any registered holder of ADRs or beneficial owner therein to obtain the benefits of credits on the basis of non-U.S. tax paid against such holder’s or beneficial owner’s income tax liability. Neither we nor the depositary shall incur any liability for any tax consequences that may be incurred by holders or beneficial owners on account of their ownership of ADRs or ADSs.
Neither the depositary nor its agents will be responsible for any failure to carry out any instructions to vote any of the deposited securities, for the manner in which any such vote is cast or for the effect of any such vote. Neither the depositary nor any of its agents shall be liable to registered holders of ADRs or beneficial owners of interests in ADSs for any indirect, special, punitive or consequential damages (including, without limitation, lost profits) of any form incurred by any person or entity, whether or not foreseeable and regardless of the type of action in which such a claim may be brought.
The depositary may own and deal in any class of our securities and in ADSs.
Disclosure of Interest in ADSs
To the extent that the provisions of or governing any deposited securities may require disclosure of or impose limits on beneficial or other ownership of deposited securities, other shares and other securities and may provide for blocking transfer, voting or other rights to enforce such disclosure or limits, you agree to comply with all such disclosure requirements and ownership limitations and to comply with any reasonable instructions we may provide in respect thereof. We reserve the right to instruct you to deliver your ADSs for cancellation and withdrawal of the deposited securities so as to permit us to deal with you directly as a holder of shares and, by holding an ADS or an interest therein, you will be agreeing to comply with such instructions.
Books of Depositary
The depositary or its agent will maintain a register for the registration, registration of transfer, combination and split-up of ADRs, which register shall include the depositary’s direct registration system. Registered holders of ADRs may inspect such records at the depositary’s office at all reasonable times, but solely for the purpose of communicating with other holders in the interest of the business of our company or a matter relating to the deposit agreement. Such register may be closed from time to time, when deemed expedient by the depositary or when reasonably requested by us in order to enable us to comply with applicable law.
The depositary will maintain facilities for the delivery and receipt of ADRs.
133
Pre-release of ADSs
In its capacity as depositary, the depositary shall not lend shares or ADSs; provided, however, that the depositary may issue ADSs prior to the receipt of shares, or a pre-release. The depositary may receive ADSs in lieu of shares to close out a pre-release (which ADSs will promptly be canceled by the depositary upon receipt by the depositary). Each such pre-release will be subject to a written agreement whereby the person or entity, or the applicant, to whom ADSs are to be delivered (a) represents that at the time of the pre-release the applicant or its customer owns the shares that are to be delivered by the applicant under such pre-release, (b) agrees to indicate the depositary as owner of such shares in its records and to hold such shares in trust for the depositary until such shares are delivered to the depositary or the custodian, (c) unconditionally guarantees to deliver to the depositary or the custodian, as applicable, such shares, and (d) agrees to any additional restrictions or requirements that the depositary deems appropriate. Each such pre-release will be at all times fully collateralized with cash, U.S. government securities or such other collateral as the depositary deems appropriate, terminable by the depositary on not more than five (5) business days’ notice and subject to such further indemnities and credit regulations as the depositary deems appropriate. The depositary will normally limit the number of ADSs involved in such pre-release at any one time to thirty percent (30%) of the ADSs outstanding (without giving effect to pre-released ADSs), provided, however, that the depositary reserves the right to change or disregard such limit from time to time as it deems appropriate. The depositary may also set limits with respect to the number of ADSs involved in pre-release with any one person on a case-by-case basis as it deems appropriate. The depositary may retain for its own account any compensation received by it in conjunction with the foregoing. Collateral provided as described above, but not the earnings thereon, shall be held for the benefit of the registered holders of ADRs (other than the applicant).
Appointment
In the deposit agreement, each registered holder of ADRs and each person holding an interest in ADSs, upon acceptance of any ADSs (or any interest therein) issued in accordance with the terms and conditions of the deposit agreement will be deemed for all purposes to:
| | • | | be a party to and bound by the terms of the deposit agreement and the applicable ADR or ADRs, and |
| | • | | appoint the depositary its attorney-in-fact, with full power to delegate, to act on its behalf and to take any and all actions contemplated in the deposit agreement and the applicable ADR or ADRs, to adopt any and all procedures necessary to comply with applicable laws and to take such action as the depositary in its sole discretion may deem necessary or appropriate to carry out the purposes of the deposit agreement and the applicable ADR and ADRs, the taking of such actions to be the conclusive determinant of the necessity and appropriateness thereof. |
Governing Law
The deposit agreement and the ADRs shall be governed by and construed in accordance with the laws of the State of New York. In the deposit agreement, we have submitted to the jurisdiction of the courts of the State of New York and appointed an agent for service of process on our behalf.
134
SHARES ELIGIBLE FOR FUTURE SALE
Upon completion of this offering, we will have 5,800,000 ADSs outstanding, representing approximately 31.1% of our outstanding ordinary shares, assuming the underwriters do not exercise their option to purchase additional ADSs. All of the ADSs sold in this offering will be freely transferable by persons other than by our “affiliates” without restriction or further registration under the Securities Act. Sales of substantial amounts of our ADSs in the public market could adversely affect prevailing market prices of our ADSs. Prior to this offering, there has been no public market for our ordinary shares or the ADSs, and although our ADSs have been approved for listing on the New York Stock Exchange, we cannot assure you that a regular trading market will develop for the ADSs. We do not expect that a trading market will develop for our ordinary shares not represented by the ADSs.
Lock-up Agreements
We have agreed that we will not (i) offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase or otherwise transfer or dispose of, directly or indirectly, or file with the SEC a registration statement under the Securities Act relating to, any ordinary shares or ADSs or any securities convertible into or exercisable or exchangeable for ordinary shares or ADSs, or publicly disclose the intention to make any offer, sale, pledge, disposition or filing, (ii) enter into any swap or other agreement that transfers, in whole or in part, any of the economic consequences of ownership of any ordinary shares or ADSs or any such other securities, whether any such transaction described in clause (i) or (ii) above is to be settled by delivery of any ordinary shares or ADSs or such other securities, in cash or otherwise, without the prior written consent of the representatives of the underwriters for a period ending 180 days after the date of this prospectus, except issuances pursuant to the exercise of employee share options outstanding on the date hereof. However, in the event that either (1) during the last 17 days of the “lock-up” period, we release earnings results or announce any material news or a material event occurs or (2) prior to the expiration of the “lock-up” period, we announce, or if the representatives determine, that we will release earnings results during the 16-day period following the last day of the “lock-up” period, then in each case the “lock-up” period will be automatically extended until the expiration of the 18-day period beginning on the date of the release of the earnings results or the announcement of the material news or the occurrence of the material event, as applicable, unless the representatives waive, in writing, such an extension.
Each of our existing shareholders, executive officers and directors and certain holders of our options and RSUs have agreed, subject to certain exceptions, not to (i) offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, or otherwise transfer or dispose of, directly or indirectly, any ADSs or ordinary shares, or any securities convertible into or exercisable or exchangeable for ADSs or ordinary shares (including without limitation, ADSs and ordinary shares which may be deemed to be beneficially owned in accordance with the rules and regulations of the SEC and securities which may be issued upon exercise of a stock option or warrant), or publicly disclose the intention to make any offer, sale, pledge or disposition, (ii) enter into any swap or other agreement that transfers, in whole or in part, any of the economic consequences of ownership of ADSs, ordinary shares or such other securities, whether any such transaction described in clause (i) or (ii) above is to be settled by delivery of ADSs, ordinary shares or such other securities, in cash or otherwise, or (iii) make any demand for or exercise any right with respect to the registration of any ADSs, ordinary shares or any security convertible into or exercisable or exchangeable for ADSs or ordinary shares, without the prior written consent of the representatives for a period ending 180 days after the date of this prospectus. However, in the event that either (1) during the last 17 days of the relevant “lock-up” period, we release earnings results or announce any material news or a material event occurs or (2) prior to the expiration of the “lock-up” period, we announce, or if the representatives determine, that we will release earnings results during the 16-day period following the last day of the “lock-up” period, then in each case the “lock-up” period will be automatically extended until the expiration of the 18-day period beginning on the date of the release of the earnings results or the announcement of the material news or the occurrence of the material event, as applicable, unless the representatives waive, in writing, such an extension.
135
After the expiration of the 180-day period, the ordinary shares or ADSs held by our existing shareholders, executive officers and directors and certain holders of our options and RSUs may be sold subject to the restrictions under Rule 144 under the Securities Act or by means of registered public offerings.
Options representing approximately 6.3 million ordinary shares will vest prior to the expiration of the “lock-up” period referenced in the preceding paragraph. Holders of such options have not signed a lock-up agreement. However, we have agreed to instruct JPMorgan Chase Bank, N.A., as depositary, not to accept any deposit of any ordinary shares by, or issue any ADSs to, our current shareholders, beneficial owners, RSU holders or option holders for 180 days after the date of this prospectus (other than in connection with this offering). See “Description of American Depositary Shares—Deposit, Withdrawal and Cancellation.”
Rule 144
All of our ordinary shares outstanding prior to this offering are “restricted securities” as that term is defined in Rule 144 under the Securities Act and may be sold publicly in the United States only if they are subject to an effective registration statement under the Securities Act or pursuant to an exemption from the registration requirement such as those provided by Rule 144 and Rule 701 promulgated under the Securities Act. In general, under Rule 144 as currently in effect, beginning 90 days after the date of this prospectus, a person (or persons whose shares are aggregated) who at the time of a sale is not, and has not been during the three months preceding the sale, an affiliate of us and has beneficially owned our restricted securities for at least six months will be entitled to sell the restricted securities without registration under the Securities Act, subject only to the availability of current public information about us, and will be entitled to sell restricted securities beneficially owned for at least one year without restriction. Persons who are our affiliates and have beneficially owned our restricted securities for at least six months may sell within any three-month period a number of restricted securities that does not exceed the greater of the following:
| | • | | 1% of the then outstanding ordinary shares, in the form of ADSs or otherwise, which will equal approximately 1,044,000 ordinary shares immediately after this offering; or |
| | • | | the average weekly trading volume of our ordinary shares, in the form of ADSs or otherwise, during the four calendar weeks preceding the date on which notice of the sale is filed with the Securities and Exchange Commission. |
Sales by our affiliates under Rule 144 are also subject to certain requirements relating to manner of sale, notice and the availability of current public information about us.
Rule 701
In general, under Rule 701 of the Securities Act as currently in effect, each of our employees, consultants or advisors who purchases our ordinary shares from us in connection with a compensatory stock plan or other written agreement executed prior to the completion of this offering is eligible to resell such ordinary shares in reliance on Rule 144, but without compliance with some of the restrictions, including the holding period, contained in Rule 144. However, the Rule 701 shares would remain subject to lock-up arrangements and would only become eligible for sale when the lock-up period expires.
Registration Rights
Upon completion of this offering, certain holders of our ordinary shares or their transferees will be entitled to request that we register their shares under the Securities Act, following the expiration of the lock-up agreements described above. See “Description of Share Capital—Registration Rights.”
136
TAXATION
The following summary of the material Cayman Islands, PRC and United States federal income tax consequences of an investment in our ADSs or ordinary shares is based upon laws and relevant interpretations thereof in effect as of the date of this prospectus, all of which are subject to change. This summary does not deal with all possible tax consequences relating to an investment in our ADSs or ordinary shares, such as the tax consequences under state, local and other tax laws. To the extent that the discussion relates to matters of Cayman Islands tax law, it represents the opinion of Conyers Dill & Pearman, our special Cayman Islands counsel. To the extent that the discussion relates to legal conclusions under current U.S. federal income tax law, and subject to qualifications herein, it represents the opinion of Skadden, Arps, Slate, Meagher and Flom LLP, our special U.S. counsel.
Cayman Islands Taxation
The Cayman Islands currently levies no taxes on individuals or corporations based upon profits, income, gains or appreciation and there is no taxation in the nature of inheritance tax or estate duty. There are no other taxes likely to be material to us levied by the government of the Cayman Islands except for stamp duties which may be applicable on instruments executed in, or brought within the jurisdiction of the Cayman Islands. The Cayman Islands is not party to any double tax treaties. There are no exchange control regulations or currency restrictions in the Cayman Islands.
People’s Republic of China Taxation
We are a holding company incorporated in the Cayman Islands, which indirectly holds, through our Hong Kong subsidiaries, 100% of our equity interests in our subsidiary in the PRC. Our operations are principally conducted through our PRC subsidiaries. The New Enterprise Income Tax Law and its implementation rules, both of which became effective on January 1, 2008, provide that China-sourced income of foreign enterprises, such as dividends paid by a PRC subsidiary to its overseas parent that is not a PRC resident enterprise, will normally be subject to PRC withholding tax at a rate of 10%, unless there are applicable treaties that reduce such rate. Under a special arrangement between China and Hong Kong, such dividend withholding tax rate is reduced to 5% if a Hong Kong resident enterprise owns over 25% of the PRC company distributing the dividends. As our Hong Kong subsidiaries owns 100% of our PRC subsidiaries, under the aforesaid arrangement, any dividends that our PRC subsidiaries pay our Hong Kong subsidiaries may be subject to a withholding tax at the rate of 5% if our Hong Kong subsidiaries are not considered to be PRC tax resident enterprises as described below. However, if our Hong Kong subsidiaries are not considered to be the beneficial owners of such dividends under a tax notice promulgated on October 27, 2009, such dividends would be subject to the withholding tax rate of 10%. See “Regulation—Regulations on Tax—Dividend Withholding Tax.”
Under the New Enterprise Income Tax Law, enterprises established under the laws of jurisdictions outside China with their “de facto management bodies” located within China may be considered to be PRC tax resident enterprises for tax purposes. If we are considered a PRC tax resident enterprise under the above definition, then our global income will be subject to PRC enterprise income tax at the rate of 25%.
The implementation rules of the New Enterprise Income Tax Law provide that (i) if the enterprise that distributes dividends is domiciled in the PRC, or (ii) if gains are realized from transferring equity interests of enterprises domiciled in the PRC, then such dividends or capital gains are treated as China-sourced income. It is not clear how “domicile” may be interpreted under the New Enterprise Income Tax Law, and it may be interpreted as the jurisdiction where the enterprise is a tax resident. Therefore, if we are considered as a PRC tax resident enterprise for tax purposes, any dividends we pay to our overseas shareholders or ADS holders as well as gains realized by such shareholders or ADS holders from the transfer of our shares or ADSs may be regarded as China-sourced income and as a result become subject to PRC withholding tax at a rate of up to 10%.
137
See “Risk Factors—Risks Related to Doing Business in China—Our global income and the dividends that we may receive from our PRC subsidiaries may be subject to PRC taxes under the PRC Enterprise Income Tax Law, which would have a material and adverse effect on our results of operations.”
Material United States Federal Income Tax Considerations
The following is a summary of the material United States federal income tax considerations relating to the acquisition, ownership and disposition of our ADSs or ordinary shares by a U.S. Holder (as defined below) that will acquire our ADSs or ordinary shares in the offering and will hold our ADSs or ordinary shares as “capital assets,” or (generally, property held for investment) under the United States Internal Revenue Code of 1986, as amended, or the Code. This summary is based upon existing United States federal tax law, including the Code, its legislative history, existing, temporary and proposed regulations thereunder, published rulings and court decisions, all of which are subject to differing interpretations or change, possibly with retroactive effect. No ruling has been sought from the IRS with respect to any United States federal income tax consequences described below, and there can be no assurance that the IRS or a court will not take a contrary position. This summary does not discuss all aspects of United States federal income taxation that may be important to particular investors in light of their individual investment circumstances, including investors subject to special tax rules (for example, financial institutions, insurance companies, regulated investment companies, real estate investment trusts, broker-dealers, traders in securities that elect mark-to-market treatment, partnerships (or other entities treated as partnerships for United States federal income tax purposes) and their partners and tax-exempt organizations (including private foundations)), holders who are not U.S. Holders, holders who own (directly, indirectly or constructively) 10% or more of our voting stock, holders who acquire their ADSs or ordinary shares pursuant to any employee share option or otherwise as compensation, investors that will hold their ADSs or ordinary shares as part of a straddle, hedge, conversion, constructive sale or other integrated transaction for United States federal income tax purposes, or investors that have a functional currency other than the United States dollar, all of whom may be subject to tax rules that differ significantly from those summarized below. This summary does not address holders of equity interests in a holder of ADSs or ordinary shares. In addition, this summary does not discuss any United States federal estate, gift or alternative minimum tax consequences or any non-United States, state or local tax considerations. Each U.S. Holder is urged to consult its tax advisor regarding the United States federal, state, local and non-United States income and other tax considerations of an investment in ADSs or ordinary shares.
General
For purposes of this summary, a “U.S. Holder” is a beneficial owner of our ADSs or ordinary shares that is, for United States federal income tax purposes, (i) an individual who is a citizen or resident of the United States, (ii) a corporation (or other entity treated as a corporation for United States federal income tax purposes) created in, or organized under the law of, the United States or any state thereof or the District of Columbia, (iii) an estate the income of which is includible in gross income for United States federal income tax purposes regardless of its source, or (iv) a trust (A) the administration of which is subject to the primary supervision of a United States court and which has one or more United States persons who have the authority to control all substantial decisions of the trust or (B) that has otherwise validly elected to be treated as a United States person under the Code.
If a partnership (or other entity treated as a partnership for United States federal income tax purposes) is a beneficial owner of our ADSs or ordinary shares, the tax treatment of a partner in the partnership will generally depend upon the status of the partner and the activities of the partnership. If a U.S. Holder is a partner of a partnership holding our ADSs or ordinary shares, the U.S. Holder is urged to consult its tax advisor regarding an investment in our ADSs or ordinary shares.
The discussion below assumes that the representations contained in the deposit agreement are true and that the obligations in the deposit agreement and any related agreement have been and will be complied with in accordance with the terms.
138
For United States federal income tax purposes, a U.S. Holder of ADSs will be treated as the beneficial owner of the underlying shares represented by the ADSs.
Passive Foreign Investment Company Considerations
A non-United States corporation, such as our company, will be classified as a “passive foreign investment company,” or “PFIC,” for United States federal income tax purposes for any taxable year, if either (i) 75% or more of its gross income for such year consists of certain types of “passive” income or (ii) 50% or more of the value of its assets (determined on the basis of a quarterly average) during such year produce or are held for the production of passive income, or the asset test. Passive income is any income that would be foreign personal holding company income under the Code including, without limitation, dividends, interest, royalties, rents, annuities, net gains from the sale or exchange of property producing such income, net gains from commodity transactions, net foreign currency gains and income from notional principal contracts. For this purpose, cash and assets readily convertible into cash are categorized as a passive asset and the company’s unbooked intangibles are taken into account. We will be treated as owning a proportionate share of the assets and earning a proportionate share of the income of any other corporation in which we own, directly or indirectly, more than 25% (by value) of the stock.
We primarily operate as a provider of pharmaceutical and biotechnology R&D outsourcing services. Based on our current income and assets and projections as to the value of our ADSs and outstanding ordinary shares pursuant to this offering, we do not expect to be classified as a PFIC for the current taxable year or the foreseeable future. While we do not anticipate becoming a PFIC, because the value of our assets for purposes of the asset test will generally be determined by reference to the market price of our ADSs or ordinary shares, fluctuations in the market price of our ADSs or ordinary shares may cause us to become a PFIC for the current or subsequent taxable years. In estimating the value of our goodwill and other unbooked intangibles, we have taken into account our anticipated market capitalization following the close of this offering. Among other matters, if our market capitalization is less than anticipated or subsequently declines, we may be or become classified as a PFIC for the current or one or more future taxable years. After the effectiveness of the registration statement, we will be required to file annual reports on Form 20-F with the Securities and Exchange Commission in which we will update our expectations as to whether or not we anticipate being a PFIC for the applicable years.
The composition of our income and our assets will also be affected by (i) future growth in activities that may potentially produce passive income, and (ii) how, and how quickly, we spend our liquid assets and the cash raised in this offering. Under circumstances where revenues from activities that produce passive income significantly increase relative to our revenues from activities that produce non-passive income or where we determine not to deploy significant amounts of cash for active purposes, our risk of becoming classified as a PFIC may substantially increase.
Furthermore, because there are uncertainties in the application of the relevant rules, it is possible that the IRS may successfully challenge our classification of certain income as non-passive income, which may result in our company becoming classified as a PFIC for the current or subsequent taxable years. Because PFIC status is a fact-intensive determination made on an annual basis and will depend upon the composition of our assets and income, and the continued existence of our goodwill, no assurance can be given that we are not or will not become classified as a PFIC. If we are classified as a PFIC for any year during which a U.S. Holder holds our ADSs or ordinary shares, we generally will continue to be treated as a PFIC for all succeeding years during which such U.S. Holder holds our ADSs or ordinary shares, unless we cease to be a PFIC and you make a “deemed sale” election with respect to the ADSs or ordinary shares.
The discussion below under “Dividends” and “Sale or Other Disposition of ADSs or ordinary shares” is written on the basis that we will not be classified as a PFIC for United States federal income tax purposes. The U.S. federal income tax rules that apply if we are classified as a PFIC for our 2010 or subsequent taxable years are generally discussed below under “Passive Foreign Investment Company Rules.”
139
Dividends
Any cash distributions (including the amount of any PRC tax withheld) paid on our ADSs or ordinary shares out of our current or accumulated earnings and profits, as determined under United States federal income tax principles, will generally be includible in the gross income of a U.S. Holder as dividend income on the day actually or constructively received by the U.S. Holder, in the case of ordinary shares, or by the depositary, in the case of ADSs. Because we do not intend to determine our earnings and profits on the basis of United States federal income tax principles, any distribution paid will generally be treated as a “dividend” for United States federal income tax purposes. For taxable years beginning before January 1, 2011, a non-corporate recipient of dividend income generally will be subject to tax on dividend income from a “qualified foreign corporation” at a maximum United States federal tax rate of 15 percent rather than the marginal tax rates generally applicable to ordinary income provided that certain holding period requirements are met. A non-United States corporation (other than a corporation that is classified as a PFIC for the taxable year in which the dividend is paid or the preceding taxable year) generally will be considered to be a qualified foreign corporation (i) if it is eligible for the benefits of a comprehensive tax treaty with the United States which the Secretary of Treasury of the United States determines is satisfactory for purposes of this provision and which includes an exchange of information program, or (ii) with respect to any dividend it pays on stock (or ADSs in respect of such stock) which is readily tradable on an established securities market in the United States. Our ADSs have been approved for listing on the New York Stock Exchange, which is an established securities market in the United States, and are expected to be readily tradable. Thus, we believe that dividends we pay on our ADSs will meet the conditions required for the reduced tax rates. Since we do not expect that our ordinary shares will be listed on an established securities market, we do not believe that dividends we pay on our ordinary shares that are not represented by ADSs will meet the conditions required for these reduced tax rates. Dividends received on our ADSs or ordinary shares will not be eligible for the deduction allowed to corporations.
In the event that we are deemed to be a PRC resident enterprise under the PRC Enterprise Income Tax Law, a U.S. Holder may be subject to PRC withholding taxes on dividends paid on our ADSs or ordinary shares. We may, however, be eligible for the benefits of the United States-PRC income tax treaty. If we are eligible for such benefits, dividends we pay on our ordinary shares, regardless of whether such shares are represented by the ADSs, would be eligible for the reduced rates of taxation applicable to qualified dividend income, as discussed above.
Dividends generally will be treated as income from foreign sources for United States foreign tax credit purposes and generally will constitute passive category income. A U.S. Holder may be eligible, subject to a number of complex limitations, to claim a foreign tax credit in respect of any foreign withholding taxes imposed on dividends received on our ADSs or ordinary shares. A U.S. Holder who does not elect to claim a foreign tax credit for foreign tax withheld, may instead claim a deduction, for United States federal income tax purposes, in respect of such withholdings, but only for a year in which such holder elects to do so for all creditable foreign income taxes. The rules governing the foreign tax credit are complex. U.S. Holders are urged to consult their tax advisors regarding the availability of the foreign tax credit under their particular circumstances.
Sale or Other Disposition of ADSs or Ordinary Shares
Subject to the discussion below under “Passive Foreign Investment Company Rules,” a U.S. Holder will generally recognize capital gain or loss upon the sale or other disposition of ADSs or ordinary shares in an amount equal to the difference between the amount realized upon the disposition and the holder’s adjusted tax basis in such ADSs or ordinary shares. Any capital gain or loss will be long-term if the ADSs or ordinary shares have been held for more than one year and will generally be United States source gain or loss for United States foreign tax credit purposes. Capital gains of non-corporate taxpayers derived from capital assets held for more than one year are currently eligible for reduced rates of taxation. In the event that gain from the disposition of the ADSs or ordinary shares is subject to tax in the PRC, such gain may be treated as PRC source gain under the United States-PRC income tax treaty. The deductibility of a capital loss is subject to limitations. U.S. Holders are urged to consult their tax advisors regarding the tax consequences if a foreign withholding tax is imposed on a
140
disposition of our ADSs or ordinary shares, including the availability of the foreign tax credit under their particular circumstances.
Passive Foreign Investment Company Rules
If we are classified as a PFIC for any taxable year during which a U.S. Holder holds our ADSs or ordinary shares, and unless the U.S. Holder makes a mark-to-market election (as described below), the U.S. Holder will generally be subject to special United States federal income tax rules that have a penalizing effect, regardless of whether we remain a PFIC, on (i) any excess distribution that we make to the U.S. Holder (which generally means any distribution paid during a taxable year to a U.S. Holder that is greater than 125 percent of the average annual distributions paid in the three preceding taxable years or, if shorter, the U.S. Holder’s holding period for the ADSs or ordinary shares), and (ii) any gain realized on the sale or other disposition, including a pledge, of ADSs or ordinary shares. Under the PFIC rules the:
| | • | | excess distribution or gain will be allocated ratably over the U.S. Holder’s holding period for the ADSs or ordinary shares; |
| | • | | amounts allocated to the current taxable year and any taxable years in the U.S. Holder’s holding period prior to the first taxable year in which we are classified as a PFIC, or a pre-PFIC year, will be taxable as ordinary income; |
| | • | | amounts allocated to each prior taxable year, other than the current taxable year or a pre-PFIC year, will be subject to tax at the highest tax rate in effect applicable to you for that year; and |
| | • | | interest charge generally applicable to underpayments of tax will be imposed on the tax attributable to each prior taxable year, other than the current taxable year or a pre-PFIC year. |
If we are a PFIC for any taxable year during which a U.S. Holder holds our ADSs or ordinary shares and any of our non-United States subsidiaries is also a PFIC, such U.S. Holder would be treated as owning a proportionate amount (by value) of the shares of each such non-United States subsidiary classified as a PFIC, each such subsidiary, a lower-tier PFIC, for purposes of the application of these rules. U.S. Holders should consult their tax advisors regarding the application of the PFIC rules to any of our subsidiaries.
As an alternative to the foregoing rules, a U.S. Holder of “marketable stock” in a PFIC may make a mark-to-market election with respect to our ADSs, but not our ordinary shares, provided that the listing of the ADSs on the New York Stock Exchange is approved and that the ADSs are regularly traded. We anticipate that our ADSs should qualify as being regularly traded, but no assurances may be given in this regard. If a U.S. Holder makes a valid mark-to-market election, the U.S. Holder will generally (i) include as ordinary income for each taxable year that we are a PFIC the excess, if any, of the fair market value of ADSs held at the end of the taxable year over the adjusted tax basis of such ADSs and (ii) deduct as an ordinary loss the excess, if any, of the adjusted tax basis of the ADSs over the fair market value of such ADSs held at the end of the taxable year, but only to the extent of the net amount previously included in income as a result of the mark-to-market election. The U.S. Holder’s adjusted tax basis in the ADSs would be adjusted to reflect any income or loss resulting from the mark-to-market election. If a U.S. Holder makes a mark-to-market election in respect of a corporation classified as a PFIC and such corporation ceases to be classified as a PFIC, the holder will not be required to take into account the gain or loss described above during any period that such corporation is not classified as a PFIC. If a U.S. Holder makes a mark-to-market election, any gain such U.S. Holder recognizes upon the sale or other disposition of our ADSs will be treated as ordinary income and any loss will be treated as ordinary loss, but only to the extent of the net amount previously included in income as a result of the mark-to-market election.
Because a mark-to-market election cannot be made for any lower-tier PFICs that we may own, a U.S. Holder may continue to be subject to the PFIC rules with respect to such U.S. Holder’s indirect interest in any investments held by us that are treated as an equity interest in a PFIC for United States federal income tax purposes.
The “QEF election” regime, which may potentially serve as a further alternative to the foregoing rules, will not be available. Subject to various limitations, a U.S. Holder may make a “qualified electing fund” election,
141
or “QEF election,” with respect to a PFIC in which the U.S. Holder directly or indirectly owns shares. Because we do not intend to provide the information necessary to enable a U.S. Holder to make a QEF election, the QEF election will not be available to U.S. Holders.
If a U.S. Holder owns our ADSs or ordinary shares during any taxable year that we are a PFIC, the holder must file an annual IRS Form 8621. In the case of a U.S. Holder who has held ADSs or ordinary shares during any taxable year in respect of which we were classified as a PFIC and continues to hold such ADSs or ordinary shares (or any portion thereof) and has not previously determined to make a mark-to-market election, and who is now considering making a mark-to-market election, special tax rules may apply relating to purging the PFIC taint of such ADSs or ordinary shares. Each U.S. Holder is urged to consult its tax advisor concerning the United States federal income tax consequences of purchasing, holding and disposing ADSs or ordinary shares if we are or become classified as a PFIC, including the possibility of making a mark-to-market election and the unavailability of the QEF election.
Information Reporting and Backup Withholding
Dividend payments with respect to the ADSs or ordinary shares and proceeds from the sale, exchange or redemption of the ADSs or ordinary shares may be subject to information reporting to the Internal Revenue Service and possible United States backup withholding at a rate of 28%. Backup withholding will not apply, however, to a U.S. Holder who furnishes a correct taxpayer identification number and makes any other required certification, or who is otherwise exempt from backup withholding. U.S Holders that are required to establish their exempt status generally must provide such certification on IRS Form W-9. We will withhold amounts from such dividend payments as required by applicable law. Under newly enacted legislation, certain individuals holding ADSs or ordinary shares other than in an account at a financial institution may be subject to additional information reporting requirements. U.S. Holders should consult their tax advisors regarding the application of the United States information reporting and backup withholding rules.
Backup withholding is not an additional tax. Amounts withheld as backup withholding may be credited against a U.S. Holder’s United States federal income tax liability, and a U.S. Holder generally may obtain a refund of any excess amounts withheld under the backup withholding rules by timely filing the appropriate claim for refund with the Internal Revenue Service and furnishing any required information.
142
UNDERWRITING
Under the terms and subject to the conditions contained in an underwriting agreement, dated the date of this prospectus, the underwriters named below, for whom Citigroup Global Markets Inc. and J.P. Morgan Securities LLC are acting as representatives, have severally agreed to purchase, and we and the selling shareholders have agreed to sell to them, the number of ADSs indicated in the table below. The address of Citigroup Global Markets Inc. is 388 Greenwich Street, New York, New York 10013. The address of J.P. Morgan Securities LLC is 383 Madison Avenue, Floor 4, New York, New York 10179.
| | | | |
Underwriter | | Number of ADSs | |
Citigroup Global Markets Inc. | | | 2,552,000 | |
J.P. Morgan Securities LLC | | | 2,552,000 | |
William Blair & Company, L.L.C. | | | 348,000 | |
Oppenheimer & Co. Inc. | | | 348,000 | |
| | | | |
Total | | | 5,800,000 | |
| | | | |
The underwriters are offering the ADSs subject to their acceptance of the ADSs from us and the selling shareholders and subject to prior sale. The underwriting agreement provides that the obligations of the several underwriters to pay for and accept delivery of the ADSs offered by this prospectus are subject to the approval of certain legal matters by their counsel and to certain other conditions. The underwriting agreement also provides that if an underwriter defaults, the purchase commitments of non-defaulting underwriters may be increased or the offering may be terminated. The underwriters are obligated to take and pay for all of the ADSs offered by this prospectus if any such ADSs are taken. However, the underwriters are not required to take or pay for the ADSs covered by the underwriters’ option described below.
We and the selling shareholders have agreed to indemnify the underwriters against, or to contribute to payments the underwriters may be required to make in respect of, liabilities arising out of an untrue or allegedly untrue statement of a material fact contained in the registration statement, prospectus and other offering documents, or the omission or alleged omission to state a material fact required to be stated therein or necessary to make the statements therein not misleading provided that such liabilities do not arise in reliance upon written information furnished to us by the underwriters expressly for use in the aforementioned documents.
The representatives have advised us and the selling shareholders that the underwriters propose initially to offer the ADSs to the public at the initial public offering price on the cover page of this prospectus and to dealers at that price less a concession not in excess of $0.63 per ADS. No further discount will be allowed to dealers or re-allowed by dealers to other dealers. After the initial public offering, the public offering price, concession and discount may be changed.
In addition, the selling shareholders have granted to the underwriters an option, exercisable for 30 days from the date of this prospectus, to purchase up to an aggregate of 870,000 additional ADSs at the initial public offering price listed on the cover page of this prospectus, less underwriter discounts and commissions. To the extent the option is exercised, each underwriter will become obligated, subject to certain conditions, to purchase additional ADSs approximately proportionate to each underwriter’s initial amount reflected in the table above.
143
The following table shows the per ADS and total underwriting discounts and commissions to be paid by us and the selling shareholders in connection with this offering. The amounts in the following table are shown assuming both no exercise and full exercise of the underwriters’ option to purchase additional ADSs as described above.
| | | | | | | | | | | | | | | | |
| | | Per ADS | | | Total | |
| | | Option Not
Exercised | | | Option
Exercised | | | Option Not
Exercised | | | Option
Exercised | |
Underwriting discounts and commissions paid by us | | $ | 1.05 | | | $ | 1.05 | | | $ | 3,360,000 | | | $ | 3,360,000 | |
Expenses payable by us | | $ | 1.14 | | | $ | 1.14 | | | $ | 3,649,447 | | | $ | 3,649,447 | |
Underwriting discounts and commissions paid by the selling shareholders | | $ | 1.05 | | | $ | 1.05 | | | $ | 2,730,000 | | | $ | 3,643,500 | |
We have agreed to reimburse the underwriters for fees and expenses of counsel to the underwriters of up to $350,000 and for out-of-pocket expenses of up to $200,000.
We have agreed that we will not (i) offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase or otherwise transfer or dispose of, directly or indirectly, or file with the SEC a registration statement under the Securities Act relating to, any ordinary shares or ADSs or any securities convertible into or exercisable or exchangeable for ordinary shares or ADSs, or publicly disclose the intention to make any offer, sale, pledge, disposition or filing, (ii) enter into any swap or other agreement that transfers, in whole or in part, any of the economic consequences of ownership of any ordinary shares or ADSs or any such other securities, whether any such transaction described in clause (i) or (ii) above is to be settled by delivery of any ordinary shares or ADSs or such other securities, in cash or otherwise, without the prior written consent of the representatives for a period ending 180 days after the date of this prospectus, except issuances pursuant to the exercise of employee share options outstanding on the date hereof. However, in the event that either (1) during the last 17 days of the “lock-up” period, we release earnings results or announce any material news or a material event occurs or (2) prior to the expiration of the “lock-up” period, we announce, or if the representatives determine, that we will release earnings results during the 16-day period following the last day of the “lock-up” period, then in each case the “lock-up” period will be automatically extended until the expiration of the 18-day period beginning on the date of the release of the earnings results or the announcement of the material news or the occurrence of the material event, as applicable, unless the representatives waive, in writing, such an extension.
Each of our existing shareholders, executive officers and directors and certain holders of our options and RSUs has agreed, subject to certain exceptions, not to (i) offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, or otherwise transfer or dispose of, directly or indirectly, any ADSs or ordinary shares, or any securities convertible into or exercisable or exchangeable for ADSs or ordinary shares (including without limitation, ADSs and ordinary shares which may be deemed to be beneficially owned in accordance with the rules and regulations of the SEC and securities which may be issued upon exercise of a stock option or warrant), or publicly disclose the intention to make any offer, sale, pledge or disposition, (ii) enter into any swap or other agreement that transfers, in whole or in part, any of the economic consequences of ownership of ADSs, ordinary shares or such other securities, whether any such transaction described in clause (i) or (ii) above is to be settled by delivery of ADSs, ordinary shares or such other securities, in cash or otherwise, or (iii) make any demand for or exercise any right with respect to the registration of any ADSs, ordinary shares or any security convertible into or exercisable or exchangeable for ADSs or ordinary shares, without the prior written consent of the representatives for a period ending 180 days after the date of this prospectus. However, in the event that either (1) during the last 17 days of the relevant “lock-up” period, we release earnings results or announce any material news or a material event occurs or (2) prior to the expiration of the “lock-up” period, we announce, or if the representatives determine, that we will release earnings results during the 16-day period following the last day of the “lock-up” period, then in each case the “lock-up” period will be automatically extended until the expiration of the 18-day period beginning on the date of the release of the earnings results or the announcement of the material news or the occurrence of the material event, as applicable, unless the representatives waive, in writing, such an extension.
144
Options representing approximately 6.3 million ordinary shares will vest prior to the expiration of the “lock-up” period referenced in the preceding paragraph. Holders of such options have not signed a lock-up agreement. However, we have agreed to instruct JPMorgan Chase Bank, N.A., as depositary, not to accept any deposit of any ordinary shares by, or issue any ADSs to, our current shareholders, beneficial owners, RSU holders or option holders for 180 days after the date of this prospectus (other than in connection with this offering). See “Description of American Depositary Shares—Deposit, Withdrawal and Cancellation.”
Our ADSs have been approved for listing on the New York Stock Exchange under the symbol “SHP.”
Before this offering, there has been no public market for our ordinary shares or ADSs. The initial public offering price was determined through negotiations among us and the representatives. Among the factors considered in determining the initial public offering price are our future prospects and those of our industry in general, our sales, earnings, certain other financial and operating information in recent periods, the price-earnings ratios, price-sales ratios and market prices of securities and certain financial and operating information of companies engaged in activities similar to ours.
An active trading market for the ADSs may not develop. It is also possible that after the offering the ADSs will not trade in the public market at or above the initial public offering price.
Until the distribution of the ADSs is completed, SEC rules may limit underwriters and selling group members from bidding for and purchasing our ADSs. However, the representatives, or any person acting for them, on behalf of the underwriters, may engage in transactions that stabilize the price of the ADSs, such as bids or purchases to peg, fix or maintain that price.
In connection with the offering, the underwriters may purchase and sell our ADSs in the open market. These transactions may include short sales, purchases on the open market to cover positions created by short sales and stabilizing transactions. Short sales involve the sale by the underwriters of a greater number of ADSs than they are required to purchase in the offering. “Covered” short sales are sales made in an amount not greater than the underwriters’ option to purchase additional ADSs in the offering. The underwriters may close out any covered short position by either exercising their option to purchase additional ADSs or purchasing ADSs in the open market. In determining the source of shares to close out the covered short position, the underwriters will consider, among other things, the price of ADSs available for purchase in the open market as compared to the price at which they may purchase ADSs through the option to purchase additional ADSs. “Naked” short sales are sales in excess of the option to purchase additional ADSs. The underwriters must close out any naked short position by purchasing ADSs in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of our ADSs in the open market after pricing that could adversely affect investors who purchase in the offering. Stabilizing transactions consist of various bids for or purchases of ADSs made by the underwriters in the open market prior to the completion of the offering.
The underwriters may also impose a penalty bid. This occurs when a particular underwriter repays to the underwriters a portion of the underwriting discount received by it because the representatives have repurchased ADSs sold by or for the account of such underwriter in stabilizing or short covering transactions.
Similar to other purchase transactions, the underwriters’ purchases to cover the syndicate short sales may have the effect of raising or maintaining the market price of our ADSs or preventing or retarding a decline in the market price of our ADSs. As a result, the price of our ADSs may be higher than the price that might otherwise exist in the open market.
Neither we nor any of the underwriters makes any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the price of the ADSs. In addition, neither we nor any of the underwriters makes any representation that the representatives will engage in these transactions or that these transactions, once commenced, will not be discontinued without notice. In connection
145
with the offering, certain of the underwriters or securities dealers may distribute prospectuses by electronic means, such as e-mail. In addition, the representatives will be facilitating internet distribution for this offering to certain of their respective internet subscription customers. An electronic prospectus may be made available on the internet website maintained by one or more of the representatives.
Other than the prospectus in electronic format, the information contained on, or that may be accessed through, the web site of any of the representatives is not part of this prospectus.
Some of the underwriters and their affiliates have engaged and may in the future engage in investment banking and other commercial dealings in the ordinary course of business with us and our affiliates.
In the third quarter of 2010, Citibank (China) Co., Ltd. Shanghai Branch, or Citibank Shanghai, an affiliate of Citigroup Global Markets Inc., provided a short-term revolving accounts receivable facility and a letter of credit facility for an aggregate of $5.0 million to Shanghai ChemExplorer Co., Ltd., or ChemExplorer, Shanghai ChemPartner Co., Ltd., or ChemPartner, and China Gateway Pharma Products (Shanghai) Limited, or Gateway. Under the terms of the facility, ChemExplorer and ChemPartner (or their direct parent companies, China Gateway Life Science (Holdings) Limited and ChemExplorer Company Limited) will assign receivables due from a pre-approved group of customers to Citibank Shanghai as security for amounts drawn under the accounts receivable facility. Amounts drawn under the accounts receivable facility have a maximum term of three months and bear interest at the three-month London Interbank Offered Rate, or LIBOR, plus 4.5%. ChemExplorer, ChemPartner and Gateway may also use this facility to obtain letters of credit. Issued letters of credit have a maximum term of one year and bear commission at a rate of 0.15% quarterly.
In the third quarter of 2010, Citibank Shanghai provided an interest-free facility for an aggregate of $7.5 million to ChemExplorer and ChemPartner for hedging and risk management derivative transactions with Citibank Shanghai.
In the third quarter of 2010, Citibank Shanghai provided ChemExplorer, ChemPartner and Gateway with a short-term revolving credit facility for an aggregate of $2.0 million. Amounts drawn under the facility have a maximum term of three months and will be secured by properties owned by Gateway. U.S. dollar amounts drawn under the facility bear interest at the three-month LIBOR plus 4.5% annually and RMB amounts drawn under the facility bear interest at 90% of the prevailing base lending rate set by the People’s Bank of China, or PBOC.
In the third quarter of 2010, Citibank Shanghai provided ChemExplorer, ChemPartner and Gateway with a three-year term loan commitment of $5.0 million. Loans provided under the commitment have a maximum term of three years and will be secured by properties owned by Gateway. Loans provided in U.S. dollars bear interest at the three-month LIBOR plus 4.5% annually and loans provided in RMB bear interest at the prevailing base lending rate set by the PBOC. Proceeds from any loans provided under the commitment may only be used for equipment purchases and plant construction.
Selling Restrictions
European Economic Area
In relation to each member state of the European Economic Area that has implemented the Prospectus Directive, each, a relevant member state, with effect from and including the date on which the Prospectus Directive is implemented in that relevant member state, or the relevant implementation date. The ADSs may not be offered to the public in that relevant member state prior to the publication of a prospectus in relation to the ADSs that has been approved by the competent authority in that relevant member state or, where appropriate,
146
approved in another relevant member state and notified to the competent authority in the relevant member state, all in accordance with the Prospectus Directive, except that with effect from and including the relevant implementation date, an offer of the ADSs to the public may be made in that relevant member state at any time:
| | • | | to legal entities that are authorized or regulated to operate in the financial markets or, if not authorized or regulated, whose corporate purpose is solely to invest in securities; |
| | • | | to any legal entity that has two or more of (i) an average of at least 250 employees during the last financial year; (ii) a total balance sheet of more than €43,000,000; and (iii) an annual net turnover of more than €50,000,000, as shown in its last annual or combined accounts; |
| | • | | to fewer than 100 natural or legal persons (other than qualified investors as defined in the Prospectus Directive) subject to obtaining the prior consent of representatives of the underwriters; or |
| | • | | in any other circumstances falling within Article 3(2) of the Prospectus Directive. |
For the purposes of the above, the expression an “offer of any ADSs to the public” in relation to any ADSs in any relevant member state means the communication in any form and by any means of sufficient information on the terms of the offer and the ADSs to be offered so as to enable an investor to decide to purchase or subscribe the ADSs, as the same may be varied in that member state by any measure implementing the Prospectus Directive in that member state and the expression Prospective Directive means Directive 2003/71/EC and includes any relevant implementing measure in each relevant member state.
United Kingdom
This prospectus is only being distributed to, and is only directed at, persons in the United Kingdom that are qualified investors within the meaning of Article 2(1)(e) of the Prospectus Directive that are also (i) investment professionals falling within Article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005, or the Order, or (ii) high net worth entities, and other persons to whom it may lawfully be communicated, falling within Article 49(2)(a) to (d) of the Order (each such person being referred to as a relevant person). This prospectus and its contents are confidential and should not be distributed, published or reproduced (in whole or in part) or disclosed by recipients to any other persons in the United Kingdom. Any person in the United Kingdom that is not a relevant person should not act or rely on this document or any of its contents.
Switzerland
This prospectus, as well as any other material relating to the ADSs that are the subject of the offering contemplated by this prospectus, do not constitute an issue prospectus pursuant to Article 652a of the Swiss Code of Obligations. The ADSs will not be listed on the SWX Swiss Exchange and, therefore, the documents relating to the ADSs, including, but not limited to, this prospectus, do not claim to comply with the disclosure standards of the listing rules of SWX Swiss Exchange and corresponding prospectus schemes annexed to the listing rules of the SWX Swiss Exchange. The ADSs are being offered in Switzerland by way of a private placement (i.e., to a small number of selected investors only, without any public offer and only to investors who do not purchase the ADSs with the intention to distribute them to the public). The investors will be individually approached by us from time to time. This prospectus, as well as any other material relating to the ADSs, is personal and confidential and do not constitute an offer to any other person. This prospectus may only be used by those investors to whom it has been handed out in connection with the offering described herein and may neither directly nor indirectly be distributed or made available to other persons without our express consent. It may not be used in connection with any other offer and shall in particular not be copied and/or distributed to the public in (or from) Switzerland.
147
Japan
The underwriters will not offer or sell any of our ADSs directly or indirectly in Japan or to, or for the benefit of any Japanese person or to others, for re-offering or re-sale directly or indirectly in Japan or to any Japanese person, except in each case, pursuant to an exemption from the registration requirements of, and otherwise in compliance with, the Securities and Exchange Law of Japan and any other applicable laws and regulations of Japan. For purposes of this paragraph, “Japanese person” means any person resident in Japan, including any corporation or other entity organized under the laws of Japan.
People’s Republic of China
This prospectus may not be circulated or distributed in the PRC and the ADSs may not be offered or sold, and will not offer or sell to any person for re-offering or resale directly or indirectly to any resident of the PRC except pursuant to applicable laws and regulations of the PRC. For the purpose of this paragraph, PRC does not include Taiwan and the special administrative regions of Hong Kong and Macau.
Taiwan
The ADSs have not been and will not be registered or filed with, or approved by, the Financial Supervisory Commission of Taiwan pursuant to relevant securities laws and regulations and may not be offered or sold in Taiwan through a public offering or in circumstances that constitute an offer within the meaning of the Securities and Exchange Act of Taiwan or relevant laws and regulations that require a registration, filing or approval of the Financial Supervisory Commission of Taiwan. No person or entity in Taiwan has been authorized to offer or sell the ADSs in Taiwan.
Hong Kong
The ADSs may not be offered or sold by means of any document other than (i) in circumstances which do not constitute an offer to the public within the meaning of the Companies Ordinance (Cap.32, Laws of Hong Kong), or (ii) to “professional investors” within the meaning of the Securities and Futures Ordinance (Cap.571, Laws of Hong Kong) and any rules made thereunder, or (iii) in other circumstances which do not result in the document being a “prospectus” within the meaning of the Companies Ordinance (Cap.32, Laws of Hong Kong), and no advertisement, invitation or document relating to the ADSs may be issued or may be in the possession of any person for the purpose of issue (in each case whether in Hong Kong or elsewhere), which is directed at, or the contents of which are likely to be accessed or read by, the public in Hong Kong (except if permitted to do so under the laws of Hong Kong) other than with respect to ADSs which are or are intended to be disposed of only to persons outside Hong Kong or only to “professional investors” within the meaning of the Securities and Futures Ordinance (Cap. 571, Laws of Hong Kong) and any rules made thereunder.
Singapore
This prospectus has not been registered as a prospectus with the Monetary Authority of Singapore. Accordingly, this prospectus and any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of the ADSs may not be circulated or distributed, nor may the ADSs be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to persons in Singapore other than (i) to an institutional investor under Section 274 of the Securities and Futures Act, Chapter 289 of Singapore, or the SFA, (ii) to a relevant person pursuant to Section 275(1), or any person pursuant to Section 275(1A), and in accordance with the conditions specified in Section 275 of the SFA or (iii) otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA, in each case subject to compliance with conditions set forth in the SFA.
148
Where the ADSs are subscribed or purchased under Section 275 of the SFA by a relevant person which is:
| | • | | a corporation (which is not an accredited investor (as defined in Section 4A of the SFA)) the sole business of which is to hold investments and the entire share capital of which is owned by one or more individuals, each of whom is an accredited investor; or |
| | • | | a trust (where the trustee is not an accredited investor) whose sole purpose is to hold investments and each beneficiary of the trust is an individual who is an accredited investor, |
shares, debentures and units of shares and debentures of that corporation or the beneficiaries’ rights and interest (howsoever described) in that trust shall not be transferred within six months after that corporation or that trust has acquired the shares pursuant to an offer made under Section 275 of the SFA except:
| | • | | to an institutional investor (for corporations, under Section 274 of the SFA) or to a relevant person defined in Section 275(2) of the SFA, or to any person pursuant to an offer that is made on terms that such shares, debentures and units of shares and debentures of that corporation or such rights and interest in that trust are acquired at a consideration of not less than S$200,000 (or its equivalent in a foreign currency) for each transaction, whether such amount is to be paid for in cash or by exchange of securities or other assets, and further for corporations, in accordance with the conditions specified in Section 275 of the SFA; |
| | • | | where no consideration is or will be given for the transfer; or |
| | • | | where the transfer is by operation of law. |
Cayman Islands
This prospectus does not constitute a public offer of the ADSs or ordinary shares, whether by way of sale or subscription, in the Cayman Islands. Each underwriter has represented and agreed that it has not offered or sold, and will not offer or sell, directly or indirectly, any ADSs or ordinary shares in the Cayman Islands.
General
No action may be taken in any jurisdiction other than the United States that would permit a public offering of the ADSs or the possession, circulation or distribution of this prospectus in any jurisdiction where action for that purpose is required. Accordingly, the ADSs may not be offered or sold, directly or indirectly, and neither the prospectus nor any other offering material or advertisements in connection with the ADSs may be distributed or published in or from any country or jurisdiction except under circumstances that will result in compliance with any applicable rules and regulations of any such country or jurisdiction.
149
EXPENSES RELATED TO THIS OFFERING
Set forth below is an itemization of the total expenses, excluding underwriting discounts and commissions, which are expected to be incurred in connection with the offer and sale of the ADSs by us. With the exception of the Securities and Exchange Commission registration fee, the New York Stock Exchange listing fee and the Financial Industry Regulatory Authority, Inc. filing fee, all amounts are estimates.
| | | | |
Securities and Exchange Commission Registration Fee | | $ | 7,847 | |
New York Stock Exchange Listing Fee | | | 150,000 | |
Financial Industry Regulatory Authority, Inc. Filing Fee | | | 11,600 | |
Printing Expenses | | | 150,000 | |
Legal Fees and Expenses | | | 1,500,000 | |
Accounting Fees and Expenses | | | 830,000 | |
Miscellaneous | | | 1,000,000 | |
| | | | |
Total | | $ | 3,649,447 | |
| | | | |
150
LEGAL MATTERS
We are being represented by Skadden, Arps, Slate, Meagher & Flom LLP with respect to certain legal matters as to United States federal securities and New York state law. The underwriters are being represented by Latham & Watkins with respect to certain legal matters as to United States federal securities and New York state law. The validity of the ordinary shares represented by the ADSs offered in this offering will be passed upon for us by Conyers Dill & Pearman. Certain legal matters as to PRC law will be passed upon for us by Fangda Partners and for the underwriters by Haiwen & Partners. Skadden, Arps, Slate, Meagher & Flom LLP may rely upon Conyers Dill & Pearman with respect to matters governed by Cayman Islands law and Fangda Partners with respect to matters governed by PRC law. Latham & Watkins may rely upon Haiwen & Partners with respect to matters governed by PRC law.
151
EXPERTS
The consolidated financial statements as of December 31, 2008 and 2009, and for each of the three years in the period ended December 31, 2009 included in this prospectus have been so included in reliance on the report of PricewaterhouseCoopers Zhong Tian CPAs Limited Company, an independent registered public accounting firm, given on the authority of the said firm as experts in auditing and accounting. The office of PricewaterhouseCoopers Zhong Tian CPAs Limited Company is located at 11th Floor, PricewaterhouseCoopers Center, 2 Corporate Avenue, 202 Hu Bin Road, Shanghai 200021, People’s Republic of China.
152
WHERE YOU CAN FIND ADDITIONAL INFORMATION
We have filed with the Securities and Exchange Commission, or the SEC, a registration statement on Form F-1, including relevant exhibits, under the Securities Act with respect to underlying ordinary shares represented by the ADSs to be sold in this offering. We have also filed with the SEC a related registration statement on F-6 to register the ADSs. This prospectus, which constitutes a part of the registration statement, does not contain all of the information contained in the registration statement. You should read the registration statement on Form F-1 and its exhibits and schedules for further information with respect to us and our ADSs.
Immediately upon the effectiveness of the registration statement we will become subject to periodic reporting and other informational requirements of the Exchange Act as applicable to foreign private issuers. Accordingly, we will be required to file reports, including annual reports on Form 20-F, and other information with the SEC. For the fiscal year ending December 31, 2010, our annual report on Form 20-F will be due within six months following the end of such fiscal year. For fiscal years ending on or after December 15, 2011, we will be required to file our annual report on Form 20-F within 120 days after the end of each fiscal year. All information filed with the SEC can be inspected and copied at the public reference facilities maintained by the SEC at 100 F Street, N.E., Washington, D.C. 20549. You can request copies of these documents, upon payment of a duplicating fee, by writing to the SEC. Please call the SEC at 1-800-SEC-0330 for further information on the operation of the public reference rooms. You may also obtain additional information over the Internet at the SEC’s website at www.sec.gov.
As a foreign private issuer, we are exempt from the rules of the Exchange Act prescribing the furnishing and content of proxy statements to shareholders, and our executive officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we will not be required under the Exchange Act to file periodic reports and financial statements with the SEC as frequently or as promptly as U.S. companies whose securities are registered under the Exchange Act. However, we intend to furnish the depositary with our annual reports, which will include a review of operations and annual audited consolidated financial statements prepared in conformity with U.S. GAAP, and all notices of shareholders’ meetings and other reports and communications generally available to our shareholders. The depositary will make such notices, reports and communications available to holders of ADSs and, upon our written request, will mail to all record holders of ADSs the information contained in any notice of a shareholders’ meeting received by the depositary from us.
153
SHANGPHARMA CORPORATION
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| | | | |
Report of Independent Registered Public Accounting Firm | | | F-2 | |
| |
Consolidated Financial Statements | | | | |
| |
Consolidated Statements of Operations for the years ended December 31, 2007, 2008 and 2009 | | | F-3 | |
| |
Consolidated Balance Sheets as of December 31, 2008 and 2009 | | | F-4 | |
| |
Consolidated Statements of Changes in Equity (Deficit) for the years ended December 31, 2007, 2008 and 2009 | | | F-5 | |
| |
Consolidated Statements of Cash Flows for the years ended December 31, 2007, 2008 and 2009 | | | F-6 | |
| |
Notes to the Consolidated Financial Statements for the years ended December 31, 2007, 2008 and 2009 | | | F-7 | |
| |
Unaudited Condensed Consolidated Financial Statements | | | | |
| |
Unaudited Condensed Consolidated Statements of Operations for the six months ended June 30, 2009 and 2010 | | | F-37 | |
| |
Unaudited Condensed Consolidated Balance Sheets as of December 31, 2009 and June 30, 2010 | | | F-38 | |
| |
Unaudited Condensed Consolidated Statements of Changes in Equity for the six months ended June 30, 2009 and 2010 | | | F-39 | |
| |
Unaudited Condensed Consolidated Statements of Cash Flows for the six months ended June 30, 2009 and 2010 | | | F-40 | |
| |
Notes to Unaudited Condensed Consolidated Financial Statements | | | F-41 | |
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders of ShangPharma Corporation:
In our opinion, the accompanying consolidated balance sheets and the related consolidated statements of operations, of changes in equity (deficit) and of cash flows present fairly, in all material respects, the financial position of ShangPharma Corporation (the “Company”) and its subsidiaries at December 31, 2008 and 2009, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2009, in conformity with accounting principles generally accepted in the United States of America. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits. We conducted our audits of these financial statements in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
/s/ PricewaterhouseCoopers Zhong Tian CPAs Limited Company
Shanghai, the People’s Republic of China
May 20, 2010
F-2
SHANGPHARMA CORPORATION
CONSOLIDATED STATEMENTS OF OPERATIONS
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
(Amount expressed in US dollars, except share data, unless otherwise stated)
| | | | | | | | | | | | | | | | |
| | | Note | | | 2007 | | | 2008 | | | 2009 | |
Net revenues | | | | | | | | | | | | | | | | |
Unrelated parties | | | 2(m) | | | | 34,670,233 | | | | 60,493,412 | | | | 72,284,657 | |
| | | | | | | | | | | | | | | | |
| | | | | | | 34,670,233 | | | | 60,493,412 | | | | 72,284,657 | |
Cost of revenues | | | | | | | | | | | | | | | | |
Unrelated parties | | | | | | | (19,027,501 | ) | | | (29,833,173 | ) | | | (40,490,139 | ) |
Related parties | | | 15 | | | | (3,787,949 | ) | | | (10,683,823 | ) | | | (7,942,831 | ) |
| | | | | | | | | | | | | | | | |
| | | | | | | (22,815,450 | ) | | | (40,516,996 | ) | | | (48,432,970 | ) |
| | | | |
Gross profit | | | | | | | 11,854,783 | | | | 19,976,416 | | | | 23,851,687 | |
| | | | | | | | | | | | | | | | |
Operating expenses: | | | | | | | | | | | | | | | | |
Selling and marketing | | | | | | | (517,075 | ) | | | (833,507 | ) | | | (1,608,584 | ) |
General and administrative | | | | | | | (3,972,427 | ) | | | (9,259,453 | ) | | | (11,994,024 | ) |
| | | | | | | | | | | | | | | | |
Total operating expenses | | | | | | | (4,489,502 | ) | | | (10,092,960 | ) | | | (13,602,608 | ) |
| | | | | | | | | | | | | | | | |
Profit from operations | | | | | | | 7,365,281 | | | | 9,883,456 | | | | 10,249,079 | |
| | | | |
Interest income | | | | | | | 213,026 | | | | 241,426 | | | | 29,621 | |
Other income | | | 2(r) | | | | 212,755 | | | | 893,161 | | | | 1,273,276 | |
Other expenses | | | 2(s) | | | | (106,094 | ) | | | (1,645,242 | ) | | | (200,938 | ) |
| | | | | | | | | | | | | | | | |
Income from operations before income taxes | | | | | | | 7,684,968 | | | | 9,372,801 | | | | 11,351,038 | |
Income taxes | | | 11 | | | | (188,179 | ) | | | (314,655 | ) | | | (1,552,223 | ) |
| | | | | | | | | | | | | | | | |
Net income attributable to ShangPharma Corporation | | | | | | | 7,496,789 | | | | 9,058,146 | | | | 9,798,815 | |
| | | | |
Allocation to preferred shareholders | | | | | | | (728,105 | ) | | | (2,280,633 | ) | | | (2,467,117 | ) |
| | | | | | | | | | | | | | | | |
Net income attributable to ShangPharma Corporation’s ordinary shareholders | | | | | | | 6,768,684 | | | | 6,777,513 | | | | 7,331,698 | |
| | | | | | | | | | | | | | | | |
Net income attributable to ShangPharma Corporation’s ordinary shareholders per share | | | 12 | | | | | | | | | | | | | |
Basic | | | | | | | 0.03 | | | | 0.03 | | | | 0.04 | |
| | | | | | | | | | | | | | | | |
Diluted | | | | | | | 0.03 | | | | 0.03 | | | | 0.04 | |
| | | | | | | | | | | | | | | | |
Weighted average ordinary shares outstanding | | | | | | | | | | | | | | | | |
Basic | | | | | | | 208,005,986 | | | | 208,005,986 | | | | 208,005,986 | |
| | | | | | | | | | | | | | | | |
Diluted | | | | | | | 230,381,122 | | | | 278,000,000 | | | | 278,051,299 | |
| | | | | | | | | | | | | | | | |
Share-based compensation included in: | | | | | | | | | | | | | | | | |
Cost of revenues | | | | | | | - | | | | (61,100 | ) | | | (178,906 | ) |
Selling and marketing | | | | | | | - | | | | - | | | | (4,274 | ) |
General and administrative | | | | | | | - | | | | (7,652 | ) | | | (68,770 | ) |
The accompanying notes are an integral part of these consolidated financial statements.
F-3
SHANGPHARMA CORPORATION
CONSOLIDATED BALANCE SHEETS
AS OF DECEMBER 31, 2008 AND 2009
(Amount expressed in US dollars, except share data, unless otherwise stated)
| | | | | | | | | | | | |
| | | Note | | | December 31, | |
| | | | 2008 | | | 2009 | |
| | | | | | | | | | |
ASSETS | | | | | | | | | | | | |
Current assets: | | | | | | | | | | | | |
Cash | | | 2(e) | | | | 15,843,577 | | | | 12,238,137 | |
Restricted cash | | | 2(f) | | | | 831,960 | | | | 146,106 | |
Investment in securities | | | 2(i) | | | | - | | | | 417,000 | |
Accounts receivable, net | | | 2(g) | | | | 9,479,157 | | | | 14,291,689 | |
Inventories | | | 3 | | | | 1,094,186 | | | | 1,145,398 | |
Prepayments and other current assets | | | 4 | | | | 1,362,043 | | | | 1,452,937 | |
Deferred tax assets | | | 11 | | | | 101,145 | | | | 374,027 | |
| | | | | | | | | | | | |
Total current assets | | | | | | | 28,712,068 | | | | 30,065,294 | |
| | | | | | | | | | | | |
Non-current assets: | | | | | | | | | | | | |
Investment in securities | | | 2(i) | | | | 300,000 | | | | - | |
Property, equipment and software, net | | | 5 | | | | 32,706,459 | | | | 35,725,474 | |
Land use right, net | | | 6 | | | | - | | | | 4,178,474 | |
| | | | | | | | | | | | |
Total non-current assets | | | | | | | 33,006,459 | | | | 39,903,948 | |
| | | | | | | | | | | | |
Total assets | | | | | | | 61,718,527 | | | | 69,969,242 | |
| | | | | | | | | | | | |
LIABILITIES | | | | | | | | | | | | |
Current liabilities: | | | | | | | | | | | | |
Accounts payable | | | | | | | 8,327,506 | | | | 4,717,038 | |
Amounts due to related parties | | | 15 | | | | 2,928,260 | | | | 1,786,796 | |
Salary and welfare payable | | | | | | | 2,059,348 | | | | 2,991,129 | |
Income taxes payable | | | | | | | 311,476 | | | | 1,588,697 | |
Advance from customers | | | | | | | 79,436 | | | | 391,908 | |
Other payables and accruals | | | 7 | | | | 3,649,244 | | | | 4,014,553 | |
| | | | | | | | | | | | |
Total current liabilities | | | | | | | 17,355,270 | | | | 15,490,121 | |
| | | | | | | | | | | | |
Total liabilities | | | | | | | 17,355,270 | | | | 15,490,121 | |
| | | | | | | | | | | | |
Commitments and contingencies | | | 16 | | | | | | | | | |
| | | |
Series A Convertible Preferred Shares (US$0.001 par value; 70,000,650 shares authorized; 69,994,014 issued and outstanding as of December 31, 2008 and 2009; none outstanding on a pro-forma basis as of December 31, 2009 (unaudited)) (liquidation value of US$45,446,441 as of December 31, 2009) | | | 9 | | | | 34,355,775 | | | | 34,355,775 | |
| | | | | | | | | | | | |
EQUITY | | | | | | | | | | | | |
Ordinary shares (US$0.001 par value; 429,999,350 shares authorized; 208,005,986 shares issued and outstanding as of December 31, 2008 and 2009; 278,000,000 outstanding on a pro-forma basis as of December 31, 2009 (unaudited)) | | | 10 | | | | 208,006 | | | | 208,006 | |
Additional paid in capital | | | | | | | 68,752 | | | | 320,702 | |
Statutory reserves | | | 2(y) | | | | 3,885,356 | | | | 5,446,416 | |
Retained earnings | | | | | | | 3,770,181 | | | | 12,007,936 | |
Accumulated other comprehensive income | | | | | | | 2,075,187 | | | | 2,140,286 | |
| | | | | | | | | | | | |
Total equity | | | | | | | 10,007,482 | | | | 20,123,346 | |
| | | | | | | | | | | | |
Total liabilities and equity | | | | | | | 61,718,527 | | | | 69,969,242 | |
| | | | | | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
F-4
SHANGPHARMA CORPORATION
CONSOLIDATED STATEMENTS OF CHANGES IN EQUITY (DEFICIT)
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
(Amount expressed in US dollars, except share data, unless otherwise stated)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Ordinary shares
(US$0.001 par value) | | | Additional
paid in
Capital | | | Statutory
reserves | | | Retained
earnings
(accumulated
deficit) | | | Accumulated
other
comprehensive
income | | | Comprehensive
income | | | Equity (deficit) | |
| | | Number
of shares | | | Par value | | | | | | | |
Balance as of January 1, 2007 | | | 208,005,986 | | | | 208,006 | | | | - | | | | - | | | | 7,728,178 | | | | 170,010 | | | | - | | | | 8,106,194 | |
Comprehensive income: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net income | | | - | | | | - | | | | - | | | | - | | | | 7,496,789 | | | | - | | | | 7,496,789 | | | | 7,496,789 | |
Other comprehensive income: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Foreign currency translation adjustment | | | - | | | | - | | | | - | | | | - | | | | - | | | | 448,904 | | | | 448,904 | | | | 448,904 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total comprehensive income | | | | | | | | | | | | | | | | | | | | | | | | | | | 7,945,693 | | | | 7,945,693 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Appropriations to statutory reserves (Note 2(y)) | | | - | | | | - | | | | - | | | | 182,382 | | | | (182,382 | ) | | | - | | | | | | | | - | |
Dividends to shareholder (Note 2(z)) | | | - | | | | - | | | | - | | | | - | | | | (2,627,576 | ) | | | - | | | | | | | | (2,627,576 | ) |
Dividend paid to the ordinary shareholders (Note 9) | | | - | | | | - | | | | - | | | | - | | | | (14,000,000 | ) | | | - | | | | | | | | (14,000,000 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance as of December 31, 2007 | | | 208,005,986 | | | | 208,006 | | | | - | | | | 182,382 | | | | (1,584,991 | ) | | | 618,914 | | | | | | | | (575,689 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Comprehensive income: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net income | | | - | | | | - | | | | - | | | | - | | | | 9,058,146 | | | | - | | | | 9,058,146 | | | | 9,058,146 | |
Other comprehensive income: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Foreign currency translation adjustment | | | - | | | | - | | | | - | | | | - | | | | - | | | | 1,456,273 | | | | 1,456,273 | | | | 1,456,273 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total comprehensive income | | | | | | | | | | | | | | | | | | | | | | | | | | | 10,514,419 | | | | 10,514,419 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Share-based compensation expense | | | - | | | | - | | | | 68,752 | | | | - | | | | - | | | | - | | | | | | | | 68,752 | |
Appropriations to statutory reserves (Note 2(y)) | | | - | | | | - | | | | - | | | | 3,702,974 | | | | (3,702,974 | ) | | | - | | | | | | | | - | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance as of December 31, 2008 | | | 208,005,986 | | | | 208,006 | | | | 68,752 | | | | 3,885,356 | | | | 3,770,181 | | | | 2,075,187 | | | | | | | | 10,007,482 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Comprehensive income: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net income | | | - | | | | - | | | | - | | | | - | | | | 9,798,815 | | | | - | | | | 9,798,815 | | | | 9,798,815 | |
Other comprehensive income: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Foreign currency translation adjustment | | | - | | | | - | | | | - | | | | - | | | | - | | | | 65,099 | | | | 65,099 | | | | 65,099 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total comprehensive income | | | | | | | | | | | | | | | | | | | | | | | | | | | 9,863,914 | | | | 9,863,914 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Share-based compensation expense | | | - | | | | - | | | | 251,950 | | | | - | | | | - | | | | - | | | | | | | | 251,950 | |
Appropriations to statutory reserves (Note 2(y)) | | | - | | | | - | | | | - | | | | 1,561,060 | | | | (1,561,060 | ) | | | - | | | | | | | | - | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance as of December 31, 2009 | | | 208,005,986 | | | | 208,006 | | | | 320,702 | | | | 5,446,416 | | | | 12,007,936 | | | | 2,140,286 | | | | | | | | 20,123,346 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
F-5
SHANGPHARMA CORPORATION
CONSOLIDATED STATEMENTS OF CASH FLOWS
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
(Amount expressed in US dollars unless otherwise stated)
| | | | | | | | | | | | | | | | |
| | | Note | | | 2007 | | | 2008 | | | 2009 | |
Cash flows from operating activities: | | | | | | | | | | | | | | | | |
Net income | | | | | | | 7,496,789 | | | | 9,058,146 | | | | 9,798,815 | |
Adjustments to reconcile net income to net cash provided by operating activities: | | | | | | | | | | | | | | | | |
Depreciation and amortization of property, equipment and software | | | | | | | 2,111,783 | | | | 4,265,014 | | | | 5,830,472 | |
Amortization of land use right | | | | | | | - | | | | - | | | | 70,822 | |
Bad debt provision | | | | | | | - | | | | 83,435 | | | | 30,000 | |
Inventory provision | | | | | | | 105,616 | | | | 83,330 | | | | 51,940 | |
Impairment in value of investment securities | | | | | | | - | | | | - | | | | 25,857 | |
Loss (gain) related to derivatives | | | | | | | - | | | | 724,755 | | | | (74,546 | ) |
Deferred taxes | | | | | | | - | | | | (101,145 | ) | | | (272,882 | ) |
Share-based compensation expense | | | | | | | - | | | | 68,752 | | | | 251,950 | |
Change in assets and liabilities: | | | | | | | | | | | | | | | | |
Increase in accounts receivable | | | | | | | (4,110,170 | ) | | | (3,832,401 | ) | | | (4,809,690 | ) |
Increase in inventories | | | | | | | (657,556 | ) | | | (290,428 | ) | | | (103,152 | ) |
(Increase) decrease in amounts due from related parties | | | | | | | 26,489 | | | | (2,317 | ) | | | - | |
Increase in prepayments and other current assets | | | | | | | (235,566 | ) | | | (696,781 | ) | | | (90,894 | ) |
Increase (decrease) in accounts payable | | | | | | | (15,663 | ) | | | 488,079 | | | | 1,541,480 | |
Increase (decrease) in amounts due to related parties | | | | | | | 1,593,359 | | | | 1,198,628 | | | | (1,141,464 | ) |
Increase in salary and welfare payable | | | | | | | 769,317 | | | | 885,672 | | | | 931,781 | |
Increase (decrease) in income taxes payable | | | | | | | 313,373 | | | | (1,710,627 | ) | | | 1,277,221 | |
Increase (decrease) in advance from customers | | | | | | | (422,163 | ) | | | (880,680 | ) | | | 312,472 | |
Increase in other payables and accruals | | | | | | | 1,511,910 | | | | 877,418 | | | | 1,089,969 | |
| | | | | | | | | | | | | | | | |
Net cash provided by operating activities | | | | | | | 8,487,518 | | | | 10,218,850 | | | | 14,720,151 | |
| | | | | | | | | | | | | | | | |
Cash flows from investing activities: | | | | | | | | | | | | | | | | |
Proceeds from repayment of loan due from a related party | | | | | | | - | | | | 287,172 | | | | - | |
Purchase of property, equipment and software | | | | | | | (8,946,306 | ) | | | (13,579,160 | ) | | | (13,969,335 | ) |
Purchase of land use right | | | | | | | - | | | | - | | | | (4,249,296 | ) |
Payment for investment in securities | | | | | | | (133,334 | ) | | | (166,666 | ) | | | (142,857 | ) |
Payment for settlement of derivatives | | | | | | | - | | | | - | | | | (650,116 | ) |
(Increase) decrease in restricted cash | | | | | | | 90,212 | | | | (625,372 | ) | | | 685,854 | |
| | | | | | | | | | | | | | | | |
Net cash used in investing activities | | | | | | | (8,989,428 | ) | | | (14,084,026 | ) | | | (18,325,750 | ) |
| | | | | | | | | | | | | | | | |
Cash flows from financing activities: | | | | | | | | | | | | | | | | |
Proceeds from issuance of Series A Convertible Preferred Shares, net of issuance cost of US$644,225 | | | 9 | | | | 20,355,775 | | | | - | | | | - | |
Dividends paid | | | 2(z) | | | | (2,627,576 | ) | | | - | | | | - | |
| | | | | | | | | | | | | | | | |
Net cash provided by financing activities | | | | | | | 17,728,199 | | | | - | | | | - | |
| | | | | | | | | | | | | | | | |
Effect of foreign exchange rate changes on cash | | | | | | | 72,460 | | | | 146,000 | | | | 159 | |
| | | | | | | | | | | | | | | | |
Net (decrease) increase in cash | | | | | | | 17,298,749 | | | | (3,719,176 | ) | | | (3,605,440 | ) |
Cash at beginning of year | | | | | | | 2,264,004 | | | | 19,562,753 | | | | 15,843,577 | |
| | | | | | | | | | | | | | | | |
Cash at end of year | | | | | | | 19,562,753 | | | | 15,843,577 | | | | 12,238,137 | |
| | | | | | | | | | | | | | | | |
Supplemental disclosure of cash flow information: | | | | | | | | | | | | | | | | |
Cash paid during the year for income taxes | | | | | | | - | | | | 2,124,539 | | | | 541,818 | |
| | | | |
Supplemental disclosure of non-cash investing and financing activities: | | | | | | | | | | | | | | | | |
Increase (decrease) in accounts payable for purchase of property, equipment and software | | | | | | | 1,444,755 | | | | 4,254,989 | | | | (5,151,948 | ) |
Deemed dividend on issuance of Series A Convertible Preferred Shares | | | 9 | | | | 14,000,000 | | | | - | | | | - | |
The accompanying notes are an integral part of these consolidated financial statements.
F-6
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009
(Amount expressed in US dollars, except share data, unless otherwise stated)
| 1. | ORGANIZATION AND NATURE OF OPERATIONS |
The accompanying consolidated financial statements include the financial statements of ShangPharma Corporation (the “Company”) and its wholly owned subsidiaries, which mainly consist of ChemExplorer Company Limited (“CEHK”), China Gateway Life Science (Holdings) Limited (“CGHK”), Shanghai ChemExplorer Co., Ltd. (“CESH”), Shanghai PharmaExplorer Co., Ltd (“PESH”), Shanghai ChemPartner Co., Ltd (“CPSH”), China Gateway Pharma Products (Shanghai) Limited (“CGNH”), Chengdu Chempartner Co., Ltd. (��CPCD”), China Gateway Technology Development (Shanghai) Ltd. (“CGTD”) and China Gateway Pharmatheutical Development Co., Ltd. (“CGFX”). The Company and its subsidiaries are collectively referred to as the "Group". The Group, as a family-owned business prior to an equity investment made by a third party investor in late 2007 (Note 9), is principally engaged in providing pharmaceutical and biotechnology research and development services.
Substantially all of the Group’s business is conducted in the People’s Republic of China (“PRC”) through its wholly owned operating subsidiaries, CESH, PESH, CPSH, CGNH, CPCD, CGTD and CGFX (collectively referred to as “PRC Operating Subsidiaries”).
CEHK and CGHK were incorporated in Hong Kong on January 3, 2003 and June 23, 2003, respectively, as direct holding companies of the PRC operating subsidiaries. CEHK and CGHK are indirectly wholly owned by two co-founders who are immediate family members prior to the restructuring undertaken in September 2007 as described below.
ShangPharma Corporation was incorporated in Cayman Islands on August 30, 2007. On September 5, 2007, the Group undertook a restructuring in anticipation of the issuance of Series A Convertible Preferred Shares to a third party investor (Note 9), whereby the Company became the ultimate holding company after all of the then existing shareholders of CEHK and CGHK exchanged their respective shares in these two entities for equivalent classes of shares of the Company on a one for one basis. The restructuring was accounted for as a legal reorganization of entities under common control in a manner similar to pooling of interest. Accordingly, the accompanying consolidated financial statements have been prepared as if the current corporate structure had been in existence from inception of the Group.
| 2. | SUMMARY OF PRINCIPAL ACCOUNTING POLICIES |
| (a) | Basis of presentation and principles of consolidations |
The accompanying consolidated financial statements have been prepared and presented in accordance with generally accepted accounting principles in the United States of America (“US GAAP”).
The consolidated financial statements include the financial statements of the Company and its subsidiaries. All significant transactions and balances between the Company and its subsidiaries have been eliminated upon consolidation. A subsidiary is an entity in which the Company, directly or indirectly, controls more than one half of the voting power; has the power to appoint or remove the majority of the members of the board of directors (“Board”); to cast a majority of votes at the meeting of the board of directors or to govern the financial and operating policies of the investee under a statue or agreement among the shareholders or equity holders.
F-7
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009 — (Continued)
(Amount expressed in US dollars, except share data, unless otherwise stated)
The preparation of financial statements in conformity with US GAAP requires management to make estimates and assumptions that affect the amounts reported in the accompanying consolidated financial statements and related disclosures. Actual results could materially differ from these estimates. Significant accounting estimates reflected in the Company's consolidated financial statements include revenue recognition, bad debt provision, inventory provision, valuation of investment in securities, useful lives and estimated residual value of property, equipment and software, valuation allowance on deferred tax assets, assumptions related to the valuation of share-based compensation.
| (c) | Fair value measurements |
The Company adopted the guidance on fair value measurements on January 1, 2008 for financial assets and liabilities. On January 1, 2009, the Company also adopted the guidance on fair value measurements for all non-financial assets and non-financial liabilities. The guidance defines fair value as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date (also referred to as an exit price). The guidance on fair value measurements establishes a hierarchy for inputs used in measuring fair value that maximizes the use of observable inputs and minimizes the use of unobservable inputs by requiring that the observable inputs be used when available. Observable inputs are inputs that market participants would use in pricing the asset or liability developed based on market data obtained from sources independent of the Company. Unobservable inputs are inputs that reflect the Company’s assumptions about the assumptions market participants would use in pricing the asset or liability developed based on the best information available in the circumstances. As such, fair value is a market-based measure considered from the perspective of a market participant who holds the asset or owes the liability rather than an entity-specific measure. The hierarchy is broken down into three levels based on the reliability of inputs as follows: (Level 1) observable inputs such as quoted prices in active markets; (Level 2) inputs other than the quoted price in active markets that are observable either directly or indirectly, or quoted prices in less active markets; and (Level 3) unobservable inputs with respect to which there is little or no market data, which require the Company to develop its own assumptions.
When available, the Company measures the fair value of financial instruments based on quoted market prices in active markets, valuation techniques that use observable market-based inputs or unobservable inputs that are corroborated by market data. Pricing information the Company obtains from third parties is internally validated for reasonableness prior to use in the consolidated financial statements. When observable market prices are not readily available, the Company generally estimates the fair value using valuation techniques that rely on alternate market data or inputs that are generally less readily observable from objective sources and are estimated based on pertinent information available at the time of the applicable reporting periods. In certain cases, fair values are not subject to precise quantification or verification and may fluctuate as economic and market factors vary and the Company’s evaluation of those factors changes. Although the Company uses its best judgment in estimating the fair value of these financial instruments, there are inherent limitations in any estimation technique. In these cases, a minor change in an assumption could result in a significant change in its estimate of fair value, thereby increasing or decreasing the amounts of the Company’s consolidated assets, liabilities, equity and net income.
F-8
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009 — (Continued)
(Amount expressed in US dollars, except share data, unless otherwise stated)
| (d) | Functional currency and foreign currency translations |
The Company and its non-PRC subsidiaries’ functional currency is the United States dollar (“US dollar” or “US$”). The Company’s PRC subsidiaries’ functional currency is the Renminbi (“RMB”), the official currency in the PRC. Transactions denominated in currencies other than the functional currencies are translated into the functional currencies at the exchange rates quoted by the People's Bank of China (the “PBOC”) prevailing at the transaction dates. Gains and losses resulting from foreign currency transactions are included in the consolidated statements of operations. Monetary assets and liabilities denominated in foreign currencies are translated into the functional currencies using the applicable exchange rates quoted by the PBOC at the balance sheet dates. All such exchange gains and losses are recognized in the consolidated statements of operations.
The reporting currency of the Group is the US dollar. Assets and liabilities are translated at the exchange rates at the balance sheet date, equity accounts are translated at historical exchange rates and revenues, expenses, gains and losses are translated using the average rate for the year. Translation adjustments are reported as cumulative translation adjustments and are shown as a separate component in the consolidated statements of equity (deficit).
Cash represents cash on hand and demand deposits placed with banks or other financial institutions. Included in the cash balance as of December 31, 2008 and 2009 are balances denominated in US$ amounting to US$13,574,493 and US$10,058,119, respectively.
Restricted cash at December 31, 2008 and 2009 includes cash held as collateral for letters of credit issued for purchases of equipment. As of December 31, 2008, restricted cash also includes cash held as collateral for foreign exchange forward contracts.
Accounts receivable are presented net of allowance for doubtful accounts. The Group provides specific provisions for bad debts when facts and circumstances indicate that the collection is doubtful and a loss is probable and estimable. For the years ended December 31, 2007, 2008 and 2009, the Group recorded allowance for doubtful accounts of nil, US$83,435 and US$30,000, respectively, and did not write off any bad debts for the years presented. Allowance for doubtful accounts was US$83,435 and US$113,435 as of December 31, 2008 and 2009, respectively.
Inventories consisting of raw materials, costs incurred prior to delivery and customer acceptance of undelivered compounds are stated at the lower of cost or market. The Company determines cost on a weighted-average basis. Cost comprises direct materials and where applicable, of direct labor and overhead that has been incurred in bringing the inventories to their present location and condition. Provisions for inventories valuation were US$105,616, US$83,330 and US$51,940 for the years ended December 31, 2007, 2008 and 2009, respectively, and once recorded, result in a new cost basis for the related inventory and such reserves are not removed until such inventory is sold or otherwise disposed.
F-9
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009 — (Continued)
(Amount expressed in US dollars, except share data, unless otherwise stated)
| (i) | Investment in securities |
Investments in debt and equity securities are, on initial recognition, classified into the three categories: held-to-maturity securities, trading securities and available-for-sale securities. Debt securities that the Company has the positive intent and ability to hold to maturity are classified as held-to-maturity securities and reported at amortized cost. Debt and equity securities that are bought and held principally for the purpose of selling them in the near term are classified as trading securities and reported at fair value, with unrealized gains and losses included in earnings. Debt and equity securities not classified as either held-to-maturity securities or trading securities are classified as available-for-sale securities and reported at fair value, with unrealized gains and losses recognized in accumulated other comprehensive income.
The Group recorded the investment in convertible and redeemable preferred shares, which are in nature of debt securities, as an available-for-sale investment. Subsequent to initial recognition, available-for-sale investment is measured at fair value with changes in fair value recognized in accumulated other comprehensive income included in shareholders’ equity. When there is objective evidence that the investment is impaired, the cumulative losses from the declines in fair value that had been recognized directly in other comprehensive income are removed from equity and recognized in the income statement. When the available-for-sale investment is sold, the cumulative fair value adjustments previously recognized in accumulated other comprehensive income are recognized in the current period operating results. The Group evaluates the investments periodically for possible other-than-temporary impairment. An other-than-temporary impairment must be recognized if an investor has the intent to sell the debt security or if it is more likely than not that it will be required to sell the debt security before recovery of its amortized cost basis.
In 2007 and 2008, the Group, through one of its subsidiaries, acquired 300,000 redeemable and convertible preferred shares of a pharmaceutical and biotechnology research and development company (��investee”) incorporated in the United States of America for a consideration of US$300,000. The Company’s investment represents 2.9% of investee’s equity interests, on an as converted basis. The preferred share is convertible into shares of the investee’s common stock at a 1:1 ratio and is redeemable, if more than 72% of the outstanding preferred shareholders request redemption on or after the fifth anniversary of the preferred share original issue date.
In 2009, the investee issued promissory notes to the Group for cash consideration of US$142,857. In connection with the promissory notes, the Group was granted warrants to purchase additional preferred shares offered in connection with a qualified financing at the lower of the per share price of the Series A redeemable and convertible preferred shares or price per preferred share issued pursuant to the qualified financing, or, Series A redeemable and convertible preferred shares at the per share price of the Series A redeemable and convertible preferred shares. The promissory notes are convertible into preferred shares offered in connection with a qualified financing at the lower of the price per share of the preferred shares the Company sells and issues pursuant to a qualified financing or the per share price of the Series A redeemable and convertible preferred shares.
In February 2010, the Group, together with other original investors of the investee, sold their investments in the investee to a third party (Note 18). According to the share purchase agreement, the Group received cash of US$351,378 in February 2010 and will receive additional cash up to US$65,714 set aside in the escrow accounts. The Group is also eligible to certain future consideration up to approximately US$1.5 million. The receipts of the future payments are contingent upon the investee achieving certain milestones defined in the
F-10
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009 — (Continued)
(Amount expressed in US dollars, except share data, unless otherwise stated)
share purchase agreement. Given total cash consideration received for the disposal of this investment was less than the carrying value of the investment as of December 31, 2009, the Company recognized an impairment of US$25,857 as of December 31, 2009. No impairment was recognized during the years ended December 31, 2007 and 2008.
As adjusted, as of December 31, 2007, 2008 and 2009, the fair value of the debt securities approximated the carrying value.
| (j) | Property, equipment and software |
Property, equipment and software are stated at cost less accumulated depreciation and amortization. Costs include amounts paid to acquire or construct the assets. Assets under construction are not depreciated until construction is completed and the assets are ready for their intended use. Assets classified as construction in progress mainly relate to the leasehold improvements, equipment which need construction or installation and the construction of new buildings for manufacturing services. Upon retirement or sale, the cost of assets disposed of and the related accumulated depreciation/amortization are removed from the accounts and any resulting gain or loss is recognized in consolidated statement of operations. Repair and maintenance costs are expensed as incurred.
Depreciation and amortization are computed using the straight-line method over the following estimated useful lives, taking into account any estimated residual value:
| | |
Item | | Useful lives |
Leasehold improvements | | Shorter of the lease term or the
estimated useful lives |
Experimental equipment | | 5-10 years |
Vehicles | | 5 years |
Office equipment and others | | 5 years |
Software licenses | | 1-10 years |
Residual value is determined based on the economic value of the property and equipment at the end of the estimated useful period, with a range from 0% to 10% of the original costs.
Land use right represents fees paid to obtain the right to use land in the PRC. Amortization is computed using the straight-line method over the terms specified in the land use right certificate of 50 years.
| (l) | Impairment of long-lived assets |
The Group reviews its long-lived assets for impairment whenever events or changes in circumstances indicate the carrying amount of an asset may not be recoverable. Recoverability of these assets is measured by comparison of its carrying amounts to the future undiscounted cash flows the assets are expected to generate. If the sum of the expected undiscounted cash flow is less than the carrying amount of the assets, the Group would recognize an impairment loss equal to the amount by which the carrying value of the asset exceeds its fair value. No impairment of long-lived assets was recognized for the years ended December 31, 2007, 2008 and 2009.
F-11
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009 — (Continued)
(Amount expressed in US dollars, except share data, unless otherwise stated)
The Group provides a broad range of integrated laboratory and manufacturing services in the drug discovery and development process to pharmaceutical and biotechnology companies. Laboratory services include contracts on full time equivalent (“FTE”) basis and fee-for-service basis.
Revenues are recognized when persuasive evidence of an arrangement exists, the sales price is fixed or determinable, delivery of the product or performance of the service has occurred and there is reasonable assurance of collection of the sales proceeds.
For laboratory services provided under a FTE basis, the customer pays a fixed rate per laboratory worker on a full time employment basis and the Group recognizes revenue as the services are provided. The FTE contracts do not require acceptance by the customer or have obligations for fixed deliverables by the Group.
Laboratory services provided on a fee-for-service often include multiple elements of deliverables. Revenue is recognized as each element is earned, provided that the fair value of the undelivered element(s) has been determined, the delivered element(s) has stand-alone value, there is no right of return on delivered element(s), and the Company is in control of the undelivered element(s). An element is considered earned upon the delivery and acceptance of the deliverables. For arrangements that include service elements, revenue is recognized for the service element using the proportionate performance method.
Under certain arrangements, the customers provide to the Company bonus payments that are contingent on the Company achieving certain milestones. Such bonus payments are recognized as revenue upon the achievement of the milestones.
Costs incurred prior to the delivery and acceptance of the deliverables are capitalized in inventory as work in progress. Cash received in advance of the delivery and acceptance of the deliverables are recorded as advance from customers.
The Group also provides manufacturing services to its customers. Revenue from the sale of manufactured products is recognized upon delivery and acceptance by the customer when title and risk of loss have been transferred. Revenue from the sale of manufactured products was not material for the years ended December 31, 2007, 2008 and 2009.
Certain laboratory services provided are subject to sales tax at a rate of 5%. Sales tax and related charges are recognized as sales taxes and are deducted from gross revenues and recorded as a liability to the taxing authorities to arrive at net revenues. The Group historically received sales tax exemptions for certain laboratory services upon the approval from local authorities. Tax exemptions for the years ended December 31, 2007, 2008 and 2009 were US$1,009,003, US$2,163,723 and US$2,598,524, respectively.
| (o) | Shipping and handling costs |
Shipping and handling costs are included in selling and marketing expenses in the consolidated statements of operations. For the years ended December 31, 2007, 2008 and 2009, shipping and handling costs were US$53,672, US$168,054 and US$183,628, respectively.
F-12
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009 — (Continued)
(Amount expressed in US dollars, except share data, unless otherwise stated)
Leases where substantially all the rewards and risks of ownership of assets remain with the leasing company are accounted for as operating leases. Payments made under operating leases net of any incentives from the lessor are charged to the consolidated statements of operations on a straight-line basis over the lease periods.
Government subsidies represent cash subsidies received from the government.
Cash subsidies which have no defined rules and regulations to govern the criteria necessary for companies to enjoy the benefits are recognized as other income when received.
Other cash subsidies received from the government, for which the Company has to meet certain obligations, are initially recorded as liabilities and recognized as other income upon fulfilling the obligations.
For the years ended December 31, 2007, 2008 and 2009, cash subsidies of nil, US$185,150 and US$645,897 were recognized as other income, respectively. The balance of the cash subsidies recorded as other payables and accruals were US$117,051 and US$398,380 as of December 31, 2008 and 2009, respectively.
Other income primarily includes rental income from the sublease of the leased office building and recognized government subsidies (Note 2(q)). Rental income was US$190,960, US$657,855 and US$420,625 for the years ended December 31, 2007, 2008 and 2009, respectively.
Other expenses primarily include foreign exchange (losses) and gains/(losses) recognized on foreign exchange forward contracts. Foreign exchange (losses) were US$(79,893), US$(843,142) and US$(125,305) for the years ended December 31, 2007, 2008 and 2009, respectively. Gains/(losses) recognized on foreign exchange forward contracts were nil, US$(724,755) and US$74,546 for the years ended December 31, 2007, 2008 and 2009, respectively.
| (t) | Share-based compensation |
Share-based compensation expense for all share-based payment awards granted is determined based on the grant date fair value. Share-based compensation expense for stock options is estimated at the grant date based on each option’s fair value as calculated by the Black-Scholes option pricing model (“Black-Scholes model”). Share-based compensation is expensed ratably on a straight-line basis for awards with service conditions only over the requisite service period, which is generally the vesting term of the share-based payment awards. The graded vesting method is applied to awards with performance conditions.
Income taxes are accounted for under the asset and liability method. Deferred taxes are determined based upon differences between the financial reporting and tax bases of assets and liabilities at currently enacted
F-13
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009 — (Continued)
(Amount expressed in US dollars, except share data, unless otherwise stated)
statutory tax rates for the years in which the differences are expected to reverse. The effect on deferred taxes of a change in tax rates is recognized in the consolidated statements of operations in the period of change. A valuation allowance is provided on deferred tax assets to the extent that it is more likely than not that such deferred tax assets will not be realized. The total income tax provision includes current tax expenses under applicable tax regulations and the change in the balance of deferred tax assets and liabilities.
The guidance on accounting for uncertain tax positions in income taxes clarifies the accounting for uncertainty in income taxes recognized in the Company’s consolidated financial statements, and prescribes a more likely than not threshold for financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. The guidance also provides guidance on de-recognition of income tax assets and liabilities, classification of current and deferred income tax assets and liabilities, accounting for interest and penalties associated with tax positions, accounting for income taxes in interim periods, and income tax disclosures.
Basic earnings per share is computed by dividing net income attributable to ordinary shareholders by the weighted average number of ordinary shares outstanding during the period using the two-class method. Under the two-class method, net income is allocated between ordinary shares and other participating securities based on their participating rights. Diluted earnings per share is calculated by dividing net income attributable to ordinary shareholders, as adjusted for the effect of dilutive ordinary equivalent shares, if any, by the weighted average number of ordinary and dilutive ordinary equivalent shares outstanding during the period. Ordinary equivalent shares consist of ordinary shares issuable upon the conversion of the Series A Preferred Shares using the if-converted method, and shares issuable upon the exercise of stock options for the purchase of ordinary shares using the treasury method. Ordinary equivalent shares are not included in the denominator of the diluted earnings per share calculation when inclusion of such stocks would be anti-dilutive.
Comprehensive income consists of two components, net income and other comprehensive income. Other comprehensive income refers to revenue, expenses, gains and losses that under generally accepted accounting principles are recorded as an element of equity (deficit) but are excluded from net income. For all periods presented, other comprehensive income represented impacts of foreign currency translation adjustments. The Group discloses this information in the consolidated statements of changes in equity (deficit).
The Company currently operates and manages its business as a single segment.
| (y) | Profit appropriation and statutory reserves in PRC |
PRC Operating Subsidiaries are wholly foreign owned enterprises incorporated in the PRC, and are required to make appropriations from after-tax profits to non-distributable reserve funds. The subsidiary, after recouping its accumulated losses, need to make appropriations to general reserve fund and staff bonus and welfare fund (“the statutory reserves”). The general reserve fund requires annual appropriations of 10% of
F-14
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009 — (Continued)
(Amount expressed in US dollars, except share data, unless otherwise stated)
after-tax profit (determined by generally accepted accounting principles in the PRC (“PRC GAAP”)) until such fund has reached 50% of the subsidiary’s registered capital; the percentage of appropriation for staff bonus and welfare fund is determined at the discretion of its Board. The statutory reserves can only be used for specific purposes, such as offsetting accumulated losses, enterprise expansion or staff welfare. These reserves are not transferrable to the Group in the form of dividends, advances or loans. Appropriations to these funds are accounted for as transfers from retained earnings to the statutory reserves. The Group made appropriations to the general reserve funds of US$182,382, US$3,702,974 and US$1,561,060 for the years ended December 31, 2007, 2008 and 2009, respectively.
Dividends are recognized when declared. Dividends of US$2,627,576 were declared and paid for the year ended December 31, 2007. No dividends were declared for the years ended December 31, 2008 and 2009.
PRC regulations currently permit payment of dividends only out of accumulated profits as determined in accordance with PRC GAAP. The Company’s PRC subsidiaries can only distribute dividends after they have met the PRC requirements for appropriation to statutory reserves (Note 2(y)).
| (aa) | Certain risks and concentration |
Financial instruments that potentially subject the Group to significant concentrations of credit risk consist primarily of cash, accounts receivables, amounts due from related parties and prepayments and other current assets. As of December 31, 2008 and 2009, substantially all of the Group’s cash was held in major financial institutions located in the PRC and in Hong Kong, which management believes are of high credit quality. Accounts receivables are typically unsecured and primarily denominated in US dollars. The Group does not require collateral or other security to support financial instruments subject to credit risks.
The Group’s customers include pharmaceutical and biotechnology companies. In the majority of circumstances, there are agreements in force with these entities that provide for the Group’s continued involvement in present research projects. However, there exists the possibility that the Group will have no further association with these entities once the ongoing projects conclude.
The following table summarizes the percentage of the Group’s revenue and accounts receivable represented by customers with balances over 10% of total revenue for the years ended December 31, 2007, 2008, and 2009, and over 10% of accounts receivable as of December 31, 2008 and 2009, respectively:
| | | | | | | | | | | | |
| | | 2007 | | | 2008 | | | 2009 | |
Revenue | | | | | | | | | | | | |
Customer A | | | 48 | % | | | 34 | % | | | 27 | % |
Customer B | | | 14 | % | | | 13 | % | | | 10 | % |
| | | | | | | | |
| | | As of December 31, | |
| | | 2008 | | | 2009 | |
Accounts receivable | | | | | | | | |
Customer A | | | 24 | % | | | 22 | % |
Customer B | | | 11 | % | | | 7 | % |
F-15
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009 — (Continued)
(Amount expressed in US dollars, except share data, unless otherwise stated)
| (ab) | Recent accounting pronouncements |
In May 2009, the Financial Accounting Standards Board (“FASB”) issued guidance on subsequent events that establishes general standards of accounting for and disclosures of events that occur after the balance sheet date but before financial statements are issued or are available to be issued. Specifically, this guidance provides (i) the period after the balance sheet date during which management of a reporting entity should evaluate events or transactions that may occur for potential recognition or disclosure in the financial statements, (ii) the circumstances under which an entity should recognize events or transactions occurring after the balance sheet date in its financial statements and (iii) the disclosures that an entity should make about events or transactions that occurred after the balance sheet date. The adoption of this guidance did not have any material impact on the consolidated financial statements. The FASB subsequent in February 2010 made an amendment to require an entity that is either a SEC filer or a conduit bond obligor for conduit debt securities that are traded in a public market is required to evaluate subsequent events through the date that the financial statements are issued. An entity that is an SEC filer is not required to disclose the date through which subsequent events have been evaluated.
In August 2009, the FASB issued guidance on Fair Value Measurements and Disclosures — Measuring Liabilities at Fair Value. The new guidance aims to provide clarification relating to the fair value measurement of liabilities, especially in circumstances where a quoted price in an active market for the identical liability is not available, a reporting entity is required to measure fair value using certain prescribed techniques. Techniques highlighted included using 1) the quoted price of the identical liability when traded as an asset, 2) quoted prices for similar liabilities when traded as assets, or 3) another valuation technique that is consistent with the principles of fair value measurements. The new guidance also clarifies that when estimating the fair value of a liability, a reporting entity is not required to include a separate input or adjustment to other inputs relating to the existence of a restriction that prevents the transfer of the liability. Finally, the guidance clarifies that both a quoted price in an active market for the identical liability and the quoted price for the identical liability when traded as an asset in an active market when no adjustment to the quoted price of the asset are required are Level 1 fair value measurements. The Company will adopt this guidance at the beginning of its fiscal year 2010, and it does not expect the adoption of this guidance will have a material impact on the Company’s consolidated financial statements.
In October 2009, the FASB issued an accounting standard update on revenue recognition relating to multiple deliverable revenue arrangements. The fair value requirements of existing accounting guidance are modified by allowing the use of the “best estimate of selling price” in addition to vendor-specific objective evidence (“VSOE”) and third-party evidence (“TPE”) for determining the selling price of a deliverable. A vendor is now required to use its best estimate of the selling price when VSOE or TPE of the selling price cannot be determined. In addition, the residual method of allocating arrangement consideration is no longer permitted. This update requires expanded qualitative and quantitative disclosure and is effective for fiscal years beginning on or after June 15, 2010 although early adoption is permitted. These updates may be applied either prospectively from the beginning of the fiscal year for new or materially modified arrangement or retrospectively. The Company is currently assessing the impact, if any, that the adoption of this update will have on its consolidated financial statements and disclosures.
In January 2010, the FASB issued an accounting standard update on improving disclosures about fair value measurements. The updated guidance amends existing disclosure requirements by adding required disclosures about items transferring into and out of Levels 1 and 2 in the fair value hierarchy; adding separate disclosures about purchase, sales, issuances, and settlements relative to Level 3 measurements; and
F-16
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009 — (Continued)
(Amount expressed in US dollars, except share data, unless otherwise stated)
clarifying, among other things, the existing fair value disclosures about the level of disaggregation. This update is effective for fiscal years beginning after December 15, 2009, except for the requirement to provide Level 3 activity of purchases, sales, issuances, and settlements on a gross basis, which is effective beginning the first quarter of 2011. The Company does not expect the adoption of this guidance will have a material impact on the Company’s financial statement.
The FASB issued an update in April 2010 that provides guidance on the criteria that should be met for determining whether the milestone method of revenue recognition is appropriate. A vendor can recognize consideration that is contingent upon achievement of a milestone in its entirety as revenue in the period in which the milestone is achieved only if the milestone meets all criteria to be considered substantive. Determining whether a milestone is substantive is a matter of judgment made at the inception of the arrangement. A milestone should be considered substantive in its entirety. An individual milestone may not be bifurcated. An arrangement may include more than one milestone, and each milestone should be evaluated separately to determine whether the milestone is substantive. A vendor’s decision to use the milestone method of revenue recognition for transactions within the scope of the amendments in the April 2010 update is a policy election. A vendor that is affected by the amendments in this update is required to provide certain disclosures. This update is effective on a prospective basis for milestones achieved in fiscal years, and interim periods within those years, beginning on or after June 15, 2010. Early adoption is permitted with certain disclosures required. A vendor may also elect, but is not required, to adopt the amendments in this update retrospectively for all prior periods. The Company is currently evaluating the impact on its consolidated financial statements of adopting this standard.
Inventories consist of the following:
| | | | | | | | |
| | | As of December 31, | |
| | | 2008 | | | 2009 | |
Raw materials | | | 624,957 | | | | 297,547 | |
Work in progress | | | 360,456 | | | | 458,484 | |
Finished goods | | | 108,773 | | | | 389,367 | |
| | | | | | | | |
| | | 1,094,186 | | | | 1,145,398 | |
| | | | | | | | |
| 4. | PREPAYMENTS AND OTHER CURRENT ASSETS |
Prepayments and other current assets consist of the following:
| | | | | | | | |
| | | As of December 31, | |
| | | 2008 | | | 2009 | |
Prepaid rental and deposit | | | 493,305 | | | | 510,608 | |
Deposit to be refunded for purchase of land use right | | | 400,000 | | | | 400,000 | |
Prepayments for purchase of raw materials | | | 270,174 | | | | 246,929 | |
Others | | | 198,564 | | | | 295,400 | |
| | | | | | | | |
| | | 1,362,043 | | | | 1,452,937 | |
| | | | | | | | |
F-17
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009 — (Continued)
(Amount expressed in US dollars, except share data, unless otherwise stated)
| 5. | PROPERTY, EQUIPMENT AND SOFTWARE, NET |
Property, equipment and software consist of the following:
| | | | | | | | |
| | | As of December 31, | |
| | | 2008 | | | 2009 | |
Leasehold improvements | | | 11,376,731 | | | | 14,230,866 | |
Experimental equipment | | | 23,190,674 | | | | 28,257,112 | |
Vehicles | | | 459,322 | | | | 534,091 | |
Office equipment and others | | | 2,225,411 | | | | 2,731,790 | |
Software licenses | | | 1,740,210 | | | | 1,803,838 | |
Construction in progress | | | 2,900,059 | | | | 3,195,624 | |
| | | | | | | | |
| | | 41,892,407 | | | | 50,753,321 | |
Less: accumulated depreciation and amortization | | | (9,185,948 | ) | | | (15,027,847 | ) |
| | | | | | | | |
Property, equipment and software, net | | | 32,706,459 | | | | 35,725,474 | |
| | | | | | | | |
Construction in progress primarily represents the construction of new buildings in Shanghai for manufacturing service business.
Depreciation and amortization expenses were US$2,111,783, US$4,265,014 and US$5,830,472 for the years ended December 31, 2007, 2008 and 2009, respectively.
The annual estimated amortization expense of software licenses for the next five years is as follows:
| | | | |
2010 | | | 143,360 | |
2011 | | | 128,930 | |
2012 | | | 108,182 | |
2013 | | | 98,580 | |
2014 | | | 98,116 | |
| | | | |
| | | 577,168 | |
| | | | |
Land use right represents fees paid to the government to obtain the right to use certain land over periods of 50 years in the PRC.
| | | | | | | | |
| | | As of December 31, | |
| | | 2008 | | | 2009 | |
Land use right | | | - | | | | 4,249,296 | |
Less: accumulated amortization | | | - | | | | (70,822 | ) |
| | | | | | | | |
| | | - | | | | 4,178,474 | |
| | | | | | | | |
Amortization expense was US$70,822 for the year ended December 31, 2009. As of December 31, 2009, estimated amortization expense in each of the next five years is US$84,986, respectively.
F-18
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009 — (Continued)
(Amount expressed in US dollars, except share data, unless otherwise stated)
| 7. | OTHER PAYABLES AND ACCRUALS |
Other payables and accruals consist of the following:
| | | | | | | | |
| | | As of December 31, | |
| | | 2008 | | | 2009 | |
Value added tax and other taxes payable | | | 1,409,213 | | | | 2,028,207 | |
Accrued rental and utilities | | | 695,374 | | | | 500,337 | |
Derivative liability- foreign-exchange forward contracts | | | 738,529 | | | | 13,868 | |
Accrued consulting and audit fee | | | 219,199 | | | | 197,245 | |
Unearned government subsidies | | | 117,051 | | | | 398,380 | |
Accrued employee annual leave | | | 87,000 | | | | 245,824 | |
Others | | | 382,878 | | | | 630,692 | |
| | | | | | | | |
| | | 3,649,244 | | | | 4,014,553 | |
| | | | | | | | |
Full-time employees of PRC Operating Subsidiaries are entitled to staff welfare benefits including medical care, welfare subsidies, unemployment insurance and pension benefits. PRC Operating Subsidiaries are required to accrue for these benefits based on certain percentages of salaries in accordance with the relevant regulations and make contributions to the state-sponsored pension and medical plans out of the amounts accrued for medical and pension benefits. The total contribution made for such employee benefits and amounts charged to the consolidated statements of operations were US$1,750,784, US$3,034,899 and US$4,248,654 for the years ended December 31, 2007, 2008 and 2009, respectively. The Chinese government is responsible for the medical benefits and ultimate pension liability to these employees.
| 9. | SERIES A CONVERTIBLE PREFERRED SHARES |
On September 7, 2007, the Company issued and sold 3,365 (prior to bonus share issuance (Note 10)) Series A Preferred Shares. The Company sold 2,019 of these shares (prior to bonus share issuance (Note 10)) to a third party investor for cash proceeds of US$21,000,000 pursuant to a Securities Purchase Agreement dated September 5, 2007, or approximately US$10,401 per share, determined to be the fair value of such shares at the date of issuance. The Company issued the remaining 1,346 (prior to bonus share issuance (Note 10)) shares to Joint Benefit Group Limited (“JBGL”), a company indirectly owned and controlled by all of the Company’s ultimate ordinary shareholders for a nominal price (such that it was a pro rata distribution to all ultimate ordinary shareholders).
Pursuant to the Securities Purchase Agreement, JBGL simultaneously sold the 1,346 (prior to bonus share issuance (Note 10)) Series A Preferred Shares to the same third party investor for cash proceeds of US$14,000,000, or approximately US$10,401 per share, on September 7, 2007. While the form of the transaction was a distribution of preferred shares to the ultimate ordinary shareholders, the Company effectively made a cash distribution of US$14,000,000 to the ultimate ordinary shareholders in connection with the sale of 3,365 Series A Preferred Shares. The Company recorded the US$14,000,000 payment as a pro rata dividend to all of the ultimate ordinary shareholders in consideration of the dilution in their ownership interest in the Company as a result of the Series A Convertible Share investment.
The initial carrying value of the Series A Preferred Shares was offset by direct issuance costs of US$644,225.
F-19
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009 — (Continued)
(Amount expressed in US dollars, except share data, unless otherwise stated)
The par value of the Company’s Series A Preferred Shares is US$0.001 per share. The significant terms are as follows:
Conversion
Each Series A Preferred Share is initially convertible into one ordinary share at any time after the date of issuance of such share, and shall be automatically converted into ordinary shares at the then-effective conversion price upon the completion of a qualifying IPO.
A qualifying IPO, as defined in the Company’s memorandum and articles of association, means an underwritten public offering of the Company’s ordinary shares on a recognized stock exchange that values the Company at no less than US$250,000,000 and that result in aggregate proceeds to the Company (net of selling expenses) of no less than US$62,500,000.
The conversion price of the Series A Preferred Shares is subject to adjustment for certain events, including but not limited to share splits, share dividends, reorganizations, mergers, consolidations, reclassifications, exchanges and substitutions. The conversion price of the Series A Preferred Shares is also subject to adjustment in the event the Company issues additional ordinary shares at a price per share that is less than such conversion price. In such case, the conversion price of the Series A Preferred Shares shall be reduced, concurrently with such issuance, to the price per share at which the additional shares are issued.
In addition to the foregoing, if the Company’s aggregate adjusted net profit after taxes for the 24 months period beginning January 1, 2007 and ending December 31, 2008 is less than US$18,000,000, the then effective conversion price of the Series A Preferred Shares shall be adjusted downwards in accordance with the formula set forth in the Company’s memorandum and articles of association. The aggregate adjusted net profit was greater than US$18,000,000 for the said 24 months and thus no adjustment event occurred.
The conversion price of the Series A Preferred Shares was adjusted to US$0.50 per share in May 2008 on a retrospective basis as a result of bonus share issuance (Note 10).
No beneficial conversion feature charge was recognized for the issuance of Series A Preferred Shares as the estimated fair value of the ordinary shares is less than the conversion price on the date of issuance and at the time of the aforementioned conversion adjustment assessment.
Voting Rights
Each Series A Preferred Share has voting rights equivalent to the number of the Company’s ordinary shares into which it is convertible.
Dividends
The holders of the outstanding Series A Preferred Shares shall be entitled to receive, out of any funds legally available, non cumulative dividends for each Series A Preferred Share held as and when declared by the Board of Directors. In the event the Company shall declare a dividend or similar distribution to the holders of ordinary shares, the holders of Series A Preferred Shares shall be entitled to a proportionate share of any such dividend or distribution as though the Series A Preferred Shares were converted into ordinary shares on the record date.
F-20
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009 — (Continued)
(Amount expressed in US dollars, except share data, unless otherwise stated)
Redemption rights
The redemption price per Series A Preferred Share is equal to the original purchase price per share (as adjusted in accordance with the Company’s memorandum and articles of association), plus an additional sum equal to a return of 12% on the purchase price, compounded quarterly from the date of the issuance to the date on which the actual payment is made, plus all accrued but unpaid dividends.
The Company has not recorded accretion as the likelihood of occurrence for the redemption event is considered remote. The Company classified the Series A Preferred Shares in the mezzanine section of the consolidated balance sheets in accordance with the provisions of Accounting Series Release No. 268 (“ASR 268”), as a result of the “deemed liquidation” redemption provisions described below under “Liquidation Preference”.
Holders of the Series A Preferred Shares have the option to redeem such shares, in whole or in part, upon the occurrence of a material breach of one or more of the investment agreements in connection with the Series A Preferred Shares, as set forth in the Company’s memorandum and articles of association.
Liquidation Preference
In the event of any liquidation, dissolution or winding up of the Company or any consolidation, amalgamation, trade sale, merger, corporate reorganization or other transaction involving the Company in which its shareholders do not retain a majority of the voting power in the surviving entity, or a sale of all or substantially all the Company’s assets (Deemed Liquidation), the holders of Series A Preferred Shares are entitled to receive an amount per Series A Preferred Share equal to 100% of the original purchase price (as adjusted for any share splits, share dividends, combinations, recapitalizations and similar transactions), plus all declared but unpaid dividends, plus an amount equal to a return of 12% on the purchase price, compounded quarterly from the date of the sale to the date on which the actual payment is made.
Form of charge agreement
On September 4, 2007, the founders agreed to pledge to the third party investor 2,673 (prior to bonus share issuance (Note 10)) ordinary shares for payment in the event of certain “enforcement events” as defined in the Securities Purchase Agreement which may result in an amount becoming payable to the third party investor by the indemnifying parties (including the Company and the founder). Pledged shares shall be released upon the earlier of the expiration of the enforcement events, a qualified public offering and/or liquidation event. During the pledge period, the founder is still entitled to the voting and dividend rights. The charged shares were all released in April 2010.
Upon incorporation of the Company, the authorized share capital was US$50,000 divided into 49,990,000 ordinary shares at a par value of US$0.001 each, and 10,000 convertible preferred shares of a par value at US$0.001 each, all of which were designated Series A Preferred Shares. The amount of issued and outstanding ordinary shares was 10,000.
On May 19, 2008, the Company effected a bonus share issuance in the form of a share split such that each holder of ordinary shares and Series A Preferred Shares received approximately 20,800 ordinary shares and Series A Preferred Shares for each ordinary share and Series A Preferred Share held, respectively. The
F-21
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009 — (Continued)
(Amount expressed in US dollars, except share data, unless otherwise stated)
amount of issued and outstanding ordinary shares and Series A Preferred Shares were increased to 208,005,986 shares and 69,994,014 shares, respectively, as a result of the bonus share issuance. The par value of both ordinary shares and Series A Preferred Shares remains unchanged at US$0.001 per share. The conversion price of Series A Preferred Shares was adjusted to US$0.50 per share as a result of the bonus share issuance without changing the aggregate liquidation value. The authorized share capital was increased to US$500,000 divided into 429,999,350 ordinary shares of a par value of US$0.001 each, and 70,000,650 convertible redeemable preferred shares of a par value of US$0.001 each, all of which are designated Series A Preferred Shares.
The effect of the bonus share issuance was retroactively adjusted to reflect the change for all years presented, as if such stock split had occurred from inception of the Group. The par value of US$207,996 related to ordinary shares first offset against the additional paid-in capital of US$2,554 and the remaining of US$205,442 was recorded against retained earnings as of January 1, 2007, the earliest balance sheet date presented.
Cayman Islands
Under the current laws of Cayman Islands, the Company is not subject to tax on income or capital gain. In addition, upon payments of dividends by the Company to its shareholders, no Cayman Islands withholding tax will be imposed.
Hong Kong
The Company's subsidiaries did not have assessable profits that were earned in or derived from Hong Kong during the years ended December 31, 2007, 2008 and 2009. Accordingly, no Hong Kong profit tax has been provided for.
PRC
Prior to January 1, 2008, the Company's subsidiaries that are incorporated in the PRC are subject to Enterprise Income Tax (“EIT”) in accordance with the Enterprise Income Tax Law and the Income Tax Law of the People’s Republic of China concerning Foreign Investment Enterprises and Foreign Enterprises and local income tax laws (collectively the “previous PRC Income Tax Laws”). Pursuant to the previous PRC Income Tax Laws and rules, enterprises were generally subject to a statutory tax rate of 33% (30% state income tax plus 3% local income tax). Subsidiaries that are registered in the Pudong New District of Shanghai are, however, subject to a 15% preferential EIT rate pursuant to the local tax preferential treatment before January 1, 2008.
In March 2007, the Chinese government enacted the Corporate Income Tax Law (the “new CIT Law”), and promulgated related regulation Implementing Regulations for the PRC Corporate Income Tax Law. The new CIT law and regulation went into effect on January 1, 2008. The new CIT Law, among other things, imposes a unified income tax rate of 25% for both domestic and foreign invested enterprises. The new CIT Law provides a five-year transitional period for those entities established before March 16, 2007, which enjoyed a favorable income tax rate of less than 25% under the previous income tax laws and rules, to gradually change their rates to 25%. In addition, on December 26, 2007, the State Council issued the Notice
F-22
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009 — (Continued)
(Amount expressed in US dollars, except share data, unless otherwise stated)
of the State Council Concerning Implementation of Transitional Rules for Corporate Income Tax Incentives (“Circular 39”). Based on Circular 39, certain specifically listed categories of corporations which enjoyed a favorable income tax rate of less than 25% under the previous income tax laws and rules are eligible for a graduated rate increase to 25% over the 5-year period beginning from January 1, 2008. Where transitional rules overlaps with preferential policies provided by the new CIT Law, a corporation may choose to implement the more favorable policy, and shall not enjoy both simultaneously. Also the policy cannot be changed once determined.
On April 14, 2008, relevant governmental regulatory authorities released qualification criteria, application procedures and assessment processes for “high and new technology enterprises,” which will be entitled to a favorable statutory tax rate of 15%. On July 8, 2008, relevant governmental regulatory authorities further clarified that new technology enterprises previously qualified under the previous income tax laws and rules as of December 31, 2007 would be allowed to enjoy grandfather treatment for the unexpired tax holidays, on condition that they were re-approved for “high and new technology enterprise” status under the regulations released on April 14, 2008.
Additionally, under the new CIT Law, 10% withholding income tax (“WHT”) will be levied on foreign investors for dividend distributions from foreign invested enterprises’ profit earned after January 1, 2008. For certain treaty jurisdictions such as Hong Kong which has signed tax arrangement with the PRC, the WHT rate is 5%.
As of December 31, 2008 and 2009, the Company did not record any withholding tax on the retained earnings of its foreign invested enterprises in the PRC, as the Company intends to indefinitely reinvest its earnings to further expand its business in mainland China, and its foreign invested enterprises do not intend to declare dividends to their immediate foreign holding companies.
CESH, PESH and CPSH, foreign invested enterprises, are located in the Shanghai Pudong Zhangjiang High-Tech Park. They were subject to an income tax rate of 15% before 2008 prior to the implementation of Corporate Income Tax Law. In 2008, the local government announced the recognition of CESH and CPSH as high-new technology enterprises. Accordingly, they are entitled to a preferential tax rate of 15%, which is effective from January 1, 2008. The high and new technology enterprise status is subject to approval and renewal every three years. In addition, CESH is entitled to tax exemption from income tax for year 2007, and a 50% reduced applicable corporate income tax rate for the subsequent three years. PESH and CPSH are entitled to a two-year exemption from income tax for the years of 2007 and 2008, and a 50% reduced applicable corporate income tax rate for the subsequent three years. Accordingly, CESH will gradually transit from income tax rate of 9% to 11% from 2008 to 2010, and PESH and CPSH will gradually transit from the income tax rate of 10% to 12% from 2009 to 2011.
CGTD, a foreign invested enterprise, is located in the Shanghai Pudong Zhangjiang High-Tech Park. It was subject to an income tax rate of 15% before 2008 prior to the implementation of Corporate Income Tax Law. CGTD will gradually transit from the income tax rate of 18% to 25% from 2008 to 2012.
CGNH, a foreign invested enterprise, is located in the Shanghai Nanhui Industrial Zone. It was subject to an income tax rate of 24% plus a local income tax 3% before 2008 prior to the implementation of Corporate Income Tax Law. CGNH transited to the uniform tax rate of 25% in 2008 from the rate of 27%.
F-23
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009 — (Continued)
(Amount expressed in US dollars, except share data, unless otherwise stated)
CPCD, a foreign invested enterprise, is located in Chengdu, China. In 2008, the local government announced the recognition of CPCD as a high-new technology enterprise. Accordingly, CPCD is entitled to a preferential tax rate of 15% (2007: 33%), which is effective from January 1, 2008. The high and new technology enterprise status is subject to approval and renewal every three years.
CGFX, a foreign invested enterprise, is located in Shanghai Fengxian, China. It is subject to an income tax rate of 25% in 2008 and 2009.
Composition of income tax expense
The current and deferred portion of income tax expense included in the consolidated statements of operations for the years ended December 31, 2007, 2008 and 2009 are as follows:
| | | | | | | | | | | | |
| | | 2007 | | | 2008 | | | 2009 | |
Current income tax expense | | | 188,179 | | | | 413,912 | | | | 1,824,879 | |
Deferred tax benefits | | | - | | | | (99,257) | | | | (272,656) | |
| | | | | | | | | | | | |
Income tax expense | | | 188,179 | | | | 314,655 | | | | 1,552,223 | |
| | | | | | | | | | | | |
Reconciliation of the differences between statutory tax rate and the effective tax rate
A reconciliation between the statutory CIT rate and the Group's effective tax rate for the years ended December 31 is as follows:
| | | | | | | | | | | | |
| | | 2007 | | | 2008 | | | 2009 | |
PRC statutory CIT rate | | | 33 | % | | | 25 | % | | | 25 | % |
Permanent differences | | | 8 | % | | | 2 | % | | | 3 | % |
Effect of differences in foreign tax rates | | | 27 | % | | | 7 | % | | | 4 | % |
Tax differential from statutory rate applicable to subsidiaries in the PRC | | | (41 | )% | | | (13 | )% | | | (6 | )% |
Late filing interest charge | | | 2 | % | | | 1 | % | | | - | |
Effects of tax holiday | | | (34 | )% | | | (32 | )% | | | (12 | )% |
Change in valuation allowances | | | 7 | % | | | 13 | % | | | - | |
| | | | | | | | | | | | |
Effective CIT rate | | | 2 | % | | | 3 | % | | | 14 | % |
| | | | | | | | | | | | |
The aggregate amount and per share effect of the tax holidays are as follows:
| | | | | | | | | | | | |
| | | 2007 | | | 2008 | | | 2009 | |
Aggregate effect | | | 2,632,579 | | | | 3,020,774 | | | | 1,369,896 | |
Basic ordinary share effect | | | 0.01 | | | | 0.01 | | | | 0.01 | |
Diluted ordinary share effect | | | 0.01 | | | | 0.01 | | | | - | |
F-24
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009 — (Continued)
(Amount expressed in US dollars, except share data, unless otherwise stated)
Significant components of deferred tax assets
The principal components of deferred tax assets are as follows:
| | | | | | | | |
| | | As of December 31, | |
| | | 2008 | | | 2009 | |
Deferred tax assets: | | | | | | | | |
Net operating loss carry-forward | | | 1,484,936 | | | | 1,578,533 | |
Accrued expense | | | 463,536 | | | | 467,737 | |
Other temporary differences | | | 107,892 | | | | 159,931 | |
Less: valuation allowance | | | (1,764,766 | ) | | | (1,787,106 | ) |
| | | | | | | | |
Total deferred tax assets, net of valuation allowance | | | 291,598 | | | | 419,095 | |
Deferred tax liabilities: | | | | | | | | |
Temporary difference related to revenue recognition | | | (134,774 | ) | | | - | |
Temporary difference related to cost of revenues | | | (55,679 | ) | | | (45,068 | ) |
| | | | | | | | |
Total deferred tax liabilities | | | (190,453 | ) | | | (45,068 | ) |
| | | | | | | | |
Deferred tax assets, net | | | 101,145 | | | | 374,027 | |
| | | | | | | | |
Movement of valuation allowances:
| | | | | | | | | | | | |
| | | 2007 | | | 2008 | | | 2009 | |
At beginning of year | | | 129,654 | | | | 512,801 | | | | 1,764,766 | |
Current year additions | | | 383,147 | | | | 1,251,965 | | | | 22,340 | |
| | | | | | | | | | | | |
At end of year | | | 512,801 | | | | 1,764,766 | | | | 1,787,106 | |
| | | | | | | | | | | | |
The Group adopted the provisions issued by FASB on accounting for uncertain tax positions effective January 1, 2007. Based on its analysis documentation, the Group made its assessment of tax liabilities for each tax position (including the potential application of interest and penalties) based on technical merits, and measured the unrecognized tax benefits associated with the tax positions. As of December 31, 2008 and 2009, the guidance did not have any material impact on the Company’s consolidated total liabilities or shareholders’ equity, thus the required rollforward of uncertain tax positions is not being provided because the impact is not material. The Group classifies interest and/or penalties related to income tax matters in income tax expense. As of December 31, 2008 and 2009, the amount of interest and penalties related to uncertain tax positions was immaterial.
Prior to 2006, the Group structure resulted in a majority of the Group’s income being recorded in offshore entities, which are exempted from income tax. In 2007, the Group reviewed its structure and concluded that such structure no longer corresponds to the Group’s operations and revised its structure. The Group voluntarily disclosed the revision with the local tax bureau and agreed to pay a total of US$1,972,634 to the tax authority in April 2008, which consisted of income tax exposure of US$1,676,209 and the late filing interest charge of US$296,425. The Group recorded US$1,676,209 as income tax expenses in year 2006 and the late interest charge of US$188,179 and US$108,246 for year 2007 and 2008 as income tax expenses in year 2007 and year 2008, respectively.
F-25
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009 — (Continued)
(Amount expressed in US dollars, except share data, unless otherwise stated)
Valuation allowances have been provided on deferred tax assets due to the uncertainty surrounding their realization. As of December 31, 2008 and 2009, valuation allowances on a majority of deferred tax assets were provided because it was more likely than not that the Group will not be able to utilize tax loss carry forwards generated by certain unprofitable subsidiaries. If events occur in the future that allow the Group to realize more of its deferred tax assets than the presently recorded amount, an adjustment to the valuation allowances will increase income when those events occur.
As of December 31, 2009, total tax losses carry forward of the Company’s subsidiaries in the PRC of approximately US$6,311,081, will expire if not used between 2011 and 2014.
Basic earnings per share and diluted earnings per share have been calculated in accordance with FASB issued guidance on computation of earnings per share for the years ended December 31, 2007, 2008 and 2009 as follows:
| | | | | | | | | | | | |
| | | 2007 | | | 2008 | | | 2009 | |
Numerator: | | | | | | | | | | | | |
Net income | | | 7,496,789 | | | | 9,058,146 | | | | 9,798,815 | |
Allocation to preferred shareholders | | | (728,105 | ) | | | (2,280,633 | ) | | | (2,467,117 | ) |
| | | | | | | | | | | | |
Net income attributable to ShangPharma Corporation’s ordinary shareholders — Basic | | | 6,768,684 | | | | 6,777,513 | | | | 7,331,698 | |
Add back of allocation to preferred shareholders | | | 728,105 | | | | 2,280,633 | | | | 2,467,117 | |
| | | | | | | | | | | | |
Net income for diluted earnings per share | | | 7,496,789 | | | | 9,058,146 | | | | 9,798,815 | |
| | | | | | | | | | | | |
Denominator: | | | | | | | | | | | | |
Denominator for basic earnings per share | | | | | | | | | | | | |
Weighted-average ordinary shares outstanding | | | 208,005,986 | | | | 208,005,986 | | | | 208,005,986 | |
Dilutive effect of share options | | | - | | | | - | | | | 51,299 | |
Dilutive effect of Series A Preferred Shares | | | 22,375,136 | | | | 69,994,014 | | | | 69,994,014 | |
| | | | | | | | | | | | |
Denominator for diluted earnings per share | | | 230,381,122 | | | | 278,000,000 | | | | 278,051,299 | |
| | | | | | | | | | | | |
Basic earnings per share | | | 0.03 | | | | 0.03 | | | | 0.04 | |
| | | | | | | | | | | | |
Diluted earnings per share | | | 0.03 | | | | 0.03 | | | | 0.04 | |
| | | | | | | | | | | | |
Allocation to preferred shareholders represents the share of net income attributable to ShangPharma Corporation by the preferred shareholders based on the preferred shareholders interests as a percentage of the total preferred and ordinary shareholders interests. No dividend has been declared to date.
For the years ended December 31, 2007 and 2008, all share equivalents related to equity based compensation have been excluded from the determination of dilutive earnings per share because the inclusion would have been anti-dilutive.
F-26
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009 — (Continued)
(Amount expressed in US dollars, except share data, unless otherwise stated)
2008 Equity and Performance Incentive Plan
In May 2008, the Company authorized an equity compensation plan (the “2008 Equity and Performance Incentive Plan”) that provides for the issuance of options to purchase up to 30,888,889 ordinary shares to the Company’s employees, non-employee directors, officers and consultants. The maximum aggregate number of ordinary shares that may be issued was increased to 36,562,358 shares on February 24, 2010 in an amendment to the 2008 Equity and Performance Incentive Plan.
In 2008 and 2009, the Company granted options to employees to purchase 21,500,600 and 8,778,000 ordinary shares, respectively, under the Company’s 2008 Equity and Performance Incentive Plan at an exercise price of US$0.50 per share. The options can be exercised within 10 years from the grant date. Pursuant to the 2008 Equity and Performance Incentive Plan, 25% of the option will vest and become exercisable on each of the four anniversaries of the date of grant. The options granted shall become immediately exercisable upon the consummation of a change of control (excluding a public offering).
The Company’s share-based compensation expense with respect to options granted to the employees was measured based on the estimated fair value of the Company’s ordinary shares at the date of grant using the Black-Scholes option pricing model and is recognized, adjusted for the estimated forfeiture, on a straight-line basis. Management utilized the results from a third party valuation firm to assist in the determination of the fair value of the ordinary shares underlying the options prepared on a retrospective basis.
Share-based compensation expense related to the options granted by the Company under the 2008 Equity and Performance Incentive Plan amounted to approximately US$68,752 and US$251,950 for the years ended December 31, 2008 and 2009, respectively.
The Company’s share option activities as of December 31, 2008 and 2009 and changes during the years then ended are presented below:
| | | | | | | | | | | | | | | | |
| | | Options
Outstanding | | | Weighted
Average
Exercise
Price | | | Weighted
Average
Remaining
Contractual
Life | | | Aggregate
Intrinsic
Value | |
Granted | | | 21,500,600 | | | | 0.5 | | | | | | | | | |
Exercised | | | - | | | | - | | | | | | | | | |
Forfeited | | | (884,900 | ) | | | 0.5 | | | | | | | | | |
Expired | | | - | | | | - | | | | | | | | | |
| | | | | | | | | | | | | | | | |
Outstanding at December 31, 2008 | | | 20,615,700 | | | | 0.5 | | | | 9.54 | | | | - | |
| | | | | | | | | | | | | | | | |
Vested and expected to vest at December 31, 2008 | | | 13,415,782 | | | | 0.5 | | | | 9.54 | | | | - | |
| | | | | | | | | | | | | | | | |
Vested and exercisable at December 31, 2008 | | | - | | | | - | | | | - | | | | - | |
| | | | | | | | | | | | | | | | |
Granted | | | 8,778,000 | | | | 0.5 | | | | | | | | | |
Exercised | | | - | | | | - | | | | | | | | | |
Forfeited | | | (2,591,825 | ) | | | 0.5 | | | | | | | | | |
Cancelled | | | (278,975 | ) | | | 0.5 | | | | | | | | | |
| | | | | | | | | | | | | | | | |
Outstanding at December 31, 2009 | | | 26,522,900 | | | | 0.5 | | | | 8.87 | | | | 1,326,145 | |
| | | | | | | | | | | | | | | | |
Vested and expected to vest at December 31, 2009 | | | 18,989,364 | | | | 0.5 | | | | 8.86 | | | | 949,468 | |
| | | | | | | | | | | | | | | | |
Vested and exercisable at December 31, 2009 | | | 4,503,975 | | | | 0.5 | | | | 8.54 | | | | 225,199 | |
| | | | | | | | | | | | | | | | |
F-27
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009 — (Continued)
(Amount expressed in US dollars, except share data, unless otherwise stated)
As the exercise price exceeds the fair value of the ordinary share of the Company as of December 31, 2008, there is no intrinsic value as of December 31, 2008.
The aggregate intrinsic value is calculated as the difference between the market value of ordinary shares in an amount of US$0.55 per share as of December 31, 2009 and the exercise prices of the options.
The weighted average grant date fair value of options granted during the two years ended December 31, 2008 and 2009 was US$0.04 and US$0.23, respectively. The total fair value of options vested during the two years ended December 31, 2008 and 2009 was nil and US$116,949, respectively.
As of December 31, 2009, there was US$1,475,198 of unrecognized compensation cost, adjusted for the estimated forfeitures, related to non-vested share-based awards granted to the Company’s employees. This cost is expected to be recognized over a weighted average period of 3.2 years. Total compensation cost may be adjusted for future changes in estimated forfeitures.
The fair value of each option granted under the Company’s 2008 Equity and Performance Incentive Plan is estimated on the date of grant using the Black-Scholes option pricing model that used assumptions noted in the following table:
| | | | | | | | |
| | | For the Years Ended
December 31, | |
| | | 2008 | | | 2009 | |
Risk-free interest rate(1) | | | 4.42-5.34% | | | | 3%-3.72% | |
Expected life (in years)(2) | | | 5-7 years | | | | 5-7 years | |
Expected dividend yield(3) | | | 0% | | | | 0% | |
Expected volatility(4) | | | 33-39% | | | | 42%-43% | |
Fair value per option at grant date | | | 0.02-0.05 | | | | 0.18-0.28 | |
| | (1) | The risk-free interest rate for periods within the contractual life of the share option is based on the rate of US$ China government bond yield in effect at the time of grant for a term consistent with the expected term of the awards. |
| | (2) | The expected term of share options granted under the 2008 Equity and Performance Incentive Plan is developed giving consideration to vesting period, contractual term, share price, employee’s level within the organization and the expected volatility of the underlying share. |
| | (3) | The company has no expectation of paying dividends on its common shares during the term of the options. |
| | (4) | Expected volatility is estimated based on the historical data volatilities of the comparable companies in similar industry as at the valuation dates. |
Founder’s 2008 Equity and Performance Incentive Plan
In May 2008, the Company authorized Founder’s 2008 Equity and Performance Incentive Plan. The founders of the Company have authorized grants of restricted share units (“RSUs”) currently owned by them to certain members of the senior management of the Company (the “Founder’s 2008 Equity and Performance Incentive Plan”). The RSUs provide for the issuance of up to 20,000,000 ordinary shares upon the vesting of RSUs.
F-28
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009 — (Continued)
(Amount expressed in US dollars, except share data, unless otherwise stated)
In 2008 and 2009, the founders granted RSUs representing the rights to receive 7,450,000 and 2,100,000 ordinary shares, respectively. Fifty-percent (50%) of the grantee’s RSUs awarded shall vest on the first anniversary of the date of grant and the remaining fifty-percent (50%) will vest on the second anniversary of the date of grant. The actual number of RSUs vesting annually is subject to certain performance criteria. If certain annual performance conditions are not met, a portion of the awards may not vest and be carried over to the third year of vesting. If the grantee remains an employee of the Company on the third anniversary of the date of grant, any RSUs that have not vested due to not meeting the performance criteria shall vest upon such date. In addition, the vesting of the RSUs is subject to the earlier of the Company completing an initial public offering or a change of control (the “First Conversion Date”). Any RSUs that have not vested as of a change of control event (excluding an initial public offering) shall become fully vested upon such date.
If the grantee’s employment is terminated for cause or he or she terminates with the Company or any subsidiary for any reason prior to the earlier of an initial public offering or change of control (the “Restriction Period”), then all vested and unvested RSUs will be forfeited and become null and void at no cost to the founders or the Company (or convert into ordinary shares only on a case-by-case basis as determined by the board of directors for a PRC employee).
No share-based compensation expense was recorded in 2008 and 2009 on the RSUs as the IPO performance condition is not considered probable until it occurs. The RSUs are accounted for as if they were unvested as of December 31, 2008 and 2009. The grantee shall have no rights as a shareholder with respect to any shares covered by the RSUs until the date shares are vested and the corresponding ordinary shares are transferred from our founders to the grantee. No adjustment shall be made for dividends, distributions or other rights for which the record date is prior to such date.
As of December 31, 2009, there was US$2.5 million of unrecognized compensation cost, adjusted for the estimated forfeitures, related to non-vested RSUs granted to the Company’s employees. Compensation costs of US$1.7 million would be recognized immediately if an IPO or change in control had occurred as of December 31, 2009.
A summary of unvested restricted share unit activities as of December 31, 2008 and 2009 is presented below:
| | | | | | | | |
Unvested Restricted Share Units | | Number of
Shares | | | Weighted Average
Grant Date Fair
Value | |
Granted | | | 7,450,000 | | | | 0.20 | |
Vested | | | - | | | | - | |
Forfeited | | | - | | | | - | |
| | | | | | | | |
Unvested at December 31, 2008 | | | 7,450,000 | | | | 0.20 | |
| | | | | | | | |
Expected to vest at December 31, 2008 | | | 7,450,000 | | | | 0.20 | |
| | | | | | | | |
Granted | | | 2,100,000 | | | | 0.47 | |
Vested | | | - | | | | - | |
Forfeited | | | - | | | | - | |
| | | | | | | | |
Unvested at December 31, 2009 | | | 9,550,000 | | | | 0.26 | |
| | | | | | | | |
Expected to vest at December 31, 2009 | | | 9,550,000 | | | | 0.26 | |
| | | | | | | | |
F-29
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009 — (Continued)
(Amount expressed in US dollars, except share data, unless otherwise stated)
The criteria established by FASB on segments disclosures of an enterprise establish standards for reporting information about operating segments in annual financial statements. It also establishes standards for related disclosures about products and services, geographic areas and major customers.
The Group currently operates and manages its business as a single segment. For the years ended December 31, 2007, 2008 and 2009, the Group’s net revenues are mainly derived from laboratory services. The Group’s net revenues from manufacturing are insignificant for the years ended December 31, 2007, 2008 and 2009.
All the long-lived assets of the Group are located in the PRC.
The Company’s net revenues by geographic region determined according to the location of the customer are as follows:
| | | | | | | | | | | | |
| | | 2007 | | | 2008 | | | 2009 | |
North America | | | 27,236,850 | | | | 47,816,027 | | | | 57,956,395 | |
Europe | | | 6,047,560 | | | | 9,428,721 | | | | 9,547,818 | |
China | | | 232,436 | | | | 1,347,841 | | | | 2,217,969 | |
Japan and other areas | | | 1,388,944 | | | | 2,263,335 | | | | 2,785,495 | |
| | | | | | | | | | | | |
| | | 34,905,790 | | | | 60,855,924 | | | | 72,507,677 | |
Sales tax | | | (235,557 | ) | | | (362,512 | ) | | | (223,020 | ) |
| | | | | | | | | | | | |
Net revenues | | | 34,670,233 | | | | 60,493,412 | | | | 72,284,657 | |
| | | | | | | | | | | | |
| 15. | RELATED PARTY TRANSACTIONS AND BALANCES |
Significant related party transactions are as follows:
| | | | | | | | | | | | |
| | | 2007 | | | 2008 | | | 2009 | |
Purchases from Lab Partner | | | 2,568,332 | | | | 9,831,380 | | | | 5,770,458 | |
Service fees charged by Lab Partner | | | 393,693 | | | | 523,409 | | | | 357,611 | |
Service fees charged by Shanghai Kehui | | | 466,458 | | | | 858,946 | | | | 813,862 | |
Office rent charged by Pharm Valley | | | 895,364 | | | | 1,246,247 | | | | 2,022,116 | |
Labpartner (Shanghai) Co., Ltd. (“Lab Partner”) acts as a raw material procurement agent for the Group since April 2007. It was owned and controlled by an immediate family member of one of the founders in 2007. In 2008, the family member disposed 50% of its equity interests in Lab Partner to two third parties. The daily operation of Lab Partner is managed by these third parties after the disposal.
Shanghai Kehui Catering Management Co., Ltd. (“Shanghai Kehui”) is a catering service provider. It was owned and controlled by one of the founders for the years ended December 31, 2007, 2008 and 2009.
The Group leases certain office facilities from Pharm Valley. The Company’s founders and their immediate family member own 20% shares of Pharm Valley.
F-30
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009 — (Continued)
(Amount expressed in US dollars, except share data, unless otherwise stated)
Balances with related parties are as follows:
| | | | | | | | |
| | | As of December 31, | |
| | | 2008 | | | 2009 | |
Amounts due to related parties: | | | | | | | | |
Lab Partner | | | 2,834,016 | | | | 1,625,056 | |
Shanghai Kehui | | | 92,049 | | | | 47,981 | |
PharmValley | | | 2,195 | | | | 113,759 | |
| | | | | | | | |
| | | 2,928,260 | | | | 1,786,796 | |
| | | | | | | | |
The Group sold its subsidiary, BioSH, to BioExplorer Company Limited (BioExplorer), a related party at that time, on December 27, 2006. BioExplorer became an unrelated party in April 2007 after it was sold to a third party. Loan receivables of US$273,800 arisen prior to 2008 from BioSH were unsecured, interest-free and had no fixed repayment terms. Loan receivables were fully collected in June 2008.
The amounts due from and due to related parties as of December 31, 2008 and 2009 mainly arose from the transactions disclosed above. They were unsecured, interest-free and had no fixed repayment terms.
| 16. | COMMITMENTS AND CONTINGENCIES |
Operating lease commitments
The Group has entered into leasing arrangements for office premises and vehicles that are classified as operating leases. Future minimum lease payments for non-cancelable operating leases at December 31, 2009 are as follows:
| | | | | | | | | | | | |
| | | Office
premises | | | Vehicle | | | Total | |
2010 | | | 2,873,032 | | | | 29,384 | | | | 2,902,416 | |
2011 | | | 1,919,918 | | | | - | | | | 1,919,918 | |
2012 | | | 997,764 | | | | - | | | | 997,764 | |
2013 | | | 555,780 | | | | - | | | | 555,780 | |
2014 and thereafter | | | - | | | | - | | | | - | |
| | | | | | | | | | | | |
| | | 6,346,494 | | | | 29,384 | | | | 6,375,878 | |
| | | | | | | | | | | | |
Rental expenses amounted to US$1,573,162, US$2,771,903 and US$3,472,379 for the years ended December 31, 2007, 2008 and 2009, respectively, and were charged to the consolidated statements of operations when incurred.
Capital commitments
As of December 31, 2009, capital commitments for new plant construction and equipment to be purchased amounted to US$6,736,152.
F-31
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009 — (Continued)
(Amount expressed in US dollars, except share data, unless otherwise stated)
Contingencies
The Group is subject to claims and legal proceedings that arise in the ordinary course of its business operations. Each of these matters is subject to various uncertainties, and it is possible that as these matters arise some may be decided unfavorably for the Group. The Group did not have any claims or legal proceedings that have a significant impact on its business, assets or operations.
| 17. | FAIR VALUE MEASUREMENTS |
The Company does not have any non-financial assets or liabilities that are recognized or disclosed at fair value in the financial statements on a recurring basis.
The Company’s financial instruments consist principally of cash, restricted cash, accounts receivable, amounts due from/to related parties, prepayments and other current assets, investment in securities, accounts payable, advance from customers, other payables and accruals and foreign exchange forward contracts. As of December 31, 2008 and 2009, the carrying values of cash, restricted cash, accounts receivable, amounts due from/to related parties, prepayments and other current assets, accounts payable, advance from customers, other payables and accruals approximated their fair values due to the short maturity of those instruments.
From time to time, the Company enters into foreign-exchange forward contracts with financial institutions to reduce volatility in the Company’s economic value from foreign currency fluctuations. These contracts are marked to market at each reporting date, with changes in fair value recognized in the consolidated statements of operations. The Company held foreign-exchange forward contracts with a total notional value of US$32,300,000 and US$15,000,000 as of December 31, 2008 and 2009, respectively. These foreign exchange forward contracts mature between 1 to 12 months. The Company used a discounted cash-flow methodology to measure fair value, which requires inputs such as interest yield curves and foreign exchange rates. The significant inputs used in the aforementioned model can be corroborated with market observable data and therefore the fair value measurements are classified as level 2.
The gains/(losses) from foreign-exchange forward contracts for the years ended December 31, 2008 and 2009 were included in other expenses (Note 2(s)).
On a recurring basis, the Company measures the investment in securities (Note 2(i)) at fair value. Since the investment in securities does not have quoted price in active markets, they are valued using valuation model. Management is responsible for determining the fair value based on Level 3 inputs. The investee company’s recent financing activities are significant to the overall fair value measurement.
F-32
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009 — (Continued)
(Amount expressed in US dollars, except share data, unless otherwise stated)
As of December 31, 2008 and 2009, information about inputs for the fair value measurements of the Company’s assets and liabilities that are measured at fair value on a recurring basis in periods subsequent to their initial recognition is as follows:
| | | | | | | | | | | | | | | | |
| | | | | | Fair Value Measurements at Reporting Date Using | |
Description | | Year ended
December 31,
2008 | | | Quoted Prices in
Active Markets for
Identical Assets
(Level 1) | | | Significant
Other Observable
Inputs
(Level 2) | | | Significant
Unobservable
Inputs
(Level 3) | |
Investment in securities | | | 300,000 | | | | - | | | | - | | | | 300,000 | |
Foreign-exchange forward contracts | | | (738,529 | ) | | | - | | | | (738,529 | ) | | | - | |
| | | | | | | | | | | | | | | | |
| | | | | | Fair Value Measurements at Reporting Date Using | |
Description | | Year ended
December 31,
2009 | | | Quoted Prices in
Active Markets for
Identical Assets
(Level 1) | | | Significant
Other Observable
Inputs
(Level 2) | | | Significant
Unobservable
Inputs
(Level 3) | |
Investment in securities | | | 417,000 | | | | - | | | | - | | | | 417,000 | |
Foreign-exchange forward contracts | | | (13,868 | ) | | | - | | | | (13,868 | ) | | | - | |
A summary of changes in the fair value of the Level 3 investment in securities for the years ended December 31, 2008 and 2009 were as follows, respectively:
| | | | |
At January 1, 2008 | | | 133,334 | |
Current year purchase | | | 166,666 | |
| | | | |
December 31, 2008 | | | 300,000 | |
| | | | |
Current year purchase | | | 142,857 | |
Realized impairment loss in value | | | (25,857 | ) |
| | | | |
December 31, 2009 | | | 417,000 | |
| | | | |
a). In October 2009, certain subsidiaries of the Company entered into an uncommitted short term revolving facility agreement with a bank, under which, the bank agrees to grant a credit facility of US$7.5 million. In consideration of the extension of the foregoing credit facility, the subsidiaries agree to assign certain of their receivables to the bank. The Group has not drawn down any amount under the revolving facility as of May 20, 2010.
b). In October 2009, certain subsidiaries of the Company signed a 60-day revolving facility offer letter with a bank, under which, the bank agrees to grant a credit facility up to US$6.5 million and to purchase the subsidiaries’ accounts receivables with full recourse. The facility bears interest at the rate of Libor + 2% p.a. per annum for a one year period through October 12, 2010. The outstanding loan balance under this facility is US$0.9 million as of May 20, 2010.
c). In January 2010, certain subsidiaries of the Company entered into a short term banking facility letter with a bank, under which, the bank agrees to grant a 90-day facility up to $3 million and to purchase the subsidiaries’ accounts receivables with full recourse. The Group has not drawn down any amount under the revolving facility as of May 20, 2010.
d). In March 2010, certain subsidiaries of the Company entered into short term revolving facility agreements with a bank, under which, the bank agrees to grant total credit facility of RMB35 million (US$5.1 million), for a one year period through March 2011. There are no outstanding loan balances under these facilities as of May 20, 2010.
F-33
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009 — (Continued)
(Amount expressed in US dollars, except share data, unless otherwise stated)
d). In February 2010, the Group sold its investment in the pharmaceutical and biotechnology research and development company to a third party (Note 2(i)).
The Company has performed an evaluation of subsequent events through May 20, 2010, which is the date the financial statements were available to be issued.
| 19. | UNAUDITED PRO FORMA EARNINGS PER SHARE FOR CONVERSION OF PREFERRED SHARES |
Each Series A Preferred Share is initially convertible into one ordinary share at any time after the date of issuance of such share, and shall be automatically converted into ordinary shares at the then-effective conversion price upon the completion of a qualifying IPO. A qualifying IPO, as defined in the Company’s memorandum and articles of association, means an underwritten public offering of the Company’s ordinary shares on a recognized stock exchange that values the Company at no less than US$250,000,000 and that result in aggregate proceeds to the Company (net of selling expenses) of no less than US$62,500,000. Accordingly, the Company has included the following pro forma financial information.
The unaudited pro-forma earnings per share for the year ended December 31, 2009 after giving effect to the conversion of the Series A Preferred Shares into ordinary shares as of inception at the conversion ratio of 1 for 1 are as follows:
| | | | |
| | | 2009 | |
Numerator: | | | | |
Net income attributable to the Company’s ordinary shareholders — Basic | | | 7,331,698 | |
Add back of allocation to preferred shareholders | | | 2,467,117 | |
| | | | |
Pro-forma net income attributable to the Company’s ordinary shareholders — Basic and diluted | | | 9,798,815 | |
| | | | |
Denominator: | | | | |
Weighted-average ordinary shares outstanding | | | 208,005,986 | |
Pro-forma effect of Series A Preferred Shares | | | 69,994,014 | |
| | | | |
Denominator for pro-forma basic calculation | | | 278,000,000 | |
Dilutive effect of share options | | | 51,299 | |
| | | | |
Denominator for pro-forma diluted calculation | | | 278,051,299 | |
| | | | |
Pro-forma basic earnings per share attributable to the Company’s ordinary shareholders | | | 0.04 | |
| | | | |
Pro-forma diluted earnings per share attributable to the Company’s ordinary shareholders | | | 0.04 | |
| | | | |
Relevant PRC laws and regulations permit PRC companies to pay dividends only out of their retained earnings, if any, as determined in accordance with PRC accounting standards and regulations. Additionally, the Company’s subsidiaries can only distribute dividends upon approval of the shareholders after they have met the PRC requirements for appropriation to statutory reserve. The statutory general reserve fund requires annual appropriations of 10% of net after-tax income should be set aside prior to payment of any dividends.
F-34
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009 — (Continued)
(Amount expressed in US dollars, except share data, unless otherwise stated)
As a result of these and other restrictions under PRC laws and regulations, the PRC subsidiaries and affiliates are restricted in their ability to transfer a portion of their net assets to the Company either in the form of dividends, loans or advances, which restricted portion amounted to approximately US$59 million, or 294% of the Company’s total consolidated net assets as of December 31, 2009. Even though the Company currently does not require any such dividends, loans or advances from the PRC subsidiaries and affiliates for working capital and other funding purposes, the Company may in the future require additional cash resources from our PRC subsidiaries and affiliates due to changes in business conditions, to fund future acquisitions and developments, or merely declare and pay dividends to or distributions to the Company shareholder.
| 21. | ADDITIONAL INFORMATION — CONDENSED FINANCIAL STATEMENTS |
The condensed financial statements of the Company have been prepared in accordance with SEC Regulation S-X Rule 5-04 and Rule 12-04.
The Company records its investment in subsidiaries under the equity method of accounting. Such investment and long-term loans to subsidiaries are presented on the balance sheet as “Interests in subsidiaries” and the profit of the subsidiaries is presented as “Equity in profit of subsidiaries” on the statement of operations.
The footnote disclosures contain supplemental information relating to the operations of the Company and, as such, these statements should be read in conjunction with the notes to the Consolidated Financial Statements of the Company. Certain information and footnote disclosures normally included in financial statements prepared in accordance with U.S. GAAP have been condensed or omitted.
As of December 31, 2008 and 2009, there were no material contingencies, significant provisions for long-term obligations, or guarantees of the Company, except for those which have been separately disclosed in the Consolidated Financial Statements, if any.
Financial information of ShangPharma Corporation
Condensed Statements of operations
| | | | | | | | | | | | |
| | | For the years ended December 31 | |
| | | 2007 | | | 2008 | | | 2009 | |
Net revenues | | | - | | | | - | | | | - | |
Cost of services | | | - | | | | - | | | | - | |
| | | | | | | | | | | | |
Gross profit | | | - | | | | - | | | | - | |
| | | | | | | | | | | | |
Total operating expenses | | | - | | | | (71,106 | ) | | | (251,950 | ) |
| | | | | | | | | | | | |
Loss from operations | | | - | | | | (71,106 | ) | | | (251,950 | ) |
| | | | | | | | | | | | |
Income before income tax expense and equity in profit of subsidiaries and equity in loss of affiliated companies | | | - | | | | (71,106 | ) | | | (251,950 | ) |
| | | | | | | | | | | | |
Income tax expense | | | - | | | | - | | | | - | |
Equity in profit of subsidiaries | | | 7,496,789 | | | | 9,129,252 | | | | 10,050,765 | |
| | | | | | | | | | | | |
Net income | | | 7,496,789 | | | | 9,058,146 | | | | 9,798,815 | |
| | | | | | | | | | | | |
F-35
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2007, 2008 AND 2009 — (Continued)
(Amount expressed in US dollars, except share data, unless otherwise stated)
Financial information of ShangPharma Corporation
Condensed Balance Sheets
| | | | | | | | |
| | | December 31, | |
| | | 2008 | | | 2009 | |
ASSETS | | | | | | | | |
Amounts due from subsidiaries | | | 21,000,000 | | | | 21,000,000 | |
Investment in subsidiaries | | | 24,009,836 | | | | 34,125,734 | |
| | | | | | | | |
Total non-current assets | | | 45,009,836 | | | | 55,125,734 | |
| | | | | | | | |
Total assets | | | 45,009,836 | | | | 55,125,734 | |
| | | | | | | | |
LIABILITIES | | | | | | | | |
Amounts due to subsidiaries | | | 646,579 | | | | 646,613 | |
| | | | | | | | |
Total liabilities | | | 646,579 | | | | 646,613 | |
| | | | | | | | |
Series A Convertible Preferred Shares (US$0.001 par value; 70,000,650 shares authorized; 69,994,014 issued and outstanding as of December 31, 2008 and 2009) (liquidation value of US$45,446,441 as of December 31, 2009) | | | 34,355,775 | | | | 34,355,775 | |
| | | | | | | | |
EQUITY | | | | | | | | |
Ordinary shares (US$0.001 par value; 429,999,350 shares authorized; 208,005,986 shares issued and outstanding as of December 31, 2008 and 2009) | | | 208,006 | | | | 208,006 | |
Additional paid-in capital | | | 3,954,108 | | | | 5,767,118 | |
Retained earnings | | | 3,770,181 | | | | 12,007,936 | |
Accumulated other comprehensive income | | | 2,075,187 | | | | 2,140,286 | |
| | | | | | | | |
Total equity | | | 10,007,482 | | | | 20,123,346 | |
| | | | | | | | |
Total liabilities and equity | | | 45,009,836 | | | | 55,125,734 | |
| | | | | | | | |
Financial information of ShangPharma Corporation
Condensed statements of Cash Flows
| | | | | | | | | | | | |
| | | For the years ended December 31 | |
| | | 2007 | | | 2008 | | | 2009 | |
Net cash provided by (used in) operating activities | | | - | | | | - | | | | - | |
| | | | | | | | | | | | |
Net cash provided by (used in) investing activities | | | - | | | | - | | | | - | |
| | | | | | | | | | | | |
Net cash provided by (used in) financing activities | | | - | | | | - | | | | - | |
| | | | | | | | | | | | |
Effect of foreign exchange rate changes on cash | | | - | | | | - | | | | - | |
| | | | | | | | | | | | |
Net increase (decrease) in cash | | | - | | | | - | | | | - | |
| | | | | | | | | | | | |
Cash at beginning of year | | | - | | | | - | | | | - | |
| | | | | | | | | | | | |
Cash at end of year | | | - | | | | - | | | | - | |
| | | | | | | | | | | | |
F-36
SHANGPHARMA CORPORATION
UNAUDITED CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
FOR THE SIX MONTHS ENDED JUNE 30, 2009 AND 2010
(Amount expressed in US dollars, except share data, unless otherwise stated)
| | | | | | | | | | |
| | | | | For the Six Months Ended June 30, | |
| | | Note | | 2009 | | | 2010 | |
Net revenues | | | | | | | | | | |
Unrelated parties | | | | | 32,661,298 | | | | 41,554,309 | |
| | | | | | | | | | |
| | | | | 32,661,298 | | | | 41,554,309 | |
Cost of revenues | | | | | | | | | | |
Unrelated parties | | | | | (18,271,156 | ) | | | (23,031,146 | ) |
Related parties | | 11 | | | (3,514,771 | ) | | | (4,177,687 | ) |
| | | | | | | | | | |
| | | | | (21,785,927 | ) | | | (27,208,833 | ) |
Gross profit | | | | | 10,875,371 | | | | 14,345,476 | |
| | | | | | | | | | |
Operating expenses: | | | | | | | | | | |
Selling and marketing | | | | | (626,730 | ) | | | (1,074,350 | ) |
General and administrative | | | | | (5,646,519 | ) | | | (6,904,301 | ) |
| | | | | | | | | | |
Total operating expenses | | | | | (6,273,249 | ) | | | (7,978,651 | ) |
| | | | | | | | | | |
Profit from operations | | | | | 4,602,122 | | | | 6,366,825 | |
Interest income | | | | | 5,192 | | | | 14,786 | |
Interest expense | | | | | - | | | | (13,486 | ) |
Other income | | 13 | | | 811,950 | | | | 1,626,397 | |
Other expenses | | | | | (109,604 | ) | | | (178,217 | ) |
| | | | | | | | | | |
Income from operations before income taxes | | | | | 5,309,660 | | | | 7,816,305 | |
Income taxes | | 8 | | | (722,551 | ) | | | (947,603 | ) |
| | | | | | | | | | |
Net income attributable to ShangPharma Corporation | | | | | 4,587,109 | | | | 6,868,702 | |
Allocation to preferred shareholders | | | | | (1,154,929 | ) | | | (1,729,381 | ) |
| | | | | | | | | | |
Net income attributable to ShangPharma Corporation’s ordinary shareholders | | | | | 3,432,180 | | | | 5,139,321 | |
| | | | | | | | | | |
Net income attributable to ShangPharma Corporation’s ordinary shareholders per share | | 9 | | | | | | | | |
Basic | | | | | 0.02 | | | | 0.02 | |
| | | | | | | | | | |
Diluted | | | | | 0.02 | | | | 0.02 | |
| | | | | | | | | | |
Weighted average ordinary shares outstanding | | | | | | | | | | |
Basic | | | | | 208,005,986 | | | | 208,005,986 | |
| | | | | | | | | | |
Diluted | | | | | 278,000,000 | | | | 210,362,840 | |
| | | | | | | | | | |
Share-based compensation included in: | | | | | | | | | | |
Cost of revenues | | | | | 60,255 | | | | 250,923 | |
Selling and marketing | | | | | 1,133 | | | | 5,994 | |
General and administrative | | | | | 23,880 | | | | 96,453 | |
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
F-37
SHANGPHARMA CORPORATION
UNAUDITED CONDENSED CONSOLIDATED BALANCE SHEETS
(Amount expressed in US dollars, except share data, unless otherwise stated)
| | | | | | | | | | | | | | |
| | | Note | | December 31,
2009 | | | June 30,
2010 | | | June 30, 2010
Pro forma
Note 15 | |
ASSETS | | | | | | | | | | | | | | |
Current assets: | | | | | | | | | | | | | | |
Cash | | | | | 12,238,137 | | | | 10,042,815 | | | | 10,042,815 | |
Restricted cash | | | | | 146,106 | | | | 693,671 | | | | 693,671 | |
Investment in securities | | | | | 417,000 | | | | - | | | | - | |
Accounts receivable, net | | | | | 14,291,689 | | | | 17,139,135 | | | | 17,139,135 | |
Inventories | | 3 | | | 1,145,398 | | | | 928,814 | | | | 928,814 | |
Prepayments and other current assets | | 4 | | | 1,452,937 | | | | 2,733,807 | | | | 2,733,807 | |
Deferred tax assets | | 8 | | | 374,027 | | | | 353,500 | | | | 353,500 | |
| | | | | | | | | | | | | | |
Total current assets | | | | | 30,065,294 | | | | 31,891,742 | | | | 31,891,742 | |
| | | | | | | | | | | | | | |
Non-current assets: | | | | | | | | | | | | | | |
Property, equipment and software, net | | 5 | | | 35,725,474 | | | | 42,185,834 | | | | 42,185,834 | |
Land use right, net | | | | | 4,178,474 | | | | 4,165,820 | | | | 4,165,820 | |
Derivative assets | | 13 | | | - | | | | 521,014 | | | | 521,014 | |
| | | | | | | | | | | | | | |
Total non-current assets | | | | | 39,903,948 | | | | 46,872,668 | | | | 46,872,668 | |
| | | | | | | | | | | | | | |
Total assets | | | | | 69,969,242 | | | | 78,764,410 | | | | 78,764,410 | |
| | | | | | | | | | | | | | |
LIABILITIES | | | | | | | | | | | | | | |
Current liabilities: | | | | | | | | | | | | | | |
Short-term bank borrowings | | 6 | | | - | | | | 2,131,313 | | | | 2,131,313 | |
Accounts payable | | | | | 4,717,038 | | | | 6,073,380 | | | | 6,073,380 | |
Amounts due to related parties | | 11 | | | 1,786,796 | | | | 1,145,352 | | | | 1,145,352 | |
Salary and welfare payable | | | | | 2,991,129 | | | | 2,365,473 | | | | 2,365,473 | |
Income taxes payable | | | | | 1,588,697 | | | | 810,017 | | | | 810,017 | |
Advance from customers | | | | | 391,908 | | | | 459,188 | | | | 459,188 | |
Other payables and accruals | | 7 | | | 4,014,553 | | | | 3,841,214 | | | | 3,841,214 | |
| | | | | | | | | | | | | | |
Total current liabilities | | | | | 15,490,121 | | | | 16,825,937 | | | | 16,825,937 | |
| | | | | | | | | | | | | | |
Total liabilities | | | | | 15,490,121 | | | | 16,825,937 | | | | 16,825,937 | |
| | | | | | | | | | | | | | |
Commitments and contingencies | | 12 | | | | | | | | | | | | |
| | | | |
Series A Convertible Preferred Shares (US$0.001 par value; 70,000,650 shares authorized; 69,994,014 issued and outstanding as of December 31, 2009 and June 30, 2010; none outstanding on a pro-forma basis as of June 30, 2010) (Liquidation value of US$48,214,129 as of June 30, 2010) | | | | | 34,355,775 | | | | 34,355,775 | | | | - | |
| | | | | | | | | | | | | | |
EQUITY | | | | | | | | | | | | | | |
Ordinary shares (US$0.001 par value; 429,999,350 shares authorized; 208,005,986 shares issued and outstanding as of December 31, 2009 and June 30, 2010; 278,000,000 outstanding on a pro-forma basis as of June 30, 2010) | | | | | 208,006 | | | | 208,006 | | | | 278,000 | |
Additional paid in capital | | | | | 320,702 | | | | 674,072 | | | | 34,959,853 | |
Statutory reserves | | | | | 5,446,416 | | | | 5,446,416 | | | | 5,446,416 | |
Retained earnings | | | | | 12,007,936 | | | | 18,876,638 | | | | 18,876,638 | |
Accumulated other comprehensive income | | | | | 2,140,286 | | | | 2,377,566 | | | | 2,377,566 | |
| | | | | | | | | | | | | | |
Total equity | | | | | 20,123,346 | | | | 27,582,698 | | | | 61,938,473 | |
| | | | | | | | | | | | | | |
Total liabilities and equity | | | | | 69,969,242 | | | | 78,764,410 | | | | 78,764,410 | |
| | | | | | | | | | | | | | |
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
F-38
SHANGPHARMA CORPORATION
UNAUDITED CONDENSED CONSOLIDATED STATEMENTS OF CHANGES IN EQUITY
FOR THE SIX MONTHS ENDED JUNE 30, 2009 AND 2010
(Amount expressed in US dollars, except share data, unless otherwise stated)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Ordinary shares | | | Additional
paid in
Capital | | | Statutory
reserves | | | Retained
earnings | | | Accumulated
other
comprehensive
income | | | Comprehensive
income | | | Total equity | |
| | | Number
of shares | | | Par value | | | | | | | |
Balance as of December 31, 2008 | | | 208,005,986 | | | | 208,006 | | | | 68,752 | | | | 3,885,356 | | | | 3,770,181 | | | | 2,075,187 | | | | | | | | 10,007,482 | |
Comprehensive income: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net income | | | - | | | | - | | | | - | | | | - | | | | 4,587,109 | | | | - | | | | 4,587,109 | | | | 4,587,109 | |
Other comprehensive income: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Foreign currency translation adjustment | | | - | | | | - | | | | - | | | | - | | | | - | | | | 27,546 | | | | 27,546 | | | | 27,546 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total comprehensive income | | | | | | | | | | | | | | | | | | | | | | | | | | | 4,614,655 | | | | 4,614,655 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Share-based compensation expense | | | - | | | | - | | | | 85,268 | | | | - | | | | - | | | | - | | | | | | | | 85,268 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance as of June 30, 2009 | | | 208,005,986 | | | | 208,006 | | | | 154,020 | | | | 3,885,356 | | | | 8,357,290 | | | | 2,102,733 | | | | | | | | 14,707,405 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance as of December 31, 2009 | | | 208,005,986 | | | | 208,006 | | | | 320,702 | | | | 5,446,416 | | | | 12,007,936 | | | | 2,140,286 | | | | | | | | 20,123,346 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Comprehensive income: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net income | | | - | | | | - | | | | - | | | | - | | | | 6,868,702 | | | | - | | | | 6,868,702 | | | | 6,868,702 | |
Other comprehensive income: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Foreign currency translation adjustment | | | - | | | | - | | | | - | | | | - | | | | - | | | | 237,280 | | | | 237,280 | | | | 237,280 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total comprehensive income | | | | | | | | | | | | | | | | | | | | | | | | | | | 7,105,982 | | | | 7,105,982 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Share-based compensation expense | | | - | | | | - | | | | 353,370 | | | | - | | | | - | | | | - | | | | | | | | 353,370 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance as of June 30, 2010 | | | 208,005,986 | | | | 208,006 | | | | 674,072 | | | | 5,446,416 | | | | 18,876,638 | | | | 2,377,566 | | | | | | | | 27,582,698 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
F-39
SHANGPHARMA CORPORATION
UNAUDITED CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
FOR THE SIX MONTHS ENDED JUNE 30, 2009 AND 2010
(Amount expressed in US dollars unless otherwise stated)
| | | | | | | | | | |
| | | | | For the Six Months Ended June 30, | |
| | | Note | | 2009 | | | 2010 | |
Cash flows from operating activities: | | | | | | | | | | |
Net income | | | | | 4,587,109 | | | | 6,868,702 | |
Adjustments to reconcile net income to net cash provided by operating activities: | | | | | | | | | | |
Depreciation and amortization of property, equipment and software | | | | | 2,904,450 | | | | 3,171,097 | |
Amortization of land use right | | | | | 28,317 | | | | 35,449 | |
Bad debt provision | | | | | 30,000 | | | | 34,058 | |
Inventory provision | | | | | 30,961 | | | | 49,046 | |
Gains related to derivatives | | | | | (85,412 | ) | | | (974,179 | ) |
Loss on disposal of fixed assets | | | | | - | | | | 9,747 | |
Deferred taxes | | | | | (115,071 | ) | | | 20,527 | |
Share-based compensation expense | | | | | 85,268 | | | | 353,370 | |
Change in assets and liabilities: | | | | | | | | | | |
Increase in accounts receivable | | | | | (1,880,439 | ) | | | (2,826,470 | ) |
(Increase) decrease in inventories | | | | | (630,741 | ) | | | 166,215 | |
(Increase) decrease in prepayments and other current assets | | | | | (565,072 | ) | | | (729,565 | ) |
Increase (decrease) in accounts payable | | | | | 643,872 | | | | (284,719 | ) |
Decrease in amounts due to related parties | | | | | (1,143,181 | ) | | | (641,444 | ) |
Decrease in salary and welfare payable | | | | | (238,830 | ) | | | (625,656 | ) |
Increase (decrease) in income taxes payable | | | | | 523,740 | | | | (778,680 | ) |
Increase (decrease) in advance from customers | | | | | 124,320 | | | | 67,280 | |
Increase (decrease) in other payables and accruals | | | | | 716,570 | | | | (159,397 | ) |
| | | | | | | | | | |
Net cash provided by operating activities | | | | | 5,015,861 | | | | 3,755,381 | |
| | | | | | | | | | |
Cash flows from investing activities: | | | | | | | | | | |
Proceeds from disposal of property, equipment and software | | | | | - | | | | 44,066 | |
Proceeds from disposal of investment in securities | | | | | - | | | | 351,378 | |
Purchase of property, equipment and software | | | | | (8,714,037 | ) | | | (7,900,557 | ) |
Purchase of land use right | | | | | (4,249,296 | ) | | | - | |
Payment for investment in securities | | | | | (85,715 | ) | | | - | |
Payment for settlement of derivatives | | | | | (480,591 | ) | | | (44,271 | ) |
(Increase) decrease in restricted cash | | | | | 283,724 | | | | (547,565 | ) |
| | | | | | | | | | |
Net cash used in investing activities | | | | | (13,245,915 | ) | | | (8,096,949 | ) |
| | | | | | | | | | |
Cash flows from financing activities: | | | | | | | | | | |
Proceeds from short-term bank borrowings | | 6 | | | - | | | | 2,886,215 | |
Repayment of short-term bank borrowings | | 6 | | | - | | | | (2,886,215 | ) |
Other borrowings, net of repayments | | 6 | | | - | | | | 2,131,313 | |
| | | | | | | | | | |
Net cash provided by financing activities | | | | | - | | | | 2,131,313 | |
| | | | | | | | | | |
Effect of foreign exchange rate changes on cash | | | | | 869 | | | | 14,933 | |
| | | | | | | | | | |
Net decrease in cash | | | | | (8,229,185 | ) | | | (2,195,322 | ) |
Cash at beginning of period | | | | | 15,843,577 | | | | 12,238,137 | |
| | | | | | | | | | |
Cash at end of period | | | | | 7,614,392 | | | | 10,042,815 | |
| | | | | | | | | | |
Supplemental disclosure of cash flow information: | | | | | | | | | | |
Cash paid during the period for income taxes | | | | | 311,526 | | | | 1,706,334 | |
Interest paid | | | | | - | | | | 13,486 | |
| | | |
Supplemental disclosure of non-cash investing and financing activities: | | | | | | | | | | |
Increase (decrease) in accounts payable for purchase of property, equipment and software | | | | | (4,062,415 | ) | | | 1,641,060 | |
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
F-40
SHANGPHARMA CORPORATION
NOTES TO THE UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
FOR THE SIX MONTHS ENDED JUNE 30, 2009 AND 2010
(Amount expressed in US dollars, except share data, unless otherwise stated)
| 1. | ORGANIZATION AND NATURE OF OPERATIONS |
The accompanying unaudited condensed consolidated financial statements include the financial statements of ShangPharma Corporation (the “Company”) and its wholly owned subsidiaries, which mainly consist of ChemExplorer Company Limited (“CEHK”), China Gateway Life Science (Holdings) Limited (“CGHK”), Shanghai ChemExplorer Co., Ltd. (“CESH”), Shanghai PharmaExplorer Co., Ltd (“PESH”), Shanghai ChemPartner Co., Ltd (“CPSH”), China Gateway Pharma Products (Shanghai) Limited (“CGNH”), Chengdu Chempartner Co., Ltd. (“CPCD”), China Gateway Technology Development (Shanghai) Ltd. (“CGTD”) and China Gateway Pharmatheutical Development Co., Ltd. (“CGFX”). The Company and its subsidiaries are collectively referred to as the “Group”. The Group, as a family-owned business prior to an equity investment made by a third party investor in late 2007, is principally engaged in providing pharmaceutical and biotechnology research and development services.
Substantially all of the Group’s business is conducted in the People’s Republic of China (“PRC”) through its wholly owned operating subsidiaries, CESH, PESH, CPSH, CGNH, CPCD, CGTD and CGFX (collectively referred to as “PRC Operating Subsidiaries”).
CEHK and CGHK were incorporated in Hong Kong on January 3, 2003 and June 23, 2003, respectively, as direct holding companies of the PRC operating subsidiaries. CEHK and CGHK are indirectly wholly owned by two co-founders who are immediate family members prior to the restructuring undertaken in September 2007 as described below.
ShangPharma Corporation was incorporated in Cayman Islands on August 30, 2007. On September 5, 2007, the Group undertook a restructuring in anticipation of the issuance of Series A Convertible Preferred Shares to a third party investor, whereby the Company became the ultimate holding company after all of the then existing shareholders of CEHK and CGHK exchanged their respective shares in these two entities for equivalent classes of shares of the Company on a one for one basis. The restructuring was accounted for as a legal reorganization of entities under common control in a manner similar to pooling of interest. Accordingly, the accompanying unaudited condensed consolidated financial statements have been prepared as if the current corporate structure had been in existence from inception of the Group.
| 2. | SUMMARY OF PRINCIPAL ACCOUNTING POLICIES |
| (a) | Basis of presentation and use of estimates |
The accompanying unaudited condensed consolidated financial statements were prepared on a basis substantially consistent with the Company’s audited consolidated financial statements for the years ended December 31, 2007, 2008 and 2009. These unaudited condensed consolidated financial statements have been prepared in accordance with US GAAP for interim financial information.
In the opinion of the Company’s management, the accompanying unaudited condensed consolidated financial statements contain all normal recurring adjustments necessary for a fair statement of the Company’s consolidated financial statements position, results of operations, and cash flows as of December 31, 2009 and June 30, 2010 and for the six-month periods ended June 30, 2009 and 2010. The December 31, 2009 condensed consolidated balance sheet data was derived from audited financial statements, but does not include all disclosures required by accounting principles generally accepted in the United States of America (“GAAP”). These unaudited condensed consolidated financial statements should be read in conjunction with the Company’s consolidated financial statements and related notes as of December 31, 2008 and 2009, and for the years ended December 31, 2007, 2008 and 2009.
F-41
SHANGPHARMA CORPORATION
NOTES TO THE UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
FOR THE SIX MONTHS ENDED JUNE 30, 2009 AND 2010 — (Continued)
(Amount expressed in US dollars, except share data, unless otherwise stated)
The preparation of unaudited condensed consolidated financial statements in conformity with US GAAP requires the Company’s management to make estimates and assumptions that affect the amounts reported in the accompanying unaudited condensed consolidated financial statements and related disclosures. Actual results could materially differ from these estimates.
| (b) | Recent accounting pronouncements |
In October 2009, the FASB issued an accounting standard update on revenue recognition relating to multiple deliverable revenue arrangements. The fair value requirements of existing accounting guidance are modified by allowing the use of the “best estimate of selling price” in addition to vendor-specific objective evidence (“VSOE”) and third-party evidence (“TPE”) for determining the selling price of a deliverable. A vendor is now required to use its best estimate of the selling price when VSOE or TPE of the selling price cannot be determined. In addition, the residual method of allocating arrangement consideration is no longer permitted. The FASB also issued an update in the same month that amends the scope of pre-existing software revenue guidance by removing from the existing guidance on software revenue recognition non-software components of tangible products and certain software components of tangible products. These updates require expanded qualitative and quantitative disclosure and they are effective for fiscal years beginning on or after June 15, 2010 although early adoption is permitted. These updates may be applied either prospectively from the beginning of the fiscal year for new or materially modified arrangement or retrospectively. The Company is currently assessing the impact that the adoption of these updates will have on its financial statements and disclosures.
In January 2010, the FASB issued an accounting standard update on improving disclosures about fair value measurements. The updated guidance amends existing disclosure requirements by adding required disclosures about items transferring into and out of Levels 1 and 2 in the fair value hierarchy; adding separate disclosures about purchase, sales, issuances, and settlements relative to Level 3 measurements; and clarifying, among other things, the existing fair value disclosures about the level of disaggregation. This update is effective for fiscal years beginning after December 15, 2009, except for the requirement to provide Level 3 activity of purchases, sales, issuances, and settlements on a gross basis, which is effective beginning the first quarter of 2011. The adoption of this update beginning on January 1, 2010 did not have a material impact on the Company’s condensed consolidated financial statements.
The FASB issued an update in April 2010 that provides guidance on the criteria that should be met for determining whether the milestone method of revenue recognition is appropriate. A vendor can recognize consideration that is contingent upon achievement of a milestone in its entirety as revenue in the period in which the milestone is achieved only if the milestone meets all criteria to be considered substantive. Determining whether a milestone is substantive is a matter of judgment made at the inception of the arrangement. A milestone should be considered substantive in its entirety. An individual milestone may not be bifurcated. An arrangement may include more than one milestone, and each milestone should be evaluated separately to determine whether the milestone is substantive. A vendor’s decision to use the milestone method of revenue recognition for transactions within the scope of the amendments in the April 2010 update is a policy election. A vendor that is affected by the amendments in this update is required to provide certain disclosures. This update is effective on a prospective basis for milestones achieved in fiscal years, and interim periods within those years, beginning on or after June 15, 2010. Early adoption is permitted with certain disclosures required. A vendor may also elect, but is not required, to adopt the amendments in this update retrospectively for all prior periods. The Company is currently evaluating the impact on its financial statements of adopting this standard.
F-42
SHANGPHARMA CORPORATION
NOTES TO THE UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
FOR THE SIX MONTHS ENDED JUNE 30, 2009 AND 2010 — (Continued)
(Amount expressed in US dollars, except share data, unless otherwise stated)
Inventories consist of the following:
| | | | | | | | |
| | | December 31,
2009 | | | June 30,
2010 | |
Raw materials | | | 297,547 | | | | 233,931 | |
Work in progress | | | 458,484 | | | | 504,509 | |
Finished goods | | | 389,367 | | | | 190,374 | |
| | | | | | | | |
| | | 1,145,398 | | | | 928,814 | |
| | | | | | | | |
| 4. | PREPAYMENTS AND OTHER CURRENT ASSETS |
Prepayments and other current assets consist of the following:
| | | | | | | | |
| | | December 31,
2009 | | | June 30,
2010 | |
Prepaid rental and deposit | | | 510,608 | | | | 579,590 | |
Deposit to be refunded for purchase of land use right | | | 400,000 | | | | 400,000 | |
Prepayments for purchase of raw materials | | | 246,929 | | | | 424,509 | |
Derivative assets- foreign-exchange forward contracts | | | - | | | | 483,492 | |
Others | | | 295,400 | | | | 846,216 | |
| | | | | | | | |
| | | 1,452,937 | | | | 2,733,807 | |
| | | | | | | | |
| 5. | PROPERTY, EQUIPMENT AND SOFTWARE, NET |
Property, equipment and software consist of the following:
| | | | | | | | |
| | | December 31,
2009 | | | June 30,
2010 | |
Leasehold improvements | | | 14,230,866 | | | | 14,807,232 | |
Experimental equipment | | | 28,257,112 | | | | 32,353,091 | |
Vehicles | | | 534,091 | | | | 549,887 | |
Office equipment and others | | | 2,731,790 | | | | 2,814,151 | |
Software licenses | | | 1,803,838 | | | | 1,836,993 | |
Construction in progress | | | 3,195,624 | | | | 7,985,661 | |
| | | | | | | | |
| | | 50,753,321 | | | | 60,347,015 | |
Less: accumulated depreciation and amortization | | | (15,027,847 | ) | | | (18,161,181 | ) |
| | | | | | | | |
Property, equipment and software, net | | | 35,725,474 | | | | 42,185,834 | |
| | | | | | | | |
Construction in progress primarily represents the construction of new buildings in Shanghai for manufacturing service business.
Depreciation and amortization expenses were US$2,904,450 and US$3,171,097 for the six months ended June 30, 2009 and 2010, respectively.
F-43
SHANGPHARMA CORPORATION
NOTES TO THE UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
FOR THE SIX MONTHS ENDED JUNE 30, 2009 AND 2010 — (Continued)
(Amount expressed in US dollars, except share data, unless otherwise stated)
In October 2009, certain subsidiaries of the Company entered into a short term revolving facility agreement with a bank. Under the agreement, the bank agreed to grant a credit facility of US$7.5 million. In consideration of the extension of the foregoing credit facility, the subsidiaries agreed to assign certain of their receivables to the bank as collateral. The Group did not draw down any amount under the revolving facility as of December 31, 2009 and June 30, 2010.
In October 2009, certain subsidiaries of the Company signed a 60-day revolving facility offer letter with a bank. Under the letter, the bank agreed to grant a credit facility up to US$6.5 million and to purchase the subsidiaries’ accounts receivables with full recourse. The facility bears interest at the rate of Libor + 2% p.a. per annum for a one year period through October 12, 2010. As of June 30, 2010, the outstanding loan balance under this facility was US$2.1 million and the related accounts receivables to be sold were US$2.6 million. The outstanding loans as of June 30, 2010 carried an interest rate from 2.43% to 2.55% per annum.
In March 2010, certain subsidiaries of the Company entered into short term revolving facility agreements with a bank. Under the agreements, the bank agreed to grant total credit facility of RMB35 million (US$5.1 million), for a one year period through March 2011. There was no outstanding loan balance under these facilities as of June 30, 2010.
| 7. | OTHER PAYABLES AND ACCRUALS |
Other payables and accruals consist of the following:
| | | | | | | | |
| | | December 31,
2009 | | | June 30,
2010 | |
Value added tax and other taxes payable | | | 2,028,207 | | | | 2,220,198 | |
Accrued rental and utilities | | | 500,337 | | | | 472,296 | |
Derivative liability- foreign-exchange forward contracts | | | 13,868 | | | | - | |
Accrued consulting and audit fee | | | 197,245 | | | | 6,527 | |
Unearned government subsidies | | | 398,380 | | | | 262,113 | |
Accrued employee annual leave | | | 245,824 | | | | 404,178 | |
Others | | | 630,692 | | | | 475,902 | |
| | | | | | | | |
| | | 4,014,553 | | | | 3,841,214 | |
| | | | | | | | |
Composition of income tax expense
The current and deferred portion of income tax expense included in the unaudited condensed consolidated statements of operations for the six months ended June 30, 2009 and 2010 were as follows:
| | | | | | | | |
| | | For the Six Months Ended
June 30, | |
| | | 2009 | | | 2010 | |
Current income tax expense | | | 837,622 | | | | 927,076 | |
Deferred tax expenses / (benefits) | | | (115,071 | ) | | | 20,527 | |
| | | | | | | | |
Income tax expense | | | 722,551 | | | | 947,603 | |
| | | | | | | | |
F-44
SHANGPHARMA CORPORATION
NOTES TO THE UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
FOR THE SIX MONTHS ENDED JUNE 30, 2009 AND 2010 — (Continued)
(Amount expressed in US dollars, except share data, unless otherwise stated)
Significant components of deferred tax assets
The principal components of deferred tax assets are as follows:
| | | | | | | | |
| | | December 31,
2009 | | | June 30,
2010 | |
Deferred tax assets: | | | | | | | | |
Net operating loss carry-forward | | | 1,578,533 | | | | 1,680,452 | |
Accrued expense | | | 467,737 | | | | 359,729 | |
Other temporary differences | | | 159,931 | | | | 86,720 | |
Less: valuation allowance | | | (1,787,106 | ) | | | (1,728,228 | ) |
| | | | | | | | |
Total deferred tax assets, net of valuation allowance | | | 419,095 | | | | 398,673 | |
Deferred tax liabilities: | | | | | | | | |
Temporary difference related to cost of revenues | | | (45,068 | ) | | | - | |
Other temporary differences | | | - | | | | (45,173 | ) |
| | | | | | | | |
Total deferred tax liabilities | | | (45,068 | ) | | | (45,173 | ) |
| | | | | | | | |
Deferred tax assets, net | | | 374,027 | | | | 353,500 | |
| | | | | | | | |
Basic earnings per share and diluted earnings per share have been calculated for the six months ended June 30, 2009 and 2010 as follows:
| | | | | | | | |
| | | For the Six Months Ended
June 30, | |
| | | 2009 | | | 2010 | |
Numerator: | | | | | | | | |
Net income | | | 4,587,109 | | | | 6,868,702 | |
Allocation to preferred shareholders | | | (1,154,929 | ) | | | (1,729,381 | ) |
| | | | | | | | |
Net income attributable to ShangPharma Corporation’s ordinary shareholders — Basic | | | 3,432,180 | | | | 5,139,321 | |
Add back allocation to preferred shareholders | | | 1,154,929 | | | | - | |
| | | | | | | | |
Net income for diluted earnings per share | | | 4,587,109 | | | | 5,139,321 | |
| | | | | | | | |
F-45
SHANGPHARMA CORPORATION
NOTES TO THE UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
FOR THE SIX MONTHS ENDED JUNE 30, 2009 AND 2010 — (Continued)
(Amount expressed in US dollars, except share data, unless otherwise stated)
| | | | | | | | |
| | | For the Six Months Ended
June 30, | |
| | | 2009 | | | 2010 | |
Denominator: | | | | | | | | |
Denominator for basic earnings per share | | | | | | | | |
Weighted-average ordinary shares outstanding | | | 208,005,986 | | | | 208,005,986 | |
Dilutive effect of share options | | | - | | | | 2,356,854 | |
Dilutive effect of Series A Preferred Shares | | | 69,994,014 | | | | - | |
| | | | | | | | |
Denominator for diluted earnings per share | | | 278,000,000 | | | | 210,362,840 | |
| | | | | | | | |
Basic earnings per share | | | 0.02 | | | | 0.02 | |
| | | | | | | | |
Diluted earnings per share | | | 0.02 | | | | 0.02 | |
| | | | | | | | |
Allocation to preferred shareholders represents the share of net income attributable to ShangPharma Corporation by the preferred shareholders based on the preferred shareholders interests as a percentage of the total preferred and ordinary shareholders interests. No dividend has been declared to date.
For the six months ended June 30, 2009, all share equivalents related to equity based compensation have been excluded from the determination of dilutive earnings per share because the inclusion would have been anti-dilutive.
For the six months ended June 30, 2010, the potentially dilutive Series A Preferred Shares were not included in the calculation of dilutive earnings per share because of their anti-dilutive effect.
2008 Equity and Performance Incentive Plan
In May 2008, the Company authorized an equity compensation plan (the “2008 Equity and Performance Incentive Plan”) that provides for the issuance of options to purchase up to 30,888,889 ordinary shares to the Company’s employees, non-employee directors, officers and consultants. The maximum aggregate number of ordinary shares that may be issued was increased to 36,562,358 shares on February 24, 2010 in an amendment to the 2008 Equity and Performance Incentive Plan.
For the six months ended June 30, 2010, the Company granted options to employees to purchase 3,841,950 ordinary shares under the Company’s 2008 Equity and Performance Incentive Plan at an exercise price of US$0.65 per share. The options can be exercised within 10 years from the grant date. Pursuant to the 2008 Equity and Performance Incentive Plan, 25% of the option will vest and become exercisable on each of the four anniversaries of the date of grant. The options granted shall become immediately exercisable upon the consummation of a change of control (excluding a public offering).
The Company’s share-based compensation expense with respect to options granted to the employees was measured based on the estimated fair value of the Company’s ordinary shares at the date of grant using the Black-Scholes option pricing model and is recognized, adjusted for the estimated forfeiture, on a straight-line basis. Management utilized the results from a third party valuation firm to assist in the determination of the fair value of the ordinary shares underlying the options prepared on a retrospective basis.
F-46
SHANGPHARMA CORPORATION
NOTES TO THE UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
FOR THE SIX MONTHS ENDED JUNE 30, 2009 AND 2010 — (Continued)
(Amount expressed in US dollars, except share data, unless otherwise stated)
Certain employees, after grant date, became consultants to the Company. The Company accounts for equity instrument issued to external consultants under the fair value method. The measurement date of the fair value of the equity instrument issued is the date on which the consultant’s performance is completed. Prior to the measurement date, the equity instruments are measured at their then-current fair value at each of the reporting date. Share-based expense is recognized over the service period using the graded-vesting method, and is adjusted to reflect changes in the fair value of the Company’s ordinary shares between the reporting periods up to the measurement date.
Share-based compensation expense related to the options granted by the Company under the 2008 Equity and Performance Incentive Plan amounted to approximately US$85,268 and US$353,370 for the six months ended June 30, 2009 and 2010, respectively.
The Company’s share option activities June 30, 2010 and changes during the period then ended are presented below:
| | | | | | | | | | | | | | | | |
| | | Options
Outstanding | | | Weighted
Average
Exercise
Price | | | Weighted
Average
Remaining
Contractual
Life | | | Aggregate
Intrinsic
Value | |
Outstanding at December 31, 2009 | | | 26,522,900 | | | | 0.5 | | | | 8.87 | | | | 1,326,145 | |
| | | | | | | | | | | | | | | | |
Granted | | | 3,841,950 | | | | 0.65 | | | | | | | | | |
Exercised | | | - | | | | - | | | | | | | | | |
Forfeited | | | (1,156,925 | ) | | | 0.5 | | | | | | | | | |
Cancelled | | | (190,775 | ) | | | 0.5 | | | | | | | | | |
| | | | | | | | | | | | | | | | |
Outstanding at June 30, 2010 | | | 29,017,150 | | | | 0.52 | | | | 8.57 | | | | 3,776,280 | |
| | | | | | | | | | | | | | | | |
Vested and expected to vest at June 30, 2010 | | | 24,626,600 | | | | 0.51 | | | | 8.46 | | | | 3,334,399 | |
| | | | | | | | | | | | | | | | |
Vested and exercisable at June 30, 2010 | | | 8,143,125 | | | | 0.5 | | | | 8.08 | | | | 1,221,469 | |
| | | | | | | | | | | | | | | | |
The aggregate intrinsic value is calculated as the difference between the market value of ordinary shares in an amount of US$0.65 per share as of June 30, 2010 and the exercise prices of the options.
The weighted average grant date fair value of options granted during the six months ended June 30, 2010 was US$0.30. The total fair value of options vested during the six months ended June 30, 2010 was US$ 264,445.
As of June 30, 2010, there was US$1,995,899 of unrecognized compensation cost, adjusted for the estimated forfeitures, related to non-vested share-based awards granted. This cost is expected to be recognized over a weighted average period of 2 years. Total compensation cost may be adjusted for future changes in estimated forfeitures.
F-47
SHANGPHARMA CORPORATION
NOTES TO THE UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
FOR THE SIX MONTHS ENDED JUNE 30, 2009 AND 2010 — (Continued)
(Amount expressed in US dollars, except share data, unless otherwise stated)
The fair value of each option granted under the Company’s 2008 Equity and Performance Incentive Plan is estimated on the date of grant using the Black-Scholes option pricing model that used assumptions noted in the following table:
| | | | |
| | | For the Six Months Ended
June 30, 2010 | |
Risk-free interest rate(1) | | | 2.96%-3.37% | |
Expected life (in years)(2) | | | 5-7 years | |
Expected dividend yield(3) | | | 0% | |
Expected volatility(4) | | | 41%-43% | |
Fair value per option at grant date | | | 0.28-0.31 | |
| | (1) | The risk-free interest rate for periods within the contractual life of the share option is based on the rate of US$ China government bond yield in effect at the time of grant for a term consistent with the expected term of the awards. |
| | (2) | The expected term of share options granted under the 2008 Equity and Performance Incentive Plan is developed giving consideration to vesting period, contractual term, share price, employee’s level within the organization and the expected volatility of the underlying share. |
| | (3) | The company has no expectation of paying dividends on its common shares during the term of the options. |
| | (4) | Expected volatility is estimated based on the historical data volatilities of the comparable companies in similar industry as at the valuation dates. |
Founder’s 2008 Equity and Performance Incentive Plan
In May 2008, the Company authorized Founder’s 2008 Equity and Performance Incentive Plan. The founders of the Company have authorized grants of restricted share units (“RSUs”) currently owned by them to certain numbers of the senior management of the Company (the “Founder’s 2008 Equity and Performance Incentive Plan”). The RSUs provide for the issuance of up to 20,000,000 ordinary shares upon the vesting of RSUs.
For the six months ended June 30, 2010, the founder granted RSUs representing the rights to receive 1,450,000 ordinary shares. Fifty-percent (50%) of the grantee’s RSUs awarded shall vest on the first anniversary of the date of grant and the remaining fifty-percent (50%) will vest on the second anniversary of the date of grant. The actual number of RSUs vesting annually is subject to certain performance criteria. If certain annual performance conditions are not met, a portion of the awards may not vest and be carried over to the third year of vesting. If the grantee remains an employee of the Company on the third anniversary of the date of grant, any RSUs that have not vested due to not meeting the performance criteria shall vest upon such date. In addition, the vesting of the RSUs is subject to the earlier of the Company completing an initial public offering or a change of control (the “First Conversion Date”). Any RSUs that have not vested as of a change of control event (excluding an initial public offering) shall become fully vested upon such date.
If the grantee’s employment is an employee and is terminated for cause or he or she terminates with the Company or any subsidiary for any reason prior to the earlier of an initial public offering or change of control (the “Restriction Period”), then all vested and unvested RSUs will be forfeited and become null and
F-48
SHANGPHARMA CORPORATION
NOTES TO THE UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
FOR THE SIX MONTHS ENDED JUNE 30, 2009 AND 2010 — (Continued)
(Amount expressed in US dollars, except share data, unless otherwise stated)
void at no cost to the founders or the Company (or convert into ordinary shares only on a case-by-case basis as determined by the board of directors for a PRC employee).
Certain employee, after grant date, became consultants to the Company. The Company accounts for equity instrument issued to external consultants under the fair value method. The measurement date of the fair value of the equity instrument issued is the date on which the consultant’s performance is completed. Prior to the measurement date, the equity instruments are measured at their then-current fair value at each of the reporting date. Share-based expense will be recognized over the service period using the graded-vesting method, and will be adjusted to reflect changes in the fair value of the Company’s ordinary shares between the reporting periods up to the measurement date.
No share-based compensation expense was recorded for the six months ended June 30, 2009 and 2010 on the RSUs as the IPO performance condition is not considered probable until it occurs. The RSUs are accounted for as if they were unvested as of June 30, 2010. The grantee shall have no rights as a shareholder with respect to any shares covered by the RSUs until the date shares are vested and the corresponding ordinary shares are transferred from the founders to the grantee. No adjustment shall be made for dividends, distributions or other rights for which the record date is prior to such date.
As of June 30, 2010, there was US$3.4 million of unrecognized compensation cost, adjusted for the estimated forfeitures, related to non-vested RSUs granted to the Company’s employees. Compensation costs of US$2.1 million would be recognized immediately if an IPO or change in control had occurred as of June 30, 2010.
A summary of unvested restricted share unit activities as of December 31, 2009 and June 30, 2010 is presented below:
| | | | | | | | |
Unvested Restricted Share Units | | Number of
Shares | | | Weighted Average
Grant Date Fair
Value | |
Unvested at December 31, 2009 | | | 9,550,000 | | | | 0.26 | |
| | | | | | | | |
Granted | | | 1,450,000 | | | | 0.65 | |
Vested | | | - | | | | - | |
Forfeited | | | - | | | | - | |
| | | | | | | | |
Unvested at June 30, 2010 | | | 11,000,000 | | | | 0.31 | |
| | | | | | | | |
Expected to vest at June 30, 2010 | | | 11,000,000 | | | | 0.31 | |
| | | | | | | | |
| 11. | RELATED PARTY TRANSACTIONS AND BALANCES |
Significant related party transactions are as follows:
| | | | | | | | |
| | | For the Six Months
Ended June 30, | |
| | | 2009 | | | 2010 | |
Purchases from Lab Partner | | | 2,324,743 | | | | 2,905,693 | |
Service fees charged by Lab Partner | | | 243,856 | | | | 207,169 | |
Service fees charged by Shanghai Kehui | | | 381,335 | | | | 402,105 | |
Office rent charged by Pharm Valley | | | 944,624 | | | | 1,068,378 | |
F-49
SHANGPHARMA CORPORATION
NOTES TO THE UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
FOR THE SIX MONTHS ENDED JUNE 30, 2009 AND 2010 — (Continued)
(Amount expressed in US dollars, except share data, unless otherwise stated)
Labpartner (Shanghai) Co., Ltd. (“Lab Partner”) acts as a raw material procurement agent for the Group since April 2007. It was owned and controlled by an immediate family member of one of the founders in 2007. In 2008, the family member disposed 50% of its equity interests in Lab Partner to two third parties. The daily operation of Lab Partner is managed by these third parties after the disposal.
Shanghai Kehui Catering Management Co., Ltd. (“Shanghai Kehui”) is a catering service provider. It was owned and controlled by one of the founders for the six months ended June 30, 2009 and 2010.
The Group leases certain office facilities from Pharm Valley. The Company’s founders and their immediate family member own 20% of Pharm Valley.
Balances with related parties are as follows:
| | | | | | | | |
| | | December 31,
2009 | | | June 30,
2010 | |
Amounts due to related parties: | | | | | | | | |
Lab Partner | | | 1,625,056 | | | | 999,113 | |
Shanghai Kehui | | | 47,981 | | | | 70,952 | |
PharmValley | | | 113,759 | | | | 75,287 | |
| | | | | | | | |
| | | 1,786,796 | | | | 1,145,352 | |
| | | | | | | | |
The amounts due to related parties as of December 31, 2009 and June 30, 2010 mainly arose from the transactions disclosed above. They were unsecured, interest-free and had no fixed repayment terms.
| 12. | COMMITMENTS AND CONTINGENCIES |
Operating lease commitments
The Group has entered into leasing arrangements for office premises and vehicles that are classified as operating leases. Future minimum lease payments for non-cancelable operating leases at June 30, 2010 are as follows:
| | | | | | | | | | | | |
| | | Office
premises | | | Vehicle | | | Total | |
For the second half of 2010 | | | 1,574,244 | | | | 122,458 | | | | 1,696,702 | |
2011 | | | 2,374,025 | | | | 40,819 | | | | 2,414,844 | |
2012 | | | 1,206,188 | | | | - | | | | 1,206,188 | |
2013 | | | 558,833 | | | | - | | | | 558,833 | |
2014 | | | - | | | | - | | | | - | |
2015 and thereafter | | | - | | | | - | | | | - | |
| | | | | | | | | | | | |
| | | 5,713,290 | | | | 163,277 | | | | 5,876,567 | |
| | | | | | | | | | | | |
Rental expenses amounted to US$1,752,237 and US$1,841,236 for the six months ended June 30, 2009 and 2010, respectively, and were charged to the consolidated statements of operations when incurred.
F-50
SHANGPHARMA CORPORATION
NOTES TO THE UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
FOR THE SIX MONTHS ENDED JUNE 30, 2009 AND 2010 — (Continued)
(Amount expressed in US dollars, except share data, unless otherwise stated)
Capital commitments
As of June 30, 2010, capital commitments for new plant construction and equipment to be purchased amounted to US$7,711,873.
Contingencies
The Group is subject to claims and legal proceedings that arise in the ordinary course of its business operations. Each of these matters is subject to various uncertainties, and it is possible that as these matters arise some may be decided unfavorably for the Group. The Group did not have any claims or legal proceedings that have a significant impact on its business, assets or operations.
| 13. | FAIR VALUE MEASUREMENTS |
From time to time, the Company enters into foreign-exchange forward contracts with financial institutions to reduce volatility in the Company’s economic value from foreign currency fluctuations. These contracts are marked to market at each reporting date, with changes in fair value recognized in the consolidated statements of operations.
The Company held deliverable foreign-exchange forward contracts with a total notional value of US$15,000,000 and US$63,000,000 as of December 31, 2009 and June 30, 2010, respectively. These foreign exchange forward contracts mature between 2 to 12 months.
In 2010, the Company also entered into non-deliverable foreign-exchange forward contracts with a well-known financial institution. Under such contracts, parties agree to exchange two currencies at a pre-determined rate at a specified date in the future without physical delivery of the two currencies. The Company held non-deliverable foreign-exchange forward contracts with notional value of US$57,000,000 as of June 30, 2010. These foreign exchange forward contracts mature between 5 to 18 months.
The fair value of the foreign-exchange forward contracts is measured using level 2 inputs other than the quoted price in active markets that are observable either directly or indirectly. As of June 30, 2010, the derivative assets related to the fair value of foreign-exchange forward contracts were classified as current assets in “Prepayments and Other Current Assets” in the amount of US$483,492 and non-current in the amount of US$521,014.
The gains from foreign-exchange forward contracts for the six months ended June 30, 2009 and 2010 were US$85,412 and US$ 974,179, respectively, and were included in other income, respectively.
On a recurring basis, the Company measures its investment in securities at fair value. Since the investment in securities do not have quoted price in active markets, they are valued using valuation model. Management is responsible for determining the fair value based on Level 3 inputs. The investee company’s recent financing activities are significant to the overall fair value measurement.
F-51
SHANGPHARMA CORPORATION
NOTES TO THE UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
FOR THE SIX MONTHS ENDED JUNE 30, 2009 AND 2010 — (Continued)
(Amount expressed in US dollars, except share data, unless otherwise stated)
As of December 31, 2009 and June 30, 2010, information about inputs for the fair value measurements of the Company’s assets and liabilities that are measured at fair value on a recurring basis in periods subsequent to their initial recognition is as follows:
| | | | | | | | | | | | | | | | |
| | | | | | Fair Value Measurements at Reporting Date Using | |
Description | | December 31,
2009 | | | Quoted Prices in
Active Markets for
Identical Assets
(Level 1) | | | Significant
Other Observable
Inputs
(Level 2) | | | Significant
Unobservable
Inputs
(Level 3) | |
Investment in securities | | | 417,000 | | | | - | | | | - | | | | 417,000 | |
Deliverable foreign-exchange forward contracts | | | (13,868 | ) | | | - | | | | (13,868 | ) | | | - | |
| | | | | | | | | | | | | | | | |
| | | | | | Fair Value Measurements at Reporting Date Using | |
Description | | June 30,
2010 | | | Quoted Prices in
Active Markets for
Identical Assets
(Level 1) | | | Significant
Other Observable
Inputs
(Level 2) | | | Significant
Unobservable
Inputs
(Level 3) | |
Deliverable foreign-exchange forward contracts | | | 232,973 | | | | - | | | | 232,973 | | | | - | |
Non-deliverable foreign-exchange forward contracts | | | 771,533 | | | | - | | | | 771,533 | | | | - | |
A summary of changes in the fair value of the Level 3 investment in securities for the six months ended June 30, 2009 and 2010 were as follows:
| | | | |
December 31, 2008 | | | 300,000 | |
| | | | |
Current period purchase | | | 85,715 | |
| | | | |
June 30, 2009 | | | 385,715 | |
| | | | |
December 31, 2009 | | | 417,000 | |
| | | | |
Current period disposal | | | (417,000 | ) |
| | | | |
June 30, 2010 | | | - | |
| | | | |
a) On July 30, 2010, the Company granted options to employees to purchase 7,404,600 ordinary shares under the Company’s 2008 Equity and Performance Incentive Plan at an exercise price of US$0.65 per share.
b) In July 2010, certain subsidiary of the Company entered into a loan agreement with a bank in relation to an one-year loan in the principal amount of RMB10 million (US$1.47 million). As of September 30, 2010, the Group has drawn down RMB5 million (US$0.75 million) bearing interest at the rate of 6.372% per annum.
F-52
SHANGPHARMA CORPORATION
NOTES TO THE UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
FOR THE SIX MONTHS ENDED JUNE 30, 2009 AND 2010 — (Continued)
(Amount expressed in US dollars, except share data, unless otherwise stated)
c) In the third quarter of 2010, certain subsidiaries of the Company entered the following arrangements with a bank: 1) A short-term revolving accounts receivable facility and a letter of credit facility for aggregate US$5 million. Amounts drawn under the accounts receivable facility have a maximum term of three months and bear interest at the rate of three-month LIBOR plus 4.5% annually. Certain receivables of the subsidiaries will be assigned to the bank as security for amounts drawn under the accounts receivable facility. Subsidiaries may also use this facility to obtain letters of credit. Issued letters of credit have a maximum term of one year and bear commission at a rate of 0.15% quarterly; 2) An interest-free facility for aggregate US$7.5 million for hedging and risk management derivative transactions with the bank; 3) A short-term revolving facility to borrow up to US$2 million, bearing interest at the rate of three-month LIBOR plus 4.5% annually for borrowings in US dollars and 90% of prevailing base lending rate set by PBOC for borrowings in RMB. Amounts drawn under the facility have a maximum term of three months and will be secured by certain property and land use right of the Group; 4) A three-year term loan in the principal amount of US$5 million, bearing interest at the rate of three-month LIBOR plus 4.5% annually for borrowings in US dollars and the prevailing base lending rate set by PBOC for borrowings in RMB. Usage is limited to purchase of equipment and construction of plants. The borrowing is secured by certain property and land use right of the Group. The Group did not draw down any amount under these facilities as of September 30, 2010.
d) On August 16, 2010, the founders granted RSUs representing the rights to receive 750,000 ordinary shares to employees under the Founder’s 2008 Equity and Performance incentive Plan.
e) On September 30, 2010, the definition of a qualified public offering in the Company’s memorandum and articles of association was amended to mean an initial public offering on a qualified exchange that values the Company at no less than US$250,000,000 immediately after the offering and that results in aggregate gross proceeds from the offering to be no less than US$75,000,000, including aggregate gross proceeds to the investor in Series A Preferred Shares of no less than US$25,000,000.
| 15. | UNAUDITED PRO FORMA BALANCE SHEET AND EARNINGS PER SHARE FOR CONVERSION OF PREFERRED SHARES |
Each Series A Preferred Share is initially convertible into one ordinary share at any time after the date of issuance of such share, and shall be automatically converted into ordinary shares at the then-effective conversion price upon the completion of a qualifying IPO. A qualifying IPO, as defined in the Company’s memorandum and articles of association, as amended on September 30, 2010, means an IPO on a qualified exchange that values the Company at no less than US$250,000,000 immediately after the IPO and that results in aggregate gross proceeds from the IPO to be no less than US$75,000,000, including aggregate gross proceeds to the investor in Series A Preferred Shares of no less than US$25,000,000 (Note 14). Accordingly, the Company has included the following pro forma financial information.
The unaudited pro-forma balance sheet as of June 30, 2010 presents an adjusted financial position as if the 69,994,014 shares of Series A Preferred Shares have been converted as of June 30, 2010, at the then conversion ratios of 1 for 1 for Series A Preferred Shares. Accordingly, the carrying value of the Series A Preferred Shares, in the amount of US$34,355,775, was reclassified from Series A Preferred Shares to ordinary shares for such pro forma adjustment.
F-53
SHANGPHARMA CORPORATION
NOTES TO THE UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
FOR THE SIX MONTHS ENDED JUNE 30, 2009 AND 2010 — (Continued)
(Amount expressed in US dollars, except share data, unless otherwise stated)
The unaudited pro-forma earnings per share for the six months ended June 30, 2010 after giving effect to the conversion of the Series A Preferred Shares into ordinary shares as of inception at the conversion ratio of 1 for 1 are as follows:
| | | | |
| | | For the Six Months Ended
June 30, 2010 | |
Numerator: | | | | |
Net income attributable to the Company’s ordinary shareholders — Basic | | | 5,139,321 | |
Add back of allocation to preferred shareholders | | | 1,729,381 | |
| | | | |
Pro-forma net income attributable to the Company’s ordinary shareholders — Basic and diluted | | | 6,868,702 | |
| | | | |
Denominator: | | | | |
Weighted-average ordinary shares outstanding | | | 208,005,986 | |
Pro-forma effect of Series A Preferred Shares | | | 69,994,014 | |
| | | | |
Denominator for pro-forma basic calculation | | | 278,000,000 | |
Dilutive effect of share options | | | 2,356,854 | |
| | | | |
Denominator for pro-forma diluted calculation | | | 280,356,854 | |
| | | | |
Pro-forma basic earnings per share attributable to the Company’s ordinary shareholders | | | 0.02 | |
| | | | |
Pro-forma diluted earnings per share attributable to the Company’s ordinary shareholders | | | 0.02 | |
| | | | |
F-54











