UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): January 21, 2014
| Thompson Designs, Inc. |
| (Exact name of registrant as specified in its charter) |
| Nevada | 000-54871 | 59-3843182 | ||
(State or other jurisdiction of incorporation) | (Commission File Number) | (IRS Employer Identification No.) |
1098 Hamilton Court Menlo Park, CA 94025 | ||
| (Address of Principal Executive Offices) |
Tel. (650) 889-5020
Fax (650) 900-4130
Registrant’s telephone number, including area code
3315 East Russell Road, Ste. A-4 129
Las Vegas, Nevada 89120
Former name or former address, if changed since last report
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
o Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
o Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
o Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
o Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
TABLE OF CONTENTS
| Item No. | Description of Item | Page No. | ||
| Item 1.01 | Entry Into a Material Definitive Agreement | 3 | ||
| Item 2.01 | Completion of Acquisition or Disposition of Assets | 4 | ||
| Item 3.02 | Unregistered Sales of Equity Securities | 18 | ||
| Item 4.01 | Changes in Registrant’s Certifying Accountant | 18 | ||
| Item 5.01 | Changes in Control of Registrant | 19 | ||
| Item 5.02 | Departure of Directors or Certain Officers; Election of Directors; Appointment of Certain Officers; Compensatory Arrangements of Certain Officers | 19 | ||
| Item 5.03 | Amendment of Articles of Incorporation or Bylaws; Change in Fiscal Year | 19 | ||
| Item 5.06 | Change in Shell Company Status | 20 | ||
| Item 9.01 | Financial Statements and Exhibits | 20 |
EXPLANATORY NOTE
This Current Report on Form 8-K is being filed by Thompson Designs, Inc. We are reporting the acquisition of a new business and providing a description of this business and its audited financial statements below.
| ● | The “Company,” “we,” “us,” or “our,” are references to the combined business of Thompson Designs, Inc. (“Thompson Designs”) and its subsidiary, BPMX. |
| ● | “BPMX” refers to BioPharmX Inc., a Delaware corporation, and our direct, wholly owned subsidiary. |
| “U.S. dollar,” “$” and “US$” refer to the legal currency of the United States. | |
| ● | “Securities Act” refers to the Securities Act of 1933, as amended. |
| ● | “Exchange Act” refers to the Securities Exchange Act of 1934, as amended. |
Share Exchange Agreement
On January 23, 2014, Thompson Designs, BPMX and stockholders of BPMX who collectively own 100% of BPMX (the “BPMX Stockholders”) entered into and consummated transactions pursuant to a Share Exchange Agreement (the “Share Exchange Agreement,” such transaction referred to as the “Share Exchange Transaction”), whereby the Company issued to the BPMX Stockholders an aggregate of 7,025,000 shares of its common stock, par value $0.001 (“Common Stock”), in exchange for 100% of the equity interests of BPMX held by the BPMX Stockholders. The shares of our Common Stock received by the BPMX Stockholders in the Share Exchange Transaction constitute approximately 77.8% of our issued and outstanding Common Stock giving effect to the issuance of shares pursuant to the Share Exchange Agreement. As a result of the Share Exchange Transaction, BPMX became a subsidiary of the Company.
The Share Exchange Agreement contains representations and warranties by us, BPMX and the BPMX Stockholders, which are customary for transactions of this type, such as, (1) with respect to the Company: organization, good standing and qualification to do business; capitalization; subsidiaries; authorization and validity of the transaction and transaction documents; consents being obtained or not required to consummate the transaction; no conflict or violation of Articles of Incorporation and By-laws, (2) with respect to BPMX: authorization; capitalization; and, title to BPMX’s common stock being exchanged, and (3) with respect to BPMX Stockholders: authorization; no conflict or violation of law; investment purpose; accredited investor status; reliance on exemption on the Company’s Common Stock to be exchanged; and, transfer or resale pursuant to the Securities Act.
3
On January 23, 2014, we completed the acquisition of BPMX pursuant to the Share Exchange Agreement. The acquisition was accounted for as a reverse merger and recapitalization effected by a share exchange. BPMX is considered the acquirer for accounting and financial reporting purposes. The assets and liabilities of the acquired entity have been brought forward at their book value and no goodwill has been recognized.
OUR CORPORATE STRUCTURE
The following diagram sets forth the structure of the Company as of the date of this Report:
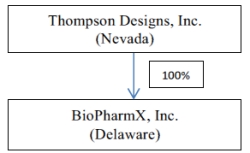
Organizational History of Thompson Designs, Inc.
Thompson Designs, Inc. was incorporated in Nevada on August 30, 2010. The business plan of the Company was originally to design and build custom signs for residential and commercial properties. Immediately after the completion of the Share Exchange Transaction, the Company discontinued its custom signs business and changed its business plan to development of novel delivery mechanisms and routes of administration for known drugs and tissues.
Organizational History of BioPharmX Inc.
BioPharmX Inc. was incorporated on August 18, 2011 in Delaware and is a development-stage biotechnology company focusing on development of novel delivery mechanisms and routes of administration for known drugs and tissues.
OUR BUSINESS
Overview
BioPharmX, Inc. (“BPMX”) is a Silicon Valley-based company, which seeks to provide innovative products through unique, proprietary platform technologies for pharmaceutical and over-the-counter (“OTC”) applications in the fast growing health and wellness markets, including women’s health, dermatology, and otolaryngology (ears, nose & throat).
BPMX is primarily a research & development (“R&D”) company focusing on the development of novel delivery mechanisms and novel routes of administration for known drugs and tissues. BPMX has expertise in formulation development, intellectual property generation, clinical trial execution, and regulatory strategy definition. BPMX’s business model is to outsource much of its manufacturing and commercialization activities in order to maintain its focus on technology sourcing, acquisitions, and partner development to create new products to address unmet needs in well-defined, multi-billion dollar markets.
Our Products
BPMX’s product pipeline includes products in three categories: prescription products, OTC products, and dietary supplements. Products will be delivered as oral, topical, inhalant, and/or injectable forms depending on the platform technology being applied and the anatomical target.
Prescription products in development include:
| ● | molecular iodine (I2) pill for the treatment of breast pain associated with fibrocystic breast disease, a woman’s health condition; |
| ● | topical antibiotics for acne and cutaneous bacterial infection; and, |
| ● | injectable filler for wrinkle reduction and volume enhancement |
In addition to OTC versions of some of our prescription products, our OTC product pipeline also includes a series of medicated bandages, nasal sprays and other products based on BPMX platform technologies.
Our supplement product pipeline includes BPMX’s breast health pill. Our initial product will be a dietary supplement distributed under the brand name VI2OLET™. The VI2OLET breast health pill is a women’s dietary supplement designed to promote breast health. The VI2OLET breast health pill includes patented technology that provides a stable solid oral dosage of molecular iodine as a safe and reliable supplement to promote breast health. Taken once a day, the VI2OLET breast health pill is intended to provide a new health measure for women to promote and enjoy a healthier life.
4
Our Market Opportunity
Our strategy begins with obtaining patented, platform technologies through in-house development, joint development, exclusive licensing, or acquisition. BPMX then develops these platform technologies into product lines and tests these products in clinical trials. BPMX frequently develops products with a bifurcated market penetration strategy, including a high-dosage, prescription version and an OTC low-dosage version. Identifying such technologies requires a strong knowledge of the markets served through technology assessment and evaluation of sell-side and buy-side opportunities through relationships with major pharmaceutical companies. By design, BPMX’s innovative products are formulated to address both market pathways and to address unmet needs in well-defined, multi-billion dollar markets. BPMX makes decisions on a product-by-product basis regarding IP licensing of its technologies or direct commercialization of its products for both pharmaceutical and OTC distribution and sales.
Strategic Partnerships/Alliances
We have existing strategic partnerships with two private companies based in the United States. We have a collaboration and licensing agreement with Iogen, LLC (“Iogen”), a biotechnology company with molecular iodine technology. Our iodine dietary supplement product and the development of our molecular iodine prescription product build upon this licensed technology. Under the agreement, BPMX received an exclusive worldwide perpetual irrevocable license to Iogen’s patented technology relating to an oral iodine pill. In consideration of the license granted under the agreement, BPMX agreed to pay to Iogen a non-refundable license issue fee of $150,000. In addition, BPMX also agreed to pay to Iogen 30% of net profit associated with direct commercialization of the product or 30% of net royalties received from any sublicensee. For other products developed and commercialized by BPMX, including the prescription iodine drug, BPMX agreed to pay to Iogen a royalty of 3% of net sales for the first 12 months of commercialization and 2% of net sales thereafter.
We also have a collaboration and supply agreement with NuTech Medical, Inc. (“NuTech”). This agreement describes the collaboration between BPMX and NuTech to develop products in the field of dermatology. Products developed under this agreement are exclusively owned by BPMX and licensed to Nutech for use in their field. In exchange for an exclusive license to Nutech’s IP in the field of dermatology, BPMX will pay to NuTech a royalty of 3% of net sales on Product sold in the field of dermatology. In exchange for granting Nutech an exclusive license to BioPharmX IP and IP developed in collaboration with NuTech in their field, BioPharmX shall receive from Nutech a royalty of 3% net sales on Products sold in their field.
Customers
Customers for our products and services include:
| ● | Pharmaceutical companies |
| ● | Dermatology and aesthetics practices |
| ● | Retail customers via retail sales channels and/or physician offices |
Suppliers
The Company has relationships with a number of suppliers for small quantities of materials to accomplish its research and development (“R&D”) objectives. In addition, the Company has relationships with contract manufacturers to supply and package its iodine dietary supplement pills for finished goods distribution. Our agreement with NuTech specifies that NuTech will supply materials for our dermatological products and associated R&D.
Manufacturing
The Company utilizes contract manufacturers to produce its products for commercial distribution. There is no plan to establish in-house manufacturing capabilities for large-scale production.
Marketing and Sales
The BPMX team has expertise in the commercialization of consumer products with channels such as drug (Walgreens, CVS), wholesale (Costco, Sam’s Club), department store (Nordstrom, Target) and specialty retail (GNC, Sephora). With years of combined experience branding and launching products both in the U.S. and Europe, the team has a deep understanding of channel strategies that include branded, private label, and licensed product strategies. BPMX plans to commercialize products in the pipeline of health and wellness markets, including women’s health, dermatology, and otolaryngology (ears, nose & throat) into various channels, beginning with our VI2OLET breast health pill.
5
BPMX has contracted with outsourced sales representatives with broad retail and pharmaceutical sales coverage and expertise. In addition, the company is leveraging outside marketing and advertising agencies to gain product awareness.
Competitive Environment
Our competitors, typically large pharmaceutical companies, vary from product to product. In the area of women’s health, many companies sell iodine supplements, mostly based on delivering iodine with iodide salts. We believe our competitive advantage is our licensed proprietary formulation, which delivers molecular iodine in a stable manner. In the areas of dermatology and aesthetics, market leaders include Valeant Pharmaceutical and Allergen.
High competitive barriers to entry include:
| ● | Imitation by competitors would require reverse engineering efforts |
| ● | Many products require time-consuming regulatory and clinical hurdles |
| ● | Exclusive partner agreements and licensing arrangements |
Technology and Intellectual Property
The Company has three proprietary platform technologies which can be used in various combinations for product development in both the prescription and OTC markets:
| ● | Iodine-based products |
| ● | Drug delivery technologies |
| ● | Injectable filler formulations |
Patents
Patent protection is an important aspect of our product portfolio development. BPMX actively develops intellectual property in-house and has several patents pending. BPMX has exclusively licensed patents for technologies related to molecular iodine.
BioPharmX Inc.
BPMX holds four U.S. provisional patent applications related to drug delivery technologies and iodine-based products.
Iogen, LLC
On March 1, 2013, BPMX entered into a collaboration and license agreement with Iogen to license certain patents, patent applications, formulations and know-how relating to molecular iodine formulations used to manufacture an oral pill based on Iogen technology. Below is a list of the U.S. patents and patent applications licensed by BPMX from Iogen.
| Title | Patent Number | |
| Treatment of iodine deficiency diseases | US 5,589,198 | |
| Methods and pharmaceutical compositions for oral delivery of molecular iodine | US 5,885,592 | |
| Stabilized oral pharmaceutical composition containing iodide and iodate and method | US 6,248,335 | |
| Non-staining topical iodine composition and method | US 6,432,426 | |
| Method for the eradication of pathogens including S. Aureus and antibiotic resistant microbes from the upper respiratory tract of mammals and for inhibiting the activation of immune cells | US 8,303,994 |
| Title | Patent Application Number | |
| Methods for inhibiting the activation of immune cells | US 2013-0039997 A1 |
6
Trademarks
BPMX has applied for trademark protection for its “BIOPHARMX” and “VI2OLET” trademarks in the U.S. and intends to apply for trademark protection in key markets outside the U.S.
Our Growth Strategy
BPMX has focused this past year on key milestones to growth, including (1) preparing for submission of an Investigational New Drug application for Phase III clinical trials for the molecular iodine pill to obtain U.S. Food and Drug Administration (FDA) approval, (2) developing commercialization plans for the low dosage version under the brand name VI2OLET, and (3) advancing the pre-clinical development and testing for the topical acne product. The required capital to grow and expand product development will depend on the company’s ability to utilize the capital markets, once publicly traded on the over-the-counter (“OTCBB”) exchange with plans to list on the NASDAQ or NYSE exchange.
Future sources of revenue are expected to include partner license fees, contracted development payments, and royalties, along with direct commercialization proceeds. Product costs of goods sold include all outsourced manufacturing costs, partner royalties, and sourcing expenses, as well as partner funded clinical trial costs associated with contracted development payments. Operating expenses include direct personnel costs, direct commercialization, and directly funded clinical costs along with associated overhead expenses.
Our overall strategy is to identify early-stage scientific research projects being done by outside individuals and organizations and develop that research into commercially viable products for prescription, OTC, and dietary supplement use within the health and wellness, dermatology, and otolaryngology markets.
Research and Development
Our core competency is providing the link between concept and commercialization through focused, practical product development based on innovative research. We employ highly-qualified scientists and consultants specializing in our various product development areas. More than 50% of our research staff have earned a Ph.D. degree and have previous experience at dynamic, high-growth companies.
As a Silicon Valley-based company, we are located in a region with many strong biotech and pharmaceutical companies, which have drawn a high caliber of scientists and scientific support staff to the region. We believe this will enable the Company to grow our product development and consultant staff. The Company’s location also provides convenient access to local formulation resources and pre-clinical test facilities.
The Company spent $31,000 and $4,000 on its research and development during the fiscal years ended December 31, 2012 and 2011, respectively. The Company spent $401,000 and $222,000 on its research and development during the nine and three months ended September 30, 2013, respectively.
Significant Accomplishments
The Company is currently preparing an IND application to enter Phase III clinical trials for the molecular iodine pill prescription product. Previously completed Phase I & II clinical trial results of over 1,500 study subjects, available through our license agreement with Iogen, will be leveraged to pursue a Phase III study that is carefully planned to achieve the most efficient and effective results towards obtaining the necessary approval.
Government Regulation
We intend to sell our products in the U.S. and foreign markets. Many of these markets are highly regulated for the distribution of drug products. Our products fall into the following key regulatory categories within the U.S.: prescription drugs, over-the-counter drugs, medical devices, and dietary supplements. Several of our products may require demonstration of safety and efficacy before being permitted to be sold. We anticipate that the majority of our products will take 3-5 years to conduct clinical studies and regulatory review before they can be sold.
7
Insurance
The Company maintains standard corporate liability insurance and plans to have standard product liability insurance prior to product launch.
Employees
The Company has 6 full-time employees as of January 24, 2014. Our employees have business and technical expertise in pharmaceutical, biological sciences, medical devices, and consumer product development, and possess extensive experience in ex-vivo/in-vitro design, preclinical and clinical development, and intellectual property generation. Quality and regulatory expertise includes knowledge of medical directives, U.S. FDA guidance for drugs and medical devices and ICH & IEC guidelines for the U.S., Canada and the European Union. The Company also utilizes consultants to provide additional expertise in niche or highly technical areas.
BPMX considers its employee relations to be good, and to date has not experienced a work stoppage due to a labor dispute. None of BPMX’s employees are represented by a labor union.
DESCRIPTION OF PROPERTY
Our principal executive office and laboratory is located at 1098 Hamilton Court, Menlo Park, California 94025, where the company occupies 10,800 sq. ft. of R&D and administration facilities that are nearby to external formulation, clinical and pre-clinical testing facilities. The lease has a term of 39 months. The monthly base rent for the facilities is $23,220 through November 30, 2014, $23,916 through November 30, 2015, and $24,634 through November 30, 2016. This excludes monthly operating expenses initially estimated at $3,261. The initial security deposit under the lease is $150,000 which is to be reduced to $50,000 after BPMX has received at least $6 million in new funding.
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND
PLAN OF OPERATIONS
This Current Report on Form 8-K contains forward-looking statements within the meaning of the federal securities laws. These include statements about our expectations, beliefs, intentions or strategies for the future, which we indicate by words or phrases such as “anticipate,” “expect,” “intend,” “plan,” “will,” “we believe,” “management believes” and similar language. Except for the historical information contained herein, the matters discussed in this “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” and elsewhere in this Current Report are forward-looking statements that involve risks and uncertainties. The cautionary language in this Current Report, provide examples of risks, uncertainties and events that may cause our actual results to differ materially from those projected. Except as may be required by law, we undertake no obligation to update any forward-looking statement to reflect events after the date of this Current Report on Form 8-K.
Plan of Operations
The Company plans to implement operations and reach its goals and objectives by hiring talented people to play key roles throughout the organization. The Company strives to hire employees with long term successful track records. It seeks to identify and hire the best talent for each open position. The Company will initiate a strong branding and marketing campaign for its first commercialized product, branded VI2OLET. While a portion of the funds that the Company intends to raise through sale of the Company's securities to finance its operations will be put into marketing, branding, and sales activities along with operational support activities, the majority of funds will be used for advancing research and development activities for new products in the pre-clinical pipeline and launching Phase III clinical studies for the molecular iodine prescription product.
Results of Operations
Nine Months ended September 30, 2013
Revenue
From August 18, 2011 through September 30, 2013, the Company has not had any revenues. The Company is in the research and development stage, but projects its molecular iodine prescription product will be released in 2014.
8
Research and Development Expenses
Research and development expenses for the nine months ended September 30, 2013, consisted primarily of compensation and benefits in the amount of $318,000 and laboratory supplies of $40,000. Research and development expenses for the period from August 18, 2011 (date of inception) through December 31, 2012 consisted of $22,000 of compensation and benefits and $5,000 in laboratory supplies. The Company has 12 full and part time employees and consultants in research and development as of September 30, 2013. The Company plans to convert some of the consultants to employees in 2014.
Sales and Marketing Expenses
Sales and marketing expenses for the nine months ended September 30, 2013, consisted primarily of compensation and benefits in the amount of $37,000 and the cost of developing marketing strategy and material in the amount of $28,000. Sales and marketing expenses for the period from August 18, 2011 (date of inception) through December 31, 2012 consisted of $7,000 primarily for the development of the company website and logo. The Company currently has no employees in sales and marketing and plans to continue to use outside consultants for this work, some of whom the Company may hire in 2014.
General and Administrative Expenses
General and administrative expenses for the nine months ended September 30, 2013, consisted primarily of compensation and benefits in the amount of $162,000, professional fees totaling $67,000 to the Company’s legal counsel and auditors, travel expense of $47,000, as well as other general and administrative expenses totaling $10,000. General and administrative expenses for the period from August 18, 2011 (date of inception) through December 31, 2012 consisted primarily of $26,000 in compensation and benefits.
Loss from Operations
Loss from operations for the nine months ended September 30, 2013 was $780,000. The loss was primarily attributable to spending on research and development with no current revenue. Loss from operations for period from August 18, 2011 (date of inception) through December 31, 2012, was $90,000.
Net Loss
Net loss for the nine months ended September 30, 2013 was $822,000. Net loss for the period from August 18, 2011 (date of inception) through December 31, 2012, was $95,000.
Inflation did not have a material impact on the Company’s operations for either of the periods. Other than the foregoing, management knows of no trends, demands, or uncertainties that are reasonably likely to have a material impact on the Company’s results of operations.
Fiscal Years Ended December 31, 2012 and 2011
Revenue
From August 18, 2011 through December 31, 2012, the Company has not had any revenues. The Company is in the research and development stage, but currently plans to have its molecular iodine prescription product to market in 2014.
Research and Development Expenses
Research and development expenses for the fiscal years ended December 31, 2012 and 2011, consisted primarily of compensation and benefits in the amount of $21,000 and $1,000, respectively. Research and development expenses for period from inception through December 31, 2012 consisted of $22,000 of compensation and benefits and $5,000 in laboratory supplies. The Company had 5 part-time consultants in research and development on December 31, 2012.
Sales and Marketing Expenses
Sales and marketing expenses for the fiscal years ended December 31, 2012 and 2011, consisted primarily of advertising and promotion in the amount of $7,000 and $150 for the cost of developing the Company’s website and logo. Sales and marketing expenses for the period from inception through December 31, 2012 consisted of $7,000 primarily for the development of the company website and logo. The Company currently has no employees in sales and marketing and uses outside consultants for this work.
9
General and Administrative Expenses
General and administrative expenses for the fiscal years ended December 31, 2012 and 2011, consisted primarily of compensation and benefits in the amount of $26,000 and zero, respectively. General and administrative expenses for the period from August 18, 2011 (date of inception) through December 31, 2012 primarily consisted of $26,000 in compensation and benefits.
Loss from Operations
Loss from operations for the fiscal years ended December 31, 2012 and 2011 was $83,000 and $7,000, respectively. The loss from operations was primarily attributable to spending on research and development with no current revenue. Loss from operations for the period from August 18, 2011 (date of inception) through December 31, 2012, was $90,000.
Net Loss
Net loss for the fiscal years ended December 31, 2012 and 2011 was $88,000 an $7,000, respectively. Net loss for the period from inception through December 31, 2012, was $95,000.
Inflation did not have a material impact on the Company’s operations for either of the periods. Other than the foregoing, management knows of no trends, demands, or uncertainties that are reasonably likely to have a material impact on the Company’s results of operations.
In January 2013, BPMX issued to an accredited investor 6% secured convertible notes in the aggregate principal amount of $500,000. The notes are secured by all the assets of BPMX pursuant to the Security Agreement dated January 3, 2014, by and between BPMX and the collateral agent, and rank senior to any other indebtedness of BPMX. The notes are automatically convertible into the shares of common stock of the Company, contingent on the completion of the reverse acquisition of the Company by BPMX and closing of a financing in the amount of at least $2,000,000 at a conversion price per share equal to 80% of the per share offering price of such financing. The investor has the right to receive at the closing of the reverse acquisition warrants to purchase shares of common stock of the Company equal to 100% of the shares of common stock underlying the respective notes.
Between September 2012 and January 2014, the Company issued 6% unsecured convertible notes to investors in the aggregate principal amount of $1,220,000. These notes have a maturity date three years from the date of issuance, with principal and interest payable at maturity. The notes are automatically convertible into the securities of the Company sold in an offering that takes place after the completion of the reverse acquisition of the Company by BPMX, contingent on the completion of the reverse acquisition and closing of such financing at a conversion price per share equal to 80% of the per share offering price of such financing.
The following table summarizes total current assets, liabilities and working capital at September 30, 2013.
September 30, 2013 | ||||
| Current Assets | $ | 166,540 | ||
| Current Liabilities | $ | 498,756 | ||
| Working Capital Deficit | $ | (332,216 | ) | |
At September 30, 2013, we had a working capital deficit of $332,000.
Net cash used for operating activities for the nine months ended September 30, 2013 was $670,000. Cash used in operating activities was primarily due to net loss for the nine months ended September 30, 2013 of $822,000 which was partially offset by changes in operating assets and liabilities of $76,000, non-cash interest expense of $42,000 and stock-based compensation of $28,000. Cash used in investing activities was primarily for acquisition of intellectual property and acquisition of fixed assets.
Net cash obtained through all financing activities for the nine months ended September 30, 2013 was $630,000. This consisted of primarily $630,000 in proceeds from issuing convertible notes payable.
Going Concern
As reflected in the accompanying financial statements, the Company has a net loss and net cash used in operations of $822,000 and $670,000, respectively, for the nine months ended September 30, 2013 and a deficit accumulated during the development stage of $917,000 as of September 30, 2013.
The ability of the Company to continue its operations is dependent on Management's plans, which include the raising of capital through debt and/or equity markets with some additional funding from other traditional financing sources, including term notes, until such time that funds provided by operations are sufficient to fund working capital requirements. The Company may need to incur additional liabilities with certain related parties to sustain the Company’s existence.
10
The Company may require additional funding to finance the growth of its current and expected future operations as well as to achieve its strategic objectives. The Company believes its current available cash along with anticipated revenues may be insufficient to meet its cash needs for the near future if it does not receive the anticipated additional funding. There can be no assurance that financing will be available in amounts or terms acceptable to the Company, if at all. In that event, the Company would be required to change its growth strategy and seek funding on that basis, if at all.
The accompanying financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. These financial statements do not include any adjustments relating to the recovery of the recorded assets or the classification of the liabilities that might be necessary should the Company be unable to continue as a going concern.
In response to the above, management will:
| ● | seek additional third party debt and/or equity financing; |
| ● | continue with the implementation of the business plan; |
| ● | seek to generate revenue through commercialization of the technology. |
To date, all of our funding has been generated from private investments. During the next twelve months, we anticipate raising funding to continue expansion; however, as of this writing, we only have sufficient funds to proceed with basic company operations only. We do not have sufficient funds to fully implement our business plan until such time that we are able to raise additional funding, to which there is no guarantee. If we do not obtain the funds necessary for us to continue our business activities we may need to curtail or cease our operations until such time as we have sufficient funds.
Recent Accounting Pronouncements
There are no recent accounting pronouncements that are expected to have a material impact on the Company’s financial statements.
Critical Accounting Policies
Our financial statements and related public financial information are based on the application of accounting principles generally accepted in the United States (“GAAP”). GAAP requires the use of estimates; assumptions, judgments and subjective interpretations of accounting principles that have an impact on the assets, liabilities, revenues and expense amounts reported. These estimates can also affect supplemental information contained in our external disclosures including information regarding contingencies, risk and financial condition. We believe our use of estimates and underlying accounting assumptions adhere to GAAP and are consistently applied. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances. Actual results may differ materially from these estimates under different assumptions or conditions. We continue to monitor significant estimates made during the preparation of our financial statements.
Our significant accounting policies are summarized in Note 3 of our audited financial statements. While all these significant accounting policies impact our financial condition and results of operations, we view certain of these policies as critical. Policies determined to be critical are those policies that have the most significant impact on our financial statements and require management to use a greater degree of judgment and estimates. Actual results may differ from those estimates. Our management believes that given current facts and circumstances, it is unlikely that applying any other reasonable judgments or estimate methodologies would cause effect on our results of operations, financial position or liquidity for the periods presented in this report.
11
We believe the following critical accounting policies and procedures, among others, affect our more significant judgments and estimates used in the preparation of our financial statements:
Stock-based compensation
The Company accounts for stock-based employee compensation arrangements which requires the recognition of compensation expense, using a fair-value based method for costs related to all employee share-based payments, including stock options. The Company estimates the fair value of share-based payment awards on the date of grant using an option pricing model. All option grants have been expensed on a straight-line basis over their vesting period. Equity instruments issued to nonemployees are recorded at their fair value on the measurement date and are subject to periodic adjustment as the underlying equity instruments vest.
For the period August 18, 2011 (date of inception) to December 31, 2012 stock-based compensation amounted to $9,000. For the nine months ended September 30, 2013, stock-based compensation was $28,000.
Off Balance Sheet Arrangements:
We do not have any off-balance sheet arrangements, financings, or other relationships with unconsolidated entities or other persons, also known as “special purpose entities” (SPEs).
DIRECTORS, EXECUTIVE OFFICERS, PROMOTERS AND CONTROL PERSONS
Directors and Executive Officers
In connection with the change in control of the Company described in Item 5.01 of this report, effective on January 21, 2014, we appointed Mr. James Pekarsky as our Chairman, Chief Executive Officer and Chief Financial Officer, and Ms. Anja Krammer as our Director and President. Ms. Kade Thompson resigned as our officer at the same time.
The following table sets forth certain information as of the Effective Date concerning our directors and executive officers. All directors hold office until the next annual meeting of stockholders or until their respective successors are elected, except in the case of death, resignation or removal:
| Name | Age | Position | ||
| James R. Pekarsky | 54 | Chief Executive Officer, Chief Financial Officer, and Chairman of the Board of Directors | ||
| Anja Krammer | 46 | President and Director |
James R. Pekarsky, age 54, is a seasoned executive who brings extensive financial and operational experience from public and private high technology and medical research companies. His background includes substantial international business experience, strategic planning, acquisitions, venture capital, bank fund raising and IPOs. Since 2008, Mr. Pekarsky has been a consultant serving as Chief Financial Officer and Chief Operating Officer to several private and public companies. Most recently, he served as Chief Financial Officer of Solar Power, Inc. (OTC Markets: SOPW) from November 2011 to August 2013. Additionally, Mr. Pekarsky served as Chief Financial Officer of MoSys, Inc., (NASDAQ: MOSY), from January 2006 to November 2007 and Virage Logic (NASDAQ:VIRL) from May 1999 to November 2003, where he led the company’s IPO. Other public companies include Mentor Graphics (NASDAQ:MENT) and Bio-Rad Laboratories (NYSE:BIO), where Mr. Pekarsky held General Manager positions based in Europe for 5 years. Mr. Pekarsky holds a B.S. in Accounting from Indiana University of Pennsylvania and an M.B.A. in Finance from Golden Gate University. We believe that Mr. Pekarsky’s credentials and extensive experience as an executive officer of publicly traded companies position him well as a member of our board of directors.
Anja Krammer, age 46, is a veteran marketing executive with over 20 years of experience in guiding healthcare and consumer enterprises in product development, sales/marketing management and commercialization strategies. Her industry background includes pharmaceuticals, medical devices, technology, and consumer products. Her therapeutic area experience includes dermatology (aesthetic/cosmetic and therapeutic drugs), cardiovascular, diabetes, consumer health, gastroenterology, and orthopedics. Ms. Krammer has served as President of BioPharmX since August 2011. Ms. Krammer previously served as Chief Marketing Officer/Founder of MBI, Inc., a management consulting firm from 2008 to 2013. Prior to joining MBI Consulting, Ms. Krammer was Vice President Global Marketing from April 2006 to August 2008 for Reliant Technologies, a venture backed startup in aesthetic medicine. From April 2004 to April 2006, Ms. Krammer served as Sr. Director of Strategic Marketing for Medtronic Corporation (NYSE: MDT). From December 2000 to September 2001, Ms. Krammer was Vice President, Solutions Marketing for Getronics Corporation (AMS: GTN), a global IT services company. From April 1999 to December 2000, Ms. Krammer served as Vice President, Indirect Channel Sales and Worldwide Industry Partnership Marketing, Itronix Division, Acterna Corporation (NASDAQ: ACTR), an optical communications company. Prior roles included, serving as Director of Worldwide Marketing and Communications Tektronix Corporation in its Color Printing and Imaging Division (NYSE: TEK) from October 1997 to April 1999. From October 1995 to October 1997, Ms. Krammer was Director of Worldwide Sales and Marketing with KeyTronic Corporation (Nasdaq: KTCC), a computer equipment manufacturer. Ms. Krammer holds a BAIS degree with a focus on Marketing/Management from University of South Carolina and an International Trade Certificate from the Sorbonne, University of Paris. We believe that Ms. Krammer's qualifications and her extensive experience as an officer of publicly traded technology companies provide a unique perspective for our Board.
12
Committees
We do not have a standing nominating, compensation or audit committee. Rather, our full board of directors performs the functions of these committees. We do not believe it is necessary for our board of directors to appoint such committees because the volume of matters that come before our board of directors for consideration permits the directors to give sufficient time and attention to such matters to be involved in all decision making. Additionally, because our Common Stock is not listed for trading or quotation on a national securities exchange, we are not required to have such committees.
Audit Committee Financial Expert
The board of directors has determined that Mr. James Pekarsky is our Audit Committee financial expert, as defined under Item 407(d)(5)(i) of Regulation S-K.
Director Independence
Our securities are not listed on a national securities exchange or in an inter-dealer quotation system which has requirements that directors be independent. No member of the Company’s board of directors qualifies as an independent director pursuant to the definition of “independent director” under the Rules of NASDAQ, Marketplace Rule 5605(a)(2). We do not have majority of independent directors.
Code of Ethics
We do not have a code of ethics but intend to adopt one in the near future that applies to all of our directors, officers and employees, including our principal executive officer, principal financial officer and principal accounting officer. The new code will address, among other things, honesty and ethical conduct, conflicts of interest, compliance with laws, regulations and policies, including disclosure requirements under the federal securities laws, confidentiality, trading on inside information, and reporting of violations of the code.
Meetings of the Board of Directors
During the Company’s fiscal year ended September 30, 2013, the board of directors did not meet on any occasion, but rather transacted business by unanimous written consent.
Board Leadership Structure and Role in Risk Oversight
Our Board recognizes that the leadership structure and combination or separation of the president and chairman roles is driven by the needs of the Company at any point in time. Currently, Mr. James Pekarsky serves as the Chief Executive Officer of the Company and the Chairman of our Board, and Ms. Anja Krammer serves as the President of the Company. We have no policy requiring the combination or separation of leadership roles and our governing documents do not mandate a particular structure. This has allowed, and will continue to allow, our Board the flexibility to establish the most appropriate structure for our company at any given time.
Immediately following the consummation of the Share Exchange Transaction, the size of our management team will be increased in order to manage our expanded operations, risks and resources.
13
Summary Compensation
The following is a summary of the compensation we paid to our executive officers, for the two fiscal years ended September 30, 2013 and 2012, and compensation paid by BPMX for the fiscal years ended December 31, 2012 and 2011. BPMX was organized on August 18, 2011.
| Name and Principal Position | Year | Salary ($) | Bonus ($) | Stock Awards ($) | Option Awards ($) | All Other Compensation ($) | Totals ($) | ||||||||||||||||||
James R. Pekarsky(1) CEO, CFO, Chairman of the Company; CEO and Director of BPMX | FY2012 FY2011 | - | - | - | - | - | - | ||||||||||||||||||
Anja Krammer(2) President and Director of the Company; President and Director of BPMX | FY2012 FY2011 | - | - | - | - | - | - | ||||||||||||||||||
Kade Thompson CEO, CFO, Director of the Company | FY2013 FY2012 | - | - | - | - | - | - | ||||||||||||||||||
_____________________
| (1) | Mr. Pekarsky was appointed as our Chief Executive Officer, Chief Financial Officer and Chairman on January 21, 2014. Mr. Pekarsky has been the Chief Executive Officer and Director of BPMX since its inception. |
| (2) | Ms. Krammer was appointed as our Director and President on January 21, 2014. Ms. Krammer has been the President and Director of BPMX since its inception. |
| (3) | Ms. Thompson resigned as our Sole Director, Chief Executive Officer, Chief Financial Officer, President, Treasurer and Secretary on January 21, 2014. |
Employment Agreements
On January 21, 2014, the Company and James Pekarsky entered into an Employment Agreement, where Mr. Pekarsky was employed as Chief Executive Officer and Chairman of the Board of the Company for a term of four years with a one-year automatic renewal term. Mr. Pekarsky is entitled to the compensation consisting of $250,000 per year for base salary and an annual bonus if performance targets are met at the discretion of the board of directors.
On January 21, 2014, the Company and Anja Krammer entered into an Employment Agreement, where Ms. Krammer was employed as President and Director of the Company for a term of four years with a one-year automatic renewal term. Ms. Krammer is entitled to the compensation consisting of $250,000 per year for base salary and an annual bonus if performance targets are met at the discretion of the board of directors.
For the fiscal year ended September 30, 2013, none of the members of our board of directors received compensation for service as a director. We do not currently have an established policy to provide compensation to members of our board of directors for their services in that capacity.
14
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDPENDENCE
Transactions with related persons
Our policy is that a contract or transaction either between the Company and a director, or between a director and another company in which he is financially interested is not necessarily void or void-able if the relationship or interest is disclosed or known to the board of directors and the stockholders are entitled to vote on the issue, or if it is fair and reasonable to our company.
On September 17, 2010, the Company issued 7,000,000 shares of common stock to Kade Thompson, its President, CEO, CFO, and sole Director, in consideration for $7,000 at a price of $0.001 per share. On December 21, 2012, the Company borrowed $3,000 from Ms. Thompson, under the terms of a Promissory Note due December 21, 2014. The note bears interest of 5% per annum payable at maturity.
On January 21, 2014, Ms. Thompson sold to BPMX 7,000,000 shares of the Company’s common stock representing approximately 77.8% of the then issued and outstanding shares of common stock and cancelled the 5% promissory note pursuant to a Stock Purchase Agreement dated as of the same date.
Since inception of BPMX, the founding executives of the company have made advances to cover short-term operating expenses. These advances are non-interest bearing. As of September 30, 2013 and December 31, 2012, related party payables of BPMX were $139,000 and $17,000, respectively.
Except for the above transactions or as otherwise set forth in this report or in any reports filed by the Company with the SEC, the Company was not a party to any transaction (where the amount involved exceeded the lesser of $120,000 or 1% of the average of our assets for the last two fiscal years) in which a Director, executive officer, holder of more than five percent of our common stock, or any member of the immediate family of any such person have or will have a direct or indirect material interest and no such transactions are currently proposed. The Company is currently not a subsidiary of any company.
The Company’s Board conducts an appropriate review of and oversees all related party transactions on a continuing basis and reviews potential conflict of interest situations where appropriate. The Board has not adopted formal standards to apply when it reviews, approves or ratifies any related party transaction. However, the Board believes that the related party transactions are fair and reasonable to the Company and on terms comparable to those reasonably expected to be agreed to with independent third parties for the same goods and/or services at the time they are authorized by the Board.
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table provides the names and addresses of each person known to us to own more than 5% of our outstanding shares of common stock as of January 23, 2014 immediately following the closing of the Share Exchange Transaction and cancellation of the 7,000,000 shares purchased by BPMX from Kade Thompson, and by the officers and directors, individually and as a group. Except as otherwise indicated, all shares are owned directly and the shareholders listed possess sole voting and investment power with respect to the shares shown. The address for each officer and director is 1098 Hamilton Court, Menlo Park, California 94025.
Name Officers and Directors | Office | Shares Beneficially Owned(1) | Percent of Class(2) | |||||||
| James Pekarsky | Chairman and CEO | 2,500,000 | 27.7 | % | ||||||
| Anja Krammer | Director and President | 2,500,000 | 27.7 | % | ||||||
| All officers and directors as a group (2 persons named above) | 5,000,000 | 55.4 | % | |||||||
| 5% Securities Holders | ||||||||||
Kin Chan 1098 Hamilton Court Menlo Park, California 94025 | 1,200,000 | 13.3 | % | |||||||
Kevin Mszanowski 211 Solana Drive Los Altos, California 94022 | 825,000 | 9.1 | % | |||||||
_______________
| (1) | Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. |
| (2) | Based on 9,025,000 shares of the Company’s common stock issued and outstanding. |
15
DESCRIPTION OF SECURITIES
General
Our authorized capital stock consists of 90,000,000 shares of common stock, with a par value of $0.001 per share, and 10,000,000 shares of preferred stock, par value $0.001 per share.
Common Stock
Our common stock is entitled to one vote per share on all matters submitted to a vote of the stockholders, including the election of directors. Except as otherwise required by law or provided in any resolution adopted by our board of directors with respect to any series of preferred stock, the holders of our common stock will possess all voting power. Generally, all matters to be voted on by stockholders must be approved by a majority (or, in the case of election of directors, by a plurality) of the votes entitled to be cast by all shares of our common stock that are present in person or represented by proxy, subject to any voting rights granted to holders of any preferred stock. Holders of our common stock representing fifty percent (50%) of our capital stock issued, outstanding and entitled to vote, represented in person or by proxy, are necessary to constitute a quorum at any meeting of our stockholders. A vote by the holders of a majority of our outstanding shares is required to effectuate certain fundamental corporate changes such as liquidation, merger or an amendment to our Articles of Incorporation. Our Articles of Incorporation do not provide for cumulative voting in the election of directors.
Subject to any preferential rights of any outstanding series of preferred stock created by our board of directors from time to time, the holders of shares of our common stock will be entitled to such cash dividends as may be declared from time to time by our board of directors from funds available therefore.
Subject to any preferential rights of any outstanding series of preferred stock created from time to time by our board of directors, upon liquidation, dissolution or winding up, the holders of shares of our common stock will be entitled to receive pro rata all assets available for distribution to such holders.
In the event of any merger or consolidation with or into another company in connection with which shares of our common stock are converted into or exchangeable for shares of stock, other securities or property (including cash), all holders of our common stock will be entitled to receive the same kind and amount of shares of stock and other securities and property (including cash). Holders of our common stock have no pre-emptive rights, no conversion rights and there are no redemption provisions applicable to our common stock.
As of the date of this report, there were 9,025,000 shares of common stock issued and outstanding.
Preferred Stock
Our board of directors may become authorized to authorize preferred shares of stock and to divide the authorized shares of our preferred stock into one or more series, each of which must be so designated as to distinguish the shares of each series of preferred stock from the shares of all other series and classes. Our board of directors is authorized, within any limitations prescribed by law and our articles of incorporation, to fix and determine the designations, rights, qualifications, preferences, limitations and terms of the shares of any series of preferred stock including, but not limited to, the following:
| 1. | The number of shares constituting that series and the distinctive designation of that series, which may be by distinguishing number, letter or title; |
| 2. | The dividend rate on the shares of that series, whether dividends will be cumulative, and if so, from which date(s), and the relative rights of priority, if any, of payment of dividends on shares of that series; |
| 3. | Whether that series will have voting rights, in addition to the voting rights provided by law, and, if so, the terms of such voting rights; |
| 4. | Whether that series will have conversion privileges, and, if so, the terms and conditions of such conversion, including provision for adjustment of the conversion rate in such events as the board of directors determines; |
| 5. | Whether or not the shares of that series will be redeemable, and, if so, the terms and conditions of such redemption, including the date or date upon or after which they are redeemable, and the amount per share payable in case of redemption, which amount may vary under different conditions and at different redemption dates; |
| 6. | Whether that series will have a sinking fund for the redemption or purchase of shares of that series, and, if so, the terms and amount of such sinking fund; |
| 7. | The rights of the shares of that series in the event of voluntary or involuntary liquidation, dissolution or winding up of the corporation, and the relative rights of priority, if any, of payment of shares of that series; |
| 8. | Any other relative rights, preferences and limitations of that series |
Options
As of January 24, 2014 there are outstanding options to purchase 2,606,000 shares of the Company’s common stock issued under the 2014 Equity Incentive Plan. The options generally vest 25% annually and expire ten years from the date of grant.
MARKET PRICE OF AND DIVIDENDS ON OUR COMMON
EQUITY AND RELATED STOCKHOLDER MATTERS
While there is no established public trading market for our Common Stock, our Common Stock is quoted on the OTC Markets OTCQB, under the symbol “TPND.” Except for one quotation dated February 14, 2013 of $0.15, there have been no reported quotations for our common stock for the two most recent fiscal years and for the interim period ended September 30, 2013 for which financial statements are included in this report.
The market price of our Common Stock is subject to significant fluctuations in response to variations in our quarterly operating results, general trends in the market and other factors, over many of which we have little or no control. In addition, broad market fluctuations, as well as general economic, business and political conditions, may adversely affect the market for our Common Stock, regardless of our actual or projected performance.
Holders
As of January 23, 2014, we had 9,025,000 shares of our common stock par value, $0.001 issued and outstanding. There were approximately 28 beneficial owners of our common stock.
16
The Transfer Agent for our capital stock is Empire Stock Transfer, located at 1859 Whitney Mesa Dr., Henderson, Nevada 89014.
Penny Stock Regulations
The Securities and Exchange Commission has adopted regulations which generally define “penny stock” to be an equity security that has a market price of less than $5.00 per share. Our Common Stock, when and if a trading market develops, may fall within the definition of penny stock and be subject to rules that impose additional sales practice requirements on broker-dealers who sell such securities to persons other than established customers and accredited investors (generally those with assets in excess of $1 million, or annual incomes exceeding $200,000 individually, or $300,000, together with their spouse).
For transactions covered by these rules, the broker-dealer must make a special suitability determination for the purchase of such securities and have received the purchaser’s prior written consent to the transaction. Additionally, for any transaction, other than exempt transactions, involving a penny stock, the rules require the delivery, prior to the transaction, of a risk disclosure document mandated by the Securities and Exchange Commission relating to the penny stock market. The broker-dealer also must disclose the commissions payable to both the broker-dealer and the registered representative, current quotations for the securities and, if the broker-dealer is the sole market-maker, the broker-dealer must disclose this fact and the broker-dealer’s presumed control over the market. Finally, monthly statements must be sent disclosing recent price information for the penny stock held in the account and information on the limited market in penny stocks. Consequently, the “penny stock” rules may restrict the ability of broker-dealers to sell our Common Stock and may affect the ability of investors to sell their Common Stock in the secondary market.
Dividend Policy
We have not paid any cash dividends to our shareholders. Any future determination as to the declaration and payment of dividends on shares of our Common Stock will be made at the discretion of our board of directors out of funds legally available for such purpose. We are under no contractual obligations or restrictions to declare or pay dividends on our shares of Common Stock. In addition, we currently have no plans to pay such dividends.
Equity Incentive Plan Information
On January 23, 2014, the Company adopted the 2014 Equity Incentive Plan (the “Plan”) which permits the Company to grant stock options to directors, officers or employees of the Company or others to purchase shares of common stock of the Company through awards of incentive and nonqualified stock options (“Option”), stock (Restricted Stock or Unrestricted Stock) and stock appreciation rights (“SARs”). 2,700,000 shares of the Company’s common stock have been authorized and reserved for the Plan, subject to an adjustment for an increase or decrease of the Company’s issued and outstanding Common Stock resulting from a stock split, change of the number of shares issued and outstanding without receipt of consideration by the Company or as the Plan administrator may determine in its discretion, provided that in no event shall the total number of shares of common stock authorized under the plan exceed 30% of the issued and outstanding shares of the Company’s common stock.
17
LEGAL PROCEEDINGS
There are no material proceedings to which any director or officer, or any associate of any such director or officer, is a party that is adverse to our Company or any of our subsidiaries or has a material interest adverse to our Company or any of our subsidiaries. No director or executive officer has been a director or executive officer of any business which has filed a bankruptcy petition or had a bankruptcy petition filed against it during the past ten years. No director or executive officer has been convicted of a criminal offense or is the subject of a pending criminal proceeding during the past ten years. No director or executive officer has been the subject of any order, judgment or decree of any court permanently or temporarily enjoining, barring, suspending or otherwise limiting his involvement in any type of business, securities or banking activities during the past ten years. No director or officer has been found by a court to have violated a federal or state securities or commodities law during the past ten years.
In addition, there are no material proceedings to which any affiliate of our Company, or any owner of record or beneficially of more than five percent of any class of voting securities of our Company, is a party that is adverse to our Company or any of our subsidiaries or has a material interest adverse to our Company or any of our subsidiaries. Currently there are no legal proceedings pending or threatened against us. We are not currently involved in any litigation that we believe could have a materially adverse effect on our financial condition or results of operations.
There is no action, suit, proceeding, inquiry or investigation before or by any court, public board, government agency, self-regulatory organization or body pending or, to the knowledge of the executive officers of our Company or any of our subsidiaries, threatened against or affecting our Company, our common stock, any of our subsidiaries or of our Company’s or our Company’s subsidiaries’ officers or directors in their capacities as such, in which an adverse decision could have a material adverse effect.
However, from time to time, we may become involved in various lawsuits and legal proceedings which arise in the ordinary course of business. Litigation is subject to inherent uncertainties, and an adverse result in these or other matters may arise from time to time that may harm our business.
RECENT SALES OF UNREGISTERED SECURITIES
Reference is made to Item 3.02 of this Current Report on Form 8-K for a description of recent sales of unregistered securities, which is hereby incorporated by reference.
INDEMNIFICATION OF OFFICERS AND DIRECTORS
Our by-laws provide for the indemnification of our directors, officers, employees, and agents, under certain circumstances, against attorney's fees and other expenses incurred by them in any litigation to which they become a party arising from their association with or activities on our behalf. We will also bear the expenses of such litigation for any of our directors, officers, employees, or agents, upon such persons promise to repay us therefore if it is ultimately determined that any such person shall not have been entitled to indemnification. This indemnification policy could result in substantial expenditures by us, which it may be unable to recoup.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons pursuant to the foregoing provisions, or otherwise, we have been advised that in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is, therefore unenforceable.
At the present time, there is no pending litigation or proceeding involving a director, officer, employee or other agent of ours in which indemnification would be required or permitted. We are not aware of any threatened litigation or proceeding which may result in a claim for such indemnification.
Please refer to Item 1.01 - “Entry into a Material Definitive Agreement” for a description of the unregistered sales of equity securities as a result of the Share Exchange Transaction, which is incorporated in its entirety into this Item 3.03.
The issuances of these securities were exempt from registration under the Securities Act pursuant to Section 4(2) of the Securities Act, Regulation D and Regulation S promulgated thereunder.
Item 4.01 Changes in Registrant’s Certifying Accountant
Previous Independent Accountants
On January 23, 2014, we dismissed Silberstein Ungar, PLLC (“SUPLLC”), as our independent accountant. The reports of SUPLLC, on our financial statements for the past two fiscal years contained no adverse opinion or a disclaimer of opinion and were not modified; however, the reports were qualified as to the uncertainty of our ability to continue as a going concern due to our dependence on a successful execution of our plan of operations and ability to raise additional financing, lack of our generation of revenues, and our stockholders’ deficit and negative working capital. The decision to change independent accountants was approved by our board of directors on January 23, 2014.
18
During our two most recent fiscal years and through the date of this report, we have had no disagreements with SUPLLC, on any matter of accounting principles or practices, financial statement disclosure, or auditing scope or procedure, which disagreements, if not resolved to the satisfaction of SUPLLC, would have caused it to make reference to the subject matter of such disagreements in its report on our financial statements for such periods.
During our two most recent fiscal years and through the date of this report on Form 8-K, there have been no reportable events as defined under Item 304(a)(1)(v) of Regulation S-K adopted by the SEC.
We provided SUPLLC, with a copy of this disclosure before its filing with the SEC. We requested that SUPLLC, provide us with a letter addressed to the SEC stating whether or not it agrees with the above statements, and we received a letter from SUPLLC, stating that it does agree with the above statements. A copy of such letter, dated as of January 27, 2014 is filed as Exhibit 16.1 to this report.
New Independent Registered Public Accounting Firm
Our board of directors appointed Burr Pilger Mayer, Inc. (“BPM”) as our new independent registered public accounting firm on January 23, 2014, which appointment was accepted by BPM effective as of January 24, 2014. During the two most recent fiscal years and through the date of our engagement, we did not consult with BPM regarding either (1) the application of accounting principles to a specified transaction, either completed or proposed, or the type of audit opinion that might be rendered on our financial statements, or (2) any matter that was either the subject of a disagreement or a reportable event (as defined in Item 304(a)(1)(v) of Regulation S-K).
On January 21, 2014, Ms. Kade Thompson, a majority shareholder of the Company, entered into a Securities Purchase Agreement (the “Purchase Agreement,” such transaction, the “Purchase Transaction”) with BPMX, pursuant to which Ms. Thompson sold to BPMX 7,000,000 shares of common stock of the Company (the “Control Shares”) for an aggregate amount of $20,000.
On January 23, 2014, the Company entered into and consummated the transactions contemplated by the Share Exchange Agreement with BPMX and its shareholders whereby the Company purchased from the shareholders of BPMX all issued and outstanding shares of BPMX’s common stock in consideration of the issuance of 7,025,000 shares of common stock of the Company. Following the issuance of such shares, the Control Shares have been cancelled by BPMX.
The Purchase Transaction and the Share Exchange Transaction resulted in (i) a change in control of the Company with the shareholders of BPMX owning approximately 77.8% of the then issued and outstanding shares of common stock of the Company, and (ii) appointment of certain nominees of the shareholders of BPMX as directors and officers of the Company and resignation of Ms. Thompson as director, chief executive officer, chief financial officer, secretary and treasurer of the Company.
Item 5.02 Departure of Directors or Principal Officers; Election of Directors; Appointment of Principal Officers.
On January 21, 2014, Kade Thompson, our former Chief Executive Officer, President and Director, resigned from each of his positions as a director and officer of the Company.
Also, on January 21, 2014, (i) James Pekarsky was appointed as the Chief Executive Officer, Chief Financial Officer, Treasurer, Director and Chairman of the Company, and (ii) Anja Krammer was appointed as the President, Secretary and Director of the Company.
On January 23, 2014, the Company adopted the accounting acquirer’s fiscal year end of December 31 as a result of the Share Exchange Transaction consummated on January 23, 2014. The Share Exchange Transaction is accounted for as a reverse merger and recapitalization with the acquired company, BPMX, becoming the acquirer in this transaction.
19
As a result of the Share Exchange Transaction as described in Items 1.01 and 2.01, which description is incorporated by reference in this Item 5.06 of this report, the Company ceased being a shell company as such term is defined in Rule 12b-2 under the Exchange Act.
(a) Financial Statements of Business Acquired.The financial statements of BPMX are appended to this Current Report beginning on page F-1, and unaudited proforma financial statements of the Company are appended to this report beginning on page F-28.
(d) Exhibits. The following exhibits are filed with this report:
| Description | ||
| 2.1 | Form of Share Exchange Agreement dated January 23, 2014 by and among the Company, BioPharmX Inc. and BioPharmX Stockholders. | |
| 4.1 | Form of Notes issued pursuant to the Stock Purchase Agreement dated January 3, 2014. | |
| 4.2 | Form of Warrant issuable pursuant to the Stock Purchase Agreement dated January 3, 2014. | |
| 10.1 | Form of Stock Purchase Agreement dated January 23, 2014 by and between Kade Thompson and BioPharmX Inc. | |
| 10.2 | Form of Employment Agreement dated January 23, 2014 by and between James Pekarsky and the Company. | |
| 10.3 | Form of Employment Agreement dated January 23, 2014 by and between Anja Krammer and the Company. | |
| 10.4 | Amended and Restated Collaboration and License Agreement dated as of March 1, 2013 by and between BioPharmX Inc. and Iogen LLC. | |
| 10.5 | Collaboration and Supply Agreement dated as of October 22, 2013 by and between BioPharmX Inc. and Nutech Medical, Inc. | |
| 10.6 | Lease Agreement dated August 23, 2013 by and between Prologis, L.P. and BioPharmX Inc. | |
| 10.7 | 2014 Equity Incentive Plan. | |
| 10.8 | Form of Securities Purchase Agreement dated January 3, 2014 by and between BPMX and the investor. | |
| 10.9 | Form of Amendment to the Securities Purchase Agreement dated January 3, 2014. | |
| 10.10 | Form of Security Agreement dated January 3, 2014 by and between BPMX and the collateral agent. | |
| 16.1 | Letter of Silberstein Ungar, PLLC to the SEC dated January 23, 2014. | |
| 21.1 | List of Subsidiaries. |
20
BioPharmX Inc.
Index to Financial Statements
| Page | ||||
| Unaudited Condensed Financial Statements as of September 30, 2013 | ||||
| Condensed Balance Sheets (Unaudited) | F-2 | |||
| Condensed Statements of Operations and Comprehensive Loss (Unaudited) | F-3 | |||
| Condensed Statements of Cash Flows (Unaudited) | F-4 | |||
| Notes to Condensed Financial Statements (Unaudited) | F-5 - F-12 | |||
| Financial Statements as of December 31, 2012 and 2011 | ||||
| Report of Independent Registered Public Accounting Firm | F-13 | |||
| Balance Sheets | F-14 | |||
Statements of Operations and Comprehensive Loss | F-15 | |||
| Statement of Stockholders’ Deficit | F-16 | |||
| Statements of Cash Flows | F-17 | |||
| Notes to Consolidated Financial Statements | F-18 - F-27 | |||
| Unaudited Proforma Financial Statements of the Company | F-28 | |||
| Proforma Balance Sheet (Unaudited) | F-29 | |||
| Proforma Statement of Operations (Unaudited) | F-30 | |||
| Notes to Proforma Financial Statements (Unaudited) | F-31 | |||
F-1
BIOPHARMX, INC. | ||||||||
| (a development stage enterprise) | ||||||||
UNAUDITED CONDENSED BALANCE SHEETS | ||||||||
| as of September 30, 2013 and December 31, 2012 | ||||||||
| ____________ | ||||||||
September 30, 2013 | December 31, 2012 | |||||||
ASSETS | ||||||||
| Current assets: | ||||||||
| Cash | $ | 24,059 | $ | 137,850 | ||||
| Prepaid expenses and other current assets | 142,481 | 1,927 | ||||||
| Total current assets | 166,540 | 139,777 | ||||||
| Property and equipment, net | 29,972 | 12,194 | ||||||
| Intangible assets, net | 150,000 | - | ||||||
| Other assets | 50,000 | - | ||||||
| Total assets | $ | 396,512 | $ | 151,971 | ||||
LIABILITIES AND STOCKHOLDERS' DEFICIT | ||||||||
| Current liabilities: | ||||||||
| Accounts payable and accrued expenses | $ | 259,476 | $ | 16,115 | ||||
| Related party payables | 139,280 | 16,530 | ||||||
| Convertible notes, short-term | 100,000 | - | ||||||
| Total current liabilities | 498,756 | 32,645 | ||||||
| Convertible notes payable | 590,207 | 162,628 | ||||||
| Other long-term liabilities | 20,717 | 2,566 | ||||||
| Total liabilities | 1,109,680 | 197,839 | ||||||
| Commitments and contingencies (Note 8) | ||||||||
| Stockholders' deficit: | ||||||||
Common stock, $0.0001 par value; 10,000,000 shares authorized; 7,025,000 and 7,400,000 shares issued and outstanding at September 30, 2013 and December 31, 2012, respectively | 703 | 740 | ||||||
| Additional paid-in capital | 202,813 | 48,487 | ||||||
| Deficit accumulated during the development stage | (916,684 | ) | (95,095 | ) | ||||
| Total stockholders' deficit | (713,168 | ) | (45,868 | ) | ||||
| Total liabilities and stockholders' deficit | $ | 396,512 | $ | 151,971 | ||||
F-2
BIOPHARMX, INC. | ||||||||||||||||||||
| (a development stage enterprise) | ||||||||||||||||||||
UNAUDITED CONDENSED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS | ||||||||||||||||||||
| for thethree and nine months ended September 30, 2013 and 2012 and, cumulatively, | ||||||||||||||||||||
| for the period from August 18, 2011 (date of inception) to September 30, 2013 | ||||||||||||||||||||
| ____________ | ||||||||||||||||||||
Three months ended September 30, | Nine months ended September 30, | Cumulative for the period from August 18, 2011 (date of inception) to | ||||||||||||||||||
| 2013 | 2012 | 2013 | 2012 | September 30, 2013 | ||||||||||||||||
| Operating expenses: | ||||||||||||||||||||
| Research and development | $ | 221,653 | $ | 1,154 | $ | 400,726 | $ | 3,499 | $ | 435,477 | ||||||||||
| Sales and marketing | 44,725 | 250 | 72,728 | 550 | 82,011 | |||||||||||||||
| General and administrative | 162,877 | 4,082 | 306,406 | 7,247 | 352,272 | |||||||||||||||
| Total operating expenses | 429,255 | 5,486 | 779,860 | 11,296 | 869,760 | |||||||||||||||
| Loss from operations | (429,255 | ) | (5,486 | ) | (779,860 | ) | (11,296 | ) | (869,760 | ) | ||||||||||
| Interest expense | (22,210 | ) | - | (41,729 | ) | - | (46,924 | ) | ||||||||||||
| Net and comprehensive loss | $ | (451,465 | ) | $ | (5,486 | ) | $ | (821,589 | ) | $ | (11,296 | ) | $ | (916,684 | ) | |||||
F-3
| (a development stage enterprise) | ||||||||||||
UNAUDITED CONDENSED STATEMENTS OF CASH FLOWS | ||||||||||||
| for the nine months ended September 30, 2013 and 2012 and, cumulatively, | ||||||||||||
| for the period from August 18, 2011 (date of inception) to September 30, 2013 | ||||||||||||
| ____________ | ||||||||||||
Nine months ended September 30, | Cumulative for the period from August 18, 2011 (date of inception) to | |||||||||||
| 2013 | 2012 | September 30, 2013 | ||||||||||
| Cash flows from operating activities: | ||||||||||||
| Net loss | $ | (821,589 | ) | $ | (11,296 | ) | $ | (916,684 | ) | |||
| Adjustments to reconcile net loss to net cash from | ||||||||||||
| operating activities: | ||||||||||||
| Stock-based compensation expense | 28,308 | 5,145 | 37,165 | |||||||||
| Depreciation expense | 6,251 | - | 6,871 | |||||||||
| Noncash interest expense | 41,729 | - | 46,923 | |||||||||
| Changes in assets and liabilities: | ||||||||||||
| Prepaid expenses and other assets | (190,554 | ) | - | (192,481 | ) | |||||||
| Accounts payable and accrued expenses | 143,361 | (2,207 | ) | 159,476 | ||||||||
| Related party payables | 122,750 | 8,880 | 139,280 | |||||||||
| Net cash provided by (used in) operating activities | (669,744 | ) | 522 | (719,450 | ) | |||||||
| Cash flows from investing activities: | ||||||||||||
| Purchases of property and equipment | (24,029 | ) | (1,631 | ) | (36,843 | ) | ||||||
| Purchase of intellectual property | (50,000 | ) | - | (50,000 | ) | |||||||
| Net cash used in investing activities | (74,029 | ) | (1,631 | ) | (86,843 | ) | ||||||
| Cash flows from financing activities: | ||||||||||||
| Repurchase of common stock | (18 | ) | - | (18 | ) | |||||||
| Proceeds from issuance of common stock | - | 370 | 370 | |||||||||
| Proceeds from issuance of convertible notes payable | 630,000 | 100,000 | 830,000 | |||||||||
| Net cash provided by financing activities | 629,982 | 100,370 | 830,352 | |||||||||
| Net increase (decrease) in cash and cash equivalents | (113,791 | ) | 99,261 | 24,059 | ||||||||
| Cash at beginning of period | 137,850 | 1,953 | - | |||||||||
| Cash at end of period | $ | 24,059 | $ | 101,214 | $ | 24,059 | ||||||
| Non-cash financing activities: | ||||||||||||
Fair value of beneficial conversion feature issued in connection with convertible notes payable | $ | 126,000 | $ | 20,000 | $ | 166,000 | ||||||
Intangible assets purchase accrued | $ | 100,000 | $ | - | $ | 100,000 | ||||||
F-4
BIOPHARMX, INC. |
(a development stage enterprise) |
NOTES TO FINANCIAL STATEMENTS
SEPTEMBER 30, 2013
| 1. | Description of Business and Basis of Presentation |
Description of Business
BioPharmX, Inc. (“BioPharmX” or the “Company”) is a Silicon Valley-based company, incorporated in Delaware on August 18, 2011, that seeks to provide innovative products through unique, patented platform technologies for pharmaceutical and over-the-counter (“OTC”) applications in the fast growing dermatology and health and wellness markets.
The strategy of the Company begins with obtaining novel, patented, platform technologies through exclusive licensing, joint development or acquisition. BioPharmX then develops platform technologies that can be developed into product lines through specialized formulation and clinical protocol development with a bifurcated market penetration strategy, prescription for the high dose prescription version and OTC consumer for the low dose version. Identifying such technologies requires a strong knowledge of the markets served through technology assessment and evaluation of sell-side and buy-side opportunities through relationships with major pharmaceutical companies. BioPharmX’s products are formulated to address both market pathways to address unmet needs in well-defined, multi-billion dollar markets for licensing or direct commercialization for both pharmaceutical and OTC distribution and sales.
Basis of Presentation and Principles of Consolidation
The accompanying financial statements have been prepared in accordance with generally accepted accounting principles in the United States of America (“U.S. GAAP”).
Preparation of these financial statements requires management to make certain judgments, estimates and assumptions. These may affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements. They also may affect the reported amounts of revenues and expenses during the reporting period. Key estimates used in the preparation of our financial statements include stock-based compensation and deferred income taxes. Actual results could differ from those estimates upon subsequent resolution of identified matters.
Unaudited Interim Financial Information
The interim balance sheet as of September 30, 2013, the interim statements of operations and comprehensive loss and cash flows for the nine months ended September 30, 2013 and 2012 are unaudited. The unaudited interim financial statements have been prepared on the same basis as the annual financial statements and, in the opinion of management, reflect all adjustments, which include only normal recurring adjustments necessary to present fairly the Company’s financial position as of September 30, 2013 and its results of operations and cash flows for the nine months ended September 30, 2013 and 2012. The financial data and other financial information disclosed in these notes to the financial statements related to the three and nine month periods are unaudited. The results of operations for the nine months ended September 30, 2013 are not necessarily indicative of the results to be expected for the year ended December 31, 2013.
Stock Split
In December 2012, the Company’s Board of Directors approved a 2-for-1 stock split of the Company’s common stock. All common stock data and stock option information in the financial statements has been adjusted to reflect the stock split.
F-5
| 2. | Going Concern Considerations and Management’s Plan |
The accompanying financial statements have been prepared assuming the Company will continue as a going concern. The Company has incurred recurring losses and negative cash flows from operations since inception. The Company has not generated revenues and has funded its operating losses through the issuance of convertible notes payable. The Company has a limited operating history and its prospects are subject to risks, expenses and uncertainties frequently encountered by companies in the industry. These risks include, but are not limited to, the uncertainty of availability of additional financing and the uncertainty of achieving future profitability. Management of the Company intends to raise additional funds through the issuance of equity securities. There can be no assurance that such financing will be available or on terms which are favorable to the Company. Failure to generate sufficient cash flows from operations, raise additional capital or reduce certain discretionary spending could have a material adverse effect on the Company’s ability to achieve its intended business objectives. These factors raise substantial doubt about the Company’s ability to continue as a going concern. The financial statements do not contain any adjustments that might result from the outcome of this uncertainty.
As shown in the accompanying financial statements, the Company incurred a net loss of $822,000 and $11,000 during the nine months ended September 30, 2013 and 2012, respectively, and has an accumulated deficit of $917,000 as of September 30, 2013. As of September 30, 2013, the Company had a working capital deficit of $332,000. As of December 31, 2012, the Company had working capital of $107,000. While management of the Company believes that it has a plan to fund on-going operations, there is no assurance that its plan will be successfully implemented. The Company is experiencing the following risks and uncertainties in the business:
In 2012, the Company initiated a financing with convertible notes to invite early investors at a 20% discount to the share price in a future offering. While the Company was able to secure a few investors, there is continued risk in the Company’s ability to attract additional early investors. Without access to continued funds for working capital the Company may not be able to execute its product strategy and pursue research and development activities on its novel platform technologies.
The discovery of key raw materials to formulate novel products depends on the Company’s ability to identify, negotiate and secure procurement of such materials. This also depends on the Company’s ability to establish comprehensive and long term vendor contracts and relationships.
The Company’s ability to compete and to achieve its product platform strategy depends on its ability to protect its proprietary discoveries and technologies. The Company currently relies on a combination of copyrights, trademarks, trade secret laws and confidentiality agreements to protect its intellectual property rights. The Company also relies upon unpatented know-how and continuing technological innovation.
The Company’s continued operations are dependent upon its ability to identify, recruit and retain adequate management personnel and contractors to perform certain jobs such as research and development, patent generation, regulatory affairs and general administrative functions. The Company requires highly trained professionals of varying levels and experience along with a flexible work force.
Research and development for novel prescription or OTC based products can be very extensive and lengthy in nature; along with the clinical trial process with the Food and Drug Administration which can require significant funding and time consuming patient studies. The competitive landscape could change significantly over the time period to complete targeted product development milestones. The current competition for BioPharmX’s products could also turn into strategic partners or potential acquirers in the future.
The significant risks and uncertainties described above could have a significant negative impact on the financial viability of BioPharmX, Inc. and raise substantial doubt about the Company’s ability to continue as a going concern. Management is working on the Company’s business model to increase working capital by managing its cash flow, securing financing and working towards bringing its first product to market.
F-6
| 3. | Summary of Significant Accounting Policies |
Cash
Cash consists of cash deposits with banks.
Fair Value of Financial Instruments
Carrying amounts of certain of the Company’s financial instruments, including cash, prepaid and other current assets, accounts payable and accrued expenses and related party payables approximate fair value due to their short maturities. Based on borrowing rates currently available to the Company for loans with similar terms, the carrying value of the convertible notes payable approximates fair value.
Fair Value Measurements
The Company recognizes and discloses the fair value of its assets and liabilities using a hierarchy that prioritizes the inputs to valuation techniques used to measure fair value. The hierarchy gives the highest priority to valuations based upon unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurements) and the lowest priority to valuations based upon unobservable inputs that are significant to the valuation (Level 3 measurements). Each level of input has different levels of subjectivity and difficulty involved in determining fair value.
| ● | Level 1 - Inputs used to measure fair value are unadjusted quoted prices that are available in active markets for the identical assets or liabilities as of the reporting date. Therefore, determining fair value for Level 1 investments generally does not require significant judgment, and the estimation is not difficult. |
| ● | Level 2 - Pricing is provided by third party sources of market information obtained through investment advisors. The Company does not adjust for or apply any additional assumptions or estimates to the pricing information received from its advisors. |
| ● | Level 3 - Inputs used to measure fair value are unobservable inputs that are supported by little or no market activity and reflect the use of significant management judgment. These values are generally determined using pricing models for which the assumptions utilize management’s estimates of market participant assumptions. The determination of fair value for Level 3 instruments involves the most management judgment and subjectivity. |
As of September 30, 2013 and December 31, 2012, the Company held no assets or liabilities with instrument valuations measured on a recurring basis.
Property and Equipment, net
Property and equipment are stated at cost, net of accumulated depreciation. Depreciation of property and equipment is computed using the straight-line method over their estimated useful lives of five years. Maintenance and repairs are charged to operations as incurred.
Impairment of Long-Lived Assets
The Company reviews long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset might not be recoverable. When such an event occurs, management determines whether there has been an impairment by comparing the anticipated undiscounted future net cash flows to the related asset’s carrying value. If an asset is considered impaired, the asset is written down to fair value, which is determined based either on discounted cash flows or appraised value, depending on the nature of the asset. The Company has not identified any such impairment losses to date.
F-7
Income Taxes
The Company accounts for income taxes using the liability method whereby deferred tax asset and liability account balances are determined based on differences between financial reporting and tax bases of assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. Valuation allowances are established to reduce deferred tax assets when management estimates, based on available objective evidence, that it is more likely than not that the benefit will not be realized for the deferred tax assets.
The Company’s policy is to recognize interest and penalties accrued on any unrecognized tax benefits as a component of income tax expense. As of the date of adoption of accounting for uncertain tax positions there was no accrued interest or penalties associated with any unrecognized tax benefits, nor was any interest expense recognized during the year.
Research and Development
Research and development costs are charged to operations as incurred. Research and development costs include, but are not limited to, payroll and personnel expenses, materials, consulting costs, and allocated overhead, including rent, equipment depreciation, and utilities.
Stock-Based Compensation
The Company accounts for stock-based employee compensation arrangements which requires the recognition of compensation expense, using a fair-value based method for costs related to all employee share-based payments, including stock options. The Company estimates the fair value of share-based payment awards on the date of grant using an option pricing model. All option grants have been expensed on a straight-line basis over their vesting period. Equity instruments issued to nonemployees are recorded at their fair value on the measurement date and are subject to periodic adjustment as the underlying equity instruments vest.
Comprehensive Loss
Comprehensive loss is the changes in equity of an enterprise, except those resulting from stockholder transactions. Accordingly, comprehensive loss includes certain changes in equity that are excluded from net loss. For the nine months ended September 30, 2013 and year ended December 31, 2012 and, cumulatively, for the period from August 18, 2011 (date of inception) to September 30, 2013, the Company’s comprehensive loss is equal to the net loss. There were no components of comprehensive loss for any of the periods presented.
| 4. | Property Equipment, net |
Property and equipment, net at September 30, 2013 and December 31, 2012 consisted of the following (in thousands):
September 30, 2013 | December 31, 2012 | |||||||
| Furniture and fixtures | $ | 11,183 | $ | 11,183 | ||||
| Lab equipment | 11,895 | 1,631 | ||||||
| Computers and equipment | 13,765 | - | ||||||
| 36,843 | 12,814 | |||||||
| Less: accumulated depreciation | (6,871 | ) | (620 | ) | ||||
| $ | 29,972 | $ | 12,194 | |||||
Depreciation expense for the nine months ended September 30, 2013 was $6,000. Depreciation expense for the cumulative period from August 18, 2011 (date of inception) to September 30, 2013 totaled $7,000.
F-8
| 5. | Related Party Payables |
Since inception, the founding executives of the Company have made advances to cover short-term operating expenses. These advances are non-interest bearing. As of September 30, 2013 and December 31, 2012, related party payables were $139,000 and $17,000, respectively.
| 6. | Convertible Notes Payable |
In September and November 2012, the Company issued convertible notes payable (“Notes”) to two individuals, respectively, in exchange for $200,000 cash. These Notes carry an interest rate of 6% per annum and mature in September and November 2015, respectively, with principal and interest payable at maturity.
During the nine months ended September 30, 2013, the Company issued Notes to nine individuals in exchange for $630,000 cash. These notes carry an interest rate of 6% per annum and mature between June 2014 and September 2016, with principal and interest payable at maturity.
The Notes automatically convert into preferred stock issued in a qualified financing at 80% of the price per share at which such preferred stock is issued in such an offering. Additionally, there is a special conversion that at maturity, unless the Company repays all outstanding principal and interest, the Notes shall be automatically converted into a number of shares of common stock of the Company at 80% of the then fair market value per share.
As a result of this beneficial conversion feature, the Company has recorded $126,000 and $40,000 as a debt discount during the nine months ended September 30, 2013 and fiscal year ended December 31, 2012. The debt discount is being amortized to interest expense over the term of the Notes. The amortization expense related to the debt discount was $24,000 and $3,000 for the nine months ended September 30, 2013 and year ended December 31, 2012, respectively and $26,000 for the cumulative period from August 18, 2011 (date of inception) to September 30, 2013. The note holders as a group also have the right to purchase up to an aggregate of $1,000,000 in shares in the event of a subsequent offer of equity securities.
| 7. | Stockholders’ Equity |
Repurchase of common stock
On March 27, 2013, the Company terminated one of the founders and repurchased 375,000 shares for $18.
F-9
Stock-based compensation
The following table summarizes the stock-based compensation by line item as follows (in thousands):
Three months ended September 30, | Nine months ended September 30, | Cumulative for the period from August 18, 2011 (date of inception) to September 30, | ||||||||||||||||||
| 2013 | 2012 | 2013 | 2012 | 2013 | ||||||||||||||||
| Research and development | $ | 8,967 | $ | 1,154 | $ | 13,202 | $ | 3,462 | $ | 17,818 | ||||||||||
| Sales and marketing | 2,568 | - | 3,649 | - | 3,828 | |||||||||||||||
| General and administrative | 5,993 | 1,683 | 11,457 | 1,683 | 15,519 | |||||||||||||||
| Total stock-based compensation expense | $ | 17,528 | $ | 2,837 | $ | 28,308 | $ | 5,145 | $ | 37,165 | ||||||||||
Stock-based compensation expense recognized in the statements of operations and comprehensive loss is based on awards ultimately expected to vest. Forfeitures are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates.
The following table summarizes stock option activity for the nine months ended September 30, 2013:
| Weighted | ||||||||||||
| Weighted | Average | |||||||||||
| Number | Average | Remaining | ||||||||||
| of | Exercise Price | Contractual | ||||||||||
| Shares | Per Share | Life (Years) | ||||||||||
| Outstanding at January 1, 2013 | 1,150,000 | $ | 0.06 | 9.3 | ||||||||
| Granted | 1,271,000 | 0.31 | 9.7 | |||||||||
| Exercised | - | - | - | |||||||||
| Forfeited | - | - | - | |||||||||
| Outstanding at September 30, 2013 | 2,421,000 | $ | 0.19 | 9.2 | ||||||||
Vested and expected to vest at September 30, 2013 | 2,421,000 | $ | 0.19 | 9.2 | ||||||||
| Vested at September 30, 2013 | 542,361 | $ | 0.07 | 8.7 | ||||||||
F-10
The following assumptions were used in the estimated grant date fair value calculations for Stock Awards during the nine months ended September 30, 2013 and the year ended December 31, 2012:
Nine months ended September 30, 2013 | Year ended December 31, 2012 | |||||||
| Expected term | 5.52 - 6.08 years | 5.52 - 6.08 years | ||||||
| Risk-free interest rate | 0.61% - 1.49 | % | 0.61% - 0.81 | % | ||||
| Volatility | 82.08 | % | 82.08 | % | ||||
| Dividend yield | - | - | ||||||
| Weighted average grant date fair value | $ | 0.19 | $ | 0.06 | ||||
The Company estimates the fair value of time-based stock options, if any, granted using the Black-Scholes-Merton option pricing formula. The fair value is then amortized on a straight-line basis over the requisite service periods of the awards, which is generally the vesting period. Time-based and performance-based options, if any, typically have a ten-year life from date of grant and vesting periods of two to four years.
| 8. | Commitments and Contingencies |
Lease Commitments
On August 23, 2013, the Company signed a lease for 10,800 square feet of office and laboratory space in Menlo Park, California. The term of the lease is 39 months from the lease commencement date of September 1, 2013. Future minimum commitments under this lease are as follows:
| Year ended December 31, | ||||
| 2013 (remainder) | $ | 23,220 | ||
| 2014 | 279,337 | |||
| 2015 | 287,717 | |||
| 2016 | 270,975 | |||
| Total | $ | 861,249 | ||
Contingencies
The Company is involved from time to time with claims or legal actions in the ordinary course of business. Management does not believe that the impact of such matters will have a material adverse effect on the Company’s financial condition or results of operations.
| 9. | Income Taxes |
No federal income taxes were provided in the nine months ended September 30, 2013, year ended December 31, 2012 or for the cumulative period from August 18, 2011 (date of inception) to September 30, 2013 due to the Company’s net losses. State minimum income and franchise taxes are included in general and administrative expenses and were immaterial for the periods presented.
At December 31, 2012, the Company had available federal net operating loss NOL carryforwards of approximately $60,000 which will begin to expire in 2031 and California state NOL carryforwards of approximately $60,000 which will begin to expire in 2021. At December 31, 2012, the net deferred tax assets of approximately $29,000, generated primarily by NOL carryforwards, have been fully reserved due to the uncertainty surrounding the realization of such benefits. The net valuation allowance increased by approximately $29,000 during the year ended December 31, 2012, respectively.
Current tax laws impose substantial restrictions on the utilization of net operating loss and credit carryforwards in the event of an “ownership change,” as defined by the Internal Revenue Code. If there should be an ownership change, the Company’s ability to utilize its carryforwards could be limited.
As of December 31, 2012 and 2011, the Company did not have any material unrecognized tax benefits. The 2012 and 2011 tax years remain open for examination by the federal and state authorities.
F-11
| 10. | Intangible Assets |
In March 2013, the Company signed a collaboration and licensing agreement (“CLA”) with a biotechnology company with molecular iodine technology (the “Licensor”). The CLA terms included a $150,000 advanced profit sharing payment in exchange for exclusive licensing and patent indemnification rights to the Licensor’s molecular iodine technology. The payment schedule included $50,000 upon signing and an additional $100,000 to be paid in monthly increments of $10,000 starting October 15, 2013. Additionally, the terms included profit sharing and royalty fees related to future revenues generated from the technology product pipeline.
As of September 30, 2013, the $150,000 advanced payment has been recorded as an intangible asset on the balance sheet. This asset will be amortized over the estimated economic life of the underlying patents, which is expected to be 3 years, commencing on the date that the Company’s molecular iodine breast health supplement is available for sale.
| 11. | Subsequent Events |
Between October 1, 2013 and January 23, 2014, the Company has issued convertible notes payable (“Notes”) to six individuals in exchange for $790,000 cash. These Notes carry an interest rate of 6% per annum and mature in September and November 2015, respectively, with principal and interest payable at maturity.
In December 2013, two related parties converted $50,000 that had been previously classified as related party payables and added them to their existing convertible notes payable. The existing convertible notes payable carry an interest rate of 5% per annum and mature in September and November 2015, respectively, with principal and interest payable at maturity. The notes are automatically convertible into the securities of the Company sold in an offering that takes place after the completion of the reverse acquisition of the Company by Thompson Designs, Inc. at a conversion price per share equal to 80% of the per share offering price of such financing.
On January 23, 2014, Thompson Designs, Inc. (“Parent”), BioPharmX and stockholders of BioPharmX who collectively own 100% of BioPharmX (the “BioPharmX Stockholders”) entered into and consummated transactions pursuant to a Share Exchange Agreement (the “Share Exchange Agreement,” such transaction referred to as the “Share Exchange Transaction”), whereby the Parent issued to the BioPharmX Stockholders an aggregate of 7,025,000 shares of its common stock, par value $0.001 (“Common Stock”), in exchange for 100% of the equity interests of BioPharmX held by the BioPharmX Stockholders. The shares of our Common Stock received by the BioPharmX Stockholders in the Share Exchange Transaction constitute approximately 77.8% of Parent’s issued and outstanding Common Stock giving effect to the issuance of shares pursuant to the Share Exchange Agreement. As a result of the Share Exchange Transaction, BioPharmX became a subsidiary of the Parent.
For financial reporting purposes, the transaction will be accounted for as a “reverse merger” rather than a business combination, because the sellers of the Company effectively control the combined companies immediately following the transaction. As such, the Company is deemed to be the accounting acquirer in the transaction and, consequently, the transaction is being treated as a reverse acquisition by the Company. Accordingly, the assets and liabilities and the historical operations that will be reflected in Parent’s ongoing financial statements will be those of the Company and will be recorded at the historical cost basis of the Company. The historical financial statements of Parent before the transaction will be replaced with the historical financial statements of the Company before the transaction in all future filings with the Securities and Exchange Commission, or SEC. The Merger is intended to be treated as a tax-free exchange under Section 368(a) of the Internal Revenue Code of 1986, as amended.
F-12

Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of
BioPharmX, Inc.
(a development stage enterprise)
We have audited the accompanying balance sheets of BioPharmX, Inc. (a development stage enterprise) (the “Company”) as of December 31, 2012 and 2011, and the related statements of operations and comprehensive loss, stockholders’ deficit and cash flows for the year ended December 31, 2012, the period from August 18, 2011 (date of inception) to December 31, 2011 and, cumulatively, for the period from August 18, 2011 (date of inception) to December 31, 2012. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of the Company’s internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of BioPharmX, Inc. (a development stage enterprise) as of December 31, 2012 and 2011, and the results of its operations and its cash flows for the year ended December 31, 2012, the period from August 18, 2011 (date of inception) to December 31, 2011 and, cumulatively, for the period from August 18, 2011 (date of inception) to December 31, 2012 in conformity with accounting principles generally accepted in the United States of America.
San Jose, California
October 18, 2013
ACCOUNTANTS & CONSULTANTS
F-13
BIOPHARMX, INC.
(a development stage enterprise)
BALANCE SHEETS
as of December 31, 2012 and 2011
____________
| 2012 | 2011 | |||||||
ASSETS | ||||||||
| Current assets: | ||||||||
| Cash | $ | 137,850 | $ | 1,953 | ||||
| Prepaid expenses and other current assets | 1,927 | 1,000 | ||||||
| Total current assets | 139,777 | 2,953 | ||||||
| Property and equipment, net | 12,194 | - | ||||||
| Total assets | $ | 151,971 | $ | 2,953 | ||||
LIABILITIES AND STOCKHOLDERS' DEFICIT | ||||||||
| Current liabilities: | ||||||||
| Accounts payable and accrued expenses | $ | 16,115 | $ | 2,227 | ||||
| Related party payables | 16,530 | 7,350 | ||||||
| Total current liabilities | 32,645 | 9,577 | ||||||
| Convertible notes payable | 162,628 | - | ||||||
| Other long-term liabilities | 2,566 | - | ||||||
| Total liabilities | 197,839 | 9,577 | ||||||
| Commitments and contingencies (Note 8) | ||||||||
| Stockholders' deficit: | ||||||||
| Common stock, $0.0001 par value; 10,000,000 shares authorized; 7,400,000 shares issued and outstanding | 740 | 740 | ||||||
| Additional paid-in capital | 48,487 | (370 | ) | |||||
| Receivable from stockholders | - | (370 | ) | |||||
| Deficit accumulated during the development stage | (95,095 | ) | (6,624 | ) | ||||
| Total stockholders' deficit | (45,868 | ) | (6,624 | ) | ||||
| Total liabilities and stockholders' deficit | $ | 151,971 | $ | 2,953 | ||||
The accompanying notes are an integral part of these financial statements.
F-14
BIOPHARMX, INC.
(a development stage enterprise)
STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
for the years ended December 31, 2012 and 2011 and, cumulatively,
for the period from August 18, 2011 (date of inception) to December 31, 2012
____________
| 2012 | 2011 | Cumulative for the period from August 18, 2011 (date of inception) to December 31, 2012 | ||||||||||
| Operating expenses: | ||||||||||||
| Research and development | $ | 30,616 | $ | 4,135 | $ | 34,751 | ||||||
| Sales and marketing | 9,133 | 150 | 9,283 | |||||||||
| General and administrative | 43,527 | 2,339 | 45,866 | |||||||||
| Total operating expenses | 83,276 | 6,624 | 89,900 | |||||||||
| Loss from operations | (83,276 | ) | (6,624 | ) | (89,900 | ) | ||||||
| Interest expense | (5,195 | ) | - | (5,195 | ) | |||||||
| Net and comprehensive loss | $ | (88,471 | ) | $ | (6,624 | ) | $ | (95,095 | ) | |||
The accompanying notes are an integral part of these financial statements.
F-15
BIOPHARMX, INC.
(a development stage enterprise)
STATEMENTS OF STOCKHOLDERS' DEFICIT
cumulative for the period from August 18, 2011 (date of inception) to December 31, 2012
____________
| Deficit | ||||||||||||||||||||||||
| Accumulated | ||||||||||||||||||||||||
| Additional | Receivable | During the | Total | |||||||||||||||||||||
| Common Stock | Paid-in | from | Development | Stockholders' | ||||||||||||||||||||
| Shares | Amount | Capital | Stockholders | Stage | Deficit | |||||||||||||||||||
| Issuance of common stock at $0.0001 per share to Founders in August 2011 | 7,400,000 | $ | 740 | $ | (370 | ) | $ | (370 | ) | $ | - | $ | - | |||||||||||
| Net and comprehensive loss | - | - | - | - | (6,624 | ) | (6,624 | ) | ||||||||||||||||
| Balance at December 31, 2011 | 7,400,000 | 740 | (370 | ) | (370 | ) | (6,624 | ) | (6,624 | ) | ||||||||||||||
| Payment of receivable for stock | - | - | - | 370 | - | 370 | ||||||||||||||||||
| Stock-based compensation | - | - | 8,857 | - | - | 8,857 | ||||||||||||||||||
| Issuance of convertible notes payable with beneficial conversion feature | - | - | 40,000 | - | - | 40,000 | ||||||||||||||||||
| Net and comprehensive loss | - | - | - | (88,471 | ) | (88,471 | ) | |||||||||||||||||
| Balance at December 31, 2012 | 7,400,000 | $ | 740 | $ | 48,487 | $ | - | $ | (95,095 | ) | $ | (45,868 | ) | |||||||||||
The accompanying notes are an integral part of these financial statements.
F-16
BIOPHARMX, INC.
(a development stage enterprise)
STATEMENTS OF CASH FLOWS
for the years ended December 31, 2012 and 2011 and, cumulatively,
for the period from August 18, 2011 (date of inception) to December 31, 2012
____________
| 2012 | 2011 | Cumulative for the period from August 18, 2011 (date of inception) to December 31, 2012 | ||||||||||
| Cash flows from operating activities: | ||||||||||||
| Net loss | $ | (88,471 | ) | $ | (6,624 | ) | $ | (95,095 | ) | |||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||||||
| Stock-based compensation expense | 8,857 | - | 8,857 | |||||||||
| Depreciation expense | 620 | - | 620 | |||||||||
| Noncash interest expense | 5,194 | - | 5,194 | |||||||||
| Changes in assets and liabilities: | ||||||||||||
| Prepaid expenses and other current assets | (927 | ) | (1,000 | ) | (1,927 | ) | ||||||
| Accounts payable and accrued expenses | 13,888 | 2,227 | 16,115 | |||||||||
| Related party payables | 9,180 | 7,350 | 16,530 | |||||||||
| Net cash provided by (used in) operating activities | (51,659 | ) | 1,953 | (49,706 | ) | |||||||
| Cash flows from investing activities: | ||||||||||||
| Purchases of property and equipment | (12,814 | ) | - | (12,814 | ) | |||||||
| Net cash used in investing activities | (12,814 | ) | - | (12,814 | ) | |||||||
| Cash flows from financing activities: | ||||||||||||
| Proceeds from issuance of common stock | 370 | - | 370 | |||||||||
| Proceeds from issuance of convertible notes payable | 200,000 | - | 200,000 | |||||||||
| Net cash provided by financing activities | 200,370 | - | 200,370 | |||||||||
| Net increase in cash and cash equivalents | 135,897 | 1,953 | 137,850 | |||||||||
| Cash at beginning of year | 1,953 | - | - | |||||||||
| Cash at end of year | $ | 137,850 | $ | 1,953 | $ | 137,850 | ||||||
| Non-cash financing activities: | ||||||||||||
| Fair value of beneficial conversion feature issued in connection with convertible notes payable | $ | 40,000 | $ | - | $ | 40,000 | ||||||
The accompanying notes are an integral part of these financial statements.
F-17
BioPharmX, Inc.
(a development stage enterprise)
Notes to Financial Statements
as of December 31, 2012 and 2011, and, cumulatively, for the period
from August 18, 2011 (date of inception) to December 31, 2012
____________
1. Description of Business and Basis of Presentation
Description of Business
BioPharmX, Inc. (“BioPharmX” or the “Company”) is a Silicon Valley-based company, incorporated in Delaware on August 18, 2011, that seeks to provide innovative products through unique, patented platform technologies for pharmaceutical and over-the-counter (“OTC”) applications in the fast growing dermatology and health and wellness markets.
The strategy of the Company begins with obtaining novel, patented, platform technologies through exclusive licensing, joint development or acquisition. BioPharmX then develops platform technologies that can be developed into product lines through specialized formulation and clinical protocol development with a bifurcated market penetration strategy, prescription for the high dose prescription version and OTC consumer for the low dose version. Identifying such technologies requires a strong knowledge of the markets served through technology assessment and evaluation of sell-side and buy-side opportunities through relationships with major pharmaceutical companies. BioPharmX’s products are formulated to address both market pathways to address unmet needs in well-defined, multi-billion dollar markets for licensing or direct commercialization for both pharmaceutical and OTC distribution and sales.
Basis of Presentation and Principles of Consolidation
The accompanying financial statements are have been prepared in accordance with generally accepted accounting principles in the United States of America (“U.S. GAAP”).
Preparation of these financial statements requires management to make certain judgments, estimates and assumptions. These may affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements. They also may affect the reported amounts of revenues and expenses during the reporting period. Key estimates used in the preparation of our financial statements include stock-based compensation and deferred income taxes. Actual results could differ from those estimates upon subsequent resolution of identified matters.
Stock Split
In December 2012, the Company’s Board of Directors approved a 2-for-1 stock split of the Company’s common stock. All common stock data and stock option information in this report has been adjusted to reflect the stock split.
F-18
BioPharmX, Inc.
(a development stage enterprise)
Notes to Financial Statements
as of December 31, 2012 and 2011, and, cumulatively, for the period
from August 18, 2011 (date of inception) to December 31, 2012
____________
| 2. | Going Concern Considerations and Management’s Plan |
The accompanying financial statements have been prepared assuming the Company will continue as a going concern. The Company has incurred recurring losses and negative cash flows from operations since inception. The Company has not generated revenues and has funded its operating losses through the issuance of convertible notes payable. The Company has a limited operating history and its prospects are subject to risks, expenses and uncertainties frequently encountered by companies in the industry. These risks include, but are not limited to, the uncertainty of availability of additional financing and the uncertainty of achieving future profitability. Management of the Company intends to raise additional funds through the issuance of equity securities. There can be no assurance that such financing will be available or on terms which are favorable to the Company. Failure to generate sufficient cash flows from operations, raise additional capital or reduce certain discretionary spending could have a material adverse effect on the Company’s ability to achieve its intended business objectives. These factors raise substantial doubt about the Company’s ability to continue as a going concern. The financial statements do not contain any adjustments that might result from the outcome of this uncertainty.
As shown in the accompanying financial statements, the Company incurred a net loss of $88,471 and $6,624 during the years ended December 31, 2012 and 2011, respectively, and has an accumulated deficit of $95,095 as of December 31, 2012. As of December 31, 2012, the Company had working capital of $107,132. As of December 31, 2011, the Company had a working capital deficit of $6,624. While management of the Company believes that it has a plan to fund on-going operations, there is no assurance that its plan will be successfully implemented. The Company is experiencing the following risks and uncertainties in the business:
In 2012, the Company initiated a financing with convertible notes to invite early investors at a 20% discount to the share price in a future offering. While the Company was able to secure a few investors, there is continued risk in the Company’s ability to attract additional early investors. Without access to continued funds for working capital the Company may not be able to execute its product strategy and pursue research and development activities on its novel platform technologies.
The discovery of key raw materials to formulate novel products depends on the Company’s ability to identify, negotiate and secure procurement of such materials. This also depends on the Company’s ability to establish comprehensive and long term vendor contracts and relationships.
The Company’s ability to compete and to achieve its product platform strategy depends on its ability to protect its proprietary discoveries and technologies. The Company currently relies on a combination of copyrights, trademarks, trade secret laws and confidentiality agreements to protect its intellectual property rights. The Company also relies upon unpatented know-how and continuing technological innovation.
The Company’s continued operations are dependent upon its ability to identify, recruit and retain adequate management personnel and contractors to perform certain jobs such as research and development, patent generation, regulatory affairs and general administrative functions. The Company requires highly trained professionals of varying levels and experience along with a flexible work force.
Research and development for novel prescription or OTC based products can be very extensive and lengthy in nature; along with the clinical trial process with the Food and Drug Administration which can require significant funding and time consuming patient studies. The competitive landscape could change significantly over the time period to complete targeted product development milestones. The current competition for BioPharmX’s products could also turn into strategic partners or potential acquirers in the future.
F-19
BioPharmX, Inc.
(a development stage enterprise)
Notes to Financial Statements
as of December 31, 2012 and 2011, and, cumulatively, for the period
from August 18, 2011 (date of inception) to December 31, 2012
____________
| 2. | Going Concern Considerations and Management’s Plan, continued |
The significant risks and uncertainties described above could have a significant negative impact on the financial viability of BioPharmX, Inc. and raise substantial doubt about the Company’s ability to continue as a going concern. Management is working on the Company’s business model to increase working capital by managing its cash flow, securing financing and working towards bringing its first product to market.
| 3. | Summary of Significant Accounting Policies |
Cash
Cash consists of only cash deposits with banks.
Fair Value of Financial Instruments
Carrying amounts of certain of the Company’s financial instruments, including cash and cash equivalents, prepaid and other current assets, accounts payable and accrued expenses and related party payables approximate fair value due to their short maturities. Based on borrowing rates currently available to the Company for loans with similar terms, the carrying value of the convertible notes payable approximates fair value.
Fair Value Measurements
The Company recognizes and discloses the fair value of its assets and liabilities using a hierarchy that prioritizes the inputs to valuation techniques used to measure fair value. The hierarchy gives the highest priority to valuations based upon unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurements) and the lowest priority to valuations based upon unobservable inputs that are significant to the valuation (Level 3 measurements). Each level of input has different levels of subjectivity and difficulty involved in determining fair value.
| · | Level 1 - Inputs used to measure fair value are unadjusted quoted prices that are available in active markets for the identical assets or liabilities as of the reporting date. Therefore, determining fair value for Level 1 investments generally does not require significant judgment, and the estimation is not difficult. |
| · | Level 2 - Pricing is provided by third party sources of market information obtained through investment advisors. The Company does not adjust for or apply any additional assumptions or estimates to the pricing information received from its advisors. |
| · | Level 3 - Inputs used to measure fair value are unobservable inputs that are supported by little or no market activity and reflect the use of significant management judgment. These values are generally determined using pricing models for which the assumptions utilize management’s estimates of market participant assumptions. The determination of fair value for Level 3 instruments involves the most management judgment and subjectivity. |
As of December 31, 2012 and 2011, the Company held no assets or liabilities with instrument valuations measured on a recurring basis.
F-20
BioPharmX, Inc.
(a development stage enterprise)
Notes to Financial Statements
as of December 31, 2012 and 2011, and, cumulatively, for the period
from August 18, 2011 (date of inception) to December 31, 2012
____________
| 3. | Summary of Significant Accounting Policies, continued |
Property and Equipment, net
Property and equipment are stated at cost, net of accumulated depreciation. Depreciation of property and equipment is computed using the straight-line method over their estimated useful lives of five years. Maintenance and repairs are charged to operations as incurred.
Impairment of Long-Lived Assets
The Company reviews long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset might not be recoverable. When such an event occurs, management determines whether there has been an impairment by comparing the anticipated undiscounted future net cash flows to the related asset’s carrying value. If an asset is considered impaired, the asset is written down to fair value, which is determined based either on discounted cash flows or appraised value, depending on the nature of the asset. The Company has not identified any such impairment losses to date.
Income Taxes
The Company accounts for income taxes using the liability method whereby deferred tax asset and liability account balances are determined based on differences between financial reporting and tax bases of assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. Valuation allowances are established to reduce deferred tax assets when management estimates, based on available objective evidence, that it is more likely than not that the benefit will not be realized for the deferred tax assets.
The Company’s policy is to recognize interest and penalties accrued on any unrecognized tax benefits as a component of income tax expense. As of the date of adoption of accounting for uncertain tax positions there was no accrued interest or penalties associated with any unrecognized tax benefits, nor was any interest expense recognized during the year.
Research and Development
Research and development costs are charged to operations as incurred. Research and development costs include, but are not limited to, payroll and personnel expenses, materials, consulting costs, and allocated overhead, including rent, equipment depreciation, and utilities.
Stock-Based Compensation
The Company accounts for stock-based employee compensation arrangements which requires the recognition of compensation expense, using a fair-value based method for costs related to all employee share-based payments, including stock options. The Company estimates the fair value of share-based payment awards on the date of grant using an option pricing model. All option grants have been expensed on a straight-line basis over their vesting period. Equity instruments issued to nonemployees are recorded at their fair value on the measurement date and are subject to periodic adjustment as the underlying equity instruments vest.
F-21
BioPharmX, Inc.
(a development stage enterprise)
Notes to Financial Statements
as of December 31, 2012 and 2011, and, cumulatively, for the period
from August 18, 2011 (date of inception) to December 31, 2012
____________
| 3. | Summary of Significant Accounting Policies, continued |
Comprehensive Loss
Comprehensive loss is the changes in equity of an enterprise, except those resulting from stockholder transactions. Accordingly, comprehensive loss includes certain changes in equity that are excluded from net loss. For the years ended December 31, 2012 and 2011 and, cumulatively, for the period from August 18, 2011 (date of inception) to December 31, 2012, the Company’s comprehensive loss is equal to the net loss. There were no components of comprehensive loss for any of the periods presented.
| 4. | Property Equipment, net |
Property and equipment, net at December 31, 2012 consisted of the following (in thousands):
| Furniture and fixtures | $ | 11,183 | ||
| Lab equipment | 1,631 | |||
| 12,814 | ||||
| Less: accumulated depreciation | (620 | ) | ||
| $ | 12,194 |
As of December 31, 2011 the Company did not hold any property and equipment. Depreciation expense for the year ended December 31, 2012 and for the cumulative period from August 18, 2011 (date of inception) to December 31, 2012 totaled $620.
| 5. | Related Party Payables |
Since inception, the founding executives of the Company have made advances to cover short-term operating expenses. These advances are non-interest bearing. As of December 31, 2012 and 2011, related party payables were $16,530 and $7,350, respectively.
| 6. | Convertible Notes Payable |
In September and November 2012, the Company issued convertible notes payable (“Notes”) to two individuals, respectively, in exchange for $200,000 cash. These Notes carry an interest rate of 6% per annum and mature in September and November 2015, respectively, with principal and interest payable at maturity.
The Notes automatically convert into preferred stock issued in a qualified financing at 80% of the price per share at which such preferred stock is issued in such an offering. Additionally, there is a special conversion that at maturity, unless the Company repays all outstanding principal and interest, the Notes shall be automatically converted into a number of shares of common stock of the Company at 80% of the then fair market value per share.
As a result of this beneficial conversion feature, the Company has recorded $40,000 as a debt discount. The debt discount is being amortized to interest expense over the term of the Notes. The amortization expense related to the debt discount was $2,628 for the year ended December 31, 2012 and for the cumulative period from August 18, 2011 (date of inception) to December 31, 2012. The note holders as a group also have the right to purchase up to an aggregate of $1,000,000 in shares in the event of a subsequent offer of equity securities.
F-22
BioPharmX, Inc.
(a development stage enterprise)
Notes to Financial Statements
as of December 31, 2012 and 2011, and, cumulatively, for the period
from August 18, 2011 (date of inception) to December 31, 2012
____________
| 7. | Stockholders’ Equity |
Issuance of common stock
On August 18, 2011, the Company sold 7,400,000 shares of its Common Stock to its four founders. The fair value of the shares was $0.0001 per share, the initial par value of the Company’s Common Stock, the date of the sale.
Equity Incentive Plan
On August 18, 2011, the Company adopted the 2011 Equity Incentive Plan (the “Plan”) which permits the Company to grant stock options to directors, officers or employees of the Company or others to purchase shares of common stock of the Company through awards of incentive and nonqualified stock options (“Options”), stock (“Restricted Stock” or “Unrestricted Stock”) and stock appreciation rights (“SARs”).
The Company currently has time-based options outstanding. The time-based options generally vest in two to four years and expire ten years from the date of grant. Total number of shares reserved and available for grant and issuance pursuant to this Plan is 2,600,000. Shares issued under the Plan will be drawn from authorized and unissued shares or shares now held or subsequently acquired by the Company. At December 31, 2012 there were 1,450,000 shares available for grant under the Plan. No options were granted during the year ended December 31, 2011.
The following table summarizes the Company’s stock option activities for the year ended December 31, 2012:
| Weighted | ||||||||||||||||
| Weighted | Average | |||||||||||||||
| Number | Average | Remaining | Aggregate | |||||||||||||
| of | Exercise Price | Contractual | Intrinsic | |||||||||||||
| Shares | Per Share | Life (Years) | Value | |||||||||||||
| Outstanding at January 1, 2012 | - | $ | - | - | $ | - | ||||||||||
| Granted | 1,150,000 | 0.06 | - | - | ||||||||||||
| Exercised | - | - | - | - | ||||||||||||
| Forfeited | - | - | - | - | ||||||||||||
| Outstanding at December 31, 2012 | 1,150,000 | $ | 0.06 | 9.3 | $ | 75,000 | ||||||||||
| Vested and expected to vest at December 31, 2012 | 1,150,000 | $ | 0.06 | 9.3 | $ | 75,000 | ||||||||||
| Vested at December 31, 2012 | - | $ | - | - | $ | - | ||||||||||
F-23
BioPharmX, Inc.
(a development stage enterprise)
Notes to Financial Statements
as of December 31, 2012 and 2011, and, cumulatively, for the period
from August 18, 2011 (date of inception) to December 31, 2012
____________
| 7. | Stockholders’ Equity, continued |
Equity Incentive Plan, continued
Information regarding options outstanding as of December 31, 2012 is as follows:
| Options Outstanding | Options Vested | |||||||||||||||||||
| Weighted | ||||||||||||||||||||
| Range | Average | Weighted | Weighted | |||||||||||||||||
| of | Remaining | Average | Average | |||||||||||||||||
| Exercise | Number | Contractual | Exercise | Number | Exercise | |||||||||||||||
| Prices | Outstanding | Life (Years) | Price | Outstanding | Price | |||||||||||||||
| $0.05 | 1,000,000 | 9.3 | $ | 0.05 | - | $ | 0.05 | |||||||||||||
| $0.13 | 150,000 | 9.9 | 0.13 | - | 0.13 | |||||||||||||||
| 1,150,000 | 9.3 | $ | 0.06 | - | $ | 0.06 | ||||||||||||||
Additional Stock Plan Information
The fair value of stock-based awards to employees is calculated through the use of the Black-Scholes option pricing model, even though such model was developed to estimate the fair value of freely tradable, fully transferable options without vesting restrictions, which differ significantly from the Company’s stock option awards. This model also requires subjective assumptions, including future stock price volatility and expected time to exercise, which greatly affect the calculated values.
The following assumptions were used in the estimated grant date fair value calculations for Stock Awards during the year ended December 31, 2012 and for the cumulative period from August 18, 2011 (date of inception) to December 31, 2012:
| Expected term | 5.52 - 6.08 years | |||
| Risk-free interest rate | 0.67% - 0.89 | % | ||
| Volatility | 82.08 | % | ||
| Dividend yield | - | |||
| Weighted average grant date fair value | $ | 0.05 | ||
Valuation and Amortization Method
The Company estimates the fair value of time-based stock options, if any, granted using the Black-Scholes-Merton option pricing formula. The fair value is then amortized on a straight-line basis over the requisite service periods of the awards, which is generally the vesting period. Time-based and performance-based options, if any, typically have a ten-year life from date of grant and vesting periods of two to four years.
F-24
BioPharmX, Inc.
(a development stage enterprise)
Notes to Financial Statements
as of December 31, 2012 and 2011, and, cumulatively, for the period
from August 18, 2011 (date of inception) to December 31, 2012
____________
| 7. | Stockholders’ Equity, continued |
Expected Term
The expected term represents the period that the Company’s stock-based awards are expected to be outstanding. For awards granted subject only to service vesting requirements, the Company utilizes the simplified method for estimating the expected term of the stock-based award, instead of historical exercise data.
Expected Volatility
The Company uses the historical volatility of the price of the common shares of selected public companies in the biotechnology sector.
Expected Dividend
The Company has never paid dividends on its common shares and currently does not intend to do so and, accordingly, the dividend yield percentage is zero for all periods.
Risk-Free Interest Rate
The Company bases the risk-free interest rate used in the Black-Scholes-Merton valuation method upon the implied yield curve currently available on U.S. Treasury zero-coupon issues with a remaining term equal to the expected term used as the assumption in the model.
The following table summarizes the stock-based compensation by line item as follows (in thousands):
| Cumulative for | ||||||||||||
| the period from | ||||||||||||
| August 18, 2011 | ||||||||||||
| (date of inception) to | ||||||||||||
| 2012 | 2011 | December 31, 2012 | ||||||||||
| Research and development | $ | 4,616 | $ | - | $ | 4,616 | ||||||
| Selling, general and administrative | 4,241 | - | 4,241 | |||||||||
| Total stock-based compensation expense | $ | 8,857 | $ | - | $ | 8,857 | ||||||
Stock-based compensation expense recognized in the statements of operations and comprehensive loss is based on awards ultimately expected to vest. Forfeitures are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates.
F-25
BioPharmX, Inc.
(a development stage enterprise)
Notes to Financial Statements
as of December 31, 2012 and 2011, and, cumulatively, for the period
from August 18, 2011 (date of inception) to December 31, 2012
____________
| 8. | Commitments and Contingencies |
Lease Commitments
During the years ended December 31, 2012 and 2011, the Company’s leased facilities were on a month-to-month basis. Total rent expense was $9,920, $1,058 and $10,978 for the years ended December 31, 2012 and 2011, and for the cumulative period from August 18, 2011 (date of inception) to December 31, 2012, respectively.
Contingencies
The Company is involved from time to time with claims or legal actions in the ordinary course of business. Management does not believe that the impact of such matters will have a material adverse effect on the Company’s financial condition or results of operations.
| 9. | Income Taxes |
No federal income taxes were provided in the years ended December 31, 2012 or 2011 or for the cumulative period from August 18, 2011 (date of inception) to December 31, 2012 due to the Company’s net losses. State minimum income and franchise taxes are included in general and administrative expenses and were immaterial for the periods presented.
At December 31, 2012, the Company had available federal net operating loss (“NOL”) carryforwards of approximately $60,000 which will begin to expire in 2031 and California state NOL carryforwards of approximately $60,000 which will begin to expire in 2021. At December 31, 2012 and 2011, the net deferred tax assets of approximately $29,000 and $0, generated primarily by NOL carryforwards, have been fully reserved due to the uncertainty surrounding the realization of such benefits. The net valuation allowance increased by approximately $29,000 and $0 during the years ended December 31, 2012 and 2011, respectively.
Current tax laws impose substantial restrictions on the utilization of net operating loss and credit carryforwards in the event of an “ownership change,” as defined by the Internal Revenue Code. If there should be an ownership change, the Company’s ability to utilize its carryforwards could be limited.
As of December 31, 2012 and 2011, the Company did not have any material unrecognized tax benefits. The 2012 and 2011 tax years remain open for examination by the federal and state authorities.
| 10. | Subsequent Events |
During the first nine months of 2013, the Company issued additional convertible notes payable to nine individuals in exchange for $630,000 cash on similar terms to the Notes issued in 2012. These convertible notes payable carry an interest rate of 6% per annum and mature at various times in 2016 with principal and interest payable at maturity.
F-26
BioPharmX, Inc.
(a development stage enterprise)
Notes to Financial Statements
as of December 31, 2012 and 2011, and, cumulatively, for the period
from August 18, 2011 (date of inception) to December 31, 2012
____________
| 10. | Subsequent Events, continued |
In March of 2013, the Company signed a collaboration and licensing agreement (“CLA”) with a biotechnology company with molecular iodine technology (the “Licensor”). The CLA terms included a $150,000 advanced profit sharing payment in exchange for exclusive licensing and patent indemnification rights to the Licensor’s molecular iodine technology. The payment schedule included $50,000 upon signing and an additional $100,000 to be paid in monthly increments of $10,000 starting October 15, 2013. Additionally, the terms included profit sharing and royalty fees related to future revenues generated from the technology product pipeline.
On August 23, 2013, the Company signed a lease for 10,800 square feet of office and laboratory space in Menlo Park, California. The term of the lease is 39 months from the lease commencement date of September 1, 3013.
The Company has evaluated all events occurring subsequent to December 31, 2012 through October 18, 2013, which is the date these financial statements were available to be issued, and did not identify any additional material recognizable subsequent events.
F-27
UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL STATEMENTS
Thompson Designs, Inc.
and
BioPharmX, Inc.
(A Development Stage Company)
Proforma Condensed Combined Balance Sheets
As of September 30, 2013 (Note 1)
(Unaudited)
BioPharmX, Inc. at September 30, 2013 | Notes | Thompson Designs Inc. at September 30, 2013 | Adjustments | Notes | Pro Forma | |||||||||||||
| ASSETS: | ||||||||||||||||||
| Cash | $ | 24,059 | $ | 162 | $ | (162 | ) | $ | 24,059 | |||||||||
| Prepaid expenses and other current assets | 142,481 | - | 142,481 | |||||||||||||||
| TOTAL CURRENT ASSETS | 166,540 | 162 | (162 | ) | 166,540 | |||||||||||||
| Property and equipment, net | 29,972 | - | 29,972 | |||||||||||||||
| Intellectual property | 150,000 | - | 150,000 | |||||||||||||||
| Other Assets | 50,000 | - | 50,000 | |||||||||||||||
| TOTAL ASSETS | $ | 396,512 | $ | 162 | $ | (162 | ) | $ | 396,512 | |||||||||
| LIABILITIES AND STOCKHOLDERS DEFICIT: | ||||||||||||||||||
| CURRENT LIABILITIES: | ||||||||||||||||||
| Accounts payable and accrued expenses | $ | 259,476 | $ | 120,032 | $ | (120,032 | ) | $ | 259,476 | |||||||||
| Related party payables | 139,280 | 116 | (116 | ) | 139,280 | |||||||||||||
| Convertible notes payable | 100,000 | - | - | 100,000 | ||||||||||||||
| TOTAL CURRENT LIABILITIES | 498,756 | 120,148 | (120,148 | ) | 498,756 | |||||||||||||
| Convertible notes payable | 590,207 | - | - | 590,207 | ||||||||||||||
| Promissory note – related party | - | 2,925 | (2,925 | ) | - | |||||||||||||
| Other long-term liabilities | 20,717 | - | - | 20,717 | ||||||||||||||
| TOTAL LIABILITIES | 1,109,680 | 123,073 | (123,073 | ) | 1,109,680 | |||||||||||||
| SHAREHOLDERS' DEFICIT | ||||||||||||||||||
| Common stock | 703 | 9,000 | (678 | ) | (2) | 9,007 | ||||||||||||
| Additional paid-in-capital | 202,813 | 13,000 | 678 | (2) | 216,509 | |||||||||||||
| Deficit accumulated during the development stage | (916,684 | ) | (144,911 | ) | 122,911 | (938,684 | ) | |||||||||||
| TOTAL SHAREHOLDERS' DEFICIT | (713,168 | ) | (122,911 | ) | 122,911 | (713,168 | ) | |||||||||||
| TOTAL LIABILITIES AND SHAREHOLDERS' DEFICIT | $ | 396,512 | $ | 162 | $ | (162 | ) | $ | 396,512 | |||||||||
F-28
Thompson Designs, Inc.
and
BioPharmX, Inc.
(A Development Stage Company)
Proforma Condensed Combined Statements of Operations
For the Six Months Ended September 30, 2013 (Note 1)
(Unaudited)
BioPharmX, Inc. Nine Months Ended September 30, 2013 | Notes | Thompson Designs Inc. Nine Months Ended September 30, 2013 | Adjustments | Notes | Pro Forma | |||||||||||||
| OPERATING EXPENSES: | ||||||||||||||||||
| Research and development | $ | 400,726 | $ | - | $ | 400,726 | ||||||||||||
| Sales and marketing | 72,728 | - | 72,728 | |||||||||||||||
| General and administrative | 306,406 | 49,524 | (49,524 | ) | 306,406 | |||||||||||||
| TOTAL OPERATING EXPENSES | 779,860 | 49,524 | (49,524 | ) | 779,860 | |||||||||||||
| Other income (expense) - Interest expense | (41,729 | ) | - | (41,729 | ) | |||||||||||||
| NET LOSS | $ | (821,589 | ) | $ | (49,524 | ) | $ | 49,524 | $ | (821,589 | ) | |||||||
| PER SHARE INFORMATION - BASIC AND FULLY DILUTED (Note 5): | ||||||||||||||||||
| Weighted average shares outstanding | 9,0250,000 | |||||||||||||||||
| Net loss per share, basic and fully diluted | $ | (0.09 | ) | |||||||||||||||
F-29
Thompson Designs, Inc.
and
BioPharmX, Inc.
(A Development Stage Company)
Proforma Condensed Combined Statements of Operations
For the period October 31, 2012 (Inception) to December 31, 2012
(Unaudited)
BioPharmX, Inc. period August 18, 2011 (Inception) to September 30, 2013 | Notes | Thompson Designs Inc. period August 30, 2010 (Inception) to September 30, 2013 | Adjustments | Notes | Pro Forma | |||||||||||||
| OPERATING EXPENSES: | ||||||||||||||||||
| Research and development | $ | 435,477 | $ | - | $ | 435,477 | ||||||||||||
| Sales and marketing | 82,011 | - | 82,011 | |||||||||||||||
| General and administrative | 352,272 | 144,419 | (144,419) | 352,272 | ||||||||||||||
| TOTAL OPERATING EXPENSES | 869,760 | 144,419 | (144,419) | 869,760 | ||||||||||||||
| Other income (expense) - Interest expense | (46,924 | ) | 3 | (46,924 | ) | |||||||||||||
| NET LOSS | $ | (916,684 | ) | $ | (144,416 | ) | $ | 144,419 | $ | (916,684 | ) | |||||||
| PER SHARE INFORMATION - BASIC AND FULLY DILUTED (Note 5): | ||||||||||||||||||
| Weighted average shares outstanding | 9,025,000 | |||||||||||||||||
| Net loss per share, basic and fully diluted | $ | (0.10 | ) | |||||||||||||||
F-30
Thompson Designs, Inc.
and
BioPharmX, Inc.
(A Development Stage Company)
Notes to Proforma Condensed Combined Financial Statements
(Unaudited)
Note 1. Financial Periods for Financial Statements
Thompson Designs Inc., a Nevada corporation (“Parent”) had a fiscal year ending September 30 during the periods presented. The most recent financial information available for Parent is for the year ended September 30, 2013. There has been minimal operating activity in Parent after September 30, 2013. As a result, the information presented for Parent as of September 30, 2013 is deemed to be current for these proforma condensed combined financial statements. As of January 23, 2014, Parent changed its fiscal year to a calendar year basis. BioPharmX, Inc., a Delaware corporation (the “Company”) reports on a calendar year basis and is utilizing financial statements as of September 30, 2013 for these proforma condensed combined financial statements.
Note 2. Reverse Merger Transaction
On January 23, 2014, the Company, BPMX and stockholders of BPMX who collectively own 100% of BPMX (the “BPMX Stockholders”) entered into and consummated transactions pursuant to a Share Exchange Agreement (the “Share Exchange Agreement,” such transaction referred to as the “Share Exchange Transaction”), whereby the Company issued to the BPMX Stockholders an aggregate of 7,025,000 shares of its common stock, par value $.001 (“Common Stock”), in exchange for 100% of equity interests of BPMX held by the BPMX Stockholders. The shares of our Common Stock received by the BPMX Stockholders in the Share Exchange Transaction constitute approximately 77.8% of our issued and outstanding Common Stock giving effect to the issuance of shares pursuant to the Share Exchange Agreement. As a result of the Share Exchange Transaction, BPMX became a subsidiary of the Company.
For financial reporting purposes, the transaction will be accounted for as a “reverse merger” rather than a business combination, because the sellers of the Company effectively control the combined companies immediately following the transaction. As such, the Company is deemed to be the accounting acquirer in the transaction and, consequently, the transaction is being treated as a reverse acquisition by the Company. Accordingly, the assets and liabilities and the historical operations that will be reflected in Parent’s ongoing financial statements will be those of the Company and will be recorded at the historical cost basis of the Company. The historical financial statements of Parent before the transaction will be replaced with the historical financial statements of the Company before the transaction in all future filings with the Securities and Exchange Commission, or SEC. The Merger is intended to be treated as a tax-free exchange under Section 368(a) of the Internal Revenue Code of 1986, as amended.
Note 3. Earnings Per Share
The proforma weighted average shares outstanding gives effect to the issuance of 9,025,000 shares of common stock in connection with the Merger as if it occurred at the beginning of the periods presented and the 9,025,000 shares outstanding in Parent post-merger.
The effect of any potentially dilutive instruments including options were anti-dilutive. Therefore, dilutive earnings per share are equivalent to basic earnings per share.
F-31
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Thompson Designs, Inc. | ||
| Date: January 27, 2014 | By: | /s/ James R Pekarsky |
| Name: James R. Pekarsky | ||
| Title: Chief Executive Officer | ||
21