Exhibit 99.1

Business Update Conference Call NYSE American: TMBR December 17, 2020

Safe Harbor Statement Certain statements contained in this PowerPoint presentation describing Timber’s technology and development program, including, without limitation, statements containing the words “expects,” “anticipates,” “believes,” and words of similar import, constitute “forward - looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. These forward - looking statements are subject to various risks and uncertainties that could cause actual future results and events to differ materially from those currently anticipated. Potential investors are cautioned not to place undue reliance on these forward - looking statements. 2 NYSE American: TMBR

3 • Rare dermatological diseases are underrepresented in drug development and pharmaceutical company sponsored trials • Many orphan and rare dermatology disorders are associated with significant quality of life impairments, particularly if the disease is insufficiently controlled • Disease visibility in particular can have a profoundly negative impact on patient confidence • Significant unmet needs exist for more efficacious and safer treatment options • Need to target underlying causes of disease rather than managing symptoms • Global premium products market size for orphan and rare dermatology therapeutics is expected to grow, from $1.64 billion in 2017 to $6.07 billion in 2024, a CAGR of 20.5%* • US premium products market size is expected to increase from $794m in 2017 to $2.73bn in 2024, a CAGR of 19.3%, accounting for just under half of the global orphan and rare dermatology market* * Source: ‘Global Orphan and Rare Dermatology Drugs Market to 2024’, GBI Research Business Intelligence, 2018 Rare Dermatologic Diseases NYSE American: TMBR

4 Timber Business Model • Focused on Orphan Drug Indications and Leveraging the 505(b)(2) pathway • Why Orphan Drugs? • Timber will be first to market by pursuing conditions for which there are no current FDA approved treatments • Requires smaller and less costly drug trials with greater flexibility from the FDA • Orphan Drug Market Exclusivity limits additional market entrants and conveys pricing power • 7 years in U.S., 10 years in E.U. • Tax advantages and potential to receive valuable priority review voucher from FDA • Why Leverage the 505(b)(2) Pathway? • De - risks the entire program • Bypasses Phase 1 trials by utilizing proven drugs and mechanisms of action • Safety and efficacy already established • Can immediately begin Phase 2 studies NYSE American: TMBR Priority Review Voucher – A program of the US Food and Drug Administration that grants a voucher for priority review to a drug d eveloper as an incentive to develop treatments for drugs that might otherwise not be profitable to develop because of a smaller pool of patients needing treatment. Voucher can be redeemed by recipient or sold to another company.
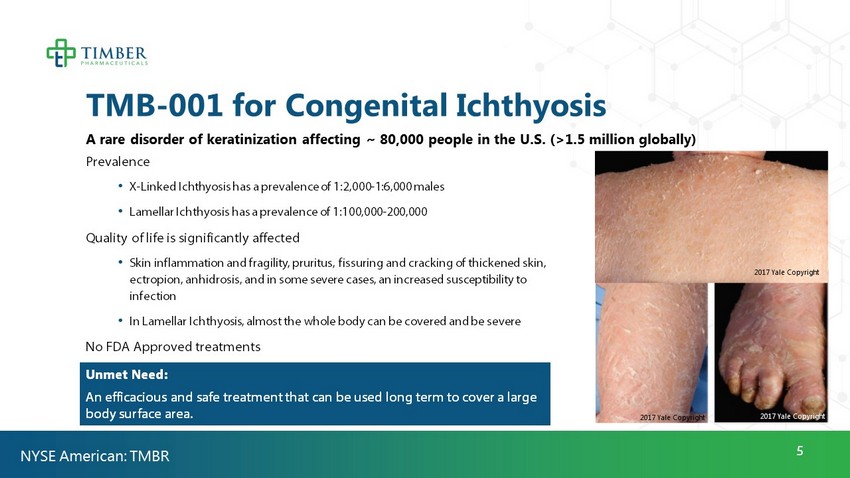
5 TMB - 001 for Congenital Ichthyosis A rare disorder of keratinization affecting ~ 80,000 people in the U.S. (>1.5 million globally) Prevalence • X - Linked Ichthyosis has a prevalence of 1:2,000 - 1:6,000 males • Lamellar Ichthyosis has a prevalence of 1:100,000 - 200,000 Quality of life is significantly affected • Skin inflammation and fragility, pruritus, fissuring and cracking of thickened skin, ectropion, anhidrosis, and in some severe cases, an increased susceptibility to infection • In Lamellar Ichthyosis, almost the whole body can be covered and be severe No FDA Approved treatments Unmet Need: An efficacious and safe treatment that can be used long term to cover a large body surface area . NYSE American: TMBR 2017 Yale Copyright 2017 Yale Copyright 2017 Yale Copyright

6 Isotretinoin is widely viewed as the most effective therapy for several skin conditions, including ichthyosis, but it is only available orally, where high dose, chronic oral therapy cannot be tolerated due to systemic toxicity TMB - 001 utilizes proprietary IPEG™ delivery system to target isotretinoin delivery to the epidermis and dermis, minimize systemic absorption and remove irritating excipients Market Opportunity: • $250 million annual estimated US market size at peak* Development Stage: • Phase 2B study in CI is currently enrolling • Phase Ib /2a POC study in CI completed in 2018 with positive data on clinically meaningful endpoints and no systemic absorption 2018 FDA Orphan Products Grant Recipient • Awarded $1.5 Million from the FDA to run the Phase 2A and Phase 2B Clinical Trials TMB - 001 Topical Isotretinoin in CI Proprietary topical formulation minimizing systemic absorption and allowing for chronic treatment NYSE American: TMBR We believe we have Strong Market Protection, with Orphan exclusivity granted (7 years in U.S., 10 years in E.U.) and additional patents pending * Based on Timber’s estimates
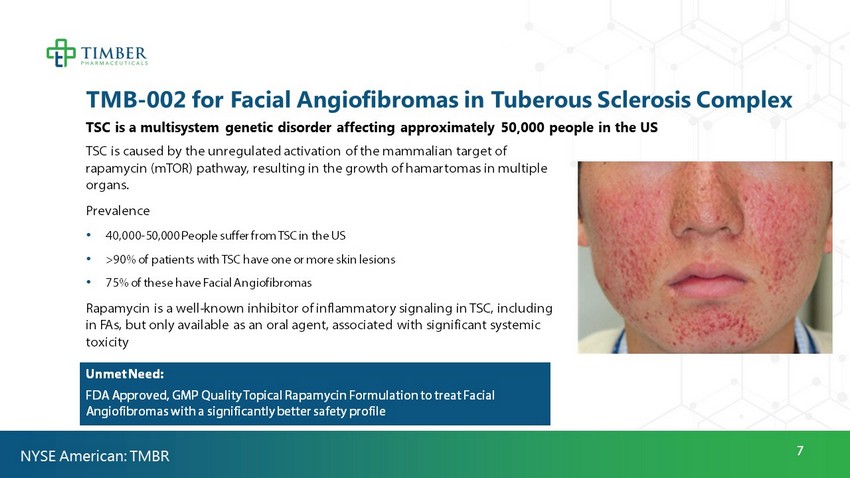
7 TMB - 002 for Facial Angiofibromas in Tuberous Sclerosis Complex TSC is a multisystem genetic disorder affecting approximately 50,000 people in the US TSC is caused by the unregulated activation of the mammalian target of rapamycin (mTOR) pathway, resulting in the growth of hamartomas in multiple organs. Prevalence • 40,000 - 50,000 People suffer from TSC in the US • >90% of patients with TSC have one or more skin lesions • 75% of these have Facial Angiofibromas Rapamycin is a well - known inhibitor of inflammatory signaling in TSC, including in FAs, but only available as an oral agent, associated with significant systemic toxicity No FDA Approved Topical Formulation of Rapamycin Unmet Need: FDA Approved, GMP Quality Topical Rapamycin Formulation to treat Facial Angiofibromas with a significantly better safety profile NYSE American: TMBR

8 TMB - 002 Topical Rapamycin (Pascomer®) Potentially the First Commercially Available, High - Quality Topical Formulation of Rapamycin with Broad Access Proprietary topical formulation of rapamycin (1.0%, 0.5%, and 0.1%) • Additional 5% strength assessed in preclinical toxicity studies Utilizes trade secret lipid crystalline vehicle system designed to improve stability and delivery of unstable APIs • Compounded formulations that are sometimes currently used lack consistent quality, potency, and homogeneity • Other topical rapamycin formulations have short shelf lives and require refrigeration • Pascomer has demonstrated room temperature stability for 0.5%, 1.0%, and 5.0% formulations Market Opportunity: • $250 million annual estimated US market size at peak* Development Stage: • Efficacy of topical rapamycin demonstrated in multiple well controlled studies and dozens of published case reports • Robust Phase 2B study (one of two pivotal studies required for registration) in FAs in TSC underway; Enrolling 120 subjects across 17 sites We believe we have Strong Market Protection, with Orphan exclusivity granted (7 years in U.S., 10 years in E.U.) and with additional patents pending NYSE American: TMBR * Based on Timber’s estimates

9 Strategic Pipeline in Rare Dermatologic Disease NYSE American: TMBR
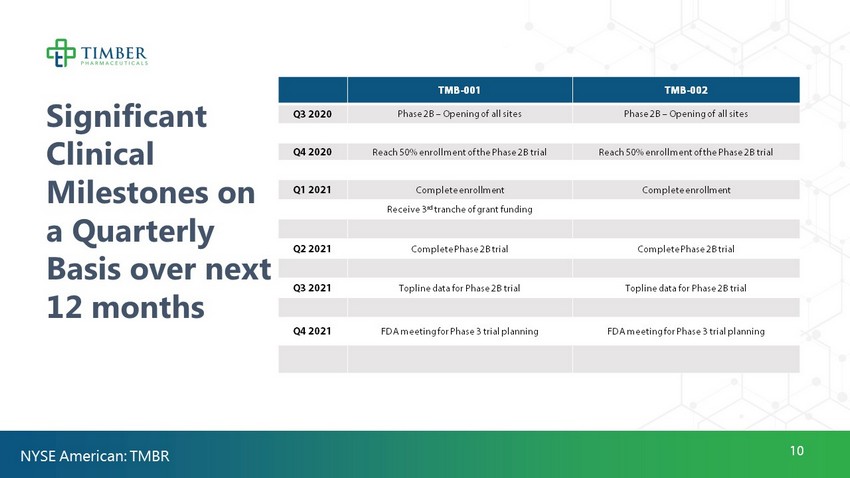
10 Significant Clinical Milestones on a Quarterly Basis over next 12 months TMB - 001 TMB - 002 Q3 2020 Phase 2B – Opening of all sites Phase 2B – Opening of all sites Q4 2020 Reach 50% enrollment of the Phase 2B trial Reach 50% enrollment of the Phase 2B trial Q1 2021 Complete enrollment Complete enrollment Receive 3 rd tranche of grant funding Q2 2021 Complete Phase 2B trial Complete Phase 2B trial Q3 2021 Topline data for Phase 2B trial Topline data for Phase 2B trial Q4 2021 FDA meeting for Phase 3 trial planning FDA meeting for Phase 3 trial planning NYSE American: TMBR
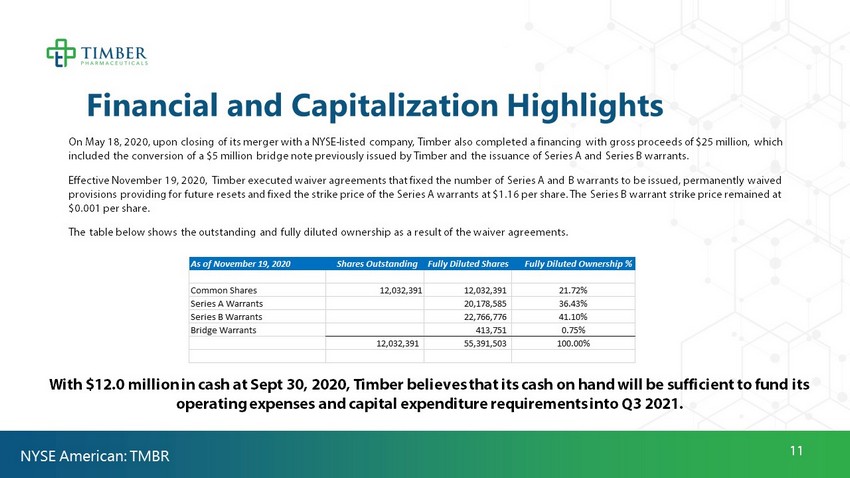
11 Financial and Capitalization Highlights On May 18, 2020, upon closing of its merger with a NYSE - listed company, Timber also completed a financing with gross proceeds of $25 million, which included the conversion of a $5 million bridge note previously issued by Timber and the issuance of Series A and Series B war ran ts. Effective November 19, 2020, Timber executed waiver agreements that fixed the number of Series A and B warrants to be issued, p ermanently waived provisions providing for future resets and fixed the strike price of the Series A warrants at $1.16 per share. The Series B w arr ant strike price remained at $0.001 per share. The table below shows the outstanding and fully diluted ownership as a result of the waiver agreements. NYSE American: TMBR With $12.0 million in cash at Sept 30, 2020, Timber believes that its cash on hand will be sufficient to fund its operating expenses and capital expenditure requirements into Q3 2021. As of November 19, 2020 Shares Outstanding Fully Diluted Shares Fully Diluted Ownership % Common Shares 12,032,391 12,032,391 21.72% Series A Warrants 20,178,585 36.43% Series B Warrants 22,766,776 41.10% Bridge Warrants 413,751 0.75% 12,032,391 55,391,503 100.00%

12 Key Takeaways • Positioned to become a leading Dermatology company with a focus on orphan diseases, led by a management team with a proven track record in development and commercialization • High potential, late stage, lower risk multi - product candidate pipeline • Proven clinical Proof of Concept (POC) • All programs qualify for Orphan Drug status and currently have no approved treatments • Large market opportunities for both lead product candidates • U.S. market for each is estimated at $250 million annually at peak* • Additional market expansion potential in broader dermatology indications • Currently enrolling patients in two Phase 2b studies with two different product candidates • Both studies on track and proceeding as expected with data readout >12 months • Actions put in place to mitigate additional risk from COVID - 19 • Lean operating model and cost structure • $12 million in cash on hand will fund Company through multiple key clinical development milestones, including topline data readout from both Phase 2b studies NYSE American: TMBR * Based on Timber estimates

Building Bridges in Medical Dermatology For additional Investor Information please contact: John Koconis Stephanie Prince CEO PCG Advisory jkoconis@timberpharma.com sprince@pcgadvisory.com (646) 863 - 6341 Thank You www.timberpharma.com












