
Gaining Momentum in Gene Therapy Exhibit 99.1

Forward-Looking Statements Statements contained in this document regarding matters that are not historical facts are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Such statements include, but are not limited to, statements regarding Avalanche Biotechnologies, Inc.’s (“Avalanche”) plans, potential opportunities, expectations, projections, goals, objectives, milestones, strategies, product pipeline, the occurrence or effect of the proposed transaction between Avalanche and Annapurna Therapeutics SAS (“Annapurna”), the sufficiency of the combined company’s resources to fund the advancement of any development program or the completion of any clinical trials, and the safety, efficacy and projected development timeline and commercial potential of products under development by Avalanche and Annapurna, all of which are based on certain assumptions made by us based on current conditions, expected future developments and other factors we believe are appropriate in the circumstances. Actual results, the timing of events, product development programs, performance or achievements of Avalanche could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without limitation, risks and uncertainties inherent in the product development and the regulatory approval process, delays in clinical trials and other matters that could affect the availability or commercial potential of product candidates, the ability to consummate the proposed transaction with Annapurna, the ability to project future cash utilization, the availability of sufficient resources to conduct or continue planned development programs, and the ability to successfully develop any of Avalanche’s or Annapurna’s product candidates. Risks and uncertainties facing Avalanche are described more fully in Avalanche’s periodic reports filed with the SEC. All forward-looking statements contained in this document speak only as of the date on which they were made. Avalanche undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made. This document contains estimates, projections and other information concerning Avalanche’s and Annapurna’s industry, business and the markets for certain drugs, including data regarding the estimated size of those markets, their projected growth rates and the incidence of certain medical conditions. Information that is based on estimates, forecasts, projections or similar methodologies is inherently subject to uncertainties and actual events or circumstances may differ materially from events and circumstances reflected in this information. Unless otherwise expressly stated, we obtained this industry, business, market and other data from reports, research surveys, studies and similar data prepared by third parties, industry, medical and general publications, government data and similar sources believed to be reliable, but the accuracy or completeness of such information is not guaranteed by, and should not be construed as representations made by, Avalanche or Annapurna.

Additional Information and Where to Find It This document not constitute a solicitation of any vote or approval. In connection with the proposed transaction, Avalanche intends to file with the SEC a proxy statement of Avalanche, as well as other relevant documents concerning the proposed transaction. INVESTORS AND SECURITYHOLDERS OF AVALANCHE ARE URGED TO READ THE PROXY STATEMENT REGARDING THE PROPOSED TRANSACTION WHEN IT BECOMES AVAILABLE AND ANY OTHER RELEVANT DOCUMENTS FILED WITH THE SEC, AS WELL AS ANY AMENDMENTS OR SUPPLEMENTS TO THOSE DOCUMENTS, BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION. A free copy of the proxy statement and other filings containing information about Avalanche may be obtained at the SEC website at www.sec.gov. You will also be able to obtain these documents, free of charge, from Avalanche by directing a written request to: Avalanche Biotechnologies, Inc., 1035 O’Brien Drive, Suite A, Menlo Park, CA 94025, Attention: Investor Relations. Investors and security holders are urged to read the proxy statement and the other relevant materials when they become available before making any voting decision with respect to the issuance of shares of the Avalanche common stock in connection with the proposed transaction and any other matters relating to the proposed transaction. Certain Information Regarding Participants Avalanche and its directors and executive officers may be deemed to be participants in the solicitation of proxies from the stockholders of Avalanche in connection with the proposed transaction and the issuance of additional Avalanche common stock. Information regarding the special interests of these directors and executive officers in the proposed transaction will be included in the proxy statement referred to above. Additional information regarding the directors and executive officers of Avalanche is also included in Avalanche’s Annual Report on Form 10-K for the year ended December 31, 2015 and the proxy statement for the Company’s 2015 Annual Meeting of Stockholders. Additional information about the interests of potential participants will be contained in the proxy statement (when filed) and other relevant materials to be filed with the SEC in connection with the proposed transaction. These documents may be obtained free of charge at the SEC web site at www.sec.gov and from Investor Relations at Avalanche in the manner set forth above.

Proposed Merger with Annapurna Therapeutics Feb 1, 2016 Avalanche and Annapurna announced proposed merger: Creating a leading gene therapy company with a diverse pipeline including new assets targeting rare genetic diseases as well as AAVL’s existing ophthalmic programs Transaction Terms: Avalanche to acquire all outstanding shares of Annapurna in exchange for ~17.6 million newly issued shares of AAVL common stock (inclusive of shares of our common stock underlying vested and unvested options, as calculated on a treasury-stock method basis) Upon completion of the proposed transaction, AAVL shareholders will own ~62.5% and Annapurna shareholders will own ~37.5% of the combined company calculated on a treasury-stock method basis as of January 28, 2016 Projected close of transaction: Q2 2016
Combined Company Highlights Diversified pipeline including 7 gene therapy product candidates in ophthalmology and rare disease ANN-001: Initiation of first-in-human trial expected in H2 2016 ANN-002: Initiation of first-in-human trial expected in 2017 Industry-leading team in gene therapy and translational medicine, including Ronald Crystal M.D. (Weill Cornell Medicine) and Mitchell Finer, Ph.D. (MPM Capital, former CSO bluebird bio) Significant capabilities and gene therapy expertise - directed evolution, vector optimization, process development and manufacturing Potentially de-risk product candidates and accelerate development processes Strong balance sheet - cash expected to fund combined company through at least 36 months
Avalanche Strategy Complete Annapurna Transaction and drive combined company programs forward Determine best path forward in wet AMD and pursue it Prioritize strategic collaboration with Regeneron- seek to leverage relationship with a leader in developing products for eye disease Conserve cash- a key strategic advantage during a time of market uncertainty Continue to build diverse gene therapy pipeline Seek to apply next generation vectors to amenable targets Prioritize indications with validated targets, well established patient populations, and defined endpoints Employ directed evolution to create and manufacture new next generation vectors with higher efficiency and greater specificity
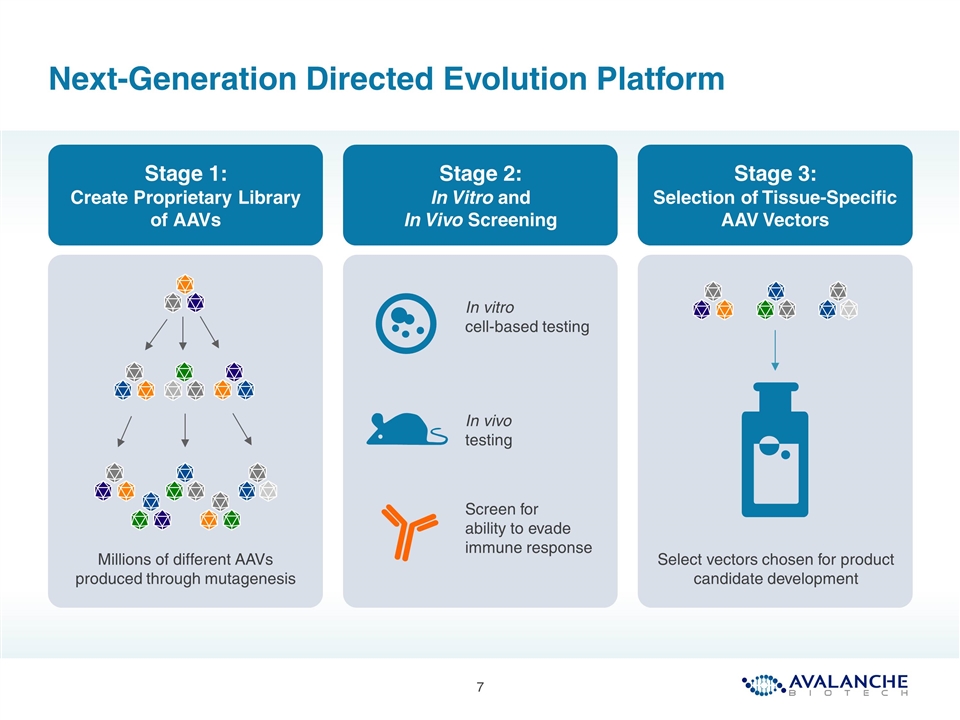
Next-Generation Directed Evolution Platform Stage 1: Create Proprietary Library of AAVs Stage 2: In Vitro and In Vivo Screening Stage 3: Selection of Tissue-Specific AAV Vectors Millions of different AAVs produced through mutagenesis In vitro cell-based testing In vivo testing Screen for ability to evade immune response Select vectors chosen for product candidate development
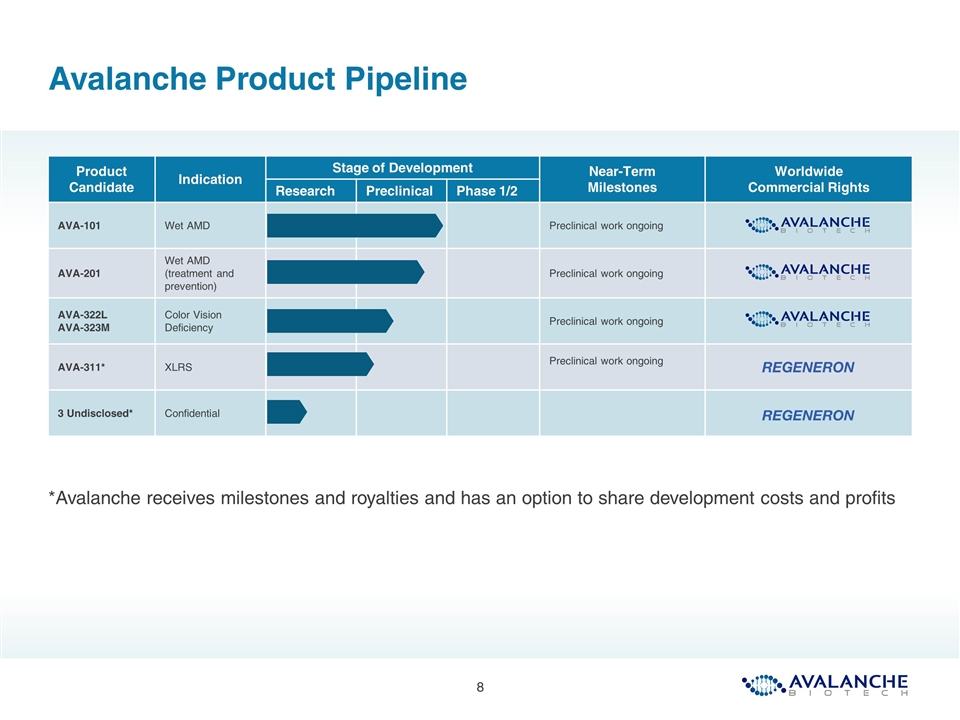
Avalanche Product Pipeline *Avalanche receives milestones and royalties and has an option to share development costs and profits Product Candidate Indication Stage of Development Near-Term Milestones Worldwide Commercial Rights Research Preclinical Phase 1/2 AVA-101 Wet AMD Preclinical work ongoing AVA-201 Wet AMD (treatment and prevention) Preclinical work ongoing AVA-322L AVA-323M Color Vision Deficiency Preclinical work ongoing AVA-311* XLRS Preclinical work ongoing 3 Undisclosed* Confidential
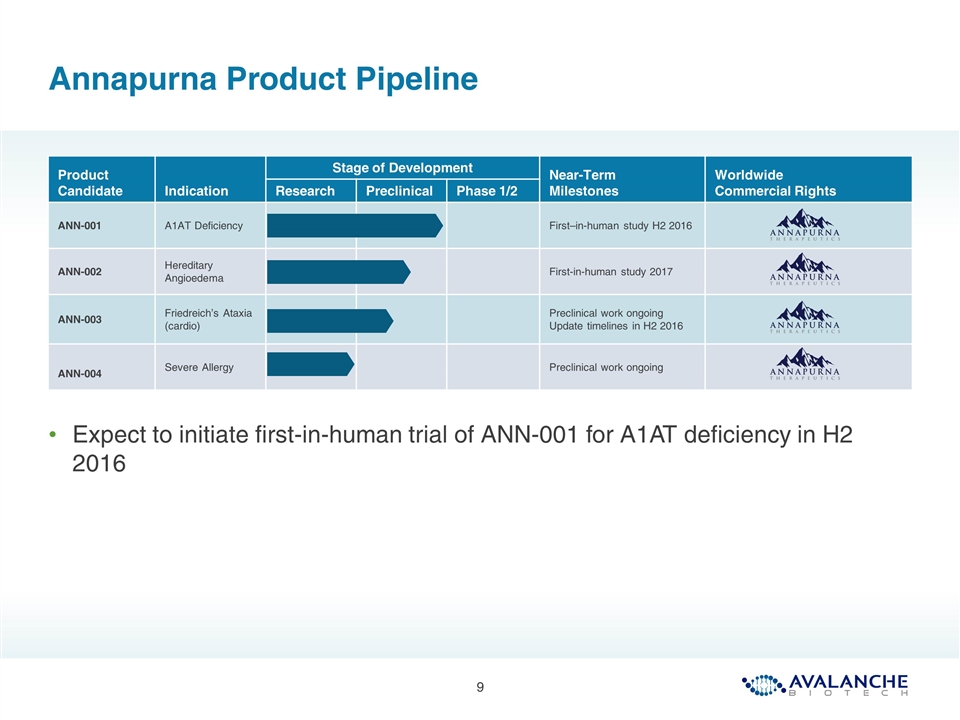
Product Candidate Indication Stage of Development Near-Term Milestones Worldwide Commercial Rights Research Preclinical Phase 1/2 ANN-001 A1AT Deficiency First–in-human study H2 2016 ANN-002 Hereditary Angioedema First-in-human study 2017 ANN-003 Friedreich’s Ataxia (cardio) Preclinical work ongoing Update timelines in H2 2016 ANN-004 Severe Allergy Preclinical work ongoing Annapurna Product Pipeline Expect to initiate first-in-human trial of ANN-001 for A1AT deficiency in H2 2016
ANN-001 for α1 Antitrypsin Deficiency (A1AT) II. ANN-002 for Hereditary Angioedema (HAE) III. ANN-003 for Friedreich’s Ataxia (FA) IV. ANN-004 for Severe Allergy Annapurna Pipeline in Detail
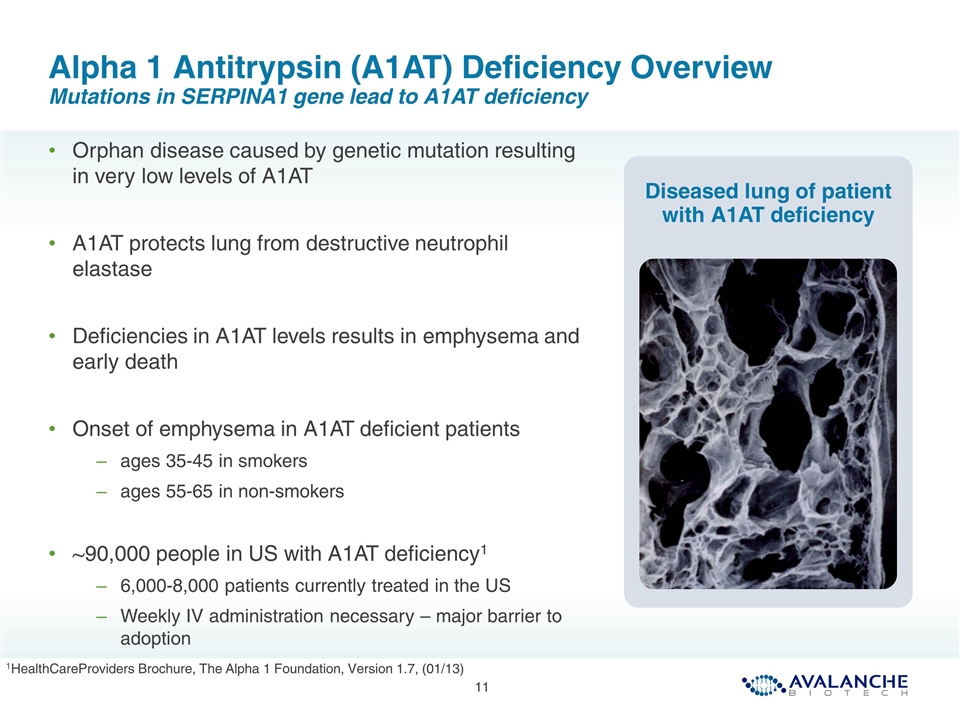
Alpha 1 Antitrypsin (A1AT) Deficiency Overview Mutations in SERPINA1 gene lead to A1AT deficiency Orphan disease caused by genetic mutation resulting in very low levels of A1AT A1AT protects lung from destructive neutrophil elastase Deficiencies in A1AT levels results in emphysema and early death Onset of emphysema in A1AT deficient patients ages 35-45 in smokers ages 55-65 in non-smokers ~90,000 people in US with A1AT deficiency1 6,000-8,000 patients currently treated in the US Weekly IV administration necessary – major barrier to adoption Diseased lung of patient with A1AT deficiency 1HealthCareProviders Brochure, The Alpha 1 Foundation, Version 1.7, (01/13)
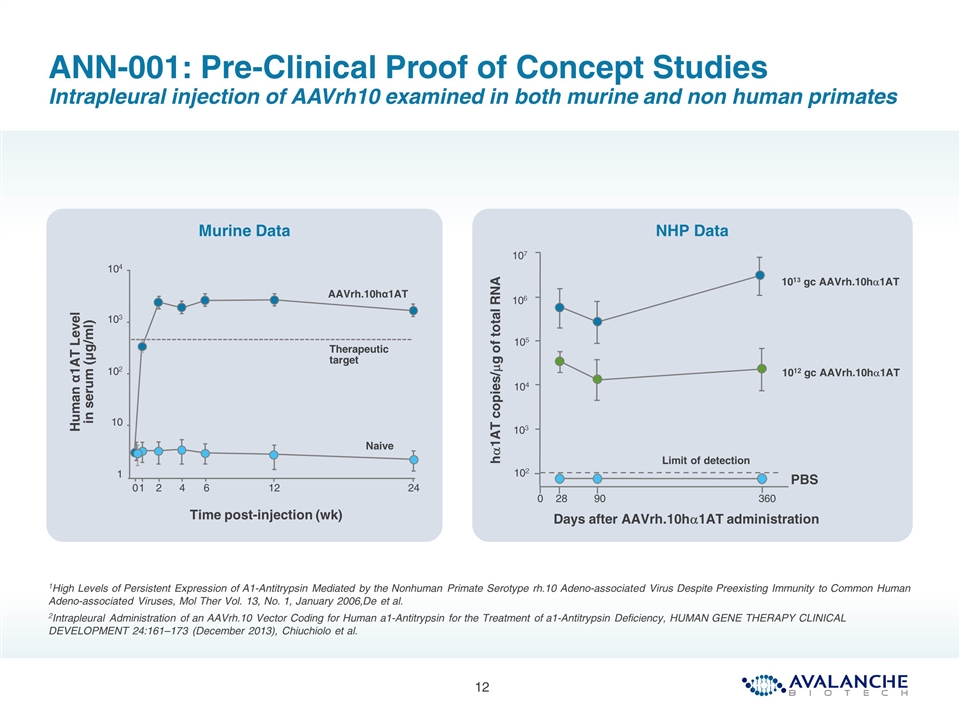
ANN-001: Pre-Clinical Proof of Concept Studies Intrapleural injection of AAVrh10 examined in both murine and non human primates 1High Levels of Persistent Expression of A1-Antitrypsin Mediated by the Nonhuman Primate Serotype rh.10 Adeno-associated Virus Despite Preexisting Immunity to Common Human Adeno-associated Viruses, Mol Ther Vol. 13, No. 1, January 2006,De et al. 2Intrapleural Administration of an AAVrh.10 Vector Coding for Human a1-Antitrypsin for the Treatment of a1-Antitrypsin Deficiency, HUMAN GENE THERAPY CLINICAL DEVELOPMENT 24:161–173 (December 2013), Chiuchiolo et al. NHP Data Murine Data Human α1AT Level in serum (μg/ml) 1 10 102 103 104 Therapeutic target AAVrh.10hα1AT Naive 0 1 2 4 6 12 24 Time post-injection (wk) Days after AAVrh.10ha1AT administration 107 105 104 103 102 28 90 360 0 Limit of detection 1013 gc AAVrh.10ha1AT 1012 gc AAVrh.10ha1AT PBS ha1AT copies/mg of total RNA 106
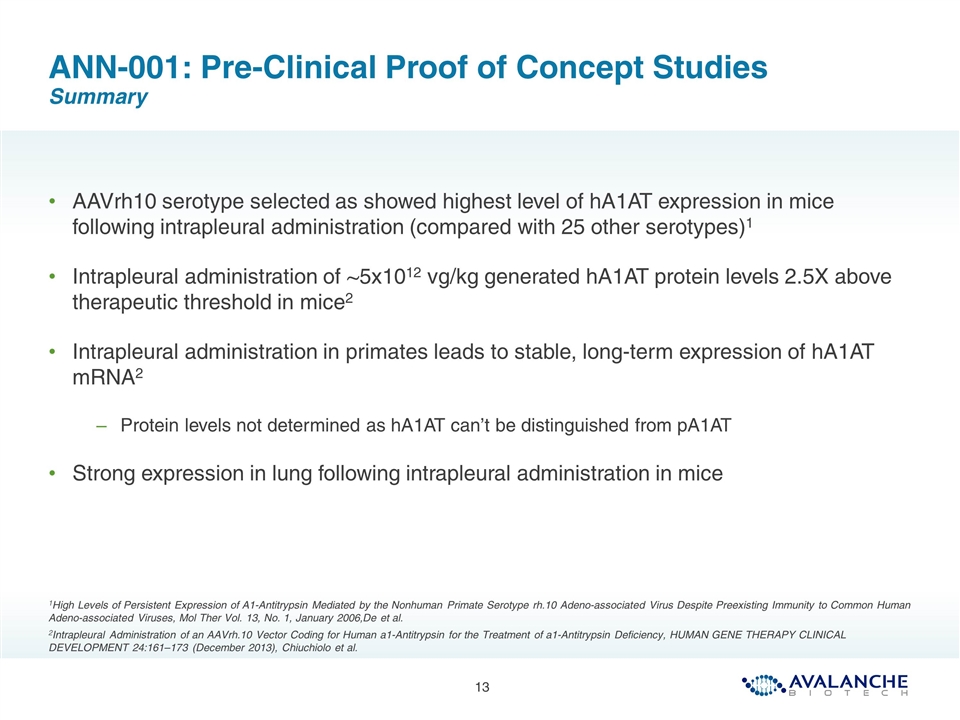
AAVrh10 serotype selected as showed highest level of hA1AT expression in mice following intrapleural administration (compared with 25 other serotypes)1 Intrapleural administration of ~5x1012 vg/kg generated hA1AT protein levels 2.5X above therapeutic threshold in mice2 Intrapleural administration in primates leads to stable, long-term expression of hA1AT mRNA2 Protein levels not determined as hA1AT can’t be distinguished from pA1AT Strong expression in lung following intrapleural administration in mice ANN-001: Pre-Clinical Proof of Concept Studies Summary 1High Levels of Persistent Expression of A1-Antitrypsin Mediated by the Nonhuman Primate Serotype rh.10 Adeno-associated Virus Despite Preexisting Immunity to Common Human Adeno-associated Viruses, Mol Ther Vol. 13, No. 1, January 2006,De et al. 2Intrapleural Administration of an AAVrh.10 Vector Coding for Human a1-Antitrypsin for the Treatment of a1-Antitrypsin Deficiency, HUMAN GENE THERAPY CLINICAL DEVELOPMENT 24:161–173 (December 2013), Chiuchiolo et al.
ANN-001 for A1AT Deficiency: Overview Validated target; disease driven by mutation in single gene Shown to provide steady levels of A1AT vs peaks and troughs in preclinical studies Rationale for Gene Therapy Approach Long term expression at therapeutically relevant levels observed in wild type mice Long term mRNA expression observed in primate lungs following intrapleural administration Proof-of-Concept Large orphan disease with ~90,000 patients in the US alone Potential to replace 1x/week IV protein infusion and further expand market (average selling price for protein therapy is greater than $100,000/year and reimbursed)) Significant Market Opportunity IND filed Patient enrollment expected to begin in H2 2016 Status

I. ANN-001 for α1 Antitrypsin Deficiency (A1AT) ANN-002 for Hereditary Angioedema (HAE) III. ANN-003 for Friedreich’s Ataxia (FA) IV. ANN-004 for Severe Allergy Annapurna Pipeline in Detail
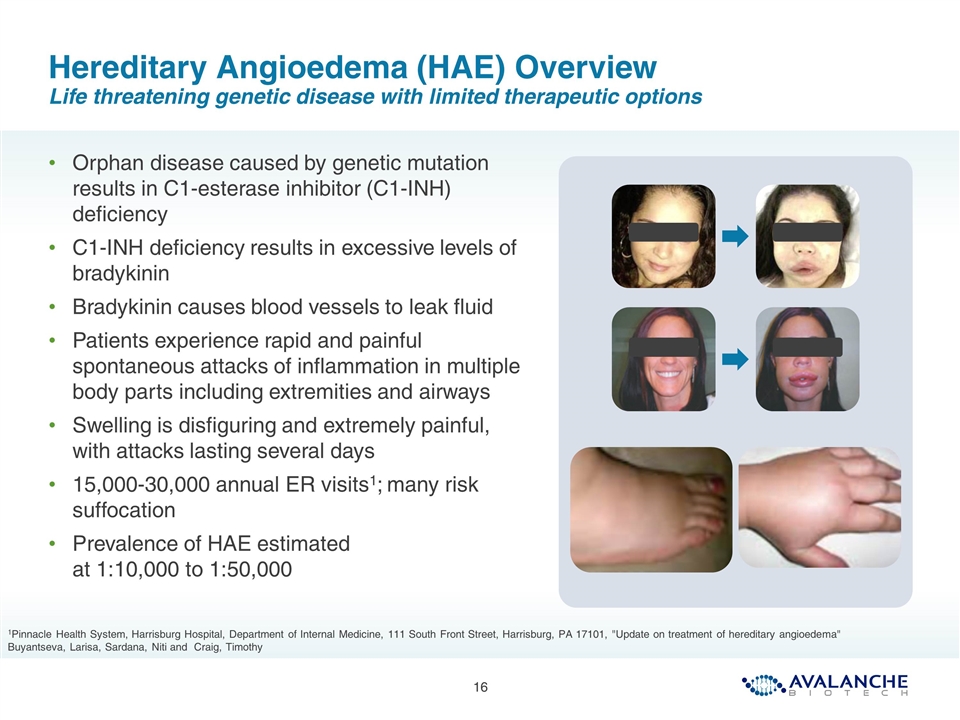
Hereditary Angioedema (HAE) Overview Life threatening genetic disease with limited therapeutic options Orphan disease caused by genetic mutation results in C1-esterase inhibitor (C1-INH) deficiency C1-INH deficiency results in excessive levels of bradykinin Bradykinin causes blood vessels to leak fluid Patients experience rapid and painful spontaneous attacks of inflammation in multiple body parts including extremities and airways Swelling is disfiguring and extremely painful, with attacks lasting several days 15,000-30,000 annual ER visits1; many risk suffocation Prevalence of HAE estimated at 1:10,000 to 1:50,000 1Pinnacle Health System, Harrisburg Hospital, Department of Internal Medicine, 111 South Front Street, Harrisburg, PA 17101, "Update on treatment of hereditary angioedema" Buyantseva, Larisa, Sardana, Niti and Craig, Timothy
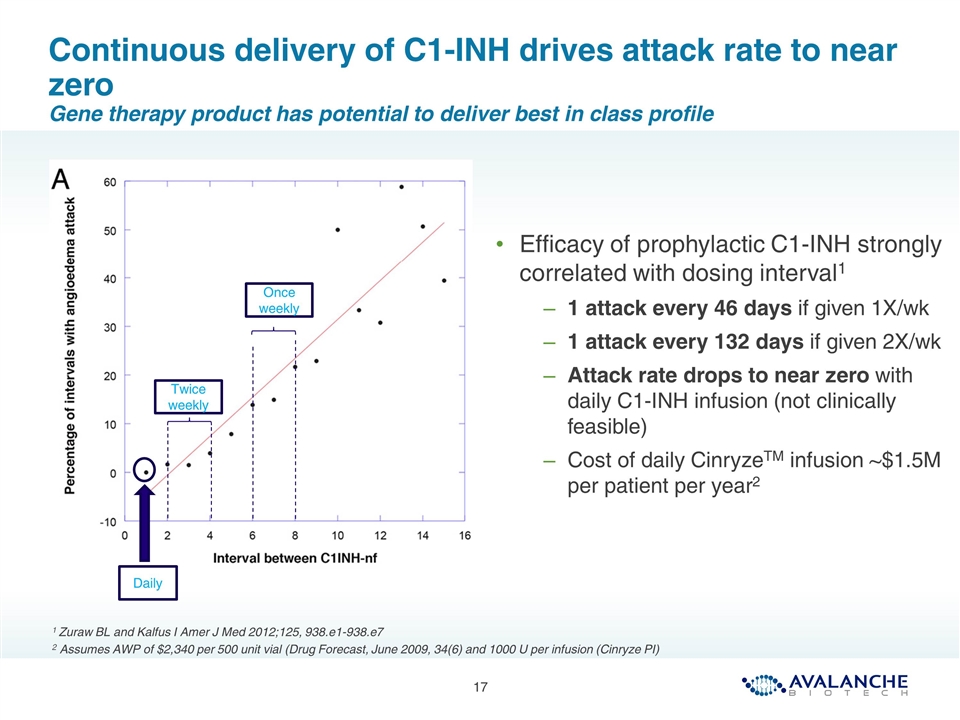
Continuous delivery of C1-INH drives attack rate to near zero Gene therapy product has potential to deliver best in class profile Once weekly Daily Efficacy of prophylactic C1-INH strongly correlated with dosing interval1 1 attack every 46 days if given 1X/wk 1 attack every 132 days if given 2X/wk Attack rate drops to near zero with daily C1-INH infusion (not clinically feasible) Cost of daily CinryzeTM infusion ~$1.5M per patient per year2 1 Zuraw BL and Kalfus I Amer J Med 2012;125, 938.e1-938.e7 2 Assumes AWP of $2,340 per 500 unit vial (Drug Forecast, June 2009, 34(6) and 1000 U per infusion (Cinryze PI) Twice weekly
ANN-002 for HAE: Overview Validated target: genetic disease caused by lack of C1-esterase inhibitor (C1-INH) Continuous levels of C1-INH in HAE patients reduce attack rate to near zero Rationale for Gene Therapy Approach Preliminary studies in relevant animal models suggest an AAV gene transfer vector may provide persistent levels of human C1-esterase inhibitor which could potentially correct the deficiency state Proof-of-Concept 5,500 patients in the US undergoing treatment1 Only ~1000 patients on prophylactic therapy, remainder treat attacks as they occur Potential to significantly expand prophylactic market Significant Market Opportunity Preclinical proof-of-concept established Patient enrollment targeted for 2017 Status 1Decision Resources Group; Wu, Jing; Anderson, Sarah. November 2015
I. ANN-001 for α1 Antitrypsin Deficiency (A1AT) ANN-003 for Friedreich’s Ataxia (FA) IV. ANN-004 for Severe Allergy II. ANN-002 for Hereditary Angioedema (HAE) Annapurna Pipeline in Detail
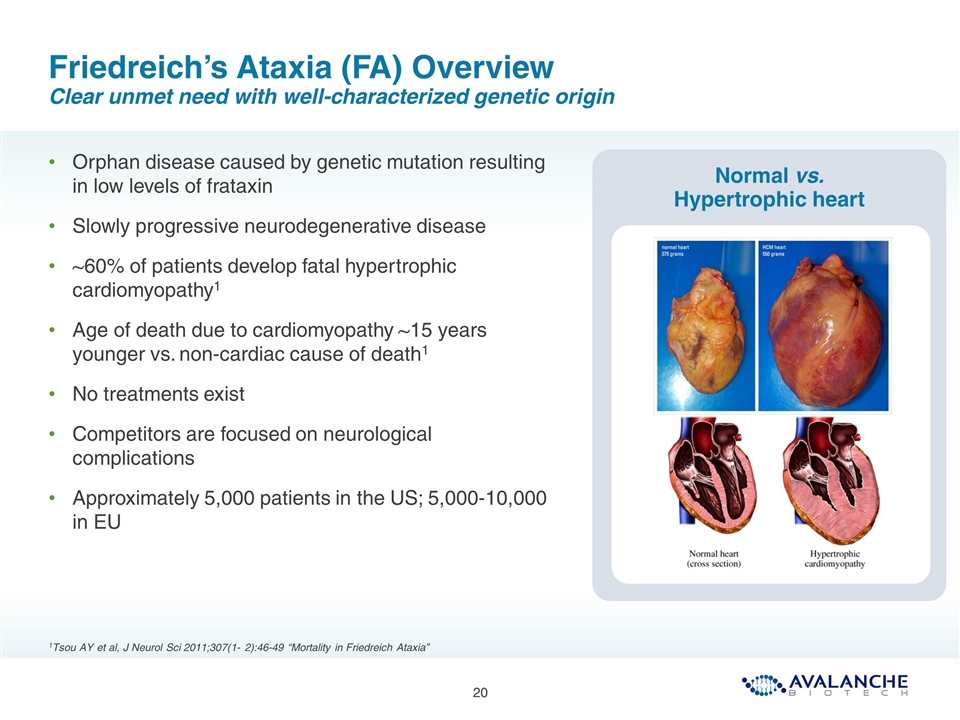
Friedreich’s Ataxia (FA) Overview Clear unmet need with well-characterized genetic origin 1Tsou AY et al, J Neurol Sci 2011;307(1- 2):46-49 “Mortality in Friedreich Ataxia” test Normal vs. Hypertrophic heart Orphan disease caused by genetic mutation resulting in low levels of frataxin Slowly progressive neurodegenerative disease ~60% of patients develop fatal hypertrophic cardiomyopathy1 Age of death due to cardiomyopathy ~15 years younger vs. non-cardiac cause of death1 No treatments exist Competitors are focused on neurological complications Approximately 5,000 patients in the US; 5,000-10,000 in EU
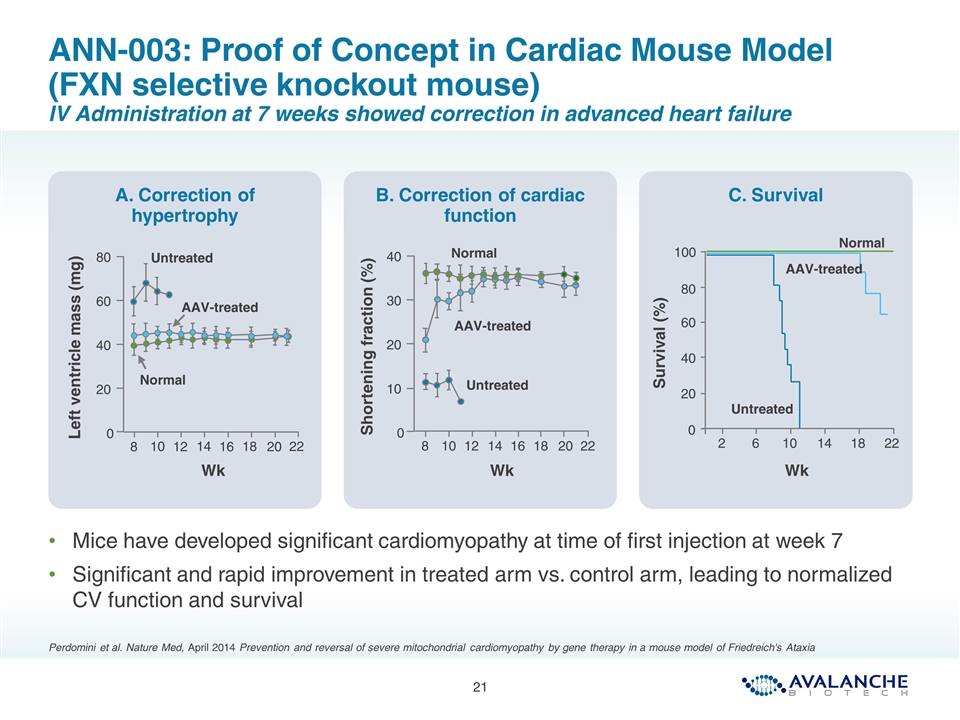
ANN-003: Proof of Concept in Cardiac Mouse Model (FXN selective knockout mouse) IV Administration at 7 weeks showed correction in advanced heart failure Mice have developed significant cardiomyopathy at time of first injection at week 7 Significant and rapid improvement in treated arm vs. control arm, leading to normalized CV function and survival Perdomini et al. Nature Med, April 2014 Prevention and reversal of severe mitochondrial cardiomyopathy by gene therapy in a mouse model of Friedreich's Ataxia A. Correction of hypertrophy B. Correction of cardiac function C. Survival Untreated AAV-treated Normal Wk 22 18 14 10 20 16 12 8 0 80 60 40 20 Left ventricle mass (mg) 22 18 14 6 10 2 100 80 40 20 0 60 Survival (%) Untreated AAV-treated Normal Wk 40 30 20 10 0 22 18 14 10 20 16 12 8 Shortening fraction (%) Untreated AAV-treated Normal Wk
ANN-003 for Friedreich's Ataxia: Overview Validated target: disease driven by mutation in single gene (FXN) AAV vector delivers FXN gene to cardiomyocytes to restore mitochondrial function Rationale for Gene Therapy Approach Pre-clinical POC established in robust cardiac mouse model Prevention of cardiomyopathy and reversal of preexisting cardiomyopathy observed Proof-of-Concept ~5,000 patients in the US, ~5,000-10,000 patients in the EU Clear unmet medical need with no currently available treatment Significant Market Opportunity Large animal studies and observational patient studies currently underway Guidance on timeline to be provided in H2 2016 Status
I. ANN-001 for α1 Antitrypsin Deficiency (A1AT) III. ANN-003 for Friedreich’s Ataxia (FA) ANN-004 for Severe Allergy II. ANN-002 for Hereditary Angioedema (HAE) Annapurna Pipeline in Detail

Severe Allergy Overview Large unmet clinical need with no well established therapies Severe allergies cause discomfort and can be fatal Most common cause of anaphylaxis in children presenting to the ER Majority of fatalities due to accidental exposure in patients with known allergies No established therapy Therapeutic approaches include: Strict avoidance Epinephrine Desensitization
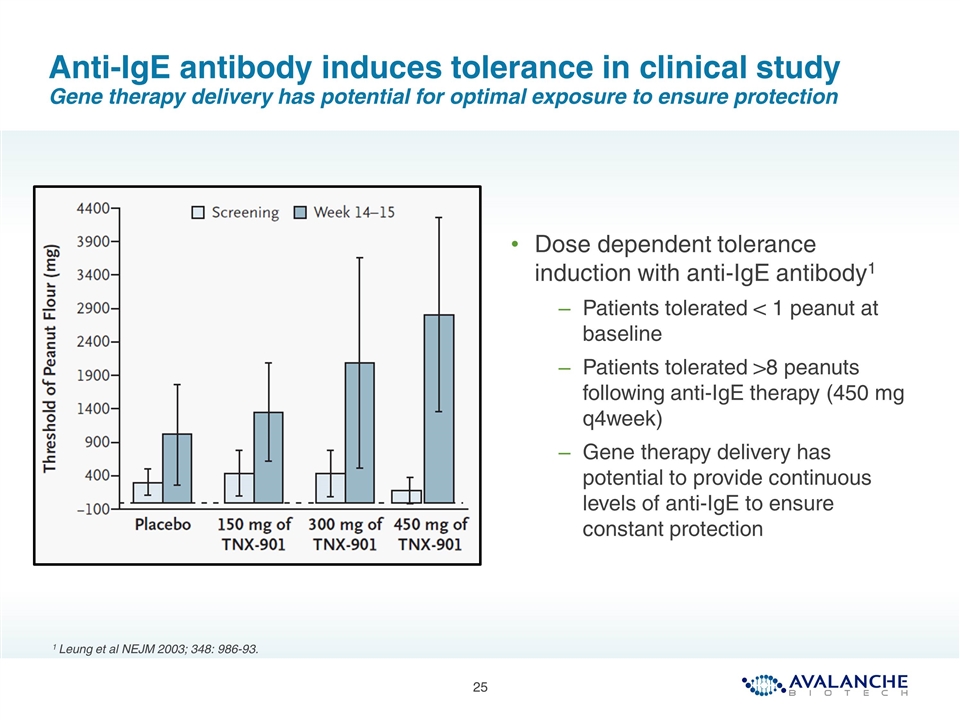
Anti-IgE antibody induces tolerance in clinical study Gene therapy delivery has potential for optimal exposure to ensure protection Dose dependent tolerance induction with anti-IgE antibody1 Patients tolerated < 1 peanut at baseline Patients tolerated >8 peanuts following anti-IgE therapy (450 mg q4week) Gene therapy delivery has potential to provide continuous levels of anti-IgE to ensure constant protection 1 Leung et al NEJM 2003; 348: 986-93.
ANN-004 for Severe Allergy: Overview Provide continuous levels of anti-IgE antibody to prevent severe reaction when patient exposed to allergen(s) Rationale for Gene Therapy Approach Clinical studies with anti-IgE antibody demonstrate induction of tolerance Protection demonstrated in fatal pre-clinical peanut allergic mouse model Proof-of-Concept Most common cause of anaphylaxis in children under 20 Approximately 20,000 children under 20 hospitalized annually in US alone due to anaphylactic shock1 Market Opportunity Preclinical studies ongoing Status 1 Kivisto et al, Allergy, 2016; FARE: Food Allergy and Statistics for the U.S.

Wet AMD
Wet AMD Gene Therapy: Next Steps Comprehensive pre-clinical wAMD research ongoing Seeking optimal vector, with optimal molecule utilizing best route of delivery Evaluating best path forward considering: Protein expression Consistent delivery Optimal method of administration Next generation vector technology Update expected mid 2016
Color Vision Deficiency Program

AVA-322L and AVA-323M Product Overview for Color Vision Deficiency 1Sharpe LT, Stockman A, Jagle H, Nathans J. Opsin genes, photopigments, color vision and color blindness. In: Gegenfurtner KR, Sharpe LT (eds.) Color Vision. Cambridge UP: Cambridge, 1999. Intravitreal injection to confer better color discrimination in patients with L-opsin or M-opsin deficiency Rationale for Gene Therapy Approach Color blind squirrel monkeys Efficient delivery to old-world monkeys following intravitreal injection Proof-of-Concept 10 million US, >20 million in US, Europe, Japan1 No treatments on market or in development Market Opportunity Ongoing preclinical studies Status

Regeneron Collaboration
Regeneron Collaboration (May 2014) Covers up to eight distinct therapeutic targets AVA-311 for juvenile X-Linked Retinoschisis (XLRS) is first collaboration program REGN has chosen 3 other candidates that remain undisclosed Avalanche option to share up to 35% on profits and development costs for 2 targets Directed Evolution Platform & Gene Therapy Expertise Proprietary Molecules & Ophthalmology Expertise
AVA-311 Product Overview for XLRS Potential long-term treatment of juvenile X-linked retinoschisis (XLRS) Optimized AAV vector delivers RS1 gene to maintain integrity of retina Rationale for Gene Therapy Approach High levels of RS1 protein expression in mouse model of XLRS Restoration of normal retina appearance Demonstration of improvement in vision after 1 month, with improvement maintained over 4 months Proof-of-Concept XLRS is an orphan disease that affects approximately 10,000 boys and young men No currently approved therapies Market Opportunity Partnered with Regeneron as part of May 2014 collaboration Status
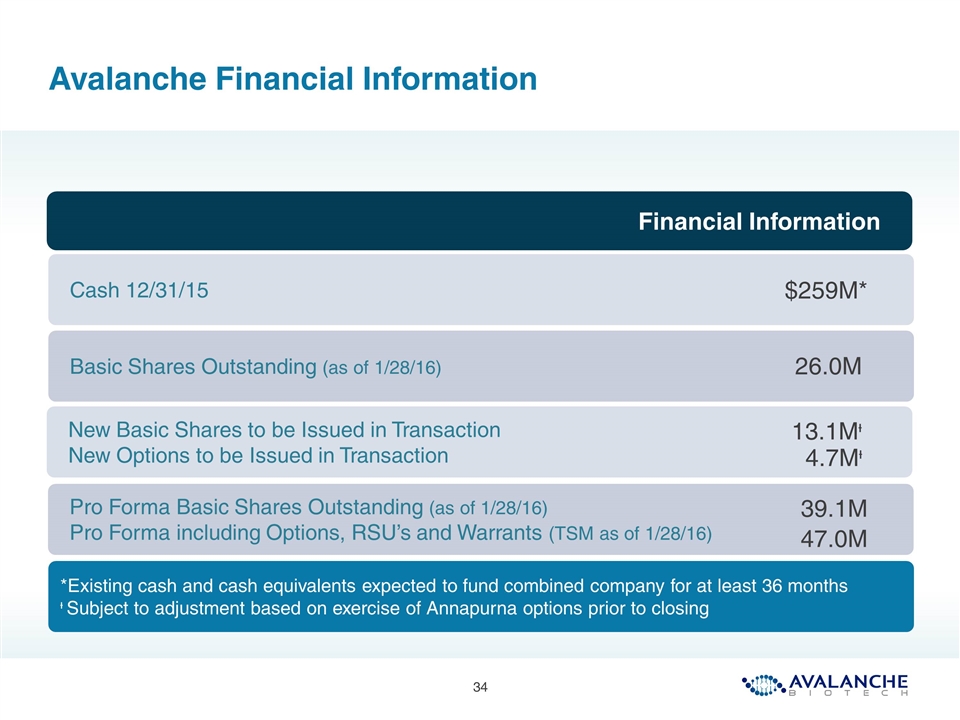
Avalanche Financial Information Basic Shares Outstanding (as of 1/28/16) New Basic Shares to be Issued in Transaction New Options to be Issued in Transaction Pro Forma Basic Shares Outstanding (as of 1/28/16) Pro Forma including Options, RSU’s and Warrants (TSM as of 1/28/16) Financial Information *Existing cash and cash equivalents expected to fund combined company for at least 36 months ᵻ Subject to adjustment based on exercise of Annapurna options prior to closing 26.0M 47.0M 13.1M Cash 12/31/15 $259M* 4.7M 39.1M
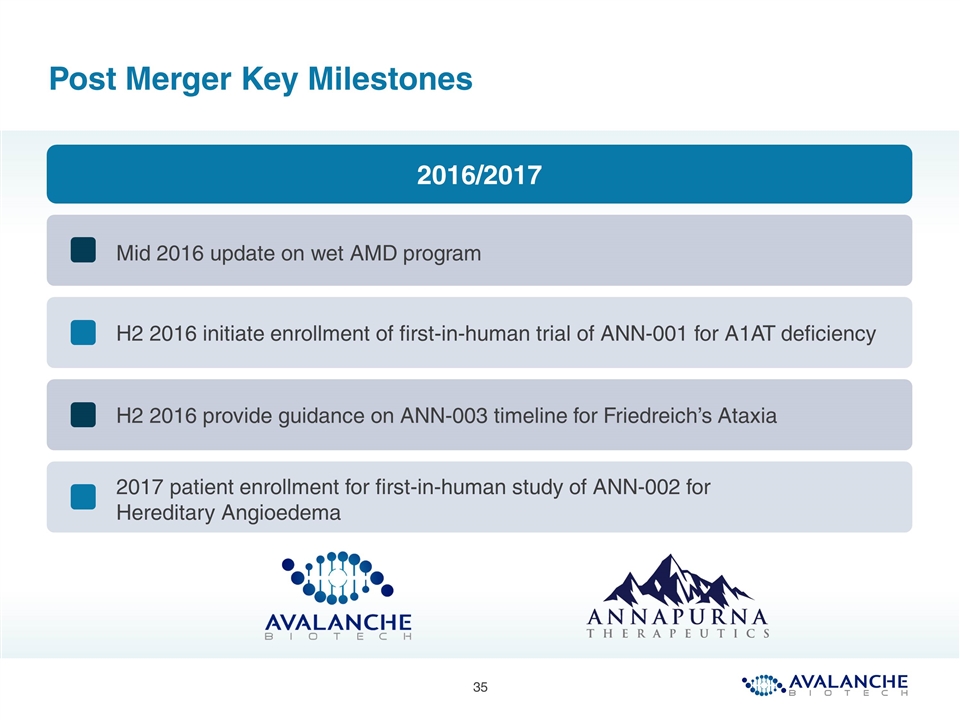
Post Merger Key Milestones Mid 2016 update on wet AMD program H2 2016 initiate enrollment of first-in-human trial of ANN-001 for A1AT deficiency H2 2016 provide guidance on ANN-003 timeline for Friedreich’s Ataxia 2016/2017 2017 patient enrollment for first-in-human study of ANN-002 for Hereditary Angioedema




































