
Gaining Momentum in Gene Therapy Exhibit 99.1

Forward-Looking Statements Statements contained in this document regarding matters that are not historical facts are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Such statements include, but are not limited to, statements regarding Adverum Biotechnologies, Inc.’s (“Adverum”) plans, potential opportunities, expectations, projections, goals, objectives, milestones, strategies, product pipeline, the sufficiency of its resources to fund the advancement of any development program or the completion of any clinical trials, and the safety, efficacy, and projected development timeline and commercial potential of products under development, all of which are based on certain assumptions made by us on current conditions, expected future developments and other factors we believe are appropriate in the circumstances. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without limitation, risks and uncertainties inherent in the product development and the regulatory approval process, delays in clinical trials and other matters that could affect the availability or commercial potential of product candidates, the ability to project future cash utilization and reserves needed for contingent future liabilities and business operations, the availability of sufficient resources for its operations and to conduct or continue planned development programs and planned clinical trials and the ability to successfully develop any of its product candidates. Risks and uncertainties facing Adverum are described more fully in Adverum’s periodic reports filed with the SEC. All forward-looking statements contained in this press release speak only as of the date on which they were made. Adverum undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made. This document contains estimates, projections and other information concerning Adverum’s industry, business and the markets for certain drugs, including data regarding the estimated size of those markets, their projected growth rates and the incidence of certain medical conditions. Information that is based on estimates, forecasts, projections or similar methodologies is inherently subject to uncertainties and actual events or circumstances may differ materially from events and circumstances reflected in this information. Unless otherwise expressly stated, we obtained this industry, business, market and other data from reports, research surveys, studies and similar data prepared by third parties, industry, medical and general publications, government data and similar sources believed to be reliable, but the accuracy or completeness of such information is not guaranteed by, and should not be construed as representations made by Adverum.
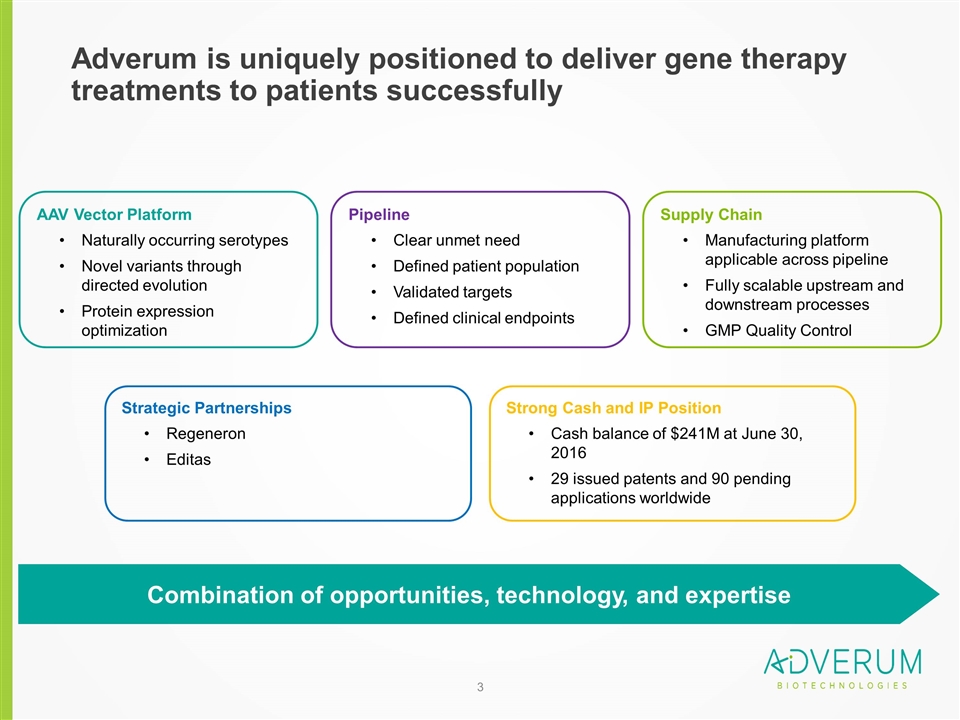
Adverum is uniquely positioned to deliver gene therapy treatments to patients successfully Supply Chain Manufacturing platform applicable across pipeline Fully scalable upstream and downstream processes GMP Quality Control Pipeline Clear unmet need Defined patient population Validated targets Defined clinical endpoints AAV Vector Platform Naturally occurring serotypes Novel variants through directed evolution Protein expression optimization Combination of opportunities, technology, and expertise Strategic Partnerships Regeneron Editas Strong Cash and IP Position Cash balance of $241M at June 30, 2016 29 issued patents and 90 pending applications worldwide
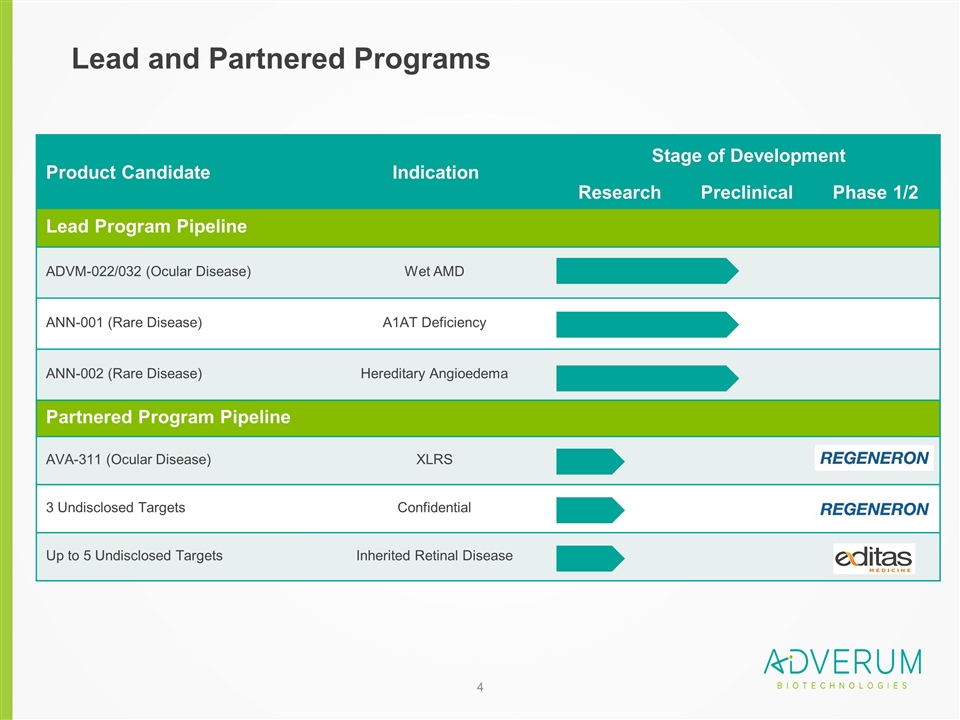
Product Candidate Indication Stage of Development Research Preclinical Phase 1/2 Lead Program Pipeline ADVM-022/032 (Ocular Disease) Wet AMD ANN-001 (Rare Disease) A1AT Deficiency ANN-002 (Rare Disease) Hereditary Angioedema Partnered Program Pipeline AVA-311 (Ocular Disease) XLRS 3 Undisclosed Targets Confidential Up to 5 Undisclosed Targets Inherited Retinal Disease Lead and Partnered Programs
Lead Programs ADVM-022 and ADVM-032 for wet Age-Related Macular Degeneration (wAMD) III. ANN-002 for Hereditary Angioedema (HAE) II. ANN-001 for Alpha-1 Antitrypsin Deficiency (A1AT)
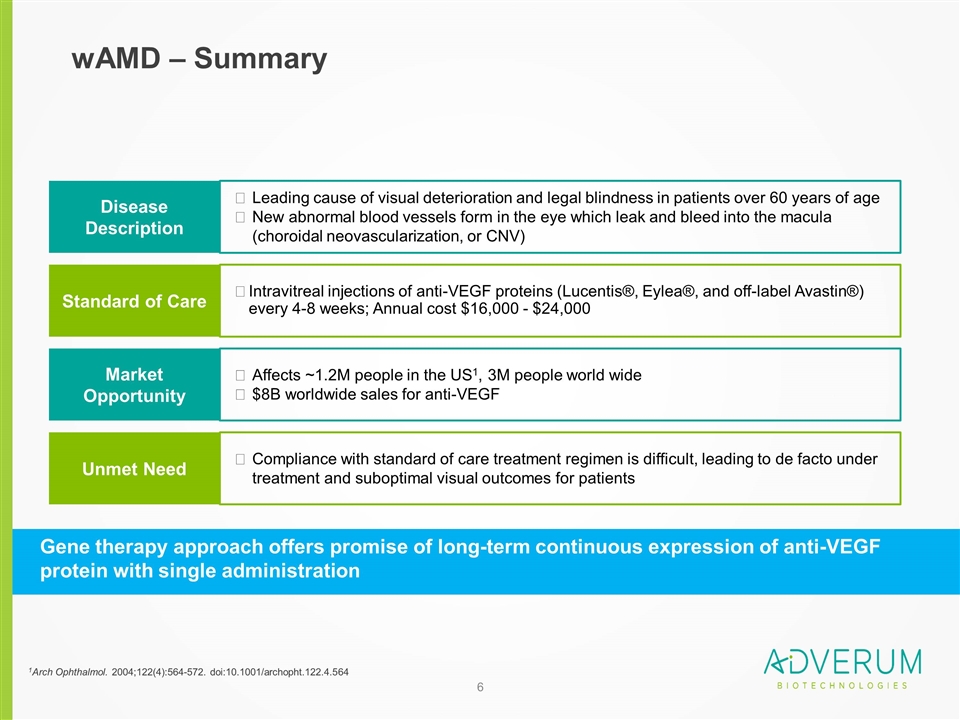
wAMD – Summary 1Arch Ophthalmol. 2004;122(4):564-572. doi:10.1001/archopht.122.4.564 Disease Description Leading cause of visual deterioration and legal blindness in patients over 60 years of age New abnormal blood vessels form in the eye which leak and bleed into the macula (choroidal neovascularization, or CNV) Standard of Care Intravitreal injections of anti-VEGF proteins (Lucentis®, Eylea®, and off-label Avastin®) every 4-8 weeks; Annual cost $16,000 - $24,000 Market Opportunity Affects ~1.2M people in the US1, 3M people world wide $8B worldwide sales for anti-VEGF Unmet Need Compliance with standard of care treatment regimen is difficult, leading to de facto under treatment and suboptimal visual outcomes for patients Gene therapy approach offers promise of long-term continuous expression of anti-VEGF protein with single administration
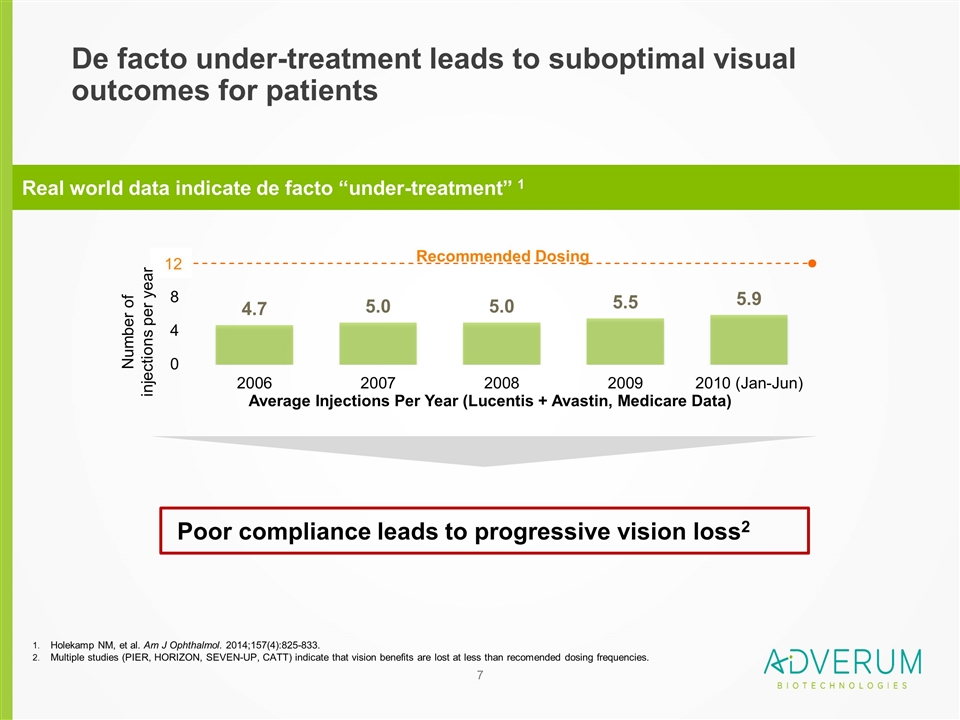
De facto under-treatment leads to suboptimal visual outcomes for patients Average Injections Per Year (Lucentis + Avastin, Medicare Data) Recommended Dosing 12 Number of injections per year Real world data indicate de facto “under-treatment” 1 Holekamp NM, et al. Am J Ophthalmol. 2014;157(4):825-833. Multiple studies (PIER, HORIZON, SEVEN-UP, CATT) indicate that vision benefits are lost at less than recomended dosing frequencies. Poor compliance leads to progressive vision loss2
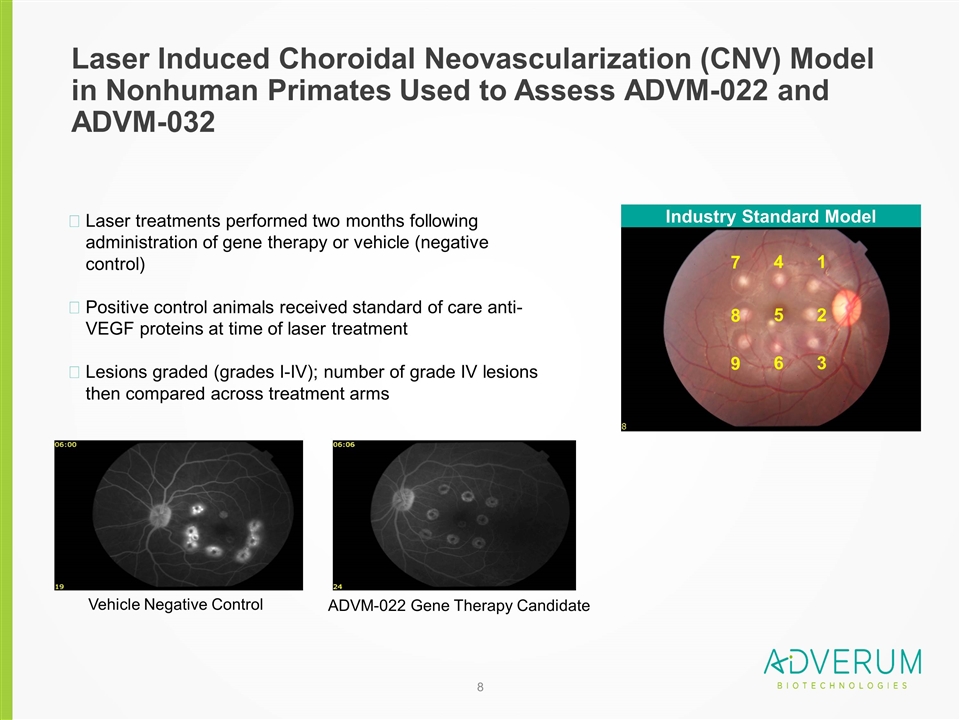
Laser Induced Choroidal Neovascularization (CNV) Model in Nonhuman Primates Used to Assess ADVM-022 and ADVM-032 Laser treatments performed two months following administration of gene therapy or vehicle (negative control) Positive control animals received standard of care anti-VEGF proteins at time of laser treatment Lesions graded (grades I-IV); number of grade IV lesions then compared across treatment arms 1 2 3 4 5 6 7 8 9 Vehicle Negative Control ADVM-022 Gene Therapy Candidate Industry Standard Model
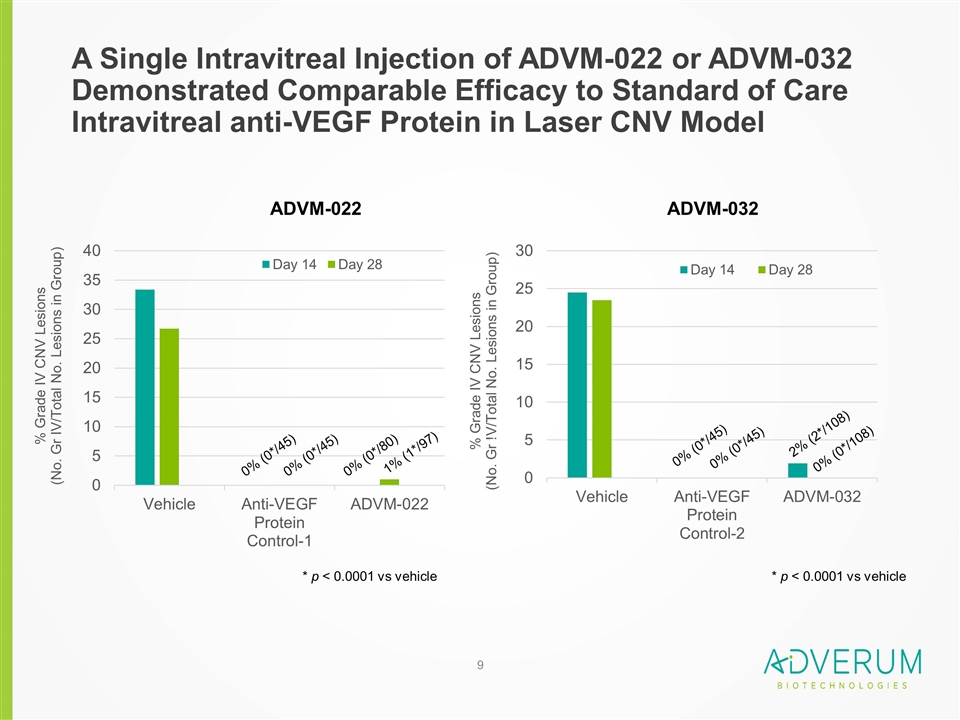
A Single Intravitreal Injection of ADVM-022 or ADVM-032 Demonstrated Comparable Efficacy to Standard of Care Intravitreal anti-VEGF Protein in Laser CNV Model ADVM-022 ADVM-032 * p < 0.0001 vs vehicle * p < 0.0001 vs vehicle
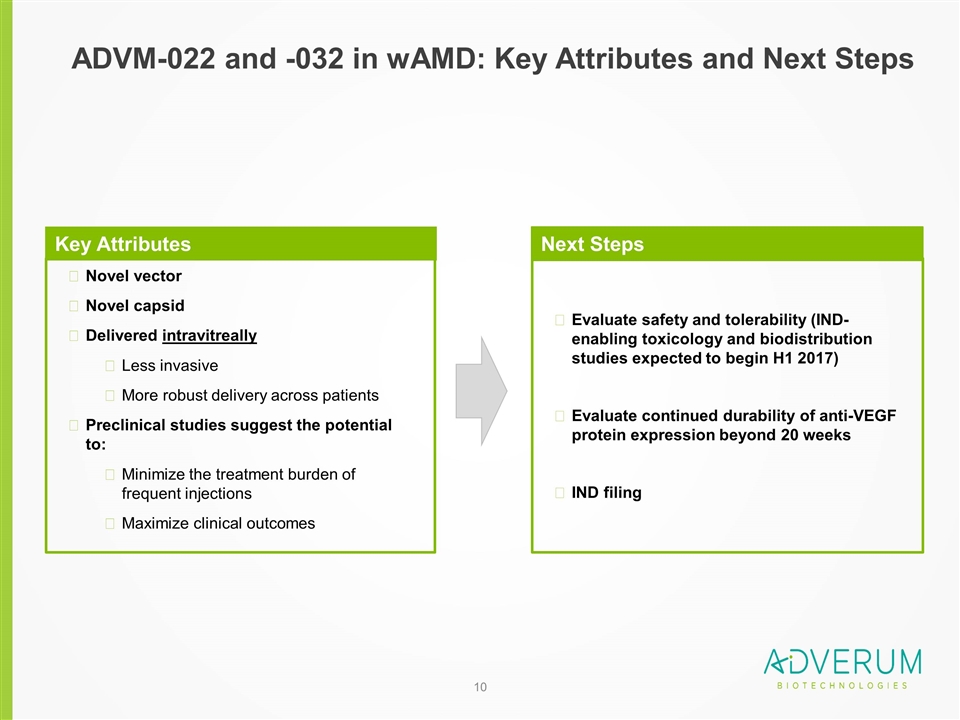
ADVM-022 and -032 in wAMD: Key Attributes and Next Steps Novel vector Novel capsid Delivered intravitreally Less invasive More robust delivery across patients Preclinical studies suggest the potential to: Minimize the treatment burden of frequent injections Maximize clinical outcomes Key Attributes Next Steps Evaluate safety and tolerability (IND-enabling toxicology and biodistribution studies expected to begin H1 2017) Evaluate continued durability of anti-VEGF protein expression beyond 20 weeks IND filing

Focus on Manufacturing to Ensure Product Supply Throughout Product Development Life Cycle and Beyond In house process development capabilities to deliver turn-key large scale process to CMO Scalable upstream process using the baculovirus/SF9 system of expression for AAV production State-of-the-art bioindustry technology for AAV purification AAV drug product formulation capabilities allowing for increased vector particle concentration Process applicable to the different AAV serotypes used across our pipeline Assay development for AAV product characterization In-house GMP Quality Control

Strong Emphasis on Discovery of Novel Vectors and Expression Cassettes to Improve on Existing Technology Next generation vectors through Directed Evolution and Rational Design of AAV capsids for better transduction efficiency and antibody neutralization profiles Discovery of improved ubiquitous and cell-specific promoters and expression cassettes for optimal transgene expression upon transduction in target tissue
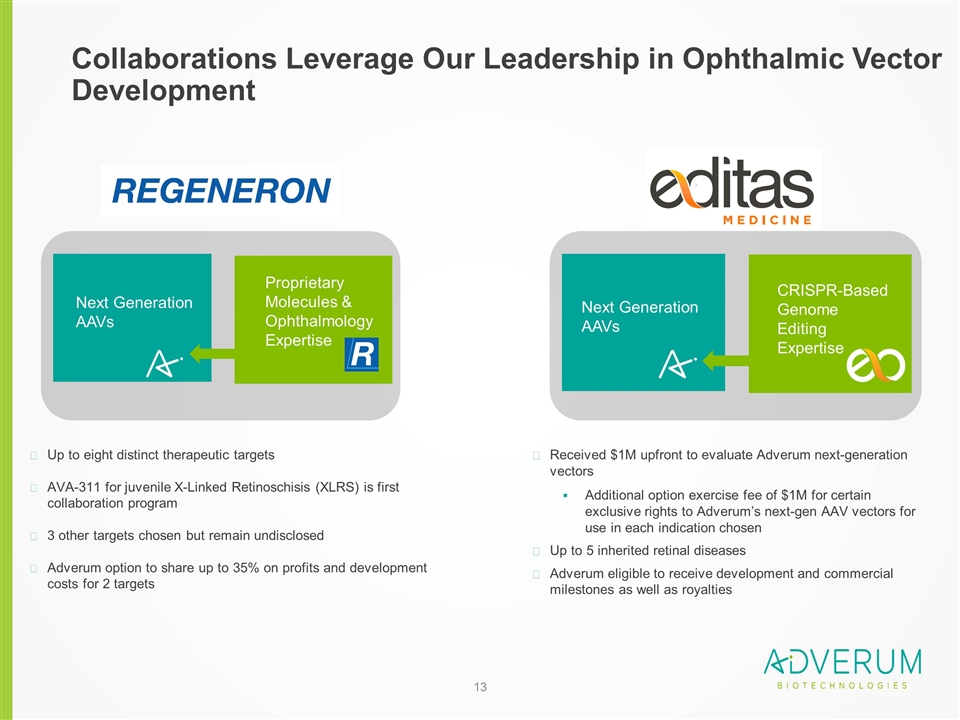
Collaborations Leverage Our Leadership in Ophthalmic Vector Development Up to eight distinct therapeutic targets AVA-311 for juvenile X-Linked Retinoschisis (XLRS) is first collaboration program 3 other targets chosen but remain undisclosed Adverum option to share up to 35% on profits and development costs for 2 targets Received $1M upfront to evaluate Adverum next-generation vectors Additional option exercise fee of $1M for certain exclusive rights to Adverum’s next-gen AAV vectors for use in each indication chosen Up to 5 inherited retinal diseases Adverum eligible to receive development and commercial milestones as well as royalties Next Generation AAVs CRISPR-Based Genome Editing Expertise Proprietary Molecules & Ophthalmology Expertise Next Generation AAVs
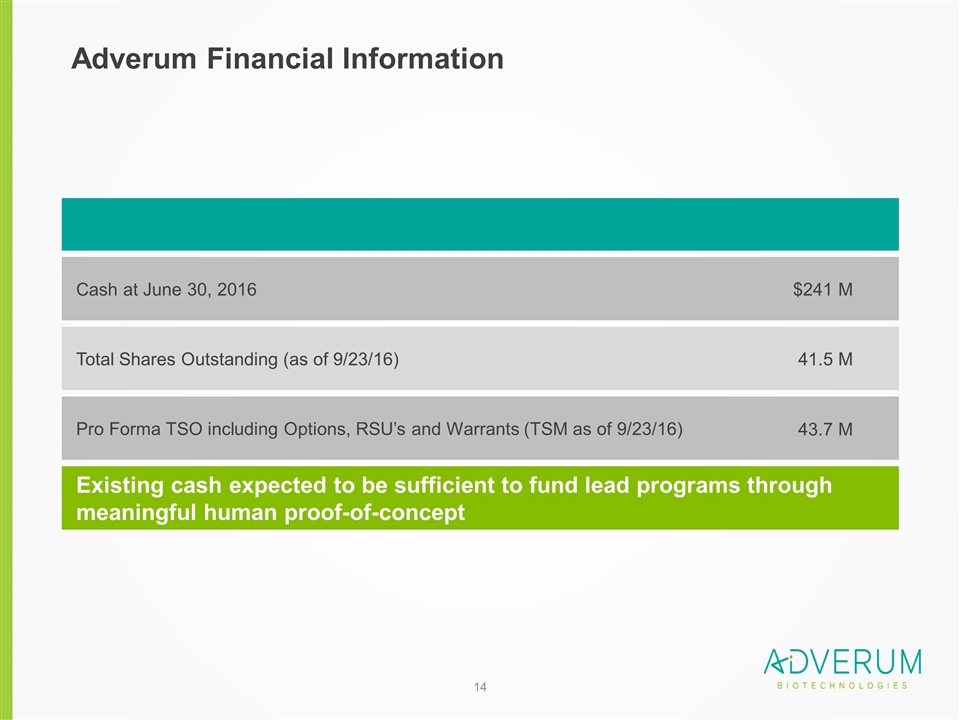
Adverum Financial Information Total Shares Outstanding (as of 9/23/16) 41.5 M Existing cash expected to be sufficient to fund lead programs through meaningful human proof-of-concept Pro Forma TSO including Options, RSU’s and Warrants (TSM as of 9/23/16) Cash at June 30, 2016 $241 M 43.7 M















