
ISOO 2022 New Developments in belzupacap sarotalocan (AU-011), an Investigational Virus-Like Drug Conjugate (VDC) in Ocular Oncology Exhibit 99.2

Legal Disclosure This presentation contains forward-looking statements, all of which are qualified in their entirety by this cautionary statement. Many of the forward-looking statements contained herein can be identified by the use of forward-looking words such as "may", "anticipate", "believe", "could', "expect", "should", "plan", "intend", "estimate", "will", "potential" and "ongoing", among others, although not all forward-looking statements contain these identifying words. These forward-looking statements include statements about the initiation, timing, progress, results, and cost of our research and development programs and our current and future preclinical studies and clinical trials, including statements regarding the timing of initiation and completion of studies or trials and related preparatory work, the period during which the results of the trials will become available, and our research and development programs; our ability to successfully manufacture our drug substances and product candidates for preclinical use, for clinical trials and on a larger scale for commercial use, if approved; the ability and willingness of our third-party strategic collaborators to continue research and development activities relating to our development candidates and product candidates; our ability to obtain funding for our operations necessary to complete further development and commercialization of our product candidates; our ability to obtain and maintain regulatory approval of our product candidates; the size and growth potential of the markets for our product candidates, and our ability to serve those markets; our financial performance; the effect of the COVID-19 pandemic, including mitigation efforts and economic effects, on any of the foregoing or other aspects of our business operations, including but not limited to our preclinical studies and clinical trials and any future studies or trials; our ability to commercialize our products, if approved; and the implementation of our business model, and strategic plans for our business and product candidates. Except as otherwise noted, these forward-looking statements speak only as of the date of this presentation, and we undertake no obligation to update or revise any of such statements to reflect events or circumstances occurring after this presentation. Because forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified and some of which are beyond our control, you should not rely on these forward-looking statements as predictions of future events. The events and circumstances reflected in our forward-looking statements may not be achieved or occur and actual results could differ materially from those projected in the forward-looking statements. We caution you not to place undue reliance on the forward-looking statements contained in this presentation. This presentation discusses product candidates that are under preclinical or clinical evaluation and that have not yet been approved for marketing by the U.S. Food and Drug Administration or any other regulatory authority. Until finalized in a clinical study report, clinical trial data presented herein remain subject to adjustment as a result of clinical site audits and other review processes. No representation is made as to the safety or effectiveness of these product candidates for the use for which such product candidates are being studied.

Aura is Dedicated to Science and Supports Collaborative Research Cadmus Rich, MD Chief Medical Officer, Head of R&D Aura Biosciences Ruben Huis in ‘t Veld, MSc Leiden University Anneli Savinainen, MS VP, Head of Preclinical R&D Aura Biosciences Rhonda Kines, PhD Principal Scientist Aura Biosciences Martine Jager, MD, PhD Professor of Ophthalmology Leiden University
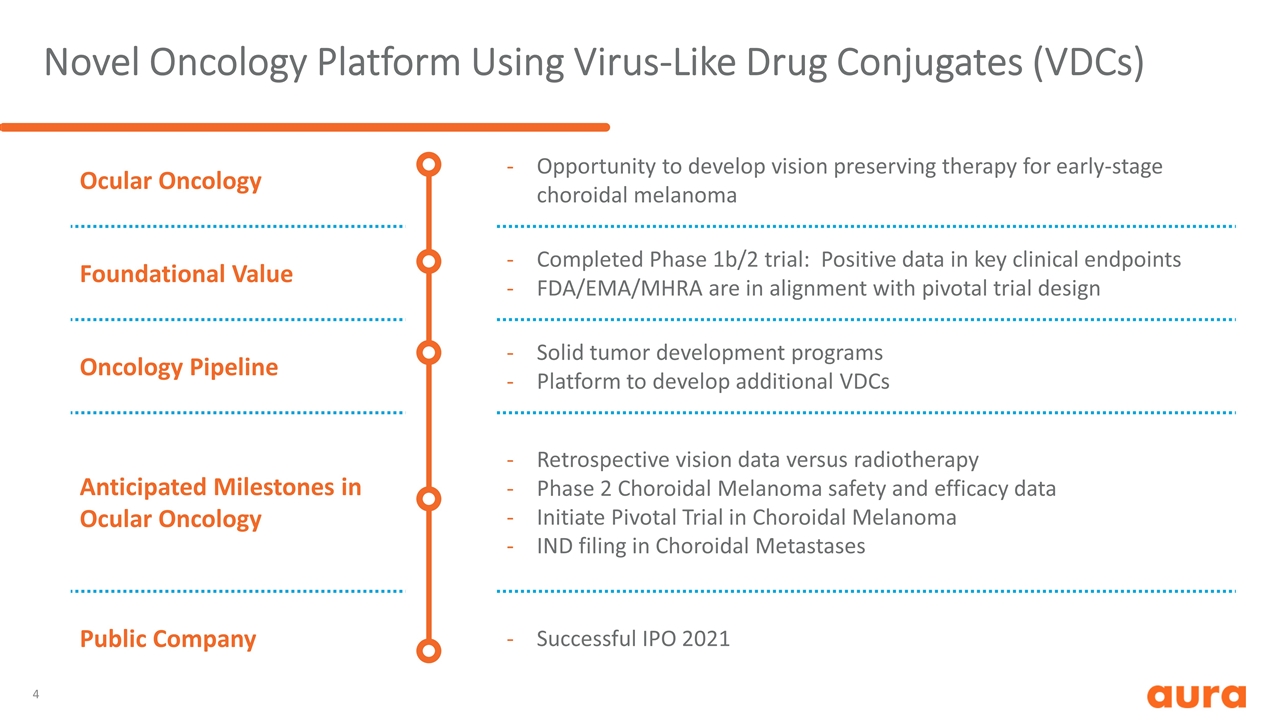
Novel Oncology Platform Using Virus-Like Drug Conjugates (VDCs) Ocular Oncology Opportunity to develop vision preserving therapy for early-stage choroidal melanoma Foundational Value Completed Phase 1b/2 trial: Positive data in key clinical endpoints FDA/EMA/MHRA are in alignment with pivotal trial design Oncology Pipeline Solid tumor development programs Platform to develop additional VDCs Anticipated Milestones in Ocular Oncology Retrospective vision data versus radiotherapy Phase 2 Choroidal Melanoma safety and efficacy data Initiate Pivotal Trial in Choroidal Melanoma IND filing in Choroidal Metastases Public Company Successful IPO 2021
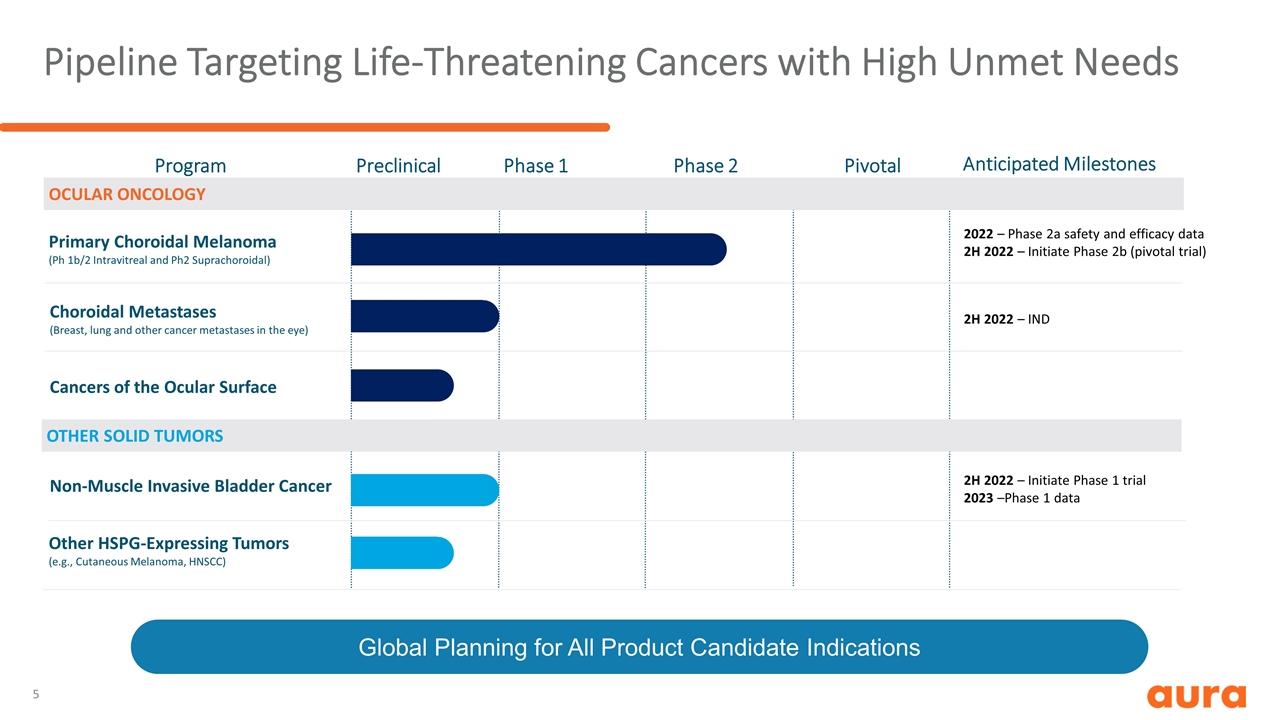
Pipeline Targeting Life-Threatening Cancers with High Unmet Needs Program OTHER SOLID TUMORS OCULAR ONCOLOGY Preclinical Phase 1 Phase 2 Pivotal Anticipated Milestones Choroidal Metastases (Breast, lung and other cancer metastases in the eye) Primary Choroidal Melanoma (Ph 1b/2 Intravitreal and Ph2 Suprachoroidal) Cancers of the Ocular Surface Non-Muscle Invasive Bladder Cancer Other HSPG-Expressing Tumors (e.g., Cutaneous Melanoma, HNSCC) 2022 – Phase 2a safety and efficacy data 2H 2022 – Initiate Phase 2b (pivotal trial) 2H 2022 – IND 2H 2022 – Initiate Phase 1 trial 2023 –Phase 1 data Global Planning for All Product Candidate Indications

Choroidal Metastasis
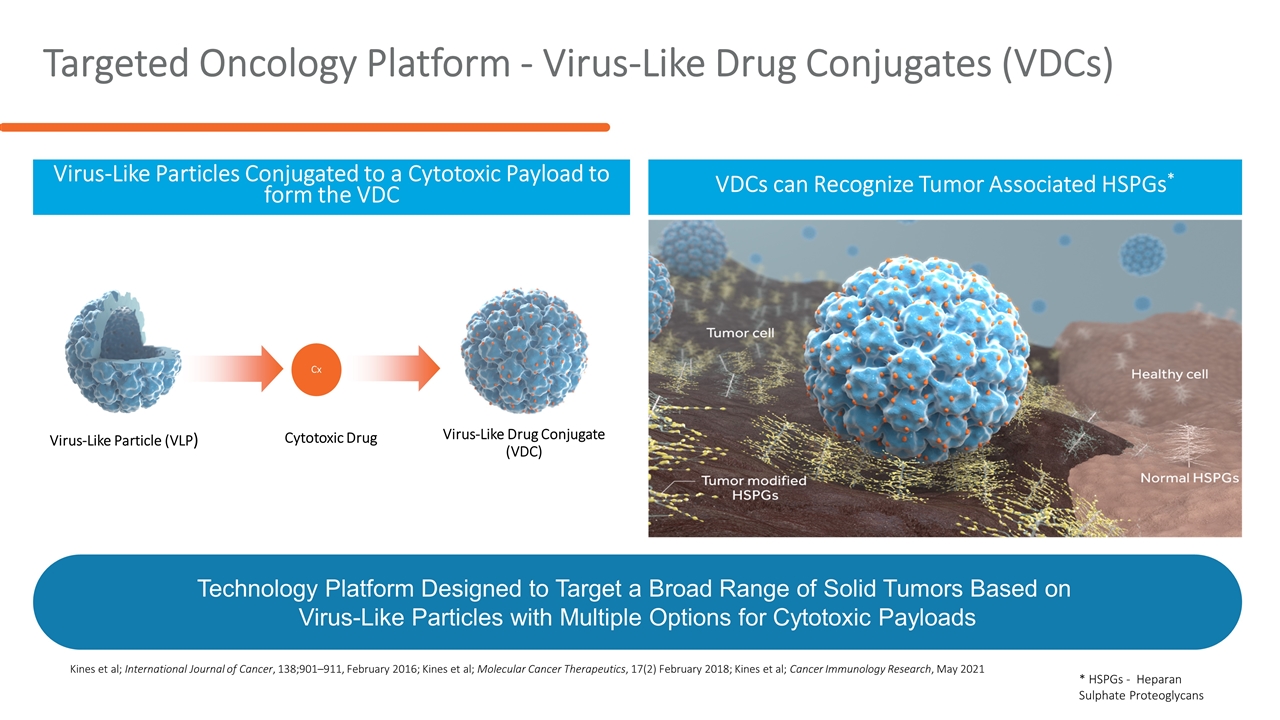
Targeted Oncology Platform - Virus-Like Drug Conjugates (VDCs) Virus-Like Particles Conjugated to a Cytotoxic Payload to form the VDC VDCs can Recognize Tumor Associated HSPGs* Virus-Like Particle (VLP) Virus-Like Drug Conjugate (VDC) Cx Cytotoxic Drug Kines et al; International Journal of Cancer, 138;901–911, February 2016; Kines et al; Molecular Cancer Therapeutics, 17(2) February 2018; Kines et al; Cancer Immunology Research, May 2021 Technology Platform Designed to Target a Broad Range of Solid Tumors Based on Virus-Like Particles with Multiple Options for Cytotoxic Payloads * HSPGs - Heparan Sulphate Proteoglycans
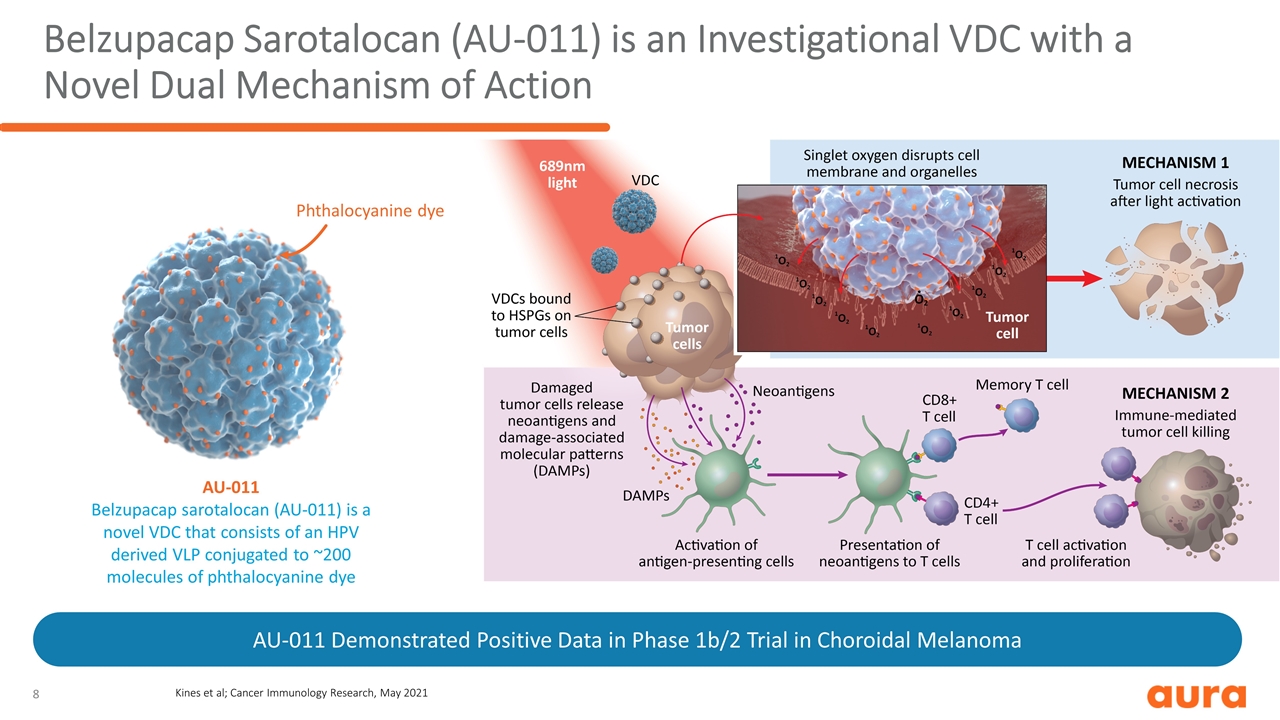
Belzupacap Sarotalocan (AU-011) is an Investigational VDC with a Novel Dual Mechanism of Action Phthalocyanine dye AU-011 Belzupacap sarotalocan (AU-011) is a novel VDC that consists of an HPV derived VLP conjugated to ~200 molecules of phthalocyanine dye AU-011 Demonstrated Positive Data in Phase 1b/2 Trial in Choroidal Melanoma Kines et al; Cancer Immunology Research, May 2021
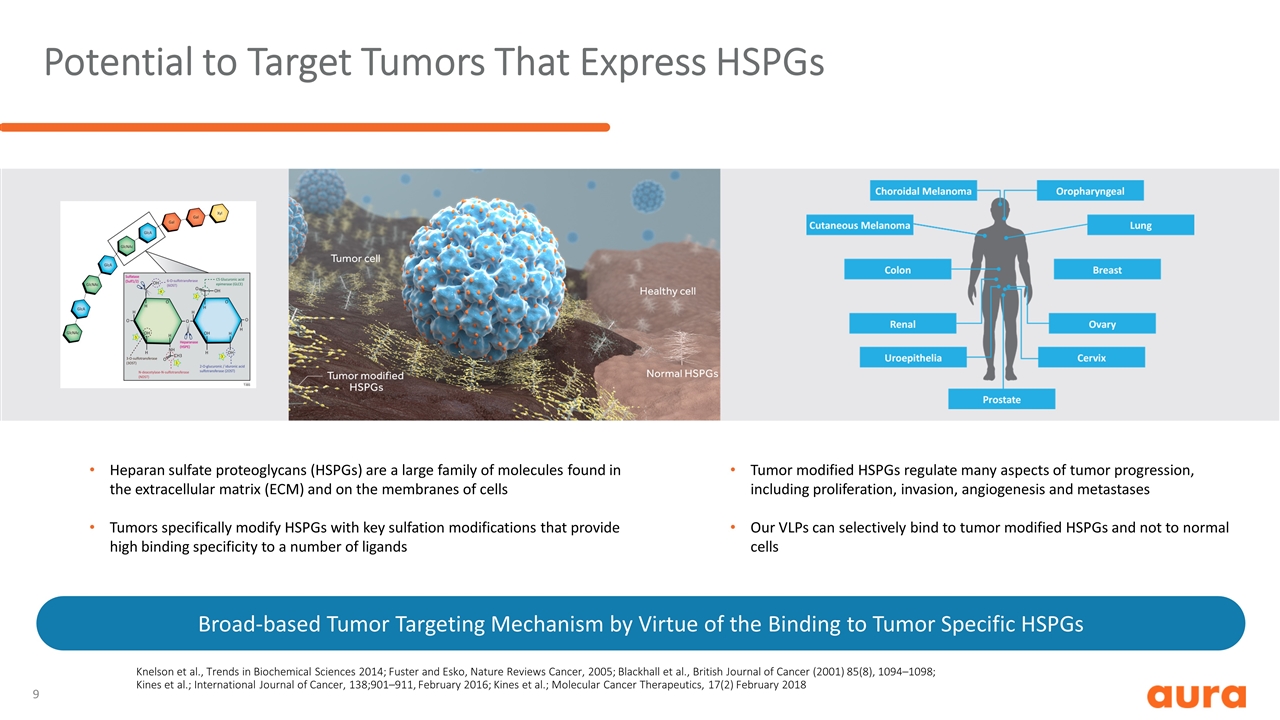
Potential to Target Tumors That Express HSPGs Broad-based Tumor Targeting Mechanism by Virtue of the Binding to Tumor Specific HSPGs Knelson et al., Trends in Biochemical Sciences 2014; Fuster and Esko, Nature Reviews Cancer, 2005; Blackhall et al., British Journal of Cancer (2001) 85(8), 1094–1098; Kines et al.; International Journal of Cancer, 138;901–911, February 2016; Kines et al.; Molecular Cancer Therapeutics, 17(2) February 2018 Heparan sulfate proteoglycans (HSPGs) are a large family of molecules found in the extracellular matrix (ECM) and on the membranes of cells Tumors specifically modify HSPGs with key sulfation modifications that provide high binding specificity to a number of ligands Tumor modified HSPGs regulate many aspects of tumor progression, including proliferation, invasion, angiogenesis and metastases Our VLPs can selectively bind to tumor modified HSPGs and not to normal cells
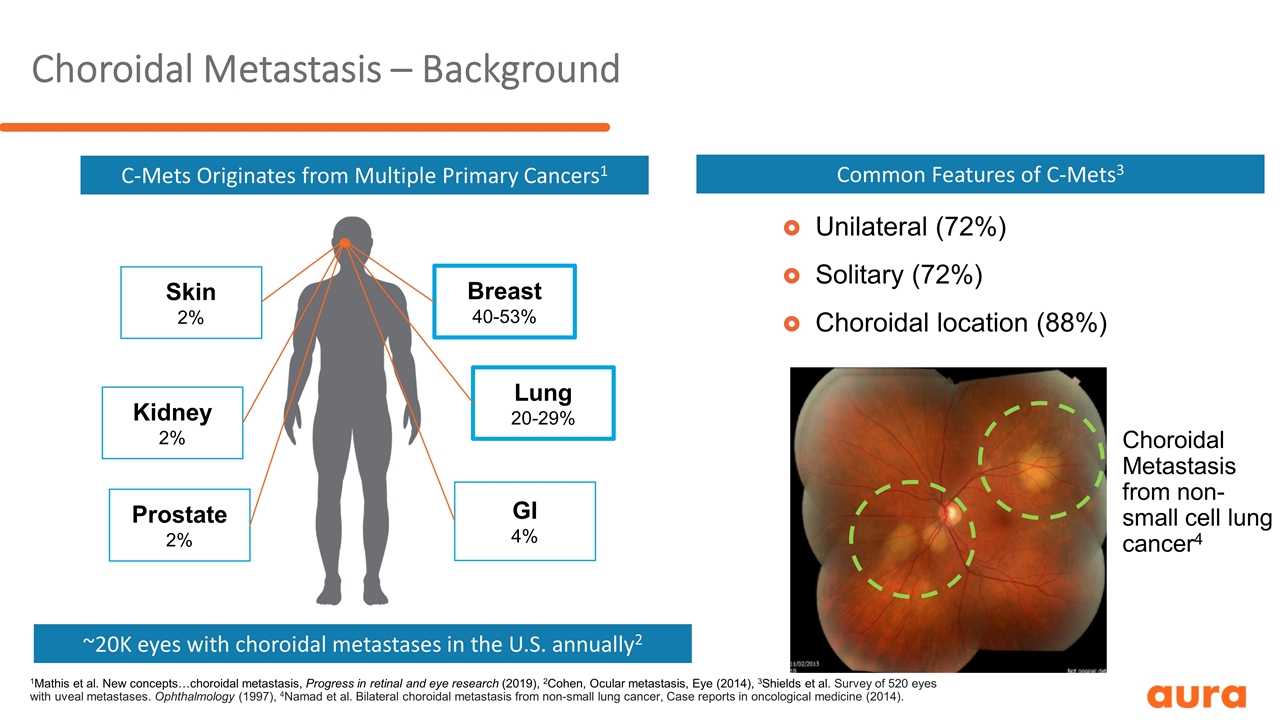
Choroidal Metastasis – Background Unilateral (72%) Unilateral (72%) Solitary (72%) Choroidal location (88%) Choroidal Metastasis from non-small cell lung cancer4 C-Mets Originates from Multiple Primary Cancers1 ~20K eyes with choroidal metastases in the U.S. annually2 1Mathis et al. New concepts…choroidal metastasis, Progress in retinal and eye research (2019), 2Cohen, Ocular metastasis, Eye (2014), 3Shields et al. Survey of 520 eyes with uveal metastases. Ophthalmology (1997), 4Namad et al. Bilateral choroidal metastasis from non-small lung cancer, Case reports in oncological medicine (2014). Breast 40-53% Lung 20-29% GI 4% Kidney 2% Prostate 2% Skin 2% Common Features of C-Mets3
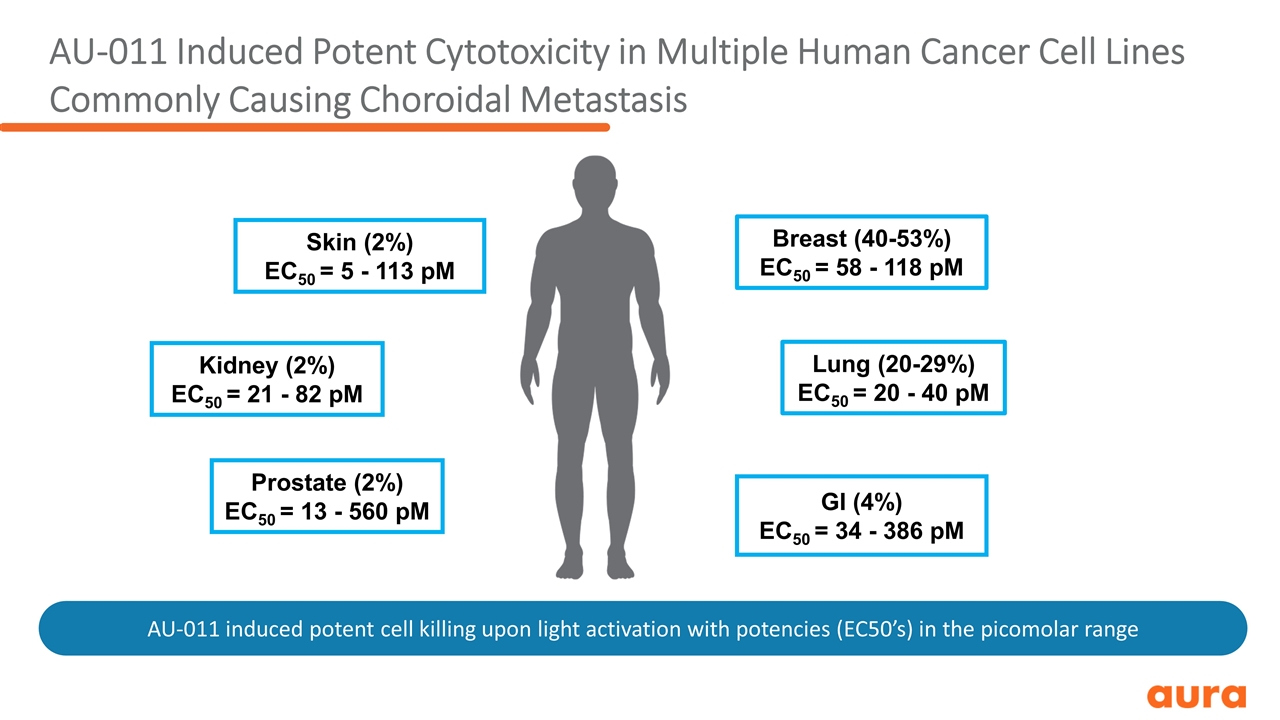
AU-011 Induced Potent Cytotoxicity in Multiple Human Cancer Cell Lines Commonly Causing Choroidal Metastasis Breast (40-53%) EC50 = 58 - 118 pM Lung (20-29%) EC50 = 20 - 40 pM GI (4%) EC50 = 34 - 386 pM Kidney (2%) EC50 = 21 - 82 pM Prostate (2%) EC50 = 13 - 560 pM Skin (2%) EC50 = 5 - 113 pM AU-011 induced potent cell killing upon light activation with potencies (EC50’s) in the picomolar range
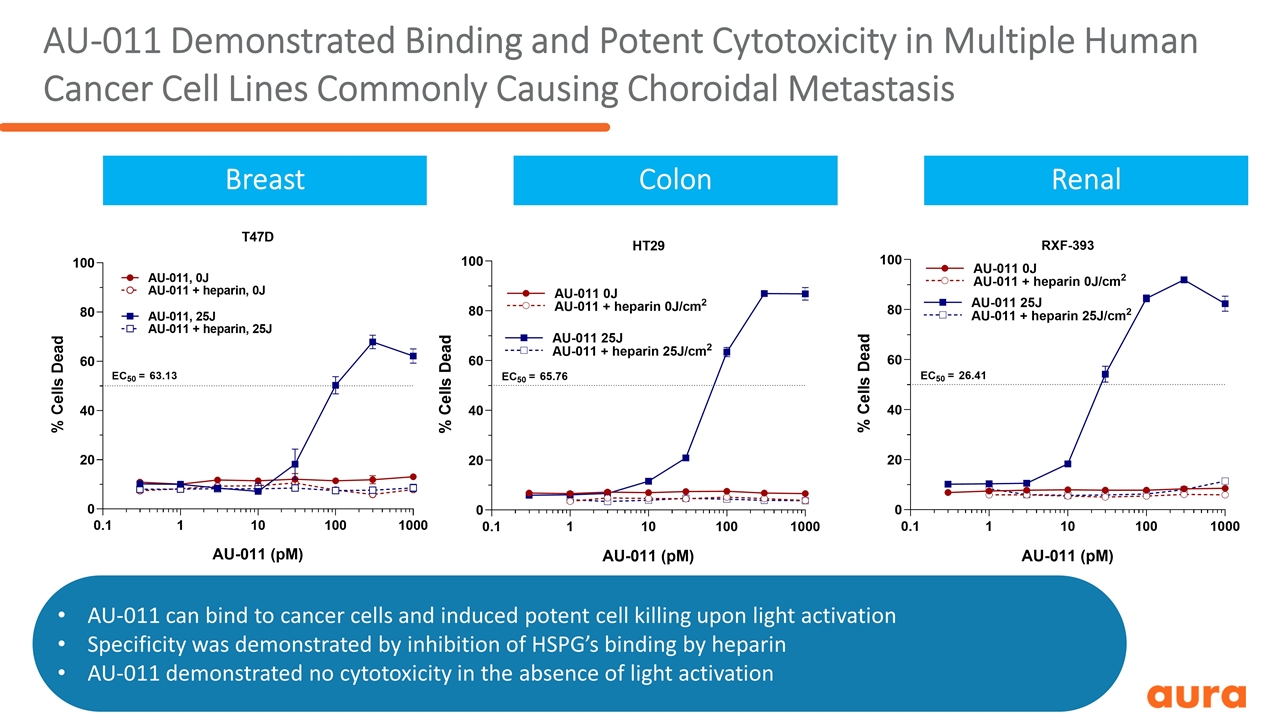
AU-011 Demonstrated Binding and Potent Cytotoxicity in Multiple Human Cancer Cell Lines Commonly Causing Choroidal Metastasis Breast Colon Renal AU-011 can bind to cancer cells and induced potent cell killing upon light activation Specificity was demonstrated by inhibition of HSPG’s binding by heparin AU-011 demonstrated no cytotoxicity in the absence of light activation
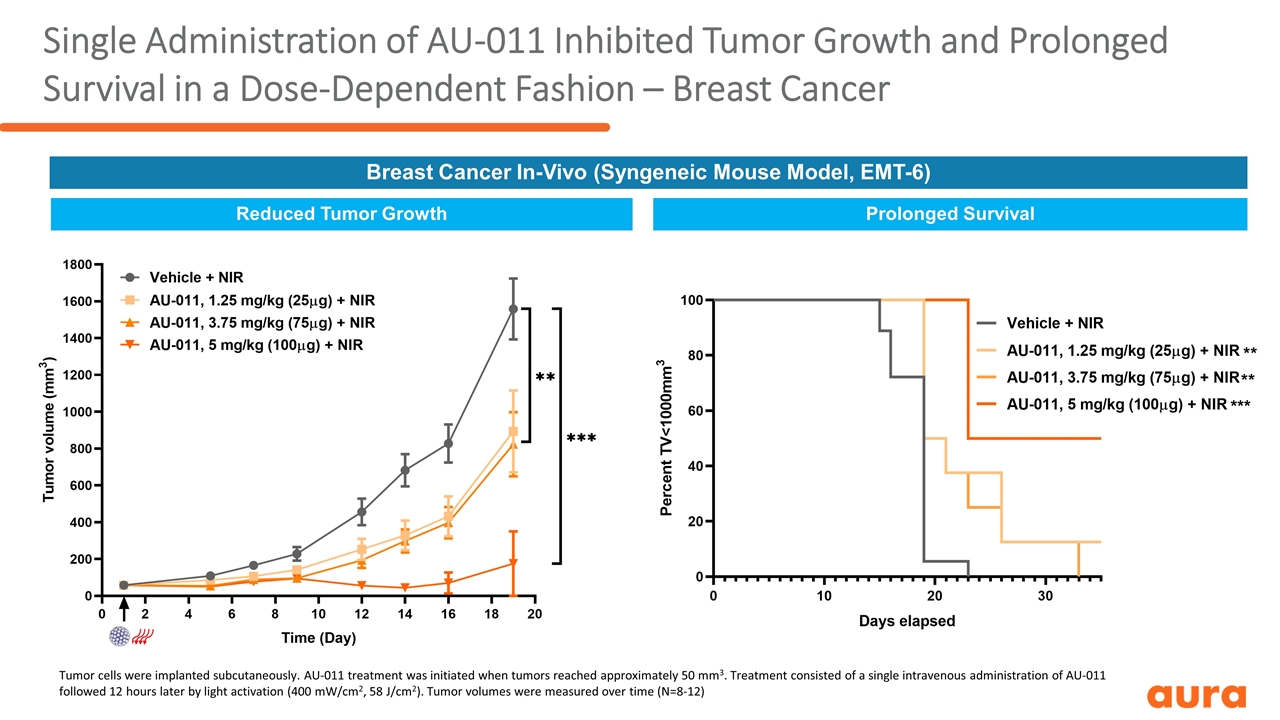
Single Administration of AU-011 Inhibited Tumor Growth and Prolonged Survival in a Dose-Dependent Fashion – Breast Cancer Reduced Tumor Growth Prolonged Survival Breast Cancer In-Vivo (Syngeneic Mouse Model, EMT-6) Tumor cells were implanted subcutaneously. AU-011 treatment was initiated when tumors reached approximately 50 mm3. Treatment consisted of a single intravenous administration of AU-011 followed 12 hours later by light activation (400 mW/cm2, 58 J/cm2). Tumor volumes were measured over time (N=8-12)
Conclusion AU-011 can bind to, and kill, tumor cells derived from the most common cancer types known to metastasize to the choroid Binds to modified HSPG’s on the surface of cancer cells No cytotoxicity in the absence of light activation was observed AU-011 showed dose-dependent activity in vivo using syngeneic mouse models for cancer types known to metastasize to the choroid Significantly inhibits tumor growth and prolongs survival Statistically significant results in multiple tumor models Study results support further evaluation of AU-011 as a potential treatment for choroidal metastasis

Retrospective Matched Case-Control Study
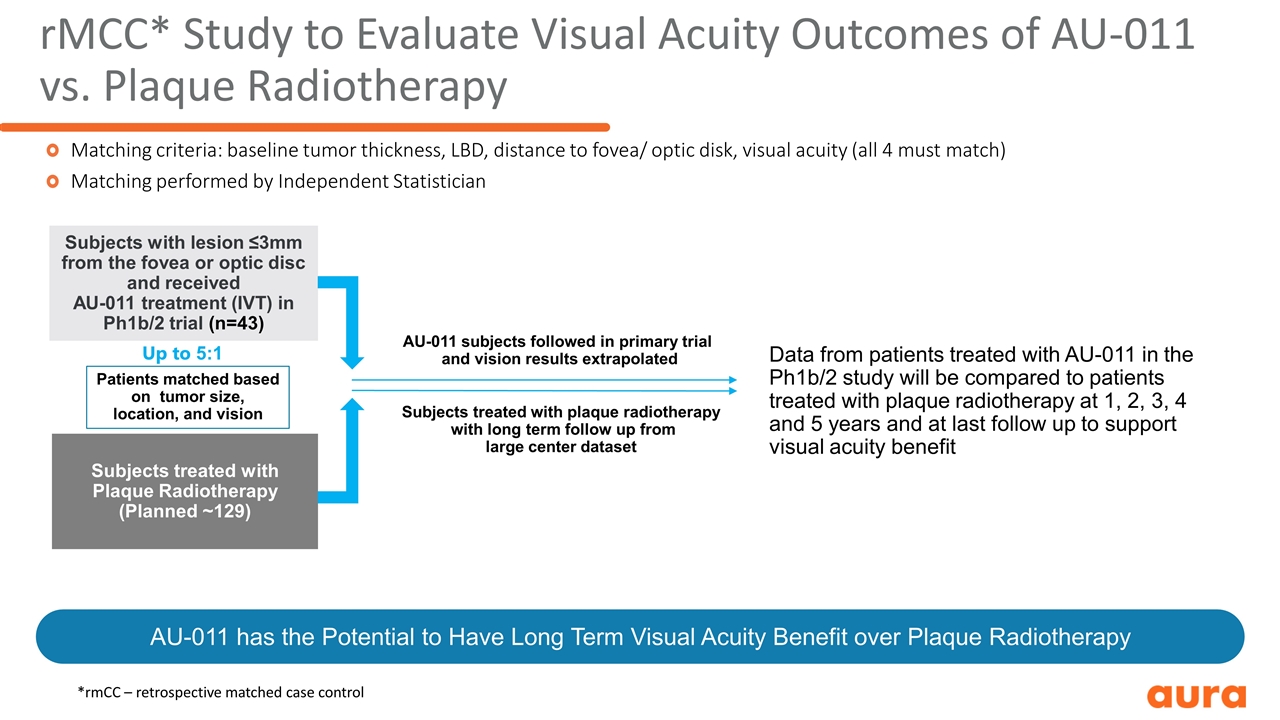
Subjects with lesion ≤3mm from the fovea or optic disc and received AU-011 treatment (IVT) in Ph1b/2 trial (n=43) Subjects treated with Plaque Radiotherapy (Planned ~129) rMCC* Study to Evaluate Visual Acuity Outcomes of AU-011 vs. Plaque Radiotherapy Matching criteria: baseline tumor thickness, LBD, distance to fovea/ optic disk, visual acuity (all 4 must match) Matching performed by Independent Statistician Patients matched based on tumor size, location, and vision Up to 5:1 AU-011 subjects followed in primary trial and vision results extrapolated Subjects treated with plaque radiotherapy with long term follow up from large center dataset Data from patients treated with AU-011 in the Ph1b/2 study will be compared to patients treated with plaque radiotherapy at 1, 2, 3, 4 and 5 years and at last follow up to support visual acuity benefit AU-011 has the Potential to Have Long Term Visual Acuity Benefit over Plaque Radiotherapy *rmCC – retrospective matched case control
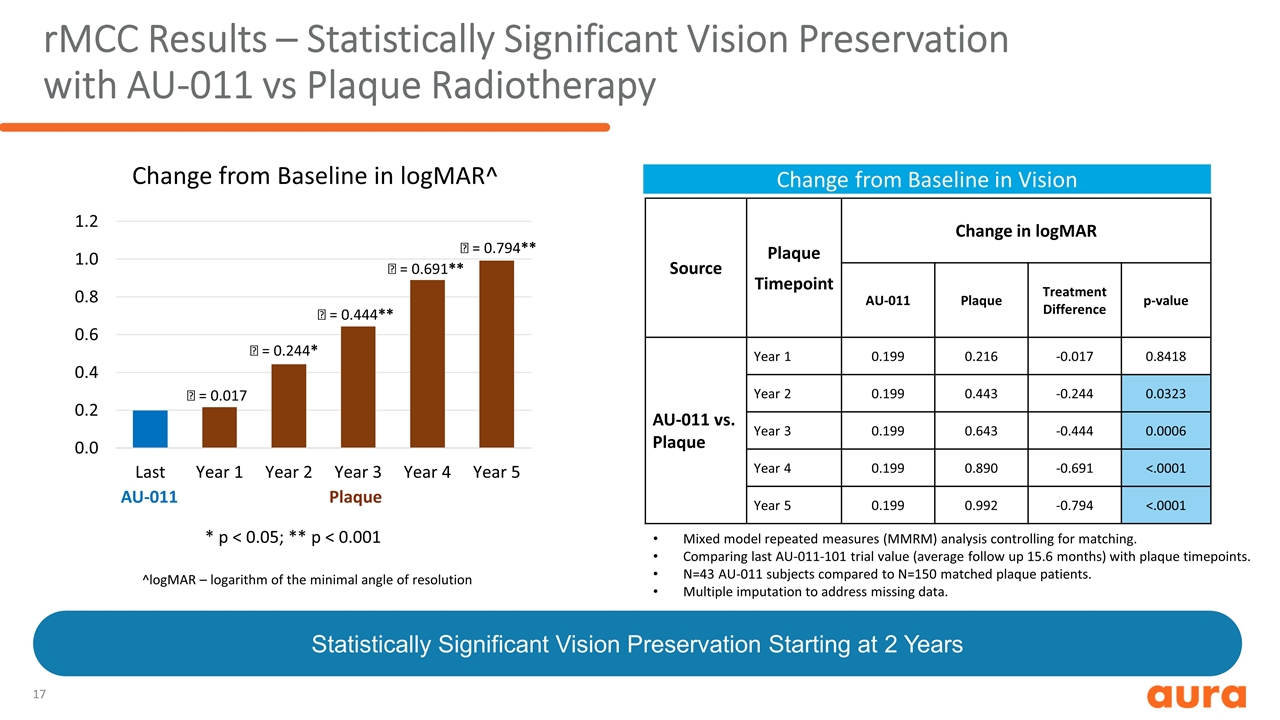
rMCC Results – Statistically Significant Vision Preservation with AU-011 vs Plaque Radiotherapy ^logMAR – logarithm of the minimal angle of resolution Change from Baseline in Vision Change from Baseline in logMAR^ AU-011 Plaque * p < 0.05; ** p < 0.001 D = 0.017 D = 0.244* D = 0.444** D = 0.691** D = 0.794** Source Plaque Timepoint Change in logMAR AU-011 Plaque Treatment Difference p-value AU-011 vs. Plaque Year 1 0.199 0.216 -0.017 0.8418 Year 2 0.199 0.443 -0.244 0.0323 Year 3 0.199 0.643 -0.444 0.0006 Year 4 0.199 0.890 -0.691 <.0001 Year 5 0.199 0.992 -0.794 <.0001 Mixed model repeated measures (MMRM) analysis controlling for matching. Comparing last AU-011-101 trial value (average follow up 15.6 months) with plaque timepoints. N=43 AU-011 subjects compared to N=150 matched plaque patients. Multiple imputation to address missing data. Statistically Significant Vision Preservation Starting at 2 Years
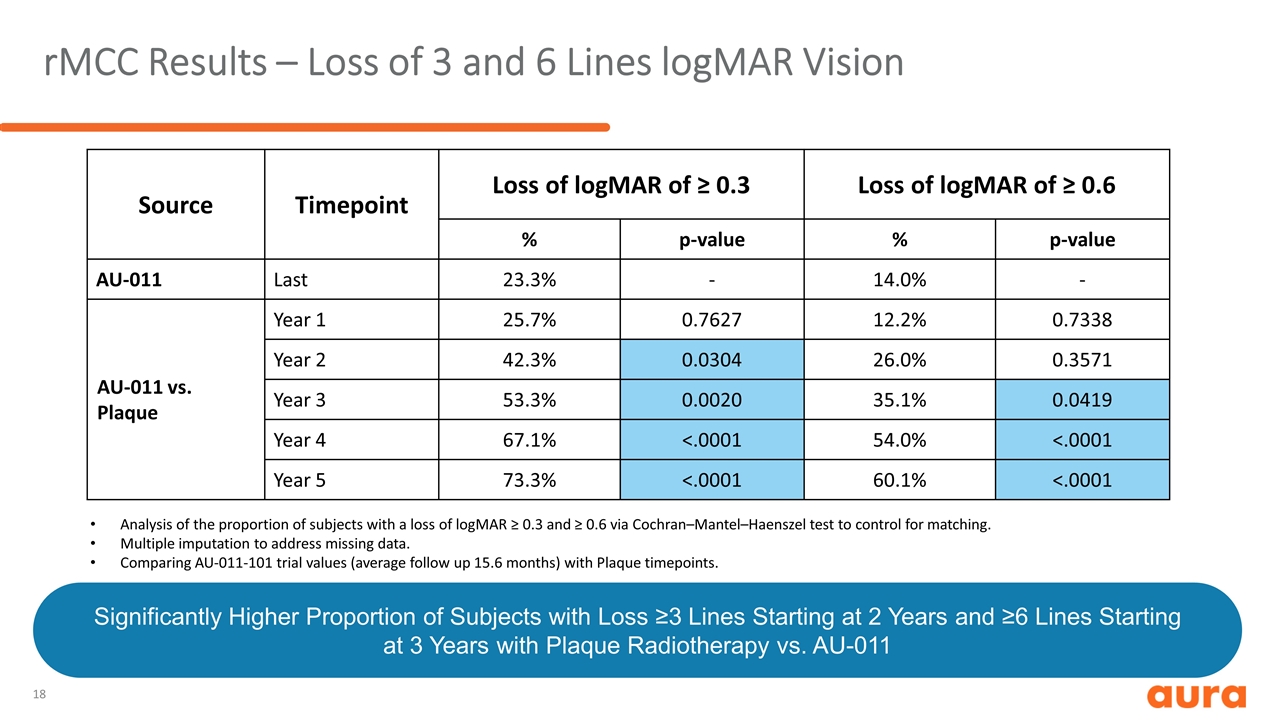
rMCC Results – Loss of 3 and 6 Lines logMAR Vision Source Timepoint Loss of logMAR of ≥ 0.3 Loss of logMAR of ≥ 0.6 % p-value % p-value AU-011 Last 23.3% - 14.0% - AU-011 vs. Plaque Year 1 25.7% 0.7627 12.2% 0.7338 Year 2 42.3% 0.0304 26.0% 0.3571 Year 3 53.3% 0.0020 35.1% 0.0419 Year 4 67.1% <.0001 54.0% <.0001 Year 5 73.3% <.0001 60.1% <.0001 Analysis of the proportion of subjects with a loss of logMAR ≥ 0.3 and ≥ 0.6 via Cochran–Mantel–Haenszel test to control for matching. Multiple imputation to address missing data. Comparing AU-011-101 trial values (average follow up 15.6 months) with Plaque timepoints. Significantly Higher Proportion of Subjects with Loss ≥3 Lines Starting at 2 Years and ≥6 Lines Starting at 3 Years with Plaque Radiotherapy vs. AU-011

AU-011 in Combination with Checkpoint Inhibitors Ruben Huis in t’ Veld
Immune checkpoint inhibition combined with targeted therapy using a novel virus-like drug conjugate Ruben Huis in ‘t Veld Research sponsored by Health Holland in collaboration with Aura Biosciences Cx Virus-Like Particle (VLP) Cytotoxic dye Virus-Like Drug Conjugate (VDC)
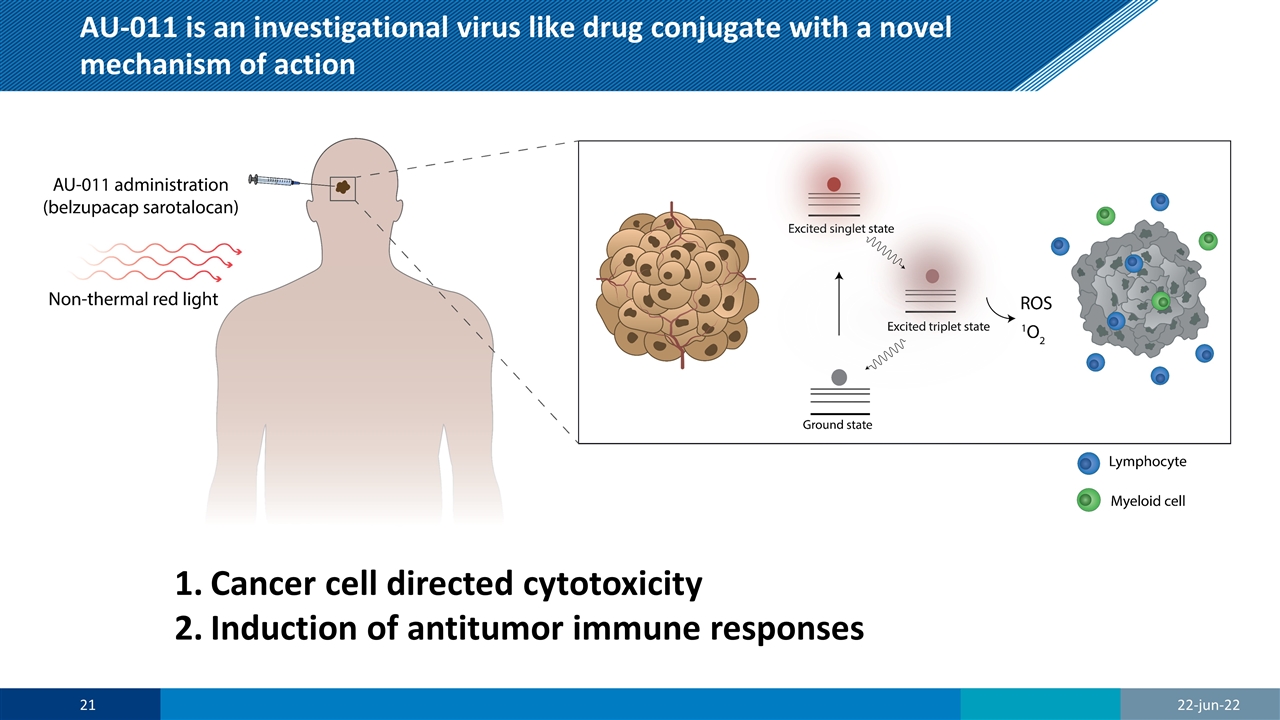
AU-011 is an investigational virus like drug conjugate with a novel mechanism of action Cancer cell directed cytotoxicity Induction of antitumor immune responses 22-jun-22
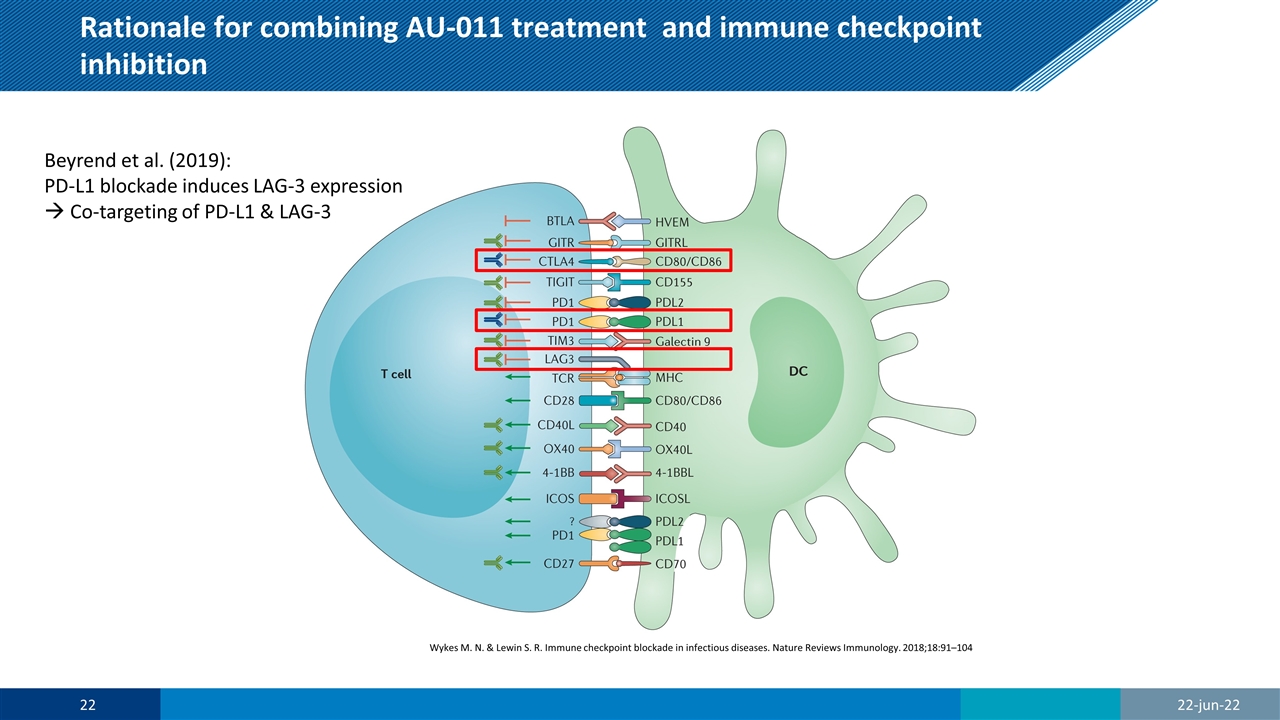
Rationale for combining AU-011 treatment and immune checkpoint inhibition Wykes M. N. & Lewin S. R. Immune checkpoint blockade in infectious diseases. Nature Reviews Immunology. 2018;18:91–104 Beyrend et al. (2019): PD-L1 blockade induces LAG-3 expression à Co-targeting of PD-L1 & LAG-3 22-jun-22
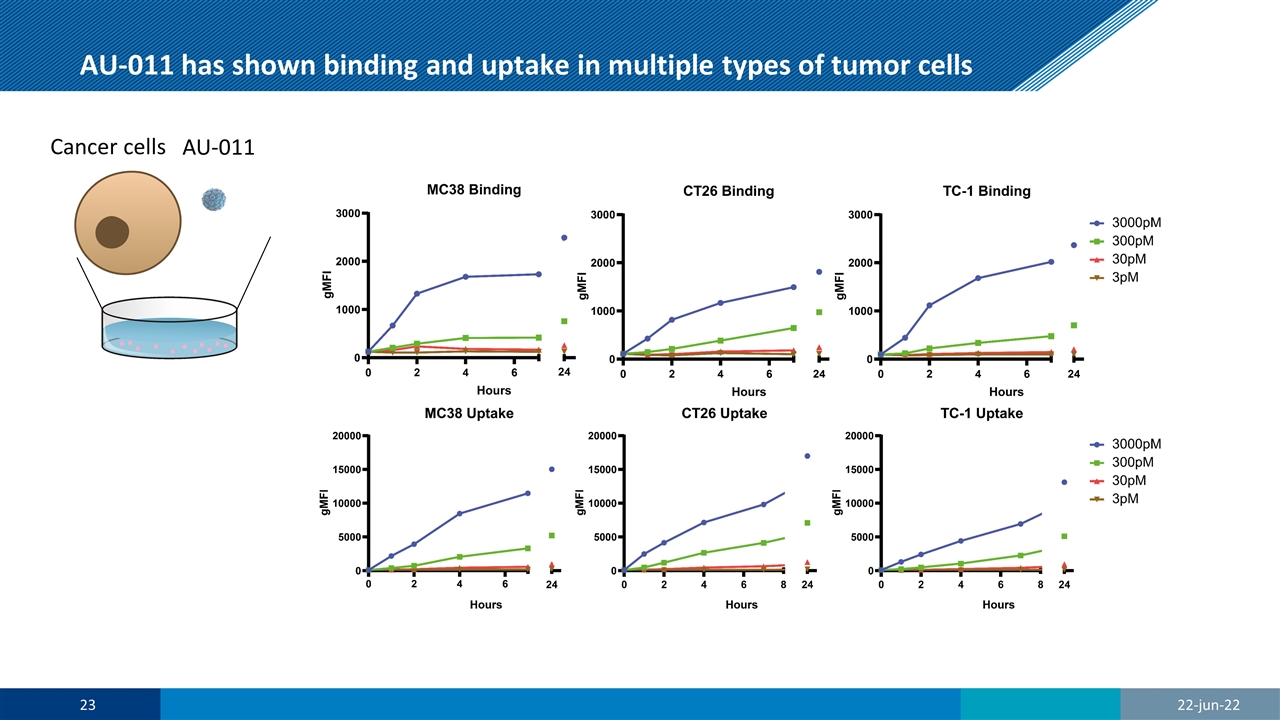
AU-011 has shown binding and uptake in multiple types of tumor cells AU-011 Cancer cells AU-011 22-jun-22
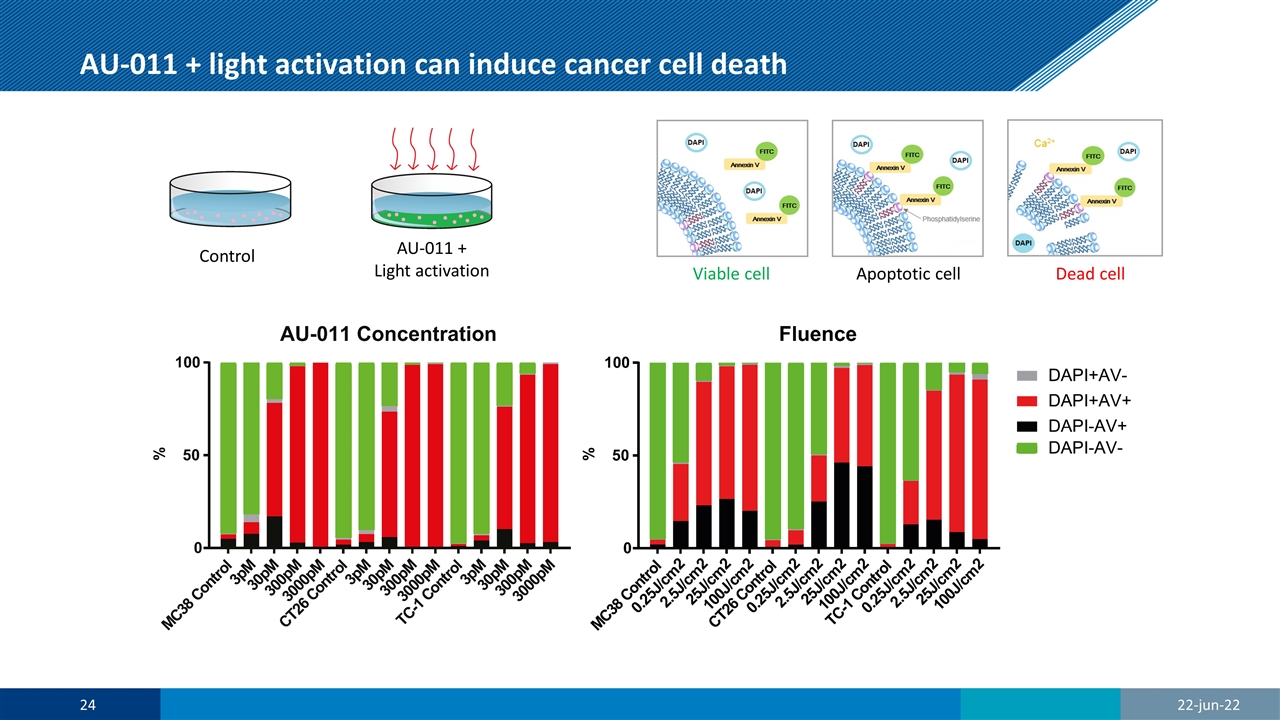
AU-011 + light activation can induce cancer cell death 22-jun-22 Viable cell Apoptotic cell Dead cell Control AU-011 + Light activation
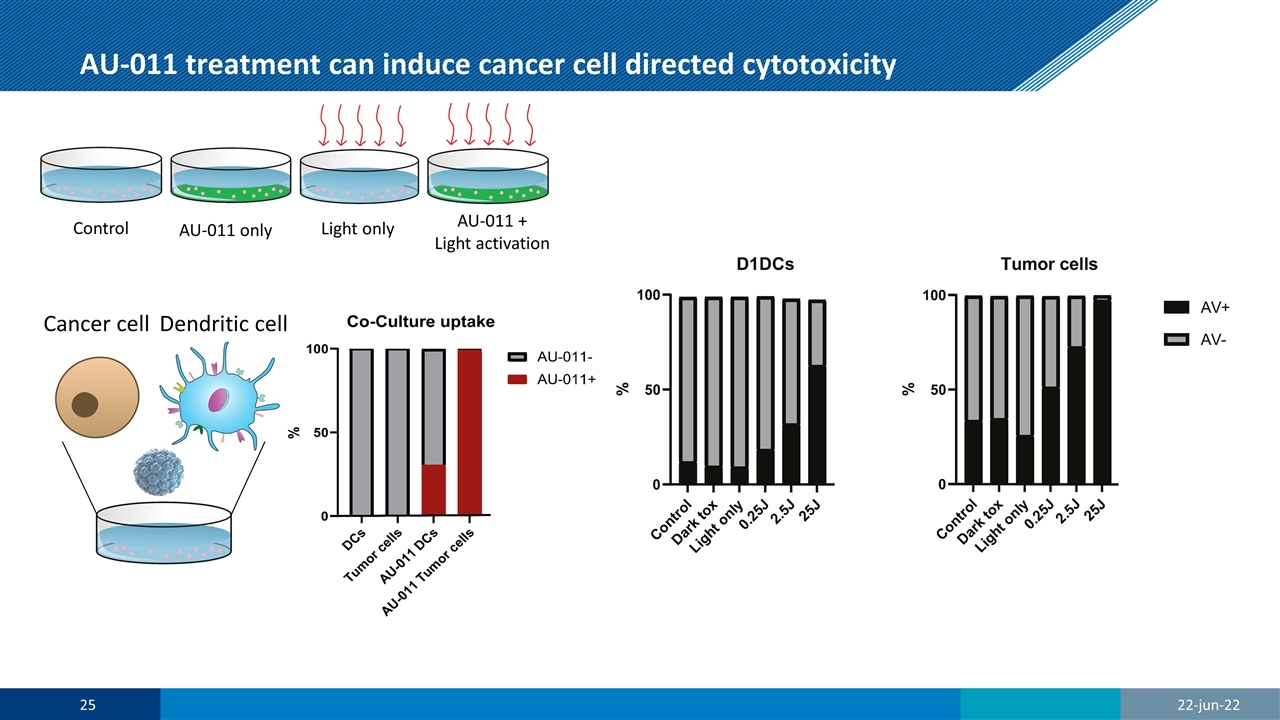
AU-011 treatment can induce cancer cell directed cytotoxicity Cancer cell Dendritic cell Control AU-011 only Light only AU-011 + Light activation
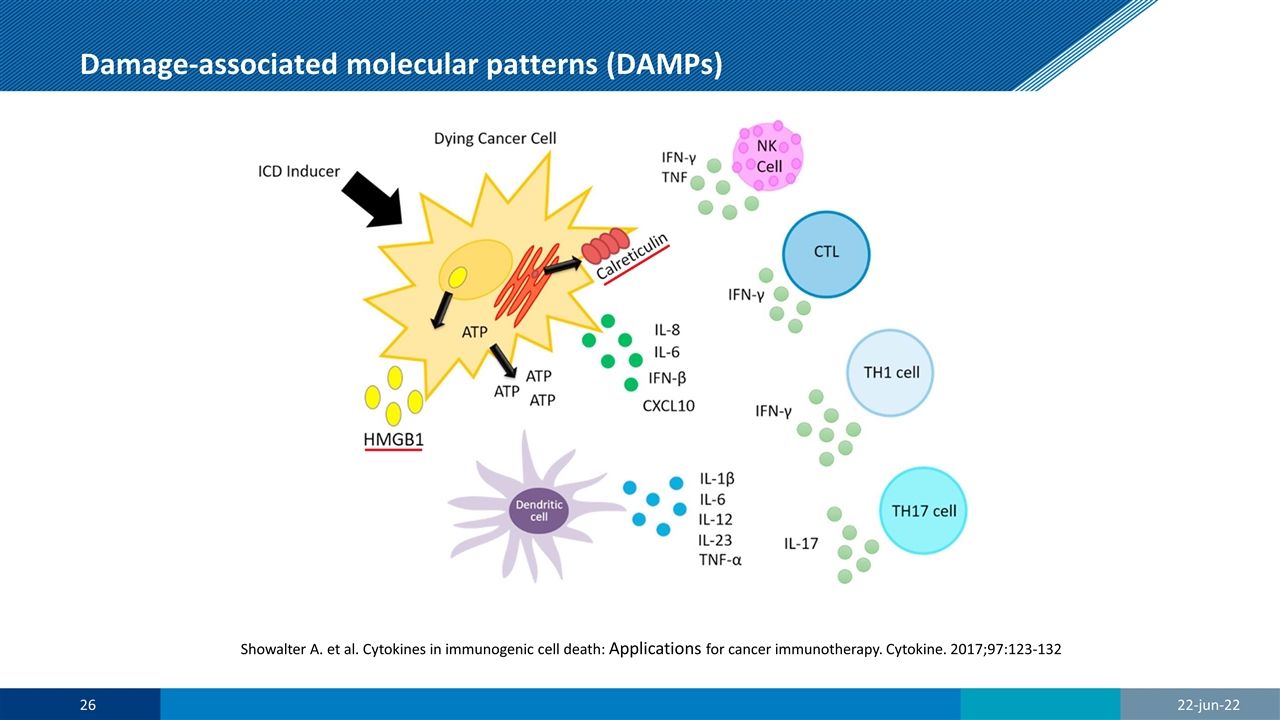
Damage-associated molecular patterns (DAMPs) Showalter A. et al. Cytokines in immunogenic cell death: Applications for cancer immunotherapy. Cytokine. 2017;97:123-132
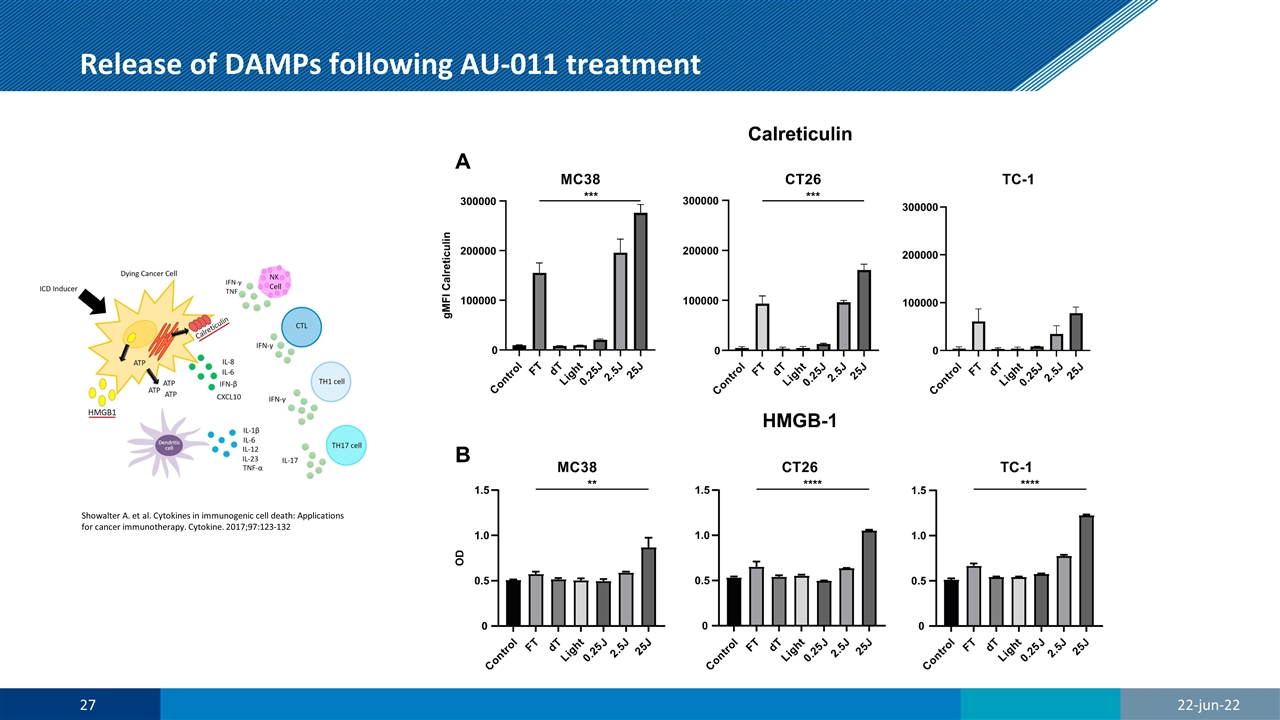
Release of DAMPs following AU-011 treatment Showalter A. et al. Cytokines in immunogenic cell death: Applications for cancer immunotherapy. Cytokine. 2017;97:123-132
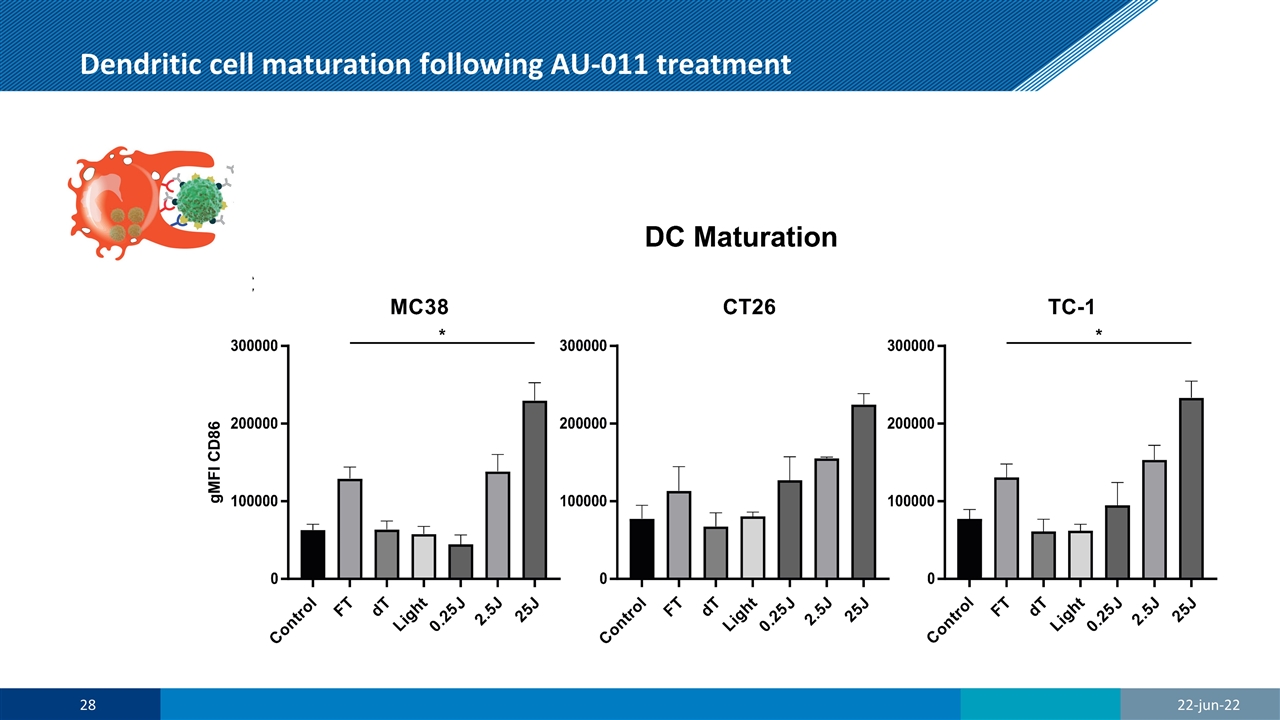
Dendritic cell maturation following AU-011 treatment
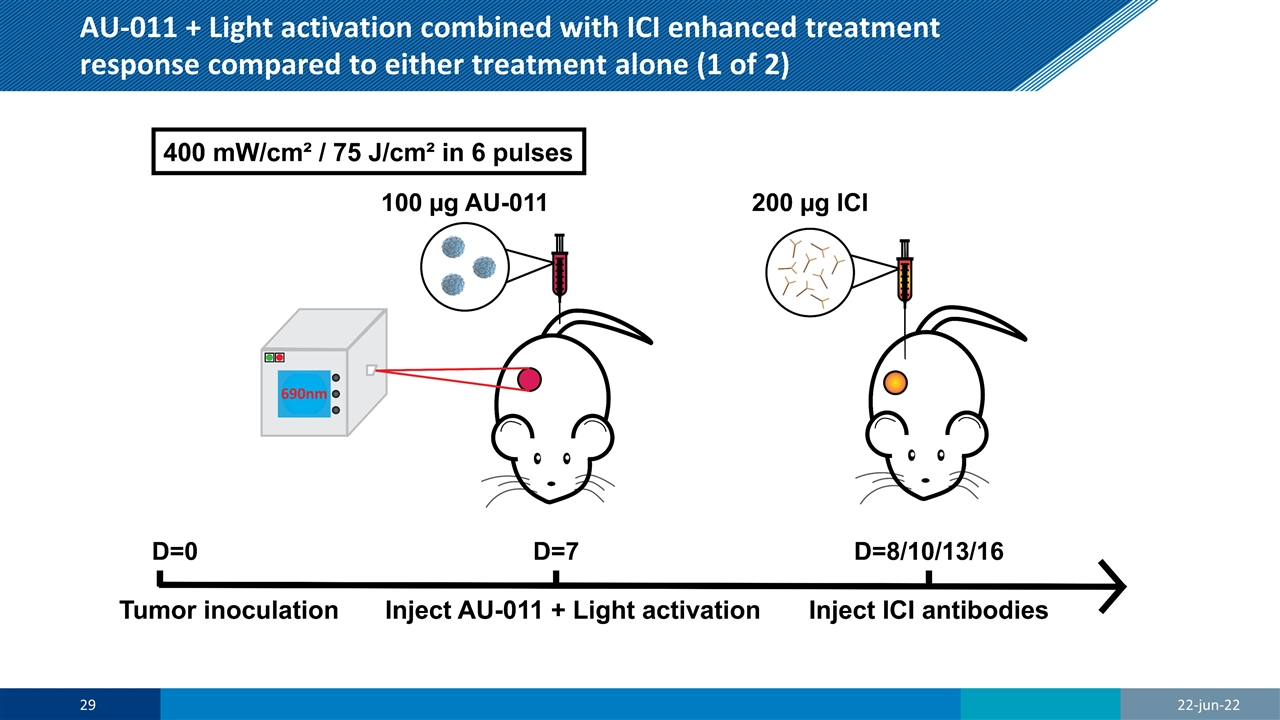
AU-011 + Light activation combined with ICI enhanced treatment response compared to either treatment alone (1 of 2) 22-jun-22
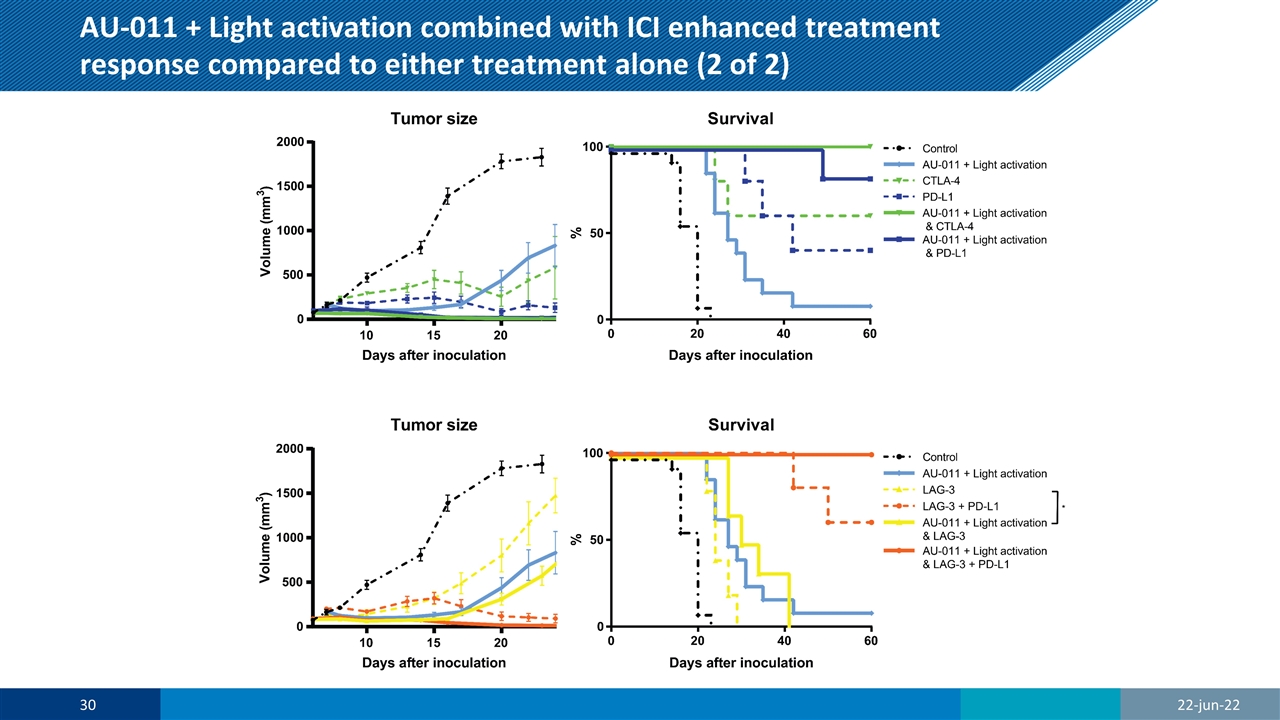
AU-011 + Light activation combined with ICI enhanced treatment response compared to either treatment alone (2 of 2)
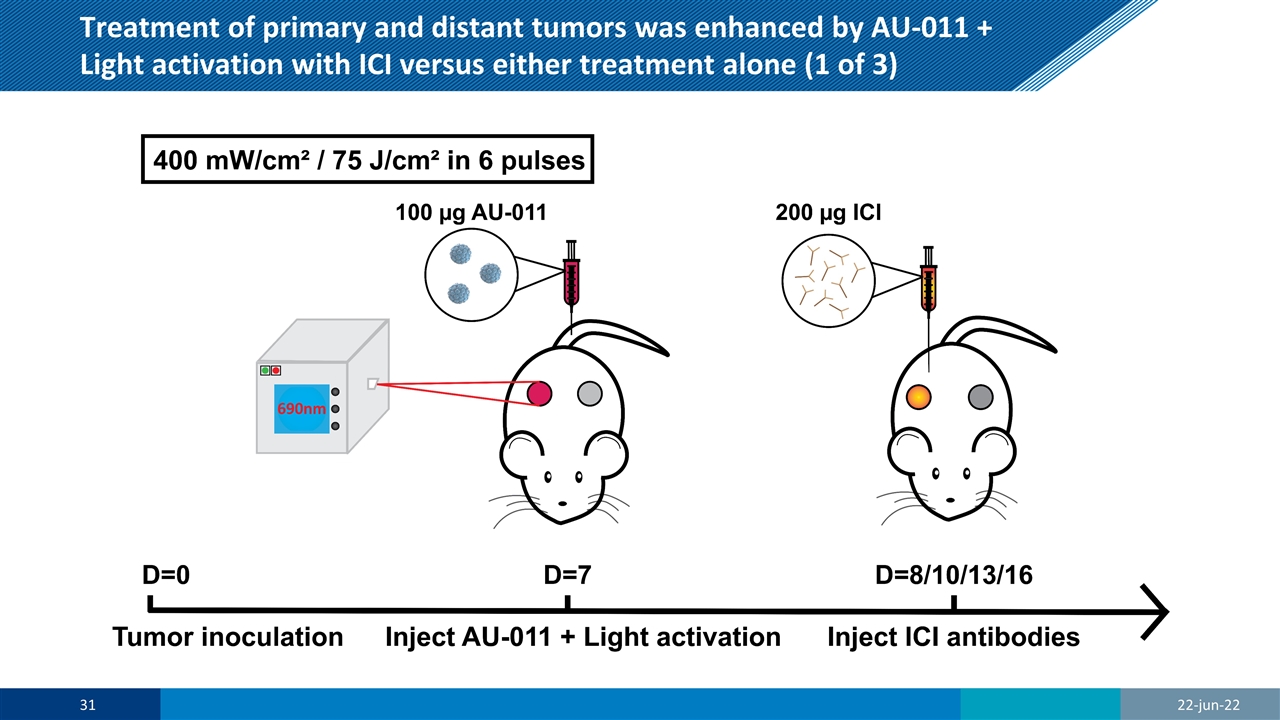
Treatment of primary and distant tumors was enhanced by AU-011 + Light activation with ICI versus either treatment alone (1 of 3) 22-jun-22
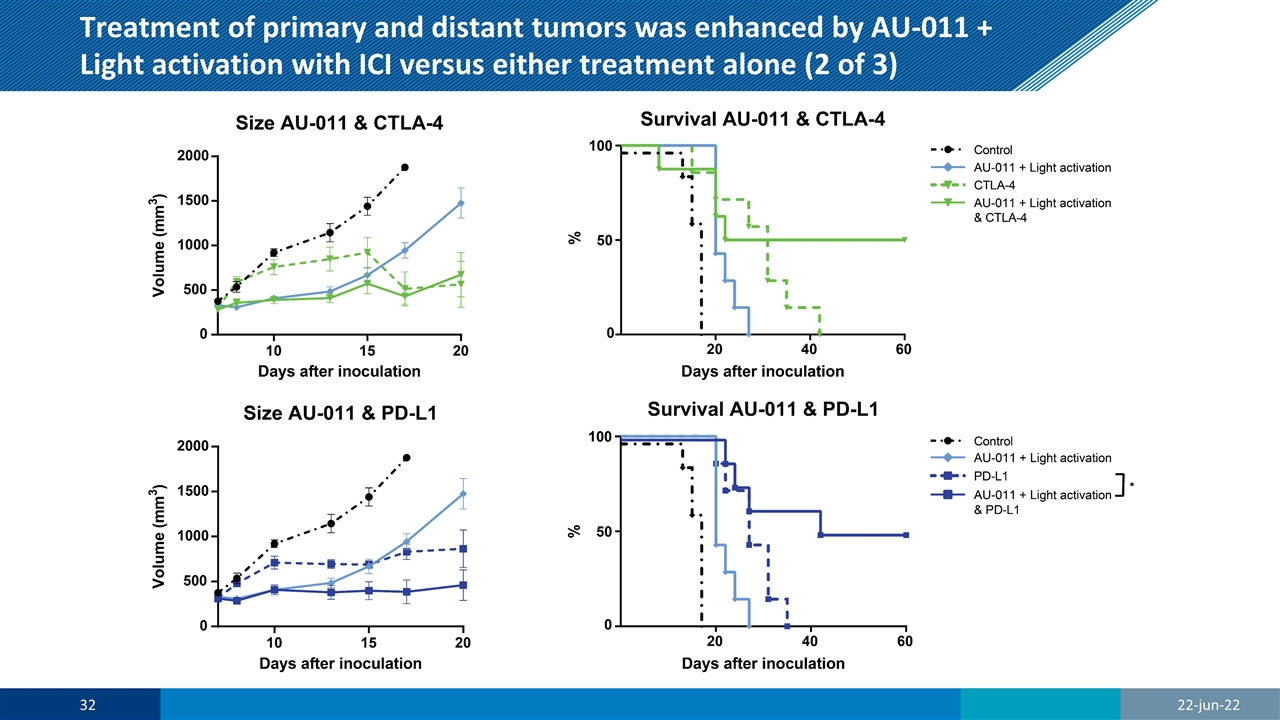
Treatment of primary and distant tumors was enhanced by AU-011 + Light activation with ICI versus either treatment alone (2 of 3) 22-jun-22
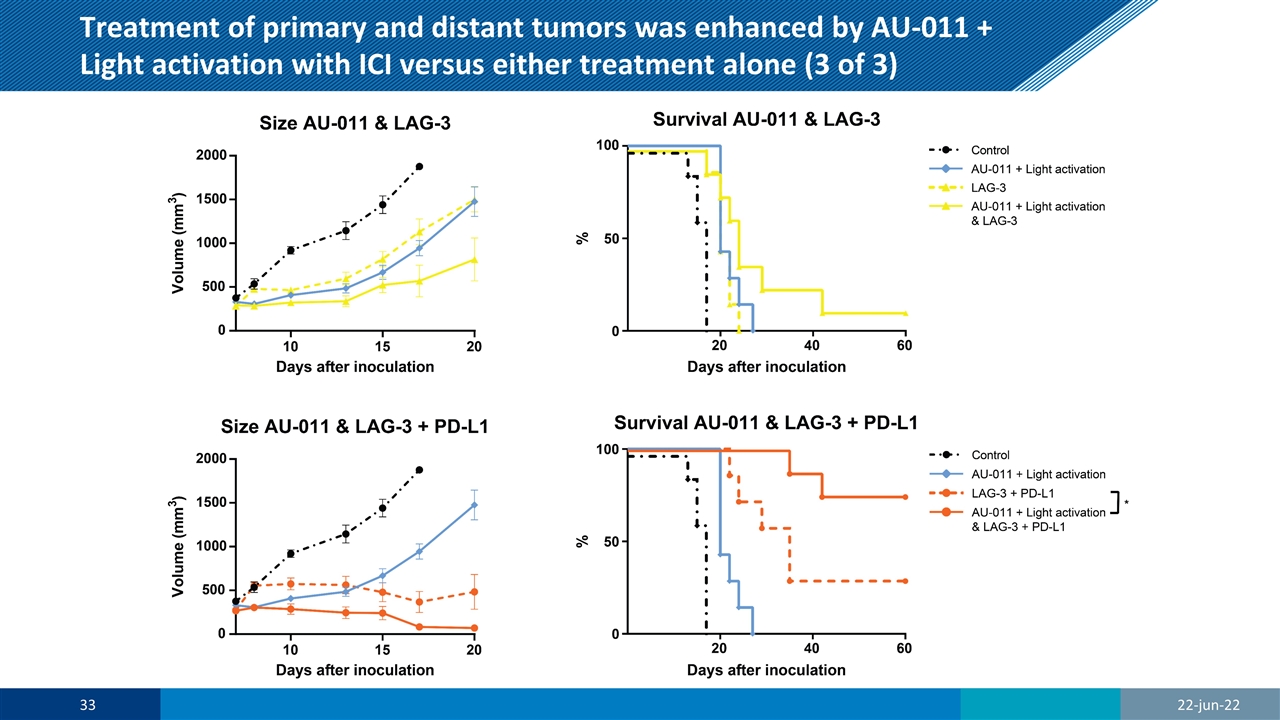
Treatment of primary and distant tumors was enhanced by AU-011 + Light activation with ICI versus either treatment alone (3 of 3) 22-jun-22
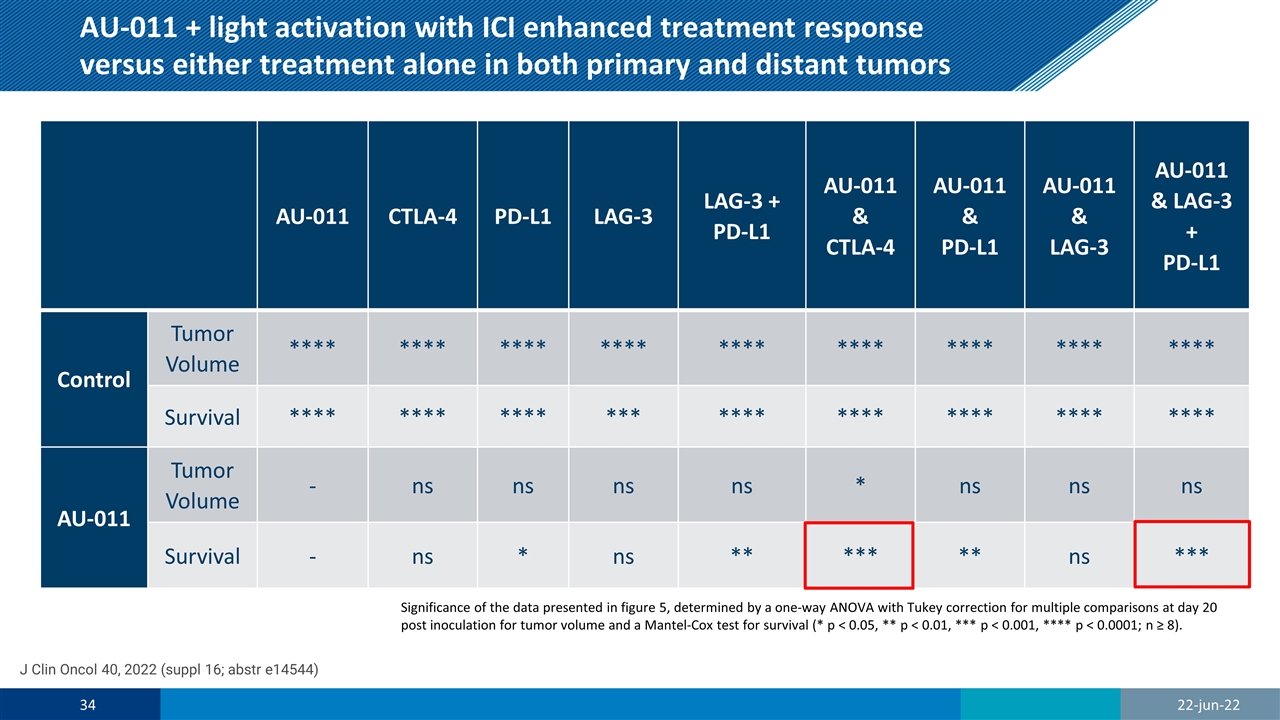
AU-011 + light activation with ICI enhanced treatment response versus either treatment alone in both primary and distant tumors AU-011 CTLA-4 PD-L1 LAG-3 LAG-3 + PD-L1 AU-011 & CTLA-4 AU-011 & PD-L1 AU-011 & LAG-3 AU-011 & LAG-3 + PD-L1 Control Tumor Volume **** **** **** **** **** **** **** **** **** Survival **** **** **** *** **** **** **** **** **** AU-011 Tumor Volume - ns ns ns ns * ns ns ns Survival - ns * ns ** *** ** ns *** Significance of the data presented in figure 5, determined by a one-way ANOVA with Tukey correction for multiple comparisons at day 20 post inoculation for tumor volume and a Mantel-Cox test for survival (* p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001; n ≥ 8). J Clin Oncol 40, 2022 (suppl 16; abstr e14544) 22-jun-22
Conclusions AU-011 + Light activation: Induced cancer cell-directed cytotoxicity Released DAMPs and induced maturation of antigen-presenting cells Combined with ICI using anti-PD-L1 & anti-LAG-3 antibodies showed potential to induce complete and lasting tumor responses in both primary and distant tumors in murine models 22-jun-22

Acknowledgements Insert > Header & footer Prof. Dr. M. J. Jager & Prof. Dr. F. Ossendorp Aura Biosciences Health Holland Leiden University Medical Center

Question & Answer

ISOO 2022 Thank you! Confidential





































