UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 20-F
(Mark One)
| ¨ | REGISTRATION STATEMENT PURSUANT TO SECTION 12(B) OR 12(G) OF THE SECURITIES EXCHANGE ACT OF 1934 |
OR
| þ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(D) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2011.
OR
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(D) OF THE SECURITIES EXCHANGE ACT OF 1934 |
OR
| ¨ | SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(D) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Date of event requiring this shell company report
For the transition period from to
Commission file number: 001-34895
SHANGPHARMA CORPORATION
(Exact name of Registrant as specified in its charter)
N/A
(Translation of Registrant’s name into English)
Cayman Islands
(Jurisdiction of incorporation or organization)
No. 5 Building, 998 Halei Road
Zhangjiang Hi-Tech Park, Pudong New Area
Shanghai, 201203
The People’s Republic of China
(Address of principal executive offices)
William Dai, Chief Financial Officer
ShangPharma Corporation
No. 5 Building, 998 Halei Road
Zhangjiang Hi-Tech Park, Pudong New Area
Shanghai, 201203
The People’s Republic of China
(86 21) 5132-0088
Facsimile: (86 21) 5132-0110
(Name, telephone, e-mail and/or facsimile number and address of company contact person)
Securities registered or to be registered pursuant to Section 12(b) of the Act:
| | |
Title of each class | | Name of exchange on which registered |
| American Depositary Shares, each of which represents 18 ordinary shares | | New York Stock Exchange |
| Ordinary shares, par value US$0.001 per share | | New York Stock Exchange* |
| * | Not for trading, but only in connection with the listing on New York Stock Exchange of the American depositary shares. |
Securities registered or to be registered pursuant to Section 12(g) of the Act:
None
(Title of Class)
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act:
None
(Title of Class)
Indicate the number of outstanding shares of each of the Issuer’s classes of capital or common stock as of the close of the period covered by the annual report. 336,109,400 ordinary shares, par value US$0.001 per share, as of December 31, 2011.
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No þ
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934. Yes ¨ No þ
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes þ No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or such shorter period that the registrant was required to submit and post such files). Yes ¨ No ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, or a non-accelerated filer. See definition of “accelerated filer and large accelerated filer” in Rule 12b-2 of the Exchange Act. (Check one):
Large accelerated filer ¨ Accelerated filer þ Non-accelerated filer ¨
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing:
| | | | | | | | |
| US GAAP | | þ | | International Financial Reporting Standards as issued by the International Accounting Standards Board ¨ | | Other | | ¨ |
If “Other” has been checked in response to the previous question, indicate by check mark which financial statement item the registrant has elected to follow.
Item 17 ¨ Item 18 ¨
If this is an annual report, indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No þ
(APPLICABLE ONLY TO ISSUERS INVOLVED IN BANKRUPTCY PROCEEDINGS DURING THE PAST FIVE YEARS)
Indicate by check mark whether the registrant has filed all documents and reports required to be filed by Sections 12, 13 or 15(d) of the Securities Exchange Act of 1934 subsequent to the distribution of securities under a plan confirmed by a court. Yes ¨ No ¨
TABLE OF CONTENTS
1
INTRODUCTION
Unless otherwise indicated and except where the context otherwise requires, references in this annual report on Form 20-F to:
| | • | | “ADSs” refers to our American depositary shares, each of which represents 18 ordinary shares, and “ADRs” refers to American depositary receipts, which, if issued, evidence our ADSs; |
| | • | | “China” or the “PRC” refer to the People’s Republic of China excluding, for the purpose of this annual report only, Hong Kong, Macau and Taiwan; |
| | • | | “RMB” or “Renminbi” refers to the legal currency of China and “$,” “dollar,” “US$” or “U.S. dollar” refers to the legal currency of the United States; |
| | • | | “shares” or “ordinary shares” refers to our ordinary shares, par value US$0.001 per share, |
| | • | | “preferred shares” refers to our Series A convertible preferred shares, par value US$0.001 per share, all of which were automatically converted into ordinary shares upon the completion of our initial public offering in October 2010, and |
| | • | | “outstanding ordinary shares” refers to our outstanding ordinary shares, excluding 17,490,600 ordinary shares that have been issued to JPMorgan Chase Bank, N.A., the depositary of our ADS program, but are reserved in anticipation of the exercise of options under our share incentive plans. |
Unless the context indicates otherwise, “we,” “us,” “our company,” “our,” and “ShangPharma” refer to ShangPharma Corporation, a Cayman Islands company, and its subsidiaries.
FORWARD-LOOKING STATEMENTS
This annual report on Form 20-F contains forward-looking statements that involve risks and uncertainties. All statements other than statements of historical facts are forward-looking statements. Known and unknown risks, uncertainties and other factors, including those listed under “Risk Factors,” that may cause our actual results, performance or achievements to be materially different from those expressed or implied by the forward-looking statements.
You can identify some of these forward-looking statements by words or phrases such as “may,” “will,” “expect,” “anticipate,” “aim,” “estimate,” “intend,” “plan,” “believe,” “likely to” or other similar expressions. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our financial condition, results of operations, business strategy and financial needs. These forward-looking statements include:
| | • | | our goals and strategies; |
| | • | | our future development, financial condition and results of operations; |
| | • | | the expected growth of the pharmaceutical and biotechnology outsourcing industry in China and globally; |
| | • | | market acceptance of our services; |
| | • | | our expectations regarding demand for our services; |
| | • | | our ability to stay abreast of market trends and technological advances; |
| | • | | our ability to effectively protect our customers’ intellectual property rights and not infringe on the intellectual property rights of others; |
| | • | | competition in the pharmaceutical and biotechnology outsourcing industry; |
2
| | • | | PRC and U.S. governmental policies and regulations relating to the pharmaceutical and biotechnology outsourcing industry; |
| | • | | litigation and government proceedings involving our company and industry; and |
| | • | | general economic and business conditions, particularly in the United States and China. |
These forward-looking statements involve various risks and uncertainties. Although we believe that our expectations expressed in these forward-looking statements are reasonable, our expectations may later be found to be incorrect. Our actual results could be materially different from our expectations. Other sections of this annual report include additional factors which could adversely impact our business and financial performance. Moreover, we operate in an evolving environment. New risk factors and uncertainties emerge from time to time and it is not possible for our management to predict all risk factors and uncertainties, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements. You should read thoroughly this annual report and the documents that we refer to in this annual report with the understanding that our actual future results may be materially different from and worse than what we expect. We qualify all of our forward-looking statements by these cautionary statements.
The forward-looking statements made in this annual report relate only to events or information as of the date on which the statements are made in this annual report. Except as required by law, we undertake no obligation to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise, after the date on which the statements are made or to reflect the occurrence of unanticipated events.
PART I.
| ITEM 1. | IDENTITY OF DIRECTORS, SENIOR MANAGEMENT AND ADVISERS |
Not applicable.
| ITEM 2. | OFFER STATISTICS AND EXPECTED TIMETABLE |
Not applicable.
A.Selected Financial Data
Selected Consolidated Financial Data
The following selected consolidated financial information for the periods and as of the dates indicated should be read in conjunction with our consolidated financial statements and related notes and “Item 5. Operating and Financial Review and Prospects” in this annual report.
Our selected consolidated statement of operations data presented below for the years ended December 31, 2009, 2010 and 2011 and our balance sheet data as of December 31, 2010 and 2011 have been derived from our audited consolidated financial statements included elsewhere in this annual report. Our audited consolidated financial statements are prepared in accordance with U.S. GAAP. Our selected consolidated statement of operations data presented below for the years ended December 31, 2007 and 2008 and our balance sheet data as of December 31, 2007, 2008 and 2009 have been derived from our audited financial statements not included in this annual report.
Our historical results do not necessarily indicate results expected for any future periods.
3
| | | | | | | | | | | | | | | | | | | | |
| | | Years Ended December 31, | |
| | | 2007 | | | 2008 | | | 2009 | | | 2010 | | | 2011 | |
| | | (in millions of US$, except for share, per share, and per ADS data) | |
Consolidated Statements of Operations Data: | | | | | | | | | | | | | | | | | | | | |
Net revenues | | | 34.7 | | | | 60.5 | | | | 72.3 | | | | 90.3 | | | | 111.6 | |
Cost of revenues | | | (22.8 | ) | | | (40.5 | ) | | | (48.4 | ) | | | (60.2 | ) | | | (75.5 | ) |
| | | | | | | | | | | | | | | | | | | | |
Gross profit | | | 11.9 | | | | 20.0 | | | | 23.9 | | | | 30.1 | | | | 36.1 | |
| | | | | | | | | | | | | | | | | | | | |
Operating expenses: | | | | | | | | | | | | | | | | | | | | |
Selling and marketing | | | (0.5 | ) | | | (0.8 | ) | | | (1.7 | ) | | | (2.3 | ) | | | (2.6 | ) |
General and administrative | | | (4.0 | ) | | | (9.3 | ) | | | (12.0 | ) | | | (17.4 | ) | | | (24.0 | ) |
Other operating income(1) | | | — | | | | — | | | | — | | | | — | | | | 0.4 | |
Other operating expenses(1) | | | — | | | | — | | | | — | | | | — | | | | (0.2 | ) |
| | | | | | | | | | | | | | | | | | | | |
Total operating expenses | | | (4.5 | ) | | | (10.1 | ) | | | (13.7 | ) | | | (19.7 | ) | | | (26.4 | ) |
| | | | | | | | | | | | | | | | | | | | |
Profit from operations | | | 7.4 | | | | 9.9 | | | | 10.2 | | | | 10.4 | | | | 9.7 | |
Interest income | | | 0.2 | | | | 0.2 | | | | * | | | | 0.1 | | | | 1.0 | |
Interest expense | | | — | | | | — | | | | — | | | | * | | | | * | |
Other income(1) | | | 0.2 | | | | 0.9 | | | | 1.3 | | | | 4.8 | | | | 4.0 | |
Other expenses | | | (0.1 | ) | | | (1.6 | ) | | | (0.2 | ) | | | (0.2 | ) | | | (1.1 | ) |
| | | | | | | | | | | | | | | | | | | | |
Income from operations before income taxes | | | 7.7 | | | | 9.4 | | | | 11.4 | | | | 15.1 | | | | 13.5 | |
Income taxes | | | (0.2 | ) | | | (0.3 | ) | | | (1.6 | ) | | | (2.1 | ) | | | (2.4 | ) |
Net income attributable to ShangPharma Corporation | | | 7.5 | | | | 9.1 | | | | 9.8 | | | | 13.0 | | | | 11.1 | |
Allocation to preferred shareholders | | | (0.7 | ) | | | (2.3 | ) | | | (2.5 | ) | | | (2.7 | ) | | | — | |
| | | | | | | | | | | | | | | | | | | | |
Net income attributable to ShangPharma Corporation’s ordinary shareholders | | | 6.8 | | | | 6.8 | | | | 7.3 | | | | 10.3 | | | | 11.1 | |
| | | | | | | | | | | | | | | | | | | | |
Net income attributable to ShangPharma Corporation’s ordinary shareholders per share | | | | | | | | | | | | | | | | | | | | |
Basic | | | 0.03 | | | | 0.03 | | | | 0.04 | | | | 0.04 | | | | 0.03 | |
| | | | | | | | | | | | | | | | | | | | |
Diluted | | | 0.03 | | | | 0.03 | | | | 0.04 | | | | 0.04 | | | | 0.03 | |
| | | | | | | | | | | | | | | | | | | | |
Net income attributable to ShangPharma Corporation’s ordinary shareholder per ADS(2) | | | | | | | | | | | | | | | | | | | | |
Basic | | | 0.59 | | | | 0.59 | | | | 0.63 | | | | 0.79 | | | | 0.60 | |
| | | | | | | | | | | | | | | | | | | | |
Diluted | | | 0.59 | | | | 0.59 | | | | 0.63 | | | | 0.78 | | | | 0.60 | |
| | | | | | | | | | | | | | | | | | | | |
Weighted average ordinary shares outstanding(3) | | | | | | | | | | | | | | | | | | | | |
Basic | | | 208,005,986 | | | | 208,005,986 | | | | 208,005,986 | | | | 233,874,361 | | | | 335,538,405 | |
| | | | | | | | | | | | | | | | | | | | |
Diluted | | | 230,381,122 | | | | 278,000,000 | | | | 278,051,299 | | | | 238,000,199 | | | | 337,885,258 | |
| | | | | | | | | | | | | | | | | | | | |
4
| (1) | Other operating income and expenses consist of research and hiring or training related government subsidies and the costs incurred related to such subsidies. Such income and expenses were recorded in other income and cost of revenues or general and administrative expenses, respectively in 2009, 2010 and the first half of 2011. Since the third quarter of 2011, as a result of an accounting policy change, the research and hiring or training related government subsidies were reclassified to other operating income, the costs related to research related subsidies were reclassified from cost of revenues to other operating expenses, and expenses related to hiring or training related subsidies continue to be recorded in general and administrative expenses. Although ASC 250 requires a retrospective application, management determined that a prospective application is deemed reasonable due to the insignificant impact on prior periods. See “—Critical Accounting Policies—Government Subsidies.” |
| (2) | Each ADS represents 18 ordinary shares. |
| (3) | In May 2008, we effected a bonus share issuance in the form of a share split by issuing an additional approximately 20,800 ordinary shares for each ordinary share outstanding and an additional approximately 20,800 preferred shares for each preferred share outstanding. As a result of the bonus share issuance, the number of ordinary shares and preferred shares increased to 208,005,986 and 69,994,014, respectively. The effect of the bonus share issuance is retroactively reflected for all periods presented herein. |
| | | | | | | | | | | | | | | | | | | | |
| | | As of December 31, | |
| | | 2007 | | | 2008 | | | 2009 | | | 2010 | | | 2011 | |
| | | (in millions of US$) | |
Consolidated Balance Sheet Data: | | | | | | | | | | | | | | | | | | | | |
Cash and cash equivalents | | | 19.6 | | | | 15.8 | | | | 12.2 | | | | 49.2 | | | | 37.8 | |
Total current assets | | | 27.5 | | | | 28.7 | | | | 30.1 | | | | 73.1 | | | | 72.3 | |
Total assets | | | 45.3 | | | | 61.7 | | | | 70.0 | | | | 138.0 | | | | 166.9 | |
Total current liabilities | | | 11.5 | | | | 17.3 | | | | 15.5 | | | | 24.4 | | | | 33.9 | |
Total liabilities | | | 11.5 | | | | 17.3 | | | | 15.5 | | | | 24.4 | | | | 33.9 | |
Series A convertible preferred shares | | | 34.4 | | | | 34.4 | | | | 34.4 | | | | — | | | | — | |
Total equity (deficit) | | | (0.6 | ) | | | 10.0 | | | | 20.1 | | | | 113.5 | | | | 133.0 | |
Exchange Rate Information
We use U.S. dollars as our reporting currency in our consolidated financial statements and in this annual report. We conduct substantially all of our operations in China. A substantial portion of our sales are denominated in U.S. dollars, while a significant portion of our costs and expenses are denominated in Renminbi. This annual report contains translations of RMB into U.S. dollars at specific rates solely for the convenience of the reader. Unless otherwise noted, all translations from RMB to U.S. dollars and from U.S. dollars to RMB in this annual report were made at a rate of RMB6.2939 to US$1.00, the exchange rate on December 30, 2011 as set forth in the H.10 statistical release of the Federal Reserve Board. We make no representation that any RMB or U.S. dollar amounts could have been, or could be, converted into U.S. dollars or RMB, as the case may be, at any particular rate, at the rates stated below, or at all. The PRC government imposes control over its foreign currency reserves in part through direct regulation of the conversion of RMB into foreign currencies and through restrictions on foreign trade. On April 20, 2012, the certified exchange rate was RMB6.3080 to US$1.00.
5
The following table sets forth information concerning exchange rates between the RMB and the U.S. dollar for the periods indicated. These rates are provided solely for your convenience and are not necessarily the exchange rates that we used in this annual report or will use in the preparation of our periodic reports or any other information to be provided to you. The source of these rates is the Federal Reserve Statistical Release.
| | | | | | | | | | | | | | | | |
| | | Exchange Rate | |
Period | | Period End | | | Average(1) | | | Low | | | High | |
2007 | | | 7.2946 | | | | 7.5806 | | | | 7.8127 | | | | 7.2946 | |
2008 | | | 6.8225 | | | | 6.9193 | | | | 7.2946 | | | | 6.7800 | |
2009 | | | 6.8259 | | | | 6.8295 | | | | 6.8470 | | | | 6.8176 | |
2010 | | | 6.6000 | | | | 6.7603 | | | | 6.8330 | | | | 6.6000 | |
2011 | | | 6.2939 | | | | 6.4475 | | | | 6.6364 | | | | 6.2939 | |
October | | | 6.3547 | | | | 6.3710 | | | | 6.3825 | | | | 6.3534 | |
November | | | 6.3765 | | | | 6.3564 | | | | 6.3839 | | | | 6.3400 | |
December | | | 6.2939 | | | | 6.3482 | | | | 6.3733 | | | | 6.2939 | |
2012 | | | | | | | | | | | | | | | | |
January | | | 6.3080 | | | | 6.3119 | | | | 6.3330 | | | | 6.2940 | |
February | | | 6.2935 | | | | 6.2997 | | | | 6.3120 | | | | 6.2935 | |
March | | | 6.2975 | | | | 6.3145 | | | | 6.3315 | | | | 6.2975 | |
April (through April 20) | | | 6.3080 | | | | 6.3052 | | | | 6.3150 | | | | 6.2975 | |
Source: Federal Reserve Statistical Release
| (1) | Annual averages are calculated from month-end rates. Monthly averages are calculated using the average of the daily rates during the relevant period. |
B.Capitalization and Indebtedness
Not applicable.
C.Reasons for the Offer and Use of Proceeds
Not applicable.
D.Risk Factors
Risks Related to Our Business and Industry
Our ability to execute projects, maintain, expand or renew existing customer engagements and obtain new customers depends largely on our ability to attract, train, motivate and retain skilled scientists.
Our success largely depends on the R&D efforts of our skilled scientists and their ability to keep pace with changes in pharmaceutical and biotechnology R&D technologies and methodologies. In particular, our customers value highly skilled Western-trained scientists, preferably with large pharmaceutical and/or biotechnology company experience. Our success also depends on the depth and quantity of our scientific personnel. We face challenges in attracting new employees and maintaining consistent quality standards throughout our employee base at our current growth rate. Our employee base increased from 1,676 as of December 2008 to 2,076 as of December 31, 2011.
We compete with pharmaceutical, biotechnology and contract research firms and academic and research institutions for qualified and experienced scientists, particularly in chemistry and biology. To effectively compete, we may be required to offer higher compensation and other benefits that could materially and adversely
6
affect our financial condition and results of operations. We may be unable to hire and retain enough skilled and experienced scientists to replace those who leave our company. Additionally, we may be unable to redeploy and retrain our professionals to keep pace with technological changes, evolving standards and changing customer preferences. Any inability to attract, train, motivate or retain skilled scientists may materially and adversely affect our business, financial condition, results of operations and prospects.
A limited number of our customers have accounted for and are expected to account for a high percentage of our revenues. The loss or significant reduction in orders from any of these customers could materially and adversely affect our business, financial condition, results of operations and prospects.
Our two largest customers in 2009 and 2010 accounted for 27% and 10% of our net revenues in 2009 and 21% and 8% of our net revenues in 2010, respectively. Our two largest customers in 2011 accounted for 18% and 11% of our net revenues in 2011, respectively. No other customer accounted for more than 10% of our net revenues in 2009, 2010 or 2011. Our top ten customers, which varied in each of the last three years, accounted for approximately 65%, 63% and 62% of our net revenues in 2009, 2010 and 2011, respectively. Furthermore, we generated a significant majority of our net revenues during the same periods from sales to customers located in the United States.
Due to our customer concentration, any of the following events, among others, may cause material revenue fluctuations or declines and materially and adversely affect our business, financial condition, results of operations and prospects:
| | • | | order or contract reduction, delay or cancellation by one or more of our significant customers and our failure to identify and acquire additional or replacement customers; and |
| | • | | a substantial reduction by one or more of our significant customers in the price they are willing to pay for our services and products. |
Any failure to retain our existing customers or expand our customer base may result in our inability to maintain or increase our revenues.
Our existing customers may not continue to generate significant revenues for us once our engagements with them are concluded and our relationships with them may not present further business opportunities. We have entered into master services agreements with some customers, the terms of which typically range from one to five years. If we fail to build new customer relationships or our relationships with existing customers terminate, we may not be able to maintain or increase our revenues.
A reduction in R&D budgets by pharmaceutical and biotechnology companies may result in a reduction or discontinued use of our services, which may adversely affect our business.
Fluctuations in the R&D budgets of pharmaceutical and biotechnology industry participants could significantly affect the demand for our services. R&D budgets fluctuate due to, among other things, pharmaceutical and biotechnology industry downturns, consolidation of pharmaceutical and biotechnology companies, general economic conditions, and changes in available resources, spending priorities and institutional budgetary policies. In addition, the global pharmaceutical and biotechnology industry has experienced significant consolidation in recent years. If pharmaceutical and biotechnology companies discontinue or decrease the use of our services due to factors such as slowdown in the overall U.S. or global economy or further industry consolidation, our revenues and earnings could be lower than we expect and our revenues may decrease or fail to grow at historical rates.
The outsourcing trend in the drug R&D industry may decrease, which could slow our growth.
The success of our business depends primarily on the number of contracts and the size of the contracts that we obtain from pharmaceutical and biotechnology companies. Over the past several years, our business has benefited from increased levels of outsourcing of drug R&D by pharmaceutical and biotechnology companies.
7
While the outsourcing trend is expected to continue for the foreseeable future, a reversal or slowing of this trend could result in diminished growth in our expected growth areas and materially adversely affect our business, financial condition, results of operations and prospects.
We have experienced and may continue to experience declining gross margin as a result of multiple factors, including appreciation of the Renminbi against the U.S. dollar, rising labor costs in China, increased depreciation and rental expenses related to our expanded facilities and equipments, and competition.
A number of factors have negatively impacted and may continue to adversely affect our gross margin. The reporting currency of our holding company, ShangPharma Corporation, is the U.S. dollar. A significant majority of our revenues are denominated in U.S. dollars and most of our costs and expenses are denominated in Renminbi. Appreciation in the value of the Renminbi relative to the U.S. dollar would increase our cost of revenues reported in U.S. dollar terms without giving effect to any underlying change in our business or results of operations. The overall rising labor costs in China and increased depreciation and rental expenses related to our expanded facilities and equipments also increases our cost of revenues. In addition, we face considerable pricing pressure on our service offerings due to intense competition both domestically and internationally and from other contract research organizations and the in-house R&D facilities in China founded by major pharmaceutical and biology companies. Primarily as a result of the foregoing, our gross margin decreased from 33.4% in 2010 to 32.3 % in 2011, and we may continue to experience declining gross margin.
If we fail to protect the intellectual property rights of our customers, we may be subject to liability for breach of contract and may suffer damage to our reputation.
Protection of intellectual property associated with pharmaceutical and biotechnology R&D services is critical to all our customers. Our customers generally retain ownership of associated intellectual property rights, including those they provide to us and those arising from the services we provide. Our success therefore depends in substantial part on our ability to protect the proprietary rights of our customers. This is particularly important for us because almost all of our operations are based in China, which has not traditionally enforced intellectual property protection to the same extent as in the United States. Despite measures we take to protect our customers’ and our own intellectual property, unauthorized parties may attempt to obtain and use information that we regard as proprietary. Any unauthorized disclosure of our customers’ proprietary information could subject us to liability for breach of contract and significantly damage our reputation, which could materially harm our business, financial condition, results of operations and prospects.
Any failure to comply with applicable regulations and industry standards or obtain various licenses and permits could harm our reputation and our business, results of operations and prospects.
A number of governmental agencies or industry regulatory bodies in China, the United States and Europe, impose strict rules, regulations and industry standards governing pharmaceutical and biotechnology R&D activities, which apply to our customers and us. Our failure to comply with such regulations could result in the termination of ongoing research, administrative penalties imposed by regulatory bodies or the disqualification of data for submission to regulatory authorities. This could harm our reputation, prospects for future work and operating results. For example, if we were to treat research animals inhumanely or in violation of international standards set out by the Association for Assessment and Accreditation of Laboratory Animal Care, or AAALAC, AAALAC could revoke any such accreditation and the accuracy of our animal research data could be questioned.
In addition, our subsidiaries in China need to obtain various licenses and permits to conduct their current operations. If any of our subsidiaries in China is unable to obtain all the requisite licenses and permits or fails to renew or update any of the licenses or permits timely, it may be subject to fines and other penalties, including suspension of its operations.
8
Compliance with environmental regulations can be expensive, and noncompliance with these regulations may result in significant monetary damages, fines and other penalties.
As our pharmaceutical and biotechnology R&D processes generate waste water, toxic and hazardous substances and other industrial wastes, we must comply with all national and local environmental laws and regulations in China. We are required to undertake environmental impact assessment procedures and pass the subsequent inspection and approval procedures before commencing our operations. We are also required to register with, or obtain approvals from, relevant environmental protection authorities for various environmental matters such as discharging waste generated in our operations. We have not completed certain environmental assessment or approval procedures for some of our facilities. We have taken remedial measures to obtain the required approvals. We had received approvals for environmental impact assessments report for our principal facilities, and are now applying for the inspection and approval of such facilities. According to relevant PRC regulations, the local environmental protection authorities are required to complete the inspection and approval procedures within several months upon receipt of an application for such inspection and approval. However, we cannot assure you that the remedial measures we are taking will be completed timely and successfully. If for any reason the relevant government authorities in China determine that we are not in compliance with environmental laws and regulations, we may be required to pay fines or damages to third parties or suspend our operations. In addition, because the requirements imposed by environmental laws and regulations may change and more stringent regulations may be adopted, we may be unable to accurately predict the cost of complying with these laws and regulations, which could be substantial.
We are subject to safety and health laws and regulations in China, and any failure to comply could adversely affect our operations.
Our operations and facilities are subject to extensive safety and health laws and regulations. We must ensure that our operations and facilities comply with PRC standards and requirements on fire prevention, hazardous and radioactive materials handling, work environment safety and employees’ health conditions. We have not completed certain procedures for some of our facilities or projects required for compliance with these laws and regulations, such as having our relevant work environments inspected for occupational hazards and preparing and filing annual evaluation report in respect of radioisotope substance. We are taking remedial measures to rectify such non-compliance. For example, we have had our work areas inspected for occupational hazards. However, we may be subject to government orders to rectify non-compliance within a specified period, pay fines or suspend our operations. In addition, we cannot eliminate the risk of accidental contamination or injury from hazardous or radioactive materials used in our operations. If we fail to prevent contamination or injury, we could be liable for any resulting damages, which may materially and adversely impact our business, financial condition, results of operations and prospects.
Any failure by us to satisfy our customers’ audits and inspections could harm our reputation and our business, financial condition, results of operations and prospects.
Our customers routinely audit and inspect our facilities, processes and practices to ensure that we meet their standards for drug discovery and development. To date, we are not aware of any material negative findings from our customers’ audits and inspections. However, we may not be able to pass all such audits and inspections to our customers’ satisfaction, and any such failure may significantly harm our reputation and materially and adversely affect our business, financial condition, results of operations and prospects.
We face increasingly intense competition. If we do not compete successfully against new and existing competitors, demand for our services and related revenues may decrease and we may be subject to increasing pricing pressure.
The global pharmaceutical and biotechnology R&D outsourcing market is highly competitive, and we expect competition to intensify. We face competition based on several factors, including the quality and scope of services, the ability to protect confidential information and intellectual property, the ability to be responsive and efficient with respect to customers’ requests, depth of customer relationship and pricing. We compete with WuXi
9
PharmaTech (Cayman) Inc., or WuXi PharmaTech, across the breadth of our service offerings and compete with some local contract research companies in China in various service areas. We also compete with industry participants in particular service areas, including Albany Molecular Research, Inc. in discovery chemistry and pharmaceutical development, and Cerep S.A. and Covance Inc. in discovery biology and preclinical development.
We expect to increasingly compete against multinational companies, both domestically and internationally, as we offer more complex and sophisticated services. Additionally, several major pharmaceutical and biotechnology companies have made in-house R&D investments in China, a trend we expect to continue. These in-house investments may result in increased competition for qualified personnel. Some of our larger competitors may have greater financial, research and other resources, broader scope of services, greater pricing flexibility, more extensive technical capabilities and greater name recognition than we do. Furthermore, consolidation within the global pharmaceutical and biotechnology R&D outsourcing markets may create stronger competitors than those we are facing today.
We also expect increased competition as new companies enter into our market and more advanced technologies become available. Our services and expertise may be rendered obsolete or uneconomical by technological advances or new approaches or technologies. Our competitors’ existing or new approaches or technologies they develop may be more effective than those we develop. Furthermore, increased competition may subject us to increasing pricing pressure and reduce demand for our services, which could reduce our margins and profitability.
We may fail to effectively expand and market our service offerings and capabilities or create demand for our newly expanded services, which may harm our growth opportunities and prospects, possibly resulting in related losses.
We intend to expand our existing services and offer new services across the drug discovery value chain. Initially focusing on discovery chemistry services, we introduced pharmaceutical development services in 2005 and discovery biology and preclinical development services in 2007. We began offering biologics services in early 2010 which has experienced rapid growth during 2011. In September 2011, we formed our first strategic partnership with Jiangsu Hengrui Medicine Co., Ltd. for the development of novel therapeutic monoclonal antibodies, under which we are heading pre-clinical research activities. We plan to construct an approximately 460,000 square-foot cGMP-quality multi-purpose manufacturing facility in Fengxian, Shanghai designed to manufacture advanced intermediates and active pharmaceutical ingredients, or APIs, for preclinical testing and clinical trials. We also plan to construct an approximately 150,000 square-foot office and laboratory services building in Fengxian, Shanghai primarily to expand our pharmaceutical development services. To successfully develop and market our new services, we must:
| | • | | accurately assess and meet customer needs and market demands; |
| | • | | optimize our drug discovery and development processes to predict and control costs; |
| | • | | hire, train and retain scientists and other personnel; |
| | • | | provide services in a timely manner; |
| | • | | increase customer awareness and acceptance of our services; |
| | • | | obtain required regulatory clearances or approvals; |
| | • | | compete effectively with other R&D outsourcing providers; |
| | • | | price our services competitively; and |
| | • | | effectively integrate customer feedback into our business planning. |
If we are unable to expand our service offerings or create demand for those newly expanded services, our business, results of operations, financial condition and prospects may be materially and adversely affected.
10
We may be unable to expand our capacity and scale up our operations as anticipated, possibly resulting in material delay, increased costs and lost business opportunities.
We plan to construct an approximately 460,000 square-foot cGMP-quality multi-purpose manufacturing facility in Fengxian, Shanghai designed to manufacture advanced intermediates and APIs for preclinical testing and clinical trials. Phase A of the multi-purpose manufacturing facility has been completed and commenced its operations in January 2011. The construction and development of the rest of the manufacturing facility will start when demanded by our future business growth. We also plan to construct an approximately 150,000 square-foot office and laboratory services building in Fengxian, Shanghai primarily for pharmaceutical development services. Approximately 32,000 square-foot of the laboratory service building has been completed and went into operation in November 2011 and the construction of the rest of the areas will start when demanded by our future business growth. In April 2011, Chengdu Chempartner Co., Ltd., our subsidiary in Chengdu moved into a 68,000 square-foot new lab and office building which replaced the original 36,000 square foot facility. In November 2011, we took over Charles River’s lease for a research facility, which provides us with an additional approximately 32,000 square-foot in-vivo research facility and approximately 10,000 square-foot lab and office space. Also, we opened an approximately 105,000 square-foot laboratory and office facility in Zhangjiang, Shanghai in December 2011, which is dedicated to supporting discovery services for one of our largest customers. We may lease or build additional animal facilities in Shanghai if the demand for our discovery biology and preclinical development services increases. Any material delay in bringing these facilities online or scaling up operations or any substantial cost increases to complete them or scale up operations could materially and adversely affect our financial condition and results of operations and result in lost business opportunities.
If we fail to effectively manage our anticipated growth and execute our growth strategies, our business, financial condition, results of operations and prospects could suffer.
Pursuing our growth strategies, including integrating and expanding our facilities and service offerings to meet our customers’ needs, has resulted in and will continue to place substantial demands on our management and resources. Managing this growth and executing our growth strategies will require, among other things:
| | • | | enhancement of our pharmaceutical and biotechnology R&D capabilities; |
| | • | | effective coordination and integration of our research facilities and teams, particularly those located in different or newly opened facilities; |
| | • | | successful personnel hiring and training; |
| | • | | effective cost controls and sufficient liquidity; |
| | • | | effective and efficient financial and management controls; |
| | • | | increased marketing and sales support activities; |
| | • | | effective quality control; and |
| | • | | our ability to manage our various vendors and suppliers and leverage our purchasing power. |
Any failure to effectively manage our anticipated growth and execute our growth strategies could materially adversely affect our business, financial condition, results of operations and prospects.
If our company or our founders grant employees share options and other share-based compensation in the future or modify any existing share-based awards, our net income may be adversely affected.
Our equity incentive plans and other similar types of incentive plans are important in order to attract and retain key personnel. As of March 31, 2012, our company and our founders have granted our employees share options in the past pursuant to our 2008 Equity and Performance Incentive Plan, or the 2008 Plan, and restricted share units pursuant to our Founder’s 2008 Equity and Performance Incentive Plan, or the 2008 Founder’s Plan, our 2010 Share Incentive Plan, or the 2010 Plan, and our 2011 Share Incentive Plan, or the 2011 Plan. As a result of the issuance of options and restricted share units, or RSUs, under these plans, we have in the past incurred and
11
expect in the future to incur share-based compensation expenses. We account for compensation costs for all share options, including share options granted to our employees, non-employee directors and officers using the fair value determined at the grant date and recognize the expense in our consolidated statement of operations. We also account for compensation costs for the conversion of RSUs into our ordinary shares. The compensation costs arising from the grant of options and restricted shares and from the modification of any existing share-based awards may materially and adversely affect our net income. Moreover, the additional expenses associated with share-based compensation may reduce the attractiveness of our equity incentive plans.
In providing our pharmaceutical and biotechnology R&D outsourcing services, we face health and safety liability and product liability risks.
In providing our services in connection with drug discovery and development, we face a range of potential liabilities, which include risks that disease models and animals infected with diseases for research interests may be harmful, or even lethal, to themselves and humans despite preventive measures we take for the quarantine and handling of animals. We also face product liability risks should the drugs we assist in developing and manufacturing become subject to product liability claims. We provide services in the development, testing and manufacturing of drugs that may ultimately be used by humans, although we do not commercially market or sell the products to end users. If any of these drugs harm people, we may be subject to litigation and may be required to pay damages to those persons.
We do not have general liability or product liability insurance covering our whole business or all of our products and services. We currently maintain general liability insurance for five contracts covering, among other things, bodily injury and property damage arising out of the products or services provided under these contracts. The aggregate net revenues derived from these contracts in 2011 accounted for 16% of our total net revenues in this period. The aggregate maximum coverage amount under the insurance policy for these contracts is US$5 million for bodily injury and property damage arising out of our products or services and economic loss sustained by persons or entities due to deficiencies in our products or services. Damages awarded in a product liability action could be substantial and, to the extent not covered by our insurance, could materially and adversely impact our business, financial condition and results of operations.
Because many of our fee-for-service based contracts are contingent on successful completion and are of a fixed price nature, we may bear risk if we do not successfully or timely develop a service or enter contracts with pricing below our estimated cost due to competitive pressures or strategic objectives, or incur overrun costs.
A significant portion of our net revenues is based on fee-for-service contracts, which are contingent on successful completion and are often for a fixed price. Therefore, we bear financial risk if we do not successfully or timely complete services or if we price our fee-for-service based contracts below our estimated cost of completing these contracts or otherwise incur cost overruns. We also face pricing pressures from some of our competitors. To capture market share, we have, in the past, intentionally priced some fee-for-service based contracts below our estimated cost of completing these contracts. Below-cost pricing or significant cost overruns could materially and adversely affect our business, financial condition, results of operations and prospects.
The loss of services of our senior management and key scientific personnel could severely disrupt our business and growth.
Our success significantly depends upon the continued service of our senior management and key scientific personnel. We are highly dependent on Mr. Michael Xin Hui, our co-founder and chief executive officer, who has managed our business, operations and sales and marketing activities and maintained personal and direct relationships with our major customers since our inception, as well as other members of our management and other key scientific personnel. The loss of any one of them, in particular Mr. Hui, would materially and adversely affect our business. Although each member of our senior management and key scientific personnel has signed a noncompete agreement with us, we may be unable to successfully enforce these provisions in the event of a dispute. If we lose the services of any of our senior management members or key scientific personnel, we may be
12
unable to locate suitable or qualified replacements, and may incur additional expenses to recruit and train new personnel, which could severely disrupt our business and growth.
A payment failure by any of our large customers could adversely affect our cash flows and profitability.
Historically, we have not experienced any significant bad debt or collection problems, but such problems may arise in the future. The failure of any of our customers to make timely payments could require us to write off accounts receivable or increase provisions made against our accounts receivable, either of which could adversely affect our cash flows and profitability.
Our limited operating history may make it difficult for you to evaluate our business and future prospects.
We commenced operations and began offering our pharmaceutical and biotechnology R&D outsourcing services in 2002. Our business model changes with the evolution of the pharmaceutical and biotechnology R&D outsourcing market in China and around the world. Accordingly, our operating history upon which you can evaluate the viability and sustainability of our business and its acceptance by industry participants is limited. These circumstances may make it difficult for you to evaluate our business and future prospects, and you should not rely on our past results or our historic growth rate as an indication of our future performance.
Failure to achieve and maintain effective internal controls over financial reporting could cause us to inaccurately report our financial result or fail to prevent fraud and have a material adverse effect on our business, results of operations and the trading price of our ADSs.
We are subject to the reporting obligations under U.S. securities laws. Section 404 of the Sarbanes-Oxley Act of 2002 and related rules require public companies to include a report of management on their internal control over financial reporting in their annual reports. This report must contain an assessment by management of the effectiveness of a public company’s internal control over financial reporting. In addition, an independent registered public accounting firm for a public company must attest to and report on management’s assessment of the effectiveness of our internal control over financial reporting. We sometimes hire a professional consultant to assist us in such efforts. Our efforts to implement standardized internal control procedures and develop the internal tests necessary to verify the proper application of the internal control procedures and their effectiveness are a key area of focus for our board of directors, our audit committee and senior management.
Our management has concluded that our internal control over financial reporting was effective as of December 31, 2011. See “Item 15. Controls and Procedures.” Our independent registered public accounting firm has issued an attestation report, which concludes that our internal control over financial reporting was effective in all material aspects as of December 31, 2011.However, if we fail to maintain effective internal control over financial reporting in the future, our management and our independent registered public accounting firm may not be able to conclude that we have effective internal control over financial reporting at a reasonable assurance level. Moreover, effective internal controls over financial reporting are necessary for us to produce reliable financial reports and are important to help prevent fraud. As a result, our failure to achieve and maintain effective internal controls over financial reporting could in turn result in the loss of investor confidence in the reliability of our financial statements and negatively impact the trading price of our ADSs. Furthermore, we have incurred and anticipate that we will continue to incur considerable costs, management time and other resources in an effort to continue to comply with Section 404 and other requirements of the Sarbanes-Oxley Act.
Our business, financial condition and results of operations may be adversely affected by the downturn in the global or Chinese economy.
The global financial markets experienced significant disruptions in 2008 and the United States, Europe and other economies went into recession. The recovery from the lows of 2008 and 2009 was uneven and it is facing new challenges, including the escalation of the European sovereign debt crisis since 2011. It is unclear whether the European sovereign debt crisis will be contained and what effects it may have. There is considerable uncertainty over the long-term effects of the expansionary monetary and fiscal policies that have been adopted by
13
the central banks and financial authorities of some of the world’s leading economies, including China’s. Economic conditions in China are sensitive to global economic conditions. Although we are uncertain about the extent to which the global financial market disruption and slowdown of the Chinese economy may impact our business in the long term, there is a risk that our business, results of operations and prospects would be materially and adversely affected by the global economic downturn and the slowdown of the Chinese economy.
Our customer agreements contain provisions that are adverse to our interests or expose us to potential liability.
Our agreements with our customers generally provide that customers can terminate the agreements or reduce the scope of services under the agreements upon short notice. In a majority of our customer agreements, our customers have the unilateral right to terminate for convenience upon one to three months’ prior notice. Although we have not been materially and adversely affected by previous contract cancellations or modifications, if a customer terminates a contract with us, we are only entitled under the terms of the contract to receive revenue earned until the date of termination. Therefore, cancellation or modification of a large contract or cancellation or modification of multiple contracts could materially and adversely affect our future business, financial condition, results of operations and prospects.
In certain of our customer agreements, we have assumed indemnification obligations for intellectual property infringement resulting from the misuse of confidential information by our employees or from the customer deliverables that we provide to the extent that we create the infringing aspect of the deliverables. As a result, we could be potentially exposed to substantial liability.
In addition, in some of our customer agreements, we agree, either by ourselves or together with third parties, not to compete with the customer. In some cases, we are required to seek the customer’s prior written consent before working for other customers on similar projects. Complying with these noncompete obligations may restrict our ability to expand certain service offerings, and failure to comply could significantly harm our business and reputation, as well as expose us to liability for breach of contract.
We use a limited number of suppliers, including a former related party, Labpartner (Shanghai) Co., Ltd., for several of our service offerings, which, if interrupted, could disrupt or delay our services, reduce our sales and force us to use more expensive supply sources.
We use a limited number of sources for our supply of certain reagents and other chemicals required in our product and service offerings. In particular, we purchased significant amounts of reagents and other chemicals from Labpartner (Shanghai) Co., Ltd., or Labpartner, a former related party, in each of 2009, 2010 and 2011. Disruptions or delays with respect to our suppliers may arise from health problems, export or import restrictions or embargoes, foreign government or economic instability, severe weather conditions, disruptions to the air travel system, contract disputes or other disruptions. If the supply of certain materials were interrupted, our services may be delayed. We also may not be able to secure alternative supply sources in a timely and cost-effective manner. If we are unable to obtain adequate supplies of required materials that meet our standards or at acceptable costs, or at all, our ability to accept and fulfill customer orders in the required quality and quantity and at the required time could be restricted, which in turn may materially and adversely affect our business, financial condition, results of operations and prospects.
We have limited insurance coverage, and any claims beyond our insurance coverage may result in our incurring substantial costs and a diversion of resources.
We maintain property insurance policies covering physical damage or loss of our equipment and office furniture, employer’s liability insurance generally covering death or work injury of employees, public liability insurance covering third party bodily injury and property damage incurred in connection with our business in China, and directors and officers liability insurance. We do not have general liability or product liability insurance covering our whole business or all of our products and services. We currently maintain general liability
14
insurance for five contracts covering, among other things, bodily injury and property damage arising out of the products or services provided under these contracts. The aggregate net revenues derived from these contracts in 2011 accounted for 16% of our total net revenues in this period. The aggregate maximum coverage amount under the insurance policy for these contracts is US$5 million for bodily injury and property damage arising out of our products or services and economic loss sustained by persons or entities due to deficiencies in our products or services. We do not maintain business disruption insurance or key man life insurance. Any business disruption or litigation, or any liability or damage to, or caused by, our facilities or our personnel beyond our insurance coverage may result in substantial costs and a diversion of resources.
Our quarterly revenues and operating results may be difficult to predict and could fall below investor expectations, which could cause the market price of our ADSs to decline.
Our quarterly revenues and operating results have fluctuated in the past and may continue to fluctuate significantly due to numerous factors, including:
| | • | | the commencement, postponement, completion or cancellation of large contracts; |
| | • | | the progress of ongoing contracts; |
| | • | | the delivery schedule of our customers; |
| | • | | budget cycles of our customers; |
| | • | | changes in the mix of our revenues from our services based on a fee-for-service or full-time-equivalent, or FTE, basis or the percentage of our fee-for-service based contracts that are contingent upon successful completion; |
| | • | | changes in the industry operating environment; |
| | • | | changes in government policies or regulations or their enforcement; |
| | • | | exchange rate fluctuations; |
| | • | | a downturn in general economic conditions in the United States, China or internationally; and |
| | • | | the timing of and charges associated with completed acquisitions or other events. |
Many of these factors are beyond our control, making our quarterly results fluctuate and difficult to predict. Fluctuations in our quarterly revenues and operating results could cause the trading price of our ADSs to decline below investor expectations.
We may need additional capital that we may be unable to obtain in a timely manner or on acceptable terms, or at all.
We may require additional capital in order to grow, remain competitive, develop new services and expand our capacity. Our ability to obtain capital is subject to a variety of uncertainties, including:
| | • | | our financial condition, results of operations and cash flows; |
| | • | | general market conditions for capital raising activities by healthcare and related companies; and |
| | • | | economic, political and other conditions in China, the United States and elsewhere. However, financing may not be available in amounts or on terms acceptable to us, if at all. |
Our capital needs may require us to sell additional equity or debt securities that may dilute our shareholders or introduce covenants that may restrict our operations or our ability to pay dividends.
Our capital needs and other business reasons could require us to sell additional equity or debt securities or obtain additional credit facilities. The sale of additional equity or equity-linked securities could dilute our
15
shareholders. The incurrence of indebtedness would increase our debt service obligations and could result in operating and financing covenants that would restrict our operations or our ability to pay dividends.
Acquisitions or investments could disrupt our ongoing business, distract our management and employees, increase our expenses and adversely affect our business.
We anticipate that a portion of any future growth of our business might be accomplished by acquiring businesses, products or technologies. The success of any acquisition will depend upon, among other things, our ability to integrate acquired personnel, operations, products and technologies into our organization effectively, to retain and motivate key personnel of the acquired businesses and to retain their clients. In addition, we might not be able to identify suitable acquisition opportunities or obtain any necessary financing on acceptable terms. We might also spend time and money investigating and negotiating with potential acquisition or investment targets, but not complete a transaction. Any acquisition could involve other risks, including the assumption of additional liabilities and expenses, issuances of potentially dilutive securities or interest-bearing debt, transaction costs, reduction in our ADS price as a result of any of these or because of market reaction to a transaction and diversion of management’s attention from other concerns.
Recent healthcare reform creates uncertainty in the need for our services.
In March 2010, the United States Congress passed the Patient Protection and Affordable Care Act. Implementation of this act, which is intended to control health care costs, may limit profits from the development of new drugs. This in turn may decrease research and development expenditures by pharmaceutical and biotechnology companies. Similar reform movements have occurred or may occur in parts of Europe and Asia. As a result, these reforms could result in decrease in the potential business opportunities available to us both in the United States and in other countries and create uncertainty in the need for our services.
Negative attention from special interest groups may impair our ability to operate our business efficiently.
Some of the services we provide, or intend to provide, involve the use of large and small animals, as well as non-human primates. Certain special interest groups categorically object to the use of animals for research purposes. Historically, our core research model activities with small animals have not been the subject of animal rights media attention, and as we expand our service biology offerings into non-GLP toxicology, we anticipate that we will work extensively with large animals and non-human primates. However, research activities with animals have been the subject of adverse attention, negatively impacting our industry. Any negative attention or threats directed against our animal research activities could impair our ability to operate our business efficiently. In addition, if regulatory authorities were to mandate a significant reduction in safety testing procedures that utilize laboratory animals, as has been advocated by certain groups, our business could be materially and adversely affected.
Contamination in our animal populations can distort or compromise the quality of our research results, cause human infection, increase costs and decrease revenue.
Our research models must be free of certain infectious agents such as certain viruses and bacteria. The presence of these infectious agents in our animal facilities could distort or compromise the quality of our research results and could adversely impact human or animal health. Removing or preventing contamination typically requires cleaning, renovating, disinfecting, retesting and restarting our animal facilities, which result in clean-up and start-up costs and reduced revenue due to lost customer confidence in the accuracy of our research results. In some cases, we may import animals carrying infectious agents capable of causing diseases in humans. As a result, there could be a possible risk of human exposure and infection. Although we have not had serious contamination in the past, contamination may occur in the future, which could materially and adversely impact our financial results.
16
For our customers’ future drugs to be marketed in the United States, we may need to obtain clearance from the FDA and our operations will need to comply with FDA standards. Any adverse action by the FDA against us would negatively impact our ability to offer our services and harm our business and prospects.
As we expand our service offerings, we may need to obtain clearance by the U.S. Food and Drug Administration, or FDA, in the event that our customers’ clinical trials reach the stage of filing a New Drug Application, or NDA, with the FDA, to grant permission to market the drug in the United States. All facilities and manufacturing techniques used to manufacture drugs and biologics in the United States must conform to standards established by the FDA. The FDA may conduct scheduled periodic inspections of our facilities to monitor our compliance with regulatory standards. If the FDA finds that we have failed to comply with the appropriate regulatory standards, it may impose fines or take other actions against us or our customers, or we may no longer be able to offer our services to U.S. customers. The resulting corrective measures may be lengthy and costly. As a result, we may be unable to fulfill our contractual obligations. Any adverse action by the FDA would materially and adversely impact our reputation and our business, financial condition, results of operations and prospects. We may or may not obtain clearance from FDA standards in the event that we are inspected, or maintain such clearance over time.
New technologies or methodologies may be developed, validated and increasingly used in the global pharmaceutical and biotechnology R&D outsourcing industry that could reduce demand for our services.
The global pharmaceutical and biotechnology R&D outsourcing industry is evolving, and we must keep pace with new technologies and methodologies in the industry to maintain our competitive position. As a result, we must invest significant human and capital resources in R&D to enhance our technology and our existing services and introduce new services utilizing advanced technologies. However, we may not be successful in adapting to or commercializing these new technologies if developed. New technologies could decrease the need for our existing technologies, and we may not be able to develop new service or technologies effectively or in a timely manner. Our failure to develop, enhance or adapt, to new technologies and methodologies could significantly reduce demand for our services and harm our business and prospects.
Our principal facilities may be vulnerable to natural disasters, other unforeseen catastrophic events or failure of information technology and communications systems.
We conduct our operations primarily at our headquarters in Shanghai Zhangjiang Hi-Tech Park and our R&D center in Chengdu. We have animal facilities in Shanghai Zhangjiang Hi-Tech Park and Fengxian, Shanghai. We commenced operations on the initial phase of Phase A of a cGMP-quality multiple-purpose manufacturing facility located in Fengxian, Shanghai in January 2011. In addition, we are developing an approximately 150,000 square-foot office and laboratory services building in Fengxian, Shanghai primarily for pharmaceutical development services. Approximately 32,000 square-foot of the laboratory service building has been completed and went into operation in November 2011 and the construction of the rest of the areas will start when demanded by our future business growth. We moved into a 68,000 square foot new lab and office building in Chengdu in April 2011. The assumed lease from Charles River in November 2011 provided us with an additional approximately 32,000 square-foot in-vivo research facility and approximately 10,000 square-foot lab and office space. We depend on our facilities for our business. Natural disasters, other unanticipated catastrophic events or failure of information technology and communications systems including power interruptions, water shortage, storms, fires, earthquakes, terrorist attacks, wars, telecommunications failures, computer viruses, hacking or other attempts to harm our computer systems, could significantly impair our ability to operate our business in its ordinary course. Our facilities and certain equipment located in these facilities would be difficult to replace in any such event and could require substantial replacement time. The occurrence of any such event may materially and adversely affect our business, financial condition, results of operations and prospects.
17
We face risks related to health epidemics and other outbreaks, which could significantly disrupt our staffing and may even result in temporary closure of our facilities.
Our business could be materially and adversely affected by the outbreak of avian influenza, severe acute respiratory syndrome, or SARS, or another epidemic. In April 2009, a new strain of influenza A virus subtype H1N1 was discovered in North America and quickly spread to other parts of the world, including China. In early June 2009, the World Health Organization declared the outbreak to be a pandemic, while noting that most of the illnesses were of moderate severity. The PRC Ministry of Health has reported a few hundred deaths caused by the influenza A (H1N1). Any outbreak of avian influenza, SARS, the influenza A (H1N1) or other adverse public health developments in China may materially and adversely affect our business operations. These occurrences could severely disrupt to our daily operations.
Risks Related to Doing Business in China
Fluctuations in exchange rates may materially and adversely affect your investment.
The conversion of Renminbi into foreign currencies, including U.S. dollars, is based on rates set by the People’s Bank of China. The PRC government allowed the Renminbi to appreciate by more than 20% against the U.S. dollar between July 2005 and July 2008. Between July 2008 and June 2010, this appreciation was halted and the exchange rate between the Renminbi and the U.S. dollar remained within a narrow band. As a consequence, the RMB fluctuated significantly during that period against other freely traded currencies, in tandem with the U.S. dollar. Since June 2010, the PRC government has allowed the Renminbi to appreciate slowly against the U.S. dollar again. It is difficult to predict how market forces or PRC or U.S. government policy may impact the exchange rate between the Renminbi and the U.S. dollar in the future. There remains significant international pressure on the Chinese government to adopt a substantial liberalization of its currency policy, which could result in further appreciation in the value of the RMB against the U.S. dollar.
The reporting and functional currency of ShangPharma Corporation is the U.S. dollar. Our offshore subsidiaries’ functional currency is the U.S. dollar. However, the functional currency of our PRC subsidiaries is the Renminbi. A significant majority of our revenues are denominated in U.S. dollars and most of our costs and expenses are denominated in Renminbi. The net proceeds from our initial public offering were denominated in U.S. dollars.
Appreciation or depreciation in the value of the Renminbi relative to the U.S. dollar would affect our financial results reported in U.S. dollar terms without giving effect to any underlying change in our business or results of operations. Fluctuations in the exchange rate will also affect the relative value of earnings from and the value of any U.S. dollar-denominated investments we make.
Limited hedging options are available in China to reduce our exposure to exchange rate fluctuations. We began hedging our U.S. dollar exposure using forward contracts from the second half of 2008 based in part on our expected monthly revenues. We may continue to enter similar or other types of hedging transactions in the future. However, the availability and effectiveness of these hedges may be limited and we may not be able to adequately hedge our exposure or at all. In addition, our currency exchange losses may be magnified by PRC exchange control regulations that restrict our ability to convert between the Renminbi and foreign currency. As a result, fluctuations in exchange rates may materially and adversely affect your investment.
Adverse changes in the political and economic policies of the PRC government could materially and adversely affect the overall economic growth of China, which could adversely affect our business.
Substantially all of our assets are located in China and all of our revenues are derived from our operations there. Accordingly, economic, political and legal developments in China significantly affect our business, financial condition, results of operations and prospects. The Chinese economy differs from the economies of most developed countries in many respects, including the amount of government involvement, level of
18
development, growth rate, control of foreign exchange and allocation of resources. While the Chinese economy has grown significantly in the past 30 years, the growth has been uneven across different periods, regions and economic sectors of China. We cannot assure you that the Chinese economy will continue to grow, or that any such growth will be steady and uniform, or that if there is a slowdown, such a slowdown will not have a negative effect on our business.
The PRC government also exercises significant control over China’s economic growth through the allocation of resources, controlling payment of foreign currency-denominated obligations, setting monetary policy and providing preferential treatment to particular industries or companies. In response to high GDP growth in recent years, beginning in early 2010, the People’s Bank of China started to take measures including increasing the statutory deposit reserve and the benchmark interest rate to lower the pace of growth of the Chinese economy. It is unclear whether the PRC government will continue such policies, or whether PRC economic policies will be effective in creating stable economic growth. Any further slowdown in the economic growth of China could reduce demand for our solutions and services, which could materially and adversely affect our business, our financial condition and results of operations.
Uncertainties with respect to the PRC legal system could adversely affect us.
We conduct our business primarily through our PRC subsidiaries. Our operations in China are governed by PRC laws and regulations. Our PRC subsidiaries are foreign-invested enterprises and are subject to laws and regulations applicable to foreign investment in China and, in particular, laws applicable to foreign-invested enterprises. The PRC legal system is largely a civil law system based on written statutes. Unlike the common law system, prior court decisions may be cited for reference but have limited precedential value.
In 1979, the PRC government began to promulgate a comprehensive system of laws and regulations governing general economic matters. The overall effect of legislation over the past two decades has significantly enhanced the protections afforded to various foreign investments in China. However, China has not developed a fully integrated legal system, and recently enacted laws and regulations may not sufficiently cover all aspects of economic activities in China. Because these laws and regulations are relatively new, and published court decisions are limited and nonbinding in nature, the interpretation and enforcement of these laws and regulations involve uncertainties. In addition, the PRC legal system is based in part on government policies and internal rules, some of which are not published on a timely basis or at all, which may have a retroactive effect. As a result, we may not be aware of our violation of these policies and rules until after the violation occurs.
Any administrative and court proceedings in China may be protracted, resulting in substantial costs and diversion of resources and management attention. However, since PRC administrative and court authorities have significant discretion in interpreting and implementing statutory and contractual terms, it may be more difficult to evaluate the outcome of administrative and court proceedings and the level of legal protection we enjoy than in more developed legal systems. These uncertainties may also impede our ability to enforce the contracts entered into by our PRC subsidiaries. As a result, these uncertainties could materially and adversely affect our business and results of operations.
SAFE regulations may limit our ability to finance our PRC subsidiaries effectively and affect the value of your investment and may make it more difficult for us to pursue growth through acquisition.
If we finance our PRC subsidiaries through additional capital contributions, the Ministry of Commerce in China or its local counterpart must approve the amount of these capital contributions. Further, any loan by us to our PRC subsidiaries cannot exceed statutory limits and must be registered with SAFE or its local counterparts. On August 29, 2008, the State Administration of Foreign Exchange, or SAFE, promulgated Circular 142, a notice regulating the conversion by a foreign-invested company of foreign currency into Renminbi by restricting how the converted Renminbi may be used. The notice requires that Renminbi converted from the foreign currency-denominated capital of a foreign-invested company may only be used for purposes within the business scope
19
approved by the applicable governmental authority and may not be used for equity investments in the PRC unless otherwise provided by laws and regulations. Further, SAFE promulgated an official notice on November 19, 2010, requiring the authenticity of the settlement of net proceeds from offshore offerings to be closely examined and the net proceeds to be settled in the manner described in the offering documents. In addition, SAFE strengthened its oversight of the flow and use of Renminbi funds converted from the foreign currency denominated capital of a foreign-invested company. The use of such Renminbi may not be changed without approval from SAFE, and may not be used to repay Renminbi loans if the proceeds of such loans have not yet been used for purposes within the company’s approved business scope. Violations of Circular 142 may result in severe penalties, including substantial fines as set forth in the Foreign Exchange Administration Regulations. We cannot assure you that we will be able to complete the necessary government registrations or obtain the necessary government approvals on a timely basis, if at all, with respect to future loans or future capital contributions by us to our PRC subsidiaries. If we fail to complete such registrations or obtain such approvals, our ability to finance our PRC operations may be negatively affected, which could adversely and materially affect our liquidity and our ability to fund and expand our business.
PRC regulations relating to the establishment of offshore special purpose companies by PRC residents may subject our PRC resident beneficial owners or our PRC subsidiaries to liability or penalties, limit our ability to inject capital into our PRC subsidiaries, limit our PRC subsidiaries’ ability to increase their registered capital or distribute profits to us, or may otherwise adversely affect us.
On October 21, 2005, SAFE issued a public circular, or Circular 75, which became effective on November 1, 2005. Circular 75 requires PRC residents to register with the local SAFE branch before establishing or controlling any company, referred to in the circular as an “offshore special purpose vehicle,” outside of the PRC for capital financing and to register again after completing an investment in or acquisition of any operating subsidiaries in the PRC, which is commonly referred to as a round-trip investment. Also, any change of shareholding or any other material capital alteration in such offshore special purpose vehicle shall be filed with the local SAFE branch within 30 days after the shareholding change or capital alteration. In May 2011, SAFE issued Circular 19 which provides detailed procedures and specific instructions for SAFE registration under the Circular 75.
In 2003, our founders, who may be deemed PRC residents for the purpose of Circular 75, established our intermediary holding companies in Hong Kong, which subsequently acquired or established their directly- owned subsidiaries in China. Circular 75 retroactively applies to our initial formation of the corporate structure as a round-trip investment and required our founders to register their respective shareholdings in our company with the local SAFE branch in Shanghai by March 31, 2006. Our founders inadvertently missed this filing deadline. We have urged our founders to, and they have been making efforts in order to, apply for a remedial SAFE registration. Although such a remedial registration is permissible under supplementary rules of Circular 75, our founders’ attempted registration has not been accepted by the local SAFE branch in Shanghai due to the lack of specific administrative procedures for remedial registrations. We will continue to urge our founders to, and our founders will continue making efforts to, apply for a remedial SAFE registration. However, we cannot assure you that our founders will be able to make the remedial registration. Even if our founders’ application for remedial registration is accepted, SAFE may still impose administrative penalties on our founders and on our PRC subsidiaries when reviewing such application. If for any reason the relevant government authorities in China determine that our founders are not in compliance with Circular 75, our founders and our PRC subsidiaries may be subject to administrative measures and penalties including, without limitation, orders to rectify and monetary fines, the SAFE registration of our PRC subsidiaries could be revoked, and any proposed foreign exchange transaction between our Hong Kong intermediary holding companies and their directly owned subsidiaries in China may be reversed and restricted, which may materially and adversely affect our business operations and liquidity in China.
20
Failure to comply with PRC regulations regarding the registration requirements for employee share ownership plans or share option plans may subject the PRC plan participants or us to fines and other legal or administrative sanctions.
Under applicable PRC regulations, all foreign exchange matters relating to employee share ownership plans, share option plans or similar plans in which Chinese citizens participate require approval from SAFE or its authorized local branch. In addition, Chinese citizens who are granted share options, shares or other equity interests such as RSUs by an offshore listed company are required, through a Chinese agent or Chinese subsidiary of the offshore listed company, to register with SAFE and complete certain other procedures. As an offshore listed company, we and our Chinese employees who have been granted share options, RSUs or shares are subject to these regulations. If we or our Chinese employees fail to comply with these regulations, we or our Chinese employees may face sanctions imposed by SAFE or other PRC government authorities, including restrictions on foreign currency conversions and additional capital contributions to our PRC subsidiaries.
We rely principally on dividends and other distributions on equity paid by our subsidiaries to fund any cash and financing requirements we may have, and any limitation on the ability of our subsidiaries entities to make payments to us could materially and adversely affect our ability to conduct our business.
We are a holding company and we rely principally on dividends from our subsidiaries in Hong Kong for our cash requirements, including any debt we may incur. We currently receive substantially all of our revenues in U.S. dollars. In future periods, however, we expect to receive an increasing portion of our revenues in RMB. Current PRC regulations permit our subsidiaries to pay dividends to us only out of their accumulated profits, if any, determined in accordance with PRC accounting standards and regulations. In addition, each of our subsidiaries in China is required to set aside a certain amount of its after-tax profits each year, if any, to fund certain statutory reserves. These reserves are not distributable as cash dividends. As of December 31, 2011, our aggregate net assets in the amount of US$90.1 million were not distributable in the form of dividends to us due to these PRC regulations. Furthermore, if our subsidiaries in China incur debt, the instruments governing the debt may restrict their ability to pay dividends or make other payments to us. In addition, the PRC tax authorities may require us to adjust our taxable income under the contractual arrangements we have in place in a manner that would materially and adversely affect our subsidiaries’ ability to pay dividends and other distributions to us. Any limitation on the ability of our subsidiaries to distribute dividends or other payments to us could materially and adversely limit our ability to grow, make investments or acquisitions that could be beneficial to our businesses, pay dividends or otherwise fund and conduct our business.
The discontinuation, reduction or delay of any of the preferential tax treatment or other government incentives available to us in the PRC or imposition of any additional PRC taxes on us could adversely affect our financial condition and results of operations.
China passed a new PRC Enterprise Income Tax Law and its implementing rules, both of which became effective on January 1, 2008. The PRC Enterprise Income Tax Law significantly curtails tax incentives granted to foreign-invested enterprises under the Foreign-Invested Enterprise Income Tax Law in effect before January 1, 2008. The PRC Enterprise Income Tax Law, however, (i) reduces the statutory rate of the enterprise income tax from 33% to 25%, (ii) permits companies established before March 16, 2007 to continue to enjoy their existing tax incentives, adjusted by certain transitional phase-out rules promulgated by the State Council on December 26, 2007, and (iii) introduces new tax incentives, subject to various qualification criteria.
The PRC Enterprise Income Tax Law and its implementing rules permit certain “high and new technology enterprises” which hold independent ownership of core intellectual property and simultaneously meet a list of other criteria, to enjoy a reduced 15% enterprise income tax rate subject to certain new qualification criteria.
The PRC governments have also used different incentives to encourage or accelerate the development of pharmaceutical and biotechnology R&D outsourcing industries. Starting from July 1, 2010 and ending on
21
December 31, 2013, government recognized Technical Advanced Service Enterprises in a number of selected cities, including Shanghai and Chengdu, may enjoy a preferential enterprise income tax rate of 15%. We have historically received sales tax exemptions for certain laboratory services upon approval from local authorities. We also benefited from government incentives in the form of cash subsidies in 2009, 2010 and 2011.
In addition, according to a preferential tax policy adopted by the PRC government in July 2011 to promote the development of China’s western regions, starting from January 1, 2011 and ending on December 31, 2020, enterprises incorporated in the western regions of China and engage in businesses which fall within certain encouraged industries may enjoy a preferential enterprise income tax rate of 15%.
Preferential tax treatment and other government incentives granted to our subsidiaries by local governmental authorities are subject to review and may be adjusted or revoked at any time. The discontinuation of any preferential tax treatment or other government incentives available to us and our wholly-owned PRC subsidiaries will cause our effective tax rate to increase or our other income to decrease, which could materially and adversely affect our financial condition and results of operations. For more information regarding the preferential tax treatment enjoyed by our Company, see “Item 5. Operating and Financial Review and Prospects—Operating Results—Selected Statements of Operations Items—Income Taxes.”
Our global income and the dividends that we may receive from our PRC subsidiaries may be subject to PRC taxes under the PRC Enterprise Income Tax Law, which would materially and adversely affect our results of operations.
Under the PRC Enterprise Income Tax Law and its implementing rules, both effective from January 1, 2008, an enterprise established outside of the PRC with “de facto management bodies” within the PRC is considered a resident enterprise and will be subject to the enterprise income tax at the rate of 25% on its global income. The implementation rules define the term “de facto management bodies” as “establishments that carry out substantial and overall management and control over the manufacturing and business operations, personnel, accounting, properties, etc. of an enterprise.” The State Tax Administration issued the Notice Regarding the Determination of Chinese-Controlled Offshore Incorporated Enterprises as PRC Tax Resident Enterprises on the Basis of De Facto Management Bodies, or SAT Circular 82, on April 22, 2009. SAT Circular 82 provides certain criteria for determining whether the “de facto management body” of an offshore-incorporated enterprise controlled by PRC enterprises is located in China. On July 27, 2011, the SAT issued Administrative Measures on Income Tax of Chinese-controlled Resident Enterprises Incorporated Overseas (Trial), or Circular 45, which became effective on September 1, 2011, to supplement Circular 82 and other tax laws and regulations. Circular 45 clarifies certain issues related to the determination of PRC resident enterprise status and post-determination administration.
Although SAT Circular 82 and Circular 45 only applies to offshore enterprises controlled by PRC enterprises and not those controlled by PRC or foreign individuals or foreign enterprises, the criteria set forth therein may reflect State Tax Administration’s general position on how the “de facto management body” test should be applied in determining the tax resident status of offshore enterprises, regardless of whether they are controlled by PRC or foreign enterprises or individuals. Accordingly, we may be considered a resident enterprise and may therefore be subject to the enterprise income tax at 25% on our global income other than dividends from our PRC subsidiaries, which could significantly increase our tax burden and materially and adversely affect our cash flow and profitability.
Pursuant to the PRC Enterprise Income Tax Law, dividends generated after January 1, 2008 and payable by a foreign-invested enterprise in China to its foreign investors that are non-PRC resident enterprises will be subject to a 10% withholding tax, unless any such foreign investor’s jurisdiction of incorporation has a tax treaty with China that provides for a different withholding arrangement. We are a Cayman Islands holding company and substantially all of our income may come from dividends we receive from our subsidiaries located in Hong Kong, which are the direct holding companies of our PRC subsidiaries. If our Hong Kong subsidiaries are considered non-PRC resident enterprises, dividends that they receive from our PRC subsidiaries may be subject
22
to withholding tax at a preferential rate of no more than 5% under certain tax arrangements between the PRC and Hong Kong. However, if our Hong Kong subsidiaries are not considered to be the beneficial owners of our PRC subsidiaries under such arrangements, such dividends would be subject to withholding tax at a rate of 10%.
Under the PRC Enterprise Income Tax Law and its implementing rules, dividends paid to a resident enterprise from its subsidiaries qualify as tax-exempt income. However, if we are treated as a PRC resident enterprise for PRC enterprise income tax purposes, there is no assurance that we would enjoy such tax-exempt treatment on dividends paid to our non-PRC holding companies, as the SAT and other PRC authorities have not yet issued guidance with respect to the processing of outbound remittances to entities that are treated as resident enterprises controlled by PRC individuals for PRC enterprise income tax purposes.
We may be required to withhold PRC income tax on the dividends we pay you (if any), and any gain you realize on the transfer of our ordinary shares and ADSs may also be subject to PRC tax if we are treated as a PRC “resident enterprise”.
If we are considered a PRC “resident enterprise” under the PRC Enterprise Income Tax Law, we may be obligated to withhold PRC income tax on payments of dividends on our ordinary shares and ADSs to investors that are non-resident enterprises of the PRC because the dividends payable on our ordinary shares and ADSs may be regarded as being derived from sources within the PRC. The withholding tax rate would generally be 10% on dividends paid to non-resident enterprises unless such non-resident enterprise is entitled to a lower rate under a tax treaty. In addition, any gain realized by investors who are non-resident enterprises of the PRC from the transfer of our ordinary shares or ADSs may be regarded as being derived from sources within the PRC and be subject to a 10% PRC tax.
Moreover, if we are treated as a PRC resident enterprise, non-resident individual investors might be subject to PRC individual income tax at a rate of 20% on dividends paid to such investors and any capital gains realized from the transfer of our ordinary shares and ADSs if such dividends and gains are deemed income derived from sources within the PRC, except in the case of individuals that qualify for a lower rate under a tax treaty. A non-resident individual is an individual who has no domicile in the PRC and does not stay within the PRC or has stayed within the PRC for less than one year. Under a tax treaty between the PRC and the United States, a lower income tax rate of 10% will apply to dividends if certain conditions are met. The foregoing PRC tax may reduce your investment return on our ordinary shares and ADSs and may also affect the price of our ordinary shares and ADSs.
The M&A Rule and other relevant rules and regulations set forth complex procedures for acquisitions conducted by foreign investors that could make it more difficult to pursue acquisitions.
The M&A Rule sets forth complex procedures and requirements that could make merger and acquisition activities by foreign investors more time-consuming and complex, including requirements in some instances that the Ministry of Commerce be notified in advance of any change-of-control transaction in which a foreign investor takes control of a PRC domestic enterprise or a foreign company with substantial PRC operations, if certain thresholds are met. In addition, the State Council issued a circular, or Circular 6, on February 3, 2011, which requires that merger and acquisition activities by foreign investors of enterprises relating to national defense and mergers and acquisitions through which foreign investors may acquirede factocontrol of domestic enterprises raising national security concerns will be subject to national security review. In August 2011, the Ministry of Commerce published a implementation rules to implement Circular 6. Complying with the requirements of the M&A Rule and other relevant rules and regulations to complete such transactions could be time-consuming, and any required approval processes, including obtaining approval from the Ministry of Commerce, may delay or inhibit our ability to complete such transactions, which could affect our ability to expand our business or maintain our market share.
23
The failure of the owners of our leased facilities to comply with PRC property related laws and other matters may materially and adversely affect our ability to use our R&D facilities.
We perform various R&D services in leased facilities in Shanghai. The owners of our leased facilities have not met a number of land- and property-related legal requirements. For example, owners of some of our leased facilities have not been able to provide us with relevant building ownership certificates. Certain properties leased by us are used for R&D purposes while they are under zoning restrictions to be used for industrial or other purposes. Certain leased properties have not been registered with the local real estate administrative authorities. In addition, certain leased facilities have been mortgaged by their respective owners.
As a result, third parties or government authorities may interfere with the use of our leased properties, we may be subject to fines by the government, our leases may be invalidated and our rights under these leases may be adversely affected. We may be forced to relocate any affected facilities. All of these consequences could materially and adversely affect our business, financial condition, results of operations and prospects.
The lack of certain certificates or permits or failure to complete certain inspection and acceptance procedures required by PRC local government for some of our facilities may materially and adversely affect our use of these facilities.
We have not obtained the title certificate for the property that we acquired to host our Chengdu R&D center. The developer and seller of the property is still in the process of obtaining the land use right certificates and certain construction related permits from local government and conducting the required inspection and acceptance procedures. In addition, Phase A of our multi-purpose manufacturing facility and the laboratory services building in Fengxian commenced its operations in January 2011 before the required inspection and acceptance procedures have been completed. The lack of these required certificates or permits or failure to complete the required inspection and acceptance procedures may subject us to various administrative penalties and restrictions, including fine and suspension of operation, which may materially and adversely affect our use of these facilities.
The audit report included in this annual report is prepared by an auditor who is not inspected by the Public Company Accounting Oversight Board and, as such, you are deprived of the benefits of such inspection.
Auditors of companies that are registered with the U.S. Securities and Exchange Commission and traded publicly in the United States, including our independent registered public accounting firm, must be registered with the Public Company Accounting Oversight Board, or the PCAOB, in the United States and are required by the laws of the United States to undergo regular inspections by the PCAOB to assess their compliance with the laws of the United States and professional standards in connection with their audits of financial statements filed with the SEC. Because our auditors are located in the People’s Republic of China, a jurisdiction where the PCAOB is currently unable to conduct inspections without the approval of the Chinese authorities, our auditors are not currently inspected by the PCAOB. This lack of PCAOB inspections in China prevents the PCAOB from regularly evaluating audits and quality control procedures of any auditors operating in China, including our auditors. The inability of the PCAOB to conduct inspections of auditors in China makes it more difficult to evaluate the effectiveness of our auditor’s audit procedures or quality control procedures as compared to auditors outside of China that are subject to PCAOB inspections. As a result, investors may be deprived of the benefits of PCAOB inspections.
Risks Related to this Our ADSs
The market price for our ADSs may be volatile.
The market price for our ADSs is likely to be highly volatile and subject to wide fluctuations in response to factors including the following:
| | • | | regulatory developments in our target markets affecting us, our customers or our competitors; |
24
| | • | | announcements of studies and reports relating to the quality of our solutions and services or those of our competitors; |
| | • | | changes in the economic performance or market valuations of other companies that provide pharmaceutical and biotechnology R&D outsourcing services; |
| | • | | actual or anticipated fluctuations in our quarterly results of operations and changes or revisions of our expected results; |
| | • | | changes in financial estimates by securities research analysts; |
| | • | | conditions in the pharmaceutical and biotechnology R&D outsourcing services industry; |
| | • | | announcements by us or our competitors of new services, acquisitions, strategic relationships, joint ventures or capital commitments; |
| | • | | additions to or departures of our senior management; |
| | • | | fluctuations of exchange rates between the Renminbi and the U.S. dollar; |
| | • | | release or expiration of lock-up or other transfer restrictions on our outstanding ordinary shares or ADSs; and |
| | • | | sales or perceived potential sales of additional ordinary shares or ADSs. |
In addition, the securities market has experienced significant price and volume fluctuations unrelated to the operating performance of any particular companies. These market fluctuations may also materially and adversely affect the market price of our ADSs.
Because we do not expect to pay dividends in the foreseeable future, you must rely on price appreciation of our ADSs for return on your investment.
We intend to retain most, if not all, of our available funds and earnings to fund the development and growth of our business. As a result, we do not expect to pay any cash dividends in the foreseeable future. Therefore, you should not rely on an investment in our ADSs as a source for any future dividend income.
Our board of directors has significant discretion as to whether to distribute dividends. Even if our board of directors decides to declare and pay dividends, the timing, amount and form of future dividends, if any, will depend on, among other things, our future results of operations and cash flow, our capital requirements and surplus, the amount of distributions, if any, received by us from our subsidiaries, our financial condition, contractual restrictions and other factors deemed relevant by our board of directors. Accordingly, the return on your investment in our ADSs will likely depend entirely upon any future price appreciation of our ADSs. There is no guarantee that our ADSs will appreciate in value or even maintain the price at which you purchased the ADSs. You may not realize a return on your investment in our ADSs and you may even lose your entire investment in our ADSs.
Substantial future sales or perceived potential sales of our ADSs in the public market could cause the price of our ADSs to decline.
Additional sales of our ADSs or ordinary shares in the public market, or the perception that these sales could occur, could cause the market price of our ADSs to decline. As of December 31, 2011, we had 336,109,400 ordinary shares issued and outstanding including 114,012,000 ordinary shares represented by ADSs. All ADSs are freely transferable without restriction or additional registration under the United States Securities Act of 1933, as amended, or the Securities Act, unless held by our “affiliates” as that term is defined in Rule 144 under the Securities Act. The remaining ordinary shares outstanding are available for sale, subject to volume and other restrictions as applicable under Rules 144 and 701 under the Securities Act. In addition, certain holders of our
25
ordinary shares have the right to cause us to register under the Securities Act the sale of their shares. Registration of these shares under the Securities Act would result in ADSs representing these shares becoming freely tradable without restriction under the Securities Act immediately upon the effectiveness of the registration. Sales of these registered shares in the public market could cause the price of our ADSs to decline.
You may not have the same voting rights as the holders of our ordinary shares and may not receive voting materials in time to be able to exercise your right to vote.
Except as described in this annual report and in the deposit agreement, holders of our ADSs will not be able to exercise voting rights attaching to the shares represented by our ADSs on an individual basis. Holders of our ADSs will appoint the depositary or its nominee as their representative to exercise the voting rights attaching to the shares represented by the ADSs. You may not receive voting materials in time to instruct the depositary to vote, and it is possible that you, or persons who hold their ADSs through brokers, dealers or other third parties, will not have the opportunity to exercise a right to vote.
Your right to participate in any rights offerings may be limited, which may cause dilution to your holdings, and you may not receive cash dividends if it is impractical to make them available to you.
We may from time to time distribute rights to our shareholders, including rights to acquire our securities. However, we cannot make rights available to you in the United States unless we register both the rights and the securities to which the rights relate under the Securities Act or an exemption from the registration requirements is available. Under the deposit agreement, the depositary will not make rights available to you unless both the rights and the underlying securities to be distributed to ADS holders are either registered under the Securities Act or exempt from registration under the Securities Act. We are under no obligation to file a registration statement with respect to any such rights or securities or to endeavor to cause such a registration statement to be declared effective and we may not be able to establish a necessary exemption from registration under the Securities Act. Accordingly, you may be unable to participate in our rights offerings and may experience dilution in your holdings.
The depositary of our ADSs has agreed to pay to ADS holders the cash dividends or other distributions it or the custodian receives on our ordinary shares or other deposited securities after deducting its fees and expenses. You will receive these distributions in proportion to the number of ordinary shares your ADSs represent. However, the depositary may, at its discretion, decide that it is inequitable or impractical to make a distribution available to any holders of ADSs. For example, the depositary may determine that it is not practicable to distribute certain property through the mail, or that the value of certain distributions may be less than the cost of mailing them. In these cases, the depositary may decide not to distribute such property to you.
You may be subject to limitations on transfer of your ADSs.
Your ADSs are transferable on the books of the depositary. However, the depositary may close its transfer books at any time or from time to time when it deems expedient in connection with the performance of its duties. In addition, the depositary may refuse to deliver, transfer or register transfers of ADSs generally when our books or the books of the depositary are closed, or at any time if we or the depositary deems it advisable to do so because of any requirement of law or of any government or governmental body, or under any provision of the deposit agreement, or for any other reason.
You may face difficulties in protecting your interests, and your ability to protect your rights through the U.S. federal courts may be limited because we are incorporated under Cayman Islands law, we conduct substantially all of our operations in China and all of our directors and officers reside outside the United States.
We are incorporated in the Cayman Islands and conduct substantially all of our operations in China through our PRC subsidiaries. All of our directors and officers reside outside the United States and a substantial portion
26
of their assets are located outside of the United States. As a result, it may be difficult or impossible for you to bring an action against us or against these individuals in the Cayman Islands or in China in the event that you believe that your rights have been infringed under the securities laws or otherwise. Even if you are successful in bringing an action of this kind, the laws of the Cayman Islands and of China may render you unable to enforce a judgment against our assets or the assets of our directors and officers.
There is no statutory recognition in the Cayman Islands of judgments obtained in the United States, although the courts of the Cayman Islands will recognize as a valid judgment, a final and conclusive judgmentin personam obtained in a federal or state court of the United States under which a sum of money is payable (other than a sum of money payable in respect of multiple damages, taxes or other charges of a like nature or in respect of a fine or other penalty) or, in certain circumstances, anin personam judgment for non-monetary relief, and would give a judgment based thereon; provided that (a) such courts had proper jurisdiction over the parties subject to such judgment; (b) such courts did not contravene the rules of natural justice of the Cayman Islands; (c) such judgment was not obtained by fraud; (d) the enforcement of the judgment would not be contrary to the public policy of the Cayman Islands; (e) no new admissible evidence relevant to the action is submitted prior to the rendering of the judgment by the courts of the Cayman Islands; and (f) there is due compliance with the correct procedures under the laws of the Cayman Islands.
Our corporate affairs are governed by our memorandum and articles of association, as amended and restated from time to time, and by the Companies Law (2011 Revision) and common law of the Cayman Islands. The rights of shareholders to take legal action against us and our directors, actions by minority shareholders and the fiduciary responsibilities of our directors are to a large extent governed by the common law of the Cayman Islands. The common law of the Cayman Islands is derived in part from comparatively limited judicial precedent in the Cayman Islands as well as from English common law, which provides persuasive, but not binding, authority on a court in the Cayman Islands. The rights of our shareholders and the fiduciary responsibilities of our directors under Cayman Islands law are not as clearly established as they would be under statutes or judicial precedents in the United States. In particular, the Cayman Islands has a less developed body of securities laws than the United States and provides significantly less protection. In addition, Cayman Islands companies may not have standing to initiate a shareholder derivative action in U.S. federal courts.
As a result, our public shareholders may have more difficulty in protecting their interests through actions against us, our management, our directors or our major shareholders than would shareholders of a corporation incorporated in a jurisdiction in the United States.
Our memorandum and articles of association contain anti-takeover provisions that could adversely affect the rights of holders of our ordinary shares and ADSs.
Our memorandum and articles of association contain certain provisions that could limit the ability of others to acquire control of our company, including a provision that grants authority to our board of directors to establish from time to time one or more series of preferred shares without action by our shareholders and to determine, with respect to any series of preferred shares, the terms and rights of that series. The provisions could have the effect of depriving our shareholders of the opportunity to sell their shares, including shares represented by ADSs, at a premium over the prevailing market price by discouraging third parties from seeking to obtain control of our company in a tender offer or similar transactions.
Our corporate actions are substantially controlled by our directors, executive officers and other principal shareholders, who can exert significant influence over important corporate matters, which may reduce the price of our ADSs and deprive you of an opportunity to receive a premium for your ADSs.
Our directors, executive officers and principal shareholders beneficially owned approximately 54.6% of our outstanding ordinary shares as of March 31, 2012. These shareholders, if acting together, could exert substantial influence over matters such as electing directors and approving material mergers, acquisitions or other business
27
combination transactions. This concentration of ownership may also discourage, delay or prevent a change in control of our company, which could have the dual effect of depriving our shareholders of an opportunity to receive a premium for their shares as part of a sale of our company and reducing the price of our ADSs. These actions may be taken even if they are opposed by our other shareholders, including the holders of our ADSs. In addition, these persons could divert business opportunities away from us to themselves or others.
We may be classified as a passive foreign investment company for United States federal income tax purposes, which could subject United States investors in our ADSs or ordinary shares to significant adverse United States federal income tax consequences.
Based on our current income and assets and projections as to the value of our ADSs and ordinary shares, we do not expect to be classified as a “passive foreign investment company,” or PFIC (as defined for United States federal income tax purposes and as described below), for the taxable year ending December 31, 2012, or for the foreseeable future. However, the application of the PFIC rules is subject to ambiguity in several respects and we will make a separate determination for each taxable year as to whether we are a PFIC (after the close of each taxable year). Depending upon the value of our assets based on the market value of our ADSs and ordinary shares and the nature of our assets and income over time, we could be or become classified as a PFIC for United States federal income tax purposes. Hence, fluctuations in the market price of our ADSs or ordinary shares may cause us to be or become classified as a PFIC for the current taxable year or any subsequent taxable year.
A non-United States corporation will be treated as a PFIC for United States federal income tax purposes for any taxable year if either (i) 75% or more of its gross income for such year consists of certain types of “passive” income, or (ii) 50% or more of the value of its assets (determined on the basis of a quarterly average) during such year is attributable to assets that produce, or are held for the production of, passive income. For this purpose, passive income means any income that would be considered foreign personal holding company income under the Internal Revenue Code of 1986, as amended, including, without limitation, dividends, interest, royalties, rents, annuities, net gains from the sale or exchange of property producing such income, net gains from commodity transactions, net foreign currency gains and income from notional principal contracts. The market value of our assets will be determined based on the market price of our ADSs and ordinary shares, which is likely to fluctuate.
If we were to be or become classified as a PFIC, a U.S. Holder (as defined in “Item 10. Additional Information—Taxation—Material United States Federal Income Tax Considerations”) may incur significantly increased United States income tax on gain recognized on the sale or other disposition of our ADSs or ordinary shares and on the receipt of distributions on our ADSs or ordinary shares to the extent such gain or distribution is treated as an “excess distribution” under the United States federal income tax rules. Further, if we are classified as a PFIC for any year during which a U.S. Holder held our ADSs or ordinary shares, we generally would continue to be treated as a PFIC for all succeeding years during which such U.S. Holder holds our ADSs or ordinary shares. We urge you to consult your tax advisor concerning the United States federal income tax consequences of acquiring, holding and disposing of ADSs or ordinary shares if we are or become classified as a PFIC. For more information. see “Item 10. Additional Information—Taxation—Material United States Federal Income Tax Considerations—Passive Foreign Investment Company Considerations.”
| ITEM 4. | INFORMATION ON THE COMPANY |
A.History and Development of the Company
We are a Cayman Islands holding company and conduct substantially all of our business through our seven operating subsidiaries in China. We commenced operations in China in 2002 and established the holding company in the Cayman Islands on August 30, 2007. We own 100% of all of our operating subsidiaries in China through two wholly-owned companies, China Gateway Life Science (Holdings) Limited and ChemExplorer Company Limited, which were incorporated in Hong Kong on June 23, 2003 and January 3, 2003, respectively. Our corporate structure reflects common practice for companies with operations in China where separate legal entities are often required or advisable for purposes of obtaining relevant tax and other incentives in the relevant jurisdictions.
28
In September 2007, in connection with our corporate restructuring and the incorporation of our Cayman Islands holding company, we issued a total of 5,000 ordinary shares, par value US$0.001 per share, to ChemExplorer Investment Holdings Ltd. and a total of 5,000 ordinary shares to ChemPartner Investment Holdings Limited. ChemExplorer Investment Holdings Ltd. and ChemPartner Investment Holdings Limited are British Virgin Islands companies ultimately owned by Mr. Michael Xin Hui and the Hui Family Trust, a British Virgin Islands trust.
In September 2007, we issued 2,019 preferred shares, par value US$0.001 per share, in a private placement to TPG Star Charisma Ltd. and TPG Biotech II Charisma Ltd., for a total amount of US$21.0 million, and an existing shareholder of our company sold 1,346 preferred shares to TPG Star Charisma Ltd. and TPG Biotech II Charisma Ltd. for a total amount of US$14.0 million in the same transaction. In May 2008, we effected a bonus share issuance in the form of a share split such that an additional 20,800 ordinary shares were issued for each ordinary share outstanding and additional approximately 20,800 preferred shares for each preferred shares outstanding. Each preferred share was automatically converted into one ordinary share upon completion of our initial public offering in October 2010. On October 19, 2010, our ADSs began trading on the New York Stock Exchange under the ticker symbol “SHP.” We issued and sold a total of 3,200,000 ADSs and our selling shareholders sold a total of 2,600,000 ADSs, representing 57,600,000 and 46,800,000 ordinary shares, respectively, at an initial offering price of US$15.00 per ADS.
In January 2011, we commenced operation on the initial phase of production at our new multi-purpose pharmaceutical development and cGMP manufacturing facility located in Fengxian, Shanghai, China. The facility operates under the name of China Gateway Pharmaceutical Development Co., Ltd., and is principally engaged in process research and development, formulation research and development, analytical method development and validation, and cGMP manufacturing of intermediates and APIs. In January 2011, our old manufacturing facility located in Nanhui, Shanghai, China under the name of China Gateway Pharma Products (Shanghai) Limited substantially discontinued its operation and is in the process of being closed.
Our principal executive offices are located at No. 5 Building, 998 Halei Road, Zhangjiang Hi-Tech Park, Pudong New Area, Shanghai, 201203, People’s Republic of China. Our telephone number at this address is (86-21) 5132-0088. Our registered office in the Cayman Islands is located at Cricket Square, Hutchins Drive, P.O. Box 2681, Grand Cayman, KY1-1111, Cayman Islands. Our agent for service of process in the United States is Law Debenture Corporate Services Inc. located at 400 Madison Avenue, 4th Floor, New York, New York 10017.
B.Business Overview
Overview
We are a leading China-based pharmaceutical and biotechnology R&D outsourcing company. We provide a broad range of high-quality, integrated services across the drug discovery and development process to international and Chinese pharmaceutical and biotechnology companies. Our services consist of discovery chemistry, discovery biology and preclinical development, pharmaceutical development and biologics services. We offer our services on an FTE basis or a fee-for-service basis. Capitalizing on our broad service offerings, the experience and expertise of our skilled scientists and our state-of-the-art facilities and equipment, we help our customers discover and develop novel drug candidates efficiently. We have a diversified and loyal global customer base. We provide services to nearly 400 customers, including many of the top pharmaceutical and biotechnology companies in the world based on revenues and a number of fast-growing biotechnology and specialty pharmaceutical companies, as well as renowned academic and research institutions in the U.S. and China. Most of our customers return to us for additional and often larger projects, and all of our top ten customers in each of 2009 and 2010 remained our customers in 2011, except that one top ten customer ceased business with us since 2010 as a result of a change in business focus after its merger with another customer.
29
Our experienced and strong R&D team contributes significantly to our market leadership. We have successfully grown our scientific research staff from 1,470 as of December 31, 2009 to 1,796 as of December 31, 2011. Over 62% of our scientific research staff has post-graduate or higher degrees, including many native Chinese scientists and managers who have returned to China after studying or working overseas with experience in global pharmaceutical and biotechnology companies and knowledge of Western business practices. Based in China and headquartered in Shanghai, we are well-positioned to attract a large number of returnees and benefit from an abundant supply of highly skilled, domestically-trained scientific talent in China. We attract, retain and motivate our employees through a comprehensive training and career development program and equity incentive plans.
We conduct our laboratory activities in five primary facilities in China: (i) an approximately 701,000 square-foot headquarters in Shanghai Zhangjiang Hi-Tech Park, including an approximately 105,000 square-foot laboratory and office facility put into use in December 2011 that is dedicated to supporting discovery services for one of our largest customers and approximately 32,000 square-foot in-vivo research facilities and approximately 10,000 square-foot lab and office space that were taken over from Charles River Laboratories International, Inc. in November 2011; (ii) an approximately 13,000 square-foot AAALAC-accredited animal facility in Shanghai Zhangjiang Hi-Tech Park and an approximately 18,000 square-foot large animal facility in Fengxian, Shanghai; (iii) an approximately 68,000 square-foot R&D center in Chengdu, which replaced our former 36,000 square-foot facilities when it became operational in April 2011; (iv) an approximately 28,000 square-foot manufacturing facility in Nanhui, Shanghai, which substantially ended operations in 2011 and is in the process of being closed; and (v) an approximately 460,000 square-foot cGMP-quality multi-purpose manufacturing facility in Fengxian, Shanghai under development. Phase A of the multi-purpose manufacturing facility commenced operations on its initial phase of production in January 2011. The construction and development of the rest of the manufacturing facility will start when demanded by our future business growth. We are also developing an approximately 150,000 square-foot office and laboratory services building in Fengxian, Shanghai primarily for pharmaceutical development services. Approximately 32,000 square-foot of the laboratory service building has been completed and went into operation in November 2011 and the construction of the rest of the areas will start when demanded by our future business growth. Our strategic location in China provides us many advantages, including relatively low-cost labor, developed infrastructure and favorable government incentives as well as a large talent pool.
We have experienced solid growth in recent years. Our net revenues increased from US$72.3 million in 2009 to US$90.3 million in 2010 and US$111.6 million in 2011, representing a Compound Annual Growth Rate, or CAGR, of 24.2%. The number of our customers increased from 134 in 2009 to 237 in 2010 and 395 in 2011. Our net income increased from US$9.8 million in 2009 to US$13.0 million in 2010 and slightly decreased to US$11.2 million in 2011, mostly due to higher share-based compensation expenses.
Customers
Our customers consist primarily of large pharmaceutical and biotechnology companies located throughout the world. We provide services to nearly 400 customers, including many of the top pharmaceutical and biotechnology companies in the world in terms of revenues and a number of fast-growing biotechnology and specialty pharmaceutical companies, as well as renowned academic and research institutions in the U.S. and China. We generated a significant majority of our total net revenues over the last three years from sales to customers located in the United States. All of our top ten customers in each of 2009 and 2010 remained our customers in 2011, except that one top ten customer ceased business with us since 2010 as a result of a change in business focus after its merger with another customer.
30
The following table sets forth our growing customer base:
| | | | | | | | | | | | |
| | | For the years ended December 31, | |
| | | 2009 | | | 2010 | | | 2011 | |
Top ten customer concentration (% of net revenues) | | | 65 | | | | 63 | | | | 62 | |
Total number of customers | | | 134 | | | | 237 | | | | 395 | |
Number of top ten customers that remain as our customers in 2011 | | | 10 | | | | 9 | (1) | | | 9 | (1) |
Average net revenues per top ten customer (millions of US$) | | | 4.7 | | | | 5.7 | | | | 6.9 | |
| (1) | Two of our top-ten customers in 2009 were merged with other customers in 2010. One continues to do business with us after its merger. The other ceased doing business with us as a result of a change in the focus of its business after its merger. |
For certain major customers, we provide dedicated teams of scientists, laboratory facilities, analytical support and independent information technology and security services. This physical and operational separation of customer projects ensures enhanced security and protection of our customers’ intellectual property. We can tailor our laboratory configuration and setup, research plan, operating procedures, information technology and security protocols to our customers’ specifications.
Our two largest customers in 2009 and 2010, accounted for 27% and 10% of our net revenues in 2009 and 21% and 8% of our net revenues in 2010, respectively. Our two largest customers in 2011, accounted for 18% and 11% of our net revenues in 2011, respectively. No other customer accounted for more than 10% of our net revenues in those periods. See “Item 3. Key Information—Risk Factors—Risks Relating to Our Business and Industry—A limited number of our customers have accounted and are expected to continue to account for a high percentage of our revenues. The loss of or significant reduction in orders from any of these customers could significantly reduce our revenues and materially and adversely affect our financial condition, results of operations and prospects.”
Our Services
We provide a broad range of high-quality, integrated services across the drug discovery and development process to international and Chinese pharmaceutical and biotechnology companies. Our services consist of discovery chemistry, discovery biology and preclinical development, pharmaceutical development and biologics services. We conduct our operations primarily at our headquarters in Shanghai Zhangjiang Hi-Tech Park, our cGMP manufacturing facility in Fengxian, Shanghai and our R&D center in Chengdu. The following chart sets forth the drug R&D process and our services:
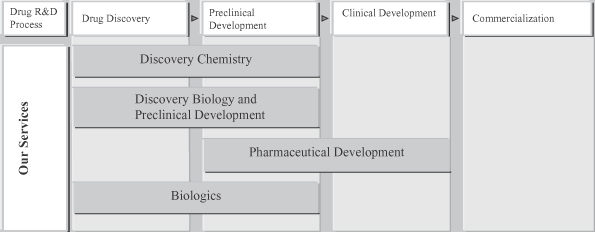
31
Discovery Chemistry
Our core service offering is discovery chemistry. In the drug discovery process, we seek to identify hits, which are chemical compounds or therapeutic antibodies or proteins that interact with a potential drug target with sufficient potency and selectivity to warrant further testing and refinement as possible drug candidates, or leads. When we identify and assess a lead, we further refine and optimize the chemical structure of the lead to improve its drug characteristics with the goal of producing a preclinical drug candidate. As of December 31, 2011, our discovery chemistry team consisted of 1,255 scientific research staff. Our discovery chemistry services include:
Medicinal chemistry. Our medicinal chemistry services include hit generation and assessment, hit-to-lead and lead optimization services. We focus on a traditional medicinal chemistry approach, a process through which a series of compounds are carefully designed with the aid of modern computational chemistry and structure-activity relationship or SAR analysis, followed by chemical synthesis and evaluations of biological activities and drug metabolism and pharmacokinetics (DMPK), absorption, distribution, metabolism and excretion (ADME) or toxicology properties. We also design and synthesize focused libraries to support SAR analysis.
Synthetic chemistry. Our synthetic chemistry services include syntheses of custom-designed compounds, as well as complex reference compounds. We have completed the syntheses of a number of complex compounds with more than 20 steps.
Library generation. Our library generation services include designing and synthesizing libraries, as well as building blocks and scaffold for library synthesis, reference compound synthesis and custom synthesis.
Analytical chemistry. We provide broad analysis and purification services, including high throughput analysis and purification, large scale compound purification, natural product isolation and structure elucidation, among other things. Our analytical chemistry services internally support synthetic chemistry, library generation and medicinal chemistry services, and offer external services focused on chiral separation, bulk material purification and high-throughput library purification.
Discovery Biology and Preclinical Development
We began providing discovery biology and preclinical development offerings in 2007. As of December 31, 2011, our discovery biology and preclinical development team consisted of 338 scientific research staff. With increasing customer demand, we anticipate that discovery biology and preclinical development services will increasingly contribute to our growth and profitability.
Discovery Biology
Our discovery biology team provides biology-based assays, models and other services for the drug discovery process, which primarily include:
| | • | | Assay development and high throughput screening. We develop biochemical and cell-based assays to examine the activity of small molecule compounds at targets such as kinases, non-kinase enzymes, G-protein-coupled receptors, nuclear receptors, transporters and ion channels. In addition, our cell biology group provides mode of action (MOA) studies in signal transduction and cell cycle pathways. |
| | • | | Pharmacology.We develop rodent and other animal disease models to evaluate compounds in various therapeutic categories including cancer, metabolic and central nervous system diseases, inflammation and infectious diseases to supportin vivo efficacy evaluation and mechanistic characterization. |
| | • | | Reagent generation. We generate and produce proteins, antibodies and cell lines for biochemical and cell-based assays. |
| | • | | Target validation and biomarker research. We provide shRNA knockdown services for target identification and validation. We also conduct biomarker analysis to support translational research. |
32
Preclinical Development
Our preclinical development team provides biology-based screening, profiling and other services for the drug preclinical development process, which primarily include:
| | • | | In Vivo. OurIn Vivo services include rodent screening based on pharmacokinetic characteristics for rapid drug candidate selection and dog and non-human primate PK for prediction of drug properties in humans. We have the expertise to administer drugs to animals orally, intravenously and by infusion and to sample various body fluids and tissues for evaluation of thein vivo behavior of new chemicals including absorption, distribution, metabolism and excretion. |
| | • | | Early formulation service. We provide discovery formulation support from initial dosing of new chemicals in animals, such as pharmacokinetic studies, to chronic dosing in efficacy or safety evaluations in rodents, including screening, identifying and optimizing proper dosing formulations and studying the pharmaceutical properties of compounds before dosing to animals. |
| | • | | In vitro ADME profiling. Ourin vitroADME profiling services include analyzing (i) the metabolic stability of drug candidates in blood and liver cells, (ii) drug-drug interaction, (iii) plasma protein binding and (iv) the manner in which drugs are transported in the body through transporter assays. |
| | • | | Metabolite identification.Our metabolite identification services include metabolite profiling, characterization, and structure elucidation as well as pharmacokinetic evaluation of parent and metabolites across various animal species in preclinical development. |
| | • | | Bioanalytical services. Our centralized bioanalytical labs provide quantitative and qualitative sample analysis for preclinical studies as well as clinical trials. In addition to internally supporting DMPK, toxicology and pharmacology studies, they offer external services such as immunoassay for protein drugs and biomarkers analysis for biologics drug development. |
| | • | | Non-GLP toxicology. We offer non-GLP toxicology services, including in vitro toxicity studies and exploratory toxicology studies in rodents, dogs and non-human primates. We operate an approximately 13,000 square-foot AAALAC-accredited animal facility in Shanghai Zhangjiang Hi-Tech Park and an approximately 18,000 square-foot large animal facility in Fengxian, Shanghai. |
Pharmaceutical Development
We provide broad, integrated pharmaceutical development services to our customers to support the drug development process. Our pharmaceutical development services focus on the early phases of the drug development process and include preformulation and formulation, process R&D, analytical development and validation and research manufacturing services. We began offering pharmaceutical development services in 2005. As of December 31, 2011, our pharmaceutical development services team consisted of 130 scientific research staff. Our pharmaceutical development services include:
| | • | | Preformulation and Formulation.Working in close collaboration with our process R&D, research manufacturing and analytical development groups, our preformulation and formulation groups offer a wide range of services for our customers’ new chemical entities in the preclinical stage. Our preformulation group provides analytical approaches to choose the right form of a drug candidate (e.g. salt and/or polymorph), evaluate its physical properties, and understand the drug candidate’s stability under various conditions per ICH guidelines. Our formulation group focuses on developing the most conventional solid or liquid dosage form or innovative dosage form and drug delivery system for the drug candidate. |
| | • | | Process R&D.Our process R&D service involves optimizing the chemical manufacturing synthesis process in order to develop a robust process to manufacture larger quantities of a drug than those needed in the previous development phases. Processes developed for small scale production of a compound may not be suitable for larger scale production because they may be too expensive, |
33
| | operationally inefficient, environmentally unacceptable or present safety concerns. Our process R&D team seeks to select and optimize the most cost-effective method of compound manufacture synthesis by, among other things, reducing the number of chemicals used in synthesis, improving the yield of the desired compound and reducing the time needed for manufacture. |
| | • | | Analytical Development and Validation.Our analytical development group provides analytical services for pharmaceutical development projects. It supports internal process R&D and offers external services focused on analytical method development, method validation, in-process quality control, reference standard qualification, release testing and stability sample storage testing. |
| | • | | Research Manufacturing.We provide manufacturing services for our customers to manufacture drug substances and drug products under cGMP regulations in sufficient quantities to conduct early stage drug development, including GLP Toxicology and Phase I Clinical Trial studies. Our cGMP kilo-lab facility located in Shanghai Zhangjiang Hi-Tech Park has the capacity to manufacture advanced intermediates and APIs in increasing quantities ranging from lab quantities, which are measured in grams, to scale-up quantities to support drug development activities, which are measured in tens to hundreds of kilograms. Our cGMP product suite facility in Shanghai Zhangjiang Hi-Tech Park has the capacity to manufacture drug products to support Phase I Clinical Trial studies. |
We are developing an approximately 460,000 square-foot cGMP-quality multi-purpose manufacturing facility in Fengxian, Shanghai designed to manufacture advanced intermediates and APIs for preclinical testing and clinical trials. Phase A of the multi-purpose manufacturing facility commenced operations on its initial phase of production in January 2011. Phase B of the Fengxian facility, comprising approximately 260,000 square feet, will include a cGMP- quality API plant and a formulation pilot plant. We plan for this facility to primarily manufacture APIs for later- stage clinical trials and potential FDA-approved drugs. The phase B construction will start when demanded by our future business growth. We are also developing an approximately 150,000 square-foot office and laboratory services building in Fengxian, Shanghai primarily for pharmaceutical development services. Approximately 32,000 square-foot of the laboratory service building has been completed and went into operation in November 2011 and the construction of the rest of the areas will start when demanded by our future business growth. We may build additional manufacturing facilities if we experience strong demand for commercial manufacturing of FDA- or EMEA-approved drugs.
Biologics
Capitalizing on the commercial success of therapeutic monoclonal antibodies and recombinant proteins in the past decade and the increasing demand for biologics contract research, we began providing biologics services in early 2010, with an initial focus on the discovery and preclinical stages, to design and implement strategies for the discovery, engineering and delivery of therapeutic antibody and recombinant protein drug candidates for clinical development. As of December 31, 2011, our biologics team consisted of 73 scientific research staff. Our current biologics services primarily include:
| | • | | Therapeutic antibody generation.We use hybridoma technology, combined with stringent screening funnels, to identify early therapeutic antibody leads with the desired biological and pharmacological properties. As part of our integrated services, we also develop surrogate antibodies forin vivostudies in animal models for target validation and proof-of-concept analysis. |
| | • | | Antibody optimization and engineering.We provide humanization design services for selected therapeutic hybridoma antibody leads, and perform laboratory tests and analysis for humanized lead selection. In addition, we perform affinity maturation to improve the binding affinity and biological potency of the humanized antibodies using phage display technology. |
| | • | | Analytical services for large molecules.We provide experimental measures to analyze the biological and physicochemical properties of therapeutic antibody or recombinant protein drug candidates, such as affinity, potency, structural integrity, glycosylation profiling, as well as pharmacokinetic analysis in |
34
| | rodents and non-human primates. We also provide pre-formulation services to address the drug-like properties of the lead candidate. Such measures include stability and solubility studies to provide a better understanding of key properties of the drug candidate formulation. |
Our biologics platform also extends to a broad spectrum of quality services across the drug discovery and development process, including general molecular biology, recombinant protein production and purification, cell line generation, epitome mapping, protein crystallography, assay development and bioanalytics. We intend to further expand our capability in biologics process development in the near future.
Marketing and Brand Promotion
We identify potential customers by actively networking with our existing customers, employees and consultants, conducting market research, participating in trade conferences and shows, and publishing marketing materials, among other activities. We also receive a significant amount of business from customer referrals.
Once we identify a potential customer and after making an initial customer contact, we seek to identify the potential customer’s needs for services and align such needs with our service capabilities either through on-site visits to, or teleconferences with, the potential customer. Subsequently, we typically conduct technical due diligence on the potential customer, make specific project proposals and, if the potential customer desires to use our services, negotiate and enter into a service agreement with the customer. We have entered into master services agreements with some customers, the terms of which typically range from one to five years.
We have expanded our sales and marketing teams in the U.S. and Europe to increase market penetration in our targeted markets and drive revenues growth.
Competition
We primarily compete with international and Chinese contract research organizations, or CROs. We anticipate that we will face increased competition as new companies enter the market and advanced technologies become available. See “Item 3. Key Information—Risk Factors—Risks Relating to Our Business and Industry—We face increasingly intense competition. If we do not compete successfully against new and existing competitors, demand for our services and related revenues may decrease and we may be subject to increasing pricing pressure.”
The pharmaceutical and biotechnology R&D outsourcing market remains highly competitive. We compete with WuXi PharmaTech across the breadth of our service offerings and compete with local Chinese contract research companies in various service areas. We also compete with industry participants in certain service areas, for example with Albany Molecular Research, Inc. in discovery chemistry and pharmaceutical development, and Cerep S.A. and Covance Inc. in discovery biology and preclinical development.
We compete primarily on the basis of the quality and scope of services, the ability to protect confidential information and intellectual property, the ability to be responsive to and efficient with respect to customers’ requests and pricing. We emphasize high-quality services and place a strategic emphasis on protection of customer intellectual property. We also compete on the basis of our relationships with customers. In addition, we compete on the basis of our turnaround time, resources and geography. We believe we compete favorably on the basis of each of these factors. However, many of our current or future competitors may have longer operating histories, better name recognition, greater levels of consumer trust, stronger management capabilities, better supplier relationships, a larger technical staff and sales force and/or greater financial, technical or marketing resources than we do. In addition, consolidation within the global pharmaceutical and biotechnology R&D outsourcing markets may create stronger competitors than those we currently face.
35
The successful recruitment and retention of highly qualified scientists is a key element in achieving our strategic goals. We believe that as competitive pressures in the drug R&D industry increase, the recruitment and retention of scientists will become increasingly competitive. To meet this challenge, we actively recruit scientists at colleges and universities and through other means. We believe the sophisticated drug R&D services that we perform and our growth prospects assist us in attracting and retaining highly qualified scientists. We also offer competitive salaries and benefits to recruit and retain highly skilled scientists.
Insurance
We maintain property insurance policies covering physical damage or loss of our equipment, and office furniture, employer’s liability insurance generally covering death or work injury of employees, public liability insurance covering third party bodily injury and property damage incurred in connection with our business within China, and directors and officers liability insurance. We do not have general liability or product liability insurance covering our whole business or all of our products and services. We currently maintain general liability insurance for five contracts covering, among other things, bodily injury and property damages arising out of the products or services provided under these contracts. The aggregate net revenues derived from these contracts in 2011 accounted for 16% of our total net revenues in this period. The aggregate maximum coverage amount under the insurance policy for these contracts is US$5 million for bodily injury and property damage arising out of our products or services and economic loss sustained by persons or entities due to deficiencies in our products or services. We do not maintain business disruption insurance or key man life insurance. We believe our insurance coverage is customary for companies of comparable size in comparable industries in China. See “Item 3. Key Information—Risk Factors—Risks Relating to Our Business and Industry—We have limited insurance coverage, and any claims beyond our insurance coverage may result in our incurring substantial costs and a diversion of resources.”
Legal Proceedings
We may from time to time be subject to various legal or administrative proceedings, either as plaintiff or defendant, arising in the ordinary course of our business. We are not currently a party to, nor are we aware of, any legal proceedings, investigation or claim that, in the view of our management, is likely to materially and adversely affect our business, financial condition or results of operations.
Regulations
Regulation of the Pharmaceutical and Biotechnology R&D Industry in China
This section summarizes the principal laws and regulations relevant to our business activities in China.
State Food and Drug Administration
In the PRC, the State Food and Drug Administration, or SFDA, is the authority that monitors and supervises the administration of pharmaceutical products and medical appliances and equipment as well as food and cosmetics.
The SFDA’s primary responsibilities include:
| | • | | monitoring and supervising the administration of pharmaceutical products, medical appliances and equipment as well as food and cosmetics in the PRC; |
| | • | | formulating administrative rules and policies concerning the supervision and administration of food, dietary supplements, cosmetics and the pharmaceutical industry; |
| | • | | evaluating, registering and approving new drugs, generic drugs, imported drugs and traditional Chinese medicine; and |
36
| | • | | approving and issuing permits for the manufacture and export/import of pharmaceutical products and medical appliances and equipment and approving the establishment of enterprises for pharmaceutical manufacture and distribution. |
PRC Drug Administration Law and Implementation Rules
The PRC Drug Administration Law promulgated by the Standing Committee of the National People’s Congress in 1984 and the Implementing Measures of the PRC Drug Administration Law promulgated by the MOH in 1989 have established the legal framework for the establishment of pharmaceutical manufacturing enterprises and pharmaceutical trading enterprises and for the administration of pharmaceutical products, which includes the development, research and manufacturing of new drugs and medical preparations by medical institutions. The PRC Drug Administration Law also regulates the packaging, trademark and advertisement of pharmaceutical products in the PRC.
Certain revisions to the PRC Drug Administration Law took effect in December 2001. They were formulated to strengthen the supervision and administration of pharmaceutical products, and to ensure the quality and safety of pharmaceutical products for human use. The revised PRC Drug Administration Law applies to entities and individuals engaged in the development, production, trade, application, supervision and administration of pharmaceutical products. It regulates and prescribes a framework for the administration of pharmaceutical manufacturers, pharmaceutical trading companies, medical preparations of medical institutions and the development, research, manufacturing, distribution, packaging, pricing and advertisement of pharmaceutical products.
The PRC Drug Administration Implementing Rules promulgated by the State Council took effect in September 2002 to provide detailed implementing regulations for the revised PRC Drug Administration Law.
Blood and Reagent Import and Export
According to the Circular on Strengthening Administration on Entry and Exit Inspection and Quarantine of Special Articles for Medical Use, import or export of medical special articles, including without limitation blood and reagents, must be inspected by the relevant inspection and quarantine authorities and be approved by the Ministry of Health or its local counterparts or other authorities, as the case may be.
Safety, Health and Environmental Matters
Our operations and properties are subject to extensive environmental protection and health and safety laws and regulations. These laws and regulations govern, among other things, the generation, storage, handling, use and transportation of hazardous or radioactive materials, the handling and disposal of hazardous, biohazardous and radioactive waste generated at our facilities, and the prevention and treatment of occupational diseases.
These laws and regulations generally impose liability regardless of the negligence or fault of a responsible party, unless such party has legally defined immunities. These laws and regulations also require us to obtain verifications, approvals or permits from governmental authorities for certain operations or facilities. If we violate or fail to comply with these laws, regulations or permits, we could be fined or otherwise sanctioned by regulators. Under certain environmental laws and regulations, we could also be held responsible for all of the costs relating to any contamination at our past or present facilities and at third party waste disposal sites.
Although we believe that our costs of complying with current and future environmental and health and safety laws, and our liabilities arising from past or future releases of, or exposure to, hazardous substances will not materially and adversely affect our business, results of operations or financial condition, they may do so. In addition, we cannot eliminate the risk of accidental contamination or injury from these hazardous materials. In that event, we could be liable for any resulting damage, which could exceed our resources and harm our results of
37
operations. We also have not completed certain procedures required for compliance with safety and health laws for some of our facilities or projects. See “Item 3. Key Information—Risk Factors—Risks Relating to Our Business and Industry—Compliance with environmental regulations can be expensive, and noncompliance with these regulations may result in significant monetary damages and fines and other penalties” and “Item 3. Key Information—Risk Factors—Risks Relating to Our Business and Industry—We are subject to safety and health laws and regulations in China, and any failure to comply could adversely affect our operations.”
Trade Secrets
According to the Anti-unfair Competition Law of the PRC, “trade secrets” refer to technical and business information that is unknown to the public, has utility and may create business interest or profit for its legal owners or holders, and which is maintained as secrets by its legal owners or holders.
Under this law, business persons are prohibited from employing the following methods to infringe trade secrets: (i) to obtain the trade secrets from the legal owners or holders by any unfair methods such as stealing, solicitation or coercion; (ii) to disclose, use or permit others to use the trade secrets obtained illegally under item (i) above; or (iii) to disclose, use or permit others to use the trade secrets in violation of any contractual agreements or any requirements of the legal owners or holders to keep such trade secrets in confidence. If a third party knows or should have known the above-mentioned illegal conduct but nevertheless obtains, uses or discloses such trade secrets of others, it may be deemed to have misappropriated others’ trade secrets. The parties being harmed may petition for administrative corrections, and competent regulatory authorities may stop any illegal activities and may fine infringing parties in the amount of RMB10,000 to RMB200,000. Alternatively, persons being harmed may file lawsuits for damages caused due to the misappropriation of the trade secrets in a competent PRC court.
The measures to protect trade secrets include oral or written agreements or other reasonable measures to require the employees of or persons in business contact with legal owners or holders to keep such trade secrets confidential. As soon as the legal owners or holders request others to keep the trade secrets confidential and adopt reasonable protection measures, the requested persons shall bear the liability to keep the trade secrets confidential.
Animal Test Permit and Other Related Regulatory Requirements
According to the Administrative Measures on Certificate for Animal Experimentation (Trial), performing experimentation on animals requires a Certificate for Use of Laboratory Animals. Applicants must satisfy the conditions as follows: (i) laboratory animals must be qualified and from entities that have Certificates for Production of Laboratory Animals; (ii) environment and facilities for laboratory animals’ living and propagating must meet state requirements; (iii) laboratory animals’ feed must meet state requirements; (iv) workers feeding or experimenting on laboratory animals must have received professional training; (v) management systems must be effective and efficient; and (vi) the applicable entity must follow other requirements as stipulated by PRC laws and regulations.
Pathogenic Microorganisms Lab Classification
According to the Administrative Rules on the Bio-safety of Pathogenic Microorganism Labs promulgated by the State Council on November 12, 2004, the labs conducting pathogenic microorganism-related experiments in China are classified into four levels based on their different bio-safety protection levels as well as in accordance with the national bio-safety standards. All of our labs are Level 1 and Level 2 labs under the Administrative Rules and are prohibited from conducting experiments relating to highly pathogenic organisms. The establishment, alteration and extension of such labs are required to be filed with the local administrative health or veterinary authority.
38
Radioisotope Use License and Other Related Regulatory Requirements
According to applicable PRC laws and regulations, it is mandatory to obtain a radiation safety license for radioisotope material handling. To apply for such license, applicants are required to meet certain conditions, including having: (i) professionals who have the relevant safety and self-protection knowledge, meet health requirements and are qualified to handle radioisotope materials; (ii) the facilities and equipment that meet national environmental, health and safety standards; (iii) a dedicated safety and monitoring committee or dedicated safety and monitoring personnel equipped with appropriate protective equipment and monitoring instruments; (iv) sound internal safety and monitoring rules and procedures and emergency response measures to prevent and minimize the damages caused by radiation accidents; and (v) the capability to treat any radioactive waste gas, liquid or solid or having a feasible treatment plan to ensure that the radioactive waste is discharged appropriately.
In addition, the purchase of radioisotope materials needs to be approved by relevant environmental protection authorities and registered with relevant environmental protection authorities after the transfer and the disposal of radioisotope material waste should be performed by qualified entities and be filed with relevant environmental protection authorities. Enterprises using radioisotopes and radial equipment should conduct yearly evaluation on the safety and maintenance conditions of these equipment, and submit such report to the relevant environmental protection authorities.
Use of Narcotic Drugs and Psychotropic Drugs
China regulates the production, distribution and use of narcotic drugs and psychotropic drugs pursuant to the Regulations on the Administration of Narcotic Drugs and Psychotropic Drugs, which became effective on November 1, 2005. Under these regulations, only those entities designated by the State Food and Drug Administration or its provincial branches may engage in the production or distribution of narcotic drugs and psychotropic drugs. In case that an entity engaged in scientific researches needs to use narcotic drugs or psychotropic drugs to carry out experiment activities, it may, upon the approval of provincial food and drug administration, purchase such drugs from those designated entities.
Regulations on Tax
PRC Enterprise Income Tax
The PRC enterprise income tax is calculated based on the taxable income determined under the PRC laws and accounting standards. On March 16, 2007, the National People’s Congress of China enacted a new PRC Enterprise Income Tax Law, which became effective on January 1, 2008. On December 6, 2007, the State Council promulgated the Implementing Rules to the PRC Enterprise Income Tax Law, or the Implementing Rules, which also became effective on January 1, 2008. On December 26, 2007, the State Council issued the Notice on Implementation of Enterprise Income Tax Transition Preferential Policy under the PRC Enterprise Income Tax Law, or the Transition Preferential Policy Circular, which became effective simultaneously with the PRC Enterprise Income Tax Law. The PRC Enterprise Income Tax Law imposes an uniform enterprise income tax rate of 25% on all domestic enterprises, including foreign-invested enterprises unless they qualify for certain exceptions, and terminates most of the tax exemptions, reductions and preferential treatments available under previous tax laws and regulations. Under the PRC Enterprise Income Tax Law and the Transition Preferential Policy Circular, enterprises established before March 16, 2007 that already enjoyed preferential tax treatments will continue to enjoy them (i) in the case of preferential tax rates, for a maximum of a five-year period starting from January 1, 2008; during the five-year period, the tax rate will gradually increase from their current preferential tax rate to 25%, or (ii) in the case of preferential tax exemption or reduction for a specified term, until the expiration of such term.
Prior to the effectiveness of the PRC Enterprise Income Tax Law on January 1, 2008, domestic companies were generally subject to an enterprise income tax at a statutory rate of 33%. However, our PRC subsidiaries in China that satisfied certain conditions enjoyed preferential tax treatment.
39
The new PRC Enterprise Income Tax Law and the Implementing Rules permit certain “high and new technology enterprises strongly supported by the state” that hold independent ownership of core intellectual property and simultaneously meet a list of other criteria, financial or non-financial, as stipulated in the Implementing Rules, to enjoy a reduced 15% enterprise income tax rate subject to certain new qualification criteria. The State Administration of Taxation, the Ministry of Science and Technology and the Ministry of Finance jointly issued the Administrative Rules for the Certification of High and New Technology Enterprises delineating the specific criteria and procedures for the “high and new technology enterprises” certification on April 14, 2008.
Moreover, under the PRC Enterprise Income Tax Law, enterprises organized under the laws of jurisdictions outside China with “de facto management bodies” located within China may be considered PRC resident enterprises and therefore subject to a 25% PRC enterprise income tax on their worldwide income. The Implementing Rules define “de facto management body” as the management body that exercises full and substantial control and overall management over the business, productions, personnel, accounts and properties of an enterprise. In addition, SAT Circular 82 provides that a foreign enterprise controlled by a PRC company or a PRC company group will be classified as a “resident enterprise” with its “de facto management bodies” located within China if the following requirements are satisfied: (i) the senior management and core management departments in charge of its daily operations function mainly in the PRC; (ii) its financial and human resource decisions are subject to determination or approval by persons or bodies in the PRC; (iii) its major assets, accounting books, company seals, and minutes and files of its board and shareholders’ meetings are located or kept in the PRC; and (iv) more than half of the enterprise’s directors or senior management with voting rights reside in the PRC. SAT Circular 45 clarifies certain issues related to determining PRC resident enterprise status and post-determination administration. Circular 45 specifies that when provided with a copy of a Chinese tax resident determination certificate issued by the competent tax authorities from an offshore incorporated PRC resident enterprise, the payer should not withhold 10% income tax when paying Chinese-sourced dividends, interest and royalties to PRC resident enterprises incorporated offshore. Although the SAT Circular 82 and Circular 45 only applies to offshore enterprises controlled by PRC enterprises and not those controlled by PRC individuals, foreigners or foreign enterprises, the determining criteria set forth in the Identification Circular may reflect the State Administration of Taxation’s general position on how the “de facto management body” test should be applied in determining the tax resident status of offshore enterprises, regardless of whether they are controlled by PRC or foreign enterprises or individuals.
Although we believe we are not a PRC resident enterprise for enterprise income tax purposes, substantial uncertainty exists. In the event that our company or any of our Hong Kong subsidiaries is considered a PRC resident enterprise, (1) we would be subject to the PRC enterprise income tax at the rate of 25% on our worldwide income; and (2) there is no assurance that dividend we receive from our subsidiaries would qualify as tax-exempt income pursuant to the PRC Enterprise Income Law as the SAT and other PRC authorities have not yet issued guidance with respect to the processing of outbound remittances to entities that are treated as resident enterprises controlled by PRC individuals for PRC enterprise income tax purposes. See “Item 3. Key Information—Risk Factors—Risks Related to Doing Business in China—Our global income and the dividends that we may receive from our PRC subsidiaries may be subject to PRC taxes under the PRC Enterprise Income Tax Law, which would materially and adversely affect our results of operations.”
Under SAT Circular 698, if a non-resident enterprise transfers the equity interests of a PRC resident enterprise indirectly via disposing of the equity interests of an overseas holding company, or Indirect Transfer, and such overseas holding company is located in a tax jurisdiction that: (i) has an effective tax rate less than 12.5% or (ii) does not tax foreign income of its residents, the foreign investor shall report to the competent tax authority of the PRC resident enterprise this Indirect Transfer. Using a “substance over form” principle, the PRC tax authority may disregard the existence of the overseas holding company if it lacks a reasonable commercial purpose and was established for the purpose of avoiding PRC tax. As a result, gains derived from such Indirect Transfer may be subject to PRC withholding tax at a rate of up to 10%. SAT Circular 698 also provides that, where a non-PRC resident enterprise transfers its equity interests in a PRC resident enterprise to its related
40
parties at a price lower than the fair market value, the relevant tax authority has the power to make a reasonable adjustment to the taxable income of the transaction. SAT Circular 698 is retroactively effective on January 1, 2008.
PRC Business Tax and Value Added Tax
Pursuant to applicable PRC laws and regulations, any entity or individual conducting business in the service industry is generally required to pay a business tax at the rate of 5% on the revenues generated from providing such services. However, if the services provided are related to technology development and transfer, such business tax may be exempted subject to the approval of relevant tax authorities.
Pursuant to the PRC Provisional Regulation on VAT, and its implementing rules, we are required to pay a VAT on the gross sales proceeds received from compound delivery services or product sales activities, less any creditable Input VAT already paid or borne. VAT is levied generally at a rate of 17%, while certain export sales are entitled to certain VAT credits.
On November 16, 2011, the Ministry of Finance and the SAT jointly issued the Circular on the Pilot Program for the Collection of Value Added Tax Instead of Business Tax, or Circular 110, and the Circular on the Pilot Program for the Collection of Value Added Tax Instead of Business Tax in the Transportation and Certain Modern Service Sectors in Shanghai, or Circular 111, which became effective on January 1, 2012. Pursuant to Circular 110 and Circular 111, a tax reform pilot program came into effect in Shanghai, which was chosen by the PRC government as the first pilot city for such reform. Starting from January 1, 2012, companies which are designated by Shanghai local tax authorities as operating in certain modern service sectors are required to pay value added tax, or VAT, in lieu of business tax. As a result of the pilot program, our PRC subsidiaries located in Shanghai are subject to VAT at the rate of 6% for sales proceeds received from certain laboratory services and 17% for sales proceeds received from compound delivery services or product sales activities. The services related to technology development and transfer remain to be tax exempted subject to the approval of relevant tax authorities.
Dividend Withholding Tax
Under the PRC tax laws effective prior to January 1, 2008, dividends paid to foreign investors by foreign-invested enterprises, such as dividends paid to us by our PRC subsidiaries, were exempted from PRC withholding tax. We are a Cayman Islands holding company and substantially all of our income may come from dividends we receive from our subsidiaries located in Hong Kong, which are the direct holding companies of our PRC subsidiaries. Pursuant to the PRC Enterprise Income Tax Law and the Implementation Rules, as well as the Arrangement between the Mainland of China and Hong Kong Special Administrative Region on the Avoidance of Double Taxation and Prevention of Fiscal Evasion with Respect to Taxes on Income, or the Arrangement, dividends generated after January 1, 2008 and distributed to our Hong Kong subsidiaries by our PRC subsidiaries may be subject to withholding tax at a preferential rate of no more than 5%, provided that our Hong Kong subsidiaries are determined by the relevant PRC tax authorities to be “non-resident enterprises” and hold at least 25% of the equity interests of our PRC subsidiaries. A non-resident enterprise under the PRC Enterprise Income Tax Law is incorporated in jurisdictions other than PRC but has an establishment or place of business (other than Management Bodies) in China or, despite the non-existence of such establishment or place in China, derives income within China. The State Administration for Taxation promulgated Notice on How to Understand and Determine the Beneficial Owners in Tax Agreement on October 27, 2009, or SAT Circular 601, which provides guidance for determining whether a resident of a contracting state is the “beneficial owner” of an item of income under China’s tax treaties and tax arrangements. According to SAT Circular 601, a beneficial owner generally must be engaged in substantive business activities. An agent or conduit company will not be regarded as a beneficial owner and, therefore, will not qualify for treaty benefits. The conduit company normally refers to a company established for the purpose of avoiding or reducing taxes or transferring or accumulating profits. If we are considered as a PRC tax resident enterprise for tax purposes, any dividends paid by us as well as gains realized by our shareholders or ADS holders from the transfer of our shares may be regarded as China-sourced
41
income and as a result subject to PRC withholding tax at a rate of up to 10% in the event that such shareholders or ADS holders are non-resident enterprises. In addition, our individual shareholders may be subject to a PRC individual income tax on such dividends and gains at a rate of 20% in accordance with the PRC Individual Income Tax Law if such dividends and gains are deemed income derived from sources within the PRC. See “Item 3. Key Information—Risk Factors—Risks Related to Doing Business in China—Our global income and the dividends that we may receive from our PRC subsidiaries may be subject to PRC taxes under the PRC Enterprise Income Tax Law, which would materially and adversely affect our results of operations and “Item 3. Key Information—Risk Factors—Risks Related to Doing Business in China—We may be required to withhold PRC income tax on the dividends we pay you (if any), and any gain you realize on the transfer of our ordinary shares and ADSs may also be subject to PRC tax if we are treated as a PRC “resident enterprise.”
Regulations on Foreign Exchange
Foreign exchange activities in China are primarily governed by the following regulations:
| | • | | Foreign Currency Administration Rules (1996), as amended, or the Exchange Rules; and |
| | • | | Administration Rules of the Settlement, Sale and Payment of Foreign Exchange (1996), or the Administration Rules. |
Under the Exchange Rules, the RMB is convertible for current account items, including the distribution of dividends, interest and royalties payments, trade and service-related foreign exchange transactions. Conversion of RMB for capital account items, such as direct investment, loan, securities investment and repatriation of investment, however, is subject to the approval of SAFE or its local counterpart.
Under the Administration Rules, foreign-invested enterprises may only buy, sell and/or remit foreign currencies at banks authorized to conduct foreign exchange business after providing valid commercial documents and, in the case of capital account item transactions, obtaining approval from SAFE or its local counterpart. Capital investments outside of China, after obtaining the required approvals of the relevant approval authorities, such as Ministry of Commerce and the National Development and Reform Commission or their local counterparts, are also required to register with SAFE or its local counterpart.
Pursuant to Circular 142, the RMB fund from the settlement of foreign currency capital of a foreign- invested enterprise must be used within the business scope as approved by the examination and approval department of the government, and cannot be used for domestic equity investment unless it is otherwise provided for. Documents certifying the purposes of the RMB fund from the settlement of foreign currency capital, including a business contract, must also be submitted for the settlement of the foreign currency. In November 2010, SAFE promulgated an official notice requiring the authenticity of settlement of the funds raised from offshore offerings to be closely examined and the settlement of funds should conform to their intended use as listed in the offering document. For the settlement of funds in excess of those intended by the offering document or for a purpose other than that listed in the offering document, resolutions by the board of directors of such enterprises relating to the use of funds shall be submitted as a separate application document. In addition, SAFE strengthened its oversight of the flow and use of the RMB capital converted from foreign currency registered capital of a foreign-invested company. The use of such RMB capital may not be altered without SAFE’s approval, and such RMB capital may not in any case be used to repay RMB loans if the proceeds of such loans have not been used. Violations of the Circular 142 could result in severe monetary fines or penalties.
Regulations on Dividend Distribution
The principal regulations governing dividend distributions of wholly foreign-owned companies include:
| | • | | the Companies Law (2005); |
| | • | | the Wholly Foreign-Owned Enterprise Law (1986), as amended; and |
| | • | | the Wholly Foreign-Owned Enterprise Law Implementing Rules (1990), as amended. |
42
Under these regulations, wholly foreign-owned companies in the PRC may pay dividends only out of their accumulated profits as determined in accordance with PRC accounting standards and regulations. In addition, these wholly foreign-owned companies are required to set aside at least 10% of their respective accumulated profits each year, if any, to fund certain reserve funds, until the accumulative amount of such fund reaches 50% of its registered capital. At the discretion of these wholly foreign-owned companies, they may allocate a portion of their after-tax profits based on PRC accounting standards to staff welfare and bonus funds. These reserve funds and staff welfare and bonus funds are not distributable as cash dividends.
Regulations on Offshore Investment by PRC Residents
Pursuant to SAFE Circular 75, issued on October 21, 2005, (i) a PRC citizen residing in the PRC or non-PRC citizen primarily residing in the PRC due to his or her economic ties to the PRC, who is referred to as a PRC resident in Circular 75, shall register with the local branch of SAFE before it establishes or controls an overseas special purpose company, for the purpose of overseas equity financing; (ii) when a PRC resident contributes the assets of or its equity interests in a domestic enterprise into an overseas special purpose company, or engages in overseas financing after contributing assets or equity interests into a special purpose company, such PRC resident shall register his or her interest in the special purpose company and the change thereof with the local branch of SAFE; and (iii) when the special purpose company undergoes a material event outside of China, such as change in share capital, creation of any security interests on its assets or merger and acquisition, the PRC resident shall, within 30 days from the occurrence of such event, register such change with the local branch of SAFE. PRC residents who are shareholders of special purpose companies established before November 1, 2005 were required to register with the local branch of SAFE before March 31, 2006.
In 2003, our founders, who may be deemed PRC residents for the purpose of Circular 75, established our intermediary holding companies in Hong Kong, which subsequently acquired or established their directly- owned subsidiaries in China. Circular 75 retroactively applies to our initial formation of the corporate structure as a round-trip investment and required our founders to register their respective shareholdings in our company with the local SAFE branch in Shanghai by March 31, 2006. Our founders inadvertently missed this filing deadline. We have urged our founders to, and they have been making efforts in order to, apply for a remedial SAFE registration. Although such a remedial registration is permissible under supplementary rules of Circular 75, the local SAFE branch in Shanghai has not accepted our founders’ respective applications due to the lack of specific administrative procedures for remedial registrations. We will urge our founders to, and our founders will continue making efforts to, apply for a remedial SAFE registration.
In May 2011, SAFE issued Circular 19 which provides for detailed operating procedures and specific instructions for SAFE registration under the Circular 75. According to Circular 19, before SAFE accepts the application for remedial registration under Circular 75, SAFE may impose administrative penalties on the PRC resident individuals and the relevant PRC domestic enterprises if: (i) the relevant offshore enterprise has undergone a material change in its assets or equity interests, (ii) such domestic enterprise made any false statement when applied for the SAFE registration, and/or (iii) the domestic enterprise directly or indirectly controlled by the offshore enterprise has made any overseas payment such as dividend distribution, equity transfer, capital decrease and repayment of shareholder loan since November 1, 2005. If for any reason the relevant government authorities in China determine that our founders do not comply with Circular 75, our founders and PRC Subsidiaries may be subject to administrative measures and penalties and any proposed foreign exchange transaction between our Hong Kong intermediary holding companies and their directly owned subsidiaries in China may be restricted. See “Item 3. Key Information—Risk Factors—Risks Related to Doing Business in China—PRC regulations relating to the establishment of offshore special purpose companies by PRC residents may subject our PRC resident beneficial owners or our PRC subsidiaries to liability or penalties, limit our ability to inject capital into our PRC subsidiaries, limit our PRC subsidiaries’ ability to increase their registered capital or distribute profits to us, or may otherwise adversely affect us.”
43
Regulations on Employee Share Options Plan
In December 2006, the People’s Bank of China promulgated the Administrative Measures of Foreign Exchange Matters for Individuals, setting forth the respective requirements for foreign exchange transactions by individuals (both PRC or non-PRC citizens) under either the current account or the capital account. In January 2007, SAFE issued implementing rules for the Administrative Measures of Foreign Exchange Matters for Individuals, which, among other things, specified approval requirements for certain capital account transactions, such as a PRC citizen’s participation in employee share ownership plans or share option plans of an overseas publicly-listed company.
On February 20, 2012, SAFE promulgated the Notices on Issues concerning the Foreign Exchange Administration for Domestic Individuals Participating in Share Incentive Plans of Overseas Listed Companies, or the Stock Option Rules, which supersedes certain rules issued by SAFE in 2007. The purpose of the Stock Option Rules is to regulate the foreign exchange administration of PRC domestic individuals who participate in employee share holding plans and share option plans or other similar share incentive plans of overseas listed companies.
According to the Stock Option Rules, PRC citizens or residents habitually residing in the PRC continuously for over one year, who have been granted shares or share options by an overseas-listed company according to its share incentive plans, are required to, through a PRC subsidiary of such overseas listed company, appoint a PRC domestic qualified agent, register with SAFE or its local counterpart and complete certain other procedures related to the share incentive plans. Concurrent with the registration with SAFE or its local counterpart, the PRC domestic qualified agent shall obtain approval from SAFE or its local counterpart for an annual foreign exchange allowance and the opening of a special foreign exchange account at a PRC domestic bank to hold the funds in connection with share holding or share option exercises. Currently, foreign exchange income of the participating PRC residents received from the sale of share and dividends distributed by the overseas listed company are required to be fully remitted into such special domestic foreign currency account before the distribution to such participants. In addition, the PRC agents are required to amend or deregister the registrations with SAFE in case of any material change in, or termination of, the share incentive plans, within the time periods provided by the Stock Option Rules. As an overseas listed company, we and our Chinese employees who have been granted share options, RSUs or shares are subject to, and intend to comply with, the foregoing requirements.
Under the Foreign Currency Administration Rules, as amended in 2008, the foreign exchange proceeds of domestic entities and individuals can be remitted into China or deposited abroad, subject to the terms and conditions to be issued by SAFE. However, the implementation rules in respect of depositing the foreign exchange proceeds abroad have not been issued by SAFE. The foreign exchange proceeds from the sales of shares can be converted into RMB or transferred to such individuals’ foreign exchange savings account after the proceeds have been remitted back to the special foreign exchange account opened at the PRC domestic bank. If share options are exercised in a cashless exercise, the PRC domestic individuals are required to remit the proceeds to special foreign exchange accounts.
Many issues with respect to the Stock Option Rules require further interpretation. We and our PRC employees who have participated in an employee share ownership plan or share option plan are subject to the Stock Option Rules. If we or our PRC employees fail to comply with the Stock Option Rules, we and our PRC employees may face sanctions imposed by the PRC foreign exchange authority or any other PRC government authorities, including restriction on foreign currency conversions and additional capital contribution to our PRC subsidiaries.
In addition, the State Administration of Taxation has issued a few circulars concerning employee share options. Under these circulars, our employees working in China who exercise share options will be subject to PRC individual income tax. Our PRC subsidiaries have obligations to file documents related to employee share options with relevant tax authorities and withhold the individual income taxes of employees who exercise their share options. If our employees fail to pay and we fail to withhold their income taxes, we may face sanctions imposed by tax authorities or any other PRC government authorities.
44
Labor Laws and Social Insurance
Pursuant to the PRC Labor Law and the PRC Labor Contract Law, employers must execute written labor contracts with employees in order to establish an employment relationship. All employers must compensate their employees with wages equal to at least the local minimum wage standards. All employers are required to establish a system for labor safety and sanitation, strictly abide by state rules and standards and provide employees with workplace safety training. Violations of the PRC Labor Contract Law and the PRC Labor Law may result in the imposition of fines and other administrative liabilities. Criminal liability may arise for serious violations. In addition, employers in China are obliged to provide employees with welfare schemes covering pension insurance, unemployment insurance, maternity insurance, work-related injury insurance, medical insurance and housing funds.
Regulations on Overseas Listing
In 2006, six PRC regulatory agencies jointly adopted the Regulations on Mergers and Acquisitions of Domestic Enterprises by Foreign Investors, or the M&A Rule. This rule requires that, if an overseas company established or controlled by PRC domestic companies or citizens intends to acquire equity interests or assets of any other PRC domestic company affiliated with the PRC domestic companies or citizens, such acquisition must be submitted to the Ministry of Commerce, rather than local regulators, for approval. In addition, this regulation requires that an overseas company controlled directly or indirectly by PRC companies or citizens and holding equity interests of PRC domestic companies needs to obtain the approval of the CSRC prior to listing its securities on an overseas stock exchange. On September 21, 2006, the CSRC, published a notice on its official website specifying the documents and materials required to be submitted by overseas special purpose companies seeking the CSRC’s approval of their overseas listings.
While the application of the M&A Rule remains unclear, based on their understanding of current PRC laws, regulations, and the notice published on September 21, 2006, our PRC counsel, Fangda Partners, has advised us that we are not required to submit an application to the CSRC for its approval for the listing and trading of our ADSs on the New York Stock Exchange, because our PRC subsidiaries were either converted into wholly foreign owned enterprises prior to September 8, 2006, the effective date of the M&A rule or were incorporated as wholly foreign owned enterprises.
Fangda has further advised us that their opinions summarized above are subject to the timing and content of any new laws, rules and regulations or clearer implementation and interpretations from the Ministry of Commerce and CSRC in any form relating to the M&A Rule.
C.Organizational Structure
We are a Cayman Islands holding company and conduct substantially all of our business through our seven operating subsidiaries in China. We commenced operations in China in 2002 and established the holding company in the Cayman Islands on August 30, 2007. We own 100% of all of our operating subsidiaries in China through two wholly-owned companies, China Gateway Life Science (Holdings) Limited and ChemExplorer Company Limited, which were incorporated in Hong Kong on June 23, 2003 and January 3, 2003, respectively. Our corporate structure reflects common practice for companies with operations in China where separate legal entities are often required or advisable for purposes of obtaining relevant tax and other incentives in the relevant jurisdictions.
45
The following diagram illustrates our corporate structure and the place of formation of our principal subsidiaries as of the date of this annual report on Form 20-F.
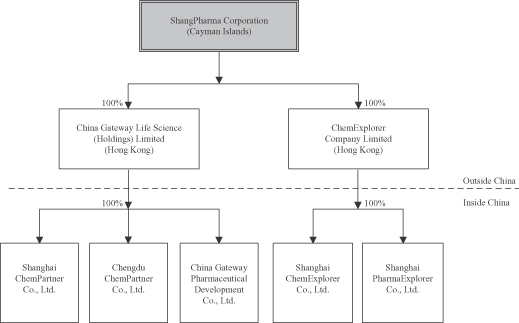
D.Property, Plants and Equipment
We are headquartered in Shanghai and conduct our business operations in five primary facilities in China:
| | • | | An approximately 701,000 square-foot headquarters in Shanghai Zhangjiang Hi-Tech Park, including an approximately 105,000 square-foot laboratory and office facility put into use in December 2011 that is dedicated to supporting discovery services for one of our largest customers and approximately 32,000 square-foot in-vivo research facilities and 10,000 square-foot lab and office space that were taken over from Charles River Laboratories International, Inc. in November 2011. |
| | • | | An approximately 13,000 square-foot AAALAC-accredited animal facility in Shanghai Zhangjiang Hi-Tech Park and an approximately 18,000 square-foot large animal facility in Fengxian, Shanghai, primarily used for discovery biology and preclinical development services. We may build or lease additional animal facilities if the demand for our discovery biology and preclinical development services increases. |
| | • | | An approximately 68,000 square-foot R&D center in Chengdu when it became operational in April 2011, which replaced our former 36,000 square-foot facility in Chengdu, to meet the increased demand for our discovery chemistry services. |
| | • | | An approximately 460,000 square-foot cGMP-quality multi-purpose manufacturing facility in Fengxian, Shanghai, which is under development, designed to manufacture advanced intermediates and APIs for preclinical testing and clinical trials. Phase A of the cGMP-quality multi-purpose manufacturing facility commenced operations on its initial phase of production in January 2011. The construction and development of the rest of the manufacturing facility will start when demanded by our future business growth. |
| | • | | An approximately 150,000 square-foot office and laboratory services building in Fengxian, Shanghai, which is under development primarily for pharmaceutical development services. Approximately 32,000 square-foot of the laboratory service building has been completed and went into operation in November 2011 and the construction of the rest of the areas will start when demanded by our future business growth. |
46
We also have an approximately 28,000 square-foot manufacturing facility, including a mini-pilot plant, a kilo-lab and a cleaning room unit, in Nanhui, Shanghai primarily used for pharmaceutical development services before our Fengxian manufacturing facility commenced its operation. The Nanhui facility substantially ended its operations in January 2011 and is in the process of being closed.
We own the Chengdu new R&D center and the manufacturing facility and the laboratory services building in Fengxian, Shanghai. Our remaining primary facilities consist of buildings leased from several property owners, including Shanghai PharmValley Corp., a related party. We have entered into a lease for each building. The leases generally have terms from two to five years and may be renewed by the parties. We generally make rental payments quarterly. Most of our leased buildings are used for office space and research activities.
| ITEM 4A. | UNRESOLVED STAFF COMMENTS |
Not applicable.
| ITEM 5. | OPERATING AND FINANCIAL REVIEW AND PROSPECTS |
You should read the following discussion and analysis of our financial condition and results of operations in conjunction with our consolidated financial statements and the related notes included elsewhere in this annual report on Form 20-F. This discussion may contain forward-looking statements based upon current expectations that involve risks and uncertainties. Our actual results may differ materially from those anticipated in these forward-looking statements as a result of various factors, including those set forth under “Item 3. Key Information—Risk Factors” or in other parts of this annual report on Form 20-F.
A.Operating Results
Overview
We are a leading China-based pharmaceutical and biotechnology R&D outsourcing company. We provide a broad range of high-quality, integrated services across the drug discovery and development process to international and Chinese pharmaceutical and biotechnology companies. Capitalizing on our broad service offerings, the experience and expertise of our skilled scientists and our state-of-the-art facilities and equipment, we help our customers discover and develop novel drug candidates efficiently. We have a strong and diversified customer base. We provide services to nearly 400 customers, including many of the top pharmaceutical and biotechnology companies in the world in terms of revenues and a number of fast-growing biotechnology and specialty pharmaceutical companies, as well as renowned academic and research institutions in the U.S. and China. We have experienced solid growth in recent years. The number of our customers increased from 134 in 2009 to 237 in 2010 and 395 in 2011, and our scientific research staff increased from 1,470 as of December 31, 2009 to 1,796 as of December 31, 2011.
We operate our business as a single segment. Our services consist of discovery chemistry, discovery biology and preclinical development, pharmaceutical development and biologics services. Our core service offering is discovery chemistry. Newer services, such as discovery biology, preclinical development and biologics services have grown at a higher rate than discovery chemistry. For example, in September 2011, we formed our first strategic partnership with Jiangsu Hengrui Medicine Co., Ltd. for the development of novel therapeutic monoclonal antibodies. Our integrated service platform allows us to cross-sell additional services to our existing customers. We saw increasing demand from our existing and new customers for integrated services, and a significant majority of our customers hired us for more than one service in each of 2009, 2010 and 2011. We offer our services on an FTE basis or a fee-for-service basis. We derive a significant majority of our net revenues from FTE-based services.
Our net revenues increased from US$72.3 million in 2009 to US$90.3 million in 2010 and US$111.6 million in 2011, representing a CAGR of 24.2%. Such increases were primarily attributable to an increase in the number of customers and resulting projects, broadened service offerings and expanded service capabilities. Our average net revenues per top ten customers increased from US$4.7 million in 2009 to US$6.9 million in 2011.
47
Our profits from operations increased from US$10.2 million in 2009 to US$10.4 million in 2010 and decreased to US$9.7 million in 2011. Our net income increased from US$9.8 million in 2009 to US$13.0 million in 2010 and decreased to US$11.2 million in 2011. The decrease in 2011 was mostly due to higher share-based compensation expenses totaled US$0.3 million, US$3.7 million, and US$6.2 million, respectively.
The global pharmaceutical and biotechnology industry has experienced significant consolidation and, during the global financial crisis, some pharmaceutical and biotechnology companies experienced financial difficulties. As a result, some of our customers reduced their R&D budgets especially in 2009 and 2010. We believe our strong growth in net revenues from 2009 to 2011 despite industry consolidation and global financial crisis reflected our customers’ recognition of our high-quality, integrated services.
Factors Affecting Our Results of Operations
We believe the following factors have had, and will continue to have, a significant effect on our results of operations:
Growth of the Global Pharmaceutical and Biotechnology R&D Outsourcing Industry.The growth of the global pharmaceutical and biotechnology R&D outsourcing industry has significantly affected, and is expected to continue to significantly affect, our results of operations. This growth has been primarily driven by the need to contain the cost of drug R&D, accelerate drug R&D process and meet the rising demand from the biotechnology industry. Many large pharmaceutical and biotechnology companies have been “offshoring” and/or outsourcing R&D activities to regions with significant resource and cost advantages, such as China. Advantages offered by China include a large talent pool in the chemistry, biology and medical sciences and related fields, relatively low-cost labor, developed infrastructure and favorable government incentives. The global pharmaceutical and biotechnology industry has experienced significant consolidation and, during the global financial crisis, some pharmaceutical and biotechnology companies experienced financial difficulties. As a result, some of our customers reduced their R&D budgets especially in 2009 and 2010. However, the trend in increasing global pharmaceutical and biotechnology R&D outsourcing activities has continued and is expected to continue in the near future.
Expansion of Our Service Offerings and Capabilities. Expansion and marketing of our service offerings and capabilities are essential to the growth of our business. We believe our customers value our ability to offer a wide range of quality services to meet their drug R&D needs, and we expect to capitalize on our strong customer base and expand our service offerings along the drug discovery and development value chain. Initially focusing on discovery chemistry services, we introduced pharmaceutical development services in 2005 and discovery biology and preclinical development services in 2007. We began offering biologics services in early 2010 which have experienced rapid growth since 2011. We have an approximately 460,000 square- foot cGMP-quality multi-purpose manufacturing facility in Fengxian, Shanghai, which is under development. Phase A of the multi-purpose manufacturing facility commenced operations on its initial phase of production in January 2011. The construction and development for the rest of the manufacturing facility will start when demanded by our future business growth. We are also developing an approximately 150,000 square-foot office and laboratory services building in Fengxian, Shanghai primarily for pharmaceutical development services. Approximately 32,000 square-foot of the laboratory service building has been completed and went into operation in November 2011 and the construction of the rest of the areas will start when demanded by our future business growth. Our business and results of operations will depend significantly on our ability to expand our service offerings and capabilities as planned and create demand for those newly expanded services or capabilities.
Labor Costs. Labor costs, which constitute a significant component of our cost of revenues, selling and marketing expenses and general and administrative expenses, significantly affect our profitability. Our employee base increased substantially from 1,713 as of December 31, 2009 to 2,076 as of December 31, 2011 and is expected to increase to meet our anticipated growth needs. In addition, our labor costs increased from 2009 to 2011 as we hired more senior scientists for newer services, such as discovery biology and preclinical development, pharmaceutical development and biologics, as well as more senior management as part of our strategy to grow our business. The build-up of our senior management team and corporate managerial and
48
supporting infrastructure was near completion at the end of 2010 and we believe that our current infrastructure will be sufficient to support our growth in the near future. The increases in labor costs were also attributable to appreciation of the Renminbi relative to the U.S. dollar, as we generally pay our employees in Renminbi, and overall rising labor costs in China. These increases were partially offset by efficiencies gained by our scientists and by our entry into higher margin services. We expect our labor costs as a percentage of total net revenues to decrease or remain relatively stable in the near future as we expect to hire more junior scientists to further expand our R&D team and leverage the experience and expertise of our senior scientists.
Fluctuations in Exchange Rates.The conversion of Renminbi into foreign currencies, including U.S. dollars, is based on rates set by the People’s Bank of China. The PRC government allowed the Renminbi to appreciate by more than 20% against the U.S. dollar between July 2005 and July 2008. Between July 2008 and June 2010, this appreciation was halted and the exchange rate between the Renminbi and the U.S. dollar remained within a narrow band. As a consequence, the RMB fluctuated significantly during that period against other freely traded currencies, in tandem with the U.S. dollar. Since June 2010, the PRC government has allowed the Renminbi to appreciate slowly against the U.S. dollar again. It is difficult to predict how market forces or PRC or U.S. government policy may impact the exchange rate between the Renminbi and the U.S. dollar in the future. There remains significant international pressure on the Chinese government to adopt a substantial liberalization of its currency policy, which could result in further appreciation in the value of the RMB against the U.S. dollar. Any further appreciation in the value of the Renminbi against the U.S. dollar will increase our cost of revenues and operating expenses and, if not passed on to our customers in the form of higher prices, adversely affect our margins.
Selected Statements of Operations Items
Net Revenues
Our net revenues comprise our total revenues from operations, less sales taxes. We offer our laboratory services on an FTE basis or a fee-for-service basis. An FTE arrangement differs from fee-for-service arrangements in that generally a team of our scientists is assigned to the needs of an individual customer for a particular period. The customer pays a fixed rate per FTE regardless of the workload or changes in the assignment, as determined in each contract, and the price generally includes some of the material costs incurred. Fees from FTE services are generally not contingent on outcomes or other deliverables or milestones. Revenues from fee-for-service contracts are typically contingent on successful performance of a service or delivery of a product and the price is often fixed in the contract. Fee-for-service contracts have different terms but are generally shorter in duration than FTE contracts. Revenues from these contracts fluctuate from period to period depending on, among other things, the nature of services and the completion schedule. The increases in our net revenues from 2009 to 2011 were attributable primarily to higher volumes from our top customers, greater cross selling of services, an increase in the size of our customer base and a broader scope of services we provided to our customers. We began providing pharmaceutical development services in 2005 and discovery biology and preclinical development services in 2007. We introduced biologics services in early 2010 which have experienced rapid growth since 2011. We derive a significant majority of our revenues from FTE contracts. The following table sets forth our FTE-based net revenues and fee-for-service-based net revenues by amount and as a percentage of our total net revenues for the periods indicated:
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | Years Ended December 31, | |
| | | 2009 | | | 2010 | | | 2011 | |
| | | Net Revenues | | | % | | | Net Revenues | | | % | | | Net Revenues | | | % | |
| | | (in millions of US$, except for percentages) | |
FTE-based net revenues | | | 56.7 | | | | 78.4 | % | | | 65.4 | | | | 72.4 | % | | | 80.7 | | | | 72.3 | % |
Fee-for-service-based net revenues | | | 15.6 | | | | 21.6 | | | | 24.9 | | | | 27.6 | | | | 30.9 | | | | 27.7 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Total net revenues: | | | 72.3 | | | | 100.0 | % | | | 90.3 | | | | 100.0 | % | | | 111.6 | | | | 100.0 | % |
| | | | | | | | | | | | | | | | | | | | | | | | |
49
We derive FTE-based revenues primarily from our discovery chemistry and discovery biology services. FTE-based revenues as a percentage of our total net revenues decreased from 78.4% in 2009 to 72.4% in 2010 and 72.3% in 2011 as we expanded our newer service offerings, such as preclinical development and biologics services, which are more typically covered by fee-for-service contracts.
We generated a majority of our net revenues in 2009, 2010 and 2011 from U.S.-based customers, with most of the remaining revenues from customers based in Europe, Japan and China. In 2011, we experienced significant revenue growth from customers based in China, including both local pharmaceutical companies and smaller domestic laboratories, as well as major multinational biotech and pharmaceutical companies’ Chinese R&D centers. In light of the increased drug development activities from domestic pharmaceutical and biotech companies and increased R&D investment into China by global pharmaceutical and biotech companies, we anticipate our China business will continue to grow and generate a larger percentage of our total revenues. A majority of our net revenues came from pharmaceutical companies, while biotechnology and specialty pharmaceutical customers contributed a significant portion of our net revenues during the same periods.
Cost of Revenues
Cost of revenues consists primarily of (i) labor costs, including salaries, bonuses and other benefits, for employees who provide laboratory services, (ii) raw material costs, including catalysts, reagents, solvents and other laboratory supplies and (iii) overhead costs, which primarily include the depreciation of equipment and owned facilities, property improvement and operating lease expenses for our leased facilities as well as utility expenses related to all our facilities.
Labor is a significant component of our cost of revenues and therefore an important factor affecting our profitability. Our headcount increased substantially from 2009 to 2011 as we expanded our service offerings and is expected to continue increasing to meet our anticipated growth needs. Our labor costs excluding share-based compensation expenses as a percentage of total net revenues remained relatively stable from 2009 to 2011 due to our hiring of more senior scientists for our newer services, appreciation of the Renminbi relative to the U.S. dollar and overall rising labor costs in China, offset by efficiencies gained by our scientists and by our entry into higher margin services.
Our raw material costs as a percentage of our total net revenues decreased from 2009 to 2010 but increased in 2011. Our newer service offerings including discovery biology, pharmaceutical development and biologics services generally have much higher raw material consumptions than our core discovery chemistry services although major expensive reagents can be reimbursed from our customers. With the strong growth of these newer service offerings in 2011, the overall raw material costs as a percentage of our total net revenues increased as a result of changes in service mix, which was partially offset by our tighter cost control measures such as improving raw material usage efficiencies, as well as our increased purchase bargaining power resulted from our economies of scale.
Our overhead costs increased from 2009 to 2011 as we expanded our facilities and purchased new equipment; for example, our overhead costs increased as a result of increasing operating lease expenses for our expanding laboratory facilities and depreciation of our new R&D center in Chengdu, our manufacturing facility and laboratory services building in Fengxian, Shanghai.
Cost of revenues also includes an allocation of our share-based compensation charges. See “—Critical Accounting Policies—Share-Based Compensation Expenses.”
Gross Profit and Margin
Gross profit is equal to net revenues less cost of revenues. Gross margin is equal to gross profit divided by net revenues. Changes in our gross profit and margin from period to period are primarily driven by an allocation
50
of our share-based compensation charges from period to period, changes in our service mix and net revenue per FTE as well as exchange rate fluctuations. Our gross margin was 33.0% in 2009, 33.4% in 2010 and 32.3% in 2011. Our gross margin was negatively impacted by the appreciation of the Renminbi relative to the U.S. dollar during these periods. Appreciation of the Renminbi, the currency in which we incur most of our expenses, in relation to the U.S. dollar, the currency in which we earn most of our revenues, puts pressure on our gross margin. Our gross margin was also negatively impacted by our increased share-based compensation costs and by increased depreciation expenses related to our expanded facilities and equipment as well as increased rental and utility expenses related to our expanded facilities. These adverse effects were partially offset by efficient material usage and improvements in operational efficiency.
Operating Expenses
Our operating expenses consist of selling and marketing expenses, general and administrative expenses and other operating income and expenses.
Selling and Marketing Expenses. Selling and marketing expenses consist primarily of salaries, bonuses, commissions and other benefits of our sales and business development personnel and promotional activities and tradeshow expenses. Our selling and marketing expenses increased from 2009 to 2011 primarily because we have built and are continuing to expand our sales and marketing teams in the U.S. and Europe during these periods. We expect that selling and marketing expenses will grow as a result of our efforts to increase market presence and drive revenue growth.
General and Administrative Expenses. General and administrative expenses consist primarily of (i) salaries, bonuses and other benefits for management and employees in our administration departments including finance, human resources, executive office, legal and information technology departments, (ii) professional service fees and (iii) other operating expenses attributable to general and administrative activities.
Our general and administrative expenses increased from 2009 to 2011 as our business expanded, including the hiring of additional executives and management staff. The build-up of our managerial and supporting infrastructure was near completion at the end of 2010 and we believe that the current infrastructure will be sufficient to support our growth in the near future. The increase of our general and administrative expenses was also attributable to the increased professional fees we incurred as a public company. We expect our general and administrative expenses to increase in future periods as our business grows but decrease or keep relatively stable in future periods as a percentage of our net revenues.
Other Operating Income and Expenses. Other operating income and expenses consist of research and hiring or training related government subsidies and the costs incurred related to such subsidies. Such income and expenses were recorded in other income and cost of revenues or general and administrative expenses, respectively in 2009, 2010 and the first half of 2011. Since the third quarter of 2011, as a result of an accounting policy change, the research and hiring or training related government subsidies were reclassified to other operating income, the costs related to research related subsidies were reclassified from cost of revenues to other operating expenses, and expenses related to hiring or training related subsidies continue to be recorded in general and administrative expenses. Although ASC 250 requires a retrospective application, management determined that a prospective application is deemed reasonable due to the insignificant impact on prior periods. See “—Critical Accounting Policies—Government Subsidies.”
Our operating expenses include an allocation of our share-based compensation charges. See “—Critical Accounting Policies—Share-Based Compensation Expenses.”
Income Taxes
Cayman Islands. We are incorporated in the Cayman Islands. Under the current laws of the Cayman Islands, we are not subject to income or capital gains tax. In addition, dividend payments are not subject to withholding tax in the Cayman Islands.
51
Hong Kong. Our subsidiaries did not have assessable profits that were earned in or derived from Hong Kong during the years ended December 31, 2009, 2010 and 2011. Accordingly, we did not pay Hong Kong profits tax during these periods.
PRC. Prior to the effectiveness of the PRC Enterprise Income Tax Law on January 1, 2008, enterprises in China were generally subject to an enterprise income tax at a statutory rate of 33% unless they qualified for certain preferential treatment. Starting January 1, 2008, the PRC Enterprise Income Tax Law has imposed a uniform enterprise income tax rate of 25% on all domestic enterprises and foreign-invested enterprises unless they qualify for certain exceptions. Enterprises established before March 16, 2007 that were entitled to the then available preferential tax treatment may continue to enjoy such treatment (i) in the case of preferential tax rates, for a maximum of a five-year period starting from January 1, 2008; during such period, the tax rate will gradually increase to 25%, or (ii) in the case of preferential tax exemption or reduction for a specified term, until the expiration of such term.
Shanghai ChemExplorer Co., Ltd. was entitled to a one-year exemption from enterprise income tax for 2007, and a 50% reduction on the otherwise applicable enterprise income tax rate for the subsequent three years, and Shanghai PharmaExplorer Co., Ltd. and Shanghai ChemPartner Co., Ltd. were entitled to a two-year exemption from enterprise income tax for 2007 and 2008, and a 50% reduction on the otherwise applicable enterprise income tax rate for the subsequent three years. Accordingly, the enterprise income tax rate applicable to Shanghai ChemExplorer Co., Ltd. increased from 10% in 2009 to 11% in 2010. The enterprise income tax rate applicable to Shanghai PharmaExplorer Co., Ltd. and Shanghai ChemPartner Co., Ltd. increased from 10% in 2009 to 12% in 2011. After the expiration of such preferential enterprise income tax rate, both Shanghai ChemExplorer Co., Ltd. and Shanghai ChemPartner Co., Ltd. will be entitled to a preferential tax rate of 15% until 2013 as approved Technical Advanced Service Enterprises, which status is effective from 2010 to 2013, subject to review by the tax authority and the approval authority. Shanghai PharmaExplorer Co., Ltd will be subject to a uniform tax rate of 25% starting 2012. In addition, Shanghai ChemExplorer Co., Ltd. and Shanghai ChemPartner Co., Ltd. were approved as high and new technology enterprises in 2008 and are entitled to a preferential tax rate of 15% effective from January 1, 2008. The high and new technology enterprise status is subject to approval and renewal every three years with current approval extending to October 19, 2014. Neither Shanghai ChemExplorer Co., Ltd. nor Shanghai ChemPartner Co., Ltd. has applied to, or obtained approval from, the local tax authority for the 15% preferential tax rate as a high and new technology enterprise since the two companies are already enjoying the 15% rate as Technical Advanced Service Enterprises.
Chengdu ChemPartner Co., Ltd. is located in Chengdu, China and was entitled to an exemption from income tax for the years 2008 and 2009 and a 50% reduced applicable corporate income tax rate for the subsequent three years (“2+3” tax holiday). In 2010, the local government approved that Chengdu ChemPartner Co., Ltd. was entitled to a 15% preferential tax rate in 2010 according to a preferential tax policy adopted to promote the development of China’s western regions. The 2+3 tax holiday and that preferential tax rate can be enjoyed at the same time. Accordingly, Chengdu ChemPartner Co., Ltd. was subject to a preferential tax rate at 7.5% in 2010. The preferential tax policy expired on December 31, 2010 and was renewed for another ten years effective January 1, 2011. As a result, Chengdu ChemPartner Co., Ltd. would be entitled to a preferential tax rate at 7.5% in 2011 as well. The entitlement to such preferential tax rate is subject to approval and renewal by local government annually. In addition, Chengdu ChemPartner Co., Ltd. was recognized as a high and new technology enterprise in 2008 and is entitled to a preferential tax rate of 15% which cannot be enjoyed at the same time with the 2+3 tax holiday. The high and new technology enterprise status is subject to approval and renewal every three years with current approval extending to October 11, 2014. Chengdu ChemPartner Co., Ltd has not applied to, or obtained approval from, the local tax authority for the 15% preferential tax rate as a high and new technology enterprise since we are already enjoying the 7.5% rate under the preference of promoting the development of China’s western regions and the 2+3 tax holiday.
Our effective enterprise tax rate increased to 18% in 2011 from 14% in 2009 and 2010, mainly as a result of these changes. See “Note 14—Taxation” to our consolidated financial statements included elsewhere in this annual report on Form 20-F.
52
Critical Accounting Policies
We prepare our consolidated financial statements in conformity with U.S. GAAP, which requires us to make estimates and assumptions that affect our reporting of, among other things, assets and liabilities, contingent assets and liabilities and net revenues and expenses. We regularly evaluate these estimates and assumptions based on the most recently available information, our historical experience and other factors that we believe to be relevant under the circumstances. Since our financial reporting process inherently relies on the use of estimates and assumptions, our actual results could differ from what we expect. This is especially true with some accounting policies that require higher degrees of judgment than others in their application. We consider the policies discussed below to be critical to an understanding of our audited consolidated financial statements because they involve the greatest reliance on our management’s judgment.
Revenue Recognition
We provide a broad range of integrated laboratory and manufacturing services in the drug discovery and development process to pharmaceutical and biotechnology companies. Laboratory services include contracts on FTE basis and fee-for-service basis.
Revenues are recognized when persuasive evidence of an arrangement exists, the sales price is fixed or determinable, delivery of the product or performance of the service has occurred and there is reasonable assurance of collection of the sales proceeds.
For laboratory services provided under a FTE basis, the customer pays a fixed rate per laboratory worker on a full time employment basis and we recognize revenue as the services are provided. The FTE contracts do not require acceptance by the customer or have obligations for fixed deliverables by us.
Prior to January 1, 2011, Laboratory services provided on a fee-for-service basis often include multiple elements of deliverables. Upon entering into such contractual agreements involving multiple elements of deliverables, we first determine whether each deliverable has standalone value to the customer. If the deliverables each have standalone value to the customer, then a separate unit of accounting is adopted to each separate deliverable. If any of the deliverables are not considered to have standalone value to the customer, then the deliverables are considered bundled and only one unit of accounting is assigned to the entire arrangement.
In September 2009, the FASB amended the accounting standards for multiple deliverables revenue arrangements. The new standard was adopted effective January 1, 2011 and requires us to use our best estimate of selling price (“BESP”) for the deliverables in an arrangement when vendor specific objective evidence (“VSOE”) or third party evidence of the selling price (“TPE”) is not available. The BESP should be determined in a way that is consistent with the price at which we would sell the deliverable if the item were to be sold separately. The residual method of allocating arrangement consideration will no longer be permitted.
We generally are not able to establish TPE or VSOE for our services, as the deliverables are highly customized and competitor pricing is not available or we are unable to reliably determine what similar competitor products’ selling prices are on a stand-alone basis. BESP for deliverables is generally established based on labor costs, reagents and supplies costs, overhead costs, risks and complexity of the work and expected profit margins developed from the competitive bidding process for customer contracts. We allocate the contractual arrangement’s value at the inception of the arrangement using relative fair value method based upon BESP. Consistent with our accounting policies prior to the adoption of this standard, revenue is recognized as each element is earned, provided that the fair value of the undelivered element(s) has been determined, the delivered element(s) has stand-alone value, there is no right of return on delivered element(s), and we are in control of the undelivered element(s). An element is considered earned upon the delivery or acceptance of the deliverables. The adoption of this standard did not have any impact on our consolidated financial statements.
For arrangements that include service elements, revenue is recognized for the service element using the proportionate performance method. We base measurement of performance on a comparison of direct labor hours through that date to the estimated total direct labor hours to complete the arrangement under the contract. We
53
believe this is the best indicator of the performance of the contractual obligations. Changes in the estimated total direct labor hours to complete a contract without a corresponding proportional change to the contract value result in a cumulative adjustment to the amount of revenue recognized in the period in which the change in estimate is determined.
Under certain arrangements, a portion of the payments owed to the Group are contingent upon successful achievement of performance standards or research and development success milestones. Revenues are recognized when the milestone achievements are accepted by the customers.
Under certain fee-for-service arrangements, the customers confirm the acceptance of the completed deliverables but require us to hold the deliverables temporarily to meet their shipping schedules. Under such arrangements, the customers also confirm that we have the rights to bill them upon the acceptance and any extra reprocessing costs due to the delay of shipping are to be burdened by them. So for such bill and hold arrangements, revenue is recognized upon acceptance of the deliverables by the customers even if delivery doesn’t occur. The amount of bill and hold sales were immaterial for 2009, 2010 and 2011. At December 31, 2011, there were no bill and hold arrangements outstanding.
In the event of changes in the scope, nature, duration, or volume of services of the contract with a customer, we negotiate to modify its existing contract or establish a new contract to reflect the changes. We recognize renegotiated amounts as revenue by revision to the total contract value resulting from the renegotiated contract.
Most of our revenue contracts can be terminated by our customers either immediately or after a specified period following notice. These contracts typically require the customer to pay the Group the fees earned through the termination date. Therefore, revenue recognized prior to cancellation generally does not require an adjustment upon cancellation.
Costs incurred prior to the delivery and acceptance of the deliverables are capitalized in inventory as work in progress. Cash received in advance of the delivery and acceptance of the deliverables is recorded as an advance from customers.
Revenues from the sale of manufactured products are recognized upon delivery and acceptance by the customer after title and risk of loss have been transferred.
Impairment of Long-Lived Assets
We review long-lived assets for impairment whenever events or changes in circumstances indicate the carrying amount of an asset may not be recoverable. Recoverability of these assets is measured by comparing their carrying amounts to the future undiscounted cash flows the assets are expected to generate. If the sum of the expected undiscounted cash flow is less than the carrying amount of the assets, we recognize an impairment loss equal to the amount by which the carrying value of the asset exceeds its fair value. We need to make judgments and estimates on expected cash flow and discount rates to derive fair value. No impairment of long-lived assets was recognized for the years ended December 31, 2009, 2010 and 2011.
Accounts Receivable
Accounts receivable are presented net of allowance for doubtful accounts. We provide specific allowance for doubtful accounts when facts and circumstances indicate that the collection is doubtful and a loss is probable and estimable. For the years ended December 31, 2009, 2010 and 2011, we recorded allowances for doubtful accounts of US$30,000, US$34,058 and US$149,783, respectively, and we wrote off doubtful accounts of nil, US$113,435 and nil, respectively.
Income Taxes
Income taxes are accounted for under the asset and liability method. Deferred taxes are determined based upon differences between the financial reporting and tax bases of assets and liabilities at currently enacted
54
statutory tax rates for the years in which the differences are expected to reverse. The effect on deferred taxes of a change in tax rates is recognized in the consolidated statements of operations in the period of change. A valuation allowance is provided on deferred tax assets to the extent that it is more likely than not that such deferred tax assets will not be realized. In assessing whether such deferred tax assets can be realized in the future, we need to make judgments and estimates on the ability to generate taxable income in future years. The total income tax provision includes current tax expenses under applicable tax regulations and the change in the balance of deferred tax assets and liabilities. We follow the guidance on accounting for uncertain tax positions in income taxes to recognize and measure our uncertain tax positions. We recognize a tax benefit from an uncertain tax position only if we believe it is more likely than not to be sustained upon examination based on the technical merits of the position. The amount of the accrual for which an exposure exists is measured as the largest amount of benefit determined on a cumulative probability basis that we believes is more likely than not to be realized upon ultimate settlement of the position. Components of the reserve are classified as either a current or long-term liability in the consolidated balance sheets based on when we expect each of the items to be settled. We accrue interest and penalties in relation to unrecognized tax benefits as a component of income tax expense.
Share-Based Compensation Expenses
2008 Equity and Performance Incentive Plan
On May 19, 2008, we adopted the 2008 Plan to attract and retain the best available personnel and provide additional incentives to employees, directors and consultants. On February 24, 2010, we amended and restated the 2008 Plan to increase the maximum aggregate number of our shares that may be issued under the 2008 Plan to 36,562,358 shares. See “Item 6. Directors, Senior Management and Employees—B. Compensation of Directors and Executive Officers—2008 Equity and Performance Incentive Plan” for a summary of the key terms of the 2008 Plan. As of December 31, 2011, options to purchase 32,593,840 ordinary shares were outstanding. The following table sets forth the options granted since the adoption of the 2008 Plan that were outstanding as of December 31, 2011:
| | | | | | | | | | | | | | | | | | | | |
Date of Grant | | Options
Outstanding | | | Exercise
Price | | | Grant Date Fair
Value of Ordinary
Shares(1) | | | Weighted
Average Fair
Value of Options | | | Grant Date
Intrinsic
Value | |
| | | (in US$, except for number of options) | |
May 19, 2008 | | | 8,525,754 | | | | 0.50 | | | | 0.20 | | | | 0.04 | | | | — | |
October 27, 2008 | | | 4,867,812 | | | | 0.50 | | | | 0.21 | | | | 0.04 | | | | — | |
May 13, 2009 | | | 2,869,200 | | | | 0.50 | | | | 0.45 | | | | 0.20 | | | | — | |
October 14, 2009 | | | 3,014,244 | | | | 0.50 | | | | 0.55 | | | | 0.27 | | | | 0.05 | |
May 24, 2010 | | | 2,917,350 | | | | 0.65 | | | | 0.65 | | | | 0.30 | | | | — | |
July 30, 2010 | | | 6,170,760 | | | | 0.65 | | | | 0.69 | | | | 0.33 | | | | 0.04 | |
November 19, 2010 | | | 300,000 | | | | 0.83 | | | | 0.63 | | | | 0.22 | | | | — | |
January 10, 2011 | | | 1,800,000 | | | | 0.69 | | | | 0.69 | | | | 0.30 | | | | — | |
June 30, 2011 | | | 595,800 | | | | 0.58 | | | | 0.58 | | | | 0.24 | | | | — | |
September 30, 2011 | | | 785,200 | | | | 0.46 | | | | 0.46 | | | | 0.19 | | | | — | |
December 30, 2011 | | | 747,720 | | | | 0.40 | | | | 0.40 | | | | 0.17 | | | | — | |
Notes:
| (1) | Prior to our initial public offering, grant date fair value for ordinary shares was determined based upon the valuation of an independent third party appraiser. After our initial public offering, grant date fair value for ordinary shares was determined based upon the closing price of our ADSs on the New York Stock Exchange on the grant date. |
Share-based compensation expense for all share-based awards granted to employees is determined based on the grant date fair value. Share-based compensation is expensed ratably on a straight-line basis for awards with vesting schedules based on service conditions only over the requisite service period, which is generally the vesting term of the share-based awards. A graded vesting method is applied to awards with vesting schedules with performance conditions.
55
Certain recipients of our share options became our consultants after their respective grant dates. We account for equity instruments issued to external consultants under the fair value method. The measurement date of the fair value of an equity instrument issued is the date on which the consultant’s performance is completed. Prior to the measurement date, the equity instrument is measured at its then-current fair value at each of the reporting dates. Share-based expense will be recognized over the service period using the graded-vesting method, and will be adjusted to reflect changes in the fair value of our ordinary shares between the reporting periods up to the measurement date.
We engaged an independent third party appraiser to assess the fair value of our share options and the ordinary shares underlying the options prior to our initial public offering. Since our initial public offering, we have assessed the fair value of our share options without the assistance of a third party appraiser. The share-based compensation expense for share options on the grant date were estimated based on each option’s fair value as calculated using the Black-Scholes option pricing model and the assumptions set forth in the following table:
| | | | | | | | | | | | |
| | | Years Ended December 31, | |
| | | 2009 | | | 2010 | | | 2011 | |
Risk-free interest rate(1) | | | 3-3.72 | % | | | 1.68%-3.37 | % | | | 1.08-2.65 | % |
Expected life (in years)(2) | | | 5-7 years | | | | 5-7 years | | | | 5-7 years | |
Expected dividend yield(3) | | | 0 | % | | | 0 | % | | | 0 | % |
Expected volatility(4) | | | 42-43 | % | | | 40%-44 | % | | | 37%-44 | % |
Fair value per option at grant date | | $ | 0.18-0.28 | | | $ | 0.20-0.35 | | | $ | 0.16-0.32 | |
Notes:
| (1) | The risk-free interest rate for the contractual term of the share option is based on the yield of China government bonds denominated in U.S. dollars in effect at the time of grant for a term consistent with the expected term of the awards. |
| (2) | The expected life of the share options granted under the 2008 Plan is based on vesting period, contractual term, share price, the employee’s level within our company and the expected volatility of the underlying shares. |
| (3) | We do not expect to pay dividends on our ordinary shares during the term of the options. |
| (4) | Expected volatility related to share options is estimated based on the average historical volatilities data of our company and our comparable companies in similar industry over a time period commensurate with the expected term of the awards as at the valuation dates. |
As we were a private company with no quoted market for our ordinary shares prior to our initial public offering, we needed to estimate the fair value of our ordinary shares at the relevant grant dates. In determining the fair values of our ordinary shares as of each award grant date, the third party appraiser considered three generally accepted valuation approaches: market, cost and income. The market approach requires market transactions of comparable assets as an indication of value. However, current comparable market transactions were not identified. The cost approach considers the cost to reproduce or replace the subject assets for similar assets at current market prices. While useful for certain purposes, the cost approach is generally considered not applicable to the valuation of a company as a going concern, as it does not capture the future earning potential of the business. In view of the above, the third party appraiser determined that the income approach is the most appropriate method to derive the fair values of our ordinary shares.
| | • | | The income approach involved applying appropriate discount rates to projected cash flows using management’s best estimates as of the valuation dates. The projected cash flows reflected, among other things, an analysis of projected revenue growth, profit margins, effective tax rates, capital expenditures and terminal multiples. The major assumptions used in determining the equity value of our company were consistent with our business plan and major milestones that we achieved. |
We and the third party appraiser also used other general inherently uncertain but best available assumptions, including the following: no material changes in the existing political, legal, fiscal and economic conditions and
56
the CRO industry in China; no significant contingent liabilities, unusual contractual obligations or substantial commitments; our ability to retain competent management and key personnel to support our ongoing operations; and no material deviation in market conditions from economic forecasts.
The valuation model then allocated the equity value between our ordinary shares and our preferred shares. The fair value of the equity interest allocated to the preferred shares was calculated using the option pricing method. Under the option pricing method, the preferred shares were treated as a call option on our equity value, with the exercise price based on the liquidation preference of the preferred shares. Because a call option was used, the option pricing method commonly used is the Black-Scholes model, which takes into account the expected life of the option, a risk-free interest rate, dividend yield and volatility. Because we were a private company prior to our initial public offering, the third party appraiser approximated volatility using the historical volatility of selected comparable publicly traded CROs listed in the U.S. In selecting such comparable companies, the third party appraiser considered factors including the nature of business, product offerings in the drug R&D process, target markets and customers, financial performance and stage of development.
Our share-based compensation expense related to the options granted by us under the 2008 Plan amounted to US$251,950 in 2009, US$930,355 in 2010 and US$1,420,531 in 2011. Determining the value of our share-based compensation expenses requires the input of highly subjective assumptions, including the expected life of the share-based awards, estimated forfeitures and the price volatility of the underlying shares. The assumptions used in calculating the fair value of share-based awards represent our best estimates, but these estimates involve inherent uncertainties and the application of our judgment. As a result, if factors change and we use different assumptions, our share-based compensation expenses could be materially different in the future.
Founder’s 2008 Equity and Performance Incentive Plan
In May 2008, our founders and our company established the 2008 Founder’s Plan to attract, motivate and retain our employees, non-employee directors and consultants. See “Item 6. Directors, Senior Management and Employees—B. Compensation of Directors and Executive Officers—Founder’s 2008 Equity and Performance Incentive Plan” for a summary of the key terms of the 2008 Founder’s Plan.
In 2009 and 2010, our founders granted RSUs convertible to 2,100,000 and 2,200,000 ordinary shares, respectively, to certain members of our senior management and other employees under the 2008 Founder’s Plan. We estimated the share-based compensation expense for RSUs granted under the 2008 Founder’s Plan based on the fair value of the RSUs on the date of grant. The vesting of RSUs was subject to the occurrence of an initial public offering or a change of control event (as defined in the 2008 Founder’s Plan), whichever occurred earlier. As a result, we did not record any share-based compensation expense in connection with the RSU grants prior to our initial public offering in October 2010 because the vesting conditions for the RSUs (the occurrence of an initial public offering or a change of control event) were not met. For the years ended December 31, 2010 and 2011, 7,541,592 and 2,426,386 RSUs became vested, respectively, and approximately US$2.8 million and US$0.8 million, respectively, were recorded as share-based compensation expense, including approximately US$2.5 million of share-based compensation expense recorded immediately upon the completion of our initial public offering.
2010 Share Incentive Plan and 2011 Share Incentive Plan
We established the 2010 Plan in December 2010 and 2011 Plan in August 2011 to promote our success and enhance value by linking the personal interests of our directors, employees and consultants to those of our shareholders and by providing such individuals with an incentive for outstanding performance to generate superior returns to our shareholders. See “Item 6. Directors, Senior Management and Employees— B. Compensation of Directors and Executive Officers—2010 Share Incentive Plan and 2011 Share Incentive Plan” for a summary of the key terms of the 2010 Plan and 2011 Plan. During 2011, we granted a total of 15,016,320 RSUs to certain of our directors, employees and consultants under the 2010 Plan and the 2011 Plan. Each RSU represents the right to receive one ordinary share upon vesting. We estimate the share-based compensation
57
expense for RSUs granted under these two plans based on the fair value of the RSUs on the date of grant. For 5,118,120 of the RSUs granted, each of the grantee’s RSUs shall vest every month up to the first to fourth anniversary of the date of grant. And for the rest of the RSUs granted, each of the grantee’s RSUs shall vest every quarter up to the tenth quarter of the date of grant. Total share-based compensation expenses related to the 2011 grant of RSUs of approximately US$9.1 million will be amortized during the vesting period from 2011 to 2014. Total compensation cost may be adjusted for future changes in estimated forfeitures.
Government Subsidies
Our government subsidies can generally be divided into (i) research related, (ii) hiring or training related, (iii) capital expenditure related and (iv) others.
For research related and hiring or training related government subsidies, we originally recognized the cash subsidies as other income when received if there is no defined rules and regulations to govern the criteria necessary for us to enjoy the benefits or initially recorded as liabilities and recognized as other income upon the obligations for earning such subsidies have been met.
For capital expenditure related government subsidies, the subsidies are initially recorded as advanced subsidies and recognized as a reduction of depreciation expenses using the straight-line method over the useful life of the assets acquired.
For other government subsidies, we recognize the cash subsidies as other income when received if there are no defined rules and regulations governing the criteria necessary for us to enjoy the benefits, or initially record them as liabilities and recognize as other income when the obligations for earning such subsidies have been met.
Effective July 1, 2011, we changed the accounting policy for research and hiring or training related government subsidies by classifying such subsidies as other operating income rather than the original other income. In addition, research related costs were reclassified from cost of revenues to other operating expenses, and hiring or training related expenses continue to be recorded in general and administrative expenses. Although ASC 250 requires a retrospective application, we determined that a prospective application is deemed reasonable due to the insignificant impact on prior periods.
Recent Accounting Pronouncements
In May 2011, FASB issued amendments to achieve common fair value measurement and disclosure requirements in US GAAP and IFRS. The amendments clarifies the application of certain existing fair value measurement guidance and expands the disclosures for fair value measurements that are estimated using significant unobservable (Level 3) inputs. The new guidance is to be applied prospectively and is effective during interim and annual periods beginning after December 15, 2011. Early application is not permitted. We will adopt this guidance effective January 1, 2012, and are currently evaluating the impact of the adoption of this standard to our consolidated financial statements.
In June 2011, the FASB issued ASU No. 2011-05, “Comprehensive Income (Topic 220): Presentation of Comprehensive Income” (ASU 2011-05). This newly issued accounting standard (1) eliminates the option to present the components of other comprehensive income as part of the statement of changes in shareholders’ equity; (2) requires the consecutive presentation of the statement of net income and other comprehensive income; and (3) requires an entity to present reclassification adjustments on the face of the financial statements from other comprehensive income to net income. The amendments in this ASU do not change the items that must be reported in other comprehensive income or when an item of other comprehensive income must be reclassified to net income nor do the amendments affect how earnings per share is calculated or presented. In December 2011, the FASB issued ASU No. 2011-12, Deferral of the Effective Date for Amendments to the Presentation of Reclassifications of Items Out of Accumulated Other Comprehensive Income in Accounting Standards Update No. 2011-05, which defers the requirement within ASU 2011-05 to present on the face of the financial statements
58
the effects of reclassifications out of accumulated other comprehensive income on the components of net income and other comprehensive income for all periods presented. During the deferral, entities should continue to report reclassifications out of accumulated other comprehensive income consistent with the presentation requirements in effect prior to the issuance of ASU 2011-05. These ASUs are required to be applied retrospectively and are effective for fiscal years, and interim periods within those years, beginning after December 15, 2011, which for us means January 1, 2012. As these accounting standards do not change the items that must be reported in other comprehensive income or when an item of other comprehensive income must be reclassified to net income, the adoption of these standards is not expected to have a material impact on our financial statements.
Results of Operations
The following table sets forth a summary of our consolidated results of operations by amount and as a percentage of our total net revenues for the periods indicated. This information should be read together with our consolidated financial statements and related notes included elsewhere in this annual report on Form 20-F. The operating results in any period are not necessarily indicative of the results that may be expected for any future period.
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | Years Ended December 31, | |
| | | 2009 | | | 2010 | | | 2011 | |
| | | Net
Revenues | | | % | | | Net
Revenues | | | % | | | Net
Revenues | | | % | |
| | | (in millions of US$, except for percentages and per share data) | |
Net revenues | | | 72.3 | | | | 100.0 | % | | | 90.3 | | | | 100.0 | % | | | 111.6 | | | | 100.0 | % |
Cost of revenues | | | (48.4 | ) | | | (67.0 | ) | | | (60.2 | ) | | | (66.6 | ) | | | (75.5 | ) | | | (67.7 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Gross profit | | | 23.9 | | | | 33.0 | | | | 30.1 | | | | 33.4 | | | | 36.1 | | | | 32.3 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Operating expenses | | | | | | | | | | | | | | | | | | | | | | | | |
Selling and marketing | | | (1.7 | ) | | | (2.2 | ) | | | (2.3 | ) | | | (2.5 | ) | | | (2.6 | ) | | | (2.4 | ) |
General and administrative | | | (12.0 | ) | | | (16.6 | ) | | | (17.4 | ) | | | (19.3 | ) | | | (24.0 | ) | | | (21.5 | ) |
Other operating income(1) | | | — | | | | — | | | | — | | | | — | | | | 0.4 | | | | 0.3 | |
Other operating expenses(1) | | | — | | | | — | | | | — | | | | — | | | | (0.2 | ) | | | (0.2 | ) |
Total operating expenses | | | (13.7 | ) | | | (18.8 | ) | | | (19.7 | ) | | | (21.8 | ) | | | (26.4 | ) | | | (23.7 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Profit from operations | | | 10.2 | | | | 14.2 | | | | 10.4 | | | | 11.5 | | | | 9.7 | | | | 8.7 | |
Interest income | | | * | | | | ** | | | | 0.1 | | | | 0.1 | | | | 1.0 | | | | 0.9 | |
Interest expense | | | — | | | | — | | | | * | | | | ** | | | | * | | | | ** | |
Other income | | | 1.3 | | | | 1.8 | | | | 4.8 | | | | 5.3 | | | | 4.0 | | | | 3.6 | |
Other expenses | | | (0.2 | ) | | | (0.3 | ) | | | (0.2 | ) | | | (0.2 | ) | | | (1.1 | ) | | | (1.0 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Income from operations before income taxes | | | 11.4 | | | | 15.7 | | | | 15.1 | | | | 16.7 | | | | 13.5 | | | | 12.1 | |
Income taxes | | | (1.6 | ) | | | (2.1 | ) | | | (2.1 | ) | | | (2.3 | ) | | | (2.4 | ) | | | (2.2 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Net income | | | 9.8 | | | | 13.6 | | | | 13.0 | | | | 14.4 | | | | 11.1 | | | | 10.0 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Allocation to preferred shareholders | | | (2.5 | ) | | | (3.5 | ) | | | (2.7 | ) | | | (3.0 | ) | | | — | | | | — | |
Net income attributable to ShangPharma Corporation’s ordinary shareholders | | | 7.3 | | | | 10.1 | % | | | 10.3 | | | | 11.4 | % | | | 11.1 | | | | 10.0 | % |
| | | | | | | | | | | | | | | | | | | | | | | | |
Net income attributable to ShangPharma Corporation’s ordinary shareholder per share | | | | | | | | | | | | | | | | | | | | | | | | |
Basic | | | 0.04 | | | | | | | | 0.04 | | | | | | | | 0.03 | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Diluted | | | 0.04 | | | | | | | | 0.04 | | | | | | | | 0.03 | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | |
59
| (1) | Other operating income and expenses consist of research and hiring or training related government subsidies and the costs incurred related to such subsidies. Such income and expenses were recorded in other income and cost of revenues or general and administrative expenses, respectively, in 2009, 2010 and the first half of 2011. Since the third quarter of 2011, as a result of an accounting policy change, the research and hiring or training related government subsidies were reclassified to other operating income, the costs related to research related subsidies were reclassified from cost of revenues to other operating expenses, and expenses related to hiring or training related subsidies continue to be recorded in general and administrative expenses. Although ASC 250 requires a retrospective application, management determined that a prospective application is deemed reasonable due to the insignificant impact on prior periods. See “—Critical Accounting Policies—Government Subsidies.” |
Year Ended December 31, 2011 Compared to Year Ended December 31, 2010
Net Revenues
Our net revenues increased by 23.6% from US$90.3 million in 2010 to US$111.6 million in 2011, primarily due to higher volumes from our top customers, greater cross selling of services and an increase in the size of the customer base. We provided services to 395 customers in 2011 compared to 237 customers in 2010. The following table sets forth our FTE-based net revenues and fee-for-service-based net revenues by amount and as a percentage of our total net revenues for the periods indicated:
| | | | | | | | | | | | | | | | |
| | | Years Ended December 31, | |
| | | 2010 | | | 2011 | |
| | | Net
Revenues | | | % | | | Net
Revenues | | | % | |
| | | (in millions of US$, except for percentages) | |
FTE-based net revenues | | | 65.4 | | | | 72.4 | % | | | 80.7 | | | | 72.3 | % |
Fee-for-service-based net revenues | | | 24.9 | | | | 27.6 | | | | 30.9 | | | | 27.7 | |
| | | | | | | | | | | | | | | | |
Total net revenues: | | | 90.3 | | | | 100.0 | % | | | 111.6 | | | | 100.0 | % |
| | | | | | | | | | | | | | | | |
Net revenues from FTE-based services increased by 23.4% from US$65.4 million in 2010 to US$80.7 million in 2011 due to increases in the average number of FTEs. Net revenues from our fee-for-service-based services increased by 24.3% from US$24.9 million in 2010 to US$30.9 million in 2011. The increase in demand for our FTE-based and fee-for-service-based services was primarily due to our continued strengthening of integrated service offerings, successful service cross-selling and rapid increased demand for services including discovery biology, preclinical development and biologics services. FTE-based revenues as a percentage of our total net revenues slightly decreased from 72.4% in 2010 to 72.3% in 2011 as we expanded our newer service offerings, such as preclinical development and biologics services, which are more typically covered by fee-for-service contracts.
Cost of Revenues
Our cost of revenues increased by 25.5% from US$60.2 million in 2010 to US$75.5 million in 2011, in line with the increase in our net revenues. The increase in our cost of revenues was primarily due to increases in labor, raw materials, overhead costs, continued appreciation of Renminbi and share-based compensation expenses. Our labor costs increased from 2010 to 2011 primarily due to the increase in our headcount to meet our business growth demand, as well as overall rising labor costs in China. Such increase was partially offset by efficiencies gained by our scientists and by our entry into higher margin services. Our raw material costs as a percentage of our total net revenues increased from 2010 to 2011 due to a shift of service mix as a result of the faster expansion of our newer service offerings such as discovery biology and biologics which generally have higher reagent consumption although major expensive reagents can be reimbursed from our customers. The impact from such shift of service mix was partially offset by our cost control measures, such as improving raw materials usage efficiencies, as well as our increased purchase bargaining power resulting from economies of
60
scale. Our overhead costs increased primarily due to our continued large capital expenditures on new equipment and the additional rental, utility and depreciation charges for our expanded facilities such as our new Chengdu R&D center, new building dedicated to supporting discovery services for one of our largest customers, assumed lease taken over from Charles River of in-vivo research facilities and office space, as well as Fengxian manufacturing facility that commenced operations in 2011.
Gross Profit and Margin
Our gross profit increased by 19.9% from US$30.1 million in 2010 to US$36.1 million in 2011. The increase in revenues and operational efficiency improvements were partially offset by continued appreciation of the Renminbi, higher share-based compensation expenses and higher materials costs as a result of changes in our services mix. Our gross margin decreased from 33.4% in 2010 to 32.3% in 2011, primarily due to the continued appreciation of Renminbi, higher share-based compensation expenses and higher material costs as a result of service mix, which were partially offset by our operational efficiency improvements.
Operating Expenses
Our total operating expenses increased by 34.0% from US$19.7 million in 2010 to US$26.4 million in 2011.
Selling and Marketing Expenses. Our selling and marketing expenses increased by 15.1% from US$2.3 million in 2010 to US$2.6 million in 2011. The increase in selling and marketing expenses in 2011 was primarily due to our continued efforts to increase market presence and drive revenue growth and higher share-based compensation expenses.
General and Administrative Expenses. Our general and administrative expenses increased by 37.5% from US$17.4 million in 2010 to US$24.0 million in 2011, primarily due to additional headcount resulting from a build-up of corporate managerial and supporting infrastructure during the second half of 2010, higher share-based compensation expenses, higher professional fees we incurred as a public company and increased operating costs related to our expanded facilities including the new building dedicated to support discovery services for one of our largest customers as well as the assumed lease of in-vivo research facilities and office space taken over from Charles River.
Other Operating Incomeand Expenses. Other operating income and expenses consist of research and hiring or training related government subsidies and the costs incurred related to such subsidies. Such income and expenses were recorded in other income and cost of revenues or general and administrative expenses, respectively in 2009, 2010 and the first half of 2011. Effective since the third quarter of 2011, as a result of an accounting policy change, research and hiring or training related government subsidies were reclassified to other operating income, the costs related to research related subsidies were reclassified from cost of revenues to other operating expenses, and expenses related to hiring or training related subsidies continue to be recorded in general and administrative expenses. See “—Critical Accounting Policies—Government Subsidies.”
Other Income
Our other income decreased from US$4.8 million in 2010 to US$4.0 million in 2011, primarily due to the accounting policy change to reclassify research and hiring or training related government subsidy income to other operating income since the third quarter of 2011 and a smaller gain recognized on foreign exchange forward contracts. The accounting policy change was not retroactively applied due to immateriality. See “—Critical Accounting Policies—Government Subsidies.”
Other Expenses
Our other expenses of US$1.1 million, which increased from US$0.2 million in 2010, primarily include a larger loss recognized on foreign exchange forward contracts, a one-time expense related to potential merger and acquisition projects and disposal losses incurred in the fourth quarter of 2011 during the closure process of our old manufacturing facility located in Nanhui, Shanghai, which was replaced by our new Fengxian manufacturing facility.
61
Income Tax Expense
Our income tax expense increased from US$2.1 million in 2010 to US$2.4 million in 2011, primarily reflecting an increase in effective tax rate. Our effective tax rate increase to 18% in 2011 from 14% in 2010. See “—Selected Statements of Operations Items—Income Taxes.”
Net Income
As a result of the foregoing, our net income decreased from US$13.0 million in 2010 to US$11.1 million in 2011.
Net income attributable to ShangPharma Corporation’s ordinary shareholders
Net income attributable to our ordinary shareholders was US$11.1 million in 2011, an increase of 8.4% from US$10.3 million in 2010.
Year Ended December 31, 2010 Compared to Year Ended December 31, 2009
Net Revenues
Our net revenues increased by 24.9% from US$72.3 million in 2009 to US$90.3 million in 2010, primarily due to a larger customer base, higher business volumes from our top customers, expanded service offering, higher FTE average rates and a favorable shift in the service mix. We provided services to 237 customers in 2010 compared to 134 customers in 2009. The following table sets forth our FTE-based net revenues and fee-for-service-based net revenues by amount and as a percentage of our total net revenues for the periods indicated:
| | | | | | | | | | | | | | | | |
| | | Years Ended December 31, | |
| | | 2009 | | | 2010 | |
| | | Net
Revenues | | | % | | | Net
Revenues | | | % | |
| | | (in millions of US$, except for percentages) | |
FTE-based net revenues | | | 56.7 | | | | 78.4 | % | | | 65.4 | | | | 72.4 | % |
Fee-for-service-based net revenues | | | 15.6 | | | | 21.6 | | | | 24.9 | | | | 27.6 | |
| | | | | | | | | | | | | | | | |
Total net revenues: | | | 72.3 | | | | 100.0 | % | | | 90.3 | | | | 100.0 | % |
| | | | | | | | | | | | | | | | |
Net revenues from FTE-based services increased by 15.5% from US$56.7 million in 2009 to US$65.4 million in 2010 due to increases in the average number of FTEs and net revenue per FTE. Net revenues from our fee-for-service-based services increased by 59.0% from US$15.6 million in 2009 to US$24.9 million in 2010. The increase in demand for our FTE-based and fee-for-service-based services was primarily due to an increase in demand for our discovery biology and preclinical development, discovery chemistry and pharmaceutical development services. FTE-based revenues as a percentage of our total net revenues decreased from 78.4% in 2009 to 72.4% in 2010 as we expanded our newer service offerings, such as discovery biology and preclinical development and pharmaceutical development, which are more typically covered by fee-for-service contracts.
Cost of Revenues
Our cost of revenues increased by 24.2% from US$48.4 million in 2009 to US$60.2 million in 2010, in line with the increase in our net revenues. The increase in our cost of revenues was primarily due to increases in labor, raw materials, overhead costs and share-based compensation expenses, including one-time RSU costs of approximately US$0.3 million that were incurred upon completion of our initial public offering. Our labor costs increased from 2009 to 2010 primarily due to the increase in our headcount and the hiring of more senior scientists for our newer services, such as discovery biology and preclinical development, as well as overall rising labor costs in China. Such increase was partially offset by efficiencies gained by our scientists and by our entry
62
into higher margin services. Our raw material costs as a percentage of our total net revenues decreased from 2009 to 2010 due to our cost control measures, such as improving raw materials usage efficiencies, as well as our increased bargaining power resulting from economies of scale. Our overhead costs increased as we expanded our facilities and purchased new equipment.
Gross Profit and Margin
Our gross profit increased by 26.2% from US$23.9 million in 2009 to US$30.1 million in 2010, primarily due to an increase in net revenues, favorable service mix, efficient material usage and operational efficiency improvement and was partially offset by continued appreciation of the Renminbi and higher share-based compensation expenses. Our gross margin increased from 33.0% in 2009 to 33.4% in 2010, primarily due to the favorable service mix, efficient material usage and operational efficiency improvement. The increase was partially offset by continued appreciation of the Renminbi and higher share-based compensation expenses.
Operating Expenses
Our total operating expenses increased by 45.0% from US$13.6 million in 2009 to US$19.7 million in 2010.
Selling and Marketing Expenses. Our selling and marketing expenses increased by 42.5% from US$1.6 million in 2009 to US$2.3 million in 2010. The increase in selling and marketing expenses in 2010 was primarily due to a build-up of corporate marketing and sales infrastructure and higher share-based compensation expenses, including one-time RSU costs of approximately US$0.2 million that were incurred upon completion of our initial public offering.
General and Administrative Expenses. Our general and administrative expenses increased by 45.3% from US$12.0 million in 2009 to US$17.4 in 2010, primarily due to higher share-based compensation including the one-time RSU costs of approximately US$2.0 million that were incurred upon completion of our initial public offering and a build-up of corporate managerial, sales and supporting infrastructure.
Other Income
Our other income increased from US$1.3 million in 2009 to US$4.8 million in 2010, primarily due to a gain on foreign exchange forward contracts of US$3.0 million, income from government grants of US$1.4 million and consideration received from the sale of an investment of US$0.3 million.
Other Expenses
Our other expenses of US$0.2 million, which were unchanged from 2009 to 2010, primarily include foreign exchange losses and losses recognized on foreign exchange forward contracts.
Income Tax Expense
Our income tax expense increased from US$1.6 million in 2009 to US$2.1 million in 2010, primarily reflecting an increase in income before taxes. Our effective tax rate remained at 14% in both 2009 and 2010. See “—Selected Statements of Operations Items—Income Taxes.”
Net Income
As a result of the foregoing, our net income increased from US$9.8 million in 2009 to US$13.0 million in 2010.
Allocation to preferred shareholders
Allocation to preferred shareholders was US$2.7 million in 2010, compared to US$2.5 million in 2009.
63
Net income attributable to ShangPharma Corporation’s ordinary shareholders
Net income attributable to our ordinary shareholders was US$10.3 million in 2010, an increase of 39.9% from US$7.3 million in 2009.
B.Liquidity and Capital Resources
Our primary sources of liquidity have been cash generated from operating and financing activities, including proceeds from our initial public offering in October 2010. As of December 31, 2011, we had approximately US$37.8 million in cash and cash equivalents. In addition, we have a bank facility comprised of a series of credit facilities including (i) one short term revolving facility not to exceed US$13.0 million subject to sublimits of US$5.0 million, US$5.0 million and US$8.0 million, respectively, for letters of credit, short-term borrowings secured by the assignment of certain of our receivables as collateral and hedging and risk-management derivative transactions; and (ii) a US$2.0 million three-month revolving facility and a US$5.0 million three-year term loan commitment, both of which will be secured by properties owned by one of our subsidiaries. No amounts had been drawn down under these facilities and term loan commitment as of December 31, 2011. As of the same date, we had a 60-day revolving facility with certain bank secured by the assignment of certain of our receivables as collateral and no amounts had been drawn down under this facility as of December 31, 2011. In July 2010, one of our subsidiaries entered into a loan agreement with a bank for a one-year working capital loan of up to RMB10.0 million (US$1.5 million). We had drawn down RMB5.0 million (US$0.75 million) under this loan, which had been fully repaid as of December 31, 2011.
We require cash to fund our ongoing business needs, particularly salary and benefits and material costs and expenses. Other cash needs include primarily the working capital for our daily operations, capital expenditures related to our expanded facilities, and purchase of equipment for our laboratory services. We believe that our cash, anticipated cash flow from operations and proceeds from our bank credit facilities will be sufficient to meet our anticipated cash needs for our operating activities, capital expenditures and other obligations for at least the next 12 months. We may, however, require additional cash resources due to changed business conditions or other future developments. We may sell additional equities or obtain credit facilities to enhance our liquidity position or to increase our cash reserves for future operations. The sale of additional equity would result in further dilution to our shareholders. The incurrence of indebtedness would result in increased fixed obligations and could result in operating covenants that would restrict our operations. We cannot assure you that financing will be available in amounts or on terms acceptable to us, if at all. The following table sets forth a summary of our cash flows for the periods indicated:
| | | | | | | | | | | | |
| | | Years Ended December 31, | |
| | | 2009 | | | 2010 | | | 2011 | |
| | | (in millions of US$) | |
Net cash provided by operating activities | | | 14.7 | | | | 18.7 | | | | 18.3 | |
Net cash used by investing activities | | | (18.3 | ) | | | (23.6 | ) | | | (28.6 | ) |
Net cash provided by (used in) financing activities | | | — | | | | 41.4 | | | | (2.4 | ) |
Effect of foreign exchange rate changes on cash and cash equivalents | | | * | | | | 0.4 | | | | 1.3 | |
Net increase (decrease) in cash and cash equivalents | | | (3.6 | ) | | | 36.9 | | | | (11.4 | ) |
Cash and cash equivalents at the beginning of the year | | | 15.8 | | | | 12.2 | | | | 49.2 | |
Cash and cash equivalents at the end of the year | | | 12.2 | | | | 49.2 | | | | 37.8 | |
Notes:
Operating Activities
Net cash provided by operating activities amounted to US$18.3 million in 2011, attributable to net income of US$11.1 million, (a) adjusted for certain non-cash expenses, primarily depreciation and amortization of
64
US$9.2 million and share-based compensation expenses of US$6.2 million, and for changes in working capital accounts that positively affected cash flow, primarily an increase in other payables and accrued liabilities of US$3.9 million, an increase in income taxes payable of US$1.3 million and an increase in accounts payable of US$1.2 million, and (b) partially offset by certain non-cash adjustments, primarily gains related to derivatives of US$1.9 million, and changes in working capital accounts that negatively affected cash flow, primarily an increase in accounts receivable of US$8.2 million and an increase in inventories of US$2.0 million. The increase in other payables and accrued liabilities is primarily due to larger unearned government subsidies, government subsidies received by us first which is necessary to be paid to certain of our entitled scientists and higher derivative liabilities for foreign-exchange forward contracts. The increase in income tax payable is due to the timing of our payments for 2011 income taxes. The increase in our accounts payable and accounts receivable were primarily due to the growth of our business and timing differences. The increase in our inventory balances were primarily due to the growth of our business, changes in completion schedule of each fee-for-service projects as well as a trend that we had more large and long-term projects with our major customers as a result of our increased customer acceptance and expanded services capabilities.
Net cash provided by operating activities amounted to US$18.7 million in 2010, primarily attributable to net income of US$13.0 million, an increase in advances from customers and salary and welfare payable of US$1.3 million and US$1.0 million, respectively, and add-back of certain non-cash adjustments such as depreciation and amortization of US$6.7 million and share-based compensation expenses of US$3.7 million, partially offset by an increase in accounts receivable and prepayment and others of US$2.6 million and US$1.1 million, respectively, and gains related to foreign exchange forward contracts of US$3.0 million.
Net cash provided by operating activities amounted to US$14.7 million in 2009, primarily attributable to net income of US$9.8 million, and add-back of certain non-cash adjustments such as depreciation and amortization of US$5.9 million and an increase of US$1.5 million in accounts payable, partially offset by an increase of US$4.8 million in accounts receivable.
Investing Activities
Net cash used in investing activities largely reflects our capital expenditures, which consist of purchases of property, equipment and software made in connection with the expansion and upgrade of our laboratory facilities and capabilities and the development of our manufacturing facility in Fengxian, Shanghai, as well as purchase of land use rights. We expect our net cash used in investing activities in 2012 to decrease as part of our manufacturing facility and laboratory service building have commenced operation in 2011 which is expected to be sufficient to support our current business growth of pharmaceutical development services. In addition, we do not expect to have large capital expenditures similar to those in 2011 which were related to our new Chengdu R&D center, our new building dedicated to supporting discovery services for one of our largest customers, in-vivo research facilities and office space acquired from Charles River as these facilities are also expected to be sufficient to support our laboratory service growth at least in 2012. The capital expenditures in 2012 are primarily expected to consist of the unpaid amounts to be paid in 2012 in connection with large projects completed in 2011 as well as general purchases of equipment and improvement of our current facilities.
Net cash used in investing activities amounted to US$28.6 million in 2011, primarily attributable to US$28.4 million in purchase of property, equipment and software, US$2.3 million in purchase of land use rights for our new Chengdu R&D center and US$1.9 million increase of restricted cash, which was partially offset by proceeds from settlement of foreign-exchange forward contracts of US$3.8 million.
Net cash used in investing activities amounted to US$23.6 million in 2010, primarily attributable to US$24.1 million in purchase of property, equipment and software.
Net cash used in investing activities amounted to US$18.3 million in 2009, primarily attributable to US$14.0 million in purchases of property, equipment and software and US$4.2 million in purchase of land use rights for our manufacturing facility in Fengxian, Shanghai.
65
Financing Activities
Net cash used in financing activities amounted to US$2.4 million for 2011, attributable to repayment of short-term borrowings of US$0.8 million, issuance costs paid for initial public offerings of US$0.7 million and repurchase of ordinary shares of US$1.1 million, which was partially offset by proceeds from issuance of ordinary shares upon exercise of options.
Net cash provided by financing activities amounted to US$41.4 million for 2010, attributable to a US$0.7 million borrowing under a one-year working capital loan and US$40.7 million in net proceeds from our initial public offering.
We did not have any inflows or outflows from financing activities in 2009.
C.Research and Development, Patents and Licenses, etc.
Research and Development
During each of the last three fiscal years, we did not spend any significant amounts on company-sponsored research and development activities.
Intellectual Property
Protection of intellectual property associated with drug R&D is critical to our customers. In our business of providing drug R&D services, our customers generally retain ownership of all associated intellectual property, including intellectual property they provide to us and intellectual property arising from the services we provide. Our success therefore depends in substantial part on our ability to protect the proprietary rights of our customers. This is particularly important because a substantial part of our operations are based in China, and China, as well as Chinese companies, have not traditionally enforced intellectual property protection to the same extent as the United States and U.S. companies.
We take necessary precautions to protect the intellectual property of our customers. We have established standard intellectual property protection procedures to control information access on a need-to know basis. We scan laboratory records into our electronic archives periodically and on an as-needed basis. We enter into agreements with all our employees under which they disown intellectual property they create during their employment, and they waive relevant intellectual property rights or claims. Our employees are also bound by confidentiality obligations and have agreed to disclose and assign to us inventions conceived by them during their respective terms of employment. During their entrance interviews, employees are required to confirm in writing that they will not disclose or use the intellectual property obtained from their previous employers and, during the exit interviews, the departing employees are required to confirm their confidentiality obligations to us and our customers in writing. Furthermore, our service agreements provide that all intellectual property generated during a project is exclusively the property of the customer for whom we are conducting the project.
Although our intellectual property rights are important to our results of operations, we believe that factors such as the technical expertise, knowledge, ability and experience of our employees are more important, and that, overall, these technical capabilities provide significant benefits to our customers.
Despite measures we take to protect intellectual property of our customers or our company, unauthorized parties may attempt to obtain and use information that we regard as proprietary. See “Item 3. Key Information—Risk Factors—Risks Relating to Our Business and Industry—If we fail to protect the intellectual property rights of our customers, we may be subject to liability for breach of contract and may suffer damage to our reputation.” To date, we are not aware of any material misuse of our proprietary information.
66
As of this annual report on Form 20-F, we had seventeen registered trademarks in China, two registered trademarks in Japan and two registered trademarks in the Europe Union. We also have five pending trademarks application in China, two pending applications in the United States, and two pending applications in India. In addition, we own 49 Internet domain names as of the date hereof.
D.Trend Information
Other than as disclosed elsewhere in this annual report, we are not aware of any trends, uncertainties, demands, commitments or events for the year 2011 that are reasonably likely to have a material adverse effect on our net revenues, income, profitability, liquidity or capital resources, or that caused the disclosed financial information to be not necessarily indicative of future operating results or financial conditions.
E.Off-Balance Sheet Arrangements
We do not have any outstanding off-balance sheet guarantees, interest rate swap transactions except for certain foreign currency forward contracts as discussed in “—Quantitative and Qualitative Disclosure about Market Risk” below. We do not engage in trading activities involving non-exchange traded contracts. In our ongoing business, we do not enter into transactions involving, or otherwise form relationships with, unconsolidated entities or financials partnerships that are established for the purpose of facilitating off-balance sheet arrangements or other contractually narrow or limited purposes.
F.Tabular Disclosure of Contractual Obligations
The following table sets forth our contractual obligations as of December 31, 2011:
| | | | | | | | | | | | | | | | | | | | |
| | | Payment Due by Period | |
| | | Total | | | Less than
1-year | | | 1-3 year | | | 3-5 year | | | More than
5 years | |
| | | (in thousands of US$) | |
Operating leases | | | 13.4 | | | | 4.8 | | | | 5.6 | | | | 2.7 | | | | 0.3 | |
Other than the obligations set forth above, we did not have any material long-term debt obligations, operating lease obligations, purchase obligations or other long-term liabilities as of December 31, 2011.
G.Safe Harbor
See “Forward-Looking information” on page 2 of this annual report on Form 20-F.
| ITEM 6. | DIRECTORS, SENIOR MANAGEMENT AND EMPLOYEES |
A.Directors and Senior Management
The following table sets forth information regarding our Directors and executive officers as of the date of this annual report on Form 20-F.
| | | | | | |
Directors and Executive Officers | | Age | | | Position/Title |
Michael Xin Hui | | | 40 | | | Chairman of the board of directors and chief executive officer |
Kevin Penghui Chen | | | 40 | | | Director |
Julian Ralph Worley | | | 67 | | | Independent director |
Yuk Lam Lo | | | 63 | | | Independent director |
Benson Tsang | | | 47 | | | Independent director |
William Dai | | | 48 | | | Chief financial officer |
Lan Xie | | | 39 | | | Vice president of finance and Investor Relations |
67
Michael Xin Hui has served as our chairman of the board of directors and chief executive officer since the inception of our company in 2002. Mr. Hui is a co-founder of our company and one of the key managerial personnel of our business, and is responsible for operations, strategic planning and business development. From 2000 to 2002, Mr. Hui was a vice president at Softbank Investment E2-Capital, a company based in Shanghai and Hong Kong, China. From 1999 to 2000, Mr. Hui worked as an investment manager of Phytomedica, Inc., a company in New York. In 1998, Mr. Hui worked as a project manager of China Innovation Center For Life Science in New York. Prior to that, Mr. Hui worked as a research scientist for American Home Products Corporation in New York. Mr. Hui holds a bachelor’s degree in chemistry from Oregon State University, a master’s degree in chemistry from Tulane University and an MBA from New York University.
Kevin Penghui Chen has served as our director since 2008. He has served as a managing director of Everbright Healthcare Investment Management Company since December 2011. He was our chief operating officer between 2008 and 2011 and our president in 2011. From September 2010 to January 2011, Mr. Chen also served as our chief financial officer. Prior to joining our company, Mr. Chen was a vice president at CITIC Capital, a China-focused private equity fund in Shanghai, China. In 2007, Mr. Chen worked for Eaton Corporation in Shanghai, China in charge of corporate development. From 2003 to 2006, Mr. Chen worked for DuPont Corporation in Shanghai as a business development manager. Prior to that, he worked as a scientist at Ligand Pharmaceuticals, Inc. Mr. Chen holds a bachelor’s degree in chemistry from Nanjing University, a master’s degree in chemistry from Tulane University and an MBA from Kellogg School of Management, Northwestern University.
Julian Ralph Worley has served as our independent director since October 2010. Mr. Worley is also the chairman of our audit Committee and a member of our compensation committee and nominating and corporate governance committee. Mr. Worley has served as an independent non-executive director of Suntech Power Holdings Co. Ltd, a leading solar energy company based in China and listed on the New York Stock Exchange, since 2006. From 2005 to February 2010, he was an independent non-executive director of Mandra Forestry Finance Limited and its holding company, Mandra Forestry Holdings Limited, an owner and operator of forestry plantations. From 2000 to 2003, he was a consultant at partner level in PricewaterhouseCoopers Philippines. He served as an audit partner in PricewaterhouseCoopers Hong Kong from 1998 to 2000 and an audit partner in Price Waterhouse Hong Kong from 1974 to 1998. He is a fellow of the Hong Kong Institute of Directors, a fellow of the Institute of Chartered Accountants in England and Wales and a fellow of the Hong Kong Institute of Certified Public Accountants. Mr. Worley holds a bachelor’s degree in economics from London School of Economics and Political Science, University of London.
Yuk Lam Lo has served as our independent director since October 2010. Mr. Lo is the chairman of our compensation committee, and a member of our audit Committee and nominating and corporate governance Committee. Mr. Lo has served as an independent director of Sinovac Biotech Limited, a biopharmaceutical company based in China and listed on NASDAQ, since 2006, an independent non-executive director of South East Group Limited, a property development and investment company listed on the Hong Kong Stock Exchange, since 2006 and as a senior director of Questmark Asia Limited, a private equity firm, since 2009. Mr. Lo has held several positions in different committees of the Hong Kong government. Mr. Lo is presently the chairman of the Chinese Manufacturers’ Association of Hong Kong—Innovation and Technology Committee, the honorary life chairman of Hong Kong Biotechnology Organization and a member of the Advisory Council for Food Safety of the Food and Health Bureau. Mr. Lo is also the honorary founding chairman of Hong Kong Bio-Organization, a member of the Advisory Committee of the Vocational Training Council and an executive vice-president of Asian College of Management. He was the former chairman of the Innovation and Technology Fund (Biotechnology Projects) Vetting Committee and the Biotechnology Committee of the Industry and Technology Development Council of the Hong Kong government. Mr. Lo is currently an adjunct professor of the Chinese University of Hong Kong, a special advisor to the Hong Kong University of Science and Technology, an honorary court member of the Hong Kong Baptist University and honorary professor in several universities in mainland China. Mr. Lo has been elected as an honorary fellow by the Hong Kong University of Science and Technology. In
68
China, he is also a consultant of the Centre for Disease Control and Prevention of China. Mr. Lo was a consultant to the Economic Bureau of Changchun and a member of the Advisory Committee of the Shenzhen Municipal Science and Technology Bureau.
Benson Tsang has served as our independent director since November 2010. Mr. Tsang is the chairman of our nominating and corporate governance committee, and a member of our audit committee and compensation committee. Since March 2010, Mr. Tsang has been the chief financial officer and financial and principal accounting officer of ATA Inc., a Nasdaq listed company. Mr. Tsang has more than 21 years of experience in accounting, financial management, and the capital markets. He has held senior financial and management positions in multinational corporations and international accounting firms, and has financial and accounting experience with companies listed on the New York Stock Exchange, the Stock Exchange of Hong Kong, and the Singapore Exchange. Mr. Tsang served as the chief financial officer of WuXi Pharmatech (Cayman) Inc. from July 2006 to February 2009. Prior to that, Mr. Tsang held senior financial and management positions in private and public companies, including PCCW Ltd. and Global Tech Holdings Ltd. He worked at PricewaterhouseCoopers and Deloitte Touche Tohmatsu from 1988 to 1996, and between March 2009 and March 2010, Mr. Tsang provided consulting services through his own firm, the Benita Consulting Company. Mr. Tsang is a member of the Canadian Institute of Chartered Accountants and the Hong Kong Institute of Certified Public Accountants. He received a Bachelor of Commerce degree and an MBA degree from McMaster University in Canada.
William Dai has served as our chief financial officer since January 2011. Mr. Dai has more than 17 years of experience in accounting, financial management, and the capital markets. Prior to ShangPharma, Mr. Dai served as chief financial officer of the New York Stock Exchange-listed China Nepster Drugstore Ltd. in Shenzhen, China since January 2009. From 2006 to 2008, Mr. Dai was the chief financial officer and vice chair of the executive committee at MicroPort Medical Co. Ltd. in Shanghai, China. From 1993 to 2005, Mr. Dai held a variety of financial and senior management roles at multinational companies in China and the United States. Mr. Dai holds a master’s degree in business administration with an emphasis in finance from Michigan State University and a bachelor’s degree in international business and English from Shanghai University’s College of International Business in Shanghai, China.
Lan Xie has served as our vice president of finance and Investor Relations since August 2011 and, prior to that, she served on various roles in our company since November 2007, including vice president of finance and operations, financial controller and vice president of finance. Prior to November 2007, Ms. Xie was a senior manager at PricewaterhouseCoopers in Shanghai, China from 2005 to 2007. From 2003 to 2005, Ms. Xie was a manager at Deloitte & Touche LLP in New York. From 1998 to 2002, Ms. Xie worked as a finance manager at Reuters Incorporated. Prior to that, Ms. Xie worked as a senior associate at PricewaterhouseCoopers LLP in Boston. Ms. Xie holds a bachelor’s degree in business administration from Boston University and an MBA from INSEAD.
Employment Agreements
We have entered into employment agreements with each of our executive officers. Under these agreements, each of our executive officers is employed for a specified period. We may terminate employment for cause, at any time, without notice or remuneration, for certain acts of the employee, such as causing material losses and damages to our company via serious neglect of duties, serious misconduct for selfish ends and fraudulent behavior and being charged with criminal offense. We may also terminate an executive officer’s employment without cause with severance payments.
Each executive officer has agreed to hold, both during and after the termination or expiration of his or her employment agreement, in strict confidence and not to use, except as required in the performance of his or her duties in connection with the employment, any of our confidential information or trade secrets, any confidential information or trade secretes of our customers or prospective customers, or the confidential or proprietary
69
information of any third party received by us and for which we have confidential obligations. In addition, each executive officer has agreed to be bound by non-competition restrictions during his or her employment and for one year after the termination of his or her employment. Specifically, each executive officer has agreed during the non-competition period (i) not to participate in R&D work at the top ten pharmaceutical and biotechnology R&D outsourcing companies in mainland China; (ii) not to provide services to, own or operate any business that provides products, services or technologies substantially similar to the business currently conducted or proposed to be conducted by us; and (iii) not to induce any of our employees or consultants to terminate his or her employment or engagement with us.
B.Compensation of Directors and Executive Officers
For the fiscal years ended December 31, 2011, we paid an aggregate of approximately RMB7.6 million ($1.2 million) in cash compensation to our executive officers and directors as a group, and paid an aggregate of approximately RMB33,200 ($5,300) in premiums for commercial medical insurance coverage for some of our executive officers. We have not set aside or accrued any amount to provide pension, retirement or other similar benefits to our executive officers and directors. Our PRC subsidiaries are required by law to make contributions equal to certain percentages of each employee’s salary for his or her pension insurance, medical insurance, housing fund, unemployment and other statutory benefits.
Scientific Advisory Board
We have established a scientific advisory board comprising leading experts in their fields. Our scientific advisors participate in advisory board meetings as well as provide ad hoc individual consulting services to management and our scientists. We regularly seek advice and input from these experienced scientific leaders on matters related to our research and development programs. Our scientific advisory board consists of experts across a range of key disciplines relevant to our programs and science. We intend to leverage the broad expertise of our advisors by seeking their counsel on important topics relating to our drug discovery and development programs.
We pay our advisors a fee for their attendance at scientific advisory board meetings and consulting services, and reimburse them for their expenses. Our current advisors are:
| | |
Name | | Professional Affiliation |
Gregory Verdine, Ph.D. (chairman) | | Erving Professor of Chemistry and Arthur Cope Scholar, Harvard University |
Robert Booth, Ph.D. | | Chief executive officer of Virobay, Inc.; former operating partner at TPG; former senior vice president at Roche; former chief scientific officer at Celera |
Paul J. Reider, Ph.D. | | Professor at Princeton University; former head of chemistry at Amgen Inc.; former vice president of process chemistry, Merck & Co., Inc. |
Malcolm MacCoss, Ph.D. | | Former vice president of basic chemistry and drug discovery science at Merck & Co., Inc.; former group vice president of chemistry at Schering-Plough; winner of two Thomas Edison Patent Awards and Arthur C. Cope Scholar Award |
Robert Kaman, Ph.D. | | Former president of Abbott Bioresearch Center and BASF Bioresearch Center |
70
2008 Equity and Performance Incentive Plan
On May 19, 2008, we adopted the 2008 Plan to attract and retain the best available personnel and provide additional incentives to employees, directors and consultants. On February 24, 2010, we amended and restated the 2008 Plan to increase the maximum aggregate number of our ordinary shares that may be issued under the 2008 Plan to 36,562,358 shares. As of December 31, 2011, options to purchase 32,593,840 ordinary shares were outstanding.
The following paragraphs describe the principal terms of the 2008 Plan.
Plan Administration. Our board of directors, a committee or a sub-committee designated by our board of directors will administer the 2008 Plan. The committee or the full board of directors, as appropriate, will determine the provisions and terms and conditions of each award grant.
Award Agreement. Awards granted under the 2008 Plan are evidenced by an award agreement that sets forth terms, conditions and limitations for each award. In addition, the award agreement may provide that securities granted are subject to a 180-day lock-up period following the effective date of a registration statement filed by us under the Securities Act, if so requested by us or any representative of the underwriters in connection with any registration of the offering of any of our securities.
Evidence of Award. Awards can be evidenced by an agreement, certificate, resolution or other type of writing or an electronic medium approved by the board of directors that sets forth the terms and conditions of the awards granted. An evidence of award, with the approval of the board of directors, need not be signed by a representative of our company or the recipient.
Eligibility. We may grant awards to any employee, non-employee director, officer or consultant of our company or any one or more of our subsidiaries, or any person who has agreed to commence serving in any of such capacities within 90 days of the date of grant.
Acceleration of Awards upon Change in Control. The outstanding awards are subject to accelerated vesting upon occurrence of a change-of-control corporate transaction, as determined by our board of directors in its sole discretion.
Exercise Price and Term of Awards. Our board of directors, or a committee designated by our board of directors, determines the exercise price, grant price and expiration date for each award.
Vesting Schedule. In general, our board of directors, or a committee designated by our board of directors, determines, or the evidence of award specifies, the vesting schedule.
Transfer Restrictions. Subject to certain exceptions, awards may not be transferred in any manner by the recipient other than by will or the laws of descent and distribution.
Termination of the 2008 Plan. Unless terminated earlier, the 2008 Plan will terminate automatically in 2018. Our board of directors has the authority to amend or terminate the 2008 Plan to the extent necessary to comply with applicable law or the rules of the principal securities exchange upon which our ADSs are traded or quoted.
Founder’s 2008 Equity and Performance Incentive Plan
In May 2008, two companies controlled by our founders, ChemExplorer Investment Holdings Ltd. and ChemPartner Investment Holdings Limited, established the 2008 Founder’s Plan to attract, motivate and retain our employees, directors and consultants. The maximum aggregate number of our ordinary shares that may be transferred by these two companies to grantees upon vesting of RSUs granted under the 2008 Founder’s Plan is 20,000,000.
71
Under the 2008 Founder’s Plan, 2,100,000, 2,200,000 and nil RSUs were granted to certain members of our senior management and other employees in 2009, 2010 and 2011, respectively. Each RSU represents the right to receive one ordinary share upon vesting. Subject to the conditions described below, fifty-percent of each grantee’s RSUs will vest on the first anniversary of the date of grant and the remaining fifty-percent will vest on the second anniversary of the date of grant. The actual number of RSUs vesting annually is subject to certain annual corporate, business unit and individual performance targets. If the annual performance targets are not met, a portion of the awards may not vest and may be carried over to the third year of vesting. Any granted RSU that has not vested due to a grantee’s failure to meet the specified performance targets will be subject to a modified vesting schedule under which the RSU will vest on the third anniversary of the date of grant if the grantee remains our employee at that time.
In addition, the vesting of RSUs was subject to the occurrence of an initial public offering or a change of control event (as defined in the 2008 Founder’s Plan), whichever occured earlier. Granted RSUs, whether vested or not, would be forfeited if a grantee’s employment with us was terminated for cause or the grantee voluntarily left our company prior to an initial public offering or a change of control event, whichever occured earlier. Pursuant to the 2008 Founder’s Plan, ChemExplorer Investment Holdings Ltd. or ChemPartner Investment Holdings Limited would transfer one ordinary share beneficially owned by them to a grantee for each vested RSU as soon as administratively practicable following the earlier of the effective date of an initial public offering or a change of control event described above.
As noted above, the vesting of RSUs was subject to the occurrence of an initial public offering or a change of control event (as defined in the 2008 Founder’s Plan), whichever occurred earlier. As a result, we did not record any share-based compensation expense in connection with the RSU grants prior to our initial public offering in October 2010 because the vesting conditions for the RSUs (the occurrence of an initial public offering or a change of control event) were not met. For the years ended December 31, 2010 and 2011, 7,541,592 and 2,426,386 RSUs became vested, respectively, and approximately US$2.8 million and US$0.8 million, respectively, were recorded as share-based compensation expense, including approximately US$2.5 million of share-based compensation expense recorded immediately upon the completion of our initial public offering. A grantee has no rights as a shareholder with respect to any RSUs held by the grantee until the date when the RSUs are vested and the corresponding ordinary shares are transferred from our founders to the grantee through ChemExplorer Investment Holdings Ltd. and ChemPartner Investment Holdings Limited. No adjustment will be made for dividends, distributions or other rights, the record date of which is prior to such conversion date.
2010 Share Incentive Plan and 2011 Share Incentive Plan
We established the 2010 Plan in December 2010 and 2011 Plan in August 2011 to promote our success and enhance value by linking the personal interests of our directors, employees and consultants to those of our shareholders and by providing such individuals with an incentive for outstanding performance to generate superior returns to our shareholders. The two plans are further intended to provide flexibility to us in our ability to motivate, attract, and retain the services of directors, employees and consultants upon whose judgment, interest, and special effort the successful conduct of our operation is largely dependent. Under the two plans, a committee of the board of directors, in its sole discretion, is authorized to grant options, restricted shares or restricted share units to recipients. Options, restricted shares and restricted share units issued under the two plans will be issued pursuant to award agreements setting forth the conditions thereto, including, as applicable, the number of options, restricted shares or restricted share units awarded, vesting conditions, restrictions on transfer and exercise price. Michael Xin Hui, our chief executive officer and one of our founders, has agreed to contribute up to 18,000,000 ordinary shares of our company beneficially owned by him or his family to our company in connection with our issuance of ordinary shares upon vesting or exercise of share incentive awards granted to our employees under the 2010 Plan. Under the 2011 Plan, the maximum aggregate number of ordinary shares authorized for issuance, or the award pool, will be replenished to 2% of the then total outstanding of ordinary shares on an as-converted basis whenever the unissued ordinary shares reserved in the award pool account for no more than 10% of the then effective award pool.
72
The following table summarizes the share options granted to certain of our directors and executive officers and to other individuals as a group that were outstanding as of December 31, 2011. These share options will vest in equal amounts on the first, second, third and fourth anniversary of their respective dates of grant.
| | | | | | | | | | |
Name | | Options
Outstanding | | Exercise Price
($/Share) | | Date of Grant | | Date of
Expiration | | Vesting
Schedule |
Kevin Penghui Chen | | * | | 0.50 | | Oct. 27, 2008 | | Oct. 27, 2018 | | 4 years |
| | * | | 0.65 | | July 30, 2010 | | July 30, 2020 | | 4 years |
William Dai | | * | | 0.69 | | Jan. 10, 2011 | | Jan. 10, 2021 | | 4 years |
Lan Xie | | * | | 0.50 | | May 19, 2008 | | May 19, 2018 | | 4 years |
| | * | | 0.65 | | July 30, 2010 | | July 30, 2020 | | 4 years |
Julian Ralph Worley | | * | | 0.83 | | Nov. 19, 2010 | | Nov. 19, 2020 | | 4 years |
Yuk Lam Lo | | * | | 0.83 | | Nov. 19, 2010 | | Nov. 19, 2020 | | 4 years |
Benson Tsang | | * | | 0.83 | | Nov. 19, 2010 | | Nov. 19, 2020 | | 4 years |
Other individuals as a group | | 8,453,754 | | 0.50 | | May 19, 2008 | | May 19, 2018 | | 4 years |
| | 3,667,812 | | 0.50 | | Oct. 27, 2008 | | Oct. 27, 2018 | | 4 years |
| | * | | 0.50 | | May 13, 2009 | | May 13, 2019 | | 4 years |
| | * | | 0.50 | | Oct. 14, 2009 | | Oct. 14, 2019 | | 4 years |
| | * | | 0.65 | | May 24, 2010 | | May 24, 2020 | | 4 years |
| | 4,711,760 | | 0.65 | | July 30, 2010 | | July 30, 2020 | | 4 years |
| | * | | 0.58 | | June 30, 2011 | | June 30, 2021 | | 4 years |
| | * | | 0.46 | | Sep. 30, 2011 | | Sep. 30, 2021 | | 4 years |
| | * | | 0.40 | | Dec, 30, 2011 | | Dec. 30, 2021 | | 4 years |
| | | | | | | | | | |
Total(1): | | 32,593,840 | | | | | | | | |
Notes:
| * | Less than 1% of our total outstanding shares. |
| (1) | Certain options have been transferred to family members of the grantees or entities established for the grantees’ estate planning purposes. The vesting of such options is subject to the same terms and conditions as if they were held by the original grantees. |
The following table summarizes, as of December 31, 2011, the RSUs granted to our directors, senior management and other employees.
| | | | | | | | |
Name | | RSU
Granted | | Date of Grant | | Date of
Expiration | | Vesting
Schedule |
Kevin Penghui Chen | | * | | May 19, 2008 | | May 19, 2018 | | 2 years |
William Dai | | * | | Jan. 10, 2011 | | Jan. 10, 2021 | | 4 years |
Lan Xie | | * | | May 19, 2008 | | May 19, 2018 | | 3 years |
| | * | | Jan. 1, 2011 | | Jan. 1, 2021 | | 2 years |
Julian Ralph Worley | | * | | May 31, 2011 | | May 31, 2021 | | 10 quarters |
Yuk Lam Lo | | * | | May 31, 2011 | | May 31, 2021 | | 10 quarters |
Benson Tsang | | * | | May 31, 2011 | | May 31, 2021 | | 10 quarters |
Other individuals as a group | | 3,800,000 | | May 19, 2008 | | May 19, 2018 | | 3 years |
| | * | | Oct. 27, 2008 | | Oct. 27, 2018 | | 3 years |
| | * | | May 13, 2009 | | May 13, 2019 | | 3 years |
| | * | | Oct. 14, 2009 | | Oct. 14, 2019 | | 3 years |
| | * | | May 24, 2010 | | May 24, 2020 | | 2 years(1) |
| | * | | Aug. 16, 2010 | | Aug. 16, 2020 | | 2 years(2) |
| | 3,805,920 | | Jan. 1, 2011 | | Jan. 1, 2021 | | 1 year(3) |
| | * | | Apr. 1, 2011 | | Apr. , 2021 | | 1 year(4) |
| | 9,498,600 | | May 31, 2011 | | May 31, 2021 | | 10 quarters(5) |
| | * | | Oct. 12, 2011 | | Oct. 12, 2021 | | Immediately |
| | | | | | | | |
Total: | | 26,766,320(6) | | | | | | |
73
Notes:
| * | Less than 1% of our total outstanding shares. |
| (1) | These RSUs were granted on May 24, 2010 and were scheduled to vest starting on May 24, 2011, the first anniversary of the grant date. |
| (2) | These RSUs were granted on August 16, 2010 and were scheduled to vest starting on August 16, 2011, the first anniversary of the grant date. |
| (3) | These RSUs were granted on January 1, 2011 and fully vested on January 1, 2012. |
| (4) | These RSUs were granted on April 1, 2011 and fully vested on April 1, 2012. |
| (5) | These RSUs were granted on May 31, 2011 and were scheduled to vest starting on August 31, 2011, the first quarter of the grant date. |
| (6) | Some of the RSUs granted in 2008, 2009 and 2010 have been transferred to the family members of the grantees or entities established for the grantees’ estate planning purposes, and the vesting of these RSUs are subject to the same terms and conditions as if they were held by the original grantees. |
C.Board Practices
Board of Directors
Our board of directors consists of five directors. A director is not required to hold any shares in our company by way of qualification. A director may vote with respect to any contract, proposed contract or arrangement in which he is materially interested provided the nature of the interest is disclosed prior to voting. A director may exercise all the powers of our company to borrow money, mortgage its undertaking, property and uncalled capital, and issue debentures or other securities whenever money is borrowed or as security for any obligation of our company or of any third party. The remuneration to be paid to the directors is determined by the board of directors. None of our non-executive directors has a service contract with us that provides for benefits upon termination of employment. There is no age limit requirement for directors.
Committees of the Board of Directors
We have established three committees under the board of directors: the Audit Committee, the Compensation Committee and the Nominating and Corporate Governance Committee. We have adopted a charter for each of these committees. Each committee’s members and functions are described below.
Audit Committee. Our Audit Committee consists of Messrs. Julian Ralph Worley, Yuk Lam Lo and Benson Tsang, and is chaired by Mr. Worley. Messrs. Worley, Lo and Tsang satisfy the “independence” requirements of Section 303A of the Corporate Governance Rules of the New York Stock Exchange and Rule 10A-3 under the Securities Exchange Act of 1934. We have determined that Messrs. Worley and Tsang qualify as “audit committee financial experts.” The Audit Committee oversees our accounting and financial reporting processes and the audits of the financial statements of our company. The Audit Committee is responsible for, among other things:
| | • | | selecting the independent auditors and pre-approving all auditing and non-auditing services permitted to be performed by the independent auditors; |
| | • | | reviewing with the independent auditors any audit problems or difficulties and management’s response; |
| | • | | reviewing and approving past or proposed related party transactions; |
| | • | | reviewing the annual audited financial statements with management and the independent auditors; |
| | • | | reviewing major issues as to the adequacy of our internal controls and any special audit steps adopted in light of material control deficiencies; |
| | • | | meeting separately and periodically with management and the independent auditors; and |
| | • | | monitoring compliance with our code of business conduct and ethics, including reviewing the adequacy and effectiveness of our procedures to ensure proper compliance. |
74
Compensation Committee. Our Compensation Committee consists of Messrs. Yuk Lam Lo, Julian Ralph Worley and Benson Tsang, and is chaired by Mr. Lo. Messrs. Lo, Worley and Tsang satisfy the “independence” requirements of Section 303A of the Corporate Governance Rules of the New York Stock Exchange. The Compensation Committee assists the board in reviewing and approving the compensation structure, including all forms of compensation, relating to our directors and executive officers. Our chief executive officer may not be present at any committee meeting during which his compensation is deliberated. The Compensation Committee is responsible for, among other things:
| | • | | reviewing and approving the total compensation package for our chief executive officer; |
| | • | | reviewing and recommending to the board the compensation of our other executive officers; |
| | • | | evaluating the compensation of our non-employee directors; and |
| | • | | reviewing periodically our general compensation plans and other employee-related plans, including incentive compensation or equity plans. |
Nominating and Corporate Governance Committee. Our Nominating and Corporate Governance Committee consists of Messrs. Benson Tsang, Julian Ralph Worley and Yuk Lam Lo, and is chaired by Mr. Benson Tsang. Messrs. Tsang, Worley and Lo satisfy the “independence” requirements of Section 303A of the Corporate Governance Rules of the New York Stock Exchange. The Nominating and Corporate Governance Committee assists the board of directors in identifying individuals qualified to become our directors and in determining the composition of the board and its committees. The Nominating and Corporate Governance Committee will be responsible for, among other things:
| | • | | identifying and recommending to the board nominees for election or re-election to the board; |
| | • | | reviewing annually with the board the composition of the board in light of the characteristics of independence, age, skills, experience and availability of service to us; |
| | • | | identifying and recommending to the board the directors to serve as members of the board of directors’ committees; and |
| | • | | advising the board periodically with respect to significant developments in the law and practice of corporate governance as well as our compliance with applicable laws and regulations, and making recommendations to the board on all matters of corporate governance and on any corrective action to be taken. |
Duties of Directors
Under Cayman Islands law, our directors have a statutory duty of loyalty to act honestly in good faith with a view to our best interests. Our directors also have a duty to exercise the skill they actually possess and such care and diligence that a reasonably prudent person would exercise in comparable circumstances. In fulfilling their duty of care to us, our directors must ensure compliance with our memorandum and articles of association. A shareholder has the right to seek damages if a duty owed by our directors is breached.
Terms of Directors and Officers
Our officers are elected by and serve at the discretion of the board of directors. Our directors are not subject to a term of office and hold office until such time as they resign or are removed from office by ordinary resolution of our shareholders or affirmative votes of at least two thirds of our directors then in office. A director will be removed from office automatically if, among other things, the director (i) becomes bankrupt or makes any arrangement or composition with his creditors; or (ii) dies or is found by our company to be or becomes of unsound mind.
75
D.Employees
We had 1,717 and 1,897 employees as of December 31, 2009 and 2010, respectively. As of December 31, 2011, we had 2,076 employees, including 1,255 in our discovery chemistry group, 338 in our discovery biology and preclinical development group, 130 in our pharmaceutical development group, 73 in our biologics group and 280 non-scientific personnel. Of the total 2,076 employees as of December 31, 2011, 1,753 worked in our headquarters in Shanghai Zhangjiang Hi-Tech Park, 172 worked in our R&D center in Chengdu, 147 worked at our manufacturing facility and laboratory services building in Fengxian, Shanghai, and 4 worked at our overseas offices. The following chart illustrates the composition of our scientific research staff by educational level as of December 31, 2011:
As of December 31, 2011, we had 1,796 scientific research staff members, over 62% of whom have post- college degrees. We recruit both recent graduates and experienced professionals. Our primary hiring strategy in China is to recruit from colleagues and universities. We also recruit overseas, primarily focusing on returnees and Ph.D.s with significant educational and/or industry background with major pharmaceutical and biotechnology companies, many of whom used to work for our customers, and through referrals, websites, advertisements in trade magazines and job fairs. We expect to hire a significant number of China-based employees during 2012.
Our future growth and profitability depend upon the research and efforts of our experienced and skilled scientists and mid-level managers, and their ability to keep pace with changes in drug discovery and development technologies. We compete vigorously with pharmaceutical firms, biotechnology firms, contract research firms and academic and research institutions to recruit scientists and mid-level managers and employees. See “Item 3. Key Information—Risk Factors—Risks Relating to Our Business and Industry—Our ability to execute projects, maintain, expand or renew existing customer engagements and obtain new customers depends largely on our ability to attract, train, motivate and retain skilled scientists.”
We recognize that our employees constitute our most valuable assets and remain focused on training and retention. We are dedicated to cultivating a corporate culture that is customer centric and people oriented through employees who exhibit a can-do attitude, teamwork and innovation. In addition to on-the-job training programs, we have a comprehensive formal training program for employees at different levels, including orientation programs for recent college graduates, management and leadership training for senior employees, and technical skills training, team building and integration training and communication skills training for domestically-trained employees. We have implemented a comprehensive review and incentive system that aligns performance and compensation as well as internal promotions, which also help us motivate and retain our employees.
We pay base salaries and bonuses to all of our employees and pay additional awards to some employees for exceptional performance. As required by PRC regulations, for our China-based employees, we participate in various employee benefit plans organized by municipal and provincial governments, including pension insurance, work-related injury benefits, maternity insurance, medical insurance, unemployment benefit plans and housing funds. We are required under PRC law to make contributions to the employee benefit plans for our China employees at specified percentages of the salaries, bonuses and certain allowances of our employees, up to a maximum amount specified by the local government from time to time.
76
We believe that we maintain a good working relationship with our employees and we have not experienced any significant labor disputes.
E.Share Ownership
The following table sets forth information with respect to the beneficial ownership of our ordinary shares, as of March 31, 2012, by:
| | • | | each of our directors and executive officers; and |
| | • | | each person known to us to own beneficially more than 5.0% of our ordinary shares. |
As of March 31, 2012, we have 336,109,400 ordinary shares outstanding. Beneficial ownership is determined in accordance with the rules and regulations of the SEC. In computing the number of shares beneficially owned by a person and the percentage ownership of that person, we have included shares that the person has the right to acquire within 60 days from March 31, 2012, including through the exercise of any option, warrant or other right, vesting of restricted share units or the conversion of any other security. These shares, however, are not included in the computation of the percentage ownership of any other person.
| | | | | | | | |
| | | Shares Beneficially Owned | |
| | | Number | | | % | |
Directors and Executive Officers: | | | | | | | | |
Michael Xin Hui(1) | | | 183,706,830 | | | | 54.5 | |
Kevin Penghui Chen | | | * | | | | * | |
Julian Ralph Worley | | | * | | | | * | |
Yuk Lam Lo | | | * | | | | * | |
Benson Tsang | | | * | | | | * | |
William Dai | | | * | | | | * | |
Lan Xie | | | * | | | | * | |
All Directors and Executive Officers as a Group | | | 184,165,894 | | | | 54.6 | |
| | |
Principal Shareholders: | | | | | | | | |
ChemExplorer Investment Holdings Ltd.(2) | | | 79,253,837 | | | | 23.5 | |
ChemPartner Investment Holdings Limited(3) | | | 104,002,993 | | | | 30.9 | |
Investment funds affiliated with TPG, represented by TPG Star Charisma Ltd. and TPG Biotech II Charisma Ltd.(4) | | | 37,234,014 | | | | 11.1 | |
Ameriprise Financial, Inc.(5) | | | 23,171,490 | | | | 6.9 | |
Notes:
| * | Less than 1% of our total outstanding shares. |
| (1) | Includes (i) 79,253,837 ordinary shares owned by ChemExplorer Investment Holdings Ltd., (ii) 104,002,993 ordinary shares owned by ChemPartner Investment Holdings Limited, and (iii) 450,000 ordinary shares issuable upon the vested RSUs that Mr. Hui holds. Each of ChemExplorer Investment Holdings Ltd. and ChemPartner Investment Holdings Limited is a British Virgin Islands company ultimately owned by Michael Xin Hui and the Hui Family Trust, a British Virgin Islands trust, as of the date of this annual report on Form 20-F. Michael Xin Hui is a director of ChemExplorer Investment Holdings Limited and ChemPartner Investment Holdings Limited. Michael Xin Hui disclaims beneficial ownership with respect to the above shares except to the extent of his pecuniary interest therein. The business address of Michael Xin Hui is No. 5 Building, 998 Halei Road, Zhangjiang Hi-tech Park, Pudong New Area, Shanghai, China, 201203. |
| (2) | ChemExplorer Investment Holdings Ltd. is a British Virgin Islands company ultimately owned by Michael Xin Hui and the Hui Family Trust, a British Virgin Islands trust. |
| (3) | ChemPartner Investment Holdings Limited is a British Virgin Islands company ultimately owned by Michael Xin Hui and the Hui Family Trust, a British Virgin Islands trust. |
77
| (4) | Includes 24,836,549 ordinary shares held by TPG Star Charisma Limited and 12,397,465 ordinary shares held by TPG Biotech II Charisma Limited. TPG Star Charisma Limited is a company incorporated in Hong Kong, whose sole shareholder is TPG Star, L.P., a Delaware limited partnership, which is managed by its general partner, TPG Star GenPar, L.P., a Delaware limited partnership, which is managed by its general partner, TPG Star Advisors, LLC, a Delaware limited liability company, whose sole member is TPG Ventures Holdings, LLC, whose managing member is TPG Ventures Partners, L.P., which is managed by its general partner, TPG Ventures Professionals, L.P., which is managed by its general partner, Tarrant Advisors, Inc., whose sole shareholder is Tarrant Capital Advisors, Inc., whose shareholders are David Bonderman and James G. Coulter. TPG Biotech II Charisma Limited is a company incorporated in Hong Kong, whose sole shareholder is TPG Biotechnology Partners II, L.P., a Delaware limited partnership, which is managed by its general partner, TPG Biotechnology GenPar II, L.P., a Delaware limited partnership, which is managed by its general partner, TPG Biotech Advisors II, LLC, a Delaware limited liability company, whose sole member is TPG Ventures Holdings, LLC, a Delaware limited liability company, whose managing member is TPG Ventures Partners, L.P., a Delaware limited partnership, which is managed by its general partner, TPG Ventures Professionals, L.P., a Delaware limited partnership, which is managed by its general partner, Tarrant Advisors, Inc., a Texas company, whose sole shareholder is Tarrant Capital Advisors, Inc., a Delaware company, whose shareholders are David Bonderman and James G. Coulter. The registered address for both of these companies is 57th Floor, Two International Finance Centre, 8 Finance Street, Central, Hong Kong. |
| (5) | Based on a Schedule 13G jointly filed on February 14, 2012 by Ameriprise Financial, Inc., which listed its business address as 145 Ameriprise Financial Center, Minneapolis, MN 55474, and Columbia Management Investment, which listed its business address as 225 Franklin St., Boston, MA 02110. |
None of our existing shareholders has different voting rights from other shareholders. We are not aware of any arrangement that may, at a subsequent date, result in a change of control of our company.
To our knowledge, 131,502,600 of our ordinary shares were held by one record holder in the United States, which was JPMorgan Chase Bank, N.A., the depositary of our ADS program. The number of beneficial owners of our ADSs in the United States is likely to be much larger than the number of record holders of our ordinary shares in the United States.
For the options granted to our directors, officers, employees and consultants, please refer to “Item 6. Directors, Senior Management and Employees—Compensation of Directors and Executive Officers.”
| ITEM 7. | MAJOR SHAREHOLDERS AND RELATED PARTY TRANSACTIONS |
A.Major Shareholders
Please refer to “Item 6. Directors, Senior Management and Employees—Share Ownership.”
B.Related Party Transactions
Investors’ Rights Agreement
In connection with the issuance and sale of our preferred shares in 2007, we entered into an investors’ rights agreement with holders of our preferred shares, holders of our ordinary shares, our founders and certain other parties. We have granted the holders of our preferred shares certain registration rights, including demand and piggyback registration rights and Form F-3 registration rights. In addition, the investors’ rights agreement provided that grants of share options pursuant to the 2008 Plan shall be subject to the condition that we achieve certain profit targets. On May 19, 2008, this agreement was amended and, among other things, this requirement was waived. On September 30, 2010, the investor’s rights agreement was further amended such that the definition of a qualified public offering has the same meaning as that in our memorandum and articles of association, as amended on September 30, 2010.
78
Transactions with Related Parties
Since April 2007, Labpartner, a company 50%-owned by immediate family members of Mr. Michael Xin Hui, has become our largest supplier for reagents and other raw materials. Labpartner follows the same bidding and selection process as all of our other suppliers. In the first half of 2007, we had four PRC subsidiaries, each of which entered into a separate framework service outsourcing agreement with LabPartner for a five year term, which may be terminated by either party upon six months’ notice. Under the framework agreements, each of these subsidiaries entered into additional agreements with LabPartner, including a purchase and sales agreement and a warehousing and logistics agreement, for the supply of reagents and other raw materials, logistics management, equipment purchase and maintenance and customs clearance. We purchase raw materials from LabPartner at prices determined through a competitive bidding process with other third party vendors. We pay LabPartner purchase prices and service fees on a monthly basis. In August 2011, all the equity interests originally owned by the immediate family members of Mr. Michael Xin Hui was disposed to a third party and Labpartner ceased to be our related party since then, although the volumes and amounts of our purchase from LabPartner remained significant. The purchase prices and service fees charged by Labpartner amounted to US$6.1 million, US$8.5 million and US$4.0 million in 2009, 2010 and seven months ended July 2011, respectively.
Shanghai Kehui Catering Management Co., Ltd., a company wholly-owned by immediate family members of Mr. Michael Xin Hui, provides employee lunches, dinners and other catering services in our company cafeteria. The services fee charged by Shanghai Kehui Catering Management Co., Ltd. amounted to US$0.8 million, US$0.9 million and US$1.1 million in 2009, 2010 and 2011, respectively.
We lease five buildings at our headquarters in Shanghai Zhangjiang Hi-Tech Park from Shanghai PharmValley Corp., a company 20%-owned by immediate family members of Mr. Michael Xin Hui. These buildings are used for office space and research activities and have an aggregate gross floor area of approximately 242,000 square feet. The office rent, utility and service fee charged by Shanghai PharmValley Corp. amounted to US$2.0 million, US$2.3 million and US$ 3.0 million in 2009, 2010 and 2011, respectively.
We started to render certain laboratory services to Shanghai Yunyi Health Management Consultancy Co., Ltd., a company wholly-owned by Mr. Michael Xin Hui and his immediate family members since 2011. The revenues generated from Shanghai Yunyi Health Management Consultancy Co., Ltd. amounted to US$0.5 million in 2011.
We believe the foregoing related party transactions were entered into on arm’s length terms.
Employment Agreements
See “Item 6—Directors, Senior Management and Employees—Directors and Senior Management—Employment Agreements.”
Equity and Performance Incentive Plan
See “Item 6—Directors, Senior Management and Employees—Directors and Senior Management—2008 Equity and Performance Incentive Plan,” “Item 6—Directors, Senior Management and Employees—Directors and Senior Management—Founder’s 2008 Equity and Performance Incentive Plan” and “Item 6—Directors, Senior Management and Employees—Directors and Senior Management—2010 Share Incentive Plan and 2011 Share Incentive Plan.”
C.Interests of Experts and Counsel
Not applicable.
79
| ITEM 8. | FINANCIAL INFORMATION |
A.Consolidated Statements and Other Financial Information
We have appended consolidated financial statements filed as part of this annual report.
Legal Proceedings
We may from time to time be subject to various legal or administrative proceedings, either as plaintiff or defendant, arising in the ordinary course of our business. We are not currently a party to, nor are we aware of, any legal proceedings, investigation or claim that, in the view of our management, is likely to materially and adversely affect our business, financial condition or results of operations.
Dividend Policy
We do not have any present plan to pay any cash dividends on our ordinary shares in the foreseeable future. We currently intend to retain most, if not all, of our available funds and any future earnings to operate and expand our business.
Our board of directors has significant discretion on whether to distribute dividends. Even if our board of directors decides to pay dividends, the form, frequency and amount will depend upon our future operations and earnings, capital requirements and surplus, general financial condition, contractual restrictions and other factors that the board of directors may deem relevant. If we pay any dividends, the depositary will distribute such payments to our ADS holders to the same extent as holders of our ordinary shares, subject to the terms of the deposit agreement, including the fees and expenses payable thereunder.
B.Significant Changes
Except as disclosed elsewhere in this annual report, we have not experienced any significant changes since the date of our audited consolidated financial statements included in this annual report.
| ITEM 9. | THE OFFER AND LISTING |
A.Offering and Listing Details
Our ADSs have been listed on the New York Stock Exchange since October 19, 2010 under the symbol “SHP”. Each ADS represents eighteen of our ordinary shares.
In 2011, the trading price of our ADSs on the New York Stock Exchange ranged from US$7.23 to US$14.11 per ADS.
80
The following table sets forth, for the periods indicated, the high and low trading prices on the New York Stock Exchange for our ADSs.
| | | | | | | | |
| | | Sales Price ($) | |
| | | High | | | Low | |
2010 | | | | | | | | |
Fourth quarter | | | 15.85 | | | | 11.19 | |
2011 | | | | | | | | |
Full year | | | 14.11 | | | | 7.23 | |
First quarter | | | 14.11 | | | | 11.46 | |
Second quarter | | | 13.00 | | | | 9.37 | |
Third quarter | | | 12.25 | | | | 7.70 | |
Fourth quarter | | | 10.42 | | | | 7.23 | |
October | | | 10.42 | | | | 7.74 | |
November | | | 9.60 | | | | 7.75 | |
December | | | 9.5 | | | | 7.23 | |
2012 | | | | | | | | |
First quarter | | | 9.58 | | | | 7.27 | |
January | | | 9.42 | | | | 7.27 | |
February | | | 9.58 | | | | 8.08 | |
March | | | 8.50 | | | | 7.58 | |
April (through April 27, 2012) | | | 8.59 | | | | 7.85 | |
B.Plan of Distribution
Not applicable.
C.Markets
Our ADSs, each of which represents eighteen of our ordinary shares, have been traded on the New York Stock Exchange since October 19, 2010. Our ADSs trade under the symbol “SHP.”
D.Selling Shareholders
Not applicable.
E.Dilution
Not applicable.
F.Expenses of the Issue
Not applicable.
| ITEM 10. | ADDITIONAL INFORMATION |
A.Share Capital
Not applicable.
B.Memorandum and Articles of Association
The following are summaries of material provisions of our second amended and restated memorandum and articles of association, as well as the Companies Law (2011 Revision), insofar as they relate to the material terms of our ordinary shares.
81
Registered Office and Objects
The Registered Office of our company is located at the offices of Codan Trust Company (Cayman) Limited, Cricket Square, Hutchins Drive, P.O. Box 2681, Grand Cayman, KY1-1111, Cayman Islands or at such other place as our board of directors may from time to time decide. The objects for which our company is established are unrestricted and we have full power and authority to carry out any object not prohibited by the Companies Law (2011 Revision), as amended from time to time, or any other law of the Cayman Islands.
Board of directors
See “Item 6. Board practices—board of directors.”
Ordinary Shares
General. All of our outstanding ordinary shares are fully paid and non-assessable. Certificates representing the ordinary shares are issued in registered form. Our shareholders who are non-residents of the Cayman Islands may freely hold and vote their ordinary shares.
Dividends. The holders of our ordinary shares are entitled to such dividends as may be declared by our board of directors subject to the Companies Law and to our second amended and restated memorandum and articles of association.
Voting Rights. Each ordinary share is entitled to one vote on all matters upon which the ordinary shares are entitled to vote. Voting at any shareholders’ meeting is by show of hands unless voting by way of a poll is required by the rules of the stock exchange where our ADSs are traded. A poll may be demanded by our chairman of such meeting or any one shareholder present in person or by proxy, or if the shareholder is a corporation, by its duly authorized representative. Voting cannot be conducted electronically.
An ordinary resolution to be passed by the shareholders requires the affirmative vote of a simple majority of the votes attaching to the ordinary shares cast in a general meeting, while a special resolution requires the affirmative vote of no less than two-thirds of the votes cast attaching to the ordinary shares. A special resolution is required for important matters such as amending our second amended and restated memorandum and articles of association. Holders of the ordinary shares may effect certain changes by ordinary resolution, including increase the amount of our authorized share capital, consolidate and divide all or any of our share capital into shares of larger amount than our existing share capital, and cancel any unissued shares.
Transfer of Shares. Subject to the restrictions contained in our second amended and restated memorandum and articles of association, any of our shareholders may transfer all or any of his or her ordinary shares by an instrument of transfer in the usual or common form or any other form approved by our board of directors.
Our board of directors may, in its sole discretion, decline to register any transfer of any ordinary share. Our directors may also decline to register any transfer of any ordinary share unless (a) the instrument of transfer is lodged with us, accompanied by the certificate for the ordinary shares to which it relates and such other evidence as our board of directors may reasonably require to show the right of the transferor to make the transfer; (b) the instrument of transfer is in respect of only one class of ordinary shares; (c) the instrument of transfer is properly stamped, if required; (d) the ordinary shares transferred are fully paid and free of any lien in favor of us; (e) in the case of a transfer to joint holders, the number of joint holders to whom the ordinary share is to be transferred does not exceed four; or (f) any fee related to the transfer has been paid to us.
If our directors refuse to register a transfer they shall, within three months after the date on which the instrument of transfer was lodged, send to each of the transferor and the transferee notice of such refusal. The registration of transfers may, after compliance with any notice requirements of the New York Stock Exchange,
82
be suspended and the register closed at such times and for such periods as our board of directors may from time to time determine, provided, however, that the registration of transfers shall not be suspended nor the register closed for more than 30 days in any year.
Liquidation.On a return of capital on winding up or otherwise (other than on conversion, redemption or purchase of shares), assets available for distribution among the holders of ordinary shares shall be distributed among the holders of the ordinary shares on a pro rata basis. If our assets available for distribution are insufficient to repay all of the paid-up capital, the assets will be distributed so that the losses are borne by our shareholders proportionately.
Redemption of Shares.Subject to the provisions of the Companies Law and other applicable law, we may issue shares on terms that are subject to redemption, at our option or at the option of the holders, on such terms and in such manner, including out of capital, as may be determined by the board of directors.
Calls on Shares and Forfeiture of Shares. Our board of directors may from time to time make calls upon shareholders for any amounts unpaid on their shares in a notice served to such shareholders at least 14 days prior to the specified time and place of payment. In the event that a call remains unpaid after it has become due and payable, our board of directors may give to the person from whom it is due not less than 14 days notice requiring payment of the amount unpaid, together with any interest that may accrue up to the date of payment, and stating that the shares in question will be forfeited if the notice is not complied with. If the requirements of any such notice are not complied with, any share subject to such notice may at any time thereafter, before payment of all calls and interest due in respect thereof have been made, be forfeited by a resolution of our board of directors to that effect.
Variations of Rights of Shares.All or any of the special rights attached to any class of shares may, subject to the provisions of the Companies Law, be varied with the sanction of a special resolution passed at a general meeting of the holders of the shares of that class. The rights conferred upon the holders of the shares of any class issued with preferred or other rights shall not, unless otherwise expressly provided by the terms of issue of the shares of that class, be deemed to be varied by the creation or issue of further shares ranking pari passu with such previously existing class of shares.
Inspection of Books and Records.Holders of our ordinary shares will have no general right under Cayman Islands law to inspect or obtain copies of our list of shareholders or our corporate records. However, we will in our second amended and restated memorandum and articles of association provide our shareholders with the right to inspect our list of shareholders and to receive annual audited financial statements. See “Where You Can Find Additional Information.”
Anti-Takeover Provisions.Some provisions of our second amended and restated memorandum and articles of association may discourage, delay or prevent a change of control of our company or management that shareholders may consider favorable, including provisions that:
| | • | | authorize our board of directors to issue preferred shares in one or more series and to designate the price, rights, preferences, privileges and restrictions of such preferred shares without any further vote or action by our shareholders; and |
| | • | | limit the ability of shareholders to call general meetings of shareholders. |
However, under Cayman Islands law, our directors may only exercise the rights and powers granted to them under our memorandum and articles of association for a proper purpose and for what they believe in good faith to be in the best interests of our company.
General Meetings of Shareholders.Shareholders’ meetings may be convened by a majority of our board of directors or our chairman. Advance notice of at least ten days is required for the convening of our annual general
83
shareholders’ meeting and any other general meeting of our shareholders. A quorum for a meeting of shareholders consists of at least two shareholders present or by proxy, representing not less than one-third in nominal value of the total issued voting shares in our company.
C.Material Contracts
We have not entered into any material contracts other than in the ordinary course of business and other than those described in “Item 4. Information on the Company” or elsewhere in this annual report on Form 20-F.
D.Exchange Controls
See “Item 4. Information on the Company—Business Overview—Regulations—Regulations on Foreign Exchange.”
E.Taxation
The following summary of the material Cayman Islands, PRC and United States federal income tax consequences of an investment in our ADSs or ordinary shares is based upon laws and relevant interpretations thereof in effect as of the date of this annual report on Form 20-F, all of which are subject to change. This summary does not deal with all possible tax consequences relating to an investment in our ADSs or ordinary shares, such as the tax consequences under state, local and other tax laws.
Cayman Islands Taxation
The Cayman Islands currently levies no taxes on individuals or corporations based upon profits, income, gains or appreciation and there is no taxation in the nature of inheritance tax or estate duty. There are no other taxes likely to be material to us levied by the government of the Cayman Islands except for stamp duties which may be applicable on instruments executed in, or brought within the jurisdiction of the Cayman Islands. The Cayman Islands is not party to any double tax treaties. There are no exchange control regulations or currency restrictions in the Cayman Islands.
People’s Republic of China Taxation
We are a holding company incorporated in the Cayman Islands, which indirectly holds, through our Hong Kong subsidiaries, 100% of our equity interests in our subsidiaries in the PRC. Our operations are principally conducted through our PRC subsidiaries. The New Enterprise Income Tax Law and its implementation rules, both of which became effective on January 1, 2008, provide that China-sourced income of foreign enterprises, such as dividends paid by a PRC subsidiary to its overseas parent that is a non-PRC resident enterprise, will normally be subject to PRC withholding tax at a rate of 10%, unless there are applicable treaties that reduce such rate. Under a special arrangement between China and Hong Kong, such dividend withholding tax rate is reduced to 5% if a Hong Kong resident enterprise owns over 25% of the PRC company distributing the dividends. As our Hong Kong subsidiaries owns 100% of our PRC subsidiaries, under the aforesaid arrangement, any dividends that our PRC subsidiaries pay our Hong Kong subsidiaries may be subject to a withholding tax at the rate of 5% if our Hong Kong subsidiaries are not considered to be PRC tax resident enterprises as described below. However, if our Hong Kong subsidiaries are not considered to be the beneficial owners of such dividends under a tax notice promulgated on October 27, 2009, such dividends would be subject to the withholding tax rate of 10%. See “Item 4. Information on the Company—Business Overview—Regulations—Regulations on Tax—Dividend Withholding Tax.”
Under the New Enterprise Income Tax Law, enterprises established under the laws of jurisdictions outside China with their “de facto management bodies” located within China may be considered to be PRC tax resident enterprises for tax purposes. If we are considered a PRC tax resident enterprise under the above definition, then our global income will be subject to PRC enterprise income tax at the rate of 25%.
84
The implementation rules of the New Enterprise Income Tax Law provide that (i) if the enterprise that distributes dividends is domiciled in the PRC, or (ii) if gains are realized from transferring equity interests of enterprises domiciled in the PRC, then such dividends or capital gains are treated as China-sourced income. It is not clear how “domicile” may be interpreted under the New Enterprise Income Tax Law, and it may be interpreted as the jurisdiction where the enterprise is a tax resident. Therefore, if we are considered as a PRC tax resident enterprise for tax purposes, any dividends we pay to our overseas shareholders or ADS holders who are non-resident enterprises as well as gains realized by such shareholders or ADS holders from the transfer of our shares or ADSs may be regarded as China-sourced income and as a result become subject to PRC withholding tax at a rate of up to 10%.
Under the PRC Individual Income Tax Law, if we are treated as a PRC resident enterprise for tax purposes, it is possible that non-resident individual investors may be subject to PRC individual income tax at a rate of 20% on any dividends paid to such investors and any capital gains realized from the transfer of our ordinary shares and/or ADSs if such dividends or capital gains are deemed income derived from sources within the PRC, unless such individuals qualify for a lower rate under a tax treaty. Under the PRC-U.S. tax treaty, a preferential income tax rate of 10% will apply to dividends, provided that certain conditions are met. See “Item 3. Key Information—Risk Factors—Risks Related to Doing Business in China—Our global income and the dividends that we may receive from our PRC subsidiaries may be subject to PRC taxes under the PRC Enterprise Income Tax Law, which would have a material and adverse effect on our results of operations.” and “Item 3. Key Information—Risk Factors—Risks Related to Doing Business in China—We may be required to withhold PRC income tax on the dividends we pay you (if any), and any gain you realize on the transfer of our ordinary shares and ADSs may also be subject to PRC tax if we are treated as a PRC “resident enterprise.”
Material United States Federal Income Tax Considerations
The following is a summary of the material United States federal income tax considerations relating to the acquisition, ownership and disposition of our ADSs or ordinary shares by a U.S. Holder (as defined below) that acquires and holds our ADSs or ordinary shares as “capital assets” (generally, property held for investment) under the United States Internal Revenue Code of 1986, as amended, or the Code.
This summary is based upon the provisions of the Code and regulations, rulings, and decisions thereunder as of the date hereof, and such authorities may be replaced, revoked, or modified, possibly with retroactive effect. This summary does not discuss all aspects of United States federal income taxation that may be important to particular investors in light of their individual investment circumstances, including investors subject to special tax rules (for example, financial institutions, insurance companies, regulated investment companies, real estate investment trusts, broker-dealers, traders in securities that elect mark-to-market treatment, partnerships (or other entities treated as partnerships for United States federal income tax purposes) and their partners, tax-exempt organizations (including private foundations), holders who are not U.S. Holders, holders who own (directly, indirectly, or constructively) 10% or more of our voting stock, holders who acquire our ADSs or ordinary shares pursuant to any employee share option or otherwise as compensation, investors that will hold our ADSs or ordinary shares as part of a straddle, hedge, conversion, constructive sale or other integrated transaction for United States federal income tax purposes, or investors that have a functional currency other than the United States dollar), all of whom may be subject to tax rules that differ significantly from those summarized below.
In addition, this summary does not discuss any state, local, or estate or gift tax considerations and, except for the limited instances where PRC tax law and potential PRC taxes are discussed below, does not discuss any non-United States tax considerations. Each U.S. Holder is urged to consult its tax advisor regarding the United States federal, state, local, and non-United States income and other tax considerations of an investment in our ADSs or ordinary shares.
85
General
For purposes of this summary, a “U.S. Holder” is a beneficial owner of our ADSs or ordinary shares that is, for United States federal income tax purposes, (i) an individual who is a citizen or resident of the United States, (ii) a corporation (or other entity treated as a corporation for United States federal income tax purposes) created in, or organized under the law of, the United States or any state thereof or the District of Columbia, (iii) an estate the income of which is includible in gross income for United States federal income tax purposes regardless of its source, or (iv) a trust (A) the administration of which is subject to the primary supervision of a United States court and which has one or more United States persons who have the authority to control all substantial decisions of the trust or (B) that has otherwise validly elected to be treated as a United States person under the Code.
If a partnership (or other entity treated as a partnership for United States federal income tax purposes) is a beneficial owner of our ADSs or ordinary shares, the tax treatment of a partner in the partnership will generally depend upon the status of the partner and the activities of the partnership. If a U.S. Holder is a partner of a partnership holding our ADSs or ordinary shares, the U.S. Holder is urged to consult its tax advisor regarding an investment in our ADSs or ordinary shares.
The discussion below assumes that the representations contained in the deposit agreement are true and that the obligations in the deposit agreement and any related agreement have been and will be complied with in accordance with the terms. For United States federal income tax purposes, a U.S. Holder of ADSs will be treated as the beneficial owner of the underlying shares represented by such ADSs.
Passive Foreign Investment Company Considerations
A non-United States corporation, such as our company, will be classified as a “passive foreign investment company,” or PFIC, for United States federal income tax purposes for any taxable year, if either (i) 75% or more of its gross income for such year consists of certain types of “passive” income or (ii) 50% or more of the value of its assets (determined on the basis of a quarterly average) during such year produce or are held for the production of passive income. For this purpose, passive income means any income which would be foreign personal holding company income under the U.S. Tax Code, including, without limitation, dividends, interest, royalties, rents, annuities, net gains from the sale or exchange of property producing such income, net gains from commodity transactions, net foreign currency gains and income from notional principal contracts. In addition, cash and assets readily convertible into cash are categorized as passive assets and our unbooked intangibles are taken into account for determining the value of our assets. We will be treated as owning a proportionate share of the assets and earning a proportionate share of the income of any other corporation in which we own, directly or indirectly, more than 25% (by value) of the stock.
We primarily operate as a provider of pharmaceutical and biotechnology R&D outsourcing services. Based on our current income and assets and the value of our ADSs and outstanding ordinary shares, we do not believe that we were a PFIC for the 2011 taxable year and we do not expect to be classified as a PFIC for the current taxable year or any subsequent taxable year. Despite our expectation, there can be no assurance that we will not be a PFIC for the current taxable year or any subsequent taxable year, as PFIC status is retested each year and depends on the actual facts in such year. We could be a PFIC, for example, if our market capitalization at any time in the future is lower than projected, or if our business and assets evolve in ways that are different from what we currently anticipate. Although we believe that a majority of our assets (by value) and the income derived from such assets do not constitute passive assets and income under the PFIC rules, there is no assurance that the U.S. Internal Revenue Service will agree with us. Additionally, the composition of our income and our assets will also be affected by future growth in activities that may potentially produce passive income and by how, and how quickly, we spend our liquid assets. Under circumstances where revenues from activities that produce passive income significantly increase relative to our revenues from activities that produce non-passive income or where we determine not to deploy significant amounts of cash for active purposes, our risk of becoming classified as a PFIC may substantially increase.
86
Furthermore, because there are uncertainties in the application of the relevant rules, it is possible that the U.S. Internal Revenue Service may successfully challenge our classification of certain income and assets as non-passive or our valuation of our tangible and intangible assets, each of which may result in our company becoming classified as a PFIC for the current or subsequent taxable years. Because PFIC status is a fact-intensive determination made on an annual basis and will depend upon the composition of our assets and income and the value of our tangible and intangible assets from time to time, no assurance can be given that we are not or will not become classified as a PFIC. If we are classified as a PFIC for any taxable year during which a U.S. Holder holds our ADSs or ordinary shares, the PFIC tax rules discussed below under “—Passive Foreign Investment Company Rules” generally will apply for such taxable year and will apply in future years even if we cease to be a PFIC in subsequent years, unless we cease to be a PFIC and the U.S. Holder makes a “deemed sale” election with respect to the ADSs or ordinary shares.
The discussion below under “—Dividends” and “—Sale or Other Disposition of ADSs or Ordinary Shares” assumes that we will not be classified as a PFIC for United States federal income tax purposes. The United States federal income tax rules that apply if we are classified as a PFIC for the current taxable year or any subsequent taxable year are discussed below under “—Passive Foreign Investment Company Rules.”
Dividends
Subject to the PFIC rules discussed below, any cash distributions (including the amount of any PRC tax withheld) paid on our ADSs or ordinary shares out of our current or accumulated earnings and profits, as determined under United States federal income tax principles, will generally be includible in the gross income of a U.S. Holder as dividend income on the day actually or constructively received by the U.S. Holder, in the case of ordinary shares, or by the depositary, in the case of ADSs. Because we do not intend to determine our earnings and profits on the basis of United States federal income tax principles, any distribution paid will generally be treated as a “dividend” for United States federal income tax purposes. For taxable years beginning before January 1, 2013, a non-corporate recipient of dividend income generally will be subject to tax on dividend income from a “qualified foreign corporation” at a maximum United States federal tax rate of 15 percent rather than the marginal tax rates generally applicable to ordinary income, provided that certain holding period requirements are met. A non-United States corporation (other than a corporation that is classified as a PFIC for the taxable year in which the dividend is paid or the preceding taxable year) generally will be considered to be a qualified foreign corporation (i) if it is eligible for the benefits of a comprehensive tax treaty with the United States which the Secretary of Treasury of the United States determines is satisfactory for purposes of this provision and which includes an exchange of information program, or (ii) with respect to any dividend it pays on shares (or ADSs in respect of such shares) which is readily tradable on an established securities market in the United States.
The U.S. Treasury Department has determined that the Agreement Between the Government of the United States of America and the Government of the People’s Republic of China for the Avoidance of Double Taxation and the Prevention of Tax Evasion with respect to Taxes on Income, or the Treaty, meets the requirements described above. We believe that we are a qualified foreign corporation for United States federal income tax purposes because our ADSs are readily tradable on the New York Stock Exchange, which is an established securities market in the United States. Accordingly, we believe that we will qualify for the benefits under the Treaty and that we are not currently, and are not likely to become in the near future, a PFIC. However, the eligibility requirements for foreign corporations are technical and uncertain and therefore, each U.S. Holder is urged to consult its tax advisor regarding the impact of these provisions and the availability of the preferential rate in its particular circumstances.
In the event that we are deemed to be a PRC resident enterprise under the PRC New Enterprise Income Tax Law, we believe that we would be eligible for benefits under the Treaty. (See “Item 10. Additional Information—Taxation—People’s Republic of China Taxation” above). If we are eligible for such benefits, dividends we pay on our ordinary shares, regardless of whether such shares are represented by our ADSs, would be eligible for the
87
reduced rates of taxation applicable to qualified dividend income, as discussed above. In the event that we are deemed to be a PRC “resident enterprise” under the PRC New Enterprise Income Tax Law, a U.S. Holder may be subject to PRC withholding taxes, if any, on dividends paid on our ADSs. Each U.S. Holder is urged to consult its tax advisors regarding the availability under the Treaty of a reduced tax rate on dividends in its particular circumstances. Dividends received on our ADSs or ordinary shares will not be eligible for the dividends received deduction allowed to corporations under the Code.
Dividends generally will be treated as income from foreign sources for United States foreign tax credit purposes and generally will constitute passive category income. Depending on its particular circumstances, a U.S. Holder may be eligible, subject to a number of complex limitations, to claim a foreign tax credit in respect of any foreign withholding taxes imposed on dividends received on our ADSs or ordinary shares. A U.S. Holder who does not elect to claim a foreign tax credit for foreign tax withheld, may instead claim a deduction, for United States federal income tax purposes, in respect of such withholdings, but only for a year in which such holder elects to do so for all creditable foreign income taxes. U.S. Holders are urged to consult their tax advisors regarding the availability of the foreign tax credit under their particular circumstances.
Sale or Other Disposition of ADSs or Ordinary Shares
Subject to the discussion below under “—Passive Foreign Investment Company Rules,” a U.S. Holder will generally recognize capital gain or loss upon the sale or other disposition of our ADSs or ordinary shares in an amount equal to the difference between the amount realized upon the disposition and such holder’s adjusted tax basis in such ADSs or ordinary shares. Any capital gain or loss will be long-term if our ADSs or ordinary shares have been held for more than one year and will generally be United States source gain or loss for United States foreign tax credit purposes. Capital gains of non-corporate taxpayers derived from capital assets held for more than one year are currently eligible for reduced rates of taxation. In the event that gain from the disposition of our ADSs or ordinary shares is subject to tax in the PRC, such gain may be treated as PRC source gain under the Treaty. The deductibility of a capital loss is subject to limitations. U.S. Holders are urged to consult their tax advisors regarding the tax consequences if a foreign withholding tax is imposed on a disposition of our ADSs or ordinary shares, including the availability of the foreign tax credit under their particular circumstances.
Passive Foreign Investment Company Rules
If we are classified as a PFIC for any taxable year during which a U.S. Holder holds our ADSs or ordinary shares, and unless the U.S. Holder makes a mark-to-market election (as described below), the U.S. Holder will generally be subject to special United States federal income tax rules that have a penalizing effect, regardless of whether we remain a PFIC, on (i) any excess distribution that we make to the U.S. Holder (which generally means any distribution paid during a taxable year to a U.S. Holder that is greater than 125 percent of the average annual distributions paid in the three preceding taxable years or, if shorter, the U.S. Holder’s holding period for our ADSs or ordinary shares), and (ii) any gain realized on the sale or other disposition, including a pledge, of our ADSs or ordinary shares. Under the PFIC rules the:
| | • | | excess distribution or gain will be allocated ratably over the U.S. Holder’s holding period for our ADSs or ordinary shares; |
| | • | | amounts allocated to the current taxable year and any taxable years in the U.S. Holder’s holding period prior to the first taxable year in which we are classified as a PFIC, or a pre-PFIC year, will be subject to tax as ordinary income; |
| | • | | amounts allocated to each prior taxable year, other than the current taxable year or a pre-PFIC year, will be subject to tax at the highest tax rate in effect applicable to you for that year; and |
| | • | | interest charge generally applicable to underpayments of tax will be imposed on the tax attributable to each prior taxable year, other than the current taxable year or a pre-PFIC year. |
88
If we are a PFIC for any taxable year during which a U.S. Holder holds our ADSs or ordinary shares and any of our non-United States subsidiaries is also a PFIC, such U.S. Holder would be treated as owning a proportionate amount (by value) of the shares of the lower-tier PFIC and would be subject to the rules described above on certain distributions by a lower-tier PFIC and a disposition of shares of a lower-tier PFIC even though such U.S. Holder would not receive the proceeds of those distributions or dispositions. Each U.S. Holder is urged to consult its tax advisor regarding the application of the PFIC rules to any of our subsidiaries.
As an alternative to the foregoing rules, a U.S. Holder of “marketable stock” in a PFIC may make a mark-to-market election with respect to our ADSs, but not our ordinary shares, provided that our ADSs continue to be listed on the New York Stock Exchange and that our ADSs are regularly traded. We believe that our ADSs qualify as regularly traded, but no assurances may be given in this regard. If a U.S. Holder makes a valid mark-to-market election, the U.S. Holder will generally (i) include as ordinary income for each taxable year that we are a PFIC the excess, if any, of the fair market value of the ADSs held at the end of the taxable year over the adjusted tax basis of such ADSs and (ii) deduct as an ordinary loss the excess, if any, of the adjusted tax basis of the ADSs over the fair market value of such ADSs held at the end of the taxable year, but only to the extent of the net amount previously included in income as a result of the mark-to-market election. The U.S. Holder’s adjusted tax basis in the ADSs would be adjusted to reflect any income or loss resulting from the mark-to-market election. If a U.S. Holder makes a mark-to-market election in respect of a corporation classified as a PFIC and such corporation ceases to be classified as a PFIC, the holder will not be required to take into account the gain or loss described above during any period that such corporation is not classified as a PFIC. If a U.S. Holder makes a mark-to-market election, any gain such U.S. Holder recognizes upon the sale or other disposition of our ADSs will be treated as ordinary income and any loss will be treated as ordinary loss, but only to the extent of the net amount previously included in income as a result of the mark-to-market election.
Because a mark-to-market election cannot be made for any lower-tier PFICs that we may own, a U.S. Holder may continue to be subject to the PFIC rules with respect to such U.S. Holder’s indirect interest in any investments held by us that are treated as an equity interest in a PFIC for United States federal income tax purposes.
We do not intend to provide information necessary for U.S. Holders to make qualified electing fund, or QEF, elections, which, if available, would result in tax treatment different from (and generally less adverse than) the general tax treatment for PFICs described above.
If a U.S. Holder holds our ADSs or ordinary shares during any taxable year that we are a PFIC, the holder must file an annual report with the U.S. Internal Revenue Service. In the case of a U.S. Holder who has held our ADSs or ordinary shares during any taxable year in respect of which we were classified as a PFIC and continues to hold such ADSs or ordinary shares (or any portion thereof) and has not previously determined to make a mark-to-market election, and who is now considering making a mark-to-market election, special tax rules may apply relating to purging the PFIC taint of such ADSs or ordinary shares. Each U.S. Holder is urged to consult its tax advisor concerning the United States federal income tax consequences of purchasing, holding and disposing of our ADSs or ordinary shares if we are or become classified as a PFIC, including the possibility of making a mark-to-market election and the unavailability of the QEF election.
Information Reporting and Backup Withholding
Individual U.S. Holders and certain entities may be required to submit to the U.S. Internal Revenue Service certain information with respect to his or her beneficial ownership of our ADSs or ordinary shares if such ADSs or ordinary shares are not held on his or her behalf by a U.S. financial institution. For example, the new law requires an individual U.S. Holder to file an attachment to his or her tax return reporting interests in specified foreign financial assets (including stock of a non- United States corporation) when the aggregate value of such interests exceed $50,000 during any taxable year. This new law also imposes penalties if an individual U.S.
89
Holder is required to submit such information to the U.S. Internal Revenue Service and fails to do so. U.S. Holders are urged to consult their tax advisors regarding their tax filing requirements with respect to an investment in our ADSs or ordinary shares.
In addition, dividend payments with respect to our ADSs or ordinary shares and proceeds from the sale, exchange or redemption of our ADSs or ordinary shares may be subject to information reporting to the U.S. Internal Revenue Service and possible United States backup withholding at a rate of 28 percent. Backup withholding will not apply, however, to a U.S. Holder who furnishes a correct taxpayer identification number and makes any other required certification, or who is otherwise exempt from backup withholding. We will withhold amounts from such dividend payments as required by applicable law. Each U.S. Holder is urged to consult its tax advisor regarding the application of the United States information reporting and backup withholding rules.
Backup withholding is not an additional tax. Amounts withheld as backup withholding may be credited against a U.S. Holder’s United States federal income tax liability, and a U.S. Holder generally may obtain a refund of any excess amounts withheld under the backup withholding rules by timely filing the appropriate claim for refund with the Internal Revenue Service and furnishing any required information.
F.Dividends and Paying Agents
Not applicable.
G.Statement by Experts
Not applicable.
H.Documents on Display
We are subject to the periodic reporting and other informational requirements of the Securities Exchange Act of 1934, as amended, or the Exchange Act. Under the Exchange Act, we are required to file reports and other information with the SEC. Specifically, we are required to file annually a Form 20-F no later than four months after the close of each fiscal year, which is December 31, for fiscal years ending on or after December 15, 2011. Copies of reports and other information, when so filed, may be inspected without charge and may be obtained at prescribed rates at the public reference facilities maintained by the SEC at 100 F Street, N.E., Room 1580, Washington, D.C. 20549, and at the regional office of the SEC located at Citicorp Center, 500 West Madison Street, Suite 1400, Chicago, Illinois 60661. The public may obtain information regarding the Washington, D.C. Public Reference Room by calling the SEC at 1-800-SEC-0330. The SEC also maintains a web site atwww.sec.gov that contains reports, proxy and information statements, and other information regarding registrants that make electronic filings with the SEC using its EDGAR system.
Our Internet website iswww.shangpharma.com. We make available free of charge on our website our annual reports on Form 20-F and any amendments to such reports as soon as reasonably practicable following the electronic filing of such report with the SEC. In addition, we provide electronic or paper copies of our filings free of charge upon request. The information contained on our website is not part of this or any other report filed with or furnished to the SEC.
As a foreign private issuer, we are exempt from the rules under the Exchange Act prescribing the furnishing and content of quarterly reports and proxy statements, and officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. Our consolidated financial statements have been prepared in accordance with U.S. GAAP.
We will furnish JPMorgan Chase Bank, N.A., the depositary of our ADSs, with our annual reports, which will include a review of operations and annual audited consolidated financial statements prepared in conformity
90
with U.S. GAAP, and all notices of shareholders’ meetings and other reports and communications that are made generally available to our shareholders. The depositary will make such notices, reports and communications available to holders of ADSs and, upon our request, will mail to all record holders of ADSs the information contained in any notice of a shareholders’ meeting received by the depositary from us.
I.Subsidiary Information
For a listing of our subsidiaries, see “Item 4. Information on the Company—Organizational Structure.”
| ITEM 11. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
Foreign Exchange Risk
Fluctuations in exchange rates affect our cost of revenues and net income and significantly impact our operating margins, because our net revenues are primarily generated from sales denominated in U.S. dollars, and a majority of our cost of revenues and operating expenses are denominated in Renminbi. In addition, we are exposed to foreign exchange risk related to cash and cash equivalents dominated in U.S. dollars.
The conversion of Renminbi into foreign currencies, including U.S. dollars, is based on rates set by the People’s Bank of China. The PRC government allowed the Renminbi to appreciate by more than 20% against the U.S. dollar between July 2005 and July 2008. Between July 2008 and June 2010, this appreciation was halted and the exchange rate between the Renminbi and the U.S. dollar remained within a narrow band. As a consequence, the RMB fluctuated significantly during that period against other freely traded currencies, in tandem with the U.S. dollar. Since June 2010, the PRC government has allowed the Renminbi to appreciate slowly against the U.S. dollar again. It is difficult to predict how market forces or PRC or U.S. government policy may impact the exchange rate between the Renminbi and the U.S. dollar in the future.
There remains significant international pressure on the Chinese government to adopt a substantial liberalization of its currency policy, which could result in further appreciation in the value of the RMB against the U.S. dollar. To the extent that we need to convert U.S. dollars into Renminbi for our operations, appreciation of the Renminbi against the U.S. dollar would have an adverse effect on the Renminbi amount that we receive from the conversion. Conversely, if we decide to convert our Renminbi into the U.S. dollar for the purpose of paying dividends on our ordinary shares or ADSs or for other business purposes, appreciation of the U.S. dollar in relation to the Renminbi would negatively affect the corresponding U.S. dollar amount available to us. Considering the amount of our cash denominated in U.S. dollars as of December 31, 2011, a 1.0% change in the exchange rates between the Renminbi and the U.S. dollar would result in an increase or decrease in the amount of approximately RMB1.0 million to our total cash denominated in U.S. dollars.
We periodically purchase derivative financial instruments, such as foreign-exchange forward contracts, to manage our exposure to Renminbi-U.S. dollar currency-exchange risk. The counterparties for these contracts are generally banks. The maturity periods of these contracts range from one to 21 months. We recorded a net gain of US$0.1 million and US$3.1 million on foreign-exchange forward contracts in 2009 and 2010, respectively. We recorded a net gain of US$1.9 million on foreign-exchange forward contracts in 2011. Our accounting policy requires us to mark to market at the end of each reporting period and to recognize the change in fair value in earnings immediately. We held foreign-exchange forward contracts with an aggregate notional amount of US$129.0 million as of December 31, 2011. As of December 31, 2011, the forward contracts had a fair value of US$0.8 million.
Interest Rate Risk
Our exposure to interest rate risk primarily relates to the interest income generated by bank deposits with original maturities of three months or less. We have not used any derivative financial instruments to manage our
91
interest risk exposure. Interest-earning instruments carry a degree of interest rate risk. We have not been exposed to material risks due to changes in interest rates. However, our future interest income may be lower than expected due to changes in market interest rates.
Inflation
Since our inception, inflation in China has not materially impacted our results of operations. According to the National Bureau of Statistics of China, the year-over-year percent changes in the consumer price index for December 2009, 2010 and 2011 were increases of 1.9%, 4.6% and 4.1%, respectively. Although we have not been materially affected by inflation, we can provide no assurance that we will not be affected in the future by higher rates of inflation in China.
| ITEM 12. | DESCRIPTION OF SECURITIES OTHER THAN EQUITY SECURITIES |
A.Debt Securities
Not applicable.
B.Warrants and Rights
Not applicable.
C.Other Securities
Not applicable.
D.American Depositary Shares
Fees and Charges Our ADS holders May Have to Pay
The depositary may charge each person to whom ADSs are issued, including, without limitation, issuances against deposits of shares, issuances in respect of share distributions, rights and other distributions, issuances pursuant to a share dividend or share split declared by us or issuances pursuant to a merger, exchange of securities or any other transaction or event affecting the ADSs or deposited securities, and each person surrendering ADSs for withdrawal of deposited securities or whose ADRs are cancelled or reduced for any other reason, US$5.00 for each 100 ADSs (or any portion thereof) issued, delivered, reduced, cancelled or surrendered, as the case may be. The depositary may sell (by public or private sale) sufficient securities and property received in respect of a share distribution, rights and/or other distribution prior to such deposit to pay such charge.
The following additional charges shall be incurred by the ADR holders, by any party depositing or withdrawing shares or by any party surrendering ADSs or to whom ADSs are issued (including, without limitation, issuance pursuant to a share dividend or share split declared by us or an exchange of shares regarding the ADRs or the deposited securities or a distribution of ADSs), whichever is applicable:
| | • | | a fee of US$1.50 per ADR or ADRs for transfers of certificated or direct registration ADRs; |
| | • | | a fee of up to US$0.05 per ADS for any cash distribution made pursuant to the deposit agreement; |
| | • | | a fee of up to US$0.05 per ADS per calendar year (or portion thereof) for services performed by the depositary in administering the ADRs (which fee may be charged on a periodic basis during each calendar year and shall be assessed against holders of ADRs as of the record date or record dates set by the depositary during each calendar year and shall be payable in the manner described in the next succeeding provision); |
92
| | • | | reimbursement of such fees, charges and expenses as are incurred by the depositary and/or any of the depositary’s agents (including, without limitation, the custodian and expenses incurred on behalf of holders in connection with compliance with foreign exchange control regulations or any law or regulation relating to foreign investment) in connection with the servicing of the shares or other deposited securities, the delivery of deposited securities or otherwise in connection with the depositary’s or its custodian’s compliance with applicable law, rule or regulation (which charge shall be assessed on a proportionate basis against holders as of the record date or dates set by the depositary and shall be payable at the sole discretion of the depositary by billing such holders or by deducting such charge from one or more cash dividends or other cash distributions); |
| | • | | a fee for the distribution of securities (or the sale of securities in connection with a distribution), such fee being in an amount equal to the fee for the execution and delivery of ADSs which would have been charged as a result of the deposit of such securities (treating all such securities as if they were shares) but which securities or the net cash proceeds from the sale thereof are instead distributed by the depositary to those holders entitled thereto; |
| | • | | share transfer or other taxes and other governmental charges; |
| | • | | cable, telex and facsimile transmission and delivery charges incurred at your request in connection with the deposit or delivery of shares; |
| | • | | transfer or registration fees for the registration of transfer of deposited securities on any applicable register in connection with the deposit or withdrawal of deposited securities; and |
| | • | | expenses of the depositary in connection with the conversion of foreign currency into U.S. dollars. |
We will pay all other charges and expenses of the depositary and any agent of the depositary (except the custodian) pursuant to agreements from time to time between us and the depositary. The charges described above may be amended from time to time by agreement between us and the depositary.
Fees and Other Payments Made by the Depositary to Us
Our depositary has agreed to reimburse us for certain expenses we incur that are related to establishment and maintenance of the ADR program, including investor relations expenses and exchange application and listing fees. In 2011, we received the following direct and indirect payments: US$0.5 million, which were recorded as a deduction of general and administrative expenses.
| ITEM 13. | DEFAULTS, DIVIDEND ARREARAGES AND DELINQUENCIES |
None.
| ITEM 14. | MATERIAL MODIFICATIONS TO THE RIGHTS OF SECURITY HOLDERS AND USE OF PROCEEDS |
See “Item 10. Additional Information” for a description of the rights of security holders, which remain unchanged.
The following “Use of Proceeds” information relates to the registration statement on Form F-1 (File number 333-169699) for our initial public offering of 5,800,000 ADSs, representing 104,400,000 ordinary shares, which registration statement was declared effective by the SEC on October 18, 2010. We issued and sold all registered ADSs at an initial offering price of US$15.00 per ADS.
93
For the period from the effective date to December 31, 2011, our expenses incurred and paid to others in connection with the issuance and distribution of the ADSs totaled US$8.0 million, which included US$3.4 million in underwriting discounts and commissions and US$4.6 million for other expenses. We received net proceeds of approximately US$40.0 million from our initial public offering.
For the period from the effective date to December 31, 2010, we used net proceeds from our initial public offering as follows:
| | • | | approximately US$5.4 million for the construction of our manufacturing facility and laboratory services building in Fengxian, Shanghai; and |
| | • | | approximately US$2.1 million for the purchase of equipment. |
For the year ended December 31, 2011, we used approximately US$25.4 million net proceeds received from our IPO for capital expenditures, including US$7.0 million for the construction of our manufacturing facility and laboratory services building in Fengxian, Shanghai; US$5.5 million for the purchase of new Chengdu R&D center; US$3.1 million for renovation of the new building dedicated to one of our largest customers; US$ 4.0 million for facilities acquired from Charles River and US$ 5.8 million for the purchase of equipment.
| ITEM 15. | CONTROLS AND PROCEDURES |
Disclosure Controls and Procedures
As of the end of the period covered by this annual report, our management, with the participation of our chief executive officer and chief financial officer, has performed an evaluation of the effectiveness of our disclosure controls and procedures within the meaning of Rules 13a-15(e) and 15d-15(e) of the Exchange Act as of December 31, 2011. Based upon this evaluation, our management has concluded that, as of the end of the period covered by this annual report, our existing disclosure controls and procedures were effective.
Disclosure controls and procedures means controls and other procedures that are designed to ensure that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the Securities Exchange Commission’s rule and forms and that such information required to be disclosed by us in the reports that we file or submit under the Exchange Act is accumulated and communicated to our management, including our principal executive and principal financial officers, or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosures.
Management’s Annual Report on Internal Control over Financial Reporting
We are responsible for establishing and maintaining adequate internal control over financial reporting. Our internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of our financial reporting and the preparation of financial statements for external purposes in accordance with U.S. GAAP and includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of our assets; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with U.S. GAAP, and that receipts and expenditures are being made only in accordance with authorizations of our management and directors; and (3) provide reasonable assurance regarding prevention or timely detection of the unauthorized acquisition, use or disposition of our assets that could have a material effect on our financial statements. Because of its inherent limitations, internal control over financial reporting can only provide reasonable assurance with respect to financial statements preparation and presentation and may not prevent or detect misstatements. In addition, projections of any evaluation of effectiveness of our internal control over financial reporting to future periods are subject to the risks that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Our management conducted an assessment of the effectiveness of our internal control over financial reporting as of December 31, 2011. In making this assessment, it used the criteria established within the Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Based on this assessment, our management has concluded that, as of December 31, 2011, our internal control over financial reporting was effective.
94
Remediation of Material Weakness
As of December 31, 2010, we did not maintain effective internal control over financial reporting due to the material weakness identified, including lack of sufficient competent personnel with appropriate levels of accounting knowledge and experience to perform period end reporting procedures, address complex U.S. GAAP accounting issues and prepare and review financial statements and related disclosures under U.S. GAAP.
A material weakness is a significant deficiency (within the meaning of Public Company Accounting Oversight Board Auditing Standard No. 5), or combination of significant deficiencies, that results in more than a remote likelihood that a material misstatement of the annual or interim financial statements will not be prevented or detected on a timely basis by employees in the normal course of their work.
For the year ended December 31, 2011, we completed the following remediation efforts specifically designed to address the material weaknesses that we identified as of December 31, 2010:
| | • | | We hired additional finance and accounting personnel, including a finance controller, a finance manager and William Dai, our new chief financial officer in early 2011, each of whom has solid knowledge of and experience with U.S. GAAP and Sarbanes Oxley compliance and are responsible for the oversight of day to day accounting and preparation of the consolidated financial statements; |
| | • | | We provided various U.S. GAAP and Sarbanes Oxley trainings to our accounting staff, finance department and internal audit department; |
| | • | | We formulated the internal policies relating to internal control over financial reporting, including the preparation of an accounting manual containing comprehensive written accounting policies and procedures to guide our financial personnel in preparing consolidated financial statements that are in compliance with U.S. GAAP and SEC requirements; |
| | • | | We established and strengthened our IT systems and controls related to the IT systems to ensure proper record keeping and; |
| | • | | We engaged a reputable internal control consultant to assist the Company in implementing internal controls and meet the compliance requirements. |
Other than these remediation measures, no significant changes have been made to our internal control over financial reporting during 2011 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
As of December 31, 2011, our management determined that applicable controls were effectively designed and operated so as to enable our management to conclude that the previously identified material weaknesses had been remediated and our internal control over financial reporting was effective.
Attestation Report of the Registered Public Accounting Firm
PricewaterhouseCoopers Zhong Tian CPAs Limited Company, our independent registered public accounting firm, has audited the effectiveness of our internal control over financial reporting as of December 31, 2011, as stated in its report, which appears on page F-2 of this Form 20-F.
Changes in Internal Control over Financial Reporting
We maintain a system of internal control over financial reporting that is designed to provide reasonable assurance that our books and records accurately reflect our transactions and that our established policies and procedures are followed. The discussion above under “Remediation of Material Weakness” includes descriptions of the material actual changes to our internal control over financial reporting in the year ended December 31, 2011 that materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
95
| ITEM 16A. | AUDIT COMMITTEE FINANCIAL EXPERT |
Our board of directors has determined that Messrs. Julian Ralph Worley and Benson Tsang, members of our Audit Committee, are audit committee financial experts.
Our board of directors has adopted a code of business conduct and ethics that applies to our directors, officers, employees and agents. We have filed our code of business conduct and ethics as an exhibit to our registration statement on Form F-1 (No. 333-169699).
| ITEM 16C. | PRINCIPAL ACCOUNTANT FEES AND SERVICES |
The following table sets forth the aggregate fees by categories specified below in connection with certain professional services rendered by PricewaterhouseCoopers Zhong Tian CPAs Limited Company, our principal external auditors, for the periods indicated. We did not pay any other fees to our auditors during the periods indicated below.
| | | | | | | | |
| | | For the Year Ended December 31, | |
| | | 2010 | | | 2011 | |
| | | (in millions of US$) | | | (in millions of US$) | |
Audit fees(1) | | | 1.0 | | | | 0.8 | |
Audit-related fees | | | * | | | | 0.2 | |
Tax fees(2) | | | 0.1 | | | | * | |
Notes:
| (1) | “Audit fees” means the aggregate fees billed for professional services rendered by our principal auditors for the audit of our annual financial statements and the review of our comparative interim financial statements. |
| (2) | “Tax fees” represents aggregate fees billed for professional services rendered by our principal auditors for tax compliance, tax advice and tax planning. |
The policy of our Audit Committee is to pre-approve all audit and non-audit services provided by PricewaterhouseCoopers Zhong Tian CPAs Limited Company, including audit services, audit-related services, tax services and other services as described above, other than those for de minimis services which are approved by the Audit Committee prior to the completion of the audit. All fees listed above were pre-approved by the Audit Committee.
| ITEM 16D. | EXEMPTIONS FROM THE LISTING STANDARDS FOR AUDIT COMMITTEES |
None.
| ITEM 16E. | PURCHASES OF EQUITY SECURITIES BY THE ISSUER AND AFFILIATED PURCHASERS |
| | | | | | | | | | | | | | | | |
Period | | Total Number of
ADS Purchased | | | Average Price
Paid Per ADS(1) | | | Total Number of
ADSs Purchased
as Part of Publicly
Announced
Plans(2) | | | Approximate
Dollar Value of
ADSs that May
Yet Be Purchased
Under the Plans(2) | |
September 2011 | | | 50,143 | | | $ | 8.71 | | | | 50,143 | | | | 9,563,417 | |
October 2011 | | | 8,533 | | | $ | 8.37 | | | | 58,676 | | | | 9,491,983 | |
November 2011 | | | 3,769 | | | $ | 8.33 | | | | 62,445 | | | | 9,460,596 | |
December 2011 | | | 61,841 | | | $ | 8.94 | | | | 124,286 | | | | 8,907,725 | |
Total | | | 124,286 | | | $ | 8.79 | | | | 124,286 | | | | 8,907,725 | |
| (1) | Each ADS represents 18 ordinary shares. |
96
| (2) | We publicly announced a share repurchase program on August 12, 2011, pursuant to which we are authorized to repurchase up to US$10 million of our ADSs within two years from August 24, 2011. |
| ITEM 16F. | CHANGE IN REGISTRANT’S CERTIFYING ACCOUNTANT |
Not applicable.
| ITEM 16G. | CORPORATE GOVERNANCE |
Because Mr. Michael Xin Hui and his family members beneficially own more than 50% of the shares of our company, and therefore hold more than 50% of the total voting power of our ordinary shares, we are a “controlled company” under the NYSE Corporate Governance Rules. We relied on certain exemptions that are available to controlled companies from NYSE corporate governance requirements, including the requirements that we have a compensation committee and a corporate governance and nominating committee that are composed entirely of independent directors.
Starting October 2011, our compensation committee and corporate governance and nominating committee have been composed of entirely of independent directors. We intend to continue to follow the applicable corporate governance standards under the NYSE Corporate Governance Rules. We are not aware of any significant differences between our current corporate governance practices and those followed by domestic companies under NYSE listing standards.
| ITEM 16H. | MINE SAFETY DISCLOSURE |
Not applicable.
PART III.
| ITEM 17. | FINANCIAL STATEMENTS |
We have elected to provide financial statements pursuant to Item 18.
| ITEM 18. | FINANCIAL STATEMENTS |
The consolidated financial statements of ShangPharma Corporation and its subsidiaries are included at the end of this annual report.
97
| | |
Exhibit Number | | Description of Document |
| 1.1 | | Second Amended and Restated Memorandum and Articles of Association of Registrant (incorporated by reference to Exhibit 3.4 from our F-1 registration statement (File No. 333-169699), as amended, initially filed with the Commission on September 30, 2010) |
| |
| 2.1 | | Specimen American Depositary Receipt of the Registrant (incorporated by reference to Exhibit 4.1 from our F-1 registration statement (File No. 333-169699), as amended, initially filed with the Commission on September 30, 2010) |
| |
| 2.2 | | Specimen Certificate for Ordinary Shares of the Registrant (incorporated by reference to Exhibit 4.2 from our F-1 registration statement (File No. 333-169699), as amended, initially filed with the Commission on September 30, 2010) |
| |
| 2.3 | | Deposit Agreement among the Registrant, the depositary and holders and beneficial holders of the American Depositary Receipts (incorporated by reference to Exhibit 4.3 from our F-1 registration statement (File No. 333-169699), as amended, initially filed with the Commission on September 30, 2010) |
| |
| 2.4 | | Investors’ Rights Agreement between the Registrant and other parties therein dated September 7, 2010 (incorporated by reference to Exhibit 4.4 from our F-1 registration statement (File No. 333-169699), as amended, initially filed with the Commission on September 30, 2010) |
| |
| 2.5 | | Waiver and Amendment Agreement No. 1 to Investors’ Rights Agreement dated as of May 19, 2008 (incorporated by reference to Exhibit 4.5 from our F-1 registration statement (File No. 333-169699), as amended, initially filed with the Commission on September 30, 2010) |
| |
| 2.6 | | Amendment Agreement No. 2 to Investors’ Rights Agreement dated as of September 30, 2010 (incorporated by reference to Exhibit 4.6 from our F-1 registration statement (File No. 333-169699), as amended, initially filed with the Commission on September 30, 2010) |
| |
| 4.1 | | 2008 Equity and Performance Incentive Plan (incorporated by reference to Exhibit 10.1 from our F-1 registration statement (File No. 333-169699), as amended, initially filed with the Commission on September 30, 2010) |
| |
| 4.2 | | Founder’s 2008 Equity and Performance Incentive Plan (incorporated by reference to Exhibit 10.2 from our F-1 registration statement (File No. 333-169699), as amended, initially filed with the Commission on September 30, 2010) |
| |
| 4.3 | | 2010 Share Incentive Plan (incorporated by reference to Exhibit 10.3 from our S-8 registration statement (File No. 333-172435), filed with the Commission on February 25, 2011) |
| |
| 4.4 | | 2011 Share Incentive Plan incorporated by reference to Exhibit 10.1 from our S-8 registration statement (File No. 333-178534), as amended, initially filed with the Commission on December 16, 2011) |
| |
| 4.5 | | Form of Indemnification Agreement between the Registrant and its Directors and Officers (incorporated by reference to Exhibit 10.3 from our F-1 registration statement (File No. 333-169699), as amended, initially filed with the Commission on September 30, 2010) |
| |
| 4.6 | | Form of Employment Agreement between the Registrant and the Officers of the Registrant (incorporated by reference to Exhibit 10.4 from our F-1 registration statement (File No. 333-169699), as amended, initially filed with the Commission on September 30, 2010) |
| |
| 4.7 | | Form of Non-Competition Agreement between the Registrant and the Officers of the Registrant (incorporated by reference to Exhibit 10.5 from our F-1 registration statement (File No. 333-169699), as amended, initially filed with the Commission on September 30, 2010) |
98
| | |
Exhibit Number | | Description of Document |
| 4.8 | | Master Laboratory Services Agreement, dated in March 2008, between Shanghai ChemPartner Co., Ltd. and Eli Lilly and Company (incorporated by reference to Exhibit 10.6 from our F-1 registration statement (File No. 333-169699), as amended, initially filed with the Commission on September 30, 2010) |
| |
| 4.9 | | Master Laboratory Services Agreement, dated in February 2009, between Shanghai ChemPartner Co., Ltd. and Eli Lilly and Company (incorporated by reference to Exhibit 10.7 from our F-1 registration statement (File No. 333-169699), as amended, initially filed with the Commission on September 30, 2010) |
| |
| 4.10 | | Amendment to Master Laboratory Services Agreement(s) with Eli Lilly and Company dated on September 18, 2009 (incorporated by reference to Exhibit 10.8 from our F-1 registration statement (File No. 333-169699), as amended, initially filed with the Commission on September 30, 2010) |
| |
| 4.11 | | Global Research and Development Outsourced Services Agreement, dated as of December 23, 2009, among Shanghai Chempartner Co., Ltd., China Gateway Life Science (Holding) Ltd. (HK) and GlaxoSmithKline Research & Development Limited ((incorporated by reference to Exhibit 10.9 from our F-1 registration statement (File No. 333-169699), as amended, initially filed with the Commission on September 30, 2010) |
| |
| 4.12 | | English translation of Service Outsourcing Agreement, dated as of June 1, 2007, between Shanghai ChemPartner Co., Ltd. and LabPartner (Shanghai Co., Ltd. (incorporated by reference to Exhibit 10.9 from our F-1 registration statement (File No. 333-169699), as amended, initially filed with the Commission on September 30, 2010) |
| |
| 4.13 | | English translation of Purchase and Sales Agreement, dated as of June 1, 2007, between Shanghai ChemPartner Co., Ltd. and LabPartner (Shanghai) Co., Ltd. (incorporated by reference to Exhibit 10.10 from our F-1 registration statement (File No. 333-169699), as amended, initially filed with the Commission on September 30, 2010) |
| |
| 4.14 | | English translation of Purchase and Sales Agreement, dated as of July 1, 2008, between Shanghai ChemPartner Co., Ltd. and LabPartner (Shanghai) Co., Ltd. (incorporated by reference to Exhibit 10.11 from our F-1 registration statement (File No. 333-169699), as amended, initially filed with the Commission on September 30, 2010) |
| |
| 4.15 | | English translation of Warehousing and Logistics Service Agreement, dated as of July 1, 2008, between Shanghai ChemPartner Co., Ltd. and LabPartner (Shanghai) Co., Ltd. (incorporated by reference to Exhibit 10.12 from our F-1 registration statement (File No. 333-169699), as amended, initially filed with the Commission on September 30, 2010) |
| |
| 4.16 | | English translation of Warehousing and Logistics Service Agreement, dated as of July 1, 2008, between Shanghai ChemPartner Co., Ltd. and LabPartner (Shanghai) Co., Ltd. (incorporated by reference to Exhibit 10.12 from our F-1 registration statement (File No. 333-169699), as amended, initially filed with the Commission on September 30, 2010) |
| |
| 4.17 | | English translation of Service Outsourcing Agreement, dated as of June 1, 2007, between Shanghai ChemExplorer Co., Ltd. and LabPartner (Shanghai) Co., Ltd. (incorporated by reference to Exhibit 10.14 from our F-1 registration statement (File No. 333-169699), as amended, initially filed with the Commission on September 30, 2010) |
| |
| 4.18 | | English translation of Warehousing and Logistics Service Agreement, dated as of July 1, 2008, between Shanghai ChemExplorer Co., Ltd. and LabPartner (Shanghai) Co., Ltd. (incorporated by reference to Exhibit 10.15 from our F-1 registration statement (File No. 333-169699), as amended, initially filed with the Commission on September 30, 2010) |
99
| | |
Exhibit Number | | Description of Document |
| 4.19 | | English translation of Service Outsourcing Agreement, dated as of April 1, 2007, between China Gateway Pharma Products (Shanghai) Limited and LabPartner (Shanghai) Co., Ltd. (incorporated by reference to Exhibit 10.16 from our F-1 registration statement (File No. 333-169699), as amended, initially filed with the Commission on September 30, 2010) |
| |
| 4.20 | | English translation of Purchase and Sales Agreement, dated as of April 1, 2007, between China Gateway Pharma Products (Shanghai) Limited and LabPartner (Shanghai) Co., Ltd. (incorporated by reference to Exhibit 10.17 from our F-1 registration statement (File No. 333-169699), as amended, initially filed with the Commission on September 30, 2010) |
| |
| 4.21 | | English translation of Warehousing and Logistics Service Agreement, effective as of April 26, 2007, between China Gateway Pharma Products (Shanghai) Limited and LabPartner (Shanghai) Co., Ltd. (incorporated by reference to Exhibit 10.18 from our F-1 registration statement (File No. 333-169699), as amended, initially filed with the Commission on September 30, 2010) |
| |
| 4.22 | | English translation of Form of Lease between the Registrant and Shanghai PharmValley Corp. (incorporated by reference to Exhibit 10.19 from our F-1 registration statement (File No. 333-169699), as amended, initially filed with the Commission on September 30, 2010) |
| |
| 4.23 | | English translation of Form of Catering Service Agreement between the Registrant and Shanghai Kehui Catering Management Co., Ltd. (incorporated by reference to Exhibit 10.20 from our F-1 registration statement (File No. 333-169699), as amended, initially filed with the Commission on September 30, 2010) |
| |
| 8.1* | | Subsidiaries of Registrant |
| |
| 11.1 | | Code of Business Conduct and Ethics of Registrant (incorporated by reference to Exhibit 99.1 from our F-1 registration statement (File No. 333-170055), as amended, initially filed with the Commission on October 20, 2010) |
| |
| 12.1* | | Chief Executive Officer Certification Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| |
| 12.2* | | Chief Financial Officer Certification Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| |
| 13.1* | | Chief Executive Officer Certification Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
| |
| 13.2* | | Chief Financial Officer Certification Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
| |
| 15.1* | | Consent of PricewaterhouseCoopers Zhong Tian CPAs Limited Company, an Independent Registered Public Accounting Firm |
| |
| 15.2* | | Consent of Fangda Partners |
| * | Filed with this Annual Report on Form 20-F. |
100
SIGNATURES
The registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this annual report on its behalf.
| | |
| SHANGPHARMA CORPORATION |
| |
| By: | | /s/ Michael Xin Hui |
| | Name: Michael Xin Hui |
| Title: | | Chief Executive Officer and Chairman of the Board |
Date: April 30, 2012
SHANGPHARMA CORPORATION
CONSOLIDATED STATEMENTS OF OPERATIONS
FOR THE YEARS ENDED DECEMBER 31, 2009, 2010 and 2011
| | | | |
| Reports of Independent Registered Public Accounting Firm | | | F-2 | |
Consolidated Financial Statements | | | | |
Consolidated Statements of Operations for the years ended December 31, 2009, 2010 and 2011 | | | F-3 | |
Consolidated Balance Sheets as of December 31, 2010 and 2011 | | | F-4 | |
Consolidated Statements of Changes in Shareholders’ Equity for the years ended December 31, 2009, 2010 and 2011 | | | F-5 | |
Consolidated Statements of Cash Flows for the years ended December 31, 2009, 2010 and 2011 | | | F-6 | |
Notes to the Consolidated Financial Statements for the years ended December 31, 2009, 2010 and 2011 | | | F-7 | |
Financial information of Shangpharma Corporation Condensed Financial Statements as of December 31, 2010 and 2011, and for the years ended December 31, 2009, 2010 and 2011 | | | F-46 | |
F-1
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Shareholders of ShangPharma Corporation:
In our opinion, the accompanying consolidated balance sheets and the related consolidated statements of operations, of changes in shareholders’ equity and of cash flows present fairly, in all material respects, the financial position of ShangPharma Corporation (“the Company”) and its subsidiaries at December 31, 2011 and 2010, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2011 in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2011, based on criteria established inInternal Control - Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). The Company’s management is responsible for these financial statements, for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in Management’s Annual Report on Internal Control over Financial Reporting appearing in Item 15 of this Form 20-F. Our responsibility is to express opinions on these financial statements and on the Company’s internal control over financial reporting based on our audits (which was an integrated audit in 2011). We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement and whether effective internal control over financial reporting was maintained in all material respects. Our audits of the financial statements included examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ PricewaterhouseCoopers Zhong Tian CPAs Limited Company
Shanghai, the People’s Republic of China
April 30, 2012
F-2
SHANGPHARMA CORPORATION
CONSOLIDATED STATEMENTS OF OPERATIONS
FOR THE YEARS ENDED DECEMBER 31, 2009, 2010 and 2011
(Amount expressed in US dollars, except share data, unless otherwise stated)
| | | | | | | | | | | | | | |
| | | Note | | 2009 | | | 2010 | | | 2011 | |
Net revenues | | | | | | | | | | | | | | |
Unrelated parties | | 2(n) | | | 72,284,657 | | | | 90,281,397 | | | | 111,149,778 | |
Related parties | | 18 | | | — | | | | — | | | | 451,911 | |
| | | | | | | | | | | | | | |
| | | | | 72,284,657 | | | | 90,281,397 | | | | 111,601,689 | |
| | | | |
Cost of revenues | | | | | | | | | | | | | | |
Unrelated parties | | | | | (40,490,139 | ) | | | (49,957,227 | ) | | | (68,893,594 | ) |
Related parties | | 18 | | | (7,942,831 | ) | | | (10,211,423 | ) | | | (6,609,829 | ) |
| | | | | | | | | | | | | | |
| | | | | (48,432,970 | ) | | | (60,168,650 | ) | | | (75,503,423 | ) |
| | | | |
Gross profit | | | | | 23,851,687 | | | | 30,112,747 | | | | 36,098,266 | |
| | | | | | | | | | | | | | |
Operating expenses: | | | | | | | | | | | | | | |
Selling and marketing | | | | | (1,608,584 | ) | | | (2,292,880 | ) | | | (2,640,095 | ) |
General and administrative | | | | | (11,994,024 | ) | | | (17,428,575 | ) | | | (23,961,054 | ) |
Other operating income | | 2(r) | | | — | | | | — | | | | 360,378 | |
Other operating expenses | | 2(r) | | | — | | | | — | | | | (193,763 | ) |
| | | | | | | | | | | | | | |
Total operating expenses | | | | | (13,602,608 | ) | | | (19,721,455 | ) | | | (26,434,534 | ) |
| | | | | | | | | | | | | | |
Profit from operations | | | | | 10,249,079 | | | | 10,391,292 | | | | 9,663,732 | |
| | | | |
Interest income | | | | | 29,621 | | | | 103,370 | | | | 966,532 | |
Interest expense | | | | | — | | | | (40,079 | ) | | | (37,792 | ) |
Other income | | 2(s) | | | 1,273,276 | | | | 4,801,609 | | | | 4,046,860 | |
Other expenses | | 2(t) | | | (200,938 | ) | | | (174,366 | ) | | | (1,081,919 | ) |
| | | | | | | | | | | | | | |
Income from operations before income taxes | | | | | 11,351,038 | | | | 15,081,826 | | | | 13,557,413 | |
Income taxes | | 14 | | | (1,552,223 | ) | | | (2,085,312 | ) | | | (2,442,562 | ) |
| | | | | | | | | | | | | | |
Net income | | | | | 9,798,815 | | | | 12,996,514 | | | | 11,114,851 | |
| | | | |
Allocation to preferred shareholders | | | | | (2,467,117 | ) | | | (2,738,594 | ) | | | — | |
| | | | | | | | | | | | | | |
Net income attributable to ShangPharma Corporation’s ordinary shareholders | | | | | 7,331,698 | | | | 10,257,920 | | | | 11,114,851 | |
| | | | | | | | | | | | | | |
Net income attributable to ShangPharma Corporation’s ordinary shareholders per share | | 15 | | | | | | | | | | | | |
Basic | | | | | 0.04 | | | | 0.04 | | | | 0.03 | |
| | | | | | | | | | | | | | |
Diluted | | | | | 0.04 | | | | 0.04 | | | | 0.03 | |
| | | | | | | | | | | | | | |
| | | | |
Weighted average ordinary shares outstanding | | | | | | | | | | | | | | |
Basic | | | | | 208,005,986 | | | | 233,874,361 | | | | 335,538,405 | |
| | | | | | | | | | | | | | |
Diluted | | | | | 278,051,299 | | | | 238,000,199 | | | | 337,885,258 | |
| | | | | | | | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
F-3
SHANGPHARMA CORPORATION
CONSOLIDATED BALANCE SHEETS
AS OF DECEMBER 31, 2010 and 2011
(Amount expressed in US dollars, except share data, unless otherwise stated)
| | | | | | | | | | |
| | | Note | | December 31, | |
| | | | 2010 | | | 2011 | |
| | | |
ASSETS | | | | | | | | | | |
Current assets: | | | | | | | | | | |
Cash and cash equivalents | | 2(f) | | | 49,160,335 | | | | 37,777,960 | |
Restricted cash | | 2(g) | | | 688,414 | | | | 2,562,475 | |
Accounts receivable, net | | 2(h) | | | 16,907,696 | | | | 23,973,518 | |
Amounts due from related parties | | 18 | | | — | | | | 503,069 | |
Inventories | | 3 | | | 1,259,163 | | | | 2,841,992 | |
Prepayments and others | | 4 | | | 4,778,625 | | | | 4,425,066 | |
Deferred tax assets | | 14 | | | 315,402 | | | | 263,627 | |
| | | | | | | | | | |
Total current assets | | | | | 73,109,635 | | | | 72,347,707 | |
| | | | | | | | | | |
Non-current assets: | | | | | | | | | | |
Derivative assets | | | | | 260,899 | | | | 6,938 | |
Property, plant, equipment and software, net | | 5 | | | 60,146,599 | | | | 86,520,808 | |
Land use right, net | | 6 | | | 4,220,508 | | | | 6,633,954 | |
Amounts due from related parties | | 18 | | | — | | | | 225,206 | |
Other long-term assets | | | | | 204,000 | | | | 558,902 | |
Deferred tax assets | | 14 | | | — | | | | 608,578 | |
Total non-current assets | | | | | 64,832,006 | | | | 94,554,386 | |
| | | | | | | | | | |
Total assets | | | | | 137,941,641 | | | | 166,902,093 | |
| | | | | | | | | | |
LIABILITIES AND SHAREHOLDERS’ EQUITY | | | | | | | | | | |
Current liabilities: | | | | | | | | | | |
Short-term bank borrowings | | 7 | | | 754,979 | | | | — | |
Accounts payable | | | | | 10,549,090 | | | | 16,306,610 | |
Amounts due to related parties | | 18 | | | 1,024,647 | | | | 122,512 | |
Salary and welfare payables | | | | | 4,039,824 | | | | 3,864,117 | |
Income taxes payable | | | | | 1,910,245 | | | | 3,241,109 | |
Advance from customers | | | | | 1,649,737 | | | | 1,595,866 | |
Other payables and accrued liabilities | | 8 | | | 4,477,213 | | | | 5,612,884 | |
| | | | | | | | | | |
Total current liabilities | | | | | 24,405,735 | | | | 30,743,098 | |
Non-current liabilities | | | | | | | | | | |
Advanced Subsidies | | | | | — | | | | 3,120,768 | |
| | | | | | | | | | |
Total non-current liabilities | | | | | — | | | | 3,120,768 | |
| | | | | | | | | | |
Total liabilities | | | | | 24,405,735 | | | | 33,863,866 | |
| | | | | | | | | | |
Commitments and contingencies | | 19 | | | | | | | | |
| | | |
Shareholders’ equity: | | | | | | | | | | |
Ordinary shares (US$0.001 par value; 500,000,000 shares authorized; 335,600,000 and 336,109,400 shares issued and outstanding as of December 31, 2010 and 2011, respectively) | | 12 | | | 335,600 | | | | 336,109 | |
Additional paid in capital | | | | | 78,989,313 | | | | 84,924,280 | |
Statutory reserves | | 2(z) | | | 6,795,484 | | | | 8,552,344 | |
Retained earnings | | | | | 23,655,382 | | | | 33,013,373 | |
Accumulated other comprehensive income | | | | | 3,760,127 | | | | 7,304,396 | |
Less: treasury stock, at cost; nil and 2,237,148 shares as of December 31, 2010 and 2011, respectively | | 13 | | | — | | | | (1,092,275 | ) |
| | | | | | | | | | |
Total shareholders’ equity | | | | | 113,535,906 | | | | 133,038,227 | |
| | | | | | | | | | |
Total liabilities and shareholders’ equity | | | | | 137,941,641 | | | | 166,902,093 | |
| | | | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
F-4
SHANGPHARMA CORPORATION
CONSOLIDATED STATEMENTS OF CHANGES IN SHAREHOLDERS’ EQUITY
FOR THE YEARS ENDED DECEMBER 31, 2009, 2010 and 2011
(Amount expressed in US dollars, except share data, unless otherwise stated)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Ordinary shares | | | | | | | | | | | | Accumulated | | | | | | Less: | | | | |
| | (US$0.001 par value) | | | Additional | | | | | | | | | Other | | | | | | Treasury stock | | | | |
| | | Number | | | | | | paid in | | | Statutory | | | Retained | | | Comprehensive | | | Comprehensive | | | Number | | | | | | Shareholders’ | |
| | of shares | | | Par value | | | Capital | | | reserves | | | earnings | | | Income | | | Income | | | of shares | | | Amount | | | Equity | |
Balance as of January 1, 2009 | | | 208,005,986 | | | | 208,006 | | | | 68,752 | | | | 3,885,356 | | | | 3,770,181 | | | | 2,075,187 | | | | | | | | — | | | | — | | | | 10,007,482 | |
Comprehensive income: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net income | | | — | | | | — | | | | — | | | | — | | | | 9,798,815 | | | | — | | | | 9,798,815 | | | | — | | | | — | | | | 9,798,815 | |
Other comprehensive income: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Foreign currency translation adjustment | | | — | | | | — | | | | — | | | | — | | | | — | | | | 65,099 | | | | 65,099 | | | | — | | | | — | | | | 65,099 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total comprehensive income | | | | | | | | | | | | | | | | | | | | | | | | | | | 9,863,914 | | | | | | | | | | | | 9,863,914 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Share-based compensation expense | | | — | | | | — | | | | 251,950 | | | | — | | | | — | | | | — | | | | | | | | — | | | | — | | | | 251,950 | |
Appropriations to statutory reserves (Note 2(z)) | | | — | | | | — | | | | — | | | | 1,561,060 | | | | (1,561,060 | ) | | | — | | | | | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance as of December 31, 2009 | | | 208,005,986 | | | | 208,006 | | | | 320,702 | | | | 5,446,416 | | | | 12,007,936 | | | | 2,140,286 | | | | | | | | — | | | | — | | | | 20,123,346 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Comprehensive income: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net income | | | — | | | | — | | | | — | | | | — | | | | 12,996,514 | | | | — | | | | 12,996,514 | | | | — | | | | — | | | | 12,996,514 | |
Other comprehensive income: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Foreign currency translation adjustment | | | — | | | | — | | | | — | | | | — | | | | — | | | | 1,619,841 | | | | 1,619,841 | | | | — | | | | — | | | | 1,619,841 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total comprehensive income | | | | | | | | | | | | | | | | | | | | | | | | | | | 14,616,355 | | | | | | | | | | | | 14,616,355 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Series A convertible preferred shares converted into ordinary shares upon initial public offering | | | 69,994,014 | | | | 69,994 | | | | 34,285,781 | | | | — | | | | — | | | | — | | | | | | | | — | | | | — | | | | 34,355,775 | |
Issuance of ordinary shares upon initial public offering, net of direct costs | | | 57,600,000 | | | | 57,600 | | | | 40,634,955 | | | | — | | | | — | | | | — | | | | | | | | — | | | | — | | | | 40,692,555 | |
Share-based compensation expense | | | — | | | | — | | | | 3,747,875 | | | | — | | | | — | | | | — | | | | | | | | — | | | | — | | | | 3,747,875 | |
Appropriations to statutory reserves (Note 2(z)) | | | — | | | | — | | | | — | | | | 1,349,068 | | | | (1,349,068 | ) | | | — | | | | | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance as of December 31, 2010 | | | 335,600,000 | | | | 335,600 | | | | 78,989,313 | | | | 6,795,484 | | | | 23,655,382 | | | | 3,760,127 | | | | | | | | — | | | | — | | | | 113,535,906 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Comprehensive income: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net income | | | — | | | | — | | | | — | | | | — | | | | 11,114,851 | | | | — | | | | 11,114,851 | | | | — | | | | — | | | | 11,114,851 | |
Other comprehensive income: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Foreign currency translation adjustment | | | — | | | | — | | | | — | | | | — | | | | — | | | | 3,544,269 | | | | 3,544,269 | | | | — | | | | — | | | | 3,544,269 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total comprehensive income | | | | | | | | | | | | | | | | | | | | | | | | | | | 14,659,120 | | | | | | | | | | | | 14,659,120 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Repurchase of treasury stock, at cost | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | | | | | (2,237,148 | ) | | | (1,092,275 | ) | | | (1,092,275 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Direct costs related to the issuance of ordinary shares upon initial public offering | | | — | | | | — | | | | (529,311 | ) | | | — | | | | — | | | | — | | | | | | | | — | | | | — | | | | (529,311 | ) |
Share-based compensation expense | | | — | | | | — | | | | 6,227,558 | | | | — | | | | — | | | | — | | | | | | | | — | | | | — | | | | 6,227,558 | |
Exercise of share options | | | 509,400 | | | | 509 | | | | 236,720 | | | | — | | | | — | | | | — | | | | | | | | — | | | | — | | | | 237,229 | |
Appropriations to statutory reserves (Note 2(y)) | | | — | | | | — | | | | — | | | | 1,756,860 | | | | (1,756,860 | ) | | | — | | | | | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance as of December 31, 2011 | | | 336,109,400 | | | | 336,109 | | | | 84,924,280 | | | | 8,552,344 | | | | 33,013,373 | | | | 7,304,396 | | | | | | | | (2,237,148 | ) | | | (1,092,275 | ) | | | 133,038,227 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
F-5
SHANGPHARMA CORPORATION
CONSOLIDATED STATEMENTS OF CASH FLOWS
FOR THE YEARS ENDED DECEMBER 31, 2009, 2010 and 2011
(Amount expressed in US dollars, except share data, unless otherwise stated)
| | | | | | | | | | | | | | |
| | | Note | | 2009 | | | 2010 | | | 2011 | |
Cash flows from operating activities: | | | | | | | | | | | | | | |
Net income | | | | | 9,798,815 | | | | 12,996,514 | | | | 11,114,851 | |
Adjustments to reconcile net income to net cash provided by operating activities: | | | | | | | | | | | | | | |
Depreciation and amortization of property, equipment and software | | | | | 5,830,472 | | | | 6,698,448 | | | | 9,185,140 | |
Amortization of land use right | | | | | 70,822 | | | | 85,758 | | | | 121,541 | |
Bad debt provision | | | | | 30,000 | | | | 34,058 | | | | 149,783 | |
Inventories provision | | | | | 51,940 | | | | 44,914 | | | | 369,100 | |
Impairment in value of investment securities | | | | | 25,857 | | | | — | | | | — | |
Gain from investment securities | | | | | — | | | | (275,929 | ) | | | — | |
Gain related to derivatives | | | | | (74,546 | ) | | | (3,033,942 | ) | | | (1,851,860 | ) |
Loss (gain) from disposal of fixed assets | | | | | — | | | | (79,237 | ) | | | 100,964 | |
Deferred taxes | | | | | (272,882 | ) | | | 58,625 | | | | (556,802 | ) |
Share-based compensation expense | | | | | 251,950 | | | | 3,747,875 | | | | 6,227,558 | |
Change in assets and liabilities: | | | | | | | | | | | | | | |
Increase in accounts receivable | | | | | (4,809,690 | ) | | | (2,584,041 | ) | | | (8,167,182 | ) |
Increase in inventories | | | | | (103,152 | ) | | | (158,679 | ) | | | (1,951,929 | ) |
Increase in amounts due from related parties | | | | | — | | | | — | | | | (705,965 | ) |
Increase in prepayments and others | | | | | (90,894 | ) | | | (1,076,004 | ) | | | (675,833 | ) |
Increase in other long-term assets | | | | | — | | | | (204,000 | ) | | | (377,213 | ) |
Increase in accounts payable | | | | | 1,541,480 | | | | 42,179 | | | | 1,234,682 | |
Decrease in amounts due to related parties | | | | | (1,141,464 | ) | | | (762,148 | ) | | | (902,135 | ) |
Increase (decrease) in salary and welfare payables | | | | | 931,781 | | | | 1,048,695 | | | | (175,707 | ) |
Increase in income taxes payable | | | | | 1,277,221 | | | | 321,548 | | | | 1,330,865 | |
Increase in advance from customers | | | | | 312,472 | | | | 1,257,829 | | | | (53,871 | ) |
Increase in advanced subsidies | | | | | — | | | | — | | | | 3,120,768 | |
Increase in other payables and accrued liabilities | | | | | 1,089,969 | | | | 492,059 | | | | 740,937 | |
| | | | | | | | | | | | | | |
Net cash provided by operating activities | | | | | 14,720,151 | | | | 18,654,522 | | | | 18,277,692 | |
| | | | | | | | | | | | | | |
Cash flows from investing activities: | | | | | | | | | | | | | | |
Purchase of property, plant, equipment and software | | | | | (13,969,335 | ) | | | (24,130,434 | ) | | | (28,430,906 | ) |
Purchase of land use right | | | | | (4,249,296 | ) | | | — | | | | (2,251,739 | ) |
Proceeds from disposal of fixed assets | | | | | — | | | | 138,206 | | | | 129,643 | |
Proceeds from disposal of investment securities | | | | | — | | | | 627,308 | | | | 41,726 | |
Payment for investment in securities | | | | | (142,857 | ) | | | — | | | | — | |
Proceeds (payments) from settlement of derivatives | | | | | (650,116 | ) | | | 319,351 | | | | 3,807,292 | |
(Increase) decrease in restricted cash | | | | | 685,854 | | | | (542,308 | ) | | | (1,874,061 | ) |
| | | | | | | | | | | | | | |
Net cash used in investing activities | | | | | (18,325,750 | ) | | | (23,587,877 | ) | | | (28,578,045 | ) |
| | | | | | | | | | | | | | |
Cash flows from financing activities: | | | | | | | | | | | | | | |
Proceeds from short-term bank borrowings | | | | | — | | | | 3,608,718 | | | | — | |
Repayment of short-term bank borrowings | | | | | — | | | | (2,871,343 | ) | | | (777,101 | ) |
Cash proceeds from initial public offering, net of issuance costs | | | | | — | | | | 40,692,555 | | | | (721,414 | ) |
Proceeds from issuance of ordinary shares upon exercise of stock options | | | | | — | | | | — | | | | 237,229 | |
Repurchase of ordinary shares | | | | | — | | | | — | | | | (1,092,275 | ) |
| | | | | | | | | | | | | | |
Net cash provided by (used in) financing activities | | | | | — | | | | 41,429,930 | | | | (2,353,561 | ) |
| | | | | | | | | | | | | | |
Effect of foreign exchange rate changes on cash and cash equivalents | | | | | 159 | | | | 425,623 | | | | 1,271,539 | |
| | | | | | | | | | | | | | |
Net (decrease) increase in cash and cash equivalents | | | | | (3,605,440 | ) | | | 36,922,198 | | | | (11,382,375 | ) |
Cash and cash equivalents at beginning of year | | | | | 15,843,577 | | | | 12,238,137 | | | | 49,160,335 | |
| | | | | | | | | | | | | | |
Cash and cash equivalents at end of year | | | | | 12,238,137 | | | | 49,160,335 | | | | 37,777,960 | |
| | | | | | | | | | | | | | |
| | | | |
Supplemental disclosure of cash flow information: | | | | | | | | | | | | | | |
Cash paid during the year for income taxes | | | | | 541,818 | | | | 1,763,764 | | | | 1,668,501 | |
Cash paid during the year for interest | | | | | — | | | | 40,079 | | | | 37,792 | |
Supplemental disclosure of non-cash investing and financing activities: | | | | | | | | | | | | | | |
Increase (decrease) in accounts payable for purchase of property, plant, equipment and software | | | | | (5,151,948 | ) | | | 5,800,744 | | | | 4,522,837 | |
The accompanying notes are an integral part of these consolidated financial statements
F-6
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2009, 2010 AND 2011
(Amount expressed in US dollars, except share data, unless otherwise stated)
1. ORGANIZATION AND NATURE OF OPERATIONS
The accompanying consolidated financial statements include the financial statements of ShangPharma Corporation (the “Company”) and its wholly owned subsidiaries, which mainly consist of ChemExplorer Company Limited (“CEHK”), China Gateway Life Science (Holdings) Limited (“CGHK”), Shanghai ChemExplorer Co., Ltd. (“CESH”), Shanghai PharmaExplorer Co., Ltd (“PESH”), Shanghai ChemPartner Co., Ltd (“CPSH”), China Gateway Pharma Products (Shanghai) Limited (“CGNH”), Chengdu Chempartner Co., Ltd. (“CPCD”), China Gateway Technology Development (Shanghai) Ltd. (“CGTD”) and China Gateway Pharmaceutical Development Co., Ltd. (“CGFX”). The Company and its subsidiaries are collectively referred to as the “Group”. The Group is principally engaged in providing pharmaceutical and biotechnology research and development services.
Substantially all of the Group’s business is conducted in the People’s Republic of China (“PRC”) through its wholly owned operating subsidiaries, CESH, PESH, CPSH, CGNH, CPCD, CGTD and CGFX (collectively referred to as “PRC Operating Subsidiaries”). CGNH, the original manufacturing facility primarily for pharmaceutical development services, was going through legal closure process as of December 31, 2011 and all substantial operations have been transferred to CGFX, the new cGMP(“certified General Manufacturing Practice”)-quality multi-purpose manufacturing facility during 2011.
CEHK and CGHK were incorporated in Hong Kong on January 3, 2003 and June 23, 2003, respectively, as direct holding companies of the PRC operating subsidiaries. CEHK and CGHK are indirectly wholly owned by two co-founders who are immediate family members prior to the restructuring undertaken in September 2007 as described below.
ShangPharma Corporation was incorporated in Cayman Islands on August 30, 2007. On September 5, 2007, the Group undertook a restructuring in anticipation of the issuance of Series A Convertible Preferred Shares to a third party investor (Note 10), whereby the Company became the ultimate holding company after all of the then existing shareholders of CEHK and CGHK exchanged their respective shares in these two entities for equivalent classes of shares of the Company on a one for one basis. The restructuring was accounted for as a legal reorganization of entities under common control in a manner similar to a pooling of interests. Accordingly, the accompanying consolidated financial statements have been prepared as if the current corporate structure had been in existence from inception of the Group.
2. SUMMARY OF PRINCIPAL ACCOUNTING POLICIES
(a) Basis of presentation and principles of consolidations
The accompanying consolidated financial statements have been prepared and presented in accordance with generally accepted accounting principles in the United States of America (“U.S. GAAP”).
The consolidated financial statements include the financial statements of the Company and its subsidiaries. All significant transactions, balances and profits between the Company and its subsidiaries have been eliminated upon consolidation. A subsidiary is an entity in which the Company, directly or indirectly, controls more than one half of the voting power; has the power to appoint or remove the majority of the members of the Board of Directors (“Board”); to cast a majority of votes at the meeting of the Board of Directors or to govern the financial and operating policies of the investee under a statute or agreement among the shareholders or equity holders.
F-7
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2009, 2010 AND 2011
(Amount expressed in US dollars, except share data, unless otherwise stated)
2. SUMMARY OF PRINCIPAL ACCOUNTING POLICIES (CONTINUED)
(b) Use of estimates
The preparation of consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, disclosure of contingent assets and liabilities at the dates of the consolidated financial statements, and the reported amounts of revenues and expenses during the reporting periods. Actual results could differ from those estimates. Significant accounting estimates reflected in the Company’s consolidated financial statements include revenue recognition, bad debt provision, inventories provision, loss contract accruals, valuation of investment in securities, valuation of derivative assets, useful lives and estimated residual value of property, equipment and software, valuation allowance on deferred tax assets, provision for uncertain tax positions and certain assumptions related to the valuation of share-based compensation.
(c) Fair value of financial instruments
The guidance on fair value measurements establishes a hierarchy for inputs used in measuring fair value that maximizes the use of observable inputs and minimizes the use of unobservable inputs by requiring that the observable inputs be used when available. Observable inputs are inputs that market participants would use in pricing the asset or liability developed based on market data obtained from sources independent of the Company. Unobservable inputs are inputs that reflect the Company’s assumptions about the assumptions market participants would use in pricing the asset or liability developed based on the best information available in the circumstances. As such, fair value is a market-based measure considered from the perspective of a market participant who holds the asset or owes the liability rather than an entity-specific measure. The hierarchy is broken down into three levels based on the reliability of inputs as follows: (Level 1) observable inputs such as quoted prices in active markets; (Level 2) inputs other than the quoted price in active markets that are observable either directly or indirectly, or quoted prices in less active markets; and (Level 3) unobservable inputs with respect to which there is little or no market data, which require the Company to develop its own assumptions.
When available, the Company measures the fair value of financial instruments based on quoted market prices in active markets, valuation techniques that use observable market-based inputs or unobservable inputs that are corroborated by market data. Pricing information the Company obtains from third parties is internally validated for reasonableness prior to use in the consolidated financial statements. When observable market prices are not readily available, the Company generally estimates the fair value using valuation techniques that rely on alternate market data or inputs that are generally less readily observable from objective sources and are estimated based on pertinent information available at the time of the applicable reporting periods. In certain cases, fair values are not subject to precise quantification or verification and may fluctuate as economic and market factors vary and the Company’s evaluation of those factors changes. Although the Company uses its best judgment in estimating the fair value of these financial instruments, there are inherent limitations in any estimation technique. In these cases, a minor change in an assumption could result in a significant change in its estimate of fair value, thereby increasing or decreasing the amounts of the Company’s consolidated assets, liabilities, shareholders’ equity and net income.
(d) Derivatives and Hedging Activities
The Company has entered into foreign exchange forward contracts with certain banks to reduce the exposure of significant changes in exchange rates between RMB and foreign currencies. These contracts are not designated as hedges and are marked to market at each reporting date, with changes in fair value recognized in
F-8
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2009, 2010 AND 2011
(Amount expressed in US dollars, except share data, unless otherwise stated)
2. SUMMARY OF PRINCIPAL ACCOUNTING POLICIES (CONTINUED)
(d) Derivatives and Hedging Activities (continued)
the consolidated statements of operations. Authoritative guidance requires companies to recognize all of the derivative financial instruments as either assets or liabilities at fair value in the consolidated balance sheets based upon quoted market prices for comparable instruments. The Company’s derivative instruments have not met the criteria for hedge accounting within the authoritative guidance. Therefore, the foreign currency forward contracts have been recorded at fair value, with gains or losses on these transactions recorded in the consolidated statements of operations within other income or other expense in the period in which they occur. The Company does not use derivative financial instruments for trading or speculative purposes. The Company used a discounted cash flow methodology to measure fair value, which requires inputs such as interest yield curves and foreign exchange rates. The significant inputs used in the aforementioned model can be corroborated with market observable data and therefore the fair value measurements are classified as level 2. Typically, any losses or gains on the forward exchange contracts are offset by re-measurement losses or gains on the underlying balances denominated in non-functional currencies. The Company’s foreign currency exchange contract is an over-the-counter instrument.
(e) Functional currency and foreign currency translations
The Company and its non-PRC subsidiaries’ functional currency is the United States dollar (“US dollar” or “US$”). The Company’s PRC subsidiaries’ functional currency is the Renminbi (“RMB”), the official currency in the PRC. Transactions denominated in currencies other than the functional currencies are translated into the functional currencies at the exchange rates quoted by the People’s Bank of China (the “PBOC”) prevailing at the transaction dates. Gains and losses resulting from foreign currency transactions are included in the consolidated statements of operations. Monetary assets and liabilities denominated in foreign currencies are translated into the functional currencies using the applicable exchange rates quoted by the PBOC at the balance sheet dates. All such exchange gains and losses are recognized in the consolidated statements of operations. Non-monetary assets and liabilities denominated in foreign currencies are translated into the functional currencies using the applicable exchange rates quoted by the PBOC at the date when the assets and liabilities occur.
The reporting currency of the Group is US dollar. Assets and liabilities are translated at the exchange rates at the balance sheet date, shareholders’ equity accounts are translated at historical exchange rates and revenues, expenses, gains and losses are translated using the average rate for the year. Translation adjustments are reported as cumulative translation adjustments and are shown, net of tax, as a separate component in the consolidated statements of changes in shareholders’ equity.
(f) Cash and cash equivalents
Cash and cash equivalents represent cash on hand, demand deposits placed with banks or other financial institutions and highly liquid investments that are unrestricted as to withdrawal and use that have original maturities of three months or less from the date of purchase. Included in the cash and cash equivalents balance as of December 31, 2010 and 2011 are balances denominated in US$ amounting to US$16,823,630 and US$15,459,546, respectively. As of December 31, 2011, US$31,443,050 and US$5,985,093 of cash and cash equivalents are under the jurisdiction of PRC and Hong Kong, respectively. As of December 31, 2010, US$44,136,696 and US$4,755,982 of cash and cash equivalents are under the jurisdiction of PRC and Hong Kong, respectively.
F-9
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2009, 2010 AND 2011
(Amount expressed in US dollars, except share data, unless otherwise stated)
2. SUMMARY OF PRINCIPAL ACCOUNTING POLICIES (CONTINUED)
(g) Restricted cash
Restricted cash at December 31, 2010 includes cash held as collateral for foreign exchange forward contracts. Restricted cash at December 31, 2011 includes cash held as collateral for foreign exchange forward contracts of US$697,409, cash in the depositary bank intended for future repurchase of shares of US$1,007,725, funds received from government eligible to certain scientists of the Company of US$753,861 and payables to employees for exercised share options of US$103,480.
(h) Accounts receivable, net
Accounts receivable are presented net of allowance for doubtful accounts. The Group provides specific provisions for bad debts when facts and circumstances indicate that the collection is doubtful and a loss is probable and estimable. For the years ended December 31, 2009, 2010 and 2011, the Group recorded allowance for doubtful accounts of US$30,000, US$34,058 and US$149,783, respectively, and wrote off bad debts of nil, US$113,435 and nil for the years presented, respectively. Allowance for doubtful accounts was US$34,058 and US$183,841 as of December 31, 2010 and 2011, respectively.
(i) Inventories
Inventories consisting of raw materials, costs incurred prior to delivery and customer acceptance of undelivered compounds are stated at the lower of cost or market. The Company determines cost on a weighted-average basis. Cost comprises direct materials and where applicable, of direct labor and overhead costs that has been incurred in bringing inventories to their present location and condition. Writedowns for inventories valuation were US$51,940, US$44,914 and US$369,100 for the years ended December 31, 2009, 2010 and 2011, respectively, and once recorded, result in a new cost basis for the related inventories and such writedowns are not reversed until such inventory are sold or otherwise disposed. For the years ended December 31, 2009, 2010 and 2011, nil, US$70,052 and US$44,211 of such reserves were removed with the sale or disposal of such inventories, respectively.
(j) Investment in securities
Investments in debt and equity securities are, on initial recognition, classified into the three categories: held-to-maturity securities, trading securities and available-for-sale securities. Debt securities that the Company has the positive intent and ability to hold to maturity are classified as held-to-maturity securities and reported at amortized cost. Debt and equity securities that are bought and held principally for the purpose of selling them in the near term are classified as trading securities and reported at fair value, with unrealized gains and losses included in earnings. Debt and equity securities not classified as either held-to-maturity securities or trading securities are classified as available-for-sale securities and reported at fair value, with unrealized gains and losses recognized in accumulated other comprehensive income.
The Group recorded the investment in convertible and redeemable preferred shares, which are in nature of debt securities, as an available-for-sale investment. Subsequent to initial recognition, available-for-sale investment is measured at fair value with changes in fair value recognized in accumulated other comprehensive income included in the consolidated statement of changes in shareholders’ equity. When there is objective evidence that the investment is impaired, the cumulative losses from the declines in fair value that had been recognized directly in other comprehensive income are removed from the consolidated statement of changes in
F-10
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2009, 2010 AND 2011
(Amount expressed in US dollars, except share data, unless otherwise stated)
2. SUMMARY OF PRINCIPAL ACCOUNTING POLICIES (CONTINUED)
(j) Investment in securities (continued)
shareholders’ equity and recognized in the consolidated statements of operations. When the available-for-sale investment is sold, the cumulative fair value adjustments previously recognized in accumulated other comprehensive income are recognized in the current period operating results. The Group evaluates the investments periodically for possible other-than-temporary impairment. An other-than-temporary impairment must be recognized if an investor has the intent to sell the debt security or if it is more likely than not that it will be required to sell the debt security before recovery of its amortized cost basis.
(k) Property, plant, equipment and software
Property, plant, equipment and software are stated at cost less accumulated depreciation and amortization. Costs include amounts paid to acquire or construct the assets. Assets under construction are not depreciated until construction is completed and the assets are ready for their intended use. As of December 31, 2010 and 2011, assets classified as construction in progress mainly relate to the construction of manufacturing facility and laboratory service building located in Fengxian for pharmaceutical development services, the leasehold improvements and equipments which need construction or installation. Upon retirement or sale, the cost of assets disposed of and the related accumulated depreciation/amortization are removed from the accounts and any resulting gains or losses are recognized in the consolidated statements of operations. Repair and maintenance costs are expensed as incurred.
Depreciation and amortization are computed using the straight-line method over the following estimated useful lives, taking into account any estimated residual values:
| | |
| Leasehold improvements | | Shorter of the lease term or the estimated useful lives |
| Building and constructions | | 20 years |
| Experimental equipment | | 3-10 years |
| Vehicles | | 5 years |
| Office equipment and others | | 5 years |
| Software licenses | | 1-10 years |
Residual value is determined based on the economic value of the property, plant and equipment at the end of the estimated useful period, with a range from 0% to 10% of the original costs.
(l) Land use right
Land use right represents fees paid to obtain the right to use land in the PRC. Amortization is computed using the straight-line method over the terms specified in the land use right certificate of 50 year or the remaining term in the certificate from the acquisition of the land use right.
(m) Impairment of long-lived assets
The Group reviews its long-lived assets for impairment whenever events or changes in circumstances indicate the carrying amount of an asset may not be recoverable. Recoverability of these assets is measured by comparison of its carrying amounts to the future undiscounted cash flows the assets are expected to generate. If
F-11
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2009, 2010 AND 2011
(Amount expressed in US dollars, except share data, unless otherwise stated)
2. SUMMARY OF PRINCIPAL ACCOUNTING POLICIES (CONTINUED)
(m) Impairment of long-lived assets (continued)
the sum of the expected undiscounted cash flow is less than the carrying amount of the assets, the Group would recognize an impairment loss equal to the amount by which the carrying value of the asset exceeds its fair value. No impairment of long-lived assets was recognized for the years ended December 31, 2009, 2010 and 2011.
(n) Revenue recognition
The Group provides a broad range of integrated laboratory and manufacturing services in the drug discovery and development process to pharmaceutical and biotechnology companies. Laboratory services include contracts on full time equivalent (“FTE”) basis and fee-for-service basis.
Revenues are recognized when persuasive evidence of an arrangement exists, the sales price is fixed or determinable, delivery of the product or performance of the service has occurred and there is reasonable assurance of collection of the sales proceeds.
For laboratory services provided under a FTE basis, the customer pays a fixed rate per laboratory worker on a full time employment basis and the Group recognizes revenue as the services are provided. The FTE contracts do not require acceptance by the customer or have obligations for fixed deliverables by the Group.
Prior to January 1, 2011, Laboratory services provided on a fee-for-service basis often include multiple elements of deliverables. Upon entering into such contractual agreements involving multiple elements of deliverables, the Group first determines whether each deliverable has standalone value to the customer. If the deliverables each have standalone value to the customer, then a separate unit of accounting is adopted to each separate deliverable. If any of the deliverables are not considered to have standalone value to the customer, then the deliverables are considered bundled and only one unit of accounting is assigned to the entire arrangement.
In September 2009, the FASB amended the accounting standards for multiple deliverables revenue arrangements. The new standard was adopted effective January 1, 2011 and requires the Group to use its best estimate of selling price (“BESP”) for the deliverables in an arrangement when vendor specific objective evidence (“VSOE”) or third party evidence of the selling price (“TPE”) is not available. The BESP should be determined in a way that is consistent with the price at which the Company would sell the deliverable if the item were to be sold separately. The residual method of allocating arrangement consideration will no longer be permitted.
The Group generally is not able to establish TPE or VSOE for its services, as the deliverables are highly customized and competitor pricing is not available or the Group is unable to reliably determine what similar competitor products’ selling prices are on a stand-alone basis. BESP for deliverables is generally established based on labor costs, reagents and supplies costs, overhead costs, risks and complexity of the work and expected profit margins developed from the competitive bidding process for customer contracts. The Group allocates the contractual arrangement’s value at the inception of the arrangement using relative fair value method based upon BESP. Consistent with the Group’s accounting policies prior to the adoption of this standard, revenue is recognized as each element is earned, provided that the fair value of the undelivered element(s) has been determined, the delivered element(s) has stand-alone value, there is no right of return on delivered element(s), and the Group is in control of the undelivered element(s). An element is considered earned upon the delivery or acceptance of the deliverables. The adoption of this standard did not have any impact on the consolidated financial statements.
F-12
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2009, 2010 AND 2011
(Amount expressed in US dollars, except share data, unless otherwise stated)
2. SUMMARY OF PRINCIPAL ACCOUNTING POLICIES (CONTINUED)
(n) Revenue recognition (continued)
For arrangements that include service elements, revenue is recognized for the service element using the proportionate performance method. The Group bases measurement of performance on a comparison of direct labor hours through that date to estimated total direct labor hours to complete the arrangement under the contract. The Group believes this is the best indicator of the performance of the contractual obligations. Changes in the estimated total direct labor hours to complete a contract without a corresponding proportional change to the contract value result in a cumulative adjustment to the amount of revenue recognized in the period in which the change in estimate is determined.
Under certain arrangements, a portion of the payments owed to the Group are contingent upon successful achievement of performance standards or research and development success milestones. Revenues are recognized when the milestone achievements are accepted by the customers.
Under certain fee-for-service arrangements, the customers confirm the acceptance of the completed deliverables but require the Group to hold the deliverables temporarily to meet their shipping schedules. Under such arrangements, the customers also confirm that the Group has the rights to bill them upon the acceptance and any extra reprocessing costs due to the delay of shipping are to be burdened by them. So for such bill and hold arrangements, revenue is recognized upon acceptance of the deliverables by the customers even if the delivery doesn’t incur. The amount of bill and hold sales were immaterial for all periods presented. At December 31, 2011, there were no bill and hold arrangements outstanding.
In the event of changes in the scope, nature, duration, or volume of services of the contract with a customer, the Group negotiates to modify its existing contract or establish a new contract to reflect the changes. The Group recognizes renegotiated amounts as revenue by revision to the total contract value resulting from the renegotiated contract.
Most of the Group’s revenue contracts can be terminated by its customers either immediately or after a specified period following notice. These contracts typically require the customer to pay the Group the fees earned through the termination date. Therefore, revenue recognized prior to cancellation generally does not require an adjustment upon cancellation.
Costs incurred prior to the delivery and acceptances of the deliverables are capitalized in inventory as work in progress. Cash received in advance of the delivery and acceptances of the deliverables are recorded as advances from customers.
The Group also provides research manufacturing services to its customers. Revenue from the sale of manufactured products is recognized upon delivery and acceptance by the customer when title and risk of loss have been transferred. Revenue from the sale of manufactured products was not material for the years ended December 31, 2009, 2010 and 2011, respectively.
(o) Sales taxes
Certain laboratory services provided are subject to sales tax at a rate of 5%. Sales tax and related charges are recognized as sales taxes and are deducted from gross revenues and recorded as a liability to the taxing
F-13
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2009, 2010 AND 2011
(Amount expressed in US dollars, except share data, unless otherwise stated)
2. SUMMARY OF PRINCIPAL ACCOUNTING POLICIES (CONTINUED)
(o) Sales taxes (continued)
authorities to arrive at net revenues. The Group historically received sales tax exemptions for certain laboratory services upon the approval from local authorities. Tax exemptions for the years ended December 31, 2009, 2010 and 2011 were US$2,598,524, US$3,566,799 and US$4,479,751, respectively.
(p) Shipping and handling costs
Shipping and handling costs are included in selling and marketing expenses in the consolidated statements of operations. For the years ended December 31, 2009, 2010 and 2011, shipping and handling costs were US$183,628, US$229,385 and US$271,409, respectively.
(q) Leases
Leases where substantially all the rewards and risks of ownership of assets remain with the leasing company are accounted for as operating leases. Payments made under operating leases net of any incentives from the lessor are charged to the consolidated statements of operations on a straight-line basis over the lease periods.
(r) Government subsidies
The Group’s government subsidies can generally be divided into 1) research related, 2) hiring and training related 3) capital expenditure related and 4) others.
For capital expenditure related government subsidies, the subsidies are initially recorded as advanced subsidies and recognized as a reduction of depreciation expenses using straight-line method over the useful life of the assets ready in use. The Group recognized nil, US$10,097 and US$444,407 as a reduction of depreciation expenses in cost of revenues for the years ended December 31, 2009, 2010 and 2011, respectively.
For other government subsidies, the Group recognizes the cash subsidies as other income when received if there are no defined rules and regulations to govern the criteria necessary for companies to enjoy the benefits, or initially record as liabilities and recognize as other income upon when the obligations for earning such subsidies have been met. Other government subsidies were immaterial for all periods presented.
For research related and hiring or training related government subsidies, the Group originally recognized the cash subsidies as other income when received if there is no defined rules and regulations to govern the criteria necessary for companies to enjoy the benefits, or initially recorded as liabilities and recognized as other income upon when the obligations for earning such subsidies have been met.
Effective on July 1, 2011, the Group changed the accounting policy for research and hiring or training related government subsidies by classifying such subsidies as operating income rather than the original non-operating income. In addition research related costs were reclassified from cost of revenues to other operating expenses, and hiring or training related expenses continue to be recorded in general and administrative expenses. Although ASC 250 requires a retrospective application, the Group determined that a prospective application is deemed reasonable due to the insignificant impact in prior periods. As a result of the change in accounting policy, effective from July 1, 2011 under the prospective application, the Group’s gross profit and profit from operations for fiscal 2011 increased by $194,000 and $360,000, respectively. Should a retrospective application
F-14
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2009, 2010 AND 2011
(Amount expressed in US dollars, except share data, unless otherwise stated)
2. SUMMARY OF PRINCIPAL ACCOUNTING POLICIES (CONTINUED)
(r) Government subsidies (continued)
be adopted, the impact on the Company’s gross profit would increase by nil, $266,000, and $120,000 for fiscal years 2009, 2010 and 2011, respectively, and the impact on the Company’s profit from operations would increase by $453,000, $607,000 and $384,000 for fiscal years 2009, 2010 and 2011, respectively. There would be no impact on the Company’s net income for fiscal years 2009, 2010 and 2011. After the accounting policy change, the cash subsidies are recognized as other operating income when received if there are no defined rules and regulations to govern the criteria necessary for companies to enjoy the benefits or initially recorded as liabilities and recognized as other operating income upon when the obligations for earning such subsidies have been met. Accordingly the costs related to such subsidies are recognized as other operating expenses to better reflect the economics of the subsidies.
The Group believes the change in accounting policy is preferable as it better reflects the substance of the government assistance which is more in line with the operating nature of the transaction. The accounting policy change is not retrospectively applied to all prior periods as well as current periods (the first and second quarter of 2011) because the financial impact of such change is assessed to be immaterial both qualitatively and quantitatively.
For the years ended December 31, 2009, 2010 and 2011, research and hiring or training related subsidies of US$453,016, US$606,445 and US$384,474 were recognized as other income, respectively. After the accounting policy change, US$360,378 of such subsidies were recognized as other operating income and US$193,763 of such subsidies related costs were recognized as other operating expenses for the year ended December 31, 2011.
The balance of the cash subsidies recorded as other payables and accrued liabilities were US$638,011 and US$151,423 as of December 31, 2010 and 2011, respectively. And the balance of the cash subsidies recorded as non-current advanced subsidies were nil and US$3,120,768, respectively.
(s) Other income
Other income primarily includes rental income from the sublease of the leased office building, recognized research and hiring or training related government subsidies before the accounting policy change effective since the third quarter of 2011(Note 2(r)), investment income from the sales of investment, foreign exchange gain, reversal of long aging accounts payable and gains recognized on foreign exchange forward contracts. The long aging accounts payables that were reversed all had aging over five years with no correspondence from vendors asking for payments during the period so the Company believes the risk and possibility to pay such liabilities are very low and it is proper to reverse such long aging balances. Rental income was US$420,625, nil and nil for the years ended December 31, 2009, 2010 and 2011, respectively. Investment income recognized for the contingent consideration upon the achievement of milestones by investee and cash receipt was nil, US$275,929 and nil for the years ended December 31, 2009, 2010 and 2011, respectively. Foreign exchange gain was nil, nil and US$770,979 for the years ended December 31, 2009, 2010 and 2011. Reversal of long aging accounts payable was nil, nil and US$276,885 for the years ended December 31, 2009, 2010 and 2011, respectively. Gains recognized on foreign exchange forward contracts were US$74,546, US$3,049,211 and US$2,445,470 for the years ended December 31, 2009, 2010 and 2011, respectively.
F-15
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2009, 2010 AND 2011
(Amount expressed in US dollars, except share data, unless otherwise stated)
2. SUMMARY OF PRINCIPAL ACCOUNTING POLICIES (CONTINUED)
(t) Other expenses
Other expenses primarily include foreign exchange losses, losses recognized on foreign exchange forward contracts, one-time fees for potential merger & acquisition projects and disposal losses during the legal closure process of CGNH. Foreign exchange losses were US$125,305, US$29,855 and nil for the years ended December 31, 2009, 2010 and 2011, respectively. Losses recognized on foreign exchange forward contracts were nil, US$15,269 and US$586,838 for the years ended December 31, 2009, 2010 and 2011, respectively. One-time fees for potential merger and acquisition projects were nil, nil and US$132,703 for the years ended December 31, 2009, 2010 and 2011. Disposal losses during the legal closure process of CGNH were nil, nil and US$196,636 for the years ended December 31, 2009, 2010 and 2011, respectively.
(u) Share-based compensation
Share-based compensation expense for all share-based payment awards granted is determined based on the grant date fair value. Share-based compensation expense for stock options is estimated at the grant date based on each option’s fair value as calculated using a Black-Scholes option pricing model (“Black-Scholes model”). Share-based compensation is expensed ratably on a straight-line basis for awards with service conditions only over the requisite service period, which is generally the vesting term of the share-based payment awards. The graded vesting method is applied to awards with performance conditions.
(v) Income tax
Income taxes are accounted for under the asset and liability method. Deferred taxes are determined based upon differences between the financial reporting and tax bases of assets and liabilities at currently enacted statutory tax rates for the years in which the differences are expected to reverse. The effect on deferred taxes of a change in tax rates is recognized in the consolidated statements of operations in the period of change. A valuation allowance is provided on deferred tax assets to the extent that it is more likely than not that such deferred tax assets will not be realized. The total income tax provision includes current tax expenses under applicable tax regulations and the change in the balance of deferred tax assets and liabilities.
The guidance on accounting for uncertain tax positions in income taxes clarifies the accounting for uncertainty in income taxes recognized in the Company’s consolidated financial statements, and prescribes a more likely than not threshold for financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. The guidance also provides guidance on de-recognition of income tax assets and liabilities, classification of current and deferred income tax assets and liabilities, accounting for interest and penalties associated with tax positions, accounting for income taxes in interim periods, and income tax disclosures. The Company’s policy is to recognize, if any, uncertain tax position related interest as interest expenses and penalties as other expenses.
(w) Earnings per share
Basic earnings per share is computed by dividing net income attributable to ordinary shareholders by the weighted average number of ordinary shares outstanding during the period using the two-class method. Under the two-class method, net income is allocated between ordinary shares and other participating securities based on their participating rights. Diluted earnings per share is calculated by dividing net income attributable to ordinary shareholders, as adjusted for the change in income or loss resulting from the assumed conversion of those participating securities, if any, by the weighted average number of ordinary and dilutive ordinary equivalent shares outstanding during the period. Ordinary equivalent shares consist of ordinary shares issuable upon the
F-16
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2009, 2010 AND 2011
(Amount expressed in US dollars, except share data, unless otherwise stated)
2. SUMMARY OF PRINCIPAL ACCOUNTING POLICIES (CONTINUED)
(w) Earnings per share (continued)
conversion of the Series A convertible preferred shares using the if-converted method, and shares issuable upon the exercise of stock options for the purchase of ordinary shares using the treasury stock method. Ordinary equivalent shares are not included in the denominator of the diluted earnings per share calculation when inclusion of such shares would be anti-dilutive.
(x) Comprehensive income
Comprehensive income consists of two components, net income and other comprehensive income. Other comprehensive income refers to revenue, expenses, gains and losses that under generally accepted accounting principles are recorded as an element of shareholders’ equity but are excluded from net income. For all periods presented, other comprehensive income represented impacts of foreign currency translation adjustments. The Group discloses this information in the consolidated statements of changes in shareholders’ equity.
(y) Segment reporting
The Company currently operates and manages its business as a single segment. In accordance with “Disclosures about Segment of an Enterprise and Related Information”, the Company’s chief operating decision-maker has been identified as the chief executive officer, who reviews operating results to make decisions about allocating resources and assessing performance for the entire company. Since the Company operates in one reportable segment, all financial segment and product information required by this statement can be found in the consolidated financial statements.
(z) Profit appropriation and statutory reserves in PRC
PRC Operating Subsidiaries are wholly foreign owned enterprises incorporated in the PRC, and are required to make appropriations from after-tax profits to non-distributable reserve funds. A subsidiary, after recouping its accumulated losses, need to make appropriations to general reserve fund and staff bonus and welfare fund (“the statutory reserves”). The general reserve fund requires annual appropriations of 10% of after-tax profit (determined by generally accepted accounting principles in the PRC (“PRC GAAP”)) until such fund has reached 50% of the subsidiary’s registered capital; the percentage of appropriation for staff bonus and welfare fund is determined at the discretion of its Board. The statutory reserves can only be used for specific purposes, such as offsetting accumulated losses, enterprise expansion or staff welfare. These reserves are not transferrable to the Group in the form of dividends, advances or loans. Appropriations to these funds are accounted for as transfers from retained earnings to the statutory reserves. The Group made appropriations to the general reserve funds of US$1,561,060, US$1,349,068 and US$1,756,860 for the years ended December 31, 2009, 2010 and 2011, respectively.
(aa) Dividends
Dividends are recognized when declared. No dividends were declared for the years ended December 31, 2009, 2010 and 2011.
PRC regulations currently permit payment of dividends only out of accumulated profits as determined in accordance with PRC GAAP. The Company’s PRC subsidiaries can only distribute dividends after they have met the PRC requirements for appropriation to statutory reserves (Note 2(z)).
F-17
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2009, 2010 AND 2011
(Amount expressed in US dollars, except share data, unless otherwise stated)
2. SUMMARY OF PRINCIPAL ACCOUNTING POLICIES (CONTINUED)
(ab) Certain risks and concentration
A majority of the Group’s operating transactions are denominated in RMB and a significant portion of the Group’s assets and liabilities is denominated in RMB. RMB is not freely convertible into foreign currencies. The value of RMB is subject to changes in the central government policies and to international economic and political developments. In the PRC, certain foreign exchange transactions are required by law to be transacted only by authorized financial institutions at exchange rates set by PBOC. Remittances in currencies other than RMB by the Group in China must be processed through PBOC or other China foreign exchange regulatory bodies which require certain supporting documentation in order to process the remittance.
Financial instruments that potentially subject the Group to significant concentrations of credit risk consist primarily of cash equivalents, accounts receivables and prepayments and other current assets. As of December 31, 2010 and 2011, substantially all of the Group’s cash equivalents were held in major financial institutions located in the PRC and in Hong Kong, which management believes are of high credit quality. China does not have an official deposit insurance program, nor does it have an agency similar to The Federal Deposit Insurance Corporation (FDIC) in the United States. However, the Group believes that the risk of failure of any of these PRC banks is remote. Bank failure is extremely uncommon in China and the Group believes that those Chinese banks that hold the Company’s cash equivalents are financially sound based on public available information. Accounts receivables are typically unsecured and primarily denominated in US dollars. The Group does not require collateral or other security to support financial instruments subject to credit risks.
The Group’s customers include pharmaceutical and biotechnology companies. In the majority of circumstances, there are agreements in force with these entities that provide for the Group’s continued involvement in present research projects. However, there exists the possibility that the Group will have no further association with these entities once the ongoing projects conclude.
The following table summarizes the percentage of the Group’s revenue and accounts receivable represented by customers with balances over 10% of total revenue for the years ended December 31, 2009, 2010, and 2011, and over 10% of accounts receivable as of December 31, 2010 and 2011, respectively:
| | | | | | | | | | | | |
| | | 2009 | | | 2010 | | | 2011 | |
Revenue | | | | | | | | | | | | |
| | | |
Customer A | | | 27 | % | | | 21 | % | | | 18 | % |
Customer B | | | 10 | % | | | 8 | % | | | 7 | % |
Customer C | | | 3 | % | | | 7 | % | | | 11 | % |
| | | | | | | | |
| | | As of December 31, | |
| | | 2010 | | | 2011 | |
Accounts receivable | | | | | | | | |
| | |
Customer A | | | 20 | % | | | 15 | % |
Customer C | | | 10 | % | | | 16 | % |
(ac) Repurchase of shares
When the Company’s shares are retired, or purchased for constructive retirement (with or without an intention to retire the stock formally in accordance with applicable laws), the excess of the purchase prices over their par value is allocated between retained earnings and additional paid-in capital subject to the limitation on the amount to be additional paid-in capital described in ASC 505-30-30-8.
F-18
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2009, 2010 AND 2011
(Amount expressed in US dollars, except share data, unless otherwise stated)
2. SUMMARY OF PRINCIPAL ACCOUNTING POLICIES (CONTINUED)
(ac) Repurchase of shares (continued)
When the Company’s shares are acquired for purposes other than retirement, the treasury stock is accounted for under the cost method.
(ad) Recent accounting pronouncements
In May 2011, FASB issued amendments to achieve common fair value measurement and disclosure requirements in US GAAP and IFRS. The amendments clarifies the application of certain existing fair value measurement guidance and expands the disclosures for fair value measurements that are estimated using significant unobservable (Level 3) inputs. The new guidance is to be applied prospectively and is effective during interim and annual periods beginning after December 15, 2011. Early application is not permitted. The Company will adopt this guidance effective January 1, 2012, and are currently evaluating the impact of the adoption of this standard to our consolidated financial.
In June 2011, the FASB issued ASU No. 2011-05, “Comprehensive Income (Topic 220): Presentation of Comprehensive Income” (ASU 2011-05). This newly issued accounting standard (1) eliminates the option to present the components of other comprehensive income as part of the statement of changes in shareholders’ equity; (2) requires the consecutive presentation of the statement of net income and other comprehensive income; and (3) requires an entity to present reclassification adjustments on the face of the financial statements from other comprehensive income to net income. The amendments in this ASU do not change the items that must be reported in other comprehensive income or when an item of other comprehensive income must be reclassified to net income nor do the amendments affect how earnings per share is calculated or presented. In December 2011, the FASB issued ASU No. 2011-12, Deferral of the Effective Date for Amendments to the Presentation of Reclassifications of Items Out of Accumulated Other Comprehensive Income in Accounting Standards Update No. 2011-05, which defers the requirement within ASU 2011-05 to present on the face of the financial statements the effects of reclassifications out of accumulated other comprehensive income on the components of net income and other comprehensive income for all periods presented. During the deferral, entities should continue to report reclassifications out of accumulated other comprehensive income consistent with the presentation requirements in effect prior to the issuance of ASU 2011-05. These ASUs are required to be applied retrospectively and are effective for fiscal years, and interim periods within those years, beginning after December 15, 2011, which for the Company means January 1, 2012. As these accounting standards do not change the items that must be reported in other comprehensive income or when an item of other comprehensive income must be reclassified to net income, the adoption of these standards is not expected to have a material impact on the Group’s financial statements.
3. INVENTORIES
Inventories consist of the following:
| | | | | | | | |
| | | As of December 31, | |
| | | 2010 | | | 2011 | |
Raw materials | | | 197,025 | | | | 376,489 | |
Work in progress | | | 997,701 | | | | 2,456,981 | |
Finished goods | | | 64,437 | | | | 8,522 | |
| | | | | | | | |
| | |
| | | 1,259,163 | | | | 2,841,992 | |
| | | | | | | | |
F-19
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2009, 2010 AND 2011
(Amount expressed in US dollars, except share data, unless otherwise stated)
4. PREPAYMENTS AND OTHERS
Prepayments and others consist of the following:
| | | | | | | | |
| | | As of December 31, | |
| | | 2010 | | | 2011 | |
Prepaid rental and deposit | | | 1,303,396 | | | | 938,025 | |
Security deposits prepaid to PRC custom’s office for importation of equipments | | | — | | | | 634,077 | |
Prepayments for purchase of raw materials | | | 447,864 | | | | 357,921 | |
Prepayment for D&O insurance | | | 105,853 | | | | 186,090 | |
Derivative assets - foreign-exchange forward contracts | | | 2,464,457 | | | | 1,377,384 | |
Receivables for transferring parts of the assets acquired from Charles River | | | — | | | | 126,966 | |
Exportation value-added tax to be refunded | | | 48,645 | | | | 100,660 | |
Unamortized large animal costs | | | — | | | | 77,678 | |
Interest receivable | | | — | | | | 83,169 | |
Others | | | 408,410 | | | | 543,096 | |
| | | | | | | | |
| | |
| | | 4,778,625 | | | | 4,425,066 | |
| | | | | | | | |
5. PROPERTY, PLANT, EQUIPMENT AND SOFTWARE, NET
Property, plant, equipment and software consist of the following:
| | | | | | | | |
| | | As of December 31, | |
| | | 2010 | | | 2011 | |
Leasehold improvements | | | 15,750,282 | | | | 27,181,270 | |
Experimental equipment | | | 38,175,211 | | | | 56,543,539 | |
Vehicles | | | 618,484 | | | | 887,087 | |
Office equipment and others | | | 3,057,229 | | | | 3,943,192 | |
Buildings and constructions | | | 11,956,223 | | | | 18,035,051 | |
Software licenses | | | 2,090,706 | | | | 2,357,324 | |
Construction in progress | | | 10,699,377 | | | | 9,149,877 | |
| | | | | | | | |
| | |
| | | 82,347,512 | | | | 118,097,340 | |
Less: accumulated depreciation and amortization | | | (22,200,913 | ) | | | (31,576,532 | ) |
| | | | | | | | |
| | |
Property, plant, equipment and software | | | 60,146,599 | | | | 86,520,808 | |
| | | | | | | | |
The balance of buildings and constructions as of December 31, 2010 represents phase A of the cGMP-quality multi-purpose manufacturing facility built up in Fengxian, Shanghai. The balance of buildings and constructions as of December 31, 2011 includes US$13.4 million related to phase A of the cGMP-quality multi-purpose manufacturing facility built up in Fengxian, Shanghai, US$1.0 million of the laboratory service building built up in Fengxian, Shanghai and US$3.7 million of the laboratory service building purchased in Chengdu.
F-20
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2009, 2010 AND 2011
(Amount expressed in US dollars, except share data, unless otherwise stated)
5. PROPERTY, PLANT, EQUIPMENT AND SOFTWARE, NET (CONTINUED)
As of October 31, 2011, the Group, through one of its main PRC Operating Subsidiaries CPSH, acquired a group of assets from Charles River Laboratories International, Inc (“Charles River”)’s Shanghai In-vivo drug research facility with a total cash consideration of US$4,954,559. The total cash consideration is allocated to each acquired equipment and renovation based on the relative fair values of the acquired assets as of the transfer date. The remaining useful life of each equipment and renovation upon acquisition is estimated to be ranged from 3 to 10 years based on the assessment of the nature, condition and usage status of each item. Included in the construction in progress as of December 31, 2011 is US$3,883,819 related to the renovation acquired from Charles River.
Depreciation and amortization expenses were US$5,830,472, US$6,698,448 and US$9,185,140 for the years ended December 31, 2009, 2010 and 2011, respectively.
The annual estimated amortization expense of software licenses for the next five years is as follows:
| | | | |
2012 | | | 159,351 | |
2013 | | | 149,265 | |
2014 | | | 148,426 | |
2015 | | | 145,069 | |
2016 | | | 139,356 | |
| | | | |
| |
| | | 741,467 | |
| | | | |
6. LAND USE RIGHT, NET
Land use rights represent fees paid to obtain the right to use certain lands over periods specified in the land use right certificate of 50 years or the remaining term in the certificate from the acquisition of the land use right.
| | | | | | | | |
| | | As of December 31, | |
| | | 2010 | | | 2011 | |
Land use right | | | 4,381,150 | | | | 6,927,438 | |
Less: accumulated amortization | | | (160,642 | ) | | | (293,484 | ) |
| | | | | | | | |
| | |
| | | 4,220,508 | | | | 6,633,954 | |
| | | | | | | | |
Amortization expenses were US$70,822, US$85,758 and US$121,541 for the years ended December 31, 2009, 2010 and 2011, respectively. As of December 31, 2011, estimated amortization expense in each of the next five years is US$140,908, respectively.
7. BORROWINGS
In October 2009, certain subsidiaries of the Company entered into a short term revolving facility agreement with a bank. Under the agreement, the bank agreed to grant a credit facility of US$7.5 million. In consideration of the extension of the foregoing credit facility, the subsidiaries agreed to assign certain of their accounts receivable to the bank as collateral. In July, 2010, the original agreement was superseded by a series of new
F-21
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2009, 2010 AND 2011
(Amount expressed in US dollars, except share data, unless otherwise stated)
7. BORROWINGS (CONTINUED)
facility agreements with a maximum borrowing capacity of US$15.0 million. In June, 2011, resulting from a renewal of the series of facility agreements, the maximum borrowing capacity increased to US$20.0 million. The detailed terms as below:
A short term revolving facility agreement not exceeding US$13.0 million, increased from US$12.5 million before the renewal
Under the renewed facility agreement, the subsidiaries can either issue letters of credit guaranteed by the bank up to US$5.0 million unchanged after the renewal through a one-year period bearing a commission at the rate of 0.15% quarterly for each letter of credit; or make financing by assigning certain of their accounts receivable as collateral up to US$5.0 million unchanged after the renewal through a three-month period bearing an interest at the rate of Libor + 4.5% per annum; or make financing up to US$8.0 million increased from US$7.5 million before the renewal with interest-free for hedging and risk management derivative transactions with the bank through a two-year period.
A pledged facility agreement in the amount of US$7.0 million unchanged
This facility agreement includes a $2.0 million three-month revolving facility bearing interest at the rate of Libor + 4.5% per annum for borrowings in US dollars and 90% of the prevailing base lending rate set by PBOC for borrowings in RMB and a $5.0 million three-year fixed term facility bearing interest at the rate of Libor + 4.5% per annum for borrowings in US dollars and the prevailing base lending rate set by PBOC for borrowings in RMB. The Group agreed to assign certain of its buildings and constructions in progress in accompany with the land use right as the pledge up to RMB 39.6 million (US$5.98 million) for any draw down under the facility agreement. In addition of the pledge, the facility agreement also contains financial covenants that require the Group to meet certain ratio for debt to earnings before interest, tax, depreciation and amortization (“EBITDA”). The financing under the three-year fixed term facility is restricted to be only used on the subsidiaries’ capital expenditure activities.
The Group did not draw down any amount under the short term revolving facility or pledged facility as of December 31, 2010 and 2011. At December 31, 2011, the Group was in compliance with the terms of the facility agreements, including covenants.
In October 2009, certain subsidiaries of the Company signed a 60-day revolving facility offer letter with a bank. Under the letter, the bank agreed to grant a credit facility up to US$6.5 million and to purchase the subsidiaries’ accounts receivable with full recourse. The facility bears interest at the rate of Libor + 2% per annum for a one year period through October 12, 2010. In September, 2011, a new 60-day revolving facility offer letter was entered into between the bank and certain subsidiaries of the Company. Under the new letter, the bank agreed to grant a credit facility up to US$3.0 million and to purchase the subsidiaries’ accounts receivable with full recourse. The facility bears interest at the rate of Libor + 2% per annum. During 2011, the Company has not used the facility and did not sell any accounts receivable. There was no outstanding loan balance under both the old and new facilities as of December 31, 2010 and 2011, respectively. At December 31, 2011, the Group was in compliance with the terms of the facility agreements.
In March 2010, certain subsidiaries of the Company entered into short term revolving facility agreements with a bank. Under the agreements, the bank agreed to grant total credit facility of RMB35.0 million (US$5.3 million), for a one year period through March 2011. The Group had drawn down RMB19.6 million (US$2.9
F-22
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2009, 2010 AND 2011
(Amount expressed in US dollars, except share data, unless otherwise stated)
7. BORROWINGS (CONTINUED)
million) bearing interest at the rate of 4.86% during 2010. All such loans have been repaid and there was no outstanding loan balance under these facilities as of December 31, 2010 and 2011, respectively.
In July 2010, certain subsidiaries of the Company entered into a loan agreement with a bank in relation to an one-year loan in the principal amount of RMB10.0 million (US$1.51 million) bearing interest at the rate of 120% of the prevailing base lending rate set by PBOC for borrowings in RMB . The Group had drawn down RMB5.0 million (US$0.75 million) in 2010 and the outstanding loan balances as of December 31, 2010 and 2011 were US$0.75 million in short-term bank borrowings on the balance sheet and nil, respectively. The actual weighted average interest rates for the years ended December 31, 2010 and 2011 were 6.672% and 7.092%, respectively. At December 31, 2011, the Group was in compliance with the terms of the loan agreement.
8. OTHER PAYABLES AND ACCRUED LIABILITIES
Other payables and accrued liabilities consist of the following:
| | | | | | | | |
| | | As of December 31, | |
| | | 2010 | | | 2011 | |
Value added tax and other taxes payable | | | 1,817,609 | | | | 1,501,662 | |
Accrued rental and utilities | | | 429,432 | | | | 804,039 | |
Derivative liability- foreign-exchange forward contracts | | | 15,269 | | | | 602,107 | |
Accrued professional fees | | | 315,758 | | | | 394,705 | |
Unearned government subsidies | | | 638,011 | | | | 151,423 | |
Accrued employee annual leave | | | 207,614 | | | | 149,980 | |
Accrued IPO bonuses and other employee incentives | | | 399,970 | | | | — | |
Accrued lab partner service fees | | | — | | | | 100,134 | |
Provision for loss contract accruals | | | — | | | | 218,951 | |
Government incentives received by the Company to be paid to the Company’s certain entitled scientists | | | — | | | | 753,861 | |
Payable to employees due to the exercise of options and vesting of RSUs | | | — | | | | 103,480 | |
Others | | | 653,550 | | | | 832,542 | |
| | | | | | | | |
| | |
| | | 4,477,213 | | | | 5,612,884 | |
| | | | | | | | |
The Company prepares a detailed budget for each individual project with contract value higher than certain threshold including budget for labor costs, reagent and supplies costs and overhead costs and true up the budget at each reporting date to reflect the actual project status and change of estimates on completion. As of each reporting date, if the project is assessed to be a loss contract according to the budget analysis, the realized loss is recorded as a write-down of inventory balances, and the unrealized future losses are accrued in other payables and accrued liabilities. As of December 31, 2010 and 2011, the provision for loss contract accruals were nil and US$218,951, respectively.
9. EMPLOYEE BENEFITS
Full-time employees of PRC Operating Subsidiaries are entitled to staff welfare benefits including medical care, welfare subsidies, unemployment insurance and pension benefits. PRC Operating Subsidiaries are required
F-23
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2009, 2010 AND 2011
(Amount expressed in US dollars, except share data, unless otherwise stated)
9. EMPLOYEE BENEFITS (CONTINUED)
to pay for these benefits based on certain percentages of salaries in accordance with the relevant regulations and make contributions to the state-sponsored pension and medical plans out of the amounts accrued for medical and pension benefits. The total contribution made for such employee benefits and amounts charged to the consolidated statements of operations were US$4,248,654, US$5,691,653 and US$8,023,850 for the years ended December 31, 2009, 2010 and 2011, respectively. The Chinese government is responsible for the medical benefits and ultimate pension liability to these employees.
10. SERIES A CONVERTIBLE PREFERRED SHARES
On September 7, 2007, the Company issued and sold 3,365 (prior to bonus share issuance (Note 12)) Series A Preferred Shares. The Company sold 2,019 of these shares (prior to bonus share issuance (Note 12)) to a third party investor for cash proceeds of US$21,000,000 pursuant to a Securities Purchase Agreement dated September 5, 2007, or approximately US$10,401 per share, determined to be the fair value of such shares at the date of issuance. The Company issued the remaining 1,346 (prior to bonus share issuance (Note 12)) shares to Joint Benefit Group Limited (“JBGL”), a company indirectly owned and controlled by all of the Company’s ultimate ordinary shareholders for a nominal price (such that it was a pro rata distribution to all ultimate ordinary shareholders).
Pursuant to the Securities Purchase Agreement, JBGL simultaneously sold the 1,346 (prior to bonus share issuance (Note 12)) Series A Preferred Shares to the same third party investor for cash proceeds of US$14,000,000, or approximately US$10,401 per share, on September 7, 2007. While the form of the transaction was a distribution of preferred shares to the ultimate ordinary shareholders, the Company effectively made a cash distribution of US$14,000,000 to the ultimate ordinary shareholders in connection with the sale of 3,365 Series A Preferred Shares. The Company recorded the US$14,000,000 payment as a pro rata dividend to all of the ultimate ordinary shareholders in consideration of the dilution in their ownership interest in the Company as a result of the Series A Convertible Share investment.
The initial carrying value of the Series A Preferred Shares was offset by direct issuance costs of US$644,225.
The par value of the Company’s Series A Preferred Shares was US$0.001 per share. The significant terms were as follows:
Conversion
Each Series A Preferred Share is initially convertible into one ordinary share at any time after the date of issuance of such share, and shall be automatically converted into ordinary shares at the then-effective conversion price upon the completion of a qualifying Initial Public Offering(“IPO”).
A qualifying IPO, as defined in the Company’s memorandum and articles of association, means an underwritten public offering of the Company’s ordinary shares on a recognized share exchange that values the Company at no less than US$250,000,000 and that result in aggregate proceeds to the Company (net of selling expenses) of no less than US$62,500,000. On September 30, 2010, the definition of a qualifying IPO was amended to mean an initial public offering on a qualified exchange that values the Company at no less than US$250,000,000 immediately after the offering and that results in aggregate gross proceeds from the offering to be no less than US$75,000,000, including aggregate gross proceeds to the investor in Series A Preferred Shares of no less than US$25,000,000.
F-24
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2009, 2010 AND 2011
(Amount expressed in US dollars, except share data, unless otherwise stated)
10. SERIES A CONVERTIBLE PREFERRED SHARES (CONTINUED)
The conversion price of the Series A Preferred Shares is subject to adjustment for certain events, including but not limited to share splits, share dividends, reorganizations, mergers, consolidations, reclassifications, exchanges and substitutions. The conversion price of the Series A Preferred Shares is also subject to adjustment in the event the Company issues additional ordinary shares at a price per share that is less than such conversion price. In such case, the conversion price of the Series A Preferred Shares shall be reduced, concurrently with such issuance, to the price per share at which the additional shares are issued.
In addition to the foregoing, if the Company’s aggregate adjusted net profit after taxes for the 24 months period beginning January 1, 2007 and ending December 31, 2008 is less than US$18,000,000, the then effective conversion price of the Series A Preferred Shares shall be adjusted downwards in accordance with the formula set forth in the Company’s memorandum and articles of association. The aggregate adjusted net profit was greater than US$18,000,000 for the said 24 months and thus no adjustment event occurred.
The conversion price of the Series A Preferred Shares was adjusted to US$0.50 per share in May 2008 on a retrospective basis (Note 12).
No beneficial conversion feature charge was recognized for the issuance of Series A Preferred Shares as the estimated fair value of the ordinary shares is less than the conversion price on the date of issuance and at the time of the aforementioned conversion adjustment assessment.
Voting rights
Each Series A Preferred Share has voting rights equivalent to the number of the Company’s ordinary shares into which it is convertible.
Dividends
The holders of the outstanding Series A Preferred Shares shall be entitled to receive, out of any funds legally available, non cumulative dividends for each Series A Preferred Share held as and when declared by the Board of Directors. In the event the Company shall declare a dividend or similar distribution to the holders of ordinary shares, the holders of Series A Preferred Shares shall be entitled to a proportionate share of any such dividend or distribution as though the Series A Preferred Shares were converted into ordinary shares on the record date.
Redemption rights
Holders of the Series A Preferred Shares have the option to redeem such shares, in whole or in part, upon the occurrence of a material breach of one or more of the investment agreements in connection with the Series A Preferred Shares, as set forth in the Company’s memorandum and articles of association.
The redemption price per Series A Preferred Share is equal to the original purchase price per share (as adjusted in accordance with the Company’s memorandum and articles of association), plus an additional sum equal to a return of 12% on the purchase price, compounded quarterly from the date of the issuance to the date on which the actual payment is made, plus all accrued but unpaid dividends.
The Company has not recorded accretion as the likelihood of occurrence for the redemption event is considered remote. The Company classified the Series A Preferred Shares in the mezzanine section of the consolidated balance sheets in accordance with the provisions of Accounting Series Release No. 268 (“ASR 268”).
F-25
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2009, 2010 AND 2011
(Amount expressed in US dollars, except share data, unless otherwise stated)
10. SERIES A CONVERTIBLE PREFERRED SHARES (CONTINUED)
Liquidation preference
In the event of any liquidation, dissolution or winding up of the Company or any consolidation, amalgamation, trade sale, merger, corporate reorganization or other transaction involving the Company in which its shareholders do not retain a majority of the voting power in the surviving entity, or a sale of all or substantially all the Company’s assets, the holders of Series A Preferred Shares are entitled to receive an amount per Series A Preferred Share equal to 100% of the original purchase price (as adjusted for any share splits, share dividends, combinations, recapitalizations and similar transactions), plus all declared but unpaid dividends, plus an amount equal to a return of 12% on the purchase price, compounded quarterly from the date of the sale to the date on which the actual payment is made.
Form of charge agreement
On September 4, 2007, the founders agreed to pledge to the third party investor 2,673 (prior to bonus share issuance (Note 12)) ordinary shares for certain enforcement events as defined in the Securities Purchase Agreement resulting in an amount becoming payable to the third party investor by the indemnifying parties (including the Company and the founder). Pledged shares shall be released upon the earlier of the expiration of the enforcement events, a qualified public offering and/or liquidation event. During the pledge period, the founder is still entitled to the voting and dividend rights. The charged shares were all released in April 2010.
All of the outstanding 69,994,014 Series A Preferred Shares were automatically converted into 69,994,014 ordinary shares on a one-for-one basis upon the completion of the qualifying IPO that occurred on October 19, 2010.
11. INVESTMENT IN SECURITIES
In 2007 and 2008, the Group, through one of its subsidiaries, acquired 300,000 Series A redeemable and convertible preferred shares of a pharmaceutical and biotechnology research and development company (“investee”) incorporated in the United States of America for a consideration of US$300,000. The Company’s investment represents 2.9% of investee’s equity interests, on an as converted basis. The preferred shares are convertible into shares of the investee’s common share at a 1:1 ratio and are redeemable, if more than 72% of the outstanding preferred shareholders request redemption on or after the fifth anniversary of the preferred share original issuance date.
In 2009, the investee issued promissory notes to the Group for cash consideration of US$142,857. In connection with the promissory notes, the Group was granted warrants to purchase additional preferred shares offered in connection with a qualified financing at the lower of the per share price of the Series A redeemable and convertible preferred shares or price per preferred share issued pursuant to the qualified financing, or, Series A redeemable and convertible preferred shares at the per share price of the Series A redeemable and convertible preferred shares. The promissory notes are convertible into preferred shares offered in connection with a qualified financing at the lower of the price per share of the preferred shares the Company sells and issues pursuant to a qualified financing or the per share price of the Series A redeemable and convertible preferred shares.
In February 2010, the Group, together with other original investors of the investee, sold their investments in the investee to a third party (Note 20). According to the share purchase agreement, the Group received cash of US$351,379 in February 2010 and is entitled to receive additional cash of up to US$65,714 set aside in an escrow account. In May 2011, US$41,726 set aside in the escrow account was received by the Group. The Group is also eligible for certain future consideration of up to approximately US$1.5 million. The receipts of the future
F-26
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2009, 2010 AND 2011
(Amount expressed in US dollars, except share data, unless otherwise stated)
11. INVESTMENT IN SECURITIES (CONTINUED)
payments are contingent upon the investee achieving certain milestones defined in the share purchase agreement. As for the years ended December 31, 2010 and 2011, US$275,929 and nil of future considerations has been recognized by the Group as other income.
12. SHARE CAPITAL
Upon incorporation of the Company, the authorized share capital was US$50,000 divided into 49,990,000 ordinary shares at a par value of US$0.001 each, and 10,000 convertible preferred shares of a par value at US$0.001 each, all of which were designated Series A Preferred Shares. The amount of issued and outstanding ordinary shares was 10,000.
On May 19, 2008, the Company affected a bonus share issuance in the form of a share split such that each holder of ordinary shares and Series A Preferred Shares received approximately 20,800 ordinary shares and Series A Preferred Shares for each ordinary share and Series A Preferred Share held, respectively. The amount of issued and outstanding ordinary shares and Series A Preferred Shares were increased to 208,005,986 shares and 69,994,014 shares, respectively, as a result of the bonus share issuance. The par value of both ordinary shares and Series A Preferred Shares remains unchanged at US$0.001 per share. The conversion price of Series A Preferred Shares was adjusted to US$0.50 per share as a result of the bonus share issuance without changing the aggregate liquidation value. The authorized share capital was increased to US$500,000 divided into 429,999,350 ordinary shares of a par value of US$0.001 each, and 70,000,650 convertible preferred shares of a par value of US$0.001 each, all of which are designated Series A Preferred Shares.
The effect of the bonus share issuance was retroactively adjusted to reflect the change for all years presented, as if such share split had occurred from inception of the Group. The par value of US$207,996 related to ordinary shares first offset against the additional paid-in capital of US$2,554 and the remaining of US$205,442 was recorded against retained earnings as of January 1, 2007.
On October 19, 2010, with the completion of the qualifying IPO, the amounts of issued and outstanding ordinary shares were increased to 335,600,000, among which 57,600,000 ordinary shares were increased from public issuance and 69,994,014 ordinary shares were automatically converted from Series A Preferred Shares.
As of December 31, 2011, the total issued and outstanding ordinary shares were increased to 336,109,400, from the exercise of share options.
13. REPURCHASE OF SHARES
In August 2011, the Board of Directors approved a share repurchase program to repurchase outstanding ADSs of the Company with an aggregate value of up to US$10.0 million within two years. The repurchase will be made from time to time depending on market conditions, share price and other factors, as well as subject to the relevant rules under United States securities regulations. The share repurchases may be made on the open market, in block trades or otherwise and may include derivative transactions. The program may be suspended or discontinued at any time.
During 2011, ShangPharma repurchased an aggregate volume of 124,286 ADSs from the open market for a total consideration of US$1,092,275. All repurchased shares were accounted for under the cost method in treasury stock as of December 31, 2011. In addition, the Company made prepayments to the depositary bank of approximately US$1.0 million, the amount is intended for future repurchases and was recorded as restricted cash.
F-27
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2009, 2010 AND 2011
(Amount expressed in US dollars, except share data, unless otherwise stated)
14. TAXATION
Cayman Islands
Under the current laws of Cayman Islands, the Company is not subject to tax on income or capital gain. In addition, upon payments of dividends by the Company to its shareholders, no Cayman Islands withholding tax will be imposed.
Hong Kong
The Company’s subsidiaries did not have assessable profits that were earned in or derived from Hong Kong during the years ended December 31, 2009, 2010 and 2011. Accordingly, no Hong Kong profit tax has been provided.
PRC
Prior to January 1, 2008, the Company’s subsidiaries that are incorporated in the PRC were subject to Enterprise Income Tax (“EIT”) in accordance with the Enterprise Income Tax Law and the Income Tax Law of the People’s Republic of China concerning Foreign Investment Enterprises and Foreign Enterprises and local income tax laws (collectively the “previous PRC Income Tax Laws”). Pursuant to the previous PRC Income Tax Laws and rules, enterprises were generally subject to a statutory tax rate of 33% (30% state income tax plus 3% local income tax). Subsidiaries that were registered in the Pudong New District of Shanghai were, however, subject to a 15% preferential EIT rate pursuant to the local tax preferential treatment before January 1, 2008.
In March 2007, the Chinese government enacted the Corporate Income Tax Law (the “new CIT Law”), and promulgated related regulation Implementing Regulations for the PRC Corporate Income Tax Law. The new CIT law and regulation went into effect on January 1, 2008. The new CIT Law, among other things, imposes a unified income tax rate of 25% for both domestic and foreign invested enterprises. The new CIT Law provides a five-year transitional period for those entities established before March 16, 2007, which enjoyed a favorable income tax rate of less than 25% under the previous income tax laws and rules, to gradually change their rates to 25%. In addition, on December 26, 2007, the State Council issued the Notice of the State Council Concerning Implementation of Transitional Rules for Corporate Income Tax Incentives (“Circular 39”). Based on Circular 39, certain specifically listed categories of corporations which enjoyed a favorable income tax rate of less than 25% under the previous income tax laws and rules are eligible for a graduated rate increase to 25% over the 5-year period beginning from January 1, 2008. Where transitional rules overlap with preferential policies provided by the new CIT Law, a corporation may choose to implement the more favorable policy, and shall not enjoy both simultaneously. Also, the policy cannot be changed once determined.
On April 14, 2008, relevant governmental regulatory authorities released qualification criteria, application procedures and assessment processes for “high and new technology enterprises,” which will be entitled to a favorable statutory tax rate of 15%. On July 8, 2008, relevant governmental regulatory authorities further clarified that new technology enterprises previously qualified under the previous income tax laws and rules as of December 31, 2007 would be allowed to enjoy grandfather treatment for the unexpired tax holidays, on condition that they were re-approved for “high and new technology enterprise” status under the regulations released on April 14, 2008.
Additionally, under the new CIT Law, 10% withholding income tax (“WHT”) will be levied on foreign investors for dividend distributions from foreign invested enterprises’ profit earned after January 1, 2008. For certain treaty jurisdictions such as Hong Kong which has signed tax arrangement with the PRC, the WHT rate is 5%.
F-28
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2009, 2010 AND 2011
(Amount expressed in US dollars, except share data, unless otherwise stated)
14. TAXATION (CONTINUED)
As of December 31, 2010 and 2011, the Company did not record any withholding tax on the retained earnings of its foreign invested enterprises in the PRC, as the Company intends to indefinitely reinvest its earnings to further expand its business in mainland China, and its foreign invested enterprises do not intend to declare dividends to their immediate foreign holding companies.
CESH, PESH and CPSH, foreign invested enterprises, are located in the Shanghai Pudong Zhangjiang High-Tech Park. They were subject to an income tax rate of 15% before 2008 prior to the implementation of Corporate Income Tax Law. In 2008, the local government announced the recognition of CESH and CPSH as high-new technology enterprises. Accordingly, they are entitled to a preferential tax rate of 15%, which is effective from January 1, 2008. The high and new technology enterprise status is subject to approval and renewal every three years. In 2011, with the approval by local government, CESH and CPSH’s high and new technology enterprise status was renewed for another three years effective from January 1, 2011. Neither CESH nor CPSH applied to, or obtained approval from the local taxing authority to enjoy the preferential tax rate applicable to high and new technology enterprises. In addition, CESH is entitled to tax exemption from income tax for year 2007, and a 50% reduced applicable corporate income tax rate for the subsequent three years. PESH and CPSH are entitled to a two-year exemption from income tax for the years of 2007 and 2008, and a 50% reduced applicable corporate income tax rate for the subsequent three years. Accordingly, CESH gradually transits from income tax rate of 9% to 11% from 2008 to 2010, and PESH and CPSH gradually transits from the income tax rate of 10% to 12% from 2009 to 2011. After the expiration of CESH and CPSH’s 50% reduced applicable corporate income tax rate preference, with the entitlement to Technical Advanced Service Enterprise (“TASE”) effective from 2010 to 2013, CESH will be subject to a preferential tax rate of 15% from 2011 to 2013 and CPSH will be subject to a preferential tax rate of 15% from 2012 to 2013. PESH will be subject to a uniform tax rate of 25% after the expiration of its 50% reduced applicable corporate income tax rate preference starting 2012.
CGTD, a foreign invested enterprise, is located in the Shanghai Pudong Zhangjiang High-Tech Park. It was subject to an income tax rate of 15% before 2008 prior to the implementation of Corporate Income Tax Law. CGTD gradually transitions from the income tax rate of 18% to 25% from 2008 to 2012.
CGNH, a foreign invested enterprise, is located in the Shanghai Nanhui Industrial Zone. It was subject to an income tax rate of 24% plus a local income tax 3% before 2008 prior to the implementation of the Corporate Income Tax Law. CGNH transitions to the uniform tax rate of 25% in 2008 from the rate of 27%.
CPCD, a foreign invested enterprise, is located in Chengdu, China. In 2008, the local government announced the recognition of CPCD as a high-new technology enterprise. Accordingly, CPCD is entitled to a preferential tax rate of 15% (2007: 33%), which is effective from January 1, 2008. The high and new technology enterprise status is subject to approval and renewal every three years. In 2011, with the approval by local government, CPCD’s high and new technology enterprise status was renewed for another three years effective from January 1, 2011. In addition, CPCD is entitled to tax exemption from income tax for years 2008 and 2009, and a 50% reduced applicable corporate income tax rate for the subsequent three years (“2+3” tax holiday). The tax preferences under high-new technology enterprise and 2+3 tax holiday cannot be enjoyed at the same time and CPCD did not apply to, or obtain approval from the local taxing authority to enjoy the preferential tax rate applicable to high and new technology enterprises. In 2010, the local government announced CPCD’s entitlement to the 15% preferential tax rate for the development of China’s western regions effectively up to 2010. The 2+3 tax holiday and preferential tax rate under the development of China’s western regions can be enjoyed at the same time. Accordingly, CPCD was subject to a preferential tax rate at 7.5% in 2010. The original preferential policies under the development of China’s western regions expired as of December 31, 2010 and in July 2011,
F-29
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2009, 2010 AND 2011
(Amount expressed in US dollars, except share data, unless otherwise stated)
14. TAXATION (CONTINUED)
the Chinese government renewed the preferential policies for another ten years effectively from January 1, 2011. As a result, with the approval by local government for the entitlement to the preferential policies in 2011, CPCD was subject to a preferential tax rate at 7.5% in 2011 as well. The entitlement to the preferential policies under the development of China’s western regions is subject to approval and renewal by local government annually.
CGFX, a foreign invested enterprise, is located in Shanghai Fengxian, China. It is subject to a uniform income tax rate of 25% since its incorporation in 2008 up to 2011 and going forward.
Composition of income tax expense
The current and deferred portion of income tax expense included in the consolidated statements of operations for the years ended December 31, 2009, 2010 and 2011 are as follows:
| | | | | | | | | | | | |
| | | 2009 | | | 2010 | | | 2011 | |
Current income tax expense | | | 1,824,879 | | | | 2,026,687 | | | | 2,999,364 | |
PRC | | | 1,824,879 | | | | 2,026,687 | | | | 2,664,180 | |
Non-PRC | | | — | | | | — | | | | 335,184 | |
Deferred tax expenses (benefits) | | | (272,656 | ) | | | 58,625 | | | | (556,802 | ) |
PRC | | | (272,656 | ) | | | 58,625 | | | | (521,450 | ) |
Non-PRC | | | — | | | | — | | | | (35,352 | ) |
| | | | | | | | | | | | |
| | | |
Income tax expense | | | 1,552,223 | | | | 2,085,312 | | | | 2,442,562 | |
| | | | | | | | | | | | |
Reconciliation of the differences between statutory tax rate and the effective tax rate
A reconciliation between the statutory CIT rate and the Group’s effective tax rate for the years ended December 31 is as follows:
| | | | | | | | | | | | |
| | | 2009 | | | 2010 | | | 2011 | |
PRC statutory CIT rate | | | 25 | % | | | 25 | % | | | 25 | % |
Permanent differences | | | 3 | % | | | (1 | %) | | | 2 | % |
Effect of differences in foreign tax rates | | | 4 | % | | | (1 | %) | | | 20 | % |
Tax differential from statutory rate applicable to subsidiaries in the PRC | | | (6 | %) | | | 2 | % | | | (3 | %) |
Effects of tax holiday | | | (12 | %) | | | (14 | %) | | | (23 | %) |
Change in valuation allowances | | | — | | | | 3 | % | | | (3 | %) |
| | | | | | | | | | | | |
| | | |
Effective CIT rate | | | 14 | % | | | 14 | % | | | 18 | % |
| | | | | | | | | | | | |
The aggregate amount and per share effect of the tax holidays are as follows:
| | | | | | | | | | | | |
| | | 2009 | | | 2010 | | | 2011 | |
Aggregate effect | | | 1,369,896 | | | | 2,096,546 | | | | 3,051,721 | |
Basic ordinary share effect | | | 0.01 | | | | 0.01 | | | | 0.01 | |
Diluted ordinary share effect | | | — | | | | 0.01 | | | | 0.01 | |
F-30
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2009, 2010 AND 2011
(Amount expressed in US dollars, except share data, unless otherwise stated)
14. TAXATION (CONTINUED)
Significant components of deferred tax assets
The principal components of deferred tax assets are as follows:
| | | | | | | | |
| | | As of December 31, | |
| | | 2010 | | | 2011 | |
Current deferred tax assets: | | | | | | | | |
Net operating loss carry-forward | | | 238,111 | | | | 521,255 | |
Accrued expense | | | 821,985 | | | | 797,954 | |
Other temporary differences | | | 135,962 | | | | 385,957 | |
Less: valuation allowance | | | (651,322 | ) | | | (503,679 | ) |
| | | | | | | | |
Sub-total | | | 544,736 | | | | 1,201,487 | |
Non-current deferred tax assets: | | | | | | | | |
Net operating loss carry-forward | | | 1,545,191 | | | | 1,438,735 | |
Deferred government subsidy income | | | 74,156 | | | | 597,744 | |
Less: valuation allowance | | | (1,619,347 | ) | | | (1,427,901 | ) |
| | | | | | | | |
Sub-total | | | — | | | | 608,578 | |
| | |
Total deferred tax assets, net of valuation allowance | | | 544,736 | | | | 1,810,065 | |
| | | | | | | | |
Current deferred tax liabilities: | | | | | | | | |
Temporary difference related to revenue recognition | | | (59,455 | ) | | | (532,037 | ) |
Temporary difference related to cost of revenues | | | (45,744 | ) | | | (178,738 | ) |
Other temporary differences | | | (124,135 | ) | | | (227,085 | ) |
| | | | | | | | |
Sub-total | | | (229,334 | ) | | | (937,860 | ) |
| | |
Total deferred tax liabilities | | | (229,334 | ) | | | (937,860 | ) |
| | | | | | | | |
| | |
Deferred tax assets, net | | | 315,402 | | | | 872,205 | |
| | | | | | | | |
Movement of valuation allowances:
| | | | | | | | | | | | |
| | | 2009 | | | 2010 | | | 2011 | |
At beginning of year | | | 1,764,766 | | | | 1,787,106 | | | | 2,270,669 | |
Current year additions | | | 22,340 | | | | 1,140,056 | | | | 963,166 | |
Current year reversals | | | — | | | | (656,493 | ) | | | (1,302,255 | ) |
| | | | | | | | | | | | |
| | | |
At end of year | | | 1,787,106 | | | | 2,270,669 | | | | 1,931,580 | |
| | | | | | | | | | | | |
The Group adopted the provisions issued by FASB on accounting for uncertain tax positions effective January 1, 2007. Based on its analysis documentation, the Group made its assessment of tax liabilities for each tax position (including the potential application of interest and penalties) based on technical merits, and measured the unrecognized tax benefits associated with the tax positions. As of December 31, 2010, the guidance did not
F-31
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2009, 2010 AND 2011
(Amount expressed in US dollars, except share data, unless otherwise stated)
14. TAXATION (CONTINUED)
have any material impact on the Company’s consolidated total liabilities or shareholders’ equity. As of December 31, 2011, the Company had approximately US$0.3 million of unrecognized tax benefits related to gains from forward foreign exchange contracts, all of which would impact its effective tax rate if recognized. The Group classifies interest and/or penalties related to income tax matters in income tax expense. As of December 31, 2010 and 2011, the amount of interest and penalties related to uncertain tax positions was immaterial. The Company does not anticipate any significant changes to its liability for unrecognized tax benefits within the next 12 months.
The following table is a roll-forward of the unrecognized tax benefits from January 1, 2010 to December 31, 2011:
| | | | |
Balance at January 1, 2010 and December 31, 2010 | | | — | |
Additions: | | | | |
Tax positions related to the current year | | | 299,832 | |
| | | | |
Balance at December 31, 2011 | | | 299,832 | |
| | | | |
Valuation allowances have been provided on deferred tax assets due to the uncertainty surrounding their realization. As of December 31, 2010 and 2011, valuation allowances on a large part of deferred tax assets were provided because it was more likely than not that the Group will not be able to utilize tax loss carry forwards generated by certain unprofitable subsidiaries. As of December 31, 2011, as determined by management after consideration of all available evidence, both positive and negative, that it is more likely than not that these deferred tax assets will be realized, US$1,302,255 valuation allowances were reversed. If events occur in the future that allow the Group to realize more of its deferred tax assets than the presently recorded amount, an adjustment to the valuation allowances will increase income when those events occur. For the years ended December 31, 2010 and 2011, the valuation allowances increased by US$1,140,056 and US$963,166, respectively. As of December 31, 2010 and 2011, a total valuation allowance of US$2,270,669 and US$1,931,580 were provided against deferred tax assets because it was more likely than not that such portion of deferred tax will not be realized based on the Company’s estimate of future taxable incomes of all its subsidiaries.
As of December 31, 2011, total tax losses carry forward of the Company’s subsidiaries in the PRC of approximately US$10,548,921, will expire if not used between 2012 and 2016.
F-32
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2009, 2010 AND 2011
(Amount expressed in US dollars, except share data, unless otherwise stated)
15. EARNINGS PER SHARE
Basic earnings per share and diluted earnings per share have been calculated in accordance with ASC issued guidance on computation of earnings per share for the years ended December 31, 2009, 2010 and 2011 as follows:
| | | | | | | | | | | | |
| | | 2009 | | | 2010 | | | 2011 | |
Numerator: | | | | | | | | | | | | |
Net income | | | 9,798,815 | | | | 12,996,514 | | | | 11,114,851 | |
Allocation to preferred shareholders | | | (2,467,117 | ) | | | (2,738,594 | ) | | | — | |
| | | | | | | | | | | | |
| | | |
Net income attributable to ShangPharma Corporation’s ordinary shareholders - Basic | | | 7,331,698 | | | | 10,257,920 | | | | 11,114,851 | |
Add back of allocation to preferred shareholders | | | 2,467,117 | | | | — | | | | — | |
| | | | | | | | | | | | |
| | | |
Net income for diluted earnings per share | | | 9,798,815 | | | | 10,257,920 | | | | 11,114,851 | |
| | | | | | | | | | | | |
| | | |
Denominator: | | | | | | | | | | | | |
Denominator for basic earnings per share | | | | | | | | | | | | |
Weighted-average ordinary shares outstanding | | | 208,005,986 | | | | 233,874,361 | | | �� | 335,538,405 | |
Dilutive effect of share options | | | 51,299 | | | | 4,125,838 | | | | 2,346,853 | |
Dilutive effect of Series A Preferred Shares | | | 69,994,014 | | | | — | | | | — | |
| | | | | | | | | | | | |
| | | |
Denominator for diluted earnings per share | | | 278,051,299 | | | | 238,000,199 | | | | 337,885,258 | |
| | | | | | | | | | | | |
| | | |
Basic earnings per share | | | 0.04 | | | | 0.04 | | | | 0.03 | |
| | | | | | | | | | | | |
| | | |
Diluted earnings per share | | | 0.04 | | | | 0.04 | | | | 0.03 | |
| | | | | | | | | | | | |
Allocation to preferred shareholders represents the share of net income attributable to ShangPharma Corporation by the preferred shareholders based on the preferred shareholders interests as a percentage of the total preferred and ordinary shareholders interests. No dividend has been declared to date. For the year ended December 31, 2010, the allocation to preferred shareholders of 55,803,447 shares has been excluded from the determination of dilutive earnings per share because the inclusion would have been anti-dilutive. For the year ended December 31, 2011, the impact of allocation to preferred shareholders was nil with the auto-conversion of all preferred shares upon the completion of the qualifying IPO that occurred on October 19, 2010.
F-33
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2009, 2010 AND 2011
(Amount expressed in US dollars, except share data, unless otherwise stated)
16. SHARE INCENTIVE PLANS
2008 Equity and Performance Incentive Plan
In May 2008, the Company authorized an equity compensation plan (the “2008 Equity and Performance Incentive Plan”) that provides for the issuance of options to purchase up to 30,888,889 ordinary shares to the Company’s employees, non-employee directors, officers and consultants. The maximum aggregate number of ordinary shares that may be issued was increased to 36,562,358 shares on February 24, 2010 in an amendment to the 2008 Equity and Performance Incentive Plan.
In 2009, 2010 and 2011, the Company granted options to employees to purchase 8,778,000, 11,539,550 and 3,928,720 ordinary shares, respectively, under the Company’s 2008 Equity and Performance Incentive Plan at a weighted average exercise price of US$0.50 per share for 2009, US$0.65 per share for 2010 and US$0.57 per share for 2011. Pursuant to the 2008 Equity and Performance Incentive Plan, 25% of the options will vest and become exercisable on each of the four anniversaries of the date of grant. The options granted shall become immediately exercisable upon the consummation of a change of control (excluding a public offering).
The Company’s share-based compensation expense with respect to options granted to the employees was measured based on the estimated fair value of the Company’s ordinary shares at the date of grant using the Black-Scholes option pricing model and is recognized, adjusted for the estimated forfeiture, on a straight-line basis. Management utilized the results from a third party valuation firm to assist in the determination of the fair value of the ordinary shares underlying the options prepared on a retrospective basis before the consummation of the qualifying IPO dated October 19, 2010.
Share-based compensation expense related to the options granted by the Company under the 2008 Equity and Performance Incentive Plan amounted to approximately US$251,950, US$930,355 and US$1,420,531 for the years ended December 31, 2009, 2010 and 2011, respectively.
F-34
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2009, 2010 AND 2011
(Amount expressed in US dollars, except share data, unless otherwise stated)
16. SHARE INCENTIVE PLANS (CONTINUED)
The Company’s share option activities as of December 31, 2010 and 2011 and changes during the years then ended are presented below:
| | | | | | | | | | | | | | | | |
| | | Options
Outstanding | | | Weighted
Average
Exercise
Price | | | Weighted
Average
Remaining
Contractual
Life | | | Aggregate
Intrinsic
Value | |
Outstanding at December 31, 2008 | | | 20,615,700 | | | | 0.50 | | | | 9.54 | | | | | |
| | | | |
Granted | | | 8,778,000 | | | | 0.50 | | | | | | | | | |
Exercised | | | — | | | | | | | | | | | | | |
Forfeited | | | (2,591,825 | ) | | | 0.50 | | | | | | | | | |
Cancelled | | | (278,975 | ) | | | 0.50 | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | | | |
Outstanding at December 31, 2009 | | | 26,522,900 | | | | 0.50 | | | | 8.87 | | | | 1,326,145 | |
| | | | | | | | | | | | | | | | |
| | | | |
Vested and expected to vest at December 31, 2009 | | | 18,989,364 | | | | 0.50 | | | | 8.86 | | | | 949,468 | |
| | | | | | | | | | | | | | | | |
| | | | |
Vested and exercisable at December 31, 2009 | | | 4,503,975 | | | | 0.50 | | | | 8.54 | | | | 225,199 | |
| | | | | | | | | | | | | | | | |
Granted | | | 11,539,550 | | | | 0.65 | | | | | | | | | |
Exercised | | | — | | | | — | | | | | | | | | |
Forfeited | | | (2,230,000 | ) | | | 0.53 | | | | | | | | | |
Cancelled | | | (498,850 | ) | | | 0.50 | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | | | |
Outstanding at December 31, 2010 | | | 35,333,600 | | | | 0.55 | | | | 8.38 | | | | 3,195,837 | |
| | | | | | | | | | | | | | | | |
| | | | |
Vested and expected to vest at December 31, 2010 | | | 30,360,591 | | | | 0.53 | | | | 8.34 | | | | 3,297,614 | |
| | | | | | | | | | | | | | | | |
| | | | |
Vested and exercisable at December 31, 2010 | | | 10,330,863 | | | | 0.50 | | | | 7.72 | | | | 1,434,842 | |
| | | | | | | | | | | | | | | | |
| | | | |
Granted | | | 3,928,720 | | | | 0.57 | | | | | | | | | |
Exercised | | | (509,400 | ) | | | 0.50 | | | | | | | | | |
Forfeited | | | (3,619,373 | ) | | | 0.56 | | | | | | | | | |
Cancelled | | | (2,539,707 | ) | | | 0.51 | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | | | |
Outstanding at December 31, 2011 | | | 32,593,840 | | | | 0.55 | | | | 7.65 | | | | — | |
| | | | | | | | | | | | | | | | |
| | | | |
Vested and expected to vest at December 31, 2011 | | | 29,978,915 | | | | 0.55 | | | | 7.60 | | | | — | |
| | | | | | | | | | | | | | | | |
| | | | |
Vested and exercisable at December 31, 2011 | | | 15,366,864 | | | | 0.52 | | | | 7.05 | | | | — | |
| | | | | | | | | | | | | | | | |
As the weighted average exercise price exceeds the fair value of the ordinary share of the Company as of December 31, 2011, there is no intrinsic value as of December 31, 2011.
F-35
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2009, 2010 AND 2011
(Amount expressed in US dollars, except share data, unless otherwise stated)
16. SHARE INCENTIVE PLANS (CONTINUED)
The aggregate intrinsic value is calculated as the difference between the fair value of ordinary shares in an amount of US$0.55, US$0.64 and US$0.40 per share as of December 31, 2009, 2010 and 2011, respectively and the exercise prices of the options.
The weighted average grant date fair value of options granted during the two years ended December 31, 2010 and 2011 was US$0.32 and US$0.24, respectively. The total fair value of options vested during the two years ended December 31, 2010 and 2011 was US$543,571 and US$1,182,809, respectively.
The options exercised during 2009, 2010 and 2011 were nil, nil and 509,400 which had an aggregate intrinsic value of nil, nil and US$69,316, respectively. In 2011, total cash received from the exercise of options amounted to US$237,229.
In 2011, the Company reached an agreement with certain employee to keep the vested portion of granted options as of resignation date still effective and exercisable for a longer period. The total options subject to such modification were 386,100. Accordingly the incremental fair value was assessed using the Black-Scholes option pricing model as of the modification dates and charged immediately upon the modification amounting to US$76,424.
As of December 31, 2011, there was US$2,771,131 of unrecognized compensation cost, adjusted for the estimated forfeitures, related to non-vested share-based awards granted to the Company’s employees. This cost is expected to be recognized over a weighted average period of 1.7 years. Total compensation cost may be adjusted for future changes in estimated forfeitures.
The fair value of each option granted under the Company’s 2008 Equity and Performance Incentive Plan is estimated on the date of grant using the Black-Scholes option pricing model that used assumptions noted in the following table:
| | | | | | |
| | | For the Years
Ended
December 31, |
| | | 2009 | | 2010 | | 2011 |
Risk-free interest rate(1) | | 3%-3.72% | | 1.68%-3.37% | | 1.08%-2.65% |
Expected life (in years)(2) | | 5-7 years | | 5-7 years | | 5-7 years |
Expected dividend yield(3) | | 0% | | 0% | | 0% |
Expected volatility(4) | | 42%-43% | | 40%-44% | | 37%-44% |
Fair value per option at grant date | | 0.18-0.28 | | 0.20-0.35 | | 0.16-0.32 |
| (1) | The risk-free interest rate for periods within the contractual life of the share option is based on the rate of US$ China government bond yield in effect at the time of grant for a term consistent with the expected term of the awards. |
| (2) | The expected term of share options granted under the 2008 Equity and Performance Incentive Plan is developed giving consideration to vesting period, contractual term, share price, employee’s level within the organization and the expected volatility of the underlying share. |
| (3) | The Company has no expectation of paying dividends on its common shares during the term of the options. |
| (4) | Expected volatility related to share options is estimated based on the average historical data volatilities of the Company and comparable companies’ ordinary shares in similar industry over a time period commensurate with the expected term of the awards as at the valuation dates. |
F-36
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2009, 2010 AND 2011
(Amount expressed in US dollars, except share data, unless otherwise stated)
16. SHARE INCENTIVE PLANS (CONTINUED)
Founder’s 2008 Equity and Performance Incentive Plan
In May 2008, the Company authorized Founder’s 2008 Equity and Performance Incentive Plan. The founders of the Company have authorized grants of restricted share units (“RSUs”) currently owned by them to certain members of the senior management of the Company (the “Founder’s 2008 Equity and Performance Incentive Plan”). The RSUs provide for the issuance of up to 20,000,000 ordinary shares upon the vesting of RSUs.
In 2009, 2010 and 2011, the founders granted RSUs representing the rights to receive 2,100,000, 2,200,000 and nil ordinary shares under the Founder’s 2008 Equity and Performance Incentive Plan, respectively. Fifty-percent (50%) of the grantee’s RSUs awarded shall vest on the first anniversary of the date of grant and the remaining fifty-percent (50%) will vest on the second anniversary of the date of grant. The actual number of RSUs vesting annually is subject to certain performance criteria. If certain annual performance conditions are not met, a portion of the awards may not vest and be carried over to the third year of vesting. If the grantee remains an employee of the Company on the third anniversary of the date of grant, any RSUs that have not vested due to not meeting the performance criteria shall vest upon such date. In addition, the vesting of the RSUs is subject to the earlier of the Company completing an initial public offering or a change of control (the “First Conversion Date”). Any RSUs that have not vested as of a change of control event (excluding an initial public offering) shall become fully vested upon such date.
If the grantee’s employment is terminated for cause or he or she terminates with the Company or any subsidiary for any reason prior to the earlier of an initial public offering or change of control (the “Restriction Period”), then all vested and unvested RSUs will be forfeited and become null and void at no cost to the founders or the Company (or convert into ordinary shares only on a case-by-case basis as determined by the Board of Directors for a PRC employee). Upon the completion of the initial public offering dated October 19, 2010, all vested RSUs became exercisable and all unvested RSUs will continue to vest based on its original vesting conditions.
No share-based compensation expense was recorded in 2009 on the RSUs as the IPO performance condition was not considered probable until it occurred. The RSUs are accounted for as if they were unvested as of December 31, 2009. The grantee shall have no rights as a shareholder with respect to any shares covered by the RSUs until the date shares are vested and the corresponding ordinary shares are transferred from our founders to the grantee. No adjustment shall be made for dividends, distributions or other rights for which the record date is prior to such date.
For the year ended December 31, 2010, compensation costs of US$2,817,520 on the RSUs were recognized after the IPO performance condition was realized, including compensation costs of US$2,478,731 were recognized immediately upon the completion of the qualifying IPO.
2010 Share Incentive Plan
In December 2010, the Company established the 2010 Share Incentive Plan (the “2010 Plan”). Under the 2010 Plan, a committee of the board of directors, in its sole discretion, is authorized to grant options, restricted shares or RSUs to recipients including directors, employees and consultants. One of the founders of the Company has agreed to contribute up to 18,000,000 ordinary shares of the Company beneficially owned by him or his family to the Company in connection with the issuance of ordinary shares upon vesting or exercise of share incentive awards granted to the directors, employees or consultants under the 2010 Plan.
F-37
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2009, 2010 AND 2011
(Amount expressed in US dollars, except share data, unless otherwise stated)
16. SHARE INCENTIVE PLANS (CONTINUED)
In 2011, the Company granted a total of RSUs representing the rights to receive 15,016,320 ordinary shares under the 2010 Plan. For 5,118,120 of the RSUs granted, each of the grantee’s RSUs shall vest every month up to the first to fourth anniversary of the date of grant. And for the rest of the RSUs granted, each of the grantee’s RSUs shall vest every quarter up to the tenth quarter of the date of grant. Any RSUs that have not vested as of a change of control event shall become fully vested upon such date.
If the grantee’s employment is terminated for cause or he or she terminates with the Company or any subsidiary for any reason prior to the earlier of each vesting date or change of control (the “Restriction Period”), then all unvested RSUs will be forfeited and become null and void at no cost to the founders or the Company.
A summary of unvested restricted share unit activities as of December 31, 2010 and 2011 is presented below:
| | | | | | | | |
Unvested Restricted Share Units | | Number of
Shares | | | Weighted Average
Grant Date Fair
Value | |
Unvested at December 31, 2008 | | | 7,450,000 | | | | 0.20 | |
| | |
Granted | | | 2,100,000 | | | | 0.47 | |
Vested | | | — | | | | — | |
Forfeited | | | — | | | | — | |
| | | | | | | | |
| | |
Unvested at December 31, 2009 | | | 9,550,000 | | | | 0.26 | |
| | | | | | | | |
| | |
Expected to vest at December 31, 2009 | | | 9,550,000 | | | | 0.26 | |
| | | | | | | | |
| | |
Granted | | | 2,200,000 | | | | 0.66 | |
Vested | | | (7,541,592 | ) | | | 0.23 | |
Forfeited | | | — | | | | — | |
| | |
Unvested at December 31, 2010 | | | 4,208,408 | | | | 0.52 | |
| | |
Expected to vest at December 31, 2010 | | | 3,716,408 | | | | 0.52 | |
| | |
Granted | | | 15,016,320 | | | | 0.64 | |
Vested | | | (7,965,962 | ) | | | 0.63 | |
Forfeited | | | (1,155,816 | ) | | | 0.57 | |
| | | | | | | | |
| | |
Unvested at December 31, 2011 | | | 10,102,950 | | | | 0.64 | |
| | | | | | | | |
| | |
Expected to vest at December 31, 2011 | | | 10,102,950 | | | | 0.64 | |
| | | | | | | | |
The fair value of each RSU granted is based on the fair market value of the underlying ordinary shares on the date of grant. Management utilized the results from a third party valuation firm to assist in the determination of the fair value of the underlying ordinary shares on a retrospective basis before the consummation of the qualifying IPO dated October 19, 2010.
The total fair value of nonvested RSUs vested for the years ended December 31, 2009, 2010 and 2011 was nil, US$1,734,566 and US$4,983,591, respectively.
F-38
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2009, 2010 AND 2011
(Amount expressed in US dollars, except share data, unless otherwise stated)
16. SHARE INCENTIVE PLANS (CONTINUED)
As of December 31, 2011, there was US$5,267,402 of total unrecognized compensation expense related to unvested RSUs under Founder’s 2008 Equity and Performance Incentive Plan and 2010 Plan, which is expected to be recognized over a weighted-average period of 2.0 years.
Share-based compensation expenses related to the RSUs granted by the Company under Founder’s 2008 Equity and Performance Incentive Plan and 2010 Plan amounted to approximately nil, US$2,817,520 and US$4,807,027 for the years ended December 31, 2009, 2010 and 2011, respectively.
2011 Share Incentive Plan
In August 2011, the Company established the 2011 Share Incentive Plan (the “2011 Plan”). Under the 2011 Plan, a committee of the board of directors, in its sole discretion, is authorized to grant options, restricted shares or RSUs to recipients including directors, employees and consultants. The maximum aggregate number of ordinary shares authorized for issuance, or the award pool, will be replenished to 2% of the then total outstanding of ordinary shares on an as-converted basis whenever the unissued ordinary shares reserved in the award pool account for no more than 10% of the award pool. As of December 31, 2011, there were no options, restricted shares or RSUs granted under the 2011 Plan.
Total share-based compensation expenses included in consolidated statements of operations is as follows
| | | | | | | | | | | | |
| | | 2009 | | | 2010 | | | 2011 | |
Share-based compensation included in: | | | | | | | | | | | | |
Cost of revenues | | | 178,906 | | | | 1,002,750 | | | | 2,146,727 | |
Selling and marketing | | | 4,274 | | | | 227,351 | | | | 290,271 | |
General and administrative | | | 68,770 | | | | 2,517,774 | | | | 3,790,560 | |
| | | | | | | | | | | | |
| | | 251,950 | | | | 3,747,875 | | | | 6,227,558 | |
| | | | | | | | | | | | |
17. SEGMENT REPORTING
The criteria established by ASC 280 on segments disclosures of an enterprise establish standards for reporting information about operating segments in annual financial statements. It also establishes standards for related disclosures about products and services, geographic areas and major customers.
The Group currently operates and manages its business as a single segment. For the years ended December 31, 2009, 2010 and 2011, the Group’s net revenues are mainly derived from laboratory services. The Group’s net revenues from manufacturing are insignificant for the years ended December 31, 2009, 2010 and 2011.
All the long-lived assets of the Group are located in the PRC.
F-39
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2009, 2010 AND 2011
(Amount expressed in US dollars, except share data, unless otherwise stated)
17. SEGMENT REPORTING (CONTINUED)
The Company’s net revenues by geographic region determined according to the location of the customer are as follows:
| | | | | | | | | | | | |
| | | 2009 | | | 2010 | | | 2011 | |
North America | | | 57,956,395 | | | | 72,953,167 | | | | 81,653,835 | |
Europe | | | 9,547,818 | | | | 8,016,135 | | | | 11,310,709 | |
China | | | 2,217,969 | | | | 4,703,941 | | | | 12,305,882 | |
Japan and other areas | | | 2,785,495 | | | | 4,817,831 | | | | 6,545,778 | |
| | | | | | | | | | | | |
| | | |
| | | 72,507,677 | | | | 90,491,074 | | | | 111,816,204 | |
Sales tax | | | (223,020 | ) | | | (209,677 | ) | | | (214,515 | ) |
| | | | | | | | | | | | |
| | | |
Net revenues | | | 72,284,657 | | | | 90,281,397 | | | | 111,601,689 | |
| | | | | | | | | | | | |
18. RELATED PARTY TRANSACTIONS AND BALANCES
Significant related party transactions are as follows:
| | | | | | | | | | | | |
| | | 2009 | | | 2010 | | | 2011 | |
Purchases from Lab Partner | | | 5,770,458 | | | | 8,076,783 | | | | 3,826,435 | |
Service fees charged by Lab Partner | | | 357,611 | | | | 401,025 | | | | 197,959 | |
Service fees charged by Shanghai Kehui | | | 813,862 | | | | 929,760 | | | | 1,118,582 | |
Office rent, utility and service fees charged by Pharm Valley | | | 2,022,116 | | | | 2,339,425 | | | | 2,978,600 | |
Laboratory service revenues from Yunyi | | | — | | | | — | | | | 451,911 | |
Labpartner (Shanghai) Co., Ltd. (“Lab Partner”) acts as a raw material procurement agent for the Group since April 2007. It was owned and controlled by an immediate family member of one of the founders in 2007. In 2008, the family member disposed 50% of its equity interests in Lab Partner to two third parties. The daily operation of Lab Partner is managed by these third parties after the disposal. In August 2011, the family member disposed the rest 50% of its equity interests in Lab Partner to one another third party and Lab Partner became an unrelated party with the Company from then on.
Shanghai Kehui Catering Management Co., Ltd. (“Shanghai Kehui”) is a catering service provider. It was owned and controlled by immediate family members of one of the founders for the years ended December 31, 2009, 2010 and 2011.
The Group leases certain office facilities and receives certain testing services from Pharm Valley. The Company’s founders and their immediate family member own 20% shares of Pharm Valley.
The Group renders certain laboratory services to Shanghai Yunyi Health Management Consultancy Co., Ltd. (“Yunyi”), which was wholly owned by the Company’s founders and their immediate family members for the years ended December 31, 2009, 2010 and 2011.
F-40
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2009, 2010 AND 2011
(Amount expressed in US dollars, except share data, unless otherwise stated)
18. RELATED PARTY TRANSACTIONS AND BALANCES (CONTINUED)
Balances with related parties are as follows:
| | | | | | | | |
| | | As of December 31, | |
| | | 2010 | | | 2011 | |
Amounts due from related parties (current): | | | | | | | | |
Shanghai Kehui | | | — | | | | 47,612 | |
PharmValley | | | — | | | | 159 | |
Yunyi | | | — | | | | 455,298 | |
| | | | | | | | |
| | | — | | | | 503,069 | |
| | | | | | | | |
| | |
Amounts due from related parties (non current): | | | | | | | | |
PharmValley | | | — | | | | 225,206 | |
| | | | | | | | |
| | |
Amounts due to related parties: | | | | | | | | |
Lab Partner | | | (817,909 | ) | | | — | |
Shanghai Kehui | | | (98,432 | ) | | | (15,772 | ) |
PharmValley | | | (108,306 | ) | | | (106,740 | ) |
| | | | | | | | |
| | | (1,024,647 | ) | | | (122,512 | ) |
| | | | | | | | |
The amounts due from and due to related parties as of December 31, 2010 and 2011 mainly arose from the transactions disclosed above. They were unsecured, interest-free and had no fixed repayment terms.
The amounts due from PharmValley amounting to US$225,206 as of December 31, 2011 represent deposits paid to PharmValley for the assumed facilities lease transfer from Charles River as part of the asset acquisition in November 2011.
19. COMMITMENTS AND CONTINGENCIES
Operating lease commitments
The Group has entered into leasing arrangements for office premises that are classified as operating leases. Future minimum lease payments for non-cancelable operating leases at December 31, 2011 are as follows:
| | | | |
| | | Office premises | |
2012 | | | 4,834,336 | |
2013 | | | 3,139,265 | |
2014 | | | 2,492,166 | |
2015 | | | 1,861,099 | |
2016 | | | 785,326 | |
Thereafter | | | 259,347 | |
| | | | |
| |
| | | 13,371,539 | |
| | | | |
Rental expenses amounted to US$3,472,379, US$3,848,296 and US$4,889,248 for the years ended December 31, 2009, 2010 and 2011, respectively, and were charged to the consolidated statements of operations when incurred.
F-41
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2009, 2010 AND 2011
(Amount expressed in US dollars, except share data, unless otherwise stated)
19. COMMITMENTS AND CONTINGENCIES (CONTINUED)
Capital commitments
As of December 31, 2011, capital commitments for laboratory and manufacturing facility improvements and equipments to be purchased amounted to US$1,564,580.
Contingencies
The Group is subject to claims and legal proceedings that arise in the ordinary course of its business operations. Each of these matters is subject to various uncertainties, and it is possible that as these matters arise some may be decided unfavorably for the Group. The Group did not have any claims or legal proceedings that could have a significant impact on its business, assets, operations or cash flows.
20 FAIR VALUE MEASUREMENTS
The guidance on fair value measurements establishes a hierarchy for inputs used in measuring fair value that maximizes the use of observable inputs and minimizes the use of unobservable inputs by requiring that the observable inputs be used when available. Observable inputs are inputs that market participants would use in pricing the asset or liability developed based on market data obtained from sources independent of the Company. Unobservable inputs are inputs that reflect the Company’s assumptions about the assumptions market participants would use in pricing the asset or liability developed based on the best information available in the circumstances. As such, fair value is a market-based measure considered from the perspective of a market participant who holds the asset or owes the liability rather than an entity-specific measure. The hierarchy is broken down into three levels based on the reliability of inputs as follows: (Level 1) observable inputs such as quoted prices in active markets; (Level 2) inputs other than the quoted price in active markets that are observable either directly or indirectly, or quoted prices in less active markets; and (Level 3) unobservable inputs with respect to which there is little or no market data, which require the Company to develop its own assumptions.
When available, the Company uses quoted market prices to determine the fair value of an asset or liability. If quoted market prices are not available, the Company measures fair value using valuation techniques that use current market-based or independently sourced market parameters, such as interest rates and currency rates, when possible.
From time to time, the Company enters into foreign-exchange forward contracts with financial institutions. These contracts are marked to market at each reporting date, with changes in fair value recognized in the consolidated statements of operations. The Company held foreign-exchange forward contracts with a total notional value of $15,000,000, $102,000,000 and $129,000,000 as of December 31, 2009, 2010 and 2011, respectively. These foreign exchange forward contracts mature between one to 21 months. The Company used a discounted cash-flow methodology to measure fair value, which requires inputs such as interest yield curves and foreign exchange rates. The significant inputs used in the aforementioned model can be corroborated with market observable data and therefore the fair value measurements are classified as level 2.
The gain or losses from foreign-exchange forward contracts for the years ended December 31, 2009, 2010 and 2011 were included in other income (Note 2(s)) and other expenses (Note 2(t)).
F-42
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2009, 2010 AND 2011
(Amount expressed in US dollars, except share data, unless otherwise stated)
20. FAIR VALUE MEASUREMENTS (CONTINUED)
As of December 31, 2010 and 2011, information about inputs for the fair value measurements of the Company’s assets and liabilities that are measured at fair value on a recurring basis in periods subsequent to their initial recognition is as follows:
| | | | | | | | | | | | | | | | |
| | | | | | Fair Value Measurements at Reporting Date Using | |
Description | | Year ended
December 31,
2010 | | | Quoted Prices in
Active Markets for
Identical Assets
(Level 1) | | | Significant
Other Observable
Inputs
(Level 2) | | | Significant
Unobservable
Inputs
(Level 3) | |
3-month time deposits | | | 25,733,543 | | | | 25,733,543 | | | | — | | | | — | |
Foreign-exchange forward contracts | | | 2,710,087 | | | | — | | | | 2,710,087 | | | | — | |
| | | | | | | | | | | | | | | | |
| | | | | | Fair Value Measurements at Reporting Date Using | |
Description | | Year ended
December 31,
2011 | | | Quoted Prices in
Active Markets for
Identical Assets
(Level 1) | | | Significant
Other Observable
Inputs
(Level 2) | | | Significant
Unobservable
Inputs
(Level 3) | |
Time deposits with maturity within 3 months | | | 14,109,524 | | | | 14,109,524 | | | | — | | | | — | |
Highly liquid and principal secured funds | | | 6,348,299 | | | | 6,348,299 | | | | — | | | | — | |
Foreign-exchange forward contracts | | | 782,215 | | | | — | | | | 782,215 | | | | — | |
As of December 31, 2010 and 2011, no items were transferred into or out of Level 1 and 2 in the fair value hierarchy.
A summary of changes in the fair value of the Level 3 investment in securities for the years ended December 31, 2010 and 2011 were as follows, respectively:
| | | | |
At January 1, 2010 | | | 417,000 | |
Current year disposal | | | (417,000 | ) |
| | | | |
| |
December 31, 2010 and 2011 | | | — | |
| | | | |
21. SUBSEQUENT EVENTS
Pursuant to Circular 110 and Circular 111, a tax reform pilot program came into effect in Shanghai, which was chosen by the PRC government as the first pilot city for such reform. Starting from January 1, 2012, companies which are designated by Shanghai local tax authorities as operating in certain modern service sectors are required to pay VAT, in lieu of business tax. As a result of the pilot program, our PRC subsidiaries located in Shanghai are subject to VAT at the rate of 6% for sales proceeds received from certain laboratory services, and 17% unchanged for sales proceeds received from compound delivery services or product sales activities. The services provided related to technology development and transfer are still tax exempted subject to the approval of relevant tax authorities. Such tax reform is not expected to have any material impact to the Company’s net revenues but the overall taxes payables are expected to become lower after the reform.
F-43
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2009, 2010 AND 2011
(Amount expressed in US dollars, except share data, unless otherwise stated)
21. SUBSEQUENT EVENTS (CONTINUED)
In March 2012, with the approval by the board of directors, the Company entered into certain RSU amendment agreements with Mr. William Dai, the chief financial officer of the Company and Mr. Julian Ralph Worley, Mr. Yuk Lam Lo and Mr. Benson Tsang, the independent directors of the Company. After the modification, Mr. William Dai’s unvested RSUs as of the modification date, which were granted during 2011 under the 2010 Plan, were cancelled along with concurrent grant of replacement award RSUs under the 2011 Plan to become vested monthly up to the third anniversary of the grant date. Mr. Julian Ralph Worley, Mr. Yuk Lam Lo and Mr. Benson Tsang’s unvested RSUs as of the modification date, which were granted during 2011 under the 2010 Plan, were cancelled along with concurrent grant of replacement award RSUs under the 2011 Plan to become vested quarterly up to the tenth quarter of the grant date. Other terms of the RSUs grants remain unchanged. The incremental fair value related to the modification was assessed based on the closing price of our ADSs traded on the NYSE as of March 30, 2012, the modification date. The Company estimated an incremental compensation charge of approximately US$0.14 million; this charge will be recognized over the remaining vesting periods of approximately twenty-two months or seven quarters, respectively.
During the first quarter of 2012, the Company granted RSUs representing the rights to receive 5,452,200 ordinary shares under the 2011 Plan, including 3,600,000 RSUs to Mr. Michael Xin Hui, the chairman of the board of directors and chief executive officer of the Company. Each of the grantee’s RSUs shall vest every quarter up to the eighth quarter of the date of grant. The weighted average grant date fair value of these RSUs is US$7.99. The total compensation costs of approximately US$3.3 million will be recognized during the vesting period from 2012 to 2014.
22. RESTRICTED NET ASSETS
Relevant PRC laws and regulations permit PRC companies to pay dividends only out of their retained earnings, if any, as determined in accordance with PRC accounting standards and regulations. Additionally, the Company’s VIE subsidiaries can only distribute dividends upon approval of the shareholders after they have met the PRC requirements for appropriation to statutory reserve under PRC GAAP. The statutory general reserve fund requires annual appropriations of 10% of net after-tax income should be set aside prior to payment of any dividends. As a result of these and other restrictions under PRC laws and regulations, the PRC subsidiaries and affiliates are restricted in their ability to transfer a portion of their net assets to the Company either in the form of dividends, loans or advances, which restricted portion amounted to approximately US$90.1 million, or 68% of the Company’s total consolidated net assets as of December 31, 2011. Even though the Company currently does not require any such dividends, loans or advances from the PRC subsidiaries and affiliates for working capital and other funding purposes, the Company may in the future require additional cash resources from our PRC subsidiaries and affiliates due to changes in business conditions, to fund future acquisitions and developments, or merely declare and pay dividends to or distributions to the Company shareholders.
23. ADDITIONAL INFORMATION—CONDENSED FINANCIAL STATEMENTS
The condensed financial statements of the Company have been prepared in accordance with SEC Regulation S-X Rule 5-04 and Rule 12-04.
The Company records its investment in subsidiaries under the equity method of accounting. Such investment and long-term loans to subsidiaries are presented on the balance sheet as “Interests in subsidiaries” and the profit of the subsidiaries is presented as “Equity in profit of subsidiaries” on the statement of operations.
F-44
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2009, 2010 AND 2011
(Amount expressed in US dollars, except share data, unless otherwise stated)
23. ADDITIONAL INFORMATION—CONDENSED FINANCIAL STATEMENTS (CONTINUED)
ShangPharma Corporation is solely a holding company and a statement of cash flows has not been presented as the company does not maintain any cash accounts. From an operating cash flow perspective, the company’s net income would be offset by the equity in profit of subsidiaries and the changes in amounts due from (to) subsidiaries. Investing and financing activities of the company are related to non-cash transactions between the company and its subsidiaries as more fully described in the footnotes to the Consolidated Financial Statements.
The footnote disclosures contain supplemental information relating to the operations of the Company and, as such, these statements should be read in conjunction with the notes to the Consolidated Financial Statements of the Company. Certain information and footnote disclosures normally included in financial statements prepared in accordance with U.S. GAAP have been condensed or omitted.
As of December 31, 2010 and 2011, there were no material contingencies, significant provisions for long-term obligations, or guarantees of the Company, except for those which have been separately disclosed in the Consolidated Financial Statements, if any.
F-45
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2009, 2010 AND 2011
(Amount expressed in US dollars, except share data, unless otherwise stated)
Financial information of Shangpharma Corporation
Condensed Statements of Operations
| | | | | | | | | | | | |
| | | For the years ended December 31 | |
| | | 2009 | | | 2010 | | | 2011 | |
Net revenues | | | — | | | | — | | | | — | |
Cost of services | | | — | | | | — | | | | — | |
| | | | | | | | | | | | |
| | | |
Gross profit | | | — | | | | — | | | | — | |
| | | | | | | | | | | | |
| | | |
Total operating expenses | | | (251,950 | ) | | | (3,747,875 | ) | | | (6,227,558 | ) |
| | | | | | | | | | | | |
| | | |
Loss from operations | | | (251,950 | ) | | | (3,747,875 | ) | | | (6,227,558 | ) |
| | | | | | | | | | | | |
| | | |
Income before income tax expense and equity in profit of subsidiaries and equity in loss of affiliated companies | | | (251,950 | ) | | | (3,747,875 | ) | | | (6,227,558 | ) |
| | | | | | | | | | | | |
| | | |
Income tax expense | | | — | | | | — | | | | — | |
Equity in profit of subsidiaries | | | 10,050,765 | | | | 16,744,389 | | | | 17,342,409 | |
| | | |
Net income | | | 9,798,815 | | | | 12,996,514 | | | | 11,114,851 | |
| | | | | | | | | | | | |
| | | |
Allocation to preferred shareholders | | | (2,467,117 | ) | | | (2,738,594 | ) | | | — | |
| | | |
Net income attributable to ShangPharma Corporation’s ordinary shareholders | | | 7,331,698 | | | | 10,257,920 | | | | 11,114,851 | |
| | | | | | | | | | | | |
F-46
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2009, 2010 AND 2011
(Amount expressed in US dollars, except share data, unless otherwise stated)
Financial information of Shangpharma Corporation
Condensed Balance Sheets
| | | | | | | | |
| | | December 31, | |
| | | 2010 | | | 2011 | |
ASSETS | | | | | | | | |
| | |
Amounts due from subsidiaries | | | 21,000,000 | | | | 21,000,000 | |
Investment in and advances to subsidiaries | | | 93,183,655 | | | | 112,687,904 | |
| | | | | | | | |
| | |
Total non-current assets | | | 114,183,655 | | | | 133,687,904 | |
| | | | | | | | |
| | |
Total assets | | | 114,183,655 | | | | 133,687,904 | |
| | | | | | | | |
| | |
LIABILITIES AND SHAREHOLDERS’ EQUITY | | | | | | | | |
| | |
Amounts due to subsidiaries | | | 647,749 | | | | 649,677 | |
| | | | | | | | |
| | |
Total liabilities | | | 647,749 | | | | 649,677 | |
| | | | | | | | |
| | |
Shareholders’ equity | | | | | | | | |
| | |
Ordinary shares (US$0.001 par value; 429,999,350 shares authorized; 335,600,000 and 336,109,400 shares issued and outstanding as of December 31, 2010 and 2011, respectively) | | | 335,600 | | | | 336,109 | |
Additional paid-in capital | | | 85,760,476 | | | | 93,476,624 | |
Retained earnings | | | 23,679,703 | | | | 33,013,373 | |
Accumulated other comprehensive income | | | 3,760,127 | | | | 7,304,396 | |
Less: treasury stock, at cost; nil and 2,237,148 shares as of December 31, 2010 and 2011, respectively | | | — | | | | (1,092,275 | ) |
| | | | | | | | |
| | |
Total shareholders’ equity | | | 113,535,906 | | | | 133,038,227 | |
| | | | | | | | |
| | |
Total liabilities and shareholders’ equity | | | 114,183,655 | | | | 133,687,904 | |
F-47
SHANGPHARMA CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2009, 2010 AND 2011
(Amount expressed in US dollars, except share data, unless otherwise stated)
Financial information of Shangpharma Corporation
Condensed Statements of Cash Flows
| | | | | | | | | | | | |
| | | For the years ended December 31 | |
| | | 2009 | | | 2010 | | | 2011 | |
Net cash provided by (used in) operating activities | | | — | | | | — | | | | — | |
| | | | | | | | | | | | |
| | | |
Net cash provided by (used in) investing activities | | | — | | | | — | | | | — | |
| | | | | | | | | | | | |
| | | |
Net cash provided by (used in) financing activities | | | — | | | | — | | | | — | |
| | | | | | | | | | | | |
| | | |
Effect of foreign exchange rate changes on cash and cash equivalents | | | — | | | | — | | | | — | |
| | | | | | | | | | | | |
| | | |
Net increase (decrease) in cash and cash equivalents | | | — | | | | — | | | | — | |
| | | | | | | | | | | | |
| | | |
Cash and cash equivalents at beginning of year | | | — | | | | — | | | | — | |
| | | | | | | | | | | | |
| | | |
Cash and cash equivalents at end of year | | | — | | | | — | | | | — | |
| | | | | | | | | | | | |
F-48


